Abstract
Background
Non‐tubal ectopic pregnancy is the implantation of an embryo at a site lying outside the uterine cavity or fallopian tubes. Sites include a caesarean scar, the cornua uteri, the ovary, the cervix, and the abdomen. There has been an increasing trend in the occurrence of these rare conditions, especially caesarean scar pregnancy (CSP).
Objectives
To evaluate the clinical effectiveness and safety of surgery, medical treatment, and expectant management of non‐tubal ectopic pregnancy in terms of fertility outcomes and complications.
Search methods
We searched the Cochrane Gynaecology and Fertility (CGF) Group Specialised Register of Controlled Trials, CENTRAL, MEDLINE, Embase, ClinicalTrials.gov, the World Health Organization (WHO) search portal and nine other databases to 12 December 2019. We handsearched reference lists of articles retrieved and contacted experts in the field to obtain additional data.
Selection criteria
We included randomized controlled trials (RCTs) published in all languages that examined the effects and safety of surgery, medical treatment, and expectant management of non‐tubal ectopic pregnancy.
Data collection and analysis
We used Cochrane standard methodological procedures. Primary outcomes were treatment success and complications.
Main results
We included five RCTs with 303 women, all reporting Caesarean scar pregnancy. Two compared uterine arterial embolization (UAE) or uterine arterial chemoembolization (UACE) plus methotrexate (MTX) versus systemic MTX and subsequent dilation and suction curettage; one compared UACE plus MTX versus ultrasonography‐guided local MTX injection; and two compared suction curettage under hysteroscopy versus suction curettage under ultrasonography after UAE/UACE.
The quality of evidence ranged from moderate to very low. The main limitations were imprecision (small sample sizes and very wide confidence intervals (CI) for most analyses), multiple comparisons with a small number of trials, and insufficient data available to assess heterogeneity.
UAE/UACE versus systemic MTX prior to suction curettage
Two studies reported this comparison. One compared UAE with systemic MTX and one compared UACE plus MTX versus systemic MTX, in both cases followed by a suction curettage.
We are uncertain whether UAE/UACE improved success rates after initial treatment (UAE: risk ratio (RR) 1.00, 95% CI 0.90 to 1.12; 1 RCT, 72 women; low‐quality evidence; UACE: RR 0.87, 95% CI 0.54 to 1.38; 1 RCT, 28 women; low‐quality evidence).
We are uncertain whether UAE/UACE reduced rates of complications (UAE: RR 0.47, 95% CI 0.13 to 1.75; 1 RCT, 72 women; low‐quality evidence; UACE: RR 0.62, 95% CI 0.26 to 1.48; 1 RCT, 28 women; low‐quality evidence).
We are uncertain whether UAE/UACE reduced adverse effects (UAE: RR 1.58, 95% CI 0.41 to 6.11; 1 RCT, 72 women; low‐quality evidence; UACE: RR 1.16, 95% CI 0.32 to 4.24; 1 RCT, 28 women; low‐quality evidence), and it was not obvious that the types of events had similar values to participants (e.g. fever versus vomiting).
Blood loss was lower in UAE/UACE groups than systemic MTX groups (UAE: mean difference (MD) –378.70 mL, 95% CI –401.43 to –355.97; 1 RCT, 72 women; moderate‐quality evidence; UACE: MD –879.00 mL, 95% CI –1135.23 to ‐622.77; 1 RCT, 28 women; moderate‐quality evidence).
Data were not available on time to normalize β‐human chorionic gonadotropin (β‐hCG).
UACE plus MTX versus ultrasonography‐guided local MTX injection
We are uncertain whether UACE improved success rates after initial treatment (RR 0.95, 95% CI 0.56 to 1.60; 1 RCT, 45 women; very low‐quality evidence).
Adverse effects: the study reported the same number of failed treatments in each arm (RR 0.88, 95% CI 0.40 to 1.92; 1 RCT, 45 women).
We are uncertain whether UACE shortened the time to normalize β‐hCG (MD 1.50 days, 95% CI –3.16 to 6.16; 1 RCT, 45 women; very low‐quality evidence).
Data were not available for complications.
Suction curettage under hysteroscopy versus under ultrasonography after UAE/UACE.
Two studies reported this comparison. One compared suction curettage under hysteroscopy versus under ultrasonography after UAE, and one compared these interventions after UACE.
We are uncertain whether suction curettage under hysteroscopy improved success rates after initial treatment (UAE: RR 0.91, 95% CI 0.81 to 1.03; 1 RCT, 66 women; very low‐quality evidence; UACE: RR 1.02, 95% CI 0.96 to 1.09; 1 RCT, 92 women; low‐quality evidence).
We are uncertain whether suction curettage under hysteroscopy reduced rates of complications (UAE: RR 4.00, 95% CI 0.47 to 33.91; 1 RCT, 66 women; very low‐quality evidence; UACE: RR 0.18, 95% CI 0.01 to 3.72; 1 RCT, 92 women; low‐quality evidence).
We are uncertain whether suction curettage under hysteroscopy reduced adverse effects (UAE: RR 3.09, 95% CI 0.12 to 78.70; 1 RCT, 66 women; very low‐quality evidence; UACE: not estimable; 1 RCT, 92 women; very low‐quality evidence).
We are uncertain whether suction curettage under hysteroscopy shortened the time to normalize β‐hCG (UAE: MD 4.03 days, 95% CI –1.79 to 9.85; 1 RCT, 66 women; very low‐quality evidence; UACE: MD 0.84 days, 95% CI –1.90 to 3.58; 1 RCT, 92 women; low‐quality evidence).
Non‐tubal ectopic pregnancy other than CSP
No studies reported on non‐tubal ectopic pregnancies in locations other than on a caesarean scar.
Authors' conclusions
For Caesarean scar pregnancies (CSP) it is uncertain whether there is a difference in success rates, complications, or adverse events between UAE/UACE and administration of systemic MTX before suction curettage (low‐quality evidence). Blood loss was lower if suction curettage is conducted after UAE/UACE than after administration of systemic MTX (moderate‐quality evidence). It is uncertain whether there is a difference in treatment success rates, complications, adverse effects or time to normalize β‐hCG between suction curettage under hysteroscopy and under ultrasonography (very low‐quality evidence). There are no studies of non‐tubal ectopic pregnancy other than CSP and RCTs for these types of pregnancy are unlikely.
Plain language summary
Interventions for non‐tubal ectopic pregnancy
Review question
How effective and safe are surgery, medical treatment, and expectant management for non‐tubal ectopic pregnancy?
Background
Non‐tubal ectopic pregnancy is the implantation of an embryo outside the womb (uterus) or fallopian tubes (which connect the uterus to the ovaries). Sites include a caesarean scar formed as the healing of an incision in the uterus after caesarean section, the cornua uteri (where the uterus and fallopian tubes meet), the ovary, the cervix, and the abdomen. There has been an increase in the occurrence of these rare conditions, especially caesarean scar pregnancy (CSP). Ectopic pregnancy makes up 80% of maternal deaths that occur in the first trimester, of which non‐tubal ectopic pregnancy deaths account for a higher rate than tubal pregnancy deaths. Early diagnosis and effective treatment are essential to reduce the immediate and delayed side effects, and to avoid significant maternal illness and death. Treatments include surgery (e.g. uterine arterial embolization (UAE; a tube delivers small particles that block the blood supply to the uterus), uterine arterial chemoembolization (UACE; a tube delivers small particles that block the blood supply to the uterus and chemotherapy), dilation and suction curettage (to remove the products of the pregnancy)); medical treatment (e.g. a medicine called methotrexate); or expectant management (wait to see if a miscarriage occurs naturally).
Study characteristics
Cochrane researchers found five clinical trials including 303 women. The evidence is current to December 2019.
Key results
Caesarean scar pregnancy
Two studies compared UAE/UACE with methotrexate (surgery) versus systemic (into a vein or muscle) methotrexate injection (medical treatment) following suction curettage. Evidence was insufficient for treatment success, complications, and side effects. Moderate‐quality evidence showed that blood loss from the treatment in the surgery group was lower than in the medical treatment group.There were no data for time for β‐hCG levels to return to normal.
One study compared UAE with local (into uterine artery) methotrexate injection (surgery) versus ultrasound‐guided local methotrexate injection (medical treatment). Evidence was insufficient for treatment success and time for β‐hCG levels to return to normal. The study reported the same number of failed treatments in each arm There were no data for complications or other side effects.
Two studies compared suction curettage under hysteroscopy (insertion of a narrow telescope with a light and camera at the end into the uterus) versus suction curettage under ultrasound after UAE/UACE with methotrexate. Evidence was insufficient for treatment success, complications, side effects, and time for β‐hCG levels to return to normal.
Non‐tubal ectopic pregnancies other than caesarean scar pregnancy
No studies reported on non‐tubal ectopic pregnancies in locations other than on a caesarean scar.
Quality of the evidence
The quality of evidence ranged from moderate to very low. The main limitations were small numbers of participants and trials, very wide range of results for most comparisons, and insufficient data to assess differences.
Summary of findings
Background
Description of the condition
Non‐tubal ectopic pregnancy is the implantation of an embryo at a site lying outside the uterine cavity or fallopian tubes. Sites include a caesarean scar, the cornua uteri, the ovary, the cervix, and the abdomen. There has been an increasing trend in the occurrence of these rare conditions, especially caesarean scar pregnancy (CSP), which poses a diagnostic and treatment challenge for clinicians (Ngu 2011; Petersen 2016). The incidence of non‐tubal ectopic pregnancy is estimated at 5.0% to 8.3% of all ectopic pregnancies, while the incidence of ectopic pregnancy is estimated at about 1% to 2% of all pregnancies (Bouyer 2002; Stucki 2008; Jurkovic 2011; Ngu 2011; Shan 2014; Yao 2005). Although relatively uncommon, these conditions are responsible for serious immediate and delayed complications (Bouyer 2002; Stucki 2008), with significant haemorrhages and a high rate of maternal fatality (Lewis 2007; Stucki 2008; Barnhart 2009; Orazulike 2013). Ectopic pregnancy makes up 80% of maternal deaths that occur in the first trimester, of which non‐tubal ectopic pregnancy deaths account for a higher rate than tubal pregnancy deaths (Stucki 2008; Jurkovic 2011). Thus, it is important to recognize that the implantation site of ectopic pregnancy affects the severity of the condition (Bouyer 2002; Kirk 2014), and early diagnosis and effective treatment are essential to reduce the immediate and delayed adverse effects, and to avoid significant maternal morbidity and mortality.
CSP has increased because of the increased rate of caesarean sections performed (Maymon 2004; Seow 2004; Shen 2012; Gibbons 2012), and may account for up to 6.1% of all ectopic pregnancies in women with a history of at least one caesarean section (Seow 2004). The criteria for ultrasonography diagnosis include: (i) no fetal parts observed in the uterine cavity or cervical canal; (ii) a gestational sac located in the anterior part of the isthmic portion separated from the endometrial cavity or fallopian tube; (iii) a gestational sac embedded within the myometrium and the fibrous tissue of the caesarean section scar with diminished healthy myometrium between the bladder and the sac; and (iv) high velocity with low impedance blood flow surrounding the gestation sac (Jurkovic 2003; Pascual 2007; Seow 2004). CSP typing according to direction of growth of the gestational sac and thickness of the myometrium between the bladder and gestational sac has been proposed, and three types (I, II, and III) were divided as different ultrasonic manifestations (Yuan 2010).
Ovarian pregnancy accounts for 1% to 6% of all ectopic pregnancies (Bouyer 2002; Comstock 2005; Tinelli 2008; Goyal 2014; Melcer 2015). Ovarian pregnancy is often confused with corpus luteum cysts. Ultrasonography examination is routinely required and laparoscopy often plays a key role in diagnosis, with confirmation by pathological examination of removed tissue. The criteria are as follows: (i) empty uterine cavity; (ii) tubes entirely intact, separated from the ovary; (iii) a gestational sac located at the normal position of the ovary; (iv) ovary and a gestational sac connected to the uterus by the ovarian ligament; and (v) trophoblastic tissue attached to the ovarian cortex in the tissue specimen (Nadarajah 2002; Comstock 2005). However, the new 'Green top' guideline summarizes that there are no agreed ultrasonography criteria for the diagnosis of ovarian pregnancy (Elson 2016).
Cornual pregnancy has a prevalence of 1.7% to 2.1% of all ectopic pregnancies (Stucki 2008; Shan 2014; Nadi 2017). The term cornual pregnancy is often used interchangeably in the literature with interstitial pregnancy, but it actually refers to a pregnancy located in the interstitial portion of a unicornuate or bicornuate uterus (Tulandi 2004; Chetty 2009). The diagnosis can be made by the following ultrasonography criteria: (i) a single interstitial portion of a fallopian tube in the main uterine body; (ii) a mobile gestational sac surrounded by myometrium but separated from the uterus; and (iii) a vascular pedicle adjoining the gestational sac to the unicornuate uterus (Jurkovic 2007; Levine 2007).
Cervical pregnancy is a rare form of ectopic pregnancy with an estimated frequency of less than 1% of all ectopic pregnancies (Chetty 2009; Faschingbauer 2011; Anev 2013). Cervical pregnancy can be diagnosed by the following ultrasonography presentations: (i) empty uterine cavity; (ii) dilated or barrel‐shaped cervix; (iii) a gestational sac within the mucosa located in the endocervical canal; and (iv) a sliding sac sign (Jurkovic 1996; Kirk 2014).
Abdominal pregnancy is one of the rarest forms of ectopic gestation and occurs in 0.3% to 1.4% of all ectopic pregnancies (Bouyer 2002; Molinaro 2007; Stucki 2008; Goyal 2014; Shan 2014). It refers to a gestational sac implanted in the omentum, abdominal vital organs, or large vessels, and is described as either primary or secondary. A primary abdominal pregnancy is due to a failure of the fimbria to pick up the ovulated follicle, while a secondary abdominal pregnancy is believed to follow early rupture or abortion of a tubal pregnancy or uterine perforation of a intrauterine pregnancy into the peritoneal cavity. The following criteria have been suggested to diagnose abdominal pregnancy: (i) empty uterine cavity; (ii) absence of a dilated fallopian tube and a complex adnexal mass; (iii) absence of myometrial tissue between the bladder and pregnancy; (iv) a gestational sac surrounded by intestinal tissue and separated by the peritoneum; and (v) a free mobility similar to fluctuation of the sac (Varma 2003; Gerli 2004; Onan 2005; Kirk 2014).
See Table 3.
1. Incidence of non‐tubal ectopic pregnancy in all ectopic pregnancies.
| Type | Incidence (%) |
| Abdominal | 0.3–1.4 |
