Abstract
Background
So far, only a few studies evaluated the correlation between CT features and clinical outcome in patients with COVID-19 pneumonia.
Purpose
To evaluate CT ability in differentiating critically ill patients requiring invasive ventilation from patients with less severe disease.
Methods
We retrospectively collected data from patients admitted to our institution for COVID-19 pneumonia between March 5th-24th. Patients were considered critically ill or non-critically ill, depending on the need for mechanical ventilation. CT images from both groups were analyzed for the assessment of qualitative features and disease extension, using a quantitative semiautomatic method. We evaluated the differences between the two groups for clinical, laboratory and CT data. Analyses were conducted on a per-protocol basis.
Results
189 patients were analyzed. PaO2/FIO2 ratio and oxygen saturation (SaO2) were decreased in critically ill patients. At CT, mixed pattern (ground glass opacities (GGO) and consolidation) and GGO alone were more frequent respectively in critically ill and in non-critically ill patients (p < 0.05). Lung volume involvement was significantly higher in critically ill patients (38.5 % vs. 5.8 %, p < 0.05). A cut-off of 23.0 % of lung involvement showed 96 % sensitivity and 96 % specificity in distinguishing critically ill patients from patients with less severe disease. The fraction of involved lung was related to lactate dehydrogenase (LDH) levels, PaO2/FIO2 ratio and SaO2 (p < 0.05).
Conclusion
Lung disease extension, assessed using quantitative CT, has a significant relationship with clinical severity and may predict the need for invasive ventilation in patients with COVID-19.
Keywords: COVID-19, SARS- CoV-2, Pneumonia, Quantitative CT, Lung volume, Invasive mechanical ventilation
1. Introduction
The novel Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) infection, referred to as COVID-19, is characterized by a large variability of clinical presentation, ranging from asymptomatic up to severe symptoms requiring endotracheal intubation [1,2]: according to the Chinese Center of Disease Control and Prevention Report, 80 % of patients experience mild symptoms, 15 % develop severe pneumonia and 5% require critical care due to severe lung dysfunction, shock or extrapulmonary organ failure [3]. The mortality rate in the intensive care unit (ICU) patients ranges between 30 and 70 % [2].
The reported risk factors for severe course and mortality are male gender, older age and comorbidities such as hypertension, atherosclerosis, diabetes, cancer, respiratory or chronic kidney disease [[3], [4], [5], [6], [7], [8]]. Previous studies have shown that levels of d-dimer and Sequential Organ Failure Assessment (SOFA) scores are higher in critically ill or fatal cases, whereas lymphopenia has been associated with poor prognosis [4,[9], [10], [11]].
An early and precise identification of the most critically ill patients is necessary, since it has been demonstrated that a transfer to ICU with a 48 -hs delay after clinical worsening has a significant impact on prognosis [12]. CT imaging allows an early detection of lung abnormalities in patients with high suspicion for SARS-CoV-2 pneumonia [13,14], thus representing a valuable diagnostic tool, with pooled sensitivity and specificity of 94 % and 37 %, respectively [15].
Initial studies have investigated the correlation between the extent of lung involvement on CT, using a visual method, and the clinical severity of SARS-CoV-2 pneumonia, with higher volumes being affected in critically ill patients [16,17]. Moreover, based on both visual and software quantification, a lower percentage of well-aerated lung has been observed in patients with poor prognosis [18].
The purpose of this study was to assess qualitative and quantitative CT data in patients with SARS-CoV-2 pneumonia and to evaluate CT ability in differentiating critically ill patients requiring invasive ventilation from patients with less severe disease.
2. Materials and methods
2.1. Patients selection criteria
The study was approved by the institutional review board of our hospital. In this retrospective study, we collected data from consecutive patients with SARS-Cov-2 pneumonia admitted to our hospital between March 5th and March 24th, 2020. Written informed consent was obtained from all study participants or from their relatives if they were unable to provide it. All patients had SARS-CoV-2 infection, confirmed by real-time reverse transcription polymerase chain reaction (rRT-PCR) assay from throat swab specimens (RealStar - Altona Diagnostic, Hamburg, Germany). We selected only patients who underwent a chest CT scan at admission. According to the internal guidelines of our hospital for the management of patients with suspected COVID-19, patients with mild symptoms do not require a CT scan, whereas they undergo only chest X-ray and clinical monitoring; therefore, these patients were excluded from our study as well as patients with known coexisting pulmonary and pleural diseases that could interfere with CT image analysis, such as chronic obstructive pulmonary disease, interstitial lung disease and pleural effusion.
