Abstract
Our objectives are to demonstrate whether the kynurenine pathway is activated in diarrhea-type irritable bowel syndrome (IBS-D) patients, and whether the neurotoxic metabolite quinolinic acid (QUIN) is out of balance with the neuroprotective metabolite kynurenic acid (KYNA), and further explore whether this can lead to increased expression of N-methyl D-aspartate receptor 2B (NMDAR2B) in the enteric nervous system and in turn leads to intestinal symptoms and mood disorders. All enrolled healthy controls and patients accepted IBS Symptom Severity Scale (IBS-SSS) score, Self-rating Depression Scale (SDS) and Self-rating Anxiety Scale (SAS) anxiety and depression scores, and also underwent colonoscopy to collect ileum and colonic mucosa specimens. The expression of NMDAR2B in intestinal mucosa was detected by immunofluorescence, and fasting serum was collected to detect the tryptophan, kynurenine (KYN), KYNA and QUIN by high performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS). Our results showed that the KYN pathway of IBS-D patients was activated. The production of QUIN and KYNA was imbalanced and resulting in an increased NMDAR2B for patients with IBS-D, which may be involved in intestinal symptoms and mood disorders of IBS-D.
Key words: Irritable bowel syndrome, kynurenine pathway, kynurenic acid, quinolinic acid, N-methyl Daspartate receptor
Introduction
Irritable Bowel Syndrome (IBS) is often associated with mental disorders with metabolic changes in 5-hydroxy tryptamine (5- HT). About 95% of tryptophan (Trp), which is a precursor of 5-HT, is metabolized by the kynurenine (KYN) pathway. In the lowinflammation state of IBS intestinal mucosa, indoleamine 2,3- dioxygenase 1 (IDO1) can promote the production of KYN by Trp along with the KYN pathway. IDO1 can also further produce the neurotoxic metabolite quinolinic acid (QUIN) and the neuroprotective metabolite kynurenic acid (KYNA). The imbalance between these two compounds can lead to excessive excitability of the intestinal neurons. In addition, the increased excitatory neurotransmitter and glutamate can act on the N-methyl-D-aspartate receptor (N-methyl-D-aspartate receptor, NMDAR) to induce NMDAR dysfunction in different subtypes. Since the NMDAR subtype with the strongest affinity for glutamate, NMDAR2B can mediate intestinal hyperactivity and high sensitivity to pain. However, its expression and significance in IBS-D patients are still unclear.
Therefore, this study aimed to investigate whether there is an imbalance between neurotoxic and neuroprotective metabolites in patients with diarrhea-type IBS (IBS-D), and whether it affects the expression of NMDAR2B in the enteric nervous system by detecting Trp, KYN, QUIN and KYNA levels in the serum and NMDAR2B expression in the intestinal mucosa. Furthermore, we also explored the relationship between abnormalities in the KYN pathway metabolism, intestinal symptoms and mood disorders in patients with IBS-D.
Materials and Methods
Patients and samples
According to the Rome IV diagnostic criteria of IBS-D, 30 IBS-D patients who were treated in the Department of Gastroenterology of Hebei General Hospital from 2018-5 to 2018-12 were selected. Those suffering from organic diseases of the digestive tract with more serious symptoms of anxiety and depression or other mental illnesses were left out. At the same time, we selected 30 healthy people at the physical examination center as the control group. All the people were graded on the Symptom Severity Scale (IBS-SSS), Self-rating Depression Scale (SDS) and Self-rating Anxiety Scale (SAS). The IBS-SSS scale can assess the severity of abdominal symptoms and its impact on life in patients with IBS, which has high sensitivity and reliability. The scoring points with 75-175, 175-300 and >300 were identified as mild, moderate and severe, respectively. SAS and SDS are used to evaluate the anxiety and depression of hospitals in general. According to the standard, SAS was divided into light, medium and heavy grades. The grade 1, 2 and 3 are related with 53-62, 63-72 and >72 scores, respectively. For SDS, grade 1, 2 and 3 are for 50-60, 61-70 and >70 scores, respectively. Then, we collected the serum specimens and stored at -80°C for later use. One piece of intestinal mucosa with a size of about 0.2 cm × 0.2 cm × 0.2 cm was separately taken with a disposable biopsy forceps at the small intestine 10 cm from the end of the ileum and transverse colon. They were fixed in formalin fixative solution, then packed, labeled, embedded in paraffin, and sliced for later use. All procedures performed were in accordance with the ethical standards of the Human Ethics Committee of Hebei General Hospital, Hebei, China, and of the Institutional Research Committee and with the 1964 Helsinki declaration and its latest amendment.
High performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS)
The HPLC-MS/MS system contains mass spectrometer (UltiMate 3000 RS, Thermo Fisher Scientific, China) coupled with chromatogram equipment (UltiMate 3000 RS, Thermo Fisher Scientific). Reference standards of Trp, KYN, KYNA, QUIN were purchased from Sigma-Aldrich (St. Louis, MO, USA). The chromatographic separation was carried out in an Hypersil GOLD (100×2.1 mm ×1.9 μm) at a flow rate of 0.30 mL/min. Water and methanol with acetonitrile formate (0.1%) and formic acid (0.1 % v/v) were used as mobile phase solvents. A gradient elution was used for the chromatographic separation of the analytes. The percentage of the organic solvent linearly changed as follows: 0 min, 5%; 4 min, 95%; 5 min, 95%; 5.1 min, 5%; 7 min, 5%. We then accurately took 50 μL serum, added with 150 μL formic acid methanol precipitation protein, swirled and shocked for 1 min, and centrifuged at 12,000 rpm for 10 min. The supernatant was used for sample analysis. Chromatogram collection and integration of the compounds were processed by software Xcilabur 3.0 (Thermo Fisher Scientific). The linear regression was performed with 1/x2 as the weighting coefficient (Figure 1).
Immunofluorescence
The paraffin sections of the mucosa were dewaxed to water, antigen retrieval, autofluorescence quenching in the circle, and serum blocking. The primary antibody was added with PBS to the sections, and the sections were placed in a wet box and incubated at 4°C overnight. The slides were immersed in PBS (pH 7.4) 3 times for 5 min each. After slightly drying, the secondary antibody with the corresponding species of the primary antibody was added dropwise in the circle to cover the tissue, and incubated for 50 min at room temperature in the dark. DAPI was used to stain the nucleus. The sections were sealed, and then examined under Nikon inverted fluorescence microscope to collect images. Use Image- Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) to convert blue/red fluorescent monochrome photos to black and white and then select the same black as the uniform standard for judging all photos positive. For each photo, analysis was performed to obtain the integral optical density (IOD) and the pixel area (AREA) of the tissue. The average optical density value (average optical, AO value) was calculated as IOD / AREA. The larger the AO value is associated with the higher the fluorescence intensity per unit area.
Statistical analysis
All data were processed by SPSS 22.0 software. Normal distribution and homogeneity of variance were tested for all measurement data. The measurement results were expressed as mean ± standard deviation. The t-test and non-parametric test were used for comparison between groups (QUIN/KYNA satisfied normality, but did not satisfied the homogeneity test of variance, so the nonparametric test was used). The correlation was analyzed by Pearson correlation. P<0.05 was considered statistically significant.
Results
Metabolite levels alternated in kynurenine pathway
The ion spectrum of Trp, KYN, KYNA and QUIN was shown in Figure 1. There was no significant difference of serum Trp between the IBS-D group and the healthy control group (P>0.05).
The serum KYN and QUIN was higher in the IBS-D group than that in the healthy control group (P<0.05), but the serum KYNA was lower in the healthy control group (P<0.05). For the ratio of metabolites, the serum KYN/Trp ratio of IBS-D group was higher than that of healthy control group (P<0.05), but KYNA/KYN ratio was lower than that of healthy control group (P<0.05). The QUIN/KYN ratio was not statistically different from healthy control group (P>0.05). The ratio of serum QUIN/KYNA in IBS-D group was higher than that in healthy control group (P<0.05) (Table 1).
Figure 1.
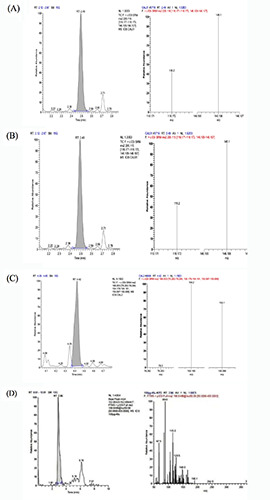
Extraction of ion chromatogram from the lower quantitative limit of (A) Trp (0.001 μg/mL), (B) KYN (0.001 μg/mL), (C) QUIN (10 ng/mL) and (D) KYNA (0.1 ng/mL). Left figure: first-order spectra; right figure: second-order spectra.
