Abstract
Background
The outbreak of a new coronavirus (SARS-CoV-2) poses a great challenge to global public health. New and effective intervention strategies are urgently needed to combat the disease.
Methods
We conducted an open-label, non-randomized, clinical trial involving moderate COVID-19 patients according to study protocol. Patients were assigned in a 1:2 ratio to receive either aerosol inhalation treatment with IFN-κ and TFF2, every 48 h for three consecutive dosages, in addition to standard treatment (experimental group), or standard treatment alone (control group). The end point was the time to discharge from the hospital. This study is registered with chictr.org.cn, ChiCTR2000030262.
Findings
A total of thirty-three eligible COVID-19 patients were enrolled from February 1, 2020 to April 6, 2020, eleven were assigned to the IFN-κ plus TFF2 group, and twenty-two to the control group. Safety and efficacy were evaluated for both groups. No treatment-associated severe adverse effects (SAE) were observed in the group treated with aerosol inhalation of IFN-κ plus TFF2, and no significant differences in the safety evaluations were observed between experimental and control groups. CT imaging was performed in all patients with the median improvement time of 5.0 days (IQR 3.0–9.0) in the experimental group versus 8.5 days (IQR 3.0–17.0) in the control group (p<0.05). In addition, the experimental group had a significant shorten median time in cough relief (4.5 days [IQR 2.0–7.0]) than the control group did (10.0 days [IQR 6.0–21.0])(p<0.005), in viral RNA reversion of 6.0 days (IQR 2.0–13.0) in the experimental group vs 9.5 days (IQR 3.0–23.0) in the control group (p < 0.05), and in the median hospitalization stays of 12.0 days (IQR 7.0–20.0) in the experimental group vs 15.0 days (IQR 10.0–25.0) in the control group (p<0.001), respectively.
Interpretation
Aerosol inhalation of IFN-κ plus TFF2 is a safe treatment and is likely to significantly facilitate clinical improvement, including cough relief, CT imaging improvement, and viral RNA reversion, thereby achieves an early release from hospitalization. These data support to explore a scale-up trial with IFN-κ plus TFF2.
Funding
National Major Project for Control and Prevention of Infectious Disease in China, Shanghai Science and Technology Commission, Shanghai Municipal Health Commission.
Keywords: COVID-19, SARS-CoV-2, TFF2, IFN-κ, Aerosol inhalation, Clinical trial, Safety and efficacy
1. Introduction
In December 2019, a novel pneumonic illness caused by a new coronavirus, SARS-CoV-2 (previously named as 2019-nCoV), emerged in Wuhan, China, which was subsequently called COVID-19 [1]. The full disease spectrum of COVID-19 ranges from a mild, moderate, self-limiting respiratory tract illness that may rapidly progress to severe progressive pneumonia, multiorgan failure, and death [2], [3], [4], [5]. As of April 26, 2020, confirmed cases have been reported in 211 countries over the world, with more than 2,800,000 confirmed cases and 193,000 deaths, according to the statistics from World Health Organization. Furthermore, at the time of this writing, the epidemic is worsening globally and impacting hundreds of thousands of persons, posing a great challenge to public health as well as global economies. At the present time, there are no specific therapeutic agents against the new virus. Recently, a trial of lopinavir–ritonavir treatment in adults hospitalized with severe COVID-19 was conducted, however, no benefit was observed beyond standard supportive care [6]. Two recent studies have shown that clinical improvement was observed in 36 of 53 severe COVID-19 patients (68%) when they received a 10-day course of remdesivir [7], there is no significant difference between a 5-day and a 10-day course of remdesivir administration [8]. Chloroquine (CQ) or hydroxychloroquine (HCQ) were considered as the most promising agents against COVID-19 at the early time of the SARS-Cov-2 pandemic. Up to date, the therapeutic efficacy of HCQ in COVID-19 patients remains controversial [9,10], clinical trials to test the efficacy of hydroxychloroquine are ongoing. Under this circumstance, effective prevention and treatment approaches are urgently needed to combat the disease.
