Abstract
Background
Currently, preoperative chemotherapy is the standard of care in locally advanced breast cancer to achieve local tumour downsizing in order to make surgery possible. Since the early 1980s, the role of preoperative chemotherapy in early stage (or operable) breast cancer has been the subject of study. Potential advantages are early introduction of systemic therapy, determination of chemosensitivity, reduction of tumour volume and downstaging of surgical requirement. Concerns exist about local control after downsized surgery and the delay of local treatment in patients with tumours resistant to chemotherapy.
Objectives
To assess the effectiveness of preoperative chemotherapy in women with operable breast cancer when compared to postoperative chemotherapy.
Search methods
The Specialised Register maintained by the Editorial Base of the Cochrane Breast Cancer Group was searched on 4th of August 2005.
Selection criteria
Randomised trials comparing preoperative chemotherapy with postoperative in women with operable breast cancer.
Data collection and analysis
Studies were assessed for eligibility and quality, and data were extracted by two independent review authors. Hazard ratios were derived for time‐to‐event outcomes directly or indirectly using the methods described by Parmar. Relative risks were derived for dichotomous outcomes. Meta‐analyses were performed using fixed effect model.
Main results
We identified 14 eligible studies which randomised a total of 5,500 women. Median follow‐up ranged from 18 to 124 months. Eight studies described a satisfactory method of randomisation.
Data, based on 1139 estimated deaths in 4620 women available for analysis, show equivalent overall survival rates with a HR of 0.98 (95% CI, 0.87 to 1.09; p, 0.67; no heterogeneity). Preoperative chemotherapy increases breast conservation rates, yet at the associated cost of increased loco regional recurrence rates. However, this rate was not increased as long as surgery remains part of the treatment even after complete tumour regression (HR, 1.12; 95% CI, 0.92 to 1.37; p, 0.25; no heterogeneity. Preoperative chemotherapy was associated with fewer adverse effects. Pathological complete response is associated with better survival than residual disease (HR, 0.48; 95% CI, 0.33 to 0.69; p, < 10‐4).
Authors' conclusions
This review suggests safe application of preoperative chemotherapy in the treatment of women with early stage breast cancer in order to down‐stage surgical requirement, to evaluate chemosensitivity and to facilitate translational research.
Plain language summary
Preoperative chemotherapy for women with operable breast cancer
Chemotherapy for patients with early stage breast cancer has been shown to improve survival. Traditionally, this therapy is given once the patient has undergone surgery. Since the early 1980's, interest has risen in administrating chemotherapy before surgery (known as preoperative or neoadjuvant chemotherapy) based on good results achieved in patients with locally advanced disease (cancer which is larger than 5cm and/or has spread to surrounding tissue or lymph nodes, or both). The rationale for preoperative chemotherapy is that an early introduction of systemic treatment (treatment that affects the whole body) will result in a decrease in the size of the tumour, hence making it possible to do more breast‐conserving surgery. For this review, we investigated the effect of the difference in timing of chemotherapy treatment for patients with early stage or operable disease.
This review identified 14 randomised controlled trials involving 5,500 women addressing this question. The analyses revealed no difference in overall survival and disease‐free survival for women who received either preoperative or postoperative chemotherapy. Preoperative treatment makes more breast‐conserving surgery possible because of shrinkage of the tumour before surgical intervention (relative risk, 0.82; 95% confidence interval, 0.76 to 0.89). However, this also results in a increase of loco‐regional recurrence (recurrence in the same area) rate (hazard ratio, 1.12; 95% confidence interval, 0.92 to 1.37). Preoperative chemotherapy provides the possibility of monitoring tumour response and making appropriate regimen changes once the tumour appears to be resistant to the primary therapy. Adverse effects, which were reported in only half of the studies, were fewer in women receiving preoperative chemotherapy. Although, postoperative complications, nausea and vomiting, and alopecia were equally distributed, events of cardiotoxicity were less likely (relative risk, 0.74; 95% confidence interval, 0.53 to 1.04) in women receiving preoperative chemotherapy. Also, serious infection (analysed in 2799 women) was less likely to occur in women receiving preoperative chemotherapy (relative risk, 0.69; 95% confidence interval, 0.56 to 0.84).
Background
Breast cancer is the most common type of cancer in women and the most common cause of cancer‐related death in women (Ferlay 2001).
During the 1970s and 1980s several clinical trials were conducted to evaluate the efficacy of postoperative adjuvant chemotherapy in terms of treatment outcome (Bonadonna 1995; Fisher 1989; Mansour 1998). Quinquennial meta‐analyses of the worldwide experience with adjuvant chemotherapy showed significant improvements in progression‐free and overall survival (EBCTCG 2005).
In the early 1980s the use of preoperative (neoadjuvant) chemotherapy was introduced in patients with locally advanced breast cancer (Hortobagyi 1983; Perloff 1982; Schick 1983) and its role in the management of locally advanced breast cancer has since been established (Hortobagyi 1997). The initial goal was to convert 'inoperable' tumours (those with classical locally advanced disease yet without overt evidence of systemic metastasis beyond breast and regional nodes) into 'operable' tumours. Inoperable breast cancers include technically unresectable advanced tumours, as well as those with characteristics indicating an extremely high risk of metastases and death despite an initial surgical resection (grave signs) (Haagensen 1943a; Haagensen 1943b). These characteristics include stage IIIB cancers (T4, N3) and patients with inflammatory carcinoma. There is a clear distinction in prognosis between stage IIIB and IIIA cancers (Hortobagyi 1988). In recent guidelines, preoperative chemotherapy for locally advanced and inflammatory breast cancer is considered as part of a multimodel treatment approach, although not based on the results of large randomised clinical trials (Deo 2003; Kaufmann 2003).
Positive results in patients with inoperable disease have largely paved the way to explore a possible extension of the role of chemotherapy delivered before surgery for patients with operable breast cancer (Bonadonna 1990; Hortobagyi 1988; Jacquillat 1989). Patients with operable breast cancer have tumours in stages I to IIIA (T1‐T3, N0‐N1,M0) and these patients can be treated with multiple therapeutic strategies.
The indications for the use of preoperative chemotherapy on earlier, operable breast cancer remain a matter of controversy. Although there is limited information available on the clinical practice worldwide, significant variations in practice are suspected. Potential advantages of the use of chemotherapy delivered before surgery include early introduction of systemic therapy, determination of the tumour's sensitivity to systemic therapy, and the potential to rapidly reduce both the tumour volume of the primary tumour and enlarged regional lymph nodes. Moreover, tumour response to preoperative chemotherapy may serve as a useful prognostic tool. Preoperative chemotherapy may permit more breast‐conserving treatment modalities, however this may introduce a problem in achieving adequate locoregional control as a result of the difficulty of assessing tumour margins after the administration of preoperative chemotherapy. A major disadvantage of preoperative chemotherapy is the potential delay for several weeks or months of appropriate local treatment for patients with tumours resistant to preoperative chemotherapy.
Breast‐conserving surgery consists of surgical removal of the tumour (with negative margins) followed by radiotherapy to eradicate any residual tumour cells. breast‐conserving therapy is associated with less morbidity and improved body image for the patient when compared to complete removal of the breast (Goodwin 2003; Kiebert 1991). Patients' choices for breast‐conserving therapy or mastectomy are based on the patient's perception of the surgeon's preference and concerns regarding breast loss and local tumour recurrence (Molenaar 2004). Long‐term results of six randomised controlled trials comparing breast‐conserving therapy and mastectomy revealed no significant difference in overall or disease‐free survival, these studies however found an increase of the loco regional recurrence rate among patients treated with breast‐conserving therapy (Arriagada 2003; Blichert‐Toft 1992; Fisher 2002; Poggi 2003; van Dongen 2000; Veronesi 2002). Established risk factors for developing loco regional recurrence after breast‐conserving therapy are positive resection margins, young age (less than 40 years), multicentric disease, and poor tumour differentiation (Fredriksson 2003). So, breast‐comnserving therapy is associated with higher local recurrence rates (rates after five and ten year follow‐up vary between 2 and 10% and between 5 and 15 %, respectively (Arriagada 2003; Elkhuizen 1998; Fisher 2002; van Dongen 2000; Veronesi 2002), yet without worsening long‐term survival. However, recently emerging data do suggest poorer long‐term prognosis after loco‐regional recurrence has occurred (Clarke 2005; Fredriksson 2002; van der Hage 2003; van Tienhoven 1999; Voogd 2005).
The aim of this review is to systematically identify and assess all of the available evidence from randomised trials as to the effectiveness of preoperative chemotherapy on treatment‐related outcomes in women with operable breast cancer.
Objectives
The major objective of this review is to assess the effectiveness of preoperative chemotherapy in women with operable breast cancer when compared to postoperative chemotherapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials.
Types of participants
Women with operable breast cancer: TNM stage T1c, T2, T3, N0 to 2, and M0 (AJCC stage I‐IIIA). We applied no restrictions to age or menopausal status.
TNM classification (Tumour, Node, Metastasis) is the global standard in cancer staging
Types of interventions
‐ Preoperative chemotherapy versus postoperative chemotherapy. ‐ Preoperative and postoperative chemotherapy versus postoperative chemotherapy.
See Table 1 for a list of chemotherapeutic agents.
1. Chemotherapeutic agents (adapted from Table 1.1 in The Chemotherapy Source Book).
| Type of Agent | Action | Includes |
| Agents that damage the DNA template | by alkylation: nitrogen mustards | cyclophosphamide |
| by alkylation: other agents | thiotepa, mitomycin C | |
| antibiotics | doxorubicin, mitoxantrone, epirubicin | |
| by platinum coordination cross‐linking | cisplatin, carboplatin | |
| Spindle poisons | vinca alkaloids | vincristine, vendesine |
| taxanes | paclitaxel | |
| Antimetabolites | thymidylate synthase | 5‐fluorouracil |
| dihydrofolate reductase | methotrexate |
Types of outcome measures
Primary outcomes: ‐ overall survival ‐ disease‐free survival ‐ loco‐regional recurrence as first event
Secondary outcomes: ‐ tumour response rate ‐ association of pathological complete response with clinical outcome ‐ type of loco‐regional treatment ‐ changes of originally planned loco‐regional treatment ‐ adverse effects ‐ quality of life
For the purpose of this review the outcomes were defined as follows. ‐ Overall survival, time from date of randomisation to date of death (any cause). ‐ Disease‐free survival, time from date of randomisation to disease relapse (including distant metastases, loco‐regional recurrences, secondary primary tumours, and contralateral breast cancers) or death, whichever came first. ‐ loco‐regional recurrence, relapse in ipsilateral breast or in ipsilateral regional lymph nodes. Time to loco‐regional recurrence was defined as time from date of randomisation to loco‐regional recurrence. Only loco‐regional recurrences as the first site of relapse were considered for analysis. ‐ Tumour response, clinical tumour response classification system according to the UICC; pathological complete response, complete disappearance of invasive carcinoma on histological examination. ‐ Type of loco‐regional treatment, we considered modified radical mastectomy and conservative treatment (breast‐conserving therapy or exclusive radiotherapy). ‐ Adverse effects, we considered World Health Organisation (WHO) grade III or IV events of serious infections, alopecia, cardiotoxicity, postoperative complications, and nausea and vomiting.
Search methods for identification of studies
The Specialised Register maintained by the Cochrane Breast Cancer Group was searched on 4th August 2005 (details of search strategies used by the group for the identification of studies and the procedure used to code references are outlined in the group's module http://www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html. The register includes both published and unpublished (including ongoing) trials and applies no language restrictions. We performed two searches, one to identify references which were coded in the specialised register as "early" and "chemo", and a second to identify those references that had been assigned the CBCG codes "locally advanced" and "chemo". In addition, we searched the reference lists of other related literature reviews (EORTC 2001, NSABP 1998, Wolff 2000).
Data collection and analysis
Trial selection The primary review author screened the abstracts of the identified references in an attempt to determine if the reference pertained to a randomised trial in women with operable breast cancer and that compared a preoperative chemotherapeutic regimen with an alternatively timed one. We obtained copies of full articles for references reporting a potentially eligible trial. For unpublished trials, we obtained information from the trial protocol or other available sources. Two review authors (SM and JH) independently applied the selection criteria on the methods sections of the selected trials and independently decided on eligibility. The review authors were blinded to all but the methods section. Any disagreements were resolved by consensus.
Quality assessment Two review authors (SM and JH) independently reviewed each included study according to its design and how the trial was conducted to assess the possibility of bias. Trial quality assessment was based on: ‐ concealment of the allocation sequence ‐ generation of the allocation sequence ‐ comparability between groups at the baseline ‐ inclusion of all randomised participants in the analysis (Intention to treat)
Allocation concealment is regarded as particularly important in protecting against bias and was graded according to the Cochrane approach: Grade A ‐ clearly adequate, Grade B ‐ possibly adequate, Grade C ‐ clearly inadequate, Grade D ‐ not used.
Data extraction At least two individuals independently extracted data from the studies identified for inclusion. Any disagreements were resolved by consensus. Data were entered and analyzed with the Cochrane Review Manager Software (RevMan 4.2). We sought missing or additional information from the authors when clarifications or extra data were needed.
Analyses Time‐to‐event data For the primary outcomes overall and disease‐free survival and time to loco‐regional recurrence the hazard ratio (HR) is the most appropriate statistic. Where possible, the HRs and associated variances were extracted directly from the trial publications. If not reported, we calculated the HR and associated statistics data (observed (O) minus expected (E) number of events and the variance) indirectly using the methods described by Parmar (Parmar 1998) and the Excel spreadsheet developed by the Matthew Sydes (Cancer Division) in collaboration with the Meta‐analysis Group of the MRC Clinical Trials Unit, London. This spreadsheet incorporates various methods combining available summary statistics (such as P‐value, number of patients analyzed and observed events in each arm) or data extracted from published Kaplan‐Meier curves to estimate the HR and associated statistics. When we had to extract data from Kaplan‐Meier curves, we inputted, where possible, the reported numbers at risk at various time‐points from the spreadsheet to adjust for censoring. However, if not reported, we adjusted the numbers at risk based on estimated minimum and maximum follow‐up times. If these were not reported in any of the reports available, minimum follow‐up was estimated using the time between the last date of accrual and the date of analysis (database lock); if date of analysis was not reported, it was estimated by subtracting six months from the date the paper was submitted for publication. Maximum follow‐up was estimated using the time between the first date of accrual and the date of analysis, as described above. Estimated follow‐up periods are recorded in the Characteristics of Included Studies table under "Notes". For each study the different estimates calculated using the spreadsheet appear in a table in order of accuracy based on which data was reported and subsequently which method was used. We used the most accurate estimates in the analyses.
If studies reported only the number of events for time‐to‐event outcomes we calculated the relative risk and accompanying P‐value and used the latter to calculate O minus E and the variance as described by Parmar.
All time‐to‐event analyses were by intention to treat. If trials did not report time‐to‐event outcomes as intention‐to‐treat then this is documented in the Characteristics of included studies table under "Notes". We did not perform statistical correction for missing data.
We obtained a pooled HR from the derived O minus E and the variance for each trial using the fixed effect model (Yusuf 1985). The pooled HR describes how many times more (or less) likely a patient was to suffer the event if they receive preoperative chemotherapy rather than postoperative chemotherapy. Ratios of treatment effects for time‐to‐event outcomes were reported so that HRs less than 1.0 favoured preoperative chemotherapy and values greater than 1.0 favoured postoperative chemotherapy. The plots are HR‐plots, although they are labelled as Peto odds ratio plots in the default mode of Meta‐view.
Tumour response to preoperative chemotherapy In general, direct tumour response assessment was only possible in patients receiving preoperative chemotherapy as the breast lumps in patients assigned to postoperative chemotherapy were already surgically removed or completely regressed after radiotherapy. Therefore, no comparisons between the two arms were possible. Instead, for every study we calculated the response rate and the associated 95% confidence interval. We summed the numerators and summed the denominators and we calculated the ratio of these. The calculated rates are based on assessable patients in the treatment arm who received preoperative chemotherapy. We have analyzed clinical complete (cCR), overall clinical (OR), and pathological complete response (pCR).
For clinical response, we have applied the classification system according to the UICC where possible (Hayward 1977). We have noted the methods (clinical examination, mammography) used by the investigators in the assessment of tumour response. A cCR was defined as complete disappearance of all clinically detectable malignant disease at the time of surgery. An OR was defined as ≥ 50% decrease in total tumour size after chemotherapy compared to the pre‐treatment size and a pCR was defined as the complete disappearance of invasive carcinoma on histological examination. We have stated differences in definition across the studies.
Association of pathological complete response with clinical outcome We analyzed the association of pCR with overall and disease‐free survival using the same techniques as described under Time‐to‐event data. We used data on assessable patients in the treatment arm who received preoperative chemotherapy and compared patients with pCR with patients who had residual disease at pathological examination (pRES).
loco‐regional treatment We analyzed the influence of preoperative chemotherapy on loco‐regional treatment. In doing so we used data from studies in which the treatment protocol allowed us to derive differences in breast conservation rates between the research and control arm. We excluded studies in which both arms had fixed loco‐regional treatment options. We used the reported numbers of radical (modified radical mastectomy (MAST)) and conservative (breast‐conserving therapy (BCT) or exclusive radiotherapy (RT)) treatment to calculate relative risks (RR) for individual trials; radical treatment was scored as an event. We excluded from the analysis patients with no information available on loco‐regional treatment.
We obtained a pooled RR across the studies using the fixed effect model (Mantel‐Haenszel). The pooled RR described how many times more (or less) likely a patient was to suffer radical surgery if they receive preoperative chemotherapy rather than postoperative chemotherapy. RR less than 1.0 favoured preoperative chemotherapy and values greater than 1.0 favoured postoperative chemotherapy.
Over time, breast conservation rates will decrease with the development of loco‐regional recurrences requiring subsequent salvage mastectomies. Where possible, we used the breast conservation rates after subsequent follow‐up for calculation of the RR. We converted the pooled RR to risk difference (RD) and to numbers‐needed‐to‐treat or needed‐to‐harm (NNT or NNH).
Changes of originally planned loco‐regional treatment We used studies reporting both the originally planned surgical requirements before randomisation and the actually performed types of loco‐regional therapy in the preoperative chemotherapy arm to analyse the changes in loco‐regional treatment requirements. We recognized six groups: no change of type of surgery (BCT → BCT and MAST → MAST), downgrading of surgical requirement (MAST → BCT; MAST → RT; BCT → RT), and conversion of conservative to radical surgery (BCT → MAST). For each trial, we stated the numbers in each group. We used the initial surgery data (not those after follow‐up; see above). We pooled the groups over studies by simple addition.
In addition, we analysed the association of down staged surgical requirement with overall survival and loco‐regional recurrence. We compared patients with down staged BCT (MAST → BCT) with patients with planned BCT (BCT → BCT) in the preoperative chemotherapy arm and we used hazard ratio's (designated as OS) or risk ratio's (LRR) and their 95% CI and we performed meta‐analyses as described above. Adverse effects We extracted the number of WHO grades III and IV events of postoperative complications, cardiotoxicity, leukopenia or neutropenia or infection, nausea and vomiting, and alopecia. We obtained a pooled RR for each subcategory using the fixed effect model (Mantel‐Haenszel).
Quality of life Data on quality of life were not reported in any of the trials.
Heterogeneity We examined heterogeneity across studies qualitatively by inspecting the distribution of point estimates for the effect measure and the overlap in their confidence intervals on the forest plot. We used the I2 statistic test to check for heterogeneity in a quantitative way (Higgins 2002). We considered a value greater than 50% as substantial heterogeneity. We explored sources of heterogeneity by subgroup and sensitivity analyses.
The initially planned subgroup analyses were not undertaken due to lack of data for these subgroups. Instead, we conducted an additional set of post‐hoc subgroup analyses which were planned after identification of the eligible trials but prior to extraction of the data. The subgroup analyses involve treatment arm (preoperative, 'sandwich'), chemotherapeutical regimen (anthracycline‐based, taxane containing), and loco‐regional treatment (breast‐conserving surgery, mastectomy, exclusive radiotherapy). To test the statistical difference of effect estimates within a subgroup analysis, we used a method described by Deeks and colleagues (Deeks 2001) and we applied a significance level of 0.05.
We performed one‐way sensitivity analyses upon heterogeneous results to explore the influence of differences in study quality based on randomisation concealment (adequate versus not adequate or unclear).
We investigated the influence of excluding outlying trials when an obvious clinical reason for the outlying result was apparent, as heterogeneity can be due to such outlying trials.
Publication bias We tested publication bias by using funnel plots; an inverted symmetrical funnel plot assumes the absence of publication bias (Egger 1997). The default graph in RevMan 4.2 uses 1/SE on the vertical axis plotted against the effect size for the particular outcome. We retrieved funnel plots from RevMan 4.2 for the following outcomes: overall survival, disease‐free survival, time to loco‐regional recurrence, and loco‐regional treatment. We did not use any statistical test for funnel plot asymmetry, but eye‐balled the plot.
Results
Description of studies
Result of search On the 4th of August 2005, the CBCG Specialised Register contained 5,749 references of which 753 were identified during our search. After detailed evaluation, we considered 19 studies for inclusion, five of which we excluded. Two studies were deemed ineligible, one study had all results stratified on an apoptotic index and we unsuccessfully attempted to contact the authors, one reported no data, and one reference reported on a subset of patients of the NSABP B‐18 trial (see Characteristics of excluded studies).
Fourteen trials met our inclusion criteria, randomizing 5,500 women: 2,752 to receive preoperative chemotherapy and 2,748 to receive chemotherapy exclusively after loco‐regional treatment. Four studies of the fourteen included were published as conference abstracts only (ABCSG 2001; ECTO 2005; Japan 1998; Lithuania 1998). Five studies reported extended follow‐up results (Bordeaux 1991; EORTC 2001; Institut Curie 1994; NSABP 1998; Royal Marsden 1998).
We attempted to contact investigators involved in the following trials for more information: ABCSG 2001, Bordeaux 1991; ECTO 2005, EORTC 2001, Institut Curie 1994; Japan 1998; Lithuania 1998; London 2001; NSABP 1998; Royal Marsden 1998; USA 2003 . In response they kindly supplied additional, unpublished data relating to the following trials: EORTC 2001, Japan 1998; London 2001, Royal Marsden 1998.
The trials varied considerably in size. Being the largest, NSABP 1998 was the largest with 1,523 participants randomised while the other studies varied in sample size from 50 to 902 participants (Japan 1998 and ECTO 2005, respectively). The three international trials (ECTO 2005; EORTC 2001; NSABP 1998) recruited 3,123 of all randomised patients (56,8%). Seven trials were carried out in a single centre (Bordeaux 1991; Edinburgh 1995; Institut Curie 1991; Institut Curie 1994; London 2001; Royal Marsden 1998; St. Petersburg 1994) and it was unclear whether one trial (Lithuania 1998) had more than one trial centre. The other three national trials involved multiple centres (ABCSG 2001; Japan 1998; USA 2003). Three trials were held in France, two in the UK, two in Europe, one in the USA and Canada, and one in each of the USA, Austria, Japan, Scotland, Russia and Lithuania. All patients were accrued in the period between November 1983 and May 2002.
For details of the selection process and a summary of the eligible trials that were included in the analyses contributing to the review questions, see Figure 1 for the study flow chart.
1.
Participants All studies included relatively healthy women with cytologically or histologically confirmed operable breast cancer and no metastatic disease. Inclusion criteria varied according to the primary objectives of individual trials. Generally, little information was reported on the hormone receptor status and histological grade of the tumours. See the Characteristics of included studies table for more details.
Interventions Figure 2 (Additional figures) gives an overview of the treatment protocols for the included studies split according to the chemotherapy strategy in the treatment arm: solely preoperative versus 'sandwich' (preoperativve and postoperative). See the Characteristics of included studies table for more details.
2.

