Abstract
The preauricular sinus (PAuS) is a congenital foramen, opening or invagination, usually located on the crus of the auricular helix and is considered a congenital malformation and component of multiple syndromes. The structure can be present unilaterally or bilaterally, with the possibility of more than one fistula present on one ear, predominantly on the auricular tags. As a well-defined and established clinical entry, PAuS has a very strictly laid-out history. However, different works of art give us a glimpse into the structure before its first true clinical description, showing that the PAuS was known to man long before it was first clinically described, such as those of Hieronymous Bosch, with the first medical descriptions being attributed to Heusinger and Virchow. In modern times, the condition is considered both an individual malformation and a component of several genetic syndromes.
Keywords: preauricular sinus, medical history, genetic syndromes, medicine in art
Introduction and background
The preauricular sinus (PAuS) is a congenital malformation characterized by a dent, dimple, or a foramen, usually located on the crus of the auricular helix [1-2]. Rarely structures with the same characteristics and clinical course can be found on the pinna, tragus, or even in the postauricular area contradictory to the established name in modern times [1-3].
The structure can be present unilaterally or bilaterally, with the possibility of more than one fistula present on one ear, predominantly on the auricular tags [3-6]. The structure presents as a simple epithelial invagination into the auricular stroma and can often become inflamed, leading to discharge from the superficial opening and discomfort [1,7]. This itself can lead to serious complications, such as facial paralysis, due to its location near the facial nerve [1,7,8]. Clinical history in such cases is important to distinguish between a fistulized abscess and an inflamed PAuS. Furthermore, chronic inflammation is a risk factor for cancerogenesis, and thus a squamous cell carcinoma ex PAuS (carcinoma from a PAuS) may develop later in life [9-11]. This is the reason why the structure evokes the interest of many otolaryngologists, specialists, and pediatricians.
As a well-defined and established clinical entry, PAuS has multiple contributors in the medical field that helped define it and its significance. However, different works of art give us a glimpse into the structure before its first true clinical description, showing that the PAuS was known to man long before it was first clinically described.
Review
Artistic representation
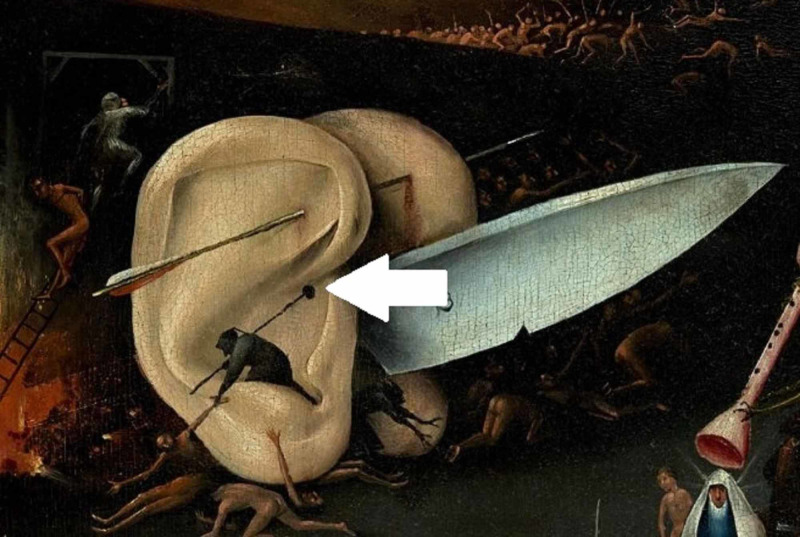
Hieronymus Bosh (c. 1450-1516) was a Dutch artist whose most famous painting is “The Garden of Earthly Delights”. The painting illustrates exterior and interior panels [11]. The exterior panel resembles the creation of the world, whereas the interior consists of three panels that represent, respectively, The Garden of Eden (left panel), The Human World (middle panel), and The Judgment Day or Hell (right panel). A small segment of the right panel illustrates a pair of ears on which a structure resembling a PAuS is seen (Figure 1) [12].
Figure 1. Hieronymous Bosch, Garden of Earthly Delights, a magnified section of the right panel of the interior section (Hell). The grotesque ear has a structure reminiscing of a PauS (arrow).
