Abstract
Background
Historically, oestrogen and progesterone were each commonly used to save threatened pregnancies. In the 1940s it was postulated that their combined use would be synergistic and thereby led to the rationale of combined therapy for women who risked miscarriage.
Objectives
To determine the efficacy and safety of combined oestrogen and progesterone therapy to prevent miscarriage.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (23 June 2013) CENTRAL (OVID) (The Cochrane Library 2013, Issue 6 of 12), MEDLINE (OVID) (1946 to June Week 2 2013), OLDMEDLINE (1946 to 1965), Embase (1974 to Week 25 2013), Embase Classic (1947 to 1973), CINAHL (1994 to 23 June 2013) and reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials that assessed the effectiveness of combined oestrogen and progesterone for preventing miscarriage. We included one stratified randomised trial and one quasi‐randomised trials. Cluster‐randomised trials were eligible for inclusion but none were identified. We excluded studies published only as abstracts.
We included studies that compared oestrogen and progesterone versus placebo or no intervention.
Data collection and analysis
Two review authors independently assessed trials for inclusion and assessed trial quality. Two review authors extracted data. Data were checked for accuracy.
Main results
Two trials (281 pregnancies and 282 fetuses) met our inclusion criteria. However, the two trials had significant clinical and methodological heterogeneity such that a meta‐analysis combining trial data was considered inappropriate.
One trial (involving 161 pregnancies) was based on women with a history of diabetes. It showed no statistically significant difference between using combined oestrogen and progestogen and using placebo for all our proposed primary outcomes, namely, miscarriage (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.32 to 2.80), perinatal death (RR 0.94, 95% CI 0.53 to 1.69) and preterm birth (less than 34 weeks of gestation) (RR 0.91, 95% CI 0.80 to 1.04). In terms of this review's secondary outcomes, use of combined oestrogen and progestogen was associated with an increased risk of maternal cancer in the reproductive system (RR 6.65, 95% CI 1.56 to 28.29). However, for the outcome of cancer other than that of the reproductive system in mothers, there was no difference between groups. Similarly, there were no differences between the combined oestrogen and progestogen group versus placebo for other secondary outcomes reported: low birthweight of less than 2500 g, genital abnormalities in the offspring, abnormalities other than genital tract in the offspring, cancer in the reproductive system in the offspring, or cancer other than of the reproductive system in the offspring.
The second study was based on pregnant women who had undergone in‐vitro fertilisation (IVF). This study showed no difference in the rate of miscarriage between the combined oestrogen and progesterone group and the no treatment group (RR 0.66, 95% CI 0.23 to 1.85). The study did not report on this review's other primary outcomes (perinatal death or rates of preterm birth), nor on any of our proposed secondary outcomes.
Authors' conclusions
There is an insufficient evidence from randomised controlled trials to assess the use of combined oestrogen and progesterone for preventing miscarriages. We strongly recommend further research in this area.
Keywords: Female; Humans; Pregnancy; Abortion, Spontaneous; Abortion, Spontaneous/prevention & control; Diethylstilbestrol; Diethylstilbestrol/administration & dosage; Drug Combinations; Estrogens; Estrogens/administration & dosage; Ethisterone; Ethisterone/administration & dosage; Fertilization in Vitro; Progesterone; Progesterone/administration & dosage; Randomized Controlled Trials as Topic
Plain language summary
Combined use of oestrogen and progesterone for preventing miscarriage
The hormones oestrogen and progesterone have established physiological roles in maintaining pregnancy. It has been suggested that supplementation of these hormones could help prevent miscarriage before 24 weeks of pregnancy, particularly in women who have low levels of the hormones, in assisted reproductive technology programs, or who have a history of repeated miscarriages. In our review of randomised controlled trials published in major scientific databases, we only identified two trials that met our inclusion criteria. The two trials involved small numbers of women. One involved 161 women with diabetes who took oral placebo or oral diethylstilboestrol and ethisterone in increasing doses from before the end of the 16th week until birth. The other trial involved 120 women with pregnancy assisted by in vitro fertilisation and embryo transfer who continued treatment until the completed 12th week of gestation.
From the little evidence available, the two trials found no evidence that combined oestrogen and progestogen can prevent miscarriage (progestogen is a major class of hormones which includes progesterone) when compared with placebo or usual care. The first of the two studies indicated an increased risk for the mothers who used hormonal therapy during pregnancy of developing cancer later in life. Diethylstilboestrol is no longer in use and poses serious adverse effects while ethisterone contains androgenic properties thought to be responsible for genital abnormalities and has been replaced by progesterone.
Overall, we acknowledge the lack of trials, especially large‐scale trials, and therefore suggest further research is needed in this area before supporting or disproving the use of combined oestrogen and progesterone for the prevention of miscarriages.
Background
Description of the condition
Definitions
Miscarriage, or 'spontaneous abortion', is defined as the loss of pregnancy under 24 weeks of gestation (RCOG 2006). 'Recurrent miscarriage' is defined as having three or more consecutive spontaneous miscarriages (RCOG 2003). Pregnant women may undergo 'threatened miscarriage', which presents as vaginal bleeding, with or without pain, within the first 20 weeks of pregnancy (Cunningham 2010). A closed cervix helps keep the fetus viable inside the uterine cavity. Once cervical dilatation occurs, a miscarriage is deemed as 'inevitable' (Cunningham 2010).
Incidence
Miscarriage is common and occurs in 10% to 15% of all clinically recognised pregnancies (Everett 1997; Liu 1991; Regan 1989; Stirrat 1990; Warburton 1964). Furthermore, an even higher miscarriage rate of 31% has been reported when undetected pregnancies are considered (Wilcox 1988). Threatened miscarriage has been reported to be present in 20% to 25% of pregnant women (Cunningham 2010; Everett 1997). Around 1% of all women suffer from recurrent miscarriage and, given that this incidence rate is higher than that expected by chance, a proportion of women with recurrent miscarriage will have particular aetiologies underlying their miscarriages (RCOG 2003).
Impact
The miscarriage process may be a traumatic event for women both physically and psychologically. Physical impact may involve sudden, considerable pain, blood loss, rapid hospitalisation and operation (Lee 1996). Furthermore, the operative process such as dilatation and curettage is known to be associated with ‐ other than surgical risks ‐ stress and emotional responses (Lee 1996). After miscarrying, the psychological impact may also include depressive symptoms, anxiety and development of obsessive‐compulsive disorder. Such decline in mental health can last up to six months or more after miscarrying (Janssen 1996).
Pathophysiology
The process of miscarriage initiates within a few weeks after the death of the embryo. Haemorrhage in the decidua basalis, which is the endometrial area that forms the base of the implanted site, together with adjacent tissue necrosis and inflammation, lead to detachment of the gestational sac and implanted ovum. The detachment stimulates uterine contractions and cervical dilation, subsequently resulting in expulsion (Porter 2008).
Aetiology
Spontaneous miscarriage
Despite numerous theories, there remains a large number of miscarriage cases in which an exact cause cannot be identified. Ultrasonography and histological investigations from cases of spontaneous miscarriages show that 70% is related to a defective ovum or fetus, the most common cause being chromosomal abnormalities (Oats 2010). In fact, chromosomal abnormalities have been reported to account for more than 50% of all miscarriages (Burgoyne 1991; Goddijin 2000; Simpson 2007). Other causes which are less common include defective implantation, systemic maternal disease (such as poorly‐controlled insulin‐dependent diabetes) (Mills 1988), uterine abnormalities and possibly psychosomatic causes, although the latter have been difficult to evaluate in studies (Oats 2010). Maternal infections are an uncommon cause (Cunningham 2010). There are many risk factors which are associated with a higher incidence of miscarriage: maternal age of greater than 35 years, previous history of miscarriage (Garcia‐Enguidanos 2002; Walch 2008), smoking, alcohol use, high caffeine use and exposure to certain environmental toxins (Cunningham 2010).
Recurrent miscarriage
The known aetiologies are similar to the causes described for spontaneous miscarriage; however there is some difference in terms of their occurrence. For instance, chromosomal abnormalities become a less likely cause for recurrent miscarriage, while uterine malformations, particularly cervical incompetence, are more likely (Oats 2010). Other aetiologies include predisposing conditions to thrombosis (such as antiphospholipid syndrome and thrombophilia), endocrinological factors (such as polycystic ovaries, thyroid dysfunction) and immunological factors (such as systemic lupus erythematosus) (Carrington 2005; Li 2002; Toth 2010). Despite all this, around 50% of recurrent miscarriages remain unexplained (Habayeb 2004; Tulppala 1993).
Description of the intervention
Progesterone and oestrogen are both female sex hormones which are essential in the maintenance of pregnancy. Progesterone is produced from the ovary by the corpus luteum after ovulation. While the corpus luteum continues progesterone synthesis up to the 10th week of gestation (Speroff 2005), the placenta concurrently begins to synthesise progesterone and by the 12th week, enough progesterone is produced to replace the corpus luteum source (Genuth 2006). Progesterone is responsible for multiple functions in the pregnancy ‐ seeHow the intervention might work ‐ and the insufficiency of progesterone during the luteal phase of the menstrual cycle and during early pregnancy are thought to be one of the many causes of miscarriage (Haas 2008). For this reason, women who have low progesterone levels of 10 ng/mL or less during early pregnancy may be supplemented with 100 mg progesterone daily until the 10th week (Speroff 2005). Women who use assisted reproductive technology may also require progesterone use before pregnancy, during the luteal phase of their menstrual cycle as a means of preparing the endometrium for successful implantation (Balasch 1987).
