Advances
-
•
All sequenced genomes of land plants and their most closely related algal ancestors Charophytes encode Receptor Kinases of the CrRLK1L family (17 members in A. thaliana), characterized by the presence of two malectin-like structures in their ectodomains.
-
•
All CrRLK1L family members studied so far are involved in controlling wall rheology of growing cells and at least one (FER) is required for normal cellular responses to mechano-stimulation.
-
•
CrRLK1L are part of multiprotein complexes which can contain several CrRLK1Ls, GPI-anchored co-receptors and other cell wall bound proteins (Leucine Rich Repeat Extensins).
-
•
At least one CrRLK1L (FER), as with yeast CWI sensing, relies on Rho GTPase signalling, which can control ROS production through the activation of plasma membrane NADPH-oxidase and the orientation of cellulose microfibrils through the reordering of microtubules.
-
•
CrRLK1Ls can also trigger Ca2+ transients, control cell wall alkalinisation through the inhibition of the plasma membrane H+-ATPase and require the activity of protein kinases and phosphatases. Gene expression can be regulated by direct sequestration and phosphorylation of transcription factors by the cytosolic kinase domain.
-
•
Several CrRLK1Ls were shown to bind to cell wall components, with a preference for de-methylesterified pectin, but also to Rapid ALkalinisation Factor (RALF) peptides, which are polycationic peptides of around 50 amino acids that are cleaved from polyanionic prodomains by subtilisin proteases.
-
•
Higher plants have large numbers of RALF peptides (36 in A. thaliana), some of which have antagonistic effects.
-
•
Autocrine RALF/CrRLK1L signalling is essential to maintain cell wall integrity and oscillatory growth of pollen tubes.
-
•
Sequestration of polycationic RALFs by polyanionic pectates in the cell wall may provide a mechanism that links free RALF levels to the methylesterification state of cell wall pectins, apoplastic pH and cell wall extensibility. This is reminiscent of the control of TGF-ß1 signaling in animal cells by sequestering latent TGF-ß1 in the ECM.
-
•
CrRLK1L FER also plays a role in plant immunity, by acting as a scaffold for the ligand-induced assembly of immune receptors with their co-receptor BAK1. This scaffold function is negatively regulated by RALF23, the levels of which are in turn controlled by its proteolytic removal from the prodomain.
Introduction
The successful colonization of the terrestrial habitat has required plants to grow in often highly fluctuating environments. Plant growth is turgor driven and in general reflects the ability of the polymer network of the cell wall to expand, ability which is expected to be very sensitive to environmental variation (Cosgrove, 2015, Cosgrove, 2016, Wolf et al., 2012). In this context, maintaining tissue growth requires the constant monitoring and adjusting of cell wall rheology. How cells sense their extracellular matrix (ECM) is intensively investigated in animals (Paluch et al., 2015, Vogel and Sheetz, 2006) and yeast (Levin, 2011) and these studies show the involvement of mechanosensing systems that convert mechanical into biochemical signals. In plants, instead, these processes remain poorly understood (Monshausen and Haswell, 2013). This is now changing rapidly with the study of a family of membrane receptor kinases that play a role in the control of cell wall integrity. This family, with the rather convoluted name «Catharanthus roseus Receptor Like Kinase 1 Like (CrRLK1L)» after its founding member identified in this species, has been extensively reviewed recently (Franck et al., 2018a). This review will focus on recent advances in our understanding of how these receptors monitor the cell wall integrity and how this affects growth, development and immunity. In addition, parallels are made with ECM sensing by yeast and animal cells.
Cell wall integrity signaling in plants
A family of putative cell wall sensors in plants
The CrRLK1L family is present in land plants (e.g. with 17 and 16 members in Arabidopsis thaliana and rice respectively) and their most closely related algal ancestors, the Charophytes (e.g. 1 member in Closterium peracerosum-strigosum–littorale) (Franck et al., 2018a). The genetic analysis of several family members shows their role in maintaining the integrity of the cell wall of growing cells (Table 1). For instance, double loss of function mutants for pollen tube-expressed isoforms ANXUR (ANX)1/2 or BHUDDA’S PAPER SEAL (BUPS)1/2, show increased growth prior to premature bursting of growing cells (Boisson-Dernier et al., 2013, Ge et al., 2017). Overexpression of ANX1 instead, leads to reduced growth with the accumulation of cell wall material at the pollen tube tip (Boisson-Dernier et al., 2013). The ERULUS (ERU) isoform seems to have the opposite role in pollen tubes and root hairs (both tip-growing cells), since in a loss of function mutant, growth is slowed down and, at least in root hairs, cell walls are thicker relative to the WT (Schoenaers et al., 2018, 2017). Another isoform, THESEUS1 (THE1), was identified by mutants and overexpression lines that respectively partially suppress or enhance the growth defect and stress responses of cellulose-deficient mutants without affecting the cellulose content (Denness et al., 2011, Hématy et al., 2007, Merz et al., 2017, Van der Does et al., 2017). In the absence of cell wall defects however, these lines do not show an obvious phenotype. THE1 therefore seems to be part of a mechanism that actively inhibits growth upon cell wall damage. THE1 appears to be partially redundant with HERK1 and HERK2 (Guo et al., 2009). In loss of function mutants of FERONIA (FER), growing root cells fail to recover from salt-induced cell wall softening and burst (Feng et al., 2018). The same Receptor Kinase (RK) is also required for normal mechanosensing as shown by the abnormal cytosolic Ca2+ and extracellular pH increase in response to cell stretching in loss of function mutants (Shih et al., 2014). Finally, a knock down mutant for the single Closterium CrRLK1L family member fails to release its gametes through the rupture of the cell wall of the conjugatory papillae, suggesting a role in the control of cell wall integrity and/or the turgor pressure (Hirano et al., 2015) also in the algal precursors of the land plants.
Table 1.
Overview of Arabidopsis CrRLKL1-related mutant phenotypes.
| Gene | Mutant allele | Mutation | Ecotype | Phenotype | References |
|---|---|---|---|---|---|
| THESEUS1 (At5g54380) | the1-1 | G37D | Col-0 | Suppressed growth defect and stress responses of cellulose deficient mutants | Hématy et al. (2007) |
| Reduced cell wall integrity responses, altered root growth direction | Denness et al., 2011, Van der Does et al., 2017 | ||||
| Altered patterning lateral root primordia | Gonneau et al. (2018) | ||||
| the1-2 | E150K | Col-0 | Suppressed growth defect and stress responses of cellulose deficient mutant | Hématy et al. (2007) | |
| the1-3 | T-DNA in promoter | WS | Idem | Hématy et al. (2007) | |
| the1-4 | T-DNA | Col-0 | Reduced responsiveness to loss of motor kinesin AtKIN13A | Fujikura et al. (2014) | |
| Enhanced response to cellulose deficiency | Merz et al. (2017) | ||||
| Enhanced sensitivity to Botrytis cinerera and altered growth adaptation to heavy metals | Qu et al., 2017, Richter et al., 2017 | ||||
| Altered patterning lateral root primordia | Gonneau et al. (2018) | ||||
| the1-6 | S53stop | Col-0 | Altered growth adaptation to heavy metals | Richter et al. (2017) | |
| Reduced isoxaben-induced stress responses | Merz et al. (2017) | ||||
| Altered patterning lateral root primordia | Gonneau et al. (2018) | ||||
| HERKULES1 (At3g46290) | herk1 | T-DNA | Col-0 | Dwarfism when combined with the1-4 | Guo et al. (2009) |
| Altered growth adaptation to heavy metals | Richter et al. (2017) | ||||
| HERKULES2 (At1g30570) | herk2-1 | T-DNA | Col-0 | Dwarfism when combined with the1-4 | Guo et al. (2009) |
| herk2-2 | T-DNA | Col-0 | Altered growth adaptation to heavy metals | Richter et al. (2017) | |
| FERONIA (At3g51550) | fer-4 | T-DNA insertion in 5‘region | Col-0 | Pollen tube overgrowth in female gametophyte | Escobar-Restrepo et al. (2007) |
| Abnormal response to mechanical stimuli, altered epidermal cell shape | Shih et al., 2014, Li et al., 2015 | ||||
| Hypersensitive to sucrose and starch accumulation | Yang et al. (2015) | ||||
| More resistant to Fusarium oxysporum | Masachis et al. (2016) | ||||
| Hypersensitive to salt, cold and heat stress and hyposensitive to osmotic stress | Chen at al. (2016) | ||||
