Abstract
Background
Some antiepileptic drugs but not others are useful in clinical practice for the prophylaxis of migraine. This might be explained by the variety of actions of these drugs in the central nervous system. The present review is part of an update of a Cochrane review first published in 2004, and previously updated (conclusions not changed) in 2007.
Objectives
To describe and assess the evidence from controlled trials on the efficacy and tolerability of topiramate for preventing migraine attacks in adult patients with episodic migraine.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2012, Issue 12), PubMed/MEDLINE (1966 to 15 January 2013), MEDLINE In‐Process (current week, 15 January 2013), and EMBASE (1974 to 15 January 2013) and handsearched Headache and Cephalalgia through January 2013.
Selection criteria
Studies were required to be prospective, controlled trials of topiramate taken regularly to prevent the occurrence of migraine attacks, to improve migraine‐related quality of life, or both.
Data collection and analysis
Two review authors independently selected studies and extracted data. For headache frequency data, we calculated mean differences (MDs) between topiramate and comparator (placebo, active control, or topiramate in a different dose) for individual studies and pooled these across studies. For dichotomous data on responders (patients with ≥ 50% reduction in headache frequency), we calculated odds ratios (ORs) and, in select cases, risk ratios (RRs); we also calculated numbers needed to treat (NNTs). We calculated MDs for selected quality of life instruments. Finally, we summarised data on adverse events from placebo‐controlled trials and calculated risk differences (RDs) and numbers needed to harm (NNHs).
Main results
Twenty papers describing 17 unique trials met the inclusion criteria. Analysis of data from nine trials (1737 participants) showed that topiramate reduced headache frequency by about 1.2 attacks per 28 days as compared to placebo (MD ‐1.20; 95% confidence interval (CI) ‐1.59 to ‐0.80). Data from nine trials (1190 participants) show that topiramate approximately doubled the proportion of responders relative to placebo (RR 2.02; 95% CI 1.57 to 2.60; NNT 4; 95% CI 3 to 6). Separate analysis of different topiramate doses produced similar MDs versus placebo at 50 mg (‐0.95; 95% CI ‐1.95 to 0.04; three studies; 520 participants), 100 mg (‐1.15; 95% CI ‐1.58 to ‐0.71; six studies; 1620 participants), and 200 mg (‐0.94; 95% CI ‐1.53 to ‐0.36; five studies; 804 participants). All three doses significantly increased the proportion of responders relative to placebo; ORs were as follows: for 50 mg, 2.35 (95% CI 1.60 to 3.44; three studies; 519 participants); for 100 mg, 3.49 (95% CI 2.23 to 5.45; five studies; 852 participants); and for 200 mg, 2.49 (95% CI 1.61 to 3.87; six studies; 1025 participants). All three doses also significantly improved three or more domains of quality of life as compared to placebo. Meta‐analysis of the three studies that included more than one dose of topiramate suggests that 200 mg is no more effective than 100 mg. With regard to mean headache frequency and/or responder rate, seven trials using active comparators found (a) no significant difference between topiramate and amitriptyline (one study, 330 participants); (b) no significant difference between topiramate and flunarizine (one study, 83 participants); (c) no significant difference between topiramate and propranolol (two studies, 342 participants); (d) no significant difference between topiramate and relaxation (one study, 61 participants); but (e) a slight significant advantage of topiramate over valproate (two studies, 120 participants). Relaxation improved migraine‐specific quality of life significantly more than topiramate. In trials of topiramate against placebo, seven adverse events (AEs) were reported by at least three studies. These were usually mild and of a non‐serious nature. Except for taste disturbance and weight loss, there were no significant differences in the frequency of AEs in general, or of the seven specific AEs, between placebo and topiramate 50 mg. AEs in general and all of the specific AEs except nausea were significantly more common on topiramate 100 mg than on placebo, with NNHs varying from 3 to 25, and the RDs versus placebo were even higher for topiramate 200 mg, with NNHs varying from 2 to 17.
Authors' conclusions
Meta‐analysis demonstrates that topiramate in a 100 mg/day dosage is effective in reducing headache frequency and reasonably well‐tolerated in adult patients with episodic migraine. This provides good evidence to support its use in routine clinical management. More studies designed specifically to compare the efficacy or safety of topiramate versus other interventions with proven efficacy in the prophylaxis of migraine are needed.
Plain language summary
Topiramate for preventing migraine attacks in adults
Various medicines, collectively termed 'antiepileptics', are used to treat epilepsy. For several years, some of these drugs have also been used for preventing migraine attacks. For the present review, researchers in The Cochrane Collaboration reviewed the evidence about the effects of topiramate in adult patients (≥ 16 years of age) with 'episodic' migraine (headache on < 15 days per month). They examined research published up to 15 January 2013 and found 17 relevant studies. Compared with placebo, topiramate reduced the frequency of migraine headaches by approximately 1.2 per month (nine studies, 1737 participants). Patients were also about twice as likely to reduce the number of their migraine headaches by 50% or more with topiramate than with placebo (nine studies, 1190 participants). Side effects associated with topiramate were common but generally mild; topiramate can, however, cause birth defects and so should be used with caution in women of childbearing age. Further research is needed comparing topiramate with other active drugs used for preventing migraine attacks.
Background
Description of the condition
Migraine is a common and disabling health problem among children and predominantly young and middle‐aged adults. Surveys from the main regions of the world suggest that the global prevalence of migraine is 14.7% (18.8% among women and 10.7% among men) (GBD 2010 Study). This disorder results in significant disability and work loss, and several studies have addressed the issue of the costs of migraine. In one of the most recent publications, aggregate direct and indirect costs to society due to migraine among adults in the European Union were estimated to amount to 50 billion Euros (67 billion US dollars) annually, or about 1222 Euros (1634 US dollars) annually per sufferer (Linde 2012).
Description of the intervention
Drug therapy for migraine falls into two categories: acute and preventive. Acute therapy aims at the symptomatic treatment of the head pain and other symptoms associated with an acute attack of migraine. The primary goals of preventive treatment are to reduce attack frequency, severity, and duration. Moreover, such therapy is commonly employed in an attempt to improve responsiveness to acute treatment, enhance functional status, and reduce disability. Evidence‐based guidelines on the drug treatment of migraine have been developed and published by the European Federation of Neurological Societies (EFNS; Evers 2009). These guidelines suggest that prophylactic therapy should be considered for patients with migraine when quality of life, business duties, or school attendance are severely impaired; when the frequency of attacks is two or more per month; when there is a lack of response to acute drug treatment; and when frequent, very long, or uncomfortable auras occur.
This review considers the evidence for the efficacy and tolerability of topiramate for preventing episodic migraine in adults. The prophylactic treatment of migraine in children is the subject of a separate Cochrane review (Victor 2003).
Topiramate is a sulphamate‐substituted monosaccharide (2,3:4,5‐Bis‐O‐(1‐methylethylidene)‐b‐D‐fructo‐pyranose sulfamate) derived from the naturally occurring sugar D‐fructose (Edvinsson 2010). The absolute bioavailability, or oral bioavailability, of topiramate is 81% to 95% and is not affected by food. The distribution volume for women is approximately 50% that of men. Topiramate is metabolised to a moderate degree (circa 20%). Six metabolites have been identified. Total clearance is low (20 to 30 mL/min) after oral intake. This occurs predominantly via renal excretion (renal clearance 10 to 20 mL/min). Topiramate has a long half‐life (19 to 25 hours). Patients with normal renal function may need four to eight days to reach steady‐state plasma concentrations. The typical dose range of topiramate used in migraine is 50 to 200 mg.
How the intervention might work
We use the term 'antiepileptics' here to refer generally to those drugs in common use for the treatment of epilepsy. The pharmacological treatment of epilepsy can be traced back as far as 1857, but the period of greatest development of antiepileptics was between 1935 and 1960, when 13 drugs were developed and marketed (Porter 1992). In recent decades, renewed interest has led to the development of several novel antiepileptics which may confer advantages in tolerability (Dalkara 2012), and these are beginning to be used in migraine also.
The use of antiepileptics for the prophylactic treatment of migraine is theoretically warranted by several known modes of action which relate either to the general modulation of pain systems or more specifically to systems involved in the pathophysiology of migraine (Silberstein 2008; Wiffen 2010). It is necessary to point out, however, that it is not currently possible to state with any certainty which particular mode or modes of action of topiramate are relevant to the prophylaxis of migraine (Edvinsson 2010). The efficacy of topiramate in migraine seems to be mediated by the interaction with multiple sites of action. It decreases the frequency of action potentials elicited by depolarising electric current, which gives expression to a blockade of voltage‐dependent Na+ channels. Topiramate modulates cortical excitability in migraineurs, but it does not appear that this alone explains the efficacy in migraine prophylaxis. Topiramate inhibits the excitatory activity of glutamate at the receptor subtype for kainate/AMPA. It has been shown to inhibit neurons of the trigeminocervical complex via a GABA‐mediated mechanism. Furthermore, topiramate inhibits the release of CGRP from prejunctional trigeminal neurons. An inhibitory effect on high‐voltage‐dependent (HVA) Ca2+ channels especially in periaqueductal grey is a possible mechanism to explain the therapeutic effect in migraine. A differential sensitivity to topiramate has been observed for HVA Ca2+ channels located on cortical neurons and those in the periaqueductal grey region (PAG). Topiramate inhibits N‐, P‐, and L‐type channels in PAG neurons, whereas in cortical neurons it modulates only P‐ and L‐type channels. Chronic, but not acute, treatment suppresses cortical spreading depression in rats. Long‐term effects on gene regulation have to be considered. Collectively this points towards a reduction in excitatory transmission and increase in inhibitory neurotransmission.
Why it is important to do this review
Some antiepileptic drugs are marketed specifically for migraine prophylaxis. The EFNS (Evers 2009) and the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society (Silberstein 2012) list topiramate among first‐line migraine prophylactics. There is a fairly substantial body of evidence from controlled trials supporting the efficacy of many of the agents used for preventing migraine, yet such therapies are used by only a small percentage of patients with migraine — 3% to 12% in various studies (Clarke 1996; Edmeads 1993; Mehuys 2012). It is hoped that this review and others like it will increase awareness of migraine prophylactic treatment options and help to provide a systematic basis for making the best possible choice of such therapy in those individuals in need of it.
The present review is part of a series of reviews which, taken together, represent an update of a Cochrane review on 'Anticonvulsant drugs for migraine prophylaxis' (Chronicle 2004; Mulleners 2008; first published in 2004, and previously updated (conclusions not changed) in 2007). The old review has been split into four separate reviews for updating:
Topiramate for the prophylaxis of episodic migraine in adults (the present review; Linde 2013a)
Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults (Linde 2013b)
Gabapentin or pregabalin for the prophylaxis of episodic migraine in adults (Linde 2013c)
Antiepileptics other than gabapentin, pregabalin, topiramate, and valproate for the prophylaxis of episodic migraine in adults (Linde 2013d)
Objectives
To describe and assess the evidence from controlled trials on the efficacy and tolerability of topiramate for preventing migraine attacks in adult patients with episodic migraine.
Methods
Criteria for considering studies for this review
Types of studies
The International Headache Society (IHS) has provided a useful document setting out guidelines for the conduct of clinical trials in migraine, to which current investigators are encouraged to adhere (Tfelt‐Hansen 2012). This document was not used as the sole basis for considering studies in this review, as too many potentially informative past studies would likely have been excluded on methodological grounds. However, many of its recommendations have been used as a basis for what follows.
Included studies were required to be prospective, controlled trials of self administered topiramate taken regularly to prevent the occurrence of migraine attacks, to improve migraine‐related quality of life, or both. We included trials only if allocation to treatment groups was randomised or pseudo‐randomised (based on some non‐random process unrelated to the treatment selection or expected response). Blinding was not required. We excluded concurrent cohort comparisons and other non‐experimental designs.
Types of participants
Study participants were required to be adults (at least 16 years of age) and to meet reasonable criteria designed to distinguish migraine from tension‐type headache. If patients with both types of headache were included in a trial, results were required to be stratified by headache diagnosis. We did not require the use of a specific set of diagnostic criteria (eg, Ad Hoc Cttee 1962; IHS Cttee 1988; ICHD‐II 2004), but migraine diagnoses had to be based on at least some of the distinctive features of migraine, eg, nausea/vomiting, severe head pain, throbbing character, unilateral location, phono/photophobia, or aura. Secondary headache disorders had to be excluded using reasonable criteria.
We anticipated that some of the trials identified would include patients described as having mixed migraine and tension‐type headaches or combination headaches, and the protocol for this review described detailed procedures for dealing with such trials. In the end, no such precautions were necessary. We excluded studies evaluating treatments for chronic daily headache, chronic migraine, and transformed migraine. The reasons for this are: (a) the definition of chronic migraine is still heavily debated, and a revision of the 2004 IHS criteria for this condition has been proposed (Olesen 2006); (b) transformed migraine and chronic daily headache, although commonly used terms, are insufficiently validated diagnoses; (c) the separation of these conditions from headache due to medication overuse is not always clear in many studies; and (d) there is some evidence that suggests that chronic migraine may be more refractory to standard prophylactic treatment than episodic migraine. We explicitly excluded trials and treatment groups including only patients with tension‐type headache.
Types of interventions
Included studies were required to have at least one arm in which topiramate (without concomitant use of other migraine prophylactic treatment) was given regularly during headache‐free intervals with the aim of preventing the occurrence of migraine attacks, improving migraine‐related quality of life, or both. Acceptable comparator groups included placebo, no intervention, active drug treatment (ie, with proven efficacy, not experimental), the same drug treatment with a clinically relevant different dose, and non‐pharmacological therapies with proven efficacy in migraine. The analysis included only drugs and dosages that are commercially available.
We recorded any data reported on treatment compliance in the Characteristics of included studies table. After examination of these data, it did not seem necessary to stratify the analysis by compliance.
We anticipated that most trials would permit the use of medication for acute migraine attacks experienced during the trial period. We therefore recorded descriptions of trial rules concerning the use of acute medication in the Characteristics of included studies table whenever such information was provided. We did not otherwise model or adjust for this factor in our analysis.
Types of outcome measures
We collected and analysed trial data on headache frequency, responders (patients with ≥ 50% reduction in headache frequency), quality of life, and adverse events.
Search methods for identification of studies
Search strategies used in our earlier review (Chronicle 2004; Mulleners 2008) are detailed in Appendix 1 (last search date 31 December 2005). For the present update, trained information specialists developed detailed search strategies for each database searched (Appendix 2). The new searches overlapped the old searches by a full year to ensure complete coverage. The last search date for all updated searches was 15 January 2013.
Databases searched for this update were:
Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library 2012, Issue 12; years searched = 2005 to 2012);
MEDLINE (via OVID), 2005 to 15 January 2013;
MEDLINE In‐Process (via OVID), current week, 15 January 2013;
EMBASE (via OVID), 2005 to 15 January 2013.
Additional strategies for identifying trials included searching the reference lists of review articles and included studies, searching books related to headache, and consulting experts in the field. We attempted to identify all relevant published trials, irrespective of language. We handsearched two journals, Headache and Cephalalgia, in their entirety through January 2013.
Data collection and analysis
Selection of studies
Two of us independently screened titles and abstracts of studies identified by the literature search for eligibility. Papers that could not be excluded with certainty on the basis of information contained in the title and/or abstract were retrieved in full for screening. Disagreements were resolved through discussion. We retrieved papers passing this initial screening process, and two of us independently reviewed the full texts. Disagreements at the full‐text stage were resolved through internal discussion and, in a few cases, through correspondence with members of the editorial staff of the Cochrane Pain, Palliative and Supportive Care Review Group. We were not blinded to study investigators' names and institutions, journal of publication, or study results at any stage of the review.
The search strategy described above identified a large number of short conference and journal abstracts. The majority of these either (a) reported partial results of ongoing trials; (b) provided insufficient information on trial design or results; (c) were early reports of included studies; or (d) were reproductions of abstracts of papers published in full (for example, the journal Headache reproduces abstracts of interest to readers, and these are found by PubMed). We agreed that short abstracts of this kind would be excluded from consideration.
Data extraction and management
Two of us independently abstracted information on patients, methods, interventions, efficacy outcomes, and adverse events from the original reports onto specially designed, pre‐tested paper forms. Disagreements were again resolved through discussion.
We anticipated that trials would vary in length, that outcomes would be measured over various units of time (eg, number of attacks per two weeks versus number of attacks per four weeks), and that results would be reported for numerous different time points (eg, four‐week headache frequency at two months versus at four months). We attempted to standardise the unit of time over which headache frequency was measured at 28 days (four weeks) wherever possible. We recorded outcomes beginning four weeks after the start of treatment and continued through all later assessment periods. We made decisions about which time points to include in the final analysis once the data had been collected.
We anticipated that outcomes measured on a continuous scale (eg, headache frequency) would be reported in a variety of ways, eg, as mean pre‐treatment, post‐treatment, and/or change scores. Among change scores, we preferred the mean of within‐patient changes (from baseline to on‐treatment in a parallel‐group trial) over the change in group means because the first both results in a lower variance (taking into account the correlation between baseline and post‐treatment scores in each patient) and adjusts for imbalances in baseline headache frequencies, while the latter has only the second advantage. When neither type of change score was reported, we compared post–treatment means between groups, assuming that baseline data would be balanced due to randomisation. We anticipated that many trials would report group means, without reporting data on the variance associated with these means. In such cases, we attempted to calculate or estimate variances based on primary data, test statistics, and/or error bars in graphs.
When efficacy outcomes were reported in dichotomous form (success/failure), we required that the threshold for distinguishing between treatment success and failure be clinically significant; for example, we interpreted a ≥ 50% reduction in headache frequency as meeting this criterion. In such cases, we recorded, for each treatment arm, the number of patients included in the analysis and the number with each outcome.
The protocol for this review specified rules for dealing with outcome data reported on an ordinal scale (eg, for reduction in headache frequency: 0%, 1% to 24%, 25% to 49%, 50% to 74%, 75% to 99%, 100%) but, in fact, none of the included trials reported ordinal data for outcomes of interest.
We envisaged that the preferred methods of collecting and presenting data on quality of life would most likely be the Migraine‐Specific Questionnaire (MSQ) and the Medical Outcomes Study 36‐item Short‐Form Health Survey (SF‐36). However, other instruments and other types of outcomes related to quality of life (eg, work absenteeism) were not excluded a priori, and these data were kept under review before specifying rules for analysing outcome data in this domain.
We recorded the proportion of patients reporting adverse events for each treatment arm wherever possible. The identity and rates of specific adverse events were also recorded. We anticipated that reporting of adverse events would vary greatly across trials with regard to the terminology used, method of ascertainment, and classification of adverse events as drug‐related or not and as severe or not.
Assessment of risk of bias in included studies
We completed a 'Risk of bias' table for each study, using assessments of random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). For new studies identified in the present update, two of us completed this assessment independently; for older studies, one of us performed the assessment and a second author reviewed and commented on it. Disagreements were resolved through discussion.
We also assessed the methodological quality of individual trials using the scale devised by Jadad and colleagues (Jadad 1996), operationalised as follows:
Was the study described as randomised? (1 = yes; 0 = no)
Was the method of randomisation well described and adequate? (0 = not described; 1 = described and adequate; ‐1 = described, but not adequate)
Was the study described as double‐blind? (1 = yes; 0 = no)
Was the method of double‐blinding well described and adequate? (0 = not described; 1 = described and adequate; ‐1 = described, but not adequate)
Was there a description of withdrawals and dropouts sufficient to determine the number of patients in each treatment group entering and completing the trial? (1 = yes; 0 = no)
Each trial thus received a score of 0 to 5 points, with higher scores indicating higher quality in the conduct or reporting of the trial. Two review authors scored the studies independently, and a consensus score was then arrived at through discussion. The consensus score is reported for each study in the Characteristics of included studies table and was not used as a weighting in statistical analyses.
Measures of treatment effect
The primary outcome considered for the efficacy analysis was headache frequency. Among headache frequency measures, we preferred number of migraine attacks to number of days with migraine. The latter measure confusingly incorporates attack duration into the measure of headache frequency. Moreover, attack duration is affected by the use of symptomatic medication, which is permitted in most trials. We also analysed headache frequency in terms of a responder rate, or the proportion of patients with a ≥ 50% reduction in headache frequency from pre‐ to post‐treatment.
As noted above (Data extraction and management), we kept patient‐reported quality of life data under review as studies were selected. For comparisons with placebo and direct dose comparisons, the data chosen for a rigorous analysis were measured by the Migraine‐Specific Questionnaire (MSQ) and the Medical Outcomes Study 36‐item Short‐Form Health Survey (SF‐36). For active treatment comparisons, we analysed data measured by the Migraine Disability Assessment (MIDAS) and the Migraine‐Specific Quality of Life Questionnaire (MSQoL). We decided not to include data measured by the less commonly used Headache Impact Test (HIT‐6), Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form (Q‐LES‐Q‐SF), and Medical Outcomes Study 12‐item Short‐Form Health Survey (SF‐12).
The analysis considered only outcome data obtained directly from the patient and not those judged by the treating physician or study personnel. Efficacy data based on contemporaneous and timed (usually daily) recording of headache symptoms were preferred to those based on global or retrospective assessments.
In addition, we tabulated adverse events for each included study.
Unit of analysis issues
In the case of cross‐over trial designs, we anticipated that the data reported would normally not permit analysis of paired within‐patient data. We therefore analysed cross‐over trials as if they were parallel‐group trials, combining data from all treatment periods. If a carry‐over effect was found and data were reported by period, then the analysis was restricted to period‐one data only. In no trial were complete within‐patient data reported, so within‐patient improvement scores were not calculated.
Dealing with missing data
Where data were missing or inadequate, we attempted to obtain these data by correspondence with study authors.
Assessment of heterogeneity
We tested estimates of efficacy (both mean differences (MDs) and odds ratios (ORs)) for homogeneity. When significant heterogeneity was present, we made an attempt to explain the differences based on the clinical characteristics of the included studies. We did not statistically combine studies that were clinically dissimilar. However, when a group of studies with statistically heterogeneous results appeared to be clinically similar, we did combine study estimates. We performed all pooled analyses using a random‐effects model.
As a sensitivity analysis, we also planned to calculate a pooled effect estimate using a fixed‐effect model for major outcomes (headache frequency, responder rate, and any adverse event) when the random‐effects result was near‐significant (0.05 ≤ P ≤ 0.15) and the pooled studies were homogeneous (heterogeneity statistics: P > 0.15/I2 < 30%). Such a sensitivity analysis would evaluate whether conclusions might differ based on the statistical model used for pooling in situations where a fixed‐effect model might reasonably be considered instead of a random‐effects model. In fact, however, no such sensitivity analyses were warranted in the present review.
