Abstract
Background
Extremely premature infants are at risk of transient hypothyroxinaemia in the first weeks after birth. These low thyroid hormone levels are associated with an increased incidence of neonatal morbidity, mortality and longer term developmental impairments. Thyroid hormone therapy might prevent these problems.
Objectives
To determine the evidence for thyroid hormone therapy in preterm infants with transient hypothyroxinaemia (low thyroid hormone level, normal TSH) for improvement of neonatal outcomes and neurodevelopment.
Search methods
Searches were performed of The Cochrane Central Register of Controlled (CENTRAL, The Cochrane Library, Issue 1, 2006), MEDLINE (1966 ‐ March 2006), PREMEDLINE (March 2006), EMBASE (1980 ‐ March 2006), previous reviews including cross references, abstracts and conference proceedings, supplemented by requests to expert informants.
Selection criteria
Trials enrolling preterm infants with transient hypothyroxinaemia (low thyroid hormone level, normal TSH level) in the neonatal period, using random or quasi‐random patient allocation to thyroid hormone therapy compared to control (placebo or no treatment).
Data collection and analysis
Independent assessment of trial quality and data extraction by each review author. Synthesis of data using relative risk (RR) and weighted mean difference (WMD) using standard methods of the Cochrane Collaboration and its Neonatal Review Group.
Main results
Only one study was eligible. Chowdhry (1984) enrolled 23 infants < 1250 g and 25 ‐ 28 weeks gestation with transient hypothyroxinaemia (serum total T4 ≤ 4 μg/dl and TSH ≤ 20 IU/L). Infants were randomised to thyroxine 10 μg/kg/day or placebo beginning on day 15 and continuing daily for seven weeks. Chowdhry (1984) reported no neonatal mortality and one infant death in each group prior to discharge. No significant difference was reported in CLD at 28 days or 36 weeks, patent ductus arteriosus, necrotising enterocolitis, retinopathy or prematurity, weight gain, growth in head circumference or length. No significant difference was reported for mean T4 levels between thyroxine and placebo treated infants on day 21, 35, 49, 63 and 77 after birth. Free T4 was not measured. Neurodevelopmental follow up was inadequate to draw any conclusions from.
Authors' conclusions
There is insufficient evidence to determine whether use of thyroid hormones for treatment of preterm infants with transient hypothyroxinaemia results in changes in neonatal morbidity and mortality, or reductions in neurodevelopmental impairments. Further research is required.
Plain language summary
Postnatal thyroid hormones for preterm infants with transient hypothyroxinaemia
A systematic overview of randomised trials does not provide sufficient evidence to determine whether thyroid hormone treatment of preterm infants with transiently low thyroid hormone levels results in changes in neonatal outcomes or reductions in developmental impairments. Extremely premature infants frequently have transiently low thyroid hormone levels in the first weeks after birth. These low thyroid hormone levels are associated with an increased incidence of complications and death in the newborn period and longer term developmental impairments. Thyroid hormone therapy might prevent these problems. One small trial comparing thyroid hormone treatment to no treatment of infants with transiently low thyroid hormone levels reported no benefit from treatment of these infants. However, this is insufficient evidence to determine if thyroid hormone treatment is effective. Further research is needed.
Background
In preterm infants, values for serum T4 and free T4 (FT4) in the first days after birth vary directly with gestation (Rooman 1996; Reuss 1997). However, unlike term infants, the concentrations of T4 and FT4 reach a nadir between day 10 and 14 after birth that is more severe at lower gestations and birth weights (Frank 1996; Rooman 1996). Thyroid hormone levels then tend to return to normal levels after three weeks, but continue to increase up to six to eight weeks after birth (van Wassenaer 1997). Reuss 1997 found the incidence of infants with severely depressed T4 values (below 4 mg/dL) ranged from 40% at 23 weeks gestation to 10.2% at 28 weeks gestation. Furthermore, the levels of T4 and FT4 found in premature infants are lower than those seen in the normal fetus at similar gestational ages (Ballabio 1989; Thorpe‐Beeston 1991; Radunovic 1991). There is no consensus definition for levels of thyroid hormones consistent with 'transient hypothyroxinaemia' in preterm infants.
This period of low thyroid hormone levels in infants born prematurely has been termed "transient hypothyroxinaemia of prematurity". Suggestions as to the cause of these low thyroid hormone values include an immaturity of the hypothalamic‐pituitary‐thyroid axis in the extremely premature infant or a form of "nonthyroidal illness" (the "sick euthyroid syndrome") reflecting the infant's response to severe illness (monograph review by Paneth 1998). Risk factors for transient hypothyroxinaemia reported in observational studies have included lower gestational age (Franklin 1986; Rooman 1996; Reuss 1997; Paul 1998; Kantor Herring 2003; Filippi 2004), maternal pre‐eclampsia with placental insufficiency (Belet 2003), fetal growth restriction (Uhrmann 1981), perinatal asphyxia (Tahirovic 1994), respiratory distress syndrome (Uhrmann 1981; Franklin 1986), more severe respiratory disease (Reuss 1997), mechanical ventilation (Reuss 1997; Kantor Herring 2003), low diastolic blood pressure (Reuss 1997) and dopamine infusions (Filippi 2004; Kantor Herring 2003). Adverse neonatal outcomes associated with transient hypothyroxinaemia have included intraventricular haemorrhage (Paul 1998; Paul 2000), chronic lung disease (Reuss 1997) and death (Reuss 1997; Paul 1998; Hsu 1999).
Transient hypothyroidism (low T4, high thyrotropin concentration) also occurs in up to 5% of neonates admitted to neonatal intensive care (Rooman 1996), 0.4% of infants with birth weights < 1500 g (Frank 1996) and 1.8% of infants born < 29 weeks gestation (Reuss 1997). There is evidence to suggest that this may be due to exposure to iodine‐containing antiseptics used in neonatal care (Linder 1997).
