Abstract
Objective
Despite the rapid adoption of transcatheter aortic valve replacement (TAVR) and worldwide interest in its implantation, TAVR valve explantation has not been well-described.
Methods
We retrospectively reviewed 1,442 consecutive patients who underwent a TAVR between 2011-2019, in which TAVR explantation was performed in 15 (1.0%) patients. In addition, two patients from outside institutions also underwent TAVR explantation at our institution. We reviewed the clinical details of these 17 patients.
Results
The frequency of TAVR explant increased over time from 0-1 in 2011-2015 to 6 in 2019. The mean age was 73.0±9.3. The majority of patients (88.2%) were in NYHA class IV heart failure. The Society of Thoracic Surgeons Predicted Risk of Mortality score was significantly higher at the time of explantation than the time of the original TAVR (3.5 vs. 9.9%, p<0.001). The indication for explantation included structural valve degeneration (23.5%), severe paravalvular leak (41.2%), TAVR procedural complications (23.5%), endocarditis (5.9%) and bridge-to-definitive surgery (5.9%). Neoendothelialization of the TAVR valve into the aortic wall requiring intense aortic endarterectomy was noted in all 5 of the TAVR valves older than 1 year, in which two (40%) required unplanned aortic root repair. There were two (11.8%) in-hospital mortalities.
Conclusions
Surgical TAVR valve explant is increasing and may become common in the near future. The clinical impact of explanting chronically-implanted valves with the potential need for aortic repair is not negligible. These data should be used to more appropriately select TAVR candidates as TAVR practices expand into younger and lower risk patients.
Introduction
Transcatheter aortic valve replacement (TAVR) is an established alternative to surgical aortic valve replacement (SAVR) for patients with severe aortic stenosis, with a growing body of evidence demonstrating the valve performance and durability of current TAVR devices [1]. However, it is known that TAVR valves degenerate in a manner similar to surgical bioprostheses [1]. TAVR was originally utilized as a less invasive alternative to allow treatment of inoperable patients [2-5]. It has rapidly advanced over the last decade from its original role in high-risk populations to those at intermediate and even low surgical risk [6-10]. Additionally, further expansion of TAVR to younger patients with bicuspid pathology is underway [11].
In contrast, a subset of patients undergoing TAVR procedure who develop serious complications with the implanted prosthesis can only be salvaged with a surgical approach [4]. Given the growing population receiving TAVR and current expansion of its indication to relatively lower risk patients, a considerable number of patients are expected to require an open surgical procedure long-term following the index TAVR procedure. In the context of the current TAVR expansion and worldwide interest in implantation, however, the discussion regarding the clinical impact of surgical explantation is lacking. The majority of patients undergoing TAVR are informed or expecting to receive a redo TAVR (TAV-in-TAV) in the future when the bioprosthesis degenerates, despite the lack of long-term outcomes and the uncertain feasibility of the TAV-in-TAV procedure.
Extremely careful thought is required to determine which patients will be best suited for TAVR or SAVR as the initial approach, since TAVR valve explantation, particularly involving older devices with endothelialization by contacting aortic tissue, is not a trivial procedure. Furthermore, there have been few cardiac surgeons who have experience with explanting a chronically-implanted TAVR valve. To the best of our knowledge, there have been only case reports in the literature describing a TAVR valve explantation procedure. Moreover, these cases mostly involve relatively fresh valves [4,10].
Although it still remains exceedingly rare, we are more frequently observing patients with transcatheter valve failure with or without structural valve degeneration (SVD), requiring device explantation with associated SAVR, rather than a TAV-in-TAV procedure. We share our important experience of surgical TAVR valve explantation in this report, as the number of patients in this clinical scenario is expected to increase over the next decade.
Methods
The University of Michigan Institutional Review Board approved all aspects of the study.
Patients and Study Design
We retrospectively reviewed 1,442 consecutive patients who underwent a TAVR (164 [11.4%] valve-in-valve TAVR) at our institution between April 28, 2011 and June 30, 2019. Of these, surgical transcatheter valve explantaton with associated SAVR +/− other concomitant procedures was performed in 15 (1.0%) patients. Two additional patients who received a TAVR at other institutions underwent surgical transcatheter valve explantation with associated SAVR at our institution. We retrospectively reviewed the clinical details of these 17 patients. Abstracted data included the following: patient demographic, clinical, and treatment variables, perioperative and follow-up echocardiographic variables, adverse events, and survival. Follow-up was complete in 100% of patients as of August 29th, 2019.
