Abstract
Purpose of Review
For over 20 years, the Women’s Ischemia Syndrome Evaluation (WISE), a program sponsored by the National Heart, Lung, and Blood Institute, has explored diverse and important aspects of ischemic heart disease in women.
Recent Findings
Women with symptoms and signs of ischemia but no significant epicardial obstructive coronary artery disease (INOCA) were documented to be at elevated risk for recurrent angina hospitalization, major adverse cardiac events, death, and health resource consumption rivaling those with obstructive coronary disease.
Summary
WISE investigators have advanced our understanding of cardiovascular outcomes, systemic manifestations, psychological variables, socioeconomic factors, genetic contributions, hormonal status, advanced imaging, coronary functional findings, biomarkers, patient-reported outcomes, and treatments pertaining to women with this disease entity. This review delves into the WISE findings subsequent to a prior review1, postulates directions for future research, and asks are we “Even ‘WISE-R?’”
Keywords: WISE, NHLBI, Non-obstructive coronary artery disease, Coronary microvascular dysfunction, Ischemic heart disease
Introduction
In 1996, the National Heart, Lung, and Blood Institute initiated the Women’s Ischemia Syndrome Evaluation (WISE), which has revolutionized our knowledge of the pathophysiological processes underlying heart disease in women and related outcomes. As described in our 2006 review [1•], the WISE collaboration explored anginal symptoms, diagnostic modalities for coronary micro- and macrovascular disease, and psychosocial and reproductive variable contributions to ischemic heart disease (IHD) in women. Ongoing WISE studies have targeted the development of innovative diagnostic and therapeutic options for IHD, both for women and increasingly men. But just how has all of this information made us “WISE-R?”
Evolution of WISE Cohorts
WISE research includes multiple cohorts over time (Fig. 1). The original WISE cohort, enrolled from 1997 to 2001 at four US sites, was comprised of women with suspected IHD, undergoing clinically indicated invasive coronary angiography, including women with and without obstructive coronary artery disease (CAD). The subsequent WISE Coronary Vascular Disease (CVD) cohort, enrolled from 2009 to 2012, enrolled exclusively women with suspected ischemia and no obstructive CAD (INOCA), defined as < 50% stenosis. The WISE-CVD cohort had fewer Caucasian women, higher levels of education, and a lower prevalence of cardiac risk factors than WISE, both cohorts showed similar patterns of disordered coronary reactivity, including a high prevalence of coronary microvascular dysfunction (CMD) [2]. Both cohorts had a high prevalence of non-obstructive CAD: however, CMD did not appear to be simply attributable to traditional atherosclerosis risk factors.
Fig. 1.

The development of various WISE cohort subgroups over time, overlying the trend in cardiovascular death rate among women; NHLBI = National Heart, Lung and Blood Institute; WTH = “Women Take Heart”, UPgh = University of Pittsburgh, Pgh = Pittsburgh, CS = Cedars-Sinai Medical Center, UF = University of Florida, RAAS = renin angiotensin aldosterone system, PDE-5 = phosphodiesterase-5, CMR = cardiac magnetic resonance imaging; CANS = Cardiac Autonomic Nervous System Study (1K23HL105787-01A1); WARRIOR = Women’s Ischemia Trial to Reduce Events In Non-Obstructive Coronary Artery Disease (NCT03417388)
Currently, WISE is enrolling women and men in 2 new cohorts: WISE - Heart Failure with Preserved Ejection Fraction (HFpEF) (NCT02582021) enrolling women and men with INOCA undergoing clinically indicated invasive functional coronary angiography (FCA), and women and men with HFpEF, and WISE - Pre-HFpEF (NCT03876223), enrolling women and men specifically undergoing clinically indicated invasive FCA, to evaluate mechanistic links between CMD and HFpEF.
INOCA Cardiovascular Outcomes
WISE studies reveal the adverse results of the underrecognized INOCA, previously labeled as benign. WISE women with 0–49% stenosis have an increased 5-year cardiovascular event rate compared with asymptomatic women in the community and adjusted for CAD risk factors [2]. In a recent analysis of 9-year mortality in the WISE cohort, 33% of the deaths occurred in women without obstructive CAD, reflecting a 13% mortality rate [3], further emphasizing the significant impact of INOCA. WISE investigations determined that CMD predicted major adverse cardiac events (MACE) including death, myocardial infarction, stroke, and heart failure hospitalization in these women [4]. Additionally, we documented that the majority of HF hospitalizations at extended follow-up were characterized by HFpEF and not associated with obstructive CAD [5].
These WISE INOCA outcomes studies underscore the critical need for further research into underlying pathophysiology, prognostic factors, diagnosis, and management strategies for INOCA and CMD.
INOCA Systemic Manifestations
Metabolic Syndrome
Recent WISE studies emphasize the association of IHD with systemic conditions. While the metabolic syndrome (MetS) is linked with CVD, WISE investigators have also observed relations with coronary atherosclerosis and arterial remodeling defined by intravascular ultrasound (IVUS) [6]. Further, this association does not depend on the full MetS cluster, but rather appears to be specifically driven by the individual factors of waist circumference and systemic blood pressure (BP). These findings support the hypothesis that dysmetabolic states and their associated inflammation may contribute to both INOCA and systemic disorders.
Renal Insufficiency
IHD has known associations with renal function, which WISE has further characterized. In WISE, the presence of mild chronic kidney disease (CKD) is an independent predictor of all-cause and cardiac mortality, regardless of CAD severity [7]. Renal insufficiency was also determined to be significantly associated with reduced coronary flow reserve (CFR) [8], lending insight into one of the possible pathogeneses for CMD.
Migraine Headache
While initial WISE analyses did not link migraine with CVD [9], subsequent longer-term follow-up showed an increased adjusted risk with MACE [10]. This higher risk was primarily driven by an associated two-fold increase in stroke risk. These findings suggest CKD, migraine, and possibly stroke may represent a broader spectrum of systemic microvascular functional disorders. CMD and these systemic diseases may also share common risk factors, which could warrant further investigation that could provide clues to better management and prevention.
Psychological Status
Higher State-Trait Anxiety Inventory scores correlate with more frequent angina and dyspnea in the WISE [11]. Depression and anxiety were also associated with elevated CVD costs [11, 12]. WISE investigators have proposed that somatic and not cognitive/affective symptoms of depression portended a worse CVD prognosis [13]. Further, negative affectivity was uniquely associated with higher body mass index (BMI) values and hostility with increased frequency of MetS [14], although neither correlated with MACE. Finally, the predictive value of depression for MACE improves when the severity of comorbid anxiety is also accounted for [12], supporting the analysis of depression and anxiety together, instead of individually.