Patients’ medical records were reviewed and admission data were collected. All patients underwent clinical examination and were screened for the presence of comorbidities such as hypertension, diabetes, ischemic heart disease, chronic kidney disease, cancer. Available laboratory data included pulse oximetry (measured in ambient air at the time of admission or by the emergency personnel if they were transported to the hospital by ambulance, before undergoing oxygen therapy or mechanical ventilation), arterial blood gas (ABG) and venous blood tests (performed with a mean interval of 1 h from admission with time-range 20 min – 2 h). Oxygen Saturation (SaO2), arterial pressure of oxygen (PaO2)/fraction of inspired oxygen (FIO2) ratio, lactate dehydrogenase (LDH) levels and lymphocytes count were registered for all patients.
CT imaging was performed immediately afterwards, compatibly with disinfection time of our dedicated imaging section.
The management of each patient was decided according to their clinical conditions and in accordance with the Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition) [19]. Patients with severe disease were treated with oxygen therapy or continuous positive airway pressure (CPAP) /non-invasive ventilation (NIV); if no clinical benefit was achieved within 2 h, patients were considered as critically ill and were transferred to the ICU for receiving invasive mechanical ventilation [12]. Patients with non-severe disease or those who had benefit from oxygen therapy or CPAP/NIV were considered as non-critically ill and were recovered in the infectious disease department.
2.2. CT imaging
CT examination of each patient at admission was reviewed. CT scan was performed using a 64-slice CT scanner (Siemens Somatom Sensation; Siemens Healthineer, Erlangen, Germany), with the patient lying in supine position at maximum inspiration. The acquired volume covered the lung from the apex to the base. Acquisition parameters were set at 120 kV, 100 mAs, pitch 1.5 and collimation 0.6 mm; all images were reconstructed with a slice thickness of 1.00 mm, 512 × 512 mm, with both a sharp and soft kernel.
CT images were evaluated by two radiologists (S.P., P.R.) with at least 10-year experience in chest imaging, in consensus; OsiriX MD 11.0 software (Pixmeo SARL, Geneva, Switzerland) was used for both image viewing and quantitative analysis.
CT images were analyzed for the typical features described for COVID-19 pneumonia [13,14]. These included: GGO, consolidations or mixed opacities (GGO and consolidation); single or multiple nodules with halo sign; crazy paving; bronchial wall thickening; vascular enlargement (defined as subsegmental vessels higher than 3 mm in diameter); linear opacities; air bronchogram and enlarged lymph nodes. The distribution of the abnormalities was also evaluated: we considered central distribution when findings were predominantly peribronchovascular, whereas subpleural and perifissural distribution was considered as peripheral.
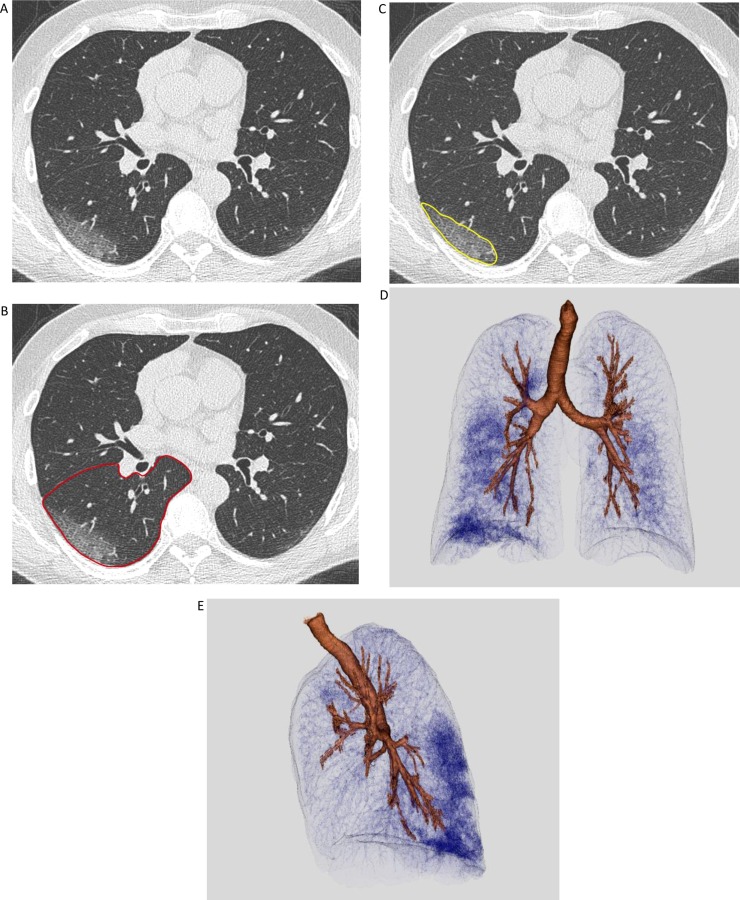
The two radiologists bordered the contour of each pulmonary lobe (right and left superior lobes, medium lobe, right and left inferior lobes), following the pleural surface and the fissures as anatomic landmarks, using a semi-automatic system (one slice every four was manually bordered whereas the others were automatically bordered); the individual volume of the lobes was automatically computed afterwards. For each lobe, areas of pulmonary disease were bordered slice-by-slice using the same semi-automatic system; the volume of affected lung parenchyma was then computed here too and was expressed in terms of percentage of lobe involved by the disease (Fig. 1 ).
