Abstract
Background and Purpose
Comparative effectiveness and safety of oral anticoagulants (OAC) in patients with atrial fibrillation (AF) and high polypharmacy are unknown.
Methods
We used Medicare administrative data to evaluate patients with new AF diagnosis from 2015 to 2017, who initiated an OAC within 90 days of diagnosis. Patients taking ≤3, 4 to 7, or ≥8 other prescription medications were categorized as having low, moderate, or high polypharmacy, respectively. Within polypharmacy categories, patients receiving apixaban 5 mg twice daily, rivaroxaban 20 mg once daily, or warfarin were matched using a 3-way propensity score matching. Study outcomes included Ischemic stroke, bleeding, and all-cause mortality.
Results
The study cohort included 6985 patients using apixaban, 3838 using rivaroxaban, and 6639 using warfarin. In the propensity-matched cohorts there was no difference in risk of ischemic stroke between the three drugs in patients with low and moderate polypharmacy. However, among patients with high polypharmacy, the risk of ischemic stroke was higher with apixaban compared to warfarin (adjusted hazard ratio [aHR] 2.34, 95% CI 1.01–5.42; p=0.05), and similar to rivaroxaban (aHR 1.38, 95% CI 0.67–2.84; p=0.4). There was no difference in risk of death between the three drugs in patients with low and moderate polypharmacy, but apixaban was associated withhigher risk of death compared to rivaroxaban (aHR 2.03, 95% CI 1.01–4.08; p=0.05) in the high polypharmacy group. Apixaban had lower bleeding risk compared to warfarin in the low polypharmacy group (aHR 0.54, 95%CI 0.32–0.90; p=0.02), but there was no difference in bleeding between the three drugs in the moderate and high polypharmacy groups.
Conclusion
Our study suggests that among patients with significant polypharmacy (>8 drugs) there may be a higher stroke and mortality risk with apixaban compared to warfarin and rivaroxaban. However, differences were of borderline significance.
Keywords: Atrial fibrillation, Polypharmacy, Anticoagulation
Introduction
Atrial fibrillation (AF) is the most common arrythmia in elderly patients in the United States 1. There is a high prevalence of multimorbidity and polypharmacy in AF patients, which is associated with worse clinical outcomes.2, 3 Apixaban and rivaroxaban were approved after the two landmark trials “Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation” (ARISTOTLE) and “The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation” (ROCKET AF) trials.4, 5 However, use of a strong CYP3A4 and P-glycoproteins (PGP) inhibitor or inducer was a key exclusion criterion for both trials.4, 5 Most of the published evidence of apixaban or rivaroxaban efficacy and safety in patients with significant polypharmacy has been from the original clinical trials, or subsequent subgroup analysis.6–9
The aim of this study was to assess the comparative effectiveness and safety of apixaban, rivaroxaban and warfarin in patients from the U.S. Medicare population with varying degrees of polypharmacy.
Methods
Data used for the study are covered under a data use agreement with the Centers for Medicare and Medicaid Services (CMS) and are not available for distribution by the authors but may be obtained from CMS with an approved data use agreement. Requests for analytic SAS code may be sent to the corresponding author.
Study cohort
The study cohort was derived from the Centers for Medicare & Medicaid Services (CMS) administrative data 5% sample for 2015 through 2017. The 5% sample includes a representative rolling cohort of Medicare beneficiaries. The following files from the 5% sample were utilized: The Beneficiary Summary File Base was used to identify patient demographic data including age, sex and race, and to identify beneficiary enrollment dates and death dates. The Pharmacy Drug Event (Part D) Files were used to identify dates of pharmacy claims for the study drugs as well as indicators of polypharmacy. The Inpatient Medicare Provider Analysis and Review (MedPAR Part A) and Carrier (Part B) Standard Analytic Files were used to identify baseline comorbidities and study outcomes. We identified 51,693 Medicare beneficiaries age 65 and older with a new diagnosis of AF during January 2015 through September 2017 who were enrolled in the Centers for Medicare & Medicaid Services Part D prescription drug plan, and initiated apixaban (5 mg or 2.5 mg) twice daily, rivaroxaban (20 mg or 15 mg) once daily, or warfarin within 90 after the AF diagnosis, and had no prior OAC use during the preceding 12 months.
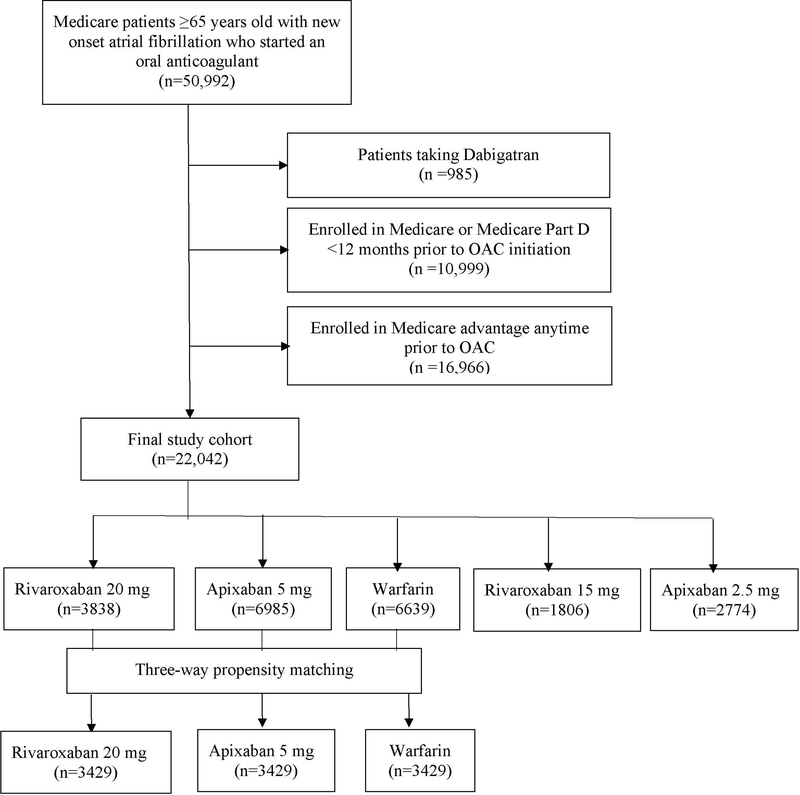
Figure 1 shows the study cohort flowchart. We excluded patients taking dabigatran due to small number (n=985). We also excluded patients who were enrolled in Medicare Part D for less than 12 months prior to OAC initiation and who were enrolled in Medicare Advantage at any time during the year preceding the study date resulting in a final cohort of 22,042. We analyzed patients taking standard dose (apixaban 5 mg twice daily or rivaroxaban 20 mg daily) or reduced dose (apixaban 2.5 mg twice daily or rivaroxaban 15 mg daily) separately. This study followed the International Society for Pharmacoeconomics and Outcomes Research reporting guidelines for comparative effective research. The institutional review board of the University of Iowa approved this study with waiver for individual informed consent.
