Abstract
Type 2 diabetes mellitus (T2DM) is associated with chronic low-grade inflammation. Carvacrol has been confirmed to possess anti-inflammatory properties, but its effect on diabetic vasculature remains unknown. The aim of the present study was to investigate the possible protective effects of carvacrol against vascular endothelial inflammation. The mice were divided into four groups (n=15 per group) as follows: Non-diabetic control mice, db/db mice, db/db mice + carvacrol (low) and db/db mice + carvacrol (high) groups. The effects of carvacrol on the pathomorphism of the thoracoab-dominal aorta in db/db mice were evaluated using hematoxylin and eosin and Masson's trichrome staining. The serum levels of insulin signaling molecules, such as phosphorylated insulin receptor, phosphorylated insulin receptor substrate-1, insulin, triglyceride (TG) and inflammatory cytokines [tumor necrosis factor-α, interleukin (IL)-1β, IL-6 and IL-8] were measured by ELISA. Furthermore, the protein levels of the toll-like receptor (TLR)4/nuclear factor (NF)-κB inflammatory signaling pathway molecules were investigated in the thoracoabdominal aorta of db/db mice and in high glucose-induced endothelial cells. Vascular endothelial cell apoptosis and viability were assessed by using flow cytometry and Cell Counting Kit-8 assays, respectively. The results demonstrated that carva-crol alleviated vascular endothelial cell injury. Carvacrol reduced the expression levels of insulin signaling molecules, insulin, TG and inflammatory cytokines in the serum of db/db mice. Moreover, carvacrol reduced the activation of the TLR4/NF-κB signaling pathway in vivo and in vitro. In vitro, carvacrol inhibited high glucose-induced endothelial cell function by promoting vascular endothelial cell apoptosis and suppressing cell viability. These findings demonstrated that carvacrol could alleviate endothelial dysfunction and vascular inflammation in T2DM.
Keywords: carvacrol, type 2 diabetes mellitus, endothelial dysfunction, vascular inflammation, toll-like receptor 4; nuclear factor-κB signaling pathway
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterized by insulin resistance and hyperglycemia (1-3). The prevalence of diabetes in adults is 6.4%, affecting 285 million adults in 2010 and expected to increase to 7.7% and 439 million adults by 2030 (4,5). Diabetes increases the risk of cardiovascular disease, such as atherosclerosis (6). Endothelial dysfunction is a hallmark of diabetes. Vascular endothelial complications, such as endothelial cell dysfunction and vascular inflammation, contribute to the morbidity and mortality of diabetes, which are common causes of limb amputation (7). Inflammation is considered as a key event in vascular dysfunction. In diabetes, the pro-inflammatory phenotype is enhanced by a variety of factors, including pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-18 and tumor necrosis factor (TNF)-α, and nuclear factor (NF)-κB (8). The main cause of diabetic vascular complications is hyperglycemia, which is associated with endothelial cell dysfunction. Hyperglycemia is a major risk factor for atherosclerosis, and atherosclerosis is the most important cause of various cardiovascular complications (9). Endothelial cells isolated from healthy subjects exposed to high glucose (HG) or isolated from diabetic patients exhibit limited proliferative capacity (10). Clinical studies have demonstrated that protecting vascular endothelial cells from damage may be a useful approach to the treatment of cardiovascular complications in diabetes. However, there is currently a lack of effective drugs for preventing the development of cardiovascular diseases in diabetic patients.
Plants have been used in traditional medicine due to their beneficial and protective properties (11). It has been reported that carvacrol (2-methyl-5-isopropylphenol), a monoterpenic phenol, has several pharmacological properties, such as anti-inflammatory (12), antioxidant (13), antiapoptotic (14), anticancer (14) and antimicrobial properties (15). Accumulating evidence from in vitro studies confirms these properties: For example, carvacrol was found to reduce the serum levels of inflammatory mediators and improve respiratory symptoms in veterans exposed to sulfur mustard (16). However, the effects of carvacrol on diabetes remain unclear.
In the present study, genetically hyperglycemic db/db mice were used as a T2DM model (17,18) to investigate whether carvacrol can alleviate vascular inflammation in diabetes.
Materials and methods
Animals
A total of 45 male C57BL/KsJ db/db mice (age, 8 weeks; weight, 32-36 g) and 15 age-matched C57BL/6J control non-diabetic db/m+ mice (age, 6 weeks; weight, 16-18 g) were purchased from Changzhou Cavans Experimental Animal Co., Ltd., (SCXK2001-0003). All mice were housed in a well-ventilated environment, with a 12-h light-dark cycle, at 23±2°C and 70±10% humidity, with free access to water and food. All animal experiments were performed strictly in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The research protocol was approved by the Traditional Chinese Medicine Guizhou University Animal Care and Ethics Committee.
Experimental design for the T2DM animal model
All mice were randomly divided into four groups as follows: i) Age-matched healthy control (n=15); ii) model control; iii) db/db model + low-dose carvacrol (5 mg/kg); and iv) db/db model + high-dose carvacrol (10 mg/kg) groups. All mice were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg). Subsequently, the db/db mice were treated with carvacrol (282197-50G, Sigma-Aldrich; Merck KGaA) daily for 6 weeks by gavage. At the same time, the normal control and model control groups were administered 0.9% saline at equal volumes. After 6 weeks, all the mice were euthanized by intraperitoneal injection of pentobarbital sodium (200 mg/kg) according to the recommendations of the animal ethics guidelines. Blood was quickly collected, centrifuged (at 3,000 × g for 10 min at 4°C) to obtain the blood serum samples, and stored at -20°C. The pancreatic tissues and skeletal muscles were removed and immediately immersed in 4% paraformaldehyde for 12 h at 4°C.
Oral glucose tolerance test (OGTT)
After 8 h of fasting, the mice were orally administered glucose solution (1.2 g/kg body weight). Blood was drawn from the tail vein, and the glucose levels were measured using a glucose monitor (Ascensia ELITE; Bayer).
Serum lipid and insulin levels
The fasting serum levels of total cholesterol, triglyceride (TG), high-density lipoprotein (HDL) and non-HDL were detected using enzymatic methods (Stanbio Laboratory). Furthermore, the serum insulin concentration was analyzed by enzyme immunoassay (Mercodia).
