Abstract
Background
Demodex blepharitis is a chronic condition commonly associated with recalcitrant dry eye symptoms though many people with Demodex mites are asymptomatic. The primary cause of this condition in humans is two types of Demodex mites: Demodex folliculorum and Demodex brevis. There are varying reports of the prevalence of Demodex blepharitis among adults, and it affects both men and women equally. While Demodex mites are commonly treated with tea tree oil, the effectiveness of tea tree oil for treating Demodex blepharitis is not well documented.
Objectives
To evaluate the effects of tea tree oil on ocular Demodex infestation in people with Demodex blepharitis.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2019, Issue 6); Ovid MEDLINE; Embase.com; PubMed; LILACS; ClinicalTrials.gov; and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We used no date or language restrictions in the electronic search for trials. We last searched the databases on 18 June 2019.
Selection criteria
We included randomized controlled trials (RCTs) that compared treatment with tea tree oil (or its components) versus another treatment or no treatment for people with Demodex blepharitis.
Data collection and analysis
Two review authors independently screened the titles and abstracts and then full text of records to determine their eligibility. The review authors independently extracted data and assessed risk of bias using Covidence. A third review author resolved any conflicts at all stages.
Main results
We included six RCTs (1124 eyes of 562 participants; 17 to 281 participants per study) from the US, Korea, China, Australia, Ireland, and Turkey. The RCTs compared some formulation of tea tree oil to another treatment or no treatment. Included participants were both men and women, ranging from 39 to 55 years of age. All RCTs were assessed at unclear or high risk of bias in one or more domains. We also identified two RCTs that are ongoing or awaiting publications.
Data from three RCTs that reported a short‐term mean change in the number of Demodex mites per eight eyelashes contributed to a meta‐analysis. We are uncertain about the mean reduction for the groups that received the tea tree oil intervention (mean difference [MD] 0.70, 95% confidence interval [CI] 0.24 to 1.16) at four to six weeks as compared to other interventions. Only one RCT reported data for long‐term changes, which found that the group that received intense pulse light as the treatment had complete eradication of Demodex mites at three months. We graded the certainty of the evidence for this outcome as very low.
Three RCTs reported no evidence of a difference for participant reported symptoms measured on the Ocular Surface Disease Index (OSDI) between the tea tree oil group and the group receiving other forms of intervention. Mean differences in these studies ranged from ‐10.54 (95% CI – 24.19, 3.11) to 3.40 (95% CI ‐0.70 7.50). We did not conduct a meta‐analysis for this outcome given substantial statistical heterogeneity and graded the certainty of the evidence as low.
One RCT provided information concerning visual acuity but did not provide sufficient data for between‐group comparisons. The authors noted that mean habitual LogMAR visual acuity for all study participants improved post‐treatment (mean LogMAR 1.16, standard deviation 0.26 at 4 weeks). We graded the certainty of evidence for this outcome as low. No RCTs provided data on mean change in number of cylindrical dandruff or the proportion of participants experiencing conjunctival injection or experiencing meibomian gland dysfunction.
Three RCTs provided information on adverse events. One reported no adverse events. The other two described a total of six participants randomized to treatment with tea tree oil who experienced ocular irritation or discomfort that resolved with re‐educating the patient on application techniques and continuing use of the tea tree oil. We graded the certainty of the evidence for this outcome as very low.
Authors' conclusions
The current review suggests that there is uncertainty related to the effectiveness of 5% to 50% tea tree oil for the short‐term treatment of Demodex blepharitis; however, if used, lower concentrations may be preferable in the eye care arena to avoid induced ocular irritation. Future studies should be better controlled, assess outcomes at long term (e.g. 10 to 12 weeks or beyond), account for patient compliance, and study the effects of different tea tree oil concentrations.
Keywords: Adult; Female; Humans; Male; Middle Aged; Anti-Infective Agents, Local; Anti-Infective Agents, Local/therapeutic use; Blepharitis; Blepharitis/drug therapy; Blepharitis/parasitology; Mite Infestations; Mite Infestations/complications; Mite Infestations/drug therapy; Randomized Controlled Trials as Topic; Tea Tree Oil; Tea Tree Oil/therapeutic use
Plain language summary
Tea tree oil for Demodex blepharitis
What was the aim of this review? To examine the effects of tea tree oil, an essential oil derived from an Australian tree, which can be applied in many different forms (eyelid wipes, eyelid shampoo, oil massages, etc.) on Demodex blepharitis. Demodex blepharitis is an inflammation of the eyelid caused by Demodex mites (frequently referred to as eyelash mites).
Key messages We are uncertain if tea tree oil is better compared to other treatments. Other factors such as dosage, ocular hygiene, and patient compliance likely affect treatment outcomes; however, more and better‐designed studies are needed to confirm these findings.
What did we study in this review? Blepharitis causes symptoms such as eye itching, burning, dryness, irritation, watering, or blurry vision, which lead people to seek medical attention. This study aimed to understand the ability of tea tree oil to improve symptoms or to treat Demodex blepharitis (or both) in comparison to no treatment or other forms of treatment containing no tea tree oil.
What were the main results of this review? This review included six studies with 562 participants (1124 eyes). They were men and women between the ages of 39 and 55 years. The included studies were conducted in the US, Korea, China, Australia, Ireland, and Turkey. Trial designs greatly varied, which limited analyses and the confidence in the results. Most studies included in this report had a high degree of bias. It is uncertain if tea tree oil (concentration ranging from 5% to 50%) is helpful for reducing the number of Demodex mites in people with Demodex blepharitis in short‐term cases. While not fully addressed in the reviewed literature, people should be educated on how to properly apply tea tree oil products because patient compliance and method of application likely affects efficacy. None of the studies in this review reported any side effects directly related to the treatment; however, one study did report irritation around the eyes on using tea tree oil, which was resolved on re‐educating the person on the application technique.
How up‐to‐date is this review? Cochrane Review authors searched for trials that had been published before 18 June 2019.
Summary of findings
Summary of findings 1. No tea tree oil group compared to tea tree oil (intervention) group for Demodex blepharitis.
| No tea tree oil group compared to tea tree oil (intervention) group for Demodex blepharitis | |||||||
|
Patient or population: Demodex blepharitis Settings: Hospital and University clinical centres Intervention: tea tree oil Comparison: no tea tree oil | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Other results | Relative effect (95% CI) | No of participants, no of eyes (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed Risk | Corresponding Risk | ||||||
| Mean change in number of Demodex mites per 8 eyelashes at 4–6 weeks | The mean change in number of Demodex mites per 8 eyelashes in the control group ranged from ‐10.7 to ‐0. 17. |
MD 0.7 higher (0.24 higher to 1.16 higher) | — | — | 215, 430 eyes (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | — |
| Mean change in participant‐reported change in symptoms at 4–6 weeks | — | — | Three trials reported sufficient data to permit calculation of between‐group difference at short term (MD 3.40, 95% CI –0.70 to 7.50 in Koo 2012; MD ‐2.60, 95% CI ‐11.94 to 6.74 in Murphy 2018; and MD –10.54, 95% CI –24.19 to 3.11 in Zhang 2019), comparing no tea tree oil group versus tea tree oil | — | 246, 492 eyes (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | The estimates of effect were inconsistent between these two trials, precluding any meta‐analysis. For Murphy 2018, we only considered data from the arms comparing lid scrubbing with and without tea tree oil. |
| Participants with an improvement in visual acuity at 4–6 weeks | — | — | One multi‐arm study reported data on visual acuity, but it did not provide individual group findings. The authors noted that mean habitual LogMAR visual acuity among all study participants improved post‐treatment (mean LogMAR 1.08, SD 0.26 at baseline; mean LogMAR 1.16, SD 0.26 at 4 weeks) | — | 86, 172 eyes (1 RCT) |
⊕⊕⊝⊝ Lowa,b | — |
| Mean change in (or mean) number of cylindrical dandruff at 4–6 weeks | — | — | — | — | 0 (0 RCTs) |
— | None of the studies addressed this outcome as defined. |
| Proportion of participants with meibomian gland dysfunction at 4–6 weeks | — | — | — | — | 0 (0 RCT) |
— | No studies addressed this outcome as defined. |
| Proportion of participants experiencing conjunctival injection (redness) at 4–6 weeks | — | — | — | — | 0 (0 RCTs) |
— | None of the studies addressed this outcome as defined. |
| Adverse events at 4‐6 weeks | — | — | One RCT reported no adverse events; one reported that five participants randomized to treatment with tea tree oil had ocular irritation that was resolved after patient re‐education on eyelid scrubbing methods; and one RCT reported initial discomfort for one participant randomized to treatment with tea tree oil that was self‐resolved on continuing the use of tea tree oil. | — | 318, (3 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c | Three of the six RCTs provided no information related to adverse events. |
| CI: confidence interval; LogMAR: logarithm of the minimum angle of resolution; MD: mean difference; RCT: randomized controlled trial; SD: standard deviation. | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded one level due to unclear or high risk of bias in at least one contributing study. bDowngraded one level due to inconsistency, substantial statistical and clinical heterogeneity. cDowngraded one level due to imprecision around effect estimates.
Background
Description of the condition
Blepharitis is a chronic inflammation of the eyelids, which may or may not involve the meibomian glands (Wolffsohn 2017). Blepharitis is characterized by eye itching, burning, dryness, irritation, or watering; the person may also experience blurry vision or the sensation of heavy eyelids (Amescua 2019; Cheng 2015; Lemp 2009). Demodex blepharitis (or demodicosis) refers to a common form of blepharitis that involves a Demodex mite infestation (Cheng 2015; Liu 2010). Two primary types of Demodex mites are known to inhabit humans (Cheng 2015).
Demodex folliculorum (0.3 mm to 0.4 mm) is primarily found at the base of the eyelashes and eyelash follicles (anterior blepharitis) (Basta‐Juzbasic 2002; Cheng 2015; Liu 2010). They feed on epithelial cells around the hair follicles, which may also cause trichiasis (inward deviation of eyelashes) or madarosis (eyelash loss) (Gao 2007).
Demodex brevis (0.2 mm to 0.3 mm) tends to inhabit the meibomian glands (posterior blepharitis) (Basta‐Juzbasic 2002; Cheng 2015; Liu 2010). They can block gland orifices, which can prevent meibum expression and induce meibomian gland dysfunction (English 1981).
Not all patients who present with Demodex mites are symptomatic, which suggests that these mites may be considered part of the normal ocular flora (Kemal 2005). Demodex mites have been associated with other skin diseases such as papulopustular rosacea, rosacea‐like eruptions of the face, and eyelid basal cell carcinomas (Erbagci 2003; Forton 2005).
Epidemiology
Blepharitis is one of the most common ocular disorders. Lemp 2009 reported that eye care providers estimate observing blepharitis in up to 47% of their patients. The prevalence of Demodex blepharitis, in particular, varies widely in the literature (29% to 100%), likely due to the lack of standardized procedures for measuring clinically significant infestations (Gao 2005a; Kemal 2005; Roth 1979). Demodex mites, however, are rarely found on the eyelids of children under 16 years old (Coston 1967). Children likely have a lower risk for Demodex infestations due to having a lower level of sebaceous gland secretions, which results in fewer secretions for the mites (Herron 2005). There does not appear to be a sex predilection for Demodex infestation (Biernat 2018).
Diagnosis
There are no specific clinical diagnostic tests for Demodex blepharitis (AAO 2018). Microscopic evaluation of epilated eyelashes may reveal Demodex mites or cylindrical dandruff/collarettes (e.g. debris generated from mites accumulating at the root of eyelashes) (Gao 2005b). Cylindrical dandruff is a pathognomonic sign for Demodex blepharitis (Gao 2005b). The microscopic evaluation involves epilating two or more random eyelashes from each eyelid; placing the eyelash on the slide; adding a drop of oil to the eyelash slide; applying a coverslip; and viewing the eyelashes with a microscope set to 25× magnification (Coston 1967; Gao 2005b). Detection and counting of Demodex eggs, larvae, and adult mites are done to grade the severity of Demodex infestation (Liu 2010). The literature reports a range of scales for grading Demodex blepharitis severity. Coston 1967 suggests that six or more Demodex mites per 16 lashes are clinically significant, while Biernat 2018 suggests that the presence of one Demodex mite, larva, or egg is clinically significant. Others have counted the number of Demodex mites present and reported them as mites per eyelash (Gao 2005b). Gao 2005b has also proposed a modified method for sampling and counting the Demodex mites that entails intentional selection of two lashes with cylindrical dandruff per eyelid, applying them to a coverslip, and counting the mites with a microscope while adding 100% alcohol to the slide to break up cylindrical dandruff. Gao and colleagues also suggest that if no cylindrical dandruff is observed, mites can still be searched for by collecting lashes as described above, adding saline to the slide instead of 100% alcohol because cylindrical dandruff will not need to be degraded.
