Abstract
Background
Animal models of traumatic brain injury suggest that induced normothermia (36.5 or 37 ºC), compared to induced hyperthermia (39 ºC), improves histopathological and neurobehavioural outcomes. Observational clinical studies of patients with TBI suggest an association between raised body temperature and unfavourable outcome, although this relationship is inconsistent.
Objectives
To assess the effects of modest cooling therapies (defined as any drug or physical therapy aimed at maintaining body temperature between 35 ºC and 37.5 ºC) when applied to patients in the first week after traumatic brain injury.
Search methods
The most recent search was run on 23rd September 2013. We searched the Cochrane Injuries Group's Specialised Register, The Cochrane Library (CENTRAL), MEDLINE (OvidSP), Embase (OvidSP), ISI WOS: SCI‐EXPANDED (1970) & CPCI‐S (1990), PubMed and trials registries together with reference checking.
Selection criteria
All completed randomised, controlled and placebo‐controlled trials published or unpublished, where modest cooling therapies were applied in the first week after traumatic brain injury.
Data collection and analysis
Two authors independently applied the selection criteria to relevant trials.
Main results
We were unable to find any randomised controlled trials of modest cooling therapies after traumatic brain injury.
Authors' conclusions
In order to further explore the preliminary findings provided by animal models and observational clinical studies that suggests there may be a beneficial effect of modest cooling for TBI, randomised trials designed to explore the effect of these interventions on patient‐centred outcomes are needed.
Plain language summary
Interventions that aim to reduce body temperature to between 35 ºC and 37.5 ºC in patients who have had a traumatic brain injury within the last week
Traumatic brain injury (an injury to the brain that occurs as a result of a direct impact, such as may occur after a road traffic accident or a fall) is a major cause of death and long‐term disability worldwide. There is some evidence from animal experiments that reducing body temperature after brain injury may improve outcomes. There is also some evidence in humans to suggest that people with a normal body temperature after traumatic brain injury may have a better outcome than those with a higher temperature.
The authors of this Cochrane review looked for evidence that reducing body temperature to between 35 ºC and 37.5 ºC would benefit patients in the week after traumatic brain injury. We looked for studies on the use of physical or drug‐induced cooling on patients with a traumatic brain injury. Physical cooling techniques include cooling blankets, use of ice, fans or other devices. Chemical cooling techniques include drugs used to reduce fever, like paracetamol (acetaminophen).
We did not find any randomised controlled trials or controlled clinical trials that we could include in this review. Such studies represent the best form of evidence to determine whether a particular therapy works, because they limit the errors that may affect the interpretation of a study. Given the lack of eligible studies, the only finding we can present is that there is no satisfactory research carried out in relation to the specific question of the risks and benefits of cooling to between 35‐37.5°C for patients with TBI. There is another Cochrane review (Hypothermia for traumatic head injury) about cooling below 35°C which includes a number of studies.
Background
Traumatic brain injury (TBI) is a major cause of death and disability amongst a predominantly young population, with an estimated 10 million people experiencing severe TBI worldwide every year (Alexander 1992). The World Health Organization Global Burdens of Disease Project identified road traffic crashes as the 4th leading cause of death in low and middle‐income countries (Mathers 2001) and road traffic injuries are consistently in the top 3 leading causes of death worldwide in the age group 5 to 44 years. TBI is an important issue that affects many people in developed and developing countries. There is, however, an important lack of coherent evidence about effective therapies for the acute care of these patients (Alderson 2004).
Some data from animal studies (Dietrich 1996; Chatzipanteli 2007) of TBI suggest that reducing body temperature by the intervention of induced normothermia (36.5 or 37 ºC), compared to artificially increasing body temperature by inducing hyperthermia (39 ºC), improves biochemical and histopathological outcomes. Observational data from clinical cohort studies also suggest that patients with an elevated body temperature (hyperthermia) may have a worse outcome than patients who have a normal body temperature (Geffroy 2004; Jiang 2002; Li 2012), but this relationship is inconsistent (Jones 1994; Stocchetti N; Andrews 2006).
The clinical utility of reducing body temperature by cooling has yet to be conclusively demonstrated in terms of reduced mortality or improved functional survival in the clinical setting of TBI. There are now some data to support the use of induced hypothermia after perinatal birth asphyxia (body temperature reduction to 35 ºC) in neonates (Gluckman 2005). Pathophysiologically, TBI differs from neonatal birth asphyxia in that the primary insult is direct neuronal trauma and this is also frequently complicated by associated secondary insults. These include disorders of airway, breathing and circulation associated with the primary trauma that may result in secondary episodes of hypoxia, hypercarbia and low cardiac output (most commonly due to haemorrhage and hypovolaemia). The treatment effects of temperature reduction after neonatal birth asphyxia has not been replicated in patients with TBI and an existing Cochrane review (Sydenham 2009) has concluded that there is no evidence that induced hypothermia (defined as interventions that reduce core body temperature to 35 ºC or less) is beneficial in the treatment of traumatic head injury. In addition, this review suggested that there is an increase in pulmonary infections with the intervention of hypothermia compared to controls.
Arguably, induced hypothermia remains a complex, unproven intervention that could only be applied to a small proportion of the more severely injured patients who suffer a TBI, where advanced pre‐hospital trauma care, intensive care facilities and skilled staff are readily available. On the other hand, we have argued that interventions that target a more modest temperature reduction may, if efficacy is demonstrated, be applicable to a much larger proportion of the global pool of patients who suffer a TBI (Andrews 2006). Examples of interventions that may achieve a more modest temperature reduction than induced hypothermia include drug therapy (for example, paracetamol, acetaminophen, non‐steroidal anti‐inflammatory drugs, etc (Price 2003)) and physical therapies (for example, bedside fans, tepid sponging, ice packs, cooling blankets, etc (Harris 2007)). We define modest cooling therapies as any intervention(s) that is (are) carried out with the intention of reducing body temperature to no less than 35 ºC. This includes interventions that are administered with the intention of lowering body temperature to below normal (less than 36 ºC but above 35 ºC), and those interventions that are given to maintain temperature within the range of greater than 36 ºC but less than 37.5 ºC (normothermia).
Why it is important to do this review
The Brain Trauma Foundation (BTF 2007) does not make recommendations regarding modest cooling therapies after TBI, reflecting the lack of evidence for this intervention. At present, we believe that modest cooling therapies, as outlined above, are used in ad hoc fashion after TBI. We endeavoured to search for randomised controlled clinical trials that explore the effects of modest cooling therapies on outcomes following TBI. This systematic review aims to assess the relationship between imposed modest changes in body temperature and outcome after TBI, and determine whether there is any clear evidence that a modest temperature reduction of any kind is beneficial, has no effect, or causes harm.
Objectives
To assess the effects of modest cooling therapies (with antipyretic drugs or physical cooling devices) when administered to patients (adult and children) admitted to hospital after TBI.
