Abstract
Background
Endovascular aortic aneurysm repair (EVAR) is used to treat aorto‐iliac and isolated iliac aneurysms in selected patients, and prospective studies have shown advantages compared with open surgical repair, mainly in the first years of follow‐up. Although this technique produces good results, anatomic issues (such as common iliac artery ectasia or an aneurysm that involves the iliac bifurcation) can make EVAR more complex and challenging and can lead to an inadequate distal seal zone for the stent‐graft. Inadequate distal fixation in the common iliac arteries can lead to a type Ib endoleak. To avoid this complication, one of the most commonly used techniques is unilateral or bilateral internal iliac artery occlusion and extension of the iliac limb stent‐graft to the external iliac arteries with or without embolisation of the internal iliac artery. However, this occlusion is not without harm and is associated with ischaemic complications in the pelvic territory such as buttock claudication, sexual dysfunction, ischaemic colitis, gluteal necrosis, and spinal cord injury.
New endovascular devices and alternative techniques such as iliac branch devices and the sandwich technique have been described to maintain pelvic perfusion and decrease complications, achieving revascularisation of the internal iliac arteries in patients not suitable for an adequate seal zone in the common iliac arteries. These approaches may also preserve the quality of life of treated individuals and may decrease other serious complications including spinal cord ischaemia, ischaemic colitis, and gluteal necrosis, thereby decreasing the morbidity and mortality of EVAR.
Objectives
To assess the effects of internal iliac artery revascularisation versus internal iliac artery occlusion during endovascular repair of aorto‐iliac aneurysms and isolated iliac aneurysms involving the iliac bifurcation.
Search methods
The Cochrane Vascular Information Specialists searched the Cochrane Vascular Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library; MEDLINE; Embase; the Cumulative Index to Nursing and Allied Health Literature (CINAHL); and the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 28 August 2019. The review authors searched Latin American Caribbean Health Sciences Literature (LILACS) and the Indice Bibliográfico Español de Ciencias de la Salud (IBECS) on 28 August 2019 and contacted specialists in the field and manufacturers to identify relevant studies.
Selection criteria
We planned to include all randomised controlled trials (RCTs) that compared internal iliac artery revascularisation with internal iliac artery occlusion for patients undergoing endovascular treatment of aorto‐iliac aneurysms and isolated iliac aneurysms involving the iliac bifurcation.
Data collection and analysis
Two review authors independently assessed identified studies for potential inclusion in the review. We used standard methodological procedures in accordance with the Cochrane Handbook for Systematic Review of Interventions.
Main results
We identified no RCTs that met the inclusion criteria.
Authors' conclusions
We found no RCTs that compared internal iliac artery revascularisation versus internal iliac artery occlusion for endovascular treatment of aorto‐iliac aneurysms and isolated iliac aneurysms involving the iliac bifurcation. High‐quality studies that evaluate the best strategy for managing endovascular repair of aorto‐iliac aneurysms with inadequate distal seal zones in the common iliac artery are needed.
Keywords: Humans; Aortic Aneurysm, Abdominal; Aortic Aneurysm, Abdominal/surgery; Embolization, Therapeutic; Embolization, Therapeutic/methods; Endovascular Procedures; Endovascular Procedures/methods; Iliac Aneurysm; Iliac Aneurysm/surgery; Iliac Artery; Iliac Artery/surgery
Plain language summary
Internal iliac artery revascularisation versus internal iliac artery occlusion for endovascular treatment of aorto‐iliac aneurysms
Background
An aorto‐iliac aneurysm is a dilatation (aneurysm) of the aorta, the main large blood vessel in the body, which carries blood out from the heart to all organs and iliac arteries (distal branches of the aorta). The aneurysm can grow and burst (rupture), which leads to severe bleeding and is frequently fatal; an estimated 15,000 deaths occur each year from ruptured aortic abdominal aneurysms in the USA alone. To avoid this complication, the aorto‐iliac aneurysm should be repaired when the maximum diameter of the aorta reaches 5 cm to 5.5 cm, or when the maximum diameter of the common iliac arteries reaches 3 cm to 4 cm.
Endovascular repair of aorto‐iliac aneurysms is one approach that is used to manage this condition: a tube (stent‐graft) is placed inside the aorto‐iliac aneurysm, so that blood flows through the stent‐graft and no longer into the aneurysm, excluding it from the circulation. To achieve a successful deployment of the stent‐graft, a good seal zone (fixation zone) is needed in the aorta (proximal) and in the common iliac arteries (distal). However, in 40% of patients, the distal seal zone in the common iliac arteries is inadequate. In these cases, most commonly the stent‐graft is extended to the external iliac artery and the internal iliac artery is blocked (occluded). However, this obstruction (occlusion) is not without harms: the internal iliac artery supplies blood to the pelvic organs (rectum, bladder, and reproductive organs) and the pelvic muscles, and occlusion is associated with complications in the pelvic area such as buttock claudication (cramping pain in the buttock during exercise), sexual dysfunction, and spinal cord injury.
New endovascular devices and techniques such as iliac branch devices have emerged to maintain blood flow into the internal iliac artery. These special stent‐grafts position the distal seal zone within the external iliac artery, and a side branch of the graft allows for revascularisation of the internal iliac artery, while excluding the aneurysm from the circulation, promoting an adequate distal seal zone, and maintaining pelvic circulation. This may also preserve the quality of life of treated individuals and may reduce serious complications including spinal cord ischaemia, ischaemic colitis, and gluteal necrosis.
This review aimed to assess the effects of internal iliac artery revascularisation compared with internal iliac artery occlusion during endovascular repair of aorto‐iliac aneurysms.
Study characteristics and key results
We searched for evidence that directly compared internal iliac artery revascularisation versus internal iliac artery occlusion for endovascular treatment of aorto‐iliac aneurysms. Our searches up to 28 August 2019 did not identify any randomised controlled trials (clinical studies in which people are randomly (by chance alone) put into one of several intervention groups) that met our criteria. Studies are needed to help vascular and endovascular surgeons choose the best option for endovascular repair of aorto‐iliac aneurysms and isolated iliac aneurysms with an inadequate distal fixation zone.
Conclusion
We found no RCTs that compared internal iliac artery revascularisation versus internal iliac artery occlusion for endovascular treatment of aorto‐iliac aneurysms. High‐quality studies that evaluate the best strategy for managing the endovascular repair of aorto‐iliac aneurysms with inadequate distal seal zones in the common iliac artery are needed.
Background
Description of the condition
An aneurysm is an abnormal dilatation of a blood vessel that exceeds 50% of the diameter of a normal vessel (Johnston 1991). In the iliac arteries, aneurysms of the common iliac artery are most prevalent, followed by aneurysms of the internal iliac artery (also known as the hypogastric artery). An ectatic or common iliac artery aneurysm is associated with an abdominal aortic aneurysm (AAA) in approximately 40% of patients (Armon 1998; Lawrence 1999). The incidence of isolated iliac artery aneurysms is less than 2% (Garland 1932; Huang 2008; Lowry 1978).
Untreated abdominal aorto‐iliac and isolated iliac aneurysms are likely to increase in size and may eventually rupture (Szilagyi 1966). An estimated 15,000 deaths in the USA each year result from ruptured AAAs ‐ the 13th leading cause of death in that country (Calero 2016). The primary determinant of rupture risk is the maximum aneurysm diameter; the decision to treat or not to treat the aneurysm is made according to the diameter and/or the presence of symptoms such as abdominal pain or distal embolisation (Chaikof 2018).