Thyroid hormones are necessary for the normal growth and maturation of the central nervous system (monograph reviews by Porterfield 1993 and Beranl 1995). Congenital hypothyroidism is strongly associated with abnormal neurodevelopment. Neurological cretinism in its severest form is characterised by profound mental retardation, deaf mutism, spastic diplegia and squint (Porterfield 1993). Even children who receive early thyroid hormone replacement therapy for congenital hypothyroidism have motor and cognitive deficits that persist to late childhood (Kooistra 1994). Infants born prematurely also have a high incidence of motor and cognitive deficits that are worse at lower gestations (Lorenz 1998). The question is whether the transient decrease in serum concentration of free T4 that occurs in some preterm infants during the first weeks after birth contributes to these neurodevelopmental problems. Three cohort studies (Lucas 1988; Lucas 1996, Meijer 1992; Den Ouden 1996; Reuss 1996) have documented an association between low thyroid hormone levels (T3 or T4) in the first weeks after birth and abnormal neurodevelopmental outcome. All three cohorts documented a measure of abnormal mental development in children who had low neonatal thyroid hormone levels. One study (Reuss 1996) found a 4.4 fold increase in risk of disabling cerebral palsy at two years. Den Ouden 1996 in the same cohort found children who had low neonatal T4 levels to have an increased risk of school failure at nine years. The associations in the cohorts persisted despite correction for potential confounders including gestation, measures of fetal growth (either birth weight or presence of growth restriction) and, in some studies, factors relating to severity of illness in preterm infants and independent risk factors for abnormal neurodevelopmental outcome.
Whether transient hypothyroxinaemia of prematurity is a causative factor for abnormal neurodevelopment is uncertain. Transient hypothyroxinaemia of prematurity is strongly correlated with low gestation and is more frequent in infants with respiratory distress syndrome and in ventilated infants. It may be that transient hypothyroxinaemia of prematurity is secondary to low gestation and/or illness severity in preterm infants. It is possible that other factors that are responsible for the abnormal neurodevelopmental outcome in preterm infants have not been taken into account adequately in the cohort studies.
This review examined the evidence from randomised and quasi‐randomised controlled trials of thyroid hormone therapy in preterm infants with transient hypothyroxinaemia (low thyroid hormone level, normal TSH) for improvement of neonatal outcomes and neurodevelopment. Thyroid hormones have effects not only on neurological development, but also on the respiratory and cardiovascular systems, and somatic growth. Therefore, studies that examined either neonatal morbidity, mortality and/or long term neurodevelopment were eligible for inclusion in this review. Separate reviews address the use of prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants (Osborn 2007a), and postnatal thyroid hormones for treatment of preterm infants with respiratory distress syndrome (Osborn 2007b).
Objectives
To assess whether thyroid hormone therapy in preterm infants with suspected hypothyroxinaemia (documented low thyroid hormone level, normal TSH) results in clinically important changes in neonatal and long term outcomes in terms of both benefits and harms.
Separate comparisons were planned to investigate the evidence for the use of different thyroid hormone preparations, doses and timing of treatment. Subgroup analysis were planned to investigate the evidence for a gestational age specific effect of treatment; and evidence for differences in effect according to study quality.
Methods
Criteria for considering studies for this review
Types of studies
Trials using random or quasi‐random patient allocation to treatment or control.
Types of participants
Studies that enrolled preterm infants (< 37 weeks gestation) with transient hypothyroxinaemia (documented low thyroid hormone levels, normal TSH) in the neonatal period (< 28 days). Levels of thyroid hormones that will be taken as consistent with the objectives of this review will include infants with levels of T4 or FT4 below the lowest quartile reported in cohorts of infants according to gestation and postnatal age, associated with a TSH ≤ 20 IU/L.
Types of interventions
Thyroid hormone therapy compared to control (placebo or no therapy). Thyroid hormone therapy could be either T4, T3 or both. Trials that compared different thyroid hormone regimens will also be included. Trials that included other co‐interventions performed differentially in the treatment and control groups were excluded.
Types of outcome measures
Primary clinical outcome measures were mortality (either neonatal or prior to discharge) and neurodevelopmental status at follow up. Neurodevelopmental outcome was categorised as:
Abnormal mental developmental after 12 months corrected age (a development or intelligence quotient > 2 standard deviations below the mean of a standardised test)
Abnormal neurological outcome (infants with either abnormal mental development or definite cerebral palsy)
Motor deficits
Sensorineural impairments including:
hearing deficit requiring aids
visual acuity < 6/60
Secondary outcome measures included important neonatal morbidities and measures of potential adverse effects of thyroid hormone treatment:
Severity of respiratory disease (e.g. duration of ventilatory support if ventilated or on CPAP at time of enrolment, duration of oxygen therapy)
Chronic lung disease defined as oxygen at 28 days post‐natal age
Chronic lung disease defined as oxygen or respiratory support at near term (36 ‐ 40 weeks) corrected age
Periventricular leucomalacia diagnosed by ultrasound or postmortem
MRI detected white matter abnormality at term
Symptomatic patent ductus arteriosus (PDA) after day three after birth treated by indomethacin or ibuprofen or ligation
Necrotising enterocolitis (at least stage 2 Bell's criteria)
Retinopathy of prematurity including all stages and severe (stage 3 or greater)
Growth including growth in weight (g/kg/day), head circumference (cm/week) and length (cm/week)
Adverse effects of thyroid hormones including tachycardia, pyrexia or cardiovascular collapse
In addition, effects of the various strategies of thyroid hormone dosing on thyroid hormone levels including total triiodothyronine (T3), total thyroxine (T4), free triiodothyronine (FT3), free thyroxine (FT4), reverse triiodothyronine (rT3) and thyrotropin (TSH) were performed.