We defined modest cooling therapies as any interventions carried out with the intention of reducing body temperature to no less than 35 ºC (this excludes studies identified in the Cochrane review titled 'Hypothermia for traumatic head injury' (Sydenham 2009) as this review assesses the effect of interventions that reduce temperature to below 35 ºC).
We wished to test the following hypotheses:
Modest cooling therapies reduce the risk of a poor outcome after traumatic brain injury (a poor outcome is defined as the composite end‐point of death or severe disability).
Modest cooling therapies increase the risk of intracranial and extracranial bleeding.
Modest cooling therapies increase the risk of pneumonia or other serious infections.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, controlled trials of interventions administered to patients within the first week after TBI, that aim to reduce body temperature to no less than 35 ºC.
Types of participants
All patients admitted to hospital following TBI (all severities).
Types of interventions
Any physical or drug therapy that reduces temperature to no less than 35 ºC.
Examples of physical therapy include bedside fans, sponging, fluid or air‐filled devices applied to body surfaces (for example, blankets, neck‐collars, helmets or hoods), ice‐water lavage or intravenous fluid administration and intravenous cooling catheters.
Examples of drug therapies include acetaminophen, paracetamol, non‐steroidal anti‐inflammatory drugs and cyclo‐oxygenase‐2 inhibitors given by any route.
Types of outcome measures
Primary outcomes
Poor outcome at the end of follow up. This is defined as death or dependency as measured by the Glasgow Outcome Score (GOS), or an equivalent method in which it is clear how many people are dependent and how many are independent at the end of the follow‐up period. This is the most important outcome as the aim of treatment should not only be to prevent death, but also to improve the quality of the patient's survival.
Secondary outcomes
Death from all causes during the follow‐up period.
Further serious intracranial haemorrhage: this is defined as the need for operative intervention (for example, evacuation of subdural or extradural haematoma) occurring during the period of intervention.
Extracranial haemorrhage: defined as the need for transfusion of greater than two units of packed cells within a 24‐hour period occurring during the period of intervention.
Abnormal Intracranial Pressure: This is defined as an intracranial pressure of more than 20mmHg as measured by an intracranial pressure monitor.
Pneumonia or other serious infections.
Information size requirement for the primary outcome: With a conventional dichotomous analysis of the GOS that compares the proportions of patients with an unfavourable outcome in the two groups (normothermia vs. control), a minimum of 716 people need to be randomised in order to have 90% power at the 5% significance level (2‐sided) to detect an absolute reduction of 12% (60% reducing to 48%) of an unfavourable outcome.
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by language, date or publication status.
Electronic searches
The Cochrane Injuries Group's Trials Search Co‐ordinator searched the following:
Cochrane Injuries Group's Specialised Register (23rd September 2013);
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library) Issue 9 of 12, 2013;
MEDLINE (OvidSP) (1950 to Sept 18th, 2013);
Embase (OvidSP) (1980 to week 37, 2013);
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to September 2013);
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to September 2013);
Pubmed (23rd September 2013);
National Research Register (issue 3, 2007);
ZETOC (http://zetoc.mimas.ac.uk/) (April 2008).
The National Research Register has now been archived and ZETOC has not retrieved any additional studies therefore these sources were not searched for this update. Their search strategies are listed in Appendix 2.
On 9 December 2013 the authors searched:
Clinicaltrials.gov (www.clinicaltrials.gov);
Current Controlled Trials MetaRegister (http://www.controlled‐trials.com/mrct/).
Searching other resources
We checked reference lists of selected studies and relevant reviews to find additional studies and contacted the authors of relevant studies to assist in the identification of unpublished studies, internal reports and conference proceedings. On 9 December 2013 we contacted the investigators, pharmaceutical companies and manufacturers of cooling equipment that are pertinent to this field (listed in Appendix 3).
Data collection and analysis
The review was conducted according to the published protocol (Saxena 2007).
Selection of studies
Two authors (MS and NH) independently screened the titles and abstracts and discarded studies that did not meet the review criteria or were not applicable; however, we initially retained studies and reviews that might include relevant data or information on ongoing trials. The authors then independently reviewed the retrieved studies, examining the abstracts and the full text to ascertain which studies satisfied the necessary inclusion criteria. We also screened references from the studies retrieved in full‐text.
Data extraction and management
The same authors independently carried out data extraction using standard data extraction forms. We translated non‐English language studies before assessment. We requested any further information required from the original author by written correspondence and considered the relevant information obtained in this manner as part of the review. We resolved disagreements in consultation with the third author (PA).
In the future the following information will be extracted from each assessed study: the title of the trial; the intervention being tested (dosage and route for drug therapy; physical cooling method for equipment); lowest body temperature attained; duration of intervention; the time interval between the onset of the initial injury and the commencement of cooling therapy; and, any neurological deterioration developing after the commencement of cooling therapy. We will also record the total number of patients randomised to intervention and placebo‐controlled groups. We will also collect data on age, gender, mechanism of injury, associated extracranial injuries and duration from injury to presentation at a hospital.
We will also record specific information regarding the number of patients in each group with the following outcomes: death and dependency, death alone, intracranial haemorrhage, extracranial haemorrhage, pneumonia, other serious infections, any other complications, and the number of patients excluded or lost to follow up.
For each study, we will also assess whether an intention‐to‐treat analysis was used according to the following criteria:
Yes ‐ specifically reported by the authors that intention‐to‐treat analysis was undertaken and this was confirmed on study assessment.
Yes ‐ not stated, but confirmed on study assessment.
No ‐ not reported and lack of intention‐to‐treat analysis confirmed on study assessment (patients who were randomised were not included in the analysis because they did not receive the study intervention, they withdrew from the study, or were not included because of protocol violation).
No ‐ stated but not confirmed upon study assessment.
Not stated.
Assessment of risk of bias in included studies
In the future if studies are included in this review, two authors will independently assess the quality of studies meeting the inclusion criteria, without blinding to authorship or journal. Discrepancies will be resolved by discussion. We will follow the most recent version of the risk of bias criteria, as described in chapter 8 of the Cochrane Handbook (Higgins 2011). We will assess the methods used to generate the randomisation sequence, concealment of the randomisation sequence (allocation concealment), blinding of investigators, participants, outcome assessors, and those involved in data analysis. We will consider there to be no blinding if the treatment group can be identified in >20% of participants because of the side effects of treatment. We will also assess how complete the outcome data are, whether there is selective reporting of the outcome measures, and any other sources of bias.
Measures of treatment effect
We will calculate the risk ratio with 95% confidence intervals for dichotomous outcomes.
For continuous outcomes measured on the same scale, we will calculate the mean difference (MD) and 95% confidence intervals. If results for continuous outcomes are reported using different scales or different versions of the same scale, we will calculate the standardised mean difference (SMD) and 95% CI.
Unit of analysis issues
The unit of analysis will be the individual patient.
