Abstract
Background
Head position during care may affect cerebral haemodynamics and contribute to the development of germinal matrix‐intraventricular haemorrhage (GM‐IVH) in very preterm infants. Turning the head toward one side may occlude jugular venous drainage while increasing intracranial pressure and cerebral blood volume. It is suggested that cerebral venous pressure is reduced and hydrostatic brain drainage improved if the infant is cared for in the supine ‘head midline’ position.
Objectives
To assess whether head midline position is more effective than other head positions for preventing (or preventing extension) of GM‐IVH in very preterm infants (< 32 weeks’ gestation at birth).
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 9), MEDLINE via PubMed (1966 to 12 September 2019), Embase (1980 to 12 September 2019), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 12 September 2019). We searched clinical trials databases, conference proceedings, and reference lists of retrieved articles.
Selection criteria
Randomised controlled trials (RCTs) comparing caring for very preterm infants in a supine head midline position versus a prone or lateral decubitus position, or undertaking a strategy of regular position change, or having no prespecified position. We included trials enrolling infants with existing GM‐IVH and planned to assess extension of haemorrhage in a subgroup of infants. We planned to analyse horizontal (flat) versus head elevated positions separately for all body positions.
Data collection and analysis
We used standard methods of Cochrane Neonatal. For each of the included trials, two review authors independently extracted data and assessed risk of bias. The primary outcomes were GM‐IVH, severe IVH, and neonatal death. We evaluated treatment effects using a fixed‐effect model with risk ratio (RR) for categorical data; and mean, standard deviation (SD), and mean difference (MD) for continuous data. We used the GRADE approach to assess the certainty of evidence.
Main results
Three RCTs, with a total of 290 infants (either < 30 weeks' gestational age or < 1000 g body weight), met the inclusion criteria. Two trials compared supine midline head position versus head rotated 90° with the cot flat. One trial compared supine midline head position versus head rotated 90° with the bed tilted at 30°. We found no trials that compared supine versus prone midline head position.
Meta‐analysis of three trials (290 infants) did not show an effect on rates of GM‐IVH (RR 1.11, 95% confidence interval (CI) 0.78 to 1.56; I² = 0%) and severe IVH (RR 0.71, 95% CI 0.37 to 1.33; I² = 0%). Neonatal mortality (RR 0.49, 95% CI 0.25 to 0.93; I² = 0%; RD −0.09, 95% CI −0.16 to −0.01) and mortality until hospital discharge (typical RR 0.50, 95% CI 0.28 to 0.90; I² = 0%; RD −0.10, 95% CI −0.18 to −0.02) were lower in the supine midline head position. The certainty of the evidence was very low for all outcomes because of limitations in study design and imprecision of estimates. We identified one ongoing study.
Authors' conclusions
We found few trial data on the effects of head midline position on GM‐IVH in very preterm infants. Although meta‐analyses suggest that mortality might be reduced, the certainty of the evidence is very low and it is unclear whether any effect is due to cot tilting (a co‐intervention in one trial). Further high‐quality RCTs would be needed to resolve this uncertainty.
Plain language summary
Head midline (central) position for preventing intraventricular haemorrhage (i.e. bleeding in the brain) in very preterm infants
Review question
Does head midline position reduce the risk of intraventricular haemorrhage (i.e. bleeding in the brain) and mortality in very preterm infants?
Background
Intraventricular haemorrhage (i.e. bleeding in the brain) occurs in 25% of very low birth weight infants and may be caused by multiple factors. Head position may affect how the blood circulates within the brain and thus may be involved in development of intraventricular haemorrhage. Turning the head toward one side may limit return of blood in the veins of the same side and may increase pressure and the amount of blood within the brain. It has been suggested that this might be avoided if the patient is in supine (lying on the back) midline (central) position, especially during the first two to three days of life, when risk of intraventricular haemorrhage is greatest.
Study characteristics
We included three small studies comparing supine midline head position versus supine head rotated 90°. The search is up to date as of 12 September 2019.
Key results
This review of trials found too little evidence to show positive or negative effects of supine (lying on the back) midline head position for prevention of intraventricular haemorrhage (i.e. bleeding within the brain) in very preterm neonates. Mortality was lower in the supine midline head position, due to one study which compared the effects of head tilting (elevating the head of the incubator upward). We found no trials that compared supine (lying on the back) versus prone (lying on the stomach) midline head position.
Conclusions
Though one of the studies reported lower mortality in the infants with head central position lying on the back and with bed tilting, results of this systematic review are consistent with beneficial or detrimental effects of a supine head midline position and do not provide a definitive answer to the review question.
Summary of findings
Summary of findings 1. Supine midline head position versus any other supine head position for preventing the occurrence or extension of germinal matrix‐intraventricular haemorrhage in preterm infants.
| Supine midline head position versus any other supine head position for preventing the occurrence or extension of germinal matrix‐intraventricular haemorrhage in preterm infants | ||||||
|
Patient or population: very preterm infants with or at risk of germinal matrix‐intraventricular haemorrhage in preterm infants
Settings: neonatal intensive care units (the included trials were conducted in Saudi Arabia and USA)
Intervention: supine midline head position Comparison: any other supine head position | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Supine midline head position versus any other supine head position | |||||
| Intraventricular haemorrhage, any grade | Study population | RR 1.11 (0.78 to 1.56) | 290 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 288 per 1000 | 320 per 1000 (225 to 449) | |||||
| Medium risk population | ||||||
| 200 per 1000 | 222 per 1000 (156 to 312) | |||||
| Intraventricular haemorrhage, grade 3 to 4 | Study population | RR 0.71 (0.37 to 1.33) | 290 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 137 per 1000 | 97 per 1000 (51 to 182) | |||||
| Medium risk population | ||||||
| 40 per 1000 | 28 per 1000 (15 to 53) | |||||
| Mortality during initial hospitalisation | Study population | RR 0.50 (0.28 to 0.90) | 290 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 205 per 1000 | 102 per 1000 (57 to 184) | |||||
| Medium risk population | ||||||
| 200 per 1000 | 100 per 1000 (56 to 180) | |||||
| Retinopathy of prematurity, > stage 3 | Study population | RR 0.90 (0.41 to 1.97) | 222 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | ||
| 109 per 1000 | 98 per 1000 (45 to 215) | |||||
| Medium risk population | ||||||
| 86 per 1000 | 77 per 1000 (35 to 169) | |||||
| Long‐term neurodevelopmental outcome ‒not reported | Study population | not estimable | no data available | |||
| See comment | See comment | |||||
| Medium risk population | ||||||
| Major neurodevelopmental disability ‒not reported | Study population | not estimable | no data available | |||
| See comment | See comment | |||||
| Medium risk population | ||||||
| Cerebellar haemorrhage on brain ultrasound ‐ not reported | Study population | not estimable | no data available | |||
| See comment | See comment | |||||
| Medium risk population | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1 limitations in study design: all studies at high or unclear risk of bias in at least one domain. 2 downgraded by one levels for imprecision: few studies and few events.
3 downgraded by one levels for inconsistency: two trials with horizontal bed; one trial with bed tilting.
Background
Description of the condition
Preterm birth remains a major risk factor for development of germinal matrix‐intraventricular haemorrhage (GM‐IVH), which occurs in 25% of very low birth weight (VLBW) infants (Canadian Neonatal Network 2014; Vermont Oxford Network 2013). Often, these haemorrhages occur during the first days of life (Dolfin 1983). Complications of GM‐IVH, including periventricular haemorrhagic infarction (PVHI), posthaemorrhagic ventricular dilatation (PHVD), and associated cerebellar haemorrhagic injury (CHI) and periventricular leukomalacia (PVL), are critical determinants of neonatal morbidity, mortality, and long‐term neurodevelopmental sequelae (Sherlock 2005). Although modern perinatal medicine has led to a significant decrease in the overall incidence of GM‐IVH among preterm infants (i.e. from 50% in the late 1970s to current rates of 15% to 25%) (Hamrick 2004; Horbar 2002; Philip 1989), GM‐IVH continues to present a significant problem in the modern neonatal intensive care unit (NICU). The origin of GM‐IVH is multifactorial, complex, and heterogeneous. Inherent fragility of the germinal matrix vasculature sets the ground for haemorrhage, and fluctuation in cerebral blood flow induces rupture of the vasculature (Romantsik 2019). Furthermore, the germinal matrix lies within an arterial end zone, and it is directly connected to the deep galenic venous system (Nakamura 1990; Pape 1979), thereby exposing it to insults of arterial ischaemia‐reperfusion and venous congestion (Pape 1979; Takashima 1978). The immature deep galenic system is prone to venous congestion and stasis, making it of potentially major importance for development of GM‐IVH and its complications (Pape 1979; Volpe 2008). It is unknown what proportion of GM‐IVH might occur because of this phenomenon. Nevertheless, many institutions adopt the practice of head midline position. Vaginal delivery, low Apgar score, severe respiratory distress syndrome, pneumothorax, hypoxia, hypercapnia, seizures, patent ductus arteriosus, infection, and other factors seem to primarily increase fluctuations in cerebral blood flow, thus representing important risk factors for development of GM‐IVH (Ballabh 2014).
Description of the intervention
It has been suggested that head position may affect cerebral haemodynamics in the preterm newborn and might be involved indirectly in development of GM‐IVH. Doppler studies in term infants have shown that turning the head toward one side functionally occluded jugular venous drainage on the ipsilateral side (Cowan 1985). Moreover, an increase in intracranial pressure (Cowan 1985; Emery 1983) and in cerebral blood volume (CBV) (Pellicer 2002) after head rotation, caused by obstruction of the homolateral jugular veins, has been reported. Thus, it has been suggested that cerebral venous pressure is reduced and hydrostatic brain drainage improved if the patient is in supine midline position with the bed tilted 30° (Cowan 1985; Emery 1983). Researchers have reported an increase in cerebral blood flow (CBF) in the supine position and an increase in partial pressure of oxygen (PO₂) in the prone position in stable preterm infants (Bembich 2012). However, Ancora's study results did not show significant changes in the tissue haemoglobin index (which is proportionate to changes in CBV) nor in oxygenation. Only infants with low gestational age (< 26 weeks) showed a reduction in CBV with head rotation (Ancora 2010). In addition, ventilatory support has been shown to influence brain haemodynamics (Cowan 1987): newborns on mechanical ventilation showed an increase in CBV during inspiration compared with those breathing spontaneously (Leahy 1982). Nasal continuous positive airway pressure (nCPAP) did not, however, seem to have an effect on CBV or on CBF among preterm infants (Dani 2007; Moritz 2008).
The definition of head midline position is complex, as the position of the body may have a relevant impact. In the supine position, the infant’s head is maintained in alignment with the midline. In the prone position, the head has to be turned to the side, so the head midline position is not feasible. In the lateral position, the midline position might be achieved if the head is kept aligned with midline. Maintenance of this position may require the presence of physical aids, such as nests or pillows, and active surveillance by nurses. It has been reported that the midline position in the lateral decubitus during kangaroo care might be associated with improved early neuromotor development as assessed by the Dubowitz score (Barradas 2006). Midline position should be kept at least when the incidence of GM‐IVH is greatest: that is, during the first two to three days of life. It is unknown, however, if strict observance of the midline position might confer any disadvantages, and if the head midline position with the infant supine is different from the head midline position with the infant lying on the side.
