Abstract
Background
Depression occurs frequently in patients with coronary artery disease (CAD) and is associated with a poor prognosis.
Objectives
To determine the effects of psychological and pharmacological interventions for depression in CAD patients with comorbid depression.
Search methods
CENTRAL, DARE, HTA and EED on The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL, ISRCTN Register and CardioSource Registry were searched. Reference lists of included randomised controlled trials (RCTs) were examined and primary authors contacted. No language restrictions were applied.
Selection criteria
RCTs investigating psychological and pharmacological interventions for depression in adults with CAD and comorbid depression were included. Primary outcomes were depression, mortality and cardiac events. Secondary outcomes were healthcare costs and health‐related quality of life (QoL).
Data collection and analysis
Two reviewers independently examined the identified papers for inclusion and extracted data from included studies. Random effects model meta‐analyses were performed to compute overall estimates of treatment outcomes.
Main results
The database search identified 3,253 references. Sixteen trials fulfilled the inclusion criteria. Psychological interventions show a small beneficial effect on depression compared to usual care (range of SMD of depression scores across trials and time frames: ‐0.81;0.12). Based on one trial per outcome, no beneficial effects on mortality rates, cardiac events, cardiovascular hospitalizations and QoL were found, except for the psychosocial dimension of QoL. Furthermore, no differences on treatment outcomes were found between the varying psychological approaches. The review provides evidence of a small beneficial effect of pharmacological interventions with selective serotonin reuptake inhibitors (SSRIs) compared to placebo on depression outcomes (pooled SMD of short term depression change scores: ‐0.24 [‐0.38,‐0.09]; pooled OR of short term depression remission: 1.80 [1.18,2.74]). Based on one to three trials per outcome, no beneficial effects regarding mortality, cardiac events and QoL were found. Hospitalization rates (pooled OR of three trials: 0.58 [0.39,0.85] and emergency room visits (OR of one trial: 0.58 [0.34,1.00]) were reduced in trials of pharmacological interventions compared to placebo. No evidence of a superior effect of Paroxetine (SSRI) versus Nortriptyline (TCA) regarding depression outcomes was found in one trial.
Authors' conclusions
Psychological interventions and pharmacological interventions with SSRIs may have a small yet clinically meaningful effect on depression outcomes in CAD patients. No beneficial effects on the reduction of mortality rates and cardiac events were found. Overall, however, the evidence is sparse due to the low number of high quality trials per outcome and the heterogeneity of examined populations and interventions.
Plain language summary
Treatments for depression in patients with coronary artery disease
This review examined clinical trials on psychological treatments and antidepressant drugs in depressed patients with coronary artery disease. The objective was to determine the effects of these treatments on depression, death rates, cardiac events such as another heart attack or surgeries, healthcare costs and quality of life. Sixteen trials were identified as relevant for the review. Seven trials investigated psychological treatments, eight trials antidepressant medications and one trial comprised both psychological and drug treatments. Psychological treatments and antidepressant drugs proved to be slightly superior to usual care or placebo (inactive drug) with regard to depressive symptoms. Furthermore, antidepressant drugs might be superior to placebo in reducing subsequent hospitalization rates and emergency room visits. In contrast, there seems to be no positive effect on death rates and cardiac events. Results regarding quality of life are inconclusive. In summary, psychological treatments and antidepressant medications may have a small yet positive effect on depression outcomes in CAD patients. However, the evidence is sparse due to the low number of trials.
Background
Coronary artery disease (CAD) is the single leading cause of death for both men and women in developed countries (Budde 2005). Similar to other chronic diseases (Baumeister 2007; Härter 2007b), a strong association between CAD and comorbid depression has been consistently reported (Baumeister 2010a; Härter 2007b; Ormel 2007; Rudisch 2003; Thombs 2006). Results from the World Mental Health Survey (Ormel 2007) indicate a twofold increased risk of depression for patients with heart disease compared to patients without heart disease. Rudisch and Nemeroff (Rudisch 2003) reported prevalence rates for depression in CAD ranging from 17% to 27%. Depending on the assessment method the prevalence rates for depression in myocardial infarction vary between 15.5% and 31.1% (Thombs 2006). Four months after discharge most patients still suffer from depressive symptoms (Thombs 2006).
The increased prevalence rates raise the issue of the impact of comorbid depression on the patients´ life and the health care system. Recent original studies and systematic reviews document a significant prognostic association between comorbid depression and increased mortality, morbidity and health care costs as well as diminished quality of life and adherence to treatment regimen (Barth 2004; Baumeister 2005; Baumeister 2011a; Frasure‐Smith 2000; Frasure‐Smith 2003; Herrmann‐Lingen 2006; Ziegelstein 2000).
Description of the condition
Coronary artery disease (CAD) is one of the most common forms of heart disease. One of the main underlying problems in cardiovascular disease is atherosclerosis, a process that plugs blood vessels with deposits of fat, cholesterol and other substances (WHO 1992). It is most serious when it restricts the blood supply to the heart (myocardial ischemia). Clinical manifestations of CAD are acute coronary syndrome comprising myocardial infarction (MI) and unstable angina (Antman 2004) as well as stable angina pectoris (Fox 2006). MI refers to what is commonly known as a "heart attack". It occurs when prolonged myocardial ischemia leads to myocardial cell death (necrosis) (Alpert 2000).
Depression is an emotional state characterised by strong feelings of sadness, worthlessness and guilt, withdrawal from others, sleeplessness and loss of appetite, sexual desire and interest in usual activities (Davison 2003). Depressive disorders can be reliably diagnosed through structured clinical interviews. The severity of depressive symptoms is usually assessed by patient‐ or clinician‐administered validated rating scales. Cut‐off scores have been validated for these scales which correspond to the likelihood of an indication of depression (Sadock 2009). Recommendations for the assessment of depression in patients with cardiovascular disease are available (Davidson 2006).
Description of the intervention
Psychological interventions comprise Cognitive Behavioural Therapy, Psychodynamic Psychotherapy, Interpersonal Psychotherapy, other approaches such as Non‐directive or Supportive Therapy and Counselling as well as single techniques of these interventions (Davison 2003). The mode of delivery comprises individual, group or family (including couple) therapy carried out by a health care professional. A comparative meta‐analysis of 53 psychotherapy studies in adults with depression Cuijpers 2008a conclude that most approaches are equally effective, with the exception of a small beneficial effect of Interpersonal Psychotherapy and a slightly lower effect of Non‐directive Supportive Therapy.
Antidepressant drugs are commonly used treatments in depressed patients. In general, the available medications do not differ in their overall efficacy and effectiveness, but differ substantially regarding short‐ and long‐term side effects (NICE 2009; Sadock 2009). Antidepressant treatment selection depends on the type of depressive disorder and the presence of comorbid somatic or mental disorders. Among many different drugs, main pharmacological classes of antidepressant medications are selective serotonin re‐uptake inhibitors (SSRIs), serotonin‐norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs). For CAD patients with moderate, severe or recurrent depression, SSRIs are viewed as safe and effective pharmacological agents (Lichtman 2008), while TCAs and MAOIs are viewed as contraindicated in these patients because of their cardiac side effects (Lichtman 2008).
How the intervention might work
Many biological and behavioural mechanisms linking CAD and depression are proposed (Härter 2007a; Joynt 2003; Musselman 1998; Skala 2006), comprising pathophysiological pathways such as decreased heart rate variability, platelet activation and endothelial dysfunction (Antman 2004) in depressed CAD patients. Furthermore, an accumulation of behavioural risk factors (smoking, physical inactivity and imbalanced diet) and comorbid medical disorders (hypertension, diabetes and obesity) in depressed patients might affect the development and course of CAD (Joynt 2003; Whooley 2008). Psychosocial stress constitutes a risk factor for both CAD and depression (Joynt 2003).
A recent review concludes that pharmacological interventions for depression might influence physiological pathways linking depression and CAD (Skala 2006). Psychological treatments may also affect physiological processes, but the interrelations between behavioural and physiological mechanisms remain unclear (Skala 2006). Psychological interventions might not only improve depression outcomes in CAD patients with comorbid depressive disorder, but may also improve medical outcome parameters by encouraging behaviour changes towards a healthier lifestyle in these patients (Rees 2004).
Why it is important to do this review
With regard to the high prevalence rates and the impact of comorbid depression on both medical and psychosocial outcomes, there is a need for effective depression treatments in CAD. In various systematic reviews, psychological and psychopharmacological interventions have proven to be effective interventions for the treatment of major depression in general (Cuijpers 2008a; Cuijpers 2008b; NICE 2009; Sadock 2009). However, as yet, there has been no systematic review on psychological and pharmacological interventions on the effects of depression treatment in CAD patients with comorbid depressive disorders.
A Cochrane review examined the effects of non‐specific psychological interventions in CAD patients in general (Rees 2004), indicating small reductions in depression and anxiety symptoms, but no significant effects of psychological interventions on all‐cause or cardiac mortality. However, the review did not study the effects of depression‐specific treatment in the population of CAD patients with comorbid depressive disorder. Furthermore, the review included non‐specific psychological interventions (mainly stress management), whereas the focus of our review is on depression‐specific psychological or pharmacological interventions explicitly used for treating depression. Some RCTs may be included in both reviews, but the research questions remain different owing to the focus of our review on the effects of depression treatments in depressed CAD patients.
The review will allow conclusions on the effects of depression treatment in CAD patients with comorbid depressive disorders. Depending on the number of primary studies, conclusions may be drawn concerning differential effects of type of intervention on depression and mortality or non‐fatal cardiac events, as well as on patients’ quality of life, thus providing a basis for treatment recommendations. Furthermore, follow‐up data may be examined concerning health care costs of the interventions. Sources of heterogeneity in the results of the primary studies can be explored and could help to provide suggestions for the design of future studies.
Objectives
To determine the effects of psychological and pharmacological interventions for depression in CAD patients with comorbid depressive disorder.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled clinical trials (RCTs) of any length of treatment and any length of follow‐up.
Types of participants
Adults (18 years or older) with CAD (ICD‐10: I20‐I25 (WHO 1992)) and comorbid depressive disorder (ICD‐10: F32/33/34.1 (WHO 1992); DSM‐IV: 296.xx; 300.4 (APA 1994); including subthreshold conditions) assessed by standardized interviews, self‐reports, medical records or physicians´ diagnosis. Inclusion of primary studies was not further limited to specific clinical subgroups in order to increase the generalisability of the results of the review.
With regard to comorbid depression, studies comprising mixed study samples (e.g. both depressed CAD patients and CAD patients with low social support (ENRICHD 2003)) were included in the review (see Subgroup analysis and investigation of heterogeneity).
Types of interventions
Psychological interventions comprise Cognitive Behavioural Therapy, Psychodynamic Psychotherapy, Interpersonal Psychotherapy, other approaches such as Non‐directive or Supportive Therapy and Counselling (Davison 2003). The mode of delivery was defined as individual, group or family (including couple) therapy carried out by a health care professional. The comparison group was 'no intervention' or 'usual care'. With regard to differential or incremental effects of different treatment approaches, trials with a control group receiving pharmacological treatment or another psychological treatment were also considered.
Pharmacological interventions included all antidepressant medications and other drug therapies used explicitly for treating depressive disorders (Sadock 2009). The control group was placebo. Pharmacological treatments compared to other pharmacological medications or psychological interventions were included to determine differential or incremental effects.
Types of outcome measures
Primary outcomes
Primary outcomes are depression (measured either dimensionally or categorically) following the intervention, as assessed by validated self‐report questionnaires or standardized interviews, all‐cause and CAD‐related mortality as well as non‐fatal cardiac events or surgery (e.g. coronary artery bypass graft (CABG), percutaneous transluminal coronary angioplasty (PTCA)).
Secondary outcomes
Secondary outcomes are health care costs or resource utilization and health‐related quality of life (QoL).
Search methods for identification of studies
Electronic searches
The following databases were searched for RCTs of treatment of depressive disorders in CAD patients on 15 July 2009:
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 2, 2009),
MEDLINE on OVID (1950 to July Week 2 2009),
EMBASE on OVID (1980 to 2009 Week 27),
PsycINFO on OVID (1806 to July Week 1 2009),
CINAHL on EBSCO (1982 to July 2009)
Database of Abstracts of Reviews of Effects (DARE), NHS Economic Evaluation Database (EED) and the Health Technology Assessment Database (HTA) (The Cochrane Library Issue 2, 2009).
RCT filters were used for MEDLINE (Higgins 2008) and EMBASE (Lefebvre 1996). Adaptations of these have been applied to the other databases. See Appendix 1 for details of the search strategies. No language restrictions were applied.
Searching other resources
The International Standard Randomized Controlled Trial Number register (ISRCTN, http://isrctn.org/) and CardioSource Registry of Randomized Cardiovascular Clinical Trials (http://www.cardiosource.com) were searched.
In addition, reference lists of all references of included trials were examined to identify other potentially relevant studies. All corresponding authors of included trials were contacted and asked about other RCTs, published or unpublished, which might be relevant to the review.
Data collection and analysis
Selection of studies
Two review authors (HB and NH) independently selected relevant studies for inclusion. A list of titles and abstracts were examined. If title and abstract contained sufficient information to determine exclusion, the article was rejected. The full papers of all remaining articles were retrieved and then reviewed by two authors (HB and NH) independently. In addition, all other potentially relevant articles identified by checking the reference lists or personal communications were also reviewed. A record of all rejected papers and the reasons for rejection was kept. Important parts of foreign‐language papers of included studies (i.e. not English or German) were translated into English. If the two review authors disagreed about the inclusion of an article, a third reviewer (JB) was asked to review the article. Disagreements were solved by consensus discussion.
Data extraction and management
Data from full copies of primary studies were independently extracted by two review authors (HB and NH) using a data extraction form. Study characteristics including participants (sample size at baseline and follow‐up, type of CAD, gender, age), type of depression (major depression, minor depression or dysthymic disorder), assessment method (standardized diagnostic interview, self‐report questionnaire, medical record or physician’s diagnosis), cut‐off used to indicate depression on self‐report questionnaire, type of intervention (type of psychological treatment versus type of pharmacological treatment), comparison group (no intervention, usual care, another psychological treatment or pharmacological treatment), length of follow‐up, descriptive statistics of primary and secondary outcomes, effect sizes and confidence intervals were extracted.
Assessment of risk of bias in included studies
Two review authors (HB and NH) independently assessed the risk of bias in included studies using the Cochrane Collaboration's tool for assessing risk of bias (Higgins 2008). Sequence generation, allocation concealment, selective outcome reporting and other sources of bias were described. With regard to psychological interventions, blinding of health care providers or patients concerning the treatment is not feasible. Trials of psychological interventions were therefore only evaluated regarding the blinding of the outcome assessors. In pharmacological trials blinding is possible for patients, personnel and outcome assessor and was evaluated accordingly.
Measures of treatment effect
Standardized mean differences (SMD) with 95% confidence intervals were computed for all continuous outcomes. Primary studies reported depression either as mean change scores from baseline to final assessment or as mean scores of final assessments. Mean differences based on change scores from baseline to final assessment can be assumed to represent the same intervention effects as mean differences based on final assessments in RCTs. However, since the standard deviations of final mean scores and mean change scores differ depending on the reliability of the measurements, final mean scores and mean change scores cannot be combined as SMD. Hence, meta‐analyses of final depression mean scores and mean depression change scores are reported separately. For dichotomous variables odds ratios (OR) with 95% confidence interval were computed.
Unit of analysis issues
The unit of analysis in the primary studies is the patient, which is randomized to either the treatment or the control group. Thus, the number of observations matches the number of units that are randomized. Multiple observations in primary studies as well as heterogeneity concerning follow‐up length between studies were analysed using different time frames, which reflect short‐term (end of treatment), medium‐term (1‐6 months after end of treatment) and long‐term (>6 months after end of treatment) follow‐up.
Dealing with missing data
Missing information from published RCTs was requested from the corresponding authors. Of fourteen authors contacted because of missing data, four replied, and three were able to provide at least some of the requested data. No imputation methods were used due to the small amount of trials per outcome.
Assessment of heterogeneity
Heterogeneity was tested for statistical significance by using the Q‐statistics with a 95% confidence interval. To examine the extent of heterogeneity, I2 was computed.
Assessment of reporting biases
Funnel plots to investigate reporting bias were not created and not tested for asymmetry due to the small amount of trials per outcome. To examine outcome reporting bias, discrepancies in reported outcomes between published protocols and original papers were analysed. Where no protocol was available, corresponding trial authors were asked for published or unpublished protocols.
Data synthesis
Random effects meta‐analyses were performed to compute overall estimates of treatment outcomes. The effect sizes of the primary studies are presented in forest plots.
Many trials used more than one tool to assess depression outcomes. Thus, we used a hierarchical approach to decide which assessment to use in the meta‐analyses. Clinician‐rated assessments were given priority over patient self‐report questionnaires, since they met the requirement for a blinded outcome assessment. If no clinician‐rated depression assessment was available, data from patient self‐report questionnaires were used.
Subgroup analysis and investigation of heterogeneity
The treatment effects were evaluated separately for the subgroups of pharmacological and psychological interventions. Subgroup analyses to determine the impact of varying study samples (e.g. depressed CAD patients vs. mixed study samples of patients with depression and/or anxiety) on results were planned but not conducted, due to the small amount of trials per outcome.
Sensitivity analysis
Sensitivity analyses to examine the impact of sex (men versus women), CAD subtype, assessment of depression diagnosis (self‐report questionnaires versus standardized diagnostic interviews), time of onset of depression (pre‐existing versus new‐onset depression), CAD severity and risk of bias of included studies on results were planned but not conducted, due to the small amount of trials per outcome.