Dealing with missing data
Missing summary data will not be a reason to exclude a study from the review. If necessary, we will contact the authors of the original papers for more information on missing data. If an eligible study is identified but provides no useable data for analysis, the study will still be included in the review. Its methods will be described in the table of included studies, and an assessment of the risk of bias will be completed.
Assessment of heterogeneity
We will consider clinical homogeneity among all included studies. For studies considered as clinically homogeneous, we will test the statistical heterogeneity using the Chi2 test and I2 statistic. We will assume statistical significance of the Chi2 test if P < 0.10. We will consider an I2 value greater than 50% to be substantial.
Assessment of reporting biases
If there are ten or more studies included in an analysis, funnel plots and linear regression tests will be used to assess for any potential reporting bias.
Data synthesis
For dichotomous outcomes we will express the results as relative risk (RR) with 95% confidence intervals (CI). We will pool data using the random‐effects model. Where continuous scales of measurement are used to assess the effects of treatment, we will use the mean difference (MD), or the standardised mean difference (SMD) if different scales have been used. We will analyse heterogeneity using a Chi‐squared test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance, and with the I2 test.
Subgroup analysis and investigation of heterogeneity
Where possible, we will explore the following:
Adults (>18 years old) versus children;
Achieved temperature reduction (35 ºC to 36 ºC versus placebo and 36 ºC to 37.5 ºC versus placebo);
Duration of temperature reduction.
We will use subgroup analysis to explore possible sources of heterogeneity. We will tabulate and assess adverse effects with descriptive techniques, as they are likely to be different for the various interventions being assessed. Where possible, we will calculate the risk difference with 95% CI for each adverse event, compared to no treatment.
Results
Description of studies
Results of the search
The electronic database searches have retrieved a total of 1395 records to September 2013. Contact with investigators, pharmaceutical companies and manufacturers of cooling equipment did not yield any further information. The updated searches in 2013 identified one new ongoing study (Figure 1; NCT01231139). This study completed recruitment in 2014, and the results will be included into this review by the end of 2015.
1.
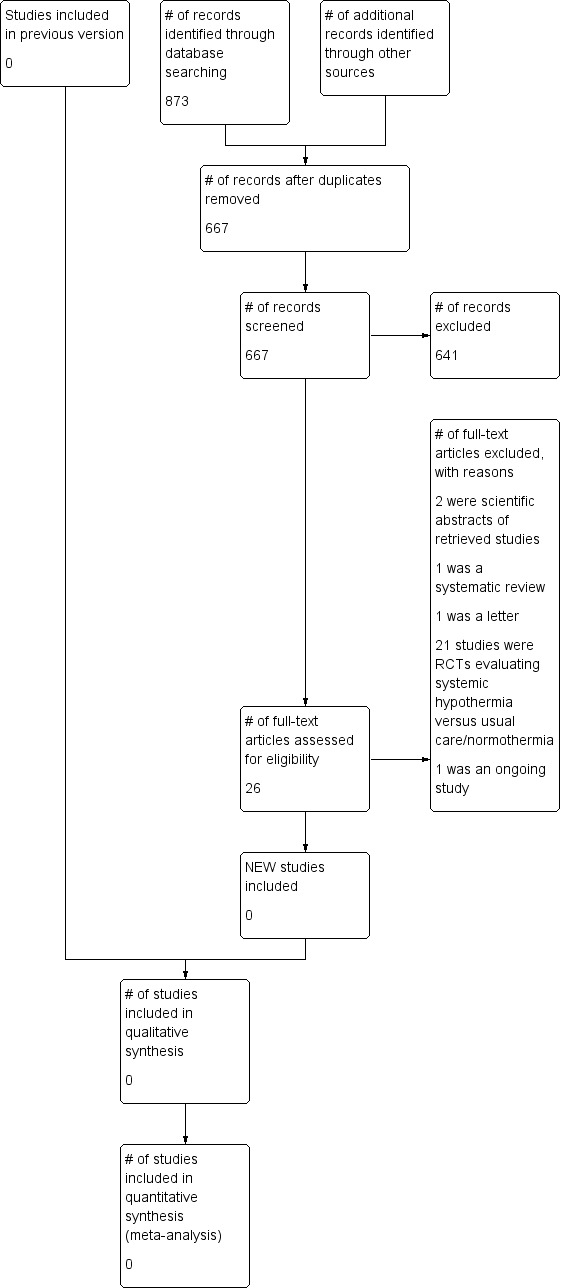
Study flow diagram
In the first version of this review, which was published in 2008, we retrieved 12 articles for further consideration. Following a complete assessment of the 12 studies, we excluded two as the intervention of selective brain cooling with a target brain temperature of 33 ºC to 35 ºC was felt by all three authors of this review to be outside the scope of our review (Qiu 2006a; Wang 2004).
After assessment of the remaining 10 studies, none were found to meet all the inclusion criteria for this review. In nine of the studies, information was provided on physiological end‐points only, without reference to patient‐centred outcomes and only four studies were placebo‐controlled.
There was one randomised, placebo‐controlled study of patients after TBI that included a group of patients with a modest temperature intervention (Qiu 2006) and reported information on patient‐centred outcomes. This study included two treatment arms (selective brain cooling to 33 ºC to 35 ºC and mild systemic hypothermia to 34.5 ºC to 36 ºC) with one control group; however, the control group was also subject to temperature cooling strategies. Following contact with the authors, this study was excluded because of methodological issues, as outcome data was lacking in 40 out of the 96 patients initially randomised in the study.
There were no studies included in the first or second versions of this review.
Risk of bias in included studies
There are no studies included in this review.
Effects of interventions
There are no studies included in this review.
Discussion
We were unable to identify any randomised, controlled trials of modest cooling after TBI meeting the inclusion criteria.
Authors' conclusions
Implications for practice.
There are no completed randomised controlled trials of modest cooling for people with TBI. Therefore, the routine application of therapy aimed at maintaining body temperature between 35 ºC and 37.5 ºC cannot be recommended at present.
Implications for research.
Trials with cooling strategies aimed at reducing body temperature to between 35 ºC and 37.5 ºC in the first week after TBI are needed.
Ideally trials should be placebo‐controlled as it is important to answer the question of whether intervening to alter the natural history of body temperature after TBI has an effect on death and disability. As physical and drug therapies may have independent mechanisms of action a factorial design is possible.
What's new
| Date | Event | Description |
|---|---|---|
| 20 October 2014 | Amended | Minor edits made to the text to improve clarity. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 21 August 2014 | Amended | All declaration of interest statements from the authors are now included in the review. |
| 31 July 2014 | New search has been performed | The search for studies has been updated to 23 September 2013. One ongoing study was identified (NCT01231139), and its results will be included into the review by the end of 2015. |
| 31 July 2014 | New citation required but conclusions have not changed | The authors of the review have changed. |
| 5 September 2007 | New citation required and major changes | Protocol first published. |
Acknowledgements
The authors would like to thank the Cochrane Injuries Group Editorial Base, the Cochrane Renal Group and the authors of the previous Cochrane reviews 'Therapeutic hypothermia for head injury' (Alderson 2004) and 'Cooling therapy for acute stroke' (Den Hertog 2009). We are grateful to Dr Doris Lam for her help in translating literature from Chinese journals.