Table 01.
Chemotherapy and endocrine therapy All included trials compared preoperative chemotherapy with a postoperative regimen. In six trials patients in the preoperative arm received all cycles prior to loco‐regional treatment. In the remaining eight trials, patients in the preoperative arm received some of the cycles after loco‐regional treatment.
A variety of chemotherapeutic regimens were administered to patients across the included trials; all regimens were made up of multiple chemotherapeutic agents. See Additional Figure 2 for the working mechanisms of the different chemotherapeutic agents. Most studies incorporated an anthracycline (doxorubicin, epirubicin, mitomycin C, or mitoxantrone) in their chemotherapy regimen. Three regimens did not contain an anthracycline: all patients in Lithuania 1998 and St. Petersburg 1994 received CMF and TMF respectively, and patients without axillary lymph node involvement in ABCSG 2001 received CMF and no anthracycline. One study randomised patients to taxane containing regimens (ECTO 2005).
Endocrine treatment was administered instead of chemotherapy to patients with tumours expressing high estrogen receptor levels in two studies (Edinburgh 1995, London 2001). Non‐responders to endocrine treatment in the preoperative arm of London 2001 crossed over to an anthracycline containing chemotherapeutic regimen after loco‐regional treatment. Tamoxifen was administered to eligible patients in seven studies (ECTO 2005, EORTC 2001, Japan 1998; Lithuania 1998; NSABP 1998, Royal Marsden 1998; USA 2003) and was mostly started after loco‐regional treatment; in one study patients in the preoperative arm started tamoxifen treatment along with chemotherapy and thus before surgery (Royal Marsden 1998).
loco‐regional treatment All trials were designed to achieve adequate local control of the tumour, however a variety of protocols were used. Five studies applied the same local treatment to all included patients (Edinburgh 1995, Japan 1998; Lithuania 1998, St. Petersburg 1994). While the other studies could vary the treatment amongst participants according to their individual requirements (for example, tumour size, nodal involvement). Three studies administrated radiotherapy before surgery (Institut Curie 1991; Institut Curie 1994, St. Petersburg 1994). Three studies treated some of the participants exclusively with radiotherapy (Bordeaux 1991; Institut Curie 1991; Institut Curie 1994).
Outcomes The outcomes measured by individual trials differed according to the trial objectives and not all the included trials provided information on all outcomes. Figure 3 (Additional figures) summaries the data available for each outcome for each trial. Any deviations from the definitions as defined for this review are noted in the Characteristics of included studies table.
3.

Table 02.
We excluded four studies from loco‐regional treatment analyses because of fixed surgical procedures in both arms: Edinburgh 1995, Japan 1998; Lithuania 1998, St. Petersburg 1994.
Only one study investigated quality of life, although the authors did not report any results due to an insufficient number of collected data (EORTC 2001).
Risk of bias in included studies
See the Characteristics of included studies table for methodological details about all included studies and Figure 4 (Additional figures) for an overview of study quality.
4.

Table 03.
Randomisation and allocation concealment Of the fourteen included studies, eight described a satisfactory method of randomisation. Four of these trials scored A for allocation concealment: in these trials allocation to treatment was either generated by computer once information about an eligible participant had been entered, or was accomplished by remote contact between the recruiting centre and the study co‐coordinating centre. In addition, two studies described randomisation by a central office (grade A), however no details of the allocation method were noted (Edinburgh 1995; USA 2003). Four studies gave no detailed information on either randomisation or allocation concealment (ABCSG 2001, Institut Curie 1991; Institut Curie 1994; Lithuania 1998). Two of the four studies lacking satisfactory description of the randomisation method and of allocation concealment showed no significant imbalances in baseline characteristics, therefore, the determined grade of allocation concealment was B (Institut Curie 1994, Institut Curie 1991). The remaining two studies reported no information on baseline characteristics and were graded D (ABCSG 2001, Lithuania 1998). In addition, Japan 1998 excluded a substantial number of patients after the randomisation and was therefore graded D.
Intention to treat, losses to follow‐up Definitions For the purpose of this review, intention to treat was defined as the analysis of all randomised participants in the groups to which they were randomised. Losses to follow‐up were defined as participants for whom the outcomes of interest were unknown (and who may or may not have had outcomes imputed in the statistical analysis).
Intention to treat Eleven studies reported time‐to‐event outcomes of which seven analysed all participants by intention to treat for those outcomes (Bordeaux 1991; ECTO 2005, EORTC 2001, Institut Curie 1991;London 2001; St. Petersburg 1994; USA 2003) and one study analysed over 98% of participants by intention to treat (NSABP 1998). The remaining three included in between 90.0% and 92.6% of randomised patients in the analyses (Institut Curie 1994; Japan 1998, Royal Marsden 1998). Overall, 98.2% of the patients included in time‐to‐event outcomes were analysed by intention to treat.
Losses to follow‐up for time‐to‐event outcomes Losses to follow‐up for time‐to‐event outcomes were low in most of the studies, with no women lost to follow‐up in six studies (Institut Curie 1994; Japan 1998, London 2001, Royal Marsden 1998; St. Petersburg 1994; USA 2003) and 0.5% to 1.0% lost in four other studies (Bordeaux 1991;EORTC 2001;Institut Curie 1991; NSABP 1998). In one study we could not retrieve if patients were lost to follow‐up (ECTO 2005).
Number of patients assessable for other outcomes For complete clinical response, data on 2114 of the 2448 women randomised were available (86%). For overall response, data on 2032 of the 2261 women randomised were available (90%). For pathological response, data on 1972 of the 2087 women randomised were available (94%). For loco‐regional treatment, data on 5292 of the 5453 women randomised were available (97%). For adverse effects, data on 3382 of the 3490 women randomised were available (97%).
Effects of interventions
Fourteen eligible studies randomised a total of 5,500 women. Median follow up ranged from 18 to 124 months. A Summary of Findings table presents the main findings of this review. This can be found in Additional Figures (Figure 5).
5.