Search methods for identification of studies
The standard search strategy of the Neonatal Review Group was used. This included searches of the Oxford Database of Perinatal Trials, Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2006), MEDLINE (1966 ‐ March 2006), PREMEDLINE (March 2006), EMBASE (1980 ‐ March 2006), previous reviews including cross references, abstracts, conferences (SPR‐PAS and PSANZ 1998 ‐ 2005), expert informants (authors of published trials and neonatologists) and journal handsearching in the English language.
MEDLINE was searched using MeSH terms '(infant‐newborn or infant‐premature) and (thyroxine or triiodothyronine)' and text words using '(hypothyroxinemia or hypothyroxinaemia or thyroxine or triiodothyronine) and [MeSH terms] (infant‐newborn or infant‐premature)'. EMBASE and PREMEDLINE using terms '(hypothyroxinemia or hypothyroxinaemia or thyroxine or triiodothyronine) and newborn. The Oxford Database of Perinatal Trials was searched using the term 'thyroid disease', and the Cochrane Central Register of Controlled Trials Register using 'thyroxine or triiodothyronine or hypothyroxinemia or hypothyroxinaemia'. No language restriction was applied. Abstracts of trials were eligible for inclusion.
Data collection and analysis
Assessment of trial quality, data extraction and synthesis of data, using relative risk (RR) and weighted mean difference (WMD), was performed using standard methods of the Cochrane Collaboration and its Neonatal Review Group. Each identified trial was assessed for methodological quality with respect to a) masking of allocation b) masking of intervention c) completeness of follow up d) masking of outcome assessment. Parveen Chowdhry (Chowdhry 1984) was contacted to obtain details of methodology. Data was synthesised using relative risk (RR), risk difference (RD) and weighted mean difference (WMD) where appropriate. From 1/RD the number needed to treat (NNT) for benefits and the number needed to harm (NNH) for adverse effects were calculated. All results given included 95% confidence intervals unless otherwise stated. Results are reported using a fixed effect analysis. Heterogeneity was looked at using the I‐squared statistic. The source of heterogeneity was explored in subgroup analysis of trials according to type of infant enrolled (gestation), thyroid hormone and dose regimen used and methodological quality of trial.
Separate comparisons were planned for the following:
Postnatal thyroid hormones (any type of preparation) versus control (no treatment or placebo), all dosing strategies
Postnatal thyroid hormones (any type of preparation) versus control (no treatment or placebo), according to dosing strategy used
Postnatal thyroid hormones versus other thyroid hormone strategy (e.g. T3 and T4 versus T4 alone)
Subgroup analysis were planned to determine if there is a gestational age effect of thyroid hormone treatment with separate analysis of trials enrolling infants:
< 28 weeks gestation
28 ‐ 31 weeks gestation
> 31 weeks gestation
Subgroup analysis was performed for those trials using good methodology as defined by the use of a random method of allocation to treatment or control, if steps are taken to ensure allocation concealment, if there was adequate blinding or treatment and if there is at least 90% follow up of survivors.
Results
Description of studies
Fourteen reports were excluded (see 'Table of Excluded Studies'). Two excluded trials (Amato 1988; Amato 1989) enrolled infants with respiratory distress syndrome and are included in the review 'Postnatal thyroid hormones for respiratory distress syndrome in preterm infants (Osborn 2007b). Four excluded studies (Smith 2000; Valerio 2004; van Wassenaer 1997; Vanhole 1997) enrolled preterm infants on gestational age criteria without documented transient hypothyroxinaemia and are included in the review 'Prophylactic postnatal thyroid hormone treatment for prevention of morbidity and mortality in preterm infants' (Osborn 2007a). Biswas 2003 was excluded as preterm infants were randomised to both thyroid hormone and hydrocortisone treatment. One study (Chowdhry 1984) enrolling infants with transient hypothyroxinaemia was included.
Infants:Chowdhry 1984 enrolled 23 preterm infants < 1250 g and 25 ‐ 28 weeks gestation with transient hypothyroxinaemia (serum total T4 ≤4 μg/dl and TSH ≤20 IU/L on 2 separate occasions).
Treatment: Infants were randomised to thyroxine 10 μg/kg/day IM or orally if tolerating feeds or placebo from day 15 for approximately seven weeks.
Outcomes: Neonatal mortality, weight gain (g/day), growth in head circumference and length (cm/week) were reported. Neurodevelopment was reported at 12 months, but 15 infants were lost to follow up so data are not reported in the review.
Risk of bias in included studies
Chowdhry 1984 reported adequate randomisation and allocation concealment procedures, blinded intervention and measurement by use of placebo and reported all infants for neonatal outcomes. However only eight (35%) infants at 12 months and four infants at 24 months had neurodevelopmental outcomes reported.
Effects of interventions
Postnatal thyroid hormones versus no thyroid hormones (all dosing strategies) (Comparison 01):
Primary outcomes:Chowdhry 1984 reported no neonatal mortality, and one infant death in each group before discharge (RR 1.09, 95% CI 0.08, 15.41). Neurodevelopmental outcomes are not reported due to excess losses. Secondary outcomes:Chowdhry 1984 reported no significant difference in CLD at 28 days (RR 0.94, 95% CI 0.45, 1.92) or CLD at 36 weeks (RR 0.73, 95% CI 0.15, 3.57), patent ductus arteriosus (RR 0.62, 95% CI 0.25, 1.56), necrotising enterocolitis (RR 0.36, 95% CI 0.02, 8.04), retinopathy or prematurity (RR 0.73, 95% CI 0.15, 3.57), weight gain (MD 0.51 g/day, 95% CI ‐2.55, 3.57), growth in head circumference (MD 0.02, 95% CI ‐0.15, 0.19) or length (MD ‐0.06, 95% CI ‐0.24, 0.12). No significant difference was reported for mean T4 levels between thyroxine and placebo treated infants on day 21, 35, 49, 63 and 77 after birth. Free T4 was not measured. Analysis according to dosing strategy used:Chowdhry 1984 randomised infants to thyroxine 10 mg/kg/day IM or orally if tolerating feeds or placebo beginning on day 15 and continuing for approximately seven weeks. No other comparisons were possible. Subgroup analyses:Chowdhry 1984 enrolled infants 25 ‐ 28 weeks gestation so the results are largely applicable to the lower gestational strata (< 28 weeks). Chowdhry 1984 met prespecified criteria for adequate methodology including adequate randmisation and allocation concealment, blinding of treatment and < 10% losses to follow up for neonatal mortality and morbidity (but not neurodevelopmental follow up).