Similarly, progestogen has also been used in cases of assisted reproductive technology (Abu‐Musa 1998; Daya 2009) and in prevention of miscarriages (Hemminki 1999; Johnson 1979), including threatened cases (Duan 2010; Thierstein 1959). One of the main concerns about maternal progestogen use has been the adverse effect of genital tract abnormalities presenting in newborns who were exposed in utero (Silver 1999; Wilkins 1960), but the association with malformations may be weak (Kullander 1976).
The second element of the intervention is oestrogen, another hormone produced from the ovaries. Historically, it was proposed that diethylstilboestrol, a synthetic oestrogen which enhanced both oestrogen and progesterone secretion, could combat the problem of hormonal deficiency in pregnancy, thereby acting as a therapeutic agent for preventing miscarriages, and perhaps hinder or lessen the impact of adverse pregnancy outcomes, such as eclampsia and preterm delivery (Smith 1948).
However, from the 1970s onwards, some adverse effects were identified in offspring who were exposed to diethylstilboestrol in utero, leading to the declaration of its contraindicated use in pregnancy by the US Food and Drug Administration in 1971 (FDA 1971). Adverse effects included premature birth and genital tract abnormalities in both male and female offspring (Bibbo 1977; Herbst 1971; Palmer 2005). For female offspring, established and documented effects include cervical adenocarcinoma (Herbst 1981), vaginal adenocarcinoma (Herbst 1971) and vaginal adenosis (Bibbo 1977; Herbst 1971). Amongst the lesser known adverse effects, one 1977 follow‐up study of prenatally exposed offspring from the early 1950s reported abnormalities, namely, irregular menstrual cycle and lower incidence of pregnancy in female offspring; and in male offspring, increased cases of pathologic semen (Bibbo 1977). Other sources describe poorer pregnancy outcomes for females exposed in utero; specifically higher rates of ectopic pregnancies, miscarriages and preterm births (Barnes 1980; Berger 1980; Goldberg 1999). Increased risk of infertility in female offspring (Palmer 2005) and slightly increased risk in males (Perez 2005) have also been postulated, while other authors have dismissed an increased risk of infertility when exposed to oestrogen or progestins (Hemminki 1999).
In recent decades, scientific literature has typically described the combined use of oestrogen and progesterone in the context of assisted reproductive technologies, in particular in‐vitro fertilisation (IVF), by which if the woman achieves pregnancy, hormonal supplementation would be continued throughout the early pregnancy period, or until the placenta has assumed the role of hormonal production (Davar 2007; Devroey 1998; Lelaidier 1992; Muasher 1991; Navot 1986; Queenan 1997; Schindler 2005). Despite the varying results over which drug protocol is best for luteal support, this review will only include trials which compare combined oestrogen and progesterone versus placebo or no intervention, where the comparison is undertaken during, but not limited to, the time of pregnancy. We also aim to clarify the effect of such therapy on preterm birth since there are both therapeutic claims and claims of preterm birth as an adverse effect from therapy.
How the intervention might work
The established roles of oestrogen and progesterone have been known to be beneficial towards maintenance of pregnancy. First, progesterone can stimulate secretory changes in the endometrial layer of the uterus, in order to create a stabilised surface for the fertilised egg to implant upon (Duan 2010; Potdar 2005). Second, progesterone keeps the myometrial layer of the uterus quiescent; that is, suppresses the uterus from contracting, which again is important for stable implantation, and important for preventing preterm labour later on in pregnancy (Duan 2010; Rao 1998). Third, progesterone is a potent modulator working in the maternal immune system to prevent the rejection of the fetus as foreign tissue (Genuth 2006; Schorge 2008; Walch 2008). By these various physiological functions, progesterone supplementation and its effects are assumed to be beneficial for pregnancy.
Oestrogen induces proliferation of the endometrial layer, which also helps to prepare for successful implantation (Genuth 2006). In addition, oestrogen stimulates continuous growth of uterine muscles (Bengtsson 1973) and influences blood flow to the uterus (Genuth 2006), all of which aim to accommodate for pregnancy. Another feature of oestrogen is the ability to increase synthesis of oestrogen receptors and progesterone receptors. This enables oestrogen to amplify its own effects on uterine growth as well as enhancing the effects of progesterone (Genuth 2006).
Why it is important to do this review
Related reviews and protocols evaluating the efficacy of hormone administration for miscarriage prevention are already available in The Cochrane Library.
Oestrogen supplementation, mainly diethylstilbestrol, for preventing miscarriages and other adverse pregnancy outcomes (Bamigboye 2003)
Progestogen for preventing miscarriage (Haas 2008)
Progestogen for treating threatened miscarriage (Wahabi 2011)
The above Cochrane reviews have only addressed the evidence of these two hormones separately, and not in combination. In all three reviews, any combination therapy used in a randomised controlled trial (RCT) resulted in the exclusion of that RCT from their analyses. However, given that for decades, both oestrogen and progesterone have been viewed as essential hormones supportive of pregnancy and given that the added presence of oestrogen can amplify the effects of progesterone (Genuth 2006), it would therefore be important to formally examine the evidence to support the efficacy of such combined use.
Objectives
To determine the efficacy and safety of combined oestrogen and progesterone as preventative therapy against miscarriage.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials that assessed the effectiveness of combined oestrogen and progesterone for preventing miscarriage. We included one stratified randomised trial and one quasi‐randomised trial. Cluster‐randomised trials were eligible for inclusion but none were identified. We excluded studies published only as abstracts.
Types of participants
We included all pregnant women, but in order for results to be meaningful in terms of clinical applicability, we categorised participants according to particular clinical conditions or particular risk factors in the subgroup analysis ‐ seeSubgroup analysis and investigation of heterogeneity.
Types of interventions
We compared oestrogen and progesterone versus placebo or no intervention. We also included studies which used a progestogen different to progesterone, due to its historic relevance and use in assisted reproductive technology ‐ seeDescription of the intervention. However, because of differences in chemistry, we had to view other progestogens as a separate intervention from progesterone. Hence, we presented data on the first two comparisons.
Combined oestrogen and progestogen (other than progesterone) versus placebo
Combined oestrogen and progesterone versus no hormonal treatment
Combined oestrogen and any progestogen versus placebo or no hormonal treatment
We do not exclude the possibilities of other comparisons arising from future updates of this review.
Studies that compare therapy with no treatment rather than with placebo have more potential for bias. This potential for bias has been addressed in the review by analysing the placebo‐based trial and no‐treatment‐based trial both separately and in conjunction.
Types of outcome measures
We included the following outcomes.
Primary outcomes
Miscarriage
Perinatal death
Preterm birth (less than 34 weeks of gestation)
Secondary outcomes
Offspring
Low birthweight of less than 2500 g
Genital tract abnormalities
Abnormalities other than of the genital tract
Cancer in the reproductive system
Cancer other than of the reproductive system
Mother
Cancer in the reproductive system
Cancer other than of the reproductive system
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (23 June 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched CENTRAL (OVID) (The Cochrane Library 2013, Issue 6 of 12), MEDLINE (OVID) (1946 to June Week 2 2013), OLDMEDLINE (1946 to 1965), Embase (1974 to Week 25 2013), Embase Classic (1947 to 1973) and CINAHL (1994 to 23 June 2013). SeeAppendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; and Appendix 6 for search strategies.
Searching other resources
We also scanned through studies referenced in three related Cochrane reviews (Bamigboye 2003; Haas 2008; Wahabi 2011) and other retrieved studies.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all potential studies identified as a result of the search strategy. We resolved any disagreement through discussion. We did not require any consultation with a third party, although if in the future, during the process of updating this review, there is disagreement that is unable to be resolved between the two review authors, we will maintain the strategy of consulting a third party.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion. We did not require any consultation with a third party, but shall maintain this strategy if required when conducting future updates. We entered data into Review Manager software (RevMan 2012) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion. We did not require consultation with a third party, but shall maintain this strategy if required when conducting future updates.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
low risk of bias (where less than 20% of the randomised population was excluded);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
No continuous data were used. However, for the purpose of future updates, we maintain the strategy of using the mean difference if outcomes are measured in the same way between trials. If appropriate in future updates of this review, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
No cluster‐randomised trials were included in this version of the review. However, in future updates of the review, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We excluded cross‐over trials due to concerns over order effects and carry‐over effects related to our proposed outcomes of interest.
In one study (MRC 1955), a minority of the randomised population proved to be cross‐over participants. We intended to collect individual participant data as far as possible, to utilise the results from the first period of the cross‐over only. However, the individual data in this minority group were unavailable, and hence we reported 'unclear risk' under the category 'other potential sources of bias'.
Other unit of analysis issues
Multiple pregnancies
For trials involving multiple pregnancies, we undertook methods described in Cochrane Pregnancy and Childbirth Group Methodological Guidelines accordingly (Gates 2009). One trial involved one set of twins in their data, which was not substantial within the randomised population, but nonetheless we analysed the fetuses as if independent and used the number of fetuses as the denominator according to Cochrane Pregnancy and Childbirth Group Methodological Guidelines accordingly (Gates 2009) and to our protocol. For the purpose of future updates, we will maintain the same strategy if it is not possible to make adjustments for the multiple pregnancies due to unavailable information.
Dealing with missing data
For included studies, we noted the levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing in an unbiased manner.