Histological examination and immunohistochemical analyses
The thoracoabdominal aorta was fixed at room temperature for 48 h in a buffer solution of 10% formalin, and then embedded in paraffin and sectioned at 20 µm. The sections were subjected to Masson's trichrome and hematoxylin and eosin (H&E) staining and observed under an optical microscope at a magnification, ×200 (BX51, Olympus Corporation). For immunohistochemical analyses, paraffin-embedded sections were incubated with anti-inhibitor of NF-κB kinase (anti-IKK; 1:100, ab32041, Abcam), anti-NF-κB inhibitor-α (anti-IKB-α; 1:100, ab32518, Abcam), anti-NALP3 (1:150, 19771-1-AP; ProteinTech Group, Inc.), anti-NF-κB (1:150, 14220-1-AP; ProteinTech Group, Inc.), anti-phosphorylated insulin receptor (anti-p-InsR; 1:100, ab60946, Abcam), anti-phosphorylated insulin receptor substrate-1 (anti-p-ISR-1; 1:100, ab3690, Abcam) and anti-toll-like receptor (TLR)4 (1:200, ab22048, Abcam) at 4°C overnight. Subsequently, the slides were incubated with secondary antibody (PV-9001; ZSGB-BIO, China) for 2 h at room temperature. The optical density was measured using ImageJ software, v1.8.0 (National Institutes of Health). The semi-quantitative results were evaluated by scoring the percentage of positive cells and staining intensity under the microscope. The percentage of positive cells was scored as follows: <5%, 0 points; 5 -25%, 1 point; 26-50%, 2 points; 51-75%, 3 points; and 76-100%, 4 points. The staining intensity was scored as follows: 0, no staining; 1, light yellow; 2, brown; and 3, tan. The product of the percentage of positive cells and the cell staining intensity was defined as the grade: 0, negative (−); 1-4, weakly positive (+); 5-8, positive (++); and 9-12, strongly positive (+++).
Reverse transcription-quantitative PCR (RT-qPCR) analysis
Total RNA was extracted from tissues or cells using a TRIzol reagent kit (15596-026, Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, the extracted RNA was reverse-transcribed into cDNA on ice using the TaqMan cDNA Synthesis kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). RT-qPCR was carried out with the miScript SYBR Green PCR kit (A25742, PowerUp™ SYBR™ Green Master Mix, Applied Biosystems; Thermo Fisher Scientific, Inc.). The conditions for RT-qPCR were as follows: One cycle of 2 min at 50°C; one cycle of 10 min at 95°C; 40 cycles of 15 sec at 95°C and 40 cycles of 1 min at 60°C. The primer sequences of the targeted genes are shown in Table I. The relative expression levels of the genes were normalized to GAPDH expression. The fold changes in expression were calculated using the 2-ΔΔCq method (PMID: 18546601).
Table I.
Primer sequence information for reverse transcription-quantitative PCR.
| Target gene | Primer sequence (5′-3′) |
|---|---|
| IKK | Forward: ACGACCTAGAGGAGCAAGCA |
| Reverse: AGCTCTGAATTGCCTGAAGC | |
| IKB-α | Forward: GGTGTTTGAATGTATTGCTGG |
| Reverse: AGGCTGTTTGGCTGAGGT | |
| NALP3 | Forward: TGGATCTAGCCACGCTAATG |
| Reverse: AAACCCATCCACTCCTCTTC | |
| NF-κB | Forward: ACCTGCCAGATACAGACGAT |
| Reverse: GAAGCTGAGCTGCGGGAA | |
| TLR4 | Forward: TCCCTGAACCCTATGAAC |
| Reverse: CTAAACCAGCCAGACCTT | |
| GAPDH | Forward: TGAGTCCTTCCACGATACCA |
| Reverse: ATCCCATCACCATCTTCCAG |
IKK, inhibitor of NF-κB kinase; IKB-α, NF-κB inhibitor-α; NF-κB, nuclear factor-κB; TLR, toll-like receptor.
Western blot analysis
Total protein was extracted from tissues or cells using RIPA lysis buffer (R0010; Solarbio) supplemented with 1% PMSF (P0100; Solarbio), and was determined with BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). A total of 120 µg protein was loaded per lane. Subsequently, the protein samples were separated by 10% SDS-PAGE and transferred to a PVDF membrane (IPVH00010; EMD Millipore). The membrane was then blocked with 5% skimmed milk at room temperature for 1 h, followed by incubation with the primary antibodies at 4°C overnight, and then incubation with the secondary antibody for 45 min. The primary antibodies included: Anti-IKK (1:1,000, ab32041; Abcam), anti-IKB-α (1:1,000, ab32518, Abcam), anti-NALP3 (1:2,000, 19771-1-AP, ProteinTech Group, Inc.), anti-NF-κB (1:2,000, 14220-1-AP, ProteinTech Group, Inc.), anti-TLR4 (1:2,000, ab22048, Abcam) and anti-β-actin (1:1,000; bs-0061R; BIOSS). β-actin was used as an internal control. Finally, the blots were visualized using an ECL kit (KGP1121; KeyGen Biotech Co., Ltd.).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were cultured for 48 h in Endothelial Cell Growth Medium-2 BulletKit™ (Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5% CO2, and divided into three groups as follows: i) Control; ii) endothelial cells + HG; and iii) endothelial cells + HG + carvacrol.
ELISA
Inflammatory cytokines (IL-1β, IL-6, IL-18 and TNF-α), insulin and TG levels in the serum were measured using a 96-well microplate and commercially available ELISA kits (cat. nos. H007, H203 and H266, respectively; all from NanJing JianCheng Bio). The optical density was read at 450 nm using a microplate reader (saf-680t; Thermo Fisher Scientific Inc.). The concentrations of IL-1β, IL-6, IL-18, TNF-α, insulin and TG were quantified based on the standard curves that were constructed by Curve Expert 1.4 software (Beijing Boleide Development of Science and Technology Co., Ltd.). Three wells from each sample were assayed in this experiment.
Flow cytometry assay of cell apoptosis
Vascular endothelial cell apoptosis was evaluated using the Annexin V-FITC Apoptosis Detection kit (BestBio). The cells were incubated with Annexin V-FITC and propidium iodide (PI) in the dark for 15 min at room temperature. The number of apoptotic cells was analyzed using flow cytometry (BD Biosciences).
Cell Counting Kit-8 (CCK-8) assay
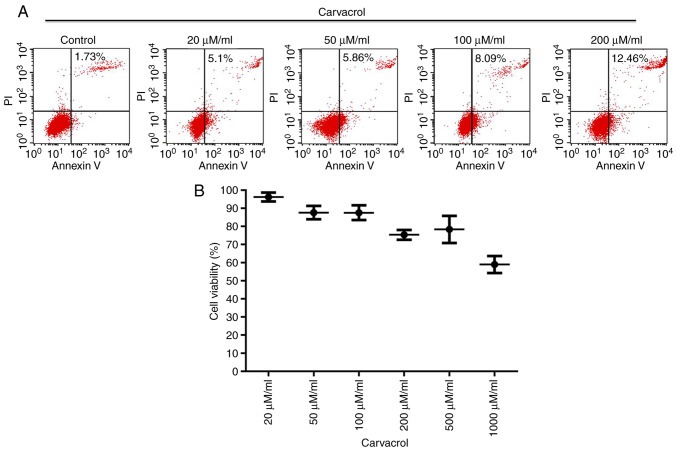
The cells were seeded in a 96-well plate (3,000 cells/well) and were cultured under different concentrations of carvacrol (20, 50, 100, 200, 500 and 1,000 µM/ml) at room temperature for 24 h. Subsequently, cell viability was evaluated using a CCK-8 Kit (Dojindo Molecular Technologies, Inc.) according to the manufacturer's instructions.