Figure 1:
Flowchart for the study population.
Polypharmacy was defined as the number of unique generic ingredients identified in Part D claims during the year prior to OAC initiation. The study cohort was classified into three groups based on degree of polypharmacy: low polypharmacy (3 or fewer generic ingredients); moderate polypharmacy (4–8 generic ingredients), and high polypharmacy (9 or more).
Study Outcomes
The primary outcome of this study was admission to an acute care inpatient setting with a primary diagnosis of ischemic stroke during follow-up. Secondary outcomes were all-cause mortality and acute inpatient admissions with a primary diagnosis of bleeding. All outcomes were specified prior to the initiation of the study. Patients were censored if they stopped taking the initial OAC (based on Part D refill data), died, or at the end of study (December 31, 2017).
Statistical Analysis
Patient characteristics were reported as mean with standard deviation or median with interquartile range for continuous variables, or as percentages for categorical variables, in each subgroup of the three OAC based on degree of polypharmacy (low, moderate and high). We reported the study outcomes as crude rates per 100 patient-years of follow-up in each subgroup. We then used the 3-way propensity matching method described by Rassen et al 10, to create equivalent groups of patients receiving apixaban, rivaroxaban, or warfarin that were balanced with respect to patient covariates. The propensity matching was done in the standard dose and the reduced dose groups separately. The matched cohorts were comprised of patients with similar observed characteristics overall and roughly equal likelihood of receiving each drug and were therefore plausible candidates for all 3 anticoagulants under study. Matching was conducted separately for patients with low, moderate, or high polypharmacy. Success of each matching algorithm was evaluated by comparing standardized differences in patient demographic characteristics, comorbidity, medication use, health services history and a frailty score which was validated in administrative databases 11, between each drug in the matched samples (Supplemental Tables I&II). Standardized differences less than 10% were considered to be robust matching.
We used multivariable Cox proportional hazards regression models to evaluate the relative hazard of each study outcome by drug. Models were generated within each polypharmacy category on the matched samples. To further control for possible differences between patients receiving alternative anticoagulants, we also adjusted for patient demographics, comorbidities, prior health services utilization, and concurrent use of specific medications including angiotensin receptor blockers, angiotensin converting enzyme inhibitors (ARB/ACEI), mineralocorticoid antagonists (MRA), beta blockers, calcium channel blockers, diuretics, insulin, oral diabetic medications, digoxin, non-steroid anti-inflammatory drugs, proton pump inhibitors, statins, antiarrhythmics, antiplatelets and selective serotonin reuptake inhibitors (SSRI). Variables were selected for final inclusion in Cox models based on relationship to the outcome, using a statistical criterion of <0.05. Models also included indicators for the type of OAC, in order to estimate the relative hazard of each outcome in patients taking apixaban (relative to warfarin), rivaroxaban (relative to warfarin), and apixaban (relative to rivaroxaban).
Data analysis was performed with SAS version 9.4 (SAS Institute, Cary, North Carolina). Two-sided P <0.05 was considered statistically significant.
Results
The study cohort included 22,042 patients with new diagnosis of AF who initiated an OAC within 90 days. In the standard dosing group, prior to matching, 3838, 6985, and 6639 patients, were treated with rivaroxaban, apixaban and warfarin respectively. Supplemental Table III shows baseline characteristics, including demographics, comorbidities, medications, and healthcare utilization, for the three study groups before propensity score matching, categorized by polypharmacy burden. On average, rivaroxaban users were younger than apixaban and warfarin users. The prevalence of most comorbidities, including heart failure, coronary artery disease, diabetes, hypertension, liver, kidney and lung disease, increased with higher grades of polypharmacy in the three groups compared to lower grades of polypharmacy. In each polypharmacy category, warfarin users had the highest prevalence of most comorbidities.
Crude rates of study outcomes
Supplemental Table IV shows unadjusted rates of study outcomes in the three study groups. Unadjusted rates of all study outcomes were highest in the moderate polypharmacy group compared to the low and high polypharmacy. Overall, prior to matching, there were 0.93, 0.60 and 1.09 ischemic stroke events per 100 patient-years in apixaban, rivaroxaban, and warfarin users, respectively, and 1.76, 2.19 and 2.55 bleeding events per 100 person-years. Mortality rates were 1.51, 1.14 and 1.68 per 100 person-years respectively.
After three-way propensity score matching, there were 1219 patients in each OAC group in the low pharmacy category, 1487 patients in each group in the moderate polypharmacy category and 723 patients in each group in the high polypharmacy category, for a total of 10,287 patients. The three groups were well matched within each polypharmacy category. Table 1 shows standardized differences in baseline characteristics, including demographics, comorbidities, medications, and healthcare utilization between the three groups within high polypharmacy patients; after propensity score matching. All characteristics were well balanced between the three drugs in each polypharmacy subgroup as evident by standardized differences <0.10.
