Abstract
Introduction
The effects of nusinersen in adults with SMA rely on neuromotor function scales and qualitative assessments. There are limited clinical or imaging data on muscle changes over time.
Methods
Two adult SMA patients underwent clinical assessments including measures of upper and lower limb function with Revised Upper Limb Module (RULM) and Hammersmith Function Motor Scale Expanded (HFMSE); both patients were also studied with whole-body muscle MRI (T1-weighted and Diffusion Tensor Imaging/DTI sequences), at baseline and after 10 and 24 months from the beginning of treatment with nusinersen.
Results
After two years of treatment, HFMSE and RULM scores were stable in both patients. DTI sequences revealed an increased number, length and organization of muscle fiber tracks, and Fractional Anisotropy (FA) values showed a significant reduction after 10 and 24 months from baseline, in their corresponding maps.
Discussion
Muscle DTI imaging seems to play an interesting role to monitor treatment effects over time in adult SMA patients.
Keywords: Spinal muscular atrophy, Nusinersen, Muscle MRI, T1-weighted sequences, Diffusion tensor imaging, Fractional anisotropy
Abbreviations: DTI, Diffusion Tensor Imaging; FA, Fractional Anisotropy; HFMSE, Hammersmith Function Motor Scale Expanded; MRC, Medical Research Council; MRI, Magnetic Resonance Imaging; RULM, Revised Upper Limb Module; SMA, Spinal Muscular Atrophy; SMN1, Survival of Motor Neuron 1 gene
Highlights
-
•
Nusinersen treatment has created great expectations in older SMA patients having long-lasting muscular atrophy.
-
•
DTI is a very sensitive technique to identify small changes in muscle architecture.
-
•
DTI shows that nusinersen treatment may have a positive effect on size, length and organization of fiber tracts.
1. Introduction
Spinal Muscular Atrophy (SMA) is an autosomal-recessive disorder caused by mutations in SMN1 gene, leading to degeneration of alpha motor neurons in the spinal cord resulting in progressive muscle weakness and disability [[1], [2], [3]]. The results from the pivotal [4] and more recent trials [5] with intrathecal nusinersen treatment, as well as the results from real world data [6], have created great expectations in older patients having long-lasting muscular atrophy and weakness; in fact there is growing evidence that treatment is at the very least stabilizing or providing some degree of improvement in motor function [7]. However, there are still some critical issues in understanding results in adults, including the lack of natural history data and outcome measures to monitor disease progression [8]. Furthermore limited data are available on muscle degeneration and neurophysiology over time, and this is often a limiting factor in interpreting the impact of any treatment in these patients. In several neuromuscular disorders, muscle MRI is increasingly used to identify a specific pattern of muscular involvement [9,10], to monitor progression [11], to quantify the effects of treatment on muscle structure [12], and in some pharmacological trials as a potential biomarker [13]. Only a few MRI studies have described the pattern of muscular involvement in the different subtypes of SMA [[14], [15], [16]]. More recently, Brogna and colleagues [17] described the pattern of muscular involvement on MRI and its large variability in both type 2 and type 3 SMA patients.
MRI performed with conventional pulse sequences (e.g., T1 and T2-weighted sequences) provides only gross information on muscle structure; more specific technique such as Dixon MRI sequence allows to quantify the amount of fat present in an area of skeletal muscle of interest [18], however, since early pathological changes often start at cellular or fascicular level, it is not able to detect early or subtle changes [19]. Diffusion tensor imaging (DTI) is a relatively new, quantitative, MRI-based technique which relies on the principles of water diffusion in tissues, which has been traditionally used mainly for fiber tracking in the central nervous system. This method is very sensitive to changes in tissue microstructure, simultaneously allowing for quantification and visualization of the macroscopic muscle architecture [20].
Here we present the data of muscle MRI imaging, including T1-weighted and DTI sequences, from two SMA siblings carrying a type 3b phenotype, at baseline and after 10 and 24 months of treatment with nusinersen.