Currently, treatment of AAA is recommended when the maximal diameter reaches 5 cm to 5.5 cm (Chaikof 2018; Moll 2011). Treatment of iliac artery aneurysms is justified when the common iliac artery has a diameter greater than 3 cm to 4 cm (Huang 2008; Santilli 2000; Williams 2014).
In 1986, Volodos described endovascular aortic aneurysm repair (EVAR) (Volodos 1986), and in 1991, Parodi and colleagues published their experience with retrograde deployment through the femoral arteries of a stent‐anchored, polyethylene terephthalate (Dacron) prosthetic graft that would act to de‐pressurise the aneurysm sac and reduce the risk of aneurysm rupture (Parodi 1991). Since that time, EVAR has gained widespread acceptance, and prospective studies have shown advantages compared with open surgical repair, mainly in the first years of follow‐up (Paravastu 2014).
Endovascular aortic aneurysm repair currently involves access via the femoral arteries and placement (deployment) of a stent‐graft device within the aorta, thereby excluding the aneurysm from the circulation and de‐pressurising the aneurysmatic sac (Moore 1995; Nelson 2014). For a successful procedure, both a correct and effective stent‐graft fixation and an adequate proximal (aorta) and distal (iliac arteries) seal zone are necessary to avoid endoleaks (defined as persistent blood flow in the aneurysm sac following stent‐graft) (Table 1).
1. Types of endoleaks.
| Type Ia | Incomplete seal at the proximal aortic attachment site |
| Type Ib | Incomplete seal at the distal iliac attachment site |
| Type II | Persistent filling of the aneurysm sac from patent aortic or iliac branches (e.g. lumbar arteries, inferior mesenteric artery, internal iliac artery) |
| Type III | Incomplete seal between components, component separation, or fabric erosion |
| Type IV | Fabric porosity |
Description of the intervention
Endovascular aortic aneurysm repair is used to treat aorto‐iliac and isolated iliac aneurysms in patients with suitable aorto‐iliac anatomy (anatomic requirements are specified in stent‐graft instructions for use) and reasonable life expectancy, resulting in less morbidity and mortality than open repair, mainly in the first years of follow‐up (Paravastu 2014; Wanhainen 2019). Although this technique produces good results, anatomic issues (such as common iliac artery ectasia or an aneurysm that involves the iliac bifurcation) can make EVAR more complex and challenging and can lead to an inadequate distal seal zone for the stent‐graft. This situation occurs in approximately 15% of individuals treated with EVAR (Bosanquet 2017). Inadequate distal fixation in the common iliac arteries leads to a type Ib endoleak (Chuter 2001).
To avoid type Ib endoleak in these patients, one of the most commonly used techniques is unilateral or bilateral internal iliac artery occlusion and extension of the iliac limb stent‐graft to the external iliac arteries with or without embolisation of the internal iliac artery (Fatima 2012; Tefera 2004; Wu 2011). Embolisation is achieved by reaching the internal iliac artery by using a catheter and deploying coils or plugs in this artery to avoid the backflow of blood (type II endoleak) (Wu 2011). However, this occlusion is not innocuous: the internal iliac artery supplies blood to the pelvic organs (rectum, bladder, and breeding organs) and pelvic muscles, and occlusion is associated with ischaemic complications in pelvic territory such as buttock claudication, sexual dysfunction, ischaemic colitis, gluteal necrosis, and spinal cord injury (Bosanquet 2017; Verzini 2009).
New endovascular devices and alternative techniques have been described to maintain pelvic perfusion and decrease complications, achieving revascularisation of the internal iliac arteries in patients not suitable for an adequate seal zone in the common iliac arteries. Iliac branch devices have emerged; these special stent‐grafts position the distal seal zone within the external iliac artery. A side branch of the graft allows for internal iliac artery revascularisation, excluding the aneurysm from the circulation, promoting an adequate distal seal zone, and maintaining pelvic circulation (Fatima 2012; Oderich 2011). Alternative techniques with the same objective such as the sandwich technique have been described (Lobato 2011). By means of transbrachial and femoral approaches, parallel stent‐grafts from one or both docking limbs of a bifurcated aortic stent‐graft are placed to preserve flow into one or both internal iliac arteries and maintain circulation to the external iliac artery (Lobato 2011). These techniques are complex, and performing them requires a high level of endovascular skill.
How the intervention might work
Abdominal aorto‐iliac and isolated iliac aneurysms involving the iliac bifurcation have unsuitable distal landing zones to allow sealing by regular bifurcated stent‐grafts, and the external iliac arteries are used to anchor the distal stent‐graft extremity, thereby preventing type Ib endoleak. In these cases, there are two options.
Internal iliac artery occlusion, which is achieved by the embolisation of one or both internal iliac arteries, or by simple coverage of one or both internal iliac arteries by iliac limb stent‐grafts. Although internal iliac artery occlusion is associated with pelvic ischaemia, some investigators have advocated that the incidence of fatal complications is low, and symptoms such as buttock claudication and sexual dysfunction are mostly benign and self‐limited (Mell 2006; Zander 2007); when both internal iliac arteries must be embolised, a staged procedure is preferred rather than simultaneous occlusion; a staged procedure involves treating the arteries at different times and then placing the stent‐graft to allow development of collateral blood vessels and to decrease ischaemic complications (Bosanquet 2017).
Internal iliac artery revascularisation with iliac branch devices or alternative techniques, which maintain flow to the internal iliac artery and pelvic circulation, aiming to decrease complications associated with artery occlusion (Parlani 2012; Taudorf 2016). These procedures are technically more challenging than conventional EVAR, and they require increased procedure time and fluoroscopy time. Tortuosity and/or stenosis in the iliac territory may increase the complexity or even prevent the deployment of internal iliac artery revascularisation devices, leading to technique failure or causing early occlusion of the branches. In such cases, some patients may develop symptoms of pelvic ischaemia (Donas 2017; Ghosh 2009).
To date, neither of these techniques has been established as the best method of treatment for iliac aneurysm.
Why it is important to do this review
Internal iliac artery occlusion is not without possible adverse effects, and pelvic ischaemia may be observed. Buttock claudication may occur in 16% to 50% of patients treated by unilateral exclusion, and in up to 80% of those undergoing bilateral exclusion (Ghosh 2009; Verzini 2009); sexual dysfunction is noted in 10% to 17% of patients (Lee 2001; Mehta 2004; Rayt 2008; Verzini 2009). Devastating complications including ischaemic colitis, gluteal necrosis, and paraplegia have also been described (Maldonado 2004). A recent review observed that buttock claudication occurred in 29.2% and 30.3% of individuals after unilateral and bilateral internal iliac artery occlusion, respectively, along with sexual dysfunction in 10% and 16.9% with occlusion of one and both internal iliac arteries, respectively; major pelvic ischaemia occurred in less than 1% (Bosanquet 2017).
New endovascular devices and alternative techniques have been developed to maintain pelvic circulation through the internal iliac arteries and decrease the complication rates of pelvic ischaemia, while maintaining the collateral network (Bischoff 2011; Lebas 2016). Internal iliac artery revascularisation makes endovascular treatment of aneurysms involving the iliac bifurcation more complex and increases both procedure time and fluoroscopy time. Potential advantages of maintaining flow into the internal iliac arteries during EVAR for aorto‐iliac aneurysms or isolated iliac aneurysms versus internal iliac artery occlusion may be decreased buttock claudication and sexual dysfunction rates, as suggested in a recent review (Kouvelos 2016). This approach may also preserve the quality of life of treated individuals, while decreasing other serious complications, including spinal cord ischaemia, ischaemic colitis, and gluteal necrosis, thereby reducing the morbidity and mortality of EVAR.
