Objectives
This is a protocol for a Cochrane Review (diagnostic). The objectives are as follows:
To determine the accuracy of laboratory tests for the diagnosis of congenital Zika virus infection.
Secondary objectives
To explore potential sources of heterogeneity, and specific factors that may influence the accuracy of diagnostic tests for congenital Zika virus infection, such as geographic area, endemicity of Zika, and the reference standard used (See Investigations of heterogeneity).
Background
Target condition being diagnosed
Zika virus is a flavivirus transmitted primarily by Aedes spp. mosquitos in tropical areas; the most competent vector species is Aedes aegypti. Temperate regions where Aedes spp. mosquitos are endemic are also at risk for vector transmission (Theel 2018). More than half of the world’s population live in areas where this species of mosquito is present (WHO 2018b). Transmission also occurs through sexual contact, blood and blood product transfusion, organ transplantation, vertically from mother to fetus, and through exposure in a laboratory or healthcare setting (WHO 2018a). Symptoms generally present 3 to 12 days post‐exposure and last between 2 and 7 days. Clinical presentation is similar to that of other flaviviruses – dengue and chikungunya – with symptoms consisting of fever, rash, conjunctivitis, malaise, muscle and joint pain, and headache (WHO 2018b).
Congenital Zika virus transmission is defined as the prenatal vertical transmission of Zika virus (CDC 2017a). Congenital Zika virus infection has been found to increase the likelihood of various complications, such as fetal loss, stillbirth, preterm birth, and Congenital Zika Virus Syndrome (CZVS) WHO 2018b). CZVS presents as various structural, neurological, and opthalmological abnormalities (Chan 2016). Affected neonates may be born with these abnormalities, or they may develop them during the first month of life (Eppes 2017). Five features that occur almost exclusively with CZVS infections are: severe microcephaly with partial collapse of the skull, decreased brain tissue with a specific pattern of brain damage (including subcortical calcifications), damage to the back of the eye (including macular scarring and focal retinal pigmentary mottling), congenital contractures (e.g. clubfoot and arthrogryposis), and hypertonia (CDC 2019). Infants born to a symptomatic mother with Zika virus are equally likely to develop symptoms of CZVS as those born to an asymptomatic mother (Eppes 2017). CZVS symptoms have been noted to be more severe when the mother becomes infected with the virus during the first trimester, suggesting that infections that arise earlier in pregnancy are more deleterious for fetal and neonatal development (Reynolds 2017). Among liveborn infants with and without birth defects, 64% and 23%, respectively, presents neuroimaging abnormalities, but it is unknown in what extent this is related to long‐term effects of CZVS and future developmental outcomes (Moore 2017).
Index test(s)
Molecular or serological assays are the diagnostic tests primarily used to determine if a person has Zika virus (Table 1). Zika virus RNA has been detected in blood, urine, cerebrospinal fluid, amniotic fluid and tissue, semen, saliva, breast milk, and cervical and vaginal mucus (Eppes 2017; WHO 2017). There are two types of tests used to determine if a person is, or has been infected with Zika: nucleic acid testing (NAT) for the detection of viral RNA particles, or serological assays for the detection of Zika virus antibodies (Eppes 2017).
1. Types of Zika virus tests.
| Zika tests | Description |
| Molecular test | A nucleic acid test (NAT) or nucleic acid amplification test (NAAT) is a technique utilized to detect a particular nucleic acid, virus, or bacteria, which acts as a pathogen in blood, tissue, urine, etc. The NAT system differs from other tests in that it detects genetic materials rather than antigens or antibodies. Detection of genetic materials allows for an early diagnosis of a disease compared to serological assays, which require time for antibodies to appear in the bloodstream. Since the amount of a specific genetic material is usually very small, and difficult to detect at normal levels, NAT includes an amplification step of the genetic material, which increases the amount of genetic material to a detectable level. There are several ways of amplification, including polymerase chain reaction (PCR), strand displacement assay (SDA), or transcription mediated assay (TMA). For symptomatic persons with Zika virus infection, Zika virus RNA can sometimes be detected early in the course of illness. RNA NAT should be performed on paired serum and urine specimens. For symptomatic pregnant women with possible exposure to Zika virus, NAT should be performed concurrently with IgM serology. For asymptomatic pregnant women with ongoing possible exposure to Zika virus, NAT is recommended three times during pregnancy. For asymptomatic pregnant women with recent possible exposure, but no ongoing Zika virus exposure (i.e. travellers), NAT may be considered on a case‐by‐case basis, using a shared physician‐patient decision‐making model, and should follow a testing algorithm for symptomatic pregnant women. |
| Serologic test | Zika virus‐specific IgM and neutralizing antibodies typically develop toward the end of the first week of illness. IgM levels are variable, but generally can be detected in an assay starting 4 days after the onset of symptoms, or in asymptomatic cases, 4 days after exposure. IgM antibodies can be detected up to 12 weeks after symptom onset or exposure, and in some cases, longer. For symptomatic pregnant women, IgM serology is performed concurrently with NAT. For asymptomatic pregnant women, if testing is conducted, IgM serology should follow a testing algorithm for symptomatic pregnant women. For pregnant women with possible exposure to Zika virus who have a fetus with prenatal ultrasound findings consistent with congenital Zika virus infection, IgM serology should be performed on maternal serum concurrently with a NAT, following a testing algorithm for symptomatic pregnant women. For symptomatic nonpregnant individuals, testing should be performed on negative NAT samples collected < 14 days after onset of symptoms, or on samples collected ≥ 14 days after onset of symptoms. IgM testing is not recommended for asymptomatic nonpregnant individuals. |
| Plaque Reduction Neutralization Test (PRNT) | PRNT is performed on specimens from women with a negative Zika virus NAT (like Real Time Polymerase chain reaction (RT‐PCR)) and non‐negative serology results, including positive, presumptive positive, possible, equivocal, or inconclusive. These tests are performed at a laboratory that has independently demonstrated proficiency to perform a PRNT. |
The NAT Real Time Polymerase chain reaction (RT‐PCR) can detect acute infections in people 7 to 22 days after symptom onset. Compared to plasma or serum samples, whole blood and urine samples can detect viral RNA for longer periods of time (Theel 2018). Prolonged viraemia and viruria has been noted in pregnant women, for unknown reasons.
