Abstract
The cell wall is a structure external to the plasma membrane that is essential for the survival of the fungi. This polysaccharidic structure confers resistance to the cell internal turgor pressure and protection against mechanical injury. The fungal wall is also responsible for the shape of these organisms due to different structural polysaccharides, such as β-(1,3)-glucan, which form fibers and confer rigidity to the cell wall. These polysaccharides are not present in animal cells and therefore they constitute excellent targets for antifungal chemotherapies. Cell wall damage leads to the activation of MAPK signaling pathways, which respond to the damage by activating the repair of the wall and the maintenance of the cell integrity. Fission yeast Schizosaccharomyces pombe is a model organism for the study morphogenesis, cell wall, and how different inputs might regulate this structure. We present here a short overview of the fission yeast wall composition and provide information about the main biosynthetic activities that assemble this cell wall. Additionally, we comment the recent advances in the knowledge of the cell wall functions and discuss the role of the cell integrity MAPK signaling pathway in the regulation of fission yeast wall.
Keywords: Cell wall; Polysaccharides; β-glucan, α-glucan; Bgs; GTPase; Pkc; MAPK
The fission yeast cell wall
The cell wall is a structure external to the plasma membrane that is present in plants, fungi and bacteria. It could be considered equivalent to the extracellular matrix in animal cells but with a higher mechanical resistance. Due to its resistance, the wall protects the cells against physical stresses and the rupture of the plasmatic membrane and prevents the disturbance of the isosmotic conditions. The cell wall is also responsible for the shape of walled organisms (Free, 2013, Gow et al., 2017). Besides its resistant and rigid nature, this structure also has a high elasticity and is remodeled in a regulated manner to permit the growth and morphological changes that occur during the life cycle of walled organisms. If the wall is eliminated by the action of lytic enzymes, the cells are converted into round protoplasts that would need to be maintained in a hyperosmotic medium to equilibrate the internal turgor pressure and avoid cell lysis.
The wall of eukaryotic organisms is mainly made of polysaccharides, and its rigid structure is due to the presence of crystalline microfibers formed by long chains of linear polysaccharides associated through hydrogen bonds. Commonly, these crystalline fibers originate from a single type of sugar bound through β-linkages; cellulose (β-(1,4) glucose) in plants, chitin (β-(1,4) N-acetylglucosamine) in fungi, or β-(1,3) glucans in both types of organisms. In addition, the cell wall includes other polysaccharides, which function as aggregation factors, and different glycoproteins (Ishihara et al., 2007, Ruiz-Herrera, 2012). The fungal cell wall usually has an internal layer that includes the structural fiber component and an outer layer composed of diverse polysaccharides and glycoproteins which are important to deal with environmental stresses.
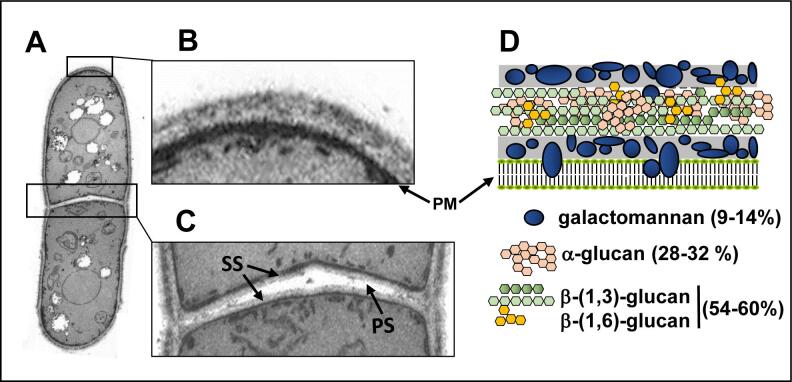
The fission yeast Schizosaccharomyces pombe is a rod-shaped unicellular fungus of 3–4 µm in diameter and 7–14 µm long. This yeast grows by elongation and divides by fission to give rise to equal daughter cells. When observed by transmission electron microscopy, the S. pombe cell wall shows a three-layer organization (Fig. 1), with two electron-dense layers in the outer and inner sides formed by galactomannan which constitutes the sugar part of wall glycoproteins (9–14% of total wall), and a central electron-transparent layer comprised of β-glucans and α-glucans, forming 54–60% and 28–32% of the total wall respectively. This layer contains crystalline fibers of β-(1,3)-glucan that provide rigidity to the cell wall, β-(1,6) glucan branches that increase the wall strength, and α-(1,3)-glucan which functions as an aggregation factor essential for cell integrity (Sugawara et al., 2003).
Fig. 1.
(A) TEM image of a fission yeast cell. Details of (B) the cell wall and (C) the septum are shown. PM: Plasma membrane, PS: primary septum, SS: Secondary septum. (D) Scheme of the fission yeast cell wall organization and percentages of the different polysaccharides.
When S. pombe cells form a division septum (Fig. 1A, C), a three layered structure is observed with a central primary septum mainly made of linear β-(1,3)-glucan that disappears upon cell separation and two outer secondary septa which initially do not have an outer layer of galactomannan. The secondary septa will later form the wall of the new tips in the daughter cells (Garcia Cortes et al., 2016). Fission yeast separation of the two daughter cells is achieved through the action of two hydrolases: Agn1 endo α-(1,3) glucanase which partially digests the lateral cell wall in the division area (Dekker et al., 2004, Garcia et al., 2005), and Eng1 endo β-(1,3) glucanase which digests the primary septum (Martin-Cuadrado et al., 2003). Eng1 contains a region at the C‐terminus that acts as a carbohydrate‐binding module with affinity for small β-(1,3)-glucan chains with a minimum degree of polymerization (Martin-Cuadrado et al., 2008).
Galactomannan
Immunoelectron microscopy using colloidal gold-labeled lectins specific against galactomannan localizes this polymer to the outer and inner sides of the cell wall. Galactomannan is the glycan part of the cell wall glycoproteins, covalently bound via N- and O-glycosidic linkages to the protein amino acids. It consists of a main chain of α-(1,6)-linked D-mannose with branches formed by α-(1,2)- or α-(1,3)-linked D-mannose and a terminal D-galactose residue in the non-reducing ends. A small amount of galactose not located at the terminal position has also been found (Horisberger et al., 1978). Although the protein content of S. pombe walls is only slightly lower than in Saccharomyces cerevisiae, they are less glycosylated and the amount of sugar bound to the proteins is 2–3 folds lower.
Fungal wall proteins are important to generate the adherence properties required to colonize different surfaces. Protein-bound N-glycan forms a tight, inflexible helix that has a hydrophobic face and causes cell surface hydrophobicity. N-glycosylation sites are abundantly present in fungal adhesins, which modulate adhesion, aggregation, biofilm formation and, in pathogenic fungi, the host-immune responses.
Most proteins found in the fungal wall are glycoproteins secreted to the medium; and only a few are covalently linked to other wall polysaccharides. The latest proteins are divided into two groups: (a) ASL (Alkali-Sensitive Linkage) or PIR (Proteins with Internal Repeats) proteins covalently attached to β-(1,3)-glucan through glutamine residues within their tandem repeat domains that can be release with a mild alkali treatment; and (b) proteins covalently attached by a C-terminal glycosyl-phosphatidylinositol (GPI) anchor to the β-(1,6)-glucan. These proteins can be removed by β-glucanase treatment (de Groot et al., 2005, Ecker et al., 2006, de Medina-Redondo et al., 2010, Klis et al., 2010).
Three of the S. pombe PIR-type proteins are similar to proteins belonging to the S. cerevisiae SUN family, with β-glucosidase activity involved in the remodeling of the cell wall during the various phases of yeast growth and in response to environmental cues. Other PIR proteins such as Asl1, are related to proteins from Aspergillus fumigatus and Ustilago maydis (de Groot et al., 2007).
The S. pombe genome contains 39 hypothetical GPI proteins with different enzymatic activities such as amilases, glucanases, proteases, etc. However, only four of them (Gas1, Gas2, Gas4, and Gas5) are β-(1,3)-glucanosyl-transferases related to S. cerevisiae Gas1, which is involved in the maturation and branching of β-(1,3)-glucan, and therefore it is essential for the cell wall assembly (Fig. 2). S. pombe Gas1 is essential for cell integrity during vegetative growth and Gas4 is essential for sporulation. Gas2 and Gas5 seem to play a minor role in the cell wall assembly (de Medina-Redondo et al., 2010).
Fig. 2.
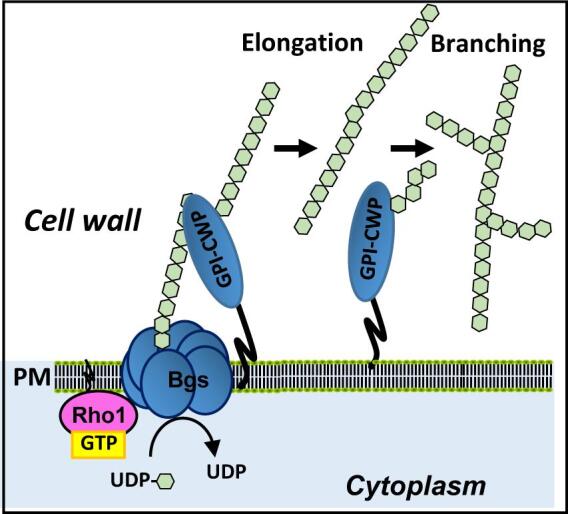
Schematic representation of the β-(1,3)-glucan synthesis by the GS complex in the plasma membrane. Then the β-(1,3)-glucan is elongated and branched through the action of cell wall proteins with glucosyltransferase activity. Glucose units ( ).