Table 1.
The level of serum kynurenine pathway metabolites in IBS-D and healthy control groups (x±s, n=30).
| Groups | Control | IBS-D | t-value | P-value |
|---|---|---|---|---|
| Trp | 5765.196±1254.989 | 5756.037±865.281 | 0.013 | 0.990 |
| KYN | 213.561±46.563 | 294.406±53.812 | -2.437 | 0.021 |
| KYNA | 13.383±2.490 | 9.812±1.665 | 2.556 | 0.016 |
| QUIN | 72.207±11.379 | 96.579±20.981 | -2.190 | 0.037 |
| KYN/Trp | 0.038±0.004 | 0.053±0.008 | -3.446 | 0.002 |
| QUIN/KYNA | 5.836±1.312 | 11.121±3.293 | -3.198 | 0.005 |
| QUIN/KYN | 0.386±0.102 | 0.328±0.045 | 1.129 | 0.268 |
| KYNA/KYN | 0.070±0.018 | 0.038±0.011 | 3.242 | 0.003 |
Table 2.
Correlation between the imbalance of kynurenine pathway metabolism and IBS-SSS score in IBS-D group (x±s, n=30).
| Variable | SSS | P-value |
|---|---|---|
| KYN/Trp | 0.388 | 0.153 |
| QUIN/KYNA | 0.795 | <0.001 |
| QUIN/KYN | 0.269 | 0.332 |
| KYNA/KYN | -0.763 | 0.001 |
Table 3.
Correlation between the imbalance of kynurenine pathway metabolism and SDS score in IBS-D group (x±s, n=30).
| Variable | SDS | P value |
|---|---|---|
| KYN/Trp | 0.559 | 0.030 |
| QUIN/KYNA | 0.655 | 0.008 |
| QUIN/KYN | 0.193 | 0.490 |
| KYNA/KYN | -0.734 | 0.002 |
Table 4.
Correlation between the imbalance of kynurenine pathway metabolism and SAS score in IBS-D group (x±s, n=30).
| Variable | SAS | P value |
|---|---|---|
| KYN/Trp | 0.487 | 0.065 |
| QUIN/KYNA | 0.691 | 0.004 |
| QUIN/KYN | 0.154 | 0.584 |
| KYNA/KYN | -0.697 | 0.004 |
Table 5.
Expression level of NMDAR2B in IBS-D and healthy control groups (x±s, n=30).
| Groups | Control | IBS-D | t-value | P-value |
|---|---|---|---|---|
| Ileum NMDAR2B | 0.008±0.003 | 0.014±0.004 | -2.478 | 0.021 |
| Colon NMDAR2B | 0.017±0.003 | 0.031±0.006 | -4.594 | <0.001 |
Table 6.
Expression level of NMDAR2B in ileum and colon mucosa (x±s, n=30).
| Groups | Ileum | Colon | t-value | P-value |
|---|---|---|---|---|
| Control | 0.008±0.003 | 0.017±0.003 | -4.529 | <0.001 |
| IBS-D | 0.014±0.004 | 0.031±0.006 | -5.188 | <0.001 |
The serum KYN and QUIN was higher in the IBS-D group than that in the healthy control group (P<0.05), but the serum KYNA was lower in the healthy control group (P<0.05). For the ratio of metabolites, the serum KYN/Trp ratio of IBS-D group was higher than that of healthy control group (P<0.05), but KYNA/KYN ratio was lower than that of healthy control group (P<0.05). The QUIN/KYN ratio was not statistically different from healthy control group (P>0.05). The ratio of serum QUIN/KYNA in IBS-D group was higher than that in healthy control group (P<0.05) (Table 1).
Rating scale changed in different groups
IBS-SSS, SAS and SDS scores in the IBS-D group were higher than those in the healthy control group (P<0.05). There was no correlation between the serum KYN/Trp ratio and the IBS-SSS symptom score in the IBS-D group (P>0.05), neither was the SAS score, but the QUIN/KYNA ratio was positively correlated with the IBSSSS symptom score and SAS score (P<0.05). The results proved that the serum KYN/Trp and QUIN/KYNA ratios in the IBS-D group were positively correlated with the SDS score (P<0.05). This also proved that KYNY and QUIN imbalance was associated with clinical symptoms and mood disorders in IBS-D patients. KYNA/KYN ratio in the IBS-D group was negatively correlated with IBS-SSS symptom score, and SAS and SDS anxiety and depression score (P<0.05). As shown, there was no significant correlation between QUIN/KYN ratio, that is, the activation of KYN in IBS-D group and IBS-SSS, SAS and SDS scores (P>0.05) (Tables 2,3 and 4).