Data synthesis
We anticipated that continuous outcome measures of headache frequency would be reported on different and often incompatible scales. Although we attempted to standardise the extraction of headache frequency data to a 28‐day (four‐week) period, this was not possible in every case. In our previous review (Chronicle 2004; Mulleners 2008), we therefore analysed these data using the standardised mean difference (SMD, with 95% confidence intervals (CIs)) rather than the mean difference (MD). The introduction of change scores in the newly included studies for some of the reviews in this series necessitated a change in the analysis plan from SMDs to MDs. The latter also has the advantage of giving a result in clinically meaningful units (ie, x fewer migraines per 28 days).
We used dichotomous data meeting our definition of a clinically significant threshold to calculate odds ratios (ORs), with 95% CIs. Although we prefer ORs because of their statistical properties, some readers may find it simpler to interpret the clinical significance of our findings using risk ratios (RRs); we have therefore calculated RRs where appropriate. We additionally computed numbers needed to treat (NNTs), with 95% CIs, as the reciprocal of the risk difference (RD) versus placebo (McQuay 1998).
In the same way, we used data on the proportion of patients reporting adverse events to calculate RDs and numbers needed to harm (NNHs).
Subgroup analysis and investigation of heterogeneity
We undertook subgroup analyses by dose where possible. We considered further subgroup analyses by method of randomisation and by completeness of blinding, but did not undertake them because of insufficient data.
Results
Description of studies
Results of the search
The PubMed search strategy for our previous review (Chronicle 2004; Mulleners 2008) yielded 1089 potentially eligible citations, while the EMBASE and CENTRAL searches yielded 290 and 6952 citations, respectively. No additional citations were retrieved from the Cochrane Pain, Palliative & Supportive Care Trials Register or from other sources. After title and abstract screening, we obtained 58 published papers on antiepileptics for full‐text scrutiny. Of these, 16 (six included, 10 excluded) investigated topiramate.
The MEDLINE search strategy for the present update (from 2005 on) yielded 188 citations as possible candidates for the current series of reviews on antiepileptic drugs for migraine prophylaxis; the search of MEDLINE In‐Process identified an additional 20 citations. The EMBASE and CENTRAL updates identified 484 and 85 citations, respectively. Three additional study reports (all unpublished and all pertaining to gabapentin) were identified from other sources. After title and abstract screening, we obtained 37 published and three unpublished papers on antiepileptics for full‐text scrutiny. Of these, 30 (14 included, 16 excluded) investigated topiramate.
Thus, for the present update, we reviewed a total of 46 papers on topiramate at the full‐text screening stage. Of these, we included 20 papers and excluded 26.
Included studies
The 20 included papers reported data from 17 unique studies. Of these, 10 compared topiramate with placebo (Brandes 2004; de Tommaso 2007; Diener 2004; Diener 2007; Edwards 2000; Gupta 2007; Lipton 2011; Mei 2004; Silberstein 2004; Storey 2001), three directly compared different doses of topiramate (Brandes 2004; Diener 2004; Silberstein 2004), and seven compared topiramate to another active intervention (Afshari 2012; Ashtari 2008; Diener 2004; Dodick 2009; Luo 2012; Shaygannejad 2006; Varkey 2011).
Two trials (Gupta 2007; Shaygannejad 2006) had a cross‐over design; the remaining 15 trials had a parallel‐group design.
The doses of topiramate investigated ranged from 50 to 200 mg/day. This can be compared to the range of doses used in epilepsy, which is 200 to 400 mg.
The duration of the treatment phase of the included trials varied from 4 to 52 weeks, with a mean of 19 weeks.
See the Characteristics of included studies for further details.
Excluded studies
Of the 46 papers on topiramate obtained for full‐text scrutiny, 26 were excluded for reasons given in the Characteristics of excluded studies table. The most common reasons for exclusion were: review article/meta‐analysis (three papers), conference abstract only (three papers), and chronic migraine only (two papers).
Risk of bias in included studies
We scored methodological quality using the Jadad scale as indicated in the Assessment of risk of bias in included studies section, with a maximum attainable score of 5. The median quality score was 3 (mean 3.6; range 2 to 5).
Of 102 risk of bias items scored for the 17 studies, the majority of ratings were either 'unclear' (52 (51%)) or 'low' (38 (37%)) (Figure 1; Figure 2); we judged nine studies (Afshari 2012; Edwards 2000; Gupta 2007; Lipton 2011; Luo 2012; Mei 2004; Shaygannejad 2006; Silberstein 2006; Varkey 2011) as having a 'high' risk of bias for at least one item (Figure 2).
1.
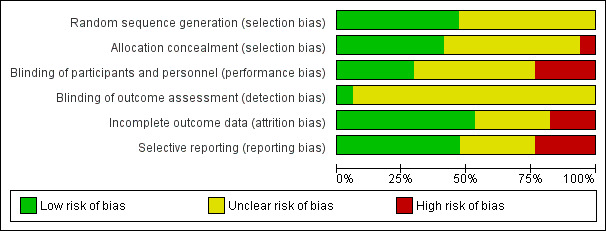
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
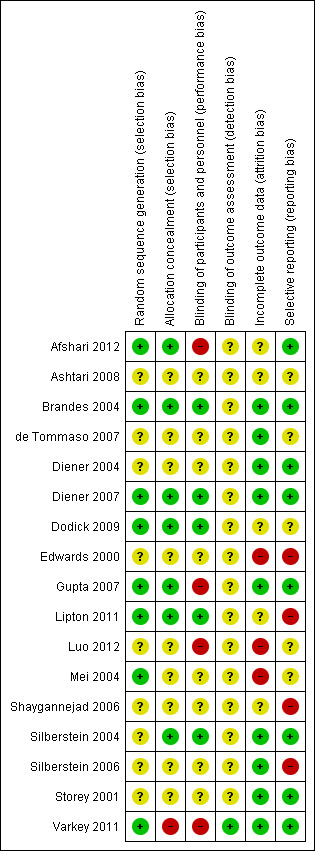
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Fewer than half of the studies (Figure 2) provided an adequate methodological description of how allocation sequences were generated. Most commonly this was achieved by a computer‐generated randomisation schedule, balanced by using permuted blocks and stratification by centre (see the Characteristics of included studies table). Likewise, fewer than half of the studies (Figure 2) provided an adequate methodological description of attempts to conceal allocation of intervention assignment. One common method was to keep sealed envelopes containing preprinted medication code labels in a limited access area until subjects qualified for participation, although interactive voice response systems were also used (see the Characteristics of included studies table). A high risk of selection bias was valued for Varkey 2011 only. Given the open nature of that study, comparing topiramate to non‐pharmacological treatments, the high number of withdrawals in the topiramate arm suggests a predetermined treatment preference among randomised subjects and a 'refusal to start' using a prophylactic drug.
Blinding
Participants and clinicians were blinded during the conduct of the majority of studies, but details of the methodology were reported for only five of them (see Figure 2 and the Characteristics of included studies table). Double‐blinding was typically achieved by packaging and labelling identical appearing tablets according to the randomisation codes. We judged four studies to have a high risk of performance bias. In Afshari 2012 it is suspected that standard medications with different appearances were provided by a third party according to allocation label. In Gupta 2007, placebo was identical in appearance and packaging to the active drug, but the lamotrigine and topiramate tablets were different in appearance, and therefore two different placebos were used. For effective blinding a double‐dummy design should have been used. Luo 2012 had an open‐label design. As Varkey 2011 compared pharmacological and non‐pharmacological treatments, blindness to treatment was not possible to achieve. Remarkably, that was the only paper clearly stating that the analyst was effectively blinded. The risk of detection bias in most studies (16 of 17; Figure 2) is unclear.
Incomplete outcome data
Completeness of data was adequately reported for nine of the 17 studies (Figure 2). Usually an intention‐to‐treat (ITT) analysis was applied (see the Characteristics of included studies table). The review authors were particularly concerned over incomplete outcome data in Luo 2012 and Mei 2004, which considered complete cases only. Furthermore, Mei 2004 presented safety data for a vaguely defined subgroup of participants only.
Selective reporting
We judged the risk of reporting bias as low in eight and unclear in five of the 17 studies (Figure 2). A major obstacle to meta‐analysis was the lack of variance measures, as in both Dodick 2009 and Silberstein 2006 (see the Characteristics of included studies table). There were other concerns over the selective availability of data, including in: Edwards 2000, which has never been published as a full report; Lipton 2011, where ≥ 50% reduction in migraine days was investigated but only reported as "higher in the topiramate group compared with the placebo treatment group"; and Shaygannejad 2006, where only two types of adverse events were reported for the topiramate‐treated participants.
Other potential sources of bias
Statistically significant results are more likely to be published than trials affirming a null result. This tendency for negative or inconclusive results to remain unpublished is inherently problematic also in the context of this review. Also, of eight corresponding authors whom we contacted and asked to provide supplementary unpublished information, only four responded with the requested information. Although it is unlikely that the requested input would have changed the conclusions of this review, the authors' support in clarifying reporting issues would undoubtedly have increased the quality and robustness of this review.
Effects of interventions
Topiramate versus placebo
Methodological considerations
Significant statistical heterogeneity was evident across trials for the efficacy outcomes. The clinical similarity of trials was therefore examined to determine whether studies should be combined for statistical meta‐analysis. Although there was methodological variation as described above (Risk of bias in included studies), the included trials were fundamentally similar with regard to basic design, patients, and measures. Note that three trials compared different doses of topiramate with placebo (50 mg, 100 mg, and 200 mg/day in Brandes 2004 and Silberstein 2004, and 100 mg and 200 mg/day in Diener 2004). In the combined analyses, these trials have contributed data only for the dose judged by trial investigators to be most clinically advisable (100 mg/day in each case). Complete data for all three doses are considered below in separate analyses.
All doses reported below are given in terms of mg/day.
Headache frequency
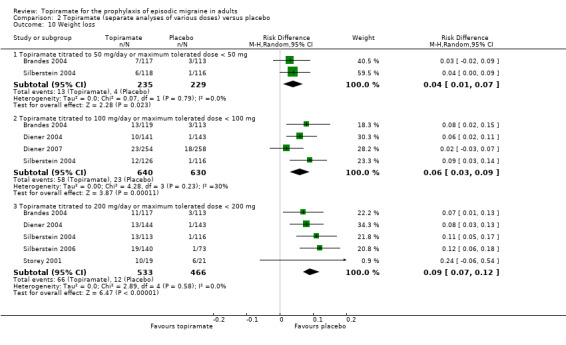
Nine trials of topiramate (Brandes 2004; de Tommaso 2007; Diener 2004; Diener 2007; Edwards 2000; Gupta 2007; Lipton 2011; Silberstein 2004; Storey 2001; 1737 patients (one study had 56 cross‐over patients)) showed a significant reduction in headache frequency (per 28‐day period) in the active group compared to the placebo group in the combined analysis (mean difference (MD) ‐1.20; 95% confidence interval (CI) ‐1.59 to ‐0.80; Figure 3). It should be noted that two of these trials (Edwards 2000; Storey 2001) reported significant reductions in migraine frequency in the active treatment group (topiramate 200 mg) compared to placebo, using analysis of covariance to control for baseline differences in frequency; by contrast, our analysis of the post‐treatment mean headache frequencies showed no statistically significant differences (Edwards 2000: MD ‐1.29; 95% CI ‐3.56 to 0.98; Storey 2001: MD ‐0.52; 95% CI ‐1.67 to 0.63). Separate analyses of all the data on the three topiramate doses studied (Figure 4) suggest similar MDs versus placebo for 50 mg (‐0.95; 95% CI ‐1.95 to 0.04; three studies; 520 participants (one study had 56 cross‐over patients)), 100 mg (‐1.15; 95% CI ‐1.58 to ‐0.71; six studies; 1620 participants), and 200 mg doses (‐0.94; 95% CI ‐1.53 to ‐0.36; five studies; 804 participants).
3.
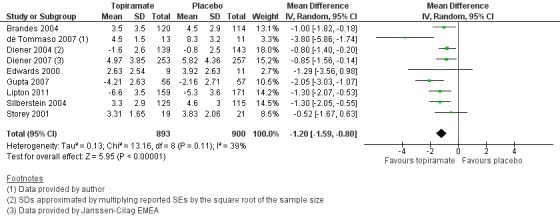
Forest plot of comparison: 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, outcome: 1.1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).
4.
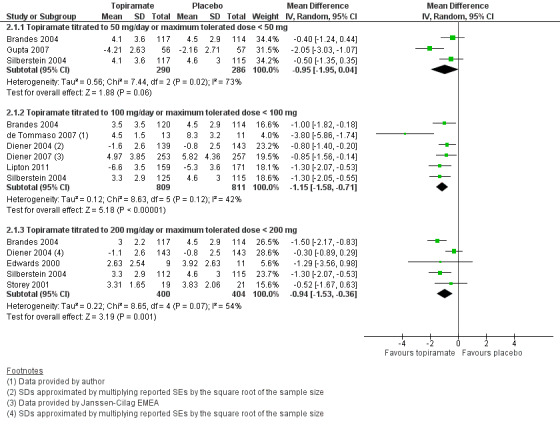
Forest plot of comparison: 2 Topiramate (separate analyses of various doses) versus placebo, outcome: 2.1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).
In clinical terms, the difference in effect between topiramate and placebo observed in the combined analysis (Figure 3) corresponds to a reduction in headache frequency of a little more than one headache per 28 days. The median baseline headache frequency in the topiramate groups of the placebo‐controlled trials was 5.6 attacks per 28 days (mean 7.0; range: 4.8 to 11.6).
Responders (patients with ≥ 50% reduction in headache frequency)
In the combined analysis of nine trials (Brandes 2004; de Tommaso 2007; Diener 2004; Edwards 2000; Gupta 2007; Mei 2004; Silberstein 2004; Silberstein 2006; Storey 2001; 1190 participants (one study had 56 cross‐over patients)) topiramate demonstrated overall superiority of treatment to placebo in the proportion of responders (odds ratio (OR) 3.18; 95% CI 2.10 to 4.82; Analysis 1.2), although there was noticeable variability in the ORs across studies. Separate analysis of all the data on the various topiramate doses studied (Analysis 2.2) showed that all three (50, 100, and 200 mg) significantly increased the proportion of responders. ORs were as follows: for 50 mg, 2.35 (95% CI 1.60 to 3.44; three studies; 520 participants (one study had 56 cross‐over participants)); for 100 mg, 3.49 (95% CI 2.23 to 5.45; five studies; 852 participants); and for 200 mg, 2.49 (95% CI 1.61 to 3.87; six studies; 1025 participants).
1.2. Analysis.
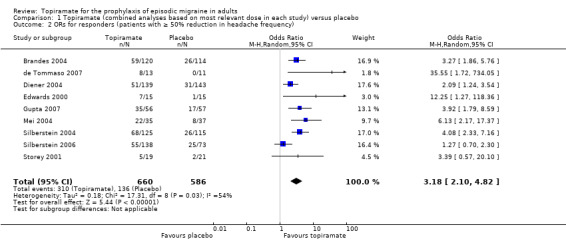
Comparison 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, Outcome 2 ORs for responders (patients with ≥ 50% reduction in headache frequency).
2.2. Analysis.
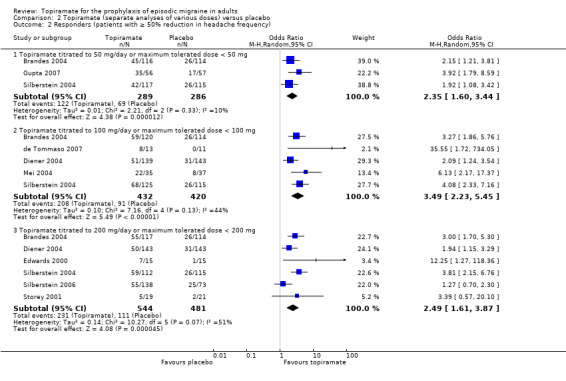
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).
In clinical terms, the effect observed in the combined analysis suggests that patients are twice as likely to experience a ≥ 50% reduction in frequency with topiramate as with placebo. Details are as follows:
The proportion of responders with topiramate was 47% (310/660; range: 26% to 63%);
The proportion of responders with placebo was 23% (136/586; range 0% to 34%);
The risk ratio (RR) for topiramate versus placebo was 2.02 (95% CI 1.57 to 2.60) (Figure 5);
The number needed to treat (NNT) for topiramate versus placebo was 4 (95% CI 3 to 6).
5.
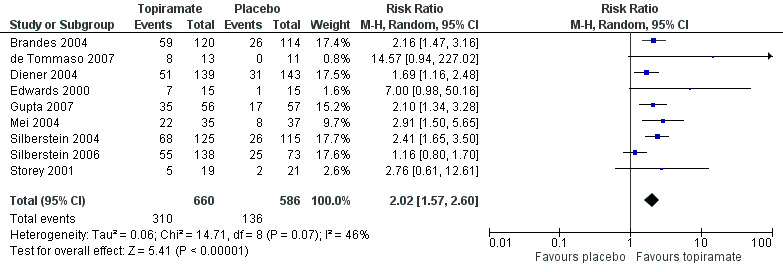
Forest plot of comparison: 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, outcome: 1.3 RRs for responders (patients with ≥ 50% reduction in headache frequency).
Quality of life
Two studies (Brandes 2004; Silberstein 2004) reported data on patient‐reported quality of life as measured by the Migraine‐Specific Questionnaire (MSQ) (three domains) and the Medical Outcomes Study 36‐item Short‐Form Health Survey (SF‐36) (eight domains). In the combined analyses of topiramate 50 mg versus placebo (463 participants), a significant difference was found for 3 of 11 domains: MSQ‐role function restrictive (Analysis 2.11), MSQ‐emotional function (Analysis 2.13), and SF‐36 bodily pain (Analysis 2.17), all favouring topiramate 50 mg. In the combined analyses of topiramate 100 mg versus placebo (474 participants), a significant difference was again found for 3 of 11 domains: MSQ‐role function restrictive (Analysis 2.11), MSQ‐role function prevention (Analysis 2.12), and MSQ‐emotional function (Analysis 2.13), all favouring topiramate 100 mg. In the combined analyses of topiramate 200 mg versus placebo (458 participants), a significant difference was found for 4 of 11 domains: MSQ‐role function restrictive (Analysis 2.11), MSQ‐role function prevention (Analysis 2.12), MSQ‐emotional function (Analysis 2.13), and SF‐36 role physical (Analysis 2.14), all favouring topiramate 200 mg.
2.11. Analysis.
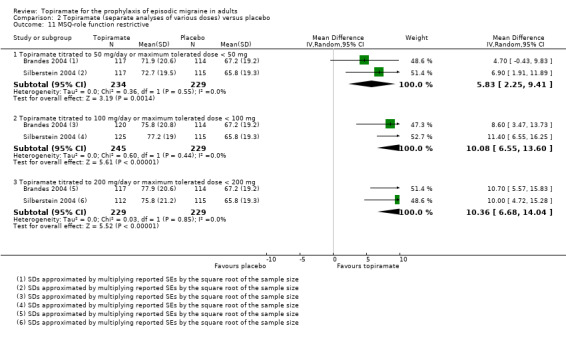
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 11 MSQ‐role function restrictive.
2.13. Analysis.
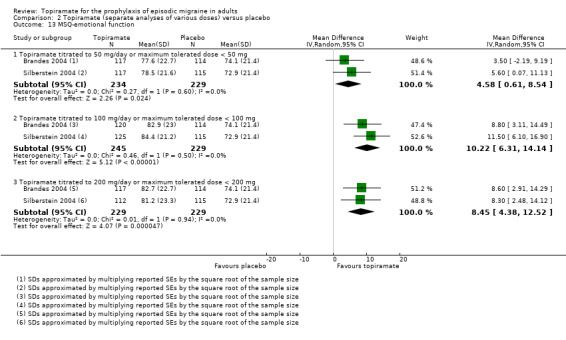
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 13 MSQ‐emotional function.
2.17. Analysis.
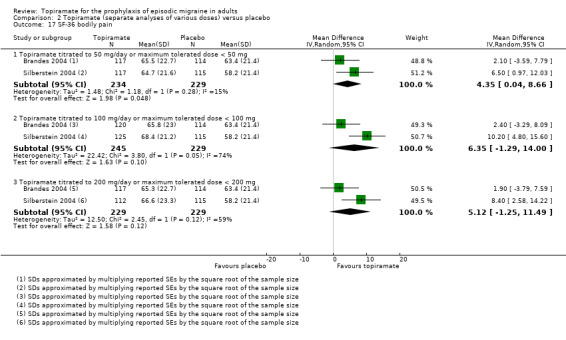
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 17 SF‐36 bodily pain.
2.12. Analysis.
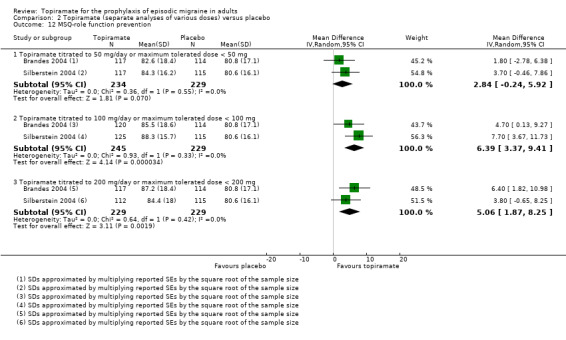
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 12 MSQ‐role function prevention.
2.14. Analysis.
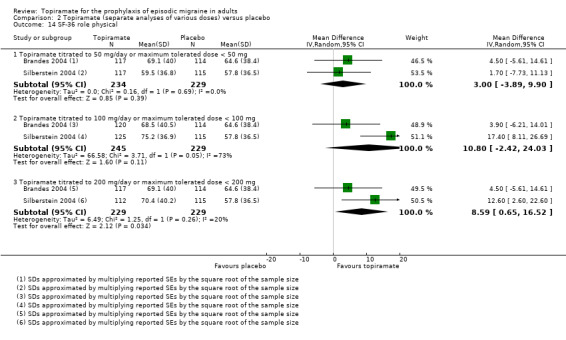
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 14 SF‐36 role physical.
In summary, the disease‐specific MSQ found better quality of life on topiramate than on placebo, but the generic SF‐36 was more equivocal (only 2 of 24 analyses pointed in this direction).
Direct dose comparisons
Three studies directly compared different doses of topiramate and reported data on headache frequency (Analysis 3.1) and responders (Analysis 3.2). Data from Brandes 2004 and Silberstein 2004 were used to compare all three doses of topiramate, with additional data from Diener 2004 contributing to the comparison between 200 mg and 100 mg. The 200 mg dose was significantly superior to 50 mg both in terms of reducing headache frequency (MD ‐0.96; 95% CI ‐1.53 to ‐0.40; 463 participants) and increasing the proportion of responders (OR 1.66; 95% CI 1.15 to 2.41; 462 participants). Likewise, 100 mg was superior to 50 mg (MD ‐0.71; 95% CI ‐1.32 to ‐0.10 (479 participants); and OR 1.80; 95% CI 1.25 to 2.60 (478 participants)). The 200 mg dose was not significantly superior to 100 mg for either outcome measure (756 participants).
