Abstract
目的
探讨呼吸道合胞病毒(RSV)感染气道上皮细胞后对表皮生长因子受体(EGFR)、紧密连接相关蛋白occludin及E-cadherin、黏蛋白MUC5AC表达的影响,并探讨可能的机制。
方法
体外实验分两组,以人气道黏液上皮细胞NCI-H292细胞为研究对象,紫外线下灭活的RSV加入NCI-H292细胞培养基中作为对照组,迅速解冻的RSV感染NCI-H292细胞为RSV感染组。48 h后通过Western blot法检测occludin、E-cadherin、磷酸化EGFR(p-EGFR)及EGFR的蛋白表达水平;采用细胞免疫荧光技术观察两组细胞occludin、E-cadherin的分布及表达;采用RT-PCR法检测两组MUC5AC mRNA的表达。
结果
RSV感染组occludin、E-cadherin的蛋白表达水平较对照组下降(P < 0.05)。RSV感染组p-EGFR及EGFR蛋白的表达水平较对照组升高(P < 0.05)。RSV感染组MUC5AC mRNA表达水平较对照组增加(P < 0.05)。
结论
RSV可破坏气道上皮细胞间的紧密连接,促进黏蛋白的分泌,影响气道的屏障功能。EGFR的磷酸化在上述过程中起重要作用。
Keywords: 呼吸道合胞病毒, 表皮生长因子受体, 紧密连接相关蛋白, 黏蛋白, 气道上皮细胞
Abstract
Objective
To study the effects of respiratory syncytial virus (RSV) infection on epidermal growth factor receptor (EGFR), tight junction association proteins and mucin in the human airway epithelial cells.
Methods
Human airway epithelial cells NCI-H292 were randomly treated by ultraviolet light-inactivated RSV (control group) or thawed RSV (RSV infection group). After 48 hours of treatment, the protein levels of occludin, E-cadherin, phosphorylated EGFR and EGFR in NCI-H292 cells were measured by Western blot. The distribution and expression levels of occludin and E-cadherin in NCI-H292 cells were examined by immunofluorescence technique. The expression levels of MUC5AC mRNA in NCI-H292 cells were assessed by RT-PCR.
Results
The protein levels of occludin and E-cadherin were signifcantly reduced in the RSV infection group compared with the control group (P < 0.05). The protein levels of phosphorylated EGFR and EGFR increased signifcantly in the RSV infection group compared with the control group (P < 0.05). The MUC5AC mRNA levels also increased signifcantly in the RSV infection group compared with the control group (P < 0.05).
Conclusions