Results
Description of studies
See: Characteristics of included studies; Table 5; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
1. Overview of study population.
| Study ID | Intervention | [n] screened | [n] randomised | [n] ITT | [n] finishing study | [%] of randomised participants finishing study | comments |
| Barth 2005 | Intervention (I): Resource‐orientated Psychotherapy Control (C): Usual Care |
Total: 1709 | I: 27 C: 32 Total: 59 |
I: 27 C: 32 Total: 59 |
I: 27 C: 28 Total: 55 |
I: 100% C: 87.5% Total: 93.2% |
|
| Brown 1993 | Intervention (I): Behavior Therapy Control (C): Person‐centered Therapy |
Total: 107 | I: NR C: NR Total: 54 |
I: NR C: NR Total: NR |
I: 20 C: 20 Total: 40 |
I: ? C: ? Total: 74.1% |
|
| CREATE 2007 | Intervention 1 (I1): Interpersonal Psychotherapy Intervention 2 (I2): Citalopram Control 1 (C1): Clinical management Control 2 (C2): Placebo |
Total: 1897 | I1: 142 I2: 142 C1: 142 C2: 142 Total: 284 |
I1: 142 I2: 142 C1: 142 C2: 142 Total: 284 |
I1: 118 I2: 124 C1: 112 C2: 106 Total: 230 |
I1: 83.1% I2: 87.3% C1: 78.9% C2: 74.6% Total: 81.0% |
2 X 2 factorial trial |
| Doering 2007 | Intervention (I): Cognitive Behavioral Therapy Control (C): Usual care |
Total: 117 | I: NR C: NR Total: NR |
I: NR C: NR Total: NR |
I: 7 C: 8 Total: 15 |
I: ? C: ? Total: ? |
|
| ENRICHD 2003 | Intervention (I): Cognitive Behavior Therapy Control (C): Usual Care |
Total: 33780 | I: 1238 C: 1243 Total: 2481 |
I: 1238 C: 1243 Total: 2481 |
I: 983 C: 985 Total: 1968 |
I: 79.4% C: 79.2% Total: 79.3% |
|
| Fang 2003 | Intervention (I): Health Education and Psychological Intervention Control (C): Usual care |
Total: ? | I: 27 C: 30 Total: 57 |
I: C: Total: |
I: C: Total: |
I: C: Total: |
|
| Freedland 2009 | Intervention 1 (I1): Cognitive Behavior Therapy Intervention 2 (I2): Supportive Stress Management Control (C): Usual Care for depression |
Total: 2955 | I1: 41 I2: 42 C1: 40 Total: 123 |
I1: 41 I2: 42 C1: 40 Total: 123 |
I1: 40 I2: 33 C1: ? Total: ? |
I1: 98% I2: 79% C1: ? Total: ? |
|
| Freeman 1986 | Intervention (I): Alprazolam Control (C): Placebo |
Total: 459 | I: 54 C: 53 Total: 107 |
I: NR C: NR Total: NR |
I: 32 C: 28 Total: 60 |
I: 59.3% C: 52.8% Total: 56.1% |
|
| Li 2005 | Intervention (I): St. John's Wort extract Control (C): Placebo |
Total: ? | I: ? C: ? Total: 87 |
I: ? C: ? Total: ? |
I: 43 C: 39 Total: 82 |
I: ? C: ? Total: 94.3% |
|
| Liu 1999 | Intervention (I): Fluoxetine Control (C): Placebo |
Total: ? | I: ? C: ? Total: ? |
I: ? C: ? Total: ? |
I: 31 C: 37 Total: ? |
I: ? C: ? Total: ? |
|
| McFarlane 2001 | Intervention (I): Sertraline Control (C): Placebo |
Total: 238 | I: 18 C: 20 Total: 38 |
I: NR C: NR Total: NR |
I: 12 C: 15 Total: 27 |
I: 66.7% C: 75.0% Total: 71.1% |
|
| McLaughlin 2005 | Intervention (I1): Telephone counseling Control (C): Usual care |
Total: 700 | I: 53 C: 47 Total: 100 |
I: NR C: NR Total: NR |
I: 45 C: 34 Total: 79 |
I: 84.9% C: 72.3% Total: 79% |
|
| MIND‐IT 2007 | Intervention (I): Mirtazapine Control (C): Placebo |
Total: 2177 | I: 47 C: 44 Total: 91 |
I: 47 C: 44 Total: 91 |
I: 22 C: 18 Total: 40 |
I: 46.8% C: 40.9% Total: 44.0% |
|
| Roose 1998 | Intervention 1 (I1): Paroxetine Intervention 2 (I2): Nortriptyline |
Total: NR | I1: 41 I2: 40 Total: 81 |
I1: 41 I2: 40 Total: 81 |
I1: 37 I2: 30 Total: 67 |
I1: 90.2% I2: 75.0% Total: 82.7% |
|
| SADHART 2002 | Intervention (I): Sertraline Control (C): Placebo |
Total: 11546 | I: 186 C: 183 Total: 369 |
I: 186 C: 183 Total: 169 |
I: 133 C: 137 Total: 270 |
I: 71.5% C: 74.9% Total: 73.1% |
|
| Strik 2000 | Intervention (I): Fluoxetine Control (C): Placebo |
Total: 556 | I: 27 C: 27 Total: 54 |
I: 27 C: 27 Total: 54 |
I: 22 C: 18 Total: 40 |
I: 81.5% C: 66.7% Total: 74.1% |
ITT = intention‐to‐treat; NR = not reported; ? = unclear
Results of the search
The database search resulted in 3253 references. See the study flowchart for details of the study selection process (Figure 1).
1.
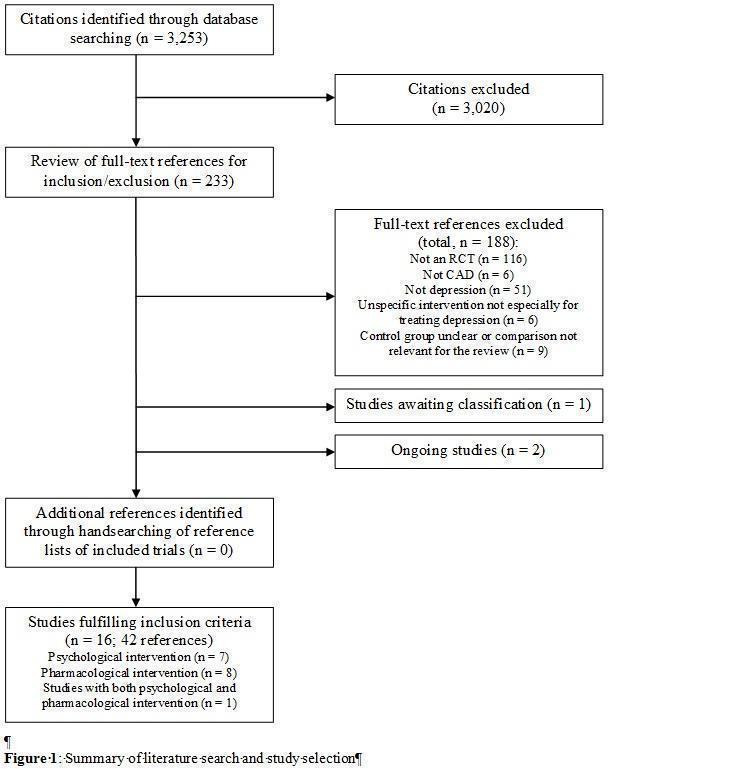
Included studies
Sixteen trials fulfilled the inclusion criteria (Barth 2005; Brown 1993; CREATE 2007; ENRICHD 2003; Doering 2007; Fang 2003; Freedland 2009; Freeman 1986; Li 2005; Liu 1999; McFarlane 2001; McLaughlin 2005; MIND‐IT 2007; Roose 1998; SADHART 2002; Strik 2000).
Seven of the included trials investigated psychological interventions comprising Cognitive Behaviour Therapy (Brown 1993; Doering 2007; ENRICHD 2003; Freedland 2009), Resource‐Orientated Psychotherapy (Barth 2005), Telephone Counseling (McLaughlin 2005) and an intervention comprising health education and various psychological treatments (Fang 2003).
Eight trials investigated effects of pharmacological depression treatments with Sertraline (McFarlane 2001; SADHART 2002), Mirtazapine (MIND‐IT 2007), Fluoxetine (Liu 1999, Strik 2000), Paroxetine and Nortriptyline (Roose 1998), Alprazolam (Freeman 1986) and St. John's Wort (Li 2005).
One trial (CREATE 2007) had a 2x2 factorial design comprising Interpersonal Psychotherapy and Citalopram.
The trial size in psychological intervention studies ranged from 15 patients in Doering 2007 to 2481 patients in ENRICHD 2003. In pharmacological intervention the trial size ranged from 27 patients in McFarlane 2001 to 369 patients in SADHART 2002.
Mean age of the participants ranged from 54.1 (Strik 2000) to 63.6 years (Brown 1993). The percentage of female participants ranged from 10% (Brown 1993) to 56% (Freedland 2009). One study was restricted to female participants (Doering 2007).
Seven studies originated from the USA (Brown 1993; Doering 2007; ENRICHD 2003; Freedland 2009; Freeman 1986; McLaughlin 2005; Roose 1998), three from China (Fang 2003; Li 2005; Liu 1999), two from Canada (CREATE 2007; McFarlane 2001), two from the Netherlands (MIND‐IT 2007; Strik 2000), one from Germany (Barth 2005) and one was a multisite study, which took place in the USA, Europe, Canada and Australia (SADHART 2002).
Six studies investigated patients with myocardial infarction (ENRICHD 2003; Fang 2003; Liu 1999; McFarlane 2001; MIND‐IT 2007; Strik 2000). CAD patients comprising myocardial infarction, angina pectoris and patients undergoing cardiac procedures were studied in six trials (Barth 2005; Brown 1993; CREATE 2007; McLaughlin 2005; Roose 1998; SADHART 2002). Four trials investigated patients after CABG (Doering 2007; Freedland 2009; Freeman 1986; Li 2005).
Furthermore, there are two ongoing trials (SPIRR‐CAD 2008; UPBEAT 2007) and one study awaiting classification (Malik 2002), which was identified as a conference abstract through the database search. Unfortunately, published data and contact information of the author were not available.
Excluded studies
A total of 16 trials (Black 1998, Bucknall 1988, Davidson 2010, Fu 2006, González‐Jaimes 2003, Kachkovskii 2006, Mohapatra 2005, Norris 2009, Oldridge 1991, Pogosova 2004,Pogosova 2009, Rollman 2009, Schrader 2005, Stern 1983, Veith 1982, Zeng 2001), which appeared to be relevant for the review, were excluded after careful examination of eligibility criteria (see Characteristics of excluded studies for reasons for exclusion).
Risk of bias in included studies
The risk of bias in included studies varied across studies (see Figure 2 and Figure 3). The available information after translating parts of the three Chinese trials (Fang 2003; Li 2005; Liu 1999) was not sufficient to make any judgments regarding risk of bias in these studies. Furthermore, many other issues of these studies remained unclear as well (see Characteristics of included studies). Hence, we decided not to report the results of the Chinese studies in this review due to the lack of information.
2.
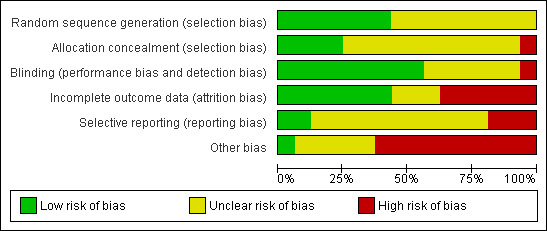
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
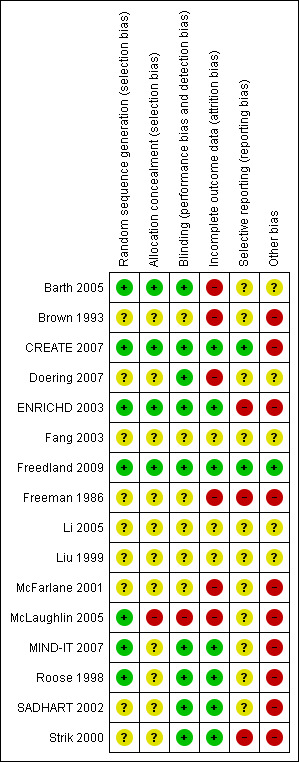
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four trials used an appropriately generated and adequately concealed randomisation procedure (Barth 2005; ENRICHD 2003; Freedland 2009; CREATE 2007). The generation of the randomisation sequence appeared to be generated appropriately in three trials, which however did not sufficiently describe the concealment of the allocation (MIND‐IT 2007; Roose 1998) or failed to conceal the allocation adequately (McLaughlin 2005). Details regarding sequence generation and allocation concealment remained unclear for the remaining nine trials (Brown 1993; Doering 2007; Fang 2003; Freeman 1986; Li 2005; Liu 1999; McFarlane 2001; SADHART 2002; Strik 2000).
Blinding
The outcome assessor was blinded in five psychological intervention trials (Barth 2005; CREATE 2007; Doering 2007; ENRICHD 2003; Freedland 2009). Two trials did not report details regarding blinding (Brown 1993, Fang 2003). One psychological trial was judged as unblinded as the outcome was assessed using patient self‐report (McLaughlin 2005).
In one pharmacological trial blinding was adequately realised and described (CREATE 2007). Four pharmacological trials stated a double‐blind method but did not describe who was blinded (MIND‐IT 2007; Roose 1998; SADHART 2002; Strik 2000). The remaining four trials did not report sufficient information regarding blinding of staff, participants and outcome assessors (Freeman 1986; Li 2005; Liu 1999; McFarlane 2001).
Incomplete outcome data
Seven trials provided intention to treat (ITT) analyses (CREATE 2007; ENRICHD 2003; Freedland 2009; MIND‐IT 2007; Roose 1998; SADHART 2002; Strik 2000) for all primary outcomes except for the depression outcomes in ENRICHD 2003. Six trials reported per‐protocol analyses (Barth 2005; Brown 1993; Doering 2007; Freeman 1986; McFarlane 2001; McLaughlin 2005). Details for the three Chinese trials were not available (Fang 2003; Li 2005; Liu 1999).
Selective reporting
Two studies were judged as free of selective reporting (CREATE 2007; Freedland 2009) based on the comparison of outcomes reported in published study protocols and original papers. Three RCTs did not report the results of all the outcomes mentioned in published protocols or methods sections (ENRICHD 2003; Freeman 1986; Strik 2000). For the remaining eleven trials no published or unpublished protocols were available (Barth 2005; Brown 1993; Doering 2007; Fang 2003; Li 2005; Liu 1999; McFarlane 2001; McLaughlin 2005; MIND‐IT 2007; Roose 1998; SADHART 2002). Thus, it remains unclear whether or not there is a risk of selective reporting in these trials.
Other potential sources of bias
One study was judged free of other sources of bias (Freedland 2009). Six pharmacological studies were sponsored by pharmaceutical industries and were thus judged as having a potential conflict of interest (CREATE 2007; Freeman 1986, MIND‐IT 2007; Roose 1998; SADHART 2002; Strik 2000). ENRICHD 2003 as a psychological study provided pharmaceutical treatment with sertraline for the subgroup of patients with no or little response to Cognitive Behavior Therapy after five weeks. Sertraline was thereby sponsored by industry (Pfizer Inc.) and ENRICHD 2003 was thus judged as having a potential conflict of interest.
The risk for other biases remains unclear for the three Chinese trials (Fang 2003; Li 2005; Liu 1999).
Barth 2005 may exhibit a performance bias because the manual adherence of therapists in the treatment group remains unclear. Furthermore, in inpatient studies therapists and clinic staff are not blind to the patients' allocation, which might impact the inpatient treatment of the intervention and the control group.
In ENRICHD 2003 QoL was not assessed at baseline and it thus remains unclear whether or not QoL was balanced in the two groups at baseline.
The study sample in Brown 1993 exhibits significant baseline differences regarding age, religion, Symptom Checklist‐90‐Revised (SCL 90‐R), Beck Depression Inventory (BDI) (with controls being more distressed on SCL 90‐R and BDI). Furthermore, no efforts for controlling therapy quality are mentioned in the publication.
Doering 2007 reported no efforts for controlling nurse therapists protocol adherence. Furthermore, while usual care comprised psychiatrists' recommendations for individualised treatment options, data on these treatments are not reported (Doering 2007).
In Freeman 1986, 60% of 459 patients met criteria for inclusion, but only 23% were included. Furthermore, in this study of CABG patients with depression and/or anxiety, the treatment group had significantly higher anxiety scores at baseline and no further information regarding comparability of groups is given (possible baseline imbalance).
In McFarlane 2001 (p. 619 and p. 620) and McLaughlin 2005 (discrepancy between text and figure of Hospital Anxiety Depression Score (HADS)) results are inconsistently reported.
Effects of interventions
Comparison 1: Psychological intervention versus usual care
Six trials with a total of 2858 patients studied the effects of a psychological intervention versus usual care (Barth 2005; ENRICHD 2003; Doering 2007, Fang 2003, Freedland 2009, McLaughlin 2005). Pooling results across different types of psychological interventions may level out specific treatment effects. However, due to the lack of trial numbers we combined these studies and conducted analyses of heterogeneity. In case of heterogeneous results, findings of primary studies are descriptively reported.
1.1 Depression score ‐ short term:
Effects of psychological interventions on short term depression scores (i.e. end of treatment) were investigated in five studies (Barth 2005; Doering 2007; Fang 2003; Freedland 2009; McLaughlin 2005). Three of these studies did not report sufficient information to compute effect sizes (Doering 2007; Fang 2003; McLaughlin 2005).
The meta‐analysis of two trials (Freedland 2009; Barth 2005) [n=127] indicated a non‐significant estimate with substantial heterogeneity (Analysis 1.1). Cognitive Behaviour Therapy was superior to usual care on the Hamilton Depression Rating Scale (HAM‐D) (Freedland 2009) [n=46] (SMD of final mean scores: ‐0.81 [‐1.26, ‐0.36]) whereas Resource‐Orientated Psychotherapy did not show a beneficial effect on the Bech Rafaelsen Melancholia Scale (BRMS) compared to usual care (Barth 2005) [n=81].
1.1. Analysis.
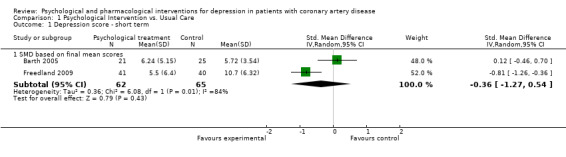
Comparison 1 Psychological Intervention vs. Usual Care, Outcome 1 Depression score ‐ short term.
1.2 Depression score ‐ medium term:
Effects of psychological interventions on medium term depression scores (i.e. one to six months after treatment) were investigated in four studies (Doering 2007; ENRICHD 2003; Freedland 2009; McLaughlin 2005). Two of these studies did not report sufficient information to compute effect sizes (Doering 2007, McLaughlin 2005).
Cognitive Behaviour Therapy was superior to usual care on HAM‐D depression in one study (ENRICHD 2003) [n=1802] (SMD of mean change scores: ‐0.19 [‐0.28, ‐0.10]), but not in another trial (Freedland 2009) [n=81] (Analysis 1.2).
1.2. Analysis.
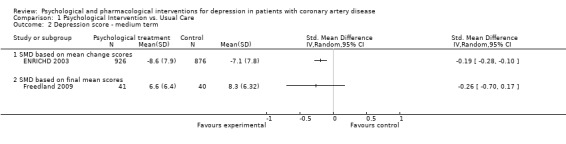
Comparison 1 Psychological Intervention vs. Usual Care, Outcome 2 Depression score ‐ medium term.
1.3 Depression score ‐ long term:
Cognitive Behaviour Therapy was superior to usual care on long term (i.e. more than six months after treatment) HAM‐D depression scores (SMD of final mean scores: ‐0.75 [‐1.20, ‐0.30]) (Freedland 2009) [n=81].
1.4 Depression remission ‐ short, medium and long term:
Only Freedland (Freedland 2009) [n=81] reported on depression remission (HAM‐D < 7). Both, in the short (i.e. end of treatment) and long term (more than six months after end of treatment) Cognitive Behaviour Therapy was beneficial compared to usual care (OR: 5.02 [1.95, 12.90]; OR: 5.06 [1.96, 13.08]). No effect was observed in the medium term (i.e. one to six months after end of treatment).
1.5 Mortality, cardiac events, and cardiovascular hospitalizations:
Only the ENRICHD trial (ENRICHD 2003) [n=2481] reported on all‐cause and cardiovascular mortality, recurrent nonfatal MI and revascularization procedures, as well as cardiovascular hospitalizations. No effect between the Cognitive Behaviour Therapy group and usual care was observed on any of these outcomes.
1.6 Quality of life ‐ short term:
Effects of psychological interventions on short term QoL were investigated in one study using mean final scores of the Medical Outcomes Study Short‐Form 36‐item Health Survey (SF‐36) (Freedland 2009) [n=81]. No effect was observed for Cognitive Behaviour Therapy compared to usual care on the Physical Component Summary (PCS) score, whereas the improvement on the Mental Component Summary (MCS) score was higher in the treatment group (SMD: 0.75 [0.30, 1.20]).
1.7 Quality of life ‐ medium term:
Effects of psychological interventions on medium term QoL were investigated in one study using mean final scores of the SF‐36 (Freedland 2009) [n=81]. No effect was observed for Cognitive Behaviour Therapy compared to usual care on the PCS score, whereas the improvement on the MCS score was higher in the treatment group (SMD: 0.61 [0.16, 1.05]). One further study did not report sufficient information to compute effects sizes regarding quality of life (ENRICHD 2003).
1.8 Quality of life ‐ long term:
Effects of psychological interventions on long term QoL were investigated in one study using mean final scores of the SF‐36 (Freedland 2009) [n=81]. No effect was observed for Cognitive Behaviour Therapy compared to usual care on the PCS score compared to usual care, whereas the improvement on the MCS score was higher in the treatment group (SMD: 0.53 [0.09, 0.98]).
