Abstract
Background
Mineralocorticoid receptor antagonist (MRA) treatment produces beneficial left ventricular (LV) remodeling in nonischemic dilated cardiomyopathy (NIDCM). This study addressed the timing of maximal beneficial LV remodeling in NIDCM when adding MRA.
Methods
We studied 12 patients with NIDCM on stable beta-blocker and angiotensin-converting enzyme inhibitor/angiotensin receptor-blocking therapy who underwent cardiac magnetic resonance imaging (CMR) before and after 6–31 months of continuous MRA therapy.
Results
At baseline, the LV ejection fraction (LVEF) was 24 (19, 27)% (median, IQR). The LV end-systolic volume index (LVESVI) was 63 (57, 76) ml, and the LV stroke volume index (LVSVI) was 19 (14, 21) ml, all depressed. After adding MRA to the HF regimen, the LVEF increased to 47 (42, 52)%, with a decrease in LVESVI to 36 (33, 45) ml, and increase in LVSVI to 36 (28, 39) ml (for each, P <0 .0001). Using generalized least squares analysis, the maximal beneficial remodeling (defined by maximal increase in LVEF, the maximal decrease in LVESVI and maximal increase in LVSVI) was achieved after approximately 12–16 months of MRA treatment.
Conclusions
Adding MRA to a standard medical regimen for NIDCM resulted in beneficial LV remodeling. The maximal beneficial remodeling was achieved with 12–16 months of MRA therapy. These results have implications for the timing of other advanced therapies, such as placing internal cardioverter-defibrillators.
Keywords: Heart failure with reduced left ventricular ejection fraction, Mineralocorticoid receptor antagonist, Cardiac magnetic resonance imaging
INTRODUCTION
Mineralocorticoid receptor antagonist (MRA) treatment produces beneficial left ventricular (LV) remodeling in heart failure with reduced ejection fraction (HFrEF) [1, 2]. MRA is thought to be beneficial via inhibition of aldosterone’s effects in multiple pathophysiologic pathways [3, 4]. We previously reported the results of adding MRA to anti-failure therapy consisting of angiotensin converting-enzyme inhibitor/angiotensin receptor blocking (ACE-I/ARB) and beta-adrenergic receptor (BB) blocking drugs [1] in patients with non-ischemic dilated cardiomyopathy (NIDCM), and that the extent of LV interstitial fibrosis is related to beneficial remodeling [5]. However, the duration of MRA therapy for which maximal beneficial remodeling can be achieved has received limited study. Because the patients in our study were evaluated at variable times after adding MRA to their anti-failure regimen, this allowed an opportunity to evaluate the optimal timing of beneficial LV remodeling in such patients with NIDCM. We also examined factors potentially related to the interaction of interstitial fibrosis and remodeling.
METHODS
Study Design and Participants
This work was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving human subjects. Informed consent was obtained for experimentation with human subjects. The study was approved by the IRB’s of both the Vanderbilt University Medical Center and the Tennessee Valley Health Care System, Nashville, TN.
We studied 12 patients who were previously described with newly diagnosed NIDCM [1, 5] using cardiac magnetic resonance (CMR) imaging before and after adding MRA to a standard HF regimen. Outpatient clinic visits with a diagnosis of HF were reviewed for inclusion/exclusion criteria. The inclusion criteria were: age ≥18, NYHA Functional Class II-IV HF, echocardiographic LV ejection fraction (LVEF) of < 35%, and serum potassium < 5.0 mg/dL. Exclusion criteria were: presence of or indication for an internal cardioverter-defibrillator (ICD); prior myocardial infarction on ECG; stress test positive for myocardial ischemia or infarction or angiographic coronary artery disease with ≥50% stenosis in a major epicardial artery. Further exclusion criteria included reversible causes of LV dysfunction such as myocarditis, post-partum cardiomyopathy, alcoholic cardiomyopathy, severe chronic obstructive airway disease, creatinine > 2.5 mg/dL or estimated glomerular filtration rate < 30 ml/min/1.73m2 (contraindication for gadolinium), uncontrolled atrial fibrillation, current MRA therapy, and physician preference. Of 16 patients enrolled, 4 did not complete the protocol because of normal LVEF on CMR (1), bronchoconstriction with adenosine (1), patient withdrawal (1), and placement of an ICD for primary prevention (1), leaving a group of 12 subjects (8 male, 4 female). Both BB and ACE-I/ARB drugs were uptitrated to stable and maximally tolerated doses for at least 3 months prior to the initial CMR study. The dose of spironolactone was 25 mg in 5 patients and 50 mg in 6 patients. One patient received eplerenone 50 mg due to intolerance of spironolactone.
