Objectives
This is a protocol for a Cochrane Review (intervention). The objectives are as follows:
Primary
To assess the comparative effectiveness of minimally invasive treatments for lower urinary tract symptoms in men with benign prostatic hyperplasia.
Secondary
To obtain an estimate of relative ranking of these minimally invasive treatments according to their effects.
Background
Description of the condition
The prostate gland is an organ approximately the size of a walnut that is located below the urinary bladder encircling the urethra (Leissner 1979). Benign prostatic obstruction (BPO) is a form of bladder outlet obstruction and may be diagnosed when the cause of outlet obstruction is known to be benign prostatic enlargement (BPE) due to benign prostatic hyperplasia (BPH); however, the latter is restricted to the histological diagnosis defined as increased numbers of epithelial and stromal cells in the prostate (Abrams 2003). BPH may or may not cause lower urinary tract symptoms (LUTS), characterised by frequency, hesitancy, and a weak stream, mainly in men over the age of 40 years, and receives clinical relevance when associated with perceived bother (Dunphy 2015). Symptom bother typically correlates with increased number and severity of symptoms, which are related to both quality of life impairment and treatment seeking (Agarwal 2014). Although we understand that LUTS is a functional unit with a multi‐factorial aetiology of associated symptoms, we considered the term BPH for this Cochrane Review due to its familiarity to the general public (EAU 2020).
The degree of bother across all LUTS can be assessed through self‐administered questionnaires, namely, the International Prostate Symptom Score (IPSS; also known as the American Urological Association (AUA) questionnaire), which includes the quality of life domain (Barry 1995). Chapple 2017 reported that increasing LUTS severity was associated with worsening men's overall distress through patient perception of the bladder condition, which is a single‐item global question (with responses ranging from 1 (causes no problems at all) to 6 (causes severe problems)).
Progression of LUTS has been observed in up to 31% of men with BPH at seven‐year follow‐up (Emberton 2008). Progression to acute urinary retention is less frequent and in men with moderate symptoms can range from 3.0/1000 person‐years in those aged 40 to 49 years to 34.7/1000 person‐years in those aged 70 to 79 years (Emberton 2008). BPH also has a negative impact on public health and reduces a person's quality of life (Kozminski 2015; Martin 2014). In Europe, 30% of men over 50 years of age, equivalent to 26 million men, are affected by bothersome LUTS, including storage symptoms (such as urinary frequency, urgency, and nocturia) or voiding symptoms (such as urinary hesitancy, weak urinary stream, straining to void, and prolonged voiding), or both. The yearly reported associated number of medical prescriptions is estimated to be around 11.6 million for 74 million people at risk from 2004 to 2008 (Cornu 2010). The prevalence of LUTS, according to an international study involving 7588 men, was 18% during ages in the 40s, 29% in the 50s, 40% in the 60s, and 56% in the 70s (Homma 1997). More recent data show the lifetime prevalence of BPH as 26.2% (95% confidence interval (CI) 22.8% to 29.6%) (Lee 2017).
Diagnosis
Initial evaluation of LUTS suggestive of BPH includes patient history, physical examination including a digital rectal examination (DRE), urinalysis, a prostate‐specific antigen (PSA) blood test if a diagnosis of prostate cancer changes management, use of a voiding diary, and IPSS (EAU 2020; McVary 2011). A DRE is performed to assess both nodules suspicious for cancer and prostate size; recently, additional imaging studies have been recommended for patients considering surgical intervention (Foster 2019).
PSA is secreted by the prostate gland and is found to be abnormally elevated in conditions such as prostate cancer, BPH, infection, or inflammation of the prostate (EAU 2020; McVary 2011). The IPSS is used to assess urinary symptom severity and quality of life. It is also used to document subjective responses to treatment (Barry 1992; EAU 2020; McVary 2011). Measurement of maximum flow rate (Qmax) and postvoid residual (PVR) is often used in diagnosis and treatment decisions (EAU 2020; McVary 2011). A low Qmax and a large PVR predict increased risk of symptom progression (Crawford 2006). Other tests such as radiological imaging, urodynamic evaluation, and cystoscopy can help the clinician determine appropriate treatment and predict treatment response (Egan 2016; McVary 2011).
Treatment
Treatment decisions are based on symptoms and the degree of symptom bother noted by the patient. Initial treatment options for BPH include conservative management (watchful waiting and lifestyle modification) and use of medications (alpha blockers, 5‐alpha reductase inhibitors, and, recently, phosphodiesterase inhibitors) (EAU 2020; McVary 2011). When patients have been refractory to conservative and medical treatment, or if BPH causes subsequent complications, such as acute urinary retention, recurrent urinary tract infection, bladder stones, haematuria, or renal insufficiency, surgical options are considered (EAU 2020; McVary 2011).
Until the 1970s, the only option available to treat this condition and relieve LUTS was open simple prostatectomy (in very large prostates) or endoscopic surgery in the form of transurethral prostatectomy, with the aim of removing or resecting prostatic tissue to open up the blocked urethra (Pariser 2015). Clinical guidelines continue to recommend monopolar or bipolar transurethral resection of the prostate (TURP) as a ('gold') reference standard treatment to provide subjective symptom relief while attaining objective improvement in urinary flow (Alexander 2019; EAU 2020; McVary 2011), but this procedure is associated with some morbidity and long‐term complications, including haematuria possibly requiring a blood transfusion, urethral stricture, urinary tract infection, and incontinence, and it usually requires at least overnight hospitalisation. Moreover, men may experience ejaculatory (65%) and erectile dysfunction (10%) related to TURP (Roehrborn 2003). Furthermore, BPH is a disease that is common among elderly men, who have increased preoperative risk for complications of general anaesthesia and surgery in general (Dunphy 2015; Yoo 2012).
Recently, several other minimally invasive treatments (MITs) that can be performed in an office setting and do not require general anaesthesia have been developed as alternatives to TURP (EAU 2020; McVary 2011), with the purpose of providing therapeutic alternatives involving lower morbidity. However, most men who consider surgical intervention do so with the expectation that this is a more definitive therapy for LUTS that will preclude the need for additional medical or surgical therapy. Given the relatively high rate of reoperation or continued use of medical therapy after surgical treatment (or both), concern has been raised about the durability of newly launched minimal invasive surgeries (NICE 2015; Strope 2015).
Description of the intervention
Minimally invasive treatments that can be performed in an office setting and do not require general anaesthesia include convective radiofrequency water vapour therapy (CRFWVT), prostatic arterial embolisation (PAE), prostatic urethral lift (PUL), a temporary implantable nitinol device (TIND), and transurethral microwave therapy (TUMT).
Convective radiofrequency water vapour therapy
The Rezūm system (NxThera Inc., Maple Grove, MN, USA) uses radiofrequency to create thermal energy in the form of water vapour to ablate prostatic tissue (Woo 2017). This system consists of two main components: a radiofrequency power supply generator and a single‐use transurethral delivery device that incorporates a standard rigid cystoscope lens, which allows the procedure to be performed under direct visualisation. Water vapour thermal energy is generated by applying a radiofrequency current against an inductive coil heater. The handheld control delivers water vapour, providing a consistent energy dose of ~ 208 calories into the prostate tissue through a retractable needle (Woo 2017). Convective radiofrequency thermal therapy is performed with the person in the dorsal lithotomy position using conscious sedation. Cystoscopic examination is performed for confirmation of the contours of the prostate and planned distribution of thermal lesions (Darson 2017; Dixon 2015; Woo 2017). The treatment needle is positioned for starting approximately 1 centimetre distal from the bladder neck and targeting the transition and central prostate adenoma by eye. Each injection of water vapour lasts approximately 9 seconds. Additional injections of vapour are delivered every 1 centimetre from the initial injection site of the prostatic urethra to the proximal edge of the verumontanum. The total number of injections in each lobe of the prostate is determined by the length of the prostatic urethra and the configuration of the prostate gland (Dixon 2015; Woo 2017). Saline flush irrigation is used to enhance visualisation and to cool the urethral surface (Woo 2017). Although most adverse events are transient and are classified as Clavien‐Dindo Grade I or II, a non‐randomised pilot study has reported 125 adverse events in 45 of 64 participants (69.2%) (Dixon 2015). The most common adverse events are postoperative urinary retention (33.8%), dysuria (21.5%), urinary urgency (20%), and suspected urinary tract infection (20%). Twelve serious adverse events were reported in 10 participants, one of which was suspected to be a procedure‐/device‐related adverse event (Clavien‐Dindo Grade IIIb urinary retention) (Dixon 2015).
