Abstract
Background
The use of high-flow nasal cannula (HFNC) and noninvasive ventilation (NIV) in patients with COVID-19 is debated.
Methods
This study was performed in four hospitals of China from January to March 2020. We retrospectively enrolled 23 and 13 COVID-19 patients who used HFNC and NIV as first-line therapy, respectively.
Results
Among the 23 patients who used HFNC as first-line therapy, 10 experienced HFNC failure and used NIV as rescue therapy. Among the 13 patients who used NIV as first-line therapy, one (8%) used HFNC as rescue therapy due to NIV intolerance. The duration of HFNC + NIV (median 7.1, IQR: 3.5–12.2 vs. 7.3, IQR: 5.3–10.0 days), intubation rate (17% vs. 15%) and mortality (4% vs. 8%) did not differ between patients who used HFNC and NIV as first-line therapy. In total cohorts, 6 (17%) patients received intubation. Time from initiation of HFNC or NIV to intubation was 8.4 days (IQR: 4.4–18.5). And the time from initiation of HFNC or NIV to termination in patients without intubation was 7.1 days (IQR: 3.9–10.3). Among all the patients, C-reactive protein was independently associated with intubation (OR = 1.04, 95% CI: 1.01–1.07). In addition, no medical staff got nosocomial infection who participated in HFNC and NIV management.
Conclusions
In critically ill patients with COVID-19 who used HFNC and NIV as first-line therapy, the duration of HFNC + NIV, intubation rate and mortality did not differ between two groups. And no medical staff got nosocomial infection during this study.
Keywords: COVID-19, High-flow nasal cannula, Noninvasive ventilation, Intubation
1. Introduction
At the end of 2019, a new coronavirus, now named severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2), was first isolated at Wuhan, China [1]. The coronavirus causes a cluster of acute respiratory illness, now named coronavirus disease 2019 (COVID-19) [2]. It has been demonstrated that the most dangerous feature of COVID-19 was person-to-person transmission [3]. With the spread of the epidemic, many countries have reported confirmed cases associated with SARS-Cov-2. On March 11, 2020, the World Health Organization (WHO) declared the outbreak of COVID-19 was a global pandemic.
In the COVID-19 population, 14% of the patients were categorized as severe cases and 5% as critical cases [4]. A systematic review and meta-analysis has pooled 31 articles involving 46,959 cases with COVID-19 and reported that the incidence of ICU admission was 29.3% [5]. At the early stage, the high-flow nasal cannula (HFNC) or noninvasive ventilation (NIV) was used in 24% of hospitalized patients [2]. And in the critical ill patients, the use of HFNC and NIV was 31% and 37%, respectively [6]. In Lombardy Region, Italy, the NIV was used in 11% of ICU patients [7]. In Seattle Region, USA, the HFNC was used in 42% of critically ill patients [8]. However, these studies failed to report how the HFNC or NIV was used. Here, we aimed to report the clinical features, settings and outcomes of HFNC and NIV in patients with COVID-19.
2. Methods
This was a retrospective observational study performed in four hospitals of China from January to March 2020 (the public health clinical center of Chengdu, the Central Hospital of Dazhou, Yongchuan Hospital of Chongqing Medical University and Chongqing Public Health Medical Center). In the suspected patients, the real-time reverse transcription polymerase chain reaction (RT-PCR) assay was performed according to the guideline made by our National Health Commission [9]. The COVID-19 was confirmed by a positive RT-PCR. All the patients with a confirmed COVID-19 were candidates to our study. We enrolled all the patients who used HFNC or NIV as first-line therapy. Among the HFNC patients, 17 cases extracted from a previous study were secondarily analyzed [10]. This study was approved by the local ethics committee and institutional review board (the First Affiliated Hospital of Chongqing Medical University, No. 20200201). Given its observational nature, informed consent was waived.
The application of NIV and HFNC was in the negative pressure ward or intensive care unit (ICU). To protect the medical staff, the N95 respirator, eye protection (mask with a visor), disposable gown, disposable surgical gloves, and disposable shoe covers were provided. Before entering the ward, all the medical staffs had wore these devices and checked each other.