2. Methods
Approval for the study was obtained by our local ethics committee. Two patients with molecularly confirmed SMA diagnosis underwent motor functional assessments including measures of upper and lower limb function using the Revised Upper Limb Module (RULM) and Hammersmith Function Motor Scale Expanded (HFMSE) respectively, at baseline and after 10 (T320) and 24 (T660) months from the first intrathecal infusion with nusinersen 12 mg. The patients were studied with MR 1.5 T scanner (Avanto, Siemens Medical Solution, Erlangen, Germany, gradient strength 45 mt/m, slew rate 200 t/m/ms) at baseline, T320 and T660.
The whole body protocol includes: axial T1-weighted images (slice thickness 10 mm; dist. Factor 10%; echo time 9.9 ms; repetition time 530 ms; averages 1; voxel size 1.1 × 1.1 × 10 mm; bandwidth 199) and axial STIR images (slice thickness 10 mm; dist. Factor 10%; echo time 94 ms; repetition time 4000 ms; inversion time 160 ms; averages 1; voxel size 1.2 × 1.1 × 10 mm; bandwidth 219).
A second MRI scan was performed on the axial plane of the calf and includes: multiecho spin-echo sequence (number of echoes 10; echo time 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 ms; echo train length 10 ms; repetition time 2860 ms; field of view 160 × 160 mm; slice thickness 4 mm; slice gap 0.44 mm; pixel bandwith 213 Hz; flip angle 90°; SENSE 2; number of averages 1; matrix 124 × 123) and DTI (slice thickness 4 mm; dist. Factor 30%; TR 7500 ms; TE 71 ms; FOV250 × 72.5; b values = 0 and 400; diffusion directions 12; bandwidth 1646 Hz/Px, averages 6).
T1w images were scored according to the modified Mercuri scale [21]: normal (score 1), mildly/moderately moth-eaten appearance with occasional/numerous scattered hyperintense areas (< 30% of the muscle - score 2, further dived in 2A and 2B); severely moth-eaten appearance with confluent hyperintense areas (> 30% and <60% of the muscle - score 3); confluent hyperintense areas (>60% of the muscle - score 4).
For the fractional anisotropy (FA) images a radiologist with seven years experience manually drown 3 different regions of interest (ROIs): ROI 1 corresponding to tibialis anterior muscle, ROI 2 gastrocnemius (medial and lateral head) muscle, ROI 3 soleus muscle; inclusion criteria for fiber tracking were: 0.2 of minimal FA, 41° of maximum rotation angle and 15 mm of minimum fiber length. Moreover the tractography pre-processing included movement correction and spatial uniformity.
3. Results
3.1. Patient 1
This is a 47-year old patient. His first symptoms started at 12–13 years of age with frequent falls and difficulty climbing stairs. A marked impaired of ambulation occurred in his early 20s and it was followed by proximal weakness in his upper limbs. At 42 years of age, the patient started to use wheel-chair for long distances but he was still able to walk for very short distances at home with bilateral support. No dysphagia was reported. DNA testing initially indicated 0 SMN1/2 SMN2 genotype, which was not consistent with his milder type 3b presentation; further DNA analysis revealed a missense variant (already reported in literature) [22] c.859G>C in exon 7 of both copies of SMN2 gene.
Neurological examination (just before starting therapy) showed normal cranial nerves. Muscle strength testing (with Medical Research Council scale/MRC) in the upper limbs showed proximal and distal mild to moderate weakness: shoulder abductors: grade 4+; elbow flexors and extensors: grade 4; wrist extensors: grade 3; wrist flexors: grade 4; and severe proximal weakness in the lower limbs: hip flexors and adductors grade 2+, knee extensors and hamstrings grade 2; ankle flexors and dorsiflexors were within normal range bilaterally (grade 5). He was able to raise his arms over his head without flexing the elbows, to roll on one side and to walk for a few meters with bilateral support and compensatory hyperlordosis. He was unable to rise from the floor independently but he was able to get up from the sitting position by widening his lower limbs and hyperextending his trunk. The remaining neurological examination was unremarkable.