How the intervention might work
An intubated preterm infant's head may be turned toward one side (facing the ventilator, e.g. a high‐frequency oscillator) for prolonged periods. As impaired venous drainage and decreased cerebral tissue oxygenation are factors implicated in the pathogenesis of IVH (Noori 2014; Osborn 2003; Takashima 2009), midline head positioning during the early transitional period has been included in recent IVH prevention bundles at many institutions, albeit without strong data to support the practice (McLendon 2003; Nankervis 2010; Obladen 2008). Midline head positioning during the early transitional period might prevent the occurrence of IVH through improved venous drainage and cerebral oxygenation.
Why it is important to do this review
As noted above, GM‐IVH occurs in 25% of VLBW infants (Canadian Neonatal Network 2014; Vermont Oxford Network 2013). Midline head positioning has been included in recent GM‐IVH prevention bundles at many institutions, albeit without strong data to support the practice (McLendon 2003; Nankervis 2010; Obladen 2008). One review recommended midline head position for preterm infants on the basis of physiological data and expert opinion; however, review authors identified no controlled trials for inclusion (Malusky 2011). Moreover, midline positioning with bed elevation of 30° has been identified as a "potentially better practice" for prevention of GM‐IVH, although review authors rated the quality of evidence as low (Carteaux 2003). We hoped that this systematic review would help clinicians and policy makers to provide specific recommendations about optimal head positioning, with an important impact on neonatal health and long‐term outcomes for the very preterm infant.
Objectives
To assess whether head midline position is more effective than other head positions for preventing (or preventing extension) of GM‐IVH in very preterm infants (< 32 weeks’ gestation at birth).
Methods
Criteria for considering studies for this review
Types of studies
We included prospective randomised clinical controlled trials, quasi‐randomised trials, and cluster‐randomised controlled trials. We excluded cross‐over trials because the intervention might have a lasting effect that compromises entry to subsequent periods of the trial.
Types of participants
We included very preterm infants (i.e. < 32 weeks' gestational age) of any birth weight, admitted to neonatal intensive care units.
We included studies enrolling infants with unknown GM‐IVH status at enrolment; if known, we planned to perform subgroup analysis on the presence of GM‐IVH at study entry.
We planned to include studies enrolling infants with existing GM‐IVH and to assess extension of haemorrhage in a subgroup of infants.
Types of interventions
Placing newborns in a head midline position compared with placing them in a prone or lateral decubitus position, or undertaking a strategy of regular position change, or having no prespecified position.
We planned to analyse horizontal (flat) versus head elevated positions separately for all body positions.
We planned to conduct the following comparisons.
-
Supine midline head position versus any other supine head position
Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0°
Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed tilted ≥ 30°
Supine midline head position with the bed tilted ≥ 30° versus supine head rotated 90° with the bed at 0°
Supine midline head position with the bed tilted ≥ 30° versus supine head rotated 90° with the bed tilted ≥ 30°
-
Supine midline head position versus any other prone head position
Supine midline head position with the bed at 0° versus prone head rotated 90° with the bed at 0°
Supine midline head position with the bed at 0° versus prone head rotated 90° with the bed tilted ≥ 30°
Supine midline head position with the bed tilted ≥ 30° versus prone head rotated 90° with the bed at 0°
Supine midline head position with the bed tilted ≥ 30° versus prone head rotated 90° with the bed tilted ≥ 30°
Supine midline head position with the bed at 0° versus supine midline head position with the bed tilted ≥ 30°
As the aim of this review is to assess the ability of head position to prevent GM‐IVH, we included trials in which the intervention is started within the first 48 hours of life.
Types of outcome measures
Primary outcomes
Any germinal matrix‐intraventricular haemorrhage: any IVH, grades 1 to 4 (according to Papile classification (Papile 1978))
Severe IVH: ultrasound diagnosis grades 3 and 4 (according to Papile classification (Papile 1978))
Neonatal death (first 28 days) or during initial hospitalisation
Secondary outcomes
Cerebellar haemorrhage on brain ultrasound in the first month of life (yes/no; Graça 2013)
Cystic periventricular leukomalacia on brain ultrasound in the first month of life
Brain magnetic resonance imaging (MRI) abnormalities at term equivalent age (yes/no), defined as white matter lesions (i.e. cavitations (Rutherford 2010); and punctate lesions (Cornette 2002)); GM‐IVH (Parodi 2015); or cerebellar haemorrhage (Fumagalli 2009; Limperopoulos 2007)
Impairment in cerebral haemodynamics during the first 3 days of life, assessed on cerebral near‐infrared spectroscopy (NIRS)
Retinopathy of prematurity: any and severe (≥ stage 3; ICROP 1984)
Long‐term neurodevelopmental outcomes (yes/no): cerebral palsy on physician assessment, developmental delay (i.e. IQ 2 standard deviations (SDs) below the mean on validated assessment tools such as Bayley Mental Developmental Index) (Bayley 1993; Bayley 2006)
Major neurodevelopmental disability: cerebral palsy, developmental delay (Bayley Mental Developmental Index (Bayley 1993; Bayley 2006) or Griffiths Mental Development Scale assessment (Griffiths 1954) > 2 SDs below the mean); intellectual impairment (IQ > 2 SDs below the mean); blindness (vision < 6/60 in both eyes); or sensorineural deafness requiring amplification (Jacobs 2013). We planned to evaluate each of these components as a separate outcome and to extract data on each long‐term outcome from studies that evaluated children after 18 months' chronological age. We planned to separately assess data on children 18 to 24 months of age and on those 3 to 5 years of age
Search methods for identification of studies
We used the standard search strategy of Cochrane Neonatal (neonatal.cochrane.org)
Electronic searches
We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 9) in the Cochrane Library; MEDLINE via PubMed (1966 to 12 September 2019); Embase (1980 to 12 September 2019); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 12 September 2019). See Appendix 1 for full search strategies for each database. We did not apply language restrictions.
Searching other resources
We searched clinical trials registries for ongoing and recently completed trials (ClinicalTrials.gov; the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en); the ISRCTN Registry).
Data collection and analysis
We used standard methods of Cochrane Neonatal, as described below.
Selection of studies
Two review authors (OR, MB) independently searched for and identified eligible trials that met the inclusion criteria of this review. We screened titles and abstracts to identify potentially relevant citations, and retrieved the full texts of all potentially relevant articles; and we independently assessed the eligibility of studies by filling out eligibility forms designed in accordance with the specified inclusion criteria. We reviewed studies for relevance by assessing study design, types of participants, interventions provided, and outcome measures reported. We resolved disagreements by discussion and, if necessary, by consultation with a third review author (MGC). We provide in the Characteristics of excluded studies table details of studies we excluded from the review, along with reasons for exclusion. We contacted trial authors if details of primary trials were not clear.
Data extraction and management
Two review authors (OR, MB) independently extracted data using a data extraction form developed ad hoc and integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group data collection checklist (Cochrane EPOC Group 2015).
We extracted the following characteristics from each included study.
Administrative details: study author(s); published or unpublished; year of publication; year in which study was conducted; details of other relevant papers cited.
Details of the study: study design; type, duration, and completeness of follow‐up (e.g. > 80%); country and location of study; informed consent; ethics approval.
Details of participants: sex; birth weight; gestational age; number of participants.
Details of interventions: initiation and duration of head midline position; co‐intervention with horizontal versus head elevated position; use of physical aids to maintain head position.
Details of outcomes as mentioned above under Types of outcome measures.
We resolved disagreements by discussion. We planned to describe ongoing studies identified by our search, when available, detailing the primary author, research question(s), methods, and outcome measures, together with an estimate of the reporting date.
When any queries arose, or when we required additional data, we contacted trial investigators/authors for clarification.
Two review authors (MGC, MB) used the Cochrane statistical tool RevMan Web for data entry.
Assessment of risk of bias in included studies
Two review authors (OR, MGC) independently assessed risk of bias in all included studies using the Cochrane 'Risk of bias' tool (Higgins 2019).
We assessed the following items.
Random sequence generation: selection bias due to inadequate generation of a randomised sequence.
Allocation concealment: selection bias due to inadequate concealment of allocations before assignment.
Blinding of participants and personnel: performance bias due to knowledge of allocated interventions by participants and personnel during the study.
Blinding of outcome assessment: detection bias due to knowledge of allocated interventions by outcome assessors.
Incomplete outcome data: attrition bias due to quantity, nature, or handling of incomplete outcome data.
Selective reporting: reporting bias due to selective outcome reporting.
Other bias: bias due to problems not covered elsewhere in the table.
We used a 'Risk of bias' graph to illustrate risk across studies. We planned to resolve disagreements by consensus and, if necessary, by consultation with a third review author (MB). See Appendix 2 for a more detailed description of risk of bias for each domain.
Random sequence generation and allocation concealment (selection bias)
Random sequence generation
For each included study, we categorised as follows the risk of bias regarding random sequence generation.
Low risk: investigators describe a random component in the sequence generation process such as referring to a random number table, using a computer random number generator, tossing a coin, shuffling cards or envelopes, throwing dice, drawing lots, and minimisation.
High risk: investigators describe a nonrandom component in the sequence generation process (sequence generated by odd or even date of birth, sequence generated by some rule based on date or day of admission, sequence generated by some rule based on hospital or clinic record number, allocation by judgment of the clinician, allocation by preference of the participant, allocation based on results of a laboratory test or a series of tests, allocation by availability of the intervention).
Unclear risk: no or unclear information is provided.
Allocation concealment
For each included study, we categorised as follows the risk of bias regarding allocation concealment.
Low risk: participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomisations), sequentially numbered drug containers or those of identical appearance, sequentially numbered sealed opaque envelopes.
High risk: participants and investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on open random allocation schedule (e.g. a list of random numbers), unsealed or non‐opaque envelopes, alternation or rotation, date of birth, case record number.
Unclear risk: no or unclear information is provided.
Blinding of study participants and personnel (performance bias)
Care providers cannot be blinded to the intervention.
Blinding of outcome assessors (detection bias)
For each included study, we categorised as follows the methods used to blind outcome assessors from knowledge of which intervention a participant received.
Criteria for a judgment of 'low risk' of bias: no blinding or incomplete blinding is described, but review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel is ensured, and it is unlikely that blinding could have been broken.
Criteria for a judgment of 'high risk' of bias: no blinding of outcome assessment is described, but review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment is described, but it is likely that blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Unclear risk: no or unclear information is provided.
Incomplete outcome data (attrition bias)
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis as follows.
-
Criteria for a judgment of 'low risk' of bias include:
no missing outcome data;
reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to introduce bias);
missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups;
for dichotomous outcome data, proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate;
for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; and
missing data imputed by appropriate methods.
