Abstract
Background
Febrile neutropenia (FN) and other infectious complications are some of the most serious treatment‐related toxicities of chemotherapy for cancer, with a mortality rate of 2% to 21%. The two main types of prophylactic regimens are granulocyte (macrophage) colony‐stimulating factors (G(M)‐CSF) and antibiotics, frequently quinolones or cotrimoxazole. Current guidelines recommend the use of colony‐stimulating factors when the risk of febrile neutropenia is above 20%, but they do not mention the use of antibiotics. However, both regimens have been shown to reduce the incidence of infections. Since no systematic review has compared the two regimens, a systematic review was undertaken.
Objectives
To compare the efficacy and safety of G(M)‐CSF compared to antibiotics in cancer patients receiving myelotoxic chemotherapy.
Search methods
We searched The Cochrane Library, MEDLINE, EMBASE, databases of ongoing trials, and conference proceedings of the American Society of Clinical Oncology and the American Society of Hematology (1980 to December 2015). We planned to include both full‐text and abstract publications. Two review authors independently screened search results.
Selection criteria
We included randomised controlled trials (RCTs) comparing prophylaxis with G(M)‐CSF versus antibiotics for the prevention of infection in cancer patients of all ages receiving chemotherapy. All study arms had to receive identical chemotherapy regimes and other supportive care. We included full‐text, abstracts, and unpublished data if sufficient information on study design, participant characteristics, interventions and outcomes was available. We excluded cross‐over trials, quasi‐randomised trials and post‐hoc retrospective trials.
Data collection and analysis
Two review authors independently screened the results of the search strategies, extracted data, assessed risk of bias, and analysed data according to standard Cochrane methods. We did final interpretation together with an experienced clinician.
Main results
In this updated review, we included no new randomised controlled trials. We included two trials in the review, one with 40 breast cancer patients receiving high‐dose chemotherapy and G‐CSF compared to antibiotics, a second one evaluating 155 patients with small‐cell lung cancer receiving GM‐CSF or antibiotics.
We judge the overall risk of bias as high in the G‐CSF trial, as neither patients nor physicians were blinded and not all included patients were analysed as randomised (7 out of 40 patients). We considered the overall risk of bias in the GM‐CSF to be moderate, because of the risk of performance bias (neither patients nor personnel were blinded), but low risk of selection and attrition bias.
For the trial comparing G‐CSF to antibiotics, all cause mortality was not reported. There was no evidence of a difference for infection‐related mortality, with zero events in each arm. Microbiologically or clinically documented infections, severe infections, quality of life, and adverse events were not reported. There was no evidence of a difference in frequency of febrile neutropenia (risk ratio (RR) 1.22; 95% confidence interval (CI) 0.53 to 2.84). The quality of the evidence for the two reported outcomes, infection‐related mortality and frequency of febrile neutropenia, was very low, due to the low number of patients evaluated (high imprecision) and the high risk of bias.
There was no evidence of a difference in terms of median survival time in the trial comparing GM‐CSF and antibiotics. Two‐year survival times were 6% (0 to 12%) in both arms (high imprecision, low quality of evidence). There were four toxic deaths in the GM‐CSF arm and three in the antibiotics arm (3.8%), without evidence of a difference (RR 1.32; 95% CI 0.30 to 5.69; P = 0.71; low quality of evidence). There were 28% grade III or IV infections in the GM‐CSF arm and 18% in the antibiotics arm, without any evidence of a difference (RR 1.55; 95% CI 0.86 to 2.80; P = 0.15, low quality of evidence). There were 5 episodes out of 360 cycles of grade IV infections in the GM‐CSF arm and 3 episodes out of 334 cycles in the cotrimoxazole arm (0.8%), with no evidence of a difference (RR 1.55; 95% CI 0.37 to 6.42; P = 0.55; low quality of evidence). There was no significant difference between the two arms for non‐haematological toxicities like diarrhoea, stomatitis, infections, neurologic, respiratory, or cardiac adverse events. Grade III and IV thrombopenia occurred significantly more frequently in the GM‐CSF arm (60.8%) compared to the antibiotics arm (28.9%); (RR 2.10; 95% CI 1.41 to 3.12; P = 0.0002; low quality of evidence). Neither infection‐related mortality, incidence of febrile neutropenia, nor quality of life were reported in this trial.
Authors' conclusions
As we only found two small trials with 195 patients altogether, no conclusion for clinical practice is possible. More trials are necessary to assess the benefits and harms of G(M)‐CSF compared to antibiotics for infection prevention in cancer patients receiving chemotherapy.
Plain language summary
Prophylactic antibiotics or G(M)‐CSF for the prevention of infections in cancer patients undergoing chemotherapy
Review question
We reviewed the existing literature examining the efficacy and safety of granulocyte (macrophage) colony‐stimulating factors (G(M)‐CSF) compared to antibiotics to prevent infections for cancer patients receiving chemotherapy.
Background
Cancer treatment with chemotherapy (anti‐cancer drugs) disrupts the immune system and lowers white blood cell counts. This increases a person's risk of infection. Both G(M)‐CSF and antibiotics can reduce the risk of infection associated with cancer treatments. The review compared the efficacy of antibiotics to G(M)‐CSFs for the prevention of infection.
Study characteristics
We searched several medical databases and identified two randomised controlled trials (RCT) that met our inclusion criteria; no new trials were identified for this review update. One trial included 40 breast cancer patients receiving high‐dose chemotherapy. Eighteen patients received G‐CSF and 22 got antibiotics (ciprofloxacin and amphotericin) to prevent infection. Another trial evaluated GM‐CSF versus antibiotics in patients with small‐cell lung cancer, with 78 patients in the GM‐CSF arm and 77 patients in the antibiotics arm.
Key results
The study that analysed G‐CSF versus antibiotics did not report all cause mortality, microbiologically or clinically documented infections, severe infections, quality of life, or adverse events. We found no evidence of a difference between the two prophylactic options for the outcomes of infection‐related mortality (no patient died because of infection), or febrile neutropenia.
The trial that assessed GM‐CSF versus antibiotics did not found any evidence of a difference in all cause mortality, trial mortality, infections, or severe infections. The only difference between the two arms was found for the adverse event thrombocytopenia, favouring patients receiving antibiotics. Quality of life was not reported in this trial.
More research is needed to determine the best prevention against infection in cancer patients.
Quality of the evidence
The quality of the evidence for infection‐related mortality and frequency of febrile neutropenia in the G‐CSF trial was very low, because of the small number of patients that were evaluated, and the study design (high risk of bias). The trial that analysed GM‐CSF versus antibiotics reported overall survival, toxic deaths, infections, severe infections, and adverse events. Because of the very small number of patients included, we judged that the overall quality for all these outcomes was low.
The evidence is current to December 2015.
Summary of findings
Background
Description of the condition
Cancer patients receiving myelosuppressive therapy or haematopoetic stem cell transplantation are at increased risk of febrile neutropenia and infectious complications. The risk of febrile neutropenia and subsequent infection is directly related to the duration and severity of neutropenia (Bodey 1966; Bodey 1986). Infectious complications constitute major dose‐limiting side effects in patients undergoing myelosuppressive therapy. Special risk circumstances, such as patient age greater than 65 years or poor performance status, impact the associated morbidity and mortality (Kuderer 2006; Pizzo 1999). The mortality rate associated with febrile neutropenia in cancer patients is between 2% and 21% (Smith 2015). In addition, infectious complications are a common cause of dose reductions during chemotherapy treatment.
Febrile neutropenia (FN) can be prevented by a prophylactic regimen. Prophylaxis started at the beginning of the first chemotherapy cycle or in parallel with documented or anticipated neutropenia is called primary prophylaxis, whereas prophylaxis given to patients who had already experienced episodes of FN in an earlier chemotherapy cycle, is referred to as secondary prophylaxis. Effective prophylaxis, using either colony‐stimulating factors (CSF) or antibiotics (or both), would decrease clinically relevant negative outcomes such as all cause mortality, infection‐related mortality, and infectious complications. Given the high costs of the consequences of FN, and also of the colony‐stimulating factors themselves, economic arguments are introduced into discussions on the best prophylactic strategy (Kuderer 2006; Leibovici 2006).
In clinical trials addressing the prevention of FN, granulocyte‐macrophage colony‐stimulating factors (GM‐CSFs) have been reported to be effective in reducing the duration and severity of chemotherapy‐induced febrile neutropenia (Johnston 2000; Holmes 2002). Prophylaxis, using antibiotics, has also been shown to be beneficial with reduced fever, incidence of infections and hospitalisations (Bucaneve 2005; Cullen 2005).
Description of the intervention
Colony‐stimulating factors (CSF)
The current American Society of Clinical Oncology (ASCO) guidelines justify the administration of CSFs in clinical settings where the expected risk of suffering FN is approximately 20% (Smith 2015). In addition to the myelotoxicity of the planned chemotherapy regimen, patient‐specific risk factors should be taken into account. Secondary prophylaxis with CSFs is recommended for patients who have developed a neutropenic complication in a previous chemotherapy cycle, and in whom a reduced dose might compromise disease‐free or overall survival, or treatment outcome. The guidelines from the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO) give similar recommendations (Vehreschild 2014).
Thus far, randomised controlled trials (Crawford 1991; Trillet‐Lenoir 1993), and subsequent meta‐analyses, have shown that primary prophylaxis with CSFs is effective in reducing FNin patients with both solid and haematological malignancies (Bohlius 2008; Hackshaw 2004; Lyman 2002; Sung 2004; Sung 2007; Wittman 2006). Furthermore, GM‐CSFs may decrease hospitalisation and the use of intravenous therapeutic antibiotics (Crawford 1991; Trillet‐Lenoir 1993). In a meta‐analysis on the use of G‐CSFs in cancer patients hospitalised with established FN, the authors observed a possible benefit of adding GM‐CSFs to antibiotic treatment on infection‐related mortality and length of hospitalisation(Clark 2005). A meta‐analysis by Kuderer 2006 showed that under certain standard dose chemotherapy regimens, early and infection‐related mortality were also reduced with primary G‐CSF prophylaxis. However, none of the meta‐analyses with less restrictive inclusion criteria were able to demonstrate that prophylactic administration of GM‐CSFs improved overall survival when compared to placebo or no treatment. None of these analyses addressed the question of GM‐CSFs versus antibiotics, which is a question closer to clinical reality. One group did a subgroup analysis of studies in which the published report mandated antibiotic prophylaxis compared to those that did not, and found no difference between the groups (Sung 2007). This may be due to the high number of trials where no information about antibiotic prophylaxis use is available. In addition, this meta‐analysis included studies that analysed cycles of chemotherapy as opposed to patients. The distorting effect of such an analysis is difficult to estimate.
Of the many meta‐analyses looking at GM‐CSF versus placebo or no treatment, only one meta‐analysis, restricted to patients with lymphoma, was published in The Cochrane Library (Bohlius 2008). This analysis found a reduction in the rate of infections (odds ratio (OR) 0.74; 95% CI 0.64 to 0.85) but no effect on infection‐related mortality (OR 1.37 favouring control; 95% CI 0.66 to 2.82).
GM‐CSF is usually well tolerated, with only a moderate number of adverse events, mostly bone pain and headaches, however, there are some hints of increased risk of acute myeloid leukaemia or myelodysplastic syndromes (Lyman 2010).
Antibiotics
During the last decade, prophylaxis with antibiotics was studied in a number of randomised clinical trials. The evidence provided was not considered to be entirely convincing, because none of the studies were sufficiently large to provide conclusive evidence on the real efficacy of prophylaxis (Bucaneve 2005; Cullen 2005; Karp 1987; Lew 1995). Subsequent meta‐analyses suggested that prophylaxis using antibiotics reduced the incidence of gram‐negative bacterial infection, total infection, fever episodes, and hospitalisation (Cruciani 2003; Engels 1998). Moreover, a meta‐analysis of data on antibiotic prophylaxis (or more specifically, fluoroquinolones) compared to placebo or no intervention demonstrated that not only infections were reduced, but all cause mortality, and infection‐related mortality were too (Gafter‐Gvili 2005; Gafter‐Gvili 2012; Leibovici 2006). One important question which is still unanswered is whether prophylaxis should be considered for all patients with cancer and neutropenia. In another meta‐analysis on antibiotic prophylaxis, the majority of patients were suffering from haematological malignancies and received high‐dose chemotherapy and bone marrow transplantation, with only a few studies focusing on solid tumours (Cullen 2005; Gafter‐Gvili 2012). Another factor possibly compromising the results of the main meta‐analysis was that studies were included that randomised chemotherapy cycles and not patients, or reported cycle‐based outcomes, as opposed to a true incidence (where the number of patients and not cycles are analysed). No information on GM‐CSFs compared to antibiotics was available from these analyses.
How the intervention might work
Colony‐stimulating factors
Granulocyte colony‐stimulating factors (G‐CSF) predominantly augment the proliferation, maturation, and release of neutrophils, resulting in a dose‐dependent increase in circulating neutrophils (Bronchud 1988; Morstyn 1988). It is a growth factor for the myeloid lineage that stimulates the growth of granulocytes and eosinophil colonies; granulocyte (macrophage) colony‐stimulating factors (GM‐CSF) also stimulate the growth of macrophages (Griffin 1990). Both colony‐stimulating factors have shown comparable results in decreasing the incidence and duration of neutropenia and fever after chemotherapy. However, there is a lack of formal comparisons between the two drugs. Probably due to the macrophage activation caused by GM‐CSF, but not G‐CSF, tolerability of GM‐CSF has been reported to be inferior. Injection site reactions in particular, seem more frequent with GM‐CSF (Alvarado 1999; Beveridge 1997; Beveridge 1998; Fischmeister 1999; Hovgaard 1992). Given the undesired additional effects of GM‐CSF and concerns of tumour stimulation by GM‐CSF, the drug has become more or less disregarded by recent clinical studies and guidelines (Smith 2015). Granulocyte (macrophage) colony‐stimulating factors is no longer commercially available in several European countries for infection prophylaxis. It is licensed for mobilisation of stem cells, and after autologous or allogeneic stem cell transplantation (Smith 2015).
Antibiotics
Antibiotic prophylaxis, most often using flouroquinolones, reduces infections by targeting potential pathogens, and in contrast to G‐CSFs it does not provoke the dose‐limiting effect of haematological toxicity. A major concern of a routine prophylactic use of antibiotics in patients with cancer and neutropenia is that it increases bacterial resistance to these agents. This, in turn, may compromise the treatment success of both current and future serious infections by expanding (multi)resistance. In addition, hypersensitivity reactions, gastrointestinal toxicities, and the promotion of fungal overgrowth after antibiotics put the patient at risk of potentially serious adverse events. These factors may limit their efficacy in reducing infection‐related morbidity or mortality (Carratala 1995; Gafter‐Gvili 2007; Somolinos 1992).
Why it is important to do this review
The best prophylactic treatment of febrile neutropenia and infections in cancer patients receiving antineoplastic therapy remains controversial, and in general, international guidelines concentrate on either antibiotics or G‐CSFs. The evidence outlined above suggests that prophylaxis with an antibiotic might be as effective as with G‐CSFs for reducing both infections and mortality.
The aim of this systematic review is to provide a comprehensive overview on the benefits and harms of G‐CSF compared to antibiotics for infection prophylaxis in cancer patients. By systematically identifying all randomised trials conducted to date and by conducting a critical review of their reliability and validity, we will mitigate the statistical limitations of individual studies.
Objectives
To compare the efficacy and safety of G‐CSF or GM‐CSF compared to antibiotics in cancer patients receiving myelotoxic chemotherapy.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs). We excluded cross‐over trials and quasi‐randomised trials. We included full‐text, abstracts, and unpublished data if sufficient information on study design, participant characteristics, interventions and outcomes was available.