Altered expression of mucosal NMDAR2B contribute to IBS-D
The expression of NMDAR2B in the ileum and colonic mucosa of the IBS-D group was higher than that in the healthy control group (P<0.05). The expression of NMDAR2B in the colon was higher than that of the ileum for both the IBS-D group and control group (P<0.05) (Figures 2 and 3) (Tables 5 and 6). There was no correlation between the expression of NMDAR2B in the ileum mucosa of IBS-D group and serum KYNA and QUIN levels (P>0.05). The expression of NMDAR2B in colonic mucosa was negatively correlated with serum KYNA level (P<0.05), but positive correlation with QUIN level (P<0.05) The expression of NMDAR2B in ileum and colonic mucosa were significantly positively correlated with QUIN/KYNA ratio (P<0.05). In addition, the expression of NMDAR2B in ileum and colonic mucosa of IBS-D group was positively correlated with the scores of IBS-SSS symptom, SAS and SDS (P<0.05) (Tables 7 and 8).
Discussion
Irritable bowel syndrome (IBS) is a common functional bowel disease with typical abdominal pain. Most of them often have emotional disorders such as depression and anxiety. There are several mechanisms to explain the pain, anxiety and depression of IBS patients. Then, euroimmune-mediated mechanism was recognized to be responsible for pain and emotional comorbidities. This provides treatment options beyond traditional antidepressant and antianxiety drugs.
In astrocytes, brain endothelial cells and neurons, KYN can produce KYNA under the action of kynurenine aminotransferases (KAT), whereas in microglia and macrophages, kynurenine-3- monooxidase (KMO) metabolizes KYN to form neurotoxic 3- hydroxy kynurenine (3-HK), 3-hydroxyanthranilic acid (3-HAA) and QUIN. Among them, 3-HK can promote the production of reactive oxygen substances (ROS), thereby increasing oxidative stress and inducing apoptosis, which is harmful to several cell types.1 QUIN exhibits neurotoxicity through at least three different mechanisms, including excitatory toxicity of NMDAR activation, ROS formation, and cytoskeletal instability. Most of its neurobehavior can be blocked by KYNA. The imbalance between QUIN and KYNA compounds is associated with a variety of diseases. There is a growing interest in the role of metabolites in the immune system, and researchers have found that they may also be involved in the pathogenesis of functional gastrointestinal diseases. Inflammation may be a common cause of abdominal pain and mood disorders in patients with IBS, which initiates the kynurenine pathway and triggers a shift from illness to mood disorder, from acute pain to chronic pain. The overproduction of QUIN enhances glutamatergic neurotransmission mediated through NMDAR.2We expect KYN pathway to be a key regulator of maintaining intestinal homeostasis. IDO is one of the major kynurenine pathway enzymes, and is immunoreactive. It is widely distributed in peripheral tissues and human intestinal tract contains relatively more IDO.3 IDO has two isoforms including IDO1 and IDO2. IDO1 is widely expressed at low levels in a variety of tissues under normal conditions and is the major isoform that causes Trp degradation. Furthermore, it is inducible and can be activated by multiple inflammatory cytokines with increased expression. The interferon- γ is the strongest stimulator. IDO2 appears to be basal expression.4 Therefore, the expression of IDO1 was significantly increased in pathological colon biopsies of patients with inflammatory bowel disease.5 Up regulation of IDO1 induces metabolic turnover, which may also involve the pathogenesis of IBS. To date, studies have shown that IDO activity has increased in both IBS male and female subjects,6 which can be expressed by KYN/Trp. And the severity of IBS disease is positively correlated with the KYN/Trp ratio. Patients with severe IBS symptoms have an increased Trp shunt along KYN pathway, which leads to abnormal 5-HT function. In addition, the altered 5-HT energy symptoms are directly related to IBS intestinal function.7 In this study, the KYN/Trp ratio was significantly increased in patients with IBS-D compared with healthy controls, suggesting that there may be low intestinal inflammation in patients with IBS-D. However, there was no correlation between the KYN/Trp ratio and the IBS-SSS score, which may be related to the small sample size. It is necessary to further expand the sample size to investigate whether the activation of KYN pathway is involved in the occurrence of intestinal symptoms. While it is still unclear how excessive circulating KYN causes anxiety and depression in IBS patients with IDO1 activation. The preclinical models confirm that IDO1 activation increases the risk of transition from disease to depression. As in neurological diseases (although to a lesser extent), increased KYN influx from the periphery increases the ratio of the neurotoxic metabolites (3-hydroxy kynurenine [3-HKYN] and QUIN) in the brain, which may increase neuronal vulnerability and adverse effects on the viability of newly produced hippocampal neurons, leading to depressive symptoms.8 Moreover, the IDO1 activation can predict the severity of depression, although IDO1 activation is usually detected in the periphery. Furthermore, the activation of IDO can also be observed when measuring Trp, KYN and kynurenine metabolites in cerebrospinal fluid.9 These findings indicate a certain degree of overlap of IDO1 activity between brain and peripheral activity. The KYN/Trp ratio in this study was positively correlated with the SDS score, but not correlated with the SAS score, indicating the activation of the KYN pathway in patients with IBS-D is associated with depression.
