Abstract
Background
Gastric cancer is the third most common cause of cancer death worldwide. Individuals infected with Helicobacter pylori have a higher likelihood of developing gastric cancer than individuals who are not infected. Eradication of H. pylori in healthy asymptomatic individuals in the general population may reduce the incidence of gastric cancer, but the magnitude of this effect is unclear.
Objectives
To assess the effectiveness of eradication of H. pylori in healthy asymptomatic individuals in the general population in reducing the incidence of gastric cancer.
Search methods
We identified trials by searching the Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 1), MEDLINE (1946 to February 2020), and EMBASE (1974 to February 2020). We handsearched reference lists from trials selected by electronic searching to identify further relevant trials. We handsearched published abstracts from conference proceedings from the United European Gastroenterology Week (published in Gut) and Digestive Disease Week (published in Gastroenterology) between 2001 and 2019. We contacted members of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Review Group and experts in the field and asked them to provide details of outstanding clinical trials and any relevant unpublished materials.
Selection criteria
We analysed randomised controlled trials comparing at least one week of H. pylori therapy with placebo or no treatment in preventing subsequent development of gastric cancer in otherwise healthy and asymptomatic H. pylori‐positive adults. Trials had to follow up participants for at least two years and needed to have at least two participants with gastric cancer as an outcome. We defined gastric cancer as any gastric adenocarcinoma, including intestinal (differentiated) or diffuse (undifferentiated) type, with or without specified histology.
Data collection and analysis
We collected data on incidence of gastric cancer, incidence of oesophageal cancer, deaths from gastric cancer, deaths from any cause, and adverse effects arising due to therapy.
Main results
Six trials met all our eligibility criteria and provided extractable data in the previous version. Following our updated search, one new RCT was identified, meaning that seven trials were included in this updated review. In addition, one previously included trial provided fully published data out to 10 years, and another previously included trial provided fully published data out to 22 years of follow‐up. Four trials were at low risk of bias, one trial was at unclear risk, and two trials were at high risk of bias. Six trials were conducted in Asian populations.
In preventing development of subsequent gastric cancer, H. pylori eradication therapy was superior to placebo or no treatment (RR 0.54, 95% confidence interval (CI) 0.40 to 0.72, 7 trials, 8323 participants, moderate certainty evidence). Only two trials reported the effect of eradication of H. pylori on the development of subsequent oesophageal cancer. Sixteen (0.8%) of 1947 participants assigned to eradication therapy subsequently developed oesophageal cancer compared with 13 (0.7%) of 1941 participants allocated to placebo (RR 1.22, 95% CI 0.59 to 2.54, moderate certainty evidence). H. pylori eradication reduced mortality from gastric cancer compared with placebo or no treatment (RR 0.61, 95% CI 0.40 to 0.92, 4 trials, 6301 participants, moderate certainty evidence). There was little or no evidence in all‐cause mortality (RR 0.97, 95% CI 0.85 to 1.12, 5 trials, 7079 participants, moderate certainty evidence). Adverse events data were poorly reported.
Authors' conclusions
We found moderate certainty evidence that searching for and eradicating H. pylori reduces the incidence of gastric cancer and death from gastric cancer in healthy asymptomatic infected Asian individuals, but we cannot necessarily extrapolate this data to other populations.
Plain language summary
Helicobacter pylori treatment for the prevention of stomach cancer
Review question
Whether testing healthy people for Helicobacter pylori and treating those infected with a course of antibiotics decreases the number of new cases of gastric cancer.
Background
Helicobacter pylori (H. pylori) is a bacteria that lives in the lining of the stomach with people usually not aware they are carrying the infection. People with H. pylori infection are more likely to develop gastric cancer than people who are not infected with the bacterium. For this reason, H. pylori is classed as carcinogenic (causing cancer) to humans. Many people worldwide die of gastric cancer every year, because by the time those affected seek the opinion of a doctor, the condition is often advanced. However, H. pylori infection is easily treatable with a one‐week course of antibiotics.
Study characteristics
A literature search up to 02 Feburary 2020 found seven trials (containing 8323 participants, four trials at low risk of bias). Six of the studies were based in Asia.
Key results
We found that antibiotics for H. pylori have a small benefit in preventing gastric cancer (68 (1.6%) of 4206 participants given treatment developed gastric cancer subsequently, compared with 125 (3.0%) of 4117 given no treatment or a placebo), and in decreasing the number of deaths from gastric cancer (36 (1.1%) of 3154, compared with 59 (1.9%) of 3147); but it is unclear whether or not they increase or decrease the number of deaths due to any cause, or increase or decrease the number of cases of oesophageal cancer. Data about side effects of treatment were poorly reported.
Quality of the evidence
Four trials were at low risk of bias, one trial was at unclear risk, and two trials were at high risk of bias. One study was at high risk of bias because no placebo was used for the active eradication therapy regimen, and so this part of the trial was unblinded, and the other study was at high risk of bias due to inconsistencies in data reporting at the two points of follow‐up. We were unable to resolve this discrepancy despite contacting the original authors. As a result, we downgraded the quality of evidence from high to moderate due to serious risk of bias.
Summary of findings
Summary of findings 1. H. pylori eradication therapy compared to control for the prevention of gastric neoplasia in healthy asymptomatic infected individuals.
| H. pylorieradication therapy compared to control for the prevention of gastric neoplasia in healthy asymptomatic infected individuals | ||||||
| Patient or population: healthy asymptomatic H. pylori‐infected individuals Settings: general population1 Intervention:H. pylori eradication therapy to prevent subsequent gastric cancer 2 Comparison: control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | H. pylorieradication therapy to prevent subsequent gastric cancer | |||||
| Incidence of gastric cancer ‐ modified ITT analysis Histological examination Follow‐up: 4 to 22 years | 30 per 1000 | 16 per 1000 (12 to 22) | RR 0.54 (0.40 to 0.72) | 8323 (7 studies) | ⊕⊕⊕⊝ moderate3,4,5,6 | Number need to treat to benefit was 72 (95% CI 55 to 118) |
|
Death from gastric cancer ‐ modified ITT analysis Follow up: 7‐22 years |
19 per 1000 | 11 per 1000 (7 to 17) | RR 0.61 (0.40 to 0.92) | 6301 (4 studies) | ⊕⊕⊕⊝ moderate4,6,7 | Number need to treat to benefit was 137 (95% CI 89 to 667) |
| Death from all causes ‐ modified ITT analysis | 92 per 1000 | 89 per 1000 (78 to 103) | RR 0.97 (0.85 to 1.12) | 7079 (5 studies) | ⊕⊕⊕⊝ moderate4,6,7 | |
|
Incidence of oesophageal squamous cell carcinoma ‐ modified ITT analysis Follow up: 7‐22 years |
7 per 1000 | 8 per 1000 (4 to 17) | RR 1.22 (0.59 to 2.54) | 3888 (2 study) | ⊕⊕⊕⊝ moderate8 | |
| Adverse events | See comment | See comment | Not estimable | 0 (0) | See comment | Adverse events were poorly reported across the studies and could not be summarised. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; ITT: intention‐to‐treat; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 As all but one study was conducted in East Asia, it is not possible to assess the effect of searching for and eradicating H. pylori in Western populations. 2 Modified ITT analysis. 3 The quality of evidence was downgraded from high to moderate due to serious risk of bias: Fourtrials were at low risk of bias, one trial was at unclear risk, and two trials were at high risk of bias. In addition, because of the factorial design of some of the trials, it is difficult to determine whether the reduction in relative risk of subsequent gastric cancer was due to H. pylori eradication therapy alone. The eradication regimens used varied considerably between the individual trials, although this reflects the fact that several of these studies were designed before the widespread adoption of proton pump inhibitor triple therapy, which was first described in 1994, as the gold standard for H. pylori eradication. 4 No significant heterogeneity was seen between studies. 5 The beneficial effect seemed to be more pronounced in the two studies that co‐administered antioxidants and vitamins to participants, but it should be noted that one of these contained the majority of gastric cancers and had the longest duration of follow‐up. There was no significant benefit of H. pylori eradication therapy in preventing subsequent occurrence of gastric cancer when only those participants either with or without preneoplastic lesions at baseline were considered in the analysis. There were no significant subgroup differences. 6 Funnel plots were not produced, as there were less than 10 studies included in the analyses. 7 The quality of evidence was downgraded from high to moderate due to serious risk of bias: one trial was at high risk of bias. 8 Only two studies were available for this outcome, with wide 95% CI.
Background
Description of the condition
Gastric cancer is the third most common cause of death from malignant disease worldwide, resulting in 750,000 deaths each year (Ferlay 2010). In most high‐income countries, the incidence of gastric cancer is falling, (Lau 2006), but the increase in age of the world population means that the total number of deaths from gastric cancer is set to rise for the foreseeable future (Forman 1998). The treatment of gastric cancer is unsatisfactory. Almost half of gastric cancers are inoperable at the time of diagnosis (Lello 2007), and the five‐year survival of these individuals is close to zero. Those undergoing operative treatment often require extensive surgery, with a 5‐year survival rate of only 20% to 30% (Cunningham 2005). Survival may be improved if the disease is diagnosed at an earlier stage (Degiuli 2006), but the cost of population screening for gastric cancer with upper gastrointestinal (GI) endoscopy would be prohibitive. Even if only those with upper GI symptoms that may be indicative of an occult gastric cancer, such as dyspepsia, were screened by endoscopy, the cost of detecting one malignant lesion has been estimated to be as high as USD 83,000. (Vakil 2009) One possible way to make a significant impact on mortality from gastric cancer could therefore be via primary prevention of the disease.
The discovery of Helicobacter pylori and the observation that it was responsible for the development of chronic gastritis, with subsequent gastric atrophy and intestinal metaplasia, raised the possibility that this organism was a necessary contributor to the carcinogenic process in most cases of gastric cancer (Correa 1975; Correa 1983; Marshall 1985; Warren 1983). Early nested case‐control studies confirmed that individuals infected with H. pylori were between three and six times more likely to develop gastric cancer, compared with uninfected controls (Forman 1991; Nomura 1991; Parsonnet 1991). This observation led the World Health Organization and the International Agency Research on Cancer to conclude that H. pylori was a class I carcinogen (IARC 1994).
A systematic review and meta‐analysis that identified 12 nested prospective case‐control studies suggested that H. pylori was associated with an almost three‐fold increase in odds of developing non‐cardia gastric cancer (HCCG 2001). A policy of screening populations at high risk of gastric cancer for H. pylori with a non‐invasive test, such as the carbon‐urea breath test, and treating those who are infected could lead, theoretically, to a reduction in the incidence of gastric cancer (Parsonnet 1996). However, healthcare providers have not seriously considered this policy, and are unlikely to do so until randomised controlled trials (RCTs) have shown such a screening programme to be effective. There are also concerns from nested case‐control studies that the risk of oesophageal adenocarcinoma is increased in people who are not infected with H. pylori, although these data are less consistent (Wu 2003; Ye 2004). These concerns stem from the theory that H. pylori eradication may induce gastro‐oesophageal reflux symptoms in some individuals, and therefore an increased risk of Barrett's oesophagus and oesophageal adenocarcinoma.
At the inception of this Cochrane review, the only fully published systematic review of RCTs reported a significant reduction in the relative risk of developing gastric cancer with H. pylori eradication therapy, compared with placebo (Fuccio 2009). However, the authors included data from the same trial twice, at two different follow‐up points (Ford 2009). When only data from one or other of these follow‐up points were included, the effect was no longer statistically significant. We therefore performed another systematic review and meta‐analysis in 2015 (Ford 2015), which demonstrated a 34% reduction in the relative risk (RR) of an incident gastric cancer, and a number needed to treat to benefit (NNTB) of 124. Given that it has been five years since the publication of this meta‐analysis, the possibility that there may now be more published trials, as well as longer duration of follow‐up in the existing trials, led us to update the review.
Description of the intervention
H. pylori eradication therapy consists of antibiotics, either alone or in combination with acid suppressant therapy, bismuth, or both. Proton pump inhibitor‐based triple therapy remains the 'gold standard' for the treatment of infection with H. pylori. With the development of accurate methods of diagnosing H. pylori infection, it has become relatively straightforward to confirm successful treatment, or eradication, of the infection.
How the intervention might work
There are biologically plausible mechanisms that may explain the association between H. pylori and gastric cancer. The infection leads to a hyperproliferative state, intragastric concentration of ascorbic acid is reduced, and the levels of mucosal reactive oxygen metabolites capable of inducing DNA damage are increased. The eradication of H. pylori normalises gastric cell turnover, luminal ascorbic acid concentrations, and the level of reactive oxygen species in the mucosa (Moayyedi 1997).
Why it is important to do this review
Population screening for and treatment of H. pylori infection may reduce the incidence of gastric cancer, particularly in populations with a high prevalence of infection with the bacterium who also have a high risk of gastric cancer. The aim of this systematic review and meta‐analysis of RCTs was to evaluate the effect of H. pylori eradication therapy in preventing gastric cancer in otherwise healthy and asymptomatic H. pylori‐positive individuals.
Objectives
To assess the effectiveness of eradication of H. pylori in healthy asymptomatic individuals in the general population in reducing the incidence of gastric cancer.
Methods
Criteria for considering studies for this review
Types of studies
We considered only parallel‐group RCTs comparing H. pylori eradication with placebo or no treatment for this review.
Types of participants
Otherwise healthy and asymptomatic adults over 16 years of age who were H. pylori‐positive, as assessed invasively by any of histology, rapid urease testing, culture (all from antrum or body biopsies obtained at endoscopy), or non‐invasively via H. pylori serology or carbon‐urea breath testing.
Types of interventions
The H. pylori eradication therapy regimen had to have an eradication rate as reported in the literature of at least 50% and was defined as any of the following, with duration of therapy of at least one week:
Proton pump inhibitor (PPI) dual therapy (PPI plus either amoxicillin or clarithromycin);
PPI triple therapy (PPI plus any two of the following: amoxicillin, macrolide, 5‐nitroimidazole);
Histamine2‐receptor antagonist (H2RA) triple therapy (H2RA plus any two of the following: amoxicillin, macrolide, 5‐nitroimidazole);
Bismuth triple therapy (bismuth salt and 5‐nitroimidazole with either amoxicillin or tetracycline);
Bismuth quadruple therapy (as bismuth triple therapy, but with the addition of a PPI);
Ranitidine bismuth citrate (RBC) dual therapy (RBC plus either amoxicillin or clarithromycin);
RBC triple therapy (RBC plus any two of the following: amoxicillin, macrolide, 5‐nitroimidazole);
Clarithromycin monotherapy.
These were compared with either placebo or no treatment.
In trials that were of factorial design that included the evaluation of dietary supplements (for example vitamin C or selenium) as well as H. pylori eradication, the main analysis included arms that randomised all participants to eradication therapy or placebo or no treatment, regardless of their allocation to these supplements.
Types of outcome measures
Participants had to have been followed up for at least two years, and trials needed to report data on subsequent incidence of gastric cancer as an outcome. We defined gastric cancer as any gastric adenocarcinoma, including intestinal (differentiated) or diffuse (undifferentiated) type, or without specified histology.
Primary outcomes
To assess the proportion of H. pylori‐positive individuals randomised to receive eradication therapy that developed gastric cancer, compared with those who received placebo or no treatment.
Secondary outcomes
We assessed the following secondary outcomes in H. pylori‐positive participants randomised to H. pylori eradication compared with placebo or no treatment:
The proportion of individuals who developed oesophageal adenocarcinoma;
The proportion of individuals who developed oesophageal squamous cell carcinoma;
The proportion of individuals who died from gastric cancer;
The proportion of individuals who died from any cause;
The proportion of adverse events (such as diarrhoea, skin rash, nausea or vomiting, headache, altered taste) dichotomised into present or absent.
Search methods for identification of studies
We conducted searches to identify all published and unpublished RCTs. We included articles published in any language.
Electronic searches
We searched the following databases in 2013, and performed an updated search on 02 February 2020:
Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library 2020, Issue 1) (Appendix 1);
MEDLINE (1946 to 02 February 2020) by Ovid (Appendix 2);
EMBASE (1974 to 02 February 2020) by Ovid (Appendix 3).
Searching other resources
We handsearched reference lists from trials selected by electronic searching to identify further relevant trials.
Abstracts
We handsearched Digestive Disease Week (published in Gastroenterology) and United European Gastroenterology Week (published in Gut) abstract books between 2001 and 2013. Conference abstracts after 2013 were indexed by Embase so no hand‐searching was needed. We contacted authors of trial reports published only as abstracts and asked them to contribute full data sets or completed papers.
Correspondence
We contacted experts in the field registered with the Cochrane Upper Gastrointestinal and Pancreatic Diseases Review Group for leads on unpublished studies.