| Caesarean scar | 6.1 |
| Cervical | < 1 |
| Cornual | 1.7–2.1 |
| Ovarian | 1–6 |
Description of the intervention
The diagnosis of, and treatment for, non‐tubal ectopic pregnancy are challenging and frequently constitute a medical emergency. Thus, prompt and effective treatment is important to avoid potentially catastrophic consequences such as massive haemorrhaging, hysterectomy, and death. The improved ultrasonography technology widely used in early pregnancy assessment and high awareness of the possibility of ectopic pregnancy have contributed to earlier diagnosis of these pregnancies. This has led to advances in their management, aiming to limit morbidity and to preserve the uterus and subsequent fertility (Molinaro 2007). Treatments include surgery, medical treatment, and expectant management. Although many treatment options have been recommended, the optimal treatment is yet to be clarified.
Surgery is the primary treatment for most ovarian, cornual, and abdominal pregnancies (Ngu 2011; Shan 2014). Management of ovarian pregnancy usually requires ovariectomy or ovarian wedge resection. Ovarian wedge resection may be considered by women with future fertility in mind, whereas ovariectomy is limited to cases of advanced gestation (Einenkel 2000; Goyal 2014). cornual pregnancy has traditionally been treated using cornual resection or hysterectomy in the cases with severely damaged uteri (Molinaro 2007). The surgery can be completed through laparoscopy or laparotomy, but laparotomy may be required for women with uterine rupture and haemoperitoneum (Tulandi 2004). Surgical management with ligation of the placental blood supply and removal of some early abdominal pregnancies implanted on a less vascular surface can be managed by laparoscopy (Molinaro 2007); in advanced pregnancies, selective arterial embolization is necessary before surgery to avoid haemorrhage (Rahaman 2004). For cervical and caesarean scar pregnancies, laparotomy and hysterectomy may be required for more advanced pregnancies (Hsieh 1998; Jin 2004; Fuchs 2015; Alammari 2017). Surgery is often performed as a life‐saving emergency measure for women with massive active bleeding or failed conservative treatment.
Improvements in the early detection of these conditions, before rupture, have led to the utilization of possible conservative treatment options. For women who desire fertility preservation, conservative measures have become the first‐line approach. These options include administration (local or systemic) of various chemotherapeutic agents (including methotrexate (MTX), etoposide, actinomycin D, and cyclophosphamide) and endoscopic removal techniques (laparoscopy or hysteroscopy), alone or as adjuvant therapies, with or without haemostatic techniques (balloon tamponade, uterine artery ligation, cerclage, and cervical stay sutures). With cervical pregnancies before 12 weeks' gestation where the women have lower serum β‐human chorionic gonadotropin (β‐hCG) levels and no fetal cardiac activity, it may seem reasonable to administer conservative treatment (Molinaro 2007). Systemic MTX administration is a common approach to cervical pregnancy and has high efficacy, but it may be associated with increased risk of primary failure for pregnancies greater than nine weeks' gestation, with β‐hCG levels above 10,000 mlU/mL, crown–rump length greater than 10 mm, and fetal cardiac activity (Hung 1998). Suction curettage with balloon tamponade or uterine arterial embolization (UAE) may be a choice for first trimester cervical pregnancy (Fylstra 2014; Hu 2016). Local or systemic injection of chemotherapy has met with various degrees of success for treatment of CSP (Marchiole 2004; Graesslin 2005; Jabeen 2018). Advances in UAE have enabled a minimally invasive approach in these women (Yu 2009; Lian 2012; Shen 2012; Zhang 2012; Zhu 2016). Dilation and suction curettage can be used after chemotherapy but should not be the first‐line approach in cervical and CSP because of the risk of perforation and catastrophic haemorrhage (Lee 1999). Medical management with MTX has been reported for ovarian pregnancy but may not be practical (Molinaro 2007). Conservative measures may be attempted for selective cornual pregnancies but carry a high risk of initial failure (Bernstein 2001; Molinaro 2007). However, Horne and colleagues reported five successful cases with gefitinib plus MTX (Horne 2014). Systemic MTX chemotherapy and ultrasonography‐guided injection of potassium chloride for management of early abdominal pregnancies has been reported (Mitra 2003).
Expectant management does not seem to be an appropriate choice for most cases and may be only suitable for women with declining β‐hCG levels and absence of fetal cardiac activity (Chetty 2009; Timor‐Tritsch 2015; Jabeen 2018). However, women should be closely monitored in case the lesion ruptures or the situation deteriorates.
How the intervention might work
Although surgery is not a routine method of diagnosis, due to improvements in the early ultrasonography diagnosis of these challenging pregnancies, it remains the main treatment option for ectopic pregnancy. Laparoscopy has been recommended in preference to laparotomy in clinically stable women because of shorter operating time, reduced recovery time, lower blood loss, and shorter duration of hospitalizations (Seinera 1997; Einenkel 2000; Shaw 2007). However, laparotomy is preferable to laparoscopy in women who have had heavy abdominal haemorrhaging and who have to undergo immediate haemostasis (Jurkovic 2011). Conservative surgery (preservation of uterus or ovary) to remove only the products of gestation or a part of the ovary is more desirable, especially for women who wish to preserve their fertility. Hysterectomy or ovariectomy is a radical modality used for urgent blood control or in women at an advanced gestational age and following misdiagnosis and failed conservative treatment or with severely damaged uteri (Ushakov 1997; Molinaro 2007; Chetty 2009).
Suction curettage can serve to extract the products of gestation and achieve haemostasis, used either as a single treatment or as an adjuvant therapy (Jurkovic 2003; Marchiole 2004).
Systemic and local administration of drugs is considered a safe and effective treatment. MTX is the most widely used chemotherapy drug, and, combined with folic acid, it is similar to treatment of gestational trophoblastic disease (Ory 1986; Sauer 1987; Liang 2010).
UAE involves blocking the arteries using gelatin beads or other materials (Shen 2012). It has been used as an adjuvant therapy to prevent excessive haemorrhage before uterine surgery or curettage procedures (Frates 1994; Honey 1999; Trambert 2005). Bilateral uterine arterial chemoembolization (UACE) is a combination of arterial embolization and local administration of chemotherapy. During the procedure, MTX is delivered directly into the gestational foci via the bilateral uterine arteries, which provide the blood supply. Then occlusive agents are injected into the delivery catheter to block the blood vessels. The procedure uses both chemotherapy and tissue ischaemia and thus uses a higher concentration of MTX leading to more effective embryocide, with fewer systemic toxic effects and reduced haemorrhage. As a minimally invasive treatment, it has been considered as a conservative method for treating CSP (Zhang 2009; Liang 2010; Li 2011a; Litwicka 2011; Lian 2012; Shen 2012).
Why it is important to do this review
To date, there has been no systematic review presenting evidence from randomized controlled trials (RCTs) to show the optimal management of non‐tubal ectopic pregnancy. The optimal treatment, treatment success rate, and complications are largely unknown. In this review, we evaluated the clinical effectiveness and safety of surgery, medical treatment, and expectant management of non‐tubal ectopic pregnancy in terms of primary treatment success and preservation of the uterus and future fertility, and collated the best evidence on interventions for non‐tubal ectopic pregnancy.
Objectives
To evaluate the clinical effectiveness and safety of surgery, medical treatment, and expectant management of non‐tubal ectopic pregnancy in terms of fertility outcomes and complications.
Methods
Criteria for considering studies for this review
Types of studies
RCTs that compared one treatment with another in the management of non‐tubal ectopic pregnancy, in all languages. The allocation to either treatment was by random allocation. We excluded quasi‐RCTs from this review.
Types of participants
Women of childbearing age with a diagnosis of caesarean scar, abdominal, cornual, cervical, or ovarian pregnancy. All these types of pregnancy were analyzed both separately and together.
Types of interventions
We made comparisons of surgery or medical treatment versus expectant management, and surgery versus medical treatment, subgrouped by location of pregnancy and specific type of intervention.
Surgery
Laparoscopic removal
Hysteroscopic evacuation
Dilation and curettage
Sac aspiration by suction, also called suction curettage
Selective uterine arterial embolization
Open surgery
Medical treatment
Systemic MTX
Local MTX
Local embryocides (potassium chloride, sodium chloride, hyperosmolar glucose, or crystalline trichosanthin under ultrasonography guidance)
Expectant management
No therapeutic intervention, only serum β‐hCG monitoring
Types of outcome measures
Primary outcomes
Treatment success (success after initial treatment) ‐ defined as an uneventful decline of serum hCG to undetectable levels by the initial treatment. Also defined as 'a steady decline in serum β‐hCG to normal levels by the initial treatment, coupled with a lack of major complications (including massive acute bleeding, recurrence of active bleeding, incomplete abortion and hysterectomy)'.
Complications (need for further treatment for reasons such as heavy bleeding, recurrence of active bleeding, pelvic infection, organ injury, hysterectomy, or death).
Secondary outcomes
Quality of life. If studies reported more than one scale, preference was given to the 36‐item Short Form (SF‐36), then other validated generic scales, and finally condition‐specific scales.
Adverse effects (blood loss, fever, nausea and vomiting, stomatitis, alopecia, pneumonitis, abdominal or pelvic pain, leukopenia, and abnormal liver or renal function).
Time to normalize β‐hCG.
Hospital stay.
Financial costs.
Future fertility (restoration of normal menstrual cycles, repeat ectopic pregnancy).
Search methods for identification of studies
We developed a comprehensive search strategy for all published and unpublished RCTs of interventions for non‐tubal ectopic pregnancy in consultation with the Information Specialist of the Cochrane Gynaecology and Fertility (CGF) Group. Two review authors (YL and HZ) independently conducted the systematic searches regardless of language or publication status.
Electronic searches
We searched the following databases:
Cochrane CGF Group Specialised Register of Controlled Trials, PROCITE platform (searched December 2019; Appendix 1);
CENTRAL, via the Cochrane Register of Studies Online (CRSO), Web platform (searched December 2019; Appendix 2);
MEDLINE, Ovid platform (searched from 1946 to December 2019; Appendix 3);
Embase, Ovid platform (searched from 1980 to December 2019; Appendix 4);
PsycINFO, Ovid platform (searched from 1806 to December 2019; Appendix 5);
CINAHL, Ebsco platform (searched from 1961 to December 2019; Appendix 6);
Chinese Biomedicine DISC (CBM), Web platform (searched from 1978 to December 2019; Appendix 7).
Other electronic sources of trials included:
the Cochrane Library – Database of Abstracts of Reviews of Effects (reference lists from non‐Cochrane reviews on similar topics; www.cochrane.org/index.htm);
ClinicalTrials.gov (a service of the US National Institutes of Health; www.clinicaltrials.gov);
World Health Organization International Trials Registry Platform search portal (apps.who.int/trialsearch/);
Citation Indexes (scientific.thomson.com/products/sci/);
Conference abstracts in the Web of Knowledge (wokinfo.com/);
LILACS database for trials from the Portuguese and Spanish‐speaking world (pesquisa.bvsalud.org/portal/);
PubMed (www.ncbi.nlm.nih.gov/pubmed/) and Google for recent trials not yet indexed in the major databases;
OpenGrey database (opengrey.eu/) and Google for grey literature.
Searching other resources
We handsearched reference lists of articles retrieved by the search and contacted experts in the field to obtain additional data. We also handsearched relevant journals and conference abstracts that are not covered in the CGF Group Specialised Register in liaison with the Information Specialist.
Data collection and analysis
Selection of studies
We downloaded the title, abstract, and keywords of every trial retrieved by electronic searching to a reference management database (EndNote) and removed duplicates. Two review authors (YL and HZ) independently screened these titles and abstracts, and retrieved the full texts of all potentially eligible studies. Two review authors (YL and HZ) independently examined the full‐text articles and selected studies for inclusion in the review. We excluded studies that clearly did not comply with the inclusion criteria and documented the reasons for exclusion. We resolved disagreements by discussion with a third review author (JF). Figure 1 documents the PRISMA flow chart.
1.
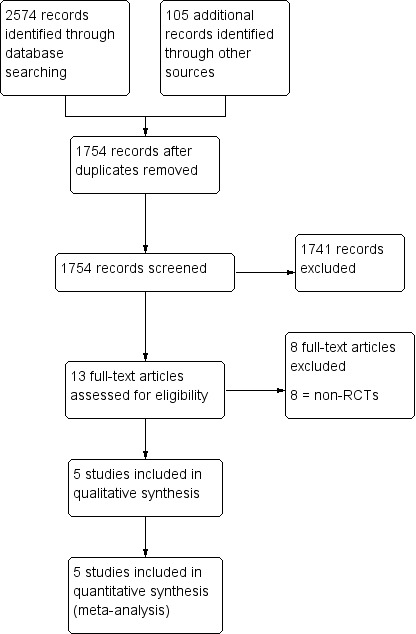
Study flow diagram.
Data extraction and management
Two review authors (YH and LS) independently extracted data from eligible studies using a data extraction form designed and pilot‐tested by the review authors.
Data extraction included:
participants' characteristics;
number of participants in each arm;
number of participants excluded from the analysis;
type of intervention;
proportion of participants who received all, part, or none of the intended treatment;
methods of randomization, blinding, and allocation concealment;
length of follow‐up;
data on outcomes.
We resolved disagreements by discussion or by an appeal to a third review author (WH). There was no blinding of review authors to article authors or journal titles. We corresponded with study authors for further information, as required.
Assessment of risk of bias in included studies
Two review authors (YL and YH) independently assessed the methodological risk of bias of each trial according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). According to the Cochrane 'Risk of bias' assessment tool, risk of bias assessment for included studies addressed seven criteria, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias; with a judgement of low risk, high risk, or unclear risk of bias. We resolved differences by discussion among the review authors or by consulting the Cochrane Menstrual Disorders and Subfertility Group. We took care to search for within‐trial selective reporting, such as trials failing to report obvious outcomes, or reporting them in insufficient detail to allow inclusion. We sought published protocols and compared the outcomes between the protocol and the final published study.
Measures of treatment effect
