Abstract
Introduction
To investigate the relationship between long-term change trajectory in body mass index (BMI) and the hazard of type 2 diabetes among Chinese adults.
Research design and methods
Data were obtained from the China Health and Nutrition Survey (CHNS). Type 2 diabetes was reported by participants themselves in each survey wave. The duration of follow-up was defined as the period from the first visit to the first time self-reported type 2 diabetes, death, or other loss to follow-up from CHNS. The patterns of change trajectories in BMI were derived by latent class trajectory analysis method. The Fine and Gray regression model was used to estimate HRs with corresponding 95% CIs for type 2 diabetes.
Results
Four patterns of the trajectories of change in BMI were identified among Chinese adults, 42.7% of participants had stable BMI change, 40.8% for moderate BMI gain, 8.9% for substantial BMI gain and 7.7% for weight loss. During the follow-up with mean 11.2 years (158 637 person-years contributed by 14 185 participants), 498 people with type 2 diabetes (3.7%) occurred. Risk of type 2 diabetes was increased by 47% among people who gained BMI more substantially and rapidly (HR: 1.47, 95% CI 1.08 to 2.02, p=0.016) and increased by 20% among those in people with the moderate BMI gain (HR: 1.20, 95% CI 0.98 to 1.48, p=0.078), compared with those with stable BMI change.
Conclusions
Long-term substantial gain of BMI was significantly associated with an increased risk of type 2 diabetes in the Chinese adults.
Keywords: BMI, type 2 diabetes, longitudinal study, Chinese
Significance of this study.
What is already known about this subject?
Overweight or obese people have a higher risk of developing type 2 diabetes. However, the association between long-term change in body mass index (BMI) and the hazard of type 2 diabetes remains limited, especially in China.
What are the new findings?
A significant number (49.7%) of Chinese adults with normal weight might become overweight or obese during middle age.
Long-term substantial gain of BMI was significantly associated with a 47% increased hazard of type 2 diabetes in the Chinese adults.
No significant association was found between long-term BMI loss and the hazard of type 2 diabetes.
How might these results change the focus of research or clinical practice?
Controlling the speed of weight gain in life course might be an effective health promotion approach, which may further reduce hazard of type 2 diabetes in the Chinese population.
Introduction
Type 2 diabetes is a serious chronic disease including a group of metabolic disorders characterized by increased blood glucose concentration.1 It is a risk factor of subsequent cardiovascular disease later in life and causes a substantial burden of premature death and disability.2 Type 2 diabetes is also an important global public health problem, which affected 415 million people around the world.1 In China, the burden of type 2 diabetes has increased dramatically over recent decades, from approximately 1% in 1980,3 to around 11% in 2013.4 Understanding the etiological study of diabetes is crucial to the control and prevention of this disease.
It is known that people who are overweight or obese have a higher risk of developing type 2 diabetes. Body mass index (BMI) is a measurement of the degree of obesity. A meta-analysis of prospective cohort studies reported that compared with people with normal weight (BMI: 18.5–24.9), people who were overweight (BMI: 25–30) or obese (BMI: >30) were more likely to have type 2 diabetes, the relative risk (RR) was 2.99 (95% CI 2.42 to 3.72) and 7.19 (95% CI 5.74 to 9.00), respectively. A longitudinal investigation among Japanese showed that the HR for 1 kg/m2 BMI increase was 1.26 (95% CI 1.24 to 1.29) for men and 1.24 (95% CI 1.20 to 1.29) for women.5 A large body of evidence has shown that the high BMI increase was an independent and dose-dependent risk for the occurrence of type 2 diabetes.6–8
Studies exploring the association between long-term BMI change and the risk of type 2 diabetes reported inconsistent results. Some studies indicated that almost any BMI/weight gain was a risk factor for type 2 diabetes.9 10 Other studies showed that modest BMI/weight loss (7%–10%) resulted in a substantial reduction (30%) in incidence of type 2 diabetes.10–12 On the other hand, several observational studies have shown inclusive and even contradictory results in the association between BMI/weight loss and the risk of type 2 diabetes.13 14
With time goes by, for exploring both the etiological study and intervention programs of type 2 diabetes, more researchers and clinical doctors are carrying out extensive research on the long-term change in BMI with type 2 diabetes development.15 Some studies have investigated the long-term change in BMI before the onset of type 2 diabetes and found that the BMI increase over time was a risk factor of type 2 diabetes using data from repeated clinical measurements during follow-up examinations of USA and Western adults.16–18 However, in the previous studies exploring the relationship between change in BMI and the risk of type 2 diabetes, the change in BMI was usually defined as the difference in BMI measures of two time points. This approach assumed that the relationship between longitudinal change in BMI during life course and the risk of type 2 diabetes was simple linear, however, this assumption might not be realistic. Instead, a more appealing way of examining the long-term change in BMI is to model group-based trajectories of change in BMI based on repeated measures during life using latent class trajectory analysis (LCTA).19 With this method, the comprehensively understanding of longitudinal physiological changes during life is advanced substantially, which can help to investigate the relationship between change in BMI and risk of type 2 diabetes. Moreover, the prevalence of obesity showed a great difference between Western and Asian populations,20 and the previous studies based on Western populations might not accurately represent the changing patterns of body weight prior to type 2 diabetes diagnosis in Asians, especially in Chinese.21 22 For the same BMI level, Chinese might have a higher risk of type 2 diabetes than Caucasians,23 thus it is necessary to investigate the relationship between change in BMI and risk of type 2 diabetes in China.
To provide a scientific evidence for the association between long-term change in BMI and the hazard of type 2 diabetes among Chinese, we used the data from a large nationwide cohort study, the China Health and Nutrition Survey (CHNS), to examine the effect of trajectories of change in BMI derived by LCTA method on the hazard of type 2 diabetes among Chinese adults.
Research design and methods
Study design and participants
Data analyzed in our study were collected from CHNS, which is an ongoing, open-cohort, internationally, collaborative project to see how the social and economic transformation of Chinese society has affected the health and nutritional status of its population. The first wave of the CHNS was conducted in 1989, and it was subsequently performed in 1991, 1993, 1997, 2000, 2004, 2006, 2009, 2011 and 2015. Data from CHNS provide us an opportunity to explore the longitudinal effect of BMI change on the hazard of type 2 diabetes. A detailed description of the design and procedures of CHNS has been published elsewhere.24
Because the data from wave 1989 did not cover the complete characteristics of participants and the data from wave 2015 have not been completely released so far, the data used in our study were based on eight waves of the CHNS conducted from 1991 to 2011. The flow diagram of the study cohort is summarized in figure 1. A total of 33 407 participants with 102 082 visits were extracted from the original surveys. However, 12 660 participants were excluded due to have less than two visits during the follow-up duration. Among the remaining 20 747 participants, we further excluded 6562 (31.6%) ones from the current analysis, including 4475 adolescents (<18 years), 352 pregnant women, 1222 participants who have a history of metabolic-related disease (eg, 178 type 2 diabetes, 948 hypertension, 44 myocardial infarctions, 33 apoplexies and 19 cancers) and 513 participants with implausible outlying data (eg, weight >300 kg or <20 kg) at baseline. Finally, a total of 14 185 participants with 62 617 visits were included in the final analytic sample. Demographic characteristics of participants in final analytic sample and excluded samples were compared (online supplementary table 1).