Fig. 1.
CT quantitative assessment of disease extension in a non-critically ill Patient with SARS-CoV-2 pneumonia. (A-C) CT image viewed at lung window, on the axial plane. On the same slice, the operator manually drew the contour of the left lower lobe (red line) and the portion of involved parenchyma in it (yellow line). The final volume was automatically computed and measured afterwards. (D,E) Volumetric representation of lung involvement (Vitrea software, version 7.10.1.20), with frontal and lateral view.
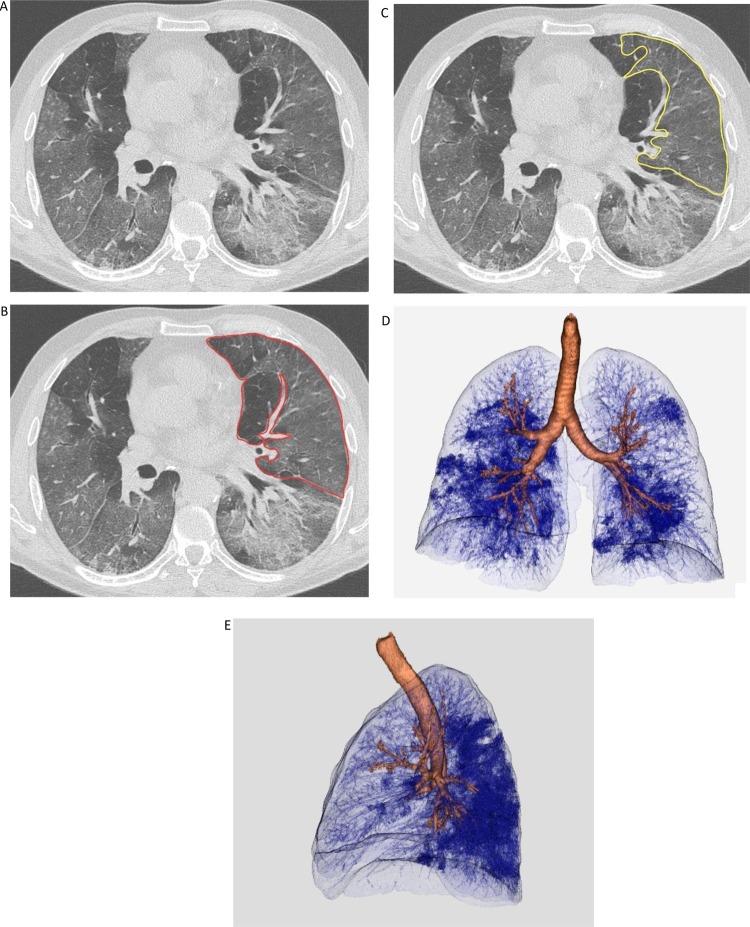
In case of multiple discrete opacities, the individual volume of each one was calculated following the same process; the overall volume of affected parenchyma was then obtained from the sum of these individual volumes. Confluent opacities were considered as a single volume: a single complex line was drawn to border the whole affected area (Fig. 2 ).
Fig. 2.
CT quantitative assessment of disease extension in a critically ill Patient with SARS-CoV-2 pneumonia. (A-C) CT image viewed at lung window, on the axial plane. On the same slice, the operator manually drew the contour of the left superior lobe (red line) and the portion of involved parenchyma in it (yellow line). The final volume was automatically computed and measured afterwards. (D,E) Volumetric representation of lung involvement (Vitrea software, version 7.10.1.20), with frontal and lateral view.
The overall volume of affected parenchyma in the right lung, in the left lung and in both lungs was calculated by adding up volumes of the respective lobes; the extent of the disease was then expressed as a percentage of the reference pulmonary volume, here too.
2.3. Outcomes
The primary aim of this study was to evaluate both qualitative and quantitative CT features of SARS-CoV-2 pneumonia in patients with distinct disease severity and to assess the value of quantitative CT in predicting the risk of critical illness. The secondary purpose was to assess the relationship between laboratory data and the extension of lung involvement.