| Reduced response to PAMPs elf18 and flg22, more susceptible to Pseudomonas syringae | Stegmann et al. (2017) | ||||
| Altered growth adaptation to heavy metals | Richter et al. (2017) | ||||
| Root cells explode during growth recovery under salinity stress | Feng et al. (2018) | ||||
| fer-2 | T-DNA insertion | Col-0 | Reduced response to PAMPs elf18 and flg22, more susceptible to Pseudomonas syringae | Stegmann et al. (2017) | |
| fer | Ds-transposon | Ler | Disrupted Calcium crosstalk during pollen tube reception | Ngo et al. (2014) | |
| ANXUR1 (At3g04690) | anx1-2 | T-DNA | Col-0 | Enhanced response to flg22 and resistance to Pseudomonas syringae | Mang et al. (2017) |
| aggie101 | A358V | Col-0 | Enhanced ETI / PTI responses, resistance to bacterial pathogens, no pollen tube growth defect | Mang et al. (2017) | |
| ANXUR2 (At5g28680) | anx2-2 | T-DNA | Col-0 | Enhanced response to flg22 and resistance to Pseudomonas syringae | Mang et al. (2017) |
| anx1-2 anx2-2 | Premature pollen tube bursting | Boisson-Dernier et al. (2009) | |||
| ERULUS (At5g61350) | eru | T-DNA | Col-0 | Reduced root hair growth and accumulation of de-esterified homogalacturonan | Schoenaers et al. (2018) |
| BUPS1 (At4g39110) | bups1-1 to bups1-5 | CRISPR/Cas9 | Col-0 | Premature bursting of pollen tubes when combined with bups2 | Ge et al. (2017) |
| bups1-T-1; bups1-T-2 | T-DNA | Col-0 | Idem | Ge et al. (2017) | |
| BUPS2 (At2g21480) | bups2-1 to bups2-5 | CRISPR/Cas9 | Col-0 | Premature bursting of pollen tubes when combined with bups1 | Ge et al. (2017) |
| RALF4 (At1g28270) | ralf4-1 to ralf4-4 | CRISPR/Cas9 | Col-0 | Premature bursting of pollen tubes when combined with ralf19 | Ge et al. (2017) |
| ralf4-1 | T-DNA | Col-0 | 50% of pollen tubes burst prematurely | Mecchia et al. (2017) | |
| RALF19 (At2g33775) | ralf19-1 to ralf19-4 | CRISPR/Cas9 | Col-0 | remature bursting of pollen tubes when combined with ralf4 | Ge et al. (2017) |
| RALF23 (At3g16570) | ralf23-3 | T-DNA | Col-0 | Enhanced response to flg22 and elf18, Pseudomonas syringae resistance | Stegmann et al. (2017) |
| RALF34 (At5g67070) | ralf34-1 | T-DNA | Col-0 | Altered patterning lateral root primordia | Murphy et al. (2016) |
| ralf34-2 | T-DNA | Ler | Idem | Murphy et al. (2016) | |
| RALF1 (At1g02900) | ralf1 | T-DNA | Col-0 | Increased root length | Haruta et al. (2014) |
| S1P (At5g19660) | s1p-3 | T-DNA | Col-0 | Enhanced response to PAMPs elf18 and flg22, more susceptible to Pseudomonas syringae | Stegmann et al. (2017) |
| s1p-6 | P612S | Col-0 | Idem | Stegmann et al. (2017) | |
| LRX8 (At3g19020), LRX9 (At1g49490), LRX10 (At2g15880) | lrx8 lrx9 lrx10 | T-DNA | Col-0 | Abnormal pollentubes with reduced gemination | Sede et al. (2018), Mecchia et al. (2017) |
| LRX1 (At1g12040), LRX2 (At1g62440) | lrx1 lrx2 | Ds-transposon | Col-0 | Root hair cell wall and growth defect | Baumberger et al. (2001) |
| LRX3 (At4g13340), LRX4 (At3g24480), LRX5 (At4g18670) | lrx3 lrx4 lrx5 | T-DNA | Col-0 | Dwarfism | Draeger et al. (2015) |
| LORELEI (At4g26466) | lre-1, lre-2 | Gamma-ray deletion | Col-0 | Pollen tube overgrowth in female gametophyte | Capron et al. (2008) |
| lre-3, lre-4 | T-DNA | Col-0 | Idem | Capron et al. (2008) | |
| LLG1 (At5g56170) | llg1-1,llg1-2 | T-DNA | Col-0 | Reduced growth, root hair and trichome defects | Li et al. (2015) |
| CML38 (At1g76650) | cml38-1, cml38-2 | T-DNA | Col-0 | Longer roots | Campos et al. (2018) |
| MARIS (At2g41970) | mri-2 | T-DNA | Col-0 | Spontaneous pollen tube and root hair bursting | Boisson-Dernier et al. (2015) |
| ATUNIS1 (At3g0558), ATUNIS2 (At5g27840) | aun1-1,aun2-1 | T-DNA | Col-0 | Reduced pollen tube and root hair growth | Franck et al. (2018) |
Col-0, Columbia-0; Ler, Landsberg erecta; Ws, Wassilewskija. EMS: ethyl methanesulfonate; PAMP: pathogen associated molecular pattern; ETI: effector triggered immunity; PTI: PAMP-triggered immunity; elf18: acetylated 18-amino acid fragment of the E. coli elongation factor Tu; flg22: 22 amino-acid peptide derived from the N-terminus of bacterial flagellin.
Together, these observations suggest: [i] that all CrRLK1L family members studied so far are involved in controlling the cell wall rheology; [ii] that different receptors can have antagonistic effects by either promoting or inhibiting wall relaxation and growth and [iii] that at least one of the receptors (FER), is required for normal cellular responses to mechano-stimulation.
CrRLK1L structure
The most salient feature of CrRLK1L’s is their ectodomain, which carries 2 regions homologous to animal malectin domains and related carbohydrate modules present in bacterial hydrolases (Franck et al., 2018a). The Xenopus malectin domain binds nigerose (α (1 > 3)-di-glucose) on N-linked glycans and plays a role in protein quality control in the ER (Schallus et al., 2010). The 3D structures of the ectodomains of ANX1 and 2 were recently determined at 1.48 and 1.1 Å resolution (Du et al., 2018, Moussu et al., 2018). The two malectin domains, each consisting of 4 antiparallel ß-strands, are tightly packed, with their ß-sandwich cores forming an angle of 85°, and connected by a ß hairpin linker, thus creating a large (30 Å) cleft. Interestingly, however, despite the structural homology with malectin domains, critical residues that form the carbohydrate binding surface area are absent in ANX1/2 and other CrRLK1L members. This suggests that the carbohydrate binding properties differ from those of animal malectin domains.
CrRLK1L ligands
Pectin
Despite the lack of the canonical carbohydrate binding sites, plasmolysis experiments in root cells showed that GFP-tagged THE1 and FER are tightly bound to the cell wall (Hématy et al., 2007, Li et al., 2018). The exact nature of the cell wall-association is not known, but heterologously produced ectodomains of FER, ANX1 and BUPS1 showed in vitro binding to pectin, with (at least for FER) a preference for de-methylesterified polygalacturonic acid relative to more highly methylesterified pectin (Feng et al., 2018, Li et al., 2018). Pectin binding also may be important for signaling as suggested by the reduced FER signaling (as measured by the activity of FER target ROP6, see below) upon in vivo reduction of the amount of de-methylesterified pectin (Li et al., 2018).
Peptides
Interestingly, several CrRLK1Ls were shown to be receptors for so-called Rapid Alkalinisation Factors (RALFs), which were named after a peptide identified in supernatants of tobacco cells, which promotes the alkalinisation of the extracellular medium (Pearce et al., 2010). The 49 amino acid highly basic active tobacco peptide is cleaved from a highly acidic prodomain by a subtilisin protease and contains 4 disulfide bond-forming cysteines. A. thaliana has 36 RALF family members (Campbell and Turner, 2017). Ge et al. showed that ANX1/2 and BUPS1/2 are receptors for pollen tube-expressed peptides RALF4 and RALF19 (Fig. 1) (Ge et al., 2017). Interestingly, the double ralf4/19 mutant shows the same pollen tube bursting phenotype as anx1/2 and bups1/2, indicating that autocrine signaling by these peptides, via ANX1-2/BUPS1-2, is essential for maintaining cell wall strength in these tip-growing cells (Ge et al., 2017). They also showed that the female-derived peptide, RALF34, competes with RALF4/19 for binding to ANX1/2 and BUPS1/2 and promotes pollen tube bursting in vitro. They proposed that RALF34 is the spatial paracrine signal from the female gametophyte that triggers pollen tube rupture and release of the sperm cells, by interfering with the autocrine cell wall integrity maintenance system (Ge et al., 2017). This scenario awaits genetic confirmation since pollen tube bursting and sperm release still occur normally in ralf34 mutants and it is possible that RALF34 is redundant with other ovule-expressed RALF peptides. Interestingly, RALF34 also has a signaling role in other cells, where it acts as a ligand for THE1, but again ralf34 mutants do not phenocopy all the aspects of the1 mutants, which also may be due to redundancy with other RALFs (Gonneau et al., 2018). Finally, RALF1 and RALF23 are both ligands for the receptor FER (Haruta et al., 2014, Stegmann et al., 2017). FER is expressed in most vegetative tissues including the female gametophyte, but not in the pollen tube. Intriguingly, in fer mutants the pollen tube fails to burst upon arrival at the female gametophyte and continues to grow inside the ovule (Escobar-Restrepo et al., 2007). This suggests that FER may act upstream of RALF34 in the triggering of pollen tube bursting, but the exact molecular link remains to be determined.
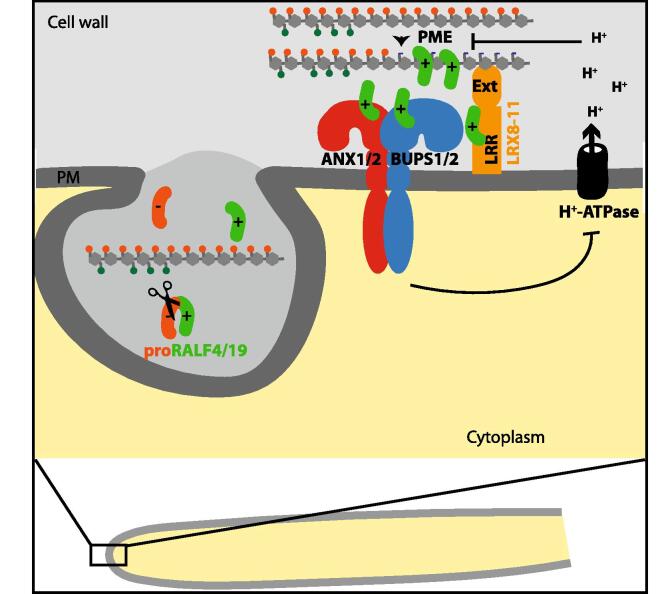
Fig. 1.