Table 1:
Baseline characteristics in the three anticoagulant groups (20 mg rivaroxaban, 5 mg apixaban, warfarin), categorized by degree of polypharmacy after propensity score matching
| High polypharmacy (>8 meds) | Medium polypharmacy (4–8 meds) | Low polypharmacy (<=3 meds) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Rivaroxaban 20 mg | Apixaban 5 mg | Warfarin | Rivaroxaban 20 mg | Apixaban 5 mg | Warfarin | Rivaroxaban 20 mg | Apixaban 5 mg | Warfarin |
| Variable | Rivaroxaban | Apixaban | Warfarin | Rivaroxaban | Apixaban | Warfarin | Rivaroxaban | Apixaban | Warfarin |
| N=723 | N=723 | N=723 | N=1487 | N=1487 | N=1487 | N=1219 | N=1219 | N=1219 | |
| Demographics | |||||||||
| Age | 74.5(6.5) | 74.8(6.6) | 74.4(6.6) | 75.2(6.5) | 75.3(6.4) | 75.3(6.7) | 75.7(6.4) | 76.0(6.4) | 76.0(6.6) |
| Female sex | 0.51(0.50) | 0.53(0.50) | 0.54(0.50) | 0.48(0.50) | 0.50(0.50) | 0.50(0.50) | 0.46(0.50) | 0.47(0.50) | 0.47(0.50) |
| Race | |||||||||
| White race | 0.86(0.35) | 0.86(0.35) | 0.86(0.35) | 0.88(0.33) | 0.87(0.33) | 0.88(0.32) | 0.90(0.31) | 0.90(0.29) | 0.90(0.30) |
| Black race | 0.04(0.20) | 0.05(0.23) | 0.04(0.19) | 0.04(0.20) | 0.04(0.19) | 0.04(0.19) | 0.04(0.19) | 0.04(0.20) | 0.04(0.20) |
| Hispanic race | 0.05(0.22) | 0.05(0.21) | 0.06(0.24) | 0.05(0.21) | 0.04(0.20) | 0.05(0.22) | 0.03(0.17) | 0.03(0.17) | 0.03(0.16) |
| Dual Medicare Medicaid eligibility | 0.23(0.42) | 0.22(0.41) | 0.22(0.42) | 0.16(0.37) | 0.17(0.37) | 0.16(0.37) | 0.12(0.33) | 0.09(0.29) | 0.12(0.33) |
| Comorbid Conditions | |||||||||
| Hypertension | 0.94(0.24) | 0.94(0.24) | 0.94(0.24) | 0.89(0.31) | 0.90(0.30) | 0.89(0.31) | 0.85(0.35) | 0.84(0.37) | 0.86(0.35) |
| Diabetes mellitus | 0.48(0.50) | 0.46(0.50) | 0.47(0.50) | 0.36(0.48) | 0.33(0.47) | 0.34(0.48) | 0.26(0.44) | 0.27(0.44) | 0.27(0.44) |
| Congestive heart failure | 0.41(0.49) | 0.44(0.50) | 0.43(0.50) | 0.30(0.46) | 0.29(0.45) | 0.31(0.46) | 0.19(0.39) | 0.20(0.40) | 0.22(0.41) |
| Prior coronary artery disease | 0.58(0.49) | 0.59(0.49) | 0.61(0.49) | 0.45(0.50) | 0.45(0.50) | 0.47(0.50) | 0.36(0.48) | 0.39(0.49) | 0.38(0.48) |
| Prior myocardial infarction | 0.11(0.32) | 0.13(0.34) | 0.15(0.35) | 0.07(0.25) | 0.08(0.27) | 0.07(0.25) | 0.04(0.20) | 0.04(0.20) | 0.04(0.18) |
| Prior cardiac device | 0.13(0.34) | 0.14(0.35) | 0.13(0.34) | 0.10(0.30) | 0.11(0.31) | 0.11(0.31) | 0.11(0.31) | 0.12(0.32) | 0.13(0.34) |
| Prior revascularization | 0.21(0.41) | 0.23(0.42) | 0.23(0.42) | 0.15(0.35) | 0.13(0.33) | 0.15(0.36) | 0.09(0.29) | 0.10(0.29) | 0.11(0.31) |
| Valvular heart disease | 0.45(0.50) | 0.43(0.50) | 0.42(0.49) | 0.38(0.49) | 0.40(0.49) | 0.38(0.49) | 0.35(0.48) | 0.37(0.48) | 0.35(0.48) |
| Renal disease | 0.20(0.40) | 0.20(0.40) | 0.20(0.40) | 0.13(0.34) | 0.13(0.33) | 0.14(0.34) | 0.10(0.30) | 0.11(0.31) | 0.11(0.31) |
| Liver disease | 0.09(0.28) | 0.09(0.29) | 0.09(0.28) | 0.05(0.23) | 0.05(0.22) | 0.05(0.21) | 0.03(0.16) | 0.03(0.18) | 0.04(0.19) |
| Hypothyroidism | 0.32(0.47) | 0.32(0.47) | 0.29(0.45) | 0.25(0.43) | 0.26(0.44) | 0.23(0.42) | 0.23(0.42) | 0.21(0.41) | 0.22(0.41) |
| Chronic pulmonary disease | 0.48(0.50) | 0.50(0.50) | 0.49(0.50) | 0.34(0.47) | 0.35(0.48) | 0.32(0.47) | 0.20(0.40) | 0.19(0.39) | 0.21(0.41) |
| Prior ischemic stroke | 0.26(0.44) | 0.26(0.44) | 0.26(0.44) | 0.18(0.38) | 0.20(0.40) | 0.19(0.40) | 0.17(0.38) | 0.18(0.39) | 0.18(0.38) |
| Prior dementia | 0.09(0.29) | 0.08(0.27) | 0.07(0.25) | 0.06(0.25) | 0.06(0.23) | 0.06(0.23) | 0.04(0.20) | 0.04(0.19) | 0.05(0.21) |
| Coagulopathy | 0.10(0.30) | 0.10(0.30) | 0.11(0.31) | 0.06(0.24) | 0.06(0.24) | 0.06(0.24) | 0.04(0.19) | 0.04(0.20) | 0.04(0.20) |
| Deficiency Anemias | 0.40(0.49) | 0.40(0.49) | 0.40(0.49) | 0.26(0.44) | 0.28(0.45) | 0.28(0.45) | 0.19(0.39) | 0.19(0.39) | 0.20(0.40) |
| Fluid and electrolyte disorders | 0.42(0.49) | 0.39(0.49) | 0.44(0.50) | 0.27(0.44) | 0.29(0.45) | 0.30(0.46) | 0.19(0.39) | 0.17(0.38) | 0.19(0.39) |
| Drug abuse | 0.02(0.14) | 0.03(0.17) | 0.04(0.19) | 0.02(0.13) | 0.01(0.12) | 0.01(0.12) | 0.01(0.09) | 0.01(0.09) | 0.00(0.06) |
| Alcohol abuse | 0.04(0.20) | 0.04(0.19) | 0.03(0.18) | 0.03(0.18) | 0.02(0.16) | 0.03(0.17) | 0.03(0.16) | 0.02(0.14) | 0.02(0.14) |
| Depression | 0.24(0.43) | 0.25(0.44) | 0.24(0.42) | 0.16(0.36) | 0.17(0.37) | 0.17(0.37) | 0.10(0.29) | 0.10(0.30) | 0.10(0.30) |
| Psychoses | 0.10(0.30) | 0.09(0.29) | 0.10(0.29) | 0.05(0.22) | 0.05(0.22) | 0.06(0.24) | 0.03(0.16) | 0.03(0.17) | 0.02(0.15) |
| Connective tissue disease | 0.11(0.31) | 0.13(0.34) | 0.10(0.31) | 0.08(0.27) | 0.09(0.28) | 0.09(0.29) | 0.04(0.19) | 0.05(0.21) | 0.04(0.19) |
| Neurological disorders | 0.25(0.43) | 0.26(0.44) | 0.24(0.43) | 0.18(0.39) | 0.17(0.38) | 0.20(0.40) | 0.14(0.35) | 0.13(0.34) | 0.14(0.35) |