NIV was managed according to current guidelines and the experts' suggestions [[11], [12], [13], [14]]. Face mask was the first choice to delivery the NIV to the patients. Optimal size of the mask was selected based on the face type in each patient. Hearted humidification was provided to improve oral or nasal dryness. The initial continuous positive airway pressure (CPAP) or positive end expiratory pressure (PEEP) was 4 cm H2O. When the patient tolerated this pressure, it was gradually increased to improve the oxygenation. The initial inspiratory pressure was 8–10 cm H2O. When the patient tolerated the initial pressure, it was gradually increased to relieve dyspnea. The fraction of inspired oxygen (FiO2) was titrated to main the peripheral oxygen saturation (SpO2) more than 93%. When the NIV intolerance occurred, HFNC could be used as a rescue therapy if the patient did not require urgent intubation.
HFNC was managed also based on current consensus and the experts' suggestions [[13], [14], [15]]. The temperature was set between 31 and 37 °C, the flow was set between 30 and 60 L/min, and the FiO2 was set to maintain the SpO2 more than 93%. When the HFNC failed to maintain the oxygenation or relieve dyspnea, the NIV as a rescue therapy was an alternative if the patient did not require urgent intubation.
When the respiratory distress was relieved and the oxygenation was improved, the intermittent use of NIV or HFNC was performed. We gradually increased the time of conventional oxygen therapy and shortened the duration of HFNC or NIV until it was totally weaned. When the respiratory distress and oxygenation progressively deteriorated, intubation for invasive mechanical ventilation was performed based on the criteria made by our Society of Critical Care Medicine [16]. However, the intubation was decided at the discretion of the attending physicians.
Before the use of HFNC or NIV, the demographics, vital signs, laboratory tests and the arterial blood gas tests were collected. The baseline PaO2/FiO2 was measured with the use of conventional oxygen therapy before the use of HFNC or NIV. We estimated the FiO2 as follows: FiO2 (%) = 21 + 4*fow (L/min) [17]. Using these data, the acute physiology and chronic health evaluation II (APACHE II) score and sequential organ failure assessment (SOFA) score were calculated.
Data were analyzed by statistical software (SPSS 17.0; IBM Corp., Armonk, NY). Student's t-test was used to analyze the normally distributed continuous variables and Mann–Whitney U test was used to analyze the non-normally distributed continuous variables. Chi-squared test or Fisher's exact test was used to analyze the categorical variables. Variables with a p value <.1 in univariate analyses were entered into logistic regression analyses to identify independent risk factors associated with intubation. The ability to predict intubation was tested by the area under the receiver operating characteristic curve (AUC). The value at maximum Youden index was selected as optimal cutoff value [18]. A p value <.05 was considered significant.
3. Results
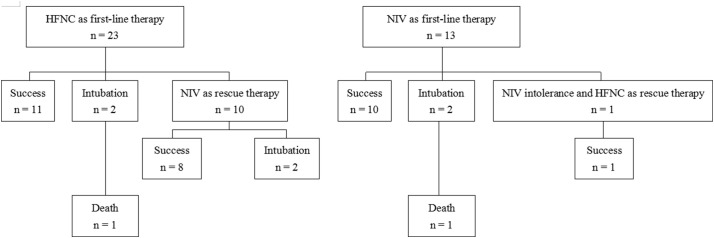
Among all the enrolled patients, 35 cases were in the negative pressure ward and one was in the ICU. Among them, HFNC was used as first-line therapy in 23 patients and NIV in 13 patients (Fig. 1 ). The clinical characteristics of the HFNC and NIV patients were summarized in Table 1 . The mean age was 65 ± 14 years in HFNC group and 50 ± 14 years in NIV group (p <0.01). The proportion of male was 52% in HFNC group and 92% in NIV group (p = .03). There were no differences in disease severity, the proportion of comorbidity, and the level of oxygenation between the two groups. Patients in HFNC group had higher chlorine, low alanine aminotransferase (ALT) and lower total bilirubin than that in NIV group. Others laboratory tests were no differences between the two groups.
Fig. 1.
The flow chart of the enrolled patients.
Table 1.
