Graphical abstract
Keywords: Streptococcus pneumoniae cell wall, Peptidoglycan hydrolases, Choline-Binding Proteins, Major autolysin LytA localization, Teichoic acids localization
Highlights
-
•
Peptidoglycan which sustains bacterial growth is targeted by b-lactam antibiotics.
-
•
Spread of antibiotic resistance requires the development of new antibacterial drugs.
-
•
The cell wall of Gram-positive bacteria carries teichoic acids and virulence factors.
-
•
Function and surface localization of virulence factors are regulated by teichoic acids.
-
•
Anti-bacterial strategy should target the localization of surface virulence factors.
Abstract
The bacterial cell wall is in part composed of the peptidoglycan (PG) layer that maintains the cell shape and sustains the basic cellular processes of growth and division. The cell wall of Gram-positive bacteria also carries teichoic acids (TAs). In this work, we investigated how TAs contribute to the structuration of the PG network through the modulation of PG hydrolytic enzymes in the context of the Gram-positive Streptococcus pneumoniae bacterium. Pneumococcal TAs are decorated by phosphorylcholine residues which serve as anchors for the Choline-Binding Proteins, some of them acting as PG hydrolases, like the major autolysin LytA. Their binding is non covalent and reversible, a property that allows easy manipulation of the system.
In this work, we show that the release of LytA occurs independently from its amidase activity. Furthermore, LytA fused to GFP was expressed in pneumococcal cells and showed different localization patterns according to the growth phase. Importantly, we demonstrate that TAs modulate the enzymatic activity of LytA since a low level of TAs present at the cell surface triggers LytA sensitivity in growing pneumococcal cells. We previously developed a method to label nascent TAs in live cells revealing that the insertion of TAs into the cell wall occurs at the mid-cell. In conclusion, we demonstrate that nascent TAs inserted in the cell wall at the division site are the specific receptors of LytA, tuning in this way the positioning of LytA at the appropriate place at the cell surface.
Introduction
The bacterial cell wall maintains the cell shape and sustains the basic cellular processes of growth and division. It is also required to resist turgor pressure and provides an interface between the cell and its environment. Because the cell wall is essential for viability and is composed by molecules not present in eukaryotic host cells, its biosynthetic enzymes have been exploited as successful targets for antibiotic development. The bacterial cell wall is composed of peptidoglycan (PG), a matrix of linear glycan chains of N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GluNAc) residues, which are cross-linked via peptides composed of l- and d-amino acids. Assembly of the PG network requires synthetic and hydrolytic enzymes. Penicillin-Binding Proteins (PBPs) polymerize the glycan chains (glycosyltransferase activity) and cross-link the stem peptides (transpeptidation activity that is inhibited by β-lactam antibiotics) (for reviews see: Sauvage and Terrak, 2016, Egan et al., 2015). Glycan chains polymerization and peptides cross-link can also be performed by enzymes other than PBPs, like RodA from Bacillus subtilis, and l,d-transpeptidases, respectively (Emami et al., 2017, Hugonnet et al., 2016). Endogenous PG hydrolases are divided into different classes according to their substrate specificity and cleave the PG polymer in order to insert new material and to allow daughter cells separation and PG maturation (for review see: Vollmer et al., 2008).
The cell wall of Gram-positive bacteria also carries teichoic acids (TAs). These glycopolymers are either attached to the PG (wall teichoic acids, WTAs) or anchored to the cytoplasmic membrane (lipoteichoic acid, LTAs) (Brown et al., 2013, Percy and Gründling, 2014). TAs are important components of the cell wall and display numerous functions, in particular in cell morphology and cell division. In many bacterial species, like lactobacilli, bacilli and staphylococci, LTAs and WTAs display different chemical structures and their biosynthesis depends on distinct biochemical pathways. WTAs synthesis is controlled by tag and tar genes (Brown et al., 2013), while LtaS is a key enzyme in the LTAs biosynthesis pathway (Percy and Gründling, 2014, Grundling and Schneewind, 2007).
A functional link between TAs and PG hydrolases during cell division has been highlighted in Bacillus subtilis and Staphylococcus aureus (Yamamoto et al., 2008, Kiriyama et al., 2014, Schlag et al., 2010) but the molecular mechanisms of this interplay are still to be deciphered. Whether TAs control PG hydrolases by regulating their enzymatic activity and/or by playing a role in their subcellular localization are important issues. We addressed these questions in the context of the Gram-positive Streptococcus pneumoniae bacterium since pneumococcal TAs exhibit specific and convenient features relevant to this study. First, pneumococcal WTAs and LTAs chains have identical repeated unit structures and length distribution, indicating that both polymers are produced by the same biosynthetic pathway (for review, see Denapaite et al., 2012). The second striking feature is the decoration of TAs by phosphorylcholine. The choline serves as an anchor for the class of Choline-Binding Proteins (CBPs), that include some PG hydrolases (Frolet et al., 2010). Their binding is non covalent and reversible, a property that allows easy manipulation of the system. Choline is not rare in bacteria but S. pneumoniae is the only known species whose growth entirely depends on exogenous choline which is exclusively integrated into the TAs. We recently took advantage of this choline growth dependency to label pneumococcal TAs with chemically-modified fluorescent choline molecules using a bioorthogonal reaction related to the click chemistry approach (Di Guilmi et al., 2017).
LytA belongs to the CBP family and is the major pneumococcal autolysin responsible for the autolysis that occurs several hours after entering the stationary phase (Tomasz, 1968, Howard and Gooder, 1974). LytA is a N-acetylmuramidase that cleaves between the d-lactate of MurNAc and the l-Ala which is the first residue in the stem peptide. LytA is only active against stationary-phase cells (Tomasz and Waks, 1975, Mellroth et al., 2012), suggesting that the arrest of the division process leads to PG sensitivity to LytA. We recently showed that PG O-acetylation also protects growing pneumococcal cells from lysis induced by LytA (Bonnet et al., 2017). In addition to the amidase catalytic domain, LytA contains a Choline-Binding Domain (CBD) that mediates the binding to TAs via the choline residues. This PG hydrolase is thus a relevant bivalent protein to exemplify the relationship between TAs and PG hydrolysis activity. Contrarly to most CBPs that contain an N-terminal signal peptide that targets them to the extracellular cell wall, LytA does not contain any recognisable motif for protein secretion (Frolet et al., 2010). This suggests that LytA should remain localized within the cytoplasm while its site of action is extracellularly. The mechanism(s) through which LytA reaches the cell wall are still unkown. One hypothesis involves programmed cell death (PCD), leading to membrane weakening and to the release of intracellular proteins. Alternatively, PCD could be induced by the production of H2O2 by SpxB leading to the release of LytA during the stationary phase (Regev-Yochay et al., 2007). PCD would also be involved in the release of pneumolysin, a potent cytotoxin also lacking a recognisable peptide signal, suggesting that H2O2 production by SpxB would induce the release of the pneumolysin during the exponential phase and of LytA during the stationary phase (Bryant et al., 2016).
We show here that during the stationary phase, LytA is released independently from its amidase activity while the cell lysis induced by LytA requires both enzymatic and TAs-binding functions. We also demonstrate that TAs are involved in the regulation of LytA activity. Firstly, the level of TAs present at the cell surface influences the sensitivity of growing pneumococcal cells to LytA and secondly, nascent TAs inserted at the division site are the specific receptors of LytA, tuning in this way the positioning of LytA at the cell surface.
Materials and methods
Plasmid construction and site-directed mutagenesis
Plasmids, and oligonucleotide primers used are listed in Table 1. The synthetic gene encoding the superfolder variant of GFP (sfGFPop) was optimized from the superfolder green fluorescent protein (sfGFP) variant (Pédelacq et al., 2006) for expression in S. pneumoniae (sfGFPop) and ordered from GeneArt (Invitrogen) (Bonnet et al., 2017). For the construction of pJB3 (Pzn-lytA-sfGFPop) plasmid, the lytA gene was amplified by PCR with pJB3For and pJB3Rev primers, digested by BssHII and BsiWI and inserted into pADG0 (Bonnet et al., 2017) between BssHII and BsiWI [bgaA::PczcD-lytA-sfGFPop] to generate pJB3. pJB31 (Pzn-lytAE87Q-sfGFPop) plasmid was contructed by PCR-based site-directed mutagenesis using ForoMJ62 and RevoMJ63 primers to indroduce the E87Q mutation in the lytA on the pJB3 plasmid. pJB33 (Pzn-lytAAMI-sfGFPop) plasmid was contructed by PCR-based site-directed mutagenesis using pJB33For and pJB33Rev primers to delete the choline binding domain sequence in the lytA on the pJB3 plasmid. pJB34 (Pzn-lytACBD-sfGFPop) plasmid was contructed by PCR-based site-directed mutagenesis using pJB34For and pJB34Rev primers to delete the amidase sequence in the lytA gene on the pJB3 plasmid. For the construction of the pADG12 plasmid, the gene encoding the superfolder GFP optimized for the expression in S. pneumoniae (sfGFPop) was cloned in a His tagged protein expression pET vector. For the construction of the pADG14 plasmid (8xHis-sfGFPop-LytA), the lytA gene was amplified by PCR with pADG14For and pADG14Rev primers, digested by HindHIII and XhoI and inserted into pADG12 between HindIII and XhoI to generate pADG14. pADG141 plasmid (8xHis-sfGFPop-LytAAMI) was contructed by PCR-based site-directed mutagenesis using pJB141For and pJB141Rev primers to delete the choline binding domain sequence in the lytA gene on the pADG14 plasmid. pADG142 plasmid (8xHis-sfGFPop-LytACBD) was contructed by PCR-based site-directed mutagenesis using pJB142For and pJB142Rev primers to delete the choline binding domain sequence in the lytA gene on the pADG14 plasmid. DNA sequencing was performed by Beckman Coulter Genomics, Genewiz.
