Abstract
Background
Anti-PD1/PD-L1 directed immune checkpoint inhibitors (ICI) are widely used to treat patients with advanced non-small-cell lung cancer (NSCLC). The activity of ICI across NSCLC harboring oncogenic alterations is poorly characterized. The aim of our study was to address the efficacy of ICI in the context of oncogenic addiction.
Patients and methods
We conducted a retrospective study for patients receiving ICI monotherapy for advanced NSCLC with at least one oncogenic driver alteration. Anonymized data were evaluated for clinicopathologic characteristics and outcomes for ICI therapy: best response (RECIST 1.1), progression-free survival (PFS), and overall survival (OS) from ICI initiation. The primary end point was PFS under ICI. Secondary end points were best response (RECIST 1.1) and OS from ICI initiation.
Results
We studied 551 patients treated in 24 centers from 10 countries. The molecular alterations involved KRAS (n = 271), EGFR (n = 125), BRAF (n = 43), MET (n = 36), HER2 (n = 29), ALK (n = 23), RET (n = 16), ROS1 (n = 7), and multiple drivers (n = 1). Median age was 60 years, gender ratio was 1 : 1, never/former/current smokers were 28%/51%/21%, respectively, and the majority of tumors were adenocarcinoma. The objective response rate by driver alteration was: KRAS = 26%, BRAF = 24%, ROS1 = 17%, MET = 16%, EGFR = 12%, HER2 = 7%, RET = 6%, and ALK = 0%. In the entire cohort, median PFS was 2.8 months, OS 13.3 months, and the best response rate 19%. In a subgroup analysis, median PFS (in months) was 2.1 for EGFR, 3.2 for KRAS, 2.5 for ALK, 3.1 for BRAF, 2.5 for HER2, 2.1 for RET, and 3.4 for MET. In certain subgroups, PFS was positively associated with PD-L1 expression (KRAS, EGFR) and with smoking status (BRAF, HER2).
Conclusions
: ICI induced regression in some tumors with actionable driver alterations, but clinical activity was lower compared with the KRAS group and the lack of response in the ALK group was notable. Patients with actionable tumor alterations should receive targeted therapies and chemotherapy before considering immunotherapy as a single agent.
Keywords: immunotherapy, lung cancer, oncogenic addiction
Key Message
Question: Is immunotherapy efficient in patients with lung cancer and harboring an oncogenic addiction?
Findings: Patients’ outcome treated with ICI monotherapy were consistent with ICI registration trials in the KRAS-subgroup but were inferior for patients with actionable driver mutations.
Meaning: ICI should thus only be considered after exhaustion of targeted and standard therapies.
Alt-text: Unlabelled Box
Introduction
The management of patients with stage 4 non-small-cell lung cancer (NSCLC) is currently undergoing significant transformation. Molecular testing, targeted therapies, and immunotherapy are now part of routine clinical care [1]. Targeted therapies are efficient in the context of oncogenic driver mutations [2]. These treatments are associated not only with high response rate, but also with unavoidable development of resistance and tumor recurrence [3]. Therapeutic options are restrained in patients after exhaustion of targeted therapies and chemotherapy. Immune checkpoint inhibitors (ICI) that block the programmed death-1 (PD-1)/programmed death ligand 1 (PD-L1) axis is a new standard of care [4., 5., 6.]. ICI response rates in general are ∼20% in unselected NSCLC, but overall survival (OS) benefit was well documented in registration trials [7., 8., 9., 10.].
Whether ICIs alone or even in combination with TKIs would offer comparable benefit in oncogene addicted subtypes of NSCLC as much as in the general unselected NSCLC population has been raised as a relevant question [11]. We may expect that immunotherapy may transform the important tumor responses achieved with targeted inhibitors in prolonged remissions. Nevertheless, data obtained from subgroups in clinical trials [9, 10, 12] and from investigators observations have shown rather weak activity of ICI in NSCLC patients harboring actionable driver mutations [13]. Therefore, the optimal use of ICI therapy in patients with actionable driver mutations remains an important field of ongoing research.
The purpose of this study was to analyze the clinical activity of ICI therapy in the context of oncogenic driver alterations. We previously conducted registry studies on targeted therapies for NSCLC with ROS1, HER2, BRAF, and RET alterations [14., 15., 16., 17., 18.]. We used our established network to perform a wide international cohort of patients with molecularly defined NSCLC. Hereinafter, we present the results for the whole cohort, and for individual molecular subgroups.
Patients and methods
Study objectives
The primary objective of our study was to describe the progression-free survival (PFS) of patients treated with PD1/PD-L1 checkpoint inhibitors (ICI) in each subgroup carrying an oncogenic driver. The secondary objectives were both the best overall response (that was not confirmed by a second measurement) and the OS for each molecular subgroup. We also analyzed the outcome of patients according to smoking status, line of treatment, and PD-L1 expression.