-
Criteria for a judgment of 'high risk' of bias include:
reasons for missing outcome data likely to be related to true outcome, with imbalance in numbers or reasons for missing data across intervention groups;
for dichotomous outcome data, proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in the intervention effect estimate;
for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size;
'as treated' analysis done with substantial departure of the intervention received from that assigned at randomisations; and
potentially inappropriate application of simple imputation.
Unclear risk: no or unclear information is provided.
Selective reporting (reporting bias)
For each included study, we described how we investigated the risk of selective outcome reporting bias and what we found. We attempted to access all protocols of included studies through clinical trials registries (e.g. ClinicalTrials.gov; the International Standard Randomised Controlled Trial Number (ISRCTN) registry (www.controlled-trials.com)) and by direct contact with study authors.
We assessed study methods as follows.
Low risk: the study protocol is available, and all of the study's prespecified (primary and secondary) outcomes of interest in the review have been reported in the prespecified way; the study protocol is not available, but it is clear that published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk: not all of the study’s prespecified primary outcomes have been reported; one or more primary outcomes were reported using measurements, analysis methods, or subsets of data (e.g. subscales) that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review were reported incompletely, so that they cannot be entered into a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear risk: no or unclear information is provided (the study protocol is not available).
Other potential sources of bias (other bias)
For each included study, we described any important concerns that we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design used).
We assessed whether each study was free of other problems that could put it at risk of bias as follows.
Low risk: the study appears to be free of other sources of bias.
High risk: the study has at least one important risk of bias (e.g. the study had a potential source of bias related to the specific study design used, was claimed to have been fraudulent, had some other problem).
Unclear risk: risk of bias may be present, but information is insufficient to assess whether an important risk of bias exists, or the rationale or evidence that an identified problem will introduce bias is insufficient.
Measures of treatment effect
We followed standard methods of Cochrane Neonatal for data synthesis. We extracted categorical data for each intervention group and calculated risk ratios (RRs) and absolute risk differences (RDs). We planned to obtain means and standard deviations for continuous data and to perform analyses using mean differences (MDs). For each measure of effect, we calculated corresponding 95% confidence intervals (CIs). We planned to present the number needed to treat for an additional beneficial outcome and the number needed to treat for an additional harmful outcome (NNTB and NNTH respectively) when we found RDs to be statistically significant (P < 0.05).
Unit of analysis issues
The unit of randomisations was the intended unit of analysis (individual neonate). If we found any cluster‐randomised controlled trials, we planned to adjust analysis for the designed effect using methods stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Dealing with missing data
We planned to obtain a dropout rate for each study. We planned to consider a dropout rate of more than 20% as significant. If we found a significant dropout rate, we planned to contact study author(s) to request additional data. We planned to perform a sensitivity analysis to evaluate overall results with and without inclusion of studies with a significant dropout rate. If a study reported outcomes only for participants completing the trial or only for participants who followed the protocol, we planned to contact study author(s) to ask them to provide additional information to facilitate an intention‐to‐treat analysis; in instances when this was not possible, we planned to perform a complete case analysis.
Assessment of heterogeneity
We planned to assess clinical heterogeneity by comparing the distribution of important participant factors between trials and trial factors (randomisations concealment, blinding of outcome assessment, loss to follow‐up, treatment type, co‐interventions). We planned to assess statistical heterogeneity by examining the I² statistic, a quantity that describes the proportion of variation in point estimates that is due to variability across studies rather than to sampling error (Higgins 2019).
We planned to interpret the I² statistic as follows, as described by Higgins 2003.
< 25%: no (none) heterogeneity.
25% to 49%: low heterogeneity.
50% to 74%: moderate heterogeneity.
≥ 75%: high heterogeneity.
We planned to consider statistical heterogeneity substantial when I² was 50% or more. In addition, we planned to employ the Chi² test of homogeneity to determine the strength of evidence that heterogeneity is genuine. We planned to explore clinical variation across studies by comparing the distribution of important participant factors among trials and trial factors (randomisations concealment, blinding of outcome assessment, loss to follow‐up, treatment types, and co‐interventions). We planned to consider a threshold of P < 0.1 as an indicator of whether heterogeneity (genuine variation in effect sizes) was present.
Assessment of reporting biases
We planned to investigate publication bias by using funnel plots if we included 10 or more clinical trials in the systematic review (Egger 1997; Higgins 2019).
Data synthesis
We summarised all eligible studies in RevMan Web. We used standard methods of Cochrane Neonatal to synthesise data using typical RR, RD, NNTB, NNTH, MD, and 95% CIs if we included more than one trial in the meta‐analysis. We performed a meta‐analysis of data from included trials by using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to present data from the following subgroups.
Gestational age (with two subgroups, < 26 weeks vs ≥ 26 weeks).
Birth weight (with two subgroups, < 1000 grams vs ≥ 1000 grams).
Intubated versus not intubated.
With or without GM‐IVH (any grade) at trial entry.
Sensitivity analysis
We planned to conduct sensitivity analyses to explore effects of the methodological quality of trials, checking to ascertain whether studies with high risk of bias will overestimate the effects of treatment.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the following (clinically relevant) outcomes: any intraventricular haemorrhage; severe intraventricular haemorrhage; death during initial hospitalisation; cerebellar haemorrhage on brain ultrasound; retinopathy of prematurity; long‐term neurodevelopmental outcome; and major neurodevelopmental disability.
Two review authors independently assessed the certainty of the evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (and two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT (Guideline Development Tool) to create a 'Summary of findings' table to report the certainty of the evidence.
The GRADE approach yields an assessment of the certainty of a body of evidence according to one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect but may be substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
We have provided results of the search for this review update in the study flow diagram (Figure 1).
1.
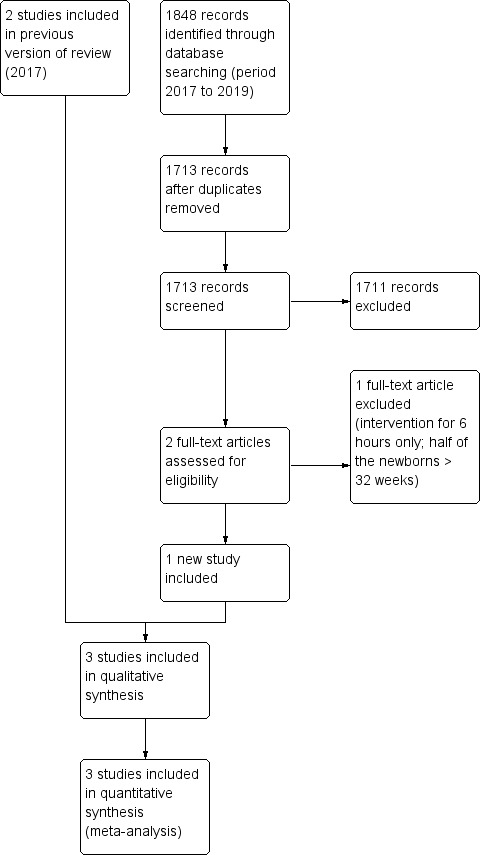
Study flow diagram.
See Table 1, Characteristics of included studies, Characteristics of excluded studies, Characteristics of ongoing studies and Characteristics of studies awaiting classification.
Results of the search
The literature searches run in September 2016 and September 2019 identified 2696 and 1713 references, respectively. After screening, we assessed seven full‐text articles for eligibility and included three trials (Al‐Abdi 2011; Al‐Abdi 2015; Kochan 2019). We excluded three trials (Antunes 2003; Imam 2019; Wu 2015); and listed one under the Studies awaiting classification heading because approximately half of the included infants had a gestational age greater than the inclusion criterion of this review and study authors reported no outcomes specified in this review (Jalali 2012).
We found one relevant study by searching clinical trial registries (NCT035430461).
Included studies
Three randomised controlled trials (RCTs) recruiting 290 very preterm infants met our inclusion criteria (Al‐Abdi 2011; Al‐Abdi 2015; Kochan 2019). We have listed the details of these trials in the Characteristics of included studies section. All trials compared supine midline head position versus supine head rotated 90°. Bed tilting was used only in the intervention group of the most recent trial (Kochan 2019). We found no trials that compared supine versus prone midline head position, and no trials that compared effects of head tilting.
Al‐Abdi 2011 was a randomised parallel clinical trial conducted at King Abdulaziz Hospital, Al‐Ahsa, Saudi Arabia, including 48 preterm infants in the period 2008 to 2009. Preterm infants were enrolled in the trial if they met the following criteria: inborn; gestational age less than 30 weeks; and postnatal age less than 2 hours. Exclusion criteria included presence of lethal congenital anomalies, hypoxic‐ischaemic encephalopathy, and need for full cardiopulmonary resuscitation at birth. Infants lying on beds at 0° were randomly assigned to be cared for in a supine midline or supine lateral head position. In the supine midline position, the infant’s chin was kept at a 90° angle to the bed. In the lateral head position, the head was tilted 90° to either side. At enrolment, it was left to the bedside nurse to place the head in the right‐tilted or left‐titled position. After that, infants’ heads were kept in their primary assigned positions throughout the first week of life. Correctness of infants’ head positions was checked every six hours by the bedside nurse. Randomisation was stratified on the basis of gender and gestational age (< 27 or 27 to 29+6 weeks). The primary outcome was rate of intraventricular haemorrhage of all grades. Two radiologists who were blinded to head position assignments independently reported laterality and grade of IVH according to Papile’s criteria (Papile 1978). If discrepancy between the two radiologists was apparent, the report of a third radiologist was used. Timing of head ultrasound examinations was: (1) at 5 to 7 days of life for stable preterm infants 32 weeks' or less gestational age (GA); (2) subsequent head ultrasound follow‐up examinations at 14 and 28 days of life, or before discharge; (3) head ultrasound examination as soon as clinical suspicion of IVH was raised; (4) if IVH was detected, a second head ultrasound examination repeated 5 to 7 days later; and (5) before commencement of indomethacin for treatment of patent ductus arteriosus. Investigators noted no statistically significant differences in the baseline characteristics of studied infants. Twenty‐three infants were cared for in the midline head position and 25 in the lateral head position. One infant in each group had no head ultrasound owing to early death, and both infants were included in the analysis. Among infants in the head midline group, 12 were cared for in a left‐tilted and 13 in a right‐tilted midline head position. During the first week of life, the incidence of IVH in the midline head position was 26% (6/23) versus 20% (5/25) in the lateral head position (risk ratio (RR) 1.30, 95% confidence interval (CI) 0.46 to 3.70; P = 0.62). Among infants who developed IVH, four in the midline and three in the lateral head position had normal first head ultrasound carried out on the second day of life before indomethacin for patent ductus arteriosus treatment. Grade 3 to 4 IVH developed in two (9%) infants in the midline head position versus one (4%) infant in the lateral head position (RR 1.4, 95% CI 0.61 to 3.37; P = 0.94). Bilateral IVH developed in three (13%) in the midline head position versus two (8%) in the lateral head position (RR 1.6, 95% CI 0.30 to 8.90; P = 0.92). Secondary analysis showed that the incidence of IVH in left‐tilted lateral head position was 25% (3/12) versus 15% (2/13) in right‐tilted lateral head position (RR 1.63, 95% CI 0.33 to 8.11; P = 0.92).
