Abstract
Trait dysphoric rumination is a transdiagnostic factor associated with depression and anxiety that has also been linked with blunted respiratory sinus arrhythmia (RSA), an index of reduced emotion regulation capacity. However, the autonomic correlates of state dysphoric rumination remain unclear. We examined the physiological correlates of state dysphoric rumination and the potential repairing effects of savoring on autonomic functioning. To provide a comprehensive assessment of autonomic correlates, we examined changes in parasympathetic (RSA) and sympathetic (cardiac pre-ejection period, PEP; and electrodermal activity, EDA) arousal independently, as well as autonomic coordination among indices. Eighty-two women (ages 18-25) completed laboratory physiological assessments, including rumination and savoring tasks, and self-report measures of trait rumination. Dysphoric rumination was associated with sympathetic activation (i.e., decreases in PEP, increases in EDA), and subsequent Savoring following a recovery period also corresponded with decreases in PEP. Trait rumination did not predict autonomic changes during state rumination. However, higher trait rumination was associated with greater sympathetic coordination (PEP-EDA correspondence) during savoring. In summary, dysphoric rumination co-occurred with sympathetic activation, and subsequent savoring successfully recruited sympathetic activity (PEP) redirected on positive moods and events. Results also emphasize the utility of examining sympathetic and parasympathetic indices, and coordination among autonomic indices to delineate autonomic activity associated with emotion regulation strategies.
Keywords: rumination, savoring, sympathetic nervous system, parasympathetic nervous system, respiratory sinus arrhythmia, pre-ejection period, electrodermal activity
1. Introduction
Dysphoric rumination is a transdiagnostic factor (McLaughlin & Nolen-Hoeksema, 2011; Watkins, 2009) implicated in the etiology of depression and anxiety (e.g., Fresco, Frankel, Mennin, Turk, & Heimberg, 2002; Gibb, Grassia, Stone, Uhrlass, & McGeary, 2012; Mellings & Alden, 2000; Nolen-Hoeksema, 2000; Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008). Rumination reflects the tendency to respond to dysphoric moods by repetitively and passively dwelling on negative moods and symptoms, their causes and implications (Nolen-Hoeksema, 1991). Instead of relieving distress, rumination has the effect of amplifying depressive and anxious moods (Blagden & Craske, 1996; Mclaughlin, Borkovec, & Sibrava, 2007; Morrow & Nolen-Hoeksema, 1990; Nolen-Hoeksema & Morrow, 1993). Thus, one mechanism by which dysphoric rumination may foster risk is by altering emotion regulation capacity over time.
Lower resting parasympathetic nervous system (PNS) tone, as indexed by reduced resting respiratory sinus arrhythmia (RSA) is proposed to reflect impaired emotion regulation capacity, and is linked to multiple forms of psychopathology, including depression and anxiety (Beauchaine, 2015). Trait ruminative tendency has also been associated with blunted RSA in the laboratory (e.g., Ottaviani et al., 2016; Williams et al., 2017; Woody, McGeary, & Gibb, 2014) and in daily life using ambulatory physiology (Carnevali, Thayer, Brosschot, & Ottaviani, 2018; Cropley et al., 2017). Thus, trait dysphoric rumination may reduce regulatory capacity via reductions in PNS activity. However, less is understood about the immediate effects of ruminating. Depression and anxiety disorders are characterized by emotion regulation deficits (Amstadter, 2008; Cisler, Olatunji, Feldner, & Forsyth, 2010; Joorman & Siemer, 2014; Rottenberg, 2005). Isolating the autonomic concomitants of state dysphoric rumination in non-clinical samples may clarify how this maladaptive emotion regulation strategy impacts ANS functioning, conferring risk for psychopathology. Therefore, the primary goal of the current study was to identify the autonomic correlates of state dysphoric rumination.
Although the majority of research focuses on a single physiological index, ANS assessments should ideally incorporate both the PNS and sympathetic nervous system (SNS), as there is evidence that changes within each branch are not necessarily always reciprocal and can occur independently (Berntson et al., 1994; Berntson, Cacioppo, & Quigley, 1991; Berntson, Norman, Hawkley, & Cacioppo, 2008). RSA, which is commonly indexed by the high frequency heart rate variability metric (HF-HRV), reflects parasympathetically-mediated variation between heartbeats that ebbs and flows across the respiration cycle (Katona & Jih, 1975). At rest, higher RSA (indicating greater parasympathetic activity) enables a range of flexible responses to environmental context suggesting greater emotion regulation capacity, whereas lower resting RSA tends to be associated with rigid or exaggerated responses (Porges, 2007). In response to stimuli, RSA withdrawal or augmentation may occur; the adaptiveness of RSA response is determined by the environmental context. For example, in reaction to a specific environmental context such as threat, RSA withdrawal (typically coupled with sympathetic augmentation), is viewed as the most adaptive response, as it enables the individual to engage in a ‘fight or flight’ response (Porges, 2007). Conversely, in a safe, relaxing environment, RSA augmentation would be the most adaptive response. Both pre-ejection period (PEP) and electrodermal activity (EDA) are thought to be relatively pure measures of sympathetic activity. PEP measures sympathetically-mediated beta-adrenergic influences on cardiac control, reflecting the time between left ventricular depolarization and ejection of blood through the aorta (Berntson et al., 1994). PEP is inversely related to SNS control (i.e., lower values representing greater sympathetic activity). In contrast, EDA captures skin conductance of sweat (Dawson, Schell, & Filion, 2007) and primarily involves cholinergic neurotransmission (Fowles, 1986; Shields, MacDowell, Fairchild, & Campbell, 1987). Therefore, to capture comprehensive changes in autonomic functioning, assessments of emotion regulation strategies should ideally monitor changes in RSA, PEP as well as EDA.
Autonomic correlates of state dysphoric rumination remain unclear, as prior research has been inconsistent in methodological approaches and operationalization of rumination. Perseverative cognition, which encompasses rumination, has been associated with lower RSA compared to mind wandering when induced in laboratory assessments (Ottaviani, Shapiro, & Couyoumdjian, 2013) and assessed via electronic diary of daily life (Ottaviani et al., 2015). Engaging in abstract rumination (largely evaluative, less concrete, unclear thinking) following a traumatic video has been associated with greater increases in heart rate compared to concrete thinking or distraction (Ehring, Szeimies, & Schaffrick, 2009). Conversely, ruminating on a future, unattained goal did not lead to a decrease in RSA, but was still associated with lower RSA compared to a mindfulness exercise (Gilbert & Gruber, 2014). Further, ruminating on a recent laboratory stressor has been associated with RSA withdrawal (LeMoult, Yoon, & Joormann, 2016) and poor cardiac recovery compared to controls, as measured by blood pressure (Robinette & Charles, 2016). Further, emotion reactivity studies did not find changes in RSA during self-reported rumination following the presentation of negative stimuli (Aldao, Mennin, & McLaughlin, 2013; Gentzler, Wheat, Palmer, & Burwell, 2013). Taken together, state rumination studies have largely focused on RSA or non-specific ANS indices such as heart rate and blood pressure (which can have both PNS and SNS influences). To our knowledge research has not yet examined SNS and PNS changes concurrently during state dysphoric rumination.
In contrast, research on state rumination that amplifies anger has revealed a largely consistent ANS pattern. This may be a result of consistent methodology across studies, which typically involve instructions to ruminate on an anger-inducing situation or past interpersonal transgressions. As opposed to dysphoric rumination which tends to induce sadness specifically (Morrow & Nolen-Hoeksema, 1990), these tasks tend to induce anger as well as a range of negative affect, including sadness and anxiety (Witvliet, DeYoung, Hofelich, & DeYoung, 2011). State ruminating tasks that induce anger have been associated with PNS withdrawal (decreases in RSA; da Silva, Witvliet, & Riek, 2016; Ottaviani & Shapiro, 2011; Ottaviani, Shapiro, Davydov, & Goldstein, 2008; Witvliet et al., 2011; Witvliet, Knoll, Hinman, & DeYoung, 2010) and SNS activation including decreases in PEP (Ottaviani & Shapiro, 2011; Ottaviani et al., 2008; Ray, Wilhelm, & Gross, 2008) and increases in EDA (Witvliet, Ludwig, & Vander Laan, 2001). However, other studies failed to find a significant change in PEP (da Silva et al., 2016) or RSA (Ray et al., 2008). Taken together, results indicate that subtypes of rumination that induce anger correspond with reciprocal effects between PNS withdrawal and SNS activation. This ANS reciprocal reactivity between PNS and SNS branches has long been viewed as an adaptive biological response to external stressors and threats, but is thought to reflect an ineffective use of resources in the absence of external stressors (Porges, 2007).