Discussion
One underpowered randomised trial reported no significant difference in mortality before discharge or any neonatal morbidity in infants with transient hypothyroxinaemia treated with thyroxine 10 μg/kg/day beginning on day 15 and continuing for seven weeks. However, neurodevelopmental follow up was inadequate so that no conclusions can be made for long term effects. The mean gestation of enrolled infants was 26.3 weeks, so infants were of a gestation that researchers hypothesise may benefit from thyroid hormone supplementation (van Wassenaer 2004). The dose of thyroxine 10 μg/kg/day was higher than reported in a trial of prophylactic thyroxine treatment (van Wassenaer 1997). However, there was no measurable effect on total T4 levels. Free T4 and free T3 levels were not measured so effects on active thyroid hormone levels are unclear. In addition, treatment was started on day 15 and mean T4 levels only reached 5 μg/dl on day 35, suggesting that infants were still exposed to a prolonged period of hypothyroxinaemia. Given the lack of clinical effect, the small size of the study, and that free T4 and free T3 levels were not measured, it is not possible to draw any conclusions about the effectiveness of thyroid hormone treatment for transient hypothyroxinaemia, or any conclusions regarding timing and dose for future trials.
A separate review (Osborn 2007a) found no significant effect of prophylactic postnatal thyroid hormone treatment in preterm infants on neonatal morbidity, mortality or neurodevelopmental outcome. Additionally, no significant effect was found from the use of thyroid hormones in preterm infants with respiratory distress syndrome, although neurodevelopmental outcomes were not reported by the two included trials (Osborn 2007b). Taken together, these reviews find no evidence of benefit from the use of postnatal thyroid hormones for the prevention (prophylactic use) or treatment of transient hypothyroxinaemia in preterm infants. In contrast, treatment of congenital hypothyroidism is essential to prevent subsequent neurodevelopmental impairment (Beranl 1995; Kooistra 1994; Porterfield 1993). However, this review found insufficient data to determine whether there is an effect on neurodevelopment from thyroid hormone treatment of preterm infants with transient hypothyroxinaemia. Further research is needed.
Authors' conclusions
Implications for practice.
There is insufficient evidence from controlled clinical trials to determine whether the use of thyroid hormones for the treatment of preterm infants with transient hypothyroxinaemia results in changes in neonatal morbidity and mortality, or reductions in neurodevelopmental impairments.
Implications for research.
Further research is required to determine what type of thyroid hormone preparation, timing and duration of treatment is required to return active thyroid hormone levels to the 'normal range', and to determine whether the use of thyroid hormones in preterm infants with transient hypothyroxinaemia results in reductions in neonatal morbidity, mortality and neurodevelopmental impairments.
What's new
| Date | Event | Description |
|---|---|---|
| 22 August 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 11 October 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Dr Parveen Chowdhry for kindly responding to the request for additional information.
Data and analyses
Comparison 1. Thyroid hormones versus no thyroid hormones.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal mortality | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Mortality to discharge | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.08, 15.41] |
| 3 CLD in survivors (oxygen at 28 days) | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.45, 1.92] |
| 4 CLD in survivors (oxygen at 36 weeks) | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.15, 3.57] |
| 5 Patent ductus arteriosus | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.25, 1.56] |
| 6 Necrotising enterocolitis | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.04] |
| 7 Retinopathy of prematurity (any grade) in survivors | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.15, 3.57] |
| 8 Weight gain (grams/day) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐2.55, 3.57] |
| 9 Growth head circumference (cm/week) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.15, 0.19] |
| 10 Growth length (cm/week) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.24, 0.12] |
1.1. Analysis.
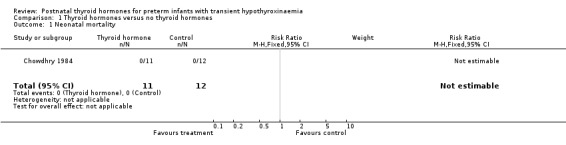
Comparison 1 Thyroid hormones versus no thyroid hormones, Outcome 1 Neonatal mortality.
1.2. Analysis.
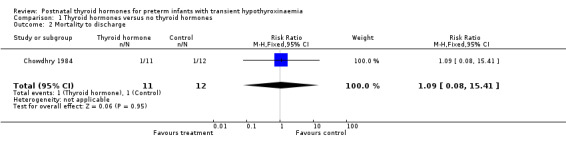
Comparison 1 Thyroid hormones versus no thyroid hormones, Outcome 2 Mortality to discharge.
1.3. Analysis.
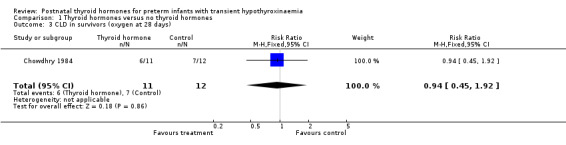
Comparison 1 Thyroid hormones versus no thyroid hormones, Outcome 3 CLD in survivors (oxygen at 28 days).
1.4. Analysis.
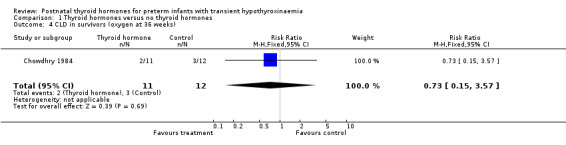
Comparison 1 Thyroid hormones versus no thyroid hormones, Outcome 4 CLD in survivors (oxygen at 36 weeks).