Immunocytochemical analyses
HUVECs were fixed in 4% paraformaldehyde at 4°C for 15 min and incubated in hydrogen peroxide for 15 min. Subsequently, the cells were blocked with goat serum (Solarbio) at 37°C for 30 min, followed by incubation with anti-IKK (1:100, ab32041; Abcam), anti-IKB-α (1:100, ab32518; Abcam), anti-NALP3 (1:150, 19771-1-AP; ProteinTech Group, Inc.), anti-NFκB (1:150, 14220-1-AP; ProteinTech Group, Inc.), anti-p-INSR (1:100, ab60946; Abcam), anti-p-ISR-1 (1:100, ab3690; Abcam) and anti-TLR4 (1:200, ab22048; Abcam) at 4°C overnight.
Statistical analysis
Statistical analyses were performed using GraphPad Prism v7.0 (GraphPad Software, Inc.). Data are expressed as means ± standard error of the mean. The differences between two groups were calculated using Student's t-test. Comparisons among multiple groups were performed using one-way analysis of variance followed by Tukey's post hoc test. P<0.05 was considered to indicate statistically significant differences.
Results
Effects of carvacrol on the pathomorphism of the thoracoabdominal aorta in db/db mice
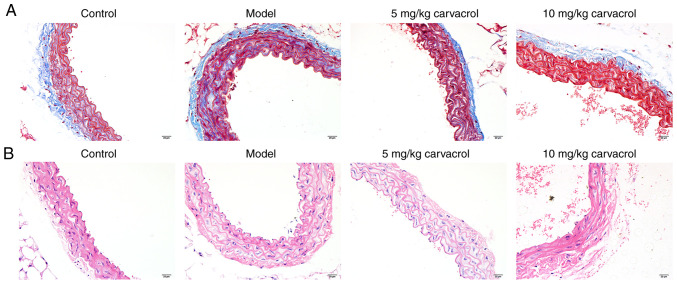
Fibrosis was assessed using Masson's trichrome staining on thoracoabdominal aortic sections (Fig. 1A). Compared with the normal control group, severe fibrosis was observed in the thoracoabdominal aorta of the db/db mice in the model group. As described in a previous study, 5 and 10 mg/kg carvacrol did not affect the normal activity and movement of the mice (19). Thus, these concentrations of carvacrol were selected for the present study. Carvacrol treatment (5 and 10 mg/kg) significantly improved vessel fibrosis in db/db mice. The model control group db/db mice exhibited severe pathological changes of the thoracoab-dominal aorta on H&E staining, such as disorderly and loosely arranged HUVECs, hypertrophied, distorted an disordered vascular smooth muscle cells with an increased number of layers, different nucleus sizes, unclear cell membrane and nuclear membrane, uneven cytoplasmic staining, and broken intracellular muscle fibers (Fig. 1B). These histological abnormalities were significantly alleviated in the carvacrol treatment groups (5 and 10 mg/kg) compared with the model group.
Figure 1.
Effects of carvacrol on the pathomorphism of the thoracoabdominal aorta of db/db mice. (A) Masson's staining of thoracoabdominal aortic sections of db/db mice. Abdominal aortic vascular smooth muscle cells and elastic fibers are stained red and collagen fibers are stained blue-green. (B) Hematoxylin and eosin staining of abdominal aortic sections of db/db mice. Magnification, ×200.
Carvacrol reduces the levels of insulin signaling molecules in the thoracoabdominal aorta of db/db mice
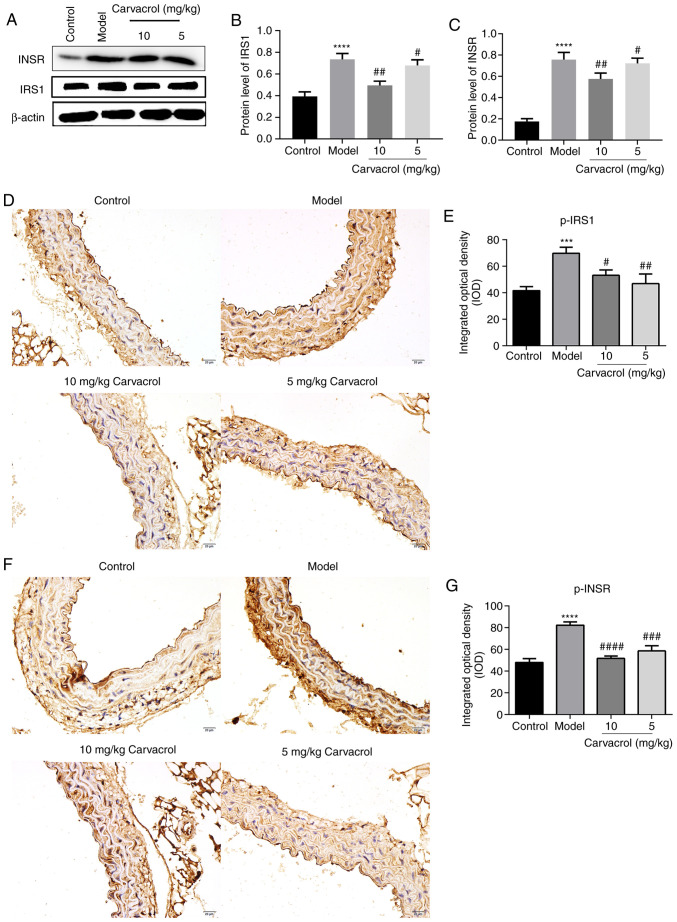
It was first observed that carvacrol decreased the levels of fasting blood glucose and serum insulin in the db/db model compared with those in the db/db model control group. Moreover, carvacrol significantly increased the Homeostatic Model Assessment of Insulin Resistance of db/db mice compared with the db/db model control group (Table II). Next, the protein expression of the insulin signaling molecules IRS-1 and InsR was found to be significantly higher in the thoracoabdominal aorta of the db/db model group compared with the control group (Fig. 2A-C). However, carvacrol treatment (5 and 10 µM/ml) significantly reduced the expression levels of these proteins (Fig. 2A-C). Similar results were observed on immunohistochemical examination (Fig. 2D-G). Thus, carvacrol reduces the expression of insulin signaling molecules in the thoracoabdominal aorta of db/db mice.
Table II.
Effect of carvacrol intervention on insulin resistance markers in db/db mice.
| Groups | Fasting blood glucose (mmol/l) | Insulin (mU/l) | HOMA-IR |
|---|---|---|---|
| Db/m+ | 6.62±3.04b | 1.20±0.15b | 0.35±0.09b |
| db/db model | 22.15±5.98 | 2.33±0.12 | 2.29±0.18 |
| db/db model + carvacrol (5 mg/kg) | 18.15±4.73 | 1.73±0.19b | 1.39±0.12b |
| db/db model + carvacrol (10 mg/kg) | 15.27±5.06a | 1.53±0.17b | 1.04±0.08b |
P<0.01 and
P<0.0001 vs. the db/db model group. HOMA-IR, Homeostatic Model Assessment of Insulin Resistance.
Figure 2.