In addition to suggesting novel ways to study the links between psychological status and CVD, WISE investigators recently sought to elucidate the mechanisms underlying these associations. Among women with suspected IHD, those with comorbid depression had 70% higher circulating serum levels of C-reactive protein (hs-CRP) and 25% higher levels of IL-6 [15]. These findings suggest that inflammation may be at least partly contributory. Another possible mediating factor is the interval development of MetS. Among women diagnosed with depression, those with comorbid MetS were at 2.6 times higher risk of CVD [16]. Vascular processes may also be responsible. Mental stress peripheral vasoreactivity was elevated in patients with CMD, suggesting a mechanism and potential treatment target for stress-induced chest pain in CMD [17].
Treatment with antidepressants, alone and in combination with anxiolytics, correlates with higher risk of CVD events and all-cause mortality in WISE women compared with untreated women in the general population [18]. While treatment of anxiety disorders correlated with less obstructive CAD, anxiolytic use was associated with more frequent chest pain, nitroglycerin use, and hospitalizations for coronary catheterization [11]. Conversely, WISE women receiving lipidlowering therapy had higher aggressive responding subscores on the Cook-Medley Hostility Scale, indicating a higher inclination towards hostility in social situations [16].
Socioeconomic Status
Sex-Based Differences
In 2006, WISE investigators proposed key working hypotheses related to sex-specific IHD for women, which have arguably set the stage for much of the investigation conducted over the past decade. Because the original WISE design (NCT00000554) exclusively studied women, WISE investigation has encouraged other researchers to also investigate women, to provide a framework exploring sex-specific IHD development. WISE investigators [19] have stressed the importance of specific study of women rather than comparison to men, in order to uncover sex-specific mechanisms and ultimately treatment strategies.
Economic Impact
Income is a significant predictor of cardiovascular death and costs, including risk-adjusted models controlling for obstructive IHD, chest pain, and cardiac risk factors [20]. WISE women with an annual income below $20,000 had the highest 5-year hospitalization and drug treatment costs. They were also more likely than higher-income women to require 2 or more anti-ischemia medications in the follow-up. When compared with women with obstructive CAD, INOCA women had more repeat catheterizations or angina hospitalizations, higher drug treatment costs, and the greatest proportion of anti-ischemic therapy costs [21].
The Role of Social Relationships and Ethnicity
When compared with women with higher Social Network Inventory scores, more isolated women experienced strokes at more than twice the rate, even following covariate adjustment [22], demonstrating a possible protective effect of social relationships. In terms of ethnicity, black women more often presented with symptoms they attributed to stomach-based pain compared to white women, irrespective of their CAD severity [23]. This was associated with higher all-cause and cardiovascular mortality among black women, suggesting that this atypical presentation may result in impairment of diagnosis and care delivery, potentially facilitating worse outcomes.
Genetic Factors
In 2008, WISE women positive for two beta-adrenergic receptor polymorphisms, ADRB1 Gly389 and ADRB3 Arg64, were found to be at increased risk for adverse cardiac events [24]. Interestingly, this risk elevation was limited to women with suspected INOCA versus those with obstructive CAD. The homozygous short variant of the 5-HTTLPR serotonin transporter gene polymorphism was also associated with increased MACE among INOCA women [25], while one genetic marker, single nucleotide polymorphism (SNP) rs2301753 on chromosome 6 in RNF39, achieved chip-wide significance for non-obstructive CAD [26].
WISE also examined the role of SNPs in genes encoding antibodies against oxidized LDL cholesterol, known to contribute to atherosclerosis [27]. SNPs in two of these genes resulted in augmented levels of IgG and IgM oxidized LDL antibodies, which led to less severe IHD, while multiple pairs of epistatic quantitative trait loci were associated with body weight, BMI, and waist and hip circumferences and ratios, using statistical modeling [28].
Hormonal Status
Endogenous Estrogen
WISE women with early onset vasomotor symptoms have higher CVD mortality and lower flow-mediated dilation than those with later onset symptoms [29]. Hypothalamic hypoestrogenism (HHE) results from inadequate ovarian stimulation by the hypothalamus in premenopausal women, resulting in low estrogen levels. HHE, specifically due to psychosocial stress, was prevalent among premenopausal WISE, which was the most powerful predictor of obstructive CAD [30]. Further, WISE women with both diabetes mellitus and HHE had higher prevalence and obstructive CAD severity [31] than those having diabetes alone. These findings further endorse that estrogen deficiency may precipitate atherosclerosis and endothelial dysfunction. However, investigators also found that estrogen exposure time has no association with angiographic CAD or other adverse outcomes [32].
Exogenous Estrogen
HT initiation before 55 years of age was associated with less obstructive CAD among WISE women with natural menopause [33]. Prior oral contraceptive (OC) use among postmenopausal women, correlated with decreased angiographic CAD severity score [34]. Also, low-dose estrogen therapy in a WISE ancillary randomized placebo-controlled trial resulted in improved chest pain, menopause symptoms, and quality of life but did not improve ischemia or endothelial dysfunction [35].
Other Hormones
Lower circulating levels of dehydroepiandrosterone sulfate (DHEA-S), associated with aging, were linked with increased cardiovascular and all-cause mortality in WISE women [36]. Phytoestrogens or dietary soy isoflavones investigation found that blood daidzein level was associated with lower triglyceride and higher high-density cholesterol (HDL-C) level, resulting in a beneficial total cholesterol/HDL-C ratio [37]. Alternatively, blood genistein levels were inversely associated with CFR, resulting in lower flow reserve with higher phytoestrogen level [38].
Menopause and Hypertension
Among premenopausal and postmenopausal women, systolic BP (SBP) and pulse pressure (PP) were found to be stronger CVD risk factors related to angiographic CAD, among premenopausal women when compared to postmenopausal women [39].
Advanced Cardiac Imaging
Cardiac Magnetic Resonance Imaging
The novel applications and limitations of imaging modalities are additional themes within recent WISE literature (Fig. 2) [40]. Due to its high spatial resolution, diagnostic accuracy, and lack of ionizing radiation, cardiac magnetic resonance imaging (CMRI) has become a powerful imaging technique for evaluating suspected IHD and other structural abnormalities among women [41].
Fig. 2.
