Abstract
Background
Allergic rhinitis is a disorder of the nasal membranes and surrounding tissues, and a worldwide cause of illness and disability. Helminths are complex tissue‐dwelling or lumen‐dwelling organisms that inhabit larger organisms and are frequently asymptomatic. Helminths modulate the natural immune responses of their human hosts, and may prevent or cure immune‐mediated or allergic diseases (e.g. allergic rhinitis) in the host. Non‐randomised studies support this hypothesis.
Objectives
To assess the safety and effectiveness of helminth therapy in people with allergic rhinitis.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; ICTRP and additional sources for published and unpublished trials. The date of the search was 24 June 2011.
Selection criteria
All randomised controlled trials where the intervention was any helminth species or combination of helminth species, administered to people with allergic rhinitis in any dose, by any route and for any duration of exposure. We accepted both intermittent and persistent allergic rhinitis patients.
Data collection and analysis
We independently extracted data and assessed eligibility and risk of bias using a standardised data collection form. We resolved any disagreement through discussion. We combined dichotomous data using risk ratio (RR) and continuous data using mean difference (MD), presenting both with 95% confidence intervals (CI).
Main results
We found five reports of two single‐centre, placebo‐controlled, double‐blinded studies (130 participants). Participants in both studies were a mix of adults with either intermittent or persistent allergic rhinitis. Both studies had a low risk of bias. One study, with 12 weeks’ follow‐up, used a single percutaneous application of 10 Necator americanus (i.e. human hookworm) larvae. The other study, with 24 weeks’ follow‐up, used three‐weekly oral dosing with 2500 Trichuris suis (i.e. pig whipworm) eggs in aqueous suspension. Of 17 outcomes evaluated in this review, eight were positive (i.e. favoured helminths). Participants taking helminths had no reduction in allergic rhinitis symptoms, percentage of well days (i.e. days with minimal symptoms and no use of medication for allergic rhinitis), lung function measures and quality of life scores. Total use of medication for allergic rhinitis (eye drops, nasal sprays, tablets) did not change; however, in the helminth group there was a statistically significant reduction in the percentage of days during the grass pollen season when participants needed to take tablets as rescue medication for their allergic rhinitis symptoms (MD –14.0%, 95% CI –26.6 to –1.40); in a typical 60‐day pollen season this 14% reduction translates into 19 days when tablets would be needed in the helminth group versus 27 days when tablets would be needed in the placebo group. Participants taking helminths percutaneously (i.e. as hookworm larvae) had local skin itching and redness in the first few days after administration. Participants taking helminths were more likely to report any gastrointestinal adverse event (RR 1.79, 95% CI 1.31 to 2.45), moderate or severe abdominal pain (RR 7.67, 95% CI 1.87 to 31.57), moderate or severe flatulence (RR 2.01, 95% CI 1.06 to 3.81) and moderate or severe diarrhoea (RR 1.99, 95% CI 1.18 to 3.37). There was no difference between the helminth and placebo groups in the incidence of serious adverse events, and in study withdrawals.
Authors' conclusions
There is currently insufficient evidence on the efficacy, tolerability and likely costs of helminth therapy to support its use in the routine management of allergic rhinitis. Administered to humans in carefully measured doses, helminths appear to be safe. More preclinical studies should be performed, before larger and extended duration trials of helminths for allergic rhinitis are carried out. Future studies should collect and report comparative data on the costs of helminth therapy versus conventional pharmacotherapy.
Keywords: Adult; Animals; Humans; Ancylostomatoidea; Ancylostomatoidea/immunology; Helminths; Helminths/immunology; Immunotherapy; Immunotherapy/methods; Randomized Controlled Trials as Topic; Rhinitis, Allergic, Perennial; Rhinitis, Allergic, Perennial/immunology; Rhinitis, Allergic, Perennial/therapy; Rhinitis, Allergic, Seasonal; Rhinitis, Allergic, Seasonal/immunology; Rhinitis, Allergic, Seasonal/therapy; Trichuris; Trichuris/immunology
Plain language summary
Helminth therapy (worms) for allergic rhinitis
Allergic rhinitis is a common health problem affecting about 500 million people worldwide; its prevalence is increasing. The symptoms of allergic rhinitis include sneezing, and an itchy, runny and blocked nose. Several classes of drugs are used to treat allergic rhinitis, but these drugs may be ineffective, and some drug classes have side effects after long‐term use. Drugs may also be relatively expensive. Helminths are complex multicellular organisms that inhabit larger organisms, and in humans are often symptomless. Helminths modulate (that is, optimally adjust) the immune systems of their hosts and it is thought that this property of helminths could be used therapeutically, to prevent or treat allergic diseases, such as allergic rhinitis. We included two well‐designed studies with a total of 130 adult participants, each study using a different species of gastrointestinal helminth (human hookworm in one study and pig whipworm in the other) as the intervention. Both studies found no significant efficacy from helminths, although one helminth species (Trichuris suis, the pig whipworm) reduced the need for participants to take tablets as ‘rescue medication’ during the grass pollen season. Adverse events such as abdominal pain and flatulence were commoner in the helminth group, but the two helminths species studied did not cause serious adverse reactions. Currently there is insufficient evidence to support the use of helminths for allergic rhinitis in routine clinical practice. More preclinical studies are needed, before larger and extended duration clinical trials of helminths for allergic rhinitis are performed.
Summary of findings
Summary of findings for the main comparison. Helminths (worms) for allergic rhinitis.
| Helminths (worms) for allergic rhinitis | ||||||
| Patient or population: patients with allergic rhinitis1 Settings: primary care, community, outpatient Intervention: helminths (worms)2 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Helminths (worms) | |||||
| All‐cause study withdrawal Investigator‐recorded Follow‐up: 12 to 24 weeks | Study population | RR 0.75 (0.18 to 3.21) | 130 (2 studies) | ⊕⊕⊕⊕ high | ||
| 62 per 1000 | 46 per 1000 (11 to 198) | |||||
| Moderate | ||||||
| 63 per 1000 | 47 per 1000 (11 to 202) | |||||
|
Change in allergic rhinitis daily symptom score during the grass pollen season
Each daily symptom score was the summation of (i) runny nose score, (ii) itchy nose score and (iii) sneezing score (each of these scores self recorded on a severity scale of 0 = best to 3 = worst, giving a maximum daily score of 9) Scale from: 0 to 9 Follow‐up: 61 days |
The mean change in allergic rhinitis daily symptom score during the grass pollen season in the intervention groups was 0 higher (0.45 lower to 0.45 higher) | 100 (1 study) | ⊕⊕⊕⊝ moderate3 | |||
|
Percentage of well days during the grass pollen season
Well days were those days with a symptom score of less than or equal to 2, and no use of rescue medication Scale from: 0 to 100 Follow‐up: 61 days |
The mean percentage of well days during the grass pollen season in the intervention groups was 3 higher (7.98 lower to 13.98 higher) | 100 (1 study) | ⊕⊕⊕⊝ moderate3 | |||
|
Total quality of life score over 12 weeks
Assessed as the total score over the entire 12‐week study period in the Juniper RQLQ, i.e. a patient‐specific test instrument derived from 28 questions drawn from 7 dimensions (sleep, non‐hay fever symptoms, practical problems, nose symptoms, eye symptoms, activities, emotions), with a maximum daily score of 168 denoting extremely poor quality of life, self reported daily and calculated at the end of the study as the log of the area under the curve Scale from: 0 to 168 Follow‐up: 12 weeks |
The mean total quality of life score over 12 weeks in the intervention groups was 0.33 higher (0.27 lower to 0.93 higher) | 30 (1 study) | ⊕⊕⊕⊝ moderate3 | |||
|
Percentage of days requiring rescue medication (i.e. as tablets) during the grass pollen season
Self recorded each day4 Scale from: 0 to 100 Follow‐up: 61 days |
The mean percentage of days requiring rescue medication (i.e. as tablets) during the grass pollen season in the intervention groups was 14 lower (26.6 to 1.4 lower) | 100 (1 study) | ⊕⊕⊕⊝ moderate3 | |||
| Hospitalisation due to any adverse event Investigator‐assessed Follow‐up: 24 weeks | Study population | RR 2.88 (0.31 to 26.69) | 96 (1 study) | ⊕⊕⊕⊝ moderate3 | ||
| 21 per 1000 | 61 per 1000 (7 to 568) | |||||
| Moderate | ||||||
| 21 per 1000 | 60 per 1000 (7 to 560) | |||||
| Any gastrointestinal adverse event Self recorded Follow‐up: 24 weeks | Study population | RR 1.79 (1.31 to 2.45) | 96 (1 study) | ⊕⊕⊕⊝ moderate3 | ||
| 489 per 1000 | 876 per 1000 (641 to 1000) | |||||
| Moderate | ||||||
| 489 per 1000 | 875 per 1000 (641 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RQLQ: Rhinoconjunctivitis Quality of Life Questionnaire; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allergic rhinitis is a disorder of the nasal membranes and surrounding tissues, and a worldwide cause of illness and disability. 2 Helminths are ubiquitous symbionts of humans and are usually asymptomatic; they modulate the natural immune responses of their human hosts. 3 Sparse data. 4 Other self recorded classes of rescue medication were: eye drops, nasal sprays.
Background
Description of the condition
Epidemiology
Allergic rhinitis is a global health problem that causes significant illness and disability worldwide. Although there is huge international variation in the incidence of allergic rhinitis, people from all countries, all ethnic groups, all socioeconomic conditions and of all ages suffer from the condition (ARIA 2008).
Allergic rhinitis was a rare condition until the 19th century, at which time it became common in both Europe and North America. The prevalence of allergic rhinitis is increasing in most countries and, at a conservative estimate, the condition afflicts over 500 million people worldwide (ARIA 2008). In North America the overall prevalence is nearly 20%, with a peak prevalence of nearly 40% occurring in childhood and adolescence (Austen 2011). In Europe around 1 in 10 adults has chronic rhinosinusitis, although there is marked regional variation in the burden of disease (Hastan 2011).
Clinical symptoms
Allergic rhinitis is defined clinically as a symptomatic disorder of the nasal membranes and surrounding tissues, induced by an IgE‐mediated inflammation and following exposure of the nasal membranes to an allergen (ARIA 2008). The disease has two phases, as follows.
An early‐phase response (also known as a type 1 or immediate hypersensitivity reaction). In this phase, histamine and other inflammatory mediators are released into the nasal mucosa by mast cells which were previously sensitised by an antigen. This causes the characteristic nasal symptoms of sneezing, pruritus (itching), rhinorrhoea (runny nose) and nasal congestion (Nasser 2010).
A late‐phase response. This phase occurs approximately four to 12 hours after antigen exposure and nasal congestion is the predominant symptom (Nasser 2010).
Classification
Historically, allergic rhinitis was classified as either seasonal (‘hay fever’) or perennial, based on any observed seasonality in the patient’s symptoms. A more modern classification, endorsed by the World Health Organization, is based on the duration of symptoms and recognises two forms of the disease, as follows.
Intermittent allergic rhinitis: lasting for less then four days per week or for less than four weeks per year (ARIA 2008).
Persistent allergic rhinitis: lasting for more than four days per week or for more than four weeks per year (ARIA 2008).
The classification is further divided, depending on the degree of disease severity and its impact on the patient’s quality of life, into mild disease, and moderate to severe disease (ARIA 2008).
Diagnosis
A diagnosis of allergic rhinitis is based on a typical history of allergic symptoms. However, as some of these symptoms may not necessarily be of allergic origin, the diagnosis may additionally be confirmed through a combination of in vitro and in vivo diagnostic tests (Al Sayyad 2007). These additional tests may include the following.
Serological testing for circulating allergen‐specific IgE. This aims to detect free or cell‐bound IgE, using enzyme allergosorbent tests (EAST) or a radioallergosorbent test (RAST).
Skin prick testing. This tests for an IgE‐mediated immediate hypersensitivity reaction, using the suspected allergens or other aeroallergens.
Nasal provocation testing (rhinomanometry). This is principally a research tool but may in time become a routine diagnostic test.
Common treatments
Current treatment approaches to allergic rhinitis include allergen avoidance (Sheikh 2010), pharmacotherapy and immunotherapy.
Commonly used drug treatments include antihistamines (Nasser 2010), topical nasal steroids (Al Sayyad 2007), anti‐leukotriene receptor antagonists, mast cell stabilisers and, in some cases, a short course of systemic steroids, or of nasal decongestants (Sur 2010). For patients whose symptoms remain uncontrolled despite these drug treatments, allergen immunotherapy is advised (Calderon 2007; Radulovic 2010).