The patient was started at age 45 on 12 mg intrathecal infusions of nusinersen as per protocol (at baseline, after 15, 30 and 60 days from baseline and then every 4 months). As a notable early finding, the patient reported subjective improvement of his muscular endurance; regarding the functional scales after 10 and 24 months of treatment, RULM and HFMSE scores have remained stable over time (Table 1 ).
Table 1.
Functional scores of the two patients, at baseline, T320 and T660 after starting treatment with nusinersen.
| Anthropometric and functional scores | Baseline (T0) | T320 | T660 | |
|---|---|---|---|---|
| Patient 1 | Age, years-old | 46 | 47 | 48 |
| Height, cm | 168 | – | – | |
| Weight, kg | 74.6 | 70 | 74 | |
| HFMSE score | 38/66 | 38/66 | 39/66 | |
| RULM score | Right 37/37 | Right 37/37 | Right 37/37 | |
| Left 33/37 | Left 36,737 | Left 37/37 | ||
| FVC, L (%) | 4.03 (92) | 3.70 (87) | 3.83 (89) | |
| FEV1, L (%) | 3.36 (95) | 3.04 (89) | 3.34 (96) | |
| PCEF, L/min | 660 | 704.4 | 527 | |
| Patient 2 | Age, years-old | 43 | 44 | 45 |
| Height, cm | 175 | – | – | |
| Weight, kg | 63.8 | 68 | 67.2 | |
| HFMSE score | 26/66 | 26/66 | 21/66 | |
| RULM score | Right 37/37 | Right 35/37 | Right 37/37 | |
| Left 35/37 | Left 34/37 | Left 34/37 | ||
| FVC, L (%) | 3.83 (89) | 3.90 (85) | 3.21 (75) | |
| FEV1, L (%) | 3.34 (96) | 3.24 (87) | 2.96 (77) | |
| PCEF, L/min | 560 | 552 | 486 |
Abbreviations. HFMSE: Hammersmith Function Motor Scale Expanded; RULM: Revised Upper Limb Module; FVC: Forced Vital Capacity; FEV1: Forced Espiratory Volume; PCEF: Peak Cough Expiratory Flow.
3.2. Patient 2
This is a 45 years-old, younger brother of the previous one. His first symptoms started at 13–14 years-old with easy fatigability of the lower limbs (especially during long walks), difficulty climbing stairs and frequent falls. Weakness progressed until 29 years of age when he became unable to climb stairs, and gradually lost ambulation. He became wheelchair-bound by age 30. At age 41, on first assessment, he was still able to rise from sitting unaided, but required full assistance for most daily activities. Genetic test for SMA revealed 0 copies of SMN1 and 2 copies of SMN2 (carrying the same rare variant 859G>C in exon 7).
Neurological examination before starting treatment showed normal cranial nerves. As reported in the former patient, muscle strength testing in the upper limbs showed proximal and distal mild to moderate weakness (shoulder abductors: grade 4-; elbow flexors and extensors: grade 4; wrist flexors and extensors: grade 4) and severe proximal weakness in the lower limbs (hip flexors, hip adductors, knee extensors and hamstrings were all grade 2). Ankle flexors and dorsiflexors were just mildly involved bilaterally (grade 4+). He had significant hip and knee contractures as well as a slight scoliosis. He was able to raise his arms over his head without flexing the elbows, to sit without support, to get from sitting position to lying position, to roll on one side, and to get from the lying to the sitting position leaning on both arms on a table. The remaining neurological examination was unremarkable.
The patient was started at age 43 on 12 mg intrathecal infusions of nusinersen as per protocol. After one year of treatment the patient reported subjective stability on motor function; looking at functional scores after 10 and 24 months of treatment, we observed that RULM scores have remained stable (Table 1) while HFMSE score decreased slightly between the first and the second follow up (the patient had to interrupt physiotherapy due to the COVID-19 pandemic).