2.4. Statistical analysis
Statistical analysis was performed using the SPSS statistical software (ver. 26.0; SPSS Inc. Chicago, IL). Continuous data are expressed by median (interquartile range). We used the χ² test and the Mann-Whitney test to compare differences in CT features and percentage of involved lung volume between the two groups. A Receiving Operator Characteristic (ROC) curve analysis was performed and the area under the ROC curve (AUC) was calculated to evaluate the differential diagnosis ability of the CT quantitative method in critically ill and non-critically ill patients. The optimal cut-off value, sensitivity, and specificity were determined by calculating the Youden index. Moreover, correlation analyses between laboratory variables and the percentage of affected lung in both groups were performed using Pearson's correlation coefficient. In all of the statistical analyses, the level of significance was set at 0.05.
3. Results
3.1. Population
Between March 5th and March 24th 2020, 262 patients were admitted to our institution for SARS-CoV-2 infection. Among these, 42 had underlying pulmonary or pleural disease; 31 patients presented with mild symptoms and did not require a CT scan at admission. Thus, 189 patients were included in the final analysis. Among these, 2 patients were intubated at the time of CT scan and 25 required invasive mechanical ventilation after CT examination. These patients (27, 14.3 %) were directed toward the ICU, while the remaining patients (162, 85.7 %) were directed toward the infectious disease department. Baseline characteristics are summarized in Table 1 . 110 out of 189 patients had comorbidities, with hypertension being the most common.
Table 1.
Patients baseline characteristics. Unless otherwise noted, data are numbers of patients, with percentages in parentheses.
| Total (N = 189) | Non-critically ill (N = 162) | Critically ill (N = 27) | p value | |
|---|---|---|---|---|
| Age (years)* | 61 (53.8−70.3) | 58.3 (51.2−68.9) | 70 (59.5−79.3) | 0.061 |
|
Gender Male Female |
121 (64.0 %) 68 (36.0 %) |
99 (61.1 %) 63 (38.9 %) |
22 (81.5 %) 5 (18.5 %) |
0.641 0.641 |
| Time from illness onset to hospital admission (days)* | 6 (5−8) | 7 (5−8) | 6 (5−7) | 0.438 |
|
Comorbidities Hypertension Diabetes Dyslipidemia Obesity Cancer Heart Disease CKD 2 Comorbidities 3 Comorbidities |
70 (37.0 %) 11 (5.8 %) 11 (5.8 %) 5 (2.6 %) 14 (7.4 %) 10 (5.3 %) 3 (1.6 %) 21 (11.1 %) 8 (4.2 %) |
57 (35.2 %) 6 (3.7 %) 6 (3.7 %) 3 (1.8 %) 12 (7.4 %) 9 (5.5 %) 3 (1.8 %) 15 (9.3 %) 6 (3.7 %) |
13 (48.1 %) 5 (18.5 %) 5 (18.5 %) 2 (7.4 %) 2 (7.4 %) 1 (3.7 %) 0 (0.0 %) 6 (22.2 %) 2 (7.4 %) |
0.197 0.002 0.002 0.096 1 0.692 0.479 0.047 0.379 |
|
Venous Blood Sample* LDH (UI/L) Lymphocytes (x 103/μL) |
336.3 (259.8−441.5) 0.8 (0.6−1.2) |
281.5 (232.5−354.2) 0.9 (0.7−1.2) |
451.4 (386.2−592.5) 0.6 (0.5−0.8) |
<0.001 0.675 |
|
ABG* SaO2 (%) PaO2/FIO2 |
97 (93−98) 323 (263−393) |
98 (97−98) 361 (322−446) |
91 (87−94) 248 (190−259) |
<0.001 <0.001 |
*Median Value, with Interquartile Range (IQR) in parenthesis.
CKD = Chronic Kidney Disease, LDH = lactate dehydrogenase, ABG = arterial blood gas, SaO2= Oxygen saturation, Pa02= arterial pressure of oxygen, FiO2=fraction of inspired oxygen.
Median LDH and median Lymphocyte count were respectively increased and decreased in both groups; ABG test revealed a reduction of SaO2 and PaO2/FIO2 ratio in critically ill patients.
3.2. Imaging features
COVID-19 CT features and their distribution in the two groups are shown in Table 2 .
Table 2.