3.1. Analysis.
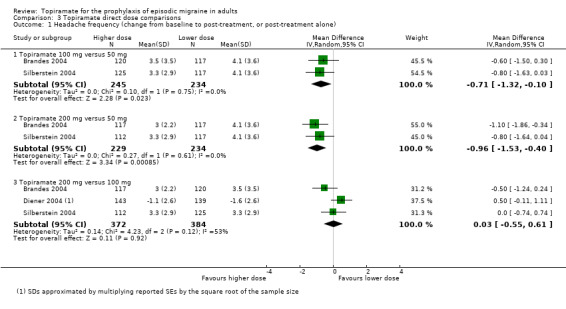
Comparison 3 Topiramate direct dose comparisons, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).
3.2. Analysis.
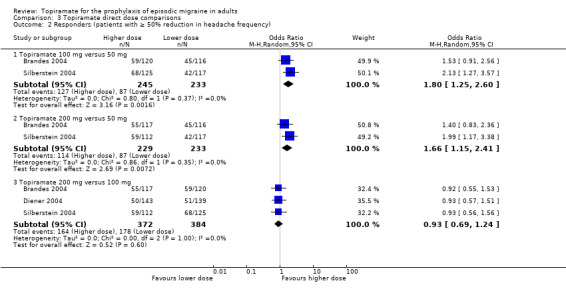
Comparison 3 Topiramate direct dose comparisons, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).
Two studies (Brandes 2004 and Silberstein 2004) directly comparing different doses of topiramate reported data on patient‐reported quality of life as measured by the MSQ (three domains) and SF‐36 (eight domains). In the combined analyses of topiramate 50 mg versus 100 mg (479 participants), a significant difference was found for 3 of 11 domains: MSQ‐role function restrictive (Analysis 3.3), MSQ‐role function prevention (Analysis 3.4), and MSQ‐emotional function (Analysis 3.5), all favouring the higher dose. In the combined analyses of topiramate 50 mg versus 200 mg (463 participants), a significant difference was found for only 1 of 11 domains: MSQ‐role function restrictive (Analysis 3.3), favouring the higher dose. In the combined analyses of topiramate 100 mg versus 200 mg (474 participants), a significant difference was found for only 1 of 11 domains: SF‐36 physical functioning (Analysis 3.8), favouring the lower dose.
3.3. Analysis.
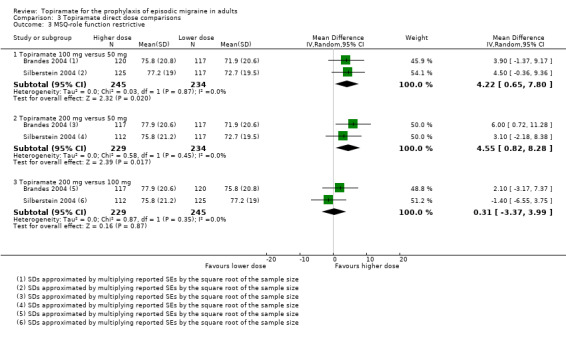
Comparison 3 Topiramate direct dose comparisons, Outcome 3 MSQ‐role function restrictive.
3.4. Analysis.
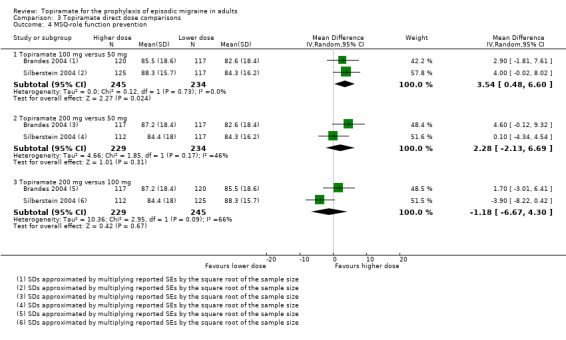
Comparison 3 Topiramate direct dose comparisons, Outcome 4 MSQ‐role function prevention.
3.5. Analysis.
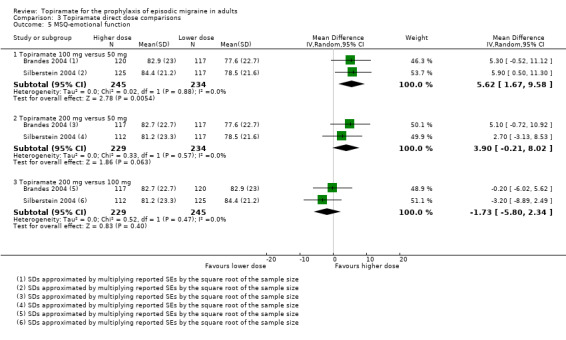
Comparison 3 Topiramate direct dose comparisons, Outcome 5 MSQ‐emotional function.
3.8. Analysis.
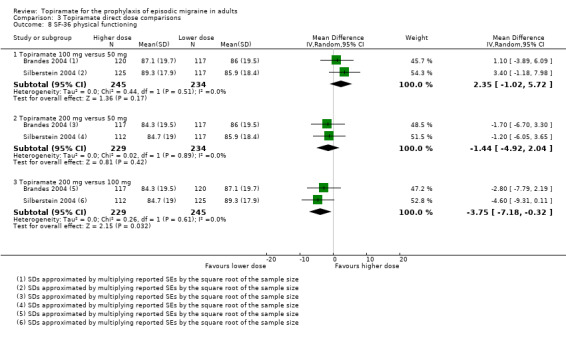
Comparison 3 Topiramate direct dose comparisons, Outcome 8 SF‐36 physical functioning.
It is important to note, however, that none of these studies was designed to have the statistical power to make comparisons between doses.
Topiramate versus active comparators
Seven trials examined topiramate versus active comparators, including:
amitriptyline (one study, 330 participants);
flunarizine (one study, 83 participants);
propranolol (two studies, 342 participants);
sodium valproate (two studies, 120 participants);
relaxation (one study, 61 participants).
Dodick 2009 compared topiramate to amitriptyline (both drugs titrated to maximum tolerated dose between 50 and 100 mg). Data were insufficient for us to calculate MDs for headache frequency, our preferred outcome measure. There was no significant difference between treatments in the proportion of responders (OR 0.68; 95% CI 0.44 to 1.05; 330 participants; Analysis 4.1) or in the change from baseline in Migraine Disability Assessment (MIDAS) scores (MD 2.10; 95% CI ‐2.93 to 7.13; 295 participants; Analysis 4.2).
4.1. Analysis.
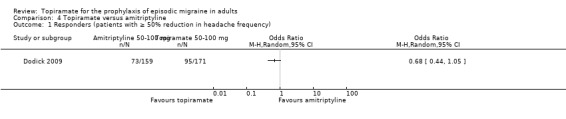
Comparison 4 Topiramate versus amitriptyline, Outcome 1 Responders (patients with ≥ 50% reduction in headache frequency).
4.2. Analysis.
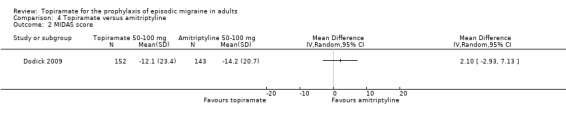
Comparison 4 Topiramate versus amitriptyline, Outcome 2 MIDAS score.
One small, open study (Luo 2012) compared topiramate titrated to 100 mg (or lower if lack of tolerance) to flunarizine 5 mg. There was no significant difference in mean headache frequency during treatment (MD 0.30; 95% CI ‐0.37 to 0.97; 83 participants; Analysis 5.1) or in the proportion of responders (OR 0.83; 95% CI 0.34 to 2.03; 83 participants; Analysis 5.2). It should be noted that the flunarizine dose used in this study (5 mg) is in the lower range of doses used in routine clinical practice (5 to 10 mg).
5.1. Analysis.
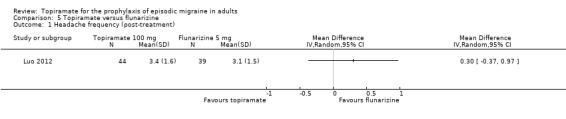
Comparison 5 Topiramate versus flunarizine, Outcome 1 Headache frequency (post‐treatment).
5.2. Analysis.
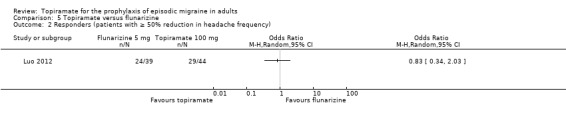
Comparison 5 Topiramate versus flunarizine, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).
In a comparison between topiramate 50 mg and propranolol 80 mg, Ashtari 2008 did not demonstrate a significant difference in mean headache frequency during treatment (MD ‐0.37; 95% CI ‐1.15 to 0.41; 60 participants; Analysis 6.1). In a larger study, Diener 2004 included an additional arm (propranolol 160 mg) in a trial of topiramate (200 mg and 100 mg) versus placebo. A comparison of propranolol with topiramate 100 mg (the dose judged by trial investigators to be most clinically advisable) showed no significant difference in the change in headache frequency from baseline (MD 0.00; 95% CI ‐0.60 to 0.60; 282 participants; Analysis 6.1) or in the proportion of responders (OR 1.32; 95% CI 0.82 to 2.13; 282 participants; Analysis 6.2). The pooled results of these two studies do not indicate a significant difference between topiramate and propranolol with regard to headache frequency (MD ‐0.14; 95% CI ‐0.61 to 0.34; 342 participants; Analysis 6.1).
6.1. Analysis.
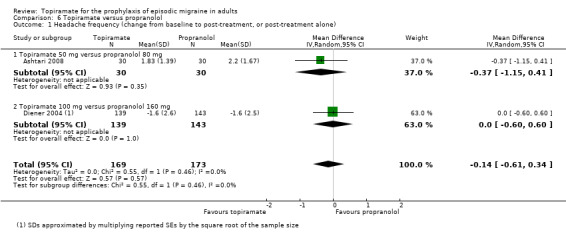
Comparison 6 Topiramate versus propranolol, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).
6.2. Analysis.
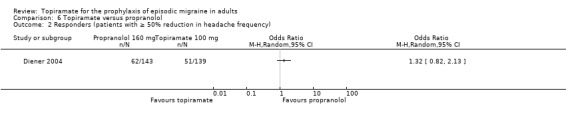
Comparison 6 Topiramate versus propranolol, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).
Two fairly small studies compared topiramate 50 mg with sodium valproate 400 mg. Afshari 2012 did not demonstrate a significant difference in mean headache frequency during treatment (MD ‐0.60; 95% CI ‐1.57 to 0.37; 56 participants; Analysis 7.1) or in MIDAS score during the treatment phase (MD ‐3.90; 95% CI ‐8.72 to 0.92; 56 participants; Analysis 7.2). On the basis of their statistical analysis, the authors of Shaygannejad 2006 found no significant differences in efficacy between the two drugs. However, our analysis of post‐treatment mean headache frequencies demonstrated a slight but significant advantage for topiramate over valproate (MD ‐1.20; 95% CI ‐2.16 to ‐0.24; 32 (cross‐over) participants; Analysis 7.1). The pooled results of these two studies indicate a significant difference between topiramate and sodium valproate, in favour of topiramate, for this outcome (MD ‐0.90; 95% CI ‐1.58 to ‐0.22; Analysis 7.1). In clinical terms, the observed effect corresponds to a reduction in headache frequency of approximately one headache per 28 days with topiramate versus sodium valproate. The median baseline headache frequency in the topiramate groups of the two trials was 6.1 headaches per 28 days (mean 6.1; range: 5.4 to 6.8). It should be noted that the doses used in these two studies are not those used in routine clinical practice for the management of migraine.
7.1. Analysis.
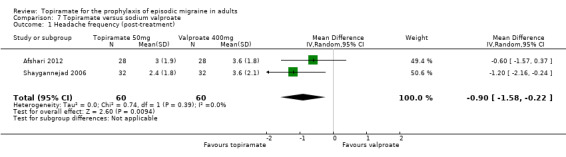
Comparison 7 Topiramate versus sodium valproate, Outcome 1 Headache frequency (post‐treatment).
7.2. Analysis.
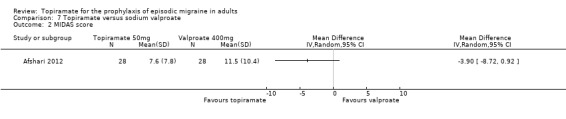
Comparison 7 Topiramate versus sodium valproate, Outcome 2 MIDAS score.
In a small, open study, Varkey 2011 compared topiramate (200 mg or maximum tolerated dose < 200 mg) to relaxation and found no significant difference in the change in headache frequency from baseline (MD ‐0.14; 95% CI ‐0.30 to 0.02; 61 participants; Analysis 8.1) or in the proportion of responders (OR 0.88; 95% CI 0.27 to 2.81; 61 participants; Analysis 8.2). There was a significant difference in the change of quality of life (Migraine‐Specific Quality of Life Questionnaire (MSQoL), 0 to 100 points) from baseline (MD ‐1.50; 95% CI ‐2.45 to ‐0.55; 61 participants; Analysis 8.3), favouring relaxation. As discussed above (Blinding (performance bias and detection bias)), there was a high risk of selection bias in this trial.
8.1. Analysis.
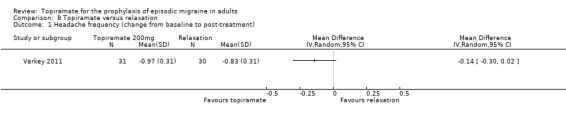
Comparison 8 Topiramate versus relaxation, Outcome 1 Headache frequency (change from baseline to post‐treatment).
8.2. Analysis.
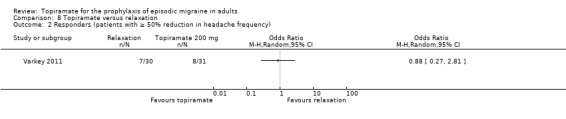
Comparison 8 Topiramate versus relaxation, Outcome 2 Responders (patients with ≥ 50% reduction in headache frequency).
8.3. Analysis.
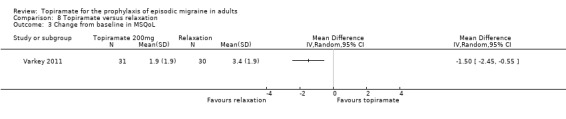
Comparison 8 Topiramate versus relaxation, Outcome 3 Change from baseline in MSQoL.
Safety
During the process of extracting safety data, it became clear that the range of adverse events (AEs) and the method of their reporting varied very considerably from trial to trial. In the nine trials of topiramate versus placebo, seven specific adverse events were reported by at least three trials. We calculated risk differences (RDs) separately for the various doses of topiramate (50 mg, 100 mg, and 200 mg) versus placebo for any adverse event (Analysis 2.3), anorexia (Analysis 2.4), fatigue (Analysis 2.5), memory problems (Analysis 2.6), nausea (Analysis 2.7), paresthesia (Analysis 2.8), taste disturbance (Analysis 2.9), and weight loss (Analysis 2.10). We then calculated numbers needed to harm (NNHs) and 95% CIs where appropriate, and these are reported in Table 1. Except for taste disturbance (Analysis 2.9) and weight loss (Analysis 2.10), there were no significant differences in the frequency of AEs in general or of the individual AEs between placebo and topiramate 50 mg. All AEs except nausea were significantly more common with topiramate 100 mg than with placebo, with NNHs varying from 3 to 25, and the RDs were even higher for topiramate 200 mg versus placebo, with NNHs varying from 2 to 17 (Table 1). RDs were generally smaller in Diener 2007, which is logical considering its design, with an initial open‐label phase (with a possibility to withdraw or adjust dosage due to AEs), followed by randomisation to the double‐blind, parallel‐group phase of prolonged topiramate treatment at individualised dose or placebo.
2.3. Analysis.
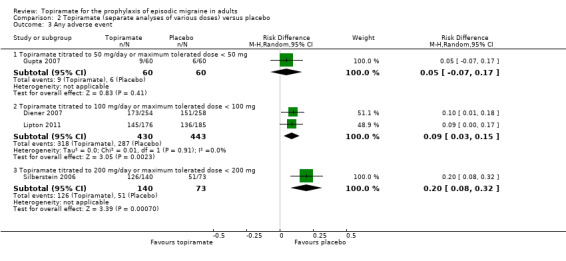
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 3 Any adverse event.
2.4. Analysis.
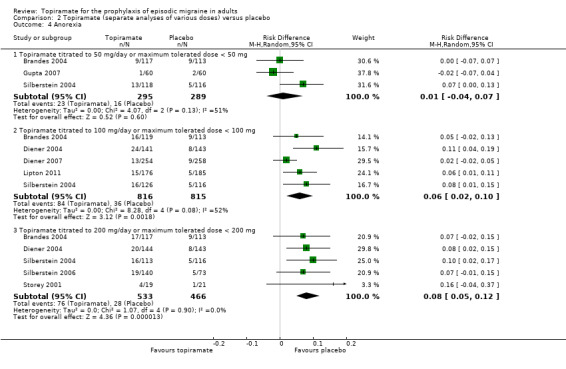
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 4 Anorexia.
2.5. Analysis.
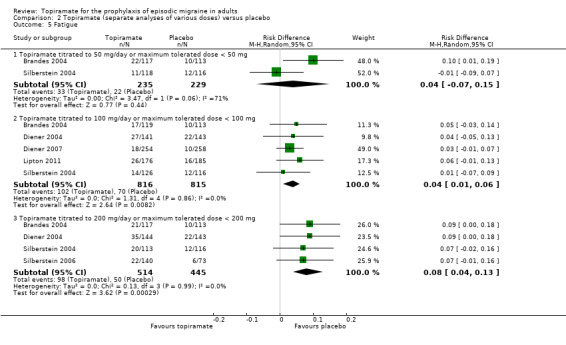
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 5 Fatigue.
2.6. Analysis.
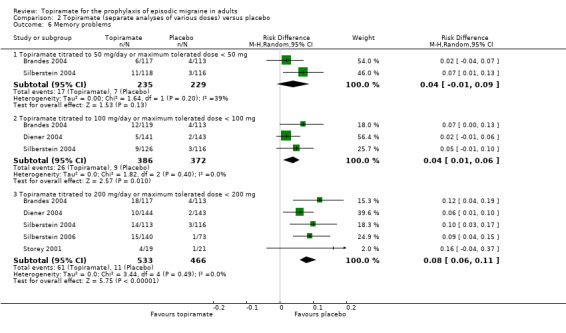
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 6 Memory problems.
2.7. Analysis.
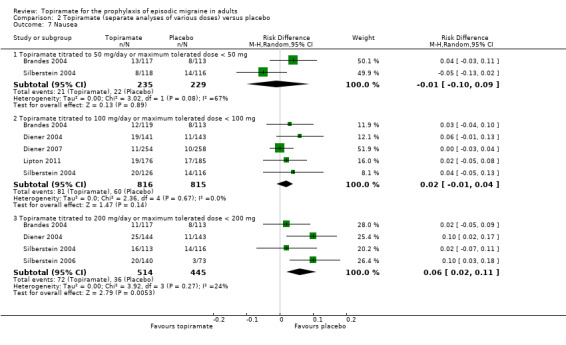
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 7 Nausea.
2.8. Analysis.
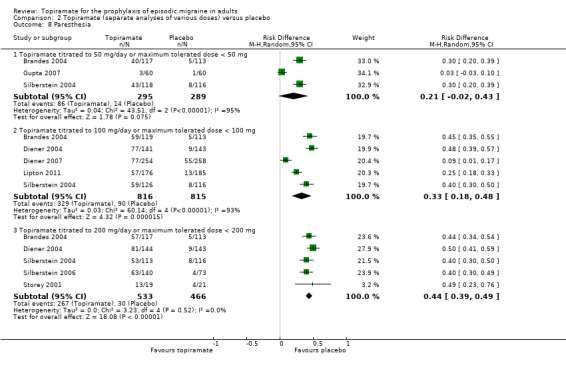
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 8 Paresthesia.
2.9. Analysis.
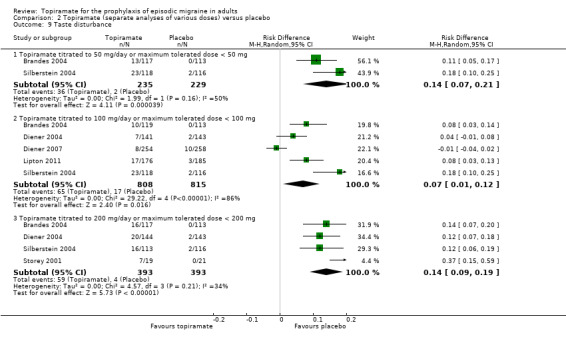
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 9 Taste disturbance.
2.10. Analysis.
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 10 Weight loss.
1. NNHs (with 95% CIs), by dose, for placebo‐controlled trials of topiramate and proportion of withdrawals in trials with active intervention or placebo as control.
| Type of AE | 50 mg/day | 100 mg/day | 200 mg/day |
| Any AE | NNH not defined* | 11 (7 to 33) | 5 (3 to 12) |
| Anorexia | NNH not defined* | 17 (10 to 50) | 12 (8 to 20) |
| Fatigue | NNH not defined* | 25 (17 to 100) | 12 (8 to 25) |
| Memory problems | NNH not defined* | 25 (17 to 100) | 12 (9 to 17) |
| Nausea | NNH not defined* | NNH not defined* | 17 (9 to 50) |
| Paresthesia | NNH not defined* | 3 (2 to 6) | 2 (2 to 3) |
| Taste disturbance | 7 (5 to 14) | 14 (8 to 100) | 7 (5 to 11) |
| Weight loss | 25 (14 to 100) | 17 (11 to 33) | 11 (8 to 14) |
| Percentage of patients in active group withdrawing because of AEs | Afshari 2012: 5%; Ashtari 2008: 3%; Brandes 2004: 17%; Gupta 2007: 2%; Silberstein 2004: 17% | Brandes 2004: 26%; de Tommaso 2007: 8%; Diener 2004: 28%; Dodick 2009: 20%; Lipton 2011: 12%; Mei 2004: 29%; Silberstein 2004: 19% | Brandes 2004: 21%; Diener 2004: 44%; Edwards 2000: 27%; Silberstein 2004: 32%; Silberstein 2006: 15%; Storey 2001: 11%; Varkey 2011: 12% |
* The 95% CI of the difference in AE rates between treatment and placebo arms (the risk difference, RD) crosses zero.