Table 04.
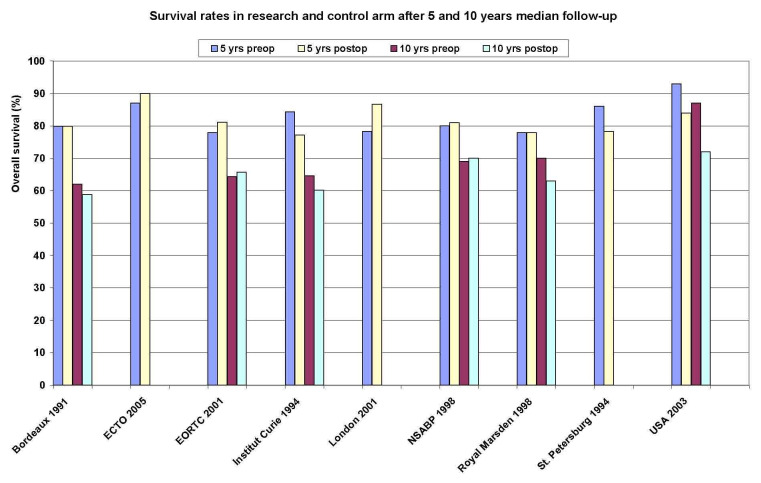
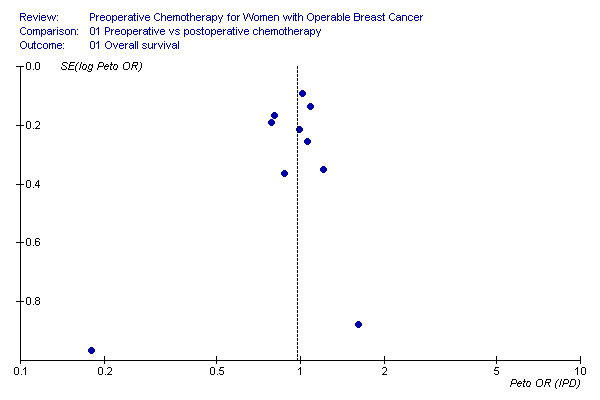
Overall survival Ten studies reported overall survival data on 4620 randomised women involving 1139 estimated deaths. Additional Figure 6 shows the survival rates of the research and control arm for each study after 5 and 10 years median follow‐up. There was no detectable difference between preoperative and postoperative chemotherapy with a HR of 0.98 (95% CI, 0.87 to 1.09; P, 0.67) and without heterogeneity across studies (I2, 0%; P, 0.61) (Figure 01.01). The associated funnel plot shows a asymmetrical distribution (Figure 7): one study with a small sample size showed a greater treatment effect (USA 2003).
6.
7.
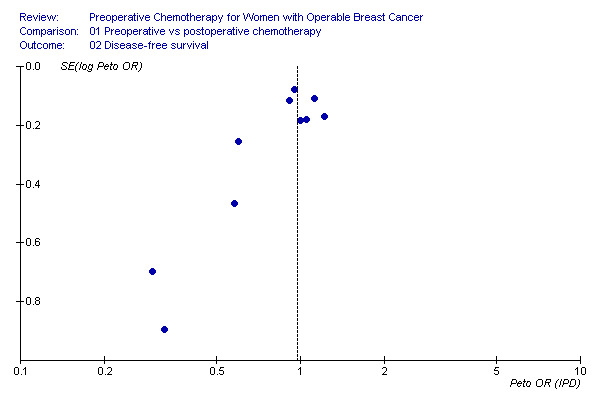
Disease‐free survival Ten studies reported disease‐free survival data on 4510 randomised women involving 1596 estimated events. There was no detectable difference between preoperative and postoperative chemotherapy with a HR of 0.97 (95% CI, 0.89 to 1.07; P, 0.58) and with moderate heterogeneity across studies (I2, 32.5%; P, 0.15) (Figure 01.02). The associated funnel plot shows an asymmetrical distribution: smaller trials show greater treatment effects (Additional Figure 8).
8.
Time to loco‐regional recurrence Eleven studies reported time to loco‐regional recurrence data on 5041 randomised women involving 558 estimated recurrences. Four studies reported loco‐regional recurrence as time‐to‐event data (EORTC 2001, Institut Curie 1994; London 2001, Royal Marsden 1998). There was a statistically significant difference in favour of postoperative chemotherapy with a HR of 1.21 (95% CI, 1.02 to 1.43; P, 0.03) and without heterogeneity across studies (I2, 7.0%; P, 0.38) (Figure 01.03). The associated funnel plot shows a symmetrical distribution (Additional Figure 9).
9.
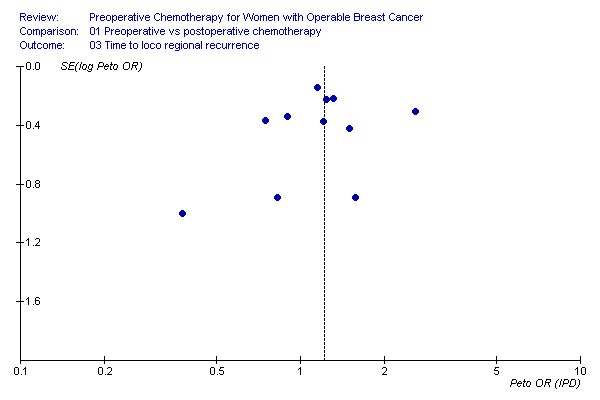
In three studies, the loco‐regional treatment for a substantial number of patients consisted of exclusive radiotherapy and no surgery. In Bordeaux 1991, 44 (33%) women received exclusive radiotherapy after preoperative chemotherapy and none in the control arm. In Institut Curie 1991, 41 (43%) women received exclusive radiotherapy after preoperative chemotherapy compared with 30 (35%) women in the control arm. In Institut Curie 1994, 102 (51%) women received exclusive radiotherapy after preoperative chemotherapy compared with 87 (46%) women in the control arm. Although these studies did not separately report loco‐recurrence rates for these patients, except for Bordeaux 1991 (13/44=29.5%), but they did show an increased overall loco‐regional recurrence rate compared to the remaining eight studies: 163/843 (19.3%) and 407/4198 (9,7%), respectively. If we excluded the three studies from the analysis, the remaining eight studies demonstrated a non‐significant difference in favour of the control arm with a HR of 1.12 (95% CI, 0.92 to 1.37; P, 0.25) and without heterogeneity (I2, 0%; P, 0.86), representing a risk difference of 2.6% (95% CI, 1.3 to 3.9; control group risk, 8.6%; NNH, 39) (Figure 08.01). This difference was non‐significantly lower compared to the three excluded trials (HR, 1.45; 95% CI, 1.06 to 1.97; P, 0.02; Chi2 for difference, 1.66; P, 0.20).
We performed a within‐study subgroup analysis with loco‐regional treatment and identified three categories: breast‐conserving surgery, mastectomy, and exclusive radiotherapy. Four studies reported recurrence rate after BCT involving 1830 women and 143 recurrences (RR, 1.13; 95% CI, 0.82 to 1.54). Four studies reported recurrence rate after mastectomy involving 1427 women and 82 recurrences (RR, 1.14; 95% CI, 0.74 to 1.75). There were no data available to compare the recurrence rate after exclusive radiotherapy between the preoperative and postoperative chemotherapy arm. In total, data for 3257 women were available involving 225 recurrences to demonstrate a non‐significant difference in favour of the control arm (HR, 1.13; 95% CI, 0.88 to 1.46; P, 0.35; risk difference, 2.3; 95% CI, 0.9 to 3.6; control group risk, 5.9%). There was no difference in loco‐regional recurrence detectable between women treated with BCT and those treated with mastectomy (Chi2 for difference, 0.01; p, 0.92) (Figure 04.01).
Tumour response to preoperative chemotherapy Eleven studies reported complete clinical response (cCR) rate in the preoperative chemotherapy arm for 1761 assessable women involving 653 cCR's. The cCR rate ranged from 0 to 64.7%. Twelve studies reported overall clinical response (OR) rate in the preoperative chemotherapy arm for 2032 assessable women involving 1384 OR's. The OR rate ranged from 11.1 to 83.3%. Seven studies reported pathological complete response (pCR) rate in the preoperative chemotherapy arm for 1972 assessable women involving 278 pCR's. The pCR rate ranged from 4.0 to 29.2%. See Table 2 and Table 3 for more details.
2. Complete (cCR) and overall (OR) clinical response.
| Study | Total number | Number of cCR | % | 95% CI | Number of OR | % | 95% CI |
| ABCSG 2001 | 214 | ‐ | ‐ | ‐ | 147 | 68.7 | 62.5‐74.9 |
| Bordeaux 1991 | 134 | 44 | 32.8 | 24.9‐40.8 | 85 | 63.4 | 55.3‐71.6 |
| ECTO 2005 | 346 | 173 | 50.0 | 44.7‐55.3 | ‐ | ‐ | ‐ |
| EORTC 2001 | 315 | 23 | 7.3 | 4.4‐10.2 | 171 | 54.3 | 48.8‐59.8 |
| Institut Curie 1991 | 76 | 10 | 13.2 | 5.6‐20.8 | 34 | 44.7 | 33.6‐55.9 |
| Institut Curie 1994 | 191 | 46 | 24.1 | 18.0‐30.1 | 126 | 66.0 | 59.2‐72.7 |
| Japan 1998 | 18 | 0 | 0 | 0 | 2 | 11.1 | ‐3.4‐25.6 |
| Lithuania 1998 | 50 | ‐ | ‐ | ‐ | 13 | 26.0 | 13.8‐38.2 |
| London 2001 | 53 | 18 | 34.0 | 21.2‐46.7 | 32 | 60.4 | 47.2‐73.5 |
| NSABP 1998 | 683 | 248 | 36.3 | 32.7‐39.9 | 543 | 79.5 | 76.5‐82.5 |
| Royal Marsden 1998 | 144 | 32 | 22.2 | 15.4‐29.0 | 120 | 83.3 | 77.2‐89.4 |
| St. Petersburg 1994 (+ primary RT) | 137 | 48 | 35.0 | 27.0‐43.0 | 98 | 71.5 | 64.0‐79.1 |
| USA 2003 | 17 | 11 | 64.7 | 42.0‐87.4 | 13 | 76.5 | 56.3‐96.6 |
| Total | 2114 / 2032 | 653 | 30.9 | 28.9‐32.6 | 1384 | 68.1 | 66.1‐70.1 |
3. Complete pathological response (pCR).
| Study | number of pCR | total number | % | 95% CI |
| ABCSG 2001 | 13 | 214 | 6.1 | 2.9‐9.3 |
| ECTO 2005 | 102 | 451 | 22.6 | 18.8‐26.5 |
| EORTC 2001 | 13 | 329 | 4.0 | 1.9‐6.1 |
| NSABP 1998 | 88 | 682 | 12.9 | 10.4‐15.4 |
| Royal Marsden 1998 | 20 | 149 | 13.4 | 8.0‐18.9 |
| St. Petersburg 1994 (+ primary RT) | 40 | 137 | 29.2 | 21.6‐36.8 |
| USA 2003 | 2 | 10 | 20.0 | ‐4.8‐44.8 |
| Total | 278 | 1972 | 14.1 | 12.6‐15.6 |
Association of pCR with clinical outcome We compared overall and disease‐free survival between women with pCR and women who had residual disease at pathological examination.
Four studies reported overall survival data for 1290 assessable women involving 381 estimated deaths. There was a statistically significant difference in favour of pCR with a HR of 0.48 (95% CI, 0.33 to 0.69; P, < 10‐4), representing a risk difference of 20.1% (95% CI, 15.7 to 24.7; control group risk, 32.0%) and without heterogeneity across studies (I2, 0%; P, 0.88) (Figure 02.01).
Five studies reported disease‐free survival data for 1741 assessable women involving 606 estimated events. There was a statistically significant difference in favour of pCR with a HR of 0.48 (95% CI, 0.37 to 0.63; P, <10‐5), representing a risk difference of 23.5% (95% CI, 20.0 to 27.3; control group risk, 38.3%) and without heterogeneity across studies (I2, 0%; P, 0.41) (Figure 02.02).
loco‐regional treatment Ten studies reported the type of loco‐regional treatment for 5292 randomised women of which 2395 underwent radical surgery (mastectomy). Three studies reported conservative treatment rates after subsequent follow‐up (Bordeaux 1991, Institut Curie 1994; Royal Marsden 1998). There was a statistically significant difference of mastectomy rate in favour of preoperative chemotherapy with a RR of 0.71 (95% CI, 0.67 to 0.75; P, <10‐5), representing a risk difference of 16.6% (95% CI, 15.1to 18.1; control group risk, 52.9%; NNT, 6) and with substantial heterogeneity across studies (I2, 83.2%; P, <10‐5) (Figure 01.04). The associated funnel plot showed a symmetrical distribution (Figure 10).
10.
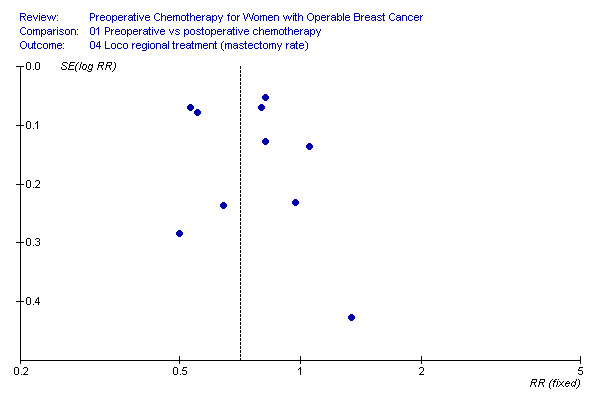
We investigated the substantial heterogeneity across studies by subgroup and sensitivity analyses. The between‐study subgroups, treatment arm and type of chemotherapy used, could not explain the heterogeneity (Figures 05.01 and 06.01); in the last subgroup three of the four categories contained only one study. We found a significant difference between the categories adequate versus not adequate or unclear methodological quality (Chi2 for difference, 21.74; P, <10‐4), however substantial heterogeneity was found in the not adequate/ unclear category (Figure 07.01). We could best explain the heterogeneity by excluding two studies for clinical reasons (Chi2 for difference, 44.07; P, <10‐5) (Figure 08.02). One study involved an intensive chemotherapy regimen including taxane and anthracycline drugs and reached a high pCR rate, allowing more conservative treatment (ECTO 2005). The second excluded study treated all patients in the control arm with mastectomy since one of the inclusion criteria was patients with tumours not suitable for conservative treatment (Bordeaux 1991). The remaining eight studies involving 1452 mastectomies in 3709 women demonstrated a statistically significant difference in favour of preoperative chemotherapy with a RR of 0.82 (95% CI, 0.76‐0.89; P, <10‐5), representing a risk difference of 8.0% (95% CI, 6.3‐9.7; control group risk, 43.1%; NNT, 13) and with moderate heterogeneity across studies (I2, 25.8%; P, 0.22) (Figure 08.02).
Change of loco‐regional treatment originally planned Five studies reported changes of loco‐regional treatment that had been originally planned in the preoperative chemotherapy arm on 1549 assessable women. Additional Table 4 lists the changes. Across studies, 397 women had their originally planned surgical treatment down staged (25.6%; 95% CI, 23.5 to 27.8), 1086 women had no change (70.1%; 95% CI, 67.8 to 72.4), and 66 women required more radical surgery than originally planned (4.3%; 95% CI, 3.3 to 5.3).
4. Changes of originally planned loco regional treatment.
| Study | BCT ‐ BCT | MAST ‐ MAST | MAST ‐ BCT | MAST ‐ RT | BCT ‐RT | BCT ‐ MAST | Total |
| Bordeaux 1991 | ‐ | 49 | 40 | 44 | ‐ | ‐ | 133 |
| EORTC 2001 | 60 | 190 | 60 | ‐ | ‐ | 14 | 324 |
| Institut Curie 1994 | ‐ | 36 | 62 | 102 | ‐ | ‐ | 200 |
| NSABP 1998 | 435 | 187 | 69 | ‐ | ‐ | 52 | 743 |
| Royal Marsen 1998 | 113 | 16 | 19 | ‐ | 1 | ‐ | 149 |
| Total | 608 | 478 | 250 | 146 | 1 | 66 | 1549 |
One study reported the association of down staged BCT compared to planned BCT in the preoperative chemotherapy arm with overall survival involving 33 deaths and 120 assessable women. There was no statistical significant difference between down staged BCT and planned BCT with a HR of 1.33 (95% CI, 0.67 to 2.63; P, 0.41; I2, not applicable) (Figure 03.01).
Two studies reported the association of down staged BCT compared to planned BCT in the preoperative chemotherapy arm with loco‐regional recurrence involving 79 local recurrences and 623 assessable women. There was a non‐significant difference in favour of planned BCT with a RR of 1.34 (95% CI, 0.85 to 2.13; P, 0.21; I2, 0; P, 0.40), representing a risk difference of 7.5% (95% CI, 1.7 to 13.2; risk control group (planned BCT in treatment arm), 11.1%; NNH, 14) (Figure 03.02).
Adverse effects A total of seven studies reported adverse effects (Figure 01.05). There was no significant difference between preoperative and postoperative chemotherapy detectable for postoperative complications, nausea/ vomiting, and alopecia. Events of cardiotoxicity were less frequently in women receiving preoperative chemotherapy (RR 0.74; 95% CI, 0.53‐1.04; p, 0.08; heterogeneity I2, 0%; P, 0.48). The four studies reporting on leucopenia/ neutropenia/ infections involving 2799 women and 327 events demonstrated a significant difference in favour of preoperative chemotherapy with a relative risk of 0.69 (95% CI, 0.56 to 0.84; P, 0.0003; heterogeneity I2, 1.1%; p, 0.39) representing a risk difference of 4.2% (95% CI, 2.3 to 5.6; control group risk, 13.8%; NNT, 24)
Discussion
In this review, we included fourteen trials randomizing 5,500 women to assess the effectiveness of preoperative chemotherapy for operable breast cancer. Study quality was generally adequate. The treatment protocols varied considerably among studies. Publication bias may be possible.