There is reason to believe that the physiological underpinnings of angry vs. dysphoric rumination may qualitatively differ, which underscores the need for research that comprehensively assesses autonomic processes underlying dysphoric rumination. Anger has been associated with greater general autonomic arousal compared to sadness (diastolic blood pressure), whereas sadness has been associated with greater SNS arousal as indexed by EDA specifically (Cacioppo, Berntson, Larsen, Poehlmann, & Ito, 2000). This is consistent with theoretical models that conceptualize anger as an approach emotion that motivates behavioral activation (Carver & Harmon-Jones, 2009). In contrast, sadness and anxiety are associated with behavioral avoidance. EDA has established links to stimuli that induce avoidance such as threats of punishment (Barlow, 2002; Bitterman & Holtzman, 1952; Lader & Wing, 1964) and thus has been conceptualized as an index of passive avoidance and behavioral inhibition (Dawson et al., 2007; Obradović & Boyce, 2012). Thus, anger appears to have greater activating effects on autonomic arousal, but we may anticipate that dysphoric rumination should also be associated with SNS activation, especially via EDA.
A secondary aim was to determine if an adaptive emotional regulation strategy, savoring, can repair the physiological and affective effects of state dysphoric rumination by focusing on positive events and upregulating positive affect. Savoring reflects the tendency to attend to, focus on, recall, appreciate and look forward to positive affect and events, which results in amplifying the intensity or duration of positive affect (Bryant & Veroff, 2007). Whereas rumination is associated with a reduction in positive affect (Mclaughlin et al., 2007), savoring enhances positive affect in the short term (McMakin, Siegle, & Shirk, 2011) and predicts greater happiness in daily life (Jose, Lim, & Bryant, 2012), even when fewer positive events occur (Hurley & Kwon, 2013). Importantly, savoring may be distinguished from other adaptive regulation strategies such as positive reappraisal, which involves reinterpreting a stressful or threatening event in a new, potentially beneficial light. Reappraisal focuses on downregulating negative affect (Gross, 1998), but may also induce positive affect (Butler, Gross, & Barnard, 2014; Shiota & Levenson, 2012). Mindfulness, which involves attending to the present (as opposed to past or future events) is a necessary precursor to either savoring or positive reappraisal (Garland, Farb, Goldin, & Fredrickson, 2015). We focus on the utility of savoring specifically as regulation strategy with cognitive and affective components incompatible with dysphoric rumination. High trait dysphoric ruminators report a greater tendency to dampen positive affect (Feldman, Joormann, & Johnson, 2008), thus their tendency to savor may be infrequent, and/or may be bolstered. Savoring has been examined as a treatment target in depression (McMakin et al., 2011), and similarly, recalling positive autobiographic memories can also reduce dysphoric affect, a process that has been termed mood repair (Daches, Yaroslavsky, & Kovacs, 2019). Given that savoring may repair the effects on dysphoric rumination on negative moods, and positive moods augment RSA levels, it is reasonable to predict that savoring may also effect changes in ANS functioning, particularly in the PNS.
Research on the ANS correlates of savoring is limited, yet there is reason to suspect that savoring may ameliorate the effects of dysphoric ruminating. In reaction to negative events and transgressions, positive reappraisal has been associated with higher or increased PNS control compared to ruminating (da Silva et al., 2016; Witvliet et al., 2011; Witvliet et al., 2010). Focusing on positive images and past experiences such as appreciating someone or meditating on love and kindness have consistently been linked with PNS augmentation (Garland, Bryan, Nakamura, Froeliger, & Howard, 2017; Kok et al., 2013; McCraty, Atkinson, Tiller, Rein, & Watkins, 1995). Unfortunately, the bulk of prior research has not assessed the impact on these regulation strategies on the SNS. PEP may be a particularly relevant index for savoring, as PEP has been found to be sensitive to reward tasks and anticipation, and thus has been conceptualized as an index of behavioral approach (Brenner, Beauchaine, & Sylvers, 2005; Fowles, 1988; Obradović & Boyce, 2012). To our knowledge, research has yet to examine changes in PEP during savoring. Since savoring is a regulation strategy that enhances positive moods, which increases motivation for behavioral approach, savoring should correspond with sympathetic activation captured by PEP specifically, not EDA. Taken together, if dysphoric rumination is associated with PNS withdrawal, then savoring may aid in the subsequent mood repair process via RSA augmentation. Although savoring may not be associated with SNS changes in EDA, successful savoring should lead to SNS arousal associated with positive affect via PEP.
In sum, the autonomic concomitants of state dysphoric rumination have not been clearly defined. It is also unknown whether ANS effects may be repaired by savoring. In addition to extending prior research by examining independent changes in RSA, PEP, and EDA, we also examine coordination between ANS indices. The autonomic space model acknowledges that changes in PNS and SNS branches may occur independently (uncoupled) or in coordination over time (coupled) (Berntson et al., 1994; Berntson et al., 1991). Autonomic coordination measures the extent to which changes in each of the two branches are coupled, or coincide over time, and thus may be a more meaningful predictor of ANS functioning than assessing each branch independently (Bauer, Quas, & Boyce, 2002; Berntson, 2019). Coordinated change can take two forms: reciprocal and non-reciprocal. Inverse changes follow the traditional reciprocal ANS model whereby withdrawal in one branch (e.g., PNS) is coupled with activation in the other (SNS). Conversely, non-reciprocal changes occur when both branches co-regulate, or change in the same direction (co-activation or co-inhibition). In addition to examining whether PNS changes (in RSA) across state dysphoric rumination and savoring tasks correlated with SNS (PEP and EDA), we also examined coordination with the SNS across tasks (changes between EDA and PEP). Because trait rumination is more common among women, we recruited female participants. Building from anger rumination and savoring enhancement protocols (McMakin et al., 2011; Ottaviani et al., 2008), participants were asked to focus on a recent event associated with sadness and joy respectively. We hypothesized that dysphoric rumination would evoke SNS activation, especially via EDA, as well as PNS withdrawal (decrease in RSA), but we did not have a specific hypothesis for PEP. We also examined whether savoring following dysphoric rumination would repair PNS via RSA augmentation, and significant SNS activation via decreases in PEP specifically. Finally, given that trait rumination has been linked with ANS alterations and dampening of positive affect, we also considered whether ANS functioning differed according to trait rumination. We expected that higher trait rumination would be associated with more pronounced ANS changes during state rumination vs. less pronounced recovery ANS changes during savoring.
2. Methods
2.1. Participants
Undergraduate women between 18 and 25 with no history of cardiovascular conditions were recruited from the research participant pool (n= 122). Fifteen participants were excluded for failing to meet inclusion criteria for the following reasons: taking medications known to influence cardiovascular functioning (antihistamines/inhalers, n =8), recent caffeine or nicotine intake (n = 5), or falling asleep during the physiological assessments (n =2). An additional 25 participants were removed in the artifact correction stage for the following reasons: abnormal ECG data due to arrhythmia or ectopic beats (n = 2); or data loss due to logistical failure to capture clear ECG (n =5), EDA (n = 2) or dz/dt (n =18) signal across all five tasks. Thus, analyses reported here focus on a final sample of 82 women (Age: M= 19.81, SD= 1.10). The majority identified as Caucasian (77%) followed by Multi-racial (10%), African American (9%) and Asian (4%); with 5% Hispanic or Latino ethnicity. Participants received course credit as compensation. We did not exclude for oral contraceptive use, and 36 participants (43.9% of the sample) reported taking oral contraceptives. The University’s Institutional Review Board approved all study procedures.
2.2. Procedure
Participants were instructed to refrain from food and caffeine for an hour and alcohol for 48 hours prior to the laboratory visit. Participants taking antihistamines or using inhalers daily were asked to refrain for 24 hours. At the laboratory visit, informed consent was first obtained. Then, after completing a brief demographic survey, physiological sensors were placed, and participants were seated in a comfortable chair. Next there was a 10-minute habituation period during which participants completed questionnaires, followed by the physiological protocol.
2.3. Physiological Protocol
Participants completed 5 tasks in the following order while physiological signals were recorded: baseline, rumination, recovery 1, savoring, recovery 2. To minimize the possibility of rumination or savoring during the baseline, participants watched a nature video (Woody, Burkhouse, Birk, & Gibb, 2015) for 5 minutes. The dysphoric rumination task was modified from an established angry rumination task (Ottaviani et al., 2008). Instead of asking participants to “focus on the causes or consequences of a recalled episode in intense anger or rage ”, participants were instructed to “recall a recent time when you felt intense sadness and think about the causes and consequences of ‘the event” for 3 minutes. Participants were asked to write a brief description of the event (1-2 sentences) in order to code the severity of the event. Next there was a 3-minute recovery period during which participants were asked to sit quietly while the next task was set-up. Then, for the savoring task, participants were asked to “recall a recent time when you felt intense joy or pride and think about the causes and consequences of ‘theevent’ for three minutes. Finally, participants were asked to sit quietly for a 3-minute recovery period.
2.4. Self-report Data
Trait rumination was assessed via the 10-item Ruminative Response Scale, which has two subscales, reflection and brooding (RRS-B: Treynor & Gonzalez, 2003). We focused on the 5-item brooding subscale (α = .78), given that brooding has consistently been found to be the more maladaptive component associated with psychopathology risk (Treynor & Gonzalez, 2003).
Depressive symptoms were assessed with the 20-item Center of Epidemiologic Studies Depression scale, which was designed to assess depression symptoms in the general population (CES-D: Radloff, 1977). Internal reliability in our sample was excellent (α = .89). Summary scores ranged from 0—44, with 24% of the sample scoring at or above the cut-off (21) for moderate depressive symptoms among college aged samples (Shean & Baldwin, 2008).