Maternal history of Zika virus infection is ideally established with a positive NAT result from a urine or serum sample, or with a combination of a positive or equivocal immunoglobulin M (IgM) serological assay result and a follow‐up plaque reduction neutralization test (PRNT), or some combination (CDC 2017b). PRNT is considered the reference standard for the diagnosis of non‐congenitally transmitted Zika virus infections, but given its technical difficulty, long turnaround time, and need for live viral culture, it is not always available (Theel 2018). It is also unable to distinguish between maternal and neonatal infection, due to transplacental antibody transfer. Maternal antibodies will wane in neonatal serum after about 18 months, and therefore, when a neonatal PRNT is positive at birth, repeating the test after 18 months can confirm or rule out infection. However, this method of testing cannot distinguish between congenital and postnatal infection, in areas with ongoing transmission of the virus (CDC 2017a).
Given the short time window for detection of NAT and the lack of widespread access to PRNT, serological assays to detect IgM antibodies are commonly used, notwithstanding their issues of cross‐reactivity with other flaviviruses. Most serological assays in Zika virus testing detect IgM class antibodies in serum or cerebrospinal fluid, and can detect antibodies starting, on average, about 10 to 14 days after symptom onset, up until 12 weeks after symptom onset. Immunoglobulin G (IgG) antibody serological assays exist and appear shortly after IgM antibodies, but test availability is limited (Eppes 2017).
Diagnostic recommendations and interpretations are dependent on the person’s clinical history, presentation, and risk of exposure (CDC 2017b; CDC 2017a). See Figure 1 for a detailed description of the diagnostic scheme for congenital Zika virus testing used by the Centers for Disease Control and Prevention (CDC) CDC 2017a). For the diagnosis of congenital Zika virus of neonates, information regarding the optimal test, timing, and sample source is unavailable. Testing for congenital Zika virus infection is performed either prenatally, using RT‐PCR to detect viral RNA in amniotic fluid, fetal, or placental tissues, or postnatally, directly testing the neonate, using RT‐PCR and IgM serology (CDC 2017b; CDC 2017a).
1.
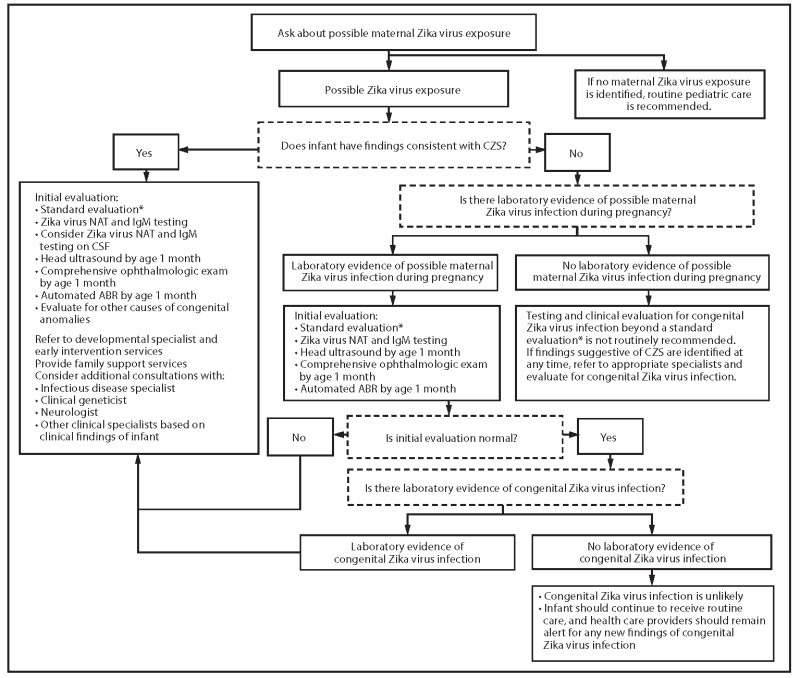
Recommendations for the evaluation of infants with possible congenital Zika virus infection, based on infant clinical findings, maternal testing results, and infant testing results; United States, October 2017 (CDC 2017a)
Prenatal and postmortem testing is performed on placental tissues, fetal tissues, and amniotic fluid, using the NAT RT‐PCR (CDC 2017b). The placenta is tested for live births of infants who present with symptoms of CZVS, born to mothers with an unspecified flavivirus, and on a case‐by‐case basis for infants without symptoms of CZVS. Fetal tissues are tested along with placental tissue, to help with diagnosis in cases of pregnancy loss, and infant death following a live birth. For cases where there was no evidence of maternal Zika virus infection, fetal and placental tissue testing is not recommended. Since there is limited information on the role of amniocentesis for the diagnosis of a fetal congenital Zika virus infection, the CDC recommends its use on a case‐by‐case basis (CDC 2017b). Information on the diagnostic accuracy of RT‐PCR testing on amniotic fluid, placenta, and post‐mortem neonatal tissues (brain, lung, and spinal cord) is unknown (Melo 2016).
Testing for congenital Zika virus infection postnatally is recommended if the neonate presents with symptoms of CZVS at birth, if the mother had a laboratory confirmed case for Zika virus, or both (CDC 2017a). See Table 2 for the laboratory interpretation of NAT and IgM serological assay results in neonates (CDC 2017a). These tests should be performed soon after birth, due to the short time window for detection RT‐PCR tests and waning IgM antibody titers, and to ensure the infection is congenital and was not acquired postnatally. Although cerebrospinal fluid has shown a higher sensitivity than blood and urine, its use in diagnostic testing is only recommended if the fluid was obtained for other reasons, because of the invasive nature of extraction procedures. The CDC does not recommend using cord blood as a test sample because of the uncertainty in distinguishing between maternal and congenital infection (CDC 2017b).
2. Interpretation of results of laboratory testing of infant’s blood, urine, or cerebrospinal fluid for evidence of congenital Zika virus infection.
| Infant test resulta | Interpretation (CDC 2017a) | |
| NAT | IgM | |
| Positive | Any result | Confirmed congenital Zika virus infectionb |
| Negative | Non‐negative | Probable congenital Zika virus infectionc,d |
| Negative | Negative | Congenital Zika virus infection unlikelyc,e |
Abbreviations: IgM = immunoglobulin M; NAT = nucleic acid test aInfant serum, urine, or cerebrospinal fluid bDistinguishing between congenital and postnatal infection is difficult in infants who live in areas where there is ongoing transmission of Zika virus and who are not tested soon after birth. If the timing of infection cannot be determined, infants should be evaluated as if they had congenital Zika virus infection. cLaboratory results should be interpreted in the context of timing of infection during pregnancy, maternal serology results, clinical findings consistent with congenital Zika syndrome, and any confirmatory testing with plaque reduction neutralization testing. dIf Zika virus plaque reduction neutralization test is negative, this suggests that the infant’s Zika virus IgM test is a false positive. eCongenital Zika virus infection is unlikely if specimens are collected within the first few days after birth and the clinical evaluation is normal; however, health care providers should remain alert for any new findings of congenital Zika virus infection.