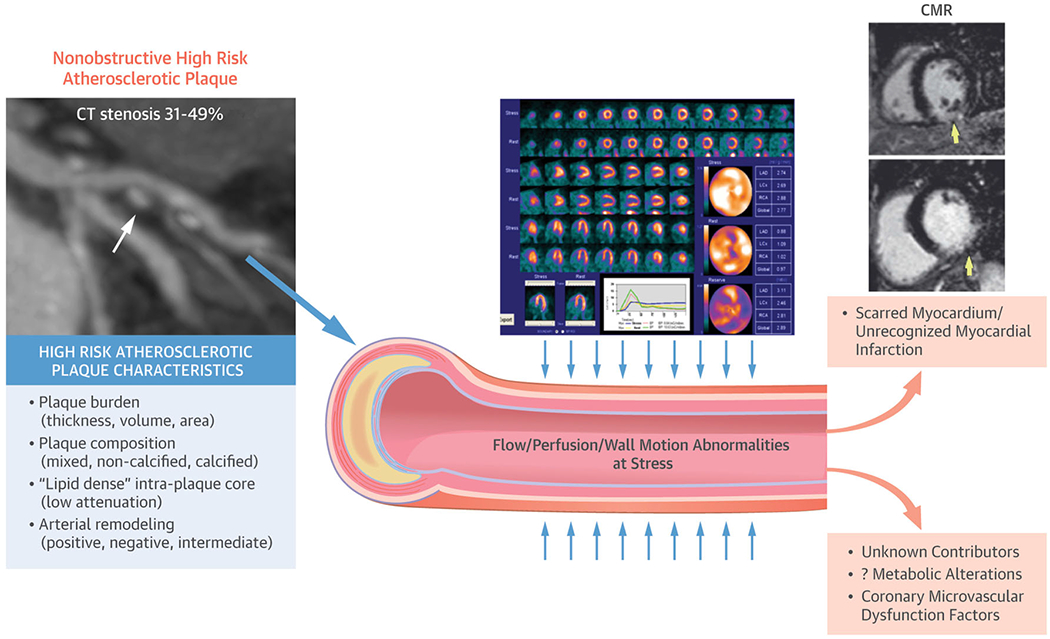
A model describing the use of CT (computed tomography), cMRI (cardiac magnetic resonance imaging), and nuclear imaging modalities to characterize pathology and stratify risk among women with ischemic heart disease. Reprinted with permission from Ref 42. Copyright 2007 Elsevier
WISE women with reduced CFR were found to have reduced time to peak filling rate (tPFR), a marker of diastolic dysfunction, on CMRI [41]. In addition, diminished coronary blood flow (CBF) change, a measure of endothelial-dependent CMD, was associated with elevated left ventricular end-diastolic pressure (LVEDP) and increased mass on CMRI. CMRI was also used to identify a previously unsuspected link between aortic flow conditions and left ventricular ejection fraction (LVEF), which may shed further light on a linkage between CMD and LV diastolic dysfunction [42].
CMRI is multifaceted with the area of myocardial perfusion imaging (MPI) evolving. Among women with INOCA, global first-pass perfusion CMRI-determined measurements of normalized uptake slope and peak signal uptake, in conjunction with LVEF, were found to predict MACE [43]. However, when tested with the non-pharmacological cold pressor method for endothelial stress, MPI reserve index (MPRI) could not identify CMD among symptomatic women, potentially due to inadequate provocative stress [44]. Further, late gadolinium enhancement (LGE) has emerged as a signal of myocardial damage and scar, now described in the setting of CMD [45]. WISE investigators developed and tested the Decisions Informed by Combining Entities (DICE) algorithm. Integrating CMRI and gated single-photon emission computed tomography (SPECT), DICE improves MPI interpretation, diagnostic, and prognostic outcomes [46]. The research benefit of evaluating CMRI and SPECT together was demonstrated by a study finding that internal energy utilization of the LV, determined independently by both modalities, has greater prognostic value than EF or the presence of a myocardial perfusion defect [47].
Positron Emission Tomography
Positron emission tomography, or PET, has emerged as another technology that can non-invasively detect and monitor CMD and its complications, in addition to subclinical and early stage atherosclerosis.
Due to its high spatial resolution, PET can accurately quantify myocardial blood flow in absolute terms and detect subtle differences in regional perfusion, critical to understanding certain cardiac disease pathologies, even in the earliest functional stages of disease before structural alterations manifest [48]. In a 2003 study [49], WISE investigators discovered that CFR was heterogeneously distributed throughout the vascular territories, suggesting that only one CFR measurement during catheterization may not accurately reflect all three vessels.
Since then, PET has become a widespread stress myocardial perfusion testing modality, which offers additional notable advantages when compared with SPECT [40]. In addition to its improved spatial and temporal resolution and optimal safety profile, PET demonstrates similar prognostic and higher diagnostic accuracy. The extent and severity of PET stress abnormalities have a directly proportional relationship with MACE. PET can also estimate both epicardial and subepicardial perfusion. The concordant use of CT with PET further allows for the correction of breast tissue attenuation, a noted limitation of SPECT among women.
The role of PET in the research of CMD treatment and associated disease pathologies has also become increasingly well-recognized. The serial measurement of MBF in response to cold exposure or pharmacologic vasodilation renders PET a useful tool for evaluating the effects of lifestyle modification and therapeutic interventions on the coronary circulation [50]. More recently, the ability for PET to concordantly measure regional myocardial blood flow patterns, myocardial oxygen, and glucose metabolism has allowed for analysis of the relationships between CMD and the development of infiltrative, hypertrophic, and other non-ischemic cardiomyopathies [48].
While PET can accurately identify changes in the coronary circulation, how these findings may affect clinical outcomes warrants further larger-scale prospective investigation.
Invasive Imaging Strategies
WISE investigation used IVUS to assess the coronary wall anatomy in a cohort of women with CMD, demonstrating preserved lumen size and evidence of positive remodeling in the setting of coronary atherosclerosis [51]. In a selected case, IVUS combined with OCT successfully identified plaque rupture as the etiology for ST elevation myocardial infarction in a woman with apical wall motion abnormality [52]. Among women with suspected INOCA, corrected TIMI frame count, an invasive angiographically derived coronary flow imaging measure, independently predicted future angina hospitalizations [53]. In a 2013 prospective study, WISE researchers developed a modified Gensini angiographic score for women, based on the presence, location, and severity of vessel stenosis and collaterals, which effectively predicted long-term MACE [54].
Invasive Functional Coronary Angiography
Functional coronary angiography (FCA) has served as a fundamental tool throughout WISE history, specifically for detecting CMD within the INOCA population [55]. Recently, WISE researchers have expanded upon the array of FCA applications, highlighting the procedure’s implications for INOCA assessment and treatment and reaffirming its diagnostic and predictive efficacy, with a published safety record [56].
WISE elaborated on the relationship between CBF change and diastolic parameters, including tPFR, end-systolic volume, and LVEF [57]. Among women with INOCA and CVD risk factors, CMD diagnosed with intra-coronary adenosine CFR improved MACE prediction, in comparison with angiographic CAD severity and CVD risk factors [58]. In a 2015 study, the WISE team utilized FCA to characterize relationships between CFR and arterial properties [59]. CFR was inversely related to aortic systolic pressure and aortic pulse wave velocity (aPWV), an index of arterial stiffness. These findings revealed a novel mechanism by which changes in arterial properties increase afterload, requiring the LV to generate additional “wasted energy” that increases myocardial oxygen demand.
The presence of brachial artery constriction, measured by non-invasive ultrasound after release of occlusion, predicted almost double the risk for MACE, after adjusting for obstructive CAD and traditional risk factors [57]. Application tonometry recording of radial artery blood pressure waveforms, among WISE women with INOCA, identified modifications in systolic wave characteristics and diastolic timing, supporting the aforementioned relationship between CFR, afterload, myocardial efficiency, and ischemia [60]. Brachial PP is a stronger predictor of MACE and CVD mortality than SBP, diastolic BP (DBP), and mean arterial pressure (MAP), with an 18% excess mortality risk for every 10 mmHg increase in PP [61].