Positive and negative affect were assessed via visual analogue scales (VAS) six times: prior to baseline and then following each task. Participants rated their levels of happiness, sadness, and anxiety and calmness on four VAS. For example, participants were asked to rate their happiness on a non-hatched VAS scale marked at one end “not at all happy” and at the other “very happy”. Scores were measured in mm with higher numbers indicating higher emotional intensity.
Rumination and savoring were assessed via self-report following the tasks where these were elicited. To capture whether participants continued to ruminate, at the end of Recovery 1 participants were also asked to rate on a VAS scale ‘How much were you just thinking about the event that made you sad?’. Similarly, to assess whether participants continue to engage in savoring, following Recovery 2 we asked them to rate ‘How much were you just thinking about the event that made you happy? on a VAS scale.
2.5. Physiological Data Acquisition and Preprocessing
All physiological data was recorded at 500Hz via BioLab 3.1 acquisition software (MindWare Technologies, Gahanna, OH). Electrocardiogram (ECG) signals were sampled with three disposable, pregeled electrodes placed in a Lead-II configuration (right clavicle, right and left lower rib-cage). Impedance cardiography was derived from the dz/dt signal obtained via a four-lead configuration, with two electrodes placed at the top and bottom of the sternum (between the collar bones and where the rib cage meets, respectively), and two electrodes on the back of the torso (one-inch above and below the corresponding front electrodes). Electrodermal activity was obtained via two disposable Ag/AgCl electrodes placed bilaterally on the palmar surface of participants’ non-dominant hand.
2.5.1. Respiratory Sinus Arrhythmia (RSA)
We used Mindware HRV 3.2 software to process the ECG data to derive RSA. Each 1-minute segment was inspected visually to ensure accurate R-wave detection. On rare occasions, suspected artifacts were corrected manually (< 1% of all beats). To calculate RSA, spectral power analysis was conducted via Fast Fourier transformations, with RSA (high frequency heart rate variability) defined as power in the 0.12 – 0.40 Hz spectral bandwidth (ms2) (see Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology, 1996). RSA was calculated per each one-minute segment, then averaged for each task.
2.5.2. Pre-ejection period (PEP)
We used Mindware IMP 3.2 to process impedance cardiography signals and derive PEP, which is computed as the time between electrical invasion of the ventricular myardium (Q-wave of the ECG) to the opening of the aortic valve (B-point of the dz/dt waveform). Due to a lack of clear and visible B points across all participants, an algorithm was used to automatically place B points at a set percentage of time between ECG and IMP wave peaks with a change in +C=4 (Lozano et al., 2007). The waveforms were visually inspected and cleaned in 1-minute segments to remove poorly defined signals from analysis, and any segment with over 30% of unusable data was removed. Individuals with less than 60% clear segments per task were excluded from analysis. PEP values were derived from the ensemble averages of each one-minute interval. Average PEP was then computed across each task.
2.5.3. Electrodermal Activity (EDA)
We used Mindware EDA 3.2 software to process the electrodermal signals. Data were collected at 500 Hz and processed with the rolling filter. All data were visually inspected in 30 second segments to ensure that phasic components (or sudden 0.05 μS changes in amplitude) were identified prior to calculation of tonic-SCL. Segments with poorly defined signal were removed from analysis. Individuals with less than 60% clear segments per task were excluded from analysis. Tonic skin conductance level (SCL) was computed as the average microsiemens level (μS) per minute then averaged across each task.
2.6. Data Analytic Plan
First, we examined whether rumination and savoring tasks induced expected changes in negative (i.e., sadness, anxiety) and positive affect (i.e., happiness, calmness). Four repeated measures ANOVA examined within subject effects across the six VAS probes (i.e., pre-baseline, post-baseline, rumination, recovery 1, savoring, recovery 2). To determine whether affect changes differed according to trait rumination, brooding rumination was entered as a continuous, between-subjects variable in both models. When significant within-person (Task) effects were found, Bonferroni pairwise post hoc comparisons were conducted to identify significant changes across tasks. Three similar repeated measures ANOVAS were run to examine changes in parasympathetic (RSA) and sympathetic (EDA and PEP) arousal across the five tasks. Given evidence that the effect of dysphoric rumination may differ between depressed and non-depressed individuals (Lyubomirsky & Nolen-Hoeksema, 1995; Morrow & Nolen-Hoeksema, 1990; Nolen-Hoeksema & Morrow, 1993) all models were re-run covarying for current depressive symptoms to determine whether effects were driven by current psychopathology. Trait rumination tends to be strongly associated with depression status, which raises the potential for multicollinearity in multivariate models. The correlation coefficient cut-off for avoiding multicollinearity is typically r ≥ .80 (Berry & Feldman, 1985). The correlation between CESD and state rumination was r = .63, p < .001. Due to expected relationships among study variables, we also examined multicollinearity diagnostics by testing the variance inflation factor (VIF) of both variables in one of the sympathetic concordance regressions. The VIF were low for CESD, VIF = 1.67, and Trait Rumination VIF = 1.63, indicating a low risk for multicollinearity (VIF<4; see O’Brien, 2007).
To examine autonomic coordination, we conducted eight multi-variate regressions to determine whether PNS changes (RSA) co-occurred with SNS changes (EDA and then PEP), across tasks. Because delta change scores are particularly problematic with physiological data, we used residual difference scores (Meyer, Lerner, De Los Reyes, Laird, & Hajcak, 2017). The primary predictor in each model was RSA residual difference scores (residuals after covarying for prior task RSA). Changes in the dependent variable (e.g., EDA) were captured by covarying for EDA levels during the prior task. To examine SNS coordination between EDA and PEP, four identical models were run, except residual difference scores was computed for EDA to examine changes in PEP. To determine whether autonomic and sympathetic coordination differed as a function of trait rumination, moderation models were tested using PROCESS SPSS Macro version 3.3 (Hayes, 2018) by entering continuous brooding rumination as a moderator of RSA residual change (autonomic coordination) or EDA residual difference scores (sympathetic coordination) in Model 1. Significant interactions were probed by examining coordination at +/− 1 SD (high and low trait rumination).
Although we were careful to remove known sources of variance on ANS activity from the sample, we also explored potential covariates. There is some evidence that ANS functioning differs according to hormonal status such as use of oral contraceptives (Armbruster, Kirschbaum, & Strobel, 2017; Milan, de Oliveira Plassa, de Abreu PhD, & Gomes, 2015), but see also (Schallmayer & Hughes, 2010; Snieder, Van Doornen, Boomsma, & Thayer, 2007; Wilczak et al., 2013). Given this mixed literature, in preliminary exploratory analyses we examined whether current oral contraceptive use (binary coded, 1=yes, 0=no) was associated with RSA, PEP, and EDA levels via independent samples t-tests. None were statistically significant (lowest p = .178). Thus, in the interest of model parsimony, we did not covary for oral contraceptive use in the final models.
3. Results
We first examined the presence of missing data as well as variable distribution in our survey measures. A few survey items had missing data (< 2%). To justify data imputation via maximum likelihood estimation, we examined if their data was missing at random (Schafer & Graham, 2002). Little’s missing completely at random test was non-significant, χ2(428)=435.324, p = .394, supporting the imputation of missing values (Little & Rubin, 1987). As the CESD was skewed, a square root transformation was applied. Descriptive statistics for all variables are presented in Table 1, with raw scores presented for clarity.
Table 1.