Patients’ selection
A global multicenter network of thoracic oncologists accrued patients in this registry. Investigators were identified via an ongoing collaboration established by our prior registries [14., 15., 16., 17., 18.]. Eligible patients had (i) a pathologic diagnosis of lung cancer; (ii) local testing positive (either direct sequencing or NGS on validated platforms) for at least one oncogenic driver mutation: EGFR (exon 18–21) activating mutation, HER2 (exon 20) activating mutation, KRAS mutation, BRAF (exon 15) mutation, MET amplification or exon 14 mutation, ALK rearrangement, ROS1 rearrangement or RET rearrangement; (iii) single agent ICI therapy with commercial anti-PD1/PD-L1-antibodies; (iv) local response assessment according to RECIST1.1 criteria; (v) follow-up with survival status. Optionally, investigators were asked to record immunotherapy-related adverse events (irAE) and PD-L1 expression in tumor cells.
PD-L1 analysis
PD-L1 analysis was carried out in each center according to local procedures. Antibodies used were E1L3N (32.8%), SP142 (31.7%), 22C3 (22.2%), SP263 (6.7%), 28-8 (5.6%), and others (1.1%). Results were provided in percentage of staining of tumor cells with three cut-off levels: 1%, 10%, and 50%.
Ethical considerations
The study was approved by the national ethics committees of France (CEPRO 2017-043, CNIL Nh22181405I) and Switzerland (Swissethics/EKNZ ID 2017-01530). Participating centers were responsible for patients’ consent and institutional approval. All contributors were trained in Good Clinical Practice. The study was a purely academic collaboration granted by both Toulouse and Lucerne Hospitals and was not funded by industry.
Data collection and response assessment
Anonymized clinical data were recorded by local investigators using electronic case report forms (eCRF) in a password-protected secure online portal from the University of Toulouse (https://ec.claudiusregaud.fr/CSOnline/). Data were centrally collected at the University of Toulouse (France). The registry was open for enrollment from May 2017 until April 2018. Best response to systemic therapies, defined as a complete or partial response achieved at least once during the course of therapy, was assessed locally using RECIST v1.1 criteria.
Statistical methods
All statistical evaluations were carried out according to the predefined plan as stated in the protocol. Data were summarized according to frequency and percentage for qualitative variables, and by median and range for quantitative variables. The 95% confidence interval for response rate was calculated using the exact binomial distribution. PFS was measured as the time from the first administration of ICI therapy to progression defined by RECIST1.1, or death due to any cause. Patients alive without progression at the time of analysis were censored at the initiation of a new therapy or last follow-up. OS was measured as the time from the first administration of ICI therapy to death due to any cause. Patients alive at the time of analysis were censored at the last follow-up. Survival data were estimated using the Kaplan–Meier method and compared using the log-rank test in overall cohort and oncogenic driver subgroups. Statistical analyses were carried out using STATA 13.1 software (StataCorp, TX).
Results
Patients’ characteristics
During an enrollment phase of almost 1 year, the registry included 551 patients from 24 centers in 10 countries. The molecular alterations involved KRAS (n = 271), EGFR (n = 125), BRAF (n = 43, V600En = 17, other n = 18), MET (n = 36, MET amplification n = 13, exon 14 skipping mutation n = 23), HER2 (n = 29), ALK (n = 23), RET (n = 16), and ROS1 (n = 7). A total of 34 patients with more than 1 driver were allocated to the dominant oncogenic driver. Details are provided in the supplementary Figure S1 and S2, available at Annals of Oncology online. Median age was 60 years (range 29–83). Gender ratio was 1 : 1. Smoking status was 28% never smokers, 51% former smokers, and 21% current smokers. The majority (96%) of tumors were adenocarcinoma. At the time of immunotherapy initiation, most patients had ECOG performance status (PS) of 1 (64%), while fewer patients were PS0 (21%), PS2 (11%), and PS3/4 (4%). All patients presented an advanced tumor stage at the beginning of immunotherapy. The clinical characteristics of each subgroup are reported in Table 1.
Supplementary Data.

Table 1.