Recent WISE findings advised the 2019 European Society FCA Guidelines [62]. Namely, in patients with persistent symptoms but angiographically normal coronary arteries or non-obstructive CAD and preserved fractional flow reserve, guidewire-based CFR and/or microcirculatory resistance measurements should be considered. Further, if coronary arteries are not significantly stenosed on angiography, intra-coronary acetylcholine with electrocardiographic monitoring can be considered. Finally, transthoracic Doppler of the left anterior descending artery, CMRI, and positron emission tomography can be considered for non-invasive CFR measurement.
Serum Biomarkers
While imaging and the various aforementioned testing modalities have a central role in CVD diagnosis and prognostication, laboratory evaluation was not as well defined.
Inflammatory Markers
As inflammation is proven to contribute to CVD, the team recently evaluated the relationships between systemic inflammatory markers and CVD outcomes. While both elevated IL-6 and serum amyloid A (SAA) protein were associated with increased all-cause mortality, only elevated IL-6 correlated with increased hospitalization [63]. A combined multi-marker testing approach, including hs-CRP, IL-6, SAA, and hemoglobin, gave predictive information beyond that provided by the Framingham Risk Score [64]. The presence of 3 or more abnormal serum biomarkers was associated with substantially increased mortality.
WISE also examined how serologic evaluation may suggest CMD. Circulating bone marrow progenitor cells (CPCs), increased in IHD, are generated by the reparative response. Decreased CFR was significantly associated with elevated CD34+ CPC levels [65]. Further studies are required to characterize the role of CPCs in obstructive versus non-obstructive disease and CVD prognostication.
Cardiac Risk Markers
Contemporary studies have also elaborated on knowledge regarding other serologic cardiac risk markers. The triglyceride/HDL-C ratio was affirmed as a strong independent predictor of all-cause mortality and MACE [66]. Interestingly, despite the strong association between CMD and the development of HFpEF, BNP did not show predictive value in women with the disease [67].
Patient-Reported Measures
Remotely collected and self-reported patient data, whether acquired by survey or wearable device technology, are increasingly popular. WISE recently investigated the prognostic and diagnostic value of such data, for higher-risk individuals.
Duke Activity Status Index
Estimated functional capacity (EFC) has been shown to relate to several major CVD outcomes. Lower EFC, based on the Duke Activity Status Index (DASI), was associated with increased long-term mortality [68] and the development of HF [69] among WISE women. Functional impairment was also predictive of MACE and indeterminate exercise stress testing [70]. Furthermore, CFR correlated directly with the DASI score [71]. This finding supports the functional importance of DASI to risk stratify women undergoing ischemic evaluation.
Aside from using DASI for EFC, self-reported data also helps characterize the joint impact of smoking history and exercise capacity upon CVD outcomes. WISE women reporting both tobacco use history and low exercise capacity had the highest MACE risk [72]. Further, all smokers had significantly greater risk, regardless of exercise capacity, even after controlling for preexisting CAD severity and other established risk factors. This study demonstrates the combined beneficial cardiovascular effects of maintaining a high exercise capacity and avoiding tobacco use, in addition to the value of information reported via validated questionnaires.
Wearable Technology
WISE assessed the capacity of Fitbit technology to determine treatment response of women with CMD to ranolazine, a late sodium channel current inhibitor. In a randomized, double-blinded crossover trial, investigators examined the difference in daily step counts after 2 weeks of therapy with ranolazine versus placebo [73]. Overall, ranolazine correlated with reduced step count. However, those patients who reported improved angina had higher step counts, objectively confirming that improved daily life activity was associated with better angina control. The predictive utility of wearable digital monitoring technology continues to be evaluated, with current exploration focusing on its association with prognostically valuable serum biomarkers, including hs-CRP, N-terminal pro-brain natriuretic peptide (NT-proBNP), and ultra-high sensitivity cardiac-specific troponin (u-hs-cTnI).
CMD Treatment
Driven by developments in understanding about mechanistic pathways, the WISE team continues to evaluate CMD treatment alternatives, including multiple pharmacologic probes involving the WISE-CMD cohort (Fig. 3). Interestingly, all of the recently studied medication classes appear to be of greatest therapeutic benefit among women with significantly decreased CFR (Table 1) [74].
Fig. 3.

A summary of the pharmacologic probes and the influence of various agents upon vascular behavior and imaging characteristics
Table 1.
Coronary microvascular dysfunction (CMD) treatment options
| Abnormal coronary vasoconstriction or endothelial inflammation |
| Angiotensin-converting enzyme inhibitors (ACE-I) |
| HMG-CoA reductase inhibitors (statins) |
| L-Arginine supplementation |
| Aerobic exercise |
| Enhanced external counterpulsation (EECP) |
| Abnormal coronary vasodilation |
| Beta-blockers/alpha-beta-blockers |
| Nitrates |
| Anti-anginal |
| Ranolazine |
| Ivabradine |
| Xanthine derivatives |
| Abnormal smooth muscle function (Prinzmetal’s angina) |
| Calcium channel blockers |
| Nitrates |
| Abnormal cardiac nociception |
| Low-dose tricyclic medication |
| Spinal cord stimulation |
| Cognitive behavioral therapy |
Renin-Angiotensin-Aldosterone System Agents
Agents that block products of the Renin-Angiotensin-Aldosterone System (RAAS) have been a significant contemporary focus. High-dose angiotensin-converting enzyme inhibitor (ACEI) quinapril correlated with improved CFR and reduced angina, over a 16-week treatment period, among women with CMD [75]. Another theory postulated that aldosterone blockade may provide a synergistic effect with ACEI upon endothelial function. However, WISE found no significant improvement when eplerenone was added to quinapril [76].
Phosphodiesterase-5 Inhibition
Phosphodiesterase-5 (PDE-5) inhibitors block degradation of cyclic GMP in vascular smooth muscle cells, thus promoting vasodilation, historically for pulmonary hypertension and erectile dysfunction. Recently, WISE investigators found that sildenafil may also acutely improve CFR among women with CMD [77].
Ranolazine
A randomized, double-blinded crossover trial of ranolazine versus placebo found that while patients with CFR under 2.5 had improved MPRI and anginal symptoms, ranolazine was not effective for milder CMD [78]. However, in a pre-specified follow-up analysis evaluating WISE women with low CFR, ranolazine improved angina and myocardial perfusion. Within this subgroup, reduced LV end-diastolic volume predicted improvement in anginal symptoms [79]. This finding supports the hypothesis that late sodium channel current blockade may be beneficial among women with more severe CMD.
Statins
Aside from the WISE probes, outside investigators have demonstrated the emerging role of statins in ameliorating CMD. The beneficial effects of statin therapy on exercise-induced ischemia and flow-mediated dilation in CMD were first shown in 2003 [80]. More recent studies have sought to elucidate the mechanisms underlying these effects. While some suggested that statins inhibit dextrose-induced superoxide anion formation [81], others reported that they downregulate arginase activity, facilitating enhanced synthesis of vasoprotective nitric oxide [82].