Data collection and analysis
Selection of studies
The lead review author screened titles and abstracts of studies that had been identified by the search strategy for articles possibly eligible for the review. The lead review author then screened the selected trials to confirm eligibility, using predesigned eligibility forms. A second review author, masked to the initial assessment, also evaluated all identified trials for eligibility. A third review author adjudicated any discrepancies, and a consensus view was taken.
Data extraction and management
The lead review author extracted data and recorded it on to specially developed forms. The second review author did a blinded check on this, and any discrepancies were resolved by consensus. Data entry into RevMan 2014 was also double‐checked. Due to the outcome of interest under study, several groups of trial investigators followed‐up trial participants at more than one time point. Where we found multiple articles for a single study, we extracted only data from the latest publication from each eligible study.
We recorded the following characteristics for each trial:
geographical location
country of origin
number of centres
method used to confirm H. pylori infection
type of eradication regimen used (including dose and schedule of individual drugs within it)
duration of treatment
eradication rate
length of follow‐up
dropouts reported and their reasons
subsequent occurrence of gastric cancer
subsequent occurrence of oesophageal cancer (adenocarcinoma or squamous carcinoma)
mortality from gastric cancer
mortality from other causes
total number of adverse events reported
In addition, as some of the trials we identified performed upper gastrointestinal endoscopy and obtained gastric biopsy specimens in all recruited individuals, we were able to obtain the number of participants in these RCTs with preneoplastic lesions at baseline (defined as presence of gastric atrophy, intestinal metaplasia, or dysplasia).
We extracted data using a modified intention‐to‐treat (ITT) analysis. In this, we excluded from the analysis individuals found to be ineligible after randomisation, and those who did not receive the intervention to which they were assigned. Due to the relatively rare nature of the primary outcome of interest, we assumed that all participants lost to follow‐up had not developed gastric cancer, but kept them in the denominator for the study. We did this because the shortest duration of follow‐up in the studies we identified was greater than or equal to four years, and therefore drop‐out rates were relatively high. We also performed a complete case analysis, as a sensitivity analysis, where we excluded all participants for whom data were missing or unavailable from the analysis altogether (Akl 2013).
Assessment of risk of bias in included studies
One review author assessed and a second review author checked study quality. We assessed the components of quality using the criteria described below. We assessed eight risk domains. We considered a study to have a low risk of bias if all risk domains were assessed as a low risk of bias; a high risk of bias if at least one domain was assessed as high risk; or an unclear risk of bias if at least one domain was assessed as unclear risk without any high risk domains.
Random sequence generation (selection bias)
We classified a study as an RCT if it was described as randomised (this includes the use of words such as randomly, random, and randomisation, etc.). We judged the study as low risk, high risk, and unclear risk according to the following:
Low risk, if the allocation sequence was generated by computer‐generated random numbers, published random number table, coin tossing, shuffling cards or envelopes, or throwing dice.
Unclear risk, if the trial was described as randomised but the method used for generation of the allocation sequence was not described.
High risk, if selection was based on patient numbers, birth dates, visit dates, or alternative allocation.
We excluded studies that described selection based on patient or clinical preference, or any selection mechanism that cannot be described as random. We also excluded studies that did not state whether the treatment was randomly allocated.
Allocation concealment (selection bias)
Low risk, if investigators were unaware of the allocation of each participant before they were entered in the trial. Acceptable methods included: central telephone randomisation schemes, pharmacy‐based schemes, sequentially numbered, opaque, sealed envelopes, or sequentially numbered drug containers of identical appearance.
Unclear risk, if the authors did not report or provide a description of an allocation concealment approach that allowed for classification as concealed or not concealed.
High risk, when investigators may have been aware of the allocation of each participant before they entered the trial, e.g. when allocation was based on patient data such as date of birth, hospital case note number, or visit dates, sealed envelopes that were not opaque, or a random number table that was not concealed from an investigator.
Blinding of participants and personnel (performance bias)
Low risk, if both participants and physicians were blinded to the treatment allocation, and it was unlikely that the blinding could have been broken.
Unclear risk, if no blinding information was available or there was insufficient information to permit a judgement of low risk or high risk.
High risk, if the authors defined the study as an open study, or no party was blinded. Either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias.
Blinding of outcome assessment (detection bias)
Low risk, if outcome assessors were blinded to the assigned treatment arm.
Unclear risk, if no information was provided for blinding of outcome assessment.
High risk, if outcome assessors were not blinded to the assigned treatment arm. Lack of blinding is likely to influence adverse events as an outcome. However, knowledge of the assigned intervention is unlikely to impact on H. pylori eradication assessment.
Incomplete outcome data (attrition bias)
We assessed attrition bias for H. pylori eradication and adverse events.
Low risk, if there were no missing outcome data; reasons for missing outcome data were unlikely to be related to true outcome; missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups; the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; missing data were imputed using appropriate methods.
Unclear risk, if insufficient reporting of attrition or exclusions to permit judgement of low risk or high risk (e.g. no reasons for missing data provided).
High risk, if reasons for missing outcome data were likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; the proportion of missing outcomes compared with observed event risk were enough to induce clinically relevant bias in intervention effect estimate; per protocol analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation.
Selective reporting (reporting bias)
Low risk, if the published reports included all expected outcomes, including those that were prespecified.
Unclear risk, if insufficient information to permit judgement of low risk or high risk.
High risk, if not all of the study’s prespecified primary outcomes were reported; the primary outcome (gastric cancer) was reported using measurements, analysis methods, or subsets of the data that were not prespecified; the primary outcome was not prespecified or was reported incompletely; or the study report failed to include results for a key outcome that would be expected to have been reported for such a study.
Other bias
Low risk, if the study appears to be free of other sources of bias.
Unclear risk, if there may be a risk of bias but there is either: insufficient information to assess whether an important risk of bias exists (e.g. limited information from a conference proceeding); or insufficient rationale or evidence that an identified problem introduces bias.
High risk, if there is at least one important risk of bias; a potential source of bias related to the specific study design used; stopped early due to a data‐dependent process (including a formal stopping rule); extreme baseline imbalance; has been claimed to have been fraudulent; or any other problem.
Measures of treatment effect
We assessed the proportion of otherwise healthy and asymptomatic H. pylori‐positive individuals randomised to receive eradication therapy who developed subsequent gastric cancer, compared with those who received placebo or no treatment.
Unit of analysis issues
We included only standard design parallel‐group RCTs with binary outcomes (incidence of gastric cancer). Cluster randomised trials, cross‐over trials, and repeated measurement were not present for the type of RCT.
Dealing with missing data
Where possible, we recorded completeness of follow‐up, with drop‐out rates by group. We attempted to contact authors for missing data. If no outcome data were available, we used the modified ITT approach, which included all eligible and randomised participants in the analysis, but we did not consider participants who were found to be ineligible after randomisation in the ITT analysis in the primary analyses. Correa 2000‐Correa 2001 and You 2006 ‐ Li 2019 both excluded participants from the analyses after randomisation (as they were subsequently found to be ineligible or did not take the treatment). We included these ineligible participants in the sensitivity analyses. Since the incidence of gastric cancer is low, we did not presume that missing participants had developed subsequent gastric cancer (worst‐case scenario).
We also performed a complete case analysis, as a sensitivity analysis, where we excluded all participants for whom data were missing or unavailable from the analysis altogether (Akl 2013). We also performed sensitivity analyses with missing data imputation based on the assumptions that: 1) incidence of gastric cancer for missing participants in both arms was the same as observed in the trial control arm; 2) incidence of gastric cancer for missing participants in the treatment arm was the same as that observed in the trial control arm, but there were no new gastric cancer cases in the control arm among those with missing data (Akl 2013).
Assessment of heterogeneity
We pooled data using a random‐effects model to give a more conservative estimate of the effect of H. pylori eradication therapy on the subsequent occurrence of gastric cancer, allowing for any heterogeneity between studies (DerSimonian 1986). We assessed heterogeneity using both the I2 statistic with a cutoff of greater than or equal to 50%, and the Chi2 test with a P value less than 0.10 used to define a significant degree of heterogeneity (Higgins 2003).
Assessment of reporting biases
We did not produce funnel plots in any of our analyses, as less than 10 studies were included in these in all cases. (Sterne 2011)
Data synthesis
For all primary and secondary outcomes, which were dichotomous, we expressed the impact of the intervention as a risk ratio (RR) together with 95% confidence intervals. We calculated the NNTB using the formula 100/(risk ratio reduction x control event rate); that is NNTB = 1/(assumed control risk (ACR) x (1 ‐ RR)), with the ACR based on the pooled control event rate from the eligible studies. There were sufficient data for the generation of a meta‐analysis for this review.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses examining the incidence of subsequent gastric cancer according to the presence or absence of preneoplastic lesions (defined as presence of gastric atrophy, intestinal metaplasia, or dysplasia) at baseline among trial participants, as judged by histopathological interpretation of gastric biopsy specimens, and according to whether trial participants were co‐administered antioxidants or vitamins during the trial.
Where we detected significant heterogeneity, we investigated possible explanations informally. We planned to explore reasons for heterogeneity according to the following predefined criteria:
eradication regimen used in the study;
geographical location of the study;
risk of bias of the study.
Sensitivity analysis
We performed a complete case analysis, where we excluded all participants for whom data were missing or unavailable from the analysis altogether (Akl 2013).
We performed missing data imputation based on the assumptions that a) incidence of gastric cancer for missing participants in both arms was the same as that observed in the trial control arm; b) incidence of gastric cancer for missing participants in the treatment arm was the same as that observed in the trial control arm, but there were no new gastric cancer cases in the control arm among those with missing data (Akl 2013).
We conducted a modified ITT analysis as well as a complete case analysis, using data from Leung 2004‐Zhou 2014 (full publication), instead of the conference abstract data presented in Zhou 2008.
We conducted a modified ITT analysis including the two celecoxib arms (anti‐H. pylori treatment and celecoxib, placebo and celecoxib) from Wong 2012b.
We included all randomised subjects, including those who were found to be ineligible or did not receive treatment after randomisation in Correa 2000‐Correa 2001 and You 2006 ‐ Li 2019.
Summary of findings and assessment of the certainty of the evidence
The results of the main outcomes from this review are presented in the Table 1. The table presents a summary of the available data as well as a evaluation of the robustness of the results according to GRADE (Grades of Recommendation, Assessment, Development and Evaluation) criteria GRADE 2011. The GRADE approach evaluates the overall quality of the evidence for a given outcome in terms of risk of bias, precision, directness of the evidence, publication bias and completeness of outcome reporting Balshem 2011. This gives an overall assessment of the confidence that can be placed in the data, which in turn can inform decision making.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies.
Results of the search
In 2013, we identified 1560 citations with six eligible RCTs. In the February 2020 updated search, we identified 716 citations with one new RCT, using the search strategy outlined above (Figure 1). We reviewed the titles and abstracts and thought 56 articles in 2013 and 12 records in 2020 to be potentially eligible for inclusion. Of these 68 articles, 16 were not RCTs (Hamajima 2002; Hsu 2007; Juibari 2003; Kamangar 2006; Kato 2006; Kim 2008; Leung 2018; Mabe 2009; Ogura 2008; Ohkusa 2001; Take 2005; Take 2007; Takenaka 2007; Uemura 2001; Uemura 2002; Yanaoka 2009); three were RCTs comparing the interventions of interest, but their primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community, and they did not report any gastric cancer data (Harvey 2004; Moayyedi 2000; Wildner‐Christensen 2003); and two were RCTs, but with no incident gastric cancers occurring during follow‐up, and therefore did not meet our eligibility criteria for inclusion (Fischbach 2001; Miehlke 2001). In the latter five studies, we contacted the lead or senior authors to ask for the most up‐to‐date information from the most recent follow‐up point of the study, in order to ensure that we were not excluding these articles injudiciously. In all cases, the authors responded and stated that there had been no incident gastric cancers reported at the last point of follow‐up. One RCT presented data on those who had H.pylori eradicated in intervention group vs those H.pylori negative in control group after 3 years, instead of presenting data in all randomized subjects; subjects were those who admitted to hospital or came to hospital for H.pylori testing, instead of general population (Tang 2010). One was a protocol of a RCT that will randomize participants to test and treat vs controls, instead of eradication vs control (Leja 2017) Another four articles were duplicate publications of studies already classified as ineligible. (Ford 2005; Imanzadeh 2004; Lane 2006; Mason 2002). Four studies were RCTs conducted among patients undergoing endoscopic mucosal resection of early gastric cancer (Choi 2014; Choi 2018a; Choi 2018b; Fukase 2008), rather than healthy asymptomatic infected participants, and the final article did not compare the interventions of interest (Fischbach 2009). In the updated search, two articles were protocols from Korea for "HEPTER study" (HELPER study) and one article was protocol from China (Pan 2016)for an ongoing randomised trial.
1.
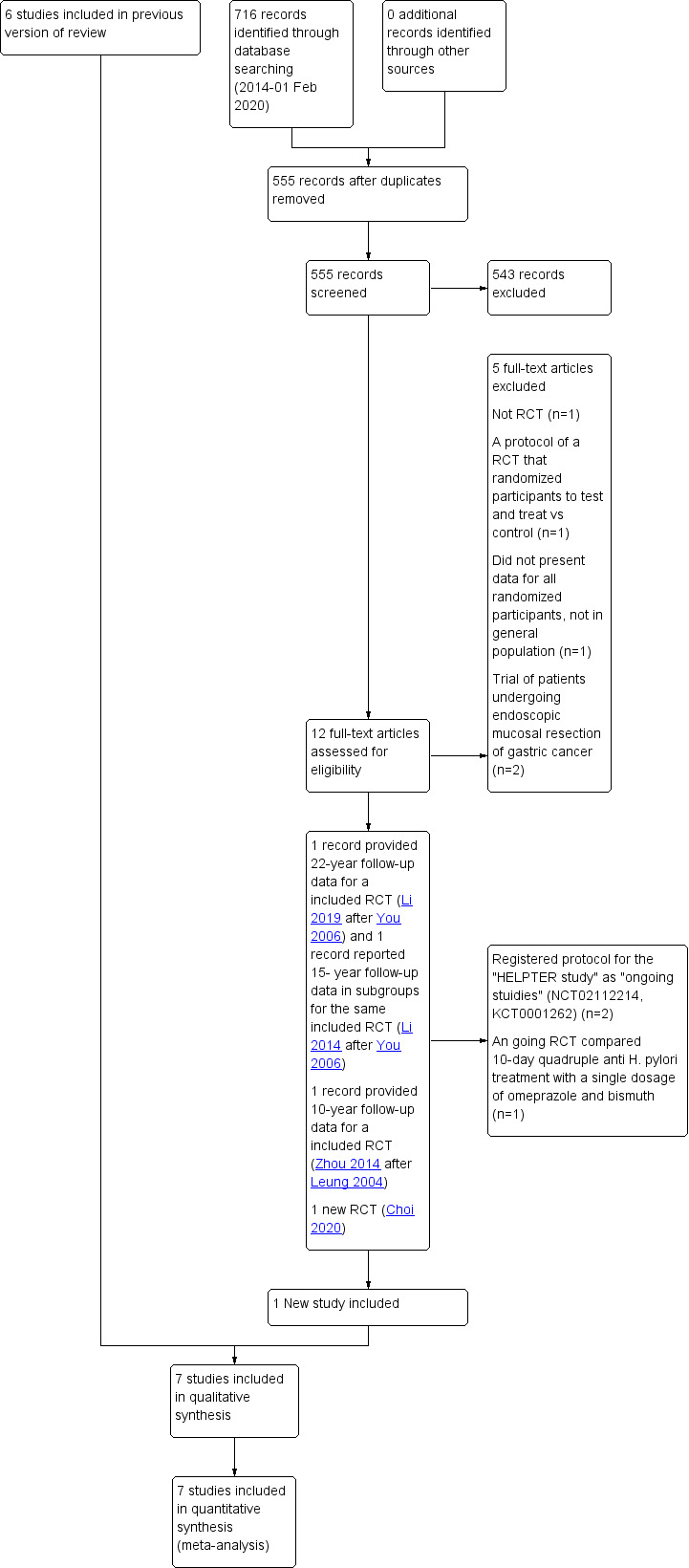
Study flow diagram: review update
This left 33 separate papers, reporting on seven separate RCTs that compared H. pylori eradication therapy with placebo or no treatment and providing data on subsequent incidence of gastric cancer, which therefore appeared to be eligible for inclusion. In the previous version of this review, 19 of 29 of these articles were preliminary or duplicate publications, or protocols of eligible RCTs, and provided no new information or did not report outcomes of interest, and were therefore excluded from the meta‐analysis (Feng 2008; Li 2013; Mera 2005; Ruiz 2001; Saito 2003; Sung 2000; Sung 2002; Wang 2009; Wong 2002; Wong 2012b; You 2001; Zhang 1998; Zhang 2006; Zhou 2003a; Zhou 2003b; Zhou 2003c; Zhou 2005a; Zhou 2005b; Zhou 2005c), leaving 10 papers that reported unique and extractable data (Correa 2000‐Correa 2001; Correa 2001; Gail 1998; Zhou 2008; Ma 2012; Saito 2005; Wong 2004; Wong 2012b; You 2006 ; Zhou 2008). One of these studies only reported adverse events data (Gail 1998). In the updated search, one article provided 22‐year follow‐up data (Li 2019) for one of the previously included RCTs and one record (Li 2014) reported 15‐ year follow‐up data in subgroups for the same RCT (You 2006) that included in published version with follow‐up data up to 14.7 years. Data from this study (You 2006 ‐ Li 2019) is being update to the 22‐year follow up data (Li 2019). For another RCT that included in the previous version (Leung 2004‐Zhou 2014), data from a conference abstract (Zhou 2008) with 10‐year follow up data were used. In the update search, the conference abstract was fully published in 2014 (Zhou 2014) but no new data is added to the meta‐analysis. One new RCT is identified in the update search (Choi 2020). Therefore, seven studies (Choi 2020; Correa 2000‐Correa 2001; Leung 2004‐Zhou 2014; Saito 2005; Wong 2004; Wong 2012a;You 2006 ‐ Li 2019) with eleven references contributed data to the analyses concerning incidence of gastric cancer in this systematic review.
