Abstract
Background
Foot ulcers in people with diabetes are non‐healing, or poorly healing, partial, or full‐thickness wounds below the ankle. These ulcers are common, expensive to manage and cause significant morbidity and mortality. The presence of a wound has an impact on nutritional status because of the metabolic cost of repairing tissue damage, in addition to the nutrient losses via wound fluid. Nutritional interventions may improve wound healing of foot ulcers in people with diabetes.
Objectives
To evaluate the effects of nutritional interventions on the healing of foot ulcers in people with diabetes.
Search methods
In March 2020 we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
We included randomised controlled trials (RCTs) that evaluated the effect of nutritional interventions on the healing of foot ulcers in people with diabetes.
Data collection and analysis
Two review authors, working independently, assessed included RCTs for their risk of bias and rated the certainty of evidence using GRADE methodology, using pre‐determined inclusion and quality criteria.
Main results
We identified nine RCTs (629 participants). Studies explored oral nutritional interventions as follows: a protein (20 g protein per 200 mL bottle), 1 kcal/mL ready‐to‐drink, nutritional supplement with added vitamins, minerals and trace elements; arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement; 220 mg zinc sulphate supplements; 250 mg magnesium oxide supplements; 1000 mg/day omega‐3 fatty acid from flaxseed oil; 150,000 IU of vitamin D, versus 300,000 IU of vitamin D; 250 mg magnesium oxide plus 400 IU vitamin E and 50,000 IU vitamin D supplements. The comparator in eight studies was placebo, and in one study a different dose of vitamin D.
Eight studies reported the primary outcome measure of ulcer healing; only two studies reported a measure of complete healing. Six further studies reported measures of change in ulcer dimension, these studies reported only individual parameters of ulcer dimensions (i.e. length, width and depth) and not change in ulcer volume.
All of the evidence identified was very low certainty. We downgraded it for risks of bias, indirectness and imprecision.
It is uncertain whether oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements, increases the proportion of ulcers healed at six months more than placebo (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.42 to 1.53). It is also uncertain whether arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement increases the proportion of ulcers healed at 16 weeks compared with placebo (RR 1.09, 95% CI 0.85 to 1.40).
It is uncertain whether the following interventions change parameters of ulcer dimensions over time when compared with placebo; 220 mg zinc sulphate supplement containing 50 mg elemental zinc, 250 mg magnesium oxide supplement, 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement, magnesium and vitamin E co‐supplementation and vitamin D supplementation. It is also uncertain whether 150,000 IU of vitamin D, impacts ulcer dimensions when compared with 300,000 IU of vitamin D.
Two studies explored some of the secondary outcomes of interest for this review. It is uncertain whether oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements, reduces the number of deaths (RR 0.96, 95% CI 0.06 to 14.60) or amputations (RR 4.82, 95% CI 0.24 to 95.88) more than placebo. It is uncertain whether arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement increases health‐related quality of life at 16 weeks more than placebo (MD −0.03, 95% CI −0.09 to 0.03). It is also uncertain whether arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement reduces the numbers of new ulcers (RR 1.04, 95% CI 0.71 to 1.51), or amputations (RR 0.66, 95% CI 0.16 to 2.69) more than placebo.
None of the included studies reported the secondary outcomes cost of intervention, acceptability of the intervention (or satisfaction) with respect to patient comfort, length of patient hospital stay, surgical interventions, or osteomyelitis incidence.
One study exploring the impact of arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement versus placebo did not report on any relevant outcomes.
Authors' conclusions
Evidence for the impact of nutritional interventions on the healing of foot ulcers in people with diabetes compared with no nutritional supplementation, or compared with a different dose of nutritional supplementation, remains uncertain, with eight studies showing no clear benefit or harm. It is also uncertain whether there is a difference in rates of adverse events, amputation rate, development of new foot ulcers, or quality of life, between nutritional interventions and placebo. More research is needed to clarify the impact of nutritional interventions on the healing of foot ulcers in people with diabetes.
Keywords: Female; Humans; Male; Middle Aged; Arginine; Arginine/administration & dosage; Diabetic Foot; Diabetic Foot/diet therapy; Dietary Proteins; Dietary Proteins/administration & dosage; Dietary Supplements; Fatty Acids, Omega-3; Fatty Acids, Omega-3/administration & dosage; Glutamine; Glutamine/administration & dosage; Magnesium; Magnesium/administration & dosage; Magnesium Oxide; Magnesium Oxide/administration & dosage; Minerals; Minerals/administration & dosage; Randomized Controlled Trials as Topic; Trace Elements; Trace Elements/administration & dosage; Valerates; Valerates/administration & dosage; Vitamins; Vitamins/administration & dosage; Wound Healing; Zinc Sulfate; Zinc Sulfate/administration & dosage
Plain language summary
Dietary supplements for treating foot ulcers in people with diabetes
What is the aim of this review?
We wanted to find out whether nutritional supplements or special diets are effective in treating foot ulcers in people with diabetes. Researchers from Cochrane collected and analysed all relevant studies (randomised controlled trials (RCTs)) to answer this question and found nine studies for inclusion. RCTs are medical studies where the treatment or care people receive is are chosen at random. This type of trial provides the most reliable health evidence about whether different approaches to treatment or care make a difference.
Key messages
Of the nine studies that we identified, eight reported the outcomes we were interested in, primarily impact on ulcer healing. Findings from five studies showed very low‐certainty evidence regarding the effect of oral nutritional supplements in tablet form on the healing of foot ulcers in people with diabetes. These five studies did not measure healing in such a way that we could be certain of the results, and they did not have enough participants for us to be certain of the effects. The results of three other studies also showed very low‐certainty evidence as to whether nutritional supplements in other forms have any impact on ulcer healing. Two of these studies showed very low‐certainty evidence as to whether nutritional supplement drinks have any impact on other outcomes such as death, likelihood of amputation, reduction in numbers of new ulcers, or people's quality of life. These studies were not well conducted and did not have enough participants involved for us to be certain of the effects.
What was studied in the review?
People with diabetes can develop foot ulcers. These are often due to reduced blood supply, reduced sensation, foot deformity, the presence of trauma, or a combination of all or some of these causes. Foot ulcers are a serious complication of diabetes and can result in serious consequences such as amputation.
It is thought that foot ulcers, like other wounds, heal better, and more quickly, if people are well‐nourished. Food supplements containing certain vitamins and protein can be given to people with foot ulcers and diabetes to help to treat their wounds.
What are the main results of the review?
We found nine relevant studies dating from 2004 to 2019, involving 629 participants, 72% were men, aged, on average, 59.2 years. Most studies took place in hospital outpatient clinics. Three studies explored a different nutritional supplement drink and compared this with a drink that looked the same but did not have any added nutritional supplement. Five studies explored the effects of different types of nutritional tablets and compared these with tablets that did not contain any active ingredient, or nutritional supplement. One study compared two different doses of a vitamin D injection. One study did not report any of the outcomes of interest for this review.
Two of the studies were sponsored by the manufacturers of the nutritional supplement, five studies were sponsored by Iranian university research funding.
Findings from eight studies are unclear as to whether nutritional interventions improve the healing of foot ulcers in people with diabetes compared with no nutritional supplementation, or compared with a different dose of nutritional supplementation. One study reported adverse events and two studies reported numbers of amputations. Results are unclear as to whether there is a difference in the numbers of amputations or deaths between nutritional supplementation and no nutritional supplementation. It is also unclear if there is a difference in health‐related quality of life or number of ulcers that recur between nutritional supplementation and no nutritional supplementation.
Overall, we judged the certainty of the evidence to be very low. None of the studies had enough participants, five did not measure outcomes in such a way that we could be certain of the results and the studies were not well conducted, so we are not very confident in the results. Additional studies at low risk of bias and of high‐certainty evidence are needed to clarify the role of nutritional interventions for the treatment of foot ulcers in people with diabetes.
How up to date is this review?
We searched for studies that had been published up to March 2020.
Summary of findings
Summary of findings 1. Oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements versus placebo for treating foot ulcers in people with diabetes.
| Oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements versus placebo for treating foot ulcers in people with diabetes | ||||||
|
Patient or population: people with diabetes and foot ulcers
Settings: diabetic foot care clinic at the department of internal medicine
Intervention: oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oral nutritional supplement | |||||
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time) |
Not reported | |||||
| Ulcer healing (proportion of ulcers healed) | Study population | RR 0.80 (0.42 to 1.53) | 53 participants (1 study) | ⊕⊝⊝⊝ Very lowa | 10/27 (37%) participants in the oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements group healed at 6 months compared with 12/26 (46%) participants in the placebo group. It is uncertain whether oral nutritional supplement increases the proportion of ulcers healed at 6 months more than placebo, because the certainty of the evidence is very low. | |
| 462 per 1000 | 369 per 1000 | |||||
| Quality of life | Not reported | |||||
| Adverse events (death) | Study population |
RR 0.96 (0.06 to 14.60) |
53 participants (1 study) | ⊕⊝⊝⊝ Very lowb | 1/27 (3%) participants in the oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements group died within the 6 months' follow‐up, and 1/26 (3%) participants in the placebo group died. It is uncertain whether oral nutritional supplement reduces the number of adverse events (deaths) more than placebo, because the certainty of the evidence is very low. | |
| 38 per 1000 | 37 per 1000 | |||||
| Development of any new foot ulcers | Not reported | |||||
| Amputation rate | Study population | RR 4.82 (0.24 to 95.88) | 53 participants (1 study) | ⊕⊝⊝⊝ Very lowc | 2/27 (7%) participants in the oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements group had an amputation within the 6 months' follow‐up, compared with none (0/26; 0%) of the participants in the placebo group. It is uncertain whether oral nutritional supplement reduces the number of amputations more than placebo, because the certainty of the evidence is very low. | |
| 0 per 1000 | 74 per 1000 | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the mean risk in the intervention group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for high risk of bias due to baseline incomparability; downgraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and two levels for very serious imprecision due to small sample size and wide confidence intervals.
bDowngraded one level for high risk of bias due to baseline incomparability; downgraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and two levels for very serious imprecision due to small sample size and wide confidence intervals.
cDowngraded one level for high risk of bias due to baseline incomparability; downgraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and two levels for very serious imprecision due to small sample size and wide confidence intervals.
Summary of findings 2. Arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement versus placebo for treating foot ulcers in people with diabetes.
| Arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement versus placebo for treating foot ulcers in people with diabetes | ||||||
|
Patient or population: people with diabetes and foot ulcers
Settings: individuals from 38 hospital and wound care centres
Intervention: arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement | |||||
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time) |
Not reported | |||||
|
Ulcer healing (proportion of ulcers healed) |
Study population |
RR 1.09 (0.85 to 1.40) |
270 participants (1 study) | ⊕⊝⊝⊝ Very lowa | 65/129 (50%) participants in the arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement group healed, compared with 65/141 (46%) participants in the placebo group. It is uncertain whether arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement increases the proportion of ulcers healed at 16 weeks compared with placebo, because the certainty of the evidence is very low. | |
| 461 per 1000 | 502 per 1000 | |||||
| Quality of life | Mean score: 0.76 ± 0.23 | Mean score: 0.73 ± 0.20 The mean health‐related quality of life in the intervention group was 0.00 higher (0.09 lower to 0.03 higher) |
MD −0.03 (−0.09 to 0.03) | 270 participants (1 study) | ⊕⊝⊝⊝ Very lowb | DFS‐SF scale 0‐100: higher scores = better health‐related quality of life In the arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement group the mean score was 0.73 ± 0.20, the mean score in the placebo group was 0.76 ± 0.23. It is uncertain whether arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement increases quality of life compared with placebo, because the certainty of the evidence is very low. |
| Adverse events | Not reported | |||||
| Development of any new foot ulcers | Study population | RR 1.04 (0.71 to 1.51 | 270 participants (1 study) | ⊕⊝⊝⊝ Very lowc | 38/129 (29.5%) participants in the arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement group developed a new ulcer compared with 40/141 (28.4%) in the placebo group. It is uncertain whether arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement reduces the number of new ulcers that develop compared with placebo, because the certainty of the evidence is very low. | |
| 284 per 1000 | 295 per 1000 | |||||
| Amputation rate | Study population | RR 0.66 (0.16 to 2.69) | 270 participants (1 study) | ⊕⊝⊝⊝ Very lowd | 3/129 (2.3%) participants in the arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement group underwent an amputation, compared with 5/141 (3.5%) in the placebo group. It is uncertain whether arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement decreases the number of amputations compared with placebo, because the certainty of the evidence is very low. | |
| 35 per 1000 | 23 per 1000 | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the mean risk in the intervention group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DFS‐SF: Diabetic Foot Ulcer Scale ‐ Short Form; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and one level for imprecision due to wide confidence intervals.
bDowngraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and one level for imprecision due to wide confidence intervals.
cDowngraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and one level for imprecision due to wide confidence intervals.
dDowngraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and one level for imprecision due to wide confidence intervals.
Summary of findings 3. 220 mg zinc sulphate supplement containing 50 mg elemental zinc versus placebo for treating foot ulcers in people with diabetes.
| 220 mg zinc sulphate supplements containing 50 mg elemental zinc versus placebo for treating foot ulcers in people with diabetes | ||||||
|
Patient or population: people with diabetes and foot ulcers
Settings: hospital clinic
Intervention: 220 mg zinc sulphate supplement containing 50 mg elemental zinc Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | 220 mg zinc sulphate supplement containing 50 mg elemental zinc | |||||
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound length reduction) |
Mean wound length reduction: −0.9 ± 1.2 | Mean wound length reduction: −1.5 ± 0.7 | MD −0.60 (−1.10 to −0.10) | 60 (1 study) | ⊕⊝⊝⊝ Very lowa | Mean wound length reduced by −1.5 ± 0.7 in the 220 mg zinc sulphate supplement containing 50 mg elemental zinc group, and −0.9 ± 1.2 in the placebo group (MD −0.60, 95% CI −1.10 to −0.10). It is uncertain whether 220 mg zinc sulphate supplement containing 50 mg elemental zinc increases the percentage change in wound length over time, because the certainty of the evidence is very low. |
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound depth reduction) |
Mean wound depth reduction: −0.3 ± 1.0 | Mean wound depth reduction: −0.8 ± 0.6 | MD −0.50 (−0.92 to −0.08) | 60 (1 study) | ⊕⊝⊝⊝ Very lowb | Mean wound depth reduced by −0.8 ± 0.6 in the 220 mg zinc sulphate supplement containing 50 mg elemental zinc group, and −0.3 ± 1.0 in the placebo group (MD −0.50, 95% CI −0.92 to −0.08). It is uncertain whether 220 mg zinc sulphate supplement containing 50 mg elemental zinc increases the percentage change in wound depth over time, because the certainty of the evidence is very low. |
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound width reduction) |
Mean wound width reduction:−0.8 ± 1.0 | Mean wound width reduction: −1.4 ± 0.8 | MD −0.60 (−1.06 to −0.14) | 60 (1 study) | ⊕⊝⊝⊝ Very lowc | Mean wound width reduced by −1.4 ± 0.8 in the 220 mg zinc sulphate supplement containing 50 mg elemental zinc group, and −0.8 ± 1.0 in the placebo group (MD −0.60, 95% CI −1.06 to −0.14). It is uncertain whether 220 mg zinc sulphate supplement containing 50 mg elemental zinc increases the percentage change in wound width over time, because the certainty of the evidence is very low. |
|
Ulcer healing (proportion of ulcers healed) |
Not reported | |||||
| Quality of life | Not reported | |||||
| Adverse events | Not reported | |||||
| Development of any new foot ulcers | Not reported | |||||
| Amputation rate | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
bDowngraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
cDowngraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
Summary of findings 4. 250 mg magnesium oxide supplement versus placebo for treating foot ulcers in people with diabetes.
| 250 mg magnesium oxide supplement versus placebo for treating foot ulcers in people with diabetes | ||||||
|
Patient or population: people with diabetes and foot ulcers
Settings: hospital clinic
Intervention: 250 mg magnesium oxide supplement Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | 250 mg magnesium oxide supplement | |||||
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound length reduction) |
Mean wound length reduction: −0.9 ± 1.1 | Mean wound length reduction:−1.8 ± 2.0 | MD −0.90 (−1.66 to −0.14) | 70 (1 study) | ⊕⊝⊝⊝ Very lowa | Mean wound length reduced by −1.8 ± 2.0 in the 250 mg magnesium oxide supplement group, and −0.9 ± 1.1 in the placebo group (MD −0.90, 95% CI −1.66 to −0.14). It is uncertain whether 250 mg magnesium oxide supplement increases the percentage change in wound length over time, because the certainty of the evidence is very low. |
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound depth reduction) |
Mean wound depth reduction: −0.3 ± 0.5 | Mean wound depth reduction: −0.8 ± 0.8 | MD −0.50 (−0.81 to −0.19) | 70 (1 study) | ⊕⊝⊝⊝ Very lowb | Mean wound depth reduced by −0.8 ± 0.8 in the 250 mg magnesium oxide supplement group, and −0.3 ± 0.5 in the placebo group (MD −0.50, 95% CI −0.81 to −0.19). It is uncertain whether 250 mg magnesium oxide supplement increases the percentage change in wound depth over time, because the certainty of the evidence is very low. |
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound width reduction) |
Mean wound width reduction: −0.8 ± 0.9 | Mean wound width reduction: −1.6 ± 2.0 | MD −0.80 (−1.53 to −0.07) | 70 (1 study) | ⊕⊝⊝⊝ Very lowc | Mean wound width reduced by −1.6 ± 2.0 in the 250 mg magnesium oxide supplement group, and −0.8 ± 0.9 in the placebo group (MD −0.80, 95% CI −1.53 to −0.07). It is uncertain whether 250 mg magnesium oxide supplement increases the percentage change in wound width over time, because the certainty of the evidence is very low. |
|
Ulcer healing (proportion of ulcers healed) |
Not reported | |||||
| Quality of life | Not reported | |||||
| Adverse events | Not reported | |||||
| Development of any new foot ulcers | Not reported | |||||
| Amputation rate | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
bDowngraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
cDowngraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
Summary of findings 5. 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement versus placebo for treating foot ulcers in people with diabetes.
| 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement versus placebo for treating foot ulcers in people with diabetes | ||||||
|
Patient or population: people with diabetes and foot ulcers
Settings: hospital clinic
Intervention: 1000 mg/day omega‐3 fatty acid from flaxseed oil supplements Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement | |||||
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound length reduction) |
Mean wound length reduction: −1.0 ± 1.1 | Mean wound length reduction: −2.1 ± 2.3 | MD −1.00 (−1.91 to −0.09) | 60 (1 study) | ⊕⊝⊝⊝ Very lowa | Mean wound length reduced by −2.1 ± 2.3 in the 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement group, and −1.0 ± 1.1 in the placebo group (MD −1.00, 95% CI −1.91 to −0.09). It is uncertain whether 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement increases the percentage change in wound length over time, because the certainty of the evidence is very low. |
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound depth reduction) |
Mean wound depth reduction: −0.50 ± 0.50 | Mean wound depth reduction: −0.80 ± 0.60 | MD −0.30 (−0.58 to −0.02) | 60 (1 study) | ⊕⊝⊝⊝ Very lowb | Mean wound depth reduced by −0.80 ± 0.60 in the 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement group, and −0.50 ± 0.50 in the placebo group (MD −0.30, 95% CI −0.58 to −0.02). It is uncertain whether 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement increases the percentage change in wound depth over time, because the certainty of the evidence is very low. |
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound width reduction) |
Mean wound width reduction: −1.0 ± 1.0 | Mean wound width reduction: −1.8 ± 1.7 | MD −0.80 (−1.51 to −0.09) | 60 (1 study) | ⊕⊝⊝⊝ Very lowc | Mean wound width reduced by −1.8 ± 1.7 in the 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement group, and −1.0 ± 1.0 in the placebo group (MD −0.80, 95% CI −1.51 to −0.09). It is uncertain whether 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement increases the percentage change in wound width over time, because the certainty of the evidence is very low. |
|
Ulcer healing (proportion of ulcers healed) |
Not reported | |||||
| Quality of life | Not reported | |||||
| Adverse events | Not reported | |||||
| Development of any new foot ulcers | Not reported | |||||
| Amputation rate | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
bDowngraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
cDowngraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
Summary of findings 6. 150,000 IU of vitamin D versus 300,000 IU of vitamin D for treating foot ulcers in people with diabetes.
| 150,000 IU of vitamin D versus 300,000 IU of vitamin D for treating foot ulcers in people with diabetes | ||||||
|
Patient or population: people with diabetes and foot ulcers
Settings: hospital clinic
Intervention: 150,000 IU of vitamin D Comparison: 300,000 IU of vitamin D | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 150,000 IU of vitamin D | 300,000 IU of vitamin D | |||||
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound area) |
Mean wound area: 5.84 ± 0.97 | Mean wound area: 5.23 ± 1.29 |
MD: 0.61 (−0.04 to 1.26) | 47 (1 study) | ⊕⊝⊝⊝ Very lowa | Mean wound area was 5.23 ± 1.29 in the 300,000 IU of vitamin D group and 5.84 ± 0.97 in the 150,000 IU of vitamin D group (MD 0.61, 95% CI −0.04 to 1.26). It is uncertain whether 150,000 IU of vitamin D when compared with 300,000 IU of vitamin D increases the percentage change in mean wound area over time, because the certainty of the evidence is very low. |
|
Ulcer healing (proportion of ulcers healed) |
Not reported | |||||
| Quality of life | Not reported | |||||
| Adverse events | Not reported | |||||
| Development of any new foot ulcers | Not reported | |||||
| Amputation rate | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to a high risk of attrition bias; downgraded one level for indirectness because baseline nutritional status of participants was very poorly reported and two levels for imprecision because of the small sample size and wide confidence intervals.
Summary of findings 7. Magnesium and vitamin E co‐supplementation versus placebo for treating foot ulcers in people with diabetes.
| Magnesium and vitamin E co‐supplementation versus placebo for treating foot ulcers in people with diabetes | ||||||
|
Patient or population: people with diabetes and foot ulcers
Settings: hospital clinic
Intervention: magnesium and vitamin E co‐supplementation Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Magnesium and vitamin E co‐supplementation | |||||
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound length) |
Mean wound length: 2.3 ± 1.3 |
Mean wound length: 1.6 ± 1.10 |
MD −0.70 (−1.33 to −0.07) | 57 (1 study) | ⊕⊝⊝⊝ Very lowa | Mean wound length was 1.6 ± 1.10 in the magnesium and vitamin E co‐supplementation group and 2.3 ± 1.3 in the placebo group (MD −0.70, 95% CI −1.33 to −0.07). It is uncertain whether magnesium and vitamin E co‐supplementation increases the percentage change in mean wound length over time, because the certainty of the evidence is very low. |
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound depth) |
Mean wound depth: 0.90 ± 0.50 |
Mean wound depth: 0.40 ± 0.30 |
MD −0.50 (−0.71 to −0.29 ) |
57 (1 study) | ⊕⊝⊝⊝ Very lowb | Mean wound depth was 0.40 ± 0.30 in the magnesium and vitamin E co‐supplementation group and 0.90 ± 0.50 in the placebo group (MD −0.50, 95% CI −0.71 to −0.29). It is uncertain whether magnesium and vitamin E co‐supplementation increases the percentage change in mean wound depth over time, because the certainty of the evidence is very low. |
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound width) |
Mean wound width: 1.8 ± 1.0 |
Mean wound width: 1.2 ± 0.90 |
MD −0.60 (−1.09 to −0.11) |
57 (1 study) | ⊕⊝⊝⊝ Very lowc | Mean wound width was 1.2 ± 0.90 in the magnesium and vitamin E co‐supplementation group and 1.8 ± 1.0 in the placebo group (MD −0.60, 95% CI −1.09 to −0.11). It is uncertain whether magnesium and vitamin E co‐supplementation increases the percentage change in mean wound width over time, because the certainty of the evidence is very low. |
|
Ulcer healing (proportion of ulcers healed) |
Not reported | |||||
| Quality of life | Not reported | |||||
| Adverse events | Not reported | |||||
| Development of any new foot ulcers | Not reported | |||||
| Amputation rate | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for high risk of attrition bias. Downgraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
bDowngraded one level for high risk of attrition bias. Downgraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
cDowngraded one level for high risk of attrition bias. Downgraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
Summary of findings 8. Vitamin D versus placebo for treating foot ulcers in people with diabetes.
| Vitamin D versus placebo for treating foot ulcers in people with diabetes | ||||||
|
Patient or population: people with diabetes and foot ulcers
Settings: hospital clinic
Intervention: vitamin D Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin D | |||||
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound length reduction |
Mean wound length reduction: −1.1 ± 0.20 | Mean wound length reduction: −2.1 ± 0.20 | MD −1.00 (−1.10 to −0.90) | 60 (1 study) | ⊕⊝⊝⊝ Very lowa | Mean wound length reduced by −2.1 ± 0.20 in the vitamin D group, and −1.1 ± 0.20 in the placebo group (MD −1.00, 95% CI −1.10 to −0.90). It is uncertain whether vitamin D increases the percentage change in wound length over time, because the certainty of the evidence is very low. |
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound depth reduction |
Mean wound depth reduction:−0.50 ± 0.10 | Mean wound depth reduction: −1.0 ± 0.10 | MD −0.50 (−0.55 to −0.45) | 60 (1 study) | ⊕⊝⊝⊝ Very lowb | Mean wound depth reduced by −1.0 ± 0.10 in the vitamin D group, and −0.50 ± 0.1 in the placebo group (MD −0.5, 95% CI −0.55 to −0.45). It is uncertain whether vitamin D increases the percentage change in wound depth over time, because the certainty of the evidence is very low. |
|
Ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound width reduction |
Mean wound width reduction: −1.1 ± 0.20 | Mean wound width reduction: −1.9 ± 0.2 | MD −0.80 (−0.90 to −0.70) | 60 (1 study) | ⊕⊝⊝⊝ Very lowc | Mean wound width reduced by −1.9 ± 0.20 in the vitamin D group, and −1.1 ± 0.20 in the placebo group (MD −0.80, 95% CI −0.90 to −0.70). It is uncertain whether vitamin D increases the percentage change in wound width over time, because the certainty of the evidence is very low. |
|
Ulcer healing (proportion of ulcers healed) |
Not reported | |||||
| Quality of life | Not reported | |||||
| Adverse events | Not reported | |||||
| Development of any new foot ulcers | Not reported | |||||
