Abstract
Background
Urinary tract infections account for about 40% of hospital‐acquired (nosocomial) infections, and about 80% of urinary tract infections acquired in hospital are associated with urinary catheters.
Objectives
To determine if certain antibiotic prophylaxes are better than others in terms of prevention of urinary tract infections, complications, quality of life and cost‐effectiveness in short‐term catheterisation in adults.
Search methods
We searched the Cochrane Incontinence Group Specialised Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and MEDLINE in Process, and handsearching of journals and conference proceedings (searched 31st October 2012). Additionally, we examined all reference lists of identified trials.
Selection criteria
All randomised and quasi‐randomised trials comparing antibiotic prophylaxis for short‐term (up to and including 14 days) catheterisation in adults.
Data collection and analysis
Data were independently extracted by all review authors and compared. Disagreements were resolved by discussion. Data were processed as described in the Cochrane Handbook for Systemtic Reviews of Interventions. Where data had not been fully reported, clarification was sought directly from the authors of the trial.
Main results
Six parallel‐group randomised controlled trials with 789 participants met the inclusion criteria. All six trials compared antibiotic prophylaxis versus no prophylaxis. Studies presented a low to unclear risk of bias with similar interventions and measured outcomes.
The primary outcome of bacteriuria was less common in the prophylaxis group amongst surgical patients with asymptomatic bacteriuria (I2 = 0; risk ratio (RR) 0.20; 95% confidence interval (CI) 0.13 to 0.31) . Two non‐surgical studies could not be combined in a meta‐analysis due to heterogeneity and only one showed significantly fewer cases of bacteriuria (RR 0.19; 95% CI 0.09 to 0.37).
Two trials of surgical patients with asymptomatic bacteriuria only (255 participants) compared one type of antibiotic prophylaxis with another and neither study showed a significant difference in cases of bacteriuria.
One study (78 participants) compared antibiotic prophylaxis in patients at catheterisation only versus antibiotic prophylaxis throughout catheterisation period with asymptomatic bacteriuria. Antibiotics at catheterisation only, resulted in significantly fewer cases of bacteriuria than giving prophylaxis throughout the catheterisation period (RR 0.29 95% CI 0.09 to 0.91).
Secondary data of pyuria were provided by two surgical studies (255 participants). When studies were pooled, pyuria occurred in significantly fewer cases in the prophylactic antibiotic group (RR 0.23, 95% CI 0.13 to 0.42). The number of gram‐negative isolates in patients' urine just before catheter removal in one study (RR 0.05, 95% CI 0.00 to 0.79) and six weeks after hospital discharge (RR 0.36, 95% CI 0.23 to 0.56) were significantly lower. There were no events in the treatment group before catheter removal. When pooled data from two studies showed significantly reduced febrile morbidity in those receiving antibiotic prophylaxis (RR 0.53 95% CI 0.31 to 0.89).
Although all studies assessed micro‐organisms isolated from the urine specimens the data were too heterogenous to pool in a meta‐analysis and have been provided in a narrative form. Further secondary data such as economic analysis, length of stay and quality of life were not covered in detail.
Authors' conclusions
The limited evidence indicated that receiving prophylactic antibiotics reduced the rate of bacteriuria and other signs of infection, such as pyuria, febrile morbidity and gram‐negative isolates in patients' urine, in surgical patients who undergo bladder drainage for at least 24 hours postoperatively. There was also limited evidence that prophylactic antibiotics reduced bacteriuria in non‐surgical patients.
Keywords: Adult; Humans; Antibiotic Prophylaxis; Catheters, Indwelling; Catheters, Indwelling/adverse effects; Drainage; Randomized Controlled Trials as Topic; Urinary Bladder; Urinary Catheterization; Urinary Catheterization/adverse effects; Urinary Tract Infections; Urinary Tract Infections/etiology; Urinary Tract Infections/prevention & control
Plain language summary
Antibiotic prophylaxis for short‐term catheter bladder drainage in adults
Catheters may be used to drain urine from the bladder in hospital for short periods of time (less than two weeks). This may cause a urinary infection, or an increase in the number of bacteria in the urine. The review found that people who had antibiotics before or during catheter use were less likely to have an infection, and less likely to have a large number of bacteria or pus cells in the urine. However, there was no evidence concerning the chance of allergic reactions or other side effects from the antibiotics. Antibiotic‐resistant bacteria were identified in most studies but there was no definite link made to the use of antibiotics.
Background
Description of the condition
Indwelling urinary catheters are commonly used for bladder drainage during hospital care. The most common complication is infection. Urinary tract infections account for about 35% of hospital‐acquired (nosocomial) infections (Klevens 2007) and about 80% of these are associated with urinary catheters (National Audit Office 2009). Such infections not only prolong hospital stay and are expensive to treat (Plowman 1997;Tambyah 2002), but also cause unpleasant symptoms such as fever and chills in up to 30% of patients. Sometimes the infection may cause life‐threatening complications including bacteraemia (Scott 2009). When used in patients who are acutely ill, the risk of a catheter‐associated infection may be higher and hence pose a greater threat to life.
Bacteria get into the catheterised bladder by direct inoculation at the time of catheter insertion and via the following routes during catheterisation: extra‐luminally by ascending from the urethral meatus along the catheter‐urethral interface, and intra‐luminally by reflux of the organisms into the catheter lumen (Warren 2001). The normal mechanical wash‐out effect of the urinary stream is interrupted when there is a urinary catheter. For this reason, micro‐organisms which enter the catheterised urinary tract multiply quickly and develop difficult to remove biofilms (Stickler 2008).
The first step in reducing catheter‐associated urinary tract infections and other complications is to avoid unnecessary catheterisation; the second is to remove the catheter as soon as possible (Pratt 2007). Alternative methods to short‐term indwelling urinary catheterisation, such as intermittent catheterisation and suprapubic catheterisation should be considered and are evaluated in another Cochrane review (Niël‐Weise 2005a).
Once a catheter is in place, the aim is to minimise the risk of infection. There are two accepted basic principles: keeping the catheter system closed and decreasing the duration it is in place (Bernard 2012). Another possible strategy to reduce the risk of infection in respect to indwelling (i.e. urethral or suprapubic) catheters is to use catheters coated with an antibacterial substance. The effect of using different types of urethral catheters has been evaluated in another Cochrane review (Schumm 2008).
Description of the intervention
A further strategy of using antibiotic prophylaxis is controversial. While it is widely accepted that systemic antibiotics reduce the risk of catheter‐associated urinary tract infection in short‐term catheterised patients (Huth 1992; Pfefferkorn 2009) it is uncertain whether this protective effect offsets the risk of developing side effects from the antibiotics and the emergence of bacterial resistance. Possible ways of reducing these unwanted effects are limiting antibiotic use to patients with a positive urine culture (usually set at a pre‐defined density of bacteria), or to those who have clinical symptoms (such as pain or fever), or to certain circumstances such as when first inserting and changing the catheter.
Why it is important to do this review
Side effects from the antibiotics and the emergence of bacterial resistance militate against prophylactic antibiotics. However, there is potential to develop optimum use of antibiotics with short‐term catheters from undertaking a systematic review of the evidence.
The aim of this review is to assess the evidence from randomised controlled trials on the effects of prophylactic systemic antibiotics, including addressing whether particular patient groups should receive systemic antibiotics when a short period of catheterisation is anticipated.
Objectives
To determine if one type of antibiotic prophylaxis is better than another or none in terms of prevention of urinary tract infections, complications, quality of life and cost‐effectiveness for short‐term catheterised adults.
Methods
Criteria for considering studies for this review
Types of studies
All randomised and quasi‐randomised trials comparing antibiotic prophylaxis for short‐term catheterisation in hospitalised adults.
Types of participants
All adults requiring short‐term urinary urethral and supra‐pubic catheterisation (up to and including 14 days) in hospital for urine monitoring, investigations, acute retention problems, acute incontinence problems. These include those suffering from general medical problems, acute illness, urinary retention and following surgery (excluding urodynamics (Foon 2012) and transurethral surgical procedures (Alsaywid 2012)).
Types of interventions
The interventions considered were:
antibiotic prophylaxis versus no prophylaxis;
antibiotic prophylaxis with antibiotic A versus giving antibiotic prophylaxis with antibiotic B;
antibiotic prophylaxis at catheterisation only versus antibiotic prophylaxis throughout the catheterisation period.
In addition, the route of administration (oral or intravenous, but not topical) was considered.
Types of outcome measures
Primary outcomes
Asymptomatic bacteriuria
Symptomatic bacteriuria/urinary tract infection (UTI)
Secondary outcomes
Secondary outcome measures
Pyuria (presence of pus in the urine)
Febrile morbidity
Organisms isolated
Adverse reaction to antibiotics
Mortality
Length of stay
Quality of life (QoL)
Generic QoL measures e.g. Short Form 36 (Ware 1992)
Psychological outcome measures e.g. Hospital Anxiety and Depression Scale (Zigmond 1983)
Economic outcomes
Costs of intervention(s)
Resource implications of differences in outcomes
Formal economic analysis (cost effectiveness, cost utility)
Other outcomes
Any other non pre‐specified outcomes judged important when performing the review
Search methods for identification of studies
We did not impose language or other restrictions on any of the searches.