Figure 1.
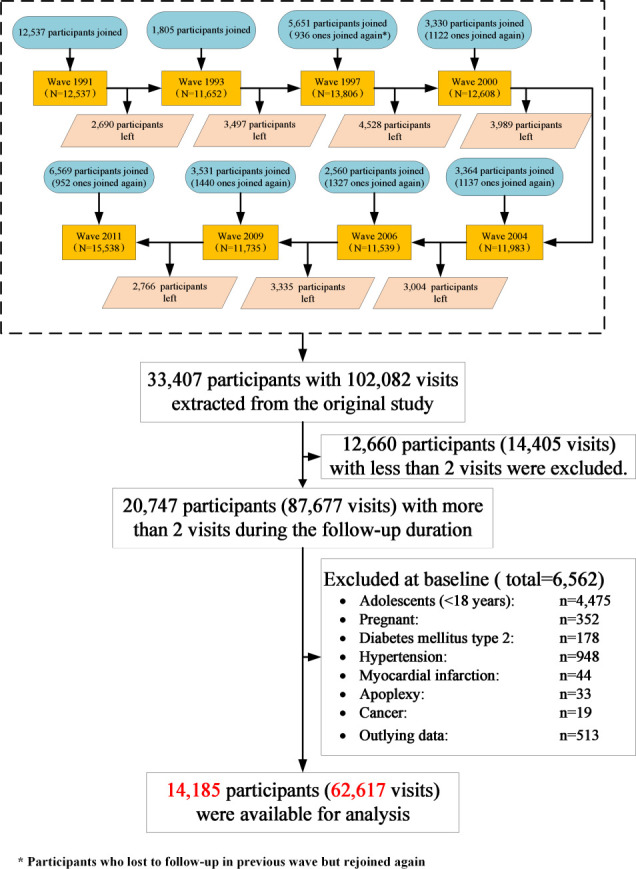
Flow diagram for cohort selection and censure.
bmjdrc-2019-000879supp001.pdf (527.2KB, pdf)
Definition of follow-up in the study
Participants included in this analysis were followed prospectively from the time of their first visit to the CHNS. As participants in this open cohort can join and leave the survey at any wave, participants lost to follow-up in one wave were still considered joining the survey in other waves. We define the duration of follow-up as the period from the first visit to the latest visit participants attended with the information about self-reported type 2 diabetes, competing risk event (death), or loss to follow-up from the CHNS study.
Self-reported diagnosed type 2 diabetes
The biomarker data were only collected from 2009 CHNS survey. Assessing diabetes according to self-report with only biomarker data available in 2009 might lead to the prevalence of diabetes being much higher in the 2009 survey than other surveys. This could bias the longitudinal association between trajectories of BMI change and the hazard risk of diabetes in our analysis. With this consideration, we finally only used self-reported diabetes information in our study. Type 2 diabetes was diagnosed by trained clinicians and reported by participants themselves in each survey wave. Participants were asked by the well-trained investigators whether they were diagnosed with type 2 diabetes by clinical measurements at each wave of this study. Answering ‘yes’ to the question ‘has a doctor ever told you that you suffer from diabetes?’ was defined as having self-reported diagnosed type 2 diabetes. Previous research supported that self-reported diabetes was a relatively valid tool to access the diabetes status of study participants in China.25 For those participants who were diagnosed with type 2 diabetes, the self-reported age of diagnosed type 2 diabetes was extracted from the question ‘How old were you when the doctor told you this? (years)’.
Anthropometry
Weight and height of the participants were collected by trained health workers using standard protocols, set by the WHO, at each wave.24 Weight was measured without shoes and wearing lightweight clothing to the nearest 0.1 kg using a calibrated beam scale, and height was measured to the nearest 0.2 cm without shoes using a portable SECA stadiometer. Three measurements were obtained per subject, and the mean value of three measurements was used in the analysis for both weight and height. BMI was calculated as weight (in kilograms) divided by the square of height (in meters).
Trajectories of change in BMI
In this study, trajectories of change in BMI were identified using LCTA, a method that has been used to identify unobserved trajectory classes in epidemiological data.26 LCTA has the advantage of identifying distinct groups with similar underlying trajectories.5 27 28 These trajectories can vary in functional form across a number of different order polynomials, allowing the best-fitting polynomial form to be specified for each trajectory separately. This property may be particularly important in our sample given the differential age-related patterns of change in BMI observed over the past 20 years in China.29 The procedure used to derive the trajectories is more fully described elsewhere.30 Briefly, LCTA was conducted using SAS V.9.4 (SAS Institute) with the TRAJ procedure.19 31 We modeled BMI change, calculated as current BMI minus baseline BMI and selected the best-fit models following standard guidelines.5 19 The best-fit models were chosen based on Bayesian Information Criteria, substantive knowledge about the BMI change over time and each trajectory containing at least 5% predicted sample size. Further information on the fitting of trajectory model and parameter estimation to describe the specific characteristics and differences of each trajectory was included in online supplementary table 2 amd 3. Once BMI change trajectories were determined, the individuals were assigned to the class with the highest posterior probability.27 Trajectory membership was then treated as an indicator variable in the study.
Covariates
Some potential covariates might confound the relation between BMI trajectories and type 2 diabetes in this study. Based on previous literatures,32–34 the demographical factors, health-related behavior factors and anthropometry (blood pressure and BMI) at baseline, which were found to be associated with type 2 diabetes, were considered as potential confounders.
Demographic variables included age, gender, education level, household income and urban-rural residence. Educational year was derived from questionnaire and divided into five categories: never, 6 years or less, 6–8 years, 9–11 years, and 12 years or higher. Household income was derived from total household income and measured in yuan.34
According to the answer ‘yes’ or ‘no’ to the question ‘Have you ever smoked cigarettes (including hand-rolled or device-rolled)’, participants were defined as smokers or non-smokers. To determine alcohol consumption, individuals were asked the question, ‘Have you consumed alcohol (beer, wine or other alcoholic beverage) during the past year (yes, no)?’. Participants were defined as drinker or non-drinker according to their answers of ‘yes’ or ‘no’.
We derived physical activity measures from a semiquantitative assessment used to determine their occupational, domestic, travel, and leisure physical activity levels. The intensity (metabolic equivalent, MET, unit kcal/kg/h) of each activity in the questionnaire was coded according to the compendium of physical activities.32 The total MET*hours/day was a combined score calculated by multiplying the frequency, duration, and intensity of physical activity.