Description of the intervention
In March 1970 Preston reported a remarkable case series of 12 naval officers who were serving in London, in government offices adjacent to a park (Preston 1970). All had suffered from allergic rhinitis (‘hay fever’) for some years, had positive skin prick tests to lime and plane tree pollen, and were afflicted with the disorder while at work in London, when the trees in the park came into pollen. The 12 patients all developed ascariasis during holidays or duty visits abroad, and the diagnosis was made by demonstrating the eggs of Ascaris lumbricoides in stools obtained at rectal examination. Subsequently:
four officers (33%) remained free of allergic rhinitis for three years;
three officers (25%) remained free of allergic rhinitis for two years;
four officers (33%) remained free of allergic rhinitis for one year; and
one officer was lost to follow‐up.
In October 1974 a researcher at the Medical Research Council laboratory at Carshalton, England, infested himself with 250 larvae of the nematode helminth Necator americanus. He had suffered for 25 years from allergic rhinitis (‘hay fever’) and had needed to take antihistamine drug treatment each summer. In September 1976 he reported: “The most pertinent finding in the context of the discussion on IgE, parasites, and allergy was that during the summers of 1975 and 1976 I remained completely free from all symptoms of hay fever” (Turton 1976).
Subsequent observational studies carried out in developing countries have found that in some poor communities helminth infestation, which is often asymptomatic, is protective against allergic rhinitis, asthma and eczema (Scrivener 2001; Huang 2002; Medeiros 2003; Haileamlak 2005). It has also been postulated that helminth infestation may protect against immune‐mediated diseases, such as inflammatory bowel disease and multiple sclerosis (Erb 2009).
The implication for clinical practice of this knowledge is that deliberately exposing patients with allergic or immune‐mediated diseases to controlled exposures of some helminth species may be a safe and effective treatment for the diseases. This concept has been tested for some common diseases through randomised controlled trials (RCTs) involving different helminth species (Summers 2005a; Blount 2009; Feary 2009b; Bager 2010; Daveson 2011). Some of these trials, though not all, have supported the concept of using helminths to treat allergic or immune‐mediated diseases.
Helminths
'Helminth' is derived from the Greek word helmins, meaning worm. The helminths of humans include species from the following four groups:
annelids (segmented worms);
nematodes (roundworms);
trematodes (flukes); and
cestodes (tapeworms).
The commonly encountered human helminths are listed in Appendix 1. The important biological characteristics of helminths are listed in Appendix 2.
The intervention to be assessed in this review is the deliberate exposure of a person with confirmed allergic rhinitis to one or more helminth species. The routes of deliberate exposure are likely to be:
oral (the human participant swallows helminth eggs or cysts); or
percutaneous (helminth larvae or cercariae are applied to the skin of the human participant, and penetrate the epidermis to reach their preferred end‐stage body structures).
The intervention may or may not be terminated through participants in the active arm taking an appropriate anthelmintic drug; we will analyse the data from studies regardless of whether or not the intervention is terminated in this way.
How the intervention might work
Helminths modulate the natural immune responses of their animal hosts, and in this way evade immune surveillance and immune challenge. An indirect effect of this immune modulation may be the remission or cure of pre‐existing allergic or immune‐mediated diseases in the host (Flohr 2008).
Why it is important to do this review
Allergic diseases were once rare but are now epidemic in affluent countries (Austen 2011). About one in five children in industrialised countries suffers from at least one of the three main allergic diseases: allergic rhinitis, asthma and eczema (ISAAC 1998). Allergy accounts for up to one‐third of school absences because of chronic illness; it is likely to be a significant, though lesser, cause of work absence also (Peakman 2009).
Current treatments for allergic rhinitis are sub‐optimal for several reasons. Allergen avoidance, the first‐line treatment for allergic rhinitis, is usually impractical (Reid 2010) and furthermore there exists considerable uncertainty around the efficacy and effectiveness of allergen avoidance in treating allergic rhinitis (Sheikh 2010). Drug treatments for allergic rhinitis may be expensive, or be ineffective, or both. The adverse effects of systemic drugs limit their usefulness (Reid 2010). Topical decongestants are associated with rebound rhinitis and with systemic responses such as hypertension (Austen 2011). Abuse of over‐the‐counter preparations may cause long‐term damage to nasal function (Nasser 2010). Allergen injection immunotherapy is resource‐intensive because it needs to be performed in the immediate presence of a physician, and administered by fully trained personnel who are experienced in the early recognition and prompt treatment of adverse reactions to the therapy (Calderon 2007).
A Cochrane review to assess the effectiveness and safety of helminth treatment for allergic rhinitis is warranted.
Objectives
To assess whether helminths are a safe and effective treatment for people with allergic rhinitis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) using adequate or quasi methods of randomisation. Single‐blind, double‐blind, triple‐blind or unblinded (i.e. ‘open label’) studies were all eligible for inclusion.
Types of participants
Participants were persons of any age with persistent or intermittent allergic rhinitis. This was confirmed by one or more of the following:
abnormal systemic levels of allergen‐specific IgE;
positive skin prick test;
positive nasal provocation test (rhinomanometry).
Where the older terminology was used we assumed ‘seasonal’ to be equivalent to ‘intermittent’ and ‘perennial’ to be equivalent to ‘persistent’.
Types of interventions
Intervention
We considered studies for inclusion where any helminth species or combination of helminth species (either tissue‐dwelling helminths or lumen‐dwelling gastrointestinal helminths) was administered to a human host:
in any dose;
by any route (oral, percutaneous, other);
for any duration of exposure (days, weeks, months); and
at any developmental stage of the organism (eggs, cercariae, larvae, adult worms).
Helminth‐derived molecular products were outside the scope of this review and were not included.
Control
The control group received placebo (i.e. sham helminth exposure), no treatment or any other active intervention.
Types of outcome measures
Primary outcomes
Allergic rhinitis symptoms, self reported (efficacy).
Well days (i.e. days with no or very mild symptoms, and no use of medication for allergic rhinitis) (efficacy).
Lung function measures (e.g. change in bronchial reactivity, forced expiratory volume in one second (FEV1), NO, acute asthma symptoms, asthma medication use) (safety).
Secondary outcomes
Use of rescue medication (i.e. non‐routine drugs taken to relieve acute exacerbations), reported validly and regardless of how recorded.
Rhinoconjunctivitis quality of life scores, self reported.
Serious adverse events (e.g. hospitalisation, death).
Other adverse events (i.e. any non‐serious systemic or regional or local adverse event).
Dropouts (i.e. all‐cause study withdrawal) (adherence).
Costs of therapy.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the search was 24 June 2011.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2011, Issue 2); PubMed; EMBASE; CINAHL; AMED; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; CNKI; ISRCTN; ClinicalTrials.gov; ICTRP and Google. We also searched the Networked Digital Library of Theses and Dissertations (NDLTD).
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in theCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 3.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, we searched PubMed, TRIPdatabase, NHS Evidence ‐ ENT & Audiology and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. We searched for conference abstracts using the Cochrane Ear, Nose and Throat Disorders Group Trials Register.
Data collection and analysis
Selection of studies
Phase one
The principal review author (AC) inspected all abstracts of studies identified as above, to determine potentially relevant reports. In addition, and to ensure reliability, PB inspected 100% all the identified abstracts.
Where disagreement existed as to the potential relevance of a particular report, we were to resolve this through discussion. Where doubt persisted, we were to retrieve the full text of the report for inspection.
Phase two
We retrieved the full text of all those reports judged to be potentially relevant for further assessment, and for a final decision on inclusion (see Criteria for considering studies for this review). Once the full texts were obtained, AC and PB in turn inspected the full reports and independently decided whether or not they met the inclusion criteria. AC and PB were not be blinded to the names of the authors, source institutions or journal of publication.
Where difficulties or disputes on study eligibility arose, we asked SK for help; if agreement was still not reached, we were to add these disputed studies to those awaiting assessment and contact the authors of the original reports for clarification.
PRISMA flow diagram
We included a PRISMA flow diagram to illustrate the results of our various searches and the process of screening and selecting studies for inclusion in the review (Moher 2009).
Data extraction and management
We designed and used a structured data collection form to record data from five key domains of each included study, as follows.
Study characteristics (study design, date of study, total study duration, number of study centres and their location, study withdrawals).
Participants (N, mean age, age range, gender distribution, sociodemographic characteristics, ethnicity, allergic rhinitis presentation, inclusion criteria, exclusion criteria).
Interventions (for each intervention: total number in intervention arm, helminth species used, developmental stage of the organism, dose of exposure, route of exposure, duration of exposure, cost).
Controls (for each control: total number in control arm; where control was an active pharmacological intervention: nature, dose, route of administration, cost).
Outcomes (outcomes specified and collected, time points reported).
For eligible studies, AC and PB extracted the data using the agreed form. AC and PB resolved discrepancies through discussion; failing resolution, we were to consult SK.
We entered the data into Review Manager (RevMan) software version 5.1 (RevMan 2011) and checked data for accuracy.
When information regarding any data item was unclear, we contacted the authors of the original reports to provide further details.
Assessment of risk of bias in included studies
AC and PB independently assessed the methodological quality of each included study using The Cochrane Collaboration’s ‘Risk of bias’ tool (Handbook 2011).
The study features we assessed were the following.
Random sequence generation
Allocation concealment
Blinding of participants and investigators
Blinding of outcome assessment
Incomplete outcome data
Selective reporting
Other bias
We recorded each of these factors as ‘low risk’, ‘high risk’ or ‘unclear risk’, with a brief overview provided in table format. If ‘unclear’, we attempted to seek clarification from the trial authors. After this process, we gave each paper an overall quality assessment grade of low, high or unclear risk of bias.
Appendix 4 gives more information about the assessment scheme that we used.
Measures of treatment effect
We performed statistical analysis using RevMan 5. We used a fixed‐effect or random‐effects model, depending on the absence or presence of heterogeneity across the studies.
Dichotomous data
We calculated risk ratios (RRs) with 95% confidence intervals for dichotomous outcomes. Where appropriate, we expressed estimated effects as NNTB (number needed to treat to benefit). The NNTB corresponds mathematically to the inverse of the risk difference, and clinically to the number of patients to be treated to achieve one desirable event. It was calculated using the pooled risk ratio.
Continuous data
For continuous variables, we calculated a mean difference (MD) or standardised mean difference (SMD), along with 95% confidence intervals, as follows:
when two or more studies presented their data as derived from the same instrument of evaluation, and with the same units of measurement, we pooled data as a mean difference (MD);
conversely, when primary studies expressed the same variables through different instruments, and with different units of measurement, we were to use the standardised mean difference (SMD).
Summary data
For those RCTs (e.g. cross‐over studies) where the only data available were summary measures of effect, along with precision estimates, we were to use the generic inverse variance method to analyse the data.
Unit of analysis issues
If any trials had multiple treatment groups, the ‘shared’ comparison group was to be divided into the number of treatment groups, and comparisons between each treatment group and the split comparison group were to be treated as independent comparisons.
Dealing with missing data
Where data were missing, we contacted trial authors directly to obtain this missing information.
For cluster‐RCTs, we were to contact study authors for an intracluster correlation coefficient (ICC) where data were not adjusted and could not be identified from the trial report. Where ICCs were neither available from trial reports nor available from trialists directly, we were to derive an average ICC based on existing information in other sources, where such information existed. This was to constitute the primary analysis. We were to perform a sensitivity analysis without the studies, and derive alternative estimates of the ICC.
For all outcomes, in all studies, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and we analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention.
For continuous data that were missing, we were to estimate standard deviations from other available data such as standard errors, or else we were to impute them using the methods suggested in the Handbook 2011. We made no assumptions about loss to follow‐up for continuous data, and we based analyses on those participants completing the trial. We performed intention‐to‐treat analyses where appropriate. We were to perform a sensitivity analysis by calculating the treatment effect including and excluding the imputed data, to see whether this altered the outcome of the analysis.
We were to investigate the effect of drop‐outs and exclusions by conducting worst versus best‐case scenario analyses.
If there was discrepancy between the number randomised and the number analysed in each treatment group, we were to calculate and report the percentage lost to follow‐up in each group.