We excluded from the analyses data from trials or outcomes that were at high risk of bias, e.g. those with high levels of missing data or a large number of participants analysed in the wrong group.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
In future updates of this review, we will carry out statistical analyses using the Review Manager software (RevMan 2012). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. We will treat the random‐effects summary as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, we will present the results as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
We did not carry out subgroup analyses given the substantial heterogeneity between the two included studies. For the purpose of future updates, we will maintain the strategy of carrying out formal subgroup analysis for the following subsets, if required.
Women with threatened miscarriage versus women without threatened miscarriage.
Women with recurrent miscarriage versus women without recurrent miscarriage.
Women using IVF versus women without IVF treatment.
We will analyse each subgroup in relation to each of the primary outcomes ‐ seePrimary outcomes.
We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2012). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
We will conduct sensitivity analyses to investigate the following effects on primary outcomes.
Inclusion/exclusion of trials with 'no intervention' as the control group.
Inclusion/exclusion of trials at high risk of bias, as determined the risk of allocation concealment.
Variations in the analysis of trial types stated in Unit of analysis issues.
Inclusion/exclusion of trials with high levels of missing data.
Fixed‐effect/random‐effects analyses for outcomes with statistical heterogeneity.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We retrieved four reports of two studies from the search of the Pregnancy and Childbirth Group's Trials Register, three reports for MRC 1955 and one from Prietl 1992). These two studies were included in our analysis.
The total number of 'hits' from searching databases was 960; nine from CENTRAL; 365 from MEDLINE; 31 from OLDMEDLINE; 470 from Embase; 20 from Embase Classic; and 65 from CINAHL. Of the 960 hits, 906 were immediately excluded due to duplicated hits of the exact same study or due to irrelevance to our research topic. We could not obtain the full texts or translations of 54 results despite our access to 12 international library systems. We postulate that this is due to the fact that the articles were published some time ago and the lack of access to non‐English titles, evident by the fact that 52 of 54 were non‐English language papers. Therefore, our list of potentially relevant studies was five.
Of the five studies, four studies were assessed and classified as excluded studies ‐ seeExcluded studies ‐ and the remaining study was assessed and classified as an included study. In addition, we scanned the references of significant reports, which resulted in one extra study. This extra study produced two reports, one report for its original study and another report for its follow‐up results of the same cohort. Hence in total, two studies were classified as included studies ‐ seeIncluded studies.
Included studies
Two trials were included, involving 281 pregnancies and 282 fetuses. One trial, MRC 1955, subsequently had two follow‐up studies performed 27 years after, on mothers and offspring respectively. The follow‐up studies involved 156 mothers and 136 children.
The MRC 1955 study was conducted across nine centres. One‐hundred and sixty‐one pregnancies from 156 women were randomised by simple stratification, however 147 were included in their analysis ‐ seeIncomplete outcome data (attrition bias). Of the 147 pregnancies, one set of twins was included hence there were 148 fetuses. All analysed participants were pregnant women under 16 weeks' gestation with a background of diabetes mellitus (duration of diabetes averaged around eight years in both groups). Participants were allocated to either oral placebo or oral diethylstilboestrol and ethisterone. Diethylstilboestrol and ethisterone were started at 50 mg/day and 25 mg/day before the end of the 16th week then the dosage increased every three weeks until birth, by which time dosage was 200 mg/day and 250 mg/day respectively ‐see Characteristics of included studies for detailed dose regimen. For the offspring, the outcomes of interest included were miscarriage, stillbirth, neonatal death, time of delivery and birthweight. For the mother, the outcomes included maternal death and pre‐eclampsia.
The same 156 women were followed up in the MRC 1955 study, however 151 were included in their analysis ‐ seeIncomplete outcome data (attrition bias). The mothers were not contacted directly. Instead, data were collected from their general practitioners, from hospital and diabetic clinics, and from Office of Population Censuses and Surveys. General practitioners were asked to complete questionnaires, which included questions about the occurrence of cancer. Outcomes of interest included death, cancer in the reproductive sites and cancers in other sites.
Twelve miscarriages occurred in the 148 fetuses in the MRC 1955 study, thus 136 offspring were included in the follow‐up study. This group included data from stillbirths and neonatal deaths. Five were excluded. Children were not contacted directly and all methodology was identical to that described for the follow‐up study of the mothers. Outcomes of interest included death under and over the age of one, urogenital abnormalities, other abnormalities, cancers, number of those who consulted for infertility, number of those married with history of miscarriage, and number of those married with at least one child. However, data for the latter three outcomes were not used in our analyses and we explain why ‐ seeIncomplete outcome data (attrition bias).
The second included trial, Prietl 1992, was a quasi‐randomised trial involving 120 pregnancies assisted by in vitro fertilisation and embryo transfer (IVF‐ET). Two participants were excluded. Women were ensured of normal endocrine profiles before IVF treatment. Despite different IVF protocols used before pregnancy, a balanced baseline in protocol types was achieved between the intervention and control group. After confirmation of pregnancy, participants were randomised, according to their odd or even year of birth, to either intramuscular injection of 500 mg 17α‐hydroxyprogesterone caproate and 10 mg oestradiol valerate in an oily vehicle (Gravibinon) twice a week, or, to no hormonal treatment. Treatment continued until the completed 12th week of gestation. The only outcome of interest was miscarriage.
Excluded studies
We excluded four studies (Berle 1977; Crowder 1950; Lightman 1999; Sathanandan 1991). An explanation for exclusion of each study is provided ‐ seeCharacteristics of excluded studies.
Risk of bias in included studies
See: Figure 1 for a summary of risk of bias assessed in our included studies. For detailed descriptions of each risk of bias, see Characteristics of included studies.
1.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation and allocation concealment was adequate in one study (MRC 1955) and inadequate in the other (Prietl 1992).
Blinding
In MRC 1955, blinding of both patients and personnel was adequate. Blinding continued throughout follow‐up period. In the other study, Prietl 1992, there was no placebo use and the sequence was known to personnel, thus blinding was deemed inadequate.
Incomplete outcome data
The MRC 1955 study performed randomisation before assessment for eligibility. After assessment for eligibility, there were 14 exclusions (10 control, four treated) of which eight were reasonably excluded, due to non‐pregnancies and miscarriage prior to intervention use. Of the remaining six, two had advanced beyond the age of 16 weeks, which still meets our review criteria, but not the criteria set by the original study and hence excluded in the original study. Finally, four were avoidable exclusions quoted to have "lacked cooperation or some other complication supervened" (MRC 1955). Given this, the latter six would have been ideally included in our analyses on the basis of intention‐to‐treat, however the intervention to which these six participants were allocated to was unknown. Despite this, attrition bias remains low because 14 exclusions out of 161 participants is only 8.7%.
Only two exclusions eventuated from Prietl 1992 due to ectopic pregnancy (one control, one treated). These are unavoidable exclusions, which remain excluded in our analyses.
The overall low levels of missing data in both studies deem low risk in attrition bias. For further details ‐ seeRisk of bias in included studies.
Selective reporting
In both studies, all pre‐specified outcomes were reported, hence we assessed both studies as being free from selective reporting bias.
Other potential sources of bias
Cross‐over participants
One study, MRC 1955, was inadequate for this risk of bias due to five cross‐over participants accounting for 10 pregnancies. This is not without concern of order effects and carry‐over effects, and ideally, only data from the first period of the cross‐over would be used in our analyses, however due to unavailable individual data, we cannot achieve this. Despite everything, we are reminded that the inclusion of five pregnancies from a second period cross‐over represents only 3.12% of the randomised population. We proceeded to include these data, but assessed the trial as 'unclear' under 'other bias'.
Effects of interventions
Justification of why meta‐analysis was not performed
Two trials met our inclusion criteria (MRC 1955; Prietl 1992). We extracted data from MRC 1955 for all three of our primary outcomes and seven of secondary outcomes. From Prietl 1992, only one primary outcome was measured. This single common primary outcome was miscarriage. We decided that the pooling of results from both trials for this common outcome was inappropriate as there is obvious clinical and methodological heterogeneity between the two trials.
One trial was on women with long histories of diabetes (MRC 1955), the other was conducted in the context of pregnant women who had undergone IVF treatment on various protocols (Prietl 1992).
Average age of the women differed approximately 10 years.
Intervention used differed in type, dosage, mode of administration, timing of use in pregnancy, and the type of control was different.
Definition of miscarriage was different.
Trial design was different.
The way trials were conducted differed in terms of allocation concealment and blinding.
Comparison 1 ‐ Combined oestrogen and progestogen (other than progesterone) versus placebo (in women with history of diabetes)
Primary outcomes
In one trial of 148 women (MRC 1955), miscarriage was not significantly different between the combined hormonal and placebo groups (risk ratio (RR) of 0.95, 95% confidence interval (CI) 0.32 to 2.80) ‐ Analysis 1.1. Other primary outcomes had similar results: perinatal death (RR 0.94, 95% CI 0.53 to 1.69 ‐ Analysis 1.2) and preterm birth less than 34 weeks (RR 0.91, 95% CI 0.80 to 1.04 ‐ Analysis 1.3).
1.1. Analysis.
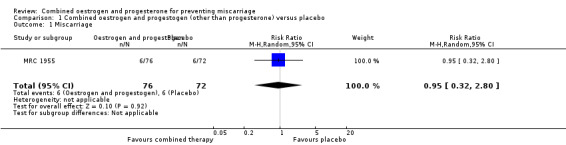
Comparison 1 Combined oestrogen and progestogen (other than progesterone) versus placebo, Outcome 1 Miscarriage.