Surgical Technique
Transcatheter valve explant and SAVR with or without concurrent procedures was performed through a median sternotomy under standard cardiopulmonary bypass support. Myocardial protection was achieved through cold blood cardioplegia delivered antegrade and/or retrograde. As described by Mangi et al. [4], direct ostial cardioplegia delivery is often difficult until the prosthesis is removed because of the limited space in the aortic root caused by the stent cage, the calcifications formed around the native and the TAVR valve, in addition to the native leaflets pushed up against the aortic wall. Transcatheter valves were exposed through an oblique or transverse aortotomy. Explanted prostheses were either Medtronic (Medtronic Inc, Minneapolis, MN) (n=13; 76.5%) or Edwards (Edwards Lifesciences, Irvine, CA)) (n=4; 23.5%) devices in the present study. When explanting the CoreValve prosthesis, the contour of the stent cage can be easily seen through the flaccid aortic wall after aortic cross-clamping (Figure 1A, Supplemental video 1). The level of the aortotomy was determined at 1.0-1.5 cm proximal to the distal edge of the stent frame (Figure 1B). For Sapien prosthesis explantation, a standard aortotomy was used to expose the valve.
Figure 1.

Intraoperative photographs of a 6.5-year-old CoreValve device explant. (A) The distal edge of the stent cage seen through the flaccid aorta (arrow). (B) Aortotomy made 1 cm distal to the stent cage with severely endothelialized transcatheter valve into the aorta (arrow). (C) Disintegrated aortic wall after aortic endarterectomy for the device removal. (D) Explanted device with structural valve degeneration and denuded aortic intima remnants attached to the stent cage.
The key instrument for the explantation of the transcatheter prostheses is a Kocher clamp regardless of the age or the valve type (Figure 2C). We do not think ice-cold saline is necessary for nitinol frame, as the stent cage remains compressed by using a crushing maneuver with a Kocher.
Figure 2.
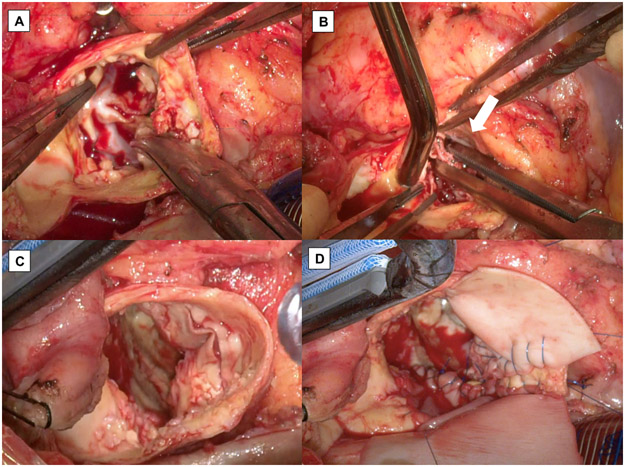
Intraoperative photographs of a 5.3-year-old Sapien device explant. (A) Severely calcified and endothelialized device with its stent cage barely visible. There were numerous calcifications and cholesterol deposits including the area adjacent to the coronary ostia. (B) Kocher clamp maneuver to facilitate liberation of the transcatheter valve. (C) Disintegrated aortic root after aortic endarterectomy and device removal. (D) Partial aortic root replacement of the non-coronary sinus segment with patch repair of the supra-coronary aorta.
Prostheses implanted less than 6 months typically had minimal adhesions between the stent frame and the aorta (Supplemental video 1, 00:59-01:05). A Kocher clamp applied to the middle body of the stent cage deformed the round shape of the prosthesis. The radial force securing the valve within the annulus easily disappeared once this stent cage “bending maneuver” was performed. The liberated valve came out effortlessly afterwards without injuring any annulus, anterior mitral leaflet, or left ventricular outflow tract structure.
As for explanting transcatheter prostheses older than 1 year, there was always dense endothelial growth of the aorta (neoendothelialization) incorporating the entire device (Figure 2A). This incorporation of the transcatheter valves into the aortic wall is much more remarkable than that of surgically implanted prosthetic valves. This neoendothelialization was most notable within the aortic root for balloon-expandable prostheses (Figure 2A) and the sinotubular junction for self-expanding prostheses (Figure 1B), both requiring careful endarterectomy of the aorta in order to separate the stent cage from the intima without compromising the aortic wall integrity. Theoretically, such endothelialization can occur on the ventricular side particularly in low TAVR valve positioning, causing anterior mitral leaflet perforation, as previously reported [12].
The process of removing these older valves started by creating an endarterectomy plane between the aortic intima and the distal half of the stent cage. A #15 scalpel blade and/or a freer elevator was used to separate the stent frame from the aortic intima. Once this was achieved circumferentially, application of a Kocher clamp to the body of the device became possible (Figure 2B). This Kocher clamp served as a handle for the remainder of the valve explant maneuver, allowing better exposure of the deeper aspect of the valve. Kocher clamping of the more proximal stent cage enabled elimination of the radial force of the prosthetic valve. For CoreValve explantation, the use of two Kocher clamps (double Kocher technique) was helpful to more effectively prevent the stent frame from expanding. Regardless of the valve age and its severity of adhesion, transcatheter valves deformed using the Kocher clamp could be liberated after circumferential removal of the neoendothelialization. Aortic replacement or patch repair was performed as needed if the aortic wall was compromised through the endarterectomy (Figures 1C, 2C and 2D). The remainder of the SAVR procedure was performed in the usual fashion.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation for normally distributed variables and medians with interquartile range (IQR) for non-normally distributed variables. Categorical variables are presented as proportion and absolute number. Differences between groups were detected using the χ2 test or Fisher's exact test for categorical variables and Student’s t test, Mann–Whitney U test or the Wilcoxon rank-sum test for continuous variables. All p-values were results of 2-tailed tests. The survival was estimated using the Kaplan-Meier method with corresponding 95% confidence intervals. The statistical analysis was performed using Stata 14.2 (StataCorp, College Station, Tx).
