Abstract
The current outbreak of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in China firstly. A rapid, highly sensitive, specific, and simple operational method was needed for the detection of SARS-CoV-2. Here, we established a real-time reverse-transcription recombinase-aided amplification assay (RT-RAA) to detect SARS-CoV-2 rapidly. The primers and probe were designed based on the nucleocapsid protein gene (N gene) sequence of SARS-CoV-2. The detection limit was 10 copies per reaction in this assay, which could be conducted within 15 min at a constant temperature (39 °C), without any cross-reactions with other respiratory tract pathogens, such as other coronaviruses. Furthermore, compared with commercial real-time RT-PCR assay, it showed a kappa value of 0.959 (p < 0.001) from 150 clinical specimens. These results indicated that this real-time RT-RAA assay may be a valuable tool for detecting SARS-CoV-2.
Keywords: SARS-CoV-2, Nucleocapsid protein, Real-time RT-RAA, Real-time RT-PCR
1. Introduction
COVID-19, caused by SARS-CoV-2, was first reported in several cases with unexplained pneumonia from Wuhan, China, in late December 2019. SARS-CoV-2 is the seventh member from coronaviruses family that infects humans. The remaining six coronaviruses are HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, SARS-CoV and MERS-CoV (Lu et al., 2020; Zhu et al., 2020). The spread of SARS-CoV-2 is more widely and quickly than initially expected. (Chen et al., 2020; Corman et al., 2020; Guo et al., 2019; Lu et al., 2020; She et al., 2020; Zhu et al., 2020). As of Feb 24, 2020, a total of 77,262 COVID-19 cases have been confirmed in mainland China with 2595 deaths, and 2069 cases outside China with 23 deaths (WHO, 2020). Rapid and simple diagnosis of SARS-CoV-2 will undoubtedly help to control the spread of the virus. Real-time RT-PCR assay rendered a specific and sensitive tool for detecting SARS-CoV-2 (Corman et al., 2020). However, its field application was impeded in resource-limited settings due to the potential contamination and the requirement of expensive equipments.
Recombinase-aided amplification (RAA) is a new isothermal amplification technology, requiring no classical thermostable enzyme. RAA has been widely used to detect microbial pathogens (Chen et al., 2018; Piepenburg et al., 2006; Zhang et al., 2017). The reaction, containing a recombinase UvsX obtained from Escherichia coli and the single strand DNA-binding protein (SSB) used in a polymerase chain reaction (PCR), can form single-stranded DNA without heating and can be finished within 30 min at a constant temperature of 39 °C. Together with reverse transcriptase and fluorescent probe system, RAA assay can also be used for the real-time detection of RNA amplicons (Shen et al., 2019; Wang et al., 2019; Yan et al., 2018).
The aim of this study was to develop a real time RT-RAA assay for the rapid and simple detection of SARS-CoV-2 and to evaluate its performance in clinical specimens.
2. Material and methods
2.1. Viral isolates and clinical specimens
The strain of SARS-CoV-2 preserved in our lab was isolated from COVID-19 patient's sample using Vero E6 cells. Pharyngeal swabs were obtained from 150 patients with acute respiratory infection within 10 days after the onset of clinical symptoms from 2019 to 2020, and stored at −70 °C before use. Other clinical specimens positive for the pathogens influenza A, influenza B, HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, respiratory syncytial virus, parainfluenza virus, rhinovirus, and human metapneumovirus, as identified by pathogen specific real-time PCR in our institute were used to evaluate the specificity of RT-RAA assay.
2.2. Primer and probe design
The nucleocapsid protein gene (N gene) of SARS-CoV-2 downloaded from the Global Initiative on Sharing All Influenza Data (GISAID) was chosen as the target region. Oligonucleotide primers and probes (Sangon Biotech, China) used in the real-time RT-RAA assays were manually designed based on the sequence of N gene (Table 1 ).
Table 1.
