Abstract
Numerous light-based diagnostic and therapeutic devices are routinely used in the clinic. These devices have a familiar look as items plugged in the wall or placed at patients’ bedsides, but recently, many new ideas have been proposed for the realization of implantable or wearable functional devices. Many advances are being fuelled by the development of multifunctional materials for photonic healthcare devices. However, the finite depth of light penetration in the body is still a serious constraint for their clinical applications. In this Review, we discuss the basic concepts and some examples of state-of-the-art implantable and wearable photonic healthcare devices for diagnostic and therapeutic applications. First, we describe emerging multifunctional materials critical to the advent of next-generation implantable and wearable photonic healthcare devices and discuss the path for their clinical translation. Then, we examine implantable photonic healthcare devices in terms of their properties and diagnostic and therapeutic functions. We next describe exemplary cases of noninvasive, wearable photonic healthcare devices across different anatomical applications. Finally, we discuss the future research directions for the field, in particular regarding mobile healthcare and personalized medicine.
Light-based healthcare has emerged as a promising approach to improve patient outcomes, owing to its multifunctionality and minimal invasiveness for biosensing, molecular imaging, surgery and therapy1–3. When biological tissues are irradiated with light, photons are absorbed and scattered by cells and proteins, with a penetration depth that depends on the wavelength (FIG. 1a). Whereas light with a wavelength of 400–500 nm can only go as deep as the epidermal layer, light with a wavelength longer than 700 nm can reach deeper tissues beyond the dermis1. Light reflected after photophysical interaction with tissues provides important biometric information, such as molecular content, morphology and microstructure4,5. In addition, molecular imaging systems can visualize the morphology and spatial distribution of tissues and organs by detecting signals from imaging probes6,7. For example, a wearable functional brain imaging system in the form of headgear has been developed by employing functional near-infrared (NIR) spectroscopy. The brain is highly transparent to NIR light of wavelength 700–900 nm, which can, thus, be used for noninvasive neuroimaging to monitor neural activity8,9. Another example is wearable pulse oximetry devices, which have been developed to measure the oxygen level within flowing blood10 (FIG. 1a). Such devices detect oxygenation and deoxygenation of haemoglobin. They can provide a noninvasive tool to monitor heart rate, peripheral oxygen saturation and respiration rate for healthcare applications.
Fig. 1 |. Fundamental mechanisms underlying representative photonic healthcare applications.
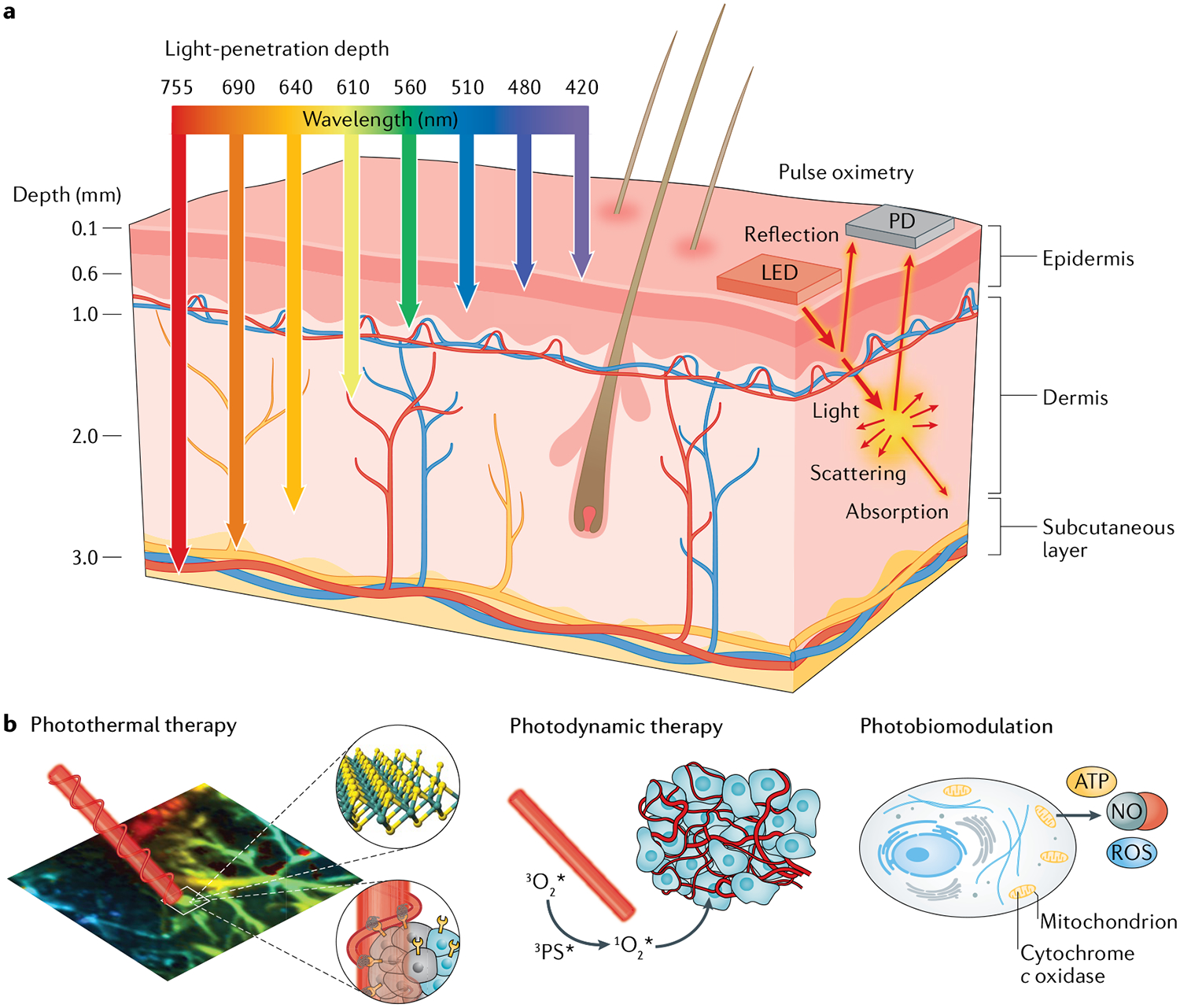
a | Schematic illustration of wavelength-dependent light-penetration depth and of photonic diagnosis via pulse oximetry, in which light absorption is detected in peripheral blood using a light-emitting diode (LED) and a photodetector (PD). b | Schematic illustrations of: photothermal therapy, in which light-triggered thermal ablation is used on diseased tissues; photodynamic therapy, which uses reactive oxygen species (ROS) generated by a photosensitizer; and photobiomodulation, which uses photonic stimulation of mitochondrial chromophores to produce nitric oxide (NO) and ROS. ATP, adenosine triphosphate.
Light-based therapy, with its minimal invasiveness, offers therapeutic benefits that can improve patient compliance. Light activates photoresponsive materials and biological tissues by inducing photochemical and biological reactions, and by generating heat11. Many kinds of therapeutic strategies have been developed (FIG. 1b), including photothermal therapy (PTT)12, photodynamic therapy (PDT)13, photobiomodulation (PBM)14 and optogenetic therapy15. In particular, PBM has been extensively investigated for emerging photonic healthcare applications16. PBM devices use red or NIR light generated by light-emitting diodes (LEDs) or lasers to stimulate wound healing and tissue regeneration. The working mechanism is based on the biological function of a mitochondrial chromophore, cytochrome c oxidase (CCO). Upon absorption of red or NIR light, CCO releases nitric oxide via photodissociation, which increases electron transport, mitochondrial metabolism and production of adenosine triphosphate16 (FIG. 1b). Light irradiation can also increase the level of reactive oxygen species (ROS) in the mitochondria, which induces cytoprotective, antiapoptotic and antioxidant effects on cells17. PBM has been used for the treatment of brain injury and neural regeneration, as well as wound healing and hair growth stimulation16–18. In a broad sense, optogenetic therapy can also be categorized as a kind of PBM. Optogenetic approaches (BOX 1) introduce light-sensitive actuators into target cells, which can then be manipulated by light illumination15. Optogenetic modulation devices have been used for the treatment of diabetes and brain, peripheral nerve and bladder diseases19–22.
Box 1 |. Basics of optogenetics.
Concept
Optogenetics enables the optical control of cells that are genetically modified to express light-sensitive ion channels. The concept of optically controllable neurons was first suggested by Francis Crick in 1979. The successful development of light-sensitive neurons through the introduction of a microbial opsin gene in 2011 initiated a wave of breakthroughs in optogenetic studies15. various ion channels responsive to light of different colours have been developed and have contributed to the diverse applications of optogenetics. optogenetic control can be realized with high temporal and spatial precision for specific cell types, and optogenetic approaches have provided probes for genetically defined targets. Such approaches can manipulate and record nervous system functions at different levels, ranging from synapses to animal behaviour, as shown in part a of the figure. The optogenetic tools can manipulate and record the activity of individual neurons. If neuronal manipulation can be set up to activate assemblies of neurons in a wide area of the brain with precise spatial resolution, it can control the behaviour of treated animals.
Steps in optogenetics studies
Introduction of the effector. Genes encoding light-sensitive ion channels are introduced into target cells on which to exert optogenetic control. These genes are introduced by either direct viral transduction or implantation of light-sensitive cells201. The most frequently used microbial opsins are bacteriorhodopsin, halorhodopsin and channelrhodopsin.
Light delivery. Light needs to be delivered to the target site to elicit optogenetic responses. This has been achieved by using waveguides19 and diverse light sources, including multiphoton lasers with an extended penetration depth202 and micro light-emitting diodes implanted at the target site20,128.
Sensing the optogenetic responses. The responses of optogenetically modified cells can be monitored by detecting the changes in the membrane potential using, for example, voltage-sensitive fluorescent proteins203, genetically encoded calcium indicators204 or patch clamps205.
Applications
Brain function control. optogenetic systems can manipulate the signal transduction of neural networks. They have been used to investigate the relationship between brain functions and activated regions of the brain128 and to analyse the pathology of neural disorders such as Alzheimer disease137 and Parkinson disease206. For example, flickering gamma light recruited microglia to the site of amyloid β (Aβ) deposition and induced Aβ phagocytosis by triggering the activated shape of microglia (part b of the figure).
Cardiac function control. optogenetic systems can regulate cardiac pacing when applied to cardiomyocytes. They can be used for heart-rate-dependent drug screening under optogenetic stimulation207 and cardiac resynchronization therapies for cardiac arrhythmia and heart failure133.
bladder function control. When opsins are introduced into bladder smooth muscle cells, bladder contraction can be controlled by light. This optogenetic method can be used for the treatment of urinary tract dysfunctions. The bladder can be wrapped with a stretchable strain gauge to monitor its volume change and be stimulated by micro light-emitting diodes on the module20 (part c of the figure).
optogenetic synthesis of therapeutics. optogenetically modified cells can be used for the light-responsive production of therapeutics; for example, glucagon-like peptide 1 can be produced under blue light to improve glucose homeostasis19.
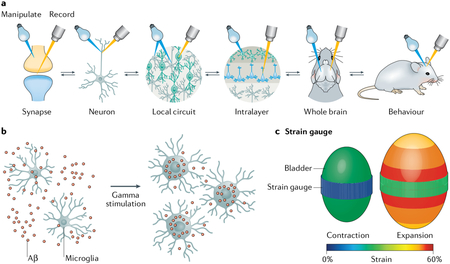
Part a of the figure is adapted from REF.136, Springer Nature limited. Part b of the figure is adapted from REF.138, Springer Nature limited. Part c of the figure is adapted from REF.20, Springer Nature limited.
Traditionally, these light-based approaches have been implemented by using instruments placed in the clinic or at patients’ bedsides. However, recent advances in materials and mobile devices are accelerating the development of implantable and wearable photonic healthcare devices3, paving the way for noninvasive point-of-care testing and personalized medicine. Multifunctional materials can induce and regulate light-based responses in biological tissues. In this Review, we provide an overview of state-of-the-art multifunctional materials for the development of implantable and wearable photonic healthcare devices and discuss the properties required for further translational applications. We also review the properties and functions of representative implantable and wearable healthcare devices. Finally, we address the future research directions for the improvement of such devices, outlining the perspectives for their use in mobile healthcare and personalized medicine.
Multifunctional materials
A wide variety of multifunctional materials have been developed for implantable and wearable photonic healthcare devices (FIG. 2). This section describes their characteristics and properties for diagnostic and therapeutic applications.
Fig. 2 |. Multifunctional material platforms for implantable and wearable photonic healthcare devices.
