Abstract
Background
Anticonvulsant therapy is sometimes used prophylactically in patients with chronic subdural haematoma, although the benefit is unclear.
Objectives
To assess the effects of prophylactic anticonvulsants in patients with chronic subdural haematoma, in both the pre‐ and post‐operative periods.
Search methods
We searched the Cochrane Injuries Group's Specialised Register, CENTRAL (The Cochrane Library), MEDLINE (OvidSP), EMBASE (OvidSP), PubMed, LILACS, and the databases clinicaltrials.gov, the WHO International Clinical Trials Registry Platform, and Current Controlled Trials. The search was through 27th March 2013.
Selection criteria
Randomised controlled trials comparing any anticonvulsant versus placebo or no intervention.
Data collection and analysis
Three authors screened the search results to identify relevant studies. No studies met the inclusion criteria for the review.
Main results
No randomised controlled trials were identified.
Authors' conclusions
No formal recommendations can be made about the use of prophylactic anticonvulsants in patients with chronic subdural haematoma based on the literature currently available. There are no randomised controlled trials on this topic, and non‐controlled studies have conflicting results. There is an urgent need for well‐designed randomised controlled trials.
Keywords: Humans; Anticonvulsants; Anticonvulsants/therapeutic use; Hematoma, Subdural, Chronic; Hematoma, Subdural, Chronic/complications; Seizures; Seizures/etiology; Seizures/prevention & control
Plain language summary
Anti‐epileptic drugs for preventing seizures in patients with long‐term bleeding around the brain (subdural haematoma)
Chronic subdural haematoma (CSH) is a serious condition in which blood collects under the thickest membrane that surrounds the brain, known as the dura mater. CSH is usually caused by minor head injuries in which a vein has torn, and this happens in particular in older patients and patients with other brain problems. A CSH may cause seizures which can be dangerous. Some doctors give patients anti‐epileptic drugs such as phenytoin or phenobarbital to try to prevent seizures. However, most patients with CSH will not have seizures and anti‐epileptic drugs can have serious side effects.
The review authors wanted to find out whether patients with a CSH who received anti‐epileptic drugs had fewer seizures than those who did not. They searched medical journals to find reports of randomised controlled trials in which one group of patients received a treatment (anti‐epileptic drugs, which could be given before or after surgery for the CSH) and were compared with a similar group of patients who received a non‐active or different treatment. The authors searched many medical journals and found no reports of randomised controlled trials on this topic. They did find other studies, but either there was no control group or the study involved looking at past patients' medical records. These un‐controlled, or retrospective, studies had conflicting results regarding the benefit of anti‐epileptic drugs.
The review authors conclude that better research needs to be done on this topic and, for now, there is no clear evidence to support the regular use of anti‐epileptic drugs for patients with long‐term subdural haematoma.
Background
Chronic subdural haematomas (CSHs) are usually caused by minor head injuries and most result from tearing of a bridging vein. The frequency of pre‐ and post‐operative seizures in these patients is not established. The overall incidence of seizures in patients with CSH has been reported to vary from 2.3% to 17% (Luxon 1979; Ohno 1993) with an incidence of post‐operative seizures reported from 1.0% to 23.4% (Grisoli 1988; Hirakawa 1972). The wide variation in these numbers is probably related to the severity of the head injury and the type of surgical procedure performed in each study.
CSH typically occurs in people with prior brain atrophy, such as elderly people or chronic alcoholics, who are also more vulnerable to the potential side effects of anticonvulsant drugs (Prabhu 2003). The efficacy of prophylactic anticonvulsive medication in this pathology has been debated and there is no consensus on its use. There is a wide variation in practice. For some specialists, the low incidence of seizures does not justify an anticonvulsant prophylaxis in patients with CSH caused by minor head injury (Ohno 1993). Others suggest that this prophylactic medication should be considered only in alcoholic patients because of their higher risk of seizure (Rubin 1993). Another study found a significant increase in morbidity and mortality associated with respiratory complications and status epilepticus in patients with new‐onset seizures. It recommended the administration of anticonvulsants for a period of six months following diagnosis in all patients with CSH (Sabo 1995).