Sequences of primers and probe for RT-RAA Assay for SARS-CoV-2.
| Primer/Probes | Sequence (5′-3′) | Genome positiona |
|---|---|---|
| SARS-CoV-2-F | CAGTTCAAGAAATTCAACTCCAGGCAGCAGTAG | 28,849-28881 |
| SARS-CoV-2-R SARS-CoV-2-P |
CAGTTTGGCCTTGTTGTTGTTGGCCTTTAC | 29,009-28980 |
| CAGACATTTTGCTCTCAAGCTGGTTCAATC [FAM-dT](THF)[BHQ-dT]CAAGCAGCAGCAAAG (C3-spacer) | 28,979-28932 | |
Note. Probe modifications: FAM: 6-Carboxyfluorescein; THF: tetrahydrofuran; BHQ: black hole quencher; C3 spacer: 3′ phosphate blocker.
The genome position refers to the nucleocapsid protein gene (N gene) from BetaCoV/Wuhan/WIV04/2019 (EPI_ISL_402,124).
2.3. Production of a standard control
Targeted N gene fragments of SARS-CoV-2 were amplified by the primers same as used in the real-time RT-RAA assay and then cloned into PUC57 vector (Sangon Biotech, China). The concentration of this plasmid was measured after extraction (MiniBEST Plasmid Purification Kit TaKaRa, China). The expected copy number of N gene was calculated according to a previous study (Min et al., 2006).
2.4. RNA extraction
Total RNA from strain and samples were extracted using a Virus RNA/DNA Extraction Kit (Tianlong, China) according to the manufacturer's instruction, and stored at −70 °C.
2.5. Real-time RT-RAA assay
The real-time RT-RAA assay was performed with an RT exo kit (Qitian Bio-technology, China), containing all the enzymes for the reverse transcription and DNA amplification. The reaction mixture contained 12.7 μL of diethyl pyrocarbonate (DEPC) H2O, 25 μL of buffer, 2.1 μL of each primer (10 μM), and 0.6 μL of probe (10 μM), finally 42.5 μL solution was transferred to a tube containing the RT-RAA lyophilized enzyme mixture (SSB, 800 ng/μL; UvsX, 120 ng/μL; DNA polymerase, 30 ng/μL), mixed gently, 5 μL of RNA template was added to the reaction mixture, and 2.5 μL of magnesium acetate (280 mM) was pipetted into the tube lids. The tube lids were closed carefully, immediately transferred to a constant temperature device QT-B6100 (Qitian Bio-technology, China), vortexed briefly, the magnesium acetate was spun down into the reaction mixture to trigger the RT-RAA reaction at 39 °C for 7 min pre-amplification. Then the tubes were transferred to a RAA fluorescence detection device QT-F1620 (Qitian Bio-technology, China) at 39 °C for 20 min. Nuclease-free water was used as a negative control.
2.6. Sensitivity and specificity of real-time RT-RAA
Serial dilutions of the plasmid standards, ranging from 1 × 101–1 × 105 copies/reaction, were used to calculate sensitivity of real-time RT-RAA. The specificity of the aasay was evaluated by testing clinical specimens positive for influenza A, influenza B, HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, respiratory syncytial virus, parainfluenza virus, rhinovirus, and human metapneumovirus.
2.7. Clinical specimen analysis
To evaluate the performance of the real-time RT-RAA assay among clinical specimens, all of the 150 pharyngeal swab specimens were subjected to the parallel analysis with a real-time RT-PCR assay (BioGem, China). The reaction mixture of the real-time RT-PCR assay contained 12 μL of qRT-PCR Buffer, 4 μL of qRT-PCR Enzyme Mix, 4 μL of primer/probe Mix, and 5 μL template, and was performed on 7900 Real-Time PCR system (ABI,USA) according to the manufacturer's instructions follows: a 50 °C for 10 min for reverse transcription, a 5 min for pre-denaturation at 95 °C followed by 40 amplification cycles of 10 s at 95 °C and 40 s at 55 °C each (annealing-extension step).
2.8. Statistical analysis
The kappa and p values were calculated by using SPSS 19.0.