Intensive Medical Therapy
Given the abundance of adverse outcomes and resource consumption associated with INOCA, WISE has initiated a study to evaluate the role of intensive medical therapy (IMT). Since women represent the fastest-growing minority population of the US military, the “Women’s Ischemia Trial to Reduce Events in Non-Obstructive CAD” (WARRIOR) trial was funded by the Department of Defense. Currently enrolling subjects, WARRIOR (NCT03417388) is a multicenter, prospective, randomized blinded outcome evaluating the influence of IMT with aspirin, a high potency statin and ACEI/ARB therapy compared with usual care on MACE in symptomatic women with INOCA, over a 3-year follow-up period. The results should facilitate clinical management guidelines for women with INOCA.
Conclusions
Based upon this review of the multifaceted endeavors of the WISE collaboration for over 2 decades, we are certainly “WISE-R”, in many ways. The knowledge shared and awareness raised by these efforts have stimulated global recognition, bringing INOCA to the forefront. Based on these results, the ESC [83] and American College of Cardiology [55] have issued position papers, outlining proposed diagnostic and treatment algorithms, as well as directions for future research. The American Heart Association has even developed a “traffic light” sequence, supporting a definitive diagnostic approach for INOCA [55].
While our understanding of INOCA has significantly advanced, many knowledge gaps and questions still remain (Fig. 4) [74]. For example, why do some individuals with certain risk factors develop INOCA, while others do not? Are there undiscovered imaging modalities that can identify INOCA earlier? Is INOCA the precursor of angiographic stenoses, or is it a separate mechanism? What processes are responsible for INOCA evolving into myocardial infarction (MINOCA) and HFpEF? According to WISE authors, further translational investigation will be necessary, specifically focused on developing a phenotypic classification for INOCA patients, generating diagnostic algorithms based upon this classification system and formulating universal guidelines [74].
Fig. 4.
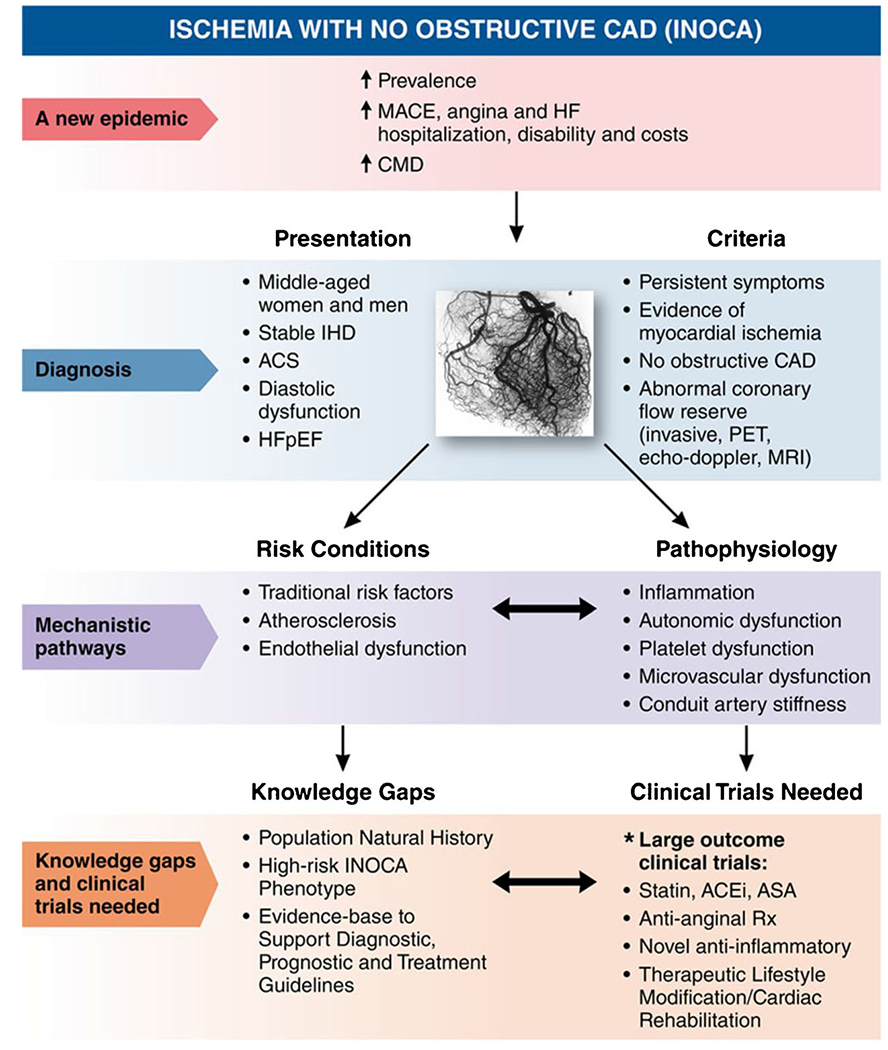
The state of our current understanding and proposed future directions for INOCA research. MACE = major adverse cardiac events, HF = heart failure, CMD = coronary microvascular disease, IHD = ischemic heart disease, ACS = acute coronary syndrome, HFpEF = heart failure with preserved ejection fraction, CAD = coronary artery disease, PET = positron emission tomography, MRI = magnetic resonance imaging, ACEi = angiotensin-converting enzyme inhibitor, ASA = aspirin, Rx = treatment. Reprinted with permission from Ref 74. Copyright 2017 Wolters Kluwer Health
Thanks to a collaborative group of investigators and inspiring research volunteer participants, we look forward to the opportunity to continue cultivating our “WISE-dom” to improve health for women and men, just on the horizon.
Acknowledgments
Funding Information This work was supported by contracts from the National Heart, Lung, and Blood Institutes nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, and N01-HV-68164; grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, and 1R03AG032631 from the National Institute on Aging; GCRC grant MO1-RR00425 from the National Center for Research Resources; the National Center for Advancing Translational Sciences Grant UL1TR000124; and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ; The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA; The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ; the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, CA; the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles; The Society for Women’s Health Research (SWHR), Washington, D.C.; the Linda Joy Pollin Women’s Heart Health Program; the Erika Glazer Women’s Heart Health Project; and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, CA.