Why it is important to do this review
To our knowledge, no systematic review of anticonvulsants for preventing seizures in patients with CSH has yet been done. The present review aimed to fulfil this need.
Objectives
To assess the effects of prophylactic anticonvulsants in patients with CSH, in both the pre‐ and post‐operative periods.
Methods
Criteria for considering studies for this review
Types of studies
All studies were required to be randomised controlled trials (RCTs) comparing any anticonvulsant versus placebo or no intervention.
Types of participants
Inclusion criteria
Patients with cranial computerized tomography (CT) or magnetic resonance imaging (MRI) compatible with an old subdural haematoma with radiological or clinical evidence of mass effect.
Exclusion criteria
History of pre‐existing seizures.
Types of interventions
Administration of any anticonvulsant drug, at any dosage, beginning at the time of the diagnosis and prolonged for any length of time, compared with placebo or no anticonvulsant. (We excluded trials comparing different anticonvulsants, different dosages, route of administration or differences in timing or duration of administration.)
Types of outcome measures
Primary outcomes
Pre‐operative and post‐operative seizures.
Secondary outcomes
Side effects (safety of the intervention).
All‐cause mortality.
Other complications: permanent neurological impairment, long term epilepsy, CSH recurrence.
Search methods for identification of studies
We did not limit searches by date, language or publication status.
Electronic searches
The Cochrane Injuries Group Trials Search Co‐ordinator searched the following electronic databases:
Cochrane Injuries Group's Specialised Register (27th March 2013);
CENTRAL (The Cochrane Library, Issue 1 of 12, 2013);
MEDLINE (OvidSP) (1946 to March Week 3 2013);
EMBASE (OvidSP) (1974 to 2013 March 27);
PubMed (27th March 2013);
LILACS (1982 to 27th March 2013).
The search strategies are documented in full in Appendix 1.
Searching other resources
The authors searched the following clinical trials registries:
Clinicaltrials.gov (http://www.clinicaltrials.gov/) (20th April 2013)
WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/) (20th April 2013)
Current Controlled Trials (http://www.controlled‐trials.com/mrct/) (20th April 2013)
For the original version of the review published in May 2005 we also carried out:
a search of the reference lists of relevant trials and review articles;
an electronic search of the database of the American Association of Neurological Surgeons (AANS);
a handsearch of the conference proceedings of the European Association of Neurosurgical Societies (EANS);
personal communication with other researchers in the field.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration, and as defined in the protocol (Ratilal 2004).
Selection of studies
Three authors (BR, LP, JC) independently assessed the studies identified by the search strategy to identify potentially suitable trials for the review according to the criteria outlined above. Had there been disagreements on the inclusion of studies, we would have had a discussion involving a fourth author (SC).
Data extraction and management
The authors independently assessed the full papers for the type of participants, the type and dose of anticonvulsant used, methodological quality, the number of patients excluded or lost to follow up, and the outcome measures stated in the protocol. We investigated sources of bias.
Assessment of risk of bias in included studies
In the future if studies are included in the review, at least two authors will assess the internal validity of individual trials, using the Cochrane Collaboration's risk of bias tool as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We plan to extract the following information for each trial: sequence generation, allocation concealment, blinding, extent of loss to follow up and description of dropouts.
The summary of findings table will include comparisons on the frequency of seizures and adverse event rates between the treatment and control groups.
Measures of treatment effect
Statistical analyses will be performed using the statistical software provided by the Cochrane Collaboration (Review Manager). Heterogeneity between trial results will be tested using a standard chi‐squared test. The results will be reported as odds ratios (and 95% confidence intervals [95% CI]) for dichotomous outcomes, using the Peto fixed‐effects method. The significance of any differences between odds ratios will be calculated using a standard method (Egger 2001). A descriptive summary of the results will be given where the outcome data from different studies cannot be combined.