Included studies
Please see Characteristics of included studies table. Three of the trials, reported in five separate publications, were of factorial design with some participants randomised to receive vitamins, antioxidants, or celecoxib in addition to H. pylori eradication therapy (Correa 2000‐Correa 2001; Wong 2012a; You 2006 ‐ Li 2019). Only one study was conducted in non‐Asians, among a population at high risk of gastric cancer in Colombia (Correa 2000‐Correa 2001). The shortest duration of follow‐up was greater than or equal to 4 years (Saito 2005), and the longest was 22 years (You 2006 ‐ Li 2019). The largest study contained 2258 participants (You 2006 ‐ Li 2019), and the smallest 513 participants (Wong 2012a).
Excluded studies
Please see Characteristics of excluded studies table.
Risk of bias in included studies
Four trials were at low risk of bias (Choi 2020; Wong 2004; Wong 2012a; You 2006 ‐ Li 2019), one trial was at unclear risk (Saito 2005), and two trials were at high risk of bias (Correa 2000‐Correa 2001; Leung 2004‐Zhou 2014) (Figure 2; Figure 3). One study was at high risk of bias because no placebo comparator was used for the active eradication therapy regimen, and therefore this part of the trial was unblinded (Correa 2000‐Correa 2001); the other study was at high risk of bias due to inconsistencies in data reporting at the two points of follow‐up, with 10 gastric cancers reported at 5 years (Leung 2004‐Zhou 2014), compared with nine at 10 years (Zhou 2014). Despite contacting the original authors, we were unable to resolve this discrepancy satisfactorily. In the case of this trial, we used data from the 10‐year follow‐up in our primary analysis, but substituted the 5‐year data in a sensitivity analysis.
2.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Random sequence generation
We considered six studies to be at low risk of bias for random sequence generation: four studies generated the allocation sequence by a computer (Choi 2020; Correa 2000‐Correa 2001; Leung 2004‐Zhou 2014; Wong 2004), and two studies generated the assignments by a company (Wong 2012; You 2006 ‐ Li 2019). We considered one study, in abstract form, to be at unclear risk of bias for random sequence generation, as no information was provided regarding the randomisation process (Saito 2005).
Allocation concealment
We considered six studies to be at low risk of bias for allocation concealment: two studies allocated participants by sealed envelopes (Leung 2004‐Zhou 2014; Wong 2004 ), one study involved the trial pharmacy keeping the randomization sequence (Choi 2020) and three studies involved central allocation (Correa 2000‐Correa 2001; Wong 2012a; You 2006 ‐ Li 2019). One study, in abstract form, had uncertain concealment (Saito 2005).
Blinding
Blinding of participants, health providers, data collectors, and outcome assessors should be possible for this type of eradication study. We considered five double‐blind, placebo‐controlled studies to be at low risk of bias for blinding of participants and personnel, as well as blinding of outcome assessors (Choi 2020; Leung 2004‐Zhou 2014; Wong 2004; Wong 2012a; You 2006 ‐ Li 2019). Pathologists were blinded in three studies (Choi 2020; Correa 2000‐Correa 2001; Leung 2004‐Zhou 2014), two of which were considered a double‐blind study because a placebo was used (Choi 2020; Leung 2004‐Zhou 2014). We considered Correa 2000‐Correa 2001 to be at high risk of bias for blinding of participants and personnel because an appropriate placebo was not available for bismuth subsalicylate, and double blinding only applied to the dietary supplements versus placebo part of the trial. We considered this study to be at low risk of bias for blinding of outcome assessment because pathologists were blinded. One study, in abstract form, had uncertain risk of bias for blinding (Saito 2005).
Incomplete outcome data
We considered one study to be at high risk of bias for incomplete outcome data (Leung 2004‐Zhou 2014). Data from Zhou 2014 were used for the main analysis due to the longer follow‐up period. However, the 10‐year follow‐up data reported in Zhou 2014 had a smaller sample size, and fewer gastric cancer cases, than those in the earlier full publication (Leung 2004). According to Leung 2004, 152 (75 vs 77) were lost to follow‐up; these participants were considered as no gastric cancer in the ITT analysis. After 10 years, 378 subjects had completed the study (Zhou 2014). Therefore, in total there would be more than 31% participants lost to follow‐up. We considered three studies to be at unclear risk of bias (Correa 2000‐Correa 2001; Saito 2005; You 2006 ‐ Li 2019). In one study, the average rate of loss was 4.3% per year over the 6‐year trial; withdrawals in the 72 months of follow‐up were 117 (26.8%) versus 104 (25%) in all H. pylori eradication arms versus control arms (Correa 2000‐Correa 2001). However, it is likely that participants who had cancer would have come back for treatment, although these individuals did not complete follow‐up. One conference proceeding did not provide detailed information (Saito 2005). We considered four studies to be at low risk of bias, because the numbers of participants who were lost to follow‐up were balanced between treatment arms and were fewer than 20% (Choi 2020; Wong 2004; Wong 2012a; You 2006 ‐ Li 2019).
Selective reporting
Four studies reported all important outcomes, and we therefore considered them to be at low risk of bias for selective reporting (Choi 2020; Wong 2004; Wong 2012a; You 2006 ‐ Li 2019). We considered three studies to be at unclear risk of bias. In one of these studies, death from gastric cancer was not reported (Correa 2000‐Correa 2001). In another study, mortality data were reported in the 2004 full publication (Leung 2004), but not in the 2014 article (Zhou 2014), which led to an inconsistent sample size between the incidence of gastric cancer and mortality analyses (Leung 2004‐Zhou 2014). One study, reported in abstract form, did not provide any mortality data (Saito 2005).
Other potential sources of bias
We considered one study to be at high risk of bias for other potential sources of bias, due to inconsistent data noted between serial publications (Leung 2004‐Zhou 2014). We identified a total of 11 publications from this study (Leung 2004; Sung 2000; Sung 2002; Zhou 2003a; Zhou 2003b; Zhou 2003c; Zhou 2005a; Zhou 2005b; Zhou 2005c; Zhou 2008; Zhou 2014), with the latest publications reporting data out to 10 years of follow‐up, Zhou 2008 and Zhou 2014, having a smaller sample size and fewer gastric cancer cases than the 2004 full publication. Specifically, 10 gastric cancer cases were reported at 5 years, compared with only nine at 10 years. We considered two studies to be at unclear risk for other potential sources of bias. One was in abstract format (Saito 2005), and the other demonstrated an inconsistent sample size between the full publication, which reported data at 7.5 years (817 versus 813) (Wong 2004), and the conference abstract, which reported data at 7 years (819 versus 809) (Wong 2002). We considered the other four studies to be at low risk of bias.
Effects of interventions
See: Table 1
Effect of H. pylori eradication therapy, compared with placebo or no therapy, on development of subsequent gastric cancer
All seven trials reported a dichotomous outcome for subsequent incidence of gastric cancer. In our primary, modified intention‐to‐treat (ITT) analysis, we included all arms in the two trials of factorial design that also randomised participants to receive antioxidants or vitamins, as well as the 10‐year follow‐up data from Zhou 2014. Overall, 68 (1.6%) of 4206 participants assigned to eradication therapy subsequently developed gastric cancer, compared with 125 (3.0%) of 4117 participants allocated to placebo or no treatment. There was no statistically significant heterogeneity between individual trial results (heterogeneity test, I2 = 0%, P = 0.61). H. pylori eradication therapy reduced the risk of gastric cancer in healthy asymptomatic infected individuals (risk ratio (RR) 0.54; 95% confidence interval (CI) 0.40 to 0.72) (NNTB = 72; 95% CI 55 to 118) (Analysis 1.1). The certainty of the evidence was moderate (Table 1) with the evidence being downgraded because one trial was at unclear risk, and two trials were at high risk of bias. In addition, because of the factorial design of some of the trials, it is difficult to determine whether the reduction in relative risk of subsequent gastric cancer was due to H. pylori eradication therapy alone.
1.1. Analysis.

Comparison 1: H. pylori eradication vs control ‐ main analyses, Outcome 1: Incidence of gastric cancer ‐ modified ITT analysis
We performed several sensitivity analyses when pooling data from these seven trials. In our complete case analysis, where all participants for whom data were missing or unavailable were excluded from the analysis altogether, the RR of developing subsequent gastric cancer was 0.53 (95% CI 0.40 to 0.71) (Analysis 1.2). When we performed a modified ITT analysis, substituting the 10‐year follow‐up data from Zhou 2014 with the 5‐year follow‐up data from Leung 2004., the RR of developing gastric cancer was 0.56 (95% CI 0.42 to 0.74) (Analysis 4.1). When we performed a complete case analysis, but substituted the 10‐year follow‐up data from Zhou 2014 with the 5‐year follow‐up data from Leung 2004, the RR was 0.55 (95% CI 0.41 to 0.74) (Analysis 4.2). When we performed a modified ITT analysis, but also included the celecoxib arms from the trial by Wong 2012, the RR was 0.55 (95% CI 0.41 to 0.74) (Analysis 4.3). When we included all randomised participants from Correa 2000‐Correa 2001 and You 2006 ‐ Li 2019 in the analysis, that is we also included participants who were found to be ineligible after randomisation or those who did not take any medication (the most strict ITT definition), the RR was 0.54 (95% CI 0.40 to 0.72) (Analysis 4.4). Finally, we performed two data imputation analyses. If we assumed the incidence of gastric cancer for missing participants in both arms was the same as that observed in the trial control arm, the RR was 0.55 (95% CI 0.41 to 0.73). If we assumed the incidence of gastric cancer for missing participants in the treatment arm was the same as that observed in the trial control arm, but there were no new gastric cancer cases in the control arm among those with missing data, the RR was 0.56 (95% CI 0.42 to 0.74) (Analysis 4.5). We can therefore be reasonably confident that our conclusions are robust, regardless of the assumptions made about missing data.
1.2. Analysis.

Comparison 1: H. pylori eradication vs control ‐ main analyses, Outcome 2: Incidence of gastric cancer ‐ complete case analysis
4.1. Analysis.

Comparison 4: H. pylori eradication vs control ‐ sensitivity analyses, Outcome 1: Incidence of gastric cancer ‐ modified ITT analysis substituting the 10‐year follow‐up data from Zhou 2014 with the 5‐year follow‐up data from Leung 2004
4.2. Analysis.

Comparison 4: H. pylori eradication vs control ‐ sensitivity analyses, Outcome 2: Incidence of gastric cancer ‐ complete case analysis substituting the 10‐year follow‐up data from Zhou 2014 with the 5‐year follow‐up data from Leung 2004
4.3. Analysis.

Comparison 4: H. pylori eradication vs control ‐ sensitivity analyses, Outcome 3: Incidence of gastric cancer‐ modified ITT analysis including the two arms of celecoxib from Wong 2012
4.4. Analysis.

Comparison 4: H. pylori eradication vs control ‐ sensitivity analyses, Outcome 4: Incidence of gastric cancer ‐ modified ITT analysis including all randomised patients from Correa 2000 and You 2006 who were found subsequently to be ineligible or did not receive treatment
4.5. Analysis.

Comparison 4: H. pylori eradication vs control ‐ sensitivity analyses, Outcome 5: Incidence of gastric cancer ‐ missing data imputation based on various assumptions
Effect of H. pylori eradication therapy, compared with placebo or no therapy, on development of subsequent gastric cancer according to presence or absence of preneoplastic lesions at baseline
We found no evidence of any benefit of H. pylori eradication therapy in preventing the subsequent occurrence of gastric cancer when we considered only those with preneoplastic lesions at baseline in the analysis. Overall, 42 (2.4%) of 1734 participants assigned to eradication therapy subsequently developed gastric cancer, compared with 57 (3.4%) of 1691 participants allocated to placebo or no treatment (RR 0.86; 95% CI 0.47 to 1.59) (Analysis 2.1). There was no statistically significant heterogeneity between individual trial results (heterogeneity test, I2 = 23%, P = 0.27). Nor was there evidence of any benefit of H. pylori eradication therapy in preventing subsequent occurrence of gastric cancer when only those participants without preneoplastic lesions at baseline were considered in the analysis. Four (0.4%) of 894 participants randomised to receive eradication therapy subsequently developed gastric cancer, compared with nine (1.0%) of 918 participants who were assigned to placebo (RR 0.42; 95% CI 0.02 to 7.69) (Analysis 2.1). There was statistically significant heterogeneity between individual trial results (heterogeneity test, I2 = 70%, P = 0.07). Three studies were included in the subgroup of "mixed" patients with and without precancerous lesions. Of them, Choi 2020 reported that they excluded patients with gastric dysplasia, but stated in the protocol that patients both with or without precancerous lesions (atrophy and intestinal metaplasia) would be included and compared. However, no such data were provided in the final report, so we considered this study to include "mixed" patients, both with and without precancerous lesions. In this subgroup analysis the pooled RR was 0.42 (95% CI 0.22 to 0.78; test for heterogeneity, I2 = 0%, P = 0.85) (Analysis 2.1). There was no significant difference between the subgroups (I2 = 0%, P = 0.85). It should be noted that there would be reduced power to detect significant differences in these subgroup analyses.
2.1. Analysis.

Comparison 2: H. pylori eradication vs control ‐ subgroup analysis according to presence or absence of pre‐neoplastic lesions at baseline, Outcome 1: Incidence of gastric cancer according to presence or absence of pre‐neoplastic lesions at baseline
Effect of H. pylori eradication therapy, compared with placebo or no therapy, on development of subsequent gastric cancer according to whether participants were co‐administered vitamins or antioxidants
We found no evidence of any benefit of H. pylori eradication therapy in preventing subsequent occurrence of gastric cancer when we considered only those participants who received eradication therapy alone in the analysis. Overall, 40 (1.3%) of 3082 participants assigned to eradication therapy alone subsequently developed gastric cancer, compared with 58 (2.0%) of 2958 participants allocated to placebo or no treatment alone (RR 0.69; 95% CI 0.41 to 1.14). There was no statistically significant heterogeneity between individual trial results (heterogeneity test, I2 = 22%, P = 0.26) (Analysis 3.1). However, when we considered those participants receiving eradication therapy in combination with antioxidants or vitamins in the analysis, 21 (1.8%) of 1178 participants randomised to receive eradication therapy and antioxidants or vitamins subsequently developed gastric cancer, compared with 41 (3.5%) of 1159 participants who were assigned to placebo or no treatment plus antioxidants or vitamins (RR 0.52; 95% CI 0.31 to 0.87). There was no statistically significant heterogeneity between individual trial results (heterogeneity test, I2 = 0%, P = 0.51) (Analysis 3.1). There was no significant difference between the subgroups (I2 = 0%, P = 0.46). Again, it should be noted that there would be reduced power to detect significant differences in these subgroup analyses, .
3.1. Analysis.

Comparison 3: H. pylori eradication vs control ‐ subgroup analysis according to use of vitamins or antioxidants, Outcome 1: Incidence of gastric cancer according to use of vitamins or anti‐oxidants
Effect of H. pylori eradication therapy, compared with placebo or no therapy, on development of subsequent oesophageal cancer
Only two trials reported these data (Wong 2004;You 2006 ‐ Li 2019). 16 (0.8%) of 1947 participants assigned to eradication therapy developed oesophageal cancer, compared with 13 (0.7%) of 1941 participants allocated to placebo (RR 1.22; 95% CI 0.59 to 2.54, P = 0.68). All three cases were squamous cell cancers in Wong 2004 but histological subtype was not clear in You 2006 ‐ Li 2019 (Analysis 1.5). The certainty of the evidence is moderate (Table 1) with the evidence being downgraded because of imprecision.