Table 1.
Strains, plasmids and oligonucleotides used in this study.
| Constructs | Genotype/description/sequence | Source |
|---|---|---|
| S. pneumoniae strains | ||
| R6 | ||
| R6 LytA-sfGFP | bgaA::PczcD –lytA (pJB3); AmpR, TetR | This work |
| R6 ΔlytA | lytA::cat; CatR | Pagliero et al. (2008) |
| ΔlytA LytA-sfGFP | lytA::cat, bgaA::PczcD –lytA (pJB3); CatR, pJB3, AmpR, TetR | This work |
| ΔlytA LytAE87Q-sfGFP | lytA::cat; bgaA::PczcD –lytAE87Q (pJB31), CatR, AmpR, TetR | This work |
| ΔlytA LytAAMI-sfGFP | lytA::cat; bgaA::PczcD –lytAAMI(pJB33); CatR, AmpR, TetR | This work |
| ΔlytA LytACBD-sfGFP | lytA::cat; bgaA::PczcD –lytACBD(pJB34); CatR, AmpR, TetR | This work |
| ftsZ-mKate | R800 ftsZ::ftsZ-mKate; StrR | Bonnet et al. (2017) |
| RH426 | ΔcomA ΔIS1167::Janus, EryRKanR | Johnsborg and Håvarstein (2009) |
| RH1 | ΔcomA::ermAM, egb::spc, EryR SpcR | Johnsborg et al. (2008) |
| RH14 | ΔcomA::ermAM, egb::spc, ΔlytA::kan, EryR SpcR KanR | Eldholm et al. (2009) |
| SPH125 | PcomR::comR, Janus between cpsO and cpsN, EryRKanR | Berg et al. (2011) |
| SPH130 | SPH125, but contains the P1 and PcomR promoters before comR | Berg et al. (2011) |
| SPH131 | SPH130, but with replacement of luc by the Janus cassette, EryRKanR | Berg et al. (2011) |
| SPH143 | SPH131 but with replacement of the Janus cassette by tarIJ (spr1149/spr1148), EryRSmR | This work |
| SPH144 | SPH143, but with replacement of native tarI and tarJ by the Janus cassette, EryRKanR | This work |
| SPH145 | SPH144, but with deletion of the Janus cassette, EryRSmR | This work |
| SPH146 | SPH145, but with replacement of lytA by a spectinomycin resistance marker (aad9), EryRSmRSpcR | This work |
| Plasmids | ||
| pADG0 | [bgaA::PZn-sfgfpopt], AmpR, TetR, AgeI, BssHII, BsiWI | Bonnet et al. (2017) |
| pJB3 | From pADG0, [bgaA::PZn-LytA-sfgfpop], AmpR, TetR | This work |
| pJB31 | From pJB3, [bgaA::PZn-LytAE87Q-sfgfpop], AmpR, TetR | This work |
| pJB33 | From pJB3, [bgaA::PZn-LytAAMI-sfgfpop], AmpR, TetR | This work |
| pJB34 | From pJB3, [bgaA::PZn-LytACBD-sfgfpop], AmpR, TetR | This work |
| pADG12 | From pET vector, 8-His-sfGFPop; Kan | This work |
| pADG14 | 8-His-sfGFPop-LytA; KanR | This work |
| pADG141 | 8-His-sfGFPop-LytAAMI; KanR | This work |
| pADG142 | 8-His-sfGFPop-LytACBD; KanR | This work |
| pET28-His-LytA | pET28a::his-lytA KanR, CmR | Philippe et al. (2015) |
| pDS-lytA | pRSET A with lytA cloned downstream of T7/lac | Berg et al. (2011) |
| Oligonucleotides | ||
| pJB3For | CGCGCGCGCATGGAAATTAATGTGAGTAAATTAAG | This work |
| pJB3Rev | GCGCGTACGTTTTACTGTAATCAAGCCATCTGG | This work |
| FORoMJ62 | ggttgaatggctttcaatcagttgaaccgctgcataggtctcagc insertion of E87Q mutation in lytA | Bonnet et al. (2017) |
| REVoMJ63 | gctgagacctatgcagcggttcaactgattgaaagccattcaacc insertion of E87Q mutation in lytA | Bonnet et al. (2017) |
| pJB33For | CAGTTTAAGCATGATATTGAGAACGGCTTGCGTACGACCGGTTCTAAAGGTGAAGAG | This work |
| pJB33Rev | CTCTTCACCTTTAGAACCGGTCGTACGCAAGCCGTTCTCAATATCATGCTTAAACTG | This work |
| pJB34For | GCGGATCTTAAGGAGGCGCGCATGACGATTGAAACAGGCTGGCAGAAG | This work |
| pJB34Rev | CTTCTGCCAGCCTGTTTCAATCGTCATGCGCGCCTCCTTAAGATCCGC | This work |
| pADG14For | CGCAAGCTT GAAATTAATGTGAGTAAATTAAGAAC | |
| pADG14REv | GCGCTCGAGTTATTATTTTACTGTAATCAAGCCATCTGG | |
| pADG141For | GCATGATATTGAGAACGGCTTGACTAATAACTC GAGCACCACCACCAC | This work |
| pADG141Rev | GTGGTGGTGGTGCTCGAGTTATTAGTCAAGCCGTTCTCAATATCATGC | This work |
| pADG142For | CCGAATTCGAGCTCCGTCGACAAGCTTGATTGAAACAGGCTGGCAGAAGAATG | This work |
| pADG142Rev | CATTCTTCTGCCAGCCTGTTTCAATCAAGCTTGTCGACGGAGCTCGAATTCGG | This work |
| khb31 | ATAACAAATCCAGTAGCTTTGG | Berg et al. (2011) |
| khb36 | TGAACCTCCAATAATAAATATAAAT | Berg et al. (2011) |
| khb33 | TTTCTAATATGTAACTCTTCCCAAT | Berg et al. (2011) |
| khb34 | CATCGGAACCTATACTCTTTTAG | Berg et al. (2011) |
| Kan484F | GTTTGATTTTTAATGGATAATGTG | Johnsborg et al. (2008) |
| LytAF | TGTATCTATCGGCAGTGTGAT | Eldholm et al. (2009) |
| LytAR | TCAACCATCCTATACAGTGAA | Eldholm et al. (2009) |
| khb90 | ATTTATATTTATTATTGGAGGTTCAATGATTTATGCAGGAATTCTTGC | This work |
| khb91 | ATTGGGAAGAGTTACATATTAGAAATTATACTTCCCACTTAAACACTG | This work |
| khb92 | AGAGACCAGCATCAGGATAC | This work |
| khb93 | CACATTATCCATTAAAAATCAAACCTATTTTCTCCTTTGTCTTAC | This work |
| khb94 | AAGTATTTTCTAGTATTATAGCACATTTAACTTTCCTTATGCTTTTGGAC | This work |
| khb95 | TTAAATGTGCTATAATACTAGAAAATACTTGTACTGGAGGTTAATTGTGGAG | This work |
| khb96 | TTCACCTTGCTCTTCCTTATAG | This work |
| khb97 | CTCCACAATTAACCTCCAGTACCTATTTTCTCCTTTGTCTTAC | This work |
| khb98 | GTAAGACAAAGGAGAAAATAGGTACTGGAGGTTAATTGTGGAG | This work |
Bacterial strains
Strain SPH143 was derived from strain SPH131 (Berg et al., 2011) by replacing its Janus cassette (Sung et al., 2001) with the genes tarI and tarJ (spr1149 and spr1148). The tarIJ fragment was amplified by PCR from S. pneumoniae RH1 using the primers khb90 and khb91. Fragments corresponding to the ∼950 bp region upstream and ∼800 bp region downstream of the Janus cassette in SPH131 were amplified using the primer pairs khb31/khb36 and khb33/khb34, respectively. These fragments were subsequently fused to the 5′ and 3′ ends of the tarIJ fragment using the primers khb31 and khb34 and overlap extension PCR as described by (Higuchi et al., 1988). The fusion product was used to transform SPH131, resulting in a strain that expresses tarIJ ectopically from PcomX when ComS∗ is added to the growth medium.
To construct the strain SPH144, the native tarIJ genes of strain SPH143 were replaced by the Janus cassette. The Janus cassette was amplified from RH426 using the primers Kan484F and khb94. Next, the primers khb92 and khb93 were used to amplify a ∼800 bp DNA fragment corresponding to the region flanking the 5′ end of tarI, whereas the primers khb95 and khb96 were used to amplify the ∼800 bp region flanking the 3′ end of tarJ. The two fragments were then fused to the 5′ and 3′ ends of the Janus cassette respectively, using the primers khb92 and khb96. Primer khb94 contains an overhang that introduced the synthetic constitutive promoter P1 downstream of the Janus cassette. This ensured that the essential licABC genes, which are situated on the same transcriptional unit as tarI and tarJ, were still expressed when the Janus cassette was inserted in place of the tarIJ genes. During transformation and subsequent selection of transformants, ComS∗ (2 µM) was added to the growth medium and agar plates to drive ectopic expression of tarIJ. Strain SPH145 was constructed by deleting the Janus cassette in SPH144. Fragments corresponding to the ∼800 bp upstream and downstream regions of the Janus cassette in strain SPH144 were amplified using the primer pairs khb92/khb97 and khb98/khb96. The fragments were joined using the primers khb92 and khb96, and the resulting fusion product was used to transform strain SPH144. This gave rise to strain SPH145, in which the regions flanking the native tarIJ genes are directly linked and normal expression of the licABC genes from their own promoter is re-established. Strain SPH146 was derived from SPH145 by replacing the lytA gene (spr1754) of SPH145 with a spectinomycin resistant marker (aad9). The lytA::SpcR fragment used to transform SPH145 was amplified from the genome of RH431 using the primers LytAF and LytAR.