This review is important because it will help identify optimal practice and will highlight where there is a need for further research on the management of aorto‐iliac aneurysms and isolated iliac aneurysms involving the iliac bifurcation.
Objectives
To assess the effects of internal iliac artery revascularisation versus internal iliac artery occlusion during endovascular repair of aorto‐iliac aneurysms and isolated iliac aneurysms involving the iliac bifurcation.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include all randomised controlled trials (RCTs) comparing internal iliac artery revascularisation (any endovascular technique) versus internal iliac artery occlusion (with or without embolisation). If some trials include more forms of treatment than are covered by this review, we planned to use only data of interest.
Types of participants
We planned to include participants who have been diagnosed with symptomatic non‐ruptured or asymptomatic thoraco‐abdominal, aorto‐iliac, or isolated iliac aneurysm (fusiform or saccular) involving the iliac bifurcation (unsuitable for a distal seal zone in the common iliac artery) who underwent endovascular aneurysm repair with distal landing zone in the external iliac artery, done by occluding or revascularising one or both internal iliac arteries. We planned to include both males and females of any age. We planned to exclude patients who have been treated with a conventional bifurcated stent‐graft with distal landing zone in the common iliac artery and those who have been treated with the bell‐bottom technique, using flared iliac stents‐grafts into an ectatic or aneurysmal common iliac artery (Fatima 2012). We planned to exclude participants with ruptured aneurysm.
Types of interventions
We planned to include trials comparing internal iliac artery revascularisation (with any iliac branch device or sandwich technique) versus internal iliac artery occlusion (with or without coil embolisation).
Types of outcome measures
Primary and secondary outcomes and the time scales chosen were guided by the reporting standards for endovascular aortic aneurysm repair (Chaikof 2002).
Primary outcomes
Mortality (all cause, procedure related, and aneurysm related): within 30 days of the procedure; within 30 days to 6 months of the procedure; within 6 months to 5 years of the procedure; beyond 5 years of the procedure.
Major pelvic ischaemia (e.g. gluteal and perineal necrosis, ischaemic colitis, spinal cord ischaemia): within 30 days of the procedure.
Minor pelvic ischaemia (e.g. buttock claudication, sexual dysfunction): within 30 days of the procedure, within 30 days up to 2 years of the procedure, beyond 2 years of the procedure.
Secondary outcomes
Technical failure (unsuccessful deployment of the endograft or the need to convert to surgical intervention, mortality, type I or III endoleaks or graft limb obstruction in the first 24 hours postoperatively, and graft obstruction within 30 days).
Endoleaks: within 30 days of the procedure; within 30 days to 6 months of the procedure; within 6 months to 5 years of the procedure; beyond 5 years of the procedure. We will consider the following endoleaks: type Ib, type II ‐ related to the internal iliac artery, and type III ‐ related to iliac branched devices.
Re‐interventions (e.g. treatment of endoleaks, limb obstruction revascularisation, stenosis angioplasty): within 30 days of the procedure; within 30 days to 6 months of the procedure; within 6 months to 5 years of the procedure; beyond 5 years of the procedure.
Leg ischaemia.
Iliac branched devices or parallel stent‐graft patency, or both.
Quality of life: we will use any validated quality of life assessments for individuals with abdominal aortic aneurysms.
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs without language, publication year, or publication status restrictions.
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 28 August 2019).
Cochrane Central Register of Controlled Trials (CENTRAL; 2019 issue 8), in the Cochrane Library, via the Cochrane Register of Studies Online (CRSO).
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily, and Ovid MEDLINE) (searched from 1 January 2017 to 28 August 2019).
Embase Ovid (searched from 1 January 2017 to 28 August 2019).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Ebsco (searched from 1 January 2017 to 28 August 2019).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. When appropriate, these strategies were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 6; Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 28 August 2019.
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We planned to check the bibliographies of all included trials for further references of relevant trials. We contacted specialists in the field, manufacturers (Cook Medical Inc., Bloomington, IN, USA; WL Gore, Newark, DE, USA), and authors of these studies for any possible unpublished data, but we identified no additional studies for inclusion.
Authors' searches
We searched the Latin American and Caribbean Health Science Information database (LILACS) and Indice Bibliográfico Español de Ciencias de la Salud (IBECS), both in lilacs.bvsalud.org/. See Appendix 2 for details of our search strategy. We did not use a filter but selected RCTs manually from the LILACS and IBECS databases. Two review authors (LHDGS and RLGF) configured this strategy, which the Cochrane Brazil Information Specialist and the other review authors revised. The review authors, in collaboration with the Cochrane Brazil Information Specialist, searched this database on 28 August 2019.
Data collection and analysis
Selection of studies
After merging the search results and removing duplicate records, two review authors (LHDGS and RLGF) independently screened the titles and abstracts of trials identified by the literature search for potential relevance. We planned to retrieve and examine the full texts of selected trials for compliance with the eligibility criteria. We planned to document the reasons for exclusion of individual trials. We planned to consult the other review authors (JCCBS and VV) in cases of disagreement.
Data extraction and management
We planned for two review authors (LHDGS and RLGF) to independently extract and collect data on a paper data extraction form. We planned to resolve discrepancies regarding the results by discussion. We planned to consult the other review authors (JCCBS and VV) in cases of disagreement. We planned to collect the following information.
Study features: publication details (e.g. year, country, authors); study design; population data (e.g. age, comorbidities, severity, duration, history concerning treatments, and responses); details of the intervention (e.g. manufacturers, material, site of insertion, open or percutaneous access for stent‐graft and branch‐graft deployment, additional procedures); number of participants randomised into each treatment group; number of participants in each group who failed treatment; numbers of participants lost to follow‐up; duration of follow‐up.
Outcomes: types of outcomes measured; timing of outcomes; adverse events.
Assessment of risk of bias in included studies
We planned for two review authors (LHDGS and RLGF) to independently assess the risk of bias of all included trials according to the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to resolve any discrepancies by discussion or by consultation with the other review authors (JCCBS and VV), if necessary. We planned to assess the following domains, rating them as having low, unclear, or high risk of bias, using Cochrane's 'Risk of bias' tool.
Random sequence generation.
Adequate concealment of allocation.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other potential threats to validity.
We planned to report assessments for each included study in the 'Risk of bias' tables located in the Characteristics of included studies section. In cases of uncertainty over data, we planned to contact study author(s) to seek clarification.
Blinding is difficult for this type of intervention. We planned to take this into consideration when assessing risk of bias in these domains.
Measures of treatment effect
We planned to calculate risk ratios (RRs) and 95% confidence intervals (CIs) for dichotomous variables. We planned to calculate mean differences (MDs) and 95% CIs for continuous outcomes that have used similar scales. We planned to calculate standardised mean differences (SMDs) and 95% CIs for continuous outcomes when different scales have been used. In the event that the necessary information is unavailable, we intended to present in additional tables any data from primary studies that are not parametric (e.g. effects reported as medians, quartiles) and data from studies without sufficient statistical information (e.g. standard deviations, numbers of participants).