Abbreviations: AE = adverse event; CI = confidence interval; NNH = number needed to harm
Table 1 also reports, by dose, the percentages of patients in active treatment groups who withdrew because of AEs in each trial. The mean percentage withdrawing because of AEs at 100 mg was 20%.
Discussion
Summary of main results
Placebo‐controlled trials
Meta‐analysis of the studies included in this review demonstrates clearly that topiramate is efficacious for the prophylaxis of migraine. Mean headache frequency was significantly reduced (by approximately 1.2 headaches per month) with topiramate as compared to placebo (nine studies with a median baseline headache frequency of 5.6 attacks per 28 days contributed to this analysis). Furthermore, and perhaps of greater clinical relevance (though less informative scientifically), patients were approximately twice as likely to have a ≥ 50% reduction in headache frequency with topiramate than with placebo (nine studies contributed to this analysis).
According to two large trials (Brandes 2004; Silberstein 2004), topiramate 50 mg gives rise to a significant increase in the proportion of responders (patients with ≥ 50% reduction in headache frequency), but not to a significant overall decrease in monthly headache frequency. In contrast, one small trial (Gupta 2007) found superiority over placebo of topiramate 50 mg both with regard to reduction of headache frequency and responder rate.
All included trials comparing topiramate 100 mg to placebo (Brandes 2004; de Tommaso 2007; Diener 2004; Diener 2007; Lipton 2011; Mei 2004; Silberstein 2004) showed unambiguous statistically significant superiority of topiramate, although one study did not report data enabling a comparison of reduction of headache frequency (Mei 2004), and two studies (Diener 2007; Lipton 2011) did not report data enabling a comparison of the proportion of responders.
The four large trials comparing topiramate 200 mg to placebo were equivocal in that all showed a statistically significant superiority for topiramate with regard to the responder rate, whereas only two studies (Brandes 2004; Silberstein 2004) also showed a statistically significant difference in reduction of headache frequency, while two do not (Diener 2004; Silberstein 2006). In two additional small trials of topiramate 200 mg (Edwards 2000; Storey 2001), the analysis for headache frequency did not reveal a significant difference versus placebo in either study, although one trial reported topiramate 200 mg to be significantly more efficacious for responder rates (Edwards 2000).
Based on the combined analyses of two studies (Brandes 2004; Silberstein 2004), all three doses of topiramate significantly improved three or more domains of quality of life as compared to placebo.
Dose comparisons
The studies including more than one dose of topiramate suggest that 200 mg is no more effective than 100 mg.
Trials with active comparators
With regard to reduction of mean headache frequency and/or responder rate, the six trials using active comparators found (a) no significant difference in efficacy between topiramate and amitriptyline (Dodick 2009); (b) no significant difference in efficacy between topiramate and flunarizine; (c) no significant difference in efficacy between topiramate and propranolol (Ashtari 2008; Diener 2004); (d) no significant difference in efficacy between topiramate and relaxation (Varkey 2011); (e) a slight significant advantage of topiramate over valproate (pooled results of Afshari 2012 and Shaygannejad 2006). Furthermore, relaxation increased migraine‐specific quality of life significantly more than topiramate (Varkey 2011). However, only three of these seven studies (Diener 2007, 95%; Dodick 2009, 85%; Varkey 2011, 80%) reported adequate power; the others were likely to have been underpowered.
Safety
Topiramate does not appear to give rise to an unexpectedly high rate of adverse events when used for migraine prophylaxis, although a large percentage of patients taking topiramate report paresthesia. Withdrawals due to adverse events were somewhat higher in trials of topiramate than would generally be expected on the basis of trials of other antiepileptic drugs, particularly sodium valproate or divalproex sodium.
Overall completeness and applicability of evidence
The studies identified were sufficient to address all of the objectives of the review. Our analysis demonstrates that topiramate is efficacious for preventing attacks in adult patients with episodic migraine, and these results fit into the context of current practice. Since the comparisons with flunarizine and valproate were in all probability underpowered, the evidence from these is incomplete. The trials comparing topiramate with amitriptyline and propranolol are of relevance since both these drugs have proven efficacy in the prophylaxis of migraine. The trial comparing topiramate with relaxation is also interesting. Further well‐designed trials of topiramate against other drug categories and non‐pharmacological interventions are desirable.
Several important issues need to be taken into account in any assessment of the efficacy of a drug for migraine prophylaxis. Diagnostic criteria, baseline headache frequency, washout periods for previous medication, rules for rescue medication, and the statistical power of the comparison were handled very variably in the 17 included studies. As investigations of the efficacy of various agents become more commonplace, it seems increasingly important that scientists and clinicians are at least aware of the trial guidelines suggested by the International Headache Society (Tfelt‐Hansen 2012). Even if these guidelines cannot — for operational or scientific reasons — be adhered to in their entirety, they provide a useful consultative framework at the early stages of trial design.
Quality of the evidence
The identified body of evidence allows a robust conclusion of an overall superiority of topiramate over placebo with regard to reduction of mean headache frequency (nine trials with 1737 participants) and the proportion of responders (nine trials with 1190 participants). The separate analyses of doses should, however, be viewed with some caution. Several of the included trials were almost certainly underpowered (de Tommaso 2007; Edwards 2000; Storey 2001). The studies including more than one dose of topiramate were generally not designed to enable direct dose comparisons, and the results of the dose comparisons reported here should therefore be viewed with some caution. It should be noted that all seven trials with active comparators are potentially problematic for reasons including lack of blinding, insufficient statistical power, and possibly incomplete statistical analysis. As usual in the context of clinical trials research, there was considerable heterogeneity in both headline results and general levels of analytical and statistical sophistication. It is fair to say that we faced several difficulties in deriving adequate information from the results of the 17 included studies. First, means and standard deviations were not always fully reported for each phase of trials. In tandem with this problem, reported measures of variability — either appearing in the text, tabulated, or as error bars in graphs — were not always adequately described or labelled. Second, methods of statistical analysis were generally under‐specified, leading in some cases to a lack of clarity as to which comparisons were significant and which were not. Third, there was considerable variability in how intention‐to‐treat analyses were performed. In a few cases, this gave rise to uncertainty about the numbers of patients continuing to each phase of the trial.
Potential biases in the review process
Of 102 risk of bias items scored for the 17 studies, the majority of ratings were either 'unclear' (52 (51%)) or 'low' (38 (37%)) (Figure 1; Figure 2). As described in detail above (Risk of bias in included studies), we judged nine trials as having a 'high' risk of bias for at least one item, as follows: allocation concealment (Varkey 2011), blinding of participants and personnel (Afshari 2012; Gupta 2007; Luo 2012; Varkey 2011), incomplete outcome data (Edwards 2000; Luo 2012; Mei 2004), and/or selective reporting (Edwards 2000; Lipton 2011; Shaygannejad 2006; Silberstein 2006). A strength of this review is that the methods used for searching and study selection make it highly likely that the absolute majority of relevant trial results in the public domain were identified. There is nevertheless an obvious risk that the reports of some trials may have been classified and thus remain unobtainable.
Agreements and disagreements with other studies or reviews
The overall conclusion in this review, that topiramate is efficacious for preventing attacks in adult patients with episodic migraine, is well in line with guideline recommendations of the European Federation of Neurological Societies (EFNS) (Evers 2009) and the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society (Silberstein 2012).
Authors' conclusions
Implications for practice.
Bearing in mind the limitations invoked by the methodological and reporting issues mentioned above, this review nevertheless helps to provide a rational framework for the application of topiramate for the preventive management of migraine headache in clinical practice. Topiramate has been investigated in 17 independent clinical trials (10 of which included placebo as a comparator), with generally consistent results. It can be concluded from this review that topiramate is of proven efficacy in migraine prevention and is suitable for routine clinical use. It must be stressed, however, that this review does not provide definite evidence for the efficacy of topiramate in the management of other aspects of the condition (eg, prodromal symptoms, aura symptoms). Likewise, the conclusions in this review cannot be extrapolated to chronic migraine, transformed migraine, or chronic daily headache. None of these conditions was considered for this review, as properly validated definitions are as yet lacking.
The seven trials allowing comparisons with another active intervention suggest that topiramate is marginally more effective than valproate, but no more effective than amitriptyline, flunarizine, propranolol, or relaxation, although these results must be viewed with caution for methodological reasons. It must be stressed that on a case‐to‐case basis, rational prescriber preferences may be justified due to differences in side effect profiles. Data from pregnancy registries indicate that infants exposed to topiramate have a higher incidence of major congenital malformations (Janssen‐Cilag 2013). For migraine, topiramate should therefore not be used during pregnancy or in women of childbearing potential not using effective contraception. Moreover, because topiramate causes increased excretion of ethinyl estradiol, low‐dose hormonal contraception may be less effective in women taking topiramate, and other means of contraception may be warranted. Although adverse events were reported by a large proportion of study participants treated with topiramate, these were usually mild and of a non‐serious nature. Thus it can be concluded that topiramate is reasonably well‐tolerated.
Implications for research.
There is a need for more studies designed specifically to compare the efficacy or safety of topiramate to other interventions with proven efficacy in the prophylaxis of migraine. Also needed are (a) better studies of dose versus effect; (b) studies of which patients do and do not respond, and why; (c) long‐term studies; (d) studies post‐withdrawal of topiramate after effective use for several months.
Future trialists should also be encouraged to follow the recommendations of the International Headache Society (Tfelt‐Hansen 2012) with regard to both trial design and reporting of data.
Little is definitely known about the mechanism of action of topiramate in migraine prophylaxis (Edvinsson 2010). A considerable amount of basic science research in both animal models and human neuroscience laboratories will be necessary in order to discover which of the many potential actions of this drug are causative in the reduction of headache frequency.
What's new
| Date | Event | Description |
|---|---|---|
| 27 May 2016 | Review declared as stable | See Published notes. |
History
Review first published: Issue 6, 2013
| Date | Event | Description |
|---|---|---|
| 8 May 2014 | Amended | Minor edit made to numbers reported in Results of the search. |
| 20 June 2013 | New citation required but conclusions have not changed | Conclusions regarding topiramate essentially unchanged. |
| 20 June 2013 | New search has been performed | Searches updated on 15 January 2013. Ten new included studies added (Afshari 2012; Ashtari 2008; de Tommaso 2007; Diener 2007; Dodick 2009; Gupta 2007; Lipton 2011; Luo 2012; Silberstein 2006; Varkey 2011). |
| 26 August 2008 | Amended | Converted to new review format. |
| 11 May 2007 | New search has been performed | May 2007 (Issue 3, 2007):
|
Notes
An updated search in May 2016 only identified one relevant study (Cady 2012). However, we did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Cady, R. K., J. Voirin, et al. (2012). "Two center, randomized pilot study of migraine prophylaxis comparing paradigms using pre‐emptive frovatriptan or daily topiramate: research and clinical implications." Headache 52(5): 749‐764.
Acknowledgements
Professor EP Chronicle, PhD, sadly passed away on 9 February 2007. We wish to acknowledge Professor Chronicle's major contribution and tremendous effort in compiling all statistical analyses and much of the text of the original review on antiepileptics (Chronicle 2004; Mulleners 2008). Without his relentless dedication it would have never seen the light of day.
The protocol for the original review was developed while Dr Chronicle was a Visiting Scholar at the University of California, Berkeley. Dr Sally Hollis, Lancaster University, and Dr Kentaro Hayashi, University of Hawaii at Manoa, provided helpful advice on statistical matters. Several pharmaceutical companies kindly provided information about trials in progress.
We thank Ruth Foxlee, Jane Hayes, and Joanne Abbott for assistance in designing search strategies and running searches; Prof Timothy Steiner for editorial guidance; and Dr Rebecca Gray for editorial assistance and technical support.
Lifting The Burden: the Global Campaign against Headache and the International Headache Society provided financial support for the editorial process (see Sources of support).
Appendices
Appendix 1. Search strategies for the previous review
For the identification of studies considered for the original review and the 2007 update (Chronicle 2004; Mulleners 2008), detailed search strategies were developed for each database searched. These were based on the search strategy for PubMed, but revised appropriately for each database. The search strategies combined the subject searches described below with the Cochrane highly sensitive search strategy for RCTs current at the time (Alderson 2004). The subject searches used a combination of controlled vocabulary and free‐text terms based on the search strategy for PubMed presented below.
Databases searched were:
Cochrane Pain, Palliative & Supportive Care Trials Register;
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2005, Issue 3);
PubMed 1966 to 31 December 2005;
EMBASE 1974 to 31 December 2005.
Additional strategies for identifying trials included searching the reference lists of review articles and included studies, searching books related to headache and consulting experts in the field. Two journals, Headache and Cephalalgia, were handsearched in their entirety, through April 2006.
Detailed descriptions of the subject search strategies used for PubMed, EMBASE, and CENTRAL are given below.
PubMed
Phase 1
#1 (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) Limits: Humans
Phase 2
#2 HEADACHE Field: MeSH Terms, Limits: Humans #3 HEADACHE DISORDERS Field: MeSH Terms, Limits: Humans #4 headache* OR migrain* OR cephalgi* OR cephalalgi* Field: All Fields, Limits: Humans #5 #2 OR #3 OR #4 Limits: Humans
Phase 3
#6 anticonvulsant* OR antiepileptic* OR acetazolamide OR carbamazepine OR chlormethiazole OR clobazam OR clonazepam OR clorazepate OR diazepam OR divalproex OR ethosuximide OR felbamate OR fosphenytoin OR gabapentin OR lamotrigine OR levetiracetam OR lidocaine OR lignocaine OR lorazepam OR mephobarbital OR methsuximide OR midazolam OR nitrazepam OR oxcarbazepine OR paraldehyde OR pentobarbital OR phenobarbital OR phenytoin OR primidone OR valproate OR tiagabine OR topiramate OR valproic OR vigabatrin OR zonisamide Field: All Fields, Limits: Humans #7 #1 AND #5 AND #6
EMBASE
#1 'migraine'/exp AND [embase]/lim #2 migrain* OR cephalgi* OR cephalalgi* AND [embase]/lim #3 headache*:ti #4 #1 OR #2 OR #3 #5 'anticonvulsive agent'/de AND [embase]/lim #6 anticonvulsant* OR antiepileptic* OR 'acetazolamide'/de OR 'carbamazepine'/de OR 'chlormethiazole'/de OR 'clobazam'/de OR 'clonazepam'/de OR 'clorazepate'/de OR 'diazepam'/de OR 'divalproex'/de OR 'ethosuximide'/de OR 'felbamate'/de OR fosphenytoin OR 'gabapentin'/de OR 'lamotrigine'/de OR 'levetiracetam'/de OR 'lidocaine'/de OR 'lignocaine'/de OR 'lorazepam'/de OR 'mephobarbital'/de OR 'methsuximide'/de OR 'midazolam'/de OR 'nitrazepam'/de OR 'oxcarbazepine'/de OR 'paraldehyde'/de OR 'pentobarbital'/de OR 'phenobarbital'/de OR 'phenytoin'/de OR 'primidone'/de OR 'valproate'/de OR 'tiagabine'/de OR 'topiramate'/de OR valproic OR 'vigabatrin'/de OR 'zonisamide'/de AND [embase]/lim #7 #5 OR #6 #8 #4 AND #7 #9 ((random*:ti,ab) OR (factorial*:ab,ti) OR (crossover*:ab,ti OR 'cross over':ab,ti OR 'cross over':ab,ti) OR (placebo*:ab,ti) OR ('double blind' OR 'double blind') OR ('single blind':ab,ti OR 'single blind':ab,ti) OR (assign*:ti,ab OR allocat*:ti,ab) OR (volunteer*:ab,ti) OR ('randomized controlled trial'/exp AND [embase]/lim) OR ('single blind procedure'/exp AND [embase]/lim) OR ('double blind procedure'/exp AND [embase]/lim) OR ('crossover procedure'/exp AND [embase]/lim)) NOT ((animal/ OR nonhuman/ OR 'animal'/de AND experiment/ AND [embase]/lim) NOT ((human/ AND [embase]/lim) AND (animal/ OR nonhuman/ OR 'animal'/de AND experiment/ AND [embase]/lim)) AND [embase]/lim) AND [embase]/lim #10 #8 AND #9
CENTRAL
(migrain* OR headache*) AND (randomized controlled trial OR controlled clinical trial) Field: All Fields
Appendix 2. Search strategies for this update
CENTRAL
#1 MeSH descriptor: [Migraine Disorders] explode all trees #2 (migrain* or cephalgi* or cephalalgi*) #3 #1 or #2 #4 MeSH descriptor: [Anticonvulsants] explode all trees #5 (anticonvulsant* or antiepileptic* or acetazolamide or carbamazepine or chlormethiazole or clobazam or clonazepam or clorazepate or diazepam or divalproex or ethosuximide or felbamate or fosphenytoin or gabapentin or lamotrigine or levetiracetam or lidocaine or lignocaine or lorazepam or mephobarbital or methsuximide or midazolam or nitrazepam or oxcarbazepine or paraldehyde or pentobarbital or phenobarbital or phenytoin or primidone or valproate or tiagabine or topiramate or valproic or vigabatrin or zonisamide or eslicarbazepine or lacosamide or perampanel or phenobarbitone or pregabalin or retigabine or rufinamide or stiripentol or *barbit*) #6 #4 or #5 #7 #3 and #6 (search limited to years 2005‐2012)
MEDLINE and MEDLINE In‐Progress (via Ovid)
exp Migraine Disorders/
(migrain* or cephalgi* or cephalalgi*).tw.
or/1‐2
exp Anticonvulsants/
(anticonvulsant* or antiepileptic* or acetazolamide or carbamazepine or chlormethiazole or clobazam or clonazepam or clorazepate or diazepam or divalproex or ethosuximide or felbamate or fosphenytoin or gabapentin or lamotrigine or levetiracetam or lidocaine or lignocaine or lorazepam or mephobarbital or methsuximide or midazolam or nitrazepam or oxcarbazepine or paraldehyde or pentobarbital or phenobarbital or phenytoin or primidone or valproate or tiagabine or topiramate or valproic or vigabatrin or zonisamide or eslicarbazepine or lacosamide or perampanel or phenobarbitone or pregabalin or retigabine or rufinamide or stiripentol or $barbit$).tw.
or/4‐5
3 and 6
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
or/8‐14
exp animals/ not humans.sh.
15 not 16
7 and 17
For MEDLINE: limited 18 to yr="2005 ‐Current" For MEDLINE In‐Process: searched current week on 15 January 2013
EMBASE (via Ovid)
exp Migraine/
(migrain* or cephalgi* or cephalalgi*).tw.
or/1‐2
exp Anticonvulsants/
(anticonvulsant* or antiepileptic* or acetazolamide or carbamazepine or chlormethiazole or clobazam or clonazepam or clorazepate or diazepam or divalproex or ethosuximide or felbamate or fosphenytoin or gabapentin or lamotrigine or levetiracetam or lidocaine or lignocaine or lorazepam or mephobarbital or methsuximide or midazolam or nitrazepam or oxcarbazepine or paraldehyde or pentobarbital or phenobarbital or phenytoin or primidone or valproate or tiagabine or topiramate or valproic or vigabatrin or zonisamide or eslicarbazepine or lacosamide or perampanel or phenobarbitone or pregabalin or retigabine or rufinamide or stiripentol or $barbit$).tw.
or/4‐5
3 and 6
random$.tw.
factorial$.tw.
crossover$.tw.
cross over$.tw.
cross‐over$.tw.
placebo$.tw.
(doubl$ adj blind$).tw.
(singl$ adj blind$).tw.
assign$.tw.
allocat$.tw.
volunteer$.tw.
Crossover Procedure/
double‐blind procedure.tw.
Randomized Controlled Trial/
Single Blind Procedure/
or/8‐22
(animal/ or nonhuman/) not human/
23 not 24
7 and 25
limit 26 to yr="2005 ‐Current"
Data and analyses
Comparison 1. Topiramate (combined analyses based on most relevant dose in each study) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) | 9 | 1793 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.59, ‐0.80] |
| 2 ORs for responders (patients with ≥ 50% reduction in headache frequency) | 9 | 1246 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [2.10, 4.82] |
| 3 RRs for responders (patients with ≥ 50% reduction in headache frequency) | 9 | 1246 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.57, 2.60] |
1.1. Analysis.
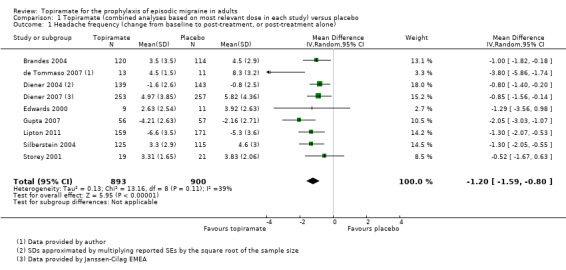
Comparison 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).
1.3. Analysis.
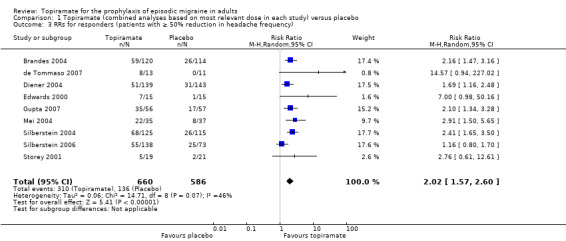
Comparison 1 Topiramate (combined analyses based on most relevant dose in each study) versus placebo, Outcome 3 RRs for responders (patients with ≥ 50% reduction in headache frequency).