We extracted both dichotomous and continuous data from the included studies. For dichotomous data (e.g. reintervention rates), we used the numbers of events in the control and intervention groups of each study to calculate risk ratios (RR). For continuous data (e.g. hospitalization duration and financial costs), we calculated mean differences (MD) between treatment groups if all studies reported using the same scales to measure outcomes. If similar outcomes are reported on different scales, we calculated the standardized mean difference (SMD). We treated ordinal data (e.g. quality of life scores) as continuous data. We calculated the 95% confidence intervals (CI) for all outcomes.
Unit of analysis issues
The primary unit of analysis was per woman randomized. We planned to report data that did not allow a valid analysis in an additional table and not include them in a meta‐analysis.
Dealing with missing data
We analyzed data on an intention‐to‐treat basis, where possible, and attempted to obtain missing data from the original investigators. Where these were unobtainable, we undertook imputation of individual values for the primary outcomes only. If studies reported sufficient details to calculate MDs but provided no information on associated standard deviations (SD), we assumed the outcome to have an SD equal to the highest SD from other studies within the same analysis. For other outcomes, we analyzed only the available data.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots and by estimation of the I2 statistic, which summarizes the percentage of heterogeneity between trials that cannot be ascribed to sampling variation. An I2 value greater than 50% indicated substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias and other reporting biases, we aimed to minimize the potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data.
Data synthesis
We performed statistical analysis in accordance with the guidelines developed by the Cochrane Gynaecology and Fertility Group. Two review authors (YL and HZ) independently analyzed data in Review Manager 5 (Review Manager 2014).
-
Surgery versus medical treatment, stratified by location of pregnancy:
caesarean scar;
abdominal;
cornual;
cervical;
ovarian pregnancy.
-
Surgery versus medical treatment, stratified by specific type of surgery and mode of administration.
-
Surgery:
laparoscopic removal;
hysteroscopic evacuation;
dilation and curettage;
sac aspiration;
selective uterine arterial embolization;
open surgery.
-
Medical treatment:
systemic MTX;
local MTX;
local embryocides (potassium chloride, sodium chloride, hyperosmolar glucose, or crystalline trichosanthin under ultrasonography guidance).
-
Surgery or medical treatment versus expectant management.
One surgery versus another surgery
For an increase in the odds of a particular outcome, which may be beneficial (e.g. treatment success) or detrimental (e.g. complications), we displayed graphically in the forest plots to the right of the centre line and a decrease in the odds of an outcome to the left of the centre line.
Subgroup analysis and investigation of heterogeneity
If we detect substantial heterogeneity, we explored possible explanations in sensitivity analyses. We took any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility and analysis of studies and data. These analyses included consideration of whether the review conclusions would have differed if:
eligibility was restricted to studies without high risk of bias;
a random‐effects model had been adopted;
alternative imputation strategies had been implemented;
the summary effect measure was odds ratio rather than RR.
Overall quality of the body of evidence: 'Summary of findings' table
We generated 'Summary of findings' tables using GRADEpro software (GRADEpro GDT). One table evaluated the overall quality of the body of evidence for the main review outcomes (treatment success and complications) using GRADE criteria (study limitations, that is risk of bias, consistency of effect, imprecision, indirectness, and publication bias), for the main review comparison UAE/UACE compared with systemic MTX. We prepared additional 'Summary of findings' tables for review comparisons UACE plus MTX versus ultrasonography‐guided local MTX, and suction curettage under hysteroscopy versus suction curettage under ultrasonography. We justified, documented, and incorporated judgements about evidence quality (high, moderate, or low) into reporting of the results for each outcome.
Results
Description of studies
Results of the search
The search retrieved 1754 articles after removing duplicates. Thirteen articles were potentially eligible and were retrieved in full text. Five studies (five articles) met our inclusion criteria. We excluded eight studies. See Characteristics of included studies table, Characteristics of excluded studies table, and the PRISMA flow chart (Figure 1).
Included studies
Study design and setting
We included five parallel‐design RCTs (Zhuang 2009; Li 2011b; Qian 2015; Wang 2015; Li 2016). All were single‐centre studies conducted in Chinese hospitals.
Participants
The studies included 371 women with CSP confirmed by ultrasonography. We found no studies reported other non‐tubal ectopic pregnancies.
Interventions
For CSP, Li 2011b compared systemic MTX alone versus UACE with MTX; Zhuang 2009 compared systemic MTX alone versus UAE, both followed by suction curettage; Wang 2015 compared UACE with MTX versus ultrasonography‐guided local MTX injection; Qian 2015 compared suction curettage under hysteroscopy versus under ultrasonography after UAE; and Li 2016 compared suction curettage under hysteroscopy versus under ultrasonography after UACE.
Outcomes
All five studies reported primary treatment success or complications, or both (our primary outcomes), four studies reported adverse effects (Zhuang 2009; Li 2011b;Li 2016;Qian 2015), three studies reported time to normalize β‐hCG (Wang 2015; Li 2016; Qian 2015), and two studies reported hospital stay (Zhuang 2009;Li 2011b).
Excluded studies
We excluded eight studies as they were not RCTs (Wei 2007; Yan 2007; Zhuang 2008; Gu 2010; Li 2010; Wu 2014; Liu 2015; Peng 2015). Two described systematic approaches in the sequence generation process (Wei 2007; Peng 2015), and five involved subjective judgement or some method of non‐random categorization of participants (Yan 2007; Zhuang 2008; Gu 2010; Li 2010; Liu 2015; Peng 2015). One was a cohort study (Wu 2014).
Risk of bias in included studies
Refer to the 'Risk of bias' tables and Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
All five included studies were at low risk of selection bias related to sequence generation, as they used computer randomization or a random numbers table.
Allocation concealment
Li 2016 applied a sealed opaque envelope with a serial number on the surface and a random number inside (low risk of bias). Zhuang 2009 also applied a system of sealed and numbered envelopes (low risk of bias). Li 2011b, Qian 2015, and Wang 2015 did not describe the method used and were at unclear risk of bias for this domain.
Blinding
Blinding of participants and personnel (performance bias)
All five included studies were at high risk of performance bias, because the blinding method could not be conducted, although most studies did not describe that.
Blinding of outcome assessors (detection bias)
Li 2016 was at low risk because data analysts were masked to group assignment. Four studies did not supply this information, so we rated them at unclear risk of bias (Zhuang 2009; Li 2011b; Qian 2015; Wang 2015).
Incomplete outcome data
Four studies analyzed all women randomized, and we judged them at low risk of attrition bias (Zhuang 2009; Li 2011b; Wang 2015; Li 2016). Qian 2015 did not clearly state attrition rates, and we rated them at unclear risk.
Selective reporting
All five included studies were at unclear risk owing to insufficient information to permit judgement.
Other potential sources of bias
We assessed all the five studies at unclear risk of other sources of bias.
Effects of interventions
Summary of findings 1. UAE/UACE compared with systemic MTX for CSP.
| UAE/UACE compared with systemic MTX for CSPa | ||||||
|
Patient or population: women with CSP Settings: department of gynaecology in China Intervention: UAE/UACE (surgery) Comparison: systemic MTX (medical treatment) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic MTX | UAE/UACE | |||||
|
Treatment success after initial treatment UAE vs systemic MTX Follow‐up: 6 months UACE plus MTX vs systemic MTX Follow‐up: 6 months |
943 per 1000 | 943 per 1000 (849 to 1056) |
RR 1.00 (0.90 to 1.12) |
72 (1) | ⊕⊕⊝⊝ Lowb,c | Defined as an decline of serum β‐hCG to undetectable levels by the initial treatment. |
| 769 per 1000 | 669 per 1000 (415 to 1062) | RR 0.87 (0.54 to 1.38) | 28 (1) | ⊕⊕⊝⊝ Lowb,c | ||
|
Complications UAE vs systemic MTX Follow‐up: 6 months UACE plus MTX vs systemic MTX Follow‐up: 6 months |
171 per 1000 | 80 per 1000 (22 to 299) |
RR 0.47 (0.13 to 1.75) |
72 (1) | ⊕⊕⊝⊝ Lowb,c | Complications including heavy bleeding, recurrence of active bleeding, and hysterectomy. |
| 538 per 1000 | 334 per 1000 (140 to 796) |
RR 0.62 (0.26 to 1.48) |
28 (1) | ⊕⊕⊝⊝ Lowb,c | ||
|
Adverse effects UAE vs systemic MTX Follow‐up: 6 months UACE plus MTX vs systemic MTX Follow‐up: 6 months |
86 per 1000 | 136 per 1000 (35 to 525) |
RR 1.58 (0.41 to 6.11) |
72 (1) | ⊕⊕⊝⊝ Lowb,c | Adverse effects in the MTX group included abnormal liver function and severe vomiting; in the UAE group included fever and pain and 1 readmitted woman. |
| 231 per 1000 | 268 per 1000 (74 to 979) |
RR 1.16 (0.32 to 4.24) |
28 (1) | ⊕⊕⊝⊝ Lowa,b | Adverse effects included mild increase in liver enzymes, mild vomiting, and moderate abdominal or pelvic pain. | |
|
Blood loss UAE vs systemic MTX Follow‐up: 6 months UACE plus MTX vs systemic MTX Follow‐up: 6 months |
The mean blood loss in the control group was 415.63 mL | The blood loss in the intervention groups was 378.70 mL lower (–401.43 to –355.97) | — | 72 (1) | ⊕⊕⊕⊝ Moderatec | Blood loss was measured using the total volume of fluid in the bottle of the vacuum aspiration equipment after suction curettage minus the volume of amniotic fluid as assessed by ultrasonography at diagnosis. |
| The mean blood loss in the control group was 471 mL | The mean blood loss in the intervention groups was 879.00 mL lower (–1135.23 to ‐622.77) | — | 28 (1) | ⊕⊕⊕⊝ Moderatec | ||
| Time to normalize β‐hCG | Not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). β‐hCG: β‐human chorionic gonadotropin;CI: confidence interval; CSP: caesarean scar pregnancy; MTX: methotrexate; RR: risk ratio; UACE: uterine arterial chemoembolization; UAE: uterine arterial embolization. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aAll the treatments were followed by suction curettage. bDowngraded one level for imprecision (very small sample sizes and no clinically meaningful difference between groups). cDowngraded one level, no data available to assess heterogeneity.
Summary of findings 2. Suction curettage under hysteroscopy compared with under ultrasonography for CSP.
| Suction curettage under hysteroscopy compared with under ultrasonography for CSP | ||||||
|
Patient or population: women with CSP Settings: department of gynaecology in China Intervention: Suction curettage under hysteroscopy Comparison: Suction curettage under ultrasonography | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Under ultrasonography | Under hysteroscopy | |||||
|
Treatment success after initial treatment after UAE Follow‐up: > 12 months after UACE Follow‐up: 2 months |
1000 per 1000 | 910 per 1000 (810 to 1030) | RR 0.91 (0.81 to 1.03) | 66 (1) | ⊕⊝⊝⊝
Very lowa,b,c |
Defined as an decline of serum β‐hCG to undetectable levels by the initial treatment. |
| 977 per 1000 | 997 per 1000 (938 to 1065) | RR 1.02 (0.96 to 1.09) | 92 (1) | ⊕⊕⊝⊝ Lowa,b | ||
|
Complications after UAE Follow‐up: > 12 months after UACE Follow‐up: 2 months |
30 per 1000 | 120 per 1000 (14 to 1017) | RR 4.00 (0.47 to 33.91) | 66 (1) | ⊕⊝⊝⊝ Verylowa,b,c | Complications in this study including recurrence of active bleeding, pelvic infection and hysterectomy. |
| 45 per 1000 | 81 per 1000 (0 to 167) | RR 0.18 (0.01 to 3.72) | 92 (1) | ⊕⊕⊝⊝ Lowa,b | Complications in this study including heavy bleeding, uterine perforation, pelvic infection, incomplete abortion and uterine adhesion. | |
|
Adverse effects after UAE Follow‐up: > 12 months after UACE Follow‐up: 2 months |
See comment | See comment | RR 3.09 (0.12 to 78.70) | 66 (1) | ⊕⊝⊝⊝ Verylowa,b,c | Adverse effects in this study including chest congestion, and on 1 experienced it in the control group. |
| See comment | See comment | Not estimable | 92 (1) | ⊕⊝⊝⊝ Verylowa,b,d | Adverse effects in this study including low fever (37.3–38.5 °C) and abdominal pain, and all the participants had these adverse effects. | |
| Blood loss | Not reported | |||||
|
Time to normalize β‐hCG after UAE Follow‐up: > 12 months after UACE Follow‐up: 2 months |
The mean time to normalize β‐hCG in the control groups was 30.15 days | The time in the intervention groups was 4.03 longer (–1.79 to 9.85) | — | 66 (1) | ⊕⊝⊝⊝ Very lowa,b,c |
— |
| The mean time to normalize β‐hCG in the control groups was 17.64 days | The time in the intervention groups was 0.84 days longer (–1.90 to 3.58) | — | 92 (1) | ⊕⊕⊝⊝ Lowa,b | — | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). β‐hCG: β‐human chorionic gonadotropin;CI: confidence interval; CSP: caesarean scar pregnancy; MTX: methotrexate; RR: risk ratio; UACE: uterine arterial chemoembolization; UAE: uterine arterial embolization. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level for imprecision (very small sample sizes and no clinically meaningful difference between groups). bDowngraded one level, no data available to assess heterogeneity. cDowngraded one level for limitations in the design and implementation of available studies. dDowngraded one level for publication bias (did not cover all the effects).
1. Surgery alone or surgery plus medical treatment versus medical treatment alone (prior to suction curettage)
For CSP, Zhuang 2009 compared medical treatment (systemic MTX) versus surgery (UAE) following suction curettage at 24 hours, Li 2011b compared medical treatment (systemic MTX) versus surgery and medical treatment (UACE plus MTX) following suction curettage, and Wang 2015 compared medical treatment (local MTX) versus surgery and medical treatment (UAE plus MTX).
1.1. Caesarean scar pregnancy
1.1.1 UAE/UACE versus systemic MTX (prior to suction curettage)
Primary outcomes
Treatment success after initial treatment
Two studies reported treatment success after initial treatment (defined as an uneventful decline of serum β‐hCG to undetectable levels by the initial treatment) between UAE (Zhuang 2009)/UACE (Li 2011b) and systemic MTX. We are uncertain whether UAE/UACE improved treatment success after initial treatment (UAE: RR 1.00, 95% CI 0.90 to 1.12; 1 RCT, 72 women; low‐quality evidence; UACE: RR 0.87, 95% CI 0.54 to 1.38; 1 RCT, 28 women; low‐quality evidence; Analysis 1.1; Figure 4).
1.1. Analysis.