In this meta‐analyses, we demonstrated comparable overall and disease‐free survival rates for preoperative and post‐operative chemotherapy, although we found a higher loco‐regional recurrence rate for patients receiving preoperative chemotherapy. However, this increase in loco‐regional recurrence rate was greatly reduced when we excluded three studies in which a substantial proportion of the study population received exclusive radiotherapy and surgery was withheld. This finding emphasizes the importance of incorporating surgery in the loco‐regional treatment regimen after the administration of preoperative chemotherapy even if the preoperative systemic treatment has lead to complete disappearance of the tumour. (It is known that radiotherapy reduces the risk of loco‐regional recurrence even after mastectomy,(Whelan 2000) however, because of limited reporting and the large variation in the radiotherapy protocols of the included studies, its role could not be analysed to a satisfactionary extent in this review.)
In this analysis, we demonstrated a substantial variation of the reported tumour response rates to preoperative chemotherapy. Different factors could have influenced the reported rates: definition of response, blinding of assessor, method and type of assessment, study population, and type of chemotherapy used. The most appropriate method of clinical tumour response assessment remains a matter of debate. Recently, new guidelines (RECIST) for solid tumours were published (Therasse 2000). Even so, clinical tumour response can be either under or overestimated due to fibrosis, weakening of the tumour margins or resolution of oedema, which suggests prognostic superiority of pathologically evaluated tumour response (Abraham 1996; Segel 1988; Veronesi 1995; Vinnicombe 1996). In addition, magnetic resonance imaging has been advocated to substitute mammography in assessing response (Pavic 2004). The superior response rate of the ECTO trial is partly explained by the incorporation of taxanes in the regimen; currently considered as very powerfull drugs in producing high response rate (Bear 2006; Felici 2005).
Notwithstanding the difficulties in achieving accuracy or reported response rates, tumour response assessment after a couple of cycles of preoperative chemotherapy offers the opportunity to modify the chemotherapeutical scheme when an insufficient response or even progression of the disease is observed. By adjusting the dose or switching to another cytotoxic agent the patient is saved the unneeded burden of the ineffective treatment and offered another appropriate systemic treatment which can be monitored in the same way as the former.
Another major topic of debate concerning preoperative chemotherapy is translational research. In vivo tumour response assessment is a useful tool in determining the predictive role of classical and molecular tumour characteristics (Fisher 1995). Furthermore, the introduction of DNA micro‐arrays and proteomics may also facilitate future tailored treatment strategies based upon custom made risk profiles rather than the classic guidelines derived from traditional randomised controlled trials (Ayers 2004; Chang 2003; Hannemann 2005; Wang 2005). Recently conducted trials investigating preoperative systemic treatments have used tumour response as a surrogate marker for prognosis of survival (Buzdar 2005; Chua 2005; Evans 2005; Smith 2005). In particular, pCR has become an important endpoint in the research of new chemotherapeutic regimens, however limited data from individual trials are available on the assumed association of pCR and overall survival. In our meta‐analyses, we demonstrated that pCR is associated with superior overall and disease‐free survival. However, the trials were not primarily designed to investigate this association, so the current findings are derived from sub‐group analyses which could introduce various forms of bias that limit the interpretation of these results.
In this analysis, we demonstrated that preoperative chemotherapy significantly reduces the number of patients undergoing radical surgery. Significant heterogeneity exists among studies which could best be explained and corrected by excluding two outlying studies. The remaining eight studies showed an increase of breast conservation rate of 8.0%. This reduction in radical surgery may be overestimated by detection bias effect: the unblinded surgeon may assess and advise the patient not quite so objective and may push more towards breast‐conserving therapy in order to increase the treatment effect and subsequently the impact of the study. Moreover, as time passes and loco‐regional recurrences occur, the subsequent salvage mastectomies will decrease the breast conservation rate. Breast conservation rates over time were poorly reported in the included studies.
Thus, we showed that higher breast conservation rates are possible after preoperative chemotherapy with limited increase in the loco‐regional recurrence rate and no increase in overall and disease‐free survival. However as discussed in the background, the follow‐up period of these early stage breast cancer trials may yet be too limited to identify differences in survival.
It has been argued that downstaging of surgical requirements after preoperative chemotherapy may introduce a higher local recurrence risk when compared to preplanned breast‐conserving therapy. We have performed subgroup analyses to explore this assumption. Based on limited data, we found a non‐significant risk increase of 7.5%. However, no adjustments were made to exclude confounding effects, which are not unlikely to have occurred since patients with down‐staged BCT in EORTC 2001 were significantly younger, hampering the interpretation of this finding. In this review, preoperative chemotherapy resulted in equivalent or even decreased rates of adverse effects. Of particular interest is the beneficial effect on serious infections: a risk reduction with preoperative chemotherapy of 4.2%. One of the proposed disadvantages of preoperative chemotherapy is alteration of the lymphatic network of the breast, hampering the accuracy of sentinel lymph node biopsy after chemotoxic treatment (Sharkey 1996). However, data from a recently published meta‐analysis that included 21 studies and 1273 women, suggest that the accuracy of sentinel lymph node biopsy after preoperative chemotherapy is as reliable as when sentinel lymph node biopsy is performed in women naïve to systemic therapy (Xing 2006). Thus, the apparent safety of this procedure after chemotherapy and the down staging effect of chemotherapy on lymph node metastases could potentially lead to decreased numbers of patients undergoing lymph node dissection and reduce the associated morbidity of that treatment modality. Whether or how this affects prognosis, particularly in clinically positive lymph node disease, remains unclear.
Authors' conclusions
Implications for practice.
This study demonstrated that preoperative chemotherapy results in an equivalent disease outcome compared to postoperative chemotherapy in terms of overall and disease‐free survival and permits more breast‐conserving therapies, yet at the associated cost of increased loco‐regional recurrence rates. However, our results suggest limited increase of this risk (approximately 2%) as long as surgery remains part of the treatment even after complete tumour regression. Moreover, this review showed decreased number of adverse effects associated with preoperative chemotherapy.
The available evidence summarised in this review suggest safe application of preoperative chemotherapy in the treatment of women with early stage breast cancer in order to achieve downstaging of surgical requirement, to evaluate chemosensitivity and to facilitate translational research. However, the prognostic significance of a loco‐regional recurrence after breast‐conserving treatment remains controversial, therefore the potential increase in risk of loco recurrence should be considered, discussed with the patient and outweighed against the burden of more radical surgery.
Implications for research.
The latest Early Breast Cancer Trials Collaboroative Group (EBCTCG) report shows the importance of an extended follow‐up (15 to 20 years) for the assessment of effectiveness of treatments for early stage breast cancer. This review also highlights the importance of this, especially in relation to loco‐regional recurrence. Thus, results after extended follow‐up of the included studies should be reported and incorporated in an updated version of the current review. Moreover, the included studies should monitor and report on salvage mastectomy rates over time.
Risk factors of loco‐regional recurrence after breast‐conserving therapy such as positive surgical margins, age younger than 40 years, high histological grade, and multicentricity should be applied in subgroup and subsequent multivariate analyses in order to determine the effect of preoperative chemotherapy in these subgroups.
Direct evidence concerning long‐term prognosis and risk of local recurrence after downstaging of surgical treatment following preoperative chemotherapy is still lacking. Indirectly derived data suggest no intrinsic risk amplification associated with downstaged breast‐conserving surgery. However, evidence from direct comparison is needed to draw valid conclusions.
What's new
| Date | Event | Description |
|---|---|---|
| 10 December 2018 | Review declared as stable | As an individual participant data meta‐analysis has been performed by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) on the same topic, we do not intend to duplicate the efforts of the EBCTCG and direct readers to their analysis on the topic (see: Lancet Oncology, 2018, volume 19, pages 27‐39). |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 13 April 2012 | Amended | Additional table linked to text |
| 7 August 2008 | Amended | Converted to new review format. |
| 14 January 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Professor John Simes, Ms Davina Ghersi, Ms Sharon Parker, Avinesh Pillai, Dr Val Gebski, Dr Sally Lord and Dr Nicolas Wilcken of the Cochrane Breast Cancer Group and the NHMRC Clinical Trials Centre for their valuable input and help during protocol and review development. We would like to thank Ms Nicole Holcroft for her work in the identification of studies through the Cochrane Breast Cancer Group Specialised Register. We also would like to thank Fergus Tai and Ms Suchaya Thongyoo (students in Health Information Management), for conducting the double time to event data extraction.
The following trial authors kindly responded to requests for additional information: ABCSG 2001, Bordeaux 1991; ECTO 2005, EORTC 2001, Institut Curie 1991; Institut Curie 1994; London 2001, Ragaz 1997, Royal Marsden 1998. Special thanks to Dr Patrick Therasse and Dr Jan Bogaerts of the EORTC for supplying updated individual patient data on the EORTC 10902 trial and to Janine van Nes, Leiden University Medical Centre, for her assistance in analysing the data.
We are indebted to the financial support of the organisations named under External sources of support listed in the cover sheet of this review.
Data and analyses
Comparison 1. Preoperative versus postoperative chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Overall survival | 10 | 4620 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.98 [0.87, 1.09] |
| 1.2 Disease‐free survival | 10 | 4510 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.97 [0.89, 1.07] |
| 1.3 Time to loco‐regional recurrence | 11 | 5041 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.21 [1.02, 1.43] |
| 1.4 Loco‐regional treatment (mastectomy rate) | 10 | 5292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.67, 0.75] |
| 1.5 Adverse effects | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 Postoperative complications | 3 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.41, 1.76] |
| 1.5.2 Cardiotoxicity | 2 | 1600 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.53, 1.04] |
| 1.5.3 Leucopenia/neutropenia/infections | 4 | 2799 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.56, 0.84] |
| 1.5.4 Nausea and vomiting | 2 | 1088 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.41] |
| 1.5.5 Alopecia | 3 | 2561 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.05] |
1.1. Analysis.