PAuS, preauricular sinus
First medical descriptions
The PAuS was first described in 1864 by C. F. Heusinger when describing the findings in a patient characteristic of the brachio-oto-renal (BOR) syndrome [13]. He detailed his finding and also referred to several already described phenomena that seemed detached from each other, such as the preliminary work by Dzondi, who described and defined congenital tracheal fistulas [13]. It is important to note that the auricula entity described by Heusinger, although morphologically identical, is its separate entity from the cervical and other brachial fistulas described before his observations [13,14].
Following Heusinger's description, Rudolph Virchow (1821-1902) also described the structure in 1864, although simply stating in his article “I also know a patient like that” [14]. Virchow, however, was the first to postulate that the PAuS is a result of a defect in the embryological fusion of the pharyngeal arches, a statement that was widely contradicted at the time (Figure 2).
Figure 2. Classical representation of PAuS (arrow).
PAuS, preauricular sinus
Sir James Paget also published on the subject in 1878, describing patients with such fistulae present not only on their ears but also on their neck [15]. Based on the description of his patients, he coined the term oto-branchial fistula [15] (Figure 3). Sir James Paget widely adapted the viewpoints of Virchow’s theory of the origin of PAuS.
Figure 3. Variant of PAuS on the auricular helix (arrow).
PAuS, preauricular sinus
During the first part of the 20th century, the PAuS became known as “natural earring holes” and “fistula auris congenita”, with several authors publishing on the subject, including its pattern of inheritance [3,15,16]. The phenomena and its transgenerational inheritance allowed for an extensive set of studies and theories on its cause, widely converging on Virchow’s original statements [17-23].
One of the most important contributions from that time period was in 1955 by Fourman and Fourman, who researched the inheritance of PAuS, constructing a genealogical tree, focusing on the inheritance of the structure [24]. They concluded that half of the siblings of the affected family have the structure and thus postulated that the sinus is inherited as a dominant trait, but with “incomplete penetrance” due to it skipping in generations or individuals [24].
Despite that initial interest, however, little to no articles were published after the late 1940s, until a small resurgence in later years, where the structure reappears under a new name - PAuS. Since then, research on the structure has seldom been carried out, and the mechanisms of its transgenerational inheritance remain widely forgotten in the literature of the 1930s and 1940s.
PAuS-associated syndromes
Melnick-Fraser Syndrome
The syndrome was first described in 1864 by Heusinger [13]. However, in 1975, Melnick et al. described a series of patients with it, and in 1980 Fraser et al. described another series of cases with the hallmarks of the syndrome in a specialized school for the deaf [25,26]. The condition is considered relatively rare, with 250 new cases of Melnick-Fraser being diagnosed in Japan in 2014 [27]. First believed to be a variant of BOR syndrome, later on, Melnick widened the syndrome into a group of nearly identical conditions dependent on the presence or absence of different hallmarks [28,29]. The term Melnick-Fraser syndrome was later coined to unify the characteristics of the separate syndromes and to completely separate it from Fraser syndrome, isolated urogenital malformations, and Frasier-Lynch syndrome, familial colorectal polyposis, with which BOR had quite often been confused as a term at that point in time. Thus, sadly Heusinger’s contribution has been widely forgotten.
Beckwith-Wiedemann Syndrome
The condition was originally described in 1963 by American pathologist John Beckwith as a combination of exomphalos, macroglossia, and gigantism, therefore referred to as exomphalos-macroglossia-gigantism (EMG) syndrome (Poster: Beckwith, JB: Extreme Cytomegaly of the adrenal Fetal Cortex, Hyperplasia of the Kidneys and Pancreas, and Leydig-Cell Hyperplasia : Another Syndrome?. Annual Meeting of Western Society for Pediatric Research, Los Angeles, CA, November 11, 1963). In 1964, however, independently of John Beckwith, the German pediatrician Hans-Rudolph Wiedemann also described several patients with the same hallmarks of the disease but also included symptoms such as adrenal hyperplasia [30]. Over time, the syndrome was dubbed Beckwith-Wiedemann syndrome, and the diagnostic criteria were expanded to include PAuS, hyperplastic kidneys, microcephaly, neonatal hypoglycemia, and hepatoblastoma developing later in life [31,32]. Mutations in 11p15 involving genes such as insulin-like growth factor 2 (IGF-2), cyclin-dependent kinase inhibitor 1C (CDKN1C), H19, and potassium voltage-gated channel subfamily Q member 1 overlapping transcript 1 (KCNQ1OT1) have been established in such patients [32]. The mechanism of inheritance is still undefined as in several patients with this condition, the exact transgenerational inheritance could not be determined, deeming it as a sporadic recessive defect. Beckwith-Wiedemann syndrome and its hallmarks are especially important to be distinguished in patients conceived with in vitro fertilization as the incidence in that population is much higher [33-35]. Beckwith-Wiedemann is considered a rare condition with an incidence of 1 per 13,700 children born, accounting for a total of about 300 children born with Beckwith-Wiedemann in the United States of America annually [36].