If drop‐outs exceeded 10% for any trial, we were to assign the worst outcome to those lost to follow‐up for dichotomous outcomes, and assess the impact of this sensitivity analysis against the results for those completing the study.
Where it was not possible to obtain missing data, we were to record this in the data collection form and report it in the ‘Risk of bias’ table.
For included studies, we noted levels of attrition. We were to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect, by using sensitivity analyses.
Assessment of heterogeneity
We assessed heterogeneity between pooled trials using:
the Chi2 test; in conjunction with
the I2 statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error, or chance (Handbook 2011).
We considered a P value of < 0.10 as statistically significant.
If enough trials were identified, we were to explore sources of heterogeneity using subgroup analyses. We displayed results graphically using forest plots, with a summary statistic presented if there was no major statistical heterogeneity (i.e. no overlap of confidence intervals in the forest plots). We used a I2 value of:
< 25% to denote low heterogeneity;
≥ 50% to denote significant heterogeneity; and
≤ 75% to denote substantial and major heterogeneity.
Assessment of reporting biases
Had been there more than 10 studies, we were to attempt to assess publication bias by preparing a funnel plot. We were to then perform a visual assessment of funnel plot asymmetry. We were to carry out exploratory analyses to investigate any suggestion of visual asymmetry in the funnel plots. We considered that our searches for trial protocols and completed trials listed in clinical trial registers would help to avoid publication bias, and assist in assessing outcome selection bias. Where necessary, we were to contact study authors in an attempt to either establish a full dataset or else obtain reasons for the non‐reporting of certain outcomes.
We were to conduct a sensitivity analysis to investigate the role of funding bias. Funding bias is defined as any bias in the design or outcome reporting of industry‐sponsored research that shows that a drug or other therapeutic product to have an apparently favourable outcome (Bekelman 2003). Relationships between industry, scientific investigators and academic institutions are widespread and often result in conflicts of interest.
Data synthesis
We pooled the results of clinically similar studies in meta‐analyses. We used adjusted summary statistics if available; otherwise we were to use unadjusted results. Pooling of data was as follows:
for dichotomous outcomes we calculated RRs for each study and then pooled these;
for continuous outcomes, we pooled the mean differences between the treatment arms at the end of follow‐up if all trials measured the outcome on the same scale (if not, then we pooled standardised mean differences);
for time‐to‐event data, we were to pool hazard ratios (HRs) using the generic inverse variance facility of RevMan 5 (Handbook 2011).
If any trials had multiple treatment groups, we were to divide the ‘shared’ comparison group into the number of treatment groups and treat comparisons between each treatment group and the split comparison group as independent comparisons.
We were to use random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986). If possible, we were to synthesise studies making different comparisons using the methods of Bucher 1997.
We assessed the quality of the body of the evidence using the approach adopted by the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group (Furlan 2009; Handbook 2011). We considered the following to represent those domains that might decrease the quality of the evidence.
The study design
Risk of bias
Inconsistency of results
Indirectness (i.e. non‐generalisability)
Imprecision (i.e. insufficient data)
Other factors (e.g. reporting bias)
We reduced the quality of the evidence by one level for each domain where poor quality was encountered. We assessed all plausible confounding factors and consider their effects as a reason to reduce any claimed effect and dose response gradient.
We defined levels of evidence as below.
High‐quality evidence
The following statement applies to all of the domains: 'Further research is very unlikely to change our confidence in the estimate of effect. There are consistent findings, that are generalisable to the population of interest, in 75% of RCTs with low risk of bias. There are sufficient data, with narrow confidence intervals. There are no known or suspected reporting biases'.
Moderate‐quality evidence
The following statement applies to one of the domains: 'Further research is likely to have an important impact on our confidence in the estimate of effect, and may change the estimate'.
Low‐quality evidence
The following statement applies to two of the domains: 'Further research is very likely to have an important impact on our confidence in the estimate of effect, and is likely to change the estimate'.
Very low‐quality evidence
The following statement applies to three of the domains: 'We are very uncertain about the estimate'.
No evidence
The following statement applies:'No RCTs were identified that measured the outcome of interest'.
We also considered a number of other factors to place the results into a wider clinical context: temporality, plausibility, strength of association, adverse events and costs.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we were to perform subgroup analyses to explore the effects of:
different helminth species or combination of helminth species;
different developmental stages of the administered helminths;
different routes of administration of the helminths;
different exposure intensities; and
different durations of exposure to the helminths.
Sensitivity analysis
Where possible, we were to perform sensitivity analyses to explore the effects of various aspects of trial and review methodology, including the effects of missing data and of whether or not allocation was concealed.
If sufficient data were available, we were to perform sensitivity analyses to determine the impact of excluding those studies with lower methodological quality, for example:
trials at high or unclear risk of bias;
unpublished studies (since these may not have been subjected to the peer review process and may have had intrinsic biases);
industry‐sponsored studies; and
trials that had not assessed adherence.
Results
Description of studies
We found five published reports, describing two studies.
Included studies
We included two studies in the review (see Characteristics of included studies for full study details). Ethics review and approval and patient consent were noted in both studies.
Fifty‐four adults with current symptoms of allergic rhinitis to any allergen were recruited, and 30 with measurable bronchial reactivity were randomised into two groups. In 15 participants 10 Necator americanus (L3) larvae in 200 μL of water were applied to an area of forearm skin and then covered for 24 hours with gauze and a waterproof adhesive dressing. The other group had 200 μL of histamine dihydrochloride solution (1.7 mg/mL) applied in the same way. The primary outcomes were the change in bronchial reactivity, calculated as the maximum fall from baseline in the provocative dose of inhaled adenosine monophosphate (AMP) required to reduce FEV1 by 10%, measured at any time over the four weeks after active or placebo infection. Secondary outcomes included the change over 12 weeks in a validated rhinoconjunctivitis quality of life score (Juniper RQLQ); and peak expiratory flow rate (PEFR) variability, change in skin wheal diameter in response to skin prick testing with various allergens, adverse event diary scores and study withdrawals.
This study was also reported in Blount 2009 and Falcone 2009.
One hundred and sixty‐two adults with grass pollen‐induced allergic rhinitis were recruited, and 100 with allergic rhinitis symptoms in the previous two grass pollen seasons or more were randomised into two groups. Before the peak of the grass pollen season, 50 participants received a total of two to five oral doses of embryonated Trichuris suis (i.e. pig whipworm) eggs in an aqueous suspension of 2500 eggs per dose, and with a 21‐day interval between doses; participants ingested eight helminth doses in total and were followed up for 24 weeks. The other group ingested a placebo solution which was similar in taste and smell. The primary outcomes were the change in mean daily total symptom score for runny, itchy or sneezing nose; and the change in the percentage of well days during the grass pollen season. Secondary outcomes included medication use, change in skin wheal diameter in response to skin prick testing with grass pollen and nine other allergens, titres of grass pollen‐specific IgE, adverse event frequencies and study withdrawals.
This study was also reported in Bager 2011.
Excluded studies
Of the 103 papers taken forward for first‐level screening, we excluded 77 on the basis of their abstracts because they were not clinical trials. We retrieved the full text of 26 papers, and excluded a further 21 papers (see Characteristics of excluded studies for details) due to inappropriateness of study setting (two papers) or of study design (19 papers). This left five papers, reporting on two studies, as described above.
The PRISMA flow diagram (Moher 2009) of the full screening process is in Figure 1.
1.
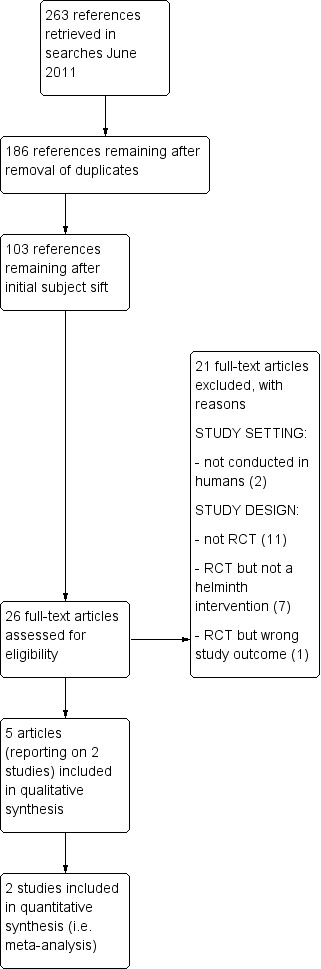
Process of screening search results and selecting studies for inclusion
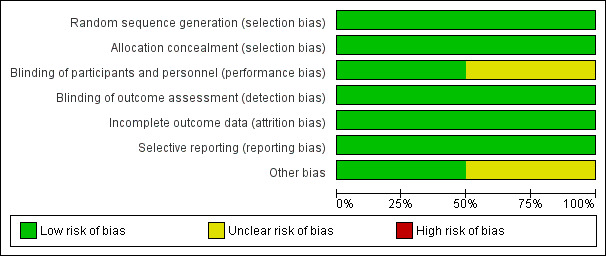
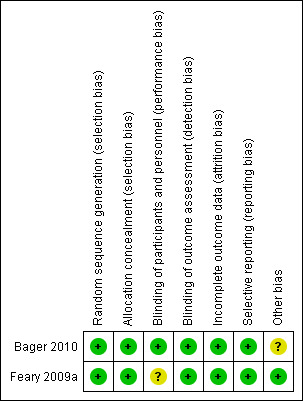
Risk of bias in included studies
Allocation
Both studies described an adequate process for random sequence generation (see Characteristics of included studies). Allocation concealment was also satisfactory and was described clearly in both study reports.
Blinding
In one study (Bager 2010) both participants and investigators were blinded and there was consequently a low risk of bias; blinding of participants was unclear in the other study (Feary 2009a).
Incomplete outcome data
All outcome data were reported adequately in both studies. Both studies reported the study drop‐outs and recorded clear explanations for the withdrawals.
Selective reporting
There was no evidence of selective outcome reporting in either study. Both studies reported additional outcomes, not central to the primary objective of the study, through multiple reports.
Other potential sources of bias
Both of the included studies were carried out at a single centre. In Bager 2010 only 5% of the participants were female.
Both studies assumed that participants were not harbouring any helminths at the time of enrolment. This assumption, if incorrect, may have introduced bias.
Imprecision may also derive from the fact that participants in both studies were a mix of adults with either intermittent allergic rhinitis or persistent allergic rhinitis.
Effects of interventions
See: Table 1
Of 17 outcomes evaluated in this review, eight were positive (i.e. favoured helminths).
Allergic rhinitis symptoms
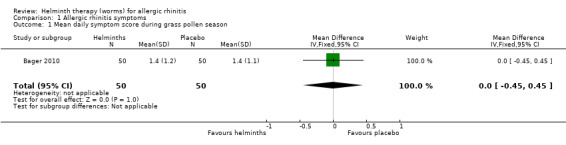
Bager 2010 reported the change in the mean daily symptom score during the grass pollen season; there was no difference between groups (mean difference (MD) 0.0, 95% confidence interval (CI) –0.45 to 0.45).
Well days
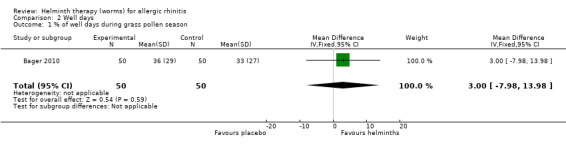
Bager 2010 reported the percentage of well days during the grass pollen season; there was no difference between groups (MD 3.0, 95% CI –7.98 to 13.98).
Lung function measures
Feary 2009a reported the bronchial reactivity change over 12 weeks; there was no difference between groups (MD 0.51, 95% CI –0.68 to 1.70).
Use of rescue medication
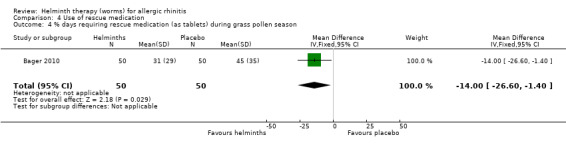
Bager 2010 reported the mean total daily medication use score during the grass pollen season; there was no difference between groups (MD –1.10, 95% CI –2.41 to 0.21). The investigators also reported the percentage of days during the grass pollen season when participants had to take rescue medication in various forms: as eye drops, nasal sprays and as tablets. There was a difference between groups only in the case of tablets: eye drops (MD –2.00%, 95% CI –12.40 to 8.40), nasal sprays (MD –3.00%, 95% CI –14.19 to 8.19) and tablets (MD –14.00%, 95% CI –26.60 to –1.40) (Figure 2).