Previous clinical trials during the SARS epidemic have shown that type I interferons (such as IFN-α, IFN-β) could effectively inhibit the replication of SARS-CoV-1 and thereby attenuated the clinical complications [11]. Recent study had shown that SARS-CoV-2 was sensitive to type I interferon pretreatment in Vero cells [12]. However, the persistent inflammatory response resulted from IFN-α/β may cause the damage to infected patients. Indeed, as animal experiments showed that IFN-α/β treatment induced influx of pathogenic inflammatory monocytes and vascular leakage, this aggravates the inflammatory damages [13]. Therefore, systemic adverse reactions of IFN-α/β would also be a concern. IFN-κ is a relatively mild type I interferon, which can effectively inhibit the replication of enveloped viruses including EMCV, IAV, HCV, and others, by activating the interferon-stimulated response element signaling pathway [14]. However, different from IFN-α2 or IFN-β, the antiviral activity of IFN-κ is cell-associated [15] and it inhibits the replication of influenza virus largely relying on IFNAR-MAPK-Fos-CHD6 axis [16] whereas IFN-α2 or IFN-β mainly employ STAT1 pathway. Recently, we further verified that IFN-κ significantly suppressed the replication of SARS-Cov-2 in Huh7 cell line (data not shown). Trefoil factor 2 (TFF2) is a secreted polypeptide with three disulfide bonds, resistant to acid, heat, and protease hydrolysis, and able to protect the gastrointestinal tract from microbial or chemical induced injury by promoting the repair of injury and regulating the immune response [17], [18], [19]. In model of asthma, TFF2 is increased in response to epithelial injury, exerts a protective role in limiting the progression of airway remodeling, and contributes to airway epithelial repair [20]. In addition, TFF2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection [21]. TFF2 also potently accelerates healing and reduces inflammation in a rat model of colitis [22]. Our researches in mice model demonstrated that TFF2 reduced the morbidity and mortality in influenza infection by attenuating the inflammatory responses and improving the pulmonary function, but not by inhibiting viral replication (Patent application No:201610104936.8). Recently, a study has shown that imbalanced host response to SARS-CoV-2, with limited IFN response coupled with exuberant inflammatory cytokines/chemokines, drives development of COVID-19 [23]. Based on these findings, we hypothesized that the combined administration of human derived IFN-κ and TFF2 would be likely synergistically to fight against SARS-CoV-2 induced respiratory pneumonia by reducing viral replication, attenuating inflammatory responses, and improving the reconstitution of respiratory tract mucosa, so as to improve the prognosis of patients with COVID-19. Therefore, to evaluate the efficacy and safety of intranasal inhalation of TFF2 and IFN-κ protein for SARS-CoV-2 infection, we conducted an open-label, non-randomized, clinical trial in adult patients hospitalized with moderate COVID-19 disease in China.
2. Methods
2.1. Study design
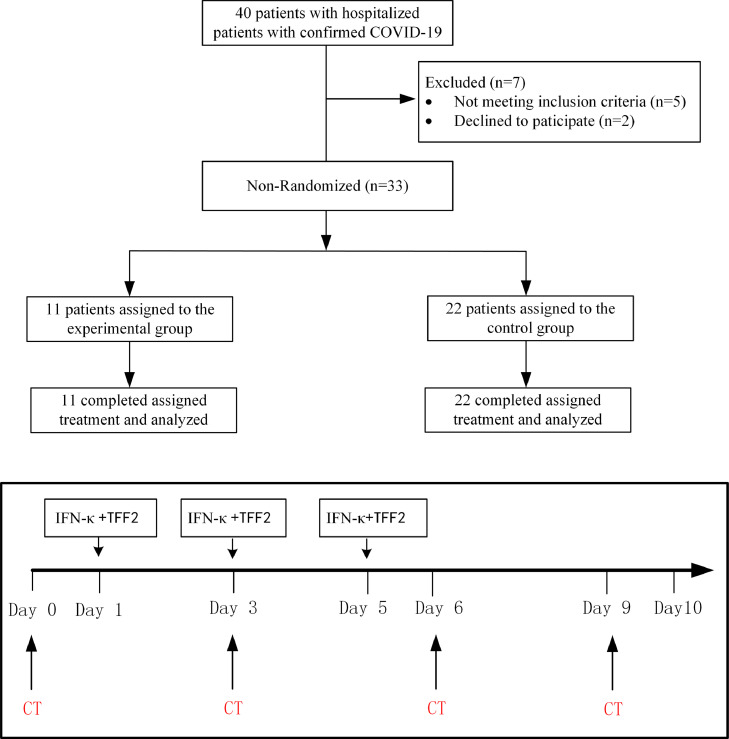
This was an open-label, non-randomized pilot clinical trial conducted from February 1, 2020 through April 6, 2020 at Shanghai Public Health Clinical Center, Shanghai, China. The protocol of the clinical treatment assigned criteria was carried out according to a stratification flowchart and treatment assignment (Fig. 1). Written informed consents were provided by all study participants, patients either accept IFN-κ plus TFF2 plus standard treatment as experimental group or receive standardized treatment alone as control group. As this is a pilot study to primarily test the safety, we recruited 11 patients in IFN-κ plus TFF2 group (a similar sample size to a Phase Ia), and set the ratio to control group as 1:2 (22 patients in control) in order to achieve reliable safety data. Standard supportive care was given according to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) released by National Health Commission & State Administration of Traditional Chinese Medicine on March 3, 2020).
Fig. 1.
Stratification flowchart and treatment assignment. Stratification flowchart of selection and follow-up of subjects with moderate COVID-19 with or without IFN-κ plus TFF2 treatment.
The primary objective of the pilot study was to evaluate the safety of aerosol inhalation of IFN-κ plus TFF2. All adverse events (AE) were based on the ICH (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use). In this trial, any AE from the beginning of aerosol inhalation to 5 days after the end of the last aerosol inhalation were taken as an adverse event during treatment (TEAE); The secondary objective of the pilot study was to evaluate the clinical efficacy of IFN-κ plus TFF2 as compared to the control group as assessed by days of hospitalization staying, CT imaging improvement and cough relief time and negative reversion of viral RNA after 10 days of treatment.