Prostatic arterial embolisation
Embolisation of the prostatic arteries has historically been used to control persistent or massive prostatic bleeding not otherwise amenable for treatment, with typical causes being BPH and locally advanced prostate cancer, or it has been performed after transurethral prostatectomy (Mitchell 1976). DeMeritt 2000 reported a case in which PAE was performed with polyvinyl alcohol particles for BPH‐induced haematuria, haematuria was immediately stopped. and the patient reported symptomatic improvement of his BPH symptoms. These researchers also found that prostate size was reduced by 52% and 62% of the initial size at 5‐month and 12‐month follow‐up, respectively. Carnevale 2010 reported positive preliminary results of PAE procedures with microspheres as a primary treatment in two patients with acute urinary retention due to BPH. For elderly patients with symptomatic BPH, PAE can be an alternative treatment performed by a femoral or radial artery puncture using conscious sedation instead of general anaesthesia. This procedure is typically performed on an outpatient basis and usually does not require catheterisation, unless the patient is experiencing urinary retention (Wang 2015). In preparation for PAE, preoperative computed tomography or magnetic resonance angiography is typically performed to evaluate the pelvic artery anatomy. Digital subtraction angiography of the right and left internal iliac arteries is performed to assess the prostatic blood supply (Martins Pisco 2012). Super‐selective microcatheterisation and embolisation are then performed on the prostatic arteries. Embolisation is typically performed to complete stasis (Carnevale 2010; Martins Pisco 2012; Wang 2015). Cone beam computed tomography can be used not only to help identify all prostatic arteries, but also to identify and avoid embolisation of vessels feeding adjacent pelvic structures (Wang 2015). Particle embolics are used almost exclusively, with wide variation in the type and size of particles (Carnevale 2010; DeMeritt 2000). Vasodilators to mitigate vasospasm once the prostatic artery is catheterised are also recommended by some researchers to avoid premature stasis (Martins Pisco 2012). Although the major complication rate is low (< 1%) (Pisco 2016), perineal pain (9.4%), haematuria (9%), and acute urinary retention (7%) are commonly reported as complications of PAE (Feng 2017). The highest prevalence of acute urinary retention amongst the included studies was 28.4% (Wang 2015). Minor complications, such as hematospermia, rectal bleeding, urinary tract infection, inguinal haematoma, and transient urinary frequency are also reported (Feng 2017; Kuang 2017; Pyo 2017; Shim 2017). However, there is inconsistency in reporting or classifying adverse events.
Prostatic urethral lift
Prostatic urethral lift (PUL) has recently become available in several countries and can be performed under local anaesthesia with oral or intravenous sedation; it can also be performed in men with blood clotting disorders or in men receiving anticoagulant therapy and therefore is being proposed and marketed for men at high risk of general anaesthesia (Chin 2012; Woo 2012). Typical inclusion criteria for PUL include prostate volume between 20 mL and 70 mL, IPSS of 12 or greater, measured Qmax of 15 mL/s or less, and PVR of less than 350 mL (McNicholas 2016). The PUL system consists of two single‐use components (a delivery device and an implant). The delivery device consists of a handheld pistol grip to which a needle‐shaped probe is attached. Each PUL implant consists of a super‐elastic nitinol capsular tab, a polyethylene terephthalate monofilament, and a stainless steel urethral endpiece. The surgeon inserts the probe into the urethra until it reaches the widest part of the prostatic urethra; a fine needle at the end of the probe then is deployed to secure an implant in a lobe of the prostate (McNicholas 2016). One end of the implant is anchored in the urethra, and the other is attached to the firm outer surface of the prostatic capsule, thus pulling the prostatic lobe away from the urethra. This is repeated on the other lobe of the prostate. Systematically, four implants for PUL are delivered ‐ two each to the right and left lateral lobes of the prostate (at the 2 o'clock and 10 o'clock positions, distally, from approximately 1.5 cm distal to the bladder neck). PUL generally is not used to treat a hypertrophied median lobe of the prostate, which causes obstructive intravesical protrusion of the prostate (McNicholas 2016); however a recent small observational study indicated that this might be feasible and effective (Rukstalis 2019). Mild adverse events, such as transient dysuria and haematuria, are commonly reported with PUL (Chin 2012; Woo 2012). Incontinence may be less prevalent with PUL (5%) than with TURP (11%) (NICE 2015). However, reoperation rates appear to be higher with PUL (8%) than with TURP (6%) (NICE 2015). In one feasibility study, implant encrustation occurred when PUL implants were placed too close to the bladder and were exposed to static urine (Chin 2012; Woo 2012).
Temporary implantable nitinol device
The temporary implantable nitinol device (TIND) labelled Medi‐Tate is a novel device that aims to provide prostatic patency. This new minimally invasive procedure can be performed in an outpatient setting under light sedation. The device is placed inside the prostatic urethra via cystoscopy and is expanded upon release (Porpiglia 2015), reshaping the bladder neck and the prostatic urethra. No catheterisation is required. The 50‐mm‐long, 33‐mm‐diameter device comprises three elongated struts and an anchoring leaflet ‐ all made of nitinol, a biocompatible super‐elastic shape‐memory alloy (Porpiglia 2015). The device is removed 5 days after placement in an outpatient setting under local anaesthesia (lidocaine gel) with retraction via a cytoscope.
A single‐arm multi‐centre observational study with 32 participants indicated that median IPSS scores decreased from 19 at baseline to 10 at 3‐week follow‐up, and to 9 at 12‐month follow‐up. Four patients suffered short‐term complications (urinary incontinence, urinary retention, urinary tract infection, and prostatic abscess) (Porpiglia 2015). A 3‐year follow‐up indicated that IPSS scores reached a median of 12 and no further complications were reported (Porpiglia 2018).
A second‐generation TIND device (iTIND) with structural differences is currently available. Only three struts are used, and the upper part of the device allows action exerted on the urethral mucosa at the level of the bladder neck, with potential avoidance of bladder mucosal injury (Bertolo 2018). A single‐arm multi‐centre observational study evaluating iTIND on 81 participants indicated that mean IPSS scores decreased from 22.5 ± 5.6 at baseline to 11.7 ± 8.0 at 1‐month follow‐up, and to 8.8 ± 6.4 at 12‐month follow‐up. Only mild complications were reported: haematuria (12.3%), micturition urgency (11.1%), pain (9.9%), dysuria (7.4%), urinary tract infection (6.2%), and urinary retention (9.9%). Only one participant required re‐intervention in the form of TURP (Porpiglia 2019). At least two ongoing randomised controlled trials are evaluating this treatment (Bertolo 2018).
Transurethral microwave therapy
Transurethral microwave thermotherapy (TUMT) uses microwave‐induced heat to ablate prostatic tissue and is designed to have fewer major complications than TURP (Walmsley 2004). The patient is treated in an outpatient setting. Once the patient's bladder is emptied by straight catheterisation, a local lidocaine gel is inserted for local anaesthesia. The treatment catheter is then placed within the urethra, and this is confirmed by return of the sterile water and by transabdominal or transrectal ultrasound; then the balloon is inflated. The catheter is composed of a curved tip, a temperature sensor, and a microwave unit. The distal port contains the bladder balloon, allowing for urine drainage and cooling. A rectal probe may be inserted and can be used to monitor rectal temperature (Rubeinstein 2003).
TUMT has evolved over past decades. The first systems worked at lower energy or heat settings, and treatment would take around an hour with minimal discomfort; however, results were disappointing. Subsequent systems incorporated catheters that provided urethral cooling, thus allowing higher energy delivery. These advancements reduced the procedure time to around 30 minutes and improved outcomes. However, higher energy leads to greater discomfort during the procedure, for which patients often require sedation and analgesia, and presents risk for urinary retention (EAU 2020; Walmsley 2004).
How the intervention might work
Convective radiofrequency water vapour therapy
Direct transfer of targeted and controlled convective thermal energy doses to the transition zone of the prostate gland to treat BPH by using sterile water vapour through tissue interstitial spaces between cells releases its stored thermal energy to create apoptosis and necrosis when in contact with hyperplastic prostatic tissue (Aoun 2015). Reportedly, no thermal effects are seen beyond the confines of the prostate, thereby leaving the urethra, bladder neck, and external sphincter unaffected (Aoun 2015; Woo 2017). In comparison, conductive ablation therapy can cause necrosis of surrounding tissues as higher temperatures and longer periods of heating are required to achieve therapeutic effects (Woo 2017).
Prostatic arterial embolisation
The underlying mechanism of PAE is the ischaemia or hypoxia that induces apoptosis, necrosis, sclerosis, and prostatic shrinkage with cystic transformation of part, or all, of the gland, resulting in a softer gland with reduced compression of the urethra (DeMeritt 2000; Sun 2008). In addition, PAE may decrease the plasma concentration of free testosterone that enters prostate cells, thereby lowering dihydrotestosterone levels in the prostate. This may result in the secondary inhibition of prostate growth (Sun 2008). Furthermore, ischaemia or hypoxia may induce prostate cell death and necrosis with a decreased number of some receptors, such as alpha‐adrenergic receptors. Therefore, the neuromuscular tone may decrease, resulting in improved clinical symptoms associated with the dynamic pathological component of BPH (Zlotta 1997).