2.
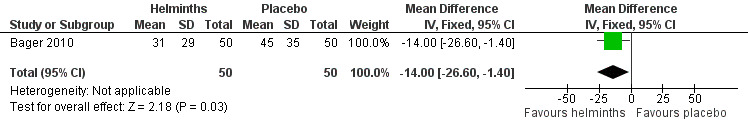
Forest plot of comparison: 3 Use of rescue medication, outcome: 3.4 % days requiring rescue medication (tablets) during grass pollen season.
Rhinoconjunctivitis quality of life score
Feary 2009a reported the total quality of life score over 12 weeks; there was no difference between groups (MD 0.33, 95% CI –0.27 to 0.93).
Serious adverse events
There were no serious adverse events in Feary 2009a. Bager 2010 reported hospitalisation due to any adverse event and hospitalisation due to any gastrointestinal adverse event, and there was no difference between groups in either category (hospitalisation due to any adverse event risk ratio (RR) 2.88, 95% CI 0.31 to 26.69; due to any gastrointestinal adverse event RR 0.32, 95% CI 0.01 to 7.66).
Other adverse events
In Feary 2009a adverse events were self reported on a 10‐point scale, instead of being dichotomised; the investigators found that the data were not distributed normally and hence were only able to report the median scores under various symptom categories. Median scores were higher for indigestion, and localised skin itching and redness – the latter two symptoms peaked on day two at the site of hookworm administration.
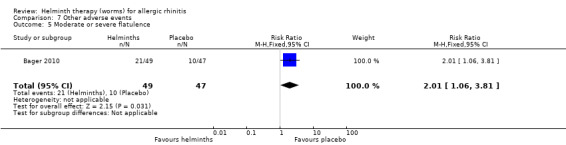
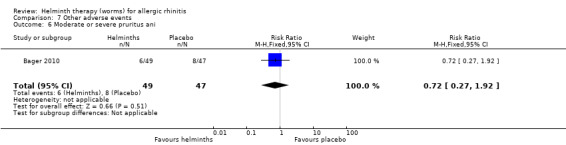
Bager 2010 reported any adverse event (RR 1.06, 95% CI 0.91 to 1.23), any gastrointestinal adverse event (RR 1.79, 95% CI 1.31 to 2.45) (Figure 3), moderate or severe abdominal pain (RR 7.67, 95% CI 1.87 to 31.57), moderate or severe diarrhoea (RR 1.99, 95% CI 1.18 to 3.37), moderate or severe flatulence (RR 2.01, 95% CI 1.06 to 3.81) and moderate or severe pruritus ani (RR 0.72, 95% CI 0.27 to 1.92). All categories of adverse event were more likely to be reported by participants taking helminths.
3.
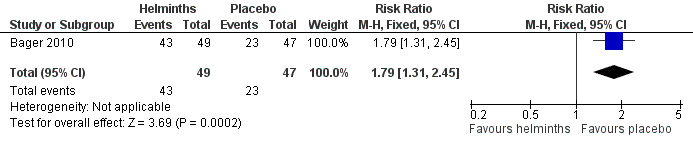
Forest plot of comparison: 5 Other adverse events, outcome: 5.2 Any gastrointestinal adverse event.
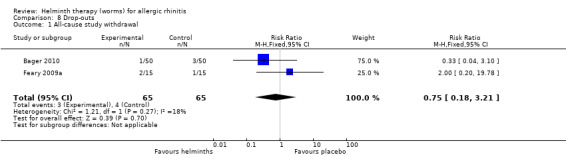
Drop‐outs
Drop‐outs were reported in both studies. There was no significant difference between groups for all‐cause study withdrawal (RR 0.75, 95% CI 0.18 to 3.21) (Figure 4).
4.
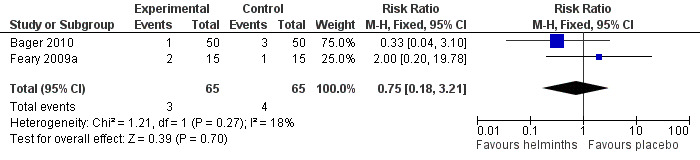
Forest plot of comparison: 1 Study withdrawal, outcome: 1.1 All‐cause withdrawal
Costs
Neither study reported data on costs (i.e. costs of helminth therapy, costs of pharmacotherapy, clinic visit costs, healthcare worker costs).
Discussion
This is the first ever systematic review of helminths for allergic rhinitis and the first Cochrane review to investigate the therapeutic use of helminths.
Summary of main results
We found five published reports, describing two studies (130 adult participants). One safety study, with 12 weeks’ follow‐up, used a single percutaneous application of 10 Necator americanus (i.e. human hookworm) larvae. One efficacy and safety study, with 24 weeks’ follow‐up, used three‐weekly oral dosing with 2500 Trichuris suis (i.e. pig whipworm) eggs in aqueous suspension.
The primary outcomes of allergic rhinitis symptoms, well days and lung function measures were not significantly different between treatment groups, nor were quality of life scores. In the helminth group there was a statistically significant reduction in the percentage of days during the grass pollen season when participants needed to take tablets as rescue medication (mean difference (MD) –14.0%, 95% confidence interval (CI) –26.6 to –1.40) (Figure 2); in a typical 60‐day pollen season this 14% reduction translates into 19 days when tablets would be needed in the helminth group versus 27 days when tablets would be needed in the placebo group. This finding may have been an artefact, however, explicable through the helminths having induced a reluctance in their human hosts to take oral medication, secondary to the transient adverse gastrointestinal effects (abdominal pain, diarrhoea, flatulence) of the helminths themselves.
Participants taking helminths were more likely to report any gastrointestinal adverse event (RR 1.79, 95% CI 1.31 to 2.45) (Figure 3), moderate or severe abdominal pain (RR 7.67, 95% CI 1.87 to 31.57), moderate or severe diarrhoea (RR 1.99, 95% CI 1.18 to 3.37) and moderate or severe flatulence (RR 2.01, 95% CI 1.06 to 3.81). Participants taking helminths percutaneously (i.e. as hookworm larvae) had local skin itching and redness in the first few days after administration. There was no difference between the helminth and the placebo groups in the incidence of serious adverse events, and of all‐cause study withdrawals (Figure 4).
Overall completeness and applicability of evidence
Outcomes in both studies were reported completely, with the exception of costs data, which were not reported in either study. Outcomes were relevant to the anticipated target groups for the intervention (Figure 5; Figure 6).
5.
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
6.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Quality of the evidence
The two included studies were small and single‐centre and had limited follow‐up. All of these study characteristics could have introduced imprecision into the estimates of effect (Handbook 2011). Imprecision may also derive from the fact that in Feary 2009a some of the participants began their exposure to helminths some weeks after the onset of the ambient allergy season, instead of before it; there is some evidence that if helminths exposure follows rather than precedes allergen exposure a potentiated allergic response may result, with a worsening of symptoms (Turner 1979; Pritchard 1992).
With the exception of study drop‐outs, different outcomes were reported by the studies. We were therefore not able to pool data in a manner that contributed greatly to what is already known on this topic.
Potential biases in the review process
PB was lead investigator of one of the studies included in this review. We know of no other potential sources of bias. On account of the comprehensive nature of our search strategy, we believe that we did not miss any studies.
Agreements and disagreements with other studies or reviews
The results of this systematic review suggest no clinical effect from helminth therapy in adults with allergic rhinitis, and do not bear out the dramatic improvement in allergic rhinitis symptoms reported in two early non‐randomised studies (Preston 1970; Turton 1976). The findings in those two non‐randomised studies, both of them highly favourable towards the therapeutic use of helminths for allergic rhinitis, may be valid findings. Alternatively, the findings may be explained by a lack of rigour in the study methodologies, or by biased reporting.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence regarding the efficacy, tolerability and likely costs of helminth therapy to support its use in the routine management of allergic rhinitis. Administered to humans in carefully measured doses, helminths appear to be safe.
Implications for research.
For each currently available specific helminth therapy, more experimental studies should be undertaken, in various models of allergy, of dose range, duration of action and mode of action, before further clinical studies are ethically justified. If the results of these further preclinical studies are positive, then large, multi‐centre randomised clinical trials are warranted.
These larger clinical trials should explore the effects of different doses of helminths, and possibly of helminth combinations, in people with allergic rhinitis. Standardised instruments should be used to assess change in allergic rhinitis symptoms and medication use, and in quality of life.
Adverse events should be measured and reported as dichotomous, not as continuous variables. Cost data for the therapies tested should be collected and included in study reports, along with costs of conventional pharmacotherapy, and clinic visit and associated healthcare staff costs. The studies should be designed with extended periods of follow‐up (up to one year), if there is experimental evidence to support this.
In future studies of helminth therapy for allergic rhinitis, participants should be categorised at the time of enrolment as suffering either from intermittent allergic rhinitis or from persistent allergic rhinitis. In seasonal allergic rhinitis trials, the onset of the intervention (i.e. exposure of the participants to helminths) should precede the usual date of onset of the pollen season in that location, by at least several months.
If helminth therapy is shown in the future to be of benefit in allergic rhinitis, and in other allergic or immune‐mediated diseases, qualitative research may be needed to identify the psychological barriers that might inhibit patients from adopting this novel mode of treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 21 June 2013 | Amended | Co‐author contact address updated. |
Acknowledgements
We thank Gemma Sandberg from the Cochrane Ear, Nose and Throat Disorders Group for her help in designing the CENTRAL search strategy; and Jenny Bellorini for her outstanding editorial assistance.
We also thank Dr Andrew Herxheimer, of the Cochrane Adverse Effects Methods Group, for his helpful advice on assessing adverse events.
Appendices
Appendix 1. Commonly encountered helminths of humans
| Phylum | Common species/definitive anatomical sites as adult worms |
| Annelids (segmented worms) | Class: Hirudinea (leeches) – ectoparasites only |
| Nematodes (roundworms) |
Class: Nematoda (roundworms) The dermis: Mansonella streptocercac The gut – small intestine: Ancylostoma duodenale (hookworm)a, Ascaris lumbricoides (roundworm)a, Capillaria philippinensisd, Necator americanus (hookworm)a, Strongyloides stercoralis (threadworm)a,Trichostrongylus orientalisd The gut – large intestine: Trichuris trichiura (whipworm)d The gut – caecum: Enterobius vermicularis (pinworm)d The lymphatic system: Brugia malayia, Brugia timoria, Wuchereria bancroftia The pericardial, peritoneal and pleural cavities: Dracunculus medinensis (Guinea worm)b,Mansonella perstansa The subcutaneous tissues: Loa loac, Mansonella ozzardic, Onchocerca volvulusc |
| Platyhelminths (flatworms) |
Class: Trematoda (flukes) The bronchi: Paragonimus sppa The gut – small intestine: Echinostoma sppd, Fasciolopsis sppd, Gastrodiscoides sppd, Heterophyes sppd, Metagonimus sppd The hepatobiliary system: Clonorchis sinensisd, Fasciola giganticaa, Fasciola hepaticaa,Opisthorchis felineusd, Opisthorchis viverrinid The venous system – mesenteric veins: Schistosoma intercalatuma, Schistosoma japonicuma, Schistosoma mansonia, Schistosoma mekongia The venous system – vesical plexus: Schistosoma haematobiuma |
|
Class: Cestoda (tapeworms) The gut – small intestine: Diphyllobothrium latum (fish tapeworm)d, Diphyllobothrium pacificumd, Dipylidium caninumd,Hymenolepsis diminuta (rat tapeworm)d,Hymenolepsis nana (dwarf tapeworm)d, Taenia saginata (beef tapeworm)d, Taenia solium (pork tapeworm)d |
a Migrates through host tissues, in larval forms.
b Migrates through host tissues, as adult worm.
c Migrates through host tissues in both larval forms and as adult worm.
d No significant tissue migration through host tissues.
Appendix 2. Biological characteristics of helminths
Helminths have the following important biological characteristics:
Helminths are complex multicellular organisms. When mature, they range in length from 2 mm (Strongyloides stercoralis adults) to 8 m (Taenia saginata adults) (Weller 2008).
Most helminth species are free‐living and inhabit either bodies of fresh water, or else warm, moist soil. The latter group of helminths are known collectively as soil‐transmitted helminths (or ‘geohelminths’).
Helminths have highly developed internal structures, including alimentary and reproductive tracts.