Clinical and biological description according to mutation type
| EGFR |
KRAS |
ALK |
BRAF |
ROS1 |
HER2 |
RET |
MET |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 125 | N = 271 | N = 23 | N = 43 | N = 7 | N = 29 | N = 16 | N = 36 | |||||||||
| Gender (n=551) | ||||||||||||||||
| Male | 48 | 38.4% | 141 | 52% | 12 | 52.2% | 24 | 55.8% | 5 | 71.4% | 15 | 51.7% | 7 | 43.8% | 21 | 58.3% |
| Female | 77 | 61.6% | 130 | 48% | 11 | 47.8% | 19 | 44.2% | 2 | 28.6% | 14 | 48.3% | 9 | 56.3% | 15 | 41.7% |
| Smoking (n=551) | ||||||||||||||||
| Never smoker | 78 | 63.4% | 12 | 4.6% | 10 | 47.6% | 11 | 26.2% | 5 | 71.4% | 14 | 51.9% | 10 | 66.7% | 8 | 23.5% |
| Former smoker | 38 | 30.9% | 168 | 64.6% | 8 | 38.1% | 22 | 52.4% | 2 | 28.6% | 12 | 44.4% | 4 | 26.7% | 15 | 44.1% |
| Current smoker | 7 | 5.7% | 80 | 30.8% | 3 | 14.3% | 9 | 21.4% | 0 | 0% | 1 | 3.7% | 1 | 6.7% | 11 | 32.4% |
| Missing | 2 | 11 | 2 | 1 | 2 | 1 | 2 | |||||||||
| Histological type (n=551) | ||||||||||||||||
| Adenocarcinoma | 121 | 96.8% | 262 | 96.7% | 21 | 91.3% | 40 | 93% | 6 | 85.7% | 28 | 96.6% | 14 | 87.5% | 34 | 94.4% |
| Squamous | 1 | 0.8% | 0 | 0% | 0 | 0% | 1 | 2.3% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| Sarcomatoid | 0 | 0% | 1 | 0.4% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 2.8% |
| Large cell carcinoma | 0 | 0% | 6 | 2.2% | 1 | 4.3% | 1 | 2.3% | 0 | 0% | 1 | 3.4% | 1 | 6.3% | 0 | 0% |
| Not specified/other/missing | 3 | 2.4% | 2 | 0.7% | 1 | 4.3% | 1 | 2.3% | 1 | 14.3% | 0 | 0% | 1 | 6.3% | 1 | 2.8% |
| Age at diagnosis (n=551) | ||||||||||||||||
| Median (year) | 60 | 59 | 55 | 61 | 45 | 62 | 54.5 | 63 | ||||||||
| Range (year) | 33–80 | 30–83 | 30–73 | 42–75 | 42–67 | 31–77 | 29–73 | 4–82 | ||||||||
Treatment characteristics and safety
Most (94%) patients received anti-PD1-antibodies (nivolumab n = 466, pembrolizumab n = 48, other n = 6), fewer patients (6%) had anti-PD-L1-antibodies (atezolizumab n = 19, durvalumab n = 11, other n = 1). ICIs were given in the first (5%), second (41%), third (26%), fourth line (13%) or in later lines (14%) of treatment (supplementary Table S3, available at Annals of Oncology online). The recording of significant (grades 3 and 4) irAE was optional. From 462 patients with available data, 50 (10.8%) had grade 3–5 irAEs, including 36 (7.8%) of grade 3, 13 (2.8%) of grade 4, and 1 of grade 5 (0.2%, endocrine disorder). The pneumonitis rate was in the expected range (13 cases, 2.8% including 8 grade 3 and 5 grade 4). No unexpected irAEs were recorded.
PD-L1 expression
PD-L1 status was available for 214 patients. The median number of positive cells was 10%. Using a 1% cut-off, one-third was negative (33.2%) and two-third was positive (66.8%). Using a 10% cut-off, half of the tumors was negative (49.7%) and half positive (50.3%). Using a 50% cut-off, one-third of the tumors was positive (33.9%). Looking into each subgroup, we found that median percentage of cells expressing PD-L1 was 0 in HER2 (n = 13), 3.5 in EGFR (n = 38), 7.5 in ALK (n = 10), 12.5 in KRAS (n = 80), 26 in RET (n = 6), 30 in MET (n = 15), 50 in BRAF (n = 9), and 90 in ROS1 (n = 5) subgroups (supplementary Table S4 and Supplementary Figure S5, available at Annals of Oncology online).
Clinical outcomes
Response rate
The rate of any partial or complete response was 19% [95% CI 16% to 23%], ranging from 0% in ALK patients to 26% in KRAS-mutated patients. If we consider the KRAS patients as a control group and exclude them from the analysis, the best response rate for patients harboring all other molecular alterations was 12.7%. We then classified the subgroups according to the rate of progressive disease (PD). PD was observed in 46% for BRAF, 50% for MET, 51% for KRAS, 67% for HER2, 67% for EGFR, 68% for ALK, 75% for RET, and 83% for ROS1. (Figure 1; supplementary Table S6, available at Annals of Oncology online). Details according to the mutation subtype are in supplementary Table S7, available at Annals of Oncology online.
Figure 1.