The trial was approved by Ethics Committee in Shanghai Public Health Clinical Center (Approval number: YJ-2020-S008-01). The trial was conducted in accordance with the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on harmonization. All participant personal information will be only accessed by the researchers and assigned members of the Hospital Ethics Committee.
2.2. Patients
Hospitalized patients with confirmed COVID-19 were enrolled in this study. Identification of SARS-CoV-2 via reverse-transcriptase-polymerase chain-reaction (RT-PCR) in a throat-swab sample was carried out by the local Center for Disease Control (CDC) or by a designated diagnostic laboratory following the recommendation of the China National Center for Disease Control. Inclusion Criteria: Patients willing and able to provide written informed consent prior to performing study procedures were included. Male and nonpregnant female patients 18 years of age or elder were eligible if they had a diagnostic specimen that was positive on RT-PCR for SARS-CoV-2. In addition, patients could be included in peripheral capillary oxygen saturation (SpO2) > 94% on room air at screening. Symptoms of infection are non-specific, and include fever, cough, and myalgia, with diarrhea, without the subsequent development of dyspnea. Patients with moderate pneumonia were then included following the diagnosis and treatment plan for the novel coronavirus disease (COVID-19) issued by the National Health Commission (NHC). Exclusion criteria: These included a physician's decision that involvement in the trial was not in the patient's best interest, presence of any condition that would not allow the protocol to be followed safely, known allergy or hypersensitivity to IFN-κ and TFF2, known severe liver disease (e.g., cirrhosis, with an alanine aminotransferase level >5 × the upper limit of the normal range or an aspartate aminotransferase level >5 × the upper limit of the normal range). Breastfeeding and pregnant patients were also excluded.
A total of 33 COVID-19 patients with moderate illness symptoms were recruited. The enrolled patients were non-randomly divided into two groups based on their options. Patients in the control group received standard of care treatment according to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) released by National Health Commission & State Administration of Traditional Chinese Medicine on March 3, 2020), including anti-fever tablets (if necessary), immune enhancers and Traditional Chinese Medicines. Anti-fever tablets include Ibuprofen Suspension, immune enhancers include Thymosin Alpha-1 for injection, Compound Vitamines of injection, Traditional Chinese Medicines include Chinese hebal Medicines, ShuFengJiDu capsule, Lianhua Qingwen capsule. In addition to standard treatment for COVID-19, patients in the experimental group also received aerosol inhalation treatment with 5 mg TFF2 protein and 1~2 mg (one million U/mg) IFN-κ. Written informed consent was signed by all enrolled patients.
2.3. Procedures
The combination of IFN-κ plus TFF2 was administrated to combat against COVID-19 since IFN-κ is able to inhibit SARS-CoV-2 replication in vitro (data not shown) while TFF2 is capable of reducing inflammation and promoting respiratory repairment (Patent application No: CN201610104936.8). Interestingly, our previous research demonstrated that IFN-κ could suppress influenza replication through a different mechanism from IFN-α2 [16]. As known, type I interferons may aggravate the inflammatory damages [13] or disrupt lung epithelial repair during recovery from viral infection [24]. Therefore, to maximize the efficacy and to minimize the risk, we employed these two molecules to synergistically treat COVID-19 patients. The preliminary effective dose of TFF2 in humans is based on the effective protection of TFF2 in influenza virus infection according to the Meeh-Rubner conversion formula. As for dosing of IFN-κ, we referred to the dosage of IFNα2b (9 × 105IU) in nasal sprays in previous study [25].
Aerosolized drug was made of purified mature TFF2 and IFN-κ protein produced under Good Manufacturing Practices (GMP) conditions, the purity was more than 99%, and the biological activities of these two proteins were verified in vitro. In addition, the drug was given to the nose inhalation only by face mask. Specifically, both proteins were dissolved in 5 mL sterilized water, and the aerosol was delivered for 20~30 min by nasal mask driven by medical compressed air atomizer (YUWELL, 403M). The aerosol inhalation treatment started from the first day of hospitalization, and included receiving the aerosol treatment 3 times every 48 h. After treatment, a survey was implemented to evaluate if there were any adverse reactions during the course of aerosol inhalation.
Patients were assessed once daily by trained doctors and nurses using diary cards that captured data in a timely fashion that recorded in an electronic medical record. Safety measures, adverse outcomes were monitored by the Good Clinical Practice office from Shanghai Public Health Clinical Center. Serial throat-swab samples were obtained at day 0 (baseline level) and day 1, 3, 6, 9, 10, and sent to Shanghai Public Health Clinical Center designated laboratory, SARS-CoV-2 viral load was assessed by real-time reverse transcription-PCR [26] until patients were discharged. Clinical data were recorded into an electronic database and validate by trial staff.
2.4. Outcomes
The primary objective of the study was to evaluate the safety of aerosol inhalation of IFN-κ plus TFF2; The secondary objectives of the study were to evaluate the clinical efficacy of IFN-κ plus TFF2 as compared to the control group as assessed by days of hospitalization stay, improvement in CT imaging, resolution of cough, and the rate of negative viral RNA after 10 days of treatment.
