Abstract
Background
Corticosteroids are commonly used in treatment of acute and chronic graft‐versus‐host disease (GvHD). Nevertheless, there has been no systematic analysis of effects of their use on the patients' survival and quality of life.
Objectives
To compare the effect of corticosteroids in treatment of patients with GvHD and to compare the effect of different regimens of corticosteroids.
Search methods
We searched MEDLINE (up to July 2008), EMBASE (up to July 2008) and the Cochrane Controlled Trials Register (up to July 2008) to identify relevant studies. All of the references were assessed in order to identify additional trials.
Selection criteria
Randomized controlled trials of any language were included in the study as long as they met any of the predefined comparisons of interest. The primary outcome in question was the overall survival of the patients. Due to lack of evidence, inclusion criteria was revised during the process of the review to include studies comparing different dosage of corticosteroids.
Data collection and analysis
All derived citations and abstracts were screened by two independent review authors for relevance. For the potentially relevant trials, the full text was obtained and reviewed by two review authors independently. Two review authors completed data extraction independently. After revising the inclusion criteria, this process was retried to ensure all relevant evidence is included in the review.
Main results
No studies met the original inclusion criteria but two studies (four articles) met the revised inclusion criteria. As they addressed different clinical questions, meta‐analysis was not performed. The outcomes of one study were in favor of efficacy of corticosteroids in inducing an earlier remission of acute GvHD, while the other study reported that early corticosteroid therapy of acute GvHD could not prevent progression of the disease to higher grades, although it was accompanied by a slightly better prognosis in the patients who responded by the fifth day of treatment.
Authors' conclusions
There is no certain study regarding appropriate use, dose and length of therapy for acute GvHD. Further studies are needed to define the appropriate use of steroids and whether other agents are appropriate as frontline therapy.
Plain language summary
Corticosteroid regimens for treatment of acute and chronic graft versus host disease (GvHD) after allogenic stem cell transplantation
Corticosteroids are commonly used to treat acute and chronic graft‐versus‐host disease (GvHD) but their effect on length and quality of life of patients has not been studied systematically. In this systematic review, we tried to compare the effect of treatment regimens used for GvHD in the absence and presence of corticosteroids, or with different doses of corticosteroids. After searching relevant sources, we located only two studies that met our criteria to be included in the study. Their results are described in detail in the text of the review. In brief, these studies are in favor earlier remission and slightly better outcome in patients but more evidence is needed in this field.
Background
Description of the condition
Several studies have documented that many patients with hematologic diseases such as hematopoietic malignancies, myelodysplastic syndrome, aplastic anemia and thalassemia can be cured by allogenic bone marrow transplantation from a HLA‐identical sibling (Locatelli 2000, Chen 2002, Sullivan 2004). A common complication of such transplantations is graft‐versus‐host disease (GvHD) which is caused by the recognition of host antigens as foreign by donor T lymphocytes. Unfortunately, GvHD remains a major source of mortality and morbidity including abnormalities of growth and development in children, sexual satisfaction and employment in adults, and functional performance status (Chen 2002). Risk factors for developing GvHD include donor‐recipient gender mismatch, donor parity, age, and source of allogenic stem cells. GvHD is categorized to acute and chronic forms, regarding the time of occurrence of disease after transplantation. It is important to delineate between acute and chronic GvHD. The cut‐off is arbitrarily set at 100 days post stem‐cell transplantation. The induction of acute GvHD may be deduced into three phases: (i) recipient conditioning, (ii) donor T cell activation and (iii) effector cells mediating. GvHD cytokines have been shown to be extremely important in the initiation and propagation of GvHD. The usual target organs are skin, gut and liver. The incidence of acute GvHD varies widely (between 10 to 90%) (Murphy 1999). Clinical grading ranges from grade I with milder symptoms to grade III with severe multi‐organ involvement, and grade IV which is life‐threatening (Alexander 2000, Sullivan 2004). Chronic GvHD occurs usually within three years following transplant in up to 60% of patients (Wingard 2002). Organ fibrosis with collagen deposition and atrophy are the hallmarks of chronic GvHD, which is generally divided into three categories: progressive onset, quiescent, and de novo, the latter having better prognosis. On the other hand patients with prior acute GvHD, liver dysfunction and thrombocytopenia have poor prognosis. Patients not responding to first and second line chronic GvHD therapy also have a poor prognosis. Regarding the prolonged or continuing use of steroids in treatment of acute and chronic GvHD, there remains the question of the effect of the duration of steroid administration, including tapering or cessation of this agent due to adverse effects.
Why it is important to do this review
Once GvHD has developed, the strategy of treatment is not well established. Most centers administer prednisone at 1 to 2 mg/kg of patient body weight or the dose equivalent of another glucocorticoid and cyclosporine A (CSA) or tacrolimus as medication of first choice (Peters 2000). Long term treatment with high dose glucocorticoids is associated with a high risk for morbidity. A regimen of cyclosporine and prednisolone administered on alternating days might have better efficacy and less toxicity than prednisolone alone for treatment of chronic GvHD (Koc 2002, Sullivan 1987).
Objectives
To determine the effect of corticosteroids used for the treatment of acute and chronic graft‐versus‐host disease (GvHD) after myeloablative allogenic stem cell transplantation in improving outcome, as well as the potential increases of undesired adverse effects such as infections (Juni 2001).
