Abstract
Background
Squamous cell carcinoma of the conjunctiva is described in the ophthalmic literature as a rare, slow‐growing tumour of the eye, normally affecting elderly men around 70 years of age. In Africa, however, the disease is different. The incidence is rising rapidly, affecting young persons (around 35 years of age), and usually affecting women. It is more aggressive, with a mean history of three months at presentation. This pattern is related to the co‐existence of the HIV/AIDS pandemic, high HPV exposure, and solar radiation in the region. Various interventions exist, but despite therapy, there is a high recurrence rate (up to 43%) and poor cosmetic results in late disease. This review was conducted to evaluate the interventions for treatment of conjunctival squamous cell carcinoma in HIV‐infected individuals.
Objectives
To evaluate the effect of interventions for treating squamous cell carcinoma of the conjunctiva in HIV‐infected individuals on local control, recurrence, death, time to recurrence, and adverse events.
Search methods
Using a sensitive search strategy, we attempted to identify all relevant trials, regardless of language or publication status, from the following electronic databases; PubMedPubMed, EMBASE and The Cochrane Library. We also searched clinical trial registries; WHO International Clinical Trials Registry Platform (ICTRP) and the US National Institutes of Health Clinicaltrials.gov. We searched the international conference proceedings of HIV/AIDS and AIDS‐related cancers from the AIDS Education Global Education System (AEGIS). Searches were conducted between January and February 2012.
Selection criteria
Randomised controlled trials (RCTs) involving HIV‐infected individuals with ocular surface squamous neoplasia.
Data collection and analysis
We independently screened the results of the search to select potentially relevant studies and to retrieve the full articles. We independently applied the inclusion criteria to the potentially relevant studies. No studies were identified that fulfilled the selection criteria.
Main results
No RCTs of interventions currently used against conjunctival squamous cell carcinoma in HIV‐infected individuals were identified.There is one ongoing RCT in Kenya that was registered in July 2012.
Authors' conclusions
Implications for practice: Current clinical practice in treatment of squamous cell carcinoma of the conjunctiva rests on a weak evidence base of case series and case reports.
Implications for research: Randomised controlled trials for treatment of this disease are needed in settings where it occurs most frequently. Preventive interventions also need to be identified. HIV/AIDS research has not focused on treatment of this tumour.
Keywords: Humans; Carcinoma, Squamous Cell; Carcinoma, Squamous Cell/therapy; Conjunctival Neoplasms; Conjunctival Neoplasms/therapy; HIV Infections; HIV Infections/complications
Plain language summary
Interventions for squamous cell carcinoma of the conjunctiva in HIV‐infected individuals
Conjunctival squamous cell carcinoma, a tumour of the thin membrane that covers the white of the eye, is becoming more common, more aggressive, and affecting more young people, especially women. This pattern is associated with the HIV/AIDS pandemic, exposure to solar radiation, and infection with human papilloma virus (HPV). Various treatment modalities exist, but the recurrence rate is high and the cosmetic outcome of late disease unsightly (Figure 1). Death may occur when the disease spreads to the surrounding structures and the brain. This review was conducted to evaluate the effects of the current interventions. No randomised controlled trials of any interventions for this cancer were found. Current clinical practice appears to be based on case series and case reports. These are weak sources of evidence for the effectiveness of a treatment. Randomised controlled clinical trials are needed.
Background
Definition Squamous cell carcinoma of the conjunctiva (SCC) is the end stage of a spectrum of conditions called ocular surface squamous neoplasia (OSSN). Histologically, OSSN varies from a benign form that includes papilloma, pseudoepitheliomatous hyperplasia and benign hereditary intraepithelial dyskeratosis, through a pre‐invasive form called conjunctival intraepithelial neoplasia (CIN) graded as I,II or III depending on depth of involvement and finally the invasive form that includes squamous cell carcinoma and mucoepidermoid carcinoma (Basti 2003). When the dysplastic changes are confined to the basal one third of the epithelium, this is termed CIN I or mild dysplasia, when they extend into the middle third of the epithelium this is termed CIN II or moderated dysplasia and when they extend into the superficial third of the epithelium this is termed CIN III or severe dysplasia. Full thickness dysplasia is also referred to as carcinoma‐in‐situ (CIS). Invasive squamous cell carcinoma of the conjunctiva refers to the infiltration through the basement membrane to involve the underlying stroma (Basti 2003; Shields 2004). Clinically, these stages may be indistinguishable except by histology. Clinical features The tumour may be asymptomatic or present with redness, irritation, severe pain, and visual loss (Tunc 1999). It commonly affects the visible area between the upper and lower eyelids (interpalpebral conjunctiva), usually on the nasal side at the margin of the conjunctiva and the cornea (limbus). This slow‐growing tumour can present as a solitary or diffuse growth. Solitary tumours can be nodular or gelatinous and may have a whitish plaque (leukoplakia). The lesion mimics benign conjunctival degenerations such as pterygium and pinguecula. Most cases are unilateral(Cervantes 2002; Chisi 2006).
The morbidity from squamous cell carcinoma of the conjunctiva relates to the effects of the disease and its treatment. It may extend into the eyeball, orbit, regional lymph nodes, surrounding paranasal sinuses and the brain. Death may result from regional or distant metastases, as well as intracranial spread.
Aetiology The aetiology of SCC has been associated with HIV infection (Ateenyi‐Agaba 1995;Waddell 1996), human papilloma virus (HPV) infection (Waddell 1996; Nakamura 1997; Newton 2002), immunosuppression in organ transplant recipients (Macarez 1999), and exposure to ultraviolet B rays of the sun (Ateenyi‐Agaba 2004; Ng 2008; ). Chronic conjunctival diseases, such as allergic conjunctivitis and trachoma, have also been implicated (Poole 1999).
The majority of affected persons in Africa are HIV‐positive; 71% in Uganda, 86% in Malawi (Waddell 1996) ,70.6% in South Africa (Mahomed 2002) and 75% in Nigeria (Osahon 2011). In a smaller study in Zimbabwe, 12 out of 13 persons with SCC or CIS (92.3%) were HIV‐positive (Porges 2003). Many ophthalmologists in Africa now consider SCC in young adults to be a marker for HIV infection. The relationship between SCC and HPV is less clear. Some case‐control studies have found an association (Moubayed 2004; de Koning 2008) while some have not (Sen 2007; Guthoff 2009). Among HIV‐infected people with SCC, cutaneous types of HPV are detected more frequently than the mucosal types (de Koning 2008; Ateenyi‐Agaba 2010). In another Ugandan study, HPV was detected more frequently in the low‐grade conjunctival neoplasias than in more advanced disease (Tornesello 2006). Ultraviolet solar radiation causes tissue damange mediated through mutation of the tp53 tumour‐suppressor gene and tissue matrix metalloproteinases (Ateenyi‐Agaba 2004; Ng 2008).