Duration of Symptoms
The duration of HF prior to the baseline CMR study was estimated by chart review for the onset of heart failure symptoms (e.g. dyspnea) or the initial date of other quantitative testing, such as echocardiography and cardiac catheterization.
CMR Imaging
CMR was performed on a 1.5-T Siemens Magnetom Avanto scanner (Erlangen, Germany), using a comprehensive study protocol which examined LV volume, performance, myocardial perfusion (both at rest and during adenosine administration), and late gadolinium enhancement imaging using a protocol that has been published in detail previously [1]. T1, the exponential time constant for CMR longitudinal relaxation, was employed as a quantitative estimate of interstitial fibrosis using the Look-Locker data acquisition protocol, yielding the value T1LL as previously described [5].
Statistical Analysis
Data are presented as median values (±IQR) and normalized for body surface area where applicable. The LV volumes, myocardial rest-stress perfusion indices and T1LL data before and after adding MRA treatment were compared using the Wilcoxon signed-rank two-sample paired test.
To examine the longitudinal trends of LV performance and metabolic efficiency over the duration of treatment, we employed generalized least squares (GLS) analysis to account for the correlations between serial measurements (in months) on the same subject, employing First Order Autoregressive AR(1) covariance structure [6]. The nonlinear relationships between months and outcomes were modeled use restricted cubic splines with 3 knots. To examine the relationship between T1LL and LV volumes, LVEF and myocardial perfusion indices, we performed regression analyses where the final measures of LV volumes, LVEF and myocardial perfusion indices were regressed on T1LL, adjusting for the baseline values, the duration of treatment and symptoms. Statistical analyses were performed in R (R Development Core Team, Vienna, Austria). Statistical significance was judged to be two-tailed P≤0.05.
RESULTS
Of the 12 patients, 5 completed their follow-up CMR studies after 6 months’ MRA treatment, and the other 7 patients completed their follow-up CMR scan after 9–31 months’ treatment. This afforded the opportunity to evaluate the timing of optimal LV remodeling after the initiation of MRA therapy. The follow-up studies were completed as feasible and not by an intentional delay in the study design.
The patients’ median age was 52 (45,55) years, and the median duration of HF was 14 (5, 32) months, based on symptoms or available cardiac test results. The median baseline LVEF on CMR was severely depressed, averaging 24%, with LV dilatation and reduced LV stroke volumes (Table 1) compared to normative data [7]. The LVEF on the original qualifying studies (11 echocardiograms and one contrast ventriculogram) averaged 21 (7) (SD) %. There was an average of 14(8) weeks between the qualifying studies and the baseline CMR study. There was no significant difference between the baseline studies and the baseline CMR study which was performed after optimizing ACE-I/ARB and BB therapy. Other baseline data have been previously published [1].
Table 1.
LV volumes, perfusion, interstitial fibrosis and metabolic indices before and after adding MRA (median, IQR)
| N | Baseline | Final | P-value | |
|---|---|---|---|---|
| LVEF (%) | 12 | 24 (19, 27) | 47 (42, 52) | <0.001 |
| LVEDVI (ml/m2) | 12 | 83 (77, 95) | 74 (62, 84) | 0.001 |
| LVESVI (ml/m2) | 12 | 63 (57, 76) | 36 (33, 45) | <0.001 |
| LVSVI (ml/m2) | 12 | 19 (14, 21) | 36 (28, 39) | <0.001 |
| Subendocardial Perfusion Index | 12 | 0.13 (0.11, 0.14) | 0.14(0.12, 16) | <0.001 |
| Subendocardial Perfusion Reserve | 12 | 1.6 (1.5, 1.7) | 1.8(1.7, 1.9) | <0.001 |
| T1LL (msec) | 12 | 369 (357, 390) | 380 (346, 418) | 0.44 |
| kmono (min−1) | 11 | 0.041(0.038, 0.043) | 0.042(0.038, 0.048) | 0.002 |
| kmono/RPP × (10−6) (min−1/beats min mmHg) | 11 | 5.5 (4.8, 6.7) | 6.4 (5.4, 7.1) | 0.003 |
| LV WMI (×106) (ml × mmHg/m2/min−1) | 11 | 3.6(2.2, 4.0) | 5.4(4.4, 5.9) | 0.001 |
Abbreviations: kmono, myocardial monoexponential [11C] acetate clearance rate; kmono/RPP, kmono/systolic rate pressure product; LV, left ventricular; LVEDVI, LV end-diastolic volume index; LVEF, LV ejection fraction; LVESVI, LV end-systolic volume index; LVSVI, LV stroke volume index; T1LL, the exponential time constant for CMR longitudinal relaxation using Look-Locker data acquisition protocol; LVWMI, LV work-metabolic index (LV stroke work/kmono).