Prostatic urethral lift
The fundamental idea of PUL consists of separation and distraction of enlarged prostatic tissue by a series of implants. The PUL system uses adjustable, permanent implants to hold excess prostatic tissue out of the way, thereby opening the narrowed urethra without cutting or removing enlarged prostatic tissue (McNicholas 2016). These implants are shaped as a double‐ended hook and aim to expand the opening of the urethra (McNicholas 2016).
Temporary implantable nitinol device
The fundamental principle of the TIND device involves 'reshaping' the prostatic urethra and bladder neck, thereby reducing urinary flow obstruction (Porpiglia 2015). This may be caused by the radial force of sustained expansion of the TIND device, causing ischaemic necrosis of the tissue and leading to bladder neck and prostatic urethra incision.
Transurethral microwave therapy
TUMT uses a special transurethral catheter that transmits heat into the prostate via electromagnetic radiation of microwaves, penetrating water‐rich tissue. Energy transferred by the microwave to the tissue in the form of heat induces coagulation necrosis, reducing prostatic volume. This mechanism may also cause denervation of receptors, decreasing the smooth muscle tone of the prostatic urethra (Walmsley 2004). Temperatures lower than 45ºC seem ineffective in causing this effect; therefore higher‐energy devices were developed to reach temperatures greater than 70ºC, causing thermoablation of the prostatic tissue (Aoun 2015).
Why it is important to do this review
The Cochrane Urology Group has developed four reviews of studies comparing each individual MIT to TURP and other therapies (Hoffman 2012; Jung 2017 (ongoing); Jung 2019; Kang 2020); however, these reviews found few head‐to‐head comparisons. Hoffman 2012, the largest review, included several trials comparing TUMT to TURP and sham procedures; Jung 2019 included two trials ‐ one comparing PUL to TURP and another one to sham; and Kang 2020 included a single trial comparing CRFWVT to a sham procedure. A recent systematic review and network meta‐analysis evaluated surgical therapies for BPH, but it covered only invasive therapies such as different forms of TURP and laser ablation (Huang 2019). We found no systematic review and network meta‐analysis to date that has used the same rigorous methods used in a Cochrane Review, which includes application of the GRADE approach and focus on patient‐important outcomes (Guyatt 2008). A network meta‐analysis could improve the precision of estimates for each pair‐wise comparison, create estimates for comparisons for which no head‐to‐head trial was found, and provide ranking of available interventions (Chaimani 2019). In contemporary practice, with the availability of numerous MITs to treat BPH, the findings of this Cochrane Review are expected to be relevant to policymakers, healthcare providers, and patients.
Objectives
Primary
To assess the comparative effectiveness of minimally invasive treatments for lower urinary tract symptoms in men with benign prostatic hyperplasia.
Secondary
To obtain an estimate of relative ranking of these minimally invasive treatments according to their effects.
Methods
Criteria for considering studies for this review
Types of studies
We will include parallel‐group randomised controlled trials (RCTs) only to avoid threatening the transitivity assumption. We will exclude cross‐over and cluster trials, as these study designs are not relevant in this setting. We will exclude single‐armed studies, quasi‐randomised trials, and observational studies. We will include RCTs regardless of their publication status or language of publication.
Types of participants
We define the eligible patient population as men over the age of 40 years with a prostate volume of 20 mL or greater (as assessed by DRE, ultrasound, and/or cross‐sectional imaging) with LUTS, as this can be determined by an IPSS of 8 or over and a Qmax less than 15 mL/s, as measured by non‐invasive uroflowmetry, invasive pressure flow studies, or both (Dunphy 2015; EAU 2020; McNicholas 2016; McVary 2011). The age limitation is based on the observation that the prevalence of BPH is increased in middle‐aged and older men, and that BPH is infrequent in younger men (Barry 1997; EAU 2020; Egan 2016). If these inclusion criteria are not fully described, we will perform a sensitivity analysis (see Sensitivity analysis).
We will exclude trials of men with active urinary tract infection; bacterial prostatitis; chronic renal failure; untreated bladder calculi or large diverticula; prostate cancer; urethral stricture disease; or prior prostate, bladder neck, or urethral surgery. We will exclude studies of men with other conditions that affect urinary symptoms, such as neurogenic bladder due to spinal cord injury, multiple sclerosis, or central nervous system disease.
We will assess the transitivity assumption by comparing characteristics of participants and the distribution of potential effect modifiers, including age, prostate volume, and severity of LUTS.
Types of interventions
We will include the following interventions.
Experimental interventions (decision set)
Convective radiofrequency water vapour therapy (CRFWVT)
Prostatic arterial embolisation (PAE)
Prostatic urethral lift (PUL)
Temporary implantable nitinol device (TIND)
Transurethral microwave therapy (TUMT)
Comparator interventions (supplementary set)
Sham control (or no intervention)
TURP (monopolar or bipolar)
Comparisons
We will include trials comparing experimental interventions versus comparator interventions or performing head‐to‐head comparisons between experimental interventions (see representation of the network in Figure 1). We will not include the comparison TURP versus sham because our primary interest is the comparative effectiveness of minimally invasive treatments. Participants in the network could in principle be randomised to any of the methods being compared, and we will verify this by comparing characteristics of study design, participants, interventions, and comparisons (Salanti 2012), while considering potential sources of clinical heterogeneity and effect modification (see Subgroup analysis and investigation of heterogeneity).
1.
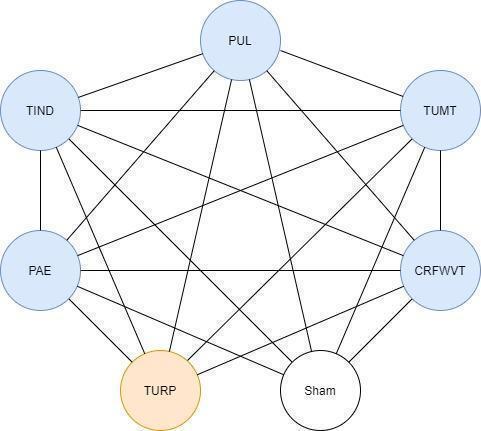
Network plot for minimal‐invasive treatments. TURP: transurethral resection of the prostate; TUMT: transurethral microwave thermotherapy of the prostate; TIND: temporary implantable nitinol device; PUL: prostatic urethral lift; CRFWVT: convective radiofrequency water vapor; PAE: prostatic artery embolisation.
Types of outcome measures
We will not use measurement of the outcomes assessed in this review as an eligibility criterion.
Primary outcomes
Urological symptom scores
Quality of life
Major adverse events
Secondary outcomes
Retreatment
Erectile function
Ejaculatory function
Minor adverse events
Acute urinary retention
Indwelling urinary catheter
Method and timing of outcome measurement
We will consider clinically important differences for all outcomes as the basis for rating the certainty of evidence for imprecision in the 'Summary of findings' tables (Jaeschke 1989; Johnston 2013).
Urological symptom scores
Mean change measured as IPSS (also known as the American Urological Association Symptom Index) or other validated scores (such as Madsen‐Iversen symptom scores). The latter would not be included in a network meta‐analysis (see Measures of treatment effect)
We will consider improvement in IPSS score of 3 points as a minimal clinically important difference (MCID) to assess efficacy and comparative effectiveness (Barry 1995). If possible, we will use different thresholds of MCID based on the severity of IPSS, with a threshold of 3 for mild LUTS, 5 for moderate LUTS, and 8 for severe LUTS (Barry 1995)
Quality of life
Mean change measured as IPSS‐quality of life
No formal threshold was established for IPSS‐quality of life. We will use an MCID of 1 to assess efficacy and comparative effectiveness (Brasure 2016; Rees 2015)
Major adverse events
Examples include postoperative haemorrhage requiring admission or intervention
We will use the Clavien–Dindo classification system to assess surgical complications and will categorise Grade III, IV, and V complications as major (Dindo 2004)
Based on Guyatt 2011a, we will consider a 25% relative change as the threshold for a clinically important difference
Retreatment
Events requiring other surgical treatment modalities (e.g. TURP) after intervention. We will consider the first retreatment and will account for repetitive events in a narrative synthesis
Based on Guyatt 2011a, we will consider a 25% relative change as the threshold for a clinically important difference
Erectile function
Mean change, measured as total score on the International Index of Erectile Function (IIEF)‐5 questionnaire (also known as the Sexual Health Inventory for Men) (Rosen 1997)
We will consider a difference in IIEF‐5 over 5 points as the MCID (Spaliviero 2010)
Ejaculatory function
Mean change, measured on the Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ‐EjD) (Rosen 2007)
We will use an MCID of 25% improvement from baseline on the MSHQ‐EjD for ejaculatory function (Nickel 2015)
Minor adverse events
Examples include postoperative fever or pain requiring medication
We will use the Clavien–Dindo classification system to assess surgical complications and will categorise Grade I and II complications as minor (Dindo 2004)
Based on Guyatt 2011a, we will consider a 25% relative change as the threshold for a clinically important difference
Acute urinary retention
Events requiring catheterisation after intervention
Based on Guyatt 2011a, we will consider a 25% relative change as the threshold for a clinically important difference
Indwelling urinary catheter
Proportion of participants with an indwelling catheter at postoperative 24 hours
Based on Guyatt 2011a, we will consider a 25% relative change as the threshold for a clinically important difference
We will consider outcomes measured up to 12 months after randomisation as short‐term, and those later than 12 months as long‐term, for urological symptom scores, quality of life, major adverse events, retreatment, erectile function, ejaculatory function, minor adverse events, and acute urinary retention. We will assess the indwelling urinary catheter over the short term only.