| Amputation rate | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
bDowngraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
cDowngraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged.
Background
Description of the condition
The International Diabetes Federation estimates that in 2017, 451 million adults worldwide had diabetes, with projections of 693 million cases by 2045. In high‐income countries, approximately 87% to 91% of all people with diabetes are estimated to have type 2 diabetes, 7% to 12% are estimated to have type 1 diabetes and 1% to 3% to have other types of diabetes (IDF 2017).
Foot ulcers in people with diabetes are non‐healing or poorly healing, partial or full‐thickness wounds below the ankle (Lavery 2008; Sanders 2015). People with diabetes may have either neuropathic (an abnormal or degenerative state of the nervous system or nerves), arterial, or venous components to their ulcer, or a combination of all three (Ackerman 2013). Long‐standing hyperglycaemia (high blood glucose level) results in nerve damage associated with autonomic (relating to, affecting, or controlled by the autonomic nervous system (Merriam‐Webster 2016)), sensory (relating to sensation or the senses (Merriam‐Webster 2016)) and motor neuropathy (relating to, concerned with, or involving muscular movement (Merriam‐Webster 2016)). Sensory neuropathy causes a loss of pain sensation; autonomic neuropathy can cause either anhydrosis (dry skin), or hyperhidrosis (excessive sweat), both of which affect skin quality; and motor neuropathy causes weakness of muscles and structural changes to the foot (Ackerman 2013).
Peripheral vascular disease (PVD) is the narrowing of the arteries and veins in the legs and is common in people with diabetes, with atherosclerosis (atheromatous deposits in the arteries) caused by hypertension, which is abnormally high arterial blood pressure that is usually indicated by an adult systolic blood pressure of 140 mm Hg or greater or a diastolic blood pressure of 90 mm Hg or greater (Merriam‐Webster 2016), being the most common cause of PVD (Marcovitch 2017).
In people with diabetes, a combination of PVD and neuropathy dramatically increases the likelihood of the development of a foot ulcer (Armstrong 2011). The landmark United Kingdom Prospective Diabetes Survey (UK PDS 1998) found in their large, multicentre study of people with newly diagnosed type 2 diabetes that 10% had some level of neuropathy and vascular disease on diagnosis, however it was not stated whether this is common in the general population, or is specifically related to those with diabetes.
Foot ulcers in people with diabetes can occur regardless of the type of diabetes; type 1 diabetes is caused by an absolute deficiency of insulin secretion and type 2 is caused by a combination of resistance to insulin action and an inadequate compensatory insulin secretory response (ADA 2008). Global prevalence of foot ulcers in people with diabetes has been estimated at 6.3% (95% confidence interval (CI) 5.4% to 7.3%), and is higher in men (4.5%, 95% CI 3.7% to 5.2%) than in women (3.5%, 95% CI 2.8% to 4.2%), and higher in people with type 2 diabetes (6.4%, 95% CI 4.6% to 8.1%) than in people with type 1 diabetes (5.5%, 95% CI 3.2% to 7.7%; Zhang 2017). Diabetes is the leading cause of non‐traumatic limb amputation in the world (Jupiter 2016). Within 18 months following amputation, almost 50% of people with diabetes will develop a foot ulcer on the other limb, and of these people, 58% have further amputations within three to five years. It is worthy of note that the three‐year mortality rate after the first amputation is between 20% and 50% (Fortington 2013).
In the UK, the mean NHS cost of wound care over 12 months was an estimated GBP 7800 per foot ulcer in a person with diabetes, ranging from GBP 2140 to GBP 8800 per healed and unhealed foot ulcer (Guest 2018). Globally in 2015, these figures were estimated to be USD 1.3 trillion. In the USA, almost one‐third of diabetes expenditure is on lower‐limb–related problems (Jeffcoate 2018).
Description of the intervention
Nutritional status is a dynamic entity reflecting physiological requirements, nutritional intake, body composition and function (BDA 2001). The presence of a wound has an impact on nutritional status due to the metabolic cost of repairing tissue damage, sepsis and nutrient losses via wound exudate (a fluid that has exuded out of a tissue or its capillaries due to injury or inflammation (Marcovitch 2017); BDA 2001). Thus, it is reasonable to assume that the nutritional status of the person with a diabetes and a foot ulcer may interfere with the healing process (Bowling 2004; Tatti 2012).
Methods to improve or maintain nutritional intake are known as nutritional support (NICE 2017), however, nutritional intervention, nutritional support, nutritional treatment and medical nutrition therapy are all interchangable terms for systematically attempting to improve a person's nutritional status.
NICE 2017 lists fortified food, additional snacks and sip feeds as methods of oral nutritional support. Some oral nutritional support products are nutritionally complete and can be taken to supplement the diet, or as a sole source of nutrition, however others only contain certain nutrients and are designed to supplement the diet (Bowling 2004). Standard oral nutritional support includes polymeric‐, peptide‐, or amino acid‐based (types of protein) supplements and also those where novel substrates have been added, such as glutamine (an amino acid synthesised within the body from glutamic acid and used in preventing immunosuppression after exercise and as an aid in recovery after a critical illness (Marcovitch 2017)), fish oils, arginine or antioxidants. We include nutrient‐based novel substrate in this review. We exclude prebiotics, probiotics and synbiotics as they are not nutrients. Schrezenmeir 2001 describes prebiotics, probiotics and synbiotics as non‐digestible food ingredients.
Categorisation of nutritional supports differ between NICE 2017, Bowling 2004 and BDA 2001, however, oral supplements, enteral and parenteral nutrition are the three common categories.
Enteral tube feeding is the delivery of a nutritionally complete feed via a tube into the stomach, duodenum or jejunum (NICE 2017).
Parenteral nutrition is the method of providing nutritional support to an individual whose gastrointestinal tract is not functioning or is inaccessible (BDA 2001). Nutrients are delivered directly into the circulatory system via a dedicated peripherally inserted central catheter (BDA 2001).
NICE 2017 advises the use of oral, enteral and parenteral nutrition alone, or in combination, for people who are either malnourished or at risk of malnutrition. However, Gottschlich 2001 argues that enteral nutrition is superior to parenteral nutrition, particularly during the early phase of wound healing.
How the intervention might work
Nutritional status may be an important predictor of wound healing (Hurd 2004; Leininger 2002; Medlin 2012). The importance of nutrition in wound healing in general is well founded in the literature (Frias Soriano 2004; Lee 2006; Medlin 2012; Omote 2005). Leininger 2002 states that the main goal of nutrition in wound healing is to provide optimum calories and nutrition to aid healing, however deficiencies in protein, albumin, vitamin D, vitamin C and zinc have all been demonstrated to decrease wound healing rates.
Hypomagnesemia (a deficiency of magnesium in the blood), is thought to contribute to development of neuropathy and abnormal platelet activity (Rodriguez‐Moran 2001), while the impact of vitamin D supplementations on wound healing arises due to its impact on stimulating phagocytosis (the process by which a cell uses its plasma membrane to engulf a large particle) and killing the bacteria by macrophages (large cells found in stationary form in the tissues, or as mobile white blood cells, especially at sites of infection; Van Etten 2004). Zinc contributes to the regulation of the different phases of wound healing including inflammation, angiogenesis and re‐epithelialisation (Lin 2017). In addition, vitamin E has been shown to improve healing through regulation of inflammation, in experimental animal studies (Shin 2017).
Nutrients are required for each phase of healing, for example, during the inflammatory phase, low serum albumin, the major circulating protein (Hurd 2004), will result in an inadequate inflammation resulting in impaired wound healing (Leininger 2002). Granulation tissue, which is formed during the proliferation stage, is largely composed of proteins of which collagen is in abundance. Indeed, collagen makes up 80% of the dry weight of the dermis and contributes to the wound's tensile strength (Martin 1992). Proteins and collagen are needed in the maturation stage to improve tissue strength (Medlin 2012). Wounds require between 1.5 g and 3 g per kg per day of protein to ensure tissue regeneration (Hurd 2004; Medlin 2012), which is up to three times the normal protein intake (Hurd 2004). However, it should be noted that people with diabetes, and especially those with renal damage, should confine their intake of protein to reduce proteinuria and improve the prognosis regarding diabetic nephropathy (an abnormal and usually degenerative state of the nervous system or nerves (Merriam‐Webster 2016); Zhang 2013). Excessive dietary intake of vitamin A has been associated with foetal malformation (Azaïs‐Braesco 2000; Rothman 1995). Therefore, it remains important that people with diabetes and foot ulcers receive adequate and correct nutrition in order to ensure successful closure of their foot ulcer, whilst having regard for the potential complications arising from the presence of diabetes itself. Further, in order to choose interventions effectively, appropriate assessment of the individual in terms of nutritional status and nutritional requirements is very important.
Why it is important to do this review
Altering nutritional intake has been shown in studies to improve wound healing in other wound types (Collins 2005; Ohura 2011). However, the precise role of nutrition in the treatment of foot ulcers in people with diabetes is as yet, unclear. Nutritional intervention may potentially improve clinical outcomes such as healing rates and healing times of foot ulcers in people with diabetes. The outcomes of this review may provide evidence to formulate such guidance, furthermore, this review may indicate areas for future research.
Objectives
To evaluate the effects of nutritional interventions on the healing of foot ulcers in people with diabetes.
Methods
Criteria for considering studies for this review
Types of studies
Published or unpublished randomised controlled trials (RCTs) and cluster‐RCTs were eligible for inclusion.
Types of participants
People of any age and sex, in any healthcare setting, with either type 1 or type 2 diabetes and an active foot ulcer.
Types of interventions
Intervention: nutritional supplementation (oral, enteral or parenteral nutrition) of any dose, or duration, or both, or special diet.
Comparison: comparisons between supplementary nutrition plus standard diet, versus standard diet alone, and between different types of supplementary nutrition (e.g. enteral versus parenteral).
Types of outcome measures
Primary outcomes
An objective measure of ulcer healing, such as:
time to complete healing;
absolute or percentage change in ulcer area, or volume, or individual parameters of ulcer dimensions over time i.e. changes in wound length, width and depth reported as separate measures (change from protocol, see Differences between protocol and review);
proportion of ulcers healed at the completion of the study period; and
healing rate at completion of the study.
Secondary outcomes
An objective measure of:
cost of intervention;
quality of life as measured by a validated scale;
acceptability of intervention (or satisfaction) with respect to patient comfort;
adverse events;
length of patient hospital stay;
development of any new foot ulcers;
amputation rate;
surgical interventions; and
osteomyelitis incidence.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
the Cochrane Wounds Specialised Register (searched 4 March 2020);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 2) in the Cochrane Library (searched 4 March 2020);
Ovid MEDLINE (1946 to 4 March 2020);
Ovid Embase (1974 to 4 March 2020);
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 4 March 2020).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision; Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL Plus searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2018). We did not impose any restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 4 March 2020);
World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/Default.aspx) (searched 4 March 2020).
Search strategies for clinical trials registries can be found in Appendix 1.
Searching other resources
We also searched the bibliographies and reference lists of all retrieved and relevant publications identified by these strategies for further studies. We contacted manufacturers of nutritional interventions used in the treatment of wounds and experts in the field to ask for information relevant to this review.
Data collection and analysis
Data collection and analysis were carried out according to methods stated in the published protocol (Corcoran 2014), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
We independently assessed titles and, where available, abstracts of the studies identified by the search strategy against the eligibility criteria for inclusion in the review. We obtained full versions of potentially relevant studies and two review authors independently screened these against the inclusion criteria. We resolved any differences in opinion by discussion and, where necessary, with reference to the Cochrane Wounds editorial base.
Data extraction and management
We extracted data from each article using a standardised data extraction sheet. We independently extracted data from eligible studies. Specifically, we extracted the following:
author;
title;
source;
date of study;
duration of study;
geographical location of study;
care setting:
inclusion/exclusion criteria;
sample size;
patient characteristics;
balance of groups at baseline;
study design details;
sources of funding;
study type;
method of randomisation;
allocation of concealment;
concurrent interventions;
wound status/category at baseline;
wound duration;
intervention details including type, dosage and duration;
control intervention details;
compliance;
outcome measures;
blinding (both patient and professional);
length of follow‐up;
loss to follow‐up;
results;
intention‐to‐treat analysis;
conclusions reported by study authors.
We resolved disagreements by discussion or, where necessary, with reference to the Cochrane Wounds editorial base. We entered and combined the data using Review Manager 5 (RevMan 5) software (Review Manager 2014).
Assessment of risk of bias in included studies
We independently assessed the included studies using the Cochrane tool for assessing risk of bias (Higgins 2017). This tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete data, selective outcome reporting and other issues (Appendix 2). We assessed blinding and completeness of outcome data for each outcome separately. We presented our assessment of risk of bias using two 'Risk of bias' summary figures; one of which is a summary of bias for each item across all studies, and the second shows a cross‐tabulation of each study by all of the 'Risk of bias' items. For studies using cluster randomisation, we would have assessed the risk of bias using the following domains: recruitment bias, baseline imbalance, loss of clusters, incorrect analysis and comparability with individually randomised trials (Higgins 2011b).
Measures of treatment effect
For dichotomous outcomes, we calculated the risk ratio (RR) with 95% confidence intervals (CIs). Risk ratio is the rate of the event of interest (e.g. wound healed) in the experimental group divided by the rate of this event in the control group and indicates the chances of wound healing for people in the experimental group compared with the control group. An RR of 1 means there is no difference in risk between the two study groups, an RR of less than 1 means the event is less likely to occur in the experimental group than in the control group and an RR of more than 1 means the event is more likely to occur in the experimental group than in the control group (Deeks 2017). For continuously distributed outcome data, we used the mean difference (MD) with 95% CIs, if all studies used the same assessment scale. The mean difference estimates the amount by which the experimental intervention changes the outcome on average compared with the control (Deeks 2017). If studies used different assessment scales, we planned to use the standardised mean difference (SMD) with 95% CIs. We planned to report time‐to‐event data (e.g. time to complete wound healing) as hazard ratios (HRs) where possible, in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
Unit of analysis issues
Unit of analysis issues may have arisen from studies including participants with diabetes and multiple foot ulcers, or in studies with individuals who were followed up and experienced recurrence of foot ulcers. We planned to record whether studies presented outcomes in relation to a wound, a foot, a participant or as multiple wounds on the same participant. We also planned to record occasions where multiple wounds on a participant were (incorrectly) treated as independent within a study, rather than having within‐patient analysis methods applied. This would have been recorded as part of the risk of bias assessment. For wound healing and amputation, unless otherwise stated, where the number of wounds appeared to equal the number of participants, we planned to treat the wound as the unit of analysis. We planned to combine studies with multiple intervention groups into one group to create a simple pair‐wise comparison, however if there was no common effect between intervention groups, we planned to split the control group into two or more groups according to the number of intervention groups (Higgins 2011b). Where possible, we planned to carry out a meta analysis of effect estimates and their standard errors from correct analyses of cluster‐randomised trials using the generic inverse‐variance method in RevMan 5 (Review Manager 2014). Where a cluster‐randomised trial had been analysed on individuals rather than the clusters, we planned to approximate the correct analyses if possible using information on:
the number of clusters (or groups) randomised to each intervention group, or the average (mean) size of each cluster;
the outcome data ignoring the cluster design for the total number of individuals (e.g. number or proportion of individuals with events, or means and standard deviations);
and an estimate of the intracluster (or intraclass) correlation coefficient (ICC).
Dealing with missing data
It is common to have data missing from study reports. Excluding participants post‐randomisation from the analysis, or ignoring those participants who are lost to follow‐up, compromises the randomisation, and potentially introduces bias into the study. In individual studies, where data on the proportion of ulcers healed were presented, we planned to assume that if randomised participants were not included in an analysis, their wound did not heal (i.e. they would have been considered in the denominator but not the numerator). Where a study did not specify participant group numbers prior to drop out, we planned to present only complete case data. In a time‐to‐healing analysis using survival analysis methods, we planned to account for dropouts as censored data. Hence all participants would have contributed to the analysis. Such analysis would assume that dropouts were missing at random (i.e. not associated with time to healing). We planned to present data for area change, and for all secondary outcomes, as a complete case analysis.
Assessment of heterogeneity
We planned to assess clinical heterogeneity by examining potential influencing factors (e.g. care setting or wound stage). We planned to assess statistical heterogeneity using I2 statistic (Higgins 2003). This examines the percentage of total variation across studies due to heterogeneity rather than chance. I2 statistic values over 75% indicate a high level of heterogeneity. We planned to carry out statistical pooling on groups of studies that we considered to be sufficiently similar. Where heterogeneity was absent or low (I2 = 0% to 25%) we planned to use a fixed‐effect model. If there was evidence of heterogeneity (I2 greater than 25%), we planned to use a random‐effects model. If heterogeneity was very high (I2 greater than 75%) we planned not to pool the data.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. Publication bias is one of a number of possible causes of 'small study effects’, that is, a tendency for estimates of the intervention effect to be more beneficial in smaller RCTs. Funnel plots allow a visual assessment of whether small study effects may be present in a meta analysis. A funnel plot is a simple scatter plot of the intervention effect estimates from individual RCTs against some measure of each study’s size or precision (Sterne 2017). We planned to present funnel plots for meta‐analyses comprising 10 RCTs or more using RevMan 5 (Review Manager 2014).
Data synthesis
We combined details of included studies in a narrative review according to type of comparator and the time point of the outcome measurement. We planned to consider clinical and methodological heterogeneity and would have undertaken pooling only when studies appeared appropriately similar in terms of population, intervention type, duration of follow‐up and outcome type.
We were unable to pre specify the amount of clinical, methodological and statistical heterogeneity in included studies but it might have been extensive. Thus, we anticipated using a random‐effects approach for meta‐analysis. Conducting meta‐analysis with a fixed‐effect model in the presence of even minor heterogeneity may provide overly narrow confidence intervals. We would only have used a fixed‐effect approach when we assessed clinical and methodological heterogeneity to be minimal, and the assumption that a single underlying treatment effect was being estimated held. We would have used the Chi2 test and I2 statistic to quantify heterogeneity but we would not have used the results of this to guide choice of model for meta‐analysis. We would have exercised caution when meta‐analysed data were at risk of small study effects, because a random‐effects model may be unsuitable. In this case, or where there were other reasons to question the selection of a fixed‐effect or random‐effects model, we would have assessed the impact of the approach using sensitivity analyses to compare results from alternate models (Thompson 1999).
We have presented data using forest plots where possible. For dichotomous outcomes, we have presented the summary estimate as a RR with 95% CI. Where continuous outcomes had been measured in the same way across studies, we planned to present a pooled MD with 95% CI. We planned to pool SMD estimates where studies measured the same outcome using different methods. For time‐to‐event data, we planned to plot (and, if appropriate, pool) estimates of HRs and 95% CIs as presented in the study reports using the generic inverse‐variance method in RevMan 5 (Review Manager 2014). Where studies analysed time to healing as a continuous measure, but it was not clear if all wounds healed, we planned to document use of the outcome in the study but we would have not summarised or used data in any meta‐analysis. We would have pooled estimates of treatment effect using RevMan 5 (Review Manager 2014).
Summary of findings and GRADE assessment of the certainty of the evidence
We have presented the main results of this review in 'Summary of findings' tables. These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined and the sum of the available data for the main outcomes (Schünemann 2017a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE approach (Schünemann 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2017b).
We have presented the following primary outcomes in the 'Summary of findings' tables:
absolute or percentage change in ulcer area, or volume, or individual parameters of changes in ulcer dimensions (change from protocol, see Differences between protocol and review);
proportion of ulcers healed at the completion of the study period.
We have also presented the following secondary outcomes in the 'Summary of findings' tables:
quality of life as measured by a validated scale;
adverse events;
development of any new foot ulcers;
amputation rate.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available we planned to undertake the following subgroup analyses:
type of setting (community, hospital, inpatient, outpatient);
type of intervention (oral, enteral, parenteral).
Sensitivity analysis
We planned to perform a sensitivity analysis by excluding studies at high or unclear risk of bias. In this sensitivity analysis, we planned to only include studies that we had assessed as having a low risk of bias in all key domains, namely adequate generation of the randomisation sequence, adequate allocation concealment and blinding of outcome assessor, for the estimates of treatment effect. For clearly understandable reasons we could not perform planned subgroup/sensitivity analyses.
Results
Description of studies
Results of the search
The search identified 270 database records and 165 clinical trial registry records. Following exclusion of duplicates, 222 abstracts remained. These abstracts underwent independent review by two review authors. Eight studies: ACTRN12612000036819; IRCT20100102002954N12; IRCT2015041321740N1; IRCT201506215623N46; IRCT2017090533941N21; NCT03679273; NCT03813927; NCT04055064 are ongoing, and one study NCT00711217 is no longer recruiting, however, no data are available for this study. We retrieved 10 citations in full text. Two review authors independently assessed the papers and applied the inclusion and exclusion criteria and deemed nine studies to be eligible for inclusion. We excluded one study as the intervention was a probiotic and did not meet our inclusion criteria (Figure 1; Moher 2009).
1.
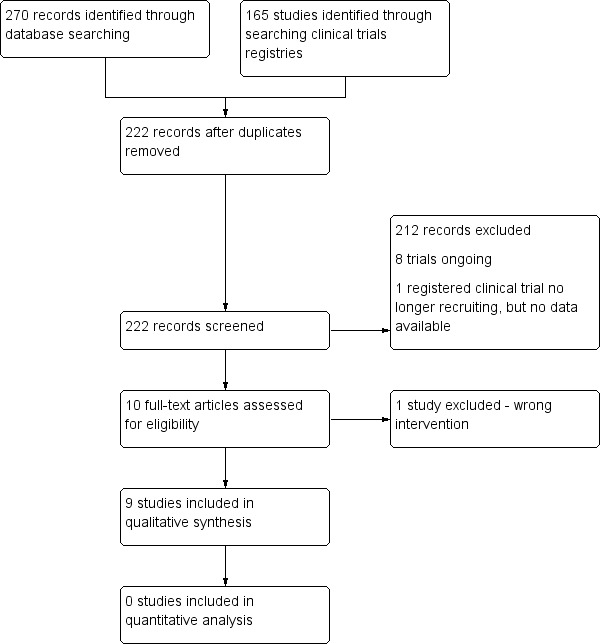
Flow diagram
Included studies
Design
All nine included studies (Armstrong 2014; Afzali 2019; Eneroth 2004; Jones 2014; Momen‐Heravi 2017; Mozaffari‐Khosravi 2017; Razzaghi 2017; Razzaghi 2018; Soleimani 2017), were RCTs.
Sample sizes
The total number of participants included in the studies was 629. The mean sample size was 76 participants (standard deviation (SD): 74; minimum nine participants (Jones 2014); maximum 270 participants (Armstrong 2014)).
Country and setting
Eneroth 2004 was undertaken in Sweden, Armstrong 2014 was undertaken in the USA (36 locations), Europe (one location) and Taiwan (one location), Jones 2014 was undertaken in the USA and Afzali 2019; Momen‐Heravi 2017; Mozaffari‐Khosravi 2017; Razzaghi 2017; Razzaghi 2018; and Soleimani 2017 were all undertaken in Iran.
The setting for Eneroth 2004 was a foot care clinic for people with diabetes at the department of internal medicine, Armstrong 2014 selected individuals from 38 hospital and wound care centres, Jones 2014 included a diabetic outpatient clinic, at the department of general surgery, Afzali 2019; Momen‐Heravi 2017; Mozaffari‐Khosravi 2017; Razzaghi 2017; Razzaghi 2018; and Soleimani 2017 selected hospital clinics.
Participants
The mean number of male participants was 50 (SD: 53; total 455) and the mean number of female participants was 19 (SD: 17; total 174). Within the nine studies, the mean age of participants was 59.2 years (SD: 6.6 years). The mean duration of ulcer in Armstrong 2014 and Eneroth 2004 was 13.25 months (SD: 14.5 months). Soleimani 2017 recorded wound duration in weeks (3.4 ± 0.8: control; 3.3 ± 0.9: intervention). The other studies (Afzali 2019; Jones 2014; Momen‐Heravi 2017; Mozaffari‐Khosravi 2017; Razzaghi 2017; Razzaghi 2018) did not provide the mean duration of ulcers. The mean baseline ulcer area size across seven studies was 10.2 cm (SD: 3.1 cm); Jones 2014 and Razzaghi 2017 did not outline the baseline wound size. The presence of malnutrition was not an inclusion criteria for any of the studies, with the interventions given randomly, irrespective of the presence or absence of malnutrition at inclusion.
Interventions
The included studies employed the following interventions:
250 mg magnesium oxide plus 400 IU vitamin E, versus placebo (Afzali 2019);
a protein (20 g protein per 200 mL bottle), 1 kcal/mL, ready to drink, nutritional supplement with added vitamins, minerals and trace elements, versus a placebo (Eneroth 2004);
arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement versus placebo (Armstrong 2014; Jones 2014);
220 mg zinc sulphate supplements, versus placebo (Momen‐Heravi 2017);
150,000 IU of vitamin D, versus 300,000 IU of vitamin D (Mozaffari‐Khosravi 2017);
50,000 IU vitamin D supplements versus placebo (Razzaghi 2017);
250 mg magnesium oxide supplements versus placebo (Razzaghi 2018); and
1000 mg/day omega‐3 fatty acid from flaxseed oil versus placebo (Soleimani 2017).
Outcomes
Eneroth 2004 reported the primary outcome as the proportion of participants who achieved complete wound healing, in addition to reporting the secondary outcomes of amputation rate and death.
Armstrong 2014 reported the primary outcome as the proportion of participants with total wound closure at 16 weeks, in addition to reporting the time to complete healing. Secondary outcomes reported were quality of life, new ulcer developed and amputation rate.
Mozaffari‐Khosravi 2017 reported the primary outcome as reduction in ulcer area (cm).
Afzali 2019, Momen‐Heravi 2017, Razzaghi 2017, Razzaghi 2018 and Soleimani 2017 reported the primary outcome of wound healing as cm reduction in wound length, width and depth at 12 weeks. None of these studies reported any of the secondary outcomes of interest.
Jones 2014 did not report the any of the primary or secondary outcomes of interest for this review.
Excluded studies
We excluded one study (Mohseni 2018), as it explored the impact of a probiotic and therefore did not meet our inclusion criteria.
Risk of bias in included studies
We have presented our assessment of risk of bias using two 'Risk of bias' summary figures; one of which is a summary of bias (Figure 2) for each item across all studies, and the second of which shows a cross‐tabulation of each study by all of the 'Risk of bias' items (Figure 3). We assessed three of the studies as being low risk of bias (Momen‐Heravi 2017; Razzaghi 2018; Soleimani 2017).
2.