Safety data included adverse events that occurred during treatment, severe adverse events, and premature discontinuation of treatment. Adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
2.5. Statistical analysis
The trial was initiated in rapid response to the COVID-19 public health emergency, at which time there was very limited information about clinical outcomes in hospitalized patients with COVID-19. Baseline comparisons of clinical and demographic characteristics according to study group allocation were done using the Mann–Whitney U test for non-parametric data. Continuous variables were compared also using the Mann–Whitney U test for non-parametric data, for both IFN-κ plus TFF2 group and the control group analyses for the outcome. Quantitative index will be described as median value with interquartile range (IQR). All statistics were conducted on a two-sided, and p-value less than or equal to 0.05 was considered statistically significant. Safety analyses were based on the patients’ actual treatment exposure. Statistical analysis was performed in Graph Pad PRISM6. This trial was registered with Chinese Clinical Trial Registry (Website: http://www.chictr.org.cn; Trial title: Clinical study for combination of anti-viral drugs and type I interferon and inflammation inhibitor TFF2 in the treatment of novel coronavirus pneumonia (COVID-19); Number: ChiCTR2000030262).
2.6. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing other report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
From February 1, 2020 to April 6, 2020, a total of 33 COVID-19 patients with moderate illness symptoms were recruited, 11 patients were assigned to the experimental group, the left 22 patients only received standard supportive care and were classified as the control group. Baseline clinical characteristics, including age, gender, demographic characteristics, laboratory test results, were shown in Table 1. No significant differences were observed between two groups except for total white blood cells (WBCs) that were significantly lower in control group than the experimental group, higher WBCs may relate to more severe disease as previously reported [27]. No discomfort complications were reported to nurses for all experimental patients during aerosol inhalation.
Table 1.
Clinical characteristics of the COVID-19 patients at baseline.
| Characteristic | Total (N = 33) | IFN-κ+TFF2 (N = 11) | Control (N = 22) | p value |
|---|---|---|---|---|
| Male sex-no. (%) | 19(57.6%) | 7(63.6%) | 12(54.5%) | |
| Age median (IQR)-yr | 50(28–58) | 45(28–52.5) | 55.5(28.3–59.8) | 0.33 |
| Underlying diseases: | ||||
| Diabetes no. (%) | 6(18·2%) | 2(18·2%) | 4(18·2%) | |
| Hypertension no. (%) | 5(15·1%) | 0(0·0%) | 5(15·1%) | |
| Hyperlipidnemid no. (%) | 1(3·0%) | 1(9·1%) | 0(0·0%) | |
| Obstrctive sleep apnea-hypopnea syndrome no. (%) | 1(3·0%) | 1(9·1%) | 0(0·0%) | |
| Cough-no. (%) | 23(69.7%) | 8(72.7%) | 15(68.2%) | |
| Ct median (IQR) | 27.3(25.1–31.3) | 28.9(27.5–31.9) | 26.7(23.8–30.6) | 0.25 |
| Body temperature, median (IQR)- °C | 37.5(36.9–38.2) | 37.5(36.8–37.6) | 37.7(37.4–38.3) | 0.1 |
| Fever-no. (%) | 22(78.6%) | 6(60%) | 16(88.9%) | |
| White-cell count (× 109/liter) -median (IQR) | 5.2(4 −6.4) | 6.4(5.4–7.2) | 4.4(3.9–5.8) | 0.04 |
| 4–10 × 109/liter-no. (%) | 26(78.8%) | 10(90.9%) | 16(72.7%) | |
| <4 × 109/liter-no. (%) | 7(21.2%) | 1(9.1%) | 6(27.8%) | |
| >10 × 109/liter-no. (%) | 0(0%) | 0(0%) | 0(0%) | |
| Lymphocyte count (× 109/liter)-median (IQR) | 1.3(1–1.6) | 1.4(1.2–1.9) | 1.2(0.9–1.5) | 0.07 |
| ≥1.0 × 109/liter-no. (%) | 25(75.8%) | 9(81.8%) | 16(72.7%) | |
| <1.0 × 109/liter-no. (%) | 8(24.2%) | 2(18.2%) | 6(27.3%) | |
| Platelet count (× 109/liter)-median (IQR) | 210(166–255) | 210(195.5–257.5) | 208(162.2–247) | 0.91 |
| ≥100 × 109/liter-no. (%) | 28(100%) | 10(100%) | 18(100%) | |