It is plausible that the aetiology involves an interaction of these factors in a yet to be determined model.
Epidemiology The epidemiology of SCC has transformed in the last few decades. It used to be an uncommon slow‐growing tumour found in elderly males (Lee 1997; McKelvie 2002), but in Africa it is becoming more common, more aggressive, and more likely to affect young persons, especially females (Ateenyi‐Agaba 1995). This pattern is related to the co‐existence of the HIV/AIDS pandemic, high HPV exposure, and solar radiation in the region. The HIV pandemic is mainly centered in Africa. In 2009 22.5 million of the 33.3 million infected people worldwide were living in Africa (UNAIDS 2010). Africa also has the highest prevalence of HPV infection in the world, with an age‐adjusted prevalence of 25.6% in women of age 15 to 74 years, followed by South America (14.3%), Asia (8.7%) and Europe (5.2%). (Clifford 2005). The equator bissects the continent and over 75% of the continent is within the tropics with consequent high ambient solar exposure all year round.
The Kampala Cancer Registry in Uganda recorded a six‐fold increase in the incidence of conjunctival squamous cell carcinoma, from an average of six per million per year between 1970 and 1988, to 35 per million per year in 1992 (Ateenyi‐Agaba 1995). A study in Australia found that 78.5% of affected persons were elderly males with a mean age of 60 years (Lee 1997). Similarly, in Britain, 77% were males and 69% of them were more than 60 years old (McKelvie 2002). In contrast, a study in Zimbabwe found that 70% of patients were young females with a median age of 35 years (Pola 2003), while in South Africa the mean age was 37 years (Mahomed 2002). In Tanzania the tumour has been observed to be more aggressive than it was previously. The mean length of history on presentation is three months (Poole 1999).
SCC is a common tumour of the ocular surface among adults in Africa. Pola 2003 In Tanzania 45.8% of 168 conjunctival biopsies seen between 1996 and 1997 were squamous cell carcinomas, while 35% were pingueculum or pterygium (Poole 1999).
The prevalence of SCC in a hospital‐based HIV‐positive population in Kenya was 7.8% (Chisi 2006).
Diagnosis Most cases are diagnosed on clinical impression after a person presents with a growth on the eyeball, which is then excised and sent for histology to get a definitive diagnosis (Shields 1997). Histopathology is the commonest mode of diagnosis in practice. Although regarded as the gold standard, histopathological diagnoses are subject to discordant interpretation (Margo 2002). Cytology has also been used, especially for the follow‐up of people treated using chemotherapy or immune therapy to monitor recurrence, since malignant cells are poorly adherent to each other (McKelvie 2001).
Other methods include the use of DNA cytometry to identify tumour‐cell regression (Nadjari 1999); immunostaining for certain molecular genetic markers, such as proliferating cells nuclear antigens (PCNA), Ki67 expression, tp53 gene mutations, and argyrophilic nucleolar organiser regions (AgNORs) (Aoki 1998); and toluidine blue staining to mark tumour margins for surgical excision (Kaji 2006). Treatment options The treatment of this condition since the pre‐HIV era has been simple: surgical excision alone or with additional adjunctive therapy. The main objective of surgical excision is complete removal of the tumour to minimise recurrence (Shields 1997). The resulting surface defect may need reconstruction using amniotic membrane transplantation (Espana 2002; Gunduz 2005) or limbal autografts (Copeland 1990). Adjunctive therapies augment surgical excision. These include cryotherapy, chemotherapy, radiotherapy, immune therapy, and amniotic membrane transplants. They work by killing residual malignant cells at the excision margins or any that may be seeded during excision, although there are also reports of their use as primary therapy (Cerezo 1990; Karp 2001; Barbazetto 2004). Cidofovir, an antiviral active against human papilloma virus, has been described as a primary therapy, and was reported to cause tumour regression (Sherman 2002). It undergoes phosphorylation to active diphosphates independent of viral enzymes, which act as both inhibitors and alternative substrates of viral DNA polymerase, thus inhibiting DNA synthesis (Safrin 2004). A list of these interventions is shown in Table 1.
1. 2 Interventions used in treatment of conjunctival squamous cell carcinoma.
| Intervention | Description | Reference |
| Surgical excision | Alcohol epitheliectomy for the corneal component and partial lamellar scleroconjunctivectomy with 3 to 4mm margins for the conjunctival component. Frozen sections may be used to ensure tumour‐free margins. Modified enucleation involves removing the affected conjunctiva with a 4mm margin together with the eyeball. Exenteration involves complete removal of orbital tissue including the periosteum of the bony walls. The eyelids may or may not be spared. | Shields 1997Shields 2004 |
| Cryotherapy | Freeze‐thaw‐freeze‐thaw (double freeze‐thaw) applying probe to the excised conjunctival margin. | Peksayar 1989 |
| Chemotherapy ‐ 5 Fluorouracil (5FU) 1% solution ‐ Mitomycin C 0.02% or 0.04% | As topical solutions to soak operated site for 2‐5 minutes, as subconjunctival injection intra‐ or post operatively or as eyedrops to be applied post‐operatively as follows 4 times daily for 1‐2 weeks or as pulsed therapy giving 4 applications for 4 days repeated every month for 4‐6 cycles 4 times daily for 2 weeks then repeat at 4‐6 weeks intervals depending on response | Yeatts 2000 Midena 2000 Frucht‐Pery 1997 |
| Radiotherapy | ‐ beta irradiation ‐ gamma irradiation ‐ photodynamic therapy | Cerezo 1990 Goldberg 1963 Barbazetto 2004 |
| Amniotic membrane transplants (AMT) | Excise tumour with a 3‐4 mm tumour‐free margin (so frozen sections needed), apply cryotherapy to the margin then to close the defect suture amniotic membrane with 8/0 vicryl sutures to adjacent conjunctiva and to adjacent cornea using 10/0 nylon sutures with epithelial side facing up. | Gunduz 2005 |
| Interferon alpha 2b | Topical drops or intralesion injection | Karp 2001 |
| Cidofovir eyedrops 2mg/ml | Applied every 2 hours for 2 weeks then 4 times daily for 2 weeks then 3 times daily for 2 weeks | Sherman 2002 |
Cryotherapy kills malignant cells by repeated cycles of freezing and thawing (Peksayar 1989). Chemotherapy involves using a class of drugs called antimetabolites. They act by interfering with the enzymes involved in the intermediary metabolism of replicating cancer cells. An example is 5‐Fluorouracil (5‐FU) and mitomycin C. The former (5‐FU), is a prodrug, which means it is converted to active molecules. One of them, 5‐ fluoro‐2'‐ deoxyuridine‐5'‐monophosphate (FdUMP), binds to the enzyme thymidylate synthetase critical for the synthesis of thymidylate, which results in the inhibition of DNA synthesis. Another one, 5‐fluorouridine‐5'‐triphosphate (FUTP) interferes with RNA synthesis (Chu 2004). It is cell‐cycle specific and acts in the S phase of the cell cycle. In one study, 1% 5‐FU eyedrops were used alone without concurrent surgery or radiotherapy to treat a recurrent tumour (Midena 2000). Mitomycin C is an antibiotic which acts by interfering with the DNA of tumour cells. It undergoes metabolic activation, which generates an alkylating agent that cross‐links DNA, making the cell unable to replicate (Chu 2004). It is a cycle‐non‐specific agent that is most effective in the G1 and S phases. Radiotherapy destroys the DNA and RNA of dividing cells (Goldberg 1963). The source of beta radiation is strontium 90 (Cerezo 1990). Photodynamic therapy combines a drug (called a photosensitizer or photosensitizing agent) with light of a specific wavelength to produce activated oxygen species that promote tumour destruction (Castano 2005). Another treatment option is amniotic membrane transplantation. It is one of the layers of the placenta and is obtained after elective Caesarean section. It has anti‐angiogenic, anti‐scarring, and anti‐inflammatory properties (Hao 2000). Immune therapy for this condition involves the use of interferon alpha. Interferon alpha is part of a group of proteins called cytokines. It has three‐pronged action with antiviral and oncostatic effects and also activates natural killer cells, which are cells of the immune system that are able to recognise and destroy tumour cells (Lake 2004). The exact mechanism of action is not known. Cidofovir, an antiviral that is active against human papilloma virus, has been reported to cause regression of this tumour (Sherman 2002). It undergoes phosphorylation to active diphosphates independent of viral enzymes, which act as both inhibitors and alternative substrates of viral DNA polymerase, thus inhibiting DNA synthesis (Safrin 2004).