Results
Patient Demographics
Patient demographics and clinical characteristics are shown in Table 1. The mean patient age at explantation was 73.0 ± 9.3 years and 6 (35.3%) were female. The median Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) and the STS-PROM with incremental risk factors [7] at the original TAVR was 3.48 (IQR 2.56-4.87) and 8.65 (IQR 5.24-13.4), respectively. Three (17.7%) were deemed extreme-risk and 7 (41.2) were high-risk. Eight (47.1%) had one or two previous sternotomies. An extensively calcified aorta (porcelain aorta) was present in five (29.4%) patients.
Table 1.
Patient demographics
| Variables | Entire cohort (n=17) |
|---|---|
| Age | 73.0 ± 9.28 |
| Female | 6 (35.5) |
| Hypertension | 14 (82.4) |
| Hyperlipidemia | 13 (76.5) |
| Diabetes | 4 (23.5) |
| Chronic kidney disease | 9 (52.9) |
| Dialysis | 2 (11.8) |
| COPD | 2 (11.8) |
| Porcelain aorta | 5 (29.4) |
| Left ventricular ejection fraction Permanent pacemaker | 44.7 ± 15.9 6 (35.3) |
| BMI | 28.0 ± 25.8 |
| BSA | 1.95 ± 0.19 |
| NYHA Functional Classification at original TAVR | |
| 1 | 0 |
| 2 | 3 (17.6) |
| 3 | 11 (64.7) |
| 4 | 3 (17.6) |
| Etiology | |
| Degenerative aortic stenosis | 13 (76.4) |
| Rheumatic | 1 (5.9) |
| Aortic root aneurysm | 2 (11.8) |
| Radiation-induced | 1 (5.9) |
| Bicuspid valve | 3 (17.6) |
| Previous cardiac surgery | 8 (47.1) |
| Redo sternotomy | |
| Isolated AVR | 2 (11.8) |
| Aortic root + ascending | 3 (17.6) |
| AVR + CABG | 1 (5.9) |
| Direct aortic TAVR | 1 (5.9) |
| Re-redo sternotomy | |
| AVR followed by AVR + ascending | 1 (5.9) |
| Valve-in-valve TAVR | 7 (41.2) |
| Recipient surgical valve | |
| Freestyle | 4 (57.1) |
| Perimount | 1 (14.3) |
| Mosaic | 1 (14.3) |
| Homograft | 1 (14.3) |
| STS-PROM at original TAVR | 3.5 (2.6-4.9) |
| With incremental factors | 8.7 (5.2-13.4) |
| Risk Classification at original TAVR | |
| Extreme risk | 3 (17.7) |
| High risk | 7 (41.2) |
| Moderate risk | 7 (41.2) |
COPD, chronic obstructive pulmonary disease; BMI, body mass index; BSA, body surface area; STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; CABG, coronary artery bypass grafting; TAVR, transcatheter aortic valve replacement; AVR, aortic valve replacement; NYHA, New York Heart Association
Indications for Transcatheter Valve Explant
The number and frequency of patients needing TAVR valve explantation has been increasing in our structural heart program (Figure 3), with 6 patients within the first half of 2019.
Figure 3.
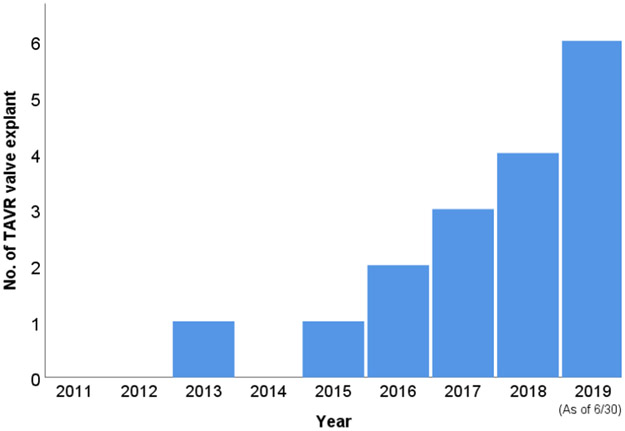
Trend of surgical transcatheter aortic prosthesis explant procedure case number by year.
TAVR, transcatheter aortic valve replacment
The indications for surgical TAVR valve explantation are summarized in Table 2. All patients were reviewed by our multidisciplinary structural heart team and deemed not suitable for TAV-in-TAV procedure. The main reasons for the exclusion from TAV-in-TAV candidacy are summarized in Table 2.