Lachiewicz-Sibley Syndrome
First described in 1985 by Lachiewicz et al., this is one of the rarest syndromes ever described [37]. The syndrome is very similar to Melnick-Fraser, though only PAuS and hypoplastic kidneys with early-onset proteinuria were found in the descendants of British and Irish immigrants settling in Ohio in the 1800s and later Nebraska. At the time of the original study, from 130 living relatives, 12 members had PAuS and hypoplastic kidneys, 10 had only hypoplastic kidneys, and 3 had only PAuS [37]. Although the exact locus of mutation remains unknown, the condition is inherited in an autosomal dominant fashion.
Twenty-first century
Today although still relatively under-researched, the PAuS is a common clinical entity with well-defined clinical strategies for treatment. Some studies have identified a genetic association between PAuS and the 8q11.1-13.1 locus; however, the results have not been reproduced widely [1,5,7,38]. Other than this individual study, no other studies have focused on the genetic reason for PauS nor have associated it with the aforementioned genetic syndromes, with genealogic studies are few and far in-between [16,24,39].
The clinical course has been well described, and treatment strategies including predominantly surgical excision have been well established in modern otorhinolaryngology and head and neck surgery [5,7,8,40]. Nowadays, patients with PAuS, not associated with a genetic syndrome, undergo a safe intervention process with few described complications, whereas patients with genetic syndromes undergo the same curative modalities, and the excision of the PauS does not affect their overall clinical course [7,40-42].
Conclusions
The PAuS, as it was first described, is often a component of several eponymous inherited syndromes such as Melnik-Fraser (BOR syndrome), Beckwith-Wiedemann, and Lachiewicz-Sibley, which also include a variety of constant kidney and other distant variable malformities such as cysts, fistulae, facial and neural defects, and others. These syndromes relatively rarely underline the importance of Virchow’s original statement that the condition is caused by malfusion of pharyngeal arches, a process taking place parallel to some stages of kidney development. However, there is no evidence in the scientific literature inferring that the presence of PAuS always concludes to the presence of any of the aforementioned syndromes. Although the exact model of inheritance of the PauS, according to modern understandings and its genetic and molecular hallmarks, are yet to be fully determined and described, some authors still consider it as an irregular dominant mutation with reduced penetrance.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.The preauricular sinus: a review of its clinical presentation, treatment, and associations. Scheinfeld NS, Silverberg NB, Weinberg JM, Nozad V. Pediatr Dermatol. 2004;21:191–196. doi: 10.1111/j.0736-8046.2004.21301.x. [DOI] [PubMed] [Google Scholar]
- 2.A case of bilateral postauricular sinuses. Kim HJ, Lee JH, Cho HS, Moon IS. Korean J Audiol. 2012;16:99–101. doi: 10.7874/kja.2012.16.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Congenital periauricular fistulas: Possible variants of the preauricular sinus. Kim JR, Kim DH, Kong SK, Gu PM, Hong TU, Kim BJ, Heo KW. Int J Pediatr Otorhinolaryngol. 2014;78:1843–1848. doi: 10.1016/j.ijporl.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Supra-auricular versus sinusectomy approaches for preauricular sinuses. Waheed El-Anwar M, Shaker Elaassar A. Int Arch Otorhinolaryngol. 2016;20:390–393. doi: 10.1055/s-0036-1583305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The preauricular sinus: a review of its aetiology, clinical presentation and management. Tan T, Constantinides H, Mitchell TE. Int J Pediatr Otorhinolaryngol. 2005;69:1469–1474. doi: 10.1016/j.ijporl.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 6.The variant type of preauricular sinus: postauricular sinus. Choi SJ, Choung Y-H, Park K, Bae J, Park HY. Laryngoscope. 2007;117:1798–1802. doi: 10.1097/MLG.0b013e3180caa1ca. [DOI] [PubMed] [Google Scholar]