Correlations and Descriptive Statistics
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 RRS-B | — | ||||||||||||||||
| 2 CESD | .62*** | — | |||||||||||||||
| 3 BaseRSA | −.16 | −.18 | — | ||||||||||||||
| 4 BasePEP | .04 | −.10 | −.06 | — | |||||||||||||
| 5 BaseEDA | .10 | .17 | .09 | −.14 | — | ||||||||||||
| 6 RumRSA | −.14 | −.20 | .84*** | .03 | .01 | — | |||||||||||
| 7 RumPEP | .10 | −.03 | −.16 | .91*** | −.18 | −.08 | — | ||||||||||
| 8 RumEDA | .05 | .11 | .13 | −.16 | .94*** | .03 | −.21 | — | |||||||||
| 9 Recov1RSA | −.17 | −.16 | .84*** | −.08 | .01 | .87*** | −.17 | .01 | — | ||||||||
| 10 Recov1PEP | .10 | −.01 | −.18 | .89*** | −.17 | −.07 | .97*** | −.18 | −.17 | — | |||||||
| 11 Recov1EDA | .08 | .16 | .11 | −.13 | .91*** | .04 | −.19 | .96*** | .01 | −.18 | — | ||||||
| 12 SavorRSA | −.18 | −.25* | .76*** | −.08 | −.12 | .87*** | −.20 | −.10 | .87*** | −.20 | −.09 | — | |||||
| 13 SavorPEP | .11 | −.01 | −.15 | .90*** | −.15 | −.04 | .97*** | −.18 | −.14 | .98*** | −.16 | −.16 | — | ||||
| 14 SavorEDA | −.01 | .06 | .16 | −.20 | .78*** | .03 | −.24 | .85*** | .03 | −.23* | .87*** | −.05 | −.23 | — | |||
| 15 Recov2RSA | −.21 | −.16 | .75*** | −.07 | −.16 | .78*** | −.16 | −.15 | .84*** | −.17 | −.16 | .87*** | −.15 | −.13 | — | ||
| 16 Recov2PEP | .09 | −.03 | −.18 | .89*** | −.16 | −.06 | .95*** | −.18 | −.15 | .97*** | −.16 | −.18 | .98*** | −.24* | −.17 | — | |
| 17 Recov2EDA | .04 | .10 | .17 | −.18 | .88*** | .03 | −.21 | .93*** | .02 | −.18 | .93*** | −.09 | −.19 | .87*** | −.15 | −.20 | — |
| Mean | 11.45 | 15.95 | 6.58 | 93.80 | 8.75 | 6.71 | 91.15 | 10.24 | 6.58 | 95.55 | 10.43 | 6.53 | 91.66 | 10.08 | 6.42 | 92.03 | 10.76 |
| SD | 3.39 | 9.66 | 1.07 | 9.33 | 4.13 | 0.97 | 8.80 | 4.06 | 0.92 | 8.53 | 4.03. | 1.02 | 8.55 | 3.85 | 1.01 | 8.46 | 4.08 |
Note: RRS-B = trait brooding rumination; CESD= depressive symptoms; Base= baseline task; Rum= state rumination task; Recover1: recovery following rumination task.; Savor= state savoring task; Recov2= recovery following savoring task. RSA = Respiratory Sinus Arrhythmia; PEP= cardiac pre-ejection period; EDA= electrodermal activity.
p <.001
p <.01
p <.05
3.1. Manipulation Check: Self-reported Affect following Dysphoric Rumination and Savoring Manipulations
As a manipulation check of our rumination and savoring inductions, we first examined changes in self-reported affect (see Table 2 and Figure 1). None of the Task × Trait Rumination interactions were significant. As expected, dysphoric rumination was associated with upregulation specifically in sadness, not anxiety. Conversely, savoring was associated with upregulation of happiness. Importantly, happiness during savoring was significantly higher than baseline, p = .001, indicating the savoring task induced positive affect and was not merely a return to baseline following rumination.
Table 2.
Repeated Measures ANOVAs testing Affective and Autonomic Changes across Tasks
| Affect DV |
Predictor | Type | F | df | p | η2 |
|---|---|---|---|---|---|---|
| Sad | Task | W | 3.68 | 5 | .003 | .04 |
| Trait Rumination | B | 25.19 | 1 | .000 | .24 | |
| Task x Trait Rumination | M | 1.20 | 5 | .307 | .02 | |
| Anxious | Task | W | 0.63 | 5 | .675 | .01 |
| Trait Rumination | B | 1.58 | 1 | .213 | .02 | |
| Task × Trait Rumination | M | 0.41 | 5 | .842 | .01 | |
| Happy | Task | W | 3.53 | 5 | .004 | .04 |
| Trait Rumination | B | 7.93 | 1 | .000 | .09 | |
| Task × Trait Rumination | M | 1.40 | 5 | .225 | .02 | |
| Calm | Task | W | 0.68 | 5 | .638 | .01 |
| Trait Rumination | B | 9.60 | 1 | .003 | .11 | |
| Task × Trait Rumination | M | 1.11 | 5 | .355 | .01 | |
| ANS DV | ||||||
| RSA | Task | W | 0.02 | 4 | .999 | .00 |
| Trait Rumination | B | 2.90 | 1 | .092 | .04 | |
| Task × Trait Rumination | M | 0.37 | 4 | .831 | .01 | |
| PEP | Task | W | 6.10 | 4 | .000 | .07 |
| Trait Rumination | B | 0.64 | 1 | .427 | .01 | |
| Task × Trait Rumination | M | 0.81 | 4 | .518 | .01 | |
| EDA | Task | W | 4.94 | 4 | .001 | .06 |
| Trait Rumination | B | 0.22 | 1 | .639 | .00 | |
| Task × Trait Rumination | M | 1.28 | 4 | .276 | .02 | |
Note: Type: Within (W), Between Subjects (B), Mixed (M) predictor.
Figure 1:
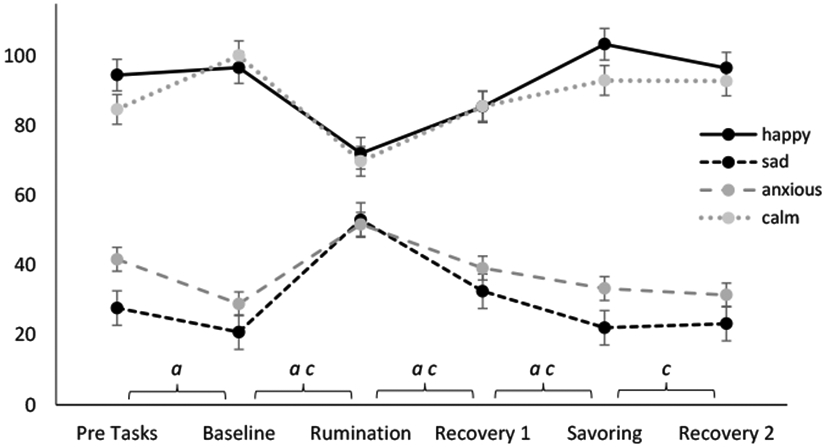
Estimated marginal means for self-reported happiness, sadness and anxiety across tasks based on visual analogue scales. Error bars represent standard error of the means. Significant change between tasks (p < .05): a = Sadness, b = Anxiety, c = Happiness, d = Calm
3.1.1. Validity Check: Self-reported Trait Rumination and State Rumination Associations
If the dysphoric rumination induction was successful, we would expect to see individuals high on trait ruminative tendency continue to ruminate at the end of the task (during recovery 1) and thus maintain sadness. As expected, trait rumination was associated with greater reported rumination during recovery 1 (r = 0.40, p < .001). During recovery 1, reported state rumination did not interact with task to predict changes in sadness, but was associated with greater sadness, F(1,80) = 38.97, p < .001, η2 = .33. Both effects were maintained after covarying for depressive symptoms.
We also expected that trait rumination would be associated with lower reported savoring and happiness during recovery 2. Trait rumination was also associated with higher reported savoring during recovery 2 (r = 0.27, p = .014). During recovery 2, reported state savoring did not predict changes in happiness, but was associated with slightly higher happiness, F(1,80) = 3.98, p = .049, η2 = .05, but this effect did not maintain significance after covarying for depressive symptoms.
3.2. PNS and SNS Activity During Rumination and Savoring Tasks
All three models (RSA, PEP, EDA) are displayed in Table 2, with the pattern of results exhibited in Figure 2. None of the trait rumination or the Task × Rumination effects were significant. Focusing first on RSA, the effect of Task was also not significant. Thus, RSA levels did not significantly change across tasks or differ according to trait rumination.
Figure 2:
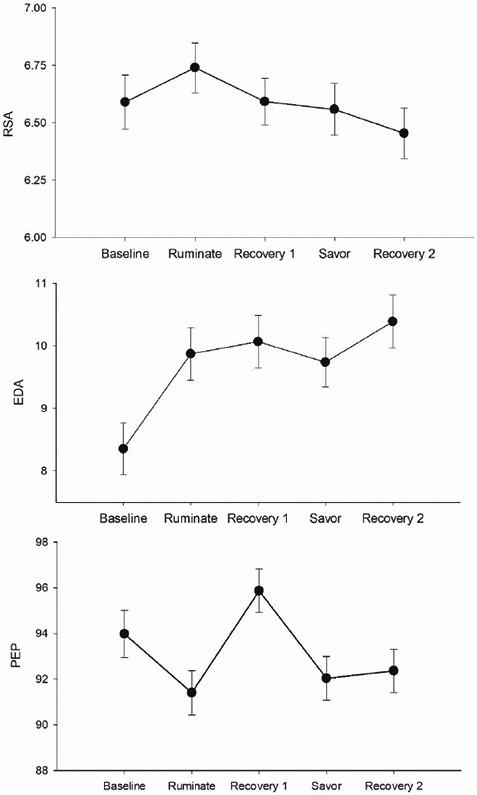
Estimated marginal means for: RSA = Respiratory Sinus Arrhythmia, EDA = Electrodermal Activity, PEP = Pre-Ejection Period across tasks. Error bars represent standard errors of the mean.
For PEP there was a significant effect of Task. Pairwise comparisons revealed significant changes across the first four tasks (all ps < .001). Specifically, PEP decreased (indicating sympathetic activation) during the rumination task, increased during recovery 1 (indicating sympathetic withdrawal) and decreased again during the savoring task.
For EDA, there was a significant effect of Task. Pairwise comparisons revealed that EDA increased during the rumination task and again during recovery 2. The pattern of results for RSA, PEP and EDA did not change when covarying for depressive symptoms.