Methods
Criteria for considering studies for this review
Types of studies
The review was aimed to include randomized controlled trials (RCTs), with or without blinding, in any language. Quasi‐randomized trials (such as those assigning participants to study groups by case record number) were not included, because they are subject to selection bias and may result in unreliable outcomes. Studies with unclear information about the method of randomization were included in the review when they appeared to be likely to be randomized.
Types of participants
Patients with acute or chronic GvHD who have undergone allogenic stem cell transplantation (SCT) either by bone marrow transplantation (BMT) or peripheral blood stem cell transplantation (PBSC) after myeloablative conditioning therapy were included. Acute GvHD is defined as GvHD occurring within the first 100 days from transplantation time. All different stages of the disease were considered as well as the different types of HLA‐matching, including HLA‐matched or ‐mismatched relatives and unrelated HLA‐matched or ‐mismatched donors.
Types of interventions
The two arms of the included studies targeting treatment of GvHD which only differed in the usage of one corticosteroid for the two arms or the dosage of one corticosteroid in the two arms. Consequently, any type of drugs or non‐chemical interventions for treatment of GvHD had been accepted until the two arms of the study had not differed in respect to such interventions. Consequently, the intervention design of included studies had to follow one of the following patterns:
Single drug versus same single drug plus corticosteroids
Multiple drug regimen versus same multiple drug regimen plus corticosteroids
We also included studies which compared the effect of different timing or dosing of corticosteroid regimen in the two arms. This addition was made during the process of the review, since we noticed the lack of studies meeting the first two criteria and the existence of studies targeting different dosage of corticosteroids in treatment of GvHD. After this addition, we rechecked all search results to ensure we include all studies meeting the new criteria.
In any of the above‐stated situations, the prevention methods for GvHD had to be balanced across the arms, or vary only due to the play of chance.
Types of outcome measures
We considered our outcome measures regarding a document supervised by EBMT statisticians (EBMT 2003).
Primary outcomes
Overall survival
Secondary outcomes
Event‐free survival (event is defined as relapse or death of any kind) following transplantation
Incidence of relapse of underlying disease (where death without relapse a competing risk) following transplantation
Non‐relapse mortality (i.e. death for causes other than the underlying disease)
Incidence of relapse of GvHD
Time to relapse of GvHD
Incidence of opportunistic infections
Incidence of other adverse effects
Quality of life
Search methods for identification of studies
Electronic searches
These databases include the Cochrane Central Register of Controlled Trials (CENTRAL) up to 30 July 2008, MEDLINE (1966 to July 2008), and EMBASE (1980 to July 2008). The search strategies for CENTRAL include disease specific keywords (see Appendix 1). The search strategies for MEDLINE (see Appendix 2) and EMBASE (see Appendix 3) focus on methodological characteristics of the studies as well. For MEDLINE database, we included the highly sensitive search strategy for identifying reports of randomized controlled trials in MEDLINE, available in Appendix 5b.2 of the Cochrane Handbook for Systematic Reviews of Intervention (Cochrane Handbook). Our search strategies for MEDLINE and EMBASE are designed for use with OVID Web Gateway.
Searching other resources
Conference proceedings
The conference proceedings (up to the end of June 2007) of the following societies were searched manually or ‐ if available ‐ electronically.
American Society of Clinical Oncology [ASCO]: Journal of Clinical Oncology (1995 to July 2008 electronically)
American Society of Hematology [ASH]: Blood (2001 to July 2008 electronically)
European Group for Blood and Marrow Transplantation [EBMT]: Bone Marrow Transplantation (2000 to July 2008 electronically)
Electronic search in databases of ongoing trials We checked databases of ongoing trials (including ClinicalTrials.gov and TrialsCentral.org) for relevant ongoing trials, and for locating potential links to other related databases and resources.
Hand searching of references The references listed in the identified trials, relevant review articles and actual treatment guidelines were hand searched for potential sources of evidence.
Data collection and analysis
Selection of studies
Two review authors (MR, MRA) studied the titles and abstracts of all of the references located by search, independently, against the inclusion criteria stated before. If required, the full texts of the references were obtained. Any study meeting the inclusion criteria was assessed for the following questions:
Was the method of randomization satisfactory?
Did the patients undergo a myeloablative conditioning therapy before transplantation?
Did one arm of patients receive corticosteroids for GvHD treatment?
Did the control group receive the same background drug therapy as the patients in the corticosteroid‐group?
Disagreements were solved in a consensus meeting or a by calling a third review author (HS). We had also planned to contact the corresponding authors in case the full text had not provided sufficient information. To ensure high quality of the systematic review, the report was based on the Quality of Reporting Meta‐analyses (QUOROM) statement (Moher 1999a).