1.2. Analysis.
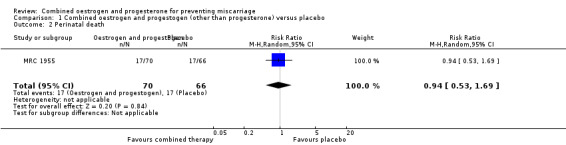
Comparison 1 Combined oestrogen and progestogen (other than progesterone) versus placebo, Outcome 2 Perinatal death.
1.3. Analysis.
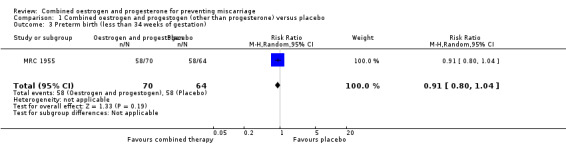
Comparison 1 Combined oestrogen and progestogen (other than progesterone) versus placebo, Outcome 3 Preterm birth (less than 34 weeks of gestation).
Secondary outcomes
For the offspring
Secondary outcomes for the offspring revealed no statistical significance between groups: low birthweight of less than 2500 g (RR 0.87, 95% CI 0.43 to 1.77 ‐ Analysis 1.4); genital tract abnormalities (RR 1.57, 95% CI 0.60 to 4.08 ‐ Analysis 1.5); abnormalities other than of the genital tract (RR 0.42, 95% CI 0.14 to 1.30 ‐ Analysis 1.6); cancer in the reproductive system of offspring (RR 2.83, 95% CI 0.12 to 68.29 ‐ Analysis 1.7) and cancer other than of the reproductive system (RR 2.83, 95% CI 0.12 to 68.29 ‐ Analysis 1.8). All of these long‐term secondary outcomes were recorded 27 years after the original study.
1.4. Analysis.
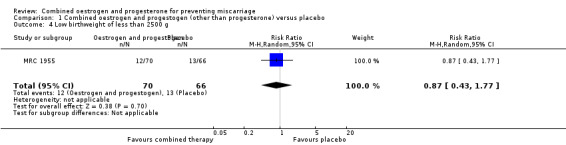
Comparison 1 Combined oestrogen and progestogen (other than progesterone) versus placebo, Outcome 4 Low birthweight of less than 2500 g.
1.5. Analysis.
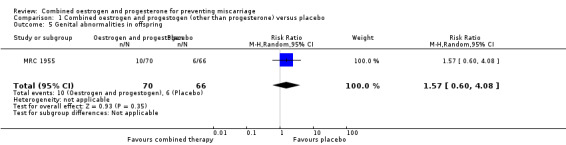
Comparison 1 Combined oestrogen and progestogen (other than progesterone) versus placebo, Outcome 5 Genital abnormalities in offspring.
1.6. Analysis.
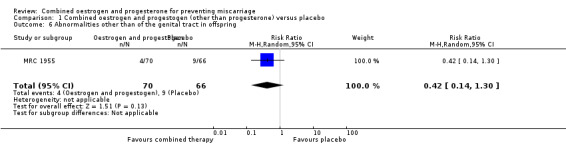
Comparison 1 Combined oestrogen and progestogen (other than progesterone) versus placebo, Outcome 6 Abnormalities other than of the genital tract in offspring.
1.7. Analysis.
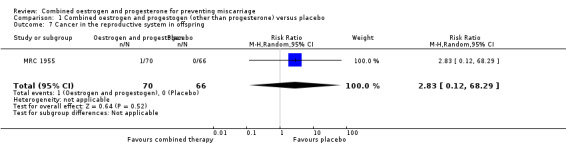
Comparison 1 Combined oestrogen and progestogen (other than progesterone) versus placebo, Outcome 7 Cancer in the reproductive system in offspring.
1.8. Analysis.
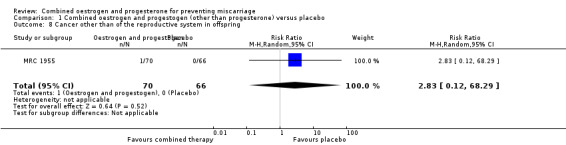
Comparison 1 Combined oestrogen and progestogen (other than progesterone) versus placebo, Outcome 8 Cancer other than of the reproductive system in offspring.
Maternal outcomes
Amongst the outcomes for the mother, the rate of cancer in the reproductive system was statistically significant (RR 6.65, 95% CI 1.56 to 28.29 ‐ Analysis 1.9). In other words, our findings suggest that maternal hormone use is associated with an increased risk of having cancer in a reproductive site 27 years later by 565%. Cancer other than of the reproductive system in mothers was not statistically significant (RR 1.90, 95% CI 0.36 to 10.07 ‐ Analysis 1.10).
1.9. Analysis.
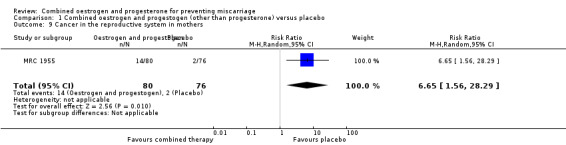
Comparison 1 Combined oestrogen and progestogen (other than progesterone) versus placebo, Outcome 9 Cancer in the reproductive system in mothers.
1.10. Analysis.
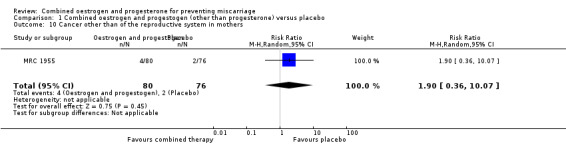
Comparison 1 Combined oestrogen and progestogen (other than progesterone) versus placebo, Outcome 10 Cancer other than of the reproductive system in mothers.
Comparison 2 ‐ Combined oestrogen and progesterone versus no hormonal treatment (in pregnant women having undergone IVF treatment)
Primary outcomes
For a single trial of 118 women (Prietl 1992), the only available outcome reported was miscarriage (RR 0.66, 95% CI 0.23 to 1.85) and there was no statistical difference between groups ‐ Analysis 2.1.
2.1. Analysis.
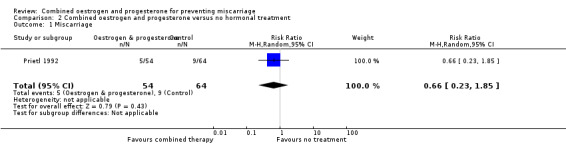
Comparison 2 Combined oestrogen and progesterone versus no hormonal treatment, Outcome 1 Miscarriage.
Secondary outcomes
None of our pre‐specified secondary outcomes were reported in the Prietl 1992 trial.
Discussion
Summary of main results
Two trials were included in our systematic review on the maternal use of combined oestrogen and progesterone. Both trials were regarded separately due to clinical and methodological heterogeneity.
One trial MRC 1955 compared combined oestrogen and progestogen versus placebo in mothers with a history of diabetes. There was no statistical difference for the following outcomes: miscarriage, perinatal death, preterm birth and low birthweight. In the follow‐up study, which was conducted 27 years later, there was no statistical difference for the following outcomes: genital abnormalities in offspring, abnormalities other than of the genital tract in offspring, cancer of the reproductive system in offspring, cancer other than of the reproductive system in offspring, cancer other than of the reproductive system in mothers. There was however, a statistical difference in cancer of the reproductive system of mothers 27 years later by 565% (RR 6.65, 95% CI 1.56 to 28.29 ‐ Analysis 1.9).
The other trial Prietl 1992 compared combined oestrogen and progesterone versus no hormonal treatment in pregnant women who had undergone IVF treatment. There was no statistical difference for the outcome of miscarriage. Other outcomes were not measured in this trial.
Overall completeness and applicability of evidence
To the best of our knowledge, this is the only systematic review on the maternal use of combined oestrogen and progesterone for preventing miscarriage. Very little on this topic was identified, with only two trials meeting our inclusion criteria. Both trials recruited small numbers of women, the pooling of which was deemed inappropriate. Therefore, there is insufficient evidence overall.
In terms of outcomes, most of our data for the pre‐specified outcomes derived from one of the two studies, MRC 1955, whereas the other study, Prietl 1992, only addressed one of our outcomes. Therefore, more evidence is needed in order to address other outcomes.
We question the clinical applicability of the MRC 1955 due to its interventions diethylstilboestrol and ethisterone. Diethylstilboestrol is no longer in use and poses serious adverse effects (Bamigboye 2003) while ethisterone contains androgenic properties thought to be responsible for genital abnormalities, hence has been commonly substituted by progesterone in modern times (Abu‐Musa 1998; Sullivan 1986).
Quality of the evidence
One of the two included studies, Prietl 1992, is at high risk of bias ‐ seeFigure 1 ‐ with its alternation sequence generation thus inadequacy in allocation concealment and lack of placebo, thus performance bias.
Potential biases in the review process
We could not obtain the full texts or translations of 55 results despite our access to 12 international library systems. We postulate that this is due to the age of the articles and the lack of access to non‐English titles, evident by the fact that 53 of 55 were non‐English language papers.
There are no other potential biases.