Helminths have complex and highly varied life cycles, with multiple developmental stages. Some developmental stages may take place in an intermediate host. Some helminth species require two distinct, successive intermediate hosts.
Helminths are highly species‐specific, in most cases with a biological dependence on a single definitive host; where they have one or more intermediate hosts, they are highly species‐specific for these also (Strickland 2000).
A very few helminth species (e.g. Enterobius vermicularis and Strongyloides stercoralis) can be transmitted directly from person to person. Generally, however, person‐to‐person transmission is not possible (and hence helminths meet minimum safety criteria as therapeutic interventions).
With the exception of leeches, which are solely ectoparasites, helminths enter their definitive, human hosts either orally (as eggs or cysts) or percutaneously (as larvae or cercariae). A specific arthropod vector such as a specific mosquito species (in lymphatic filariasis) or a specific species of biting fly or midge (in loiasis, onchocerciasis and mansonellosis) may be necessary for the helminth to achieve successful percutaneous penetration of the host.
Multiple infections with different helminth species are common in endemic areas (Finch 2009).
The larval and adult forms of helminths are always motile. Many helminths species have a larval migratory phase in their human hosts, before taking up residence as adult worms in their definitive anatomical site (see Appendix 1). Some adult helminths species migrate also. Eosinophilia and elevated serum IgE levels are features of many helminth infestations (Weller 2008).
Once established in their definitive anatomical site, adult helminths may be very long‐lived (up to 30 years in the case of the schistosomes) (Finch 2009). However, because most helminth parasites do not self replicate, the acquisition of a heavy burden of adult worms requires repeated exposure to the parasite in its infectious stage, whether egg or larva. Hence clinical disease, as opposed to asymptomatic infection, generally develops only with prolonged residence in an endemic area (Weller 2008).
On account of their large size, helminths are solely extracellular; hence they are sometimes referred to as ‘macroparasites’ (Olano 2006).
Because of their size, and their prolonged life cycles and generation times, helminths have limited capacity for genetic alteration, compared to smaller, simpler microbes or ‘microparasites’ (Olano 2006).
Appendix 3. Search strategies
| CENTRAL | PubMed | EMBASE (Ovid) | CINAHL (EBSCO) |
| #1 MeSH descriptor Rhinitis explode all trees with qualifier: PS #2 MeSH descriptor Rhinitis explode all trees #3 (rhiniti* OR rhinopath* OR rhinosinusit* OR rhinoconjunctivitis OR ozena* OR hayfever OR hay NEXT fever OR pollinosis OR pollenosis OR pollonosis) #4 (allerg* :ti OR hypersensitiv*:ti) #5 (nose:ti OR nasal*:ti OR cat*:ti OR dander:ti OR mite*:ti OR dust*:ti OR dog*:ti OR ragweed:ti OR pollen:ti OR grass*:ti OR cedar:ti OR alder:ti OR willow:ti OR birch:ti OR mugwort:ti OR tree*:ti OR weed*:ti OR rapeseed*:ti OR perennial*:ti OR season*:ti OR spring:ti OR summer:ti OR respiratory:ti OR SAR:ti OR PAR:ti)) #6 #4 AND #5 #7 #2 OR #3 OR #6 #8 MeSH descriptor Helminths explode all trees #9 MeSH descriptor Antigens, Helminth explode all trees #10 MeSH descriptor Antibodies, Helminth explode all trees #11 MeSH descriptor Parasitology explode all trees #12 (helminth* OR anti‐helminth* OR antihelminth* OR anthelmint* OR aschelminth* OR soil‐transmitted helminth* OR geohelminth* OR parasit*) #13 (annelid* OR hirudine* OR leech*) #14 (nematod* OR roundworm* OR hookworm* OR pinworm* OR threadworm* OR whipworm* OR ancylostom* OR ascari* OR brugia OR enterobi* OR loa OR mansonell* OR onchocerc* necator OR strongyl* OR toxocar* OR trichin* OR trichur* OR wuchereria) #15 ((filarial AND worm*) OR filariasis OR onchocerca OR onchocerciasis OR loa‐loa OR loiasis OR wuchereria OR brugia OR mansonella OR mansonellosis ) #16 (dracuncul* OR (guinea AND worm*)) #17 (platyhelminth* OR flatworm* OR trematod* OR fluke* OR clonorchis OR echinostom* fasciol* OR gastrodiscoid* OR heterophy* OR metagonim* OR opisthorch* OR paragonim* OR schistosom*) #18 (cestod* OR tapeworm* OR diphyllobothrium OR hymenolepis OR taenia* OR tenia* OR cysticerc*) #19 #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 #20 #7 AND #19 #21 #1 OR #20 | #1 "Rhinitis/parasitology"[Mesh] #2 “Rhinitis” [Mesh] OR rhiniti* [tiab] OR rhinopath* [tiab] OR rhinosinusit* [tiab] OR rhinoconjunctivitis [tiab] OR ozena* [tiab] OR hayfever [tiab] OR (hay [tiab] AND fever [tiab] ) OR pollinosis [tiab] OR pollenosis [tiab] OR pollonosis [tiab] #3 ((allerg* [ti] OR hypersensitiv*[ti]) AND (nose[ti] OR nasal*[ti] OR cat*[ti] OR dander[ti] OR mite*[ti] OR dust*[ti] OR dog*[ti] OR ragweed[ti] OR pollen[ti] OR grass*[ti] OR cedar[ti] OR alder[ti] OR willow[ti] OR birch[ti] OR mugwort[ti] OR tree*[ti] OR weed*[ti] OR rapeseed*[ti] OR perennial*[ti] OR season*[ti] OR spring[ti] OR summer[ti] OR respiratory[ti] OR SAR[ti] OR PAR[ti])) #4 #2 OR #3 #5 “Helminths” [Mesh] OR “Antigens, Helminth” [Mesh] OR “Antibodies, Helminth” [Mesh] OR “Parasitology” [Mesh] #6 (helminth* OR anti‐helminth* OR antihelminth* OR anthelmint* OR aschelminth* OR soil‐transmitted helminth* OR geohelminth* OR parasit* OR annelid* OR hirudine* OR leech* OR nematod* OR roundworm* OR hookworm* OR pinworm* OR threadworm* OR whipworm* OR ancylostom* OR ascari* OR brugia OR enterobi* OR loa OR mansonell* OR onchocerc* necator OR strongyl* OR toxocar* OR trichin* OR trichur* OR wuchereria OR (filarial AND worm*) OR filariasis OR onchocerca OR onchocerciasis OR loa‐loa OR loiasis OR wuchereria OR brugia OR mansonella OR mansonellosis OR dracuncul* OR (guinea AND worm*) #7 (platyhelminth* OR flatworm* OR trematod* OR fluke* OR clonorchis OR echinostom* fasciol* OR gastrodiscoid* OR heterophy* OR metagonim* OR opisthorch* OR paragonim* OR schistosom* OR cestod* OR tapeworm* OR diphyllobothrium OR hymenolepis OR taenia* OR tenia* OR cysticerc*) #8 #5 OR #6 OR #7 #9 #4 AND #8 #10 #1 OR #9 | 1 exp rhinitis/ 2 (rhiniti* or rhinopath* or rhinosinusit* or rhinoconjunctivitis or ozena* or hayfever or (hay and fever) or pollinosis or pollenosis or pollonosis).tw. 3 ((allerg* or hypersensitiv*) and (nose or nasal* or cat* or dander or mite* or dust* or dog* or ragweed or pollen or grass* or cedar or alder or willow or birch or mugwort or tree* or weed* or rapeseed* or perennial* or season* or spring or summer or respiratory or SAR or PAR)).ti. 4 1 or 2 or 3 5 exp helminth/ 6 exp parasite antigen/ 7 exp parasite antibody/ 8 (helminth* or anti‐helminth* or antihelminth* or anthelmint* or aschelminth* or geohelminth* or parasit* or annelid* or hirudine* or leech* or nematod* or roundworm* or hookworm* or pinworm* or threadworm* or whipworm* or ancylostom* or ascari* or brugia or enterobi* or loa or mansonell* or onchocerc* or necator or strongyl* or toxocar* or trichin* or trichur* or wuchereria or worm* or filariasis or onchocerca or onchocerciasis or loa‐loa or loiasis or wuchereria or brugia or mansonella or mansonellosis or dracuncul* or platyhelminth* or flatworm* or trematod* or fluke* or clonorchis or echinostom* or fasciol* or gastrodiscoid* or heterophy* or metagonim* or opisthorch* or paragonim* or schistosom* or cestod* or tapeworm* or diphyllobothrium or hymenolepis or taenia* or tenia* or cysticerc*).tw. 9 5 or 6 or 7 or 8 10 4 and 9 | S1 (MH "Rhinitis+") S2 TX (rhiniti* or rhinopath* or rhinosinusit* or rhinoconjunctivitis or ozena* or hayfever or (hay and fever) or pollinosis or pollenosis or pollonosis) S3 TI ((allerg* or hypersensitiv*) and (nose or nasal* or cat* or dander or mite* or dust* or dog* or ragweed or pollen or grass* or cedar or alder or willow or birch or mugwort or tree* or weed* or rapeseed* or perennial* or season* or spring or summer or respiratory or SAR or PAR)) S4 S1 or S2 or S3 S5 (MH "Helminths") S6 TX (helminth* or anti‐helminth* or antihelminth* or anthelmint* or aschelminth* or geohelminth* or parasit* or annelid* or hirudine* or leech* or nematod* or roundworm* or hookworm* or pinworm* or threadworm* or whipworm* or ancylostom* or ascari* or brugia or enterobi* or loa or mansonell* or onchocerc* or necator or strongyl* or toxocar* or trichin* or trichur* or wuchereria or worm* or filariasis or onchocerca or onchocerciasis or loa‐loa or loiasis or wuchereria or brugia or mansonella or mansonellosis or dracuncul* or platyhelminth* or flatworm* or trematod* or fluke* or clonorchis or echinostom* or fasciol* or gastrodiscoid* or heterophy* or metagonim* or opisthorch* or paragonim* or schistosom* or cestod* or tapeworm* or diphyllobothrium or hymenolepis or taenia* or tenia* or cysticerc*) S7 S5 or S6 S8 S4 and S7 |
| Cochrane ENT Disorders Group Trials Register (ProCite database) | Web of Science/BIOSIS Previews (Web of Knowledge) | CAB Abstracts | ICTRP |
| (helminth* OR anti‐helminth* OR antihelminth* OR anthelmint* OR aschelminth* OR geohelminth* OR parasit* OR annelid* OR hirudine* OR leech* OR nematod* OR roundworm* OR hookworm* OR pinworm* OR threadworm* OR whipworm* OR ancylostom* OR ascari* OR brugia OR enterobi* OR loa OR mansonell* OR onchocerc* OR necator OR strongyl* OR toxocar* OR trichin* OR trichur* OR wuchereria OR worm* OR filariasis OR onchocerca OR onchocerciasis OR loa‐loa OR loiasis OR wuchereria OR brugia OR mansonella OR mansonellosis OR dracuncul* OR platyhelminth* OR flatworm* OR trematod* OR fluke* OR clonorchis OR echinostom* OR fasciol* OR gastrodiscoid* OR heterophy* OR metagonim* OR opisthorch* OR paragonim* OR schistosom* OR cestod* OR tapeworm* OR diphyllobothrium OR hymenolepis OR taenia* OR tenia* OR cysticerc*) | #1 TS=(rhiniti* or rhinopath* or rhinosinusit* or rhinoconjunctivitis or ozena* or hayfever or (hay and fever) or pollinosis or pollenosis or pollonosis) #2 TI=((allerg* or hypersensitiv*) and (nose or nasal* or cat* or dander or mite* or dust* or dog* or ragweed or pollen or grass* or cedar or alder or willow or birch or mugwort or tree* or weed* or rapeseed* or perennial* or season* or spring or summer or respiratory or SAR or PAR)) #3 #2 OR #1 #4 TS=(helminth* or anti‐helminth* or antihelminth* or anthelmint* or aschelminth* or geohelminth* or parasit* or annelid* or hirudine* or leech* or nematod* or roundworm* or hookworm* or pinworm* or threadworm* or whipworm* or ancylostom* or ascari* or brugia or enterobi* or loa or mansonell* or onchocerc* or necator or strongyl* or toxocar* or trichin* or trichur*) #5 TS=(wuchereria or worm* or filariasis or onchocerca or onchocerciasis or loa‐loa or loiasis or wuchereria or brugia or mansonella or mansonellosis or dracuncul* or platyhelminth* or flatworm* or trematod* or fluke* or clonorchis or echinostom* or fasciol* or gastrodiscoid*) #6 TS=(heterophy* or metagonim* or opisthorch* or paragonim* or schistosom* or cestod* or tapeworm* or diphyllobothrium or hymenolepis or taenia* or tenia* or cysticerc*) #7 #6 OR #5 OR #4 #8 #7 AND #3 | 1 (rhiniti* or rhinopath* or rhinosinusit* or rhinoconjunctivitis or ozena* or hayfever or (hay and fever) or pollinosis or pollenosis or pollonosis).tw. 2 ((allerg* or hypersensitiv*) and (nose or nasal* or cat* or dander or mite* or dust* or dog* or ragweed or pollen or grass* or cedar or alder or willow or birch or mugwort or tree* or weed* or rapeseed* or perennial* or season* or spring or summer or respiratory or SAR or PAR)).ti. 3 1 or 2 4 (helminth* or anti‐helminth* or antihelminth* or anthelmint* or aschelminth* or geohelminth* or parasit* or annelid* or hirudine* or leech* or nematod* or roundworm* or hookworm* or pinworm* or threadworm* or whipworm* or ancylostom* or ascari* or brugia or enterobi* or loa or mansonell* or onchocerc* or necator or strongyl* or toxocar* or trichin* or trichur* or wuchereria or worm* or filariasis or onchocerca or onchocerciasis or loa‐loa or loiasis or wuchereria or brugia or mansonella or mansonellosis or dracuncul* or platyhelminth* or flatworm* or trematod* or fluke* or clonorchis or echinostom* or fasciol* or gastrodiscoid* or heterophy* or metagonim* or opisthorch* or paragonim* or schistosom* or cestod* or tapeworm* or diphyllobothrium or hymenolepis or taenia* or tenia* or cysticerc*).tw. 5 3 and 4 | rhinti* AND helminth* OR rhiniti* AND worm* OR rhiniti* AND parasit* OR hayfever AND helminth* OR hayfever AND worm* OR hayfever AND parasit* OR pollenosis AND helminth* OR pollenosis AND worm* OR pollenosis AND parasit* |
Appendix 4. 'Risk of bias' assessment
Strategy to assess risk of bias
We will assess each of the following study features and record them as 'Yes', 'No' or 'Unclear'.