Advanced disease where the tumour has spread requires removal of the eyeball (modified enucleation) or the entire orbital contents (exenteration) in an attempt to save life (Shields 1991).
Despite therapy, up to 43% of treated patients experience recurrence at variable times (but usually within two years) (Peksayar 1989; Tabin 1997; Yeatts 2000). Simple excision has a higher rate of recurrence. A study in Australia reported 23% (Lee 1997). In the USA, it was 28.5%, but was 7.7% when combined with cryotherapy (Sudesh 2000). In one series of 17 cases treated with mitomycin C eye drops, 15 of them had undergone one to four surgical procedures before the mitomycin C treatment and six (35%) recurred within six months (Frucht‐Pery 1997). The efficacies of treatment reported in the literature need to be interpreted cautiously, since some of the treated cases have recurrences from prior surgical excision (Frucht‐Pery 1997; Midena 2000). The recurrence rate may be higher in Africa due to late presentation, exposure to solar radiation, and lack of many of the adjunctive therapies. Only cryotherapy and chemotherapy are likely to be found in advanced centres.
It is not clear whether the efficacy of these interventions is different in people with HIV infection. The effect, if any, of highly active antiretroviral therapy (HAART) on this condition is also not clearly known.
Clinical and public health importance of this review Although the number of people living with HIV infection worldwide seems to have stabilized Africa remains the global epicentre of the AIDS pandemic (UNAIDS 2010). The region also has the highest prevalence of HPV in the world and high solar ultraviolet exposure year‐round (Clifford 2005). If this triad of risk factors remains, the number of cases of conjunctival squamous cell carcinoma is expected to remain high. The effect of HPV vaccination in children on OSSN if any will take a long time to determine.
Recurrences still occur after surgical excision. Most of the current adjunctive therapies described are not readily available in low‐income countries. They are expensive and inconvenient to use. Cryotherapy requires special probes and canisters of liquid nitrogen gas that need refilling. Amniotic membrane transplantation requires tissue banks to process and preserve the transplant material. Medications for chemotherapy are not widely available, but are probably the easiest to obtain and use in such settings, since they are packed in small vials and do not require stringent storage conditions.
The cosmetic outcome of advanced disease and its treatment is very poor and leaves patients with an empty socket (Figure 1). Patients who undergo orbital exenteration often experience social problems because of their facial disfigurement leading to limited social interaction (Bonanno 2010). Facilities for cosmetic surgery and orbital implants in low‐income countries are often unavailable.
1.
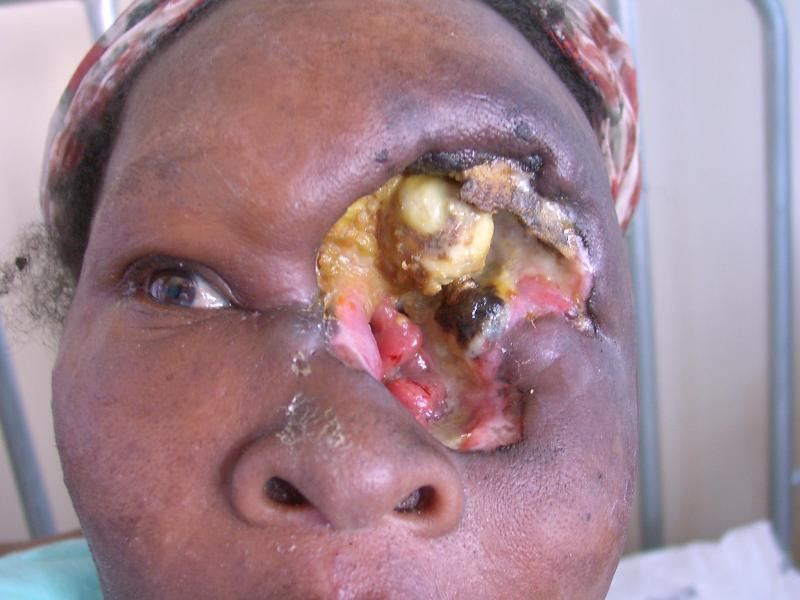
With antiretroviral therapy, HIV‐infected people can now enjoy an improved physical appearance and quality of life, but unsightly lesions on the face such as those caused by conjunctival squamous cell carcinoma unmask what would otherwise be a 'hidden' HIV infection. This potentially exposes the affected persons to the stigma and discrimination associated with HIV infection.
Effective evidence‐based interventions that allow early diagnosis and treatment are therefore needed.
Objectives
To evaluate interventions for treating squamous cell carcinoma of the conjunctiva in HIV‐infected individuals (see Table 1 for list of interventions).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCT).
Types of participants
HIV‐positive people with ocular surface squamous neoplasia (OSSN), whose diagnosis of HIV infection is based on either antibody or antigen tests, and whose OSSN is determined by histology, conjunctival impression cytology, or immunostaining.
