Abstract
Background
Bronchiolitis is one of the most common respiratory problems in the first year of life. The sputum of infants with bronchiolitis has increased deoxyribonucleic acid (DNA) content, leading to mucous plugging and airway obstruction. Recombinant human deoxyribonuclease (rhDNase), an enzyme that digests extracellular DNA, might aid the clearance of mucus and relieve peripheral airway obstruction.
Objectives
To determine the effect of nebulised rhDNase on the severity and duration of viral bronchiolitis in children younger than 24 months of age in the hospital setting.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 7 which includes the Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to July Week 4, 2012), EMBASE (1974 to August 2012) and LILACS (1982 to August 2012).
Selection criteria
Randomised controlled trials (RCTs) using nebulised rhDNase alone or with concomitant therapy in children younger than 24 months of age hospitalised with acute bronchiolitis.
Data collection and analysis
Two review authors independently performed literature searches, assessed trial quality and extracted data. We obtained unpublished data from trial authors. We used Review Manager 5.1 to pool treatment effects expressed as the mean difference (MD) or standardised mean difference (SMD) with 95% confidence intervals (CI).
Main results
Three RCTs (333 participants) were identified, two of which were multicentre trials comprising only participants positive for respiratory syncytial virus (RSV). The other trial enrolled participants clinically diagnosed with bronchiolitis from a hospital in Italy. All studies used 2.5 mL (1 mg/mL) of nebulised rhDNase compared with placebo either as a daily or a twice daily dose. Adjunctive therapy included nebulised salbutamol, steroids, supplemental oxygen, intravenous fluids or tube feeding, nasal washing, nasal decongestants and antibiotics.
Overall, nebulised rhDNase showed no benefit in clinically meaningful outcomes. Meta‐analysis favoured the control group with a shorter duration of hospital stay (MD 0.50; 95% CI 0.10 to 0.90, P = 0.01) and better clinical score improvement (SMD ‐0.24; 95% CI ‐0.50 to 0.01, P = 0.06). The largest trial showed no difference in supplemental oxygen use or intensive care unit (ICU) admission.
In one RCT, four out of 11 patients in the treatment group had atelectasis. Two of these patients showed distinctive clinical improvement after nebulised rhDNase.
There was no significant difference in adverse events. These included temporary desaturation, temporary coughing, increased coughing, facial rash, hoarseness, dyspnoea and bad taste, reported in a total of 11 patients from both treatment groups.
Authors' conclusions
The results based on the three included studies in this review did not support the use of nebulised rhDNase in children under 24 months of age hospitalised with acute bronchiolitis. In these patients, treatment did not shorten the length of hospitalisation or improve clinical outcomes. It might have a role in severe bronchiolitis complicated by atelectasis, but further clinical studies would need to be performed.
Keywords: Humans; Infant; Administration, Inhalation; Bronchiolitis, Viral; Bronchiolitis, Viral/drug therapy; Deoxyribonucleases; Deoxyribonucleases/administration & dosage; Nebulizers and Vaporizers; Pulmonary Atelectasis; Pulmonary Atelectasis/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Nebulised deoxyribonuclease for viral bronchiolitis in children younger than 24 months
Bronchiolitis is the most common respiratory illness leading to hospitalisations in infants. Viral infections, particularly respiratory syncytial virus, are the usual cause, which lead to blockage of the small airways of the lungs due to inflammation and increased mucus production. Afflicted children have fever, cough, wheezing and difficulty breathing. Treatment is usually supportive. In bronchiolitis, the mucus produced contains large amounts of DNA, which makes it thicker and stickier. Removal of this DNA facilitates clearance of the mucus. RhDNase is an enzyme that breaks down DNA and hence may improve symptoms. We performed this review to assess the effect of rhDNase delivered through a nebuliser in children under 24 months old hospitalised for bronchiolitis.
We identified three randomised controlled trials involving 333 children up to 24 months of age hospitalised with bronchiolitis. All three studies compared nebulised rhDNase with placebo. Any additional treatments were given to both groups. Overall, the studies did not show that nebulised rhDNase shortened the duration of hospital admission, or improved the severity of symptoms. No serious side effects were reported by any of the studies.
One study showed that in patients suffering from atelectasis, a severe complication of bronchiolitis wherein the lung does not expand completely, nebulised rhDNase treatment resulted in a distinct improvement within two days. To confirm this beneficial effect, further clinical studies in patients with severe bronchiolitis are needed. Currently, the use of this treatment in young children hospitalised with bronchiolitis is not recommended.
Background
Description of the condition
Bronchiolitis is one of the most common respiratory problems in the first year of life. It is usually self limiting, with only a small proportion of affected children needing hospital admission. Despite this, it is a major cause of morbidity and mortality in this age group and is the leading cause of infant hospitalisation in the USA (Leader 2003). In Europe and the USA, up to 3% of infants under 12 months of age are hospitalised with bronchiolitis (Deshpande 2003; Hall 2009). Most cases are viral in origin, respiratory syncytial virus (RSV) being the most common cause (Manoha 2007).
The predominant pathological feature of acute bronchiolitis is inflammation of the respiratory and terminal bronchioles (Calogero 2007; Wohl 2003). Viral infection results in death of respiratory epithelial cells. The epithelium sloughs off and together with inflammatory cells, produces cellular debris to form a thick mucous plug. Combined with oedema and cellular infiltration around the airway, the viscous mucus is responsible for obstructing the airway and disrupting normal airflow. Bronchiolitis is diagnosed clinically by fever, cough, increased respiratory rate, accessory muscle use, expiratory wheezing, inspiratory crackles and increased mucus production.
Although much is known about the mechanism and manifestation of bronchiolitis, treatment remains controversial. Currently the management of this condition is mainly supportive, including supplemental oxygen, nasal washing, adequate fluid intake, a suitable thermal environment to minimise oxygen consumption and mechanical ventilation when necessary. Interventions aimed at reducing mucus production and increasing clearance of airway secretions, such as bronchodilators, corticosteroids and chest physiotherapy, are also widely used. However, recent reviews have failed to show a consistently significant benefit in the routine use of these symptomatic treatments (Corneli 2007; Gadomski 2010; Fernandes 2010; Perrotta 2007). At present there are promising reports that epinephrine (Hartling 2011) and nebulised hypertonic saline (Zhang 2011) may be beneficial in reducing the length of hospital stay but further research is required to confirm these findings.
Description of the intervention
In bronchiolitis, degenerating leukocytes and epithelial cells release large amounts of DNA (Merkus 2001). DNA has an inherent tendency to form a viscous gel, contributing to increased viscosity and adhesiveness of the mucus (Armstrong 1950). Removal of this DNA facilitates clearance of the mucus. RhDNase is an enzyme that digests extracellular DNA. Initially developed for patients with cystic fibrosis, rhDNase greatly reduces the viscosity of purulent sputum (Shak 1990). It is delivered in the nebulised form, wherein liquid rhDNase is converted to a fine mist for inhalation. Despite being expensive, rhDNase is now an established treatment in cystic fibrosis (Suri 2002).