RSV may down-regulate the tight junction association proteins and upregulate the expression of MUC5AC in airway epithelial cells, which contributes to epithelial barrier dysfunction. EGFR phosphorylation may play an important role in regulation of airway barrier.
Keywords: Respiratory syncytial virus, Epidermal growth factor receptor, Tight junction association protein, Mucin, Airway epithelial cell
气道上皮细胞间的顶端连接复合物与其表面的黏液层构成了抵御病原微生物入侵的屏障。顶端连接复合物由紧密连接与黏附连接组成,在调控细胞周围的通透性及维持细胞的极化上起重要作用[1]。紧密连接是跨膜蛋白(如occludin、claudins)和胞质蛋白(如ZO-1/2、cingulin)组成[2]。参与黏附连接的主要有上皮钙黏蛋白(E-cadherin)、β-连环蛋白(β-catenin)及α-连环蛋白(α-catenin)[1]。气道黏液层的重要组分为黏蛋白MUC5AC[3]。哮喘、肺囊性纤维病等疾病状态时,气道上皮细胞均受累。表皮生长因子受体(epidermal growth factor receptor, EGFR)可参与上皮细胞的生长、修复和瘤变,在黏蛋白的分泌、细胞间紧密连接的调控中起重要作用[3-6]。
病毒可影响气道的紧密连接,如腺病毒感染上皮细胞后产生纤维蛋白,破坏细胞间的黏附,实现病毒逃逸[7];人鼻病毒可改变气道上皮细胞紧密连接蛋白的表达,致细胞屏障功能受损[8]。呼吸道合胞病毒(RSV)感染是五岁以下儿童发病和死亡的主要原因之一[9],目前RSV对气道紧密连接的影响仍不明确。气道上皮细胞是RSV最主要的靶细胞。RSV附于气道上皮细胞后可启动固有免疫反应,进而诱发病理改变[10]。研究显示EGFR的活化可抑制气道上皮细胞的抗病毒防御功能[11]。因此,明确RSV对EGFR的影响可为机体增强病毒防御能力、降低病毒感染提供可能的方法。
基于上述背景,本实验将探讨RSV感染气道上皮细胞后对EGFR、紧密连接相关蛋白(occludin、E-cadherin)、MUC5AC表达的影响并探讨可能的机制。
1. 材料与方法
1.1. 主要试剂与仪器
NCI-H292细胞株(武汉博士德生物工程有限公司);RSV病毒Long株(美国模式培养物保藏所);Alexa Fluor 488标记二抗(美国BioLegend公司);occludin抗体、E-cadherin抗体、EGFR抗体、p-EGFR抗体、GAPDH抗体、辣根过氧化物酶标记二抗(北京博奥森生物技术有限公司);ECL化学发光液(北京康为世纪生物科技有限公司);RIPA裂解液(上海碧云天生物技术有限公司);高纯总RNA快速提取试剂盒(离心柱型)、RT-PCR Kit(北京百泰克生物技术有限公司);RPMI培养基1640、胎牛血清(美国Gibco公司)。全自动激光共聚焦显微镜(日本Olympus公司);Step-one PCR仪(美国ABI公司);G:BOX型凝胶成像系统(英国Syngene公司)。
1.2. NCI-H292细胞培养及分组
NCI-H292细胞培养于RPMI1640培养基,其中含10%胎牛血清及终浓度为100 U/mL的青霉素和100 mg/L的链霉素。将NCI-H292细胞接种于六孔板,细胞密度为1.2×106/mL,培养体积3 mL。当细胞于5%CO2、95%湿度、37℃的培养箱中生长至70%,分为对照组及RSV感染组。对照组:弃上清,加入3 mL不含双抗及胎牛血清的RPMI1640培养液,再加入紫外灭活的RSV;感染组:弃上清,加入500 μL不含双抗及胎牛血清的培养基,再加入迅速解冻的5 μL RSV(感染复数=1.0)。每30 min摇晃六孔板1次,2 h后加2.5 mL不含双抗及胎牛血清的培养基。孵育48 h后收集细胞进行实验。
1.3. 免疫荧光技术检测occludin、E-cadherin表达