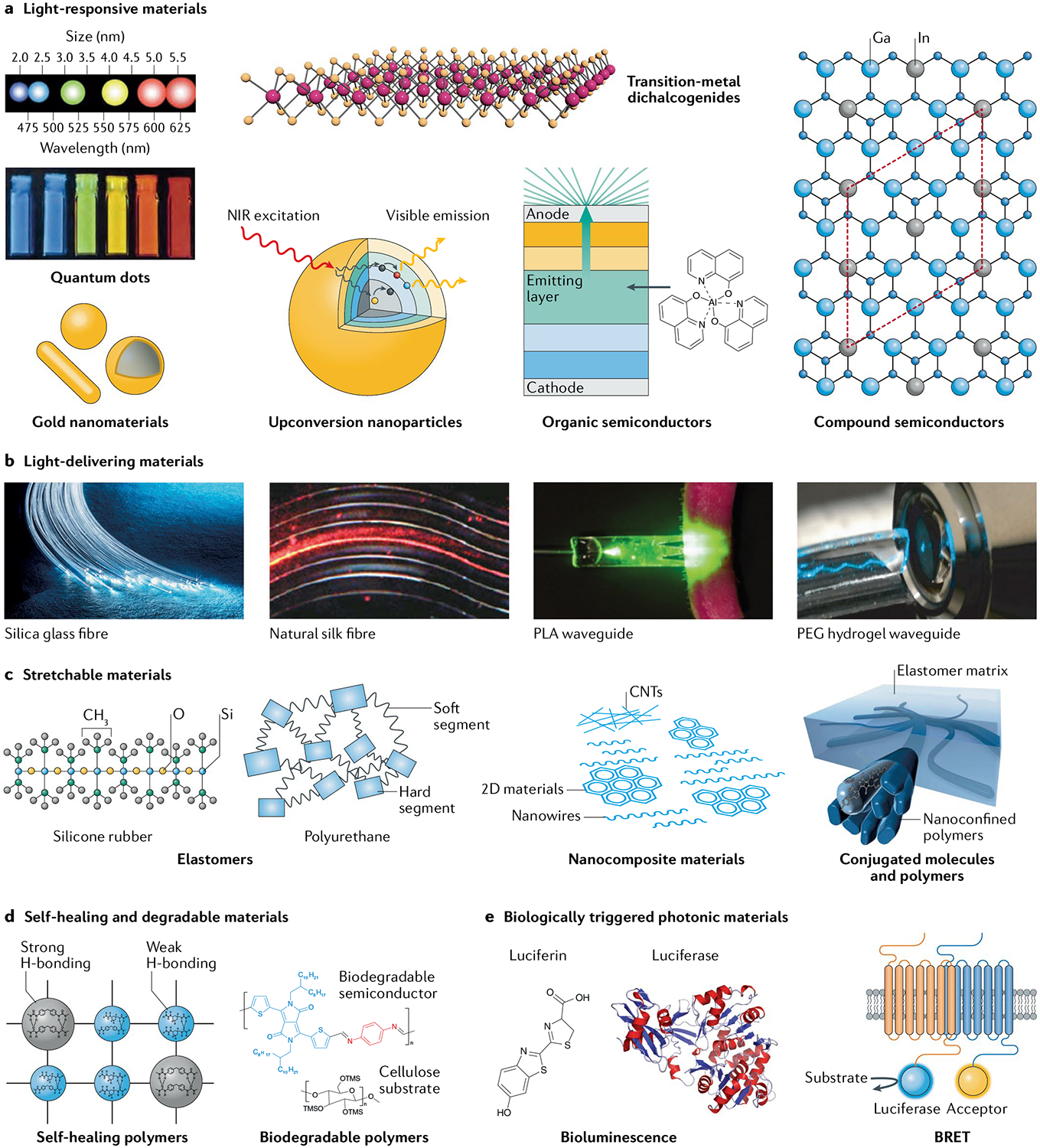
Materials platforms include: a | light-responsive materials, b | light-delivering materials, c | stretchable electronic materials, d | self-healing materials and biodegradable materials and e | biologically triggered photonic materials. BRET, bioluminescence resonance energy transfer; CNT, carbon nanotube; NIR, near infrared; PEG, polyethylene glycol; PLA, poLy(l-Lactic acid). Part a is adapted from REFs24,35, Springer Nature Limited, and with permission from REF.3, Wiley-VCH. Part b is reproduced from Valentyn Volkov/Alamy Stock Photo and adapted from REFs19,50, Springer Nature Limited, and with permission from REF.48, Wiley-VCH. In panel c, the image of conjugated polymers is adapted with permission from REF.73, AAAS. Part d (left) is adapted with permission from REF.76, Wiley-VCH. Part d (right) is adapted with permission from REF.81, National Academy of Sciences. Part e is adapted with permission from REF.208, Elsevier and REF.93, Frontiers.
Light-responsive materials.
Light-responsive materials absorb light and exhibit optical responses such as fluorescence, phosphorescence, and plasmonic and photothermal effects. The past decade has seen dramatic progress in the understanding, design and fabrication of light-responsive materials. They now play a central role in the development of implantable and wearable diagnostic and therapeutic devices. Examples of light-responsive materials include quantum dots (QDs)23–26, plasmonic gold nanostructures27–29, transition-metal dichalcogenides30,31, upconversion nanoparticles32–34, organic semiconductors35–40 and compound semiconductors41,42 (FIG. 2a).
QDs are semiconductor nanocrystals, typically a few nanometres in size, known for their ability to emit light of a specific colour or with a narrow spectral bandwidth23,24. The colour of the emitted light depends on the quantum confinement effect (and, thus, on the QD size) and on their chemical composition, which makes QDs suitable for light-mediated diagnosis and therapy25,26.
Plasmonic gold nanostructures exhibit a surface plasmon resonance, that is, a resonant oscillation of free electrons stimulated by the incident light, which considerably enhances light absorption27,28. Such absorption enhancement has been harnessed to increase the contrast of medical images and to amplify spectroscopic signals, which is essential for the miniaturization of healthcare devices29.
Transition-metal dichalcogenides are a class of 2D semiconductors formed by a layer of transition-metal atoms sandwiched between two hexagonal lattices of chalcogenide atoms. They have a direct band gap, which allows them to interact with light through quantum mechanical interactions30, and have been used in fluorescence imaging and PTT of cancers31.
Upconversion nanoparticles are composed of lanthanide-doped transition metals and convert lower-energy photons into higher-energy photons32. They can increase the light-penetration depth in tissues; most importantly, they make visible light available in the deep tissues33.
Organic semiconductors are carbon-based materials that include conjugated small molecules and polymers35, carbon nanotubes36, nanodiamonds37, graphene oxides38 and carbon dots39 and have unique mechanical properties, such as flexibility and stretchability, and tuneable optical properties. These materials have received much attention owing to their biocompatibility and, in some cases, biodegradability, and have been extensively investigated for biosensing, biomedical imaging, drug delivery, PTT and optogenetics39,40.
Compound semiconductors are semiconducting materials conventionally composed of elements from groups III and V or groups II and VI on the periodic table41. They have been recognized for their high electron mobility and sensitivity, and have been used in LEDs and photodetectors for decades42.
Light-delivering materials.
Often, light-based healthcare devices, especially those working deep within tissues, require waveguides to manipulate and transfer light to target sites. Optical waveguides should constrain the expansion of light in one or two dimensions to deliver it with minimal loss. Accordingly, they are generally highly transparent and composed of a core with a high refractive index surrounded by cladding materials with a low refractive index43,44. Silica fibres are commonly used in conventional applications45 but the development of flexible, biocompatible and biodegradable waveguides (FIG. 2b) has attracted great attention for implantable healthcare device applications. Light waveguides should employ biomaterials with a higher refractive index than that of surrounding biological tissues, ranging from 1.34 to 1.47 (REF.46). Natural fibrous materials with a high refractive index such as cellulose47 and silk48 have been actively investigated as optical waveguides owing to their biocompatibility. Biodegradable polymers have also been developed as optical waveguides in the forms of fibres49, films50 and hydrogels19,51,52. For example, a transparent and flexible poly(l-lactic acid) waveguide (FIG. 2b) was fabricated by a melt-pressing technique for photochemical tissue bonding50. The waveguide was tuned to exhibit a reduced refractive index for light transmission through its surface. In addition, hydrogel waveguides have been developed by using polyethylene glycol19,51, agarose52 and gelatin53 and endowed with additional functions or properties through the encapsulation of biomolecules or cells. For example, hydrogel waveguides encapsulating optogenetically modified cells have been used as a multifunctional platform for sensing and delivering therapies in vivo19.
Stretchable electronic materials.
To maximize conform-ability, durability and performance, all the components of implantable or wearable photonic healthcare devices must be highly deformable and, ideally, stretchable for proper and seamless function within or alongside the body54,55. For the development of such systems, the key components of photonic devices should meet a minimum threshold of deformability. Many efforts have been made to develop stretchable materials for all components, including insulators, conductors and semiconductors (FIG. 2c); in this section, we discuss some notable examples.
Elastomers have been used as substrates, encapsulating and insulating layers. These materials should exhibit biocompatibility as well as adequate mechanical and insulating properties to avoid foreign-body reactions, because they interface with human tissues and organs56. Silicone rubbers such as poly(dimethylsiloxane) (PDMS) and Ecoflex, and other elastomers such as poly[styrene-block-(ethylene-co-butylene)-block-styrene] and polyurethane have been successfully used57.
Stretchable conductors are one of the essential elements of implantable and wearable systems. They are used for stretchable interconnections between rigid devices or intrinsically stretchable devices to provide stretchability to the overall system. Thin-film metals or conductive polymers such as poly(3,4-ethylenedioxy-thiophene):polystyrene sulfonate become stretchable when made into serpentine58 and wrinkle10,59 structures. Intrinsically stretchable conductors have also been extensively investigated, because they enable more seamless, higher-resolution integration of stretchable systems. Such materials have been prepared as composites of elastomers and conductive fillers or networks, such as metallic nanoparticles, nanowires, flakes and carbon nanotubes60–63. Poly(3,4-ethylenedioxythiophene):polystyrene sulfonate could also be modified to exhibit high stretchability by mixing it with either plasticizers64,65 or ionic additives66. Noticeably, liquid metals such as eutectic gallium-indium, which has high conductivity and high stretchability, can also be used for implantable and wearable electronic devices67,68.
Stretchable semiconductors are challenging to produce but have been made by using networks of single-walled carbon nanotubes69 and by modifying polymer semiconductors chemically or physically, without compromising their electrical performance. Three chemical strategies can be used to prepare stretchable polymeric semiconductors: utilizing less rigid backbones and side chains70; adding non-covalent dynamic crosslinkers that allow energy dissipation upon strain71; and introducing crosslinkers with amorphous oligomers, such as PDMS72. A physical strategy leverages the nanoconfinement effect that modulates the intrinsic ductility of conjugated polymers by enhancing chain dynamics and suppressing the growth of large crystalline domains73. Using these strategies, stretchable polymer semiconductors have achieved a charge-carrier mobility comparable to or higher than that of amorphous Si transistors, even at a strain of 100%.
Self-healing and biodegradable materials.
Self-healing capability and biodegradability (FIG. 2d) are crucial properties for next-generation implantable and wearable devices with an optimized lifetime. When wearable devices are exposed to unexpected damage, the ability to self-heal can significantly enhance their reliability74. Self-healing is achieved by dynamic molecular interactions, such as metal-ligand coordination75, hydrogen bonding76, π-π interaction77 and electrostatic interaction78. In addition, it can be obtained by using a self-healing matrix with conductive fillers and self-healable conductors79.
Biodegradability is greatly beneficial for implantable devices because no additional surgery is needed to remove the device after it has performed its function. The degraded materials should be bioabsorbable and/or excretable without causing any toxic and negative side effects in the body. In this context, biopolymers based on silk80, cellulose81 and gelatin82, and synthetic polymers based on poly(lactic-co-glycolic acid) (PLGA)83 and poly(1,8-octanediol-co-citrate)84 have been harnessed as biodegradable insulating polymers and substrates in electronic and photoelectronic systems. Biodegradable, implantable electronic devices have also been developed using organic materials based on imine chemistry81 and inorganic materials based on physiologically safe metals, such as iron85 and transient magnesium80.
Biologically triggered photonic materials.
Luciferin, a light-emitting compound, is oxidized in the presence of the enzyme luciferase and, as a result, it releases energy in the form of light (FIG. 2e). Luciferin and luciferase are very diverse and are found in fireflies, snails, bacteria, dinoflagellates and fungi. Their reaction mechanisms are different in different organisms, but every reaction between luciferin and luciferase requires oxygen to proceed86. These reactions have mainly been exploited for bioimaging87,88 applications in which only one cell or one biological feature is analysed at a time. To enable the imaging of more features, d-luciferin has been designed to show various colours for bioimaging. The natural substrates of d-luciferin and the luciferine coelenterazine are common pairs with luciferase enzymes (Firefly, Renilla reniformis and Gaussia luciferases)89,90. Because coelenterazine is not suitable for practical applications owing to its low stability and bioavailability, many studies have focused on making customized luciferins and mutant luciferases. A general strategy was developed for evolving and defining Firefly luciferase mutations that could accept chemically modified luciferin87. The resulting additional orthogonal pairs contributed to the practical application of luciferin-luciferase bioimaging.