The total energy intake was estimated by both the household and individual levels.34 Household food consumption was determined by conducting a detailed examination of changes in inventory from the beginning to the end of each day for 3 consecutive days in combination with a weighing technique. Individual dietary intake for 3 consecutive days was collected for every household member.
Furthermore, smoking, alcohol consumption, physical activity and dietary energy intake were predisposed as time-variant variables in current prospective cohort studies.35 In this analysis, we divided participants into four categories based on their changes in smoking and alcohol consumption behavior during follow-up period: (1) neither smoking/drinking at baseline nor at latest survey (never smoking/drinking); (2) smoking/drinking at latest survey but not at baseline (change to be a smoker/drinker); (3) smoking/drinking at baseline but not at latest survey (quit smoking/drinking); and (4) both smoking/drinking at baseline and latest survey (keep smoking/drinking). Change in physical activity and energy intake level from baseline to latest survey was classified as two categories (increase or decrease) by calculating their values at current minus those at baseline.
In order to avoid possible overadjustment, we used the directed acyclic graph (DAG) and change-in-estimation measures to identify the minimum set of variables to enter into the adjusted model.36 37 After selecting the model using the previously described DAG (online supplementary figure 1), the covariate for which removal caused the change in the exposure HR less than 0.1 (a 10% change) was removed. Seven variables (age in years as continues variable; gender, change in physical activity and change in dietary energy intake as binary variables; BMI at baseline, change in smoking, and change in alcohol consumption as categorical variables) were included in final analysis.
Statistical analysis
Sociodemographic and health-related characteristics of the study participants were summarized using the mean±SD for continuous variables, and count (proportion) for binary and categorical variables. Analysis of variance and χ2 tests were used to test differences between continuous variables and binary/categorical variables, respectively.
During the follow-up period, some participants might die before they were diagnosed with diabetes. Considering this assumption, we used a competing risk framework with deaths as competing events to estimate the cumulative incidence of type 2 diabetes and examine the association between patterns of trajectories of change in BMI and the hazards of self-reported type 2 diabetes. The Fine and Gray regression model was used to calculate the subdistribution HR for the occurrence of type 2 diabetes with corresponding 95% CIs,38 with time since entry into the study as the underlying timescale (model 1). We adjusted for covariates at baseline in several steps. We gradually added to the crude model those covariates considered to be potential confounders for the type 2 diabetes. Four adjusted models were built: (1) model 2 was adjusted for age and gender based on the crude model (model 1); (2) model 3 further adjusted change in smoking (never smoking, change to be a smoker, quit smoking and keep smoking), alcohol consumption (never drinking, change to be a drinker, quit drinking, keep drinking) and physical activity (increase and decrease) based on the model 2; (3) model 4 further adjusted for change in dietary energy intake (increase and decrease) based on the model 3. In addition, we used inverse probability of censorship weighting (IPCW) approach in model 5 to adjust for potential bias from loss to follow-up.39 40
Additional subgroup analyses were performed separately according to baseline characteristics as gender, BMI status at baseline, urbanization status (urban vs rural), age group (≤35 years vs >35 years) and change in health-related behaviors (smoking, alcohol consumption, physical activity and dietary energy intake), which would explore the stability and heterogeneity of association between the changing BMI and self-reported type 2 diabetes. Possible effect modification by those variables of the association between different patterns of BMI trajectories and the risk of type 2 diabetes was tested using Wald χ2 tests with adding each variable as primary predictor interaction terms. Effect estimates for the interaction term are shown only for models with a p<0.10.
Furthermore, to evaluate the statistical effect of missing covariates data on the association between covariates and type 2 diabetes, the multivariate analyses were also performed with multiple imputations by the chained equations method. First, missing data were assumed to be missing at random mechanism. We included BMI change trajectory patterns, self-reported type 2 diabetes and the other variables from model 5 in the imputation models. Discriminant functions for binary/categorical covariates and linear regression for continuous covariates were used to impute values. Since the maximum amount of missing among those variables was less than 15%, we generated 20 imputed data sets. The Fine and Gray’s model as done in aforementioned analysis was repeated using each of the augmented data sets, parameter estimates were averaged across the 20 analyses, and their SEs were computed using the Rubin method. Second, we used a pattern mixture method to evaluate the sensitivity of our results under the missing not at random (MNAR) assumption.41
All statistical tests were two sided, and p values less than 0.05 were considered statistically significant. Statistical analyses were performed using SAS V.9.4 (SAS Institute). Fine and Gray’s model was performed by using SAS PROC PHREG. Multiple imputation and MNAR sensitivity analysis were performed by using SAS PROCs MI and MIANALYZE.
Results
Pattern of BMI change trajectories and baseline information of participants of different BMI change trajectory pattern
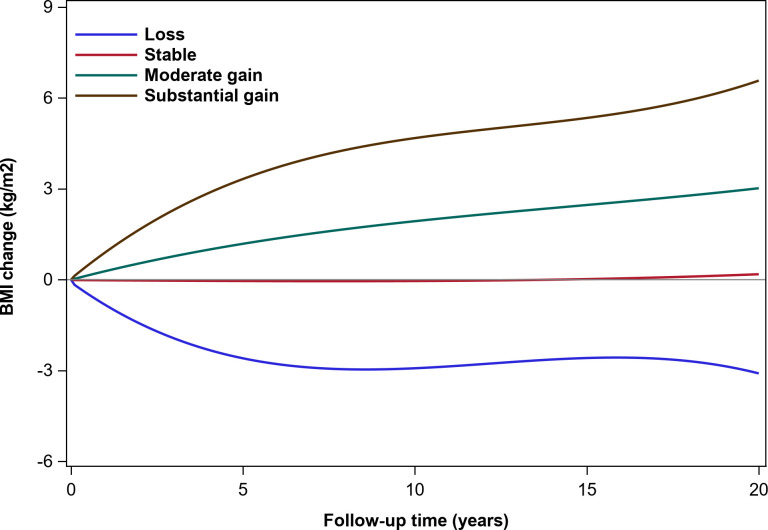
Among 14 185 participants with 62 509 BMI measurements, we identified four distinct patterns of trajectories of change in BMI (figure 2). The latent class pattern characterized by a stable BMI change indicated a total average change of nearly 0 kg/m2 during the entire follow-up and was termed as ‘Stable’, this pattern represented most of the participants (n=5858, 42.7%). The second pattern had a total average gain of almost 3 kg/m2 during follow-up and was termed as ‘Moderate gain’ (n=5609, 40.8%). The third pattern termed as ‘Substantial gain’ was characterized by a rapidly total average gain of nearly 6 kg/m2 (n=1217, 8. 9%). The fourth pattern had an average decrease of nearly 3 kg/m2 during the follow-up and was labeled ‘Loss’ (n=1052, 7.7%).
Figure 2.
Trajectory modeling identified four distinct body mass index (BMI) gain patterns.