Table 2.
The indications for surgical transcatheter valve explant procedure. (A) The main indications for valve explant. (B) The main reasons for exclusion from redo transcatheter valve replacement.
| (A) Clinical indications for valve replacement | Entire cohort (n=17) |
|---|---|
| Paravalvular leak | 7 (41.2) |
| Structural valve degeneration | 4 (23.5) |
| Intraoperative valve migration | 2 (11.8) |
| Intraoperative coronary obstruction | 2 (11.8) |
| Prosthetic valve endocarditis | 1 (5.9) |
| Bridge-to-definitive open procedure | 1 (5.9) |
| (B) Reasons for exclusion from repeat transcatheter valve replacement | Entire cohort (n=17) |
| Need for other concomitant procedures | 6 (35.3) |
| Intraoperative conversion to open procedure | 4 (23.5) |
| Anticipated difficulty positioning with mitral impingement | 3 (17.6) |
| Coronary height | 2 (11.8) |
| Prosthetic valve endocarditis | 6 (35.3) |
| Bridge-to-definitive open procedure | 1 (5.9) |
The leading indication for valve explantation was severe symptomatic paravalvular leak (PVL) (7 patients; 41.2%), consisting of 4 Evolut R, 2 CoreValve and one Sapien. Of these, two (28.6%) had resultant severe mitral regurgitation due to device migration with or without severe tricuspid regurgitation and one (14.3%) had a ventricular septal defect. The median time from the index TAVR to the surgical TAVR valve explant in patients with PVL was 96 days (IQR 69-438). The second leading indication was SVD (4 patients; 23.5%). The median time to SVD was 3.34 years (IQR 0.74-6.18). The patient whose TAVR valve degeneration occurred within 1 year was dialysis-dependent. Most of the patients (90.9%) with either severe PVL or SVD presented with NYHA class IV heart failure. Four (23.5%) had an intraoperative conversion to SAVR due to coronary obstruction with hemodynamic instability in 2 cases and valve migration into the left ventricle in 2 cases during the TAVR. Intraoperative conversion to SAVR represents 0.3% (n=4/1,442) of the entire TAVR series at our institution. Both cases with valve migration were TAV-in-SAV TAVRs within a stentless bioprosthesis. Additionally, there was one patient (5.9%) who developed prosthetic valve endocarditis of the TAVR device and resultant severe aortic inefficiency 2 months after the procedure. One TAVR valve (5.9%) was intentionally implanted as a bridge-to-definitive surgical intervention in a 72-year-old male with history of homograft aortic root replacement who developed severe aortic insufficiency, NYHA class IV heart failure and stroke in the presence of 6 cm ascending aortic aneurysm. He was originally deemed too high risk for redo aortic repair due to a significant stroke the day prior to his scheduled open surgical procedure. Redo aortic repair with TAVR device explant and SAVR was undertaken 273 days after the TAVR procedure and full recovery from the stroke.
Reasons for Exclusion from TAV-in-TAV Candidacy
Most patients had more than one reason for TAV-in-TAV exclusion. The main reasons (Table 2) included the necessity for other associated procedures other than the prosthetic valve problem (35.3%), intraoperative conversion to open procedure (23.5%), potential device malposition with or without anterior mitral impingement (17.6%), low coronary height (11.8%), prosthetic valve endocarditis (5.9%) and bridge-to-definitive open procedure (5.9%).
In contrast to our 17 open surgical patients, there have been 5 patients (0.4%), who have undergone a TAV-in-TAV procedure due to severe PVL (80%) and SVD (20%), with a median 63-day (IQR 9-625) interval following the index TAVR. In other words, only 25% of patients who needed an aortic valve re-intervention following the index TAVR underwent a redo TAVR procedure and the majority required surgical TAVR explant/SAVR.
Operative Data
The operative data is summarized in Table 2. The majority of patients (88.2%) were in NYHA class IV heart failure at time of surgery. The STS-PROM with or without incremental risk factors was significantly higher than the time of original TAVR (p<0.001; Figure 4). Explanted devices comprised CoreValve (Medtronic Inc; 4 (23.5%)), Evolut R (Medtronic Inc; 6 (35.3%)), Evolut PRO (Medtronic Inc; 3 (17.6%)), Sapien (Edwards Lifesciences; 1 (5.9%)) and Sapien 3 (Edwards Lifesciences; 3 (17.6%)). The incidence of device explant in each device type was 0.9% (4/454 implants), 0.9% (6/688 implants), 3.4% (3/88 implants), 2.6% (3/116 implants) and 1.0% (1/94 implants), respectively. There was no Sapien XT (Edwards Lifesciences) device explant. The median TAVR valve age was 195 days (69-486) and older than 1 year in 5 (29.4%) patients. Two valves (11.8%) were older than 5 years. The oldest valve was a 29 mm CoreValve, which was 6.5 years old (Figure 1A-D).
Figure 4.

Box plots of the Society of Thoracic Surgeons Predicted Risk of Mortality at time of index transcatheter aortic valve replacement and surgical transcatheter valve removal with/without incremental risk factors.