Comparison 1: Preoperative versus postoperative chemotherapy, Outcome 1: Overall survival
1.2. Analysis.

Comparison 1: Preoperative versus postoperative chemotherapy, Outcome 2: Disease‐free survival
1.3. Analysis.

Comparison 1: Preoperative versus postoperative chemotherapy, Outcome 3: Time to loco‐regional recurrence
1.4. Analysis.

Comparison 1: Preoperative versus postoperative chemotherapy, Outcome 4: Loco‐regional treatment (mastectomy rate)
1.5. Analysis.

Comparison 1: Preoperative versus postoperative chemotherapy, Outcome 5: Adverse effects
Comparison 2. Pathological complete response (pCR) vs residual disease (pRES).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Overall survival | 4 | 1290 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.48 [0.33, 0.69] |
| 2.2 Disease‐free survival | 5 | 1741 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.48 [0.37, 0.63] |
2.1. Analysis.

Comparison 2: Pathological complete response (pCR) vs residual disease (pRES), Outcome 1: Overall survival
2.2. Analysis.

Comparison 2: Pathological complete response (pCR) vs residual disease (pRES), Outcome 2: Disease‐free survival
Comparison 3. Downstaged vs planned breast conserving surgery in treatment arm.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Overall survival | 1 | 120 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.33 [0.67, 2.63] |
| 3.2 Loco‐regional recurrence | 2 | 623 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.85, 2.13] |
3.1. Analysis.

Comparison 3: Downstaged vs planned breast conserving surgery in treatment arm, Outcome 1: Overall survival
3.2. Analysis.

Comparison 3: Downstaged vs planned breast conserving surgery in treatment arm, Outcome 2: Loco‐regional recurrence
Comparison 4. Preoperative vs postoperative chemotherapy (Subgroup Local treatment).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Loco‐regional recurrence | 6 | 3257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.88, 1.46] |
| 4.1.1 Breast conserving surgery | 4 | 1830 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.82, 1.54] |
| 4.1.2 Mastectomy | 4 | 1427 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.74, 1.75] |
| 4.1.3 RT only | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
4.1. Analysis.

Comparison 4: Preoperative vs postoperative chemotherapy (Subgroup Local treatment), Outcome 1: Loco‐regional recurrence
Comparison 5. Preoperative vs postoperative chemotherapy (Subgroup Treatment arm).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Loco‐regional treatment (mastectomy rate) | 10 | 5292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.67, 0.75] |
| 5.1.1 Preop vs postop chemo | 6 | 4185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.66, 0.75] |
| 5.1.2 Preop and postop ('sandwich') vs postop chemo | 4 | 1107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.61, 0.91] |
5.1. Analysis.

Comparison 5: Preoperative vs postoperative chemotherapy (Subgroup Treatment arm), Outcome 1: Loco‐regional treatment (mastectomy rate)
Comparison 6. Preoperative vs postoperative chemotherapy (Subgroup Chemotherapy regimens).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Loco‐regional treatment (mastectomy rate) | 10 | 5292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.67, 0.75] |
| 6.1.1 Taxane and anthracycline containing | 1 | 1313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.46, 0.61] |
| 6.1.2 Anthracycline containing | 7 | 3346 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.72, 0.83] |
| 6.1.3 Proportion of participants receiving anthracycline | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.58, 3.11] |
| 6.1.4 No anthracycline containing | 1 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.63, 1.05] |
6.1. Analysis.

Comparison 6: Preoperative vs postoperative chemotherapy (Subgroup Chemotherapy regimens), Outcome 1: Loco‐regional treatment (mastectomy rate)
Comparison 7. Preoperative vs postoperative chemotherapy (Subgroup Methodological quality).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Loco‐regional treatment (mastectomy rate) | 10 | 5292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.67, 0.75] |
| 7.1.1 Adequate | 5 | 2715 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.74, 0.88] |
| 7.1.2 Not adequate or Unclear | 5 | 2577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.57, 0.68] |
7.1. Analysis.

Comparison 7: Preoperative vs postoperative chemotherapy (Subgroup Methodological quality), Outcome 1: Loco‐regional treatment (mastectomy rate)
Comparison 8. Preoperative versus postoperative chemotherapy (Excluding outlying studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Time to loco regional recurrence | 11 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | Subtotals only | |
| 8.1.1 Included | 8 | 4198 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.12 [0.92, 1.37] |
| 8.1.2 Excluded | 3 | 843 | Peto Odds Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.45 [1.06, 1.97] |
| 8.2 Loco‐regional treatment (mastectomy rate) | 10 | 5292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.67, 0.75] |
| 8.2.1 Included | 8 | 3709 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.76, 0.89] |
| 8.2.2 Excluded | 2 | 1583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.48, 0.60] |
8.1. Analysis.

Comparison 8: Preoperative versus postoperative chemotherapy (Excluding outlying studies), Outcome 1: Time to loco regional recurrence
8.2. Analysis.