A slit‐lamp biomicroscope‐based method may be as effective as these epilation‐based methods. When performing the slit‐lamp biomicroscope‐based method, the eyelashes should be evaluated by situating the patient at the slit‐lamp biomicroscope (Murphy 2020). The examiner should then select an eyelash with a collarette (Murphy 2020). If a collarette is not present, a random eyelash should be selected (Murphy 2020). The selected lash should then be rotated four times counter‐clockwise followed by four times clockwise with sterile forceps, and the number of mites associated with that eyelash should be counted (Murphy 2020). Since the slit‐lamp biomicroscope‐based method is comparable to the epilation‐based methods, the slit‐lamp biomicroscope method may be preferable because it inflicts less discomfort on the patient than epilating an eyelash (Murphy 2020). Other work suggests that smart phones may also soon play a role in diagnosing Demodex blepharitis (Kaya 2018).
Treatment options
Treatment of Demodex blepharitis is geared towards mite eradication (Fromstein 2018). Current treatment approaches include eyelid hygiene, 1% sulfur ointment, 1% mercury oxide ointment, pilocarpine gel, iodized solutions, warm compresses, intense pulsed light, ivermectin, and tea tree oil (Coston 1967; Filho 2011; Fromstein 2018; Liu 2010; Zhang 2018). Of all the treatments investigated, tea tree oil is the most promising option for killing Demodex mites (Liu 2010). Tea tree oil therapies may be more effective as they are known to have antibacterial, antifungal, and anti‐inflammatory properties (Liu 2010).
Description of the intervention
Tea tree oil is an essential oil derived via distillation from the leaves and terminal branches of a small paper‐barked tree known as Australian Melaleuca alternifolia (Lam 2018; Swords 1978). Tea tree oil contains over 100 different components (Hammer 2006; Swords 1978), although only 15 of the most common components are included in the 2017 International Organization for Standardization (ISO) tea tree oil standard (ISO‐4730; see Table 2). Tea tree oil is typically applied topically to the eyelid in the form of a scrub via eyelid wipes or foam when attempting to fight ocular Demodex infestations (Cheng 2015). However, it should never be taken orally because it is highly toxic if ingested (Hammer 2006). It has been recommended to use tea tree oil treatments for at least two Demodex mite life cycles (i.e. approximately six weeks) in order to ensure the adequate killing of the parasite (Cheng 2015).
1. Components of International Organization for Standardization Tea Tree Standard.
| Molecule | Minimum concentration | Maximum concentration |
| Terpinen‐4‐ol R isomer ratio S isomer ratio | 35.00 67.00 29.00 | 48.00 71.00 33.00 |
| γ‐Terpinene | 14.00 | 28.00 |
| α‐Terpinene | 6.00 | 12.00 |
| 1,8‐Cineole | < 0.01 | 10.00 |
| p‐Cymene | 0.50 | 8.00 |
| Terpinolene | 1.50 | 5.00 |
| α‐Terpineol | 2.00 | 5.00 |
| α‐Pinene | 1.00 | 4.00 |
| Sabinene | < 0.01 | 3.50 |
| Aromadendrene | 0.20 | 3.00 |
| Ledene | 0.10 | 3.00 |
| δ‐Cadinene | 0.20 | 3.00 |
| Limonene | 0.50 | 1.50 |
| Globulol | < 0.01 | 1.00 |
| Viridiflorol | < 0.01 | 1.00 |
ISO‐4730: 2017; reproduced from Lam 2018.
How the intervention might work
Although tea tree oil likely has multiple modes of action and likely contains multiple molecular species with antimicrobial properties, since it has action against bacteria, fungus, and parasites (Cheng 2015; Li 2017; Schelz 2006), terpinen‐4‐ol is the most active tea tree oil molecular compound against Demodex mites (Tighe 2013). In fact, data from Tighe 2013 suggest that terpinen‐4‐ol is the only component in the 2004 ISO tea tree oil standard that can effectively kill Demodex mites at a 1% concentration when diluted in mineral oil. Determining that terpinen‐4‐ol alone has high antiparasitic properties at a 1% concentration is an essential finding because higher concentrations of tea tree oil have been associated with ocular irritation and high concentrations of oxidation products have been associated with allergic reactions, which are the primary adverse effects associated with tea tree oil treatment (Hammer 2006). Tighe 2013 also found that α‐terpineol, 1,8‐cineole, sabinene, limonene, terpinolene, and α‐terpinene are effective at killing Demodex mites when used at therapeutic concentrations (all greater than 2.5%), and they found that the action of terpinen‐4‐ol could be enhanced by the inclusion of terpinolene and inhibited by the inclusion of α‐terpineol, suggesting that the components within tea tree oil interact in a complex manner.
While the full mechanism of tea tree oil action against Demodex mites is unknown, tea tree oil causes the Demodex mites to migrate out of the skin, which may make it easier for treatments to take action against them (Liu 2010). Tea tree oil, or more specifically terpinen‐4‐ol and 1,8‐cineole within tea tree oil, may also act against Demodex by competitively inhibiting acetylcholinesterases (Lam 2018). Demodex mites also have the ability to carry bacteria (internally or externally), which may further promote blepharitis and a Demodex infestation‐associated immune reaction (Liu 2010); therefore, the antibacterial effects (e.g. increased membrane permeability) of tea tree oil may also contribute to its therapeutic effects (Lam 2018). Tea tree oil has also been found to have anti‐inflammatory properties, as it has been shown that terpinen‐4‐ol is able to suppress monocyte‐derived pro‐inflammatory proteins (e.g. tumor necrosis factor alfa, interleukin‐8) and oxygen‐derived reactive species production (superoxide) (Brand 2001; Hart 2000), which may further promote resolution of the condition.
Why it is important to do this review
Recalcitrant, multifactorial, dry eye commonly occurs in people with ocular Demodex infestations (Cheng 2015; Post 1963). While there has been a general systematic review on chronic blepharitis (Lindsley 2012), this review is out of date, and there has yet to be a systematic review focusing on the treatment of ocular Demodex infestations with tea tree oil. While the use of tea tree oil for the treatment of ocular Demodex infestations is clinically accepted (Cheng 2015), the evidence supporting this practice is not fully formed. This review is, therefore, important because it summarizes the current understanding of the use of tea tree oil for treating ocular Demodex infestations along with the clinical trials that either support or refute the clinical utility of tea tree oil.
Objectives
To evaluate the effects of tea tree oil on ocular Demodex infestation in people with Demodex blepharitis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐RCTs. We defined quasi‐RCTs as trials that did not use randomization to allocate participants to treatment groups but that attempted to use a non‐biased method of treatment assignments such as birth date, Social Security number, or medical record number of a consecutive sample of eligible patients.
We included trials using a cross‐over design if it was possible to determine that the treatment sequence was randomly or quasi‐randomly assigned.
Types of participants
We included trials that enrolled adults aged 18 years or older diagnosed with ocular Demodex blepharitis.
Types of interventions
We compared the treatment of Demodex blepharitis with any form of tea tree oil (any concentration or formulation) to another treatment (e.g. baby shampoo, eyelid scrubs, antimicrobial, anti‐inflammatory, antiallergic medications, or a combination of these) or no treatment (e.g. no treatment, placebo).
We also included studies comparing different concentrations of tea tree oil to each other.
Types of outcome measures
Primary outcomes
Mean change in number of Demodex mites per eight eyelashes from baseline to four to six weeks, measured by any method.
Mean change in participant‐reported symptoms, including, but not limited to, irritation, burning, tearing, itching, eyelid sticking, photophobia, and increased frequency of blinking, from baseline; measured using participant symptom reports, questionnaires, interviews, or visual analog scale (VAS) at four to six weeks. Although it is ideal for studies to use validated scales, we considered all scales used in included studies since standardized information was unavailable. We planned to conduct sensitivity analyses to examine the impact of any assumptions made in this regard.
Secondary outcomes
Mean change in number of Demodex mites per eight eyelashes from baseline to 10 to 12 weeks, measured by any method.
Mean change in participant‐reported symptoms from baseline to 10 to 12 weeks, measured by any method.
Participants with an improvement in visual acuity (i.e. improvement of 2 or more lines), measured on a visual acuity chart with a LogMAR scale (or equivalent) at four to six weeks and 10 to 12 weeks. When continuous LogMAR data were available, we analyzed the mean change in visual acuity from baseline.
Mean change in number of cylindrical dandruff by eyelid from baseline to four to six weeks and 10 to 12 weeks, as measured by the reduction in the number of collarettes compared to baseline.
Proportion of participants with meibomian gland dysfunction as defined by study investigators (e.g. meibum quality or expressibility, or both) from baseline to four to six weeks and 10 to 12 weeks.
Proportions of participants experiencing conjunctival injection (redness) (as defined by study investigators) from baseline to four to six weeks and 10 to 12 weeks.
Proportion of participants with mites eradicated (percent eradication) at four to six weeks and 10 to 12 weeks.
Patient compliance as defined by investigators.
Adverse events, as reported by study investigators.
For continuous outcomes, when the mean change from baseline was not available, we used mean difference (MD) at a follow‐up time point instead. For simplicity, we referred to the four‐ to six‐week time point as 'short term' and the 10‐ to 12‐week time point as 'long term.'
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and quasi‐RCTs. There were no language or publication year restrictions. The electronic databases were last searched on 18 June 2019.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019; Issue 6) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 18 June 2019) (Appendix 1).
MEDLINE Ovid (1946 to 18 June 2019) (Appendix 2).
Embase.com (1947 to 18 June 2019) (Appendix 3).
PubMed (1948 to 18 June 2019) (Appendix 4).
Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to 18 June 2019) (Appendix 5).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 18 June 2019) (Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 18 June 2019) (Appendix 7).
Searching other resources
We searched the reference lists of included trials for any additional trials not identified by the electronic searches. We contacted experts in the field for information on current, past, or unpublished trials.
Data collection and analysis
Selection of studies
After removing duplicates from the search results, two review authors (ADP, KS) independently screened titles and abstracts of all records identified by the search using a web‐based review management software (Covidence). The review authors classified each record as either relevant (a vote of 'yes') or not relevant (a vote of 'no') for full‐text review. Two review authors retrieved and then independently reviewed the full texts of all studies identified as relevant during the title and abstract screening to determine if the studies met the inclusion criteria (a vote of 'include') or not (a vote of 'exclude'). We did not need to contact trial authors to clarify any details needed to make a complete assessment of eligibility. A third review author (JL) resolved discrepancies that occurred at all stages.
Data extraction and management
Two review authors independently extracted data using a web‐based electronic data collection form. We extracted the information as described in Appendix 8, including: study setting, countries where recruitment took place, sample size, study duration and follow‐up time, study design, analysis choice, sources of funding, and potential conflicts of interests; characteristics of participants (e.g. inclusion/exclusion criteria), underlying disease conditions, and medical history; interventions (e.g. dose and duration of tea tree oil), comparators, outcomes (e.g. domain, specific measurement, specific metric, method of aggregation, and the time frame); and quantitative results.
We compared the extracted data and resolved any discrepancies by discussion. Two review authors (APD and KS) completed data entry into Review Manager 5 (Review Manager 2014), and a third review author (JL) verified the data entered.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in included trials following the guidance described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). Specific items for consideration included: random sequence generation and allocation concealment (selection bias), masking of participants and study personnel (performance bias), masking of outcome assessors who assessed participant‐reported changes in symptoms and number of mites (detection bias), missing data and intention‐to‐treat analysis (attrition bias), and selective outcome reporting (reporting bias).
We assigned each item as having 'low risk,' 'high risk,' or, if the information provided was insufficient to make an assessment, 'unclear risk.' We documented reasons for our assessments and resolved any discrepancies through discussion. We presented the overall assessments in the 'Risk of bias' summary figure and graph (Higgins 2017).
Measures of treatment effect
We treated ordinal outcomes and scales measuring participant‐reported symptoms as continuous data or dichotomous data as appropriate, depending on the length of the scale used and the manner in which the outcomes are reported.
We reported MDs in change from baseline with 95% confidence intervals (CIs) for continuous outcomes (i.e. in number of Demodex mites, participant‐reported change in symptoms, visual acuity, number of collarettes, meibomian gland dysfunction, and conjunctival infections) and risk ratios (RRs) with 95% CIs for any dichotomous outcomes (i.e. proportion of participants reporting change in symptoms and proportion of participants with improvements in visual acuity).
Unit of analysis issues
When the unit of analysis was one study eye per participant, accounting for non‐independence of eyes is not necessary. If any trials compared eyes within individuals (e.g. one eye was randomized to the treatment while the other was randomized to no treatment), then we noted whether or not the study investigators included statistical methods accounting for the correlation between eyes belonging to the same individual. We used the estimates that had accounted for the correlation. We recognized that the unit of analysis is the eyelash for some outcomes and the individual participant for others, and we, therefore, exercised caution when extracting the data and summarized any unit of analysis issues that we encountered. When data from multi‐arm studies were available, we selected the pair of interventions that best represented the comparison of tea tree oil versus no tea tree oil (e.g., excluding comparators that involve devices as adjuvant treatment or more invasive procedures).