1.5. Analysis.

Comparison 1: H. pylori eradication vs control ‐ main analyses, Outcome 5: Incidence of oesophageal squamous cell carcinoma ‐ modified ITT analysis
Effect of H. pylori eradication therapy, compared with placebo or no therapy, on death from gastric cancer
Four studies, containing 6301 participants, provided data on mortality from gastric cancer (Choi 2020; Leung 2004‐Zhou 2014; Wong 2004; You 2006 ‐ Li 2019). Follow‐up ranged from 5 years to 22 years. Overall, there were 36 deaths (1.1%) from gastric cancer among 3154 participants randomised to eradication therapy, compared with 59 (1.2%) deaths in 3147 participants receiving placebo. There was no statistically significant heterogeneity between individual trial results (heterogeneity test, I2 = 0%, P = 0.95). There was evidence of benefit of H. pylori eradication therapy in preventing death from gastric cancer in healthy asymptomatic infected individuals (RR 0.61; 95% CI 0.40 to 0.92) (Analysis 1.3). The NNTB was 137 (95% CI 89 to 667).Certainty of the evidence is moderate (Table 1) and this was downgraded .
1.3. Analysis.

Comparison 1: H. pylori eradication vs control ‐ main analyses, Outcome 3: Death from gastric cancer ‐ modified ITT analysis
Effect of H. pylori eradication therapy, compared with placebo or no therapy, on all‐cause mortality
Five studies, containing 7079 participants, provided data on all‐cause mortality (Choi 2020; Correa 2000‐Correa 2001; Wong 2004; Wong 2012a; You 2006 ‐ Li 2019). Follow‐up ranged from 6 to 22 years. Overall, 315 (8.9%) of 3551 participants receiving eradication therapy were dead at last point of follow‐up, compared with 323 (9.2%) of 3528 participants receiving placebo or no treatment. There was no statistically significant heterogeneity between individual trial results (heterogeneity test, I2 = 0%, P = 0.50). There was no evidence of any benefit of H. pylori eradication therapy in preventing death from any cause in healthy asymptomatic infected individuals (RR 0.97; 95% CI 0.85 to 1.12) (Analysis 1.4). Certainty of the evidence is moderate (Table 1) and downgraded because three of the studies were either unclear or high risk of bias.
1.4. Analysis.

Comparison 1: H. pylori eradication vs control ‐ main analyses, Outcome 4: Death from all causes ‐ modified ITT analysis
Adverse events with H. pylori eradication therapy, compared with placebo or no therapy
Only two of the studies we identified reported individual adverse events data with eradication therapy compared with placebo or no treatment (Choi 2020; Gail 1998). Gail 1998 reported that there was no difference in the incidence of adverse events with eradication therapy, with the exception of skin rash, which occurred in 3.1% of those receiving eradication therapy compared with 0.1% of those allocated placebo. Choi 2020 reported that any adverse event was higher in the eradication group (53.0% vs 19.1% P <0.001), and that any adverse event ≥ grade 3 occurred in 0.8% vs 0.1% (P = 0.04). Another study reported that side effects were monitored closely, and none of any clinical importance were detected, although no dichotomous data were reported to support this statement (Correa 2000‐Correa 2001).
Discussion
Summary of main results
This systematic review and meta‐analysis suggests that searching for and eradicating H. pylori infection in otherwise healthy and asymptomatic infected individuals reduces the subsequent incidence of gastric cancer. The risk of a subsequent gastric cancer with eradication therapy was reduced by 46%, and the NNTB to prevent one case of gastric cancer was 72. The effect size we observed remained robust through the majority of sensitivity analyses we performed. We were unable to confirm or refute whether any benefit of H. pylori eradication therapy depended on the presence or absence of preneoplastic lesions at baseline. However, it is important to highlight that there would be reduced power to detect significant differences in most of the subgroup analyses we conducted, and the original trials were not powered for secondary endpoints, such as mortality from gastric cancer or effect on oesophageal cancer. Finally, there were few cases of subsequent oesophageal cancer, and adverse events data were poorly reported in the studies we identified. The data now suggest an effect of H. pylori eradication in reducing gastric cancer‐related mortality, with a NNTB of 137, but trials still show no impact of H. pylori eradication on all cause mortality. This would be the most robust end‐point to evaluate but as gastric cancer accounts for only a small proportion of the overall death rate the sample size for trials would need to be extremely large.
Overall completeness and applicability of evidence
As all but one of the eligible trials we identified were conducted in Asian populations, and the other trial in a South American population, it is not possible to assess the effect of screening and treatment of H. pylori in healthy and asymptomatic individuals in Western populations. In addition, none of the RCTs we identified reported individual adverse events data, which means that we were unable to assess the balance of benefits and harms if population screening and treatment for H. pylori infection were to be adopted as a public health measure. Most trials recruited participants with a mean age of approximately 50 years of age with and age range of 20‐65 years. It would be helpful to know the optimum age when H. pylori should be eradicated as preneoplastic changes will be minimized if treatment is offered in younger age groups but in these groups it will take longer before any benefit of the intervention is realized so this may be less cost‐effective. Elucidating this issue would be helpful for policy makers. Currently most guidelines do not suggests population H. pylori eradication and treatment Chey 2017 although this changing and now some are recommending this approach in high gastric cancer risk countries (IARC 2014; Fock 2009; Malfertheiner 2017).
Quality of the evidence
Only four of the RCTs we identified were at low risk of bias (Choi 2020; Wong 2004; Wong 2012a; You 2006 ‐ Li 2019), one trial was at unclear risk because it was reported in abstract form (Saito 2005), and two trials were at high risk of bias (Correa 2000‐Correa 2001; Leung 2004‐Zhou 2014). In Correa 2000‐Correa 2001, this was because no placebo comparator was used for the active eradication therapy regimen, and therefore this part of the trial was unblinded, and in Leung 2004‐Zhou 2014 it was due to inconsistencies in data reporting at the two follow‐up points, with 10 gastric cancers reported at 5 years, compared with nine at 10 years (Zhou 2008). Despite contacting the original authors, we were unable to resolve this discrepancy satisfactorily. Certainty of the evidence is downgraded from high to moderate (Table 1).
Potential biases in the review process
There were limitations of this review due to the quality and characteristics of the published literature identified, which we have highlighted above. Because of the factorial design of some of the trials, it was also difficult to ascertain whether the significant reduction in the risk of subsequent gastric cancer was due to H. pylori eradication therapy alone, or to the antioxidants or vitamins that were co‐administered in some of the trials. Certainly, the beneficial effect of eradication therapy appeared to be more pronounced in the two studies that co‐administered antioxidants and vitamins to participants, suggesting that there may have been some additive benefit derived from these supplements, although power to demonstrate effect modification due to these different treatments is again limited. However, it should be noted that one of these trials contained the majority of gastric cancers, and had the longest duration of follow‐up of almost 22 years.
Agreements and disagreements with other studies or reviews
A previous systematic review and meta‐analysis that examined this issue 11 years ago reported that there was a benefit of eradicating H. pylori to prevent future development of gastric cancer (Fuccio 2009). The magnitude of this effect was less than that we observed, with a RR of subsequent gastric cancer of 0.65 (95% CI 0.43 to 0.98),due to longer follow‐up in one of the trials (You 2006 ‐ Li 2019), plus the inclusion of the newly identified trial (Choi 2020) .
Although these data suggest that population H. pylori screening and treatment may reduce the incidence of gastric cancer, the 95% CIs are relatively wide, and the result is somewhat dependent on one study (You 2006 ‐ Li 2019). However, there was still a significant effect of eradication therapy, compared with placebo or no treatment, in preventing subsequent occurrence of gastric cancer when this trial was excluded from the analysis (RR = 0.56; 95% CI 0.35 to 0.92), with no heterogeneity between studies (I2 = 0%, P = 0.49), and a NNTB of 144. In addition, there are data from other sources that support our findings. Three RCTs have suggested that H. pylori eradication can reduce the future incidence of a metachronous cancer among people who have had an endoscopic mucosal resection of gastric cancer (Fukase 2008; Choi 2018a; Choi 2018b). On Matsu Island in Taiwan, where population screening and treatment was adopted in 2004, H. pylori prevalence fell from 63% at baseline to less than 14% during the subsequent 4 years, and prevalence of gastric atrophy fell from almost 60% to less than 14% (Lee 2013). At the same time, the 5‐year average incidence of gastric cancer declined from 40.3 per 100,000 person‐years to 30.4, yielding a rate ratio of 0.75 (95% CI 0.37 to 1.52), at a time when the incidence of gastric cancer elsewhere in Taiwan remained unchanged. However, the intervention did not affect the incidence of intestinal metaplasia, adding support to the theory that there is a 'point of no return' in the histological changes induced by H. pylori beyond which cancer prevention by eradication of the infection is no longer possible. There was no significant effect on mortality from gastric cancer observed during the period of this study, although follow‐up was limited to four years, which is shorter than all but one of the studies included in our meta‐analysis. Finally, the prevalence of erosive oesophagitis at upper gastrointestinal endoscopy increased from 14% at baseline to 27% by 2008, suggesting possible deleterious effects of population screening and treatment for H. pylori infection, due to a potential for an increased risk of oesophageal adenocarcinoma in the long term. This was something we aimed to assess in our meta‐analysis, but incomplete reporting of oesophageal cancers among our included studies prevented us from achieving this.
Authors' conclusions
Implications for practice.
These data provide moderate certainty evidence that searching for and eradicating H. pylori can reduce the future incidence of gastric cancer in healthy asymptomatic people who are infected with the bacterium. However, as the only trial conducted in a non‐Asian population failed to demonstrate any benefit of such an approach, these findings may not necessarily apply to the rest of the world.
The findings of this systematic review and meta‐analysis add to the increasing evidence that eradicating H. pylori in the general population has the potential to prevent gastric cancer. International guidelines for the management of H. pylori infection may change as a result.
Implications for research.
Given that any population‐based approach to mass screening for H. pylori, with eradication of the infection in positive individuals, will involve healthy subjects, there needs to be more information on any potential harms of H. pylori eradication before such a strategy can be advocated as a means of preventing gastric cancer. Further trials are therefore needed in different populations to extend the evidence base, and these should report on both the benefits and harms of such an approach.
What's new
| Date | Event | Description |
|---|---|---|
| 19 March 2020 | New citation required and conclusions have changed | Updated evidence suggests eradicating H. pylori reduces the incidence of gastric cancer as well as death from gastric cancer in healthy asymptomatic infected Asian individuals |
| 2 February 2020 | New search has been performed | Updated search on 2 Feb 2020, 22‐year and 10‐year follow‐up data are reported for two previously included RCTs. One new RCT included. Analyses are updated. |
History
Protocol first published: Issue 1, 2006 Review first published: Issue 7, 2015
| Date | Event | Description |
|---|---|---|
| 30 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Drs Jaw‐Town Lin, Mitchell H Gail, Sarah Rhodes and Ms. Marilyn Walsh for their constructive comments on our review version 2015.
We thank Professor Morris Gordon and Colin W. Howden, MD for their comments on the 2020 updated of the review.
Appendices
Appendix 1. CENTRAL search strategy (via OvidSP)
exp Helicobacter/ or exp Helicobacter Infections/
(helicobacter or campylobacter).tw,kw.
(pylori or pyloridis or HP).tw,kw.
or/1‐3
exp Stomach Neoplasms/
exp Lymphoma, B‐Cell, Marginal Zone/
(mucosa associated lymphoid tissue lymphoma or MALT).tw,kw.
((stomach or gastric) adj3 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or lymphoma* or adenocarcinoma* or malign*)).tw,kw.
or/5‐8
4 and 9
exp Proton Pump Inhibitors/
proton pump inhibitor*.mp.
(PPI or PPIs).tw,kw.
(omeprazole or h 16868 or losec or prilosec or rapinex or zegerid).mp.
(lansoprazole or lanzoprazole or ag 1749 or agopton or bamalite or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).mp.
(pantoprazole or by 1023 or protium or protonix or skf‐96022).mp.
(esomeprazole or nexium).mp.
(rabeprazole or aciphex or dexrabeprazole or e 3810 or ly‐307640 or pariet).mp.
(dexlansoprazole or Kapidex or Dexilant or AGN 20194* or AGN20194* or dexrabeprazole).mp.
(tenatoprazole or CAS 113712‐98‐4 or STU‐Na or TAK‐390* or TAK390* or TAK‐438 or TAK438 or AZD0865 or "AZD 0865").mp.
or/11‐20
exp Histamine H2 Antagonists/
((histamine or H2 or H‐2 or H2R or H 2 R) adj3 (antagonist* or blocker* or blockage* or blockader*)).tw,kw.
(H2RA or H2RAs or H2‐RA or H2RAs).tw,kw.
(antihistaminic* adj2 (H2 or H‐2)).tw,kw.
(Cimetidine or Tagamet or altramet or biomet or biomet400 or eureceptor or histodil or skf 92334 or skf92334).tw,kw.
(ranitidine or zantac or ah 19065 or ah19065 or biotidin or ranisen or ranitidine or sostril or zantic).tw,kw.
(Famotidine or Pepcid or mk 208 or mk208 or ym 11170 or ym11170).tw,kw.
(Nizatidine or Axid or axid or ly 139037 or ly139037).tw,kw.
(Roxatidine or Rotane or Zorpe).tw,kw.
or/22‐30
exp Bismuth/
exp Amoxicillin/
exp Clarithromycin/
exp Nitroimidazoles/
exp Macrolides/
(metronidazole or tinidazole or amoxicillin* or amoxycillin*).tw,kw.
(clarithromycin or azithromycin or roxithromycin).tw,kw.
(bismuth or nitroimidazole* or macrolide*).tw,kw.
or/32‐39
21 or 31 or 40
10 and 41
Appendix 2. MEDLINE search strategy (via OvidSP)
exp Helicobacter/ or exp Helicobacter Infections/
(helicobacter or campylobacter).tw,kw.
(pylori or pyloridis or HP).tw,kw.
or/1‐3
exp Stomach Neoplasms/
exp Lymphoma, B‐Cell, Marginal Zone/
(mucosa associated lymphoid tissue lymphoma or MALT).tw,kw.
((stomach or gastric) adj3 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or lymphoma* or adenocarcinoma* or malign*)).tw,kw.
or/5‐8
4 and 9
randomized controlled trial.pt.
controlled clinical trial.pt.
placebo.ab.
drug therapy.fs.
random*.mp.
trial.ab.
groups.ab.
or/11‐17
exp animals/ not humans/
18 not 19
10 and 20
exp Proton Pump Inhibitors/
proton pump inhibitor*.mp.
(PPI or PPIs).tw,kw.
(omeprazole or h 16868 or losec or prilosec or rapinex or zegerid).mp.
(lansoprazole or lanzoprazole or ag 1749 or agopton or bamalite or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).mp.
(pantoprazole or by 1023 or protium or protonix or skf‐96022).mp.
(esomeprazole or nexium).mp.
(rabeprazole or aciphex or dexrabeprazole or e 3810 or ly‐307640 or pariet).mp.
(dexlansoprazole or Kapidex or Dexilant or AGN 20194* or AGN20194* or dexrabeprazole).mp.
(tenatoprazole or CAS 113712‐98‐4 or STU‐Na or TAK‐390* or TAK390* or TAK‐438 or TAK438 or AZD0865 or "AZD 0865").mp.
or/22‐31
exp Histamine H2 Antagonists/
((histamine or H2 or H‐2 or H2R or H 2 R) adj3 (antagonist* or blocker* or blockage* or blockader*)).tw,kw.
(H2RA or H2RAs or H2‐RA or H2RAs).tw,kw.
(antihistaminic* adj2 (H2 or H‐2)).tw,kw.
(Cimetidine or Tagamet or altramet or biomet or biomet400 or eureceptor or histodil or skf 92334 or skf92334).tw,kw.
(ranitidine or zantac or ah 19065 or ah19065 or biotidin or ranisen or ranitidine or sostril or zantic).tw,kw.
(Famotidine or Pepcid or mk 208 or mk208 or ym 11170 or ym11170).tw,kw.
(Nizatidine or Axid or axid or ly 139037 or ly139037).tw,kw.
(Roxatidine or Rotane or Zorpe).tw,kw.
or/33‐41
exp Bismuth/
exp Amoxicillin/
exp Clarithromycin/
exp Nitroimidazoles/
exp Macrolides/
(metronidazole or tinidazole or amoxicillin* or amoxycillin*).tw,kw.
(clarithromycin or azithromycin or roxithromycin).tw,kw.
(bismuth or nitroimidazole* or macrolide*).tw,kw.
or/43‐50
32 or 42 or 51
21 and 52
Note: Lines 11‐20, Cochrane hanboodk RCT filter: “Box 6.4.c: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format”. We made the following minor revisions: we used “random*” instead of “randomized.ab” or “randomly.ab.” to capture word variations such as “randomised, randomization, random”.