Data extraction and management
Two review authors (SB,MRA) independently studied the trials to extract their data, using a form including the following fields:
General information: title, authors, source, contact address, country, year of publication, setting, funding
Trials characteristics: design, method of randomization, concealment of allocation, blinding of patients, blinding of clinicians, blinding of outcome assessors, method of GvHD‐diagnosis (clinical or histopathological)
Patients: inclusion and exclusion criteria, underlying disease demanding SCT, type of conditioning regimen, type of stem cell source, type and extent of HLA‐matching (i. e. HLA‐identical sibling, all extents of HLA‐mismatched related and HLA‐matched and mismatched unrelated donor), sample size, baseline characteristics (e.g. median age), withdrawals, losses to follow‐up
Interventions: type of drug regimens, dose, day of initiation and duration of treatments
Outcomes: event‐free survival, relapse incidence, non‐relapse mortality, incidence of relapse of GvHD, time to relapse of GvHD, incidence of opportunistic infections, incidence of other adverse effects, and quality of life
We contacted corresponding authors for unpublished data, but no more evidence was retrieved this way. Results based on Intention To Treat (ITT) analysis were accepted, if available; otherwise, the most conservative results were used. Any probable disagreement was solved in a consensus meeting and, if necessary, by calling a third reviewer.
Assessment of risk of bias in included studies
Two review authors (MR,HS) assessed the methodological quality of each eligible reference, independently, using the following questionnaire which is based on CONSORT Questionnaire (Standards of Reporting Trials, Moher 2001a,Moher 2001b):
Was the treatment allocation concealed?
Were the patients blinded to the treatment?
Were the clinicians blinded to the treatment?
Were the outcome assessors blinded to the treatment?
Were the numbers and reasons of withdrawals, drop‐outs and lost to follow‐up stated for each group?
Did the analysis include an intention‐to‐treat‐analysis?
Disagreements were solved in a consensus meeting or a third review author (AS) was called.
Data synthesis
We planned to use RevMan Analysis 1.0 software (included in the Cochrane Collaboration's Review Manager 4.2.8 package) for meta‐analysis and generation of graphs. However, our review did not result in a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
The following subgroups were defined to be compared in the subgroup analysis:
Underlying disease
Transplant type (bone marrow vs. peripheral blood)
Conditioning regimen
Timing of corticosteroid use
HLA matching criteria
Prevention methods used before the occurrence of GvHD
However, sufficient information was not available in the included studies to undertake a subgroup analysis.
Sensitivity analysis
Analysis based on the following items was planned upon development of the review protocol, but our review did not include a sensitivity analysis due to lack of evidence.
Method of GvHD diagnosis
Study size
Funding of the trial
The level of loss to follow up
Results
Description of studies
Results of the search
After removing duplicated results, 3986 records were resulted from the electronic searching in CENTRAL, MEDLINE, and EMBASE; searching conference proceedings didn't yield any relevant studies. Two of us (HS, MR) screened through these records and selected relevant studies based on their titles and abstracts. We kept both RCTs and reviews, since further studies could be found by tracking the references of reviews. Full texts of 21 studies were retrieved for full text scrutiny, and two more references were identified by reference tracking. Finally, four articles reporting two studies were identified to meet the inclusion criteria (for QUOROM diagram see Figure 1). Characteristics of these studies are provided in the table 'Characteristics of included studies'. In the meantime, several studies were excluded from the study for reasons such as lack of appropriate randomization, uncontrolled design or because they addressed a different question (e.g. a comparison that was not in the interest of our study). Details of these studies are described in the table 'Characteristics of excluded studies'.
1.
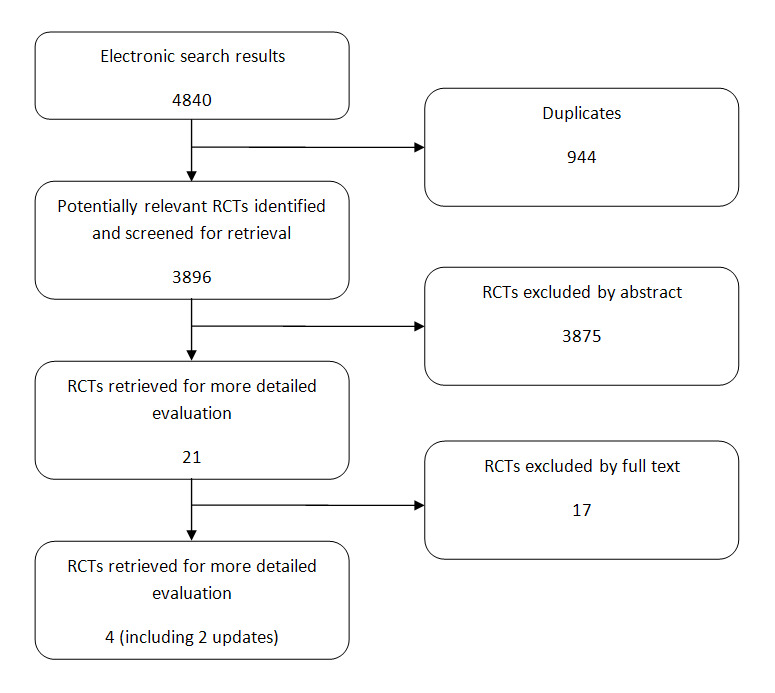
QUOROM flowchart for study selection process
Included studies
Study design
All included studies were randomized controlled trials. Interventions
One article (Hings 1993) reported a study comparing 14 patients receiving short taper regimen versus 16 patients receiving long taper regimen of methylprednisolone (MP). Of the remaining three articles, two (Van Lint 1995,Van Lint 1997) were preliminary reports of the third article (Van Lint 1998) which compared 47 patients receiving a low dose regimen of 6‐methylprednisolone against 48 patients receiving a high dose regimen of the same drug. Outcomes
Both studies reported the rate of response to treatment, as well as a measure of survival. The details of the outcome measure of each study are described below.