Carvacrol reduced the levels of insulin signaling molecules, including IRS-1, InsR, p-IRS-1 and p-InsR in the abdominal aorta of db/db mice. (A) Western blot analysis of protein expression in the abdominal aorta of db/db mice. (B and C) Western blotting demonstrated the expression levels of IRS-1 and InsR in the abdominal aorta of db/db mice with and without carvacrol treatment. (D and E) Immunohistochemical staining for p-IRS-1 in the abdominal aorta of db/db mice; magnification, ×200. (F and G) Immunohistochemical staining for p-InsR in the abdominal aorta of db/db mice; magnification, ×200. Compared with control: ***P<0.001 and ****P<0.0001. Compared with model: #P<0.05, ##P<0.01, ###P<0.001 and ####P<0.0001. InsR, insulin receptor; IRS-1, insulin receptor substrate-1; p-, phosphorylated; IKK, inhibitor of NF-κB kinase; IKB-α, NF-κB inhibitor-α; NF-κB, nuclear factor-κB; TLR, toll-like receptor. Scale bar, 20 µm.
Carvacrol reduces the expression levels of insulin, TG and markers of insulin resistance in the serum of db/db mice
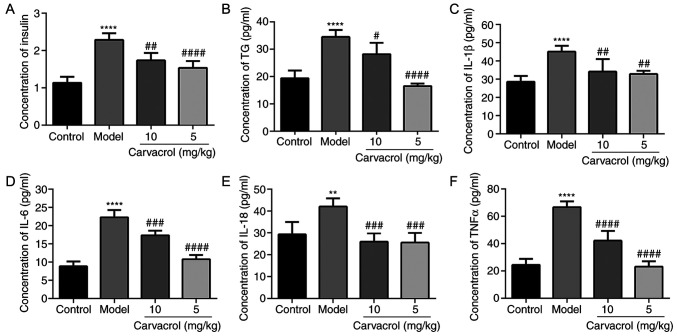
As shown in Fig. 3A, the serum insulin level in the db/db mice group was significantly higher compared with that in the normal control group, and carvacrol treatment (5 and 10 mg/kg) markedly decreased the serum insulin level compared with the db/db mice of the model control group. It was observed that the serum TG level was significantly higher in db/db mice compared with that in the normal control group (Fig. 3B). However, carvacrol treatment (5 and 10 mg/kg) markedly reduced the TG level compared with that in the db/db mouse control group.
Figure 3.
Carvacrol reduced the expression levels of insulin, TG and markers of insulin resistance in the serum of db/db mice according to ELISA. (A) Insulin; (B) TG; (C) IL-1β; (D) IL-6; (E) IL-18; and (F) TNF-α. Compared with control: **P<0.01 and ****P<0.0001. Compared with model: #P<0.05, ##P<0.01, ###P<0.001 and ####P<0.0001. TG, triglyceride; IL, interleukin; TNF, tumor necrosis factor.
Inflammatory markers, such as IL-1β, IL-6, IL-18 and TNF-α, have been confirmed as the main cause of insulin resistance in diabetic patients. Therefore, the levels of IL-1β, IL-6, IL-18 and TNF-α were measured in the serum of db/db mice. It was observed that the serum levels of IL-1β, IL-6, IL-18 and TNF-α in the db/db mice of the model group were significantly higher compared with those in the normal control group (Fig. 3C-F). However, carvacrol treatment (5 and 10 mg/kg) obviously decreased the serum levels of IL-1β, IL-6, IL-18 and TNF-α compared with those in the db/db mice of the model control group.
Effects of carvacrol on the TLR4/NF-κB signaling pathway in the thoracoabdominal aorta of db/db mice
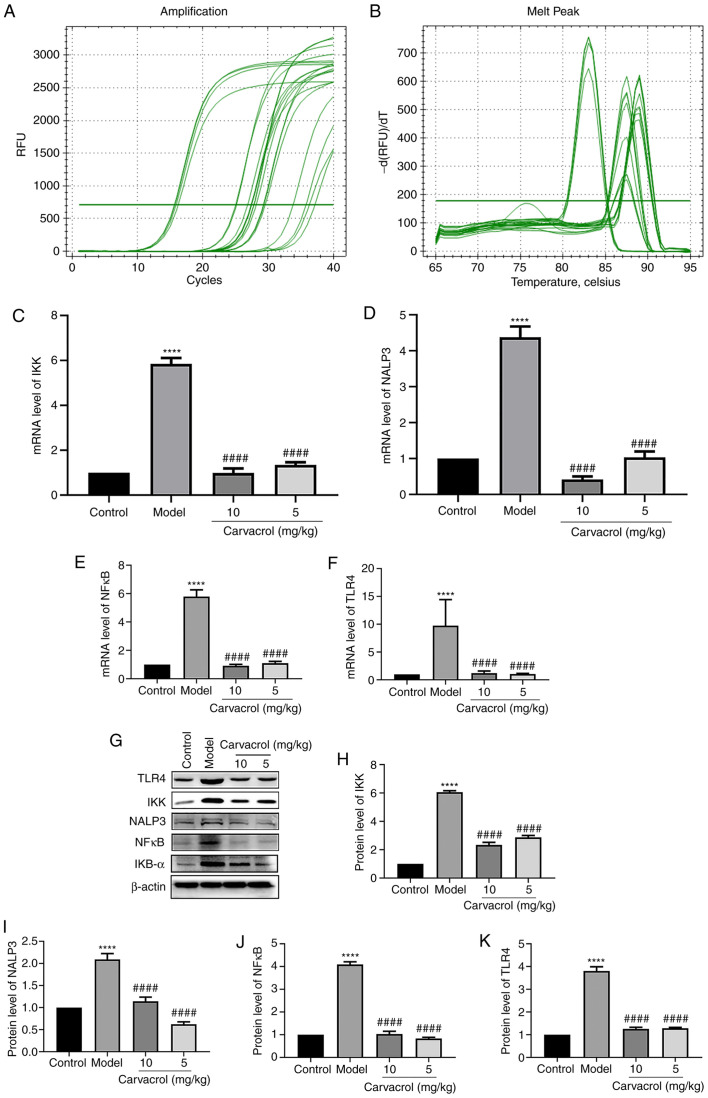
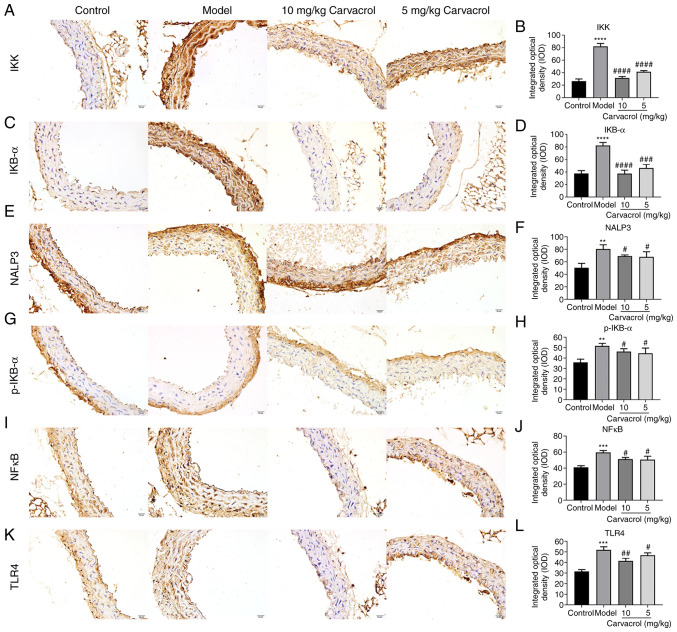
It has been demonstrated that the TLR4/NF-κB signaling pathway is involved in the regulation of vascular inflammatory responses (20,21). To explore the potential underlying mechanism, the expression levels of the TLR4/NF-κB pathway molecules, including IKK, IKB-α, NALP3, NF-κB and TLR4, were measured in the thoracoabdominal aorta of db/db mice. The mRNA and protein levels of IKK, NALP3, NF-κB and TLR4 were found to be significantly higher in the db/db mice of the model group compared with those in the normal control group (Fig. 4A-K). However, carvacrol treatment (5 and 10 mg/kg) markedly decreased the levels of IKK, NALP3, NFκB and TLR4 compared with the db/db mice of the model control group (Fig. 4A-K). The results of immunohistochemical analysis were consistent with the results mentioned above (Fig. 5A-L). Moreover, it was observed that the IKB-α and p-IKB-α levels were higher in the thoracoabdominal aorta of db/db mice compared with those in the normal control group, whereas carvacrol treatment (5 and 10 kg/kg) decreased the levels of IKB-α and p-IKB-α.