Types of participants
We planned to include paediatric and adult, male and female patients with a confirmed diagnosis of any type of cancer who were undergoing myelotoxic chemotherapy. Both solid and haematological malignancies were eligible.
Types of interventions
We included trials comparing G‐CSF or GM‐CSF to antibiotics in the primary prophylaxis of infection‐related complications. Trials that examined pegylated G‐CSF (pegfilgrastim) were eligible, provided pegfilgrastim was given once, 24 hours after the completion of chemotherapy.
Comparison 1
G‐CSF versus antibiotics
Comparison 2
GM‐CSF versus antibiotics
Trials looking at secondary prophylaxis, defined as prophylaxis in a patient who suffered from FN in an earlier course of chemotherapy, were also eligible, but a subgroup analysis was planned. However, we did not identify any trial evaluating secondary prophylaxis.
We included studies in which the intended chemotherapy regimen and supportive care did not differ between study arms. Therefore, we excluded studies that compared dose‐intensified, dose‐accelerated, or dose‐dense regimens with standard chemotherapy, as this resulted in different chemotherapy protocols in the arm that received antibiotic prophylaxis and the arm that received CSF prophylaxis. Trials with more than two arms were included, provided at least two arms with the relevant comparison had the same chemotherapy protocol.
We excluded trials using G‐CSF, GM‐CSF, or antibiotics to treat febrile neutropenia, fever, or infections.
Types of outcome measures
Primary outcomes
Overall survival
All cause mortality (including infection‐related, treatment‐related, or on‐trial mortality)
Infection‐related mortality
Studies focusing solely on the efficacy of prophylaxis will most likely have short‐term follow‐up only, mainly providing information on early mortality. Determining the cause of death in severely ill patients can be associated with measurement bias. Therefore, we extracted all cause mortality, comprising infection‐related and treatment‐related mortality.
Secondary outcomes
-
Microbiologically or clinically documented infections, or both
We accepted any definition of clinically documented or microbiologically documented infections given by authors. If available, we extracted data on all, not only severe, clinically or microbiologically documented infections. Microbiologically documented infections were required to have some kind of cultural confirmation of the infection. Infections reported without information on microbiological confirmation were considered to be clinically documented infections.
Severe infections
Frequency of febrile neutropenia (FN; any definition of fever and neutropenia accepted)
Quality of life (QoL; if measured with a validated QoL instrument)
Adverse events
Search methods for identification of studies
For this updated review, we revised the search strategy used for the first review. We used search strategies based on those described in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We did not use any language constraints.
Electronic searches
We searched the following electronic databases:
Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library, December 2015; see Appendix 1)
MEDLINE (1980 to December 2015; for search strategy see Appendix 2)
EMBASE (1980 to January 2008; for search strategy see Appendix 3)
Since we revised our searches, we re‐ran them for CENTRAL and MEDLINE for the entire period, i.e. 1980 to 2015.
Searching other resources
We searched conference proceedings of the following annual meetings, which were not included in CENTRAL for abstracts:
American Society of Hematology (ASH) from 2000 to 2015
American Society of Clinical Oncology (ASCO) from 2000 to 2015
European Hematology Association (EHA) from 2000 to 2015
We electronically searched the database of ongoing trials:
Metaregister of controlled trials
We handsearched the following references:
References of all identified trials, relevant review articles and current treatment guidelines
Data collection and analysis
Selection of studies
Two review authors (NS, OB) independently screened the results of the search strategies for eligibility by reading the abstracts. In the case of disagreement, we obtained the full‐text publication. If no consensus could be reached, we consulted a third review author, in accordance with Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We documented the study selection process in a flow chart as recommended in the PRISMA statement (Moher 2009), showing the total numbers of retrieved references and the numbers of included and excluded studies.
Data extraction and management
Two review authors independently extracted the data according to the guidelines proposed by Higgins 2011b. If required, we contacted authors of individual studies for additional information. We used a standardised data extraction form containing the following items:
General information: author; title; source; publication date; country; language; duplicate publications.
Quality assessment ('Risk of bias' assessment): sequence generation; allocation concealment; blinding (participants, personnel, outcome assessors); incomplete outcome data; selective outcome reporting; other potential sources of bias.
Study characteristics: trial design; aims; setting and dates; source of participants; inclusion and exclusion criteria; comparability of groups; subgroup analysis; statistical methods; power calculations; treatment cross‐overs; compliance with assigned treatment; length of follow‐up; time point of randomisation.
Participant characteristics: age; diagnosis; stage of disease; prior treatments; number of participants recruited, allocated, and evaluated; participants lost to follow‐up; noticeable differences in risk factors for developing FN.
Interventions: duration; type; dose and timing of GM‐CSF, G‐CSF, antibiotics, and other infection prophylaxes (e.g. antimycotics); concomitant treatment (setting, duration, type of chemotherapy); and supportive care (e.g. type of empirical antibiotic therapy).
Outcomes: all cause mortality; infection‐related mortality; microbiologically or clinically documented infections, or both; severe infections; QoL; frequency of FN; adverse events.
Assessment of risk of bias in included studies
Two review authors (NS and OB) independently assessed the risk of bias for each study using the following criteria outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions(Higgins 2011a).
Sequence generation
Allocation concealment
Blinding (participants, personnel, outcome assessors)
Incomplete outcome data
Selective outcome reporting
Other potential sources of bias
We made a judgement for every criterion, using one of three categories.
'Low risk': if the criterion was adequately fulfilled in the study, i.e. the study was at a low risk of bias for the given criterion.
'High risk': if the criterion was not fulfilled in the study, i.e. the study was at high risk of bias for the given criterion.
'Unclear': if the study report did not provide sufficient information to allow for a judgement of 'Yes' or 'No', or if the risk of bias was unknown for one of the criteria listed above.
Measures of treatment effect
We used intention‐to‐treat data. For binary outcomes, we calculated risk ratios (RRs) with 95% confidence intervals (CIs) for each comparison. We did not identify or extract time‐to‐event or continuous outcomes.
Unit of analysis issues
We evaluated the number of patients with events rather than number of episodes, as the second one could be biased (e.g. a patient with one episode of febrile neutropenia is at increased risk to have a second episode of febrile neutropenia).
Dealing with missing data
As suggested in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), there were many potential sources of missing data that had to be taken into account: at the study level, outcome level, and summary data level. It is important to distinguish between 'missing at random' and 'not missing at random'. As we only identified one trial without missing data, we did not contact the original investigators.
Assessment of heterogeneity
As we only found two trials, which we did not meta‐analyse, we did not assess heterogeneity of treatment effects between trials.
Assessment of reporting biases
In meta‐analyses with at least 10 trials included for one outcome, we would have explored potential publication bias by generating a funnel plot and statistically testing this by using a linear regression test (Sterne 2011). We would have considered a P value of less than 0.1 to be significant for this test. However, as we analysed two trials only, we did not generate a funnel plot.
Data synthesis
As we only identified one trial for each comparison, we could not pool data. However, to analyse data for individual studies we entered data into Review Manager (RevMan) 5.3.
Moreoever, we created 'Summary of findings' tables for each comparison on absolute risks in each group with the help of the GRADE approach, and will use it to summarise the evidence of all cause mortality, infection‐related mortality, quality of life, incidence of febrile neutropenia, incidence of severe infections and adverse events.
Subgroup analysis and investigation of heterogeneity
We had considered performing subgroup analyses using the following characteristics:
Different types of underlying malignant disease;
Different baseline risk for febrile neutropenia or infection;
Study setting (in‐patients or out‐patients);
Different type of treatment (e.g. haematologic stem cell transplantation versus standard chemotherapy);
Different types of G‐CSFs used;
Age (<18 versus ≥ 18 years); and
According to whether regimens included antimycotic prophylaxis.
However, as we had insufficient data to meta‐analyse, we could not perform these analyses.
Sensitivity analysis
We had considered performing sensitivity analyses using the following quality criteria:
Quality components with regard to low and high risk of bias;
Fixed‐effect modelling versus random‐effects modelling;
Duration of study; and
full‐text publication versus abstract publication only.
Again, as we identified only two trials, which were too heterogenous to pool, we could not perform these analyses.
Results
Description of studies
Results of the search
The literature search was designed to find all relevant articles where G‐CSFs, GM‐CSFs, or antibiotics were used as prophylactic agents. For this update, we set up a new search covering all time periods, i.e. after removing duplicates, we screened titles and abstracts of 11,785 references and excluded 11,696 at the initial stage. We assessed the full text of the remaining 89 references and excluded 87 references with reasons (see Excluded studies). As we identified no new trial fitting the inclusion criteria for this review update, we included the two already known trials in this review. See Figure 1 for study flow diagram.
1.
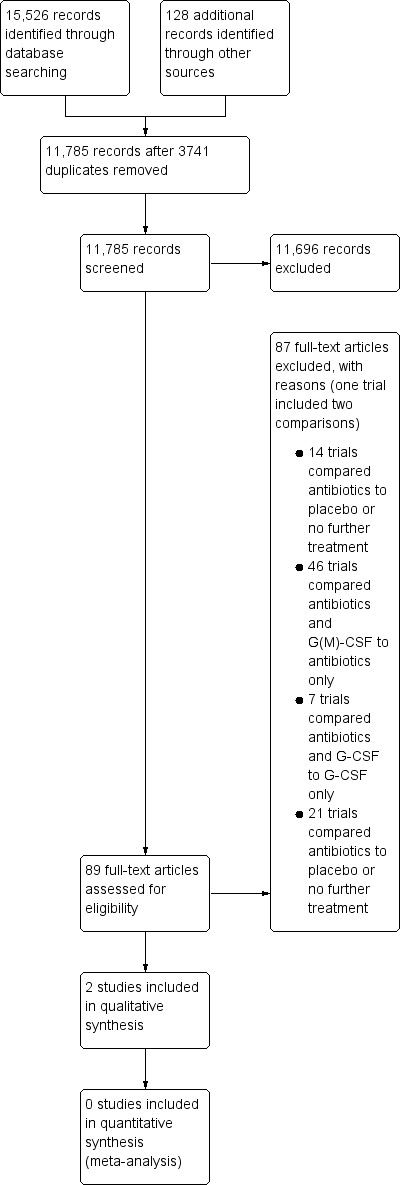
Study flow diagram.
Included studies
Two studies fulfilled the inclusion criteria of this review. One study involved adults with breast cancer receiving high‐dose chemotherapy, and compared prophylaxis for at least six cycles (Schroder 1999). The other trial evaluated patients with small‐cell lung cancer receiving accelerated chemotherapy (Sculier 2001). For more details see Characteristics of included studies.
Design
Schroder 1999 was an open‐label randomised (1:1) study. Sculier 2001 was a three‐arm trial, two arms of which could be analysed in this review. The third arm evaluated standard chemotherapy without any infectious prophylaxis.
Sample sizes
Schroder 1999 included 40 patients, 18 in the G‐CSF prophylaxis arm and 22 in the antibiotics arm. Sculier 2001 included 243 patients, 233 of whom were eligible. However, 78 of these patients received an intervention not applicable for this review, therefore 155 patients were analysed in this review.
Locations
Location is not reported by Schroder 1999, the Sculier 2001 trial took place in several European countries.
Participants
Schroder 1999 randomised chemotherapy‐naive patients with breast cancer who received three, three‐week courses of intravenous cyclophosphamide (1500 mg/m²), epirubicin (80 mg/m²), and 5‐fluouracil (1500 or 1000 mg/m²) given on day one; followed by three cycles of intravenous cyclophosphamide (1500 mg/m²), 5‐fluouracil (600 mg/m²) on day one and intravenous methotrexate (1500 mg/m²) on day two. Sculier 2001 included patients with small‐cell lung cancer receiving six courses of EVI (epirubicin 90 mg/m², vindesine 3 mg/m² and ifosfamide 5 g/m²) every 14 days.
Interventions
In the G‐CSF arm in the Schroder 1999 trial, patients received 263 µg subcutaneous of G‐CSF (lenograstim) on days 3 through to day 12 of each cycle. Patients in the antibiotics arm received two oral prophylactic agents, a combination of ciprofloxacin (250 mg twice daily) and amphotericin B (500 mg four times per day) on days 3 through to day 17 of each cycle, without blinding of the study participants. Patients in the Sculier 2001 study received either GM‐CSF as a daily subcutaneous dose of 5 µg/kg, from day 3 through to day 13 or until the neutrophil count reached ≥ 4000 mm³ after nadir, or cotrimoxazole (160 mg trimethoprim plus 800 mg sulfamethoxazole). This was administered orally every 12 hours from day three until the end of the courses of chemotherapy.
Outcomes
Schroder 1999 evaluated infection‐related mortality, episodes of hospitalisation for febrile neutropenia, duration of hospitalisation for febrile neutropenia, grade IV leucopenia, and analysed costs of prophylaxis. Sculier 2001 assessed overall survival, tumour response, absolute and relative dose intensity, incidence of infections and severe infection, and adverse events. None of the trials evaluated quality of life.
Conflict of interest
Funding not reported.
Excluded studies
We excluded 87 trials with reasons (one trial included two comparisons (Tjan‐Heijnen 2003):
14 trials compared antibiotics to placebo or no further treatment (Attal 1991; Carlson 1997; Cullen 2005; Dickgreber 2009; Karp 1986; Lamy 1993; Lee 2002; Petersen 1988; Pignon 1990; Rafecas 1989; Schuette 2011; Talbot 1993; Tjan‐Heijnen 2003; Yamada 1993).
46 trials compared antibiotics and G‐CSF or GM‐CSF to antibiotics only (Aarts 2013; Alonzo 2002; Altman 1996; Ardizzoni 1994; Bishop 2000; Bradstock 2001; Burton 2006; Clarke 1999; Dibenedetto 1995; Ernst 2008; Faber 2006; Garcia 2000; Garcia‐Saenz 2002; Geissler 1997; Gonzalez‐Vicent 2004; Greenberg 1996; Gulati 1992; Heath 2003; Hecht 2010; Heil 1997; Joshi 2003; Ladenstein 2010; Lee 1998; Lehrnbecher 2007; Little 2002; Maiche 1993; McQuaker 1997; Michel 2000; Miles 1994; Nemunaitis 1995; Nolan 2007; Ojeda 1999; Ottmann 1995; Pettengell 1992; Piccirillo 1999; Przepiorka 2001; Pui 1997; Schmitz 2004; Spitzer 1994; Stahel 1994; Timmer‐Bonte 2005; Trigg 2000; Welte 1996; Witz 1998; Yau 1996; Zinzani 1997a).
7 trials compared antibiotics and G‐CSF to G‐CSF only (Eleutherakis‐Papaiakovou 2010; Feng 2014; Kim 2005; Lalami 2004; Lee 1998; Suh 2008; Tjan‐Heijnen 2003).
21 trials compared G‐CSF to placebo or no further treatment (Bennett 2001; Björkholm 1999; Brugger 2009; Chevallier 1995; Crawford 1997; Doorduijn 2005; Dunlop 1996; Fridrik 1997; Godwin 1998; Hartmann 1997; Holowiecki 2002; Kosaka 2015; Larson 1998; Michon 1998; Osby 2003; Patte 2002; Romieu 2007; Seymour 1995; Trillet‐Lenoir 1993; Veyret 2006; Vogel 2005).
Risk of bias in included studies
See Figure 2 and Figure 3 for risk of bias summary.
2.