在六孔板内提前放入灭菌处理的盖玻片,用上述方法培养及感染NCI-H292细胞,每组制作3张细胞爬片。48 h后,PBS室温环境下冲洗细胞并弃去PBS,加入4%多聚甲醛(PFA)固定细胞10 min,PBS冲洗3次。加入1%BSA室温封闭60 min,PBS冲洗5 min。分别加入E-cadherin(1 : 100)、occludin(1 : 100)一抗,4℃孵育过夜,PBS冲洗3次,加入Alexa Fluor 488标记的二抗(1 : 500),避光1 h,PBS冲洗3次,晾干封片。在全自动激光共聚焦显微镜下观察。细胞免疫荧光图像用Image J软件分析,结果用平均免疫荧光强度表示。实验独立重复3次。
1.4. 蛋白免疫印迹检测occludin、E-cadherin、EGFR、p-EGFR的表达
按每孔加入150 μL裂解液的比例提取总蛋白,冰上裂解处理30 min,收集裂解液,4℃、12 000 r/min离心5 min,取上清。BCA法测蛋白浓度。取40 μg总蛋白与2×SDS-Loading buffer混合,95℃变性5 min后上样,10% SDS-PAGE凝胶电泳70 min,半干式转膜至PVDF膜(25 V,30 min),5%脱脂牛奶室温封闭1 h,分别加入E-cadherin(1 : 200)、occludin(1 : 200)、EGFR(1 : 500)、p-EGFR(1 : 200)、GAPDH(1 : 1 000)一抗,4℃孵育过夜,TBST洗膜3次,加入辣根过氧化物酶标记的二抗(1 : 50 000),室温平稳摇动1 h,TBST洗膜3次,ECL显色,G:BOX型凝胶成像系统进行扫描。成像的蛋白条带经Image J软件分析灰度值,结果以目的蛋白与内参蛋白GAPDH灰度值的比值表示蛋白相对表达量。实验独立重复3次。
1.5. RT-PCR法检测MUC5AC表达
依据试剂盒方法提取总RNA,并据试剂盒反应体系及条件进行反转录,测cDNA的浓度。采用Primer3.0软件设计引物并由上海赛百盛基因技术有限公司合成。MUC5AC上游引物序列:5'-TGATCATCCAGCAGCAGGGCT-3',下游引物序列:5'-CCGGCTCAGAGGACATATGGG-3',片段长度409 bp;内参GAPDH上游引物序列:5'-AGGTCGGAGTCAACGGATTTG-3',下游引物序列:5'-GTGATGGCATGGACTGTGGT-3',片段长度532 bp。反应体系(25 μL):10×PCR buffer 2.5 μL,dNTP 2 μL,Taq DNA聚合酶0.2 μL,模板DNA 5 μL,上、下游引物各1 μL,ddH2O 13.3 μL。反应条件:94℃预变性2 min;94℃45 s,60℃45 s,72℃1 min,35个循环;72℃延伸5 min。采用3%琼脂糖凝胶电泳,应用UVP凝胶成像系统记录结果。图像经Image J软件分析灰度值,结果用MUC5AC与GAPDH灰度值的比值表示mRNA相对表达量。实验独立重复3次。
1.6. 统计学分析
采用SPSS 13.0统计软件对数据进行统计分析。计量资料采用均数±标准差(x±s)表示,两组间比较采用两独立样本t检验,P < 0.05为差异有统计学意义。
2. 结果
2.1. 两组occludin及E-cadherin蛋白表达的变化
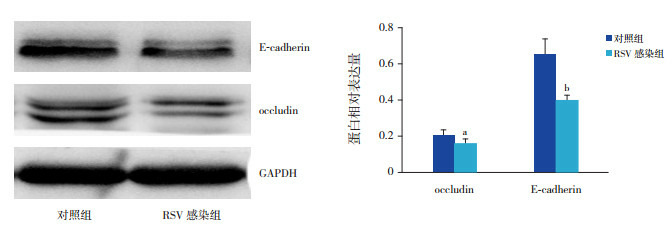
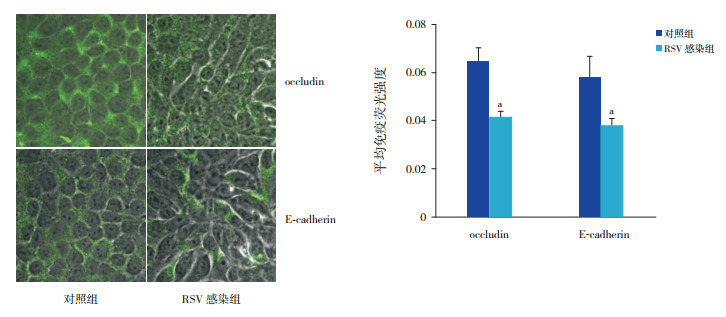
RSV感染NCI-H292细胞48 h,occludin、E-cadherin在蛋白水平的表达减少,与对照组比较,差异有统计学意义(P < 0.05,图 1)。通过免疫荧光技术观察两组细胞,RSV感染组occludin、E-cadherin的荧光强度较对照组下降(P < 0.01),同时RSV感染组细胞形态发生改变(图 2)。
1.
Western blot法检测两组occludin及E-cadherin的蛋白表达变化
左图为蛋白电泳条带图。右图为两组目的蛋白表达统计图(n=3),a示与对照组比较,P < 0.05;b示与对照组比较,P < 0.01。
2.