1.5. Analysis.
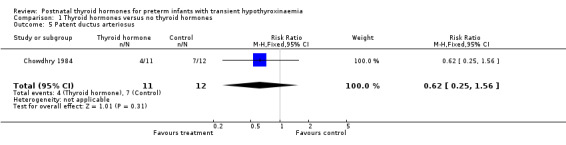
Comparison 1 Thyroid hormones versus no thyroid hormones, Outcome 5 Patent ductus arteriosus.
1.6. Analysis.
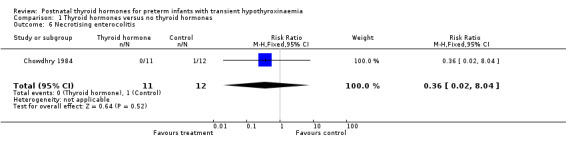
Comparison 1 Thyroid hormones versus no thyroid hormones, Outcome 6 Necrotising enterocolitis.
1.7. Analysis.
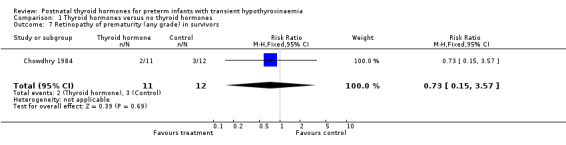
Comparison 1 Thyroid hormones versus no thyroid hormones, Outcome 7 Retinopathy of prematurity (any grade) in survivors.
1.8. Analysis.
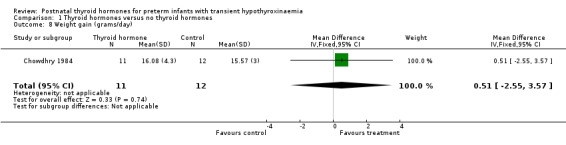
Comparison 1 Thyroid hormones versus no thyroid hormones, Outcome 8 Weight gain (grams/day).
1.9. Analysis.
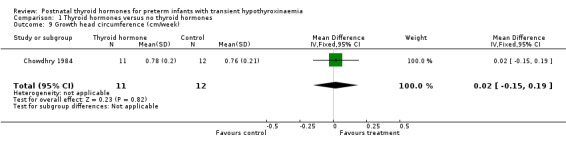
Comparison 1 Thyroid hormones versus no thyroid hormones, Outcome 9 Growth head circumference (cm/week).
1.10. Analysis.
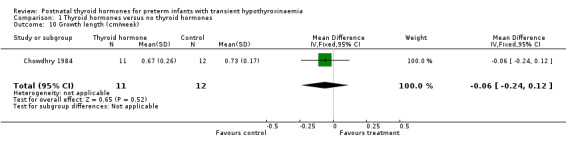
Comparison 1 Thyroid hormones versus no thyroid hormones, Outcome 10 Growth length (cm/week).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chowdhry 1984.
| Methods | Random study: yes ‐ randomised in pharmacy by "drawing cards". Single center. Blinding of randomization: yes. Blinding of intervention: yes, placebo controlled. Complete follow up: All infants had mortality, thyroid function and growth assessed. 15 (65%) lost to neurodevelopmental follow up. Blinding of outcome measurement: yes. Power calculation performed: not stated. | |
| Participants | Twenty three preterm infants < 1250g and 25‐28 weeks' gestation with T4 <= 4 microg/dl and TSH <= 20 IU/L on two occasions. Treated group: mean birth weight (+/‐ sd) 834+/‐182g, mean gestation 26.5+/‐10.6 weeks (n = 11). Control group: mean birth weight 738+/‐162g, mean gestation 26.3+/‐3.4 weeks (n = 12). | |
| Interventions | Treatment (n = 11): from day 15, approximately 7 weeks treatment with thyroxine 10 microg/kg/day IM (orally when tolerating feeds). Increased to 15 micrograms/kg/day if no increase in serum T4 after 1 week. Controls (n = 12): placebo. | |
| Outcomes | Neurodevelopment at 12 months: Bayley Mental Development Index and Bayley Psychomotor Index. Neonatal mortality. Mean time to T4 > 5 microg/dl. Weight gain (mean daily weight gain = g/day). Growth of head circumference = cm/week. Growth of length = cm/week. | |
| Notes | Developmental data not included: only 5 treated and 3 controls evaluated at 12 months; 2 treated and 2 control infants evaluated at 24 months. Author provided additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amato 1988 | Enrolled infants with RDS. |
| Amato 1989 | Enrolled infants with RDS. |
| Bettendorf 2000 | Enrolled infants after cardiac surgery. |
| Biswas 2003 | Randomised trial of triiodothyronine and hydrocortisone versus placebo preterm infants <30 weeks gestation. |
| Cassio A 2003 | Enrolled infants with congenital hypothyroidism. |
| Chowdhury 2001 | Enrolled infants after cardiac surgery. |
| Eggermont 1984 | Non‐randomized cohort comparison. Thyroxine given to 'sick' preterm infants and compared to a control group of 'non‐sick' preterm infants. |
| Schonberger 1981 | Inadequate randomisation. Used alternation and included 5 infants who were not alternated as controls. |
| Selva 2002 | Enrolled infants with congenital hypothyroidism. |
| Smith 2000 | Enrolled infants < 32 weeks gestation, birthweight 600‐1500g, not on basis of thyroid function. |
| Valerio 2004 | Enrolled infants < 28 weeks gestation not on basis of thyroid function. |
| van Wassenaer 1993 | Not a randomised study. Used historical controls to compare the effect of three different doses of thyroxine on neonatal thyroid hormone levels. Dose was varied over 3 consecutive time periods (6, 8 and 10 micrograms/kg/day). |
| van Wassenaer 1997 | Enrolled preterm infants 25‐29 weeks gestation not on basis of thyroid function. |
| Vanhole 1997 | Enrolled infants <28 weeks gestation not on basis of thyroid function. |
Characteristics of ongoing studies [ordered by study ID]
Golombek.
| Trial name or title | Hypothyroxemia trial |
| Methods | |
| Participants | Very preterm infants (<28 weeks) |
| Interventions | Thyroid hormones ‐ type and dose to be determined |
| Outcomes | Include neurodevelopment |
| Starting date | To be announced |
| Contact information | SERGIO_GOLOMBECK@NYMC.EDU |
| Notes |
Contributions of authors
Both authors contributed to all components of protocol and review. Both authors independently assessed trial eligibility and quality and extracted data.