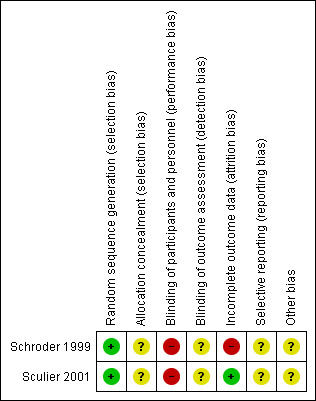
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
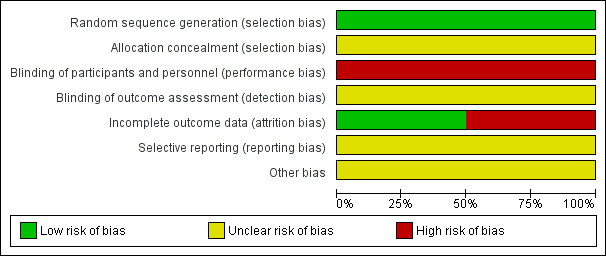
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Both trials were described as randomised, but the randomisation procedure was not reported. Therefore, we judged the risk of selection bias as unclear.
Blinding
There was no blinding of the participants or personnel due to the use of either an oral antibiotic or subcutaneous injections of GM‐CSF; no information was given about whether or not the assessors were blinded. Therefore we judged potential risk of performance bias as high and of detection bias as unclear.
Incomplete outcome data
As 23 courses from seven patients from the antibiotics group, who switched to rhG‐CSF, were not included in the analysis by Schroder 1999, we judged the risk of attrition bias as high in this trial. All patients in the Sculier 2001 trial were evaluated as randomised, reasons for ten patients not being eligible after randomisation were given. Therefore, we judged risk of attrition bias for this trial as low.
Selective reporting
As we did not identify study protocols; it is unclear if all the planned outcomes are reported. We judged the risk of reporting bias as unclear.
Other potential sources of bias
As no other potential source of bias was reported, we judged this bias as "unclear".
Effects of interventions
for the main comparison.
| G‐CSF compared with antibiotics for the prevention of infections and improvement of survival in cancer patients receiving myelotoxic chemotherapy | ||||||
|
Patient or population: cancer patients receiving myelotoxic chemotherapy Intervention: G‐CSF Comparison: antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Antibiotics | G‐CSF | |||||
| All cause mortality | see comment | not reported | ||||
| Infection‐related mortality | see comment | 40 (1 RCT) |
⊕⊝⊝⊝1,2 very low | no patient died of infectious causes during the 18‐week duration of the trial | ||
| Quality of life | see comment | not reported | ||||
| Incidence of febrile neutropenia | 318 per 1000 |
388 per 1000 (169 to 904) |
RR 1.22 (0.53 to 2.84) |
40 (1 RCT) |
⊕⊝⊝⊝1,2 very low | |
| Incidence of severe infections | see comment | not reported | ||||
| Adverse events | see comment | not reported | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 due to the low number of very low number of events, the result is highly imprecise (downgraded by 2 points)
2 high risk of performance bias (neither patients nor physicians blinded) and detection bias (no intention to treat analysis) (downgraded by 1 point)
2.
| GM‐CSF compared with antibiotics for the prevention of infections and improvement of survival in cancer patients receiving myelotoxic chemotherapy | ||||||
|
Patient or population: cancer patients receiving myelotoxic chemotherapy Intervention: GM‐CSF Comparison: antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Antibiotics | GM‐CSF | |||||
| All cause mortality | see comment | 115 (1 RCT) |
⊕⊕⊝⊝1 low | Two‐year survival times were 6% (0 to 12%) in both arms | ||
| Infection‐related mortality | see comment | not reported | ||||
| Quality of life | see comment | not reported | ||||
| Incidence of febrile neutropenia | see comment | not reported | ||||
| Incidence of severe infections (Grade III or IV) |
182 per 1000 |
282 per 1000 (156 to 509) |
RR 1.55 (0.86 to 2.80) |
115 (1 RCT) |
⊕⊕⊝⊝1 low | not reported |
| Adverse events Toxic deaths |
39 per 1000 |
51 per 1000 (12 to 222) |
RR 1.32 (0.30 to 5.69) |
115 (1 RCT) |
⊕⊕⊝⊝1 low | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 due to the low number of very low number of events, the result is highly imprecise (downgraded by 2 points)
Comparison 1: G‐CSF versus antibiotics
Overall survival
Not reported by Schroder 1999.
All cause mortality (including infection‐related, treatment‐related, or on‐trial mortality)
Not reported by Schroder 1999.
Infection‐related mortality
Infection‐related mortality was the same in both groups of the Schroder 1999 trial: no patient died of infectious causes during the 18‐week duration of the trial.
Microbiologically or clinically documented infections
Not reported.
Incidence of severe infections
Not reported
Quality of life (QoL)
Not reported.
Incidence of febrile neutropenia (FN)
Schroder 1999 reported febrile neutropenia in 7/18 patients receiving G‐CSF and in 7/22 patients receiving ciprofloxacin and amphotericin B (relative risk (RR) 1.22; 95% confidence interval (CI) 0.53 to 2.84).
Adverse events
Not reported.
Comparison 2: GM‐CSF versus antibiotics
Overall survival
There was no evidence of a difference in median survival time, with 264 (95% CI 220 to 308) days for patients in the GM‐CSF arm and 264 (95% CI 223 to 305 days) in the antibiotics arm (Sculier 2001). Two‐year survival times were 6% (0 to 12%) in both arms.
All cause mortality (including infection‐related, treatment‐related, or on‐trial mortality)
There were four toxic deaths in the GM‐CSF arm (5.1%) and three in the antibiotics arm (3.8%), without evidence for a difference (RR 1.32; 95% CI 0.30 to 5.69; P = 0.71).
Infection‐related mortality
This outcome was not reported in Sculier 2001.
Microbiologically or clinically documented infections
There were 22 grade III or IV infections (28%) in the GM‐CSF arm in the Sculier 2001 trial and 14 infections (18%) in the antibiotics arm, without any evidence of a difference (RR 1.55; 95% CI 0.86 to 2.80; P = 0.15).
Incidence of severe infections
There were 5 episodes out of 360 cycles (1.3%) of grade IV infections in the GM‐CSF arm and 3 episodes out of 334 cycles in the cotrimoxazole arm (0.8%), without evidence of a difference (RR 1.55; 95% CI 0.37 to 6.42; P = 0.55).
Quality of life (QoL)
Not reported.
Incidence of febrile neutropenia (FN)
Not reported.
Adverse events
There was no significant difference between the two arms for non‐haematological toxicities like diarrhoea, stomatitis, infections, neurologic, respiratory or cardiac adverse events. Grade III and IV thrombopenia occurred significantly more frequently in the GM‐CSF arm (60.8%) compared to the antibiotics arm (28.9%); with a RR 2.10; 95% CI 1.41 to 3.12; P = 0.0002.
Discussion
Summary of main results
The striking finding of this review is that there is only one very small study comparing granulocyte colony‐stimulating factors (G‐CSF) to antibiotics for infection prophylaxis in cancer patients receiving myelosuppressive chemotherapy, and one trial with 155 patients evaluating granulocyte macrophage colony‐stimulating factors (GM‐CSF) versus antibiotics. The trial evaluating G‐CSF did not report all cause mortality, incidence of documented or severe infections, quality of life, or adverse events. We did not find evidence of a difference in infection‐related mortality (none of the 40 included patients died because of infection), or in incidence of febrile neutropenia.
The trial that evaluated GM‐CSF reported overall survival, toxic deaths, infections and severe infections and non‐haematological adverse events, without any evidence of a difference between the GM‐CSF arm and the antibiotics arm. Patients in the antibiotics arm had fewer thrombopenic adverse events. Quality of life was not reported.
Overall completeness and applicability of evidence
As only two small trials were identified, it is not possible to come to a final conclusion regarding the best prophylactic regimen in cancer patients at risk of neutropenia. Therefore, this clinically important question remains unanswered. Moreover, the trial assessing G‐CSF evaluated only a few of the outcomes of interest (incidence of febrile neutropenia and infection‐related mortality), but all cause mortality, incidence of documented or severe infections, quality of life, and adverse events were not assessed.
The trial evaluating GM‐CSF versus antibiotics reported more of the outcomes of interest (overall survival, toxic deaths, infections and severe infections and adverse events), however, due to the small sample size, there was no evidence of a difference, except for the adverse event, thrombocytopenia.
The 41 trials that were excluded because they evaluated the influence of the combination of GM‐CSF and antibiotics compared to GM‐CSF or antibiotics only, underline the huge imbalance between the number of direct comparisons of the two drugs we evaluated in this review, and the number of trials that were conducted in this field.
Quality of the evidence
The risk of bias in Schroder 1999 was high, as this trial was not blinded and not all patients of the included 40 patients were analysed as randomised (seven of 22 patients from the antibiotics arm crossed‐over to G‐CSF and were excluded from analysis). The risk of bias for Sculier 2001 could be considered to be moderate, as risk of performance bias was high, but risk of selection and attrition bias was low.
As only two trials could be included in this review, one evaluating G‐CSF, the other evaluating GM‐CSF, no meta‐analysis was possible.The trial evaluating G‐CSF reported infection‐related mortality and incidence of febrile neutropenia. We judged the quality of evidence for both outcomes to be very low, due to the small number of events, which lead to high imprecision (downgraded by two levels), and the high risk of bias (downgraded by one level).
The trial that analysed GM‐CSF versus antibiotics reported overall survival, toxic deaths, infections, severe infections and adverse events. Because of the very small number of patients included, we downgraded overall quality of the evidence for all outcomes by two levels (high imprecision). As risk of bias was moderate in this trial, we did not downgrade the quality of evidence for this reason. Therefore, overall quality for all the outcomes mentioned above was considered to be low.
Potential biases in the review process
To prevent bias within the review, we considered only RCTs and performed all relevant processes in duplicate. We developed a sensitive search strategy, and searched all relevant data from international cancer congresses by hand to minimise potential publication bias. We are not aware of any obvious deficiencies in our review process. The small number of trials included in this review could lead to publication bias as a funnel plot could not be generated.
Agreements and disagreements with other studies or reviews
One comprehensive meta‐analysis of GM‐CSF versus control includes 148 trials with more than 16,000 patients (Sung 2007). However, in this publication it is not reported how many patients received additional antibiotics, and how many patients received either G‐CSF or GM‐CSF. Similarly, the most comprehensive antibiotics versus control meta‐analysis includes 49 trials with more than 6000 patients (for the outcome all cause mortality; Gafter‐Gvili 2005). The low number of trials directly comparing antibiotics to G‐CSFs is surprising, considering the higher cost of GM‐CSFs compared to standard antibiotics. However, a high number of trials comparing GM‐CSFs to control received funding from pharmaceutical companies that produce GM‐CSFs. As there are only two small trials directly comparing G‐CSF or GM‐CSF versus antibiotics, no final conclusion on the best prophylactic regimen is possible. Clearly, more trials with larger numbers of patients are required to answer this question, in particular, with regard to early all cause and infection‐related mortality. In addition, GM‐CSF is no longer commercially available for infection prophylaxis in several European countries; it is licensed instead for mobilisation of stem cells or after autologous or allogeneic stem cell transplantation (Smith 2015).
Authors' conclusions
Implications for practice.
There is insufficient direct evidence from randomised controlled trials to recommend one prophylaxis (G‐CSFs, GM‐CSFs, or antibiotics) over the other for cancer patients receiving myelotoxic chemotherapy.
Implications for research.
Large high quality trials comparing antibiotic prophylaxis to infection prophylaxis using G‐CSFs or GM‐CSFs are necessary in a wide range of cancer patients, to evaluate clinically important outcomes, like all cause and infection‐related mortality, incidence of febrile neutropenia, quality of life and adverse events.
What's new
| Date | Event | Description |
|---|---|---|
| 28 August 2015 | New citation required but conclusions have not changed | New search |
| 28 August 2015 | New search has been performed | New search, inclusion criteria adapted, RoB adapted |
Notes
Some passages in this review, especially in the methods part, are from the standard template of the Cochrane Haematological Malignancies Review Group.
Acknowledgements
We would like to thank the authors of the first published version of this review, Christine Herbst, Frauke Naumann‐Winter, Eva‐Brigitta Kruse, Julia Bohlius and Holger Schulz. We also thank Olaf Weingart and Andrea Will of the Cochrane Haematological Malignancies Group (CHMG) Editorial Base as well as the Content Editor and the Statistic Editor for commenting on this review. We also thank the Copy‐Editors Janet Wale and Vicki Pennick.