Comparison 8: Preoperative versus postoperative chemotherapy (Excluding outlying studies), Outcome 2: Loco‐regional treatment (mastectomy rate)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ABCSG 2001.
| Study characteristics | ||
| Methods | National (Austria), multicentre RCT, Accrual 10/1991 ‐ 10/1999. No information on allocation concealment reported. Treatment allocation method not reported. Baseline comparability: not reported. | |
| Participants | 423 Women with breast cancer. Till 1996, 301 receptor‐negative pts were accrued. From 1996, 122 receptor‐positive pts with tumours larger than 3 cm were regardless of nodal status added to the study. No information on patients' characteristics available. | |
| Interventions | Preop and postop vs postop CMF/EC Arm A: 3 cycles of preoperative Cyclophosphamide 600 mg/m2, Methotrexate 40 mg/m2, Fluorouracil 600 mg/m2 IV on days 1 and 8. Followed by 3 cycles of postoperative CMF (same as above) for node‐negative pts or 3 cycles of EC (Epirubicin 60 mg/m2, Cyclophosphamide 600 mg/m2 on day 1) for node‐positive pts. Arm B: idem as for A, all postoperative Additional treatment modalities: Surgery: not specified (including breast‐conserving interventions) |
|
| Outcomes | Response rate, according to UICC. pCR definition unclear. Type of surgery | |
| Notes | Results are from abstract form (ASCO 2001). Authors contacted for further information. Received reaction, however no additional data is provided as publication is pending.
Median follow‐up: not reported
No information on postrandom exclusions or pts lost to follow‐up.
Unclear number of patients assessed for response analysis. This study is graded as D as no information was available on allocation concealement or on baseline characteristics |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Bordeaux 1991.
| Study characteristics | ||
| Methods | Single centre (France) RCT, accrual 1/1985 ‐ 4/1989. No information on allocation concealment reported. Treatment allocation was by stratification on ER‐status. Baseline comparability: no significant imbalance reported. Arm B slightly larger tumours and more clinically node involvement. | |
| Participants | 272 Women with histologically confirmed (drill biopsy), operable breast cancer (T2 >3cm, T3, N0‐1, M0). Age < 70 yrs. No bilateral BC. No slow‐growing tumours. Residence not too far from the hospital and not a medical professional. 526 Pts assessed for eligibility. After randomisation 3 pts refused surgery. Mean age: 53. Premenopausal: 37%. T2: 83%; T3: 17%. Clinically lymph node involvement: 56%. | |
| Interventions | Preop vs postop EVM + MTV Arm A: 3 cycles of preoperative Epirubicin 50 mg/m2, Vincristine 1 mg/m2, Methotrexate 20 mg/m2 every 3 weeks followed by 3 cycles of preoperative Mitomycin C 10 mg/m2, Thiotepa 20 mg/m2, Vindesine 4 mg/m2 every 3 weeks. Followed within 3 weeks by loco‐regional treatment which was based on tumour regression: ‐ Complete regression: exclusive RT of breast (50 Gy + 20‐24 Gy boost) and axilla, internal mammary, supraclavicular node areas (50 Gy + 10 Gy boost on axilla if positive prechemotherapy). ‐ Residual < 2cm: lumpectomy + breast irradiation (50 Gy + 10 Gy boost). ‐ Residual > 2cm: modified radical mastectomy (Patey) without RT. Arm B: Patey mastectomy within 15 days after randomisation. Followed by chemotherapy as for arm A if histological axillary node involvement or negative ER/PR, otherwise no adjuvant chemotherapy. |
|
| Outcomes | Overall survival, calculated from the date of randomisation. Disease‐free survival, time to local recurrence or metastasis. Loco regional recurrence, first site of relapse. Response rate, exact method not reported. Type of surgery, after 10 yr f/u (arm B all mastectomy). Adverse effects | |
| Notes | Extended follow‐up results presented in 1993 (Lyon Chir) and 1999 (Ann Oncol). Initial study published in 1991 (Ann Oncol). Authors reacted upon query, however no additional data is provided. Median follow‐up: 34 (1991), 84 (1993; est f/u: 37‐89) and 124 (1999; rep f/u: 47‐148) months. 2 Pts in arm B who refused surgery were lost to follow‐up. Survival, response and adverse effects calculated for all randomised patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ECTO 2005.
| Study characteristics | ||
| Methods | International (Europe), multicentre, RCT, accrual 1996 ‐ 5/2002. No information on allocation concealment reported. Treatment allocation was by stratification. Baseline comparability: no significant imbalance reported. | |
| Participants | 1355 Women with breast cancer tumours larger than 2 cm in its maximum diameter as assessed by mammography. No locally advanced or metastatic disease. Pts naïve to breast cancer treatment. Not pregnant or nursing. No active infection. No history of second malignancy except BCC or in situ cancer of cervix. Age 18‐70. 31 Pts did not receive allocated intervention because of ineligibility (8) or refusal (23). Age: <50: 45%, >50: 55%. T <4 cm 80%, >4 cm 20%. Grade I: 12%, II: 56%, III: 32%. Clinically lymph node involvement: not reported. | |
| Interventions | 3 Arms: Preop AT‐CMF vs postop AT‐CMF vs postop A‐CMF Arm A: 4 cycles of preoperative Doxorubicin 60 mg/m2, Paclitaxel 200 mg/m2 IV every 3 weeks followed by 4 cycles of Cyclophosphamide 600 mg/m2, Methotrexate 40 mg/m2, Fluorouracil 600 mg/m2 IV on days 1 and 8 every 4 weeks. Arm B: idem as for A, all postoperative. Arm C: 4 cycles of postoperative Doxorubicin 75 mg/m2 IV every 3 weeks followed by 4 cycles of CMF IV on days 1 and 8 every 3 weeks. Additional treatment modalities: ‐ mastectomy or BCT + radiotherapy ‐ RT for pts treated with mastectomy and pT4. RT delivered within 4 weeks after completing chemotherapy and surgery. ‐ Tamoxifen: all pts are candidate (20 mg daily for 5 yrs) |
|
| Outcomes | Overall survival (Arm A vs Arm B), from date of randomisation Disease‐free survival (Arm A vs Arm B), to first evidence of BC progression or relapse Loco regional recurrence (Arm A vs Arm B & C), from date of surgery to date of first evidence of local breast recurrence. Response rate (pCR, cCR), absence of invasive carcinoma in breast. Association of pCR with DFS. Type of surgery Adverse effects | |
| Notes | Results are from abstract form (ASCO 2003, 2005) and accompanied presentation (ASCO 2005). Additional information available from protocol published on Cancernet (12/1997). Authors reacted upon query, however no additional data is provided as publication is in preparation. Median f/u: 50 mths 138 pts did not complete chemotherapy. Overall, DFS, response analysis and adverse effects calculated for all randomised patients (ITT). Time to LRR and loco regional treatment for 1313 pts (97%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Edinburgh 1995.
| Study characteristics | ||
| Methods | Single centre (Scotland), RCT, accrual period unclear. Randomisation was by central office. Treatment allocation method not reported. Baseline comparability: no significant imbalance apparent or reported. | |
| Participants | 79 Women with histologically confirmed (FNA) operable breast cancer > 4 cm. No metastatic disease. No postrandom exclusions. Mean age: 51. Mean t‐size: 4.9 cm. | |
| Interventions | Preop and postop vs postop CAP or endocrine treatment Arm A (40): ‐ ER ‐ pts and non‐responding ER+ pts (23): 4 cycles of preoperative Cyclophosphamide 1000 mg/m2, Doxorubicin 50 mg/m2, Prednisolone 40 mg for 5 days every 3 weeks. Followed by 2 cycles of postoperative cycles of CAP. ‐ Responding ER+ pts (14): endocrine treatment * Premenopausal: Goserelin (leuteinising hormone releasing hormone (LHRH) agonist) injection s.c. monthly for 12 weeks. Followed by oophorectomy. * Postmenopausal: Tamoxifen 20 mg daily for 12 weeks and continued postoperatively. Arm B (39): appropriate adjuvant therapy: NFS. Additional treatment modalities: Modified radical mastectomy with level III axillary clearance for all pts within 3 weeks after last cycle of chemotherapy or after study entry. |
|
| Outcomes | Adverse effects | |
| Notes | Authors contacted for further information without reaction. Adverse effects calculated for all randomised patients. 4 Protocol violations: 3 pts in arm A received postoperative systemic treatment and 1 pt in arm B received primary systemic treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
EORTC 2001.
| Study characteristics | ||
| Methods | International (Europe), multicentre RCT, Accrual 4/1991 ‐ 5/1999. Randomisation was by telephone call to central office. Treatment allocation was by stratification. Baseline comparability: no significant imbalance apparent or reported. | |
| Participants | 698 Women with histologically confirmed (FNA, core needle biopsy) primary, operable breast cancer (T1c‐T3, T4b, N0‐1, M0). No bilateral BC. No previous or current cancers (except adeq treated BCC, SCC or cervix). WHO performance status 1‐2. Absence of active cardiac disease. Not pregnant or lactating. Pts naïve to breast cancer treatment. 33 pts did not receive allocated intervention because of ineligibility (16), refusion of further cooperation (8), postoperative complications (2), and unknown reasons (7). Median age (range; standard deviation): 48.5 (25‐70; 9.33) Premenopausal status assessed on basis of age (<50 yr): 55%. T1: 14%; T2: 58; T3: 21; T4: 5. Clinically lymph node involvement: 48%. | |
| Interventions | Preop vs postop FEC Arm A: 4 cycles of preoperative Fluorouracil 600 mg/m2, Epirubicin 60 mg/m2, Cyclophosphamide 600 mg/m2 IV every 3 weeks. Arm B: idem as for A, all postoperative (first cycle administered within 36 hrs after surgery). Additional treatment modalities: ‐ Surgery: modified radical mastectomy or BCT (wide local excision or quadrantectomy/axillary dissection and RT); followed within 4 wks of the fourth course of chemotherapy in arm A. ‐ RT: administered after surgery or completion of chemotherapy. After BCT and after non‐radical surgery. 50 Gy in 5 weeks at target volumes. Chest wall/parasternal: pts with initial tumour of 5 cm or more. Infra‐ and supraclavicular fossa: pts with positive infraclavicular node after LN dissection. ‐ Tamoxifen: pts >50 yrs (regardless of ER/nodal status) received 20 mg daily for at least 2 yrs. |
|
| Outcomes | Overall survival, calculated from the date of randomisation. Disease‐free survival, disease relapse or death. Loco regional recurrence, ipsilateral breast or regional lymph nodes, incl supraclavicular nodes. Response rate, according to UICC, palpation and mammography. pCR, no signs of residual malignant cells in primary site and axillary LN. Association of pCR with OS and DFS Type of surgery Change of originally planned type of surgery Adverse effects | |
| Notes | Extended follow‐up data (individual patient data) supplied by EORTC data centre. Initial study published in 2001 (JCO). Median follow‐up: 5 (2001) and 9.75 (IPD 2005) yrs. Nine pts lost to follow‐up with unknown reasons. 19 Pts discontinued chemotherapy. 36 (5%) Pts had T4b tumours. Time‐to‐event outcomes calculated for all randomised patients. 315 Pts assessed for clinical response analysis: 16 not assessable. 19 received no preop chemo (6 ineligible, 10 refused, 3 received postop chemo). 329 Pts assessed for pathological response. All pts assessed for adverse effects |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Institut Curie 1991.
| Study characteristics | ||
| Methods | Single centre (France) RCT, accrual 11/1983 ‐ 3/1986. No information on allocation concealment reported. Treatment allocation method not reported. Baseline comparability: no significant imbalance apparent or reported. Arm B slightly older and more T3N1b. | |
| Participants | 196 Women with operable breast cancer (T2‐3, N0,1b, M0). No prior cancer, no serious concomitant illness, and age <65 yrs. 15 Postrandom exclusions because of errors of randomisation, poor pts' or physicians' compliance or treatment outside institution. Postoperative chemotherapy was withheld in 21 pts in arm B and in 18 pts in arm A because of N‐ at surgery. Median age: 50 Premenopausal: 61%. T2: 40%; T3: 60. Clinically lymph node involvement: 72%. | |
| Interventions | Preop and postop vs postop FAC/AMVT Arm A: 2 cycles of preoperative 5‐Fluorouracil 500 mg/m2 IV or i.m. on days 1, 3, 5, 8, Doxorubicin 25 mg/m2 and Cyclophosphamide 400 mg/m2 IV on day 1 and 8 every 4 wks. Good responders received a following 4 cycles of FAC as above after loco regional treatment. Non‐responders (n=8) received 4 cycles of Doxorubicin 20 mg/m2, Methotrexate 25 mg/m2, Vindesine 3 mg/m2, Thiotepa 7 mg/m2 on days 1 and 8 for pts with an initial poor response. Arm B: 6 cycles of postoperative FAC as above. Additional treatment modalities Primary radiation therapy: 55 Gy in 6 weeks to breast and inferior axillary nodes + 45 Gy to supraclavicular nodes and internal mammary chain. A boost to tumour bed (totalling 75‐80 Gy) was given to pts who had a regression of the tumour at 55 Gy. Surgery (mastectomy or lumpectomy) was limited to pts presenting with a persisting mass after RT. |
|
| Outcomes | Loco regional recurrence, tumour presence at or after 9 months from the start of treatment. Response rate, according to UICC. Type of surgery | |
| Notes | Authors reacted upon query, however no additional data is provided. Median follow‐up: 54 months. 2 Pts lost to follow‐up. 76 Pts (received planned treatment) assessed for response analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Institut Curie 1994.
| Study characteristics | ||
| Methods | Single centre (France) RCT, accrual 10/1986 ‐ 6/1990. No information on allocation concealment reported. Treatment allocation method not reported. Baseline comparability: no significant imbalance apparent or reported. | |
| Participants | 414 Premenopausal women with histologically confirmed (drill biopsy) breast cancer (T2‐3, N0‐1, M0). T‐size: 3‐7 cm. No bilateral, inflammatory or locally advanced disease. No prior cancer, no serious concomitant illness. 24 Postrandom exclusions because pts opted out. 45 Pts did not receive their allocated chemotherapy regimen due to protocol violations or errors of randomisations (7 in A; 12 in B) or because chemotherapy was withheld (24 pts in B were pN‐ after surgery and 2 pts in A, no reasons provided). Mean age: 45 yrs. Premenopausal: 100%. T2: 73%; T3: 27%. Clinically lymph node involvement: 59%. | |
| Interventions | Preop vs postop FAC Arm A: 2 cycles of preoperative 5‐Fluorouracil 500 mg/m2 on days 1, 3, 5, 8, Doxorubicin 25 mg/m2 and Cyclophosphamide 400 mg/m2 IV on day 1 and 8 every 4 wks. Followed by response assessment: Good responders: 2 additional cycles of preoperative FAC Non‐responders: loco‐regional treatment Arm B: 4 cycles of FAC within 2 weeks of ending loco‐regional treatment. Additional treatment modalities: Primary irradiation: 54 Gy in 6 weeks to breast and axillary nodes + 45 Gy to supraclavicular nodes and internal mammary chain. Pts with CR or near CR received a boost to tumour bed (totalling 75‐80 Gy) and had no surgery. N+ pts received a 10‐15 Gy boost to inferior axilla if no surgery was performed. Surgery (mastectomy or lumpectomy) was limited to pts presenting with a persisting mass after 54 Gy. 20 Pts in A and 4 in B underwent mastectomy without RT. |
|
| Outcomes | Overall survival, calculated from the date of randomisation. Disease‐free survival Loco‐regional recurrence Response rate, appears to be according to UICC. Type of surgery, after 5 yr f/u Adverse effects | |
| Notes | Extended follow‐up results presented in 1999 (Br Ca Res & Tr). Initial study published in 1994 (EJC). Detailed report on tumour response (1995, EJC). Authors reacted upon query, however no additional data is provided. Median follow‐up: 54 (1994; est f/u 8‐78) and 105 (1999; rep f/u 27‐135) months. No losses to follow‐up. 390 Pts assessed for survival analyses and adverse effects. Assessable for response: 191 (after 2 cycles) and 153 (after 4 cycles) pts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Japan 1998.
| Study characteristics | ||
| Methods | National (Japan), multicentre RCT, accrual 4/95 ‐ 12/97. No information on allocation concealment reported. Treatment allocation was by minimisation method. Baseline comparability: no significant imbalance reported. | |
| Participants | 50 Women with histologically confirmed breast cancer. Stage II with tumour size >4 cm and stage III. 5 Postrandom exclusions because of ineligibility (3 stage IV, 1 sarcoma, and 1 unknown reason). 2 Pts refused further cooperation but were included in the analyses. | |
| Interventions | Preop and post op vs postop EC and UFT Arm A: 2 cycles of preoperative Epirubicin 50 mg/m2, Cyclophosphamide 200 mg/m2 every 3 weeks followed by 3 cycles of postoperative EC. Daily administration of 400 mg UFT. Arm B: idem as for A, all postoperative. Additional treatment modalities: ‐ surgery: mastectomy for all patients ‐ tamoxifen: 20 mg for 2 yrs. |
|
| Outcomes | Overall survival Disease‐free survival Loco regional recurrence Clinical response rate, method unclear. | |
| Notes | Announcement of presenting interim‐results on St. Gallen conference 1998 (abstract). Authors provided additional information and data.
Median follow‐up is unclear (±18 months).
Survival calculated for all eligible patients (n=45).