Clinical pathway
Following the CDC guidelines, Figure 1 shows the clinical pathway and presents the context in which tests for Zika might be used postnatally (CDC 2017a), and Table 3 shows the context in which tests for Zika might be used prenatally for placental, fetal, or infant autopsy tissues. Amniocentesis is also recommended on a case‐by‐case situation for prenatal diagnostic purposes (CDC 2017b).
3. CDC guidance for Zika virus testinga of placentalb, fetal, or infant autopsy tissues for completed pregnancies with possible Zika virus exposure during pregnancyc.
| Pregnancy outcome | Maternal Zika virus test results on non‐tissue, clinical specimens (e.g. serum, urine) | |||
| Acute Zika virus infectiond |
Zika virus infection; timing of infection cannot be determinede Flavivirus infection; timing of infection cannot be determined |
> 12 weeks after symptom onset or exposure,f with either negative maternal Zika virus IgM, or no maternal testing conducted | No evidence of Zika virus infectiong | |
| Testing of placental tissues | ||||
| Live birth, possible Zika virus–associated birth defectsh | Not indicatedi | Should be considered an aid in maternal diagnosis | Not indicatedi | |
| Live birth, no obvious Zika virus–associated birth defects at birth | Not indicated | May be considered an aid in maternal diagnosis on a case‐by‐case and jurisdictional basis. Not routinely recommended for asymptomatic women with possible Zika virus exposure, but without ongoing possible exposure | Not indicated | |
| Testing of placental and fetal tissues | ||||
| Pregnancy loss, possible Zika virus–associated birth defects | May be considered an aid in fetal diagnosis | May be considered an aid in fetal and maternal diagnosis | Not indicatedi | |
| Pregnancy loss, no obvious Zika virus–associated birth defects | May be considered an aid in fetal diagnosis | May be considered an aid in fetal and maternal diagnosis | Not indicatedi | |
| Testing of placental and infant autopsy tissues | ||||
| Infant death following live birth | Should be considered an aid in infant diagnosis | Should be considered an aid in infant and maternal diagnosis | Not indicatedi | |
Abbreviations: IHC = immunohistochemistry; NAT = nucleic acid test; RT‐PCR = reverse‐transcription polymerase chain reaction aZika virus testing on formalin‐fixed, paraffin embedded tissue specimens is conducted at CDC’s Infectious Diseases Pathology Branch (IDPB) and includes Zika virus RT‐PCR on placental and fetal or infant tissues. Zika virus IHC may be performed on placental tissues into the second trimester, fetal tissues from any gestational age, and infant autopsy tissues. bPlacental tissues include placental disc, umbilical cord, and fetal membranes. Zika virus RNA can be focal within placental tissues, and testing of three sections of placenta, one section of umbilical cord, and one section of fetal membrane is recommended (www.cdc.gov/zika/laboratories/test-specimens-tissues.html). For pregnancy losses and infant deaths, submission of placental tissues, in addition to fetal or infant autopsy tissues, if available, is preferred, but if not available, will not preclude placental testing. cPossible Zika virus exposure includes travel to, or residence in an area with risk of Zika virus transmission during pregnancy or the periconceptional period (8 weeks before conception, or 6 weeks before the last menstrual period), or sex without a condom, during pregnancy or the periconceptional period, with a partner who travelled to, or resides in an area with risk of Zika virus transmission (www.cdc.gov/zika/geo/index.html). Zika virus testing is not routinely recommended for asymptomatic pregnant women with recent possible Zika virus exposure, but without ongoing exposure, and who have a fetus or infant without Zika virus–associated birth defects. dIn the event of a confirmed maternal acute Zika virus infection, or confirmed congenital Zika virus infection in the infant (e.g. a positive NAT), placental testing from live birth is not indicated. Currently, placental testing does not routinely provide additional diagnostic information in the setting of a maternal or infant diagnosis of acute or congenital Zika virus infection. eFor women with no possible Zika virus exposure before the current pregnancy, a positive IgM result likely represents acute Zika virus infection, and placental testing is not indicated. fAll, or part of possible maternal Zika virus exposure, or symptom onset occurred > 12 weeks before maternal serum specimen was collected. gIncludes pregnant women with negative Zika virus NAT, and negative Zika virus IgM ≤ 12 weeks after symptom onset or exposure. hPossible Zika virus–associated birth defects that meet the CDC surveillance case definition include the following: brain abnormalities, or microcephaly (or both), intracranial calcifications, ventriculomegaly, neural tube defects and other early brain malformations, eye abnormalities, or other consequences of central nervous system dysfunction, including arthrogryposis (joint contractures), congenital hip dysplasia, and congenital deafness (www.cdc.gov/zika/geo/pregnancy-outcomes.html). In all cases, infants or fetuses with possible Zika virus–associated birth defects should also be evaluated for other etiologies of congenital anomalies. iTesting may be considered on a case‐by‐case basis; consult CDC for case‐specific questions at www.cdc.gov/zika/laboratories/test-specimens-tissues.html.
Laboratory and clinical evaluation for congenital Zika virus infection are recommended for neonates if there is laboratory confirmation of maternal infection during pregnancy, the neonate presents with CZVS, or both. Laboratory testing is not recommended for neonates who do not present with CZVS, or those whose mothers were exposed during pregnancy, but do not have laboratory evidence of the Zika virus (CDC 2017a). During the initial examination, clinicians search for low birth weight, reduced fetal movement, arthrogryposis, brainstem dysfunction, brain atrophy, and optic nerve abnormalities (Chan 2016).
During the initial evaluation, performed within the first few days of birth, clinicians conduct nucleic acid testing on a sample of the neonate’s serum and urine, and serological assay for IgM antibodies. Infants who do not present with CZVS, but have a positive laboratory test for congenital Zika virus infection, will undergo the additional screening for symptomatic infants (CDC 2017a). Treatment for infants with CZVS primarily focuses on monitoring and addressing the signs and symptoms associated with CZVS. There is no cure for congenital Zika virus infection, nor the complications associated with it. However, it is still crucial to monitor all infected infants, including those who do not present with symptoms of CZVS at birth, to ensure that the infant's growth is optimized by identifying and addressing complications with therapy and a multidisciplinary team of medical support. For infants confirmed for congenital Zika virus infection or that present with CZVS, the recommended protocol is to monitor the neonate's health with neurodevelopmental evaluations at two to four weeks, three months, six months, nine months, 12 months, 18 months, and 24 months (WHO 2017).