In addition, nebulised rhDNase has been used in the treatment of other lung diseases with significant mucous plugging or impaired mucociliary clearance (Boogaard 2007a). Studies performed in children with asthma (Greally 1995; Patel 2000; Puterman 1997), severe atelectasis (Erdeve 2007; Hendriks 2005; Kupeli 2003), primary ciliary dyskinesia (ten Berge 1999) and RSV bronchiolitis (Merkus 2001) have reported varying degrees of improvement in lung function, sputum volume, oxygen need and chest X‐ray (CXR) appearance.
How the intervention might work
One of the predominant pathological features in bronchiolitis is mucous plugging. The sputum of infants with bronchiolitis has increased DNA content (Wohl 2003). RhDNase, by digesting DNA, might aid the clearance of mucus and relieve peripheral airway obstruction. Recent studies on rhDNase have shown improvement of radiologic abnormalities seen in RSV bronchiolitis (Merkus 2001; Nasr 2001).
Why it is important to do this review
The mucolytic property of rhDNase has been well documented in cystic fibrosis, and case reports have shown promising results in other respiratory diseases such as bronchiolitis. Nevertheless, it has not yet been established whether rhDNase improves clinical outcomes in viral bronchiolitis.
Objectives
To determine the effect of nebulised rhDNase on the severity and duration of viral bronchiolitis in children younger than 24 months of age in the hospital setting.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
We included children younger than 24 months of age with documented bronchiolitis in the hospital setting. Acute bronchiolitis was defined as the first episode of acute wheezing associated with rhinorrhoea, sneezing, cough, fever or tachypnoea. Confirmation of viral aetiology was not necessary for study inclusion.
We excluded participants who were born before 32 weeks of gestation, had low birth weight (< 2.5 kg), chronic lung disease or heart disease.
Types of interventions
Nebulised rhDNase alone versus control.
Nebulised rhDNase plus any form of concomitant therapy (intervention) versus the same form of concomitant therapy alone (control).
Types of outcome measures
Primary outcomes
Duration of hospitalisation (days)
Secondary outcomes
Clinical score
Respiratory rate
Wheezing
Accessory muscle use
Oxygen saturation and duration of supplemental oxygen
Number of intensive care admissions
Radiological score and findings
Adverse events
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 7, part of The Cochrane Library, www.thecochranelibrary.com (accessed 3 August 2012), which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to July Week 4, 2012), EMBASE (1974 to August 2012) and LILACS (1982 to August 2012).
We used the following search terms to search CENTRAL and MEDLINE. We adapted these terms to search EMBASE (see Appendix 1) and LILACS (see Appendix 2). There were no language or publication restrictions.
MEDLINE (Ovid)
1. Exp Bronchiolitis 2. bronchiolit*.tw. 3. respiratory syncytial viruses/ or respiratory syncytial virus, human/ 4. Respiratory Syncytial Virus Infections/ 5. (respiratory syncytial virus* or rsv).tw. 6. adenoviridae/ or exp mastadenovirus/ 7. adenoviridae infections/ or adenovirus infections, human/ 8. adenovir*.tw. 9. Influenza, Human/ 10. (influenza or flu).tw. 11. exp Paramyxoviridae Infections/ 12. parainfluenza*.tw. 13. or/1‐12 14. exp Deoxyribonucleases/ 15. exp Deoxyribonuclease I/ 16. deoxyribonucleas*.tw. 17. dna nucleas*.tw. 18. dnase.tw. 19. rhdnase.tw. 20. or/14‐19 21. 20 and 13
Searching other resources
We contacted experts in this field, checked conference abstracts and consulted www.clinicalstudyresults.org for unpublished studies. We checked reference lists of all relevant articles to identify other relevant studies. We contacted the authors of any identified abstracts to ascertain the nature of the study design and outcome measures. We only included abstracts with sufficient information on the study design and outcome measures.
Data collection and analysis
Selection of studies
Two review authors (IWC, AE) independently identified the studies and assessed whether they met the inclusion criteria. The third review author (CM) was designated to resolve any discrepancies. We identified studies for the review based on their abstracts. We retrieved the full‐text articles if there was insufficient information in the abstract.
Data extraction and management
Two review authors (IWC, AE) independently performed data extraction. We collected the following data using a standardised data extraction form for each included study.
Study characteristics: title of the study, names of authors, publication status, setting.
Method: method of allocation, concealment of randomisation and specification of who was blinded (clinicians caring for the patients, assessors, data managers or the care giver).
Participants: age, demographic factors, inclusion and exclusion criteria, withdrawal or loss to follow‐up.
Disease: diagnostic criteria of bronchiolitis, duration of illness, RSV status.
Intervention: type, dose, duration, route and co‐interventions.
Control: type, dose, duration, route and co‐interventions.
Outcome: we extracted the mean, standard deviation and the number of participants studied in each group for continuous outcomes. We extracted the total number of participants per group and the number of participants experiencing the event for dichotomous outcomes.
We entered the extracted data into RevMan 2011, The Cochrane Collaboration's software program.
Assessment of risk of bias in included studies
Two review authors (IWC, AE) independently assessed the methodological quality of all included studies using the Cochrane Collaborations' tool for assessing risk of bias (Higgins 2011). Two review authors (IWC, AE) independently assessed the following six domains in each study.
1. Sequence generation (selection bias)
Low risk: sequence generated by random number table, computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots or minimisation. Unclear risk: insufficient information about the sequence generation process to permit judgement but did mention 'randomisation'. High risk: sequence generated by odd or even dates of birth, date of admission or hospital or clinic record number, judgement of the clinician, availability of the intervention, results of laboratory tests or preference of the participant.
2. Allocation concealment (selection bias)
Low risk: participant and investigators could not foresee assignment because of central allocation, drug container of identical appearance or sealed, opaque envelopes. Unclear risk: insufficient information about the allocation concealment to permit judgement but did mention 'randomisation'. High risk: participant and investigators could foresee assignment because of open random allocation schedule, alteration or rotation of allocation, unsealed or non‐opaque envelopes, or allocation based on date of birth, case record number or any other explicitly unconcealed procedure.
3. Blinding of outcome assessment (detection bias)
Low risk: blinding of key study personnel and participants or no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding. Unclear risk: insufficient information to permit judgement of 'low risk' or 'high risk' or the study did not address this outcome. High risk: no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
4. Incomplete outcome data addressed (attrition bias)
Low risk: no missing outcome data, reasons for missing outcome data unlikely to be related to true outcome, missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups, for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate, for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size or missing data have been imputed using appropriate methods. Unclear risk: insufficient or no reporting of missing outcome data. High risk: reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size, 'as‐treated' analysis done with substantial departure of the intervention received from that assigned at randomisation or potentially inappropriate application of simple imputation.