Self-reported rumination and savoring did not predict changes in or levels of RSA, PEP, or EDA during recovery 1 or recovery 2 respectively
3.3. Autonomic Coordination across State Rumination and Savoring Tasks
RSA residuals did not predict changes in PEP across any of the tasks (lowest p = .246). Similarly, RSA residuals did not predict changes in EDA across tasks (lowest p = .630). Trait rumination did not moderate any of these associations (lowest p = .191).
3.4. SNS Coordination across State Rumination and Savoring Tasks
Next, we examined SNS coordination by testing whether EDA residuals predicted changes in PEP across tasks. Significant effects emerged during two tasks: Recovery 1, and Savoring. During Recovery 1, there was a significant effect of residual EDA on PEP, β= −0.08, t = −2.95, p = .004, semi-partial correlation sr= −0.32 indicating increases in one index corresponded with decreases in the other. Since there was a decrease in sympathetic arousal during Recovery 1 as indexed by PEP, we can infer this association indicates concordant sympathetic withdrawal (increases in PEP corresponded with decreases in EDA). During savoring, the residual EDA × Rumination interaction trended to significance, β= −0.09, t = −2.02, p = .047. Probing the interaction there was a negative association between PEP and EDA changes during savoring among high trait ruminators, β= −0.47, t = −2.34, p = .022. Given PEP significantly decreased during savoring, we may infer that sympathetic co-activation occurred among high-trait ruminators (greater decreases in PEP corresponded with larger increases in EDA). Among low trait-ruminators, there was no significant association between changes in EDA and PEP, β= 0.14, t = 0.87, p = .386. When covarying for depressive symptoms, the EDA × Rumination interaction reached statistical significance, p = .038.
4. Discussion
Dysphoric rumination is a transdiagnostic factor implicated in anxiety and depressive disorders, and has maladaptive effects on emotion regulation capacity, which may be captured via ANS assessment. Clarifying the biological underpinnings of dysphoric rumination may advance etiology and maintenance models of internalizing disorders. To date though, the autonomic correlates of state dysphoric rumination has been largely limited to PNS assessments. The aim of the current study therefore, was to carry out a comprehensive ANS assessment of state dysphoric rumination on PNS and SNS, and examine the potential repairing effects of post-rumination savoring in a non-clinical sample. We extend prior research by assessing individual changes in RSA, PEP, and EDA, as well as ANS and SNS coordination, as a function of trait rumination and post-rumination savoring. State dysphoric rumination corresponded with SNS activation via both EDA and PEP. Cholinergic-driven sympathetic activity (EDA) was maintained for the remainder of the laboratory protocol. In contrast, savoring resulted in sympathetic activation driven by beta-adrenergic neurotransmission (PEP). The current results highlight the utility of comprehensive ANS assessments for delineating the effects of specific emotion regulation strategies, such as dysphoric rumination, and results indicate that sympathetic effects may be at least redirected towards savoring.
The autonomic correlates of state dysphoric rumination we observed partially aligned with hypotheses. We had expected dysphoric rumination to coincide with SNS activation driven by EDA. Instead, we found SNS activation across both measures (i.e., there was a large association between decreases in PEP as co-occurring with increases in EDA). SNS activation associated with dysphoric rumination aligns with prior research supporting decreases in PEP during angry rumination (Ottaviani & Shapiro, 2011; Ottaviani et al., 2008; Ray et al., 2008). To our knowledge, this is the first study to simultaneously assess EDA and PEP during laboratory-induced dysphoric rumination. Thus, finding activation across both indices extends prior research by indicating that dysphoric rumination is associated with sympathetic activation driven by both beta-adrenergic and cholinergic neurotransmission. The lack of PNS changes during dysphoric rumination contrasts prior research demonstrating PNS withdrawal during rumination inductions that upregulate anger (da Silva et al., 2016; Ottaviani & Shapiro, 2011; Ottaviani et al., 2008; Witvliet et al., 2011; Witvliet et al., 2010) and rumination following a laboratory stressor (LeMoult et al., 2016). We found that state dysphoric rumination was associated with upregulation of sadness specifically (i.e., not anxiety), which is consistent with prior work that dysphoric rumination amplifies sadness, not anxiety or hostility (Morrow & Nolen-Hoeksema, 1990). One potential explanation is that dysphoric rumination is not as activating as rumination that amplifies anger. Another possibility is that the effects of dysphoric rumination are more salient when currently dysphoric or anxious. Future research is needed to assess the ANS correlates of dysphoric rumination following a negative mood induction to determine differential effects with rumination that amplifies anger.
To examine autonomic correlates of mood repair processes, we also examined the physiological effect of savoring following dysphoric rumination. We found that savoring a positive autobiographical event corresponded with decreases in PEP (i.e., increases in SNS activation), but no changes in RSA or EDA. PEP levels had increased during the recovery period following rumination and was higher than baseline levels prior to savoring. The current pattern of results aligns with research supporting that PEP indexes sympathetic activity in response to rewards (Brenner et al., 2005; Fowles, 1988; Obradović & Boyce, 2012) and provides initial evidence that following dysphoric rumination, savoring is effective at recruiting beta-adrenergic driven sympathetic activity among both high and low trait ruminators.
The lack of change in RSA is more puzzling and contrasts prior evidence that related strategies such as mindfulness meditations corresponds with RSA activation (Ditto, Eclache, & Goldman, 2006; Garland et al., 2017; Kok et al., 2013; McCraty et al., 1995). Prior work indicates that positive reappraisal of transgressions is associated with higher HRV compared to rumination that induces anger, but rumination in these inductions was associated with HRV withdrawal (da Silva et al., 2016; Witvliet et al., 2011; Witvliet et al., 2010). Our results suggest that savoring, as a mood repair process may be less effective at recruiting PNS activation following dysphoric rumination. Rather, perhaps RSA is only amplified during savoring when it results in enhancing an already positive mood state.
The current study is the first to assess PEP changes associated with savoring, and our observation that PEP decreases during savoring suggests that post-ruminative savoring is associated with SNS arousal driven by beta-adrenergic neurotransmission (Larsen, Schneiderman, & Pasin, 1986). Given that savoring increased happiness above baseline levels, the current results correspond with literature conceptualizing PEP as an index of behavioral approach (Brenner et al., 2005; Gendolla, 2012; Kelsey, 2012) and reward sensitivity (Obradović & Boyce, 2012). We also observed PEP decreasing during dysphoric rumination, which co-occurred with increased sadness, an emotion typical of behavioral inhibition. Taken together, this pattern may suggest that PEP functioned as a general index of emotional arousal (i.e., not valence specific) that is associated with personally significant emotionally laden events in the current study. After an initial increase during dysphoric rumination, EDA levels were maintained across the remaining tasks. Thus, we may either infer that savoring was not effective at reducing cholinergic-driven SNS activation, or that a longer recovery period was needed to capture EDA’s return to baseline following dysphoric rumination.
In addition to examining changes within RSA, EDA and PEP, we also examined whether changes in ANS indices co-occurred across tasks. There was a marginal effect, such that savoring was associated with SNS coordination among individuals higher in trait rumination, exhibiting concordance between decreases in PEP and increases in EDA. In contrast, SNS coordination did not occur among lower trait ruminators. This pattern should be interpreted with caution pending replication but has potential implications. Competitive coordination between parasympathetic and sympathetic branches has been conceptualized as adaptive vs. ineffective expenditure of resources, depending on the demands of the task (Gatzke-Kopp & Ram, 2018). We extend this rationale to coordination within the sympathetic branch during savoring. We suggest that SNS concordance displayed by high trait ruminators may reflect a less effective attempt to up-regulate positive affect as it required multiple sources of sympathetic activation. In contrast, low trait ruminators who may be better at savoring positive affect were able to enhance positive affect by recruiting specific sympathetic resources (increases in PEP only). SNS concordance also occurred among everyone in this non-clinical sample during the first recovery period, supporting concordant changes in sympathetic withdrawal (increases in PEP and decreases in EDA). In this non-clinical sample, we suggest that general SNS withdrawal may reflect adaptive recovery from rumination and may differ in depressed individuals. However, research is limited on sympathetic coordination, and future work is needed to interpret adaptive vs. ineffective coordination within the SNS.
We were surprised to find that trait rumination was not associated with differences in SNS or PNS arousal during state dysphoric rumination in the laboratory. Prior research has linked trait rumination with exaggerated PNS withdrawal in non-clinical samples (Borelli, Hilt, West, Weekes, & Gonzalez, 2014; Woody et al., 2015) vs. augmentation in clinical and high-risk samples (Aldao et al., 2013; Gentzler et al., 2013) in reaction to negative stimuli. Results of the current study indicate that trait dysphoric rumination does not explain parasympathetic or sympathetic variance during dysphoric rumination. However, we recruited a general sample (rather than high vs. low ruminators). Thus, it is possible that moderation analyses were underpowered due to relatively low levels of trait rumination in our sample (only 22% of participants reported a mean of three or higher on the RRS, indicating a tendency to frequently ruminate). Further, there is evidence that the impact of dysphoric rumination on negative affect differs according to depression status (Lyubomirsky & Nolen-Hoeksema, 1995; Morrow & Nolen-Hoeksema, 1990; Nolen-Hoeksema & Morrow, 1993). Therefore, an important next step will be to examine differences in the autonomic correlates of dysphoric rumination among depressed and non-depressed samples.
