Abstract
Background
Mild cognitive impairment (MCI) is an intermediate state between normal cognition and dementia in which daily function is largely intact. This condition may present an opportunity for research into the prevention of dementia. Carbohydrate is an essential and easily accessible macronutrient which influences cognitive performance. A better understanding of carbohydrate‐driven cognitive changes in normal cognition and mild cognitive impairment may suggest ways to prevent or reduce cognitive decline.
Objectives
To assess the effectiveness of carbohydrates in improving cognitive function in older adults with normal cognition or mild cognitive impairment.
Search methods
We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group Specialized Register, on 6 April 2012 using the terms: carbohydrates OR carbohydrate OR monosaccharides OR disaccharides OR oligosaccharides OR polysaccharides OR CARBS. ALOIS contains records from all major healthcare databases (The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL, LILACS) as well as from many trial databases and grey literature sources.
Selection criteria
All randomised controlled trials (RCT) examining the effect of any form of carbohydrates on the cognition or daily functioning of adults aged 55 years or over with normal cognition or MCI.
Data collection and analysis
One review author selected and retrieved relevant articles for further assessment. The remaining authors independently assessed whether any of the retrieved trials should be included. Disagreements were resolved by discussion.
Main results
One study was included. It involved 44 adults aged 60 to 80 years and compared a glucose drink with a saccharin drink, given on only a single occasion. Those receiving the glucose drink were significantly faster in completing the switching condition of the modified Stroop test (F 1, 41 = 10.47; P < 0.01) compared to those receiving the saccharin drink. Participants in the glucose group also showed a significantly smaller dual‐task cost in a computerised test of divided attention compared to the placebo group (F 1, 38 = 8.49; P < 0.01, ƞ2 = 0.18). As a glucose drink was administered only once, safety, global function, behaviour disturbance, and activities of daily living were not investigated in the study.
Authors' conclusions
With only one RCT included, there is insufficient evidence to base any recommendations about the use of any form of carbohydrate for enhancing cognitive performance in older adults with normal cognition or mild cognitive impairment. More studies of many different carbohydrates are needed to tease out complex nutritional issues and to further evaluate memory improvement.
Keywords: Aged, Humans, Cognition, Cognition Disorders, Cognition Disorders/drug therapy, Dietary Carbohydrates, Dietary Carbohydrates/therapeutic use, Independent Living
Plain language summary
There is insufficient evidence for the use of carbohydrates to improve cognitive performance in older adults with normal or mild cognitive impairment
Carbohydrates consist of sugars, oligosaccharides and polysaccharides. These components are found in a large range of foods and have variable effects on digestion, blood sugar levels, and their impact on health. Despite the evidence accumulated from biological and epidemiological (observational) studies and non‐randomised clinical trials, only one randomised, controlled trial could be included in this review. This study had 44 participants. Participants who were given a single glucose drink showed possible momentary enhancement of cognitive performance compared to those given a saccharin drink. A safety assessment was not reported. We need more studies on different types of carbohydrates, particularly those from fruit, vegetable and whole grain sources, for older adults with normal cognition and mild cognitive impairment to understand the role of this nutrient type in the prevention or reduction of cognitive decline.
Background
Description of the condition
Cognition encompasses the range of higher brain functional domains that include memory, perception, language, and reasoning. Mild cognitive impairment (MCI) represents an intermediate state of cognitive decline along a continuum of normal to demented (Winblad 2004). In the intermediate state preceding dementia, some memory function falls below the level expected for an individual’s education and age but does not noticeably impact daily living. This is in contrast to dementia, which inhibits independent daily functioning (Dodge 2006; McGuire 2006; Morala 2006; Scanlan 2007). In the absence of a cure, the burden dementia places on individuals, families, and communities is a tremendous challenge (Prince 2004; WHO 2003).
The confluence of the increasing prevalence of chronic diseases, the ageing population, and the resulting increase in prevalence of cognitive decline and dementia among older adults is the current concern. Between 2001 and 2010, a 100% increase was projected in cases of dementia in developed countries and a 300% increase in Asia and Asia‐Pacific countries (Ferri 2005). Even small reductions in the incidence, or delays in the onset, are likely to have significant effects on the prevalence of dementia and the associated enormous public health burden. Evidence from human epidemiologic studies has shown that one important factor which can influence cognitive performance is the nutritional components of food and patterns of their intake in the diet. While simple carbohydrates such as sucrose (glucose and fructose) may have an immediate effect on cognition, diets high in complex carbohydrates, such as fruits, legumes, vegetables, and cereals, are associated with better cognitive function and a lower risk of dementia in the longer term (Dai 2006; Gu 2010; Hughes 2010; Kang 2006; Morris 2006). In contrast, diets high in fats, both trans and saturated fats, may adversely affect cognition (Eskelinen 2008; Laitinen 2006; Morris 2004).
Description of the intervention
Carbohydrates are essential (Lucas 2000) and easily accessible macronutrients. The important components of carbohydrates found in the diet are simple sugars, oligosaccharides, and polysaccharides (IUPAC 1996). Simple sugars such as sucrose are usually found in processed foods, for example soft drinks and biscuits. These are easily digestible and cause an immediate rise in blood glucose levels. On the other hand, oligo‐ and polysaccharides are commonly found in fruits, vegetables, whole grains, and dairy foods; and they may have variable times of digestion or indigestibility. Fruits, vegetables, whole grains, and vegetable‐enriched diets are also the best nutritional sources of phytonutrients, vitamins, and minerals and hence have important roles in overall health maintenance. Formulary dietary foods containing carbohydrates are also used in medical nutritional therapy.
The impact of acute glucose (simple carbohydrate) ingestion on cognitive performance has received much attention. A substantial body of evidence supports a relationship between glucose availability and changes in cognition. Variable improvements in cognitive tasks, as well as adverse effects of glucose and carbohydrate‐rich foods, have been demonstrated in both human and animal models across the age spectrum (Foster 1998; Hall 1989; Manning 1990). Progressive glucoregulatory dysfunction with ensuing hyperglycaemia and hyperinsulinaemia, common among older adults, may occur at least a decade before the presentation of type 2 diabetes mellitus (T2DM) (Shulman 1999). The more consistent improvement of cognitive performance following glucose ingestion in healthy elderly individuals (Hall 1989; Manning 1993 ; Manning 1997) and people with Alzheimer’s disease (AD) (Craft 1993; Meneilly 1993) than in healthy young individuals suggests a link between poor memory and deranged glucose regulation. This connection is supported by the decline in glucose metabolic activities in the hippocampus that have been demonstrated with advanced imaging techniques in those at risk of age‐related cognitive decline or with MCI (Fouquet 2009; Hunt 2007; Li 2008; Mosconi 2008). For those who showed no progression of cognitive deficits, there was a suggestion of compensatory hypermetabolism of glucose with neuronal disconnection in various regions of the brain connecting to the hippocampus (Fouquet 2009). In addition, the decrease in cognitive deficits reported after improvements in glycaemic control in patients with T2DM supports the argument that metabolic disturbances can contribute to possible reversible cognitive deficits (Cooray 2010; Ryan 2006).
Preliminary cohort studies have demonstrated an association between the Mediterranean diet and a significantly lower risk of incident AD. These studies, with follow‐up times of four to seven years, report significant effects with a suggestion of dose‐response patterns. Among the important characteristics of the Mediterranean diet are a proportionately high intake of vegetables, legumes, fruits, and cereals. Data for the three studies in the USA were from the cohort of the Washington Heights‐Inwood Columbia Aging Project (WHICAP). However, each analysis addressed slightly different outcomes. These were the association between AD and the Mediterranean diet (Scarmeas 2006); the association between the progression from MCI to AD and the Mediterranean diet (Scarmeas 2009a); and the association between AD and the Mediterranean diet combined with physical activity (Scarmeas 2009b).
Sampling techniques were used to minimize selection bias and baseline characteristics were compared according to exposure level. However, details of the selection of participants to the exposure group were not provided in some of the publications. Despite methodological limitations in these three studies, there were fairly consistent results regarding the association between higher compliance with a Mediterranean diet and a significantly lower risk of incident AD. In these studies an informant report was not part of the diagnostic process of AD. However, the association of higher compliance with a Mediterranean diet and a reduced risk of MCI may be influenced by the lack of informant reports (Scarmeas 2006; Scarmeas 2009b) and retrospective application of diagnostic criteria for MCI (Scarmeas 2009a). Studies have demonstrated the discriminative and predictive power of these proxy informant reports in differentiating between MCI and AD (Isella 2006) as well as for increasing the accuracy of a diagnosis of AD (Hancock 2009; Morales 1995). A separate study in a European community did not replicate the finding of an association between adherence to a Mediterranean diet and a reduced risk of dementia (Feart 2009). This was due to a lack of statistical power.
Five cohort studies have suggested a significant protective association between higher amounts of vegetables in the diet and lower rates of cognitive decline or AD; the association was the strongest for green leafy vegetables (Dai 2006; Gu 2010; Hughes 2010; Kang 2006; Morris 2006). Three studies stated that the participants were non‐demented at baseline (Dai 2006; Gu 2010; Morris 2006); the other studies did not provide adequate information about baseline cognitive levels (Kang 2006; Morris 2006). However, most participants were assumed to be non‐demented at baseline in the latter studies. Only one study included informant reports in the diagnostic process (Dai 2006). The length of follow up ranged from two to 31.5 years. All the studies used sample selection methods to minimize selection bias. The analyses appear to be generally appropriate and were controlled for relevant potential confounders. Although the results of these studies are consistent in that they suggest a protective effect on cognition associated with eating vegetables, the actual differences in mean scores between the groups are small.
Vegetables, legumes, cereals, fruits, and nuts are potential sources of saccharides. Several saccharides and their glycoforms are found in the brain structure (Albach 2001). Clinical and placebo‐controlled studies have reported positive effects of some of these saccharides on cognitive performance over the longer term in younger and middle aged adults (Best 2008; Best 2009; Best 2010; Wang 2004). The intervention studies have included proprietary saccharide supplements and saccharides in the diet. The results from these studies suggest the possibility of functional roles of the saccharides in cognitive performance.
How the intervention might work
Carbohydrates are utilised as energy sources while some are important structural and functional components of the brain. Only glucose has been extensively studied. Many of the mechanisms of carbohydrate utilisation at the molecular level remain unclear.
Several mechanisms by which dietary carbohydrates might influence cognitive function have been proposed. These include the following.
Glucose as an energy substrate.
Neurotransmitter synthesis.
Effects via insulin.
Effects via cortisol.
Peripheral mechanisms.
1. Glucose as an energy substrate
The brain has a high level of energy consumption but low storage capacity. Distribution of energy resources between the brain and blood is strictly regulated (Fehm 2006; Peters 2004). Glucose is the only substrate for brain metabolism in a non‐starved state. Mental function is related to blood glucose levels by a narrow, inverted U‐shaped dose‐response curve (Parsons 1992). There is a deterioration of cognitive function at either end of the optimal range of blood glucose levels.
2. Neurotransmitter synthesis
A number of neurotransmitters (for example acetylcholine and gamma‐aminobutyric acid) are products of glucose metabolism. Besides modulating cognitive function and cortical plasticity (Arendt 1986; Bigl 1998; Schliebs 1996), neurotransmitters play critical physiological roles in controlling cerebral blood flow (Sato 2004) and the sleep‐wake cycle (de Leon 1997).
3. Effects via insulin
Insulin is a growth factor for all cells, including neurons in the brain. In healthy brains, insulin is involved in the regulation of various processes such as neuronal survival, energy metabolism, and plasticity associated with learning and memory. Direct positive effects of insulin on cognition are supported by several studies utilising intranasal (Benedict 2010; Reger 2006; Reger 2008), intravenous (Craft 2003; Hallschmid 2010), and intracerebroventricular insulin (Park 2000). In contrast, chemical depletion of insulin in rats reduces the size of the brain and induces neurological changes similar to those seen in AD (Lester‐Coll 2006). Elevated adiposity increases peripheral insulin resistance (Luchsinger 2009). In turn, insulin resistance and hyperinsulinaemia cause a reduction in brain insulin (Nelson 2008). The resulting deranged blood glucose levels, as well as the degenerative processes in the brain (Li 2007), may lead to abnormal beta‐amyloid peptide formation similar to that found in the brains of patients with AD at post‐mortem (de la Monte 2009; Westerman 2002). This may also contribute to the higher risk of dementia, including AD, along the continuum of obesity, metabolic disorders, and T2DM seen in epidemiologic studies (Fitzpatrick 2009; Knopman 2009; Muller 2007; Stewart 2005; Yaffe 2009).
4. Effects via cortisol
The corticosteroid hormone cortisol has also been suggested as a potential mediator of an association between glucose and cognition (Comijs 2010; Huang 2009; Souza‐Talarico 2010). Cortisol responses to glucose load are complex and may vary with circumstances. In healthy adults, glucose stimulates cortisol release only during demanding tasks (Gonzalez‐Bono 2002). Although glucose ingestion prior to a stressful task specifically provokes a greater cortisol response to the task it is the response on plasma glucose levels that determines the extent of the rise in cortisol (Kirschbaum 1997). In both animal and some human studies there is, as with glucose, evidence for an inverted U‐shaped relationship between the cortisol dose and cognitive performance (Abercrombie 2003). There is a deterioration of cognitive function, especially memory, at both ends of the cortisol spectrum. The presence of a high level of cortisol and a raised concentration of cortisol‐binding receptors in the hippocampus induces memory impairment (Het 2005; Newcomer 1994). This may explain the association between high morning plasma cortisol levels and raised cortisol following a post‐prandial rise in blood glucose with adverse memory performance in patients with impaired glucose tolerance and T2DM (Benton 2003; Reynolds 2010; Rosmond 2000).
5. Peripheral mechanisms
Attenuation of the memory‐enhancing effects of peripherally administered hormones following vagotomy in rats suggests that gastrointestinal (GI) hormones (for example cholecystokinin, gastric‐releasing peptide, and bombesin) could relay signals to the central nervous system via the vagus nerve to influence cognitive processes (Flood 1987; Flood 1988). Circulating ghrelin, released following the consumption of significant proportions of complex carbohydrates, crosses the blood–brain barrier (Diano 2006) adversely influencing cognitive function (Spitznagel 2010). The extent of the actual effects of all these hormones on the brain is unclear.
Finally, other saccharides, such as mannose, galactose, fucose, xylose, n‐acetyl‐glucosamine, n‐acetyl‐neuraminic acid, n‐acetyl‐galactosamine, and their glycoforms, are involved in the functioning of synapses and neurotransmitters (Miller 2009; Yamaguchi 2002). They are involved in modulating the electrical activity of neurons (Kleene 2004), the general integrity of the central nervous system (Bandtlow 2000; Maeda 2010), and play a role in mood regulation (Dutton 2008). An involvement of saccharides in cognition is also likely. There is still much to learn of exact mechanisms.
Why it is important to do this review
The predicted acceleration in the demographic shift towards an aged population will result in greater increases in the number of elderly people living in less developed countries, where malnutrition or inappropriate nutrition is more common, compared to developed countries (UN 2007). Furthermore, an anticipated extended lifespan does not imply extended disease‐free or disability‐free life expectancy as it once did in developed countries, such as Japan (WHO 2003). A better understanding of factors that may prevent or reduce cognitive decline beyond MCI could ease the public burden and enable the development of effective strategies to improve public health.