Risk of bias graph
3.

Risk of bias summary
Allocation
All studies stated that the participants were randomly allocated to the study groups. Seven of the studies stated they had undertaken randomisation. Armstrong 2014 described randomisation as follows, "subjects were prospectively randomised (1:1 ratio) within each site to receive either the control, or experimental intervention". Six studies stated that they had randomised participants using computer‐generated random numbers. Thus, we deemed Afzali 2019; Armstrong 2014; Momen‐Heravi 2017; Mozaffari‐Khosravi 2017; Razzaghi 2017; Razzaghi 2018; and Soleimani 2017 at low risk of bias for this domain. Eneroth 2004 and Jones 2014, did not clearly describe the randomisation process. Further, in Eneroth 2004 statistically significant differences between the groups at baseline were noted for the following parameters; palpable pulses, dorsalis pedis or tibialis: control 10/27 (37%); placebo 3/23 (13%); P = 0.05; and protein energy malnutrition present at commencement of the study: control 12/27 (44%); intervention 5/26 (19%); P = 0.05. In Jones 2014 there was also a difference between the study groups at baseline for diabetic control, as measured through HbA1c, where this control was noted to be better in the intervention group (median HbA1c: control 7.8 (minimum 6.4 to maximum 14); intervention 9.0 (minimum 7.2 to max 9.3). We therefore judged both Eneroth 2004; Jones 2014 to be at high risk of bias for this domain.
Four studies stated that they had concealed allocation from the participants and researchers until the final analyses were completed. Thus, we deemed Momen‐Heravi 2017; Razzaghi 2017; Razzaghi 2018 and Soleimani 2017 to be at low risk of bias for this domain. None of the other five studies stated whether they had achieved allocation concealment, and thus we consider them to be at unclear risk of bias in this domain.
Blinding
Eight of the studies described successfully blinding participants and staff. This was because either the intervention and placebo looked the same, or because each set of packages used for the participant had a number on them, indicating whether it was a placebo or intervention and neither the participant nor the individual responsible for handing out the package knew what the numbers represented. Jones 2014 stated that physicians and laboratory technicians who worked with the study participants, and the participants themselves, were blinded to whether the participants were assigned to treatment or placebo. Mozaffari‐Khosravi 2017 did not provide any information, thus we consider it to be at unclear risk of bias in this domain.
Seven studies outlined that blinded outcome assessment was achieved, with Eneroth 2004 stating that the investigators were blinded to treatment until the study end. Jones 2014 stated that researchers involved in the study were also blinded to whether the participants were assigned to treatment or placebo. Five studies stated that allocation was concealed from the researchers until the final analyses were completed. Thus, we judged these studies as being of low risk of detection bias (Afzali 2019; Momen‐Heravi 2017; Razzaghi 2017; Razzaghi 2018; Soleimani 2017). Armstrong 2014 and Mozaffari‐Khosravi 2017 did not state if the outcome assessors were blinded to treatment allocation and thus we judged them to be of unclear risk of detection bias.
Incomplete outcome data
Six studies provided data for all participants enrolled into their studies and thus, we judged them to be at low risk of attrition bias.
Armstrong 2014 provided data for all participants enrolled into the study for the outcomes of number of wounds healed, new ulcers developed and amputation rates. However, they did not provide data for the outcomes of mean wound size at week 16 and health‐related quality of life at week 16 for all participants enrolled. Thus, we judged Armstrong 2014 to be at unclear risk of attrition bias.
In Afzali 2019and Mozaffari‐Khosravi 2017 a number of participants were lost to follow‐up, thus, we judged these studies to be at high risk of attrition bias.
Selective reporting
All studies provided data for all outcomes and thus we judged them to be at low risk of reporting bias.
Other potential sources of bias
We did not identify any additional sources of bias in the studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
Comparison 1: oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements versus placebo (one study, 53 participants)
One study compared oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements with placebo (Eneroth 2004).
Primary outcome: ulcer healing (proportion of ulcers healed at the completion of the study period)
It is uncertain whether oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements, increases the proportion of ulcers healed at six months compared with placebo, because the certainty of the evidence is very low (RR 0.80, 95% CI 0.42 to 1.53; downgraded one level for high risk of bias; downgraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and two levels for very serious imprecision due to small sample size and wide confidence intervals; Analysis 1.1), see Table 1.
1.1. Analysis.