Appendices
Appendix 1. CENTRAL search strategy
January 2008
#1 MeSH descriptor Anti‐Bacterial Agents explode all trees
#2 (antibacterial*) OR (anti‐bacterial*)
#3 (antibio*)
#4 (antimicrobial*) OR (anti‐microbial*) OR (anti‐mycobacterial*) OR (antimyocobacterial*) OR (bacteriocid*) OR (selective NEAR/3 decontaminat*)
#5 MeSH descriptor Antibiotic Prophylaxis explode all trees
#6 MeSH descriptor Quinolones explode all trees
#7 (fluoroquinilones) OR (ciprofloxa*in*) OR (ofloxa*in*) OR (norfloxa*in*) OR (enoxa*in*) OR (pefloxa*in*)
#8 MeSH descriptor Trimethoprim explode all trees
#9 (trimethoprim) OR (sulfamethoxazol*) OR (trimethoprim‐sulfamethoxazol*, (trimethoprim NEAR/3 sulfamethoxazol*)) OR (tmp‐smz*)
#10 MeSH descriptor Polymyxins explode all trees
#11 (colistin) OR (nalidixic NEAR/3 acid) OR (polymyxin)
#12 MeSH descriptor Aminoglycosides explode all trees
#13 MeSH descriptor Gentamicins explode all trees
#14 MeSH descriptor Nebramycin explode all trees
#15 MeSH descriptor Neomycin explode all trees
#16 MeSH descriptor Vancomycin explode all trees
#17 (gentami*in) OR (tobramy*in) OR (meomy*in)
#18 MeSH descriptor Roxithromycin explode all trees
#19 MeSH descriptor Rifampin explode all trees
#20 (vancomy*in) OR (roxithromy*in) OR (rifampin*,rifampicin*)
#21 MeSH descriptor beta‐Lactams explode all trees
#22 MeSH descriptor Penicillins explode all trees
#23 MeSH descriptor Amoxicillin explode all trees
#24 MeSH descriptor Cephalothin explode all trees
#25 MeSH descriptor Ceftriaxone explode all trees
#26 MeSH descriptor Ticarcillin explode all trees
#27 (beta‐lactam*) OR (peni*illin) OR (amoxi*illin*) OR (cephalot*in*,cefalot*in*) OR (ceftriaxone*)
#28 (tica*illin*) OR (framycetin)
#29 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28)
#30 MeSH descriptor Colony‐Stimulating Factors explode all trees
#31 MeSH descriptor Colony‐Stimulating Factors, Recombinant explode all trees
#32 MeSH descriptor Granulocyte Colony Stimulating Factor, Recombinant explode all trees
#33 MeSH descriptor Granulocyte Colony‐Stimulating Factor explode all trees
#34 MeSH descriptor Macrophage Colony‐Stimulating Factor explode all trees
#35 MeSH descriptor Granulocyte‐Macrophage Colony‐Stimulating Factor explode all trees
#36 (rhg*csf*,rhgm*csf*) OR (rmethug*,rhmethug*) OR (rhug*,rhugm*) OR (gcsf*,g‐csf*) OR (gm‐csf*,gmcsf*)
#37 (granulo*yt* NEAR/3 fa*tor*) OR (ma*rophag* NEAR/5 fa*tor*) OR (csf.ti) OR (filgrastim*) OR (neupogen*)
#38 (lenograstim*) OR (euprotin*) OR (peg*filgrastim*) OR (neulasta*) OR (leukine)
#39 (molgramostine*) OR (mielogen*) OR (leucomax*) OR (granocyte)
#40 MeSH descriptor Filgrastim explode all trees
#41 (#30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40)
#42 MeSH descriptor Leukopenia, this term only
#43 .MeSH descriptor Agranulocytosis explode all trees
#44 (granulocytopen*) OR (agranulocyto*) OR (neutropen*) OR (leu*open*) OR (aplasia, aplastic, aplasion)
#45 (leukocyt* NEAR/5 nadir) OR (neutrophil NEAR/5 nadir)
#46 MeSH descriptor Infection explode all trees
#47 (infect*)
#48 MeSH descriptor Sepsis explode all trees
#49 (septicemia, septicaemia) OR (bacteraem*, bacterem*) OR (fever*) OR (pyrexia) OR (fever NEAR/4 (unknown NEAR/3 origin))
#50 MeSH descriptor Fever explode all trees
#51 MeSH descriptor Fever of Unknown Origin, this term only
#52 (pneumonia) OR (lung inflammation) OR (pulmonary inflammation) OR (pneumonitis)
#53 (#42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52)
#54 MeSH descriptor Neoplasms by Histologic Type explode all trees
#55 MeSH descriptor Neoplasms by Site explode all trees
#56 (neoplas*) OR (krebs,cancer*) OR (malignan*)
#57 (leukaem*,leukem*) OR (lymphom*) OR (melano*) OR (metastas*) OR (mesothelio*,mesotelio*)
#58 (gliom,glioblastom*) OR (osteo*sarcom*) OR (carcinomatos*) OR (blastom*) OR (neuroblastom*)
#59 MeSH descriptor Pneumonia explode all trees
#60 (#54 OR #55 OR #56 OR #57 OR #58 OR #59)
#61 (#53 OR #59)
#62 (#29 AND #41 AND #61)
December 2015
ID Search
#1 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees
#2 (antibacterial* or anti‐bacterial*)
#3 antibio*
#4 (antimicrobial* or anti‐microbial*)
#5 (anti‐Mycobacterial* or antimycobacterial*)
#6 bacteriocid*
#7 (selective* near/3 decontaminat*)
#8 MeSH descriptor: [Antibiotic Prophylaxis] explode all trees
#9 MeSH descriptor: [Quinolones] explode all trees
#10 Fluoroquinolones*
#11 ciprofloxa*in*
#12 ofloxa*in*
#13 norfloxa*in*
#14 Enoxa*in*
#15 pefloxa*in*
#16 MeSH descriptor: [Trimethoprim] explode all trees
#17 trimethoprim*
#18 sulfamethoxazol*
#19 Trimethoprim‐Sulfamethoxazol*
#20 tmp‐smz*
#21 MeSH descriptor: [Polymyxins] explode all trees
#22 colistin*
#23 (Nalidixic* near/3 acid*)
#24 Polymyxin*
#25 MeSH descriptor: [Aminoglycosides] explode all trees
#26 MeSH descriptor: [Gentamicins] explode all trees
#27 Gentami*in*
#28 MeSH descriptor: [Nebramycin] explode all trees
#29 Tobramy*in*
#30 MeSH descriptor: [Neomycin] explode all trees
#31 Neomy*in*
#32 MeSH descriptor: [Vancomycin] explode all trees
#33 Vancomy*in*
#34 MeSH descriptor: [Roxithromycin] explode all trees
#35 Roxithromy*in*
#36 MeSH descriptor: [Rifampin] explode all trees
#37 (rifampin* or rifampicin*)
#38 MeSH descriptor: [beta‐Lactams] explode all trees
#39 MeSH descriptor: [Penicillins] explode all trees
#40 MeSH descriptor: [Amoxicillin] explode all trees
#41 MeSH descriptor: [Cephalothin] explode all trees
#42 MeSH descriptor: [Ceftriaxone] explode all trees
#43 MeSH descriptor: [Ticarcillin] explode all trees
#44 (beta‐lactam* or beta* lactam*)
#45 Peni*illin*
#46 Amoxi*illin*
#47 (Cephalot*in* or cefalot*in*)
#48 Ceftriaxone*
#49 Ticar*illin*
#50 framycetin*
#51 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50
#52 MeSH descriptor: [Colony‐Stimulating Factors] explode all trees
#53 MeSH descriptor: [Granulocyte Colony‐Stimulating Factor] explode all trees
#54 MeSH descriptor: [Granulocyte‐Macrophage Colony‐Stimulating Factor] explode all trees
#55 MeSH descriptor: [Macrophage Colony‐Stimulating Factor] explode all trees
#56 RHG*CSF* or RH‐G*CSF* or RHGM*CSF* or RH‐GM*CSF*
#57 RMETHUG* or RHMETHUG* or R‐METHUG* or RH‐METHUG*
#58 RHUG* or RHUGM*
#59 GCSF* or G‐CSF*
#60 GM‐CSF* or GMCSF*
#61 GRANULO*YT* near/3 FA*TOR*
#62 MA*ROPHAG* near/5 FA*TOR*
#63 FILGRASTIM*
#64 neupogen*
#65 religrast*
#66 nugraf*
#67 LENOGRASTIM*
#68 Granocyte*
#69 Euprotin*
#70 PEG*FILGRASTIM*
#71 Neulasta*
#72 LEUKINE*
#73 sagramostim*
#74 MOLGRAMOSTIN*
#75 macrogen*
#76 Mielogen*
#77 Leucomax*
#78 nartograstim*
#79 pegnartograstim*
#80 ecogramostim*
#81 regramostim*
#82 leridistim*
#83 #52 or #53 or #54 or #55 or #56 or #57 or #58 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79 or #80 or #81 or #82
#84 biograstim*
#85 ratiograstim*
#86 XM02*
#87 immunex*
#88 granulokin*
#89 nivestim*
#90 tevagrastim*
#91 zarzio*
#92 #84 or #85 or #86 or #87 or #88 or #89 or #90 or #91
#93 #83 or #92
#94 #51 or #93
#95 MeSH descriptor: [Neoplasms by Histologic Type] explode all trees
#96 MeSH descriptor: [Neoplasms by Site] explode all trees
#97 neoplas*
#98 tumor* or tumour*
#99 (Krebs* or cancer*)
#100 malignan*
#101 (carcino* or karzino*)
#102 karzinom*
#103 sarcom*
#104 leukem* or leukaem*
#105 lymphom*
#106 melano*
#107 metastas*
#108 (mesothelio* or mesotelio*)
#109 carcinomatos*
#110 osteo*sarcom*
#111 (blastom* or neuroblastom*)
#112 carcinomatos*
#113 (gliom* or glioblastom*)
#114 osteo*sarcom*
#115 (blastom* or neuroblastom*)
#116 #95 or #96 or #97 or #98 or #99 or #100 or #101 or #102 or #103 or #104 or #105 or #106 or #108 or #112 or #113 or #114 or #115
#117 #94 and #116
#118 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #17 or #18 or #19 or #20 or #22 or #23 or #24 or #25 or #27 or #28 or #29 or #31 or #33 or #35 or #37 or #43 or #44 or #45 or #46 or #47 or #48 or #50
#119 #118 or #93
#120 #116 and #119 Publication Date from 1985 to 2014, in Trials
#121 #118 and #93
#122 #116 and #121 Publication Date from 1985 to 2015
Appendix 2. MEDLINE search strategy
From 1980 to 20 January 2008
1 exp ANTI‐BACTERIAL AGENTS/
2 (antibacterial$ or anti‐bacterial$).tw,kf,ot.
3 antibio$.tw,kf,ot.
4 (antimicrobial$ or anti‐microbial$).tw,kf,ot.
5 (anti‐mycobacterial$ or antimycobacterial$).tw,kf,ot.
6 bacteriocid$.tw,kf,ot.
7 (selective$ adj3 decontaminat$).tw,kf,ot.
8 ANTIBIOTIC PROPHYLAXIS/
9 exp QUINOLONE/
10 fluoroquinolones$.tw,kf,ot.
11 ciprofloxa#in$.tw,kf,ot.
12 ofloxa#in$.tw,kf,ot.
13 norfloxa#in$.tw,kf,ot.
14 enoxa#in$.tw,kf,ot.
15 pefloxa#in$.tw,kf,ot.
16 exp TRIMETHOPRIM/
17 trimethoprim$.tw,kf,ot.
18 sulfamethoxazol$.tw,kf,ot.
19 trimethoprim‐sulfamethoxazol$.tw,kf,ot.
20 tmp‐smz$.tw,kf,ot.
21 exp POLYMYXINS/
22 colistin$.tw,kf,ot.
23 (nalidixic$ adj3 acid$).tw,kf,ot.
24 polymyxin$.tw,kf,ot.
25 AMINOGLYCOSIDES/
26 GENTAMICINS/
27 gentami#in$.tw,kf,ot.
28 exp NEBRAMYCIN/
29 tobramy#in$.tw,kf,ot.
30 NEOMYCIN/
31 neomy#in$.tw,kf,ot.
32 VANCOMYCIN/.
33 vancomy#in$.tw,kf,ot.
34 ROXITHROMYCIN/
35 roxithromy#in$.tw,kf,ot.
36 RIFAMPIN/
37 (rifampin$ or rifampicin$).tw,kf,ot.
38 BETA‐LACTAMS/
39 beta‐lactam$.tw,kf,ot.
40 PENICILLINS/
41 peni#illin$.tw,kf,ot.
42 AMOXICILLIN/
43 amoxi#illin$.tw,kf,ot.
44 CEPHALOTHIN/
45 (cephalot?in$ or cefalot?in$).tw,kf,ot.
46 CEFTRIAXONE/
47 ceftriaxone$.tw,kf,ot.
48 TICARCILLIN/
49 ticar#illin$.tw,kf,ot.
50 framycetin$.tw,kf,ot.
51 or/1‐50
52 COLONY‐STIMULATING FACTORS/
53 exp COLONY‐STIMULATING FACTORS, RECOMBINANT/
54 exp GRANULOCYTE COLONY STIMULATING FACTOR, RECOMBINANT/
55 exp GRANULOCYTE COLONY‐STIMULATING FACTOR/
56 exp GRANULOCYTE‐MACROPHAGE COLONY‐STIMULATING FACTOR/
57 MACROPHAGE COLONY‐STIMULATING FACTOR/
58 (rhg?csf$ or rhgm?csf$).tw,kf,ot.
59 (rmethug$ or rhmethug$).tw,kf,ot.
60 (rhug$ or rhugm$).tw,kf,ot.
61 (gcsf$ or g‐csf$).tw,kf,ot.
62 (gm‐csf$ or gmcsf$).tw,kf,ot.
63 (granulo?yt$ adj3 fa#tor$).tw,kf,ot.
64 (ma#rophag$ adj5 fa#tor$).tw,kf,ot.
65 csf.ti.
66 FILGRASTIM$.tw,hw,nm,kf.
67 NEUPOGEN$.tw,hw,nm,kf.
68 LENOGRASTIM$.tw,hw,nm,kf.
69 GRANOCYTE$.tw,hw,nm,kf.
70 EUPROTIN$.tw,hw,nm,kf.
71 PEG?FILGRASTIM$.tw,hw,nm,kf.
72 NEULASTA$.tw,hw,nm,kf.
73 LEUKINE$.tw,hw,nm,kf.
74 MOLGRAMOSTIN$.tw,hw,nm,kf.
75 Mielogen$.tw,kf,ot.
76 LEUCOMAX$.tw,hw,nm,kf.
77 or/52‐76
78 51 or 77
79 *LEUKOPENIA/
80 exp AGRANULOCYTOSIS/
81 granulocytopen$.tw,kf,ot.
82 agranulocyto$.tw,kf,ot.
83 neutropen$.tw,kf,ot.
84 leu#open$.tw,kf,ot.
85 (aplasia or aplastic or aplasion).tw,kf,ot.
86 (leukocyt$ adj5 nadir).tw,ot.
87 (neutrophil$ adj5 nadir).tw,ot.
88 INFECTION/
89 infect$.tw,kf,ot.
90 SEPSIS/
91 (septicem$ or septicaem$).tw,kf,ot.
92 (bacteraem$ or bacterem$).tw,kf,ot.
93 FEVER/
94 fever$.tw,kf,ot.
95 pyrexia$.tw,kf,ot.
96 "Fever of Unknown Origin"/
97 (fever adj4 (unknown adj3 origin)).tw,kf,ot.
98 PNEUMONIA/
99 (lung$ or pulmon$) and inflammation$).tw,kf,ot.
100 pneumonit$.tw,kf,ot.
101 engraftment$.tw,kf,ot.
102 (neutrophil$ adj3 recover$).tw,kf,ot.
103 (haematolog$ adj3 recover$).tw,kf,ot.
104 (hematolog$ adj3 recover$).tw,kf,ot.
105 or/79‐104
106 exp NEOPLASMS BY HISTOLOGIC TYPE/
107 exp NEOPLASMS BY SITE/
108 neoplas$.tw,kf,ot.
109 tumo?r$.tw,kf,ot.
110 (krebs$ or cancer$).tw,kf,ot.
111 malignan$.tw,kf,ot.
112 (carcino$ or karzino$).tw,kf,ot.
113 karzinom$.tw,kf,ot.
114 sarcom$.tw,kf,ot.
115 leuk#?m$.tw,kf,ot.
116 lymphom$.tw,kf,ot.
117 melano$.tw,kf,ot.
118 metastas$.tw,kf,ot.
119 (mesothelio$ or mesotelio$).tw,kf,ot.
120 carcinomatos$.tw,kf,ot.
121 (gliom$ or glioblastom$).tw,kf,ot.
122 osteo?sarcom$.tw,kf,ot.
123 (blastom$ or neuroblastom$).tw,kf,ot.
124 or/106‐123
125 105 and 124
126 78 and 125
127 randomized controlled trial.pt.
128 controlled clinical trial.pt.
129 RANDOMIZED CONTROLLED TRIALS/
130 RANDOM ALLOCATION/
131 DOUBLE BLIND METHOD/
132 SINGLE BLIND METHOD/
133 or/127‐132
134 (ANIMALS not HUMANS)/
135 133 not 134
136 clinical trial.pt.
137 exp CLINICAL TRIALS/
138 (clin$ adj25 trial$).ti,ab.
139 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
140 PLACEBOS/
141 placebo$.ti,ab.
142 random$.ti,ab.
143 RESEARCH DESIGN/
144 or/136‐143
145 144 not 134
146 145 not 135
147 COMPARATIVE STUDY/
148 exp EVALUATION STUDIES/
149 FOLLOW UP STUDIES/
150 PROSPECTIVE STUDIES/
151 (control$ or prospectiv$ or volunteer$).ti,ab.
152 or/143‐147
153 152 not 134
154 153 not (135 or 146)
155 135 or 146 or 154
156 126 and 155
Update search January 2008 to 3 December 2015
1 exp ANTI‐BACTERIAL AGENTS/
2 (antibacterial$ or anti‐bacterial$).tw,kf,ot.
3 Antibio$.tw,kf,ot.
4 (antimicrobial$ or anti‐microbial$).tw,kf,ot.
5 (Anti‐Mycobacterial$ or antimycobacterial$).tw,kf,ot.
6 Bacteriocid$.tw,kf,ot.
7 (selective adj3 decontaminat$).tw,kf,ot.
8 Antibiotic Prophylaxis/
9 exp QUINOLONE/
10 Fluoroquinolones$.tw,kf,ot.
11 ciprofloxa#in$.tw,kf,ot.
12 ofloxa#in$.tw,kf,ot.
13 norfloxa#in$.tw,kf,ot.
14 Enoxa#in.tw,kf,ot.
15 pefloxa#in$.tw,kf,ot.
16 exp TRIMETHOPRIM/
17 trimethoprim.tw,kf,ot.
18 sulfamethoxazol$.tw,kf,ot.
19 Trimethoprim‐Sulfamethoxazol$.tw,kf,ot.
20 tmp‐smz$.tw,kf,ot.
21 exp POLYMYXINS/
22 colistin$.tw,kf,ot.
23 (Nalidixic$ adj3 acid$).tw,kf,ot.