For the gene depletion studies of tarIJ we used the ComRS system described previously (Berg et al., 2011). In brief, strain SPH146 (tarIJ-depletion) was grown in the presence of 2 µM ComS∗. The cells were collected by centrifugation and washed once in fresh C medium to remove excess ComS∗. Then the cells were resuspended in fresh C medium without ComS∗ to an OD492 of 0.05 before they were twofold diluted in eppendorf tubes containing C medium. The dilution series were then transferred to a 96-well NBS clear-bottom plate (Corning) containing 2 µM or 0 µM ComS∗ and incubated in a FluoStar Optima plate-reader (BMG Labtech) at 37 °C. The OD492 was measured automatically every 10 min. In the experiments where expression of tarIJ was fine-tuned in a dose-dependent manner, cultures of SPH146 were diluted to OD492∼0.05 in C medium with 2 µM ComS∗ and grown for 1 h. Then the cells were harvested, washed, and transferred to C medium containing a 1.25-fold dilution series of ComS∗ (0.125, 0.1, 0.08, 0.064, 0.0512, 0.041, 0.033 0.026 and 0.021 µM). Then the cells were transferred to a NBS clear-bottom plate for OD492-measurments. When LytA-sensitivity was examined, LytA (5 µg ml−1) was added to selected wells in the plate at the start of the experiment, or in the exponential growth phase at OD492∼0.25.
WT lytA-sfGFP, ΔlytA-lytA-sfGFP, ΔlytA-lytAE87Q-sfGFP, ΔlytA-lytAAMI-sfGFP and ΔlytA-lytACBD-sfGFP strains were obtained by transformation with pJB3 (lytA-sfGFP), pJB31 (lytAE87Q-sfGFP), pJB33 (-lytAAMI-sfGFP) or pJB34 (lytACBD-sfGFP) plasmid (TetR), respectively in the appropriate genetic background. The strains, plasmids, and oligonucleotide primers used are listed in Table 1. Allelic replacements were performed using the Janus method, a two-step procedure based on a bicistronic kan-rpsL cassette called Janus (Sung et al., 2001). This method avoids polar effects and allows expression of the fusion proteins at physiological levels.
Growth conditions, media, and transformation
Liquid cultures of S. pneumoniae strains were grown at 37 °C/5%CO2 in Cmedium (Cmed) supplemented with 0.5% yeast extract (Lacks and Hotchkiss, 1960) (CY), Chemically Defined Medium containing 5 or 10 µg/ml choline (CDM-Cho-5, CDM-Cho-10) or in Todd Hewitt medium (TH; BD Sciences). For strains harboring a zinc-inducible plasmid, CY was supplemented with 0.15 mM ZnCl2 to induce protein expression. For transformation, about 250 ng of DNA was added to cells treated with synthetic competence stimulating peptide 1 in TH medium at pH 8.0 supplemented with 1 mM CaCl2. Cells were grown for 2 h at 37 °C/5%CO2, and transformants were selected on Columbia (BD Sciences) blood (4%) agar plates containing the appropriate antibiotics (tetracyclin 250 µg/ml).
Continuous growth was followed by inoculating 2.5 ml of CY, CDM-Cho-5 or CDM-Cho-10 in 24-wells plates, which were sealed and incubated at 37 °C in a FLUOstar plate reader (BMG Labtech) equipped with a 595-nm filter. The OD595 was recorded every 10 to 20 min after shaking. Each point was done in triplicate and experiments repeated three independent times. LytA sensitivity was determined by adding the recombinant His-LytA at 10 µg/mL at OD595nm 0.3–0.4.
The localizations of sfGFPop-LytA, sfGFPop-LytAAMI and sfGFPop-LytACBD were performed as follow: the recombinant proteins were added at 10 µg/ml in CY pneumococcal cultures at OD595 nm 0.3–0.4. The incubations lasted from 4 min to 30 min at room temperature. After two washes in CY to discard unbound fluorescent proteins, the cells were resuspended into CY and immediately observed.
For labeling of newly synthesized PG, cells were grown in CY at OD595 nm 0.4 at 37 °C/5%CO2, pelleted at room temperature for 2 min at 4500 g, resuspended and incubated for 3 min in CY supplemented with 2 mM HADA (Kuru et al., 2012). After two washes in CY to discard unbound dye, the cells were resuspended into CY and immediately observed.
For pulse-labelling of newly synthesized teichoic acids, cells were grown in Cmed supplemented with 4 μg/ml choline at 37 °C/5%CO2 until OD595 nm 0.4, then pelleted at room temperature for 2 min at 4500 g, resuspended and incubated for 5 min in Cmed supplemented with 4–10 μg/ml choline-alkyl (manuscript submitted) and 25 μM DIBO (Click-IT™ Alexa Fluor™ 594 DIBO Alkyne, for copper free click chemistry detection of azide – C10407). After two washes in Cmed to discard unbound dye, the cells were resuspended into CY and immediately observed.
In pulse-chase experiment, pulse-labelling of newly synthesized teichoic acids was performed as described in the above paragraph. sfGFPop-LytA recombinant protein at 10 µg/ml was added at the same time than the choline-alkyl and DIBO molecules. After resuspension into CY and immediate observation of a first aliquot (time 0), cells were incubated at 37 °C/5%CO2 and aliquots were observed after 20 and 40 min.
Protein expression and purification
Strain BL21(DE3) Rosetta pLysS Rare (CmR) of E. coli was transformed with the plasmids pET28-His-LytA (KanR), pADG14 (KanR), pADG141 (KanR), or pADG142 (KanR). Cells were grown in Luria Bertani medium and protein expression was induced at OD595nm 0.6 with 0.5 mM IPTG (isopropyl β-D-thiogalactopyranoside) at 25 °C overnight. Cells from 2-litres cultures were harvested by centrifugation, resuspended in 50 ml of a buffer containing 50 mM Tris pH 8.0, 500 mM NaCl, 25 mM imidazole, 10% glycerol and a protease inhibitor cocktail (Complete EDTA free, Sigma–Aldrich) and lyzed using a Microfluidize M-110P (Microfluidics). The lysate was clarified by centrifugation (20 min at 39,191 × g at 4 °C) and loaded onto a 10-ml Ni-nitrilotriacetic acid (NTA) column (Qiagen). His-LytA proteins were eluted with a 25 mM to 500 mM imidazole gradient. Pooled fractions were concentrated and further purified by size exclusion chromatography using a Superdex S75 10/300 GL column (GE Healthcare) in 25 mM Tris-HCl (pH 8) and 150 mM NaCl.
Fluorescence microscopy image acquisition and analysis
Cells were grown at 37 °C/5%CO2 in CY to OD595 nm 0.3, transferred to microscope slides, and observed at 37 °C on an Olympus BX61 microscope equipped with a UPFLN 100O-2PH/1.3 objective and a QImaging Retiga-SRV 1394 cooled charge-coupled-device camera or an Olympus IX81 microscope equipped with a UPFLN 100O-2PH/1.3 objective and a Hamamtsu Orca Flash4 camera.
Image acquisition was performed using the software packages Volocity. Images were analyzed using the open-source softwares MicrobeTracker (Sliusarenko et al., 2011) and Oufti (Paintdakhi et al., 2016) and processed with Adobe Photoshop CS5 and Image J. Demographs integrate the signal values in each cell. The cells were sorted by their length value and the fluorescence values were plotted as a heat map.
Time lapse microscopy was performed using the automated CellASIC ONIX Microfluidic Platform (EMD Millipore Corporation). Briefly, a glycerol stock of WT strain was inoculated in 5 ml of CY medium and grown to an OD600nm of 0.05. Bacterial cells were loaded into an ONIX B04A-03 microfluidic plate (EMD Millipore Corporation) at 0.25 psi and a constant flow of CY medium at 1 psi was subsequently applied during 1 h. A rapid injection of sfGFPop-LytA recombinant protein at 50 µg/mL was done during 10 min at 3 psi followed by a washing of 5 min at 2 psi, then the flow was reduced to 0.25 during the chase. Images were acquired every 5 min during 1 h using an automated inverted epifluorescence microscope Nikon Ti-E/B equipped with the “perfect focus system” (PFS, Nikon), a phase contrast objective (CFI Plan Apo Lambda DM 100X, NA1.45), a Semrock filter set for GFP (Ex: 482BP35; DM: 506; Em: 536BP40) a LED light source (Spectra X Light Engine, Lumencor), a sCMOS camera (Neo sCMOS, Andor) and a chamber thermostated at 37 °C. Fluorescence images were capturated and processed using Nis-Elements AR software (Nikon). GFP fluorescence images were false colored green and overlaid on phase contrast images.
CBP elution by 2%choline and LytA quantification by immunoblotting
For quantification of the cell-wall anchored LytA, cells were grown at 37 °C in 50 ml of filtered CY and every 40 min, the OD595nm was measured and culture samples of 1 ml were withdrawn. The cells were centrifigated for 2 min at 5000 g and the pellet was resuspended into 10 μl of CY containing or not 2% Choline (Yother and White, 1994). After an incubation period of 20 min under gentle mixing, the samples were centrifugated for 3 min at 5000 g and the supernatant containing the CBP eluted by 2% choline was withdrawn. Fractions treated and non-treated with choline were analyzed by immunoblot and quantified with ImageJ. The samples were boiled in Laemmli for 5 min, loaded on SDS-PAGE gel and run at 200 V for 30 min. The gel was transferred to a nitrocellulose membrane which was then blocked with 5% milk in PBS-T (phosphate-buffered saline with 0.1% Tween) (saturation solution) for 1 h at room temperature. The membrane was incubated with an anti-LytA polyclonal rabbit antibody diluted in the saturation solution for 1 h at room temperature. After three washes in PBS-T, the membrane was incubated with a goat anti-rabbit immunoglobulin coupled to the G-horseradish peroxidase) for 1 h30 at room temperature. Protein was detected with the ECL horseradish peroxidase Western blotting detection kit. Quantitative analysis was realized with ImageJ.