Clinical characteristics of the enrolled patients at initiation of HFNC and NIV
| Total cohort |
HFNC |
NIV |
p | |
|---|---|---|---|---|
| N = 36 | N = 23 | N = 13 | ||
| Age, years | 60 ± 16 | 65 ± 14 | 50 ± 14 | <0.01⁎ |
| Male (%) | 24 (67%) | 12 (52%) | 12 (92%) | 0.03⁎ |
| APACHE II score | 9 ± 4 | 10 ± 5 | 8 ± 2 | 0.24 |
| SOFA score | 4 ± 1 | 4 ± 2 | 4 ± 1 | 0.85 |
| The level of PaO2/FiO2 | ||||
| >200 mmHg | 12 (33%) | 9 (39%) | 3 (23%) | 0.47 |
| 150–200 mmHg | 14 (39%) | 10 (44%) | 4 (31%) | 0.50 |
| 100–150 mmHg | 10 (28%) | 4 (17%) | 6 (46%) | 0.12 |
| Comorbidity | ||||
| Hypertension | 9 (25%) | 6 (26%) | 3 (23%) | >0.99 |
| Diabetes mellitus | 4 (11%) | 4 (17%) | 0 (0%) | 0.27 |
| Chronic heart disease | 6 (17%) | 6 (27%) | 0 (0%) | 0.07 |
| Chronic respiratory disease | 1 (3%) | 1 (4%) | 0 (0%) | >0.99 |
| Laboratory tests | ||||
| White blood cell counts, ×109/L | 6.3 ± 2.6 | 5.8 ± 2.2 | 7.2 ± 3.0 | 0.12 |
| Neutrophil percentage, % | 78 ± 9 | 77 ± 9 | 81 ± 8 | 0.16 |
| Lymphocyte count, ×109/L | 0.81 ± 0.36 | 0.78 ± 0.36 | 0.84 ± 0.37 | 0.63 |
| Platelet counts, ×109/L | 187 ± 101 | 176 ± 95 | 206 ± 112 | 0.39 |
| Hemoglobin, mg/dL | 123 ± 19 | 120 ± 19 | 128 ± 14 | 0.18 |
| Albumin, g/L | 35 ± 4 | 35 ± 3 | 37 ± 4 | 0.08 |
| Potassium, mmol/L | 3.8 ± 0.5 | 3.8 ± 0.5 | 3.9 ± 0.6 | 0.56 |
| Sodium, mmol/L | 137 ± 5 | 138 ± 5 | 135 ± 4 | 0.14 |
| Chlorine, mmol/L | 102 ± 5 | 103 ± 5 | 100 ± 3 | 0.03⁎ |
| Urea nitrogen, mmol/L | 4.9 ± 3.4 | 5.2 ± 3.9 | 4.3 ± 2.1 | 0.47 |
| Creatinine, μmol/L | 66 (55–80) | 62 (54–71) | 77 (58–83) | 0.16 |
| ALT, U/L | 32 (21–63) | 29 (18–41) | 55 (28–113) | 0.03⁎ |
| AST, U/L | 37 (27–62) | 35 (26–48) | 39 (34–150) | 0.08 |
| Total bilirubin, μmol/L | 14 ± 5 | 12 ± 4 | 17 ± 5 | <0.01⁎ |
| C-reactive protein, mg/L | 56 ± 45 | 52 ± 40 | 62 ± 53 | 0.56 |
| Procalcitonin, ng/mL | 0.07 (0.04–0.10) | 0.07 (0.04–0.09) | 0.08 (0.04–0.19) | 0.76 |
HFNC = high flow nasal cannula, NIV = noninvasive ventilation, APACHE II = acute physiology and chronic health evaluation II, SOFA = sequential organ failure assessment, ALT = alanine aminotransferase, AST = aspartate aminotransferase.
p < .05 for comparison between HFNC and NIV.
Table 2 shows the settings of the HFNC and NIV at the initial 24 h. In HFNC group, the temperature was around 35 °C, flow was around 40 L/min, and FiO2 was around 40%. In NIV group, four (31%) patients used continuous positive airway pressure (CPAP) at 1–2 h of NIV but one transitioned to bi-level positive pressure ventilation at 12 h of NIV. The other 9 patients (69%) used bi-level positive pressure ventilation. At 1–2 h of NIV, the inspiratory pressure was 12 cmH2O (interquartile range [IQR]: 12–15), the positive end expiratory pressure (PEEP) or CPAP was 6 cmH2O (IQR: 6–9), and the FiO2 was 40% (IQR: 40–50%). The similar settings were used at 12 h and 24 h of NIV.
Table 2.