Types of interventions
Any intervention used for treating OSSN, including the following:
surgical excision, modified enucleation, and exenteration
adjunctive therapies such as cryotherapy, chemotherapy, radiotherapy, amniotic membrane transplants , and immune therapies, such as interferon therapy
cidofovir
different regimens of HAART
combinations of the above
We were interested in trials that compared any of the interventions with another intervention or a placebo, or trials comparing different dosages or regimens of the same intervention.
Types of outcome measures
Primary outcomes
local control, defined as a tumour‐free period of two years
recurrence at six months, then at six‐month intervals thereafter until two years after treatment, diagnosed by histopathology, conjunctival impression cytology, or immunostaining
death
Secondary outcomes
time to recurrence
adverse events
Search methods for identification of studies
The HIV/AIDS review group search methods were used. We searched electronic databases and abstracts of conference proceedings. There were no language restrictions. The key words used in the search included:
ocular surface squamous neoplasia, squamous cell carcinoma, conjunctiva, carcinoma in situ, conjunctival intraepithelial neoplasia, HIV, AIDS, excision, enucleation, exenteration, chemotherapy, cryotherapy, interferon, immunetherapy, radiotherapy, A search had been conducted in December 2009 using search terms from the first edition of this review and found no trials. We conducted an updated search beginning January 2010.
Electronic databases
The following electronic databases were searched. 1. PubMed ‐ from January 2010 to 27th January 2012 2. EMBASE ‐ from January 2010 to 10th February 2012 3. The Cochrane Library ‐ from January 2010 to 3rd February 2012
Clinical trial registers
We searched the WHO International Clinical Trials Registry Platform (ICTRP) on 10th February 2012 (WHO ICTRP 2012). We also searched the clinical trial register of the US National Institutes of Health (NIH) www.clinicaltrials.gov website (NIH 2012) on 12th February 2012. This database includes trials registered with the US National Eye Institute (NEI) and US National Cancer Institute (NCI).
Conference proceedings
We searched abstracts of the proceedings of the HIV/AIDS conferences covered in the AIDS Education Global Education System (AEGIS) database (http://www.aegis.com/search).
This search covered all the following conferences which also shows the latest conference abstracts available in the AEGIS database:
1. International AIDS conference by the the International AIDS Society (IAS) upto the conference held from 18th‐23rd August 2010
2. International AIDS Society (IAS) HIV Pathogenesis and Treatment upto the 5th conference held from 19th‐22nd July 2009
3. International Congress on Drug Therapy in HIV infection upto the 9th meeting on 9th‐13th November 2008
4. International Workshops on Adverse Drug Reactions and Lipodystrophy in HIV upto the 11th workshop on 26th‐28th October 2009
5. Conference on Retroviruses and Opportunistic Infections (CROI) upto the 15th conference on 3rd‐6th February 2008
6. Centers for Disease Control and Prevention (CDC) National HIV Prevention conference upto the meeting on 12th‐15th June 2005
7. The International Workshops on HIV Drug Resistance and Treatment Strategies upto the 5th workshop on 4th‐8th June 2001
8. British HIV Association (BHIVA) upto the 17th conference held on 6th to 8th April 2011
9. European AIDS Clinical Society upto the 11th conference held on 24th‐27th October 2007
10. National HIV/AIDS Update Conference upto the 17th conference on 10th‐13th April 2005
Reference lists
We checked the reference lists of all studies identified by the above methods for additional relevant records.
Data collection and analysis
We independently screened the results of the search to select potentially relevant studies and to retrieve the full articles (Table 2). Where there was any uncertainty we obtained the full article. We independently applied the inclusion criteria using a specially designed eligibility form to the potentially relevant studies. No randomised controlled trials met the inclusion criteria.
2. 1 Review methods.
| Study selection | The titles and abstracts of search results are scanned and compared to predetermined selection criteria for studies. Articles written in foreign languages are translated to English. Two authors working independently select trials. The authors are blinded to the trial uthors and institutions by asking the trial search co‐ordinator to block this out when sending abstracts. Where there is uncertainty about inclusion, the full text of the article is obtained and read. If additional information is needed about the trial in question, it is categorized as awaiting assessment until this is obtained. If differences arise regarding trial inclusion, they are discussed and if still unresolved, Dr Taryn Young at the South African Cochrane Centre is asked to take a decision. |
| Data extraction | Data are collected and recorded in similar data extraction forms. The following information is extracted; citation details, study eligibility, study quality and study characteristics. The study characteristics include methods, participants, interventions and outcomes. |
| Assessment of methodological quality of included studies | The methodological quality of the included studies is assessed to determine validity. Selection bias is checked by assessing whether the generation of a random allocation sequence and allocation concealment were performed. Randomization is considered adequate if the allocation sequence is generated from a table of random numbers or by computer. Allocation concealment is deemed adequate if undertaken by means of sequentially prenumbered sealed opaque envelopes, a centralised system or prenumbered coded identical containers. In addition, assessment of blinding, losses to follow‐up and whether the analysis was by intention‐to‐treat (ITT) is undertaken. The definition of ITT is the requirement that participants be analyzed in the groups to which they were randomized, regardless of which intervention they actually received. |
| Data analysis | Measures of treatment effect:The effect measures of choice here are relative risk (RR) for dichotomous data and hazard ratio (HR) for time‐to‐event data with a 95% confidence interval. |
| Dichotomous data include: ‐ number of people who experience local control in each comparison group ‐ number of people who experience recurrence during the specified time periods in each comparison group ‐ number of deaths in each comparison group ‐ number of people who experience adverse events in each comparison group ‐ number of people who experience local control in each comparison group ‐ number of people who experience recurrence during the specified time periods in each comparison group ‐ number of deaths in each comparison group ‐ number of people who experience adverse events in each comparison group | |
| Time‐to‐event data include: ‐ time to recurrence | |
| Dealing with missing data: Participants lost to follow up are censored in the survival analysis. | |
| Assessment of heterogeneity: To identify statistical heterogeneity we look at the forest plot for overlapping confidence intervals and test it using the Cochrane Q test with a p‐value of 0.10. The impact of heterogeneity on the meta‐analysis is measured using the I‐squared test. If I‐squared is greater than 70% we will investigate by checking the trials for data entry errors and looking for existence of subgroups. Subgroup analysis by age, sex, geographical location and diagnostic method will be done. These factors have been shown to influence the occurrence of OSSN and we anticipate that they may also influence treatment effects. If no explanation for heterogeneity is found or its correction is not possible, meta‐analysis will not be done. | |
| Meta‐analysis: A fixed‐effect model is used for meta‐analysis but when there is heterogeneity that cannot be readily explained, a random‐effects model is incorporated. | |
| Sensitivity analysis: We examine how the magnitude of effect differs according to study quality or trial size and also the results of per‐protocol analysis with those of intention‐to‐treat analysis. | |
| Assessment of reporting bias: Publication bias is assessed by using a funnel plot to look for asymmetry. |
Results
Description of studies
No studies were identified that fulfilled the selection criteria. The PRISMA flowchart in Figure 2 summarizes the results (Figure 2). There is an ongoing study in Kenya that was registered in the PanAfrican Clinical Trials Register on 19th July 2012. This register feeds into the WHO International Clinical Trials Registry Platform (ICTRP).