Sources of support
Internal sources
RPA Newborn Care, Royal Prince Alfred Hospital, Sydney, Australia.
Department of Neonatal Medicine, Royal Children's Hospital, Melbourne, Australia.
External sources
Centre for Perinatal Health Services Research, University of Sydney, Australia.
NHMRC Grant ID 216757, Australia.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Chowdhry 1984 {published and unpublished data}
- Chowdhry P, Scanlon JW, Auerbach R, Abbassi V. Results of controlled double‐blind study of thyroid replacement in very low‐birth‐weight premature infants with hypothyroxinemia. Pediatrics 1984;73:301‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Amato 1988 {published data only}
- Amato M, Pasquier S, Carasso A, Muralt G. Postnatal thyroxine administration for idiopathic respiratory distress syndrome in preterm infants. Hormone Research 1988;29:27‐30. [DOI] [PubMed] [Google Scholar]
Amato 1989 {published data only}
- Amato M, Guggisberg C, Schneider H. Postnatal triiodothyronine replacement and respiratory distress syndrome of the preterm infant. Hormone Research 1989;32(5‐6):213‐7. [DOI] [PubMed] [Google Scholar]
Bettendorf 2000 {published data only}
- Bettendorf M, Schmidt KG, Grulich‐Henn J, Ulmer HE, Heinrich UE. Tri‐iodothyronine treatment in children after cardiac surgery: a double‐blind, randomised, placebo‐controlled study. Lancet 2000;356:529‐34. [DOI] [PubMed] [Google Scholar]
Biswas 2003 {published data only}
- Biswas S, Buffery J, Enoch H, Bland JM, Walters D, Markiewicz M. A longitudinal assessment of thyroid hormone concentrations in preterm infants younger than 30 weeks' gestation during the first 2 weeks of life and their relationship to outcome. Pediatrics 2002;109:222‐7. [DOI] [PubMed] [Google Scholar]
- Biswas S, Buffery J, Enoch H, Bland M, Markiewicz M, Walters D. Pulmonary effects of triiodothyronine (T3) and hydrocortisone (HC) supplementation in preterm infants less than 30 weeks gestation: results of the THORN trial‐‐thyroid hormone replacement in neonates. Pediatric Research 2003;53:48‐56. [DOI] [PubMed] [Google Scholar]
Cassio A 2003 {published data only}
- Cassio A, Cacciari E, Cicognani A, Damiani G, Missiroli G, Corbelli E, Balsamo A, Bal M, Gualandi S. Treatment for congenital hypothyroidism: thyroxine alone or thyroxine plus triiodothyronine?. Pediatrics 2003;111:1055‐60. [DOI] [PubMed] [Google Scholar]
Chowdhury 2001 {published data only}
- Chowdhury D, Ojamaa K, Parnell VA, McMahon C, Sison CP, Klein I. A prospective randomized clinical study of thyroid hormone treatment after operations for complex congenital heart disease. Journal of Thoracic & Cardiovascular Surgery 2001;122:1023‐5. [DOI] [PubMed] [Google Scholar]
Eggermont 1984 {published data only}
- Eggermont E, Vanderschueren Lodeweyckx M, Nayer P, Smeets E, Vanacker G, Cornette C, Jaeken J, Devlieger H, Eeckels R, Beckers C. The thyroid‐system function in preterm infants of postmenstrual ages of 31 weeks or less: evidence for a "transient lazy thyroid system". Helvetica Paediatrica Acta 1984;39:209‐22. [PubMed] [Google Scholar]
Schonberger 1981 {published data only}
- Schonberger W, Grimm W, Emmrich P, Gempp W. Reduction of mortality rate in premature infants by substitution of thyroid hormones. European Journal of Pediatrics 1981;135:245‐53. [DOI] [PubMed] [Google Scholar]
Selva 2002 {published data only}
- Selva KA, Mandel SH, Rien L, Sesser D, Miyahira R, Skeels M, Nelson JC, Lafranchi SH. Initial treatment dose of L‐thyroxine in congenital hypothyroidism. Journal of Pediatrics 2002;141:786‐92. [DOI] [PubMed] [Google Scholar]
Smith 2000 {published data only}
- Smith LM, Leake RD, Berman N, Villanueva S, Brasel JA. Postnatal thyroxine supplementation in infants less than 32 weeks' gestation: effects on pulmonary morbidity. Journal of Perinatology 2000;20:427‐31. [DOI] [PubMed] [Google Scholar]
Valerio 2004 {published data only}
- Valerio PG, Wassenaer AG, Kok JH. A randomized, masked study of T3 plus T4 administration in preterm infants less than 28 weeks of gestational age: hormonal and clinical effects. Pediatric Research. 2002; Vol. 51:125A. [DOI] [PubMed]
- Valerio PG, Wassenaer AG, Vijlder JJ, Kok JH. A randomized, masked study of triiodothyronine plus thyroxine administration in preterm infants less than 28 weeks of gestational age: hormonal and clinical effects. Pediatric Research 2004;55:248‐53. [DOI] [PubMed] [Google Scholar]
van Wassenaer 1993 {published data only}
- Wassenaer AG, Kok JH, Endert E, Vulsma T, Vijlder JJ. Thyroxine administration to infants of less than 30 weeks' gestational age does not increase plasma triiodothyronine concentrations. Acta Endocrinologica 1993;129:139‐46. [DOI] [PubMed] [Google Scholar]
van Wassenaer 1997 {published and unpublished data}