Alternative test(s)
Neonates suspected of having congenital Zika virus infection will undergo additional tests to investigate signs and symptoms related to CZVS. These tests include a comprehensive ophthalmological examination, head ultrasound, and an automated auditory brainstem response evaluation (CDC 2017a). These tests are screening for complications related to congenital Zika virus infection, and alone, do not confirm a congenital infection. As previously stated, these symptoms are not fully understood and may develop postnatally, which means that infants who present with signs and symptoms not yet associated with CZVS, or infants who develop CZVS later in life, may not be diagnosed properly (Eppes 2017; Moore 2017). Therefore, the focus of this review will be on tests that can provide a definitive diagnosis of congenital Zika virus infection, rather than the additional tests that only screen for CZVS complications.
Rationale
There is limited information on the diagnostic accuracy of available laboratory tests for congenital Zika virus in fetuses and neonates. The World Health Organization (WHO) has established the sensitivity of laboratory tests, and the role these tests play in the diagnosis of infants affected by CZVS, as a research priority (WHO 2017). At present, there are no systematic reviews that address the accuracy of tests for congenital Zika virus.
The evidence must be assessed in light of the numerous limitations noted for the diagnosis of congenital Zika virus infection. RT‐PCR and IgM testing might only detect the Zika virus, on average, within 12 weeks after symptom onset; an affected neonate who was infected earlier in the pregnancy might test negative at birth. Thus, many infants may have been infected with the Zika virus in utero, and be at risk for developing complications associated with CZVS, but not be monitored for these complications since they tested negative at birth. As previously stated, the optimal assay, specimen, and timing for performing diagnostic assays on infants postnatally, remains unknown (CDC 2017a).The most accurate specimen source is cerebrospinal fluid, but for a neonatal population, this is not ideal (CDC 2017a). Thus, it is important to evaluate all sample sources to understand which sample source and assay is most suited for neonates.
This review aims to provide diagnostic accuracy information on the available tests, in order to improve the diagnostic pathway, and further understand the role of laboratory diagnostics for congenital Zika virus. Benefits of improving this diagnostic process will facilitate the rapid management of affected neonates, to ensure their optimal growth and development, especially those who do not present with signs and symptoms at birth but develop them later. This will also lead to studies that aim to understand the full clinical picture of CZVS.
Objectives
To determine the accuracy of laboratory tests for the diagnosis of congenital Zika virus infection.
Secondary objectives
To explore potential sources of heterogeneity, and specific factors that may influence the accuracy of diagnostic tests for congenital Zika virus infection, such as geographic area, endemicity of Zika, and the reference standard used (See Investigations of heterogeneity).
Methods
Criteria for considering studies for this review
Types of studies
We will include test accuracy studies that allow the comparison of results of one or more index test for congenital Zika virus infection versus a reference standard, including prospective and retrospective studies, studies in which all participants concurrently receive more than one index test and a reference standard, and studies that recruit a series of participants unselected by true disease status. We will exclude studies in which it is not possible to derive a 2 x 2 table of the number of true positives, false positives, false negatives, and true negatives; diagnostic case control studies; or studies that reported preliminary experimental findings, i.e. laboratory‐based studies.
Participants
We will include studies about fetuses, stillborn babies, or live birth newborns under suspicion of congenital Zika virus infection.
Index tests
We will assess current tests used for the laboratory diagnosis of congenital Zika virus infection in a fetus or neonate, or both, including the following:
Nucleic acid tests (NAT)
Immunoglobulin M (IgM) antibody assays
We will not evaluate serological assays that cannot distinguish between maternal and neonatal antibodies. We will include any threshold for deciding test positivity, either qualitative or quantitative.
Target conditions
The target condition for this review is congenital Zika virus infection, defined as the prenatal vertical transmission of Zika virus (CDC 2017a).
Reference standards
Given that there is not yet an established reference standard for the diagnosis of congenital Zika virus infection in neonates and fetuses, the following reference standards were developed, using an informal consensus‐building process with experts in the field of Zika diagnostics and congenital Zika virus infection (Appendix 1). After discussion, two sets of criteria were proposed, depending upon the availability of clinical and epidemiological evidence of infection. Reference standard A is to be used for those cases who only meet epidemiologic criteria, and Reference standard B is to be used for cases that meet both clinical and epidemiologic criteria. We also propose a Reference standard C to define a case of fetal death due to Zika virus infection. These clinical and epidemiologic criteria are outlined in Appendix 1.
Reference standard A for congenital Zika virus infectiona
An infant who does not meet the clinical criteria, but meets the epidemiologic criteria and has laboratory evidence of Zika virus infectionb, including:
Zika virus, viral antigen, or viral RNA detected in infant tissue, cerebrospinal fluid, blood, or urine collected within two weeks after birth;
Zika virus IgM and neutralizing antibodies detected in cerebrospinal fluid collected within two weeks after birth;
Zika virus IgM and neutralizing antibodies detected in blood collected within two weeks after birth, and Zika virus neutralizing antibodies in blood collected at ≥18 months of age.
Reference standard B for congenital Zika virus infection
An infant who meets the clinical and epidemiologic criteria, and has laboratory evidence of Zika virus infectionb, including:
Zika virus, viral antigen, or viral RNA detected in infant tissue, cerebrospinal fluid, blood, or urine collected within two weeks after birth;
Zika virus IgM and neutralizing antibodies detected in cerebrospinal fluid or blood collected within two weeks after birth;
Zika virus neutralizing antibodies detected in blood collected at ≥ 18 months of age.
Reference standard C for fetal deaths due to Zika virus
A fetal death that occurs after 20 weeks' gestation, or the fetus weighs > 500 grams, meets the clinical and epidemiologic criteria, and has Zika virus, viral antigen, or viral RNA in amniotic fluid, placental tissue, or fetal tissue.