Imaging findings. Data are number of patients with percentages in parentheses. p values were calculated by χ2 test.
| Total (N = 189) | Non-critically ill (N = 162) | Critically ill (N = 27) | p value | |
|---|---|---|---|---|
| Findings | ||||
| GGO | 50 (26.5 %) | 48 (29.6 %) | 2 (7.4 %) | 0.023 |
| Consolidation | 17 (9.0 %) | 15 (9.3 %) | 2 (7.4 %) | 0.78 |
| GGO + consolidation | 113 (59.8 %) | 90 (55.6 %) | 23 (85.2 %) | 0.008 |
| Halo sign | 3 (1.6 %) | 3 (1.9 %) | – | 0.477 |
| Crazy paving | 96 (50.8 %) | 72 (44.4 %) | 24 (88.9 %) | <0.0001 |
| Bronchial wall thickening | 154 (81.5 %) | 129 (79.6 %) | 25 (92.6 %) | 0.134 |
| Air bronchogram | 88 (46.6 %) | 69 (42.6 %) | 19 (70.4 %) | 0.018 |
| Bronchial distortion | 41 (21.7 %) | 30 (18.5 %) | 11 (40.7 %) | 0.031 |
| Linear opacities | 89 (47.1 %) | 76 (469%) | 13 (49.7 %) | 0.243 |
| Vascular enlargement | 34 (18.0 %) | 24 (14.8 %) | 10 (37.0 %) | 0.023 |
| Distribution | ||||
| Central | 6 (3.17 %) | 6 (3.7 %) | – | 0.311 |
| Peripheral | 135 (71.4 %) | 123 (75.9 %) | 12 (44.4 %) | 0.005 |
| Central + peripheral | 42 (22.2 %) | 27 (16.7 %) | 15 (55.6 %) | <0.0001 |
| Lymph node involvement | 44 (23.3 %) | 30 (18.5 %) | 14 (51.9 %) | 0.002 |
GGO = ground glass opacities.
GGO alone was significantly more frequent in non-critically ill patients (29.6 % vs. 7.4 %, p < 0.05), whereas the mixed pattern (GGO with consolidation) was significantly more frequent in critically ill patients (85.2 % vs. 55.6 %, p < 0.05). “Crazy paving” pattern, bronchial wall thickening and vascular enlargement were significantly more frequent in critically ill patients (respectively 88.9 % vs. 44.4 %, 70.4 % vs. 42.6 % and 37.0 % vs. 14.8 %, p < 0.05).
Peripheral distribution was more frequently observed in non-critically ill patients (75.9 % vs. 44.4 %), p < 0.05), whereas both peripheral and central distribution was more frequent in patients with critical disease (55.6 % vs. 16.7 %, p < 0.05).
Lymph node involvement was significatively more frequent in critically ill patients (51.9 % vs 18.5 %, p < 0.05).
Among non-critically ill patients, 6 subjects had no CT features consistent with pneumonia; no patients with a normal CT were observed in the critically ill group.
3.3. Quantitative analysis of lung volume involvement
The time required for the measurement was influenced by the learning curve of the operators and the actual volume of disease extension. However, the overall duration of calculation for each patient ranged between 18 and 39 min, with a median value of 23 min.
The median percentage of lung involvement was 6.0 % (IQR = 2.7–12.1) in non-critically ill patients and 39.9 % (IQR = 28.2–53.8) in critically ill patient, with a significant difference between the two groups (p < 0.01) (Table 3 ).
Table 3.
Volume findings. Data are Median Value of percentages (%), with Interquartile Range (IQR) in parenthesis. p values were calculated by Mann-Whitney test.
| Total (N = 189) | Non-critically ill (N = 162) | Critically ill (N = 27) | p value | |
|---|---|---|---|---|
| RUL | 2.6 (0.5–7.8) | 2.1 (0.4–5.3) | 38.3 (30.1–53.4) | |
| RML | 2.4 (0.4–8.3) | 1.9 (0.3–6.5) | 37.0 (20.4–57.6) | |
| RLL | 14.8 (5.6–27.0) | 10.50 (4.6–21.0) | 268 (8,7–47.2) | |
| Right lung | 8.5 (2.6–18.9) | 6.80 (2.3–13.7) | 55.5 (39.6–79.1) | |
| LUL | 3.8 (0.4–14.3) | 3.1 (0.3–7.9) | 49.25 (27.4–57.8) | |
| LLL | 9.1 (2.4−29.8) | 6.8 (1.88–21.9) | 31.06 (18.6–45.5) | |
| Left lung | 6.9 (2.7–20.8) | 6.43 (1.9–12.7) | 54.1 (39.7–69.0) | |
| Right + left lung | 7.8 (3.6–15.6) | 6.0 (2.7–12.1) | 39.9 (28.2–53.8) | <0.0001 |
RUL = Right Upper Lobe; RML = Right Middle Lobe; RLL = Right Lower Lobe; LUL = left Upper Lobe;; LLL = left Lower Lobe.