).
The fission yeast genome also codes for 14 putative adhesins that, unlike those previously characterized, do not contain detectable glycosyl phosphatidyl inositol (GPI) anchor signals or glutamine residues within tandem repeat domains to mediate their attachment to the cell wall, suggesting the existence of a novel cell wall attachment mechanism (Linder and Gustafsson, 2008). Map4 is one of those adhesins which can be extracted from the cell walls either with SDS/mercaptoethanol or with mild alkali solutions but not with β-glucanases (Sharifmoghadam and Valdivieso, 2008). The α-glucan might contribute to the attachment of some S. pombe wall glycoproteins since the outer layer of galactomannan was significantly reduced in cells depleted of Ags1, the α-glucan synthase (Cortes et al., 2012).
β-glucans and β-glucan synthases
The β-glucans are polysaccharides composed of D-glucose monomers linked by β-(1,3) or β-(1,6) bonds. Traditionally, these β-glucans have been classified by the type of bonding of the main chain. S. pombe β-glucans include three types: linear β-(1,3)-glucan, branched β-(1,3)-glucan with branches of β-(1,3)-glucan attached via β-(1,6) bonds to the main chain, and β-(1,6)-glucan with branches of β-(1,6)-glucan linked via β-(1,3) bonds (Manners and Meyer, 1977, Humbel et al., 2001, Magnelli et al., 2005). Antibodies specific against different β-glucans define the organization of these polymers in the S. pombe wall and septum. Linear β-(1,3)-glucan is presents almost exclusively in the primary septum and a small amount is also observed in the cell wall of the tips (Humbel et al., 2001, Cortés et al., 2007). Branched β-(1,3)-glucan, the major β-glucan, is located throughout the cell wall and the septa. Branched β-(1,6)-glucan forms part of the inner cell wall layer but close to the outer surface of galactomannoproteins (Humbel et al., 2001).
Scanning electron microscopy during wall regeneration in S. pombe protoplasts showed that this structure is formed initially by a network of fibers that group into bundles interconnected and surrounded by amorphous particles. β-(1,3)-glucan is the primary component of the fiber structure. Initially, it is deposited on the plasma membrane as particles that subsequently extend to generate a network of microfibers that cover the whole surface; this network constitutes the frame on which the other polymers are deposited. As the protoplast grows, β-(1,3)-glucan fibers allow the restoration of the fission yeast cylindrical form (Osumi, 1998).
Linear β-(1,3)-glucan forms linear chains arranged in a single helix with a small proportion of triple-helix structures. This glucan is the polysaccharide responsible for the primary septum structure, and is recognized with higher affinity than the rest of S. pombe wall polysaccharides by the fluorochrome Calcofluor White, being responsible for the strong labeling of the septum with this compound (Cortés et al., 2007). The single helix conformation makes the primary septum susceptible to degradation by β-(1,3)-glucanases permitting the separation of the cells after cytokinesis (Martin-Cuadrado et al., 2008).
Branched β-(1,3)-glucan is the most abundant polymer in the S. pombe wall. It is essential for cell integrity and shape, and plays a crucial role during cytokinesis in the formation of the secondary septum and in the link of the cell wall to the plasma membrane and the contractile actomyosin ring (Muñoz et al., 2013).
Branched β-(1,6)-glucan is a minor polysaccharide in the wall of fission yeast. It is formed by a main chain of β-(1,6)-linked glucose and 75% of β-(1,3)-linked branches. This percentage of branches is much higher than that of the β-(1,6)-glucan in other yeasts like S. cerevisiae or Candida albicans. Because of the abundance of both β-(1,6) and β-(1,3) links it is also called “diglucan” and represents 5–10% of the wall (Sugawara et al., 2004, Magnelli et al., 2005). There is no information about how β-(1,6)-glucan is synthesized and incorporated into the fission yeast cell wall. Indeed, no β-(1,6)-glucan synthase protein has been identified in any fungal species, although several S. cerevisiae proteins, like Kre1, Kre5, Kre6 and Skn1, Kre9, Kre11, or Knh1, which are conserved in other fungi, are required for normal levels of cell wall β-(1,6)-glucan (Aimanianda et al., 2009). Some of these proteins form part of the secretory pathway, suggesting that β-(1,6)-glucan synthesis may occur in intracellular organelles. Indeed, immunoelectron microscopy showed the presence of β(1,6)-glucan particles associated with the Golgi apparatus in S. pombe suggesting that initial biosynthesis of this polymer might occur in the Golgi whereas the β-(1,3)-glucan binding is achieved at the cell surface (Humbel et al., 2001).
The biosynthesis of β-(1,3)-glucan is catalyzed by an enzyme complex conserved in fungi and plants called β-(1,3)-glucan synthase (GS) (EC 2.4.1.34, UDP-glucose:1,3-β-D-glucan 3-β-D-glucosyltransferase). GS is located in the inner side of the plasma membrane (Shematek et al., 1980) and uses UDP-glucose as substrate. This enzyme synthesizes in vitro linear chains of 60–80 glucoses, which is a considerably smaller length compared to that of the cell wall glucan chains. In S. cerevisiae and other fungi, linear β-(1,3)-glucan is elongated and branched further by GPI-bound Gas proteins, which belong to the glycosidase/transglycosidases 72 family (Ragni et al., 2007) (Fig. 2A). β(1,3)-glucanosyl-transferase activity is essential for viability in fission yeast, being Gas1 required to maintain cell integrity during vegetative growth (de Medina-Redondo et al., 2010).
The GS complex is composed of at least two subunits: an integral membrane protein corresponding to the catalytic subunit; and the small GTPase Rho1, which acts as the regulatory subunit (Arellano et al., 1996, Drgonova et al., 1996). Direct activation by Rho1 GTPase is a general mechanism in fungal GS biosynthesis (Cabib et al., 1998). Small GTPases are GTP-binding proteins with biochemical similarity to the α-subunit of heterotrimeric G proteins, whose molecular conformation changes depend on the nucleotide they are bound to (Wennerberg et al., 2005) (Fig. 3). Bound to GTP they are active and can interact with effector proteins, but they have an intrinsic GTPase activity and hydrolyze GTP to GDP, which leads to their inactivation. The transition from GDP- to GTP-bound Rho proteins is promoted by guanine nucleotide exchange factors (GEFs) (Bos et al., 2007). On the other hand, GTPase activating proteins (GAPs) facilitate the hydrolysis of GTP bound to Rho proteins (Mishra and Lambright, 2016). Rho proteins are also regulated by Rho GDIs (guanine nucleotide dissociation inhibitor), which extract the GTPases from the membrane by masking their isoprenoid group, thus preventing their activation by Rho GEFs (Fig. 3) (Garcia-Mata et al., 2011). S. pombe Rho1 localizes to sites of active growth (Arellano et al., 1997) where it can be activated by three GEFs: Rgf1, Rgf2, and Rgf3, which are similar to S. cerevisiae Rom1 and Rom2 (Mutoh et al., 2005). Rgf1 is the main activator of Rho1 during all the mitotic cycle, Rgf2 is essential during the sporulation process and plays a redundant function with Rgf1 during polarized growth, and Rgf3 is essential in cytokinesis (García et al., 2006, Roncero and Sanchez, 2010). An additional GEF named Gef2 also plays a role in cytokinesis. Gef2 localizes to the contractile ring and the precursor cortical nodes through the adaptor protein Nod1 and binds to purified GTPases Rho1, Rho4, and Rho5 in vitro but it is not known yet if it plays a role as activator of these GTPases (Zhu et al., 2013). Rho1 is negatively regulated by at least three GAPs: Rga1 is the major regulator of Rho1 and cells lacking Rga1 are sick and have a thick cell wall (Nakano et al., 2001); Rga5 plays a role in cytokinesis (Calonge et al., 2003) and Rga8 participates in the cross-talk between Rho1 and Cdc42 but its function is not known yet.
Fig. 3.
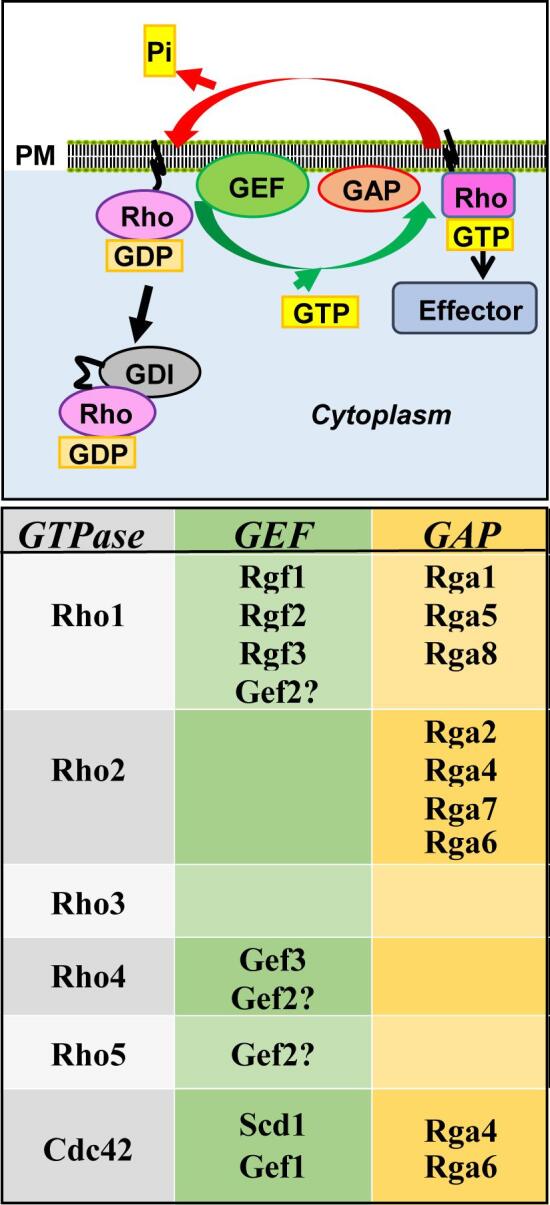
Scheme of Rho GTPases activation cycle. Top, GDP-bound Rho GTPases are activated by GEFs, which are proteins that promote the substitution of GDP by GTP. Active GTP-bound Rho GTPases promote the activation of different effectors. The intrinsic GTPase activity is stimulated by GAPs and causes the hydrolysis of the GTP into GDP and the return of the Rho GTPase to the GDP-bound inactive state. The GDI proteins extract GDP-bound Rho GTPases from the membrane. Bottom, the different S. pombe Rho GEFs and GAPs described to date are shown.