Sacculi purification and transmission electron microscopy
Sacculi from S. pneumoniae R6 strain were prepared as described previously (McPherson and Popham, 2003). 10 µL of sacculi were incubated at 37 °C for 1 min with 0 (Control) or 5 µg/mL of recombinant purified LytA for 1 min at 37 °C in 100 µL PBS. Samples were then applied to the clean side of carbon on mica (carbon/mica interface) and negatively stained with 2% (wt/vol) sodium silicotungstate (pH 7.4). A grid was placed on top of the carbon film, which was subsequently air-dried. Images were taken under low dose conditions with a CM12 Phillips electron microscope at 120 kV and a calibrated nominal magnification of 13,000 using an ORIUS SC1000 camera (Gatan, Inc.).
Scanning and transmission electron microscopy
To deplete tarIJ in SPH146 cells, ComS∗ was removed from the growth medium as described by Berg et al. (2013). During depletion, growth was followed spectrophotometrically, and 25 ml samples of cell culture were collected when the growth rate of the tarIJ-depleted cells were significantly reduced compared to SPH146 cells grown in the presence of 2 µM ComS∗. The collected cells were fixed and prepared for SEM and TEM as described previously (Straume et al., 2017).
Results
Reduced choline concentration in the culture medium triggers sensitivity to LytA in growing pneumococci
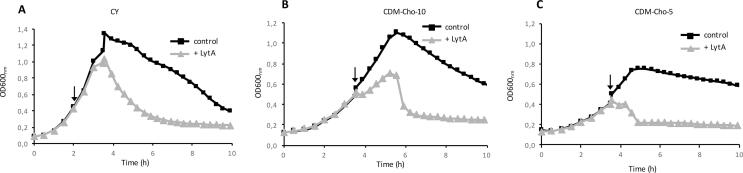
To test if TAs would drive the activity of LytA, we first asked whether the pattern of TAs associated to the cell wall, or the level of choline decoration, might affect PG sensitivity to LytA. S. pneumoniae cells were cultured in media containing different concentrations of choline. CY medium (C-medium supplemented with 0.5% of yeast extract) contains a concentration of choline superior to 5 µg/ml, indeed, the C-medium contains 5 µg/ml of choline but the quantity of choline present in yeast extract is unknown. To control the concentration of choline, Chemically-Defined Medium (CDM) was prepared and supplemented with 5 µg/ml or 10 µg/ml of choline, CDM-Cho-5 and CDM-Cho-10 conditions, respectively. Growth rates in these media are reduced compared to CY medium (Fig. 1A, B, C, black curves). The most important effect is observed in CDM-Cho-5 (Fig. 1C, black curve) since exponential phase ended at OD600 nm 0.8 compared to OD600 nm 1.2–1.4 in CY and CDM-Cho-10.
Fig. 1.
Effect of choline on pneumococci sensitivity to LytA-induced lysis. A. Growth of wild-type pneumococci in CY, a choline-rich medium, in the absence or in the presence of exogenous LytA at 10 µg/ml. B. Growth in CDM medium, containing a choline concentration of 10 µg/ml, in the absence or in the presence of exogenous LytA at 10 µg/ml. C. Growth in CDM medium, containing a choline concentration of 5 µg/ml, in the absence or in the presence of exogenous LytA at 10 µg/ml. The addition of exogenous LytA is indicated by an arrow in all three panels.
As shown by other investigators and ourselves (Tomasz and Waks, 1975, Mellroth et al., 2012, Bonnet et al., 2017), the presence of exogenous LytA in pneumococcal culture does not affect the exponential growth phase rate but accelerates the onset of the lytic phase (Fig. 1A, grey curve). These experiments were performed with the pneumococcal wild-type strain to compare the lytic effect of exogenous LytA in respect to the endogenous one. We then investigated whether the concentration of choline in the medium would influence the sensitivity of growing cells to LytA. Pneumococcal cultures grown in CDM-Cho-10 and CDM-Cho-5 were supplemented with LytA (10 µg/ml final concentration) at mid-exponential phase (OD600 nm 0.5) (Fig. 1B and C, grey curves). Immediately after addition of LytA, the growth rate dropped for about 30 min in both conditions and resumed in CDM-Cho-10 but not in CDM-Cho-5 where total cell lysis occurred. These data suggest that the quantity of choline present in the culture medium affects the sensitivity of PG to LytA cleavage. These results also suggest that choline starvation might lead to the production of TAs displaying a decreased level of choline decoration or to a reduced amount of TAs exposed at the cell surface, since only TAs substituted with choline are exported to the cell surface (Damjanovic et al., 2007).
Reduced expression of tarIJ triggers LytA sensitivity in growing pneumococci
To test this second hypothesis, i.e., whether the amount of TAs at the cell surface would influence the sensitivity of pneumococcus to LytA, we used a depletion system to construct a mutant strain tarIJ, in which the amount of TAs produced can be controlled by the extracellular concentration of an 8-amino acid peptide designated ComS∗ (Berg et al., 2011). The genes, tarI and tarJ encode a ribitol 5-phosphate cytidylyl transferase and a ribulose 5-phosphate reductase, both required for the synthesis of the TAs precursor cytidine 5′-diphosphate (CDP)-ribitol (Baur et al., 2009). When CDP-ribitol is no longer available in the cell, WTAs and LTAs syntheses stop, resulting in growth arrest and cell death. The depletion of the tarI and tarJ genes was performed in a pneumococcal strain deleted for lytA (SPH146). Depletion of tarIJ alters the sensitivity of pneumococci to LytA (see below), and the presence of endogenously produced LytA might therefore influence the results of our experiments. First, the lowest ComS∗ concentrations that supports normal or nearly normal growth of the SPH146 strain were determined. When grown in the presence of 2 μM ComS∗, the strain SPH146 grew normally and showed normal morphology (Fig. 2A and D). SPH146 cells grown in the presence of 2 μM ComS∗ were harvested by centrifugation, washed once with plain medium and resuspended to OD492 nm 0.05 in the same medium containing concentrations of ComS∗ ranging from 0.04 to 0.125 μM (Fig. 3A). Cultures grown in medium containing 0.1 μM and 0.125 μM ComS∗ grew well whereas cultures containing lower concentrations of ComS∗ displayed impaired growth (Fig. 3A). TAs-depleted cells developed an elongated shape and appeared to have several septa (Fig. 2B and C). Transmission electron microscopy revealed that they contained several false or aborted septa initiations, demonstrating that they struggled to divide (Fig. 2E). These results show that TAs play a crucial role in septum formation in S. pneumoniae.
Fig. 2.
Scanning (A, B and C) and transmission (D and E) electron micrographs of normal (A and D) and tarIJ depleted (B, C and E) S. pneumoniae cells. Cells grown in the presence of 2 µM ComS* (A and D) displayed the typical ovococcal shape of wild-type pneumococci. Depletion of tarIJ expression by removal of ComS* from the growth medium (B, C and E) gave rise to severe morphological abnormalities and multiple false or aborted division zones (indicated by arrows). All cell samples were collected during exponential growth. Scale bars in A, B, C: 1 µm; in D and E: 0.5 µm.
Fig. 3.
Reduced expression of tarIJ triggers LytA sensitivity in growing S. pneumoniae. A. Impact of decreasing TarIJ expression on the growth of SPH146. TarIJ expression was regulated by growing the SPH146 cells in medium containing concentration of ComS* ranging from 0.125 to 0.041 µM. B. LytA-sensitivity of SPH146 cells growing exponentially in medium containing 0.125, 0.100 and 0.080 µM ComS*. Purified LytA (5 µg ml−1) was added to the cultures when they reached OD492 0.2–0.3, as indicated by the arrow.
The LytA sensitivity of tarIJ-depleted cells was investigated by adding exogenous LytA to exponentially growing cultures at OD492 nm 0.25. As expected, tarIJ cells grown at the highest ComS∗ concentration (0.125 μM) were resistant to LytA during exponential phase, while those grown at 0.08 μM were sensitive (Fig. 3B). At 0.1 μM only a portion of the cells was affected when LytA was added at OD492nm 0.25, but after reaching OD492nm 0.45 the whole culture lysed. These experiments indicate that actively growing pneumococci are sensitive to exogenous LytA when the amount of TAs in their cell envelope is reduced to a critical level. We have previously demonstrated the effect of MurNAc O-acetylation on the formation of robust and mature PG and in the protection of PG from LytA activity (Bonnet et al., 2017). These results, together with the data presented here, indicate that the structure of PG (probed by the accessibility of the MurNAc – l-alanine amide bond to LytA) shoud depend on both the MurNAc O-acetylation and the level of TAs present at the cell surface.
Localization of LytA varies according to pneumococcal growth phase
The data obtained so far indicate that the amount of TAs in the cell wall affects the lytic activity of LytA. We next asked if this effect could be correlated to TAs and LytA respective distribution at the cell surface. We first analyzed the cellular localization of LytA over the cell cycle.