Appendices
Appendix 1. Search strategies
Cochrane Injuries Group Specialised Register
((((hypotherm* or cryotherap*) OR (temperature and reduc*) OR (“ice‐water lavage”) OR (cold or cool*)) and (blanket* or helmet* or hood* or fluid* or catheter* or "neck collar")) OR ((intravenous) and (cold or cool*) and (fluid* or catheter*))) AND (INREGISTER) [REFERENCE] [STANDARD]
Cochrane Central Register of Controlled Trials (CENTRAL)
#1MeSH descriptor: [Hypothermia] explode all trees #2MeSH descriptor: [Hypothermia, Induced] explode all trees #3MeSH descriptor: [Cryotherapy] explode all trees #4hypotherm* or cryotherap*:ti,ab,kw (Word variations have been searched) #5(cool* or cold) near/3 (therap* or device* or equipment):ti,ab,kw (Word variations have been searched) #6(temperature near/3 reduc*):ti,ab,kw (Word variations have been searched) #7intravenous near/3 (cold or cool*):ti,ab,kw (Word variations have been searched) #8fluid* or catheter*:ti,ab,kw (Word variations have been searched) #9#7 and #8 #10(cool* or cold*) near/3 (blanket* or neck collar* or helmet* or hood*):ti,ab,kw (Word variations have been searched) #11ice‐water lavage:ti,ab,kw (Word variations have been searched) #12(#1 or #2 or #3 or #4 or #5 or #6 or #9 or #10 or #11) #13MeSH descriptor: [Analgesics, Non‐Narcotic] explode all trees #14analgesic* near/3 (non‐narcotic* or non‐opioid*):ti,ab,kw (Word variations have been searched) #15MeSH descriptor: [Acetaminophen] explode all trees #16antipyretic*:ti,ab,kw (Word variations have been searched) #17Aspirin or Meclofenamate sodium or Niflumic acid or Tiaprofenic acid or Tolfenamic acid or Acemetacine or Naproxen or Diclofenac or Etodolac or Fentiazac or Etofenamato or Fenbufen or Fentiazac or Nimesulid or Flurbiprofen or Ibuprofen or Indomethacin or Sulindac or ketoprofen or Lonazolac or Piroxicam or Tenoxicam or Proglumetacina or acetaminophen or paracetamol or acetamidophenol or hydroxyacetanilide or apap or acamol or acephen or acetaco or acetaminophen or algotropyl or anacin‐3 or datril or n‐4‐hydroxyphenyl acetanilide or n‐acetyl‐p‐aminophenol or panadol or Tylenol or p‐acetamidophenol:ti,ab,kw (Word variations have been searched) #18MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees #19(analgesic* or non‐steroidal) near/3 (anti‐inflammator*):ti,ab,kw (Word variations have been searched) #20NSAID* or anti‐rheumatic* or aspirin‐like:ti,ab,kw (Word variations have been searched) #21(#13 or #14 or #15 or #16 or #17 or #18 or #19 or #20) #22MeSH descriptor: [Body Temperature] explode all trees #23MeSH descriptor: [Body Temperature Regulation] explode all trees #24body near/3 temperature:ti,ab,kw (Word variations have been searched) #25body near/3 (cool* or cold*):ti,ab,kw (Word variations have been searched) #26(#22 or #23 or #24 or #25) #27(#21 and #26) #28MeSH descriptor: [Craniocerebral Trauma] explode all trees #29MeSH descriptor: [Cerebrovascular Trauma] explode all trees #30MeSH descriptor: [Brain Edema] explode all trees #31MeSH descriptor: [Glasgow Coma Scale] explode all trees #32MeSH descriptor: [Glasgow Outcome Scale] explode all trees #33MeSH descriptor: [Unconsciousness] explode all trees #34glasgow near/3 scale*:ti,ab,kw (Word variations have been searched) #35Unconscious* or coma* or concuss* or 'persistent vegetative state':ti,ab,kw (Word variations have been searched) #36Rancho Los Amigos Scale:ti,ab,kw (Word variations have been searched) #37(head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) near/3 (injur* or trauma* or damag* or wound* or fracture* or contusion*):ti,ab,kw (Word variations have been searched) #38Diffuse axonal injur*:ti,ab,kw (Word variations have been searched) #39(#37 or #38) #40(#32 or #33 or #34 or #35 or #36) #41(#39 and #40) #42(#28 or #29 or #30 or #39 or #41)
MEDLINE (OvidSP) 1. exp CRANIOCEREBRAL TRAUMA/ 2. exp Cerebrovascular Trauma/ 3. exp BRAIN EDEMA/ 4. exp GLASGOW COMA SCALE/ 5. exp GLASGOW OUTCOME SCALE/ 6. exp UNCONSCIOUSNESS/ 7. (glasgow adj3 scale*).ab,ti. 8. (Unconscious* or coma* or concuss* or 'persistent vegetative state').ab,ti. 9. Rancho Los Amigos Scale.ab,ti. 10. ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) adj3 (injur* or trauma* or damag* or wound* or fracture* or contusion*)).ab,ti. 11. Diffuse axonal injur$.ab,ti. 12. 10 or 11 13. 4 or 5 or 6 or 7 or 8 or 9 14. 12 and 13 15. (therapy or surgery or rehabilitation or diet therapy or drug therapy or Prevention & Control).fs. 16. 12 and 15 17. 1 or 2 or 3 or 14 or 16 18. exp Hypothermia/ 19. exp Hypothermia, Induced/ 20. exp Cryotherapy/ 21. (hypotherm$ or cryotherap$).ab,ti. 22. ((cool* or cold) adj3 (therap* or device* or equipment)).ab,ti. 23. (temperature adj3 reduc*).ab,ti. 24. (intravenous adj3 (cold or cool*) adj3 (fluid* or catheter*)).ab,ti. 25. ((cool* or cold*) adj3 (blanket* or neck collar* or helmet* or hood*)).ab,ti. 26. ice‐water lavage.ab,ti. 27. or/18‐26 28. exp Analgesics, Non‐Narcotic/ 29. (analgesic* adj3 (non?narcotic* or non?opioid*)).ab,ti. 30. exp Acetaminophen/ 31. antipyretic$.ab,ti. 32. (Aspirin or Meclofenamate sodium or Niflumic acid or Tiaprofenic acid or Tolfenamic acid or Acemetacine or Naproxen or Diclofenac or Etodolac or Fentiazac or Etofenamato or Fenbufen or Fentiazac or Nimesulid or Flurbiprofen or Ibuprofen or Indomethacin or Sulindac or ketoprofen or Lonazolac or Piroxicam or Tenoxicam or Proglumetacina or acetaminophen or paracetamol or acetamidophenol or hydroxyacetanilide or apap or acamol or acephen or acetaco or acetaminophen or algotropyl or anacin‐3 or datril or n‐4‐hydroxyphenyl acetanilide or n‐acetyl‐p‐aminophenol or panadol or Tylenol or p‐acetamidophenol).ab,ti. 33. exp Anti‐Inflammatory Agents, Non‐Steroidal/ 34. ((analgesic* or non?steroidal) adj3 anti?inflammator*).ab,ti. 35. (NSAID* or anti?rheumatic* or aspirin‐like).ab,ti. 36. or/28‐35 37. exp Body Temperature/ 38. exp Body Temperature Regulation/ 39. (body adj3 temperature).ab,ti. 40. (body adj3 (cool* or cold*)).ab,ti. 41. or/37‐40 42. 36 and 41 43. 27 or 42 44. 17 and 43 45. randomi?ed.ab,ti. 46. randomized controlled trial.pt. 47. controlled clinical trial.pt. 48. placebo.ab. 49. clinical trials as topic.sh. 50. randomly.ab. 51. trial.ti. 52. or/45‐51 53. (animals not (humans and animals)).sh. 54. 52 not 53 55. 44 and 54