Conflict of Interest Drs. Barsky, Sopko, Doyle, Reis, Rogers, Rutledge, Shufelt, Shaw, and Wei have nothing to disclose. Dr. Bairey Merz reports personal fees from iRhythm, other from Sanofi, other from Abbott Diagnostics, during the conduct of the study. Dr. Handberg reports grants from NIH/NHLBI, during the conduct of the study; grants from Aastom Biosciences, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Brigham and Women’s Hospital, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Duke Clinical Research Institute, East Carolina University, Everyfit Inc., Gilead, Ionis, Medtronic, Merck & Co., Mesoblast, PCORI, Relypsa, Sanofi Aventis, outside the submitted work. Dr. Pepine reports grants from NIH/NHLBI, during the conduct of the study; grants from NIH/NCATS, grants from BioCardia BC-14-001-02; Mesoblast, Inc. MSB-MPC-CHF001; Ventrix, Inc.; Athersys Inc. AMI MultiStem; Verily Life Sciences LLC-Project Baseline OSMB; Ironwood MSB-MPC-CHF00-DMC, Imbria Pharmaceuticals Inc.; Milestone Pharmaceuticals Inc.; Caladrius Biosciences, Inc.; Gatorade Trust; and McJunkin Family Foundation, outside the submitted work.
Footnotes
Human and Animal Rights and Informed Consent All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.•.Ahmed B, Bairey Merz CN, Sopko G. Are we ‘WISE’r? Findings from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation Study. Womens Health (Lond). 2006;2(1):57–64. [DOI] [PubMed] [Google Scholar]; This article examines how impaired microvascular function can result in increased hazard of mortality, major adverse cardiac events and angina hospitalization.
- 2.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169(9):843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenkre TS, Malhotra P, Johnson BD, Handberg EM, Thompson DV, Marroquin OC, et al. Ten-year mortality in the WISE Study (Women’s Ischemia Syndrome Evaluation). Circ Cardiovasc Qual Outcomes. 2017;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, et al. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol. 2019;73(6): 684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakir M, Nelson MD, Jones E, Li Q, Wei J, Sharif B, et al. Heart failure hospitalization in women with signs and symptoms of ischemia: a report from the women’s ischemia syndrome evaluation study. Int J Cardiol. 2016;223:936–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalig A, Johnson BD, Anderson R, Bavry A, Cooper-DeHoff R, Handberg E, et al. Relationships between components of metabolic syndrome and coronary intravascular ultrasound (IVUS) atherosclerosis measures in women without obstructive coronary artery disease: the NHLBI-Sponsored Women’s Ischemia Syndrome (WISE). Study Journal of the American College of Cardiology. 2012;59(13 Supplement):E1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohandas R, Segal M, Srinivas TR, Johnson BD, Wen X, Handberg EM, et al. Mild renal dysfunction and long-term adverse outcomes in women with chest pain: results from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2015;169(3):412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohandas R, Segal MS, Huo T, Handberg EM, Petersen JW, Johnson BD, et al. Renal function and coronary microvascular dysfunction in women with symptoms/signs of ischemia. PLoS One. 2015;10(5):e0125374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed B, Bairey Merz CN, McClure C, Johnson BD, Reis SE, Bittner V, et al. Migraines, angiographic coronary artery disease and cardiovascular outcomes in women. Am J Med. 2006;119(8): 670–5. [DOI] [PubMed] [Google Scholar]
- 10.Rambarat CA, Elgendy IY, Johnson BD, Reis SE, Thompson DV, Sharaf BL, et al. Migraine headache and long-term cardiovascular outcomes: an extended follow-up of the Women’s Ischemia Syndrome Evaluation. Am J Med. 2017;130(6):738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutledge T, Kenkre TS, Bittner V, Krantz DS, Thompson DV, Linke SE, et al. Anxiety associations with cardiac symptoms, angiographic disease severity, and healthcare utilization: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation. Int J Cardiol. 2013;168(3):2335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutledge T, Vaccarino V, Johnson BD, Bittner V, Olson MB, Linke SE, et al. Depression and cardiovascular health care costs among women with suspected myocardial ischemia: prospective results from the WISE (Women’s Ischemia Syndrome Evaluation) Study. J Am Coll Cardiol. 2009;53(2):176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linke SE, Rutledge T, Johnson BD, Vaccarino V, Bittner V, Cornell CE, et al. Depressive symptom dimensions and cardiovascular prognosis among women with suspected myocardial ischemia: a report from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Arch Gen Psychiatry. 2009;66(5):499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whittaker KS, Krantz DS, Rutledge T, Johnson BD, Wawrzyniak AJ, Bittner V, et al. Combining psychosocial data to improve prediction of cardiovascular disease risk factors and events: the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation study. Psychosom Med. 2012;74(3):263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50(21):2044–50. [DOI] [PubMed] [Google Scholar]
- 16.Vaccarino V, McClure C, Johnson BD, Sheps DS, Bittner V, Rutledge T, et al. Depression, the metabolic syndrome and cardiovascular risk. Psychosom Med. 2008;70(1):40–8. [DOI] [PubMed] [Google Scholar]
- 17.Mehta PK, Hermel M, Nelson MD, Cook-Wiens G, Martin EA, Alkhoder AA, et al. Mental stress peripheral vascular reactivity is elevated in women with coronary vascular dysfunction: results from the NHLBI-sponsored Cardiac Autonomic Nervous System (CANS) study. Int J Cardiol. 2018;251:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krantz DS, Whittaker KS, Francis JL, Rutledge T, Johnson BD, Barrow G, et al. Psychotropic medication use and risk of adverse cardiovascular events in women with suspected coronary artery disease: outcomes from the Women’s Ischemia Syndrome Evaluation (WISE) study. Heart. 2009;95(23):1901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shufelt C, Braunstein GD, Pepine CJ, Bairey Merz CN. Recognizing sex similarities in cardiovascular disease research. J Am Coll Cardiol. 2015;65(19):2152–3. [DOI] [PubMed] [Google Scholar]
- 20.Shaw LJ, Merz CN, Bittner V, Kip K, Johnson BD, Reis SE, et al. Importance of socioeconomic status as a predictor of cardiovascular outcome and costs of care in women with suspected myocardial ischemia. Results from the National Institutes of Health, National Heart, Lung and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Womens Health (Larchmt). 2008;17(7):1081–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114(9):894–904. [DOI] [PubMed] [Google Scholar]
- 22.Rutledge T, Linke SE, Olson MB, Francis J, Johnson BD, Bittner V, et al. Social networks and incident stroke among women with suspected myocardial ischemia. Psychosom Med. 2008;70(3):282–7. [DOI] [PubMed] [Google Scholar]
- 23.Eastwood JA, Johnson BD, Rutledge T, Bittner V, Whittaker KS, Krantz DS, et al. Anginal symptoms, coronary artery disease, and adverse outcomes in black and white women: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. J Women’s Health (Larchmt). 2013;22(9):724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacanowski MA, Zineh I, Li H, Johnson BD, Cooper-DeHoff RM, Bittner V, et al. Adrenergic gene polymorphisms and cardiovascular risk in the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation. J Transl Med. 2008;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park K, Egelund E, Huo T, Merz CNB, Handberg EM, Johnson BD, et al. Serotonin transporter gene polymorphism in women with suspected ischemia: a report from the NHLBI-sponsored WISE. Gender and the Genome. 2018;2(1):8–15. [Google Scholar]