Dealing with missing data
We planned to address missing study data for the outcomes of interest or any unclear information by writing to study investigators. We planned to wait two weeks for investigators to reply before considering multiple imputations or other imputation approaches for missing data. In the event that the quality of the available data prevented any meaningful analysis, we omitted the study from the analyses and noted this decision in the Discussion.
Assessment of heterogeneity
We evaluated clinical and methodological heterogeneity by examining participant characteristics, types or dosing of tea tree oil, and outcomes by carefully reviewing the available data and taking into consideration the potential risk of bias. We examined statistical heterogeneity using forest plots and the I² statistic (Deeks 2017). The I² statistic describes the proportion of total variation across trials that is due to heterogeneity rather than chance (Higgins 2017). We considered an I² greater than 70% as the cut‐off point to identify the presence of considerable heterogeneity (Higgins 2017). We considered the consistency of the effect estimates. For example, if all effect estimates were in the same direction, we reported a meta‐analysis even though there might have been considerable statistical heterogeneity. We did not conduct a meta‐analysis if there were considerable clinical, methodological, and statistical heterogeneity issues.
Assessment of reporting biases
We examined selective outcome reporting as part of the 'Risk of bias' assessment by comparing the outcomes reported in the included studies and the outcomes listed in study registration or study protocols (where available). We did not examine funnel plots of intervention effect estimates for evidence of asymmetry.
Data synthesis
We followed Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions for data analysis (Deeks 2017). In the absence of considerable clinical and methodological heterogeneity, we used a random‐effects model to compute a quantitative synthesis. If the number of studies included in the quantitative synthesis was fewer than three with no evidence of substantial statistical heterogeneity, we used a fixed‐effect meta‐analysis. We provided a descriptive, qualitative synthesis of included trials and their results.
Subgroup analysis and investigation of heterogeneity
We planned to consider a subgroup analysis by severity (mild versus moderate, as defined by study investigators) of Demodex infestation. The effects of tea tree oil may vary based on the severity of infestation. If sufficient data were available, we planned to conduct subgroup analyses based on types of comparators, for example, no treatment, placebo, or other non‐tea tree oil treatments (such as baby shampoo, eyelid scrubs, antimicrobial, anti‐inflammatory, antiallergic medications, or a combination of these).
Sensitivity analysis
We planned to conduct two sensitivity analyses to determine the effect of excluding studies at a high risk of bias for incomplete outcome data (i.e. the amount or distribution of missing outcomes differed between treatment groups) (Higgins 2017); and the effect of excluding studies that were quasi‐randomized trials.
Summary of findings and assessment of the certainty of the evidence
We prepared a 'summary of findings' table for the seven outcomes prespecified in our protocol (Savla 2019).
Mean change in number of Demodex mites per eight eyelashes from baseline to four to six weeks (and 10 to 12 weeks if available), measured by any method.
Mean change in participant‐reported symptoms, including but not limited to irritation, burning, tearing, itching, eyelid sticking, photophobia, and increased frequency of blinking, from baseline; measured using participant symptom reports, questionnaires, interviews, or VAS at four to six weeks (and 10 to 12 weeks if available).
Participants with an improvement in visual acuity (i.e. improvement of 2 or more lines), measured on a visual acuity chart with a LogMAR scale (or equivalent) at four to six weeks (and 10 to 12 weeks if available).
Mean change in number of cylindrical dandruff by eyelid from baseline to four to six weeks (and 10 to 12 weeks if available), as measured by the reduction in the number of collarettes compared to baseline.
Proportion of participants with meibomian gland dysfunction as defined by study investigators (e.g. meibum quality or expressibility, or both) from baseline at four to six weeks (and 10 to 12 weeks if available).
Proportions of participants experiencing conjunctival injection (redness) (as defined by study investigators) from baseline at four to six weeks (and 10 to 12 weeks if available).
Adverse events, as reported by study investigators.
We assessed the certainty of the evidence using the GRADE approach with GRADEpro GDT software (GRADEpro GDT).
Results
Description of studies
Results of the search
The electronic search yielded 71 records (Figure 1). After excluding one duplicate, we screened the remaining 70 records and excluded 56 records based on information in the title and abstract. We obtained 14 full‐text records that appeared to be relevant for detailed investigation. We included six reports of six studies (see Characteristics of included studies table) and excluded six reports of five studies (see Characteristics of excluded studies table). We identified two ongoing studies that appear to meet the inclusion criteria of our review. These studies will be assessed when data become available (see Characteristics of ongoing studies table for further details).
1.
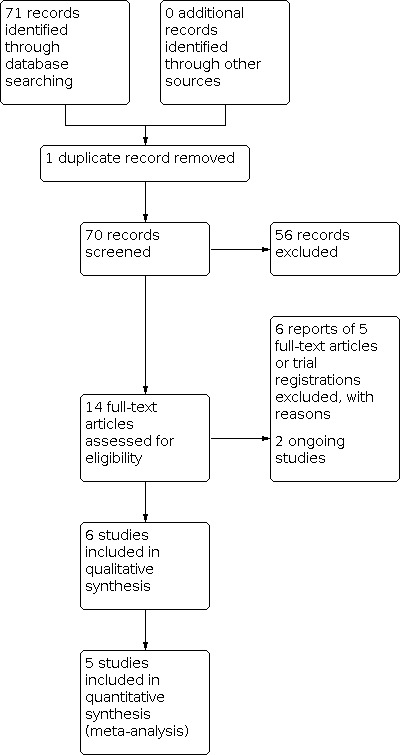
Study flow diagram.
Included studies
We included six RCTs (Karakurt 2018; Koo 2012; Murphy 2018; NCT01647217; Wong 2019; Zhang 2019), among which five RCTs were included in one or more meta‐analyses. Four RCTs had two parallel comparison groups (Karakurt 2018; Koo 2012; NCT01647217; Zhang 2019). Wong 2019 compared left versus right eyes. Murphy 2018 compared three parallel groups, and we did not include this RCT in any meta‐analysis due to concerns with methodological heterogeneity. Specifically, Murphy 2018 included "control" participants who did not have Demodex blepharitis in each of the three arms and one of the comparators involves treatment with a microblepharoexfoliation device. All RCTs were single‐site and took place in either the US, Korea, China, Australia, Ireland, or Turkey. Koo 2012 enrolled participants between 2009 and 2011. Zhang 2019 and Murphy 2018 did not specify the enrolment periods, and the remaining three trials had enrolment time frames ranging between 2014 and 2016. All the trials had a follow‐up duration of at least four to six weeks (short term) with Zhang 2019, having a three months follow‐up period (long term). Five trials reported receiving financial support (Karakurt 2018; Murphy 2018; NCT01647217; Wong 2019; Zhang 2019). Financial support for NCT01647217 came from Tissue Tech Inc.; Karakurt 2018 reported financial support from Erzincan University; Zhang 2019 reported financial support from Program of Shanghai Technology Research Leader; Wong 2019 had non‐financial (treatment devices) support from OptiMed Pty Ltd.; and Murphy 2018 had non‐financial (treatment devices) support from Scope Ophthalmics and Dr. Organic Ltd.
Type of participants
The six RCTs enrolled 562 participants (1124 eyes; range 17 to 281 participants per study). Participants were both men and women with a mean age of 39 to 55 years when reported. No study enrolled participants who were younger than 15 years of age. The most common justification for excluding children was the low prevalence of Demodex mites in younger people (Coston 1967; Herron 2005). There was no significant baseline difference in demographic characteristics between the intervention and control groups for any of the included studies. The inclusion criteria for all included studies was a clinical diagnosis of ocular demodicosis/Demodex blepharitis. Karakurt 2018 and NCT01647217 required participants to have had a previous diagnosis of Demodex blepharitis and regular follow‐ups since being diagnosed with the condition. Most of the other studies excluded participants using other forms of treatments for Demodex mites or having other ocular or systemic diseases.
Type of interventions
The six included RCTs compared some form of tea tree oil intervention and application regimen with another Demodex blepharitis treatment or no treatment. Koo 2012 compared weekly in‐clinic eyelid scrubs (50% tea tree oil) plus daily at‐home eyelid scrubs (10% tea tree oil) as an intervention group with weekly in‐clinic eyelid scrubs (0% tea tree oil) plus daily at‐home eyelid scrubs using saline solution as the control group. NCT01647217 compared pads with terpinen‐4‐ol one or two times per day for six weeks with placebo pads one or two times per day for six weeks. Karakurt 2018 compared tea tree oil containing eyelash shampoo (7.5% tea tree oil; Blefaroshampoo, Teka, Turkey) two times per day for four weeks to tea tree oil‐free eyelash shampoo (0% tea tree oil; Blepharitis Shampoo, Jeomed, Turkey) two times per day for four weeks. Wong 2019 compared Blephadex Eyelid Wipes once daily on either right or left eye for 30 days with no treatment for the contralateral eye for 30 days.
The remaining two RCTs compared tea tree oil to another treatment. Zhang 2019 compared daily 5% tea tree oil lid massage for 15 minutes with in‐clinic intense pulsed light treatment for 90 days delivered at 30, 60, and 90 days. Murphy 2018 compared Dr. Organic Tea Tree Face Wash containing 38% terpinen‐4‐ol daily at night for four weeks, OcuSoft Lid Scrub Plus containing 0.5% 1,2‐octanediol at night for four weeks, and one in‐clinic microblepharoexfoliation treatment at baseline visit using BlephEx.
Type of outcomes
Three RCTs (Koo 2012; NCT01647217; Zhang 2019), including 430 eyes, reported the mean change in the number of Demodex mites per eight eyelashes from baseline in the short term. Murphy 2018 provided information on the "quantity of Demodex folliculorum" but did not report this data in a way that could be meta‐analyzed. The remaining two RCTs (Karakurt 2018; Wong 2019) did not report this primary outcome. Three RCTs (246 participants) reported the mean score in participant‐reported symptoms (Koo 2012; Zhang 2019; Murphy 2018); these were measured using the Ocular Surface Disease Index (OSDI) score. NCT01647217 did not report any data on the mean change in participant‐reported symptoms.
For our secondary outcomes, only Zhang 2019 (80 eyes), reported long‐term data for the mean change in the number of Demodex mite per eight eyelashes and long‐term mean score in the participant‐reported symptoms. Zhang 2019 did not report the proportions of participants with meibomian gland dysfunction or the proportion of participants with conjunctival congestion. However, they did report a mean score on the meibomian gland expressibility, meibum quality, and conjunctival congestion at both short term and long term. Murphy 2018 (172 eyes) reported participant's improvement in visual acuity in LogMAR. One RCT (135 participants) reported a subjective mean score for cylindrical dandruff and eye redness before and after the intervention (Karakurt 2018). NCT01647217 (30 eyes) reported a change in lid margin redness and bulbar conjunctival hyperemia at six weeks (0 to 6 scale). Koo 2012 only reported that Demodex‐infested participants had a conjunctival injection prevalence of 9.9%. Koo 2012, Murphy 2018, and Karakurt 2018 (total of 762 eyes) reported the short‐term percentage of participants with the eradication of Demodex mites; Zhang 2019 (80 eyes) reported the percentage of participants with the eradication in the long term. Koo 2012 and Wong 2019 reported short‐term compliance rates.
Three of the six included RCTs (Koo 2012; NCT01647217; Wong 2019) did not report any serious adverse events. Wong 2019 reported initial participant discomfort in one tea tree oil user that resolved on continued use of tea tree oil. Koo 2012 reported ocular irritation in five tea tree oil users that were resolved after re‐educating the participants on eyelid scrubbing methods. NCT01647217 reported zero adverse events. Karakurt 2018, Murphy 2018, and Zhang 2019 did not provide information related to adverse events. However, Karakurt 2018's conclusion stating that 7.5% tea tree oil eyelash shampoo was effective for Demodex reduction without adverse effects.
Excluded studies
Of the 14 records we selected for full‐text review, we excluded five studies. ChiCTR1800019466, ChiCTR‐OON‐16010205, and Maher 2018 were not RCTs. IRCT2013111313567N5 did not address the patient population described in our eligibility criteria. Ngo 2018 did not study the treatment of Demodex blepharitis but the patient comfort for various treatments on a single day. See the Characteristics of excluded studies table.
Risk of bias in included studies
The risk of bias in the included RCTs is summarized in Figure 2. We contacted the study authors to clarify risk of bias if it was unclear, but received no replies. Therefore, the risk of bias was left as unclear for all studies.
2.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Random sequence generation
Wong 2019 reported using Graphpad software for random sequence generation; we considered this method to have a low risk of bias. Four RCTs (Koo 2012; Murphy 2018; NCT01647217; Zhang 2019) did not report the sequence generation method; we assessed these studies at unclear risk of bias. Karakurt 2018 did not report the method used for random sequence generation; however, the participant population was selected retrospectively, and because of this, this study was assessed at high risk of bias.
Allocation concealment
Wong 2019 used a sealed envelope technique to inform the participants which eye was going to be receiving the intervention. Accordingly, we assessed the risk of bias for allocation concealment as low. Five RCTs did not describe the methods that they used for allocation concealment, so we assessed the five trials at unclear risk of bias.