| Obesity | 0.33(0.47) | 0.34(0.47) | 0.33(0.47) | 0.26(0.44) | 0.24(0.43) | 0.27(0.44) | 0.20(0.40) | 0.20(0.40) | 0.19(0.39) |
| Paralysis | 0.06(0.23) | 0.05(0.22) | 0.06(0.24) | 0.05(0.21) | 0.05(0.21) | 0.05(0.21) | 0.03(0.18) | 0.03(0.18) | 0.04(0.19) |
| Pulmonary hypertension | 0.14(0.35) | 0.14(0.35) | 0.14(0.34) | 0.08(0.27) | 0.08(0.27) | 0.09(0.28) | 0.05(0.21) | 0.05(0.21) | 0.05(0.22) |
| Metastatic cancer | 0.04(0.19) | 0.04(0.19) | 0.04(0.20) | 0.02(0.15) | 0.03(0.17) | 0.02(0.13) | 0.01(0.11) | 0.01(0.10) | 0.01(0.10) |
| Solid tumor without metastasis | 0.16(0.36) | 0.15(0.36) | 0.14(0.35) | 0.14(0.34) | 0.13(0.34) | 0.14(0.35) | 0.13(0.33) | 0.11(0.32) | 0.11(0.31) |
| Unexplained Weight loss | 0.11(0.31) | 0.08(0.27) | 0.11(0.32) | 0.05(0.23) | 0.06(0.23) | 0.08(0.27) | 0.05(0.21) | 0.04(0.19) | 0.05(0.21) |
| Peripheral vascular disease | 0.33(0.47) | 0.37(0.48) | 0.35(0.48) | 0.25(0.44) | 0.27(0.44) | 0.28(0.45) | 0.19(0.39) | 0.20(0.40) | 0.20(0.40) |
| Prior intracerebral hemorrhage | 0.01(0.11) | 0.02(0.13) | 0.02(0.12) | 0.01(0.08) | 0.01(0.08) | 0.00(0.07) | 0.01(0.09) | 0.01(0.09) | 0.01(0.09) |
| Prior GI bleeding | 0.10(0.30) | 0.09(0.29) | 0.11(0.31) | 0.08(0.27) | 0.09(0.29) | 0.08(0.28) | 0.06(0.23) | 0.05(0.22) | 0.04(0.20) |
| Prior VTE | 0.04(0.19) | 0.03(0.16) | 0.04(0.19) | 0.03(0.17) | 0.02(0.16) | 0.03(0.16) | 0.01(0.12) | 0.01(0.11) | 0.01(0.12) |
| Healthcare utilization in prior year | |||||||||
| Acute stay Days | 7.24(10.10) | 7.37(10.15) | 8.10(9.87) | 4.13(7.24) | 4.42(7.84) | 4.62(6.84) | 2.13(4.92) | 2.12(4.43) | 2.28(4.55) |
| Long-Term Days | 0.69(4.25) | 0.57(3.70) | 0.54(3.61) | 0.40(3.63) | 0.50(3.75) | 0.47(2.73) | 0.23(2.01) | 0.21(1.74) | 0.33(2.59) |
| SNF Days | 5.07(23.52) | 4.35(15.72) | 4.66(17.86) | 2.70(12.55) | 2.70(13.12) | 2.83(11.70) | 1.59(8.99) | 1.04(6.01) | 1.48(8.22) |
| Concurrent Medications at time of initial OAC prescription | |||||||||
| Angiotensin receptor blockers | 0.25(0.43) | 0.26(0.44) | 0.27(0.44) | 0.23(0.42) | 0.24(0.43) | 0.22(0.42) | 0.19(0.39) | 0.18(0.38) | 0.18(0.39) |
| ACE inhibitors | 0.35(0.48) | 0.32(0.47) | 0.35(0.48) | 0.34(0.47) | 0.32(0.47) | 0.35(0.48) | 0.32(0.47) | 0.31(0.46) | 0.32(0.46) |
| Beta blockers | 0.73(0.44) | 0.73(0.44) | 0.71(0.45) | 0.67(0.47) | 0.69(0.46) | 0.69(0.46) | 0.62(0.48) | 0.64(0.48) | 0.63(0.48) |
| Calcium channel blockers | 0.22(0.42) | 0.23(0.42) | 0.25(0.43) | 0.22(0.41) | 0.21(0.41) | 0.22(0.42) | 0.15(0.35) | 0.14(0.35) | 0.16(0.36) |
| Diuretics | 0.37(0.48) | 0.37(0.48) | 0.37(0.48) | 0.27(0.44) | 0.28(0.45) | 0.28(0.45) | 0.15(0.35) | 0.15(0.35) | 0.17(0.37) |
| Oral diabetic medications | 0.27(0.44) | 0.24(0.43) | 0.25(0.44) | 0.18(0.39) | 0.18(0.39) | 0.18(0.39) | 0.13(0.34) | 0.13(0.34) | 0.13(0.34) |
| Digoxin | 0.11(0.32) | 0.11(0.31) | 0.12(0.32) | 0.07(0.25) | 0.06(0.23) | 0.07(0.26) | 0.05(0.21) | 0.04(0.20) | 0.05(0.21) |
| Insulin | 0.11(0.31) | 0.12(0.32) | 0.12(0.33) | 0.06(0.24) | 0.07(0.25) | 0.05(0.22) | 0.02(0.15) | 0.03(0.17) | 0.03(0.17) |
| NSAIDs | 0.20(0.40) | 0.16(0.37) | 0.19(0.39) | 0.13(0.33) | 0.12(0.33) | 0.13(0.33) | 0.06(0.25) | 0.06(0.25) | 0.07(0.25) |
| Proton pump inhibitors | 0.40(0.49) | 0.41(0.49) | 0.37(0.48) | 0.28(0.45) | 0.27(0.45) | 0.27(0.44) | 0.18(0.38) | 0.17(0.38) | 0.18(0.38) |
| SSRI | 0.23(0.42) | 0.24(0.43) | 0.24(0.43) | 0.16(0.37) | 0.16(0.36) | 0.17(0.37) | 0.12(0.32) | 0.11(0.31) | 0.12(0.33) |
| Statins | 0.58(0.49) | 0.60(0.49) | 0.64(0.48) | 0.56(0.50) | 0.58(0.49) | 0.56(0.50) | 0.51(0.50) | 0.51(0.50) | 0.50(0.50) |
| Antiarrhythmics | 0.35(0.48) | 0.32(0.47) | 0.34(0.47) | 0.25(0.43) | 0.27(0.44) | 0.24(0.43) | 0.16(0.37) | 0.16(0.37) | 0.17(0.37) |
| Antiplatelets | 0.16(0.37) | 0.19(0.40) | 0.16(0.36) | 0.12(0.33) | 0.13(0.34) | 0.13(0.33) | 0.08(0.27) | 0.09(0.29) | 0.08(0.27) |
| PGP inhibitors | 0.27(0.44) | 0.26(0.44) | 0.29(0.45) | 0.19(0.39) | 0.20(0.40) | 0.18(0.39) | 0.10(0.30) | 0.11(0.31) | 0.12(0.32) |
| Mineralocorticoid inhibitors | 0.08(0.27) | 0.08(0.27) | 0.07(0.25) | 0.04(0.20) | 0.05(0.21) | 0.05(0.22) | 0.03(0.16) | 0.03(0.18) | 0.03(0.18) |
| Health Status Risk scores | |||||||||
| Gagne Comorbidity Score | 4.66(3.11) | 4.62(3.02) | 4.71(3.18) | 3.27(2.64) | 3.26(2.65) | 3.37(2.55) | 2.33(2.22) | 2.31(2.11) | 2.42(2.18) |
| Frailty score | 16.4(11.9) | 15.9(11.4) | 16.3(11.8) | 11.1(9.9) | 11.2(9.5) | 11.1(9.5) | 7.6(7.8) | 7.6(7.8) | 7.6(7.8) |
| CHA2DS2-VASc | 5.03(1.65) | 5.10(1.63) | 5.10(1.65) | 4.48(1.60) | 4.52(1.57) | 4.55(1.57) | 4.14(1.60) | 4.21(1.56) | 4.25(1.54) |
| HAS Bled score | 3.15(1.09) | 3.13(1.03) | 3.14(1.07) | 2.74(1.00) | 2.75(0.97) | 2.76(1.00) | 2.47(0.91) | 2.48(0.94) | 2.49(0.92) |
All values are presented as mean (standard deviation).