The settings of HFNC and NIV
| 1–2 h | 12 h | 24 h | |
|---|---|---|---|
| HFNC | |||
| Temperature,°C | 35 (34–35) | 35 (34–35) | 34 (34–35) |
| Flow, L/min | 40 (33–40) | 40 (36–40) | 40 (40–40) |
| FiO2, % | 40 (40–46) | 40 (40–41) | 40 (35–42) |
| NIV | |||
| Use of CPAP mode, % | 4/13 (31%) | 3/13 (23%) | 3/12 (25%) |
| Inspiratory pressure (above zero), cmH2O | 12 (12–15) | 12 (12–16) | 12 (12–16) |
| PEEP/CPAP, cmH2O | 6 (6–9) | 6 (6–9) | 6 (6–10) |
| FiO2, % | 40 (40–50) | 40 (40–48) | 40 (40–49) |
HFNC = high flow nasal cannula, NIV = noninvasive ventilation, CPAP = continuous positive airway pressure, PEEP = positive end expiratory pressure.
There were no differences in vital signs and arterial blood gas tests between HFNC and NIV groups except the respiratory rate (Table 3 ). At 1–2 h, 12 h and 24 h, the respiratory rate was lower in HFNC group than that in NIV group. The outcomes between the two groups were summarized in Table 4 . In HFNC group, 10 (43%) patients used NIV as a rescue therapy. In NIV group, one (8%) used HFNC due to NIV intolerance. There was no difference in the duration of HFNC + NIV between the patients who used HFNC and NIV as first-line therapy (Median 7.1 days, IQR: 3.5–12.2 vs. 7.3 days, IQR: 5.3–10.0, p = .67). There was also no difference in intubation rate (17% vs. 15%, p >0.99) and mortality (4% vs. 8%, p >0.99) between the two groups.
Table 3.
Vital signs and arterial blood gas tests at baseline, 1–2 h, 12 h, and 24 h of intervention.
| HFNC |
NIV |
p | |
|---|---|---|---|
| N = 23 | N = 13 | ||
| Baseline | |||
| Respiratory rate, breaths/min | 26 ± 5 | 27 ± 6 | 0.48 |
| Heart rate, beats/min | 89 ± 16 | 89 ± 16 | 0.99 |
| Mean arterial pressure, mmHg | 90 ± 12 | 91 ± 7 | 0.77 |
| pH | 7.44 ± 0.04 | 7.47 ± 0.07 | 0.19 |
| PaCO2, mmHg | 35 ± 5 | 35 ± 5 | 0.74 |
| PaO2/FiO2, mmHg | 196 ± 46 | 165 ± 48 | 0.06 |
| 1–2 h | |||
| Respiratory rate, breaths/min | 22 ± 2 | 27 ± 5 | <0.01⁎ |
| Heart rate, beats/min | 88 ± 18 | 85 ± 16 | 0.57 |
| Mean arterial pressure, mmHg | 88 ± 9 | 90 ± 6 | 0.49 |
| pH | 7.45 ± 0.04 | 7.48 ± 0.05 | 0.07 |
| PaCO2, mmHg | 36 ± 5 | 35 ± 4 | 0.59 |
| PaO2/FiO2, mmHg | 210 ± 84 | 211 ± 78 | 0.97 |
| 12 h | |||
| Respiratory rate, breaths/min | 21 ± 2 | 24 ± 3 | 0.02⁎ |
| Heart rate, beats/min | 78 ± 16 | 81 ± 14 | 0.62 |
| Mean arterial pressure, mmHg | 86 ± 8 | 89 ± 8 | 0.22 |
| pH | 7.45 ± 0.05 | 7.47 ± 0.04 | 0.18 |
| PaCO2, mmHg | 37 ± 5 | 34 ± 4 | 0.19 |
| PaO2/FiO2, mmHg | 212 ± 60 | 203 ± 61 | 0.70 |
| 24 h | |||
| Respiratory rate, breaths/min | 22 ± 2 | 25 ± 3 | <0.01⁎ |
| Heart rate, beats/min | 79 ± 12 | 82 ± 16 | 0.56 |
| Mean arterial pressure, mmHg | 89 ± 9 | 90 ± 11 | 0.83 |
| pH | 7.45 ± 0.04 | 7.46 ± 0.05 | 0.72 |
| PaCO2, mmHg | 36 ± 5 | 36 ± 5 | 0.91 |
| PaO2/FiO2, mmHg | 224 ± 92 | 202 ± 65 | 0.48 |
HFNC = high flow nasal cannula, NIV = noninvasive ventilation.
p < .05 for comparison between HFNC and NIV.