Bioluminescence resonance energy transfer (FIG. 2e) occurs between a bioluminescent donor substance that generates light and an acceptor that receives energy, which is transferred through non-radioactive (dipole-dipole) transport91–94. The energy-transfer efficiency is inversely proportional to the sixth power of the distance between the donor and the acceptor; thus, the phenomenon occurs mainly when the donor-acceptor distance is less than 10 nm. When energy is transmitted, the acceptor generates light with a longer wavelength than that of the light emitted by the donor. Through this phenomenon, it is possible to monitor real-time protein-protein interactions in living cells, cell extracts or purified preparations. QDs have also been used as acceptors for the detection of peptidases95.
Translational research.
As we discussed, a wide variety of multifunctional materials have been investigated for the development of implantable and wearable photonic healthcare devices. The types, properties and applications of these materials are summarized in TABLE 1. Light-responsive materials and biologically triggered photonic materials can be used as light-emitting sources under excitation and mediate light-triggered reactions in biological tissues. Light-delivering materials can improve the light-penetration depth by providing efficient routes for light to travel through tissues. Moreover, stretchable materials and self-healing materials can be used for the fabrication of flexible healthcare devices, enhancing their wearability.
Table 1 |.
Types, photonic properties and characteristics of multifunctional photonic materials and their applications
| Material | Photonic properties | Characteristics | Applications | Refs |
|---|---|---|---|---|
| Light-responsive materials | ||||
| Quantum dots | Broad absorption; sharp emission | Tuneable colours; narrow spectral bandwidth | Flexible and/or stretchable LEDs and PDs; bioimaging | 23–26 |
| Gold nanomaterials | NIR absorption | Surface plasmon resonance; local heating | Photothermal therapy | 27–29 |
| Transition-metal dichalcogenides | Light absorption; fluorescence | 2D semiconductors with a direct bandgap | Photothermal therapy; bioimaging | 30,31 |
| Upconversion nanoparticles | Upconversion of photon energy | Deep-tissue illumination | Photodynamic therapy; photochemical tissue bonding | 32–34,197 |
| Organic semiconductors | Light absorption; light emission | Tuneable colours; flexibility and stretchability; biocompatibility and biodegradability | Flexible and/or stretchable LEDs and PDs; health monitoring; photodynamic therapy | 35–40 |
| Compound semiconductors | Light emission | High efficiency; high brightness | Miniaturized LEDs; photodynamic therapy | 41,42 |
| Silicon semiconductors | Light absorption | Wide absorption spectrum | Miniaturized PDs; health monitoring | 105,142,143 |
| Light-delivering materials | ||||
| Inorganic fibres | Transfer light from its source to the target site | High light-delivery rate; generally inflexible | Photochemical tissue bonding; optogenetic therapy | 45 |
| Natural fibres | Transfer light from its source to the target site | Biocompatible | Photochemical tissue bonding; optogenetic therapy | 47,48 |
| Polymer waveguides | Transfer light from its source to the target site | Flexible and biodegradable | Photochemical tissue bonding; optogenetic therapy | 49,50 |
| Hydrogel waveguides | Transfer light from its source to the target site | Biocompatible and biodegradable; encapsulation of biomolecules or cells | Photochemical tissue bonding; optogenetic therapy | 19,51–53 |
| Biologically triggered photonic materials | ||||
| Luciferase and luciferin | Bioluminescence | No photoexcitation; biocompatible and biodegradable; unstable |
Bioimaging; BRET | 86–95 |
BRET, bioluminescence resonance energy transfer; LED, light-emitting diode; NIR, near infrared; PDs, photodetectors.
To bring photonic healthcare devices to the clinic, it is essential to assess the biocompatibility and clinical feasibility of the materials and of the required light energy. The first thing to consider is foreign-body reaction. When a device is implanted in the body, it comes into contact with tissues and induces acute and/or chronic inflammation96. Natural materials such as silk48, gelatin53 and hyaluronate97, as well as some synthetic materials such as PLGA and polyethylene glycol98, have been investigated to reduce foreign-body reaction. In addition, there have been technical efforts to reduce foreign-body reaction by coating the devices with non-inflammatory materials like zwitterionic hydrogels99 and incorporating anti-inflammatory drugs100. The second issue is toxicity in the body. For example, QDs are promising fluorescent agents owing to their high quantum yield, facile chemical modification and tuneable optical properties26. However, their medical use has been highly limited because of their cytotoxicity. A further toxicity issue specifically critical for photonic devices is light toxicity. High optical power can damage tissues by inducing the denaturation of proteins and DNA, especially in the case of ultraviolet and blue light101. Thus, devices should be optimized to perform their function with the minimally required light intensity. Finally, biodegradability is a critical issue for translational applications. Several kinds of bioresorbable devices have been developed by using biodegradable polymers such as PLGA and biodegradable metallic components such as Mg to simplify treatment and improve patient compliance102–104.
Implantable photonic healthcare devices
This section discusses implantable photonic healthcare devices in the form of miniaturized, stretchable and transient devices for photonic diagnosis and for photodynamic and optogenetic therapies (FIG. 3).
Fig. 3 |. Implantable photonic healthcare devices.
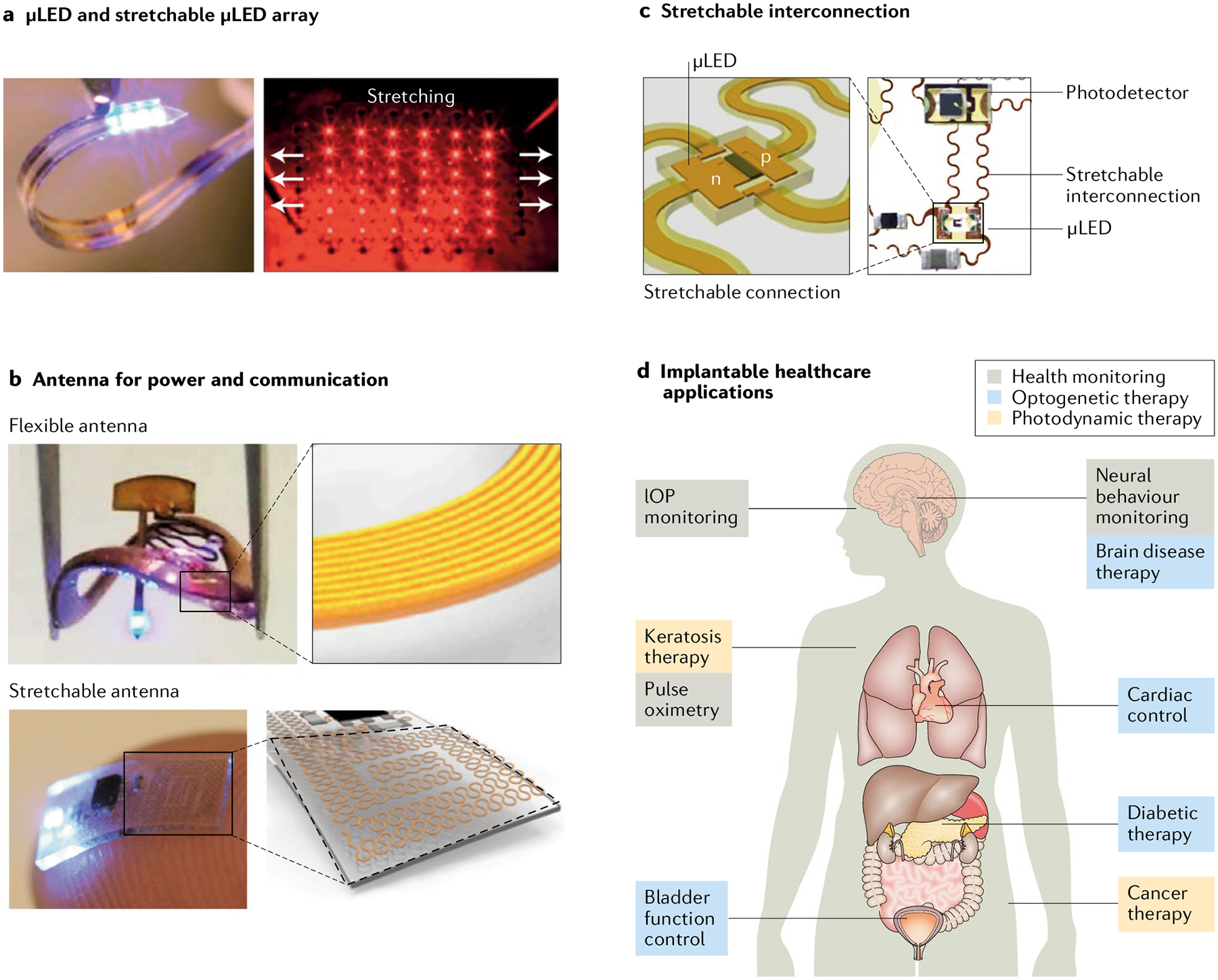
The essential components include micro light-emitting diodes μLEDs) and their stretchable arrays (part a), flexible and stretchable antennas for wireless power and communication (part b) and stretchable interconnections between the components (part c). Photonic healthcare devices can be used for different applications, including health monitoring and photodynamic and optogenetic therapies (part d). IOP, intraocular pressure. Part a is adapted from REF.129, Springer Nature Limited, and with permission from REF.109, Elsevier. Part b is adapted from REF.21, Springer Nature Limited, and with permission from REF.22, Elsevier. Part c is adapted from REF.129, Springer Nature Limited and with permission from REF.142, AAAS.
Miniaturized photonic devices.
Implantable photonic devices have been widely investigated for pulse oximetry105, intraocular pressure (IOP) monitoring106, PDT107,108 and optogenetics20–22,109–111. These photonic devices should be miniaturized for facile implantation into the body. For IOP sensing, resonance peak shifts in the optical-cavity structure have been exploited to avoid the need to include electrical-circuit systems106. In general, however, miniaturized implantable photonic devices have to be prepared by microscale fabrication of inorganic LEDs and photodetectors, ensuring small-size device control and fine patterning112,113 with wireless power transmission and remote data communication. Photonic devices fabricated with micro LEDs (μLEDs, FIG. 3a) have many advantages compared with conventional devices using optical fibres. In particular, they can be used for diagnosis and therapy inside the body without an external light source. Moreover, μLEDs allow effective thermal management, reduce tissue damage and minimize long-term inflammation114. μLEDs have been fabricated and transferred to a flexible substrate for implantation113. They are very thin (around 6 μm) and have a length and width of several tens to hundreds of micrometres, suitable for implantation in the body. Precise transfer and localization of such small and fragile devices in the desired position is important. A chip with a thickness of 6 μm was transferred to a desired location using an elastomer without causing any damage115. By contrast, commercially available μLEDs are much thicker (~150 μm).
A properly designed power system is also very important for the operation of devices implanted in the body. Power systems can be classified into two types: electrochemical power systems of super capacitor and battery, and wireless energy-transfer systems. Because battery and super capacitor systems require heavy and toxic materials and a big space, miniaturized devices generally use wireless power-transfer systems. Such systems can supply enough electrical power to operate μLEDs and other energy-demanding components. Implantable photonic devices can be operated by near-field resonant inductive coupling, near-field capacitive coupling, ultrasonic transcutaneous energy transfer, mid-field wireless power transfer and far-field electromagnetic coupling116. A triboelectric device combined with ultrasonic transcutaneous energy transfer has also been proposed as a noninvasive wireless power system for an implant device117.
Wireless power-transmission systems have been used in commercially available implant devices, such as a hearing supplement cochlear device118 and a visual acuity improvement device119 that uses near-field resonant inductive coupling. A very small implantable photonic device with a weight of 30 mg and a volume of 15 mm3 was developed for the treatment of cancer107. The device receives a radio frequency of 1–1.5 GHz and its μLED emits light with two wavelengths of 400 nm and 660 nm. Photosensitizers injected into the tumour are activated by the light and generate ROS for PDT. Retinal prosthetic devices to help to restore sight utilizing wireless power transmission have been also developed and are used in humans120.
Miniaturized photonic devices have also been effectively harnessed for optogenetic applications. A μLED with a wavelength of 470 nm was fabricated to stimulate the spinal cord and peripheral nerves using wireless power at the radio frequency of 2.34 GHz21. The photonic device was 0.7 mm thick, 3.8 mm wide and 6 mm long, with a weight of 16 mg. The motor cortex in the brain has been stimulated by photonic devices with a volume of 10–25 mm3 and a weight of 20–50 mg containing μLEDs with a wavelength of 450–470 nm using 1.5-GHz radio frequency110. In another report111, μLEDs with a wavelength of 540 nm embedded in a photonic device ~10 mm in length and width were operated at a frequency of 13.56 MHz to treat bladder pain.