Effects of Adding MRA on Beneficial Remodeling
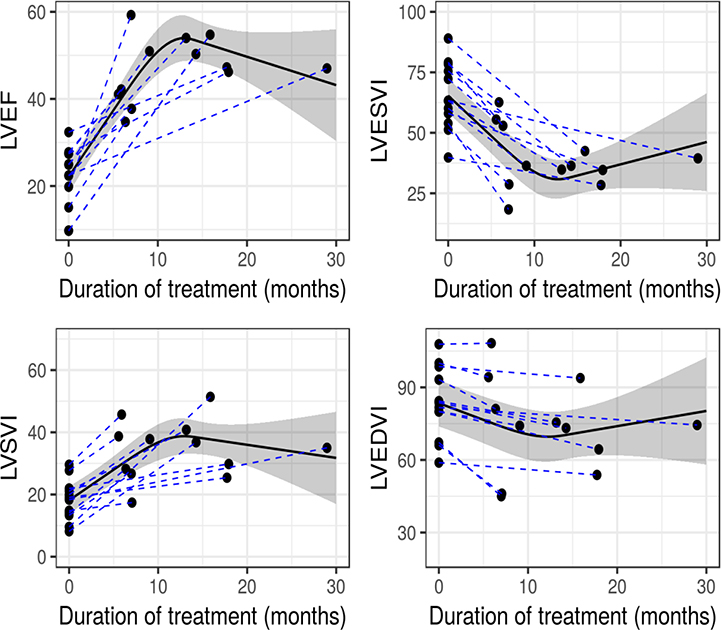
Following the addition of MRA, the LVEF increased by a median of 22 (15, 32) EF units (P < 0.001), and the LVESVI decreased by 24 (21,30) ml (P <0.001). The LVEF increased after 6 months MRA therapy compared to baseline and increased further after 12–16 months treatment (Fig. 1). The model produced by GLS analysis demonstrated a significant, nonlinear relationship between the duration of treatment and LVEF (χ295.2, df=2, p < 0.001) and a plateau of the increase in LVEF at 12–16 months. The GLS analysis again demonstrated similarly non-linear, significant relations between the duration of treatment and LVESVI (χ2=110, df=2), LV stroke volume index (χ2=18.9, df=2) and LV end-diastolic volume index (χ2=41.2, df=2), also consistent with beneficial LV remodeling (all P ≤ 0.001). The changes in LVEF and LVESVI were the most prominent.
Figure 1. Beneficial LV remodeling over time.
GLS analysis demonstrates a statistically significant, nonlinear, progressive increase in LVEF, with apparent plateau at 12–16 months of treatment. This improvement is related to sharp decrease in LVESVI, increase in LVSVI and lesser change in LVEDVI (all P≤0.001). Solid line= model-predicted values. Shaded band= pointwise 95% confidence limits of predicted values. GLS=generalized least squares analysis; LVEF=left ventricular ejection fraction; LVESVI=LV stroke volume index; LVEDVI=LV end-diastolic volume index.
Duration of Symptoms and Relation to Interstitial Fibrosis
As shown previously [5], the baseline T1LL has a significant relation to the decrease in LVESVI after adding MRA. Thus, we compared the baseline T1LL to the duration of symptoms in 11 of 12 patients, excluding one who had previously used cocaine. There was a significant relation between the duration of symptoms and T1LL, such that a shorter duration of symptoms was associated with greater values for T1LL, the latter signifying less interstitial fibrosis (p=0.03) (b=−1.03, 95% CI=−1.92 to −0.15, P=0.03) (Fig. 2).