Comparison 1: Oral nutritional supplement with 1 kcal/mL versus placebo, Outcome 1: Ulcer healing (proportion of ulcers healed at 6 months)
Secondary outcomes
Adverse events
It is uncertain whether oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements, impacts on the number of adverse events (deaths) compared with placebo, because the certainty of the evidence is very low (RR 0.96, 95% CI 0.06 to 14.60; downgraded one level for high risk of bias; downgraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and two levels for very serious imprecision due to small sample size and wide confidence intervals; Analysis 1.2), see Table 1.
1.2. Analysis.

Comparison 1: Oral nutritional supplement with 1 kcal/mL versus placebo, Outcome 2: Adverse events (death)
Amputation rate
It is uncertain whether oral nutritional supplement with a protein, 1 kcal/mL, ready‐to‐drink, with added vitamins, minerals and trace elements, impacts on the number of amputations compared with placebo, because the certainty of the evidence is very low (RR 4.82, 95% CI 0.24 to 95.88; downgraded one level for high risk of bias ; downgraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and two levels for very serious imprecision due to small sample size and wide confidence intervals; Analysis 1.3), see Table 1.
1.3. Analysis.

Comparison 1: Oral nutritional supplement with 1 kcal/mL versus placebo, Outcome 3: Amputation
Eneroth 2004 did not report the secondary outcomes cost of intervention, quality of life as measured by a validated scale, acceptability of the intervention (or satisfaction) with respect to patient comfort, length of patient hospital stay, development of any new foot ulcers, surgical interventions and osteomyelitis incidence.
Comparison 2: arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement versus placebo (one study, 270 participants)
Two studies compared arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement with placebo (Armstrong 2014; Jones 2014). One study Jones 2014 reported none of the outcomes of interest for this review.
Primary outcome: ulcer healing (time to complete healing)
Armstrong 2014 reported no difference in the time to complete healing between the study groups, however they did not provide any data for this outcome.
Primary outcome: ulcer healing (proportion of ulcers healed at the completion of the study period)
It is uncertain whether arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement increases the proportion of ulcers healed at 16 weeks compared with placebo, because the certainty of the evidence is very low (RR 1.09, 95% CI 0.85 to 1.40; downgraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and one level for imprecision due to wide confidence intervals; Analysis 2.1), see Table 2.
2.1. Analysis.

Comparison 2: Mixed oral nutritional supplementation versus control, Outcome 1: Ulcer healing (proportion of ulcers healed at 16 weeks)
Secondary outcomes
Quality of life as measured by a validated scale
Armstrong 2014 measured quality of life using the Diabetic Foot Ulcer Scale ‐ Short version (DFS‐SF) index score at 16 weeks, with a higher score indicating a better health‐related quality of life. It is uncertain whether arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement increases health‐related quality of life at 16 weeks more than placebo, because the certainty of the evidence is very low (MD −0.03, 95% CI −0.09 to 0.03; downgraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and one level for imprecision due to wide confidence intervals; Analysis 2.2), see Table 2.
2.2. Analysis.

Comparison 2: Mixed oral nutritional supplementation versus control, Outcome 2: Health‐related quality of life ‐ higher score = better health‐related quality of life
Development of any new foot ulcers
It is uncertain whether arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement reduces the number of new foot ulcers developed compared with placebo because the certainty of the evidence is very low (RR 1.04, 95% CI 0.71 to 1.51; downgraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and one level for imprecision due to wide confidence intervals; Analysis 2.3), see Table 2.
2.3. Analysis.

Comparison 2: Mixed oral nutritional supplementation versus control, Outcome 3: New ulcers developed
Amputation rate (at 16 weeks)
It is uncertain whether arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement reduces amputation rate more than placebo because the certainty of the evidence is very low (RR 0.66, 95% CI 0.16 to 2.69; downgraded one level for indirectness because baseline nutritional status of participants was very poorly reported, and one level for imprecision due to wide confidence intervals; Analysis 2.4), see Table 2.
2.4. Analysis.

Comparison 2: Mixed oral nutritional supplementation versus control, Outcome 4: Amputation
Armstrong 2014 did not report the secondary outcomes cost of intervention, acceptability of the intervention (or satisfaction) with respect to patient comfort, adverse events, length of patient hospital stay, surgical interventions or osteomyelitis incidence.
Comparison 3: 220 mg zinc sulphate supplement containing 50 mg elemental zinc versus placebo (one study, 60 participants)
One study compared 220 mg zinc sulphate supplement containing 50 mg elemental zinc with placebo (Momen‐Heravi 2017).
Primary outcome: ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm reduction in wound length, depth and width)
Momen‐Heravi 2017 evaluated wound healing as mean (SD) cm reduction in wound length, depth and width. It is uncertain whether 220 mg zinc sulphate supplement containing 50 mg elemental zinc increases the absolute change in individual parameters of ulcer dimensions over time: mean cm reduction in wound length, depth and width, because the certainty of the evidence is very low (downgraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume; it would be possible for one or more of these to change and the total volume of the wound (for example) would remain unchanged), see Analysis 3.1; Analysis 3.2; Analysis 3.3 and Table 3.
3.1. Analysis.

Comparison 3: Zinc sulphate supplements versus placebo, Outcome 1: Mean wound length reduction
3.2. Analysis.

Comparison 3: Zinc sulphate supplements versus placebo, Outcome 2: Mean wound depth reduction
3.3. Analysis.

Comparison 3: Zinc sulphate supplements versus placebo, Outcome 3: Mean wound width reduction
Secondary outcomes
Momen‐Heravi 2017 did not report any of the secondary outcomes: cost of intervention, quality of life as measured by a validated scale, acceptability of the intervention (or satisfaction) with respect to patient comfort, adverse events, length of patient hospital stay, development of any new foot ulcers, amputation rate, surgical interventions, or osteomyelitis incidence.
Comparison 4: 250 mg magnesium oxide supplement versus placebo (one study, 70 participants)
One study compared 250 mg magnesium oxide supplement with placebo (Razzaghi 2018).
Primary outcome: ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm reduction in wound length, depth and width)
Razzaghi 2018 evaluated wound healing as mean (SD) cm reduction in wound length, depth and width. It is uncertain whether 250 mg magnesium oxide supplement increases the absolute change in individual parameters of ulcer dimensions over time: mean cm reduction in wound length, depth and width, because the certainty of the evidence is very low (downgraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume; it would be possible for one or more of these to change and the total volume of the wound (for example) would remain unchanged), see Analysis 4.1; Analysis 4.2; Analysis 4.3 and Table 4.
4.1. Analysis.

