Abstract
To investigate the environmental impacts of reclaimed asphalt pavement (RAP) while it was freshly processed (i.e. hot mixed asphalt or HMA) and after being subjected to weathering, three RAP materials, namely north-RAP, central-RAP, south-RAP, from three plants and one fresh HMA loose mix samples (Fresh-HMA) throughout New Jersey, USA underwent four different weathering processes including: UV and precipitation weathering on unbounded RAP, UV and precipitation weathering on compacted RAP, weathering by heat and moisture cycles, and groundwater flow-through leaching. Batch experiments were conducted to mimic releasing of trace elements in weak acidic leachate from landfills. North-RAP and central-RAP released levels of Pb greater than the United States Environmental Protection Agency (USEPA) primary drinking water maximum contaminant level (MCL) of 15 μg/L. Novel two-column experiments (a RAP column followed by a soil column) were conducted to investigate the release of trace elements from RAP and the attenuation effect of soil on potential pollutants. The results of these experiments showed that pollutants released from RAPs such as Mn and Ni were largely attenuated in the soil. The results suggest that RAP can be used as an unbound material in environments except those acidic (i.e., pH < 5 as in mines with sulfur-containing minerals and landfills with acidic environment).
Keywords: reclaimed asphalt pavement, trace element, metal, column experiment, batch extraction, soil attenuation
Graphical Abstract

1. Introduction
Asphalt has been extensively used for paved roads. In the United States, approximately 94% of the more than 2.7 million miles of paved roads and highways are surfaced with asphalt (NAPA, 2020). Reclaimed asphalt pavement (RAP) is obtained through milling and removal of existing pavement surfaces. Recycling asphalt pavements optimizes the use of natural resources, reduces the cost in the production of new asphalt, and benefits the environment in the reduction of construction debris. Studies have confirmed the performance of pavements containing up to 30% reclaimed asphalt pavement (RAP) is as good as that of virgin asphalt (Copeland, 2011). A new Federal Highway Administration survey has found a very high asphalt recycling rate of more than 99% (Hansen & Copeland, 2017), with more than 97% being put back to use in new pavements and approximately 3% being used as aggregates for unbound base materials, aggregates for stabilized base materials, pothole filler materials, and roadway shoulder materials. For those RAP materials that are not being reused right away, they have to be stored on site which could be for long periods of time and often costly. There was still a minor fraction, 0.012%, that was estimated being disposed of in waste landfills (NAPA, 2019).
Recent studies have also revealed the potential environmental risk of recycling or storing recycled materials or debris due to the release of trace elements such as heavy metals (cadmium, chromium, copper, nickel, lead and zinc) and organic compounds such as polycyclic aromatic hydrocarbons (PAHs) into groundwater and soil (Azizian et al, 2003; Brantley & Townsend, 1999; Chen et al, 2013; Kayhanian et al, 2009; Legret et al, 2005; Liu & Borst, 2018) in various environments, including unsealed and sealed asphalt concrete pavements (Bernot et al, 2011), parking lots (Boving et al, 2008), roofing materials (Gwenzi et al, 2015), and in agricultural soil up to 50 m from highway edges (Kibblewhite, 2018). Some state agencies such as New Jersey Department of Environmental Protection (NJDEP) have prevented the use of RAP materials as unbound base materials, stabilized base materials, shoulder materials, or in parking lots due to the potential toxic pollutants mentioned above that might leach from RAP used in these applications.
One of the limitations from previous studies is that trace elements were typically associated with soil particles when transported by surface and/or ground water (Azizian et al, 2003) but previous experiments have mostly estimated the release of trace elements from RAP or soil separately. This made it difficult to differentiate the fractions of potential pollutants coming from RAP and soil. Natural weathering processes by sunlight, air and precipitation typically oxidize the major components of asphalt and hence result in the release of toxic compounds into aqueous phase. Soil could either be a source to release toxic trace elements or act as a sink to attenuate these compounds (Azizian et al, 2003; Page et al, 2015) depending on soil properties and water chemistry. This study conducted batch extraction and novel two-column (RAP and soil) flow-through experiments to investigate the release or attenuation of trace elements and environmental impacts of unbound RAP using both fresh hot mix asphalt (HMA) loose sample while it is freshly processed and RAP samples after being subjected to accelerated weathering processes. This study also explored potential engineering solutions to meet federal and state environmental standards or guidelines for drinking water aquifers when recycling asphalt.
2. Material and methods
2.1. Asphalt samples
The reclaimed asphalt pavement (RAP) materials in this study were obtained from three asphalt plants in northern, central, and southern New Jersey (NJ), USA. A fresh asphalt mix was also obtained from the plant in northern NJ. North-RAP, central-RAP, south-RAP, and Fresh-HMA are used hereafter for the northern, central, southern RAP samples and HMA samples, respectively (Table 1). A gradation analysis showed that the majority of the asphalt samples were smaller than 13 mm (½ inch) and larger than 0.6 mm (Sieve No. 30) and the binder contents in the asphalt samples ranged from 2.9 to 4.5 percent (He et al, 2018).
Table 1.