Results
Description of studies
The search strategy yielded a total of 126 references. We found no trials that were eligible for inclusion in the review (Figure 1).
1.
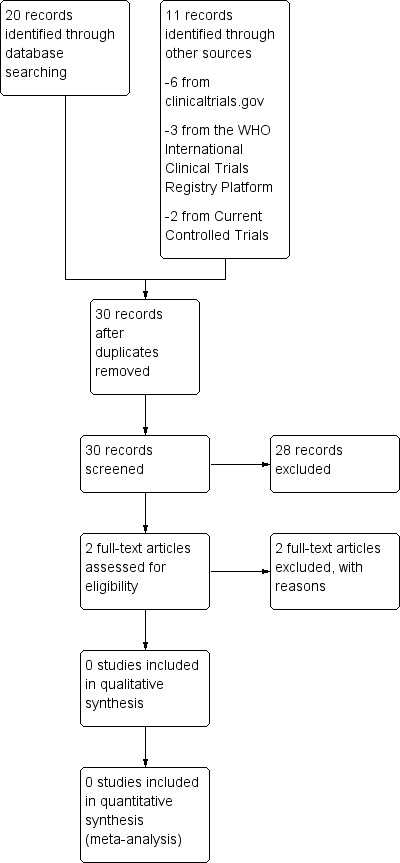
This study flow diagram reflects only the most recent updated version of this review (2013).
Risk of bias in included studies
No studies could be assessed.
Effects of interventions
As no studies are included in the review, no data could be analysed.
Discussion
There are no randomised controlled trials of anticonvulsants for preventing seizures in patients with chronic subdural haematoma.
Through the search for studies we identified three retrospective studies with a control group.
Out of the 138 patients studied retrospectively by Rubin 1993, 83 were given anti‐epileptic drugs. Seizures were noted in 4.5% (n = 4) of this population. The rest of the population (n = 55) were not treated with anti‐epileptic drugs and the incidence of seizures in this population was 3.4% (n = 2). According to the authors, antiepileptic drugs should not be administered prophylactically in patients with CSH because the risk of epilepsy is not high enough to balance the morbidity caused by the anticonvulsants.
In the retrospective analysis of Ohno 1993, 129 patients treated for CSH between August 1980 and March 1992 were studied. Patients were usually given phenobarbital pre‐operatively. Until December 1987, 56 of 59 patients were given anticonvulsants post‐operatively. Prophylactic use of these drugs was subsequently discontinued, except in those patients with severe head injury (17 of 70 received prophylaxis). None of the 73 patients in total who were given prophylactic antiepileptic drug treatment developed seizures. Only two of 56 patients not given prophylaxis developed early post‐operative seizures. A total of four patients had seizures, none of whom had received anticonvulsant drugs. The incidence of seizures was considered low and similar to that previously reported for minor head injury. The trialists suggest that routine use of antiepileptic prophylaxis is not justified in patients with CSH related with minor injuries.
Sabo 1995 reported a retrospective analysis with historical controls of 98 patients treated surgically for CSH and examined the prevalence of seizure activity, morbidity, mortality and the side effects of anticonvulsant medication. Of the 92 patients without pre‐existing seizure, 42 (46%) received adequate prophylactic phenytoin. Adequate prophylaxis included an initial dose of phenytoin (15 mg/kg) and daily medication to adjust serum drug levels within therapeutic range. The administration of this drug was associated with three non‐serious dermatological reactions. One (2.4%) patient among the 42 who received prophylactic anticonvulsants experienced seizure activity in comparison to 16 (32%) of 50 patients who did not receive adequate prophylaxis (P < 0.001). The onset of new seizures was found in 17 (18.5%) of the 92 patients and was associated with increases in morbidity (P = 0.036) and mortality (P < 0.005). Therefore, they recommend the use of phenytoin prophylaxis in patients treated surgically for CSH for six months following the diagnosis.