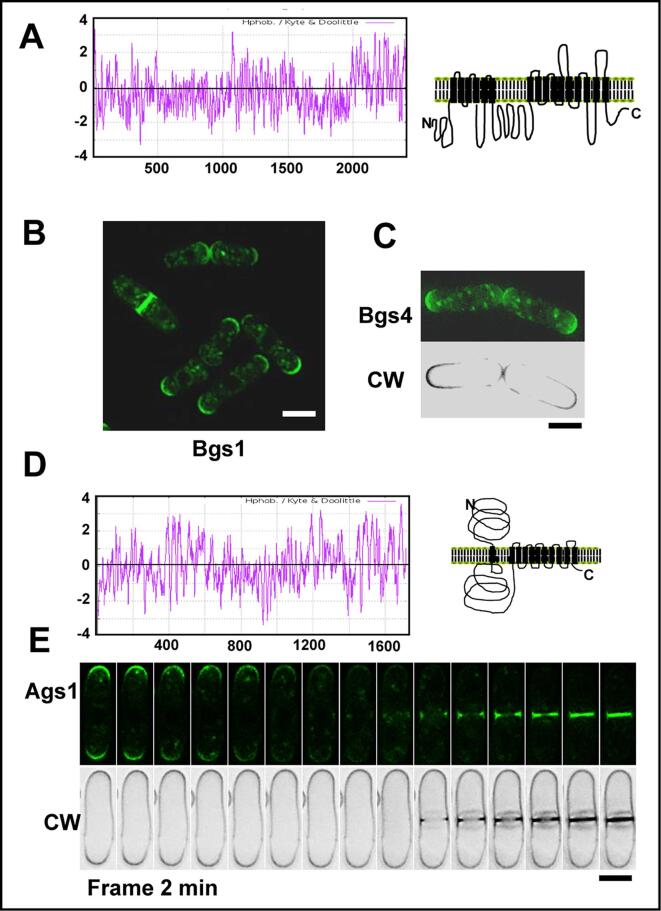
All the fungal GS catalytic subunits are large proteins with 15–16 predicted transmembrane domains divided into two hydrophobic regions separated by a central hydrophilic region essential for its function (Fig. 4A). S. pombe has four genes, bgs1+ to bgs4+, encoding four putative GS catalytic subunits. All these subunits perform essential non-overlapping roles in the synthesis of β-glucans during vegetative cell growth and septum assembly (Bgs1, 3 and 4), and during spore wall maturation (Bgs2) (Garcia Cortes et al., 2016, Estravis et al., 2017).
Fig. 4.
(A) Kite and Doolittle hydropathy plot and model of the Bgs proteins structure with 15–16 transmembrane domains. (B) Fluorescence micrographs showing the localization of GFP-Bgs1 in exponentially growing S. pombe cells. (C) Localization of GFP-Bgs4 in calcofluor-stained growing cells. (D) Kite and Doolittle hydropathy plot and model of the Ags1 protein structure. (E) Time-lapse micrographs showing Ags1-GFP in exponentially growing cells stained with calcofluor (lower panels). CW: calcofluor white. The bar is 5 μm.
The gene bgs1+ was first identified by complementation of the cps1-12 mutant strain which is multiseptated and hypersensitive to a spindle poison (Ishiguro et al., 1997). Two other mutants with different alleles of the same gene, swl1-N12 (cps1-N12) and drc1-191 (cps1-191), assembled contractile actomyosin rings but did not form division septa (Le Goff et al., 1999, Liu et al., 1999, Liu et al., 2000), demonstrating that Bgs1 is a GS essential for septum formation. Bgs1 localizes to the membrane of the division area and the growing poles (Cortes et al., 2002, Liu et al., 2002) (Fig. 4B). Immunoelectron microscopy with gold-labeled anti-β-(1,3)-glucan antibodies of the septa formed in bgs1Δ germinating spores established that Bgs1 is responsible for the synthesis of the linear β-(1,3)-glucan that forms the primary septum (Cortés et al., 2007).
The gene bgs2+ is induced during sporulation. Bgs2 localizes to the ascospore periphery and is required for ascospore wall maturation and survival (Liu et al., 2000, Martín et al., 2000).
The gene bgs3+ was identified as a suppressor of a mutant hypersensitive to the GS-specific antifungal compound echinocandin (Carnero et al., 2000). Although Bgs3 is essential, its function still remains unknown. Like Bgs1 and Bgs4 (Fig. 4B and C), Bgs3 was found in the membrane of the growing areas (Martín et al., 2003). Spores lacking Bgs3 fail to elongate and to maintain longitudinal growth and polarity. Similarly, vegetative cells become rounded and stop growth upon repression of bgs3+ expression (Martín et al., 2003).
The gene bgs4+ codes for the GS catalytic subunit that synthesizes the major cell wall β-(1,3)-glucan, and it is essential for the maintenance of cell shape and integrity during cell growth, and septation (Cortés et al., 2005). Bgs4 depletion promotes cell lysis mainly at the onset of cell separation (Cortés et al., 2005, Muñoz et al., 2013). Mutations in Bgs4 are responsible for the resistance to specific glucan synthase inhibitors (Martins et al., 2011). In some bgs4 mutant strains part of the galactomannan is lost from their walls, suggesting that some wall glycoproteins are bound to the polymer made by Bgs4 (Ribas et al., 1991).
The Bgs enzymes are membrane proteins and must be delivered to the cell surface in order to exert their function. They also need actin polymerization for their internalization from the plasma membrane in order to recycle or degrade (Cortes et al., 2002, Cortés et al., 2007). However, there is not much information about the trafficking of glucan synthases by the vesicle trafficking mechanisms. A thermosensitive strain expressing the cdc42L160S mutant allele demonstrated that Cdc42 GTPase regulates polarized secretion and participates in membrane trafficking, endosome recycling, and vacuole formation (Estravis et al., 2011). This strain has a defective cell wall with a significant decrease in β-(1,3)-glucan, and the growth defect of this strain at 36 °C can be suppressed by the presence of an osmotic stabilizer (Estravis et al., 2012). Analysis of Bgs1 and Bgs4 localization in cdc42L160S cells unveiled a difference in the traffic of both enzymes (Estravis et al., 2012). The use of a mutant strain with reduced levels of the clathrin light chain which do not lead to a general defect in protein secretion, showed that glucan synthesis is clathrin-dependent (de Leon et al., 2013).
Additionally, it has been suggested that the F-BAR protein Cdc15 delivers Bgs1 from the Golgi to the plasma membrane of the central region (Arasada and Pollard, 2014), and that the protein Sbg1 plays an earlier and more important role than Cdc15 in Bgs1 trafficking and localization (Davidson et al., 2016). Sbg1 is a type II integral membrane proteins with a SKN1/KRE6 domain and its loss results in an unstable actomyosin ring and a significant reduction of the β-(1,3)-glucan in the cell wall. This phenotype is similar to that observed in the cps1-191 mutant (Sethi et al., 2016). Sbg1 and Bgs1 physically interact and are dependent on each other to localize to the division site (Sethi et al., 2016). The SKN1/KRE6 domain is conserved in Golgi proteins of different fungal species which have putative roles in β-(1,6)-glucan synthesis (Free, 2013, Gow et al., 2017).
α-(1,3)-glucan and α-glucan synthases
This polysaccharide is a major wall component of filamentous and dimorphic fungi (Yoshimi et al., 2017). It is a linear polysaccharide of approximately 260 glucose residues, formed by a tandem of two linear chains, each consisting of about 120–130 residues of α-(1,3)-bound D-glucose, connected by around 10 α-(1,4)-linked glucose units located at the reducing end of each single chain (Grun et al., 2005).
α-(1,3)-glucan functions as an aggregation factor for hyphae and conidiain some Aspergillus species because defects in this polymer lead to hyphal and conidial dispersion (de Medina-Redondo et al., 2010). In S. pombe α-(1,3)-glucan has an essential role in the structure and rigidity of the cell wall, as treatment with α-glucanases in addition to β-glucanases is required to produce round protoplasts (Alfa et al., 1993). Moreover, mutant strains defective in the synthesis of α-(1,3)-glucan maintain the fiber structure but are unable to acquire a cylindrical shape (Osumi, 1998).
During cytokinesis α-(1,3)-glucan is part of the secondary septum and plays an important role in the strength of adhesion between primary and secondary septa needed to support the physical force of the internal turgor pressure during cell separation (Cortes et al., 2012). It is believed that α-(1,3)-glucan is localized with the branched β-(1,3)-glucan in the less electron-dense region of the cell wall and in both primary and secondary septa (Cortes et al., 2012), although it has not been detected yet by immunoelectron microscopy.