Comparison 1: UAE/UACE versus systemic MTX, both + suction curettage, Outcome 1: Treatment success after initial treatment
4.

Forest plot of comparison: 1 UAE/UACE versus systemic MTX, outcome: 1.1 Treatment success after initial treatment.
Complications
Two studies reported rates of complications (heavy bleeding, recurrence of active bleeding, and hysterectomy) between UAE (Zhuang 2009)/UACE (Li 2011b) and systemic MTX. We are uncertain whether UAE/UACE reduced rates of complications (UAE: RR 0.47, 95% CI 0.13 to 1.75; 1 RCT, 72 women; low‐quality evidence; UACE: RR 0.62, 95% CI 0.26 to 1.48; 1 RCT, 28 women; low‐quality evidence; Analysis 1.2; Figure 5).
1.2. Analysis.

Comparison 1: UAE/UACE versus systemic MTX, both + suction curettage, Outcome 2: Complications
5.

Forest plot of comparison: 1 UAE/UACE versus systemic MTX, outcome: 1.2 Complications.
Secondary outcomes
Quality of life
No study reported this outcome.
Adverse effects (overall)
One study comparing UAE with systemic MTX reported rates of adverse effects (UAE: including fever and pain and one readmitted woman; MTX group: including abnormal liver function and severe vomiting) (Zhuang 2009). The other study compared rates of adverse effects (mild increase in liver enzymes, mild vomiting, and moderate abdominal or pelvic pain) between UACE and systemic MTX (Li 2011b). We are uncertain whether UAE/UACE reduced adverse effects (UAE: RR 1.58, 95% CI 0.41 to 6.11; 1 RCT, 72 women; low‐quality evidence; UACE: RR 1.16, 95% CI 0.32 to 4.24; 1 RCT, 28 women; low‐quality evidence; Analysis 1.3; Figure 6). It was not obvious that the types of events had similar values to participants (e.g. fever versus vomiting).
1.3. Analysis.

Comparison 1: UAE/UACE versus systemic MTX, both + suction curettage, Outcome 3: Adverse effects (overall)
6.

Forest plot of comparison: 1 UAE/UACE versus systemic MTX, outcome: 1.3 Adverse effects (overall).
Blood loss
Two studies comparing UAE (Zhuang 2009)/UACE (Li 2011b) with systemic MTX reported blood loss. It was lower in the UAE/UACE groups than in the systemic MTX groups (UAE: MD –378.70 mL, 95% CI –401.43 to –355.97; 1 RCT, 72 women; moderate‐quality evidence; UACE: MD –879.00 mL, 95% CI –1135.23 to ‐622.77; 1 RCT, 28 women; moderate‐quality evidence; Analysis 1.4; Figure 7).
1.4. Analysis.

Comparison 1: UAE/UACE versus systemic MTX, both + suction curettage, Outcome 4: Blood loss
7.

Forest plot of comparison: 1 UAE/UACE versus systemic MTX, outcome: 1.4 Blood loss [mL].
Time to normalize β‐hCG
No study reported this outcome.
Hospital stay
Two studies comparing UAE (Zhuang 2009)/UACE (Li 2011b) with systemic MTX reported hospital stay. It was shorter in the UAE/UACE groups than in the systemic MTX group (UAE: MD –27.90 days, 95% CI –29.44 to –26.36; 1 RCT, 72 women; low‐quality evidence; MD –23.00 days, 95% CI –27.80 to –18.20; 1 RCT, 28 women; low‐quality evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: UAE/UACE versus systemic MTX, both + suction curettage, Outcome 5: Hospital stay
Financial costs
No study reported this outcome.
Future fertility
No study reported this outcome.
1.2. Ovarian pregnancy
No study reported on ovarian pregnancy.
1.3. Cornual pregnancy
No study reported on cornual pregnancy.
1.4. Cervical pregnancy
No study reported on cervical pregnancy.
1.5. Abdominal pregnancy
No study reported on abdominal pregnancy.
2. Surgery plus medical treatment versus medical treatment alone
2.1. Caesarean scar pregnancy
2.1.1 UACE plus MTX versus ultrasonography‐guided local MTX injection
Primary outcomes
Treatment success after initial treatment
One study reported treatment success after initial treatment (defined as an uneventful decline of serum β‐hCG to undetectable levels by the initial treatment) (Wang 2015). We are uncertain whether UACE improved treatment success after initial treatment compared with local MTX injection (RR 0.95, 95% CI 0.56 to 1.60; 1 RCT, 45 women; very low‐quality evidence; Analysis 2.1; Figure 8).
2.1. Analysis.

Comparison 2: UACE plus MTX versus ultrasonography‐guided local MTX injection, Outcome 1: Treatment success after initial treatment
8.