Agreements and disagreements with other studies or reviews
The authors of MRC 1955 and Prietl 1992 could not support the benefit of hormone treatment for the prevention of miscarriages (as defined by their own definition), however Prietl 1992 claimed that the significantly higher rate of preclinical pregnancies in their control group, which towards the end resulted in significantly lower ongoing pregnancy rate reflected the ability of hormone treatment in salvaging early pregnancies. This claim however, lacks support from other IVF studies of the non‐randomised type. These studies involved comparison of natural cycle IVF and programmed hormone cycle IVF, and the latter intervention always implied continuous hormone support throughout early pregnancy. Three such studies were identified, of which two had dealt with very small numbers (de Ziegler 1990; Schmidt 1989) and the third, a large retrospective study (Queenan 1994) showed almost identical rates in pregnancies, clinical pregnancy losses and ongoing pregnancies. Studies not dealing with IVF similarly refuted the hypothesis of better salvage rates with hormonal therapy (Crowder 1950; Nesbitt 1965).
In our analyses, the only outcome that showed statistical significance was the higher rate of cancer in reproductive sites of treated mothers. Our data for this outcome derived from the follow‐up study of MRC 1955, and the authors of MRC 1955 investigated for a possible dose‐response relationship between the total amount of diethylstilboestrol taken during pregnancy and the occurrence of such cancers (there was no explanation of why the authors called it 'diethylstilboestrol dose‐response' when presumably combined diethylstilbostrol and ethisterone were given). Nonetheless, a convincing dose‐response relationship was not established. A 25‐year follow‐up study focusing on mothers who were exposed to diethylstilboestrol only (Bibbo 1978) followed up a much larger population but its original trial involved lower amounts of diethylstilboestrol exposure than MRC 1955. When we interpreted the data from Bibbo 1978 we found that, in contrast to our findings, there was no significant difference in reproductive site cancers between exposed and unexposed mothers. Hence, the relationship between hormonal exposure during pregnancy and rate of cancer in reproductive sites remains a gap in research.
Authors' conclusions
Implications for practice.
There is insufficient evidence to assess the effectiveness and safety of the maternal use of combined oestrogen and progesterone for the prevention of miscarriage. One small study suggests that combined hormonal use was associated with increased risk of reproductive cancer for the mother, however, this increased risk could not be supported by evidence in other scientific trials. We conclude that more research is needed prior to establishing any implications for practice.
Implications for research.
There is an insufficient number of trials to support or refute the use of combined oestrogen and progesterone in preventing miscarriages. Ideally, trials should recruit a large number of participants, use randomised allocation, use placebo, remain blinded, minimise drop‐out rates and report the results of all pre‐specified outcomes. For information of some of the secondary outcomes, we recommend that a follow‐up study of original participants and their offspring be conducted in the long term.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL Search Strategy
MeSH descriptor Abortion, Spontaneous explode all trees
miscarriag* in Clinical Trials
spontaneous abortion in Clinical Trials
spontaneous pregnancy loss in Clinical Trials
f?etal death in Clinical Trials
recurrent ADJ abortion in Clinical Trials
recurrent ADJ pregnancy loss in Clinical Trials
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
MeSH descriptor Progestins explode all trees
progesteron* in Clinical Trials
progestin* in Clinical Trials
progest?gen* in Clinical Trials
MeSH descriptor Estrogens explode all trees
?estrogen* in Clinical Trials
?estr?diol* in Clinical Trials
(#9 OR #10 OR #11 OR #12)
(#13 OR #14 OR #15)
(#8 AND #16 AND #17)
Appendix 2. CINAHL Search Strategy
Limiters/Expanders: All terms were expanded. Search mode: Boolean/Phrase
(MM "Abortion, Spontaneous+") OR (MH "Abortion, Habitual")
miscarriag*
abortion*
pregnan* N3 los*
(f?etal or f?etus*) and (death* or die* or dead or decease*)
recurrent N2 abort*
S1 or S2 or S3 or S4 or S5 or S6
(MM "Progesterone+")
progesteron*
(MM "Progestational Hormones+")
progest?gen*
S8 or S9 or S10 or S11
(MH "Estrogens+") OR (MM "Phytoestrogens+")
?estrogen*
?estr?di?l*
S13 or S14 or S15
(MH "Clinical Trials") OR (MH "Preventive Trials") OR (MH "Intervention Trials") OR (MH "Random Assignment")
PT clinical trial*
AB randomi?ed control* trial*
AB control* clinical trial*
AB randomi?ed
AB placebo*
AB randomly
TI trial
random* N2 allocat*
(MH "Quantitative Studies") OR (MH "Experimental Studies") OR (MH "Comparative Studies") OR (MH "Double‐Blind Studies") OR (MH "Triple‐Blind Studies")
S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26
(MM "Animals") OR (MM "Animals, Laboratory") OR (MM "Animal Studies")
S27 not S28
S7 and S12 and S16 and S29
Appendix 3. Embase Search Strategy
exp SECOND TRIMESTER ABORTION/ or exp SPONTANEOUS ABORTION/ or exp IMMINENT ABORTION/ or exp RECURRENT ABORTION/
miscarriag$.tw.
miscarriage/
abortion$.tw.
(pregnan$ adj3 los$).tw.
((f?etal or f?etus$) adj3 (death$ or die$ or dead or decease$)).tw.
(recurrent adj miscarriag$).tw.
(recurrent adj abort$).tw.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
exp PROGESTERONE/ or exp ESTRADIOL BENZOATE PLUS PROGESTERONE/ or exp PROGESTERONE DERIVATIVE/
progesteron$.tw.
progestin$.tw.
progest?gen$.tw.
10 or 11 or 12 or 13
exp ESTROGENS/
?estrogen$.tw.
?estr?di?l$.tw.
15 or 16 or 17
exp controlled clinical trial/
randomi?ed control$ trial$.mp.
randomi?ed.ti,ab,sh.
randomly.ti,ab,sh.
placebo$.ti,ab,sh.
trial.ti.
(random$ adj allocat$).ti,ab,sh.
clinical trials as topic/
control$ clinical trial$.mp.
19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
animals/ not (humans/ and animals/)
28 not 29
9 and 14 and 28 and 30
Appendix 4. Embase Classic (1947 to 1973) Search Strategy
exp RECURRENT ABORTION/ or exp SECOND TRIMESTER ABORTION/ or exp IMMINENT ABORTION/ or exp ABORTION/ or exp SPONTANEOUS ABORTION/
miscarriag$.tw
miscarriage/
abortion$.tw.
(pregnan$ adj3 los$).tw.
(f?etal adj3 (death$ or die$ or dead or decease$)).tw.
1 or 2 or 3 or 4 or 5 or 6
exp PROGESTERONE/ or exp ESTRADIOL BENZOATE PLUS PROGESTERONE/
progesteron$.tw.
progestin$.tw.
progest?gen$.tw.
8 or 9 or 10 or 11
exp CONJUGATED ESTROGEN/ or exp CATECHOL ESTROGEN/ or exp ESTROGEN/ or exp ESTROGEN THERAPY/
?estrogen$.tw.
?estr?di?l$.af.
13 or 14 or 15
controlled study/
exp controlled clinical trial/
exp PROSPECTIVE STUDY/ or exp QUANTITATIVE STUDY/ or exp REPLICATION STUDY/ or exp COMPARATIVE STUDY/ or exp EXPERIMENTAL STUDY/ or exp CLINICAL STUDY/ or exp IN VIVO STUDY/ or exp PREVENTION STUDY/ or exp INTERVENTION STUDY/
17 or 18 or 19
animal/ not (human/ and animal/)
20 not 21
7 and 12 and 16 and 22
Appendix 5. MEDLINE Search Strategy
exp Abortion, Habitual/ or exp Abortion, Spontaneous/
miscarriag$.tw.
miscarriage/
abortion$.tw.
(pregnan$ adj3 los$).tw.
((f?etal or f?etus*) adj3 (death$ or die$ or dead or decease$)).tw.
(recurrent adj abort$).tw.
1 or 2 or 3 or 4 or 5 or 6 or 7
exp Progesterone Congeners/ or exp Progesterone/
exp Progestins/
progesteron$.tw.
progestin$.tw.
progest?gen$.tw.
9 or 10 or 11 or 12 or 13
exp "Estrogens, Conjugated (USP)"/ or exp "Estrogens, Esterified (USP)"/ or exp Estrogens/ or exp Estrogens, Catechol/ or exp Estrogens, Non‐Steroidal/
?estrogen$.tw.
exp Ethinyl Estradiol/ or exp Estradiol/ or exp Estradiol Congeners/ or exp Ethinyl Estradiol‐Norgestrel Combination/
?estr?di?l$.tw.
15 or 16 or 17 or 18
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
exp animals/ not humans.sh
28 not 29
8 and 14 and 19 and 28
Appendix 6. OLDMEDLINE (1946 to 1965) Search Strategy
exp Abortion, Spontaneous/ or exp Abortion, Threatened/ or exp Abortion, Habitual/
miscarriag*.tw.
miscarriage/
abortion$.tw.
(pregnan$ adj3 los$).tw.
((f?etal or f?etus*) adj3 (death$ or die$ or dead or decease$)).tw.
(recurrent adj abort$).tw.
1 or 2 or 3 or 4 or 5 or 6 or 7
exp Progesterone/
exp Progestins/
progesteron*.tw.
progestin*.tw.
progest?gen$.tw.
9 or 10 or 11 or 12 or 13
exp "Estrogens, Conjugated (USP)"/ or exp Estrogens/
exp Estradiol Congeners/ or exp Estradiol/ or exp Ethinyl Estradiol/
?estrogen$.tw.
?estr?di?l$.tw.