Most characteristics were significantly different between the analytic and excluded samples (online supplementary table 1). The mean age of participants at baseline was 41.4±15.0 years, 53.2% of the participants were women. Approximately 45% of them had an education level of more than 9 years and about 64% lived in rural areas. Over 30% of participants were smoker or drinker. The mean physical activity was 6.7±10.9 MET/day. According to the pattern of trajectories of change in BMI, the comparison of the characteristics between the participants who were enrolled in present analysis was presented in table 1. Mean age, education level, urbanization, BMI/weight and diastolic blood pressure at baseline were significantly different among the patterns, whereas their gender, household income, change in smoking, change in alcohol consumption, change in physical activity level, dietary energy intake and duration of follow-up were similar.
Table 1.
Characteristics of participants among four groups of BMI gain trajectories (n=14 185*)
| Loss | Stable | Moderate gain | Substantial gain | P value | |
| Participants (n) | 1052 | 5858 | 5609 | 1218 | |
| BMI measurements during follow-up | 4.5±2.03 | 4.4±2.1 | 4.5±2.1 | 4.4±2.0 | 0.361 |
| Age (years) | 40.6±14.7 | 41.6±14.9 | 41.5±15.1 | 42.0±15.0 | <0.0001 |
| Female (%) | 559 (53.1) | 3128 (53.4) | 2981 (53.2) | 670 (55.1) | 0.980 |
| Education (years) | 6.9±4.2 | 6.6±4.2 | 6.4±4.3 | 6.5±4.3 | 0.004 |
| Never | 143 (15.0%) | 935 (17.7%) | 988 (19.5%) | 197 (18.2%) | 0.066 |
| <6 | 200 (21.0%) | 1109 (21.0%) | 1078 (21.3%) | 237 (21.9%) | |
| 6–8 | 177 (18.6%) | 939 (17.8%) | 894 (17.6%) | 189 (17.4%) | |
| 9–11 | 298 (31.3%) | 1639 (31.1%) | 1467 (28.9%) | 309 (28.5%) | |
| >12 | 134 (14.1%) | 649 (12.3%) | 647 (12.8%) | 152 (14.0%) | |
| Rural (%) | 605 (57.5) | 3590 (61.3) | 3796 (67.7) | 752 (61.8) | <0.001 |
| Household income (¥) | 6460.4±7203.9 | 6459.1±10 654.2 | 6194.3±11 095.0 | 6005.3±7415.9 | 0.453 |
| Anthropometry at baseline | |||||
| Height (cm) | 161.1±8.2 | 160.4±8.5 | 160.4±8.3 | 160.9±8.3 | 0.014 |
| Weight (kg) | 58.9±10.7 | 57.4±10.2 | 57.1±10.0 | 57.9±10.3 | <0.001 |
| BMI (kg/m2) | 22.6±3.3 | 22.3±3.1 | 22.1±3.0 | 22.3±3.2 | <0.001 |
| Lean (<18.5) | 77 (7.3%) | 481 (8.2%) | 485 (8.7%) | 97 (8.0%) | 0.004 |
| Normal (18.5–23.9) | 658 (62.6%) | 3875 (66.2%) | 3802 (67.8%) | 809 (66.5%) | |
| Overweight (24–27.9) | 249 (23.7%) | 1216 (20.8%) | 1066 (19.0%) | 250 (20.5%) | |
| Obesity (≥28) | 68 (6.5%) | 286 (4.9%) | 256 (4.6%) | 61 (5.0%) | |
| Systolic BP (mm Hg) | 117.2±16.6 | 116.9±17.6 | 116.2±18.0 | 117.1±18.1 | 0.114 |
| Diastolic BP (mm Hg) | 76.3±10.5 | 76.1±10.9 | 75.6±11.4 | 76.3±11.8 | 0.029 |
| Initial health-related behavior at baseline | |||||
| Cigarette smoker (%) | 328 (31.7) | 1891 (32.8) | 1812 (32.8) | 390 (32.6) | 0.922 |
| Alcohol drinker (%) | 406 (38.8) | 2168 (37.3) | 2020 (36.2) | 413 (34.2) | 0.081 |
| Physical activities (MET-hours/day) | 6.9±10.6 | 6.6±10.7 | 6.9±11.4 | 6.7±10.5 | 0.462 |
| Dietary total energy (kcal) | 2490.5±733.3 | 2475.2±780.0 | 2481.2±746.5 | 2446.9±710.5 | 0.493 |
| Latest health-related behavior | |||||
| Cigarette smoker (%) | 286 (28.1) | 1584 (28.3) | 1541 (28.7) | 310 (26.6) | 0.509 |
| Alcohol drinker (%) | 337 (33.0) | 1808 (32.2) | 1681 (31.3) | 341 (29.3) | 0.540 |
| Physical activities (MET-hours/day) | 20.2±18.5 | 19.1±19.8 | 18.8±19.8 | 19.4±19.5 | 0.184 |
| Dietary total energy (kcal) | 2147.9±951.4 | 2096.5±949.1 | 2070.1±897.8 | 2085.5±684.0 | 0.059 |
| Change in time-varying health-related behavior | |||||
| Smoking | |||||
| Never smoking | 640 (64.6%) | 3553 (64.6%) | 3358 (63.8%) | 749 (65.8%) | 0.338 |
| Change to be a smoker | 51 (5.2%) | 238 (4.3%) | 275 (5.2%) | 41 (3.6%) | |
| Quit smoking | 76 (7.7%) | 415 (7.6%) | 421 (8.0%) | 93 (8.2%) | |
| Keep smoking | 224 (22.6%) | 1291 (23.5%) | 1211 (23.0%) | 256 (22.5%) | |
| Alcohol consumption | |||||
| Never drinking | 538 (53.0%) | 3014 (54.0%) | 2951 (55.3%) | 669 (57.8%) | 0.501 |
| Change to be a drinker | 90 (8.9%) | 538 (9.6%) | 513 (9.6%) | 104 (9.0%) | |
| Quit drinking | 141 (13.9%) | 764 (13.7%) | 714 (13.4%) | 149 (12.9%) | |
| Keep drinking | 246 (24.2%) | 1263 (22.6%) | 1161 (21.8%) | 235 (20.3%) | |
| Physical activity change (MET-hours/day) | 13.17±20.12 | 12.25±21.12 | 11.60±21.14 | 12.30±21.04 | 0.121 |
| Increase | 666 (65.1%) | 3638 (64.5%) | 3449 (63.9%) | 776 (66.3%) | 0.619 |
| Decrease | 357 (34.9%) | 2000 (35.5%) | 1946 (36.1%) | 394 (33.7%) | |
| Dietary total energy level (kcal) | −341.57±1093.37 | −380.36±1142.08 | −411.34±1079.06 | −365.65±873.41 | 0.168 |
| Increase | 341 (33.3%) | 1774 (31.5%) | 1639 (30.4%) | 372 (31.8%) | 0.130 |
| Decrease | 682 (66.7%) | 3864 (68.5%) | 3756 (69.6%) | 798 (68.2%) | |
| Type 2 diabetes | 35 (3.3%) | 190 (3.2%) | 219 (3.9%) | 54 (4.4%) | 0.067 |
| Censored | 953 (90.6%) | 5236 (89.4%) | 4952 (88.3%) | 1079 (88.7%) | |
| Competing risk (death) | 64 (6.1%) | 432 (7.4%) | 438 (7.8%) | 84 (6.9%) | |
| Duration of follow-up (years) | 11.4±6.7 | 11.0±6.8 | 11.3±6.8 | 10.9±6.7 | 0.053 |
Values in the table are mean±SD or n (%); missing data are handled in the analysis.