This review examines the effects of carbohydrates on cognitive performance in both older adults with normal cognition and with MCI. An enhanced understanding of the role of carbohydrates in driving cognitive changes in these groups may suggest future research directions which focus on the relationship between nutrition, the brain, and those cognitive functions which are necessary to sustain independent living.
Objectives
To assess the effectiveness of carbohydrates in improving cognitive function and the ability to perform activities of daily living.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were included without restrictions on the basis of language, date of trial, publication status, or duration.
Two‐period crossover studies with randomisation and absence of carryover effects were potentially eligible for inclusion.
Types of participants
Studies of people aged over 55 years, of either gender, who showed no evidence of cognitive impairment or who had mild cognitive impairment and were living in the community (non‐institutionalised) were considered.
The diagnosis of mild cognitive impairment (MCI) was based on the consensus criteria of Winblad 2004. These criteria are as follows.
Not normal but not demented; do not meet criteria (DSM IV, ICD 10) for a dementia syndrome (APA 1994; ICD 1993).
Cognitive decline is determined by self or an informant report; impairment on objective cognitive tasks or evidence of decline over time on objective cognitive tasks.
Preserved basic activities of daily living or minimal impairment in complex instrumental functions.
Participants with impaired glucose tolerance and type 2 diabetes mellitus (T2DM) were included. Those with neurological diseases (including in the central nervous system) and on active medications affecting cognition were also excluded. Some examples of active medications affecting cognition are the licensed drugs used in dementia (donepezil, rivastigmine, galantamine, and memantine), benzodiazepines and other sedative‐hypnotics, antipsychotic drugs, glucocorticoids, and anticholinergics.
Types of interventions
All forms of intervention with carbohydrates, with or without other nutrients or substances, were considered. Monosaccharide, disaccharide, oligosaccharide, and polysaccharide interventions were eligible. These interventions could be for any time duration. Only the oral mode of administration was considered. Studies with placebo or no intervention comparison groups were eligible for inclusion, though the latter were considered separately. Studies were eligible if the intervention consisted of a provided diet and the comparator was the usual diet. Trials where the intervention was compared to another active intervention and not to a placebo were excluded.
Types of outcome measures
Primary outcomes
The primary outcomes of interest were as follows.
Cognitive function: this included any cognitive outcome measure that is sensitive to change in those people with normal cognition or MCI. Such measures may test the individual cognitive domains, a combination of cognitive domains, or overall global function (e.g. Cambridge Cognitive Examination (CAMCOG)).
Functional performance: any outcome measures of the activities of daily living, instrumental activities of daily living (IADL), and higher hierarchy of functions were to be included. Examples are Functional Capacities for Activities of Daily Living, or FC‐ADL (an error‐based ADL measure) (Jefferson 2008), standardised timed IADL measure (Wadley 2008), Everyday Problems Test (a measure of everyday problem solving indexing IADLs) (Burton 2009), global driving ratings (Wadley 2009), and the Financial Capacity Instrument (Triebel 2009).
Secondary outcomes
These outcomes were also considered, if reported:
behavioural or affective disorders;
quality of life;
glycaemic control;
safety and adverse effects.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), which is the Cochrane Dementia and Cognitive Improvement Group (CDCIG) Specialized Register (6 April 2012). The search terms used were: carbohydrates OR carbohydrate OR monosaccharides OR disaccharides OR oligosaccharides OR polysaccharides OR CARBS.
ALOIS is maintained by the Cochrane Dementia Group's Trials Search Co‐ordinator and contains studies in the areas of dementia prevention, dementia treatment, and cognitive enhancement in healthy people. The studies are identified from the following.
Monthly searches of a number of major healthcare databases: MEDLINE, EMBASE, CINAHL, PsycINFO, and LILACS.
Monthly searches of a number of trials registers: ISRCTN; UMIN (Japan's Trials Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials; and the Netherlands National Trials Register plus others).
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library).
Six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS, see 'About ALOIS' on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL, and conference proceedings can be viewed in the ‘Methods used in reviews’ section within the editorial information about the CDCIG. Additional searches were performed in many of the sources listed above to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen in Appendix 1. Details of an earlier search for trials to be included in this review can be seen in Appendix 2.
The search of May 2008 retrieved a total of 419 results. After a first assessment and de‐duplication of these results, the authors were left with 26 references for further assessment. In June 2010, the search retrieved a total of 1469 results. After a first assessment and de‐duplication of these results the authors were left with 39 references to further assess. In the subsequent updated search of April 2012, 1343 results were retrieved. After the first assessment and removal of duplicate publications, 25 references remained for further assessment.
Searching other resources
The electronic search was complemented by a manual search for recent, relevant articles in medical libraries. Reference lists of retrieved articles were also screened for additional trials. Additional relevant information was also searched for using the Internet search engine Google. Three additional references were found from these searches.
Data collection and analysis
Selection of studies
One review author (CPO) discarded obviously irrelevant publications based on the title of the publication and its abstract. In the presence of any suggestion that an article could be relevant, it was retrieved for further assessment. The review authors independently assessed the trials from the resulting culled list for exclusion or inclusion. Full papers were reviewed to resolve queries about data and to clarify questions of concern. One author was contacted for clarification. Disagreements were resolved by discussion between the authors or consultation with a third party.
Data extraction and management
Methodological characteristics of each trial were entered in a table with information on the country of study; the number, age and gender of participants randomised to the groups; the method used to exclude dementia; inclusion and exclusion criteria; the methods of randomisation and allocation concealment; the method of measurement of cognitive performance used in the study; the methods of measurement of outcomes; and the number of participants lost during treatment.
Summary statistics that were reported in each study were to be extracted. For continuous data, this was to be the mean change from baseline, the standard deviation of the mean change, and the number of patients for each treatment group at each assessment. Where changes from baseline were not reported, the mean, standard deviation, and the number in each treatment group at each time point were to be extracted. For binary outcomes, the number in each treatment group and the numbers experiencing the outcome of interest or end‐point of clinical relevance were to be extracted. Where available, intention‐to‐treat (ITT) data were to be used. In the absence of ITT data, data for on‐treatment analysis were to be extracted, and indicated as such.
Assessment of risk of bias in included studies
Methodological quality criteria were developed, with input from all of the authors, based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). The intention was for two authors to independently assess and score the studies according to the following criteria: method of randomisation; concealment of treatment allocation; similarity of groups at baseline with regard to the most important prognostic indicators; details of eligibility criteria, blinding, presence of point estimates and measures of variability for the primary outcome measures; and the presence of an ITT analysis.
When the description of the randomisation process was unclear or missing, the corresponding author of the study was contacted in an attempt to retrieve the required information. Trials at high risk of bias due to inadequate methods of randomisation or allocation concealment would be excluded. Trials with inadequate concealment have been shown to overestimate the treatment effect (Chalmers 1983; Schulz 1995). Using the recommended approach outlined in Section 8.7 of the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.0.2), the risk of bias for important outcomes within and across studies would be included in a summary assessment.
Data analysis
No raw data were available in the report of the included trial. The discussion in subsequent paragraphs is based on the reported analyses.
Results
Description of studies
The CDCIG database search identified 26 trials from the first search in May 2008, 39 trials from the search in June 2010, and a further 25 trials from the search in April 2012. In addition, three trials were identified from other searches. One duplicated study was removed. A total of 92 trials were screened. All of the studies except for two were published in English. After examining the abstracts, full‐text articles were obtained for assessment when it was unclear if the criteria for inclusion were met. Authors from the selected trials were contacted for further details . Only one author replied. These processes are summarised in Figure 1.
1.
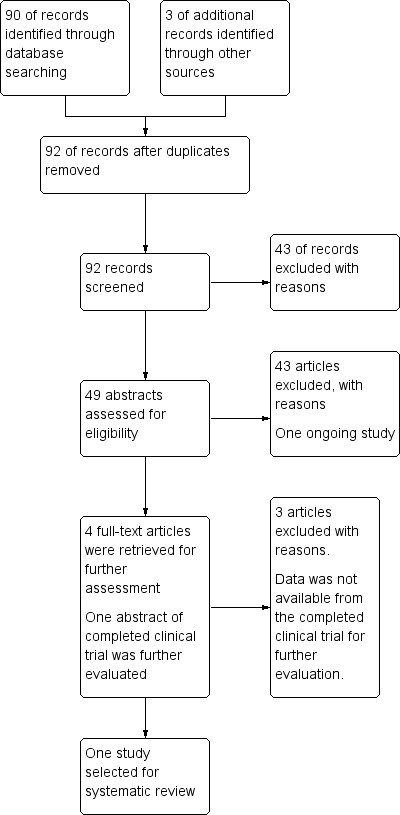
Adapted PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow‐chart of study selection.
Included studies
There was only one published RCT that fulfilled the inclusion criteria (Gagnon 2010). For full details of the trial, see the 'Characteristics of included studies' table. In this between‐participants double‐blind, randomised controlled trial, a glucose drink was compared with a placebo drink. The intervention consisted of 50 g of glucose mixed with 290 mL of water and 10 mL of lemon juice (Xenex Labs©); while 23.7 mg of saccharin, 290 mL of water, and 10 mL of lemon juice (Hermesetas©) were used to make the placebo drink. Each of the participan ts were tested once with either the intervention drink or the placebo drink.
A total of 44 people who met the protocol criteria were recruited. Inclusion criteria were participants without diabetes as well as medical conditions that could affect cognition and bias data interpretation (for example, absence of general anaesthesia in the past six months; absence of neurological disease, stroke, etc). Participants were excluded if they obtained scores lower than 27 on the mini‐mental state examination (MMSE) ( Folstein 1975) and if their fasting blood glucose levels were equal to or greater than 7.0 mmol/L. The mean age of the participants was 67.7 years. The glucose group and the placebo group did not differ across age, gender, years of education, body mass index, MMSE, digit span forward, digit span backward, matrix reasoning, and digit symbol substitution at baseline.
Outcome measures
Modified Stroop test
This test was based on the protocol developed by Lezak 1995. The colour‐ related tests were carried out in four different conditions (reading, naming, inhibition, switching). Under each condition, the participant was given instructions to execute the task as fast and as accurately as possible. The colour reading and naming conditions evaluate the processing speed. Testing the inhibition condition involves naming the colour in which a colour word is printed, and that differs from the original meaning (for example, RED printed in green ink). Alternating between identifying the colour in which the colour word is printed and reading the word when it is placed in a rectangle test flexibility and switching.
Trial making test, part A
This task assessed processing speed and visuo‐spatial search. Numbers one to 25 are placed randomly on the testing sheet. The participant is instructed to trace a line linking the numbers in increasing order as fast as possible.
Trial making test, part B
This task measured controlled attention and flexibility. Numbers and letters are placed randomly on a sheet of paper. The participant is instructed to link alternately between letters and numbers, in alphabetical and increasing numerical orders.
Computerised dual‐tasks
This consisted of a set of visual discrimination tasks that test the divided attention. E‐Prime 1 stimuli were viewed at approximately 45 cm from a personal computer (PC) monitor. Green or yellow colour discrimination tasks and B or C letters discrimination tasks were performed alone and concurrently. In a single‐pure trial, one of the tasks was executed consecutively; while in the dual‐mixed trial, both tasks were performed concurrently. One of the tasks, single‐mixed trial, was presented alone in between the concurrent trials. These trials were used to control for memory load incurred by maintaining different response alternatives as well as to isolate the cost of co‐ordinating both tasks. The calculated attentional costs provide information on cognitive processes involved in dual‐task situations.
Excluded studies
On screening the 92 trials, there were 71 RCTs and the remaining 21 records were non‐randomised or controlled clinical trials. A total of 22 RCTs and 21 non‐randomised or controlled clinical trials did not meet any of the selection criteria of this review and were excluded. Of the 49 remaining RCTs, two studies are ongoing (ACTRN12606000475549; NCT01427231 2011). Four full‐text articles were retrieved for further assessment. The reasons for exclusion of 46 studies were as follows.
Three studies used a counter‐balanced crossover design (Riby 2004; Riby 2006; Riby 2009). Random assignment could not be confirmed in any of the three trials. Furthermore, Riby 2004 and Riby 2006 did not use accepted consensus criteria for diagnosis of MCIs and did not use DSM IV (APA 1994) or ICD 10 (ICD 1993) criteria for exclusion of dementia.
The participants of 32 studies were either younger than the specified age criterion (Benton 2003; Benton 2004; Best 2008; Best 2010; Brandt 2010; Brinkworth 2009; Cheatham 2009; Corsica 2002; Ford 2002; Halyburton 2007; Kennedy 2004; Lloyd 1994; Maridakis 2009a; Maridakis 2009b; Markus 2007; Markus 2008; Meikle 2004; Morgan 2009; Nabb 2006; NCT00980408; Rohleder 2009; Salinsky 2005; Scholey 2006; Scholey 2009; Smith 2009; Wang 2004; Wells 1998; Winder 1998), were acutely ill patients in hospital (Gariballa 2006; Garriballa 2007), or had dementia on entry to the trial (Ban 1991; Young 2004). ACTRN12610000624088 2010 included patients aged 45 to 60 years but separate data were not available for those aged over 55 years.
Two studies used fatty acids, not carbohydrates, for interventions. These were ketasyn, a medium‐chain triglyceride (Henderson 2006), and NeoBee 895 (Stepan, Inc.), an emulsified medium‐chain triglyceride (Reger 2004).
One study used choline alphoscerate, a membrane phospholipid, for intervention (Levin 2009). Another study used tryptophan for intervention (Markus 2006).
Two studies used pharmacological agents, repaglinide, glibenclamide (Abbatecola 2009), gluisine, and glargine (Bergenstal 2008), for interventions.
In two study, the interventions were ketogenic and non‐ketogenic diets of low energy contents (Krikorian 2012; Wing 1995).
The primary and secondary end‐points of three studies were not cognition or cognitive performance. The primary outcomes of one study were antioxidant biomarkers with no measures of cognition (Amagase 2009). In the second study, the end‐points were behaviour changes pertaining to life‐styles and not cognition (Block 2008). The participants in the third study had seasonal affective disorder (Mischoulon 2010). There were no exclusion criteria for MCI and dementia as well as no cognitive outcome measures. Another study analysed only the relationship between glycaemic control and cognition (Cukierman‐Yaffe 2009).
Ongoing classification
There were two ongoing studies: ACTRN12606000475549 and NCT01427231 2011. Although NCT01427231 2011 fulfilled the inclusion criteria and data collection was complete, no data were available for analysis. Details of the ongoing studies are given in the Table 'Characteristics of ongoing studies'.
Risk of bias in included studies
There was only one RCT included in this review (Gagnon 2010). This study was a between‐participants, double‐blind, randomised controlled trial. Although the study design was confirmed by the author, no further details were provided. Interventions in both the study and control arm were carried out only once. There were a number of methodological limitations which must be considered. In total, 44 participants (32 women and 12 men) took part in the study. All have an education of a mean of 15.2 years. The inclusion and exclusion criteria are summarised in Characteristics of included studies. Only one participant failed the screening, due to a fasting blood glucose of greater than 7 mmol/L. There were no withdrawals during the trial. Please refer to Figure 2 and Figure 3.