免疫荧光染色法检测RSV感染NCI-H292细胞48 h后occludin及E-cadherin的表达变化(×300)
左图为细胞免疫荧光图,绿色荧光为目的蛋白的阳性表达;RSV感染组occludin及E-cadherin的阳性表达均弱于对照组。右图为两组目的蛋白平均荧光强度统计图(n=3),a示与对照组比较,P < 0.01。
2.2. 两组p-EGFR及EGFR蛋白表达的变化
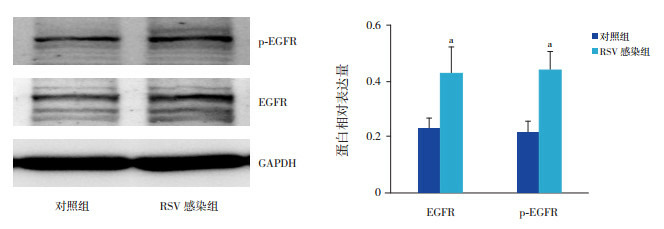
RSV感染NCI-H292细胞48 h,EGFR磷酸化水平及EGFR的蛋白表达水平均较对照组增高(P < 0.05),见图 3。
3.
Western blot法检测两组p-EGFR及EGFR蛋白的表达变化
左图为蛋白电泳条带图;右图为两组目的蛋白表达统计图(n=3),a示与对照组比较,P < 0.05。
2.3. 两组MUC5AC mRNA的表达变化
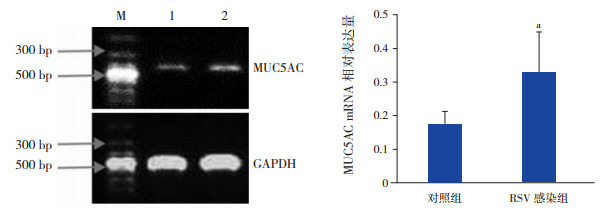
RSV感染NCI-H292细胞48 h,MUC5AC mRNA的相对表达量高于对照组,差异有统计学意义(P < 0.05),见图 4。
4.
RT-PCR法检测两组MUC5AC mRNA的表达
左图为PCR扩增产物的凝胶电泳图,M:Marker,1:对照组,2:RSV感染组;右图为MUC5AC mRNA相对表达量统计图(n=3),a示与对照组比较,P < 0.05。
3. 讨论
RSV感染组EGFR的磷酸化水平明显增加,提示RSV可致EGFR活化。EGFR定位于细胞膜基底外侧,其胞内区有酪氨酸激酶活性,当配体与EGFR结合导致受体自身的酪氨酸残基磷酸化,可募集相关信号转运蛋白,激活下游信号通路。RSV介导的EGFR活化可导致下游ERK活性增加,促进IL-8释放并抑制RSV感染细胞的凋亡[12];可抑制干扰素调节因子1依赖性干扰素-λ的产生,增加RSV的感染滴度,而抑制EGFR活化能降低病毒的感染滴度[13]。上述发现提示抑制EGFR的活化是降低RSV感染程度的方法。
EGFR的胞外区与E-cadherin连接。E-cadherin是广泛分布于上皮细胞表面的跨膜糖蛋白。在胞内,E-cadherin与β-catenin、α-catenin形成连接复合物,连接肌动蛋白、细胞骨架蛋白,促进细胞间的黏附[14]。实验中RSV感染上皮细胞后促进EGFR磷酸化,而β-catenin是EGFR磷酸化后的作用底物。因此,当EGFR磷酸化后E-cadherin/β-catenin连接复合物解体,E-cadherin相关的细胞间屏障功能受损[15]。随着紧密连接的受损,E-cadherin/EGFR胞外段的连接受损,EGFR可易位至胞膜顶端,此时更易与配体结合、活化,进一步破坏紧密连接[1]。利用siRNA沉默E-cadherin后,引起EGFR的磷酸化增加,其下游MEK/ERK-1/2和p38 MAPK通路激活,导致Th2型炎症反应趋化因子表达增加,同时E-cadherin参与的细胞间连接减弱[16]。体内实验显示,小鼠支气管上皮细胞E-cadherin表达缺失时,紧密连接蛋白occludin、ZO-1及claudins发生易位[17]。因此可推断,RSV感染气道上皮细胞后下调E-cadherin的表达,致紧密连接相关蛋白易位,导致气道黏膜屏障功能受损。EGFR磷酸化是导致E-cadherin介导的屏障功能受损的重要因素。
RSV感染组occludin的蛋白表达水平下调。occludin是定位于紧密连接部位的跨膜蛋白,它的羧基末端通过ZO-1/2与细胞骨架蛋白结合,维持细胞间的黏附[18]。研究显示,机械性肺损伤、镉、甲醛暴露等理化因素刺激时,气道上皮细胞的occludin下调,而磷酸化occludin上调,PKC通路激活,同时影响连接复合物的完整性,导致屏障功能受损[19-21]。本实验结果提示除上述理化因素外,RSV亦可下调occludin的表达。Chen等[22]证实:EGFR及下游p38MAPK/NF-κB信号通路的活化,可致occludin在转录水平、蛋白水平的表达均下调,而加入EGFR抑制剂时上述效应减弱。RSV感染组的occludin表达下调伴EGFR活化,可能与Chen等[22]发现的机制相关。
RSV感染组黏蛋白MUC5AC在转录水平的表达上调。实验中MUC5AC表达的增高与EGFR及下游生长因子信号通路的活化有关[23];另外,与紧密连接对黏蛋白基因的调控有关。气道上皮细胞间紧密连接受损时可释放β-catenin,β-catenin与LEF1相互作用后转入核内,能增强MUC5AC启动子的活性[24]。
综上所述,RSV感染气道上皮细胞可活化EGFR,下调气道上皮细胞紧密连接相关蛋白E-cadherin、occludin的表达,上调黏蛋白MUC5AC的表达,破坏气道黏膜的屏障功能。EGFR的磷酸化在上述过程中起重要作用。本文还存在不足,EGFR相关信号通路在RSV影响气道上皮细胞紧密相关连接蛋白表达中的作用仍需进一步的研究。
Biography
刘娟娟, 女, 硕士, 住院医师。Email:6512035@zju.edu.cn
References