In addition, we obtained data for the following outcomes directly from trial authors. Death: one infant in the midline group and five in the lateral group (all deaths occurred within the first 2 weeks of life); retinopathy of prematurity, any stage: 10 infants in the midline group and four in the lateral head position group; retinopathy of prematurity, severe (≥ stage 3): three infants in the midline group and one in the lateral head position group; cystic periventricular leukomalacia: one infant in the midline group and none in the lateral head position group. No data on long‐term follow‐up were available.
Al‐Abdi 2015 was a multicentre (Saudi Arabia) randomised parallel clinical trial that included 62 preterm neonates (< 30 weeks' gestation) within two hours of life and without IVH at 12 hours of life. Exclusion criteria included the following: outborn; lethal congenital anomalies; hypoxic‐ischaemic encephalopathy; and external cardiac compression or epinephrine administration at birth. Head ultrasound scan was performed within 12 hours of life, at seven days of life, and at the physician's discretion. Head ultrasound scan was reported by three radiologists who were blinded to assignment of head position. Infants lying on 0° beds were randomly assigned to be cared for in a supine midline position (n = 31) or supine lateral head position (n = 31) throughout the first week of life. Severity score of IVH was calculated according to an IVH severity score defined by one of the study authors (Al Abdi's score). The planned sample size was 600 (alpha 5%; power 80%). However, the study was prematurely terminated when only 71 neonates (12%) had been recruited owing to low accrual rate. The trial was registered on ClinicalTrials.gov, and findings were presented at the Pediatric Academic Societies (PAS) Meeting in 2015. Risk of IVH was exactly the same in both groups (6/31 (19.4%); RR 1.0, 95% CI 0.36 to 2.76; P > 0.99). Risk ratio of left‐sided IVH in flat midline versus flat right lateral groups was 2.0 (95% CI 0.94 to 10.13; P = 0.39). Risk ratio of right‐sided IVH groups was 1.0 (95% CI 0.32 to 3.11; P = 0.39). Risk ratio of bilateral IVH in flat midline versus flat right lateral was 3.0 (95% CI 0.33 to 27.29; P = 0.31). Median of IVH severity score was 3.0 in flat midline versus 1.0 in flat right lateral (P = 0.21). We obtained data for the following outcomes directly from trial authors. Neonatal death: three newborns (1 due to sepsis) in each group; any GM‐IVH: six newborns in each group; and severe IVH: one newborn in each group.
Kochan 2019 was a randomised parallel clinical trial, conducted at the Children’s Hospital of The King’s Daughters in Norfolk, Virginia (USA) and including 180 extremely low birth weight (< 1000 g) infants admitted to the NICU in 2012 to 2015. Only infants that could be randomised, placed into the midline or lateral position, and undergo an admission cranial ultrasound within four hours of birth were included in the trial. Infants with congenital anomalies were excluded. By a mean age of three hours of life infants were randomised, for the first four days of life, to either supine midline with 30° bed tilting with a custom wedge‐shaped frame (n = 90); or supine, head rotated 90° right or left every four hours with flat bed (n = 90). After the fourth day all infants were placed in the flat position. Cranial ultrasounds were performed immediately after randomisations, daily for the first four days of life and on day 7. Normal scans were repeated at four weeks of age, abnormal scans were repeated weekly. The sample size (n = 180; alpha 5%; power 80%) was calculated on an incidence of GM‐IVH any grade of 40% and anticipated 20% difference in its occurrence. Protocol variations were documented in 26 infants randomised to the group with supine midline with bed tilting. Nineteen infants had short periods of flat positioning during procedures. Data from all infants were maintained in their original randomisations group. Mortality before hospital discharge was lower in the supine midline with bed tilting (12%) than in the head rotated 90° with flat bed (24%) (P = 0.033). There were no significant differences in the incidence of GM‐IVH, sepsis, urinary tract infection, necrotizing enterocolitis, intestinal perforation, retinopathy of prematurity and length of hospital stay between the two groups.
We identified one ongoing trial (NCT035430461). Inborn infants with gestational age of less than 31 weeks and with less than 3 hours of life from birth are to be randomised to either midline head position, supine or slightly side‐lying, with a bed elevation between 15° and 30° or midline head position with the aid of nesting rolls (or both) with a bed elevation between 15° and 30° (see Characteristics of ongoing studies).
Excluded studies
We excluded three trials because of characteristics of their interventions and populations (Antunes 2003; Imam 2019; Wu 2015). Antunes 2003 adopted the two different head positions at 48 hours of life, whereas in this review we included trials in which the intervention was started within the first 48 hours of life (as specified in Types of interventions). We excluded Wu 2015 because mean birth weight was more than 2.5 kg, suggesting that included newborns were not of 32 weeks' or less gestational age, as specified in this review (Types of participants). We excluded Imam 2019 because the intervention was provided for six hours only; moreover, half of the newborns were more than 32 weeks' gestational age.
Risk of bias in included studies
We present information on risk of bias in the included trials in the 'Risk of bias graph' (Figure 2) and in the 'Risk of bias summary' (Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Al‐Abdi 2011 reported adequate sequence generation and randomisations in blocks to ensure balanced combinations of positions and finally an identification number in a sealed envelope. Al‐Abdi 2015 did not provide sufficient information on how the randomisations sequence was generated and concealed, and we judged this study to be at unclear risk of selection bias. In Kochan 2019 infants were randomised using a block randomisations table; allocation concealment is unclear.
Blinding
Owing to the nature of the intervention, blinding was not possible in the included studies; blinding was, however, provided for outcome assessors.
Incomplete outcome data
The three studies reported outcomes for all randomised infants.
Selective reporting
The Al‐Abdi 2015 study protocol was registered and there did not appear to be any differences between the published protocol and the full report.
The study protocols were not available to us for the studies by Al‐Abdi 2011 and Kochan 2019, so we cannot assess if there were any deviations from the study as planned and the final report.
Other potential sources of bias
Al‐Abdi 2011 did not carry out calculation of sample size (pilot study). Al‐Abdi 2015 performed the calculation but ended the study prematurely due to a low accrual rate (71 infants were enrolled instead of 600, corresponding to 12%).
In Kochan 2019 the incidence of pre‐eclampsia was higher in the midline group (36/90, 40%) than in the control (22/90, 24%) (P = 0.026); whereas the incidence of prolonged rupture of membranes was lower in the midline group (9/90, 10%) than in the control (21/90, 23%) (P = 0.018). However multivariate analysis of maternal pre‐eclampsia and prolonged rupture of membranes showed no significant effect on outcomes. Protocol variations were reported in 26 infants in the midline group (data were reported according to intention‐to‐treat analysis).
Effects of interventions
See: Table 1
We planned to conduct multiple comparisons as described in Types of interventions. However, all three included trials could be grouped in the first type of prespecified comparison: supine midline head position versus any other supine head position. More precisely, two trials compared supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0° (Al‐Abdi 2011; Al‐Abdi 2015); and in one trial bed tilting was provided in the head midline position group (Kochan 2019). We present meta‐analyses of the included trials both as totals (all three trials); and sub‐totals (the two trials with no bed tilting and the one with bed tilting separately).
Supine midline head position versus any other supine head position (comparison 1)
Three trials (Al‐Abdi 2011; Al‐Abdi 2015; Kochan 2019), with a total of 290 infants, met the eligibility criteria (see Table 1).
Primary outcomes
GH‐IVH: any severity (grade 1 to 4) (Outcome 1.1)
See Analysis 1.1 and Figure 4.
1.1. Analysis.

Comparison 1: Supine midline head position versus any other supine head position, Outcome 1: Intraventricular haemorrhage, any grade
4.

Forest plot of comparison: 1 Supine midline head position versus any other supine head position, outcome: 1.1 Intraventricular haemorrhage, any grade.
All trials included in the primary comparison reported this outcome (typical RR 1.11, 95% CI 0.78 to 1.56; typical RD 0.03, 95% CI −0.07 to 0.14; I² = 0% for both RR and RD; 3 studies, 290 infants) (Al‐Abdi 2011; Al‐Abdi 2015; Kochan 2019). The certainty of the evidence (GRADE) for this outcome was very low due to limitations in study design and imprecision (see Table 1).
Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0°
Two trials reported this outcome (Al‐Abdi 2011; Al‐Abdi 2015). Supine midline head position did not alter the risk of developing any GM‐IVH (typical RR 1.14, 95% CI 0.55 to 2.35; typical RD 0.03, 95% CI −0.13 to 0.18; I² = 0% for both RR and RD; 2 studies, 110 infants).
For Al‐Abdi 2015, we obtained data for this outcome directly from trial authors.
Supine midline head position with the bed tilted ≥ 30° versus supine head rotated 90° with the bed at 0°
Only Kochan 2019 reported this outcome (RR 1.10, 95% CI 0.74 to 1.62; RD 0.03, 95% CI −0.11 to 0.17; 1 study, 180 participants). The test for heterogeneity was not applicable.
Severe IVH: (grades 3 and 4) (Outcome 1.2)
See Analysis 1.2 and Figure 5.
1.2. Analysis.

Comparison 1: Supine midline head position versus any other supine head position, Outcome 2: Intraventricular haemorrhage, grade 3 to 4
5.

Forest plot of comparison: 1 Supine midline head position versus any other supine head position, outcome: 1.2 Intraventricular haemorrhage, grade 3 to 4.
All trials included in the primary comparison reported this outcome (typical RR 0.71, 95% CI 0.37 to 1.33; typical RD −0.04, 95% CI −0.11 to 0.03; I² = 0% for both RR and RD; 3 studies, 290 infants) (Al‐Abdi 2011; Al‐Abdi 2015; Kochan 2019). The certainty of the evidence (GRADE) for this outcome was very low due to limitations in study design and imprecision (see Table 1).
Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0°
Two trials reported this outcome (Al‐Abdi 2011; Al‐Abdi 2015). Supine midline head position did not alter the risk of developing any severe IVH (typical RR 1.57, 95% CI 0.28 to 8.98; typical RD 0.02, 95% CI −0.06 to 0.10; I² = 0% for both RR and RD; 2 studies, 110 infants).
For Al‐Abdi 2015, we obtained data for this outcome directly from trial authors.
Supine midline head position with the bed tilted ≥ 30° versus supine head rotated 90° with the bed at 0°
Only Kochan 2019 reported this outcome (RR 0.61, 95% CI 0.31 to 1.22; RD −0.08, 95% CI −0.18 to 0.03; 1 study, 180 participants). The test for heterogeneity was not applicable.
Neonatal mortality (within 28 days) (Outcome 1.3)
See Analysis 1.3 and Figure 6.