Forest plot of comparison: 2 UACE plus MTX versus ultrasonography‐guided local MTX injection, outcome: 2.1 Treatment success after initial treatment.
Complications
No study reported this outcome.
Secondary outcomes
Quality of life
No study reported this outcome.
Adverse effects (overall)
The study reported the same number of failed treatments in each arm ((RR 0.88, 95% CI 0.40 to 1.92; 1 RCT, 45 women).
No study reported this outcome.
Time to normalize β‐hCG
One study reported time to normalize β‐hCG (Wang 2015). We are uncertain whether UACE shortened the time to normalize β‐hCG (MD 1.50 days, 95% CI –3.16 to 6.16; 1 RCT, 45 women; very low‐quality evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: UACE plus MTX versus ultrasonography‐guided local MTX injection, Outcome 2: Time to normalize β‐hCG
Hospital stay
No study reported this outcome.
Financial costs
No study reported this outcome.
Future fertility
No study reported this outcome.
2.2. Ovarian pregnancy
No study reported on ovarian pregnancy.
2.3. Cornual pregnancy
No study reported on cornual pregnancy.
2.4. Cervical pregnancy
No study reported on cervical pregnancy.
2.5. Abdominal pregnancy
No study reported on abdominal pregnancy.
3. Surgery versus another surgery
3.1. Caesarean scar pregnancy
3.1.1. Suction curettage under hysteroscopy versus under ultrasonography after UAE/UACE
Primary outcomes
Treatment success rates after initial treatment
Two studies reported treatment success after initial treatment (defined as an uneventful decline of serum β‐hCG to undetectable levels by the initial treatment (Qian 2015; Li 2016). We are uncertain whether suction curettage under hysteroscopy improved treatment success after initial treatment compared with under ultrasonography (UAE: RR 0.91, 95% CI 0.81 to 1.03; 1 RCT, 66 women; very low‐quality evidence; UACE: RR 1.08, 95% CI 0.96 to 1.09; 1 RCT, 92 women; low‐quality evidence; Analysis 4.1; Figure 9).
Complications
One study reported rates of complications including heavy bleeding, recurrence of actively bleeding, pelvic infection, and hysterectomy (Qian 2015). The other study reported rates of complications including heavy bleeding, uterine perforation, pelvic infection, incomplete abortion, and uterine adhesion (Li 2016). We are uncertain whether suction curettage under hysteroscopy reduced rates of complications after UAE(Qian 2015) or UACE(Li 2016) compared with under ultrasonography (UAE: RR 4.00, 95% CI 0.47 to 33.91; 1 RCT, 66 women; very low‐quality evidence; UACE: RR 0.18, 95% CI 0.01 to 3.72; 1 RCT, 92 women; low‐quality evidence; Analysis 4.2; Figure 10).
Secondary outcomes
Quality of life
No study reported this outcome.
Adverse effects (overall)
One study reported rates of adverse effects (chest congestion) (Qian 2015), and the other study reported all the participants had a low fever (37.3–38.5 °C) and abdominal pain (Li 2016). We are uncertain whether suction curettage under hysteroscopy reduced adverse effects after UAE (Qian 2015)/UACE (Li 2016) compared with under ultrasonography (UAE: RR 3.09, 95% CI 0.12 to 78.70; 1 RCT, 66 women; very low‐quality evidence; UACE: not estimable; 1 RCT, 92 women; very low‐quality evidence; Analysis 4.3; Figure 11).
Time to normalize β‐hCG
Two studies reported time to normalize β‐hCG (Qian 2015; Li 2016). We are uncertain whether suction curettage under hysteroscopy shortened the time to normalize β‐hCG after UAE (Qian 2015)/UACE (Li 2016) compared with under ultrasonography (UAE: MD 4.03 days, 95% CI –1.79 to 9.85; 1 RCT, 66 women; very low‐quality evidence; UACE: MD 0.84 days, 95% CI –1.90 to 3.58; 1 RCT, 92 women; low‐quality evidence; Analysis 4.4).
Hospital stay
No study reported this outcome.
Financial costs
No study reported this outcome.
Future fertility
No study reported this outcome.
3.2. Ovarian pregnancy
No study reported on ovarian pregnancy.
3.3. Cornual pregnancy
No study reported on cornual pregnancy.
3.4. Cervical pregnancy
No study reported on cervical pregnancy.
3.5. Abdominal pregnancy
No study reported on abdominal pregnancy.
4. Surgery versus expectant management
No study compared surgery versus expectant management.
Other analyses
Data were too few for review authors to conduct planned subgroup and sensitivity analyses, or to construct a funnel plot to assess reporting bias.
Discussion
Summary of main results
Caesarean scar pregnancy
Most of our included studies performed suction curettage for CSP, and there was no evidence showing a preference for using hysteroscopy or ultrasonography‐guided surgery, after systemic MTX, or after UAE/UACE for success, complications, and effects; however, hospital stay of the surgery group was shorter than in the medical treatment group and blood loss was less. Results were based on very limited evidence and thus should be interpreted with caution.
Non‐tubal ectopic pregnancy other than caesarean scar pregnancy
RCTs are probably not feasible owing to low incidence and because it is usually an emergency surgery.
Overall completeness and applicability of evidence
The included studies partially answered the review question. We found few studies meeting our criteria, and among them, results showed a lack of consistency in methods of interventions and outcome measures, which leads to difficulties in combining data in a suitable meta‐analysis, thus making it difficult for review authors to draw clinically relevant conclusions. Before suction curettage, UAE/UACE showed less blood loss than systemic MTX, but more evidence is needed. As all the included studies were conducted in China, we consider there could be a specific concern about generalization of the results.
Quality of the evidence
All five studies were RCTs with adequately described randomization, and four studies reported allocation concealment. The interventions could not be blinded, but there was a limited impact on our primary outcomes. However, most outcomes in the comparisons had very low/low‐quality evidence, except blood loss which was of moderate quality. The main limitations were serious imprecision (associated with small sample sizes and very wide CIs for most comparisons), the issue of multiple comparisons with a small number of trials, and no data available for heterogeneity. In addition, some outcomes were downgraded for indirectness or publication bias.
Potential biases in the review process
We made every effort to minimize biases in the review process. We aimed to identify all relevant studies by performing comprehensive database searches. We followed Cochrane methods in the review process. In addition, we emailed the authors of included and excluded articles.
Agreements and disagreements with other studies or reviews
One non‐Cochrane review concluded that an interventional rather than a medical approach is better for CSP based on efficacy and safety, but their recommendations were primarily based on case series (Petersen 2016). Our review did not draw a similar conclusion although UAE/UACE showed less blood loss than systemic MTX. We consider more robust evidence is needed.
Authors' conclusions
Implications for practice.
It is uncertain whether there is a difference in success, complications, and adverse effects between UAE/UACE and administration of systemic MTX before suction curettage (low‐quality evidence).
Blood loss may be lower if suction curettage is conducted after uterine arterial embolization (UAE)/uterine arterial chemoembolization (UACE) than after administration of systemic methotrexate (MTX) (moderate‐quality evidence).
It is uncertain whether there is a difference in success, time to normalize β‐hCG (low‐quality evidence), complications, or adverse effects (very low‐quality evidence) between suction curettage under hysteroscopy and under ultrasonography.
Implications for research.
Population: subgroup according to caesarean scar pregnancy type (size and direction of growth of the gestational sac and thickness of the myometrium between bladder and gestational sac) could be considered (Jin 2016). More studies could be conducted in countries other than China.
Intervention: researchers could focus on UAE/UACE and suction curettage under monitoring.
Comparison: researchers could focus on surgery versus medical and between different surgeries.
Outcome: important complications such as heavy bleeding, recurrence of actively bleeding, serious pelvic infection, and hysterectomy could be more specific. Researchers could continue to follow‐up future pregnancy outcomes and quality of life after treatment.
History
Protocol first published: Issue 7, 2014 Review first published: Issue 7, 2020
| Date | Event | Description |
|---|---|---|
| 16 July 2014 | Amended | Name of author Shiyuan Yang incorrect in published protocol |
Acknowledgements
The authors thank Helen Nagels (Managing Editor), Marian Showell (Information Specialist), and the editorial board of the Cochrane Gynaecology and Fertility Group for their invaluable assistance in developing this review. We thank Dr Qiushi Wang, Dr Shiyuan Yang, and Prof Taixiang Wu who contributed to the protocol (Shen 2014).
We thank also Drs Annika Strandell, Prathima Dimitrova and Demián Glujovsky for peer review comments on the draft full review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility (CGF) Group Specialised Register search strategy
PROCITE platform
Searched 12 December 2019
Keywords CONTAINS "ectopic pregnancies" or "ectopic pregnancy" or "ectopic pregnancy‐outcome" or "ectopic pregnancy rate" or "ectopic rate" or "caesarean scar pregnancy" or Title CONTAINS "ectopic pregnancies" or "ectopic pregnancy" or "ectopic pregnancy‐outcome" or "ectopic pregnancy rate" or "ectopic rate" or "caesarean scar pregnancy"
(263 records)
Appendix 2. CENTRAL Register of Studies ONLINE (CRSO) search strategy
Web platform
Searched 12 December 2019
#1 MESH DESCRIPTOR Pregnancy, Ectopic EXPLODE ALL TREES 159
#2 (cornual adj3 pregnanc*):TI,AB,KY 1
#3 (heterotopic adj3 pregnanc*):TI,AB,KY 2
#4 (ectopic adj3 pregnanc*):TI,AB,KY 639
#5 (abdomin* adj3 pregnanc*):TI,AB,KY 33
#6 (extrauterine adj3 pregnanc*):TI,AB,KY 8
#7 (interstitial adj3 pregnanc*):TI,AB,KY 2
#8 (cervi* adj3 pregnanc*):TI,AB,KY 188
#9 (ovar* adj3 pregnanc*):TI,AB,KY 888
#10 (cesarean scar adj3 pregnanc*):TI,AB,KY 23
#11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 1778
#12 MESH DESCRIPTOR Hysteroscopy EXPLODE ALL TREES 382
#13 MESH DESCRIPTOR Laparoscopy EXPLODE ALL TREES 5347
#14 MESH DESCRIPTOR Hysterectomy EXPLODE ALL TREES 1722
#15 MESH DESCRIPTOR Uterine Artery Embolization EXPLODE ALL TREES 47
#16 MESH DESCRIPTOR Dilatation and Curettage EXPLODE ALL TREES 357
#17 MESH DESCRIPTOR Salpingectomy EXPLODE ALL TREES 44
#18 MESH DESCRIPTOR Salpingostomy EXPLODE ALL TREES 39
#19 MESH DESCRIPTOR Methotrexate EXPLODE ALL TREES 3756
#20 MESH DESCRIPTOR Potassium Chloride EXPLODE ALL TREES 319
#21 MESH DESCRIPTOR Sodium Chloride EXPLODE ALL TREES 2453
#22 (expectant management):TI,AB,KY 619
#23 (wait adj1 see):TI,AB,KY 182
#24 (watchful wait*):TI,AB,KY 708
#25 (medical treatment*):TI,AB,KY 5430
#26 (medical therap*):TI,AB,KY 4355
#27 (hysteroscop* or laparoscop*):TI,AB,KY 19570
#28 hysterectom*:TI,AB,KY 5845
#29 (uterine artery emboli?ation or UAE):TI,AB,KY 552
#30 (Dilatation adj3 Curettage):TI,AB,KY 364
#31 (High‐intensity focused ultraso*):TI,AB,KY 219
#32 Methotrexate:TI,AB,KY 10515
#33 (Potassium Chloride):TI,AB,KY 840
#34 (Sodium Chloride):TI,AB,KY 7972
#35 (sac adj3 aspirat*):TI,AB,KY 0
#36 (open surg*):TI,AB,KY 2381
#37 (conservative treatment*):TI,AB,KY 4560
#38 (conservative management):TI,AB,KY 937
#39 embryocide*:TI,AB,KY 0
#40 (hyperosmolar glucose):TI,AB,KY 13
#41 (crystalline trichosanthin):TI,AB,KY 0
#42 (Salpingostom* or salpingectom*):TI,AB,KY 293
#43 MESH DESCRIPTOR High‐Intensity Focused Ultrasound Ablation EXPLODE ALL TREES 66
#44 (ultrasound ablation):TI,AB,KY 72
#45 MESH DESCRIPTOR Laparotomy EXPLODE ALL TREES 714
#46 laparotom*:TI,AB,KY 3142
#47 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 61887
#48 #11 AND #47 427
Appendix 3. MEDLINE search strategy
Ovid platform
Searched from 1946 to 12 December 2019
1 pregnancy, ectopic/ or exp pregnancy, abdominal/ or pregnancy, heterotopic/ (11763) 2 (cornual adj3 pregnanc$).tw. (304) 3 (heterotopic adj3 pregnanc$).tw. (641) 4 ectopic pregnanc$.tw. (9946) 5 (abdomin$ adj3 pregnanc$).tw. (1832) 6 (extrauterine adj3 pregnanc$).tw. (1302) 7 (interstitial adj3 pregnanc$).tw. (629) 8 (cervi$ adj3 pregnanc$).tw. (2678) 9 (ovar$ adj3 pregnanc$).tw. (2879) 10 (cesarean scar adj3 pregnanc$).tw. (454) 11 or/1‐10 (21375) 12 exp hysteroscopy/ or laparoscopy/ or hand‐assisted laparoscopy/ (87508) 13 exp hysterectomy/ or uterine artery embolization/ (30831) 14 exp "Dilatation and Curettage"/ (3036) 15 Methotrexate/ (37195) 16 Potassium Chloride/ (17780) 17 Sodium Chloride/ (58018) 18 expectant management.tw. (2467) 19 (wait adj1 see).tw. (5) 20 medical treatment.tw. (44126) 21 medical therap$.tw. (29916) 22 (hysteroscop$ or laparoscop$).tw. (126734) 23 hysterectom$.tw. (35236) 24 uterine artery emboli?ation.tw. (1667) 25 (Dilatation and Curettage).tw. (1366) 26 High‐intensity focused ultrasound.tw. (2795) 27 Methotrexate.tw. (39821) 28 Potassium Chloride.tw. (6061) 29 Sodium Chloride.tw. (16652) 30 sac aspiration.tw. (25) 31 high‐intensity focused ultrasound ablation/ or laparotomy/ (20142) 32 open surg$.tw. (23722) 33 conservative treatment.tw. (28616) 34 conservative management.tw. (14395) 35 embryocide$.tw. (10) 36 hyperosmolar glucose.tw. (72) 37 crystalline trichosanthin.tw. (1) 38 or/12‐37 (461512) 39 11 and 38 (5837) 40 randomized controlled trial.pt. (495635) 41 controlled clinical trial.pt. (93449) 42 randomized.ab. (462685) 43 randomised.ab. (92495) 44 placebo.tw. (208784) 45 clinical trials as topic.sh. (189357) 46 randomly.ab. (322897) 47 trial.ti. (209094) 48 (crossover or cross‐over or cross over).tw. (82695) 49 or/40‐48 (1318313) 50 exp animals/ not humans.sh. (4648880) 51 49 not 50 (1213123) 52 39 and 51 (266)
Appendix 4. Embase search strategy
Ovid platform
Searched from 1980 to 12 December 2019
1 exp ectopic pregnancy/ (17716) 2 (cornual adj3 pregnanc$).tw. (500) 3 (heterotopic adj3 pregnanc$).tw. (877) 4 ectopic pregnanc$.tw. (12257) 5 (abdomin$ adj3 pregnanc$).tw. (1627) 6 (extrauterine adj3 pregnanc$).tw. (852) 7 (interstitial adj3 pregnanc$).tw. (676) 8 (cervi$ adj3 pregnanc$).tw. (2828) 9 (ovar$ adj3 pregnanc$).tw. (2882) 10 (cesarean scar adj3 pregnanc$).tw. (684) 11 or/1‐10 (24654) 12 exp hysteroscopy/ (11521) 13 exp laparoscopy/ or exp hand assisted laparoscopy/ (151525) 14 exp hysterectomy/ or exp abdominal hysterectomy/ (66893) 15 exp uterine artery embolization/ (3546) 16 exp curettage/ (12543) 17 "Dilatation and Curettage".tw. (1421) 18 exp methotrexate/ (164785) 19 potassium chloride/ (24218) 20 sodium chloride/ (172253) 21 exp conservative treatment/ (545564) 22 expectant management.tw. (3664) 23 (wait adj1 see).tw. (15) 24 medical treatment.tw. (61737) 25 medical therap$.tw. (45496) 26 (hysteroscop$ or laparoscop$).tw. (202457) 27 hysterectom$.tw. (52580) 28 uterine artery emboli?ation.tw. (2683) 29 (Dilatation and Curettage).tw. (1554) 30 High‐intensity focused ultrasound.tw. (3997) 31 Methotrexate.tw. (62532) 32 Potassium Chloride.tw. (6376) 33 Sodium Chloride.tw. (17647) 34 sac aspiration.tw. (20) 35 exp high intensity focused ultrasound/ (5266) 36 exp laparotomy/ (73782) 37 open surg$.tw. (36210) 38 conservative treatment.tw. (35831) 39 conservative management.tw. (19782) 40 embryocide$.tw. (10) 41 hyperosmolar glucose.tw. (79) 42 crystalline trichosanthin.tw. (1) 43 or/12‐42 (1381398) 44 11 and 43 (10377) 45 Clinical Trial/ (949432) 46 Randomized Controlled Trial/ (576494) 47 exp randomization/ (85116) 48 Single Blind Procedure/ (37225) 49 Double Blind Procedure/ (164383) 50 Crossover Procedure/ (61279) 51 Placebo/ (329309) 52 Randomi?ed controlled trial$.tw. (216303) 53 Rct.tw. (34912) 54 random allocation.tw. (1950) 55 randomly allocated.tw. (33705) 56 allocated randomly.tw. (2480) 57 (allocated adj2 random).tw. (807) 58 Single blind$.tw. (23682) 59 Double blind$.tw. (196984) 60 ((treble or triple) adj blind$).tw. (1056) 61 placebo$.tw. (293710) 62 prospective study/ (565394) 63 or/45‐62 (2106769) 64 case study/ (65626) 65 case report.tw. (386274) 66 abstract report/ or letter/ (1070162) 67 or/64‐66 (1512139) 68 63 not 67 (2055003) 69 44 and 68 (809)
Appendix 5. PsycINFO search strategy
Ovid platform
Searched from 1806 to 12 December 2019
1 (cornual adj3 pregnanc$).tw. (1) 2 (heterotopic adj3 pregnanc$).tw. (1) 3 ectopic pregnanc$.tw. (97) 4 (abdomin$ adj3 pregnanc$).tw. (9) 5 (extrauterine adj3 pregnanc$).tw. (3) 6 (cervi$ adj3 pregnanc$).tw. (13) 7 (ovar$ adj3 pregnanc$).tw. (40) 8 (cesarean scar adj3 pregnanc$).tw. (0) 9 or/1‐8 (156) 10 exp Surgery/ (69481) 11 exp Hysterectomy/ (441) 12 exp Drug Therapy/ or exp Induced Abortion/ (145074) 13 expectant management.tw. (30) 14 (wait adj1 see).tw. (2) 15 medical treatment.tw. (6116) 16 medical therap$.tw. (1427) 17 (hysteroscop$ or laparoscop$).tw. (503) 18 hysterectom$.tw. (811) 19 uterine artery emboli?ation.tw. (4) 20 (Dilatation and Curettage).tw. (11) 21 High‐intensity focused ultrasound.tw. (10) 22 Methotrexate.tw. (352) 23 Potassium Chloride.tw. (332) 24 Sodium Chloride.tw. (957) 25 sac aspiration.tw. (0) 26 open surg$.tw. (95) 27 conservative treatment.tw. (287) 28 conservative management.tw. (149) 29 embryocide$.tw. (0) 30 hyperosmolar glucose.tw. (0) 31 crystalline trichosanthin.tw. (0) 32 or/10‐31 (217544) 33 9 and 32 (36) 34 random.tw. (56898) 35 control.tw. (435200) 36 double‐blind.tw. (22534) 37 clinical trials/ (11506) 38 placebo/ (5429) 39 exp Treatment/ (1023681) 40 or/34‐39 (1412737) 41 33 and 40 (35)
Appendix 6. CINAHL search strategy
Ovid platform
Searched from 1961 to 12 December 2019
| # | Query | Results |
| S56 | S41 AND S55 | 229 |
| S55 | S42 OR S43 or S44 or S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 | 1,363,333 |
| S54 | TX allocat* random* | 11,181 |
| S53 | (MH "Quantitative Studies") | 23,926 |
| S52 | (MH "Placebos") | 11,504 |
| S51 | TX placebo* | 60,127 |
| S50 | TX random* allocat* | 11,181 |
| S49 | (MH "Random Assignment") | 56,448 |
| S48 | TX randomi* control* trial* | 179,148 |
| S47 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 1,038,136 |
| S46 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 249 |
| S45 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 249 |
| S44 | TX clinic* n1 trial* | 254,707 |
| S43 | PT Clinical trial | 86,318 |
| S42 | (MH "Clinical Trials+") | 270,305 |
| S41 | S11 AND S40 | 1,621 |
| S40 | S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 | 120,857 |
| S39 | TX laparotomy | 7,133 |
| S38 | TX (hyperosmolar glucose) | 12 |
| S37 | TX embryocide* | 1 |
| S36 | TX (conservative management) | 4,101 |
| S35 | TX (conservative treatment) | 6,871 |
| S34 | TX (open surg*) | 9,564 |
| S33 | (MM "Laparotomy") | 1,172 |
| S32 | TX sac aspiration | 17 |
| S31 | TX Sodium Chloride | 7,215 |
| S30 | TX Potassium Chloride | 1,011 |
| S29 | TX Methotrexate | 7,669 |
| S28 | TX (High‐intensity focused ultrasound) | 574 |
| S27 | TX (Dilatation and Curettage) | 814 |
| S26 | TX (uterine artery emboli?ation) | 994 |
| S25 | TX hysterectom* | 10,490 |
| S24 | TX (hysteroscop* or laparoscop*) | 34,538 |
| S23 | TX medical therap* | 19,973 |
| S22 | TX medical treatment | 22,335 |
| S21 | TX (wait N1 see) | 475 |
| S20 | TX expectant management | 949 |
| S19 | (MM "Sodium Chloride") | 1,358 |
| S18 | (MM "Potassium Chloride") | 145 |
| S17 | (MM "Methotrexate") | 2,319 |
| S16 | (MM "Dilatation and Curettage") | 367 |
| S15 | (MH "Uterine Artery Embolization") | 516 |
| S14 | (MM "Hysterectomy") OR (MM "Hysterectomy, Vaginal") | 3,852 |
| S13 | (MM "Laparoscopy") OR (MM "Surgery, Laparoscopic") | 14,762 |
| S12 | (MM "Hysteroscopy") | 959 |
| S11 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 | 4,582 |
| S10 | TX (cesarean scar N3 pregnanc*) | 297 |
| S9 | TX (ovar* N3 pregnanc*) | 452 |
| S8 | TX (cervi* N3 pregnanc*) | 723 |
| S7 | TX (interstitial N3 pregnanc*) | 158 |
| S6 | TX (extrauterine N3 pregnanc*) | 52 |
| S5 | TX (abdomin* N3 pregnanc*) | 441 |
| S4 | TX (ectopic pregnanc*) | 3,190 |
| S3 | TX (heterotopic N3 pregnanc*) | 172 |
| S2 | TX (cornual N3 pregnanc*) | 99 |
| S1 | (MM "Pregnancy, Ectopic") OR (MM "Pregnancy, Heterotopic") | 1,909 |
Appendix 7. CBM search strategy
Web platform
Searched from 1978 to 12 December 2019
1 Mesh:Pregnancy, Ectopic/All Trees/AL/DH/DT/NU/PC/QL/RH/RT/SU/TH/XL/ZD/ZH/ZJ/ZL
2 (Cervical Pregnancy) or (Cervical Pregnancies) or (Pregnancy, cervical) ‐limitation:Clinical Trials; Randomized Controlled Trials; Multicenter Studies
3 (Cesarean Scar Pregnancy) or (Cesarean Scar Pregnancies) or (Pregnancy, Cesarean Scar) ‐limitation:Clinical Trials; Randomized Controlled Trials; Multicenter Studies
4 (Interstitial Pregnancy) or (Interstitial Pregnancies) or (Pregnancy, Interstitial)‐limitation:Clinical Trials; Randomized Controlled Trials; Multicenter Studies
5 (Cornual Pregnancy) or (Cornual Pregnancies) or (Pregnancy, Cornual)‐limitation:Clinical Trials; Randomized Controlled Trials; Multicenter Studies
6 (Ovarian Pregnancy) or (Ovarian Pregnancies) or (Pregnancy, Ovarian)‐limitation:Clinical Trials; Randomized Controlled Trials; Multicenter Studies
7 (Abdominal Pregnancy) or (Abdominal Pregnancies) or (Pregnancy, Abdominal)‐limitation:Clinical Trials; Randomized Controlled Trials; Multicenter Studies
8 (Non‐tubal Pregnancy) or (Non‐tubal Pregnancies) or (Pregnancy, Non‐tubal)‐limitation:Clinical Trials; Randomized Controlled Trials; Multicenter Studies
9 (Unusual Located Pregnancy) or (Unusual Located Pregnancies) or (Pregnancy, Unusual Located)‐limitation:Clinical Trials; Randomized Controlled Trials; Multicenter Studies
10 #8 or #9
11 # 1 and # 2
12 # 1 and # 3
13 # 1 and # 4
14 # 1 and # 5
15 # 1 and # 6
16 # 1 and # 7
17 # 1 and # 10
18 #11 or # 12 or # 13 or # 14 or # 15 or # 16 or #17
Data and analyses
Comparison 1. UAE/UACE versus systemic MTX, both + suction curettage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Treatment success after initial treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 UAE vs systemic MTX | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| 1.1.2 UACE plus MTX vs systemic MTX | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.54, 1.38] |
| 1.2 Complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 UAE vs systemic MTX | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.75] |
| 1.2.2 UACE plus MTX vs systemic MTX | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.26, 1.48] |
| 1.3 Adverse effects (overall) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 UAE vs systemic MTX | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.41, 6.11] |
| 1.3.2 UACE plus MTX vs systemic MTX | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.32, 4.24] |
| 1.4 Blood loss | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.4.1 UAE vs systemic MTX | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐378.70 [‐401.43, ‐355.97] |
| 1.4.2 UACE plus MTX vs systemic MTX | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐879.00 [‐1135.23, ‐622.77] |
| 1.5 Hospital stay | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.5.1 UAE vs systemic MTX | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐27.90 [‐29.44, ‐26.36] |
| 1.5.2 UACE plus MTX vs systemic MTX | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐23.00 [‐27.80, ‐18.20] |
Comparison 2. UACE plus MTX versus ultrasonography‐guided local MTX injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Treatment success after initial treatment | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.56, 1.60] |
| 2.2 Time to normalize β‐hCG | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [‐3.16, 6.16] |
| 2.3 Adverse effects (overall) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.40, 1.92] |
2.3. Analysis.