Comparison 4: 250 mg magnesium oxide supplements versus placebo, Outcome 1: Mean wound length reduction
4.2. Analysis.

Comparison 4: 250 mg magnesium oxide supplements versus placebo, Outcome 2: Mean wound depth reduction
4.3. Analysis.

Comparison 4: 250 mg magnesium oxide supplements versus placebo, Outcome 3: Mean wound width reduction
Secondary outcomes
Razzaghi 2018 did not report any of the secondary outcomes: cost of intervention, quality of life as measured by a validated scale, acceptability of the intervention (or satisfaction) with respect to patient comfort, adverse events, length of patient hospital stay, development of any new foot ulcers, amputation rate, surgical interventions, or osteomyelitis incidence.
Comparison 5: 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement versus placebo (one study, 60 participants)
One study compared 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement with placebo (Soleimani 2017).
Primary outcome: ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm reduction in wound length, depth and width)
Soleimani 2017 evaluated wound healing as mean (SD) cm reduction in wound length, depth and width. It is uncertain whether 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement increases the absolute change in individual parameters of ulcer dimensions over time: mean cm reduction in wound length, depth and width, because the certainty of the evidence is very low (downgraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume; it would be possible for one or more of these to change and the total volume of the wound (for example) would remain unchanged) see Analysis 5.1; Analysis 5.2; Analysis 5.3 and Table 5.
5.1. Analysis.

Comparison 5: 1000 mg/day omega‐3 fatty acid from flaxseed oil supplements versus placebo, Outcome 1: Mean wound length reduction
5.2. Analysis.

Comparison 5: 1000 mg/day omega‐3 fatty acid from flaxseed oil supplements versus placebo, Outcome 2: Mean wound depth reduction
5.3. Analysis.

Comparison 5: 1000 mg/day omega‐3 fatty acid from flaxseed oil supplements versus placebo, Outcome 3: Mean wound width reduction
Secondary outcomes
Soleimani 2017 did not report any of the secondary outcomes: cost of intervention, quality of life as measured by a validated scale, acceptability of the intervention (or satisfaction) with respect to patient comfort, adverse events, length of patient hospital stay, development of any new foot ulcers, amputation rate, surgical interventions, or osteomyelitis incidence.
Comparison 6: 150,000 IU of vitamin D, versus 300,000 IU of vitamin D (one study, 47 participants)
One study compared 150,000 IU of vitamin D with 300,000 IU of vitamin D (Mozaffari‐Khosravi 2017).
Primary outcome: ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound area)
It is uncertain whether 150,000 IU of vitamin D when compared with 300,000 IU of vitamin D increases the absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound area, because the certainty of the evidence is very low (downgraded one level due to a high risk of attrition bias; downgraded one level for indirectness because baseline nutritional status of participants was very poorly reported and downgraded two levels for imprecision because of the small sample size and wide confidence intervals), see Analysis 6.1 and Table 6.
6.1. Analysis.

Comparison 6: 150,000 IU of vitamin D versus 300,000 IU of vitamin D, Outcome 1: Mean wound area
Secondary outcomes
Mozaffari‐Khosravi 2017 did not report any of the secondary outcomes: cost of intervention, quality of life as measured by a validated scale, acceptability of the intervention (or satisfaction) with respect to patient comfort, adverse events, length of patient hospital stay, development of any new foot ulcers, amputation rate, surgical interventions, or osteomyelitis incidence.
Comparison 7: magnesium and vitamin E co‐supplementation versus placebo (one study, 57 participants)
One study compared magnesium and vitamin E co‐supplementation with placebo (Afzali 2019).
Primary outcome: ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm wound length, depth and width)
Afzali 2019 evaluated wound healing as mean (SD) cm wound length, depth and width. It is uncertain whether magnesium and vitamin E co‐supplementation increases the absolute change in individual parameters of ulcer dimensions over time: mean (SD) wound length, depth and width, because the certainty of the evidence is very low (downgraded one level for high risk of attrition bias; downgraded two levels for imprecision because of the small sample size and wide confidence intervals; downgraded two levels for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume; it would be possible for one or more of these to change and the total volume of the wound (for example) would remain unchanged), see Analysis 7.1; Analysis 7.2; Analysis 7.3 and Table 7.
7.1. Analysis.

Comparison 7: Magnesium and vitamin E co‐supplementation versus placebo, Outcome 1: Mean wound length
7.2. Analysis.

Comparison 7: Magnesium and vitamin E co‐supplementation versus placebo, Outcome 2: Mean wound depth
7.3. Analysis.

Comparison 7: Magnesium and vitamin E co‐supplementation versus placebo, Outcome 3: Mean wound width
Afzali 2019 did not report any of the secondary outcomes: cost of intervention, quality of life as measured by a validated scale, acceptability of the intervention (or satisfaction) with respect to patient comfort, adverse events, length of patient hospital stay, development of any new foot ulcers, amputation rate, surgical interventions, or osteomyelitis incidence.
Comparison 8: vitamin D versus placebo (one study, 60 participants)
One study compared vitamin D with placebo (Razzaghi 2017).
Primary outcome: ulcer healing (absolute change in individual parameters of ulcer dimensions over time: mean (SD) cm reduction in wound length, depth and width)
Razzaghi 2017 evaluated wound healing as mean (SD) cm reduction in wound length, depth and width. It is uncertain whether vitamin D increases the absolute change in individual parameters of ulcer dimensions over time: mean cm reduction in wound length, depth and width, because the certainty of the evidence is very low (downgraded twice for imprecision because of the small sample size and wide confidence intervals; downgraded twice for indirectness because baseline nutritional status of participants was very poorly reported and the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume; it would be possible for one or more of these to change and the total volume of the wound (for example) would remain unchanged), see Analysis 8.1; Analysis 8.2; Analysis 8.3 and Table 8.
8.1. Analysis.

Comparison 8: Vitamin D versus placebo, Outcome 1: Mean wound length reduction
8.2. Analysis.

Comparison 8: Vitamin D versus placebo, Outcome 2: Mean wound depth reduction
8.3. Analysis.