1.3. Analysis.

Comparison 1: Supine midline head position versus any other supine head position, Outcome 3: Neonatal mortality (within 28 days of life)
6.

Forest plot of comparison: 1 Supine midline head position versus any other supine head position, outcome: 1.3 Neonatal mortality (within 28 days of life).
All trials included in the primary comparison reported this outcome (typical RR 0.49, 95% CI 0.25 to 0.93; typical RD −0.09, 95% CI −0.16 to −0.01; I² = 0% for both RR and RD; 3 studies, 290 infants) (Al‐Abdi 2011; Al‐Abdi 2015; Kochan 2019).
Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0°
Two trials reported this outcome (typical RR 0.52, 95% CI 0.16 to 1.65; I² = 28%; typical RD −0.07, 95% CI −0.18 to 0.05; I² = 44%; 2 studies, 110 infants) (Al‐Abdi 2011; Al‐Abdi 2015). For both trials, we obtained data for this outcome directly from trial authors.
Supine midline head position with the bed tilted ≥ 30° versus supine head rotated 90° with the bed at 0°
Only Kochan 2019 reported this outcome (RR 0.47, 95% CI 0.21 to 1.03; RD −0.10, 95% CI −0.20 to 0.00; 1 study, 180 participants). The test for heterogeneity was not applicable. We obtained data for this outcome directly from trial authors.
Mortality during initial hospitalisation (Outcome 1.4)
See Analysis 1.4 and Figure 7.
1.4. Analysis.

Comparison 1: Supine midline head position versus any other supine head position, Outcome 4: Mortality during initial hospitalization
7.

Forest plot of comparison: 1 Supine midline head position versus any other supine head position, outcome: 1.4 Mortality during initial hospitalisation.
All trials included in the primary comparison reported this outcome (typical RR 0.50, 95% CI 0.28 to 0.90; I² = 0%; typical RD −0.10, 95% CI −0.18 to −0.02; I² = 14%; 3 studies, 290 participants) (Al‐Abdi 2011; Al‐Abdi 2015; Kochan 2019). The certainty of the evidence (GRADE) for this outcome was very low due to limitations in study design and imprecision (see Table 1).
Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0°
Two trials reported this outcome (Al‐Abdi 2011; Al‐Abdi 2015). Supine midline head position did not alter the risk of neonatal mortality (typical RR 0.52, 95% CI 0.16 to 1.65; I² = 28%; typical RD −0.07, 95% CI −0.18 to 0.05; I² = 44%; 2 studies, 110 infants). For both trials, we obtained data for this outcome directly from trial authors.
Supine midline head position with the bed tilted ≥ 30° versus supine head rotated 90° with the bed at 0°
Only Kochan 2019 reported this outcome (RR 0.50, 95% CI 0.26 to 0.97; RD −0.12, 95% CI −0.23 to −0.01; 1 study, 180 infants). The test for heterogeneity was not applicable.
Secondary outcomes
Cystic periventricular leukomalacia (Outcome 1.5)
See Analysis 1.5.
1.5. Analysis.

Comparison 1: Supine midline head position versus any other supine head position, Outcome 5: Cystic periventricular leukomalacia
Al‐Abdi 2011 and Kochan 2019 reported this outcome (typical RR 3.25, 95% CI 0.14 to 76.01; typical RD 0.01, 95% CI −0.02 to 0.04; 2 studies, 228 infants).
Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0°
One trial reported that one infant in the supine midline group (1/23) versus no infants in the control group (0/25) received a diagnosis of cystic periventricular leukomalacia; this difference was not significant (RR 3.25, 95% CI 0.14 to 76.01; RD 0.04, 95% CI −0.07 to 0.15) (Al‐Abdi 2011). The test for heterogeneity was not applicable.
We obtained data for this outcome directly from trial authors.
Supine midline head position with the bed tilted ≥ 30° versus supine head rotated 90° with the bed at 0°
Kochan 2019 (180 infants) reported no event.
Retinopathy of prematurity, any stage (Outcome 1.6)
See Analysis 1.6.
1.6. Analysis.

Comparison 1: Supine midline head position versus any other supine head position, Outcome 6: Retinopathy of prematurity, any stage
Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0°
One trial reported a diagnosis of retinopathy of prematurity in 10 infants in the supine midline group (10/22) versus four infants in the control group (4/20); this difference was not significant (RR 2.27, 95% CI 0.85 to 6.11; RD 0.25, 95% CI −0.02 to 0.53) (Al‐Abdi 2011). The test for heterogeneity was not applicable.
We obtained data for this outcome directly from trial authors.
Retinopathy of prematurity, ≥ stage 3 (Outcome 1.7)
See Analysis 1.7.
1.7. Analysis.

Comparison 1: Supine midline head position versus any other supine head position, Outcome 7: Retinopathy of prematurity, ≥ stage 3
Al-Abdi 2011 and Kochan 2019 reported this outcome (typical RR 0.90, 95% CI 0.41 to 1.97; I² = 19%; typical RD −0.01, 95% CI −0.09 to 0.07; I² = 32%; 2 studies, 228 infants). The certainty of the evidence (GRADE) for this outcome was very low due to limitations in study design and imprecision (see Table 1).
Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0°
One trial reported that retinopathy of prematurity of stage 3 or more was diagnosed in three infants in the supine midline group (3/22) versus one infant in the control group (1/20); this difference was not significant (RR 2.73, 95% CI 0.31 to 24.14; RD 0.09, 95% CI −0.09 to 0.26) (Al‐Abdi 2011). The test for heterogeneity was not applicable.
We obtained data for this outcome directly from trial authors.
Supine midline head position with the bed tilted ≥ 30° versus supine head rotated 90° with the bed at 0°
One trial reported this outcome (RR 0.73, 95% CI 0.31 to 1.72; RD −0.03, 95% CI −0.12 to 0.06; 1 study, 180 participants) (Kochan 2019). The test for heterogeneity was not applicable.
Additional outcomes
We found no data on the following: cerebellar haemorrhage; brain MRI abnormalities; impairment in cerebral haemodynamics; long‐term neurodevelopmental outcomes; and major neurodevelopmental disability.
Supine midline head position versus any other prone head position (comparison 2)
We found no trials comparing supine midline head position versus any other prone head position.
Supine midline head position with the bed at 0° versus supine midline head position with the bed tilted ≥ 30° (comparison 3)
We found no trials comparing supine midline head position with the bed at 0° versus supine midline head position with the bed tilted ≥ 30°.
Subgroup analysis
We were unable to conduct any of the planned subgroup analyses, as we included only two small trials.
Discussion
Summary of main results
We evaluated the benefits and harms of midline position for prevention of germinal matrix‐intraventricular haemorrhage (GM‐IVH) in very preterm infants. Three trials, with a total of 290 preterm infants with gestational age less than 30 weeks, met the inclusion criteria of our review (Al‐Abdi 2011; Al‐Abdi 2015; Kochan 2019). The two trials by Al‐Abdi and colleagues compared supine midline head position versus supine head rotated 90°; Al‐Abdi 2015 was prematurely terminated owing to low accrual rate when only 71 neonates (12%) had been recruited. Kochan 2019 compared supine midline head position with the bed tilted 30° or more versus supine head rotated 90° with the bed at 0°.
Supine midline head position was not better than supine head rotated for reducing GM‐IVH any grade or severe IVH. Neonatal mortality and mortality until hospital discharge were lower in the supine midline head position: this was mainly due to Kochan 2019. However, given the imprecision of our estimates (i.e. wide confidence intervals, small sample size), results of this systematic review were consistent either with a beneficial or detrimental effect of head midline positioning. We rated the certainty of evidence as very low (GRADE) for all outcomes because of limitations in study design of most included trials and imprecision of estimates (few and small studies). Optimal information size was not achieved for any of the outcomes of this review. Furthermore, we found no benefit of midline over lateral head position for secondary outcomes, such as retinopathy of prematurity or cystic periventricular leukomalacia. None of the studies reported the long‐term neurodevelopmental outcome.
We identified one trial to list under Studies awaiting classification because most infants had a gestational age greater than that specified in the inclusion criteria of this review; moreover, study authors reported none of the outcomes specified in this review (Jalali 2012). We found one ongoing study on clinical trials registries with estimated study completion date for May 2021 (NCT035430461).
Overall completeness and applicability of evidence
Three randomised trials (290 newborns) assessed this study question, and one of them was stopped early after recruiting only 12% of the planned sample size (Al‐Abdi 2015). Two trials compared supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0° (Al‐Abdi 2011; Al‐Abdi 2015); and one trial compared supine midline head position with the bed tilted 30° or more versus supine head rotated 90° with the bed at 0° in infants with gestational age (GA) less than 30 weeks (Kochan 2019). Available evidence is insufficient to show whether head midline position is an effective and safe intervention for preventing GM‐IVH and reduce neonatal mortality in very preterm neonates. Meta‐analysis of mortality showed 50% reduction in the group with supine midline head position. This was due mainly to the data from the largest study, Kochan 2019. We cannot ascertain whether the reduced mortality (in Kochan 2019 only) was a consequence of the bed tilting (in Al‐Abdi 2011 and Al‐Abdi 2015 the bed was placed at 0° in both arms), of the greater vulnerability due to the lower gestational age (mean gestational age 26 weeks in Kochan 2019) or of other factors.
We found no trials that compared supine versus prone midline head position; thus we cannot draw any conclusions regarding their comparative effectiveness. Only one of the three included trials enrolled a considerable number of infants with GA less than 26 weeks — the group at highest risk for GM‐IVH (Kochan 2019). Available data were insufficient for assessment of the primary outcomes of this review as well as other important outcomes such as retinopathy of prematurity and long‐term neurodevelopmental outcomes.
Quality of the evidence
According to the GRADE approach, we rated the overall certainty of evidence for clinically relevant outcomes as very low (see Table 1). We downgraded the overall certainty of evidence for critical outcomes because of imprecision of results (small number of participants; few events) that could be a source of random error risk, and premature stopping of participant enrolment. Random error is closely related to imprecision, as results of smaller studies are subject to greater sampling variation and hence are less precise (Higgins 2019). Given the nature of the intervention, it was not possible to blind personnel. The certainty of the included trials, although they included small samples, was moderate in general and they showed low risk of bias (Figure 2 and Figure 3).
Potential biases in the review process
We used standard methods of Cochrane Neonatal in conducting this systematic review. Our inclusive search strategy theoretically would have included all relevant studies. For example, availability of the full text was not imperative as in the systematic review on the same topic by de Bijl‐Marcus and colleagues, which included one randomised controlled trial only (de Bijl‐Marcus 2016). We minimised potential biases, although selection of criteria applied in considering studies for inclusion in this review (namely, Types of participants and Types of interventions) led to the exclusion of three trials (Antunes 2003; Imam 2019; Wu 2015). In Antunes 2003 interventions were started not earlier than 48 hours of life; in Wu 2015 newborns had gestational age of more than 32 weeks; in Imam 2019 the intervention was provided for six hours only (and most of the newborns had gestational age > 32 weeks).