- 1.Ganesan S, Comstock AT, Sajjan US. Barrier function of airway tract epithelium. Tissue Barriers. 2013;1(4):e24997. doi: 10.4161/tisb.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazzoni G, Martinez-Estrada OM, Orsenigo F, et al. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occluding. J Biol Chem. 2000;275(27):20520–20526. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- 3.Bonser LR, Erle DJ. Airway mucus and asthma:the role of MUC5AC and MUC5B. J Clin Med. 2017;6(12):112. doi: 10.3390/jcm6120112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seo GS, Jiang WY, Park PH, et al. Hirsutenone reduces deterioration of tight junction proteins through EGFR/Akt and ERK1/2 pathway both converging to HO-1 induction. Biochem Pharmacol. 2014;90(2):115–125. doi: 10.1016/j.bcp.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Petecchia L, Sabatini F, Usai C, et al. Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway. Lab Invest. 2012;92(8):1140–1148. doi: 10.1038/labinvest.2012.67. [DOI] [PubMed] [Google Scholar]
- 6.Terakado M, Gon Y, Sekiyama A, et al. The Rac1/JNK pathway is critical for EGFR-dependent barrier formation in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300(1):L56–L63. doi: 10.1152/ajplung.00159.2010. [DOI] [PubMed] [Google Scholar]
- 7.Walters RW, Freimuth P, Moninger TO, et al. Adenovirus fber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell. 2002;110(6):789–799. doi: 10.1016/S0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- 8.Looi K, Troy NM, Garratt LW, et al. Effect of human rhinovirus infection on airway epithelium tight junction protein disassembly and transepithelial permeability. Exp Lung Res. 2016;42(7):380–395. doi: 10.1080/01902148.2016.1235237. [DOI] [PubMed] [Google Scholar]
- 9.Mazur NI, Martinón-Torres F, Baraldi E, et al. Lower respiratory tract infection caused by respiratory syncytial virus:current management and new therapeutics. Lancet Respir Med. 2015;3(11):888–900. doi: 10.1016/S2213-2600(15)00255-6. [DOI] [PubMed] [Google Scholar]
- 10.Farrag MA, Almajhdi FN. Human respiratory syncytial virus:role of innate immunity in clearance and disease progression. Viral Immunol. 2016;29(1):11–26. doi: 10.1089/vim.2015.0098. [DOI] [PubMed] [Google Scholar]
- 11.Ueki IF, Min-Oo G, Kalinowski A, et al. Respiratory virusinduced EGFR activation suppresses IRF1-dependent interferon λ and antiviral defense in airway epithelium. J Exp Med. 2013;210(10):1929–1936. doi: 10.1084/jem.20121401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monick MM, Cameron K, Staber J, et al. Activation of the epidermal growth factor receptor by respiratory syncytial virus results in increased inflammation and delayed apoptosis. J Biol Chem. 2005;280(3):2147–2158. doi: 10.1074/jbc.M408745200. [DOI] [PubMed] [Google Scholar]