The enzyme responsible for the synthesis of the cell wall α-(1,3)-D-glucan in S. pombe is Ags1, also called Mok1, which is essential for cell integrity (Hoschstenbach et al., 1998, Katayama et al., 1999). Ags1 orthologs are not found in budding yeasts but are widely extended in filamentous, dimorphic, and pathogenic fungi (Yoshimi et al., 2017). No α-(1,3)-D-glucan synthase activity has been detected in vitro yet. The predicted synthase, Ags1, is a large integral membrane protein of 272 kDa, with a cytoplasmic synthase domain, a hydrophobic region with multiple transmembrane domains and an extracellular transglycosidase domain (Fig. 4D). Like the Bgs proteins, Ags1 is found in the membrane of growing poles and septa (Konomi et al., 2003, Cortes et al., 2012) (Fig. 4E).
Ags1 cytoplasmic domain shares amino acid sequence motifs with bacterial glycogen synthases and plant starch synthases (Vos et al., 2007). It has been proposed that this domain adds glucose residues to the non-reducing end of an α-(1,3)-glucan chain. On the other hand, the large extracellular N-terminal region might cross-link the newly synthesized α-(1,3)-glucan to other cell wall components (Vos et al., 2007).
S. pombe contains four additional ags1+ paralogs (mok11+- mok14+), which are induced in a sequential manner and are required at different stages during sexual differentiation (García et al., 2006). Mok12 and Mok13 are probably involved in the synthesis of the α-(1,3)-glucan necessary for spore wall maturation and resistance to environmental stress while Mok14 is necessary for the synthesis of the iodine-reactive amyloid polymer characteristic of the external layer of S. pombe spores (García et al., 2006). Deletion of Mok11 did not show any obvious sporulation defect.
Cell integrity signaling in fission yeast
Extracellular environmental changes cause different types of stress in all the organisms and can seriously affect the viability of the cells. All cells have signaling pathways required to transmit the stress signals from outside and to develop the response and gene expression changes required to adapt and survive. Mitogen activated protein kinase (MAPK) cascades, are major signaling pathways in the transduction of the signals from the cell surface to the nucleus (Martinez-Soto and Ruiz-Herrera, 2017). They include sensors that detect environmental changes and transmit the signal to other proteins that in turn activate the MAPK module. This module includes three conserved kinases: the MAP kinase kinase kinase (MAPKKK); the MAP kinase kinase (MAPKK), and the MAP kinase (MAPK). Sequential phosphorylation of these proteins transmit the signal (Levin, 2011). Then the MAPK phosphorylates different target proteins, including transcription factors that promote the gene expression changes required to respond to the stimuli. S. pombe has three MAPK pathways: the pheromone signaling pathway with Spk1 as MAPK (Shiozaki and Russell, 1995), the stress-activated pathway (SAP) with Sty1/Spc1 as MAPK (Toda et al., 1993), and the cell integrity pathway (CIP) with Pmk1/Spm1 as MAPK that responds to numerous stress conditions, such as hypo- or hyper-osmotic pressure, absence of glucose, cell wall damage, and oxidative stress, and that also participates in cytokinesis, and ion homeostasis (Toda et al., 1996, Sengar et al., 1997, Zaitsevskaya-Carter and Cooper, 1997, Bone et al., 1998, Sugiura et al., 1999, Loewith et al., 2000, Madrid et al., 2006, Barba et al., 2008). The cell integrity MAPK is conserved in all fungi and shows a high degree of similarity to mammalian ERK1/2 and ERK5 (Perez and Cansado, 2010, Levin, 2011, Martinez-Soto and Ruiz-Herrera, 2017).
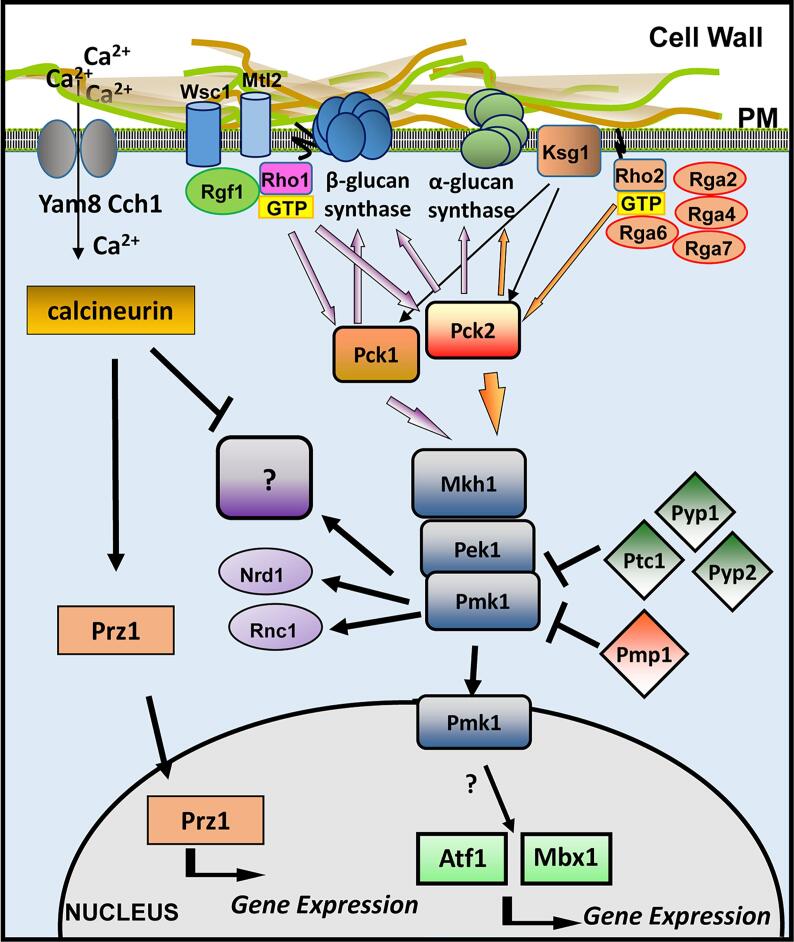
The CIP includes sensors that detect changes in the cell wall or membrane caused by different stresses and transmit the stimulus to the MAPK module. S. cerevisiae sensors are transmembrane proteins encoded by the genes WSC1-4, MID2, and MTL1 (Rodicio and Heinisch, 2010). The corresponding proteins occupy different microdomains at the plasma membrane (Kock et al., 2016), and upon detection of a cell surface stress, they transmit the stimulus to Rom2, a GEF that activates Rho1. Then, this GTPase activates the PKC ortholog Pkc1 and the CIP MAPK cascade (Heinisch and Rodicio, 2018). The sensors contain an N-terminal serine-threonine-rich domain and a C-terminal cytoplasmic tail (Levin, 2011). Atomic force microscopy (AFM) studies suggest that the serine-threonine-rich domain behaves like a linear spring and demonstrated that disulfide bridges are required for clustering of the sensors (Dupres et al., 2009, Dupres et al., 2011). S. pombe Wsc1 and Mtl2 proteins are similar to the sensors described in S. cerevisiae (Cruz et al., 2013). Both cause activation of Rho1 through two GEFs, Rgf1 or Rgf3, and regulate the wall assembly. Remarkably, they do it independently of the CIP MAPK, whose activity is not affected in cells lacking Wsc1 (Cruz et al., 2013).
Rho2 is another GTPase that localizes to the growth sites and is involved in the control of cell polarity, reorganization of the actin cytoskeleton, and biosynthesis of the cell wall (Hirata et al., 1998, Calonge et al., 2000). Rho1 is essential while Rho2 is not, and its deletion only produces rounded cells that are more sensitive to treatment with β -glucanases (Hirata et al., 1998). GTP-bound Rho1 and Rho2 interact with and regulate the two redundant fission yeast PKC orthologs Pck1 and Pck2 (Arellano et al., 1999, Villar-Tajadura et al., 2008). Pck1 and Pck2 are unstable proteins with N-terminal PEST sequences and their interaction with the GTPases increases their stability (Arellano et al., 1999, Villar-Tajadura et al., 2008). Additionally, a single S. pombe gene, ksg1+, codes for a phospholipid-dependent kinase (PDK) (Niederberger and Schweingruber, 1999) that activates Pck1 and Pck2 (Graub et al., 2003). These two kinases share overlapping roles in cell viability and partially complement each other (Arellano et al., 1999). They regulate the in vitro activity of the β-(1,3)-D-glucan synthase although the specific mechanism is not known (Arellano et al., 1999). Therefore, Rho1 regulates the biosynthesis of β-(1,3)-glucan through a direct regulation of the Bgs enzymes and also indirectly through the activation of Pck2 and Pck1. On the other hand Rho2 also activates Pck2, but does not affect the Bgs enzymes. Instead, Rho2 regulates the activity of the α-glucan synthase Mok1 exclusively through Pck2 (Calonge et al., 2000). Additionally, Rho2 and Pck2 are the main activators of the CIP, whose central element is the MAPK Pmk1 (Ecker et al., 2006) (Fig. 5). There is no Rho2 GEF described so far. This GTPase is negatively regulated by four GAPs, Rga2, Rga4, Rga7 and Rga6, all of which also downregulate the Pmk1 MAPK signal (Soto et al., 2010).
Fig. 5.