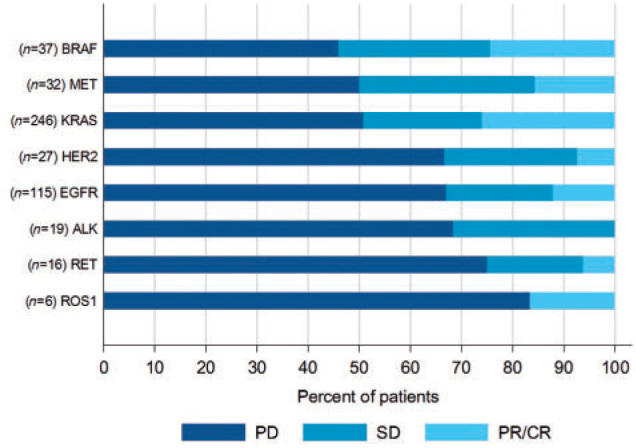
Best response to ICI according to RECIST criteria (PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response).
Overall survival
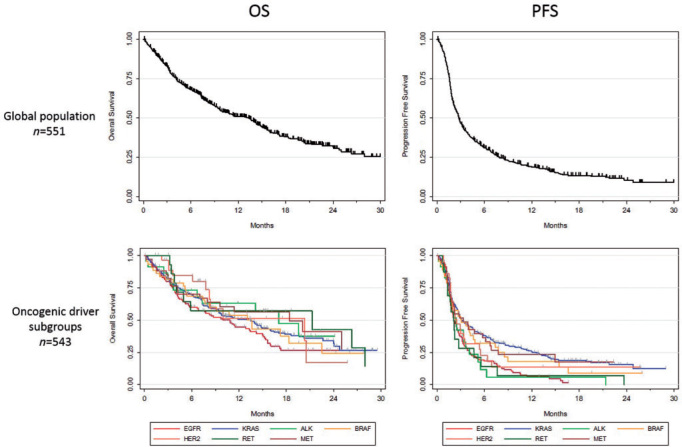
In the entire cohort, median follow-up was 16.1 months, and median OS from start of ICI therapy was 13.3 months [10.0–14.9] (Figure 2). Median OS (in months) for individual molecular subgroups was 10.0 [6.7; 14.2] for EGFR mutated patients, 13.5 [9.4; 15.6] for KRAS, 17.0 [3.6; NR] for ALK, 13.6 [7.4; 22.5] for BRAF, 20.3 [7.8; NR] for HER2, 21.3 [3.8; 28.0] for RET, and 18.4 [7.0; NR] for MET (supplementary data S7, available at Annals of Oncology online). In the univariate analysis, OS did not correlate with gender, age, smoking, number of prior therapies, or PD-L1 expression (supplementary Table S8, available at Annals of Oncology online).
Figure 2.
Overall survival (on the left) and progression-free survival (on the right) in the whole cohort (upper figures) and in each subgroup (lower figures).
Progression-free survival
In the entire cohort, median PFS was 2.8 months [95% CI 2.5–3.1]. Median PFS (in months) for individual molecular subgroups was 2.1 [1.8; 2.7] for EGFR, 3.2 [2.7; 4.5] for KRAS, 2.5 [1.5; 3.7] for ALK, 3.1 [1.8; 4.6] for BRAF, 2.5 [1.8; 3.5] for HER2, 2.1 [1.3; 4.7] for RET, and 3.4 [1.7; 6.2] for MET (Figure 2). Long-term responders were more frequent in KRAS (12 months PFS: 25.6%), MET (23.4%), and BRAF (18.0%) subgroups, than in EGFR (6.4%), ALK (5.9%), HER2 (13.6%), and RET (7.0%) subgroups (Table 2). If we exclude KRAS patients from the analysis (n = 279 patients with all other alterations), median PFS was 2.4 months.
Table 2.
PFS according to primary oncogenic driver from initiation of ICI
| EVT/N | Median PFS [95% CI] (months) | 6-month PFS [95% CI] | 12-month PFS [95% CI] | |
|---|---|---|---|---|
| KRAS | 208/271 | 3.2 [2.7; 4.5] | 37.9 [32.1; 49.8] | 25.6 [20.2; 31.3] |
| EGFR | 117/125 | 2.1 [1.8; 2.7] | 18.4 [12.1; 25.6] | 6.4 [2.7; 12.1] |
| BRAF | 34/43 | 3.1 [1.8; 4.6] | 32.1 [18.3; 46.6] | 18.0 [7.2; 32.7] |
| HER2 | 23/29 | 2.5 [1.8; 3.5] | 22.7 [8.9; 40.2] | 13.6 [3.6; 30.1] |
| MET | 26/36 | 3.4 [1.7; 6.2] | 36.5 [20.7; 52.4] | 23.4 [10.6; 39.0] |
| ALK | 21/23 | 2.5 [1.5; 3.7] | 11.8 [2.2; 30.2] | 5.9 [ 0.4; 23.0] |
| ROS1 | – | – | – | – |
| RET | 15/16 | 2.1 [1.3; 4.7] | 14.1 [2.3; 35.9] | 7.0 [0.4; 27.1] |
EVT, event; N, number.