All standardized differences between the three drugs in each polypharmacy group were <0.10 indicating well balanced groups after propensity score matching.
Ischemic stroke
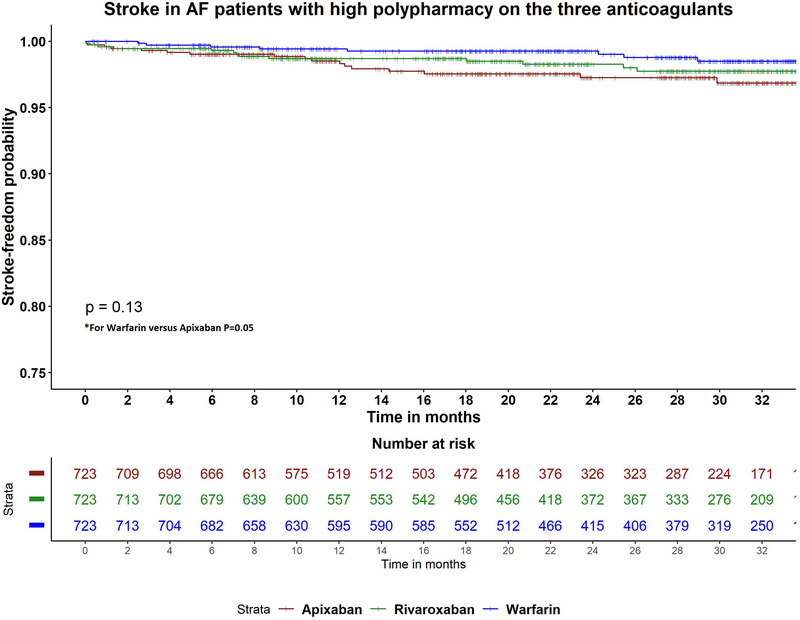
Table 2 shows adjusted hazards ratios for ischemic stroke with (apixaban versus warfarin), (rivaroxaban versus warfarin) and (apixaban versus rivaroxaban), by polypharmacy level. In the low and moderate polypharmacy categories, all three drugs were associated with similar risk of ischemic stroke in pairwise comparisons. However, in the high polypharmacy category, apixaban was associated with higher risk of ischemic stroke compared to warfarin (aHR 2.34, 95% CI 1.01–5.42; p=0.05), and similar risk of stroke compared to rivaroxaban (aHR 1.38, 95% CI 0.67–2.84; p=0.4) (Figure 2A).
Table 2.
Relative hazard of stroke, bleeding or death in propensity matched samples of standard dose anticoagulation, by polypharmacy level
| Apixaban 5 mg vs Warfarin | Rivaroxaban 20 mg vs Warfarin | Apixaban 5 mg vs Rivaroxaban 20 mg | |
|---|---|---|---|
|
Low polypharmacy (≤3drugs) N=1219 in each drug |
|||
| Ischemic Stroke | 1.06 (0.54–2.08; p=0.86) | 0.87 (0.43–1.77; p=0.71) | 1.22 (0.60–2.47; p=0.59) |
| Bleeding | 0.54 (0.32–0.90; p=0.02) | 0.76 (0.48–1.20; p=0.24) | 0.71 (0.41–1.22; p=0.21) |
| GI Hemorrhage | 0.50 (0.27–0.93; p=0.03) | 0.81 (0.48–1.39; p=0.45) | 0.62 (0.32–1.38; p=0.14) |
| Intracranial | 0.63 (0.18–2.14; p=0.46) | 0.62 (0.18–2.11; p=0.44) | 1.02 (0.45–4.06; p=0.98) |
| Death | 0.69 (0.38–1.27; p=0.24) | 0.51 (0.27–1.00; p=0.05) | 1.35 (0.66–2.78; p=0.42) |
|
Medium polypharmacy (4–8 drugs) N=1487 in each drug |
|||
| Ischemic Stroke | 1.07 (0.55–2.10; p=0.84) | 1.00 (0.50–1.98; p=0.99) | 1.08 (0.54–2.13; p=0.83) |
| Bleeding | 0.85 (0.59–1.24; p=0.40) | 0.95 (0.66–1.37; p=0.79) | 0.80 (0.54–1.19; p=0.27) |
| GI Hemorrhage | 1.07 (0.69–1.67; p=0.76) | 1.05 (0.67–1.63; p=0.83) | 1.02 (0.66–1.59; p=0.93) |
| Intracranial | 0.92 (0.28–3.00; p=0.88) | 0.90 (0.27–2.94; p=0.86) | 1.02 (0.30–3.52; p=0.98) |
| Death | 1.52 (0.92–2.57; p=0.10) | 1.28 (0.75–2.17; p=0.37) | 1.20 (0.74–1.96; p=0.46) |
|
High polypharmacy (>8 drugs) N=723 in each drug |
|||
| Ischemic Stroke | 2.34 (1.01–5.42; p=0.05) | 1.70 (0.71–4.10; p=0.24) | 1.38 (0.67–2.84; p=0.39) |
| Bleeding | 0.93 (0.56–1.54; p=0.77) | 1.06 (0.65–1.73; p=0.81) | 0.87 (0.53–1.44; p=0.59) |
| GI Hemorrhage | 1.37 (0.73–2.59; p=0.33) | 1.46 (0.78–2.71; p=0.24) | 0.94 (0.53–1.68; p=0.84) |
| Intracranial | 0.64 (0.27–9.84; p=0.59) | 1.62 (0.58–13.48; p=0.53) | 0.63 (0.15–2.63; p=0.53) |
| Death | 1.46 (0.79–2.70; p=0.24) | 0.72 (0.35–1.49; p=0.37) | 2.03 (1.01–4.08; p=0.05) |
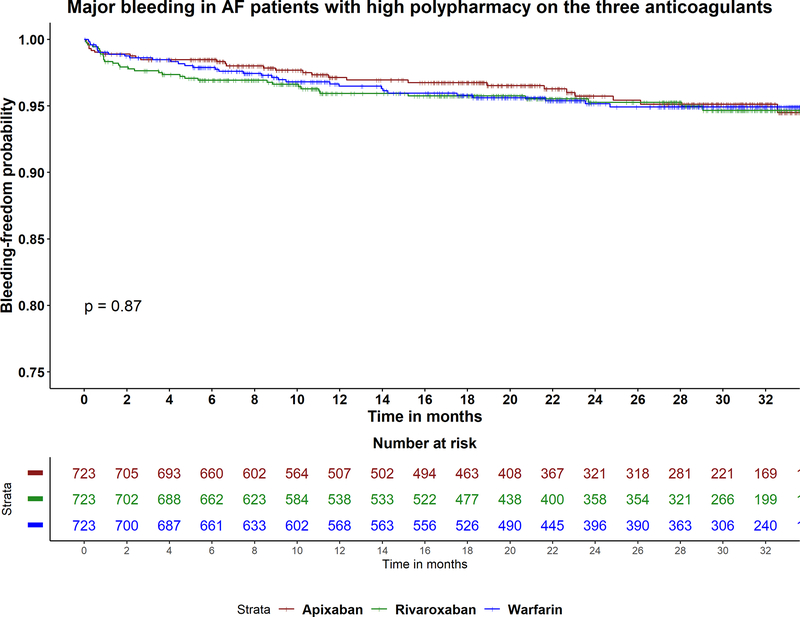
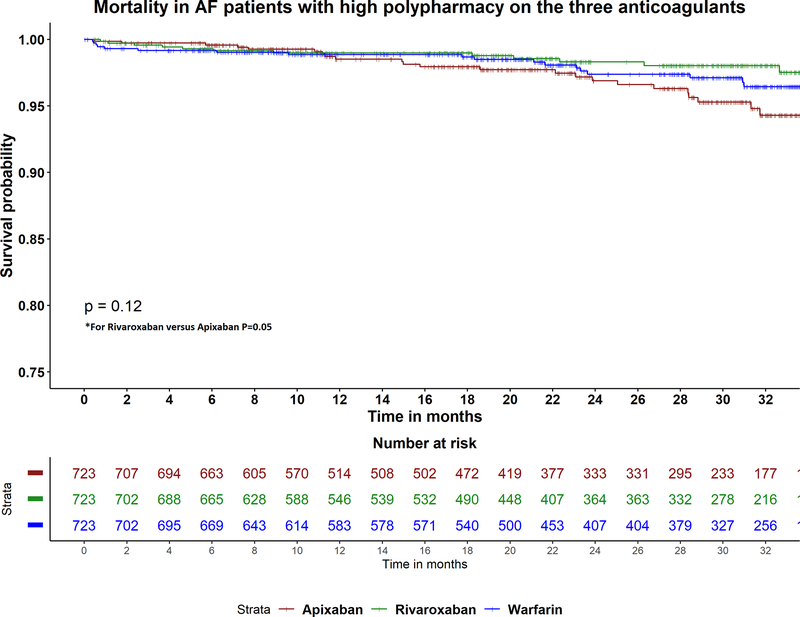