Figure 2.
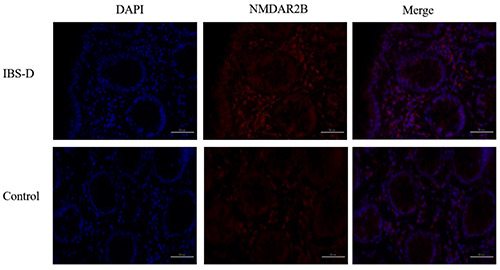
Immunofluorescence of NMDAR2B in the colonic mucosa of IBS-D and control groups (400x).
Table 7.
Correlation between NMDAR2B expression in the ileum mucosa and imbalance of kynurenine metabolism and IBS-SS, SDS and SAS scores in IBS-D group (x±s, n=30).
| Variable | Ileum NMDAR2B | P-value |
|---|---|---|
| KYNA | -0.499 | 0.058 |
| QUIN | 0.596 | 0.019 |
| QUIN/KYNA | 0.823 | <0.001 |
| SSS | 0.748 | 0.001 |
| SDS | 0.545 | 0.036 |
| SAS | 0.766 | 0.001 |
Table 8.
Correlation between NMDAR2B expression in the colonic mucosa and imbalance of kynurenine metabolism and IBS-SSS, SDS and SAS scores in IBS-D group (x±s, n=30).
| Variable | Colon NMDAR2B | P-value |
|---|---|---|
| KYNA | -0.796 | <0.001 |
| QUIN | 0.453 | 0.090 |
| QUIN/KYNA | 0.893 | <0.001 |
| IBS-SSS | 0.579 | 0.024 |
| SDS | 0.561 | 0.030 |
| SAS | 0.651 | 0.009 |
The inflammatory response associated with the KYN pathway may also be involved in the pathogenesis of IBS patients based on the balance between pro-inflammatory, excitotoxic QUIN and antiinflammatory, neuroprotective KYNA, which may have profound effects on the excitability of intestinal neurons. The effects may affect bowel movements and sensory function. Excitotoxicity is a neuronal death caused by excessive or long-term activation of excitatory amino acid receptors. The main player in this process is glutamate, which acts on ionic NMDAR, a ligand gated ion channel that is highly permeable to Ca2+. NMDAR has four subtypes including NMDAR1, NMDAR2 and NMDAR3. Among them, NMDAR2 has two subtypes of NMDAR2A, NMDAR2B, NMDAR2C and NMDAR2D. Only the heteropolymer formed by NRNMDAR2 and NMDAR1 has the function of NMDAR, while glutamic acid has a strong affinity for NR2B, so the heteropolymer formed by NMDAR1 and NMDAR2B plays the most important role in nociceptive transmission. Increased glutamate leads to Ca2+ imbalance of homeostasis, mitochondrial dysfunction, and production of ROS and reactive nitrogen. It also increases neuronal excitability through the Ca2+-PKC-NO pathway, which is increasing and maintaining pain signals, and leading to central sensitization. 10 In addition, pain stimuli can induce different changes in NMDAR expression in different subtypes, such as increased expression of NMDAR2A and decreased expression of NMDAR2C. NMDAR2B mediates intestinal function as the most potent NMDAR subtype of glutamate, over-exercise and high pain sensitivity.11 However, its expression and significance in IBS-D patients is not clear. Therefore, this study further explored the changes of NBDAR2B expression in the intestinal nervous system and its relationship with intestinal symptoms and mood disorders.