1. Sequence generation for randomisation
Was the allocation sequence adequately generated, e.g. coin toss, random number tables, computer‐generated, other?
2. Allocation concealment
Was allocation adequately concealed in a way that would not allow both the investigators and the participants to know or influence the intervention group before an eligible participant is entered into the study, e.g. central randomisation or sequentially numbered, opaque, sealed envelopes?
3. Blinding
Were investigators blinded to the helminth interventions they were administering?
Were participants blinded to the helminth interventions they were receiving?
Were assessors blinded to the effects they were assessing?
For each of the three groups, we will record blinding as: 'Yes', 'No', 'Not possible' or 'Unclear'. We will record the study as double‐blind if both the investigators and participants were blinded, and as triple‐blind if all three groups were blinded.
4. Incomplete outcome data
Were incomplete outcome data adequately addressed?
If any withdrawals occurred, were these withdrawals described and reported by treatment group?
Were clear explanations recorded for withdrawals and drop‐outs in treatment groups?
Incomplete outcome data essentially include attrition, exclusions and missing data. An example of an adequate method to address incomplete outcome data is the use of intention‐to‐treat analysis (ITT).
5. Selective reporting of outcomes
Are reports of the study free from any suggestion of selective outcome reporting?
If 'Yes', this will be interpreted as no evidence that statistically non‐significant results might have been selectively withheld from publication (e.g. through selective under‐reporting of data, or through selective reporting of a subset of the data).
6. Other potential sources of bias
Was the study apparently free of other defects that could put it at a high risk of bias (e.g. baseline imbalance, or the use of an insensitive instrument to measure outcomes)?
Data and analyses
Comparison 1. Allergic rhinitis symptoms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean daily symptom score during grass pollen season | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.45, 0.45] |
1.1. Analysis.
Comparison 1 Allergic rhinitis symptoms, Outcome 1 Mean daily symptom score during grass pollen season.
Comparison 2. Well days.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 % of well days during grass pollen season | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐7.98, 13.98] |
2.1. Analysis.
Comparison 2 Well days, Outcome 1 % of well days during grass pollen season.
Comparison 3. Lung function measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bronchial reactivity change | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐0.68, 1.70] |
3.1. Analysis.
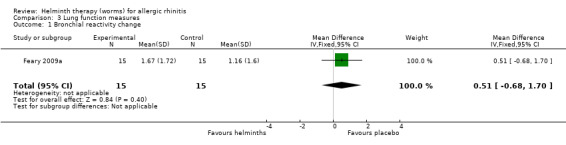
Comparison 3 Lung function measures, Outcome 1 Bronchial reactivity change.
Comparison 4. Use of rescue medication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean total daily medication use score during grass pollen season | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.41, 0.21] |
| 2 % days requiring rescue medication (as eye drops) during grass pollen season | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐12.40, 8.40] |
| 3 % days requiring rescue medication (as nasal sprays) during grass pollen season | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐14.19, 8.19] |
| 4 % days requiring rescue medication (as tablets) during grass pollen season | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐14.0 [‐26.60, ‐1.40] |
4.1. Analysis.
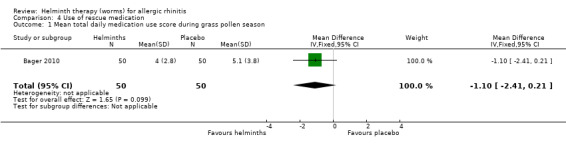
Comparison 4 Use of rescue medication, Outcome 1 Mean total daily medication use score during grass pollen season.
4.2. Analysis.
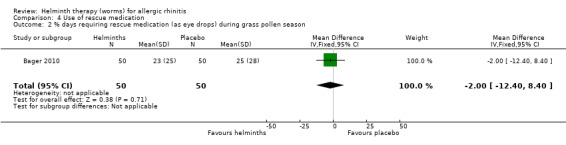
Comparison 4 Use of rescue medication, Outcome 2 % days requiring rescue medication (as eye drops) during grass pollen season.
4.3. Analysis.
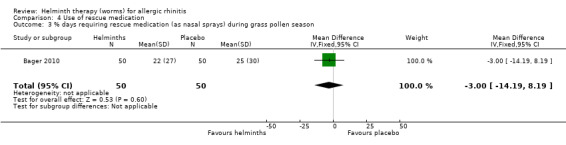
Comparison 4 Use of rescue medication, Outcome 3 % days requiring rescue medication (as nasal sprays) during grass pollen season.
4.4. Analysis.
Comparison 4 Use of rescue medication, Outcome 4 % days requiring rescue medication (as tablets) during grass pollen season.
Comparison 5. Rhinoconjunctivitis quality of life score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total quality of life score over 12 weeks | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.27, 0.93] |
5.1. Analysis.
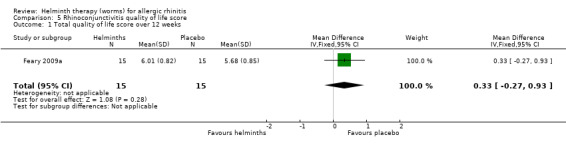
Comparison 5 Rhinoconjunctivitis quality of life score, Outcome 1 Total quality of life score over 12 weeks.
Comparison 6. Serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospitalisation due to any adverse event | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.31, 26.69] |
| 2 Hospitalisation due to any gastrointestinal adverse event | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.66] |
6.1. Analysis.
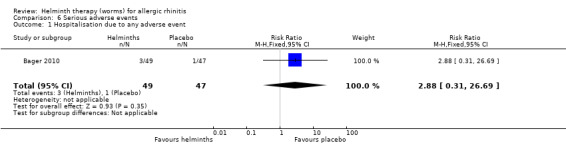
Comparison 6 Serious adverse events, Outcome 1 Hospitalisation due to any adverse event.
6.2. Analysis.
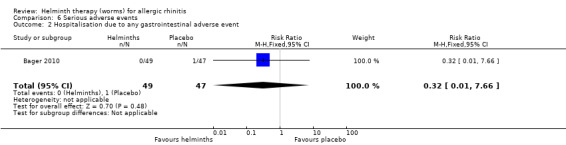
Comparison 6 Serious adverse events, Outcome 2 Hospitalisation due to any gastrointestinal adverse event.
Comparison 7. Other adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any adverse event | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.91, 1.23] |
| 2 Any gastrointestinal adverse event | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.31, 2.45] |
| 3 Moderate or severe abdominal pain | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.67 [1.87, 31.57] |
| 4 Moderate or severe diarrhoea | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.18, 3.37] |
| 5 Moderate or severe flatulence | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.06, 3.81] |
| 6 Moderate or severe pruritus ani | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.27, 1.92] |
7.1. Analysis.
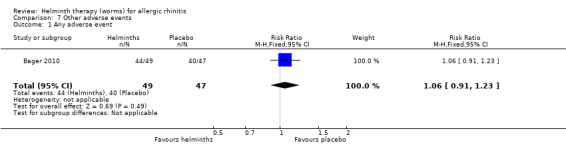
Comparison 7 Other adverse events, Outcome 1 Any adverse event.
7.2. Analysis.
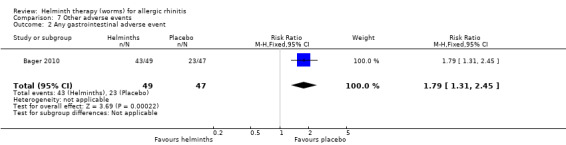
Comparison 7 Other adverse events, Outcome 2 Any gastrointestinal adverse event.
7.3. Analysis.
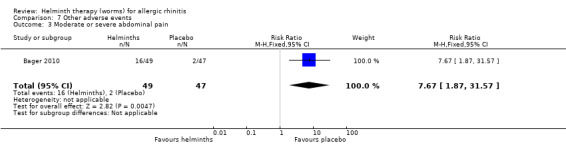
Comparison 7 Other adverse events, Outcome 3 Moderate or severe abdominal pain.
7.4. Analysis.
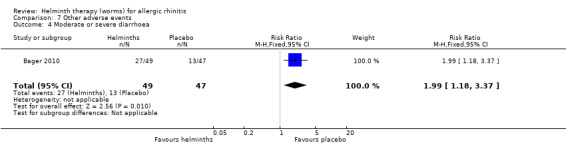
Comparison 7 Other adverse events, Outcome 4 Moderate or severe diarrhoea.
7.5. Analysis.
Comparison 7 Other adverse events, Outcome 5 Moderate or severe flatulence.
7.6. Analysis.
Comparison 7 Other adverse events, Outcome 6 Moderate or severe pruritus ani.