Risk of bias in included studies
Although the application of randomization was described in both of the studies, the method was not mentioned in the reports. Although the comparison of the treatments in both studies could be accomplished with the interventions 'masked', none of them reported any level of blinding to be applied. Also, allocation of the patients to the study groups was not declared to be concealed in any of the studies. Intention To Treat method was used to analyze the data in one study (Van Lint 1998) but not in the other one. The authors did not provide further information when they were contacted. While assessing the quality of the studies using Jadad scoring (Jadad 1996) based on the available information, both studies got a score of one. A trial with a Jadad score of less than three is considered to be of a poor quality (Moher 1999b).
Effects of interventions
Since the two studies included in this review addressed different questions, the results are presented per article. In cases where the authors had not reported the P values, we re‐analyzed the data. A)Hings 1993 study:
This study was carried out by recruiting 68 patients who had developed moderate to severe acute GvHD after receiving allogenic transplantations, and were eligible for treatment with Prednisolone (PRED) at 60 mg/m2/day. After 14 days, if they had responded or had stable disease, they were defined as eligible to be randomized to either short taper or long taper of PRED. 38 patients were not eligible for randomization for reasons like death or progression to chronic GvHD. 14 patients were allocated to short taper group, and 16 were allocated to long taper group. The differences between baseline characteristics of the patients in the two groups were not statistically significant. Patients were assessed on a weekly basis, and a scoring system, which was a modification of Seattle clinical staging score for GvHD, was used to evaluate the patients' response to therapy. All patients were followed for a minimum of one year post‐transplantation.
The two treatment arms differed in the amount of steroids administered (6300 mg/m2 for long taper and 2275 mg/m2 for the short taper group) and the prescribed duration of the treatment (21 versus 1 weeks, respectively). At the end of eight weeks, three of the 13 evaluable patients in the short taper group, and two of the 13 evaluable patients in the long taper group still had active GvHD. Although the overall response rate was similar in the two study arms, patients receiving the long taper achieved more rapid resolution, compared to the short taper group (with the median of 30 days versus 42 days, respectively; P value = 0.01). When analyzed with respect to total cumulative PRED dose, patients in both groups responded after receiving approximately the same dose (median dose was 1300 mg/m2 for long taper and 188 mg/m2 for short taper group, P value = 0.5). No statistically significant differences were seen in the rate of flares of acute GvHD, incidence of chronic GvHD, date of onset of chronic GvHD post‐transplantation or post‐randomization and, in the number of days hospitalization in the first six months post‐transplantation (P values being greater than 0.5 in all of the above). Survival of 6 months post‐transplantation was 12 of 14 and 13 of 16 for short and long taper patients, respectively (P value = 0.87). 19 episodes of infectious complications were observed in the long taper group, compared to 17 episodes in the short taper group; the difference was not significant. The incidence and timing of the infection were reported to be similar in both groups, as well as the distribution of infectious organisms. Also, no difference was observed in the incidence of non‐infectious complications related to steroid therapy.
Overall, the authors concluded that a long taper regimen of steroids could result in a slightly earlier remission of acute GvHD symptoms, but no overall improvement in the rate of control of GvHD. Also, more rapid cessation of steroids did not improve either morbidity or mortality in the first six months post‐transplantation, and did not reduce the incidence of steroid related complications.
B) Van Lint 1998 study:
We identified three reports of the same study conducted by Van Lint et al. The first two (Van Lint 1995, Van Lint 1997) were preliminary reports, confined to a summary of the methods and results of the study. Here we describe the study based on the most complete report (Van Lint 1998).
The study was a multi‐center clinical trial, aimed to compare the effect of high versus low dose 6‐Methylprednisolone (6MP). 95 patients with hematological neoplastic and non‐neoplastic diseases who had received HLA‐identical bone marrow transplants from sibling donors and had developed acute GvHD were randomized to receive either 2 mg/kg/d (low dose) or 6 mg/kg/d (high dose) of 6MP. Patients who showed progression or poor response after 5 days on the low dose regimen, were shifted to the high dose regimen. The results were analyzed as Intention To Treat. 47 patients were allocated to the low dose regimen, and 48 to the high dose arm. Baseline characteristics were similar in both groups; the study arms were also matched for some factors which could confound the results, including age, GvHD prophylaxis and diagnosis.
Comparison of the given doses of 6MP within different intervals showed that the dose of 6MP did not differ significantly except for the first 10 days of therapy. Patients were classified to responders and non‐responders, based on the regression or progression of GvHD. Death due to transplant‐related complications occurred in 10 of 66 responders and 17 of 27 non‐responders (P value < 0.00001) while the response rate was similar in the two groups (68% in low dose and 71% in high dose group, P value = 0.9). Also no difference was observed in the proportion of responders to non‐responders, when patients were stratified based on their age (P value = 0.9), GvHD prophylaxis regimen (P value = 0.9), conditioning regimen (P value = 0.5), and the phase of the disease (P value = 0.7). The average cumulative dose of 6MP was indifferent between the two groups (25mg/kg in non‐responders versus 28mg/kg in responders on days 1 through 5, P value = 0.6; 17mg/kg in non‐responders versus 15mg/kg in non‐responders on days six through ten, P value = 0.5).