Appendix 3. EMBASE search strategy (via OvidSP)
exp Helicobacter/ or exp Helicobacter infection/
(helicobacter or campylobacter).tw,kw.
(pylori or pyloridis or HP).tw,kw.
or/1‐3
exp stomach tumor/
exp marginal zone lymphoma/
(mucosa associated lymphoid tissue lymphoma or MALT).tw,kw.
((stomach or gastric) adj3 (cancer* or carcinoma* or neoplas* or tumor* or tumour* or lymphoma* or adenocarcinoma* or malign*)).tw,kw.
or/5‐8
4 and 9
random*.mp.
placebo*.mp.
double‐blind*.mp.
clinical trial:.mp.
blind*.tw.
or/11‐15
exp animal/ not exp human/
16 not 17
10 and 18
exp proton pump inhibitor/
proton pump inhibitor*.mp.
(PPI or PPIs).tw,kw.
(omeprazole or h 16868 or losec or prilosec or rapinex or zegerid).mp.
(lansoprazole or lanzoprazole or ag 1749 or agopton or bamalite or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).mp.
(pantoprazole or by 1023 or protium or protonix or skf‐96022).mp.
(esomeprazole or nexium).mp.
(rabeprazole or aciphex or dexrabeprazole or e 3810 or ly‐307640 or pariet).mp.
(dexlansoprazole or Kapidex or Dexilant or AGN 20194* or AGN20194* or dexrabeprazole).mp.
(tenatoprazole or CAS 113712‐98‐4 or STU‐Na or TAK‐390* or TAK390* or TAK‐438 or TAK438 or AZD0865 or "AZD 0865").mp.
or/20‐29
exp histamine H2 receptor antagonist/
((histamine or H2 or H‐2 or H2R or H 2 R) adj3 (antagonist* or blocker* or blockage* or blockader*)).tw,kw.
(H2RA or H2RAs or H2‐RA or H2RAs).tw,kw.
(antihistaminic* adj2 (H2 or H‐2)).tw,kw.
(Cimetidine or Tagamet or altramet or biomet or biomet400 or eureceptor or histodil or skf 92334 or skf92334).tw,kw.
(ranitidine or zantac or ah 19065 or ah19065 or biotidin or ranisen or ranitidine or sostril or zantic).tw,kw.
(Famotidine or Pepcid or mk 208 or mk208 or ym 11170 or ym11170).tw,kw.
(Nizatidine or Axid or axid or ly 139037 or ly139037).tw,kw.
(Roxatidine or Rotane or Zorpe).tw,kw.
or/31‐39
exp bismuth citrate/ or exp bismuth salt/ or exp bismuth/ or exp bismuth salicylate/ or exp ranitidine bismuth citrate/ or exp colloidal bismuth compound/ or exp bismuth citrate plus metronidazole plus tetracycline/
exp amoxicillin plus clarithromycin plus lansoprazole/ or exp clarithromycin/ or exp clarithromycin derivative/
exp amoxicillin/
exp nitroimidazole/
exp macrolide/
(metronidazole or tinidazole or amoxicillin* or amoxycillin*).tw,kw.
(clarithromycin or azithromycin or roxithromycin).tw,kw.
(bismuth or nitroimidazole* or macrolide*).tw,kw.
or/41‐48
30 or 40 or 49
19 and 50
Note: Lines 11‐18. RCT filter: Combined high sensitivity, high specificty and minimum difference RCT filter: https://hiru.mcmaster.ca/hiru/hedges/All‐EMBASE.htm
Appendix 4. Glossary of terms
Antibiotics: amoxicillin, clarithromycin, macrolide, 5‐nitroimidazole
Antrum: the bottom section of the stomach
Ascorbic acid: vitamin C
Asymptomatic: without symptoms
Bismuth: a medication that reduces stomach acid
Carbon‐urea breath test: a diagnostic test that uses an isotope of carbon to detect H. pylori
Carcinogenic: causing cancer
Chronic: long term
Dyspepsia: indigestion
Endoscopy: a diagnostic test using a telescope to look into the stomach and intestines
Gastric atrophy: loss of the normal glands in the stomach
Gastric metaplasia: a change in the cells in the stomach from one type to another
Gastric: stomach
Gastritis: inflammation of the stomach
Gastrointestinal: involving the stomach and intestines
Histamine2‐receptor antagonist: a medication that reduces stomach acid
Histology: the appearance of biopsies from an organ under the microscope
Luminal: in the cavity of a hollow organ, such as the stomach or intestine
Malignant: cancerous
Mortality: death
Mucosa: in the lining of a hollow organ, such as the stomach or intestine
Neoplasia: abnormal growth of tissue, often cancerous
Nested case‐control study: variation of a case‐control study in which only a subset of controls from the cohort are compared to the cases
Non‐cardia: involving the antrum or body of the stomach
Occult: unobvious or hidden
Proton pump inhibitor: a medication that reduces stomach acid
Ranitidine bismuth citrate: a medication that reduces stomach acid
Rapid urease testing: a biopsy test from the mucosa of the stomach, which is placed in a special liquid, to detect H. pylori
Serology: a blood test to detect antibodies against an infection such as H. pylori
Data and analyses
Comparison 1. H. pylori eradication vs control ‐ main analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incidence of gastric cancer ‐ modified ITT analysis | 7 | 8323 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.40, 0.72] |
| 1.2 Incidence of gastric cancer ‐ complete case analysis | 7 | 7698 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.40, 0.71] |
| 1.3 Death from gastric cancer ‐ modified ITT analysis | 4 | 6301 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.40, 0.92] |
| 1.4 Death from all causes ‐ modified ITT analysis | 5 | 7079 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.12] |
| 1.5 Incidence of oesophageal squamous cell carcinoma ‐ modified ITT analysis | 2 | 3888 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.59, 2.54] |
Comparison 2. H. pylori eradication vs control ‐ subgroup analysis according to presence or absence of pre‐neoplastic lesions at baseline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Incidence of gastric cancer according to presence or absence of pre‐neoplastic lesions at baseline | 7 | 8307 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| 2.1.1 Patients with precancerous lesions | 4 | 3425 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.59] |
| 2.1.2 Patients without precancerous lesions | 2 | 1812 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.02, 7.69] |
| 2.1.3 Mixed patients with and without precancerous lesions or ulcer (can't separate) | 3 | 3070 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.22, 0.78] |
Comparison 3. H. pylori eradication vs control ‐ subgroup analysis according to use of vitamins or antioxidants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Incidence of gastric cancer according to use of vitamins or anti‐oxidants | 7 | 8323 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.43, 0.87] |
| 3.1.1 Without antioxidants | 7 | 5986 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.41, 1.14] |
| 3.1.2 With antioxidants | 2 | 2337 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.87] |
Comparison 4. H. pylori eradication vs control ‐ sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Incidence of gastric cancer ‐ modified ITT analysis substituting the 10‐year follow‐up data from Zhou 2014 with the 5‐year follow‐up data from Leung 2004 | 7 | 8358 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.42, 0.74] |
| 4.2 Incidence of gastric cancer ‐ complete case analysis substituting the 10‐year follow‐up data from Zhou 2014 with the 5‐year follow‐up data from Leung 2004 | 7 | 7747 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.74] |
| 4.3 Incidence of gastric cancer‐ modified ITT analysis including the two arms of celecoxib from Wong 2012 | 7 | 8834 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.74] |
| 4.4 Incidence of gastric cancer ‐ modified ITT analysis including all randomised patients from Correa 2000 and You 2006 who were found subsequently to be ineligible or did not receive treatment | 7 | 8474 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.40, 0.72] |
| 4.5 Incidence of gastric cancer ‐ missing data imputation based on various assumptions | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.5.1 Assuming incidence of gastric cancer for missing participants in both arms same as observed in the trial control arm | 7 | 8323 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.73] |
| 4.5.2 Assuming incidence of gastric cancer for missing participants in treatment arm same as observed in the trial control arm, but no new gastric cancer cases in the control arm among those with missing data | 7 | 8323 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.42, 0.74] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Choi 2020.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Country: Korea, Single centre | |
| Interventions | 1.lansoprazole 30 mg. amoxicillin 1000 mg, clarithromycin 500 mg, twice daily for 7 days (n=912) 2.placebo, same number of pills, identical in appearance and taste for 7 days (n=914) Follow‐up: median duration of follow‐up for gastric cancer development =9.2 years (IQR, 6.2 to 10.6; maximum, 14.1), and the median duration of follow‐up for overall survival was 10.2 years (interquartile range, 8.9 to 11.6) |
|
| Outcomes | Development of gastric cancer; development of gastric cancer according to H. pylori eradication status during the follow‐up period; overall survival, and the development of adenoma | |
| Notes | H. pylori eradication status was evaluated in 1587 participants during the follow‐up period. Eradication rate (70.1% (551/786) in the treatment group and 7.1% (57/801) in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization sequence" |
| Allocation concealment (selection bias) | Low risk | “computer‐generated randomization sequence was kept at the trial pharmacy and was not accessible to the investigators who enrolled participants”. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | ”Through‐out the trial, participants and investigators, including the endoscopist, pathologist, physician, research nurse, and statistician, were unaware of trial‐group assignments“. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ”Through‐out the trial, participants and investigators, including the endoscopist, pathologist, physician, research nurse, and statistician, were unaware of trial‐group assignments“. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow up: 8.8% (80/912) vs 7.7% (70/914), balance between two arms. |
| Selective reporting (reporting bias) | Low risk | Reported prespecified outcomes. |
| Other bias | Low risk | No other risk of bias is noted. |
Correa 2000‐Correa 2001.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Country: Colombia, two communities in Narino Province. 976 participants with confirmed histologic diagnoses of multifocal non‐metaplastic atrophy and/or intestinal metaplasia, two precancerous lesions. Mean age 51.1 years (range 29‐69 years), 46.1% male. Method to confirm presence of H. pylori: histological examination of gastric biopsies obtained at upper GI endoscopy. Only 852 out of 976 participants were eligible and treated. Study period: started in 1991 |
|
| Interventions | 1. Bismuth subsalicylate 262 mg, amoxicillin 500 mg, and metronidazole 375 mg 3 times daily for 2 weeks, including regimens with or without dietary supplements (n = 437). 2. Placebo, including regimens with or without dietary supplements (n = 415). Participants assigned to anti‐H. pylori treatment who tested positive for H. pylori at 36 months were treated again for 14 days with amoxicillin (1 g twice a day), clarithromycin (500 mg twice a day), and either omeprazole (20 mg twice a day) or lansoprazole (30 mg twice a day). Factorial design: eight different regimens: H. pylori eradication with or without one of the four dietary supplements of beta‐carotene (30 mg once per day) and/or ascorbic acid (1 g twice a day) or placebo: A) placebo only; B) anti‐H. pylori; C) beta‐carotene (BC); D) ascorbic acid (AA); E) H. pylori eradication + BC; F) H. pylori eradication + AA; G) BC + AA; and H) H. pylori eradication + BC + AA. Last point of follow‐up 6 years. |
|
| Outcomes | Histological examination of gastric biopsies obtained at upper GI endoscopy at 6 years. Primary outcome was "progression of preneoplastic lesions". Other outcomes: relative risks of progression, no change, and regression from multifocal non‐metaplastic atrophy and intestinal metaplasia. | |
| Notes | 1. High‐risk participants (all with confirmed histologic diagnoses of multifocal non‐metaplastic atrophy and/or intestinal metaplasia, two precancerous lesions), primary outcome was "progression of preneoplastic lesions". 2. Randomised to anti‐H. pylori triple therapy and/or dietary supplementation with AA, BC, or their corresponding placebos. 3. 976 were randomised, but only 852 were eligible and treated after randomisation (7 refused and 117 ineligible). 852 were included in the ITT analyses, and all randomised participants were included in the sensitivity analyses. 631 participants completed the trials and were included in the complete case analyses. 4. Details of gastric cancer data were reported in Correa 2001(a letter). 5. H. pylori eradication rate 58.0% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated lists produced in New Orleans and applied in the field in Pasto, three strata: atrophy (without metaplasia), intestinal metaplasia, or dysplasia. |
| Allocation concealment (selection bias) | Low risk | Central randomisation, therefore allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No double‐blinding for eradication vs non‐eradication (because an appropriate placebo was not available for bismuth subsalicylate), double‐blinding only applied to supplements vs placebo: "After a factorial design, a double‐blind approach—i.e., study investigators and subjects were unaware of treatment assignments, supplements and placebos were provided in identical coded tablets by Hoffmann‐La Roche Inc" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At the end of the study, a single experienced pathologist, blinded to treatment assignment and all other study variables, examined all biopsy specimens collected at baseline and after 72 months of follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 976 were randomised but only 852 were eligible and treated after randomisation (7 refused and 117 ineligible). 852 were included in the ITT analyses, and all randomised participants were included in the sensitivity analyses. 631 participants completed the trial and were included in the complete case analysis. 221 participants withdrew before their 72‐month evaluation: 102 quit treatment, 59 were lost to follow‐up, 34 dropped out of the study because of pregnancy and other medical conditions, 18 died of causes unrelated to gastric cancer, and eight developed cancer other than gastric cancer. The average rate of loss was 4.3% per year over the 6‐year trial. Withdrew in 72 months = 117 (26.8%) vs 104 (25%) in all H. pylori eradication arms vs control arms. However, it is likely those who had cancer would have come back for treatment although these individuals did not complete the follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No data were reported for deaths from gastric cancer or adverse events. |
| Other bias | Low risk | No other risk of bias is noted. |
Leung 2004‐Zhou 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Country: China. 11 villages in Yantai County, Shandong Province. 587 volunteers underwent upper endoscopy with biopsy specimens obtained from the antrum and corpus. H. pylori–infected volunteers with or without symptoms of dyspepsia were randomised. Mean (range) age 52.0 (35‐75) years, 47.8% men. Method to confirm H. pylori infection: histological examination and rapid urease testing. 33.7% participants with preneoplastic lesions at baseline. Study period: screened participants in 1996 In the Zhou 2014 10‐year follow‐up report, data are only reported for "552 subjects who had both Warthin‐Starry (W‐S) staining and UBT results that were positive". |
|
| Interventions | 1. Omeprazole 20 mg, amoxicillin 1 g, clarithromycin 500 mg twice daily for 1 week (n = 295 in Leung 2004 and 276 in Zhou 2014). 2. Placebo twice daily for 1 week (n = 292 in Leung 2004 and 276 in Zhou 2014). Sample size 587 in Leung 2004 ; 552 in Zhou 2014 . Follow‐up: 5 years in Leung 2004; 10 years in Zhou 2008 (as conference abstract) and Zhou 2014 (as full publication). |
|
| Outcomes | Histological examination at 2, 5, 8, and 10 years. | |
| Notes | 1. Data from Zhou 2014 (276 vs 276) rather than Leung 2004 (295 vs 292) were entered in the main analysis, because Zhou 2014 abstract had 10 years of follow‐up. Inconsistent data were noted between Zhou 2014 and Leung 2004. Zhou et al. had a series of publications but with smaller sample size and fewer gastric cancers than those reported by Leung 2004. . Zhou 2008 that was included in the previous published version was a conference abstract at that time, and was fully published in 2014. No new data analysis was needed after including data from Zhou 2014. 2. In Zhou 2005a and Zhou 2005b, participants were regrouped as eradicated group and group (included those failing eradication or controls) as 246 vs 306 (276 + 30 vs 276 ‐ 30). 3. According to Leung 2004, 152 (75 vs 77) were lost to follow‐up; these participants were considered as no gastric cancer in the ITT analysis. 4. Mortality data were available in Leung 2004 but not in Zhou 2014 (except reporting four patients died of gastric cancer) , therefore a larger sample size and larger number of participants with gastric cancer in Leung 2004. 5. Zhou 2014 reported data based on baseline atrophy observed at the gastric antrum and corpus, but we were not able to extract the exact number of patients with baseline atrophy from table 1 (reported separately for antrum and corpus). 6. H. pylori eradication rate 55.6% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by instructions in sealed envelopes. The instructions were constructed according to a random‐number list generated by computer. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used, central randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used. Medications had identical appearances. Both participants and physicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Slides were coded in a random manner such that the pathologist was blinded to the identity of participants, treatment assignment, and year at which biopsies were obtained. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data from Zhou 2014 were used for the main analyses due to the longer follow‐up period. However, The 10‐year follow‐up data reported in Zhou 2014 had a smaller sample size and fewer gastric cancer cases than those in the full publication (Leung 2004). According to Leung 2004, 152 (75 vs 77) were lost to follow‐up; these participants were considered as no gastric cancer in the ITT analysis. After 10 years, 378 subjects had completed the study (Zhou 2014) |
| Selective reporting (reporting bias) | Unclear risk | Reported prespecified outcomes. Mortality data were available in Leung 2004 but not in Zhou 2014 , leading therefore to different sample sizes in these analyses. |
| Other bias | High risk | Inconsistent data were noted between Zhou 2014 and Leung 2004. Zhou et al. had a series of publications but with smaller sample size and fewer gastric cancers than reported by Leung 2004 (inconsistencies in data reporting at the two follow‐up points, with 10 gastric cancers reported at 5 years, compared with nine at 10 years). |