In the univariate analysis, PFS significantly correlated with smoking (median PFS: 2.5, 2.8, and 3.5 months for never smokers, former smokers, and current smokers, respectively, P < 0.0001), and with PD-L1 expression (3.0 versus 4.2 months for negative and positive expression of PD-L1, P = 0.02). However, PFS did not correlate with gender (P = 0.5), age (P = 0.3), or number of previous lines of treatment (P = 0.08) (supplementary Table S9 and S10, available at Annals of Oncology online). Interestingly, a higher rate of rapid progression (within 2 months) was observed for EGFR (44.8%), ALK (45.5%), ROS1 (42.9%), and RET (43.8%) patients than for KRAS (36%) (supplementary Table S11, available at Annals of Oncology online), respectively.
Molecular subgroup analyses
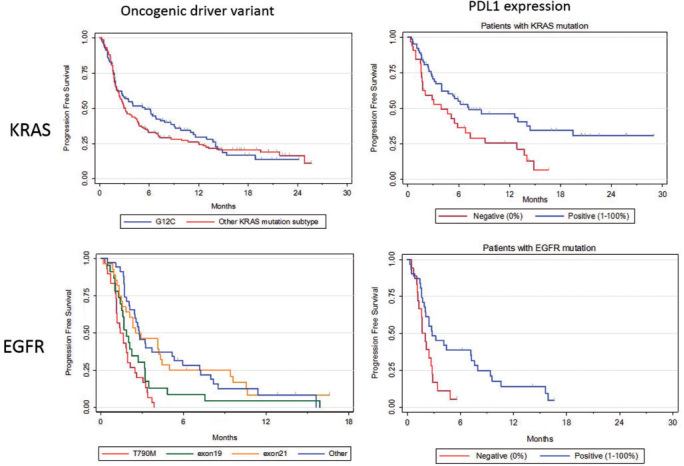
KRAS mutations were identified in 271 patients. PFS was not significantly different regarding KRAS mutation subtype if we compare G12C (n = 100) to other mutations (n = 143, P = 0.47) or G12D (n = 39) versus other KRAS mutations (n = 204, P = 0.40). PFS did also not correlate with smoking (P = 0.98), or with the number of previous lines of treatment. In patients with available PD-L1 expression data (n = 95), PD-L1 positive expression was significantly (P = 0.01) correlated with a longer PFS (median PFS: 7.2 versus 3.9 months) (Figure 3). We also separate patients harboring KRAS transition (G12D, G13D, G12S) from KRAS transversion (G12C, G12A, G12V, G13C). PFS was not impacted by the nature of KRAS alteration (2.9 months for transition, 4.0 for transversion, P = 0.27; supplementary Table S12, available at Annals of Oncology online).
Figure 3.
PFS according to oncogenic drivers’ variants and PDL1 expression.
PFS was significantly different across EGFR molecular subgroups ranging from 1.4 month in T790M and complex mutations subgroup to 1.8 for exon 19, 2.5 for exon 21, and 2.8 for other mutations (P < 0.001). PFS correlated neither with smoking (P = 0.06), nor with the number of previous lines of treatment. PD-L1 positivity was significantly correlated with a longer PFS (2.8 months versus 1.7, P = 0.01) (Figure 3).
For BRAF patients, PFS was significantly higher in smokers versus never smokers (4.1 versus 1.9 months, P = 0.03). Median PFS was numerically shorter in the V600E subgroup (1.8 months) compared with other BRAF mutations (4.1 months, P = 0.20).
MET molecular alterations were found in 36 patients. Median PFS correlated neither with alteration subtype (exon 14 skipping mutation versus other MET alterations, P = 0.09), nor with smoking.
HER2 mutations were identified in 29 patients. PFS correlated with smoking (3.4 months for smokers versus 2.0 months for never smokers, P = 0.04).
Due to a low number of patients, ALK, ROS1, and RET were analyzed together in a subgroup termed ‘rearrangements’. Median PFS was only slightly higher in never smokers (2.6 months) than in smokers (1.8 months, P = 0.03). PD-L1 was not available in enough patients but no tumor response was reported in patients from this group in the context of PD-L1 positivity (supplementary Table S13 and Supplementary Figure S5, available at Annals of Oncology online). Main results for all cohorts are presented in supplementary Figure S14, available at Annals of Oncology online.