In 17% of patients in low dose group and 20% from the high dose arm, acute GvHD progressed to grade III‐IV (P value=0.6). The progression risk was similar in patients treated early (within the first 12 days) and those treated later (after the 12th day.) Among the 68 patients evaluable for chronic GvHD, the number of patients who had developed limited or extensive chronic GvHD was similar in the two study arms (P value=0.8). The probability of relapse was not statistically different in the two groups of patients (P value = 0.1).
The overall actuarial transplant‐related mortality rate was 28% in the low dose group and 32% in the high dose group (P value = 0.7) and the mortality rate was not different in patients stratified for age, GvHD prevention regimen, conditioning regimen, or disease status at transplant (early versus advanced). However, it was significantly lower in patients with acute GvHD of grade I‐II versus patients who experienced acute GvHD of grade III‐IV (23% versus 58%, P value < 0.0001). In the low dose arm, the patients who did not respond by day five of therapy, had a transplant‐related mortality rate of 46%, which was significantly larger than the 16% transplant‐related mortality rate for patients who responded by day five (P value = 0.007).
The actuarial 3‐year survival of patients allocated to the low dose and high dose arms were 63% and 62% respectively (P value = 0.9). Causes of death included leukemia recurrence, acute GvHD, infections, interstitial pneumonia, multiorgan failure, hepatitis, heart failure, and hemorrhage, and their rates were similar in the two groups (P value = 0.6). Infectious side effects of therapy were not statistically different in the two study arms.
All together, the authors conclusion was that early treatment of acute GvHD with high dose 6MP could not prevent progression of the disease to grade III‐IV, and treatment with a low dose regimen would have a good prognosis in patients who responded by the fifth day of treatment.
Discussion
Corticosteroids have been used for prevention and treatment of GvHD for many years. Although advancements in the treatment of GvHD using immuno‐modulators and other immuno‐suppressor drugs have proven many other therapeutic modalities to be effective, corticosteroids are still being widely used in the treatment protocol of this disease (Bacigalupo 2007; Van Lint 2006).
Two studies met the inclusion criteria of this systematic review. The outcomes for this review were not uniformly reported in the included studies, and the two trials did not address questions similar enough for results to be pooled. Blinding was not undertaken in any of the two studies and the quality of reporting of these trials was also generally quite poor, according to CONSORT. The included studies emphasized on overall survival, remission and relapse rates, and did not describe the quality of life of the survivors. Both trials had a small sample size, hence a questionable statistical power.
Hings et al reported that a long taper regimen of corticosteroids could result in a slightly earlier remission of acute GvHD symptoms, but the overall rate of control of GvHD was not affected (Hings 1993). Van Lint et al concluded that early treatment of acute GvHD with high dose 6MP could not prevent progression of the disease to grade III‐IV, but treatment with a low dose regimen would result in a better prognosis in patients who responded by the fifth day of treatment (Van Lint 1998).
This review highlights the paucity of information on the role of corticosteroids in treatment of Graft versus Host Disease. Also, there was a lack of evidence supporting the efficacy of corticosteroids for treatment of chronic GvHD, which should be addressed by future randomized controlled trials.
One could argue that the use of corticosteroids in treatment of GvHD is obviously mandatory, so there is no interest in assessing it through trials. It should be noted that corticosteroids are also used in prevention of GvHD, and several studies have assessed this approach. In a recently published systematic review, Quellmann et al. reported that by pooling the results of five RCTs it could be concluded that the addition of corticosteroids to GvHD prophylaxis regimens reduces the risk of acute GvHD, although, based on the randomized trials currently available there is no evidence that this benefit improves long‐term outcomes (Quellmann 2008).
In brief, there is a lack of RCT data on which to base definitive conclusions.
Authors' conclusions
Implications for practice.
There is not enough evidence supporting the use of corticosteroids to achieve a faster or better response in treatment of GvHD, and the currently available evidence does not explain its impact on the survival of the patients. The use of corticosteroids is accompanied by a higher risk of fungal infections and other corticosteroid related adverse reactions. However, there is little evidence from randomized controlled trials to evaluate its effect on the patients' quality of life, which is particularly important for patients suffering chronic GvHD.
Implications for research.
There is a need for well structured research into treatment options of GvHD with a larger sample size, reporting outcomes such as disease free survival, measures of quality of life, and cost‐effectiveness measures, to provide more strong recommendations supporting or disproving use of corticosteroids for treatment of GvHD. Current evidence does not address the effect of corticosteroids in treatment of chronic GvHD. This review suffers from the great heterogeneity concerning definition of study arms and study conduct.
What's new
| Date | Event | Description |
|---|---|---|
| 27 November 2009 | Amended | Typos corrected |
Acknowledgements
We would like to thank Thilo Kober (former Co‐ordinator of the Cochrane Haematological Malignancies Group) for his aids in materializing the research plan. We would also like to thank Susanne Quellmann (Cochrane review author) truly, for her timely and invaluable help in development of our search strategy.