2.

PRISMA study flow diagram.
Risk of bias in included studies
No studies were identified that fulfilled the selection criteria.
Effects of interventions
No studies were identified that fulfilled the selection criteria. The search strategy yielded 12 records, which were all excluded (see table of excluded studies).
Discussion
In this review no randomised controlled trials on the effectiveness of interventions against conjunctival squamous cell carcinoma in HIV‐infected individuals were identified. No randomised controlled trials were found, even in the HIV‐uninfected populations. Current practice is based on the results of interventional case series and case reports (Table 1). The quality of evidence from these study designs ranks low by various systems for grading evidence. It is graded level four by the Oxford Centre for Evidence‐Based Medicine (OECBM) recommendations (Phillips 2001) and low‐grade by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group (Atkins 2004). According to GRADE, low means that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. These studies are also mainly from western countries, where the participants are usually elderly males, who have a different disease profile from participants in developing countries. They have a less aggressive form of the disease and few are HIV‐infected (Ateenyi‐Agaba 1995; Waddell 1996; Lee 1997; Poole 1999; Mahomed 2002; McKelvie 2002). We could not explain why there are no trials, but postulate that perhaps it reflects differences in research priorities, since the prevalence of this tumour is low among HIV‐infected individuals living in the western world.
There is a need to conduct randomised controlled trials to provide unbiased evidence for the effectiveness of currently used interventions for treating conjunctival squamous cell carcinoma. Ideally, the disease would be studied in its early stages, before it has spread, since in the later stages the treatment is more costly, can only be provided in tertiary care centres, and would tend to be individually tailored depending on the extent of spread. Studies are needed in the setting where the greatest burden of disease is found. The typical setting involves young HIV‐infected individuals, mostly females with aggressive disease and HIV co‐infections, in the context of high HIV prevalence, high solar exposure, and limited resources.
Investigating the effectiveness of simple surgical excision versus wide excision with or without adjunctive antimetabolite eye drops would be relevant as these are relatively simple interventions that can be easily translated in developing countries. The effect of new health technologies used in the developed world, such as cryotherapy, amniotic membrane transplants, and photodynamic therapy, should also be assessed in RCTs.
Preventive interventions directed against the mutagenic effects of solar ultraviolet B radiation and the oncogenic viruses associated with this disease also need to be identified.
There is also an important implication for diagnostic tests for early disease. Ethical issues that may arise include, for instance, whether participants should be subjected to the application of potentially dangerous cytotoxic antimetabolites as an adjunct to excision biopsy before there is histological confirmation of tumour. Treatment of conjunctival squamous cell carcinomas with topical mitomycin C and/or radiation has been associated with induction of atypical cell changes, such as conjunctival DNA‐polyploidy afterward (Cartsburg 2001). More sensitive diagnostic tests, such as cytology or immunostaining, may thus need to be used pre‐operatively.
Treatment of opportunistic tumours in HIV/AIDS, such as squamous cell carcinoma of the conjunctiva, is part and parcel of HIV care. Untreated squamous cell carcinoma of the conjunctiva threatens survival. Not paying attention to this disease may compromise the gains from other care availed to affected individuals.
Authors' conclusions
Implications for practice.
Current clinical practice in treatment of squamous cell carcinoma of the conjunctiva is based on case series and case reports. This is a weak evidence base.
Implications for research.
RCTs for treatment of this disease are needed in the settings where it occurs most frequently. Preventive interventions, such as HPV vaccination, also need to be identified once scientific knowledge of the serotypes involved is established. HIV/AIDS research has not focused on treatment of this tumour.
What's new
| Date | Event | Description |
|---|---|---|
| 11 December 2012 | New citation required but conclusions have not changed | New comprehensive searches, review updated. |
| 11 December 2012 | New search has been performed | Updated |
History
Protocol first published: Issue 1, 2006 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 29 October 2008 | Amended | Converted to new review format. |
| 19 February 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Dr S Gichuhi was awarded a Reviews for Africa Programme Fellowship (www.mrc.ac.za/cochrane/rap.htm), funded from the Nuffield Commonwealth Programme, through The Nuffield Foundation. We would like to acknowledge the support provided by the staff at the South African Cochrane Centre and the Cochrane HIV/AIDS Review Group mentoring programme.
Appendices
Appendix 1. PubMed search strategy and results
Title: Interventions for squamous cell carcinoma of the conjunctiva in HIV‐infected individuals
Database: PubMed (2010 – 2012)
Date: 27 January 2012
| Search | Query | Items found |
| #13 | Search #1 AND #2 AND #11 AND ("2010/10/01"[Date ‐ Publication] : "2012/01/27"[Date ‐ Publication]) | 4 |
| #12 | Search #1 AND #2 AND #11 | 23 |
| #11 | Search #9 OR #10 | 2290 |
| #10 | Search conjunctival neoplasms[mh] | 1729 |