3. Results
3.1. Analytical sensitivity of the real-time RT-RAA assay
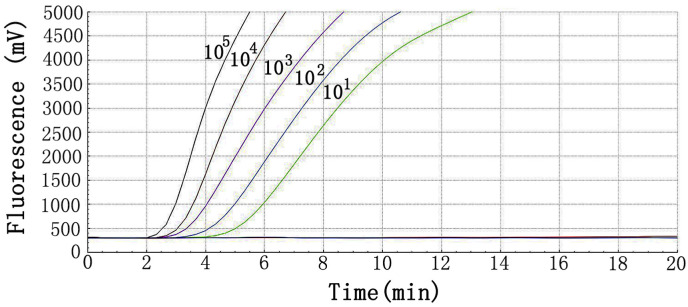
The sensitivity of RT-RAA assay for SARS-CoV-2 was determined using a panel of serially diluted recombinant plasmid ranging from 101 to 105 copies per reaction. The repeatability of the method was evaluated by testing each dilution 8 times (Table 2 ). The minimum detection limit of real-time RAA assay was 10 copies/reaction. As presented in Fig. 1 , the reactions with higher concentration of the virus target plasmid showed earlier peaks.
Table 2.
The reproducibility of real-time RT-RAA with a dilution series of plasmid DNA.
| Concentration | No. of replicates tested | No. of detection | Means Tt value (min) | Detection rate (%) |
|---|---|---|---|---|
| 105 | 8 | 8 | 2 | 100 |
| 104 | 8 | 8 | 2.33 | 100 |
| 103 | 8 | 8 | 2.33 | 100 |
| 102 | 8 | 8 | 3 | 100 |
| 101 | 8 | 8 | 4 | 100 |
Tt: time threshold.
Fig. 1.
Amplification plots of real-time RT-RAA for 10-fold serial dilutions of plasmid.
3.2. Analytical specificity of real-time RT-RAA assay
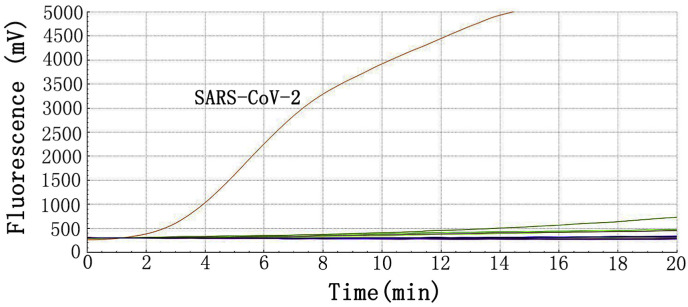
The specificity of RT-RAA assay was evaluated by samples positive for other pathogens that could cause similar respiratory symptoms. Only the extracts from SARS-CoV-2 strain and COVID-19 patient's samples showed positive reactions. And all negative controls and extracts from other pathogens samples were negative in the real-time RT-RAA assays (Fig. 2 ). Thus, it demonstrated high specificity for the target gene of SARS-CoV-2. These results indicate that the analytical specificity of the real-time RT-RAA assay was 100%.
Fig. 2.
Specificity of the real-time RT-RAA assay for SARS-Cov-2.
3.3. Comparison of real-time RT-RAA assay and real-time RT-PCR
As showed in Table 3 , of all 150 samples, 68 pharyngeal swabs had a positive amplification by real-time RT-PCR assay with a Ct value ranging from 21.0 to 36.5. Of the 68 positive samples in real-time PCR, 65 showed positive results with the real-time RT-RAA assay (Table S1), resulting in a sensitivity of 95.6%. Of the 82 samples without amplification in real-time RT-PCR, all were also showed negative result by the real-time RT-RAA assay, providing a specificity of 100%. Compared with the real-time RT-PCR, the kappa value of this assay was 0.959 (p < 0.001). No significant difference between them was observed.
Table 3.