2.
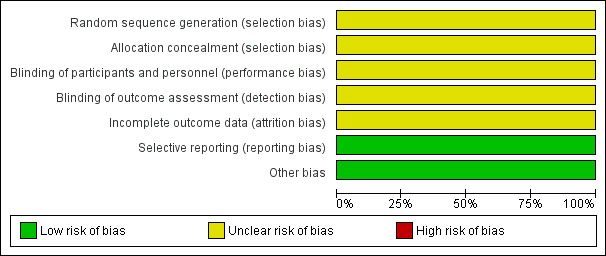
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
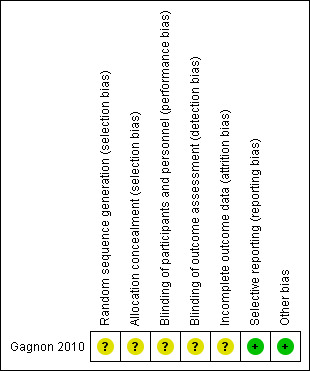
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The study did not report adequately on randomisation and allocation concealment.
Blinding
Although the study was described as double‐blinded, there was no reporting of the double‐blinding procedure.
Incomplete outcome data
Data were either incomplete or missing in both the glucose and placebo groups. Data were unavailable for one male participant in the computerised task because he was unable to discriminate between the colours green and yellow. Further, one female participant in the glucose group was not able to complete the task. In the trial making tests A and B, one female participant was excluded because she misunderstood the instructions. There was no detail on how these missing data were managed.
Selective reporting
None detected
Other potential sources of bias
None detected
Effects of interventions
Outcomes
Modified Stroop test
This outcome was reported by the authors but no further data were available for detailed examination. Four conditions of the task were included in the analysis and were subjected to two‐way analysis of variance with factors of treatment arm. One participant was unable to distinguish colours and was excluded. The glucose arm compared to the placebo arm was significantly faster to complete the switching condition of the modified Stroop test (F 1, 41 = 10.47; P < 0.01). For the three other conditions, there were no significant differences between the two arms. These results corresponded to the analyses for errors. Significant difference in errors between groups were found only on the switching condition (F 1, 41 = 6.26; P < 0.05), with more errors for participants in the placebo group (M = 4.38) compared to those in the glucose group (M = 2.05). This meant that faster execution times for participants in the glucose group were not accompanied by increased errors.
Trial tests, part A and B
The Trial Making Test consisted of part A and B tasks. There was a significant shorter execution time in the glucose group compared to the placebo group in the part A task (F 1, 41 = 6.81; P < 0.05). However, there were no significant group differences in the performances of part B as well as both the difference score (B‐A) and a ratio score ((B‐A)/A). The difference score (B‐A) was derived from a calculation to isolate the cost incurred by Trial Making Test, part B. The ratio or proportional score ((B‐A)/A)) was derived by controlling for visuo‐spatial and speeded components of both parts, A and B.
Computerised dual‐tasks
The analysis by the authors showed only a statistically significant smaller dual‐task cost for participants in the glucose group on the non‐prioritised task when compared to the placebo group (F 1, 38 = 8.49; P < 0.01, ƞ2 = 0.18). This smaller dual‐task cost remained significant when the researchers controlled for speed of processing.
Safety and tolerability
There was no report of adverse effects.
Heterogeneity
Heterogeneity was not assessed in view of only one study being available.
Subgroup analysis
Subgroup analysis was not performed due to insufficient data.
Sensitivity analyses
Sensitivity analyses was not performed due to insufficient studies and data.
Publication and small study bias
A funnel plot was not possible due to insufficient studies and data.
Discussion
There was one study included in this review (Gagnon 2010). In view of the limited availability of studies and data, there is insufficient evidence to confirm the effect of carbohydrates on cognitive function and the ability to perform daily activities in older adults with normal cognition or mild cognitive impairment (MCI). Previous reviews have focused on animal and human experimental trials but not on RCTs.
Any benefits of the short‐term or prolonged use of glucose are yet to be established. A high intake of simple carbohydrate, such as sucrose (glucose and fructose), is a risk factor for obesity, metabolic syndrome, and associated impaired glucose tolerance (IGT) and type 2 diabetes mellitus (T2DM) (Malik 2010). In turn, obesity, metabolic syndrome, IGT, and T2DM are risk factors for cognitive decline (Gatto 2008; Roriz‐Filho 2009). Therefore, interventions containing high proportions of simple carbohydrates may have the potential to exacerbate risk factors for cognitive decline. Further, the association between caloric restrictions and enhancement of memory performance seen in epidemiological observational studies (Beydoun 2008; Wilcox 2007) as well as in an experimental study of ageing brains (Witte 2009) support an adverse effect of prolonged consumption of high levels of simple carbohydrate.
On the other hand, increased total vegetable intake (Dai 2006; Gu 2010; Hughes 2010; Kang 2006; Morris 2006) and adherence to the 'Mediterranean diet' have been associated with reduced risk of dementia (Feart 2009; Scarmeas 2006; Scarmeas 2009a; Scarmeas 2009b; Trichopoulou 2003). Vegetables, legumes, and cereals form major components of the latter diet. These foods are rich in complex carbohydrates such as dietary fibres. Other components of the Mediterranean diet, such as omega‐3 fatty acids or flavonoids, may also have neuroprotective benefits. However, not all studies with omega‐3‐fatty acids have demonstrated benefits for cognition (Kalmijn 1997; Morris 2005; Solfrizzi 2006). Furthermore, though flavonoids have demonstrated effective antioxidant behaviour in cellular models (Stein 2006), the results of preventive clinical trials with vitamin E, an antioxidant, have not found any benefits for cognition and it may even be detrimental (Isaac 2008; Lloret 2009).
Authors' conclusions
Implications for practice.
There is insufficient evidence to support the use of carbohydrates in maintaining or improving cognitive competence, which impacts on independent living in older adults with normal cognition or mild cognitive impairment.
Implications for research.
The implications for research are two‐fold. First, there is a continuing potential for development of interventions for cognitive enhancement from the diversity of carbohydrate sources that are available. The second is the quality of the available studies.
The wide range of carbohydrates and their many apparent effects on cognitive functions mean that, at first sight, they might appear suitable as interventions to improve failing cognitive function in the elderly. However, identifying an effect of any individual nutrient in healthy diets is challenging because of known synergies between nutrients, the difficulty of controlling and recording dietary intake over a long period, the occurrence of insulin resistance and associated diabetes, as well as the effect of overall nutritional status.
Since evidence available on the protective associations of a Mediterranean diet and high vegetable intake has focused on limited population groups in developed countries, confirmation of these findings in other populations, particularly in Asians, is necessary. Small effect sizes, the inappropriate use of multiple cognitive measures, and the possibility of residual confounders all need to be considered in designing these studies. If a protective association is confirmed, it is necessary to identify potential interventions that are of high impact and cost‐effective in reducing the burden of dementia in the coming years. The effectiveness of such interventions at a population versus clinical level needs to be clarified. The impact of co‐morbid conditions, the presence of other modifiable risk factors for cognitive decline, and the optimal age for initiation of potential interventions are also among the important issues that need to be addressed.
What's new
| Date | Event | Description |
|---|---|---|
| 6 April 2012 | New search has been performed | An update search was performed for this review on 6 April 2012 |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 4, 2011
| Date | Event | Description |
|---|---|---|
| 22 June 2010 | New search has been performed | 1st Updated search |
| 25 April 2008 | Amended | First search |
Acknowledgements
The review authors would like to acknowledge the Cochrane Dementia and Cognitive Improvement Group for their help as well as Anne Lydiatt for her contribution as consumer editor. Special thanks to Professor Jackie Ho for her kind advice and support during the initial stage of this review and to Miranda Cumpston (Australasian Cochrane Centre) for her kind advice on the finer technical points of the review.
Appendices
Appendix 1. Search June 2010
| Source | Search strategy | Hits |
| ALOIS (CDCIG SR) (www.medicine.ox.ac.uk/alois) | Keyword search: carbohydrates OR carbohydrate OR monosaccharides OR disaccharides OR oligosaccharides OR polysaccharides OR CARBS | June 2010: 22 April 2012: 22 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (OvidSP) | 1. "cognit* impair*".mp. 2. exp *Cognition Disorders/ 3. MCI.ti,ab. 4. ACMI.ti,ab. 5. ARCD.ti,ab. 6. SMC.ti,ab. 7. CIND.ti,ab. 8. BSF.ti,ab. 9. AAMI.ti,ab. 10. MD.ti,ab. 11. LCD.ti,ab. 12. QD.ti,ab. 13. AACD.ti,ab. 14. MNCD.ti,ab. 15. MCD.ti,ab. 16. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 17. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 18. "preclinical AD".mp. 19. "pre‐clinical AD".mp. 20. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 21. (aMCI or MCIa).ti,ab. 22. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 23. ("GDS 3" or "stage 3 GDS").ti,ab. 24. ("global deterioration scale" and "stage 3").mp. 25. "Benign senescent forgetfulness".ti,ab. 26. "mild neurocognit* disorder*".ti,ab. 27. (prodrom* adj2 dement*).ti,ab. 28. (episodic* adj2 memory).mp. 29. ("preclinical dementia" or "pre‐clinical dementia").mp. 30. (healthy adj2 (elderly or aged or old* or senior)).mp. 31. "community dwelling".mp. 32. "independent living".mp. 33. (adult* adj2 (old or elderly or aged)).mp. 34. ("healthy participants" or "healthy persons").mp. 35. or/1‐34 36. exp Dietary Carbohydrates/ 37. exp *Carbohydrates/ 38. (carbs or carbohydrate*).ti,ab. 39. Monosaccharides/ 40. Disaccharides/ 41. Oligosaccharides/ 42. Polysaccharides/ 43. (monosaccharide* or disaccharide* or oligosaccharide* or polysaccharide*).ti,ab. 44. saccharide*.mp. 45. (sugar* or starch*).ti,ab. 46. or/36‐45 47. 35 and 46 48. randomized controlled trial.pt. 49. controlled clinical trial.pt. 50. randomi?ed.ab. 51. placebo.ab. 52. drug therapy.fs. 53. randomly.ab. 54. trial.ab. 55. groups.ab. 56. or/48‐55 57. (animals not (humans and animals)).sh. 58. 56 not 57 59. 47 and 58 60. (2006* or 2007* or 2008* or 2009* or "2010").ed. 61. 59 and 60 [for the 2010 search] |
June 2010: 346 April 2012: 281 |
| EMBASE 1980‐2010 week 51 (OvidSP) |
1. "cognit* impair*".mp. 2. exp cognitive defect/ 3. exp mild cognitive impairment/ 4. MCI.ti,ab. 5. ACMI.ti,ab. 6. ARCD.ti,ab. 7. SMC.ti,ab. 8. CIND.ti,ab. 9. BSF.ti,ab. 10. AAMI.ti,ab. 11. MD.ti,ab. 12. LCD.ti,ab. 13. QD.ti,ab. 14. AACD.ti,ab. 15. MNCD.ti,ab. 16. MCD.ti,ab. 17. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 18. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 19. "preclinical AD".mp. 20. "pre‐clinical AD".mp. 21. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 22. (aMCI or MCIa).ti,ab. 23. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 24. ("GDS 3" or "stage 3 GDS").ti,ab. 25. ("global deterioration scale" and "stage 3").mp. 26. "Benign senescent forgetfulness".ti,ab. 27. "mild neurocognit* disorder*".ti,ab. 28. (prodrom* adj2 dement*).ti,ab. 29. "age‐related symptom*".mp. 30. (episodic adj2 memory).mp. 31. ("pre‐clinical dementia" or "preclinical dementia").mp. 32. (healthy adj2 (elderly or aged or old* or senior)).mp. 33. "community dwelling".mp. 34. "independent living".mp. 35. (adult* adj2 (old or elderly or aged)).mp. 36. ("healthy participants" or "healthy persons").mp. 37. or/1‐36 38. exp *carbohydrate/ 39. (carbs or carbohydrate*).ti,ab. 40. monosaccharide/ 41. disaccharide/ 42. oligosaccharide/ 43. polysaccharide/ 44. saccharide*.mp. 45. (monosaccharide* or disaccharide* or oligosaccharide* or polysaccharide*).ti,ab. 46. (sugar* or starch*).ti,ab. 47. or/38‐46 48. 37 and 47 49. randomized controlled trial/ 50. controlled clinical trial/ 51. randomi?ed.ab. 52. placebo.ab. 53. randomly.ab. 54. trial.ab. 55. groups.ab. 56. "double‐blind*".ti,ab. 57. or/49‐56 58. 48 and 57 59. (2008* or 2009* or 2010*).em. 60. 58 and 59 |
June 2010: 524 April 2012: 800 |
| Psyc INFO 1806‐June week 2 2010 (OvidSP) |
1. "cognit* impair*".mp. 2. exp Cognitive Impairment/ 3. MCI.ti,ab. 4. ACMI.ti,ab. 5. ARCD.ti,ab. 6. SMC.ti,ab. 7. CIND.ti,ab. 8. BSF.ti,ab. 9. AAMI.ti,ab. 10. MD.ti,ab. 11. LCD.ti,ab. 12. QD.ti,ab. 13. AACD.ti,ab. 14. MNCD.ti,ab. 15. MCD.ti,ab. 16. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 17. ((cognit* or memory or cerebr* or mental*) adj3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*)).ti,ab. 18. "preclinical AD".mp. 19. "pre‐clinical AD".mp. 20. ("preclinical alzheimer*" or "pre‐clinical alzheimer*").mp. 21. (aMCI or MCIa).ti,ab. 22. ("CDR 0.5" or "clinical dementia rating scale 0.5").ti,ab. 23. ("GDS 3" or "stage 3 GDS").ti,ab. 24. ("global deterioration scale" and "stage 3").mp. 25. "Benign senescent forgetfulness".ti,ab. 26. "mild neurocognit* disorder*".ti,ab. 27. (prodrom* adj2 dement*).ti,ab. 28. "age‐related symptom*".mp. 29. (episodic adj2 memory).mp. 30. ("pre‐clinical dementia" or "preclinical dementia").mp. 31. (healthy adj2 (elderly or aged or old* or senior)).mp. 32. "community dwelling".mp. 33. "independent living".mp. 34. (adult* adj2 (old or elderly or aged)).mp. 35. ("healthy participants" or "healthy persons").mp. 36. or/1‐35 37. exp Carbohydrates/ 38. (carbs or carbohydrate*).ti,ab. 39. exp *Sugars/ 40. (monosaccharide* or disaccharide* or oligosaccharide* or polysaccharide*).ti,ab. 41. saccharide*.mp. 42. (sugar* or starch*).ti,ab. 43. or/37‐42 44. 36 and 43 45. exp Clinical Trials/ 46. random*.mp. 47. placebo.ti,ab. 48. trial.ab. 49. groups.ab. 50. "double‐blind*".mp. 51. or/45‐50 52. 44 and 51 |
June 2010: 90 April 2012: 23 |
| CINAHL (EBSCOhost) | S1 TX "cognit* impair*" S2 TX "cognit* defect*" S3 (MH "Cognition Disorders+") S4 TX MCI S5 TX ACMI S6 TX ARCD S7 TX SMC S8 TX CIND S9 TX BSF S10 TX AAMI S11 AB MD S12 AB LCD S13 AB QD OR "questionable dementia" S14 TX AACD S15 TX MNCD S16 TX "N‐MCI" or "A‐MCI" or "M‐MCI" S17 TX "preclinical AD" S18 TX "pre‐clinical AD" S19 TX "preclinical alzheimer*" or "pre‐clinical alzheimer*" S20 TX aMCI OR MCIa S21 TX "CDR 0.5" or "clinical dementia rating scale 0.5" S22 TX "GDS 3" OR "stage 3 GDS" S23 TX "global deterioration scale" AND "stage 3" S24 TX "Benign senescent forgetfulness" S25 TX "mild neurocognit* disorder*" S26 TX prodrom* N2 dement* S27 TX "age‐related symptom*" S28 TX cognit* N2 deficit* S29 TX cognit* N2 deteriorat* S30 TX cognit* N2 declin* S31 TX cognit* N2 degenerat* S32 TX cognit* N2 complain* S33 TX cognit* N2 disturb* S34 TX cognit* N2 disorder* S35 TX memory N2 episod* or TX memory N2 los* or TX memory N2 impair* or TX memory N2 complain* S36 TX memory N2 disturb* or TX memory N2 disorder* or TX cerebr* N2 impair* or TX cerebr* N2 los* S37 TX cerebr* N2 complain* or TX cerebr* N2 deteriorat* or TX cerebr* N2 disorder* or TX cerebr* N2 disturb* S38 TX mental* N2 declin* or TX mental* N2 los* or TX mental* N2 impair* or TX mental* N2 deteriorat* S39 TX "pre‐clinical dementia" or TX "preclinical dementia" S40 TX healthy N2 elderly S41 TX healthy N2 old* S42 TX healthy N2 aged S43 TX healthy N2 senior S44 TX adult N2 old* S45 TX adult N2 elderly S46 TX adult N2 aged S47 TX "community dwelling" S48 TX "independent living" S49 TX "healthy participants" OR "healthy persons" S50 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 S51 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 S52 TX carbs OR carbohydrate* S53 (MH "Monosaccharides") or (MH "Disaccharides") or (MH "Oligosaccharides") S54 TX monosaccharide* or disaccharide* or oligosaccharide* or polysaccharide* S55 TX saccharide* S56 TX sugar* or starch* S57 S51 or S52 or S53 or S54 or S55 or S56 S58 S50 and S57 S59 (MH "Clinical Trials+") S60 AB random* S61 AB trial S62 AB placebo S63 AB "control group" S64 AB "double‐blind*" S65 S59 or S60 or S61 or S62 or S63 or S64 S66 S58 and S65 |