| #9 | Search #5 AND #8 | 1099 |
| #8 | Search #6 OR #7 | 546016 |
| #7 | Search carcinoma[tiab] OR carcinomas[tiab] OR neoplasia[tiab] OR neoplasm[tiab] OR neoplasms[tiab] | 514787 |
| #6 | Search carcinoma, squamous cell[mh] | 92643 |
| #5 | Search #3 OR #4 | 23573 |
| #4 | Search conjunctiva[tiab] OR conjunctivas[tiab] OR conjunctival[tiab] | 18906 |
| #3 | Search conjunctiva[MeSH Terms] | 11829 |
| #2 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | 2499392 |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH:noexp] | 282439 |
Appendix 2. EMBASE search strategy and results
Title: Interventions for squamous cell carcinoma of the conjunctiva in HIV‐infected individuals
Database: PubMed (2010 – 2012)
Date: 10 February 2012
| No. | Query | Results |
| #13 | #1 AND #2 AND #11 AND [humans]/lim AND [embase]/lim AND [1‐2‐2012]/sd NOT [10‐2‐2012]/sd | 0 |
| #12 | #1 AND #2 AND #11 | 4 |
| #11 | #9 OR #10 | 2964 |
| #10 | 'conjunctiva tumor'/syn | 1802 |
| #9 | #5 AND #8 | 1796 |
| #8 | #6 OR #7 | 646277 |
| #7 | carcinoma:ab,ti OR carcinomas:ab,ti OR neoplasia:ab,ti OR neoplasm:ab,ti OR neoplasms:ab,ti | 617186 |
| #6 | 'carcinoma, squamous cell'/syn | 110025 |
| #5 | #3 OR #4 | 56567 |
| #4 | conjunctiva:ab,ti OR conjunctivas:ab,ti OR conjunctival:ab,ti | 22041 |
| #3 | 'conjunctiva'/syn | 51682 |
| #2 | random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR cross?over:ab OR crossover*:ti OR crossover*:ab OR placebo*:ti OR placebo*:ab OR (doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab) OR (singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab) OR assign*:ti OR assign*:ab OR volunteer*:ti OR volunteer*:ab OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR 'randomized controlled trial'/de OR 'randomized controlled trial' OR allocat*:ti OR allocat*:ab | 1186635 |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus' OR hiv:ti OR hiv:ab OR 'hiv‐1':ti OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab OR 'human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab OR 'human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab OR 'acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab OR 'acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR 'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab | 371108 |
Appendix 3. The Cochrane Library search strategy and results
Title: Interventions for squamous cell carcinoma of the conjunctiva in HIV‐infected individuals
Database: CLIB (2010 – 2012)
Date: 3 February 2012
| ID | Search | Hits |
| #1 | MeSH descriptor HIV Infections explode all trees | 6499 |
| #2 | MeSH descriptor HIV explode all trees | 2172 |
| #3 | hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN IMMUNE‐DEFICIENCY VIRUS OR HUMAN IMMUNO‐DEFICIENCY VIRUS OR HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED IMMUNO‐DEFICIENCY SYNDROME OR ACQUIRED IMMUNE‐DEFICIENCY SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME | 10597 |
| #4 | MeSH descriptor Lymphoma, AIDS‐Related, this term only | 21 |
| #5 | MeSH descriptor Sexually Transmitted Diseases, Viral, this term only | 22 |
| #6 | (#1 OR #2 OR #3 OR #4 OR #5) | 10671 |
| #7 | MeSH descriptor Conjunctiva, this term only | 473 |
| #8 | conjunctiva*:ti,ab,kw | 1906 |
| #9 | (#7 OR #8) | 1906 |
| #10 | MeSH descriptor Carcinoma, Squamous Cell, this term only | 1818 |
| #11 | carcinoma*:ti,ab,kw OR neoplasia:ti,ab,kw OR neoplasm*:ti,ab,kw | 42921 |
| #12 | (#10 OR #11) | 42921 |
| #13 | (#6 AND #9 AND #12) | 2 |
| #14 | (#6 AND #9 AND #12), from 2010 to 2012 | 0 |
Appendix 4. WHO International Clinical Trials Registry Platform (ICTRP) search terms and results
Title: Interventions for squamous cell carcinoma of the conjunctiva in HIV‐infected individuals
Database: WHO International Clinical Trials Registry Platform (ICTRP) 2010 ‐ 2012
Date: 10 February 2012
No results were found for: hiv AND carcinoma AND conjunctiva
Appendix 5. National Institutes for Health (NIH) Clinical Trials registry search terms and results
Title: Interventions for squamous cell carcinoma of the conjunctiva in HIV‐infected individuals
Database: Clinicaltrials.gov
Date: 10 February 2012
Found no studies with search of: "squamous cell carcinoma" AND conjunctiva AND hiv
Characteristics of studies
Characteristics of ongoing studies [ordered by study ID]
Gichuhi 2012.
| Trial name or title | Adjuvant topical 5fluorouracil (5FU) for ocular surface squamous neoplasia |
| Methods | In this randomized controlled trial tumours involving less than 2 quadrants of the conjunctiva that are suspected to be OSSN will be surgically excised |
| Participants | Adults with histologically proven OSSN whose tumour excision site has healed |
| Interventions | Topical 1% 5‐Fluorouracil in artificial tears vs artificial tears applied 6 hourly for one month |
| Outcomes | Primary: Development of histopathologically confirmed recurrent OSSN anytime during the first year after the primary surgery. Secondary: Adverse effects of using topical 5‐FU 1% four times daily for one month. |
| Starting date | 23/8/2012 |
| Contact information | sgichuhi@uonbi.ac.ke |
| Notes |
Contributions of authors
SG developed the review topic idea. JI looked through search outputs, provided input, guidance and mentoring.
Sources of support
Internal sources
University of Nairobi, Department of Ophthalmology, Kenya.
External sources
South African Cochrane Centre HIV/AIDS Mentoring Programme, South Africa.
Declarations of interest
Stephen Gichuhi has received funding to conduct a trial in this field (Gichuhi 2012).
New search for studies and content updated (no change to conclusions)
References
References to ongoing studies
Gichuhi 2012 {unpublished data only}
- Adjuvant topical 5fluorouracil (5FU) for ocular surface squamous neoplasia. Ongoing study 23/8/2012.