Comparison 8: Vitamin D versus placebo, Outcome 3: Mean wound width reduction
Razzaghi 2017 did not report any of the secondary outcomes: cost of intervention, quality of life as measured by a validated scale, acceptability of the intervention (or satisfaction) with respect to patient comfort, adverse events, length of patient hospital stay, development of any new foot ulcers, amputation rate, surgical interventions, or osteomyelitis incidence.
Discussion
Summary of main results
Nine studies, exploring the effect of nutritional interventions for treating foot ulcers in people with diabetes were eligible and included in this review. Eight studies compared different oral nutritional supplements with placebo (Table 1; Table 2; Table 3; Table 4; Table 5; Table 7; Table 8). One study compared two different doses of vitamin D (Table 6). Armstrong 2014 and Eneroth 2004 reported the primary outcome of interest, whereas, Afzali 2019; Momen‐Heravi 2017; Mozaffari‐Khosravi 2017; Razzaghi 2017; Razzaghi 2018 and Soleimani 2017 reported the surrogate outcomes of change in wound size or volume. Jones 2014 did not report any of the primary or secondary outcomes of interest for this review.
Primary outcome
It is uncertain whether there is a difference in proportion of ulcers healed at the completion of the study period, or absolute change in individual parameters of ulcer dimensions, or ulcer area, over time, for those treated, or not treated with oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements; arginine, glutamine and β‐hydroxy‐β‐methylbutyrate supplement; 220 mg zinc sulphate supplement containing 50 mg elemental zinc; 250 mg magnesium oxide supplement; 1000 mg/day omega‐3 fatty acid from flaxseed oil supplement; 150,000 IU of vitamin D versus 300,000 IU of vitamin D; magnesium and vitamin E co‐supplementation or vitamin D alone, because the certainty of evidence is very low.
Secondary outcomes
Armstrong 2014 reported the secondary outcomes quality of life, development of new ulcers and amputation, and it is uncertain whether there is a difference in these outcomes for those treated or not treated with nutritional interventions because the certainty of the evidence is very low. Eneroth 2004 reported the secondary outcomes amputation and adverse events (death), and it is uncertain whether there is a difference in these outcomes for those treated or not treated with oral nutritional supplement with 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements, because the certainty of the evidence is very low.
None of the included studies reported the secondary outcomes: cost of intervention, acceptability of the intervention (or satisfaction) with respect to patient comfort, length of patient hospital stay, surgical interventions, or osteomyelitis incidence.
In summary, evidence for the effectiveness of nutritional interventions remains unclear, with eight studies showing no clear benefit or harm, and one study that did not report any of the outcomes for this review. Further information is required to clarify the effect of nutritional interventions on the treatment of foot ulcers in people with diabetes.
Overall completeness and applicability of evidence
In Armstrong 2014, the inclusion criteria were individuals with type 1 or type 2 diabetes undergoing pharmacological treatment for glycaemic control who had at least one University of Texas grade 1A foot ulcer (Lavery 1996). The Texas grade 1A indicates a superficial wound not involving tendon, capsule, or bone. Given that Zhang 2013 has shown that nutritional status deteriorates as the severity of a foot ulcer increases, it could be argued that those with grade 1A ulcers are not necessarily those most reflective of the diabetic population requiring nutritional interventions.
In Eneroth 2004, the inclusion criteria were individuals aged over 60 years with diabetes mellitus and a Wagner grade 1–2 foot ulcer (Wagner 1981) of over four weeks' duration. A European study suggests that the prevalence of more severe foot ulcers in people with diabetes is greater that previously understood (Prompers 2008). Thus, Wagner Grade 1‐2 ulcers may not necessarily be representative of the severity of ulcers of those most in need of focused interventions to enhance wound‐healing outcomes. However, Afzali 2019; Momen‐Heravi 2017; Mozaffari‐Khosravi 2017; Razzaghi 2017; Razzaghi 2018and Soleimani 2017 included grade 3 foot ulcers in people with diabetes, categorised according to 'Wagner‐Meggitt's' criteria (Wagner 1981; Meggitt 1976) and therefore these participants may be more representative of those individuals requiring nutritional interventions.
The studies were carried out in a relatively wide range of countries; USA (37 locations), Europe (Sweden and Spain), Taiwan, and Iran (6 studies). However, despite this spread of geographical locations, there were only two studies from Europe, with none from (for example) the UK, Germany, France, Ireland, Italy and the Netherlands. This should be considered when assessing the applicability of findings to the European setting, as this may limit the external validity of the findings from this review (Kennedy‐Martin 2015).
The nutritional status of the participants was not an inclusion/exclusion criteria in the studies included in the review. As such, the supplement was generally given randomly irrespective of the presence or absence of malnutrition at inclusion. Quality or quantity of dietary intake plays a major part in the clinical application of these oral nutritional supplements (Albahrani 2016), thus, the baseline nutritional status of the participants is important to understand. Further, in this review, this may be affected by the low, middle or high income of the country of residence. Importantly, given the risk of toxicity, appropriate patient assessment is essential prior to administering any fat‐soluble vitamin supplements (Albahrani 2016).
Outcomes such as complete wound healing and adverse events are important, however, these were inconsistently reported, or not reported at all in the included studies. This is not unique to the current review, as it has been well documented in the literature that a major issue in the synthesis of clinical study data relates to the fact that outcomes are often not clearly defined, leading to poor reproducibility (Gottrup 2010). None the less, this lack of consistency in outcome reporting is a challenge as it adversely influences the generation of meaningful information (O'Connor 2013).
Quality of the evidence
We used GRADE to assess the quality of the evidence (Schünemann 2011), which includes explicit criteria for this assessment, namely, study design, risk of bias, imprecision, inconsistency, indirectness, and magnitude of effect (Guyatt 2011a). In this review, we assessed the certainty of the evidence as very low, arising mainly due to risk of bias, indirectness and imprecision.
We deemed Afzali 2019and Mozaffari‐Khosravi 2017 to be at high risk of attrition bias and Eneroth 2004 and Jones 2014 to be at high risk of other bias due to baseline incomparability of study groups.
Attrition bias arises when some of the participants allocated to the study groups leave before the completion of the study and are lost to follow‐up. This may result in there being systematic differences between those who continue in the study and those who leave (Bell 2013). Attrition bias can be a problem, for example Jüni 2005 suggest that results from studies with patient exclusions tend to show more beneficial effects of the experimental treatment than seen when all, or most patients randomised are included in the analysis. Further, a systematic review by Akl 2012 concluded that outcomes of participants lost to follow‐up could change the interpretation of results. Thus, it is important to consider the potential impact that attrition may have on study outcomes and for this reason we downgraded Afzali 2019and Mozaffari‐Khosravi 2017 for risk of attrition bias.
One of the intentions of randomisation is to ensure that the characteristics of the participants are evenly distributed among the study groups, so that any differences identified in the study outcomes can be attributed to the intervention and not to some specific characteristic of one of the study groups (Roberts 1998). Thus, baseline incomparability of study groups is considered to be a problem in clinical studies because variances in the study groups, arising, for example, due to age or severity of disease may have an impact on the outcomes measured over and above the intervention itself (Roberts 1999). The CONSORT 2010 guidelines identify the importance of identification of the baseline characteristics of the participants so that the potential for generalisation can be assessed. Further, though not a guarantee of comparability of study groups, randomisation should ensure that any differences will have arisen due to chance rather than bias. In this review, two studies presented study groups that were different at baseline, therefore, we downgraded Eneroth 2004 for risk of other bias. Jones 2014 did not report any of the primary or secondary outcomes of interest for this review
We downgraded all the included studies for indirectness arising due to the fact that baseline nutritional status of participants was very poorly reported. The nutritional status of participants is fundamental to interpretation because the nutritional status of the individual affects metabolism and as such the impact of the nutritional interventions employed within the included studies. Thus, given that the nutritional status of the participants in the studies is unclear, this affects the external generalisability of the findings. A further issue related to indirectness in this review related to the use of a surrogate outcome measures. The problem in this review was that the surrogate outcome was not a direct measure of the outcome of interest which was pre‐stipulated in the review protocol. In a number of studies in this review, the outcomes reported were individual parameters of ulcer dimensions and not ulcer area or volume, it would be possible for one or more of these to change and have the total volume of the wound (for example) remain unchanged. This required downgrading the certainty of evidence because it reduces the confidence that one may have in the estimates of effect (Guyatt 2011b).
Imprecision was also a problem within this review, however, is an important element in the grading of the confidence in the estimate (Castellini 2018). In assessing imprecision, Guyatt 2011c suggests that one should assess whether the CI around the estimate of treatment effect is sufficiently narrow. If it is not, Guyatt 2011c recommends downgrading the evidence. In this review, all studies presented with very wide CIs and small sample sizes (mean: 76 participants). Therefore, we downgraded the certainty of the evidence due to this imprecision.
Potential biases in the review process
We followed clearly described procedures to prevent potential bias in the review process. This included a careful literature search and the methods we used were transparent and reproducible. It is possible that studies published in journals that were outside our search strategy may have been missed. Although there is a wide geographical spread, albeit only two from Europe, we identified and included in this review only studies published in English. This raises the possibility that a selection bias based on language may be a potential consideration. Although we did not set out to include only studies published in the English language, Egger 1997 in one review of RCTs conducted in Germany, found that authors were more likely to report their findings in English language journals when their results were statistically significant. As such, to ensure completeness of the data included and to avoid bias in reviews, Egger 1997 argues that it is important to include non‐English language studies.
We had prespecified “absolute or percentage change in ulcer area or volume over time” as a primary outcome in our protocol. However the outcomes reported in some of the studies were individual parameters of ulcer dimensions and it is possible that this discrepancy may have caused bias. Kirkham 2010 identified that there was an increased risk of obtaining a significant result in the meta‐analysis if there was either an inclusion, or an upgrade of primary outcomes, compared with no discrepancy between the protocol and the full systematic review (RR 1.66, 95% CI 1.10 to 2.49; P = 0.02).
Agreements and disagreements with other studies or reviews
Due to the lack of studies and reviews in this area in general, we are unable to conclude whether this review agrees or disagrees with other studies or reviews.
Authors' conclusions
Implications for practice.
There is very low‐certainty evidence from randomised controlled trials on the effectiveness of nutritional interventions for treating foot ulcers in people with diabetes. Results are unclear as to whether there is a difference in the healing, amputations or deaths between nutritional supplementation and no nutritional supplementation. It is also unclear if there is a difference in health‐related quality of life or number of ulcers that recur between nutritional supplementation and no nutritional supplementation. Thus, there is insufficient evidence to support or refute the use of nutritional interventions for treating foot ulcers in people with diabetes.
Implications for research.
Overall, more research is needed to clarify the impact of nutritional interventions on the healing of foot ulcers in people with diabetes. Further studies are justified based on the incidence of foot ulcers in people with diabetes and the high costs associated with foot ulcer management. Future research should focus on use of direct measures of wound healing such as time to complete healing or proportion of ulcers healed at the completion of the study period, rather than indirect measures of wound healing, such as changes in wound parameters (length, depth and width). These studies should be large enough and designed to a high standard. They should also include patient‐related outcomes such as product acceptability, adverse events and health‐related quality of life, and economic evaluations to assist healthcare managers to make rational decisions.
History
Protocol first published: Issue 11, 2014 Review first published: Issue 7, 2020
Acknowledgements
The review authors would like to acknowledge peer referees: Magnus Ågren, Malcolm Brewster, Janet Gunderson, Fiona Paton, Richard Kirubakaran and Uwe Wollina for their comments on the protocol and Janet Yarrow, Sorrel Burden and Gill Norman for their comments on the review. Thanks also to copy editors Clare Dooley and Denise Mitchell.
MA Corcoran is supported by a Health Research Board (Ireland) Cochrane Training Fellowship.
Appendices
Appendix 1. Search strategies
Cochrane Wounds Specialised Register
1. MESH DESCRIPTOR Nutrition Therapy EXPLODE ALL AND INREGISTER
2 (nutrition* or diet*) AND INREGISTER
3 ((enteral or parenteral or tube) near5 (feed* or nutrition* or administrat*)) AND INREGISTER
4 MESH DESCRIPTOR Dietary Supplements EXPLODE ALL AND INREGISTER
5 MESH DESCRIPTOR Micronutrients EXPLODE ALL AND INREGISTER
6 MESH DESCRIPTOR Dietary Proteins EXPLODE ALL AND INREGISTER
7 MESH DESCRIPTOR Dietary Carbohydrates AND INREGISTER
8 MESH DESCRIPTOR Dietary Fats AND INREGISTER
9 MESH DESCRIPTOR Energy Intake AND INREGISTER
10 (diet* near3 (supplement* or fortification or capsule* or tablet* or liquid*)) AND INREGISTER
11 (nutrient* near3 (supplement* or fortification or capsule* or tablet* or liquid*)) AND INREGISTER
12 ((micronutrient* or micro‐nutrient* or vitamin* or multivitamin* or mineral* or trace next element* or zinc or iodine or iron or cobalt or chromium or copper or manganese or fluoride or sodium or selenium or molybdenum) near3 (supplement* or fortification or capsule* or tablet* or liquid*)) AND INREGISTER
13 ((macronutrient* or macro‐nutrient* or protein* or amino next acid* or carbohydrate* or calorie* or energ* or fat* or lipid*) near3 (supplement* or fortification or capsule* or tablet* or liquid*)) AND INREGISTER
14 ((food or diet) near3 (intake or fortif*)) AND INREGISTER
15 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 AND INREGISTER
16 MESH DESCRIPTOR Foot Ulcer EXPLODE ALL AND INREGISTER
17 (diabet* near3 ulcer*) AND INREGISTER
18 (diabet* near3 (foot or feet)) AND INREGISTER
19 (diabet* near3 wound*) AND INREGISTER
20 (diabet* near3 defect*) AND INREGISTER
21 #16 OR #17 OR #18 OR #19 OR #20 AND INREGISTER
22 #15 AND #21 AND INREGISTER
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Nutrition Therapy] explode all trees
#2 (nutrition* or diet*):ti,ab,kw
#3 ((enteral or parenteral or tube) near/5 (feed* or nutrition* or administrat*)):ti,ab,kw
#4 MeSH descriptor: [Dietary Supplements] explode all trees
#5 MeSH descriptor: [Micronutrients] explode all trees
#6 MeSH descriptor: [Dietary Proteins] explode all trees
#7 MeSH descriptor: [Dietary Carbohydrates] explode all trees
#8 MeSH descriptor: [Dietary Fats] explode all trees
#9 MeSH descriptor: [Energy Intake] explode all trees
#10 (diet* near/3 (supplement* or fortification or capsule* or tablet* or liquid*)):ti,ab,kw
#11 (nutrient* near/3 (supplement* or fortification or capsule* or tablet* or liquid*)):ti,ab,kw
#12 ((micronutrient* or micro‐nutrient* or vitamin* or multivitamin* or mineral* or trace next element* or zinc or iodine or iron or cobalt or chromium or copper or manganese or fluoride or sodium or selenium or molybdenum) near/3 (supplement* or fortification or capsule* or tablet* or liquid*)):ti,ab,kw
#13 ((macronutrient* or macro‐nutrient* or protein* or amino next acid* or carbohydrate* or calorie* or energ* or fat* or lipid*) near/3 (supplement* or fortification or capsule* or tablet* or liquid*)):ti,ab,kw
#14 ((food or diet) near/3 (intake or fortif*)):ti,ab,kw
#15 {or #1‐#14}
#16 MeSH descriptor: [Foot Ulcer] explode all trees
#17 (diabet* near/3 ulcer*):ti,ab,kw
#18 (diabet* near/3 (foot or feet)):ti,ab,kw
#19 (diabet* near/3 wound*):ti,ab,kw
#20 (diabet* near/3 defect*):ti,ab,kw
#21 {or #16‐#20}
#22 {and #15, #21} in Trials
Ovid MEDLINE
1 exp Nutrition Therapy/
2 (nutrition* or diet*).tw.
3 ((enteral or parenteral or tube) adj5 (feed* or nutrition* or administrat*)).tw.
4 exp Dietary Supplements/
5 exp Micronutrients/
6 exp Dietary Proteins/
7 exp Dietary Carbohydrates/
8 exp Dietary Fats/
9 exp Energy Intake/
10 (diet* adj3 (supplement* or fortification or capsule* or tablet* or liquid*)).tw.
11 (nutrient* adj3 (supplement* or fortification or capsule* or tablet* or liquid*)).tw.
12 ((micronutrient* or micro‐nutrient* or vitamin* or multivitamin* or mineral* or trace next element* or zinc or iodine or iron or cobalt or chromium or copper or manganese or fluoride or sodium or selenium or molybdenum) adj3 (supplement* or fortification or capsule* or tablet* or liquid*)).tw.
13 ((macronutrient* or macro‐nutrient* or protein* or amino next acid* or carbohydrate* or calorie* or energ* or fat* or lipid*) adj3 (supplement* or fortification or capsule* or tablet* or liquid*)).tw.
14 ((food or diet) adj3 (intake or fortif*)).tw.
15 or/1‐14
16 exp Foot Ulcer/
17 (diabet* adj3 ulcer*).tw.
18 (diabet* adj3 (foot or feet)).tw.
19 (diabet* adj3 wound*).tw.
20 (diabet* adj3 defect*).tw.
21 or/16‐20
22 and/15,21
23 randomized controlled trial.pt.
24 controlled clinical trial.pt.
25 randomi?ed.ab.
26 placebo.ab.
27 clinical trials as topic.sh.
28 randomly.ab.
29 trial.ti.
30 or/23‐29
31 exp animals/ not humans.sh.
32 30 not 31
33 22 and 32
Ovid Embase
1 food intake/
2 (nutrition* or diet*).tw.
3 ((enteral or parenteral or tube) adj5 (feed* or nutrition* or administrat*)).tw.
4 exp Dietary Supplements/
5 exp Micronutrients/
6 exp Dietary Proteins/
7 exp Dietary Carbohydrates/
8 exp Dietary Fats/
9 exp Energy Intake/
10 (diet* adj3 (supplement* or fortification or capsule* or tablet* or liquid*)).tw.
11 (nutrient* adj3 (supplement* or fortification or capsule* or tablet* or liquid*)).tw.
12 ((micronutrient* or micro‐nutrient* or vitamin* or multivitamin* or mineral* or trace next element* or zinc or iodine or iron or cobalt or chromium or copper or manganese or fluoride or sodium or selenium or molybdenum) adj3 (supplement* or fortification or capsule* or tablet* or liquid*)).tw.
13 ((macronutrient* or macro‐nutrient* or protein* or amino next acid* or carbohydrate* or calorie* or energ* or fat* or lipid*) adj3 (supplement* or fortification or capsule* or tablet* or liquid*)).tw.
14 ((food or diet) adj3 (intake or fortif*)).tw.
15 or/1‐14
16 exp Foot Ulcer/
17 (diabet* adj3 ulcer*).tw.
18 (diabet* adj3 (foot or feet)).tw.
19 (diabet* adj3 wound*).tw.
20 (diabet* adj3 defect*).tw.
21 or/16‐20
22 and/15,21
23 Randomized controlled trials/
24 Single‐Blind Method/
25 Double‐Blind Method/
26 Crossover Procedure/
27 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab.
28 (doubl* adj blind*).ti,ab.
29 (singl* adj blind*).ti,ab.
30 or/23‐29
31 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
32 human/ or human cell/
33 and/31‐32
34 31 not 33
35 30 not 34
36 22 and 35
EBSCO CINAHL Plus
S44 S30 AND S43
S43 S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42
S42 TI allocat* random* or AB allocat* random*
S41 MH "Quantitative Studies"
S40 TI placebo* or AB placebo*
S39 MH "Placebos"
S38 TI random* allocat* or AB random* allocat*
S37 MH "Random Assignment"
S36 TI randomi?ed control* trial* or AB randomi?ed control* trial*
S35 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
S34 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
S33 TI clinic* N1 trial* or AB clinic* N1 trial*
S32 PT Clinical trial
S31 MH "Clinical Trials+"
S30 S23 AND S29
S29 S24 OR S25 OR S26 OR S27 OR S28
S28 TI diabet* N3 defect* OR AB diabet* N3 defect*
S27 TI diabet* N3 wound* OR AB diabet* N3 wound*
S26 TI ( diabet* N3 (foot or feet) ) OR AB ( diabet* N3 (foot or feet) )
S25 TI diabet* N3 ulcer* OR AB diabet* N3 ulcer*