Other retrospective studies considered the incidence of seizures in patients with CSH but have not included a control group for anticonvulsant therapy (Hirakawa 1972; Luxon 1979; Kotwica 1991; McKissock 1960).
Authors' conclusions
Implications for practice.
No randomised controlled trials have been identified, and this review has no included studies. No conclusions can be reached about the use of prophylactic anticonvulsants in patients with CSH from the information currently available. Clinicians must balance potential benefit against the possible risk of complications in each case.
Implications for research.
Randomised controlled trials of prophylactic anticonvulsants in patients with CSH are required in order to gain an understanding of the effects of this treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 23 May 2013 | New citation required but conclusions have not changed | The search was updated to 27 March 2013. No new studies were identified; the conclusions have not changed. No studies are included in this review. |
| 23 May 2013 | New search has been performed | The search for studies has been updated to 27 March 2013. |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 15 March 2009 | New search has been performed | The search was updated to 15 March 2009. No new trials were identified. The results and conclusions remain the same. |
| 16 June 2008 | Amended | Converted to new review format. |
Acknowledgements
We are grateful all members of the Cochrane Injuries Group in London, namely Paul Chinnock and Katharine Ker for their continued assistance, and to Karen Blackhall for conducting some of the electronic searches.
Appendices
Appendix 1. Search strategy
For the most recent update the search strategies have been modified according to database search interface changes. Also PubMed was searched to capture references, such as those supplied by publisher, not indexed in MEDLINE.
Cochrane Injuries Group's Specialised Register (subdural or subepidural or pachymening* or extracran* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed*) and Anticonvuls* or antiepilep* or Carbamazep* or Carbazepin* or carbatrol or Finlepsin or Neurotol or Epitol or Amizepine or Tegretol or Phenytoin or fosphenyt* or diphenylhydant* or fenitoi* or antisacer or difenin or dihydan or dilantin or Cerebyx or Phenytek or epamin or epanutin or hydantol or valproic* or valproat* or divalproex or propylpentanoic* or ergenyl or convulsofin or depakote or divalproex or dipropyl* or depakene or depakine or depakote or depacon or vupral or clonazepam or antelepsin or rivotril or Primidone or desoxyphenobarbital or misodine or primaclone or sertan or mizodin or mysoline or Ethosuximide or ethosuccimid or ethylmethylsuccimide or suksilep or suxilep or zarontin or gabapent* or neurontin or gabamox or lamotrigin* or lamictal or topiramat* or topamax or Vigabatrin or Sabril or tiagabin* or gabitril or felbamat* or felbatol or levetiracet* or keppra or zonisamid* or zonegran or oxcarbazep* or trileptal
Cochrane Central Register of Controlled Trials (The Cochrane Library) #1MeSH descriptor: [Hematoma, Subdural, Acute] explode all trees #2hematom* or haematom* or hemorrhag* or haemorrhag*:ti,ab,kw (Word variations have been searched) #3#1 or #2 #4MeSH descriptor: [Anticonvulsants] explode all trees #5MeSH descriptor: [Carbamazepine] this term only #6MeSH descriptor: [Phenytoin] this term only #7MeSH descriptor: [Valproic Acid] this term only #8MeSH descriptor: [Clonazepam] this term only #9MeSH descriptor: [Ethosuximide] this term only #10MeSH descriptor: [Vigabatrin] this term only #11(Anticonvuls* or antiepilep* or Carbamazep* or Carbazepin* or carbatrol or Finlepsin or Neurotol or Epitol or Amizepine or Tegretol or Phenytoin or fosphenyt* or diphenylhydant* or fenitoi* or antisacer or difenin or dihydan or dilantin or