Electronic searches
This review has drawn on the search strategy developed for the Incontinence Group as a whole. Relevant trials were primarily identified from the Cochrane Incontinence Group Specialised Register. The methods used to derive this, including the search strategy, are described under the Group's module in The Cochrane Library. The register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in Process, CINAHL and handsearching of journals and conference proceedings.
The Incontinence Group Specialised Register was searched using the Group's own keyword system. The search terms used were:
(design.rct* or design.cct*) AND ({intvent.mech.cath*} or {intvent.mech.device*} or {intvent.mech.sheaths.} or {intvent.prevent.antibiotics*} or {intvent.prevent.antinfect.*} or {intvent.prevent.cath*} or {intvent.prevent.cleaning fluids*} or {intvent.prevent.surg*} or {intvent.surg.intraoperativemanagement*} or {intvent.surg.postsurgman*} or {intvent.surg.presurgman*.} or {intvent.surg.urethrotomy.})
(All searches were of the keyword field of Reference Manager 12, Thomson Reuters). Date of the most recent search of the register for this review: 31st October 2012. Most of the trials in the Incontinence Group Specialised Register are also contained in CENTRAL.
Searching other resources
Additionally we searched the reference lists of all identified trials.
Data collection and analysis
Selection of studies
The update review authors independently assessed all titles and abstracts identified by the search. Where there was any possibility that the study might be included, the full paper was obtained. If required, any disagreements that could not be resolved by discussion would have been resolved in consultation with an independent person.
Data extraction and management
Data were independently extracted by all review authors and compared. Where the data in trials had not been fully reported, we sought clarification from the trialists.
Included trial data were processed as described in the Cochrane Handbook for Systemtic Reviews of Interventions (Higgins 2011). When appropriate, meta‐analysis was undertaken. For categorical outcomes the numbers reporting an outcome were related to the numbers at risk in each group to derive a risk ratio (RR). For continuous variables, we planned to use means and standard deviations to derive a mean difference (MD).
The likelihood of important clinical heterogeneity was considered in each meta‐analysis. This took into account the results of the Chi2 test for heterogeneity and the I2 statistic. As a general rule, the outcome data were combined using a fixed‐effect model to calculate pooled estimates and their 95% confidence intervals. However, the use of a random‐effect model was considered where there were concerns that heterogeneity might have been complicating an analysis.
If the data allowed, the intention was to perform sensitivity analyses to assess the impact of study quality, such as adequate versus poor allocation concealment, and subgroup analyses for different diagnostic groups, or according to the sex of participants.
Assessment of risk of bias in included studies
The risk of bias in the included studies was assessed using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). This included:
sequence generation;
allocation concealment;
blinding of participants or therapists;
blinding of outcome assessors;
completeness of outcome data;
selective outcome reporting;
other potential sources of bias.
All three review authors independently assessed these domains. Any differences of opinion were resolved by consensus or by consulting an independent person.
Measures of treatment effect
Analyses were based on available data from all included trials relevant to the comparisons and outcomes of interest. For trials with multiple publications, only the most up‐to‐date or complete data for each outcome were included. Meta‐analysis was undertaken where data were available from more than one study assessing the same outcome. A fixed‐effect model was used for calculations of pooled estimates and their 95% confidence intervals. For categorical outcomes, we related the numbers reporting an outcome to the numbers at risk in each group to calculate a risk ratio (RR) with 95% confidence intervals (CI). For continuous variables, we planned to use means and standard deviations to calculate a mean difference (MD) with 95% confidence intervals. If similar outcomes had been reported on different scales, we planned to calculate the standard mean difference (SMD). We reversed the direction of effect, if necessary to ensure consistency across trials. If data to calculate RRs were not given, we utilised the most detailed numerical data available to calculate the actual numbers or mean and standard deviations (e.g. test statistics, P values).
Unit of analysis issues
The primary analysis was per patient randomised.
Dealing with missing data
The data were analysed on an intention‐to‐treat (ITT) basis as far as possible, meaning that all participants were analysed in the groups to which they were randomised. If this was not the case, we considered whether the trial should be excluded. Attempts were made to obtain missing data from the original trialists. However, if this was not possible, data were reported as given in the studies, except if there was evidence of differential loss to follow‐up from the randomised groups. In that case, the use of imputation of missing data was considered. If trials reported sufficient detail to calculate mean differences but no information on associated standard deviation (SD), the outcome was assumed to have a standard deviation equal to the highest SD from other trials within the same analysis.
Assessment of heterogeneity
Trials were only combined if they were thought to be clinically similar. Heterogeneity between studies was assessed by visual inspection of plots of the data, the χ2 test for heterogeneity and I2statistic (Higgins 2003). We defined the thresholds for interpretation of the I2 statistic according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, the review authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data.
Data synthesis
Trials were combined if interventions were similar based on clinical criteria. To combine trial data, a meta‐analysis was conducted and a fixed‐effect approach to the analysis was used unless there was evidence of heterogeneity across studies.
Subgroup analysis and investigation of heterogeneity
Data were subgrouped if possible by the extent of bacteriuria in the following:
antibiotic A versus antibiotic A (i.e. same antibiotic);
antibiotic A versus antibiotic B (i.e. different antibiotic);
men only,
women only;
surgical patients only;
non‐surgical patients only.
We planned that if heterogeneity between trials was sufficiently large, we would investigate possible causes. The investigation of heterogeneity was planned to address: populations and interventions in the individual trials. The investigation could also include subgroup analyses, meta‐regression and sensitivity analyses. If heterogeneity remained after appropriate investigation and possible removal of outlying trials, a random‐effects model would have been used in the meta‐analysis.
Sensitivity analysis
We planned to investigate the effects of including or excluding trials at high risk of bias by means of sensitivity analyses.
Results
Description of studies
Six parallel‐group randomised controlled trials with 789 participants met the inclusion criteria. All six trials compared antibiotic prophylaxis versus no prophylaxis. Studies presented a low to unclear risk of bias with similar interventions and measured outcomes.
Results of the search
Altogether for this review 821 records were screened for eligibility. In the original review (Niël‐Weise 2005b), 10 studies fulfilled the initial selection criteria (Britt 1977; Gasser 1996; Little 1974; Mountokalakis 1985; Meyer 1975; Ragnaud 1983; Romanelli 1990; Shohet 1983; Stricker 1988; van der Wall 1992). Cross‐referencing of these 10 studies yielded another two references (Jaffe 1985; Verbrugh 1988). Of these 12 reports, six were excluded after reading the whole article (Little 1974; Meyer 1975; Ragnaud 1983; Shohet 1983; Stricker 1988; Verbrugh 1988). The reasons for exclusion are listed in the table of Characteristics of excluded studies. The six trials included in the review were all parallel‐group randomised controlled trials (Britt 1977; Gasser 1996; Jaffe 1985; Mountokalakis 1985; Romanelli 1990; van der Wall 1992).
For this update, we found two studies Esposito 2006 and a study by Petronella 2012. On correspondence with the author, the latter study was found to be a second report of the same trial and so only data from the original study by Esposito 2006 were used. One study in the original review was excluded (Gasser 1996) as it no longer met the inclusion criteria. The flow of literature through the assessment process is shown in the PRISMA flowchart (Figure 1).
1.
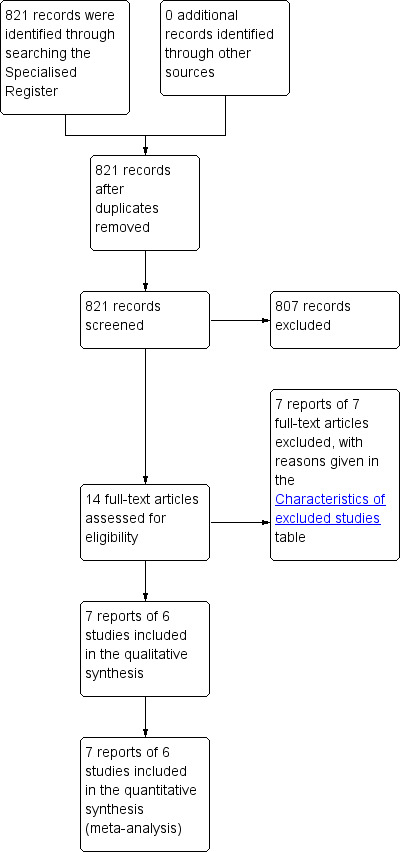
PRISMA study flow diagram.
Included studies
Details of the six included trials are given in the Characteristics of included studies table.
Participants
The trials' populations varied.
Four trials included hospitalised adults undergoing non‐urological surgery and who had postoperative bladder drainage (Britt 1977; Esposito 2006; Jaffe 1985; van der Wall 1992). Patients had a urethral catheter for three to 14 days (Esposito 2006), 24 hours (Jaffe 1985), a urethral or a suprapubic catheter for at least 24 hours (Britt 1977), or a urethral or a suprapubic catheter for three to 18 days (van der Wall 1992). Two of these trials included only female patients (Britt 1977; Jaffe 1985) and one trial included mainly female patients, with somewhat more women in the control group (van der Wall 1992).
Two trials included adult hospitalised patients who needed indwelling urethral catheterisation for at least seven days mainly because of bladder dysfunction associated with neurological disorders (Mountokalakis 1985; Romanelli 1990). Both trials included men and women, in which respect the trial groups were not quite balanced (ratio male/female: treatment group (I) 1:1.2, control group 1:1 (Mountokalakis 1985); treatment group 1:0.8, control group 1:0.7 (Romanelli 1990).