STS-PROM, The Society of Thoracic Surgeons Predicted Risk of Mortality
TAVR valve explant was extremely difficult in patients with older valves (Figure 1A-D, Figure 2A-D, Supplemental video 1), while relatively fresh valves were easily explanted without any special instruments, techniques, or cold saline irrigation. Intense endarterectomy of the aortic root/the sinotubular junction was required in all patients with TAVR valves older than 1 year (p<0.001). In addition, the aortic root space was always “very crowed” in the patients with older TAVR valves because of the endothelialization and severe calcification of the TAVR valve in addition to the native valves with calcifications or the original surgical bioprosthetic valves left behind at time of TAVR (Figure 2D). Of these older valves, one patient required an unplanned aortic root replacement, another required an unplanned aortic patch repair with partial aortic root replacement of the non-coronary sinus segment due to near-totally disintegrated aortic wall from the TAVR valve explantation (Figures 2C and 2D) and one required multiple tacking stitches to repair the destroyed aortic wall from the endarterectomy (Figure 1C). Other than these aortic issues, certain patients also required other associated procedures. Two (11.8%) required extracorporeal membrane oxygenation support for 48 hours following the SAVR due to cardiorespiratory failure and two (11.8%) required intraaortic balloon pump insertion. In contrast, there was no tissue damage in the left ventricular outflow tract.
Postoperative Outcomes
The early postoperative outcomes are summarized in Supplemental Table 1. There were two (11.8%) in-hospital mortalities. The cause of death was ischemic bowel in one patient with a porcelain aorta and sudden cardiac arrest in the intensive care unit on the 13th postoperative day in the other. There was no stroke or bleeding requiring surgical exploration. New or worsening renal failure [13] occurred in all patients with explant of TAVR valves older than 1 year (p=0.004) except for one who had end-stage renal failure preoperatively. Additionally, the intensive care unit stay was significantly longer in patients with older TAVR valves compared to those with younger TAVR valves (p=0.027). Among those without preoperative pacemaker, 66.7% of patients with older TAVR valves required permanent pacemaker implantation, versus only one (7.7%) in the other group. At 3 years, the estimated survival was 68.0 ± 12.2% (Supplemental Figure 1).
Discussion
This study represents the first comprehensive analysis describing surgical transcatheter valve explantation to provide insights that have not previously been considered with the rapid expansion of TAVR. The primary findings of interest in this study were: (1) the overall frequency of surgical TAVR valve explantation among post-TAVR patients was 1% over the 8 years; (2) the number of patients undergoing TAVR explant is increasing; (3) STS-PROM in these patients was much higher at time of valve explant than at the time of TAVR implant; (4) the surgical valve explant for TAVR valves older than 1 year was extremely challenging due to the neoendothelialization between the aorta and the device, requiring intensive aortic endarterectomy with or without unplanned aortic replacement; (5) explantation of relatively young TAVR valves did not require any special technique, equipment, or aortic procedure regardless of the valve type.
The field of TAVR has been rapidly expanding over the last decade, with > 500,000 procedures performed in > 70 countries with a case volume growing by 40% annually, evolving from a challenging intervention to a simple, safe, and standardized procedure [14]. However, despite this global paradigm shift in aortic valve practice studies highlighting the impact of surgical transcatheter valve explantation are very few. This paucity of reports may be due to the lack of long-term follow-up or underreporting of TAVR explantation. In our program, approximately 1% of patients who received a TAVR required a surgical valve explant, while only 0.4% received a TAV-in-TAV. Barbanti and associates reviewed the data from 14 centers including 13,876 post-TAVR patients at participating centers [1]. There were 50 (0.4%) patients who underwent a TAV-in-TAV procedure mostly due to PVL. Interestingly, the frequency of TAV-in-TAV procedure in their report is almost identical to the number in the present study. We speculate that there were patients who received surgical TAVR valve explantation and SAVR in their series, although the authors did not describe any of surgical valve explant data. Reviewing these surgical explant cases and knowing the implications of TAVR explantation is of paramount importance, as it allows us to more appropriately select TAVR candidates than the current guideline recommendations.
As for previous TAVR valve explant and recommended surgical technique in the literature, we have identified 11 case reports that involved description of valve explant technique from 2008 to 2019 [4,10,15-23]. The time interval between TAVR implant and explant was from immediate to 7 years (median 187 days (IQR 21-913)). Surgical explantation of older TAVR valve cases are increasingly reported recently, being exclusively after 2015 [4,10,15-17]. The description of the valve or the aortic finding in these reports is in line with our observations. Aortic endarterectomy is seemingly mandatory with respect to valves older than a year after implantation and “unplanned” aortic replacement appears frequently necessary in these old valve cases.