Schematic representation of S. pombe cell wall integrity pathway. Rho1 is activated by the sensors Wsc1 and Mtl2, which detect and transmit signals coming from the cell wall through the GEF Rgf1. Rho1 activates y the β-(1,3)-glucan synthase and stabilizes the kinases Pck1 and Pck2. Both kinases are activated by the Phospholipid-dependent kinase Ksg1 and in turn activate the β-(1,3)-glucan synthase. Rho2 is not activated by Wsc1 or Mlt2, but regulates the α-(1,3)-glucan synthase via Pck2. Additionally, Rho2-Pck2 are the main activators of the MAPK cell integrity pathway. Signaling of this pathway is transmitted through the MAPK module to Atf1 and Mbx1 transcription factors in the nucleus and to different targets such as the RNA-binding proteins Rnc1 or Ndr1 in the cytoplasm. Pmk1 also activates the Yam8/Cch1 calcium channel that in turns activates the phosphatase complex calcineurin. This phosphatase antagonizes the function of MAPK pathway in response to several stimuli. Other phosphatases including Pmp1, and the SAP-dependent phosphatases Ptc1, Pyp1 and Pyp2 negatively regulate Pmk1 activation.
Pck1 plays a minor role in the CIP regulation while Rho1 activates the CIP only in response to certain stimuli such as cell wall damage (Sanchez-Mir et al., 2014). As mentioned above, Rho1 is activated by the membrane sensors Wsc1 and Mlt2 but the signals of these sensors does not regulate the CIP activity (Cruz et al., 2013). The GEF Rgf1 regulates Pmk1 activation in a process that involves the activation of Rho1 and Pck2 (Garcia et al., 2009). Other Rho1 GEFs such as Rgf2 or Rgf3 can also activate the cell integrity MAPK when overexpressed but it is not clear if they activate this pathway when expressed at physiological levels (Garcia et al., 2009).
The MAPK module of the S. pombe CIP includes Mkh1 (MAPKKK), Pek1/Shk1 (MAPKK), and Pmk1/Spm1 (MAPK) (Fig. 5). The activation of Pmk1 is completely dependent on Mkh1 and Pek1 function (Madrid et al., 2006). Deletion of any gene encoding the MAPK module causes similar defects in cytokinesis, vacuole fusion, and sensitivity to potassium ions and β-glucanases (Toda et al., 1996, Zaitsevskaya-Carter and Cooper, 1997, Bone et al., 1998, Sugiura et al., 1999, Loewith et al., 2000). Mkh1 and Pek1 are cytoplasmic proteins and they also localize to the membrane of the division septum. Pmk1 is localized in both the cytoplasm and the nucleus as well as in the mitotic spindle and in the septum membrane during cytokinesis (Madrid et al., 2006). Pmk1 activation takes place in the cytoplasm and/or at the septum membrane. This MAPK is phosphorylated by Pek1 in Thr-186 and Tyr-188, two conserved residues of the activation motif (Sugiura et al., 1999). However monophosphorylated Trh186 Pmk1 was able to execute most of the biological functions of this kinase (Vazquez et al., 2015).
Most MAPKs translocate to the nucleus upon activation and phosphorylate transcription factors in response to the stimulus. However, Pmk1 is constitutively localized in both cytoplasm and nucleus and can cross the nuclear membrane without being activated (Madrid et al., 2006). Moreover, its localization pattern is not affected in response to stress and most of its functions can be accomplished without traveling to the nucleus (Sanchez-Mir et al., 2012). Among the few known Pmk1 cytoplasmic targets are Rnc1 and Nrd1, two RNA-binding proteins. Nrd1 stabilizes different mRNAs, like myo2+ mRNA, and could participate in the Pmk1 regulation of cytokinesis (Satoh et al., 2009). Rnc1 stabilizes the mRNA of Pmp1, a dual-specificity phosphatase that is the main negative regulator of Pmk1 (Sugiura et al., 1998). This negative feedback loop provides a regulatory mechanism for fine-tuning the Pmk1 pathway (Sugiura et al., 2003).
Pmk1 also activates the Yam8/Cch1 calcium channel that in turns activates the phosphatase complex calcineurin. This phosphatase plays a major role antagonizing the function of MAPK pathway in response to several stimuli (Sugiura et al., 1998, Sugiura et al., 2002, Ma et al., 2011, Viana et al., 2013). On the contrary, in S. cerevisiae calcineurin cooperates with the CIP MAPK in the induction of the GS gene FKS2 (Zhao et al., 1998).
Although most Pmk1 functions do not require nuclear targeting and the number of genes induced by Pmk1 activation is very low (Chen et al., 2008), the cell wall integrity might be somehow regulated by Pmk1 at a transcriptional level (Takada et al., 2007). The transcription factor Atf1, which is phosphorylated by the SAP MAPK Sty1 (Wilkinson et al., 1996), is also phosphorylated by Pmk1 when the cell wall is damage (Takada et al., 2007). However, while the role of S. cerevisiae CIP MAPK pathway in the production of various cell wall polysaccharides as well as in their polarized delivery to the site of cell wall remodeling is well established (Levin, 2011), a similar role has never been shown for the fission yeast Pmk1 MAPK. Indeed, inactivation of Pmk1 improved the growth of a rho1-596 thermosensitive strain which displays severe cell wall defects and needs the function of calcineurin to survive (Viana et al., 2013).
In summary, CIP is very well conserved in the fungal kingdom but its biological significance in the regulation of the fission yeast cell wall biosynthesis is still not clearly defined.
Concluding remarks
The fungal cell wall is an ideal target for therapeutic intervention against fungal infections since it is essential for these organisms, and most of the components and the enzymes necessary for cell wall biosynthesis and remodeling are not present in mammalian cells. However, many questions remain to be solved before we can have a complete view of the mechanisms governing fungal wall synthesis. Fission yeast studies during the last two decades have unveiled many aspects of the glucan synthases and its regulation. Moreover, the recent data available on the β-(1,3)-glucan synthases Bgs4 and Bgs1 regarding their role in stabilizing the contractile ring during cytokinesis (Muñoz et al., 2013, Arasada and Pollard, 2014, Cortes et al., 2015) set the basis for new studies on the molecular mechanisms of septation such as: the connection between the cell wall β-(1,3)-glucan and the actomyosin ring in order to form the cleavage furrow; the role of Bgs1 in maintaining other glucan synthases in the division area; the requirements for the transport and recycling of the glucan synthases at the site of cell division; etc. Underscoring the relevance of the cell wall in cytokinesis and in other cellular processes, such as polarized growth, will likely reveal the existence of conserved regulatory circuits among fungi and other eukaryotes. Comparative studies in a broad range of fungi will help to identify novel pathways that coordinate the cell wall synthesis with the major physiological processes and the adaptive response of fungal cells to different stresses.
Conflicts of interest
We wish to confirm that there are no known conflicts of interest associated with this publication.
Acknowledgements
We thank D.M. Posner for language revision. This work was supported by grants BIO2015-69958-P and BFU2017-82423-P from MINECO, Spain and CSI068P17 from Junta de Castilla y León. The authors wish to acknowledge the many other studies on cell wall and apologize for not citing every important contribution in this review due to space limitations.