Types of intervention
In all trials, except one (Mountokalakis 1985), participants were randomised to prophylactic antibiotics or to a matching placebo. Patients in the control group of this trial did not receive any treatment.
There were six different types of antibiotics, with different doses used:
levofloxacin 250 mg (a fluoroquinolone) (Esposito 2006);
ciprofloxacin 500 mg (a fluoroquinolone) (Esposito 2006);
ciprofloxacin 250 mg or 500 mg (a fluoroquinolone) (van der Wall 1992);
cefazolin 500 mg (a cephalosporin) (Britt 1977);
co‐trimoxazole 1.2 g/240 mg (a sulphamethoxazole‐trimethoprim combination) (Jaffe 1985);
ampicillin 3 g or 1 g (a beta‐lactam) (Mountokalakis 1985);
aztreonam 2 g (a monobactam) (Romanelli 1990).
The numbers of doses of study medication given varied between:
a single dose (Jaffe 1985; Romanelli 1990);
three doses over eight hours or eight hourly for seven days (Mountokalakis 1985);
nine doses over three days even when the catheter had been removed (Britt 1977);,
once or twice a day (Esposito 2006; van der Wall 1992).
In three studies antibiotics were continued as long as the catheter was in place (Esposito 2006; Mountokalakis 1985; van der Wall 1992).
Timing of outcome assessment
All studies had varied time points at which outcomes were measured, but these were comparable within studies.
Outcome measures
Primary outcome
Five trials measured bacteriuria (asymptomatic and symptomatic) as the primary outcome. The definition of significant bacteriuria varied from:
>103 colony forming units per mL (Esposito 2006; van der Wall 1992) to
>105 colony forming units per mL (Britt 1977; Mountokalakis 1985; Romanelli 1990).
In one trial (Jaffe 1985), the definition of urinary tract infection involved bacteriuria (>105 colony forming units per mL) accompanied by urinary symptoms including febrile morbidity.
With regard to bacteriuria, there were considerable differences in the times when urine samples were tested.
In one trial (Jaffe 1985), bacteriuria was measured at the time of catheter removal (24 hours after surgery) and on the 3rd and 6th postoperative day.
One trial assessed bacteriuria on the 3rd postoperative day (Britt 1977).
Two trials assessed bacteriuria just before the catheter was removed (Esposito 2006; van der Wall 1992).
In another two trials, bacteriuria was assessed just before catheter removal or after a maximum of seven days follow‐up (Mountokalakis 1985; Romanelli 1990).
Secondary outcomes
Pyuria
Two trials investigated pyuria, which was defined as >10 leucocytes/mm3 or > 3 leucocytes/microscopic field (Esposito 2006) and 8 leucocytes/µL (van der Wall 1992). Both trials assessed pyuria alongside bacteriuria. In one trial this was within 24 hrs of catheter insertion, just before the catheter was removed, if requested by a physician and from a clean catch specimen obtained at six‐weeks follow‐up (van der Wall 1992). In the second trial, this was at the end of treatment and during the follow‐up period of four to six weeks (Esposito 2006).
Febrile morbidity
Two surgical trials assessed febrile morbidity (Britt 1977; Jaffe 1985). In one trial, febrile morbidity was defined as an oral temperature of 38oC or greater for at least two consecutive days (excluding the first 24 hours), with blood cultures taken if the oral temperature was greater than 38.3oC (Britt 1977). One trial diagnosed febrile morbidity if a temperature reading of 38oC or more was observed on at least two occasions four hours apart (excluding the first 24 hours) but the site for taking the temperature was not stated (Jaffe 1985).
Organisms isolated
All trials assessed micro‐organisms isolated from the urine specimens and all except one (Britt 1977) investigated their resistance patterns against the trial drugs. One trial cultured for all organisms in the urine then selected specific microbes for analysis against resistance to the trial drugs (Esposito 2006). Another trial considered gram‐negative bacilli and/or Enterococci as these were not considered to reflect contamination (Britt 1977). One trial reported all the micro‐organisms found and commented on poly‐microbic bacteriuria in both the treatment and placebo group (Mountokalakis 1985). One trial reported the micro‐organisms recovered on the first, third and seventh day of sampling and according to whether the patient was diabetic or not (Romanelli 1990). In another study, all coagulase negative Staphylococci were considered to be Staphylococci epidermidis and they defined multi‐resistance as resistance to at least ampicillin, cefamandole and one of the aminoglycoside antibiotics (van der Wall 1992).
Adverse reaction to antibiotics
One trial reported no adverse side effects of the antibiotic (Jaffe 1985). Another reported that three patients on the trial drug experienced moderate gastrointestinal symptoms on the second day of prophylaxis and so the antibiotic was discontinued (van der Wall 1992). Esposito 2006 listed the adverse events but stated that none were judged to be related to the study treatment.
Mortality
No trials investigated or reported on mortality.
Length of stay
Three trials reported the mean length of hospital stay of the treatment group in comparison with the placebo group (Britt 1977; Esposito 2006; Jaffe 1985). Jaffe 1985 also reported the prolonged mean length of hospital stay in those with febrile morbidity and urinary tract infection. One trial reported the mean pre‐surgical and post‐surgical length of stay rather than mean from hospitalisation to discharge (Esposito 2006).
Quality of life
No trials investigated or reported on quality of life.
Costs associated with urinary tract infection (UTI)
One trial stated that short‐term systemic antibiotic prophylaxis at the time of catheterisation was a low‐cost approach but did not provide any evidence for this statement (Mountokalakis 1985).
Excluded studies
Details of the excluded studies are given in the Characteristics of excluded studies table.
In total, in this update seven studies were excluded, either because they were not randomised controlled trials or they did not provide relevant data for the review. One previously included study was excluded (Gasser 1996). Initially, the update review authors retrieved numerous other trials, which had the potential to be included based on the criteria used to include Gasser 1996 in the original version of the review. However, a systematic review that aims to review and assess the benefits and harms of antibiotic prophylaxis for reducing the risk of UTIs following transurethral urological procedures is being undertaken and it was decided that antibiotic prophylaxis for transurethral urological procedures would be better explored separately (Alsaywid 2012).
Risk of bias in included studies
Overall, the risk of bias in the included trials was moderate with 'low risk' for random sequence generation, attrition bias and timing of outcome assessment, 'unclear' for allocation concealment, blinding and selective reporting and 'high risk' or 'unclear' incomplete outcome data (ITT analysis). See Figure 2; Risk of Bias summary and Figure 3; Risk of bias graph and Characteristics of included studies.
2.
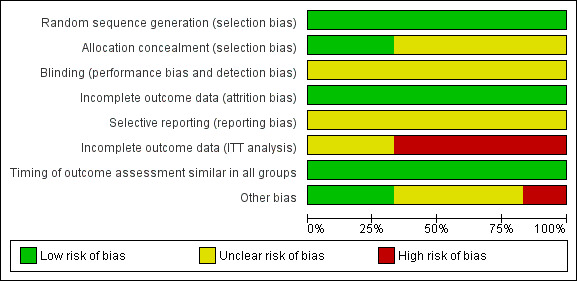
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
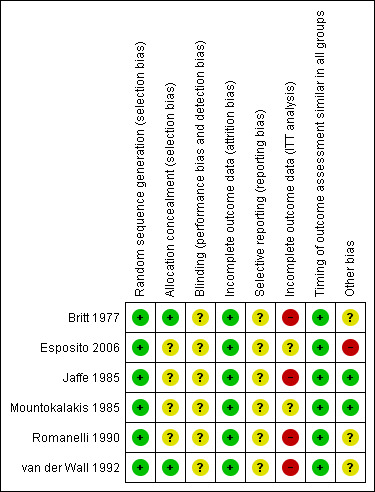
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Randomisation was adequately stated in all studies.
Allocation concealment
This was not stated and thus unclear in the majority of studies with only two trials giving details of allocation concealment of a hospital pharmacy randomisation schedule (Britt 1977) and all tablets being identical (van der Wall 1992).
Blinding
Blinding was unclear for participants, care givers and outcome assessors in three studies. No studies blinded all three. One study was stated as double blind, but with no further detail (Romanelli 1990), one study did not blind outcome assessors (van der Wall 1992) and in one study one arm was double blind and one was single blind with no further detail (Esposito 2006). Clinical staff and patients were not blinded in the trial of Mountokalakis: the participants in the control group did not receive a placebo. In addition, the author did not report whether or not the outcome assessor was blinded (Mountokalakis 1985). No blinding was reported in Jaffe 1985 and blinding of patients was not stated in Britt 1977.
Incomplete outcome data
Loss to follow‐up was judged as low risk as withdrawals and exclusions after randomisation were explained and were 16% or less in all studies.
Incomplete outcome data (ITT analysis)
Intention‐to‐treat analysis was classified as high risk in four studies as it was not stated (Britt 1977; Jaffe 1985; Romanelli 1990; van der Wall 1992). Intention‐to‐treat was stated but unclear in Esposito 2006 and not stated in Mountokalakis 1985 although there were no drop outs.
Selective reporting
We were not able to access the protocols of any of the included studies and so selective reporting was judged as unclear in the published papers.
Other potential sources of bias
Several studies had sponsorship (Britt 1977; Esposito 2006; van der Wall 1992) or assistance ( Romanelli 1990) from pharmaceutical organisations. It is not known to what extent this could have increased the risk of bias. As far as could be ascertained no studies were stopped early for any reason.