Because these reference standards can be considered imperfect, we will conduct a latent class analysis (LCA (Baughman 2008; Black 2002; Dendukuri 2012)). In this case, a latent class model recognizes that the true disease status (i.e. the congenital Zika virus infection) is 'latent', or not observed. We present more information about LCA in the Statistical analysis and data synthesis section.
aIncludes infants who do not present with sufficient clinical criteria (Appendix 1) i.e. infants who do not present with clinical findings, and those who present with clinical findings that are less specific for congenital Zika virus syndrome (CZVS), such as other brain anomalies (e.g. ventriculomegaly, corpus callosum agenesis, cerebellar hypoplasia, mild microcephaly, or postnatal onset microcephaly), other eye anomalies (e.g. microphthalmia, cataracts, chorioretinal atrophy, or optic nerve hypoplasia), and neurological sequelae (e.g. hypertonia, dystonia, tremors, swallowing dysfunction, intellectual disability, hearing loss, or visual impairment). bLaboratory evidence of Zika virus infection in the infant excludes testing on amniotic fluid, placenta, umbilical cord, or cord blood.
Search methods for identification of studies
We have developed electronic searches, assisted by the Cochrane Neonatal's Information Specialist. We will use the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register). We will search for errata or retractions from included studies published in full‐text on PubMed (www.ncbi.nlm.nih.gov/pubmed), and report the date this was done in the review.
Electronic searches
A comprehensive and sensitive search strategy, using Cochrane Neonatal's standard search strategy for neonates, combined with terms for Zika virus, mothers, and fetuses, will be applied to search the following databases (see Appendix 2):
Cochrane Central Register of Controlled Trials (CENTRAL, current issue) in the Cochrane Library
PubMed and MEDLINE Ovid SP (1946 to current)
Embase Elsevier Ovid SP (1982 to current)
Latin American and Caribbean Health Science Information database (LILACS) iAH English BIREME (1982 to current)
CINAHL EBSCO (1984 to current)
We will not apply language restrictions. We will search clinical trial registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International (WHO)Trials Registry and Platform; and theISRCTN Registry).
Searching other resources
We will search for related additional studies among the references of all relevant studies. We will search references from the International Congress on Infectious Diseases over the last five years. We will contact experts in this topic to check if there are additional studies to consider besides our included studies. In addition, we will screen the following institutional websites and repositories for additional information:
Center for Infectious Disease Research and Policy (CIDRAP), University of Minnesota. This organization only deals with infectious diseases and is an excellent source for the latest news and research. Links are provided to information culled from all of the other organizations listed here—and more (see research and literature; www.cidrap.umn.edu; search Zika virus).
Centers for Disease Control and Prevention (CDC). The direct link to the Zika page will be checked for pregnant or travelling women. See 'What’s New' for the latest news regarding Zika virus. There are answers on this page to many questions, available in both English and Spanish (www.cdc.gov/zika/).
National Center for Advancing Translational Sciences (NCATS, National Institutes of Health), Genetic and Rare Diseases Information Center (GARD). This provides information about tests, treatment, and research, and visitors to the web page can even submit a question (rarediseases.info.nih.gov/. search Zika virus).
National Institute of Allergy and Infectious Diseases (NIAID, National Institutes of Health). NIAID is the lead institute at NIH for Zika (www.niaid.nih.gov; under Health and Research Topics A‐Z, choose Zika virus).
Pan American Health Organization (PAHO). PAHO serves as the Regional Office of the Americas for the WHO. It links to information from WHO, but also provides information specifically on the Americas in English or Spanish (www.paho.org/hq/; search Zika virus).
WHO. Information is provided in six languages: Arabic, Chinese, French, English, Russian, and Spanish (www.who.int/en/; search Zika virus).
Data collection and analysis
Selection of studies
Three review authors (MC, SM, SG) will independently screen the titles and abstracts retrieved from the database searches, excluding irrelevant articles. The full text of the remaining articles will then be reviewed by authors SN and SM for eligibility. All disagreements will be resolved through discussion, and if necessary, they will consult a third review author (AC) as an arbitrator. We will use Covidence to facilitate the process. When a study does not present enough relevant data to create a 2 × 2 table, we will contact the study authors directly, to request further information.
Data extraction and management
Review authors SM and SN will independently extract the following data from every article, using a pre‐designed data extraction form that will be piloted with at least five studies, if available, before using. This form will extract the following information:
Study ID, authors, year of publication
Geographical region
Study design and setting
Study participant recruitment method
Characteristics of study participants
Laboratory characteristics
Sample(s) tested
Reference standard
Index test(s)
True positive (TP), true negative (TN), false positive (FP), false negative (FN)
Indeterminate test results
We will resolve discrepancies in data extraction by discussion. If necessary, we will consult review author AC.
We will cross‐tabulate the numerical information in 2 × 2 tables for the index test results (positive or negative) against the target condition (positive or negative), and display results in Review Manager 5 (RevMan 5) tables (Review Manager 2014).
Assessment of methodological quality
We will assess the risk of bias of the included studies using the QUality Assessment of Diagnostic Accuracy Studies‐2 tool (QUADAS‐2) Whiting 2011). This tool is comprised of four domains: participant selection, index test, reference standard, and participant flow. Two review authors (SM and SG), who will be blinded to each other’s scores, will independently perform the 'QUADAS‐2' assessment. We will resolve any disagreements by discussion, or, if necessary, we will consult a third review author (AC), who will act as an arbitrator. We will assess each domain for risk of bias, and also consider the first three domains for applicability concerns. We will also pilot the tool on two included studies, and refine if needed. In Appendix 3, we describe the components of each of these domains, and how we will make judgements concerning risk of bias.
Statistical analysis and data synthesis
We will use a template for 2 x 2 tables, describing the TP, FP, FN, and TN results for each included study. From this, we will calculate sensitivity and specificity, with 95% confidence intervals (CIs), at the individual study level. We will present individual study results graphically, by plotting estimates of sensitivities and specificities in forest plots and summary receiver operating characteristic (ROC) plots. These analyses will enable visual assessment of the variation between studies, and will also facilitate investigations of heterogeneity to explore the effect of certain characteristics on test performance. We will enter data into Review Manager 5 software, and check it for accuracy (Review Manager 2014).
If there are sufficient data, we will perform meta‐analysis for each index test, using hierarchical models as recommended in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Macaskill 2010). If studies report a common threshold, we will perform meta‐analyses, using the bivariate model to estimate summary points (summary sensitivities and specificities (Chu 2006; Reitsma 2005)). We will fit the bivariate model using the 'meqrlogit' command in Stata version 15 (Stata). For each meta‐analysis, we will copy parameter estimates into Review Manager 2014 5 to produce a summary receiver operating characteristics (SROC) plot, showing the summary point (summary sensitivity and specificity), along with 95% CI and 95% prediction regions. We will consider fitting a univariate random‐effects model in a case of non‐convergence of the bivariate model estimation due to the small number of available studies (Takwoingi 2017). If thresholds vary considerably between the included studies, we will plot the sensitivity and specificity for each study in ROC space, and use the hierarchical summary receiver operating characteristic (HSROC) model to estimate a SROC curve. The HSROC model will be fitted using the PROC NLMIXED command in SAS software (SAS).