Comparison 2: UACE plus MTX versus ultrasonography‐guided local MTX injection, Outcome 3: Adverse effects (overall)
Comparison 3. Suction curettage under hysteroscopy versus under ultrasonography.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Treatment success after initial treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1.1 Suction curettage after UAE | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.03] |
| 3.1.2 Suction curettage after UACE | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.96, 1.09] |
| 3.2 Complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.2.1 Suction curettage after UAE | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.47, 33.91] |
| 3.2.2 Suction curettage after UACE | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.72] |
| 3.3 Adverse effects (overall) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.3.1 Suction curettage after UAE | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.12, 78.70] |
| 3.3.2 Suction curettage after UACE | 1 | 92 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.4 Time to normalize β‐hCG | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 Suction curettage after UAE | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 4.03 [‐1.79, 9.85] |
| 3.4.2 Suction curettage after UACE | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [‐1.90, 3.58] |
3.1. Analysis.

Comparison 3: Suction curettage under hysteroscopy versus under ultrasonography, Outcome 1: Treatment success after initial treatment
3.2. Analysis.

Comparison 3: Suction curettage under hysteroscopy versus under ultrasonography, Outcome 2: Complications
3.3. Analysis.

Comparison 3: Suction curettage under hysteroscopy versus under ultrasonography, Outcome 3: Adverse effects (overall)
3.4. Analysis.