*448 participants were failed to be classified into BMI trajectory groups.
BMI, body mass index; BP, blood pressure; MET, metabolic equivalent.
Association between BMI gain trajectories and type 2 diabetes
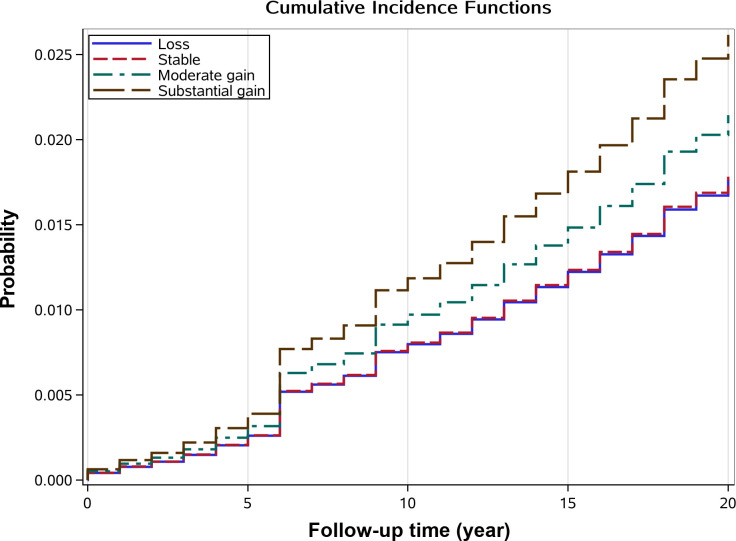
During the mean of 11.2 years (158 637 person-years) of follow-up, there were 498 self-reported type 2 diabetes (3.7%), 1071 deaths as competing risk (7.4%) and 12 167 (85.8%) censoring occurrence totally. Among those participants with self-reported type 2 diabetes, 190 were in the ‘Stable’ pattern (3.2%), 219 in the ‘Moderate gain’ pattern (3.9%), 54 in the ‘Substantial gain’ pattern (4.4%) and 35 in the ‘Loss’ pattern (3.3%). Cumulative hazard of self-reported type 2 diabetes is shown in figure 3. Compared with ‘Stable’ pattern, ‘Substantial gain’ (HR: 1.38, 95% CI 1.02 to 1.87, p=0.036) pattern was associated with higher hazard of type 2 diabetes. No significant associations were shown in both ‘Moderate gain’ (HR: 1.18, 95% CI 0.97 to 1.43, p=0.103) and ‘Loss’ patterns (HR: 1.01, 95% CI 0.70 to 1.45, p=0.965) with the risk of type 2 diabetes. Table 2 further summarized the Fine and Gray’s multiple regression analysis for the associations between different patterns of trajectories of change in BMI and the hazard of type 2 diabetes; the ‘Substantial gain’ pattern was still associated with the hazard of type 2 diabetes after adjusting for potential covariates with HR varying from 1.36 (95% CI 1.01 to 1.84, p=0.047 in model 2) to 1.50 (95% CI 1.10 to 2.02, p=0.010 in model 5).
Figure 3.
Cumulative incidence event risk of type 2 diabetes among different patterns of body mass index (BMI) change trajectory.
Table 2.
Multivariate Cox regression analysis for the associations between different patterns of BMI trajectories and the risk of self-reported type 2 diabetes
| BMI trajectory change pattern | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||
| HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | HR (95% CI) |
P value | |
| Loss | 1.01 (0.70 to 1.45) | 0.965 | 1.07 (0.75 to 1.53) | 0.721 | 1.05 (0.72 to 1.54) | 0.792 | 1.06 (0.72 to 1.55) | 0.768 | 0.99 (0.67 to 1.45) | 0.949 |
| Stable | Reference | Reference | Reference | Reference | Reference | |||||
| Moderate gain | 1.18 (0.97 to 1.43) | 0.103 | 1.16 (0.96 to 1.41) | 0.125 | 1.16 (0.94 to 1.42) | 0.169 | 1.15 (0.94 to 1.41) |
0.182 | 1.15 (0.94 to 1.42) | 0.174 |
| Substantial gain | 1.38 (1.02 to 1.87) | 0.036 | 1.36 (1.01 to 1.84) | 0.047 | 1.49 (1.09 to 2.03) | 0.012 | 1.49 (1.09 to 2.03) | 0.012 | 1.50 (1.10 to 2.05) | 0.010 |
Model 1 includes only the category of BMI trajectory change pattern.
Model 2 adds age and gender to model 1.
Model 3 includes covariates in model 2 plus smoke, drink, and physical activity.
Model 4 includes covariates in model 3 plus dietary energy intake.
Model 5 IPCW method adjusted for potential bias from loss to follow-up.
Bold font indicates significant association.
BMI, body mass index; IPCW, inverse probability of censorship weighting.
Exploring analysis
Additional subgroup analyses were presented in online supplementary figure 2, with a focus on exploring differences according to gender, BMI at baseline, urbanization, age and change in health-related behavior variables (smoking, drinking, physical activity and dietary energy intake) for the risk of type 2 diabetes. Those analyses assessed whether the effect of change in BMI trajectory was similar across subgroups. Subgroup analyses showed only effect on appreciable modification of BMI at baseline was significant (interaction p value <0.05). Furthermore, a sensitivity analysis showed that these trends were still unchanged in multiple imputation analysis (online supplementary table 5).
Discussion
Main findings
In the present study, we investigated the trajectories of change in BMI in China using LCTA method based on a 20-year longitudinal sample from the CHNS study. Four patterns of the trajectories of change in BMI were identified among Chinese adults. About 4 in 10 Chinese adults could keep their BMI stable and only a handful of adults had a decline in BMI. It is worth noting that 49.7% of Chinese adults had moderate/substantial BMI gain, which meant a total average increment of more than 3 kg/m2 of BMI during the entire period of follow-up. It implied that a significant number of Chinese adults with normal weight might become overweight or obese during the middle age. Additionally, we investigated the associations of the hazard of type 2 diabetes with those patterns using competing risk analysis and found that ‘Substantial gain’ trajectory of longitudinal change in BMI might increase significantly the risk of type 2 diabetes in Chinese adults compared with ‘Stable’ trajectory, even after adjustment for age, gender, initial BMI at baseline and change in health-related behaviors during the follow-up period.