Embase (OvidSP) 1. exp Brain Injury/ 2. exp Brain Edema/ 3. exp Glasgow Coma Scale/ 4. exp Glasgow Outcome Scale/ 5. exp Rancho Los Amigos Scale/ 6. exp Unconsciousness/ 7. ((brain or cerebral or intracranial) adj3 (oedema or edema or swell*)).ab,ti. 8. ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) adj3 (injur* or trauma* or damag* or wound* or fracture* or contusion*)).ab,ti. 9. (Glasgow adj3 (coma or outcome) adj3 (scale* or score*)).ab,ti. 10. Rancho Los Amigos Scale.ab,ti. 11. (Unconscious* or coma* or concuss* or 'persistent vegetative state').ab,ti. 12. Diffuse axonal injur*.ab,ti. 13. ((head or crani* or cerebr* or brain* or intra‐cran* or inter‐cran*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressure)).ab,ti. 14. or/1‐13 15. exp INDUCED HYPOTHERMIA/ 16. exp HYPOTHERMIA/ 17. exp cryotherapy/ 18. (hypotherm$ or cryotherap$).ab,ti. 19. ((cool$ or cold) adj3 (therap$ or device$ or equipment)).ab,ti. 20. (temperature adj3 reduc$).ab,ti. 21. (intravenous adj3 (cold or cool$) adj3 (fluid$ or catheter$)).ab,ti. 22. ((cool$ or cold$) adj3 (blanket$ or neck collar$ or helmet$ or hood$)).ab,ti. 23. ice‐water lavage.ab,ti. 24. or/15‐23 25. (analgesic$ adj3 (non?narcotic$ or non?opioid$)).ab,ti. 26. exp Paracetamol/ 27. antipyretic$.ab,ti. 28. (Aspirin or Meclofenamate sodium or Niflumic acid or Tiaprofenic acid or Tolfenamic acid or Acemetacine or Naproxen or Diclofenac or Etodolac or Fentiazac or Etofenamato or Fenbufen or Fentiazac or Nimesulid or Flurbiprofen or Ibuprofen or Indomethacin or Sulindac or ketoprofen or Lonazolac or Piroxicam or Tenoxicam or Proglumetacina or acetaminophen or paracetamol or acetamidophenol or hydroxyacetanilide or apap or acamol or acephen or acetaco or acetaminophen or algotropyl or anacin‐3 or datril or n‐4‐hydroxyphenyl acetanilide or n‐acetyl‐p‐aminophenol or panadol or Tylenol or p‐acetamidophenol).ab,ti. 29. exp Nonsteroid Antiinflammatory Agent/ 30. exp Antipyretic Analgesic Agent/ 31. ((analgesic$ or non?steroidal) adj3 anti?inflammator$).ab,ti. 32. (NSAID$ or anti?rheumatic$ or aspirin‐like).ab,ti. 33. or/25‐32 34. exp body temperature/ 35. exp Thermoregulation/ 36. (body adj3 temperature).ab,ti. 37. (body adj3 (cool$ or cold$)).ab,ti. 38. or/34‐37 39. 33 and 38 40. 24 or 39 41. 14 and 40 42. exp Randomized Controlled Trial/ 43. exp controlled clinical trial/ 44. randomi?ed.ab,ti. 45. placebo.ab. 46. Clinical Trial/ 47. randomly.ab. 48. trial.ti. 49. or/42‐48 50. exp animal/ not (exp human/ and exp animal/) 51. 49 not 50 52. 41 and 51
ISI Web of Science (SCI‐EXPANDED; CPCI‐S)
#35 #34 AND #29 #34 #33 AND #32 #33 TS=((human*)) #32 #31 OR #30 #31 TS=((randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial)) OR TS=((controlled clinical trial OR controlled trial OR clinical trial OR placebo)) #30 TS=(((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*))) #29 #28 AND (#19 or #9) #28 #27 OR #25 #27 #26 AND #25 #26 #22 OR #21 OR #20 #25 #24 OR #23 #24 TS=("Diffuse axonal injur*") #23 TS=((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) near/3 (injur* or trauma* or damag* or wound* or fracture* or contusion*)) #22 TS=(Rancho Los Amigos Scale) #21 TS=(Unconscious* or coma* or concuss* or 'persistent vegetative state') #20 TS=(glasgow near/3 scale*) #19 #18 AND #15 #18 #17 OR #16 #17 TS=(body near/3 (cool* or cold*)) #16 TS=(body near/3 temperature) #15 #14 OR #13 OR #12 OR #11 OR #10 #14 TS=(NSAID* or anti‐rheumatic* or aspirin‐like) #13 TS=((analgesic* or non‐steroidal) near/3 (anti‐inflammator*)) #12 TS=(Aspirin or Meclofenamate sodium or Niflumic acid or Tiaprofenic acid or Tolfenamic acid or Acemetacine or Naproxen or Diclofenac or Etodolac or Fentiazac or Etofenamato or Fenbufen or Fentiazac or Nimesulid or Flurbiprofen or Ibuprofen or Indomethacin or Sulindac or ketoprofen or Lonazolac or Piroxicam or Tenoxicam or Proglumetacina or acetaminophen or paracetamol or acetamidophenol or hydroxyacetanilide or apap or acamol or acephen or acetaco or acetaminophen or algotropyl or anacin‐3 or datril or n‐4‐hydroxyphenyl acetanilide or n‐acetyl‐p‐aminophenol or panadol or Tylenol or p‐acetamidophenol) #11 TS=(antipyretic*) #10 TS=(analgesic* near/3 (non‐narcotic* or non‐opioid*)) #9 #8 OR #7 OR #6 OR #3 OR #2 OR #1 #8 TS=(ice‐water lavage) #7 TS=((cool* or cold*) near/3 (blanket* or "neck collar*" or helmet* or hood*)) #6 #5 AND #4 #5 TS=(fluid* or catheter*) #4 TS=(intravenous near/3 (cold or cool*)) #3 TS=((temperature near/3 reduc*)) #2 TS=((cool* or cold) near/3 (therap* or device* or equipment)) #1 TS=(hypotherm* or cryotherap*)
PubMed