Furthermore, biocompatible photonic devices have been made using living cells121,122. A green living laser was fabricated by inserting cells expressing enhanced green fluorescent protein as an optical gain material into a high-quality microcavity resonator121. An intracellular microlaser was also developed122 and might possibly be applied to optogenetic diabetes therapy19. In addition, a miniaturized transient healthcare device was fabricated using a biodegradable PLGA substrate and a biodegradable Mg electrode123. The transient device was completely decomposed in phosphate-buffered saline at 37 °C within 25 days.
Stretchable photonic devices.
As well as being miniaturized, implantable photonic healthcare devices need to be integrated on a stretchable platform with elasticity comparable to that of organs and tissues124,125. Because implantable photonic devices generally consist of many functional components occupying a considerably large area, they should be stretchable while maintaining the function of each component125,126. In particular, an antenna for power transfer and data communication is a critical requirement for an implantable system functioning inside the body without the need of wires (FIG. 3b). Bluetooth wireless communication can use a small antenna inside an integrated circuit chip but it consumes an unacceptably large amount of power. Thus, most stand-alone implantable photonic systems use a large antenna127,128. Stretchable devices have been fabricated by including stretchable interconnections in the form of serpentine-structured metal electrodes among rigid functional components, such as LEDs, photodetectors and integrated-circuit chips, combined with stretchable elastomers as substrate and encapsulation materials125,126 (FIG. 3c).
Some applications require several photonic devices, such as LED arrays129 or multiple LEDs with different colours22,108,128. In these cases, the circuitries, which include multiple photonic devices and other components, should be configured through stretchable interconnections on a stretchable platform. Implantable photonic devices should be highly biocompatible and stably attached for continuous local light delivery inside the body without causing any damage or disturbance to tissues and organs. A well-known elastomer, PDMS, is commonly used as a biocompatible stretchable-substrate and encapsulation material. Remarkably, stable adhesion onto tissues could be achieved by using a polydopamine-modified PDMS nanosheet108.
Some implantable photonic devices use an external light to stimulate cells adjacent to the device130 or to transfer power for device operation131. These devices have sensor arrays or multiple components, which are fabricated on very thin biocompatible plastic substrates prepared with Parylene. They can be conformally attached to organs and compressively deformed as a pseudo-stretchable system.
Optogenetic devices.
Optogenetics has been actively investigated to optically control the function of living cells15,132 (BOX 1). Although optogenetics has been implemented in a variety of fields, including cardiology133, immunology134 and urology135, most of the advances have been achieved in neurology and psychobiology. The approach, because of its high spatial and temporal resolution, is a promising strategy to control biological systems15,136.
For example, a fibre-pigtailed hydrogel waveguide was implanted in freely moving mice for cell-based optogenetic nanotoxicity sensing and optogenetic diabetes therapy19. Cytotoxicity reporter cells were encapsulated in the hydrogel waveguide and emitted green fluorescence under cytotoxic stress, to detect the toxicity of QDs in situ in live mice. The data were consistent with ex vivo data. Optogenetic cells were also encapsulated in a hydrogel waveguide to produce glucagon-like peptide 1 under blue light, which improved glucose homeostasis in mice with chemically induced diabetes.
Progress in miniaturized and stretchable devices has greatly contributed to the development of implantable optogenetic devices, including a device with injectable μLEDs and a multichannel stretchable antenna128. The μLEDs were fabricated on a needle-shaped substrate for use in the deep brain. The antenna could transmit radio-frequency energy and remotely control multiple μLEDs. This optogenetic device was used to investigate the brain regions involved in sleep arousal and preference and aversion. An optogenetic device with a stimulation-and-sensing module and a wireless control-and-power module was also developed for the neuromodulation of bladder function20. The bladder could be stimulated by μLEDs on the stimulation-and-sensing module in vivo. The wireless control-and-power module recorded the response monitored by a stretchable strain gauge, controlled the μLEDs and transmitted wireless power by resonant magnetic coupling.
Gamma waves in the brain are disrupted in mouse models of Alzheimer disease137. Optogenetically driven gamma oscillations were used to reduce the level of amyloid β (Aβ); in addition, flickering gamma light (40 Hz) applied for 1 h reduced the level of Aβ in the visual cortex and attenuated the plaque load via optogenetic stimulation. Gamma oscillations appeared to recruit microglia to the site of Aβ deposition and induce Aβ phagocytosis by triggering the activated shape of microglia138. These findings might be helpful for future optogenetic treatments of neurological disorders.
Despite all these works, the control of optogenetic gene expression is a big challenge for the clinical translation of optogenetic therapies. Most optogenetic approaches include the use of viral delivery systems for introducing genes and suffer from the toxicity of viral vectors and the long-term instability of gene expression136. Although transgenic animals have been used for preclinical studies136, transgenic approaches cannot be used in clinical applications. Thus, more advanced optogenetic gene-delivery systems are required.
Concerning photonic devices for optogenetic applications, the goal is to integrate all the components of optogenetics, including gene-delivery systems, actuators and sensors, into one miniaturized implantable device. Moreover, optogenetic modulation should be achieved in large areas, covering substantial portions of tissues and organs. For example, neuronal manipulation should be performed within a wide area of the brain with precise spatial resolution to activate populations of neurons, mimicking natural neuronal modulation. To this end, novel optogenetic methods using spatial light modulators to regulate multiple individual neurons simultaneously have been investigated139.
Translational optoelectroceutical applications.
Electroceutical is a term used to indicate an electronic device or system used to alleviate pathologies in the body; examples are deep-brain stimulation devices and pacemakers140,141. Most electroceuticals stimulate nerves by applying an electrical signal to affect biological systems. Optoelectroceuticals are electronic devices that affect biological systems through light (FIG. 3d). PDT for the treatment of cancer and keratosis is a good example of an optoelectroceutical treatment using photonic devices. Conventionally, PDT has suffered from limited light penetration in opaque biological tissues. Light has generally been transmitted to a deep-region organ using optical fibres. By implanting a photonic device, light could be transmitted directly without any obstruction, which was successfully applied to cancer PDT107,108.
When a photonic device is implanted, it should be sutured to the surrounding tissue to fix it inside the body. For this purpose, PDMS was modified with a bio-adhesive polydopamine underlayer108. A metronomic PDT with a small dose of light was performed by implanting μLEDs. As a result, the photonic device showed a local anticancer effect via the emission of low-intensity light for an extended period of time. Owing to the direct proximity of the irradiation, the light intensity was 1,000 times lower than that used in conventional PDT. Importantly, it was possible to treat delicate tissues such as brain tissue through polydopamine-based adhesion, without the need to suture the device to the underlying tissue108.
Wearable photonic healthcare devices
This section describes wearable photonic devices fabricated with rigid or thin-film electronic components for health monitoring and health intervention, including skin rejuvenation, wound treatment, mental healthcare and brain-disease therapy (FIG. 4).
Fig. 4 |. Wearable photonic healthcare devices.
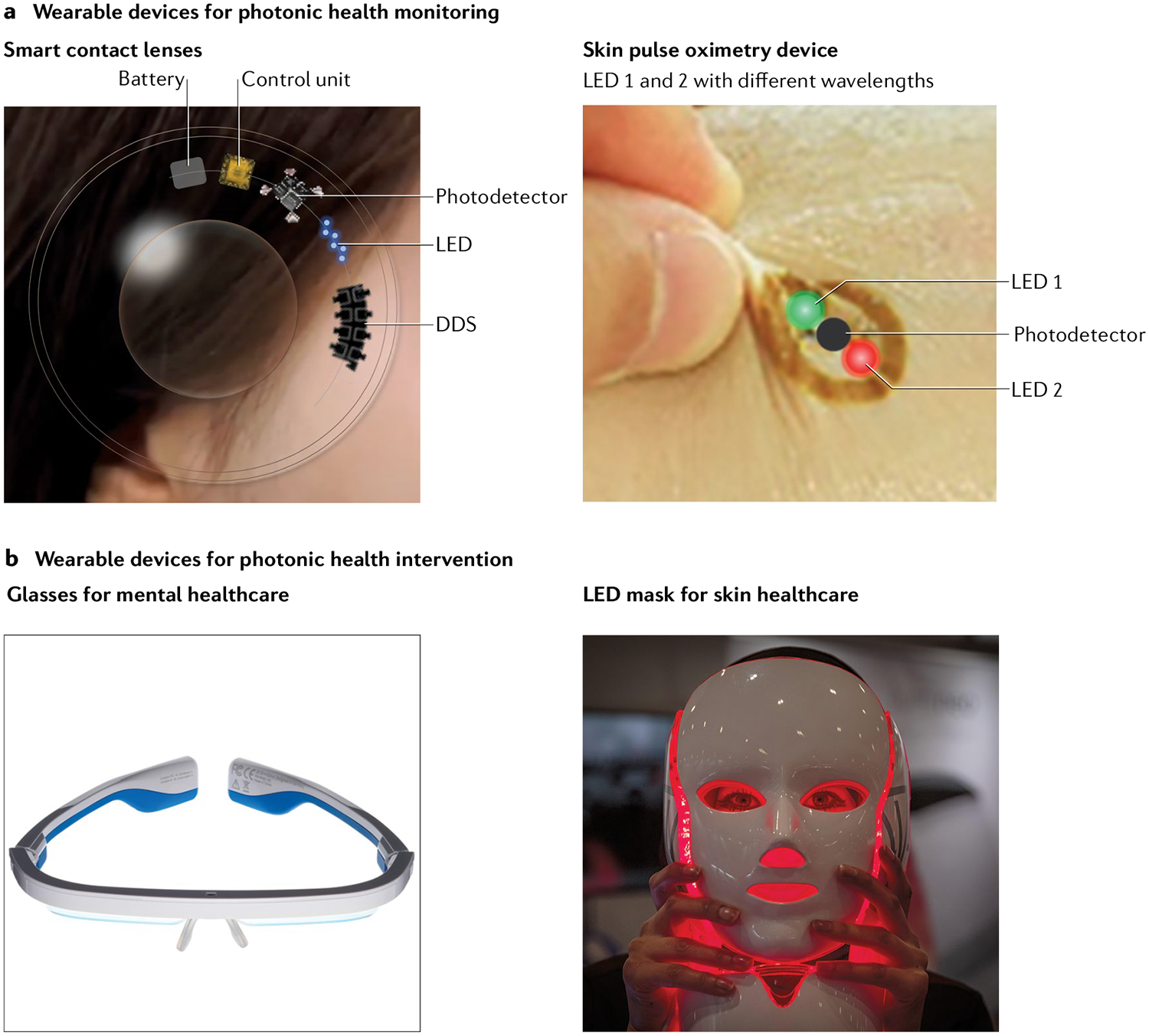
Applications of wearable photonic devices for photonic health monitoring using smart contact lenses and skin pulse oximetry devices (part a) and health intervention for mental and skin disorders (part b). DDS, drug-delivery system (can also be used for theranostic applications); LED, light-emitting diode. The skin device in part a is adapted with permission from REF.142, AAAS. Part b is reprinted from Hugh Threlfall/Alamy Stock Photo and Guy Corbishley/Alamy Stock Photo.
Evolution of wearable photonic devices.
Wearable photonic devices have been extensively investigated for healthcare applications: wrist bands, patches, nails, eyeglasses and contact lenses have been tested. Photonic devices can be prepared in several ways, with the goal of achieving seamless integration in the human body. One method is to integrate rigid-component photonic devices and peripherals on a thin and stretchable platform, similar to the strategy described above for implantable devices. This method utilizes rigid electronic components such as LEDs, photodetectors and integrated-circuit chips connected by serpentine electrodes that provide stretchable electrical connections between the components142, 143. Rigid but smaller components such as μLEDs can provide higher stretchability and versatility to the system. Another possibility is to use flexible or stretchable thin-film photonic devices, which enable uniform illumination over a large area close to the device and provide conformal coverage. Buckling and wavy structures have been introduced into thin-film devices to make stretchable light sources and detectors144–146. For example, small-molecule organic LEDs (OLEDs) with high performance were fabricated, achieving a luminous current efficiency of ~70 cd/A with a stretchability of up to 100%147,148. Stretchable photonic devices have also been fabricated using intrinsically stretchable materials. The emitting layer was prepared with polymers, nanoparticles or their composites, and stretchable transparent electrodes were prepared with polymers, liquid metals and composites of elastomers and conductive networks149–151. Moreover, a self-healable and stretchable light-emitting capacitor was developed using a Cu-doped emissive layer incorporating ZnS particles and self-healable transparent electrodes152. Photonic devices based on such intrinsically stretchable materials could be stretched up to 400%153. Despite these advancements, performance and stability still need to be improved for practical applications of intrinsically stretchable photonic healthcare devices.