Cerebyx or Phenytek or epamin or epanutin or hydantol or valproic* or valproat* or divalproex or propylpentanoic* or ergenyl or convulsofin or depakote or divalproex or dipropyl* or depakene or depakine or depakote or depacon or vupral or clonazepam or antelepsin or rivotril or Primidone or desoxyphenobarbital or misodine or primaclone or sertan or mizodin or mysoline or Ethosuximide or ethosuccimid or ethylmethylsuccimide or suksilep or suxilep or zarontin or gabapent* or neurontin or gabamox or lamotrigin* or lamictal or topiramat* or topamax or Vigabatrin or Sabril or tiagabin* or gabitril or felbamat* or felbatol or levetiracet* or keppra or zonisamid* or zonegran or oxcarbazep* or trileptal):ti,ab,kw (Word variations have been searched) #12#4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 #13#3 and #12
MEDLINE (OvidSP) 1. exp Subdural Hematoma/ 2. (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 3. (subdural or subepidural or pachymening* or extracran*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 4. 2 and 3 5. 1 or 4 6. exp Anticonvulsive Agent/ 7. (Anticonvuls* or antiepilep* or Carbamazep* or Carbazepin* or carbatrol or Finlepsin or Neurotol or Epitol or Amizepine or Tegretol or Phenytoin or fosphenyt* or diphenylhydant* or fenitoi* or antisacer or difenin or dihydan or dilantin or Cerebyx or Phenytek or epamin or epanutin or hydantol or valproic* or valproat* or divalproex or propylpentanoic* or ergenyl or convulsofin or depakote or divalproex or dipropyl* or depakene or depakine or depakote or depacon or vupral or Clonazepam or antelepsin or rivotril or Primidone or desoxyphenobarbital or misodine or primaclone or sertan or mizodin or mysoline or Ethosuximide or ethosuccimid or ethylmethylsuccimide or suksilep or suxilep or zarontin or gabapent* or neurontin or gabamox or lamotrigin* or lamictal or topiramat* or topamax or Vigabatrin or Sabril or tiagabin* or gabitril or felbamat* or felbatol or levetiracet* or keppra or zonisamid* or zonegran or oxcarbazep* or trileptal).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 8. 6 or 7 9. 5 and 8 10. randomized controlled trial.pt. 11. controlled clinical trial.pt. 12. clinical trials as topic.sh. 13. randomi?ed.ab,ti. 14. placebo.ab. 15. random*.ab. 16. trial.ti. 17. Comparative study.pt. 18. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19. (animals not (humans and animals)).sh. 20. 18 not 19 21. 9 and 20
Embase Classic + Embase (OvidSP) 1.exp Subdural Hematoma/ 2.(haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 3.(subdural or subepidural or pachymening* or extracran*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 4.3 and 2 5.4 or 1 6.exp Anticonvulsive Agent/ 7.(Anticonvuls* or antiepilep* or Carbamazep* or Carbazepin* or carbatrol or Finlepsin or Neurotol or Epitol or Amizepine or Tegretol or Phenytoin or fosphenyt* or diphenylhydant* or fenitoi* or antisacer or difenin or dihydan or dilantin or Cerebyx or Phenytek or epamin or epanutin or hydantol or valproic* or valproat* or divalproex or propylpentanoic* or ergenyl or convulsofin or depakote or divalproex or dipropyl* or depakene or depakine or depakote or depacon or vupral or Clonazepam or antelepsin or rivotril or Primidone or desoxyphenobarbital or misodine or primaclone or sertan or mizodin or mysoline or Ethosuximide or ethosuccimid or ethylmethylsuccimide or suksilep or suxilep or zarontin or gabapent* or neurontin or gabamox or lamotrigin* or lamictal or topiramat* or topamax or Vigabatrin or Sabril or tiagabin* or gabitril or felbamat* or felbatol or levetiracet* or keppra or zonisamid* or zonegran or oxcarbazep* or trileptal).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 8.6 or 7 9.8 and 5 10.exp Randomized Controlled Trial/ 11.exp controlled clinical trial/ 12.randomi?ed.ab,ti. 13.placebo.ab. 14.*Clinical Trial/ 15.randomly.ab. 16.trial.ti. 17.10 or 11 or 12 or 13 or 14 or 15 or 16 18.exp animal/ not (exp human/ and exp animal/) 19.17 not 18 20.19 and 9