June 2010: 132 April 2012: 0 |
| Web of Science with Conference Proceedings (1945 to present) (Web of Knowledge) | #1 Topic=(mci OR "mild cognitive impairment") Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S Timespan=All Years #2 Topic=(aami OR "age associated memory impairment") #3 Topic=("minor memory complaint*" OR aacd OR "age related memory impairment" OR "age associated cognitive decline") #4 Topic=("age related cognitive decline" OR "cognit* impair*") #5 Topic=("cind" OR "smc" OR "subjective memory complaint*") #6 Topic=("preclinical ad" OR "pre‐clinical ad" OR "preclinical alzheimer*" OR "pre‐clinical alzheimer*") #7 Topic=("episodic memory" OR "dementia prodrome" OR "incipient dementia") #8 Topic=("prevent* dement*" OR "prevent* alzheimer*") #9 Topic=((cognit* OR memory* OR cerebral OR "cognit* function*" OR "brain activity") NEAR (improv* OR enhanc* OR perform* OR process* OR "lessen deterioration")) #10 Topic=(carbohydrate* OR monosaccharide* OR disaccharide* OR oligosaccharide* OR polysaccharide* OR saccharide*) #11 Topic=("double‐blind*" OR random* OR "clinical trial" OR placebo) #12 #9 OR #8 OR #7 OR #6 OR #4 OR #3 OR #2 OR #1 #13 #12 AND #10 #14 #13 AND #11 |
June 2010: 135 April 2012: 37 |
| LILACS (BIREME) | (mci OR "mild cognitive impairment" OR "aami" OR "age associated memory impairment$" OR "minor memory complaint$" OR "aacd" OR "age related memory impairment" OR "age associated cognitive decline" OR "mc la" OR "cognit$ impair$" OR healthy OR elderly OR "cind" OR "smc" OR "subjective memory complaint$" OR "preclinical ad" OR "pre‐clinical ad" OR "preclinical alzheimer$" OR "pre‐clinical alzheimer$" OR ((cognit$ OR memory$ OR cerebral OR "cognit$ function$" OR "brain activity") AND (improv$ OR enhanc$ OR perform$ OR process$ OR "lessen deterioration"))) [Palavras] and (carbohydrate$ OR monosaccharide$ OR disaccharide$ OR oligosaccharide$ OR polysaccharide$ OR sugar$ OR starch$) | June 2010: 59 |
| CENTRAL (The Cochrane Library) | #1 "cognit* impair*" #2 MeSH descriptor Cognition Disorders explode all trees #3 MCI #4 ACMI #5 ARCD #6 SMC #7 CIND #8 BSF #9 AAMI #10 LCD #11 QD OR "questionable dementia" #12 AACD #13 MNCD #14 MCD #15 "N‐MCI" or "A‐MCI" or "M‐MCI" #16 (cognit* or memory or cerebr* or mental*) NEAR/3 (declin* or impair* or los* or deteriorat* or degenerat* or complain* or disturb* or disorder*) #17 "preclinical AD" #18 "pre‐clinical AD" #19 "preclinical alzheimer*" or "pre‐clinical alzheimer*" #20 aMCI OR MCIa #21 "CDR 0.5" OR "clinical dementia rating scale 0.5" #22 "GDS 3" OR "stage 3 GDS" #23 "global deterioration scale" AND "stage 3" #24 "Benign senescent forgetfulness" #25 "mild neurocognit* disorder*" #26 (prodrom* NEAR/2 dement*) #27 episodic* NEAR/2 memory #28 "preclinical dementia" OR "pre‐clinical dementia" #29 episodic NEAR/2 memory #30 "pre‐clinical dementia" OR "preclinical dementia" #31 (healthy NEAR/2 (elderly or aged or old* or senior)) #32 "community dwelling" #33 "independent living" #34 "healthy participants" OR "healthy persons" #35 MeSH descriptor Carbohydrates explode all trees #36 carbs or carbohydrate* #37 MeSH descriptor Monosaccharides, this term only #38 MeSH descriptor Disaccharides, this term only #39 MeSH descriptor Oligosaccharides, this term only #40 MeSH descriptor Polysaccharides explode all trees #41 monosaccharide* OR disaccharide* OR oligosaccharide* OR polysaccharide* #42 saccharide* #43 sugar* OR starch* #44 (#35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43) #45 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34) #46 (#45 AND #44), from 2008 to 2010 |
June 2010: 102 April 2012: 104 |
| Clinicaltrials.gov | Interventional Studies | cognitive OR cognition OR elderly OR aged OR memory | carbohydrates OR Monosaccharides OR Disaccharides OR Oligosaccharides OR Polysaccharides | received from 01/01/2008 to 06/22/2010 | June 2010: 37 April 2012: 53 |
| ICTRP Search Portal (WHO) | Advanced search: Interventional Studies | cognitive OR cognition OR elderly OR aged OR memory | carbohydrates OR Monosaccharides OR Disaccharides OR Oligosaccharides OR Polysaccharides | received from 01/01/2008 to 22/06/2010 | June 2010: 22 April 2012: 21 |
| Total | June 2010: 1469 April 2012: 1343 |
|
| Total after first‐assess | June 2010: 40 April 2012: 25 |
|
Appendix 2. Search May 2008
| Source searched | Search strategy | Hits after first assess |
| MEDLINE (OvidSP) | 1. (mci or "mild$ cognit$ impair$").mp. 2. ("aa mi" or "age‐associated memory impairment").mp. 3. "minor memory complaint$".mp. 4. ("aa cd" or "age related memory impairment" or "age‐associated cognitive decline").mp. 5. ("age related" adj3 "cognitive decline").mp. [mp=title, original title, abstract, name of substance word, subject heading word] 6. ("mc la" or "cognit$ impair$").mp. 7. ("ci nd" or "smc" or "subjective memory complaint$").mp. 8. ("preclinical ad" or "pre‐clinical ad" or "preclinical alzheimer$" or "pre‐clinical alzheimer$").mp. 9. "episodic memory".mp. 10. "dementia prodrome".mp. 11. "incipient dementia".mp. 12. ("prevent$ dement$" or "prevent$ alzheimer$").mp. 13. ((cognit$ or memory$ or cerebral or "cognit$ function$" or "brain activity") adj4 (improv$ or enhanc$ or perform$ or process$ or "lessen deterioration")).mp. 14. alzheimer disease/pc or dementia/pc 15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 16. Carbohydrates/ or carbohydrate$.mp. 17. Monosaccharides/ or monosaccharide$.mp. 18. disaccharide$.mp. or Disaccharides/ 19. oligosaccharide$.mp. or Oligosaccharides/ 20. Polysaccharides/ or polysaccharide$.mp. 21. 16 or 17 or 18 or 19 or 20 22. 15 and 21 23. randomized controlled trial.pt. 24. controlled clinical trial.pt. 25. randomized.ab. 26. placebo.ab. 27. drug therapy.fs. 28. randomly.ab. 29. trial.ab. 30. groups.ab. 31. 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 32. humans.sh. 33. 31 and 32 34. 22 and 33 | 48 |
| EMBASE (OvidSP) | 1. (mci or "mild$ cognit$ impair$").mp. 2. ("aa mi" or "age‐associated memory impairment").mp. 3. "minor memory complaint$".mp. 4. ("aa cd" or "age related memory impairment" or "age‐associated cognitive decline").mp. 5. ("age related" adj3 "cognitive decline").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. ("mc la" or "cognit$ impair$").mp. 7. ("ci nd" or "smc" or "subjective memory complaint$").mp. 8. ("preclinical ad" or "pre‐clinical ad" or "preclinical alzheimer$" or "pre‐clinical alzheimer$").mp. 9. "episodic memory".mp. 10. "dementia prodrome".mp. 11. "incipient dementia".mp. 12. ("prevent$ dement$" or "prevent$ alzheimer$").mp. 13. ((cognit$ or memory$ or cerebral or "cognit$ function$" or "brain activity") adj4 (improv$ or enhanc$ or perform$ or process$ or "lessen deterioration")).mp. 14. alzheimer disease/pc or dementia/pc 15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 16. Carbohydrates/ or carbohydrate$.mp. 17. Monosaccharides/ or monosaccharide$.mp. 18. disaccharide$.mp. or Disaccharides/ 19. oligosaccharide$.mp. or Oligosaccharides/ 20. Polysaccharides/ or polysaccharide$.mp. 21. 16 or 17 or 18 or 19 or 20 22. 15 and 21 23. (random$ or factorial$).mp. 24. (cross over$ or crossover$).mp. 25. placebo$.mp. 26. (doubl$ adj5 blind$).mp. 27. (singl$ adj5 blind$).mp. 28. (assign$ or allocat$ or volunteer$).mp. 29. crossover‐procedure/ or dounble‐blind procedure/ or single‐blind procedure/ 30. randomized controlled trial/ 31. 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 32. 22 and 31 | 38 |
| PsycINFO (OvidSP) | 1. (mci or "mild$ cognit$ impair$").mp. 2. ("aa mi" or "age‐associated memory impairment").mp. 3. "minor memory complaint$".mp. 4. ("aa cd" or "age related memory impairment" or "age‐associated cognitive decline").mp. 5. ("age related" adj3 "cognitive decline").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. ("mc la" or "cognit$ impair$").mp. 7. ("ci nd" or "smc" or "subjective memory complaint$").mp. 8. ("preclinical ad" or "pre‐clinical ad" or "preclinical alzheimer$" or "pre‐clinical alzheimer$").mp. 9. "episodic memory".mp. 10. "dementia prodrome".mp. 11. "incipient dementia".mp. 12. ("prevent$ dement$" or "prevent$ alzheimer$").mp. 13. ((cognit$ or memory$ or cerebral or "cognit$ function$" or "brain activity") adj4 (improv$ or enhanc$ or perform$ or process$ or "lessen deterioration")).mp. 14. alzheimer disease/pc or dementia/pc 15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 16. Carbohydrates/ or carbohydrate$.mp. 17. Monosaccharides/ or monosaccharide$.mp. 18. disaccharide$.mp. or Disaccharides/ 19. oligosaccharide$.mp. or Oligosaccharides/ 20. Polysaccharides/ or polysaccharide$.mp. 21. 16 or 17 or 18 or 19 or 20 22. 15 and 21 23. random$.tw. 24. factorial$.tw. 25. crossover$.tw. 26. cross over$.tw. 27. placebo$.tw. 28. (doubl$ adj blind$).tw. 29. (sing$ adj blind$).tw. 30. Clinical Trials/ 31. 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 32. 31 and 22 | 13 |
| CINAHL (OvidSP) | 1. (mci or "mild$ cognit$ impair$").mp. 2. ("aa mi" or "age‐associated memory impairment").mp. 3. "minor memory complaint$".mp. 4. ("aa cd" or "age related memory impairment" or "age‐associated cognitive decline").mp. 5. ("age related" adj3 "cognitive decline").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. ("mc la" or "cognit$ impair$").mp. 7. ("ci nd" or "smc" or "subjective memory complaint$").mp. 8. ("preclinical ad" or "pre‐clinical ad" or "preclinical alzheimer$" or "pre‐clinical alzheimer$").mp. 9. "episodic memory".mp. 10. "dementia prodrome".mp. 11. "incipient dementia".mp. 12. ("prevent$ dement$" or "prevent$ alzheimer$").mp. 13. ((cognit$ or memory$ or cerebral or "cognit$ function$" or "brain activity") adj4 (improv$ or enhanc$ or perform$ or process$ or "lessen deterioration")).mp. 14. alzheimer disease/pc or dementia/pc 15. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 16. Carbohydrates/ or carbohydrate$.mp. 17. Monosaccharides/ or monosaccharide$.mp. 18. disaccharide$.mp. or Disaccharides/ 19. oligosaccharide$.mp. or Oligosaccharides/ 20. Polysaccharides/ or polysaccharide$.mp. 21. 16 or 17 or 18 or 19 or 20 22. 15 and 21 23. random$.tw. 24. factorial$.tw. 25. crossover$.tw. 26. cross over$.tw. 27. placebo$.tw. 28. (doubl$ adj blind$).tw. 29. (sing$ adj blind$).tw. 30. Clinical Trials/ 31. 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 32. 31 and 22 | 4 |
| LILACS (Bireme) | (mci OR "mild cognitive impairment" OR "aa mi" OR "age associated memory impairment$" OR "minor memory complaint$" OR "aa cd" OR "age related memory impairment" OR "age associated cognitive decline" OR "mc la" OR "cognit$ impair$" OR "ci nd" OR "smc" OR "subjective memory complaint$" OR "preclinical ad" OR "pre‐clinical ad" OR "preclinical alzheimer$" OR "pre‐clinical alzheimer$" OR ((cognit$ OR memory$ OR cerebral OR "cognit$ function$" OR "brain activity") AND (improv$ OR enhanc$ OR perform$ OR process$ OR "lessen deterioration"))) [Palavras] and (carbohydrate$ OR monosaccharide$ OR disaccharide$ OR oligosaccharide$ OR polysaccharide$) | 3 |
| The Cochrane Library | #1. mci OR "mild cognitive impairment" #2. "aa mi" OR "age‐associated memory impairment" #3. "aa cd" OR "age‐related memory impairment" OR "age‐associated cognitive decline" OR "age‐related cognitive decline" #4. "mc la" OR "cognit* impair*" #5. "ci nd" OR "smc" OR "subjective memory complaint*" #6. "preclinical ad" OR "pre‐clinical ad" OR "preclinical alzheimer*" OR "pre‐clinical alzheimer*" #7. "episodic memory" OR "dementia prodrome" OR "incipient dementia" #8. "prevent* dement*" OR "prevent* alzheimer*" #9. ((cognit* OR memory* OR cerebral OR "cognit* function*" OR "brain activity") NEAR (improv* OR enhanc* OR perform* OR process* OR "lessen deterioration")) #10. Carbohydrates (MeSH) #11. Monosaccharides (MeSH) #12. Disaccharides (MeSH) #13. Oligosaccharides (MeSH) #14. Polysaccharides (MeSH) #15. Carbohydrate* (Search all Text) #16. Monosaccharides (Search all Text) #17. Disaccharides (Search all Text) #18. Oligosaccharides (Search all Text) #19. Polysaccharides (Search all Text) #20. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 #21. #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 #22. #20 AND #21 |
27 |
| CDCIG Specialized Register | carbohydrates OR monosaccharides OR disaccharides OR oligosaccharides OR polysaccharides | 7 |
| ISI Conference Proceedings | #1. Topic=(mci OR "mild cognitive impairment") #2. Topic=("aa mi" OR "age associated memory impairment") #3. Topic=("minor memory complaint*" OR "aa cd" OR "age related memory impairment" OR "age associated cognitive decline") #4. Topic=("age related cognitive decline" OR "mc la" OR "cognit* impair*") #5. Topic=("ci nd" OR "smc" OR "subjective memory complaints") #6. Topic=("preclinical ad" OR "pre‐clinical ad" OR "preclinical alzheimer*" OR "pre‐clinical alzheimer*") #7. Topic=("episodic memory" OR "dementia prodrome" OR "incipient dementia") #8. Topic=("prevent* dement*" OR "prevent* alzheimer*") #9. Topic=(((cognit* OR memory* OR cerebral OR "cognit* function*" OR "brain activity") NEAR (improv* OR enhanc* OR perform* OR process* OR "lessen deterioration"))) #10. Topic=(carbohydrate* OR monosaccharide* OR disaccharide* OR oligosaccharide* OR polysaccharide*) #11. Topic=("randomized controlled trial" OR "randomised controlled trial" OR "clinical trial") #12. #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #13. #12 AND #11 AND #10 |
136 |
| ClinicalTrials.gov | (carbohydrates OR monosaccharides OR disaccharides OR oligosaccharides OR polysaccharides) AND (cognition OR "mild cognitive impairment" OR "age‐associated memory impairment" OR "age‐related memory impairment" OR "age‐associated cognitive decline" OR "age‐related cognitive decline" OR "cognitive impairment not dementia" OR "subjective memory complaints" OR "preclinical AD" OR "pre‐clinical AD" OR "preclinical Alzheimer" OR "episodic memory" OR "dementia prodrome" OR "incipient dementia") | 1 |
| metaRegister of Controlled Trials: ISRCTN Register Action Medical Research Medical Research Council (UK) HTA NIH The Wellcome Trust UK Clinical Trials Gateway |
(carbohydrate% OR monosaccharides OR disaccharides OR oligosaccharides OR polysaccharides) AND (cognit% OR "mild cognitive impairment" OR "age‐associated memory impairment" OR "age‐related memory impairment" OR "age‐associated cognitive decline") | 168 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gagnon 2010.