Main outcomes for 'Summary of findings' tables
We will present 'Summary of findings' tables reporting the following outcomes listed according to priority.
Urological symptom scores.
Quality of life.
Major adverse events.
Retreatment.
Erectile function.
Ejaculatory function.
Search methods for identification of studies
We will perform a comprehensive search with no restrictions on language of publication nor publication status.
Electronic searches
We will retrieve relevant studies from existing Cochrane Reviews for each individual treatment (Hoffman 2012; Jung 2017; Jung 2019; Kang 2020). We will update searches for each of the individual Cochrane Reviews assessing each minimally invasive treatment. We will perform a comprehensive search for TIND from the inception of each of the following databases (see Appendix 1).
-
Cochrane Library via Wiley.
Cochrane Database of Systematic Reviews.
Cochrane Central Register of Controlled Trials.
Database of Abstracts of Reviews of Effects.
Health Technology Assessment Database.
MEDLINE via Ovid (from 1946).
Embase via Elsevier (from 1974).
Scopus (from 1966).
Web of Science (from 1900).
Latin American and the Caribbean Health Sciences Literature (LILACS; www.bireme.br/, from 1982).
We will also search the following.
ClinicalTrials.gov (www.clinicaltrials.gov/).
World Health Organization (WHO) International Clinical Trials Registry Platform search portal (apps.who.int/trialsearch/).
Grey literature repository from the current Grey Literature Report (www.greylit.org/).
Searching other resources
We will try to identify other potentially eligible trials and ancillary publications by searching the reference lists of retrieved included trials, reviews, meta‐analyses, and health technology assessment reports. We will contact study authors of included trials to identify further studies that we may have missed. We will contact drug/device manufacturers for ongoing or unpublished trials. We will search abstract proceedings of relevant meetings of the American Urological Association, the European Association of Urology, and the International Continence Society for 2017 to 2020 for unpublished studies.
Data collection and analysis
Selection of studies
We will use EndNote 2016 reference management software to identify and remove potential duplicate records. Two review authors (JVAF, LG) will independently scan abstracts, titles, or both to determine which studies should be assessed further using Covidence 2013 software. Two review authors (of JVAF, LG, JHJ, MI, and MIO) will investigate all potentially relevant records as full text, will map records to studies, and will classify studies as included studies, excluded studies, studies awaiting classification, or ongoing studies in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We will resolve any discrepancies through consensus or recourse to a third review author (PD). We will document reasons for exclusion. We will present an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
We will develop a dedicated data abstraction form that we will pilot‐test ahead of time. Because we will retrieve relevant studies from existing Cochrane Reviews for each individual treatment for which study characteristics, outcome data, and risk of bias assessments were done by members of our review team (Hoffman 2012; Jung 2017; Jung 2019; Kang 2020), the following sections will apply only to new studies identified by our search methods.
For studies that fulfilled inclusion criteria, two review authors (of JVAF, LG, and JHJ) will independently abstract the following information.
Study design.
Study dates.
Study settings and country.
Participant inclusion and exclusion criteria (e.g. age, baseline IPSS).
Participant details, baseline demographics (e.g. age, prostate size, IPSS).
Numbers of participants by study and by study arm.
Details of relevant experimental intervention (e.g. size of cystoscope, energy‐generating device, embolisation agent, delivery device) and comparator intervention (e.g. monopolar versus bipolar energy, specifications of the sham procedure).
Definitions of relevant outcomes and methods (e.g. type of instrument, such as IPSS) and timing of outcome measurement (e.g. in months), as well as relevant subgroups (e.g. based on age, prostate volume, severity of LUTS).
Study funding sources.
Declarations of interest by primary investigators.
We will extract outcome data relevant to this Cochrane Review as needed for calculation of summary statistics and measures of variance. For dichotomous outcomes, we will present numbers of events and totals for populations in a 2 × 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we will obtain means and standard deviations or data necessary to calculate this information.
We will resolve any disagreements by discussion or, if required, by consultation with a third review author (PD).
We will provide in tables information, including the trial identifier, about potentially relevant studies.
We will contact authors of included studies to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary study, we will maximise yield of information by mapping all publications to unique studies and collating all available data. We will use the most complete data set aggregated across all known publications. In case of doubt, we will give priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (JVAF and LG) will independently assess the risk of bias of each included study. We will resolve disagreements by consensus, or by consultation with a third review author (PD). We will present a 'Risk of bias' summary figure to illustrate these findings. We will further summarise the risk of bias across domains for each outcome in each included study, as well as across studies and domains, for each outcome in accordance with the approach for summary assessments of risk of bias presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in randomised controlled trials
We will assess risk of bias using Cochrane's 'Risk of bias' assessment tool (Higgins 2011). We will assess the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other sources of bias.
We will judge risk of bias domains as 'low risk', 'high risk', or 'unclear risk' and will evaluate individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For selection bias (random sequence generation and allocation concealment), we will evaluate risk of bias at a trial level.
For performance bias (blinding of participants and personnel), we will consider all outcomes similarly susceptible to performance bias.
For detection bias (blinding of outcome assessment), we will group outcomes as susceptible to detection bias (subjective) or not susceptible to detection bias (objective) outcomes.
We define the following endpoints as subjective outcomes.
Urological symptom scores.
Quality of life.
Major adverse events.
Erectile function.
Ejaculatory function.
Minor adverse events.
We define the following endpoints as objective outcomes.
Retreatment.
Acute urinary retention.
Indwelling urinary catheter.
We will assess attrition bias (incomplete outcome data) on an outcome‐specific basis and will present the judgement for each outcome separately when reporting our findings in 'Risk of bias' tables.
For reporting bias (selective reporting), we will evaluate risk of bias at a trial level.
Measures of treatment effect
Relative treatment effect
We will express dichotomous data as risk ratios (RRs) with 95% confidence interval (CIs) to enhance the interpretability of results. We will express continuous data as mean differences (MDs) with 95% CIs. We will prioritise post‐intervention over change from baseline measurements. We anticipate that different scales might be used for urological symptom scores (e.g. Madsen symptom score in few older studies), in which case we will include outcome data using the preferred scale for this outcome (i.e. IPSS) and will report outcome data from other scales separately in a narrative synthesis of quantitative data. In the presence of binary and continuous data for the same outcome, we will perform analysis for continuous data, and if sufficient data are available for each comparison, we will perform analysis for binary data.
Relative treatment ranking
We will obtain a treatment hierarchy using P scores (Rücker 2015). P scores allow ranking of treatments on a continuous zero to 1 scale in a frequentist framework network meta‐analysis.
Unit of analysis issues
The unit of analysis will be the individual participant. When multiple trial arms are reported in a single trial, we will include only the arms with comparisons relevant to the network (see Figure 1).
Dealing with missing data
We will obtain missing data (e.g. missing standard deviation) from study authors and will perform intention‐to‐treat analyses if data are available. We will investigate attrition rates (e.g. dropouts, losses to follow‐up, withdrawals) and will critically appraise issues of missing data. We will not impute missing data.
Assessment of heterogeneity
Pair‐wise meta‐analysis
We will identify heterogeneity through visual inspection of forest plots to assess the amount of overlap of CIs and the I² statistic, which quantifies between‐study variation across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). We will interpret the I² statistic as follows (Deeks 2011).
0% to 40%: may not be important.
30% to 60%: may indicate moderate heterogeneity.
50% to 90%: may indicate substantial heterogeneity.