The 5′ end of the lytA gene was fused to the superfolder green fluorescent protein optimized for expression in S. pneumoniae (sfGFPop) (Fig. S1A) and placed under the control of a Zn-inducible promoter (Eberhardt et al., 2009). Pneumococcal lytA mutant strain carrying the construction LytA-sfGFPop was grown in the presence of Zn. Expression level of the ectopic copy of lytA induced by Zn is lower than the endogenous expression level (Fig. S1B) as already observed for other pneumococcal genes (Eberhardt et al., 2009, Morlot et al., 2013). Although added at low concentration (0.15 mM), addition of Zn accelerates the onset of cell lysis (Fig. S1C, grey curves). As expected, the mutant strain deleted for lytA does not display lytic phase, independently of the presence of Zn (Fig. S1D). In the complemented strain, the expression of LytA-sfGFPop restored the lytic phase, although to a lesser extend than in the lytA mutant strain considering the LytA lower expression level induced by Zn compared to the endogenous lytA gene (Fig. S1D). Altogether, these data indicate that the expression of an ectopic copy of LytA fused to sfGFPop is functional in pneumococcal cells. The phenotype of the cells expressing LytA-sfGFPop was analyzed in a lytA deletion background to ascertain that the localization and lytic effect observed raised only from the LytA-sfGFPop, excluding then any side effect generated by the endogenous LytA.
We monitored the localization of LytA-sfGFPop at different phases of the pneumococcal growth by epifluorescence microscopy (Fig. 4A). LytA-sfGFPop appeared homogenously localized in the cytoplasm of growing cells (exponential phase) but concentrated into foci in cells that stopped growing (stationary phase), referred hereafter as “heterogeneous protein organization”. Surprisingly, during the lytic phase, all the intact cells exhibited discrete LytA-sfGFPop labeling at mid-cell. Quantification of the fluorescence distribution indicate that all growing cells display the homogenous LytA labeling (Fig. 4B). The proportion of this cell population decreases during the stationary growth phase in favour to an increasing amount of cells harboring the heterogeneous LytA pattern. During the lytic phase, LytA is positioned at mid-cell in the large majority of non-lyzed cells. The representation of the fluorescence signal profile arranged by increasing cell length (demograph) of a growing cell population and of cells withdrawn during the lytic phase illustrate the homogenous and the mid-cell patterns of LytA-sfGFPop (Fig. 4C).
Fig. 4.
Localization of LytA varies according to the culture growth phase. A. Fluorescence images of LytA-sfGFPop expressed in ΔlytA cells withdrawn at different growth phase. Phase contrast (grey), GFP fluorescent signal (green) and merge (right) images are shown. Scale bars: 2 µm. B. Distribution of bacteria displaying homogeneous, heterogeneous and mid-cell localization of LytA-sfGFPop during exponential phase (OD600 nm 0,40), stationary phase (OD600 nm 0,97), early (OD600 nm 0,76) and late (OD595 nm 0,23) lytic phase. N = 98–170 bacteria per OD point were analyzed. C. Demograph representation of the fluorescence signal profile of LytA-sfGFPop in cells sorted by increasing cell length (MicrobeTracker, MATLAB). Images analysis was performed on cells withdrawn during the exponential phase (upper demograph) and the late lytic phase (bottom demograph). N = 30–120 bacteria per demograph were analyzed.
LytA released in the medium binds to the surface of neighboring cells at mid-cell position
To test the subcellular localization of LytA (cytoplasmic or extracellular), cells were incubated with 2% choline, a quantity of choline sufficient to elute CBPs (like LytA) from the cell surface (Yother and White, 1994). Cells were withdrawn in lytic phase and the percentage of homogenous, heterogeneous and mid-cell fluorescence signal provided by the expression of LytA-sfGFPop was determined before and after treatment with 2% choline (Fig. 5A). Total loss of the mid-cell pattern after choline treatment was observed while the heterogeneous profile remained unchanged. These results reveal that LytA-sfGFPop positioned at mid-cell is extracellularly exposed in contrast to the heterogeneous pattern, which corresponds to a cytoplasmic localization. After choline treatment, we observed that all cells still exhibited fluorescence suggesting that cells with a surface fluorescent signal still contain cytoplasmic LytA-sfGFPop. LytA-sfGFPop release arises at the end of the linear growth phase (Fig. S2) and it significantly increases during the lytic phase reaching up to 20% of the total protein expressed, in accordance with published results (Mellroth et al., 2012).
Fig. 5.
LytA is released in the medium and binds at the surface of non-lyzed cells at mid-cell. A. Distribution of bacteria displaying homogeneous, heterogeneous and mid-cell localization of LytA-sfGFPop during lytic phase before and after treatment with 2% choline. B. Pneumococcal wild-type cells expressing LytA-sfGFPop were mixed with cells deleted from lytA and expressing FtsZ fused to a red fluorescent protein (mKate2) at the ratio 2:1. The growth of the mixed culture was pursued at 37 °C until and aliquots were withdrawn when the culture entered the lytic phase. Phase contrast (grey), GFP fluorescent signal (green), mKate2 (red) and merge (right) images are shown. Scale bars: 2 µm. C. Growth curves of the ΔlytA strain transformed with the Zn-inducible plasmid encoding the inactive form of LytA, LytAE87Q, in the absence or the presence of 0.15 mM Zn. D. Growth curves of the ΔlytA strain transformed with the Zn-inducible plasmid encoding the amidase domain of LytA, in the absence or the presence of 0.15 mM Zn. E. Growth curves of the ΔlytA strain transformed with the Zn-inducible plasmid encoding the CBD domain of LytA, in the absence or the presence of 0.15 mM Zn. (For interpretation of the references to colour in this figure caption, the reader is referred to the web version of this article.)
To test whether extracellular LytA remains associated to the “exporter” cell or binds to sibling cells, cells expressing LytA-sfGFPop and cells expressing FtsZ fused to a red fluorescent protein (FtsZ-mKate2) were grown to mid-exponential phase and mixed to a ratio of 2:1. Growth of the mixed cultures was pursued at 37 °C until the lytic phase was reached. Imaging of the mixed populations showed that LytA-sfGFPop was associated to FtsZ-mKate2 expressing cells indicating that LytA-sfGFPop associates in trans to sibling non-lyzed cells (Fig. 5B).
Release of LytA is independent of the lytic activity but both amidase and CBD domains are required
We addressed the question of the molecular determinants responsible for the release and the lytic activity of LytA in pneumococcal cells. To do so, different variants of LytA fused to sfGFPop were expressed in the ΔlytA mutant strain and the growth profiles of each strain were analyzed. As expected, the inactive form LytAE87Q-sfGFPop did not complement the lytic phenotype, confirming that the enzymatic activity of LytA is necessary to induce cell lysis (Fig. 5C). However, this strain displayed the same 3-phases localization pattern as the wild-type strain (Fig. S3). To further investigate the functional properties of LytA, the amidase (Ami) and CBD domains were individually fused to sfGFPop and expressed in the ΔlytA mutant strain. Neither LytAAMI-sfGFPop nor LytACBD-sfGFPop were able to induce cell lysis (Fig. 5D, 5E). Altogether, these data indicate that i), the release of LytA is not dependent from the amidase enzymatic activity and ii), LytA should be anchored to TAs by the CBD to exert cell lysis function.
LytA localization at the cell surface is driven by the CBD
Since the lytic activity of LytA is dependent on its binding to TAs, we analyzed the molecular determinants driving the localization of LytA at the cell surface. To do so, we exploited the property of the CBPs to associate to TAs when added exogenously to pneumococcal cells. The full-length LytA and the individual CBD and amidase domains were fused to the sfGFPop at the N-terminal ends and expressed in Escherichia coli. The recombinant proteins sfGFPop-LytA (Fig. S1A), sfGFPop-LytACDB and sfGFPop-LytAAMI were purified to homogeneity. No amidase activity was detected in the fusion sfGFPop-LytA probably because the catalytic domain is constrained by the sfGFPop proximity. We took advantage of this feature to focus only on the binding property of LytA and not on the cleavage of the PG MurNAc – l-alanine amide bond. The fluorescent proteins were tested for their ability to associate to growing pneumococcal cells (Fig. 6A). No binding of sfGFPop-LytAAMI was observed confirming that the isolated catalytic domain is unable to attach to the cell surface (data not shown). The protein sfGFPop-LytACBD was shown to associate to the cell surface with a pattern similar to the one of the full-length sfGFPop-LytA (Fig. 6A). The demograph representations of the signal profiles of sfGFPop-LytA and sfGFPop-LytACBD in cells arranged by increasing cell length clearly show a localization at mid-cell as well as at a single pole (Fig. 6A). These data indicate that TAs recognition depends on CBD and that the specific localization of LytA is only dependent on the TAs-CBD interaction. We then sought to analyze the localization pattern of LytA in relation with its enzymatic activity. Purified sacculi prepared from pneumococcal growing cells were purified and digested with recombinant LytA (Fig. 6B). Electron microscopy observations indicate that cleavage of the sacculi occurred at the mid-cell and polar sites, which correspond to the positions occupied by sfGFPop-LytA on live growing cells.
Fig. 6.
Localization of LytA and cell wall cleavage sites. A. The CBD is sufficient to trigger localization of LytA. Wild-type cells in exponential growth phase were incubated with sfGFPop-LytA or sfGFPop-LytACBD recombinant proteins (10 µg/mL). Merge image of fluorescence and phase contrast views are showed. Scale bars: 2 µm. Demograph representation of the signal profile of sfGFPop-LytA and sfGFPop-LytACBD in cells sorted by increasing cell length (MicrobeTracker, MATLAB). B. Electron micrographs of S. pneumoniae sacculi incubated 1 min at 37 °C with buffer or with 5 µg/mL of recombinant purified LytA. Cleavage of the cell wall by LytA at both sides of the mid-cell sites are indicated by arrows and at the cell pole by arrow heads.