Effects of interventions
Comparison 1: Antibiotic prophylaxis versus no prophylaxis
All six studies compared antibiotic prophylaxis with no prophylaxis. Data on bacteriuria were available for all six eligible trials. The data from six trials could not be combined in a meta‐analysis because of clinical heterogeneity. Asymptomatic bacteriuria was reported in five studies (Britt 1977; Esposito 2006; Mountokalakis 1985; Romanelli 1990; van der Wall 1992). Three surgical studies (Britt 1977; Esposito 2006; van der Wall 1992) showed significantly fewer cases of bacteriuria in those patients receiving antibiotic prophylaxis. The trials were combined in a meta‐analysis as they were judged to be sufficiently similar (I2 = 0% risk ratio (RR) 0.20; 95% confidence interval (CI) 0.13 to 0.31) (Analysis 1.1.1). One surgical study (Jaffe 1985) showed significantly fewer cases of symptomatic bacteriuria/UTI in surgical patients receiving antibiotic prophylaxis versus no prophylaxis (RR 0.20 95% CI 0.06 to 0.66) (Analysis 1.3.1). Two non‐surgical studies (Mountokalakis 1985; Romanelli 1990) could not be combined in a meta‐analysis due to heterogeneity (Analysis 1.2). Mountokalakis 1985 (RR 0.63; 95% CI 0.34 to 1.13) and Romanelli 1990 (RR 0.19; 95% CI 0.09 to 0.37) (Analysis 1.2).
1.1. Analysis.
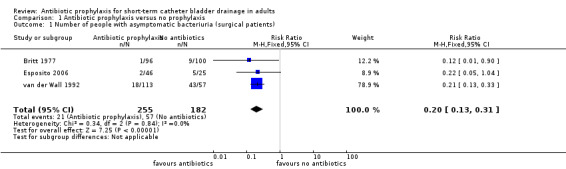
Comparison 1 Antibiotic prophylaxis versus no prophylaxis, Outcome 1 Number of people with asymptomatic bacteriuria (surgical patients).
1.3. Analysis.
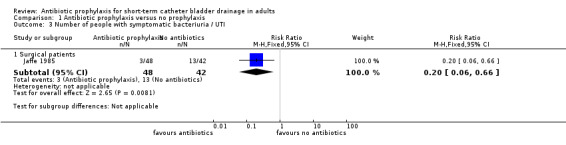
Comparison 1 Antibiotic prophylaxis versus no prophylaxis, Outcome 3 Number of people with symptomatic bacteriuria / UTI.
1.2. Analysis.
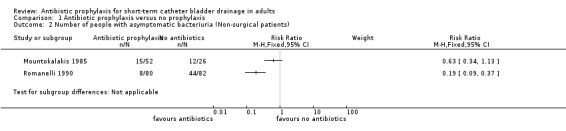
Comparison 1 Antibiotic prophylaxis versus no prophylaxis, Outcome 2 Number of people with asymptomatic bacteriuria (Non‐surgical patients).
Comparison 2: Antibiotic prophylaxis with antibiotic A versus antibiotic B
Two trials of surgical patients compared one type of antibiotic prophylaxis with another (255 participants). Esposito 2006 compared the use of levofloxacin versus ciprofloxacin (RR 4.23 95% CI 0.21 to 83.53) (Analysis 2.1.1). van der Wall 1992 compared ciprofloxacin 250 mg versus 1000 mg (RR 1.37 95% CI 0.58 to 3.21) (Analysis 2.1.2). In comparing patients with asymptomatic bacteriuria, neither study showed a significant difference in cases of bacteriuria.
2.1. Analysis.
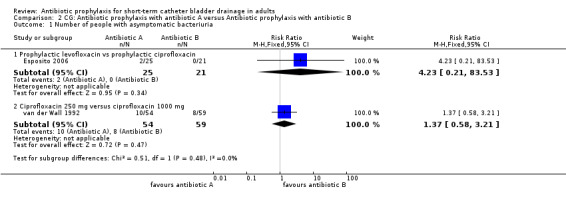
Comparison 2 CG: Antibiotic prophylaxis with antibiotic A versus Antibiotic prophylaxis with antibiotic B, Outcome 1 Number of people with asymptomatic bacteriuria.
Comparison 3: Antibiotic prophylaxis at catheterisation only versus antibiotic prophylaxis throughout catheterisation period
One study (78 participants) compared antibiotic prophylaxis in patients with asymptomatic bacteriuria (Mountokalakis 1985). Antibiotics at catheterisation only, resulted in significantly fewer cases of bacteriuria than giving antibiotic prophylaxis throughout the catheterisation period (RR 0.29 95% CI 0.09 to 0.91) (Analysis 3.1).
3.1. Analysis.
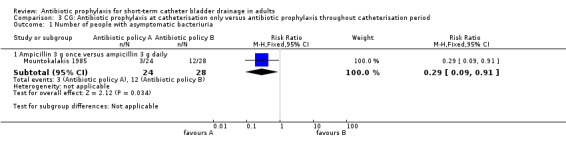
Comparison 3 CG: Antibiotic prophylaxis at catheterisation only versus antibiotic prophylaxis throughout catheterisation period, Outcome 1 Number of people with asymptomatic bacteriuria.
Other outcome measures
Pyuria
Pyuria data were provided by two surgical studies (255 participants) (Esposito 2006; van der Wall 1992). When studies were pooled, pyuria occurred in significantly fewer cases in the prophylactic antibiotic, surgical patient group (RR 0.23, 95% CI 0.13 to 0.43) (Analysis 1.5.1). The number of gram‐negative isolates in patients' urine just before catheter removal in van der Wall 1992 (RR 0.05, 95% CI 0.00 to 0.79, Analysis 1.6.1) and six weeks after hospital discharge (RR 0.36, 95% CI 0.23 to 0.56, Analysis 1.6.2) were significantly lower. There were no events in the treatment group before catheter removal.
1.5. Analysis.
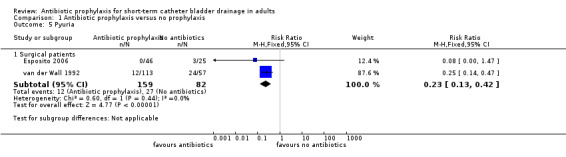
Comparison 1 Antibiotic prophylaxis versus no prophylaxis, Outcome 5 Pyuria.
1.6. Analysis.
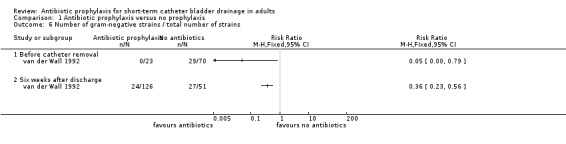
Comparison 1 Antibiotic prophylaxis versus no prophylaxis, Outcome 6 Number of gram‐negative strains / total number of strains.
Febrile morbidity
Febrile morbidity was assessed in two surgical studies (Britt 1977; Jaffe 1985) and when pooled, data showed significantly reduced febrile morbidity in those receiving antibiotic prophylaxis (RR 0.53 95% CI 0.31 to 0.89) (Analysis 1.4.2). In the meta‐analysis I2 = 53% which showed a degree of heterogeneity.
1.4. Analysis.
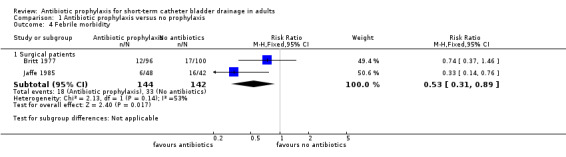
Comparison 1 Antibiotic prophylaxis versus no prophylaxis, Outcome 4 Febrile morbidity.
Organisms isolated
Although all studies assessed micro‐organisms isolated from the urine specimens, the data were too heterogenous to pool in a meta‐analysis and have therefore been provided in a narrative form. Certain organisms are known to be uropathogens associated with UTI. Identifying these types in the studies helps to understand the effectiveness of the prophylaxis and its role in resistance development. In this review, a number of organisms were resistant to the trial drugs leading to prophylaxis failure.
Investigation by Britt 1977 was limited to gram‐negative bacilli and/or enterococci and two enterococci and one indole‐negative Proteus were isolated in the antibiotic group and eight E. coli and one Enterobacter isolated in the placebo group. No data on antibiotic resistance were reported.
Esposito 2006 obtained 59 organisms from culture of the urine and distal catheter after removal (17 strains in the levofloxacin group, 22 strains in the ciprofloxacin group and 20 strains in the placebo group). The most frequently occurring bacteria were Enterococcus species (spp.), Staphylococcus spp. and Pseudomonas spp.. Analysis was then performed on selected bacteria isolated during the study to determine their susceptibility to the trial drugs. Eleven isolates were obtained (nine E. faecalis and two E. coli), all of which were sensitive to the trial drugs.
Jaffe 1985 tested for all organisms and isolated three organisms in the antibiotic group (two E.coli and one Klebsiella spp.) with one of the E. coli being resistant to the trial drug. In the placebo group 13 organisms were recovered (two Proteus mirabilis, two Klebsiella spp., one Streptococcus faecalis, one Psuedomonas aeruginosa and five E. coli), while oneKlebsiella and one Proteus were resistant to the trial drug.