Discussion
The standard of care for patients with actionable driver alterations is a targeted therapy. After exhaustion of targeted agents and chemotherapy, immunotherapy may be considered as a salvage treatment. Nevertheless, evidence to support the role of ICI in this setting is controversial, as EGFR and ALK alterations have been associated with low ICI efficacy in prior studies [19]. To address this issue, we conducted a global ‘real world’ study. Our study was retrospective and had other limitations, including reporting bias, lack of central molecular and radiologic assessment, and variable scanning intervals. Nevertheless, we obtained new findings of clinical relevance.
In the overall cohort, the best response with ICI therapy by RECIST was 19%, and median PFS was 2.8 months. This result was mainly driven by the large KRAS-subgroup, and it is in concordance with registration trials testing immunotherapy in pretreated patients, regardless EGFR or ALK status [9, 10]. Regarding molecular subgroups, we confirmed that patients with KRAS-mutant NSCLC derived a greater benefit from ICI than EGFR-mutant NSCLC, as previously reported [9]. It has been reported that KRAS-mutant NSCLC are more likely to express PD-1 and PD-L1 [20]. In our study, we have not been able to detect a significant correlation between KRAS mutation subtypes and PFS, but we confirmed that PD-L1 expression is associated with a better outcome. The limited number of patients with available PDL1 status and the heterogeneity of the tests did not allow us to draw a definitive conclusion on its potential interest. Recently, STK11/LKB1 co-mutation in KRAS-mutant NSCLC was reported as a new predictive marker for tumor resistance to ICI therapy [21]. STK11 was not part of routine testing and our study did not include tissue collection, therefore, future studies will have to validate this interesting finding in a larger cohort. ICI are thus an adequate treatment of KRAS-mutated patients.
Concerning patients with EGFR mutation, the role of ICI therapy is still controversial. Recent studies showed an inverse relationship between PD-L1 expression and EGFR mutations. Moreover, an uninflamed tumor microenvironment is often reported in the context of oncogenic addiction [22, 23]. Gainor et al. also suggested that a dearth of tumor-infiltrating CD8+ lymphocytes, may explain the low response rate to PD-1 axis inhibitors observed amongst EGFR- and ALK-driven NSCLC [24]. A recent meta-analysis including three randomized trials of immunotherapy in TKI-pretreated patients reported that ICI do not improve OS compared with docetaxel in patients with EGFR-mutant NSCLC [25]. In addition, a recent phase II trial of pembrolizumab in TKI-naive patients with PD-L1 positive EGFR-mutant NSCLC showed no RECIST responses in the first 11 patients [26]. In the phase II trial ATLANTIC of durvalumab in EGFR/ALK mutant NSCLC, response rate was 3.6% for PD-L1 <25%, and 12.2% for PD-L1 >25%. Median PFS was 1.9 month [19]. Benefit has, however, been reported in patients with EGFR mutations with the combination of carboplatin, paclitaxel, bevacizumab, and atezolizumab in the IMpower150 trial [5].
BRAF mutations were associated with slightly better outcomes compared with EGFR mutations (RR 24% and PFS 3.1 months). The potential efficacy of immunotherapy in BRAF-mutant melanoma has already been suggested [27]. Recently, Dudnik et al. reported frequent expression of PDL1 and comparable PFS (3.7 months) in BRAF V600E-mutated patients [28]. In our study, PFS in patients with BRAF-mutant NSCLC was positively associated with smoking status. It thus appears that immunotherapy may be considered in BRAF positive patients after targeted therapy and one line of chemotherapy.
ALK, ROS1, and RET translocation represent a small subgroup of NSCLC. In our study, PD-L1 expression was relatively high in those cases. However, most tumors were refractory to ICI therapy. These observations were consistent with other studies, namely with ATLANTIC for ALK, and with a cohort study from MSKCC for RET [29]. Although these data are preliminary, we do not recommend ICI as single agents in patients with ALK/ROS1/RET rearranged NSCLC.
In conclusion, patients’ outcome treated with ICI monotherapy overall were consistent with ICI registration trials, based on the large KRAS-subgroup in our study. However, outcomes for patients with actionable driver mutations (EGFR, ALK, ROS1) were inferior and ICI should only be considered after exhaustion of targeted therapies and in some cases, potentially in all other therapies including standard and salvage chemotherapies. We think that there are two ways to optimize the use of immunotherapy in the context of oncogenic addiction. The first one is to combine immunotherapy with other drugs such as chemotherapy and antiangiogenic agents. The second one is to identify new relevant biomarkers besides PD-L1 expression and TMB considering the complex molecular biology of NSCLC.
Acknowledgements
We thank all participating centers for supporting this study and the eCRF team.
Funding
This work was supported by public funding from Toulouse University Hospital (France) and Lucerne Cantonal Hospital (Switzerland). The research was carried out with no industry support and the paper was written by the authors without editorial assistance. No grant number is applicable.