Performance of real-time RAA assay in comparison to the reference method, real-time RT-PCR, for detecting SARS-CoV-2 in clinical samples.
| Real-time PCR |
Performance characteristics |
|||||
|---|---|---|---|---|---|---|
| Positive | Negative | Sensitivity (%) | Specificity (%) | Kappa | ||
| Real-time RAA | Positive | 65 | 0 | 95.6 | 100 | 0.959 |
| Negative | 3 | 82 | ||||
| Total | 68 | 82 | ||||
4. Discussion
Although viral isolation was time-consuming and dependent on skilled technician, it is still the gold standard for SARS-CoV-2 identification (NHC, 2020). Molecular techniques have been used successfully to identify infectious agents for many years. Real-time RT-PCR and high-throughput sequencing are currently main tools for the identification of SARS-CoV-2 in China (Armstrong et al., 2019; Palacios et al., 2008). Recently, nucleic acid isothermal amplification (NAIA) has developed rapidly. RAA is easier and cheaper in comparison to other isothermal amplification techniques, such as rolling circle amplification (RCA), nucleic acid sequence-based amplification (NASBA), loop-mediated isothermal amplification (LAMP), and single primer isothermal amplification (SPIA). Similarly with recombinase polymerase amplification (RPA), but use the different recombinase which comes from bacteria or fungi, both of them can react at lower temperature.
In the present study, a novel molecular method was established to detect N gene of SARS-CoV-2 by using real-time RT-RAA. Since RT-RAA assay can be conducted at 39 °C, and scanned by a portable real-time fluorescence device without thermal cycler. It is useful for molecular diagnosis of SARS-CoV-2 infection, especially in low-equipment setting. The highly sensitive molecular diagnostic method for SARS-CoV-2 in the study had a limit of 10 copies per reaction. Results of clinical sample analysis showed that 3 of the 68 real-time RT-PCR-positive samples were negative as detected by RT-RAA. This might due to the viral load in these samples is below the detection limit of RT-RAA (the Ct value were >35 tested by real-time RT-PCR), or the viral genomes in these samples might mutate at the position of RAA primers or probe. If more clinical samples were included in this study, there might also be samples positive for RT-RAA but negative for real-time RT-PCR. Indeed, a multiple-centre clinical evaluation of another RT-RAA assay targeting the ORF1ab gene of SARS-CoV-2 we established showed there were some samples positive by real-time RT-PCR but missed by RT-RAA, and some samples positive by RT-RAA but missed by real-time RT-PCR (Wang et al., 2020).
The longer primers (>30 base oligonucleotides) and probes (>45 base oligonucleotides) enhanced the efficient recombinase-DNA complex and the specificity of the RAA assay, without any cross-reaction with other respiratory pathogens, including coronaviruses. Due to simultaneous chain lysis and amplification, million copies of target gene can be amplified within 20 min. All specimens could be correctly diagnosis within 8 min. If the 7 min pre-amplification was included, the results could be acquired within 15 min. In addition, all the enzymes was included in the lyophilized pellets and added into a tube, which can be closed for fast, easy transportation (without cold chain) and operation to minimize the contamination (Wang et al., 2019).
However, this study has a few limitations. Only the single N gene of SARS-CoV-2 is targeted, studies with larger sample size are needed to verify the accuracy of the established assay. Additionally, to evaluate the sensitivity of the RT-RAA assay, we used serially diluted recombinant plasmid but not RNA standard which might better reflect the detection sensitivity of the assay.
In summary, a rapid, highly sensitive, specific, and simply operational real-time RT-RAA method was developed to detect the N gene of SARS-CoV-2. This assay may be useful in low-resource settings.
Author Contributions
Tao Wu: participated in the design and conceived of the study, written-original draft, written- review & editing. Yiyue Ge: funding acquisition, written-review & editing. Kangchen Zhao, Xiaojuan Zhu, Yin Chen: collected the samples and performed the experiments. Bin Wu: funding acquisition, participated in the statistical analysis. Fengcai Zhu: project administration, supervision. Baoli Zhu: project administration, supervision. Lunbiao Cui: funding acquisition, project administration, helped to draft the manuscript, written-review & editing
Declaration of competing interest
None.