Comparisons with other studies and implications of findings
The hazard of self-reported type 2 diabetes (3.7%, from 3.2% to 4.4% in each pattern) in our study was lower than those reported in other studies in the world (9.0% in men and 7.9% in women at 2014) and in China (11.2%).42 43 Variation in reported risk of type 2 diabetes might be attributed to the different assessment methods of diabetes between our study and other studies. As we described in the Research Design and Methods section, we finally decided to assess diabetes according to self-report, which could underestimate the prevalence of diabetes in our study. It was sufficiently discussed in the Study Strengths and Limitations section.
Our results showed that people experiencing long-term substantial gain of BMI were more likely to have type 2 diabetes. This was in line with previous studies in different cultural and socioeconomic setting population from Western to Asian countries.16–18 44 45 A prospective cohort study with 24 years of follow-up in the USA (n=114 824) indicated that BMI increase over time has been consistently shown as a risk factor of type 2 diabetes, and the women who had weight gain of 5.0–7.9 kg had 90% higher risk of type 2 diabetes.16 Another national cohort study in the USA (n=14 407) reported that increase in BMI may portend an increase in the incidence of type 2 diabetes.17 In addition, a prospective cohort study of university alumni in the USA (n=20 187) with over 30 years of follow-up indicated that BMI gain significantly increased the risk of type 2 diabetes with a positive dose–response relation.45 Recently, an observational prospective cohort study from the UK (n=6705) showed that nearly all adults who developed type 2 diabetes were with gradual increases in BMI (0.13 kg/m2 per year) over an 18-year observation period.18 In Asian population, a retrospective cohort study from Japan revealed that individuals who eventually progressed to type 2 diabetes experienced increasing levels of BMI in the early stage of the disease development. Their mean BMI changed from 23.7±2.3 kg/m2 at 10 years before diagnosis to 24.5±3.0 kg/m2 at 8 years before diagnosis, and then maintained stable over the 8 years before the diagnosis of type 2 diabetes.44 Our study analyzed the data of repeated BMI measurements during 20 years of follow-up and confirmed that the long-term substantial excess BMI gain could be a risk factor of type 2 diabetes in China. With controlling those key confounders reported in previous studies in our multivariate competing risk model step by step, our result was independent of differences in baseline characteristics, including age, gender, BMI at baseline and time-related variation of health-related behavior factors.
In addition to these similarities with the published literature, we further identified unique patterns of longitudinal change in BMI associated with hazard of type 2 diabetes. Four patterns of the trajectories of change in BMI were found among Chinese adults with LCTA method. Forty per cent of Chinese adults could keep their stable BMI in the course of life but an important finding was that near half of Chinese adults could experience moderate or substantial BMI gain in the course of life, which implied that they would be caught in the risk of having chronic diseases, such as hypertension.46 Our study found that hazard of type 2 diabetes was increased by 47% among people who gained BMI more substantially and rapidly (HR: 1.47, 95% CI 1.08 to 2.02) and increased by 20% among those in people with the moderate level of BMI gain (HR: 1.20, 95% CI 0.98 to 1.48), compared with those with stable BMI change. It has previously been proposed that more rapid weight gain may increase the hazard of type 2 diabetes in Japanese.47 Our results provide preliminary evidence that rapid and substantial BMI gain might place Chinese individuals at greater risk for type 2 diabetes. This finding implied that controlling the weight from early adulthood and restricting the speed of weight gain may be more effective for reducing the hazard of type 2 diabetes in middle-aged Chinese adults.
Beyond our expectation, there was not significant association found between long-term BMI loss and the hazard of type 2 diabetes in this study. This was inconsistent with previous studies in Western countries. The result from the Health Professionals Follow-up Study (n=22 171) in the USA showed that compared with men whose weight remained stable (±2 kg), men who lost weight more than 6 kg in 10 years had 50% lower risk of type 2 diabetes (RR: 0.5, 95% CI 0.3 to 0.9). Another prospective study that followed up 7176 British men during 20 years indicated that BMI loss was associated with lower risk of type 2 diabetes than the stable group (RR: 0.62, 95% CI 0.42 to 0.90).10 Recently, a pooled analysis of data from three European trial cohorts (n=739) reported participants with ≥5% BMI loss at 1 year had 65% lower risk of type 2 diabetes (HR: 0.35, 95% CI 0.22 to 0.56); maintaining ≥5% BMI loss longer could further reduce type 2 diabetes incidence.48 However, our results were consistent with some previous studies that long-term BMI loss did not decrease the risk of type 2 diabetes,13 14 especially in Asians populations.49 One of the possible mechanisms of the association between long-term substantial gain of BMI with the hazard of type 2 diabetes would be the altered adipose tissue distribution in individuals with more rapid weight gain resulting in higher insulin concentration associated.47 Further investigation is needed to explore the impact of long-term substantial gain of BMI on the pathology of type 2 diabetes.
Study strengths and limitations
Our study had some unique strengths worthy to mention. First, while a cluster randomized trial study (Da Qing Diabetes Prevention Study) has suggested that weight loss related to lifestyle intervention was an effective approach to prevent diabetes in China,50 there remains significant uncertainty of whether these interventions can reduce weight, especially given that participants in this intervention study were high-risk group (impaired glucose tolerance). Our population-based long-term follow-up study, with participants randomly selected from the mainland China and followed up nearly for 20 years, provides generalizable evidence of the long-term harmful impact of substantial and rapid weight gain to support public health programs. Second, with the design of cohort study, we were able to clearly indicate the temporal sequence association between longitudinal exposure of BMI change and the risk of type 2 diabetes. Finally, instead of using difference in BMI at only two time points in previous studies,13 15 17 we modeled the trajectory of change in BMI over time to represent the long-term tendency of BMI gain and loss. It could reflect the whole face of BMI change and its effect on occurrence of diabetes. This framework of modeling BMI trajectory could be a unique economic tool which may facilitate public health intervention, and could be widely used in primary health system for identifying person at high risk of diabetes.