Figure 3.
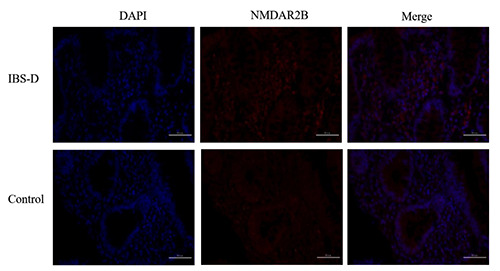
Immunofluorescence of NMDAR2B in the ileum mucosa of IBS-D and control groups (400x).
Under normal conditions, QUIN is the major end product of the KYN pathway in monocytes, including tissue macrophages,12 which act primarily through NMDAR-mediated excitotoxicity. QUIN is a weak endogenous agonist of NMDAR that acts selectively and involves receptor subtypes containing the NR2A and NR2B subunits. QUIN causes the greatest excitotoxic damage in the brain regions rich with NMDAR containing NMDAR2A and NMDAR2B subunits, which is mainly in the striatum and hippocampus. In addition, it can increase the release of glutamate from neurons to inhibit the uptake of glutamate by astrocytes, maintain elevated levels of glutamate, continuously stimulate NMDAR, and eventually lead to excitotoxicity. But, KYNA has neuroprotective effects. In the present study, serum QUIN was higher than healthy controls, and the QUIN/KYNA ratio was higher than that in the healthy control group, indicating that the metabolic branching enzyme activity of QUIN was enhanced. Moreover, this experiment demonstrated that the expression level of NMDAR2B in ileum and colonic mucosa of IBS-D patients was higher than that of the control, and it was positively correlated with the IBS-SSS symptom severity score. Those results indicated that overproduction of QUIN may lead to excessive excitatory neurotransmitter glutamate. While the body may mediate neuronal excitability by compensatory increasing the expression of NMDAR2B and cause abdominal pain symptoms and excessive intestinal movement in IBS-D patients. Expression of NMDAR2B mediates neuronal excitability and leads to abdominal pain symptoms and excessive intestinal movement in patients with IBS-D. Moreover, this study demonstrates that the expression of NMDAR2B in the colonic mucosa of IBS-D patients and healthy controls is higher than that of the ileum, and it is related to the effect of nutrient absorption on the ileum for the first time. In addition, ketamine, an NMDAR antagonist, can rapidly and significantly improve mood in patients with refractory depression and can target inflammation-induced depression.13 This is consistent with the findings of this study. The expression of NMDAR2B in the intestinal mucosa is directly proportional to the SAS and SDS anxiety and depression scores of patients. This finding further supports the imbalance of kynurenine metabolites participate in the occurrence of emotional disorders in patients with IBS-D by compensatory increasing NMDAR2B expression.
Studies have shown that KYNA reduced colonic motility in rats and dogs,14 but its exact role in intestinal motility is still largely unknown. KYNA is present in intestinal glutamatergic neurons and may cause intestinal peristalsis. Glutamate is likely to act as an excitatory and may regulate cholinergic transmission in ENS.15 Recent studies have shown that KYNA can reduce colonic movement, even if KYNA is moderately increased. It will lead to a rapid decrease in extracellular glutamate. The inhibition of KYNA synthesis will increase acetylcholine levels, thus speeding up intestinal movement.16 In addition, KYNA may also play a role in intestinal sensitivity mechanisms because it also acts as a glutamate antagonist, a sensitive mechanism that inhibits mucosal and vagal afferent tone.17 Therefore, KYNA may play an important role in regulating intestinal motility and pain sensitivity, which may be involved in the pathogenesis of IBS-D. Inhibition of intestinal NMDAR by KYNA or its analogues may lead to a decrease in glutamate release, which may affect the intestine. The expression of NMDAR2B, which has the strongest affinity for glutamate on neurons, provides a new option for intestinal hyperactivity and inflammatory processes. In this study, serum neuroprotective KYNA levels and KYNA/KYN ratios were significantly lower in the IBS-D group than in the control group, and negatively correlated with IBS-SSS intestinal symptom scores and SAS and SDS anxiety and depression scores, indicating the imbalance of KYNA and QUIN is involved in the development of intestinal symptoms and mood disorders in patients with IBS-D. In addition, the synergistic effect of KYNA and QUIN can increase the expression of NMDAR2B in intestinal neurons, and may increase the excitability of intestinal neurons through the Ca2+-PKC-NO pathway. Maintaining the pain threshold in patients with IBS-D leads to high sensitivity to pain and further involvement in intestinal and mood disorders in IBS-D patients.