Mountokalakis 1985 identified 10 species of organisms. There were four organisms of three strains in ampicillin group 1 (two E. coli, one Proteus mirabilis and oneCandida albicans) withProteus being resistant to the trial drug. In ampicillin group 2, 21 organisms of 14 strains were isolated (three E. coli, three Proteus spp., three Providencia spp., three Enterobacter spp., twoKlebsiella spp., three Serratia spp., one Citrobacter freundii, one Psuedomonas aeruginosa and twoCandida albicans) with two organisms being resistant to the prolonged course of the antibiotic (one Providencia rettgeri and one Serratia marcescens). Fifteen organisms of nine strains were isolated in the control group (six E. coli, oneProteus vulgaris, one Providencia stuartii, one Enterobacter cloacae, three Staphylococcus spp., one Psuedomonas aeruginosa and twoCandida albicans) with four being resistant to the antibiotic (one E. coli, oneProteus spp., one Providencia spp. and onePsuedomonas aeruginosa).
Romanelli 1990 identified nine organisms of four strains in the antibiotic group from the first to the seventh day of catheterisation (three E. coli, threeProteus mirabilis, twoKlebsiella spp., oneSerratia spp). In the placebo group, 44 organisms of six strains were obtained from the first to the seventh day of catheterisation (nine E. coli, thirteenProteus spp., five Klebsiella spp, five Serratia spp. and twelve Psuedomonas aeruginosa) were isolated. The results of organisms recovered are also reported by diabetic and non‐diabetic group from the first, third and seventh catheter day. Romanelli 1990 also reported that antibiotic treatment failed in nine participants in the intervention group. In all cases gram‐negative bacilli were isolated in the urine with one Kelbsiella species identified as resistant to the trial drug.
van der Wall 1992 identified 13 organisms of 13 strains in ciprofloxacin group 1 before catheter removal (one Diptheroids, six S.epidermis, twoE. faecalis, one Beta streptococcus and threeCandida spp.), nine of which were resistant to the antibiotic drug (one Diptheroids, five S.epidermis and threeCandida spp). In ciprofloxacin group 2, 10 organisms of two strains were isolated before catheter removal (five S.epidermis, fiveCandida spp.) with all 10 organisms being resistant to the antibiotic. In the placebo group, 70 organisms were isolated before catheter removal (20 E.coli, six otherEnterobacteriaceae, twoPsuedomonas spp. and one other glucose non‐fermenting organism, one Diptheroid, 18 Staphylococcus spp., 14 Enterococcus spp., four Streptococcus spp., two Lactobacillus spp. and twoCandida spp.). Of these, seven were resistant to the trial drug (one E. faecalis, twoBeta Streptococcus, twoLactobacillus spp., twoCandida spp.).
All trials reported resistance to the trial drug where Candida was isolated. However, this would be an expected resistance given it is a fungus rather than a bacteria.
Adverse reaction to antibiotics
Three studies reported adverse reaction to antibiotics but the data were too heterogenous to pool in a meta‐analysis (Esposito 2006; Jaffe 1985; van der Wall 1992). Esposito 2006 reported 23 adverse events. All of the adverse events were judged as not related to the study treatment by the investigators and there were no serious adverse events. Jaffe 1985 reported no adverse side effects to co‐trimoxazole. van der Wall 1992 reported that three patients on the trial drug experienced moderate gastrointestinal symptoms on the second day of prophylaxis and so the drug was discontinued.
Mortality
No trials investigated or reported on mortality.
Length of stay
Three trials reported the mean length of hospital stay of the treatment group in comparison to the placebo group. The data did not include standard deviations and P values and so could not be pooled (Britt 1977; Esposito 2006; Jaffe 1985). Esposito 2006 calculated hospital stay in pre‐surgery and post‐surgery phases. The mean pre‐surgical stay was higher in the placebo group (5.9 +/‐7.5 days) compared with the levofloxacin (3.9 +/‐ 3.6 days) and ciprofloxacin (3.3 +/‐ 3.7 days) groups. The mean post‐surgical stay was higher in the placebo group (7.6 +/‐6.6 days) compared with the ciprofloxacin (7.4 +/‐ 5.4 days) and levofloxacin (6.0 +/‐ 4.2 days) groups.
In Jaffe 1985, the mean hospital stay for the placebo group was 8 (+/‐ 1.4) days and 7 (+/‐ 1.2 days) in the intervention group. Febrile morbidity and urinary tract infection prolonged hospitalisation significantly to a mean stay of 9.2 (+/‐ 1.6) days (P < 0.05). In Britt 1977, average hospital stay was six days and 5.6 days for abdominal hysterectomy and 6.1 days and 7.6 days for vaginal hysterectomy patients in the prophylaxis group and placebo groups respectively.
Quality of life
No trials investigated or reported on quality of life.
Costs associated with UTI
One trial (Mountokalakis 1985) stated that short‐term systemic antibiotic prophylaxis at the time of catheterisation was a low‐cost approach but did not provide any evidence for this statement.
Discussion
Summary of main results
There was broad consistency across the trials that, as might be expected, prophylactic antibiotics reduced the frequency of bacteriuria and other markers of urinary tract infection. However, the review identified few data to describe unwanted effects of prophylaxis, such as adverse drug reactions and development of antibiotic resistance. Despite the frequency of urinary catheterisation, only six eligible trials were identified involving 789 participants that addressed the issue of whether or not to use antibiotics. The only measure of outcome that was consistently reported was bacteriuria; data for other outcomes were either not reported or available for single trials only. Only one trial (Jaffe 1985) defined the primary outcome of urinary tract infection involving bacteriuria accompanied by urinary symptoms (including febrile morbidity).
Overall completeness and applicability of evidence
Definitions now widely used for surveillance purposes for the diagnosis of symptomatic urinary tract infection (UTI) include primarily symptoms e.g. dysuria, frequency, fever, supra pubic tenderness, bacteriuria and at least one other criteria. In asymptomatic UTI the definition is bacteriuria and a positive blood culture. There must be at least one matching uropathogen micro‐organism to the urine culture, or at least two matching blood cultures drawn on separate occasions if the matching pathogen is a common skin commensal (CDC 2008). Using bacteriuria alone as a criteria for diagnosing UTI is limiting and may lead to unnecessary antibiotic therapy in catheterised patients. Although one trial (Britt 1977) included taking blood cultures as part of their protocol in those with fever, the results of any pathogens isolated were not reported and so their relationship to the bacteriuria is unknown. All but one trial (Mountokalakis 1985), were placebo‐controlled and appear to have been satisfactorily performed. The trials involved two broad groups: post‐surgical (non‐urological) and non‐surgical patients. The trials also varied in the antibiotic used for prophylaxis and in the frequency with which the antibiotics were administered. However, given the general consistency of the results, this variation enhances the generalisability of the review findings.
Types of bacteria
There were variations in protocols between the trials in the organisms tested for and reported on. Four trials cultured and reported on all bacteria isolated (Jaffe 1985; Mountokalakis 1985; Romanelli 1990; van der Wall 1992), one cultured all then selected a number of bacteria isolated during the study for further analysis (Esposito 2006). One trial identified gram‐negative bacteria and/or Enteroccoci only (Britt 1977), therefore, reporting only on organisms most likely to represent uropathogens that are commonly associated with UTI .
Resistance to antibiotics
A major concern with employing prophylactic antibiotics is the development of antibiotic‐resistant strains of bacteria and antibiotic prophylaxis has come in for particular scrutiny because it involves treating those who are asymptomatic. Over‐prescribing and unnecessary use of antibiotics increases the chance of resistance developing as seen with Methicillin‐resistant Staphylococcus aureus which is now endemic in acute healthcare settings. Clostridium difficile is also linked to over‐prescribing and particular groups of antibiotics such as cephalosporins and fluoroquinolones (especially ciprofloxacin). Extended Spectrum Beta Lactamase (ESBL)‐producing organisms include a range of common uropathogenic bacteria which have also developed resistance to cephalosporins. As a result of increasing resistance to broad spectrum antibiotics, prescription of these antibiotic groups are now being restricted.
One trial used cefazolin but did not report on resistance (Britt 1977). Two trials used ciprofloxacin (Esposito 2006; van der Wall 1992) one (van der Wall 1992) reporting significant resistance to the trial drug in both intervention groups. The other trial reported that the selected bacteria analysed were sensitive to the trial drug but this trial only analysed 11 out of the 59 organisms obtained (Esposito 2006). Increasing resistance of common uropathogens to ampicillin has also seen an increase in first treatment failure requiring the prescription of second line courses of antibiotics for UTI. One trial (Mountokalakis 1985) identified ampicillin‐resistant organisms in all groups.
Adverse effects
Another concern about antibiotic prophylaxis is the possibility of an allergic adverse drug reaction. None of the reports provided any information about this.
Alternative strategies
One possible approach to limiting the use of prophylactic antibiotics is through serial urine culture, with antibiotic treatment if asymptomatic bacteriuria is identified and infection is diagnosed according to the Center for Disease Control (CDC) definition above (CDC 2008). Another approach is to limit the use of antibiotic prophylaxis to patients who are at high risk for complications of UTI, for example, patients with implants, immunosuppression or diabetes. One trial (Romanelli 1990) reported results for diabetics and non‐diabetics but no trial identified serial urine cultures or selection criteria for high risk groups to limit antibiotic use.
Quality of life
No trials investigated or reported on quality of life.