Blinding
Masking of participants and personnel (performance bias)
Mean change in the number of Demodex mites and the mean change in participant‐reported symptoms are the primary outcomes for this review. Hence, ideally, both the investigator and the participant must be masked. For two of the RCTs (Wong 2019; Zhang 2019), the participants were aware of the intervention they were receiving, and only the investigators were masked. Murphy 2018 had three intervention groups: the participants were aware of the treatment they were receiving in all three groups. Examiners of two groups were masked; however, the examiners of the groups receiving microblepharoexfoliation as an intervention were unmasked as this was an in‐clinic procedure. For Karakurt 2018 the participants were masked but the investigators were not. Accordingly, we assessed Karakurt 2018, Murphy 2018, Wong 2019, and Zhang 2019 to all have a high risk of bias. During Koo 2012, the investigator was masked, but it is unclear if the participants were masked. Hence, we assessed Koo 2012 at unclear risk of bias. We assessed NCT01647217 at unclear risk of bias as it reported quadruple masking (participant, care provider, investigator, and outcomes assessor); however, there was no information on how masking was achieved.
Masking of outcome assessment (detection bias)
In one trial (Koo 2012), a single masked examiner conducted outcome assessments, which was deemed to have a low risk of bias. NCT01647217 reported that the outcome assessor was masked, but there was no information on how this was done. Hence, we assessed NCT01647217 to have an unclear risk of bias. Four RCTs were at high risk of bias for masking: Murphy 2018 and Karakurt 2018 did not mask the investigator's ability to ascertaining outcome measurements, and participants in Wong 2019 and Zhang 2019 were aware of their treatment assignments.
Incomplete outcome data
Wong 2019 and Zhang 2019 were at low risk of incomplete outcome data because there were no missing data on the outcomes of our review. NCT01647217 was at unclear risk of bias because there was only the trial registry record. We also evaluated Karakurt 2018 and Murphy 2018 as low risk of bias since data was provided for all participants that were randomized and all outcomes that were measured. Koo 2012 had a loss to follow‐up of participants in both the intervention and control group, and there was no information comparing the data for participants who completed the study and those who did not complete the study, so we judged this study at high risk of bias.
Selective reporting
Two RCTs (Wong 2019; Zhang 2019) were at low risk of bias as all the outcomes specified in the protocol had been reported. We evaluated three RCTs (Karakurt 2018; Koo 2012; Murphy 2018) at unclear risk of bias as there was no published protocol to assess if all outcomes had been reported. NCT01647217 was at unclear risk of bias as the only report was the trial registry record.
Effects of interventions
See: Table 1
Mean change in number of Demodex mites per eight eyelashes
Three RCTs (Koo 2012; NCT01647217; Zhang 2019), a total of 430 eyes, reported mean change in number of Demodex mites per eight eyelashes at short term, among which one reported a statistically significant, larger mean reduction in the group that received tea tree oil (MD 0.68, 95% CI 0.22 to 1.14) (Koo 2012). We conducted a meta‐analysis of the three studies with meta‐analyzable data and found no evidence of a difference in mean change in number of Demodex mites per eight eyelashes between the intervention and comparator groups at short term (MD 0.70, 95% CI 0.24 to 1.16; I² = 0%; Figure 3). The certainty of the evidence was very low, downgraded for risk of bias, inconsistency, and imprecision.
3.

Forest plot of comparison: 1 No tea tree oil group versus tea tree oil (intervention) group, outcome: 1.1 Mean change in (or mean) number of Demodex mites per 8 eyelashes.
At long term, for the secondary outcome, one study reported results for 80 eyes showing no difference in mean change in number of Demodex mites per eight eyelashes between the two treatment groups (MD –2.00, 95% CI –6.79 to 2.79) (Zhang 2019).
Mean change in (or mean) participant‐reported symptoms
Three RCTs (Koo 2012; Murphy 2018; Zhang 2019), a total of 246 participants, reported results for this primary outcome at short term, with results showing no statistically significance difference between the tea tree oil and no tea tree groups (Figure 4). For Murphy 2018, we only considered the data from the participants who were demodex positive within the two arms comparing lid scrubbing with and without tea tree oil. Given concerns with considerable methodological and statistical heterogeneity observed, we did not conduct a meta‐analysis. We graded the certainty of the evidence as low, downgrading for risk of bias and inconsistency.
4.

Forest plot of comparison: 1 No tea tree oil group versus tea tree oil (intervention) group, outcome: 1.2 Mean change in (or mean) participant‐reported symptoms.
For the secondary outcome, one study (40 participants) found no difference in mean change in participant‐reported symptoms in the long term (MD –10.07, 95% CI –28.30 to 8.16) (Zhang 2019).
Participants with an improvement in visual acuity
One RCT (Murphy 2018), which included 172 eyes, reported results for visual acuity with their statistical analysis indicating that visual acuity was worse in study population for all three groups combined (LogMAR; 1.08, standard deviation [SD] 0.26 at baseline, 1.12, SD 0.26 at 2 weeks, and 1.16, SD 0.26 at 4 weeks). Murphy 2018, however, did not report individual group findings. Further, we note that the change in visual acuity from baseline at four weeks for their collective study population, while statistically significant, was not clinically meaningful. We graded the certainty of the evidence as low, downgrading for risk of bias and inconsistency.
Mean change in number of cylindrical dandruff
No studies provided information about mean change in number of clinical dandruff.
One study (Karakurt 2018) provided a patient‐reported cylindrical dandruff ocular "symptom score" for 135 participants. The study found that only the tea tree oil intervention group had a statistically significant improvement in ocular symptoms post‐treatment (1.83 for the tea tree oil group versus 1.77 for the no tea tree oil group; no SDs provided).
Proportion of participants with meibomian gland dysfunction
No studies provided information about the proportion of participants experiencing meibomian gland dysfunction.
One RCT (Zhang 2019), which included 80 eyes, did note that "meibum quality" using an investigator‐derived score "were significantly decreased after treatment" with intense pulsed light involving tea tree oil.
Proportions of participants experiencing conjunctival injection (redness)
No studies provided information about the proportion of participants experiencing conjunctival injection.
However, three studies examined conjunctival injection using redness 'scores' as an ordinal measurement. Karakurt 2018 reported a statistically significant difference in conjunctival injection between groups with the tea tree oil group having lower redness post‐treatment (post‐treatment score: 0.11 in tea tree oil group versus 1.82 in no tea tree oil group; no SDs provided). Two studies observed no difference between groups (NCT01647217: MD –0.80, 95% CI –2.69 to 1.09; Zhang 2019: MD –0.30, 95% CI –0.63 to 0.03 at short term; MD –0.20, 95% CI –0.55 to 0.15 at long term). The certainty of the evidence was very low, downgraded for risk of bias, inconsistency, and imprecision.
Proportion of participants with mites eradicated (percent eradication)
Three RCTs (670 eyes) reported proportion of participants with mites eradicated, two in the short term (Karakurt 2018; Koo 2012), and one in the long term (Zhang 2019). Our meta‐analysis of results at the short‐term time point suggested that participants who received intervention with tea tree oil were more likely to have mites eradicated than those who did not (RR 0.32, 95% CI 0.17 to 0.59; I² = 0%; Figure 5).
5.

Forest plot of comparison: 1 No tea tree oil group versus tea tree oil (intervention) group, outcome: 1.3 Proportion of participants eradicated of mites (post‐hoc addition).
At long term, one study, which included 80 eyes, observed that the participants who received intense pulsed light were more likely to have mites eradicated than those who received tea tree oil intervention (RR 1.32, 95% CI 1.02 to 1.72) (Zhang 2019).
Patient compliance as defined by investigators
Wong 2019 (20 participants) assessed compliance by counting the number of unused wipes the participants returned, finding that only 5% of their participants were more than 10% non‐compliant. Koo 2012 (160 participants) grouped participants who received treatment with tea tree oil categorically (i.e. good, moderate, and poor compliance) depending on the number of times the participants scrubbed their eyelids per week; Koo 2012 found that good and moderate compliance was associated with a statistically significant reduction in the Demodex mite counts and OSDI scores whereas poor compliance was associated with no improvement in these metrics.
Adverse events
Three RCTs (318 participants) reported information for adverse events. NCT01647217 reported no adverse events. Koo 2012 reported that five tea tree oil users had ocular irritation that was resolved after patient re‐education on eyelid scrubbing methods. Wong 2019 reported initial discomfort for one tea tree oil user that resolved on continuing use of tea tree oil. Three RCTs did not provide any information related to adverse events (Karakurt 2018; Murphy 2018; Zhang 2019). The certainty of the evidence was very low, downgraded for risk of bias, inconsistency, and imprecision.
Discussion
Demodex mites are a common cause of Blepharitis (Cheng 2015; Liu 2010), with the condition mostly affecting adults of either sex (Biernat 2018; Herron 2005). Not all people with Demodex mites are symptomatic, which suggests that the mites may be part of the normal flora of the eye (Kemal 2005). The literature suggests that tea tree oil (primary active ingredient terpinen‐4‐ol) is a promising treatment of Demodex blepharitis (Tighe 2013). Tea tree oil or terpinen‐4‐ol are commonly prescribed in many different forms (e.g. scrubs, lid‐wipes, lid‐massages, lid shampoo, etc.) and many different concentrations (5% to 50%). The data from this meta‐analysis suggest that there is uncertainty to whether tea tree oil is an effective means for treating people who have Demodex blepharitis as compared to other treatments. Nevertheless, Koo 2012 suggests that regular treatment for at least one month is beneficial for improving signs and symptoms. While one study found that other treatments such as intense pulsed light treatment are also effective for mitigating the signs and symptoms of Demodex blepharitis (Zhang 2019), the use of tea tree oil‐containing products are economical, convenient, patient‐friendly, and requires little attention from a clinician. Unfortunately, this systematic review was only able to recover six studies, most of which had a high degree of bias. Therefore, this report should be considered inconclusive for using tea tree oil for Demodex blepharitis and preliminary in nature.
Summary of main results
This systematic review identified six RCTs that compared a tea tree oil intervention to control or different form of Demodex blepharitis treatment. This review found uncertainty related to the topical use of tea tree oil for the treatment of Demodex mites. It is also unclear if tea tree oil can mitigate the ocular symptoms associated with the condition. The meta‐analysis only found a short‐term mean change in the number of Demodex mites per eight eyelashes where the use of tea tree oil was favored over other interventions; however, the certainty of this evidence was very low.
We observed statistical heterogeneity suggesting methodological or clinical differences between the studies included in the meta‐analysis. For example, we noted that Koo 2012 recruited a much larger sample size, with 281 participants who were randomized to receive either eyelid scrub with tea tree oil (141 participants) or eyelid scrub without tea tree oil (140 participants), compared to NCT01647217 (17 participants) and Zhang 2019 (40 participants), which had a much smaller sample size. Additionally, Koo 2012 performed weekly in‐clinic eyelid scrubs, which could have also been a major factor that improved participant compliance and treatment efficacy. Zhang 2019 and NCT01647217 only reported end of the month interaction with the participants.
Since there were a limited number of studies uncovered during our search, this systematic review is unable to provide a clear understanding of the method of application or dosage of tea tree oil. While other forms of treatment, such as intense pulsed light treatment, also show some promising results (Zhang 2019), tea tree oil appears to be one of the most commonly prescribed and effective means for treating Demodex blepharitis. This statement is highlighted by Karakurt 2018 and Koo 2012, who found improvements in signs and symptoms in people with Demodex blepharitis who were treated with tea tree oil; however, not all the included studies found tea tree oil to be the most beneficial. For example, Zhang 2019 found that people with Demodex blepharitis who were treated with intense pulsed light were able to achieve eradication for all participants, whereas not all participants using tea tree oil eradicated Demodex mites after three months of treatment. These between‐study differences can likely be attributed to the characteristics of participants, the treatment and control groups used, and the concentrations of tea tree oil administered. These issues, along with the sparsity of high‐quality studies, suggest the need for additional research in this field.
Overall completeness and applicability of evidence
This systematic review synthesized data from six RCTs with very different methodological considerations. Because of this, it is necessary to consider how these studies differed when we interpret the findings. Some of the primary issues are that the methods used to diagnose Demodex blepharitis varied for each trial, the technique applied to quantify mites was unique to each trial, and there was no consistency among the RCTs on the method of tea tree oil application or concentrations.
Very few trials reported data on treatment compliance or adverse outcomes. These issues are especially problematic because the small between‐group difference can have a large impact on the primary outcomes. Koo 2012 and NCT01647217 both had loss to follow‐up. NCT01647217 only lost one participant from both the treatment and control group. Koo 2012 lost 35 and 86 participants from the treatment and control groups, respectively. They reported no explanation for large to follow‐up from the control group. This large amount of missing data from the control group could have significantly affected the outcomes of the study. These data overall suggest that future studies should be performed in a much more rigorous manner, so the collected data can be more easily translated to other studies and clinical practice.