24 Polymyxin$.tw,kf,ot.
25 AMINOGLYCOSIDES/
26 GENTAMICINS/
27 Gentami#in$.tw,kf,ot.
28 exp NEBRAMYCIN/
29 Tobramy#in$.tw,kf,ot.
30 NEOMYCIN/
31 Neomy#in$.tw,kf,ot.
32 VANCOMYCIN/
33 Vancomy#in$.tw,kf,ot.
34 ROXITHROMYCIN/
35 Roxithromy#in$.tw,kf,ot.
36 RIFAMPIN/
37 (rifampin$ or rifampicin$).tw,kf,ot.
38 BETA‐LACTAMS/
39 PENICILLINS/
40 AMOXICILLIN/
41 CEPHALOTHIN/
42 CEFTRIAXONE/
43 TICARCILLIN/
44 (beta‐lactam$ or beta$ lactam$).tw,kf,ot.
45 Peni#illin$.tw,kf,ot.
46 Amoxi#illin$.tw,kf,ot.
47 (Cephalot?in$ or cefalot?in$).tw,kf,ot.
48 Ceftriaxone$.tw,kf,ot.
49 Ticar#illin$.tw,kf,ot.
50 framycetin$.tw,kf,ot.
51 or/1‐50
52 COLONY‐STIMULATING FACTORS/
53 exp GRANULOCYTE COLONY‐STIMULATING FACTOR/
54 exp GRANULOCYTE‐MACROPHAGE COLONY‐STIMULATING FACTOR/
55 MACROPHAGE COLONY‐STIMULATING FACTOR/
56 (RHG?CSF$ or RH‐G?CSF$ or RHGM?CSF$ or RH‐GM?CSF$).tw.
57 (RMETHUG$ or RHMETHUG$ or R‐METHUG$ or RH‐METHUG$).tw.
58 (RHUG$ or RHUGM$).tw.
59 (GCSF$ or G‐CSF$).tw.
60 (GM‐CSF$ or GMCSF$).tw.
61 (GRANULO?YT$ adj3 FA#TOR$).tw.
62 (MA#ROPHAG$ adj5 FA#TOR$).tw.
63 CSF.ti.
64 FILGRASTIM$.tw,hw,nm,kf.
65 neupogen$.tw,hw,nm,kf.
66 LENOGRASTIM$.tw,hw,nm,kf.
67 Granocyte.tw,hw,nm,kf.
68 Euprotin.tw,hw,nm,kf.
69 PEG?FILGRASTIM$.tw,hw,nm,kf.
70 Neulasta.tw,hw,nm,kf.
71 LEUKINE.tw,hw,nm,kf.
72 sagramostim$.tw,kf,nm,ot.
73 MOLGRAMOSTIN$.tw,hw,nm,kf.
74 Mielogen$.tw,kf,nm,ot.
75 Leucomax$.tw,hw,nm,kf.
76 nartograstim$.tw,kf,nm,ot.
77 pegnartograstim$.tw,kf,nm,ot.
78 ecogramostim$.tw,kf,nm,ot.
79 regramostim$.tw,kf,nm,ot.
80 leridistim$.tw,kf,ot.
81 or/52‐80
82 biograstim$.mp.
83 ratiograstim$.mp.
84 XM02$.mp.
85 immunex$.mp.
86 granulokin$.mp.
87 nivestim$.mp.
88 tevagrastim$.mp.
89 zarzio$.mp.
90 or/82‐89
91 81 or 90
92 51 or 91
93 exp NEOPLASMS BY HISTOLOGIC TYPE/
94 exp NEOPLASMS BY SITE/
95 neoplas$.tw,kf,ot.
96 tumo?r$.tw,kf,ot.
97 (Krebs$ or cancer$).tw,kf,ot.
98 malignan$.tw,kf,ot.
99 (carcino$ or karzino$).tw,kf,ot.
100 karzinom$.tw,kf,ot.
101 sarcom$.tw,kf,ot.
102 leuk#?m$.tw,kf,ot.
103 lymphom$.tw,kf,ot.
104 melano$.tw,kf,ot.
105 metastas$.tw,kf,ot.
106 (mesothelio$ or mesotelio$).tw,kf,ot.
107 carcinomatos$.tw,kf,ot.
108 (gliom$ or glioblastom$).tw,kf,ot.
109 osteo?sarcom$.tw,kf,ot.
110 (blastom$ or neuroblastom$).tw,kf,ot.
111 or/93‐110
112 92 and 111
113 randomized controlled trial.pt.
114 controlled clinical trial.pt.
115 randomi?ed.ab.
116 placebo.ab.
117 clinical trials as topic.sh.
118 randomly.ab.
119 trial.ti.
120 or/113‐119
121 humans.sh.
122 120 and 121
123 112 and 122
Appendix 3. EMBASE search strategy
From 1980 to 20 January 2008
1 exp ANTI‐BACTERIAL AGENTS/
2 (antibacterial? OR anti‐bacterial?).tw.
3 antibio?.tw.
4 (antimicrobial? OR anti‐microbial?).tw.
5 (anti‐mycobacterial? OR antimyocobacterial?).tw.
6 bacteriocid?.tw.
7 (selective ADJ3 decontaminat?).tw.
8 ANTIBIOTIC PROPHYLAXIS/
9 exp QUINOLONE/
10 fluoroquinilones?.tw.
11 ciprofloxa#in?.tw.
12 ofloxa#in?.tw.
13 norfloxa#in?.tw.
14 enoxa#in?.tw.
15 pefloxa#in?.tw.
16 exp TRIMETHOPRIM/
17 trimethoprim?.tw.
18 sulfamethoxazol?.tw.
19 (trimethoprim‐sulfamethoxazol? OR (trimethoprim ADJ3 sulfamethoxazol?)).tw.
20 tmp‐smz?.tw.
21 exp POLYMYXIN/
22 colistin?.tw.
23 (nalidixic? ADJ3 acid?).tw.
24 polymyxin?.tw.
25 AMINOGLYCOSIDE/
26 GENTAMICIN/
27 gentami#in?.tw.
28 exp NEBRAMYCIN/
29 tobramy#in?.tw.
30 NEOMYCIN/
31 neomy#in?.tw.
32 VANCOMYCIN/
33 vancomy#in?.tw.
34 ROXITHROMYCIN/
35 roxithromy#in?.tw.
36 RIFAMPIN/
37 (rifampin? OR rifampicin?).tw.
38 BETA‐LACTAMS/
39 PENICILLINS/
40 AMOXICILLIN/
41 CEPHALOTHIN/
42 CEFTRIAXONE/
43 TICARCILLIN/
44 (beta‐lactam? OR beta$ lactam$).tw.
45 peni#illin?.tw.
46 amoxi#illin?.tw.
47 (cephalot#in? OR cefalot#in?).tw.
48 ceftriaxone?.tw.
49 ticar#illin?.tw.
50 framycetin?.tw.
51 OR/ 1‐50
52 COLONY‐STIMULATINGING FACTORS/
53 exp COLONY‐STIMULATING FACTORS, RECOMBINANT/
54 exp GRANULOCYTE COLONY STIMULATING FACTOR, RECOMBINANT/
55 exp GRANULOCYTE COLONY‐STIMULATING FACTOR/
56 GRANULOCYTE‐MACROPHAGE COLONY‐STIMULATING FACTOR/
57 MACROPHAGE COLONY‐STIMULATING FACTOR/
58 (rhg#csf? OR rhgm#csf?).tw.
59 (rmethug? OR rhmethug?).tw.
60 (rhug? OR rhugm?).tw.
61 (gcsf? OR g‐csf?).tw.
62 (gm‐csf? OR gmcsf?).tw.
63 (granulo#yt? ADJ3 fa#tor?).tw.
64 (ma#rophag? ADJ5 fa#tor?).tw.
65 csf.ti
66 filgrastim?.tw.
67 neupogen?.tw.
68 lenograstim?.tw.
69 euprotin?.tw.
70 granocyte?.tw.
71 peg#filgrastim?.tw.
72 neulasta?.tw.
73 leukine?.tw.
74 molgramostine?.tw.
75 mielogen?.tw.
76 leucomax?.tw.
77 OR/ 52‐76
78 * LEUKOPENIA/
79 exp AGRANULOCYTOSIS/
80 granulocytopen?.tw.
81 agranulocyto?.tw.
82 neutropen?.tw.
83 leu#open?.tw.
84 (aplasia OR aplastic OR aplasion).tw.
85 leukocyt? ADJ5 nadir).tw.
86 (neutrophil? ADJ5 nadir).tw.
87 INFECTION/
88 infect?.tw.
89 SEPSIS/
90 (septicemia? OR septicaemia?).tw.
91 (bacteraem? OR bacterem?).tw.
92 FEVER/
93 pyrexia.tw.
94 fever?.tw.
95 FEVER OF UNKNOWN ORIGIN/
96 (fever ADJ4 (unknown ADJ3 origin)).tw.
97 PNEUMONIA/
98 ((lung? OR pulmonary?) AND inflammation?).tw.
99 pneumonitis?.tw.
100 engraftment?.tw.
101 (neutrophil? ADJ3 recover?).tw.
102 (hematolog? ADJ3 recover?).tw.
103 (haematology? ADJ3 recover?).tw.
104 OR/ 78‐103
105 exp NEOPLASMS BY HISTOLOGIC TYPE/
106 exp NEOPLASMS BY SITE/
107 neoplas?.tw.
108 (tumor? OR tumour?).tw.
109 (krebs? OR cancer?).tw.
110 malignan?.tw.
111 (carcino? OR karzino?).tw.
112 karzinom?.tw.
113 sarcom?.tw.
114 (leukaem? OR leukem?).tw
115 lymphom?.tw.
116 melano?.tw.
117 metastas?.tw.
118 (mesothelio? OR mesotelio?).tw.
119 carcinomatos?.tw.
120 (gliom? OR glioblastom?).tw.
121 osteo?sarcom?.tw.
122 OR/ 105‐121
123 CLINICAL TRIAL/
124 RANDOMIZED CONTROLLED TRIALS/
125 RANDOM ALLOCATION/
126 SINGLE‐BLIND METHOD/
127 DOUBLE‐BLIND METHOD/
128 CROSS‐OVER STUDIES/
129 PLACEBOS/
130 Randomi?ed controlled trial$.tw.
131 RCT.tw.
132 random allocation.tw.
133 randomly allocated.tw.
134 Allocated randomly.tw.
135 (allocated ADJ2 random).tw.
136 (allocated ADJ2 random).tw.
137 single blind$.tw.
138 double blind$.tw.
139 ((treble or triple) ADJ blind$).tw.
140 placebo$.tw.
141 PROSPECTIVE STUDIES/
142 OR/ 123‐141
143 CASE STUDY/
144 case report.tw.
145 ABSTRACT REPORT/ OR LETTER/
146 OR/ 143‐145
147 142 NOT 146
148 ANIMAL/
149 HUMAN/
150 148 NOT 149
151 147 NOT 150
152 51 OR 77
153 104 AND 122
154 152 AND 153
155 154 AND 51
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Schroder 1999.
| Methods | Randomisation
Recruitment Period
Median follow‐up time
|
|
| Participants | 40 patients randomised
Inclusion criteria
Mean age in years
Metastases
Country
|
|
| Interventions | All patients
G‐CSF arm
Antibiotics arm
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Before chemotherapy, patients were randomized to group I or II." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label trial (subcutaneous injection of G‐CSF versus oral antibiotics) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessor not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Not included in the analyses were 23 courses from seven patients from group II (antibiotics), who switched to rhG‐CSF. Of these seven patients, three patients stopped, because of disease progression or death from the disease, after having received a total of nine courses; therefore 11 more courses were not administered and not included in the analyses." |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, therefore unclear, if all the planned outcomes are reported |
| Other bias | Unclear risk | Not reported |
Sculier 2001.
| Methods | Randomisation
Recruitment Period
Median follow‐up time
|
|
| Participants | 243 patients randomised, 233 eligible
Inclusion criteria
Mean age in years
Stage
Brain metastases
Countries
|
|
| Interventions | All patients
GM‐CSF arm
Antibiotics arm
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "eligible patients were randomised" |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label trial (subcutaneous injection of GM‐CSF versus oral antibiotics) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessor not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "In the 233 eligible patients, 14 were nonassessable for response (2 in arm A, 6 in arm B, and 6 in arm C) for the following reasons: too long delay between 2 courses of chemotherapy (1), early death unrelated to cancer or treatment complications (9), protocol violation (2), death prior to starting treatment (1), no work‐up at evaluation (1)" |
| Selective reporting (reporting bias) | Unclear risk | No study protocol identified, therefore unclear if all the planned outcomes are reported |
| Other bias | Unclear risk | Not reported |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aarts 2013 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Alonzo 2002 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Altman 1996 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Ardizzoni 1994 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Attal 1991 | Comparison of antibiotics versus placebo |
| Bennett 2001 | Comparison of G‐CSF versus placebo |
| Bishop 2000 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Björkholm 1999 | Comparison of G‐CSF versus placebo |
| Bradstock 2001 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Brugger 2009 | Comparison of G‐CSF versus placebo |
| Burton 2006 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Carlson 1997 | Comparison of antibiotics versus placebo |
| Chevallier 1995 | Comparison of G‐CSF versus placebo |
| Clarke 1999 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Crawford 1997 | Comparison of G‐CSF versus placebo |
| Cullen 2005 | Comparison of antibiotics versus placebo |
| Dibenedetto 1995 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Dickgreber 2009 | Comparison of antibiotics versus placebo |
| Doorduijn 2005 | Comparison of G‐CSF versus placebo |
| Dunlop 1996 | Comparison of G‐CSF versus placebo |
| Eleutherakis‐Papaiakovou 2010 | Comparison of G‐CSF plus antibiotics versus G‐CSF alone |
| Ernst 2008 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Faber 2006 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Feng 2014 | Comparison of G‐CSF plus antibiotics versus G‐CSF alone |
| Fridrik 1997 | Comparison of G‐CSF versus placebo |
| Garcia 2000 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Garcia‐Saenz 2002 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Geissler 1997 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Godwin 1998 | Comparison of G‐CSF versus placebo |
| Gonzalez‐Vicent 2004 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Greenberg 1996 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Gulati 1992 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Hartmann 1997 | Comparison of G‐CSF versus placebo |
| Heath 2003 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Hecht 2010 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Heil 1997 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Holowiecki 2002 | Comparison of G‐CSF versus placebo |
| Jones 1996 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Joshi 2003 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Karp 1986 | Comparison of antibiotics versus placebo |
| Kim 2005 | Comparison of G‐CSF plus antibiotics versus G‐CSF alone |
| Kosaka 2015 | Comparison of G‐CSF versus placebo |
| Ladenstein 2010 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Lalami 2004 | Comparison of GM‐CSF plus antibiotics versus GM‐CSF alone |
| Lamy 1993 | Comparison of antibiotics versus placebo |
| Larson 1998 | Comparison of G‐CSF versus placebo |
| Lee 1998 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Lee 2002 | Comparison of antibiotics versus placebo |
| Lehrnbecher 2007 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Little 2002 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Maiche 1993 | Comparison of GM‐CSF plus antibiotics versus GM‐CSF alone |
| McQuaker 1997 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Michel 2000 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Michon 1998 | Comparison of G‐CSF versus placebo |
| Miles 1994 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Nemunaitis 1995 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Nolan 2007 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Ojeda 1999 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Osby 2003 | Comparison of G‐CSF versus placebo |
| Ottmann 1995 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Patte 2002 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Petersen 1988 | Comparison of antibiotics versus placebo |
| Pettengell 1992 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Piccirillo 1999 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Pignon 1990 | Comparison of antibiotics versus placebo |
| Przepiorka 2001 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Pui 1997 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Rafecas 1989 | Comparison of antibiotics versus placebo |
| Romieu 2007 | Comparison of G‐CSF versus placebo |
| Schmitz 2004 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Schuette 2011 | Comparison of antibiotics versus placebo |
| Seymour 1995 | Comparison of G‐CSF versus placebo |
| Spitzer 1994 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Stahel 1994 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Suh 2008 | Comparison of G‐CSF plus antibiotics versus G‐CSF alone |
| Talbot 1993 | Comparison of antibiotics versus placebo |
| Timmer‐Bonte 2005 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Tjan‐Heijnen 2003 | Comparison of G‐CSF plus antibiotics versus antibiotics alone |
| Trigg 2000 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Trillet‐Lenoir 1993 | Comparison of G‐CSF versus placebo |
| Veyret 2006 | Comparison of G‐CSF versus placebo |
| Vogel 2005 | Comparison of G‐CSF versus placebo |
| Welte 1996 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Witz 1998 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
| Yamada 1993 | Comparison of antibiotics versus placebo |
| Yau 1996 | Comparison of GM‐CSF plus antibiotics versus antibiotics alone |
Differences between protocol and review
Outcomes:
We did not evaluate secondary prophylaxis as we identified only two trials, assessing G‐CSF or GM‐CSF and antibiotics for primary prophylaxis.