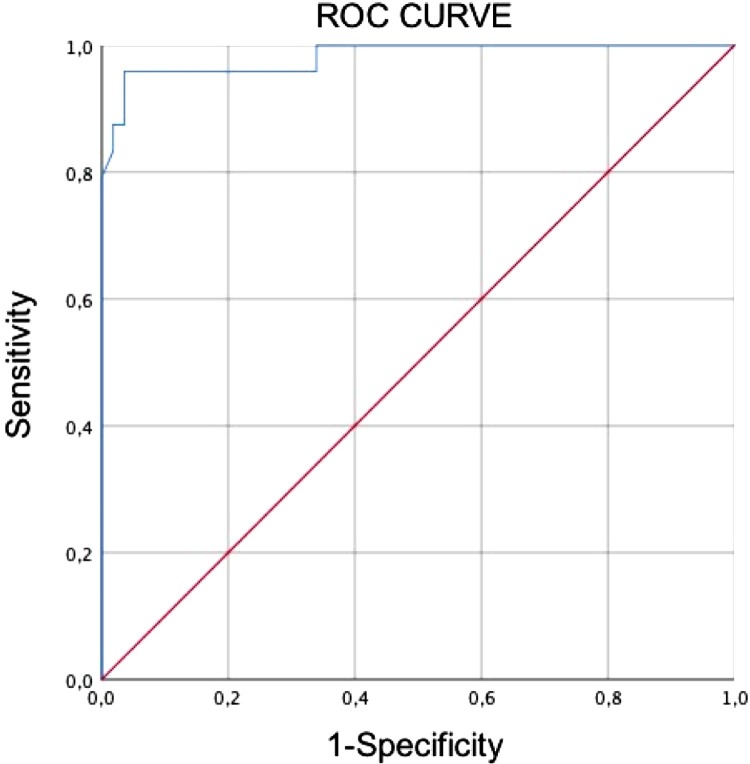
The percentage of lung volume involvement calculation yielded an AUC value of 0.982 (CI 95 % = 0.953–1.000). The cut-off of 23.0 % of lung involvement had 96 % sensitivity and 96 % specificity in distinguishing critically ill from non-critically ill patients (Fig. 3 ).
Fig. 3.
ROC curve analysis evaluating the differential diagnosis ability of quantitative CT in critically ill and non-critically ill patients. The area under the curve (AUC) of affected lung (%) for diagnosing critically ill disease was 0.982 (95 %CI 0.953–1.000). The cut-off of 23.0 % had 96 % sensitivity and 96 % specificity.
In the overall population, Pearson’s correlation coefficient revealed a positive relationship between the percentage of lung involvement and the LDH level (r = 0.718, p < 0.05) and a negative relationship between the percentage of lung involvement and SaO2 (r=-0.690, p < 0.05) and PaO2/FiO2 ratio (r=-0.684, p < 0.05).
4. Discussion
4.1. Population
In comparison to other studies [20], we found a higher percentage of critically ill patients requiring ICU management and invasive mechanical ventilation (27/189, 14 %). This result likely reflects different factors: a) a lower number of swabs performed in asymptomatic/mildly symptomatic individuals; b) this study was carried out during the beginning of COVID-19 outbreak in our region, when ICU beds and furniture were relatively widely available in our center, that was entirely dedicated to COVID-19 patients; c) differently from first experiences in China, endotracheal intubation is now considered as a proactive support tool rather than a salvage therapy, so a more extensive use is encouraged [12]; d) CT-scan was not offered to all COVID-19 patients admitted in our emergency department: patients with mild clinical presentation underwent only chest X-ray as imaging examination, so they were excluded from this study.
In this study, we mainly focused on laboratory parameters reflecting tissue damage or impaired respiratory physiology; for that reason, we did not include d-dimer measurement in our analysis, even though it has been shown to be associated with an increased risk of thromboembolic events in patients with COVID-19 [21,22] and it is considered as an important parameter for patients stratification [11]. Overall, results from laboratory tests are comparable with available data from literature: PaO2/FIO2 ratio, commonly used as reference parameters for the assessment of acute respiratory distress syndrome (ARDS) severity [12,23], is significantly lower in critically ill patients, while LDH (proposed in several studies as a parameter that could be correlated to the severity of the disease [11]) is significantly higher. Lymphopenia has been previously identified as a typical feature in patients with COVID-19 and it has been associated with poor prognosis [11]; however, we did not find any relationship between lymphocyte count and extension of lung involvement.
4.2. Imaging features
The evaluation of imaging features highlighted a higher prevalence of GGO pattern in non-critically ill patients, and a higher frequency of mixed pattern (GGO + consolidation), “crazy paving” and vascular enlargement in critically ill patients. These results are in phase with previous studies investigating the evolution of CT features over time in patients with pneumonia caused by SARS-CoV-2 [14,[24], [25], [26]] and other human Coronaviruses (SARS-CoV that caused the 2002−03 epidemic and Middle East Respiratory Syndrome-Coronavirus [MERS-CoV]) [27]: the CT features observed in patients with critical disease reflect a more advanced and severe presentation and are related with a greater reduction of respiratory exchanges; in particular, the combined presence of consolidation and GGO is a sign of alveolar damage at different stages, while interlobular thickening is a sign of interstitial pathology; on the other hand, GGO alone depicts an incomplete alveolar filling, that is usually present in the earlier and less severe phases of the disease [24].