5. Selective outcome reporting (reporting bias)
Low risk: the study protocol may or may not be available but all of the study's expected primary or secondary outcomes have been reported in the pre‐specified way. Unclear risk: insufficient information to permit judgement about outcome reporting. High risk: not all of the study's pre‐specified primary outcomes have been reported, one or more of the reported outcomes were not pre‐specified, one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis or the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
6. Other bias
Low risk: the study appears to be free of other sources of bias. Unclear risk: there may be a risk of bias but there is insufficient information to assess whether an important risk of bias exists. High risk: there is at least one important risk of bias, has been claimed to have been fraudulent or had some other problem.
We defined high‐quality trials as those with low risk in sequence generation, allocation concealment, blinding and loss to follow‐up.
Measures of treatment effect
We measured the outcomes obtained as continuous variables. We used RevMan 2011 to pool treatment effects. For continuous variables measured on the same scale, we calculated the mean difference (MD) with 95% confidence intervals (CI). We combined clinical scores assessed with different scales using the standardised mean difference (SMD) with 95% CI.
Dealing with missing data
We contacted the trial authors directly if there were missing data or insufficient information was presented in the published trial. We were able to obtain further information regarding the interventions used from two of the trials (Boogaard 2007b; Nenna 2009). To enable us to combine study outcomes, we obtained raw data for the duration of hospitalisation and for the separate components of the clinical scores from one of the trials (Boogaard 2007b). We then calculated the mean and SD for these variables prior to performing the meta‐analyses.
Assessment of heterogeneity
We measured heterogeneity by using the I2 statistic, with a value greater than 50% considered to be substantial (Higgins 2011).
Assessment of reporting biases
We would have used a funnel plot to detect any publication bias. This was not necessary due to the small number of included trials.
Data synthesis
We calculated a weighted treatment effect across trials using RevMan 2011 based on a fixed‐effect model. For continuous outcomes, we calculated the MD or the SMD, expressing the pooled treatment effects with 95% CIs.
Subgroup analysis and investigation of heterogeneity
We did not perform subgroup analysis.
Sensitivity analysis
We performed sensitivity analysis for methodological quality. No additional sensitivity analysis was conducted as no other issues were identified during the review process.
Results
Description of studies
Results of the search
The electronic searches retrieved 1686 citations.
We identified three RCTs and three conference abstracts, which were reviewed in full text. The three abstracts corresponded to the three RCTs, which met all the criteria for study selection for this review (see Characteristics of included studies table). No other studies were identified from other resources.
Included studies
Population
All three studies were randomised, double‐blind, placebo‐controlled clinical trials involving inpatients with documented bronchiolitis. Two were multicentre trials, one from the Netherlands (Boogaard 2007b: 10 hospitals) and one from the USA (Nasr 2001: two hospitals). The other trial enrolled inpatients from one hospital in Rome (Nenna 2009). The multicentre trials only included patients with RSV detected from nasopharyngeal samples; the other trial used clinical diagnosis later supported with viral studies. One study enrolled participants with gestational ages from 32 weeks (Boogaard 2007b), while the remaining two studies enrolled subjects born after 37 weeks of gestation. The upper age limits used were six months (Nenna 2009), 12 months (Boogaard 2007b) and 24 months (Nasr 2001).
Intervention
All studies used 2.5 mL (1 mg/mL) of rhDNAse delivered by jet nebulisation. For the control participants, two of the studies gave nebulised saline (Boogaard 2007b; Nenna 2009). The other study gave rhDNase in a solution made with 150 mM sodium chloride and 1.5 mM calcium chloride (Nasr 2001). This excipient was given to the control group.
One trial (Nasr 2001) gave all participants nebulised salbutamol as part of their bronchiolitis protocol, with some of the participants also receiving steroids for three to five days. In the other two studies additional treatment included supplemental oxygen, intravenous fluids or tube feeding, nasal washing, nasal decongestants, antibiotics and bronchodilators. Two trials used a daily dose for up to five days (Nasr 2001; Nenna 2009) and the other gave the dose twice daily until discharge (Boogaard 2007b).
Outcome measures
All the studies compared length of hospital stay (LOS). To report their results, the largest trial (Boogaard 2007b) used geometric means while the other two trials calculated arithmetic means. In order to combine the LOS results from all three studies, we obtained the raw data from the authors of the largest trial and calculated the arithmetic means.
The same clinical scoring system was used in two trials (Boogaard 2007b; Nasr 2001) on admission and at discharge. Initially described by Wang (Wang 1992), the score rated respiratory rate, wheezing, retraction and general condition from zero to three (the higher score corresponding to increased severity). One of these studies also compared CXRs taken during admission and at the completion of the study (Nasr 2001). The other multicentre trial compared duration of supplemental oxygen use and intensive care unit (ICU) admission (Boogaard 2007b).
For one of the trials (Boogaard 2007b), we used the unpublished raw data provided by the trial authors to calculate the change in clinical score between the day of admission (day one) and day three; and also to determine individual scores (respiratory rate, wheezing and retraction) on an intention‐to‐treat (ITT) basis. If a score was missing, we assumed complete patient recovery and thus assigned a score of zero for each parameter. For the respiratory rate score, there were 28 participants in the control group and 28 participants in the treatment group with missing data; for the wheezing score, there were 38 participants in the control group and 52 participants in the treatment group with missing data; and for the retraction score, there were 39 participants in the control group and 51 participants in the treatment group with missing data.
One trial (Nenna 2009) used a different scoring system. The total clinical score was based on oxygen saturation, retractions, respiratory rate, feeding and chest X‐ray findings.
Only one study reported adverse events (Boogaard 2007b).
Excluded studies
We excluded one case series (Merkus 2001) describing the effect of nebulised rhDNase on five infants with severe RSV bronchiolitis and atelectasis.
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 1.
1.
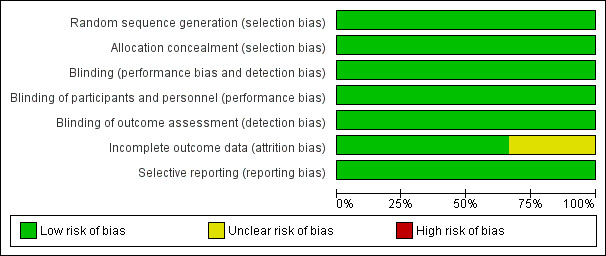
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All the studies clearly described randomisation and allocation concealment methods.
Blinding
Each trial appropriately outlined blinding.
Incomplete outcome data
Descriptions of withdrawals were adequate in all the trials. In the largest study (Boogaard 2007b), two (one from each study arm) out of 224 participants were not included in the ITT analysis as consent was withdrawn after the first dose. 30 participants were excluded from the per‐protocol analysis for varying reasons as specified in the study.
Another trial had 11 out of 86 enrolled participants excluded from the study because of missing data (Nasr 2001) and the smallest trial (Nenna 2009) had no patient loss to follow‐up with all 22 participants included.
Selective reporting
No reporting bias was identified. There were no statistically significant positive results reported by any of the published trials.
Other potential sources of bias
Analysis from one of the studies was based on ITT (Boogaard 2007b) for all randomised participants and a separate per‐protocol analysis was performed which excluded participants violating the study protocol. Results from both analyses were similar.