- 26.Weng L, Taylor KD, Chen YD, Sopko G, Kelsey SF, Bairey Merz CN, et al. Genetic loci associated with nonobstructive coronary artery disease in Caucasian women. Physiol Genomics. 2016;48(1): 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Q, Reis SE, Kammerer C, Craig W, McNamara DM, Holubkov R, et al. Association of anti-oxidized LDL and candidate genes with severity of coronary stenosis in the Women’s Ischemia Syndrome Evaluation study. J Lipid Res. 2011. ;52(4):801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Wu R, Lin M, Terra S, Pepine C, McGorray S, et al. Epistatic control of human obesity as revealed by linkage disequilibrium mapping: a report from the NHLBI-sponsored WISE study. Current Genomics - CURR GENOMICS. 2006;7:463–8. [Google Scholar]
- 29.Thurston RC, Johnson BD, Shufelt CL, Braunstein GD, Berga SL, Stanczyk FZ, et al. Menopausal symptoms and cardiovascular disease mortality in the Women’s Ischemia Syndrome Evaluation (WISE). Menopause. 2017;24(2):126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bairey Merz CN, Johnson BD, Sharaf BL, Bittner V, Berga SL, Braunstein GD, et al. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am CollCardiol. 2003;41(3): 413–9. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed B, Bairey Merz CN, Johnson BD, Bittner V, Berga SL, Braunstein GD, et al. Diabetes mellitus, hypothalamic hypoestrogenemia, and coronary artery disease in premenopausal women (from the National Heart, Lung, and Blood Institute sponsored WISE study). Am J Cardiol. 2008;102(2):150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merz CN, Johnson BD, Berga SL, Braunstein GD, Azziz R, Yang Y, et al. Total estrogen time and obstructive coronary disease in women: insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Women’s Health (Larchmt). 2009;18(9):1315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shufelt CL, Johnson BD, Berga SL, Braunstein GD, Reis SE, Bittner V, et al. Timing of hormone therapy, type of menopause, and coronary disease in women: data from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Menopause. 2011. ;18(9):943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merz CN, Johnson BD, Berga S, Braunstein G, Reis SE, Bittner V, et al. Past oral contraceptive use and angiographic coronary artery disease in postmenopausal women: data from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Fertil Steril. 2006;85(5):1425–31. [DOI] [PubMed] [Google Scholar]
- 35.Merz CNB, Olson MB, McClure C, Yang Y-C, Symons J, Sopko G, et al. A randomized controlled trial oflow-dose hormone therapy on myocardial ischemia in postmenopausal women with no obstructive coronary artery disease: results from the National Institutes of Health/National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). American Heart Journal. 2010;159(6):987.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shufelt C, Bretsky P, Almeida CM, Johnson BD, Shaw LJ, Azziz R, et al. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: results from the National Institutes of Health—National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women’s Ischemia Syndrome Evaluation (WISE). The Journal of Clinical Endocrinology & Metabolism. 2010;95(11): 4985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bairey Merz CN, Johnson BD, Braunstein GD, Pepine CJ, Reis SE, Paul-Labrador M, et al. Phytoestrogens and lipoproteins in women. The Journal of Clinical Endocrinology & Metabolism. 2006;91(6): 2209–13. [DOI] [PubMed] [Google Scholar]
- 38.Pepine CJ, von Mering GO, Kerensky RA, Johnson BD, McGorray SP, Kelsey SF, et al. Phytoestrogens and coronary microvascular function in women with suspected myocardial ischemia: a report from the Women’s Ischemia Syndrome Evaluation (WISE) study. J Women’s Health (Larchmt). 2007;16(4):481–8. [DOI] [PubMed] [Google Scholar]
- 39.Gierach GL, Johnson BD, Bairey Merz CN, Kelsey SF, Bittner V, Olson MB, et al. Hypertension, menopause, and coronary artery disease risk in the Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 2006;47(3 Suppl):S50–8. [DOI] [PubMed] [Google Scholar]
- 40.Baldassarre LA, Raman SV, Min JK, Mieres JH, Gulati M, Wenger NK, et al. Noninvasive imaging to evaluate women with stable ischemic heart disease. JACC Cardiovasc Imaging. 2016;9(4): 421–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wei J, Mehta PK, Shufelt C, Yang Y, Gill E, Kahlon R, et al. Diastolic dysfunction measured by cardiac magnetic resonance imaging in women with signs and symptoms of ischemia but no obstructive coronary artery disease. Int J Cardiol. 2016;220:775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doyle M, Pohost GM, Bairey Merz CN, Farah V, Shaw LJ, Sopko G, et al. Aortic flow conditions predict ejection efficiency in the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). Cardiovasc Diagn Ther. 2017;7(3):288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doyle M, Weinberg N, Pohost GM, Bairey Merz CN, Shaw LJ, Sopko G, et al. Prognostic value ofglobal MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Imaging. 2010;3(10):1030–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landes S, Dela Cruz S, Wei J, AlBadri A, Shufelt C, Mehta P, et al. Cold pressor stress cardiac magnetic resonance myocardial flow reserve is not useful for detection of coronary endothelial dysfunction in women with signs and symptoms of ischemia and no obstructive CAD. PLoS One. 2017;12(1):e0169818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakir M, Wei J, Thomson L, Petersen J, Li Q, Jones E, et al. Prevalence of myocardial scar in women with signs and symptoms of ischemia but no obstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation. J Am Coll Cardiol. 2015;65(10):A1580. [Google Scholar]
- 46.Doyle M, Pohost GM, Merz CN, Shaw LJ, Sopko G, Rogers WJ, et al. Improved diagnosis and prognosis using decisions informed by combining entities (DICE): results from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). Cardiovasc Diagn Ther. 2013;3(4):216–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doyle M, Weinberg N, Pohost GM, Merz CN, Shaw LJ, Sopko G, et al. Left ventricular energy model predicts adverse events in women with suspected myocardial ischemia: results from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Cardiovasc Diagn Ther. 2013;3(2):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bravo PE, Di Carli MF, Dorbala S. Role of PET to evaluate coronary microvascular dysfunction in non-ischemic cardiomyopathies. Heart Fail Rev. 2017;22(4):455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marroquin OC, Holubkov R, Edmundowicz D, Rickens C, Pohost G, Buchthal S, et al. Heterogeneity of microvascular dysfunction in women with chest pain not attributable to coronary artery disease: implications for clinical practice. Am Heart J. 2003;145(4):628–35. [DOI] [PubMed] [Google Scholar]
- 50.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3(6): 623–40. [DOI] [PubMed] [Google Scholar]
- 51.Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Interv Cardiol. 2010;23(6):511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quesada O, Ahmed A, Cantu S, Wei J, Azarbal B, Merz CNB. Use of intravascular ultrasound and optimal coherence tomography for etiological diagnosis in a woman presenting with St-segment elevation myocardial infarction, apical wall motion abnormality and non-obstructive coronary artery disease. Journal of the American College of Cardiology. 2018;71(11):2204. [Google Scholar]