| <100 × 109/liter-no. (%) | 0(0%) | 0(0%) | 0(0%) | |
| Serum creatinine (μmol/liter)-median (IQR) | 70.7(53.2–78.1) | 71.7(50.5–77.7) | 69.4(55.9–82) | 0.39 |
| ≤133 μmol/liter-no. (%) | 32(97%) | 11(100%) | 21 (95.2%) | |
| >133 μmol/liter-no. (%) | 1(3%) | 0(0%) | 1(4.8%) | |
| AST(U/liter)-median (IQR) | 21(17–24) | 19(14–22) | 22(19.3–28) | 0.35 |
| ≤40 U/liter-no. (%) | 30(90.9%) | 10(90.9%) | 20(90.9%) | |
| >40 U/liter-no. (%) | 3(9.1%) | 1(9.1%) | 2(9.1%) | |
| ALT(U/liter)-median (IQR) | 23(15–32) | 21(13.5–31.5) | 25(19.3–31.8) | 0.44 |
| ≤50 U/liter-no. (%) | 29(87.9%) | 10(90.9%) | 19(86.4%) | |
| >50 U/liter-no. (%) | 4(12.1%) | 1(9.1%) | 3(13.6%) | |
| LDH (U/liter)-median (IQR) | 195(172–226) | 182(157–227) | 195.5(175–225.5) | 0.50 |
| ≤245 U/liter-no. (%) | 25(75.8%) | 8(72.7%) | 17(77.3%) | |
| >245 U/liter-no. (%) | 8(24.2%) | 3(27.3%) | 5(22.7%) | |
| CK(U/liter)-median (IQR) | 80(59.8–104.8) | 79(45.5–110.3) | 80(64.8–102.8) | 0.42 |
| ≤185 U/liter-no. (%) | 32(97%) | 11(100%) | 21(95.5%) | |
| >185 U/liter-no. (%) | 1(3%) | 0(0%) | 1(4.5%) |
All enrolled COVID-19 patients were not allergic to aerosol inhalation of IFN-κ plus TFF2, with no serious organ disease, including heart, lung, kidney, brain, blood, and also no serious neurological or mental illness. All female patients were not pregnant. IQR, interquartile range; AST, Aspartate amino transferase; ALT, Alanine amino transferase; LDH, Lactate dehydrogenase; CK, Creatine kinase. P value was evaluated between the control group and IFN-κ plus TFF2 group by unpair t-test. p < 0.05 was denoted as significance.
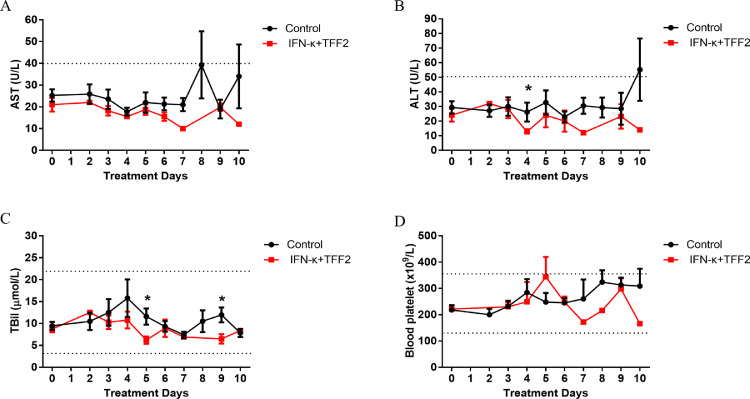
Compared with the control group, ALT, AST, total bilirubin (TBil) and platelets showed a trend of lower levels in the experimental group, but no significances were reached and they all fall into normal ranges in both groups (Fig. 2). These results indicate that IFN-κ plus TFF2 treatment have no significant negative impact on liver, gallbladder, and platelets.
Fig. 2.
Biochemical Indexes of COVID-19 patients between the control and IFN-κ plus TFF2 Groups. AST(A), ALT(B), TBil(C), and Blood Platelet(D) were detected, and showed a downward trend in the IFN-κ plus TFF2 group (N = 11), compared to the control group (N = 22), while they were covering the normal range value. Specially, the level of ALT and TBil were significantly decreased during the inhalation in the IFN-κ plus TFF2 group.
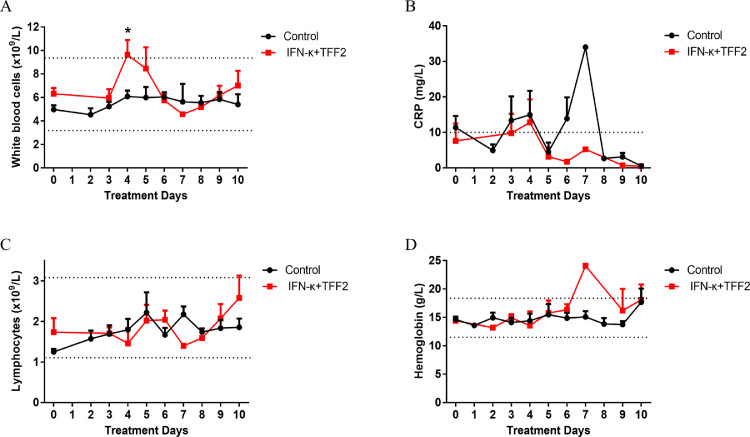
We then analyzed the kinetic changes of effectiveness index in the peripheral blood of the control group and experimental group. The absolute counts of total white blood cells (WBCs) at day 4 of IFN-κ plus TFF2 group were significantly higher than control group during the IFN-κ plus TFF2 treatment period. Once the treatment was completed (from day 6 to day 10), WBCs level returned to normal range value (Fig. 3A), implicating the administration of IFN-κ plus TFF2 may attenuate the lung inflammation and thereby interrupt the migration of WBCs into lung and result in accumulation in blood. Furthermore, CRP level was much lower in the blood of the IFN-κ plus TFF2 group than that in the control group (Fig. 3B), and plasma TNF-α, IL-6 and IL-8 rapidly declined and then remained stable at a low level in the IFN-κ plus TFF2 group when comparing with that in the control group from day 0 to day 10 (Supplementary Fig. 1), which further implied that the experimental group produced less inflammatory and stress responses. In addition, no significant difference in the numbers of lymphocytes, and the concentration of hemoglobin between these two groups were observed, which suggested that IFN-κ plus TFF2 treatment had no effect on the survivals of lymphocytes and the function of red blood cells (Fig. 3C, 3D). After treatment for 6 days, all blood index returned to normal range value.