PubMed ((((publisher[sb])) OR (PubMed in process))) AND (((((((haematoma*[title/abstract] OR hematoma*[title/abstract] OR haemorrhag*[title/abstract] OR hemorrhag*[title/abstract] OR bleed*[title/abstract])) AND (subdural[title/abstract] OR subepidural[title/abstract] OR pachymening*[title/abstract] OR extracran*[title/abstract]))) OR ("Hematoma, Subdural"[Mesh]))) AND ((("Anticonvulsants"[Mesh])) OR (Anticonvuls*[title/abstract] OR antiepilep*[title/abstract] OR Carbamazep*[title/abstract] OR Carbazepin*[title/abstract] OR carbatrol[title/abstract] OR Finlepsin[title/abstract] OR Neurotol[title/abstract] OR Epitol[title/abstract] OR Amizepine[title/abstract] OR Tegretol[title/abstract] OR Phenytoin[title/abstract] OR fosphenyt*[title/abstract] OR diphenylhydant*[title/abstract] OR fenitoi*[title/abstract] OR antisacer[title/abstract] OR difenin[title/abstract] OR dihydan[title/abstract] OR dilantin[title/abstract] OR Cerebyx[title/abstract] OR Phenytek[title/abstract] OR epamin[title/abstract] OR epanutin[title/abstract] OR hydantol[title/abstract] OR valproic*[title/abstract] OR valproat*[title/abstract] OR divalproex[title/abstract] OR propylpentanoic*[title/abstract] OR ergenyl[title/abstract] OR convulsofin[title/abstract] OR depakote[title/abstract] OR divalproex[title/abstract] OR dipropyl*[title/abstract] OR depakene[title/abstract] OR depakine[title/abstract] OR depakote[title/abstract] OR depacon[title/abstract] OR vupral[title/abstract] OR Clonazepam[title/abstract] OR antelepsin[title/abstract] OR rivotril[title/abstract] OR Primidone[title/abstract] OR desoxyphenobarbital[title/abstract] OR misodine[title/abstract] OR primaclone[title/abstract] OR sertan[title/abstract] OR mizodin[title/abstract] OR mysoline[title/abstract] OR Ethosuximide[title/abstract] OR ethosuccimid[title/abstract] OR ethylmethylsuccimide[title/abstract] OR suksilep[title/abstract] OR suxilep[title/abstract] OR zarontin[title/abstract] OR gabapent*[title/abstract] OR neurontin[title/abstract] OR gabamox[title/abstract] OR lamotrigin*[title/abstract] OR lamictal[title/abstract] OR topiramat*[title/abstract] OR topamax[title/abstract] OR Vigabatrin[title/abstract] OR Sabril[title/abstract] OR tiagabin*[title/abstract] OR gabitril[title/abstract] OR felbamat*[title/abstract] OR felbatol[title/abstract] OR levetiracet*[title/abstract] OR keppra[title/abstract] OR zonisamid*[title/abstract] OR zonegran[title/abstract] OR oxcarbazep*[title/abstract] OR trileptal[title/abstract]))) LILACS (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed*) AND (subdural or subepidural or pachymening* or extracran*) AND (Anticonvuls* or antiepilep* or Carbamazep* or Carbazepin* or carbatrol or Finlepsin or Neurotol or Epitol or Amizepine or Tegretol or Phenytoin or fosphenyt* or diphenylhydant* or fenitoi* or antisacer or difenin or dihydan or dilantin or Cerebyx or Phenytek or epamin or epanutin or hydantol or valproic* or valproat* or divalproex or propylpentanoic* or ergenyl or convulsofin or depakote or divalproex or dipropyl* or depakene or depakine or depakote or depacon or vupral or Clonazepam or antelepsin or rivotril or Primidone or desoxyphenobarbital or misodine or primaclone or sertan or mizodin or mysoline or Ethosuximide or ethosuccimid or ethylmethylsuccimide or suksilep or suxilep or zarontin or gabapent* or neurontin or gabamox or lamotrigin* or lamictal or topiramat* or topamax or Vigabatrin or Sabril or tiagabin* or gabitril or felbamat* or felbatol or levetiracet* or keppra or zonisamid* or zonegran or oxcarbazep* or trileptal)
Differences between protocol and review
In the future, the risk of bias will be assessed through the Cochrane Collaboration's risk of bias tool (Higgins 2011).