Hypothetical mechanism for cell wall integrity control in growing pollen tubes through CrRLK1L-dependent autocrine signaling. RALF4/19 are polycationic mature peptides that are released from a polyanionic prodomain by a subtilisin protease presumably in a secretory compartment of the pollen tube. Due to their polycationic nature, these peptides are expected to be sequestered by de-methylesterified anionic pectin domains. High PME activity in an alkaline environment would thus reduce the amount of peptide available for binding to the BUPS1/2-ANX1/2 receptor complex leading to the de-repression of the H+ ATPase and cell wall acidification, which in turn would inhibit PME activity. Secretion of more methylesterified pectin and RALF peptide would again activate the receptor complex and promote wall alkalinisation. This provides a hypothetical mechanism for the observed oscillations in growth rate, cell wall pH, cytosolic Ca2+, ROS and de-methylesterified pectin in pollen tubes. RALF4/19 also bind the LRR domain of pollen-expressed LRX8, LRX9, LRX10 and LRX11, which are tightly anchored to the cell wall through their extensin-like domain. RALF4/19-LRX sequestering could also contribute to the control of free RALF concentrations in the apoplast. Alternatively, LRX proteins could be an integral part of the CrRLK1L signaling complex. Abbreviations: ANX1/2, ANXUR1 and 2; BUPS1/2, BUDDHA’S PAPER SEAL1 and 2; LRX8/9/10/11, LEUCINE RICH REPEAT (LRR)-EXTENSIN (Ext) GLYCOPROTEIN8, 9, 10 and 11; PM, plasma membrane; PME, PECTIN METHYLESTERASE; RALF4/19, RAPID ALKALINIZATION FACTOR4 and19.
CrRLKL1 signaling complex
CrRLK1Ls are part of multiprotein complexes, as already shown in pollen tubes where binding studies show that ANX- and BUPS-type CrRLK1Ls (both represented by redundant protein pairs) bind each other (Ge et al., 2017). In addition, they both bind to RALF4/19 and are required for normal growth and RALF4/19 responsiveness, indicating that the signaling complex consists of heteromers of at least ANX and BUPS (Ge et al., 2017).
FER also forms a complex with glycosylphosphatidylinositol-anchored proteins (GPI-AP), which can differ in different cell types (e.g. LORELEI (LRE) in ovules and LRE-LIKE GPI-AP (LLG1) in vegetative tissues, 4 isoforms in A. thaliana) (Capron et al., 2008, Li et al., 2015). LRE and LLG1 are essential for FER signaling since the corresponding loss of function mutants show exactly the same phenotypes as fer in the respective tissues. GFP-tagged FER is retained in the ER in llg1 mutants, suggesting a role for LLG1 as a chaperone (Li et al., 2015). It is not known whether other CrRLK1Ls also interact with such GPI-anchored proteins.
Leucine-rich repeat extensins (LRXs) are extracellular proteins with an N-terminal leucine-rich repeat (LRR) domain and a C-terminal extensin domain that anchors to the cell wall presumably through covalent cross-links (Draeger et al., 2015, Ringli, 2010). A link with CrRLKL1 signaling was shown by the inability of RALF4 to repress pollen tube growth in triple lrx mutants (lrx8-10, lrx9-11) and the demonstration that RALF4 binds to the LRR domain of at least one LRX (LRX8), although with a lower affinity (KD > 10 µM) than for ANX1/2 or BUPS1/2 ectodomains (1 µM KD range) (Fig. 1) (Mecchia et al., 2017). LRXs also come in different flavors in different cell types, with specific isoforms in root hairs (LRX1-2), pollen tubes (LRX8-11) (Sede et al., 2018) and aerial organs (LRX3-5) (Baumberger et al., 2003). It is intriguing that RALF4 (and perhaps other RALFs) can bind to two totally unrelated classes of proteins. The determination of the 3D structure of the LRX4-RALF4 complex will reveal whether it shares structural determinants with CrRLKs ANX/BUPs or whether the different protein partners bind to different parts of the same peptide.
Interestingly, RALF1 was also shown to bind to CML38, a cell wall calmodulin-like protein (Campos et al., 2018). In a cml38 mutant, RALF1 does not bind to the cell wall and fails to inhibit root growth. RALF1 binding to CML38 only occurs in the presence of Ca2+ and at a low pH (Campos et al., 2018). Finally, given their poly-cationic nature (iso-electric points range from 7.8 to 10.7) (Campbell and Turner, 2017), mature RALF peptides are expected to show electrostatic interactions with poly-anionic de-methylesterified pectin. Potential implications of the multitude of binding partners of RALF peptides will be discussed below.
CrRLKL1 output
To understand the role of CrRLK1L signaling in the maintenance of cell wall integrity of growing cells, it is important to take into account our current understanding of the mechanism of plant cell expansion. The walls of growing plant cells consist of a hydrated gel of cross-linked polymers (Lampugnani et al., 2018). Cellulose microfibrils are the main loadbearing components. The orientation of the microfibrils is determined by the orientation of the cortical microtubules, which guide the movement of the cellulose synthase complexes in the plasma membrane (Paredez et al., 2006). Cortical microtubules themselves orient along the stress patterns in the tissue through a so far unknown mechano-responsive mechanism depending on microtubule severing by katanin (Uyttewaal et al., 2012). Together, this creates a feedback loop that reinforces the cell walls along the stress patterns. Structural proteins and the matrix polysaccharides hemicellulose and pectin are synthesized in the Golgi apparatus and secreted into the apoplast, where they form extensive crosslinks (Lampugnani et al., 2018). Cellulose microfibrils are cross-linked at discrete positions (so-called “biomechanical hotspots”) by xyloglucan, where they appear to act as a double-sided glue (Park and Cosgrove, 2015). The cell wall protein expansin is thought to remove these crosslinks, thus promoting irreversible expansion of the cell wall (Park and Cosgrove, 2015). Pectins are also connected to cellulose through the side chains of the block polymer rhamnogalacturonan (RG)-I (Ralet et al., 2016, Zykwinska et al., 2005). Pectins are important regulators of cell wall rheology since they have a strong impact on the charge density of the cell wall matrix. Homogalacturonan (HG), a linear polymer of GalA, is the most abundant pectic component representing around 20% of the primary cell wall mass (Zablackis et al., 1995). HG is secreted in a highly methylesterified form, which can undergo selective de-methylesterification by Pectin Methyl Esterases (PMEs) (Wolf et al., 2009a). The thus exposed carboxyl groups in turn attract hydronium ions and promote local cell wall acidification and hydration. Longer de-methylesterified stretches (of ∼10 GalA residues) can form cooperative Ca2+ cross-links, referred to as “egg boxes”, which contribute to wall stiffening (Cabrera et al., 2008). Another pectic polymer, RGII, forms borate diester crosslinks, which are also essential for the integrity of the cell wall (Funakawa and Miwa, 2015). Finally, oxidative cross-linking between tyrosine residues of extensins or, in grasses, between arabinoxylan-linked ferulic acid residues also contributes to cell wall strength (Held et al., 2004, Lindsay and Fry, 2008).
Given the gel-like nature of the cell wall it can be expected that environmental factors, such as temperature, water availability, cation concentration, pH and mechanical stresses, can profoundly affect its rheological properties, and that, in order to maintain growth, the cell wall composition needs to be constantly adapted through the insertion of new polymers but also through the in situ enzymatic modification of the polymers (Voxeur and Hofte, 2016). The cell wall pH is an important regulator of this process. A decrease in pH promotes cell wall compliance in particular through the removal of cellulose-xyloglucan cross links by expansin, which has a low pH optimum (Cosgrove, 2015, Cosgrove, 2016). Many cell wall hydrolases and transglycosylases also have an acidic pH optimum but, so far, their contribution to the control of cell wall rheology has yet to be clarified. Increasing the cell wall pH promotes PME activity (Wolf et al., 2009a). This in turn increases the negative charge density of the wall, which might contribute to the re-acidification of the cell wall. Pectin de-methylesterification can also promote wall stiffening through crosslinking depending on the Ca2+ concentration in the cell wall (Cabrera et al., 2008).
All RALF peptides tested so far promote surface alkalinisation, when applied externally (Morato do Canto et al., 2014). This is likely the result of the inhibition of the plasma membrane proton pump, since RALF1 was shown to induce, within 5 min, the phosphorylation of H+-ATPase AHA2 (Haruta et al., 2014). RALF1 and RALF34 were also shown to induce, within a minute, a transient increase in cytosolic Ca2+ in A. thaliana seedlings (Gonneau et al., 2018, Haruta et al., 2014). Oscillations in cytosolic Ca2+ levels occur simultaneously in the growing pollen tube and the receiving synergid cells of the ovule and these oscillations are perturbed in a fer-4 mutant suggesting a role for this receptor in this crosstalk (Ngo et al., 2014).
RALF1 also induces, within 30 min, changes in gene expression (Cabrera et al., 2008). Downstream signaling components include, at least in tip growing cells, RECEPTOR LIKE CYTOPLASMIC KINASE (RLCK) MARIS (MRI) (Boisson-Dernier et al., 2015) and TYPE ONE PROTEIN PHOSPHATASES (TOPP) ATUNIS (AUN)1/2 (Franck et al., 2018b), respectively as positive and negative regulators of the ANX1/2-RALF4/19 pathway in pollen tubes and of the FER pathway in root hairs. Finally, the phosphatase ABI2 opposes the effect of RALF peptides by binding to and dephosphorylating FER (Yu et al., 2012). ABA in turn inhibits ABI2 upon binding to its PYR/PYL/RCAR receptor.