Smart contact lenses and eyeglasses.
In the field of wearable photonic devices, smart contact lenses are especially promising for healthcare applications, because the eye can be an excellent interface between an electronic device and the body154. In particular, smart contact lenses have been used for the continuous monitoring of tear glucose concentration155–158 and IOP159. Glucose sensors were fabricated on a contact lens using glucose oxidase to measure the glucose concentration in tears, which is strongly correlated with blood glucose concentration. Moreover, μLEDs have been attached to the contact lens to act as a display: when the glucose concentration in the tear decreases below a certain threshold, a high current flows through the μLED (instead of through the sensor) and the μLED turns on156.
Smart contact lenses have also been developed using volume-changing158,160–162 or colour-changing163–166 materials, which do not require an electrical power source. The glucose concentration in tears was measured from a change in the colour of the photonic device using phenylboronic acid158. When phenylboronic acid binds to glucose, the volume of phenylboronic-acid hydrogels increases. The glucose concentration in tears could be measured with a sensitivity of 12 nm mmol−1 within a concentration range of 0–50 mM.
Despite the progress in the development of smart contact lenses for tear glucose monitoring, the lag time between blood and tear glucose concentration profiles remains a major challenge167,168. Thus, a smart photonic contact lens that measures glucose concentration in eye blood vessels rather than in tears has been developed169 (FIG. 4a). A μLED on the contact lens irradiates NIR light to detect glucose in conjunctival blood vessels and the reflected light is analysed by a photodetector to determine glucose concentrations in real time. The photonic current is proportional to the glucose concentration in the sample. Smart photonic contact lenses thus hold promise for real-time blood glucose monitoring in diabetes.
In addition to photonic diagnosis, light can be used for the treatment of ocular diseases. Most treatments are based on PBM applied to diseases such as diabetic retin-opathy170, methanol-induced retinal toxicity171 and light-induced photoreceptor degeneration172. For example, for the treatment of diabetic retinopathy, light with a wavelength of 670 nm was transmitted for 240 s per day with an output of 25 mW cm−2, and a total light delivery of 6.0 J cm−2 per day. The PBM resulted in the effective reduction of superoxide production, leucostasis and intercellular adhesion molecule 1.
Although most eyeglass-type wearable devices have been developed for applications in virtual reality and augmented reality, recent studies have shown that photonic glasses can be used for phototherapy with comfortable accessibility to the eye (FIG. 4b). Blue light can control circadian rhythms173–177 and prevent seasonal affective disorder (SAD)178–181. Circadian rhythms are significantly impaired by the absence of cytochrome Y1 and cytochrome Y2, members of the blue-light photoreceptor family175. Moreover, human hormone secretion is also influenced by the intensity and wavelength of light. Thus, many patients with SAD easily become depressed in autumn and winter, when light has a relatively low intensity. One approach to alleviate the problem is to shine more light, in a controlled way, onto the eye. AYO and Luminette are commercial eyeglasses fitted with blue LEDs that transmit blue light to the user’s eyes to modulate the circadian rhythm and treat SAD182–184.
Health-monitoring skin devices.
Photonic skin devices can be utilized for health-monitoring applications. The most common example is photoplethysmography (PPG), which monitors blood perfusion to the dermis and subcutaneous tissues by measuring the temporal change in photon absorption, which is related to the blood-volume change associated with cardiac cycles. The monitoring system in general is composed of light-emitting devices and photodetectors, and has been used to acquire a spectrum of healthcare information, such as heart rate, saturation of peripheral oxygen (SpO2) and respiration rate. There have also been efforts to estimate blood pressure via PPG, but more work is still needed to establish a firm link between them185,186. Pulse oximeters for heart rate and SpO2 already play a critical role in hospitals and in personal mobile healthcare. Most of the current wearable products, including smart watches and bands, have a PPG sensor based on rigid LEDs, photodetectors and other components. Flexible and stretchable PPG patch systems using rigid components for LEDs and photodetectors, and interconnections with serpentine electrodes or liquid metals have been developed for on-nail and on-skin applications142,143,187 (FIG. 4a). In addition, fully flexible and stretchable wearable PPG systems have been developed using thin-film photonic devices58,188,189. A very thin PPG sensor system with a total thickness of 3 μm has not only enabled conformal integration onto the skin but also improved PPG sensing performance189. Thin-film photonic devices with a ring-shaped organic photodetector surrounding circular OLEDs have significantly reduced the power consumption of pulse oximeters, bringing it down to ~24 μW, which is important for the continuous monitoring of health signals with small-capacity batteries190. A printed array of flexible polymer LEDs and photodetectors could obtain a map of SpO2 levels from the skin191. Wearable devices will enable the collection of healthcare information throughout the day in a noninvasive way192.
Health-intervention skin devices.
Light illumination can impact human health in many ways, collectively classified as PBM14. Light with red or NIR wavelength is absorbed by the chromophores in mitochondria and increases the activity of CCO193. Photonic healthcare devices based on LEDs and low-power lasers, such as wearable mask-shaped facial skin-care and wound-care devices, are already in commercial use (FIG. 4b). If such devices were developed on a seamless wearable platform, they would enable photonic health intervention at any time in daily life. Flexible OLEDs with a wavelength of 600–700 nm were proposed as a band-type wound-care device and have shown visible improvement of fibroblast migration for the recovery of deep wounds194. Flexible μLED arrays with a wavelength of 650 nm have been applied to promote hair growth195; in addition, ultraviolet B LED illumination showed a statistically significant effect on vitamin-D formation196. Seamlessly attachable wearable devices with high performance, low power consumption and low heat generation that minimize thermal damage to cells for long-term use on the skin might provide a new paradigm for healthcare applications. Uniform light illumination over a wide area is also essential for photonic skin devices. Although some PBM technologies have been clinically validated and obtained Food and Drug Administration clearance, further studies are required to elucidate the exact mechanism of PBM in the body.
Future perspectives
We have provided an overview of implantable and wearable photonic devices for smart healthcare using multifunctional materials platforms (TABLE 2). As diagnostic tools, optical sensing and imaging probes have been developed for the optical detection of biomarkers and for the molecular imaging and spectroscopic analysis of biological tissues. Light-based therapy has evolved in terms of the specificity of the interactions of light with biological matters. Light can induce heat generation from photothermal agents for PTT and production of ROS for PDT. In addition, light can initiate biological reactions, which have been exploited for PBM and optogenetic therapies. Progress in multifunctional materials has improved their light responsiveness, stretchability, biodegradability and ability to deliver light into deep tissues, enhancing the efficacy of wireless photonic healthcare systems and patient compliance.
Table 2 |.
Types, working principles and applications of implantable and wearable photonic devices for smart healthcare
| Device | Main mechanism | Working principle | Applications | Refs |
|---|---|---|---|---|
| Implantable photonic devices | ||||
| Health-monitoring device | Pulse oximetry | Comparison of the optical absorption of Hb and HbO2 | Monitoring neural behaviour | 105 |
| Optical resonance of cavity structure | Monitoring the reflected resonance peak shift via the distance change of the optical cavity caused by IOP | IOP monitoring | 106 | |
| Photodynamic device | Photonic ROS generation | Photosensitizers generate ROS for cancer therapy | Cancer therapy | 107,108 |
| Optogenetic device | Optogenetics | Genetically modified cells express light-sensitive ion channels | Treatment of diabetes, brain, peripheral nerve and bladder diseases | 19–22,114,128 |
| Wearable photonic devices | ||||
| Contact lens | Structural colour change of photonic crystal | Volume change resulting from the binding of glucose to boronic acid | Tear glucose level monitoring | 158,160,161 |
| Volume change resulting from drug release from the lens | Ocular drug-release monitoring | 162 | ||
| Colorimetric change of glucose reagents | Optical change resulting from the binding of glucose to boronic acid or concanavalin A | Tear glucose level monitoring | 163–166 | |
| Diffuse reflection of NIR light | Light scattering depending on the glucose concentration change in ocular blood vessels | Blood glucose level monitoring | 169 | |
| Smart glasses | Photoreceptor stimulation | Modulating hormone secretion with blue-light LEDs | SAD and circadian rhythm control | 182–184 |
| Skin patch | Pulse oximetry | Comparison of the optical absorption of Hb and HbO2 | SpO2 level monitoring |
142,143,187, 188,190,191 |
| Photoplethysmography | Detecting the volumetric change of peripheral blood | Cardiac function monitoring | 145,189 | |
| Photobiomodulation | Stimulating mitochondrial chromophores for cell proliferation | Wound healing, skin care and hair growth | 194,195 | |
Hb, haemoglobin; HbO2, oxygenated haemoglobin; IOP, intraocular pressure; LED, light-emitting diode; NIR, near infrared; ROS, reactive oxygen species; SAD, seasonal affective disorder; SpO2, saturation of peripheral oxygen.
Photonic healthcare has a great potential to be beneficial with minimal use of chemical or biological drugs. Whereas optogenetic therapy requires genetic modification to introduce light-sensitive ion channels, PBM activates natural chromophores and photoreceptors. PBM can affect circadian rhythm, SAD and pathological responses such as tissue regeneration without the administration of drugs. However, although light-based therapies are attractive for patients, there are still big technical hurdles for their clinical translation. The mechanisms underlying some light-based therapies are still under investigation, and their therapeutic effect should be optimized to be comparable or superior to that obtained with conventional medicines. In addition, light-based therapy is currently available only for certain applications. Further studies on multifunctional materials for implantable and wearable photonic healthcare devices will expand their clinical feasibility.
In the fabrication of photonic devices, the choice of the type of light source is important and depends on the desired biomedical photonic application. Light sources include lasers, LEDs, μLEDs and OLEDs and determine the light spectrum and temperature change, resulting in different biological effects (TABLE 3). In a laser, light with a single wavelength is concentrated at one point, which is suitable for strong light irradiation of a narrow area197,198. LEDs and μLEDs provide strong point illumination; thus, they are suitable for applications in miniaturized implants20,21,107. Because OLEDs are thin-film devices using an organic emitter, they can be used as flexible and stretchable surface light sources, which are effectively used for wearable devices190,194. QD LEDs and perovskite LEDs have high colour purity and are suitable for emitting light of a desired wavelength with a very narrow spectrum23,151. However, their biophotonic applications are limited owing to their low external quantum efficiency and stability. In addition, some light sources can cause problematic heating in the body, which can be reduced by increasing the luminescence efficiency. Because the optical power depends on the light source, radiation time, radiation pulse and light wavelength, its optimal value should be determined after careful consideration of the purpose of the photonic diagnosis or therapy. In the case of PDT, the choice of optical system should also be made with the type of photosensitizer in mind.
Table 3 |.
Properties of optical systems used in photonic diagnosis and therapy
| Light source | Wavelength | Optical power | Irradiation time | Application | Device type | Refs |
|---|---|---|---|---|---|---|
| Laser (UCNPa) | 980 nm (540 nm) | 500 mW cm−2 (1.5 mW cm−2) | 20 min | Photochemical tissue bonding | Portable | 197 |
| Laser | 670 nm | 10 mW | 40–60 min | Photobiomodulation (Parkinson disease) | Implantable | 198 |
| LEDb | 660 nm | 1.3 mW | 30 min | Photodynamic therapy (cancer) | Implantable | 107 |
| Micro LEDc | 630 nm or 530 nm | 33 μW cm−2 or 70 μW cm−2 | 240 h | Photodynamic therapy (cancer) | Implantable | 108 |
| Micro LED | 540 nm | 44 ± 11 μW | – | Optogenetic therapy (neuromodulation) | Implantable | 20 |
| Micro LED | 470 nm | 10 mW mm−2 | – | Optogenetic therapy (pain modulation) | Implantable | 21 |
| Micro LED | 625 nm or 540 nm | – | – | Pulse oximetry | Implantable | 105 |
| OLED | 612 nm or 725 nm | 0.2 mW or 0.9 mW | – | Pulse oximetry | Wearable | 191 |
| OLED | 630–690 nm | 5 mW cm−2 | 10–30 min | Photobiomodulation (wound healing) | Wearable | 194 |
LED, light-emitting diode; OLED, organic light-emitting diode; UCNP, upconversion nanoparticle.
Photosensitizer: Rose Bengal.
Photosensitizer: chlorin e6.
Photosensitizer: Photofrin.