Comparison 2. Topiramate (separate analyses of various doses) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 576 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.95, 0.04] |
| 1.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 6 | 1620 | Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.58, ‐0.71] |
| 1.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 804 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.53, ‐0.36] |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 575 | Odds Ratio (M‐H, Random, 95% CI) | 2.35 [1.60, 3.44] |
| 2.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 852 | Odds Ratio (M‐H, Random, 95% CI) | 3.49 [2.23, 5.45] |
| 2.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 6 | 1025 | Odds Ratio (M‐H, Random, 95% CI) | 2.49 [1.61, 3.87] |
| 3 Any adverse event | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.07, 0.17] |
| 3.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 873 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.03, 0.15] |
| 3.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 1 | 213 | Risk Difference (M‐H, Random, 95% CI) | 0.20 [0.08, 0.32] |
| 4 Anorexia | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 584 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.07] |
| 4.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 4.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.05, 0.12] |
| 5 Fatigue | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.07, 0.15] |
| 5.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.06] |
| 5.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 4 | 959 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.04, 0.13] |
| 6 Memory problems | 5 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.01, 0.09] |
| 6.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 3 | 758 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.06] |
| 6.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.06, 0.11] |
| 7 Nausea | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 7.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.04] |
| 7.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 4 | 959 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.11] |
| 8 Paresthesia | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 3 | 584 | Risk Difference (M‐H, Random, 95% CI) | 0.21 [‐0.02, 0.43] |
| 8.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1631 | Risk Difference (M‐H, Random, 95% CI) | 0.33 [0.18, 0.48] |
| 8.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.44 [0.39, 0.49] |
| 9 Taste disturbance | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.07, 0.21] |
| 9.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 5 | 1623 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.01, 0.12] |
| 9.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 4 | 786 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.09, 0.19] |
| 10 Weight loss | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 464 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.01, 0.07] |
| 10.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 4 | 1270 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.03, 0.09] |
| 10.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 5 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.07, 0.12] |
| 11 MSQ‐role function restrictive | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 5.83 [2.25, 9.41] |
| 11.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 10.08 [6.55, 13.60] |
| 11.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 10.36 [6.68, 14.04] |
| 12 MSQ‐role function prevention | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.84 [‐0.24, 5.92] |
| 12.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 6.39 [3.37, 9.41] |
| 12.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 5.06 [1.87, 8.25] |
| 13 MSQ‐emotional function | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 4.58 [0.61, 8.54] |
| 13.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 10.22 [6.31, 14.14] |
| 13.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 8.45 [4.38, 12.52] |
| 14 SF‐36 role physical | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 3.00 [‐3.89, 9.90] |
| 14.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 10.80 [‐2.42, 24.03] |
| 14.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 8.59 [0.65, 16.52] |
| 15 SF‐36 vitality | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.08 [‐4.68, 8.84] |
| 15.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 4.48 [‐7.77, 16.73] |
| 15.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐4.52, 7.24] |
| 16 SF‐36 physical functioning | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐3.28, 4.36] |
| 16.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 2.78 [‐3.29, 8.86] |
| 16.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐5.27, 3.35] |
| 17 SF‐36 bodily pain | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 4.35 [0.04, 8.66] |
| 17.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 6.35 [‐1.29, 14.00] |
| 17.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 5.12 [‐1.25, 11.49] |
| 18 SF‐36 general health | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 1.45 [‐2.18, 5.08] |
| 18.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 4.18 [‐1.21, 9.57] |
| 18.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐1.00, 6.15] |
| 19 SF‐36 social functioning | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.00 [‐1.92, 5.92] |
| 19.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 3.13 [‐3.73, 9.99] |
| 19.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 1.94 [‐2.07, 5.96] |
| 20 SF‐36 role emotional | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.30 [‐4.56, 9.16] |
| 20.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 4.64 [‐3.39, 12.68] |
| 20.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 2.75 [‐4.99, 10.48] |
| 21 SF‐36 mental health | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 Topiramate titrated to 50 mg/day or maximum tolerated dose < 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐4.59, 6.98] |
| 21.2 Topiramate titrated to 100 mg/day or maximum tolerated dose < 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐5.65, 10.81] |
| 21.3 Topiramate titrated to 200 mg/day or maximum tolerated dose < 200 mg | 2 | 458 | Mean Difference (IV, Random, 95% CI) | 1.57 [‐4.21, 7.35] |
2.1. Analysis.
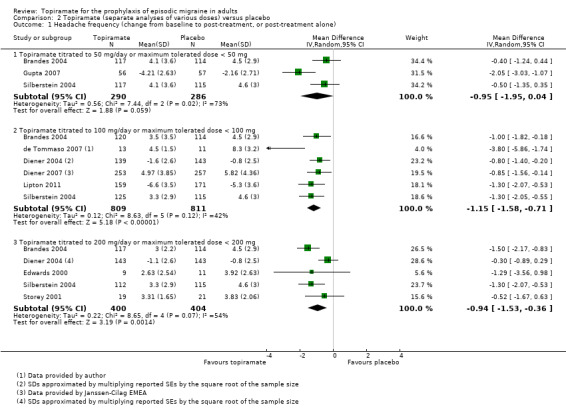
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone).
2.15. Analysis.
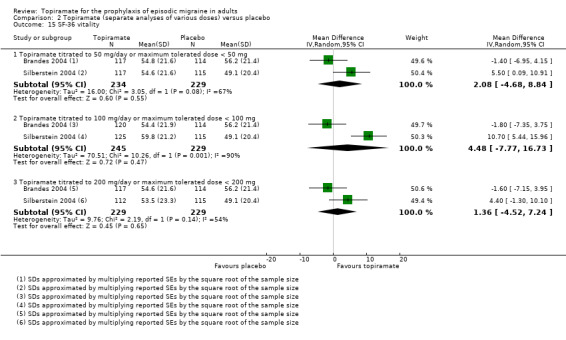
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 15 SF‐36 vitality.
2.16. Analysis.
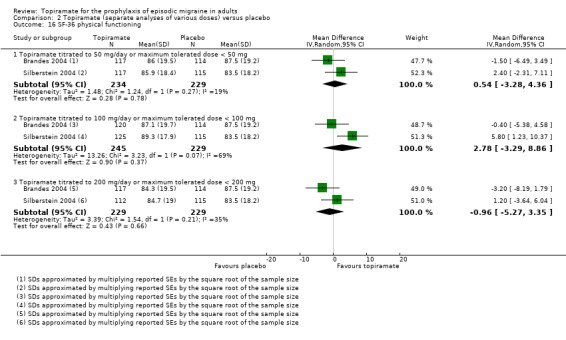
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 16 SF‐36 physical functioning.
2.18. Analysis.
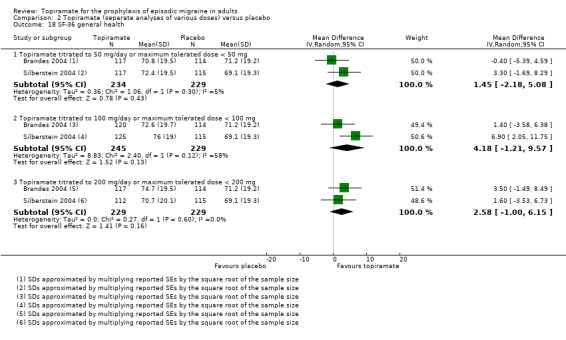
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 18 SF‐36 general health.
2.19. Analysis.
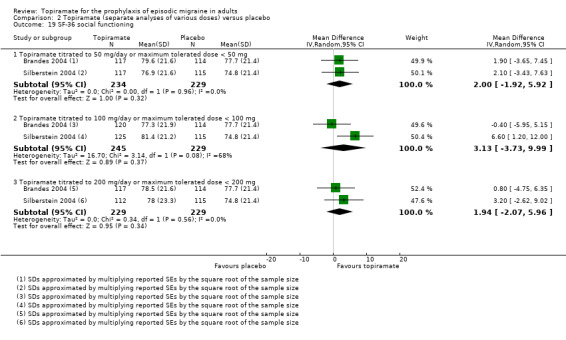
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 19 SF‐36 social functioning.
2.20. Analysis.
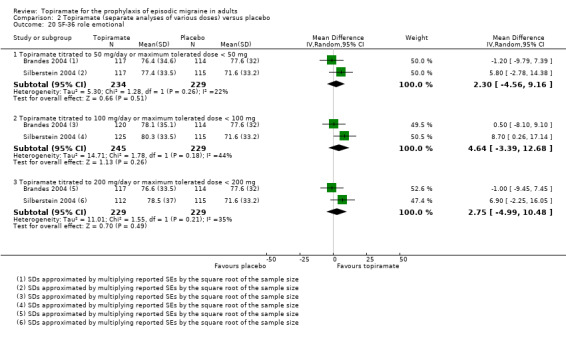
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 20 SF‐36 role emotional.
2.21. Analysis.
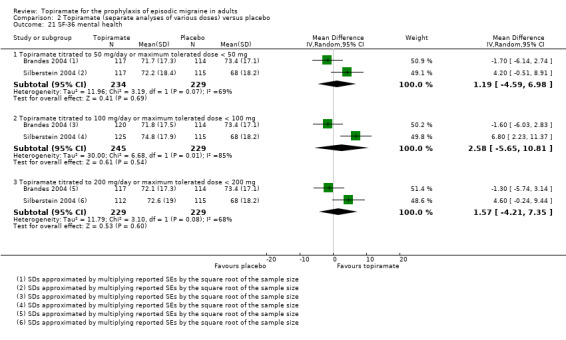
Comparison 2 Topiramate (separate analyses of various doses) versus placebo, Outcome 21 SF‐36 mental health.
Comparison 3. Topiramate direct dose comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.32, ‐0.10] |
| 1.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.53, ‐0.40] |
| 1.3 Topiramate 200 mg versus 100 mg | 3 | 756 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.55, 0.61] |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Topiramate 100 mg versus 50 mg | 2 | 478 | Odds Ratio (M‐H, Random, 95% CI) | 1.80 [1.25, 2.60] |
| 2.2 Topiramate 200 mg versus 50 mg | 2 | 462 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [1.15, 2.41] |
| 2.3 Topiramate 200 mg versus 100 mg | 3 | 756 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.24] |
| 3 MSQ‐role function restrictive | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 4.22 [0.65, 7.80] |
| 3.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 4.55 [0.82, 8.28] |
| 3.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | 0.31 [‐3.37, 3.99] |
| 4 MSQ‐role function prevention | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 3.54 [0.48, 6.60] |
| 4.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 2.28 [‐2.13, 6.69] |
| 4.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐6.67, 4.30] |
| 5 MSQ‐emotional function | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 5.62 [1.67, 9.58] |
| 5.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐0.21, 8.02] |
| 5.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐5.80, 2.34] |
| 6 SF‐36 role physical | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 7.70 [‐8.27, 23.67] |
| 6.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 5.51 [‐5.17, 16.19] |
| 6.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐9.31, 4.90] |
| 7 SF‐36 vitality | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.44 [‐3.05, 7.92] |
| 7.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐4.64, 3.39] |
| 7.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐3.01 [‐9.38, 3.35] |
| 8 SF‐36 physical functioning | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.35 [‐1.02, 5.72] |
| 8.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐4.92, 2.04] |
| 8.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐3.75 [‐7.18, ‐0.32] |
| 9 SF‐36 bodily pain | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.13 [‐1.83, 6.08] |
| 9.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.85 [‐3.27, 4.96] |
| 9.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐5.23, 2.91] |
| 10 SF‐36 general health | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.72 [‐0.76, 6.21] |
| 10.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐4.36, 6.62] |
| 10.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐8.85, 5.65] |
| 11 SF‐36 social functioning | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐5.53, 7.79] |
| 11.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐4.07, 3.96] |
| 11.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐5.56, 3.46] |
| 12 SF‐36 role emotional | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 2.33 [‐3.79, 8.45] |
| 12.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐5.69, 6.94] |
| 12.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐7.92, 4.63] |
| 13 SF‐36 mental health | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Topiramate 100 mg versus 50 mg | 2 | 479 | Mean Difference (IV, Random, 95% CI) | 1.31 [‐1.87, 4.49] |
| 13.2 Topiramate 200 mg versus 50 mg | 2 | 463 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.87, 3.67] |
| 13.3 Topiramate 200 mg versus 100 mg | 2 | 474 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐4.10, 2.36] |
3.6. Analysis.
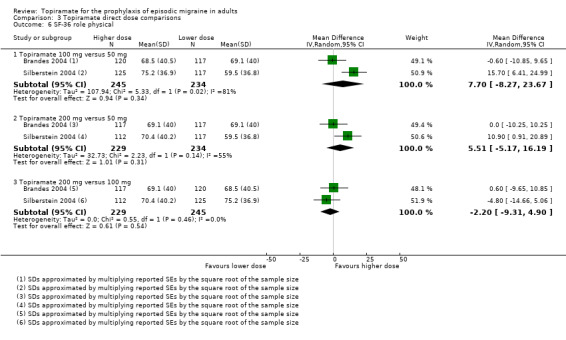
Comparison 3 Topiramate direct dose comparisons, Outcome 6 SF‐36 role physical.
3.7. Analysis.
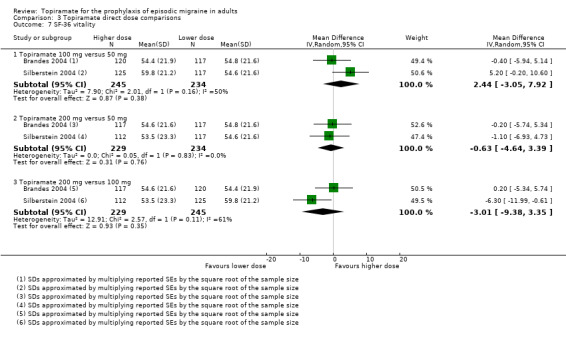
Comparison 3 Topiramate direct dose comparisons, Outcome 7 SF‐36 vitality.
3.9. Analysis.
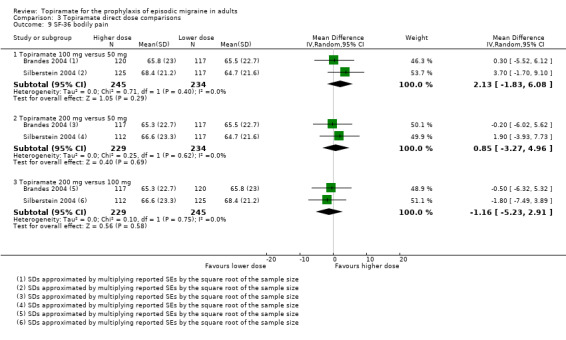
Comparison 3 Topiramate direct dose comparisons, Outcome 9 SF‐36 bodily pain.
3.10. Analysis.
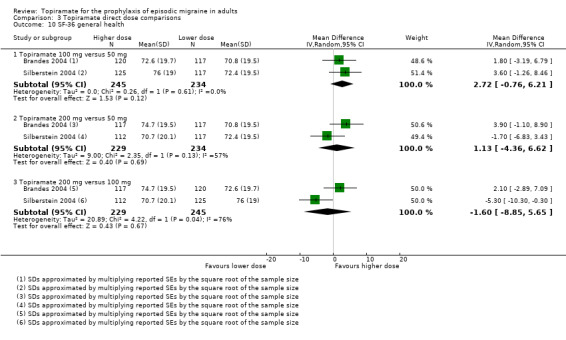
Comparison 3 Topiramate direct dose comparisons, Outcome 10 SF‐36 general health.
3.11. Analysis.
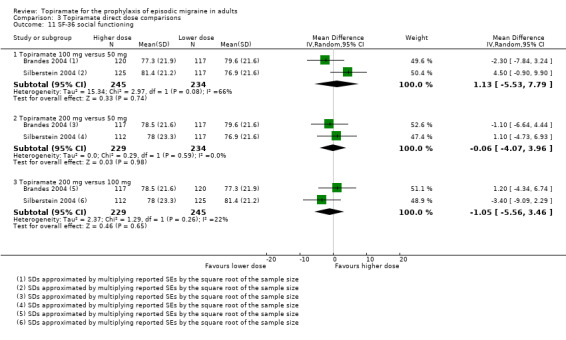
Comparison 3 Topiramate direct dose comparisons, Outcome 11 SF‐36 social functioning.
3.12. Analysis.
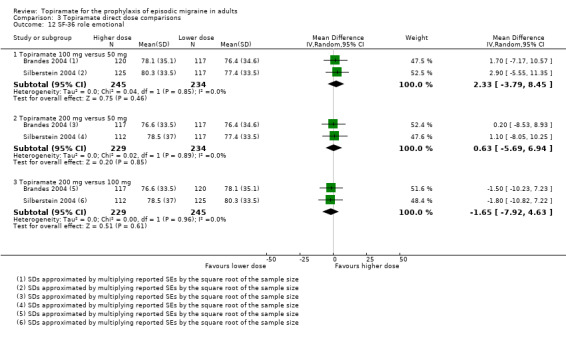
Comparison 3 Topiramate direct dose comparisons, Outcome 12 SF‐36 role emotional.
3.13. Analysis.
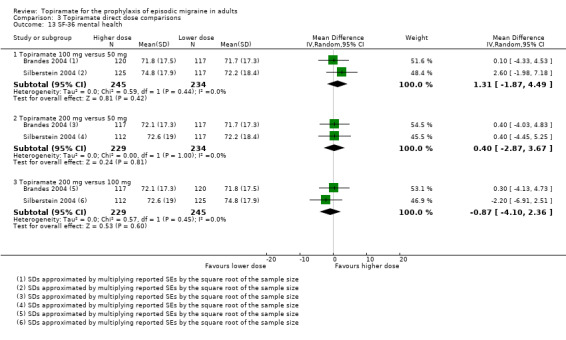
Comparison 3 Topiramate direct dose comparisons, Outcome 13 SF‐36 mental health.
Comparison 4. Topiramate versus amitriptyline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Responders (patients with ≥ 50% reduction in headache frequency) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 MIDAS score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. Topiramate versus flunarizine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache frequency (post‐treatment) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Topiramate versus propranolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache frequency (change from baseline to post‐treatment, or post‐treatment alone) | 2 | 342 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.61, 0.34] |
| 1.1 Topiramate 50 mg versus propranolol 80 mg | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐1.15, 0.41] |
| 1.2 Topiramate 100 mg versus propranolol 160 mg | 1 | 282 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.60, 0.60] |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Topiramate versus sodium valproate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache frequency (post‐treatment) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.58, ‐0.22] |
| 2 MIDAS score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 8. Topiramate versus relaxation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache frequency (change from baseline to post‐treatment) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Responders (patients with ≥ 50% reduction in headache frequency) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Change from baseline in MSQoL | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Afshari 2012.
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. The study consisted of a 4‐week baseline period (possibly retrospective) and a prospective treatment period of 12 weeks Discontinuation rate: topiramate 30%, sodium valproate 22% Compliance (adherence) data: not available Rule for use of acute medication: during acute attacks, patients were allowed to use acetaminophen, NSAIDs, ergotamine, triptans, and opioids Methodological quality score: 3 |
|
| Participants | Inclusion: migraine with or without aura according to ICHD‐II; migraine onset at least 6 months prior to study and before age 50; migraine frequency 4 to 10 attacks per month; attacks separated by 48 h pain‐free interval. Ages 18 to 65. Non‐pregnant, non‐lactating adequate contraception. Migraine prophylaxis withdrawn at least 1 month prior to study entry Exclusion: non‐migraine headaches; > 8 treatment days/month of ergots, NSAIDs, or triptans. No rule reported for exclusion of CDH Other exclusions: alcohol/drug dependence. Hemiplegic, basilar, or ophthalmoplegic migraine. Serious medical conditions Setting: single‐centre Country: Iran Intention‐to‐treat analysis of 56 patients. Of these, 9 had migraine with aura and 47 migraine without aura (ie, not stated that some had both). 44 females and 12 males included in the ITT analysis; mean age among ITT participants treated with topiramate 32.1 ± 10.2; mean age among ITT participants treated with sodium valproate 29.2 ± 9.6. 40 allocated to receive topiramate; 36 allocated to receive sodium valproate |
|
| Interventions | Topiramate 50 mg/day versus sodium valproate 400 mg/day (12 weeks). Topiramate initiated with 25 mg/day for 1 week, thereafter 50 mg/day until study end. Dosing frequency not stated. Sodium valproate initiated with 200 mg/day for 1 week, thereafter 400 mg/day until study end. Dosing frequency not stated | |
| Outcomes | Headache frequency (4 weeks). Headache severity. Duration of episode. Weight. MIDAS at baseline and 8 weeks. HIT‐6 at baseline and 8 weeks. Responder rate Time point(s) considered in the review: last (third) month of double‐blind phase for frequency; entire double‐blind phase for MIDAS |
|
| Notes | A migraine attack persisting longer than 72 hours was counted as a new distinct migraine period. This outcome measure runs the risk of confounding reductions in migraine frequency with reductions in attack duration. Since it is unclear if the baseline was prospective, change scores from baseline were excluded from the analyses of this review. Complementary information requested by email (twice) and ordinary letter (once) but not provided by corresponding author Funders of the trial: Kermanshah University of Medical Sciences, Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Medication prescribed with preprinted medication code labels |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Stated that both participants and clinicians were blinded by the use of preprinted medication code labels. However, there is no mention of equally appearing tablets. It is thus possible that standard medication was provided by third party according to allocation label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20 randomised patients did not contribute to the ITT analysis: 8 AEs; 10 lack of efficacy (whereof 8 were allocated to topiramate); 2 moved |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, or analyses |
Ashtari 2008.
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. Retrospective baseline (presumably 1 month) followed by 8 weeks prospective treatment phase Discontinuation rate in double‐blind phase: topiramate 3%, propranolol 3% Compliance (adherence) data: not available Rule for use of acute medication: no mention Methodological quality score: 3 |
|
| Participants | Inclusion: migraine with or without aura according to ICHD‐I; migraine onset at least 1 year prior to study and before age 50; migraine frequency 3 or more attacks per month during the 3 months prior to study entry; pain‐free interval of at least 48 h between attacks; concomitant migraine prophylactics withdrawn 1 month prior to study entry. Ages 18 to 65 Exclusion: no adequate rule reported for exclusion of CDH or overuse of acute medication. Other exclusions: pregnancy; breast feeding; general and neurologic diseases Setting: single‐centre Country: Iran Complete case analysis of 60 patients. Not reported how many had migraine with aura. 49 females and 11 males; mean age in topiramate group 31.7 ± 8; mean age in propranolol group 29.9 ± 9. 31 allocated to receive topiramate and 31 allocated to receive propranolol |
|
| Interventions | Topiramate 50 mg/day versus propranolol 80 mg/day (8 weeks). Topiramate initiated with 25 mg/day for 1 week, thereafter 50 mg/day until study end. Dosing frequency not stated. Propranolol initiated with 40 mg/day for 1 week, thereafter 80 mg/day until study end. Dosing frequency not stated | |
| Outcomes | Migraine attack frequency per 4 weeks; attack duration (hours); headache intensity (VAS, not mentioned if at prespecified time points or maximum); AEs Time point(s) considered in the review: last (second) month of double‐blind phase |
|
| Notes | Since the baseline was retrospective, change scores from baseline were excluded from the analyses of this review. Complementary information requested twice but not provided by corresponding author Funders of the trial: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was randomised (1:1). Presumably sequence generation was by a lottery procedure (see below under 'Allocation concealment') |
| Allocation concealment (selection bias) | Unclear risk | After selection of 1 of 62 sealed envelopes, half of which contained medication codes for topiramate and the other half those for propranolol, a tear‐off label was removed, revealing the randomisation number. The medication code was removed from the envelope. It is not clear who delivered the study medication according to the medication code |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Both participants and clinicians were blinded. It is unclear how this was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 subject dropped out in each group (both due to AEs) and they were excluded from the data available for this review |
| Selective reporting (reporting bias) | Unclear risk | AEs of propranolol (not included in this review) are inadequately reported |
Brandes 2004.