S24 (MH "Foot Ulcer+")
S23 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22
S22 TI ((food or diet) N3 (intake or fortif*)) OR AB ((food or diet) N3 (intake or fortif*))
S21 TI ((macronutrient* or macro‐nutrient* or protein* or "amino acid*" or carbohydrate* or calorie* or energ* or fat* or lipid*) N3 (supplement* or fortification or capsule* or tablet* or liquid*)) OR AB ((macronutrient* or macro‐nutrient* or protein* or "amino acid*" or carbohydrate* or calorie* or energ* or fat* or lipid*) N3 (supplement* or fortification or capsule* or tablet* or liquid*))
S20 TI ((micronutrient* or micro‐nutrient* or vitamin* or multivitamin* or mineral* or "trace element*" or zinc or iodine or iron or cobalt or chromium or copper or manganese or fluoride or sodium or selenium or molybdenum) N3 (supplement* or fortification or capsule* or tablet* or liquid*)) OR AB ((micronutrient* or micro‐nutrient* or vitamin* or multivitamin* or mineral* or "trace element*" or zinc or iodine or iron or cobalt or chromium or copper or manganese or fluoride or sodium or selenium or molybdenum) N3 (supplement* or fortification or capsule* or tablet* or liquid*))
S19 TI (nutrient* N3 (supplement* or fortification or capsule* or tablet* or liquid*)) OR AB (nutrient* N3 (supplement* or fortification or capsule* or tablet* or liquid*))
S18 TI (diet* N3 (supplement* or fortification or capsule* or tablet* or liquid*)) OR AB (diet* N3 (supplement* or fortification or capsule* or tablet* or liquid*))
S17 (MH "Energy Intake")
S16 (MH "Trace Elements+")
S15 (MH "Minerals+")
S14 (MH "Vitamins+")
S13 (MH "Micronutrients")
S12 (MH "Macronutrients")
S11 (MH "Dietary Carbohydrates+")
S10 (MH "Dietary Fats+")
S9 (MH "Dietary Proteins+")
S8 (MH "Dietary Supplementation")
S7 (MH "Dietary Supplements+")
S6 TI ((enteral or parenteral or tube) N5 (feed or nutrition* or administrat*)) or AB ((enteral or parenteral or tube) N5 (feed or nutrition* or administrat*))
S5 TI (nutrition* or diet*) or AB (nutrition* or diet*)
S4 MH "Parenteral Nutrition+"
S3 (MH "Enteral Nutrition")
S2 (MH "Nutritional Support+")
S1 MH "Diet Therapy"
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
nutrition OR diet OR enteral OR parenteral OR feed OR food | Foot Ulcer
nutrition OR diet OR enteral OR parenteral OR feed OR food | Diabetic Foot Ulcer
nutrition OR diet OR enteral OR parenteral OR feed OR food | Diabetic Foot
World Health Organization International Clinical Trials Registry Platform
foot AND ulcer [Title] AND nutrition OR diet OR enteral OR Parenteral OR feed OR food [Intervention]
foot AND ulcer [Condition] AND nutrition OR diet OR enteral OR Parenteral OR feed OR food [Intervention]
diabetic foot ulcer [Title] AND nutrition OR diet OR enteral OR Parenteral OR feed OR food [Intervention]
diabetic foot ulcer [condition] AND nutrition OR diet OR enteral OR Parenteral OR feed OR food [Intervention]
diabetic AND foot [Title] AND nutrition OR diet OR enteral OR Parenteral OR feed OR food [Intervention]
diabetic AND foot [Condition] AND nutrition OR diet OR enteral OR Parenteral OR feed OR food [Intervention]
Appendix 2. Risk of bias
'Risk of bias' assessment (individually randomised controlled trials)
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
no blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding;
blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken;
either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following:
no blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding;
blinding of key study participants and personnel attempted, but likely that the blinding could have been broken;
either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Either of the following:
Insufficient information to permit judgement of low or high risk of bias;
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
no missing outcome data;
reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias);
missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups;
for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate;
for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size;
missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups;
for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate;
for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size;
‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
potentially inappropriate application of simple imputation.
Unclear
Either of the following:
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided);
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
the study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way;
the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
not all of the study’s pre‐specified primary outcomes have been reported;
one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified;
one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect);
one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis;
the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Oral nutritional supplement with 1 kcal/mL versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Ulcer healing (proportion of ulcers healed at 6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Adverse events (death) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Amputation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Mixed oral nutritional supplementation versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Ulcer healing (proportion of ulcers healed at 16 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Health‐related quality of life ‐ higher score = better health‐related quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3 New ulcers developed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4 Amputation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Zinc sulphate supplements versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mean wound length reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2 Mean wound depth reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3 Mean wound width reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. 250 mg magnesium oxide supplements versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Mean wound length reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2 Mean wound depth reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.3 Mean wound width reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. 1000 mg/day omega‐3 fatty acid from flaxseed oil supplements versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Mean wound length reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2 Mean wound depth reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3 Mean wound width reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. 150,000 IU of vitamin D versus 300,000 IU of vitamin D.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Mean wound area | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐0.04, 1.26] |
Comparison 7. Magnesium and vitamin E co‐supplementation versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Mean wound length | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.33, ‐0.07] |
| 7.2 Mean wound depth | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐0.71, ‐0.29] |
| 7.3 Mean wound width | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.09, ‐0.11] |
Comparison 8. Vitamin D versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Mean wound length reduction | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐1.10, ‐0.90] |
| 8.2 Mean wound depth reduction | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐0.55, ‐0.45] |
| 8.3 Mean wound width reduction | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐0.90, ‐0.70] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Afzali 2019.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Setting/location: Department of Infectious Disease, School of Medicine, Kashan University of Medical Sciences, Kashan, Iran Study duration: 12 weeks |
|
| Participants |
Sample size: n = 60 participants randomised, 57 analysed Inclusion criteria:
Exclusion criteria:
Participant characteristics:
|
|
| Interventions |
Intervention:
Control:
|
|
| Outcomes |
Primary outcome
|
|
| Notes | Funded by a grant from the Vice Chancellor for Research, KAUMS, and Iran | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomization sequence was developed by an expert statistician using blocks of various length in random sequence." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote "Participants and researchers dispensing the capsules and involved in the trial were blinded to the bottle content" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote "All laboratory analyses were blind procedures" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 participants in the control group and 1 participant in the intervention group were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Study registered and all outcomes reported |
| Other bias | Low risk | None detected |
Armstrong 2014.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Setting/location: community‐dwelling individuals from 38 hospitals and wound care centres in the USA (36), Europe (1) and Taiwan (1). Study duration: 16 weeks |
|
| Participants |
Sample size: n = 270 participants Inclusion criteria:
Exclusion criteria:
Participant characteristics:
|
|
| Interventions |
Intervention:
Control:
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | funded by Abbott Nutrition (Columbus, OH, USA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were prospectively randomised (1:1 ratio) within each site to receive either the control or experimental intervention." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" "both products were identical in packaging, appearance, dissolving characteristics and weight." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data for wound area at 16 weeks and HRQoL at 16 weeks, not presented for all participants |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None detected |
Eneroth 2004.
| Study characteristics | ||
| Methods |
Study design: prospective RCT Setting/location: diabetic foot‐care clinic at the Department of Internal Medicine, Lund University Hospital, Sweden Study duration: 6 months |
|
| Participants |
Sample size: n = 53 participants Inclusion criteria: aged ≥ 60 years; diabetes mellitus; Wagner grade 1 or 2 foot ulcer of at least four weeks' duration; distal blood pressure must have been measured in the previous 3 months; agreed to participate in the study. Exclusion criteria: active chronic inflammatory intestinal disease; malignancy; immunosuppressive treatment; decreased kidney function; severe heart disease; psychiatric, addictive or any other disorder compromising the patient’s ability to participate in the study or to give truly informed consent Participant characteristics:
|
|
| Interventions |
Intervention: 20 g protein per 200 mL bottle, 1 kcal/mL, nutritional supplement with added vitamins, minerals and trace elements (n = 26); taken orally, once daily Control: 400 mL placebo daily (n = 27); taken orally, once daily |
|
| Outcomes |
Complete wound healing: 12/26 intervention; 10/27 placebo; P > 0.05 Amputation: 2/26 intervention, none reported in the placebo group Death: 1/26 intervention and 1/27 control |
|
| Notes | This study was sponsored by a grant from Nutricia AB, Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Statistically significant differences between the groups at baseline for the following parameters:
|
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "each set of packages used for one patient had a number on it, indicating whether it was a placebo or intervention. Neither the patient nor the individual responsible for handing out the package knew what the numbers represented." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote "the participants and investigator were blinded to treatment until the trial end." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Wound healing outcomes were provided for the participants who dropped out of the study |
| Selective reporting (reporting bias) | Low risk | Reported: complete wound healing, amputation and death |
| Other bias | Low risk | None detected |
Jones 2014.
| Study characteristics | ||
| Methods |
Study design: prospective RCT (pilot study) Setting/location: diabetic outpatient clinic at the department of general surgery, University of Nevada School of Medicine, Las Vegas, Nevada, USA Study duration: 2 weeks |
|
| Participants |
Sample size: n = 9 participants Inclusion criteria:
Exclusion criteria:
Participant characteristics:
|
|
| Interventions |
Intervention:
Control:
|
|
| Outcomes |
Primary outcome
|
|
| Notes | No data provided for any of the primary or secondary outcomes as outlined a priori in the review protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Differences between the groups at baseline for the following parameter:
|
| Allocation concealment (selection bias) | Unclear risk | Quote: "were assigned randomly from a blinded third party" Comment: however, how this was undertaken is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "physicians and laboratory technicians who worked with the study participants, the participants themselves, and researchers involved in the study were also blinded to whether the patients were assigned to treatment or placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "physicians and laboratory technicians who worked with the study participants, the participants themselves, and researchers involved in the study were also blinded to whether the patients were assigned to treatment or placebo." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for all 6 participants |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None detected |
Momen‐Heravi 2017.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Setting/location: Shahid Beheshti Clinic in Kashan, Iran Date: August 2015‐November 2015 Study duration: 12 weeks |
|
| Participants |
Sample size: n = 60 participants Inclusion criteria:
Exclusion criteria:
Participant characteristics:
|
|
| Interventions |
Intervention:
Control:
|
|
| Outcomes |
Primary outcome
|
|
| Notes | The study was funded by a grant from the Vice Chancellor for Research, Kashan University of Medical Sciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization assignment was conducted by using computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization and allocation were concealed from the researchers and participants until the final analyses were completed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "allocation was concealed from the participants until the final analyses were completed" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "allocation was concealed from the researchers until the final analyses were completed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All those randomised were analysed according to ITT |
| Selective reporting (reporting bias) | Low risk | Study registered and all outcomes reported |
| Other bias | Low risk | None detected |
Mozaffari‐Khosravi 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Setting/location: Diabetic center of Hamadan University of Medical Sciences Study duration: 4 weeks |
|
| Participants |
Sample size: n = 47 participants Inclusion criteria:
Exclusion criteria:
Participant characteristics:
|
|
| Interventions |
Group A received 150,000 IU of vitamin D through intramuscular injection Group B received 300,000 IU of vitamin D through intramuscular injection |
|
| Outcomes |
Primary outcome Wound healing: wound area reduction |
|
| Notes | No funding declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients were randomly divided into two groups A and B according to the table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 participant excluded in group A and 2 participants excluded in group B |
| Selective reporting (reporting bias) | Low risk | Study registered and all outcomes reported |
| Other bias | Low risk | None detected |
Razzaghi 2017.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Setting/location: Department of Infectious Disease, School of Medicine, Kashan University of Medical Sciences, Kashan, Iran Study duration: 12 weeks |
|
| Participants |
Sample size: n = 60 participants Inclusion criteria:
Exclusion criteria:
Participant characteristics:
|
|
| Interventions |
Intervention:
Control:
|
|
| Outcomes |
Primary outcome
|
|
| Notes | Funded by a grant from the Vice Chancellor for Research, KUMS, and Iran | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Randomization assignment was performed using computer generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote "Randomization and allocation were concealed from the researchers and participants until the final analyses were completed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Vitamin D supplements and placebo capsules were similar in shape and size |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote "Randomization and allocation were concealed from the researchers and participants until the final analyses were completed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All those randomised were analysed at end of study |
| Selective reporting (reporting bias) | Low risk | Study registered and all outcomes reported |
| Other bias | Low risk | None detected |
Razzaghi 2018.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Setting/location: Naghavi Hospital in Kashan, Iran Date: December 2016‐February 2017 Study duration: 12 weeks |
|
| Participants |
Sample size: n = 70 participants Inclusion criteria:
Exclusion criteria:
Participant characteristics:
|
|
| Interventions |
Intervention:
Control:
|
|
| Outcomes |
Primary outcome
|
|
| Notes | The study was funded by a grant from the Vice Chancellor for Research, Kashan University of Medical Sciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization assignment was conducted by using computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization and allocation were concealed from the researchers and participants until the final analyses were completed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "allocation was concealed from the participants until the final analyses were completed" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "allocation was concealed from the researchers until the final analyses were completed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All those randomised were analysed according to ITT |
| Selective reporting (reporting bias) | Low risk | Study registered and all outcomes reported |
| Other bias | Low risk | None detected |
Soleimani 2017.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial Setting/location: an infectious clinic affiliated to Kashan University of Medical Sciences (KUMS), Kashan, Iran Date: April 2016‐July 2016 Study duration: 12 weeks |
|
| Participants |
Sample size: n = 60 participants Inclusion criteria:
Exclusion criteria:
Participant characteristics:
|
|
| Interventions |
Intervention:
Control:
|
|
| Outcomes |
Primary outcome
|
|
| Notes | The study was funded by a grant from the Vice Chancellor for Research, Kashan University of Medical Sciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization assignment was conducted by using computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization and allocation were concealed from the researchers and participants until the final analyses were completed" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "allocation was concealed from the participants until the final analyses were completed" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "allocation was concealed from the researchers until the final analyses were completed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All those randomised were analysed according to ITT |
| Selective reporting (reporting bias) | Low risk | Study registered and all outcomes reported |
| Other bias | Low risk | None detected |
BMI: body mass index; CFU/g: colony‐forming unit, is a unit used to estimate the number of viable bacteria or fungal cells in a sample; D: depth; DFU: diabetic foot ulcer; EQ‐5D: EuroQoL‐5D; HbA1c: measurement of blood glucose; HMB: β‐hydroxy‐β‐methylbutyrate; HRQoL: health‐related quality of life; ITT: intention‐to‐treat; L: length; PEM: protein‐energy malnutrition; QoL: quality of life; RCT: randomised controlled trial; W: width
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Mohseni 2018 | Explored the impact of a probiotic, therefore did not meet our inclusion criteria |
Characteristics of studies awaiting classification [ordered by study ID]
NCT00711217.
| Methods | RCT |
| Participants | Individuals with DFUs |
| Interventions | Medical food versus placebo |
| Outcomes | Primary: wound healing (time frame: 16 weeks) |
| Notes |
DFU: diabetic foot ulcer; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12612000036819.
| Study name | The effect of nutritional supplementation on the healing of diabetic foot ulcers |
| Methods | RCT |
| Participants | Individuals with DFUs |
| Interventions | commercial arginine powder |
| Outcomes | Healing rate of the foot ulcer |
| Starting date | 30th March 2012 |
| Contact information | tim.crowe@deakin.edu.au |
| Notes |
IRCT20100102002954N12.
| Study name | Evaluation of the efficacy of vitamin D in the treatment of diabetic foot ulcers |
| Methods | RCT |
| Participants | Individuals with DFUs |
| Interventions | 50,000 units of vitamin D per week versus usual care |
| Outcomes | DFU area reduction |
| Starting date | 23 September 2018 |
| Contact information | Mahsa Azimi; m.azimi458@gmail.com |
| Notes |
IRCT2015041321740N1.
| Study name | Comparison effect of two different doses of vitamin d injection supplements on foot ulcer status in type II diabetic patients with vitamin d deficiency |
| Methods | RCT |
| Participants | Individuals with DFUs |
| Interventions | vitamin D injection |
| Outcomes | Foot ulcers scale |
| Starting date | 17th March 2015 |