We identified a trial to list under Studies awaiting classification because most infants had a gestational age greater than that specified in the inclusion criteria of this review (Jalali 2012).
Agreements and disagreements with other studies or reviews
At least two other reviews have addressed the clinical question of the use of head midline position to prevent GM‐IVH in very preterm neonates, with conflicting conclusions (Carteaux 2003; de Bijl‐Marcus 2016). Carteaux and colleagues recommended implementing a plan of care that includes midline head positioning for premature infants (Carteaux 2003). The rationale for their decision — which was made before any randomised controlled trial had been conducted — is unclear, and we disagree with their conclusions. In contrast, another systematic review (de Bijl‐Marcus 2016) was published after both Al‐Abdi 2011 and Al‐Abdi 2015 had been reported. However, Al‐Abdi 2015 was published as an abstract only and therefore was not included in de Bijl‐Marcus 2016, for which review authors specified that "availability of the full text was imperative." An additional, minor difference between our review and de Bijl‐Marcus 2016 consisted of assessment of reporting bias for Al‐Abdi 2011, which we scored as unclear because the study protocol was not available. Moreover, we succeeded in contacting Dr. Al‐Abdi to retrieve additional data on relevant outcomes for both trials (Al‐Abdi 2011; Al‐Abdi 2015). Overall, we agree with de Bijl‐Marcus 2016 that evidence is insufficient to allow conclusions on effects of head positioning or tilting (or both) on the incidence of GMH‐IVH in very preterm infants. It is noteworthy that regional cerebral saturations measured by near‐infrared spectroscopy in extremely preterm infants during the first three days of life remained within normal range when the head was turned from the midline position to either side (Liao 2015). Other studies have compared the supine versus prone position in terms of decreased cerebral haemoglobin oxygenation and cerebral blood volume in supine versus prone position (Pichler 2001); and decreased volume of down‐side ventricles versus volume of up‐side ventricles (Nagdyman 1999). Nevertheless, these findings may not justify the recommendation — adopted in several neonatal intensive care units worldwide and endorsed by Carteaux 2003 — to maintain the head in midline position for prevention of GM‐IVH. Head and body position may, however, have important effects on relevant outcomes such as pulmonary function. Two Cochrane Reviews have been published on the effects of body positioning on respiratory morbidity, that is in spontaneously breathing (Bredemeyer 2012) and ventilated infants (Rivas‐Fernandez 2016). In the review on body positioning for spontaneously breathing preterm infants with apnoea, review authors reported that evidence is insufficient to show effects of body positioning on apnoea, bradycardia, oxygen desaturation, and oxygen saturation (Bredemeyer 2012). However, the review on position of neonates receiving mechanical ventilation concluded that evidence of low to moderate certainty favours the prone position for slightly improved oxygenation in neonates undergoing mechanical ventilation (Rivas‐Fernandez 2016). However these two reviews had different objectives — they did not focus on GM‐IVH — hence different populations and interventions as compared with those of this review.
Authors' conclusions
Implications for practice.
We found limited data on the effects of head midline position on germinal matrix‐intraventricular haemorrhage in very preterm neonates. The largest of the three included trials reported reduced mortality in the supine midline head position with the bed titled 30° or more as compared to the group with supine head rotated 90° with the bed at 0°. We cannot ascertain whether the reduced mortality in this trial was a consequence of the bed tilting (in the other two included trials the bed was placed at 0° in both arms). None of the three studies showed an effect on germinal matrix‐intraventricular haemorrhage. Given the imprecision of our estimates, results of this systematic review are consistent with either a beneficial or detrimental effect of head midline position, and do not provide a definitive answer to the review question. Limited evidence is available on other clinically relevant outcomes. The choice between prone and supine position might as well be based on available evidence from studies focusing on the impact of position on respiratory morbidity (Bredemeyer 2012; Rivas‐Fernandez 2016).
Implications for research.
Multiple studies have investigated different positions of the head and body of the preterm newborn, mainly focusing on effects on respiratory morbidity. Future trials should report on short‐ and long‐term outcomes, including germinal matrix‐intraventricular haemorrhage, cerebellar haemorrhage, retinopathy of prematurity, and neurodevelopmental disability. Supine midline head position might be compared with supine head rotated 90°; prone midline head position; or use of head tilting (see Types of interventions).
What's new
| Date | Event | Description |
|---|---|---|
| 12 September 2019 | New search has been performed | We updated searches in 2019 and found one new eligible study for inclusion |
| 12 September 2019 | New citation required and conclusions have changed | We included one new study and made changes to the main conclusions |
History
Protocol first published: Issue 9, 2016 Review first published: Issue 7, 2017
Acknowledgements
We would like to thank the following members of Cochrane Neonatal who provided editorial and administrative support: Colleen Ovelman, Managing Editor; Jane Cracknell, Assistant Managing Editor; Roger Soll, Co‐coordinating editor; and Bill McGuire, Co‐coordinating Editor.
We thank Jason Elliot‐Smith (Oxford, UK) for his diligent proofreading of the review.
We thank Maria Björklund (Library and ICT services, Lund University) for defining and running the search strategy for the 2019 update.
Appendices
Appendix 1. Search strategy
CENTRAL
Head midline position OR midline OR head position OR head tilt* OR bed tilt* OR supine OR lateral OR prone OR horizontal position OR elevated position OR rotated OR rotation OR central position
AND
MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET OR infant or infants or infant’s or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET
PubMed (12 September 2019)
(((((((Head midline position OR midline OR head position OR head tilt* OR bed tilt* OR supine OR lateral OR prone OR horizontal position OR elevated position OR rotat* OR central position)))))
AND
((((((((((((((((((((infant, newborn[MeSH Terms]) OR newborn*[Title/Abstract]) OR new born[Title/Abstract]) OR new borns[Title/Abstract]) OR newly born[Title/Abstract]) OR baby*[Title/Abstract]) OR babies*[Title/Abstract]) OR premature[Title/Abstract]) OR prematurity[Title/Abstract]) OR preterm[Title/Abstract]) OR pre term[Title/Abstract]) OR "low birth weight"[Title/Abstract]) OR "low birthweight"[Title/Abstract]) OR VLBW[Title/Abstract]) OR LBW[Title/Abstract]) OR infan*[Title/Abstract]) OR neonat*[Title/Abstract])))
AND (((((((((randomised controlled trial[Publication Type]) OR controlled clinical trial[Publication Type]) OR randomised[Title/Abstract]) OR placebo[Title/Abstract]) OR drug therapy[MeSH Subheading]) OR randomly[Title/Abstract]) OR trial[Title/Abstract]) OR groups[Title/Abstract] NOT (animals[mh] NOT humans[mh]))))) AND ( "2016/01/01"[PDat]: "2019/12/31"[PDat] ))
Embase
'head midline position':ab,ti OR midline:ab,ti OR 'head position':ab,ti OR 'head tilt*':ab,ti OR 'bed tilt*':ab,ti OR supine:ab,ti OR lateral:ab,ti OR prone:ab,ti OR 'horizontal position':ab,ti OR 'elevated position':ab,ti OR rotat*:ab,ti OR 'central position':ab,ti
AND
'newborn*':ab,ti OR 'new born':ab,ti OR 'new borns':ab,ti OR 'newly born baby*':ab,ti OR 'babies':ab,ti OR 'premature':ab,ti OR 'prematurity':ab,ti OR 'preterm':ab,ti OR 'pre term':ab,ti OR 'low birth weight':ab,ti OR 'low birthweight':ab,ti OR 'vlbw':ab,ti OR 'lbw':ab,ti OR 'infant':ab,ti OR 'infants':ab,ti OR 'infantile':ab,ti OR 'infancy':ab,ti OR 'neonat*':ab,ti
AND
randomized AND controlled AND trial OR (controlled AND clinical AND trial) OR randomized OR placebo OR (clinical AND trials AND as AND topic) OR randomly OR (clinical AND trial)
NOT
(human NOT animal)
CINAHL
(Head midline position OR midline OR head position OR head tilt* OR bed tilt* OR supine OR lateral OR prone OR horizontal position OR elevated position OR rotat* OR central position)
AND
(infant or infants or infant’s or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW)
AND
(randomized controlled trial OR controlled clinical trial OR randomized OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
ClinicalTrials.gov
Search Head position
Filter Child (Birth‐17)
Appendix 2. Risk of bias
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk.
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk; or
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Supine midline head position versus any other supine head position.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Intraventricular haemorrhage, any grade | 3 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.78, 1.56] |
| 1.1.1 Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0° | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.55, 2.35] |
| 1.1.2 Supine midline head position with the bed tilted ≥ 30° versus supine head rotated 90° with the bed at 0° | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.74, 1.62] |
| 1.2 Intraventricular haemorrhage, grade 3 to 4 | 3 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.37, 1.33] |
| 1.2.1 Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0° | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.28, 8.98] |
| 1.2.2 Supine midline head position with the bed at ≥30° versus supine head rotated 90° with the bed at 0° | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.31, 1.22] |
| 1.3 Neonatal mortality (within 28 days of life) | 3 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.25, 0.93] |
| 1.3.1 Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0° | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.65] |
| 1.3.2 Supine midline head position with the bed tilted ≥ 30° versus supine head rotated 90° with the bed at 0° | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.21, 1.03] |
| 1.4 Mortality during initial hospitalization | 3 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.28, 0.90] |
| 1.4.1 Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0° | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.65] |
| 1.4.2 Supine midline head position with the bed tilted ≥ 30° versus supine head rotated 90° with the bed at 0° | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.26, 0.97] |
| 1.5 Cystic periventricular leukomalacia | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [0.14, 76.01] |
| 1.5.1 Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0° | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [0.14, 76.01] |
| 1.5.2 Supine midline head position with the bed at ≥30° versus supine head rotated 90° with the bed at 0° | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.6 Retinopathy of prematurity, any stage | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.27 [0.85, 6.11] |
| 1.7 Retinopathy of prematurity, ≥ stage 3 | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.41, 1.97] |
| 1.7.1 Supine midline head position with the bed at 0° versus supine head rotated 90° with the bed at 0° | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.31, 24.14] |
| 1.7.2 Supine midline head position with the bed tilted ≥ 30° versus supine head rotated 90° with the bed at 0° | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.31, 1.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Abdi 2011.