RAP samples (n = 20) used in batch extraction and flow-through column experiments
| Weathering processes |
Unbound QUV 25 Cycles |
Compacted QUV 25 Cycles |
Heat & Moisture 60 cycles |
Groundwater Leaching |
|---|---|---|---|---|
| Engineering applications |
Uncovered Fill |
Guiderail Base |
Guiderails Subbase |
Covered Fill |
| north -RAP | north-RAP-uv | north -RAP-uvc | north -RAP-hm | north -RAP-gwl |
| central-RAP | central -RAP-uv | central -RAP-uvc | central -RAP-hm | central -RAP-gwl |
| south-RAP | south -RAP-uv | south -RAP-uvc | south -RAP-hm | south -RAP-gwl |
| Fresh-HMA | Fresh-HMA -uv | Fresh-HMA -uvc | Fresh-HMA -hm | Fresh-HMA -gwl |
These four sets of RAP and HMA samples underwent four different weathering processes in an environmental chamber at the Department of Civil Engineering and Engineering Mechanics of Columbia University, including (1) solar UV and precipitation weathering on unbounded asphalt samples (uv-), (2) UV and precipitation weathering on compacted asphalt samples (uvc-), (3) weathering on unbounded asphalt samples by heat and moisture cycles (hm-), and (4) groundwater flow-through leaching of unbounded asphalt samples (gwl-). The first three weathering treatments mimic medium to long-term (over months or years) outdoor aging and degradation of pavement in an accelerated setting of a few days to weeks, and were done in a Q-Lab UV Tester under controlled UV light from a fluorescent UV lamp (UVA 340 nm) and elevated moisture content and temperatures. The last weathering treatment simulates the leaching of pavement by natural rainwater and was done using a continuous flow-through column. Detailed weathering experiments have been reported elsewhere (He et al, 2018).
2.2. Soil sample
A soil sample was used to evaluate the release of trace elements from soil and the adsorption capability of soil particles for pollutants eluted from asphalt. The soil sample was collected from a New Jersey Department of Transportation (NJDOT) project site on Route 35. This soil sample has a specific gravity of 2.650 and a soil texture of sandy loam with 75 percent sand, 16 percent silt and 9 percent clay according to the United States Department of Agriculture (USDA) classification system. This type of sandy, granular soil is highly desirable and widely used in road construction of embankment or fill (Federal_Highway_Administration, 2016).
2.3. Artificial rainwater
Certain RAP applications, such as non-vegetative cover under guiderail, can be exposed to rainwater. Artificial New Jersey rainwater was thus used as a flow-through solution in column experiments. The 2014 annual averaged chemical composition of rainwater from three NJ monitoring sites in Atlantic, Ocean, and Mercer Counties from the National Atmospheric Deposition Program (NADP, 2019) was averaged and used to make the artificial NJ rainwater by dissolving chemicals of calcium chloride (CaCl2), magnesium chloride hexahydrate (MgCl2·6H2O), potassium chloride (KCl), ammonium sulfate ((NH4)2SO4), sodium nitrate (NaNO3) and sodium chloride (NaCl) into Milli-Q water (>18.1 MΩ) (Table 2). This reaction solution underwent 2 hours of continuous air bubbling from the bottom for air saturation before used in column experiments. The air saturated artificial rainwater had an approximate pH of 5.0, which was very close to the pH range of 5.04 – 5.09 in the actual rainwater.
Table 2.
Chemical composition of artificial New Jersey rainwater
| Na | Mg | K | Ca | nh4+ | no3− | Cl− | SO42- | pH | ||
|---|---|---|---|---|---|---|---|---|---|---|
| actual NJ rainwater average | 629 | 79 | 59 | 72 | 169 | 590 | 1136 | 580 | μg/L | 5.07 |
| artificial NJ rainwater based on stoichiometry | 630 | 81 | 61 | 74 | 217 | 593 | 1055 | 579 | μg/L | |
| artificial NJ rainwater from analysis | NA | 78 | 73 | 75 | NA | NA | NA | NA | μg/L | ~ 5.0 |
2.4. Batch extraction experiments
Water-soluble fraction of trace elements in the RAP samples was extracted using a modified United States Environmental Protection Agency (USEPA) Method 1311 (USEPA, 1992). The batch extraction experiment procedure involved preparing a water-based extraction fluid (pH 4.93 ± 0.05) consisting of acetic acid (CH3COOH) and sodium hydroxide (NaOH) to extract RAP samples at a 20:1 liquid to solid ratio (w/w). Samples were placed on an orbital shaker at 350 rpm for 18 ± 2 hours, after which the liquid extract was separated from the solids by filtration through a 0.7 μm glass fiber filter. The filtered liquid extract was acidified to 1 percent nitric acid (HNO3, Optima grade) for element analysis.
2.5. Two-column flow-through experiments
A novel two-column flow-through experiment was designed to investigate the release of metals from RAP samples and the attenuation effect of soil on these potential pollutants before they could enter groundwater systems. The setup of the two-column experiment is shown in Figure 1. The columns were made of Teflon to minimize the possible release of metals or organic compounds from the column, and customized made at Lamont-Doherty Earth Observatory (LDEO) with an inner diameter of 50.8 mm and a filling length of 265 mm, which made an inner volume of 537 mL for each column. All the other tubing and connection parts were made of Teflon with an exception that the Tee was made of stainless steel.