((((((((((intravenous[title/abstract] AND (cold[title/abstract] OR cool*[title/abstract]) AND (fluid*[title/abstract] OR catheter*[title/abstract]))) OR ice‐water lavage[Title/Abstract]) OR ((temperature[Title/Abstract]) AND reduc*[Title/Abstract])) OR ((hypotherm*[Title/Abstract]) OR cryotherap*[Title/Abstract])) OR ((((((((blanket*[Title/Abstract]) OR helmet*[Title/Abstract]) OR hood*[Title/Abstract]) OR fluid*[Title/Abstract]) OR catheter*[Title/Abstract]) OR neck collar*[Title/Abstract])) AND ((cool*[Title/Abstract]) OR cold*[Title/Abstract])))) AND (((((((("Comparative Study"[Publication Type]) OR "Randomized Controlled Trial"[Publication Type]) OR "Controlled Clinical Trial"[Publication Type])) OR (((((((randomized[Title/Abstract]) OR randomised[Title/Abstract]) OR placebo[Title/Abstract]) OR randomly[Title/Abstract]) OR trial[Title/Abstract]) OR groups[Title/Abstract]) OR group[Title/Abstract]))) NOT (("Animals"[Mesh]) NOT ("Animals"[Mesh] AND "Humans"[Mesh]))))) AND ((((((((((((head[Title/Abstract]) OR crani*[Title/Abstract]) OR cerebr*[Title/Abstract]) OR capitis[Title/Abstract]) OR brain*[Title/Abstract]) OR forebrain*[Title/Abstract]) OR skull*[Title/Abstract]) OR hemispher*[Title/Abstract]) OR intracran*[Title/Abstract]) OR intercran*[Title/Abstract]) OR (((haematoma*[Title/Abstract] OR hematoma*[Title/Abstract] OR haemorrhag*[Title/Abstract] OR hemorrhag*[Title/Abstract] OR bleed*[Title/Abstract] OR pressur*[Title/Abstract])) AND (head[Title/Abstract] OR crani*[Title/Abstract] OR cerebr*[Title/Abstract] OR brain*[Title/Abstract] OR intra?cran*[Title/Abstract] OR inter?cran*[Title/Abstract] OR intracran*[Title/Abstract] OR intercran*[Title/Abstract])) OR (((glasgow outcome scale[Title/Abstract]) OR glasgow coma scale[Title/Abstract]) OR rancho los amigos scale[Title/Abstract]) OR ((diffuse axonal injury[Title/Abstract]) OR diffuse axonal injuries[Title/Abstract]) OR ((brain[Title/Abstract] OR cerebral[Title/Abstract] OR intracranial[Title/Abstract]) AND (oedema[Title/Abstract] OR edema[Title/Abstract] OR swell*[Title/Abstract])) OR ((unconscious*[Title/Abstract] OR coma*[Title/Abstract] OR concuss*[Title/Abstract]) AND (injur*[Title/Abstract] OR trauma*[Title/Abstract] OR damag*[Title/Abstract] OR wound*[Title/Abstract] OR fracture*[Title/Abstract] OR contusion*[Title/Abstract] OR haematoma*[Title/Abstract] OR hematoma*[Title/Abstract] OR haemorrhag*[Title/Abstract] OR hemorrhag*[Title/Abstract] OR pressur*[Title/Abstract])) OR (((Coma[Mesh])) AND (injur*[title/abstract] OR trauma*[title/abstract] OR damag*[title/abstract] OR wound*[title/abstract] OR fractur*[title/abstract] OR contusion*[title/abstract] OR haematoma*[title/abstract] OR hematoma*[title/abstract] OR haemorrhag*[title/abstract] OR hemorrhag*[title/abstract] OR pressur*[title/abstract] OR lesion*[title/abstract] OR destruction*[title/abstract] OR oedema*[title/abstract] OR edema*[title/abstract] OR contusion*[title/abstract] OR concus*[title/abstract])))))) AND publisher[sb]
Current Controlled Trials (http://www.controlled‐trials.com/mrct/) ((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) and (injur* or trauma* or damag* or wound* or fracture* or contusion*)) and (hypothermia or cooling)
ClinicalTrials.gov (http://www.clinicaltrials.gov) (head OR brain) AND (hypothermia OR cooling)
Appendix 2. Searches for previous versions
National Research Register 2007, issue 3 #1 MeSH descriptor Hypothermia explode all trees #2 MeSH descriptor Hypothermia, Induced explode all trees #3 MeSH descriptor Cryotherapy explode all trees #4 hypotherm* or cryotherap* #5 (cool* or cold) near3 (therap* or device* or equipment) #6 (temperature near3 reduc*) #7 intravenous near3 (cold or cool*) #8 fluid* or catheter* #9 (#7 AND #8) #10 (cool* or cold*) near3 (blanket* or neck collar* or helmet* or hood*) #11 ice‐water lavage #12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #9 OR #10 OR #11) #13 MeSH descriptor Analgesics, Non‐Narcotic explode all trees #14 analgesic* near3 (non‐narcotic* or non‐opioid*) #15 MeSH descriptor Acetaminophen explode all trees #16 antipyretic* #17 Aspirin or Meclofenamate sodium or Niflumic acid or Tiaprofenic acid or Tolfenamic acid or Acemetacine or Naproxen or Diclofenac or Etodolac or Fentiazac or Etofenamato or Fenbufen or Fentiazac or Nimesulid or Flurbiprofen or Ibuprofen or Indomethacin or Sulindac or ketoprofen or Lonazolac or Piroxicam or Tenoxicam or Proglumetacina or acetaminophen or paracetamol or acetamidophenol or hydroxyacetanilide or apap or acamol or acephen or acetaco or acetaminophen or algotropyl or anacin‐3 or datril or n‐4‐hydroxyphenyl acetanilide or n‐acetyl‐p‐aminophenol or panadol or Tylenol or p‐acetamidophenol #18 MeSH descriptor Anti‐Inflammatory Agents, Non‐Steroidal explode all trees #19 (analgesic* or non‐steroidal) near3 (anti‐inflammator*) #20 NSAID* or anti‐rheumatic* or aspirin‐like #21 (#13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20) #22 MeSH descriptor Body Temperature explode all trees #23 MeSH descriptor Body Temperature Regulation explode all trees #24 body near3 temperature #25 body near3 (cool* or cold*) #26 (#22 OR #23 OR #24 OR #25) #27 (#21 AND #26) #28 MeSH descriptor Craniocerebral Trauma explode all trees #29 MeSH descriptor Cerebrovascular Trauma explode all trees #30 MeSH descriptor Brain Edema explode all trees #31 MeSH descriptor Glasgow Coma Scale explode all trees #32 MeSH descriptor Glasgow Outcome Scale explode all trees #33 MeSH descriptor Unconsciousness explode all trees #34 glasgow near3 scale* #35 Unconscious* or coma* or concuss* or 'persistent vegetative state' #36 Rancho Los Amigos Scale #37 (head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) near3 (injur* or trauma* or damag* or wound* or fracture* or contusion*) #38 Diffuse axonal injur* #39 (#37 OR #38) #40 (#32 OR #33 OR #34 OR #35 OR #36) #41 (#39 AND #40) #42 (#28 OR #29 OR #30 OR #39 OR #41) #43 (#27 AND #42)
ZETOC (April 2008) head injur* cool* OR brain injur* cool* OR head injur* mild hypotherm* OR brain injur* mild hypotherm* OR head injur* modest hypotherm* OR brain injur* modest hypotherm*
Appendix 3. Organisations contacted
We contacted the following pharmaceutical companies on 9 December 2013:
Reckitt Benckiser (aspirin and ibuprofen) Merck Sharpe Dome (indomethacin and sulindac) Novartis (diclofenac) Mylan (meclofenamate sodium) Cayman (neflumic acid) Sanofi‐Aventis (ketoprofen and tiaprofenic acid) Galen (tolfenimic acid) Pharma 24 (acemetacine) Roche (naproxen and tenoxicam) Wyeth‐Lederle (etodolac, fentiazac and fenbufen) Helsinn healthcare (nimesulide) Pfizer (flurbiprofen and piroxicam) Rotta (proglumetacin) Johnson and Johnson (acetaminophan) Bristol‐Myers‐Squibb (Paracetmol)
We also contacted the following manufacturers of cooling equipment on 9 December 2013: Coolgard Cool line catheter (Alsius CA) Tyco healthcare (Warmtouch 5800) Gaymar USA (MTA 4702) Arizant (Polar Air™ Model 600 Air Cooling System) Cincinnati, Ohio (subzero) Medivance (arctic sun temperature management system)
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrews 2005 | Mixed patient cohort (9/15 TBI) Physiological end‐point (brain temperature) |
| Cormio 2007 | Mixed patient cohort (12/22 TBI) Physiological end‐point (body temperature) No placebo control group |
| Diringer 2004 | Mixed patient cohort (82/296 TBI) Physiological end‐point (body temperature) No placebo control group |
| Harris 2007 | Mixed patient cohort (8/12 TBI) Physiological end‐point (brain temperature) |
| Hinz 2007 | Mixed patient cohort (5/26 TBI) Physiological end‐point (body temperature) No placebo control group |
| Hoedemaekers 2007 | Physiological end‐point (body temperature) Mixed patient cohort (11/25 TBI) No placebo control group |
| Mayer 2001 | Mixed patient cohort (27/220 TBI) Physiological end‐point (body temperature) No placebo control group |
| Mayer 2004 | Mixed patient cohort (2/47 TBI) Physiological end‐point (body temperature) No placebo control group |
| Price 2003 | Mixed patient cohort (TBI number not specified, total 67) Physiological end‐point (body temperature) |
| Qiu 2006 | No intention‐to‐treat analysis was carried out. After reviewing the study we contacted the authors and they confirmed that they did not carry out an intention‐to‐treat analysis. Loss to follow up: 40 out of the 96 patients randomised did not have death or disability outcome data reported. |
| Qiu 2006a | Intervention: selective brain cooling to brain temperature of 33 ºC to 35 ºC |
| Wang 2004 | Intervention: selective brain cooling to brain temperature of 33 ºC to 35 ºC |
TBI = traumatic brain injury
Characteristics of ongoing studies [ordered by study ID]
NCT01231139.
| Trial name or title | The Paracetamol AfteR Traumatic Brain InjurY Study (PARITY) |
| Methods | Randomised Controlled Trial |
| Participants | Age > 18 and < 65 |
| Interventions | Intervention: Paracetamol dissolved in 0.9% Sodium Chloride. Intravenous paracetamol, 1 gram (100mls), administered over 30 minutes (every 4 hours for 3 days). Control: 0.9% Sodium Chloride. 100mls saline given intravenous over 30 minutes (every 4 hours for 3 days). |
| Outcomes | Primary outcome: Core body temperature: bladder temperature probe (Time Frame: 30 minutes after final dose of study drug has been administered) Secondary outcome measures: (1) Blood Pressure (systolic and mean arterial pressure) measured by intra‐arterial pressure monitor (Time Frame: 6 hourly during study treatment) (2) Liver function test (Time Frame: daily from first dose of study treatment to the 7th day) (3) Serum Paracetamol levels (blood analysis) after a single dose of study drug and after final dose of study drug. (Time Frame: baseline, 30, 45, 90, 240 minutes after single dose. 240 minutes after final dose of study drug) (4) Temperature (bladder and tympanic) (Time Frame: Hourly from first study drug treatment until 4 hours after final study drug treatment) (Time‐weighted mean, area under the 3‐day temperature curve, daily maximum and minimum temperature) (5) The use of physical cooling interventions (Time Frame: hourly during the period of study intervention) (6) Intracranial pressure (Time Frame: 6 hourly during the period of study intervention) (Mean daily intracranial pressure for day 1, 2 and 3.) (7) Incidence of cerebral hypoperfusion (Time Frame: During study intervention period) (8) Systolic blood pressure < 90 mmHg or Mean arterial pressure < 50 mmHg for > 15 minutes Cerebral perfusion pressure < 50 mmHg for > 15 minutes |
| Starting date | October 2010 |
| Contact information | Manoj Saxena m.saxena@unsw.edu.au Parisa Glass pglass@george.org.au |
| Notes | As of 1 August 2014, the study has completed and the results will be included in the review within one year. |
Contributions of authors
Dr Manoj Saxena: conception and design of the review; development of all stages of the review; drafting the review and revising the review according to comments; contact with pharmaceutical companies; final version to be published.
Naomi Hammond and Kiran Deol: assessing the trials for inclusion criteria and extracting data; drafting and revising the review.
Andrew Cheng: assessing the trials for inclusion and extracting data (original review), drafting and revising the review.
Professor Peter Andrews: conception of the review; assessing the trials for inclusion criteria; drafting and revising the review.
Sources of support
Internal sources
-
Professor Peter Andrews, UK.
Supported by the University of Edinburgh
External sources
No sources of support supplied
Declarations of interest
Dr Manoj Saxena: I am engaged in an investigator‐initiated research program to answer the question of whether normothermia reduces death and disability after acute neurological illnesses (PARITY Study).
Naomi Hammond: None known.
Kiran Deol: I am an Intensive Care Doctor involved in the treatment of patients with head injury. I am also involved in other academic research in the area of head injury.
Andrew Cheng: None known.
Professor Peter Andrews: I am chief investigator of an international clinical trial of titrated therapeutic hypothermia to reduce intracranial pressure after traumatic brain injury (NIHR HTA Funded).