Quality of the evidence
The RCTs included in this review primarily had unclear or high bias. Except for one trial (Wong 2019), all the other trials were at high or unclear risk for the method for random sequence generation or the method for allocation concealment. There was a high and unclear risk of bias in all the RCTs as the participants or personnel were not masked to the intervention, with Koo 2012 being the only trial that mentioned masking of the outcomes assessor. Attrition bias and reporting bias was likewise reported to be high or unclear for all the RCTs except with Wong 2019 and Zhang 2019. Additionally, the meta‐analysis was only possible for the mean change in the number of Demodex mites per eight eyelashes because of the clinical and methodological differences between studies we included in the review. These data overall suggest a low quality of data and the need for better‐designed studies.
Potential biases in the review process
The information specialist designed a comprehensive search of the relevant literature by queuing multiple databases and the applicable clinical trial registries to ensure that all pertinent data was captured. Two investigators independently completed the review process as per the criteria and processes described in the Methods section of this review and the protocol (Savla 2019). None of the authors involved in this review have conflicts of interest related to this topic. Thus, the bias involved in this report is expected to be minimal.
Agreements and disagreements with other studies or reviews
Our search detected 10 reviews related to the treatment of Demodex blepharitis with topical tea tree oil. Two of these reviews were systematic reviews that contained a meta‐analysis (Lindsley 2012; Navel 2019), while one was a systematic review that did not contain a meta‐analysis (Bitton 2019). The Lindsley 2012, which was the inspiration for the current review, was a systematic review of anterior and posterior blepharitis. Lindsley 2012 did not specifically analyze tea tree oil for the treatment of Demodex blepharitis. Navel 2019 performed a meta‐analysis of studies that used tea tree oil for the treatment of Demodex blepharitis, though their review contained RCTs and non‐RCTs. Bitton 2019 performed a systematic review of general eyelid hygiene products, though there was no meta‐analysis performed, both RCTs and non‐RCTs were included, products beyond tea tree oil were studied, and studies included those that were not focused on treating Demodex blepharitis.
The seven other reviews had no in‐depth product comparisons. Czepita 2007 performed a basic literature review of Demodex blepharitis, though this review did not discuss the use of tea tree oil. Cheng 2015; Fromstein 2018; Lam 2018; Liu 2010; and Nicholls 2017 all performed general literature reviews on Demodex blepharitis with some authors including other conditions, which all only included a discussion of using tea tree oil for the treatment of Demodex blepharitis. Jones 2017 provided a comprehensive overall of all dry eye treatment, which included a brief discussion of how tea tree oil is used to treat Demodex blepharitis. Sabeti 2020 performed a literature review describing the treatment of meibomian gland dysfunction. Sabeti 2020 only provided a general overview of the treatment of Demodex blepharitis with tea tree oil.
While the above reviews focused on a variety of topics, all the reviews detected during our search indicated that tea tree oil was an effective means for treating Demodex blepharitis.
Authors' conclusions
Implications for practice.
The current review suggests that there is uncertainty related to the effectiveness of 5% to 50% tea tree oil for the short‐term treatment of Demodex blepharitis, though lower concentrations may be preferable in the eye care arena to avoid induced ocular irritation. The certainty of evidence is very low for reducing number of mites and moderate for participant‐reported symptoms. The prevalence of Demodex blepharitis is high (Koo 2012); therefore, it might be reasonable to consider screening people with recalcitrant dry eye. Recent advances in slit‐lamp‐based mite identification have made it feasible for the everyday practitioners to screen for and manage people with Demodex blepharitis. Although not examined as an outcome in this review, patient compliance has been associated with effectiveness of treatment (Koo 2012). When tea‐tree oil containing products are prescribed, patients should be regularly educated on how to use them properly.
Implications for research.
This review identified areas of research that need to be thoroughly vetted.
Standard methods for accurate diagnosis and classifying the severity of Demodex blepharitis (Murphy 2018), which should include multiple factors (e.g. number of Demodex mites/larvae/eggs per eyelash, presence of clinical dandruff, patient‐reported ocular symptoms).
A complete understanding of the prevalence of Demodex blepharitis among different populations and age groups.
A systematic comparison between the different forms of treatments and a comparison of different tea tree oil concentrations to determine the concentrations that are safe and effective.
Further research into the best form of delivery of the treatment and the appropriate dosage for different treatment options, also, assessing the effect of combination therapy.
A better understanding of how compliance affects treatment outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2022 | Review declared as stable | This review is no longer being updated as the intervention is no longer used. |
History
Protocol first published: Issue 6, 2019 Review first published: Issue 6, 2020
Acknowledgements
Cochrane Eyes and Vision (CEV) created and executed the electronic search strategies. We are grateful to the following peer reviewers for their time and comments on the protocol: Jennifer Harthan (Illinois College of Optometry) and Justin Kwan (Professional Eye Care Center). The authors are also grateful to the following peer reviewers for their time and comments: Rebecca Petris (Dry Eye Zone), and also to the two peer reviewers who wish to remain anonymous.
This review was managed by CEV@US and was signed off for publication by Tianjing Li and Richard Wormald.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Blepharitis] explode all trees #2 blephariti* #3 demodicosis or demodicidosis or demodecosis or "cylindrical dandruff" #4 demodex or "d. folliculorum" or "d. brevis" #5 blepharoconjunctivitis #6 ocular near (rosacea or mites) #7 MeSH descriptor: [Meibomian Glands] explode all trees #8 meibomian near gland* #9 ocular near gland* #10 (eye* or ocular) near inflamm* #11 (eye* or ocular) near infect* #12 eye* near seborrheic #13 eye* near staphylococcal #14 {OR #1‐#13} #15 MeSH descriptor: [Tee Tree Oil] explode all trees #16 "Tea tree" OR TTO OR Melaleuca #17 Cliradex #18 ISO4730 OR "ISO 4730" OR "4‐Terpineol" OR "terpinene‐4‐ol" or terpin* OR "T4O" #19 {OR #15‐#18} #20 #14 AND #19
Appendix 2. MEDLINE Ovid search strategy
1. exp BLEPHARITIS/ 2. blephariti*.tw. 3. (demodicosis or demodicidosis or demodecosis or "cylindrical dandruff").tw. 4. (demodex or "d. folliculorum" or "d. brevis").tw. 5. blepharoconjunctivitis.tw. 6. (ocular adj3 (rosacea or mites)).tw. 7. exp Meibomian Glands/ 8. (meibomian adj3 gland*).tw. 9. (ocular adj3 gland*).tw. 10. ((eye* or ocular) adj3 inflamm*).tw. 11. ((eye* or ocular) adj3 infect*).tw. 12. (eye* adj3 seborrheic).tw. 13. (eye* adj3 staphylococcal).tw. 14. or/1‐13 15. exp "Tea Tree Oil"/ 16. ("Tea tree" or TTO or Melaleuca).tw. 17. Cliradex.tw. 18. (ISO4730 or "ISO 4730" or terpin* or "T4O").tw. 19. or/15‐18 20. 14 and 19
Appendix 3. Embase.com search strategy
#1 'blepharitis'/exp #2 blephariti*:ti,ab,kw #3 demodicosis:ti,ab,kw OR demodicidosis:ti,ab,kw OR demodecosis:ti,ab,kw OR 'cylindrical dandruff':ti,ab,kw #4 demodex:ti,ab,kw OR 'd. folliculorum':ti,ab,kw OR 'd. brevis':ti,ab,kw #5 blepharoconjunctivitis.:ti,ab,kw #6 (ocular NEAR/3 (rosacea OR mites)):ab,ti,kw #7 'meibomian gland'/exp #8 (meibomian NEAR/3 gland*):ab,ti,kw #9 (ocular NEAR/3 gland*):ti,ab,kw #10 ((eye* OR ocular) NEAR/3 inflamm*):ti,ab,kw #11 ((eye* OR ocular) NEAR/3 infect*):ti,ab,kw #12 (eye* NEAR/3 seborrheic):ti,ab,kw #13 (eye* NEAR/3 staphylococcal):ti,ab,kw #14 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 #15 'tea tree oil'/exp #16 'tea tree':ti,ab,kw,tn OR tto:ti,ab,kw,tn OR melaleuca:ti,ab,kw,tn #17 cliradex:ti,ab,kw,tn #18 iso4730:ti,ab,kw,tn OR 'iso 4730':ti,ab,kw,tn OR terpin*:ti,ab,kw,tn OR 't4o':ti,ab,kw,tn #19 #15 OR #16 OR #17 OR #18 #20 #14 AND #19
Appendix 4. PubMed search strategy
#1 blephariti*[tw] #2 (demodicosis[tw] OR demodicidosis[tw] OR demodecosis[tw] OR "cylindrical dandruff"[tw]) #3 (demodex[tw] OR "d. folliculorum"[tw] OR "d. brevis"[tw]) #4 Blepharoconjunctivitis[tw] #5 (ocular[tw] AND (rosacea[tw] OR mites[tw])) #6 (Meibomian[tw] AND gland*[tw]) #7 (ocular[tw] AND gland*[tw]) #8 ((eye*[tw] OR ocular[tw]) AND inflamm*[tw]) #9 ((eye*[tw] OR ocular[tw]) AND infect*[tw]) #10 (eye*[tw] AND seborrheic[tw]) #11 (eye*[tw] AND staphylococcal[tw]) #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 ("Tea tree"[tw] OR TTO[tw] OR Melaleuca[tw]) #14 Cliradex[tw] #15 (ISO4730[tw] OR "ISO 4730"[tw] OR terpin*[tw] OR "T4O"[tw]) #16 #13 OR #14 OR #15 #17 #12 AND #16 #18 Medline[sb] #19 #17 NOT #18
Appendix 5. LILACS search strategy
(MH:C11.338.133$ OR blephariti$ OR blefariti$ OR blefarit$ OR demodicosis OR demodicidosis OR demodecosis OR "cylindrical dandruff" OR demodex OR "d. folliculorum" OR "d. brevis" OR blepharoconjunctivitis OR (ocular rosacea) OR (ocular mites) OR (meibomian gland$) OR (Glándulas Tarsale$) OR (Glândulas Tarsai$) OR MH:A09.371.337.614$ OR MH:A10.336.827.600$ OR (ocular gland$) OR (eye$ inflamm$) OR (ocular inflamm$) OR (eye$ infect$) OR (ocular infect$) OR (eye$ seborrheic) OR (eye$ staphylococcal)) AND (MH:D10.627.675.775$ OR MH:D10.627.700.940$ OR MH:D20.215.784.750.940$ OR "tea tree" OR TTO OR Melaleuca OR "Árbol de Té" OR Cliradex OR ISO4730 OR "ISO 4730" OR terpin$ OR "T4O")
Appendix 6. ClinicalTrials.gov search strategy
(blepharitis OR demodicosis OR demodicidosis OR demodecosis OR "cylindrical dandruff" OR demodex OR "d. folliculorum" OR "d. brevis" OR blepharoconjunctivitis OR meibomian gland OR ocular gland OR ocular rosacea OR ocular mites OR eye inflammation OR eye infection OR ocular inflammation OR ocular infection) AND ("Tea tree" OR TTO OR Melaleuca OR Cliradex OR ISO4730 OR "ISO 4730" OR terpinen OR terpinene OR terpinolene OR "T4O")
Appendix 7. WHO ICTRP search strategy
Blepharitis AND tea tree OR demodex AND tea tree OR blepharoconjunctivitis AND tea tree OR meibomian gland AND tea tree OR eye infection AND tea tree OR Blepharitis AND MELALEUCA OR demodex AND MELALEUCA OR blepharoconjunctivitis AND MELALEUCA OR meibomian gland AND MELALEUCA OR eye infection AND MELALEUCA OR Blepharitis AND Cliradex OR demodex AND Cliradex OR blepharoconjunctivitis AND Cliradex OR meibomian gland AND Cliradex OR eye infection AND Cliradex OR Blepharitis AND terpinen OR demodex AND terpinen OR blepharoconjunctivitis AND terpinen OR meibomian gland AND terpinen OR eye infection AND terpinen
Appendix 8. Data on study characteristics
| Mandatory items | Optional items | |
| Methods | ||
| Study design |
|
Exclusions after randomization Losses to follow‐up Number randomized/analyzed How were missing data handled? e.g. available case analysis, imputation methods Reported power calculation (Y/N), if yes, sample size and power Unusual study design/issues |
| Eyes or Unit of randomization/ unit of analysis |
|
|
| Participants | ||
| Country | Setting Ethnic group Equivalence of baseline characteristics (Y/N) |
|
| Total number of participants | This information should be collected for total study population recruited into the study. If these data are reported for the people who were followed up only, please indicate. | |
| Number (%) of men and women | ||
| Average age and age range | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Interventions | ||
| Intervention (n = ) Comparator (n = ) See MECIR 65 and 70 |
|
|
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports See MECIR R70 |
List outcomes Adverse events reported (Y/N) Length of follow‐up and intervals at which outcomes assessed |
Planned/actual length of follow‐up |
Data and analyses
Comparison 1. No tea tree oil group versus tea tree oil (intervention) group.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mean change in (or mean) number of Demodex mites per 8 eyelash | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 4–6 weeks (mean change) | 3 | 215 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.24, 1.16] |
| 1.1.2 10–12 weeks (mean change) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐6.79, 2.79] |
| 1.2 Mean change in (or mean) participant‐reported change in symptoms | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 4–6 weeks (mean) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.2 10–12 weeks (mean) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Proportion of participants eradicated of mites (post‐hoc addition) | 3 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 4–6 weeks | 2 | 295 | Risk Ratio (IV, Fixed, 95% CI) | 0.32 [0.17, 0.59] |
| 1.3.2 10–12 weeks | 1 | 40 | Risk Ratio (IV, Fixed, 95% CI) | 1.32 [1.02, 1.72] |
| 1.4 Patient compliance as defined by investigators (post‐hoc addition) | 2 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4.1 Baseline | 2 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1: No tea tree oil group versus tea tree oil (intervention) group, Outcome 1: Mean change in (or mean) number of Demodex mites per 8 eyelash