Figure 2A-C:
Kaplan Meier curves for the study outcomes A) ischemic stroke, B) major bleeding, C) all-cause mortality in atrial fibrillation patients with high polypharmacy on apixaban, rivaroxaban and warfarin. P-value for comparison between apixaban and warfarin in stroke =0.5. P-value for comparison between apixaban and rivaroxaban in mortality =0.5.
Bleeding
Table 2 shows adjusted hazards ratios for any bleeding, gastrointestinal and intracranial bleeding with (apixaban versus warfarin), (rivaroxaban versus warfarin) and (apixaban versus rivaroxaban) in each degree of polypharmacy. Among patients with low polypharmacy, apixaban was associated with lower major bleeding (aHR 0.54, 95% CI 0.32–0.90; p=0.02), and lower gastrointestinal bleeding (aHR 0.50, 95% CI 0.27–0.93; p=0.03), compared to warfarin. There was no difference between the three drugs in rates of bleeding, including gastrointestinal and intracranial bleeding, in the moderate and high polypharmacy patients (Figure 2B).
All-cause mortality
Table 2 shows adjusted hazards ratios for all-cause mortality in (apixaban versus warfarin), (rivaroxaban versus warfarin) and (apixaban versus rivaroxaban) by polypharmacy level. There was no difference in risk of mortality between the three drugs in low and moderate polypharmacy patients. However, in patients with high polypharmacy, apixaban was associated with higher risk of mortality compared to rivaroxaban (aHR 2.03, 95% CI 1.01–4.08; p=0.05), but similar risk when compared to warfarin (aHR 1.46, 95% CI 0.79–2.70; p=0.2) (Figure 2C). There was no difference in risk of mortality between rivaroxaban and warfarin in high polypharmacy group (aHR 0.72, 95% CI 0.35–1.49; p=0.4).
Reduced Dose Analysis
Before matching, there were 1806 patients taking reduced dose of rivaroxaban and 2774 patients taking reduced dose of apixaban. Table 3 shows adjusted hazards ratios for the study outcomes in pairwise comparisons of the three drugs by polypharmacy level. In the low polypharmacy group, similar to the standard dose analysis, apixaban was associated with lower risk of major and gastrointestinal bleeding compared to warfarin. However, rivaroxaban was also associated with lower risk of major bleeding compared to warfarin. Apixaban was also associated with lower risk of stroke compared to warfarin. In the high polypharmacy group, there was no difference in risk of stroke between the three different anticoagulants. However, apixaban was associated with lower risk of major and gastrointestinal bleeding compared to both warfarin and rivaroxaban. In the reduced dose analysis, within the high polypharmacy group, apixaban remained associated with higher risk of death compared to rivaroxaban.
Table 3.