Rho1 Of Plants (ROP) proteins play also a critical role in CrRLK1L signaling. Indeed, FER and THE1 were shown to bind to ROPGEFs, which catalyse the exchange of GDP by GTP in ROPs (Duan et al., 2010, Qu et al., 2017). A. thaliana has 14 ROPGEF and 11 ROP proteins, which act on a variety of downstream effectors with different targets (Feiguelman et al., 2017). For instance, FER is required for auxin-induced ROS production in the root, possibly through GTP-ROP2-mediated activation of the plasma membrane NADPH-oxidase RbohD (Duan et al., 2010) and FER-dependent activation of ROP11 promotes ABI2 activity (Chen et al., 2016). Interestingly, ROP signaling was also shown to be involved in symmetry breaking and the formation of membrane micro-domains in a number of cases (Feiguelman et al., 2017, Yang and Lavagi, 2012). This is illustrated by the generation of the puzzle-shaped epidermal pavement cells of A. thaliana leaves (Chen et al., 2015). The interdigitations are the result of growth promotion at the lobe and growth restriction at the indenting region, through the local activation of ROP2 and ROP6, respectively. ROP2 binds its effector RIC4, which promotes actin nucleation and outgrowth, possibly by targeting secretory vesicles to the growing lobe. ROP6, instead, binds RIC1, which binds katanin and promotes its microtubule severing activity (Lin et al., 2013). This favors the reorientation of microtubules perpendicular to the indentation and growth restriction. ROP2/RIC4 inhibits ROP6/RIC1 and vice versa and this mutual inhibition is part of a self-organizing mechanism underlying the subcellular spatial separation of ROP2 and ROP6 activity domains (Chen et al., 2015). Interestingly, a recent study showed that FER binds to ROPGEF14 in pavement cells and activates ROP6 upon binding to de-methylesterified pectin (Li et al., 2018). Given the fact that FER activation also promotes cell wall alkalinisation, which in turn promotes pectin methylesterase activity (PMEs have a high pH optimum), this raises the interesting possibility that FER is part of the self-reinforcing activation loop of a Turing’s reaction-diffusion scheme (Turing, 1990) underlying the emergence of the ROP2-ROP6 activity domains. It will be interesting to see whether RALF peptides and/or mechanosensing also play a role in this process.
CrRLK1Ls and homeostasis of cell wall rheology
A recent study showed that FER is essential for the adaptation of the wall rheology to high salt concentrations (Feng et al., 2018). Exposure of roots to high salt induces cell wall softening, presumably because monovalent cations interfere with Ca2+ crosslink formation. Normal wall stiffness is re-established after some 5 h in wild type roots. This process is associated with a high frequency of cytosolic Ca2+ transients in recovering cells. Interestingly, in fer loss of function mutants, root cells do not show such an increase in Ca2+ transients, fail to re-establish normal wall stiffness and eventually burst. This fer cell bursting phenotype is mimicked in mur1 mutants (in which cell walls are weakened due to a reduced number of RG-II-borate diester cross links) and cell bursting in fer can be prevented by supplementing Ca2+ and borate to the medium, which are thought to reinforce the cell wall. How FER triggers recovery of cell wall stiffness is not understood, but it may involve FER-mediated cell wall alkalinisation and PME activity and/or ROS-induced oxidative crosslinking of cell wall polymers. In a similar way, hypocotyls of the1 mutants show an altered growth adaptation to the presence of heavy metals, some of which are also thought to interfere with Ca2+-pectate crosslinking (Richter et al., 2017).
The study of tip-growing root hairs and pollen tubes showed that FER and other CrRLK1Ls also have a role in normal cell growth control. Both root hairs and pollen tubes show oscillations in growth rate with the same period (pollen tubes ≥ 25 s, root hairs ∼1 min) as cytosolic Ca2+ levels, cell wall deposition, surface pH and ROS levels, but with a different phase (Bascom et al., 2018). The analysis of the phase relationship in root hairs shows that growth slows down or accelerates with increasing or decreasing surface pH and ROS levels respectively. In addition, artificially lowering or increasing surface pH or ROS levels leads to root hair bursting or growth inhibition respectively. In fer and llg1 mutants, root hairs burst prematurely, consistent with a role for the FER/LLG1 signaling module in the alkalization and/or ROS production at the cell surface (Duan et al., 2010, Li et al., 2015). The CrRLK1L ERU is specifically expressed in tip-growing cells and seems to act antagonistically to FER since loss-of-function mutant root hairs show reduced growth rate, thicker cell walls and high PME activity (Schoenaers et al., 2018). Growth rate and cell wall thickness also oscillate in eru but with a reduced frequency and a much larger amplitude, confirming observations in pollen tubes showing that cell wall secretion precedes the increase in growth rate and the extent of the cell wall deposition predicts the magnitude of the subsequent growth response (Bascom et al., 2018). The eru root hair phenotype is rescued in the presence of a PME inhibitor suggesting that uncontrolled PME activity (in principle associated with high surface pH) and premature Ca2+ eggbox formation may explain the growth inhibition. Increased phosphorylation of the AHA1/2 proton pump suggests a perturbation of the cell wall pH in eru (Schoenaers et al., 2018). So far, no RALF ligand for ERU has been identified. Finally, FER also plays a role in the intra-organ coordination of the expansion of diffusely growing cells as shown by the chaotic cell expansion pattern in fer roots and hypocotyls (Bastien et al., 2016, Shih et al., 2014).
Dual function for CrRLK1Ls in growth/development and immunity
FER also can act as a scaffold for immune signalling (Fig. 2). Indeed, in fer mutants, the ligand-induced association between membrane-bound immune receptors (such as the flagellin 22 receptor FLS2) and their co-receptor BAK1 is reduced, which compromises Pathogen Associated Molecular Pattern (PAMP)-triggered immune (PTI)-signaling (Stegmann et al., 2017). It is conceivable that FER organizes PTI signaling nanoclusters in the plasma membrane (Bucherl et al., 2017). The FER ligand RALF23 prevents, like the absence of FER, FLS2-BAK1 interaction and inhibits immune signaling, indicating that the availability of RALF23 in the apoplast negatively regulates the scaffold function of FER. This availability is in part regulated by the cleavage of the propeptide by the subtilisin protease S1P, presumably in an intracellular compartment. The S1P activity in turn is upregulated as part of the PTI response (Stegmann et al., 2017). Together, this constitutes a negative feedback loop, which is expected to contribute to keeping the immune signaling response in check. The RALF-induced cytosolic Ca2+ transients and the alkalinisation of the cell surface (at least those induced by FER ligand RALF1) do not require BAK1 and therefore do not seem to depend on the scaffold function of FER (Dressano et al., 2017). The emerging picture from these studies is that FER has a dual role as a scaffold for immune signaling and as a platform for the control of the homeostasis of the cell wall rheology in growing cells and that RALF binding controls the switch between these two functions. Interestingly, the fungal pathogen Fusarium oxysporum produces an effector that mimics the effect of RALF peptides and thus manipulates plant immunity (Masachis et al., 2016). Finally, the RLKs ANX1 and ANX2, besides their role in pollen tube growth, also negatively regulate PTI and effector-triggered immunity in Arabidopsis leaves (Mang et al., 2017).
Fig. 2.
Dual role for FERONIA (FER) in immunity and growth control. (Left panel): FER in cooperation with LLG1 serves as a scaffold for the interaction of pathogen-associated molecular pattern (PAMP)-Recognition Receptors (PRR) with their coreceptor BAK1. This is exemplified here by flg22-induced FLS2-BAK1 association, which triggers immune signaling including the promotion of ROS production by RboHD. In addition, the cytoplasmic domain of FER binds to a ROP-GEF, which promotes the GDP to GTP exchange on ROP2. GTP-ROP2 binds to RboHD and activates ROS production. flg22 activation of FLS2-BAK1 also promotes the processing of proRALF23 by the subtilisin protease S1P in a secretory compartment. Mature RALF23 binds to FER-LLG1 and thus promotes the FLS2-BAK1 disassembly. This constitutes a negative feedback loop contributing to keeping PAMP-triggered immune signaling in check. (Right panel): RALF23 binding to FER-LLG1 also triggers cytosolic Ca2+ transients, the inhibition of the AHA1/2 proton pump and the katanin-dependent promotion of microtubule reordering through the ROP-GEF activation of ROP6. The FER ectodomain also binds to de-methylesterified pectin domains (methyl and acetyl groups are represented by red and green dots), which are generated by PME at high pH. This may also contribute to regulating FER signaling. Abbreviations: AHA1/2, ARABIDOPSIS H+ -ATPASE1 and 2; BAK1, BRASSINOSTEROID INSENSITIVE1 ASSOCIATED KINASE 1; flg22, flagellin epitope 22; FLS2, FLAGELLIN SENSING2, GEF, GUANINE NUCLEOTIDE EXCHANGE FACTOR; LLG1, LORELEI-LIKE CPI-ANCHORED PROTEIN1; PM, plasma membrane; PME, PECTIN METHYLESTERASE; RALF23, RAPID ALKALINIZATION FACTOR 23; RbohD; respiratory burst oxidase homolog D; ROS: reactive oxygen species; ROP2/6, RHO-GTPASE OF PLANTS2 and 6; S1P, SITE-1 PROTEASE.
How do CrRLK1Ls detect cell wall changes?
A major unanswered question concerns the molecular mechanism of cell wall integrity sensing. So far the following features of the system have been identified:
-
(1)
It involves complexes of CrRLK1L receptor kinases, GPI-anchored glycoproteins and intracellular signaling components including protein kinases, phosphatases and ROPGEFs/ROPs, which are connected to the cell wall through pectin- and/or RALF-binding.