Appendices
Appendix 1. CENTRAL search strategy
1. GRAFT VS HOST DISEASE (MeSH) 2. GRAFT VS HOST REACTION explode (MeSH) 3. graft versus host* 4. graft‐versus host* 5. graft‐versus‐host* 6. graft vs host* 7. graft‐vs host* 8. graft‐vs‐host* 9. graft v host* 10. graft‐v host* 11. graft‐v‐host* 12. GvHD 13. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
Appendix 2. MEDLINE search strategy
1. RANDOMIZED CONTROLLED TRIAL.pt. 2. CONTROLLED CLINICAL TRIAL.pt. 3. RANDOMIZED CONTROLLED TRIALS.sh. 4. RANDOM ALLOCATION.sh. 5. DOUBLE BLIND METHOD.sh. 6. SINGLE BLIND METHOD.sh. 7. or/1‐6 8. (ANIMALS not HUMAN).sh. 9. 7 not 8 10. CLINICAL TRIAL.pt. 11. exp CLINICAL TRIALS/ 12. (clin$ adj25 trial$).ti,ab. 13. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 14. PLACEBOS.sh. 15. placebo$.ti,ab. 16. random$.ti,ab. 17. RESEARCH DESIGN.sh. 18. or/10‐17 19. 18 not 8 20. 19 not 9 21. COMPARATIVE STUDY.sh. 22. exp EVALUATION STUDIES/ 23. FOLLOW UP STUDIES.sh. 24. PROSPECTIVE STUDIES.sh. 25. (control$ or prospective$ or volunteer$).ti,ab. 26. or/21‐25 27. 26 not 8 28. 27 not (9 or 20) 29. 9 or 20 or 28 30. Graft vs Host Disease/ 31. graft versus host$.ti,ab. 32. graft vs host$.ti,ab. 33. graft v host$.ti,ab. 34. GvHD.ti,ab. 35. or/30‐34 36. 29 and 35
Appendix 3. EMBASE search strategy
1. randomized controlled trial/ 2. random allocation/ 3. controlled study/ 4. multicenter study/ 5. Phase 1 Clinical Trial/ 6. Phase 2 Clinical Trial/ 7. Phase 3 Clinical Trial/ 8. phase 4 clinical trial/ 9. double blind procedure/ 10. single blind procedure/ 11. (RANDOM$ or CROSS?OVER$ or FACTORIAL$ or PLACEBO$ or VOLUNTEER$).ti,ab. 12. ((SINGL$ or DOUBL$ or TREBL$ or TRIPL$) adj (BLIND$ or MASK$)).ti,ab. 13. or/1‐12 14. graft versus host reaction/ 15. graft‐versus‐host$.ti,ab. 16. graft versus host$.ti,ab. 17. graft vs host$.ti,ab. 18. graft v host$.ti,ab. 19. GvHD.ti,ab. 20. or/14‐19 21. 13 and 20 22. limit 21 to human
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hings 1993.
| Methods | Randomized controlled trial | |
| Participants | Moderate/severe acute GvHD patients who responded to primary therapy with corticosteroids | |
| Interventions | Long taper vs short taper of corticosteroids | |
| Outcomes | Clinical response Relapse of acute GvHD Onset of chronic GvHD Complications Survival | |
| Notes | Jadad = 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Van Lint 1995.
| Methods | Randomized controlled trial | |
| Participants | Acute GvHD after allogenic BMT, not responding to 48 hours of 0.5 mg/kg of 6‐MP | |
| Interventions | High dose (10mg/kg) 6‐MP vs low dose (2mg/kg) 6‐MP, both reduced every 5 days | |
| Outcomes | Clinical response Evolution to aGvHD III‐IV CMV infection actuarial TRM Relapse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Van Lint 1997.
| Methods | Randomized controlled trial | |
| Participants | Acute GvHD after allogenic BMT, not responding to 48 hours of 0.5 mg/kg of 6‐MP | |
| Interventions | High dose (10mg/kg) 6‐MP vs low dose (2mg/kg) 6‐MP, both reduced every 5 days | |
| Outcomes | Clinical response Evolution to aGvHD III‐IV CMV infection actuarial TRM Relapse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Van Lint 1998.