Effects of interventions
A total of 333 inpatients were enrolled by the three randomised controlled trials (RCTs) and data from 319 participants were analysed. The treatment was easy to administer.
Duration of hospital stay
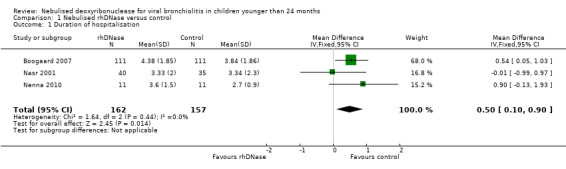
Each individual trial reported that rhDNase treatment had no statistically significant effect on the duration of hospital stay. Meta‐analysis showed that the duration of hospital stay was significantly shorter in the control group (Figure 2), with a pooled mean difference (MD) of 0.50 days (95% confidence interval (CI) 0.10 to 0.90, P = 0.01). There was no significant heterogeneity in results between studies (I2 statistic = 0%).
2.
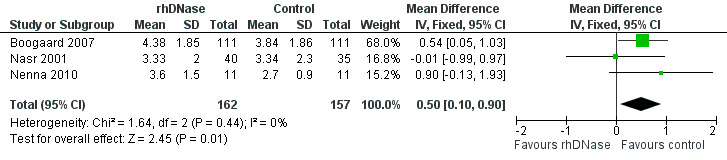
Forest plot of comparison: duration of hospitalisation
Clinical score
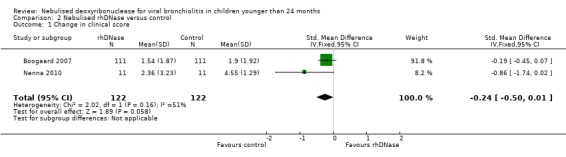
None of the trials found any statistically significant difference in clinical scores between the control and intervention groups. We pooled data from two trials (Boogaard 2007b; Nenna 2009) comparing clinical scores on admission (assigned as day one) and day three. Although not statistically significant, Figure 3 shows that the control group overall had a higher improvement in clinical score than the rhDNase group (standardised mean difference (SMD) ‐0.24; 95% CI ‐0.50 to 0.01, P = 0.06, I2 statistic = 65%).
3.
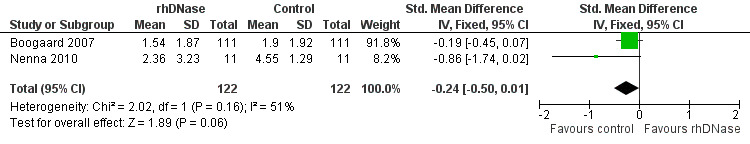
Forest plot of comparison: change in total clinical score
These studies used different overall scoring systems and had different lengths of stay. The smaller study (22 participants, Nenna 2009) evaluated clinical score differences between the study and placebo groups every day until day four only, while the larger study recorded clinical scores until discharge. Nenna 2009 reported that two participants in the intervention group showed a worsening of clinical scores, perhaps due to their young age (both one‐month old males). This may explain the apparent favouring of the control group in this RCT, which may in turn account for the significant heterogeneity between the studies (in addition to the use of different clinical scoring systems).
Respiratory rate
The individual scores (respiratory rate score, wheezing score and chest retraction score) were combined from two studies (Boogaard 2007b; Nasr 2001), both of which used the same scoring system (Wang 1992). The differences between the individual scores in the larger study (Boogaard 2007b) were derived from the raw data on day of admission and day three. The differences between the individual scores in the other study (Nasr 2001) were based on the scores on the day of admission and the day of discharge (the average length of stay being 3.34 days in the control group and 3.33 days in the intervention group).
Meta‐analysis of the differences in respiratory rate score (MD 0.06; 95% CI ‐0.15 to 0.28, P = 0.55, I2 statistic = 0%), favoured the rhDNase group but not in a statistically significant manner (Figure 4).
4.
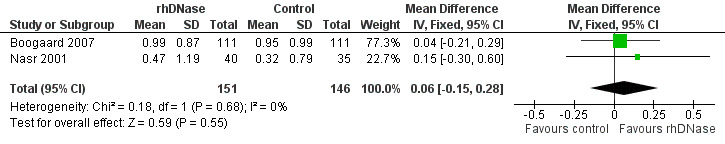
Forest plot of comparison: change in respiratory rate score
Wheezing
Meta‐analysis of the differences in wheezing score (MD ‐0.03; 95% CI ‐0.21 to 0.16, P = 0.78, I2 statistic = 0%) favoured the control group; but this was not statistically significant (Figure 5).
5.
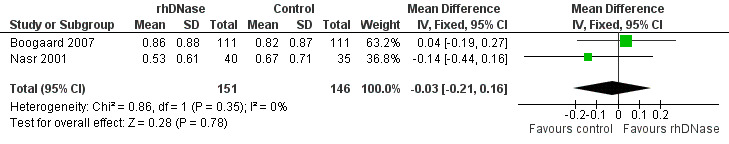
Forest plot of comparison: change in wheezing score
Accessory muscle use
The studies assessed accessory muscle use, with severity ranging from none to severe chest retractions with nasal flare (Wang 1992). Meta‐analysis (Figure 6) showed that the differences in chest retraction score (MD ‐0.02; 95% CI ‐0.19 to 0.14, P = 0.77, I2 statistic = 0%) were higher in the control group is favoured; but this was not statistically significant.
6.
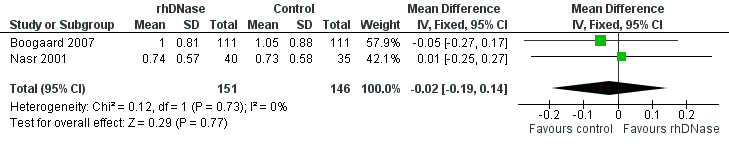
Forest plot of comparison: change in retraction score
Oxygen saturation and duration of supplemental oxygen
One of the studies (Nenna 2009) incorporated oxygen saturation in calculating the clinical severity score. A score of zero was given for levels higher than 95%; one for saturations of 90% to 94%; and two for levels below 89%. The actual data was not reported individually.
In the largest trial (Boogaard 2007b), supplemental oxygen was administered once oxygen saturation fell consistently below 93%, and was discontinued once it was consistently over 92%. There was no significant difference (expressed as a ratio of the geometric means of rhDNase and placebo groups with 95% CI) in the duration of supplemental oxygen use (1.29; 95% CI 0.99 to 1.67, P = 0.053).
Number of intensive care admissions
In the same study (Boogaard 2007b), seven children (3.2%) required transfer to the intensive care unit (ICU), five of whom received mechanical ventilation, with no significant differences observed between the control and intervention groups in terms of the proportion of individuals requiring intensive care (P = 1.0) or mechanical ventilation (P = 0.43).