Toulouse University Hospital
Lucerne Cantonal Hospital
Novartis
Roche/Genentech
Pfizer
Astrazeneca
Disclosure
JM reported consulting advisory role for Novartis, Roche/Genentech, Pfizer, BMS, E Lilly/ImClone, MSD, Astrazeneca; research funding from Roche, BMS, Astrazeneca; travel fees from Pfizer, Roche, BMS. AM reported honoraria from Roche, BMS; consulting advisory role from Roche BMS, Boerhinger, travel fees from BMS. AD reported honoraria from Bayer, Lilly, Ariad/Millenium, BergenBio, Hengrui, Exelixis, Tyra, Verastem, GSK, Teva, Taiho, PharmaMar, Merck, Puma, Foundation Medicine, Wolters Kluwer. FB reported honoraria from Astrazeneca, Boerhinger, E Lilly, Merck, MSD, Novartis, P Fabre, Pfizer, Roche, Takeda; consulting advisory role from Astrazeneca, Boerhinger, E Lilly, Merck, MSD, Novartis, P Fabre, Pfizer, Roche, Takeda; research funding from Astrazeneca, BMS, P Fabre, Roche. PB reported honoraria from BMS, Boerhinger. ABC reported honoraria from Astra Zeneca, BMS, MSD, Roche, Pfizer, Novartis, Takeda; consulting advisory role from Astrazeneca, Novartis, Pfizer, Roche; research funding from Merck Serrono, Novartis; travel fees from Roche, Pfizer, Astra Zeneca. SC reported honoraria from Pfizer, Astrazeneca, MSD, Novartis, Boerhinger, BMS, E Lilly, Merck Serrono, Chugai Pharma; research funding from Roche, Pfizer, Astrazeneca, Boerhinger. VG reported honoraria from MSD; consulting advisory role from Astrazeneca, Roche, Boerhinger, BMS, Abbvie; travel accommodation from Pfizer. AD reported consulting advisory role from Ignyta, Loxo, TP Therapeutics, Astrazeneca, Pfizer, Blueprint Medicines, Roche/Genentech, Takeda, Helsinn Therapeutics, BeiGene. SIO reported honoraria from Pfizer, Roche, Genentech, Takeda, Novartis, Astrazeneca, Foundation Medicine; consulting advisory role from Pfizer, Roche, Novartis, Astrazeneca, takeda, Foundation medicine, TP Therapeutics, Ignyta; speakers bureau from Genentech, Astrazeneca; Takeda; research funding from Pfizer, Roche, Astrazeneca, MedImmune, Clovis Oncology, ARIAD, Ignyta, Peregrine Pharma, GSK, Astellas Pharma, Chugai Pharma. AC-F reported consulting advisory role from Roche, Boerhinger, BMS, Pfizer, Astrazeneca, MSD, Takeda. JN reported consulting advisory role from Takeda, Astrazeneca, Genentech, Lilly; research funding from Genentech, Merck, Novartis, Boerhinger, Exelixis, Takeda, Nektar Therapeutics. TLN reported honoraria from Takeda, Ariad, Boerhinger. SN reported Speakers Bureau from Astrazeneca, MSD, BMS, Roche, Pfizer, Lilly, Takeda. NP reported honoraria from Astrazeneca, Boerhinger, BMS, Lilly, MSD, Novartis, Pfizer, Roche, NovellusDx, FMI, Gaurdants360; consulting advisory role from Astrazeneca, Boerhinger, BMS, Lilly, MSD, Novartis, Pfizer, Roche, NovellusDx, FMI, Gaurdants360; research funding from Astrazeneca, Boerhinger, BMS, Lilly, MSD, Novartis, Pfizer, Roche, NovellusDx, FMI, Gaurdants360; travel fees from Astrazeneca, Boerhinger, BMS, Lilly, MSD, Novartis, Pfizer, Roche, NovellusDx, FMI, Gaurdants360. SIR reported consulting advisory role from BMS, Astrazeneca, Lilly, Boerhinger, Eisai, Roche, Novartis, Merck, MSD, Astellas, Bayer, Pfizer, Takeda; research funding from Boerhinger, Astrazeneca, BMS, Eisai, Merck, Expert Testimony from Roche, Astrazeneca, BMS, Roche, Lilly, Astrazeneca, Amgen. TLN reported Honoraria from Ariad, consulting advisory role from Boerhinger. RV reported honoraria and consulting advisory role from Boerhinger, MSD, BMS, Astrazeneca; research funding from Roche, BMS, Takeda, Abbvie, Pfizer, Merck. HW reported honoraria from Novartis, Astrazeneca; research funding from Genentech, Pfizer, Lilly, Celgene, astrazeneca, exelixis, Novartis, Clovis, Xcovery, BMS, Gilead, Pharmacyclics, ACEA biosciences; travel fees from Astrazeneca. MW reported travel fees from Illumina, Astrazenca. VWZ reported Stock from TP therapeutics; honoraria from Astrazeneca, consulting advisory role from Astrazeneca, Takeda, TP therapeutics; speakers bureau from Astrazeneca, Roche. AAT, OG, MVdH, MLT, AL, CM, LM, JM, LM, SP, RR, DS, DRC, GZ, BS, MF, BB have declared no conflicts of interest.