15 or 16 or 17 or 18
8 and 14 and 19
Data and analyses
Comparison 1. Combined oestrogen and progestogen (other than progesterone) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Miscarriage | 1 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.32, 2.80] |
| 2 Perinatal death | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.69] |
| 3 Preterm birth (less than 34 weeks of gestation) | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.04] |
| 4 Low birthweight of less than 2500 g | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.43, 1.77] |
| 5 Genital abnormalities in offspring | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.60, 4.08] |
| 6 Abnormalities other than of the genital tract in offspring | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.14, 1.30] |
| 7 Cancer in the reproductive system in offspring | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.12, 68.29] |
| 8 Cancer other than of the reproductive system in offspring | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.12, 68.29] |
| 9 Cancer in the reproductive system in mothers | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 6.65 [1.56, 28.29] |
| 10 Cancer other than of the reproductive system in mothers | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.9 [0.36, 10.07] |
Comparison 2. Combined oestrogen and progesterone versus no hormonal treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Miscarriage | 1 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.23, 1.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
MRC 1955.
| Methods | Simple, stratified randomisation in a multicentre trial. Stratified by age and parity. Timing of randomisation: occurred before assessment for eligibility. Baseline characteristics in each intervention group were established and made comparable. |
|
| Participants | From across 9 centres, 161 pregnancies of women with diabetes in their 16th week of gestation or less were randomised. 14 were excluded (8 not pregnant prior to intervention use, 2 whose gestational age surpassed their '16 weeks or less' criteria, 4 "lacked cooperation or some other complication supervened"). Remaining 147 were analysed (71 control, 76 intervention). Age: all participants were aged 40 or below. Treatment group mean age was 22.4. Control group mean age was 20.4. Country: UK. Date of study: July 1950 to January 1953. |
|
| Interventions | Control: placebo tablets identical to intervention. Intervention: oral diethylstilboestrol (Stilbestrol) and ethisterone in increasing doses. Dosage and duration: (in mg/day)
|
|
| Outcomes | Miscarriage (defined as fetal death before 28 weeks of gestation). Stillbirth (defined as expulsion after 28 weeks' gestation without breath or showing any signs of life). Neonatal death (defined as death after showing signs of life). Living children (defined as children surviving for at least 1 month. This group was followed up for at least 6 months). Maternal death. Preterm birth (not defined, but delivery times were tabulated by week of delivery). Birthweight (not defined, but weights were tabulated by whole pounds). Congenital abnormalities (not defined). Pregnancy complications: oedema, albuminuria, toxaemia and hydramnios. Outcomes from follow‐up study on mothers Death in later life. Cancer in the reproductive system. Cancer other than of the reproductive system. Outcomes from follow‐up study on offspring Death in later life. Cancer in the reproductive system. Cancer other than of the reproductive system. |
|
| Notes | UK's Medical Research Council appointed a conference committee to conduct study. Report was prepared by DD Reid. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified by age and parity, and simple with an 1:1 allocation ratio. Baseline characteristics in both groups were similar. |
| Allocation concealment (selection bias) | Low risk | Sequence was controlled by a central office. Participants given a series number which was attached to their clinical record sheet and bottle of tablets. Treatment tablets, placebo tablets and packaging were made identical such that both participant and personnel would not know which intervention was received. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment tablets, placebo tablets and packaging were made identical such that both participant and personnel would not know which intervention was received. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Detection bias is low risk since the outcome is miscarriage. The outcome measurement is not likely to be influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 exclusions (10 control, 4 treated) from entire analysis were explained: 5 miscarried before commencing intervention, 3 were non‐pregnancies, 2 had advanced beyond the 16 weeks age criteria, 4 "lacked cooperation or some other complication supervened". For the outcomes of miscarriage, there were no further exclusions. For the outcome of preterm birth, 1 set of twins was excluded (2 control group). For the outcome of low birth weight, 1 stillbirth was excluded (treated group) due to unstated reason. Exclusions in follow‐up study of mothers A total of 136 women were included. For outcomes of death, genital tract cancer and non‐genital tract cancer, 5 exclusions were due to emigration (1 control), untraceable medical records/GP reluctant to fill out questionnaire /'not traced' (3 controls, 1 treated). Exclusions in follow‐up study of offspring A total of 136 offspring, including stillbirths, were included. For outcomes of genital tract abnormalities and non‐genital tract abnormalities, none were excluded. At long‐term follow‐up, 5 were excluded due to emigration (1 control, 1 treated) and adoption (1 control, 2 treated). For outcomes of genital tract cancer and non‐genital tract cancer, 12 were excluded (same 5 exclusions lost to follow‐up, 8 probably due to incomplete questionnaires from GP). Of the 8, 4 were control, 4 were treated. |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all pre‐specified outcomes were reported. |
| Other bias | Unclear risk | It is probable that 5 women were cross‐over participants whom each had 2 pregnancies during the original trial. In all 5 cases, their second pregnancy was allocated to the opposite intervention to that of the first pregnancy. This is not without concerns of order effects and carry‐over effects, but individual data on the 5 participants were not available. |
Prietl 1992.
| Methods | Quasi‐randomised trial. TIming of randomisation: once pregnancy was confirmed by rising HCG levels from day 13 to day 15 since oocyte retrieval in IVF. Baseline characteristics in each intervention group were established and deemed comparable. |
|
| Participants | 120 women having undergone IVF‐ET were allocated once pregnancy was confirmed (65 control, 55 intervention). Age: treatment group mean age was 31.7 +/‐ 0.7; age range was 25 to 39. Control group mean age was 32.8 +/‐ 0.7; age range was 26 to 40. Country: Germany. Date of study: September 1989, but end time not stated. |
|
| Interventions | Control group: no hormonal treatment during pregnancy. No placebo was given. Intervention group: intramuscular injection of 500 mg 17α‐hydroxyprogesterone caproate and 10 mg oestradiol valerate in an oily vehicle (Gravibinon) twice a week. Duration: from confirmation of pregnancy until the end of the 12th week of gestation. |
|
| Outcomes | Miscarriage (defined as loss between 7th and 12th week of gestation, confirmed by ultrasound and decrease in HCG). | |
| Notes | Sources of funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation sequence was by year of birth. However, baseline characteristics in each intervention group were established and deemed comparable. |
| Allocation concealment (selection bias) | High risk | Sequence known to personnel. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo was given. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Detection bias is low risk since the outcome is miscarriage. The outcome measurement is not likely to be influenced by the lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants (1 control, 1 treated) were excluded due to ectopic pregnancies. |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all pre‐specified outcomes were reported. |
| Other bias | Unclear risk | Sources of funding not stated. |
HCG: human chorionic gonadotropin IVF‐ET: in‐vitro fertilisation and embryo transfer
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berle 1977 | This study was about treating established threatened miscarriage, rather than the prevention of miscarriage. |
| Crowder 1950 | This study was about treating established threatened miscarriage, rather than the prevention of miscarriage. This study randomised before accurate eligibility assessment, leading to the exclusion of over 20% of randomised participants. This study compared oestrogen and standard treatment versus standard treatment only. Although some in the oestrogen group also received progesterone, the criteria of selection for such added progesterone was not mentioned. Progesterone dosage was low (30 mg/day) and duration was short (hospitalisation period) whereas oestrogen use continued until the 28th week, hence the authors considered the progesterone component negligible. |
| Lightman 1999 | This study introduced intervention prior to established pregnancy. This study did not have a placebo/no treatment group. This study compared intramuscular progesterone and oestrogen versus vaginal progesterone and oestrogen. |
| Sathanandan 1991 | This study introduced intervention prior to established pregnancy. This study was semi‐randomised and did not assess any of our specified outcomes. |
Differences between protocol and review
Two changes were made from the protocol. In this review, we changed the criteria for included studies so that quasi‐randomised trials were included, but in keeping with the protocol, any quasi‐randomised trial included was labelled as high risk of bias in its sequence generation. The second change from our original protocol was that search strategies in OLDMEDLINE, MEDLINE, Embase Classic and Embase were based upon results that started from an earlier year of publication. This change reflected the newly default ranges of publication dates set within the mentioned databases. Nonetheless, since earlier dates were used in our searches we can only be more confident that more literature was reviewed rather than less.
Contributions of authors
Danforn Lim (DL) is guarantor for the review and developed the concept of topic. The topic was conceived by LC and FW. DL and KH completed the independent assessment of studies for inclusion, assessment of trial quality and data extraction. DL, LC, and KH were involved in developing the protocol and final review. All authors reviewed the final version. LC provided a methodological and statistical perspective and FW provided a clinical perspective.
Declarations of interest
None known.