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. 28‐day baseline period. Duration of treatment: 8 weeks titration, then 18 weeks stable dosage, followed by open‐label extension Discontinuation rate: dropout reported as 47% for combined active treatment groups; 48% for placebo, but unclear how many patients completed the entire trial Compliance (adherence) data: only reported as percentage of patients achieving target dose Rule for use of acute medication: analgesics, ergot derivatives, triptans and opioids allowed Methodological quality score: 5 |
|
| Participants | Inclusion: IHS migraine criteria; migraine frequency of 3 to 12 in 28‐day baseline phase; women practising adequate contraception or unable to bear children Exclusion: secondary headaches, daily headache, and analgesic overuse headache were all adequately excluded. Other exclusions: failure to respond to more than 2 previous migraine‐prophylactic regimens, migraine onset after age 50, continued use of various CNS‐active and other drugs, history of nephrolithiasis, previous exposure to topiramate, use of experimental drug or device within 30 days of screening Setting: multicentre Country: 52 North American clinical centres Intention‐to‐treat analysis of 468 patients. Patients both with and without aura recruited, but percentages not reported. 406 females and 62 males; age range 12 to 65. 117 received 50 mg/day dose, 120 received 100 mg/day dose, 117 received 200 mg/day dose and 114 received placebo |
|
| Interventions | Topiramate 50 mg/day versus topiramate 100 mg/day versus topiramate 200 mg/day versus placebo (18 weeks). Dosage started at 25 mg/day and increased by 25 mg each week to reach assigned dose or maximum tolerated dose | |
| Outcomes | Headache frequency per 28 days. Proportion of responders (50% reduction in frequency). Severity and duration of attacks. Month of onset of drug action. Quality of life (average maintenance AUC of MSQ and SF‐36 scores). AEs Time points considered in the review: through entire double‐blind phase (migraine frequency, response rate, AEs); week 8 to 26 of double‐blind phase (MSQ, SF‐36) |
|
| Notes | Lowest allowable age was 12 years; hence some patients not adult. Headache frequency defined as the number of migraine periods per 28 days, where a migraine period is any occurrence of migraine headache that started, ended, or recurred with 24 hours. A migraine attack persisting into a second 24‐hour period was counted as a new distinct migraine period. This outcome measure runs the risk of confounding reductions in migraine frequency with reductions in attack duration Funders of the trial: Johnson & Johnson Pharmaceutical Research and Development, LLC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule balanced by using permutated blocks of 4 and stratified by centre |
| Allocation concealment (selection bias) | Low risk | An interactive voice response system was used to assign randomisation numbers to patients |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and clinicians were blinded to study medication. Study medication packaged and labelled according to a medication code schedule generated before the trial. Each bottle had a 2‐part, tear‐off label; study medication identification was concealed and could be revealed only in case of emergency |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Treatment assignments were not revealed to investigators or study monitors until all patients had completed therapy and the database had been finalised. Not clearly stated that blinding included the stage of analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis excluded 15 patients who did not provide any post‐baseline data, out of 483 randomised |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, subgroups, or analyses |
de Tommaso 2007.
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. Two months baseline period. Duration of treatment: 2 months Discontinuation rate: topiramate 20%, placebo 27%, levetiracetam 0% Compliance (adherence) data: "non‐compliance" reported as the most common reason for dropping out (6/7), but the extent to which patients took medications as prescribed is not reported Rule for use of acute medication: not reported Methodological quality score: 3 |
|
| Participants | Inclusion: migraine without aura according to ICHD‐II; attack frequency not specified. Ages 18 to 49. Consent to additional neurophysiological tests Exclusion: no adequate rule reported for exclusion of secondary headaches, daily headache, or analgesic overuse headache. However, no included patient had CDH or other headache than migraine according to correspondence with the first author. Other exclusions: psychoactive drugs, general/neurological/psychiatric disorders Setting: single‐centre Country: Italy Intention‐to‐treat analysis of 39 patients. Patients with aura not recruited. 35 females and 10 males included; mean age 37.9 ± 12.4, age range 18 to 49. 15 received topiramate, 15 received placebo, and 15 received levetiracetam |
|
| Interventions | Topiramate 100 mg/day (50 mg BID, presumably tablets) versus placebo versus levetiracetam (8 weeks). Dose escalation strategy not reported | |
| Outcomes | Migraine days per month. 50% or greater reduction in headache frequency. Flicker frequency dependent α‐rhythm phase synchronisation (phase synchronisation index). Mean iCNV amplitude and iCNV habituation index Time point(s) considered in the review: through entire treatment period (2 months) |
|
| Notes | Levetiracetam arm of trial excluded as comparator from this review, since the intervention is experimental. Study was not principally designed to obtain clinical outcome data of the interventions Funders of the trial: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study was double‐blind, but methodological description is lacking |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No concern among the review authors over incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Means (SD) for migraine frequency during treatment period not presented in publications but provided by first author after request. No safety data reported for placebo‐group |
Diener 2004.
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. 14‐day washout period then 28‐day baseline period. Duration of treatment: 8 weeks titration then 18 weeks maintenance Discontinuation rate: dropouts: 32% for topiramate 100 mg, 55% for topiramate 200 mg, 29% for propranolol, 31% for placebo Compliance (adherence) data: daily dose recorded; plasma concentration of topiramate recorded Rule for use of acute medication: aspirin, paracetamol, NSAIDs, ergot compounds, triptans, and opioids permitted Methodological quality score: 4 |
|
| Participants | Inclusion: IHS migraine criteria, migraine onset more than 1 year prior to study, migraine frequency 3 to 12 per month during 28‐day baseline phase. Ages 12 to 65. No mixed or combination headaches included Exclusion: daily headache was adequately excluded; no information given on the exclusion of secondary headache and analgesic overuse headache. Other exclusions: failure to respond to more than 2 previous migraine‐prophylactic regimens, asthma, bradyarrhythmia, uncontrolled diabetes, contraindications to beta‐blockers Setting: multicentre Country: 13 countries in Europe, Asia, Australia, and Africa Intention‐to‐treat analysis of 568 patients. Patients both with and without aura recruited, but percentages not reported. 453 females and 115 males; age range 12 to 65. 139 received topiramate 100 mg/day, 143 received topiramate 200 mg/day, 143 received propranolol 160 mg/day, and 143 received placebo |
|
| Interventions | Topiramate 100 mg/day versus topiramate 200 mg/day versus propranolol 160 mg/day versus placebo (18 weeks). Dosages started at 25 mg/day (topiramate) and 20 mg/day (propranolol) and increased by 25 mg (topiramate) or 20 mg (propranolol) each week to reach assigned dose or maximum tolerated dose | |
| Outcomes | Headache frequency per 28 days. Change in number of migraine days per month. Change in average monthly rate of rescue medication. Proportion of responders (50% reduction in frequency). Month of onset of drug action. Average duration Time point(s) considered in the review: through the core double‐blind phase |
|
| Notes | Lowest allowable age was 12 years; hence some patients not adult. Headache frequency defined as the number of migraine periods per 28 days, where a migraine period is any occurrence of migraine headache that started, ended, or recurred with 24 hours. A migraine attack persisting into a second 24‐hour period was counted as a new distinct migraine period. This outcome measure runs the risk of confounding reductions in migraine frequency with reductions in attack duration Funders of the trial: Johnson & Johnson Pharmaceutical Research and Development, LLC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation, in equal proportions, to 1 of 4 treatment groups. Method not described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and clinicians were blinded. Method not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis excluded 7 patients who did not provide any post‐baseline data, out of 575 randomised |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, subgroups, or analyses |
Diener 2007.
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. 26‐week open‐label phase at target dose of topiramate 100 mg/day (allowing 50 to 200 mg/day), then randomising to 26‐week, double‐blind, parallel‐group phase of prolonged topiramate treatment at individualised dose or placebo Discontinuation rate in double‐blind phase: topiramate 18%, placebo 20% Compliance (adherence) data: not available Rule for use of acute medication: individuals with medication overuse not included; triptans, ergots, opiates, and other analgesics thereafter permitted Methodological quality score: 5 |
|
| Participants | Inclusion: migraine with or without aura according to ICHD‐II; history of migraine at least 1 year; migraine frequency of ≥ 4 attacks/month. Ages 18 to 80 Exclusion: overuse of acute medication. Acceptable exclusion of secondary headaches. Other exclusions: prophylaxis in month preceding entry (3 months for flunarizine); prior poor response on > 2 prophylactics; pregnancy and breastfeeding Setting: 88 centres Country: 21 countries in Europe and the Middle East Intention‐to‐treat analysis of 507 patients. Patients both with and without aura recruited, but percentages not reported. 445/512 females and 67/512 males; mean age among allocated to continuing on topiramate 40.1 ± 10.6; mean age among allocated to switching to placebo 40.1 ± 10.7, age range 18 to 69. 255 allocated to continuing on topiramate and 259 allocated to switching to placebo |
|
| Interventions | Topiramate 100 mg/day versus placebo (26 weeks). Topiramate target dose 100 mg/day (tablets 50 mg BID), individualised according to efficacy and tolerability between 50 and 200 mg/day; dose remaining stable 4 weeks prior to randomisation. Mean dose last month of double‐blind phase: 103 ± 37 mg/day. Matching placebo BID; topiramate meanwhile tapered out by 100 mg weekly. β‐blockers and amitriptyline were allowed in both groups for indications other than migraine | |
| Outcomes | Number of days with migraine headache per 28 days. Duration and severity of migraines. Number of days with acute medication. Patient satisfaction over double‐blind phase. Proportion of responders (50% reduction in frequency) not investigated. MIDAS. HIT‐6. SF‐12 Time point(s) considered in the review: last 4 weeks of the double‐blind phase, ie, weeks 17 to 26 |
|
| Notes | CDH was not an exclusion criterion, but the migraine frequencies (migraine days per 28 days) during the last month of the open‐label phase (group continuing with topiramate: 4.9 ± 3.7 (data provided by Janssen‐Cilag); group that switched to placebo: 4.6 ± 4.0) confirm that the absolute majority had episodic migraine Funders of the trial: Janssen‐Cilag, EMEA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation, medication randomised in blocks of 4; blocks provided per study centre |
| Allocation concealment (selection bias) | Low risk | Subjects sequentially allocated to next available medication number within block at randomisation (= entry into double‐blind phase) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both clinicians and patients blinded. Medication provided by number. Use of identical appearing tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No concern among the review authors over incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Mean monthly migraine frequency during the last month of the double‐blind phase only given in publication for the group that switched to placebo but provided by drug company for the group that continued with topiramate. Variance measures for change in MIDAS scores only roughly indicated in a graph (Fig. 4) |
Dodick 2009.
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. 14 to 28‐day washout period then 28‐day baseline period. Duration of treatment: 4 weeks titration then 22 weeks maintenance followed by up to 2 weeks taper/exit phase Discontinuation rate: topiramate 43%, amitriptyline 44% Compliance (adherence) data: not available Rule for use of acute medication: use of acute headache medications including over‐the‐counter analgesics, NSAIDs, triptans, ergot derivatives, and dihydroergotamine mesylate, was permitted for symptomatic relief of headaches throughout the study, but was not to exceed 4 days per week Methodological quality score: 5 |
|
| Participants | Inclusion: migraine with or without aura according to ICHD‐II, migraine onset at least 6 months prior to study, migraine frequency of 3 to 12 attacks/month during 3 months prior to screening and during baseline period. Ages 18 and above Exclusion: CDH during baseline, analgesic overuse (> 15 treatment days per month with abortive medication). Acceptable exclusion of secondary headaches. Other exclusions: failed > 2 adequate trials of migraine preventive medication, prior lack of efficacy for topiramate and/or amitriptyline, migraine onset after the age of 50 years, aura without headache only, history of cluster headache, progressive neurological disorder, condition more painful than migraine, contraindication for amitriptyline, unstable medical condition within the past 2 years, major psychiatric disorder within the past 6 months, drugs/alcohol abuse within the past 2 years, nephrolithiasis, active liver disease, liver function tests ≥ 2 times the upper limit of normal, pregnancy, lactation, inadequate contraception Setting: 32 centres Country: USA Intention‐to‐treat analysis of 331 patients. Patients both with and without aura recruited, but percentages not reported. 281 females and 50 males; mean age 38.8 ± 11.0, age range 18 to 70. 178 received topiramate and 169 received amitriptyline |
|
| Interventions | Topiramate 100 mg/day versus amitriptyline 100 mg/day (26 weeks). Dosages started at 25 mg/day and increased by 25 mg each week to reach 50 mg BID (topiramate) and 100 mg at night with morning placebos (amitriptyline) or the maximum tolerated dose. A stable dose of at least 50 mg/day was required | |
| Outcomes | Mean 28‐day rate of migraine episodes defined as the period from the onset to the cessation of painful migraine symptoms, not to exceed 24 h. Response rates (≥ 25%, ≥ 50%, ≥ 75%, or 100% reduction) on 28‐day migraine and headache days (migraine and non‐migrainous headache). Mean 28‐day rate of days with headache, abortive medication use, migraine duration, migraine severity, severity of migraine‐associated symptoms, frequency of migraine‐associated vomiting. Severity of functional disability (MIDAS, MSQ, Q‐LES‐Q‐SF). Mean change in weight and BMI Time point(s) considered in the review: through entire treatment period (26 weeks) |
|
| Notes | If painful migraine symptoms lasted > 24 hours, this was considered a new and distinct migraine episode. Such a definition runs the risk of confounding reduction in headache frequency with reduction in attack duration Funders of the trial: Ortho‐McNeil Janssen |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated 5‐digit subject numbers and 4‐digit medication code numbers. Randomisation in permuted blocks of 4 by site |
| Allocation concealment (selection bias) | Low risk | Numbers were assigned as subjects qualified for participation; assigned medication code was retained for duration of study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy. Capsules of identical appearance. Topiramate: 2 active capsules BID + 2 placebo capsules evening. Amitriptyline: 2 placebo capsules morning + 4 active capsules evening. Treatment assigned by medication code |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many participants in the topiramate group contributed to the endpoint ≥ 50% reduction in headache frequency. Complementary data requested twice but not provided by corresponding author |
| Selective reporting (reporting bias) | Unclear risk | Within‐group variance measures lacking for changes in least squares mean of migraine frequencies and MSQ scores. Complementary data requested twice but not provided by corresponding author |
Edwards 2000.
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. 4‐week baseline period. Duration of treatment: 6 weeks titration then 8 weeks stable dosage Discontinuation rate: dropouts: 6 of 15 in topiramate group; 4 of 15 in placebo group Compliance (adherence) data: compliance data reported as number of patients reaching target dose (11 of 15) Rule for use of acute medication: acute medication permitted; allowed types not specified Methodological quality score: 3 |
|
| Participants | Inclusion: IHS migraine criteria, migraine onset before age 50, migraine for more than 1 year, migraine frequency 2 to 8 per month, negative pregnancy test Exclusion: daily headaches and analgesic abuse headaches were adequately excluded. Other exclusions: pregnancy or lactation; substance‐related disorder in 3 months prior to study; Axis I disorders; other relevant medical conditions; history of renal calculi; participant in any other clinical trial within 30 days of study onset Setting: not reported (appears to be single‐centre) Country: USA 30 patients recruited and analysed; various analyses undertaken. Patients with and without aura recruited but percentages not reported. 29 females and 1 male; age range 30 to 62. 15 received topiramate and 15 received placebo |
|
| Interventions | Topiramate 200 mg/day versus placebo (14 weeks). Dosage started at 25 mg/day and increased by 25 mg each week to reach target dose | |
| Outcomes | Number of migraine attacks per 28 days in entire double‐blind period. Number of migraine attacks per 28 days in last 10 weeks of study. Proportion of responders (50% reduction in frequency). Severity and disability scores Time point(s) considered in the review: through entire double‐blind period |
|
| Notes | Funders of the trial: Ortho‐McNeil Pharmaceutical | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and clinician were blinded, and placebo was used. No more information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data available only from abstract and poster presentation |
| Selective reporting (reporting bias) | High risk | Data available only from abstract and poster presentation |
Gupta 2007.
| Methods | Prospective, randomised, double‐blind, triple cross‐over trial. Study duration 23 weeks (although stated 20 weeks); 4 weeks prospective baseline followed by 4 weeks with first intervention, 1‐week washout, cross‐over to 4 weeks with second intervention, 1‐week washout, cross‐over to 4 weeks with third intervention, 1‐week washout, and finally cross‐over to 4 weeks with fourth intervention. Each subject received all treatments in a specified order Discontinuation rate: topiramate 7%, lamotrigine 7%, topiramate placebo 7%, lamotrigine placebo 7% Compliance (adherence) data: not available Rule for use of acute medication: patients were allowed to take tablets with a combination of paracetamol and diclofenac potassium (supplied to them) at their choice Methodological quality score: 2 |
|
| Participants | Inclusion: migraine with or without aura according to ICHD‐I; migraine frequency of 4 to 10 attacks/month, history of migraine at least 1 year, debut of migraine before age 50, at least 48 h pain free interval between attacks. Ages 18 to 65 Exclusion: headaches other than migraines. > 8 days/month of NSAIDs, ergots, or triptans. Paracetamol overuse and CDH not mentioned as exclusion criteria. Other exclusions: migraine prophylactic drug (or drug with such potential) last month, antipsychotic/antidepressant drug last 3 months, alcohol, or other drug dependence, nephrolithiasis, participated in earlier study of lamotrigine or topiramate, used lamotrigine or topiramate 2 weeks or longer, used experimental drug last month Setting: single‐centre Country: India Intention‐to‐treat analysis of 57 patients. 32% (19/60) of included patients had migraine with aura. 47 females and 13 males; mean age 29.4 ± 7.7 years (range 16 to 48). 57 received topiramate, 57 received lamotrigine, 57 received topiramate placebo, and 57 received lamotrigine placebo |
|
| Interventions | Topiramate 50 mg/day versus topiramate placebo versus lamotrigine 50 mg/day versus lamotrigine placebo (4 weeks). Topiramate and lamotrigine were given as 25 mg tablet BID (stable dosage), and placebos as 1 tablet BID (stable dosage) | |
| Outcomes | Change in migraine frequency per 28 days compared to baseline. Proportion of responders (50% reduction in frequency). Responder rate migraine intensity. Attack frequency. Attack duration. Intensity on VAS. Phonophobia. Photophobia. Rescue medication use. Response to rescue medication. Aura frequency. "Reports of AEs communicated historically during visits, as transcribed on headache diaries" Time point(s) considered in the review: through entire treatment period (4 weeks) |
|
| Notes | Lamotrigine (and lamotrigine placebo) data excluded as comparator from this review, since the intervention is experimental. Unclear if any participants had CDH. Shorter treatment periods (1 month) than recommended (3 months) by IHS for evaluation of efficacy in clinical trials. Since 2 potentially active drugs were used, there is an obvious risk of carry‐over effect (analysis for order effects lacking) Funders of the trial: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule allocating participants to 1 of 4 treatment arms: LTG – LPLAC‐TOP‐TPLAC or LPLAC‐LTG‐TPLAC‐TOP or TOP‐TPLAC‐LTG‐LPLAC or TPLAC‐TOP‐LPLAC‐LTG |
| Allocation concealment (selection bias) | Low risk | Preprinted medication code labels. Sealed envelopes containing the code labels with a tear‐off label concealing the randomisation number were provided to the investigator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients and clinicians were blinded. Placebo was identical in appearance and packaging to active drug, but since the lamotrigine and topiramate tablets were different in appearance, 2 different placebos were used. For effective blinding a double‐dummy design would be required. Study medication was packaged and labelled according to a medication code schedule generated before the trial. Each package had all 4 medications numbered according to the phase of the trial. Each bottle had a 2‐part, tear‐off label; study medication identification was concealed and could be revealed only in case of emergency |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Investigators were blinded but not clearly stated that this included the stage of analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No concern among the review authors over incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, subgroups, or analyses |
Lipton 2011.