Fig. 1.

A schematic illustration of the two-column flow-through experiment setup
The aforementioned artificial NJ rainwater was introduced by a peristaltic pump at 2 mL/min flow rate from the bottom into the RAP-filled column. The outflow from the RAP column was then split using a Tee, with one end connected to a fraction collector for automatically and continuously collecting solution samples after the RAP column (referred to as “RAP solution” hereafter) into sterile glass vials at a frequency of 1 hour per sample, and the other end was driven by another peristaltic pump to flow into the soil-filled column from the bottom at a flow rate of 1 mL/min. At this flow rate, the flow velocity in the soil column was 0.5 mm/min, which was slightly higher but comparable to the typical infiltration rate of 0.4–0.8 inch/hour or 0.17–0.34 mm/min in a sandy loam soil (NRCCA, 2010) and higher than the suggested minimum infiltration rate of 0.2 mm/min for the soils underlying a porous pavement by National Asphalt Pavement Association (NAPA, 2020). The solution after the soil column (referred to as “soil solution” hereafter) was collected by another fraction collector also at the frequency of 1 hour per sample.
For quality control, before running column experiments, an “Equipment Blank Test” of running Milli-Q water through the two columns without filling RAP or soil was conducted for baseline definition and comparison. This Equipment Blank Test was run at a flow rate of 2 mL/min through RAP column (without filling of RAP) and 1 mL/min through soil column (without filling of soil) for 3 hours before 180 mL filtered water samples, equivalent to 1 pore volume (PV) of the column if filled with 30% porosity, were collected after RAP (sample ID B-R-01) and soil (sample ID B-S-01) columns, respectively.
To characterize the levels of metals that could be released from the soil sample, a “Soil Blank Test” was conducted by running artificial rainwater through the two columns with only the 2nd column filled with the soil sample. First set of filtered water samples S-R-01 (the second letter R refers to samples after the RAP column, hereafter) and S-S-01 (the second letter S refers to samples after the soil column, hereafter) were collected after continuous flow of 1 PV of the soil column, i.e. 160 ml. Second set of filtered water samples S-R-02 and S-S-02 were collected after continuous flow of 15 PVs of the soil column, i.e. 2,400 ml.
Based on levels of metals in artificial rainwater and in soil leachates from blank tests, samples including Fresh-HMA, north-RAP, Fresh-HMA-uvc, and north-RAP-uv were selected for flow-through column experiments (see Table 1 for details on weathering processes of these samples). Fresh-HMA was the only pre-application sample that had not been utilized on roadways hence selected for comparison with RAPs. north-RAP sample had much higher extractable Pb concentration (13.5 μg/L) than central-RAP and south-RAP, thus was selected for column experiments. Fresh-HMA-uvc was selected to compare with Fresh-HMA because it showed higher concentrations of lead, cobalt, chromium, iron, and potassium than other weathered Fresh-HMA samples. North-RAP-uv was selected because it showed higher extractable lead concentration (50.1 μg/L, exceeding drinking water standard of 15 μg/L) than other north-RAP weathering treatments. Overall, a total of 6 sets of flow-through column experiments were conducted (Table 3).
Table 3.
Summary of flow-through column experiment setup
| Experiment | RAP sample |
Packed RAP Column | Soil sample |
Packed Soil Column | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RAP (g) |
Water (g) |
Porosity (%) |
Flow Rate (ml/min) | Soil (g) |
Water (g) |
Porosity (%) |
Flow Rate (ml/min) | |||
| Equipment blank | N/A | NA | 537 | NA | 2.0 | No | NA | 537 | NA | 1.0 |
| Soil blank | N/A | NA | 537 | NA | 3.0 | Yes | 889 | 159 | 30 | 0.60 |
| Fresh-HMA | Fresh-HMA | 605 | 234 | 44 | 1.9 | Yes | 929 | 123 | 23 | 0.75 |
| North-RAP | North-RAP | 760 | 174 | 32 | 2.0 | Yes | 890 | 152 | 28 | 0.79 |
| Weathered fresh-HMA | Fresh-HMA-uvc | 531 | 281 | 52 | 2.0 | Yes | 945 | 132 | 25 | 0.21* |
| Weathered north-RAP | North-RAP-uv | 675 | 226 | 42 | 1.9 | Yes | 861 | 175 | 33 | 0.77 |
Soil column was clogged after 9 hours.
Most of the column experiments were conducted at relatively constant flow rates throughout the experiment period. An exception was “weathered Fresh-HMA-uvc + soil” column experiment in which the soil column was clogged after 9 hours of continuous flow and resulted a gradually reduced flow rate to 0.11 mL/min after 52 hours. As a consequence, multiple samples were combined together to make enough solution for one sample for this experiment.