In non-growing cells withdrawn in stationary phase, sfGFPop-LytA positions only at mid-cell corresponding to the former site of active cell wall synthesis (Fig. S4), a pattern reminiscent to the one observed with the endogenous form of fluorescent LytA when bound to neighbouring non-lyzed cells (Fig. 5B). Although neither TAs nor PG are synthesized during stationary phase, LytA might keep the capability to recognize TAs species formerly synthesized during the exponential phase.
LytA binds to newly synthesized cell wall
To investigate in more details the distribution pattern of LytA at the cell surface, we followed the position of the division machinery using FtsZ-mKate2 and of the regions of active PG synthesis using labelled D-amino acids (HADA) (Kuru et al., 2012) together with the localization of sfGFPop-LytA. Of note, the morphogenetic process of ovococcal bacteria like S. pneumoniae is distinct from that of cocci like S. aureus and rod-shape organisms exemplified by B. subtilis since it results from two modes of PG insertion, one allowing division by synthesizing a septum (septal or cross-wall synthesis), the other allowing elongation by synthesizing peripheral cell wall (peripheral synthesis). sfGFPop-LytA and HADA were added to cells expressing FtsZ-mKate2. The incubation lasted for 4 min before washing and observation (Fig. 7). At the pre-division stage (i), FtsZ-mKate2 is positioned at the mid-cell site although no PG has been yet synthesized and sfGFPop-LytA is mainly detected at one cell pole. The early division stage (ii) is launched when cross-wall PG synthesis at the division site initiates cell constriction, this septal site is also occupied by FtsZ-mKate2 and sfGFPop-LytA. As the peripheral growth progresses (iii), sfGFPop-LytA is still present at the septal site, probably on both sides of the cross-wall site since the labeled zone appears slightly enlarged. FtsZ-mKate2 is then repositioned at the equator of the daughter cells which is the future site of division (iv) before de novo association of sfGFPop-LytA and HADA to these new division sites (v). In the meantime, a population of sfGFPop-LytA remains associated to the former parental division site, which corresponds to the new poles of the daughter cells (v).
Fig. 7.
LytA is associated to newly synthesized cell wall in growing cells. sfGFPop-LytA (10 µg/ml) and fluorescent amino-acid HADA (500 µM) were added to pneumococcal cells (OD600 nm 0.3) expressing FtsZ-mKate2 and incubated for 4 min at 37 °C before washing and fluorescence microscopy imaging. Phase contrast (grey) and fluorescent signals of mKate2 (red), GFP (green) and HADA images are shown. Scale bars: 1 µm. Cell division process is divided into i to v stages. (For interpretation of the references to colour in this figure caption, the reader is referred to the web version of this article.)
We also investigated in real-time the localization of sfGFPop-LytA during cell division in a microfluidic system. sfGFPop-LytA was pulse-injected on cells before extensive wash with CY medium. The localization of sfGFPop-LytA is followed on a single cell throughout a complete cell cycle (Fig. 8 and Supplemental video S1). A comparable pattern was observed: in early division stage (Fig. 8 frame 4 min) sfGFPop-LytA localizes at the equator of the cell which is the site of the newly synthesized cell wall when cell undergoes division process (Fig. 8 frames 9–14 min) and remains associated to this position, which under the progress of the cell cycle gives rise to the cell poles (Fig. 8 frames 24 min to 64 min). Faint labeling at the new division site of daughter cells is also detected suggesting that sfGFPop-LytA from the initial labeled site can relocalize to the new cell wall synthesis sites (Fig. 8 frames 34 min and 44 min).
Fig. 8.
Association of LytA to the nascent cell wall. Time-lapse images of WT cells labelled by sfGFPop-LytA (green) in a microfluidic system. sfGFPop-LytA was pulse-injected at 50 µg/ml on cells before extensive wash with CY medium (see Methods). The movie shows an overlay of GFP (green) and phase-contrast (grey) images. Scale bar: 1 µm. (For interpretation of the references to colour in this figure caption, the reader is referred to the web version of this article.)
In conclusion, localization of sfGFPop-LytA at the division site follows the PG synthesis site as reported by HADA and remains associated to the cell poles. This observation is consistent with the timing of TAs insertion in the cell wall which persists at the division site after PG synthesis has ceased (Bonnet et al., submitted).
LytA binds to newly synthesized TAs
To investigate whether sfGFPop-LytA might recognize and bind to newly inserted TAs, TAs have been mapped on the surface of live pneumococcal cells. We have developed a method to metabolically label TAs by click chemistry. The choline-growth dependency of S. pneumoniae has been exploited to incorporate choline molecules bearing small functional groups ready for click chemistry when incorporated into the TAs polymers. Using Cu(I)-catalyzed azide-alkyne cycloaddition, fluorescent reporters were grafted to TAs decorated with the chemically modified choline molecules in a specific and selective manner (Di Guilmi et al., 2017). This method has been adapted to live cells using copper-free bioorthogonal click reaction and fluorescent reporter such as DIBO-Alexa Fluor 594 (Bonnet et al., submitted). Briefly, pneumococcal cells grown in the presence of normal choline were washed and incubated for 4 min with choline-N3, DIBO-Alexa594 and sfGFPop-LytA at 37 °C. After two washes, cell growth was pursued in C-medium supplemented with 4 μg/ml choline at 37 °C for different periods of time. Microscopy observations were performed immediately after the 4-min pulse period (0 min) and after a chase duration of 20 min and 40 min (Fig. 9). At t0, sfGFPop-LytA is perfectly co-localized with the newly cell wall-inserted and labeled TAs, positioned at the parental division site (yellow arrow) and daughter cells equators (cyan arrows). After a 20-min chase period, the cell wall-inserted and labeled TAs equators had split into two rings (viewed side-on) that move apart as PG synthesis elongates the cell while sfGFPop-LytA remained associated at mid-cell. After a 40-min chase period, once the cell division is complete, the cell wall-inserted and labeled TAs had moved to the newly equatorial sites. If cells have separated after their division, the TAs labeling is found on free hemispheres. Similar observations were achieved with sfGFPop-LytACBD (Fig. S5) showing that the dynamics of LytA localization is independent from the amidase activity. These data show that sfGFPop-LytA associates to newly cell wall-inserted TAs at the division site. Considering the dynamics of the cell wall synthesis, we hypothesize that sfGFPop-LytA binds to newly cell wall-inserted TAs and then dissociates from those TAs once they move apart due to cell wall elongation.
Fig. 9.
LytA binds to newly synthesized TAs positioned at the septal cross-wall site. Pneumococcal cells grown in the presence of normal choline were washed at OD600 nm 0.3 and incubated for 4 min with choline-N3, DIBO and sfGFPop-LytA. After two washes, cell growth was pursued in CY at 37 °C. Microscopy observations were performed immediately after the 4-min pulse period (0 min) and after a chase duration of 20 min and 40 min. Phase contrast (grey), fluorescent signals of TAs labelled with DIBO-Alexa594 (red), GFP (green) and merge images are shown. The parental cross-wall septal site is indicated by yellow arrows and the daughter new septal site by cyan arrows. Scale bars: 2 µm. (For interpretation of the references to colour in this figure caption, the reader is referred to the web version of this article.)
Discussion
A characteristic of the pneumococcal growth in laboratory culture condition is the occurrence of autolysis (Tomasz 1968), launched several hours after the beginning of the stationary phase and induced by the amidase LytA, which cleaves the amide bond between MurNAc and l-alanine (Howard and Gooder, 1974). Pneumococcal cells are protected from LytA lysis during the exponential growth phase but these protective features are lost upon entry into the stationary phase (Tomasz and Waks, 1975, Mellroth et al., 2012). Despite the long-standing identification of the role of LytA as the major pneumococcal autolysin, the molecular mechanisms of its regulation are still poorly understood.
In this work, we show by two complementary approaches that the sensitivity of growing pneumococcal cells to LytA is challenged by the level of TAs present at the cell surface. Firstly, the modulation of the concentration of choline into the culture medium impacts the activity of LytA since depletion of choline increases the sensitivity of cells to LytA-induced lysis. One would expect that in conditions where TAs would be poorly decorated by choline (when choline is supplemented at low concentration), less LytA molecules would bind, thereby reducing the LytA-induced lysis. However, our data show the opposite effect indicating that the hydrolase activity of LytA is not directly correlated to the quantity of protein bound at the cell surface. It has been proposed that the flippase TacF would specifically transports TAs loaded in choline across the membrane (Damjanovic et al., 2007, González et al., 2008, Kharat et al., 2008, Denapaite et al., 2012 for review). Consequently, the choline depletion could negatively impact TAs synthesis and/or TAs translocation across the membrane. Secondly, we generated viable pneumococcal strain depleted in tarI and tarJ genes which should produce reduced amount of TAs. This strain also displays an increased sensitivity towards LytA in a comparable manner to the condition of choline starvation. These results indicate that when the amount of TAs is strongly reduced in the cell wall, the structure of PG might be modified to favor the access of LytA to the targeted MurNAc – l-alanine amide bond. We described a similar effect when MurNAc residues are not O-acetylated (Bonnet et al., 2017). Altogether, these data suggest that the structuration of the PG network can be regulated, for example by MurNAc O-acetylation and/or by the proximity of TAs.