Comparison 2: Psychological intervention versus psychological intervention
In three trials with a total of 461 participants the effects of a specific psychological intervention were compared with the effects of another psychological intervention (Brown 1993; CREATE 2007; Freedland 2009). Pooled estimates will not be reported for this comparison due to the heterogeneous interventions and comparators examined in these trials.
2.1 Depression score ‐ short term:
Effects of psychological interventions compared to another psychological intervention on short term depression scores (i.e. end of treatment) were investigated in three studies (Brown 1993; CREATE 2007; Freedland 2009).
No effect was observed for Behavior Therapy compared to Person‐Centered Therapy on the BDI (Brown 1993) [n=40] and for Cognitive Behaviour Therapy compared to Supportive Stress Management on the HAM‐D (Freedland 2009) [n=83]. Interpersonal Psychotherapy showed a beneficial effect compared to Clinical Management on the HAM‐D (SMD of mean change scores: ‐0.23 [‐0.46, 0.00]) (CREATE 2007) [n=284] (Analysis 2.1).
2.1. Analysis.
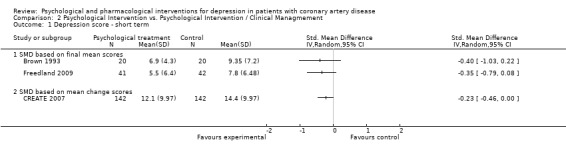
Comparison 2 Psychological Intervention vs. Psychological Intervention / Clinical Managmement, Outcome 1 Depression score ‐ short term.
2.2 Depression score ‐ medium term:
Effects of psychological interventions compared to another psychological intervention on medium term depression scores (i.e. one to six months after treatment) were investigated in two studies (Brown 1993; Freedland 2009).
Cognitive Behaviour Therapy was not superior to Supportive Stress Management on the HAM‐D depression score (Freedland 2009) [n=83]. Behavior Therapy showed a beneficial effect compared to Person‐Centered Therapy on the BDI (SMD of final mean scores = ‐0.65 [‐1.28, ‐0.01]) (Brown 1993) [n=40] (Analysis 2.2).
2.2. Analysis.
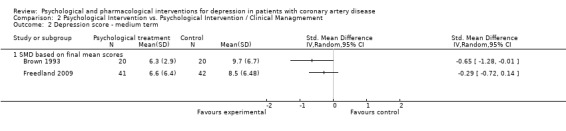
Comparison 2 Psychological Intervention vs. Psychological Intervention / Clinical Managmement, Outcome 2 Depression score ‐ medium term.
2.3 Depression score ‐ long term:
Effects of psychological interventions compared to another psychological intervention on long term depression scores (i.e. more than six months after treatment) were investigated in two studies (Brown 1993; Freedland 2009).
Cognitive Behaviour Therapy was not superior compared to Supportive Stress Management on the HAM‐D (Freedland 2009) [n=83]. Behavior Therapy was superior to Person‐Centered Therapy on the BDI (SMD of final mean scores = ‐0.69 [‐1.33, ‐0.05]) (Brown 1993) [n=40] (Analysis 2.3).
2.3. Analysis.
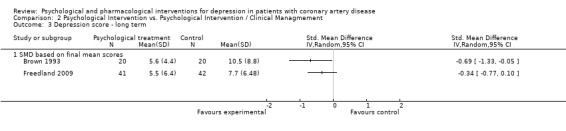
Comparison 2 Psychological Intervention vs. Psychological Intervention / Clinical Managmement, Outcome 3 Depression score ‐ long term.
2.4 Depression remission ‐ short term:
Effects of psychological interventions compared to another psychological intervention on short term depression remission (i.e. end of treatment) were investigated in two studies (CREATE 2007; Freedland 2009).
No effect was observed for Interpersonal Psychotherapy compared to Clinical Management on the HAM‐D (CREATE 2007) [n=284] and Cognitive Behaviour Therapy compared to Supportive Stress Management on the same instrument (Freedland 2009) [n=83] (Analysis 2.4).
2.4. Analysis.
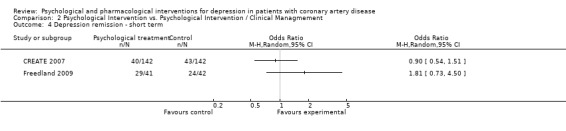
Comparison 2 Psychological Intervention vs. Psychological Intervention / Clinical Managmement, Outcome 4 Depression remission ‐ short term.
2.5 Depression remission ‐ medium and long term:
No effect was observed for Cognitive Behaviour Therapy compared to Supportive Stress Management on HAM‐D depression remission in one study (Freedland 2009) [n=83] in the medium (i.e. one to six months after end of treatment) and the long term (i.e. more than six months after end of treatment).
2.6 Cardiac events:
Recurrent nonfatal MI, congestive heart failure and recurrent angina pectoris were investigated in one study (CREATE 2007) [n=284] and did not show significantly different rates between Cognitive Behaviour Therapy and Clinical Management.
2.7 Quality of life ‐ short, medium and long term:
Only Freedland (Freedland 2009) [n=83] reported on QoL using mean final scores of the SF‐36. No effects were observed for Cognitive Behaviour Therapy compared to Supportive Stress Management in the short (i.e. end of treatment), medium (i.e. one to six months after end of treatment) and long term (i.e. more than six months after end of treatment).
Comparison 3: Pharmacological intervention versus placebo
Eight trials with a total of 1098 patients studied the effects of a pharmacological intervention versus placebo (CREATE 2007; Freeman 1986; Li 2005; Liu 1999; McFarlane 2001; MIND‐IT 2007; SADHART 2002; Strik 2000). Pooling results across different types of pharmacological interventions may level out specific treatment effects. However, due to the lack of trial numbers we combined these studies and conducted analyses of heterogeneity. In case of heterogeneous results, findings of primary studies are descriptively reported.
3.1 Depression score ‐ short term:
Effects of pharmacological interventions on short term depression scores (i.e. end of treatment) were investigated in eight studies (CREATE 2007; Freeman 1986; Li 2005; Liu 1999; McFarlane 2001; MIND‐IT 2007; SADHART 2002; Strik 2000). Five trials did not report sufficient information to compute effects sizes (Freeman 1986; Li 2005; Liu 1999; McFarlane 2001; MIND‐IT 2007).
The meta‐analysis of three studies (CREATE 2007; SADHART 2002; Strik 2000) [n=707] indicated a beneficial effect of pharmacologic interventions versus placebo with an estimate of SMD = ‐0.24 [‐0.38, ‐0.09] and no between‐study heterogeneity (Analysis 3.1). Citalopram showed a beneficial effect compared to placebo on the HAM‐D (CREATE 2007) [n=284] (SMD of mean change scores: ‐0.33 [‐0.56, ‐0.10]). Sertraline (SADHART 2002) [n=369] and Fluoxetine (Strik 2000) [n=54] did not show a beneficial effect compared to placebo.
3.1. Analysis.
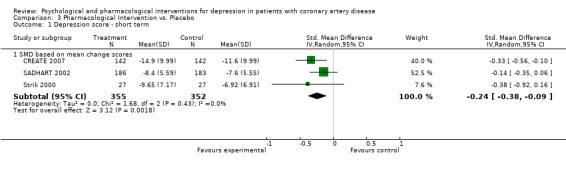
Comparison 3 Pharmacological Intervention vs. Placebo, Outcome 1 Depression score ‐ short term.
3.2 Depression remission ‐ short term:
Effects of pharmacological interventions on short term depression remission (i.e. end of treatment) were investigated in three studies (CREATE 2007; MIND‐IT 2007; Strik 2000).
The meta‐analysis of three studies (CREATE 2007; MIND‐IT 2007; Strik 2000) [n=429] indicated a beneficial effect of pharmacologic interventions versus placebo with an estimate of OR = 1.80 [1.18, 2.74] and no between‐study heterogeneity (Analysis 3.2). Citalopram showed a beneficial effect compared to placebo on the HAM‐D (OR: 1.93 [1.14, 3.25]) (CREATE 2007) [n=284]. Mirtazapine (MIND‐IT 2007) [n=91] and Fluoxetine (Strik 2000) [n=54] did not show a beneficial effect compared to placebo.
3.2. Analysis.
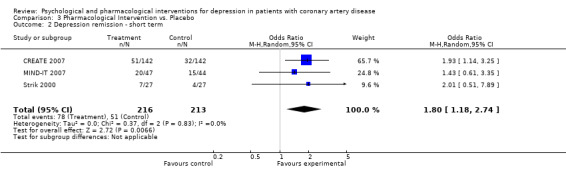
Comparison 3 Pharmacological Intervention vs. Placebo, Outcome 2 Depression remission ‐ short term.
3.3 All‐cause mortality:
All‐cause mortality was investigated in four studies (Liu 1999; McFarlane 2001; MIND‐IT 2007; SADHART 2002), whereas one did not report sufficient information to compute an effect size (Liu 1999). No deaths occurred in two studies (MIND‐IT 2007 [n=91], (McFarlane 2001) [n=27]) and no effect was observed in one trial (SADHART 2002) [n=369] (Analysis 3.3).
3.3. Analysis.
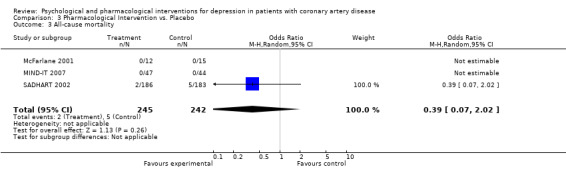
Comparison 3 Pharmacological Intervention vs. Placebo, Outcome 3 All‐cause mortality.
3.4 Cardiac events:
Cardiac events were investigated in three studies (CREATE 2007; SADHART 2002; MIND‐IT 2007) (Analysis 3.4).
3.4. Analysis.
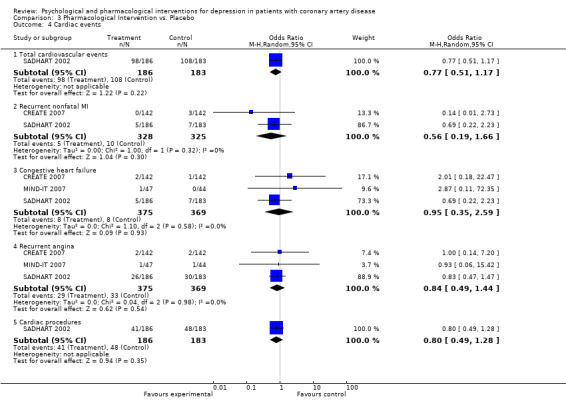
Comparison 3 Pharmacological Intervention vs. Placebo, Outcome 4 Cardiac events.
Total cardiovascular events were not significantly decreased in one trial of Sertraline compared to placebo (SADHART 2002) [n=369].
The meta‐analysis of two studies (CREATE 2007; SADHART 2002) [n=653] regarding recurrent nonfatal MI indicated a non‐significant estimate (Analysis 3.4). Recurrent nonfatal MI was not significantly decreased in two trials of Sertraline (SADHART 2002) [n=369] and Citalopram (CREATE 2007) [n=284].
The meta‐analysis of three studies (CREATE 2007; MIND‐IT 2007; SADHART 2002) [n=744] regarding congestive heart failure indicated a non‐significant estimate (Analysis 3.4). Congestive heart failure was not significantly decreased in three trials of Sertraline (SADHART 2002) [n=369], Mirtazapine (MIND‐IT 2007) [n=91] and Citalopram (CREATE 2007) [n=284].
The meta‐analysis of three studies (CREATE 2007; MIND‐IT 2007; SADHART 2002) [n=744] regarding recurrent angina pectoris indicated a non‐significant estimate (Analysis 3.4). Recurrent angina pectoris was not significantly decreased in three trials of Sertraline (SADHART 2002) [n=369], Mirtazapine (MIND‐IT 2007) [n=91] and Citalopram (CREATE 2007) [n=284].
Cardiac procedures were not significantly decreased in one trial of Sertraline compared to placebo (SADHART 2002) [n=369].
3.5 Resource utilization:
Resource utilization was investigated in three studies (MIND‐IT 2007; SADHART 2002; Strik 2000).
The meta‐analysis of three studies (MIND‐IT 2007; SADHART 2002; Strik 2000) [n=514] indicated reduced hospitalizations in pharmacological interventions versus placebo (OR = 0.58 [0.39, 0.85]) (Analysis 3.5). Hospitalizations were significantly reduced in a trial of Sertraline (OR = 0.59 [0.38, 0.91] SADHART 2002) [n=369], whereas no effect was observed in the trials of Mirtazapine (MIND‐IT 2007) [n=91] and Fluoxetine (Strik 2000) [n=54].
3.5. Analysis.
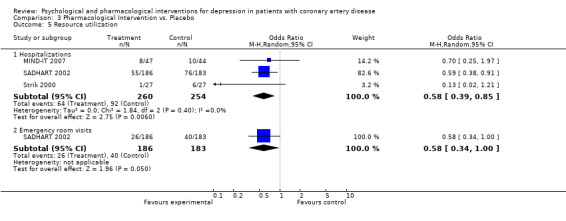
Comparison 3 Pharmacological Intervention vs. Placebo, Outcome 5 Resource utilization.
Emergency room visits were significantly reduced in a trial of Sertraline (OR = 0.58 [0.34, 1.00] SADHART 2002) [n=369].
3.6 Healthcare costs:
Healthcare costs excluding antidepressant medication with Sertraline were not significantly reduced in SADHART 2002 [n=369].
3.7 Quality of life ‐ short term:
SADHART 2002 [n=369] investigated quality of life using the QoL Enjoyment and Satisfaction scale (Q‐LES‐Q) and Medical Outcomes Study Short‐Form 36 (SF‐36) comparing Sertraline with placebo. Data for the SF‐36 were not reported sufficiently to compute effects sizes. No effect was observed for the Q‐LES‐Q.
Comparison 4: Pharmacological intervention versus pharmacological intervention
One study with 81 patients compared the effects of Paroxetine with Nortriptyline on depression outcomes in CAD patients (Roose 1998). Using the clinician‐rated HAM‐D no differences were observed between the groups on short term depression scores or short term depression remission (HAM‐D < 9).
Subgroup and sensitivity analyses
Subgroup and sensitivity analyses were planned to take into account variables such as type of intervention, population, sex, CAD subtype, assessment of depression diagnosis, time of onset of depression, CAD severity and risk of bias. As yet, these analyses are not feasible due to the lack of primary data. However, Cochrane reviews are planned to be updated on a regular basis. Hence, updates of our review might comprise these analyses.
Discussion
The present systematic review investigated the effects of psychological and pharmacological interventions on depression outcomes, mortality, cardiac events, healthcare costs and health‐related quality of life in CAD patients with comorbid depressive disorder. Based on a comprehensive search strategy 16 RCTs fulfilling the inclusion criteria were identified from a set of 3,253 references. Seven trials compared psychological interventions, eight trials pharmacological interventions and one trial had a 2x2 factorial design comprising psychological and pharmacological interventions.
Summary of main results
The results of the present review provide some evidence of a small beneficial effect of psychological interventions compared to usual care on depression severity and remission rates. The psychosocial dimension of health‐related QoL showed a small beneficial effect in one trial. No beneficial effects on the reduction of mortality rates, rates of cardiac events, cardiovascular hospitalizations and the physical dimension of health‐related quality of life were found. However, the latter findings are in each case based on only one trial.
The evidence base regarding the comparison of psychological interventions versus other psychological interventions is sparse. Based on three trials there seem to be no significant differences between the varying approaches on treatment outcomes.
With regard to the comparison of pharmacological interventions versus placebo the review provides evidence of a small beneficial effect of selective serotonine reuptake inhibitors (SSRIs) on depression outcomes. The evidence regarding hospitalisation rates and emergency room visits is sparse but points in the direction of a beneficial effect of SSRIs compared to placebo. Effects of tricyclic antidepressants (TCAs) were studied in only one small trial where no effect on any of the investigated outcomes was observed. No evidence regarding a positive effect on mortality and cardiac events was found for either class of antidepressants.
The comparison of pharmacological interventions versus other pharmacological interventions comprises only one trial, which showed no evidence of a superior effect of Paroxetine (SSRI) versus Nortriptyline (TCA) regarding depression. No other outcomes relevant to this review were studied in this trial.
Overall, the evidence base is small and does not allow for conclusions about the effects of psychological and pharmacological interventions on most outcomes. Moreover, the settings, samples, interventions and outcome measures are heterogeneous across the included trials, hampering the meta‐analytical synthesis of the results. The planned subgroup and sensitivity analyses were not feasible due to the low number of studies per outcome and methodological heterogeneity between the studies.
Overall completeness and applicability of evidence
The review summarizes the evidence regarding depression treatments in a variety of settings. The included trials comprise different CAD samples (myocardial infarction, angina pectoris, patients undergoing surgery), investigate various types of psychological and pharmacologic interventions and were located in different countries with different health care systems, thus increasing the generalisability of the results. However, the overall completeness is limited and the applicability of evidence restricted due to the following four aspects.
Firstly, most outcomes were investigated insufficiently. For example in psychological interventions mortality, cardiac events and health care costs were investigated in only two trials. Furthermore, QoL was investigated solely in one psychological and one pharmacological trial. Hence, evidence of treatment effects on these outcomes need to be interpreted carefully. Moreover, most trials were underpowered to detect effects of depression treatments on outcomes, which rarely occur such as mortality and specific cardiac events.
Secondly, no studies comparing psychological and pharmacological interventions were found. Consequently, no conclusions can be drawn on the differential effects of these treatment approaches. A meta‐analysis of comparative studies on depression treatments in general concludes that pharmacologic treatments are more effective than psychological interventions for dysthymia, while the differences between pharmacologic treatments and psychological interventions are clinically insignificant for major depression (Cuijpers 2008b). The NICE guideline on depression in adults with a chronic physical health problem, however, favours to use psychological interventions as first‐line interventions in patients with minor and mild to moderate depression due to adverse effects of antidepressants and the resulting poor risk‐benefit ratio (NICE 2009). To what extent this recommendation holds true for depressed CAD patients cannot be decided based on the results of the present review.
Thirdly, the samples of included trials most likely differ regarding subtypes and severity of depression. The included trials comprised participants with a wide range of depressive symptomatology and different etiology (e.g. dysthymia, minor and major depression, adjustment disorder with depressed mood). Depressive disorders were present immediately following the cardiac event or up to 12 months after the event. Furthermore, diverse methods and cut‐off points to diagnose depression were used. These mixed samples of depressed patients may have levelled potential effects of depression treatments in patients with specific subtypes of depression. For example, the onset of depression was previously shown to be a moderator of treatment outcomes in CAD patients (Dickens 2008). Another trial on depression treatment in general highlighted differential responses to psychotherapy versus pharmacotherapy in chronic depressed patients with childhood trauma compared to patients without a history of childhood trauma (Nemeroff 2003). Moreover, a recent patient‐level meta‐analysis concludes that the effects of antidepressant medication is associated with the severity of depressive symptoms showing minimal effects in mild to moderate depression and substantial benefit in severe depression (Fournier 2010). Sensitivity analyses to examine these differential effects of different depression subtypes were, however, not feasible in the present review, due to the small number of trials per outcome. Thus, conclusions regarding differential treatment effects depending on depression subtypes or severity cannot be drawn.
Finally, the length of psychotherapies examined in the included trials ranged from four sessions (Barth 2005) to 12 sessions (Brown 1993; Freedland 2009). The minimum number of sessions needed to show substantial benefit in psychotherapy, however, should rather be around 20 sessions (Harnett 2010). Hence, the small effects found in the included psychological intervention trials may partly be due to an insufficient number of sessions.