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. Total study duration up to 266 days; consisting of a pretreatment phase of up to 70 days (screening/washout period, followed by a 28 to 35‐day baseline period), a 26‐week double‐blind phase (6‐week titration followed by 20‐week maintenance), and 1‐week taper/exit phase Discontinuation rate: topiramate 37%, placebo 44% Compliance (adherence) data: not available Rule for use of acute medication: subjects were permitted to take acute headache medication as indicated. The type and method of acute headache medication use was as consistent as possible with that used by the subject prior to enrolment Methodological quality score: 5 |
|
| Participants | Inclusion: migraine with or without aura according to ICHD‐II; migraine frequency of 9 to 14 days/month; history of migraine at least 1 year; onset of migraine before age 50. Ages 18 to 65. Other inclusion criteria: good health, capable of taking oral medication, no risk of pregnancy Exclusion: < 15 total headache days/month; had used a combination of acute headache medications for any reason for > 4 days/week on a regular basis during the 3 months before baseline period. Secondary headaches were adequately excluded. Other exclusions: previously failed > 2 adequate trials of migraine prophylactic drugs; use of migraine prophylactic drugs in the 6 weeks before baseline period; previously discontinued topiramate therapy due to lack of efficacy or AE; exclusively migraine aura without headache; other equally painful condition; cluster headache; basilar or hemiplegic migraine; progressive neurological disorder other than migraine; malignancy; significant medical history or medical condition of neurological, cardiovascular, hepatic, or renal disease; nephrolithiasis; unstable medical condition that may have impaired participation in the study or necessitate the use of drugs not permitted; abnormal renal, liver, or blood tests (specified); suicidality and/or psychiatric disease; drug or alcohol abuse within the past 2 years and positive urine drug screen Setting: 87 centres Country: not reported (all authors from USA) Intention‐to‐treat analysis of 330 "efficacy‐evaluable" (EE) patients. Patients both with and without aura recruited, but percentages not reported. 294 females and 36 males; mean age topiramate group 39.6 ± 10.6; mean age placebo group 40.9 ± 11.2; age range not reported. 188 received topiramate and 197 received placebo |
|
| Interventions | Topiramate 100 mg/day versus placebo (26 weeks). Topiramate initiated with a single 25 mg tablet in the evening day 1 to 7, then increased each week by a single 25 mg tablet/day until a total dosage of 100 mg/day (two 25 mg tablets BID). The titration was adjusted at the discretion of the investigator on the basis of subject tolerability. Subjects must have maintained a dose of at least 3 tablets/day beginning at day 42 and throughout the study period. The mean dose used during maintenance period was 89.5 ± 14.2 mg/day. Placebo initiated with a single tablet in the evening day 1 to 7, then increased each week by a single tablet per day until a total dosage of 2 tablets BID. The titration was adjusted at the discretion of the investigator on the basis of subject tolerability. Subjects must have maintained a dose of at least 3 tablets/day beginning at day 42 and throughout the study period | |
| Outcomes | Headache frequency per 28 days. Proportion of responders (≥ 50% and ≥ 75% reduction in headache days and migraine days). ≥ 15 headache days per 28‐day period (CDH) at month 6. CDH during the last 28‐day period of the double‐blind phase for those subjects that had completed at least 28 days of the double‐blind phase. Time to first reporting of CDH. CDH of which at least half of days with migraine headache. Time to first reporting of CDH of which at least half of days with migraine headache. 28‐day rate of headache days. 28‐day rate of acute medication days. Change in the 28‐day frequency of nausea, photophobia, and phonophobia. MSQ. MIDAS Time point(s) considered in the review: through the 26‐week double‐blind phase |
|
| Notes | Funders of the trial: Ortho‐McNeil Janssen Scientific Affairs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subjects were assigned to either of the 2 treatment groups based on a computer‐generated predetermined randomisation schedule prepared by the sponsor before the study. Randomisation sequences were generated for each site |
| Allocation concealment (selection bias) | Low risk | Medication code numbers were preprinted on study medication labels and assigned as subjects qualified for the study and were randomised to treatment. Sealed envelopes containing the study medication identification (ie, active or placebo) were provided to the investigator and kept in a limited access area |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians were blinded. The double‐blind study medication tablets were identical in appearance and packaged in identically appearing bottles |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Efficacy only reported for the subgroup (EE) of ITT participants who completed at least 28 days of the double‐blind phase |
| Selective reporting (reporting bias) | High risk | ≥ 50% and ≥ 75% reduction in headache days and migraine days were collected but only reported as "higher in the topiramate group compared with the placebo treatment group". For MSQ and MIDAS results, the authors refer to www.clinicaltrials.gov (study identifier: NCT00212810). More than 5 years after study completion, no results from this study have yet been posted there. Corresponding author requested twice about the numbers of subjects with 50% or greater reduction in 28‐day migraine day frequency in both groups without providing data |
Luo 2012.
| Methods | Prospective, randomised, open, parallel‐group trial. Total study duration 13 months; 1‐month baseline period and 12‐month treatment period Discontinuation rate: topiramate 12%, flunarizine 22% Compliance (adherence) data: not available Rule for use of acute medication: subjects were permitted to take acute headache medication as indicated. Allowed rescue drugs included aspirin, acetaminophen, oral NSAIDs, ergot derivatives, triptans and opioids Methodological quality score: 2 |
|
| Participants | Inclusion: migraine with aura, migraine without aura, and/or chronic migraine according to ICHD‐II; at least 2 attacks/month that produce disability lasting 3 or more days per month despite the use of acute treatment; history of migraine at least 1 year. Ages 18 to 65 Exclusion: other primary headache including TTH (confirmed by corresponding author); overuse of analgesics, triptans, or other specific agents for the acute treatment of migraine, including simple analgesics > 15 days/month and combined analgesics > 10 days/month. Secondary headaches were adequately excluded. Other exclusions: use of migraine prophylactic medications in the month before trial entry or flunarizine in the 3 months before trial entry. Prior poor or no efficacy of > 2 migraine prophylactic medications. History of depressive illness, extrapyramidal disorders, chronic obstructive pulmonary disease, bronchospasm, asthma, heart failure, sinus bradycardia, second‐degree atrioventricular block, hypotension or peripheral vascular disease, serious diseases (diabetes, serious hepatic, renal, cardiovascular, respiratory, or malignant illness). Pregnancy, lactation, or childbearing potential without adequate contraception. History of allergy to flunarizine or topiramate Setting: single‐centre Country: China Total number of randomised participants: 150, of which 50 assigned to topiramate (44 contributed to results), 50 to flunarizine (39 contributed to results), and 50 to topiramate + flunarizine. No information on proportion with migraine with aura. Among topiramate completers 30 were females and 14 males; among flunarizine completers 29 were females and 10 males; mean age topiramate completers 42.2 ± 12.4 (range 21 to 65); mean age flunarizine completers 43.2 ± 13.9 (range 20 to 64) |
|
| Interventions | Topiramate 100 mg/day versus flunarizine 5 mg/day versus a combination of both (12 months). Topiramate (presumably tablets) were initiated at 25 mg/day and thereafter increased weekly by 25 mg until reaching target a dose of 100 mg/day. If there was any significant AE, patients were instructed to decrease to the previously tolerated dose. Mean topiramate dose was 62.5 ± 24.4 mg/day. Flunarizine (presumably capsules) was given in a 5 mg/day dose | |
| Outcomes | The primary efficacy parameter was the reduction in mean monthly migraine frequency of at least 50% as compared with baseline. Secondary efficacy parameters were mean monthly frequency of attacks, accumulated monthly migraine duration, and severity of head pain. Weight. Other AEs Time point(s) considered in the review: third month of treatment phase |
|
| Notes | According to the publication, only patients who had chronic migraine were to be included, but the corresponding author reports that only a small minority (7/126 completers; 6%) had chronic migraine according to ICHD‐II Funders of the trial: National Natural Science Foundation, Science and Technology Item of Guandong Province of China, and Natural Science Foundation of Guandong Province of China |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on method for random sequence generation except for resulting 1:1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Subjects who discontinued prematurely (6 in topiramate group whereof 5 due to AEs and 1 lost to follow‐up; 9 in flunarizine group whereof 8 due to lack of efficacy and 3 lost to follow‐up) were excluded from efficacy analyses |
| Selective reporting (reporting bias) | Unclear risk | In Tables 1 and 2, migraine frequency data are mislabelled. Corresponding author confirmed it should be attacks (not days) per month in Table 1 and absolute means (not change) in Table 2. AEs (not included in this review) are inadequately reported |
Mei 2004.
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. One month baseline period. Duration of treatment: 4 weeks titration, then 12 weeks stable dosage Discontinuation rate: dropout 35% for active treatment group, 40% for placebo Compliance (adherence) data: no description of how compliance was assessed Rule for use of acute medication: NSAID and triptan use monitored; unclear as to whether other drugs were permitted Methodological quality score: 4 |
|
| Participants | Inclusion: IHS migraine criteria, migraine frequency of 2 to 6 per month Exclusion: renal pathology, women taking oral contraceptives, women with the possibility of becoming pregnant during the study period, commencement of any migraine prophylactic medication in the 2 months prior to the trial Setting: single‐centre Country: Italy Complete case analysis of 72 patients. Percentages of patients with aura: 23% in active treatment group, 16% in placebo. 39 females and 33 males; age range 20 to 60. 35 received 100 mg/day topiramate, 37 received placebo |
|
| Interventions | Topiramate 100 mg/day versus placebo (16 weeks). Dosage started at 25 mg/day then increased by 25 mg each week until 100 mg dose reached | |
| Outcomes | Headache frequency per 28 days. Proportion of responders (50% reduction in frequency). Severity and duration of attacks, consumption of rescue medications, days of disability Time point(s) considered in the review: last 4 weeks of the double‐blind phase |
|
| Notes | Funders of the trial: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation (ratio 1:1) in balanced blocks of 2 using a computer‐generated random number scheme |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study was double‐blinded, and placebo was used. No more information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Analysis of responder rate appears to consider complete cases only, and safety data are only presented for a vaguely defined subgroup of participants |
| Selective reporting (reporting bias) | Unclear risk | Standard deviations lacking for migraine frequency during treatment phase |
Shaygannejad 2006.
| Methods | Prospective, randomised, double‐blind, double‐cross‐over trial. 1‐month baseline period. Duration of treatment: 1 week titration, followed by 7 weeks stable dose of first drug. 2 months washout, then 1‐week titration, followed by 7 weeks stable dose of second drug Discontinuation rate: no dropouts were recorded Compliance (adherence) data: compliance was reported as good, but no details or results of compliance measurement are given Rule for use of acute medication: unspecified analgesics allowed, but not more than once per day. No other details provided Methodological quality score: 3 |
|
| Participants | Inclusion: IHS migraine criteria, migraine for at least 6 months prior to trial, migraine frequency 3 or more per month in the 3 months prior to trial Exclusion: no clear details given on the exclusion of secondary headache, daily headache, or analgesic overuse headache. Other exclusions: concurrent medical treatment; concurrent serious medical problems; other neurological disease; lactating or pregnant Setting: single neurology clinic Country: Iran Complete case analysis of 64 patients. Patients with and without aura recruited, but percentages not reported. 36 males and 28 females; age range 14 to 57 years |
|
| Interventions | Topiramate 50 mg/day versus sodium valproate 400 mg/day (8 weeks); repeat in cross‐over phase. Topiramate dose started at 25 mg/day and was incremented to 50 mg/day; sodium valproate was started at 200 mg/day and incremented to 400 mg/day | |
| Outcomes | Headache frequency per month; migraine intensity; migraine duration Time point(s) considered in the review: last (second) month of stable dosage treatment phase |
|
| Notes | Study appears to use doses of both topiramate and valproate that are lower than normal clinical doses Funders of the trial: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients and clinicians were blinded, but method description is lacking |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts are acknowledged |
| Selective reporting (reporting bias) | High risk | Only 2 types of AEs are reported for topiramate |
Silberstein 2004.
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. 28‐day baseline period. Duration of treatment: 8 weeks titration, then 18 weeks stable dosage Discontinuation rate: dropout reported as 47% for combined active treatment groups; 41% for placebo. It is unclear how many patients contributed to efficacy data but in fact discontinued the study early Compliance (adherence) data: compliance data reported only as percentage of patients achieving target dose Rule for use of acute medication: analgesics, ergot derivatives, triptans, and opioids allowed Methodological quality score: 5 |
|
| Participants | Inclusion: IHS migraine criteria; migraine frequency of 3 to 12 in 28‐day baseline phase; women practicing adequate contraception or unable to bear children Exclusion: secondary headaches, daily headache, and analgesic overuse headache were all adequately excluded. Other exclusions: failure to respond to more than 2 previous migraine‐prophylactic regimens, migraine onset after age 50, continued use of various CNS‐active and other drugs, history of nephrolithiasis, previous exposure to topiramate, use of experimental drug or device within 30 days of screening Setting: multicentre Country: USA Intention‐to‐treat analysis of 469 patients. Patients both with and without aura recruited, but percentages not reported. 416 females and 53 males; age range 12 to 65. 117 received 50 mg/day dose, 125 received 100 mg/day dose, 112 received 200 mg/day dose, and 115 received placebo |
|
| Interventions | Topiramate 50 mg/day versus topiramate 100 mg/day versus topiramate 200 mg/day versus placebo (18 weeks). Dosage started at 25 mg/day and increased by 25 mg each week to reach assigned dose or maximum tolerated dose | |
| Outcomes | Headache frequency per 28 days. Proportion of responders (50% reduction in frequency). Number of days requiring rescue medication. Time to onset of drug action. Quality of life (average maintenance AUC of MSQ and SF‐36 scores). AEs Time point(s) considered in the review: through entire double‐blind phase (migraine frequency, response rate, AEs); week 8 to 26 of double‐blind phase (MSQ, SF‐36) |
|
| Notes | Lowest allowable age was 12 years; hence some patients not adult. Headache frequency defined as the number of migraine periods per 28 days, where a migraine period is any occurrence of migraine headache that started, ended, or recurred with 24 hours. A migraine attack persisting into a second 24‐hour period was counted as a new distinct migraine period. This outcome measure runs the risk of confounding reductions in migraine frequency with reductions in attack duration Funders of the trial: Johnson & Johnson Pharmaceutical Research and Development, LLC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation in permutation blocks of 4 stratified by centre |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing study drug information were provided to investigators in case such information was required on unblinding a patient |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and clinicians were blinded to study medication with preprinted medication code labels. Placebo was identical in appearance and packaging to active drug |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis excluded 18 patients who did not provide any post‐baseline data, out of 487 randomised |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, subgroups, or analyses |
Silberstein 2006.
| Methods | Prospective, randomised, double‐blind, parallel‐group trial. Study duration up to 7 months; up to 1 month screening/washout, 1 month prospective baseline, and 5 months double‐blind phase (2 months titration and 3 months maintenance) Discontinuation rate: topiramate 36%, placebo 18% Compliance (adherence) data: not available Rule for use of acute medication: use of acute medications was allowed for the symptomatic relief of breakthrough migraine pain Methodological quality score: 3 |
|
| Participants | Inclusion: migraine with or without aura according to ICHD‐I; average migraine frequency of 3 to 8 migraine episodes/month for 3 months before screening; history of migraine at least 1 year; migraine onset before age 50. Ages 18 to 65 Exclusion: > 15 headache days/month during the 3 months before screening, during screening, or during the prospective baseline period; overused acute migraine treatment (eg, triptan use on > 8 days/month); transformed migraine. Secondary headaches acceptably excluded. Other exclusions: previously failed to respond to topiramate therapy; preventive medication within 2 weeks of the start of baseline period; cluster headache; basilar, ophthalmoplegic, or hemiplegic migraine; migraine aura exclusively (without headache); previously failure to respond to > 2 adequately dosed migraine preventive medications; receipt of injected corticosteroids, local anaesthetics, or botulinum toxin within 60 days before screening; risk of pregnancy; lactation; serum alanine and/or aspartate aminotransferase levels > 2 times the upper limit of the normal range; active liver disease Setting: 27 centres Country: USA Intention‐to‐treat analysis of 211 patients. 75 subjects (36%) in ITT group had migraine with aura. 181 females and 30 males; mean age 40.5 ± 11.1; age range 18 to 64. 140 received topiramate 73 received placebo |
|
| Interventions | Topiramate 200 mg/day versus placebo (20 weeks). Topiramate (presumably tablet) 25 mg/day for the first week, followed by weekly increases of 25 mg to a maximum of 100 mg BID or the maximum tolerated dose at week 8. Mean dosage during maintenance period: 161 ± 53 mg/day. Placebo (tablet?) 1/day for the first week, followed by weekly increases of 1 to a maximum of 4 BID or the maximum tolerated dose at week 8 | |
| Outcomes | Headache frequency per 28 days. Proportion of responders (those with ≥ 50%, ≥ 75%, or 100% reduction in monthly migraine frequency). Safety assessments included measurement of vital signs, physical examinations, clinical laboratory test, and evaluation of AEs Time point(s) considered in the review: through the 20‐week double‐blind phase |
|
| Notes | Funders of the trial: Ortho‐McNeil Neurologics Inc., Titusville, New Jersey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information in publication except for 2:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients and clinicians were blinded. No description of method except for the use of placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No concern among the review authors over incomplete outcome data |
| Selective reporting (reporting bias) | High risk | Data on mean migraine frequencies during the double‐blind period lacking (only changes in least squares means without variance measures given in publication). Supplementary information requested twice from corresponding author, but no reply |
Storey 2001.
| Methods | Prospective, randomised, double‐blind, parallel trial. Total duration: 20 weeks. 4‐week baseline period, 8 weeks titration, 8‐week maintenance period Discontinuation rate: dropout 16% for active treatment; 10% for placebo Compliance (adherence) data: no compliance data reported Rule for use of acute medication: abortive medications permitted (no further specification) Methodological quality score: 3 |
|
| Participants | Inclusion: IHS migraine criteria; migraine onset at least 1 year prior to trial; 2 or more attacks per month for previous 12 months; adequate contraception for women; negative pregnancy test 72 hours prior to trial Exclusion: secondary headaches, daily headaches, and analgesic overuse headaches were adequately excluded. Other exclusions: substance‐related disorders, psychiatric disorder, carbonic anhydrase inhibitors, other experimental interventions, history of renal calculi, diagnosis of multiple sclerosis, other contraindications Setting: single neurology clinic Country: USA 40 migraine patients participated; numbers with and without aura not reported. 39 females and 1 male; allowed age range 18 to 65 years |
|
| Interventions | Topiramate versus placebo (16 weeks). Dosage titrated and maintained at 200 mg/day or maximum tolerated dose. Mean actual dose 125 mg/day | |
| Outcomes | Number of migraine attacks per 28 days. Migraine severity (3‐point scale). Change in body weight Time point(s) considered in the review: through entire double‐blind phase |
|
| Notes | Unusual feature of trial: concomitant migraine prophylactics were allowed if patients had been on stable dose for 3 months prior to start of trial, and no changes in dose took place during trial Funders of the trial: Ortho‐McNeil Pharmaceutical, Raritan, New Jersey |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information except 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients and clinicians were blinded. No description of method except for the use of placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No concern among the review authors over incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, subgroups, or analyses |
Varkey 2011.
| Methods | Prospective, randomised, open, parallel‐group trial. Total study duration 10 to 12 months: 1 to 3 months baseline, 3 months treatment period, and 6 months additional follow‐up Discontinuation rate: topiramate 32%, relaxation 13% Compliance (adherence data on file provided by corresponding author): 77% in topiramate group (defined as using the drug > 2 months in accordance with the prescription and measured using self reports), 87% in relaxation group (defined as participating in 6 or more sessions at the clinic plus verbal confirmation of practice at home) Rule for use of acute medication: medication overuse headache was an exclusion criterion. No restrictions were thereafter placed on the use of concomitant acute medication. Acute medication use (doses/month) was documented during the whole treatment period Methodological quality score: 3 |
|
| Participants | Inclusion: migraine with or without aura according to ICHD‐II; 2 to 8 migraine attacks/month; history of migraine at least 1 year; onset of migraine before 50 years of age. Ages 18 to 65 Exclusion: medication overuse headache according to ICHD‐II. Other secondary headaches adequately excluded. CDH not an exclusion criterion (1 patient had chronic migraine). Other exclusions: interval headaches not distinguishable from migraine; regular exercise (once or more per week during the 12 weeks prior to the study); earlier regular practice of relaxation; pregnancy; breastfeeding; use of daily migraine prophylaxis in the 12 weeks prior to the study; inability to understand Swedish; use of antipsychotic or antidepressive medication in the 12 weeks prior to the study; drug or alcohol abuse; topiramate intolerance Setting: single‐centre Country: Sweden Intention‐to‐treat analysis of 91 patients; 7 had migraine with aura only, 44 had migraine without aura only, and 40 had both migraine with and without aura. 82 females and 9 males; mean age 44.3 ± 10.6; allowed age range 18 to 65 years. 31 received topiramate, 30 received relaxation and 30 received exercise |
|
| Interventions | Topiramate 200 mg/day versus relaxation versus exercise (36 weeks). Topiramate tablets started by 25 mg at night and thereafter increased weekly by 25 mg in dialogue with a neurologist until reaching the highest tolerable dose with a maximum of 100 mg BID. Participants in relaxation arm had to attend a scheduled individual appointment for relaxation with a registered physiotherapist once a week. The relaxation programme (Larsson 2002) is based on relaxation, breathing, and stress‐management techniques and includes a series of 6 exercises, each of which is based on the one before. Each relaxation exercise lasted for between 5 and 20 minutes, and verbal and written information was given before the introduction of a new relaxation exercise. After each session there was an opportunity for the participant to discuss their progress with the physiotherapist. If they were absent, they were contacted and informed about how to continue on their own. Between the scheduled sessions, the participants practised at home every day with a compact disc | |
| Outcomes | Migraine attack frequency per 28 days. Proportion of responders (≥ 50% and 25% to 49% reduction in migraine attack frequency). Migraine days per 28 days. Mean pain intensity (VAS 0 to 100). Acute medication use (doses per 28 days). Quality of life (MSQoL, 0 to 100 points). Level of physical activity (MET‐minutes/week). Sedentary hours/day. Oxygen uptake. AEs Time point(s) considered in the review: third month of treatment |
|
| Notes | Exercise arm of trial excluded as comparator from this review, since the intervention is experimental Funders of the trial: The Swedish Research Council; The Gothenburg Research and Development Council; Praktikertjänst Inc, Stockholm, Sweden; The Minnesfonden at the Swedish Association of Registered Physiotherapists; The Renée Eander Fund; The Neurological Research Foundation; The Olle Engkvist Byggmästare Foundation; GlaxoSmithKline; AstraZeneca |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation procedure was conducted by an independent person (separate from clinician and patient) according to a lottery procedure. Six pieces of paper, 2 for each group (n = 3), were folded twice and put into an opaque envelope. One piece of paper was taken each time a patient entered the study. After 6 participants had been included, the procedure started again |
| Allocation concealment (selection bias) | High risk | High number of withdrawals in topiramate arm ("refusal to start") suggests treatment selection bias by the subjects (predetermined treatment preference). Given the open nature of the study this may have influenced outcome reporting |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As this study compared pharmacological and non‐pharmacological treatments, blindness to treatment was not possible to achieve |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The completed assessment forms were encoded and returned to the study secretary in sealed envelopes. The evaluator was effectively blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No concern among the review authors over incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | No suspicion of selective reporting of outcomes, time points, subgroups, or analyses |
Abbreviations: AE = adverse event; AUC = area under the curve; BID = twice (two times) a day; BMI = body mass index; CDH = chronic daily headache; CNS = central nervous system; h = hour; HIT‐6 = Headache Impact Test; ICHD‐I/ICHD‐II = International Classification of Headache Disorders, 1st/2nd Edition; iCNV = initial contingent negative variation; IHS = International Headache Society; ITT = intention‐to‐treat; MET = metabolic equivalents; MIDAS = Migraine Disability Assessment; MSQ = Migraine‐Specific Questionnaire; MSQoL = Migraine‐Specific Quality of Life Questionnaire; NSAIDs = non‐steroidal anti‐inflammatory drugs; Q‐LES‐Q‐SF = Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form; SD = standard deviation; SF‐12 = Medical Outcomes Study 12‐item Short‐Form Health Survey; SF‐36 = Medical Outcomes Study 36‐item Short‐Form Health Survey; TTH = tension‐type headache; VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bartolini 2005 | Reports data on chronic migraine only |
| Bavrasad 2010 | Serious flaws including selective outcome reporting and concerns about data integrity |
| Cady 2012 | Comparator not used prophylactically but taken early in each attack during premonitory symptoms |
| Cazares 2008 | Poor reporting with many details lacking and important data conflicting in tables, graphs, and text |
| Cutrer 2001 | Basic science paper |
| Di Trapani 2000 | No control group |
| Edwards 2003 | Combined analysis of data from 2 included trials (Edwards 2000 and Storey 2001) |
| Freitag 2003 | Conference abstract only |
| Garcia‐Monco 2007 | Not randomised or pseudo‐randomised |
| Gode 2010 | Highly selected and small sample of women with vertigo. Potential effect of inadequate randomisation procedure cannot be weighted (patient characteristics are missing). Inclusion of patients with CDH. Lack of estimates of variance |
| Hart 2003 | Conference abstract only |
| Huntington 2005 | Not controlled trial (brief information sheet) |
| Keskinbora 2008 | All participants had depression. Since the comparator (amitriptyline) is an antidepressant, the results are not valid for migraineurs without psychiatric morbidity |
| Krymchantowski 2011 | Not randomised or pseudo‐randomised |
| Krymchantowski 2012 | No treatment arm in which topiramate alone was given |
| Li 2002 | No treatment arm in which topiramate alone was given |
| Li 2007 | No control group |
| Luykx 2009 | Meta‐analysis of data on adverse drug reactions. Of 6 migraine studies analysed, 5 are included in this review (Brandes 2004; Diener 2004; Mei 2004; Silberstein 2004; Silberstein 2006), while the 6th is excluded (Silvestrini 2003) |
| Millan‐Guerrero 2008 | Comparator is experimental (subcutaneous histamine) |
| Mohammadianinejad 2011 | Comparator is experimental (zonisamide) |
| Naegel 2010 | Review article |
| Reuter 2010 | Post hoc analysis of Diener 2007 (included) |
| Rodríguez‐Leyva 2010 | Data obtained retrospectively by using MIDAS, which is not designed to measure migraine attack frequency. Means and variance thus lacking for migraine frequency, as are responder rates |
| Silberstein 2002 | Conference abstract only |
| Silberstein 2003 | Review article |
| Silvestrini 2003 | Reports data on chronic migraine only |
Abbreviations: CDH = chronic daily headache; MIDAS = Migraine Disability Assessment
Differences between protocol and review
After reviewing the variety of methods used for calculating headache index, it was decided that no systematic analysis of headache index data would be undertaken, for two principal reasons. First, rarely was sufficient information given to allow a clear understanding of how the index was calculated, and second, even when indexes were clearly described, they were not always useful — for example, because they confounded severity scores with frequency scores. Avoiding the use of headache index measures is consistent with the recommendations of the International Headache Society (Tfelt‐Hansen 2012).