Saito 2005.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Country: Japan, 145 centres 692 healthy volunteers between 20 to 59 years with H. pylori infection. Mean age not reported (range 20‐59 years), proportion men not reported. Number of participants with preneoplastic lesions at baseline was not reported, although the aim was "the regression/progression of atrophy by one grade or more". Study period: not clear. |
|
| Interventions | 1. Lansoprazole 30 mg, amoxicillin 1.5 g, clarithromycin 400 mg once daily for 1 week (n = 379) 2. Non‐eradication group Method to confirm H. pylori infection was not reported. Follow‐up: ≥ 4 years |
|
| Outcomes | Histological examination ≥ 4 years; regression/progression of atrophy by 1 grade or more in the eradicated and non‐eradicated groups in participants followed ≥ 4 years. | |
| Notes | 1. Updated searched on Feb 2020: still no follow‐up full publication. 2. Original study planned two outcomes, but finally decided to only evaluate "the prevention of the onset and progression of atrophy of gastric mucosa by H. pylori elimination" and did not evaluate "comparative study on the frequency of stomach cancer". 3. H. pylori eradication rate 74.4% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Conference proceeding, participants were "randomised", but the sample size is imbalanced (379 vs 313). |
| Allocation concealment (selection bias) | Unclear risk | Conference proceeding, no detailed information was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Conference proceeding, no detailed information was provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Conference proceeding, no detailed information was provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Conference proceeding, no detailed information for losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Conference proceeding, no detailed information was provided. |
| Other bias | Unclear risk | Conference proceeding, no detailed information was provided. |
Wong 2004.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Country: China. seven villages in Changle County, Fujian Province. 2423 healthy participants recruited for a screening endoscopic study. Participants without endoscopic lesions and positive for H. pylori infection (n = 1628) were randomised. Those with peptic ulcer were excluded. Mean age 42.2 (range 35‐65) years, 54.0% men. Method to confirm H. pylori infection: histological examination and rapid urease testing. 37.7% participants with preneoplastic lesions at baseline (gastric atrophy, intestinal metaplasia, dysplasia). Study period: 1994 to Jan 2002 |
|
| Interventions | 1. Omeprazole 20 mg, amoxicillin/clavulanic acid 750 mg, metronidazole 400 mg twice daily for 2 weeks (n = 817) 2. Placebo (n = 813) Follow‐up: 7.5 years |
|
| Outcomes | Incidence of gastric cancer: gastric cancer in participants with or without precancerous lesions at baseline was the secondary outcome. Histological examination at 7.5 years or, if diagnosed before 7.5 years, review of clinical records and pathology specimens by 3 blinded clinicians. | |
| Notes | This was the first study targeted at a general population (less than 40% with precancerous lesions); a few discrepancies were seen between Wong 2002 abstract and Wong 2004 full publication. Inconsistent sample size between the full publication followed up at 7.5 years (817 vs 813) (Wong 2004) and the conference abstract followed up at 7 years (819 vs 809) (Wong 2002). We used data from the full publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation generated by computer. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by drawing a sealed envelope that contained a pre‐assigned random treatment generated by computer. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study, endoscopists were blinded and participants were followed by blinded clinical team, likely participants were blinded as well. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Endoscopists and histologists were blinded, clinical team in Hong Kong who reviewed the gastric cancer cases were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 735/817 vs 703/813 followed at 7.5 years, losses to follow‐up with reasons were balanced between two groups (7.7% vs 11.4%); 62% had follow‐up endoscopy, but those who refused endoscopy were followed up in clinics. |
| Selective reporting (reporting bias) | Low risk | Reported prespecified outcomes. |
| Other bias | Unclear risk | Inconsistent sample size between the full publication followed up at 7.5 years (817 vs 813) (Wong 2004) and the conference abstract followed up at 7 years (819 vs 809) (Wong 2002). |
Wong 2012a.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Country: China. 12 villages in Linqu County, Shandong Province. 1024 participants with H. pylori infection and advanced gastric lesions (severe chronic atrophic gastritis, intestinal metaplasia, indefinite dysplasia, or dysplasia); mean age 53.0 (range 35 to 64) years, 46.4% men. Method to confirm H. pylori infection:13Carbon‐urea breath testing. Histology was also performed. 100% participants with preneoplastic lesions at baseline Study period: 2002‐2009 |
|
| Interventions | Anti‐H. pylori treatment and/or COX‐2 inhibitor or placebo in a 2x2 factorial design: 1. Omeprazole 20 mg, amoxicillin 1 g, clarithromycin 500 mg + placebo twice daily for 1 week (n = 255) 2. Placebo (n = 258) 3. Omeprazole 20 mg, amoxicillin 1 g, clarithromycin 500 mg twice daily for 1 week + celecoxib (n = 255) 4. Celecoxib + placebo (n = 256) Follow‐up: 5 years |
|
| Outcomes | Gastric cancer: histological examination at 5 years. Regression or progression of advanced gastric lesions. | |
| Notes | 2x2 factorial design, in the main analysis we did not include data from the two arms that used celecoxib, only data for H. pylori eradication only vs placebo only (n = 513). The celecoxib arms were included in a sensitivity analysis. Eradication rate: 63.5% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised treatment assignments were generated blindly by Westat Inc, (Rockville, MD, USA) after eligibility was determined. |
| Allocation concealment (selection bias) | Low risk | Central randomisation was used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial. All placebos were identical in number, size, and colour to the original medications. Both participants and investigators were blinded to treatment assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Endoscopists and pathologists were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 234/258 (90.7%) vs 233/255 (91.4%) participants had follow‐up gastric biopsy data and the authors reported outcomes for these, losses to follow‐up with reasons were balanced and provided, 89.7% completed the repeat upper endoscopy and histology. |
| Selective reporting (reporting bias) | Low risk | Reported prespecified outcomes. |
| Other bias | Low risk | No other risk of bias was noted. |
You 2006 ‐ Li 2019.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Country: China. 13 villages in Linqu County, Shandong Province. 2258 participants were randomly selected in villages and given baseline endoscopy. Mean age 46.8 (range 35 to 64) years, 50.0% men. Excluded participants who were too ill or who refused. Method to confirm H. pylori infection: serological testing 64% participants with preneoplastic lesions at baseline Study period: 1994‐2010 |
|
| Interventions | 1. Omeprazole 20 mg and amoxicillin 1 g twice daily for 2 weeks, with or without vitamin or garlic supplements (n = 1130). Participants who had continued evidence of infection after 3 months received a repeat course of treatment for 2 weeks unless they had previously developed rashes or other evidence of allergy to the initial treatment. 2. Placebo, with or without vitamin or garlic supplements (n = 1128)
2x2x2 factorial design: H. pylori eradication; dietary supplementation with capsules containing vitamin C, vitamin E, and selenium; dietary supplementation with capsules containing steam‐distilled garlic oil and Kyolic aged garlic extract. Garlic and vitamin supplements were not given in June and July 1999, and garlic supplements were not given in September 2002 because of interruptions in the availability of the supplement. Follow‐up: 14.7 years presented in Ma 2012, 15 years presented in Li 2014; 22 years presented in Li 2019 |
|
| Outcomes | Gastric cancer: Histological examination, clinical, laboratory, or pathological data and cause‐specific mortality. Prevalence of dysplasia and other precancerous gastric lesions: prevalence of dysplasia or gastric cancer (score > 6); prevalence of severe chronic atrophic gastritis, intestinal metaplasia, dysplasia, or gastric cancer; and average severity score, effects of one‐time H. pylori treatment and long‐term vitamin or garlic supplements in reducing the prevalence of advanced precancerous gastric lesions. Secondary endpoints: rates of transition from baseline to final histopathologic states and the effects of treatments on these rates of transition; evidence of the effectiveness of amoxicillin and omeprazole in eradicating H. pylori; and blood pressure at the time of the final examination. |
|
| Notes | 1. You 2006: no gastric cancer data for those who did not receive dietary supplement; some participants were ineligible after randomisation and were excluded in the main analyses, these were included in the sensitivity analyses. 2. Some data were obtained from the authors. In the 2013 version, subgroup analysis data according to use of vitamins or anti‐oxidants were based on data provided by the author Dr. Mitchell H. Gail using follow‐up data to 01 August 2010 on Oct 2013, with a smaller number of deaths compared with Li 2019. 3. Since no subgroup data related to precancerous lesions, or use of vitamins or anti‐oxidants, were clearly reported in Li 2019, data for subgroup analyses for this study are not changed from 2013. 4. Inconsistent sample size between Gail 1998 protocol (3411 randomised = 2285 H. pylori‐positive and 1126 H. pylori‐negative); and 3365 randomised (2258 H. pylori‐positive vs 1107 H. pylori‐negative) in You 2006. The supplement in Li 2019 suggested 2258 patients of 2285 patients were eligible for analysis. According to figure 1 in Li 2019, pre‐randomization eligibility violations were excluded, therefore, we used 2258 in the meta‐analyses. 5. Eradication rate: 73.2%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We masked both the subjects and the researchers to treatment assignment. After confirming the eligibility of subjects, we assigned treatments randomly at Westat, Inc. in the United States and used this assignment to distribute coded bottles of capsules from the pharmacy in the city of Weifang in Shandong Province" |
| Allocation concealment (selection bias) | Low risk | Central randomisation, with distribution of coded bottles of capsules from the pharmacy. Pill bottles bearing codes corresponding to those assignments were then distributed to the study participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Look‐alike placebo capsules containing lactose and starch for amoxicillin and sucrose and starch for omeprazole were given to serologically positive controls and to all seronegative participants. To protect blinding, the investigators randomly selected an equal number of participants of the placebo arm from the same village and 10‐year age range for retreatment with placebo. Look‐alike placebo capsules were also used for supplements. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded, only 1 person had the authority to break the code when necessary (e.g. toxicity). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2285 H. pylori‐positive participants randomised in Gail 1998, 2258 H. pylori‐positive participants were analysed in You 2006 ‐ Li 2019. Overall, only 13% of participants did not have final gastric biopsy data. Only three people were lost to follow‐up for vital status in Li 2019, but unclear from which group. |
| Selective reporting (reporting bias) | Low risk | Reported prespecified outcomes, some data were obtained from the authors. |
| Other bias | Low risk | No other risk of bias was noted. |
GI: gastrointestinal ITT: intention‐to‐treat RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Choi 2014 | Trial of participants undergoing endoscopic mucosal resection of gastric cancer |
| Choi 2018a | Trial of participants undergoing endoscopic mucosal resection of gastric cancer |
| Choi 2018b | Trial of participants undergoing endoscopic mucosal resection of gastric cancer |
| Fischbach 2001 | No incident gastric cancers detected during follow‐up |
| Fischbach 2009 | Did not compare the interventions of interest |
| Ford 2005 | No gastric cancer data, follow‐up study of Moayyedi 2000 whose primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community |
| Fukase 2008 | Trial of participants undergoing endoscopic mucosal resection of gastric cancer |
| Hamajima 2002 | Not a RCT |
| Harvey 2004 | No gastric cancer data. Primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community |
| Hsu 2007 | Prospective follow‐up study, not RCT |
| Imanzadeh 2004 | No gastric cancer data |
| Juibari 2003 | Not a RCT |
| Kamangar 2006 | Case control study, not RCT |
| Kato 2006 | Cohort study, not RCT |
| Kim 2008 | Cohort study, not RCT |
| Lane 2006 | No gastric cancer data, duplicate publication of Harvey 2004, whose primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community |
| Leja 2017 | A protocol of a RCT that will randomize participants to test and treat vs controls, instead of eradication vs control |
| Leung 2018 | Cohort study, not RCT |
| Mabe 2009 | Cohort study, not RCT |
| Mason 2002 | No gastric cancer data, cost‐effectiveness analysis of Moayyedi 2000, whose primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community |
| Miehlke 2001 | No incident gastric cancers detected during follow‐up |
| Moayyedi 2000 | No gastric cancer data. Primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community |
| Ogura 2008 | Cohort study, not RCT |
| Ohkusa 2001 | Not RCT, reported data for glandular atrophy and intestinal metaplasia |
| Take 2005 | Cohort study, not RCT |
| Take 2007 | Cohort study, not RCT |
| Takenaka 2007 | Cohort study, not RCT |
| Tang 2010 | Presented data on those who had H.pylori eradicated in intervention group vs those H.pylori negative in control group after 3 years, instead of presenting data in all randomized subjects; subjects were those who admitted to hospital or came to hospital for H.pylori testing, instead of general population. (This Chinese‐lanagure study was not found in our literature search, but was found in the references cited in a review published in Chinese in 2019) |
| Uemura 2001 | Cohort study, not RCT |
| Uemura 2002 | Cohort study, not RCT |
| Wildner‐Christensen 2003 | No gastric cancer data. Primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community |
| Yanaoka 2009 | Cohort study, not RCT |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
HELPER study.
| Study name | HELPER study (Helicobacter Pylori Eradication for Gastric Cancer Prevention in the General Population) |
| Methods | RCT |
| Participants | Men and women aged 40‐65 who are invited to participate in the National Cancer Screening Program and receive upper endoscopy |
| Interventions | 10‐day bismuth‐based quadruple therapy (10‐day bismuth‐based quadruple therapy Bismuth 300 mg (4 times a day), lansoprazole
30 mg (twice a day), metronidazole 500 mg (3 times a day), and tetracycline 500 mg (4 times a day) for 10 days) vs placebo |
| Outcomes | Primary outcome: The incidence of gastric cancer between the intervention and placebo groups [ Time Frame: Up to 10 years After H. pylori eradication ] Secondary outcomes: 1. Incidence of gastric dysplasia 2. Occurrence of adverse events caused by antibiotic treatment 3. Incidence and mortality from other medical conditions such as obesity, diabetes, circulatory diseases, oesophageal diseases as well as other cancers and cognitive impairment 4. Mortality from gastric cancer 5. All‐cause mortality 6. Modification of atrophy score |
| Starting date | June 2014 |
| Contact information | National Cancer Center, Korea. Il Ju Choi, M.D., Ph.D. +82‐31‐920‐2282 cij1224@ncc.re.kr International Agency for Research on Cancer. Rolando Herrero, M.D., Ph.D. +33 4 72 73 86 83 HerreroR@iarc.fr |
| Notes | Estimated Study Completion Date: June 2029 |
Pan 2016.
| Study name | |
| Methods | RCT |
| Participants | In March 2011, 347 811 residents aged 25–54 years identified from a roster of 980 villages in 10 townships of Linqu were invited. A total of 184 786 residents aged 25–54 years were enrolled in this trial and received 13 C‐urea breath test. |
| Interventions | 10‐day quadruple anti‐H. pylori treatment with omeprazole 20 mg twice daily, tetracycline 750 mg three time a day, metronidazole
400 mg three time a day and bismuth citrate 300 mg twice daily, vs look alike placebos of tetracycline and metronidazole together with single dosages of 20 mg omeprazole and 300 mg bismuth citrate |
| Outcomes | Primary outcome: the incidence of gastric cancer. Secondary outcomes: gastric cancer mortality and other health effects related to H. pylori eradication. |
| Starting date | The field work of this intervention trial was launched in March 2011 and completed in September 2013 (30 months), The trial participants will prospectively undergo follow‐up for at least 7 years after the treatment. |
| Contact information | Dr Wei‐cheng You, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Cancer Epidemiology, Peking University Cancer Hospital & Institute, 52 Fu‐cheng Road, Hai‐dian District, Beijing.100142, P. R. China; weichengyou@yahoo.com. Dr. Meinhard Classen, Technische Universität, München, International, Digestive Cancer Alliance, 81675 Munich, Germany; meinhard.classen@lrz.tum.de |
| Notes |
Differences between protocol and review
The only substantive change from the protocol lies in a slight change in our primary endpoint. The protocol had intended that the systematic review and meta‐analysis focus on the effect of H. pylori eradication therapy in preventing gastric neoplasia in the general population. While the population we have reported on is the same, we did not report the effect of H. pylori eradication therapy on all types of gastric neoplasia, but rather on gastric adenocarcinoma only. This was because only one of the six trials that were eligible for the review reported any cases of gastric neoplasia that were not a gastric adenocarcinoma (Saito 2005). This was a gastric mucosa‐associated lymphoid tissue lymphoma, occurring in a single participant, and was not included in any of our analyses.
Methods > Types of interventions
Although it had been omitted in the protocol, we included the intervention 'RBC triple therapy (RBC plus any two of the following: amoxicillin, macrolide, 5‐nitroimidazole)' because it is a recognised eradication therapy regimen.