One of the most novel aspects of the current study was our examination of autonomic coordination of PNS and SNS across dysphoric rumination and post-ruminative savoring. The lack of significant results was surprising. It is likely that the null results of autonomic coordination in the current sample is due to lack of variance in RSA across tasks. Prior research on angry rumination supports individual changes in both PNS and SNS arousal (RSA withdrawal and PEP activation) (Ottaviani & Shapiro, 2011; Ottaviani et al., 2008). Thus, we may expect cooperative coordination between changes in RSA and PEP/EDA during dysphoric rumination (such that greater SNS withdrawal corresponds with greater sympathetic activation), but coordination cannot be assumed based on changes in individual branches. This is the first study to examine autonomic coordination during dysphoric rumination and savoring. Null results should be interpreted cautiously pending replication.
The current study benefitted from a comprehensive ANS assessment that included RSA, PEP and EDA. ANS functioning was delineated by examining changes within individual indices, as well as coordination between PNS and SNS branches and coordination within SNS. That said, there are several limitations worth emphasizing. First, to assess natural perseverative rumination during the first recovery period, we used a general thought probe that did not distinguish between rumination subtypes. Thus, it is possible that participants engaged in reflective as well as brooding rumination. Replication studies may assess internal thought content via recently validated state rumination procedures (Marchetti, Mor, Chiorri, & Koster, 2018). Second, as we were interested in savoring as a mood repair process, we did not counterbalance the savoring and rumination tasks. Therefore, future research with comprehensive autonomic assessments are needed to delineate the autonomic correlates of savoring across a variety of contexts. Third, we recruited a general sample of undergraduate women. Future research may further clarify ANS correlates of dysphoric rumination by comparing distinct populations (e.g., individuals high or low on trait rumination), clinical populations (depressed vs. non-depressed) given evidence that rumination functions differently when currently depressed (Nolen-Hoeksema & Morrow, 1993), as well as age and biological sex differences. Fourth, as there is evidence that parasympathetic tone as measured by HF-HRV is impacted by hormonal changes during women’s menstrual cycle phase (Schmalenberger et al., 2020; Schmalenberger et al., 2019), future research should assess factors such as pregnancy, breastfeeding status, menstruation timing, and use of oral contraceptives on ANS functioning. Further, as there is evidence of biological sex differences in resting HF-HRV (Jarczok et al., 2018; Koenig & Thayer, 2016), as well as links between HF-HRV and emotion regulation difficulties and neural activation in limbic regions implicated in emotional reactivity (Nugent, Bain, Thayer, Sollers, & Drevets, 2011; Williams et al., 2019), research should examine biological sex differences in autonomic coordination during rumination and savoring.
In conclusion, the current study extends research on the ANS correlates of dysphoric rumination and potential repairing effects of savoring. Results indicate that dysphoric rumination coincides with cholinergic and beta-adrenergic driven SNS activation. Subsequent savoring was associated with successful recruitment of beta-adrenergic SNS activation and increase in positive affect. The pattern of SNS activation across rumination and subsequent savoring highlights that changes in SNS arousal is driven by multiple underlying sources. Thus, SNS assessments should ideally include PEP as well as EDA. We also found SNS coordination differed according to trait rumination during savoring. This pattern highlights the need for future research to include assessments of ANS and SNS coordination, as coordination cannot be inferred from changes across individual indices. The changes in SNS arousal specifically are linked to an emotion regulation strategy implicated in depression and anxiety disorders risk and maintenance. Future research on dysphoric rumination and savoring is needed to determine how the influence on ANS functioning over time and to examine whether ANS patterns differ in clinical samples.
Highlights.
State dysphoric rumination corresponded with sympathetic activation (EDA and PEP)
Post-rumination savoring led to sympathetic activation (PEP), successful mood repair
Dysphoric rumination and savoring did not induce parasympathetic change (RSA)
Autonomic assessments should include multiple indices
Acknowledgments
Funding: Dr. Bylsma receives research funding support from MH118218.
Footnotes
Competing Interests statement: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldao A, Mennin DS, & McLaughlin KA (2013). Differentiating worry and rumination: Evidence from heart rate variability during spontaneous regulation. Cognitive Therapy Research, 37(3), 613–619. doi: 10.1007/s10608-012-9485-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter A (2008). Emotion regulation and anxiety disorders. Journal of Anxiety Disorders, 22, 211–221. doi: 10.1016/j.janxdis.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster D, Kirschbaum C, & Strobel A (2017). The not-so-bitter pill: effects of combined oral contraceptives on peripheral physiological indicators of emotional reactivity. Hormones and Behavior, 94, 97–105. doi: 10.1016/j.yhbeh.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Barlow DH (2002). Anxiety and its disorders—The nature and treatment of anxiety and panic (2nd ed.). New York, NY: Guilford Press. [Google Scholar]
- Bauer AM, Quas JA, & Boyce WT (2002). Associations between physiological reactivity and children's behavior: Advantages of a multisystem approach. Journal of Developmental and Behavioral Pediatrics, 23(2), 102–113. doi: 10.1097/00004703-200204000-00007 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory sinus arrhythmia: A transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology, 3, 43–47. doi: 10.1016/j.copsyc.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG (2019). Presidential address 2011: Autonomic modes of control and health. Psychophysiology, 56(1), e13306. doi: 10.1111/psyp.13306 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, & Fieldstone A (1994). Autonomic cardiac control. III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology, 31(6), 599–608. doi: 10.1111/j.1469-8986.1994.tb02352.x [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1991). Autonomic determinism: The modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychological Review, 98(4), 459–487. doi: 10.1037/0033-295X.98.4.459 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, & Cacioppo JT (2008). Cardiac autonomic balance vs. cardiac regulatory capacity. Psychophysiology, 45(4), 643–652. doi: 10.1111/j.1469-8986.2008.00652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry WD, & Feldman S (1985). Multiple Regression in Practice (Quantitative Applications in the Social Sciences). Thousand Oaks. CA: SAGE Publications. [Google Scholar]
- Bitterman ME, & Holtzman WH (1952). Conditioning and extinction of the galvanic skin response as a function of anxiety. The Journal of Abnormal and Social Psychology, 47(3), 615–623. doi: 10.1037/h0057190 [DOI] [PubMed] [Google Scholar]
- Blagden JC, & Craske MG (1996). Effects of active and passive rumination and distraction: A pilot replication with anxious mood. Journal of Anxiety Disorders, 10, 243–252. doi: 10.1016/0887-6185(96)00009-6 [DOI] [Google Scholar]
- Borelli JL, Hilt LM, West JL, Weekes NY, & Gonzalez MC (2014). School-aged children's depressive rumination is associated with their reactivity to sadness but not fear. Journal of Clinical Child and Adolescent Psychology, 43(5), 799–812. doi: 10.1080/15374416.2013.814542 [DOI] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP, & Sylvers PD (2005). A comparison of psychophysiological and self-report measures of BAS and BIS activation. Psychophysiology, 42, 108–115. doi: 10.1111/j.1469-8986.2005.00261.x [DOI] [PubMed] [Google Scholar]
- Bryant FB, & Veroff J (2007). Savoring: A new model of positive experience. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Butler EA, Gross JJ, & Barnard K (2014). Testing the effects of suppression and reappraisal on emotional concordance using a multivariate multilevel model. Biological Psychology, 98, 6–18. doi: 10.1016/j.biopsycho.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, & Ito TA (2000). The psychophysiology of emotion. Handbook of emotions In Lewis M & Haviland-Jones JM (Eds.), Handbook of emotions (pp. 172–191). New York, NY: Guildford Press. [Google Scholar]
- Carnevali L, Thayer JF, Brosschot JF, & Ottaviani C (2018). Heart rate variability mediates the link between rumination and depressive symptoms: A longitudinal study. International Journal of Psychophysiology, 131, 131–138. doi: 10.1016/j.ijpsycho.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Carver CS, & Harmon-Jones E (2009). Anger Is an approach-related Affect: Evidence and implications. Psychological Bulletin, 135(2), 183–204. doi: 10.1037/a0013965 [DOI] [PubMed] [Google Scholar]