Because photonic healthcare devices, particularly wearable devices, can be easily used in households, they can contribute to point-of-care testing and personalized medicine, which can be particularly important for anticancer199 and diabetic treatment200. For personalized medicine, real-time diagnosis and statistical analysis of case studies are necessary to provide appropriate remedies (FIG. 5). Point-of-care testing provides readily accessible diagnosis at the bedside, and the data can be wirelessly sent to a patient’s mobile phone and/or to providers at hospitals and medical centres via transmission systems compliant with the secure Health Insurance Portability and Accountability Act of 1996 (HIPAA or the Kennedy-Kassebaum Act). This way, patients can be treated by telemedicine, either synchronously or asynchronously, in the comfort of their homes with the aid of their smartphones or similar products. While store-and-forward-type telemedicine has been established by connecting traditional tabletop diagnostics and computers, the advent of ubiquitous smartphones with round-the-clock Internet connectivity now enables the continuous collection of vast amounts of data on individual patients’ biometrics that may guide diagnosis and targeted therapies. How to cull, interpret and use such volumes of data for clinical care remains an active area of enquiry to which artificial intelligence and machine learning undoubtedly hold the key.
Fig. 5 |. Schematic illustration of mobile health and personalized telemedicine.
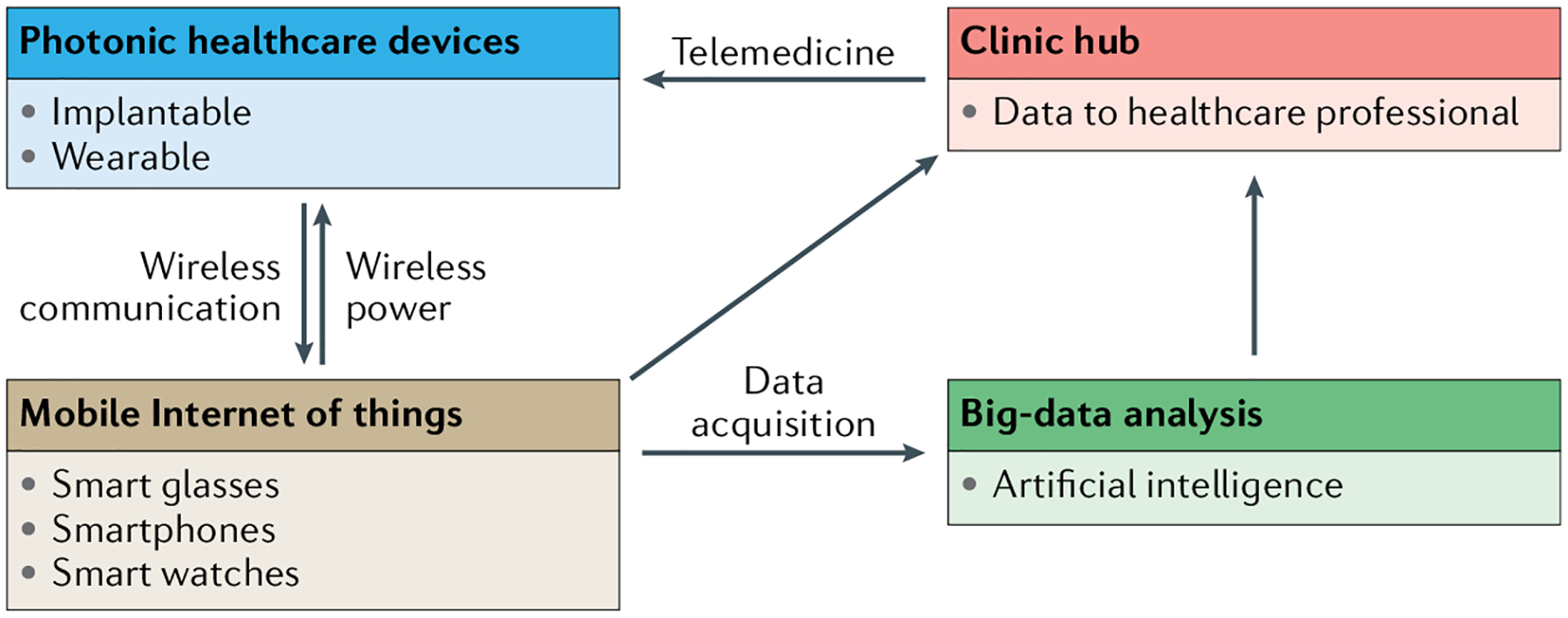
Implantable and wearable healthcare devices, combined with the Internet of things, big data and artificial intelligence, will, in the future, enable mobile and personalized telemedicine.
Acknowledgements
This research was supported by the Center for Advanced Soft Electronics (Global Frontier Project, CASE-2015M3A6A5072945), Engineering Research Center (ERC) Program (grant no. NRF-2017R1A5A1014708) and the Basic Science Research Program (2017R1E1A1A03070458) of the National Research Foundation (NRF) funded by the Ministry of Science and ICT, Korea. This work was also supported by the World Class 300 Project (S2482887) of the Small and Medium Business Administration (SMBA), Korea. The Stanford researchers acknowledge support from Stanford Catalyst for Collaborative Solutions Program and Stanford Bio-X seed funding. The Stanford researchers acknowledge support from Department of Defense Air Force Office of Scientific Research (FA9550-15-1-0106), Samsung Electronics and Stanford Catalyst for Collaborative Solutions Program.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yun SH & Kwok SJJ Light in diagnosis, therapy and surgery. Nat. Biomed. Eng 1, 0008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This review paper describes the fundamental concept of light, its interactions with biological matters and its applications to diagnosis, therapy and surgery.
- 2.Van Soest G, Regar E & van der Steen AFW Photonics in cardiovascular medicine. Nat. Photonics 9, 626–629 (2015). [Google Scholar]
- 3.Kim H et al. Multifunctional photonic nanomaterials for diagnostic, therapeutic, and theranostic applications. Adv. Mater 30, 1701460 (2018). [DOI] [PubMed] [Google Scholar]; This review paper describes the fundamental science, synthetic concepts, characteristics and biomedical applications of multifunctional photonic nanomaterials that can interact through light with biological systems.
- 4.Helmchen F & Denk W Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Tuchin VV Polarized light interaction with tissues. J. Biomed. Opt 21, 071114 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Weisleder R Molecular imaging in cancer. Science 312, 1168–1171 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Lee M-Y et al. Biodegradable photonic melanoidin for theranostic applications. ACS Nano 10, 822–831 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Tak S, Uga M, Flandin G, Dan I & Penny WD Sensor space group analysis for fNIRS data. J. Neurosci. Methods 264, 103–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horton NG et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photonics 7, 205–209 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokota T et al. Ultraflexible organic photonic skin. Sci. Adv 2, e1501856 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee D-E et al. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev 41, 2656–2672 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Shao J et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun 7, 12967 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu K et al. Simple peptide-tuned self-assembly of photosensitizers towards anticancer photodynamic therapy. Angew. Chem. Int. Ed 55, 3036–3039 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Hamblin MR, Huang Y-Y & Heiskanen V Non-mammalian hosts and photobiomodulation: do all life-forms respond to light? Photochem. Photobiol 95, 126–139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deisseroth K Optogenetics. Nat. Methods 8, 26–29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuffler DP Photobiomodulation in promoting wound healing: a review. Regen. Med 11, 107–122 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Huang Y-Y, Wang Y, Lyu P & Hamblin MR Photobiomodulation of human adipose-derived stem cells using 810 nm and 980 nm lasers operates via different mechanisms of action. Biochim. Biophys. Acta 1861, 441–449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamblin MR Shining light on the head: photobiomodulation for brain disorders. Biochim. Biophys. Acta Clin 6, 113–124 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi MH et al. Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo. Nat. Photonics 7, 987–994 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes light-guiding hydrogel implants for in vivo optogenetic nanotoxicity sensing and optogenetic therapy in diabetic mice.
- 20.Mickle AD et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature 565, 361–365 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes a wireless, miniaturized bio-optoelectronic implant for the optogenetic modulation of the peripheral nervous system.
- 21.Park SI et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol 33, 1280–1286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin G et al. Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics. Neuron 93, 509–521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirasaki Y, Supran GJ, Bawendi MG & Bulovic V Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 7, 13–23 (2013). [Google Scholar]
- 24.Biju V, Itoh T, Anas A, Sujith A & Ishikawa M Semiconductor quantum dots and metal nanoparticles: syntheses, optical properties, and biological applications. Anal. Bioanal. Chem 391, 2469–2495 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Michalet X et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–544 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medintz IL, Uyeda HT, Goldman ER & Mattoussi H Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater 4, 435–446 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Hutter E & Fendler JH Exploitation of localized surface plasmon resonance. Adv. Mater 16, 1685–1706 (2004). [Google Scholar]
- 28.Yang X, Yang M, Pang B, Vara M & Xia Y Gold nanomaterials at work in biomedicine. Chem. Rev 115, 10410–10488 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Giljohann DA et al. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed 49, 3280–3294 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manzeli S, Ovchinnikov D, Pasquier D, Yazyev OV & Kis A 2D transition metal dichalcogenides. Nat. Rev. Mater 2, 17033 (2017). [Google Scholar]
- 31.Xu M, Liang T, Shi M & Chen H Graphene-like two-dimensional material. Chem. Rev 113, 3766–3798 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Haase M & Schäfer H Upconverting nanoparticles. Angew. Chem. Int. Ed 50, 5808–5829 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Zhou B, Shi B, Jin D & Liu X Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol 10, 924–936 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Tian G et al. Mn2+ dopant-controlled synthesis of NaYF4:Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery. Adv. Mater 24, 1226–1231 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Friend RH et al. Electroluminescence in conjugated polymers. Nature 397, 121–128 (1999). [Google Scholar]; This review paper describes the background science, materials fabrication and semiconductor physics of electroluminescent conjugated polymers.
- 36.Bernholc J, Brenner D, Nardelli MB, Meunier V & Roland C Mechanical and electrical properties of nanotubes. Annu. Rev. Mater. Res 32, 347–375 (2002). [Google Scholar]
- 37.Mochalin VN, Shenderova O, Ho D & Gogotsi Y The properties and applications of nanodiamonds. Nat. Nanotechnol 7, 11–23 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Dreyer DR, Park S, Bielawski CW & Ruoff RS The chemistry of graphene oxide. Chem. Soc. Rev 39, 228–240 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Park Y, Yoo J, Lim B, Kwon W & Rhee S-W Improving the functionality of carbon nanodots: doping and surface functionalization. J. Mater. Chem. A 4, 11582–11603 (2016). [Google Scholar]
- 40.Kim H et al. Dual-color-emitting carbon nanodots for multicolor bioimaging and optogenetic control of ion channels. Adv. Sci 4, 1700325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Alamo JA Nanometre-scale electronics with III–V compound semiconductors. Nature 479, 317–323 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Chen K et al. Direct growth of single-crystalline III–V semiconductors on amorphous substrates. Nat. Commun 7, 10502 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto K Fundamentals of Optical Waveguides 2nd edn Ch. 1 (Academic, 2006). [Google Scholar]
- 44.Parker GJ in Encyclopedia of Materials: Science and Technology 2nd edn (eds Buschow KHJ et al. ) 3703–3707 (Pergamon, 2001). [Google Scholar]
- 45.Sparta DR et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protoc 7, 12–23 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humar M et al. Toward biomaterial-based implantable photonic devices. Nanophotonics 6, 414–434 (2017). [Google Scholar]
- 47.Dupuis A et al. Prospective for biodegradable microstructured optical fibers. Opt. Lett 32, 109–111 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Parker ST et al. Biocompatible silk printed optical waveguides. Adv. Mater 21, 2411–2415 (2009). [Google Scholar]
- 49.Shan D et al. Flexible biodegradable citrate-based polymeric step-index optical fiber. Biomaterials 143, 142–148 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nizamoglu S et al. Bioabsorbable polymer optical waveguides for deep-tissue photomedicine. Nat. Commun 7, 10374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo J, Zhou M & Yang C Fluorescent hydrogel waveguide for on-site detection of heavy metal ions. Sci. Rep 7, 7902 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain A, Yang AHJ & Erickson D Gel-based optical waveguides with live cell encapsulation and integrated microfluidics. Opt. Lett 37, 1472–1474 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Manocchi AK, Domachuk P, Omenetto FG & Yi H Facile fabrication of gelatin-based biopolymeric optical waveguides. Biotechnol. Bioeng 103, 725–732 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Oh JY, Xu J, Tran H & Bao Z Skin-inspired electronics: an emerging paradigm. Acc. Chem. Res 51, 1033–1045 (2018). [DOI] [PubMed] [Google Scholar]; This review paper describes intrinsically stretchable materials for conductors, semiconductors and insulators, and futuristic self-healable or biodegradable electronic materials.