New
References
References to studies included in this review
MRC 1955 {published data only}
- Beral V, Colwell L. Randomised trial of high doses of stilboestrol and ethisterone in pregnancy: long‐term follow‐up of mothers. British Medical Journal 1980;281:1098‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beral V, Colwell L. Randomised trial of high doses of stilboestrol and ethisterone therapy in pregnancy: long‐term follow‐up of the children. Journal of Epidemiology & Community Health 1981;35:155‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council. The use of hormones in the management of pregnancy in diabetics. Lancet 1955;2:833‐6. [PubMed] [Google Scholar]
Prietl 1992 {published data only}
- Prietl G, Diedrich K, Ven HH, Luckhaus J, Krebs D. The effect of 17alpha‐hydroxyprogesterone caproate/oestradiol valerate on the development and outcome of early pregnancies following in vitro fertilization and embryo transfer: a prospective and randomized controlled trial. Human Reproduction 1992;7(1):1‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Berle 1977 {published data only}
- Berle P, Behnke K. The treatment of threatened abortion [Uber Behandlungserfolge der drohenden Fehlgeburt]. Geburtshilfe und Frauenheilkunde 1977;37(2):139‐42. [PubMed] [Google Scholar]
Crowder 1950 {published data only}
- Crowder RE, Bills ES, Broadbent JS. The management of threatened abortion: a study of 100 cases. American Journal of Obstetrics and Gynecology 1950;60(4):896‐9. [DOI] [PubMed] [Google Scholar]
Lightman 1999 {published data only}
- Lightman A, Kol S, Itskovitz‐Eldor J. A prospective randomized study comparing intramuscular with intravaginal natural progesterone in programmed thaw cycles. Human Reproduction 1999;14(10):2596‐9. [DOI] [PubMed] [Google Scholar]
Sathanandan 1991 {published data only}
- Sathanandan M, Macnamee MC, Rainsbury P, Wick K, Brinsden P, Edwards RG. Replacement of frozen‐thawed embryos in artificial and natural cycles: a prospective semi‐randomized study. Human Reproduction 1991;6(5):685‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Abu‐Musa 1998
- Abu‐Musa A, Hannoun A, Khalil A, Masaad Z, Karam K. Artificial preparation for oocyte donation using synthetic estrogen and progestogen. Clinical and Experimental Obstetrics and Gynecology 1998;25(3):83‐5. [PubMed] [Google Scholar]
Balasch 1987
- Balasch J. Luteal phase insufficiency: clinical aspects. Journal of Steroid Biochemistry 1987;27(1‐3):393‐7. [DOI] [PubMed] [Google Scholar]
Bamigboye 2003
- Bamigboye AA, Morris J. Oestrogen supplementation, mainly diethylstilbestrol, for preventing miscarriages and other adverse pregnancy outcomes. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004353] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 1980
- Barnes AB, Colton T, Gundersen J, Noller KL, Tilley BC, Strama T, et al. Fertility and outcome of pregnancy in women exposed in utero to diethylstilbestrol. New England Journal of Medicine 1980;302(11):609‐13. [DOI] [PubMed] [Google Scholar]
Bengtsson 1973
- Bengtsson LP. Hormonal effects on human myometrial activity. Vitamins and Hormones 1973;31:257‐303. [DOI] [PubMed] [Google Scholar]
Berger 1980
- Berger MJ, Goldstein DP. Impaired reproductive performance in DES‐exposed women. Obstetrics & Gynecology 1980;55(1):25‐7. [PubMed] [Google Scholar]
Bibbo 1977
- Bibbo M, Gill WB, Azizi F, Blough R, Fang VS, Rosenfield RL, et al. Follow‐up study of male and female offspring of DES‐exposed mothers. Obstetrics & Gynecology 197;49(1):1‐8. [PubMed] [Google Scholar]
Bibbo 1978
- Bibbo M, Haenszel WM, Wied GL, Hubby M, Herbst AL. A twenty‐five‐year follow‐up study of women exposed to diethylstilbestrol during pregnancy. New England Journal of Medicine 1978;298(14):763‐6. [DOI] [PubMed] [Google Scholar]
Burgoyne 1991
- Burgoyne PS, Holland K, Stephens R. Incidence of numerical chromosome anomalies in human pregnancy estimation from induced and spontaneous abortion data. Human Reproduction 1991;6(4):555‐65. [DOI] [PubMed] [Google Scholar]
Carrington 2005
- Carrington B, Sacks G, Regan L. Recurrent miscarriage: pathophysiology and outcome. Current Opinion in Obstetrics and Gynecology 2005;17:591‐7. [DOI] [PubMed] [Google Scholar]
Cunningham 2010
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY. Chapter 9. Abortion. Williams Obstetrics. 23rd Edition. New York: McGraw‐Hill Companies, 2010. [Google Scholar]
Davar 2007
- Davar R, Eftekhar M, Tayebi N. Transfer of cryopreserved‐thawed embryos in a cycle using exogenous steroids with or without prior gonadotrophin‐releasing hormone agonist. Journal of Medical Sciences 2007;7(5):880‐3. [Google Scholar]
Daya 2009
- Daya S. Luteal support: progestogens for pregnancy protection. Maturitas. Supplementum 2009;65:S29‐S34. [DOI] [PubMed] [Google Scholar]
de Ziegler 1990
- Ziegler D, Frydman R. Different implantation rates after transfers of cryopreserved embryos originating from donated oocytes or from regular in vitro fertilization. Fertility and Sterility 1990;54(4):682‐8. [DOI] [PubMed] [Google Scholar]
Devroey 1998
- Devroey P, Pados G. Preparation of endometrium for egg donation. Human Reproduction 1998;4(6):856‐61. [DOI] [PubMed] [Google Scholar]
Duan 2010
- Duan L, Yan D, Zeng W, Yang X, Wei Q. Effect of progesterone treatment due to threatened abortion in early pregnancy for obstetric and perinatal outcomes. Early Human Development 2010;86:41‐3. [DOI] [PubMed] [Google Scholar]
Everett 1997
- Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. BMJ 1997;315(7099):32‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 1971
- FDA Drug Bulletin. Diethylstilboestrol Contraindicated in Pregnancy. US Dept of Health, Education and Welfare, 1971. [Google Scholar]
Garcia‐Enguidanos 2002
- Garcia‐Enguidanos A, Calle ME, Valero J, Luna S, Dominguez‐Rojas V. Risk factors in miscarriage: a review. European Journal of Obstetrics & Gynecology and Reproductive Biology 2002;102:111‐9. [DOI] [PubMed] [Google Scholar]
Gates 2009
- Gates S. Cochrane Pregnancy and Childbirth Group Methodological Guidelines. http://pregnancy.cochrane.org/sites/pregnancy.cochrane.org/files/uploads/MethodologicalGuidelines%20%28January%202010%29.doc (accessed 2010).
Genuth 2006
- Genuth SM. Chapter 52: Female reproduction. In: Levy MN, Stanton BA, Koeppen BM editor(s). Berne & Levy Principles of Physiology. 4th Edition. Philadelphia: Elsevier Mosby, 2006:725‐46. [Google Scholar]
Goddijin 2000
- Goddijn M, Leschot NJ. Genetic aspects of miscarriage. Clinical Obstetrics and Gynecology 2000;14(5):855‐65. [DOI] [PubMed] [Google Scholar]
Goldberg 1999
- Goldberg JM, Falcone T. Effect of diethylstilbestrol on reproductive function. Fertility and Sterility 1999;72(1):1‐7. [DOI] [PubMed] [Google Scholar]
Haas 2008
- Haas DM, Ramsey PS. Progestogen for preventing miscarriage. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD003511.pub2] [DOI] [PubMed] [Google Scholar]
Habayeb 2004
- Habayeb OMH, Konje JC. The one‐stop recurrent miscarriage clinic: an evaluation of its effectiveness and outcome. Human Reproduction 2004;19(12):2952‐8. [DOI] [PubMed] [Google Scholar]
Hemminki 1999
- Hemminki E, Gissler M, Merilainen J. Reproductive effects of in utero exposure to estrogen and progestin drugs. Fertility and Sterility 1999;71(6):1092‐8. [DOI] [PubMed] [Google Scholar]
Herbst 1971
- Herbst AL, Ulfelder H, Poskanzer DC. Association of maternal stilbestrol therapy with tumor appearance in young women. New England Journal of Medicine 1971;284(16):878‐81. [DOI] [PubMed] [Google Scholar]
Herbst 1981
- Herbst AL. Clear cell adenocarcinoma and the current status of DES‐exposed females. Cancer 1981;48(2):484‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Janssen 1996
- Janssen HJEM, Cuisinier MCJ, Hoogduin KAL, Graauw KPHM. Controlled perspective study on the mental health of women following pregnancy loss. American Journal of Psychiatry 1996;153(2):226‐30. [DOI] [PubMed] [Google Scholar]
Johnson 1979
- Johnson LD, Driscoll SG, Hertig AT, Cole PT, Nickerson RJ. Vaginal adenosis in stillborns and neonates exposed to diethylstilbestrol and steroidal estrogens and progestins. Obstetrical and Gynecological Survey 1979;34(11):845‐6. [DOI] [PubMed] [Google Scholar]
Kullander 1976
- Kullander S, Kallen B. A prospective study of drugs and pregnancy. 3. Hormones. Acta Obstetricia et Gynecologica Scandinavica 1976;55(3):221‐4. [DOI] [PubMed] [Google Scholar]
Lee 1996
- Lee C, Slade P. Miscarriage as a traumatic event: a review of the literature and new implications for intervention. Journal of Psychosomatic Research 1996;40(3):235‐44. [DOI] [PubMed] [Google Scholar]