Table 4.
Outcomes
| HFNC |
NIV |
p | |
|---|---|---|---|
| N = 23 | N = 13 | ||
| Duration of HFNC, days | 3.6 (1.6–8.4) | 7.0# | − |
| Duration of NIV, days | 5.1 (2.3–7.5) | 6.8 (4.5–10.0) | 0.26 |
| Duration of HFNC + NIV, days | 7.1 (3.5–12.2) | 7.3 (5.3–10.0) | 0.67 |
| HFNC as rescue therapy, % | − | 1 (8%) | − |
| NIV as rescue therapy, % | 10 (43%) | − | − |
| Intubation, % | 4 (17%) | 2 (15%) | >0.99 |
| Mortality, % | 1 (4%) | 1 (8%) | >0.99 |
HFNC = high flow nasal cannula, NIV = noninvasive ventilation.
#only one patient used HFNC due to NIV intolerance.
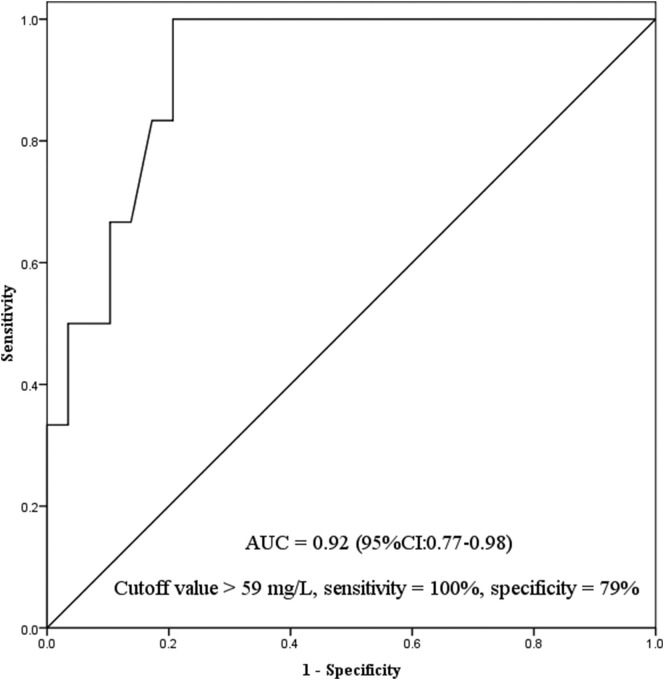
Among all the patients, 6 received intubation and 30 avoided intubation (Table 5 ). In multivariate analyses, only C-reactive protein was independently associated with intubation (Table 6 ). The AUC for prediction of intubation was 0.92 (95% confidence interval [CI]: 0.77–0.98) (Fig. 2 ). Using C-reactive protein of 59 mg/L as cutoff value to predict intubation, the sensitivity was 100% and specificity was 79%. In addition, no medical staff got nosocomial infection in our study.
Table 5.