Data analysis:
We did not identify time‐to‐event outcomes and continuous data. For time‐to‐event outcomes, we would have extracted hazard ratios (HRs) from published data according to Parmar and Tierney (Parmar 1998; Tierney 2007). We would have calculated continuous outcomes as standardised mean differences.
As we included only one trial in each comparison, we could not pool the data. If we identify more trials for future updates, we will check whether the data are sufficiently similar to be combined. Then, we will pool results by applying meta‐analyses using the fixed‐effect model, and the random‐effects model as a sensitivity analysis.
If the trials are too clinically heterogeneous to combine, we will only perform subgroup analyses, without calculating an overall estimate. We will analyse data according to Cochrane recommendations (Deeks 2011), and will use the Cochrane statistical package in Review Manager 5 for analysis (Review Manager (RevMan)).
Assessment of heterogeneity:
As we did not meta‐analyse the data, we did not assess heterogeneity. If we perform a meta‐analysis in a future update, we will identify heterogeneity by using a Chi² test with a significance level at P < 0.1. We will use the I² statistic to quantify possible heterogeneity (I² > 30% moderate heterogeneity, I² > 75% considerable heterogeneity; Deeks 2011). Moreover, we will explore potential causes of heterogeneity by sensitivity and subgroup analyses.
Contributions of authors
Nicole Skoetz: data extraction and analysis, drafting of final review
Julia Bohlius: content input
Ina Monsef: database search
Oliver Blank: data extraction and analysis
Andreas Engert: clinical expertise and content input
Jörg Janne Vehreschild: clinical expertise
Sources of support
Internal sources
-
Köln Fortune, Germany.
Funding programme “Köln Fortune” , Medical Faculty University of Cologne
External sources
-
BMBF, Germany.
Project grant application NO 01KG1209, Federal Ministry of Education and Research (BMBF)
Declarations of interest
Nicole Skoetz: none known
Julia Bohlius: none known
Ina Monsef: none known
Oliver Blank: none known
Andreas Engert: none known
Jörg Janne Vehreschild: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Schroder 1999 {published data only}
- Schroder CP, Vries EG, Mulder NH, Willemse PH, Sleijfer DT, Hospers GA, et al. Prevention of febrile leucopenia after chemotherapy in high‐risk breast cancer patients: no significant difference between granulocyte‐colony stimulating growth factor or ciprofloxacin plus amphotericin B. Journal of Antimicrobial Chemotherapy 1999;43(5):741‐3. [DOI] [PubMed] [Google Scholar]
Sculier 2001 {published data only}
- Sculier JP, Paesmans M, Lecomte J, Cutsem O, Lafitte JJ, Berghmans T, et al. A three‐arm phase III randomised trial assessing, in patients with extensive‐disease small‐cell lung cancer, accelerated chemotherapy with support of haematological growth factor or oral antibiotics. British Journal of Cancer 2001;85(10):1444‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aarts 2013 {published data only}
- Aarts MJ, Peters FP, Mandigers CM, Dercksen MW, Stouthard JM, Nortier HJ, et al. Primary granulocyte colony‐stimulating factor prophylaxis during the first two cycles only or throughout all chemotherapy cycles in patients with breast cancer at risk for febrile neutropenia. Journal of Clinical Oncology 2013;31(34):4290‐6. [DOI: 10.1200/JCO.2012.44.6229.] [DOI] [PubMed] [Google Scholar]
Alonzo 2002 {published data only}
- Alonzo TA, Kobrinsky NL, Aledo A, Lange BJ, Buxton AB, Woods WG. Impact of granulocyte colony‐stimulating factor use during induction for acute myelogenous leukemia in children: a report from the Children's Cancer Group. Journal of Pediatric Hematology/Oncology 2002;24(8):627‐35. [DOI] [PubMed] [Google Scholar]
Altman 1996 {published data only}
- Altman A, Steinherz P, Trigg M, Halpern S, Jhanwar S, Pieters R, et al. A prospective randomized trial of granulocyte colony‐stimulating factor (G‐CSF) during induction or consolidation phases of intensive chemotherapy for acute lymphocytic leukemia (ALL) [abstract]. Journal of Clinical Oncology. 1996:542, Abstract 1760.
Ardizzoni 1994 {published data only}
- Ardizzoni A, Venturini M, Sertoli MR, Giannessi PG, Brema F, Danova M, et al. Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) allows acceleration and dose intensity increase of CEF chemotherapy: a randomised study in patients with advanced breast cancer. British Journal of Cancer 1994;69(2):385‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Attal 1991 {published data only}
- Attal M, Schlaifer D, Rubie H, Huguet F, Charlet JP, Bloom E, et al. Prevention of gram‐positive infections after bone marrow transplantation by systemic vancomycin: a prospective, randomized trial. Journal of Clinical Oncology 1991;9(5):865‐70. [DOI] [PubMed] [Google Scholar]
Bennett 2001 {published data only}
- Bennett CL, Hynes D, Godwin J, Stinson TJ, Golub RM, Appelbaum FR, et al. Economic analysis of granulocyte colony stimulating factor as adjunct therapy for older patients with acute myelogenous leukemia (AML): estimates from a Southwest Oncology Group clinical trial. Cancer Investigation 2001;19(6):603‐10. [DOI] [PubMed] [Google Scholar]
Bishop 2000 {published data only}
- Bishop MR, Tarantolo SR, Geller RB, Lynch JC, Bierman PJ, Pavletic ZS, et al. A randomized, double‐blind trial of filgrastim (granulocyte colony‐stimulating factor) versus placebo following allogeneic blood stem cell transplantation. Blood 2000;96(1):80‐5. [PubMed] [Google Scholar]
Björkholm 1999 {published data only}
- Björkholm M, Osby E, Hagberg H, Kvaloy S, Teerenhovi L, Myhre J. Randomized trial of r‐metHu granulocyte colony‐stimulating factor (G‐CSF) as adjunct to CHOP or CNOP treatment of elderly patients with aggressive non‐Hodgkin's lymphoma [abstract]. Blood. 1999:599a.
Bradstock 2001 {published data only}
- Bradstock K, Matthews J, Young G, Lowenthal R, Baxter H, Cetal A. Effects of glycosylated recombinant human granulocyte colony‐stimulating factor after high‐dose cytarabine‐based induction chemotherapy for adult acute myeloid leukaemia. Leukemia 2001;15(9):1331‐8. [DOI] [PubMed] [Google Scholar]
Brugger 2009 {published data only}
- Brugger W, Bacon P, Lawrinson S, Romieu G. Neutrophil recovery in elderly breast cancer patients receiving adjuvant anthracycline‐containing chemotherapy with pegfilgrastim support. Critical Reviews in Oncology/Hematology 2009;72(3):265‐9. [DOI] [PubMed] [Google Scholar]
Burton 2006 {published data only}
- Burton C, Linch D, Hoskin P, Milligan D, Dyer MJ, Hancock B, et al. A phase III trial comparing CHOP to PMitCEBO with or without G‐CSF in patients aged 60 plus with aggressive non‐Hodgkin's lymphoma. British Journal of Cancer 2006;94(6):806‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlson 1997 {published data only}
- Carlson JW, Fowler JM, Mitchell SK, Carson LF, Mayer AR, Copeland LJ. Chemoprophylaxis with ciprofloxacin in ovarian cancer patients receiving paclitaxel: a randomized trial. Gynecologic Oncology 1997;65(2):325‐9. [DOI] [PubMed] [Google Scholar]
Chevallier 1995 {published data only}
- Chevallier B, Chollet P, Merrouche Y, Roche H, Fumoleau P, Kerbrat P, et al. Lenograstim prevents morbidity from intensive induction chemotherapy in the treatment of inflammatory breast cancer. Journal of Clinical Oncology 1995;13(7):1564‐71. [DOI] [PubMed] [Google Scholar]
Clarke 1999 {published data only}
- Clarke V, Dunstan FD, Webb DK. Granulocyte colony‐stimulating factor ameliorates toxicity of intensification chemotherapy for acute lymphoblastic leukemia. Medical & Pediatric Oncology 1999;32(5):331‐5. [DOI] [PubMed] [Google Scholar]
Crawford 1997 {published data only}
- Crawford J, Kreisman H, Garewal H, Jones SE, Shoemaker D, Pupa MR, et al. The impact of filgrastim schedule variation on hematopoietic recovery post‐chemotherapy. Annals of Oncology 1997;8(11):1117‐24. [DOI] [PubMed] [Google Scholar]
Cullen 2005 {published data only}
- Cullen M, Steven N, Billingham L, Gaunt C, Hastings M, Simmonds P, et al. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. New England Journal of Medicine 2005;353(10):988‐98. [DOI] [PubMed] [Google Scholar]
Dibenedetto 1995 {published data only}
- Dibenedetto SP, Ragusa R, Ippolito AM, Lo NL, Cataldo A, D'Amico S, et al. Assessment of the value of treatment with granulocyte colony‐stimulating factor in children with acute lymphoblastic leukemia: a randomized clinical trial. European Journal of Haematology 1995;55(2):93‐6. [DOI] [PubMed] [Google Scholar]
Dickgreber 2009 {published data only}
- Dickgreber N, Nagel S, Roscher K, Schuette W. Randomised, placebo‐controlled phase III trial of docetaxel plus carboplatin with or without levofloxacin prophylaxis in elderly patients with advanced non‐small cell lung cancer: The APRONTA Trial. [19th European Congress of Clinical Microbiology and Infectious Diseases]. Clinical Microbiology and Infection 2009;15:S519. [Google Scholar]
Doorduijn 2005 {published data only}
- Doorduijn J, Buijt I, Holt B, Steijaert M, Uyl‐de Groot C, Sonneveld P. Self‐reported quality of life in elderly patients with aggressive non‐Hodgkin's lymphoma treated with CHOP chemotherapy. European Journal of Haematology 2005;75(2):116‐23. [DOI] [PubMed] [Google Scholar]
Dunlop 1996 {published data only}
Eleutherakis‐Papaiakovou 2010 {published data only}
- Eleutherakis‐Papaiakovou E, Kostis E, Migkou M, Christoulas D, Terpos E, Gavriatopoulou M, et al. Prophylactic antibiotics for the prevention of neutropenic fever in patients undergoing autologous stem‐cell transplantation: results of a single institution, randomized phase 2 trial. American Journal of Hematology 2010;85(11):863‐7. [DOI] [PubMed] [Google Scholar]
Ernst 2008 {published data only}
- Ernst P, Bacigalupo A, Ringden O, Ruutu T, Kolb HJ, Lawrinson S, et al. A phase 3, randomized, placebo‐controlled trial of filgrastim in patients with haematological malignancies undergoing matched‐related allogeneic bone marrow transplantation. Archives of Drug Information 2008;1(3):89‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faber 2006 {published data only}
- Faber E, Pytlik R, Slaby J, Zapletalova J, Kozak T, Raida L, et al. Individually determined dosing of filgrastim after autologous peripheral stem cell transplantation in patients with malignant lymphoma‐‐results of a prospective multicentre controlled trial. European Journal of Haematology 2006;77(6):493‐500. [DOI] [PubMed] [Google Scholar]
Feng 2014 {published data only}
- Feng X, Ruan Y, He Y, Zhang Y, Wu X, Liu H, et al. Prophylactic first‐line antibiotics reduce infectious fever and shorten hospital stay during chemotherapy‐induced agranulocytosis in childhood acute myeloid leukemia. Acta Haematologica 2014;132(1):112‐7. [DOI] [PubMed] [Google Scholar]
Fridrik 1997 {published data only}
- Fridrik MA, Greil R, Hausmaninger H, Krieger O, Oppitz P, Stoger M, et al. Randomized open label phase III trial of CEOP/IMVP‐Dexa alternating chemotherapy and filgrastim versus CEOP/IMVP‐Dexa alternating chemotherapy for aggressive non‐Hodgkin's lymphoma (NHL). A multicenter trial by the Austrian Working Group for Medical Tumor Therapy.[Erratum appears in Ann Hematol. 2014 Mar;93(3):539‐40]. Annals of Hematology 1997;75(4):135‐40. [DOI] [PubMed] [Google Scholar]
Garcia 2000 {published data only}
- Garcia G. Immediate vs. delayed imipenem treatment in cancer patients with profound neutropenia induced by high‐dose chemotherapy: Results of a randomized study. Revista Espanola de Quimioterapia 2000; Vol. 15:257‐63. [PubMed]
Garcia‐Saenz 2002 {published data only}
- Garcia‐Saenz JA, Martin M, Casado A, Perez‐Segura P, Manrique I, Flores L, et al. Immediate vs. delayed imipenem treatment in cancer patients with profound neutropenia induced by high‐dose chemotherapy: results of a randomized study. Revista Espanola de Quimioterapia 2002;15(3):257‐63. [PubMed] [Google Scholar]
Geissler 1997 {published data only}
- Geissler K, Koller E, Hubmann E, Niederwieser D, Hinterberger W, Geissler D, et al. Granulocyte colony‐stimulating factor as an adjunct to induction chemotherapy for adult acute lymphoblastic leukemia‐‐a randomized phase‐III study. Blood 1997;90(2):590‐6. [PubMed] [Google Scholar]
Godwin 1998 {published data only}
- Godwin JE, Kopecky KJ, Head DR, Willman CL, Leith CP, Hynes HE, et al. A double‐blind placebo‐controlled trial of granulocyte colony‐stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest oncology group study (9031). Blood 1998;91(10):3607‐15. [PubMed] [Google Scholar]
Gonzalez‐Vicent 2004 {published data only}
- Gonzalez‐Vicent M, Madero L, Sevilla J, Ramirez M, Diaz MA. A prospective randomized study of clinical and economic consequences of using G‐CSF following autologous peripheral blood progenitor cell (PBPC) transplantation in children. Bone Marrow Transplantation 2004;34(12):1077‐81. [DOI] [PubMed] [Google Scholar]
Greenberg 1996 {published data only}
- Greenberg P, Advani R, Keating A, Gulati SC, Nimer S, Champlin R, et al. GM‐CSF accelerates neutrophil recovery after autologous hematopoietic stem cell transplantation. Bone Marrow Transplantation 1996;18(6):1057‐64. [PubMed] [Google Scholar]
Gulati 1992 {published data only}
- Gulati SC, Bennett CL. Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) as adjunct therapy in relapsed Hodgkin disease. Annals of Internal Medicine 1992;116(3):177‐82. [DOI] [PubMed] [Google Scholar]
Hartmann 1997 {published data only}
- Hartmann LC, Tschetter LK, Habermann TM, Ebbert LP, Johnson PS, Mailliard JA, et al. Granulocyte colony‐stimulating factor in severe chemotherapy‐induced afebrile neutropenia. New England Journal of Medicine 1997;336(25):1776‐80. [DOI] [PubMed] [Google Scholar]
Heath 2003 {published data only}
- Heath JA, Steinherz PG, Altman A, Sather H, Jhanwar S, Halpern S, et al. Human granulocyte colony‐stimulating factor in children with high‐risk acute lymphoblastic leukemia: a Children's Cancer Group Study. Journal of Clinical Oncology 2003;21(8):1612‐7. [DOI] [PubMed] [Google Scholar]
Hecht 2010 {published data only}
- Hecht JR, Pillai M, Gollard R, Heim W, Swan F, Patel R, et al. A randomized, placebo‐controlled phase II study evaluating the reduction of neutropenia and febrile neutropenia in patients with colorectal cancer receiving pegfilgrastim with every‐2‐week chemotherapy. Clinical Colorectal Cancer 2010;9(2):95‐101. [DOI] [PubMed] [Google Scholar]
Heil 1997 {published data only}
- Heil G, Hoelzer D, Sanz MA, Lechner K, Liu Yin JA, Papa G, et al. A randomized, double‐blind, placebo‐controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia. The International Acute Myeloid Leukemia Study Group. Blood 1997;90(12):4710‐8. [PubMed] [Google Scholar]
Holowiecki 2002 {published data only}
- Holowiecki J, Giebel S, Krzemien S, Krawczyk‐Kulis M, Jagoda K, Kopera M, et al. G‐CSF administered in time‐sequenced setting during remission induction and consolidation therapy of adult acute lymphoblastic leukemia has beneficial influence on early recovery and possibly improves long‐term outcome: a randomized multicenter study. Leukemia & Lymphoma 2002;43(2):315‐25. [DOI] [PubMed] [Google Scholar]
Jones 1996 {published data only}
- Jones SE, Schottstaedt MW, Duncan LA, Kirby RL, Good RH, Mennel RG, et al. Randomized double‐blind prospective trial to evaluate the effects of sargramostim versus placebo in a moderate‐dose fluorouracil, doxorubicin, and cyclophosphamide adjuvant chemotherapy program for stage II and III breast cancer. Journal of Clinical Oncology 1996;14(11):2976‐83. [DOI] [PubMed] [Google Scholar]
Joshi 2003 {published data only}
- Joshi SS, Bishop MR, Lynch JC, Tarantolo SR, Abhyankar S, Bierman PJ, et al. Immunological and clinical effects of post‐transplant G‐CSF versus placebo in T‐cell replete allogeneic blood transplant patients: results from a randomized double‐blind study. Cytotherapy 2003;5(6):542‐52. [DOI] [PubMed] [Google Scholar]
Karp 1986 {published data only}
- Karp JE, Merz WG, Hendricksen C, Laughon B, Redden T, Bamberger BJ, et al. Infection management during antileukemia treatment‐induced granulocytopenia: the role for oral norfloxacin prophylaxis against infections arising from the gastrointestinal tract. Scandinavian Journal of Infectious Diseases Supplement 1986;48:66‐78. [PubMed] [Google Scholar]
Kim 2005 {published data only}
- Kim S, Cho YH, Ko OB, Koo JE, Lee D, Chong YP, et al. Beneficial prophylactic antimicrobial use against infectious complications during autologous stem cell transplantation: a result of randomized phase II study in multiple myeloma and non‐Hodgkin's lymphoma patients. Blood. 2005; Vol. 11.