75% to 100%: considerable heterogeneity.
We will also use Cochran’s Q test to assess for heterogeneity of estimated effect sizes from individual studies. However, we will cautiously interpret these results considering both the low power to detect true heterogeneity when the number of studies is small, and the excessive power needed to detect negligible heterogeneity when the number of studies is high (Huedo‐Medina 2006; Pereira 2010).
Network meta‐analysis
Assessment of the transitivity assumption
Before conducting a network meta‐analysis, we will assess the transitivity assumption. Network meta‐analysis rests on the assumption of transitivity, that is, that effect modifiers have a comparable distribution across treatment comparisons in a network (Cipriani 2013; Jansen 2013). To assess the plausibility of this assumption, we will visually inspect the comparability of distributions of age, prostate volume, and urological symptom score severity (IPSS), time point of outcome assessment, and risk of bias (randomisation, allocation concealment, and blinding to risk of bias) as potential treatment effect modifiers across comparisons (Salanti 2014). We will assess the similarity of inclusion and exclusion criteria of all studies, including participants, treatments, and outcomes, to evaluate whether they impact treatment effects.
Assessment of statistical consistency
Lack of transitivity in a network can threaten the validity of the consistency assumption, that is, statistical agreement between direct and indirect evidence (Caldwell 2005; Lu 2004). Results can be misleading in the presence of inconsistency in the network. We will evaluate the presence of inconsistency both locally and globally. We will evaluate each network locally using the loop‐specific method by generating an inconsistency factor along with a 95% CI for each closed loop (Veroniki 2013). This way, we will identify which piece of evidence will be responsible for inconsistency, and we will explore this further. We will also apply a global assessment for consistency in each network by applying the design‐by‐treatment interaction model (White 2012a). It has been shown that inconsistency tests have low power to detect true inconsistency (Song 2012; Veroniki 2014). Hence, we will assess transitivity even in the absence of evidence for inconsistency. If inconsistency is found, we will follow the guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Section 11.4.4.4; Chaimani 2019).
Assessment of reporting biases
We will attempt to obtain study protocols to assess for selective outcome reporting.
We will use comparison‐adjusted funnel plots to assess small‐study effects (Chaimani 2013). Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials), and publication bias. We will, therefore, interpret results carefully.
Data synthesis
Methods for direct treatment comparisons
We will perform analyses according to recommendations provided in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), and we will use the statistical software of Cochrane ‐ Review Manager 2014 ‐ for analysis. If appropriate, we will perform these standard pair‐wise meta‐analyses using a random‐effects model because we anticipate methodological and clinical heterogeneity across studies. We will calculate corresponding 95% CIs for all analyses, and we will graphically present the results using forest plots. When trials are clinically too heterogeneous to be combined, we will perform only subgroup analyses without calculating an overall estimate.
Methods for indirect and network comparisons
We will fit a random‐effects network meta‐analysis model because we anticipate methodological and clinical heterogeneity across studies. We will assume a common within‐network heterogeneity estimate across comparisons, and we will estimate this using the restricted maximum likelihood (REML) method (Veroniki 2016). This is a reasonable assumption given that all treatments included in the network are of the same nature. An advantage of this approach is that treatment comparisons informed by a single study can borrow strength from the rest of the studies in the network (Higgins 1996; Salanti 2008). Each network meta‐analysis treatment effect estimate will be presented along with a 95% CI and a 95% predictive interval (PrI). A PrI is an interval within which the treatment effect estimate of a future study is expected to lie, accounting for both uncertainty of the treatment effect and between‐study variance estimates (Higgins 2009; Riley 2011). We will conduct network meta‐analysis by using the network suite of commands in Stata (White 2012; White 2015).
Relative treatment ranking
We will estimate the ranking probabilities that all treatments will be at each possible rank for each intervention. We will use the surface under the cumulative ranking (SUCRA) curve to rank effectiveness and safety of minimally invasive interventions (Salanti 2011). SUCRA accounts for both effect size magnitude and uncertainty around the underlying effect size. We will use the rank‐heat plot to present SUCRA values for all outcomes in a single plot (Veroniki 2016). We will display (network plot and league table) results using the 'network graph package' in Stata (Chaimani 2015).
Subgroup analysis and investigation of heterogeneity
When we find important heterogeneity and/or inconsistency, we will explore possible sources for primary outcomes. When sufficient studies are available, we will perform subgroup analysis by using the following potential effect modifiers as possible sources of inconsistency and/or heterogeneity.
Patient age (younger than 65 years versus 65 years and older).
Prostate volume (≤ 40 mL or > 40 mL).
Severity of LUTS based on IPSS (score ≤ 19 (moderately symptomatic) versus > 19 (severely symptomatic)).
These subgroup analyses are based on the following observations.
Age is a well‐known risk factor for BPH surgery. Older people have a higher rate of postoperative complications compared with younger people (Bhojani 2014; Pariser 2015). The age cut‐off is based on the World Health Organization (WHO) definition of old age (WHO 2002).
Outcomes and complications of minimally invasive procedures, such as TURP, correlate with prostate volume (Reich 2008). Prostate volume cut‐off greater than 40 mL is based on this being the most commonly used threshold to distinguish 'small' from 'large' for the indication of treatment with a 5‐alpha reductase inhibitor (EAU 2020).
The relationship between changes in IPSS scores and patient global ratings of improvement is influenced by baseline scores (Barry 1995).
We plan to perform subgroup analyses limited to the primary outcomes.
Sensitivity analysis
We plan to perform sensitivity analyses limited to the primary outcomes to explore the influence of the following factors (when applicable) on effect size.
Restricting the analysis in RCTs by taking into account risk of bias, by excluding studies at 'high risk' or 'unclear risk' (studies with at least one domain at 'high risk' or 'unclear risk' of bias for the analysed outcome).
Restricting the analysis to RCTs with adequately described inclusion criteria (prostate size, age, IPSS value, and Qmax).
'Summary of findings' tables
We will use 'Summary of findings' (SoF) tables to summarise key results of the Review, using the Confidence in Network Meta‐analysis (CINeMA) framework and software (Chaimani 2019; CINEMA 2017; Salanti 2014). We will include the following outcomes.
Urological symptom scores.
Quality of life.
Major adverse events.
Retreatment.
Erectile function.
Ejaculatory function.
We will use the five GRADE criteria (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to evaluate the quality of a body of evidence as it relates to studies that contributed data to the meta‐analysis for each pre‐specified outcome (Guyatt 2008). Two review authors (JVAF and LG) will independently make judgements about quality of the evidence (high, moderate, low, or very low) and will resolve disagreements by discussion or consultation with a third review author (PD). We will create an SoF table for each outcome using the approach presented by Yepes‐Nuñez 2019 (see Table 1 and Table 2).
1. Dummy summary of findings table (dichotomous outcomes).
| Dichotomous outcomes | ||||||
|
Patient or population: Interventions: Comparator (reference): sham procedure Outcome: Setting: hospital procedure – outpatient follow‐up (network plot on the right) * | ||||||
|
Total studies Total participants |
Anticipated absolute effect (95% CI) *** | Certainty of evidence |
Ranking (95% CI) **** |
|||
| Relative effect** | Without intervention | With Intervention | Difference | |||
| MIT 1 | ||||||
| MIT 2 | ||||||
| MIT 3 | ||||||
| MIT 4 | ||||||
| MIT 5 | ||||||
| TURP | ||||||
| Sham procedure | ||||||
| MIT: minimally invasive treatment, CI: confidence interval. Network meta‐analysis summary of findings table definitions. * Lines in the network graphic represent direct comparisons. ** Estimates are reported as odds ratio and CI. *** Anticipated absolute effect compares two risks by calculating the difference between risks of the intervention group and risk of the control group. **** Ranking and confidence intervals for efficacy outcomes are presented. Rank statistics is defined as the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. | ||||||
|
GRADE Working Group grades of evidence (or certainty of the evidence). High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| Explanatory footnotes | ||||||
2. Dummy summary of findings table (continuous outcomes).
| Continuous outcomes | |||||
|
Patient or population: Interventions: Comparator (reference): sham procedure Outcome: Setting: hospital procedure – outpatient follow‐up (network plot on the right) * | |||||
|
Total studies Total participants |
Anticipated absolute effect (95% CI) ** | Certainty of evidence |
Ranking (95% CI) *** |
||
| Relative effect | Without intervention | With Intervention | |||
| MIT 1 | |||||
| MIT 2 | |||||
| MIT 3 | |||||
| MIT 4 | |||||
| MIT 5 | |||||
| TURP | |||||
| SHAM | |||||
| MIT: minimally invasive treatment, CI: confidence interval. Network meta‐analysis summary of findings table definitions. * Lines in the network graphic represent direct comparisons. ** Estimates are reported as mean difference and confidence interval (CI). *** Ranking and confidence intervals for efficacy outcome are presented. Rank statistics is defined as the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. | |||||
|
GRADE Working Group grades of evidence (or certainty of the evidence). High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
| Explanatory footnotes | |||||
History
Protocol first published: Issue 6, 2020
Notes
We based portions of the Methods section of this review on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which was modified and adapted for use by Cochrane Urology. General concepts of benign prostatic hyperplasia and review methods has been adapted from one of the reviews from the suite on this topic (Hoffman 2012; Jung 2017; Jung 2019; Kang 2020).