Fig. 3.
The Blood Indices of COVID-19 Patients between the Control and IFN-κ plus TFF2 Groups.(A). The absolute counts of total white blood cells (WBCs) of IFN-κ plus TFF2 group (N = 11) were significantly higher than that of control group (N = 22) during the inhalation. Once the treatment was finished, WBCs level returned to normal range value. There was no significant difference in CRP (B), Lymphocytes(C), and Hemoglobin(D) between these two group. .
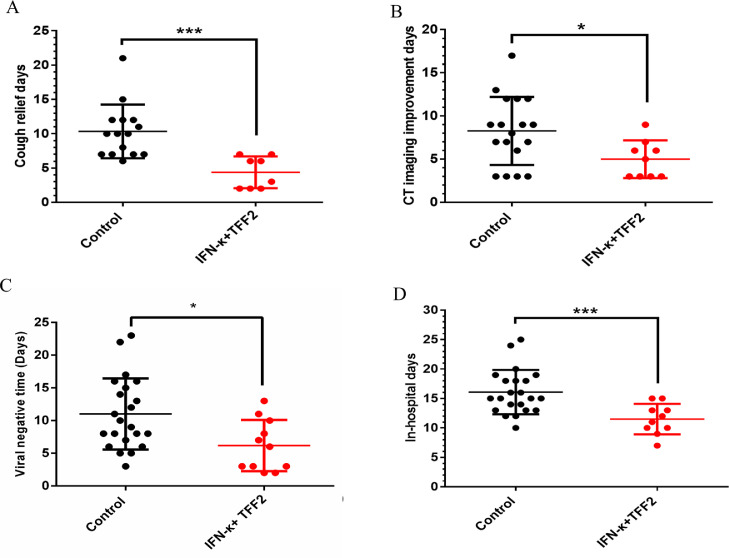
Clinical data, including cough relief, CT imaging improvement, viral RNA negative reversion, and hospitalization days, were collected and analyzed according to the protocol. For cough, 8 patients in the IFN-κ plus TFF2 group and 15 patients in the control group had a cough symptom at baseline. An average cough relief median time of 4.5 days (IQR 2.0–7.0) in the experimental group versus 10.0 days (IQR 6.0–21.0) in the control group (p < 0.005) was observed (Fig. 4A). Pulmonary CT imaging is defined as three levels: exacerbated, unchanged, and improved (Lesion reduction on CT imaging). After 6 days of treatment, the rates of improvement in CT imaging in experimental group and the control group were 63.9% (7/11) and 22.7% (5/22), respectively, then further increased to 82% (9/11) vs 59% (13/22) on day 10, finally reached an improvement median time of 5.0 days (IQR 3.0–9.0) for experimental group vs 8.5 days (IQR 3.0–17.0) for control group (p < 0.05) (Fig. 4B). The median times of viral RNA reversion were 6.0 days (IQR 2.0–13.0) in the experimental group vs 9.5 days (IQR 3.0–23.0) in the control group (p < 0.05) (Fig. 4C). In addition, IFN-κ plus TFF2 treatment significantly shorten the hospitalization of patients as 12.0 days (IQR 7.0–20.0) in the experimental group vs 15 days (IQR 10.0–25.0) in the control group (p < 0.001) (Fig. 4D).
Fig. 4.
Time to negative transformation of viral nucleic acid and clinical improvement from COVID-19 patients in the treatment of control and IFN-κ plus TFF2 Group. (A). The time of cough relief time. (B). The time of CT imaging improvement days (C). The viral negative time. (D). The days for the enrolled COVID-19 patients staying at hospital.
4. Discussion
This open-label, non-randomized clinical trial found that aerosol inhalation of IFN-κ plus TFF2 is a safe treatment for moderate COVID-19 patients. Both IFN-κ and TFF2 are the small proteins and could be induced to respond to respiratory viral infections. In this trial, safety evaluations, including WBCs level, lymphocytes, CRP, hemoglobin, ALT, AST, blood platelet and TBil, all were fallen in normal ranges, and no aerosol inhalation treatment of IFN-κ plus TFF2 related severe adverse events were noticed. Interestingly, the adjustment of treatment regimens in control group may result in fluctuation of those evaluation, for example, on the day 9 of standard care treatment, ulinastatin was employed to improve the coagulation function in partial patients because the fibrinogen of the patient was dropped; Indeed, the administration of ulinastatin could cause the increase of ALT and AST as shown on the 10th day. In contrast, in IFN-κ plus TFF2 group, less fluctuation was observed, which probably implicates patients in IFN-κ plus TFF2 group are more stable. In addition, aerosol inhalation is a non-invasive and easy-to-use approach for the patients, and it is also applicable for delivery to COVID-19 patients with ventilator through oxygen pipe. Our clinical survey has shown that there are no discomfort responses during the aerosol inhalation.