Edited (no change to conclusions)
References
References to studies excluded from this review
Andrews 2005 {published data only}
- Andrews PJ, Harris B, Murray GD. Randomized controlled trial of effects of the airflow through the upper respiratory tract of intubated brain‐injured patients on brain temperature and selective brain cooling. British Journal of Anaesthesia 2005;94(3):330‐5. [DOI] [PubMed] [Google Scholar]
Cormio 2007 {published data only}
- Cormio M, Citerio G. Continuous low dose diclofenac sodium infusion to control fever in neurosurgical critical care. Neurocritical Care 2007;6(2):82‐9. [DOI] [PubMed] [Google Scholar]
Diringer 2004 {published data only}
- Diringer MN for the Neurocritical Care Fever Reduction Trial Group. Treatment of fever in the neurologic intensive care unit with a catheter‐based heat exchange system. Critical Care Medicine 2004;32(2):559‐64. [DOI] [PubMed] [Google Scholar]
Harris 2007 {published data only}
- Harris BA, Andrews PJ, Murray GD. Enhanced upper respiratory tract airflow and head fanning reduce brain temperature in brain‐injured, mechanically ventilated patients: a randomized, crossover, factorial trial. British Journal of Anaesthesia 2007;98(1):93‐9. [DOI] [PubMed] [Google Scholar]
Hinz 2007 {published data only}
- Hinz J, Rosmus M, Popov A, Moerer O, Frerichs I, Quintel M. Effectiveness of an intravascular cooling method compared with a conventional cooling technique in neurologic patients. Journal of Neurosurgical Anesthesiology 2007;19(2):130‐5. [DOI] [PubMed] [Google Scholar]
Hoedemaekers 2007 {published data only}
- Hoedemaekers CW, Ezzahti M, Gerritsen A, Hoeven J. Comparison of cooling methods to induce and maintain normo‐ and hypothermia in intensive care unit patients: a prospective intervention study. Critical Care 2007;11:R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayer 2001 {published data only}
- Mayer SA, Commichau C, Scarmeas N, Presciutti M, Bates J, Copeland D. Clinical trial of an air‐circulating cooling blanket for fever control in critically ill neurologic patients. Neurology 2001;56(3):292‐8. [DOI] [PubMed] [Google Scholar]
Mayer 2004 {published data only}
- Mayer SA, Kowalski RG, Presciutti M, Osiapkovich ND, McGann E, Fitzsimmons BF, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Critical Care Medicine 2004;32(12):2508‐15. [DOI] [PubMed] [Google Scholar]
Price 2003 {published data only}
- Price T, McGloin S, Izzard J, Gilchrist M. Cooling strategies for patients with severe cerebral insult in ICU (Part 2). Nursing in Critical Care 2003;8(1):37‐45. [DOI] [PubMed] [Google Scholar]
Qiu 2006 {published and unpublished data}
- Qiu WS, Wang WM, Du HY, Liu W, Shen H, Shen L, et al. Thrombocytopaenia after therapeutic hypothermia in severe traumatic brain injury. Chinese Journal of Traumatology 2006;9(4):238‐41. [PubMed] [Google Scholar]
Qiu 2006a {published data only (unpublished sought but not used)}
- Qiu W, Shen H, Zhang Y, Wang W, Liu W, Jiang Q, et al. Non invasive selective brain cooling by head and neck cooling is protective in severe traumatic brain injury. Journal of Clinical Neurosciences 2006;13:995‐1000. [DOI] [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang H, Olivero W, Lanzino G, Elkins W, Rose J, Honings D, et al. Rapid and selective cerebral hypothermia achieved using a cooling helmet. Journal of Neurosurgery 2004;100:272‐7. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01231139 {published data only}
- The Paracetamol AfteR Traumatic Brain InjurY Study (PARITY). Ongoing study October 2010.
Additional references
Alderson 2004
- Alderson P, Gadkary C, Signorini DF. Therapeutic hypothermia for head injury. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD001048.pub2] [DOI] [PubMed] [Google Scholar]
Alexander 1992
- Alexander E Jr. Global Spine and Head Injury Prevention Project (SHIP). Surgical Neurology 1992;38:478‐9. [DOI] [PubMed] [Google Scholar]
Andrews 2006
- Andrews PJD, Anderson EL, Saxena MK. Cooling therapies after neuronal injury: direct brain cooling and systemic hypothermia. Intensive Care Yearbook 2006.
BTF 2007
- Brain Trauma Foundation. Guidelines for the management of severe traumatic brain injury. Journal of Neurotrauma 2007;24:s1‐117. [DOI] [PubMed] [Google Scholar]
Chatzipanteli 2007
- Chatzipanteli K, Alonso OF, Kraydieh S, Dietrich WD. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immunocytochemical studies. Journal of Cerebral Blood Flow & Metabolism 2000;20:531‐42. [DOI] [PubMed] [Google Scholar]
Den Hertog 2009
- Hertog HM, Worp HB, Tseng MC, Dippel DWJ. Cooling therapy for acute stroke. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001247.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dietrich 1996
- Dietrich WD, Alonso O, Halley M, Busto R. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery 1996;38:533‐41. [DOI] [PubMed] [Google Scholar]
Geffroy 2004
- Geffroy A, Bronchard R, Merckx P, et al. Severe traumatic head injury in adults: which patients are at risk of early hyperthermia?. Intensive Care Medicine 2004;30:785‐90. [DOI] [PubMed] [Google Scholar]
Gluckman 2005
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, David Edwards A, Ferriero M, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663‐70. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jiang 2002
- Jiang JY, Gao GY, Li WP, Yu MK, Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. Journal of Neurotrauma 2002;19:869‐74. [DOI] [PubMed] [Google Scholar]
Jones 1994
- Jones P, Andrews P, Midgley S. Measuring the burden of secondary insults in head‐injured patients during intensive care. Journal of Neurosurgical Anesthesiology 1994;6:4‐14. [PubMed] [Google Scholar]
Li 2012
- Li J, Jiang JY. Chinese Head Trauma Databank: effect of hyperthermia on the outcome of acute head trauma patients. Journal of Neurotrauma 2012;29:96‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathers 2001
- Mathers CD, Lopez AD, Murray CJL. The burden of disease and mortality by condition: data, methods and results from 2001. (Chapter 3). The burden of disease and risk factors. New York: Oxford University Press, 2006. [PubMed] [Google Scholar]
Stocchetti N
- Stocchetti N, Rossi S, Zanier ER, et al. Pyrexia in head‐injured patients admitted to intensive care. Intensive Care Med 2002;28:1555‐62. [DOI] [PubMed] [Google Scholar]
Sydenham 2009
- Sydenham E, Roberts R, Alderson O. Hypothermia for traumatic head injury. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD001048.pub4] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Saxena 2007
- Saxena M, Andrews PJD, Cheng A. Modest temperature reduction for traumatic brain injury. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006811] [DOI] [Google Scholar]