Additional references
Aoki 1998
- Aoki S, Kubo E, Nakamura S, Tsuzuki A, Tsuzuki S, Takahashi Y, et al. Possible prognostic markers in conjunctival dysplasia and squamous cell carcinoma. Japanese Journal of Ophthalmology 1998;42(4):256‐61. [DOI] [PubMed] [Google Scholar]
Ateenyi‐Agaba 1995
- Ateenyi‐Agaba C. Conjunctival squamous cell carcinoma associated with HIV infection in Kampala, Uganda. Lancet 1995;345(8951):695‐6. [DOI] [PubMed] [Google Scholar]
Ateenyi‐Agaba 2004
- Ateenyi‐Agaba C, Dai M, Calvez F, Katongole‐Mbidde E, Smet A, Tommasino M, et al. TP53 mutations in squamous cell carcinomas of the conjuntiva: evidence for UV‐induced mutagenesis. Mutagenesis 2004;19(5):399‐401. [DOI] [PubMed] [Google Scholar]
Ateenyi‐Agaba 2010
- Ateenyi‐Agaba C, Franceschi S, Wabwire‐Mangen F, Arsian A, Othieno E, Binta‐Kahwa J, Doorn LJ, Kleter B, Weiderpass E. Human papillomavirus infection and squamous cell carcinoma of the conjunctiva. British Journal of Cancer 2010;102:262‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strengths of recommendations. BMJ 2004;328(7454):1490‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbazetto 2004
- Barbazetto IA, Lee TC, Abramson DH. Treatment of conjunctival squamous cell carcinoma with photodynamic therapy. American Journal of Ophthalmology 2004;138(2):183‐9. [DOI] [PubMed] [Google Scholar]
Basti 2003
- Basti S, Macsai MS. Ocular surface squamous neoplasia: a review. Cornea 2003;22(7):687‐704. [DOI] [PubMed] [Google Scholar]
Bonanno 2010
- Bonanno A, Esmaeli B, Fingere MC, Nelson DV, Randal S, Weber RS. Social challenges of cancer patients with orbitofacial disfigurement. Ophthalmic Plastic and Reconstructive Surgery 2010;26:18‐22. [DOI] [PubMed] [Google Scholar]
Cartsburg 2001
- Cartsburg O, Kallen C, Hillenkamp J, Sundmacher R, Pomjanski N, Bocking A. Topical mitomycin C and radiation induce conjunctival DNA‐polyploidy. Analytical Cellular Pathology 2001;23(2):65‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castano 2005
- Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: Part three ‐ photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis and Photodynamic Therapy 2005;2(2):91‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cerezo 1990
- Cerezo L, Otero J, Aragon G, Polo E, Torre A, Valcarcel F, et al. Conjunctival intraepithelial and invasive squamous cell carcinomas treated with strontium‐90. Radiotherapy and oncology Journal of the European Society for Therapeutic Radiology and Oncology 1990;17(3):191‐7. [DOI] [PubMed] [Google Scholar]
Cervantes 2002
- Cervantes G, Rodriguez AA Jr, Leal AG. Squamous cell carcinoma of the conjunctiva: clinicopathological features in 287 cases. Canadian Journal of Ophthalmology 2002;37(1):14‐9. [DOI] [PubMed] [Google Scholar]
Chisi 2006
- Chisi SK, Kollmann MKH, Karimurio J. Conjunctival squamous cell carcinoma in patients with Human Immunodeficiency Virus infection seen at two hospitals in Kenya. East African Medical Journal 2006;83(5):267‐70. [DOI] [PubMed] [Google Scholar]
Chu 2004
- Chu T, Sartorelli AC. Cancer chemotherapy. In: Katzung BG editor(s). Basic and clinical pharmacology. 9th Edition. Boston: McGraw Hill, 2004:898‐930. [Google Scholar]
Clifford 2005
- Clifford GM, Gallus S, Herrero R, Munoz N, Snijders PJF, Vaccarella S, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet 2005;366(9490):991‐8. [DOI] [PubMed] [Google Scholar]
Copeland 1990
- Copeland RA. Limbal autograft for reconstruction after conjunctival squamous cell carcinoma. American Journal of Ophthalmology 1990;4(110):412‐5. [DOI] [PubMed] [Google Scholar]
de Koning 2008
- Koning MN, Waddell K, Magyezi J, Purdie K, Proby C, Harwood C, Lucas S, Downing R, Quint WG, Newton R. Genital and cutaneous human papillomavirus (HPV) types in relation to conjunctival squamous cell neoplasia: a case‐control study in Uganda. Infectious agents and cancer 2008;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Espana 2002
- Espana EM, Prabhasawat P, Grueterich M, Solomon A, Tseng SCG. Amniotic membrane transplantation for reconstruction after excision of large ocular surface neoplasias. British Journal of Ophthalmology 2002;86(6):640‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frucht‐Pery 1997
- Frucht‐Pery J, Sugar J, Baum J, Sutphin JE, Pe'er J, Savir H, et al. Mitomycin C treatment for conjunctival‐corneal intraepithelial neoplasia: a multicenter experience. Ophthalmology 1997;104(12):2085‐93. [DOI] [PubMed] [Google Scholar]
Goldberg 1963
- Goldberg JR, Becker SC, Rosenbaum HD. Gamma radiation in the treatment of squamous cell carcinoma of the limbus. American Journal of Ophthalmology 1963;55:811‐5. [PubMed] [Google Scholar]
Gunduz 2005
- Gunduz K, Ucakhan OO, Kanpolat A, Gunalp I. Non‐preserved human amniotic membrane transplantation for conjunctival reconstruction after excision of extensive ocular surface neoplasia. Eye 2005;20(3):351‐7. [DOI] [PubMed] [Google Scholar]
Guthoff 2009
- Guthoff R, Marx A, Stroebel P. No evidence for a pathogenic role of human papillomavirus infection in ocular surface squamous neoplasia in Germany. Current Eye Research 2009;34:666‐71. [DOI] [PubMed] [Google Scholar]
Hao 2000
- Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 2000;19(3):348‐52. [DOI] [PubMed] [Google Scholar]
Kaji 2006
- Kaji Y, Hiraoka T, Oshika T. Vital staining of squamous cell carcinoma of the conjunctiva using toluidine blue. Acta Ophthalmologica Scandinavica 2006;84(6):825‐6. [DOI] [PubMed] [Google Scholar]
Karp 2001
- Karp CL, Moore JK, Rosa RH Jr. Treatment of conjunctival and corneal intraepithelial neoplasia with topical interferon alpha‐2b. Ophthalmology 2001;108(6):1093‐8. [DOI] [PubMed] [Google Scholar]
Lake 2004
- Lake DF, Briggs AD, Akporiaye ET. Immunopharmacology. In: Katzung BG editor(s). Basic and clinical pharmacology. 9th Edition. Boston: McGraw Hill, 2004:931‐57. [Google Scholar]
Lee 1997
- Lee GA, Hirst LW. Retrospective study of ocular surface squamous neoplasia. Australian and New Zealand Journal of Ophthalmology 1997;25(4):269‐76. [DOI] [PubMed] [Google Scholar]
Macarez 1999
- Macarez R, Bossis S, Robinet A, Callonnec A, Charlin JF, Colin J. Conjunctival epithelial neoplasias in organ transplant patients receiving cyclosporine therapy. Cornea 1999;18(4):495‐7. [DOI] [PubMed] [Google Scholar]
Mahomed 2002
- Mahomed A, Chetty R. Human immunodeficiency virus infection, Bcl‐2, p53 protein, and Ki‐67 analysis in ocular surface squamous neoplasia. Archives of Ophthalmology 2002;120(5):554‐8. [DOI] [PubMed] [Google Scholar]
Margo 2002
- Margo CE, Harman LE, Mulla ZD. The reliability of clinical methods in ophthalmology. Survey of Ophthalmology 2002;47(4):375‐86. [DOI] [PubMed] [Google Scholar]
McKelvie 2001
- McKelvie PA, Daniell M. Impression cytology following mitomycin C therapy for ocular surface squamous neoplasia. British Journal of Ophthalmology 2001;85(9):1115‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKelvie 2002
- McKelvie PA, Daniell M, McNab A, Loughnan M, Santamaria JD. Squamous cell carcinoma of the conjunctiva: a series of 26 cases. British Journal of Ophthalmology 2002;86(2):168‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Midena 2000
- Midena E, Angeli CD, Valenti M, Belvis V, Boccato P. Treatment of conjunctival squamous cell carcinoma with topical 5‐fluorouracil. British Journal of Ophthalmology 2000;84(3):268‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moubayed 2004
- Moubayed P, Mwakyoma H, Schneider DT. High frequency of Human Papillomavirus 6/11, 16, and 18 infections in precancerous lesions and squamous cell carcinoma of the conjunctiva in subtropical Tanzania. American Journal of Clinical Pathology 2004;122:938‐43. [DOI] [PubMed] [Google Scholar]
Nadjari 1999
- Nadjari B, Kersten A, Ross B, Motherby H, Krallmann R, Sundmacher R, et al. Cytologic and DNA cytometric diagnosis and therapy monitoring of squamous cell carcinoma in situ and malignant melanoma of the cornea and conjunctiva. Analytical and Quantitative Cytology and Histology 1999;21(5):387‐96. [PubMed] [Google Scholar]
Nakamura 1997
- Nakamura Y, Mashima Y, Kameyama K, Mukai M, Oguchi Y. Detection of human papillomavirus infection in squamous tumours of the conjunctiva and lacrimal sac by immunohistochemistry, in situ hybridisation and polymerase chain reaction. British Journal of Ophthalmology 1997;81(4):308‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newton 2002
- Newton R, Ziegler J, Ateenyi‐Agaba C, Bousarghin L, Casabonne D, Beral V, et al. The epidemiology of conjunctival squamous cell carcinoma in Uganda. British Journal of Cancer 2002;87(3):301‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2008
- Ng J, Coroneo MT, Wakefield D, Girolamo N. Ultraviolet radiation and the role of matrix metalloproteinases in the pathogenesis of ocular surface squamous neoplasia. Investigative Ophthalmology & Visual Science 2008;49:5295‐5306. [DOI] [PubMed] [Google Scholar]
NIH 2012
- U.S. National Institutes of Health. Information on clinical trials and human research studies. www.clinicaltrials.gov (accessed 10 February 2012).