Along with these strengths above, our study has several limitations. First, as we mentioned previously, assessment of type 2 diabetes in our study was based on participants’ self-report rather than medical record or diagnosis tests, which could induce the reporting/recalling bias. In view of the low diagnosis rate of type 2 diabetes in China, some actual patients who had not been diagnosed by the doctor reported their conditions as without type 2 diabetes. It might lead to underestimation of the prevalence of type 2 diabetes in our study (online supplementary table 4). To evaluate the degree of underestimation of diabetes in our study, we assessed diabetes based on self-report of diabetes and biomarker data in 2009 wave survey and found the prevalence of diabetes in 2009 was 8.6%, which was similar to the average prevalence of diabetes in the world and in China reported by other studies.42 43 It indicated that our studies might underestimate 50% risk of diabetes. However, with the consideration of that assessing diabetes according to self-report with only one biomarker datum collected in 2009 might bias the longitudinal association between trajectories of BMI change and the hazard risk of diabetes in our analysis, we finally only used self-reported diabetes information in our study. Second, although we reduced the possibility of confounding by controlling for relevant factors, it was still possible that the real effect could be overestimated or underestimated due to confounding by some genetic and environment factors not included in the present analysis, such as ethnicity, food security, sugar availability, impaired glucose tolerance and visceral fat distribution.1 51 Further studies are needed to explore the influence of those unmeasured confounders on the association between long-term physiological changes in the natural population and the hazard of type 2 diabetes. Third, subjects excluded from our analysis were seemed to be younger, higher household income level and higher education level (online supplementary table 1). We hope but did not have an opportunity to estimate the effect of BMI trajectory on the hazard of type 2 diabetes in those subjects. Thus, our results might not be generalizable to young adults, and high-income and highly educated people. Fourth, informative censoring due to loss to follow-up might limit the interpretation of our result.52 IPCW model was used to limit the bias of loss to follow-up and our main findings were unchanged in exploratory analyses (eg, subgroup analysis and multiple imputation), indicating the robustness of our study. Finally, competing risk bias could impact the results,53 because those who died during follow-up would have their outcomes censored, and it is unclear whether there is an association between death and weight trajectory groups. To reduce this bias, we used the Fine and Gray’s subdistribution hazard model with deaths as competing events to estimate the cumulative incidence of type 2 diabetes, which means that our findings are reliable.
Implication
Our study indicated that the long-term substantial gain of BMI was significantly associated with an increased hazard of type 2 diabetes in the Chinese adults. Our findings provided an implication that controlling the speed of weight gain in life course might be an effective health promotion approach, and further may reduce hazard of type 2 diabetes in the Chinese population. Further studies are needed to evaluate the potential biological pathways between long-term weight gain and type 2 diabetes pathogenesis.
Acknowledgments
This research uses data from the China Health and Nutrition Survey (CHNS). We thank the National Institute of Nutrition and Food Safety, the Chinese Center for Disease Control and Prevention, the Carolina Population Center, the University of North Carolina at Chapel Hill, the National Institutes of Health (NIH; R01-HD30880, DK056350, and R01-HD38700), and the Fogarty International Center, NIH, for statement of authors’ contributions to manuscript financial support for the CHNS data collection and analysis files from 1991 to 2011.
Footnotes
Contributors: BM, YZ, SD and HY designed the research. BM, CW, XG, YZ and SD conducted the study. BM, CW, XG, WW and JD analyzed the data. BM, CW, YZ, DW and SD wrote the initial draft of the manuscript. BM had the primary responsibility for the final content. All authors read and approved the final manuscript.
Funding: This work was supported by the Chinese National Key Research and Development Program of China (grant numbers: 2017YFC0907200 and 2017YF0907201), the National Natural Science Foundation of China (grant number: 81230016), the Natural Science Basic Research Plan of the Shaanxi Province (grant Number: 2020JQ-090), the Fundamental Research Funds for the Central Universities (grant Number: xzy032020033) and the Shaanxi Health and Family Planning Commission (grant number: Sxwsjswzfcght2016-013).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Informed consent was obtained from each participant, and the survey was approved by the institutional review committees of the University of North Carolina at Chapel Hill, the National Institute of Nutrition and Food Safety, the Chinese Center for Disease Control and Prevention, and the China-Japan Friendship Hospital, Ministry of Health. The current study was approved by the Human Research Ethics Committee of Xi’an Jiaotong University Health Science Center (No: XJTU 2020-32) and conducted according to the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. Original data are available in Carolina Population Center of the University of North Carolina at Chapel Hill (https://www.cpc.unc.edu/projects/china). Please contact the corresponding author to access the analytic data set relevant to this study.
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. . IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017;128:40–50. 10.1016/j.diabres.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in Diabetes-2016 abridged for primary care providers. Clin Diabetes 2016;34:3–21. 10.2337/diaclin.34.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danaei G, Finucane MM, Lu Y, et al. . National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. The Lancet 2011;378:31–40. 10.1016/S0140-6736(11)60679-X [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Gao P, Zhang M, et al. . Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017;317:2515–23. 10.1001/jama.2017.7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagin DS. Group-Based modeling of development. Harvard University Press, 2005. [Google Scholar]
- 6.Abdullah A, Peeters A, de Courten M, et al. . The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract 2010;89:309–19. 10.1016/j.diabres.2010.04.012 [DOI] [PubMed] [Google Scholar]
- 7.Sanada H, Yokokawa H, Yoneda M, et al. . High body mass index is an important risk factor for the development of type 2 diabetes. Intern Med 2012;51:1821–6. 10.2169/internalmedicine.51.7410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tirosh A, Shai I, Afek A, et al. . Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med 2011;364:1315–25. 10.1056/NEJMoa1006992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schienkiewitz A, Schulze MB, Hoffmann K, et al. . Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2006;84:427–33. 10.1093/ajcn/84.2.427 [DOI] [PubMed] [Google Scholar]
- 10.Wannamethee SG, Shaper AG, Walker M. Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes. J Epidemiol Community Health 2005;59:134–9. 10.1136/jech.2003.015651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing RR, Venditti E, Jakicic JM, et al. . Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care 1998;21:350–9. 10.2337/diacare.21.3.350 [DOI] [PubMed] [Google Scholar]
- 12.Long SD, O'Brien K, MacDonald KG, et al. . Weight loss in severely obese subjects prevents the progression of impaired glucose tolerance to type II diabetes. A longitudinal interventional study. Diabetes Care 1994;17:372–5. 10.2337/diacare.17.5.372 [DOI] [PubMed] [Google Scholar]
- 13.Kaneto C, Toyokawa S, Miyoshi Y, et al. . Long-Term weight change in adulthood and incident diabetes mellitus: my health up study. Diabetes Res Clin Pract 2013;102:138–46. 10.1016/j.diabres.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 14.Higgins M, D'Agostino R, Kannel W, et al. . Benefits and adverse effects of weight loss. observations from the Framingham study. Ann Intern Med 1993;119:758–63. 10.7326/0003-4819-119-7_Part_2-199310011-00025 [DOI] [PubMed] [Google Scholar]