If data permit, we will compare test accuracy by including covariate terms in bivariate models, to estimate differences in sensitivity and specificity. We will assess the statistical significance of these differences by using likelihood ratio tests to compare models with, and without the covariate terms. If we estimate summary curves because studies reported different thresholds, we will compare summary curves instead of summary points, by including covariate terms in a HSROC model to assess differences in accuracy, threshold, shape of the curves, or a combination.
In additional analyses, we will use latent class meta‐analysis that allows for adjustment due to an imperfect reference standard, and accommodates different reference standards across studies. Latent class analysis (LCA) is a statistical modelling technique that allows the estimation of test accuracy in the absence of an adequate reference standard to define the presence or absence of disease (Black 2002; van Smeden 2014). The LCA model estimates the sensitivity and specificity of a set of diagnostic tests, based on observed frequencies in test patterns. As such, this technique provides a model‐based estimate of the reference standard classification (Baughman 2008; Dendukuri 2012). These analyses will be done using WinBUGS (Lunn 2000), or R version 3.4.2 (R).
Investigations of heterogeneity
Initially, we will investigate heterogeneity by visual inspection of the forest plots for sensitivity and specificity, and by evaluating the individual results of the studies in the ROC space, to examine the variability between studies, and the presence of a correlation between both indices (threshold effect). Assuming that a sufficient number of studies report study‐level covariates, we will investigate the effect of these, by including each of these factors as covariates in the model implemented for statistical analysis (detailed above). Anticipated sources of heterogeneity are:
Geographic area (WHO regions)
Zika endemicity (endemic versus non‐endemic regions)
Symptomatic versus asymptomatic cases
Reference standard used (see Reference standards)
Sensitivity analyses
To examine the robustness of the results to the decisions we made in the review process, we will conduct analyses with the following alternative decisions.
Analysis of studies that have a low risk of bias for the four QUADAS‐2 domains (participant selection, index test, reference standard, and flow and timing)
An analysis of the different definitions of laboratory history of maternal Zika virus infection, as defined by the authors
Assessment of reporting bias
We will not investigate reporting bias by means of statistical tests, due to the current uncertainty about how it operates in diagnostic test accuracy studies, and the interpretation of existing analytical tools, such as funnel plots. Instead, we will consult available study protocols to assess the reporting of preplanned outcomes.
History
Protocol first published: Issue 7, 2020
Acknowledgements
The Academic Editor is Professor Yemisi Takwoingi.
The editorial base of the Cochrane Infectious Diseases Group (CIDG) is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
We acknowledge Dr Fernando Althabe and Dr Nandini Dendukuri for their expert advice. We acknowledge the CIDG and Cochrane Neonatal Group, as well as the Cochrane Diagnostic Test Accuracy Editorial Team, for their help and editorial advice during the preparation of this systematic review protocol. We acknowledge the experts from the CDC Division of Vector‐borne Diseases, Dr Susan Hills, Dr Marc Fischer, Dr J Erin Staples, and Dr Cynthia Moore, as well as the representative from the WHO, Dr Ingrid Rabe, for their collaboration and contribution in the informal consensus‐building process to establish a reference standard for this review.
Appendices
Appendix 1. Procedure to establish a reference standard definition
This reference standard was developed using an informal consensus‐building process with experts in the field of Zika diagnostics and congenital Zika virus infection. These experts hold affiliations with United States Centers for Disease Control and Prevention and the World Health Organization. Experts were contacted via email and invited to participate in the construction of a reference standard for the purpose of this review. Once accepted, an introductory phone call was held. The purpose of this introductory call was to explain the objectives and methodology of this systematic review, the consensus‐building process, and their role. Participants were then given approximately one month to hold discussions and construct a reference standard definition, based on current clinical and laboratory knowledge. This definition was then sent to the systematic review team for commentary. An online meeting was held between the experts and the systematic review team to discuss this definition and come to a consensus. The meeting notes were compiled, and edits to this definition were made. This draft was then sent to the expert participants for their review. Comments on this draft were then made electronically, and returned to the systematic review team, who then made appropriate changes, and returned a final draft to all expert participants for their review and approval.
After discussion, two sets of criteria were proposed, depending upon the availability of clinical and epidemiological evidence of infection. Here are the clinical and epidemiological criteria.
| Definitions | |
| Clinical criteria |
|
| Epidemiologic criteria |
|
aSevere microcephaly is defined as an occipital frontal circumference > 3 standard deviations below the mean for age and sex. Mild microcephaly is defined as an occipital frontal circumference 2 to 3 standard deviations below the mean for age and sex. bWe accept the CDC Screening tool for possible Zika virus exposure during pregnancy www.cdc.gov/pregnancy/documents/zika-provider-screening-p.pdf
During your most recent pregnancy:
Did you travel to any area where the spread of Zika was a concern?c
Did you live in any area where spread of Zika was a concern?c
Did you have sex without a condom with someone who lives in or travelled to an area where the spread of Zika was a concern?c
If 'yes' to any of the questions above – did you have any symptoms of Zika (e.g. rash, fever, joint or muscle pain, headache, red eyes)?
If the mother answers 'yes' or 'unsure' to any of these questions, the mother had possible Zika virus exposure, and more detailed history or tests are warranted.
cAreas with risk of Zika can be found at the following website: wwwnc.cdc.gov/travel/page/zika-information. Please note that there is information for both USA and international locations.