Lelaidier 1992
- Lelaidier C, Ziegler D, Gaetano J, Hazout A, Fernandez H, Frydman R. Controlled preparation of the endometrium with exogenous oestradiol and progesterone: a novel regimen not using a gonadotrophin‐releasing hormone agonist. Human Reproduction 1992;7(10):1353‐6. [DOI] [PubMed] [Google Scholar]
Li 2002
- Li TC, Makris M, Tomsu M, Tuckerman E, Laird S. Recurrent miscarriage: aetiology, management and prognosis. Human Reproduction Update 2002;8(5):463‐81. [DOI] [PubMed] [Google Scholar]
Liu 1991
- Liu H‐C, Rosenwaks Z. Early pregnancy wastage in IVF (in vitro fertilization) patients. Journal of in Vitro Fertilization and Embryo Transfer 1991;8(2):65‐72. [DOI] [PubMed] [Google Scholar]
Mills 1988
- Mills JL, Simpson JL, Driscoll SG, Jovanovic‐Peterson L, Allen MV, Aarons JH, et al. Incidence of spontaneous abortion among normal women and insulin‐dependent diabetic women whose pregnancies were identified within 21 days of conception. New England Journal of Medicine 1988;319(25):1617‐23. [DOI] [PubMed] [Google Scholar]
Muasher 1991
- Muasher SJ, Kruithoff C, Simonetti S, Oehninger S, Acosta AA, Jones GS. Controlled preparation of the endometrium with exogenous steroids for the transfer of frozen‐thawed pre‐embryos in patients with anovulatory or irregular cycles. Human Reproduction 1991;6(3):443‐5. [DOI] [PubMed] [Google Scholar]
Navot 1986
- Navot DN, Laufer NL, Kopolovic J, Rabinowitz R, Birkenfeld A, Lewin A, et al. Artificially induced endometrial cycles and establishment of pregnancies in the absence of ovaries. New England Journal of Medicine 1986;314:806‐11. [DOI] [PubMed] [Google Scholar]
Nesbitt 1965
- Nesbitt RE Jr, Aubry RH, Goldberg EM, Jacobs RD. Correlated hormone excretion patterns and cytohormone variations in normal and complicated pregnancies: Influence of administration of ovarian steroids or placebo in relation to outcome of pregnancy. American Journal of Obstetrics and Gynecology 1965;93(5):702‐19. [PubMed] [Google Scholar]
Oats 2010
- Oats J, Abraham S. Chapter 11 Miscarriage and abortion. In: Oats J, Abraham S editor(s). Llewellyn‐Jones Fundamentals of Obstetrics and Gynaecology. 9th Edition. Elsevier Limited, 2010:99‐106. [Google Scholar]
Palmer 2005
- Palmer JR, Wise LA, Robboy SJ, Titus‐Ernstoff L, Noller KL, Herbst AL, et al. Hypospadias in sons of women exposed to diethylstilbestrol in utero. Epidemiology 2005;16(4):583‐6. [DOI] [PubMed] [Google Scholar]
Perez 2005
- Perez KM, Titus‐Ernstoff L, Hatch EE, Troisi R, Wactawski‐Wende J, Palmer JR, et al. Reproductive outcomes in men with prenatal exposure to diethylstilbestrol. Fertility and Sterility 2005;84(6):1649‐56. [DOI] [PubMed] [Google Scholar]
Porter 2008
- Porter TF, Branch DW, Scott JR. Early pregnancy loss. In: Gibbs RS, Karlan BY, Haney AF, Nygaard IE editor(s). Danforth's Obstetrics & Gynecology. 9th Edition. Philadelphia: Lippincott Williams & Wilkins, 2008:61‐71. [Google Scholar]
Potdar 2005
- Potdar N, Konje JC. The endocrinological basis of recurrent miscarriages. Current Opinion in Obstetrics and Gynecology 2005;17:424‐8. [DOI] [PubMed] [Google Scholar]
Queenan 1994
- Queenan JT, Weeck LL, Seltman HJ, Muasher SJ. Transfer of cryopreserved‐thawed pre‐embryos in a natural cycle or a programmed cycle with exogenous hormonal replacement yields similar pregnancy results. Fertility and Sterility 1994;62(3):545‐50. [DOI] [PubMed] [Google Scholar]
Queenan 1997
- Queenan JT, Ramey JW, Seltman HJ, Eure L, Veeck LL, Muasher SJ. Transfer of cryopreserved‐thawed pre‐embryos in a cycle using exogenous steroids without prior gonadotrophin‐releasing hormone agonist suppression yields favourable pregnancy results. Human Reproduction 1997;12(6):1176‐80. [DOI] [PubMed] [Google Scholar]
Rao 1998
- Rao CV. Novel concepts in neuroendocrine regulation of reproductive tract functions. In: Bazer FW editor(s). Endocrinology of Pregnancy. Totowa: Humana Press Inc, 1998:125‐44. [Google Scholar]
RCOG 2003
- Regan L, Backos MJ, Rai R. The investigation and treatment of couples with recurrent miscarriage. Royal College of Obstetricians and Gynaecologists Green‐top Guideline No. 17. London: RCOG, 2003. [Google Scholar]
RCOG 2006
- Hinshaw K, Fayyad A, Munjuluri P. The management of early pregnancy loss. Royal College of Obstetricians and Gynaecologists Green‐top Guideline No. 25. London: RCOG, 2006. [Google Scholar]
Regan 1989
- Regan L, Braude PR, Trembath PL. Influence of past reproductive performance on risk of spontaneous abortion. BMJ 1989;299:541‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schindler 2005
- Schindler AE. Endocrinology of pregnancy: consequences for the diagnosis and treatment of pregnancy disorders. Journal of Steroid Biochemistry and Molecular Biology 2005;97:386‐8. [DOI] [PubMed] [Google Scholar]
Schmidt 1989
- Schmidt CL, Taney FH, Ziegler D, Kuhar MJ, Gagliardi CL, Colon JM, et al. Transfer of cryopreserved‐thawed embryos: the natural cycle versus controlled preparation of the endometrium with gonadotropin‐releasing hormone agonist and exogenous estradiol and progesterone (GEEP). Fertility and Sterility 1989;52(4):609‐16. [DOI] [PubMed] [Google Scholar]
Schorge 2008
- Schorge JO, Schaffer JI, Halvorson LM, Hoffman BL, Bradshaw KD, Cunningham FG. Chapter 15. Reproductive endocrinology. In: Schorge JO, Schaffer JI, Halvorson LM, Hoffman BL, Bradshaw KD, Cunningham FG editor(s). Williams Gynecology. New York: McGraw‐Hill Companies, 2008. [Google Scholar]
Silver 1999
- Silver RI, Rodriguez R, Chang TSK, Gearhart JP. In vitro fertilization is associated with an increased risk of hypospadias. Journal of Urology 1999;161:1954‐7. [PubMed] [Google Scholar]
Simpson 2007
- Simpson JL. Causes of fetal wastage. Clinical Obstetrics and Gynecology 2007;50(1):10‐30. [DOI] [PubMed] [Google Scholar]
Smith 1948
- Smith OW. Diethylstilboestrol in the prevention and treatment of complications of pregnancy. American Journal of Obstetrics and Gynecology 1948;56:821‐34. [PubMed] [Google Scholar]
Speroff 2005
- Speroff L, Fritz MA. Steroid hormones in pregnancy. In: Speroff L, Fritz MA editor(s). Clinical Gynecologic Endocrinology and Infertility. 7th Edition. Philadelphia: Lippincott Williams & Wilkins, 2005:259‐72. [Google Scholar]
Stirrat 1990
- Stirrat GM. Recurrent miscarriage I: definition and epidemiology. Lancet 1990;336:673‐5. [DOI] [PubMed] [Google Scholar]
Sullivan 1986
- Sullivan FM. Problems in the long‐term assessment of risks from drugs used in pregnancy maintenance. Human Reproduction 1986;1(7):459‐61. [DOI] [PubMed] [Google Scholar]
Thierstein 1959
- Thierstein ST, Rodler DEH. Norlutin and stilbestrol compared to progesterone and stilbestrol in treating threatened and repeated abortion. Nebraska State Medical Journal 1959;44:433‐41. [PubMed] [Google Scholar]
Toth 2010
- Toth B, Jeschk U, Rogenhofer N, Scholz C, Würfel W, Thaler CJ, et al. Recurrent miscarriage: current concepts in diagnosis and treatment. Journal of Reproductive Immunology 2010;85:25‐32. [DOI] [PubMed] [Google Scholar]
Tulppala 1993
- Tulppala M, Palosuo T, Ramsay T, Miettinen A, Salonen R, Ylikorkala O. A prospective study of 63 couples with a history of recurrent spontaneous abortion: contributing factors and outcome of subsequent pregnancies. Human Reproduction 1993;8(5):764‐70. [DOI] [PubMed] [Google Scholar]
Wahabi 2011
- Wahabi HA, Abed Althagafi NF, Elawad M, Al Zeidan RA. Progestogen for treating threatened miscarriage. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD005943.pub3] [DOI] [PubMed] [Google Scholar]
Walch 2008
- Walch KT, Huber JC. Progesterone for recurrent miscarriage: truth and deceptions. Best Practice and Research in Clinical Obstetrics and Gynaecology 2008;22(2):375‐89. [DOI] [PubMed] [Google Scholar]
Warburton 1964
- Warburton D, Fraser FC. Spontaneous abortion risks in man: data from reproductive histories collected in a medical genetics unit. Human Genetics 1964;15(1):1‐25. [PMC free article] [PubMed] [Google Scholar]
Wilcox 1988
- Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. New England Journal of Medicine 1988;319(4):189‐94. [DOI] [PubMed] [Google Scholar]
Wilkins 1960
- Wilkins L. Masculinization of female fetus due to use of orally given progestins. JAMA 1960;172(10):1028‐32. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lim DCE 2011
- Lim DCE, Cheng LNC, Ho KKW, Wong FWS. Combined oestrogen and progesterone for preventing miscarriage. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD009278] [DOI] [PMC free article] [PubMed] [Google Scholar]