Radiological score and findings
The trial by Nasr 2001 measured radiological changes before and after treatment. Analysing the treatment and control arms separately, they reported a significant improvement (chest X‐ray difference score was 0.46, P = 0.02) in the intervention group, compared with a significant deterioration (chest X‐ray difference score was ‐0.60, P = 0.02) in the placebo group. The authors then performed a one‐way analysis of covariance using the hospital discharge chest X‐ray scores as the dependent variable and the hospital admission score as the covariate. This revealed that there was a significant difference between the groups (P = 0.01) in spite of the fact that group assignment was random: chest X‐ray and respiratory rate variables showed trends suggesting that the rhDNase group (40 participants) was more severely affected than the placebo group (35 participants) at the beginning of the trial.
In another trial (Nenna 2009), chest X‐ray showed atelectasis in four out of 11 children in the rhDNase group. The authors reported a distinct reduction in clinical score for two of these patients (‐80% and ‐67%) during the first two days of the trial. Both of these patients' symptoms were due to respiratory syncytial virus (RSV).
Adverse events
One study (Boogaard 2007b) described adverse events, which included temporary desaturation, temporary coughing, increased coughing, increased mucus, facial rash, hoarseness, bad taste and dyspnoea. However, no statistical difference was observed between the intervention (eight events in total) and control (three events in total) groups. The other studies specified that no adverse events were registered (Nasr 2001; Nenna 2009). In particular, no airway hyperactivity or bronchospasm were observed.
Discussion
Summary of main results
The results based on the three included studies in this review did not show benefit with nebulised recombinant human deoxyribonuclease (rhDNase) treatment. In these trials involving children under 24 months of age hospitalised with bronchiolitis, the treatment did not shorten the length of hospital stay, improve clinical scores, decrease supplemental oxygen use or reduce intensive care unit (ICU) admission. Our analyses showed that children in the control group had significantly shorter hospitalisation (mean difference (MD) 0.50; 95% confidence interval (CI) 0.10 to 0.90, P = 0.01) and better clinical outcomes (standardised mean difference (SMD) ‐0.24; 95% CI ‐0.50 to 0.01, P = 0.06).
One study found significant improvement in radiological appearances in the intervention group compared to deterioration in the control group. In another randomised controlled trial (RCT) four out of 11 patients in the treatment group had atelectasis. Two of these patients with atelectasis showed distinctive clinical improvement after nebulised rhDNase.
No statistically significant adverse events were reported with nebulised rhDNase.
Overall completeness and applicability of evidence
There was no significant heterogeneity among the trials, all of which delivered the same amount of rhDNase by jet nebulisation using the corresponding excipient as control. One trial used twice daily dosing (Boogaard 2007b) rather than once daily. Another one co‐administered salbutamol as part of their protocol and notably did not report any adverse events (Nasr 2001).
The two multicentre trials enrolled only patients with respiratory syncytial virus (RSV)‐proven bronchiolitis (Boogaard 2007b; Nasr 2001), thus the results may not necessarily be applicable to non‐RSV bronchiolitis.
The outcomes measured across the three studies had similar results, making them relevant to children under 24 months of age hospitalised with acute RSV bronchiolitis.
In one study (Nasr 2001), the participants in the intervention group were found to be more severely affected than those in the control group, despite randomisation. This trial indicated radiological improvement after nebulised rhDNase treatment. Chest X‐ray changes are a surrogate endpoint and no significant benefit was shown in clinically relevant outcomes such as symptom improvement. Hence the clinical significance of chest X‐ray improvement is unclear.
The trials excluded children with risk factors for severe bronchiolitis and those who required intensive care at admission, so that most of the patients enrolled only had mild airway obstruction. The authors of the original studies suggested that this might help explain the lack of benefit seen after rhDNase treatment.
Indeed, the noticeable improvement observed in participants with atelectasis suggests that nebulised rhDNase may be more beneficial in severe bronchiolitis. However, subgroup analysis performed in the largest trial (Boogaard 2007b) showed that even in patients with more severe symptoms, rhDNase treatment did not curtail hospital stay or improve clinical outcomes.
In addition, none of the patients received airway clearance therapy, which is usually given concurrently to children with cystic fibrosis to help evacuate liquefied mucus (Boogaard 2007b). Especially in the much younger children who were unable to cough as effectively, this may have been a useful adjunct to potentiate the effect of nebulised rhDNase.
Quality of the evidence
We assessed all trials included to be of high quality, scoring 'low risk' in sequence generation, allocation concealment, blinding and loss to follow‐up.
Potential biases in the review process
There was no disagreement between the two review authors regarding any of the assessment parameters. There was 100% agreement regarding the trials included and excluded.
Agreements and disagreements with other studies or reviews
The beneficial effect of rhDNase on two patients with atelectasis (Nenna 2009) is consistent with the improvement seen in an earlier case series (Merkus 2001). They reported rapid and marked clinical and radiological improvement after administration of nebulised rhDNase in patients with atelectasis secondary to severe RSV bronchiolitis. Mechanical ventilation was averted in two infants and the three on artificial ventilation made a speedy recovery.
Authors' conclusions
Implications for practice.
The results from the three included studies do not support the use of nebulised rhDNase in previously healthy children under the age of 24 months hospitalised with mild to moderately severe bronchiolitis. While safe and easy to administer, this intervention did not reduce the duration of hospital stay or accelerate the rate of clinical improvement in such patients.
Implications for research.
While respiratory syncytial virus (RSV) remains the most common cause of bronchiolitis in infants, the efficacy of nebulised recombinant human deoxyribonuclease (rhDNase) in complicated bronchiolitis caused by non‐RSV pathogens will need to be clarified.
Nebulised rhDNase is more likely to be of benefit in patients with atelectasis. Clinical studies on patients with severe bronchiolitis, such as those requiring mechanical ventilation or intensive care, are needed to establish any effects. Future studies could also determine if in combination with airway clearance therapy, rhDNase might decrease duration of ICU stay, artificial ventilation and steroid dose, and reduce complications such as secondary bacterial infections (from retained mucus).
History
Protocol first published: Issue 3, 2010 Review first published: Issue 11, 2012
| Date | Event | Description |
|---|---|---|
| 9 September 2010 | Amended | Contact details updated. |
Acknowledgements
The review authors wish to thank the following people. For commenting on the draft protocol: Anne Lyddiatt, Carla Perrotta, Ruben Boogaard, Hema Patel, Nelcy Rodriguez and Anca Zalmanovici. For providing ongoing assistance regarding protocol and review submissions: Liz Dooley (Managing Editor, Cochrane Acute Respiratory Infections Group). For assisting with the electronic searches: Sarah Thorning (Trials Search Co‐ordinator, Cochrane Acute Respiratory Infections Group). For commenting on the draft review: Manal Kassab, Carla Perrotta, Ruben Boogaard, Elaine Beller and Anca Zalmanovici Trestioreanu.