-
(2)
At least one CrRLK1L (FER) is required for normal mechano-sensing.
-
(3)
Autocrine RALF peptide ligands are essential for the receptor function in growth regulation (at least for ANX1/2 and BUPS1/2).
-
(4)
RALFs are produced as propeptides consisting of a highly acidic prodomain and a highly basic mature peptide. The two domains are proteolytically separated by subtilisin proteases. After removal of the neutralizing prodomain, mature peptides are prone to electrostatic interactions with negatively charged pectin in addition to binding to CrRLK1Ls and cell wall-bound LRXs.
-
(5)
Different RALFs and CrRLK1Ls can have antagonistic effects on cell wall strength and growth.
-
(6)
CrRLKLs can influence wall rheology through pH, ROS, ROP-mediated orientation of cortical microtubules, and potentially actin polymerization and secretion of wall polymers.
To gain more insight into possible mechanisms, it is interesting to briefly review what is known about feedback signaling from the extracellular matrix in fungi or animal cells.
Yeast cell wall integrity sensing by mechanosensors
Fungi have, like plant cells, strong but elastic walls, the synthesis and remodelling of which needs to be precisely coordinated. Genetic analysis has identified components of a cell wall integrity signaling network, which plays a critical role in the response to abiotic stresses (heat, osmotic stress) and chemicals that induce wall stress but also in pheromone-induced formation of a «shmoo» or mating projection, a polar outgrowth, of the cell, which also depends on cell wall synthesis and remodelling (Fig. 3A) (Kock et al., 2015, Levin, 2011). The stress signals are transmitted to Rho1 GTPase, which controls, through a variety of effectors, the synthesis and polarized delivery of cell wall polymers to sites of cell wall remodeling. Five membrane spanning glycoproteins (Wsc1-3, Mid1 and Mtl1) sense cell surface stress. These sensors consist of a cytoplasmic domain that binds the Rho1 GDP/GTP exchange factor Rom2; a membrane-spanning domain; a highly mannosylated Ser/Thr rich (STR) region of varying length and an N-terminal headgroup. The N-term domain contains 8 disulfide bond-forming cysteines in Wsc1-3, or an essential glycosylated asparagine in Mid2 and Mtl1. AFM studies on single molecules (Kock et al., 2015) showed that the STR region behaves like a Hookean spring, which stretches or contracts with the deformation of the cell wall. It is not understood how stretching of the STR region promotes Rom2 activation, but it presumably involves the deformation of the plasma membrane since cell wall integrity signaling can also be triggered by chemical perturbation of the plasma membrane (Kock et al., 2015). Interestingly, a tagged version of the Wsc1 sensor forms clusters, the size of which increases upon heat or osmotic stress. The ability to cluster requires the disulfide bonds in the headgroup. The mechanism of the cell wall stress-induced clustering and to what extent this affects downstream signaling remains to be determined (Kock et al., 2015). Interestingly, force-induced formation of adhesion nanodomains has been observed using single molecule AFM experiments on a covalently anchored cell wall protein (Als5p) in Candida albicans (Alsteens et al., 2010). Even more strikingly, the clustering spontaneously propagated across the entire cell surface, even after killing the cells. The authors hypothesize that the exposure of hydrophobic domains by stretching the protein promotes self-association and amyloid-like propagation of the clusters to neighboring proteins (Alsteens et al., 2010). In this way, force-induced cluster formation of adhesion molecules is a plausible mechanism governing the adhesion strength of C. albicans cells.
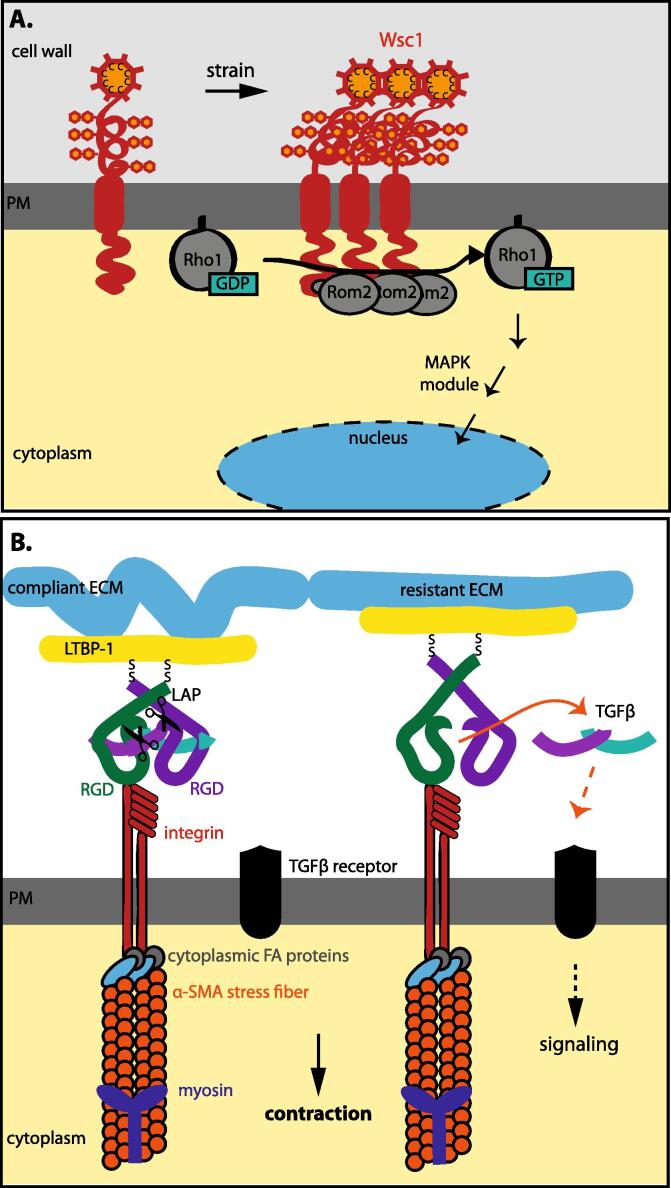
Fig. 3.
Sensing of the cell wall/extracellular matrix (ECM) in yeast and animal cells. A. Wsc1 is a cell wall integrity (CWI) sensor in yeast. Features shared by all five known CWI sensors are the presence of a head group, which is a cysteine-rich domain for Wsc1, a highly mannosylated serine/threonine-rich (STR) domain, a transmembrane domain and a short cytosolic tail, which interacts directly with the GDP/GTP exchange factor Rom1/2. The latter activates the small GTPase Rho1. Rho1-GTP then interacts with protein kinase C, which in turn activates a MAPK signalling cascade leading to changes in the expression of genes controlling cell cycle progression and cell wall deposition and remodelling. The STR domain acts a Hookean spring, the elastic stretching of which, as a result of cell wall deformation, leads to the activation of the signalling pathway. Cell wall deformation also promotes the clustering of the Wsc1 sensor, which enhances the signalling capacity of the system (modified from Kock et al., 2015). B. Mechanical activation of latent Transforming Growth Factor (TGF) β in animal fibroblast cells. TGF-ß1 is secreted as a homodimer together with its latency-associated propeptide (LAP), which is cleaved in the trans-Golgi, but remains non-covalently attached also after secretion (Shi et al., 2011). This association keeps TGF-ß1 in a latent state. The LAP domain dimer is, with one end, covalently attached (disulfide bonds) to a 120–160 kDa latent TGF-β-binding protein (LTBP-1 in fibroblasts), which is part of the ECM, and with its opposite end, connected to the actin cytoskeleton, since it associates, through its RGD sequence, with αν integrins. In the presence of a compliant ECM, actin-myosin contraction will not induce a conformation change in the LAP and TGFβ remains latent (left panel). In the presence of a stiff ECM, however, a strain-induced conformational change of the LAP domain will occur, which triggers the release of active TGF-ß1 and te activation of its receptor (adapted from Wipff et al., 2007).
Mechanosensing by molecular stretching in animal cells
Force-induced reinforcement of extracellular matrix (ECM) adhesion sites is also well described in animal tissues, where the adhesion strength is proportional to stiffness of the ECM (Changede and Sheetz, 2017, Lecuit and Yap, 2015, Van Helvert et al., 2018). These adhesion sites are the location of the signaling from externally or internally generated forces. The well-characterized integrin adhesion sites contain some 400 force-dependent differentially bound proteins (Schiller and Fässler, 2013). Integrins are transmembrane proteins with ectodomains that bind the tripeptide RGD in ECM proteins and cytosolic domains that bind a variety of proteins, in particular the mechanosensor talin (Van Helvert et al., 2018). Upon ligand binding, integrins form 100 nm clusters that recruit FHOD1 formin, which promotes the polymerisation of actin attached to the talin head. On a stiff ECM, mechanical stretching of the actin fibres promotes their association with bipolar myosin II, which probes the stiffness of the substrate by generating traction on the cluster. In response to this force, talin, which makes the connection between integrin and actin fibres, stretches thus exposing cryptic binding sites for vinculin, the binding of which reinforces the adhesions (Schiller and Fässler, 2013). Other signaling proteins are also force activated, including transcription factors that shuttle to the nucleus to regulate transcription. Sustained force leads to the maturation of strong focal adhesions and the formation of actin stress fibres. In contrast, on a soft substrate, where no force can be generated, adhesions fail to mature and integrins are endocytosed (Van Helvert et al., 2018).