The tarIJ pneumococcal mutant strain displays shape defects confirming the roles of TAs in cell morphology and cell division. Indeed, Gram-positive bacteria that display reduced amounts of TAs (through inactivation of WTAs or LTAs encoding genes) present abnormal cell shapes and sizes, irregular cell wall thickness and abnormal septa positioning, strongly supporting a tight coordination between PG and TAs biosynthesis to build the cell wall. In addition, the failure of mutants to efficiently divide and separate during cell division implicates a role of TAs in coordinating the localization, stability, and/or activity of PG hydrolases. Inactivation of genes encoding WTAs or LTAs was achieved in B. subtilis and S. aureus by disrupting the first-acting genes involved in the respective biosynthetic pathways, while the combination of the two deletions appeared to be lethal, indicating that TAs are essential for the formation of the cell wall and/or for cell survival (D’Elia et al., 2006a, D’Elia et al., 2006b, Grundling and Schneewind, 2007, Schirner et al., 2009, Oku et al., 2009). B. subtilis WTAs mutants appear affected in cell elongation (D’Elia et al., 2006b) while septation and cell division are abnormal in LTAs mutants (Schirner et al., 2009) suggesting that WTAs and LTAs would be specialized for elongation and division processes, respectively. These observations seem to be restricted to bacilli species since S. aureus blocked in WTAs synthesis have extensive defects in septation and cell separation (Campbell et al., 2011). Further evidences suggesting that TAs synthesis is coupled to cell division come from the observation that the localization of proteins involved in the WTAs and LTAs synthesis in B. subtilis displayed a pattern similar to that of nascent PG at the division site (Formstone et al., 2008, Schirner et al., 2009).
A functional link between TAs and PG hydrolases during cell division has been reported in B. subtilis and S. aureus (Yamamoto et al., 2008, Kiriyama et al., 2014, Schlag et al., 2010). Cell separation in B. subtilis depends on the cleavage of PG by DL-endopeptidases among which LytF plays an active role. It has been shown that LytF binding to PG as well as its sidewall subcellular localization are inhibited both by WTAs (Yamamoto et al., 2008) and a comparable effect by LTAs has also been described (Kiriyama et al., 2014). The staphylococcal bifunctional autolysin Atl is localized at the septal region where it exerts its PG hydrolytic activities to efficiently allow cell separation. The positioning of Atl is regulated by WTAs: by preventing binding of Atl to the old cell wall but not to the cross-wall site, WTAs favors the separation of daughter cells (Schlag et al., 2010). Lastly, WTAs by contributing to the proton-binding capacity of S. aureus cell walls, influence the autolysis rate via the major autolysin Atl (Biswas et al., 2012). The sum of these data indicates that TAs control PG remodeling, and therefore impact cell morphogenesis, by directing the subcellular localization of key PG hydrolytic enzymes. Whether i) PG hydrolases directly bind to TAs and ii) TAs guide the enzymes to the right place at the right time during the cell cycle is still unclear.
We addressed these questions by investigating the localization of the LytA-sfGFPop form expressed in the pneumococcal cells and observed that it varies according to growth-phase. LytA was diffuse into the cytoplasm during the exponential phase. During the progression to stationary phase, the fluorescence was reorganized into micro-domains, either associated to the inner face of the cytoplasmic membrane or randomly distributed into the cytoplasm. When released during the lytic phase, LytA-sfGFPop binds at the surface of non-lyzed neighbouring cells at mid-cell position. Our data regarding LytA subcellular distribution confirmed the data previously published (Mellroth et al., 2012). Indeed, lytA starts to be released at the end of the exponential phase and growing amount of the protein is present in the medium and associates to neighboring cells until a threshold is reached during stationary phase leading to the lysis of the entire population. The inactive form of LytA displayed a localization pattern and an association level at the cell surface comparable to the LytA active form, indicating that the release of LytA does not depend on its amidase activity. However, both the amidase and CBD domains are required to exert the lytic activity, including the release from the cytoplasm and the binding to TAs.
We exploited the characteristic of the CBPs to bind to choline-associated TAs when added exogenously to pneumococcal cells. The binding pattern of LytA fused to sfGFPop was followed in diverse conditions. First, the full-length LytA and the isolated CBD proteins were compared. Similar behaviors were observed, indicating that the localization of LytA at the cell surface is triggered by the association to TAs through the CBD. We observed that LytA is present in the hot spot of active cross-wall synthesis. Since LytA is specifically associated to TAs, we propose that LytA binds to newly synthesized TAs incorporated into the nascent cross-wall. In support of this idea, our pulse-chase experiments reveal that the synthesis of TAs occurs at the division site, confirming pioneering work performed by Briles and Tomasz in 1970. Co-localization data show that LytA binds to TAs inserted at the division site where PG cross-wall is also formed. Although TAs are present all over the cell surface (Briles and Tomasz, 1970; Bonnet et al., submitted), no association of LytA to TAs present in the peripheral cell wall has been observed. This observation suggests that (i) different TAs species are present in the septal and the peripheral walls, (ii) septal TAs might be submitted to a modification/maturation process very soon after its synthesis (or translocation), hence impeding LytA recognition and binding. The molecular determinants that make neo-synthesized TAs specifically recognized by LytA remain to be deciphered. Whether this population is enriched in LTAs or WTAs or display particular degree of choline decoration are some of the many important questions to be addressed in the near future.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work used the platforms of the Grenoble Instruct-ERIC centre (ISBG; UMS 3518 CNRS-CEA-UGA-EMBL) with support from FRISBI (ANR-10-INSB-05-02) and GRAL (ANRS-10-LABX-49-01) within the Grenoble Partnership for Structural Biology (PSB). The electron microscopy facility is supported by the Rhône-Alpes Region, the Fondation pour la Recherche Médicale (FRM), the fonds FEDER, and the GIS-Infrastructures en Biologie Santé et Agronomie (IBISA). J.B. received a PhD fellowship from the French Ministry of Education and Research. We thank Kousik Sundararajan for assistance with TEM (Hilde Kolstad, Norway) Yves Brun, Erkin Kuru and Michael van Nieuwenzhe (Bloomington University) for providing HADA and Guy Schoehn for support. We are grateful to André Zapun for helpful discussions.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.tcsw.2018.05.001.
Appendix A. Supplementary data
References
- Baur S., Marles-Wright J., Buckenmaier S., Lewis R.J., Voller W. Synthesis of CDP-activated ribitol for teichoic acid precursors in Streptococcus pneumoniae. J. Bacteriol. 2009;191:1200–1210. doi: 10.1128/JB.01120-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K.H., Biørnstad T.J., Straume D., Håvarstein L.S. Peptide-regulated gene depletion system developed for use in Streptococcus pneumoniae. J. Bacteriol. 2011;193:5207–5215. doi: 10.1128/JB.05170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K.H., Stamsås G.A., Straume D., Håvarstein L.S. Effects of low PBP2b levels on cell morphology and peptidoglycan composition in Streptococcus pneumoniae R6. J. Bacteriol. 2013;195:4342–4354. doi: 10.1128/JB.00184-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas R., Martinez R.E., Göhring N., Schlag M., Josten M., Xia G. Proton-binding capacity of Staphylococcus aureus Wall Teichoic Acid and its role in controlling autolysin activity. PLoS One. 2012;7:e41415. doi: 10.1371/journal.pone.0041415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet J., Durmort C., Jacq M., Mortier-Barrière I., Campo N., VanNieuwenhze M.S., Brun Y.V., Arthaud C., Gallet B., Moriscot C., Morlot C., Vernet T., Di Guilmi A.M. Peptidoglycan O-acetylation is functionally related to cell wall biosynthesis and cell division in Streptococcus pneumoniae. Mol. Microbiol. 2017;106:832–846. doi: 10.1111/mmi.13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles E.B., Tomasz A. Radioautographic evidence for equatorial wall growth in a Gram-positive bacterium. Segregation of choline-3H-labeled teichoic acid. J. Cell. Biol. 1970;47:786–790. doi: 10.1083/jcb.47.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Santa Maria J.P., Jr., Walker S. Wall teichoic acids of Gram-positive bacteria. Annu. Rev. Microbiol. 2013;67:313–336. doi: 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant J.C., Dabbs R.C., Oswalt K.L., Brown L.R., Rosch J.W., Seo K.S., Donaldson J.R., McDaniel L.S., Thornton J.A. Pyruvate oxidase of Streptococcus pneumoniae contributes to pneumolysin release. BMC Microbiol. 2016;16:271. doi: 10.1186/s12866-016-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J., Singh A.K., Santa Maria J.P., Jr., Kim Y., Brown S., Swoboda J.G., Mylonakis E., Wilkinson B.J., Walker S. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem. Biol. 2011;6:106–116. doi: 10.1021/cb100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanovic M., Kharat A.S., Eberhardt A., Tomasz A., Vollmer W. The essential tacF gene is responsible for the choline-dependent growth phenotype of Streptococcus pneumoniae. J. Bacteriol. 2007;189:7105–7111. doi: 10.1128/JB.00681-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Elia M.A., Pereira M.P., Chung Y.S., Zhao W., Chau A., Kenney T.J., Sulavik M.C., Black T.A., Brown E.D. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J. Bacteriol. 2006;188:4183–4189. doi: 10.1128/JB.00197-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Elia M.A., Millar K.E., Beveridge T.J., Brown E.D. Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J. Bacteriol. 2006;188:8313–8316. doi: 10.1128/JB.01336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denapaite D., Brückner R., Hakenbeck R., Vollmer W. Biosynthesis of teichoic acids in Streptococcus pneumoniae and closely related species: lessons from genomes. Microbial Drug Resistance. 2012;18:344–358. doi: 10.1089/mdr.2012.0026. [DOI] [PubMed] [Google Scholar]