Appendix 2. Search strategies
PubMed
| Search | Query |
| #21 | Search (#5 AND #20) |
| #20 | Search (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) |
| #19 | Search Mother*[tiab] |
| #18 | Search Mothers[Mesh] OR "Pregnancy"[Mesh] OR "Pregnancy Complications, Infectious"[Mesh] OR pregnan*[TIAB] |
| #17 | Search newborn*[TIAB] OR "new born"[TIAB] OR "new borns"[TIAB] OR "newly born"[TIAB] OR baby*[TIAB] OR babies[TIAB] OR premature[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR "pre term"[TIAB] OR “low birth weight”[TIAB] OR "low birthweight"[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR infant[TIAB] OR infants[TIAB] OR infantile[TIAB] OR infancy[TIAB] |
| #16 | Search Neonat*[tiab] |
| #15 | Search Infant, Newborn[Mesh] |
| #14 | Search Maternofetal[tiab] |
| #13 | Search Materno Fetal[tiab] |
| #12 | Search Maternal[tiab] |
| #11 | Search Intrauterine[tiab] |
| #10 | Search Congenital[tiab] |
| #9 | Search Fetomaternal[tiab] OR fetal[TIAB] OR fetus*[TIAB] OR "Fetus"[Mesh] |
| #8 | Search Mother‐to‐Child[tiab] |
| #7 | Search Vertical[tiab] |
| #6 | Search Infectious Disease Transmission, Vertical[Mesh] |
| #5 | Search (#1 OR #2 OR #3 OR #4) |
| #4 | Search Zika Virus Infection[Mesh] |
| #3 | Search Zika[tiab] |
| #2 | Search ZikV[tiab] |
| #1 | Search Zika Virus[Mesh] |
Embase
| No. | Query |
| #22 | #5 AND #21 |
| #21 | #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 |
| #20 | mother*:ti,ab |
| #19 | 'mother'/exp |
| #18 | 'newborn'/exp |
| #17 | newborn*:ti,ab |
| #16 | neonate*:ti,ab |
| #15 | 'newborn'/exp |
| #14 | maternofetal:ti,ab |
| #13 | 'materno fetal':ti,ab |
| #12 | maternal:ti,ab |
| #11 | intrauterine:ti,ab |
| #10 | congenital:ti,ab |
| #9 | fetomaternal:ti,ab |
| #8 | 'mother to child':ti,ab |
| #7 | vertical:ti,ab |
| #6 | 'vertical transmission'/exp |
| #5 | #1 OR #2 OR #3 OR #4 |
| #4 | 'zika fever'/exp |
| #3 | zika:ti,ab |
| #2 | zikv:ti,ab |
| #1 | 'zika virus'/exp |
LILACS (iAH English)
(MH Zika Virus OR ZikV OR Zika OR MH Zika Virus Infection) AND (MH Infectious Disease Transmission, Vertical OR Vertical OR Fetomaternal OR Congenit$ OR Intrauter$ OR Maternal OR MH Infant, Newborn OR Neonat$ OR Newborn$ OR Nacido$ OR Nascido$) [Words]
CINAHL EBSCO
| # | Query |
| S21 | S5 AND S20 |
| S20 | S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 |
| S19 | TI Mother* OR AB Mother* |
| S18 | (MH "Mothers+") |
| S17 | TI Newborn* OR AB Newborn* |
| S16 | TI Neonate* OR AB Neonate* |
| S15 | (MH "Infant, Newborn+") |
| S14 | TI Maternofetal OR AB Maternofetal |
| S13 | TI Materno Fetal OR AB Materno Fetal |
| S12 | TI Maternal OR AB Maternal |
| S11 | TI Intrauterine OR AB Intrauterine |
| S10 | TI Congenital OR AB Congenital |
| S9 | TI Fetomaternal OR AB Fetomaternal |
| S8 | TI Mother to Child OR AB Mother to Child |
| S7 | TI Vertical OR AB Vertical |
| S6 | (MH "Disease Transmission, Vertical") |
| S5 | S1 OR S2 OR S3 OR S4 |
| S4 | (MH "Zika Virus Infections") |
| S3 | TI Zika OR AB Zika |
| S2 | TI ZikV OR AB ZikV |
| S1 | (MH "Zika Virus") |
Appendix 3. QUality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) tool
| Domain | Participant selection | Index test | Reference standard | Flow and timing |
| Description | Describe methods of participant selection: describe included participant's (prior testing, presentation, intended use of index test and setting) | Describe the index test and how it was conducted and interpreted (where the laboratory test was applied, by whom, who interpreted the results of the test, what was considered a positive result) | Describe the reference standard and how it was conducted and interpreted (what is the reference standard used, definition of positive result, who interpreted the results of the reference standard, etc) | Describe any participants who did not receive the index test(s) or reference standard, or both, or who were excluded from the 2 × 2 table (refer to flow diagram): describe the time interval and any interventions between index test(s) and reference standard |
| Signalling questions (yes/no/unclear) | Was a consecutive or random sample of neonates enrolled? Yes = a consecutive or random sample of neonates was enrolled Unclear = insufficient information provided No = selected sample of neonates were analysed |
Were the congenital Zika virus test results interpreted without knowledge of the results of the reference standard? Yes = the index test was interpreted without knowledge of the reference standard findings Unclear = insufficient information provided No = the index test was interpreted with knowledge of the reference standard findings, or the index test was part of the reference standard |
Is the reference standard likely to correctly classify the target condition? Yes = a reference standard from those listed in Methods was applied Unclear = insufficient information provided No = other criteria, even isolated findings, were applied to diagnose CZV |
Was there an appropriate interval between index test(s) and reference standard? Yes = the index test and the reference standard are administered within 2 weeks after birth, or at the same time Unclear = insufficient information provided No = the index test and the reference standard were administered in different times after birth (> 2 weeks) |
| Did the study avoid inappropriate exclusions? Yes = all neonates who were candidates to be tested were included Unclear = insufficient information provided No = the study excluded neonates who were candidates for testing |
If a threshold was used, was it prespecified? Yes = the positivity threshold was established before the administration of the index test Unclear = insufficient information provided No = the positivity threshold was not established after the administration of the index test results (i.e. optimal cut‐off) |
Were the reference standard results interpreted without knowledge of the results of the index test? Yes = the reference standard was interpreted without knowledge of the index test results Unclear = insufficient information provided No = the reference standard was interpreted with knowledge of the index test results, or the index test was part of the reference standard |
Did all participants receive a reference standard? Yes = a reference standard was applied regardless of the index test results Unclear = insufficient information provided No = only a sample of neonates received the reference standard (for example, those with positive index test results) |
|
| Did all participants receive the same reference standard? Yes = a unique reference standard was applied, regardless of the index test results Unclear = insufficient information provided No = more than one reference standard was applied, mostly depending on index test results | ||||
| Were all participants included in the analysis? Yes= All neonates who were enrolled were analysed Unclear = insufficient information provided No = the study excluded participants from final analysis (i.e. indeterminate results) | ||||
| Risk of bias (high/low/unclear) | Could the selection of participants have introduced bias? | Could the conduct or interpretation of the index test have introduced bias? | Could the reference standard, its conduct, or its interpretation have introduced bias? | Could the participant flow have introduced bias? |
| Concerns regarding applicability (high/low/unclear) | Are there concerns that the included participants did not match the review question? | Are there concerns that the index test, its conduct, or interpretation differed from the review question? | Are there concerns that the target condition, as defined by the reference standard, did not match the review question? |
Contributions of authors
All authors contributed to drafting the protocol, and read and approved the final version for publication.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
-
Instituto de Efectividad Clínica y Sanitaria (IECS‐CONICET), Argentina
Librarian, Technical support
External sources
-
Department for International Development, UK
Project number 300342‐104
Declarations of interest
AC has no conflicts of interest to declare.