It has been suggested that the enlargement of subsegmental vessels could reflect the hyperemia induced by SARS-CoV-2 infection and might be the result of pro-inflammatory factors release (15); however, there is still no clear evidence on the actual role of this imaging feature, nor on the reason why it is more frequent on critically ill patients.
Combined central and peripheral distribution was significantly more frequent in critical disease: since no significant difference was found between the two groups for peripheral or central distribution alone, we believe that this result may simply represent the involvement of larger volumes in patients with more severe disease.
In our study about a half of patients with critical disease had lymph node enlargement. Caruso et al., from the same region of Italy, already reported a high percentage (59 %) of mediastinal lymphadenopathies in patients with COVID-19, which was not described in other studies worldwide [27]. Some authors described a cytokine release syndrome (CRS), which is more frequent in critically ill patients and might explain the volumetric increase of lymph nodes [28].
4.3. Quantitative analysis of lung volume involvement
COVID-19 disease has a highly variable clinical presentation, from asymptomatic up to acute respiratory distress symptoms and death [2,3]. Currently, there are only a few studies evaluating the relationship between the extension of lung disease and the need for ICU management [16,17]; these studies, based on limited samples of patients, rely on a visual method for the assessment of pulmonary involvement. By direct calculation of lung volumes, the introduction of a quantitative analysis for the assessment of disease extension may offer a more accurate estimation and may overcome the disadvantages of a visual method, including the interobserver variability [13,29].
Our results demonstrate that the percentage of lung volume involvement has a high AUC value in ROC curve analysis, therefore this parameter may be useful in predicting critical illness and the need for ICU in COVID-19 patients: greater volumes of affected parenchyma are associated with more widespread impairment of alveolar and interstitial space, and more prominent reduction of the surface for respiratory exchanges. This aspect is also in accordance with pulse oximetry, ABG and LDH values.
The median value of lung involvement in critically ill patients was 39.95 %. However, some patients who required invasive mechanical ventilation had a low percentage of affected lung (10–20 %). Noteworthy, differently from other forms of ARDS [30], in SARS-CoV-2 pneumonia a severe hypoxemia may occur also in patients with a well-preserved lung gas volume, as reported by Gattinoni et al. [31]. The presence of a subgroup of patients with a low extension of lung disease among critically ill subjects can explain the low cut-off value derived from the ROC curve analysis (23.0 % of lung involvement).
4.4. Limitations
This study has several limitations. First of all, this is a retrospective study carried out on a relatively limited number of patients: subjects with underlying pulmonary and pleural diseases (that have been associated with poor prognosis) were excluded from the analysis, since these conditions could compromise the quantitative assessment of lung volumes; at the same time, patients with mild disease did not undergo CT at admission, according to our hospital internal protocol, and thus were excluded from the study. Besides, the CT quantitative analysis is a relatively time-consuming process for manual calculation: however, the increasing introduction of automatic systems might allow the integration of this method as a support for decision making and clinical management of patients with SARS-CoV-2 infection. Moreover, there were no available data on d-dimer values, which are considered as a prognostic factor [11]. Finally, our assessment was based on imaging and laboratory findings acquired during patient admission, and we did not evaluate the evolution of different parameters over time with follow-up.
Conclusion
Our study demonstrates that the extension of lung disease assessed using a quantitative method has a significant relationship with disease severity and may predict the need for invasive mechanical ventilation in patients with SARS-CoV-2 infection.
CRediT authorship contribution statement
Andrea Leonardi: Conceptualization, Methodology, Formal analysis, Investigation, Writing - original draft. Roberto Scipione: Methodology, Formal analysis, Investigation, Writing - original draft. Giulia Alfieri: Methodology, Formal analysis, Writing - original draft, Visualization. Roberta Petrillo: Resources, Data curation, Writing - original draft. Miriam Dolciami: Resources, Data curation, Writing - original draft. Fabio Ciccarelli: Resources, Data curation, Writing - original draft. Stefano Perotti: Methodology, Validation, Writing - review & editing. Gaia Cartocci: Resources, Data curation, Writing - original draft. Annarita Scala: Resources, Data curation, Writing - original draft. Carmela Imperiale: Resources, Writing - original draft. Franco Iafrate: Formal analysis, Data curation, Writing - review & editing. Marco Francone: Formal analysis, Data curation, Writing - review & editing. Carlo Catalano: Supervision, Writing - review & editing. Paolo Ricci: Conceptualization, Writing - review & editing, Supervision.