Comparisons between patients with and without intubation
| Intubation |
Without intubation |
p | |
|---|---|---|---|
| N = 6 | N = 30 | ||
| Age, years | 72 ± 14 | 57 ± 15 | 0.03⁎ |
| Male (%) | 3 (50%) | 21 (70%) | 0.38 |
| APACHE II score | 13 ± 7 | 8 ± 3 | <0.01⁎ |
| SOFA score | 5 ± 2 | 3 ± 1 | <0.01⁎ |
| From HFNC or NIV initiation to termination, days | 8.4 (4.4–18.5) | 7.1 (3.9–10.3) | 0.52 |
| Laboratory tests | |||
| White blood cell counts, ×109/L | 4.7 ± 2.0 | 6.6 ± 2.6 | 0.08 |
| Neutrophil percentage, % | 71 ± 9 | 80 ± 8 | 0.02⁎ |
| Lymphocyte count, ×109/L | 0.95 ± 0.42 | 0.78 ± 0.35 | 0.30 |
| Platelet counts, ×109/L | 106 (66–129) | 159 (125–275) | <0.01⁎ |
| Hemoglobin, mg/dL | 120 ± 13 | 124 ± 19 | 0.62 |
| Albumin, g/L | 33 ± 3 | 36 ± 4 | 0.11 |
| Potassium, mmol/L | 3.6 ± 0.7 | 3.9 ± 0.5 | 0.22 |
| Sodium, mmol/L | 139 ± 7 | 136 ± 4 | 0.15 |
| Chlorine, mmol/L | 103 ± 5 | 102 ± 5 | 0.68 |
| Creatinine, μmol/L | 80 (52–116) | 65 (56–77) | 0.33 |
| ALT, U/L | 35 (16–58) | 32 (22–65) | 0.63 |
| AST, U/L | 62 (44–81) | 35 (26–49) | 0.03⁎ |
| Total bilirubin, μmol/L | 10 (7–15) | 13 (11–18) | 0.11 |
| C-reactive protein, mg/L | 113 (72–162) | 35 (22–57) | <0.01⁎ |
| Procalcitonin, ng/mL | 0.07 (0.04–0.11) | 0.07 (0.04–0.12) | >0.99 |
| Vital signs collected at baseline | |||
| Respiratory rate, breaths/min | 30 ± 9 | 25 ± 4 | 0.06 |
| Heart rate, beats/min | 93 ± 17 | 88 ± 16 | 0.54 |
| Mean arterial pressure, mmHg | 83 ± 10 | 92 ± 10 | 0.04⁎ |
| pH | 7.46 ± 0.07 | 7.45 ± 0.06 | 0.87 |
| PaCO2, mmHg | 36 ± 5 | 35 ± 5 | 0.48 |
| PaO2/FiO2, mmHg | 180 ± 47 | 186 ± 49 | 0.81 |
| Vital signs collected at1–2 h of HFNC or NIV | |||
| Respiratory rate, breaths/min | 24 ± 6 | 24 ± 4 | 0.90 |
| Heart rate, beats/min | 91 ± 16 | 86 ± 18 | 0.52 |
| Mean arterial pressure, mmHg | 86 ± 8 | 89 ± 8 | 0.37 |
| pH | 7.45 ± 0.06 | 7.46 ± 0.05 | 0.71 |
| PaCO2, mmHg | 37 ± 7 | 35 ± 4 | 0.26 |
| PaO2/FiO2, mmHg | 179 ± 55 | 217 ± 86 | 0.30 |
HFNC = high flow nasal cannula, NIV = noninvasive ventilation, APACHE II = acute physiology and chronic health evaluation II; SOFA = sequential organ failure assessment, ALT = alanine aminotransferase, AST = aspartate aminotransferase,
p < .05 for comparison between patients with and without intubation.
Table 6.
Univariate and multivariate analysis for intubation
| Univariate analysis |
p | Multivariate analysis |
p | |
|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | |||
| Age, years | 1.08 (1.00–1.16) | 0.05 | − | − |
| APACHE II score | 1.28 (1.03–1.59) | 0.02 | − | − |
| SOFA score | 2.23 (1.10–4.52) | 0.03 | − | − |
| Neutrophil percentage, % | 0.87 (0.76–1.00) | 0.04 | − | − |
| Platelet counts | 0.96 (0.92–1.00) | 0.04 | − | − |
| C-reactive protein, mg/L | 1.04 (1.01–1.07) | <0.01 | 1.04 (1.01–1.07) | <0.01 |
| Respiratory rate at baseline, breaths/min | 1.16 (0.98–1.36) | 0.08 | − | − |
| Mean arterial pressure at baseline, mmHg | 0.89 (0.79–1.00) | 0.05 | − | − |
OR = odds ratio, CI = confidence internal, APACHE II = acute physiology and chronic health evaluation II, SOFA = sequential organ failure assessment.
Fig. 2.
Prediction of intubation tested by C-reactive protein.
4. Discussion
Nearly half of the patients who used HFNC as first-line therapy were transitioned to NIV as a rescue therapy. However, only fewer patients who used NIV as a first-line therapy were transitioned to HFNC. The intubation rate and mortality were relatively low. The duration of HFNC + NIV, intubation rate and mortality did not differ between the two groups. Among all the patients, C-reactive protein was independently associated with intubation. And it had high distinguishing power to predict intubation.