| Methods | Randomized controlled trial | |
| Participants | Acute GvHD after allogenic BMT, not responding to 48 hours of 0.5 mg/kg of 6‐MP | |
| Interventions | High dose (10mg/kg) 6‐MP vs low dose (2mg/kg) 6‐MP, both reduced every 5 days | |
| Outcomes | Clinical response Evolution to aGvHD III‐IV CMV infection Actuarial TRM Relapse Survial | |
| Notes | Jadad = 1 Intention to treat analysis applied most of the time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bacigalupo | Uncontrolled study. |
| Deeg 1985 | Quasi‐randomization was used (alternation) |
| Doney 1981 | The study comparison didn't meet inclusion criteria (Corticosteroids vs Anti‐thymocyte globulin.) |
| Gilman 2000 | Study type is letter and it doesn't contain information about a relevant comparison. |
| Kanojia 1984 | Uncontrolled study. |
| Michallet 1999 | Retrospective analysis. |
| Oblen 1992 | Uncontrolled study. |
| Prentice 1980 | Uncontrolled study. |
| Srivanasan 2004 | Retrospective analysis. |
| Sullivan 1987 | The study comparison didn't meet inclusion criteria (it was prednisolone + placebo vs prednisolone + azathioprine) |
| Sullivan 1993 | The study comparison didn't meet inclusion criteria (it was prednisolone vs alternating day cyclosporine/prednisolone) |
Differences between protocol and review
During the searching phase, we noted that there are a few studies comparing different dosage of corticosteroids in treatment of GvHD. As we found no studies to meet our original inclusion criteria, we updated our inclusion criteria and other relevant sections accordingly. The words "regimens" was added to the title of the review to indicate that different dosages is also reviewed. A new item was added to the inclusion criteria, to include studies that compare different timing and/or dosage of corticosteroids. Search results were screened with scrutiny to identify all studies meeting this new criterion. Results section reflects the outcome of this process. References of newly included studies were also rechecked to ensure all relevant studies are found and included.
Contributions of authors
Mersedeh Rohanizadegan: Searching for trials, Study selection, Quality assessment
Mahtab Rabbani‐Anari: Study selection, Data extraction
Setareh Banihosseini: Data extraction
Hojjat Salmasiam: Contact reviewer, Drafting of manuscripts, Searching for trials, Study appraisal
Raheleh Rahimi Darabad: Searching gray literature
Alia SHakiba: Searching gray literature
James L.M. Ferrara: Scientific support, Revision of manuscript
Theresa Hahn: Statistical support
Phillip McCarthy: Scientific support, Revision of manuscript
Sources of support
Internal sources
-
Tehran University of Medical Sciences, Iran.
Full texts of some of the articles were obtained using the access provided by Tehran University of Medical Sciences.
External sources
No sources of support supplied
Declarations of interest
The protocol is not supported by any pharmaceutical company or any other funding. Two of our team members (JF, PM) had been involved in bone marrow transplantation programs held by their institutes in the past. None of them were involved in such programs at the time of development of this systematic review.
Edited (no change to conclusions)
References
References to studies included in this review
Hings 1993 {published data only}
- Hings IM, Filipovich AH, Miller WJ, Blazar BL, McGlave PB, Ramsay NK, et al. Prednisone therapy for acute graft‐versus‐host disease: short‐ versus long‐term treatment. A prospective randomized trial. Transplantation 1993;56(3):577‐80. [DOI] [PubMed] [Google Scholar]
Van Lint 1995 {published data only}
- Lint MT. Treatment of acute graft versus host disease with high vs low dose methylprednisolone: a randomized GITMO study. Bone Marrow Transplantation 1995;15 suppl 2:S143. [Google Scholar]
Van Lint 1997 {published data only}
- Lint MT. Treatment of acute graft versus host disease with high vs low dose 6‐methylprednisolone (6MP): A randomized study. Bone Marrow Transplantation 1997;19 (suppl):S192. [Google Scholar]
Van Lint 1998 {published data only}
- Lint MT, UderzoC, Locasciulli A, Majolino I, Scime R, Locatelli F, et al. Early treatment of acute graft‐versus‐host disease with high‐ or low‐dose 6‐methylprednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood 1998;92(7):2288‐93. [PubMed] [Google Scholar]
References to studies excluded from this review
Bacigalupo {published data only}
- Bacigalupo A, Lint MT, Frassoni F, Podesta M, Veneziano G, Avanzi G, et al. High dose bolus methylprednisolone for the treatment of acute graft versus host disease. Blut 1983;46:125‐32. [DOI] [PubMed] [Google Scholar]
Deeg 1985 {published data only}
- Deeg HJ, Loughran JTP, Storb R. Treatment of human acute graft‐versus‐host disease with antithymocyte globulin and cyclosporine with or without methylprednisolone. Transplantation 1985;40(2):162‐6. [DOI] [PubMed] [Google Scholar]
Doney 1981 {published data only}
- Doney KC, Weiden PL, Storb R, Thomas ED. Treatment of graft‐versus‐host disease in human allogeneic marrow graft recipients: a randomized trial comparing antithymocyte globulin and corticosteroids. American Journal of Hematology 1981;11(1):1‐8. [DOI] [PubMed] [Google Scholar]
Gilman 2000 {published data only}
- Gilman AL, Schultz KR. Treatment of chronic GVHD. Bone Marrow Transplantation 2000;25(7):689‐96. [DOI] [PubMed] [Google Scholar]