Figure 4.
Effects of carvacrol on the TLR4/NF-κB signaling pathway in the thoracoabdominal aorta of db/db mice. (A and B) Reverse transcription-quantitative PCR analysis. mRNA levels of (C) IKK, (D) NALP3, (E) NF-κB and (F) TLR4 in the thoracoabdominal aortic tissues of db/db mice. (G) Western blot analysis. Protein levels of (H) IKK, (I) NALP3, (J) NF-κB and (K) TLR4 in the thoracoabdominal aortic tissues of db/db mice. Compared with control: ****P<0.0001. Compared with model: ####P<0.0001. IKK, inhibitor of NF-κB kinase; IKB-α, NF-κB inhibitor-α; NF-κB, nuclear factor-κB; TLR, toll-like receptor.
Figure 5.
Immunohistochemical analyses results showing the expression levels of (A and B) IKK, (C and D) IKB-α, (E and F) NALP3, (G and H) IKB-α, (I and J) NF-κB and (K and L) TLR4 in the thoracoabdominal aortic tissues of db/db mice (magnification, ×200). Compared with control: **P<0.01; ***P<0.001 and ****P<0.0001. Compared with model: #P<0.05, ##P<0.01, ###P<0.001 and ####P<0.0001. IKK, inhibitor of NF-κB kinase; IKB-α, NF-κB inhibitor-α; NF-κB, nuclear factor-κB; TLR, toll-like receptor. Scale bar, 20 µm.
Carvacrol promotes apoptosis and inhibits the viability of HUVECs
Endothelial cell apoptosis and viability were then investigated. Flow cytometry was employed to evaluate HUVEC apoptosis. It was observed that carvacrol promoted vascular endothelial cell apoptosis in a dose-dependent manner (Fig. 6A). Cell viability was assessed with the CCK-8 assay. The results demonstrated that carvacrol decreased endothelial cell viability in a dose-dependent manner (Fig. 6B).
Figure 6.
Carvacrol promoted apoptosis and inhibited viability of HUVECs. (A) Flow cytometry assay results showing the apoptosis of HUVECs under different concentrations of carvacrol (20, 50, 100 and 200 µM/ml). (B) Cell Counting Kit-8 assay demonstrated endothelial cell viability (20, 50, 100, 500 and 1,000 µM/ml carvacrol). HUVECs, human umbilical vein endothelial cells.
Carvacrol reduces the levels of insulin signaling molecules in HG-induced HUVECs
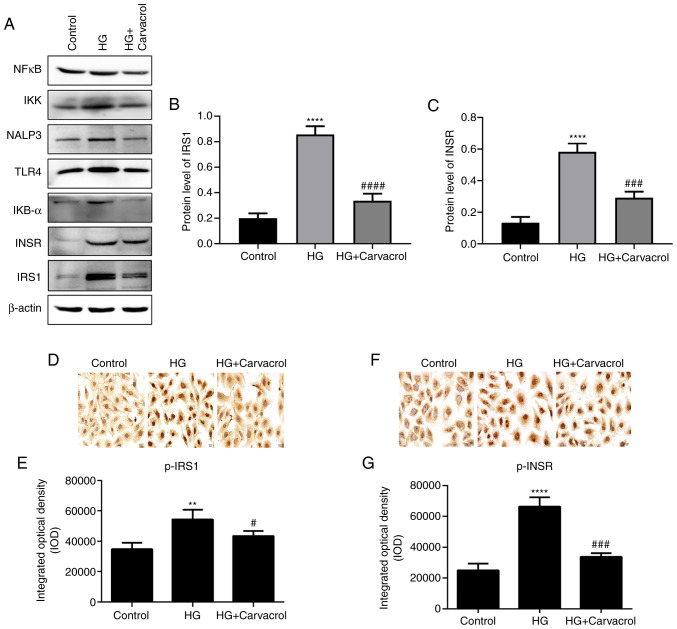
The protein levels of IRS-1 and InsR were found to be significantly higher in HG-induced HUVECs compared with those in the HUVECs control group (Fig. 7A-C), and carvacrol treatment (5 and 10 mg/kg) significantly reduced the levels of p-IRS-1 and p-InsR in HG-induced HUVECs (Fig. 7D-G). The results mentioned above indicate that carvacrol reduces the levels of insulin signaling molecules in HG-induced HUVECs.
Figure 7.
Carvacrol reduced the levels of insulin signaling molecules in HG-induced HUVECs. (A) Western blotting of different proteins in HUVECs. (B) Expression of IRS-1 in the HUVECs according to western blotting analysis. (C) Expression of InsR in HUVECs according to western blotting analysis. (D and E) Immunohistochemical staining for p-IRS-1 in HG-induced HUVECs; magnification, ×200. (F and G) Immunohistochemical staining for p-InsR in HG-induced HUVECs; magnification, ×200. Compared with control: **P<0.01 and ****P<0.0001. Compared with model: #P<0.05, ###P<0.001 and ####P<0.0001. HUVECs, human umbilical vein endothelial cells; HG, high glucose; p-InsR, phosphorylated insulin receptor; p-IRS-1, phosphorylated insulin receptor substrate-1; IKK, inhibitor of NF-κB kinase; IKB-α, NF-κB inhibitor-α; NF-κB, nuclear factor-κB; TLR, toll-like receptor.