References
- Aimanianda V., Clavaud C., Simenel C., Fontaine T., Delepierre M., Latge J.P. Cell wall b-(1,6)-glucan of Saccharomyces cerevisiae: structural characterization and in situ synthesis. J. Biol. Chem. 2009;284:13401–13412. doi: 10.1074/jbc.M807667200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfa C., Fantes P., Hyams J., McLeod M., Warbrick E., editors. Experiments with Fission Yeast: A Laboratory Course Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 1993. [Google Scholar]
- Arasada, R., Pollard, T.D., 2014. Contractile Ring Stability in S. pombe Depends on F-BAR Protein Cdc15p and Bgs1p Transport from the Golgi Complex. Cell reports. [DOI] [PMC free article] [PubMed]
- Arellano M., Duran A., Perez P. Rho 1 GTPase activates the (1–3)beta-D-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. EMBO J. 1996;15:4584–4591. [PMC free article] [PubMed] [Google Scholar]
- Arellano M., Duran A., Perez P. Localization of the Schizosaccharomyces pombe rho1 GTPase and its involvement in the organization of the actin cytoskeleton. J. Cell Sci. 1997;110:2547–2555. doi: 10.1242/jcs.110.20.2547. [DOI] [PubMed] [Google Scholar]
- Arellano M., Valdivieso M.H., Calonge T.M., Coll P.M., Durán A., Pérez P. Schizosaccharomyces pombe protein kinase C homologues, pck1p and pck2p, are targets of rho1p and rho2p and differentially regulate cell integrity. J. Cell Sci. 1999;112:3569–3578. doi: 10.1242/jcs.112.20.3569. [DOI] [PubMed] [Google Scholar]
- Barba G., Soto T., Madrid M., Nunez A., Vicente J., Gacto M., Cansado J. Activation of the cell integrity pathway is channelled through diverse signalling elements in fission yeast. Cell Signal. 2008;20:748–757. doi: 10.1016/j.cellsig.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Bone N., Millar J.B., Toda T., Armstrong J. Regulated vacuole fusion and fission in Schizosaccharomyces pombe: an osmotic response dependent on MAP kinases. Curr. Biol. 1998;8:135–144. doi: 10.1016/s0960-9822(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Bos J.L., Rehmann H., Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Cabib E., Drgonova J., Drgon T. Role of small G proteins in yeast cell polarization and wall biosynthesis. Ann. Rev. Biochem. 1998;67:307–333. doi: 10.1146/annurev.biochem.67.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calonge T.M., Arellano M., Coll P.M., Perez P. Rga5p is a specific Rho1p GTPase-activating protein that regulates cell integrity in Schizosaccharomyces pombe. Mol. Microbiol. 2003;47:507–518. doi: 10.1046/j.1365-2958.2003.03312.x. [DOI] [PubMed] [Google Scholar]
- Calonge T.M., Nakano K., Arellano M., Arai R., Katayama S., Toda T., Mabuchi I., Perez P. Schizosaccharomyces pombe Rho2 GTPase regulates the cell wall a-glucan biosynthesis, through the protein kinase Pck2p. Mol. Biol. Cell. 2000;11:4393–4401. doi: 10.1091/mbc.11.12.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero E., Ribas J.C., García B., Durán A., Sánchez Y. Schizosaccharomyces pombe Ehs1p is involved in maintaining cell wall integrity and in calcium uptake. Mol. Gen. Genet. 2000;264:173–183. doi: 10.1007/s004380000318. [DOI] [PubMed] [Google Scholar]
- Cortes J.C., Ishiguro J., Duran A., Ribas J.C. Localization of the (1,3)beta-D-glucan synthase catalytic subunit homologue Bgs1p/Cps1p from fission yeast suggests that it is involved in septation, polarized growth, mating, spore wall formation and spore germination. J. Cell Sci. 2002;115:4081–4096. doi: 10.1242/jcs.00085. [DOI] [PubMed] [Google Scholar]
- Cortés J.C., Konomi M., Martins I.M., Munoz J., Moreno M.B., Osumi M., Durán A., Ribas J.C. The (1,3)b-D-glucan synthase subunit Bgs1p is responsible for the fission yeast primary septum formation. Mol. Microbiol. 2007;65:201–217. doi: 10.1111/j.1365-2958.2007.05784.x. [DOI] [PubMed] [Google Scholar]
- Cortes J.C., Pujol N., Sato M., Pinar M., Ramos M., Moreno B., Osumi M., Ribas J.C., Perez P. Cooperation between Paxillin-like Protein Pxl1 and Glucan Synthase Bgs1 Is essential for actomyosin ring stability and septum formation in fission yeast. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J.C., Sato M., Munoz J., Moreno M.B., Clemente-Ramos J.A., Ramos M., Okada H., Osumi M., Duran A., Ribas J.C. Fission yeast Ags1 confers the essential septum strength needed for safe gradual cell abscission. J. Cell. Biol. 2012;198:637–656. doi: 10.1083/jcb.201202015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés J.C.G., Carnero E., Ishiguro J., Sánchez Y., Durán A., Ribas J.C. The novel fission yeast (1,3)b-D-glucan synthase catalytic subunit Bgs4p is essential during both cytokinesis and polarized growth. J. Cell Sci. 2005;118:157–174. doi: 10.1242/jcs.01585. [DOI] [PubMed] [Google Scholar]
- Cruz S., Munoz S., Manjon E., Garcia P., Sanchez Y. The fission yeast cell wall stress sensor-like proteins Mtl2 and Wsc1 act by turning on the GTPase Rho1p but act independently of the cell wall integrity pathway. Microbiologyopen. 2013;2:778–794. doi: 10.1002/mbo3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Wilkinson C.R., Watt S., Penkett C.J., Toone W.M., Jones N., Bahler J. Multiple pathways differentially regulate global oxidative stress responses in fission yeast. Mol. Biol. Cell. 2008;19:308–317. doi: 10.1091/mbc.E07-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R., Pontasch J.A., Wu J.Q. Sbg1 Is a novel regulator for the localization of the beta-glucan synthase Bgs1 in fission yeast. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot P.W., Ram A.F., Klis F.M. Features and functions of covalently linked proteins in fungal cell walls. Fungal Genet. Biol. 2005;42:657–675. doi: 10.1016/j.fgb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- de Groot P.W., Yin Q.Y., Weig M., Sosinska G.J., Klis F.M., de Koster C.G. Mass spectrometric identification of covalently bound cell wall proteins from the fission yeast Schizosaccharomyces pombe. Yeast (Chichester, England) 2007;24:267–278. doi: 10.1002/yea.1443. [DOI] [PubMed] [Google Scholar]
- de Leon N., Sharifmoghadam M.R., Hoya M., Curto M.A., Doncel C., Valdivieso M.H. Regulation of cell wall synthesis by the clathrin light chain is essential for viability in Schizosaccharomyces pombe. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Medina-Redondo M., Arnaiz-Pita Y., Clavaud C., Fontaine T., del Rey F., Latge J.P., Vazquez de Aldana C.R. beta(1,3)-glucanosyl-transferase activity is essential for cell wall integrity and viability of Schizosaccharomyces pombe. PLoS One. 2010;5 doi: 10.1371/journal.pone.0014046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker N., Speijer D., Grun C.H., van den Berg M., de Haan A., Hochstenbach F. Role of the alpha-glucanase Agn1p in fission-yeast cell separation. Mol. Biol. Cell. 2004;15:3903–3914. doi: 10.1091/mbc.E04-04-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgonova J., Drgon T., Tanaka K., Kollar R., Chen G.C., Ford R.A., Chan C.S., Takai Y., Cabib E. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 1996;272:277–279. doi: 10.1126/science.272.5259.277. [DOI] [PubMed] [Google Scholar]
- Dupres V., Alsteens D., Wilk S., Hansen B., Heinisch J.J., Dufrene Y.F. The yeast Wsc1 cell surface sensor behaves like a nanospring in vivo. Nat. Chem. Biol. 2009;5:857–862. doi: 10.1038/nchembio.220. [DOI] [PubMed] [Google Scholar]
- Dupres V., Heinisch J.J., Dufrene Y.F. Atomic force microscopy demonstrates that disulfide bridges are required for clustering of the yeast cell wall integrity sensor Wsc1. Langmuir. 2011;27:15129–15134. doi: 10.1021/la203679s. [DOI] [PubMed] [Google Scholar]
- Ecker M., Deutzmann R., Lehle L., Mrsa V., Tanner W. Pir proteins of Saccharomyces cerevisiae are attached to b-1,3-glucan by a new protein-carbohydrate linkage. J. Biol. Chem. 2006;281:11523–11529. doi: 10.1074/jbc.M600314200. [DOI] [PubMed] [Google Scholar]
- Estravis M., Rincon S., Perez P. Cdc42 regulation of polarized traffic in fission yeast. Commun. Integr. Biol. 2012;5:370–373. doi: 10.4161/cib.19977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estravis, M., Rincon, S.A., Portales, E., Perez, P., and Santos, B., 2017. Cdc42 activation state affects its localization and protein levels in fission yeast. Microbiology. [DOI] [PubMed]
- Estravis M., Rincon S.A., Santos B., Perez P. Cdc42 regulates multiple membrane traffic events in fission yeast. Traffic. 2011;12:1744–1758. doi: 10.1111/j.1600-0854.2011.01275.x. [DOI] [PubMed] [Google Scholar]
- Free S.J. Fungal cell wall organization and biosynthesis. Adv. Genet. 2013;81:33–82. doi: 10.1016/B978-0-12-407677-8.00002-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata R., Boulter E., Burridge K. The 'invisible hand': regulation of RHO GTPases by RHOGDIs. Nat. Rev. Mol. Cell. Biol. 2011;12:493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Cortes J.C., Ramos M., Osumi M., Perez P., Ribas J.C. The cell biology of fission yeast septation. Microbiol. Mol. Biol. Rev. 2016;80:779–791. doi: 10.1128/MMBR.00013-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I., Jimenez D., Martin V., Duran A., Sanchez Y. The alpha-glucanase Agn1p is required for cell separation in Schizosaccharomyces pombe. Biol. Cell. 2005;97:569–576. doi: 10.1042/BC20040096. [DOI] [PubMed] [Google Scholar]