Appendices
Appendix 1. Embase.com search strategy
#22. #18 AND #21 9 4 Aug 2010 #21. #19 OR #20 795,476 4 Aug 2010 #20. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR volunteer*:ab,ti OR allocat*:ab,ti OR assign*:ab,ti OR ((singl* OR doubl*) NEAR/2 (blind* OR mask*)):ab,ti AND [embase]/lim 757,735 4 Aug 2010 #19. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim 221,080 4 Aug 2010 #18. #13 AND #17 137 4 Aug 2010 #17. #14 OR #15 OR #16 8,858 4 Aug 2010 #16. deoxyribonucleas*:ab,ti OR 'dna nuclease':ab,ti OR dnase:ab,ti AND rhdnase:ab,ti AND [embase]/lim 143 4 Aug 2010 #15. 'deoxyribonuclease i'/de AND [embase]/lim 3,853 4 Aug 2010 #14. 'deoxyribonuclease'/de AND [embase]/lim 5,016 4 Aug 2010 #13. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 116,105 4 Aug 2010 #12. paramyxovir*:ab,ti AND [embase]/lim 1,930 4 Aug 2010 #11. 'paramyxovirus infection'/exp AND [embase]/lim 1,615 4 Aug 2010 #10. influenza*:ab,ti OR flu:ab,ti OR parainfluenza*:ab,ti OR 'para‐influenza':ab,ti AND [embase]/lim 55,378 4 Aug 2010 #9. 'parainfluenza virus infection'/exp OR 'parainfluenza virus'/exp AND [embase]/lim 4,112 4 Aug 2010 #8. 'influenza'/de OR 'influenza virus'/de OR 'influenza virus a'/exp OR 'influenza virus b'/exp AND [embase]/lim 38,100 4 Aug 2010 #7. adenovir*:ab,ti AND [embase]/lim 31,327 4 Aug 2010 #6. 'adenovirus'/de OR 'mastadenovirus'/de AND [embase]/lim 17,385 4 Aug 2010 #5. 'human adenovirus infection'/exp OR 'adenovirus infection'/de AND [embase]/lim 374 4 Aug 2010 #4. 'respiratory syncytial virus':ab,ti OR 'respiratory syncytial viruses':ab,ti OR rsv:ab,ti AND [embase]/lim 8,755 4 Aug 2010 #3. 'respiratory syncytial pneumovirus'/de OR 'respiratory syncytial virus infection'/exp AND [embase]/lim 8,450 4 Aug 2010 #2. bronchiolit*:ab,ti AND [embase]/lim 6,201 4 Aug 2010 #1. 'bronchiolitis'/exp AND [embase]/lim 8,370 4 Aug 2010
Appendix 2. LILACS (BIREME) search strategy
Mh Bronchiolitis OR Tw bronchiolit$ OR Tw bronquiolit$ OR Mh respiratory syncytial viruses OR Mh respiratory syncytial virus, human OR Mh respiratory syncytial virus infections OR Tw respiratory syncytial virus$ OR Tw rsv OR Tw virus sincitiales respiratorios OR Tw virus sinciciais respiratorios OR Mh adenoviridae OR Mh mastadenovirus OR Mh adenoviridae infections OR Mh adenovirus infections, human OR Tw adenovir$ OR Mh influenza, human OR Tw influenza$ OR Tw flu OR Tw gripe humana OR Mh paramyxoviridae infections OR Tw parainfluenza OR Tw paramyxovirid$ [Words] and Mh deoxyribonuclease OR Mh deoxyribonuclease 1 OR Tw deoxyribonucleas$ OR Tw dna nucleas$ OR Tw dnase OR Tw rhdnase [Words]
Data and analyses
Comparison 1. Nebulised rhDNase versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of hospitalisation | 3 | 319 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [0.10, 0.90] |
1.1. Analysis.
Comparison 1 Nebulised rhDNase versus control, Outcome 1 Duration of hospitalisation.
Comparison 2. Nebulised rhDNase versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in clinical score | 2 | 244 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.50, 0.01] |
2.1. Analysis.
Comparison 2 Nebulised rhDNase versus control, Outcome 1 Change in clinical score.
Comparison 3. Nebulised rhDNase versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in respiratory rate score | 2 | 297 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.15, 0.28] |
3.1. Analysis.
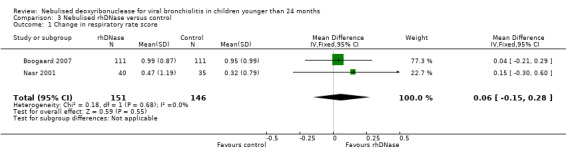
Comparison 3 Nebulised rhDNase versus control, Outcome 1 Change in respiratory rate score.
Comparison 4. Nebulised rhDNase versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in wheezing score | 2 | 297 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.21, 0.16] |
4.1. Analysis.
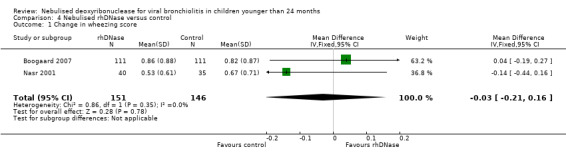
Comparison 4 Nebulised rhDNase versus control, Outcome 1 Change in wheezing score.
Comparison 5. Nebulised rhDNase versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in retraction score | 2 | 297 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.19, 0.14] |
5.1. Analysis.
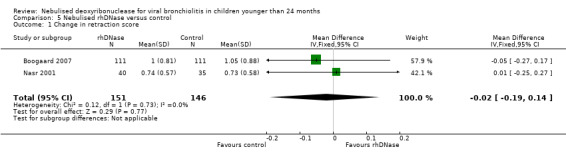
Comparison 5 Nebulised rhDNase versus control, Outcome 1 Change in retraction score.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boogaard 2007.
| Methods | Multicentre, randomised, double‐blind, controlled clinical trial | |
| Participants | Age: < 12 months Gender: male 109, female 113 Inclusion criteria: proven RSV bronchiolitis requiring supplemental oxygen admitted to participating hospitals (10 in total) Exclusion criteria: gestational age < 32/40; infants with cardiopulmonary disease or immunodeficiency; systemic steroids at time of hospital admission; ICU admission before parental consent for study Diagnostic criteria (case definition): RSV by direct immunofluorescence of nasopharyngeal aspirate (NPA) sample Duration of disorder: at admission: days sick 0 to > = 6 |
|
| Interventions | 1. Type: nebulised rhDNase 2. Dose: 2.5 mg twice daily (a 2.5 mL solution of 1 mg/mL rhDNase) 3. Duration: until discharge 4. Compared with: placebo (2.5 mL of sodium chloride 0.9%) 5. Additional treatment: supportive care according to the hospital guidelines. This included nasal washings, nasal decongestants, supplemental oxygen and tube feeding or IV fluids when necessary; antibiotics or bronchodilators |
|
| Outcomes | Length of hospital stay Secondary end points were ‐ duration of supplemental oxygen (supplemental oxygen was started when oxygen saturation was consistently < 93% and stopped when saturation was consistently > 92%) ‐ improvement in symptom score ‐ number of intensive care admissions Adverse events The clinical assessment scoring described by Wang 1992 was utilised |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table sample with blocks of 4 numbers made by the study statistician |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Physicians, nurses, parents and the trial co‐ordinator remained unaware of the intervention assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data from all randomised participants were analysed on an intention‐to‐treat basis. A separate per‐protocol analysis was conducted in which participants who violated the study protocol were excluded 2 participants withdrew from the study after the first dose of study medication (1 in each group) and consequently had no follow‐up data available |
| Selective reporting (reporting bias) | Low risk | |
Nasr 2001.