| Methods | This randomised controlled trial (RCT) was of between‐subjects design. The methods of randomisation and allocation concealment were not mentioned in the article. The authors had not responded to our query on this matter. Both the participants and investigators were blind to the nature of the drink. Intention‐to‐treat analysis was not mentioned. | |
| Participants | 40 healthy community dwelling adults of both genders aged between 60 and 80 years. Inclusion criteria: no diabetes as well as medical conditions that could affect cognition and bias data interpretation (e.g., absence of general anaesthesia in the past 6 months, absence of neurological disease, stroke, etc). Exclusion criteria: MMSE (Folstein 1975) less than 27, diabetes, fasting blood glucose levels equal to or greater than 7.0 mmol/L, a history of any medical conditions that could affect cognition (e.g., general anaesthesia in the past 6 months, neurological disease, stroke, etc), unable to complete tasks due to auditory or visual impairments. |
|
| Interventions | Each participant received a glucose drink (50 g of glucose mixed with 290 mL of water and 10 mL of lemon juice (Xenex Labs©)) or a placebo drink (23.7 mg of saccharin, 290 mL of water, and 10 mL of lemon juice (Hermesetas©)).
Intervention: a glucose drink consisting 50 g glucose mixed with 290 mL of water and 10 mL of lemon juice (Xenex Labs©)
Control: a placebo drink consisting of 23.7 mg of saccharin, 290 mL of water, and 10 mL of lemon juice (Hermesetas©). Participants fasted for 10‐12 hours prior to the intervention. |
|
| Outcomes | Blood glucose level following drink ingestion at 0, 15, 30, 45 and 90 minutes; attentional functions: neuropsychological tests (trail A and B, modified Stroop) and a computerized dual‐task. | |
| Stated aims of study | To determine the overall acute effects of glucose ingestion on different forms of attention and attentional control mechanisms in fasting healthy older adults. | |
| Notes | Original research article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although the author confirmed the presence of random sequence generation, further information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Although the author confirmed the presence of allocation concealment, further information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although the author confirmed the presence of blinding, further information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although the author confirmed the presence of blinding, further information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were either incomplete or missing from the following groups.
There was no information on how these missing data were managed. |
| Selective reporting (reporting bias) | Low risk | Nothing detected. |
| Other bias | Low risk | Nothing detected. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbatecola 2009 | Although this was a randomised controlled trial two pharmacological agents, repaglinide and glibenclamide, were the interventions used. |
| ACTRN12610000624088 2010 | The ages of the participants ranged from 45 to 60 years. There is no specific data on participants aged 55 or over for meeting the criteria for inclusion in the systematic review. |
| Amagase 2009 | Although this was a randomised controlled trial, the end‐points were antioxidant biomarkers with no cognitive measures. |
| Ban 1991 | Although this was a randomised controlled trial, the participants had dementia. |
| Benton 2003 | Although this was a randomised controlled trial, the participants were young adults and there was no placebo control. |
| Benton 2004 | Although this was a randomised controlled trial, the participants were young adults. |
| Bergenstal 2008 | Although this was a randomised controlled trial two pharmacological agents, glulisine and glargine, were the interventions used. There was no outcome measure of cognition. |
| Best 2008 | Although this was a randomised controlled trial, the mean age of the study sample (N=45) was below 55 (mean age 52.1 ± 5.86 years). |
| Best 2010 | Although this was a randomised controlled trial, the mean age of the study sample (N=109) was below 55. Mean ages for the placebo and treatment groups were 52.44 ± 4.16 and 53.36 ± 4.58 years respectively. |
| Block 2008 | Although this was a randomised controlled trial, behaviour changes pertaining to life‐style were the outcome measures and not cognition. |
| Brandt 2010 | The participants were young adults. |
| Brinkworth 2009 | Although this was a randomised controlled trial, the mean age of the study sample (N=106) was below 55 (mean age 50.0 0.8 years). |
| Cheatham 2009 | Although this was a randomised controlled trial, the participants were young adults. |
| Conti 1989 | There was no randomisation and the particpants had dementia. There was also no placebo control. |
| Corsica 2002 | Although this was a randomised controlled trial, the participants were young females of age between 20‐45 years. |
| Craft 1994 | There was no randomised allocation sequence. |
| Cukierman‐Yaffe 2009 | Although this was a randomised controlled trial, only the relationship between glycaemic control and cognition was analysed. |
| DeFrance 1997 | There was no randomisation and the participants were young adults. The intervention was amino acids. |
| Finnigan 1998 | The participants were young adults. There is inadequate information of randomised allocation of participants. |
| Fischer 2001 | There was no randomisation and the participants were young adults. |
| Fischer 2002 | There was no randomisation and the participants were young adults. |
| Ford 2002 | Although this was a randomised controlled trial, the participants were young adults. |
| Gariballa 2006 | Although this was a randomised controlled trial and of appropriate age, the participants were hospitalised and acutely ill. |
| Garriballa 2007 | Although this was a randomised controlled trial and of appropriate age, the participants were hospitalised and acutely ill. |
| Halyburton 2007 | Although this was a randomised controlled trial, the mean age of the study sample was below 55 years. |
| Henderson 2006 | Although this was a randomised controlled trial, the was no placebo or non‐intervention control. |
| Kaplan 2000 | There was no randomisation. |
| Kaplan 2001 | There was no randomisation. |
| Kennedy 2004 | Although this was a randomised controlled trial, the participants were young adults. |
| Krikorian 2012 | The study assessed the potential cognitive benefit of dietary ketosis in older adults with mild memory decline. |
| Lee 2001 | This was not a randomised controlled trial, with no control or non‐intervention group. |
| Levin 2009 | Although this was a randomised controlled trial, the intervention was choline alphoscerate (cereton), a membrane phospholipid. |
| Lloyd 1994 | Although this was a randomised controlled trial, the participants were young adults. |
| Malacco 1992 | The intervention was dihydroergocristine. |
| Maridakis 2009a | Although this was a randomised controlled trial, the participants were young adults. |
| Maridakis 2009b | Although this was a randomised controlled trial, the participants were young adults. |
| Markus 1999 | Although this was a randomised controlled trial, the participants were young adults and there was no placebo or non‐interventional control. |
| Markus 2002 | Although this was a randomised controlled trial, the participants were young adults. In addition, the intervention was alpha‐lactalbumin, instead of carbohydrates. |
| Markus 2006 | Although this was a randomised controlled trial, the intervention was dietary tryptophan, instead of carbohydrates. |
| Markus 2007 | Although this was a randomised controlled trial, the participants were young adults. |
| Markus 2008 | Although this was a randomised controlled trial, the participants were young adults. In addition, the interventions were hydrolyzed protein and alpha‐lactalbumin whey protein, instead of carbohydrates. |
| Meikle 2004 | Although this was a randomised controlled trial, the participants were young adults. |
| Messier 2010 | There was no randomisation. |
| Mischoulon 2010 | Although this was a randomised controlled trial, the participants had seasonal affective disorder. There were no exclusion criteria for mild cognitive impairment and dementia as well as no cognitive outcome measures. |
| Morgan 2009 | Although this was a randomised controlled trial, the participants were young adults. |
| Nabb 2006 | Although this was a randomised controlled trial, the participants were young adults and there was no placebo or non‐interventional control. |
| NCT00980408 | Although this is a randomised ongoing controlled trial, the participants are young adults. |
| Nilsson 2009 | There was inadequate information on randomised allocation of participants in the counterbalanced design. |
| Pradignac 1995 | This was not a randomised controlled trial, with no control or non‐intervention group. |
| Reger 2004 | Although this was a randomised controlled trial, the intervention was a medium chain triglyceride. |
| Riby 2004 | Counterbalanced design but no evidence of randomisation. |
| Riby 2006 | Counterbalanced design but no evidence of randomisation. |
| Riby 2008 | There was inadequate information on randomised allocation of participants in the counterbalanced design. The participants were younger than specified age. |
| Riby 2009 | Counterbalanced design but no evidence of randomisation. |
| Rohleder 2009 | Although this was a randomised controlled trial, the participants were young adults. The intervention was oral cortisol and not carbohydrates. |
| Salinsky 2005 | Although this was a randomised controlled trial, the participants were young adults. |
| Sayegh 1995 | There was no randomisation and the participants were young adults. |
| Scholey 2006 | The participants were young adults. |
| Scholey 2009 | Although this was a randomised controlled trial, the participants were young adults. |
| Smit 2004 | There was no randomisation and the participants were young adults. |
| Smith 2009 | There was randomised allocation to groups but the participants were young adults. |
| Vamosi 1976 | There was no randomisation and no control or non‐intervention group. |
| Verger 1998 | There was no randomisation and the intervention consisted of protein. |
| Wang 2004 | Although this was a randomised controlled trial, the participants were young adults. |
| Wells 1998 | Although this was a randomised controlled trial, the participants were young adults. The outcome measures were mood, energy expenditure, and heart rate. |
| Winder 1998 | Although this was a randomised controlled trial, the participants were 55 years or less. |
| Wing 1995 | Although this was a randomised controlled trial, the participants were young adults. |
| Young 2004 | Although this was a randomised controlled trial, the participants were from an institution and had probable Alzheimer's dementia. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12606000475549.
| Trial name or title | High Protein Diets, Weight Loss and Diabetes Study 2007 Or A 12 week randomised parallel trial comparing 2 different high protein weight loss diets on blood cholesterol levels, blood sugar control and on kidney and cognitive function in Type 2 Diabetes and Obesity: http://www.anzctr.org.au/ACTRN12606000475549.aspx |
| Methods | Randomised controlled trial, non‐blinding |
| Participants | 80 participants with type 2 diabetes mellitus, obesity |
| Interventions | Two different high protein weight loss diets (12 week dietary intervention of daily high protein 34%, low dietary cholesterol 230mg, fat 26%, carbohydrate 37% diet for 3 meals per day) |
| Outcomes | Primary outcome measures: low density lipoprotein (LDL); high density lipoprotein (HDL); total cholesterol and triglycerides; fasting ApoB; glucose tolerance test (GTT) (0, 2hours); post‐prandial glucose using CGMS (36hr); HbA1C. Secondary outcome measures: nutrient intakes before and during weight loss; plasma folate, homocysteine; cognitive function; satiety profiles; weight loss (including lean and fat loss as assessed by bioelectrical impedance); renal function (urinary urea, plasma creatinine and microalbuminuria); plasma lutein zeaxanthein and other carotenoids. |
| Starting date | February 2007 |
| Contact information | http://www.anzctr.org.au/ACTRN12606000475549.aspx . 2006 |
| Notes | ongoing |
NCT01427231 2011.
| Trial name or title | Short‐term Effect of Glucose and Sacharose Ingestion on Cognitive Performance and Mood in Elderly |
| Methods | Interventional randomised double‐blind crossover controlled trial |
| Participants | Healthy adults, male and female 70 years and over |
| Interventions | Interventions: glucose drink or saccharose drink Control: placebo with sweetener |
| Outcomes | Primary outcome measures: Performance on the paired associate recall test Secondary outcome measures:
|
| Starting date | August 2011 |
| Contact information | NCT01427231 |
| Notes | completed but no publication or data available http://clinicaltrials.gov/ct2/show/NCT01427231 |
Differences between protocol and review
Our approach to data collection and extraction was revised based on the methodology in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.0.2).