Footnotes
Note: The preliminary results were previously presented at the ASCO-SITC meeting (26 January 2018, San Francisco, abstract no. 172) and at the ASCO Annual Meeting (1 June 2018, abstract no. 9010, oral communication in Clinical Science Symposium).
References
- 1.Doroshow D.B., Herbst R.S. Treatment of advanced non-small cell lung cancer in 2018. JAMA Oncol. 2018;4(4):569–570. doi: 10.1001/jamaoncol.2017.5190. [DOI] [PubMed] [Google Scholar]
- 2.Resources N. NCCN Framework for Resource Stratification of NCCN Guidelines (NCCN Framework™) 2019 https://www.nccn.org/framework/ [Google Scholar]
- 3.Cortot A.B., Janne P.A. Molecular mechanisms of resistance in epidermal growth factor receptor-mutant lung adenocarcinomas. Eur Respir Rev. 2014;23(133):356–366. doi: 10.1183/09059180.00004614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reck M., Rodriguez-Abreu D., Robinson A.G. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 5.Socinski M.A., Jotte R.M., Cappuzzo F. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi L., Rodriguez-Abreu D., Gadgeel S. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 7.Brahmer J., Reckamp K.L., Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borghaei H., Brahmer J. Nivolumab in nonsquamous non-small-cell lung cancer. N Engl J Med. 2016;374(5):493–494. doi: 10.1056/NEJMc1514790. [DOI] [PubMed] [Google Scholar]
- 9.Borghaei H., Paz-Ares L., Horn L. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst R.S., Baas P., Kim D.W. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 11.Gettinger S., Politi K. PD-1 axis inhibitors in EGFR- and ALK-driven lung cancer: lost cause? Clin Cancer Res. 2016;22(18):4539–4541. doi: 10.1158/1078-0432.CCR-16-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehrenbacher L., Spira A., Ballinger M. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 13.Naidoo J., Schindler K., Querfeld C. Autoimmune Bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383–389. doi: 10.1158/2326-6066.CIR-15-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gautschi O., Milia J., Cabarrou B. Targeted Therapy for Patients with BRAF-Mutant Lung Cancer: Results from the European EURAF Cohort. J Thorac Oncol. 2015;10:1451–1457. doi: 10.1097/JTO.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 15.Mazieres J., Zalcman G., Crino L. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33:992–999. doi: 10.1200/JCO.2014.58.3302. [DOI] [PubMed] [Google Scholar]
- 16.Mazieres J., Barlesi F., Filleron T. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol. 2016;27:281–286. doi: 10.1093/annonc/mdv573. [DOI] [PubMed] [Google Scholar]
- 17.Gautschi O., Milia J., Filleron T. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 2017;35(13):1403–1410. doi: 10.1200/JCO.2016.70.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazieres J., Peters S., Lepage B. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31:1997–2003. doi: 10.1200/JCO.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 19.Garassino M.C., Cho B.C., Kim J.H. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen N., Fang W., Lin Z. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother. 2017;66(9):1175–1187. doi: 10.1007/s00262-017-2005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skoulidis F., Goldberg M.E., Greenawalt D.M. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822–835. doi: 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soo R.A., Lim S.M., Syn N.L. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: current controversies and future directions. Lung Cancer. 2018;115:12–20. doi: 10.1016/j.lungcan.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Dong Z.Y., Zhang J.T., Liu S.Y. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6(11):e1356145. doi: 10.1080/2162402X.2017.1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gainor J.F., Shaw A.T., Sequist L.V. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C.K., Man J., Lord S. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer—a meta-analysis. J Thorac Oncol. 2017;12(2):403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Lisberg A., Cummings A., Goldman J.W. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138–1145. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh S.J., Rizos H., Scolyer R.A., Long G.V. Resistance to combination BRAF and MEK inhibition in metastatic melanoma: where to next? Eur J Cancer. 2016;62:76–85. doi: 10.1016/j.ejca.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Dudnik E., Peled N., Nechushtan H. BRAF mutant lung cancer: programmed death ligand 1 expression, tumor mutational burden, microsatellite instability status, and response to immune check-point inhibitors. J Thorac Oncol. 2018;13(8):1128–1137. doi: 10.1016/j.jtho.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Sabari J.K., Leonardi G.C., Shu C.A. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol. 2018;29(10):2085–2091. doi: 10.1093/annonc/mdy334. [DOI] [PMC free article] [PubMed] [Google Scholar]