Figure 2. Relation of interstitial fibrosis to duration of symptoms.
Shorter duration of symptoms was associated with greater T1LL (less interstitial fibrosis) (b=−1.0, 95% CI=−1.9, −0.1, p=0.03). See Figure 1 for statistical explanation. T1LL= exponential time constant for cardiac magnetic resonance longitudinal relaxation using Look-Locker data acquisition protocol.
Although the subendocardial perfusion reserve increased following the addition of MRA to HF treatment (Table 1), this was not correlated with interstitial fibrosis as estimated by the baseline T1LL. Also, there was no relation between T1LL and the final LVEF after MRA, after adjusting for baseline LVEF and the duration of treatment (b=0.14, P=0.19).
Effect of MRA Dosage on LVEF
We examined the relation of the dosage of MRA to changes in LVEF. With 25 mg spironolactone the LVEF increased by 20 (10) EF units (n=5), and with 50 mg the LVEF increased by 27 (13) units (n=6) (P=NS), excluding the one patient receiving eplerenone.
DISCUSSION
Duration of Treatment and Effect of MRA
The primary finding of this study is that the LVEF increased progressively for at least one year after adding MRA to ACE-I/ARB and BB treatment in NIDCM. Two factors were identified that appeared to mediate this beneficial remodeling following the addition of MRA therapy: 1) the amount of interstitial fibrosis at enrollment and 2) the duration of HF symptoms prior to entering the study.
The use of MRA has been incorporated into HF Guidelines because of improvement in clinical status and LVEF [8]. With regard to LVEF, we reviewed the literature in patients with chronic heart failure with HFrEF, in which MRA treatment was added to other HF therapy with documentation of LVEF, excluding studies after acute myocardial infarction. A significant increase in LVEF vs. placebo was observed in 9 of 12 studies, ranging 3–11 EF units [2] [9–16], excluding the present work in which LVEF increased by a median of 22 units. These studies lasted 4–12 months, with the current study lasting longer. Among the remaining 3 studies, 2 lasted only 2 and 3 months, respectively, showing no significant change in LVEF [17, 18], and the other showed no significant change over an intermediate term of 9 months[19].
The work of Chan et al. has the greatest relevance to the present study [9]. These investigators employed CMR to study NIDCM patients treated with MRA. Similar to our findings, there was a 5 unit increase in LVEF at 6 months MRA treatment and an 11 unit increase at 12 months (p=0.01). The time-dependent increase in LVEF is similar to our results. The present study extends this work, and the GLS analysis we supply demonstrates an apparent plateau in the improvement in LVEF after 12–16 months of MRA treatment.
Factors Affecting LV Remodeling
In the current study, the LVESVI decreased (beneficial remodeling), and GLS analysis showed the maximal improvement occurred at 12–16 months. The decrease in LVESVI was responsible for the increase in LVEF, and such reductions in LVESVI are consistent with improved contractility [20], reduced systemic vascular resistance or both.
In the present study, all patients had beneficial LV remodeling. This is a likely consequence of having no replacement fibrosis and little interstitial fibrosis [5, 21], which is likely related to an early stage of non-ischemic heart failure. In this study we showed that the interstitial fibrosis estimated by T1LL was related to the duration of HF, with the least fibrosis associated with the shortest duration of symptoms. The magnitude of beneficial remodeling in this study may be an example of a near-maximal, expectable effect of anti-failure therapy in recent onset NIDCM. The median duration of HF symptoms in our patients was 14 (5, 32) months before adding MRA therapy to already-established ACE-I/ARB and BB regimens. In our review of the MRA literature, we found no quantitative information relating the duration of HF to changes in LVEF in any published studies [2, 9–19]. Since the present study involved CMR, the selection criteria favored enrolling patients with new onset HF, because patients with chronically depressed LVEF often have internal cardiac defibrillators. Thus, the detection and prompt treatment of new onset NIDCM was likely associated with finding patients with the least interstitial fibrosis and the greatest potential for beneficial remodeling. In addition to its direct myocardial effects, spironolactone enhances nitric oxide in the peripheral vasculature, which might reduce vascular impedance and further augment LVEF [22].