An interesting example of a mechanosensing mechanism outside the cell is the control of TGF-ß1 growth factor activity as a function of ECM stiffness in fibroblast cells (Fig. 3B) (Hinz, 2015, Van Helvert et al., 2018). TGF-ß1 is secreted as a homodimer together with its latency-associated propeptide (LAP), which is cleaved in the trans-Golgi, but remains non-covalently attached also after secretion (Shi et al., 2011). This association keeps TGF-ß1 in a latent state. The LAP domain dimer is, with one end, covalently attached (disulfide bonds) to a 120–160 kDa latent TGF-β-binding protein (LTBP), which is part of the ECM, and with its opposite end, connected to the actin cytoskeleton, since it associates, through its RGD sequence, with αν integrins. The trick is that a strain-induced conformational change of the LAP domain triggers the release of active TGF-ß1. The link with the stiffness of the ECM can now be easily understood since, as long as the ECM network is in a relaxed state, LAP-TGF-ß1 in the large latent complex will not show substantial strain upon cellular traction. However, once the ECM polymers are maximally pre-strained, even a small traction will induce the conformational change required for TGF-ß1 release. TGF-ß1 then binds its receptor, which in turn signals increased cytoskeletal contractility and additional TGF-ß1 release. In conclusion, ECM stiffness controls TGF-ß1 release by contraction and hence restricts the autocrine maintenance of the cellular state to the appropriate mechanical microenvironment (Hinz, 2015).
Conclusions and perspectives
These short overviews show that cell wall/ECM sensing mechanisms in fungi and animal cells share some common features. In both cases they involve the conversion of mechanical signals into biochemical signals through strain-induced reversible conformational changes of sensor proteins. The sensitivity of the system is dependent on the stiffness of the ECM and a wide sensitivity range is obtained through clustering of the sensors.
It remains to be seen whether cell wall integrity sensing in plants also involves strain-induced conformational changes of the CrRLKL1 receptors and/or RALF-sequestering cell wall components. Candidates for the latter could be cell wall-bound LRX proteins (Baumberger et al., 2001, Mecchia et al., 2017) or even loadbearing pectates, which, like cellulose microfibrils, were shown to undergo strain-induced stiffening (Zhang et al., 2017), associated with conformational changes (Boyer, 2016).
An interesting possibility is that sequestering of the positively charged RALFs to de-methylesterified pectins is part of a feedback loop underlying the oscillations in cell wall pH and growth as observed in pollen tubes and root hairs (Bascom et al., 2018). Indeed, secretory organelles contain cell wall matrix polymers, including highly methylesterified HG but also non-charged proRALF and inactive proPME. Interestingly, the prodomains of at least some RALFs and PMEs are removed by the same S1P subtilisin protease (Srivastava et al., 2009, Stegmann et al., 2017, Wolf et al., 2009), presumably in a late secretory compartment. Upon arrival in the cell wall, mature RALF peptides presumably bind to CrRLKL1 receptors and promote cell wall alkalinisation and as a result PME activity. The thus generated negatively charged pectate could sequester hydronium ions but also RALFs, which would contribute to a local decrease in cell wall pH. It is interesting in this context that the binding of RALF34 to THE1 diminished with decreasing pH (Gonneau et al., 2018). The lowering of the pH leads to wall relaxation upon which another round of secretion of pectins and RALFs could again promote wall alkalinisation and so forth. It remains to be shown to what extent strain and the delivery of secretory vesicles are coupled, but it also may involve CrRLKL1 ROP signaling. Finally, the observation that RALF1 activity depends on its co-ligand CML38 (Campos et al., 2018), which only binds to RALF1 at high Ca2+ levels and low pH, might provide a means to integrate information on cell wall pH and extracellular Ca2+ levels, which are critical for pectin cross linking and cell wall viscosity.
Together, these considerations show that exciting times lie ahead for the study of wall integrity signaling in plants.
Outstanding questions
-
•
The structural determinants of the interaction of RALF peptides with CrRLKLs, LLGs and LRXs but also with polyanionic cell wall polymers.
-
•
The diversity of the RALF peptides and to what extent they are redundant or reflect differences in binding properties, including dependence on pH, Ca2+ concentration, and CML38 interaction.
-
•
Possible post-translational modification of RALF peptides, including the regulation of the processing of the propeptides and the dependence of disulfide bonds on the cell wall redox levels.
-
•
The regulation of the cell wall pH by PME activity.
-
•
The elucidation of signalling pathways, including the role of protein kinases, phosphatases and transcription factors.
-
•
The role of CrRLKL-dependent ROP signaling, similar to the activation Rho1-like GTPases by ROM1/2 GEFs in yeast. This includes the identification of the ROP effectors involved in the control of cytoskeleton dynamics and secretory vesicle delivery and the role of ROP GTPases in the self-organisation of subcellular activity domains.
-
•
The connection between strain in the cell wall and RALF-CrRLKL signaling, can mechanical stress affect RALF availability to its receptor?
-
•
The nature of the antagonistic effects of different CrRLKL1 receptors on growth (e.g. ANX vs ERU).
-
•
The dual role of CrRLKL receptors as a scaffold for PTI signaling and the control of cell wall integrity.
Conflict of interest
None.
Acknowledgements
This work was partially supported by the French National Agency for Research (ANR) grants “Pectosign” and “Goodvibrations” to HH. VGD is an Advanced Postdoc Fellow from Swiss National Science Foundation. The IJPB benefits from the support of the LabEx Saclay Plant Sciences-SPS (ANR-10-LABX-0040-SPS).
References
- Alsteens D., Garcia M.C., Lipke P.N., Dufrene Y.F. Force-induced formation and propagation of adhesion nanodomains in living fungal cells. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20744–20749. doi: 10.1073/pnas.1013893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom C.S., Hepler P.K., Bezanilla M. Interplay between ions, the cytoskeleton, and cell wall properties during tip growth. Plant Physiol. 2018;176:28–40. doi: 10.1104/pp.17.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien R., Legland D., Martin M., Fregosi L., Peaucelle A., Douady S., Moulia B., Höfte H. KymoRod: a method for automated kinematic analysis of rod-shaped plant organs. Plant J. 2016;88 doi: 10.1111/tpj.13255. [DOI] [PubMed] [Google Scholar]
- Baumberger N., Doesseger B., Guyot R., Diet A., Parsons R.L., Clark M.A., Simmons M.P., Bedinger P., Goff S.A., Ringli C., Keller B. Whole-genome comparison of leucine-rich repeat extensins in Arabidopsis and rice. A conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant Physiol. 2003;131:1313–1326. doi: 10.1104/pp.102.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N., Ringli C., Keller B. The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis in Arabidopsis thaliana. Genes Dev. 2001;15:1128–1139. doi: 10.1101/gad.200201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A., Franck C.M., Lituiev D.S., Grossniklaus U. Receptor-like cytoplasmic kinase MARIS functions downstream of CrRLK1L-dependent signaling during tip growth. Proc. Natl. Acad. Sci. U.S.A. 2015;112:12211–12216. doi: 10.1073/pnas.1512375112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A., Lituiev D.S., Nestorova A., Franck C.M., Thirugnanarajah S., Grossniklaus U. ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J.S. Enzyme-less growth in chara and terrestrial plants. Front. Plant Sci. 2016;7:866. doi: 10.3389/fpls.2016.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucherl C.A., Jarsch I.K., Schudoma C., Segonzac C., Mbengue M., Robatzek S., MacLean D., Ott T., Zipfel C. Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains. Elife. 2017;6 doi: 10.7554/eLife.25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera J.C., Boland A., Messiaen J., Cambier P., Van Cutsem P. Egg box conformation of oligogalacturonides: the time-dependent stabilization of the elicitor-active conformation increases its biological activity. Glycobiology. 2008;18:473–482. doi: 10.1093/glycob/cwn027. https://doi.org/cwn027[pii]10.1093/glycob/cwn027. [DOI] [PubMed] [Google Scholar]
- Campbell L., Turner S.R. A comprehensive analysis of RALF proteins in green plants suggests there are two distinct functional groups. Front. Plant Sci. 2017;8(37) doi: 10.3389/fpls.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos W.F., Dressano K., Ceciliato P.H.O., Carlos Guerrero-Abad J., Leonir Silva A., Fiori C.S., Do Canto A.M., Bergonci T., Claus L.A.N., Silva-Filho M.C., Moura D.S. Arabidopsis thaliana rapid alkalinization factor 1-mediated root growth inhibition is dependent on calmodulin-like protein 38. J. Biol. Chem. 2018;293:2159–2171. doi: 10.1074/jbc.M117.808881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron A., Gourgues M., Neiva L.S., Faure J.-E., Berger F., Pagnussat G., Krishnan A., Alvarez-Mejia C., Vielle-Calzada J.-P., Lee Y.-R., Liu B., Sundaresan V. Maternal control of male-gamete delivery in arabidopsis involves a putative GPI-anchored protein encoded by the LORELEI gene. Plant Cell Online. 2008;20:3038–3049. doi: 10.1105/tpc.108.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changede R., Sheetz M. Integrin and cadherin clusters: a robust way to organize adhesions for cell mechanics. BioEssays. 2017;39:1–12. doi: 10.1002/bies.201600123. [DOI] [PubMed] [Google Scholar]