Comparison 3: Suction curettage under hysteroscopy versus under ultrasonography, Outcome 4: Time to normalize β‐hCG
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Li 2016.
| Study characteristics | ||
| Methods | Randomization using sealed, opaque and numbered envelopes, with a randomization table | |
| Participants | 92 women with CSP | |
| Interventions | All participants underwent UACE (MTX 50 mg infused bilaterally into the gelatin sponge particles). Within 24–48 hours, suction curettage under hysteroscopy monitoring (31A,17B. 48 cases, age: > 35y, n = 29; < 35y, n = 19), or suction curettage under ultrasonography monitoring (28A,16B. 44 cases, age: > 35y, n = 31; < 35y, n = 13) (A: diameter of the gestational sac ≤ 3.0 cm; B: diameter of the gestational sac > 3.0cm) |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a sealed opaque envelope with a serial number on the surface and a random number inside." |
| Allocation concealment (selection bias) | Low risk | Quote: "The random number had been packed in the serially numbered envelopes by hospital staff members who had no contact with patients, clinicians, and investigators." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Clinicians were not masked to group assignment, but participants and data analysts were." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Clinicians were not masked to group assignment, but participants and data analysts were." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed. |
Li 2011b.
| Study characteristics | ||
| Methods | Randomization using a randomization table | |
| Participants | 28 women with CSP | |
| Interventions | Systemic MTX intravenously 50 mg/m2 of body surface area (size of sac/mass: 129.6 ± 96.7 cm3; 13 cases, age:33.2 ± 4.5y) treatment vs UACE plus MTX 40 mg and gelatin sponge particles (size of sac/mass: 161.1 ± 102.1 cm3; 15 cases, age:34.2 ± 5.5y) suction curettage performed when β‐hCG decreased to < 50 mIU/mL in systemic MTX group suction curettage performed after 24 hours in UACE group |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "By using a randomization table." |
| Allocation concealment (selection bias) | Unclear risk | No data available for this specific type of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No data available for this specific type of bias, and blinding was not possible with this methodology. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No data available for this specific type of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed. |
Qian 2015.
| Study characteristics | ||
| Methods | Used computerized randomization | |
| Participants | 66 women with CSP who had no internal bleeding and were haemodynamically stable, unruptured, and were at < 10 weeks of gestation. | |
| Interventions | All women underwent UAE After 24 hours, hysteroscopy surgery (gestational sac diameter: 2.97 ± 1.53 cm. 33 cases, age: 32.00 ± 4.15y) vs suction curettage under transabdominal sonography guidance (gestational sac diameter: 2.79 ± 1.50 cm. 33 cases, age:30.79 ± 4.29y) |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using computerized randomization." |
| Allocation concealment (selection bias) | Unclear risk | No data available for this specific type of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No data available for the specific type of bias, and blinding was not possible with this methodology. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No data available for this specific type of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data available for this specific type of bias. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed. |
Wang 2015.
| Study characteristics | ||
| Methods | Randomization using a randomization list generated by a random number generator | |
| Participants | 45 women with CSP who were haemodynamically stable and eligible for conservative treatment | |
| Interventions | Ultrasonography‐guided local injection of MTX into the gestational sac (15A, 6B. 21 cases, age: 29.52 ± 4.88y) vs UAE with local MTX injection (18A, 6B. 24 cases, age: 29.96 ± 4.14y) (A: diameter of the gestational sac ≤ 5 cm, B: diameter of the gestational sac > 5 cm) Another dose of systemic MTX (50 mg/m2 body surface area, intramuscularly) was administered if serum β‐hCG levels failed to decrease by 15% between days 4 and 7. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized according to a randomization list, generated by a random number generator." |
| Allocation concealment (selection bias) | Unclear risk | No data available for this specific type of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No data available for the specific type of bias, and blinding was not possible with this methodology. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No data available for this specific type of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "46 patients were recruited, only one participant was lost to follow up." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed. |
Zhuang 2009.
| Study characteristics | ||
| Methods | Randomization using sealed and numbered envelopes, with a randomization table | |
| Participants | 72 women with CSP | |
| Interventions | Infusion of MTX intravenously (50 mg/m2 body surface area) (35 cases, age: 32.88 ± 0.98y) vs UAE (37 cases, age: 32.23 ± 0.65y) suction curettage performed when β‐hCG decreased to < 50 mIU/mL in MTX group suction curettage performed after 24 hours in UACE group |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | Data of primary outcome (serum β‐hCG level) not shown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned in a 1:1 allocation ratio using a randomization table." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was conducted via a system of sealed and numbered envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No data available for this specific type of bias, and blinding was not possible with this methodology. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No data available for this specific type of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias existed. |
β‐hCG: β‐human chorionic gonadotropin; CSP: caesarean scar pregnancy; MTX: methotrexate; PVA: polyvinyl acetate; UACE: uterine arterial chemoembolization; UAE: uterine arterial embolization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gu 2010 | Not an RCT (involving method of non‐random categorization of participants) |
| Li 2010 | Not an RCT (via emailing the author) |
| Liu 2015 | Not an RCT (grouping based on subjective judgement) |
| Peng 2015 | Not an RCT (systematic approaches in the sequence generation process) |
| Wei 2007 | Not an RCT (systematic approaches in the sequence generation process) |
| Wu 2014 | Not an RCT (cohort study) |
| Yan 2007 | Not an RCT (via emailing the author) |
| Zhuang 2008 | Not an RCT (group based on subjective judgement) |
RCT: randomized controlled trial.
Differences between protocol and review
There was a change of authorship. Qiushi Wang, Shiyuan Yang, and Taixiang Wu contributed to the protocol (Shen 2014), but not the review. Ying Long and Yuanyuan Hu joined the team at the review stage.
Data were too few for review authors to conduct planned subgroup and sensitivity analyses, or to construct a funnel plot to assess reporting bias.
We have removed reference to interstitial pregnancy (included in the protocol) as this is a tubal pregnancy and thus does not meet this review's inclusion criteria.
One comparison between different surgical methods was added to the review.
We added the following definition of treatment success: 'a steady decline in serum β‐hCG to normal levels by the initial treatment, coupled with a lack of major complications (including massive acute bleeding, recurrence of active bleeding, incomplete abortion and hysterectomy)'.
We added a secondary outcome: 'time to normalise β‐hCG'.
Contributions of authors
YL: screened titles and abstracts, retrieved and examined the full texts, selected studies, assessed risk of bias.
HZ: screened titles and abstracts, retrieved and examined the full texts, selected studies.
YH: extracted data, assessed risk of bias.
LS: extracted data.
JF: resolved disagreements on selection of studies.
WH: resolved disagreements on data extraction and management.
All review authors contributed to the review development.
Sources of support
Internal sources
-
All authors, China
None of the authors received any specific source of support.
External sources
National Natural Science Foundation of China (81601259), China
Sichuan Science and Technology Program (2017SZ0115), China
Declarations of interest
HZ, YH, LS, JF, WH, QW, and SY had no conflicts to declare.
YL's institution received grants from National Natural Science Foundation of China and Science and Technology Department of Sichuan Province, Sichuan Science and Technology with respect to this Cochrane Review.
This author contributed equally to this work
This author contributed equally to this work
New
References
References to studies included in this review
Li 2011b {published data only}
- Li C, Li C, Feng D, Jia C, Liu B, Zhan X. Transcatheter arterial chemoembolization versus systemic methotrexate for the management of cesarean scar pregnancy. International Journal of Gynaecology and Obstetrics 2011;113(3):178-82. [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li Y, Gong L, Wu X, Gao H, Zheng H, Lan W. Randomized controlled trial of hysteroscopy or ultrasonography versus no guidance during D&C after uterine artery chemoembolization for cesarean scar pregnancy. International Journal of Gynaecology and Obstetrics 2016;135(2):158-62. [DOI] [PubMed] [Google Scholar]
Qian 2015 {published data only}
- Qian Z, Huang L, Zhu X. Curettage or operative hysteroscopy in the treatment of cesarean scar pregnancy. Archives of Gynecology and Obstetrics 2015;292(5):1055-61. [DOI] [PubMed] [Google Scholar]
Wang 2015 {published data only}
- Wang M, Yang Z, Li Y, Chen B, Wang J, Ma X, et al. Conservative management of cesarean scar pregnancies: a prospective randomized controlled trial at a single center. International Journal of Clinical and Experimental Medicine 2015;8(10):18972-80. [PMC free article] [PubMed] [Google Scholar]
Zhuang 2009 {published data only}
- Zhuang Y, Huang L. Uterine artery embolization compared with methotrexate for the management of pregnancy implanted with in a cesarean scar. American Journal of Obstetrics and Gynecology 2009;201(2):152.e1-3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gu 2010 {published data only}
- Gu W, Wang H, Wan J, Zhang L, Wang Y, Wang W, et al. Intra-arterial infusion of MTX for the treatment of cesarean scar pregnancy: a comparative study between different doses [不同剂量MTX经动脉灌注治疗切口妊娠疗效的分析]. Journal of Interventional Radiology 2010;19(7):568-71. [Google Scholar]
Li 2010 {published data only}
- Li Y, Tan S, Qu Q, Liu F. Clinical randomized controlled study on the efficacy and safety of curettage and conservative treatment of cervical pregnancy [宫颈妊娠保守治疗后清宫与单纯保守治疗疗效及安全性的临床随机对照研究]. Journal of Sichuan University (Medical Edition) 2010;41(2):370-1. [Google Scholar]
Liu 2015 {published data only}
- Liu B, Cao M, Zhang Y, Zhu Z. Uterine artery embolization and uterine artery chemoembolization for the treatment of cesarean scar pregnancy: a comparative study [子宫动脉栓塞与化疗栓塞治疗瘢痕妊娠 疗效比较]. Journal of Interventional Radiology 2015;24(7):588-91. [Google Scholar]
Peng 2015 {published data only}
- Peng P, Gui T, Liu X, Chen W, Liu Z. Comparative efficacy and safety of local and systemic methotrexate injection in cesarean scar pregnancy. Therapeutics and Clinical Risk Management 2015;11:137-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2007 {published data only}
- Wei X, Li M, Shao H, Ni Q, Shao J. A comparative study of tieing vs suturing on laparoscopic management of interstitial tubal pregnancy [腹腔镜下套圈法与缝合法治疗输卵管问质部妊娠的对照研究]. Chinese Primary Health Care 2007;21(1):84-5. [Google Scholar]
Wu 2014 {published data only}
- Wu X, Xue X, Wu X, Lin R, Yuan Y, Wang Q, et al. Combined laparoscopy and hysteroscopy vs. uterine curettage in the uterine artery embolization-based management of cesarean scar pregnancy: a retrospective cohort study. International Journal of Clinical and Experimental Medicine 2014;7(9):2793-803. [PMC free article] [PubMed] [Google Scholar]
Yan 2007 {published data only}
- Yan B. Application of laparoscopy in the treatment of cornual pregnancy [腹腔镜在宫角妊娠治疗中的应用]. Guangzhou Medical Journal 2007;38(4):43-5. [Google Scholar]
Zhuang 2008 {published data only}
- Zhuang Y, Wei L, Wang W, Huang L. Treatment of pregnancy in a previous caesarean section scar with uterine artery embolization: analysis of 60 cases [应用子宫动脉栓塞术治疗剖宫产瘢痕处妊娠]. Zhonghua Yi Xue za Zhi [Chinese Medical Journal; Free China Ed] 2008;88(33):2372-4. [PubMed] [Google Scholar]
Additional references
Alammari 2017
- Alammari R, Thibodeau R, Harmanli O. Vaginal hysterectomy for treatment of cervical ectopic pregnancy. American College of Obstetricians and Gynecologists 2017;129(1):63-5. [DOI] [PubMed] [Google Scholar]
Anev 2013
- Anev I, Wang J, Palep-Singh M, Seif MW. Monochorionic diamniotic twin cervical ectopic pregnancy following assisted conception: a case report. Journal of Reproductive Medicine 2013;58(9-10):445-7. [PubMed] [Google Scholar]
Barnhart 2009
- Barnhart KT. Clinical practice. Ectopic pregnancy. New England Journal of Medicine 2009;361(4):379-87. [DOI] [PubMed] [Google Scholar]
Bernstein 2001
- Bernstein HB, Thrall MM, Clark WB. Expectant management of intramural ectopic pregnancy. Obstetrics and Gynecology 2001;97(5 Pt 2):826-7. [DOI] [PubMed] [Google Scholar]
Bouyer 2002
- Bouyer J, Coste J, Fernandez H, Pouly JL, Job-Spira N. Sites of ectopic pregnancy: a 10 year population-based study of 1800 cases. Human Reproduction 2002;17(12):3224-30. [DOI] [PubMed] [Google Scholar]
Chetty 2009
- Chetty M, Elson J. Treating non-tubal ectopic pregnancy. Best Practice & Research. Clinical Obstetrics & Gynaecology 2009;23(4):529-38. [DOI] [PubMed] [Google Scholar]
Comstock 2005
- Comstock C, Huston K, Lee W. The ultrasonographic appearance of ovarian ectopic pregnancies. Obstetrics and Gynecology 2005;105(1):42-5. [DOI] [PubMed] [Google Scholar]
Einenkel 2000