Acknowledgements
We are very grateful to Cochrane Urology, especially the Managing Editor Robert Lane, as well as Cochrane Urology Korea for supporting this review. Furthermore, we are grateful to Gretchen Kuntz for revising and providing feedback on the search strategies. We also thank Marco Blanker, Sevann Helo, and Murad Mohammad for their peer‐review input of the protocol.
Appendices
Appendix 1. Search strategy
| Cochrane Library (via Wiley) |
| #1 MeSH descriptor: [Prostatic Hyperplasia] explode all tree #2 MeSH descriptor: [Prostatism] explode all trees #3 MeSH descriptor: [Urinary Bladder Neck Obstruction] explode all trees #4 (Prostat* near/3 hyperplasia*):ti,ab,kw #5 (Prostat* near/3 hypertroph*):ti,ab,kw #6 (Prostat* near/3 adenoma*):ti,ab,kw #7 (BPH OR BPO OR BPE):ti,ab,kw #8 (prostat* near/3 enlarg*):ti,ab,kw #9 (Prostatism):ti,ab,kw #10 (Bladder* near/3 obstruct*):ti,ab,kw #11 (BOO):ti,ab,kw #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 (Nitinol):ti,ab,kw #14 (TIND):ti,ab,kw #15 (iTIND):ti,ab,kw #16 #13 OR #14 OR #15 #17 #12 AND #16 |
| MEDLINE (via Ovid) |
| #1 exp Prostatic Hyperplasia/ #2 exp Prostatism/ #3 exp Urinary Bladder Neck Obstruction/ #4 (Prostat* adj3 hyperplasia*).tw. #5 (Prostat* adj3 hypertroph*).tw. #6 (Prostat* adj3 adenoma*).tw. #7 (BPH or BPO or BPE).tw. #8 (prostat* adj3 enlarg*).tw. #9 Prostatism.tw.(590) #10 (Bladder* adj3 obstruct*).tw. #11 BOO.tw. #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 Nitinol.tw. #14 TIND.tw. #15 iTIND.tw. #16 #13 OR #14 OR #15 #17 #12 AND #16 |
| Embase (via Elsevier) |
| #1. 'prostate hypertrophy'/exp #2. 'prostatism'/exp #3. 'bladder obstruction'/exp #4. (prostat* NEAR/3 hyperplasia*):ti,ab,kw #5. (prostat* NEAR/3 hypertroph*):ti,ab,kw #6. (prostat* NEAR/3 adenoma*):ti,ab,kw #7. bph:ti,ab,kw OR bpo:ti,ab,kw OR bpe:ti,ab,kw #8. (prostat* NEAR/3 enlarg*):ti,ab,kw #9. prostatism:kw,ti,ab #10. (bladder* NEAR/3 obstruct*):ti,ab,kw #11. boo:ti,ab,kw #12. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13. 'nitinol'/exp #14. nitinol:ta,ab,kw #15. tind:ta,ab,kw #16. itind:ta,ab,kw #17. #13 OR #14 OR #15 OR #16 #18. #12 AND #17 |
| LILACS |
| tw:(“prostatic hyperplasia” OR “hiperplasia prostática” OR prostat* OR “urinary bladder neck obstruction” OR “obstrucción del cuello de la vejiga urinaria” OR “obstrução do colo da bexiga urinária” OR bph OR bpo OR bpe) AND tw:(Nitinol OR TIND OR DNIT) |
| Scopus |
| TITLE‐ABS‐KEY ( "Prostatic Hyperplasia" OR prostat* OR "Urinary Bladder Neck Obstruction" ) AND TITLE‐ABS‐KEY ( nitinol OR tind OR itind ) |
| Web of Science |
| #1 TI=("Prostatic Hyperplasia" OR Prostat* OR "Urinary Bladder Neck Obstruction") #2 TS=("Prostatic Hyperplasia" OR Prostat* OR "Urinary Bladder Neck Obstruction") #3 #1 OR #2 #4 TS=(nitinol OR tind OR itind ) OR TI=( nitinol OR tind OR itind) #5 #4 AND #3 |
Contributions of authors
JVAF: conception and study design and drafting the protocol.
JHJ: drafting the protocol.
MI: drafting the protocol.
MB: drafting the protocol.
SY: revising the protocol
MIO: drafting the protocol.
CMEL: creating search strategies and searching for trials.
AAV: drafting the protocol.
LG: drafting the protocol.
PD: conception and study design, providing clinical and methodological advice on the protocol.
Sources of support
Internal sources
-
Instituto Universitario Hospital Italiano, Argentina
Salary support for Juan Franco, Luis Garegnani, Camila Micalea Escobar Liquitay
-
Department of Urology, Yonsei University Wonju College of Medicine, Korea, South
Salary support for Jae Hung Jung
-
Minneapolis VA Health Care System, USA
Salary support for Philipp Dahm
-
Department of Urology, University of Minnesota, USA
Support in kind for Philipp Dahm
External sources
-
None, Argentina
N/A
Declarations of interest
JVAF: none known.
JHJ: none known.
MI: none known.
MB: Boston Scientific (consultant for endourology and stone management), Auris Health (consultant for robotic surgery and endourology).
MIO: none known.
CMEL: none known.
AAV: none known.
LG: none known.
PD: none known.
New
References
Additional references
Abrams 2003
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003; 61(1):37-49. [PMID: ] [DOI] [PubMed] [Google Scholar]
Agarwal 2014
- Agarwal A, Eryuzlu LN, Cartwright R, Thorlund K, Tammela TL, Guyatt GH, et al. What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. European Urology 2014; 65(6):1211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alexander 2019
- Alexander CE, Scullion MMF, Omar MI, Yuan Y, Mamoulakis C, N'Dow JMO, et al. Bipolar versus monopolar transurethral resection of the prostate for lower urinary tract symptoms secondary to benign prostatic obstruction. Cochrane Database of Systematic Reviews 2019, Issue 12. [DOI: 10.1002/14651858.CD009629.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aoun 2015
- Aoun F, Marcelis Q, Roumeguère T. Minimally invasive devices for treating lower urinary tract symptoms in benign prostate hyperplasia: technology update. Research and Reports in Urology 2015; 7:125-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barry 1992
- Barry MJ, Fowler FJ Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. Journal of Urology 1992; 148(5):1549-57; discussion 1564. [DOI] [PubMed] [Google Scholar]
Barry 1995
- Barry MJ, Williford WO, Chang Y, Machi M, Jones K, Walker-Corkery E, et al. Benign prostatic hyperplasia-specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? Journal of Urology 1995; 154(5):1770-4. [DOI] [PubMed] [Google Scholar]
Barry 1997
- Barry MJ, Fowler FJ Jr, Bin L, Pitts JC 3rd, Harris CJ, Mulley AG Jr. The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. Journal of Urology 1997; 157(1):10-4; discussion 14-5. [PubMed] [Google Scholar]
Bertolo 2018
Bhojani 2014
- Bhojani N, Gandaglia G, Sood A, Rai A, Pucheril D, Chang SL, et al. Morbidity and mortality after benign prostatic hyperplasia surgery: data from the American College of Surgeons national surgical quality improvement program. Journal of Endourology 2014; 28(7):831-40. [DOI] [PubMed] [Google Scholar]
Brasure 2016
- Brasure M, MacDonald R, Dahm P, Olson CM, Nelson VA, Fink HA, et al. AHRQ comparative effectiveness reviews. In: Newer Medications for Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: A Review. Rockville (MD): Agency for Healthcare Research and Quality (US), 2016. [PubMed] [Google Scholar]
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. British Medical Journal 2005; 331(7521):897-900. [DOI] [PMC free article] [PubMed]
Carnevale 2010
- Carnevale FC, Antunes AA, da Motta Leal Filho JM, Oliveira Cerri LM, Baroni RH, Marcelino AS, et al. Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia: preliminary results in two patients. Cardiovascular and Interventional Radiology 2010; 33(2):355-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2015
- Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata Journal 2015; 15(4):905-50.