Quality of the evidence
The included trials differed with regard to methodological shortcomings (see Risk of bias in included studies) and quality of reporting. Many trials did not adequately describe design aspects such as randomisation procedure, allocation concealment and blinding. Furthermore, many trials did not report ITT analyses, missing data was common and selective reporting may have occurred because published protocols were not available for most studies. Low‐quality studies have been associated with exaggerated effects (Cuijpers 2010; Moher 1999). Thus, treatment effects summarized in this review may be overestimated due to poor methodological quality of some of the included trials.
Furthermore, most pharmacological studies were sponsored by pharmaceutical companies and were thus judged as having a conflict of interest. It has been shown that studies sponsored by pharmaceutical companies were more likely to have outcomes favouring the sponsor than were studies with other sponsors (Higgins 2008). Furthermore, selective reporting of null findings in industry‐funded RCTs of antidepressant trials was previously documented (Turner 2008). Despite our comprehensive search strategy, there may be unpublished trials with non‐significant results.
Another bias results from selective reporting of negative findings for prespecified primary outcomes while emphasizing positive results from secondary or new outcomes of antidepressant medication trials (Pigott 2010). We were not able to obtain published protocols for most included trials in this review and thus were not able to judge the risk of selective reporting for these studies.
Finally, meta‐analyses regarding depressive outcomes were hampered because depressive symptoms were assessed by a heterogeneous set of clinician‐rated tools and self‐report questionnaires. Furthermore, the included trials reported either final mean scores or mean change scores from baseline to final assessment or did not report sufficient information to compute effect estimates for these trials.
Potential biases in the review process
In the review process we decided to consider the ENRICHD 2003 and Barth 2005 studies as psychological intervention trials, neglecting the fact that participants in these trials were allowed to receive pharmacologic treatments additional to the assigned psychological intervention. Hence, it remains unclear to which degree the effects in these studies were impacted by additional pharmacological treatments. Similarly, the results might be biased by our decision to include mixed study samples of CAD patients with depression and/or low social support (ENRICHD 2003) and patients with depression and/or anxiety (Brown 1993; Freeman 1986; McLaughlin 2005). By including these trials with mixed study samples the treatment effects might rather be underestimated than exaggerated in this review.
A second potential bias may result from the translation process of the included Chinese trials (Fang 2003; Li 2005; Liu 1999). Despite our efforts to translate the Chinese trials accurately, the translations did not result in unambiguous and interpretable results. Thus, we decided not to make any judgments regarding risk of bias and not to report outcome data of these trials in the review.
Agreements and disagreements with other studies or reviews
Differences in included trials in this review compared to previous reviews are attributable to our focus on trials investigating depression treatments in depressed CAD patients. Two psychological intervention trials included in the present review (CREATE 2007; ENRICHD 2003) are also included in the review of Van Straten 2010, which investigated the effects of psychological treatments on depressive symptoms in medical diseases. The authors conducted a meta‐analysis of 23 studies with 10 different medical diseases and concluded that depressive symptoms could be effectively treated with psychological interventions. Results from our review point in the same direction for CAD patients with depression. However, the evidence is sparse and further studies are needed.
In a recent Cochrane review, Rayner et al. (Rayner 2010) systematically reviewed trials investigating the effects of antidepressant medication in treating depression in physically ill people. In a meta‐analysis of 51 studies they concluded that antidepressants are superior to placebo. The evidence of the present review agrees with this finding for the specific group of CAD patients with comorbid depression. Four trials are included in both reviews (CREATE 2007; MIND‐IT 2007; SADHART 2002; Strik 2000).
Finally, Brown 1993 and ENRICHD 2003 are trials which are also included in a Cochrane review on the effects of psychological interventions in CAD patients in general (i.e. not restricted to depressed CAD patients) (Rees 2004). Overall, psychological interventions had no effect on total or cardiac mortality and small effects on depression and anxiety (Rees 2004). This results are in line with the findings in the present review.
Authors' conclusions
Implications for practice.
Psychological interventions and pharmacological interventions with SSRIs in CAD patients may have small yet positive effects on depression outcomes, the former for both short and long term and the latter for short term measures. The NICE guideline on depression in adults with a chronic physical health problem, however, favours to use psychological interventions as first‐line interventions in patients with minor and mild to moderate depression due to adverse effects of antidepressants and the resulting poor risk‐benefit ratio (NICE 2009). In the primary studies of the present review antidepressant medications compared to placebo were associated with increased rates of dizziness, diarrhoea, somnolence, sweating, palpitations, libido reduction or sexual difficulties in CREATE 2007, fatigue, appetite changes and weight gain in MIND‐IT 2007 as well as nausea and diarrhoea in SADHART 2002. Nortriptyline had a higher rate of adverse events compared to Paroxetine in Roose 1998. These side‐effects have to be weighted against the positive effects on depression outcomes when considering to initiate pharmacological treatment in depressed CAD patients.
The evidence for more specific recommendations is scarce. There is no evidence to recommend a specific psychological intervention on the basis of this review. With regard to pharmacological interventions recommendations on benefits and risks of SSRIs versus TCAs for the treatment of depression in CAD patients cannot be made due to the lack of an adequate number of studies investigating TCAs and the small evidence base regarding cardiac end points in the included studies. However, TCAs are viewed as highly cardiotoxic in overdose and may therefore worsen outcome in CAD patients (Taylor 2008, Lichtman 2008).
Implications for research.
The presence of depression in CAD patients is associated with a high additional burden and a negative medical prognosis (Barth 2004; Baumeister 2005, Baumeister 2011a; Frasure‐Smith 2000; Frasure‐Smith 2003; Herrmann‐Lingen 2006; Ziegelstein 2000). All the more, the rather small improvements in depression outcomes as well as the sparse evidence regarding other outcomes are unsatisfying. Accordingly, there is a need for further trials focusing on outcomes not yet sufficiently examined. This applies at least to medium and long term depression, quality of life, mortality, cardiac events and health care costs. Moreover, to examine differential effects of depression treatments, more comparative trials of psychological and pharmacological interventions are needed. Finally, there is a need for trials of psychological interventions examining the minimum dose needed for a clinical meaningful treatment response.
Another conclusion based on the present review might be that single intervention approaches are insufficient in CAD patients with comorbid depression. Larger effect sizes have been shown for multimodal and collaborative care interventions for depression (De Maat 2007; Unützer 2002). First results in depressed CAD patients are promising (Davidson 2010; Rollman 2009) and collaborative care interventions should be further examined in future trials and systematic reviews.
With regard to the small effects of both psychological and pharmacological interventions for depression in CAD patients, however, there might also be the need for a change of the current research agenda away from including all depressed patients regardless of their specific depression subtype and severity (Baumeister 2009a; Baumeister 2009b; Bech 2010; Pigott 2010). As highlighted above and summarized earlier (Baumeister 2010c; Lichtenberg 2010) the effectiveness of depression treatments may vary depending on depression subtypes. The evidence of depression treatment in general emphasizes that treatment effectiveness should at least be examined for different levels of depression severity (Baumeister 2011b; Fournier 2010; NICE 2009) taking clinical significance of depression into account (Baumeister 2008; Baumeister 2010b; Wakefield 2010). Finally, in CAD patients the need for subtyping depression might particularly apply to the differentiation of new onset depression versus recurrent depression (Dickens 2008).
Acknowledgements
We would like to thank the German Federal Ministry of Education and Research for funding the research project (grant number: 01KG0809), the Cochrane Heart Group for the comprehensive support and the German Cochrane Centre Freiburg for providing a very helpful workshop for review authors. We gratefully acknowledge Erla Magnusdottir and Tatjana Alexander for translating Chinese and Russian papers.
Appendices
Appendix 1. Search strategy
CENTRAL, DARE, HTA and EED on The Cochrane Library
#1 MeSH descriptor myocardial ischemia explode all trees #2 MeSH descriptor Myocardial Revascularization explode all trees #3 (ischemi* in All Text near/3 heart in All Text) #4 (ischaemi* in All Text near/3 heart in All Text) #5 (coronary in All Text near/3 disease* in All Text) #6 angina in All Text #7 myocardial next infarct* in All Text #8 heart next infarct* in All Text #9 (coronary in All Text near/3 bypass in All Text) #10 (heart in All Text near/3 disease in All Text) #11 (cardiac in All Text near/3 disease in All Text) #12 chd in All Text #13 cad in All Text #14 (coronary in All Text near/3 angioplasty in All Text) #15 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10) #16 (#11 or #12 or #13 or #14) #17 (#15 or #16) #18 MeSH descriptor depression explode all trees #19 MeSH descriptor Depressive Disorder explode all trees #20 MeSH descriptor Mood Disorders this term only #21 "depression" in Keywords #22 "depressive" in Keywords #23 "Dysthymia" in Keywords #24 dysthymi* in All Text #25 (depressi* in All Text near/3 disorder* in All Text) #26 (depressi* in All Text near/3 symptom* in All Text) #27 mood next disorder* in All Text #28 depression in Record Title #29 antidepress* in All Text #30 anti‐depress* in All Text #31 (#18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27) #32 (#28 or #29 or #30) #33 (#31 or #32) #34 (#17 and #33)
MEDLINE (OVID)
1 exp Myocardial Ischemia/ 2 exp Myocardial Revascularization/ 3 (isch?emi$ adj3 heart).tw. 4 (coronary adj3 disease).tw. 5 angina.tw. 6 myocardial infarct$.tw. 7 heart infarct$.tw. 8 (coronary adj3 bypass$).tw. 9 (heart adj3 disease).tw. 10 (cardiac adj3 disease).tw. 11 chd.tw. 12 CAD.tw. 13 (coronary adj3 angioplasty).tw. 14 or/1‐13 15 Depression/ 16 exp Depressive Disorder/ 17 Mood Disorders/ 18 dysthymi$.tw. 19 (depressi$ adj3 disorder$).tw. 20 (depressi$ adj3 symptom$).tw. 21 mood disorder$.tw. 22 affective disorder$.tw. 23 antidepress$.tw. 24 anti‐depress$.tw. 25 or/15‐24 26 14 and 25 27 randomized controlled trial.pt. 28 controlled clinical trial.pt. 29 randomized.ab. 30 placebo.ab. 31 drug therapy.fs. 32 randomly.ab. 33 trial.ab. 34 groups.ab. 35 or/27‐34 36 (animals not humans).sh. 37 35 not 36 38 26 and 37
EMBASE (OVID)
1 exp ischemic heart disease/ 2 exp coronary artery surgery/ 3 exp percutaneous coronary intervention/ 4 (isch?emi$ adj3 heart).tw. 5 (coronary adj3 disease).tw. 6 angina.tw. 7 myocardial infarct$.tw. 8 heart infarct$.tw. 9 (coronary adj3 bypass$).tw. 10 (heart adj3 disease).tw. 11 (cardiac adj3 disease).tw. 12 chd.tw. 13 CAD.tw. 14 (coronary adj3 angioplasty).tw. 15 or/1‐14 16 exp depression/ 17 affective neurosis/ 18 Mood Disorder/ 19 dysthymi$.tw. 20 (depressi$ adj3 disorder$).tw. 21 (depressi$ adj3 symptom$).tw. 22 mood disorder$.tw. 23 affective disorder$.tw. 24 antidepress$.tw. 25 anti‐depress$.tw. 26 or/16‐25 27 15 and 26 28 controlled clinical trial/ 29 random$.tw. 30 randomized controlled trial/ 31 follow‐up.tw. 32 double blind procedure/ 33 placebo$.tw. 34 placebo/ 35 factorial$.ti,ab. 36 (crossover$ or cross‐over$).ti,ab. 37 (double$ adj blind$).ti,ab. 38 (singl$ adj blind$).ti,ab. 39 assign$.ti,ab. 40 allocat$.ti,ab. 41 volunteer$.ti,ab. 42 Crossover Procedure/ 43 Single Blind Procedure/ 44 or/28‐43 45 (exp animals/ or nonhuman/) not human/ 46 44 not 45 47 27 and 46
PsycINFO
1 exp heart disorders/ 2 heart surgery/ 3 (isch?emi$ adj3 heart).tw. 4 (coronary adj3 disease).tw. 5 angina.tw. 6 myocardial infarct$.tw. 7 heart infarct$.tw. 8 (coronary adj3 bypass$).tw. 9 (heart adj3 disease).tw. 10 (cardiac adj3 disease).tw. 11 chd.tw. 12 CAD.tw. 13 (coronary adj3 angioplasty).tw. 14 or/1‐13 15 exp affective disorders/ 16 "depression (emotion)"/ 17 dysthymi$.tw. 18 (depressi$ adj3 disorder$).tw. 19 (depressi$ adj3 symptom$).tw. 20 mood disorder$.tw. 21 affective disorder$.tw. 22 antidepress$.tw. 23 anti‐depress$.tw. 24 or/15‐23 25 14 and 24 26 random$.tw. 27 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or dummy or mask$)).tw. 28 placebo$.tw. 29 crossover.tw. 30 assign$.tw. 31 allocat$.tw. 32 ((clin$ or control$ or compar$ or evaluat$ or prospectiv$) adj25 (trial$ or studi$ or study)).tw. 33 placebo/ 34 treatment effectiveness evaluation/ 35 mental health program evaluation/ 36 experimental design/ 37 clinical trials/ 38 or/26‐37 39 25 and 38
CINAHL (EBSCO)
( (MH "Affective Disorders+") or (TI depression) or dysthymi* or (mood disorder*) or (affective disorder*) or antidepress* or anti‐depress* or (depressi* N3 disorder*) or (depressi* N3 symptom*) ) and ( (MH "Myocardial Ischemia+") or (MH "Myocardial Revascularization+") or Angina or (myocardial infarct*) or (heart infarct*) or coronary or cardiac or chd or CAD or (heart disease) ) and ( (MH "Clinical Trials+") or randomi* or randomly or placebo* or trial )
Data and analyses
Comparison 1. Psychological Intervention vs. Usual Care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression score ‐ short term | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 SMD based on final mean scores | 2 | 127 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.27, 0.54] |
| 2 Depression score ‐ medium term | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 SMD based on mean change scores | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 SMD based on final mean scores | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Depression score ‐ long term | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 SMD based on final mean scores | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Depression remission ‐ short, medium and long term | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 short term | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 medium term | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 long term | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Mortality, cardiac events and cardiovascular hospitalizations | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 All‐cause mortality | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Cardiovascular mortality | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Recurrent nonfatal MI | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Revascularization procedures | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Cardiovascular hospitalizations | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Quality of life ‐ short term | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 SF‐36 PCS | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 SF‐36 MCS | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of life ‐ medium term | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 SF‐36 PCS | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 SF‐36 MCS | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Quality of life ‐ long term | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 SF‐36 PCS | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 SF‐36 MCS | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.3. Analysis.
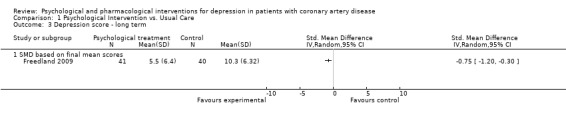
Comparison 1 Psychological Intervention vs. Usual Care, Outcome 3 Depression score ‐ long term.
1.4. Analysis.
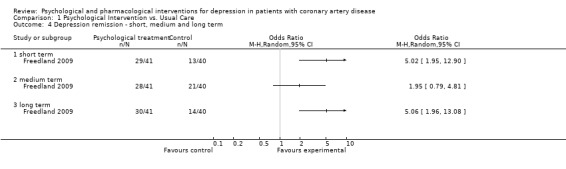
Comparison 1 Psychological Intervention vs. Usual Care, Outcome 4 Depression remission ‐ short, medium and long term.
1.5. Analysis.
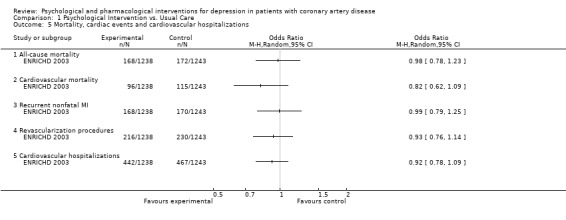
Comparison 1 Psychological Intervention vs. Usual Care, Outcome 5 Mortality, cardiac events and cardiovascular hospitalizations.
1.6. Analysis.
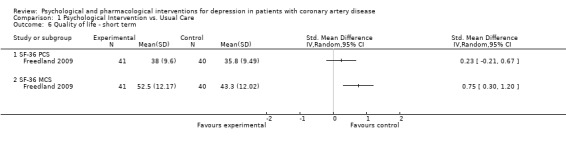
Comparison 1 Psychological Intervention vs. Usual Care, Outcome 6 Quality of life ‐ short term.
1.7. Analysis.
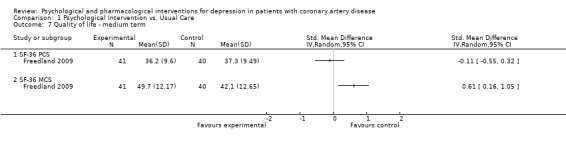
Comparison 1 Psychological Intervention vs. Usual Care, Outcome 7 Quality of life ‐ medium term.
1.8. Analysis.
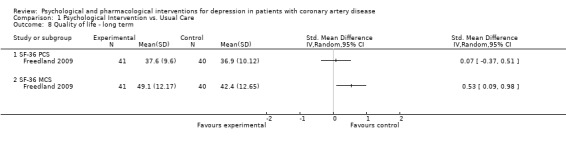
Comparison 1 Psychological Intervention vs. Usual Care, Outcome 8 Quality of life ‐ long term.
Comparison 2. Psychological Intervention vs. Psychological Intervention / Clinical Managmement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression score ‐ short term | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 SMD based on final mean scores | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 SMD based on mean change scores | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Depression score ‐ medium term | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 SMD based on final mean scores | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Depression score ‐ long term | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 SMD based on final mean scores | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Depression remission ‐ short term | 2 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Depression remission ‐ medium and long term | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 medium term | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 long term | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Cardiac events | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Recurrent nonfatal MI | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Congestive heart failure | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Recurrent angina | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of life | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 SF‐36 PCS ‐ short term | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 SF‐36 MCS ‐ short term | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 SF‐36 PCS ‐ medium term | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 SF‐36 MCS ‐ medium term | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 SF‐36 MCS ‐ long term | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 SF‐36 PCS ‐ long term | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.5. Analysis.
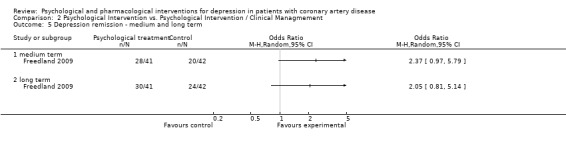
Comparison 2 Psychological Intervention vs. Psychological Intervention / Clinical Managmement, Outcome 5 Depression remission ‐ medium and long term.
2.6. Analysis.
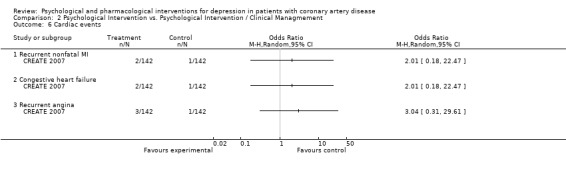
Comparison 2 Psychological Intervention vs. Psychological Intervention / Clinical Managmement, Outcome 6 Cardiac events.
2.7. Analysis.
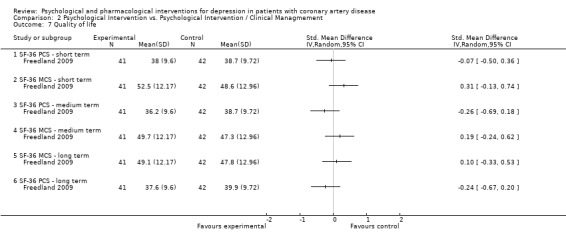
Comparison 2 Psychological Intervention vs. Psychological Intervention / Clinical Managmement, Outcome 7 Quality of life.