- 55.Oh JY & Bao Z Second skin enabled by advanced electronics. Adv. Sci 6, 1900186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irimia-Vladu M “Green” electronics: biodegradable and biocompatible materials and devices for sustainable future. Chem. Soc. Rev 43, 588–610 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Kong D et al. Capacitance characterization of elastomeric dielectrics for applications in intrinsically stretchable thin film transistors. Adv. Funct. Mater 26, 4680–4686 (2016). [Google Scholar]
- 58.Kim D-H et al. Materials and noncoplanar mesh designs for integrated circuits with linear elastic responses to extreme mechanical deformations. Proc. Natl Acad. Sci. USA 105, 18675–18680 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuhisa N, Chen X, Bao Z & Someya T Materials and structure designs of stretchable conductors. Chem. Soc. Rev 48, 2946–2966 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Park M et al. Highly stretchable electric circuits from a composite material of silver nanoparticles and elastomeric fibres. Nat. Nanotechnol 7, 803–809 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Matsuhisa N et al. Printable elastic conductors by in situ formation of silver nanoparticles from silver flakes. Nat. Mater 16, 834–840 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Park M, Park J & Jeong U Design of conductive composite elastomers for stretchable electronics. Nano Today 9, 244–260 (2014). [Google Scholar]
- 63.Lipomi DJ et al. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol 6, 788–792 (2011). [DOI] [PubMed] [Google Scholar]
- 64.Vosgueritchian M, Lipomi DJ & Bao Z Highly conductive and transparent PEDOT:PSS films with a fluorosurfactant for stretchable and flexible transparent electrodes. Adv. Funct. Mater 22, 421–428 (2012). [Google Scholar]
- 65.Oh JY, Kim S, Baik HK & Jeong U Conducting polymer dough for deformable electronics. Adv. Mater 28, 4455–4461 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Wang Y et al. A highly stretchable, transparent, and conductive polymer. Sci. Adv 3, e1602076 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dickey MD Stretchable and soft electronics using liquid metals. Adv. Mater 29, 1606425 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Yan J, Lu Y, Chen G, Yang M & Gu Z Advances in liquid metals for biomedical applications. Chem. Soc. Rev 47, 2518–2533 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Xu F et al. Highly stretchable carbon nanotube transistors with ion gel gate dielectrics. Nano Lett 14, 682–686 (2014). [DOI] [PubMed] [Google Scholar]
- 70.Müller C et al. Tough, semiconducting polyethylene-poly(3-hexylthiophene) diblock copolymers. Adv. Funct. Mater 17, 2674–2679 (2007). [Google Scholar]
- 71.Oh JY et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 539, 411–415 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Wang GJN et al. Inducing elasticity through oligosiloxane crosslinks for intrinsically stretchable semiconducting polymers. Adv. Funct. Mater 26, 7254–7262 (2016). [Google Scholar]
- 73.Xu J et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 355, 59–64 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Kang J, Tok JB-H & Bao Z Self-healing soft electronics. Nat. Electron 2, 144–150 (2019). [Google Scholar]
- 75.Rao Y-L et al. Stretchable self-healing polymeric dielectrics cross-linked through metal-ligand coordination. J. Am. Chem. Soc 138, 6020–6027 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Kang J et al. Tough and water-insensitive self-healing elastomer for robust electronic skin. Adv. Mater 30, 1706846 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Hart LR et al. Perylene as an electron-rich moiety in healable, complementary π-π stacked, supramolecular polymer systems. Polymer 69, 293–300 (2015). [Google Scholar]
- 78.Hohlbein N, Shaaban A & Schmidt A Remote-controlled activation of self-healing behavior in magneto-responsive ionomeric composites. Polymer 69, 301–309 (2015). [Google Scholar]
- 79.Tee BC, Wang C, Allen R & Bao Z An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nat. Nanotechnol 7, 825–832 (2012). [DOI] [PubMed] [Google Scholar]
- 80.Hwang S-W et al. A physically transient form of silicon electronics. Science 337, 1640–1644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lei T et al. Biocompatible and totally disintegrable semiconducting polymer for ultrathin and ultralightweight transient electronics. Proc. Natl Acad. Sci. USA 114, 5107–5112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Irimia-Vladu M et al. Biocompatible and biodegradable materials for organic field-effect transistors. Adv. Funct. Mater 20, 4069–4076 (2010). [Google Scholar]
- 83.Bettinger CJ & Bao Z Organic thin-film transistors fabricated on resorbable biomaterial substrates. Adv. Mater 22, 651–655 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hwang S-W et al. Biodegradable elastomers and silicon nanomembranes/nanoribbons for stretchable, transient electronics, and biosensors. Nano Lett 15, 2801–2808 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Boutry CM et al. A sensitive and biodegradable pressure sensor array for cardiovascular monitoring. Adv. Mater 27, 6954–6961 (2015). [DOI] [PubMed] [Google Scholar]
- 86.Hastings JW Biological diversity, chemical mechanisms, and the evolutionary origins of bioluminescent systems. J. Mol. Evol 19, 309–321 (1983). [DOI] [PubMed] [Google Scholar]
- 87.Jones KA et al. Orthogonal luciferase-luciferin pairs for bioluminescence imaging. J. Am. Chem. Soc 139, 2351–2358 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yeh H-W et al. Red-shifted luciferase-luciferin pairs for enhanced bioluminescence imaging. Nat. Methods 14, 971–974 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maguire CA et al. Triple bioluminescence imaging for in vivo monitoring of cellular processes. Mol. Ther. Nucl. Acids 2, e99 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bhaumik S & Gambhir SS Optical imaging of Renilla luciferase reporter gene expression in living mice. Proc. Natl Acad. Sci. USA 99, 377–382 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pfleger KDG & Eidne KA Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET). Nat. Methods 3, 165–174 (2006). [DOI] [PubMed] [Google Scholar]
- 92.James JR, Oliveira MI, Carmo AM, Iaboni A & Davis SJ A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nat. Methods 3, 1001–1006 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Salahpour A et al. BRET biosensors to study GPCR biology, pharmacology, and signal transduction. Front. Endocrinol 3, 105 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kobayashi H, Picard LP, Schönegge AM & Bouvier M Bioluminescence resonance energy transfer-based imaging of protein-protein interactions in living cells. Nat. Protoc 14, 1084–1107 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Yao H, Zhang Y, Xiao F, Xia Z & Rao J Quantum dot/bioluminescence resonance energy transfer based highly sensitive detection of proteases. Angew. Chem. Int. Ed 46, 4346–4349 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Sarkar K, Xue Y & Sant S in The Immune Response to Implanted Materials and Devices (ed. Corradetti B) 81–105 (Springer, 2017). [Google Scholar]
- 97.Singh A et al. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater 13, 988–995 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Merrill EW in Poly(ethylene glycol) Chemistry: Biotechnical and Biomedical Applications (ed. Harris JM) 199–220 (Plenum, 1992). [Google Scholar]
- 99.Zhang L et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol 31, 553–556 (2013). [DOI] [PubMed] [Google Scholar]
- 100.Kastellorizios M, Papadimitrakopoulos F & Burgess DJ Multiple tissue response modifiers to promote angiogenesis and prevent the foreign body reaction around subcutaneous implants. J. Control. Rel 214, 103–111 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Laissue PP, Alghamdi RA, Tomancak P, Reynaud EG & Shroff H Assessing phototoxicity in live fluorescence imaging. Nat. Methods 14, 657–661 (2017). [DOI] [PubMed] [Google Scholar]
- 102.Kang S-K et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–79 (2016). [DOI] [PubMed] [Google Scholar]
- 103.Salles GN et al. A novel bioresorbable device as a controlled release system for protecting cells from oxidative stress from Alzheimer’s disease. Mol. Neurobiol 54, 6827–6838 (2017). [DOI] [PubMed] [Google Scholar]
- 104.Yu KJ et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater 15, 782–791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang H et al. Wireless, battery-free optoelectronic systems as subdermal implants for local tissue oximetry. Sci. Adv 5, eaaw0873 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee JO et al. A microscale optical implant for continuous in vivo monitoring of intraocular pressure. Microsyst. Nanoeng 3, 17057 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bansal A, Yang F, Xi T, Zhang Y & Ho JS In vivo wireless photonic photodynamic therapy. Proc. Natl Acad. Sci. USA 115, 1469–1474 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamagishi K et al. Tissue-adhesive wirelessly powered optoelectronic device for metronomic photodynamic cancer therapy. Nat. Biomed. Eng 3, 27–36 (2019). [DOI] [PubMed] [Google Scholar]; This paper describes an implantable optoelectronic device to overcome the light-penetration issue in the application of photodynamic therapy.
- 109.Jeong J-W et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Montgomery KL et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods 12, 969–974 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Samineni VK et al. Optogenetic silencing of nociceptive primary afferents reduces evoked and ongoing bladder pain. Sci. Rep 7, 15865 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park S-I et al. Printed assemblies of inorganic light-emitting diodes for deformable and semitransparent displays. Science 325, 977–981 (2009). [DOI] [PubMed] [Google Scholar]
- 113.Kim H-S et al. Unusual strategies for using indium gallium nitride grown on silicon (111) for solid-state lighting. Proc. Natl Acad. Sci. USA 108, 10072–10077 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim T-I et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim S et al. Microstructured elastomeric surfaces with reversible adhesion and examples of their use in deterministic assembly by transfer printing. Proc. Natl Acad. Sci. USA 107, 17095–17100 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Agarwal K, Jegadeesan R, Guo YX & Thakor NV Wireless power transfer strategies for implantable bioelectronics. IEEE Rev. Biomed. Eng 10, 136–161 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Hinchet R et al. Transcutaneous ultrasound energy harvesting using capacitive triboelectric technology. Science 365, 491–494 (2019). [DOI] [PubMed] [Google Scholar]
- 118.Djourno A & Eyries C Auditory prosthesis by means of a distant electrical stimulation of the sensory nerve with the use of an indwelt coiling. Presse Med 65, 1417 (1957). [PubMed] [Google Scholar]
- 119.Brindley GS & Lewin WS The sensations produced by electrical stimulation of the visual cortex. J. Physiol 196, 479–493 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kelly SK & Rizzo J in Artificial Vision (ed. Gabel VP) 85–97 (Springer, 2017). [Google Scholar]
- 121.Gather MC & Yun SH Single-cell biological lasers. Nat. Photonics 5, 406–410 (2011). [Google Scholar]
- 122.Humar M & Yun SH Intracellular microlasers. Nat. Photonics 9, 572–576 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koo J et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med 24, 1830–1836 (2018). [DOI] [PubMed] [Google Scholar]
- 124.Reiner R & Dvir T Tissue-electronics interfaces: from implantable devices to engineered tissues. Nat. Rev. Mater 3, 17076 (2017). [Google Scholar]
- 125.Hong YJ, Jeong H, Cho KW, Lu N & Kim D-H Wearable and implantable devices for cardiovascular healthcare: from monitoring to therapy based on flexible and stretchable electronics. Adv. Funct. Mater 29, 1808247 (2019). [Google Scholar]
- 126.Xu H, Yin L, Lui C, Sheng X & Zhao N Recent advances in biointegrated optoelectronic devices. Adv. Mater 30, 1800156 (2018). [DOI] [PubMed] [Google Scholar]
- 127.Samineni VK et al. Fully implantable, battery-free wireless optoelectronic devices for spinal optogenetics. Pain 158, 2108–2116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park SI et al. Stretchable multichannel antennas in soft wireless optoelectronic implants for optogenetics. Proc. Natl Acad. Sci. USA 113, E8169–E8177 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim R-H et al. Waterproof AlInGaP optoelectronics on stretchable substrates with applications in biomedicine and robotics. Nat. Mater 9, 929–937 (2010). [DOI] [PubMed] [Google Scholar]
- 130.Lee W et al. Transparent, conformable, active multielectrode array using organic electrochemical transistors. Proc. Natl Acad. Sci. USA 114, 10554–10559 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Park S et al. Self-powered ultra-flexible electronics via nano-grating-patterned organic photovoltaics. Nature 561, 516–521 (2018). [DOI] [PubMed] [Google Scholar]
- 132.Nihongaki Y, Kawano F, Nakajima T & Sato M Photoactivatable CRISPR-Cas9 for optogenetic genome editing. Nat. Biotechnol 33, 755–760 (2015). [DOI] [PubMed] [Google Scholar]
- 133.Nussinovitch U & Gepstein L Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat. Biotechnol 13, 750–754 (2015). [DOI] [PubMed] [Google Scholar]
- 134.Tan P, He L, Han G & Zhou Y Optogenetic immunomodulation: shedding light on antitumor immunity. Trends Biotechnol 35, 215–226 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park JH et al. Optogenetic modulation of urinary bladder contraction for lower urinary tract dysfunction. Sci. Rep 7, 40872 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Häusser M Optogenetics: the age of light. Nat. Methods 11, 1012–1014 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Iaccarino HF et al. Gamma frequency entertainment attenuates amyloid load and modifies microglia. Nature 540, 230–235 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates the feasibility of optogenetic therapy to control brain signals with a gamma frequency of 40 Hz for the treatment of Alzheimer disease.