| Study characteristics | ||
| Methods | Randomised parallel clinical trial, conducted at King Abdulaziz Hospital, Al‐Ahsa, Saudi Arabia, and including 48 preterm infants in 2008 to 2009. Randomisation was stratified on the basis of gender and gestational age (< 27 or 27 to 29+6 weeks). Randomisation was carried out according to a predetermined computer‐generated randomisation sequence with consecutively numbered sealed envelopes. In the case of eligible multiple births, each infant was randomly assigned. An intention‐to‐treat analysis was used throughout this study. Among infants in the head midline group, 12 were cared for in a left‐tilted and 13 in a right‐tilted midline head position | |
| Participants | Preterm infants were enrolled in the trial if they met the following criteria: (1) inborn; (2) gestational age < 30 weeks; and (3) postnatal age < 2 hours. Exclusion criteria included the presence of lethal congenital anomalies, hypoxic‐ischaemic encephalopathy, and the need for full cardiopulmonary resuscitation at birth | |
| Interventions | Infants lying on beds at 0° were randomly assigned to be cared for in a supine midline (23 infants) or supine lateral (25 infants) head position. In the supine midline position, the infant’s chin was kept at a 90° angle to the bed. In the lateral head position, the head was tilted 90° to either side. At enrolment, it was left to the bedside nurse to place the head in the right‐tilted or left‐titled position | |
| Outcomes | Primary outcome: rate of intraventricular haemorrhage of all grades Secondary outcomes: rate of intraventricular haemorrhage, grade 3 to 4; neonatal mortality; retinopathy of prematurity (any stage; ≥ stage 3); cystic periventricular leukomalacia |
|
| Notes | This was a pilot study (underpowered) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out according to a predetermined computer‐generated randomisations sequence |
| Allocation concealment (selection bias) | Low risk | Envelopes for randomisations were opaque (we obtained data for this outcome directly from trial authors) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Head ultrasound scans were assessed by 2 radiologists who were blinded to assignment of head position |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Other bias | Unclear risk | Trial was underpowered (pilot) |
Al‐Abdi 2015.
| Study characteristics | ||
| Methods | Multicentre randomised (block size of 4) parallel clinical trial. Head ultrasound scan was performed within 12 hours of life, at 168 hours of life, and at the physician's discretion. Head ultrasound scan was reported by 3 radiologists who were blinded to assignment of head position. The primary analysis was by intention‐to‐treat | |
| Participants | Preterm neonates (< 30 weeks' gestation) without intraventricular haemorrhage at 12 hours of life scan Exclusion criteria: outborn; lethal congenital anomalies; hypoxic‐ischaemic encephalopathy; external cardiac compression; or epinephrine administration at birth |
|
| Interventions | Heads of 62 preterm neonates were randomly placed in flat midline (n = 31) or flat right lateral position (n = 31) throughout the first 168 hours of life | |
| Outcomes | The only outcome reported in the abstract was intraventricular haemorrhage | |
| Notes | Although the planned sample size was 600 (alpha 5%; power 80%), the study was prematurely terminated owing to low accrual rate when only 71 neonates (12%) had been recruited Trial was registered on ClinicalTrials.gov/ct2/show/NCT01584375 and findings presented at the Pediatric Academic Societies (PAS) Meeting in 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisations not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Head ultrasound scans were reported by 3 radiologists who were blinded to assignment of head position |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol available |
| Other bias | High risk | Trial lacks power because only 71 infants were enrolled (instead of 600) |
Kochan 2019.
| Study characteristics | ||
| Methods | Randomised parallel clinical trial, conducted at the Children’s Hospital of The King’s Daughters in Norfolk, Virginia (USA) and including 180 extremely low birth weight infants in 2012 to 2015. Block randomised using a block randomisations table. |
|
| Participants | Extremely low birth weight infants (< 1000 g) admitted to the NICU Baseline characteristics
Multivariate analysis of maternal pre‐eclampsia and prolonged rupture of membranes showed no significant effect on outcomes. Infants with congenital anomalies were excluded. |
|
| Interventions | Infants admitted to the NICU were placed supine in the incubator while umbilical lines were placed and radiographs obtained.
Cranial US were performed immediately after randomisations by a mean (SD) age of 2.9 h (1); there was no significant difference in the age at the time of admission between the two study groups (P = 0.35). Follow‐up ultrasounds were performed daily for the first 4 days of life and on day 7. Normal scans were repeated at 4 weeks of age, abnormal scans were repeated weekly. All cranial ultrasound studies were interpreted by paediatric radiologists all blinded to the randomisations group. Outcome data were reported according to intention‐to‐treat analysis. Protocol variations were reported in 26 infants in the midline group as follows.
After the fourth day, all infants were placed in the flat position. |
|
| Outcomes | Primary outcome: incidence of GM‐IVH any grade Secondary outcomes: severe IVH; survival on the first 4 days and to discharge; cystic periventricular leukomalacia on brain ultrasound in the first month of life; retinopathy of prematurity requiring treatment |
|
| Notes | The nursing staff documented protocol variations in 26 infants randomised to the ELEV group. Data from all infants were maintained in their original randomisations group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Infants were randomized using a block randomizations table" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible for the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote. "All cranial ultrasound studies were interpreted by one of five pediatric radiologists all blinded to the randomizations group". All main outcomes likely to be assessed by physicians unaware of the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not registered on an online database (information provided by study authors). We cannot judge whether there were any deviations from the protocol |
| Other bias | Low risk | Appears free of other bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Antunes 2003 | Intervention was started after 48 hours of life |
| Imam 2019 | Intervention was provided for 6 hours only |
| Wu 2015 | Mean birth weight was > 2.5 kg (2.75 ± 0.58 in intervention group and 2.92 ± 0.64 in control group), suggesting that included newborns were not ≤ 32 weeks' gestational age as specified in this review |
Characteristics of studies awaiting classification [ordered by study ID]
Jalali 2012.
| Methods | Randomised clinical trial conducted at 17 Shahrivar Children's Hospital of Rasht (Rasht Iran), including 31 intubated newborns in the period 2010 to 2011 |
| Participants | Inclusion criteria: gestational age ≥ 28 weeks; tracheal intubation at postnatal age < 48 hours; absence of congenital malformations Patients were excluded if they were given a diagnosis of congenital sepsis or pneumonia, or could not be maintained in mechanical ventilation for 5 days |
| Interventions | Neonates were randomised to be nursed in supine (n = 16) or lateral position (n = 15) |
| Outcomes | No outcomes specified in our review were reported by study authors |
| Notes | The objective of this trial was to evaluate the influence of lateral and supine position on bacterial colonization of the endotracheal tube in ventilated neonates. Approximately half of the included infants had a gestational age greater than that specified by the inclusion criterion of this review. We tried to contact trial authors but received no response. Study authors reported on none of the outcomes specified in this review |
Characteristics of ongoing studies [ordered by study ID]
NCT035430461.
| Study name | Tortle Midliner and Intraventricular haemorrhage |
| Methods | Prospective randomised parallel controlled trial |
| Participants | Inborn infants with gestational age ≤ 30 weeks 6 days and with < 3 hours of life from birth |
| Interventions |
|
| Outcomes | Primary outcome: GM‐IVH |
| Starting date | August 2018 |
| Contact information | Adrienne C Alexander, Orlando, Florida, USA |
| Notes | Estimated enrolment: 150 Estimated primary completion date: December 2020 |
Differences between protocol and review
Standard neonatal search methods were updated in September 2019.
Contributions of authors
OR and MB reviewed the literature and wrote the review.
MGC commented on and reviewed the review.
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden
to OR and MB
-
Istituto Giannina Gaslini, Genoa, Italy
to MGC
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
OR has no interest to declare.
MGC has no interest to declare.
MB has no interest to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Al‐Abdi 2011 {published data only}
- Al-Abdi SY, Nojoom MS, Alshaalan HM, Al-Aamri MA. Pilot-randomized study on intraventricular hemorrhage with midline versus lateral head positions. Saudi Medical Journal 2011;32(4):420-1. [PMID: ] [PubMed] [Google Scholar]
Al‐Abdi 2015 {published data only}
- Al-Abdi S, Alallah J, Al Omran A, Al Alwan Q, Al Hashimi H, Haidar S. The risk of intraventricular hemorrhage with flat midline versus flat right lateral head positions: a prematurely terminated multicenter randomized clinical trial. In: The Pediatric Academic Societies (PAS). 2015.
Kochan 2019 {published data only}
- Kochan M, Leonardi B, Firestine A, McPadden J, Cobb D, Shah TA, et al. Elevated midline head positioning of extremely low birth weight infants: effects on cardiopulmonary function and the incidence of periventricular-intraventricular hemorrhage. Journal of Perinatology 2019;39(1):54-62. [DOI: 10.1038/s41372-018-0261-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Antunes 2003 {published data only}
- Antunes LC, Rugolo LM, Crocci AJ. Effect of preterm infant position on weaning from mechanical ventilation [Efeito da posição do prematuro no desmame da ventilação mecânica]. Jornal de Pediatria 2003;79(3):239-44. [PMID: ] [PubMed] [Google Scholar]
Imam 2019 {published data only}
- Imam SS, Shinkar DM, Mohamed NA, Mansour HE. Effect of right lateral position with head elevation on tracheal aspirate pepsin in ventilated preterm neonates: randomized controlled trial. Journal of Maternal-fetal & Neonatal Medicine 2019;32(22):3741-6. [DOI: 10.1080/14767058.2018.1471674] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wu 2015 {published data only}
- Wu J, Zhai J, Jiang H, Sun Y, Jin B, Zhang Y, et al. Effect of change of mechanical ventilation position on the treatment of neonatal respiratory failure. Cell Biochemistry and Biophysics 2015;72(3):845-9. [DOI: 10.1007/s12013-015-0547-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Jalali 2012 {published data only}
- Jalali SZ, Mojtabaei SH, Heidarzadeh A, Aghamahdi F, Ahmad-Soltani M. The influence of lateral and supine position on bacterial colonization of endotracheal tube in neonates admitted to neonatal intensive care unit. Iranian Journal of Pediatrics 2012;22(4):499-504. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT035430461 {unpublished data only}
- NCT03543046. Tortle midliner and intraventricular hemorrhage. clinicaltrials.gov/ct2/show/NCT03543046 (first received 2 May 2018).
Additional references
Ancora 2010
- Ancora G, Maranella E, Aceti A, Pierantoni L, Grandi S, Corvaglia L, et al. Effect of posture on brain hemodynamics in preterm newborns not mechanically ventilated. Neonatology 2010;97(3):212-7. [DOI: 10.1159/000253149] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ballabh 2014
- Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clinics in Perinatology 2014;41(1):47-67. [DOI: 10.1016/j.clp.2013.09.007 ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barradas 2006
- Barradas J, Fonseca A, Guimarães CL, Lima GM. Relationship between positioning of premature infants in Kangaroo Mother Care and early neuromotor development. Jornal de Pediatria 2006;82(6):475-80. [DOI: 10.2223/JPED.1565 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development. 2nd edition. San Antonio, TX: The Psychological Corporation, 1993. [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd edition. San Antonio, TX: Harcourt Assessment, 2006. [Google Scholar]
Bembich 2012
- Bembich S, Oretti C, Travan L, Clarici A, Massaccesi S, Demarini S. Effects of prone and supine position on cerebral blood flow in preterm infants. Journal of Pediatrics 2012;160(1):162-4. [DOI: 10.1016/j.jpeds.2011.08.056] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bredemeyer 2012
- Bredemeyer SL, Foster JP. Body positioning for spontaneously breathing preterm infants with apnoea. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD004951.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Canadian Neonatal Network 2014
- Shah P, Yoon EW, Chan P, The Canadian Neonatal Network. Annual report 2014. www.canadianneonatalnetwork.org (accessed prior to 21 November 2019).