- 15.Ye M, Robson PJ, Eurich DT, et al. . Changes in body mass index and incidence of diabetes: a longitudinal study of Alberta's tomorrow project cohort. Prev Med 2018;106:157–63. 10.1016/j.ypmed.2017.10.036 [DOI] [PubMed] [Google Scholar]
- 16.Colditz GA, Willett WC, Rotnitzky A, et al. . Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995;122:481–6. 10.7326/0003-4819-122-7-199504010-00001 [DOI] [PubMed] [Google Scholar]
- 17.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol 1997;146:214–22. 10.1093/oxfordjournals.aje.a009256 [DOI] [PubMed] [Google Scholar]
- 18.Vistisen D, Witte DR, Tabák AG, et al. . Patterns of obesity development before the diagnosis of type 2 diabetes: the Whitehall II cohort study. PLoS Med 2014;11:e1001602. 10.1371/journal.pmed.1001602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 2001;29:374–93. 10.1177/0049124101029003005 [DOI] [Google Scholar]
- 20.Yoon K-H, Lee J-H, Kim J-W, et al. . Epidemic obesity and type 2 diabetes in Asia. Lancet 2006;368:1681–8. 10.1016/S0140-6736(06)69703-1 [DOI] [PubMed] [Google Scholar]
- 21.Chan JCN, Malik V, Jia W, et al. . Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129–40. 10.1001/jama.2009.726 [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Ma W, Yuan Z, et al. . Association between obesity indices and type 2 diabetes mellitus among middle-aged and elderly people in Jinan, China: a cross-sectional study. BMJ Open 2016;6:e012742. 10.1136/bmjopen-2016-012742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Wang DD, Ley SH, et al. . Time trends of dietary and lifestyle factors and their potential impact on diabetes burden in China. Diabetes Care 2017;40:1685–94. 10.2337/dc17-0571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popkin BM, Du S, Zhai F, et al. . Cohort Profile: The China Health and Nutrition Survey--monitoring and understanding socio-economic and health change in China, 1989-2011. Int J Epidemiol 2010;39:1435–40. 10.1093/ije/dyp322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan X, Liu T, Wu L, et al. . Validity of self-reported diabetes among middle-aged and older Chinese adults: the China health and retirement longitudinal study. BMJ Open 2015;5:e006633. 10.1136/bmjopen-2014-006633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Croudace TJ, Jarvelin M-R, Wadsworth MEJ, et al. . Developmental typology of trajectories to nighttime bladder control: epidemiologic application of longitudinal latent class analysis. Am J Epidemiol 2003;157:834–42. 10.1093/aje/kwg049 [DOI] [PubMed] [Google Scholar]
- 27.Andruff H, Carraro N, Thompson A, et al. . Latent class growth modelling: a tutorial. Tutor Quant Methods Psychol 2009;5:11–24. 10.20982/tqmp.05.1.p011 [DOI] [Google Scholar]
- 28.Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods 1999;4:139–57. 10.1037/1082-989X.4.2.139 [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Du S, Zhai F, et al. . Trends in the distribution of body mass index among Chinese adults, aged 20–45 years (1989–2000). Int J Obes 2007;31:272–8. 10.1038/sj.ijo.0803416 [DOI] [PubMed] [Google Scholar]
- 30.Paynter L, Koehler E, Howard AG, et al. . Characterizing long-term patterns of weight change in China using latent class trajectory modeling. PLoS One 2015;10:e0116190. 10.1371/journal.pone.0116190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociol Methods Res 2007;35:542–71. 10.1177/0049124106292364 [DOI] [Google Scholar]
- 32.Ainsworth BE, Haskell WL, Herrmann SD, et al. . 2011 compendium of physical activities: a second update of codes and Met values. Med Sci Sports Exerc 2011;43:1575–81. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 33.Jones-Smith JC, Popkin BM. Understanding community context and adult health changes in China: development of an urbanicity scale. Soc Sci Med 2010;71:1436–46. 10.1016/j.socscimed.2010.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paeratakul S, Popkin BM, Keyou G, et al. . Changes in diet and physical activity affect the body mass index of Chinese adults. Int J Obes Relat Metab Disord 1998;22:424–31. 10.1038/sj.ijo.0800603 [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y, Li Y, Satija A, et al. . Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ 2019;365:l2110. 10.1136/bmj.l2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, et al. . Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol 2017;30:dyw341–94. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 37.Weng H-Y, Hsueh Y-H, Messam LLM, et al. . Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol 2009;169:1182–90. 10.1093/aje/kwp035 [DOI] [PubMed] [Google Scholar]
- 38.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 39.Pollock BD, Stuchlik P, Harville EW, et al. . Life course trajectories of cardiovascular risk: impact on atherosclerotic and metabolic indicators. Atherosclerosis 2019;280:21–7. 10.1016/j.atherosclerosis.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeon J, Jung KJ, Kimm H, et al. . Changes in secondhand smoke exposure levels and risk of type 2 diabetes in middle age: the Korean genome and epidemiology study (KoGES). BMJ Open Diabetes Res Care 2019;7:e000859-e:e000859 10.1136/bmjdrc-2019-000859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Buuren S. Flexible imputation of missing data. Chapman and Hall/CRC, 2018. [Google Scholar]
- 42.Li Y, Teng D, Shi X, et al. . Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American diabetes association: national cross sectional study. BMJ 2020;369:m997. 10.1136/bmj.m997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou B, Lu Y, Hajifathalian K, et al. . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30. 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heianza Y, Arase Y, Kodama S, et al. . Trajectory of body mass index before the development of type 2 diabetes in Japanese men: Toranomon Hospital Health Management Center Study 15. J Diabetes Investig 2015;6:289–94. 10.1111/jdi.12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oguma Y, Sesso HD, Paffenbarger RS, et al. . Weight change and risk of developing type 2 diabetes. Obes Res 2005;13:945–51. 10.1038/oby.2005.109 [DOI] [PubMed] [Google Scholar]
- 46.Ahn S, Zhao H, Smith ML, et al. . Bmi and lifestyle changes as correlates to changes in self-reported diagnosis of hypertension among older Chinese adults. J Am Soc Hypertens 2011;5:21–30. 10.1016/j.jash.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Yatsuya H, Li Y, et al. . Long-Term weight-change slope, weight fluctuation and risk of type 2 diabetes mellitus in middle-aged Japanese men and women: findings of Aichi workers' cohort study. Nutr Diabetes 2017;7:e252. 10.1038/nutd.2017.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Penn L, White M, Lindström J, et al. . Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European diabetes prevention study RCT. PLoS One 2013;8:e57143. 10.1371/journal.pone.0057143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishikawa-Takata K, Ohta T, Moritaki K, et al. . Obesity, weight change and risks for hypertension, diabetes and hypercholesterolemia in Japanese men. Eur J Clin Nutr 2002;56:601–7. 10.1038/sj.ejcn.1601364 [DOI] [PubMed] [Google Scholar]
- 50.Li G, Zhang P, Wang J, et al. . The long-term effect of lifestyle interventions to prevent diabetes in the China dA Qing diabetes prevention study: a 20-year follow-up study. Lancet 2008;371:1783–9. 10.1016/S0140-6736(08)60766-7 [DOI] [PubMed] [Google Scholar]
- 51.Kuwahara K, Honda T, Nakagawa T, et al. . Body mass index trajectory patterns and changes in visceral fat and glucose metabolism before the onset of type 2 diabetes. Sci Rep 2017;7:43521. 10.1038/srep43521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howe CJ, Cole SR, Lau B, et al. . Selection bias due to loss to follow up in cohort studies. Epidemiology 2016;27:91–7. 10.1097/EDE.0000000000000409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Walraven C, McAlister FA. Competing risk bias was common in Kaplan-Meier risk estimates published in prominent medical journals. J Clin Epidemiol 2016;69:170–3. 10.1016/j.jclinepi.2015.07.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-000879supp001.pdf (527.2KB, pdf)