Relative hazard of stroke, bleeding or death in propensity matched samples of low dose anticoagulation, by polypharmacy level
| Apixaban 2.5 mg vs Warfarin | Rivaroxaban 15 mg vs Warfarin | Apixaban 2.5 mg vs Rivaroxaban 15 mg | |
|---|---|---|---|
| Low polypharmacy (≤3drugs) | |||
| Ischemic Stroke | 0.28 (0.09–0.85, P=0.02) | 0.48 (0.20–1.19, P=0.1) | 0.58 (0.17–1.98, P=0.4) |
| Bleeding | 0.54 (0.29–1.00, P=0.05) | 0.49 (0.26–0.93, P=0.03) | 1.10 (0.53–2.28, P=0.8) |
| GI Hemorrhage | 0.33 (0.14–0.76, P=0.01) | 0.55 (0.27–1.10, P=0.09) | 0.60 (0.24–1.52, P=0.7) |
| Intracranial | 1.54 (0.41–5.74, P=0.5) | 0.29 (0.03–2.55, P=0.2) | 5.39 (0.63–46.18, P=0.1) |
| Death | 0.94 (0.52–1.69, P=0.8) | 0.98 (0.55–1.74, P=0.9) | 0.96 (0.52–1.75, P=0.9) |
| Medium polypharmacy (4–8 drugs) | |||
| Ischemic Stroke | 1.28 (0.61–2.69, P=0.5) | 0.72 (0.31–1.67, P=0.4) | 1.79 (0.78–4.09, P=0.2) |
| Bleeding | 0.87 (0.51–1.49, P=0.6) | 0.97 (0.58–1.62, P=0.9) | 0.90 (0.52–1.55, P=0.7) |
| GI Hemorrhage | 1.28 (0.69–2.37, P=0.4) | 1.32 (0.72–2.41, P=0.4) | 0.97 (0.55–1.74, P=0.9) |
| Intracranial | NA | 1.02 (0.21–5.08, P=0.9) | NA |
| Death | 1.09 (0.68–1.74, P=0.7) | 1.01 (0.63–1.60, P=0.9) | 1.08 (0.68–1.74, P=0.7) |
| High polypharmacy (>8 drugs) | |||
| Ischemic Stroke | 2.09 (0.71–6.12, P=0.2) | 1.00 (0.29–3.47, P=1.0) | 2.08 (0.71–6.10, P=0.2) |
| Bleeding | 0.20 (0.09–0.44, P<0.001) | 0.65 (0.38–1.09, P=0.1) | 0.31 (0.13–0.71, P=0.006) |
| GI Hemorrhage | 0.25 (0.10–0.61, P=0.002) | 0.83 (0.46–1.51, P=0.5) | 0.30 (0.12–0.74, P=0.009) |
| Intracranial | NA | NA | NA |
| Death | 1.80 (0.94–3.43, P=0.07) | 0.81 (0.38–1.72, P=0.6) | 2.23 (1.11–4.46, P=0.02) |
Discussion
In this comparative effectiveness study of three different anticoagulants in patients with AF and polypharmacy, we demonstrate several important findings. First, in patients with low degree of polypharmacy, there was no difference in the risk of ischemic stroke with apixaban, rivaroxaban or warfarin. However, apixaban was associated with a safety benefit as evident by the lower risk of bleeding compared to warfarin. Second, in patients with a high degree of polypharmacy (≥8 medications), bleeding risk was similar between apixaban, warfarin and rivaroxaban. However, apixaban was associated with a higher risk of ischemic stroke compared to warfarin. Furthermore, apixaban was associated with higher risk of mortality compared to rivaroxaban. Third, reduced dose apixaban and rivaroxaban were both associated with lower risk of bleeding compared to warfarin in the high polypharmacy group. However, apixaban remained associated with higher risk of death compared to rivaroxaban.
Polypharmacy is a major problem that is highly prevalent in patients with AF, with studies showing that more than 75% of AF patients have polypharmacy.12 Polypharmacy is associated with negative clinical outcomes in several ways.13 First, there is the risk of drug-drug interaction. This interaction may result either in lower efficacy for a given drug, or in a toxic level increasing side effects.13 Second, patients with polypharmacy frequently miss taking their medications, or end up taking the wrong dosage or frequency.13, 14 Polypharmacy is more prevalent in elderly patients with higher prevalence of multimorbidity, dementia and cognitive impairment 15. In patients with AF, polypharmacy burden increases with higher multimorbidity which in turn increases the risk of stroke and the risk of bleeding.3, 7, 8, 12.
Rivaroxaban and apixaban were approved for stroke prevention in AF patients after the two landmark trials, ROCKET AF and ARISTOTLE respectively.4, 5 A post hoc analysis of the ROCKET AF study showed that there was no difference in rivaroxaban outcomes compared to warfarin in patients with different degrees of polypharmacy.8 For the ARISTOTLE trial, a post hoc analysis demonstrated that in patients with polypharmacy, apixaban was more effective than warfarin in stroke prevention and at least as safe as warfarin in incidence of major bleeding. 7 However, it is important to note that the use of strong CYP3A4 or P-glycoprotein (P-GP) inhibitors was a key exclusion criterion for both trials. 4, 5 Furthermore, in the post hoc analysis, there were concerns about misclassification of drugs and non-representativeness of strong enzyme inducers or inhibitors. 16 Most of the published evidence regarding the efficacy or safety of apixaban and rivaroxaban in patients with significant polypharmacy has been from these original two trials, or subsequent subgroup analysis.6–9. Post hoc and subgroup analysis of randomized controlled trials data is constrained by the potential for bias and error 17. Our aim was to investigate the comparative effectiveness and safety of these anticoagulants in a very high polypharmacy population with from the real world setting within the United States. Among all the countries involved in the Aristotle trial, the United States was the country with the highest prevalence of polypharmacy among their enrolled patients. 7
In our study, we found that apixaban is associated with a higher risk of ischemic stroke when compared to warfarin and a higher risk of mortality when compared to rivaroxaban. One potential explanation is that apixaban is dosed twice daily, while warfarin and rivaroxaban are dosed once daily. The dosing frequency of a medication affects a patient’s adherence to that prescribed medication. A previous study showed that adherence to a twice daily drug is 69% compared to 79% in a medication that is dosed once daily. 18 In another study, simplified dosing regimens increased adherence by up to 20%, while patient education interventions were largely unsuccessful. 14 Another explanation for our findings could be the use of a warfarin anticoagulation clinic model, which enables frequent INR monitoring and dosing adjustments, and has been shown to be associated with higher adherence in prior research.19
The mechanism of action for both rivaroxaban and apixaban is factor X inhibition. While both medications are metabolized through several pathways including the cytochrome P450 system, hepatic metabolism and renal excretion, they differ in lipid solubility and in volume of distribution in the body, with rivaroxaban having a higher volume of distribution (50 liters) compared to apixaban (21 liters).20–22 The intestinal absorption of both apixaban and rivaroxaban is dependent on P-Glycoprotein (P-GP) transporters, and the effect of P-GP inhibitors and inducers on apixaban and rivaroxaban efficacy has not been well-studied.23 One previous study found that the bleeding benefit of DOACs was lost when they are used concomitantly with P-GP inhibitors. 24 In the ARISTOTLE trial, P-GP inhibitor prevalence was 25%. 7 Conversely, within our study of “real world” patients, the concomitant use of P-GP inhibitors with apixaban and rivaroxaban was high, and the most commonly involved P-GP inhibitor was amiodarone. We performed a robust three-way propensity score matching analysis where patient characteristics - including concurrent use of P-GP inhibitors - were balanced across the three drug groups. The question of whether the higher apixaban mortality in our study is due medication non-adherence leading to higher stroke risk or caused by drug interaction with strong P-GP inhibitors, could not be answered with our analysis and requires further studies.