Given the results of our study, a major concern was raised whether the “TAVR as the first valve strategy” is appropriate, particularly in younger patients who are likely to require repeat valve interventions in the future. Current ongoing discussion regarding TAVR indication is not taking this scenario of future morbid open surgery for explantation into account. This information is critical for relatively healthy young patients who have both TAVR and SAVR options. Furthermore, there is no long-term data on the durability of TAVR valve or the feasibility of a reproducible safe TAV-in-TAV procedure. In fact, patients who received a TAV-in-TAV in the present study represents less than half of patients who received surgical valve explantation due to various clinical reasons precluding TAV-in-TAV (Table 2). Despite the unclear feasibility of TAV-in-TAV, lack of data on TAVR durability or TAVR explant safety, many patients have been informed that future repeat TAVR is expected.
Tang et al. conducted an excellent study, investigating the feasibility of TAV-in-TAV procedure after primary Sapien 3 implantation based on the intraoperative angiographic coplanar 3-cusp view. The authors concluded that the TAV-in-TAV procedure is unfeasible in 21.4% of TAVR cases [24]. However, this number may be significantly underestimated, as this study does not consider aortic neoendothelialization, leaflet thrombus formation, cholesterol deposition growth, or severe calcification of the native or the prosthetic valve, which were all seen in the present study (Figures 1 and 2). Very little is known about the natural history of the integration of the TAVR valve into the aorta and left ventricular outflow tract [25]. It is of critical importance to note that significant endothelialization with more calcifications and thrombus formation always occur within the aortic root, as observed in the present study. These deposits and materials attached to the leaflets can narrow the orifice of the prosthesis, cause incomplete TAV-in-TAV valve expansion, and obstruct or embolize the coronary arteries. Furthermore, severe mitral with/without tricuspid and coronary artery pathology in these patients in the presence of failed transcatheter prosthesis was very common in the present study, precluding the TAV-in-TAV option.
After witnessing the “crowded” aortic root and experiencing the endothelialized TAVR device at time of valve explant, we feel the need to reconsider patient selection for TAVR. Indeed, it is our opinion that surgical TAVR explant, particularly old valves, even in virgin chest is technically much more challenging than standard redo SAVR procedures. This experience has clearly impacted on our practice with respect to patients aged 70 or younger with minimal medical comorbidities being offered for SAVR rather than TAVR, considering “lifetime management strategy” of relatively young patients. Morbid valve explant with unplanned aortic replacement in patients who are much older than at their index TAVR procedure is not a trivial undertaking. In fact, 30% of patients in the present study were in their 80’s. None of our post-TAVR patients was expecting surgical re-interventions except for one who underwent TAVR as a bridge-to-definitive open surgery. For this reason, we do not advocate TAVR as the first valve strategy in relatively young patients who have both SAVR and TAVR options and require additional procedures in their lifetime.
Study Limitations
This study has several limitations inherent to its retrospective nature with small sample size. In view of the large numbers of TAVR devices implanted worldwide, further investigation involving other institutions using standardized methodology and diagnostic criteria is highly warranted.
In summary, the evolution of TAVR over the past decade has been undoubtedly unprecedented. Presently, TAVR, rather than SAVR, is the treatment of choice in the extreme or high-risk patients in relatively elderly population if anatomically suitable. However, as the role of TAVR continues to expand in the treatment of progressively lower risk patients with aortic stenosis, the increased frequency of TAVR has been accompanied by a number of device failures that require open surgical intervention [19], as demonstrated in this study. Patients must be informed regarding the uncertainty of the TAV-in-TAV feasibility and possible future surgical explantation involving extensive aortic endarterectomy with aortic repair. In this context, judicious clinical judgment is extremely crucial and full multidisciplinary collaboration is of paramount importance for the TAVR candidate selection process.
Supplementary Material
Central Picture.

A 5.3-year-old balloon expandable device and post-device explant disintegrated aorta.
Table 3.