We also observed that IFN-κ plus TFF2 added to standard treatment was effective in clearing viral throat carriage of SARS-CoV-2 in absence of significant elevated inflammation responses in COVID-19 patients. 90% patients in IFN-κ plus TFF2 group turned to undetectable viral RNA whereas 59% shown in control group on day 10 after administration. A recent report showed that the median duration of viral shedding in COVID-19 was 20 days in patients with severe illness and could be as long as 37 days [1].There data suggested that the IFN-κ plus TFF2 might inhibit SARS-Cov-2 replication in vivo, and thereby promoted negative reversion of viral RNA, similar to hydroxychloroquine treatment [28]. The prognosis of COVID-19 patients has been associated with the levels of pro-inflammatory cytokines in the peripheral blood [27], IL-6 receptor blocking antibodies, Tocilizumab therapy have been applied to counteract the cytokine storm in severe COVID-19 patients [29]. Thus, it is critical to examine how the treatment of IFN-κ plus TFF2 influences the inflammatory cytokines. In this study, CRP was monitored as an indicator of inflammatory responses, and shown a lower level in the experimental group than that in the control group, indicating that treatment with IFN-κ plus TFF2 may eventually reduce inflammatory responses, which was further supported by the observation on gradually increased white blood cells in blood in the course of treatment but not in the control group, as it is rationalized that the local aerosol inhalation may attenuate respiratory inflammation and results in the migration of white blood cells from respiratory tissues back to blood. Since only one administration applied every 48 h, we proposed that once every 24 h with prolonged period may further improve the efficacy of treatment.
Given this is an open label trial, to minimize the risk of bias, all radiologists, clinical doctors and nurses were blinded from grouping information to interpret clinical data or to make clinical decisions. CT imaging was carried out and evaluated by radiologists, the release from of hospital was determined by clinical doctors according the discharging standards, and cough symptom was recorded by the nurses. All data from electronic medical records were collected and analyzed by the researchers. In accordance with the earlier suppression of viral RNA, a significant improvement in clinical manifestations after TFF2 and IFN-κ treatment was observed, including earlier cough relief and CT imaging improvement, and shortened hospitalization stay, similar to the report from clinical trial of hydroxychloroquine [28]. Therefore, these results suggested the efficacy of TFF2 plus IFN-κ treatment in moderate COVID-19 patient and a study to scale up will be crucial to further validate our pilot study.
As this is small sample sized, non-randomized, not double-blind study, though we took measures to minimize the bias, patients psychological influence and clinical practices from known grouping people may exert their powers on statistical bias. However, due to non-randomized study with respect to patient assignment, the power is probably lower due to the small sample size, the p-values may be meaningless. In addition, the estimated duration of viral shedding is influenced by the frequency of respiratory specimen collection, lack of quantitative viral RNA assay, and relatively low positive rate of SARS-CoV-2 RNA detection in throat-swabs. Furthermore, all recruited subjects were moderate COVID-19 cases, it remains unknown whether this treatment is applicable to severe and critical cases.
Our pilot study indicated that IFN-κ plus TFF2 is likely to be a safe treatment, and might be beneficial to COVID-19 patients. Further to scale-up study will be necessary to determine its efficacy on COVID-19. In addition, considering that this unique treatment potentially to facilitate clinical improvement, and thereby achieves an early release from hospitalization, the synergistic effect with other medicines such as HCQ [10], azithromycin [9], remdesivir [8], high dosage intravenous immunoglobulin (IVIG) [30], or high dose vitamin C [31] should be further explored.
Funding
This work was supported by National Natural Science Foundation of China (81771704), the National Major Project for Control and Prevention of Infectious Disease in China (2018ZX10301403–003), The Second Batch of Emergency Science and Technology Projects of Shanghai Science and Technology Commission (20411950200), and Shanghai Municipal Health Commission Special Emergency Project for Prevention and Treatment of Novel Coronavirus Pneumonia by Traditional Chinese Medicine (2020NCP001). We thank all patients who participated in this trial and their families. We thank all the clinical, technical and paramedical staffs of hospitalization units and laboratories for this support in this difficult context.
Contributors
XZ, TZ, JX, and HL designed the study and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. WF and YL contributed to writing of the report. JX contributed to critical revision of the report. YL and WF contributed to the statistical analysis, JX, XZ and WF provided the purified proteins. LX, MW, ZY, JC, LL, YL, FS are the clinical doctors, responsible for carrying out this trial by communicating with the patients and their family, ML, LW, LZ, RJ, JY are the nurses, responsible for the implementation of patients' aerosol inhalation. ZS, HH, XC, XM are responsible for providing the data of viral load, and collecting clinical characteristics of the enrolled patients. CS check the language. All authors reviewed and approved the final version.