18 Pts assessed for response analysis (excluding 2 refusals). This study is graded as D as a number of participants were excluded post randomisation thereby increasing the risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Lithuania 1998.
| Study characteristics | ||
| Methods | RCT (Lithuania), accrual 3/1994 ‐ 9/1997. No information on allocation concealment reported. Treatment allocation method not reported. Baseline comparability: not reported. | |
| Participants | 100 Women with breast cancer. Stage II (T2, N0‐1). Age range: 28‐50. T2: 100%. | |
| Interventions | Preop and postop vs postop CMF Arm A: 2 cycles of preoperative Cyclophosphamide, Methotrexate, Fluorouracil: NFS. Arm B: idem as for A, all postoperative. Additional treatment modalities: Conservative surgery (plastic quadranectomy), RT, adjuvant chemo/hormonotherapy (NFS). |
|
| Outcomes | Disease‐free survival Loco regional recurrence Response rate, method unknown. | |
| Notes | Results are from abstract form (St Gallen 1998).
Authors contacted for further information without reaction.
Follow‐up: 3.5 years.
No information on postrandom exclusions or losses to follow‐up. This study is graded as D as no information was available on allocation concealement or on baseline characteristics |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
London 2001.
| Study characteristics | ||
| Methods | Single centre (UK) RCT, accrual 1990 ‐ 1993. Randomisation was by serially numbered envelope, pts sequentially allocated in order of presentation. Treatment allocation was by random number table. Baseline comparability: no significant imbalance apparent or reported. | |
| Participants | 210 Women with histologically confirmed (Tru‐cut biopsy) primary breast cancer. T1‐4, N0‐1, M0. No significant cardiac or renal impairment. No previous history of cancer. No postrandom exclusions. Median age (range): 54 (30‐69). Premenopausal: 37 %. T1‐2: 76%; T3‐4: 24%. Clinically lymph node involvement: 18%. ER+ (>30% positive cells using immunohistochemistry): 51%. | |
| Interventions | Preop and postop vs postop treatment. Receiving either chemo‐ or endocrine therapy based on ER‐status. Arm A (N): ‐ ER+ pts (47): endocrine treatment * Premenopausal (13): Goserelin (leuteinising hormone releasing hormone (LHRH) agonist) 3,75 mg s.c. monthly for 12 weeks. * Postmenopausal (34): Formestane (4‐hydroxyandrosenedione) 250 mg i.m. every 2 weeks for 12 weeks. ‐ ER ‐ pts (53): 4 cycles in 12 weeks of preoperative Mitozantrone 7 mg/m2 every 3 weeks, Mitomycin C 7 mg/m2 every 6 weeks, Methotrexate 30 mg/m2 every 3 weeks with foninic acid rescue 15 mg 4 times for 24 hours, starting 24 hrs after chemotherapy. ‐ After clinically assessing tumour response and surgery/radiotherapy: ‐ Responders: received a total of 8 cycles MMM or 18 months Goserelin or Formestane (doses as above). ‐ Non‐responders: * ER + pts: 8 cycles of MMM (as above) * ER ‐ pts: 8 cycles of 5‐Fluorouracil 600 mg/m2, Epirubicin 50 mg/m2, Cyclophosphamide 600 mg/m2 every 3 weeks. Arm B: ‐ ER+ pts (60): endocrine therapy * Premenopausal (10): Goserelin as above for 18 months. * Postmenopausal (50): Formestane as above for 18 months. ‐ ER ‐ pts (50): 8 cycles of MMM as above. Additional treatment modalities: Primary surgery (mastectomy or conservative surgery (wide local excision and Level I axillary dissection/RT to breast + boost to scar) or primary radiotherapy. Pts with involved axillary nodes: RT to axilla and supraclavicular fossa. Pts with tumours in medial half of breast: RT to ipsilateral mammary chain. When primary RT did not produce a response, a mastectomy was performed. |
|
| Outcomes | Overall survival, calculated from the date after primary treatment. Time to loco regional treatment Response rate, according to UICC by mammography of breast. Type of surgery | |
| Notes | Authors provided additional information on the randomisation process. Rep min f/u: 60 months, est max f/u: 108. No losses to follow‐up. Survival calculated for all randomised patients. Response data for all 53 pts who received chemotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
NSABP 1998.
| Study characteristics | ||
| Methods | International (USA, Canada), multicentre RCT, accrual 10/1988 ‐ 4/1993. Randomisation was by central office. Treatment allocation was by biased‐coin minimization algorithm. Baseline comparability: no significant imbalance apparent or reported. | |
| Participants | 1523 Women with histologically confirmed (FNA, CNS; open biopsy not permitted) primary, palpable, operable breast cancer (T1‐3, N0‐1, M0). No locally advanced disease. 21 Postrandom exclusions because of ineligibility (6 advanced disease, 3 no consent, 3 open biopsy, 9 others reasons). 38 Pts did not receive allocated chemotherapy (12 because of no invasive cancer in surgical specimen, 10 of protocol violations). Median age (standard deviation): 50 (11). Pre‐ and perimenopausal: 50%. T1: 28%; T2: 59; T3: 13. Clinically lymph node involvement: 26%. | |
| Interventions | Preop vs postop AC Arm A: 4 cycles of preoperative Doxorubicin 60 mg/m2, Cyclophosphamide 600 mg/m2 IV every 3 weeks. Women with progressive disease before completion of all 4 courses received the remaining courses after surgery. Arm B: idem as for A, all postoperative. Additional treatment modalities: ‐ Modified radical mastectomy or lumpectomy and axillary node dissection. ‐ Irradiation was followed after lumpectomy and started within 4 weeks after lumpectomy or last course of chemotherapy. ‐ Tamoxifen: pts >50 yrs (regardless of ER/nodal status) received 10 mg twice daily for 5 yrs, beginning on the day after the last dose of chemotherapy. |
|
| Outcomes | Overall survival, death from any cause; calculated from the date of randomisation. Disease‐free survival, events include loco regional or distant treatment failure, contralateral BC, second primary cancer, or death with no evidence of cancer. Pts who became inoperable before surgery or in whom the tumour cld not be completely resected were counted as local treatment failures. Loco regional recurrence, n of events, as site of first relapse. Response rate, according to UICC. pCR, absence of invasive carcinoma in the surgical breast specimen. Only complete clinical responders were evaluated for pCR. Type of surgery Change of originally planned type of surgery Association of pCR with OS and DFS Adverse effects | |
| Notes | Extended follow‐up results presented in 2001 (JNCI‐M). Initial study published in 1998 (JCO). Authors contacted for further information without reaction. Mean follow‐up: 6 (1998) and 9.5 (2001) yrs. Est f/u range 87‐144). 9 Pts lost to follow‐up with unknown reasons. 53 Pts discontinued chemotherapy. Survival calculated for all eligible patients with follow‐up (N= 1493). Data on breast tumour response available for 683 pts. Not evaluated tumours were not monitored according to protocol or were subject of protocol deviation. Data on adverse effects available for 1473 pts, regardless of eligibility. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Royal Marsden 1998.
| Study characteristics | ||
| Methods | Single centre (UK) RCT, accrual 2/1990 ‐ 8/1995. Randomisation was by telephone call to central office. Treatment allocation was by variable sized permuted blocks. Baseline comparability: no significant imbalance apparent or reported. | |
| Participants | 309 Women with histologically confirmed (cytology, Tru‐cut biopsy) primary, operable breast cancer (T1‐4, N0‐1, M0). No premenopausal pts who wished to consider further pregnancy. No clinical evidence myocardial dysfunction. 16 Postrandom exclusions because of ineligibility. 7 Pts refused further cooperation. Median age (range): 56 (27‐69). Premenopausal: 33% T1: 12%; T2: 82; T3: 5; T4: 2. Clinically lymph node involvement: 19% | |
| Interventions | Preop and postop vs postop MM(M) Arm A: 4 cycles of preoperative Mitomycin C 7 mg/m2 every 6 weeks, Mitoxantrone 7 mg/m2 every 3 weeks, Methotrexate 35 mg/m2 every 3 weeks or 2M (same as 3M, with the exclusion of Mitomycin C and increased dose of Mitoxantrone to 11 mg/m2) followed by 4 cycles postoperative of 3M or 2M. Arm B: idem as for A, all postoperative. Additional treatment modalities: Mastectomy or BCT/radiotherapy (54 Gy to breast + 10 Gy boost to scar). Clinically involved lymph nodes: Level II axillary lymph node dissection. No axillary dissection for clinically node negative pts. RT to axilla and supraclavicular fossa was only given to those pts with palpable nodes at presentation, who did not have axillary dissection. RT started 4‐6 weeks after surgery and was given concurrently with chemotherapy. Tamoxifen: 20 mg daily for 5 years simultaneously started with chemotherapy. |
|
| Outcomes | Overall survival, calculated from the date of primary diagnosis. Disease‐free survival, censored at death without recurrence. Time to loco regional recurrence. Response rate, according to UICC, palpation. pCR, breast tumour only, including DCIS. Association of pCR with OS and DFS. Type of surgery, after 4 yr f/u. Change of originally planned type of surgery | |
| Notes | Extended follow‐up results presented in 2005 (Ann Oncol). Initial study published in 1998 (Ann Oncol). Authors provided additional information on randomisation process. First 115 pts received 3M chemotherapy, thereafter 2M. Two (1%) pts had T4 tumour. Median follow‐up: 112 (rep f/u: 12‐145) months. Survival calculated for 286 women (excluding 16 postrandom exclusions and 7 refusals). Assessable for clinical and pathological response, 144 and 149, respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
St. Petersburg 1994.
| Study characteristics | ||
| Methods | Single centre (Russia) RCT, accrual 1/1985 ‐ 1/1990. No information on allocation concealment reported. Treatment allocation was by table of random numbers. Baseline comparability: no significant imbalance apparent or reported. Arm A slightly younger (mean 49.7 vs 51.2). | |
| Participants | 271 Women with histologically confirmed (FNA) breast cancer (Stage IIb‐IIIa: T3N0,1; T2N1; T1,2N2; M0). Age < 55 yrs. No postrandom exclusions. Mean age (range): 50 (27‐55). T1‐2: 18%; T3: 82%. Clinically lymph node involvement: 69%. | |
| Interventions | Preop and postop vs postop TMF Arm A: 1 or 2 cycle(s) (N=94 and 43, resp.) of preoperative Thiotepa 20 mg/m2 i.m. on days 1,3,5,7,9,11, Methotrexate 40 mg/m2, 5‐Fluorauracil IV on days 1 and 8 every 4 weeks. Followed, starting during mastectomy, by 4‐5 cycles of TMF. Arm B: 6 cycles of postoperative TMF. Both arms: Preoperative RT: 60 Gy (2 Gy daily) to mammary gland + 40 Gy to axillary area, supra‐ and subclavicular areas followed after 3‐4 weeks by modified radical mastectomy. |
|
| Outcomes | Overall survival, calculated from the date of initial treatment. Disease‐free survival, relapse, metastase, or death. Response rate, according to UICC, assessed 2 wks after RT, mammography. pCR, complete disappearance in breast and LN. Association of pCR with OS and DFS | |
| Notes | 14 Pts presented with Stage N2 (T1 or 2).
150 Pts received the total 6 cycles of TMF (53% in A, 58% in B); 5 cycles in 22% and 19%; 4 cycles in 14% and 18%; 3 or less in 12% and 5%. No reasons provided for this decrease. Median follow‐up: 53 months. Est f/u range: 32‐92 months. No losses to follow‐up. Survival calculated for all randomised patients. All pts assessed for response analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
USA 2003.
| Study characteristics | ||
| Methods | National (USA), multicentre RCT, accrual 1990 ‐ 11/1998. Pts randomised by central randomisation office. Treatment allocation was not reported. Baseline comparability: no significant imbalance apparent or reported. Arm A is slightly older. | |
| Participants | 53 Women with histologically confirmed, stage II breast cancer (T1N1, T2N0, T2N1). Absence of chronic cardiac or pulmonary disease and pregnancy. 1 pt in arm A refused chemotherapy after randomisation. Median age; range: 49 (arm A), 43 (arm B); 28‐68. Premenopausal: 60%. T1: 4%; T2: 96%. Clinically lymph node involvement: 28%. | |
| Interventions | Preop vs postop FLAC + G(M)‐CSF Arm A: 5 cycles of preoperative 5‐Fluorouracil 400 mg/m2, Leucovorin 500 mg/m2 (given 1 hour before 5‐FU), Doxorubicin 15 mg/m2 IV on days 1,2,3 and Cyclophosphamide 600 mg/m2 IV on day 1 every 3 wks. Granulocyte‐macrophage colony stimulation factor 10 µg/kg SC daily on days 4‐16 (for first 27 pts). G‐CSF 5 µg/kg SC daily on days 4‐18 (for remaining pts). Arm B: idem as for A, all postoperative (2‐3 wks after surgery) Additional treatment modalities: ‐ Patey modified radical mastectomy or breast segmentectomy/axillary lymph node dissection/whole‐breast radiotherapy. ‐ Radiotherapy given after completion of chemotherapy. Pts with negative axilla received a minimum dose of 50.4 Gy to the breast. Positive axilla pts received additional irradiation to the supraclavicular nodes (50.4 Gy). Pts with extranodal extension received 50.4 Gy to the posterior axillary field. All pts received an additional 10‐Gy boost to the surgical bed. ‐ Tamoxifen for ER+/PR+ pts: 10 mg twice daily for 5 yrs, beginning with completion of local therapy and chemotherapy. |
|
| Outcomes | Overall survival, calculated from the date of randomisation. Disease‐free survival Loco‐regional recurrence Response rate, according to The Breast Cancer Task Force Treatment Committee, NCI, 1978; we only used data on breast response. pCR, definition unclear. Type of surgery Adverse effects | |
| Notes | Trial was designed to recruit 130 pts, but accrual was terminated early because of slow enrollment. GM‐CSF was replaced by G‐CSF after the first 27 pts because of an improved toxicity profile of G‐CSF. Authors contacted for further information, without reaction. Median follow‐up: 9.0 yrs (rep f/u 17‐137 months). No losses to follow‐up. Survival and adverse effects calculated for all randomised patients. 17 Pts assessed for response analysis, 9 pts not assessable (excisional biopsy before therapy and negative nodes). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Deo 2003 | Randomised controlled trial consisting of 101 women with operable locally advanced disease (T4b, N0‐2, M0) |
| Hyams 1993 | Abstract of conference proceeding. Reported a subset of patients part of NSABP B‐18 study. |
| Ragaz 1997 | Abstract of conference proceeding. No data available. Publication pending. |
| Shao 1999 | Randomised controlled trial comparing preoperative with postoperative chemotherapy. Relevant data stratified to apoptotic index. No response from authors. |
| Stauffer 1993 | Abstract of conference proceeding. Not properly randomised (of the 98 analyzed patients only 87 were included in a randomized prospective fashion) |
Contributions of authors
SM and JH selected the studies and extracted the data. SM performed the statistical analyses and drafted the review. CV and JH provided clinical input and reviewed and commented on the review.
Sources of support
Internal sources
No sources of support provided
External sources
KWF Kankerbestrijding, Netherlands
Dr Hendrik Muller Vaderlandsch Fonds, Netherlands
Schuurman Schimmel‐van Outeren Studiebeurs, Netherlands
Jo Keur Studiefonds, Netherlands
Leiden International Study Foundation, Netherlands
Nijbakker‐Morra Stichting, Netherlands
Declarations of interest
CV and JH were involved in the EORTC 10902 study. None of the authors who contributed to this article have any financial or personal relationships with people or organisations that could inappropriately influence the data published.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
ABCSG 2001 {published data only}
- Jakesz R. Comparison of pre- vs. postoperative chemotherapy in breast cancer patients: Four-year results of Austrian breast & colorectal cancer study group (ABCSG) trial 7. In: American Society of Clinical Oncology. Annual Meeting. 2001:Abstract 125.
Bordeaux 1991 {published data only}
- Mauriac L, Durand M, Avril A, Dilhuydy JM. Effects of primary chemotherapy in conservative treatment of breast cancer patients with operable tumors larger than 3 cm. Results of a randomized trial in a single centre. Annals of Oncology 1991;2(5):347-54. [DOI] [PubMed] [Google Scholar]
- Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Annals of Oncology 1999;10(1):47-52. [DOI] [PubMed] [Google Scholar]
ECTO 2005 {published data only}
- Eiermann W, Sabadell D, Baselga J, Vazquez V, Guillem Porta V, Semiglazov V et al. European cooperative trial in operable breast cancer (ECTO): No increased risk of local breast tumor recurrence (LBR) as first and only event after primary systemic therapy (PST). In: American Society of Clinical Oncology. Vol. 22. 2003:Abstract 37.
- Gianni L, Baselga J, Eiermann W, Guillem Porta V, Semiglazov V, Lluch A et al. European cooperative trial in operable breast cancer (ECTO): Improved freedom from progression (FFP) from adding paclitaxel (T) to doxorubicin (A) followed by cyclophosphamide methotrexate and fluorouracil (CMF). Journal of Clinical Oncology, ASCO Annual Meeting Proceedings 2005;23(Suppl 16):Abstract 513. [Google Scholar]
Edinburgh 1995 {published data only}
- Forouhi P, Dixon JM, Leonard RC, Chetty U. Prospective randomized study of surgical morbidity following primary systemic therapy for breast cancer. British Journal of Surgery 1995;82(1):79-82. [DOI] [PubMed] [Google Scholar]