| Methods | Randomised, double‐blind, placebo‐controlled investigation | |
| Participants | 1. Age: < = 2 years of age 2. Gender: male 47, female 28 3. Inclusion criteria: < = 2 years of age; previously healthy full‐term neonates; hospitalised for proven RSV infection 4. Diagnostic criteria (case definition): specimens for viral isolation and quantitation were obtained from a nasopharyngeal swab and assayed for antigen detection using indirect immunofluorescent antibody staining technique. The criteria for hospitalisation of these participants were decided by the emergency department attending physician at both institutions |
|
| Interventions | 1. Type: rhDNase was provided as a solution (1 mg/mL) in 2.5 mL of excipient (150 mM sodium chloride, 1.5 mM calcium chloride, pH 6.0) 2. Dose: 2.5 mg daily 3. Duration: for up to 5 days 4. Compared with: the placebo was excipient alone Additional treatment: all participants in the 2 groups received albuterol nebulised treatment as part of the RSV protocol in the 2 institutions. 19 (6 in control/13 in intervention) participants received a steroid dose of 2 mg/kg/d for 3 to 5 days as a burst |
|
| Outcomes | Length of stay Difference measures between hospital admission and discharge: ‐ respiratory rate score ‐ wheezing score ‐ retraction score ‐ CXR score The clinical assessment scoring described by Wang 1992 was utilised No adverse events |
|
| Notes | CXR and respiratory rate variables showed trends that suggest that the rhDNase group was more ill than the placebo group in spite of the fact that group assignment was random Significant CXR score improvement after rhDNase versus significant worsening with placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was conducted by the University of Michigan Investigational Drug Service using a random table sample with blocks of 4. All CXRs were coded and randomised |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both physicians and parents were blinded with respect to the treated and placebo groups. The statistician was unaware of treatment status coding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were examined twice daily by a paediatric pulmonologist or study co‐ordinator; all were blinded to the patient’s assignment. 2 paediatric radiologists reviewed the CXRs and were blinded to each patient’s study assignment, identity and date of examination (hospital admission versus discharge) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 participants were excluded from the analysis because of missing data (75 participants were included in the analysis). 11 more participants did not receive hospital admission or discharge CXRs and were excluded from the analysis of CXR scoring (64 participants) |
| Selective reporting (reporting bias) | Low risk | |
Nenna 2010.
| Methods | Randomised, double‐blind, placebo‐controlled study | |
| Participants | 1. Age: < 6 months 2. Gender: 12 males, 10 females 3. Inclusion criteria: previously healthy full‐term neonates; 6 months of age or less at the time of the observation; clinical severity score > = 4; enrolled in a time span of 24 hours from admission to hospital 4. Diagnostic criteria (case definition): clinical diagnosis of bronchiolitis; viral isolation and quantification from NPA; PCR assays for viral detection 5. Duration of disorder: 1 to 4 days before hospitalisation |
|
| Interventions | 1. Type: nebulised rhDNAse 2. Dose: 2.5 mL daily 3. Duration: 3 days 4. Compared with: placebo (saline) 5. Additional treatment: oxygen, intravenous fluids, nebulised salbutamol All participants received the drug with the same nebulising equipment |
|
| Outcomes | Length of hospital stay Days until participants were ready for discharge Days for weight recovery Daily clinical score reduction ‐ SaO2 ‐ Presence of retractions ‐ Respiratory rate ‐ Feeding evaluation ‐ Auscultatory rale presence No adverse effects |
|
| Notes | 4 out of 11 patients in the treatment group had atelectasis; 2 out of the 4 patients with atelectasis showed rapid improvement after rhDNase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table sample with each number corresponding to a pack of 3 doses of the drug/placebo |
| Allocation concealment (selection bias) | Low risk | The allocation codes were not opened until the trial was completed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Throughout the study, both physicians/nurses and parents were blinded in respect to the study or placebo groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants interrupted the study |
| Selective reporting (reporting bias) | Low risk | |
CXR: chest X‐ray d: day ICU: intensive care unit IV: intravenous NPA: nasopharyngeal aspirate PCR: polymerase chain reaction RSV: respiratory syncytial virus rhDNase: recombinant human deoxyribonuclease SaO2: oxygen saturation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Merkus 2001 | Case series only |
Differences between protocol and review
We did not perform subgroup analyses according to patients' age (children under 12 months of age versus children under 24 months of age) and viral aetiology (RSV‐positive versus RSV‐negative). We did not assess any binary or dichotomous outcomes (for which we would have calculated a risk ratio (RR)).
Contributions of authors
Annabelle Enriquez (AE) was responsible for writing the protocol background and review. I‐Wen Chu (IWC) was responsible for writing the protocol methods. AE and IWC were responsible for study selection, quality assessment, data collection and data analysis. Craig Mellis (CM), Wan‐Yu Lin (WYL) and AE are responsible for providing general advice on the protocol. CM was responsible for resolving any disagreements between AE and IWC and for providing general guidance on the review. AE and WYL were responsible for the meta‐analysis and statistical analysis of the raw data. All review authors approved the final version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Rong Sing Medical Foundation, Taiwan.
Research Grant for Dr. I‐Wen Chu
Declarations of interest
None known.