Contributions of authors
CPO: drafting of review, search for trials, obtaining copies of trial reports, selection of trials for inclusion or exclusion, extraction of data, entry of data (into RevMan), interpretation of data analyses
ZY: selection of trials for inclusion or exclusion, extraction of data, entry of data (into RevMan)
HT‐A: searching for trials, interpretation of data analysis
LSC: searching for trials, obtaining copies of trial reports, selection of included trials and exclusion of trials, extraction of data, interpretation of data analysis
Sources of support
Internal sources
Institute of Gerontology, University Putra Malaysia, Malaysia.
External sources
No sources of support supplied
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Gagnon 2010 {published data only}
References to studies excluded from this review
Abbatecola 2009 {published data only}
- Abbatecola AM, Rizzo MR, Barbieri M, et al. Postprandial plasma glucose excursions and cognitive functioning in aged type 2 diabetics. Neurology 2006;67(2):235‐40. [DOI] [PubMed] [Google Scholar]
ACTRN12610000624088 2010 {published data only}
- ACTRN12610000624088. Effects of saccharides on high demand cognitive performance and psychological well‐being in healthy, middle‐aged Australian adults between 45 and 60 years of age. ANZCTR: http://www.anzctr.org.au/ACTRN12610000624088.aspx 2010.
Amagase 2009 {published data only}
- Amagase H, Sun B, Borek C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutrition Research 2009;29(1):19‐25. [DOI] [PubMed] [Google Scholar]
Ban 1991 {published data only}
- Ban TA, Morey LC, Aguglia E, et al. Glycosaminoglycan polysulfate in the treatment of old age dementias. Progress in Neuro‐psychopharmacology & Biological Psychiatry 1991;15(3):323‐42. [DOI] [PubMed] [Google Scholar]
Benton 2003 {published data only}
- Benton D, Ruffin MP, Lassel T, et al. The delivery rate of dietary carbohydrates affects cognitive performance in both rats and humans. Psychopharmacology (Berl) 2003;166(1):86‐90. [DOI] [PubMed] [Google Scholar]
Benton 2004 {published data only}
- Benton D, Donohoe RT. The influence on cognition of the interactions between lecithin, carnitine and carbohydrate. Psychopharmacology (Berl) 2004;175(1):84‐91. [DOI] [PubMed] [Google Scholar]
Bergenstal 2008 {published data only}
- Bergenstal RM, Johnson M, Powers MA, et al. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care 2008;31(7):1305‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Best 2008 {published data only}
- Best T, Bryan J, Burns N. An investigation of the effects of saccharides on the memory performance of middle‐aged adults. Journal of Nutrition, Health & Aging 2008;12(9):657‐62. [DOI] [PubMed] [Google Scholar]
Best 2010 {published data only}
- Best T, Kemps E, Bryan J. Saccharide effects on cognition and well‐being in middle‐aged adults: a randomized controlled trial. Developmental Neuropsychology 2010;35(1):66‐80. [DOI] [PubMed] [Google Scholar]
Block 2008 {published data only}
- Block G, Sternfeld B, Block CH, et al. Development of Alive! (A Lifestyle Intervention Via Email), and its effect on health‐related quality of life, presenteeism, and other behavioral outcomes: randomized controlled trial. Journal of Medical Internet Research 2008;10(4):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandt 2010 {published data only}
- Brandt KR, Sunram‐Lea SI, Jenkinson PM, et al. The effects of glucose dose and dual‐task performance on memory for emotional material. Behavioural Brain Research 2010;211(1):83‐8. [DOI] [PubMed] [Google Scholar]
Brinkworth 2009 {published data only}
- Brinkworth GD, Buckley JD, Noakes M, et al. Long‐term effects of a very low‐carbohydrate diet and a low‐fat diet on mood and cognitive function. Archives of Internal Medicine 2009;169(20):1873‐80. [DOI] [PubMed] [Google Scholar]
Cheatham 2009 {published data only}
- Cheatham RA, Roberts SB, Das SK, et al. Long‐term effects of provided low and high glycemic load low energy diets on mood and cognition. Physiology and Behavior 2009;98(3):374‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conti 1989 {published data only}
- Conti L, Re F, Lazzerini F, et al. Glycosaminoglycan polysulfate (Ateroid) in old‐age dementias: effects upon depressive symptomatology in geriatric patients. Progress in Neuro‐psychopharmacology & Biological Psychiatry 1989;13(6):977‐81. [DOI] [PubMed] [Google Scholar]
Corsica 2002 {published data only}
- Corsica JA. Carbohydrate self‐administration, mood, and cognition in carbohydrate cravers. United States ‐‐ Illinois: Dissertation Abstracts International: Section B: The Sciences and Engineering, The Herman M. Finch University of Health Sciences ‐ The Chicago Medical School 2002.
Craft 1994 {published data only}
- Craft S, Murphy C, Wemstrom J. Glucose effects on complex memory and nonmemory tasks: The influence of age, sex, and glucoregulatory response. Psychobiology 1994;22(2):95‐105. [Google Scholar]
Cukierman‐Yaffe 2009 {published data only}
- Cukierman‐Yaffe T, Gerstein HC, Williamson JD, et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes‐memory in diabetes (ACCORD‐MIND) trial. Diabetes Care 2009;32(2):221‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeFrance 1997 {published data only}
- DeFrance JF, Hymel C, Trachtenberg MC, et al. Enhancement of attention processing by Kantroll in healthy humans: a pilot study. Clinical EEG (Electroencephalography ) 1997;28(2):68‐75. [DOI] [PubMed] [Google Scholar]
Finnigan 1998 {published data only}
- Finnigan F, Hammersley R, Millar K. Effects of meal composition on blood alcohol level, psychomotor performance and subjective state after ingestion of alcohol. Appetite 1998;31(3):361‐75. [DOI] [PubMed] [Google Scholar]
Fischer 2001 {published data only}
- Fischer K, Colombani PC, Langhans W, et al. Cognitive performance and its relationship with postprandial metabolic changes after ingestion of different macronutrients in the morning. British Journal of Nutrition 2001;85(3):393‐405. [DOI] [PubMed] [Google Scholar]
Fischer 2002 {published data only}
- Fischer K, Colombani PC, Langhans W, Wenk C. Carbohydrate to protein ratio in food and cognitive performance in the morning. Physiology and Behavior 2002;75(3):411‐23. [DOI] [PubMed] [Google Scholar]
Ford 2002 {published data only}
- Ford CE, Scholey AB, Ayre G, et al. The effect of glucose administration and the emotional content of words on heart rate and memory. Journal of Psychopharmacology 2002;16(3):241‐4. [DOI] [PubMed] [Google Scholar]
Gariballa 2006 {published data only}
- Gariballa S, Forster S, Walters S, et al. A randomized, double‐blind, placebo‐controlled trial of nutritional supplementation during acute illness. American Journal of Medicine 2006;119(8):693‐9. [DOI] [PubMed] [Google Scholar]
Garriballa 2007 {published data only}
- Gariballa S, Forster S. Dietary supplementation and quality of life of older patients: a randomised, double‐blind, placebo‐controlled trial. Journal of the American Geriatric Society 2007;55(12):2030‐4. [DOI] [PubMed] [Google Scholar]
Halyburton 2007 {published data only}
- Halyburton AK, Brinkworth GD, Wilson CJ, et al. Low‐ and high‐carbohydrate weight‐loss diets have similar effects on mood but not cognitive performance. American Journal of Clinical Nutrition 2007;86(3):580‐7. [DOI] [PubMed] [Google Scholar]
Henderson 2006 {published data only}
- Henderson S. A double‐blind, placebo‐controlled study of Ketasyn (AC‐1202) administered for ninety days in subjects with age‐associated memory impairment. ClinicalTrials.gov 10th October 2008. [Identifier:NCT00355550]
Kaplan 2000 {published data only}
- Kaplan RJ, Greenwood CE, et al. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. American Journal of Clinical Nutrition 2000;72(3):825‐36. [DOI] [PubMed] [Google Scholar]
Kaplan 2001 {published data only}
- Kaplan RJ, Greenwood CE, et al. Dietary protein, carbohydrate, and fat enhance memory performance in the healthy elderly. American Journal of Clinical Nutrition 2001;74(5):687‐93. [DOI] [PubMed] [Google Scholar]
Kennedy 2004 {published data only}
- Kennedy DO, Scholey AB. A glucose‐caffeine 'energy drink' ameliorates subjective and performance deficits during prolonged cognitive demand. Appetite 2004;42(3):331‐3. [DOI] [PubMed] [Google Scholar]
Krikorian 2012 {published data only}
- Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiology of Aging 2012; Vol. 33, issue 2:425.e19‐27. [DOI] [PMC free article] [PubMed]
Lee 2001 {published data only}
- Lee L, Kang SA, Lee HO, et al. Relationships between dietary intake and cognitive function level in Korean elderly people. Public Health 2001;115(2):133‐8. [DOI] [PubMed] [Google Scholar]
Levin 2009 {published data only}
- Levin OS, Batukaeva LA, Anikina MA, et al. Efficacy and tolerability of choline alphoscerate (cereton) in patients with Parkinson's disease with cognitive disorders. Zhurnal Nevrologii i Psikhiatrii Imeni S. S. Korsakova 2009;109(11):42‐6. [PubMed] [Google Scholar]
Lloyd 1994 {published data only}
- Lloyd HM, Green MW, Rogers PJ. Mood and cognitive performance effects of isocaloric lunches differing in fat and carbohydrate content. Physiology & Behavior 1994;56(1):51‐7. [DOI] [PubMed] [Google Scholar]
Malacco 1992 {published data only}
- Malacco E, Cesare F. Effects of dihydroergocristine treatment on carbohydrate tolerance and cognitive function in patients with non‐insulin‐dependent diabetes. Current Therapeutic Research 1992;51(4):515‐23. [Google Scholar]
Maridakis 2009a {published data only}
- Maridakis V, Herring MP, O'Connor PJ. Sensitivity to change in cognitive performance and mood measures of energy and fatigue in response to differing doses of caffeine or breakfast. International Journal of Neuroscience 2009;119(7):975‐94. [DOI] [PubMed] [Google Scholar]
Maridakis 2009b {published data only}
- Maridakis V, O'Connor PJ, Tomporowski PD. Sensitivity to change in cognitive performance and mood measures of energy and fatigue in response to morning caffeine alone or in combination with carbohydrate. International Journal of Neuroscience 2009;119(8):1239‐58. [DOI] [PubMed] [Google Scholar]
Markus 1999 {published data only}
- Markus CR, Panhuysen G, et al. Carbohydrate intake improves cognitive performance of stress‐prone individuals under controllable laboratory stress. British Journal of Nutrition 1999;82(6):457‐67. [PubMed] [Google Scholar]
Markus 2002 {published data only}
- Markus CR, Olivier B, Haan EH. Whey protein rich in alpha‐lactalbumin increases the ratio of plasma tryptophan to the sum of the other large neutral amino acids and improves cognitive performance in stress‐vulnerable subjects. American Journal of Clinical Nutrition 2002;75(6):1051‐6. [DOI] [PubMed] [Google Scholar]
Markus 2006 {published data only}
- Markus CR, Jonkman LM, Lammers JH, et al. Evening dietary tryptophan improves post‐sleep behavioral and brain measures of memory function in healthy subjects. Current Topics in Nutraceutical Research 2006;4(2):79‐88. [Google Scholar]
Markus 2007 {published data only}
- Markus CR. Effects of carbohydrates on brain tryptophan availability and stress performance. Biological Psychology 2007;76(1‐2):83‐90. [DOI] [PubMed] [Google Scholar]
Markus 2008 {published data only}
- Markus CR, Firk C, Gerhardt C, et al. Effect of different tryptophan sources on amino acids availability to the brain and mood in healthy volunteers. Psychopharmacology (Berl) 2008;201(1):107‐14. [DOI] [PubMed] [Google Scholar]
Meikle 2004 {published data only}
- Meikle A, Riby LM, Stollery B. The impact of glucose ingestion and gluco‐regulatory control on cognitive performance: a comparison of younger and middle aged adults. Human Psychopharmacology 2004;19(8):523‐35. [DOI] [PubMed] [Google Scholar]
Messier 2010 {published data only}
- Messier C, Tsiakas M, Gagnon M, et al. Effect of age and glucoregulation on cognitive performance. Journal of Clinical and Experimental Neuropsychology 2010;32(8):809‐21. [DOI] [PubMed] [Google Scholar]
Mischoulon 2010 {published data only}
- Mischoulon D, Pedrelli P, Wurtman J, et al. Report of two double‐blind randomized placebo‐controlled pilot studies of a carbohydrate‐rich nutrient mixture for treatment of seasonal affective disorder (SAD). CNS Neuroscience & Therapeutics 2010;16(1):13‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2009 {published data only}
- Morgan CA, Hazlett G, Southwick S, et al. Effect of carbohydrate administration on recovery from stress‐induced deficits in cognitive function: a double‐blind, placebo‐controlled study of soldiers exposed to survival school stress. Military Medicine 2009;174(2):132‐8. [DOI] [PubMed] [Google Scholar]
Nabb 2006 {published data only}
- Nabb SL, Benton D. The effect of the interaction between glucose tolerance and breakfasts varying in carbohydrate and fibre on mood and cognition. Nutritional Neuroscience 2006;9(3‐4):161‐8. [DOI] [PubMed] [Google Scholar]
NCT00980408 {published data only}
- ClinicalTrials.gov identifier: NCT00980408. The Influence of Glutamate on Memory in Humans. University Hospital, Bonn 2009; Vol. September 18.