Solution samples collected from column experiments were combined from every 3 samples into one sample to obtain enough volume for analysis, which reduced the number of samples from 96 to 32 in each of the four sets of RAP + soil column experiment. Half of the 32 combined samples were analyzed for trace elements, including all 8 combined samples from the first day, 4 from the second day, 2 from the third day and 2 from the fourth day. Combined solution samples from column experiments were filtered through 0.7 μm pore space glass fiber filter and then acidified to 1 percent HNO3 (Optima grade) for analysis.
2.6. Sample analysis
Both batch extraction solution samples and column experiment solution samples were analyzed for 32 elements (i.e., silver (Ag), aluminum (Al), arsenic (As), barium (Ba), beryllium (Be), calcium (Ca), cadmium (Cd), cobalt (Co), chromium (Cr), cesium (Cs), copper (Cu), iron (Fe), potassium (K), lanthanum (La), lithium (Li), magnesium (Mg), manganese (Mn), molybdenum (Mo), nickel (Ni), phosphorous (P), lead (Pb), rubidium (Rb), sulfur (S), antimony (Sb), selenium (Se), tin (Sn), strontium (Sr), titanium (Ti), thallium (Tl), uranium (U), vanadium (V), and zinc (Zn)) on high-resolution inductively coupled plasma mass spectrometry (HR ICP-MS, Thermo Scientific Element XR, Waltham, MA, USA) at LDEO following a modified USEPA method 200.8 (Cheng et al, 2004). External (NIST 1643e) and internal (LDEO) standard solutions were used for QA/QC and the recovery ratios for the analyzed elements were within 100±10%.
3. Results and discussion
3.1. Water-soluble trace elements from RAPs in batch extraction experiments
Element analysis results (Figure 2 and Appendix Table A.1) from the water-soluble extraction experiments showed overall no elements exceeded USEPA primary drinking water Maximum Contaminant Levels (MCL), in fact most of elements showed concentrations orders of magnitude lower than MCLs, except lead (Pb) in groundwater leached north-RAP-gwl sample (16.1 μg/L) and unbound QUV 25 cycles weathered north-RAP-uv sample (50.1 μg/L). In fact, Pb in leachates of north-RAP and its weathered products are all close to or higher than the EPA primary drinking water MCL of 15 μg/L, most likely from the historical usage of tetraethyl lead and white paint on the road (Lewis, 1985). Previous studies also found Pb concentrations exceeded the MCL, e.g. milled RAP from six locations was found to contain up to 1.80 mg/L of leachable Pb (Kriech, 1991), six samples of RAP collected from asphalt plants throughout Florida showed up to 26.5 ug/L of Pb in leaching experiments (Brantley & Townsend, 1999), and at University of Wisconsin - Madison field site Pb was observed in asphalt leachate solutions with a peak concentration of 35.2 μg/L (Chen et al, 2013). Secondary MCLs (SMCL) are set by the EPA as guidelines for management of aesthetic considerations of water quality (USEPA, 2019). SMCLs are not legally enforceable. Levels of Al, Fe and particularly Mn are higher than SMCLs, possibly due to increased dissolution of minerals under acidic condition. This is consistent with other leaching studies, e.g. Azizian et al (2003) found approximately 1.5 – 2 mg/L Al in a water-asphalt ratio of 4:1 leaching experiment, while in this study the Al concentrations in 20:1 water-asphalt ratio leachates averaged at 0.47 mg/L. More reducing conditions in the extraction experiments may also favor the release of Mn and Fe from solid to aqueous phase (Lovley, 1991). The relatively high rock components (e.g., Al and Ca) are mainly due to the mineral dissolution by acidic leachate solution. Ag, Zn and S (as in sulfate) showed concentrations orders of magnitude lower than SMCLs.
Fig. 2.