Data sharing
Data are available on various websites and have been made publicly available, Additional materials may be required after approval from the corresponding author and National Health Commission.
Declaration of Competing Interest
We have applied for the patent 202010239633.3 (pending), and the patent PCT/CN2020/082195 (pending) base on these data. All authors reviewed the signed the conflict of interest forms.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100478.
Contributor Information
Xiaoyan Zhang, Email: zhangxiaoyan@shphc.org.cn.
Tongyu Zhu, Email: zhutongyu@shphc.org.cn.
Jianqing Xu, Email: xujianqing@shphc.org.cn.
Hongzhou Lu, Email: luhongzhou@shphc.org.cn.
Appendix. Supplementary materials
References
- 1.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;23(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu K., Fang Y.Y., Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao B., Wang Y., Wen D. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grein J., Ohmagari N., Shin D. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman J.D., Lye D., Hui D.S. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. The Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Moriguchi H., Sato C. Treatment of SARS with human interferons. LANCET. 2003;362(9390):1159. doi: 10.1016/S0140-6736(03)14484-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lokugamage K.G., Schindewolf C., Menachery V.D. SARS-CoV-2 sensitive to type I interferon pretreatment. bioRxiv. 2020:2020–2023. doi: 10.1101/2020.03.07.982264. [DOI] [Google Scholar]
- 13.Channappanavar R., Fehr A.R., Vijay R. Dysregulated Type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaFleur D.W., Nardelli B., Tsareva T. Interferon-kappa, a novel type I interferon expressed in human keratinocytes. J Biol Chem. 2001;276(43):39765–39771. doi: 10.1074/jbc.M102502200. [DOI] [PubMed] [Google Scholar]
- 15.Buontempo P.J., Jubin R.G., Buontempo C.A., Wagner N.E., Reyes G.R., Baroudy B.M. Antiviral activity of transiently expressed IFN-kappa is cell-associated. J Interferon Cytokine Res. 2006;26(1):40–52. doi: 10.1089/jir.2006.26.40. [DOI] [PubMed] [Google Scholar]
- 16.He Y., Fu W., Cao K. IFN-kappa suppresses the replication of influenza A viruses through the IFNAR-MAPK-Fos-CHD6 axis. Sci Signal. 2020;13(626) doi: 10.1126/scisignal.aaz3381. [DOI] [PubMed] [Google Scholar]
- 17.Poulsen S.S., Thulesen J., Christensen L., Nexo E., Thim L. Metabolism of oral trefoil factor 2 (TFF2) and the effect of oral and parenteral TFF2 on gastric and duodenal ulcer healing in the rat. GUT. 1999;45(4):516–522. doi: 10.1136/gut.45.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baus-Loncar M., Schmid J., Lalani E. Trefoil factor 2 (TFF2) deficiency in murine digestive tract influences the immune system. Cell Physiol Biochem. 2005;16(1–3):31–42. doi: 10.1159/000087729. [DOI] [PubMed] [Google Scholar]
- 19.Farrell J.J., Taupin D., Koh T.J. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109(2):193–204. doi: 10.1172/JCI12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royce S.G., Lim C., Muljadi R.C. Trefoil factor-2 reverses airway remodeling changes in allergic airways disease. Am J Respir Cell Mol Biol. 2013;48(1):135–144. doi: 10.1165/rcmb.2011-0320OC. [DOI] [PubMed] [Google Scholar]
- 21.Wills-Karp M., Rani R., Dienger K. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med. 2012;209(3):607–622. doi: 10.1084/jem.20110079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran C.P., Cook G.A., Yeomans N.D., Thim L., Giraud A.S. Trefoil peptide TFF2 (spasmolytic polypeptide) potently accelerates healing and reduces inflammation in a rat model of colitis. GUT. 1999;44(5):636–642. doi: 10.1136/gut.44.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniel B.M., Benjamin N.P., Liu W.C. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. (Pre-Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Major J., Crotta S., Llorian M. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020:c2061. doi: 10.1126/science.abc2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao L., Yu S., Chen Q. A randomized controlled trial of low-dose recombinant human interferons alpha-2b nasal spray to prevent acute viral respiratory infections in military recruits. Vaccine. 2010;28(28):4445–4451. doi: 10.1016/j.vaccine.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amrane S., Tissot-Dupont H., Doudier B. Rapid viral diagnosis and ambulatory management of suspected COVID-19 cases presenting at the infectious diseases referral hospital in Marseille, France, - January 31st to March 1st, 2020: a respiratory virus snapshot. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J., Li S., Liu J. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. medRxiv. 2020:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao X., Ye F., Zhang M. In vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med ViroL. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao W., Liu X., Bai T. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with Coronavirus disease 2019. Open Forum Infect Dis. 2020;7(3) doi: 10.1093/ofid/ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov. 2020;5 doi: 10.1016/j.medidd.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.