Nilsson 2009 {published data only}
- Nilsson A, Radeborg K, Bjorck I. Effects of differences in postprandial glycaemia on cognitive functions in healthy middle‐aged subjects. European Journal of Clinical Nutrition 2009;63(1):113‐20. [DOI] [PubMed] [Google Scholar]
Pradignac 1995 {published data only}
- Pradignac A, Schlienger JL, Velten M, et al. Relationships between macronutrient intake, handicaps, and cognitive impairments in free living elderly people. Aging (Milano) 1995;7(1):67‐74. [DOI] [PubMed] [Google Scholar]
Reger 2004 {published data only}
- Reger MA, Henderson ST, et al. Effects of beta‐hydroxybutyrate on cognition in memory‐impaired adults. Neurobiology of Aging 2004;25(3):311‐4. [DOI] [PubMed] [Google Scholar]
Riby 2004 {published data only}
- Riby LM, Meikle A, Glover C. The effects of age, glucose ingestion and gluco‐regulatory control on episodic memory. Age and Ageing 2004;33(5):483‐7. [DOI] [PubMed] [Google Scholar]
Riby 2006 {published data only}
- Riby LM, McMurtrie H, Smallwood J, Ballantyne C, Meikle A, Smith E. The facilitative effects of glucose ingestion on memory retrieval in younger and older adults: is task difficulty or task domain critical?. British Journal of Nutrition 2006;95(2):414‐20. [DOI] [PubMed] [Google Scholar]
Riby 2008 {published data only}
- Riby LM, McLaughlin J, Riby DM, et al. Lifestyle, glucose regulation and the cognitive effects of glucose load in middle‐aged adults. British Journal of Nutrition 2008;100(5):1128‐34. [DOI] [PubMed] [Google Scholar]
Riby 2009 {published data only}
- Riby LM, Marriott A, Bullock R, Hancock J, Smallwood J, McLaughlin J. The effects of glucose ingestion and glucose regulation on memory performance in older adults with mild cognitive impairment. European Journal of Clinical Nutrition 2009;63(4):566‐71. [DOI] [PubMed] [Google Scholar]
Rohleder 2009 {published data only}
- Rohleder N, Wolf JM, Kirschbaum C, et al. Effects of cortisol on emotional but not on neutral memory are correlated with peripheral glucocorticoid sensitivity of inflammatory cytokine production. International Journal of Psychophysiology 2009;72(1):74‐80. [DOI] [PubMed] [Google Scholar]
Salinsky 2005 {published data only}
- Salinsky MC, Storzbach D, Spencer DC, et al. Effects of topiramate and gabapentin on cognitive abilities in healthy volunteers. Neurology 2005;64(5):792‐8. [DOI] [PubMed] [Google Scholar]
Sayegh 1995 {published data only}
- Sayegh R, Schiff I, Wurtman J, et al. The effect of a carbohydrate‐rich beverage on mood, appetite, and cognitive function in women with premenstrual syndrome. Obstetrics and Gynecology 1995;86(4 Pt 1):520‐8. [DOI] [PubMed] [Google Scholar]
Scholey 2006 {published data only}
- Scholey AB, Laing S, Kennedy DO. Blood glucose changes and memory: effects of manipulating emotionality and mental effort. Biological Psychology 2006;71(1):12‐9. [DOI] [PubMed] [Google Scholar]
Scholey 2009 {published data only}
- Scholey AB, Sunram‐Lea SI, Greer J. Glucose administration prior to a divided attention task improves tracking performance but not word recognition: evidence against differential memory enhancement?. Psychopharmacology (Berl) 2009;202(1‐3):549‐58. [DOI] [PubMed] [Google Scholar]
Smit 2004 {published data only}
- Smit HJ, Cotton JR, Hughes SC, et al. Mood and cognitive performance effects of "energy" drink constituents: caffeine, glucose and carbonation. Nutritional Neuroscience 2004;7(3):127‐39. [DOI] [PubMed] [Google Scholar]
Smith 2009 {published data only}
- Smith AP, Wilds A. Effects of cereal bars for breakfast and mid‐morning snacks on mood and memory. International Journal of Food Sciences and Nutrition 2009;60 Suppl 4:63‐9. [DOI] [PubMed] [Google Scholar]
Vamosi 1976 {published data only}
- Vamosi B, Molnar L, Demeter J, et al. Comparative study of the effect of ethyl apovincaminate and xantinol nicotinate in cerebrovascular diseases. Immediate drug effects on the concentrations of carbohydrate metabolites and electrolytes in blood and CSF. Arzneimittelforschung 1976;26(10a):1980‐4. [PubMed] [Google Scholar]
Verger 1998 {published data only}
- Verger P, Lagarde D, Batejat D, et al. Influence of the composition of a meal taken after physical exercise on mood, vigilance, performance. Physiology & Behavior 1998;64(3):317‐22. [DOI] [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang C, Szabo JS, Dykman RA. Effects of a carbohydrate supplement upon resting brain activity. Integrative Physiological and Behavioral Science 2004;39(2):126‐38. [DOI] [PubMed] [Google Scholar]
Wells 1998 {published data only}
- Wells AS, Read NW, Macdonald IA. Effects of carbohydrate and lipid on resting energy expenditure, heart rate, sleepiness, and mood. Physiology & Behavior 1998;63(4):621‐8. [DOI] [PubMed] [Google Scholar]
Winder 1998 {published data only}
- Winder R, Borrill J. Fuels for memory: The role of oxygen and glucose in memory enhancement. Psychopharmacology 1998;136(4):349‐56. [DOI] [PubMed] [Google Scholar]
Wing 1995 {published data only}
- Wing RR, Vazquez JA, Ryan CM. Cognitive effects of ketogenic weight‐reducing diets. International Journal of Obesity and Related Metabolic Disorders 1995;19(11):811‐6. [PubMed] [Google Scholar]
Young 2004 {published data only}
- Young KW, Greenwood CE, et al. Providing nutrition supplements to institutionalized seniors with probable Alzheimer's disease is least beneficial to those with low body weight status. Journal of the American Geriatric Society 2004;52(8):1305‐12. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12606000475549 {published data only}
- High Protein Diets, Weight Loss and Diabetes Study 2007. ALOIS 2007.
NCT01427231 2011 {published data only}
- NCT01427231. Short‐term Effect of Glucose and Sacharose Ingestion on Cognitive Performance and Mood in Elderly. ClinicalTrials.gov 2011.
Additional references
Abercrombie 2003
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behavioral Neuroscience 2003;117(3):505‐16. [DOI] [PubMed] [Google Scholar]
Albach 2001
- Albach C, Klein RA, Schmitz B. Do rodent and human brains have different N‐glycosylation patterns?. Biological Chemistry 2001;382(2):187–94. [DOI] [PubMed] [Google Scholar]
APA 1994
- American Pschological Association (APA). Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Press, 1994. [Google Scholar]
Arendt 1986
- Arendt T, Bigl V. Alzheimer plaques and cortical cholinergic innervation. Neuroscience 1986;17(1):277‐9. [DOI] [PubMed] [Google Scholar]
Bandtlow 2000
- Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiological Reviews 2000;80(4):1267‐90. [DOI] [PubMed] [Google Scholar]
Benedict 2010
- Benedict C, Frey WH, Schioth HB, Schultes B, Born J, Hallschmid M. Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Experimental Gerontology 2010;Sep 14(ISSN 1873‐6815 (Electronic) 0531‐5565 (Linking)):DOI S0531‐5565(10)00270‐6 [pii] 10.1016/j.exger.2010.08.026 [doi]. [DOI] [PubMed] [Google Scholar]
Best 2009
- Best T, Kemps E, Bryan J. Association between dietary saccharide intake and self‐reported memory performance in middle‐aged adults. The British Journal of Nutrition 2009;101(1):93‐9. [DOI] [PubMed] [Google Scholar]
Beydoun 2008
- Beydoun MA, Beydoun HA, et al. Obesity and central obesity as risk factors for incident dementia and its subtypes: A systematic review and meta‐analysis. Obesity Review 2008;9(3):204‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bigl 1998
- Bigl V, Schliebs R. Simulation of cholinergic deficits ‐ a novel experimental approach to study pathogenetic aspects of Alzheimer's disease. Journal of Neural Transmission 1998;53 Suppl:237‐47. [DOI] [PubMed] [Google Scholar]
Burton 2009
- Burton CL, Strauss E, Bunce D, Hunter MA, Hultsch DF. Functional abilities in older adults with mild cognitive impairment. Gerontology 2009;55(5):570‐81. [DOI] [PubMed] [Google Scholar]
Chalmers 1983
- Chalmers TC, Celano P, Sacks HS, Smith H. Bias in treatment assignment in controlled clinical trials. New England Journal of Medicine 1983;309(22):1358‐61. [DOI] [PubMed] [Google Scholar]
Comijs 2010
- Comijs HC, Gerritsen L, Penninx BW, Bremmer MA, Deeg DJ, Geerlings MI. The association between serum cortisol and cognitive decline in older persons. American Journal of Geriatric Psychiatry 2010;18(1):42‐50. [DOI] [PubMed] [Google Scholar]
Cooray 2010
- Cooray G, Nilsson E, Wahlin A, Laukka EJ, Brismar K, Brismar T. Effects of intensified metabolic control on CNS function in type 2 diabetes. Psychoneuroendocrinology 2010;Epub Jul 23(ISSN 1873‐3360 (Electronic) 0306‐4530 (Linking)):DOI S0306‐4530(10)00151‐4 [pii] 10.1016/j.psyneuen.2010.06.009 [doi]. [DOI] [PubMed] [Google Scholar]
Craft 1993
- Craft S, Dagogo‐Jack SE, et al. Effects of hyperglycemia on memory and hormone levels in dementia of the Alzheimer type: a longitudinal study. Behavioral Neuroscience 1993;107(6):926‐40. [DOI] [PubMed] [Google Scholar]
Craft 2003
- Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K, et al. Insulin dose‐response effects on memory and plasma amyloid precursor protein in Alzheimer's disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology 2003;28(6):809‐22. [DOI] [PubMed] [Google Scholar]
Dai 2006
- Dai Q, Borenstein, AR, Wu Y, Jackson JC, Larson EB. Fruit and vegetable juices and Alzheimer's disease: the Kame Project. The American Journal of Medicine 2006;119(9):751‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
de la Monte 2009
- Monte SM. Insulin resistance and Alzheimer's disease. BMB Reports 2009;42(8):475‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Leon 1997
- Leon MJ, McRae T, Rusinek H, Convit A, Santi S, Tarshish C. Cortisol reduces hippocampal glucose metabolism in normal elderly, but not in Alzheimer's disease. The Journal of Clinical Endocrinology and Metabolism 1997;82(10):3251‐9. [DOI] [PubMed] [Google Scholar]
Diano 2006
- Diano S, Farr SA, Benoit SC, McNay EC, Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Neuroscience 2006;9(3):381‐8. [DOI] [PubMed] [Google Scholar]
Dodge 2006
- Dodge HH, Du Y, Saxton JA, Ganguli M. Cognitive domains and trajectories of functional independence in nondemented elderly persons. The Journals of Gerontology. Series A, Biological Science and Medical Science 2006;61(12):1330‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dutton 2008
- Dutton AC, Massoura AN, Dover TJ, Andrews NA, Barnes NM. Identification and functional significance of N‐glycosylation of the 5‐ht5A receptor. Neurochemistry International 2008;52(3):419‐25. [DOI] [PubMed] [Google Scholar]
Eskelinen 2008
- Eskelinen MH, Ngandu T, Helkala EL, Tuomilehto J, Nissinen A, Soininen H, et al. Fat intake at midlife and cognitive impairment later in life: a population‐based CAIDE study. International Journal of Geriatric Psychiatry 2008;23(7):741‐7. [DOI] [PubMed] [Google Scholar]
Feart 2009
- Feart C, Samieri C, Rondeau V, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009;302(6):638‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fehm 2006
- Fehm HL, Kern W, Peters A. The selfish brain: competition for energy resources. Progress in Brain Research 2006;153:129‐40. [DOI] [PubMed] [Google Scholar]
Ferri 2005
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366(9503):2112‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzpatrick 2009
- Fitzpatrick A, Kuller H, Lopez O, Diehr P, O’Meara E, Longstreth WJ, et al. Midlife and late‐life obesity and the risk of dementia: cardiovascular health study. Archives of Neurology 2009;66(3):336‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flood 1987
- Flood JF, Smith GE, et al. Modulation of memory processing by cholecystokinin: dependence on the vagus nerve. Science 1987;236(4803):832‐4. [DOI] [PubMed] [Google Scholar]
Flood 1988
- Flood JF, Morley JE. Effects of bombesin and gastrin‐releasing peptide on memory processing. Brain Research 1988;460(2):314‐22. [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189‐98. [PUBMED: 1202204] [DOI] [PubMed] [Google Scholar]
Foster 1998
- Foster JK, Lidder PG, Sunram SI. Glucose and memory: fractionation of enhancement effects?. Psychopharmacology (Berl) 1998;137(3):259‐70. [DOI] [PubMed] [Google Scholar]
Fouquet 2009
- Fouquet M, Desgranges B, Landeau B, Duchesnay E, Mezenge F, Sayette V, et al. Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimer's disease. Brain 2009;132(Pt 8):2058‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gatto 2008
- Gatto NM, Henderson VW, John JA, McCleary C, Hodis HN, Mack WJ. Metabolic syndrome and cognitive function in healthy middle‐aged and older adults without diabetes. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 2008;15(5):627‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez‐Bono 2002
- Gonzalez‐Bono E, Rohleder N, Hellhammer DH, Salvador A, Kirschbaum C. Glucose but not protein or fat load amplifies the cortisol response to psychosocial stress. Hormones and Behavior 2002;41(3):328‐33. [DOI] [PubMed] [Google Scholar]
Gu 2010
- Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer disease risk: a protective diet. Archives of Neurology 2010;67(6):699‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 1989
- Hall JL, Gonder‐Frederick LA, et al. Glucose enhancement of performance on memory tests in young and aged humans. Neuropsychologia 1989;27(9):1129‐38. [DOI] [PubMed] [Google Scholar]
Hallschmid 2010
- Hallschmid M, Jauch‐Chara K, Korn O, Molle M, Rasch B, Born J, et al. Euglycemic infusion of insulin detemir compared with human insulin appears to increase direct current brain potential response and reduces food intake while inducing similar systemic effects. Diabetes 2010;59(4):1101‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hancock 2009
- Hancock P, Larner AJ. Diagnostic utility of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) and its combination with the Addenbrooke's Cognitive Examination‐Revised (ACE‐R) in a memory clinic‐based population. International Psychogeriatrics 2009;21(9):526‐30. [DOI] [PubMed] [Google Scholar]
Het 2005
- Het S, Ramlow G, Wolf OT. A meta‐analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology 2005;30(8):771‐84. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. Chichester, UK:: John Wiley & Sons, Ltd.