New
References
References to studies included in this review
Boogaard 2007 {published and unpublished data}
- Boogaard R, Hulsmann AR, Hop WCJ, Merkus PJF. Efficacy of recombinant human deoxyribonuclease (Rh‐DNase) in infants hospitalised with respiratory syncytial virus bronchiolitis a multicentre randomised double blind clinical trial [Abstract]. European Respiratory Journal 2006;28(Suppl 50):758s [4365]. [Google Scholar]
- Boogaard R, Hulsmann AR, Veen L, Vaessen‐Verberne AAPH, Yap YN, Sprij AJ, et al. Recombinant human deoxyribonuclease in infants with respiratory syncytial virus bronchiolitis. Chest 2007;131(3):788‐95. [DOI] [PubMed] [Google Scholar]
Nasr 2001 {published data only (unpublished sought but not used)}
- Nasr S, Strouse P, Soskolne E, Maxvold N, Garver K, Rubin B, et al. Efficacy of recombinant human DNase I in the management of RSV bronchiolitis [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A339. [Google Scholar]
- Nasr SZ, Strouse PJ, Soskolne E, Maxvold N, Garver KA, Rubin BK, et al. Efficacy of recombinant human deoxyribonuclease I in the hospital management of respiratory syncytial virus bronchiolitis. Chest 2001;120:203‐8. [DOI] [PubMed] [Google Scholar]
Nenna 2010 {published and unpublished data}
- Corazzesi E, Nenna R, Tromba V, Tuccinardi R, Piacenti S, Scalercio F, et al. Recombinant human deoxyribonuclease treatment in hospital management of infants with moderate to severe bronchiolitis. European Respiratory Journal 2006;28(Suppl 50):758s [4367]. [Google Scholar]
- Nenna R, Tromba V, Berardi R, Angelis D, Papoff P, Sabbatino G, et al. Recombinant human deoxyribonuclease treatment in hospital management of infants with moderate‐severe bronchiolitis. European Journal of Inflammation 2009;7(3):169‐74. [Google Scholar]
References to studies excluded from this review
Merkus 2001 {published data only}
- Merkus PJFM, Hoog M, Gent R, Jongste JC. DNase treatment for atelectasis in infants with severe respiratory syncytial virus bronchiolitis. European Respiratory Journal 2001;18(4):734‐7. [PubMed] [Google Scholar]
Additional references
Armstrong 1950
- Armstrong JB, White JC. Liquefaction of viscous purulent exudate by deoxyribonuclease. Lancet 1950;256(6641):739‐42. [DOI] [PubMed] [Google Scholar]
Boogaard 2007a
- Boogaard R, Jongste JC, Merkus PJ. Pharmacotherapy of impaired mucociliary clearance in non‐CF pediatric lung disease. A review of the literature. Pediatric Pulmonology 2007;42(11):989‐1001. [PUBMED: 17902149] [DOI] [PubMed] [Google Scholar]
Boogaard 2007b
- Boogaard R, Hulsmann AR, Veen L, Vaessen‐Verberne AAPH, Yap YN, Sprij AJ, et al. Recombinant human deoxyribonuclease in infants with respiratory syncytial virus bronchiolitis. Chest 2007;131(3):788‐95. [DOI] [PubMed] [Google Scholar]
Calogero 2007
- Calogero C, Sly PD. Acute viral bronchiolitis: to treat or not to treat ‐ that is the question. Journal of Pediatrics 2007;151(3):235‐7. [DOI] [PubMed] [Google Scholar]
Corneli 2007
- Corneli HM, Zorc JJ, Mahajan P, Shaw KN, Holubkov R, Reeves SD, et al. Bronchiolitis Study Group of the Pediatric Emergency Care Applied Research Network (PECARN). A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. New England Journal of Medicine 2007;357(4):331‐9. [PUBMED: 17652648] [DOI] [PubMed] [Google Scholar]
Deshpande 2003
- Deshpande SA, Northern V. The clinical and health economic burden of respiratory syncytial virus disease among children under 2 years of age in a defined geographical area. Archives of Disease in Childhood 2003;88(12):1065‐9. [PUBMED: 14670770] [DOI] [PMC free article] [PubMed] [Google Scholar]
Erdeve 2007
- Erdeve O, Uras N, Atasay B, Arsan S. Efficacy and safety of nebulized recombinant human DNase as rescue treatment for persistent atelectasis in newborns: case‐series. Croatian Medical Journal 2007;48:234‐9. [PMC free article] [PubMed] [Google Scholar]
Fernandes 2010
- Fernandes RM, Bialy LM, Vandermeer B, Tjosvold L, Plint AC, Patel H, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD004878.pub2] [DOI] [PubMed] [Google Scholar]
Gadomski 2010
- Gadomski AM, Brower M. Bronchodilators for bronchiolitis. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD001266.pub2] [DOI] [PubMed] [Google Scholar]
Greally 1995
- Greally P. Human recombinant DNase for mucus plugging in status asthmaticus. Lancet 1995;346:1423‐4. [DOI] [PubMed] [Google Scholar]
Hall 2009
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. New England Journal of Medicine 2009;360(6):588‐98. [PUBMED: 19196675] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartling 2011
- Hartling L, Bialy LM, Vandermeer B, Tjosvold L, Johnson DW, Plint AC, et al. Epinephrine for bronchiolitis. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD003123.pub2] [DOI] [PubMed] [Google Scholar]
Hendriks 2005
- Hendriks T, Hoog M, Lequin MH, Devos AS, Merkus PJ. DNase and atelectasis in non‐cystic fibrosis pediatric patients. Critical Care 2005;9(4):341‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kupeli 2003
- Kupeli S, Teksam O, Dogru D, Yurdakok M. Use of recombinant human DNase in a premature infant with recurrent atelectasis. Pediatrics International 2003;45:584‐6. [DOI] [PubMed] [Google Scholar]
Leader 2003
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. Journal of Pediatrics 2003;143(Suppl 5):127‐32. [DOI] [PubMed] [Google Scholar]
Manoha 2007
- Manoha C, Espinosa S, Aho SL, Huet F, Pothier P. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. Journal of Clinical Virology 2007;38(3):221‐6. [DOI] [PubMed] [Google Scholar]
Nenna 2009
- Nenna R, Tromba V, Berardi R, Angelis D, Papoff P, Sabbatino G, et al. Recombinant human deoxyribonuclease treatment in hospital management of infants with moderate‐severe bronchiolitis. European Journal of Inflammation 2009;7(3):169‐74. [Google Scholar]
Patel 2000
- Patel A, Harrison E, Durward A, Murdoch IA. Intratracheal recombinant human deoxyribonuclease in acute life‐threatening asthma refractory to conventional treatment. British Journal of Anaesthesia 2000;84:505‐7. [DOI] [PubMed] [Google Scholar]
Perrotta 2007
- Perrotta C, Ortiz Z, Roque M. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004873.pub3] [DOI] [PubMed] [Google Scholar]
Puterman 1997
- Puterman AS, Weinberg EG. rhDNase in acute asthma. Pediatric Pulmonology 1997;23:316‐7. [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Shak 1990
- Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL. Recombinant human DNase reduces the viscosity of cystic fibrosis sputum. Proceedings of the National Academy of Sciences of the United States of America 1990;87(23):9188‐92. [PUBMED: 2251263] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suri 2002
- Suri R, Grieve R, Normand C, Metcalfe C, Thompson S, Wallis C, et al. Effects of hypertonic saline, alternate day and daily rhDNase on healthcare use, costs and outcomes in children with cystic fibrosis. Thorax 2002;57(10):841‐6. [PUBMED: 12324668] [DOI] [PMC free article] [PubMed] [Google Scholar]
ten Berge 1999
- Berge M, Brinkhorst G, Kroon AA, Jongste JC. DNase treatment in primary ciliary dyskinesia ‐ assessment by nocturnal pulse oximetry. Pediatric Pulmonology 1999;27:59‐61. [DOI] [PubMed] [Google Scholar]
Wang 1992
- Wang EE, Milner RA, Navas L, Maj H. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. American Review of Respiratory Disease 1992;145:106‐9. [DOI] [PubMed] [Google Scholar]
Wohl 2003
- Wohl ME, Chernick V. Treatment of acute bronchiolitis. New England Journal of Medicine 2003;349:82‐3. [DOI] [PubMed] [Google Scholar]
Zhang 2011
- Zhang L, Mendoza‐Sassi RA, Wainwright C, Klassen TP. Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD006458.pub2] [DOI] [Google Scholar]