Comparison 8. Drop‐outs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause study withdrawal | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.21] |
8.1. Analysis.
Comparison 8 Drop‐outs, Outcome 1 All‐cause study withdrawal.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bager 2010.
| Methods | RCT (setting: single centre; country: Denmark; length of follow‐up: 24 weeks) Ethical approval: Danish Ethics Committee |
|
| Participants | Number randomised: 100 (helminths = 50, placebo = 50) Mean age: 35 years (helminths), 39 years (placebo) Gender (M/F): 48/2 (helminths), 47/3 (placebo) |
|
| Interventions | 8 successive oral doses of Trichuris suis (i.e. pig whipworm) with a 21‐day interval between doses; each dose comprising a 15 mL pH‐neutral aqueous suspension containing 2500 embryonated T. suis eggs | |
| Outcomes | Mean daily symptom score during the 61‐day grass pollen season, each daily symptom score being the summation of (i) runny nose score, (ii) itchy nose score and (iii) sneezing score (each of these scores recorded on a severity scale of 0 = best to 3 = worst, giving a total maximum daily score of 9) Percentage of well days during the 61‐day grass pollen season (well days = days with symptom score of ≦ 2, and no use of rescue medication) Mean daily score for medication use during the 61‐day grass pollen season, with a maximum daily score of 32 Percentage of days during the 61‐day grass pollen season when rescue medication for allergic rhinitis was required, recorded by medication class (i.e. eye drops, nasal sprays, tablets) Measurable change (mm) in diameter of skin wheal, between prick testing with grass pollen allergen at enrolment, and repeat testing after final treatment Adverse events (self recorded and categorised in intensity as 0 = none, 1 = mild, 2 = moderate, 3 = severe) Participants hospitalised due to adverse events Drop‐outs (i.e. all‐cause study withdrawal) |
|
| Notes | The pollen season was defined as starting after 3 consecutive days with a pollen count of ≧ 10 pollen grains/m3, and lasted 61 days (i.e. from 28 May 2008 to 27 July 2008, with the peak day on 9 June 2008); all dosing took place between March and October, starting 4 to 13 weeks before the peak day Participants were men (or women not of childbearing potential) aged 18 to 65 years with (i) symptoms of grass pollen‐induced allergic rhinitis in the previous 2 pollen seasons, (ii) a wheal diameter of ≧ 3 mm on skin prick testing with grass pollen allergen, (iii) a specific IgE level against grass pollen allergen of ≧ 0.7 kilo units antigen per litre (kUA/L), (iv) a spirometric FEV1 of ≧ 70% of predicted, and (v) no significant asthma Participants were supplied with standardised classes and quantities of rescue medication at the start of the trial, and this rescue medication was replenished every 3 weeks Placebo was 15 mL of a pH‐neutral oral solution similar in taste and smell to the intervention solution, administered at 21‐day intervals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The sponsor electronically randomized subjects 1 to 1 in blocks of 10 to receive 8 treatments of placebo or TSO with an interval of 21 days" |
| Allocation concealment (selection bias) | Low risk | Quote: "The taste, smell and appearance of TSO and placebo were similar" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Treatment assignment was blinded to all personnel at the trial clinic [and to] subjects... for the duration of the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Treatment assignment was blinded to... data management personnel for the duration of the trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "98% of treatments and 100% of sampling visits were performed as scheduled" Withdrawals were few and were described and reported adequately |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting Adverse events were reported in a publication (Bager 2011) separate to the main report (Bager 2010) |
| Other bias | Unclear risk | Very low numbers of female participants 100% of participants were Caucasian The study assumed that participants were not harbouring any helminths at the time of enrolment Participants in both studies were a mix of adults with either intermittent allergic rhinitis or persistent allergic rhinitis The study was carried out at a single centre Statens Serum Institute, Copenhagen acted as the (non‐commercial) sponsor |
Feary 2009a.
| Methods | RCT (setting: single centre; country: UK; length of follow‐up: 12 weeks) Ethical approval: Nottingham Research Ethics Committee | |
| Participants | Number randomised: 30 Mean age: 30 years (helminths), 33 years (placebo) Gender (M/F): 9/15 (helminths), 9/15 (placebo) |
|
| Interventions | 10 Necator americanus (L3) larvae in 200 μL of distilled water, applied to an area of forearm skin and then covered for 24 hours with gauze and a waterproof adhesive dressing | |
| Outcomes | Change in bronchial reactivity, expressed as the change between Week 0 and Week 4 in the in the number of provocation doubling doses of inhaled adenosine monophosphate required to reduce FEV1 by 10% (and where a fall in the number of doubling doses = a better clinical outcome) PEFR self recorded each morning and evening as the best of 3 attempts; PEF variability then calculated for the 4 weeks of the intervention and expressed as the mean of the 2 lowest PEFR values during the entire 4‐week period, as a percentage of the 4‐week PEFR period mean (and with a value of 100 indicating no variability, i.e. perfect control) Measurable change (mm) in diameter of skin wheal, between prick testing at enrolment with various allergens (cat fur, Dermatophagoides pteronyssinus, grass pollen) and repeat testing after final treatment Total score over the entire 12‐week study period in Juniper Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ), with the score calculated at the end of the study by the investigators, as the log of the area under the curve Self recorded daily symptom scores, each score based on a visual analogue scale of 0 = none to 10 = maximal, for a range of 12 pre‐specified likely adverse effects of helminths (i.e. localised skin itching/redness, wheeze, cough, breathlessness, nausea, diarrhoea, abdominal pain, flatulence, indigestion, loss of appetite, tiredness), investigator‐assessed during the predicted period of high risk for helminth adverse effects Drop‐outs (i.e. all‐cause study withdrawal) |
|
| Notes | Recruitment and enrolment took place between February to August 2006 Participants were men and non‐pregnant women aged ≧ 18 years, with current symptoms of allergic rhinoconjunctivitis and with subclinical asthma (i.e. with measurable airway responsiveness to inhaled adenosine monophosphate, but no clinical diagnosis of asthma) Juniper RQLQ (Juniper 1991) was interviewer‐administered at baseline, then again at 2‐weekly clinic visits; this is a patient‐specific test instrument derived from 28 questions drawn from 7 dimensions (sleep, non‐hay fever symptoms, practical problems, nose symptoms, eye symptoms, activities, emotions) and with a maximum score of 168, denoting extremely poor quality of life Placebo was 200 μL of histamine dihydrochloride solution (at a strength of 1.7 mg/mL) applied to an area of forearm skin and then covered for 24 hours with gauze and a waterproof adhesive dressing |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects [were randomised to] active or placebo infection, allocated in blocks of four according to a computer generated random code" |
| Allocation concealment (selection bias) | Low risk | Quote: "the solutions were administered by an independent member of the research team who was not involved in any of the study measurements, to ensure that the clinical researcher carrying out the protocol measures remained blind to treatment allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "of the 14 individuals in the placebo group who completed the study, three correctly thought they had received placebo... Of the 13 with hookworm infection who completed the study, eight correctly thought they had received hookworm" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To maintain blinding of the clinical researcher, subjects were asked to cover their arm at the site of the dressing application during [assessment] visits in case of local skin redness and to discuss any queries relating to the study with a different member of the research team" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 95% of assessments were carried out at the appropriate time Withdrawals were few and were described and reported adequately |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting Additional outcomes were reported in two publications (Blount 2009; Falcone 2009) separate to the main report (Feary 2009a) |
| Other bias | Low risk | Some participants used antihistamine (loratadine 10 mg tablets) daily or as rescue medication; one took a daily steroid nasal spray throughout the study The study assumed that participants were not harbouring any helminths at the time of enrolment Participants in both studies were a mix of adults with either intermittent allergic rhinitis or persistent allergic rhinitis The study was carried out at a single centre Two of the investigators declared a conflict of interest as being "inventors of patents supporting the use of molecules derived from helminths as immune modulatory agents" |
F: female
FEV1: forced expiratory volume in one second
IgE: class E circulating immunoglobulin
Juniper RQLQ: Juniper Rhinoconjunctivitis Quality of Life Questionnaire (Juniper 1991), a patient‐specific test instrument derived from 28 questions drawn from 7 dimensions (sleep, non‐hay fever symptoms, practical problems, nose symptoms, eye symptoms, activities, emotions), with a maximum score of 168 denoting extremely poor quality of life
L3: third stage larval moult
M: male
PEFR: peak expiratory flow rate
RCT: randomised controlled trial
RQLQ: Rhinoconjunctivitis Quality of Life Questionnaire
TSO: Trichuris suis ova
UK: United Kingdom
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abreu 2008 | Not RCT |
| Baqueiro 2007 | Not RCT |
| Bousquet 2003 | Not a helminth intervention |
| Corne 1997 | Not a helminth intervention |
| Daniluk 2008 | Not RCT |
| Diesel 2011 | Study not conducted in humans |
| Fernandes 2010 | Not RCT |
| Gaspar‐Sobrinho 2010 | Not RCT |
| Guerrier 1991 | Not RCT |
| Hamid 2011 | Wrong study outcome |
| Hepworth 2010 | Not RCT |
| Herrera 1992 | Not RCT |
| Horak 2009 | Not a helminth intervention |
| Hrdlickova 2009 | Not RCT |
| Mortemousque 2004 | Not a helminth intervention |
| Nagata 1995 | Not RCT |
| Ollerstam 2002 | Study not conducted in humans |
| Snyman 1998 | Not a helminth intervention |
| Souza 1985 | Not a helminth intervention |
| van Bever 1992 | Not a helminth intervention |
| Yuasa 1981 | Not RCT |
RCT: randomised controlled trial
Differences between protocol and review
Between drafting the protocol and writing the review we added some outcomes, and renamed or reordered others. This is unusual in a Cochrane review but we felt it to be necessary as this was the first review of its kind in this field and a logical structure for the important outcomes associated with helminth therapy only emerged after some considerable reflection and discussion between the authors. Our intention was not to manipulate the results in any particular direction.
We extracted the data for but did not systematically analyse a number of physiological measurements (change in diameter of skin wheal on skin prick testing, assessment of basophil activation) and laboratory values (serum antibodies, serum haemoglobin, total histamine). These data were of interest to the investigators but not important to patients, and hence were not central to this review.
PB inspected 100% all the identified abstracts, instead of a 10% sample as proposed in the protocol.
Contributions of authors
AC conceived and co‐ordinated the review, registered the title, wrote the protocol, helped design the CENTRAL search strategy, searched for studies, carried out initial screening and quality assessment, wrote to authors, performed data extraction and data analysis, and wrote the review text.
PB helped develop the protocol, carried out initial screening and quality assessment, and helped write the review text.
SK helped develop the protocol, helped with data analysis and helped write the review text.
Sources of support
Internal sources
Commander Regional Forces, UK.
External sources
None, Not specified.
Declarations of interest
PB was lead investigator in one of the studies included in this review. He has signed a consultancy contract with Colorado Biosciences Inc, who hold the license for Trichuris suis ova in the USA.
There are no other financial conflicts of interest and the review authors declare that they have no other association with any manufacturer or promoter of pharmaceutical or helminth products, and no association with any parties who may have vested interests in the results of this review.