SM has no conflicts of interest to declare.
MLC has no conflicts of interest to declare.
DC has no conflicts of interest to declare.
LG has no conflicts of interest to declare.
SNG has no conflicts of interest to declare.
PB has no conflicts of interest to declare.
IAR has no conflicts of interest to declare.
New
References
Additional references
Baughman 2008
- Baughman AL, Bisgard KM, Cortese MM, Thompson WW, Sanden GN, Strebel PM. Utility of composite reference standards and latent class analysis in evaluating the clinical accuracy of diagnostic tests for pertussis. Clinical and Vaccine Immunology 2008;15(1):106-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 2002
- Black MA, Craig BA. Estimating disease prevalence in the absence of a gold standard. Statistics in Medicine 2002;21(18):2653-69. [DOI] [PubMed] [Google Scholar]
CDC 2017a
- Adebanjo T, Godfred-Cato S, Viens L Fischer M, Staples JE, Kuhnert-Tallman W, et al. Update: Interim Guidance for the Diagnosis, Evaluation, and Management of Infants with Possible Congenital Zika Virus Infection — United States, (Including US Territories), July 2017. MMWR Morbidity and Mortality Weekly Report 2017;66(29):1089-99. [DOI: 10.15585/mmwr.mm6641a1 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2017b
- Oduyebo T, Polen KD, Walke HT, Reagan-Steiner S, Lathrop E, Rabe IB, et al. Update: Interim guidance for health care providers caring for pregnant women with possible Zika virus exposure – United States (Including U.S. Territories), July 2017. MMWR. Morbidity and Mortality Weekly Report 2017;66(29):781-93. [DOI: 10.15585/mmwr.mm6629e1] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2019
- Centers for Disease Control and Prevention (CDC). Congenital Zika Syndrome & Other Birth Defects. www.cdc.gov/pregnancy/zika/testing-follow-up/zika-syndrome-birth-defects.html (accessed prior to February 27, 2020).
Chan 2016
- Chan JF, Choi GK, Yip CC, Cheng VC, Yuen KY. Zika fever and congenital Zika syndrome: an unexpected emerging arboviral disease. Journal of Infection 2016;72(5):507-24. [DOI: 10.1016/j.jinf.2016.02.011] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiolgy 2006;59(12):1331-2. [DOI: 10.1016/j.jclinepi.2006.06.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed prior to 22 January 2018. Melbourne, Australia: Veritas Health Innovation.Available at www.covidence.org.
Dendukuri 2012
- Dendukuri N, Schiller I, Joseph L, Pai M. Bayesian meta-analysis of the accuracy of a test for tuberculous pleuritis in the absence of a gold standard reference. Biometrics 2012;68(4):1285-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eppes 2017
- Eppes C, Rac M, Dunn J, Versalovic J, Murray KO, Suter MA, et al. Testing for Zika virus infection in pregnancy: key concepts to deal with an emerging epidemic. American Journal of Obstetrics and Gynecology 2017;216(3):209-25. [DOI: 10.1016/j.ajog.2017.01.020] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lunn 2000
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS. A Bayesian modelling framework: concepts, structure, and extensibility. Statistics and Computing 2000;10:325-37. [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In Deeks JJ, Bossuyt PM, Gatsonis C, editor(s), Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2010. Available from srdta.cochrane.org.
Melo 2016
- Melo AS, Aguiar RS, Amorim MM, Arruda MB, Melo FO, Ribeiro ST, et al. Congenital Zika virus infection: beyond neonatal microcephaly. JAMA Neurology 2016;73(12):1407-16. [DOI: 10.1001/jamaneurol.2016.3720] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moore 2017
- Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatrics 2017;171(3):288-95. [DOI: 10.1001/jamapediatrics.2016.3982] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
R [Computer program]
- R Foundation for Statistical Computing R: A language and environment for statistical computing. Version 3.4.2. Vienna, Austria: R Foundation for Statistical Computing, 2017.Available at www.R-project.org.
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiolgy 2005;58(10):982-90. [DOI: 10.1016/j.jclinepi.2005.02.022] [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reynolds 2017
- Reynolds MR, Jones AM, Petersen EE, Lee EH, Rice ME, Bingham A, et al. Vital signs: update on Zika virus-associated birth defects and evaluation of all U.S. infants with congenital Zika virus exposure - U.S. Zika Pregnancy Registry, 2016. MMWR Morbidity and Mortality Weekly Report 2017;66(13):366-73. [DOI: 10.15585/mmwr.mm6613e1] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
SAS [Computer program]
- SAS Institute SAS software. Version 9.4. Cary (NC): SAS Institute, 2014.Available at www.sas.com/en_ca/home.html.
Stata [Computer program]
- Stata. Version 15. College Station, TX, USA: StataCorp, 2017.Available at www.stata.com.
Takwoingi 2017
- Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta‐analysis of diagnostic test accuracy with few studies or sparse data. Statistical Methods in Medical Research 2017;26(4):1896-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Theel 2018
- Theel ES, Hata DJ. Diagnostic testing for Zika virus: a postoutbreak update. Journal of Clinical Microbiology 2018;56(4):e01972-17. [DOI: 10.1128/JCM.01972-17] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Smeden 2014
- Smeden M, Naaktgeboren CA, Reitsma JB, Moons KGM, Groot JAH. Latent class models in diagnostic studies when there is no reference standard – a systematic review. American Journal of Epidemiology 2014;179(4):423-31. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. [DOI: 10.7326/0003-4819-155-8-201110180-00009] [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2017
- World Health Organization (WHO). WHO toolkit for the care and support of people affected by complications associated with Zika virus. Geneva: World Health Organization, 2017. [Google Scholar]
WHO 2018a
- World Health Organization (WHO). Zika virus. www.who.int/news-room/fact-sheets/detail/zika-virus (accessed prior to 22 January 2019).
WHO 2018b
- World Health Organization (WHO). Mosquito-borne diseases. www.who.int/neglected_diseases/vector_ecology/mosquito-borne-diseases/en/ (accessed prior to 22 January 2019).