Costs associated with UTI
No trials reported an economic evaluation. Three trials reported the mean length of hospital stay although the data did not include standard deviations and P values and so could not be pooled. One trial stated that short‐term systemic antibiotic prophylaxis at the time of catheterisation was a low‐cost approach but did not provide any evidence for this statement. Length of stay data were provided by some studies.
In Jaffe 1985 the mean hospital stay for the placebo group was 8 (+/‐ 1.4) days and 7 (+/‐ 1.2 days) in the intervention group. Febrile morbidity and urinary tract infection prolonged hospitalisation significantly to a mean stay of 9.2 (+/‐ 1.6) days (P < 0.05). In Britt 1977 average hospital stay was six days and 5.6 days for abdominal hysterectomy and 6.1 days and 7.6 days for vaginal hysterectomy patients in the prophylaxis group and placebo groups respectively.
Quality of the evidence
The quality of the evidence was generally fair. Most trials were at low risk of bias regarding randomisation, attrition bias and timing of outcome assessment. Allocation concealment methods, blinding and selective reporting were at medium risk of bias and incomplete outcome data was at high risk of bias.
Agreements and disagreements with other studies or reviews
We are unaware of another systematic review on this topic apart from the original version of this review (Niël‐Weise 2005b). This first update differs substantially from the original review in that the update review authors have changed the population by removing all studies on urodynamics (Foon 2012) and transurethral procedures (Alsaywid 2012). The intervention categories have also been changed to a more simple categorisation. In addition, the outcomes were changed to reflect those of urinary tract infection.
Authors' conclusions
Implications for practice.
Weak evidence suggested that antibiotic prophylaxis compared to giving antibiotics when microbiologically indicated reduced the rate of bacteriuria in surgical patients with a urinary catheter for 24 hours. Limited evidence indicated that receiving antibiotics for the first three postoperative days or from postoperative day two until catheter removal reduced the rate of bacteriuria and other signs of infection, such as pyuria, febrile morbidity and gram‐negative isolates in patients' urine in surgical patients undergoing bladder drainage for at least 24 hours postoperatively. There was also limited evidence that prophylactic antibiotics reduced bacteriuria in non‐surgical patients.
Because of the limited evidence and the few data on adverse effects and development of antibiotic resistance, the results should be interpreted with caution.
Implications for research.
More randomised controlled trials are needed, especially for the comparison of giving antibiotics if microbiologically indicated versus treating with antibiotics if clinically indicated. The antibiotics selected for prophylaxis should be those that do not increase the risk of multi‐resistance development or be likely to cause complications of treatment such as Clostridium difficile. Patients who are at high risk for complications of urinary tract infection should particularly be included.
Consistency in reporting for the period a patient will be catheterised, type and dose of antibiotics and the point in time bacteriuria will be measured would strengthen research policies. Urinary tract infection should be defined according to standardised definitions for catheterised patients rather than bacteriuria alone. Trials should be sufficiently powered to enable subgroup analysis in general surgical patients, urological or gynaecological surgery patients and non‐surgical patients.
What's new
| Date | Event | Description |
|---|---|---|
| 11 June 2013 | New search has been performed | Review updated with one RCT removed as not fitting the criteria for the review (Gasser 1996). One new RCT included (Esposito 2006). Outcome measures changed to primary and secondary and rewritten to reflect the outcomes of the intervention (antibiotics) and not the population (patients with a short‐term catheter). |
| 11 June 2013 | New citation required but conclusions have not changed | Review updated with one RCT removed as not fitting the criteria for the review (Gasser 1996). One new RCT included (Esposito 2006). Outcome measures changed to primary and secondary and rewritten to reflect the outcomes of the intervention (antibiotics) and not the population (patients with a short‐term catheter). |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 12 September 2008 | Amended | Converted to new review format. |
| 29 May 2006 | New search has been performed | Update ‐ conclusions not changed |
| 25 May 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The review authors would like to acknowledge the contributions of the previous review authors Barbara S Niël‐Weise and Peterhans J van den Broek for their contribution. The review authors would like to acknowledge the Univesity of South Wales for giving them the time and the resources to undertake this review.
We would like to thank the author Dr S. Esposito for clarifying the original data set for the study (Esposito 2006).
Data and analyses
Comparison 1. Antibiotic prophylaxis versus no prophylaxis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people with asymptomatic bacteriuria (surgical patients) | 3 | 437 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.13, 0.31] |
| 2 Number of people with asymptomatic bacteriuria (Non‐surgical patients) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Number of people with symptomatic bacteriuria / UTI | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Surgical patients | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.66] |
| 4 Febrile morbidity | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Surgical patients | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.89] |
| 5 Pyuria | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Surgical patients | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.13, 0.42] |
| 6 Number of gram‐negative strains / total number of strains | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Before catheter removal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Six weeks after discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. CG: Antibiotic prophylaxis with antibiotic A versus Antibiotic prophylaxis with antibiotic B.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people with asymptomatic bacteriuria | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Prophylactic levofloxacin vs prophylactic ciprofloxacin | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.23 [0.21, 83.53] |
| 1.2 Ciprofloxacin 250 mg versus ciprofloxacin 1000 mg | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.58, 3.21] |
Comparison 3. CG: Antibiotic prophylaxis at catheterisation only versus antibiotic prophylaxis throughout catheterisation period.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of people with asymptomatic bacteriuria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Ampicillin 3 g once versus ampicillin 3 g daily | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.09, 0.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Britt 1977.
| Methods | Placebo‐controlled RCT | |
| Participants | 215 female patients entered into the study and randomised Inclusion criteria: Undergoing elective gynaecological surgery and who received a urinary catheter or supra‐pubic catheter at the time of surgery for at least one day Exclusion criteria: Rapidly or ultimately fatal disease, active clinical infection, allergy to penicillins or cephalosporins Losses post randomisation: |
|
| Interventions | Group 1 (n = 96): Cefazolin sodium 500 mg was given at the time of operation, and each subsequent dose was given at 8‐hour intervals, IV or IM, for a total of nine doses (= three days). Group 2 (n = 100): Placebo was given at the time of operation, and each subsequent dose was given at 8‐hour intervals, IV or IM, for a total of nine doses (= three days). |
|
| Outcomes | Bacteriuria during period of antibiotic prophylaxis (days 1‐3) (102> CFU/mL for urinary catheter and 105> CFU/mL for MSU):
Group 1 1/96 Group 2 9/100 Secondary outcomes: febrile morbidity, catheter days, types of organisms |
|
| Notes | A urine sample was obtained at the time of catheter insertion and daily from the drainage tube. A clean‐voided specimen was taken on discharge when the catheter was already removed. Remark: Group 1: 6 supra pubic/90 urinary catheters (0.07) Group 2: 12 supra pubic/88 urinary catheters (0.14) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Hospital pharmacy provided a randomisation schedule. |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacy provided a randomisation schedule. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote "Neither the attending physicians, study personnel, nor nursing personnel were aware of the treatment regime assigned by the pharmacy". Blinding by patients not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.8% attrition (19 patients were excluded: 14 patients were bacteriuric at the time of catheter insertion, five patients were catheterised for less than one day). |
| Selective reporting (reporting bias) | Unclear risk | Reports all prespecified outcomes but we were unable to access the trial protocol. |
| Incomplete outcome data (ITT analysis) | High risk | No ITT analysis stated. |
| Timing of outcome assessment similar in all groups | Low risk | All participants assessed on third day. |
| Other bias | Unclear risk | Public Health Service grant plus a grant for Ely Lilly & Co. Indianapolis, Ind. |
Esposito 2006.
| Methods | Placebo‐controlled RCT | |
| Participants | 82 patients enrolled and randomised Inclusion criteria: Male or female patients over 18 years Planned surgical intervention requiring catheterisation Informed consent Catheter for 3‐14 days Exclusion criteria: None stated |
|
| Interventions | Group 1: Levofloxacin 250 mg orally once a day until catheter removed or for maximum of 13 days (n = 25) Group 2: Placebo drug orally once a day until catheter removed or for maximum of 13 days (n = 25) Group 3: Ciprofloxacin 500 mg orally twice a day until catheter removed or for maximum of 13 days (n = 21) Follow‐up : daily until end of prophylaxis (visit 3) maximum of 13 days the follow‐up 4‐6 weeks after end of treatment was not included in the analysis |
|
| Outcomes | Bacteriuria (103> CFU/mL with one isolated pathogen for urinary catheter) Group 1 2/25 Group 2 5/25 Group 3 0/21 Secondary outcomes: pyuria, type of organism, adverse events Pyuria 103> leucocytes/mL or 3 > leucocytes/microscopic field Group 1 0/25 Group 2 3/25 Group 3 0/21 |
|
| Notes | M‐ITT data are reported which excluded patients with bacteriuria at randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "patients were randomized into three groups, according to a randomization list". |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information given on method of allocation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote " The design of the study was double blind for the LVFX and placebo group and single blind for the CPFX group". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13.4% attrition. Quote "82 enrolled patients" and 71 patients were evaluable at the end of prophylaxis. |
| Selective reporting (reporting bias) | Unclear risk | Reports all prespecified outcomes but we were unable to access the trial protocol. |
| Incomplete outcome data (ITT analysis) | Unclear risk | Quote "ITT: patients with at least one dose of study drug taken and Modified‐ITT patients with negative bacteriuria at visit 2". |
| Timing of outcome assessment similar in all groups | Low risk | All participants measured at visit 3, end of prophylaxis. |
| Other bias | High risk | Quote "This study was supported with a financial grant by GlaxoSmithKline S.p.A." |
Jaffe 1985.