- Einenkel J, Baier D, Horn L-C, Alexander H. Laparoscopic therapy of an intact primary ovarian pregnancy with ovarian hyperstimulation syndrome. Human Reproduction 2000;15(9):2037-40. [DOI] [PubMed] [Google Scholar]
Elson 2016
- Elson CJ, Salim R, Potdar N, Chetty M, Ross JA, Kirk EJ on behalf of the Royal College of Obstetricians and Gynaecologists. Diagnosis and Management of Ectopic Pregnancy. BJOG 2016;123(13):e15. [Google Scholar]
Faschingbauer 2011
- Faschingbauer F, Mueller A, Voigt F, Beckmann MW, Goecke TW. Treatment of heterotopic cervical pregnancies. Fertility and Sterility 2011;95(5):1787.e9-13. [DOI] [PubMed] [Google Scholar]
Frates 1994
- Frates MC, Benson CB, Doubilet PM, Disalvo DN, Brown DC, Laing FC. Cervical ectopic pregnancy: results of conservative treatment. Radiology 1994;191:773-5. [DOI] [PubMed] [Google Scholar]
Fuchs 2015
- Fuchs N, Manoucheri E, Verbaan M, Einarsson JI. Laparoscopic management of extrauterine pregnancy in caesarean section scar: description of a surgical technique and review of the literature. BJOG 2015;122(1):137. [DOI] [PubMed] [Google Scholar]
Fylstra 2014
- Fylstra DL. Cervical pregnancy: 13 cases treated with suction curettage and balloon tamponade. American Journal of Obstetrics and Gynecology 2014;210(6):581.e1-5. [DOI] [PubMed] [Google Scholar]
Gerli 2004
- Gerli S, Rossetti D, Baiocchi G, Clerici G, Unfer V, Carlo Di Renzo G. Early ultrasonographic diagnosis and laparoscopic treatment of abdominal pregnancy. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2004;113(1):103-5. [DOI] [PubMed] [Google Scholar]
Gibbons 2012
- Gibbons L, Belizan JM, Lauer JA, Betran AP, Merialdi M, Althabe F. Inequities in the use of cesarean section deliveries in the world. American Journal of Obstetrics and Gynecology 2012;206:331.e1-19. [DOI] [PubMed] [Google Scholar]
Goyal 2014
- Goyal DL, Tondon R, Goel P, Sehgal A. Ovarian ectopic pregnancy: a 10 years’ experience and review of literature. International Journal of Reproductive Biomedicine 2014;12(12):825-30. [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 12 February 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).Available at gradepro.org.
Graesslin 2005
- Graesslin O, Dedecker F, Quereux C, Gabriel R. Conservative treatment of ectopic pregnancy in a cesarean scar. Obstetrics and Gynecology 2005;105(4):869-71. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Honey 1999
- Honey L, Leader A, Claman P. Uterine artery embolization – a successful treatment to control bleeding cervical pregnancy with a simultaneous intrauterine gestation. Human Reproduction 1999;14(2):553-5. [DOI] [PubMed] [Google Scholar]
Horne 2014
- Horne AW, Skubisz MM, Tong S, Duncan WC, Neil P, Wallace EM, et al. Combination gefitinib and methotrexate treatment for non-tubal ectopic pregnancies: a case series. Human Reproduction 2014;29(7):1375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsieh 1998
- Hsieh YY, Chang CC, Tsai HD, Yeh LS, Hsu TY, Yang TC. Intramural pregnancy with negative b-hCG. Journal of Reproductive Medicine 1998;43:468-70. [PubMed] [Google Scholar]
Hu 2016
- Hu J, Tao X, Yin L, Shi Y. Successful conservative treatment of cervical pregnancy with uterine artery embolization followed by curettage: a report of 19 cases. BJOG 2016;123(S3):97. [DOI] [PubMed] [Google Scholar]
Hung 1998
- Hung TH, Shau WY, Hsieh TT, Hsu JJ, Soong YK, Jeng CJ. Prognostic factors for an unsatisfactory primary methotrexate treatment of cervical pregnancy: a quantitative review. Human Reproduction 1998;13(9):2636-42. [DOI] [PubMed] [Google Scholar]
Jabeen 2018
- Jabeen K, Karuppaswamy J. Non-surgical management of caesarean scar ectopic pregnancy – a five-year experience. Journal of Obstetrics and Gynaecology 2018;38(8):1121-7. [DOI] [PubMed] [Google Scholar]
Jin 2004
- Jin H, Zhou J, Yu Y, Dong M. Intramural pregnancy, a report of two cases. Journal of Reproductive Medicine 2004;49(7):569-72. [PubMed] [Google Scholar]
Jin 2016
- Jin L, Chen WL, Zhou YF. Expert consensus on diagnosis and treatment of caesarean scar pregnancy (2016) [剖宫产术后子宫瘢痕妊娠诊治专家共识 (2016)]. Chinese Journal of Obstetrics and Gynecology 2016;51(8):568-72. [Google Scholar]
Jurkovic 1996
- Jurkovic D, Hackett E, Campbell S. Diagnosis and treatment of early cervical pregnancy: a review and a report of two cases treated conservatively. Ultrasound in Obstetrics & Gynecology 1996;8(6):373-80. [DOI] [PubMed] [Google Scholar]
Jurkovic 2003
- Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound in Obstetrics & Gynecology 2003;21(3):220-7. [DOI] [PubMed] [Google Scholar]
Jurkovic 2007
- Jurkovic D, Marvelos D. Catch me if you can: ultrasound diagnosis of ectopic pregnancy. Ultrasound in Obstetrics & Gynecology 2007;30(1):1-7. [DOI] [PubMed] [Google Scholar]
Jurkovic 2011
- Jurkovic D, Wilkinson H. Diagnosis and management of ectopic pregnancy. BMJ 2011;342:d3397. [DOI] [PubMed] [Google Scholar]
Kirk 2014
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Human Reproduction Update 2014;20(2):250-61. [DOI] [PubMed] [Google Scholar]
Lee 1999
- Lee CL, Wang C, Chao A, Yen C-F, Soong Y-K. Laparoscopic management of an ectopic pregnancy in a previous caesarean section scar. Human Reproduction 1999;14(5):1234-6. [DOI] [PubMed] [Google Scholar]
Levine 2007
- Levine D. Ectopic pregnancy. Radiology 2007;245(2):385-97. [DOI] [PubMed] [Google Scholar]
Lewis 2007
- Lewis G. Saving mothers' lives: reviewing maternal deaths to make motherhood safer – 2003-2005. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. London, UK: CEMACH; 2007.
Li 2011a
- Li H, Guo H, Han J, Wang J, Xiong G, Shen J, et al. Endoscopic treatment of ectopic pregnancy in a cesarean scar. Journal of Minimally Invasive Gynecology 2011;18(1):31-5. [DOI] [PubMed] [Google Scholar]
Lian 2012
- Lian F, Wang Y, Chen W, Li J, Zhan Z, Ye Y, et al. Uterine artery embolization combined with local methotrexate and systemic methotrexate for treatment of cesarean scar pregnancy with different ultrasonographic pattern. Cardiovascular and Interventional Radiology 2012;35(2):286-91. [DOI] [PubMed] [Google Scholar]
Liang 2010
- Liang FB, He J. Methotrexate-based bilateral uterine arterial chemoembolization for treatment of cesarean scar pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2010;89(12):1592-4. [DOI] [PubMed] [Google Scholar]
Litwicka 2011
- Litwicka K, Greco E. Caesarean scar pregnancy: a review of management options. Current Opinion in Obstetrics & Gynecology 2011;23(6):415-21. [DOI] [PubMed] [Google Scholar]
Marchiole 2004
- Marchiole P, Gorlero F, De Caro G. Intramural pregnancy embedded in a previous cesarean section scar treated conservatively. Ultrasound in Obstetrics & Gynecology 2004;23(3):305-9. [DOI] [PubMed] [Google Scholar]
Maymon 2004
- Maymon R, Halperin R, Mendlovic S, Schneider D, Herman A. Ectopic pregnancies in a Caesarean scar: review of the medical approach to an iatrogenic complication. Human Reproduction Update 2004;10(6):515-23. [DOI] [PubMed] [Google Scholar]
Melcer 2015
- Melcer Y, Smorgick N, Vaknin Z, Mendlovic S, Raziel A, Maymon R. Primary ovarian pregnancy: 43 years experience in a single institute and still a medical challenge. Israel Medical Association Journal 2015;17(11):687-90. [PubMed] [Google Scholar]
Mitra 2003
- Mitra AG, LeQuire MH. Minimally invasive management of 14.5-week abdominal pregnancy without laparotomy: a novel approach using percutaneous sonographically guided feticide and systemic methotrexate. Journal of Ultrasound in Medicine 2003;22(7):709-14. [DOI] [PubMed] [Google Scholar]
Molinaro 2007
- Molinaro TA, Barnhart KT. Ectopic pregnancies in unusual locations. Seminars in Reproductive Medicine 2007;25(2):123-30. [DOI] [PubMed] [Google Scholar]
Nadarajah 2002
- Nadarajah S, Sim LN, Loh SF. Laparoscopic management of an ovarian pregnancy. Singapore Medical Journal 2002;43(2):95-6. [PubMed] [Google Scholar]
Nadi 2017
- Nadi M, Richard C, Filipuzzi L, Bergogne L, Douvier S, Sagot P. Interstitial, angular and cornual pregnancies: diagnosis, treatment and subsequent fertility [Grossesse interstitielle, angulaire et cornuale: diagnostic, traitement et futur obstetrical]. Gynecologie Obstetrique Fertilite & Senologie 2017;45:340-7. [DOI] [PubMed] [Google Scholar]
Ngu 2011
- Ngu SF, Cheung VY. Non-tubal ectopic pregnancy. International Journal of Gynaecology and Obstetrics 2011;115(3):295-7. [DOI] [PubMed] [Google Scholar]
Onan 2005
- Onan MA, Turp AB, Saltik A, Akyurek N, Taskiran C, Himmetoglu O. Primary omental pregnancy: case report. Human Reproduction 2005;20(3):807-9. [DOI] [PubMed] [Google Scholar]
Orazulike 2013
- Orazulike NC, Konje JC. Diagnosis and management of ectopic pregnancy. Women's Health (London, England) 2013;9(4):373-85. [DOI] [PubMed] [Google Scholar]
Ory 1986
- Ory SJ, Alelei L, Villanueva AL, Sand PK, Tamura RK. Conservative treatment of ectopic pregnancy with methotrexate. American Journal of Obstetrics and Gynecology 1986;154(6):1299-306. [DOI] [PubMed] [Google Scholar]
Pascual 2007
- Pascual MA, Hereter L, Graupera B, Tresserra F, Fernandez-Cid M, Simon M. Three-dimensional power Doppler ultrasound diagnosis and conservative treatment of ectopic pregnancy in a cesarean section scar. Fertility and Sterility 2007;88(3):706.e5-7. [DOI] [PubMed] [Google Scholar]
Petersen 2016
- Petersen BK, Hoffmann E, Larsen RC. Cesarean scar pregnancy: a systematic review of treatment studies. Fertility and Sterility 2016;105(4):958-67. [DOI] [PubMed] [Google Scholar]
Rahaman 2004
- Rahaman J, Berkowitz R, Mitty H, Gaddipati S, Brown B, Nezhat F. Minimally invasive management of an advanced abdominal pregnancy. Obstetrics and Gynecology 2004;103(5 Pt 2):1064-8. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sauer 1987
- Sauer MV, Gorill MJ, Rodi IA, Yeko TR, Greenberg LH, Bustillo M, et al. Nonsurgical management of unruptured pregnancy: an extended trial. Fertility and Sterility 1987;48:752-5. [PubMed] [Google Scholar]
Seinera 1997
- Seinera P, Di Gregorio A, Arisio R, Decko A, Crana F. Ovarian pregnancy and operative laparoscopy: report of eight cases. Human Reproduction 1997;12(3):608-10. [DOI] [PubMed] [Google Scholar]
Seow 2004
- Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Cesarean scar pregnancy: issues in management. Ultrasound in Obstetrics & Gynecology 2004;23(3):247-53. [DOI] [PubMed] [Google Scholar]
Shan 2014
- Shan N, Dong D, Deng W, Fu Y. Response to unusual ectopic pregnancies: a retrospective analysis of 65 cases. Journal of Obstetrics and Gynaecology Research 2014;40(1):147-54. [DOI] [PubMed] [Google Scholar]
Shaw 2007
- Shaw SW, Hsu JJ, Chueh HY, Han CM, Chen FC, Chang YL, et al. Management of primary abdominal pregnancy: twelve years of experience in a medical centre. Acta Obstetricia et Gynecologica Scandinavica 2007;86(9):1058-62. [DOI] [PubMed] [Google Scholar]
Shen 2012
- Shen L, Tan A, Zhu H, Guo C, Liu D, Huang W. Bilateral uterine artery chemoembolization with methotrexate for cesarean scar pregnancy. American Journal of Obstetrics and Gynecology 2012;207(5):386.e1-6. [DOI] [PubMed] [Google Scholar]
Stucki 2008
- Stucki D, Buss J. The ectopic pregnancy, a diagnostic and therapeutic challenge. Journal of Medicine and Life 2008;1(1):40-8. [PMC free article] [PubMed] [Google Scholar]
Timor‐Tritsch 2015
- Timor-Tritsch IE, Khatib N, Monteagudo A, Ramos J, Berg B, Kovács S. Cesarean scar pregnancies: experience of 60 cases. Journal of Ultrasound in Medicine 2015;34(4):601-10. [DOI] [PubMed] [Google Scholar]
Tinelli 2008
- Tinelli A, Hudelist G, Malvasi A, Tinelli R. Laparascopic management of ovarian pregnancy. JSLS : Journal of the Society of Laparoendoscopic Surgeons 2008;12(2):169-72. [PMC free article] [PubMed] [Google Scholar]
Trambert 2005
- Trambert JJ, Einstein M, Banks E, Frost A, Goldberg G. Uterine artery embolization in the management of vaginal bleeding from cervical pregnancy. Journal of Reproductive Medicine 2005;50(11):844-50. [PubMed] [Google Scholar]
Tulandi 2004
- Tulandi T, Al-Jaroudi D. Interstitial pregnancy: results generated from The Society of Reproductive Surgeons Registry. Obstetrics and Gynecology 2004;103(1):47-50. [DOI] [PubMed] [Google Scholar]
Ushakov 1997
- Ushakov FB, Elchalal U, Aceman PJ, Schenker JG. Cervical pregnancy: past and future. Obstetrical & Gynecological Survey 1997;52(1):45-59. [DOI] [PubMed] [Google Scholar]
Varma 2003
- Varma R, Mascarenhas R, Jame D. Successful outcome of advanced abdominal pregnancy with exclusive omental insertion. Ultrasound in Obstetrics & Gynecology 2003;21(2):192-4. [DOI] [PubMed] [Google Scholar]
Yao 2005
- Yao Y, Hu LN. The incidence of ectopic pregnancy and the main causes of misdiagnosis [异位妊娠发病率及误诊的主要原因]. Journal of Practical Obstetrics and Gynecology 2005;21(6):321-2. [Google Scholar]
Yu 2009
- Yu B, Douglas NC, Guarnaccia MM, Sauer MV. Uterine artery embolization as an adjunctive measure to decrease blood loss prior to evacuating a cervical pregnancy. Archives of Gynecology and Obstetrics 2009;279(5):721-4. [DOI] [PubMed] [Google Scholar]
Yuan 2010
- Yuan Y, Dai Q, Cai S, Lyu K, Liu X, Jin L, et al. Value of sonography in the diagnosis of caesarean scar pregnancy [超声对剖宫产瘢痕妊娠的诊断价值]. Chinese Journal of Ultrasonography 2010;19(4):321-4. [Google Scholar]
Zhang 2009
- Zhang YL, Huang LL. Uterine artery embolization compared with methotrexate for the management of pregnancy implanted with in a cesarean scar. American Journal of Obstetrics and Gynecology 2009;201(2):52.e1‐3. [DOI] [PubMed] [Google Scholar]
Zhang 2012
- Zhang B, Jiang Z, Huang M, Guan S, Zhu K, Qian J, et al. Uterine artery embolization combined with methotrexate in the treatment of cesarean scar pregnancy: results of a case series and review of the literature. Journal of Vascular and Interventional Radiology 2012;23(12):1582-8. [DOI] [PubMed] [Google Scholar]
Zhu 2016
- Zhu X, Deng X, Xiao S, Wan Y, Xue M. A comparison of high-intensity focused ultrasound and uterine artery embolisation for the management of caesarean scar pregnancy. International Journal of Hyperthermia 2016;32(2):144. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Shen 2014
- Shen L, Fu J, Huang W, Zhu H, Wang Q, Yang S, Wu T. Interventions for non‐tubal ectopic pregnancy. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD011174] [DOI] [PMC free article] [PubMed] [Google Scholar]