Kanojia 1984 {published data only}
- Kanojia MD, Anagnostou AA, Zander AR, Vellekoop L, Spitzer G, Verma DS, et al. High‐dose methylprednisolone treatment for acute graft‐versus‐host disease after bone marrow transplantation in adults. Transplantation 1984;37(3):246‐9. [DOI] [PubMed] [Google Scholar]
Michallet 1999 {published data only}
- Michallet M, Perrin MC, Belhabri A, Molina L, Nicolini F, Tigaud JD, et al. Impact of cyclosporine and methylprednisolone dose used for prophylaxis and therapy of graft‐versus‐host disease on survival and relapse after allogeneic bone marrow transplantation. Bone Marrow Transplantation 1999;23(2):145‐50. [DOI] [PubMed] [Google Scholar]
Oblen 1992 {published data only}
- Oblen DJ, Felker D, Coyle K, Myers L. High‐dose methylprednisolone therapy for acute graft‐versus‐host disease associated with matched unrelated donor bone marrow transplantation. Bone Marrow Transplantation 1992;10:355‐7. [PubMed] [Google Scholar]
Prentice 1980 {published data only}
- Prentice HG, Bateman SM, Bradstock KF, Hoffbrand AV. High dose methyl prednisolone therapy in established acute graft‐versus‐host disease. Blood 1980;41:175‐7. [Google Scholar]
Srivanasan 2004 {published data only}
- Srinivasan R, Chakrabarti S, Walsh T, Igarashi T, Takahashi Y, Kleiner D, et al. Improved survival in steroid‐refractory acute graft versus host disease after non‐myeloablative allogeneic transplantation using a daclizumab‐based strategy with comprehensive infection prophylaxis. British Journal of Haematology 2004;124(6):777‐88. [DOI] [PubMed] [Google Scholar]
Sullivan 1987 {published data only}
- Sullivan KM, Witherspoon RP, Deeg HJ, Sanders J, Doney K, Appelbaum F, et al. Treatment of chronic graft‐versus‐host disease (GVHD): long term follow‐up of a randomized clinical trial. Blood 1987;5 suppl 1:315A. [Google Scholar]
Sullivan 1993 {published data only}
- Sullivan KM, Gooley T, Nims J, Flowers M, Lin D, Anasetti C. Comparison of cyclosporine (CSP), prednisone (PRED) or alternating‐day CSP/PRED in patients with standard and high‐risk chronic graft‐versus‐host disease (GVHD). Blood 1993;82:215A. [Google Scholar]
Additional references
Bacigalupo 2007
- Bacigalupo A. Management of acute graft‐versus‐host disease. British Journal of Haematology 2007;137:87‐98. [DOI] [PubMed] [Google Scholar]
Chen 2002
- Chen BJ, Cui X, Liu C, Chao NJ. Prevention of graft‐versus‐host disease while preserving graft‐versus‐leukemia effect after selective depletion of host‐reactive T cells by photodynamic cell purging process. Blood 2002;99(9):3083‐8. [DOI] [PubMed] [Google Scholar]
Cochrane Handbook
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 4.2.5. Chichester, UK: John Wiley & Sons, Ltd, 2005:185‐8. [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koc 2002
- Koc S, Leisenring W, Flowers MED, Anasetti C, Deeg HJ, Nash RA, et al. Therapy for chronic graft‐versus‐host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood 2002;100(1):48‐51. [DOI] [PubMed] [Google Scholar]
Locatelli 2000
- Locatelli F, Bruno B, Zecca M, Van‐Lint MT, McCann S, Arcese W, et al. Cyclosporin A and short‐term methotrexate versus cyclosporin A as graft versus host disease prophylaxis in patients with severe aplastic anemia given allogeneic bone marrow transplantation from an HLA‐identical sibling: results of a GITMO/EBMT randomized trial. Blood 2000;96(5):1690‐7. [PubMed] [Google Scholar]
Moher 1999a
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [DOI] [PubMed] [Google Scholar]
Moher 1999b
- Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al. Assessing the quality of reports of randomized trials: implications for the conduct of meta‐analyses. Health Technology Assessment 1999;3:1‐98. [PubMed] [Google Scholar]
Moher 2001a
- Moher D, Schulz KF, Altman DG, CONSORT. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Medical Research Methodology 2001;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2001b
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [PubMed] [Google Scholar]
Murphy 1999
- Murphy WJ, Blazar BR. New strategies for preventing graft‐versus‐host disease. Current Opinion in Immunology 1999;11(5):509‐15. [DOI] [PubMed] [Google Scholar]
Peters 2000
- Peters C, Minkov M, Gadner H, Klingebiel T, Vossen J, Locatelli F, et al. Statement of current majority practices in graft‐versus‐host disease prophylaxis and treatment in children. Bone Marrow Transplantation 2000;26(4):405‐11. [DOI] [PubMed] [Google Scholar]
Quellmann 2008
- Quellmann S, Schwarzer G, Hübel K, Engert A, Bohlius J. Corticosteroids in the prevention of graft‐vs‐host disease after allogeneic myeloablative stem cell transplantation: a systematic review and meta‐analysis. Leukemia 2008;22(9):1801‐3. [DOI] [PubMed] [Google Scholar]
Sullivan 2004
- Sullivan KM. Graft‐vs‐host disease. In: Blume KG, Forman SJ, Appelbaum FR editor(s). Thomas' Hematopoietic Stem Cell Transplantation. Wiley‐Blackwell, 2004:635‐64. [Google Scholar]
Van Lint 2006
- Lint MT, Milone G, Leotta S, Uderzo C, Scime R, Dallorso S, et al. Treatment of acute graft‐versus‐host disease with prednisolone: significant survival advantage for day5 responders and no advantage for non‐responders receiving anti–thymocyte globulin. Ttransplantation 4177‐81. [DOI] [PubMed] [Google Scholar]