- Briet JM, Wassenaer AG, Dekker FW, Vijlder JJ, Baar A, Kok JH. Neonatal thyroxine supplementation in very preterm children: developmental outcome evaluated at early school age. Pediatrics 2001;107:712‐8. [DOI] [PubMed] [Google Scholar]
- Briet JM, Wassenaer AG, Baar A, Dekker FW, Kok JH. Evaluation of the effect of thyroxine supplementation on behavioural outcome in very preterm infants. Developmental Medicine and Child Neurology 1999;41:87‐93. [DOI] [PubMed] [Google Scholar]
- Smit BJ, Kok JH, Vries LS, Wassenaer AG, Dekker FW, Ongerboer de Visser BW. Somatosensory evoked potentials in very preterm infants in relation to L‐thyroxine supplementation. Pediatrics 1998;101:865‐9. [DOI] [PubMed] [Google Scholar]
- Smit BJ, Kok JH, Vries LS, Wassenaer AG, Dekker FW, Ongerboer de Visser BW. Motor nerve conduction velocity in very preterm infants in relation to L‐thyroxine supplementation. Journal of Pediatrics 1998;132:64‐9. [DOI] [PubMed] [Google Scholar]
- Wassenaer AG, Kok JH, Briet JM, Pijning AM, Vijlder JJ. Thyroid function in very preterm newborns: possible implications. Thyroid 1999;9:85‐91. [DOI] [PubMed] [Google Scholar]
- Wassenaer AG, Briët JM, Baar A, Smit BJ, Tamminga P, Vijlder JJM, Kok JH. Free thyroxine levels during the first weeks of life and neurodevelopmental outcome until the age of 5 years in very preterm infants. Pediatrics 2002;109:534‐9. [DOI] [PubMed] [Google Scholar]
- Wassenaer AG, Kok JH, Briet JM, Baar A, Vijlder JJ. Thyroid function in preterm newborns; is T4 treatment required in infants <27 weeks' gestational age?. Experimental and Clinical Endocrinology & Diabetes 1997;105:12‐8. [DOI] [PubMed] [Google Scholar]
- Wassenaer AG, Kok JH, Dekker FW, Endert E, Vijlder JJ. Thyroxine administration to infants of less than 30 weeks gestational age decreases plasma tri‐iodothyronine concentrations. European Journal of Endocrinology 1998;139:508‐15. [DOI] [PubMed] [Google Scholar]
- Wassenaer AG, Kok JH, Vijlder JJ, Briet JM, Smit BJ, Tamminga P, Baar A, Dekker FW, Vulsma T. Effects of thyroxine supplementation on neurologic development in infants born at less than 30 weeks' gestation. The New England Journal of Medicine 1997;336:21‐6. [DOI] [PubMed] [Google Scholar]
- van Wassenaer, Kok JH, Dekker FW, Vijlder JJ. Thyroid function in very preterm infants: influences of gestational age and disease. Pediatric Research 1997;42:604‐9. [DOI] [PubMed] [Google Scholar]
Vanhole 1997 {published and unpublished data}
- Vanhole C, Aerssens P, Devlieger H, Zegher F. L‐Thyroxine treatment of preterm newborns. Pediatric Research 1996;40:555. [DOI] [PubMed] [Google Scholar]
- Vanhole C, Aerssens P, Naulaers G, Casneuf A, Devlieger H, Berghe G, Zegher F. L‐thyroxine treatment of preterm newborns: clinical and endocrine effects. Pediatric Research 1997;42:87‐92. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Golombek {unpublished data only}
- Hypothyroxemia trial. Ongoing study To be announced.
Additional references
Ballabio 1989
- Ballabio M, Nicolini U, Jowett T, Ruiz de Elvira MC, Ekins RP, Rodeck CH. Maturation of thyroid function in normal human foetuses. Clinical Endocrinology 1989;31:565‐71. [DOI] [PubMed] [Google Scholar]
Belet 2003
- Belet N, Imdat H, Yanik F, Kucukoduk S. Thyroid function tests in preterm infants born to preeclamptic mothers with placental insufficiency. Journal of Pediatric Endocrinology 2003;16:1131‐5. [DOI] [PubMed] [Google Scholar]
Beranl 1995
- Beranl J, Nunez J. Thyroid hormones and brain development. European Journal of Endocrinology 1995;133:390‐8. [DOI] [PubMed] [Google Scholar]
Den Ouden 1996
- Ouden AL, Kok JH, Verkerk PH, Brand R, Verloove‐Vanhorick SP. The relation between neonatal thyroxine levels and neurodevelopmental outcome at age 5 and 9 years in a national cohort of very preterm and/or very low birth weight infants. Pediatric Research 1996;39:142‐5. [DOI] [PubMed] [Google Scholar]
Filippi 2004
- Filippi L, Cecchi A, Tronchin M, Dani C, Pezzati M, Seminara S, et al. Dopamine infusion and hypothyroxinaemia in very low birth weight preterm infants. European Journal of Pediatrics 2004;163:7‐13. [DOI] [PubMed] [Google Scholar]
Frank 1996
- Frank JE, Faix JE, Hermos RJ, Mullaney DM, Rojan DA, Mitchell ML, et al. Thyroid function in very low birth weight infants: effects on hypothyroidism screening. Journal of Pediatrics 1996;128:548‐54. [DOI] [PubMed] [Google Scholar]
Franklin 1986
- Franklin RC, Purdie GL, O'Grady CM. Neonatal thyroid function: prematurity, prenatal steroids, and respiratory distress syndrome. Archives of Disease in Childhood 1986;61:589‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 1999
- Hsu CH, Chang JH, Lee YJ, Hung HY, Kao HA, Huang FY. Thyroid function in the sick very low‐birth‐weight infants. Acta Paediatrica Taiwanica 1999;40:237‐42. [PubMed] [Google Scholar]
Kantor Herring 2003
- Kantor Herring MJ, Leef KH, Locke RG, Stefano JL, Bartoshesky L, Paul DA. Are perinatal risk factors helpful in predicting and optimizing treatment strategies for transient hypothyroxinemia in very‐low‐birth‐weight infants?. American Journal of Perinatology 2003;20:333‐9. [DOI] [PubMed] [Google Scholar]