- 7.The clinical efficacy of early intervention for infected preauricular sinus. Choo OS, Kim T, Jang JH, Choung YH. Int J Pediatr Otorhinolaryngol. 2017;95:45–50. doi: 10.1016/j.ijporl.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 8.Association of recurrence of infected congenital preauricular cysts following incision and drainage vs fine-needle aspiration or antibiotic treatment. Rataiczak H, Lavin J, Levy M, Bedwell J, Preciado D, Reilly BK. JAMA Otolaryngol Neck Surg. 2017;143:131–134. doi: 10.1001/jamaoto.2016.2988. [DOI] [PubMed] [Google Scholar]
- 9.Congenital preauricular cysts and fistulas. Pastore PN, Erich JB. Arch Otolaryngol - Head Neck Surg. 1942;36:120–125. [Google Scholar]
- 10.Methylene blue staining and probing for fistula resection: application in a case of bilateral congenital preauricular fistulas. Martín-Granizo R, Pérez-Herrero MC, Sánchez-Cuéllar A. Int J Oral Maxillofac Surg. 2002;31:439–441. doi: 10.1054/ijom.2001.0062. [DOI] [PubMed] [Google Scholar]
- 11.Demographics of head and neck cancer patients: a single institution experience. Stoyanov GS, Kitanova M, Dzhenkov DL, Ghenev P, Sapundzhiev N. Cureus. 2017;9:0. doi: 10.7759/cureus.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hieronymus Bosch’s stamps: realistic, satyrical, grotesque and otorhinolaryngological. Mudry A. http://www.historiaorl.com/wp-content/uploads/2017/12/Albert-Mudry-Hieronymus-Bosch%C2%B4s-Stamps.pdf Hist Otorhinolaryngol. 2017;1:189–213. [Google Scholar]
- 13.Hals-Kiemen-Fisteln von noch nicht beobachteter form. Heusinger CF. https://www.semanticscholar.org/paper/Hals-Kiemen-Fisteln-von-noch-nicht-beobachteter-Heusinger/7edd094c5995a067ae89fc4eb36f787a8e8fe318 Arch Pathol Anat Physiol Klin Med. 1864;29:358–380. [Google Scholar]
- 14.Ueber missbildungen am ohr und im bereiche des ersten kiemenbogens. Virchow R. Arch Pathol Anat Physiol Klin Med. 1864;30:221–234. [Google Scholar]
- 15.Cases of branchial fistulæ in the external ears. Paget J. Med Chir Trans. 1878;61:41–50. doi: 10.1177/095952877806100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fistula auris congenita. Donald GF. Australas J Dermatol. 1952;1:253–255. doi: 10.1111/j.1440-0960.1952.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 17.Natural “ear-ring” holes. Edmonds HW, Keeler CE. J Hered. 1940;31:507–510. [Google Scholar]
- 18.Inheritance of a pit in the skin of the left ear. Kindred JE. J Hered. 1921;12:366–367. [Google Scholar]
- 19.On the inheritance of ear pit. McDonough ES. J Hered. 1941;32:169–170. [Google Scholar]
- 20.Three generations of ear pits. Whitney DD. J Hered. 1939;30:323–324. [Google Scholar]
- 21.The inheritance of ear pits in six generations of a family. Connon FE. J Hered. 1941;32:413–416. [Google Scholar]
- 22.The development of the external ear. Wood-Jones F, I-Chuan W. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1249008/pdf/janat00545-0095.pdf. J Anat. 1934;68:525–533. [PMC free article] [PubMed] [Google Scholar]
- 23.Ear pit and its inheritance. Quelprud T. J Hered. 1940;31:379–384. [Google Scholar]
- 24.Hereditary deafness in family with ear-pits (fistula auris congenita) Fourman P, Fourman J. Br Med J. 1955;2:1354–1356. doi: 10.1136/bmj.2.4952.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Autosomal dominant branchiootorenal dysplasia. Melnick M, Bixler D, Silk K, Yune H, Nance WE. https://pubmed.ncbi.nlm.nih.gov/1218203/ Birth Defects Orig Artic Ser. 1975;11:121–128. [PubMed] [Google Scholar]
- 26.Frequency of the branchio-oto-renal (BOR) syndrome in children with profound hearing loss. Fraser FC, Sproule JR, Halal F, Optiz JM. Am J Med Genet. 1980;7:341–349. doi: 10.1002/ajmg.1320070316. [DOI] [PubMed] [Google Scholar]