Kosaka 2015 {published data only}
- Kosaka Y, Rai Y, Masuda N, Takano T, Saeki T, Nakamura S, et al. Phase III placebo‐controlled, double‐blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy. Support Care Cancer 2015;23(4):1137‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ladenstein 2010 {published data only}
- Ladenstein R, Valteau‐Couanet D, Brock P, Yaniv I, Castel V, Laureys G, et al. Randomized Trial of prophylactic granulocyte colony‐stimulating factor during rapid COJEC induction in pediatric patients with high‐risk neuroblastoma: the European HR‐NBL1/SIOPEN study. Journal of Clinical Oncology 2010;28(21):3516‐24. [DOI] [PubMed] [Google Scholar]
Lalami 2004 {published data only}
- Lalami Y, Paesmans M, Aoun M, Munoz‐Bermeo R, Reuss K, Cherifi S, et al. A prospective randomised evaluation of G‐CSF or G‐CSF plus oral antibiotics in chemotherapy‐treated patients at high risk of developing febrile neutropenia. Supportive Care in Cancer 2004;12(10):725‐30. [DOI] [PubMed] [Google Scholar]
Lamy 1993 {published data only}
- Lamy T, Michelet C, Dauriac C, Grulois I, Donio PY, Prisé PY. Benefit of prophylaxis by intravenous systemic vancomycin in granulocytopenic patients: a prospective, randomized trial among 59 patients. Acta Haematologica 1993;90(3):109‐13. [DOI] [PubMed] [Google Scholar]
Larson 1998 {published data only}
- Larson RA, Dodge RK, Linker CA, Stone RM, Powell BL, Lee EJ, et al. A randomized controlled trial of filgrastim during remission induction and consolidation chemotherapy for adults with acute lymphoblastic leukemia: CALGB study 9111. Blood 1998;92(5):1556‐64. [PubMed] [Google Scholar]
Lee 1998 {published data only}
- Lee SM, Radford JA, Dobson L, Huq T, Ryder WD, Pettengell Retal. Recombinant human granulocyte colony‐stimulating factor (filgrastim) following high‐dose chemotherapy and peripheral blood progenitor cell rescue in high‐grade non‐Hodgkin's lymphoma: clinical benefits at no extra cost. British Journal of Cancer 1998;77(8):1294‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2002 {published data only}
- Lee DG, Choi SM, Choi JH, Yoo JH, Park YH, Kim YJ, et al. Selective bowel decontamination for the prevention of infection in acute myelogenous leukemia: a prospective randomized trial. Korean Journal of Internal Medicine 2002;17(1):38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehrnbecher 2007 {published data only}
- Lehrnbecher T, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Creutzig U. Prophylactic human granulocyte colony‐stimulating factor after induction therapy in pediatric acute myeloid leukemia. Blood 2007;109(3):936‐43. [DOI] [PubMed] [Google Scholar]
Little 2002 {published data only}
- Little MA, Morland B, Chisholm J, Hole A, Shankar A, Devine T, et al. A randomised study of prophylactic G‐CSF following MRC UKALL XI intensification regimen in childhood ALL and T‐NHL. Medical & Pediatric Oncology 2002;38(2):98‐103. [DOI] [PubMed] [Google Scholar]
Maiche 1993 {published data only}
- Maiche AG, Muhonen T. Granulocyte colony‐stimulating factor (G‐CSF) with or without a quinolone in the prevention of infection in cancer patients. European Journal of Cancer 1993;29A(10):1403‐5. [DOI] [PubMed] [Google Scholar]
McQuaker 1997 {published data only}
- McQuaker IG, Hunter AE, Pacey S, Haynes AP, Iqbal A, Russell NH. Low‐dose filgrastim significantly enhances neutrophil recovery following autologous peripheral‐blood stem‐cell transplantation in patients with lymphoproliferative disorders: evidence for clinical and economic benefit (Structured abstract). Journal of Clinical Oncology 1997;15(2):451‐7. [DOI] [PubMed] [Google Scholar]
Michel 2000 {published data only}
- Michel G, Landman‐Parker J, Auclerc MF, Mathey C, Leblanc T, Legall E, et al. Use of recombinant human granulocyte colony‐stimulating factor to increase chemotherapy dose‐intensity: a randomized trial in very high‐risk childhood acute lymphoblastic leukemia. Journal of Clinical Oncology 2000;18(7):1517‐24. [DOI] [PubMed] [Google Scholar]
Michon 1998 {published data only}
- Michon JM, Hartmann O, Bouffet E, Meresse V, Coze C, Rubie H, et al. An open‐label, multicentre, randomised phase 2 study of recombinant human granulocyte colony‐stimulating factor (filgrastim) as an adjunct to combination chemotherapy in paediatric patients with metastatic neuroblastoma. European Journal of Cancer 1998;34(7):1063‐9. [DOI] [PubMed] [Google Scholar]
Miles 1994 {published data only}
- Miles DW, Fogarty O, Ash CM, Rudd RM, Trask CW, Spiro SG, et al. Received dose‐intensity: a randomized trial of weekly chemotherapy with and without granulocyte colony‐stimulating factor in small‐cell lung cancer. Journal of Clinical Oncology 1994;12(1):77‐82. [DOI] [PubMed] [Google Scholar]
Nemunaitis 1995 {published data only}
- Nemunaitis J, Rosenfeld CS, Ash R, Freedman MH, Deeg HJ, Appelbaum F, et al. Phase III randomized, double‐blind placebo‐controlled trial of rhGM‐CSF following allogeneic bone marrow transplantation. Bone Marrow Transplantation 1995;15(6):949‐54. [PubMed] [Google Scholar]
Nolan 2007 {published data only}
- Nolan L, Lorigan P, Chilton S, Newman J, Else R, Smith P, et al. Low‐dose lenograstim is as effective as standard dose in shortening neutrophil engraftment time following myeloablative chemotherapy and peripheral blood progenitor cell rescue. British Journal of Haematology 2007;137(5):436‐42. [DOI] [PubMed] [Google Scholar]
Ojeda 1999 {published data only}
- Ojeda E, Garcia‐Bustos J, Aguado M, Arrieta R, Quevedo E, Yuste VJ, et al. A prospective randomized trial of granulocyte colony‐stimulating factor therapy after autologous blood stem cell transplantation in adults. Bone Marrow Transplantation 1999;24(6):601‐7. [DOI] [PubMed] [Google Scholar]
Osby 2003 {published data only}
- Osby E, Hagberg H, Kvaløy S, Teerenhovi L, Anderson H, Cavallin‐Stahl E, et al. CHOP is superior to CNOP in elderly patients with aggressive lymphoma while outcome is unaffected by filgrastim treatment: results of a Nordic Lymphoma Group randomized trial. Blood 2003;101(10):3840‐8. [DOI] [PubMed] [Google Scholar]
Ottmann 1995 {published data only}
- Ottmann OG, Hoelzer D, Gracien E, Ganser A, Kelly K, Reutzel R, et al. Concomitant granulocyte colony‐stimulating factor and induction chemoradiotherapy in adult acute lymphoblastic leukemia: a randomized phase III trial. Blood 1995;86(2):444‐50. [PubMed] [Google Scholar]
Patte 2002 {published data only}
- Patte C, Laplanche A, Bertozzi AI, Baruchel A, Frappaz D, Schmitt C, et al. Granulocyte colony‐stimulating factor in induction treatment of children with non‐Hodgkin's lymphoma: a randomized study of the French Society of Pediatric Oncology. Journal of Clinical Oncology 2002;20(2):441‐8. [DOI] [PubMed] [Google Scholar]
Petersen 1988 {published data only}
- Petersen F, Thornquist M, Buckner C, Counts G, Nelson N, Meyers J, et al. The effects of infection prevention regimens on early infectious complications in marrow transplant patients: a four arm randomized study. Infection 1988;16(4):199‐208. [DOI] [PubMed] [Google Scholar]
Pettengell 1992 {published data only}
- Pettengell R, Gurney H, Radford JA, Deakin DP, James R, Wilkinson PM, et al. Granulocyte colony‐stimulating factor to prevent dose‐limiting neutropenia in non‐Hodgkin's lymphoma: a randomized controlled trial. Blood 1992;80(6):1430‐6. [PubMed] [Google Scholar]
Piccirillo 1999 {published data only}
- Piccirillo N, Sica S, Laurenti L, Chiusolo P, Barbera EO, Sora F, et al. Optimal timing of G‐CSF administration after CD34+ immunoselected peripheral blood progenitor cell transplantation. Bone Marrow Transplantation 1999;23(12):1245‐50. [DOI] [PubMed] [Google Scholar]
Pignon 1990 {published data only}
- Pignon B, Thiriet L, Aubert D, Pennaforte JL, Vilque JP, Lartigue B, et al. Evaluation of the efficacy of prophylactic intravenous antibiotherapy with ceftriaxone in post‐chemotherapy agranulocytic patients. Nouvelle Revue Française d'Hématologie 1990;32(4):249‐52. [PubMed] [Google Scholar]
Przepiorka 2001 {published data only}
- Przepiorka D, Smith TL, Folloder J, Anderlini P, Chan KW, Korbling M, et al. Controlled trial of filgrastim for acceleration of neutrophil recovery after allogeneic blood stem cell transplantation from human leukocyte antigen‐matched related donors. Blood 2001;97(11):3405‐10. [DOI] [PubMed] [Google Scholar]
Pui 1997 {published data only}
- Pui CH, Boyett JM, Hughes WT, Rivera GK, Hancock ML, Sandlund JT, et al. Human granulocyte colony‐stimulating factor after induction chemotherapy in children with acute lymphoblastic leukemia. New England Journal of Medicine 1997;336(25):1781‐7. [DOI] [PubMed] [Google Scholar]
Rafecas 1989 {published data only}
- Rafecas FJ, Gil E, Martín G, Martínez J, Sanz G, Sempere A, et al. Oral ciprofloxacin in the prophylaxis of bacterial infection in neutropenic patients. A randomized, double‐blind, comparative clinical study. Revista Española de Quimioterapia 1989;2:174‐7. [Google Scholar]
Romieu 2007 {published data only}
- Romieu G, Clemens M, Mahlberg R, Fargeot P, Constenla M, Schutte M, et al. Pegfilgrastim supports delivery of FEC‐100 chemotherapy in elderly patients with high risk breast cancer: a randomized phase 2 trial. Critical Reviews in Oncology/hematology 2007;64(1):64‐72. [DOI] [PubMed] [Google Scholar]
Schmitz 2004 {published data only}
- Schmitz N, Ljungman P, Cordonnier C, Kempf C, Linkesch W, Alegre A, et al. Lenograstim after autologous peripheral blood progenitor cell transplantation: results of a double‐blind, randomized trial. Bone Marrow Transplantation 2004;34(11):955‐62. [DOI] [PubMed] [Google Scholar]
Schuette 2011 {published data only}
- Schuette W, Nagel S, Weikersthal LF, Pabst S, Schumann C, Deuss B, et al. Randomized phase III trial of docetaxel plus carboplatin with or without levofloxacin prophylaxis in elderly patients with advanced non‐small cell lung cancer: the APRONTA trial. Journal of Thoracic Oncology 2011;6(12):2090‐6. [DOI] [PubMed] [Google Scholar]
Seymour 1995 {published data only}
- Seymour AM, Campos E, Thatcher N, Greve J, Cunningham D, Howell A, et al. A single‐blind, randomised, vehicle‐controlled dose‐finding study of recombinant human granulocyte colony‐stimulating factor (lenograstim) in patients undergoing chemotherapy for solid cancers and lymphoma. European Journal of Cancer 1995;31A(13‐14):2157‐63. [DOI] [PubMed] [Google Scholar]
Spitzer 1994 {published data only}
- Spitzer G, Adkins DR, Spencer V, Dunphy FR, Petruska PJ, Velasquez WS, et al. Randomized study of growth factors post‐peripheral‐blood stem‐cell transplant: neutrophil recovery is improved with modest clinical benefit. Journal of Clinical Oncology 1994;12(4):661‐70. [DOI] [PubMed] [Google Scholar]
Stahel 1994 {published data only}
- Stahel RA, Jost LM, Cerny T, Pichert G, Honegger H, Tobler A, et al. Randomized study of recombinant human granulocyte colony‐stimulating factor after high‐dose chemotherapy and autologous bone marrow transplantation for high‐risk lymphoid malignancies. Journal of Clinical Oncology 1994;12(9):1931‐8. [DOI] [PubMed] [Google Scholar]
Suh 2008 {published data only}
- Suh Y, Chun C, Oh S. Prevention of infection after TAC chemotherapy for node‐positive breast cancer as an adjuvant therapy with or without ciprofloxacin. Annals of Oncology 2008;26(15S):81. [Google Scholar]
Talbot 1993 {published data only}
- Talbot GH, Cassileth PA, Paradiso L, Correa‐Coronas R, Bond L. Oral enoxacin for infection prevention in adults with acute nonlymphocytic leukemia. The Enoxacin Prophylaxis Study Group. Antimicrobial Agents & Chemotherapy 1993;37(3):474‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Timmer‐Bonte 2005 {published data only}
- Timmer‐Bonte JN, Boo TM, Smit HJ, Biesma B, Wilschut FA, Cheragwandi SA, et al. Prevention of chemotherapy‐induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colony‐stimulating factor in small‐cell lung cancer: a Dutch Randomized Phase III Study. Journal of Clinical Oncology 2005;23(31):7974‐84. [DOI] [PubMed] [Google Scholar]
Tjan‐Heijnen 2003 {published data only}
- Tjan‐Heijnen VC, Caleo S, Postmus PE, Ardizzoni A, Burghouts JT, Buccholz E, et al. Economic evaluation of antibiotic prophylaxis in small‐cell lung cancer patients receiving chemotherapy: an EORTC double‐blind placebo‐controlled phase III study (08923). Annals of Oncology 2003;14(2):248‐57. [DOI] [PubMed] [Google Scholar]
Trigg 2000 {published data only}
- Trigg ME, Peters C, Zimmerman MB. Administration of recombinant human granulocyte‐macrophage colony‐stimulating factor to children undergoing allogeneic marrow transplantation: a prospective, randomized, double‐masked, placebo‐controlled trial. Pediatric Transplantation 2000;4(2):123‐31. [DOI] [PubMed] [Google Scholar]