3.3. MRI findings
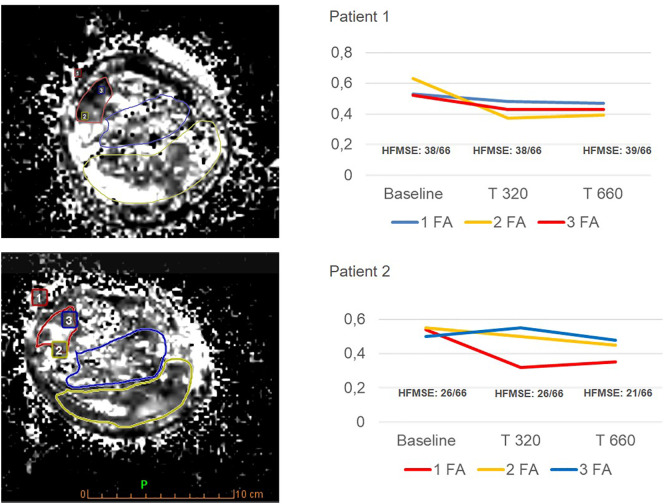
Whole-body muscle MRI T1 performed at baseline revealed a marked fatty replacement of muscles of the pelvic girdle and thigh, and a less severe involvement of the scapular girdle, paraspinal and leg muscles in both of patients (Fig. 1 ); no major differences after 10 (T320) and 24 months (T660) of treatment were detected on MRI wT1 sequences compared to baseline. The elaborated tractography revealed an increased number, length and organization of muscle fiber tracks (Fig. 2 ), after 10 months which can be detected visually on the generated maps, and which seem to remain stable at 24 months in both patients. More specifically, for patient 1, the quantitative DTI evaluation showed decreased FA values in all compartments compared to the baseline examination at 10 months (T320), that remained unchanged at 24 months (T660) (Fig. 3 ). For patient 2, DTI sequences revealed significant decrease of FA values from the baseline examination to the first follow up (10 months – T320), with a mild further decrement at 24 months (T660).
Fig. 1.
Whole body muscle MRI.
Patient 1. 1A - Upper limb and paraspinal muscles: according to Mercuri scale scores: Right arm (anterior compartment 2A, posterior compartment 2B). Left arm (anterior and posterior compartment 2B). Paraspinal muscles 2A. 1B - Pelvic girdle: severe bilateral involvement of gluteus medius (4) and gluteus maximus (3), with relative sparing of the gluteus minimus (2A on the right, 2B on the left). Severe fatty replacement of all remaining muscles of the pelvic girdle, with a relative sparing of the right tensor fasciae latae (2B). Both psoas muscles show severe fatty replacement (4), while erector spinae muscle was less affected (2B). 1C - Thigh: diffuse and severe fatty replacement of muscles belonging to both anterior and posterior compartment of thigh (4), with a relative minimal sparing of the right vastus laterals (3). 1D - Leg: asymmetrical fatty infiltration, more pronounced on the left side, where triceps surae muscle shows an end stage appearance (4). On the right side there is an intermediate involvement of the medial head of the gastrocnemius and soleus muscle (3), as well as the lateral head of gastrocnemius (2B). Peroneal muscles are both markedly involved (4). Tibialis anterior shows intermediate bilateral involvement (3) while extensor digitorum and tibialis posterior present with minimal fatty infiltration (2A).
Patient 2. 1E - Upper limb and paraspinal muscles: moderate fatty infiltration of the anterior compartment of both arms (2A), whereas the posterior compartment shows an intermediate involvement (2B). Upper paraspinal muscles show a mild asymmetry with right side being more preserved (2A) compared to left side (2B). 1F - Pelvic girdle severe bilateral fatty replacement of gluteus medius (4) and gluteus maximus (3). Severe involvement of all remaining muscles of the pelvic girdle, including obturator internus, piriformis, tensor fasciae latae, sartorius and both psoas (4). Erector spinae muscle shows an intermediate involvement (2B). 1G - Thigh: severe fatty replacement of posterior compartment muscles (3), while the anterior compartment seems less affected especially at both vastus lateralis (2B). 1H - Leg: fatty replacement is more pronounced at posterior compartment especially at medial head of gastrocnemius and soleus (3), while lateral head of gastrocnemius being more preserved, especially on the right side (2B). Flexor and extensor muscles in the anterior compartment present with moderate grade of fatty replacement, ranging from 2B (flexor digitorum longus, flexor hallucis longus, peroneus longus) to 2A (tibialis anterior, extensor hallucis and digitorum).