Operative data
| Variables | Entire cohort (n=17) |
>1-year-old TAVR (n=5) |
<1-year-old TAVR (n=12) |
p- value |
|---|---|---|---|---|
| STS-PROM* (IQR) | 9.9 (6.2-21.4) | 17.6 (7.9-53.3) | 9.24 (5.6-20.1) | 0.21 |
| STS-PROM with incremental risk factors* (IQR) | 16.6 (12.3-33.5) | 39.6 (12.4-62.3) | 14.7 (12.2-23.6) | 0.25 |
| Redo sternotomy | 8 (47.1) | 2 (40.0) | 6 (50.0) | 1.00 |
| First time redo | 7 (41.2) | 2 (40.0) | 5 (41.7) | |
| Second time redo | 1 (5.9) | 0 (20.0) | 1 (8.3) | |
| TAVR device explanted | ||||
| CoreValve | 4 (23.5) | 2 (40.0) | 2 (16.7) | 0.54 |
| Evolut R | 6 (35.3) | 2 (40.0) | 4 (33.3) | 1.00 |
| Evolut PRO | 3 (17.6) | 0 | 3 (25.0) | 0.52 |
| Sapien | 1 (5.9) | 1 (20.0) | 1 (8.3) | 0.52 |
| Sapien 3 | 3 (17.6) | 0 | 3 (25.0) | 0.52 |
| TAVR valve age (Days) | 195 (69-486) | 1135 (438-2150) | 72 (59-170) | 0.001 |
| Cardiopulmonary bypass time (minutes) | 184 (138-246) | 202 (160-205) | 162 (134-302) | 0.92 |
| Aortic cross-clamp time (minutes) | 137 (91-188) | 173 (123-175) | 126 (85-226) | 0.75 |
| Circulatory arrest | 1 (5.9) | 0 | 1 (8.3) | 1.00 |
| Valve size (mm) | 25 (23-27) | 23 (21-24) | 25 (25-27) | 0.011 |
| Implanted valve | ||||
| Magna Ease | 9 (52.9) | 5 (100) | 9 (33.3) | 0.52 |
| Trifecta | 5 (29.4) | 0 | 5 (41.7) | 0.25 |
| Freestyle | 2 (11.8) | 0 | 2 (16.7) | 1.00 |
| St Jude Regent | 1 (5.9) | 0 | 1 (8.3) | 1.00 |
| Concomitant procedure(s) | ||||
| Root enlargement | 3 (17.6) | 2 (40.0) | 1 (8.3) | 0.19 |
| Mitral | 4 (23.5) | 2 (40.0) | 2 (16.6) | 0.55 |
| Tricuspid | 3 (17.6) | 1 (20.0) | 2 (16.6) | 1.00 |
| Mitral + Tricuspid | 2 (11.8) | 1 (20.0) | 1 (8.3) | 0.52 |
| Aortic surgery | 6 (35.3) | 5 (100) | 1 (8.3) | 0.001 |
| Ascending | 1 (5.9) | 1 (20.0) | 1 (8.3) | 0.52 |
| Partial arch | 1 (5.9) | 0 | 1 (8.3) | 1.00 |
| Root | 2 (11.8) | 1 (20.0) | 1 (8.3) | 0.52 |
| Endarterectomy | 5 (29.4) | 5 (100) | 0 | <0.001 |
| Root patch repair | 1 (5.9) | 1 (20.0) | 0 | 0.29 |
| CABG | 1 (5.9) | 1 (20.0) | 1 (8.3) | 0.29 |
| ECMO | 2 (11.8) | 1 (20.0) | 1 (8.3) | 0.52 |
| IABP | 2 (11.8) | 0 | 2 (16.7) | 1.00 |
Bold indicates statistically significant (p<0.05).
The median of the Calculated using STS Adult Cardiac Surgery Database Version 2.9 only for isolated AVR or AVR + CABG despite multiple other concurrent procedures in some patients.
STS-PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; IQR, interquartile range; CABG, coronary artery bypass grafting; TAVR, transcatheter aortic valve replacement; AVR, aortic valve replacement; ECMO, extracorporeal membrane oxygenation; IABP, intraaortic balloon pumping
Central message.
With an increasing frequency of surgical transcatheter valve explantation, judicious clinical judgment is crucial in appropriately selecting transcatheter valve therapy as an initial valve strategy.
Perspective Statement.
The evolution of TAVR over the past decade has been unprecedented resulting in an increase of surgical TAVR explantation scenarios. The clinical impact of explanting older valves with the potential need for unplanned aortic repair is not negligible. Providers should be judicious in selecting TAVR candidates as it continues to expand into lower risk and younger candidates.
Acknowledgments
Sources of Funding: None.
Abbreviations
- TAVR
transcatheter aortic valve replacement
- SAVR
surgical aortic valve replacement
- STS-PROM
The Society of Thoracic Surgeons Predicted Risk of Mortality
- CABG
coronary artery bypass grafting
- IQR
interquartile range
- COPD
chronic obstructive pulmonary disease
- PVL
paravalvular leak
- SVD
structural valve degeneration
- TAV-in-TAV
transcatheter aortic valve replacement in transcatheter aortic valve
- NYHA
New York Heart Association
- ECMO
extracorporeal membrane oxygenation
- IABP
intraaortic balloon pump
- ICU
intensive care unit
Footnotes
Conflict of Interest Statement: S.F. serves as a consultant for Terumo Aortic. A.A.B. is supported by the National Research Service Award postdoctoral fellowship (No. 5T32HL076123). B.Y. is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health and Phil Jenkins and Darlene & Stephen J. Szatmari Funds. Others have no relevant disclosures.