- García I., Tajadura V., Martín V., Toda T., Sánchez Y. Synthesis of a-glucans in fission yeast spores is carried out by three a-glucan synthase paralogues, Mok12p, Mok13p and Mok14p. Mol. Microbiol. 2006;59:836–853. doi: 10.1111/j.1365-2958.2005.04995.x. [DOI] [PubMed] [Google Scholar]
- Garcia P., Tajadura V., Sanchez Y. The Rho1p exchange factor Rgf1p signals upstream from the Pmk1 mitogen-activated protein kinase pathway in fission yeast. Mol. Biol. Cell. 2009;20:721–731. doi: 10.1091/mbc.E08-07-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow N.A.R., Latge J.P., Munro C.A. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.funk-0035-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graub R., Hilti N., Niederberger C., Schweingruber M.E. Ksg1, a homologue of the phosphoinositide-dependent protein kinase 1, controls cell wall integrity in Schizosaccharomyces pombe. J. Basic Microbiol. 2003;43:473–482. doi: 10.1002/jobm.200310287. [DOI] [PubMed] [Google Scholar]
- Grun C.H., Hochstenbach F., Humbel B.M., Verkleij A.J., Sietsma J.H., Klis F.M., Kamerling J.P., Vliegenthart J.F. The structure of cell wall a-glucan from fission yeast. Glycobiology. 2005;15:245–257. doi: 10.1093/glycob/cwi002. [DOI] [PubMed] [Google Scholar]
- Heinisch J.J., Rodicio R. Protein kinase C in fungi-more than just cell wall integrity. FEMS Microbiol. Rev. 2018;42 doi: 10.1093/femsre/fux051. [DOI] [PubMed] [Google Scholar]
- Hirata D., Nakano K., Fukui M., Takenaka H., Miyakawa T., Mabuchi I. Genes that cause aberrant cell morphology by overexpression in fission yeast: a role of a small GTP-binding protein Rho2 in cell morphogenesis. J. Cell Sci. 1998;111:149–159. doi: 10.1242/jcs.111.2.149. [DOI] [PubMed] [Google Scholar]
- Horisberger M., Vonlanthen M., Rosset J. Localization of a-galactomannan and of wheat germ agglutinin receptors in Schizosaccharomyces pombe. Arch. Microbiol. 1978;119:107–111. doi: 10.1007/BF00964260. [DOI] [PubMed] [Google Scholar]
- Hoschstenbach F., Klis F.M., Van den Ende H., Van Donselaar E., Peters P.J., Klausner R.D. Identification of a putative alpha-glucan synthase essential for cell wall construction and morphogenesis in fission yeast. Proc. Natl. Acad. Sci. USA. 1998;95:9161–9166. doi: 10.1073/pnas.95.16.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbel B.M., Konomi M., Takagi T., Kamasawa N., Ishijima S.A., Osumi M. In situ localization of b-glucans in the cell wall of Schizosaccharomyces pombe. Yeast (Chichester, England) 2001;18:433–444. doi: 10.1002/yea.694. [DOI] [PubMed] [Google Scholar]
- Ishiguro J., Saitou A., Durán A., Ribas J.C. cps1+, a Schizosaccharomyces pombe gene homolog of Saccharomyces cerevisiae FKS genes whose mutation confers hypersensitivity to cyclosporin A and papulacandin B. J. Bacteriol. 1997;179:7653–7662. doi: 10.1128/jb.179.24.7653-7662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara S., Hirata A., Nogami S., Beauvais A., Latge J.P., Ohya Y. Homologous subunits of 1,3-b-glucan synthase are important for spore wall assembly in Saccharomyces cerevisiae. Eukaryot. Cell. 2007;6:143–156. doi: 10.1128/EC.00200-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama S., Hirata D., Arellano M., Pérez P., Toda T. Fission yeast a-glucan synthase Mok1 requires the actin cytoskeleton to localize the sites of growth and plays an essential role in cell morphogenesis downstream of protein kinase C function. J. Cell. Biol. 1999;144:1173–1186. doi: 10.1083/jcb.144.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis F.M., Brul S., De Groot P.W. Covalently linked wall proteins in ascomycetous fungi. Yeast (Chichester, England) 2010;27:489–493. doi: 10.1002/yea.1747. [DOI] [PubMed] [Google Scholar]
- Kock C., Arlt H., Ungermann C., Heinisch J.J. Yeast cell wall integrity sensors form specific plasma membrane microdomains important for signalling. Cell. Microbiol. 2016;18:1251–1267. doi: 10.1111/cmi.12635. [DOI] [PubMed] [Google Scholar]
- Konomi M., Fujimoto K., Toda T., Osumi M. Characterization and behaviour of a-glucan synthase in Schizosaccharomyces pombe as revealed by electron microscopy. Yeast (Chichester, England) 2003;20:427–438. doi: 10.1002/yea.974. [DOI] [PubMed] [Google Scholar]
- Le Goff X., Woollard A., Simanis V. Analysis of the cps1 gene provides evidence for a septation checkpoint in Schizosaccharomyces pombe. Mol. Gen. Genet. 1999;262:163–172. doi: 10.1007/s004380051071. [DOI] [PubMed] [Google Scholar]
- Levin D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics. 2011;189:1145–1175. doi: 10.1534/genetics.111.128264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder T., Gustafsson C.M. Molecular phylogenetics of ascomycotal adhesins–a novel family of putative cell-surface adhesive proteins in fission yeasts. Fungal Genet. Biol. 2008;45:485–497. doi: 10.1016/j.fgb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Liu J., Tang X., Wang H., Balasubramanian M. Bgs2p, a 1,3-b-glucan synthase subunit, is essential for maturation of ascospore wall in Schizosaccharomyces pombe. FEBS Lett. 2000;478:105–108. doi: 10.1016/s0014-5793(00)01828-7. [DOI] [PubMed] [Google Scholar]
- Liu J., Tang X., Wang H., Oliferenko S., Balasubramanian M.K. The localization of the integral membrane protein cps1p to the cell division site is dependent on the actomyosin ring and the septation- inducing network in Schizosaccharomyces pombe. Mol. Biol. Cell. 2002;13:989–1000. doi: 10.1091/mbc.01-12-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wang H., McCollum D., Balasubramanian M.K. Drc1p/Cps1p, a 1,3-b-glucan synthase subunit, is essential for division septum assembly in Schizosaccharomyces pombe. Genetics. 1999;153:1193–1203. doi: 10.1093/genetics/153.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith R., Hubberstey A., Young D. Skh1, the MEK component of the Mkh1 signaling pathway in Schizosaccharomyces pombe. J. Cell Sci. 2000;113:153–160. doi: 10.1242/jcs.113.1.153. [DOI] [PubMed] [Google Scholar]
- Ma Y., Sugiura R., Koike A., Ebina H., Sio S.O., Kuno T. Transient receptor potential (TRP) and Cch1-Yam8 channels play key roles in the regulation of cytoplasmic Ca2+ in fission yeast. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid M., Soto T., Khong H.K., Franco A., Vicente J., Perez P., Gacto M., Cansado J. Stress-induced response, localization, and regulation of the Pmk1 cell integrity pathway in Schizosaccharomyces pombe. J. Biol. Chem. 2006;281:2033–2043. doi: 10.1074/jbc.M506467200. [DOI] [PubMed] [Google Scholar]
- Magnelli P.E., Cipollo J.F., Robbins P.W. A glucanase-driven fractionation allows redefinition of Schizosaccharomyces pombe cell wall composition and structure: assignment of diglucan. Anal. Biochem. 2005;336:202–212. doi: 10.1016/j.ab.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Manners D.J., Meyer M.T. The molecular structures of some glucans from the cell walls of Schizosaccharomyces pombe. Carbohydr. Res. 1977;57:189–203. [Google Scholar]
- Martin-Cuadrado A.B., Duenas E., Sipiczki M., Vazquez de Aldana C.R., del Rey F. The endo-beta-1,3-glucanase eng1p is required for dissolution of the primary septum during cell separation in Schizosaccharomyces pombe. J. Cell Sci. 2003;116:1689–1698. doi: 10.1242/jcs.00377. [DOI] [PubMed] [Google Scholar]
- Martin-Cuadrado A.B., Encinar del Dedo J., de Medina-Redondo M., Fontaine T., del Rey F., Latge J.P., Vazquez de Aldana C.R. The Schizosaccharomyces pombe endo-1,3-beta-glucanase Eng1 contains a novel carbohydrate binding module required for septum localization. Mol. Microbiol. 2008;69:188–200. doi: 10.1111/j.1365-2958.2008.06275.x. [DOI] [PubMed] [Google Scholar]
- Martín V., García B., Carnero E., Durán A., Sánchez Y. Bgs3p, a putative 1,3-b-glucan synthase subunit, is required for cell wall assembly in Schizosaccharomyces pombe. Eukaryot. Cell. 2003;2:159–169. doi: 10.1128/EC.2.1.159-169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín V., Ribas J.C., Carnero E., Durán A., Sánchez Y. bgs2+, a sporulation-specific glucan synthase homologue is required for proper ascospore wall maturation in fission yeast. Mol. Microbiol. 2000;38:308–321. doi: 10.1046/j.1365-2958.2000.02118.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Soto D., Ruiz-Herrera J. Functional analysis of the MAPK pathways in fungi. Rev. Iberoam. Micol. 2017;34:192–202. doi: 10.1016/j.riam.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Martins I.M., Cortés J.C.G., Muñoz J., Moreno M.B., Ramos M., Clemente-Ramos J.A., Durán A., Ribas J.C. Differential activities of three families of specific b(1,3)glucan synthase inhibitors in wild-type and resistant strains of fission yeast. J. Biol. Chem. 2011;286:3484–3496. doi: 10.1074/jbc.M110.174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A.K., Lambright D.G. Invited review: small GTPases and their GAPs. Biopolymers. 2016;105:431–448. doi: 10.1002/bip.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz J., Cortés J.C., Sipiczki M., Ramos M., Clemente-Ramos J.A., Moreno M.B., Martins I.M., Pérez P., Ribas J.C. Extracellular cell wall beta(1,3)glucan is required to couple septation to actomyosin ring contraction. J. Cell. Biol. 2013;203:265–282. doi: 10.1083/jcb.201304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh T., Nakano K., Mabuchi I. Rho1-GEFs Rgf1 and Rgf2 are involved in formation of cell wall and septum, while Rgf3 is involved in cytokinesis in fission yeast. Genes Cells. 2005;10:1189–1202. doi: 10.1111/j.1365-2443.2005.00908.x. [DOI] [PubMed] [Google Scholar]