Kooistra 1994
- Kooistra L, Laane C, Vulsma T, Schellekens JMH, Meere JJ, Kalverboer AF. Motor and cognitive development in children with congenital hypothyroidism: a long‐term evaluation of the effects of neonatal treatment. Journal of Pediatrics 1994;124:903‐9. [DOI] [PubMed] [Google Scholar]
Linder 1997
- Linder N, Davidovitch N, Reichman B, Kuint J, Lubin D, Meyerovitch J, et al. Topical iodine‐containing antiseptics and subclinical hypothyroidism in preterm infants. Journal of Pediatrics 1997;131:434‐9. [DOI] [PubMed] [Google Scholar]
Lorenz 1998
- Lorenz JM, Wooliever DE, Jetton JR, Paneth N. A quantitative review of mortality and developmental disability in extremely premature newborns. Archives of Pediatric and Adolescent Medicine 1998;152:425‐35. [DOI] [PubMed] [Google Scholar]
Lucas 1988
- Lucas A, Rennie J, Baker BA, Morley R. Low plasma triiodothyronine and outcome in preterm infants. Archives of Disease in Childhood 1988;63:1201‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucas 1996
- Lucas A, Morley R, Fewtrell MS. Low triiodothyronine concentration in preterm infants and subsequent intelligence quotient (IQ) at 8 year follow up. British Medical Journal 1996;312:1132‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meijer 1992
- Meijer WJ, Verloove‐Vanhorick SP, Brand R, Brande JL. Transient hypothyroxinaemia associated with developmental delay in very preterm infants. Archives of Disease in Childhood 1992;67:944‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborn 2007a
- Osborn DA, Hunt RW. Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005948.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborn 2007b
- Osborn DA, Hunt RW. Postnatal thyroid hormones for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005946.pub2] [DOI] [PubMed] [Google Scholar]
Paneth 1998
- Paneth N. Does transient thyroxinemia cause abnormal neurodevelopment in premature infants?. Clinics in Perinatology 1998;25:627‐43. [PubMed] [Google Scholar]
Paul 1998
- Paul DA, Leef KH, Stefano JL, Bartoshesky L. Low serum thyroxine on initial newborn screening is associated with intraventricular hemorrhage and death in very low birth weight infants. Pediatrics 1998;101:903‐7. [DOI] [PubMed] [Google Scholar]
Paul 2000
- Paul DA, Leef KH, Stefano JL, Bartoshesky L. Thyroid function in very‐low‐birth‐weight infants with intraventricular hemorrhage. Clinics in Pediatrics 2000;39:651‐6. [DOI] [PubMed] [Google Scholar]
Porterfield 1993
- Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development ‐ current perspectives. Endocrine Reviews 1993;14:94‐106. [DOI] [PubMed] [Google Scholar]
Radunovic 1991
- Radunovic N, Dumez Y, Nastic D, Mandelbrot L, Dommergues M. Thyroid function in fetus and mother during the second half of normal pregnancy. Biology of the Neonate 1991;59:139‐48. [DOI] [PubMed] [Google Scholar]
Reuss 1996
- Reuss ML, Paneth N, Pinto‐Martin JA, Lorenz JM, Susser M. The relation of transient hypothyroxinemia in preterm infants to neurologic development at two years of age. New England Journal of Medicine 1996;334:821‐7. [DOI] [PubMed] [Google Scholar]
Reuss 1997
- Reuss ML, Leviton A, Paneth N, Susser M. Thyroxine values from newborn screening of 919 infants born before 29 weeks' gestation. American Journal of Public Health 1997;87:1693‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rooman 1996
- Rooman RP, Du Caju MVL, Beeck LO, Docx M, Reempts P, Acker KJ. Low thyroxinaemia occurs in the majority of very preterm newborns. European Journal of Pediatrics 1996;155:211‐5. [DOI] [PubMed] [Google Scholar]
Tahirovic 1994
- Tahirovic HF. Transient hypothyroxinemia in neonates with birth asphyxia delivered by emergency cesarean section. Journal of Pediatric Endocrinology 1994;7:39‐41. [DOI] [PubMed] [Google Scholar]
Thorpe‐Beeston 1991
- Thorpe‐Beeston JG, Nicolaides KH, Felton CV, Butler J, McGregor AM. Maturation of the secretion of thyroid hormone and thyroid stimulating hormone in the fetus. New England Journal of Medicine 1991;324:532‐6. [DOI] [PubMed] [Google Scholar]
Uhrmann 1981
- Uhrmann S, Marks KH, Maisels MJ, Kulin HE, Kaplan M, Utiger R. Frequency of transient hypothyroxinaemia in low birthweight infants. Potential pitfall for neonatal screening programmes. Archives of Disease in Childhood 1981;56:214‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Wassenaer 1997
- Wassenaer AG, Kok JH, Dekker FW, Vijlder JJ. Thyroid function in very preterm infants: influences of gestational age and disease. Pediatric Research 1997;42:604‐9. [DOI] [PubMed] [Google Scholar]
van Wassenaer 2004
- Wassenaer AG, Kok JH. Hypothyroxinaemia and thyroid function after preterm birth. Seminars in Neonatology 2004;9:3‐11. [DOI] [PubMed] [Google Scholar]