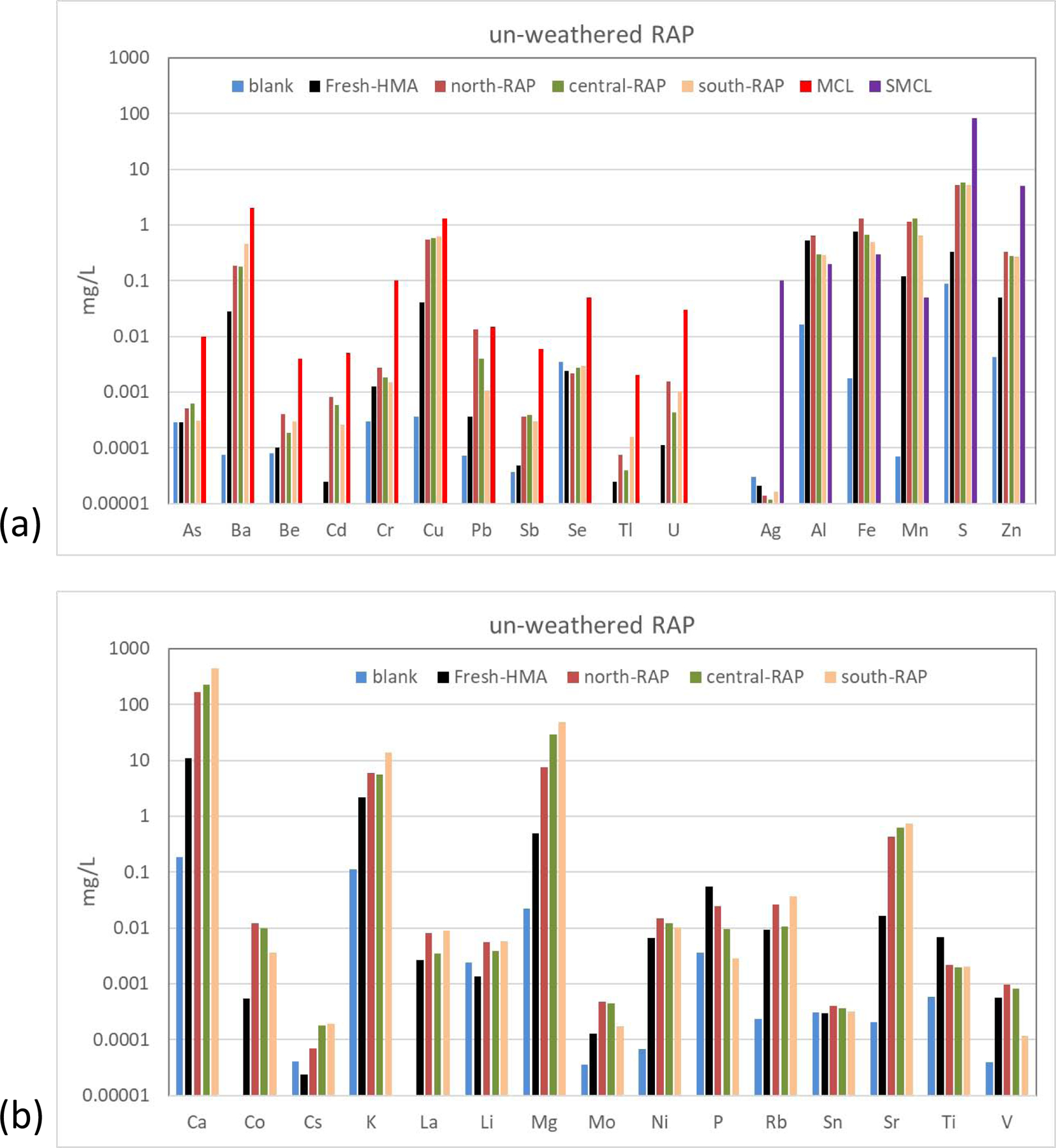
Water extractable concentrations of 32 elements in un-weathered RAP samples: (a) elements with (S)MCLs, and (b) elements without (S)MCLs
* Blank stands for batch extraction method blank concentrations.
* (S)MCL stands for USEPA drinking water (Secondary) Maximum Contaminant Levels.
For un-weathered RAP samples (Figure 2 and Appendix Table A.1), Fresh-HMA showed significantly lower concentrations for most elements including Ba, Be, Ca, Cd, Co, Cs, Cu, K, Li, Mg, Mn, Mo, Ni, Pb, S, Sb, Sr, U, and Zn in comparison to other RAPs that underwent road exposure (Figure 3), indicating the source of these elements from road material, vehicle emission and dust deposition. Weathered Fresh-HMA showed consistently low levels for most elements (Figure 3a), indicating aging did not lead to apparent release of trace elements or pose potential contamination to groundwater sources. This study also did not show weathering or aging of asphalt led to apparent reduction of leached metallic elements in batch extraction experiments.
Fig. 3.

Water extractable concentrations of 32 elements in un-weathered & weathered RAP samples: (a) fresh-HMA, (b) north-RAP, (c) central-RAP, and (d) south-RAP
* In each plot, left are elements with MCL, middle are elements with SMCL, right are elements without (S)MCL.
Water-extractable levels of most elements were comparable between un-weathered RAPs and their weathered counterparts (Figure 3) except titanium (Ti). For example, for Ca, Mg, Al, K, and Li, their water extractable levels in un-weathered and weathered products are almost the same, demonstrating that UV exposure and precipitation have very limited effects on these elements that are mainly from mineral aggregates (rock and sand). This was anticipated since those rocky elements would need much longer and extensive weathering to show weathering effects. Among all four asphalt samples (Fresh-HMA, north-RAP, central-RAP, and south-RAP), Ti levels in un-weathered RAPs were consistently and significantly higher than weathered products. This may be due to the loss of TiO2 typically used in surface coating of asphalt pavements during weathering (Osborn et al, 2014).
Asphalt binder (from asphalt in crude oil) can contain elevated level of Ni and V and the two elements in aerosol particulate matter have been used in NYC as a source indicator of burning of bunk oil in boilers (Hays et al, 2008). Interestingly, their levels in fresh-HMA were similar to those in used RAPs and the weathering process appears to have little to no effect on their water-extractable levels. The low solubility of these elements in water may be the reason.
North-RAP showed a higher Pb level than fresh-HMA and two other used RAPs (central-RAP and south-RAP). In addition, Pb levels in north-RAP samples were not apparently increased or reduced by weathering processes (Figure 3b).