IBS is a common functional gastrointestinal disease. This study further demonstrates that the KYN pathway is activated in IBS-D patients, and the neurotoxic metabolites are imbalanced with neuroprotective metabolites and serum QUIN levels are increased. However, the KYNA content is reduced. The synergistic effect of the two aspects causes an increase in the release of glutamate in the intestinal neurons, which may increase the intestinal dysmotility, high sensitivity of pain and mood disorders in patients with IBS-D by compensatory increasing the expression of NMDAR2B in the enteric nervous system. Therefore, the KYN pathway metabolic disorder may participate in the pathogenesis of IBS-D through NMDAR2B, as a medium for brain-gut interaction. It is helpful to further understand the neuroimmune mechanism behind the emotional disorder and abdominal pain complication in IBS-D patients, and produce effective drug targets.
References
- 1.Mole DJ, Webster SP, Uings I, Zheng X, Binnie M, Wilson K, et al. Kynurenine-3-monooxygenase inhibition prevents multiple organ failure in rodent models of acute pancreatitis. Nat Med 2016;22:202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker AK, Kavelaars A, Heijnen CJ, Dantzer R. Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev 2014;66:80-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamazaki F, Kuroiwa T, Takikawa O, Kido R. Human indolylamine 2,3-dioxygenase. Its tissue distribution, and characterization of the placental enzyme. Biochem J 1985;230:635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heitkemper MM, Han CJ, Jarrett ME, Gu H, Djukovic D, Shulman RJ, et al. Serum tryptophan metabolite levels during sleep in patients with and without irritable bowel syndrome (IBS). Biol Res Nurs 2016;18:193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf AM, Wolf D, Rumpold H, Moschen AR, Kaser A, Obrist P, et al. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol 2004;113: 47-55. [DOI] [PubMed] [Google Scholar]
- 6.Clarke G, McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. A distinct profile of tryptophan metabolism along the kynurenine pathway downstream of toll-like receptor activation in irritable bowel syndrome. Front Pharmacol 2012; 3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keszthelyi D, Troost FJ, Jonkers DM, Kruimel JW, Leue C, Masclee AA. Decreased levels of kynurenic acid in the intestinal mucosa of IBS patients: relation to serotonin and psycho- logical state. J Psychosom Res 2013;74:501-4. [DOI] [PubMed] [Google Scholar]
- 8.Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation 2011;8:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry 2010;15:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, et al. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci 2002;22:6208-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaszaki J, Erces D, Varga G, Szabo A, Vecsei L, Boros M. Kynurenines and intestinal neurotransmission: the role of Nmethyl- D-aspartate receptors. J Neural Transm (Vienna) 2012;119:211-23. [DOI] [PubMed] [Google Scholar]
- 12.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol 2003;81:247-65. [DOI] [PubMed] [Google Scholar]
- 13.Bohar Z, Toldi J, Fulop F, Vecsei L. Changing the face of kynurenines and neurotoxicity: therapeutic considerations. Int J Mol Sci 2015;16:9772-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez L, Roberts LD, LaRosa J, Heinz N, Gerszten R, Nurko S, et al. Relationship between postprandial metabolomics and colon motility in children with constipation. Neurogastroenterol Motil 2013;25:420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirchgessner AL. Glutamate in the enteric nervous system. Curr Opin Pharmacol 2001;1:591-6. [DOI] [PubMed] [Google Scholar]
- 16.Kaszaki J, Palasthy Z, Erczes D, Racz A, Torday C, Varga G, et al. Kynurenic acid inhibits intestinal hypermotility and xanthine oxidase activity during experimental colon obstruction in dogs. Neurogastroenterol Motil 2008;20:53-62. [DOI] [PubMed] [Google Scholar]
- 17.Slattery JA, Page AJ, Dorian CL, Brierley SM, Blackshaw LA. Potentiation of mouse vagal afferent mechanosensitivity by ionotropic and metabotropic glutamate receptors. J Physiol 2006;577:295-306. [DOI] [PMC free article] [PubMed] [Google Scholar]