- Cisler JM, Olatunji BO, Feldner MT, & Forsyth JP (2010). Emotion regulation and the anxiety disorders: An integrative review. Journal of Psychopathology and Behavioral Assessment, 32, 68–82. doi: 10.1007/s10862-009-9161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley M, Plans D, Morelli D, Sütterlin S, Inceoglu I, Thomas G, & Chu C (2017). The association between work-related rumination and heart rate variability: A field study. Frontiers in Human Neuroscience, 11. doi: 10.3389/fnhum.2017.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva SP, Witvliet CVO, & Riek B (2016). Self-forgiveness and forgiveness-seeking in response to rumination: Cardiac and emotional responses of transgressors. Journal of Positive Psychology, 12(4), 362–372. doi: 10.1080/17439760.2016.1187200 [DOI] [Google Scholar]
- Daches S, Yaroslavsky I, & Kovacs M (2019). The persistence of hedonically-based mood repair among young offspring at high- and low-risk for depression. Cognition and Emotion, 1–13. doi: 10.1080/02699931.2019.1660622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, & Filion DL (2007). The electrodermal system In Cacioppo JT, Tassinary LG, & Berntson GG (Eds.), Handbook of Psychophysiology (3rd ed., pp. 159–181). New York, NY: Cambridge University Press. [Google Scholar]
- Ditto B, Eclache M, & Goldman N (2006). Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Annals of Behavioral Medicine, 32(3), 227–234. [DOI] [PubMed] [Google Scholar]
- Ehring T, Szeimies AK, & Schaffrick C (2009). An experimental analogue study into the role of abstract thinking in trauma-related rumination. Behavior Research and Therapy, 47(4), 285–193. doi: 10.1016/j.brat.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Feldman GC, Joormann J, & Johnson SL (2008). Responses to positive affect: A self-report measure of rumination and dampening. Cognitive Therapy and Research, 32(4), 507–525. doi: 10.1007/s10608-006-9083-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC (1986). The eccrine system and electrodermal activity In Coles MGH, Donchin E, & Porges SW (Eds.), Psychophysiology: Systems, processes, and applications, (pp. 51–96). New York, NY: Guilford. [Google Scholar]
- Fowles DC (1988). Psychophysiology and psychopathology: A motivational approach. Psychophysiology, 25, 373–391. doi: 10.1111/j.1469-8986.1988.tb01873.x [DOI] [PubMed] [Google Scholar]
- Fresco DM, Frankel AN, Mennin DS, Turk CL, & Heimberg RG (2002). Distinct and overlapping features of rumination and worry: The relationship of cognitive production to negative affective states. Cognitive Therapy and Research, 26(2), 179–188. doi:doi: 10.1023/A:1014517718949 [DOI] [Google Scholar]
- Garland EL, Bryan CJ, Nakamura Y, Froeliger B, & Howard MO (2017). Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology, 234(4), 621–629. doi: 10.1007/s00213-016-4494-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Farb NA, Goldin PR, & Fredrickson BL (2015). The mindfulness-to-meaning theory: extensions, applications, and challenges at the attention–appraisal–emotion interface. Psychological Inquiry, 26(4), 377–387. doi: 10.1080/1047840X.2015.1092493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzke-Kopp L, & Ram N (2018). Developmental dynamics of autonomic function in childhood. Psychophysiology, 55(11), e13218. doi: 10.1111/psyp.13218 [DOI] [PubMed] [Google Scholar]
- Gendolla GH (2012). Implicit affect primes effort: A theory and research on car-diovascular response. International Journal of Psychophysiology, 86, 123–135. doi: 10.1016/j.ijpsycho.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Gentzler AL, Wheat AL, Palmer CA, & Burwell RA (2013). Children’s responses to cognitive challenge and links to self-reported rumination. Cognition & Emotion, 27(2), 305–317. doi: 10.1080/02699931.2012.716394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Grassia M, Stone LB, Uhrlass DJ, & McGeary JE (2012). Brooding rumination and risk for depressive disorders in children of depressed mothers. Journal of Abnormal Child Psychology, 40(2), 317–326. doi: 10.1007/s10802-011-9554-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert K, & Gruber J (2014). Emotion regulation of goals in bipolar disorder and major depression: A comparison of rumination and mindfulness. Cognitive Therapy and Research, 38(4), 375–388. doi: 10.1007/s10608-014-9602-3 [DOI] [Google Scholar]
- Gross JJ (1998). Antecedent-and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology, 74( 1), 224. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2018). Introduction to mediation, moderation, and conditional process analysis. Retrieved from http://www.guilford.com/p/hayes3 [DOI] [PubMed] [Google Scholar]
- Hurley DB, & Kwon P (2013). Savoring helps most when you have little: Interaction between savoring the moment and uplifts on positive affect and satisfaction with life Journal of Happiness Studies, 14, 1261–1271. doi: 10.1007/s10902-012-9377-8 [DOI] [Google Scholar]
- Jarczok MN, Aguilar-Raab C, Koenig J, Kaess M, Borniger JC, Nelson RJ, … Fischer JE (2018). The Heart´s rhythm ‘n’blues: Sex differences in circadian variation patterns of vagal activity vary by depressive symptoms in predominantly healthy employees. Chronobiology International, 35(7), 896–909. doi: 10.1080/07420528.2018.1439499 [DOI] [PubMed] [Google Scholar]
- Joorman J, & Siemer M (2014). Emotion Regulation in Mood Disorders In Gross JJ (Ed.), Handbook of Emotion Regulation (pp. 413–427). New York, NY: Guildford Press. [Google Scholar]
- Jose PE, Lim BT, & Bryant FB (2012). Does savoring increase happiness? A daily diary study. The Journal of Positive Psychology, 7(3), 176–187. doi: 10.1080/17439760.2012.671345 [DOI] [Google Scholar]
- Katona PG, & Jih F (1975). Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. Journal of Applied Physiology, 39(5), 801–805. doi: 10.1152/jappl.1975.39.5.801 [DOI] [PubMed] [Google Scholar]
- Kelsey RM (2012). Beta-adrenergic cardiovascular reactivity and adaptation tostress: The cardiac pre-ejection period as an index of effort In Wright RA & Gendolla GHE (Eds.), How motivation affects cardiovascular response: Mechanisms and applications (pp. 43–60). Washington, DC: American Psychological Association. [Google Scholar]
- Koenig J, & Thayer JF (2016). Sex differences in healthy human heart rate variability: a meta-analysis. Neuroscience and Biobehavioral Reviews, 64, 288–310. doi: 10.1016/j.neubiorev.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Kok BE, Coffey KA, Cohn MA, Catalino LI, Vacharkulksemsuk T, Algoe SB, … Fredrickson BL (2013). How positive emotions build physical health: perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychological Science, 24(7), 1123–1132. doi: 10.1177/0956797612470827 [DOI] [PubMed] [Google Scholar]
- Lader MH, & Wing L (1964). Habituation of the psycho-galvanic reflex in patients with anxiety states and in normal subjects. Journal Neurology Neurosurgery and Psychiatry, 27(3), 210–218. doi:doi: 10.1136/jnnp.27.3.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Schneiderman N, & Pasin RD (1986). Physiological bases of cardiovascular psychophysiology In Coles MGH, Donchin E, & Porges SW (Eds.), Psychophysiology: Systems, processes, and applications (pp. 122–165). New York, NY: Guilford. [Google Scholar]
- LeMoult J, Yoon KL, & Joormann J (2016). Rumination and cognitive distraction in major depressive disorder: An examination of respiratory sinus arrhythmia. Journal of Psychopathology and Behavioral Assessment, 38(1), 20–29. doi: 10.1007/s10862-015-9510-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA, & Rubin DB (1987). Statistical Analysis with Missing Data. New York, NY, US: J. Wiley & Sons. [Google Scholar]
- Lozano DL, Norman GJ, Knox D, Wood BL, Miller BD, Emery CF, & Berntson GG (2007). Where to B in dZ/dt. Psychophysiology, 44, 113–119. doi: 10.1111/j.1469-8986.2006.00468.x [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, & Nolen-Hoeksema S (1995). Effects of self-focused rumination on negative thinking and interpersonal problem solving. Journal of Personality and Social Psychology, 69(1), 176–190. doi: 10.1037/0022-3514.69.1.176 [DOI] [PubMed] [Google Scholar]
- Marchetti I, Mor N, Chiorri C, & Koster EHW (2018). The brief state rumination inventory (BSRI): Validation and psychometric evaluation. Cognitive Therapy and Research, 42(4), 447–460. doi:doi: 10.1007/s10608-018-9901-1 [DOI] [Google Scholar]
- McCraty R, Atkinson M, Tiller WA, Rein G, & Watkins AD (1995). The effects of emotions on short-term power spectrum analysis of heart rate variability American Journal of Cardiology, 76, 1089–1092. doi: 10.1016/S0002-9149(99)80309-9 [DOI] [PubMed] [Google Scholar]
- Mclaughlin KA, Borkovec TD, & Sibrava NJ (2007). The effects of worry and rumination on affect states and cognitive activity. Behavior Therapy, 38, 23–38. doi: 10.1016/j.beth.2006.03.003 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, & Nolen-Hoeksema S (2011). Rumination as a transdiagnostic factor in depression and anxiety. Behaviour Research and Therapy, 49(3), 186–193. doi: 10.1016/j.brat.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Siegle GJ, & Shirk SR (2011). Positive affect stimulation and sustainment (PASS) module for depressed mood: A preliminary investigation of treatment-related effects. Cognitive Therapy and Research, 35(3), 217–226. doi: 10.1007/s10608-010-9311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellings TM, & Alden LE (2000). Cognitive processes in social anxiety: The effects of self-focus, rumination and anticipatory processing. Behaviour Research and Therapy, 38(3), 243–257. doi: 10.1016/S0005-7967(99)00040-6 [DOI] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, & Hajcak G (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54(1), 114–122. doi: 10.1111/psyp.12664 [DOI] [PubMed] [Google Scholar]