Acknowledgements
This study was funded by the National Major Science & Technology Projects for Infectious Disease Control and Prevention (2017ZX10103008, 2017ZX10302301), National Natural Science Foundation of China (81871666, 31700035), Natural Science Foundation of Jiangsu Province (BK20191489), Jiangsu Provincial Key Medical Discipline of Epidemiology (ZDXKA2016008), and the Key Research and Development Project of Jiangsu Province (BE2019761).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virol.2020.07.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Armstrong G.L., Maccannell D., Taylor J., Carleton H.A., Neuhaus E.B., Bradbury R.S., Posey J.E., Gwinn M. Pathogen genomics in public health. N. Engl. J. Med. 2019;381:2569–2580. doi: 10.1056/NEJMsr1813907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Li X., Li G., Zhao L., Duan S., Yan T., Feng Z., Ma X. Use of a rapid reverse-transcription recombinase aided amplification assay for respiratory syncytial virus detection. Diagn. Microbiol. Infect. Dis. 2018;90:90–95. doi: 10.1016/j.diagmicrobio.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brunink S., Schneider J., Schmidt M.L. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Wei D., Zhang X., Wu Y., Li Q., Zhou M., Qu J. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B.S., Noh Y.J., Shin J.H., Baek S.Y., Min K.I., Ryu S.R., Kim B.G., Park M.K., Choi S.E., Yang E.H. Assessment of the quantitative real-time polymerase chain reaction using a cDNA standard for human group A rotavirus. J. Virol Methods. 2006;137:280–286. doi: 10.1016/j.jviromet.2006.06.028. [DOI] [PubMed] [Google Scholar]
- NHC . 2020. Notice on Novel Coronavirus Pneumonia Diagnosis and Treatment Plan (Trial Version Fifth Revision) [Google Scholar]
- Palacios G., Druce J., Du L., Tran T., Birch C., Briese T., Conlan S., Quan P., Hui J., Marshall J.C. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 2008;358:991–998. doi: 10.1056/NEJMoa073785. [DOI] [PubMed] [Google Scholar]
- Piepenburg O., Williams C.H., Stemple D.L., Armes N.A. DNA detection using recombination proteins. PLoS Biol. 2006;4:1115–1121. doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She J., Jiang J., Ye L., Hu L., Bai C., Song Y. 2019 novel coronavirus of pneumonia in Wuhan, China: emerging attack and management strategies. Clin. Transl. Med. 2020;9:19. doi: 10.1186/s40169-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Qiu F., Shen L., Yan T., Zhao M., Qi J., Chen C., Zhao L., Wang L., Feng Z. A rapid and sensitive recombinase aided amplification assay to detect hepatitis B virus without DNA extraction. BMC Infect. Dis. 2019;19:229. doi: 10.1186/s12879-019-3814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Cai K., He X., Shen X., Wang J., Liu J., Xu J., Qiu F., Lei W., Cui L., Ge Y., Wu T., Zhang Y., Yan H., Chen Y., Yu J., Ma X., Shi H., Zhang R., Li X., Gao Y., Niu P., Tan W., Wu G., Jiang Y., Xu W., Ma X. the official publication of the European Society of Clinical Microbiology and Infectious Diseases; 2020. Multiple-centre Clinical Evaluation of an Ultrafast Single-Tube Assay for SARS-CoV-2 RNA. Clinical Microbiology and Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.Y., Fan L.I., Shen X.X., Fu S.H., Ying H.E., Lei W.W., Liang G.D., Wang H.Y., Ma X.J. A reverse-transcription recombinase-aided amplification assay for the rapid detection of the far-eastern subtype of tick-borne encephalitis virus. Biomed. Environ. Sci. 2019;32:357–362. doi: 10.3967/bes2019.047. [DOI] [PubMed] [Google Scholar]
- Who . 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 35. [Google Scholar]
- Yan T., Li X., Wang L., Chen C., Duan S., Qi J., Li L., Ma X. Development of a reverse transcription recombinase-aided amplification assay for the detection of coxsackievirus A10 and coxsackievirus A6 RNA. Arch. Virol. 2018;163:1455–1461. doi: 10.1007/s00705-018-3734-9. [DOI] [PubMed] [Google Scholar]
- Zhang X., Guo L., Ma R., Cong L., Wu Z., Wei Y., Xue S., Zheng W., Tang S. Rapid detection of Salmonella with recombinase aided amplification. J. Microbiol. Methods. 2017;139:202–204. doi: 10.1016/j.mimet.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.