Unit of analysis issues
We planned to use the individual participant as our unit of analysis (unit to be randomised for interventions to be compared), that is, the number of observations in the analysis should match the number of individuals randomised. For trials that considered multiple interventions in the same group, we planned to analyse only partial data of interest.
Dealing with missing data
We planned to contact study authors for additional information regarding missing or unavailable data. In cases of non‐response, irrespective of the type of data, we planned to report dropout rates in the Characteristics of included studies tables and to use intention‐to‐treat analysis.
Assessment of heterogeneity
We planned to quantify inconsistency among pooled estimates using the I² statistic (where I² = (Q ‐ df)/Q) × 100%, where Q is the Chi² statistic and 'df' represents the degree of freedom). This illustrates the percentage of variability in effect estimates resulting from heterogeneity rather than sampling error (Higgins 2011). We planned to interpret thresholds for the I² statistic as follows: 0% to 25% is low heterogeneity; 25% to 75% is moderate heterogeneity; and more than 75% is substantial heterogeneity (Higgins 2003).
Assessment of reporting biases
We planned to assess reporting biases or small‐study effects by drawing a funnel plot (trial effect versus trial size) if a sufficient number of studies (more than 10) were included in the review (Higgins 2011).
Data synthesis
We planned to compute pooled estimates of the intervention effect for each outcome using a fixed‐effect model employing Review Manager 5 software If we did not identify substantial heterogeneity (I² > 75%) (RevMan 2014). We planned to perform a random‐effects model analysis if we identified substantial heterogeneity. If it is not possible to carry out a meta‐analysis, we planned to report study results narratively.
Subgroup analysis and investigation of heterogeneity
If possible, we planned to perform subgroup analyses for trials examining the effects of internal iliac artery revascularisation versus internal iliac artery occlusion with or without embolisation. We also intended to perform subgroup analyses to consider the following.
Age.
Gender.
Symptomatic versus asymptomatic aneurysms.
Occlusion of one versus both internal iliac arteries.
Occlusion versus revascularisation of the remaining internal iliac artery in participants who have one internal iliac artery occluded by atherosclerotic disease before intervention.
Staged occlusion of both internal iliac arteries versus revascularisation of one internal iliac artery and occlusion of the opposite one.
Staged occlusion of both internal iliac arteries versus revascularisation of both internal iliac arteries.
Intervention material (e.g. iliac branch devices versus parallel stents).
If we find substantial heterogeneity, and if data are sufficient, we planned to investigate possible causes by further exploring the impact of the condition of individuals and interventions (i.e. participant characteristics, intervention material) using subgroup analysis. We planned to test for subgroup differences by using interaction tests.
Sensitivity analysis
We planned to perform sensitivity analysis based on separation of studies according to blinding of outcome assessment (high, low, or unclear risk of bias). Because blinding is difficult for this type of intervention, if necessary we planned to exclude from the meta‐analysis trials with high or unclear risk of bias in these domains. We planned to present these results and to compare them with the overall findings
Summary of findings and assessment of the certainty of the evidence
We planned to use GRADEpro GDT to prepare a 'Summary of findings' table to present the key information presented in this review (GRADEpro GDT). We have included a draft table in this protocol (Table 2). The population will consist of males and females of any age who have been diagnosed with aorto‐iliac or isolated iliac aneurysm involving the iliac bifurcation (unsuitable for distal seal zone in the common iliac artery) who underwent endovascular aneurysm repair with distal landing zone in the external iliac artery, by occluding or revascularising one or both internal iliac arteries. We planned to compare internal iliac artery revascularisation (with any iliac branch device or sandwich technique) with internal iliac artery occlusion (with or without coil embolisation). For each comparison, we planned to include the following outcomes: mortality, major pelvic ischaemia (e.g. gluteal and perineal necrosis, ischaemic colitis, spinal cord ischaemia), minor pelvic ischaemia (e.g. buttock claudication, sexual dysfunction), technical failure, endoleaks, re‐interventions, and quality of life. We intended to present these data at the most relevant time point. We planned to use the GRADE approach to assess the certainty of evidence for each outcome (Atkins 2004). We intended to assign one of four levels of certainty: high, moderate, low, or very low, based on overall risk of bias, directness of the evidence, inconsistency of results, precision of the estimates, and risk of publication bias, as previously described (Higgins 2011). We intended to base this table on methods described in Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions, and to justify any departures from standard methods (Atkins 2004; Higgins 2011).
2. Example 'Summary of findings' table: internal iliac artery revascularisation versus internal iliac artery occlusion for endovascular repair of iliac aneurysms.
| Internal iliac artery revascularisation versus internal iliac artery occlusion for endovascular repair of iliac aneurysms | ||||||
|
Patient or population: people with aorto‐iliac or isolated iliac aneurysm involving the iliac bifurcation Setting: hospital Intervention: internal iliac artery revascularisationa Comparison: internal iliac artery occlusionb | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with internal iliac artery occlusion | Risk with internal iliac artery revascularisation | |||||
|
Mortality (follow‐up) |
Study population | RR [value] ([value] to [value]) | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 | [value] per 1000 | |||||
|
Major pelvic ischaemia including gluteal and perineal necrosis, ischaemic colitis, spinal cord ischaemia (follow‐up) |
Study population | RR [value] ([value] to [value]) | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 | [value] per 1000 | |||||
|
Minor pelvic ischaemia including buttock claudication, sexual dysfunction (follow‐up) |
Study population | RR [value] ([value] to [value]) | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 | [value] per 1000 ([value] to [value]) | |||||
|
Technical failure (follow‐up) |
Study population | RR [value] ([value] to [value]) | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 | [value] per 1000 ([value] to [value]) | |||||
|
Endoleaks (follow‐up) |
Study population | RR [value] ([value] to [value]) | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 | [value] per 1000 ([value] to [value]) | |||||
|
Re‐interventions (follow‐up) |
Study population | RR [value] ([value] to [value]) | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| [value] per 1000 | [value] per 1000 ([value] to [value]) | |||||
|
Quality of life (follow‐up) |
The mean [outcome] ranged across control groups from [value][measure] | The mean [outcome] in the intervention groups was [value] [lower/higher] [(value to value lower/higher)] | [value] ([value]) | ⊕⊝⊝⊝
very low ⊕⊕⊝⊝ low ⊕⊕⊕⊝ moderate ⊕⊕⊕⊕ high |
||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aEndovascular aneurysm repair with distal landing zone in the external iliac artery, revascularising one or both internal iliac arteries. bEndovascular aneurysm repair with distal landing zone in the external iliac artery, occluding one or both internal iliac arteries.
Results
Description of studies
See Figure 1.
1.
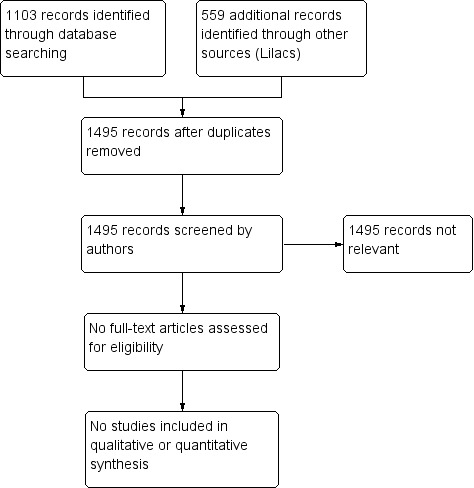
Study flow diagram.
For this review, we found no RCTs that met the inclusion criteria.