- 27.Branchio-oto-renal syndrome: comprehensive review based on nationwide surveillance in Japan. Morisada N, Nozu K, Iijima K. Pediatr Int. 2014;56:309–314. doi: 10.1111/ped.12357. [DOI] [PubMed] [Google Scholar]
- 28.Branchio-oto-renal dysplasia and branchio-oto dysplasia: two distinct autosomal dominant disorders. Melnick M, Hodes ME, Nance WE, Yune H, Sweeney A. Clin Genet. 1978;13:425–442. doi: 10.1111/j.1399-0004.1978.tb04142.x. [DOI] [PubMed] [Google Scholar]
- 29.Hereditary hearing loss and ear dysplasia-renal adysplasia syndromes: syndrome delineation and possible pathogenesis. Melnick M. https://pubmed.ncbi.nlm.nih.gov/7213985/ Birth Defects Orig Artic Ser. 1980;16:59–72. [PubMed] [Google Scholar]
- 30.[Familial malformation complex with umbilical hernia and macroglossia - a new syndrome] Wiedemann HR. https://pubmed.ncbi.nlm.nih.gov/14231762/ J Genet Hum. 1964;13:223–232. [PubMed] [Google Scholar]
- 31.Clinical features and natural history of Beckwith-Wiedemann syndrome: presentation of 74 new cases. Elliott M, Bayly R, Cole T, Temple IK, Maher ER. Clin Genet. 2008;46:168–174. doi: 10.1111/j.1399-0004.1994.tb04219.x. [DOI] [PubMed] [Google Scholar]
- 32.The clinical course of an overgrowth syndrome, from diagnosis in infancy through adulthood: the case of Beckwith-Wiedemann syndrome. Pappas JG. Curr Probl Pediatr Adolesc Health Care. 2015;45:112–117. doi: 10.1016/j.cppeds.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Association between Beckwith-Wiedemann syndrome and assisted reproductive technology: a case series of 19 patients. Chang AS, Moley KH, Wangler M, Feinberg AP, DeBaun MR. Fertil Steril. 2005;83:349–354. doi: 10.1016/j.fertnstert.2004.07.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckwith-Wiedemann syndrome and assisted reproduction technology (ART) Maher ER, Brueton LA, Bowdin SC, et al. J Med Genet. 2003;40:62–64. doi: 10.1136/jmg.40.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.In vitro fertilization may increase the risk of Beckwith-Wiedemann syndrome related to the abnormal imprinting of the KCN1OT gene. Gicquel C, Gaston V, Mandelbaum J, Siffroi J-P, Flahault A, Le Bouc Y. https://www.cell.com/ajhg/pdf/S0002-9297(07)60663-6.pdf. Am J Hum Genet. 2003;72:1338–1341. doi: 10.1086/374824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Exomphalos-macroglossia-gigantism syndrome in Jamaican infants. Thorburn MJ, Wright ES, Miller CG, Smith-Read EH. Am J Dis Child. 1970;119:316–321. doi: 10.1001/archpedi.1970.02100050318006. [DOI] [PubMed] [Google Scholar]
- 37.Hereditary renal disease and preauricular pits: report of a kindred. Lachiewicz AM, Sibley R, Michael AF. J Pediatr. 1985;106:948–950. doi: 10.1016/s0022-3476(85)80248-1. [DOI] [PubMed] [Google Scholar]
- 38.A locus for congenital preauricular fistula maps to chromosome 8q11.1-q13.3. Zou F, Peng Y, Wang X, et al. J Hum Genet. 2003;48:155–158. doi: 10.1007/s100380300024. [DOI] [PubMed] [Google Scholar]
- 39.Analysis of incidence and genetic predisposition of preauricular sinus. An SY, Choi HG, Lee JS, Kim JH, Yoo SW, Park B. Int J Pediatr Otorhinolaryngol. 2014;78:2255–2257. doi: 10.1016/j.ijporl.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 40.Preauricular sinuses in the pediatric population: techniques and recurrence rates. Gan EC, Anicete R, Tan HKK, Balakrishnan A. Int J Pediatr Otorhinolaryngol. 2013;77:372–378. doi: 10.1016/j.ijporl.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 41.Preauricular sinus: incidence and inheritance. Stoyanov GS, Renjilian A, Matev B, et al. Scri Sci Med. 2018;50:24–29. [Google Scholar]
- 42.Association of preauricular sinuses and renal anomalies. Leung AKC, Robson WLM. Urology. 1992;40:259–261. doi: 10.1016/0090-4295(92)90487-h. [DOI] [PubMed] [Google Scholar]