- 138.Aron L & Yankner BA Neural synchronization in Alzheimer’s disease. Nature 540, 207–208 (2016). [DOI] [PubMed] [Google Scholar]
- 139.Kim CK, Adhikari A & Deisseroth K Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci 18, 222–235 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu Y et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng 3, 58–68 (2019). [DOI] [PubMed] [Google Scholar]
- 141.Famm K, Litt B, Tracey KJ, Boyden ES & Slaoui M Drug discovery: a jump-start for electroceuticals. Nature 496, 159–161 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim J et al. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv 2, e1600418 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim J et al. Miniaturized battery-free wireless systems for wearable pulse oximetry. Adv. Funct. Mater 27, 1604373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.White MS et al. Ultrathin, highly flexible and stretchable PLEDs. Nat. Photonics 7, 811–816 (2013). [Google Scholar]
- 145.Kim T et al. Fully stretchable optoelectronic sensors based on colloidal quantum dots for sensing photoplethysmographic signals. ACS Nano 11, 5992–6003 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Kaltenbrunner M Ultrathin and lightweight organic solar cells with high flexibility. Nat. Commun 3, 770 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yin D et al. Efficient and mechanically robust stretchable organic light-emitting devices by a laser-programmable buckling process. Nat. Commun 7, 11573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Han T-H et al. Extremely efficient flexible organic light-emitting diodes with modified graphene anode. Nat. Photonics 6, 105–110 (2012). [Google Scholar]
- 149.Liang J, Li L, Niu X, Yu Z & Pei Q Elastomeric polymer light-emitting devices and displays. Nat. Photonics 7, 817–824 (2013). [Google Scholar]
- 150.Liang J et al. Silver nanowire percolation network soldered with graphene oxide at room temperature and its application for fully stretchable polymer light-emitting diodes. ACS Nano 8, 1590–1600 (2014). [DOI] [PubMed] [Google Scholar]
- 151.Bade SGR et al. Stretchable light-emitting diodes with organometal-halide-perovskite-polymer composite emitters. Adv. Mater 29, 1607053 (2017). [DOI] [PubMed] [Google Scholar]
- 152.Son D et al. An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat. Nanotechnol 13, 1057–1065 (2018). [DOI] [PubMed] [Google Scholar]
- 153.Larson C et al. Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science 351, 1071–1074 (2016). [DOI] [PubMed] [Google Scholar]
- 154.Takei K, Honda W, Harada S, Arie T & Akita S Toward flexible and wearable human-interactive health-monitoring devices. Adv. Healthc. Mater 4, 487–500 (2015). [DOI] [PubMed] [Google Scholar]
- 155.Senior M Novartis signs up for Google smart lens. Nat. Biotechnol 3, 856 (2014). [DOI] [PubMed] [Google Scholar]
- 156.Park J et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv 4, eaap9841 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes smart contact lenses that can measure tear glucose concentration and display it using light-emitting diodes powered wirelessly.
- 157.Yao H, Shum AJ, Cowan M, Lähdesmäki I & Parviz BA A contact lens with embedded sensor for monitoring tear glucose level. Biosens. Bioelectron 26, 3290–3296 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Elsherif M, Hassan MU, Yetisen AK & Butt H Wearable contact lens biosensors for continuous glucose monitoring using smartphones. ACS Nano 12, 5452–5462 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leonardi M, Pitchon EM, Bertsch A, Renaud P & Mermoud A Wireless contact lens sensor for intraocular pressure monitoring: assessment on enucleated pig eyes. Acta Ophthalmol 87, 433–437 (2009). [DOI] [PubMed] [Google Scholar]
- 160.Kim J, Campbell AS, de Ávila BE-F & Wang J Wearable biosensors for healthcare monitoring. Nat. Biotechnol 37, 389–406 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Domschke A, March WF, Kabilan S & Lowe C Initial clinical testing of a holographic non-invasive contact lens glucose sensor. Diabetes Technol. Ther 8, 89–93 (2006). [DOI] [PubMed] [Google Scholar]
- 162.Deng J et al. Self-reporting colorimetric analysis of drug release by molecular imprinted structural color contact lens. ACS Appl. Mater. Interfaces 10, 34611–34617 (2018). [DOI] [PubMed] [Google Scholar]
- 163.March WF, Mueller A & Herbrechtsmeier P Clinical trial of a noninvasive contact lens glucose sensor. Diabetes Technol. Ther 6, 782–789 (2004). [DOI] [PubMed] [Google Scholar]
- 164.Badugu R, Lakowicz JR & Geddes CD Noninvasive continuous monitoring of physiological glucose using a monosaccharide-sensing contact lens. Anal. Chem 76, 610–618 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Badugu R, Lakowicz JR & Geddes CD A glucose-sensing contact lens: from bench top to patient. Curr. Opin. Biotechnol 16, 100–107 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang J, Hodge W, Hutnick C & Wang X Noninvasive diagnostic devices for diabetes through measuring tear glucose. J. Diabetes Sci. Technol 5, 166–172 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Khalil OS Spectroscopic and clinical aspects of noninvasive glucose measurements. Clin. Chem 45, 165–177 (1999). [PubMed] [Google Scholar]
- 168.Maruo K, Tsurugi M, Tamura M & Ozaki Y In vivo noninvasive measurement of blood glucose by near-infrared diffuse-reflectance spectroscopy. Appl. Spectrosc 57, 1236–1244 (2003). [DOI] [PubMed] [Google Scholar]
- 169.Hahn SK, Lee G-H, Sim JY, Koo JH & Keum DH, Smart remotely controlled contact lens Korean Patent 10-2017-0059225, PCT/KR2017/015243, Korean Patent 10-1956701 (2019).
- 170.Tang J et al. (2013). Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: in vivo and in vitro. Investig. Ophthalmol. Vis. Sci 54, 3681–3690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Eells JT et al. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl Acad. Sci. USA 100, 3439–3444 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Albarracin R, Eells JT & Valter K Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Investig. Ophthalmol. Vis. Sci 52, 3582–3592 (2011). [DOI] [PubMed] [Google Scholar]
- 173.Cashmore AR, Jarillo JA, Wu YJ & Liu D Cryptochromes: blue light receptors for plants and animals. Science 284, 760–765 (1999). [DOI] [PubMed] [Google Scholar]
- 174.Thresher RJ et al. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science 282, 1490–1494 (1998). [DOI] [PubMed] [Google Scholar]
- 175.van der Horst GT et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630 (1999). [DOI] [PubMed] [Google Scholar]
- 176.Kim W-Y et al. ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature 449, 356–360 (2007). [DOI] [PubMed] [Google Scholar]
- 177.Emery P, So WV, Kaneko M, Hall JC & Rosbash M CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95, 669–679 (1998). [DOI] [PubMed] [Google Scholar]
- 178.Strong RE et al. Narrow-band blue-light treatment of seasonal affective disorder in adults and the influence of additional nonseasonal symptoms. Depress. Anxiety 26, 273–278 (2009). [DOI] [PubMed] [Google Scholar]
- 179.Gordijn MC & Meesters Y The effects of blue-enriched light treatment compared to standard light treatment in seasonal affective disorder. J. Affect. Disord 136, 72–80 (2012). [DOI] [PubMed] [Google Scholar]
- 180.Glickman G, Byrne B, Pineda C, Hauck WW & Brainard GC Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs). Biol. Psychiatry 59, 502–507 (2006). [DOI] [PubMed] [Google Scholar]
- 181.Anderson JL, Glod CA, Dai J, Cao Y & Lockley SW Lux vs. wavelength in light treatment of seasonal affective disorder. Acta Psychiatr. Scand 120, 203–212 (2009). [DOI] [PubMed] [Google Scholar]
- 182.Bragard I & Coucke PA Impact of the use of Luminette on well-being at work in a radiotherapy department. Cancer Radiother 17, 731–735 (2013). [DOI] [PubMed] [Google Scholar]
- 183.Langevin RH, Laurent A & Sauvéc A Preliminary assessment on the effectiveness of the Luminette in adolescents with a delayed sleep phase syndrome (DSPS): randomized single blind placebo-controlled study. Méd. Sommeil 11, 91–97 (2014). [Google Scholar]
- 184.Kirschbaum-Lesch I, Gest S, Legenbauer T & Holtmann M Feasibility and efficacy of bright light therapy in depressed adolescent inpatients. Z. Kinder Jugendpsychiatr. Psychother 46, 423–429 (2018). [DOI] [PubMed] [Google Scholar]
- 185.Elgendi M On the analysis of fingertip photoplethysmogram signals. Curr. Cardiol. Rev 8, 14–25 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chua C-PE, Redmond SJ, McDarby G & Heneghan C Towards using photo-plethysmogram amplitude to measure blood pressure during sleep. Ann. Biomed. Eng 38, 945–954 (2010). [DOI] [PubMed] [Google Scholar]
- 187.Kim M-G, Kim C, Alrowais H & Brand O Multiscale and uniform liquid metal thin-film patterning based on soft lithography for 3D heterogeneous integrated soft microsystems: additive stamping and subtractive reverse stamping. Adv. Mater. Technol 3, 1800061 (2018). [Google Scholar]
- 188.Han D et al. Flexible blade-coated multicolor polymer light-emitting diodes for optoelectronic sensors. Adv. Mater 29, 1606206 (2017). [DOI] [PubMed] [Google Scholar]
- 189.Park S et al. Ultraflexible near-infrared organic photodetectors for conformal photoplethysmogram sensors. Adv. Mater 30, 1802359 (2018). [DOI] [PubMed] [Google Scholar]
- 190.Lee H et al. Toward all-day wearable health monitoring: an ultralow-power, reflective organic pulse oximetry sensing patch. Sci. Adv 4, eaas9530 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes thin-film photonic devices for pulse oximetry with significantly reduced power consumption, enabling all-day wearable healthcare.
- 191.Khan Y et al. A flexible organic reflectance oximeter array. Proc. Natl Acad. Sci. USA 115, E11015–E11024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chu B, Burnett W, Chung JW & Bao Z Bring on the bodynet. Nature 549, 328–330 (2017). [DOI] [PubMed] [Google Scholar]; This Comment article describes stretchable sensors, circuits and batteries to fabricate wearable devices on the bodynet, a network of sensors, screens and smart devices woven into our clothing, worn on our skin and implanted in our bodies.
- 193.Karu TI Cellular mechanisms of low-power laser therapy. Proc. SPIE 5149, 60–66 (2003). [Google Scholar]
- 194.Jeon Y et al. A wearable photobiomodulation patch using a flexible red-wavelength OLED and its in vitro differential cell proliferation effects. Adv. Mater. Technol 3, 1700391 (2018). [Google Scholar]
- 195.Lee HE et al. Trichogenic photostimulation using monolithic flexible vertical AlGaInP light-emitting diodes. ACS Nano 12, 9587–9595 (2018). [DOI] [PubMed] [Google Scholar]
- 196.Kalajian TA, Aldoukhi A, Veronikis AJ, Persons K & Holick MF Ultraviolet B light emitting diodes (LEDs) are more efficient and effective in producing vitamin D3 in human skin compared to natural sunlight. Sci. Rep 7, 11489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Han S et al. Upconversion nanoparticles/hyaluronate-rose bengal conjugate complex for noninvasive photochemical tissue bonding. ACS Nano 11, 9979–9988 (2017). [DOI] [PubMed] [Google Scholar]
- 198.Darlot F et al. Near-infrared light is neuroprotective in a monkey model of Parkinson disease. Ann. Neurol 79, 59–75 (2016). [DOI] [PubMed] [Google Scholar]
- 199.Friedman AA, Letai A, Fisher DE & Flaherty KT Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 15, 747–756 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Collins FS & Varmus H A new initiative on precision medicine. N. Engl. J. Med 372, 793–795 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Deisseroth K Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci 18, 1213–1225 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Papagiakoumou E et al. Scanless two-photon excitation of channelrhodopsin-2. Nat. Methods 7, 848–854 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gong Y et al. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science 350, 1361–1366 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Berlin S et al. Photoactivatable genetically encoded calcium indicators for targeted neuronal imaging. Nat. Methods 12, 852–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kyung T et al. Optogenetic control of endogenous Ca2+ channels in vivo. Nat. Biotechnol 33, 1092–1096 (2015). [DOI] [PubMed] [Google Scholar]
- 206.Steinbeck JA et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat. Biotechnol 33, 204–209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lapp H et al. Frequency-dependent drug screening using optogenetic stimulation of human iPSC-derived cardiomyocytes. Sci. Rep 7, 9629 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.PDB ID 1LCI. Conti E, Franks NP & Brick P Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes. Structure 4, 287–298 (1996). [DOI] [PubMed] [Google Scholar]