Several variables displayed in Table 1 were associated with beneficial remodeling, but only T1LL was quantitatively related to the improvement in LVESVI. Our prior work has shown that improvement in myocardial perfusion reserve, myocardial oxidative metabolism and LV systolic efficiency (LV work-metabolic index) accompany beneficial remodeling [1]. Neglia et.al showed that myocardial perfusion reserve is impaired in NIDCM using PET [23] and that it is prognostically important [24]. In the present study, adding MRA to anti-failure therapy was associated with an increase in subendocardial perfusion reserve, but we found no significant relations between T1LL and the subendocardial perfusion index, subendocardial perfusion reserve or the change in LVEF. Tsagalou et. al. showed that reduced coronary blood flow reserve is related to greater intercapillary distance and that interstitial fibrosis was increased in patients with NIDCM, but they also found no relationship between interstitial fibrosis and coronary blood flow reserve [25]. Possibly, little interstitial fibrosis is a permissive factor for beneficial remodeling although not quantitatively related to the degree of remodeling.
An additional factor relating to improved LVEF in HFrEF is the dose of MRA. Cicoira et. al. showed a dose-response relation between the dose of spironolactone and improvement in LVEF [16]. Our results were directionally similar, but not statistically significant in this small number of patients.
Limitations
We studied a relatively small number of patients, but we employed CMR, which has excellent accuracy and thus allows the study of fewer patients than needed with echocardiography to document significant changes in LV dimensions and LVEF [26]. The present study was not a placebo controlled study, but the patients were compared against themselves, with findings that were highly statistically significant, directionally similar to the previous placebo controlled studies of MRA [2, 9–19] and in conformity with HF treatment guidelines [8] which advise treatment with MRA. While this study did not include placebo-treated control subjects, we believe that spontaneous recovery of LV function is very unlikely because we excluded patients with possible causes of transient LV dysfunction, such as myocardial ischemia and myocarditis.
Conclusions
The greater than average improvement in LV remodeling we found in comparison to other studies may have been due to the relatively prompt treatment of new onset NIDCM with anti-failure therapy. We propose a mechanism for optimal beneficial remodeling, such that the likelihood of the greatest beneficial remodeling is related to the least interstitial fibrosis. The statistical modeling of our results confirmed that a longer duration of treatment including MRA may be useful for improving LVEF in recent-onset NIDCM and suggested that a longer duration of therapy may be considered before proceeding to other therapies, such as the use of internal cardioverter-defibrillators for primary prevention. Further studies to confirm these findings may be useful.
Acknowledgement
The authors thank Adam Stein, AS, RT, Francesca Sabo, BS, RT Donald CiFelli, BS, RT, Barbara Konz, RN, Amber Brock, RN, Debra Rassel, RN, Linda Howerton, RN, Brenda White, RN and Rebecca Hung, MD for their contributions. The authors thank the Vanderbilt Heart Advisory Council Fund for their support. The Gd-DTPA was used off-label.
Funding: Supported in part by a Discovery Grant from the Vanderbilt University Medical Center and by the Vanderbilt CTSA grant UL1RR024975 NCRR/NIH, CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences and U01HL100398. Drs. Adkisson and Bell were supported by T32 HL 07411–31. Dr. Bell is supported by K12 HD 043483–11 from NIH/NICHD, Paul B. Beeson K23AG048347 award from NIA and by the Eisenstein Women’s Heart Fund. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
Clinical trials Registration: http://www.clinicaltrials.gov ID NCT00574119.