Huang 2009
- Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer's disease. Journal of Clinical Neuroscience 2009;16(10):1283‐6. [DOI] [PubMed] [Google Scholar]
Hughes 2010
- Hughes TF, Andel R, Small BJ, Borenstein AR, Mortimer JA, Wolk A, et al. Midlife fruit and vegetable consumption and risk of dementia in later life in Swedish twins. American Journal of Psychiatry 2010;18(5):413‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunt 2007
- Hunt A, Schonknecht P, Henze M, Seidl U, Haberkorn U, Schroder J. Reduced cerebral glucose metabolism in patients at risk for Alzheimer's disease. Psychiatry Research 2007;155(2):147‐54. [DOI] [PubMed] [Google Scholar]
ICD 1993
- ICD. The ICD‐10 Classificiation of Mental and Behavioural Disorders: Diagnostic Criteria for Research. International Classification of Disease‐10. Geneva: World Health Organization, 1993. [Google Scholar]
Isaac 2008
- Isaac MG, Quinn R, Tabet N. Vitamin E for Alzheimer's disease and mild cognitive impairment. Cochrane Database of Systematic Reviews 2008;3:CD002854. [DOI] [PubMed] [Google Scholar]
Isella 2006
- Isella V, Villa L, Russo A, Regazzoni R, Ferrarese C, Appollonio IM. Discriminative and predictive power of an informant report in mild cognitive impairment. Journal of Neurology, Neurosurgery, and Psychiatry 2006;77(2):166‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
IUPAC 1996
- McNaught AD. IUPAC‐IUBMB Joint Commission on Biochemical Nomenclature (JCBN): Nomenclature of Carbohydrate. Pure and Applied Chemistry 1996;68(10):1919‐2008. [Google Scholar]
Jefferson 2008
- Jefferson AL, Byerly LK, Vanderhill S, Lambe S, Wong S, Ozonoff A, et al. Characterization of activities of daily living in individuals with mild cognitive impairment. American Journal of Geriatric Psychiatry 2008;16(5):375‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalmijn 1997
- Kalmijn S, Launer LJ, et al. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Annals of Neurology 1997;42(5):776‐82. [DOI] [PubMed] [Google Scholar]
Kang 2006
- Kang JH, Ascherio A, Grodstein F. Fruit and vegetable and cognitive decline in aging women. Annals of Neurology 2006;57(5):713‐20. [DOI] [PubMed] [Google Scholar]
Kirschbaum 1997
- Kirschbaum C, Gonzalez Bono E, Rohleder N, Gessner C, Pirke KM, Salvador A, et al. Effects of fasting and glucose load on free cortisol responses to stress and nicotine. The Journal of Clinical Endocrinology and Metabolism 1997;82(4):1101‐5. [DOI] [PubMed] [Google Scholar]
Kleene 2004
- Kleene R, Schachner M. Glycans and neural cell interactions. Nature Review: Neuroscience 2004;5(3):195‐208. [DOI] [PubMed] [Google Scholar]
Knopman 2009
- Knopman D, Mosley T, Catellier D, et al. Fourteen‐year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimer's & Dementia 2009;5(3):207‐14. [DOI] [PubMed] [Google Scholar]
Laitinen 2006
- Laitinen MH, Ngandu T, Rovio S, Helkala EL, Uusitalo U, Viitanen M, et al. Fat intake at midlife and risk of dementia and Alzheimer's disease: a population‐based study. Dementia and Geriatric Cognitive Disorders 2006;22(1): 99‐107. [DOI] [PubMed] [Google Scholar]
Lester‐Coll 2006
- Lester‐Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer's disease. Journal of Alzheimer's Disease 2006;9(1):13‐33. [DOI] [PubMed] [Google Scholar]
Lezak 1995
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th Edition. Oxford University Press, 1995. [Google Scholar]
Li 2007
- Li L, Holscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Research Reviews 2007;56(2):384‐402. [DOI] [PubMed] [Google Scholar]
Li 2008
- Li Y, Rinne JO, Mosconi L, Pirraglia E, Rusinek H, DeSanti S, et al. Regional analysis of FDG and PIB‐PET images in normal aging, mild cognitive impairment, and Alzheimer's disease. European Journal of Nuclear Medicine and Molecular Imaging 2008;35(12):2169‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lloret 2009
- Lloret A, Badia MC, Mora NJ, Pallardo FV, Alonso MD, Vina J. Vitamin E paradox in Alzheimer's disease: it does not prevent loss of cognition and may even be detrimental. Journal of Alzheimer's Disease 2009;17(1):143‐9. [DOI] [PubMed] [Google Scholar]
Lucas 2000
- Lucas J A, Orshan S A, et al. Determinants of health‐promoting behavior among women ages 65 and above living in the community. Scholarly Inquiry for Nursing Practice 2000;14(1):77‐100 (discussion 101‐9). [PubMed] [Google Scholar]
Luchsinger 2009
- Luchsinger JA, Gustafson DR. Adiposity and Alzheimer's disease. Current Opinion in Clinical Nutrition and Metabolic Care 2009;12(1):15‐21. [PUBMED: 19057182] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maeda 2010
- Maeda N, Fukazawa N, Ishii M. Chondroitin sulfate proteoglycans in neural development and plasticity. Frontiers in Bioscience 2010;15:626‐44. [DOI] [PubMed] [Google Scholar]
Malik 2010
- Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar‐sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta‐analysis. Diabetes Care 2010;33(11):2477‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manning 1990
- Manning C, Hall J, Gold P. Glucose effects on memory and other neuropsychological tests in elderly humans. Psychological Science 1990;1(5):307‐11. [Google Scholar]
Manning 1993
- Manning CA, Ragozzino ME, et al. Glucose enhancement of memory in patients with probable senile dementia of the Alzheimer's type. Neurobiology of Aging 1993;14(6):523‐8. [DOI] [PubMed] [Google Scholar]
Manning 1997
- Manning CA, Parsons MW, et al. Glucose effects on declarative and nondeclarative memory in healthy elderly and young adults. Psychobiology 1997;25(2):103‐8. [Google Scholar]
McGuire 2006
- McGuire LC, Ford ES, Ajani UA. Cognitive functioning as a predictor of functional disability in later life. The American Journal of Geriatric Psychiatry 2006;14(1):36‐41. [DOI] [PubMed] [Google Scholar]
Meneilly 1993
- Meneilly G, Hill A. Alterations in glucose metabolism in patients with Alzheimers disease. Journal of the American Geriatrics Society 1993;41(7):710‐4. [DOI] [PubMed] [Google Scholar]
Miller 2009
- Miller MC, Nesmelova IV, Platt D, Klyosov A, Mayo KH. The carbohydrate‐binding domain on galectin‐1 is more extensive for a complex glycan than for simple saccharides: implications for galectin‐glycan interactions at the cell surface. Biochemical Journal 2009;421(2):211‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morala 2006
- Morala DT, Shiomi T, Maruyama H. Factors associated with the functional status of community‐dwelling elderly. Journal of Geriatric Physical Therapy 2006;29(3):101‐6. [DOI] [PubMed] [Google Scholar]
Morales 1995
- Morales JM, Gonzalez‐Montalvo JI, Bermejo F, Del‐Ser T. The screening of mild dementia with a shortened Spanish version of the "Informant Questionnaire on Cognitive Decline in the Elderly". Alzheimer Disease and Associated Disorders 1995;9(2):105‐11. [DOI] [PubMed] [Google Scholar]
Morris 2004
- Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Dietary fat intake and 6‐year cognitive change in an older biracial community population. Neurology 2004;62(9):1573‐9. [DOI] [PubMed] [Google Scholar]
Morris 2005
- Morris MC, Evans DA, et al. Fish consumption and cognitive decline with age in a large community study. Archives of Neurology 2005;62(12):1849‐53. [DOI] [PubMed] [Google Scholar]
Morris 2006
- Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age‐related cognitive change. Neurology 2006;67(8):1370‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosconi 2008
- Mosconi L, Santi S, Li J, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiology of Aging 2008;29(5):676‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller 2007
- Muller M, Tang M, Schupf N, Manly J, Mayeux R, Luchsinger J. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dementia and Geriatric Cognitive Disorders 2007;24(3):185‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 2008
- Nelson TJ, Sun MK, Hongpaisan J, Alkon DL. Insulin, PKC signaling pathways and synaptic remodeling during memory storage and neuronal repair. European Journal of Pharmacology 2008;585(1):76‐87. [DOI] [PubMed] [Google Scholar]
Newcomer 1994
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid‐induced impairment in declarative memory performance in adult humans. Journal of Neuroscience 1994;14(4):2047‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2000
- Park CR, Seeley R J, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive‐avoidance task. Physiology & Behavior 2000;68(4):509‐14. [DOI] [PubMed] [Google Scholar]
Parsons 1992
- Parsons MW, Gold PE. Glucose enhancement of memory in elderly humans: an inverted‐U dose‐response curve. Neurobiology of Aging 1992;13(3):401‐4. [DOI] [PubMed] [Google Scholar]
Peters 2004
- Peters A, Schweiger U, Pellerin L, Hubold C, Oltmanns KM, Conrad M, et al. The selfish brain: competition for energy resources. Neuroscience and Biobehavioral Reviews 2004;28(2):143‐80. [DOI] [PubMed] [Google Scholar]
Prince 2004
- Prince M. Care arrangements for people with dementia in developing countries. International Journal of Geriatric Psychiatry 2004;19(2):170‐7. [DOI] [PubMed] [Google Scholar]
Reger 2006
- Reger MA, Watson GS, Frey WH, Baker LD, Cholerton B, Keeling ML, et al. Effects of intranasal insulin on cognition in memory‐impaired older adults: modulation by APOE genotype. Neurobiology of Aging 2006;27(3):451‐8. [DOI] [PubMed] [Google Scholar]
Reger 2008
- Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, et al. Intranasal insulin improves cognition and modulates beta‐amyloid in early AD. Neurology 2008;70(6):440‐8. [DOI] [PubMed] [Google Scholar]
Reynolds 2010
- Reynolds RM, Strachan MW, Labad J, Lee AJ, Frier BM, Fowkes FG, et al. Morning cortisol levels and cognitive abilities in people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care 2010;33(4):714‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roriz‐Filho 2009
- Roriz‐Filho JS, Sa‐Roriz TM, Rosset I, Camozzato AL, Santos AC, Chaves ML, et al. (Pre)diabetes, brain aging, and cognition. Biochimica et Biophysica Acta 2009;1792(5):432‐43. [DOI] [PubMed] [Google Scholar]
Rosmond 2000
- Rosmond R, Holm G, Bjorntorp P. Food‐induced cortisol secretion in relation to anthropometric, metabolic and haemodynamic variables in men. International Journal of Obesity and Related Metabolic Disorders 2000;24(4):416‐22. [DOI] [PubMed] [Google Scholar]
Ryan 2006
- Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care 2006;29(2):345‐51. [DOI] [PubMed] [Google Scholar]
Sato 2004
- Sato A, Sato Y, Uchida S. Activation of the intracerebral cholinergic nerve fibres originating in the basal forebrain increases regional cerebral blood flow in the rat's cortex and hippocampus. Neuroscience Letters 2004;361(1‐3):90‐3. [DOI] [PubMed] [Google Scholar]
Scanlan 2007
- Scanlan JM, Binkin N, Michieletto F, Lessig M, Zuhr E, Borson S. Cognitive impairment, chronic disease burden, and functional disability: a population study of older Italians. American Journal of Geriatric Psychiatry 2007;15(8):716‐24. [DOI] [PubMed] [Google Scholar]
Scarmeas 2006
- Scarmeas N, Stern Y, Tang MX, et al. Mediterranean diet and risk for Alzheimer's disease. Annals of Neurology 2006;59(6):912‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scarmeas 2009a
- Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Archives of Neurology 2009;66(2):216‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scarmeas 2009b
- Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009;302(6):627‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schliebs 1996
- Schliebs R, Robner S, Bigl V. Immunolesion by 192IgG‐saporin of rat basal forebrain cholinergic system ‐ a useful tool to produce cortical cholinergic dysfunction. Progress in Brain Research 1996;109:253‐64. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman D. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐1. [DOI] [PubMed] [Google Scholar]
Shulman 1999
- Shulman GI. Cellular mechanisms of insulin resistance in humans. American Journal of Cardiology 1999;84(1A):3J‐10J. [DOI] [PubMed] [Google Scholar]
Solfrizzi 2006
- Solfrizzi V, Colacicco AM, et al. Dietary fatty acid intakes and rate of mild cognitive impairment. The Italian Longitudinal Study on Aging. Experimental Gerontology 2006;41(6):619–27. [DOI] [PubMed] [Google Scholar]
Souza‐Talarico 2010
- Souza‐Talarico JN, Chaves EC, Lupien SJ, Nitrini R, Caramelli P. Relationship between cortisol levels and memory performance may be modulated by the presence or absence of cognitive impairment: evidence from healthy elderly, mild cognitive impairment and Alzheimer's disease subjects. Journal of Alzheimer's Disease 2010;19(3):839‐48. [DOI] [PubMed] [Google Scholar]
Spitznagel 2010
- Spitznagel MB, Benitez A, Updegraff J, et al. Serum ghrelin is inversely associated with cognitive function in a sample of non‐demented elderly. Psychiatry and Clinical Neurosciences 2010;64(6):608‐11. [DOI] [PubMed] [Google Scholar]
Stein 2006
- Stein D, Sano M. Anti‐oxidant drugs in the treatment and prevention of Alzheimer’s disease and cognitive loss. Therapeutic strategies. Hertforshire, UK: Dementia Atlas Medical Publishing Ltd, 2006. [Google Scholar]
Stewart 2005
- Stewart R, Masaki K, Xue Q, Peila R, Petrovitch H, White L, et al. A 32‐year prospective study of change in body weight and incident dementia: The Honolulu‐Asia Aging Study. Archives of Neurology 2005;62(1):55‐60. [DOI] [PubMed] [Google Scholar]
Trichopoulou 2003
- Trichopoulou A, Costacou T, et al. Adherence to a Mediterranean diet and survival in a Greek population. New England Journal of Medicine 2003;348(26):2599–608. [DOI] [PubMed] [Google Scholar]
Triebel 2009
- Triebel KL, Martin R, Griffith HR, Marceaux J, Okonkwo OC, Harrell L, et al. Declining financial capacity in mild cognitive impairment: A 1‐year longitudinal study. Neurology 2009;73(12):928‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
UN 2007
- UN 2007. World Population Prospects: The 2006 Revision. Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat 2007.
Wadley 2008
- Wadley VG, Okonkwo O, Crowe M, Ross‐Meadows LA. Mild cognitive impairment and everyday function: evidence of reduced speed in performing instrumental activities of daily living. American Journal of Geriatric Psychiatry 2008;16(5):416‐24. [DOI] [PubMed] [Google Scholar]
Wadley 2009
- Wadley VG, Okonkwo O, Crowe M, Vance DE, Elgin JM, Ball KK, et al. Mild cognitive impairment and everyday function: an investigation of driving performance. Journal of Geriatric Psychiatry and Neurology 2009;22(2):87‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Westerman 2002
- Westerman MA, Cooper‐Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, et al. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. Journal of Neuroscience 2002;22(5):1858‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Shaping the future. World Health Report. Geneva: World Health Organization, 2003. [Google Scholar]
Wilcox 2007
- Wilcox BJ, et al. Caloric restriction, the traditional Okinawan diet, and healthy aging: The diet of the world’s longest‐lived people and its potential impact on morbidity and life span. Annals of the New York Academy of Sciences 2007;1114(Oct):434–55. [DOI] [PubMed] [Google Scholar]
Winblad 2004
- Winblad B Palmer K, Kivipelto M. Mild cognitive impairment‐‐beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine 2004;256(3):240‐6. [DOI] [PubMed] [Google Scholar]
Witte 2009
- Witte M, Fobkerb R, et al. Caloric restriction improves memory in elderly humans. PNAS 2009;106(4):1255‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yaffe 2009
- Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Archives of Neurology 2009;66(3):324‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamaguchi 2002
- Yamaguchi Y. Glycobiology of the synapse: the role of glycans in the formation, maturation, and modulation of synapses. Biochimica et Biophysica Acta 2002;1573(3):369‐76. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ooi 2011
- Ooi CP, Loke SC, Yassin Z, Hamid TA. Carbohydrates for improving the cognitive performance of independent‐living older adults with normal cognition or mild cognitive impairment. Cochrane Database of Systematic Reviews (Online) 2011;4:CD007220. [PUBMED: 21491398] [DOI] [PMC free article] [PubMed] [Google Scholar]