Contributions of authors
All correspondence: BR Drafting the review: BR, LP, JC, CS Search for trials: BR, LP, JC Obtaining copies of trial reports: BR, LP, JC Selection of trials for inclusion/exclusion: BR, LP, JC, CS
Sources of support
Internal sources
Clinical Therapeutics Institute, Lisbon Faculty of Medicine, Portugal.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
Additional references
Egger 2001
- Egger M, Smith GD, Altman DG. Meta‐analysis in context. Systematic Reviews in Health Care. 2. BMJ Books, 2001. [Google Scholar]
Grisoli 1988
- Grisoli F, Graziani N, Peragut JC, Vincentelli F, Fabrizi AP, Caruso G, et al. Perioperative lumbar injection of Ringer's lactate solution in chronic subdural hematomas: a series of 100 cases. Neurosurgery 1988;23(5):616‐21. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirakawa 1972
- Hirakawa K, Hashizume K, Fuchinoue T, Takahashi H, Nomura K. Statistical analysis of chronic subdural hematoma in 309 adult cases. Neurologia medico‐chirurgica (Tokyo) 1972;12(0):71‐83. [DOI] [PubMed] [Google Scholar]
Kotwica 1991
- Kotwica Z, Brzezinski J. Epilepsy in chronic subdural haematoma. Acta Neurochirurgica (Wien) 1991;113:118‐20. [DOI] [PubMed] [Google Scholar]
Luxon 1979
- Luxon LM, Harrison MJG Luxon LM, Harrison MJG. Chronic subdural haematoma. Quarterly Journal of Medicine 1979;48(189):43‐53. [PubMed] [Google Scholar]
McKissock 1960
- McKissock W, Richardson A, Bloom WH. Subdural haematoma. A review of 389 cases. Lancet 1960;1:1365‐9. [Google Scholar]
Ohno 1993
- Ohno K, Maehara T, Ichimura K, Suzuki R, Hirakawa K, Monma S. Low incidence of seizures in patients with chronic subdural haematoma. Journal of Neurology, Neurosurgery and Psychiatry 1993;56(11):1231‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prabhu 2003
- Prabhu S, Zauner A, Bullock M. Surgical management of traumatic brain injury. In: H. Richard Winn editor(s). Youmans Neurological Surgery. 5th Edition. Vol. 4, Philadelphia: Saunders, 2003:5145‐80. [Google Scholar]
Review Manager [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan). Version Version 5.2. Copenhagen: The Cochrane Collaboration, 2012.
Rubin 1993
- Rubin G, Rappaport ZH. Epilepsy in chronic subdural haematoma. Acta Neurochirurgica (Wien) 1993;123(1‐2):39‐42. [DOI] [PubMed] [Google Scholar]
Sabo 1995
- Sabo RA, Hanigan WC, Aldag JC. Chronic subdural hematomas and seizures: the role of prophylactic anticonvulsive medication. Surgical Neurology 1995;43(6):579‐82. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ratilal 2004
- Ratilal BO, Costa J, Sampaio C. Anticonvulsants for preventing seizures in patients with chronic subdural haematoma. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004893] [DOI] [PubMed] [Google Scholar]