Carteaux 2003
- Carteaux P, Cohen H, Check J, George J, McKinley P, Lewis W, et al. Evaluation and development of potentially better practices for the prevention of brain hemorrhage and ischemic brain injury in very low birth weight infants. Pediatrics 2003;11(4 Pt 2):e489-96. [PMID: ] [PubMed] [Google Scholar]
Cochrane EPOC Group 2015
- Effective Practice and Organisation of Care (EPOC). Data extraction and management. EPOC resources for review authors. epoc.cochrane.org/epoc-specific-resources-review-authors (accessed prior to 21 November 2019).
Cornette 2002
- Cornette LG, Tanner SF, Ramenghi LA, Miall LS, Childs AM, Arthur RJ, et al. Magnetic resonance imaging of the infant brain: anatomical characteristics and clinical significance of punctate lesions. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;86(3):F171-7. [DOI: 10.1136/fn.86.3.f171] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cowan 1985
- Cowan F, Thoresen M. Changes in superior sagittal sinus blood velocities due to postural alterations and pressure on the head of the newborn infant. Pediatrics 1985;75(6):1038–47. [PMID: ] [PubMed] [Google Scholar]
Cowan 1987
- Cowan F, Thoresen M. The effect of intermittent positive pressure ventilation on cerebral arterial and venous blood velocities in the newborn infant. Acta Paediatrica Scandinavica 1987;76(2):239-47. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dani 2007
- Dani C, Bertini G, Cecchi A, Corsini I, Pratesi S, Rubaltelli FF. Brain hemodynamic effects of nasal continuous airway pressure in preterm infants of less than 30 weeks’ gestation. Acta Pediatrica (Oslo, Norway : 1992) 2007;96(10):1421-5. [DOI: 10.1111/j.1651-2227.2007.00453.x ] [PMID: ] [DOI] [PubMed] [Google Scholar]
de Bijl‐Marcus 2016
- Bijl-Marcus KA, Brouwer AJ, Vries LS, Wezel-Meijler G. The effect of head positioning and head tilting on the incidence of intraventricular hemorrhage in very preterm infants: a systematic review. Neonatology 2016;111(3):267-79. [DOI: 10.1159/000449240] [PMID: 27923236 ] [DOI] [PubMed] [Google Scholar]
Dolfin 1983
- Dolfin T, Skidmore MB, Fong KW, Hoskins EM, Shennan AT. Incidence, severity, and timing of subependymal and intraventricular hemorrhages in preterm infants born in a perinatal unit as detected by serial real-time ultrasound. Pediatrics 1983;71(4):541-6. [PMID: ] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Emery 1983
- Emery JR, Peabody JL. Head position affects intracranial pressure in newborn infants. Journal of Pediatrics 1983;103(6):950–3. [DOI: 10.1016/s0022-3476(83)80728-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Fumagalli 2009
- Fumagalli M, Ramenghi LA, Righini A, Groppo M, Bassi L, De Carli A, et al. Cerebellar hemorrhages and pons development in extremely low birth weight infants. Frontiers in Bioscience (Elite Edition) 2009;1:537-41. [DOI: 10.2741/e50] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 21 November 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014.Available at gradepro.org.
Graça 2013
- Graça AM, Geraldo AF, Cardoso K, Cowan FM. Preterm cerebellum at term age: ultrasound measurements are not different from infants born at term. Pediatric Research 2013;74(6):698-704. [DOI: 10.1038/pr.2013.154] [PMID: ] [DOI] [PubMed] [Google Scholar]
Griffiths 1954
- Griffiths R. The Abilities of Babies: A Study in Mental Measurement. New York (NY): McGraw-Hill, 1954. [Google Scholar]
Hamrick 2004
- Hamrick SE, Miller SP, Leonard C, Glidden DV, Goldstein R, Ramaswamy V, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. Journal of Pediatrics 2004;145(5):593-9. [DOI: 10.1016/j.jpeds.2004.05.042] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.. [DOI] [PMC free article] [PubMed]
Horbar 2002
- Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al, Members of the Vermont Oxford Network. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics 2002;110(1 Pt 1):143-51. [DOI: 10.1542/peds.110.1.143] [PMID: ] [DOI] [PubMed] [Google Scholar]
ICROP 1984
- International Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Pediatrics 1984;74(1):127-33. [PMID: ] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leahy 1982
- Leahy FA, Durand M, Cates D, Chernick V. Cranial blood volume changes during mechanical ventilation and spontaneous breathing in newborn infants. Journal of Pediatrics 1982;101(6):984-7. [DOI: 10.1016/s0022-3476(82)80026-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liao 2015
- Liao SM, Rao R, Mathur AM. Head position change is not associated with acute changes in bilateral cerebral oxygenation in stable preterm infants during the first 3 days of life. American Journal of Perinatology 2015;32(7):645-52. [DOI: 10.1055/s-0034-1390348] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Limperopoulos 2007
- Limperopoulos C, Bassan H, Gauvreau K, Robertson RL Jr, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 2007;120(3):584-93. [DOI: 10.1542/peds.2007-1041 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Malusky 2011
- Malusky S, Donze A. Neutral head positioning in premature infants for intraventricular hemorrhage prevention: an evidence-based review. Neonatal Network : NN 2011;30(6):381-96. [DOI: 10.1891/0730-0832.30.6.381 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
McLendon 2003
- McLendon D, Check J, Carteaux P, Michael L, Moehring J, Secrest JW, et al. Implementation of potentially better practices for the prevention of brain hemorrhage and ischemic brain injury in very low birth weight infants. Pediatrics 2003;111(4 Pt 2):e497–503. [PMID: ] [PubMed] [Google Scholar]
Moritz 2008
- Moritz B, Fritz M, Mann C, Simma B. Nasal continuous positive airway pressure (nCPAP) does not change cardiac output in preterm infants. American Journal of Perinatology 2008;25(2):105-9. [DOI: 10.1055/s-2008-1040341] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nagdyman 1999
- Nagdyman N, Walka MM, Kampmann W, Stöver B, Obladen M. 3-D ultrasound quantification of neonatal cerebral ventricles in different head positions. Ultrasound in Medicine & Biology 1999;25(6):895-900. [DOI: 10.1016/s0301-5629(99)00030-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nakamura 1990
- Nakamura Y, Okudera T, Fukuda S, Hashimoto T. Germinal matrix hemorrhage of venous origin in preterm neonates. Human Pathology 1990;21(10):1059-62. [DOI: 10.1016/0046-8177(90)90256-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nankervis 2010
- Nankervis CA, Martin EM, Crane ML, Samson KS, Welty SE, Nelin LD. Implementation of a multidisciplinary guideline-driven approach to the care of the extremely premature infant improved hospital outcomes. Acta Paediatrica 2010;99(2):188-93. [DOI: 10.1111/j.1651-2227.2009.01563.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Noori 2014
- Noori S, McCoy M, Anderson MP, Ramji F, Seri I. Changes in cardiac function and cerebral blood flow in relation to peri/intraventricular hemorrhage in extremely preterm infants. Journal of Pediatrics 2014;164(2):264-70. [DOI: 10.1016/j.jpeds.2013.09.045] [PMID: ] [DOI] [PubMed] [Google Scholar]
Obladen 2008
- Obladen M, Metze B, Henrich W, Aktas A, Czernik C, Schulz-Baldes A. Interdisciplinary surveillance of intraventricular hemorrhage associated conditions in infants <1000 g. Acta Paediatrica 2008;97(6):731-7. [DOI: 10.1111/j.1651-2227.2008.00812.x ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Osborn 2003
- Osborn DA, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics 2003;112(1 Pt 1):33–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pape 1979
- Pape KE, Wigglesworth JS. Haemorrhage, Ischaemia and the Perinatal Brain. Cambridge (UK): Cambridge University Press, 1979. [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [DOI: 10.1016/s0022-3476(78)80282-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Parodi 2015
- Parodi A, Morana G, Severino MS, Malova M, Natalizia AR, Sannia A, et al. Low-grade intraventricular hemorrhage: is ultrasound good enough? Journal of Maternal-fetal & Neonatal Medicine 2015;28 Suppl 1:2261-4. [DOI: 10.3109/14767058.2013.796162 ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pellicer 2002
- Pellicer A, Gaya F, Madero R, Quero J, Cabanas F. Noninvasive continuous monitoring of the effects of the head position on brain hemodynamic in ventilated infants. Pediatrics 2002;109(3):434-40. [DOI: 10.1542/peds.109.3.434] [PMID: ] [DOI] [PubMed] [Google Scholar]
Philip 1989
- Philip AG, Allan WC, Tito AM, Wheeler LR. Intraventricular hemorrhage in preterm infants: declining incidence in the 1980s. Pediatrics 1989;84(5):797-801. [PMID: ] [PubMed] [Google Scholar]
Pichler 2001
- Pichler G, Schmölzer G, Müller W, Urlesberger B. Body position-dependent changes in cerebral hemodynamics during apnea in preterm infants. Brain & Development 2001;23(6):395-400. [DOI: 10.1016/s0387-7604(01)00245-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan Web [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019.Available at revman.cochrane.org.
Rivas‐Fernandez 2016
- Rivas-Fernandez M, Roqué i Figuls M, Diez-Izquierdo A, Escribano J, Balaguer A. Infant position in neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD003668.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romantsik 2019
- Romantsik O, Bruschettini M, Ley D. Intraventricular hemorrhage and white matter injury in preclinical and clinical studies. NeoReviews 2019;20(11):e636-52. [DOI: 10.1542/neo.20-11-e636] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rutherford 2010
- Rutherford MA, Supramaniam V, Ederies A, Chew A, Bassi L, Groppo M, et al. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology 2010;52(6):505-21. [DOI: 10.1007/s00234-010-0700-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sherlock 2005
- Sherlock RL, Anderson PJ, Doyle LW, Victorian Infant Collaborative Study Group. Neurodevelopmental sequelae of intraventricular hemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Human Development 2005;81(11):909-16. [DOI: 10.1016/j.earlhumdev.2005.07.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Takashima 1978
- Takashima S, Tanaka K. Microangiography and vascular permeability of the subependymal matrix in the premature infant. Canadian Journal of Neurological Sciences [Journal Canadien des Sciences Neurologiques] 1978;5(1):45-50. [PMID: ] [PubMed] [Google Scholar]
Takashima 2009
- Takashima S, Itoh M, Oka A. A history of our understanding of cerebral vascular development and pathogenesis of perinatal brain damage over the past 30 years. Seminars in Pediatric Neurology 2009;16(4):226–36. [DOI: 10.1016/j.spen.2009.09.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vermont Oxford Network 2013
- Vermont Oxford Network. Database of very low birth weight infants born in 2012. Nightingale Internet Reporting System. public.vtoxford.org (accessed 4 April 2014).
Volpe 2008
- Volpe JV. Neurology of the Newborn. 5th edition. Philadelphia (PA): Saunders, 2008. [Google Scholar]