Results of the search
From the titles and abstracts of identified records, we classified all 1662 studies identified (167 duplicates) for this review as not relevant because they were not RCTs, or they did not compare the specific types of interventions that we had planned to assess.
Included studies
We found no studies that met the inclusion criteria.
Excluded studies
We excluded no studies from this review.
Risk of bias in included studies
It was not possible to assess methodological quality, as we found no studies that met the inclusion criteria.
Effects of interventions
We identified no RCTs that compared internal iliac artery revascularisation versus internal iliac artery occlusion during endovascular repair of aorto‐iliac aneurysms and isolated iliac aneurysms involving the iliac bifurcation.
Discussion
Endovascular repair of aorto‐iliac aneurysms with inadequate distal seal zone in the common iliac artery leads to type Ib endoleak (Chuter 2001). This situation occurs in approximately 15% of individuals treated with endovascular aortic aneurysm repair (EVAR) (Bosanquet 2017). One of the most commonly used techniques in these cases is unilateral or bilateral internal iliac artery occlusion and extension of the iliac limb stent‐graft to the external iliac arteries with or without embolisation of the internal iliac artery (Fatima 2012; Tefera 2004; Wu 2011). However, this occlusion is not without harms and is associated with ischaemic complications in the pelvic territory such as buttock claudication, sexual dysfunction, ischaemic colitis, gluteal necrosis, and spinal cord injury (Bosanquet 2017; Verzini 2009).
New endovascular devices and alternative techniques such as iliac branch devices and the sandwich technique have been described to revascularise the internal iliac artery and decrease pelvic ischaemic complications in patients not suitable for an adequate seal zone in the common iliac arteries.
This review aimed to assess effects of internal iliac artery revascularisation versus internal iliac artery occlusion during endovascular repair of aorto‐iliac aneurysms and isolated iliac aneurysms involving the iliac bifurcation.
Summary of main results
We found no randomised controlled trials (RCTs) comparing internal iliac artery revascularisation with internal iliac artery occlusion for endovascular treatment of aorto‐iliac aneurysms and isolated iliac aneurysms involving the iliac bifurcation.
Overall completeness and applicability of evidence
We found no RCTs that compared internal iliac artery revascularisation with internal iliac artery occlusion for endovascular treatment of aorto‐iliac aneurysms and isolated iliac aneurysms involving the iliac bifurcation. For this review, we identified 1662 study reports, which we classified as not relevant because they were not RCTs, or because they did not compare the specific types of interventions that we had planned to assess.
We acknowledge that designing and conducting an appropriate study for this topic is difficult, given that the overall incidence of aorto‐iliac aneurysms with inadequate distal seal zone is low. New endovascular devices and alternative techniques such as iliac branch devices and the sandwich technique have been used to revascularise the internal iliac artery, although no evidence‐based RCTs have been done to support their use. This fact reinforces the importance of this review and serves as an incentive for further investigation.
Quality of the evidence
We found no RCTs that were eligible for this review.
Potential biases in the review process
We identified no RCTs that were eligible for this review. The Cochrane Vascular Information Specialist performed a comprehensive search of the literature, and we performed study selection according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Agreements and disagreements with other studies or reviews
A systematic review of the effect of internal iliac artery exclusion included 61 publications (35 cohort studies and 26 case series) and observed that buttock claudication occurred in 29.2% and 30.3% of individuals after unilateral and bilateral internal iliac artery occlusion, respectively; sexual dysfunction occurred in 10% and 16.9% with occlusion of one and both internal iliac arteries, respectively; and major pelvic ischaemia occurred in less than 1% (Bosanquet 2017).
A systematic review of observational studies published in 2016 assessed the effects of internal iliac artery occlusion and internal iliac artery revascularisation during endovascular treatment of aorto‐iliac aneurysms. Review authors included 30 studies about occlusion and 27 about revascularisation of the internal iliac artery and found 36.5% and 4.1% of buttock claudication in the occlusion group and the revascularisation group, respectively (Kouvelos 2016).
Verzini 2009 was a retrospective study comparing patients treated with occlusion of the internal iliac artery and patients treated with revascularisation of this artery during endovascular treatment of iliac aneurysms involving the iliac bifurcation. Verzini 2009 observed a 22% buttock claudication rate in the occlusion group and a 4% buttock claudication rate in patients treated by internal iliac revascularisation.
Taudorf 2016 retrospectively analysed 112 patients, of whom 84 had unilateral iliac aneurysm and 28 had bilateral iliac aneurysm. In total, 140 limbs were treated (115 by occlusion and 25 by revascularisation of the internal iliac artery). Taudorf 2016 observed buttock claudication in 37% of patients treated by occlusion. None of the patients treated with revascularisation techniques had this complication (Taudorf 2016).
The pELVIS registry analysed 575 patients treated with iliac branch devices between 2005 and 2015 and showed a high success rate (97.6%) and low 30‐day mortality (1.9%). Buttock claudication was observed in only one patient (Donas 2017).
American (2018) and European (2019) guidelines recommend revascularisation of at least one internal iliac artery during endovascular treatment of aorto‐iliac aneurysms and iliac aneurysms involving the iliac bifurcation (Chaikof 2018; Wanhainen 2019).
Authors' conclusions
Implications for practice.
We found no RCTs that compared internal iliac artery revascularisation versus internal iliac artery occlusion for endovascular treatment of aorto‐iliac aneurysms. Currently, evidence is insufficient to show the risks and benefits of internal iliac artery revascularisation during EVAR compared with internal iliac artery occlusion. Until more evidence is available, selection of internal iliac artery management strategies for repair should depend on other sources of information, for example, registries, reviews of cohort studies and case series, and society guidelines.
Implications for research.
High‐quality RCTs that compare internal iliac artery revascularisation versus internal iliac artery occlusion for endovascular treatment of aorto‐iliac aneurysms are needed.
There is a need for RCTs with high methodological quality (i.e. adequate report of randomisation, allocation concealment, blinding, etc.) assessing effects on these patients prospectively in an unconfounded randomised study of endovascular treatment for aorto‐iliac aneurysms and inadequate distal seal zone in the common iliac artery.
Calculation of the sample size to verify differences in new‐onset pelvic ischaemia symptoms between the two groups (occlusion versus revascularisation of the internal iliac artery) is difficult due to lack of data. A recent systematic review of observational studies shows risk of pelvic ischaemia of 29.2% versus 4.1% for patients undergoing internal iliac artery occlusion and internal iliac revascularisation during EVAR, respectively (Kouvelos 2016). Therefore, a randomised controlled trial would require around 26 participants in each arm for 80% power and 5% two‐sided significance. Given possible dropouts of 10% over the whole study, the required sample size in each study arm would be 29 participants.
The most important outcomes to be measured are death and pelvic ischaemia symptoms. Other important issues to be considered are technical failure, presence of endoleaks, and quality of life.
In addition to selected outcomes from this review, a cost analysis should be considered as an outcome in future studies.
History
Protocol first published: Issue 11, 2018 Review first published: Issue 7, 2020
Notes
Parts of this protocol are based on a standard template established by Cochrane Vascular.
Acknowledgements
We would like to thank Cochrane Vascular, Cochrane Brazil, and the Division of Vascular and Endovascular Surgery, Universidade Federal de Sao Paulo, Brazil, for their support.
The review authors and the Cochrane Vascular editorial base wish to thank the following peer reviewers for their input: Mr Shahab Hajibandeh, Glan Clwyd Hospital, Bodelwyddan, UK; Rengarajan Rajagopal, India (consumer reviewer).