Fig. 2.
DTI tractography images.
Diffusion Tensor Imaging (DTI) tractography of legs demonstrating rarefied appearance of the muscle fibers before the treatment (A, B) and the increased number, length and organization of fiber tracks after the pharmacological treatment at T320 (C, D) which seem to stabilize at T660 (E and F) in the two patients studied. For the analysis the same slice for each patient at proximal third of the leg has been considered.
Fig. 3.
DTI fractional anisotropy (FA).
Trends and regional variation in fractional anisotropy (FA) values before and after the treatment (T320 and T660). 3 ROIs (1 tibialis anterior; 2 gastrocnemius - medial and lateral head; 3 soleus) were drawn on FA map for patient 1 and patient 2. Corresponding graphics of both patients show a decrease in FA values on follow-up (as known FA is a scalar value between zero and one that describes the degree of anisotropy of a diffusion process: zero means that diffusion is isotropic, in all directions, one that the diffusion is confined in only one direction; FA values close to 0.28 ± 0.05 represent normal fibers).
4. Discussion
The cases reported above might suggest further evidence on the effects of nusinersen in stabilizing the disease course in adult SMA patients [4]; in fact HFMSE and RULM scores have remained stable after two years of therapy and these results are in line with what reported by Yeo and colleagues who observed a substantial stability of these scores in a cohort of 6 SMA type 3 patients followed for 12 months during treatment with nusinersen.
However there are still limited data of the effects of motor neuron rescue on muscle, and functional scales (such as HFMSE and RULM) used to monitor patients over time, may not capture subjective improvements in muscle strength or small changes perceived by the patients; in addition RULM and HFMSE can display stable results at least over one year of follow up even in not-treated SMA 3 patients [23].
In the last years muscle MRI is emerging as an interesting tool in identifying the pattern of muscular involvement in neuromuscular diseases, and also in spinal muscular atrophy [14]. However, despite T1w sequences can help to analyze the pattern and the grade of muscular involvement, little can be provided about small changes in the muscle structure with this technique.
Recent studies have demonstrated the role of DTI in the detection and quantification of muscle fibers fat replacement [20,Table 1]; FA is a parameter used to quantify the directional orientation of water molecules within the fibers; FA values are ranged between 0 and 1: in principle, when [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]] tissues are intact, water is forced to move in a specific direction and the FA value is close to 1; unexpectedly patients with severe fat replacement show positive correlation with FA values and negative correlation with Apparent Diffusion Coefficient (ADC); a possible explanation is the artificial increase of FA values in patients with more than 40–45% fat muscle infiltration [25]. In our cases DTI showed that nusinersen treatment may have a positive effect on size, length and organization of fiber tracts raising the potential neurogenic rescue even in long-standing chronic SMA patients. The decrease of FA values in both patients is due to the involution or stabilization of the muscle fat replacement. T1w sequences did not show any changes in muscular structure or in the degree of fatty replacement, probably due to the important extent of muscular involvement at baseline and the long disease duration which couldn't allow also to identify a specific pattern of muscular involvement.
It is important to consider that the data presented are limited to two patients only, with a SMA type 3 phenotype with slow progression and, although interesting, the results of the muscle imaging do not allow to establish whether this technique will correlate to disease progression and clinical findings. Further studies will be needed to verify whether muscle DTI imaging may have a role in determining the impact of nusinersen or any other treatment in a non-invasive way in SMA population; indeed the major limit of our study is the small sample size, which is due not only to the application of this “exploring technique” but also to the rarity of their specific genotype; in fact, it is important to consider that these patients carry a genetic variant that acts as a positive modifier of the clinical phenotype, displaying a milder SMA phenotype compared to what would have been more likely predicted by the 0 SMN1/2 SMN2 genotype.