Effects of carvacrol on the TLR4/NF-κB signaling pathway in HG-induced HUVECs
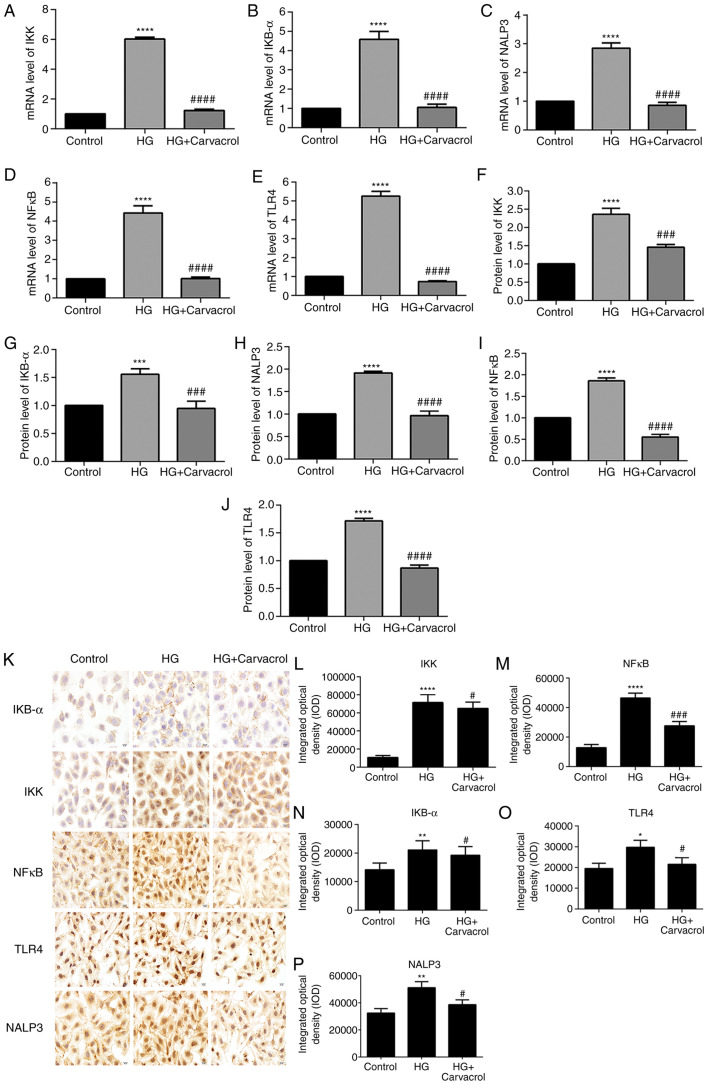
It was inferred that carvacrol reduces the activation of the TLR4/NF-κB signaling pathway in HG-induced HUVECs. As expected, the mRNA and protein expression of IKK, IKB-α, NALP3, NF-κB and TLR4 were found to be increased in HG-induced HUVECs compared with those in the control group (Fig. 8A-J). However, carvacrol significantly decreased the expression of IKK, IKB-α, NALP3, NF-κB and TLR4 compared with the control group (Fig. 8A-J). These data indicated that carvacrol may be involved in the activation of the TLR4/NF-κB signaling pathway in HG-induced HUVECs. In addition, immunohistochemical analysis was performed. As expected, the results of immunohistochemistry were consistent with the results mentioned above (Fig. 8K-O). The hypothesis diagram of the present study is shown in Fig. 9.
Figure 8.
Effects of carvacrol on the TLR4/NF-κB signaling pathway in HG-induced HUVECs. Reverse transcription-quantitative PCR analysis results showing the mRNA expression levels of (A) IKK, (B) IKB-α, (C) NALP3, (D) NF-κB and (E) TLR4. Western blotting results showing the protein expression levels of (F) IKK, (G) IKB-α, (H) NALP3, (I) NF-κB and (J) TLR4. (K) Representative images of immunohistochemical analyses results magnification, ×200. Expression levels of (L) IKK, (M) NF-κB, (N) IKB-α, (O) TLR4 and (P) NALP3 in HG-induced HUVECs according to the immunohistochemical analysis results. Compared with control: *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. Compared with model: #P<0.05, ###P<0.001 and ####P<0.0001. IKK, inhibitor of NF-κB kinase; IKB-α, NF-κB inhibitor-α; NF-κB, nuclear factor-κB; TLR, toll-like receptor; HUVECs, human umbilical vein endothelial cells; HG, high glucose.
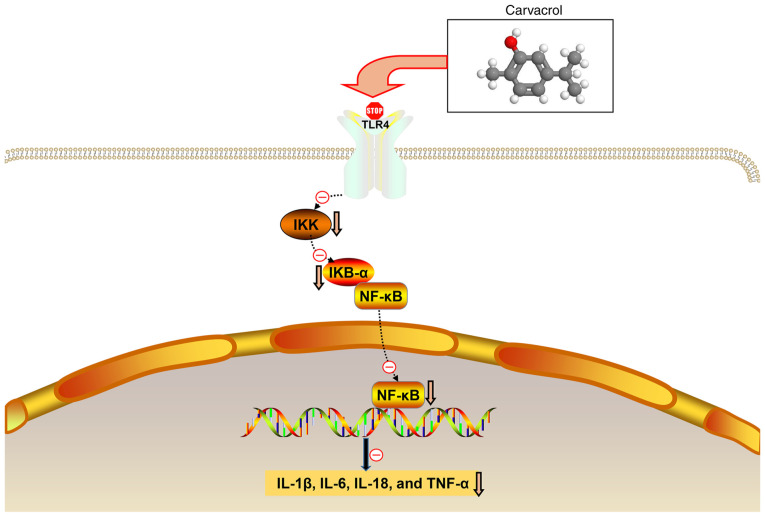
Figure 9.
Hypothesis diagram of the present study. IKK, inhibitor of NF-κB kinase; IKB-α, NF-κB inhibitor-α; NF-κB, nuclear factor-κB; TLR, toll-like receptor; IL, interleukin; TNF, tumor necrosis factor.
Discussion
The optimal antidiabetic drug would be expected to improve insulin resistance in diabetic patients without causing any side effects (22). However, the currently available antidiabetic drugs are associated with long-term side effects. Therefore, in an attempt to find safe and effective antidiabetic drugs, the effects of carvacrol on a db/db mouse model were investigated. The results of the present study suggested that carvacrol may alleviate endothelial cell dysfunction and vascular inflammation in T2DM.
In the present study, db/db mice were used to construct a T2DM model. It was observed that the db/db model mice exhibited major pathological changes. The db/db mice were treated with low-dose carvacrol (5 mg/kg) or high-dose carvacrol (10 mg/kg), and it was demonstrated that both doses significantly alleviated the histological abnormalities of the abdominal aorta in the db/db mouse model. It is well known that T2DM-related disorders are often associated with insulin resistance (23-25). Peripheral target tissues exhibit reduced sensitivity to insulin, leading to abnormal insulin secretion and even hyperglycemia. Insulin resistance is considered to be a major therapeutic target in T2DM (26). It was herein demonstrated that both low- and high-dose carvacrol improved insulin resistance via suppressing the phosphorylation of the insulin signaling molecules p-IRS-1 and p-InsR in vitro as well as in vivo, indicating that carvacrol may be of value in the treatment of T2DM.