References
- 1.Barbanti M, Webb JG, Tamburino C, Van Mieghem NM, Makkar RR, Piazza N et al. Outcomes of Redo Transcatheter Aortic Valve Replacement for the Treatment of Postprocedural and Late Occurrence of Paravalvular Regurgitation and Transcatheter Valve Failure. Circ Cardiovasc Interv. 2016;9. [DOI] [PubMed] [Google Scholar]
- 2.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. [DOI] [PubMed] [Google Scholar]
- 3.Popma JJ, Adams DH, Reardon MJ, Yakubov SJ, Kleiman NS, Heimansohn D, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–81. [DOI] [PubMed] [Google Scholar]
- 4.Mangi AA, Ramchandani M, Reardon M Surgical Removal and Replacement of Chronically Implanted Transcatheter Aortic Prostheses: How I Teach It. Ann Thorac Surg. 2018;105:12–14. [DOI] [PubMed] [Google Scholar]
- 5.Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–91. [DOI] [PubMed] [Google Scholar]
- 6.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609–20. [DOI] [PubMed] [Google Scholar]
- 7.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2019;380:1695–1705.30883058 [Google Scholar]
- 8.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380:1695–1705. [DOI] [PubMed] [Google Scholar]
- 9.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380:1706–1715. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen TC, Rice RD, Umana-Pizano JB, Loyalka P. Minimally invasive removal of an infected Edwards S3. J Thorac Cardiovasc Surg. 2019. (In-press) [DOI] [PubMed] [Google Scholar]
- 11.Makkar RR, Yoon SH, Leon MB, Chakravarty T, Rinaldi M, Shah PB, et al. Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke. JAMA. 2019;321:2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong DR, Boone RH, Thompson CR, Allard MF, Altwegg L, Carere RG, et al. Mitral valve injury late after transcatheter aortic valve implantation. J Thorac Cardiovasc Surg. 2009;137:1547–9. [DOI] [PubMed] [Google Scholar]
- 13.https://www.sts.org/sites/default/files/documents/ACSD_DataSpecificationsV2_9.pdf (Last accessed on 9/1/2019)
- 14.Costa G, Barbanti M, Tamburino C. Trends in European TAVI Practice. CARDIAC INTERVENTIONS TODAY (https://citoday.com/pdfs/cit0318_F4_Tamburino.pdf) (Last accessed on September 3rd, 2019)
- 15.Belhaj SR, Anselmi A, Tomasi J, Verhoye JP. Late surgical explantation of a transcatheter heart valve in a patient with a porcelain aorta. Eur J Cardiothorac Surg. 2019;55:1008–1011. [DOI] [PubMed] [Google Scholar]
- 16.Wang LW, Granger EK, McCourt JA, Pye R, Kaplan JM, Muller DW, Late surgical explantation and aortic valve replacement after transcatheter aortic valve implantation. Ann Thorac Surg. 2015;99:1434–6. [DOI] [PubMed] [Google Scholar]
- 17.Marti D, Rubio M, Escribano N, de Miguel R, Rada I, Moris C. Very late thrombosis of a transcatheter aortic valve-in-valve. JACC Cardiovasc Interv 2015;8:e151–e153. [DOI] [PubMed] [Google Scholar]
- 18.Ninomiya Y, Hamasaki S, Nomoto Y, Kawabata T, Fukumoto D, Yoshimura A, et al. A case of acute coronary syndrome caused by delayed coronary ischemia after transcatheter aortic valve implantation. J Cardiol Cases. 2017;17:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thyregod HG, Lund JT, Engstrøm T, Steinbrüchel DA. Transcatheter aortic valve prosthesis surgically replaced 4 months after implantation. Eur J Cardiothorac Surg. 2010;37:494–6. [DOI] [PubMed] [Google Scholar]
- 20.Bloom JP, Kwon MH, Tolis G Jr. Early Distal Migration of a Self-Expanding Aortic Valve Prosthesis Causing Myocardial Infarction. Ann Thorac Surg. 2019;108:e1–e3. [DOI] [PubMed] [Google Scholar]
- 21.Notaristefano F, Reccia MR, Notaristefano S, Annunziata R, Sclafani R, Ambrosio G, et al. Bailout surgical explantation of a transcatheter valve-in-valve for subacute thrombosis: When there is no time for anticoagulation: Case report and literature review. Cardiovasc Revasc Med. 2018;19:536–539. [DOI] [PubMed] [Google Scholar]
- 22.Weymann A, Patil NP, Karck M, Resolution of heart block after surgical correction of failed transcatheter aortic valve implantation. Ann Thorac Surg. 2015;99:1437–9. [DOI] [PubMed] [Google Scholar]
- 23.Litzler PY, Cribier A, Zajarias A, Comte D, Eltchaninoff H, Tron C, Haas-Hubscher C, Bessou JP Surgical aortic valve replacement after percutaneous aortic valve implantation: what have we learned? Circ Cardiovasc Interv. 2008;1:155–8. [DOI] [PubMed] [Google Scholar]
- 24.Tang GHL, Zaid S, Gupta E, Ahmad H, Khan A, Kovacic JC, et al. Feasibility of Repeat TAVR After SAPIEN 3 TAVR: A Novel Classification Scheme and Pilot Angiographic Study. JACC Cardiovasc Interv. 2019;12:1290–1292. [DOI] [PubMed] [Google Scholar]
- 25.Linke A, Höllriegel R, Walther T, Schierle K, Wittekind C, Ender J, et al. Ingrowths of a percutaneously implanted aortic valve prosthesis (corevalve) in a patient with severe aortic stenosis. Circ Cardiovasc Interv. 2008;1:155–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