- Nakano K., Mutoh T., Mabuchi I. Characterization of GTPase-activating proteins for the function of the Rho-family small GTPases in the fission yeast Schizosaccharomyces pombe. Genes Cells. 2001;6:1031–1042. doi: 10.1046/j.1365-2443.2001.00485.x. [DOI] [PubMed] [Google Scholar]
- Niederberger C., Schweingruber M.E. A Schizosaccharomyces pombe gene, ksg1, that shows structural homology to the human phosphoinositide-dependent protein kinase PDK1, is essential for growth, mating and sporulation. Mol. Gen. Genet. 1999;261:177–183. doi: 10.1007/s004380050955. [DOI] [PubMed] [Google Scholar]
- Osumi M. The ultrastructure of yeast: cell wall structure and formation. Micron. 1998;29:207–233. doi: 10.1016/s0968-4328(97)00072-3. [DOI] [PubMed] [Google Scholar]
- Perez P., Cansado J. Cell integrity signaling and response to stress in fission yeast. Curr. Protein Pept. Sci. 2010;11:680–692. doi: 10.2174/138920310794557718. [DOI] [PubMed] [Google Scholar]
- Ragni E., Fontaine T., Gissi C., Latge J.P., Popolo L. The gas family of proteins of Saccharomyces cerevisiae: characterization and evolutionary analysis. Yeast (Chichester, England) 2007;24:297–308. doi: 10.1002/yea.1473. [DOI] [PubMed] [Google Scholar]
- Ribas J.C., Roncero C., Rico H., Duran A. Characterization of a Schizosaccharomyces pombe morphological mutant altered in the galactomannan content. FEMS Microbiol. Lett. 1991;63:263–267. doi: 10.1016/0378-1097(91)90096-s. [DOI] [PubMed] [Google Scholar]
- Rodicio R., Heinisch J.J. Together we are strong–cell wall integrity sensors in yeasts. Yeast (Chichester, England) 2010;27:531–540. doi: 10.1002/yea.1785. [DOI] [PubMed] [Google Scholar]
- Roncero C., Sanchez Y. Cell separation and the maintenance of cell integrity during cytokinesis in yeast: the assembly of a septum. Yeast (Chichester, England) 2010;27:521–530. doi: 10.1002/yea.1779. [DOI] [PubMed] [Google Scholar]
- Ruiz-Herrera J. CRC Press. Taylor & Francis Group; Boca Raton FL: 2012. Fungal Cell Wall Structure, Synthesis, and Assembly; pp. 33487–42742. [Google Scholar]
- Sanchez-Mir L., Franco A., Madrid M., Vicente-Soler J., Villar-Tajadura M.A., Soto T., Perez P., Gacto M., Cansado J. Biological significance of nuclear localization of mitogen-activated protein kinase Pmk1 in fission yeast. J. Biol. Chem. 2012;287:26038–26051. doi: 10.1074/jbc.M112.345611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mir L., Soto T., Franco A., Madrid M., Viana R.A., Vicente J., Gacto M., Perez P., Cansado J. Rho1 GTPase and PKC ortholog Pck1 are upstream activators of the cell integrity MAPK pathway in fission yeast. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh R., Morita T., Takada H., Kita A., Ishiwata S., Doi A., Hagihara K., Taga A., Matsumura Y., Tohda H., Sugiura R. Role of the RNA-binding protein Nrd1 and Pmk1 mitogen-activated protein kinase in the regulation of myosin mRNA stability in fission yeast. Mol. Biol. Cell. 2009;20:2473–2485. doi: 10.1091/mbc.E08-09-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengar A.S., Markley N.A., Marini N.J., Young D. Mkh1, a MEK kinase required for cell wall integrity and proper response to osmotic and temperature stress in Schizosaccharomyces pombe. Mol. Cell. Biol. 1997;17:3508–3519. doi: 10.1128/mcb.17.7.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K., Palani S., Cortes J.C., Sato M., Sevugan M., Ramos M., Vijaykumar S., Osumi M., Naqvi N.I., Ribas J.C., Balasubramanian M. A new membrane protein Sbg1 links the contractile ring apparatus and septum synthesis machinery in fission yeast. PLoS Genet. 2016;12:e1006383. doi: 10.1371/journal.pgen.1006383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifmoghadam M.R., Valdivieso M.H. The Schizosaccharomyces pombe Map4 adhesin is a glycoprotein that can be extracted from the cell wall with alkali but not with beta-glucanases and requires the C-terminal DIPSY domain for function. Mol. Microbiol. 2008;69:1476–1490. doi: 10.1111/j.1365-2958.2008.06375.x. [DOI] [PubMed] [Google Scholar]
- Shematek E.M., Braatz J.A., Cabib E. Biosynthesis of yeast cell wall. I. Preparation and properties of b(1–3)glucan synthetase. J. Biol. Chem. 1980;255:888–894. [PubMed] [Google Scholar]
- Shiozaki K., Russell P. Counteractive roles of protein phosphatase 2C (PP2C) and a MAP kinase kinase homolog in the osmoregulation of fission yeast. EMBO J. 1995;14:492–502. doi: 10.1002/j.1460-2075.1995.tb07025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto T., Villar-Tajadura M.A., Madrid M., Vicente J., Gacto M., Perez P., Cansado J. Rga4 modulates the activity of the fission yeast cell integrity MAPK pathway by acting as a Rho2 GTPase-activating protein. J. Biol. Chem. 2010;285:11516–11525. doi: 10.1074/jbc.M109.071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T., Sato M., Takagi T., Kamasaki T., Ohno N., Osumi M. In situ localization of cell wall a-1,3-glucan in the fission yeast Schizosaccharomyces pombe. J. Electron Microsc. (Tokyo) 2003;52:237–242. doi: 10.1093/jmicro/52.2.237. [DOI] [PubMed] [Google Scholar]
- Sugawara T., Takahashi S., Osumi M., Ohno N. Refinement of the structures of cell-wall glucans of Schizosaccharomyces pombe by chemical modification and NMR spectroscopy. Carbohydr. Res. 2004;339:2255–2265. doi: 10.1016/j.carres.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Sugiura R., Kita A., Shimizu Y., Shuntoh H., Sio S.O., Kuno T. Feedback regulation of MAPK signalling by an RNA-binding protein. Nature. 2003;424:961–965. doi: 10.1038/nature01907. [DOI] [PubMed] [Google Scholar]
- Sugiura R., Sio S.O., Shuntoh H., Kuno T. Calcineurin phosphatase in signal transduction: lessons from fission yeast. Gen. Cells. 2002;7:619–627. doi: 10.1046/j.1365-2443.2002.00557.x. [DOI] [PubMed] [Google Scholar]
- Sugiura R., Toda T., Dhut S., Shuntoh H., Kuno T. The MAPK kinase Pek1 acts as a phosphorylation-dependent molecular switch. Nature. 1999;399:479–483. doi: 10.1038/20951. [DOI] [PubMed] [Google Scholar]
- Sugiura R., Toda T., Shuntoh H., Yanagida M., Kuno T. pmp1+, a suppressor of calcineurin deficiency, encodes a novel MAP kinase phosphatase in fission yeast. EMBO J. 1998;17:140–148. doi: 10.1093/emboj/17.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Nishimura M., Asayama Y., Mannse Y., Ishiwata S., Kita A., Doi A., Nishida A., Kai N., Moriuchi S., Tohda H., Giga-Hama Y., Kuno T., Sugiura R. Atf1 is a target of the mitogen-activated protein kinase Pmk1 and regulates cell integrity in fission yeast. Mol. Biol. Cell. 2007;18:4794–4802. doi: 10.1091/mbc.E07-03-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Dhut S., Superti-Furga G., Gotoh Y., Nishida E., Sugiura R., Kuno T. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol. Cell. Biol. 1996;16:6752–6764. doi: 10.1128/mcb.16.12.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Shimanuki M., Yanagida M. Two novel protein kinase C-related genes of fission yeast are essential for cell viability and implicated in cell shape control. EMBO J. 1993;12:1987–1995. doi: 10.1002/j.1460-2075.1993.tb05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez B., Soto T., del Dedo J.E., Franco A., Vicente J., Hidalgo E., Gacto M., Cansado J., Madrid M. Distinct biological activity of threonine monophosphorylated MAPK isoforms during the stress response in fission yeast. Cell Signal. 2015;27:2534–2542. doi: 10.1016/j.cellsig.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Viana R.A., Pinar M., Soto T., Coll P.M., Cansado J., Perez P. Negative functional interaction between cell integrity MAPK pathway and Rho1 GTPase in fission yeast. Genetics. 2013;195:421–432. doi: 10.1534/genetics.113.154807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar-Tajadura M.A., Coll P.M., Madrid M., Cansado J., Santos B., Perez P. Rga2 is a Rho2 GAP that regulates morphogenesis and cell integrity in S. pombe. Mol. Microbiol. 2008;70:867–881. doi: 10.1111/j.1365-2958.2008.06447.x. [DOI] [PubMed] [Google Scholar]
- Vos A., Dekker N., Distel B., Leunissen J.A., Hochstenbach F. Role of the synthase domain of Ags1p in cell wall alpha-glucan biosynthesis in fission yeast. J. Biol. Chem. 2007;282:18969–18979. doi: 10.1074/jbc.M605147200. [DOI] [PubMed] [Google Scholar]
- Wennerberg K., Rossman K.L., Der C.J. The Ras superfamily at a glance. J. Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- Wilkinson M.G., Samuels M., Takeda T., Toone W.M., Shieh J.C., Toda T., Millar J.B., Jones N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- Yoshimi A., Miyazawa K., Abe K. Function and biosynthesis of cell wall alpha-1,3-glucan in fungi. J Fungi (Basel) 2017;3 doi: 10.3390/jof3040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsevskaya-Carter T., Cooper J.A. Spm1, a stress-activated MAP kinase that regulates morphogenesis in S.pombe. EMBO J. 1997;16:1318–1331. doi: 10.1093/emboj/16.6.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Jung U.S., Garrett-Engele P., Roe T., Cyert M.S., Levin D.E. Temperature-induced expression of yeast FKS2 is under the dual control of protein kinase C and calcineurin. Mol. Cell. Biol. 1998;18:1013–1022. doi: 10.1128/mcb.18.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.H., Ye Y., Wu Z., Wu J.Q. Cooperation between Rho-GEF Gef2 and its binding partner Nod1 in the regulation of fission yeast cytokinesis. Mol. Biol. Cell. 2013;24:3187–3204. doi: 10.1091/mbc.E13-06-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]