Edited (no change to conclusions)
References
References to studies included in this review
Bager 2010 {published data only}
- Bager P, Arnved J, Rønborg S, Wohlfahrt J, Poulsen LK, Westergaard T, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double‐blind, placebo‐controlled clinical trial. Journal of Allergy and Clinical Immunology 2010;125(1):123‐30. [DOI] [PubMed] [Google Scholar]
- Bager P, Kapel C, Roepstorff A, Thamsborg S, Arnved J, Rønborg S, et al. Symptoms after ingestion of pig whipworm Trichuris suis eggs in a randomized placebo‐controlled double‐blind clinical trial. PLoS One 2011;6(8):e22346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feary 2009a {published data only}
- Blount D, Hooi D, Feary J, Venn A, Telford G, Brown A, et al. Immunologic profiles of persons recruited for a randomized, placebo‐controlled trial of hookworm infection. American Journal of Tropical Medicine and Hygiene 2009;81(5):911‐6. [DOI] [PubMed] [Google Scholar]
- Falcone FH, Telford G, Hooi D, Brown AP, Seabra R, Feary J, et al. Antigen‐driven basophil activation is indicative of early Necator americanus infection in IgE‐seronegative patients. Journal of Allergy and Clinical Immunology 2009;124(6):1343‐50. [DOI] [PubMed] [Google Scholar]
- Feary J, Venn A, Brown A, Hooi D, Falcone FH, Mortimer K, et al. Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo‐controlled feasibility study. Clinical and Experimental Allergy 2009;39(7):1060‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abreu 2008 {published data only}
- Abreu RR, Rocha RL, Lamounier JA, Guerra AF. Etiology, clinical manifestations and concurrent findings in mouth‐breathing children. Jornal de Pedatria 2008;84(6):529‐35. [DOI] [PubMed] [Google Scholar]
Baqueiro 2007 {published data only}
- Baqueiro T, Pontes de Carvalho L, Carvalho FM, Santos NM, Alcantara‐Neves NM. Asthma and rhinitis symptoms in individuals from different socioeconomic levels in a Brazilian city. Allergy and Asthma Proceedings 2007;28(3):362‐7. [DOI] [PubMed] [Google Scholar]
Bousquet 2003 {published data only}
- Bousquet J, Ndiaye M, Ait‐Khaled N, Annesi‐Maesano I, Vignola AM. Management of chronic respiratory and allergic diseases in developing countries. Focus on sub‐Saharan Africa. Allergy 2003;58(4):265‐83. [DOI] [PubMed] [Google Scholar]
Corne 1997 {published data only}
- Corne J, Djukanovic R, Thomas L, Warner J, Botta L, Grandordy B, et al. The effect of intravenous administration of a chimeric anti‐IgE antibody on serum IgE levels in atopic subjects: efficacy, safety and pharmacokinetics. Journal of Clinical Investigation 1997;99(5):879‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daniluk 2008 {published data only}
- Daniluk U, Kaczmarski M, Sidor K. Chosen factors and high total concentration of immunoglobulin E (IgE) in children. Polish Journal of Environmental Studies 2008;17(4):473‐8. [Google Scholar]
Diesel 2011 {published data only}
- Disel A, Deboer DJ. Serum allergen‐specific immunoglobulin E in atopic and healthy cats: comparison of a rapid screening immunoassay and complete‐panel analysis. Veterinary Dermatology 2011;22(1):39‐45. [DOI] [PubMed] [Google Scholar]
Fernandes 2010 {published data only}
- Fernandes JF, Taketomi EA, Mineo JR, Miranda DO, Alves R, Resende RO, et al. Antibody and cytokine responses to house dust mite allergens and Toxoplasma gondii antigens in atopic and non‐atopic Brazilian subjects. Clinical Immunology 2010;136(1):148‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaspar‐Sobrinho 2010 {published data only}
- Gaspar‐Sobrinho FP, Souza‐Machado A, Santos SB, Orge G, Lessa HA, Cruz AA, et al. Clinical and immunological features of patients with atopy and concomitant HTLV‐1 infection. Brazilian Journal of Medical and Biological Research 2010;43(12):1167‐72. [DOI] [PubMed] [Google Scholar]
Guerrier 1991 {published data only}
- Guerrier G, Noiret A. Cockroach allergy in children ‐ clinical laboratory and environmental characteristics. Revue Française d'Allergie et d'Immunologie Clinique 1991;31(3):153‐6. [Google Scholar]
Hamid 2011 {published data only}
- Hamid A. A longitudinal study of allergy and intestinal helminth infections in semi urban and rural areas of Flores, Indonesia (ImmunoSPIN Study). BMC Infectious Diseases 2011;11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hepworth 2010 {published data only}
- Hepworth MR, Hamelmann E, Lucius R, Hartmann S. Looking into the future of Trichuris suis therapy. Journal of Allergy and Clinical Immunology 2010;125(3):768‐9. [DOI] [PubMed] [Google Scholar]
Herrera 1992 {published data only}
- Herrera JS, Herrera MA, Hurtado H, Herrera S. Stress and lambliasis in patients with respiratory allergic disease. Stress Medicine 1992;8(2):105‐10. [Google Scholar]
Horak 2009 {published data only}
- Horak F, Jaeger S, Worm M, Melac M, Didier A. Implementation of pre‐seasonal sublingual immunotherapy with a five‐grass pollen tablet during optimal dosage assessment. Clinical and Experimental Allergy 2009;39(3):394‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hrdlickova 2009 {published data only}
- Hrdlickova B, Izakovicova‐Holla L. Association of single nucleotide polymorphisms in the eosinophil peroxidase gene with allergic rhinitis in the Czech population. International Archives of Allergy and Immunology 2009;150(2):184‐91. [DOI] [PubMed] [Google Scholar]
Mortemousque 2004 {published data only}
- Mortemousque B, Jacquet A, Richard C, Depont F, Colin J, Moore N. Randomised double masked trial comparing the efficacy and tolerance of 0.05% mequitazine eye drops versus 0.05% levocabastine and placebo in allergic conjunctivitis induced by a conjunctival provocation test with Dermatophagoides pteronyssinus. British Journal of Ophthalmology 2004;88(3):336‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagata 1995 {published data only}
- Nagata M, Sedgwick JB, Busse WW. Differential effects of granulocyte‐macrophage colony‐stimulating factor on eosinophil and neutrophil superoxide anion generation. Journal of Immunology 1995;155(10):4948‐54. [PubMed] [Google Scholar]
Ollerstam 2002 {published data only}
- Ollerstam O, Rohfritsch O, Hoglund S, Larsson S. A raid hypersensitive response associated with resistance in the willow Salix viminalis against the gall midge Dasineura marginemtorquens. Entomologia Experimentalis et Applicata 2002;102(2):153‐62. [Google Scholar]
Snyman 1998 {published data only}
- Snyman JR, Sommers K. Levasimole's effect on the cutaneous hypersensitivity reaction and eosinophil function in patients allergic to grass pollen. Medical Science Research 1998;26(3):151‐3. [Google Scholar]
Souza 1985 {published data only}
- Souza JC, Paiva LJ, Miniti A. Comparative trial of astemizole with dextro chlorpheniramine in the treatment of allergic rhinitis. Folha Medica 1985;91(4):319‐25. [Google Scholar]
van Bever 1992 {published data only}
- Bever HP, Stevens WJ. Effect of hyposensitization upon the immediate and late asthmatic reaction and upon histamine reactivity in patients allergic to house dust mite (Dermatophagoides pteronyssinus). European Respiratory Journal 1992;5(3):318‐22. [PubMed] [Google Scholar]
Yuasa 1981 {published data only}
- Yuasa T, Shimomura Y, Yamamoto Y, Tada R, Nakagawa Y. Lymphocyte transformation test to mite antigens in allergic conjunctival disorders. Nippon Ganka Gakkai Zasshi 1981;85(8):1060‐5. [PubMed] [Google Scholar]
Additional references
Al Sayyad 2007
- Al Sayyad JJ, Fedorowicz Z, Alhashimi D, Jamal A. Topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD003163.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
ARIA 2008
- Bousquet J, Khlataev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al in collaboration with the World Health Organization. Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy 2008;63(Suppl 86):8‐160. [DOI] [PubMed] [Google Scholar]
Austen 2011
- Austen KF. Allergies, anaphylaxis and systemic mastocytosis. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J editor(s). Harrison’s Principles of Internal Medicine. 18th Edition. New York: McGraw‐Hill, 2011:2707‐18. [Google Scholar]
Bager 2011
- Bager P, Kapel C, Roepstorff A, Thamsborg S, Arnved J, Rønborg S, et al. Symptoms after ingestion of pig whipworm Trichuris suis eggs in a randomized placebo‐controlled double‐blind clinical trial. PLoS One 2011;6(8):e22346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bekelman 2003
- Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA 2003;289(19):454‐65. [DOI] [PubMed] [Google Scholar]
Blount 2009
- Blount D, Hooi D, Feary J, Venn A, Telford G, Brown A, et al. Immunologic profiles of persons recruited for a randomized, placebo‐controlled clinical trial of hookworm infection. American Journal of Tropical Medicine and Hygiene 2009;81(5):911‐6. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomised controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Calderon 2007
- Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001936.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daveson 2011
- Daveson JA, Jones DM, Gaze S, McSorley H, Clouston A, Pascoe A, et al. Effect of hookworm infection on wheat challenge in celiac disease – a randomised double‐blind placebo controlled trial. PLoS One 2011;6(3):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Erb 2009
- Erb KJ. Can helminths or helminth‐derived products be used in humans to prevent or treat allergic diseases?. Trends in Immunology 2008;30(2):75‐82. [DOI] [PubMed] [Google Scholar]
Falcone 2009
- Falcone FH, Telford G, Hooi D, Brown AP, Seabra R, Feary J, et al. Antigen‐driven basophil activation is indicative of early Necator americanus infection in IgE‐seronegative patients. Journal of Allergy and Clinical Immunology 2009;124:1343‐50. [DOI] [PubMed] [Google Scholar]
Feary 2009b
- Feary JR, Venn AJ, Mortimer K, Brown AP, Hooi D, Falcone FH, et al. Experimental hookworm infection: a randomized placebo‐controlled trial in asthma. Clinical and Experimental Allergy 2009;40(2):299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finch 2009
- Finch RG, IrvingWL, Moss P, Anderson J. Infectious diseases, tropical medicine and sexually transmitted infection. In: Kumar P, Clark M editor(s). Kumar and Clark’s Clinical Medicine. 7th Edition. London: Saunders, 2009:79‐206. [Google Scholar]
Flohr 2008
- Flohr C, Quinnell RJ, Britton J. Do helminth parasites protect against atopy and allergic disease?. Clinical and Experimental Allergy 2008;39(1):20‐32. [DOI] [PubMed] [Google Scholar]
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Tulder M. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929‐41. [DOI] [PubMed] [Google Scholar]
Haileamlak 2005
- Haileamlak A, Dagoye D, Williams H, Venn AJ, Hubbard R, Britton J, et al. Early life risk factors for atopic dermatitis in Ethiopian children. Journal of Allergy and Clinical Immunology 2005;15(2):370‐6. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hastan 2011
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe: an underestimated disease. A GA²LEN study. Allergy 2011;66(9):1216‐23. [DOI] [PubMed] [Google Scholar]
Huang 2002
- Huang SL, Tsai PF, Yeh YF. Negative association of Enterobius infestation with asthma and rhinitis in primary school children in Taipei. Clinical and Experimental Allergy 2002;32(7):1029‐32. [DOI] [PubMed] [Google Scholar]
ISAAC 1998
- The ISAAC Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet 1998;351(9111):1225‐32. [PubMed] [Google Scholar]
Juniper 1991
- Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clinical and Experimental Allergy 1991;21(1):77‐83. [DOI] [PubMed] [Google Scholar]
Medeiros 2003
- Medeiros M Jr, Figueiredo JP, Almeida MC, Matos MA, Araújo MI, Cruz AA, et al. Schistosoma mansoni infection is associated with a reduced course of asthma. Journal of Allergy and Clinical Immunology 2003;111(5):947‐51. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nasser 2010
- Nasser M, Fedorowicz Z, Aljufairi H, McKerrow W. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD006989.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olano 2006
- Olano JP, Weller PF, Guerrant RL, Walker DH. Principles of parasitism: host‐parasite interaction. In: Guerrant RL, Walker DH, Weller PF editor(s). Tropical Infectious Diseases: Principles, Pathogens and Practice. 2nd Edition. London: Churchill Livingstone, 2006:1‐12. [Google Scholar]
Peakman 2009
- Peakman M. The immune system and disease. In: Kumar P, Clark M editor(s). Kumar and Clark's Clinical Medicine. 7th Edition. London: Saunders, 2009:53‐77. [Google Scholar]
Preston 1970
- Preston PJ. The biology of the atopic response. Journal of the Royal Naval Medical Service 1970;61(3):229‐35. [PubMed] [Google Scholar]
Pritchard 1992
- Pritchard DI. Parasites and allergic disease: a review of the field and experimental evidence for a ‘cause‐and‐effect’ relationship. In: Moqbel R editor(s). Allergy and Immunity to Helminths. London: Taylor and Francis, 1992:38‐50. [Google Scholar]
Radulovic 2010
- Radulovic S, Calderon MA, Wilson D, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD002893.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2010
- Reid PT, Innes JA. Respiratory disease. In: Colledge NR, Walker BR, Ralston SH editor(s). Davidson's Principles and Practice of Medicine. 21st Edition. London: Churchill Livingstone, 2010:641‐730. [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Scrivener 2001
- Scrivener S, Yemaneberhan H, Zebenigus M, Tilahun D, Girma S, Ali S, et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case‐control study. Lancet 2001;358(9292):1493‐9. [DOI] [PubMed] [Google Scholar]
Sheikh 2010
- Sheikh A, Hurwitz B, Nurmatov U, Schayck CP. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD001563.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strickland 2000
- Strickland GT. General principles. In: Strickland GT editor(s). Hunter's Tropical Medicine and Emerging Infectious Diseases. 9th Edition. Philadelphia, PA: Saunders, 1999:713‐6. [Google Scholar]
Summers 2005a
- Summers RW, Elliott DE, Urban JF, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 2005;128:825‐32. [DOI] [PubMed] [Google Scholar]
Sur 2010
- Sur DK, Scandale S. Treatment of allergic rhinitis. American Family Physician 2010;81:1440‐6. [PubMed] [Google Scholar]
Turner 1979
- Turner KJ, Feddema L, Quinn EH. Non‐specific potentiation of IgE by parasitic infections in man. International Archives of Allergy and Applied Immunology 1979;58(2):232‐6. [DOI] [PubMed] [Google Scholar]
Turton 1976
- Turton JA. IgE, parasites, and allergy. Lancet 1976;2(7987):686. [DOI] [PubMed] [Google Scholar]
Weller 2008
- Weller PF, Nutman TB. Intestinal nematodes. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al. editor(s). Harrison’s Principles of Internal Medicine. New York: McGraw‐Hill, 2008:1739‐44. [Google Scholar]