3.2. Release and attenuation of trace elements in column experiments
No major or trace elements were found exceeding the USEPA’s primary drinking water MCLs from RAP columns, only Al and Fe from soil columns exceeded the secondary MCLs in very few samples (Appendix Table A.2). Compared to strong dissolution capability of the acidic batch extraction fluid, the artificial rainwater used in column experiments is less capable to elute elements. The average water to RAP ratio for flow-through column experiments can be calculated as below:
Average RAP mass = 643 g.
So the average water to RAP ratio was 11,520/643 = 18:1, which is similar to the water to RAP ratio of 20:1 in batch extraction experiments.
However, average concentrations of most elements in the batch extraction solutions were orders of magnitude higher than those in the solutions after RAP columns, although the contact time between batch extraction and column experiments was different. Average Pb concentration in RAP extracted solutions was 6.52 μg/L, or 160 times than the average in solutions after RAP column of 0.04 μg/L. Fe and Mn concentrations in RAP extracted solutions were 494 and 273 times higher than those in flow-through column experiments, respectively. As and Mo were exceptions and showed higher concentrations in column solutions than in batch extraction solutions, which is probably because that the typically higher pH conditions in column experiments or asphalt leachates, e.g. pH 8–11 in asphalt leachates (Chen et al, 2020; Chen et al, 2018), and averaged pH of 8.3–8.5 in north-RAP column solutions in this study, favored the desorption and dissociation of As and Mo from iron oxyhydroxides (Dixit & Hering, 2003), although they both had relatively low concentrations of < 5 μg/L in all experiments.
Major elements including Ca, K, Mg, and S showed 4–7 times higher concentrations in soil effluents than in RAP effluents (Appendix Table A.2). Most trace elements including Al, As, Ba, Cu, Fe, Mo, P, Pb, U, and Zn also showed a major source from soil, while Mn and Ni showed higher release from some asphalt samples but were attenuated by the soil column. The first few samples from the leachates of unweathered T-RAP column showed As concentrations higher than those after the soil column, which might be due to the enhanced adsorption of As onto iron oxyhydroxides and/or clay minerals in the soil when the high pH leachate solution after RAP was reduced to relatively lower pH conditions (Chen et al, 2020) that favored As adsorption (Dixit & Hering, 2003; Manning & Goldberg, 1997).
Time-series concentrations of selected elements in all 6 sets of flow-through column experiments are shown in Figure 4. Major elements including Ca, K, Mg, and S were leached out fast, i.e. hours to less than 1 day, in both fresh-HMA and north-RAP samples, and in both RAPs and soil columns, consistent with the rapid decrease of electrical conductivity (EC) of column solutions and very similar to those found in previous studies (Brantley & Townsend, 1999; Legret et al, 2005). In comparison, many trace elements showed slower leaching during the 4 days of experiment.
Fig. 4.

Time-series concentrations of selected elements in flow-through column experiments using fresh-HMA, north-RAP and their weathered counterparts
Weathering or aging of asphalt showed an apparent reduction effect on the leachable metals in the flow-through column experiments, such as As, Cr, Cu, Ca, K, Li, Mg, Mn, V, which is consistent with previous studies (Chen et al, 2018). Fe showed reduced concentrations in weathered HMA leachates than in fresh-HMA leachates, but the weathered north-RAP did not show reduced leachable Fe than un-weathered north-RAP.
3.3. Implications on reuse and environmental impact assessment of RAPs
This study explored potential engineering solutions through which RAP materials can be used in an unbound form while also meeting federal and state environmental standards and guidelines.
RAP batch extraction experiments demonstrated that levels of most metals including As, Ba and U in leachate are below EPA drinking water MCL. Flow-through column experiments showed all major and trace element concentrations lower than primary and secondary MCLs in solutions after RAP columns. Acidic leaching (e.g., in landfills where organic materials decompose creating an acidic and reducing environment) could lead to elution of Pb at levels higher than MCLs, and release of Fe and Mn much higher than Secondary drinking water MCLs. These suggest that RAP can be used as unbound aggregates in most surface, base, and subbase conditions such as parking lots, farm roads, or pathways; for quarry reclamation; as non-vegetative cover underneath guiderails; and mixed with other materials for subbase or base materials; and for the current uses in hot mix asphalt applications, except acidic environments including but not limited to mines with sulfur-containing minerals or landfills where other materials may decompose creating an acidic environment.
Contaminated soil could be a source for toxic elements, as column elution experiments showed that NJ’s natural soil sample was the main source for trace elements leaching out of the experiments including Al, As, Ba, Cu, Fe, Mo, P, Pb, U, and Zn, thus soil testing for metal is necessary before usage. Testing of samples collected at various times during column elution experiments (i.e., time-series samples) showed that the natural NJ soil, utilized in these experiments, had significantly higher levels of Ca, K, Mg, and S (major elements) than the evaluated RAP materials. The rate at which these major elements leached out of both materials (i.e., RAP and NJ soil) was relatively fast, with the majority of the leachable part getting into solution in <1 to 2 days.