Comparison 3. Pharmacological Intervention vs. Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression score ‐ short term | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 SMD based on mean change scores | 3 | 707 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.38, ‐0.09] |
| 2 Depression remission ‐ short term | 3 | 429 | Odds Ratio (M‐H, Random, 95% CI) | 1.80 [1.18, 2.74] |
| 3 All‐cause mortality | 3 | 487 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.07, 2.02] |
| 4 Cardiac events | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Total cardiovascular events | 1 | 369 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.51, 1.17] |
| 4.2 Recurrent nonfatal MI | 2 | 653 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.66] |
| 4.3 Congestive heart failure | 3 | 744 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.35, 2.59] |
| 4.4 Recurrent angina | 3 | 744 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.49, 1.44] |
| 4.5 Cardiac procedures | 1 | 369 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.49, 1.28] |
| 5 Resource utilization | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Hospitalizations | 3 | 514 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.39, 0.85] |
| 5.2 Emergency room visits | 1 | 369 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.34, 1.00] |
| 6 Healthcare costs | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Excluding antidepressant medication costs | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of life ‐ short term | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Q‐LES‐Q | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.6. Analysis.
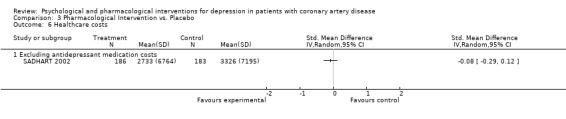
Comparison 3 Pharmacological Intervention vs. Placebo, Outcome 6 Healthcare costs.
3.7. Analysis.
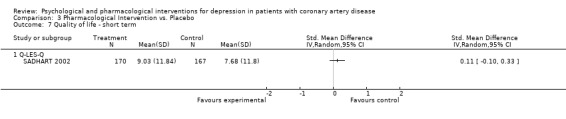
Comparison 3 Pharmacological Intervention vs. Placebo, Outcome 7 Quality of life ‐ short term.
Comparison 4. Pharmacological Intervention vs. Pharmacological Intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression score ‐ short term | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 SMD based on mean change scores | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Depression remission ‐ short term | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected |
4.1. Analysis.
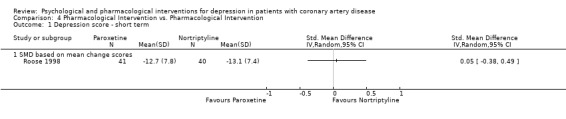
Comparison 4 Pharmacological Intervention vs. Pharmacological Intervention, Outcome 1 Depression score ‐ short term.
4.2. Analysis.
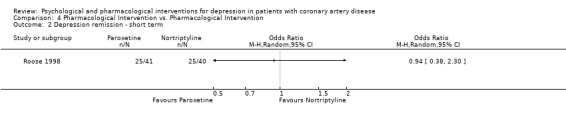
Comparison 4 Pharmacological Intervention vs. Pharmacological Intervention, Outcome 2 Depression remission ‐ short term.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barth 2005.
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 59 Length of follow‐up: No follow‐up Analysis: Per‐protocol (4 patients in the control group dropped‐out) |
|
| Participants | Location: Germany Number of study centres and setting: 3 cardiac inpatient rehabilitation clinics CAD criteria: Patients with Myocardial Infarction (MI), Coronary Artery Bypass Grafting (CABG), Percutaneous Transluminal Coronary Angioplasty (PTCA), Unstable Angina Pectoris; diagnosis based on physician's report; time to randomisation unclear Depression criteria: Major depression, dysthymia and depressive adjustment disorder assessed in a 2‐stage procedure: 1) Hospital Depression and Anxiety Scale (HADS) and 2) Structured Clinical Interview for DSM‐IV in all patients with a HADS score of 17 or higher Other entry criteria: None stated Exclusion criteria: Poor general health, language and cognitive deficits, bipolar disorder, psychotherapy at residence, psychotic symptoms Treatment: 27 (18.5% female, mean age: 60.8 (SD: 11.1)) Control: 32 (28.1% female, mean age: 55.6 (SD: 10.1)) Comparability of groups: No significant baseline differences |
|
| Interventions | Treatment: Brief, individualized, Resource‐orientated Psychotherapy (4 to 6 sessions of 50 minutes each) comprising patient education, motivation, goal setting, crisis management, modification of dysfunctional cognitions and behaviour, and written recommendations for further outpatient treatment; patients with severe depression were treated additionally with sertraline Control: Usual care Duration of treatment: 3 to 4 weeks during inpatient rehabilitation |
|
| Outcomes | Review outcomes: Bech Rafaelsen Melancholia Scale (BRMS), Beck Depression Inventory (BDI) score, HADS depression score Other outcomes: HADS anxiety score |
|
| Funding | Ministry for Education and Research, Germany, Federal Insurance Authority, Baden‐Württemberg, Germany | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Randomisation carried out by methodology center (independent from study staff) |
| Allocation concealment (selection bias) | Low risk | Comment: By sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Blinded interviewers for BRMS |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: Table 2 (p. 6/7): "Only patients with data at both assessments were included in the analysis." |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcomes as stated in methods section Comment: No protocol or design paper available |
| Other bias | Unclear risk | Comment: Possible performance bias with regard to manual adherence of therapists in treatment group, which remains unclear. Furthermore, in inpatient studies therapists and clinic staff are not blind to the patients' allocation, which might impact the inpatient treatment of the intervention and the control group. |
Brown 1993.
| Methods | RCT design: 2‐arm parallel group trial Total N randomised: 54 Length of follow‐up: 15 months Analysis: per‐protocol, 14 drop‐outs at 15 months |
|
| Participants | Location: USA Number of study centres and setting: Patients recruited from 5 cardiac rehabilitation departments of medical centres and by newspaper advertisements CAD diagnosis: Myocardial infarction and/or coronary bypass surgery 4 to 24 months before study; diagnosis based on physician's report; prognosis of no worse than 3.3 based on criteria of the New York Heart Association for moderately compromised cardiac status; stable cardiac status with no medical contraindications to increased physical activity according to their physician´s report Depression diagnosis: New‐onset depression and/or anxiety on the Schedule of Affective Disorders and Schizophrenia (SADS); scores of >13 on the Beck Depression Inventory (BDI) or >70 on the Symptom Checklist 90‐Revised (SCL 90‐R) Other entry criteria: Spouses, friends, or relative who were willing to participate; age between 43 and 75 years Exclusion criteria: Unstable medical condition, chronic, severe depression and/or anxiety preceding the cardiac event, suicidal ideation, changes in county residence, unwillingness or inability to include a partner, preexisting psychiatric disorder Treatment N: 20 (45% female, mean age: 63.55 (SD: 7.43)) Control N: 20 (10% female, mean age: 57.65 (SD: 7.82)) Comparability of groups: Significant baseline differences regarding age, religion, SCL 90‐R, BDI (with control being more distressed on SCL 90‐R and BDI) |
|
| Interventions | Treatment: Behavior therapy for patients and their partners by Lewinsohn (weekly 1‐hour sessions), in which patients should increase and intensify adaptive behaviors (pleasant activities, relaxation, cognitive restructuring, assertion/anger management, time management) and partners practice positive reinforcement of adaptive behaviors and ignored maladaptive behaviors Control: Person‐centered therapy by Rogers (weekly 1‐hour sessions) Duration of treatment: 12 sessions (treatment and control) |
|
| Outcomes | Outcomes: BDI score Other outcomes: SADS‐C, SCL 90‐R, Minnesota Multiphasic Personality Inventory‐168 (MMPI‐168), Pleasant Events Schedule (PES), Unpleasant Events Schedule (UES), Locke Wallace Marital Adjustment Test, Katz Adjustment Scale |
|
| Funding | Study supported in part by a grant from the American Heart Association | |
| Notes | Study investigated effects of behavior therapy of patients and their partners on depression and/or anxiety in comparison to person‐centered therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: Blinding of patients not stated Quote: Regarding Schedule of Affective Disorders and Schizophrenia (SADS) "None of the therapists conducted the post‐treatment interviews." (p.203) Comment: All other outcomes patient self‐report |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Per‐protocol analysis and no drop‐out analysis (14 of 54 patients dropped‐out from baseline to 15‐month follow‐up) Comment: Missing data present |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcomes reported as stated in the methods section Comment: No protocol or design paper available |
| Other bias | High risk | Comment: No efforts regarding therapy quality mentioned Comment: Significant baseline imbalance |
CREATE 2007.
| Methods | RCT design: 2 x 2 factorial trial Total N randomised: 284 Length of follow‐up: No follow‐up Analysis: Intention‐to‐treat (ITT) with last‐observation‐carried‐forward applied for missing data |
|
| Participants | Location: Canada Number of study centres and setting: 9 hospitals with patients being referred from physicians, responded to media advertisements or targeted posters CAD diagnosis: Evidence of CAD based on hospital chart evidence of a previous hospitalization for acute myocardial infarction, or coronary angiographic evidence of 50% or more blockage in at least one major coronary artery, or previous revascularization; patients were not randomised less than 1 week following discharge Depression diagnosis: Current major depressive episode based on the Structured Clinical Interview for Depression (SCID) with at least 4 weeks' duration; baseline score of >19 on the Hamilton Depression Rating Scale (HAM‐D) Other entry criteria: Adult patients (18 years or older), stable CAD according to physician's clinical judgement Exclusion criteria: Coronary bypass surgery planned during the next 4 months, Canadian Cardiovascular Society Angina Class (CCS) = 4, bipolar disorder, major depression with psychotic features, or evidence of substance abuse or dependency during the previous 12 months, serious suicide risk based on clinical judgement, use of antidepressants, lithium, or anticonvulsants for mood disorder, currently undergoing any form of psychotherapy, absence of response to a previous adequate trial of citalopram or IPT, two previous unsuccessful trials of treatment for depression for the index episode, lifetime history of early termination (<8 weeks) of citalopram because of adverse events or side effects, lifetime history of early termination (<8 weeks) of two other SSRI antidepressants because of adverse events or side effects, significant cognitive problems, depression due to a general medical condition based on clinical judgement, participation in other trials, inability to speak English or French, unable or willing to comply with the study regimen Treatment 1 N: 142 (31.0% female, mean age: 59.0 (SD: 9.81)) Treatment 2 N: 142 (23.2% female, mean age: 57.9 (SD: 9.15)) Control 1 N: 142 (18.3 % female, mean age: 57.3 (SD: 8.35)) Control 2 N: 142 (26.1% female, mean age: 58.4 (SD: 9.16)) Comparability of groups: Significantly more women in IPT compared to CM |
|
| Interventions | Treatment 1: Interpersonal Psychotherapy (IPT) + CM provided weekly by certified therapists following published treatment guidelines dealing with common problems in CAD patients, including interpersonal conflicts, life transitions, grief, loss, and social isolation Treatment 2: Citalopram + CM (20‐mg/d to 40 mg/d, tablets) Control 1: Clinical management (CM) with 20‐ to 25‐minute visits including information about depression and medication use, reassurance, and encouragement of adherence to medication and the study protocol, review of side effects and progress Control 2: Placebo administration matched to citalopram condition Duration of treatment: 12 weeks |
|
| Outcomes | Outcomes: HAM‐D score, depression remission (HAM‐D <= 8), Beck Depression Inventory II (BDI‐II), cardiac events Other outcomes: Interpersonal Relationships Inventory (IPRI), Functional Performance Inventory (FPI), ECG, blood pressure |
|
| Funding | Canadian Institute of Health Research; Fondation du Centre Hospitalier de l'Université de Montréal; Fondation de l'Institut de Cardiologie de Montréal; Citalopram and matching placebo donated by Lundbeck Canada Inc. | |
| Notes | Factorial design allowed for two randomised comparisons of main effects: 1) IPT vs. CM, 2) Citalopram vs. Placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer generated block randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "Concealed in sequentially numbered, site‐specific, sealed opaque envelopes stored at the coordinating center until randomization." (p. 369) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Pharmacological intervention arm: Therapists, patients, site psychiatrists, telephone raters for primary outcome, and other personnel blinded to assignment regarding Citalopram treatment Comment: Psychological intervention arm: Telephone rater for primary outcome assessment blinded to patients' allocation to IPT vs. CM |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Intention‐to‐treat (ITT) analysis with last‐observation‐carried‐forward |
| Selective reporting (reporting bias) | Low risk | Comment: Primary and secondary outcomes reported in accordance with the study protocol (ISRCTN15858091) |
| Other bias | High risk | Comment: Conflicting interests: Citalopram and matching placebo donated by Lundbeck Canada Inc. |
Doering 2007.
| Methods | RCT design: 2‐arm parallel group trial Total N randomised: Not stated Length of follow‐up: 4 months Analysis: Per‐protocol |
|
| Participants | Location: USA Number of study centres and setting: 2 urban medical centres CAD diagnosis: Patients undergoing first‐time Coronary Artery Bypass Grafting (CABG); time to randomization not specified Depression diagnosis: Patients with Mini‐Mental State Examination (MMSE) score of >= 24 were interviewed with the Diagnostic Interview and Structured Hamilton (DISH); diagnosis of major depression during inpatient treatment or 2 to 4 weeks after hospital admission or minor depression at both interviews Other entry criteria: <=75 years, English‐speaking, available for 6 months follow‐up Exclusion criteria: Malignancies or autoimmune disorders Treatment N: 7 (100% female, mean age: 58.6 (SD: 7.6)) Control N: 8 (100% female, mean age: 60.9 (SD: 9.4)) Comparability of groups: Treatment patients with a significantly higher rate of depression history |
|
| Interventions | Treatment: Cognitive Behavioral Therapy (weekly 1‐hour sessions) by a trained nurse therapist including establishing therapeutic relationship, behavioral activation, active problem‐solving, identification of automatic thoughts, reframing automatic thoughts, learning self‐therapy and relapse prevention Control: Usual care comprising usual medical and nursing follow‐up after CABG and an assessment by a psychiatrist who recommended individualised treatment options Duration of treatment: 8 weeks |
|
| Outcomes | Review outcomes: Beck Depression Inventory (BDI) score Other outcomes: Postoperative illnesses measured by a Modified Health Review (MHR), Natural killer cell cytotoxicity (NK cytotoxicity), Interleukin (IL‐6), C‐reactive protein (CRP) |
|
| Funding | Not stated | |
| Notes | Study investigated depressed post‐CABG women | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Outcome assessed by a blinded research assistant |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Only those patients who completed all study measures were included in this report." (p. 19) |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcomes reported as stated in the methods section Comment: No protocol or design paper available |
| Other bias | Unclear risk | Comment: No efforts regarding nurse therapists protocol adherence reported Comment: Usual care comprised psychiatrists' recommendations for individualised treatment options, but utilised treatments of control patients were not assessed |
ENRICHD 2003.
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 2481 Length of follow‐up: Evaluations after 6 months and annually thereafter (follow‐up duration 18 to 54 months) Analysis: Intention‐to‐treat (93 treatment patients did not receive intervention) |
|
| Participants | Location: USA Number of study centres and setting: Outpatients from 73 hospitals affiliated with 8 clinical centres CAD criteria: Acute myocardial infarction (MI) with elevation in one or more biomarker as well as MI‐compatible symptoms or characteristic ECG ST‐T changes or new Q waves; randomisation within 28 days after MI Depression criteria: Major depression or dysthymia diagnosis based on the Depression Interview and Structured Hamilton (DISH) according to modified DSM‐IV criteria Other entry criteria: Low perceived social support assessed through the ENRICHD Social Support Instrument (ESSI) Exclusion criteria: Patients with acute MI following Percutaneous Coronary Intervention (PCI) or Coronary Artery Bypass Grafting (CABG), receiving psychotherapy or taking an antidepressant for longer than 14 days but remained depressed, noncardiac conditions likely to be fatal within 1 year, too ill to participate, participating in another trial, major psychiatric disorder (including schizophrenia, bipolar disorder, severe dementia, or active substance abuse), at risk for suicide, refusal of participation or physician disallowed participation, could not be enrolled within 28 days, inaccessible for intervention or follow‐up Treatment: 1238 (43% female, mean age: 61 (SD: 12.6)) Control: 1243 (44% female, mean age: 61 (SD: 12.5)) Comparability of groups: No significant baseline differences except for the use of angiotensin‐converting enzyme (ACE) inhibitors |
|
| Interventions | Treatment: Individual (at least 6 1‐hour sessions weekly) and group (weekly 2‐hour sessions) Cognitive Behavior Therapy (CBT) by Beck supplemented with techniques based on social learning theory for patients with low perceived social support; patients with scores >24 on the Hamilton Rating Scale for Depression (HAM‐D) or those with less than 50% reduction in Beck Depression Inventory (BDI) score after 5 weeks referred to study psychiatrist for consideration of pharmacotherapy with sertraline (50 to 200 mg/d) Control: Usual care Duration of treatment: Individual behavioral intervention up to 6 months with additional 12 weeks for group therapy, adjunctive pharmacotherapy up to 12 months |
|
| Outcomes | Review outcomes: Combined end point of all‐cause mortality and nonfatal reinfarction, all‐cause mortality, cardiovascular mortality, revascularization procedures, cardiovascular hospitalizations, depression (change in HRSD and BDI scores from baseline to 6 months), health‐related quality of life (SF‐12 PCS and MCS) Other outcomes: Social support and social networks, life satisfaction, change in cardiac risk factor profile, perceived stress, self‐efficacy |
|
| Funding | National Heart, Lung, and Blood Institute; Pfizer Inc. | |
| Notes | Mixed study sample (patients with depression and/or low perceived social support were enrolled) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Automated telephone randomization system using permuted blocks with varying sizes, stratified by clinical center; test for selection bias potentially resulting from unmasking of previous assignments (participants and interventionists were unblinded) with nonsignificant results |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation obtained by an automated telephone randomization system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: Participants and interventionists unmasked Quote: "Staff who collected, verified, or classified end point data or follow‐up assessments were masked as much as possible" (Berkman, 2003). Quote: "End point data collection, verification and classification, and follow‐up psychosocial assessments are conducted by staff who are blinded to treatment assignment." (Hoskings, 2000) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Depression outcomes analysed per protocol, all other outcomes ITT |
| Selective reporting (reporting bias) | High risk | Comment: Results of all main outcomes reported as described in the design papers of the trial except for change in cardiac risk factor profile, perceived stress and self‐efficacy |
| Other bias | High risk | Comment: Therapy quality and adherence to treatment protocol were monitored by the Beck Institute Comment: QoL was not assessed at baseline and it thus remains unclear whether or not QoL was balanced in the two groups at baseline Comment: Conflicting interests: Funded by Pfizer (Inc.) |
Fang 2003.
| Methods | RCT design: Parallel‐group trial Total N randomised: 57 Length of follow‐up: 8 weeks Analysis: unclear |
|
| Participants | Location: China Number of study centres: Patients selected from 2 hospitals CAD diagnosis: Myocardial infarction (MI) confirmed by an electronic radiograph Depression diagnosis: Sung's self‐rating depressive scale (SDS) score > 43 Other entry criteria: Sung's self‐rating anxiety scale (SAS) score > 38 Exclusion criteria: unclear Treatment N: 27 (sex and age distribution unclear) Control N: 30 (sex and age distribution unclear) Comparability of the groups: unclear |
|
| Interventions | Treatment: Health education and psychological intervention in addition to standard medication. Health education included basic myocardial infarction knowledge and related subjects such as healthy diet, exercise, cholesterol control. Psychological intervention comprised support (5 times a week, 30 ‐ 40 minutes per meeting), various psychological treatments tailored according to the patients needs (twice a week for 30 ‐ 40 minutes), and mind and body relaxation time using breathing exercises, and various relaxation techniques (twice daily, 15 ‐ 20 minutes) Control: Usual care Duration of treatment: 8 weeks |
|
| Outcomes | Review outcomes: Sung's self‐rating depressive scale (SDS) score Other outcomes: Sung's self‐rating anxiety scale (SAS) score, New York Heart Association (NYHA) functional class, Left Ventricular Ejection Fraction (LVEF) |
|
| Funding | unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not applicable |
| Allocation concealment (selection bias) | Unclear risk | not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not applicable |
| Selective reporting (reporting bias) | Unclear risk | not applicable |
| Other bias | Unclear risk | not applicable |
Freedland 2009.