- Milan RC, de Oliveira Plassa B, de Abreu PhD LC, & Gomes RL (2015). Oral contraceptives attenuate cardiac autonomic responses to musical auditory stimulation: Pilot study. Alternative Therapies in Health and Medicine, 21(5), 37. [PubMed] [Google Scholar]
- Morrow J, & Nolen-Hoeksema S (1990). Effects of responses to depression on the remediation of depressive affect. Journal of Personality and Social Psychology, 58(3), 519–527. doi: 10.1037/0022-3514.58.3.519 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (1991). Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology, 100(4), 569–582. doi: 10.1037/0021-843X.100.4.569 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (2000). The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology, 109(3), 504–511. doi: 10.1037/0021-843X.109.3.504 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, & Morrow J (1993). Effects of rumination and distraction on naturally occurring depressed mood. Cognition and Emotion, 7(6), 561–570. doi: 10.1080/02699939308409206 [DOI] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, & Lyubomirsky S (2008). Rethinking Rumination. Perspectives on Psychological Science, 3, 400–424. doi: 10.1111/j.1745-6924.2008.00088.x [DOI] [PubMed] [Google Scholar]
- Nugent AC, Bain EE, Thayer JF, Sobers JJ, & Drevets WC (2011). Sex differences in the neural correlates of autonomic arousal: a pilot PET study. International Journal of Psychophysiology, 80(3), 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RM (2007). A caution regarding rules of thumb for variance inflation factors. Quality & quantity, 41(5), 673–690. [Google Scholar]
- Obradović J, & Boyce WT (2012). Physiological measures of emotion from a developmental perspective: State of the science: Developmental psychophysiology of emotion processes. Monographs of the Society for Research in Child Development, 77(2), 120–128. doi: 10.1111/j.1540-5834.2011.00670.x [DOI] [Google Scholar]
- Ottaviani C, Shahabi L, Tarvainen M, Cook I, Abrams M, & Shapiro D (2015). Cognitive, behavioral, and autonomic correlates of mind wandering and perseverative cognition in major depression. Frontiers in Neuroscience, 8, 433. doi: 10.3389/fnins.2014.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C, & Shapiro D (2011). Do we need a stressor to be stressed? Insights from cardiac regulation. Japanese Psychological Research, 53(2), 155. doi: 10.1111/j.1468-5884.2011.00462.x [DOI] [Google Scholar]
- Ottaviani C, Shapiro D, & Couyoumdjian A (2013). Flexibility as the key for somatic health: From mind wandering to perseverative cognition. Biological Psychology, 94( 1), 38–43. doi: 10.1016/j.biopsycho.2013.05.003 [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Shapiro D, Davydov DM, & Goldstein IB (2008). Autonomic stress response modes and ambulatory heart rate level and variability. Journal of Psychophysiology, 22, 28–40. doi: 10.1027/0269-8803.22.1.23 [DOI] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, & Brosschot JF (2016). Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychological Bulletin, 142(3), 231–259. doi: 10.1037/bul0000036 [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. doi: 10.1016/j.biopsycho.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement, 1(3), 385–401. doi:10.1177_014662167700100306 [Google Scholar]
- Ray RD, Wilhelm FH, & Gross JJ (2008). All in the mind's eye? Anger rumination and reappraisal. Journal of Personality and Social Psychology, 94(1), 133–145. doi: 10.1037/0022-3514.94.1.133 [DOI] [PubMed] [Google Scholar]
- Robinette JW, & Charles ST (2016). Age, rumination, and emotional recovery from a psychosocial stressor. The Journals of Gerontology: Series B, 71(2), 265–274. doi: 10.1093/geronb/gbu097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J (2005). Mood and emotion in major depression. Current Directions in Psychological Science, 14(3), 167–170. doi:10.1111_j.0963-7214.2005.00354.x [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: Our view of the state of the art. Psychological Methods, 7(2), 147–177. doi: 10.1037/1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- Schallmayer S, & Hughes BM (2010). Impact of oral contraception and neuroticism on cardiovascular stress reactivity across the menstrual cycle. Psychology, Health & Medicine, 15(1), 105–115. [DOI] [PubMed] [Google Scholar]
- Schmalenberger KM, Eisenlohr-Moul TA, Jarczok MN, Eckstein M, Schneider E, Brenner IG, … Ditzen B (2020). Menstrual cycle changes in vagally-mediated heart rate variability are associated with progesterone: Evidence from two within-person studies Journal of Clinical Medicine, .9(3), 617. doi: 10.3390/jcm9030617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalenberger KM, Eisenlohr-Moul TA, Würth L, Schneider E, Thayer JF, Ditzen B, & Jarczok MN (2019). A systematic review and meta-analysis of within-person changes in cardiac vagal activity across the menstrual cycle: Implications for female health and future studies. Journal of Clinical Medicine, 8, 1946. doi: 10.3390/jcm8111946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shean G, & Baldwin G (2008). Sensitivity and specificity of depression questionnaires in a college-age sample. Journal of Genetic Psychology, 169(3), 281–292. doi: 10.3200/GNTP.169.3.281-292 [DOI] [PubMed] [Google Scholar]
- Shields S, MacDowell KA, Fairchild SB, & Campbell ML (1987). Is mediation of sweating cholinergic, adrenergic, or both? A comment on the literature. Psychophysiology, 24(3), 312–319. doi: 10.1111/j.1469-8986.1987.tb00301.x [DOI] [PubMed] [Google Scholar]
- Shiota MN, & Levenson RW (2012). Turn down the volume or change the channel? Emotional effects of detached versus positive reappraisal. Journal of Personality and Social Psychology, 103(3), 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snieder H, Van Doornen LJ, Boomsma DI, & Thayer JF (2007). Sex differences and heritability of two indices of heart rate dynamics: a twin study. Twin Research and Human Genetics, 10(2), 364–372. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. (1996). Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation, 93, 1043–1065. doi: 10.1161/01.CIR.93.5.1043 [DOI] [PubMed] [Google Scholar]
- Treynor W, & Gonzalez R, Nolen-Hoeksema S (2003). Rumination Reconsidered: A Psychometric Analysis ∣ SpringerLink. Cognitive Therapy and Research, 27(3), 247–259. doi: 10.1023/A:1023910315561 [DOI] [Google Scholar]
- Watkins ER (2009). Depressive rumination and co-morbidity: Evidence for brooding as a transdiagnostic process. Journal of Rational-Emotive and Cognitive-Behavior Therapy, 27(3), 160–175. doi:doi: 10.1007/s10942-009-0098-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczak A, Marciniak K, Kłapciński M, Rydlewska A, Danel D, & Jankowska EA (2013). Relations between combined oral contraceptive therapy and indices of autonomic balance (baroreflex sensitivity and heart rate variability) in young healthy women. Ginekologia Polska, 84(11). [DOI] [PubMed] [Google Scholar]
- Williams DP, Feeling NR, Hill LK, Spangler DP, Koenig J, & Thayer JF (2017). Resting heart rate variability, facets of rumination and trait anxiety: Implications for the perseverative cognition hypothesis. Frontiers in Human Neuroscience, 11. doi: 10.3389/fnhum.2017.00520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DP, Tracy LM, Gerardo GM, Rahman T, Spangler DP, Koenig I, & Thayer JF (2019). Sex moderates the relationship between resting heart rate variability and self-reported difficulties in emotion regulation. Emotion, 19(6), 992. [DOI] [PubMed] [Google Scholar]
- Witvliet CVO, DeYoung NJ, Hofelich AJ, & DeYoung PA (2011). Compassionate reappraisal and emotion suppression as alternatives to offense-focused rumination: Implications for forgiveness and psychophysiological well-being. Journal of Positive Psychology, 286–299. doi: 10.1080/17439760.2011.577091 [DOI] [Google Scholar]
- Witvliet CVO, Knoll RW, Hinman NG, & DeYoung PA (2010). Compassion-focused reappraisal, benefit-focused reappraisal, and rumination after an interpersonal offense: Emotion-regulation implications for subjective emotion, linguistic responses, and physiology. The Journal of Positive Psychology, 5(3), 226–242. doi: 10.1080/17439761003790997 [DOI] [Google Scholar]
- Witvliet CVO, Ludwig TE, & Vander Laan KL (2001). Granting Forgiveness or Harboring Grudges: Implications for Emotion, Physiology, and Health:. Psychological Science, 12(2), 117–123. doi:10.1111_1467-9280.00320 [DOI] [PubMed] [Google Scholar]
- Woody ML, Burkhouse KL, Birk SB, & Gibb BE (2015). Brooding rumination and cardiovascular reactivity to a laboratory- based interpersonal stressor. Psychophysiology, 52(5), 722–725. doi: 10.1111/psyp.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ML, McGeary JE, & Gibb BE (2014). Brooding rumination and heart rate variability in women at high and low risk for depression: Group differences and moderation by. Journal of Abnormal Psychology, 123(1), 61–67. doi: 10.1037/a0035450 [DOI] [PMC free article] [PubMed] [Google Scholar]