T2DM is considered to be a chronic inflammatory disease. Inflammation and immune cell dysfunction have been shown to be associated with insulin resistance and secretion dysfunction (27). In diabetes, vascular inflammation and endothelial cell dysfunction play a major role in the development of vascular disease (28). Pro-inflammatory cytokines promote vascular dysfunction in diabetes by promoting endothelial cell inflammation (21). For example, the pro-inflammatory cytokine IL-1β has been reported to be a driving factor for β-cell dysfunction (29,30). IL-1β secretion is increased in the islets in response to high levels of glucose, which promotes the recruitment and activation of macrophages, thereby maintaining the islet inflammatory response (30). IL-6 has been confirmed to be highly expressed in the serum of patients with T2DM, which induces insulin resistance (31). It was previously demonstrated that serum IL-18 levels are positively correlated with the pathogenesis and development of T2DM (32). Furthermore, TNF-α is mainly produced in adipocytes or peripheral tissues. Elevated TNF-α levels impair insulin signaling through serine phosphorylation, which induces insulin resistance in adipocytes and peripheral tissues (33). It has been demonstrated that insulin resistance in diabetic patients can increase the serum levels of inflammatory mediators (including IL-1β, IL-6, IL-18 and TNF-α) and plays an important role in regulating glucose homeostasis (22). The results of the present study indicated that carvacrol effectively reduced the serum levels of inflammatory mediators, including IL-1β, IL-6, IL-18 and TNF-α, in db/db mice. These findings indicated that carvacrol may alleviate insulin resistance of db/db mice by inhibiting the expression of these pro-inflammatory cytokines.
IKK activates the nuclear translocation of NF-κB by the degradation inhibitor IκBα (34). In the present study, db/db mice exhibited increased expression of IKK and IκBα. Increased IKK expression indicated the activation of NF-κB signaling. Furthermore, increased IκBα expression may be an adaptive response to this activation. However, carvacrol significantly reduced IKK and IκBα expression in db/db mice. Therefore, carvacrol may reduce vascular inflammation by suppressing the NF-κB signaling pathway. The TLR4/NF-κB signaling pathway is involved in the inflammatory response of diabetes. To explore whether carvacrol also exerts anti-inflammatory effects through the TLR4/NF-κB signaling pathway, the expression levels of relevant markers in the TLR4/NF-κB signaling pathway in the abdominal aorta were examined. TLR4 and NF-κB levels were significantly upregulated in db/db mice, but they were reduced by carvacrol at both the transcriptional and translational levels. Therefore, the results mentioned above indicate that carvacrol may exert its anti-inflammatory effects through inactivation of the TLR4/NF-κB signaling pathway.
Endothelial cells are sensitive to changes in blood glucose levels (35). Endothelial cell injury has been identified as an early event in the development of atherosclerosis. Diabetes, as one of the risk factors for atherosclerosis, can damage the vascular endothelium. There is growing evidence that diabetic atherosclerosis is associated with hyperglycemia. Both in vivo and in vitro studies have demonstrated that hyperglycemia can contribute to HUVEC damage and dysfunction, ultimately leading to atherosclerosis (36,37). In the present study, the results revealed that carvacrol promoted apoptosis of HG-induced HUVECs in a dose-dependent manner. As expected, the in vitro experiment results demonstrated that the protein levels of the TLR4/NF-κB signaling pathway molecules were elevated in HG-induced vascular endothelial cells. Moreover, carvacrol significantly suppressed the levels of relevant markers in the TLR4/NF-κB signaling pathway. These results indicated that carvacrol may protect HG-induced HUVECs through inactivation of the TLR4/NF-κB signaling pathway.
In conclusion, the present study demonstrated that carvacrol alleviated the histological abnormalities of the abdominal aorta in a db/db mouse model. Furthermore, the anti-inflammatory effect of carvacrol was confirmed in the db/db mouse model. Further research into the underlying mechanism demonstrated that carvacrol reduced the activation of the TLR4/NF-κB inflammatory response signaling pathway in vitro and in vivo. Thus, these findings indicated that carvacrol may reduce vascular inflammation and endothelial cell dysfunction, and it may be of value in the treatment of T2DM.
Acknowledgments
Not applicable.
Abbreviations
- T2DM
type 2 diabetes mellitus
- H&E
hematoxylin and eosin
- p-InsR
phosphorylated insulin receptor
- p-IRS-1
phosphorylated insulin receptor substrate-1
- TG
triglyceride
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-18
interleukin-18
- TNF-α
tumor necrosis factor-α
- NF-κB
nuclear factor-κB
- CCK-8
Cell Counting Kit-8
- RT-qPCR
reverse transcription-quantitative PCR
- ELISA
enzyme-linked immunosorbent assay
Funding
The present study was funded by grants from the National Natural Science Foundation of China (81760813) and the Science and Technology Cooperation Plan of Guizhou [Qiankehe LH (2016) No.7127].
Availability of data and materials
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
WZ conceived and designed the study. CD and QH conducted most of the experiments and data analysis, and wrote the manuscript. HX and YC participated in collecting data and helped with the drafting the manuscript. All authors have reviewed and approved the final version of the manuscript.
Ethics approval and consent to participate
All animal experiments were performed strictly in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The research protocol was approved by the Traditional Chinese Medicine Guizhou University Animal Care and Ethics Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.van der Schaft N, Schoufour JD, Nano J, Kiefte-de Jong JC, Muka T, Sijbrands EJG, Ikram MA, Franco OH, Voortman T. Dietary antioxidant capacity and risk of type 2 diabetes mellitus, prediabetes and insulin resistance: The rotterdam study. Eur J Epidemiol. 2019;34:853–861. doi: 10.1007/s10654-019-00548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Z, Jardine M, Perkovic V, Matthews DR, Mahaffey KW, de Zeeuw D, Fulcher G, Desai M, Oh R, Simpson R, et al. Canagliflozin and fracture risk in individuals with type 2 diabetes: Results from the CANVAS program. Diabetologia. 2019;62:1854–1867. doi: 10.1007/s00125-019-4955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y, Yang Y, Dong B, Zheng H, Lin X, Du Y, Li X, Zhao L, Gao H. Metabonomic profiles delineate potential role of glutamate-glutamine cycle in db/db mice with diabetes-associated cognitive decline. Mol Brain. 2016;9:40. doi: 10.1186/s13041-016-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Petersen C, Bharat D, Cutler BR, Gholami S, Denetso C, Mueller JE, Cho JM, Kim JS, Symons JD, Anandh Babu PV. Circulating metabolites of strawberry mediate reductions in vascular inflammation and endothelial dysfunction in db/db mice. Int J Cardiol. 2018;263:111–117. doi: 10.1016/j.ijcard.2018.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawada N, Jiang A, Takizawa F, Safdar A, Manika A, Tesmenitsky Y, Kang KT, Bischoff J, Kalwa H, Sartoretto JL, et al. Endothelial PGC-1α mediates vascular dysfunction in diabetes. Cell Metab. 2014;19:246–258. doi: 10.1016/j.cmet.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan E, Wang B, McClelland A, Mohan M, Marai M, Beuscart O, Derouiche S, Gray S, Pickering R, Tikellis C, et al. Protective effect of let-7 mirna family in regulating inflammation in diabetes-associated atherosclerosis. Diabetes. 2017;66:2266–2277. doi: 10.2337/db16-1405. [DOI] [PubMed] [Google Scholar]