Appendices
Appendix 1. Database searches
| Source | Search strategy | Hits retrieved |
| CENTRAL via CRSO | #1 MESH DESCRIPTOR Iliac Artery EXPLODE ALL TREES 159 #2 MESH DESCRIPTOR Iliac Aneurysm EXPLODE ALL TREES 12 #3 iliac:TI,AB,KY 1590 #4 aortoiliac:TI,AB,KY 131 #5 aorto‐iliac:TI,AB,KY 45 #6 #1 OR #2 OR #3 OR #4 OR #5 1668 #7 hypogastric:TI,AB,KY 115 #8 #6 AND #7 25 #9 MESH DESCRIPTOR Endovascular Procedures EXPLODE ALL TREES 7561 #10 MESH DESCRIPTOR Stents EXPLODE ALL TREES 3788 #11 MESH DESCRIPTOR Vascular Surgical Procedures 598 #12 MESH DESCRIPTOR Blood Vessel Prosthesis EXPLODE ALL TREES 432 #13 MESH DESCRIPTOR Blood Vessel Prosthesis Implantation EXPLODE ALL TREES 434 #14 endovasc*:TI,AB,KY 2503 #15 endostent*:TI,AB,KY 1 #16 endoluminal:TI,AB,KY 171 #17 endoprosthe*:TI,AB,KY 296 #18 (graft or endograft*):TI,AB,KY 18680 #19 percutaneous*:TI,AB,KY 14230 #20 stent*:TI,AB,KY 11115 #21 EVAR:TI,AB,KY 206 #22 TEVAR:TI,AB,KY 43 #23 #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 43033 #24 iliac and aneurysm 133 #25 aortoiliac and aneurysm 41 #26 aorto‐iliac and aneurysm 12 #27 #1 OR #2 OR #24 OR #25 OR #26 284 #28 #23 AND #27 186 #29 #8 OR #28 204 |
12.11.18 ‐ 204 28.8.19 ‐ 23 |
| ClinicalTrials.gov | aorto‐iliac aneurysm OR Iliac Aneurysm OR Iliac Artery OR iliac OR aortoiliac OR aorto‐iliac | Stent OR Blood Vessel Prosthesis OR Endovascular Procedures OR Vascular Surgical Procedures | 12.11.18 ‐ 60 28.8.19 ‐ 12 |
| ICTRP Search Portal | aorto‐iliac aneurysms OR Iliac Aneurysms OR aortoiliac aneurysms AND Stent OR Blood Vessel Prosthesis OR Endovascular Procedures OR Vascular Surgical Procedures | 12.11.18 ‐ 5 28.8.19 ‐ 0 |
| MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily, and Ovid MEDLINE®) 1946 to present 2017, 2018, and 2019 only |
1 exp Iliac Artery/ 2 exp Iliac Aneurysm/ 3 iliac.ti,ab. 4 aortoiliac.ti,ab. 5 aorto‐iliac.ti,ab. 6 or/1‐5 7 hypogastric.ti,ab. 8 6 and 7 9 exp Endovascular Procedures/ 10 exp STENTS/ 11 exp Vascular Surgical Procedures/ 12 exp Blood Vessel Prosthesis/ 13 exp Blood Vessel Prosthesis Implantation/ 14 endovasc*.ti,ab. 15 endostent*.ti,ab. 16 endoluminal.ti,ab. 17 endoprosthe*.ti,ab. 18 (graft or endograft*).ti,ab. 19 percutaneous*.ti,ab. 20 stent*.ti,ab. 21 EVAR.ti,ab. 22 TEVAR.ti,ab. 23 or/9‐22 24 (iliac and aneurysm).ti,ab. 25 (aortoiliac and aneurysm).ti,ab. 26 (aorto‐iliac and aneurysm).ti,ab. 27 1 or 2 or 24 or 25 or 26 28 23 and 27 29 8 or 28 9336 30 randomized controlled trial.pt. 31 controlled clinical trial.pt. 32 randomized.ab. 33 placebo.ab. 34 drug therapy.fs. 35 randomly.ab. 36 trial.ab. 444273 37 groups.ab. 38 or/30‐37 39 exp animals/ not humans.sh. 40 38 not 39 41 29 and 40 |
12.11.18 ‐ 94 28.8.19 ‐ 60 |
| Embase 2017, 2018, and 2019 only | 1 exp iliac artery/ 2 exp iliac artery aneurysm/ 3 iliac.ti,ab. 4 aortoiliac.ti,ab. 5 aorto‐iliac.ti,ab. 6 or/1‐4 7 hypogastric.ti,ab. 8 6 and 7 9 exp endovascular surgery/ 10 exp stent/ 11 exp vascular surgery/ 12 exp blood vessel prosthesis/ 13 endovasc*.ti,ab. 14 endostent*.ti,ab. 15 endoluminal.ti,ab. 16 endoprosthe*.ti,ab. 17 (graft or endograft*).ti,ab. 18 percutaneous*.ti,ab. 19 stent*.ti,ab. 20 EVAR.ti,ab. 21 TEVAR.ti,ab. 22 or/9‐21 23 (iliac and aneurysm).ti,ab. 24 (aorto‐iliac and aneurysm).ti,ab. 25 (aortoiliac and aneurysm).ti,ab. 26 1 or 2 or 23 or 24 or 25 27 22 and 26 28 8 or 27 29 randomized controlled trial/ 30 controlled clinical trial/ 31 random$.ti,ab. 32 randomization/ 33 intermethod comparison/ 34 placebo.ti,ab. 35 (compare or compared or comparison).ti. 36 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 37 (open adj label).ti,ab. 38 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 39 double blind procedure/ 40 parallel group$1.ti,ab. 41 (crossover or cross over).ti,ab. 42 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 43 (assigned or allocated).ti,ab. 44 (controlled adj7 (study or design or trial)).ti,ab. 45 (volunteer or volunteers).ti,ab. 46 trial.ti. 47 or/29‐46 48 28 and 47 |
12.11.18 ‐ 353 28.8.19 ‐ 231 |
| CINAHL 2017, 2018, and 2019 only |
S39 S26 AND S38 S38 S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 S37 MH "Random Assignment" S36 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" S35 MH "Crossover Design" S34 MH "Factorial Design" S33 MH "Placebos" S32 MH "Clinical Trials" S31 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S30 TX crossover OR "cross‐over" S29 AB placebo* S28 TX trial* S27 TX "latin square" S26 S7 OR S25 S25 S20 AND S24 S24 S1 OR S2 OR S21 OR S22 OR S23 S23 TX aorto‐iliac and aneurysm S22 TX aortoiliac and aneurysm S21 TX iliac and aneurysm S20 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 S19 TX TEVAR S18 TX EVAR S17 TX stent* S16 TX percutaneous* S15 TX graft or endograft* S14 TX endoprosthe* S13 TX endoluminal S12 TX endostent* S11 TX endovasc* S10 MH "Blood Vessel Prosthesis" S9 MH "Vascular Surgery+" S8 MH "Stents+" S7 S5 AND S6 S6 TX hypogastric S5 S1 OR S2 OR S3 OR S4 S4 TX aorto‐iliac S3 TX aortoiliac S2 TX iliac S1 MH "Iliac Artery" |
12.11.18 ‐ 37 28.8.19 ‐ 28 |
Appendix 2. LILACS and IBECS search strategy
((MH:(Iliac Artery) OR (Arteria Ilíaca) OR (Artéria Ilíaca) OR MH:(Aorta Abdominal) OR (Aorta Abdominal) OR (Aorta Abdominal) OR MH:(Aneurysm) OR (Aneurisma) OR (Aneurisma) OR (Saccular Aneurysm) OR MH:(Aortic Aneurysm Abdominal) OR (Aneurisma de la Aorta Abdominal) OR (Aneurisma da Aorta Abdominal) OR (C14.907.055.239.075*) OR (C14.907.109.139.075*) OR MH:(Iliac Aneurysm) OR (Aneurisma Ilíaco) OR (Aneurisma Ilíaco) OR (Aneurysm Iliac) OR MH:(Aortic Aneurysm) OR (Aneurisma de la Aorta) OR (Aneurisma Aórtico) OR (C14.907.055.239*) OR (C14.907.109.139*) OR MH:(Aneurysm Infected) OR (Aneurisma Infectado) OR (Aneurisma Infectado) OR (Aneurysm Bacterial) OR (Aneurysm Mycotic) OR (Mycotic Aneurysm) OR (C01.539.069*) OR (C14.907.055.131*) OR MH:(Endoleak) OR (Endofuga) OR (Endoleak) OR (Endofuga) OR (Endovazamento) OR (Escape Endovascular) OR (Fuga Endovascular) OR (Fuga Vascular Interna) OR (Vazamento Endovascular) OR (Vazamento pós‐Tratamento Endovascular de Aneurisma) OR (Vazamento Vascular Interno) OR (C14.907.055.501*) OR (C23.550.414.941.500) OR (C23.550.767.850.500*)) AND (MH:(Therapeutic Occlusion) OR (Oclusión Terapéutica) OR (Oclusão Terapêutica) OR MH:(Embolization Therapeutic) OR (Embolización Terapéutica) OR (Embolização Terapêutica) OR (Embolotherapy) OR (E02.520.360*) OR (E02.926.500*) OR MH:(Balloon Occlusion) OR (Oclusión con Balón) OR (Oclusão com Balão) OR (Balloon Tamponade) OR (E02.148.106*) OR (E02.520.392.500*) OR (E02.926.500.074*) OR (E05.157.063*) OR MH:(Chemoembolization Therapeutic) OR (Quimioembolización Terapéutica) OR (Quimioembolização Terapêutica) OR (E02.520.360.150*) OR (E02.926.500.150*) OR MH:(Graft Occlusion Vascular) OR (Oclusión de Injerto Vascular) OR (Oclusão de Enxerto Vascular) OR (Vascular Graft Occlusion) OR (Graft Restenosis Vascular) OR (Vascular Graft Restenosis) OR MH:(Endovascular Procedures) OR (Procedimientos Endovasculares) OR (Procedimentos Endovasculares) OR (E04.100.814.529*) OR (E04.502.382*) OR MH:(Stents) OR (Stents) OR (Stents))) AND ( db:("LILACS" OR "IBECS"))
Differences between protocol and review
As suggested by the reviewers, we excluded allocation concealment from the sensitivity analysis. Allocation concealment could be easily achievable with an appropriate method of randomisation.
Contributions of authors
LHDGS: contact person with the editorial base; co‐ordinated contributions from co‐authors; wrote the final draft of the review; drafted the clinical sections of the Background; responded to clinical, methodological, and statistical comments of referees; and is the guarantor of the final review. JCCBS: worked on the Methods section and contributed to writing of the review. VV: worked on the Methods section and contributed to writing of the review. RLGF: worked on the Methods section and contributed to writing of the review. LCUN: contributed to writing of the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office
Declarations of interest
LHDGS: none known. JCCBS: none known. VV: none known. RLGF: none known. LCUN: none known.
New
References
Additional references
Armon 1998
- Armon MP, Wenham PW, Whitaker SC, Gregson RHS, Hopkinson BR. Common iliac artery aneurysms in patients with abdominal aortic aneurysms. European Journal of Vascular and Endovascular Surgery 1998;15(3):255-7. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff 2011
- Bischoff MS, Di Luozzo G, Griepp EB, Griepp RB. Spinal cord preservation in thoracoabdominal aneurysm repair. Perspectives in Vascular Surgery and Endovascular Therapy 2011;23(3):214-22. [DOI] [PubMed] [Google Scholar]
Bosanquet 2017
- Bosanquet DC, Wilcox C, Whitehurst L, Cox A, Williams IM, Twine CP, et al. Systematic review and meta-analysis of the effect of internal iliac artery exclusion for patients undergoing EVAR. European Journal of Vascular and Endovascular Surgery 2017;53(4):534-48. [DOI] [PubMed] [Google Scholar]
Calero 2016
- Calero A, Illig KA. Overview of aortic aneurysm management in the endovascular era. Seminars in Vascular Surgery 2016;29(1-2):3-17. [DOI] [PubMed] [Google Scholar]
Chaikof 2002
- Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, et al. Reporting standards for endovascular aortic aneurysm repair. Journal of Vascular Surgery 2002;35:1048-60. [DOI] [PubMed] [Google Scholar]
Chaikof 2018
- Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. Journal of Vascular Surgery 2018;67(1):2-77. [DOI] [PubMed] [Google Scholar]
Chuter 2001
- Chuter TAM, Faruqi RM, Sawhney R, Reilly LM, Kerlan RB, Canto CJ, et al. Endoleak after endovascular repair of abdominal aortic aneurysm. Journal of Vascular Surgery 2001;34(1):98-105. [DOI] [PubMed] [Google Scholar]
Donas 2017
- Donas KP, Inchingolo M, Cao P, Pratesi C, Pratesi G, Torsello G, et al. Secondary procedures following iliac branch device treatment of aneurysms involving the iliac bifurcation: the pELVIS registry. Journal of Endovascular Therapy 2017;24(3):405-10. [DOI] [PubMed] [Google Scholar]
Fatima 2012
- Fatima J, Correa MP, Mendes BC, Oderich GS. Pelvic revascularization during endovascular aortic aneurysm repair. Perspectives in Vascular Surgery and Endovascular Therapy 2012;24(2):55-62. [DOI] [PubMed] [Google Scholar]
Garland 1932
- Garland HG. The pathology of aneurysm: a review of 167 autopsies. Journal of Pathology and Bacteriology 1932;35(3):333-50. [Google Scholar]
Ghosh 2009
- Ghosh J, Murray D, Paravastu S, Farquharson F, Walker MG, Serracino-Inglott F. Contemporary management of aorto-iliac aneurysms in the endovascular era. European Journal of Vascular and Endovascular Surgery 2009;37(2):182-8. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 31 October 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huang 2008
- Huang Y, Gloviczki P, Duncan AA, Kalra M, Hoskin TL, Oderich GS, et al. Common iliac artery aneurysm: expansion rate and results of open surgical and endovascular repair. Journal of Vascular Surgery 2008;47(6):1203-10. [DOI] [PubMed] [Google Scholar]
Johnston 1991
- Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Disease Prevention. Journal of Vascular Surgery 1991;13(3):452-8. [DOI] [PubMed] [Google Scholar]
Kouvelos 2016
- Kouvelos GN, Katsargyris A, Antoniou GA, Oikonomou K, Verhoeven ELG. Outcome after interruption or preservation of internal iliac artery flow during endovascular repair of abdominal aorto-iliac aneurysms. European Journal of Vascular and Endovascular Surgery 2016;52(5):621-34. [DOI] [PubMed] [Google Scholar]
Lawrence 1999
- Lawrence PF, Gazak C, Bhirangi L, Jones B, Bhirangi K, Oderich G, et al. The epidemiology of surgically repaired aneurysms in the United States. Journal of Vascular Surgery 1999;30(4):632-40. [DOI] [PubMed] [Google Scholar]
Lebas 2016
- Lebas B, Galley J, Legall M, Gerges C, Chaufour X. Preservation of the internal iliac arteries with branched iliac stent-grafts (ZBIS): 5 years of experience. Annals of Vascular Surgery 2016;33:18-22. [DOI] [PubMed] [Google Scholar]
Lee 2001
- Lee WA, O'Dorisio J, Wolf YG, Hill BB, Fogarty TJ, Zarins CK. Outcome after unilateral hypogastric artery occlusion during endovascular aneurysm repair. Journal of Vascular Surgery 2001;33(5):921-6. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6. Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lobato 2011