It may therefore be argued that the response observed at DTI cannot be generalized to all SMA subtypes, but that it may be related to a potentially additive effect of nusinersen treatment, acting on exon splicing of SMN2 (as known the c.859G>C variant in exon 7 of SMN2 acts to partially restore normal exon splicing and produces more SMN2 full-length transcript).
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical publication statement
Authors confirm that they have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Declaration of Competing Interest
None.
References
- 1.Rudnik-Schoneborn S., De Visser M., Zerres K. Spinal muscular atrophies. In: Engel E., Frazini-Amstrong C., editors. Myology. vol. 2. McGraw-Hill; New York: 2004. [Google Scholar]
- 2.Munsat T.L., Davies K.E. International SMA consortium meeting. Neuromuscul. Disord. 1992;2:423–428. doi: 10.1016/s0960-8966(06)80015-5. [DOI] [PubMed] [Google Scholar]
- 3.Zerres K., Rudnik-Schoneborn S. Natural history in proximal spinal muscular atrophy: clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch. Neurol. 1995;52:518–523. doi: 10.1001/archneur.1995.00540290108025. [DOI] [PubMed] [Google Scholar]
- 4.Yeo C.J.J., Simeone S.D., Townsend E.L., Zhang R.Z., Swoboda K.J. Prospective cohort study of nusinersen treatment in adults with spinal muscular atrophy. J. Neuromuscul. Dis. 2020;7:257–268. doi: 10.3233/JND-190453. [DOI] [PubMed] [Google Scholar]
- 5.Jochmann E., Steinbach R., Jochmann T. Experiences from treating seven adult 5q spinal muscular atrophy patients with Nusinersen. Ther. Adv. Neurol. Disord. 2020;13 doi: 10.1177/1756286420907803. 1756286420907803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pane M., Coratti G., Sansone V.A., Messina S., Bruno C., Catteruccia M. Nusinersen in type 1 spinal muscular atrophy: twelve-month real-world data. Ann. Neurol. 2019;86:443–451. doi: 10.1002/ana.25533. [DOI] [PubMed] [Google Scholar]
- 7.Walter M.C., Wenninger S., Thiele S., Stauber J., Hiebeler M., Greckl E. Safety and treatment effects of nusinersen in longstanding adult 5q-SMA type 3 - a prospective observational study. J. Neuromuscul. Dis. 2019;6:453–465. doi: 10.3233/JND-190416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sansone V.A., Walter M.C., Attarian S. Measuring outcomes in adults with spinal muscular atrophy - challenges and future directions - meeting report. J. Neuromuscul. Dis. 2020;10:3233. doi: 10.3233/JND-200534. [DOI] [PubMed] [Google Scholar]
- 9.Barp A., Laforet P., Bello L., Tasca G., Vissing J., Monforte M. European muscle MRI study in limb girdle muscular dystrophy type R1/2A (LGMDR1/LGMD2A) J. Neurol. 2019;267:45–56. doi: 10.1007/s00415-019-09539-y. [DOI] [PubMed] [Google Scholar]
- 10.Paoletti M., Pichiecchio A., Cotti Piccinelli S., Tasca G., Berardinelli A.L., Padovani A. Advances in quantitative imaging of genetic and acquired myopathies: clinical applications and perspectives. Front. Neurol. 2019;10:78. doi: 10.3389/fneur.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gidaro T., Reyngoudt H., Le Loue J., Behin A., Toumi F., Villeret M. Quantitative nuclear magnetic resonance imaging detects subclinical changes over 1 year in skeletal muscle of GNE myopathy. J. Neurol. 2019;267:228–238. doi: 10.1007/s00415-019-09569-6. [DOI] [PubMed] [Google Scholar]