- 9.Ren Y, Tao S, Zheng S, Zhao M, Zhu Y, Yang J, Wu Y. Salvianolic acid B improves vascular endothelial function in diabetic rats with blood glucose fluctuations via suppression of endothelial cell apoptosis. Eur J Pharmacol. 2016;791:308–315. doi: 10.1016/j.ejphar.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Kang H, Ma X, Liu J, Fan Y, Deng X. High glucose-induced endothelial progenitor cell dysfunction. Diab Vasc Dis Res. 2017;14:381–394. doi: 10.1177/1479164117719058. [DOI] [PubMed] [Google Scholar]
- 11.Kara M, Uslu S, Demirci F, Temel HE, Baydemir C. Supplemental carvacrol can reduce the severity of inflammation by influencing the production of mediators of inflammation. Inflammation. 2015;38:1020–1027. doi: 10.1007/s10753-014-0066-0. [DOI] [PubMed] [Google Scholar]
- 12.Somensi N, Rabelo TK, Guimarães AG, Quintans-Junior LJ, de Souza Araújo AA, Moreira JCF, Gelain DP. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-kB pathway. Int Immunopharmacol. 2019;75:105743. doi: 10.1016/j.intimp.2019.105743. [DOI] [PubMed] [Google Scholar]
- 13.Manouchehrabadi M, Farhadi M, Azizi Z, Torkaman-Boutorabi A. Carvacrol protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro models of parkinson's disease. Neurotox Res. 2020;37:156–170. doi: 10.1007/s12640-019-00088-w. [DOI] [PubMed] [Google Scholar]
- 14.Khan F, Singh VK, Saeed M, Kausar MA, Ansari IA. Carvacrol induced program cell death and cell cycle arrest in androgen-independent human prostate cancer cells via inhibition of notch signaling. Anticancer Agents Med Chem. 2019;19:1588–1608. doi: 10.2174/1871520619666190731152942. [DOI] [PubMed] [Google Scholar]
- 15.Shoorei H, Khaki A, Khaki AA, Hemmati AA, Moghimian M, Shokoohi M. The ameliorative effect of carvacrol on oxidative stress and germ cell apoptosis in testicular tissue of adult diabetic rats. Biomed Pharmacother. 2019;111:568–578. doi: 10.1016/j.biopha.2018.12.054. [DOI] [PubMed] [Google Scholar]
- 16.Khazdair MR, Boskabady MH. The effect of carvacrol on inflammatory mediators and respiratory symptoms in veterans exposed to sulfur mustard, a randomized, placebo-controlled trial. Respir Med. 2019;150:21–29. doi: 10.1016/j.rmed.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: Molecular characterization and effects of Western diet feeding. Metabolism. 2000;49:22–31. doi: 10.1016/S0026-0495(00)90588-2. [DOI] [PubMed] [Google Scholar]
- 18.Peng BY, Wang Q, Luo YH, He JF, Tan T, Zhu H. A novel and quick PCR-based method to genotype mice with a leptin receptor mutation (db/db mice) Acta Pharmacol Sin. 2018;39:117–123. doi: 10.1038/aps.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmoodi M, Amiri H, Ayoobi F, Rahmani M, Taghipour Z, Ghavamabadi RT, Jafarzadeh A, Sankian M. Carvacrol ameliorates experimental autoimmune encephalomyelitis through modulating pro- and anti-inflammatory cytokines. Life Sci. 2019;219:257–263. doi: 10.1016/j.lfs.2018.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Wu S, Chen C, Xie B, Fang Z, Hu W, Chen J, Fu H, He H. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation. 2017;14:143. doi: 10.1186/s12974-017-0917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang ZH, Peng J, Ren Z, Yang J, Li TT, Li TH, Wang Z, Wei DH, Liu LS, Zheng XL, Jiang ZS. New role of PCSK9 in athero-sclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis. 2017;262:113–122. doi: 10.1016/j.atherosclerosis.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Sharma BR, Kim HJ, Rhyu DY. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes. J Transl Med. 2015;13:62. doi: 10.1186/s12967-015-0412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogata S, Ito S, Masuda T, Ohtsuki S. Changes of blood-brain barrier and brain parenchymal protein expression levels of mice under different insulin-resistance conditions induced by high-fat diet. Pharm Res. 2019;36:141. doi: 10.1007/s11095-019-2674-8. [DOI] [PubMed] [Google Scholar]
- 24.Pivari F, Mingione A, Brasacchio C, Soldati L. Curcumin and type 2 diabetes mellitus: Prevention and treatment. Nutrients. 2019;11:1837. doi: 10.3390/nu11081837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Zhou H, Palyha O, Mu J. Restoration of insulin receptor improves diabetic phenotype in T2DM mice. JCI Insight. 2019;4:e124945. doi: 10.1172/jci.insight.124945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 27.Kammoun HL, Allen TL, Henstridge DC, Barre S, Coll RC, Lancaster GI, Cron L, Reibe S, Chan JY, Bensellam M, et al. Evidence against a role for NLRP3-driven islet inflammation in db/db mice. Mol Metab. 2018;10:66–73. doi: 10.1016/j.molmet.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollack RM, Donath MY, LeRoith D, Leibowitz G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care. 2016;39(Suppl 2):S244–S52. doi: 10.2337/dcS15-3015. [DOI] [PubMed] [Google Scholar]
- 29.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI200215318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herder C, Dalmas E, Böni-Schnetzler M, Donath MY. The IL-1 pathway in type 2 diabetes and cardiovascular complications. Trends Endocrinol Metab. 2015;26:551–563. doi: 10.1016/j.tem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Rehman K, Akash MSH, Liaqat A, Kamal S, Qadir MI, Rasul A. Role of interleukin-6 in development of insulin resistance and type 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr. 2017;27:229–236. doi: 10.1615/CritRevEukaryotGeneExpr.2017019712. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang H, Han J, Cheng L, Liu SL. A positive causal influence of IL-18 levels on the risk of T2DM: A mendelian randomization study. Front Genet. 2019;10:295. doi: 10.3389/fgene.2019.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akash MSH, Rehman K, Liaqat A. Tumor necrosis factor-alpha: Role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2018;119:105–110. doi: 10.1002/jcb.26174. [DOI] [PubMed] [Google Scholar]
- 34.Babu PV, Si H, Liu D. Epigallocatechin gallate reduces vascular inflammation in db/db mice possibly through an NF-κB-mediated mechanism. Mol Nutr Food Res. 2012;56:1424–1432. doi: 10.1002/mnfr.201200040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silambarasan M, Tan JR, Karolina DS, Armugam A, Kaur C, Jeyaseelan K. MicroRNAs in hyperglycemia induced endothelial cell dysfunction. Int J Mol Sci. 2016;17:518. doi: 10.3390/ijms17040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao X, Dong Y, Zhong J, Cao R, Zhao X, Wen G, Liu J. Adiponectin protects endothelial cells from the damages induced by the intermittent high level of glucose. Endocrine. 2011;40:386–393. doi: 10.1007/s12020-011-9531-9. [DOI] [PubMed] [Google Scholar]
- 37.Torimoto K, Okada Y, Mori H, Tanaka Y. Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12:1. doi: 10.1186/1475-2840-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.