| Methods | Placebo‐controlled RCT | |
| Participants | 98 female patients enrolled and randomised Inclusion criteria: Patients undergoing elective abdominal hysterectomy for benign conditions Exclusion criteria: Patients on antibiotic therapy during the two weeks before surgery, known hypersensitivity to co‐trimoxazole |
|
| Interventions | Group 1: 200 mg sulphamethoxazole and 240 mg trimethoprim diluted in 500 mL saline in a slow intravenous infusion during the last 30 minutes before surgery (n = 50). Group 2: 200 mg placebo diluted in 500 mL saline in a slow intravenous infusion during the last 30 minutes before surgery (n = 48). Urine samples for culture were obtained at the time of catheter insertion, 24 hours after surgery at the time of catheter removal and on the 3rd and 6th postoperative day. |
|
| Outcomes | Urinary tract infection (defined as the presence of 105 CFU/mL accompanied by urinary symptoms) on the 6th postoperative day:
Group 1: 3/48; Group 2:13/42 Febrile morbidity (reading of > 38oC was observed on at least two occasions 4 hours or more apart excluding the first 24 postoperative hours) Group 1: 6/48 Group 2: 16/42 Secondary outcomes: febrile morbidity, hospital stay, types of organisms |
|
| Notes | A urethral catheter was inserted one hour before surgery and removed 24 hours later. Urine samples for culture were obtained at the time of catheter insertion, at the time of catheter removal and on the 3rd and 6th postoperative day. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Patients were randomly assigned to either the treatment or placebo group". |
| Allocation concealment (selection bias) | Unclear risk | Randomisation stated but no information given on method of allocation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No blinding of care givers, participants or outcome assessors reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.1% attrition. Two patients were excluded due to positive preoperative urine cultures. In the control group 3 patients were excluded due to positive preoperative urine cultures, 2 due to malignancy found in the uterus and 1 because of a mistake in following the protocol. |
| Selective reporting (reporting bias) | Unclear risk | Reports all prespecified outcomes but we were unable to access the trial protocol. |
| Incomplete outcome data (ITT analysis) | High risk | No ITT analysis stated. |
| Timing of outcome assessment similar in all groups | Low risk | Urine samples for culture were obtained at the time of catheter insertion, at the time of catheter removal and on the 3rd and 6th postoperative day. |
| Other bias | Low risk | No sponsorship stated. |
Mountokalakis 1985.
| Methods | Non‐placebo RCT | |
| Participants | 78 patients enrolled and randomised Inclusion criteria: Newly hospitalised patients (number of patients randomised not reported) with recent stroke in whom placement of an indwelling urethral catheter was used for treatment of urinary incontinence for at least seven days. Exclusion criteria: Known urological disease, any urinary tract instrumentation in the previous year, history of urinary tract infection, antimicrobial treatment in the previous 15 days, bacteriuric at the time of catheter insertion |
|
| Interventions | Group 1: 3 g ampicillin IM, divided in three equal doses: 1 hour before, at the time of, and 6 hours after insertion of the catheter (n = 24) Group 2: 3 x 1 g ampicillin IM daily (n = 28) Group 3: Control (No prophylactic antibiotics) (n = 26) Follow‐up : CSU day 1‐7 or until significant bacteriuria |
|
| Outcomes | Significant bacteriuria was defined as 105 > CFU/mL:
Group 1: 3/24; Group 2: 12/28; Group 3:12/26 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "assigned randomly to 3 treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No blinding of care givers, participants or outcome assessors reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop outs reported. |
| Selective reporting (reporting bias) | Unclear risk | Reports all prespecified outcomes but we were unable to access the trial protocol. |
| Incomplete outcome data (ITT analysis) | Unclear risk | No ITT analysis stated, but no drop outs reported. |
| Timing of outcome assessment similar in all groups | Low risk | Quote "The study was completed in each patient at the end of an observation period of 7 days or when a urine culture revealed significant bacteria". |
| Other bias | Low risk | Sponsorship not stated. |
Romanelli 1990.
| Methods | Placebo‐controlled RCT | |
| Participants | 169 patients enrolled and randomised Inclusion criteria: Elderly hospitalised patients who needed indwelling urethral catheterisation for at least seven days (mainly neurological disorders associated with bladder dysfunction) Exclusion criteria: None stated |
|
| Interventions | Group 1: A single dose of Aztreonam IM (2 g in 4 mL lidocaine 2%) three hours before catheterisation (n = 80) Group 2: A single dose of placebo (4 mL lidocaine 2%) three hours before catheterisation (n = 82) Follow‐up : Urine culture on days 1, 3 and 7 |
|
| Outcomes | Bacteriuria was defined as 105> CFT/mL: At the first day of catheterisation: Group 1: 0/80; Group 2:19/82 Bacteriuria at the third day of catheterisation: Group 1: 8/80; Group 2: 44/82 Bacteriuria at the seventh day of catheterisation: Group 1: 9/80; Group2: 44/82 Secondary outcomes: Types of organisms, results in diabetic patients |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "patients were randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote "double blind" but not stated whether blinding applies to participants, care givers or outcome. assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% attrition. Seven were excluded due to positive urine culture prior to catheterisation. |
| Selective reporting (reporting bias) | Unclear risk | Reports all prespecified outcomes but we were unable to access the trial protocol. |
| Incomplete outcome data (ITT analysis) | High risk | No ITT analysis stated. |
| Timing of outcome assessment similar in all groups | Low risk | All participants urine culture measurement on 1st, 3rd and 7th day. |
| Other bias | Unclear risk | Quote " The authors are grateful to Prof. G Patrizi and Dr L Ventriglia (Squibb, Italy) for their helpful technical assistance". |
van der Wall 1992.
| Methods | Placebo‐controlled RCT | |
| Participants | 202 patients enrolled Inclusion criteria: Surgical patients from two hospitals who had postoperative bladder drainage scheduled to last for 3 to 14 days (vaginal repair, total hip replacement, rectal surgery); at least 18 years old; had given informed consent Exclusion criteria: Pregnancy, impaired renal or hepatic function, symptomatic UTI, fever, antibiotic use within 48 hours before study inclusion |
|
| Interventions | Group 1: 250 mg ciprofloxacin per day from 2nd postoperative until catheter removal (n = 59) Group 2: 500 mg ciprofloxacin twice daily from 2nd postoperative until catheter removal (n = 64) Group 3: Placebo from 2nd postoperative until catheter removal (n = 61) Follow‐up :CSU within 24 hours of insertion, CSU just before removal if requested by a physician, clean catch at 6‐week follow‐up |
|
| Outcomes | Significant bacteriuria was defined as 103> CFU/mL of CSU and 105> CFT/mL in clean‐catch urine Bacteriuria 103> CFU/mL at the time of catheter removal: Group 1 (250 mg):10/54; Group 2 (1000 mg):8/59; Group 3: 43/57; Group 1 & 2 (250 mg or 1000 mg):18/113; Bacteriuria 105> CFU/mL at the time of catheter removal Group 1 (250 mg):4/54; Group 2 (1000 mg): 2/59; Group 3: 40/57; Secondary outcomes: types of organisms, pyuria, febrile illness, hospital acquired infection, symptomatic UTI Pyuria was defined as more than 8 leucocytes/µL Pyuria at the time of catheter removal: Group 1 & 2 (250 mg or 1000 mg):12/113; Group 3: 24/57 |
|
| Notes | Suprapubic vs urethral bladder drainage:
Group 1: 16/50 (0.3)
Group 2:12/56 (0.2)
Group 3: 17/51 (0.3) Different 24‐hour perioperative antibiotic prophylactic regimens were given. 3 patients had side effects of ciprofloxacin (moderate gastrointestinal symptoms). two hospitals Remark: Of the 170 patients evaluated for bacteriuria, 11% or less in each group were bacteriuric at the time of catheter insertion (data not shown). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Randomisation was achieved with separate lists with permuted blocks of 12 random numbers for each of the two hospitals and for each hospital service (gynaecology, surgery, orthopaedics) participating in the study". |
| Allocation concealment (selection bias) | Low risk | Quote "All tablets were identical and contained either 250mg ciprofloxacin or no active drug". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote "Patients and the doctors and nurses involved in their care were all unaware of the nature of the medication being given". Blinding by outcome assessors not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16% attrition 18 excluded prior to randomisation because of protocol errors (16) or because they refused further participation (2) and "14 patients were not evaluable at the time of catheter removal" because a urine specimen was not obtained at the time of catheter removal. The excluded patients were evenly distributed over the three study arms. |
| Selective reporting (reporting bias) | Unclear risk | Reports all prespecified outcomes but we were unable to access the trial protocol. |
| Incomplete outcome data (ITT analysis) | High risk | No ITT analysis stated. |
| Timing of outcome assessment similar in all groups | Low risk | Until catheter removal: Median (range) days:
Group 1 (250 mg):7.0 (3‐18); Group 2 (1000 mg):7.0 (3‐15); Group 3 (placebo): 8.0 (3‐16) |
| Other bias | Unclear risk | Quote " a clinical research grant from Bayer AG, Leverkussen, Germany" |
CFU: colony‐forming unit CPFX: Ciprofloxacin CSU: catheter specimen of urine IM: intramuscular ITT: intention‐to‐treat IV: intravenous LVFX: levofloxacin M‐ITT: modified intention‐to‐treat MSU: midstream specimen of urine RCT: randomised controlled trial URI: urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gasser 1996 | Primary purpose of antibiotic administration was to prevent surgical site infection. |
| Little 1974 | Not randomised trial. |
| Meyer 1975 | Another question was answered. |
| Ragnaud 1983 | Another question was answered. |
| Shohet 1983 | Not randomised trial. |
| Stricker 1988 | Another question was answered. |
| Verbrugh 1988 | Not randomised trial. |
Differences between protocol and review
The title of the review was changed to reflect the population, intervention and outcomes stated.