Trillet‐Lenoir 1993 {published data only}
- Trillet‐Lenoir V, Green J, Manegold C, Pawel J, Gatzemeier U, Lebeau B, et al. Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. European Journal of Cancer 1993;29A(3):319‐24. [DOI] [PubMed] [Google Scholar]
Veyret 2006 {published data only}
- Veyret C, Levy C, Chollet P, Merrouche Y, Roche H, Kerbrat P, et al. Inflammatory breast cancer outcome with epirubicin‐based induction and maintenance chemotherapy: ten‐year results from the French Adjuvant Study Group GETIS 02 Trial. Cancer 2006;107(11):2535‐44. [DOI] [PubMed] [Google Scholar]
Vogel 2005 {published data only}
- Vogel CL, Wojtukiewicz MZ, Carroll RR, Tjulandin SA, Barajas‐Figueroa LJ, Wiens BL, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double‐blind, placebo‐controlled phase III study. Journal of Clinical Oncology 2005;23(6):1178‐84. [DOI] [PubMed] [Google Scholar]
Welte 1996 {published data only}
- Welte K, Reiter A, Mempel K, Pfetsch M, Schwab G, Schrappe M, et al. A randomized phase‐III study of the efficacy of granulocyte colony‐stimulating factor in children with high‐risk acute lymphoblastic leukemia. Berlin‐Frankfurt‐Munster Study Group. Blood 1996;87(8):3143‐50. [PubMed] [Google Scholar]
Witz 1998 {published data only}
- Witz F, Sadoun A, Perrin MC, Berthou C, Briere J, Cahn JY, et al. A placebo‐controlled study of recombinant human granulocyte‐macrophage colony‐stimulating factor administered during and after induction treatment for de novo acute myelogenous leukemia in elderly patients. Groupe Ouest Est Leucemies Aigues Myeloblastiques (GOELAM). Blood 1998;91(8):2722‐30. [PubMed] [Google Scholar]
Yamada 1993 {published data only}
- Yamada T, Dan K, Nomura T. Prevention of bacterial and fungal infections in acute leukemia patients: a new and potent combination of oral norfloxacin and amphotericin B. Internal Medicine (Tokyo, Japan) 1993;32(9):710‐5. [DOI] [PubMed] [Google Scholar]
Yau 1996 {published data only}
- Yau JC, Neidhart JA, Triozzi P, Verma S, Nemunaitis J, Quick DP, et al. Randomized placebo‐controlled trial of granulocyte‐macrophage colony‐stimulating‐factor support for dose‐intensive cyclophosphamide, etoposide, and cisplatin. American Journal of Hematology 1996;51(4):289‐95. [DOI] [PubMed] [Google Scholar]
Additional references
Alvarado 1999
- Alvarado Ibarra ML, Borbolla Escoboza JR, Lopez‐Hernandez MA, Gonzalez‐Avante CM, FloresChapa JD, Trueba Christy E, et al. Neutrophil recovery time and adverse side effects in acute leukemia patients treated with intensive chemotherapy and concomitant G or GM‐CSF. Revista de Investigacion Clinica 1999;51(2):77‐80. [PUBMED: 10410585] [PubMed] [Google Scholar]
Beveridge 1997
- Beveridge RA, Miller JA, Kales AN, Binder RA, Robert NJ, Heisrath‐Evans J, et al. Randomized trial comparing the tolerability of sargramostim (yeast‐derived RhuGM‐CSF) and filgrastim (bacteria‐derived RhuG‐CSF) in cancer patients receiving myelosuppressive chemotherapy. Supportive Care in Cancer 1997;5(4):289‐98. [PUBMED: 9257425] [DOI] [PubMed] [Google Scholar]
Beveridge 1998
- Beveridge RA, Miller JA, Kales AN, Binder RA, Robert NJ, Harvey JH, et al. A comparison of efficacy of sargramostim (yeast‐derived RhuGM‐CSF) and filgrastim (bacteria‐derived RhuG‐CSF) in the therapeutic setting of chemotherapy‐induced myelosuppression. Cancer Investigation 1998;16(6):366‐73. [PUBMED: 9679526] [DOI] [PubMed] [Google Scholar]
Bodey 1966
- Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Annals of Internal Medicine 1966;64(2):328‐40. [DOI] [PubMed] [Google Scholar]
Bodey 1986
- Bodey GP. Infection in cancer patients. A continuing association. American Journal of Medicine 1986;81(1A):11‐26. [DOI] [PubMed] [Google Scholar]
Bohlius 2008
- Bohlius J, Herbst C, Reiser M, Schwarzer G, Engert A. Granulopoiesis‐stimulating factors to prevent adverse effects in the treatment of malignant lymphoma. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003189.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bronchud 1988
- Bronchud MH, Potter MR, Morgenstern G, Blasco MJ, Scarffe JH, Thatcher N, et al. In vitro and in vivo analysis of the effects of recombinant human granulocyte colony‐stimulating factor in patients. British Journal of Cancer 1988;58(1):64‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bucaneve 2005
- Bucaneve G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. New England Journal of Medicine 2005;353(10):977‐87. [DOI] [PubMed] [Google Scholar]
Carratala 1995
- Carratala J, Fernandez‐Sevilla A, Tubau F, Callis M, Gudiol F. Emergence of quinolone‐resistant Escherichia coli bacteremia in neutropenic patients with cancer who have received prophylactic norfloxacin. Clinical Infectious Diseases 1995;20(3):557‐60. [DOI] [PubMed] [Google Scholar]
Clark 2005
- Clark OA, Lyman GH, Castro AA, Clark LG, Djulbegovic B. Colony‐stimulating factors for chemotherapy‐induced febrile neutropenia: a meta‐analysis of randomized controlled trials. Journal of Clinical Oncology 2005;23(18):4198‐214. [DOI] [PubMed] [Google Scholar]
Crawford 1991
- Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, et al. Reduction by granulocyte colony‐stimulating factor of fever and neutropenia induced by chemotherapy in patients with small‐cell lung cancer. New England Journal of Medicine 1991;325(3):164‐70. [DOI] [PubMed] [Google Scholar]
Cruciani 2003
- Cruciani M, Malena M, Bosco O, Nardi S, Serpelloni G, Mengoli C. Reappraisal with meta‐analysis of the addition of Gram‐positive prophylaxis to fluoroquinolone in neutropenic patients. Journal of Clinical Oncology 2003;21(22):4127‐37. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].The Cochrane Collaboration. Available from www.cochrane‐handbook.org. 2011. [Google Scholar]
Engels 1998
- Engels EA, Lau J, Barza M. Efficacy of quinolone prophylaxis in neutropenic cancer patients: a meta‐analysis. Journal of Clinical Oncology 1998;16(3):1179‐87. [DOI] [PubMed] [Google Scholar]
Fischmeister 1999
- Fischmeister G, Kurz M, Haas OA, Micksche M, Buchinger P, Printz D, et al. G‐CSF versus GM‐CSF for stimulation of peripheral blood progenitor cells (PBPC) and leukocytes in healthy volunteers: comparison of efficacy and tolerability. Annals of Hematology 1999;78(3):117‐23. [PUBMED: 10211753] [DOI] [PubMed] [Google Scholar]
Gafter‐Gvili 2005
- Gafter‐Gvili A, Fraser A, Paul M, Leibovici L. Meta‐Analysis: Antibiotic prophylaxis reduces mortality in neutropenic patients. Annals of Internal Medicine 2005;142(12 Pt 1):979‐95. [DOI] [PubMed] [Google Scholar]
Gafter‐Gvili 2007
- Gafter‐Gvili A, Paul M, Fraser A, Leibovici L. Effect of quinolone prophylaxis in afebrile neutropenic patients on microbial resistance: systematic review and meta‐analysis. Journal of Antimicrobial Chemotherapy 2007;59(1):5‐22. [DOI] [PubMed] [Google Scholar]
Gafter‐Gvili 2012
- Gafter‐Gvili A, Fraser A, Paul M, Vidal L, Lawrie TA, Wetering M D, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD004386.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffin 1990
- Griffin JD, Cannistra SA, Sullivan R, Demetri GD, Ernst TJ, Kanakura Y. The biology of GM‐CSF: regulation of production and interaction with its receptor. International Journal of Cell Cloning 1990;8 Suppl 1:35‐44. [DOI] [PubMed] [Google Scholar]
Hackshaw 2004
- Hackshaw A, Sweetenham J, Knight A. Are prophylactic haematopoietic growth factors of value in the management of patients with aggressive non‐Hodgkin's lymphoma?. British Journal of Cancer 2004;90(7):1302‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. 2011. [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. 2011. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].The Cochrane Collaboration. Available from www.cochrane‐handbook.org. 2011. [Google Scholar]
Holmes 2002
- Holmes FA, Jones SE, O'Shaughnessy J, Vukelja S, George T, Savin M, et al. Comparable efficacy and safety profiles of once‐per‐cycle pegfilgrastim and daily injection filgrastim in chemotherapy‐induced neutropenia: a multicenter dose‐finding study in women with breast cancer. Annals of Oncology 2002;13(6):903‐9. [DOI] [PubMed] [Google Scholar]
Hovgaard 1992
- Hovgaard DJ, Nissen NI. Effect of recombinant human granulocyte‐macrophage colony‐stimulating factor in patients with Hodgkin's disease: a phase I/II study. Journal of Clinical Oncology 1992;10(3):390‐7. [PUBMED: 1740678] [DOI] [PubMed] [Google Scholar]
Johnston 2000
- Johnston E, Crawford J, Blackwell S, Bjurstrom T, Lockbaum P, Roskos L, et al. Randomized, dose‐escalation study of SD/01 compared with daily filgrastim in patients receiving chemotherapy. Journal of Clinical Oncology 2000;18(13):2522‐8. [DOI] [PubMed] [Google Scholar]
Karp 1987
- Karp JE, Merz WG, Hendricksen C, Laughon B, Redden T, Bamberger BJ, et al. Oral norfloxacin for prevention of gram‐negative bacterial infections in patients with acute leukemia and granulocytopenia. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1987;106(1):1‐7. [DOI] [PubMed] [Google Scholar]
Kuderer 2006
- Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006;106(10):2258‐66. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, Available from www.cochrane‐handbook.org. 2011. [Google Scholar]
Leibovici 2006
- Leibovici L, Paul M, Cullen M, Bucaneve G, Gafter‐Gvili A, Fraser A, et al. Antibiotic prophylaxis in neutropenic patients: new evidence, practical decisions. Cancer 2006;107(8):1743‐51. [DOI] [PubMed] [Google Scholar]
Lew 1995
- Lew MA, Kehoe K, Ritz J, Antman KH, Nadler L, Kalish LA, et al. Ciprofloxacin versus trimethoprim/sulfamethoxazole for prophylaxis of bacterial infections in bone marrow transplant recipients: a randomized, controlled trial. Journal of Clinical Oncology 1995;13(1):239‐50. [DOI] [PubMed] [Google Scholar]
Lyman 2002
- Lyman GH, Kuderer NM, Djulbegovic B. Prophylactic granulocyte colony‐stimulating factor in patients receiving dose‐intensive cancer chemotherapy: A meta‐analysis. American Journal of Medicine 2002;112(5):406‐11. [DOI] [PubMed] [Google Scholar]
Lyman 2010
- Lyman GH, Dale DC, Wolff DA, Culakova E, Poniewierski MS, Kuderer NM, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony‐stimulating factor: a systematic review. Journal of Clinical Oncology 2010;28(17):2914‐24. [PUBMED: 20385991] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐Analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [PUBMED: 19631508] [DOI] [PubMed] [Google Scholar]
Morstyn 1988
- Morstyn G, Campbell L, Souza LM, Alton NK, Keech J, Green M, et al. Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet 1988;1(8587):667‐72. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pizzo 1999
- Pizzo PA. Fever in immunocompromised patients. New England Journal of Medicine 1999;341(12):893‐900. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Smith 2015
- Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. Journal of Clinical Oncology 2015;33(28):3199‐212. [DOI] [PubMed] [Google Scholar]
Somolinos 1992
- Somolinos N, Arranz R, Rey MC, Jimenez ML. Superinfections by Escherichia coli resistant to fluoroquinolones in immunocompromised patients. Journal of Antimicrobial Chemotherapy 1992;30(5):730‐1. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, Available from www.cochrane‐handbook.org.. 2011. [Google Scholar]
Sung 2004
- Sung L, Nathan PC, Lange B, Beyene J, Buchanan JR. Prophylactic granulocyte colony‐stimulating factor and granulocyte‐macrophage colony‐stimulating factor decrease febrile neutropenia after chemotherapy in children with cancer: A meta‐analysis of randomized controlled trials. Journal of Clinical Oncology 2004;22(16):3350‐6. [DOI] [PubMed] [Google Scholar]
Sung 2007
- Sung L, Nathan PC, Alibhai SM, Tomlinson GA, Beyene J. Meta‐analysis: effect of prophylactic hematopoietic colony‐stimulating factors on mortality and outcomes of infection. Annals of Internal Medicine 2007;147(6):400‐11. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;June 7(8):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vehreschild 2014
- Vehreschild JJ, Bohme A, Cornely OA, Kahl C, Karthaus M, Kreuzer KA, et al. Prophylaxis of infectious complications with colony‐stimulating factors in adult cancer patients undergoing chemotherapy‐evidence‐based guidelines from the Infectious Diseases Working Party AGIHO of the German Society for Haematology and Medical Oncology (DGHO). Annals of Oncology 2014;25(9):1709‐18. [PUBMED: 24631945] [DOI] [PubMed] [Google Scholar]
Wittman 2006
- Wittman B, Horan J, Lyman GH. Prophylactic colony‐stimulating factors in children receiving myelosuppressive chemotherapy: a meta‐analysis of randomized controlled trials. Cancer Treatment Reviews 2006;32(4):289‐303. [DOI] [PubMed] [Google Scholar]