Experiments showed that Mn and Ni were the main trace elements leaching out from some RAP samples, however, these elements were attenuated by the soil columns. This illustrates that soil layers beneath asphalt pavement systems can be used to improve water quality (Page et al, 2015). Previous studies have found most heavy metals in rainfall runoff from urban roadways were attenuated in the top 20-cm soil layer (Turer et al, 2001).
Due to the inconsistent pollutant levels found among the three RAP stockpiles evaluated in this study, it is also recommended, as a precautionary measure, to determine the releasable levels of metals for RAP stockpiles before using in acidic environments by extracting leachate samples using batch experiments and measuring metal pollutant levels. If the releasable levels of metals exceed US EPA drinking water standards, it is recommended to investigate into the soil mineralogy and assess the time duration that the soil layer between the RAP and the groundwater aquifer is able to attenuate the metals leached from RAPs (Chen et al, 2020). It is important to note that the sandy loam soil used in this study might represent a relatively lower capacity for metal attenuation compared to more finely sized silty or clayey soils. However, it was beyond the scope of this study to determine the type and thickness of the soil layer that is appropriate for the use of RAP.
3.4. Limitations
Only dissolved concentrations of element were used in discussion in this study. However, suspended particulates can contribute significantly to the total concentrations of some elements and could cause harm to health once digested into human body. Unfiltered samples were collected and frozenly stored, but additional study is needed to address this issue. Further studies are also needed to quantify the release time and rates of metals of concern from RAPs and the soil attenuation capacity, coefficient, and rate. More studies are needed to evaluate trace element release if planning to use RAP in strong acidic environments such as mines (pH < 3). The release and environmental impacts of organic compounds such as polycyclic aromatic hydrocarbons (PAHs) is discussed in another publication.
4. Conclusions
This study investigated the environmental impacts of RAP, with a focus on trace element release from RAPs and potential retention of some metals by natural soil. The RAPs in this study included 1 un-aged fresh hot mix asphalt (HMA) and 3 weathered RAP materials. This study also explored potential engineering solutions through which RAP materials can be used in an unbound form while also meeting federal and state environmental standards and guidelines. Batch experiments were conducted to mimic releasing of trace elements in weak acidic leachate from landfills. North-RAP and central-RAP released levels of Pb greater than the USEPA primary drinking water MCL of 15 μg/L. Novel two-column experiments (a RAP column followed by a soil column) were conducted to investigate the release of trace elements from RAP and the attenuation effect of soils on potential pollutants. The results of these experiments showed that potential pollutants released from RAPs such as Mn and Ni could be largely attenuated in the soil column. The results suggest that RAP can be used as an unbound material in environments except those acidic (i.e., pH < 5 as in mines with sulfur-containing minerals and landfills with acidic environment).
Supplementary Material
Highlights:
Fresh hot mix asphalt and reclaimed asphalt pavement were environmentally assessed.
Water extractable element concentrations were below MCLs except lead (Pb).
Few potential pollutants released from RAPs were largely attenuated by soil column.
RAPs can be used as unbound material in non-acidic environments (pH ≥ 5).
Acknowledgements
This work was supported by New Jersey Department of Transportation [NJDOT201313] and U.S. National Institute of Environmental Health Sciences [ES009089]. The authors thank Masha Pitiranggon and James Ross of LDEO for sample analysis, Ryan Harris and Tom Protus of LDEO for making the columns. The authors also thank two anonymous reviewers for comprehensive reviews and comments. This is LDEO contribution #8417.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.trd.2020.102415.
References
- Azizian MF, Nelson PO, Thayumanavan P & Williamson KJ (2003) Environmental impact of highway construction and repair materials on surface and ground waters: Case study: crumb rubber asphalt concrete. Waste Management, 23(8), 719–728. [DOI] [PubMed] [Google Scholar]
- Bernot MJ, Calkins M, Bernot RJ & Hunt M (2011) The Influence of Different Urban Pavements on Water Chemistry. Road Materials and Pavement Design, 12(1), 159–176. [Google Scholar]
- Boving TB, Stolt MH, Augenstern J & Brosnan B (2008) Potential for localized groundwater contamination in a porous pavement parking lot setting in Rhode Island. Environmental Geology, 55(3), 571–582. [Google Scholar]
- Brantley AS & Townsend TG (1999) Leaching of pollutants from reclaimed asphalt pavement. Environmental Engineering Science, 16(2), 105–116. [Google Scholar]
- Chen J, Sanger M, Ritchey R, Edil TB & Ginder-Vogel M (2020) Neutralization of high pH and alkalinity effluent from recycled concrete aggregate by common subgrade soil. Journal of Environmental Quality, 49(1), 172–183. [DOI] [PubMed] [Google Scholar]
- Chen J, Tinjum JM & Edil TB (2013) Leaching of Alkaline Substances and Heavy Metals from Recycled Concrete Aggregate Used as Unbound Base Course. Transportation Research Record, 2349(1), 81–90. [Google Scholar]
- Chen J, Wang H, Wu JT & Xu GJ (2018) Evaluation of Asphalt Effect on Water Quality Using Leaching Test and Molecular Simulation. Journal of Testing and Evaluation, 46(5), 2121–2129. [Google Scholar]
- Cheng Z, Zheng Y, Mortlock R & van Geen A (2004) Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem, 379, 512–518. [DOI] [PubMed] [Google Scholar]
- Copeland A (2011) Reclaimed Asphalt Pavement in Asphalt Mixtures: State of The Practice U.S. Department of Transportation, the Federal Highway Administration, Publication # FHWA-HRT-11–021. [Google Scholar]
- Dixit S & Hering JG. (2003) Comparison of Arsenic(V) and Arsenic(III) Sorption onto Iron Oxide Minerals: Implications for Arsenic Mobility. Environmental Science & Technology, 37(18), 4182–4189. [DOI] [PubMed] [Google Scholar]
- Federal_Highway_Administration (2016) User Guidelines for Waste and Byproduct Materials in Pavement Construction, FHWA-RD-97–148.