Chaimani 2019
- Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Chapter 11. Undertaking network meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions, Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook, 2019. [Google Scholar]
Chapple 2017
- Chapple C, Castro-Diaz D, Chuang YC, Lee KS, Liao L, Liu SP, et al. Prevalence of lower urinary tract symptoms in China, Taiwan, and South Korea: results from a cross-sectional, population-based study. Advances in Therapy 2017; 34(8):1953-65. [DOI] [PMC free article] [PubMed]
Chin 2012
- Chin PT, Bolton DM, Jack G, Rashid P, Thavaseelan J, Yu RJ, et al. Prostatic urethral lift: two-year results after treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urology 2012; 79(1):5-11. [DOI] [PubMed] [Google Scholar]
CINEMA 2017 [Computer program]
- Institute of Social and Preventive Medicine, University of Bern CINeMA: Confidence in Network Meta-Analysis. Institute of Social and Preventive Medicine, University of Bern, 2017.Available from cinema.ispm.unibe.ch.
Cipriani 2013
- Cipriani A, Higgins JPT, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013; 159(2):130-7. [DOI] [PubMed] [Google Scholar]
Cornu 2010
- Cornu JN, Cussenot O, Haab F, Lukacs B. A widespread population study of actual medical management of lower urinary tract symptoms related to benign prostatic hyperplasia across Europe and beyond official clinical guidelines. European Urology 2010; 58(3):450-6. [DOI] [PubMed] [Google Scholar]
Covidence 2013 [Computer program]
- Veritas Health Innovation Covidence. Version accessed 16 August 2017. Melbourne, Australia: Veritas Health Innovation, 2013.Available at www.covidence.org.
Crawford 2006
- Crawford ED, Wilson SS, McConnell JD, Slawin KM, Lieber MC, Smith JA, et al. Baseline factors as predictors of clinical progression of benign prostatic hyperplasia in men treated with placebo. Journal of Urology 2006; 175(4):1422-6; discussion 1426-7. [DOI] [PubMed] [Google Scholar]
Darson 2017
- Darson MF, Alexander EE, Schiffman ZJ, Lewitton M, Light RA, Sutton MA, et al. Procedural techniques and multicenter postmarket experience using minimally invasive convective radiofrequency thermal therapy with Rezūm system for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Research and Reports in Urology 2017; 9:159-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9. Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DeMeritt 2000
- DeMeritt JS, Elmasri FF, Esposito MP, Rosenberg GS. Relief of benign prostatic hyperplasia-related bladder outlet obstruction after transarterial polyvinyl alcohol prostate embolization. Journal of Vascular and Interventional Radiology 2000; 11(6):767-70. [DOI] [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004; 240(2):205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dixon 2015
- Dixon CM, Cedano ER, Pacik D, Vit V, Varga G, Wagrell L, et al. Two-year results after convective radiofrequency water vapor thermal therapy of symptomatic benign prostatic hyperplasia. Research and Reports in Urology 2016; 8:207-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunphy 2015
- Dunphy C, Laor L, Te A, Kaplan S, Chughtai B. Relationship between depression and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Reviews in Urology 2015; 17(2):51-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
EAU 2020
- European Association of Urology. Management of Non-Neurogenic Male Lower Urinary Tract Symptoms (LUTS), incl. Benign Prostatic Obstruction (BPO). uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/ç (accessed 14 April 2020).
Egan 2016
- Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urologic Clinics of North America 2016; 43(3):289-97. [DOI] [PubMed] [Google Scholar]
Emberton 2008
- Emberton M, Cornel EB, Bassi PF, Fourcade RO, Gómez JMF, Castro R. Benign prostatic hyperplasia as a progressive disease: a guide to the risk factors and options for medical management. International Journal of Clinical Practice 2008; 62(7):1076-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote 2016 [Computer program]
- EndNote. Version 7.5. Philadelphia (PA): Clarivate Analytics, 2016.Available at endnote.com/.
Feng 2017
- Feng S, Tian Y, Liu W, Li Z, Deng T, Li H, et al. Prostatic arterial embolization treating moderate-to-severe lower urinary tract symptoms related to benign prostate hyperplasia: a meta-analysis. CardioVascular and Interventional Radiology 2017; 40(1):22-32. [DOI] [PubMed] [Google Scholar]
Foster 2019
- Foster HE, Dahm P, Kohler TS, Lerner LB, Parsons JK, Wilt TJ, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2019. Journal of Urology 2019; 202(3):592-8. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann HJ, et al. GRADE: what is "quality of evidence" and why is it important to clinicians? BMJ (Clinical Research Ed) 2008; 336(7651):995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence – imprecision. Journal of Clinical Epidemiology 2011; 64(12):1283-93. [DOI] [PubMed] [Google Scholar]
Higgins 1996
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002; 21(11):1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed) 2003; 327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. Journal of the Royal Statistical Society. Series A: Statistics in Society 2009; 172(1):137-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions, Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hoffman 2012
- Hoffman RM, Monga M, Elliott SP, MacDonald R, Langsjoen J, Tacklind J, et al. Microwave thermotherapy for benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD004135.pub3] [DOI] [PubMed] [Google Scholar]
Homma 1997
- Homma Y, Kawabe K, Tsukamoto T, Yamanaka H, Okada K, Okajima E, et al. Epidemiologic survey of lower urinary tract symptoms in Asia and Australia using the international prostate symptom score. International Journal of Urology 1997; 4(1):40-6. [DOI] [PubMed] [Google Scholar]
Huang 2019
- Huang SW, Tsai CY, Tseng CS, Shih MC, Yeh YC, Chien KL, et al. Comparative efficacy and safety of new surgical treatments for benign prostatic hyperplasia: systematic review and network meta-analysis. BMJ (Clinical Research Ed.) 2019; 367:l5919. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huedo‐Medina 2006
- Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I² index? Psychological Methods 2006/06;11(2):193-206. [DOI] [PubMed] [Google Scholar]
Jaeschke 1989
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989; 10(4):407-15. [DOI] [PubMed] [Google Scholar]
Jansen 2013
- Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Medicine 2013; 11(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2013
- Johnston BC, Patrick DL, Busse JW, Schünemann HJ, Agarwal A, Guyatt GH. Patient-reported outcomes in meta-analyses – Part 1: assessing risk of bias and combining outcomes. Health and Quality of Life Outcomes 2013; 11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 2017
- Jung JH, Shin TY, McCutcheon KA, Borofsky M, Narayan V, Young S, et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD012867] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 2019
- Jung JH, Reddy B, McCutcheon KA, Borofsky M, Narayan V, Kim MH, et al. Prostatic urethral lift for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2019, Issue 5. [DOI: 10.1002/14651858.CD012832.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2020
- Kang TW, Jung JH, Hwang EC, Borofsky M, Kim MH, Dahm P. Convective radiofrequency water vapour thermal therapy for lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2020, Issue 3. [DOI: 10.1002/14651858.CD013251.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kozminski 2015
- Kozminski MA, Wei JT, Nelson J, Kent DM. Baseline characteristics predict risk of progression and response to combined medical therapy for benign prostatic hyperplasia (BPH). BJU International 2015; 115(2):308-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuang 2017
- Kuang M, Vu A, Athreya S. A systematic review of prostatic artery embolization in the treatment of symptomatic benign prostatic hyperplasia. Cardiovascular and Interventional Radiology 2017; 40(5):655-63. [DOI] [PubMed] [Google Scholar]
Lee 2017
- Lee SWH, Chan EMC, Lai YK. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta-analysis. Scientific Reports 2017; 7(1):7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leissner 1979
- Leissner KH, Tisell LE. The weight of the human prostate. Scandinavian Journal of Urology 1979; 13(2):137-42. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009; 6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2004
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 2004; 23(20):3105-24. [DOI] [PubMed] [Google Scholar]
Martin 2014
- Martin S, Lange K, Haren MT, Taylor AW, Wittert G. Risk factors for progression or improvement of lower urinary tract symptoms in a prospective cohort of men. Journal of Urology 2014; 191(1):130-7. [DOI] [PubMed] [Google Scholar]
Martins Pisco 2012
- Martins Pisco J, Pereira J, Rio Tinto H, Fernandes L, Bilhim T. How to perform prostatic arterial embolization. Techniques in Vascular and Interventional Radiology 2012; 15(4):286-9. [DOI] [PubMed] [Google Scholar]
McNicholas 2016
- McNicholas TA. Benign prostatic hyperplasia and new treatment options – a critical appraisal of the UroLift system. Medical Devices 2016; 9:115-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
McVary 2011
- McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. Journal of Urology 2011; 185(5):1793-803. [DOI] [PubMed] [Google Scholar]
Mitchell 1976
- Mitchell ME, Waltman AC, Athanasoulis CA, Kerr WS Jr, Dretler SP. Control of massive prostatic bleeding with angiographic techniques. Journal of Urology 1976; 115(6):692-5. [DOI] [PubMed] [Google Scholar]
NICE 2015
- National Institute for Health and Care Excellence. UroLift for treating lower urinary tract symptoms of benign prostatic hyperplasia. www.nice.org.uk/guidance/mtg26 (accessed 28 September 2017).