- Lobato AC. Sandwich technique for aortoiliac aneurysms extending to the internal iliac artery or isolated common/internal iliac artery aneurysms: a new endovascular approach to preserve pelvic circulation. Journal of Endovascular Therapy 2011;18(1):106-11. [DOI] [PubMed] [Google Scholar]
Lowry 1978
- Lowry SF, Kraft RO. Isolated aneurysms of the iliac artery. Archives of Surgery 1978;113(11):1289-93. [DOI] [PubMed] [Google Scholar]
Maldonado 2004
- Maldonado TS, Rockman CB, Riles E, Douglas D, Adelman MA, Jacobowitz GR, et al. Ischemic complications after endovascular abdominal aortic aneurysm repair. Journal of Vascular Surgery 2004;40(4):703-9; discussion 709-10. [DOI] [PubMed] [Google Scholar]
Mehta 2004
- Mehta M, Veith FJ, Darling RC, Roddy SP, Ohki T, Lipsitz EC, et al. Effects of bilateral hypogastric artery interruption during endovascular and open aortoiliac aneurysm repair. Journal of Vascular Surgery 2004;40(4):698-702. [DOI] [PubMed] [Google Scholar]
Mell 2006
- Mell M, Tefera G, Schwarze M, Carr S, Acher C, Hoch J, et al. Absence of buttock claudication following stent-graft coverage of the hypogastric artery without coil embolization in endovascular aneurysm repair. Journal of Endovascular Therapy 2006;13(3):415-9. [DOI] [PubMed] [Google Scholar]
Moll 2011
- Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms: clinical practice guidelines of the European Society for Vascular Surgery. European Journal of Vascular and Endovascular Surgery 2011;41(Suppl 1):S1-58. [DOI] [PubMed] [Google Scholar]
Moore 1995
- Moore WS. The role of endovascular grafting technique in the treatment of infrarenal abdominal aortic aneurysm. Cardiovascular Surgery (London) 1995;3(2):109-14. [DOI] [PubMed] [Google Scholar]
Nelson 2014
- Nelson PR, Kracjer Z, Kansal N, Rao V, Bianchi C, Hashemi H, et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). Journal of Vascular Surgery 2014;59(5):1181-93. [DOI] [PubMed] [Google Scholar]
Oderich 2011
- Oderich GS, Greenberg RK. Endovascular iliac branch devices for iliac aneurysms. Perspectives in Vascular Surgery and Endovascular Therapy 2011;23(3):166-72. [DOI] [PubMed] [Google Scholar]
Paravastu 2014
- Paravastu S, Chandra V, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD004178. [DOI: 10.1002/14651858.CD004178.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parlani 2012
- Parlani G, Verzini F, De Rango P, Brambilla D, Coscarella C, Ferrer C, et al. Long-term results of iliac aneurysm repair with iliac branched endograft: a 5-year experience on 100 consecutive cases. European Journal of Vascular and Endovascular Surgery 2012;43(3):287-92. [DOI] [PubMed] [Google Scholar]
Parodi 1991
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Annals of Vascular Surgery 1991;5(6):491-9. [DOI] [PubMed] [Google Scholar]
Rayt 2008
- Rayt HS, Bown MJ, Lambert KV, Fishwick NG, McCarthy MJ, London NJM, et al. Buttock claudication and erectile dysfunction after internal iliac artery embolization in patients prior to endovascular aortic aneurysm repair. Cardiovascular and Interventional Radiology 2008;31(4):728-34. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Santilli 2000
- Santilli SM, Wernsing SE, Lee ES. Expansion rates and outcomes for iliac artery aneurysms. Journal of Vascular Surgery 2000;31(1 Pt 1):114-21. [DOI] [PubMed] [Google Scholar]
Szilagyi 1966
- Szilagyi DE, Smith RF, DeRusso FJ, Elliott JP, Sherrin FW. Contribution of abdominal aortic aneurysmectomy to prolongation of life. Annals of Surgery 1966;164(4):678-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taudorf 2016
- Taudorf M, Gronvall J, Schroeder TV, Lonn L. Endovascular aneurysm repair treatment of aortoiliac aneurysms: can iliac branched devices prevent gluteal claudication? Journal of Vascular and Interventional Radiology 2016;27(2):174-80. [DOI] [PubMed] [Google Scholar]
Tefera 2004
- Tefera G, Turnipseed WD, Carr SC, Pulfer KA, Hoch JR, Acher CW. Is coil embolization of hypogastric artery necessary during endovascular treatment of aortoiliac aneurysms? Annals of Vascular Surgery 2004;18(2):143-6. [DOI] [PubMed] [Google Scholar]
Verzini 2009
- Verzini F, Parlani G, Romano L, De Rango P, Panuccio G, Cao P. Endovascular treatment of iliac aneurysm: concurrent comparison of side branch endograft versus hypogastric exclusion. Journal of Vascular Surgery 2009;49(5):1154-61. [DOI] [PubMed] [Google Scholar]
Volodos 1986
- Volodos NL, Shekhanin VE, Karpovich IP, Troian VI, Gur'ev IA. A self-fixing synthetic blood vessel endoprosthesis. Vestnik Khirurgii Imeni I.I.Grekova 1986;137(11):123-5. [PubMed] [Google Scholar]
Wanhainen 2019
- Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. European Journal of Vascular and Endovascular Surgery 2019;57(1):8-93. [DOI] [PubMed] [Google Scholar]
Williams 2014
- Williams SK, Campbell WB, Earns JJ. Survey of management of common iliac artery aneurysms by members of the Vascular Society of Great Britain and Ireland. Annals of the Royal College of Surgeons of England 2014;96(2):116-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2011
- Wu Z, Raithel D, Ritter W, Qu L. Preliminary embolization of the hypogastric artery to expand the applicability of endovascular aneurysm repair. Journal of Endovascular Therapy 2011;18(1):114-20. [DOI] [PubMed] [Google Scholar]
Zander 2007
- Zander T, Baldi S, Rabellino M, Rostagno R, Isaza B, Llorens R, et al. Bilateral hypogastric artery occlusion in endovascular repair of abdominal aortic aneurysms and its clinical significance. Journal of Vascular and Interventional Radiology 2007;18(12):1481-6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sousa 2018
- Sousa LHDG, Baptista‐Silva JCC, Vasconcelos V, Flumignan RLG. Internal iliac artery revascularisation versus internal iliac artery occlusion for endovascular treatment of aorto-iliac aneurysms. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD013168. [DOI: 10.1002/14651858.CD013168] [DOI] [PMC free article] [PubMed] [Google Scholar]