| Contact information | Haratianmohsen@yahoo.com |
| Notes |
IRCT201506215623N46.
| Study name | Effects of zinc supplementation on metabolic profiles, inflammatory factors and biomarkers of oxidative stress in patients with diabetic foot |
| Methods | RCT |
| Participants | Individuals with DFUs |
| Interventions | zinc supplements |
| Outcomes | Mean ulcer area |
| Starting date | 2nd June 2015 |
| Contact information | asemi_r@yahoo.com |
| Notes |
IRCT2017090533941N21.
| Study name | Clinical trial of the effect of combined magnesium and vitamin E supplementation compared with the placebo on metabolic profiles in patients with diabetic foot ulcer |
| Methods | RCT |
| Participants | Individuals with DFUs |
| Interventions | combined magnesium and vitamin E supplements |
| Outcomes | Decrease of the wound size relative to original size: ulcer length (cm), ulcer width (cm), Ulcer depth (cm) |
| Starting date | 20th October 2017 |
| Contact information | asemi_z@kaums.ac.ir |
| Notes |
NCT03679273.
| Study name | Nutritional supplement on wound healing in diabetic foot |
| Methods | RCT |
| Participants | Individuals with DFUs |
| Interventions | Abound supplement versus traditional supplement |
| Outcomes | Measurement of wound size change |
| Starting date | 1 October 2018 |
| Contact information | Yu‐Yao Huang; yyh@gmail.com |
| Notes |
NCT03813927.
| Study name | Vitamin D treatment of diabetic patients with foot ulcers |
| Methods | RCT |
| Participants | Individuals with DFUs |
| Interventions | Supplementation with tablet 170 μg Vitamin D each day versus placebo |
| Outcomes | Wound healing (time frame: 48 weeks or wound healing) |
| Starting date | 31 December 2017 |
| Contact information | Peter Max Halschou‐Jensen, MD; Zealand University Hospital |
| Notes |
NCT04055064.
| Study name | The effects of nutrition supplementation and education on the healing of diabetic foot ulcers (DFU) |
| Methods | RCT |
| Participants | Individuals with DFUs |
| Interventions | Dietary Supplement: glucose control nutritional shake, nutrition education |
| Outcomes | Improvement in wound healing rate |
| Starting date | May 23, 2017 |
| Contact information | Raedeh Basiri, Florida State University |
| Notes |
DFU: diabetic foot ulcer; RCT: randomised controlled trial
Differences between protocol and review
We prespecified “absolute or percentage change in ulcer area or volume over time” as a primary outcome. However the outcomes reported in some of the studies are individual parameters of ulcer dimensions. In order to ensure that relevant studies are captured in this review, we have included these have reported them as relevant in the main text and in the 'Summary of findings' tables. Where these outcomes are included we have downgraded the evidence for indirectness.
We have amended the title of the review to remove the term 'systemic' in line with feedback from peer reviewers.
Contributions of authors
Zena Moore: conceived, designed and co‐ordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; secured funding; approved the final review prior to submission and is guarantor of the review.
Meave Corcoran: conceived, designed and co‐ordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; secured funding; and approved the final review prior to submission;
Declan Patton: extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; and approved the final review prior to submission.
Contributions of editorial base:
Joan Webster, Editor: edited the protocol; advised on methodology, interpretation and protocol content. Approved the final protocol prior to submission.
Jo Dumville, Editor: edited the review; advised on methodology, interpretation and review content. Approved the final review prior to submission.
Gill Rizzello (Managing Editor): co‐ordinated the editorial process. Advised on interpretation and content. Edited the review.
Naomi Shaw and Sophie Bishop (Information Specialists): ran the searches and edited the search methods section of the review.
Ursula Gonthier and Tom Patterson (Editorial Assistants): edited the Plain language summary and reference sections of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Health Research Board, Ireland
-
National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Declarations of interest
Zena Moore: received an honorarium for speaking at professional meetings for Smith & Nephew, and Molnlycke. Neither of these companies manufacture or market the intervention of interest, or a potential comparator relevant to the topic of this review.
Meave Corcoran: received a Cochrane Fellowship grant from the Health Research Board of Ireland to undertake this review.
Declan Patton: none known.
New
References
References to studies included in this review
Afzali 2019 {published data only}
- Afzali H, Jafari Kashi AH, Momen-Heravi M, Razzaghi R, Amirani E, Bahmani F, et al. The effects of magnesium and vitamin E co-supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Wound Repair and Regeneration 2019;27(3):277-84. [DOI] [PubMed] [Google Scholar]
Armstrong 2014 {published data only}
- Armstrong DG, Hanft JR, Driver VR, Smith AP, Lazaro-Martinez JL, Reyzelman AM, et al, Diabetic Foot Nutrition Study Group. Effect of oral nutritional supplementation on wound healing in diabetic foot ulcers: a prospective randomized controlled trial. Diabetic Medicine 2014;31(9):1069-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eneroth 2004 {published data only}
- Eneroth M, Larsson J, Oscarsson DC, Apelqvist J. Nutritional supplementation for diabetic foot ulcers: the first RCT. Journal of Wound Care 2004;13(6):230-4. [DOI] [PubMed] [Google Scholar]
Jones 2014 {published data only}
- Jones MS, Rivera M, Puccinelli CL, Wang MY, William SJ, Barber AE. Targeted amino acid supplementation in diabetic foot wounds: pilot data and a review of the literature. Surgical Infections 2014;15(6):708-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Momen‐Heravi 2017 {published data only}
- Momen-Heravi M, Barahimi E, Razzaghi R, Bahmani F, Gilasi HR, Asemi Z. The effects of zinc supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Wound Repair and Regeneration 2017;25:512-20. [DOI] [PubMed] [Google Scholar]
Mozaffari‐Khosravi 2017 {published data only}
- Mozaffari-Khosravi H, Haratian-Arab M, MoeinTavakkoli H, Nadjarzadeh A. Comparative effect of two different doses of vitamin D on diabetic foot ulcer and inflammatory indices among the type 2 diabetic patients: a randomized clinical trial. Iranian Journal of Diabetes and Obesity 2017;8(4):164-71. [Google Scholar]
Razzaghi 2017 {published data only}
- Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, et al. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Journal of Diabetes and its Complications 2017;31(4):766-72. [DOI] [PubMed] [Google Scholar]
Razzaghi 2018 {published data only}
- Razzaghi R, Pidar F, Momen-Heravi M, Bahmani F, Akbari H, Asemi Z. Magnesium supplementation and the effects on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Biological Trace Element Research 2018;181:207-15. [DOI] [PubMed] [Google Scholar]
Soleimani 2017 {published data only}
- Soleimani Z, Hashemdokht F, Bahmani F, Taghizadeh M, Memarzadeh MR, Asemi Z. Clinical and metabolic response to flaxseed oil omega-3 fatty acids supplementation in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Journal of Diabetes and its Complications 2017;31:1394-400. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Mohseni 2018 {published data only}
- Mohseni S, Bayani M, Bahmani F, Tajabadi-Ebrahimi M, Bayani MA, Jafari P, et al. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Diabetes/Metabolism Research and Reviews 2018;34(3):e2970. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00711217 {published data only}
- NCT00711217. Evaluation of a medical food for chronic wounds. clinicaltrials.gov/ct2/show/NCT00711217 (first received 8 July 2008).
References to ongoing studies
ACTRN12612000036819 {published data only}
- ACTRN12612000036819. The effect of nutritional supplementation on the healing of diabetic foot ulcers. org.au/Trial/Registration/TrialReview.aspx?id=347920 (first received 9th January 2012).
IRCT20100102002954N12 {published data only}
- IRCT20100102002954N12. Evaluation of the efficacy of vitamin D in the treatment of diabetic foot ulcers. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20100102002954N12 (first received 22 September 2018).
IRCT2015041321740N1 {published data only}
- IRCT2015041321740N1. Comparison effect of two different doses of vitamin D injection supplements on foot ulcer status in type II diabetic patients with vitamin D deficiency. en.irct.ir/trial/18968 (first received 16th May 2015).
IRCT201506215623N46 {published data only}
- IRCT201506215623N46. Effects of zinc supplementation on metabolic profiles, inflammatory factors and biomarkers of oxidative stress in patients with diabetic foot. en.irct.ir/trial/6076?revision=6076 (first received 3rd July 2015).
IRCT2017090533941N21 {published data only}
- IRCT2017090533941N21. Clinical trial of the effect of combined magnesium and vitamin E supplementation compared with the placebo on metabolic profiles in patients with diabetic foot ulcer. en.irct.ir/trial/26063 (first received 16th November 2017).
NCT03679273 {published data only}
- NCT03679273. Nutritional supplement on wound healing in diabetic foot. clinicaltrials.gov/ct2/show/NCT03679273 (first received 20 September 2018).
NCT03813927 {published data only}
- NCT03813927. Vitamin D treatment of diabetic patients with foot ulcers. clinicaltrials.gov/ct2/show/NCT03813927 (first received 23 January 2019).
NCT04055064 {published data only}
- NCT04055064. The effects of nutrition supplementation and education on the healing of diabetic foot ulcers (DFU). clinicaltrials.gov/ct2/show/NCT04055064 (first received 13th August 2019).
Additional references
Ackerman 2013
- Ackerman PW, Hart DA. Influence of comorbidities: neuropathy, vasculopathy, and diabetes on healing response quality. Advances in Wound Care 2013;2:410-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
ADA 2008
- American Diabetes Association (ADA). Diagnosis and classification of diabetes mellitus. Diabetes Care 2008;31 Suppl 1:S55-60. [DOI] [PubMed] [Google Scholar]
Akl 2012
- Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW, et al. Potential impact on estimated treatment effects of information lost to follow-up in randomised controlled trials (LOST-IT): systematic review. BMJ 2012;344:e3469. [DOI] [PubMed] [Google Scholar]
Albahrani 2016
- Albahrani AA, Greaves RF. Fat-soluble vitamins: clinical indications and current challenges for chromatographic measurement. Clinical Biochemist Reviews 2016;37(1):27-47. [PMC free article] [PubMed] [Google Scholar]
Armstrong 2011
- Armstrong DG, Cohen K, Courric S, Bharara M, Marston W. Diabetic foot ulcers and vascular insufficiency: our population has changed, but our methods have not. Journal of Diabetes Science and Technology 2011;5(6):1591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azaïs‐Braesco 2000
- Azaïs-Braesco V, Pascal G. Vitamin A in pregnancy: requirements and safety limits. American Journal of Clinical Nutrition 2000;7(5):1325-33. [DOI] [PubMed] [Google Scholar]
BDA 2001
- The British Dietetic Association (BDA). Manual of Dietetic Practice. 3rd edition. Oxford (UK): Blackwell, 2001. [Google Scholar]
Bell 2013
- Bell ML, Kenward MG, Fairclough DL, Horton NJ. Differential dropout and bias in randomised controlled trials: when it matters and when it may not. BMJ 2013;346:e8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowling 2004
- Bowling T, editor(s). Nutritional Support for Adults and Children: A Handbook for Hospital Practice. Abingdon (UK): Radcliffe Medical Press, 2004. [Google Scholar]
Castellini 2018
- Castellini G, Bruschettini M, Gianola S, Gluud C, Moja L. Assessing imprecision in Cochrane systematic reviews: a comparison of GRADE and trial sequential analysis. Systematic Reviews 2018;7:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2005
- Collins CE, Kershaw J, Brockington S. Effect of nutritional supplements on wound healing in home-nursed elderly: a randomized trial. Nutrition 2005;21(2):147-55. [DOI] [PubMed] [Google Scholar]
CONSORT 2010
- Consolidated Standards of Reporting Trials (CONSORT). Allocation concealment. www.consort-statement.org/resources/glossary#A (accessed 16 February 2018).
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Egger 1997
- Egger M, Zellweger-Zähner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet 1997;350(9074):326–9. [DOI] [PubMed] [Google Scholar]
Fortington 2013
- Fortington LV, Geertzen JH, Van Netten JJ, Postema K, Rommers GM, Dijstra PU. Short and long term mortality rates after a lower limb amputation. European Journal of Vascular and Endovascular Surgery 2013;46:124-31. [DOI] [PubMed] [Google Scholar]
Frias Soriano 2004
- Frias Soriano L, Lage Vazquez MA, Perez-Portabella Maristany C, Xandri Graupera JM, Wouters-Wesseling W, Wagenaar L. The effectiveness of oral nutritional supplementation in the healing of pressure ulcers. Journal of Wound Care 2004;13(8):319-22. [DOI] [PubMed] [Google Scholar]
Gottrup 2010
- Gottrup F, Apelqvist J, Price P. Outcomes in controlled and comparative studies on non-healing wounds: recommendations to improve the quality of evidence in wound management. Journal of Wound Care 2010;19:237-68. [DOI] [PubMed] [Google Scholar]
Gottschlich 2001
- Gottschlich MM. The Science and Practice of Nutrition Support: A Case-Based Core Curriculum. Dubuque (IA): Kendall Hunt, 2001. [Google Scholar]
Guest 2018
- Guest JF, Fuller GW, Vowden P. Diabetic foot ulcer management in clinical practice in the UK: costs and outcomes. International Wound Journal 2018;15:43-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxmanc AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64:383-94. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence--indirectness. Journal of Clinical Epidemiology 2011;64:1303-10. [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Brozeka J, Alonso-Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011;64:1283-93. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.training.cochrane.org/handbook.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.training.cochrane.org/handbook.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hurd 2004
- Hurd TA. Nutrition and wound care management/prevention. Wound Care Canada 2004;2(2):20-4. [Google Scholar]
IDF 2017
- International Diabetes Federation (IDF). IDF Diabetes Atlas. Eighth edition 2017. www.diabetesatlas.org/ (accessed 5 June 2019).
Jeffcoate 2018
- Jeffcoate WJ, Vileikyte L, Boyko EJ, Armstrong DG, Boulton AJ. Current challenges and opportunities in the prevention and management of diabetic foot ulcers. Diabetes Care 2018;41:645-52. [DOI] [PubMed] [Google Scholar]
Jupiter 2016
- Jupiter DC, Thorud JC, Buckley CJ, Shibuya N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. International Wound Journal 2016;13:892-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jüni 2005
- Jüni P, Egger M. Commentary: empirical evidence of attrition bias in clinical trials. International Journal of Epidemiology 2005;34(1):87-8. [DOI] [PubMed] [Google Scholar]
Kennedy‐Martin 2015
- Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials 2015;16:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Altman DG, Williamson PR. Bias due to changes in specified outcomes during the systematic review process. PLoS One 2010;5(3):e9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavery 1996
- Lavery LA, Armstrong DG, Harkless LB. Classification of diabetic foot wounds. Journal of Foot and Ankle Surgery 1996;35(6):528-31. [DOI] [PubMed] [Google Scholar]
Lavery 2008
- Lavery LA, Peters EJ, Williams JR, Murdoch DP, Hudson A, Lavery DC. Re-evaluating the way we classify the diabetic foot. Diabetes Care 2008;31:154-6. [DOI] [PubMed] [Google Scholar]
Lee 2006
- Lee SK, Posthauer ME, Dorner B, Redovian V, Maloney MJ. Pressure ulcer healing with a concentrated, fortified, collagen protein hydrolysate supplement: a randomized controlled trial. Advances in Skin and Wound Care 2006;19(2):92-6. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Leininger 2002
- Leininger S. The role of nutrition in wound healing. Critical Care Nursing Quarterly 2002;1:13-21. [Google Scholar]
Lin 2017
- Lin PH, Sermersheim M, Li H, Lee PH, Steinberg SM, Ma J. Zinc in wound healing modulation. Nutrients 2017;10(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marcovitch 2017
- Marcovitch H, editor(s). Black's Medical Dictionary. 43rd edition. London (UK): Bloomsbury, 2017. [Google Scholar]
Martin 1992
- Martin P, Hopkinson-Woolley J, McCluskey J. Growth factors and cutaneous wound repair. Progress in Growth Factor Research 1992;4(1):25-44. [DOI] [PubMed] [Google Scholar]
Medlin 2012
- Medlin S. Nutrition for wound healing. British Journal of Nursing 2012;21(12):11-5. [DOI] [PubMed] [Google Scholar]
Meggitt 1976
- Meggitt B. Surgical management of the diabetic foot. British Journal of Hospital Medicine 1976;16:227-32. [Google Scholar]
Merriam‐Webster 2016
- Merriam-Webster, editor(s). Merriam-Webster's Medical Dictionary. Martinsburg (WV): Merriam-Webster, 2016. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Medicine 6;7:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2017
- National Institute for Health and Care Excellence (NICE). Nutrition support in adults: oral nutrition support, enteral tube feeding and parenteral nutrition. Clinical guideline [CG32]. Updated August 2017. www.nice.org.uk/guidance/CG32 (accessed 16 February 2018).
O'Connor 2013
- O'Connor DP, Brinker MR. Challenges in outcome measurement: clinical research perspective. Clinical Orthopaedics and Related Research 2013;471(11):3496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohura 2011
- Ohura T, Nakajo T, Okada S, Omura K, Adachi K. Evaluation of effects of nutrition intervention on healing of pressure ulcers and nutritional states (randomized controlled trial). Wound Repair and Regeneration 2011;19:330-6. [DOI] [PubMed] [Google Scholar]
Omote 2005
- Omote S, Sugama J, Sanada H, Konya C, Okuwa M, Kitagawa A. Healing process of pressure ulcers after a change in nutrition of bedridden elderly: a case series. Japan Journal of Nursing Science 2005;2(2):85-93. [Google Scholar]
Prompers 2008
- Prompers L, Huijberts M, Schaper N, Apelqvist J, Bakker K, Edmonds M, et al. Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale Study. Diabetologia 2008;51:1826-34. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 1998
- Roberts C, Torgerson D. Randomisation methods in controlled trials. BMJ 1998;317:1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 1999
- Roberts C, Torgerson DJ. Baseline imbalance in randomised controlled trials. BMJ 1999;319(7203):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez‐Moran 2001
- Rodriguez-Moran M, Guerrero-Romero F. Low serum magnesium levels and foot ulcers in subjects with type 2 diabetes. Archives of Medical Research 2001;31:300-3. [DOI] [PubMed] [Google Scholar]
Rothman 1995
- Rothman KJ, Moore LL, Singer MR, Nguyen UD, Mannino S, Milunsky A. Teratogenicity of high vitamin A intake. New England Journal of Medicine 1995;333(21):1369-73. [DOI] [PubMed] [Google Scholar]
Sanders 2015
- Sanders LJ. Johns Hopkins diabetes guide: foot ulcers. www.hopkinsguides.com/hopkins/view/Johns_Hopkins_Diabetes_Guide/547054/all/Foot_Ulcers (accessed 16 February 2018).
Schrezenmeir 2001
- Schrezenmeir J, De Vrese M. Probiotics, prebiotics and synbiotics: approaching a definition. American Journal of Clinical Nutrition 2001;73(2):361-4. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2017a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al, on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Schünemann 2017b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al, on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Shin 2017
- Shin J, Yang SJ, Lim Y. Gamma-tocopherol supplementation ameliorated hyper-inflammatory response during the early cutaneous wound healing in alloxan-induced diabetic mice. Experimental Biological Medicine 2017;242:502-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2018
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/search-filters.html (accessed 6 July 2018).
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Tatti 2012
- Tatti P, Barber A. The use of a specialized nutritional supplement for diabetic foot ulcers reduces the use of antibiotics. Journal of Endocrinology and Metabolism 2012;2(1):26-31. [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18:2693-708. [DOI] [PubMed] [Google Scholar]
UK PDS 1998
- United Kingdom Prospective Diabetes Study Group (UK PDS). Intensive blood glucose control with sulphonylurea or insulin compared with conventional treatment and the risk of complications in type 2 diabetes. Lancet 1998;352(9144):837-53. [PubMed] [Google Scholar]
Van Etten 2004
- Van Etten E, Decallonne B, Bouillon R, Mathieu C. NOD bone marrow derived dendritic cells are modulated by analogs of 1,25-dihydroxyvitamin D3. Journal of Steroid Biochemistry and Molecular Biology 2004;89-90:457-9. [DOI] [PubMed] [Google Scholar]
Wagner 1981
- Wagner FW. The dysvascular foot: a sytem of diagnosis and treatment. Foot and Ankle 1981;2(2):64-122. [DOI] [PubMed] [Google Scholar]
Zhang 2013
- Zhang S, Tang Z, Fang P, Qian H, Xu L, Ning G. Nutritional status deteriorates as the severity of diabetic foot ulcer increases and independently associates with prognosis. Experimental and Therapeutic Medicine 2013;5:215-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2017
- Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Annals of Medicine 2017;49(2):106-16. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Corcoran 2014
- Corcoran MA, Moore ZE. Systemic nutritional interventions for treating foot ulcers in people with diabetes. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD011378] [DOI] [PMC free article] [PubMed] [Google Scholar]