Nickel 2015
- Nickel JC, Brock GB, Herschorn S, Dickson R, Henneges C, Viktrup L. Proportion of tadalafil-treated patients with clinically meaningful improvement in lower urinary tract symptoms associated with benign prostatic hyperplasia – integrated data from 1,499 study participants. BJU International 2015; 115(5):815-21. [DOI] [PubMed] [Google Scholar]
Pariser 2015
- Pariser JJ, Pearce SM, Patel SG, Bales GT. National trends of simple prostatectomy for benign prostatic hyperplasia with an analysis of risk factors for adverse perioperative outcomes. Urology 2015; 86(4):721-5. [DOI] [PubMed] [Google Scholar]
Pereira 2010
- Pereira TV, Patsopoulos NA, Salanti G, Ioannidis JPA. Critical interpretation of Cochran's Q test depends on power and prior assumptions about heterogeneity. Research Synthesis Methods 2010/04;1(2):149-61. [DOI] [PubMed] [Google Scholar]
Pisco 2016
- Pisco JM, Bilhim T, Pinheiro LC, Fernandes L, Pereira J, Costa NV, et al. Medium- and long-term outcome of prostate artery embolization for patients with benign prostatic hyperplasia: results in 630 patients. Journal of Vascular and Interventional Radiology 2016; 27(8):1115-22. [DOI] [PubMed] [Google Scholar]
Porpiglia 2015
- Porpiglia F, Fiori C, Bertolo R, Garrou D, Cattaneo G, Amparore D. Temporary implantable nitinol device (TIND): a novel, minimally invasive treatment for relief of lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH): feasibility, safety and functional results at 1 year of follow-up. BJU International 2015; 116(2):278-87. [DOI] [PubMed] [Google Scholar]
Porpiglia 2018
- Porpiglia F, Fiori C, Bertolo R, Giordano A, Checcucci E, Garrou D, et al. 3-Year follow-up of temporary implantable nitinol device implantation for the treatment of benign prostatic obstruction. BJU International 2018; 122(1):106-12. [DOI] [PubMed] [Google Scholar]
Porpiglia 2019
- Porpiglia F, Fiori C, Amparore D, Kadner G, Manit A, Valerio M, et al. Second-generation of temporary implantable nitinol device for the relief of lower urinary tract symptoms due to benign prostatic hyperplasia: results of a prospective, multicentre study at 1 year of follow-up. BJU International 2019; 123(6):1061-9. [DOI] [PubMed] [Google Scholar]
Pyo 2017
- Pyo JS, Cho WJ. Systematic review and meta-analysis of prostatic artery embolisation for lower urinary tract symptoms related to benign prostatic hyperplasia. Clinical Radiology 2017; 72(1):16-22. [DOI] [PubMed] [Google Scholar]
Rees 2015
- Rees J. Patients not P values. BJU international 2015; 115(5):678-9. [DOI] [PubMed] [Google Scholar]
Reich 2008
- Reich O, Gratzke C, Bachmann A, Seitz M, Schlenker B, Hermanek P, et al. Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. Journal of Urology 2008; 180(1):246-9. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011; 342(7804):964-7. [DOI] [PubMed] [Google Scholar]
Roehrborn 2003
- Roehrborn C, McConnell J, Barry M, Benaim E, Bruskewitz R, Blute ML, et al. American Urological Association guideline: management of benign prostatic hyperplasia (BPH). www.auanet.org/documents/education/clinical-guidance/Benign-Prostatic-Hyperplasia.pdf (accessed 28 September 2017).
Rosen 1997
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997; 49(6):822-30. [DOI] [PubMed] [Google Scholar]
Rosen 2007
- Rosen RC, Catania JA, Althof SE, Pollack LM, O'Leary M, Seftel AD, et al. Development and validation of four-item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology 2007; 69(5):805-9. [DOI] [PubMed] [Google Scholar]
Rubeinstein 2003
Rukstalis 2019
- Rukstalis D, Grier D, Stroup SP, Tutrone R, deSouza E, Freedman S, et al. Prostatic urethral lift (PUL) for obstructive median lobes: 12 month results of the MedLift study. Prostate Cancer and Prostatic Diseases 2019; 22(3):411-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2008
Salanti 2011
- Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011; 64(2):163-71. [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012; 3(2):80-97. [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE 2014; 9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shim 2017
- Shim SR, Kanhai KJ, Ko YM, Kim JH. Efficacy and safety of prostatic arterial embolization: systematic review with meta-analysis and meta-regression. Journal of Urology 2017; 197(2):465-79. [DOI] [PubMed] [Google Scholar]
Song 2012
- Song F, Clark A, Bachmann MO, Maas J. Simulation evaluation of statistical properties of methods for indirect and mixed treatment comparisons. BMC Medical Research Methodology 2012; 12(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spaliviero 2010
- Spaliviero M, Strom KH, Gu X, Araki M, Culkin DJ, Wong C. Does Greenlight HPS (™) laser photoselective vaporization prostatectomy affect sexual function? Journal of Endourology 2010; 24(12):2051-7. [DOI] [PubMed] [Google Scholar]
Strope 2015
- Strope SA, Vetter J, Elliott S, Andriole GL, Olsen MA. Use of medical therapy and success of laser surgery and transurethral resection of the prostate for benign prostatic hyperplasia. Urology 2015; 86(6):1115-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2008
- Sun F, Sanchez FM, Crisostomo V, Lima JR, Luis L, Garcia-Martinez V, et al. Benign prostatic hyperplasia: transcatheter arterial embolization as potential treatment - preliminary study in pigs. Radiology 2008; 246(3):783-9. [DOI] [PubMed] [Google Scholar]
Veroniki 2013
- Veroniki AA, Higgins Haris SV, Salanti G. Evaluation of inconsistency in networks of interventions. International Journal of Epidemiology 2013; 42(1):332-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veroniki 2014
- Veroniki AA, Mavridis D, Higgins JPT, Salanti G. Characteristics of a loop of evidence that affect detection and estimation of inconsistency: a simulation study. BMC Medical Research Methodology 2014; 14(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veroniki 2016
- Veroniki AA, Straus SE, Fyraridis A, Tricco AC. The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes. Journal of Clinical Epidemiology 2016; 76:193-9. [DOI] [PubMed] [Google Scholar]
Walmsley 2004
- Walmsley K, Kaplan SA. Transurethral microwave thermotherapy for benign prostate hyperplasia: separating truth from marketing hype. Journal of Urology 2004; 172:1249-55. [DOI] [PubMed] [Google Scholar]
Wang 2015
- Wang MQ, Guo LP, Zhang GD, Yuan K, Li K, Duan F, et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms due to large (> 80 mL) benign prostatic hyperplasia: results of midterm follow-up from Chinese population. BMC Urology 2015; 15:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Research Synthesis Methods 2012; 3(2):111-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2012a
- White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Research Synthesis Methods 2012; 3(2):111-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2015
- White IR. Network meta-analysis. Stata Journal 2015; 15(4):951-85.
WHO 2002
- World Health Organization. Proposed working definition of an older person in Africa for the MDS project. www.who.int/healthinfo/survey/ageingdefnolder/en (accessed 17 August 2017).
Woo 2012
- Woo HH, Bolton DM, Laborde E, Jack G, Chin PT, Rashid P, et al. Preservation of sexual function with the prostatic urethral lift: a novel treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Journal of Sexual Medicine 2012; 9(2):568-75. [DOI] [PubMed] [Google Scholar]
Woo 2017
- Woo HH, Gonzalez RR. Perspective on the Rezūm® System: a minimally invasive treatment strategy for benign prostatic hyperplasia using convective radiofrequency water vapor thermal therapy. Medical Devices 2017; 10:71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yepes‐Nuñez 2019
- Yepes-Nuñez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. Journal of clinical epidemiology 2019; 115:1-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yoo 2012
- Yoo TK, Cho HJ. Benign prostatic hyperplasia: from bench to clinic. Korean Journal of Urology 2012; 53(3):139-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zlotta 1997
- Zlotta AR, Raviv G, Peny MO, Noel JC, Haot J, Schulman CC. Possible mechanisms of action of transurethral needle ablation of the prostate on benign prostatic hyperplasia symptoms: a neurohistochemical study. Journal of Urology 1997; 157(3):894-9. [PubMed] [Google Scholar]