- 53.Petersen JW, Johnson BD, Kip KE, Anderson RD, Handberg EM, Sharaf B, et al. TIMI frame count and adverse events in women with no obstructive coronary disease: a pilot study from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). PLoS One. 2014;9(5):e96630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharaf B, Wood T, Shaw L, Johnson BD, Kelsey S, Anderson RD, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166(1):134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139(18):e891–908. [DOI] [PubMed] [Google Scholar]
- 56.Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5(6):646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaya M, Mehta P, Mohammadi AH, Agarwal M, Sedlak T, Johnson BD, et al. Microvascular coronary dysfunction, left ventricular volumes and mass: results from the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction Study. Journal of the American College of Cardiology. 2013;61(10): E1442–E. [Google Scholar]
- 58.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55(25):2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols WW, Denardo SJ, Davidson JB, Huo T, Bairey Merz CN, Pepine CJ. Association of aortic stiffness and wave reflections with coronary flow reserve in women without obstructive coronary artery disease: an ancillary study from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2015;170(6):1243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nichols WW, Denardo SJ, Johnson BD, Sharaf BL, Bairey Merz CN, Pepine CJ. Increased wave reflection and ejection duration in women with chest pain and nonobstructive coronary artery disease: ancillary study from the Women’s Ischemia Syndrome Evaluation. J Hypertens. 2013;31 (7) :1447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson RD, Sizemore BC, Barrow GM, Johnson BD, Merz CN, Sopko G, et al. Pulse pressure and adverse outcomes in women: a report from the Women’s Ischemia Syndrome Evaluation (WISE). Am J Hypertens. 2008;21(11):1224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3): 407–77. [DOI] [PubMed] [Google Scholar]
- 63.AlBadri A, Lai K, Wei J, Landes S, Mehta PK, Li Q, et al. Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: a report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). PLoS One. 2017;12(5):e0177684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arant CB, Wessel TR, Ridker PM, Olson MB, Reis SE, Delia Johnson B, et al. Multimarker approach predicts adverse cardiovascular events in women evaluated for suspected ischemia: results from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Clin Cardiol. 2009;32(5):244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mekonnen G, Hayek SS, Mehta PK, Li Q, Mahar E, Mou L, et al. Circulating progenitor cells and coronary microvascular dysfunction: results from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation - Coronary Vascular Dysfunction Study (WISE-CVD). Atherosclerosis. 2016;253:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bittner V, Johnson BD, Zineh I, Rogers WJ, Vido D, Marroquin OC, et al. The triglyceride/high-density lipoprotein cholesterol ratio predicts all-cause mortality in women with suspected myocardial ischemia: a report from the Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2009;157(3):548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones E, Wei J, Mehta P, Shufelt C, Minissian M, Pepine C, et al. B-type natriuretic peptide does not correlate with invasive or noninvasive measures of coronary microvascular dysfunction in women with preserved ejection fraction: a report from the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction (Wise-Cvd) Study. Journal of the American College of Cardiology. 2014;63(12):A1411–A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elgendy IY, Mansoor H, Li Q, Guo Y, Handberg EM, Bairey Merz CN, et al. Long-term mortality and estimated functional capacity among women with symptoms of ischemic heart disease: from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation. Am Heart J. 2018;206:123–6. [DOI] [PubMed] [Google Scholar]
- 69.Leong DW, Zarrini P, Cook-Wiens G, Elgendy I, Reis SE, Handberg EM, et al. Abstract 13301: Decreased functional capacity using the Duke Activity Status Index (DASI) predicts development of heart failure in women with ischemia and no obstructive coronary artery disease (INOCA): results from the Women’s Ischemia Syndrome Evaluation (WISE) long-term follow-up. Circulation. 2018;138(Suppl_1):A13301–A. [Google Scholar]
- 70.Shaw LJ, Olson MB, Kip K, Kelsey SF, Johnson BD, Mark DB, et al. The value of estimated functional capacity in estimating outcome: results from the NHBLI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 2006;47(3 Suppl):S36–43. [DOI] [PubMed] [Google Scholar]
- 71.Handberg E, Johnson BD, Arant CB, Wessel TR, Kerensky RA, von Mering G, et al. Impaired coronary vascular reactivity and functional capacity in women: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 2006;47(3 Suppl):S44–9. [DOI] [PubMed] [Google Scholar]
- 72.Linke SE, Rutledge T, Johnson BD, Olson MB, Bittner V, Cornell CE, et al. The joint impact of smoking and exercise capacity on clinical outcomes among women with suspected myocardial ischemia: the WISE study. J Women’s Health (Larchmt). 2009;18(4): 443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Birkeland K, Khandwalla RM, Kedan I, Shufelt CL, Mehta PK, Minissian MB, et al. Daily activity measured with wearable technology as a novel measurement of treatment effect in patients with coronary microvascular dysfunction: substudy of a randomized controlled crossover trial. JMIR Res Protoc. 2017;6(12):e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merz CNB, Pepine CJ, Walsh MN, Fleg JL, Camici PG, Chilian WM, et al. Ischemia and no obstructive coronary artery disease (INOCA). Circulation. 2017;135(11):1075–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J. 2011. ;162(4): 678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bavry AA, Handberg EM, Huo T, Lerman A, Quyyumi AA, Shufelt C, et al. Aldosterone inhibition and coronary endothelial function in women without obstructive coronary artery disease: an ancillary study of the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Am Heart J. 2014;167(6):826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Denardo SJ, Wen X, Handberg EM, Bairey Merz CN, Sopko GS, Cooper-DeHoff RM, et al. Effect of phosphodiesterase type 5 inhibition on microvascular coronary dysfunction in women: a Women’s Ischemia Syndrome Evaluation (WISE) ancillary study. Clin Cardiol. 2011;34(8):483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bairey Merz CN, Handberg EM, Shufelt CL, Mehta PK, Minissian MB, Wei J, et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. 2016;37(19):1504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rambarat CA, Elgendy IY, Handberg EM, Bairey Merz CN, Wei J, Minissian MB, et al. Late sodium channel blockade improves angina and myocardial perfusion in patients with severe coronary microvascular dysfunction: Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction ancillary study. Int J Cardiol. 2019;276:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kayikcioglu M, Payzin S, Yavuzgil O, Kultursay H, Can LH, Soydan I. Benefits of statin treatment in cardiac syndrome-X1. Eur Heart J. 2003;24(22):1999–2005. [DOI] [PubMed] [Google Scholar]
- 81.Haas MJ, Horani MH, Parseghian SA, Mooradian AD. Statins pre-vent dextrose-induced endothelial barrier dysfunction, possibly through inhibition of superoxide formation. Diabetes. 2006;55(2): 474–9. [DOI] [PubMed] [Google Scholar]
- 82.Holowatz LA, Santhanam L, Webb A, Berkowitz DE, Kenney WL. Oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolaemic humans. J Physiol. 2011;589(Pt 8):2093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 2017;38(3):143–53. [DOI] [PubMed] [Google Scholar]