EORTC 2001 {published and unpublished data}
- Hage JA, de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. Journal of Clinical Oncology 2001;19(22):4224-37. [DOI] [PubMed] [Google Scholar]
Institut Curie 1991 {published data only}
- Scholl SM, Asselain B, Palangie T, Dorval T, Jouve M, Garcia Giral E et al. Neoadjuvant chemotherapy in operable breast cancer. European Journal of Cancer 1991;27(12):1668-71. [DOI] [PubMed] [Google Scholar]
Institut Curie 1994 {published data only}
- Broet P, Scholl SM, la Rochefordiere A, Fourquet A, Moreau T, De Rycke Y et al. Short and long-term effects on survival in breast cancer patients treated by primary chemotherapy: an updated analysis of a randomized trial. Breast Cancer Res Treat 1999;58(2):151-56. [DOI] [PubMed] [Google Scholar]
- Scholl SM, Fourquet A, Asselain B, Pierga JY, Vilcoq JR, Durand JC et al. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: preliminary results of a randomised trial: S6. European Journal of Cancer 1994;30A(5):645-52. [DOI] [PubMed] [Google Scholar]
- Scholl SM, Pierga JY, Asselain B, Beuzeboc P, Dorval T, Garcia-Giralt E et al. Breast tumour response to primary chemotherapy predicts local and distant control as well as survival. European Journal of Cancer 1995;31A(12):1969-75. [DOI] [PubMed] [Google Scholar]
Japan 1998 {published and unpublished data}
- Enomoto K, Ikeda T, Matsui A, Kitajima M, Koh J, Masamura S et al. Neoadjuvant therapy in stage II with T>=4CM and stage III breast cancer. In: 6th International Conference Adjuvant therapy for Primary Breast Cancer St Gallen. 1998:P73.
Lithuania 1998 {published data only}
- Ostapenko V, Pipiriene T, Valuckas K. Primary chemotherapy in conservative treatment of stage II breast cancer. In: In 6th International Conference Adjuvant therapy for Primary Breast Cancer St Gallen. 1998:Abstract P78.
London 2001 {published data only}
- Gazet JC, Ford HT, Gray R, McConkey C, Sutcliffe R, Quilliam J et al. Estrogen-receptor-directed neoadjuvant therapy for breast cancer: results of a randomised trial using formestane and methotrexate, mitozantrone and mitomycin C (MMM) chemotherapy. Annals of Oncology 2001;12(5):685-91. [DOI] [PubMed] [Google Scholar]
NSABP 1998 {published data only}
- Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. Journal of Clinical Oncology 1998;16(8):2672-85. [DOI] [PubMed] [Google Scholar]
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18.. Journal of the National Cancer Institute. Monographs 2001;30:96-102. [DOI] [PubMed] [Google Scholar]
Royal Marsden 1998 {published data only}
- Cleator SJ, Makris A, Ashley SE, Lal R, Powles TJ. Good clinical response of breast cancers to adjuvant chemoendocrine therapy is associated with improved overall survival. Annals of Oncology 2005;16:267-72. [DOI] [PubMed] [Google Scholar]
- Makris A, Powles TJ, Ashley SE, Chang J, Hickish T, Tidy VA, Nash AG, Ford HT. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Annals of Oncology 1998;9(11):1179-84. [DOI] [PubMed] [Google Scholar]
St. Petersburg 1994 {published data only}
- Semiglazov VF, Topuzov EE, Bavli JL, Moiseyenko VM, Ivanova OA, Seleznev IK et al. Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb-IIIa breast cancer. Annals of Oncology 1994;5(7):591-5. [DOI] [PubMed] [Google Scholar]
USA 2003 {published data only}
- Danforth DN Jr, Cowan K, Altemus R, Merino M, Chow C, Berman A, et al. Preoperative FLAC/granulocyte-colony-stimulating factor chemotherapy for stage II breast cancer: a prospective randomized trial. Annals of Surgical Oncology 2003;10(6):635-44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Deo 2003 {published data only}
- Deo SV, Bhutani M, Shukla NK, Raina V, Rath GK, Purkayasth J. Randomized trial comparing neo-adjuvant versus adjuvant chemotherapy in operable locally advanced breast cancer (T4b N0-2 M0). Journal of Surgical Oncology 2003;84(4):192-7. [DOI] [PubMed] [Google Scholar]
Hyams 1993 {published data only}
- Hyams DM, Fieler MA, Nobis G. An institutional experience with preoperative systematic chemotherapy in early stage breast cancer. Breast Cancer Research & Treatment 1993;27(1/2):147 (Abstract 68). [Google Scholar]
Ragaz 1997 {published data only}
- Ragaz J, Baird R, Rebbeck P, Trevisan C, Goldie J, Coldman A. Preoperative (neoadjuvant - PRE) versus postoperative (POST) adjuvant chemotherapy (CT) for stage i-II breast cacner (SI-II BC). Long-term analysis of British Columbia randomized trial. In: American Society of Clinical Oncology. Annual Meeting. 1997:Abstract 499.
Shao 1999 {published data only}
- Shao ZM, Li J, Wu J, Han QX, Shen ZZ, Fontana JA et al. Neo-adjuvant chemotherapy for operable breast cancer induces apoptosis. Breast Cancer Research and Treatment 1999;53(3):263-9. [DOI] [PubMed] [Google Scholar]
Stauffer 1993 {published data only}
- Stauffer JS, Allred DC, Aust JB, Cruz AB. Preoperative versus postoperative adjuvant chemotherapy in early operable breast cancer. Breast Cancer Research & Treatment 1993;27(1/2):148 (Abstract 148). [Google Scholar]
Additional references
Abraham 1996
- Abraham DC, Jones RC, Jones SE, Cheek JH, Peters GN, Knox SM et al. Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging. Cancer 1996;78(1):91-100. [DOI] [PubMed] [Google Scholar]
Arriagada 2003
- Arriagada R, Le MG, Guinebretiere JM, Dunant A, Rochard F, Tursz T. Late local recurrences in a randomised trial comparing conservative treatment with total mastectomy in early breast cancer patients. Annals Of Oncology 2003;14(11):1617-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ayers 2004
- Ayers M, Symmans WF, Stec J, Damokosh AI, Clark E, Hess K et al. Gene expression profiles predict complete pathologic response to neoadjuvant paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide chemotherapy in breast cancer. J Clin Oncol 2004;22(12):2284-93. [DOI] [PubMed] [Google Scholar]
Bear 2006
- Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer:National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2006;24(13):2019-27. [DOI] [PubMed] [Google Scholar]
Blichert‐Toft 1992
- Blichert-Toft M, Rose C, Andersen JA, Overgaard M, Axelsson CK, Andersen KW et al. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. Journal National Cancer Institute Monographs 1992;11:19-25. [PubMed] [Google Scholar]
Bonadonna 1990
- Bonadonna G, Veronesi U, Brambilla C, Ferrari L, Luini A, Greco M, et al. Primary chemotherapy to avoid mastectomy in tumors with diameters of three centimeters or more. Journal of the National Cancer Institute 1990;82(19):1539-45. [DOI] [PubMed] [Google Scholar]
Bonadonna 1995
- Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: The results of 20 years of follow-up. New England Journal of Medicine 1995;332:901-6. [DOI] [PubMed] [Google Scholar]
Buzdar 2005
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. Journal of Clinical Oncology 2005;23(16):3676-85. [DOI] [PubMed] [Google Scholar]
Chang 2003
- Chang JC, Wooten EC, Tsimelzon A, Hilsenbeck SG, Gutierrez MC, Elledge R et al. Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet 2003;362(9381):362-9. [DOI] [PubMed] [Google Scholar]
Chua 2005
- Chua S, Smith IE, A'Hern RP, Coombes GA, Hickish TF, Robinson AC et al. Neoadjuvant vinorelbine/epirubicin (VE) versus standard adriamycin/cyclophosphamide (AC) in operable breast cancer: analysis of response and tolerability in a randomised phase III trial (TOPIC 2). Annals of Oncology 2005;16(9):1435-41. [DOI] [PubMed] [Google Scholar]
Clarke 2005
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366(9503):2087-2106. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. London: BMJ Publication Group, 2001. [Google Scholar]
EBCTCG 2005
- Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365(9472):1687-1717. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elkhuizen 1998
- Elkhuizen PH, de Vijver MJ, Hermans J, Zonderland HM, de Velde CJ, Leer JW. Local recurrence after breast-conserving therapy for invasive breast cancer: high incidence in young patients and association with poor survival. International Journal of Radiation Oncology Biology Physics 1998;40(4):859-67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Evans 2005
- Evans TR, Yellowlees A, Foster E, Earl H, Cameron DA, Hutcheon AW et al. Phase III randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: an anglo-celtic cooperative oncology group study. Journal of Clinical Oncology 2005;23(13):2988-2995. [DOI] [PubMed] [Google Scholar]
Felici 2005
- Felici A, Bria E, Ferretti G, Carlini P, Ciccarese M, Cecere FL, et al. Taxanes as neoadjuvant chemotherapy for breast cancer: Pooled-analysis of 3120 patients enrolled in 10 randomized trials. J Clin Oncol ASCO Annual Meeting Proceedings 2005;16S:682. [Google Scholar]
Ferlay 2001
- Ferlay J, Bray F, Pisani P, Parkin DM. Cancer Incidence, Mortality and Prevalence Worldwide. GLOBOCAN, Version 1.0. IARC CancerBase No. 5. Lyon, IARCPress, 2001.
Fisher 1989
- Fisher B, Redmond C, Dimitrov NV, Bowman D, Legault-Poisson S, Wickerham DL, et al. A randomized clinical trial evaluating sequential methotrexate and fluorouracil in the treatment of patients with node-negative breast cancer who have estrogen-receptor-negative tumors. New England Journal of Medicine 1989;320:473-8. [DOI] [PubMed] [Google Scholar]
Fisher 1995
- Fisher B, Mamounas EP. Preoperative chemotherapy: a model for studying the biology and therapy of primary breast cancer. J Clin Oncol 1995;13(3):537-540. [DOI] [PubMed] [Google Scholar]
Fisher 2002
- Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. The New England Journal Of Medicine 2002;347(8):567-575. [DOI] [PubMed] [Google Scholar]
Fredriksson 2002
- Fredriksson I, Liljegren G, Arnesson L-G, Emdin SO, Palm-Sjovall M, Fornander T et al. Local recurrence in the breast after conservative surgery--a study of prognosis and prognostic factors in 391 women. European Journal of Cancer 2002;38(14):1860-1870. [DOI] [PubMed] [Google Scholar]
Fredriksson 2003
- Fredriksson I, Liljegren G, Palm-Sjovall M, Arnesson LG, Emdin SO, Fornander T et al. Risk factors for local recurrence after breast-conserving surgery. Br J Surg 2003;90(9):1093-1102. [DOI] [PubMed] [Google Scholar]
Goodwin 2003
- Goodwin PJ, Black JT, Bordeleau LJ, Ganz PA. Health-related quality-of-life measurement in randomized clinical trials in breast cancer--taking stock. JNCI Cancer Spectrum 2003;95(4):263-281. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haagensen 1943a
- Haagensen CD, Stout AP. Carcinoma of the breast. Criteria of inoperability. The American surgeon 1943;118:859-66. [Google Scholar]
Haagensen 1943b
- Haagensen CD, Stout AP. Carcinoma of the breast. Criteria of inoperability. The American surgeon 1943;118:1032-40. [Google Scholar]
Hannemann 2005
- Hannemann J, Oosterkamp HM, Bosch CA, Velds A, Wessels LF, Loo C et al. Changes in gene expression associated with response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2005;23(15):3331-3342. [DOI] [PubMed] [Google Scholar]
Hayward 1977
- Hayward JL, Carbone PP, Heuson JC, Kumaoka S, Segaloff A, Rubens RD. Assessment of response to therapy in advanced breast cancer: a project of the Programme on Clinical Oncology of the International Union Against Cancer, Geneva, Switzerland. Cancer 1977;39(3):1289-1294. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-1558. [DOI] [PubMed] [Google Scholar]
Hortobagyi 1983
- Hortobagyi GN, Blumenschein GR, Spanos W, Montague ED, Buzdar AU, Yap HY, et al. Multimodal treatment of locoregionally advanced breast cancer. Cancer 1983;51(5):763-8. [DOI] [PubMed] [Google Scholar]
Hortobagyi 1988
- Hortobagyi GN, Ames FC, Buzdar AU, Kau SW, McNeese MD, Paulus D, et al. Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer 1988;62(12):2507-16. [DOI] [PubMed] [Google Scholar]
Hortobagyi 1997
- Hortobagyi GN, Buzdar AU. Locally advanced breast cancer. In: Bonadonna G, Hortobagyi GN, Gianni AM, editors(s). Textbook of Breast Cancer: A Clinical Guide to Therapy. London, UK: Martin Dunitz Ltd, 1997:155-68. [Google Scholar]
Jacquillat 1989
- Jacquillat C, Weil M, Auclerc G, Auclerc MF, Khayat D, Baillet F. Neoadjuvant chemotherapy in the conservative management of breast cancer: a study of 252 patients. Recent Results in Cancer Research 1989;115:36-42. [DOI] [PubMed] [Google Scholar]
Kaufmann 2003
- Kaufmann M, Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. Journal of Clinical Oncology 2003;21(13):2600-8. [DOI] [PubMed] [Google Scholar]
Kiebert 1991
- Kiebert GM, Haes JC, de Velde CJ. The impact of breast-conserving treatment and mastectomy on the quality of life of early-stage breast cancer patients: a review. J Clin Oncol 1991;9(6):1059-1070. [DOI] [PubMed] [Google Scholar]
Mansour 1998
- Mansour EG, Gray R, Shatila AH, Tormey DC, Cooper MR, Osborne CK, et al. Survival advantage of adjuvant chemotherapy in high-risk node-negative breast cancer: Ten-year analysis--an intergroup study. Journal of Clinical Oncology 1998;16:3486-92. [DOI] [PubMed] [Google Scholar]
Molenaar 2004
- Molenaar S, Oort F, Sprangers M, Rutgers E, Luiten E, Mulder J et al. Predictors of patients' choices for breast-conserving therapy or mastectomy: a prospective study. Br J Cancer 2004;90(11):2123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Pavic 2004
- Pavic D, Koomen MA, Kuzmiak CM, Lee YH, Pisano ED. The role of magnetic resonance imaging in diagnosis and management of breast cancer. Technol Cancer Res Treat 2004;3(6):527-541. [DOI] [PubMed] [Google Scholar]
Perloff 1982
- Perloff M, Lesnick GJ. Chemotherapy before and after mastectomy in stage III breast cancer. Archives of surgery 1982;117:879-81. [DOI] [PubMed] [Google Scholar]
Poggi 2003
- Poggi MM, Danforth DN, Sciuto LC, Smith SL, Steinberg SM, Liewehr DJ et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer 2003;98(4):697-702. [DOI] [PubMed] [Google Scholar]
Schick 1983
- Schick P, Goodstein J, Moor J, Butler J, Senter KL. Preoperative chemotherapy followed by mastectomy for locally advanced breast cancer. Journal of Surgical Oncology 1983;22(4):278-82. [DOI] [PubMed] [Google Scholar]
Segel 1988
- Segel MC, Paulus DD, Hortobagyi GN. Advanced primary breast cancer: assessment at mammography of response to induction chemotherapy. Radiology 1988;169(1):49-54. [DOI] [PubMed] [Google Scholar]
Sharkey 1996
- Sharkey FE, Addington SL, Fowler LJ, Page CP, Cruz AB. Effects of preoperative chemotherapy on the morphology of resectable breast carcinoma. Mod Pathol 1996;9(9):893-900. [PubMed] [Google Scholar]
Smith 2005
- Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23(22):5108-5116. [DOI] [PubMed] [Google Scholar]
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-216. [DOI] [PubMed] [Google Scholar]
van der Hage 2003
- Hage JA, Putter H, Bonnema J, Bartelink H, Therasse P, de Velde CJ, EORTC Breast Cancer Group. Impact of locoregional treatment on the early-stage breast cancer patients: a retrospective analysis. Eur J Cancer 2003;39(15):2192-2199. [DOI] [PubMed] [Google Scholar]
van Dongen 2000
- Dongen JA. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European organization for research and treatment of cancer 10801 trial. J Natl Cancer Inst 2000;92(14):1143-1150. [DOI] [PubMed] [Google Scholar]
van Tienhoven 1999
- Tienhoven G, Voogd AC, Peterse JL, Nielsen M, Andersen KW, Mignolet et al. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). EORTC Breast Cancer Cooperative Group and the Danish Breast Cancer Cooperative Group. Eur J Cancer 1999;35(1):32-38. [DOI] [PubMed] [Google Scholar]
Veronesi 1995
- Veronesi U, Bonadonna G, Zurrida S, Galimberti V, Greco M, Brambilla C et al. Conservation surgery after primary chemotherapy in large carcinomas of the breast. Ann Surg 1995;222(5):612-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veronesi 2002
- Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. New England Journal of Medicine 2002;347(16):1227-1232. [DOI] [PubMed] [Google Scholar]
Vinnicombe 1996
- Vinnicombe SJ, MacVicar AD, Guy RL, Sloane JP, Powles TJ, Knee G et al. Primary breast cancer: mammographic changes after neoadjuvant chemotherapy, with pathologic correlation. Radiology 1996;198(2):333-340. [DOI] [PubMed] [Google Scholar]
Voogd 2005
- Voogd AC, Oost FJ, Rutgers EJT, Elkhuizen PHM, Geel AN, Scheijmans LJEE et al. Long-term prognosis of patients with local recurrence after conservative surgery and radiotherapy for early breast cancer. European Journal of Cancer 2005;41(17):2637-2644. [DOI] [PubMed] [Google Scholar]
Wang 2005
- Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 2005;365(9460):671-679. [DOI] [PubMed] [Google Scholar]
Whelan 2000
- Whelan TJ, Julian J, Wright J, Jadad AR, Levine ML. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. Journal of Clinical Oncology 2000;18(6):1220-1229. [DOI] [PubMed] [Google Scholar]
Wolff 2000
- Wolff AC, Davidson NE. Primary systemic therapy in operable breast cancer. J Clin Oncol 2000;18(7):1558-1569. [DOI] [PubMed] [Google Scholar]
Xing 2006
- Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg 2006;93(5):539-546. [DOI] [PubMed] [Google Scholar]
Yusuf 1985
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Progress in Cardiovascular Diseases 1985;27:335-371. [DOI] [PubMed] [Google Scholar]