- Chen J., Wang F., Zheng S., Xu T., Yang Z. Pavement cells: a model system for non-transcriptional auxin signalling and crosstalks. J. Exp. Bot. 2015;66:4957–4970. doi: 10.1093/jxb/erv266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Yu F., Liu Y., Du C., Li X., Zhu S., Wang X., Lan W., Rodriguez P.L., Liu X., Li D., Chen L., Luan S. FERONIA interacts with ABI2-type phosphatases to facilitate signaling cross-talk between abscisic acid and RALF peptide in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 2016;113:E5519–E5527. doi: 10.1073/pnas.1608449113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D.J. Plant expansins: diversity and interactions with plant cell walls. Curr. Opin. Plant Biol. 2015;25:162–172. doi: 10.1016/j.pbi.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D.J. Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016;67:463–476. doi: 10.1093/jxb/erv511. [DOI] [PubMed] [Google Scholar]
- Denness L., McKenna J.F., Segonzac C., Wormit A., Madhou P., Bennett M., Mansfield J., Zipfel C., Hamann T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 2011;156:1364–1374. doi: 10.1104/pp.111.175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draeger C., Ndinyanka Fabrice T., Gineau E., Mouille G., Kuhn B.M., Moller I., Abdou M.T., Frey B., Pauly M., Bacic A., Ringli C. Arabidopsis leucine-rich repeat extensin (LRX) proteins modify cell wall composition and influence plant growth. BMC Plant Biol. 2015;15:1–11. doi: 10.1186/s12870-015-0548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressano K., Ceciliato P.H.O., Silva A.L., Guerrero-Abad J.C., Bergonci T., Ortiz-Morea F.A., Bürger M., Silva-Filho M.C., Moura D.S. BAK1 is involved in AtRALF1-induced inhibition of root cell expansion. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1007053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S., Qu L.J., Xiao J. Crystal structures of the extracellular domains of the CrRLK1L receptor-like kinases ANXUR1 and ANXUR2. Protein Sci. 2018;27:886–892. doi: 10.1002/pro.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q., Kita D., Li C., Cheung A.Y., Wu H.M. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. U.S.A. 2010;107:17821–17826. doi: 10.1073/pnas.1005366107. https://doi.org/1005366107[pii]10.1073/pnas.1005366107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Restrepo J.M., Huck N., Kessler S., Gagliardini V., Gheyselinck J., Yang W.C., Grossniklaus U. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;80(317):656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- Feiguelman G., Fu Y., Yalovsky S. ROP GTPases structure-function and signaling pathways. Plant Physiol. 2017;176:01415.2017. doi: 10.1104/pp.17.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W., Kita D., Peaucelle A., Cartwright H.N., Doan V., Duan Q., Liu M.C., Maman J., Steinhorst L., Schmitz-Thom I., Yvon R., Kudla J., Wu H.M., Cheung A.Y., Dinneny J.R. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 2018;28:666–675.e5. doi: 10.1016/j.cub.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck C.M., Westermann J., Boisson-Dernier A. Plant malectin-like receptor kinases: from cell wall integrity to immunity and beyond. Annu. Rev. Plant Biol. 2018;69 doi: 10.1146/annurev-arplant-042817-040557. annurev-arplant-042817-040557. [DOI] [PubMed] [Google Scholar]
- Franck C.M., Westermann J., Burssner S., Lenz R., Lituiev D.S., Boisson-Dernier A. The protein phosphatases atunis1 and Atunis2 regulate cell wall integrity in tip-growing cells. Plant Cell. 2018 doi: 10.1105/tpc.18.00284. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikura U., Elsaesser L., Breuninger H., Sanchez-Rodriguez C., Ivakov A., Laux T., Findlay K., Persson S., Lenhard M. Atkinesin-13A modulates cell-wall synthesis and cell expansion in Arabidopsis thaliana via the THESEUS1 pathway. PLoS Genet. 2014;10(9) doi: 10.1371/journal.pgen.1004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakawa H., Miwa K. Synthesis of borate cross-linked rhamnogalacturonan II. Front. Plant Sci. 2015;6:223. doi: 10.3389/fpls.2015.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z., Bergonci T., Zhao Y., Zou Y., Du S., Liu M.C., Luo X., Ruan H., García-Valencia L.E., Zhong S., Hou S., Huang Q., Lai L., Moura D.S., Gu H., Dong J., Wu H.M., Dresselhaus T., Xiao J., Cheung A.Y., Qu L.J. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science. 2017;80(358):1596–1600. doi: 10.1126/science.aao3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonneau M., Desprez T., Martin M., Doblas V.G., Bacete L., Miart F., Sormani R., Hématy K., Renou J., Landrein B., Murphy E., Van De Cotte B., Vernhettes S., De Smet I., Höfte H. Receptor kinase THESEUS1 is a rapid alkalinisation factor 34 receptor in arabidopsis. Curr. Biol. 2018 doi: 10.1016/j.cub.2018.05.075. [DOI] [PubMed] [Google Scholar]
- Guo H., Li L., Ye H., Yu X., Algreen A., Yin Y. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 2009;106:7648–7653. doi: 10.1073/pnas.0812346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M., Sabat G., Stecker K., Minkoff B.B., Sussman M.R. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science. 2014;80(343):408–411. doi: 10.1126/science.1244454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held M.A., Tan L., Kamyab A., Hare M., Shpak E., Kieliszewski M.J. Di-isodityrosine is the intermolecular cross-link of isodityrosine-rich extensin analogs cross-linked in vitro. J. Biol. Chem. 2004;279:55474–55482. doi: 10.1074/jbc.M408396200. [DOI] [PubMed] [Google Scholar]
- Hématy K., Sado P.-E., Van Tuinen A., Rochange S., Desnos T., Balzergue S., Pelletier S., Renou J.-P., Höfte H., Hematy K., Sado P.-E., Van Tuinen A., Rochange S., Desnos T., Balzergue S., Pelletier S., Renou J.-P., Hofte H. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 2007;17:922–931. doi: 10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Hinz B. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol. 2015 doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Hirano N., Marukawa Y., Abe J., Hashiba S., Ichikawa M., Tanabe Y., Ito M., Nishii I., Tsuchikane Y., Sekimoto H. A receptor-like kinase, related with cell wall sensor of higher plants, is required for sexual reproduction in the unicellular charophycean alga, closterium peracerosum-strigosum-littorale complex. Plant Cell Physiol. 2015 doi: 10.1093/pcp/pcv065. [DOI] [PubMed] [Google Scholar]
- Kock C., Dufrene Y.F., Heinisch J.J. Up against the wall: is yeast cell wall integrity ensured by mechanosensing in plasma membrane microdomains? Appl. Environ. Microbiol. 2015;81:806–811. doi: 10.1128/AEM.03273-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani E.R., Khan G.A., Somssich M., Persson S. Building a plant cell wall at a glance. J. Cell Sci. 2018;131:jcs207373. doi: 10.1242/jcs.207373. [DOI] [PubMed] [Google Scholar]
- Lecuit T., Yap A.S. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol. 2015;17:533–539. doi: 10.1038/ncb3136. [DOI] [PubMed] [Google Scholar]
- Levin D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics. 2011;189:1145–1175. doi: 10.1534/genetics.111.128264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Yeh F.L., Cheung A.Y., Duan Q., Kita D., Liu M.C., Maman J., Luu E.J., Wu B.W., Gates L., Jalal M., Kwong A., Carpenter H., Wu H.M. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. Elife. 2015;4 doi: 10.7554/eLife.06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Tang W., Anderson C.T., Yang Z. FERONIA’s sensing of cell wall pectin activates ROP GTPase signaling in 1 Arabidopsis. BioRxiv. 2018;2018:12–13. doi: 10.1093/cid/ciy020/4797620. [DOI] [Google Scholar]
- Lin D., Cao L., Zhou Z., Zhu L., Ehrhardt D., Yang Z., Fu Y. Rho GTPase signaling activates microtubule severing to promote microtubule ordering in arabidopsis. Curr. Biol. 2013 doi: 10.1016/j.cub.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Lindsay S.E., Fry S.C. Control of diferulate formation in dicotyledonous and gramineous cell-suspension cultures. Planta. 2008;227:439–452. doi: 10.1007/s00425-007-0630-z. [DOI] [PubMed] [Google Scholar]
- Mang H., Feng B., Hu Z., Boisson-Dernier A., Franck C., Meng X., Huang Y., Zhou J., Xu G., Wang T., Shan L., He P. Differential regulation of two-tiered plant immunity and sexual reproduction by ANXUR receptor-like kinases. Plant Cell. 2017 doi: 10.1105/tpc.17.00464. tpc.00464.2017. 10.1105/tpc.17.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masachis S., Segorbe D., Turrà D., Leon-Ruiz M., Fürst U., El Ghalid M., Leonard G., López-Berges M.S., Richards T.A., Felix G., Di Pietro A. A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat. Microbiol. 2016;1:16043. doi: 10.1038/nmicrobiol.2016.43. [DOI] [PubMed] [Google Scholar]
- Mecchia M.A., Santos-Fernandez G., Duss N.N., Somoza S.C., Boisson-Dernier A., Gagliardini V., Martínez-Bernardini A., Fabrice T.N., Ringli C., Muschietti J.P., Grossniklaus U. RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science. 2017;80(358):1600–1603. doi: 10.1126/science.aao5467. [DOI] [PubMed] [Google Scholar]