1.2. Analysis.

Comparison 1: No tea tree oil group versus tea tree oil (intervention) group, Outcome 2: Mean change in (or mean) participant‐reported change in symptoms
1.3. Analysis.

Comparison 1: No tea tree oil group versus tea tree oil (intervention) group, Outcome 3: Proportion of participants eradicated of mites (post‐hoc addition)
1.4. Analysis.

Comparison 1: No tea tree oil group versus tea tree oil (intervention) group, Outcome 4: Patient compliance as defined by investigators (post‐hoc addition)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Karakurt 2018.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Power/Sample size calculation: NR Authors also stated that this was a retrospective study. |
|
| Participants |
Baseline characteristics No TTO group Number of participants: 60 Age – mean: 55.15 (SD 13.97) years Women – n (%): 35 (58.3) Demodex count per eyelash – mean: 10.82 OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR TTO (intervention) group Number of participants: 75 Age – mean: 57.52 (SD 14.22) years Women – n (%): 40 (53.3) Demodex count per eyelash – mean: 10.25/eyelash OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Overall Number of participants: 135 Age – mean: 56.47 (SD 14.11) years Women – n (%): 75 (55.6) Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Inclusion criteria: diagnosed with demodectic blepharitis; history of regular application of eyelash‐based treatments for Demodex; and regular follow‐up in the clinic. Exclusion criteria: any ocular or systemic disease other than demodectic blepharitis; had undergone ocular surgery; treated using systemic or topical treatments and people without treatment follow‐up. Pretreatment: no statistically significant differences in terms of age or sex. Additionally, per the authors, quote: "no statistically significant differences were determined between the groups with regard to pre‐treatment Demodex positivity." |
|
| Interventions |
Intervention characteristics No TTO group
TTO (intervention) group
|
|
| Outcomes | Demodex positivity (number/total); mean number of Demodex mites per eye; total number of Demodex mites per eye, and ocular symptoms (including itching, burning, feeling of a foreign body in the eye, eye redness, and cylindrical dandruff) in all patients before and after the treatment course. | |
| Identification |
Sponsorship source: Erzincan University Country: Turkey Setting: hospital Comments: none Authors name: Erhan Zeytun Institution: Health Services Vocational School, Erzincan University Address: 24100 Erzincan Turkey Clinical trial registry/registration number: NR |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Judgment comment: randomization method not described. They reported that participants were randomly assigned to the groups, though they also stated at the beginning of the methods section that this was a retrospective review. |
| Allocation concealment (selection bias) | Unclear risk | Judgment comment: authors did not describe allocation concealment. They only stated that this was a 'single‐blinded' study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The present study was single‐blinded, and the patient did not know whether their shampoo contained TTO." Judgment comment: the investigator was not masked to the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgment comment: they did not describe if the person counting the mites was masked. The person collecting the patient data was unmasked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgment comment: data provided for all participants randomized and all outcomes in the methods section. |
| Selective reporting (reporting bias) | Unclear risk | Judgment comment: all outcomes in the methods were reported; however, they did not have a prepublished protocol, so it is unclear if they selectively reported data. |
Koo 2012.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Power/Sample size calculation: NR |
|
| Participants |
Baseline characteristics No TTO group Number of participants: 140 Age – mean: 55.6 (SD 11.3) years Women: 63% Demodex count per 8 eyelashes – mean: 4.3 (SD 2.7) OSDI score – mean: 35.3 (SD 11.6) Visual acuity: NR Number of cylindrical dandruff: NR TTO (intervention) group Number of participants: 141 Age – mean: 53.7 (SD 10.3) years Women: 66% Demodex count per 8 eyelashes – mean: 4.0 (SD 2.5) OSDI score – mean: 34.5 (SD 10.7) Visual acuity: NR Number of cylindrical dandruff: NR Overall Number of participants: 281 Age – mean: 55.7 (SD 12.4) years Women: 69.0% Demodex count per 8 eyelashes – mean: 3.4 (SD 3.3) OSDI score – mean: 20.1 (SD 11.9) Visual acuity: NR Number of cylindrical dandruff: NR Inclusion criteria: people with ocular surface discomfort, such as dryness, pruritus, ocular pain, or visual disturbance were enrolled; people with ocular Demodex were randomized. Exclusion criteria: any type of eye surgery in last 6 months; currently using eyedrops other than artificial tears; eyelid scrubbing treatment previously or currently Pretreatment: 104 men and 231 women recruited |
|
| Interventions |
Intervention characteristics No TTO group
TTTO (intervention) group
|
|
| Outcomes | Mean ocular Demodex count; mean OSDI score; and compliance to treatment | |
| Identification |
Sponsorship source: authors indicated no financial support or financial conflict of interest. Country: Korea Setting: Department of Ophthalmology, Chung‐Ang University Hospital Comments: none Authors name: Jae Chan Kim, MD Institution: Chung‐Ang University Hospital Address: 102 Heuksok‐ro, Dongjak‐gu, Seoul 156‐755, Korea Clinical trial registry/registration number: no |
|
| Notes | Participant‐reported change in symptoms was reported in the paper as mean in participant‐reported change in symptoms (OSDI scores) at 1 month. Mean number of Demodex mites at 4–6 weeks was not per eyelash but instead per 8 cilia or eyelashes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgment comment: authors did not discuss the sequence generation method. They reported that they randomly assigned the participants to groups. |
| Allocation concealment (selection bias) | Unclear risk | Judgment comment: authors did not specify how the sequence was concealed. They reported that the participants were randomly assigned to groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Ocular demodecosis and treatment response were confirmed in a blind manner by single examiner with microscopic examination of epilated lashes." Judgment comment: Demodex testing was masked. The authors did not specifically say how the test product and control product were masked, so we do not fully know if the participants were masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Ocular demodecosis and treatment response were confirmed in a blind manner by single examiner with microscopic examination of epilated lashes." Judgment comment: masking for the primary outcome was clearly stated in the paper. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgment comment: both groups had a high loss to follow‐up. There was no comparison of completed and non‐completed participants. No trial number was given, so we could not check to see if they followed a predetermined protocol. |
| Selective reporting (reporting bias) | Unclear risk | Judgment comment: no trial number was given; therefore, we could not check if the authors followed their protocol. The authors did list data for all the tests in the methods section, though they did minimal testing, so they may have left information out of the manuscript. |
Murphy 2018.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group; multi‐arm Power/Sample size calculation: NR |
|
| Participants |
Baseline characteristics Group A: Dr. Organic TTFW Number of participants: 22 Age – mean: 49.6 (SD 17.1) years Women %: NR Demodex count per 4 eyelashes – mean: 4.9 (range: 0–21) OSDI score – mean: 27.4 (SD 16.7) Visual acuity: NR Number of cylindrical dandruff: NR Group B: OcuSoft Lid Scrub Plus Number of participants: 24 Age – mean: 49.6 (SD 16.9) years Women %: NR Demodex count per 4 eyelashes – mean: 3.8 (range: 0–11) OSDI score – mean: 28.6 (SD 23.6) Visual acuity: NR Number of cylindrical dandruff: NR Group C: BlephEx microblepharoexfoliation device Number of participants: 23 Age – mean: 49.86 (SD 19.7) years Women %: NR Demodex count per 4 eyelashes – mean: 6.5 (range: 1–25) OSDI score – mean: 30.1 (SD 19.8) Visual acuity: NR Number of cylindrical dandruff: NR Overall Number of participants: NR Age: NR Women %: NR Demodex count per 4 eyelashes: NR OSDI score: NR Visual acuity: 1.08 (SD 0.26) Number of cylindrical dandruff: NR Inclusion criteria: aged > 18 years Exclusion criteria: ocular surgery in the past 6 months, or undergoing current ophthalmic treatment Pretreatment: individual groups seemed to be balanced; however, their unorthodox control groups were small and younger than the people within their respective groups. |
|
| Interventions |
Intervention characteristics Group A: Dr. Organic TTFW
Group B: OcuSoft Lid Scrub Plus
Group C: BlephEx microblepharoexfoliation device
|
|
| Outcomes | Mean severity of symptoms score (via modified version of the OSDI dry eye questionnaire); habitual visual acuity (LogMAR); slit lamp exam to assess and grade presence of cylindrical dandruff; and assessment for presence of Demodex. | |
| Identification |
Sponsorship source: materials used in study supplied by Scope Ophthalmics and Dr. Organic Ltd. Country: Ireland Setting: Dublin Institute of Technology Comments: none Authors name: Orla Murphy Institution: Dublin Institute of Technology Address: NR Clinical trial registry/registration number: NR |
|
| Notes | Each of the three arms also includes "control" participants who did not have Demodex follicurlorum | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgment comment: participants were asked to choose a number from a list. Each number corresponded to a treatment. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each subject chose a number from the list, which corresponded to the treatment assigned to the subject." Judgment comment: information not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The examiner was blind to the treatment throughout all stages of the study for group A and group B. The examiner performed the BlephEx™ treatment on subjects from group C and was therefore not blind to the treatment in this group." Judgment comment: examiner knew who was in 1 of the groups, and the participants knew which group they were in. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The examiner performed the BlephEx™ treatment on subjects from group C and was therefore not blind to the treatment in this group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgment comment: there is sufficient explanation provided for all analysis. No missing data reported |
| Selective reporting (reporting bias) | Unclear risk | Judgment comment: no prepublished protocol listed, so we could not determine if there was selective reporting. |
NCT01647217.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Power/Sample size calculation: NR |
|
| Participants |
Baseline characteristics Placebo pad group Number of participants: 9 Age – mean: 49 (SD 18.5) years Women – n: 6 Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Terpinen‐4‐ol treatment group Number of participants: 8 Age – mean: 48.6 (SD 21.2) years Women – n: 4 Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Overall Number of participants: 17 Age – mean: 48.8 (SD 19.1) years Women – n: 10 Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Inclusion criteria: people with symptomatic Demodex blepharitis for duration of ≥ 3 months; aged 15–80 years; both genders and all ethnic groups comparable with the local community; able to understand and willing to sign a written informed consent; able and willing to co‐operate with the investigational plan; able and willing to complete all mandatory follow‐up visits. Exclusion criteria: currently engaged in another clinical trial; unwilling or unable to give consent, accept randomization, or return for scheduled visits; aged < under 15 years; pregnant women or expecting to be pregnant during the study; systemic immune deficient conditions such as AIDS or under systemic immunosuppressant; concomitant use of ophthalmic topical medications (excluding non‐preserved tear substitutes); concomitant use of systemic antibiotics or steroids; contact lens wear (unless discontinued for ≥ 30 days before randomization); active ocular infection or allergy; unable to close eyes or uncontrolled blinking; presence of aqueous tear‐deficient dry eye defined by the fluorescein clearance test as < 3 mm wetting in 1 minute; Schirmer test with anesthesia; previous allergic reaction to TTO‐containing products or cosmetic fragrance. Pretreatment: unable to determine from the trial registry. |
|
| Interventions |
Intervention characteristics Placebo pads group
Terpinen‐4‐ol treatment group
|
|
| Outcomes | Primary: reduction in mite count after treatment compared to the baseline data. Secondary: change in lid margin redness and bulbar conjunctival hyperemia. |
|
| Identification |
Sponsorship source: Tissue Tech Inc. Country: US Setting: Biotech Company Comments: none Authors name: Scheffer CG Tseng, MD, PhD Institution: Tissuetech Inc Address: NR Clinical trial registry/registration number:NCT01647217 |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgment comment: information available only as trial registry record. |
| Allocation concealment (selection bias) | Unclear risk | Judgment comment: information available only as trial registry record. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgment comment: information available only as trial registry record. Investigators noted quadruple (participant, care provider, investigator, outcomes assessor) masking but did not provide information on how this was implemented. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgment comment: information available only as trial registry record. Investigators noted quadruple (participant, care provider, investigator, outcomes assessor) masking but did not provide information on how this was implemented. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgment comment: information available only as trial registry record. |
| Selective reporting (reporting bias) | Unclear risk | Judgment comment: information available only as trial registry record. |