Osahon 2011
- Osahon AI, Ukponmwan CU, Uhunmwangho OM. Prevalence of HIV seropositivity among patients with squamous cell carcinoma of the conjunctiva. Asian Pacific Journal of Tropical Biomedicine 2011;1(2):150‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peksayar 1989
- Peksayar G, Soyturk MK, Demiryont M. Long‐term results of cryotherapy on malignant epithelial tumours of the conjunctiva. American Journal of Ophthalmology 1989;107(4):337‐49. [DOI] [PubMed] [Google Scholar]
Phillips 2001
- Phillips B, Ball C, Sackett D, Badenoch D, Straus S, Haynes B, et al. Levels of evidence and grades of recommendation. www.cebm.net/levels_of_evidence.asp (accessed 14 June 2006).
Pola 2003
- Pola EC, Masanganise R, Rusakaniko S. The trend of ocular surface squamous neoplasia among ocular surface tumour biopsies submitted for histology from Sekuru Kaguvi Eye Unit, Harare between 1996 and 2000. Central African Journal of Medicine 2003;49:1‐4. [PubMed] [Google Scholar]
Poole 1999
- Poole TR. Conjunctival squamous cell carcinoma in Tanzania. British Journal of Ophthalmology 1999;83(2):177‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Porges 2003
- Porges Y, Groisman GM. Prevalence of HIV with conjunctival squamous cell neoplasia in an African provincial hospital. Cornea 2003;22(1):1‐4. [DOI] [PubMed] [Google Scholar]
Safrin 2004
- Safrin S. Antiviral agents. In: Katzung BG editor(s). Basic and clinical pharmacology. 9th Edition. Boston: McGraw Hill, 2004:801‐27. [Google Scholar]
Sen 2007
- Sen S, Sharma A, Panda A. Immunohistochemical localization of human papilloma virus in conjunctival neoplasias: a retrospective study. Indian Journal of Ophthalmology 2007;55:361‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 2002
- Sherman MD, Feldman KA, Farahmand SM, Margolis TP. Treatment of conjunctival squamous cell carcinoma with topical cidofovir. American Journal of Ophthalmology 2002;134(3):432‐3. [DOI] [PubMed] [Google Scholar]
Shields 1991
- Shields JA, Shields CL, Suvarnamani C, Tantisira M, Shah P. Orbital exenteration with eyelid sparing: indications, technique and results. Ophthalmic surgery 1991;22(5):292‐7. [PubMed] [Google Scholar]
Shields 1997
- Shields JA, Shields CL, Potter P. Surgical management of conjunctival tumours: The 1994 Lynn B. McMahan Lecture. Archives of Ophthalmology 1997;115(6):808‐15. [DOI] [PubMed] [Google Scholar]
Shields 2004
- Shields JA, Shields CL. Tumors of the conjunctiva and cornea. In: McLeod SD editor In: Tasman W, Jaeger EA editor(s). Duane's clinical ophthalmology CD‐ROM. Revised 2004. Vol. 4, Philadelphia: Lippincott William and Wilkins, 2004:Chapter 10. [Google Scholar]
Sudesh 2000
- Sudesh S, Rapuano CJ, Cohen EJ, Eagle RC Jr, Laibson PR. Surgical management of ocular surface squamous neoplasms: the experience from a cornea center. Cornea 2000;19(3):278‐83. [DOI] [PubMed] [Google Scholar]
Tabin 1997
- Tabin G, Levin S, Snibson G, Loughnan M, Taylor H. Late recurrences and the necessity for long‐term follow‐up in corneal and conjunctival neoplasia. Ophthalmology 1997;104(3):485‐92. [DOI] [PubMed] [Google Scholar]
Tornesello 2006
- Tornesello ML, Duraturo ML, Waddell KM, Biryahwaho B, Downing R, Balinandi S, et al. Evaluating the role of human papillomaviruses in conjunctival neoplasia. British Journal of Cancer 2006;94(3):446‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tunc 1999
- Tunc M, Char DH, Crawford B, Miller T. Intraepithelial and invasive squamous cell carcinoma of the conjunctiva: analysis of 60 cases. British Journal of Ophthalmology 1999;83(1):98‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
UNAIDS 2010
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Global report: UNAIDS report on the global AIDS epidemic 2010. http://www.unaids.org/globalreport/global_report.htm 2010.
Waddell 1996
- Waddell KM, Lewallen S, Lucas SB, Atenyi‐Agaba C, Herrington CS, Liomba G. Carcinoma of the conjunctiva and HIV infection in Uganda and Malawi. British Journal of Ophthalmology 1996;80(6):503‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO ICTRP 2012
- WHO ICTRP. WHO International Clinical Trials Registry Platform. www.who.int/ictrp/ (accessed on 10 February 2012).
Yeatts 2000
- Yeatts RP, Engelbrecht NE, Curry CD, Ford JG, Walker KA. 5‐Fluorouracil for the treatment of intraepithelial neoplasia of the conjunctiva and cornea. Ophthalmology 2000;107(12):2190‐5. [DOI] [PubMed] [Google Scholar]