After publication of the protocol, we decided not to extract trial data on pain intensity, duration of attacks, or associated symptoms of migraine (nausea, vomiting, photophobia, phonophobia). The reasons were that such information was rarely given, and that the methods used were not standardised.
Our methods for assessing and dealing with heterogeneity have evolved over time in line with changing Cochrane methods. The protocol for the original review specified that we would test estimates of efficacy for homogeneity, use a fixed‐effect model to combine homogenous estimates, and use a random‐effects model to combine estimates when a group of studies with statistically heterogeneous results appeared to be clinically similar. In the original review itself, and in the 2007 update (Chronicle 2004; Mulleners 2008), we in fact used a random‐effects model throughout for pooled analyses. In the present review, we again use a random‐effects model for pooling, but we have added a possible fixed‐effect sensitivity analysis in select cases; see Assessment of heterogeneity for details.
Contributions of authors
Prof Linde: Designing the review. Co‐ordinating the review. Data collection for the review. Screening search results. Organising retrieval of papers. Screening retrieved papers against eligibility criteria. Appraising quality of papers. Extracting data from papers. Writing to authors of papers for additional information. Providing additional data about papers. Data management for the review. Entering data into RevMan. Analysis of data. Interpretation of data. Providing a clinical perspective. Writing the review.
Dr Mulleners: Conceiving the review. Designing the review. Data collection for the review. Screening search results. Organising retrieval of papers. Screening retrieved papers against eligibility criteria. Appraising quality of papers. Extracting data from papers. Interpretation of data. Providing a clinical perspective.
Prof Chronicle: Performing previous work that was the foundation of the current review.
Assoc Prof McCrory: Analysis of data. Interpretation of data. Providing a methodological perspective. Providing general advice on the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
International Headache Society, UK.
Funding for administrative costs associated with editorial and peer review of the original and updated reviews
-
Lifting The Burden: the Global Campaign against Headache, UK.
Funding for administrative costs associated with editorial and peer review of the updated review
Declarations of interest
Prof Linde: During the process of preparing this review the author received a travel grant from Allergan in Sweden and was involved as an investigator in a clinical trial in Norway sponsored by AstraZeneca and comparing candesartan, propranolol, and placebo in the prophylaxis of migraine. He was senior investigator in one of the included studies (Varkey 2011).
Dr Mulleners: The author was a paid consultant for the Merck Dutch Migraine Advisory Board and received a speaker's fee from Merck Sharp & Dohme Corp.
Prof Chronicle: Author deceased. During the process of preparing the original review the author was a paid consultant for Johnson & Johnson and NPS Pharmaceuticals in the USA.
Assoc Prof McCrory: During 2008, the author was a paid expert witness for the plaintiffs in a legal action against the manufacturer of Neurontin (gabapentin). In this capacity, he prepared a systematic review examining previously confidential research reports obtained from the manufacturer (through discovery), along with published trial reports of gabapentin for migraine prophylaxis, and testified at trial.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Afshari 2012 {published data only (unpublished sought but not used)}
- Afshari D, Rafizadeh S, Rezaei M. A comparative study of the effects of low‐dose topiramate versus sodium valproate in migraine prophylaxis. International Journal of Neuroscience 2012;122(2):60‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ashtari 2008 {published data only}
- Ashtari F, Shaygannejad V, Akbari M. A double‐blind, randomized trial of low‐dose topiramate vs propranolol in migraine prophylaxis. Acta Neurologica Scandinavica 2008;118(5):301‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brandes 2004 {published data only}
- Brandes JL, Kudrow DB, Rothrock JF, Rupnow MFT, Fairclough DL, Greenberg SJ. Assessing the ability of topiramate to improve the daily activities of patients with migraine. Mayo Clinic Proceedings 2006;81(10):1311‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brandes JL, Saper JR, Diamond M, Couch JR, Lewis DW, Schmitt J, et al. Topiramate for migraine prevention: a randomized controlled trial. JAMA 2004;291(8):965‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Tommaso 2007 {published and unpublished data}
- Tommaso M, Guido M, Sardaro M, Serpino C, Vecchio E, Stefano G, et al. Effects of topiramate and levetiracetam vs placebo on habituation of contingent negative variation in migraine patients. Neuroscience Letters 2008;442(2):81‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tommaso M, Marinazzo D, Nitti L, Pellicoro M, Guido M, Serpino C, et al. Effects of levetiracetam vs topiramate and placebo on visually evoked phase synchronization changes of alpha rhythm in migraine. Clinical Neurophysiology 2007;118(10):2297‐304. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Diener 2004 {published data only}
- Diener HC, Tfelt‐Hansen P, Dahlöf C, Lainez MJA, Sandrini G, Wang SJ, et al. Topiramate in migraine prophylaxis: results from a placebo‐controlled trial with propranolol as an active control. Journal of Neurology 2004;251(8):943‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Diener 2007 {published and unpublished data}
- Diener HC, Agosti R, Allais G, Bergmans P, Bussone G, Davies B, et al. Cessation versus continuation of 6‐month migraine preventive therapy with topiramate (PROMPT): a randomised, double‐blind, placebo‐controlled trial. Lancet Neurology 2007;6(12):1054‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dodick 2009 {published data only (unpublished sought but not used)}
- Dodick DW, Freitag F, Banks J, Saper J, Xiang J, Rupnow M, et al. for the CAPSS‐277 Investigator Group. Topiramate versus amitriptyline in migraine prevention: a 26‐week, multicenter, randomized, double‐blind, double‐dummy, parallel‐group noninferiority trial in adult migraineurs. Clinical Therapeutics 2009;31(3):542‐59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Edwards 2000 {published data only}
- Edwards KR, Glantz MJ, Norton JA, Cross N. Prophylactic treatment of episodic migraine with topiramate: a double‐blind, placebo‐controlled trial in 30 patients. Cephalalgia 2000;20(4):316. [DOI: 10.1046/j.1468-2982.2000.00204.x] [DOI] [Google Scholar]
Gupta 2007 {published data only}
- Gupta P, Singh S, Goyal V, Shukla G, Behari M. Low‐dose topiramate versus lamotrigine in migraine prophylaxis (the Lotolamp study). Headache 2007;47(3):402‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lipton 2011 {published data only (unpublished sought but not used)}
- Lipton RB, Silberstein S, Dodick D, Cady R, Freitag F, Mathew N, et al. Topiramate intervention to prevent transformation of episodic migraine: the topiramate INTREPID study. Cephalalgia 2011;31(1):18‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Luo 2012 {published and unpublished data}
- Luo N, Di W, Zhang A, Wang Y, Ding M, Qi W, et al. A randomized, one‐year clinical trial comparing the efficacy of topiramate, flunarizine, and a combination of flunarizine and topiramate in migraine prophylaxis. Pain Medicine 2012;13(1):80‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mei 2004 {published data only}
- Mei D, Capuano A, Vollono C, Evangelista M, Ferraro D, Tonali P, et al. Topiramate in migraine prophylaxis: a randomised double‐blind versus placebo study. Neurological Sciences 2004;25(5):245‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shaygannejad 2006 {published data only}
- Shaygannejad V, Janghorbani M, Ghorbani A, Ashtary F, Zakizade N, Nasr V. Comparison of the effect of topiramate and sodium valproate in migraine prevention: a randomized blinded crossover study. Headache 2006;46(4):642‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Silberstein 2004 {published data only}
- Silberstein SD, Loder E, Forde G, Papadopoulos G, Fairclough D, Greenberg S. The impact of migraine on daily activities: effect of topiramate compared with placebo. Current Medical Research & Opinion 2006;22(6):1021‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Neto W, Schmitt J, Jacobs D, for the MIGR‐001 Study Group. Topiramate in migraine prevention: results of a large controlled trial. Archives of Neurology 2004;61(4):490‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Silberstein 2006 {published data only (unpublished sought but not used)}
- Silberstein SD, Hulihan J, Karim MR, Wu SC, Jordan D, Karvois D, et al. Efficacy and tolerability of topiramate 200 mg/d in the prevention of migraine with/without aura in adults: a randomized, placebo‐controlled, double‐blind, 12‐week pilot study. Clinical Therapeutics 2006;28(7):1002‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Storey 2001 {published data only}
- Storey JR, Calder CS, Hart DE, Potter DL. Topiramate in migraine prevention: a double‐blind, placebo‐controlled study. Headache 2001;41(10):968‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Varkey 2011 {published and unpublished data}
- Varkey E, Cider Å, Carlsson J, Linde M. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia 2011;31(14):1428‐38. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bartolini 2005 {published data only}
- Bartolini M, Silvestrini M, Taffi R, Lanciotti C, Luconi R, Capecci M, et al. Efficacy of topiramate and valproate in chronic migraine. Clinical Neuropharmacology 2005;28(6):277‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bavrasad 2010 {published data only}
- Bavrasad R, Nejad SEM, Yarahmadi AR, Sajedi SI, Rahim F. Assessment of the middle dose of topiramate in comparison with sodium valproate for migraine prophylaxis: a randomized‐double‐blind study. International Journal of Pharmacology 2010;6(5):670‐5. [EMBASE: 2010449325] [Google Scholar]
Cady 2012 {published data only}
- Cady RK, Voirin J, Farmer K, Browning R, Beach ME, Tarrasch J. Two center, randomized pilot study of migraine prophylaxis comparing paradigms using pre‐emptive frovatriptan or daily topiramate: research and clinical implications. Headache 2012;52(5):749‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cazares 2008 {published data only}
- Cazares M, Leal R, Cardiel M, Ibarra O, Garcia M. Essay controlled by drawing, crossed, to evaluate the efficiency and safety of amitriptyline against topiramate in the prevention of the migraine [Ensayo controlado por sorteo, cruzado, para evaluar la eficacia y seguridad de amitriptilina contra topiramato en la prevención de la migraña]. Revista Mexicana de Neurociencia 2008;9(6):475‐9. [Google Scholar]
Cutrer 2001 {published data only}
- Cutrer FM. Antiepileptic drugs: how they work in headache. Headache 2001;41 Suppl 1:S3‐S10. [DOI] [PubMed] [Google Scholar]
Di Trapani 2000 {published data only}
- Trapani G, Mei D, Marra C, Mazza S, Capuano A. Topiramate as prophylactic treatment in migraine [Topiramate nella terapia di profilassi dell'emicrania]. Acta Medica Romana 2000;38(1‐2):164‐8. [Google Scholar]
Edwards 2003 {published data only}
- Edwards KR, Potter DL, Wu SC, Kamin M, Hulihan J. Topiramate in the preventive treatment of episodic migraine: a combined analysis from pilot, double‐blind, placebo‐controlled trials. CNS Spectrums 2003;8(6):428‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Freitag 2003 {published data only}
- Freitag FG. Topiramate prophylaxis in patients suffering from migraine with aura: results from a randomized, double‐blind, placebo‐controlled trial. Advanced Studies in Medicine 2003;3(6C):S562‐4. [Google Scholar]
Garcia‐Monco 2007 {published data only}
- Garcia‐Monco JC, Foncea N, Bilbao A, Ruiz De Velasco I, Gomez‐Beldarrain M. Impact of preventive therapy with nadolol and topiramate on the quality of life of migraine patients. Cephalalgia 2007;27(8):920‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gode 2010 {published data only}
- Gode S, Celebisoy N, Kirazli T, Akyuz A, Bilgen C, Karapolat H, et al. Clinical assessment of topiramate therapy in patients with migrainous vertigo. Headache 2010;50(1):77‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hart 2003 {published data only}
- Hart CE, Dodick DW, Brandes JL, Rothrock JF, Jacobs D, Neto W, et al. Migraine prophylaxis with topiramate: results of double‐blind, placebo‐controlled, dose‐response trials. Epilepsia 2003;44 Suppl 9:106‐7. [Google Scholar]
Huntington 2005 {published data only}
- Huntington J, Yuan CL. Topiramate (Topamax) for migraine prevention. American Family Physician 2005;72(8):1563‐5. [EMBASE: 2006032188] [Google Scholar]
Keskinbora 2008 {published data only (unpublished sought but not used)}
- Keskinbora K, Aydinli I. A double‐blind randomized controlled trial of topiramate and amitriptyline either alone or in combination for the prevention of migraine. Clinical Neurology & Neurosurgery 2008;110(10):979‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krymchantowski 2011 {published data only}
- Krymchantowski AV, Jevoux CC. Topiramate vs divalproex sodium in the preventive treatment of migraine: a prospective "real‐world" study. Headache 2011;51(4):554‐8. [EMBASE: 2011188025] [DOI] [PubMed] [Google Scholar]
Krymchantowski 2012 {published data only}
- Krymchantowski AV, Cunha Jevoux C, Bigal ME. Topiramate plus nortriptyline in the preventive treatment of migraine: a controlled study for nonresponders. Journal of Headache & Pain 2012;13(1):53‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2002 {published data only}
- Li ZX, Li XG, Xiong XJ, Zhang ZL. A clinical study of sibelium combining topiramate in prophylactic treatment of migraine [original in Chinese]. Chinese Journal of Modern Applied Pharmacy 2002;19(5):419‐21. [Google Scholar]
Li 2007 {published data only}
- Li HL, Kwan P, Leung H, Yu E, Tsoi TH, Hui ACF, et al. Topiramate for migraine prophylaxis among Chinese population. Headache 2007;47(4):616‐9. [EMBASE: 2007195566] [DOI] [PubMed] [Google Scholar]
Luykx 2009 {published data only}
- Luykx J, Mason M, Ferrari MD, Carpay J. Are migraineurs at increased risk of adverse drug responses? A meta‐analytic comparison of topiramate‐related adverse drug reactions in epilepsy and migraine. Clinical Pharmacology & Therapeutics 2009;85(3):283‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Millan‐Guerrero 2008 {published data only}
- Millan‐Guerrero RO, Isais‐Millan R, Barreto‐Vizcaino S, Gutierrez I, Rivera‐Castano L, Trujillo‐Hernandez B, et al. Subcutaneous histamine versus topiramate in migraine prophylaxis: a double‐blind study. European Neurology 2008;59(5):237‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mohammadianinejad 2011 {published data only (unpublished sought but not used)}
- Mohammadianinejad SE, Abbasi V, Sajedi SA, Majdinasab N, Abdollahi F, Hajmanouchehri R, et al. Zonisamide versus topiramate in migraine prophylaxis: a double‐blind randomized clinical trial. Clinical Neuropharmacology 2011;34(4):174‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Naegel 2010 {published data only}
- Naegel S, Obermann M. Topiramate in the prevention and treatment of migraine: efficacy, safety and patient preference. Neuropsychiatric Disease and Treatment 2010;6(1):17‐28. [EMBASE: 2010222500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reuter 2010 {published data only}
- Reuter U, Rio MS, Diener HC, Allais G, Davies B, Gendolla A, et al. Migraines with and without aura and their response to preventive therapy with topiramate. Cephalalgia 2010;30(5):543‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rodríguez‐Leyva 2010 {published data only (unpublished sought but not used)}
- Rodríguez‐Leyva I, Sánchez‐Aguilar M, Hernández‐Sierra JF, Mandeville PB, Rodríguez Leyva MDR, Shiguetomi‐Medina JM, et al. Topiramate vs. amitriptyline in prophylactic treatment of migraine: a controlled clinical trial. Revista Mexicana de Neurociencia 2010;11(5):338‐42. [EMBASE: 2011173413] [Google Scholar]
Silberstein 2002 {published data only}
- Silberstein S, Karim R, Kamin M, Jordan D, Hulihan J. Topiramate prophylaxis in patients suffering from migraine with aura: results from a randomized, double‐blind placebo‐controlled trial. European Journal of Neurology 2002;9 Suppl 2:44. [Google Scholar]
Silberstein 2003 {published data only}
- Silberstein SD. Efficacy and safety of topiramate in migraine prevention: a dose‐ranging, placebo‐controlled, double‐blind, multicenter study. Advanced Studies in Medicine 2003;3(6C):S565‐8. [Google Scholar]
Silvestrini 2003 {published data only}
- Silvestrini M, Bartolini M, Coccia M, Baruffaldi R, Taffi R, Provinciali L. Topiramate in the treatment of chronic migraine. Cephalalgia 2003;23(8):820‐4. [DOI] [PubMed] [Google Scholar]
Additional references
Ad Hoc Cttee 1962
- Ad Hoc Committee on the Classification of Headache of the National Institute of Neurological Diseases and Blindness. Classification of headache. JAMA 1962;179(9):717‐8. [Google Scholar]
Alderson 2004
- Alderson P, Green S, Higgins JPT, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Reviewers' Handbook 4.2.2 [updated December 2003]; Appendix 5b. In: The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons, Ltd.
Clarke 1996
- Clarke CE, MacMillan L, Sondhi S, Wells NE. Economic and social impact of migraine. QJM 1996;89(1):77‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dalkara 2012
- Dalkara S, Karakurt A. Recent progress in anticonvulsant drug research: strategies for anticonvulsant drug development and applications of antiepileptic drugs for non‐epileptic central nervous system disorders. Current Topics in Medicinal Chemistry 2012;12(9):1033‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Edmeads 1993
- Edmeads J, Findlay H, Tugwell P, Pryse‐Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension‐type headache on life‐style, consulting behaviour, and medication use: a Canadian population survey. Canadian Journal of Neurological Sciences 1993;20(2):131‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Edvinsson 2010
- Edvinsson L, Linde M. New drugs in migraine treatment and prophylaxis: telcagepant and topiramate. Lancet 2010;376(9741):645‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Evers 2009
- Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, et al. European Federation of Neurological Societies. EFNS guideline on the drug treatment of migraine—revised report of an EFNS task force. European Journal of Neurology 2009;16(9):968‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GBD 2010 Study
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013;380(9859):2163‐96. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICHD‐II 2004
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:1‐160. [DOI] [PubMed] [Google Scholar]
IHS Cttee 1988
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl 7:1‐96. [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Janssen‐Cilag 2013
- Janssen‐Cilag. Topimax product information. LIF—the research based pharmaceutical industry; 2013. www.fass.se (accessed 15 March 2013).
Larsson 2002
- Larsson B, Andrasik F. Relaxation treatment of recurrent headaches in children and adolescents. In: Guidetti V, Russell G, Sillanpää M, Winner P editor(s). Headache and Migraine in Childhood and Adolescence. London: Martin Dunitz, 2002:307‐16. [Google Scholar]
Linde 2012
- Linde M, Gustavsson A, Stovner LJ, Steiner TJ, Barré J, Katsarava Z, et al. The cost of headache disorders in Europe: the Eurolight project. European Journal of Neurology 2012;19(5):703‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Linde 2013a
- Linde M, Mulleners WM, Chronicle EP, McCrory DC. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010610] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2013b
- Linde M, Mulleners WM, Chronicle EP, McCrory DC. Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010611] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2013c
- Linde M, Mulleners WM, Chronicle EP, McCrory DC. Gabapentin or pregabalin for the prophylaxis of episodic migraine in adults. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010609] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2013d
- Linde M, Mulleners WM, Chronicle EP, McCrory DC. Antiepileptics other than gabapentin, pregabalin, topiramate, and valproate for the prophylaxis of episodic migraine in adults. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010608] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 1998
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [Google Scholar]
Mehuys 2012
- Mehuys E, Paemeleire K, Hees T, Christiaens T, Bortel LM, Tongelen I, et al. Self‐medication of regular headache: a community pharmacy‐based survey. European Journal of Neurology 2012;19(8):1093‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Olesen 2006
- Olesen J, Bousser M‐G, Diener H‐C, Dodick D, First M, Goadsby PJ, et al. Headache Classification Committee. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia 2006;26(6):742‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Porter 1992
- Porter RJ, Meldrum BS. Antiepileptic drugs. In: Katzung BG editor(s). Basic and Clinical Pharmacology. 5th Edition. Norwalk, CT: Appleton & Lange, 1992:331‐49. [Google Scholar]
Silberstein 2008
- Silberstein S, Saper J, Berenson F, Somogyi M, McCague K, D'Souza J. Oxcarbazepine in migraine headache: a double‐blind, randomized, placebo‐controlled study. Neurology 2008;70(7):548‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Silberstein 2012
- Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E, Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence‐based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012;78(17):1337‐45. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tfelt‐Hansen 2012
- Tfelt‐Hansen P, Pascual J, Ramadan N, Dahlöf C, D'Amico D, Diener HC, et al. International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 2012;32(1):6‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Victor 2003
- Victor S, Ryan S. Drugs for preventing migraine headaches in children. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD002761] [DOI] [PubMed] [Google Scholar]
Wiffen 2010
- Wiffen PJ, Collins S, McQuay HJ, Carroll D, Jadad A, Moore RA. Anticonvulsant drugs for acute and chronic pain. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001133.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Chronicle 2004
- Chronicle EP, Mulleners WM. Anticonvulsant drugs for migraine prophylaxis. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003226.pub2] [DOI] [PubMed] [Google Scholar]
Mulleners 2008
- Mulleners WM, Chronicle EP. Anticonvulsants in migraine prophylaxis: a Cochrane review. Cephalalgia 2008;28(6):585‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