- 12.Arpan I., Willcocks R.J., Forbes S.C., Finkel R.S., Lott D.J., Rooney W.D. Examination of effects of corticosteroids on skeletal muscles of boys with DMD using MRI and MRS. Neurology. 2014;83:974–980. doi: 10.1212/WNL.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlier P.G., Marty B., Scheidegger O., Loureiro de Sousa P., Baudin P.Y., Snezhko E. Skeletal muscle quantitative nuclear magnetic resonance imaging and spectroscopy as an outcome measure for clinical trials. J. Neuromuscul. Dis. 2016;3:1–28. doi: 10.3233/JND-160145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durmus H., Yilmaz R., Gulsen-Parman Y., Oflazer-Serdaroglu P., Cuttini M., Dursun M. Muscle magnetic resonance imaging in spinal muscular atrophy type 3: selective and progressive involvement. Muscle Nerve. 2017;55:651–656. doi: 10.1002/mus.25385. [DOI] [PubMed] [Google Scholar]
- 15.Liu G.C., Jong Y.J., Chiang C.H., Yang C.W. Spinal muscular atrophy: MR evaluation. Pediatr. Radiol. 1992;22:584–586. doi: 10.1007/BF02015357. [DOI] [PubMed] [Google Scholar]
- 16.Mercuri E., Pichiecchio A., Allsop J., Messina S., Pane M., Muntoni F. Muscle MRI in inherited neuromuscular disorders: past, present, and future. Magn. Reson. Imaging. 2007;25:433–440. doi: 10.1002/jmri.20804. [DOI] [PubMed] [Google Scholar]
- 17.Brogna C., Cristiano L., Verdolotti T., Pichiecchio A., Cinnante C., Sansone V. MRI patterns of muscle involvement in type 2 and 3 spinal muscular atrophy patients. J. Neurol. 2020;267:898–912. doi: 10.1007/s00415-019-09646-w. [DOI] [PubMed] [Google Scholar]
- 18.Burakiewicz J., Sinclair C.D.J., Fischer D., Walter G.A., Kan H.E., Hollingsworth K.G. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J. Neurol. 2017;264:2053–2067. doi: 10.1007/s00415-017-8547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oudeman J., Nederveen A.J., Strijkers G.J., Maas M., Luijten P.R., Froeling M. Techniques and applications of skeletal muscle diffusion tensor imaging: a review. J. Magn. Reson. Imag. 2016;43:773–788. doi: 10.1002/jmri.25016. [DOI] [PubMed] [Google Scholar]
- 20.Chianca V., Albano D., Messina C., Cinnante C.M., Triulzi F.M., Sardanelli F. Diffusion tensor imaging in the musculoskeletal and peripheral nerve systems: from experimental to clinical applications. Eur. Radiol. Exp. 2017;1:12. doi: 10.1186/s41747-017-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mercuri E., Talim B., Moghadaszadeh B. Clinical and imaging findings in six cases of congenital muscular dystrophy with rigid spine syndrome linked to chromosome 1p (RSMD1) Neuromuscul. Disord. 2002;12:631–638. doi: 10.1016/s0960-8966(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 22.Prior T.W., Krainer A.R., Hua Y., Swoboda K.J., Snyder P.C., Bridgeman S.J. A positive modifier of spinal muscular atrophy in the SMN2 gene. Am. J. Hum. Genet. 2009;85:408–413. doi: 10.1016/j.ajhg.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mercuri E., Finkel R., Montes J. Patterns of disease progression in type 2 and 3 SMA: implications for clinical trials. Neuromuscul. Disord. 2016;26:126–131. doi: 10.1016/j.nmd.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponrartana S., Ramos-Platt L., Wren T.A. Effectiveness of diffusion tensor imaging in assessing disease severity in Duchenne muscular dystrophy: preliminary study. Pediatr. Radiol. 2015;45:582–589. doi: 10.1007/s00247-014-3187-6. [DOI] [PubMed] [Google Scholar]
- 25.Williams S.E., Heemskerk A.M., Welch E.B., Li K., Damon B.M., Park J.H. Quantitative effects of inclusion of fat on muscle diffusion tensor MRI measurements. J. Magn. Reson. Imaging. 2013;38:1292–1297. doi: 10.1002/jmri.24045. [DOI] [PMC free article] [PubMed] [Google Scholar]