Contributions of authors
All reviewers contributed equally to writing the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Incontinence Group.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Britt 1977 {published data only}
- Britt MR, Garibaldi RA, Miller WA, Hebertson RM, Burke JP. Antimicrobial prophylaxis for catheter‐associated bacteriuria. Antimicrobial Agents & Chemotherapy 1977;11:240‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esposito 2006 {published data only}
- Esposito S, Noviello S, Leone S, Marvaso A, Drago L, Marchetti F, for the LEVT06 Study Group. A pilot study on prevention of catheter‐related urinary tract infections with fluoroquinolones. Journal of Chemotherapy 2006;18(5):494‐501. [DOI] [PubMed] [Google Scholar]
- Petronella P, Scorzeli M, Fiore A, Corbisiero MC, Agresti E, Esposito S, et al. Antibiotic prophylaxis in catheter‐associated urinary infections. New Microbiologica 2012;35(2):191‐8. [PubMed] [Google Scholar]
Jaffe 1985 {published data only}
- Jaffe R, Altaras M, Fejgin M, Ben‐Aderet N. Prophylactic single‐dose co‐trimoxazole for prevention of urinary tract infection after abdominal hysterectomy. Chemotherapy 1985;31(6):476‐9. [DOI] [PubMed] [Google Scholar]
Mountokalakis 1985 {published data only}
- Mountokalakis T, Skounakis M, Tselentis J. Short‐term versus prolonged systemic antibiotic prophylaxis in patients treated with indwelling catheters. Journal of Urology 1985;134(3):506‐8. [DOI] [PubMed] [Google Scholar]
Romanelli 1990 {published data only}
- Romanelli G, Giustina A, Cravarezza P, Bossoni S, Bodini C, Girelli A, et al. A single dose of aztreonam in the prevention of urinary tract infections in elderly catheterized patients. Journal of Chemotherapy 1990;2(3):178‐81. [DOI] [PubMed] [Google Scholar]
van der Wall 1992 {published data only}
- Wall E, Verkooyen RP, Mintjes‐de Groot J, Oostinga J, Dijk A, Hustinx WN, et al. Prophylactic ciprofloxacin for catheter‐associated urinary‐tract infection [see comments]. Lancet 1992;339(8799):946‐51. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gasser 1996 {published data only}
- Gasser TC, Wisard M, Frei R. Oral fleroxacin prophylaxis in transurethral surgery. Journal of Urology 1996;156:146‐8. [PubMed] [Google Scholar]
Little 1974 {published data only}
- Little PJ, Pearson S, Peddie BA, Greenslade NF, Utley WL. Amoxicillin in the prevention of catheter‐induced urinary infection. Journal of Infectious Diseases 1974;129(Suppl 2):S241‐2. [DOI] [PubMed] [Google Scholar]
Meyer 1975 {published data only}
- Meyer FP, Walther H, Matzke G. The need for early therapy in gynecologic patients with postoperative indwelling catheters [German]. Zentralblatt fur Gynakologie 1975;97(6):333‐42. [PubMed] [Google Scholar]
Ragnaud 1983 {published data only}
- Ragnaud JM, Gin H, Tauzin‐Fin P, Ballanger P, Ballanger R, Aubertin J. Prevention of post‐operative infections in urologic surgery. Comparative study of cotrimoxazole and cefazolin. [French]. Pathologie Biologie 1983;31:434‐7. [PubMed] [Google Scholar]
Shohet 1983 {published data only}
- Shohet I, Alagam M, Shafir R, Tsur H, Cohen B. Postoperative catheterization and prophylactic antimicrobials in children with hypospadias. Urology 1983;22(4):391‐3. [DOI] [PubMed] [Google Scholar]
Stricker 1988 {published data only}
- Stricker PD, Grant AB. Relative value of antibiotics and catheter care in the prevention of urinary tract infection after transurethral prostatic resection. British Journal of Urology 1988;61(6):494‐7. [DOI] [PubMed] [Google Scholar]
Verbrugh 1988 {published data only}
- Verbrugh HA, Mintjes‐de Groot AJ, Andriesse R, Hamersma K. Postoperative prophylaxis with norfloxacin in patients requiring bladder catheters. European Journal of Clinical Microbiology of Infectious Diseases 1988;7(4):490‐4. [DOI] [PubMed] [Google Scholar]
Additional references
Alsaywid 2012
- Alsaywid BS, Deshpande AV, Smith GH, Farnsworth RH, Webb NR. Antibiotic prophylaxis for transurethral urological procedures. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010074] [DOI] [Google Scholar]
Bernard 2012
- Bernard MS, Hunter KF, Moore KN. A review of strategies to decrease the duration of indwelling urethral catheters and potentially reduce the incidence of catheter‐associated urinary tract infections. Urological Nursing 2012;32(1):29‐37. [PubMed] [Google Scholar]
CDC 2008
- Horan TC, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. American Journal of Infection Control 2008;36(5):309‐32. [DOI] [PubMed] [Google Scholar]
Foon 2012
- Foon R, Toozs‐Hobson P, Latthe P. Prophylactic antibiotics to reduce the risk of urinary tract infections after urodynamic studies. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD008224.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systemtic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester: John Wiley & Sons, 2011.
Huth 1992
- Huth TS, Burke JP, Larsen RA, Classen DC, Stevens LE. Randomized trial of meatal care with silver sulfadiazine cream for the prevention of catheter‐associated bacteriuria. Journal of Infectious Diseases 1992;165(1):14‐8. [MEDLINE: ; 15405] [DOI] [PubMed] [Google Scholar]
Klevens 2007
- Klevens RM, Edwards JR, Richards CL Jr, Horan TC, Gaynes RP, Pollock DA, et al. Estimating health care‐associated infections and deaths in U.S. hospitals. Public Health Reports 2007;122(2):160‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
National Audit Office 2009
- National Auidt Office. Reducing Healthcare Associated Infections in Hospitals in England. London Stationery Office. London: NAO, 2009.
Niël‐Weise 2005a
- Niël‐Weise BS, Broek PJ. Urinary catheter policies for short‐term bladder drainage in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004203.pub2] [DOI] [PubMed] [Google Scholar]
Petronella 2012
- Petronella P, Scorzeli M, Fiore A, Corbisiero MC, Agresti E, Esposito S, et al. Antibiotic prophylaxis in catheter‐associated urinary infections. New Microbiologica 2012;35(2):191‐8. [PubMed] [Google Scholar]
Pfefferkorn 2009
- Pfefferkorn U, Lea S, Moldenhauer J, Peterli R, Flue M, Ackerman C. Antibiotic prophylaxis at urinary catheter removal prevents urinary tract infections: a prospective randomized trial. Annals of Surgery 2009;249(4):573‐5. [DOI] [PubMed] [Google Scholar]
Plowman 1997
- Plowman RP, Graves N, Roberts JA. Hospital Acquired Infection. Office of Health Economics, London. 1997.
Pratt 2007
- Pratt RJ, Pellowe CM, Wilson JA, Loveday HP, Harper PJ, Jones SR, et al. epic2: National evidence‐based guidelines for preventing healthcare‐associated infections in NHS hospitals in England. Journal of Hospital Infection 2007;65(Suppl 1):S1‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumm 2008
- Schumm K, Lam TBL. Types of urethral catheters for management of short‐term voiding problems in hospitalised adults. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD004013.pub3] [DOI] [PubMed] [Google Scholar]
Scott 2009
- Scott RD. The direct medical costs of healthcare‐associated infections in U.S. hospitals and the benefits of prevention. Altanta, GA: Division of Healthcare Quality Promotion, National Center for Preparedness, Detection, and Control of Infectious Diseases, Coordinating Center for Infectious Diseases, Centers for Disease Control and Prevention, 2009. Available from: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. [Google Scholar]
Stickler 2008
- Stickler DJ, Morgan SD. Observations on the development of the crystalline bacterial biofilms that encrust and block Foley catheters. Journal of Hospital Infection 2008;69(4):350‐60. [DOI] [PubMed] [Google Scholar]
Tambyah 2002
- Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter‐associated urinary tract infection in the era of managed care. Infection Control and Hospital Epidemiology 2002;23(1):27‐31. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [MEDLINE: ] [PubMed] [Google Scholar]
Warren 2001
- Warren JW. Catheter‐associated urinary tract infections. International Journal of Antimicrobial Agents 2001;17:299‐303. [MEDLINE: ; 15414] [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Niël‐Weise 2005b
- Niël‐Weise BS, Broek PJ. Antibiotic policies for short‐term catheter bladder drainage in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005428] [DOI] [PubMed] [Google Scholar]