Methods > Data synthesis
The protocol did not pre specify the methods used to handle missing data and sensitivity analysis (for example modified intention‐to‐treat approach, complete case analysis, imputation). We amended methods for investigating heterogeneity (for example the cutoff for statistical significance of I2 statistic changed from P less than 0.2 to less than 0.1).
Methods > Subgroup analysis and investigation of heterogeneity
The protocol did not pre specify subgroup analysis 'according to whether trial participants were co‐administered antioxidants or vitamins within the trial'. We added this due to the nature of the design of some of the included RCTs, which were factorial trials.
Contributions of authors
Conceived and designed the study: all review authors Analysed and interpreted the data: all review authors Drafted the review: ACF and YY Approved the final draft of the review: all review authors
Sources of support
Internal sources
McMaster University, Canada
External sources
No sources of support supplied
Declarations of interest
ACF: none known.
YY: none known.
DF: none known.
RH: none known.
PM is a joint co‐ordinating editor of Cochrane Gut, however editorial decisions about this review were made independently by another joint Co‐ordinating Editor of Cochrane Gut (Professor Gordon), and the Senior and Associate Editors of the Cochrane Abdomen and Endocrine network.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Choi 2020 {published data only}
- Choi IJ, Kim CG, Lee JY, Kim YI, Kook MC, Park B, Joo J. Family History of Gastric Cancer and Helicobacter pylori Treatment. New England Journal of Medicine 2020;382(5):427-436. [DOI] [PubMed] [Google Scholar]
Correa 2000‐Correa 2001 {published data only}
- Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacterpylori therapy. Journal of the National Cancer Institute 2000;92(23):1881-8. [DOI] [PubMed] [Google Scholar]
- Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Re: Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. Journal of the National Cancer Institute 2001;93(7):559-60. [DOI] [PubMed] [Google Scholar]
- Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut 2005;54(11):1536-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz B, Garay J, Correa P, Fontham ET, Bravo JC, Bravo LE, et al. Morphometric evaluation of gastric antral atrophy: improvement after cure of Helicobacter pylori infection. American Journal of Gastroenterology 2001;96(12):3281-7. [DOI] [PubMed] [Google Scholar]
Leung 2004‐Zhou 2014 {published data only}
- Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut 2004;53(9):1244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JJ, Lin SR, Ching JY, Zhou LY, To KF, Wang RT, et al. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterology 2000;119(1):7-14. [DOI] [PubMed] [Google Scholar]
- Sung JJ, Lin SR, Leung W, Ng EK, Ching JY, To KF, et al. Does eradication of H. pylori prevent deterioration of gastric atrophy and intestinal metaplasia? A 5-year follow-up. In: Gastroenterology. Vol. 122(Suppl 4). 2002:A170. Abstract S1142.
- Zhou L, Lin S, Ding S, Huang X, Jin Z, Cui R, et al. Relationship of Helicobacter pylori eradication with gastric cancer and gastric mucosal histological changes: a 10-year follow-up study. Chinese medical journal 2014;127(8):1454-8. [PMID: ] [PubMed] [Google Scholar]
- Zhou L, Sung JJ, Lin S, Jin Z, Ding S, Huang X, et al. A five-year follow-up study on the pathological changes of gastric mucosa after H. pylori eradication. Chinese Medical Journal 2003;116(1):11-4. [PMID: ] [PubMed] [Google Scholar]
- Zhou L. Ten-year follow-up study on the incidence of gastric cancer and the pathological changes of gastric mucosa after H. pylori eradication in China. In: Gastroenterology. Vol. 134 (4, suppl 1). 2008:A233. Abstract S1606.
- Zhou LY, Lin SR, Ding SG, Huang SB, Guo CJ, Zhang L, et al. Eight-year follow-up study on prevalence of gastric cancer and the histological changes of gastric mucosa after H.pylori eradication [除幽门螺杆菌对胃癌患病率及胃黏膜组织学变化的八年随访研究]. Chinese Journal of Digestion 2005;25(6):324-7. [Google Scholar]
- Zhou LY, Lin SR, Ding SG, Huang SB, Guo CJ, Zhang L, et al. Eight-year follow-up study on the morbidity of gastric cancer and the pathological changes of gastric mucosa after H.pylori eradication [除幽门螺杆菌对胃癌患病率及胃黏膜组织学变化的八年随访研究]. www.paper.edu.cn/scholar/downpaper/zhouliya-3 (accessed 10 June 2015).
- Zhou LY, Lin SR, Ding SG, Huang XB, Zhang L, Meng LM, et al. The changing trends of the incidence of gastric cancer after Helicobacter pylori eradication in Shandong area. Chinese Journal of Digestive Diseases 2005;6(3):114-5. [DOI] [PubMed] [Google Scholar]
- Zhou LY, Lin SR, Shen ZY, Zhong SZ, Ding SG, Huang XB, et al. Five-year follow-up study after Helicobacter pylori eradication: Reinfection and peptic ulcer status. Chinese Journal of Digestive Diseases 2003;4(1):45-8. [Google Scholar]
- Zhou LY, Sung JJ, Lin SR, Jin Z, Ding SG, Huang XB, et al. Changes of gastric mucosa histopathology after Helicobacterpylori eradication [根除幽门螺杆菌对胃黏膜炎症变化的人群随访研究]. Zhonghua Nei Ke za Zhi [Chinese Journal of Internal Medicine] 2003;42(3):162-4. [PubMed]
Saito 2005 {published data only}
- Saito D, Boku N, Fujioka T, Fukuda Y, Matsushima Y, Sakaki N, et al. Impact of H. pylori eradication on gastric cancer prevention: endoscopic results of the Japanese Intervention Trial (JITHP-Study). A randomized multi-center trial. In: Gastroenterology. Vol. 128 4 (Supp2). 2005:A4. Abstract 23.
- Saito D. Present state of Japanese intervention trial of H. pylori. Nihon Rinsho 2003;61(1):50-5. [PubMed] [Google Scholar]
Wong 2004 {published data only}
- Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004;291(2):187-94. [DOI] [PubMed] [Google Scholar]
- Wong BCY, Lam S, Wong WM, et al. Eradication of Helicobacter pylori infection significantly slows down the progression of precancerous lesions in high risk populations: a 5 year prospective randomized study. In: Gastroenterology. Vol. 122. 2002:A588. Abstract W1216.
Wong 2012a {published data only}
- Feng GS, Ma JL, Wong BC, Zhang L, Liu WD, Pan KF, et al. Celecoxib-related gastroduodenal ulcer and cardiovascular events in a randomized trial for gastric cancer prevention. World Journal of Gastroenterology 2008;14(28):4535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BC, Zhang L, Ma JL, Pan KF, Li JY, Shen L, et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut 2012;61(6):812-8. [DOI] [PubMed] [Google Scholar]
- Wong BC. Eradication of Helicobacter pylori for prevention of gastric cancer. In: Journal of Gastroenterology and Hepatology. Vol. 27 (Suppl. 5). 2012:5-6. Abstract UA01-05.
You 2006 ‐ Li 2019 {published data only}
- Gail MH, You WC, Chang YS, Zhang L, Blot WJ, Brown LM, et al. Factorial trial of three interventions to reduce the progression of precancerous gastric lesions in Shandong, China: design issues and initial data. Controlled Clinical Trials 1998;19(4):352-69. [DOI] [PubMed] [Google Scholar]
- Li WQ, Ma JL, Zhang L, Brown LM, Li JY, Shen L, et al. Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. Journal of the National Cancer Institute 2014;106(7). [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WQ, Zhang JY, Ma JL, Li ZX, Zhang L, Zhang Y, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ 2019;366:l5016. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WQ, Zhang L, Ma JL, Brown L, Li JY, Shen L, et al. Fifteen-year effects of Helicobacter pylori treatment on gastric cancer incidence and mortality among participants with different baseline gastric lesions and ages. American Journal of Epidemiology 2013;177 (Suppl 11):S61. [Google Scholar]
- Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. Journal of the National Cancer Institute 2012;104(6):488-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang L, Moslehi R, Ma J, Pan K, Zhou T, et al. Long-term garlic or micronutrient supplementation, but not anti-Helicobacter pylori therapy, increases serum folate or glutathione without affecting serum vitamin B-12 or homocysteine in a rural Chinese population. Journal of Nutrition 2009;139(1):106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. Journal of the National Cancer Institute 2006;98(14):974-83. [DOI] [PubMed] [Google Scholar]
- You WC, Chang YS, Heinrich J, Ma JL, Liu WD, Zhang L, et al. An intervention trial to inhibit the progression of precancerous gastric lesions: compliance, serum micronutrients and S-allyl cysteine levels, and toxicity. European Journal of Cancer Prevention 2001;10(3):257-63. [DOI] [PubMed] [Google Scholar]
- Zhang L, Gail MH, Wang YQ, Brown LM, Pan KF, Ma JL, et al. A randomized factorial study of the effects of long-term garlic and micronutrient supplementation and of 2-wk antibiotic treatment for Helicobacter pylori infection on serum cholesterol and lipoproteins. American Journal of Clinical Nutrition 2006;86(4):912-9. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma JL, Liu WD, Jiang J, Pan KF, Pan YS, et al. A randomized multi-intervention trial to inhibit gastric cancer in Shandong (progress report). Chinese Journal of Clinical Oncology 1998;25(5):338-40. [Google Scholar]
References to studies excluded from this review
Choi 2014 {published data only}
- Choi J, Kim SG, Yoon H, Im JP, Kim JS, Kim WH, et al. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clinical Gastroenterology and Hepatology 2014;12(5):793-800. [DOI] [PubMed] [Google Scholar]
Choi 2018a {published data only}
- Choi JM, Kim SG, Choi J, Park JY, Oh S, Yang HJ, Lim JH, Im JP, Kim JS, Jung HC. Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: a randomized controlled trial. Gastrointest Endosc 2018;88(3):475-485.e2. [DOI: 10.1016/j.gie.2018.05.009] [Clinical trial registration number: NCT01510730] [DOI] [PubMed] [Google Scholar]
Choi 2018b {published data only}
- Choi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, Park B, Nam BH. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N Engl J Med 2018;378(12):1085-1095. [DOI: 10.1056/NEJMoa1708423] [ClinicalTrials.gov number, NCT02407119] [DOI] [PubMed] [Google Scholar]
Fischbach 2001 {published data only}
- Fischbach LA, Correa P, Ramirez H, Realpe JL, Collazos T, Ruiz B, et al. Anti-inflammatory and tissue-protectant drug effects: results from a randomized placebo-controlled trial of gastritis patients at high risk for gastric cancer. Alimentary Pharmacology & Therapeutics 2001;15(6):831-41. [DOI] [PubMed] [Google Scholar]
Fischbach 2009 {published data only}
- Fischbach LA, Bravo LE, Zarama GR, Bravo JC, Ojha RP, Priest EL, et al. A randomized clinical trial to determine the efficacy of regimens containing clarithromycin, metronidazole, and amoxicillin among histologic subgroups for Helicobacter pylori eradication in a developing country. Helicobacter 2009;14(2):100-8. [DOI] [PubMed] [Google Scholar]
Ford 2005 {published data only}
- Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. A community screening program for Helicobacter pylori saves money: 10-year follow-up of a randomized controlled trial. Gastroenterology 2005;129(6):1910-7. [DOI] [PubMed] [Google Scholar]
Fukase 2008 {published data only}
- Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. The Lancet 2008;372(9636):392-7. [DOI] [PubMed] [Google Scholar]
Hamajima 2002 {published data only}
- Hamajima N, Matuo K, Watanabe Y, Suzuki T, Nakamura T, Matsuura A, et al. A pilot study to evaluate stomach cancer risk reduction by Helicobacter pylori eradication. American Journal of Gastroenterology 2002;97(3):764-5. [DOI] [PubMed] [Google Scholar]
Harvey 2004 {published data only}
- Harvey RF, Lane JA, Murray LJ, Harvey IM, Donovan JL, Nair P, et al. Randomised controlled trial of effects of Helicobacter pylori infection and its eradication on heartburn and gastro-oesophageal reflux: Bristol Helicobacter project. BMJ 2004;328(7453):1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 2007 {published data only}
- Hsu PI, Lai KH, Hsu PN, Lo GH, Yu HC, Chen WC, et al. Helicobacter pylori infection and the risk of gastric malignancy. American Journal of Gastroenterology 2007;102(4):725-30. [DOI] [PubMed] [Google Scholar]
Imanzadeh 2004 {published data only}
- Imanzadeh F, Sayyari AA, Akbari MR. Histopathology of the stomach mucosa after treatment for Helicobacter pylori: A multi-centric study. In: Gut. Vol. 53 (Suppl VI). 2004:A292.
Juibari 2003 {published data only}
- Juibari M, Moayyedi R, Gholampour M, Bijarchi R, Peter K, Koucharian C, et al. Risk of precancerous pathologic changes in the stomach after appropriate eradication of Helicobacter pylori: A multi-centric study from a developing country. In: Gut. Vol. 52 (Suppl VI). 2003:A148.
Kamangar 2006 {published data only}
- Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. Journal of the National Cancer Institute 2006;98(20):1445-52. [DOI] [PubMed] [Google Scholar]
Kato 2006 {published data only}
- Kato M, Asaka M, Nakamura T, Azuma T, Eomita A, Kamoshida T, et al. H.pylori eradication prevents the development of gastric cancer- results of a long-term retrospective study in Japan. Alimentary Pharmacology & Therapeutics 2006;24(Suppl 4):203-6. [Google Scholar]
Kim 2008 {published data only}
- Kim N, Park RY, Cho SI, Lim SH, Lee KH, Lee W, et al. Helicobacter pylori infection and development of gastric cancer in Korea: long-term follow up. Journal of Clinical Gastroenterology 2008;42(5):448-54. [DOI] [PubMed] [Google Scholar]
Lane 2006 {published data only}
- Lane JA, Murray LJ, Noble S, Egger M, Harvey IM, Donovan JL, et al. Impact of Helicobacter pylori eradication on dyspepsia, health resource use, and quality of life in the Bristol Helicobacter project: randomised controlled trial. BMJ 2006;332(7535):199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leja 2017 {published data only}
- Leja M, Park JY, Murillo R, Liepniece-Karele I, Isajevs S, Kikuste I, et al. Multicentric randomised study of Helicobacter pylori eradication and pepsinogen testing for prevention of gastric cancer mortality: the GISTAR study. BMJ open 2017;7(8):e016999. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leung 2018 {published data only}
- Leung WK, Wong IOL, Cheung KS, Yeung KF, Chan EW, Wong AYS, et al. Effects of Helicobacter pylori Treatment on Incidence of Gastric Cancer in Older Individuals. Gastroenterology 2018;155(1):67-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mabe 2009 {published data only}
- Mabe K, Takahashi M, Oizumi H, Tsukuma H, Shibata A, Fukase K, et al. Does Helicobacter pylori eradication therapy for peptic ulcer prevent gastric cancer? World Journal of Gastroenterology 2009;15(34):4290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2002 {published data only}
- Mason J, Axon AT, Forman D, Duffett S, Drummond M, Crocombe W, et al. The cost-effectiveness of population Helicobacter pylori screening and treatment: a Markov model using economic data from a randomized controlled trial. Alimentary Pharmacology & Therapeutics 2002;16(3):559-68. [DOI] [PubMed] [Google Scholar]
Miehlke 2001 {published data only}
- Miehlke S, Kirsch C, Dragosics B, Gschwantler M, Oberhuber G, Antos D, et al. Helicobacter pylori and gastric cancer: current status of the Austrian Czech German gastric cancer prevention trial (PRISMA Study). World Journal of Gastroenterology 2001;7(2):243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moayyedi 2000 {published data only}
- Moayyedi P, Feltbower R, Brown J, Mason S, Mason J, Nathan J, et al. Effect of population screening and treatment for Helicobacter pylori on dyspepsia and quality of life in the community: a randomised controlled trial. Leeds HELP Study Group. The Lancet 2000;355(9213):1665-9. [DOI] [PubMed] [Google Scholar]
Ogura 2008 {published data only}
- Ogura K, Hirata Y, Yanai A, Shibata W, Ohmae T, Mitsuno Y, et al. The effect of Helicobacter pylori eradication on reducing the incidence of gastric cancer. Journal of Clinical Gastroenterology 2008;42(3):279-83. [DOI] [PubMed] [Google Scholar]
Ohkusa 2001 {published data only}
- Ohkusa T, Fujiki K, Takashimizu I, Kumagai J, Tanizawa T, Eishi Y, et al. Improvement in atrophic gastritis and intestinal metaplasia in patients in whom Helicobacter pylori was eradicated. Annals of Internal Medicine 2001;134(5):380-6. [DOI] [PubMed] [Google Scholar]
Take 2005 {published data only}
- Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Yokota K, et al. The effect of eradicating Helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. American Journal of Gastroenterology 2005;100(5):1037-42. [DOI] [PubMed] [Google Scholar]
Take 2007 {published data only}
- Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Yokota K, et al. Baseline gastric mucosal atrophy is a risk factor associated with the development of gastric cancer after Helicobacter pylori eradication therapy in patients with peptic ulcer diseases. Journal of Gastroenterology 2007;42(Suppl 17):21-7. [DOI] [PubMed] [Google Scholar]
Takenaka 2007 {published data only}
- Takenaka R, Okada H, Kato J, Makidono C, Hori S, Kawahara Y, et al. Helicobacter pylori eradication reduced the incidence of gastric cancer, especially of the intestinal type. Alimentary Pharmacology & Therapeutics 2007;25(7):805-12. [DOI] [PubMed] [Google Scholar]
Tang 2010 {published data only}
- Tang JM, Chen Y, Lu HL, Zhao GM, Xu H, Su RH. Histologic changes and incidence of gastric cancer after Helicobacter pylori eradication: a 3-year follow-up study [根除幽门螺杆菌对胃黏膜组织学变化及胃癌患病率的 3 年随访研究]. Modern Digestion Intervention (现代消化及介入诊疗) 2010;15(1):47-49. [Google Scholar]
Uemura 2001 {published data only}
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. The New England Journal of Medicine 2001;345(11):784-9. [DOI] [PubMed] [Google Scholar]
Uemura 2002 {published data only}
- Uemura N, Okamoto S, Yamamoto S. H. pylori infection and the development of gastric cancer. Keio Journal of Medicine 2002;51(Suppl 2):63-8. [DOI] [PubMed] [Google Scholar]
Wildner‐Christensen 2003 {published data only}
- Wildner-Christensen M, Møller Hansen J, Schaffalitzky De Muckadell OB. Rates of dyspepsia one year after Helicobacter pylori screening and eradication in a Danish population. Gastroenterology 2003;125(2):372-9. [DOI] [PubMed] [Google Scholar]
Yanaoka 2009 {published data only}
- Yanaoka K, Oka M, Ohata H, Yoshimura N, Deguchi H, Mukoubayashi C, et al. Eradication of Helicobacter pylori prevents cancer development in subjects with mild gastric atrophy identified by serum pepsinogen levels. International Journal of Cancer. Journal International du Cancer 2009;125(11):2697-703. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
HELPER study {published data only}
- KCT0001262. Helicobacter Pylori Eradication for Gastric Cancer Prevention in the General Population. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=KCT0001262 . [http://cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=5307]
- NCT02112214. Helicobacter Pylori Eradication for Gastric Cancer Prevention in the General Population (HELPER). https://clinicaltrials.gov/ct2/show/nct02112214 .