- 13.Kalinowski A, Galen BT, Ueki IF, et al. Respiratory syncytial virus activates epidermal growth factor receptor to suppress interferon regulatory factor 1-dependent interferon-lambda and antiviral defense in airway epithelium. Mucosal Immunol. 2018;11(3):958–967. doi: 10.1038/mi.2017.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta S, Nijhuis A, Kumagai T, et al. Defects in the adherens junction complex (E-cadherin/β-catenin) in inflammatory bowel disease. Cell Tissue Res. 2015;360(3):749–760. doi: 10.1007/s00441-014-1994-6. [DOI] [PubMed] [Google Scholar]
- 15.邢 荣春, 郑 军, 刘 伟, et al. E-cadherin在EGFR分子靶向治疗中作用的研究进展. 肝胆胰外科杂志. 2013;25(2):175–177. doi: 10.3969/j.issn.1007-1954.2013.02.031. [DOI] [Google Scholar]
- 16.Heijink IH, Kies PM, Kauffman HF, et al. Down-regulation of E-cadherin in human bronchial epithelial cells leads to epidermal growth factor receptor-dependent Th2 cell-promoting activity. J Immunol. 2007;178(12):7678–7685. doi: 10.4049/jimmunol.178.12.7678. [DOI] [PubMed] [Google Scholar]
- 17.Tunggal JA, Helfrich I, Schmitz A, et al. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005;24(6):1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuse M, Itoh M, Hirase T, et al. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127(6):1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arican RY, Sahin Z, Ustunel I, et al. Effects of formaldehyde inhalation on the junctional proteins of nasal respiratory mucosa of rats. Exp Toxicol Pathol. 2009;61(4):297–305. doi: 10.1016/j.etp.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Cao X, Lin H, Muskhelishvili L, et al. Tight junction disruption by cadmium in an in vitro human airway tissue model. Respir Res. 2015;16(1):30. doi: 10.1186/s12931-015-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, Gu C, Wang Y. Upregulation of the tight junction protein occludin:effects on ventilation-induced lung injury and mechanisms of action. BMC Pulm Med. 2014;14:94. doi: 10.1186/1471-2466-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen F, Hori T, Ohashi N, et al. Occludin is regulated by epidermal growth factor receptor activation in brain endothelial cells and brains of mice with acute liver failure. Hepatology. 2011;53(4):1294–1305. doi: 10.1002/hep.24161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeyama K, Dabbagh K, Lee HM, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999;96(6):3081–3086. doi: 10.1073/pnas.96.6.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young HW, Williams OW, Chandra D, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5' elements. Am J Respir Cell Mol Biol. 2007;37(3):273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