- Gwenzi W, Dunjana N, Pisa C, Tauro T & Nyamadzawo G (2015) Water quality and public health risks associated with roof rainwater harvesting systems for potable supply: Review and perspectives. Sustainability of Water Quality and Ecology, 6, 107–118. [Google Scholar]
- Hansen KR & Copeland A (2017) Asphalt Pavement Industry Survey on Recycled Materials and Warm-Mix Asphalt Usage: 2016.
- Hays MD, Beck L, Barfield P, Lavrich RJ, Dong YJ & Vander Wal RL (2008) Physical and chemical characterization of residential oil boiler emissions. Environmental Science & Technology, 42(7), 2496–2502. [DOI] [PubMed] [Google Scholar]
- He X, Hochstein D, Ge Q, Ali AW, Chen F & Yin H (2018) Accelerated Aging of Asphalt by UV Photo-Oxidation Considering Moisture and Condensation Effects. Journal of Materials in Civil Engineering, 30(1), 04017261. [Google Scholar]
- Kayhanian M, Vichare A, Green PG & Harvey J (2009) Leachability of dissolved chromium in asphalt and concrete surfacing materials. Journal of Environmental Management, 90(11), 3574–3580. [DOI] [PubMed] [Google Scholar]
- Kibblewhite MG (2018) Contamination of agricultural soil by urban and peri-urban highways: An overlooked priority? Environmental Pollution, 242, 1331–1336. [DOI] [PubMed] [Google Scholar]
- Kriech AS (1991) Evaluation of RAP for Use as Clean Fill. Heritage Research Group report. [Google Scholar]
- Legret M, Odie L, Demare D & Jullien A (2005) Leaching of heavy metals and polycyclic aromatic hydrocarbons from reclaimed asphalt pavement. Water Research, 39(15), 3675–3685. [DOI] [PubMed] [Google Scholar]
- Lewis J (1985) Lead Poisoning: A Historical Perspective. EPA Journal, 1985(5). [Google Scholar]
- Liu JY & Borst M (2018) Performances of metal concentrations from three permeable pavement infiltrates. Water Research, 136, 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiological Reviews, 55(2), 259–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BA & Goldberg S (1997) Adsorption and Stability of Arsenic(III) at the Clay Mineral−Water Interface. Environmental Science & Technology, 31(7), 2005–2011. [Google Scholar]
- NADP (2019) National Atmospheric Deposition Program. http://nadp.slh.wisc.edu/data/sites/list/?net=NTN.
- NAPA (2019) Asphalt Pavement Industry Survey on Recycled Materials and Warm-Mix Asphalt Usage: 2018.
- NAPA (2020) Engineering Overview from National Asphalt Pavement Association, 2020. Available online: [Accessed.
- NRCCA (2010) Northeast Region Certified Crop Adviser (NRCCA) Study Resources, Soil and Water Management, Competency Area 2: Soil hydrology AEM, Cornell University; (https://nrcca.cals.cornell.edu/soil/CA2/CA0211.1.php), 2010. Available online: [Accessed. [Google Scholar]
- Osborn D, Hassan M, Asadi S & White JR (2014) Durability Quantification of TiO2 Surface Coating on Concrete and Asphalt Pavements. Journal of Materials in Civil Engineering, 26(2), 331–337. [Google Scholar]
- Page JL, Winston RJ & Hunt WF (2015) Soils beneath suspended pavements: An opportunity for stormwater control and treatment. Ecological Engineering, 82, 40–48. [Google Scholar]
- Turer D, Maynard JB & Sansalone JJ (2001) Heavy metal contamination in soils of urban highways: Comparison between runoff and soil concentrations at Cincinnati, Ohio. Water Air and Soil Pollution, 132(3–4), 293–314. [Google Scholar]
- USEPA (1992) Toxicity Characteristic Leaching Procedure. https://www.epa.gov/sites/production/files/2015-12/documents/1311.pdf.
- USEPA (2019) 2019: Secondary Drinking Water Standards (https://www.epa.gov/dwstandardsregulations/secondary-drinking-water-standards-guidance-nuisance-chemicals). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