| Methods | RCT design: 3‐arm parallel‐group trial Total N randomised: 123 Length of follow‐up: 6 months Analysis: Intention‐to‐treat (ITT) |
|
| Participants | Location: USA Number of study centres and setting: Patients who had undergone Coronary Artery Bypass Surgery (CABG) from 3 hospitals CAD diagnosis: Coronary Artery Bypass Surgery (CABG), randomisation within 12 months after surgery Depression diagnosis: Beck Depression Inventory (BDI) score of 10 or higher and current major or minor depressive episode assessed with the Depression Interview and Structured Hamilton (DISH) Other entry criteria: Patients aged 21 years or older Exclusion criteria: Severe psychiatric comorbidities (schizophrenia or bipolar disorder), active alcoholism or substance abuse, severe cognitive impairment, noncardiac illnesses with a poor 1‐year prognosis, being too medically ill or living too far away to participate, being unable to communicate in English, or for receiving ongoing psychotherapeutic services Treatment 1 (CBT) N: 41 (56% female, mean age: 62 (SD: 11)) Treatment 2 (SSM) N: 42 (50% female, mean age: 59 (SD: 10)) Control N: 40 (43% female, mean age: 61 (SD: 9)) Comparability of groups: Proportion of African American participants in treatment 2 (SSM) significantly higher than in the other arms |
|
| Interventions | Treatment 1: Individual Cognitive Behavior Therapy (CBT) (weekly 1‐hour sessions) including target problem identification, problem solving, behavioral activation, cognitive techniques (challenging distressing automatic thoughts, changing dysfunctional attitudes), self‐therapy and relapse‐prevention skills Treatment 2: Supportive Stress Management (SSM) (weekly 1‐hour sessions) including patient education regarding stress and coping, practice in progressive muscle relaxation training, controlled breathing and relaxing imagery Control: Usual care for depression Duration of treatment: 12 weeks |
|
| Outcomes | Review outcomes: Hamilton Depression Rating Scale depression remission (HAM‐D < 7), HAM‐D score, BDI depression remission, BDI score, Medical Outcomes Study Short‐Form 36‐item Health Survey (SF‐36) Other outcomes: Beck Anxiety Inventory, Beck Hopelessness Scale, Perceived Stress Scale, Heart Surgery Questionnaire (HSQ), Digit Symbol test, part B of the Trailmaking Test, a paragraph recall test, and the Short Blessed Test to assess cognitive impairment after CABG surgery |
|
| Funding | National Institute of Mental Health, USA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Computer‐generated random allocation sequence with block sizes of 3 and 6 |
| Allocation concealment (selection bias) | Low risk | Comment: Concealed in sealed envelopes and revealed to the study coordinator immediately after the participant completed all of the baseline assessments |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The outcome assessors were masked to the participants' group assignments" (p. 389) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: Missing data "plausibly missing at random" (p. 389) Comment: Missing outcome data imputed |
| Selective reporting (reporting bias) | Low risk | Comment: Outcomes reported in accordance to the study protocol |
| Other bias | Low risk | |
Freeman 1986.
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 107 Length of follow‐up: no follow‐up Analysis: Per‐protocol (60 of 107 patients completed the trial) |
|
| Participants | Location: USA Number of study centres and setting: Patients from 1 hospital CAD diagnosis: Patients undergoing Coronary Artery Bypass Surgery (CABG) (assessment method and time to randomisation not specified) Depression diagnosis: A score of 13 or greater on the Center for Epidemiological Studies ‐ Depression Scale (CES‐D) and/or a score of 36 or greater on the Spielberger State Anxiety Inventory (SSAI); presence of clinically significant anxiety or depression was confirmed by a semistructured psychiatric interview Other entry criteria: Under 65 years of age Exclusion criteria: Females of childbearing potential, patients with a history of sensitivity to benzodiazepines, patients with prior or existing evidence for substance abuse, antisocial personality, psychosis, significant uncontrolled systemic disease, cerebral infarction, dementia, or insufficient English Treatment N: 32 (sex and age distribution unclear) Control N: 28 (sex and age distribution unclear) Comparability of groups: Treatment group significantly higher anxiety scores at baseline; no further information regarding comparability of groups |
|
| Interventions | Treatment: Alprazolam (tablets, 2.5mg/d at bedtime, maximum dose 4.5mg/d) Control: Placebo Duration of treatment: 1 month |
|
| Outcomes | Review outcomes: CES‐D score, Zung Self‐Rating Depression Scale (Zung SDS) score Other Outcomes: SSAI score, Zung Self‐Rating Anxiety Scale (Zung SAS), Physician's Global Impression Scale, signs and symptoms of psychosis, cognitive dysfunction, depression, anxiety, and somatization (psychiatric semistructured interviews) |
|
| Funding | Upjohn Company | |
| Notes | Mixed study sample (patients with depression and/or anxiety) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: No details reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Only 60 of 107 patients completed the trial Comment: 22 early dropouts in the alprazolam group (with noncompleters being less distressed than completers preoperatively) Comment: 25 early dropouts in the placebo group (with noncompleters being more distressed than completers preoperatively) |
| Selective reporting (reporting bias) | High risk | Comment: No protocol or design paper available Comment: Signs and symptoms of psychosis, cognitive dysfunction, depression, anxiety, and somatization (psychiatric semistructured interviews) assessed but not reported |
| Other bias | High risk | Comment: Possible selection bias: 60% of 459 patients met entrance criteria, but only 23% were included. "The remainder were excluded from entering the drug trial by semistructured interview or were rendered ineligible because of surgical complications or withdrawal of consent." (p. 39) Comment: Possible baseline imbalance: Treatment group significantly higher anxiety scores at baseline; no further information regarding comparability of groups Comment: Conflicting interests: Funding through Upjohn Company |
Li 2005.
| Methods | RCT design: Parallel‐group trial Total N randomised: 87 Length of follow‐up: 6 weeks Analysis: unclear (2 cases lost in the treatment group, 3 cases lost in the control group) |
|
| Participants | Location: China Number of study centres and setting: Hospitalized patients (number of centres unclear) CAD diagnosis: Patients after Coronary Artery Bypass Grafting (CABG) Depression diagnosis: Self‐rated Hamilton Depression Rating Scale (HAM‐D) score >18 Other entry criteria: unclear Exclusion criteria: unclear Treatment N: 43 (sex and age distribution unclear) Control N: 39 (sex and age distribution unclear) Comparability of groups: unclear |
|
| Interventions | Treatment: St. John's Wort extract (300 mg, 3 times a day) Control: Placebo Duration of treatment: 6 weeks |
|
| Outcomes | Review outcomes: HAM‐D score Other outcomes: Ventricular function (Tei‐Index), side‐effects |
|
| Funding | unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not applicable |
| Allocation concealment (selection bias) | Unclear risk | not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not applicable |
| Selective reporting (reporting bias) | Unclear risk | not applicable |
| Other bias | Unclear risk | not applicable |
Liu 1999.
| Methods | RCT design: Parallel‐group trial Total N randomised: unclear Length of follow‐up: 4 weeks Analysis: unclear |
|
| Participants | Location: China Number of study centres and setting: Patients from 1 hospital CAD diagnosis: Myocardial infarction (MI) as confirmed by electrocardiography Depression diagnosis: Center for Epidemiologic Studies Depression Scale (CES‐D), Hamilton Depression Rating Scale (HAMD), diagnosis according to Chinese Classification of Mental Disorders, Second Edition, Revised (CCMD‐2‐R) Other entry criteria: unclear Exclusion criteria: unclear Treatment N: 31 (32% female, mean age unclear) Control N: 37 (27% female, mean age unclear) Comparability of groups: No significant differences |
|
| Interventions | Treatment: Fluoxetine Control: Placebo Duration of treatment: 4 weeks |
|
| Outcomes | Review outcomes: HAM‐D, Mortality Other Outcomes: Ventricular Tachycardia (VT), Heart Rate Variability (HRV) |
|
| Funding | unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not applicable |
| Allocation concealment (selection bias) | Unclear risk | not applicable |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | not applicable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not applicable |
| Selective reporting (reporting bias) | Unclear risk | not applicable |
| Other bias | Unclear risk | not applicable |
McFarlane 2001.
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 38 Length of follow‐up: No follow‐up Analysis: Per‐protocol (27 completers, 6 drop‐outs in the treatment group, 5 drop‐outs in the control group) |
|
| Participants | Location: Canada Number of study centres and setting: Patients admitted to 1 coronary care unit CAD diagnosis: Acute myocardial infarction; assessment method and time to randomisation not specified Depression diagnosis: Score > 15 on the Inventory to Diagnose Depression (IDD) questionnaire on two occasions (just before hospital discharge and two weeks later) Other entry criteria: None stated Exclusion criteria: Predischarge 24‐hour Holter recordings showing either atrial fibrillation or ventricular etopic beats greater than 100 per hour, congestive heart failure, any life‐threatening comorbid condition, inability to complete the questionnaire, and taking antidepressant medication Treatment N: 12 (33% female, mean age: 56 (SD: 11)) Control N: 15 (47% female, mean age: 56 (SD: 12)) Comparability of groups: No significant baseline differences |
|
| Interventions | Treatment: Sertraline (50 mg/d) Control: Placebo Duration of treatment: 22 weeks |
|
| Outcomes | Review outcomes: IDD depression score Other outcomes: Time dependent changes in both time and frequency domain parameters of Heart Rate Variability (HRV) (heart rate, SD of all 24‐hour N‐N intervals (SDNN), root mean square of the SD of successive N‐N intervals (rMSSD), LF/HF ratio, LF power nu) |
|
| Funding | Heart and Stroke Foundation of Ontario, Canada | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: No details reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 11 drop‐outs (3 had side‐effects, 7 non‐compliant, 1 with ectopy) |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol or design paper available Comment: Outcomes reported according to methods section |
| Other bias | High risk | Comment: Inconsistent description of results (p. 619 and p. 620) |
McLaughlin 2005.
| Methods | RCT design: 2‐arm parallel group trial Total N randomised: 100 Length of follow‐up: 4 months Analysis: Per‐protocol (8 treatment patients dropped out, 12 control patients (1 death) dropped out) |
|
| Participants | Location: USA Number of study centres and setting: Patients with Acute Coronary Syndrome (ACS) from 2 Hospitals CAD diagnosis: Acute Coronary Syndrome (ACS) assessed by medical chart review in the coronary care unit; time to randomisation not specified Depression diagnosis: Score of 7 and more on either of the subscales of the Hospital Anxiety Depression Scale (HADS) Other entry criteria: 35 years of age or older, able to speak English, access to a touch‐tone phone Exclusion criteria: Mental health care in the prior 3 months, psychoactive drug use during the past year, and diagnosis of substance abuse during the past year Treatment N: 45 (31.1% female, mean age: 59.9 (SD: 10.2)) Control N: 34 (35.3% female, mean age: 60.7 (SD: 9.8)) Comparability of the groups: Significantly higher anger scores among females in the treatment group, significantly more patients with Myocardial Infarction (MI) in the treatment group |
|
| Interventions | Treatment: 6 telephone counselling sessions (30 minutes each) with clinicians (psychiatrist, clinical psychologist, internist) comprising review of common fears experienced by those living with chronic medical conditions and identification and management of barriers to adjustment to medical illness; patients with HADS score > 15 were referred for emergent care Control: Usual care (received a booklet on coping with cardiac illness typical of those given at hospital discharge and were instructed to contact their primary care physician if they experienced any warning signs of depression; advised to continue follow‐up with their primary care and specialist physicians) Duration of treatment: 8 weeks |
|
| Outcomes | Review outcomes: HADS depression score Other outcomes: HADS anxiety score, Work and Social Adjustment Scale (WSAS), State‐Trait Anger Expression Inventory (STAXI), Clinical Global Impressions (CGI) scale |
|
| Funding | National Institute of Mental Health, USA; Robert Wood Johnson Foundation | |
| Notes | Mixed study sample (patients with depression and/or anxiety) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "by coin flip" (p. 1085 in McLaughlin et al., 2005) |
| Allocation concealment (selection bias) | High risk | Quote: "The study coordinator conducted the coin flip and assigned patients to a treatment arm when she contacted study participants by telephone and enrolled consenting participants." (p. 540 in Bambauer et al., 2005) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: Patient self‐report (HADS) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: The authors describe that multiple imputation methods were used to examine if data were missing at random. But all analyses were reported for the final cohort of 79 patients |
| Selective reporting (reporting bias) | Unclear risk | Comment: Outcomes consistent in methods and results sections Comment: No protocol and design paper available |
| Other bias | High risk | Comment: Results reported inconsistently (discrepancy between text and figure regarding HADS score) |
MIND‐IT 2007.
| Methods | RCT design: Nested, 2‐arm parallel‐group trial Total N randomised: 91 Length of follow‐up: No follow‐up Analysis: Intention‐to‐treat (10 drop‐outs in the intervention group compared to 3 in the placebo group during the first 8‐week acute treatment phase, 23 drop‐outs in the intervention group compared to 15 in the placebo group during the entire treatment (24 weeks)) |
|
| Participants | Location: Netherlands Number of study centres and setting: Patients with a Myocardial Infarction (MI) from 8 hospitals CAD criteria: MI with typical clinical picture, increase of cardiac enzymes, electrocardiographic (ECG) changes and chest pain for > 20 minutes; time to randomisation 3 to 12 months (to exclude adjustment disorders) Depression criteria: 2‐stage procedure, in which those with 1) score of 10 or more on the Beck Depression Inventory (BDI) were 2) interviewed with the Composite International Diagnostic Interview (CIDI) for major or minor depression diagnosis (psychiatrist confirmed CIDI diagnosis) Other entry criteria: age >= 18 years Exclusion criteria: Occurence of MI while hospitalized for another reason, except for unstable angina pectoris, lacking capability to participate in study procedures, any disease likely to influence short‐term survival, already receiving psychiatric treatment for depressive disorder, participation in any clinical trial that might intervene with the study, hyperthyroidism, suicidality Treatment N: 47 (12.8% female, mean age: 56.6 (SD: 11.1)) Control N: 44 (18.2% female, mean age: 57.9 (SD: 9.7)) Comparability of groups: No significant baseline differences |
|
| Interventions | Treatment: Mirtazapine (30 to 45 mg/d); patients who did not respond and patients with relapse were offered open treatment with citalopram Control: Placebo Duration of treatment: 24 weeks (8 weeks acute plus 16 weeks continuation treatment) |
|
| Outcomes | Review outcomes: Hamilton Depression Rating Scale (HAM‐D) score, depression remission (HAM‐D <= 7), BDI score, depression scale of the Symptom Checklist 90 item (dSCL‐90), cardiac events Other outcomes: Clinical Global Impression (CGi), side effects, concurrent medication, weight, heart rate, blood pressure, PR interval, QRS interval, QT interval |
|
| Funding | Netherlands Heart Foundation; Organon (Netherlands); Lundbeck (Denmark) | |
| Notes | MIND‐IT trial investigated antidepressant treatment in general versus usual care in patients following myocardial infarction (N = 331). The intervention arm consisted of double‐blind Mirtazapine, open pharmacological treatment, non‐pharmacological treatment or no treatment. The care as usual arm comprised pharmacological treatment, non‐pharmacological treatment or no treatment. Data of the nested trial investigating mirtazapine versus placebo (n = 91) was used in this review due to the pre‐defined comparisons (i.e. pharmacological intervention vs. placebo). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Central randomization service (computer‐generated blocks of four) |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" (p. 607) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: ITT with last observation carried forward |
| Selective reporting (reporting bias) | Unclear risk | Comment: Results and methods section consistent Comment: No protocol or design paper available |
| Other bias | High risk | Comment: Conflicting interests: Funded by Organon (Netherlands) and Lundbeck (Denmark) |
Roose 1998.
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 81 Length of follow‐up: No follow‐up Analysis: Intention‐to‐treat (4 Paroxetine patients discontinued, 10 Nortriptyline patients discontinued) |
|
| Participants | Location: USA Number of study centres and setting: Outpatients from 4 hospitals CAD criteria: Myocardial infarction (MI), coronary artery bypass grafting, coronary angioplasty, positive stress test, or angiographic evidence of a 75% or greater luminal narrowing of a major coronary artery; time to randomisation unclear Depression criteria: Meeting DSM‐IV criteria for major depressive disorder, unipolar subtype, with a score of 16 or greater on the 17‐item Hamilton Rating Scale for Depression (HAM‐D) Other entry criteria: Age >= 18 Exclusion criteria: MI within the past 3 months, a baseline QTc interval of 460 milliseconds or greater, unstable or crescendo angina, receiving drugs with class 1 antiarrhythmic activity or warfarin Treatment 1 N: 41 (12% female, mean age: 57.8 (SD: 11.0)) Treatment 2 N: 40 (22% female, mean age: 57.9 (SD: 12.7)) Comparability of groups: No significant baseline differences |
|
| Interventions | Treatment 1: Paroxetine (+ dummy placebo at night) (age < 65: 20 mg/d for the first 3 weeks; age > 65: 10 mg/d for the first week, 20 mg/d for week 2 and 3; if no response (HAM‐D reduction 50% or HAM‐D <= 8) 30 mg/d at week 4 and 40 mg/d at end of week 5) Treatment 2: Nortriptyline (+ dummy placebo in the morning) (25 mg for the first 2 days; 50 mg on day 3; on day 7 plasma level measurement and adjustment of the dose to achieve a nortriptyline plasma level between 203 and 456 nmol/L (80‐120 ng/mL)) Duration of treatment: 6 weeks |
|
| Outcomes | Review outcomes: HAM‐D depression score, depression remission rate (HAM‐D score of 8 or less) Other outcomes: Heart rate, blood pressure, PR interval, QRS interval, QT interval, heart rate variability (SDNN, pNN50), adverse events |
|
| Funding | Smith‐Kline Beecham Pharmaceuticals (GlaxoSmithKline) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized by permuted blocks of 10" (p. 288) |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Double dummy technique" (p. 288) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: ITT with last observation carried forward |
| Selective reporting (reporting bias) | Unclear risk | Comment: Results and methods section consistent Comment: No protocol or design paper available |
| Other bias | High risk | Comment: Conflicting interests: Funded by Smith‐Kline Beecham Pharmaceuticals |
SADHART 2002.