- Di Guilmi A.M., Bonnet J., Peiβert S., Durmort C., Gallet B., Vernet T., Gisch N., Wong Y.S. Specific and spatial labeling of choline-containing teichoic acids in Streptococcus pneumoniae by click chemistry. Chem. Commun. (Camb). 2017;53:10572–10575. doi: 10.1039/c7cc05646j. [DOI] [PubMed] [Google Scholar]
- Eberhardt A., Wu L.J., Errington J., Vollmer W., Veening J.W. Cellular localization of choline-utilization proteins in Streptococcus pneumoniae using novel fluorescent reporter systems. Mol. Microbiol. 2009;74:395–408. doi: 10.1111/j.1365-2958.2009.06872.x. [DOI] [PubMed] [Google Scholar]
- Egan A.J.F., Biboy J., van’t Veer I., Breukink E., Vollmer W. Activities and regulation of peptidoglycan synthases. Phil. Trans. R. Soc. B. 2015;370:20150031. doi: 10.1098/rstb.2015.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldholm V., Johnsborg O., Haugen K., Ohnstad H.S., Håvarstein L.S. Fratricide in Streptococcus pneumoniae: Contributions and role of the cell wall hydrolases CbpD, LytA and LytC. Microbiology. 2009;155:2223–2234. doi: 10.1099/mic.0.026328-0. [DOI] [PubMed] [Google Scholar]
- Emami K., Guyet A., Kawai Y., Devi J., Wu L.J., Allenby N. RodA as the missing glycosyltransferase in Bacillus subtilis and antibiotic discovery for the peptidoglycan polymerase pathway. Nat. Microbiol. 2017;2:16253. doi: 10.1038/nmicrobiol.2016.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formstone A., Carballido-Lopez R., Noirot P., Errington J., Scheffers D.J. Localization and interactions of teichoic acid synthetic enzymes in Bacillus subtilis. J. Bacteriol. 2008;190:1812–1821. doi: 10.1128/JB.01394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolet C., Beniazza M., Roux L., Gallet B., Noirclerc-Savoye M., Vernet T., Di Guilmi A.M. New adhesin functions of surface-exposed pneumococcal proteins. BMC Microbiology. 2010;10:190. doi: 10.1186/1471-2180-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsborg O., Eldholm V., Bjørnstad M.L., Håvarstein L.S. A predatory mechanism dramatically increases the efficiency of lateral gene transfer in Streptococcus pneumoniae and related commensal species. Mol. Microbiol. 2008;69:245–253. doi: 10.1111/j.1365-2958.2008.06288.x. [DOI] [PubMed] [Google Scholar]
- Johnsborg O., Håvarstein L.S. Pneumococcal LytR, a protein from the LytR-CpsA-Psr family, is essential for normal septum formation in Streptococcus pneumoniae. J. Bacteriol. 2009;191:5859–5864. doi: 10.1128/JB.00724-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A., Llull D., Morales M., García P., García E. Mutations in the tacF gene of clinical strains and laboratory transformants of Streptococcus pneumoniae: impact on choline auxotrophy and growth rate. J. Bacteriol. 2008;190:4129–4138. doi: 10.1128/JB.01991-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundling A., Schneewind O. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc. Natl. Acad. Sci. U.S.A. 2007;104:8478–8483. doi: 10.1073/pnas.0701821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R.K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucl. Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L.V., Gooder H. Specificity of the autolysin of Streptococcus (Diplococcus) pneumoniae. J. Bacteriol. 1974;117:796–804. doi: 10.1128/jb.117.2.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugonnet, J.E., Mengin-Lecreulx, D., Monton, A., den Blaauwen, T., Carbonnelle, E., Veckerlé, C., Brun, Y.V., van Nieuwenhze, M., Bouchier, C., Tu, K., Rice, L.B., Arthur, M., 2016. Factors essential for L,D-transpeptidase-mediated peptidoglycan cross-linking and β-lactam resistance in Escherichia coli. Elife e19469. doi:10.7554/eLife.19469. [DOI] [PMC free article] [PubMed]
- Kharat A.S., Denapaite D., Gehre F., Brückner R., Vollmer W., Hakenbeck R., Tomasz A. Different pathways of choline metabolism in two choline-independent strains of Streptococcus pneumoniae and their impact on virulence. J. Bacteriol. 2008;190:5907–5914. doi: 10.1128/JB.00628-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriyama Y., Yazawa K., Tanaka T., Yoshikawa R., Yamane H., Hashimoto M., Sekiguchi J., Yamamoto H. Localization and expression of the Bacillus subtilis DL-endopeptidase LytF are influenced by mutations in LTA synthases and glycolipid anchor synthetic enzymes. Microbiology. 2014;160:2639–2649. doi: 10.1099/mic.0.080366-0. [DOI] [PubMed] [Google Scholar]
- Kuru E., Velocity Hughes H., Brown P.J., Hall E., Tekkam S., Cava F. In situ probing of newly synthesized peptidoglycan in live bacteria with fluorescent D-amino acids. Angew. Chem. Int. Ed. Engl. 2012;51:12519–12523. doi: 10.1002/anie.201206749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Hotchkiss K.D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim. Biophy. Acta. 1960;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- McPherson D.C., Popham D.L. Peptidoglycan synthesis in the absence of class A penicillin-binding proteins in Bacillus subtilis. J. Bacteriol. 2003;185:1423–1431. doi: 10.1128/JB.185.4.1423-1431.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellroth P., Daniels R., Eberhardt A., Rönnlund D., Blom H., Widengren J. LytA, major autolysin of Streptococcus pneumoniae, requires access to nascent peptidoglycan. J. Biol. Chem. 2012;287:11018–11029. doi: 10.1074/jbc.M111.318584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlot C., Bayle L., Jacq M., Fleurie A., Tourcier G., Galisson F. Interaction of Penicillin-Binding Protein 2x and Ser/Thr protein kinase StkP, two key players in Streptococcus pneumoniae R6 morphogenesis. Mol. Microbiol. 2013;90:88–102. doi: 10.1111/mmi.12348. [DOI] [PubMed] [Google Scholar]
- Oku Y., Kurokawa K., Matsuo M., Yamada S., Lee B.L., Sekimizu K. Pleiotropic roles of polyglycerolphosphate synthase of lipoteichoic acid in growth of Staphylococcus aureus cells. J. Bacteriol. 2009;191:141–151. doi: 10.1128/JB.01221-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintdakhi A., Parry B., Campos M., Irnov I., Elf J., Surovtsev I. Oufti: an integrated software package for high-accuracy, high-throughput quantitative microscopy analysis. Mol. Microbiol. 2016;99:767–777. doi: 10.1111/mmi.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliero E., Dublet B., Frehel C., Dideberg O., Vernet T., Di Guilmi A.M. The inactivation of a new peptidoglycan hydrolase Pmp23 leads to abnormal septum formation in Streptococcus pneumoniae. The Open Microbiol. J. 2008;2:107–114. doi: 10.2174/1874285800802010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pédelacq J.D., Cabantous S., Tran T., Terwilliger T.C., Waldo G.S. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 2006;24:79–88. doi: 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- Percy M.G., Gründling A. Lipoteichoic acid synthesis and function in Gram-positive bacteria. Annu. Rev. Microbiol. 2014;68:81–100. doi: 10.1146/annurev-micro-091213-112949. [DOI] [PubMed] [Google Scholar]
- Philippe J., Gallet B., Morlot C., Denapaite D., Hakenbeck R., Chen Y. Mechanism of β-lactam action in Streptococcus pneumoniae: the piperacillin paradox. Antimicrob. Agents Chemother. 2015;59:609–621. doi: 10.1128/AAC.04283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev-Yochay G., Trzcinski K., Thompson C.M., Lipsitch M., Malley R. SpxB is a suicide gene of Streptococcus pneumoniae and confers a selective advantage in an in vivo competitive colonization model. J. Bacteriol. 2007;189:6532–6539. doi: 10.1128/JB.00813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage E., Terrak M. Glycosyltransferases and Transpeptidases/Penicillin-Binding Proteins: valuable targets for new antibacterials. Antibiotics. 2016;5:12. doi: 10.3390/antibiotics5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirner K., Marles-Wright J., Lewis R.J., Errington J. Distinct and essential morphogenic functions for wall- and lipoteichoic acids in Bacillus subtilis. EMBO J. 2009;28:830–842. doi: 10.1038/emboj.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlag M., Biswas R., Krismer B., Kohler T., Zoll S., Yu W., Schwarz H., Peschel A., Götz F. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol. Microbiol. 2010;75:864–873. doi: 10.1111/j.1365-2958.2009.07007.x. [DOI] [PubMed] [Google Scholar]
- Sliusarenko O., Heinritz J., Emonet T., Jacobs-Wagner C. High-throughput, subpixel precision analysis of bacterial morphogenesis and intracellular spatio-temporal dynamics. Mol. Microbiol. 2011;80:612–627. doi: 10.1111/j.1365-2958.2011.07579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straume D., Stamsås G.A., Salehian Z., Håvarstein L.S. Overexpression of the fratricide immunity protein ComM leads to growth inhibition and morphological abnormalities in Streptococcus pneumoniae. Microbiology. 2017;163:9–21. doi: 10.1099/mic.0.000402. [DOI] [PubMed] [Google Scholar]
- Sung C.K., Li H., Claverys J.P., Morrison D.A. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 2001;67:5190–5196. doi: 10.1128/AEM.67.11.5190-5196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of pneumococcus: chain formation, loss of transformability, and loss of autolysis. Proc. Natl. Acad. Sci. U.S.A. 1968;59:86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc. Natl. Acad. Sci. U.S.A. 1975;72:4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer W., Joris B., Charlier P., Foster S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 2008;32:259–286. doi: 10.1111/j.1574-6976.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Miyake Y., Hisaoka M., Kurosawa S., Sekiguchi J. The major and minor wall teichoic acids prevent the sidewall localization of vegetative dl-endopeptidase LytF in Bacillus subtilis. Mol. Microbiol. 2008;70:297–310. doi: 10.1111/j.1365-2958.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- Yother J., White J.M. Novel surface attachment mechanism of the Streptococcus pneumoniae protein PspA. J. Bacteriol. 1994;176:2976–2985. doi: 10.1128/jb.176.10.2976-2985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.