REFERENCES
- 1.Bell SP, Adkisson DW, Lawson MA, et al. Antifailure therapy including spironolactone improves left ventricular energy supply-demand relations in nonischemic dilated cardiomyopathy. J Am Heart Assoc. 2014;3: pii: e000883. doi: 10.1161/JAHA.114.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsutamoto T, Wada A, Maeda K, et al. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol. 2001;37:1228–33. [DOI] [PubMed] [Google Scholar]
- 3.Pitt B, Pedro Ferreira J, Zannad F. Mineralocorticoid receptor antagonists in patients with heart failure: current experience and future perspectives. Eur Heart J Cardiovasc Pharmacother. 2017;3:48–57. [DOI] [PubMed] [Google Scholar]
- 4.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–97. [DOI] [PubMed] [Google Scholar]
- 5.Bradham WS, Bell SP, Adkisson DW, et al. Myocardial T1 Measurement Predicts Beneficial LV Remodeling After Long-Term Heart Failure Therapy. J Card Fail. 2017;23:262–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrell FE Jr. Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis. Springer; 2001. [Google Scholar]
- 7.Salton CJ, Chuang ML, O’Donnell CJ, et al. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J Am Coll Cardiol. 2002;39:1055–60. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017. [DOI] [PubMed] [Google Scholar]
- 9.Chan AK, Sanderson JE, Wang T, et al. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J Am Coll Cardiol. 2007;50:591–6. [DOI] [PubMed] [Google Scholar]
- 10.Feola M, Menardi E, Ribichini F, et al. Effects of the addition of a low dose of spironolactone on brain natriuretic peptide plasma level and cardiopulmonary function in patients with moderate congestive heart failure. Med Sci Monit. 2003;9:Cr341–5. [PubMed] [Google Scholar]
- 11.Vizzardi E, D’Aloia A, Giubbini R, et al. Effect of spironolactone on left ventricular ejection fraction and volumes in patients with class I or II heart failure. Am J Cardiol. 2010;106:1292–6. [DOI] [PubMed] [Google Scholar]
- 12.Vizzardi E, Sciatti E, Bonadei I, et al. Effects of spironolactone on ventricular-arterial coupling in patients with chronic systolic heart failure and mild symptoms. Clin Res Cardiol. 2015;104:1078–87. [DOI] [PubMed] [Google Scholar]
- 13.Boccanelli A, Mureddu GF, Cacciatore G, et al. Anti-remodelling effect of canrenone in patients with mild chronic heart failure (AREA IN-CHF study): final results. Eur J Heart Fail. 2009;11:68–76. [DOI] [PubMed] [Google Scholar]
- 14.Li MJ, Huang CX, Okello E, et al. Treatment with spironolactone for 24 weeks decreases the level of matrix metalloproteinases and improves cardiac function in patients with chronic heart failure of ischemic etiology. Can J Cardiol. 2009;25:523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasama S, Toyama T, Kumakura H, et al. Effect of spironolactone on cardiac sympathetic nerve activity and left ventricular remodeling in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2003;41:574–81. [DOI] [PubMed] [Google Scholar]
- 16.Cicoira M, Zanolla L, Rossi A, et al. Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 2002;40:304–10. [DOI] [PubMed] [Google Scholar]
- 17.Barr CS, Lang CC, Hanson J, et al. Effects of adding spironolactone to an angiotensin-converting enzyme inhibitor in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1995;76:1259–65. [DOI] [PubMed] [Google Scholar]
- 18.Akbulut M, Ozbay Y, Ilkay E, et al. Effects of spironolactone and metoprolol on QT dispersion in heart failure. Jpn Heart J. 2003;44:681–92. [DOI] [PubMed] [Google Scholar]
- 19.Udelson JE, Feldman AM, Greenberg B, et al. Randomized, double-blind, multicenter, placebo-controlled study evaluating the effect of aldosterone antagonism with eplerenone on ventricular remodeling in patients with mild-to-moderate heart failure and left ventricular systolic dysfunction. Circ Heart Fail. 2010;3:347–53. [DOI] [PubMed] [Google Scholar]
- 20.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005;289:H501–12. [DOI] [PubMed] [Google Scholar]
- 21.Masci PG, Schuurman R, Andrea B, et al. Myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy: a contrast-enhanced cardiovascular magnetic study. Circ Cardiovasc Imaging. 2013;6:790–9. [DOI] [PubMed] [Google Scholar]
- 22.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000;101:594–7. [DOI] [PubMed] [Google Scholar]
- 23.Neglia D, Parodi O, Gallopin M, et al. Myocardial blood flow response to pacing tachycardia and to dipyridamole infusion in patients with dilated cardiomyopathy without overt heart failure. A quantitative assessment by positron emission tomography. Circulation. 1995;92:796–804. [DOI] [PubMed] [Google Scholar]
- 24.Neglia D, Michelassi C, Trivieri MG, et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation. 2002;105:186–93. [DOI] [PubMed] [Google Scholar]
- 25.Tsagalou EP, Anastasiou-Nana M, Agapitos E, et al. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2008;52:1391–8. [DOI] [PubMed] [Google Scholar]
- 26.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. [DOI] [PubMed] [Google Scholar]