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 369 Length of follow‐up: No follow up Analysis: Intention‐to‐treat (53 discontinued treatment, 46 discontinued placebo) |
|
| Participants | Location: USA, Europe, Canada, Australia Number of study centres and setting: Outpatients from 40 cardiology centres and psychiatry clinics CAD criteria: Patients hospitalized for Myocardial Infarction (MI) or unstable angina in the past 30 days. Criteria for acute MI: at least 1 criterion from each of the following 2 categories: Category A: 1) creatine kinase isoenzyme MB (CK‐MB) level greater than the upper limit of normal, 2) CK or troponin T or troponin 1 level more than 2 times the upper limit of normal, 3) a total lactate dehydrogenase (LDH) level more than 1.5 times the upper limit of normal (with LDH 1 greater than LDH 2). Category B: 1) typical ischemic symptoms (chest pain or shortness of breath) lasting for more than 10 minutes, 2) ECG evidence of ischemic ST‐segment depression, ST‐segment elevation, or new pathological Q waves. Criteria for unstable angina: 1) experienced angina of anginal equivalent symptoms at rest, with episodes lasting for at least 10 minutes and leading to hospitalization, and had ECG documentation of transient ST‐segment elevation or depression of more than 0.5 mm, or had T wave inversion of greater han 1 mm within 12 hours of an episode of chest pain; 2) were hospitalized for symptoms of unstable angina and had known CAD with a documented history of a prior MI, had undergone a prior revascularization procedure, or had documented coronary artery stenosis greater than 75% in one of the major epicardial vessels. Depression criteria: Major depression according to structured Diagnostic Interview Schedule (DIS) for DSM‐IV, Beck Depression Inventory (BDI) score of 10 or greater Other entry criteria: None Exclusion criteria: Uncontrolled hypertension, cardiac surgery anticipated during the next 6 months, MI or unstable angina developed less than 3 months after CABG, resting heart rate of less than 40/min, MI or unstable angina of nonatherosclerotic etiology, Killip class III or IV status, persistent clinically significant laboratory abnormalities, renal dysfunction, hepatic dysfunction, other significant noncardiac disease, women of childbearing potential not using adequate contraception, current use of class 1 antiarrhythmic medications, use of reserpine, guanethidine, clonidine, methyldopa, anticonvulsants, neuroleptics, antidepressants, benzodiazepines, initiation of psychotherapy in the 3 months prior to study entry, alcohol or substance abuse or dependence in past 6 months, psychotic symptoms, history of psychosis, bipolar disorder, organic brain syndrome, dementia, significant suicide risk Treatment N: 186 (37% female, mean age: 56.8 (SD: 11.1)) Control N: 183 (36% female, mean age: 57.6 (SD: 10.4)) Comparability of groups: No significant baseline differences |
|
| Interventions | Treatment: Sertraline 50 mg/d for the first 6 weeks, up to 100 mg/d for weeks 6‐10, up to 150 mg/d for weeks 10‐12, up to 200 mg/d for weeks 12‐24 Control: Placebo Duration of treatment: 24 weeks |
|
| Outcomes | Review outcomes: Cardiac events, mortality, Hamilton Rating Scale for Depression (HAM‐D) score, direct medical costs (inpatient hospitalizations, emergency room visits, cardiac procedures), quality of life (Quality of Life Enjoyment and Satisfaction scale (Q‐LES‐Q) and Medical Outcomes Study Short‐Form 36 (SF‐36)) Other outcomes: Left Ventricular Dysfunction (LVEF), heart rate, blood pressure, standard ECG, heart rate variability, Clinical Global Impression ‐Severity (CGI‐S) and ‐Improvement (CGI‐I), ventricular premature complexes |
|
| Funding | Pfizer Inc.; Suzanne C. Murphy Foundation; Thomas and Caroline Royster Research Fund; Perry and Martin Granoff Family Foundation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" (p. 702) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Last observation carried forward |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol or design paper available |
| Other bias | High risk | Comment: Conflicting interests: Funded by Pfizer Inc. |
Strik 2000.
| Methods | RCT design: 2‐arm parallel‐group trial Total N randomised: 54 Length of follow‐up: No follow‐up Analysis: Intention‐to‐treat for primary outcomes (9 withdrawn in control, 5 withdrawn in treatment group), per‐protocol for cardiologic safety variables |
|
| Participants | Location: Netherlands Number of study centres and setting: Patients from 2 hospitals CAD criteria: Myocardial Infarction (MI) diagnosed by a cardiologist with a clinical picture typical of MI, electrocardiographic changes specific for MI, and a maximum plasma concentration of aspartate aminotransferase (ASAT) of twice the upper normal range (80 U/liter); enrolment 3 to 12 months after MI Depression criteria: Patients with a score above the cut‐off on the SCL‐90 Depression Scale (>22 for men and > 28 for women) were interviewed with the Schedules for Clinical Assessment in Neuropsychiatry; patients meeting DSM‐III‐R criteria for major depressive episode and having a Hamilton Rating Scale for Depression (HAM‐D) score of >17 were included Other entry criteria: 18 to 75 years Exclusion criteria: Any concurrent psychosocial or therapeutic intervention, psychotic symptomatology, a second psychiatric diagnosis, history of mania, current pregnancy or lactation, life‐threatening noncardiac physical illness, concurrent use of psychotropic drugs, hypersensitivity to fluoxetine, liver or severe kidney dysfunction, right ventricular filling pressure >30 mm Hg and a low systolic volume or an ATVI <10 cm Treatment N: 27 (22% female, mean age: 54.1 (SD: 11.3)) Control N: 27 (37% female, mean age: 58.7 (SD: 10.1)) Comparability of groups: No significant baseline differences |
|
| Interventions | Treatment: Fluoxetine (acute treatment period of 9 weeks, and continuation period of 16 weeks; 20 to 60 mg/d) Control: Placebo Duration of treatment: Maximum of 25 weeks |
|
| Outcomes | Review outcomes: Depression remission defined as a HAM‐D end‐point score of < 7, death, rehospitalization due to cardiac events Other outcomes: SCL‐90 Hostility Scale score, concurrent use of medications, cognitive performance, non‐cardiac life‐threatening disease, blood pressure, electrocardiographic variables (heart rate, PR interval, QRS interval, QT interval), echocardiographic variables (LVEF, ATVI, E/A ratio) |
|
| Funding | Eli Lilly, Dutch Prevention Fund; Maastricht University Hospital Research Fund | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: No details reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" (p. 785) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: 14 patients meeting inclusion criteria were not included, but did not differ from participants in age, gender, or maximum ASAT Comment: Intention‐to‐treat for primary outcomes |
| Selective reporting (reporting bias) | High risk | Comment: Many outcomes not or only partially reported Comment: No protocol or design paper available |
| Other bias | High risk | Comment: Conflicting interests: Funded by Eli Lilly |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Black 1998 | Study investigated psychologically distressed patients (depression was not explicitly assessed) |
| Bucknall 1988 | Study investigated a sample of heart disease patients, which was not restricted to CAD |
| Davidson 2010 | Study investigated patient‐preference, stepped‐care depression treatment (including no treatment, problem‐solving therapy and/or pharmacotherapy) versus usual care, which is not a pre‐defined comparison of this review |
| Fu 2006 | Control group unclear (treatment with "Shierkang tablets") |
| González‐Jaimes 2003 | Study investigated patients with acute myocardial infarction and adjustment disorder with depressed mood (DSM‐IV: 309.0), but not depression |
| Kachkovskii 2006 | Control group unclear |
| Mohapatra 2005 | Study compares pharmacological treatment versus usual care, which is not a pre‐defined comparison of this review |
| Norris 2009 | Study investigated effectiveness of providing follow‐up information regarding mental health services to depressed patients after cardiac catheterization |
| Oldridge 1991 | Intervention not specifically for treating depression |
| Pogosova 2004 | Study compared pharmacological treatment versus usual care, which is not a pre‐defined comparison of this review |
| Pogosova 2009 | Study compared pharmacological treatment versus usual care, which is not a pre‐defined comparison of this review |
| Rollman 2009 | Study investigated telephone‐delivered collaborative care versus usual care, which is not a pre‐defined comparison of this review |
| Schrader 2005 | Study investigated effectiveness of different forms of communication between hospital psychiatric services and general practitioners of depressed cardiac patients Sample of heart disease patients not restricted to CAD |
| Stern 1983 | Study compared counselling versus exercise therapy, which is not a pre‐defined comparison of this review |
| Veith 1982 | Sample of heart disease patients not restricted to CAD |
| Zeng 2001 | Study compared pharmacological treatment versus usual care, which is not a pre‐defined comparison of this review |
Characteristics of studies awaiting assessment [ordered by study ID]
Malik 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Conference abstract identified through database search. No published data available. No contact information available. |
Characteristics of ongoing studies [ordered by study ID]
SPIRR‐CAD 2008.
| Trial name or title | A Stepwise Psychotherapy intervention for Reducing Risk in Coronary Artery Disease ‐ a randomized controlled trial (SPIRR‐CAD) ISRCTN76240576 |
| Methods | RCT design: 2‐arm parallel‐group trial Target number of participants: 569 Length of follow‐up: 24 months |
| Participants | Location: Germany Inclusion criteria: Patients with recent coronary heart disease and elevated Hospital Anxiety and Depression Scale (HADS) depression subscale score (> 7) |
| Interventions | Treatment: Stepwise, individual and group psychotherapy in addition to usual cardiological care Control: Usual cardiological care |
| Outcomes | Primary outcome: Changes from baseline to year 1 in depressive symptoms (HADS‐D) |
| Starting date | 01/12/2008 |
| Contact information | Prof. Christoph Herrmann‐Lingen: cherma@gwdg.de |
| Notes | Funding: German Research Foundation |
UPBEAT 2007.
| Trial name or title | Understanding prognostic benefits of exercise and antidepressant therapy (UPBEAT) NCT00302068 |
| Methods | RCT design: 3‐armed parallel‐group trial Target number of participants: 200 Length of follow‐up: 12 months |
| Participants | Location: USA Inclusion criteria: Patients with coronary heart disease (CHD) and depressive symptoms (Beck Depression Inventory (BDI‐II) score 9 or greater) |
| Interventions | Treatment: Aerobic exercise Active comparator: Sertraline Control: Placebo |
| Outcomes | Primary outcome: Hamilton Rating Scale for Depression (HAM‐D) |
| Starting date | July 2006 |
| Contact information | James A. Blumenthal, PhD: blume003@mc.duke.edu Andrew Sherwood, PhD: sherw002@mc.duke.edu |
| Notes | Funding: National Heart, Lung, and Blood Institute, USA |
Differences between protocol and review
Subgroup analyses and sensitivity analyses have been omitted due to the small amount of trials investigating the various outcomes.
For the same reason funnel plots were not created and not tested for asymmetry.
Contributions of authors
Harald Baumeister: drafting of protocol, developing of search strategy, trials search and selection, data extraction, entering data into RevMan, data analysis, drafting the review, updating the review
Nico Hutter: drafting of protocol, developing of search strategy, trials search and selection, data extraction, entering data into RevMan, data analysis, drafting the review
Jürgen Bengel: drafting of protocol, trials search and selection, drafting the review
Sources of support
Internal sources
No sources of support supplied
External sources
-
German Federal Ministry of Education and Research, Germany.
Funding the review project
Declarations of interest
None known
New
References
References to studies included in this review
Barth 2005 {published and unpublished data}
- Barth J, Paul J, Härter M, Bengel J. Inpatient psychotherapeutic treatment for cardiac patients with depression in Germany: short‐term results. GMS Psycho‐Social‐Medicine 2005;2:1‐8. [PMC free article] [PubMed] [Google Scholar]
Brown 1993 {published data only (unpublished sought but not used)}
- Brown MA, Munford AM, Munford PR. Behavior therapy of psychological distress in patients after myocardial infarction or coronary bypass. Journal of Cardiopulmonary Rehabilitation and Prevention 1993;13:201‐10. [Google Scholar]
CREATE 2007 {published data only}
- Frasure‐Smith N, Koszycki D, Swenson JR, Baker B, Zyl L. Design and Rationale for a Randomized, Controlled Trial of Interpersonal Psychotherapy and Citalopram for Depression in Coronary Artery Disease (CREATE): Erratum. Psychosomatic Medicine 2007;69(2):216. [DOI] [PubMed] [Google Scholar]
- Frasure‐Smith N, Koszycki D, Swenson JR, Baker B, Zyl LT, Laliberté MA, et al. Design and rationale for a randomized, controlled trial of interpersonal psychotherapy and citalopram for depression in coronary artery disease (CREATE). Psychosomatic Medicine 2006;68:87‐93. [DOI] [PubMed] [Google Scholar]
- Lespérance F, Frasure‐Smith N, Koszycki D, Laliberté MA, Zyl LT, Baker B, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007;29:367‐79. [DOI] [PubMed] [Google Scholar]
- Zyl LT, Lesperance F, Frasure‐Smith N, Malinin AI, Atar D, Laliberte MA, et al. Platelet and endothelial activity in comorbid major depression and coronary artery disease patients treated with citalopram: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy Trial (CREATE) biomarker sub‐study. Journal of Thrombosis & Thrombolysis 2009;27(1):48‐56. [DOI] [PubMed] [Google Scholar]
Doering 2007 {published data only (unpublished sought but not used)}
- Doering LV, Cross R, Vredevoe D, Martinez‐Maza O, Cowan MJ. Infection, depression, and immunity in women after coronary artery bypass: a pilot study of cognitive behavioral therapy. Alternative Therapies in Health and Medicine 2007;13:18‐21. [PubMed] [Google Scholar]
ENRICHD 2003 {published data only (unpublished sought but not used)}
- Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106‐16. [DOI] [PubMed] [Google Scholar]
- ENRICHD Investigators. Enhancing Recovery in Coronary Heart Disease (ENRICHD) study intervention: rationale and design. Psychosomatic Medicine 2001;63(5):747‐55. [PubMed] [Google Scholar]
- ENRICHD Investigators. Enhancing recovery in coronary heart disease (ENRICHD): baseline characteristics. The American Journal of Cardiology 2001;88(3):316‐22. [DOI] [PubMed] [Google Scholar]
- ENRICHD Investigators. Enhancing recovery in coronary heart disease patients (ENRICHD): study design and methods. American Heart Journal 2000;139:1‐9. [DOI] [PubMed] [Google Scholar]
- Mendes de Leon CF, Czajkowski SM, Freedland KE, Bang H, Powell LH, Wu C, et al. The effect of a psychosocial intervention and quality of life after acute myocardial infarction: the Enhancing Recovery in Coronary Heart Disease (ENRICHD) clinical trial. Journal of Cardiopulmonary Rehabilitation 2006;26:9‐13. [DOI] [PubMed] [Google Scholar]
Fang 2003 {published data only}
- Fang R, Jiang Y, Song J, Cheng G, Xue G. Control study of general psychological intervention for patients with myocardial infarction [Chinese]. Chinese Journal of Clinical Rehabilitation 2003;7:1382‐3. [Google Scholar]
Freedland 2009 {published data only}
- Freedland KE. Treatment of depression after coronary bypass surgery. http://www.controlled‐trials.com/mrct/trial/438737 (accessed 14 December 2009).
- Freedland KE, Skala JA, Carney RM, Rubin EH, Lustman PJ, Dávila‐Roman VG, et al. Treatment of depression after coronary artery bypass surgery: a randomized controlled trial. Archives of General Psychiatry 2009;66:387‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 1986 {published data only (unpublished sought but not used)}
- Freeman AM, Fleece L, Folks DG, Sokol RS, Hall KR, Pacifico AD, et al. Alprazolam treatment of postcoronary bypass anxiety and depression. Journal of Clinical Psychopharmacology 1986;6:39‐41. [PubMed] [Google Scholar]
Li 2005 {published data only}
- Li YM, Li GY, Wang XL, Yu LF. Rehabilitation effect of San John's Wort extract on depression and myocardial function after coronary artery bypass grafting: a randomized grouping, placebo‐control and blind evaluation [Chinese]. Zhongguo Linchuang Kangfu 2005;9:38‐9. [Google Scholar]
Liu 1999 {published data only}
- Liu JD, Zheng H. Efficacy of fluoxetine in the treatment of depression in patients with acute myocardial infarction [Chinese]. Journal of Clinical Psychological Medicine 1999;9:210‐1. [Google Scholar]
McFarlane 2001 {published data only (unpublished sought but not used)}
- McFarlane A, Kamath MV, Fallen EL, Malcolm V, Cherian F, Norman G. Effect of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction. American Heart Journal 2001;142(4):617‐23. [DOI] [PubMed] [Google Scholar]
McLaughlin 2005 {published data only (unpublished sought but not used)}
- Bambauer KZ, Aupont O, Stone PH, Locke SE, Mullan MG, Colagiovanni J, et al. The effect of a telephone counseling intervention on self‐rated health of cardiac patients. Psychosomatic Medicine 2005;67:539‐45. [DOI] [PubMed] [Google Scholar]
- McLaughlin TJ, Aupont O, Bambauer KZ, Stone P, Mullan MG, Colagiovanni J. Improving psychologic adjustment to chronic illness in cardiac patients: the role of depression and anxiety. Journal of General Internal Medicine 2005;20:1084‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
MIND‐IT 2007 {published data only (unpublished sought but not used)}
- Honig A, Kuyper AM, Schene AH, Melle JP, Jonge P, Tulner DM, et al. Treatment of post‐myocardial infarction depressive disorder: a randomized, placebo‐controlled trial with mirtazapine. Psychosomatic Medicine 2007;69:606‐13. [DOI] [PubMed] [Google Scholar]
- Schins A, Hamuly KK, Scharp S, Lousberg R, Melle J, Crijns H, et al. Whole blood serotonin and platelet activation in depressed post‐myocardial infarction patients. Life Sciences 2004;76(6):637‐50. [DOI] [PubMed] [Google Scholar]
- Melle JP, Jonge P, Honig A, Schene AH, Kuyper AM, Crijns HJ, et al. Effects of antidepressant treatment following myocardial infarction. The British Journal of Psychiatry 2007;190:460‐6. [DOI] [PubMed] [Google Scholar]
- Brink RH, Melle JP, Honig A, Schene AH, Crijns HJ, Lambert FP, et al. Treatment of depression after myocardial infarction and the effects on cardiac prognosis and quality of life: rationale and outline of the Myocardial INfarction and Depression‐Intervention Trial (MIND‐IT). American Heart Journal 2002;144:219‐25. [PubMed] [Google Scholar]
Roose 1998 {published data only (unpublished sought but not used)}
- Nelson JC, Kennedy JS, Pollock BG, Laghrissi‐Thode F, Narayan M, Nobler MS, et al. Treatment of major depression with nortriptyline and paroxetine in patients with ischemic heart disease. The American Journal of Psychiatry 1999;156:1024‐8. [DOI] [PubMed] [Google Scholar]
- Pollock BG, Laghrissi‐Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. Journal of Clinical Psychopharmacology 2000;20(2):137‐40. [DOI] [PubMed] [Google Scholar]
- Roose S, Pollock B, Kennedy J, Nelson J, Gergel I, McCafferty J. Paroxetine in the treatment of depressed patients with ischaemic heart disease. Sixth World Congress of Biological Psychiatry, Nice, France. June 22 27. 1997.
- Roose S, Pollock B, Kennedy J, Nelson J, McCafferty J, Gergel I. Paroxetine versus nortriptyline in ischemic disease. 150th Annual Meeting of the American Psychiatric Association San Diego, California, USA 17 22 May. 1997.