Pan 2016 {published data only}
- Pan KF, Zhang L, Gerhard M, Ma JL, Liu WD, Ulm K, et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut 2016;65(1):9-18. [PMID: ] [DOI] [PubMed] [Google Scholar]
Additional references
Akl 2013
- Akl EA, Johnston BC, Alonso-Coello P, Neumann I, Ebrahim S, Briel M, et al. Addressing dichotomous data for participants excluded from trial analysis: a guide for systematic reviewers. PLoS One 2013;8(2):e57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann H, Oxman AD, Kunz R et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI] [PubMed] [Google Scholar]
Chey 2017
- Chey WD, Leontiadis GI, Howden DW, Moss S. ACG guideline: treatment of Helicobacter pylori infection. American Journal of Gastroenterology 2017;112(2):212-39. [DOI] [PubMed] [Google Scholar]
Correa 1975
- Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. The Lancet 1975;2(7924):58-60. [DOI] [PubMed] [Google Scholar]
Correa 1983
- Correa P. The gastric precancerous process. Cancer Surveys 1983;2:437-50. [Google Scholar]
Correa 2001
- Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Re: Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. Journal of the National Cancer Institute 2001;93(7):559-60. [DOI] [PubMed] [Google Scholar]
Cunningham 2005
- Cunningham SC, Kamangar F, Kim MP, Hammoud S, Haque R, Maitra A, et al. Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution. Journal of Gastrointestinal Surgery 2005;9(5):718-25. [DOI] [PubMed] [Google Scholar]
Degiuli 2006
- Degiuli M, Calvo F. Survival of early gastric cancer in a specialized European center. Which lymphadenectomy is necessary? World Journal of Surgery 2006;30(12):2193-203. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Feng 2008
- Feng GS, Ma JL, Wong BC, Zhang L, Liu WD, Pan KF, et al. Celecoxib-related gastroduodenal ulcer and cardiovascular events in a randomized trial for gastric cancer prevention. World Journal of Gastroenterology 2008;14(28):4535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferlay 2010
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. Journal International du Cancer 2010;127:2839-917. [DOI] [PubMed] [Google Scholar]
Fock 2009
- Fock KM, Katelaris P, Sugano K, Ang TL, Hunt RH et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori eradication. Journal of Gastroenterology and Hepatology 2009;24:1587-600. [DOI] [PubMed] [Google Scholar]
Ford 2009
- Ford AC, Moayyedi P. Redundant data in the meta-analysis on Helicobacter pylori eradication. Annals of Internal Medicine 2009;151(7):513-4. [DOI] [PubMed] [Google Scholar]
Ford 2011
- Ford AC. Chemoprevention for gastric cancer. Best Practice & Research Clinical Gastroenterology 2011;25(4-5):581-92. [DOI] [PubMed] [Google Scholar]
Forman 1991
- Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ 1991;302:1302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forman 1998
- Forman D. Helicobacter pylori: the gastric cancer problem. Gut 1998;43 Suppl 1:S33-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fuccio 2009
- Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Annals of Internal Medicine 2009;151(2):121-8. [DOI] [PubMed] [Google Scholar]
Gail 1998
- Gail MH, You WC, Chang YS, Zhang L, Blot WJ, Brown LM, et al. Factorial trial of three interventions to reduce the progression of precancerous gastric lesions in Shandong, China: design issues and initial data. Controlled Clinical Trials 1998;19(4):352-69. [DOI] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
HCCG 2001
- Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001;49(3):347-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
IARC 1994
- International Agency for Research on Cancer. Schistosomes, liver flukes and Helicobacter pylori. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans 1994;61:177-241. [PMC free article] [PubMed] [Google Scholar]
IARC 2014
- IARC Helicobacter pylori Working Group. Helicobacter pylori eradication as a strategy for preventing gastric cancer. IARC Working Group Reports 8.
Lau 2006
- Lau M, Le A, El-Serag HB. Noncardia gastric adenocarcinoma remains an important and deadly cancer in the United States: secular trends in incidence and survival. American Journal of Gastroenterology 2006;101(11):2485-92. [DOI] [PubMed] [Google Scholar]
Lee 2013
- Lee YC, Chen TH, Chiu HM, Shun CT, Chiang H, Liu TY, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 2013;62(5):676-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lello 2007
- Lello E, Furnes B, Edna TH. Short and long-term survival from gastric cancer. A population-based study from a county hospital during 25 years. Acta Oncologica 2007;46(3):308-15. [DOI] [PubMed] [Google Scholar]
Leung 2004
- Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut 2004;53(9):1244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2013
- Li WQ, Zhang L, Ma JL, Brown L, Li JY, Shen L, et al. Fifteen-year effects of Helicobacter pylori treatment on gastric cancer incidence and mortality among participants with different baseline gastric lesions and ages. American Journal of Epidemiology 2013;177 (Suppl 11):S61. [Google Scholar]
Li 2014
- Li WQ, Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, Liu WD, Hu Y, Han ZX, Crystal-Mansour S, Pee D, Blot WJ, Fraumeni JF Jr, You WC, Gail MH. Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. J Natl Cancer Inst 2014;106(7):pii: dju116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2019
- Li WQ, Zhang JY, Ma JL, Li ZX, Zhang L, Zhang Y, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ 2019;366:l5016. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2012
- Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. Journal of the National Cancer Institute 2012;104(6):488-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malfertheiner 2017
- Malfertheiner P, Megraud F, OMorain CA, Gisbert JP, Kuipers EJ et al. Management of Helicobacter pylori infection- the Maastricht V Florence Consensus report. Gut 2017;66:6-30. [DOI] [PubMed] [Google Scholar]
Marshall 1985
- Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Medical Journal of Australia 1985;142(8):436-9. [DOI] [PubMed] [Google Scholar]
Mera 2005
- Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut 2005;54(11):1536-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moayyedi 1997
- Moayyedi P, Dixon MF. Significance of Helicobacter pylori infection and gastric cancer: implications for screening. Gastrointestinal Endoscopy Clinics of North America 1997;7(1):47-64. [PubMed] [Google Scholar]
Nomura 1991
- Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. The New England Journal of Medicine 1991;325(16):1132-6. [DOI] [PubMed] [Google Scholar]
Parsonnet 1991
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. The New England Journal of Medicine 1991;325(16):1127-31. [DOI] [PubMed] [Google Scholar]
Parsonnet 1996
- Parsonnet J, Harris RA, Hack HM, Owens DK. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. The Lancet 1996;348(9021):150-4. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ruiz 2001
- Ruiz B, Garay J, Correa P, Fontham ET, Bravo JC, Bravo LE, et al. Morphometric evaluation of gastric antral atrophy: improvement after cure of Helicobacter pylori infection. American Journal of Gastroenterology 2001;96(12):3281-7. [DOI] [PubMed] [Google Scholar]
Saito 2003
- Saito D. Present state of Japanese intervention trial of H. pylori. Nihon Rinsho 2003;61(1):50-5. [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Sung 2000
- Sung JJ, Lin SR, Ching JY, Zhou LY, To KF, Wang RT, et al. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterology 2000;119(1):7-14. [DOI] [PubMed] [Google Scholar]
Sung 2002
- Sung JJ, Lin SR, Leung W, Ng EK, Ching JY, To KF, et al. Does eradication of H. pylori prevent deterioration of gastric atrophy and intestinal metaplasia? A 5-year follow-up. In: Gastroenterology. Vol. 122(Suppl 4). 2002:A170. Abstract S1142.
UGPD Module 2015
- Forman D, Delaney B, Kuipers E, Malthaner R, Moayyedi P, Gardener E, et al. Cochrane Upper GI and Pancreatic Diseases Group module. http://onlinelibrary.wiley.com/o/cochrane/clabout/articles/UPPERGI/sect0-meta.html (accessed 10 June 2015).
Vakil 2009
- Vakil N, Talley N, Zanten SV, Flook N, Persson T, Björck E, et al. Cost of detecting malignant lesions by endoscopy in 2741 primary care dyspeptic patients without alarm symptoms. Clinical Gastroenterology and Hepatology 2009;7(7):756-61. [DOI] [PubMed] [Google Scholar]
Wang 2009
- Wang Y, Zhang L, Moslehi R, Ma J, Pan K, Zhou T, et al. Long-term garlic or micronutrient supplementation, but not anti-Helicobacter pylori therapy, increases serum folate or glutathione without affecting serum vitamin B-12 or homocysteine in a rural Chinese population. Journal of Nutrition 2009;139(1):106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warren 1983
- Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. The Lancet 1983;1(8336):1273-5. [PubMed] [Google Scholar]
Wong 2002
- Wong BCY, Lam S, Wong WM, et al. Eradication of Helicobacter pylori infection significantly slows down the progression of precancerous lesions in high risk populations: a 5 year prospective randomized study. In: Gastroenterology. Vol. 122. 2002:A588. Abstract W1216.
Wong 2012
- Wong BC, Zhang L, Ma JL, Pan KF, Li JY, Shen L, et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut 2012;61(6):812-8. [DOI] [PubMed] [Google Scholar]
Wong 2012b
- Wong BC. Eradication of Helicobacter pylori for prevention of gastric cancer. In: Journal of Gastroenterology and Hepatology. Vol. 27 (Suppl 5). 2012:5-6. Abstract UA01-05.
Wu 2003
- Wu AH, Crabtree JE, Bernstein L, Hawtin P, Cockburn M, Tseng CC, et al. Role of Helicobacter pylori CagA+ strains and risk of adenocarcinoma of the stomach and esophagus. International Journal of Cancer 2003;103:815-21. [DOI] [PubMed] [Google Scholar]
Ye 2004
- Ye W, Held M, Lagergren J, Engstrand L, Blot WJ, McLaughlin JK, et al. Helicobacter pylori infection and gastric atrophy: risk of adenocarcinoma and squamous-cell carcinoma of the esophagus and adenocarcinoma of the gastric cardia. Journal of the National Cancer Institute 2004;96(5):388-96. [DOI] [PubMed] [Google Scholar]
You 2001
- You WC, Chang YS, Heinrich J, Ma JL, Liu WD, Zhang L, et al. An intervention trial to inhibit the progression of precancerous gastric lesions: compliance, serum micronutrients and S-allyl cysteine levels, and toxicity. European Journal of Cancer Prevention 2001;10(3):257-63. [DOI] [PubMed] [Google Scholar]
You 2006
- You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. Journal of the National Cancer Institute 2006;98(14):974-83. [DOI] [PubMed] [Google Scholar]
Zhang 1998
- Zhang L, Ma JL, Liu WD, Jiang J, Pan KF, Pan YS, et al. A randomized multi-intervention trial to inhibit gastric cancer in Shandong (progress report). Chinese Journal of Clinical Oncology 1998;25(5):338-40. [Google Scholar]
Zhang 2006
- Zhang L, Gail MH, Wang YQ, Brown LM, Pan KF, Ma JL, et al. A randomized factorial study of the effects of long-term garlic and micronutrient supplementation and of 2-wk antibiotic treatment for Helicobacter pylori infection on serum cholesterol and lipoproteins. American Journal of Clinical Nutrition 2006;86(4):912-9. [DOI] [PubMed] [Google Scholar]
Zhou 2003a
- Zhou L, Sung JJ, Lin S, Jin Z, Ding S, Huang X, et al. A five-year follow-up study on the pathological changes of gastric mucosa after H. pylori eradication. Chinese Medical Journal 2003;116(1):11-4. [PubMed] [Google Scholar]
Zhou 2003b
- Zhou LY, Lin SR, Shen ZY, Zhong SZ, Ding SG, Huang XB, et al. Five-year follow-up study after Helicobacter pylori eradication: Reinfection and peptic ulcer status. Chinese Journal of Digestive Diseases 2003;4(1):45-8. [Google Scholar]
Zhou 2003c
- Zhou LY, Sung JJ, Lin SR, Jin Z, Ding SG, Huang XB, et al. Changes of gastric mucosa histopathology after Helicobacterpylori eradication [根除幽门螺杆菌对胃黏膜炎症变化的人群随访研究]. Zhonghua Nei Ke za Zhi [Chinese Journal of Internal Medicine] 2003;42(3):162-4. [PubMed]
Zhou 2005a
- Zhou LY, Lin SR, Ding SG, Huang SB, Guo CJ, Zhang L, et al. Eight-year follow-up study on prevalence of gastric cancer and the histological changes of gastric mucosa after H.pylori eradication [除幽门螺杆菌对胃癌患病率及胃黏膜组织学变化的八年随访研究]. Chinese Journal of Digestion 2005;25(6):324-7. [Google Scholar]
Zhou 2005b
- Zhou LY, Lin SR, Ding SG, Huang SB, Guo CJ, Zhang L, et al. Eight-year follow-up study on the morbidity of gastric cancer and the pathological changes of gastric mucosa after H.pylori eradication [除幽门螺杆菌对胃癌患病率及胃黏膜组织学变化的八年随访研究]. www.paper.edu.cn/scholar/downpaper/zhouliya-3 (accessed 10 June 2015).
Zhou 2005c
- Zhou LY, Lin SR, Ding SG, Huang XB, Zhang L, Meng LM, et al. The changing trends of the incidence of gastric cancer after Helicobacter pylori eradication in Shandong area. Chinese Journal of Digestive Diseases 2005;6(3):114-5. [DOI] [PubMed] [Google Scholar]
Zhou 2008
- Zhou L. Ten-year follow-up study on the incidence of gastric cancer and the pathological changes of gastric mucosa after H. pylori eradication in China. In: Gastroenterology. Vol. 134 (4, suppl 1). 2008:A233. Abstract S1606.
Zhou 2014
- Zhou L, Lin S, Ding S, Huang X, Jin Z, Cui R, et al. Relationship of Helicobacter pylori eradication with gastric cancer and gastric mucosal histological changes: a 10-year follow-up study. Chinese medical journal 2014;127(8):1454-8. [PMID: ] [PubMed] [Google Scholar]
References to other published versions of this review
Ford 2014
- Ford A, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 2014;348:g3174. [DOI: 10.1136/bmj.g3174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 2015
- Ford AC, Forman D, Hunt R, Yuan Y, Moayyedi P. Helicobacter pylori eradication for the prevention of gastric neoplasia. The Cochrane database of systematic reviews 2015;(7):CD005583. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]