Our study has several limitations. First, our patients were all older than 65 years old because the source of our study cohort was Medicare data, which is primarily composed of elderly patients. Whether our findings are generalizable to younger patients is unknown. Second, our study lacked information on creatinine and glomerular filtration values, factors which could affect drug excretion and plasma levels. Third, we assessed the degree of polypharmacy and use of different medications at baseline in our patients, but we were unable to adjust concurrent medication use for subsequent prescription changes. Finally, any comparative-effectiveness analysis using observational data is subject to potential bias caused by treatment assignment. We used a rigorous propensity matching approach for this current study in an attempt to minimize potential bias. Nonetheless, propensity matching, and other similar statistical algorithms are only able to control for differences in observable patient characteristics across treatment groups. If unobservable differences remain across treatment groups, such differences may impact our results. For example, if patients who initiate apixaban have more unobservable characteristics that increase stroke risk compared to patients who initiate rivaroxaban or warfarin, our finding that apixaban is associated with higher stroke risk may be biased.
In conclusion, significant polypharmacy is prevalent among AF patients from the US. While apixaban was associated with fewer bleeding events in patients with low polypharmacy, bleeding risk was similar between apixaban, rivaroxaban and warfarin in patients with high polypharmacy. Our study suggests that among patients with significant polypharmacy (>8 drugs) there may be a higher stroke and mortality risk with apixaban compared to warfarin and rivaroxaban. However, differences were of borderline significance.
Supplementary Material
Acknowledgments
Funding: Dr. Mentias received support from National Institute of Health NRSA institutional grant (T32 HL007121) at the Abboud Cardiovascular Research Center. Dr. Vaughan Sarrazin receives support from the National Institute on Aging (R01 AG055663), and the Health Services Research and Development Service (HSR&D) of the Department of Veterans Affairs.
Footnotes
Disclosures: All authors do not have any conflicts of interest or financial disclosures.
References
- 1.Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: The current epidemic. J Geriatr Cardiol. 2017;14:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mentias A, Shantha G, Adeola O, Barnes GD, Narasimhan B, Siontis KC, et al. Role of diabetes and insulin use in the risk of stroke and acute myocardial infarction in patients with atrial fibrillation: A medicare analysis. Am Heart J. 2019;214:158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mentias A, Shantha G, Chaudhury P, Vaughan Sarrazin MS. Assessment of outcomes of treatment with oral anticoagulants in patients with atrial fibrillation and multiple chronic conditions: A comparative effectiveness analysis. JAMA Netw Open. 2018;1:e182870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891 [DOI] [PubMed] [Google Scholar]
- 5.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992 [DOI] [PubMed] [Google Scholar]
- 6.Flaker G, Lopes RD, Hylek E, Wojdyla DM, Thomas L, Al-Khatib SM, et al. Amiodarone, anticoagulation, and clinical events in patients with atrial fibrillation: Insights from the aristotle trial. J Am Coll Cardiol. 2014;64:1541–1550 [DOI] [PubMed] [Google Scholar]
- 7.Jaspers Focks J, Brouwer MA, Wojdyla DM, Thomas L, Lopes RD, Washam JB, et al. Polypharmacy and effects of apixaban versus warfarin in patients with atrial fibrillation: Post hoc analysis of the aristotle trial. BMJ. 2016;353:i2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccini JP, Hellkamp AS, Washam JB, Becker RC, Breithardt G, Berkowitz SD, et al. Polypharmacy and the efficacy and safety of rivaroxaban versus warfarin in the prevention of stroke in patients with nonvalvular atrial fibrillation. Circulation. 2016;133:352–360 [DOI] [PubMed] [Google Scholar]
- 9.Steinberg BA, Hellkamp AS, Lokhnygina Y, Halperin JL, Breithardt G, Passman R, et al. Use and outcomes of antiarrhythmic therapy in patients with atrial fibrillation receiving oral anticoagulation: Results from the rocket af trial. Heart Rhythm. 2014;11:925–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rassen JA, Shelat AA, Franklin JM, Glynn RJ, Solomon DH, Schneeweiss S. Matching by propensity score in cohort studies with three treatment groups. Epidemiology. 2013;24:401–409 [DOI] [PubMed] [Google Scholar]
- 11.Kundi H, Popma JJ, Reynolds MR, Strom JB, Pinto DS, Valsdottir LR, et al. Frailty and related outcomes in patients undergoing transcatheter valve therapies in a nationwide cohort. Eur Heart J. 2019;40:2231–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaikh F, Pasch LB, Newton PJ, Bajorek BV, Ferguson C. Addressing multimorbidity and polypharmacy in individuals with atrial fibrillation. Curr Cardiol Rep. 2018;20:32. [DOI] [PubMed] [Google Scholar]
- 13.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure-lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med. 2004;164:722–732 [DOI] [PubMed] [Google Scholar]
- 15.Mortazavi SS, Shati M, Keshtkar A, Malakouti SK, Bazargan M, Assari S. Defining polypharmacy in the elderly: A systematic review protocol. BMJ Open. 2016;6:e010989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouatou Y, El Biali M, Samer CF. Letter by bouatou et al. regarding article, “polypharmacy and the efficacy and safety of rivaroxaban versus warfarin in the prevention of stroke in patients with nonvalvular atrial fibrillation”. Circulation. 2016;134:e3–4 [DOI] [PubMed] [Google Scholar]
- 17.Desai M, Pieper KS, Mahaffey K. Challenges and solutions to pre- and post-randomization subgroup analyses. Curr Cardiol Rep. 2014;16:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310 [DOI] [PubMed] [Google Scholar]
- 19.Barnes GD, Nallamothu BK, Sales AE, Froehlich JB. Reimagining anticoagulation clinics in the era of direct oral anticoagulants. Circ Cardiovasc Qual Outcomes. 2016;9:182–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009;15 Suppl 1:9S–16S [DOI] [PubMed] [Google Scholar]
- 21.Piccini JP, Patel MR, Mahaffey KW, Fox KA, Califf RM. Rivaroxaban, an oral direct factor xa inhibitor. Expert Opin Investig Drugs. 2008;17:925–937 [DOI] [PubMed] [Google Scholar]
- 22.Raghavan N, Frost CE, Yu Z, He K, Zhang H, Humphreys WG, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81 [DOI] [PubMed] [Google Scholar]
- 23.Stollberger C, Finsterer J. Relevance of p-glycoprotein in stroke prevention with dabigatran, rivaroxaban, and apixaban. Herz. 2015;40 Suppl 2:140–145 [DOI] [PubMed] [Google Scholar]
- 24.Kim IS, Kim HJ, Yu HT, Kim TH, Uhm JS, Kim JY, et al. Non-vitamin k antagonist oral anticoagulants with amiodarone, p-glycoprotein inhibitors, or polypharmacy in patients with atrial fibrillation: Systematic review and meta-analysis. J Cardiol. 2019;73:515–521 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.