- Roose SP, Laghrissi‐Thode F, Kennedy JS, Nelson JC, Bigger JT, Pollock BG, et al. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA 1998;279:287‐91. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Pesce V, Jayaraman A, Roose S. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on long‐term heart rate variability measures. Biological Psychiatry 2002;52(5):418‐29. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Roose S, Mallavarapu M, Radhakrishna RK, Pesce V. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on measures of nonlinearity and chaos of heart rate. Neuropsychobiology 2002;46(3):125‐35. [DOI] [PubMed] [Google Scholar]
SADHART 2002 {published data only (unpublished sought but not used)}
- Glassman AH, O'Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT Jr, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002;288:701‐9. [DOI] [PubMed] [Google Scholar]
- Glassman AH, O'Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT Jr, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina: ERRATUM. JAMA: The Journal of the American Medical Association 2002;288(14):1720. [DOI] [PubMed] [Google Scholar]
- Lattanzio F, Cherubini A, Furneri G, Bari M, Marchionni N. Sertraline treatment for depression associated with acute coronary syndromes: a cost analysis from the viewpoint of the Italian Healthcare System. Aging‐Clinical & Experimental Research 2008;20(1):76‐80. [DOI] [PubMed] [Google Scholar]
- O'Connor CM, Glassman AH, Harrison DJ. Pharmacoeconomic analysis of sertraline treatment of depression in patients with unstable angina or a recent myocardial infarction. The Journal of Clinical Psychiatry 2005;66:346‐52. [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Glassman AH, Malinin AI, Nemeroff CB, Musselman DL, Zyl LT, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003;108(8):939‐44. [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Suckow RF, Cooper TB, O'Connor CM, Malinin AI, Krishnan KR, et al. Relationship between release of platelet/endothelial biomarkers and plasma levels of sertraline and N‐desmethylsertraline in acute coronary syndrome patients receiving SSRI treatment for depression. American Journal of Psychiatry 2005;162(6):1165‐70. [DOI] [PubMed] [Google Scholar]
- Swenson JR, O'Connor CM, Barton D, Zyl LT, Swedberg K, Forman LM, et al. Influence of depression and effect of treatment with sertraline on quality of life after hospitalization for acute coronary syndrome. The American Journal of Cardiology 2003;92:1271‐6. [DOI] [PubMed] [Google Scholar]
Strik 2000 {published data only (unpublished sought but not used)}
- Strik JJ, Honig A, Klinkenberg E, Dijkstra J, Jolles J. Cognitive performance following fluoxetine treatment in depressed patients post myocardial infarction. Acta Neuropsychiatrica 2006;18(1):1‐6. [DOI] [PubMed] [Google Scholar]
- Strik JJ, Honig A, Lousberg R, Lousberg AH, Cheriex EC, Tuynman‐Qua HG, et al. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double‐blind, placebo‐controlled trial. Psychosomatic Medicine 2000;62:783‐9. [DOI] [PubMed] [Google Scholar]
- Strik JJ, Praag HM, Honig A. Depression after first myocardial infarction. A prospective study on incidence, prognosis, risk factors and treatment. Tijdschrift Voor Gerontologie en Geriatrie 2003;34(3):104‐12. [PubMed] [Google Scholar]
References to studies excluded from this review
Black 1998 {published data only}
- Black JL, Allison TG, Williams DE, Rummans TA, Gau GT. Effect of intervention for psychological distress on rehospitalization rates in cardiac rehabilitation patients. Psychosomatics 1998;39:134‐43. [DOI] [PubMed] [Google Scholar]
Bucknall 1988 {published data only}
- Bucknall C, Brooks D, Curry PVL, Bridges PK, Bouras N, Ankier SI. Mianserin and trazodone for cardiac patients with depression. European Journal of Clinical Pharmacology 1988;33:565‐9. [DOI] [PubMed] [Google Scholar]
Davidson 2010 {published data only}
- Burg MM, Lespérance F, Rieckmann N, Clemow L, Skotzko C, Davidson KW. Treating persistent depressive symptoms in post‐ACS patients: the project COPES phase‐I randomized controlled trial. Contemporary Clinical Trials 2008;29:231‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson KW, Rieckmann N, Clemow L, Schwartz JE, Shimbo D, Medina V, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms. Archives of Internal Medicine 2010;170:600‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fu 2006 {published data only}
- Fu YX, Meng HQ, Luo QH. Interventional effect of fluoxetine on patients with acute myocardial infarction accompanied by anxiety and depression [Chinese]. Chinese Journal of Clinical Rehabilitation 2006;10:16‐7. [Google Scholar]
González‐Jaimes 2003 {published data only}
- Gonzalez‐Jaimes EI, Turnbull‐Plaza B. Selection of psychotherapeutic treatment for adjustment disorder with depressive mood due to acute myocardial infarction. Archives of Medical Research 2003;34:298‐304. [DOI] [PubMed] [Google Scholar]
Kachkovskii 2006 {published data only}
- Kachkovskii MA, Kriukov NN. Treatment of depression in patients with myocardial infarction with tianeptine [Russian]. Kardiologiia 2006;46:21‐6. [PubMed] [Google Scholar]
Mohapatra 2005 {published data only}
- Mohapatra PK, Kar N, Kar GC, Behera M. Effectiveness of sertraline in treatment of depression in a consecutive sample of patients with acute myocardial infarction: six month prospective study on outcome. Clinical Practice and Epidemiology in Mental Health 2005;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norris 2009 {published data only}
- Norris CE, Patterson L, Galbraith D, Hegadoren KM. All you have to do is call; a pilot study to improve the outcomes of patients with coronary artery disease. Applied Nursing Research 2009;22:133‐7. [DOI] [PubMed] [Google Scholar]
Oldridge 1991 {published data only}
- Oldridge N, Guyatt G, Jones N, Crowe J, Singer J, Feeny D, et al. Effects on quality of life with comprehensive rehabilitation after acute myocardial infarction. The American Journal of Cardiology 1991;67:1084‐9. [DOI] [PubMed] [Google Scholar]
- Oldridge N, Streiner D, Hoffmann R, Guyatt G. Profile of mood states and cardiac rehabilitation after acute myocardial infarction. Medicine and Science in Sports and Exercise 1995;27:900‐5. [PubMed] [Google Scholar]
Pogosova 2004 {published data only}
- Pogosova GV, Zhidko NI, Krasnitsky VB, Tikhomirova EA, Odintsova AS, Akhmedjanov NM, et al. Clinical efficacy of tianeptine in patients with ischemic heart disease and comorbid depression [Russian]. Kardiologiia 2004;44:20‐4. [PubMed] [Google Scholar]
Pogosova 2009 {published data only}
- Pogosova GV, Koltunov IE, Karpova AV, Eliseeva NA, Sapunova ID. Clinical efficacy of escitalopram in patients with ischemic heart disease and comorbid depression [Russian]. Kardiologiia 2009;49:4‐8. [PubMed] [Google Scholar]
Rollman 2009 {published data only}
- Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Houck PR, Counihan PJ, et al. Telephone‐delivered collaborative care for treating post‐CABG depression: a randomized controlled trial. JAMA 2009;302:2095‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Schulberg HC, Reynolds CF III. The Bypassing the Blues treatment protocol: stepped collaborative care for treating post‐CABG depression. Psychosomatic Medicine 2009;71:217‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schrader 2005 {published data only}
- Cheok F, Schrader G, Banham D, Marker J, Hordacre AL. Identification, course, and treatment of depression after admission for a cardiac condition: rationale and patient characteristics for the Identifying Depression As a Comorbid Condition (IDACC) project. American Heart Journal 2003;146:978‐84. [DOI] [PubMed] [Google Scholar]
- Schrader G, Cheok F, Hordacre AL, Marker J, Wade V. Effect of psychiatry liaison with general practitioners on depression severity in recently hospitalised cardiac patients: a randomised controlled trial. The Medical Journal of Australia 2005;182:272‐6. [DOI] [PubMed] [Google Scholar]
- Wade V, Cheok F, Schrader G, Hordacre AL, Marker J. Depression after cardiac hospitalisation‐‐the Identifying Depression as a Comorbid Condition (IDACC) study. Australian Family Physician 2005;34:985‐9. [PubMed] [Google Scholar]
Stern 1983 {published data only}
- Stern MJ, Gorman PA, Kaslow L. The group counseling v exercise therapy study. A controlled intervention with subjects following myocardial infarction. Archives of Internal Medicine 1983;143:1719‐25. [PubMed] [Google Scholar]
Veith 1982 {published data only}
- Veith RC, Raskind MA, Caldwell JH, Barnes RF, Gumbrecht G, Ritchie JL. Cardiovascular effects of tricyclic antidepressants in depressed patients with chronic heart disease. The New England Journal of Medicine 1982;306:954‐9. [DOI] [PubMed] [Google Scholar]
Zeng 2001 {published data only}
- Zeng W, Ma H, Liang Q, Dong Y, Ye H, Zhang Y. The influence of antidepressive therapy on short‐term prognosis in elderly patients with unstable angina and depression [Chinese]. Zhonghua Nei Ke Za Zhi 2001;40:809‐10. [PubMed] [Google Scholar]
References to studies awaiting assessment
Malik 2002 {published data only}
- Malik NA. Comparison of treatment to influence depression as a risk factor for ischemic heart disease with new generation antidepressants. XII World Congress of Psychiatry, Yokohama, Japan. Aug 24 9, 2002.
References to ongoing studies
SPIRR‐CAD 2008 {published data only}
- ISRCTN76240576. A Stepwise Psychotherapy Intervention for Reducing Risk in Coronary Artery Disease ‐ a randomised controlled trial (SPIRR‐CAD). http://www.controlled‐trials.com/isrctn/pf/76240576 (accessed 10 August 2009).
UPBEAT 2007 {published data only}
- Blumenthal JA, Sherwood A, Rogers SD, Babyak MA, Doraiswamy PM, Watkins L, et al. Understanding prognostic benefits of exercise and antidepressant therapy for persons with depression and heart disease: the UPBEAT study‐‐rationale, design, and methodological issues. Clinical Trials 2007;4:548‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alpert 2000
- Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined‐‐a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Journal of the American College of Cardiology 2000;36:959‐69. [DOI] [PubMed] [Google Scholar]
Antman 2004
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004;44:e82‐e292. [PubMed] [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV. 4th Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
Barth 2004
- Barth J, Schumacher M, Herrmann‐Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta‐analysis. Psychosomatic Medicine 2004;66:802‐13. [DOI] [PubMed] [Google Scholar]
Baumeister 2005
- Baumeister H, Balke K, Härter M. Psychiatric and somatic comorbidities are negatively associated with quality of life in physically ill patients. Journal of Clinical Epidemiology 2005;58:1090‐100. [DOI] [PubMed] [Google Scholar]
Baumeister 2007
- Baumeister H, Härter M. Mental disorders in patients with obesity in comparison with healthy probands. International Journal of Obesity 2007;31:1155‐64. [DOI] [PubMed] [Google Scholar]
Baumeister 2008
- Baumeister H, Morar V. The impact of clinical significance criteria on subthreshold depression prevalence rates. Acta Psychiatrica Scandinavica 2008;118:443‐50. [DOI] [PubMed] [Google Scholar]
Baumeister 2009a
- Baumeister H, Maercker A, Casey P. Adjustment disorders with depressed mood: A critique of its DSM‐IV and ICD‐10 conceptualization and recommendations for the future. Psychopathology 2009;42:139‐47. [DOI] [PubMed] [Google Scholar]
Baumeister 2009b
- Baumeister H, Kufner K. It is time to adjust the adjustment disorder category. Current Opinion in Psychiatry 2009;22:409‐12. [DOI] [PubMed] [Google Scholar]
Baumeister 2010a
- Baumeister H, Kriston L, Bengel J, Härter M. High agreement of self‐report and physician‐diagnosed somatic conditions yields limited bias in examining mental‐physical comorbidity. Journal of Clinical Epidemiology 2010;63:558‐65. [DOI] [PubMed] [Google Scholar]
Baumeister 2010b
- Baumeister H. A clinical significance criterion is essential for diagnosing subthreshold depression. American Journal of Psychiatry 2010;167:866. [DOI] [PubMed] [Google Scholar]
Baumeister 2010c
- Baumeister H, Parker G. A second thought on subtyping major depression. Psychotherapy & Psychosomatics 2010;79:388‐9. [DOI] [PubMed] [Google Scholar]
Baumeister 2011a
Baumeister 2011b
Bech 2010
- Bech P. Is the antidepressive effect of second‐generation antidepressants a myth?. Psychological Medicine 2010;40:181‐6. [DOI] [PubMed] [Google Scholar]
Budde 2005
- Budde T, Breithardt G. Coronary Artery Disease [Koronare Herzkrankheit]. In: Greten H editor(s). Innere Medizin: Verstehen‐Lernen‐Anwenden. Stuttgart: Thieme, 2005:34‐54. [Google Scholar]
Cuijpers 2008a
- Cuijpers P, Straten A, Andersson G, Oppen P. Psychotherapy for depression in adults: a meta‐analysis of comparative outcome studies. Journal of Consulting and Clinical Psychology 2008;76:909‐22. [DOI] [PubMed] [Google Scholar]
Cuijpers 2008b
- Cuijpers P, Straten A, Andersson G, Oppen P. Are psychological and pharmacologic interventions equally effective in the treatment of adult depressive disorders? A meta‐analysis of comparative studies. Journal of Clinical Psychiatry 2008;69:1675‐85. [DOI] [PubMed] [Google Scholar]
Cuijpers 2010
- Cuijpers P, Straten A, Bohlmeijer E, Hollon SD, Andersson G. The effects of psychotherapy for adult depression are overestimated: a meta‐analysis of study quality and effect size. Psychological Medicine 2010;40:211‐23. [DOI] [PubMed] [Google Scholar]
Davidson 2006
- Davidson KW, Kupfer DJ, Bigger JT, Califf RM, Carney RM, Coyne JC, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute working group report. Annals of Behavioral Medicine 2006;32:121‐6. [DOI] [PubMed] [Google Scholar]
Davison 2003
- Davison GC, Neale JM. Abnormal Psychology. New York: John Wiley & Sons, 2003. [Google Scholar]
De Maat 2007
- Maat SM, Dekker J, Schoevers RA, Jonghe F. Relative efficacy of psychotherapy and combined therapy in the treatment of depression: a meta‐analysis. European Psychiatry 2007;22:1‐8. [DOI] [PubMed] [Google Scholar]
Dickens 2008
- Dickens C, McGowan L, Percival C, Tomenson B, Cotter L, Heagerty A, et al. New onset depression following myocardial infarction predicts cardiac mortality. Psychosomatic Medicine 2008;70:450‐5. [DOI] [PubMed] [Google Scholar]
Fournier 2010
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity. A patient‐level meta‐analysis. JAMA 2010;303:47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2006
- Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. European Heart Journal 2006;27:1341‐81. [DOI] [PubMed] [Google Scholar]
Frasure‐Smith 2000
- Frasure‐Smith N, Lesperance F, Gravel G, Masson A, Juneau M, Talajic M, et al. Depression and health‐care costs during the first year following myocardial infarction. Journal of Psychosomatic Research 2000;48:471‐8. [DOI] [PubMed] [Google Scholar]
Frasure‐Smith 2003
- Frasure‐Smith N, Lesperance F. Depression and other psychological risks following myocardial infarction. Archives of General Psychiatry 2003;60:627‐36. [DOI] [PubMed] [Google Scholar]
Harnett 2010
- Harnett P, O'Donovan A, Lambert MJ. The dose response relationship in psychotherapy: implications for social policy. Clinical Psychologist 2010;14:39‐44. [Google Scholar]
Herrmann‐Lingen 2006
- Herrmann‐Lingen C, Buss U. Anxiety and depression in patients with coronary heart disease. In: Jordan J, Bardé B, Zeiher AM editor(s). Contributions toward evidence‐based psychocardiology: a systematic review of the literature. Washington, DC: American Psychological Association, 2006:125‐57. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. [Google Scholar]
Härter 2007a
- Härter M, Baumeister H. Etiology of mental disorders in chronic somatic diseases [Ätiologie psychischer Störungen bei chronischen körperlichen Erkrankungen]. In: Härter M, Baumeister H, Bengel J editor(s). Psychische Störungen bei körperlichen Erkrankungen. Heidelberg: Springer, 2007:1‐13. [Google Scholar]
Härter 2007b
- Härter M, Baumeister H, Reuter K, Jacobi F, Höfler M, Bengel J, et al. Increased 12‐month prevalence rates of mental disorders in patients with chronic somatic diseases. Psychotherapy and Psychosomatics 2007;6:354‐60. [DOI] [PubMed] [Google Scholar]
Joynt 2003
- Joynt KE, Whellan DJ, O'Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biological Psychiatry 2003;54:248‐61. [DOI] [PubMed] [Google Scholar]
Lefebvre 1996
- Lefebvre C, McDonald S. Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE. Fourth International Cochrane Colloquium, Adelaide, Australia. 20‐24 October 1996.
Lichtenberg 2010
- Lichtenberg P, Belmaker RH. Subtyping Major Depressive Disorder. Psychotherapy & Psychosomatics 2010;79:131‐5. [DOI] [PubMed] [Google Scholar]
Lichtman 2008
- Lichtman JH, Bigger JT, Blumenthal JA, Frasure‐Smith N, Kaufmann PG, Lesperance F, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American HeartAssociation Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation 2008;118:1768‐75. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Jadad AR. Assessing the quality of reports of randomised trials: implications for the conduct of meta‐analyses. Health Technology Assessment 1999;29:823‐32. [PubMed] [Google Scholar]
Musselman 1998
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Archives of General Psychiatry 1998;55:580‐92. [DOI] [PubMed] [Google Scholar]
Nemeroff 2003
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. PNAS 2003;100:14293‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Clinical Excellence. Depression: the treatment and management of depression in adults (update). Clinical Guideline 90 2009. http://guidance.nice.org.uk/CG90 (accessed 26 July 2011). [PubMed]
Ormel 2007
- Ormel J, Korff M, Burger H, Scott K, Demyttenaere K, Huang YQ, et al. Mental disorders among persons with heart disease ‐ results from World Mental Health surveys. General Hospital Psychiatry 2007;29:325‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pigott 2010
- Pigott HE, Leventhal AM, Alter GS, Boren JJ. Efficacy and effectiveness of antidepressants: current status of research. Psychotherapy and Psychosomatics 2010;79:267‐79. [DOI] [PubMed] [Google Scholar]
Rayner 2010
- Rayner L, Price A, Evans A, Valsraj K, Higginson IJ, Hotopf M. Antidepressants for depression in physically ill people. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD007503.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rees 2004
- Rees K, Bennett P, West R, Davey Smith G, Ebrahim S. Psychological interventions for coronary heart disease. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD002902.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rudisch 2003
- Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biological Psychiatry 2003;54:227‐40. [DOI] [PubMed] [Google Scholar]
Sadock 2009
- Sadock BJ, Sadock VA, Ruiz P (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry. 9th Edition. Philadelphia, Pa, London: Lippincott Williams & Wilkins, 2009. [Google Scholar]
Skala 2006
- Skala JA, Freedland KE, Carney RM. Coronary heart disease and depression: A review of recent mechanistic research. Canadian Journal of Psychiatry 2006;51:738‐45. [DOI] [PubMed] [Google Scholar]
Taylor 2008
- Taylor D. Antidepressant drugs and cardiovascular pathology: a clinical overview of effectiveness and safety. Acta Psychiatrica Scandinavica 2008;118:434‐42. [DOI] [PubMed] [Google Scholar]
Thombs 2006
- Thombs BD, Bass EB, Ford DE, Stewart KJ, Tsilidis KK, Patel U, et al. Prevalence of depression in survivors of acute myocardial infarction. Journal of General Internal Medicine 2006;21:30‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2008
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. The New England Journal of Medicine 2008;358:252‐60. [DOI] [PubMed] [Google Scholar]
Unützer 2002
- Unutzer J, Katon W, Callahan CM, Williams JW Jr, Hunkeler E, Harpole L, et al. Collaborative care management of late‐life depression in the primary care setting: a randomized controlled trial. JAMA 2002;288:2836‐45. [DOI] [PubMed] [Google Scholar]
Van Straten 2010
- Straten A, Geraedts A, Verdonck‐de Leeuw I, Andersson G, Cuijpers P. Psychological treatment of depressive symptoms in patients with medical disorders: A meta‐analysis. Journal of Psychosomatic Research 2010;69:23‐32. [DOI] [PubMed] [Google Scholar]
Wakefield 2010
- Wakefield JC, Schmitz MF, Baer JC. Does the DSM‐IV clinical significance criterion for major depression reduce false positives? Evidence from the National Comorbidity Survey Replication. American Journal of Psychiatry 2010;167:298‐304. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The ICD‐10 Classification of Mental and Behavioral Disorders. Clinical description and diagnostic guidelines. 10th Edition. Geneva: World Health Organization, 1992. [Google Scholar]
Whooley 2008
- Whooley MA, Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 2008;300:2379‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziegelstein 2000
- Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Archives of Internal Medicine 2000;160:1818‐23. [DOI] [PubMed] [Google Scholar]