In China, use of HFNC ranged from 21% to 31% (pooled incidence: 26%) among the critically ill patients, and the use of NIV ranged from 14% to 37% (pooled incidence: 28%) [6,19,20]. However, the use of HFNC and NIV were largely different between China and other countries. In Lombardy Region, Italy, NIV was used in 11% of ICU patients but no patients used HFNC [7]. In Seattle Region, USA, the HFNC was used in 42% of critically ill patients but none used NIV [8]. Maybe, the availability of the HFNC and NIV, and suggestions or recommendations made by experts or consensus were various between different countries.
In China, the experts have suggested that the HFNC and NIV can be used in COVID-19 patients with PaO2/FiO2 ≥ 150 mmHg, and NIV can be cautiously used in those with PaO2/FiO2 between 100 and 150 mmHg [13,14]. The Asian Critical Care Clinical Trials Group has suggested that the HFNC and NIV only can be used in COVID-19 patients with mild acute respiratory distress syndrome (ARDS) [21]. The Surviving Sepsis Campaign COVID-19 subcommittee (most of the experts came from European and USA) has suggested that the HFNC is superior to NIV in COVID-19 patients with acute hypoxemic respiratory failure, and the NIV can be tried with close monitoring if the HFNC is unavailable [22]. However, most of the recommendations were based on the experts' suggestion. The level of evidence is weak. To the best of our knowledge, there were no studies to focus on the use of HFNC or NIV in COVID-19 patients except our previous one paper [10]. Different with the experts' suggestions, 10 patients (17% in HFNC and 46% in NIV) with PaO2/FiO2 between 100 and 150 mmHg have used HFNC or NIV as a first-line therapy. And among all the patients, the duration of HFNC + NIV, intubation rate and mortality were similar between two groups. These data provide an important reference for clinical physicians to select respiratory support device on patients with COVID-19.
Among all the patients who used NIV as first-line therapy, the intubation rate was 92% in patients with Middle East respiratory syndrome, 30% in patients with severe acute respiratory syndrome, and 59% in patients with influenza pneumonia [[23], [24], [25]]. In hypoxemic patients whose respiratory failure caused by other reasons, the intubation rate was 36% [26]. However, in our study, the intubation rate was only 15% in COVID-19 patients who used NIV as a first-line intervention. Among the patients who used NIV as a rescue therapy, the intubation rate was 20%. And no medical staff in our study was infected by the airborne transmission. Therefore, the NIV is an alternative respiratory support for COVID-19 patients but all safety measures should be taken.
Early identification of the high-risk patients and early application of intubation decreased mortality [27]. On the contrary, delayed intubation significantly lead to mortality increase both in patients with HFNC and NIV [28,29]. In our study, we found that the C-reactive protein collected at the initiation of HFNC or NIV had high distinguishing power to predict intubation. Therefore, HFNC and NIV should be cautiously used in patients with high level of C-reactive protein. Close monitoring should be taken to avoid delayed intubation.
Fear of aerosolized transmission was the major problem during the use of HFNC or NIV in COVID-19 patients. In theory, NIV generates more aerosols than HFNC as NIV usually generates higher pressure than HFNC [30]. In our study, use of HFNC and NIV was in the negative pressure ward or ICU, adequate protective supplies (N95 respirator, eye protector, disposable gown, disposable surgical gloves, disposable shoe covers, etc.) were provided to all the medical staffs, and strict training was applied before contact with the COVID-19 patients. Therefore, no medical staff got nosocomial infection who managed the critically ill patients in our study. Our experience provides a reference on the prevention and control of nosocomial infection when the HFNC or NIV was used in COVID-19 patients.
This study has several limitations. First, this is a retrospective study, the use of the HFNC and NIV was mainly decided based on the experts' suggestions and the physicians' experience. Second, we only enrolled 39 patients in our study. The small sample size may skew the results. Further, studies with larger sample size are required to confirm our results. Third, the median time from initiation of HFNC or NIV to intubation was 8.4 days. As intubation beyond 1–2 days after the initiation of HFNC or NIV is associated with increased mortality [[27], [28], [29]], delayed intubation may occur in our study. Close monitoring is needed to avoid delayed intubation.