Wong 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: investigators randomized the treatment between right and left eyes, so the comparisons was within the same participant. Power/Sample size calculation: “Post‐hoc power calculation based on a sample size of 20 determined that a numerical reduction in Demodex of 3.2 (0.5 ± 0.75 log units) could be detected with a power of 80% and at a 5% level of significance.” The investigators randomized the treatment between right and left eyes, so the comparison was within the same subject. |
|
| Participants |
Baseline characteristics No TTO group Number of participants: 20 eyes (contralateral) Age: NR Women %: NR Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR TTO (intervention) group Number of participants: 20 eyes Age: NR Women %: NR Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Overall Number of participants: 20 Age – median: 63.5 (range: 48–76) years Women – n (%): 14 (70) Demodex count per eyelash– median: 5 (range: NR) OSDI score – median: 9 (range: NR) Visual acuity: NR Number of cylindrical dandruff: NR Inclusion criteria: aged ≥ 45 years; similar vision in each eye determined by having monocular best corrected visual acuity of each eye within 2 lines of each other on a logMAR chart at 6 m distance; to ensure an unbiased perception of ocular comfort, as previous evidence suggests that reduced visual quality increases subjective ocular discomfort symptoms. Exclusion criteria: any active anterior segment disease, with the exception of blepharitis; participants who had started ocular medications or systemic medications that might affect tear film or ocular microbiota (including corticosteroids, antibiotics, immunosuppressants, fish oil, flaxseed oil and other omega‐3 supplements) < 3 months prior to the baseline visit or if change in dosage was anticipated during the study; history of ocular surgery in last 6 months; use of contact lenses within last 6 months; pregnancy or lactation; known allergy to TTO, coconut oil or fluorescein dye; heavy makeup users (defined as regular use of eye makeup including mascara, eyeliner, eye shadow, and eye creams); history of epilepsy or migraines exacerbated by flashing lights. Pretreatment: no between‐group differences because the treatment was randomized by eye. VAS Overall Comfort: NR VAS Dryness: NR OSDI: NR |
|
| Interventions |
Intervention characteristics No TTO group
TTO (intervention) group
|
|
| Outcomes | Median Demodex number; median bacterial colony count; median lipase clearance zone (mm); mean non‐invasive tear breakup time score; mean lipiview; median tear volume; median OSDI score; median itching score (measured using VAS ranging from 0 to 100); overall discomfort score (measured using a VAS ranging from 0 to 100); and mean dryness score (measured using VAS ranging from 0 to 100). | |
| Identification |
Sponsorship source: OptiMed Pty Ltd supplied BlephadexTM Eyelid Wipes at no cost. However, OptiMed Pty Ltd had no other role in the study and none of the investigators received benefits or had any financial interest with OptiMed Pty Ltd. No conditions imposed on use, ownership of results, or material by OptiMed Pty Ltd or any other party. Country: Australia Setting: School of Optometry and Vision Science, UNSW Sydney Comments: study settings not clearly mentioned. Authors name: Katherine Wong Institution: School of Optometry and Vision Science, University of New South Wales Address: Gate 14 Barker St, UNSW, Sydney, NSW 2052, Australia Clinical trial registry/registration number: ACTRN12618001368224 |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Upon completion of the baseline visit, eligible participants were randomly assigned to use the BlephadexTM Eyelid Wipes once daily on either the right or the left eye for 30 days using the web based GraphPad software (https://www.graphpad.com/), to generate the randomisation sequence." |
| Allocation concealment (selection bias) | Low risk | Quote: "A sealed envelope system was employed to notify the participant which eye was allocated to the treatment, and the designated eye was also labelled on the inside of the box of lid wipes by an unmasked investigator." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Whilst investigators were masked to the treatment allocation for the duration of the study, it was impossible to mask participants." Judgment comment: since the participants were not blinded to the intervention the risk of bias was high. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgment comment: methods such as Demodex counts were masked and likely to have low bias from outcome assessors; however, the participants knew which eye was being treated, so monocular symptoms were likely biased by participant knowledge. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgment comment: all 20 participants completed the study. All methods in the method section had results. |
| Selective reporting (reporting bias) | Low risk | Judgment comment: all methods listed on the trial registry (ACTRN12618001368224) were reported. |
Zhang 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Power/Sample size calculation: “Our hypothesis was that there would be a 25% relative difference in [demodex count] between the IPL group and the control group, which meant that a sample size of 20 patients in each group was needed to get a power of 80% for a significance level of 5% with a two‐tailed test.” |
|
| Participants |
Baseline characteristics IPL Number of participants: 20 Age – mean: 39.15 (SD 10.98) years Women – n (%): 8 (40) Demodex count per 8 eyelashes ‐ mean: 13.05 (SD 8.49) OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR 5% TTO treatment control Number of participants: 20 Age – mean: 38.25 (SD 12.34) years Women – n (%): 6 (30) Demodex count per 8 eyelashes ‐ mean: 12.85 (SD 6.49) OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Overall Number of participants: NR Age: NR Women – n (%): 14 (35) Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Inclusion criteria: people with blepharitis experienced ocular demodicosis. Exclusion criteria: acute episodes of ocular surface or facial skin diseases; history of sun exposure or allergic disease within 1 month; any topical or systemic diseases that could affect results (e.g. facial skin cancer, recurrent herpes simplex, graft‐versus‐host disease, systemic lupus erythematosus); eye surgery and medical treatment; any treatment that could affect IPL treatment and results. Pretreatment: none reported |
|
| Interventions |
Intervention characteristics IPL
5% TTO treatment control
|
|
| Outcomes | Ocular demodicosis confirmed by light microscopic exam (mean Demodex counts); lid margin abnormalities (scored as 0–4 based on presence of 4 criteria: irregular lid margins, vascular engorgement, plugging of meibomian gland orifices, and shift of the mucocutaneous junction); conjunctival congestion assessment graded as 0 (no congestion), 1 (congestion confined to the fornix with bright red blood vessels), 2 (obvious congestion that reached the palpebral fissure with crimson and fuzzy blood vessels), or 3 (diffuse congestion); OSDI; slit‐lamp biomicroscopic exam; conjunctival congestion; fluorescein tear film break‐up time; corneal fluorescein staining; modified Schirmer I test with anesthetic; Meibomian gland assessment including MG expressibility; meibum quality. | |
| Identification |
Sponsorship source: Program of Shanghai Technology Research Leader (No 18XD1424500) and Science and Technology Support Project (No 18441902500) of Shanghai Science and Technology Commission. Country: China Setting: EENT Hospital of Fudan University, Shanghai, China Comments: none Authors name: Lan Gong Institution: The Eye & ENT Hospital of Fudan University. Address: Room 405, No.83 Fenyang Road, Shanghai 200031, China Clinical trial registry/registration number:ChiCTR‐OON‐16010205 |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgment comment: authors noted that "Forty patients were randomly divided into two groups" but did not provide information on how this was implemented. |
| Allocation concealment (selection bias) | Unclear risk | Judgment comment: no information provided about allocation concealment; the authors stated that the participants were randomly divided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This is a simple blind, randomized controlled clinical trial and the examiner was masked to the treatment groups." Judgment comment: investigators were masked; however, participants were not masked (not possible with intervention), which may have resulted in information bias. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This is a simple blind, randomized controlled clinical trial and the examiner was masked to the treatment groups." Judgment comment: most outcomes were masked; however, symptoms were reported by the participants, and their responses may have been biased by knowing their groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgment comment: all participants completed the study. All outcomes appeared to be reported. This is also a registered trial: ChiCTR‐OON‐16010205, and all outcomes in the registration are reported. |
| Selective reporting (reporting bias) | Low risk | Judgment comment: all participants completed the study. All outcomes appeared to be reported. This is also a registered trial: ChiCTR‐OON‐16010205, and all outcomes in the registration are reported. |
IPL: intense pulsed light; NA: not applicable; NR: not reported; OSDI: Ocular Surface Disease Index; SD: standard deviation; TTFW: tea tree face wash; TTO: tea tree oil; VAS: visual analog scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ChiCTR1800019466 | Not a randomized clinical trial. |
| ChiCTR‐OON‐16010205 | Not a randomized clinical trial. |
| IRCT2013111313567N5 | Patient population did not meet our eligibility criteria. |
| Maher 2018 | Not a randomized clinical trial. |
| Ngo 2018 | Intervention did not meet our eligibility criteria. |
Characteristics of ongoing studies [ordered by study ID]
NCT01073150.
| Study name | Tea tree oil in the treatment of chronic blepharitis |
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
| Participants |
Baseline characteristics No TTO group Number of participants: NR Age: NR Women %: NR Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR TTO (intervention) group Number of participants: NR Age: NR Women %: NR Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Overall Number of participants: NR Age: NR Women %: NR Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Inclusion criteria: chronic blepharitis without previous treatment Exclusion criteria: aged < 18 years; pregnant woman; ocular surface disease (severe dry eye, lagoftalmo, entropium, ectropium) Pretreatment: NR |
| Interventions |
Intervention characteristics No TTO group
TTO (intervention) group
|
| Outcomes | NR |
| Starting date | April, 2009 |
| Contact information | clinicaltrials.gov/show/nct01073150 |
| Notes |
NCT03422146.
| Study name | Antimicrobial and clinical efficacy of Cliradex® as compared with I‐Lid'n Lash® hygiene in treating blepharitis |
| Methods |
Study design: randomized controlled trial Study grouping: parallel group |
| Participants |
Baseline characteristics I‐Lid 'n Lash® Hygiene control group Number of participants: NR Age: NR Women %: NR Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Cliradex® eyelid hygiene (intervention) group Number of participants: NR Age: NR Women %: NR Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Overall Number of participants: NR Age: NR Women %: NR Demodex count per eyelash: NR OSDI score: NR Visual acuity: NR Number of cylindrical dandruff: NR Inclusion criteria: symptomatic blepharitis for ≥ 3 months. Men and women aged > 18 years; ethnic groups comparable with the local community; able and willing to co‐operate with the investigational plan; able and willing to complete all postoperative follow‐up visits; able to understand and willing to sign a written informed consent. Exclusion criteria: currently engaged in another clinical trial; unwilling or unable to give consent; unwilling to accept randomization; unable to return for scheduled visits; children aged < 18 years; concomitant use of ophthalmic topical medications (excluding non‐preserved tear substitutes); concomitant use of systemic antibiotics or steroids; active ocular infection or allergy; previous surgery on the eyelids such as blepharoplasty; abraded skin on or around the eyelids; unable to close eyes or uncontrolled blinking; previous allergic reaction to TTO‐containing products or cosmetic fragrance. Pretreatment: unable to determine from the registration. This study is still recruiting. |
| Interventions |
Intervention characteristics I‐Lid 'n Lash Hygiene control group
Cliradex eyelid hygiene (intervention) group
|
| Outcomes | Primary: change in the number of colony‐forming units after 2 weeks' treatment Secondary: improvement in signs and symptoms of blepharitis Other: evidence of continued microbiologic improvement after discontinuing blepharitis treatment; evidence of continued improvement in signs and symptoms of blepharitis, based on grading scale, after discontinuing blepharitis treatment; evidence of continued improvement in signs and symptoms of blepharitis, based on participant questionnaire, after discontinuing blepharitis treatment. |
| Starting date | February, 2017 |
| Contact information | clinicaltrials.gov/show/nct03422146 |
| Notes |
NR: not reported; OSDI: Ocular Surface Disease Index; TTO: tea tree oil.
Differences between protocol and review
There were minor deviations from the published protocol that included the post‐hoc addition of two outcomes: proportion of participants eradicated of Demodex mites and patient compliance with treatment. These outcomes were added because, after we began collecting data, we found that studies were reporting these two outcomes, and we felt that this information would be valuable for judging the effectiveness of tea tree oil on Demodex blepharitis. Proportion of participants with Demodex mites eradication was added since it was found that during data collection that this metric was often presented in place of the mean change in number of Demodex mites for judging the effectiveness of tea tree oil. Patient compliance was added because Demodex blepharitis treatments were being evaluated at four weeks or longer, and the review authors considered that compliance may affect the effectiveness of the treatments being considered. The primary outcome was revised from 'Mean change in number of Demodex mites per eyelash' to 'Mean change in number of Demodex mites per eight eyelashes.' The change was made to align the outcome to the same unit as the one used to measure data in the studies.
Contributions of authors
Conception and design of protocol and review: ADP, JTL.
Drafting the protocol/review or commenting on them critically for intellectual content: KS, ADP, JTL.