Contributor Information
Andrea Leonardi, Email: andrea.leonardi1988@gmail.com.
Roberto Scipione, Email: robertoscipione90@gmail.com.
Giulia Alfieri, Email: giuliaalfieri@hotmail.it.
Roberta Petrillo, Email: roberta.petrillo@hotmail.it.
Miriam Dolciami, Email: miriam.dolciami@gmail.com.
Fabio Ciccarelli, Email: fabio_ciccarelli@hotmail.it.
Stefano Perotti, Email: stefano.perotti@uniroma1.it.
Gaia Cartocci, Email: gaia.cartocci@uniroma1.it.
Annarita Scala, Email: aritascala@gmail.com.
Carmela Imperiale, Email: carmelaimperiale2@gmail.com.
Franco Iafrate, Email: francoiafrate@gmail.com.
Marco Francone, Email: marco.francone@uniroma1.it.
Carlo Catalano, Email: carlo.catalano@uniroma1.it.
Paolo Ricci, Email: paolo.ricci@uniroma1.it.
References
- 1.2020. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19 -. 11 March 2020.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-.11-march-2020 (accessed 5 April 2020) [Google Scholar]
- 2.Thomas-Rüddel D., Winning J., Dickmann P. Coronavirus disease 2019 (COVID-19): update for anesthesiologists and intensivists March 2020. Anaesthesist. 2020:1–10. doi: 10.1007/s00101-020-00758-x. [DOI] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M., J. M Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Resp Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q., Yang K., Wang W. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao J., Hu X., Cheng W., Yu L. Clinical features and short-term outcomes of 18 patients with coronavirus disease 2019 in intensive care unit. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ESICM Webinar. Of Intensive Care Medicine. 2020. Medicine ESOIC, ESICM webinar—update on coronavirus. [Google Scholar]
- 9.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng L., Qiu H., Wan L. Intubation and ventilation amid the COVID-19 outbreak: wuhan’s experience. Anesthesiology. 2020 doi: 10.1097/ALN.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernheim A., Mei X., Huang M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H., Hong H., Yoon S.H. Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology. 2020:201343. doi: 10.1148/radiol.2020201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao W., Zhong Z., X Xie. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am. J. Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 17.Li K., Fang Y., Li W. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur. Radiol. 2020:1–10. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombi D., Bodini F.C., Petrini M., Maffi G., Morelli N., Milanese G., Michieletti E. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020:201433. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.7th ed. 2020. China National Health Commission, Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment.http://kjfy.meetingchina.org/msite/news/show/cn/3337.html (Accessed April 14 2020) [Google Scholar]
- 20.Guan W.J., Liang W.H., Zhao Y. Comorbidity and its impact on 1590 patients with Covid-19 in China: a Nationwide Analysis. Eur. Respir. J. 2020 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danzi G.B., Loffi M., Galeazzi G. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotzinger D.C., Beigelman-Aubry C., von Garnier C., Qanadli S.D. Pulmonary embolism in patients with COVID-19: time to change the paradigm of computed tomography. Thromb. Res. 2020 doi: 10.1016/j.thromres.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broccard A.F. Making sense of the pressure of arterial oxygen to fractional inspired oxygen concentration ratio in patients with acute respiratory distress syndrome. OA Crit Care. 2013;1:1. [Google Scholar]
- 24.Shi H., Han X., Zheng C. Evolution of CT manifestations in a patient recovered from 2019 novel coronavirus (2019-nCoV) pneumonia in Wuhan, China. Radiology. 2020;295 doi: 10.1148/radiol.2020200269. 20-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding X., Xu J., Zhou J., Long Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur. J. Radiol. 2020;109009 doi: 10.1016/j.ejrad.2020.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Q., Liu Q., Xu H., Lu H., Liu S., Li H. Imaging of coronavirus disease 2019: a Chinese expert consensus statement. Eur. J. Radiol. 2020:109008. doi: 10.1016/j.ejrad.2020.109008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caruso D., Zerunian M., Polici M. Chest CT Features of COVID-19 in Rome, Italy. Radiology. 2020;2020:201237. doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Y., Wang Y., Shao C. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ooi G.C., Khong P.L., Müller N.L. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology. 2004;230(3):836–844. doi: 10.1148/radiol.2303030853. [DOI] [PubMed] [Google Scholar]
- 30.Xie J., Tong Z., Guan X. 2020. Critical Care Crisis and Some Recommendations During the COVID-19 Epidemic in China. Intensive Care Medicine; pp. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gattinoni L., Chiumello D., Caironi P. COVID-19 pneumonia: different respiratory treatment for different phenotypes? Intensive Care Med. 2020 doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]