5. Conclusions
In critically ill patients with COVID-19, the duration of HFNC + NIV, intubation rate and mortality did not differ between patients who used HFNC and NIV as a first-line therapy. Among all the patients, C-reactive protein was independently associated with intubation. And it had high distinguishing power to predict intubation. In addition, no medical staff got nosocomial infection in our study who managed the HFNC and NIV patients with COVID-19.
Authors' contributions
JD and KW conceived the study, and joined in data collection, data analysis, data interpretation and manuscript preparation, and take responsibility for the content of the manuscript. BXC, XYL, WWS, WZ, JL YSL, YLH and LFP joined in data collection, data analysis, data interpretation and manuscript preparation. All the authors revised the manuscript and approved the final revision.
Declaration of Competing Interest
We declare that we have no competing interests.
Acknowledgments
This study was supported by special project for emergency clinical research on novel coronavirus pneumonia in Chongqing Medical University (No.13).
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y., Liu X., Xiong L., et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. http://www.nhc.gov.cn/wjw/index.shtml
- 10.Wang K., Zhao W., Li J., et al. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10:37. doi: 10.1186/s13613-020-00653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keenan S.P., Sinuff T., Burns K.E., et al. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ. 2011;183:E195–E214. doi: 10.1503/cmaj.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochwerg B., Brochard L., Elliott M.W., et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50 doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 13.Critical care committee of Chinese Association of Chest Physician Conventional respiratory support therapy for Severe Acute Respiratory Infections (SARI): Clinical indications and nosocomial infection prevention and control. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:189–194. doi: 10.3760/cma.j.issn.1001-0939.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Yuan X., Mu J.S., Mo G.X., et al. Respiratory support for severe 2019-nCoV pneumonia suffering from acute respiratory failure: time and strategy. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:177–180. doi: 10.3760/cma.j.issn.1001-0939.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Respiratory & Critical Care Medicine Group of Chinese Thoracic Society Expert consensus of high flow nasal cannula oxygen therapy on clinical application regularity. Zhonghua Jie He He Hu Xi Za Zhi. 2019;42:83–91. doi: 10.3760/cma.j.issn.1001-0939.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Society of Critical Care Medicine of Chinese Medical Association Practical guidelines for mechanical ventilation (2006) Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007;19:65–72. [Google Scholar]
- 17.Branson R.D., Hess D.R., Chatbum R.L. J.B. Lippincott Company; Philadelphia: 1995. Respiratory care equipment; pp. 55–62. [Google Scholar]
- 18.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y., Ling Y., Bai T., et al. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phua J., Weng L., Ling L., et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhazzani W., Moller M.H., Arabi Y.M., et al. Surviving Sepsis campaign: guidelines on the Management of Critically ill Adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alraddadi B.M., Qushmaq I., Al-Hameed F.M., et al. Noninvasive ventilation in critically ill patients with the Middle East respiratory syndrome. Influenza Other Respi Viruses. 2019;13:382–390. doi: 10.1111/irv.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung T.M., Yam L.Y., So L.K., et al. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest. 2004;126:845–850. doi: 10.1378/chest.126.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masclans J.R., Perez M., Almirall J., et al. Early non-invasive ventilation treatment for severe influenza pneumonia. Clin Microbiol Infect. 2013;19:249–256. doi: 10.1111/j.1469-0691.2012.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan J., Chen L., Liang G., et al. Noninvasive ventilation failure in patients with hypoxemic respiratory failure: the role of sepsis and septic shock. Ther Adv Respir Dis. 2019;13 doi: 10.1177/1753466619888124. (1753466619888124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan J., Han X., Bai L., et al. Assessment of heart rate, acidosis, consciousness, oxygenation, and respiratory rate to predict noninvasive ventilation failure in hypoxemic patients. Intensive Care Med. 2017;43:192–199. doi: 10.1007/s00134-016-4601-3. [DOI] [PubMed] [Google Scholar]
- 28.Kang B.J., Koh Y., Lim C.M., et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41:623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 29.Carrillo A., Gonzalez-Diaz G., Ferrer M., et al. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med. 2012;38:458–466. doi: 10.1007/s00134-012-2475-6. [DOI] [PubMed] [Google Scholar]
- 30.Hui D.S., Chow B.K., Lo T., et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53:1802339. doi: 10.1183/13993003.02339-2018. [DOI] [PubMed] [Google Scholar]