- Merz D., Richter J., Gonneau M., Sanchez-Rodriguez C., Eder T., Sormani R., Martin M., Hématy K., Hofte H., Hauser M.T. T-DNA alleles of the receptor kinase THESEUS1 with opposing effects on well wall integrity signaling. J. Exp. Bot. 2017;68:4583–4593. doi: 10.1093/jxb/erx263. https://doi.org/doi:10.1093/jxb/erx263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen G.B., Haswell E.S. A force of nature: molecular mechanisms of mechanoperception in plants. J. Exp. Bot. 2013;64:4663–4680. doi: 10.1093/jxb/ert204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morato do Canto A., Ceciliato P.H., Ribeiro B., Ortiz Morea F.A., Franco Garcia A.A., Silva-Filho M.C., Moura D.S. Biological activity of nine recombinant AtRALF peptides: implications for their perception and function in Arabidopsis. Plant Physiol. Biochem. 2014;75:45–54. doi: 10.1016/j.plaphy.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Moussu S., Augustin S., Roman A.O., Broyart C., Santiago J. Crystal structures of two tandem malectin-like receptor kinases involved in plant reproduction. BioRxiv. 2018 doi: 10.1101/251959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo Q.A., Vogler H., Lituiev D.S., Nestorova A., Grossniklaus U. A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev. Cell. 2014;29:491–500. doi: 10.1016/j.devcel.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Paluch E.K., Nelson C.M., Biais N., Fabry B., Moeller J., Pruitt B.L., Wollnik C., Kudryasheva G., Rehfeldt F., Federle W. Mechanotransduction: use the force(s) BMC Biol. 2015;13:47. doi: 10.1186/s12915-015-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredez A.R., Somerville C.R., Ehrhardt D.W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science. 2006;80(312):1491–1495. doi: 10.1126/science.1126551. [DOI] [PubMed] [Google Scholar]
- Park Y.B., Cosgrove D.J. Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol. 2015;56:180–194. doi: 10.1093/pcp/pcu204. [DOI] [PubMed] [Google Scholar]
- Pearce G., Yamaguchi Y., Munske G., Ryan C.A. Structure-activity studies of RALF, rapid alkalinization factor, reveal an essential–YISY–motif. Peptides. 2010;31:1973–1977. doi: 10.1016/j.peptides.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Qu S., Zhang X., Song Y., Lin J., Shan J., Shan X. THESEUS1 positively modulates plant defense responses against Botrytis cinerea through GUANINE EXCHANGE FACTOR4 signaling. J. Integr. Plant Biol. 2017:797–804. doi: 10.1111/jipb.12565. in press. [DOI] [PubMed] [Google Scholar]
- Ralet M.C., Crepeau M.J., Vigouroux J., Tran J., Berger A., Salle C., Granier F., Botran L., North H.M. The affinity of xylan branches on rhamnogalacturonan I for cellulose provides the structural driving force for mucilage adhesion to the Arabidopsis seed coat. Plant Physiol. 2016 doi: 10.1104/pp.16.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J., Ploderer M., Mongelard G., Gutierrez L., Hauser M.-T. Role of CrRLK1L cell wall sensors HERCULES1 and 2, THESEUS1, and FERONIA in growth adaptation triggered by heavy metals and trace elements. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringli C. The hydroxyproline-rich glycoprotein domain of the Arabidopsis LRX1 requires Tyr for function but not for insolubilization in the cell wall. Plant J. 2010;63:662–669. doi: 10.1111/j.1365-313X.2010.04270.x. https://doi.org/TPJ4270 [pii]10.1111/j.1365-313X.2010.04270.x. [DOI] [PubMed] [Google Scholar]
- Schallus T., Feher K., Sternberg U., Rybin V., Muhle-Goll C. Analysis of the specific interactions between the lectin domain of malectin and diglucosides. Glycobiology. 2010;20:1010–1020. doi: 10.1093/glycob/cwq059. https://doi.org/cwq059 [pii]10.1093/glycob/cwq059. [DOI] [PubMed] [Google Scholar]
- Schiller H.B., Fässler R. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 2013;14:509–519. doi: 10.1038/embor.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenaers S., Balcerowicz D., Breen G., Hill K., Zdanio M., Mouille G., Holman T.J., Oh J., Wilson M.H., Nikonorova N., Vu L.D., De Smet I., Swarup R., De Vos W.H., Pintelon I., Adriaensen D., Grierson C., Bennett M.J., Vissenberg K. The auxin-regulated CrRLK1L kinase ERULUS controls cell wall composition during root hair tip growth. Curr. Biol. 2018;28:722–732.e6. doi: 10.1016/j.cub.2018.01.050. [DOI] [PubMed] [Google Scholar]
- Schoenaers S., Balcerowicz D., Costa A., Vissenberg K. The kinase ERULUS controls pollen tube targeting and growth in arabidopsis thaliana. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.01942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sede A.R., Borassi C., Wengier D.L., Mecchia M.A., Estevez J.M., Muschietti J.P. Arabidopsis pollen extensins LRX are required for cell wall integrity during pollen tube growth. FEBS Lett. 2018 doi: 10.1002/1873-3468.12947. [DOI] [PubMed] [Google Scholar]
- Shi M., Zhu J., Wang R., Chen X., Mi L., Walz T., Springer T.A. Latent TGF-β structure and activation. Nature. 2011;474:343–351. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih H.W., Miller N.D., Dai C., Spalding E.P., Monshausen G.B. The receptor-like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Curr. Biol. 2014;24:1887–1892. doi: 10.1016/j.cub.2014.06.064. [DOI] [PubMed] [Google Scholar]
- Srivastava R., Liu J.X., Guo H., Yin Y., Howell S.H. Regulation and processing of a plant peptide hormone, AtRALF23, in Arabidopsis. Plant J. 2009;59:930–939. doi: 10.1111/j.1365-313X.2009.03926.x. [DOI] [PubMed] [Google Scholar]
- Stegmann M., Monaghan J., Smakowska-Luzan E., Rovenich H., Lehner A., Holton N., Belkhadir Y., Zipfel C. The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science. 2017;80(355):287–289. doi: 10.1126/science.aal2541. [DOI] [PubMed] [Google Scholar]
- Turing A.M. The chemical basis of morphogenesis. 1953. Bull. Math. Biol. 1990;52:153–197. doi: 10.1098/rstb.1952.0012. [DOI] [PubMed] [Google Scholar]
- Uyttewaal M., Burian A., Alim K., Landrein B., Borowska-Wykret D., Dedieu A., Peaucelle A., Ludynia M., Traas J., Boudaoud A., Kwiatkowska D., Hamant O. Mechanical stress acts via katanin to amplify differences in growth rate between adjacent cells in Arabidopsis. Cell. 2012;149:439–451. doi: 10.1016/j.cell.2012.02.048. [DOI] [PubMed] [Google Scholar]
- Van der Does D., Boutrot F., Engelsdorf T., Rhodes J., McKenna J.F., Vernhettes S., Koevoets I., Tintor N., Veerabagu M., Miedes E., Segonzac C., Roux M., Breda A.S., Hardtke C.S., Molina A., Rep M., Testerink C., Mouille G., Höfte H., Hamann T., Zipfel C. The Arabidopsis leucine-rich repeat receptor kinase MIK2/LRR-KISS connects cell wall integrity sensing, root growth and response to abiotic and biotic stresses. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Helvert S., Storm C., Friedl P. Mechanoreciprocity in cell migration. Nat. Cell Biol. 2018;20:8–20. doi: 10.1038/s41556-017-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V., Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006 doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Voxeur A., Hofte H. Cell wall integrity signaling in plants: “To grow or not to grow that’s the question”. Glycobiology. 2016 doi: 10.1093/glycob/cww029. [DOI] [PubMed] [Google Scholar]
- Wipff P.-J., Rifkin D.B., Meister J.-J., Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J. Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S., Hématy K., Höfte H., Hematy K., Hofte H. Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 2012;63:381–407. doi: 10.1146/annurev-arplant-042811-105449. [DOI] [PubMed] [Google Scholar]
- Wolf S., Mouille G., Pelloux J. Homogalacturonan methyl-esterification and plant development. Mol Plant. 2009;2:851–860. doi: 10.1093/mp/ssp066. https://doi.org/ssp066 [pii]10.1093/mp/ssp066. [DOI] [PubMed] [Google Scholar]
- Wolf S., Rausch T., Greiner S. The N-terminal pro region mediates retention of unprocessed type-I PME in the Golgi apparatus. Plant J. 2009;58:361–375. doi: 10.1111/j.1365-313X.2009.03784.x. [DOI] [PubMed] [Google Scholar]
- Yang T., Wang L., Li C., Liu Y., Zhu S., Qi Y., Liu X., Lin Q., Luan S., Yu F. Receptor protein kinase FERONIA controls leaf starch accumulation by interacting with glyceraldehyde-3-phosphate dehydrogenase. Biochem. Biophys. Res. Commun. 2015;465:77–82. doi: 10.1016/j.bbrc.2015.07.132. [DOI] [PubMed] [Google Scholar]
- Yang Z., Lavagi I. Spatial control of plasma membrane domains: ROP GTPase-based symmetry breaking. Curr. Opin. Plant Biol. 2012;15:601–607. doi: 10.1016/j.pbi.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Qian L., Nibau C., Duan Q., Kita D., Levasseur K., Li X., Lu C., Li H., Hou C., Li L., Buchanan B.B., Chen L., Cheung A.Y., Li D., Luan S. FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc. Natl. Acad. Sci. U.S.A. 2012;109:14693–14698. doi: 10.1073/pnas.1212547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablackis E., Huang J., Muller B., Darvill A.G., Albersheim P. Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol. 1995;107:1129–1138. doi: 10.1104/pp.107.4.1129. https://doi.org/107/4/1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T., Vavylonis, D., Durachko, D.M., Cosgrove, D.J., 2017. Nanoscale movements of cellulose microfibrils in primary cell walls 3, 17056. https://doi.org/10.1038/nplants.2017.56https://www.nature.com/articles/nplants201756#supplementary-information. [DOI] [PMC free article] [PubMed]
- Zykwinska A.W., Ralet M.C., Garnier C.D., Thibault J.F. Evidence for in vitro binding of pectin side chains to cellulose. Plant Physiol. 2005;139:397–407. doi: 10.1104/pp.105.065912. [DOI] [PMC free article] [PubMed] [Google Scholar]