Final approval of the document to be published: KS, ADP, JTL.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Eye Institute (NEI), National Institutes of Health (NIH), USA
Cochrane Eyes and Vision US Project (and JL during his time with the Project) is supported by cooperative agreement UG1EY020522, NEI, NIH
-
National Institute for Health Research (NIHR), UK
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
KS, JTL, and ADP have no financial interests in the subject matter of this systematic review. This Cochrane Review was prepared while JTL was a methodologist at the Johns Hopkins Bloomberg School of Public Health. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the US government.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Karakurt 2018 {published data only}
- Karakurt Y, Zeytun E. Evaluation of the efficacy of tea tree oil on the density of Demodex mites (Acari: Demodicidae) and ocular symptoms in patients with demodectic blepharitis. Journal of Parasitology 2018;104(5):473-8. [DOI] [PubMed] [Google Scholar]
Koo 2012 {published data only}
- Koo H, Kim TH, Kim KW, Wee SW, Chun YS, Kim JC. Ocular surface discomfort and Demodex: effect of tea tree oil eyelid scrub in Demodex blepharitis. Journal of Korean Medical Science 2012;27(12):1574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2018 {published data only}
- Murphy O, O'Dwyer V, Lloyd-McKernan A. The efficacy of tea tree face wash, 1,2-octanediol and microblepharoexfoliation in treating Demodex folliculorum blepharitis. Contact Lens & Anterior Eye 2018;41(1):77-82. [DOI] [PubMed] [Google Scholar]
NCT01647217 {published data only}
- NCT01647217. Demodex Blepharitis Treatment Study (DBTS). clinicaltrials.gov/ct2/show/NCT01647217 (first received 23 July 2012).
Wong 2019 {published data only}
- Wong K, Flanagan J, Jalbert I, Tan J. The effect of Blephadex™ Eyelid Wipes on Demodex mites, ocular microbiota, bacterial lipase and comfort: a pilot study. Contact Lens & Anterior Eye 2019;42(6):652-7. [DOI] [PubMed] [Google Scholar]
Zhang 2019 {published data only}
- Zhang X, Song N, Gong L. Therapeutic effect of intense pulsed light on ocular demodicosis. Current Eye Research 2019;44(3):250-6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ChiCTR1800019466 {published data only}
- ChiCTR1800019466. Eradication of Demodex folliculorum by okra eyelid patch in the treatment of demodectic blepharitis. www.chictr.org.cn/showprojen.aspx?proj=32773(first received 12 November 2018).
ChiCTR‐OON‐16010205 {published data only}
- ChiCTR-OON-16010205. The effect of orbital intense pulsed light treatment on blepharokeratoconjunctivitis. www.chictr.org.cn/com/25/showproj.aspx?proj=17380 (first received 21 December 2016).
IRCT2013111313567N5 {published data only}
- IRCT2013111313567N5. Impact of tea tree oil on dry eye treatment. en.irct.ir/trial/13390 (first received 21 June 2015).
Maher 2018 {published data only}
- Maher TN. The use of tea tree oil in treating blepharitis and meibomian gland dysfunction. Oman Journal of Ophthalmology 2018;11(1):11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngo 2018 {published data only}
- Ngo W, Jones L, Bitton E. Short-term comfort responses associated with the use of eyelid cleansing products to manage Demodex folliculorum. Eye & Contact Lens 2018;44(Suppl 2):S87-S92. [DOI] [PubMed] [Google Scholar]
- Ngo W, Jones LW, Srinivasan S, Bitton E. Discomfort over time associated with various ocular Demodex treatment products. Investigative Ophthalmology and Visual Science 2016;57(12):ARVO E-abstract 2838. [Google Scholar]
References to ongoing studies
NCT01073150 {published data only}
- NCT01073150. Tea tree oil in the treatment of chronic blepharitis. clinicaltrials.gov/show/nct01073150 (first received 23 February 2010).
NCT03422146 {published data only}
- NCT03422146. Antimicrobial and clinical efficacy of Cliradex® as compared with I-Lid'n Lash® hygiene in treating blepharitis. clinicaltrials.gov/show/nct03422146 (first received 5 February 2018).
Additional references
AAO 2018
- American Academy of Ophthalmology. Blepharitis – preferred practice pattern. www.aao.org/preferred-practice-pattern/blepharitis-ppp-2018 (accessed prior to 17 April 2019).
Amescua 2019
- Amescua G, Akpek EK, Farid M, Garcia-Ferrer FJ, Lin A, Rhee MK, et al. Blepharitis preferred practice pattern. Ophthalmology 2019;26(1):P56-93. [DOI] [PubMed] [Google Scholar]
Basta‐Juzbasic 2002
- Basta-Juzbasic A, Subic JS, Ljubojevic S. Demodex folliculorum in development of dermatitis rosaceiformis steroidica and rosacea-related diseases. Clinics in Dermatology 2002;20(2):135-40. [DOI] [PubMed] [Google Scholar]
Biernat 2018
- Biernat MM, Rusiecka-Ziolkowska J, Piatkowska E, Helemejko I, Biernat P, Gosciniak G. Occurrence of Demodex species in patients with blepharitis and in healthy individuals: a 10-year observational study. Japanese Journal of Ophthalmology 2018;62(6):628-33. [DOI] [PubMed] [Google Scholar]
Bitton 2019
- Bitton E, Ngo W, Dupont P. Eyelid hygiene products: a scoping review. Contact Lens & Anterior Eye 2019;42(6):591-7. [DOI] [PubMed] [Google Scholar]
Brand 2001
- Brand C, Ferrante A, Prager RH, Riley TV, Carson CF, Finlay-Jones JJ, et al. The water-soluble components of the essential oil of Melaleuca alternifolia (tea tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflammation Research 2001;50(4):213-9. [DOI] [PubMed] [Google Scholar]
Cheng 2015
- Cheng AM, Sheha H, Tseng SC. Recent advances on ocular Demodex infestation. Current Opinion in Ophthalmology 2015;26(4):295-300. [DOI] [PubMed] [Google Scholar]
Coston 1967
- Coston TO. Demodex folliculorum blepharitis. Transactions of the American Ophthalmological Society 1967;65:361-92. [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne: Veritas Health Innovation, accessed prior to 9 April 2019. Available at www.covidence.org.
Czepita 2007
- Czepita D, Kuzna-Grygiel W, Czepita M, Grobelny A. Demodex folliculorum and Demodex brevis as a cause of chronic marginal blepharitis. Annales Academiae Medicae Stetinensis 2007;53(1):63-7. [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
English 1981
- English FP, Nutting WB. Demodicosis of ophthalmic concern. American Journal of Ophthalmology 1981;91(3):362-72. [DOI] [PubMed] [Google Scholar]
Erbagci 2003
- Erbagci Z, Erbagci I, Erkilic S. High incidence of demodicidosis in eyelid basal cell carcinomas. International Journal of Dermatology 2003;42(7):567-71. [DOI] [PubMed] [Google Scholar]
Filho 2011
- Filho PA, Hazarbassanov RM, Grisolia AB, Pazos HB, Kaiserman I, Gomes JA. The efficacy of oral ivermectin for the treatment of chronic blepharitis in patients tested positive for Demodex spp. British Journal of Ophthalmology 2011;95(6):893-5. [DOI] [PubMed] [Google Scholar]
Forton 2005
- Forton F, Germaux MA, Brasseur T, De Liever A, Laporte M, Mathys C, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. Journal of the American Academy of Dermatology 2005;52(1):74-87. [DOI] [PubMed] [Google Scholar]
Fromstein 2018
- Fromstein SR, Harthan JS, Patel J, Opitz DL. Demodex blepharitis: clinical perspectives. Clinical Optometry 2018;10:57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2005a
- Gao YY, Di Pascuale MA, Li W, Baradaran-Rafii A, Elizondo A, Kuo CL, et al. In vitro and in vivo killing of ocular Demodex by tea tree oil. British Journal of Ophthalmology 2005;89(11):1468-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gao 2005b
- Gao YY, Di Pascuale MA, Li W, Liu DT, Baradaran-Rafii A, Elizondo A, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Investigative Ophthalmology and Visual Science 2005;46(9):3089-94. [DOI] [PubMed] [Google Scholar]
Gao 2007
- Gao YY, Di Pascuale MA, Elizondo A, Tseng SC. Clinical treatment of ocular demodecosis by lid scrub with tea tree oil. Cornea 2007;26(2):136-43. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed prior to 9 April 2019. Hamilton (ON): McMaster University (developed by Evidence Prime Inc), 2015. Available at gradepro.org.
Hammer 2006
- Hammer KA, Carson CF, Riley TV, Nielsen JB. A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food and Chemical Toxicology 2006;44(5):616-25. [DOI] [PubMed] [Google Scholar]
Hart 2000
- Hart PH, Brand C, Carson CF, Riley TV, Prager RH, Finlay-Jones JJ. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflammation Research 2000;49(11):619-26. [DOI] [PubMed] [Google Scholar]
Herron 2005
- Herron MD, O'Reilly MA, Vanderhooft SL. Refractory Demodex folliculitis in five children with acute lymphoblastic leukemia. Pediatric Dermatology 2005;22(5):407-11. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Jones 2017
- Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II management and therapy report. Ocular Surface 2017;15(3):575-628. [DOI] [PubMed] [Google Scholar]
Kaya 2018
- Kaya A, Gurdal C. Office-based diagnosis of Demodex using smartphone. Eye & Contact Lens 2018;44(6):e25-6. [DOI] [PubMed] [Google Scholar]
Kemal 2005
- Kemal M, Sumer Z, Toker MI, Erdogan H, Topalkara A, Akbulut M. The prevalence of Demodex folliculorum in blepharitis patients and the normal population. Ophthalmic Epidemiology 2005;12(4):287-90. [DOI] [PubMed] [Google Scholar]
Lam 2018
- Lam NS, Long XX, Griffin RC, Chen MK, Doery JC. Can the tea tree oil (Australian native plant: Melaleuca alternifolia Cheel) be an alternative treatment for human demodicosis on skin? Parasitology 2018;145(12):1510-20. [DOI] [PubMed] [Google Scholar]
Lemp 2009
- Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocular Surface 2009;7(2):S1-14. [DOI] [PubMed] [Google Scholar]
Li 2017
- Li Y, Shao X, Xu J, Wei Y, Xu F, Wang H. Tea tree oil exhibits antifungal activity against Botrytis cinerea by affecting mitochondria. Food Chemistry 2017;234:62-7. [DOI: 10.1016/j.foodchem.2017.04.172] [DOI] [PubMed] [Google Scholar]
Lindsley 2012
- Lindsley K, Matsumura S, Hatef E, Akpek EK. Interventions for chronic blepharitis. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD005556. [DOI: 10.1002/14651858.CD005556.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2010
- Liu J, Sheha H, Tseng SC. Pathogenic role of Demodex mites in blepharitis. Current Opinion in Allergy and Clinical Immunology 2010;10(5):505-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2020
- Murphy O, O'Dwyer V, Lloyd-McKernan A. The clinical use of eyelash manipulation in the diagnosis of Demodex folliculorum blepharitis. Eye & Contact Lens 2020;46(Suppl 1):S33-8. [DOI] [PubMed] [Google Scholar]
Navel 2019
- Navel V, Mulliez A, Benoist d'Azy C, Baker JS, Malecaze J, Chiambaretta F, et al. Efficacy of treatments for Demodex blepharitis: a systematic review and meta-analysis. Ocular Surface 2019;17(4):655-69. [DOI] [PubMed] [Google Scholar]
Nicholls 2017
- Nicholls SG, Oakley CL, Tan A, Vote BJ. Demodex species in human ocular disease: new clinicopathological aspects. International Ophthalmology 2017;37(1):303-12. [DOI] [PubMed] [Google Scholar]
Post 1963
- Post CF, Juhlin E. Demodex folliculorum and blepharitis. Archives of Dermatology 1963;88(3):298-302. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roth 1979
- Roth AM. Demodex folliculorum in hair follicles of eyelid skin. Annals of Ophthalmology 1979;11(1):37-40. [PubMed] [Google Scholar]
Sabeti 2020
- Sabeti S, Kheirkhah A, Yin J, Dana R. Management of meibomian gland dysfunction: a review. Survey of Ophthalmology 2020;65(2):205-17. [DOI] [PubMed] [Google Scholar]
Schelz 2006
- Schelz Z, Molnar J, Hohmann J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia 2006;77(4):279-85. [DOI] [PubMed] [Google Scholar]
Swords 1978
- Swords G, Hunter GL. Composition of Australian tea tree oil (Melaleuca alternifolia). Journal of Agricultural and Food Chemistry 1978;26(3):734-7. [Google Scholar]
Tighe 2013
- Tighe S, Gao YY, Tseng SC. Terpinen-4-ol is the most active ingredient of tea tree oil to kill Demodex mites. Translational Vision Science and Technology 2013;2(7):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolffsohn 2017
- Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II diagnostic methodology report. Ocular Surface 2017;15(3):539-74. [DOI] [PubMed] [Google Scholar]
Zhang 2018
- Zhang X, Song N, Gong L. Therapeutic effect of intense pulsed light on ocular demodicosis. Current Eye Research 2019;44(3):250-6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Savla 2019
- Savla K, Le JT, Pucker AD. Tea tree oil for Demodex blepharitis. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD013333. [DOI: 10.1002/14651858.CD013333] [DOI] [PMC free article] [PubMed] [Google Scholar]


