Abstract
Background
Venous thromboembolism (VTE) is the term given to any thromboembolic event (blocking of a blood vessel by a blood clot) occurring in the venous system. The current treatment recommended for VTE is anticoagulation (reduction of the blood's ability to clot). The aim of this review is to summarize results from randomized controlled trials (RCTs) for the effectiveness of anticoagulants (heparins, including low molecular weight heparins and vitamin K antagonists) in the treatment of VTE, compared to non‐steroidal anti‐inflammatory drugs (NSAIDs) or placebo.
Objectives
To examine the randomized controlled evidence for the effectiveness and safety of anticoagulant treatment compared to NSAIDs or placebo in patients with VTE on the incidence of fatal and non‐fatal pulmonary emboli (PE) and the recurrence or extension of deep vein thrombosis (DVT).
Search methods
The Cochrane Peripheral Vascular Diseases (PVD) Group searched their Specialized Register (last searched 14 May 2008) and the Cochrane Central Register of Controlled Trials (CENTRAL) database (last searched Issue 2, 2008). In addition, DKC also searched reference lists and contacted pharmaceutical companies and experts in the field.
Selection criteria
All randomized trials of anticoagulants versus NSAIDs or placebo in the initial treatment of VTE (DVT or PE or both).
Data collection and analysis
DKC and JM independently assessed trial quality and extracted data. JCP (biostatistician) analyzed the design elements and feasibility of a future randomized controlled trial to determine definitively efficacy and safety of anticoagulants in VTE treatment.
Main results
Two RCTs were included. Data were not pooled because of heterogeneity between the studies. The two RCTs were too small to determine any difference in mortality, occurrence of pulmonary emboli, progression or return of DVT between patients treated with anticoagulation and those receiving no anticoagulation.
Authors' conclusions
The limited evidence from RCTs of anticoagulants versus NSAIDs or placebo is inconclusive regarding the efficacy and safety of anticoagulants in VTE treatment. The use of anticoagulants is widely accepted in clinical practice, so a further RCT comparing anticoagulants to placebo could not ethically be carried out.
Plain language summary
Anticoagulants compared with anti‐inflammatory drugs or placebo for treating people who have venous blood clots
A blood clot can block a venous blood vessel to cause what is known as a thromboembolism. This most often occurs in a leg (deep vein thrombosis) or in the lungs (pulmonary embolism), which can be fatal. Once formed, a blood clot in a leg can increase in size or can move to the lungs and the recommended treatment is to give drugs that thin the blood (anticoagulants). These include heparins and drugs that inhibit the action of vitamin K (warfarin, phenprocoumon, and acenocoumarol). The possible harms caused by anticoagulants include bleeding in the gut or brain and anticoagulant‐induced clotting. The review authors made a thorough search of the medical literature looking for controlled studies on people with blood clots in their veins comparing blood thinning drugs (anticoagulants) with drugs to reduce inflammation (non‐steroidal anti‐inflammatory drugs) or dummy treatment (placebo). Only two small studies with a total of 113 participants treated over three months were identified, which gave inconclusive results. Since the use of anticoagulants is widely accepted in clinical practice, designing and implementing other similar studies would not be ethical.
Background
Venous thromboembolism (VTE) is the term given to any thromboembolic event (blocking of a blood vessel by a blood clot) occurring in the venous system. This includes deep vein thrombosis (DVT) (economy class syndrome, when clots lodge in a limb) and pulmonary embolism (PE) (when clots lodge in the lungs, which can be fatal). Venous thromboembolism is a common problem in the general population (Hansson 1997; Nordstrom 1992), and an important cause of death in hospitalized patients (Rubinstein 1988).
In 1960, Barritt and Jordan reported the first randomized, placebo‐controlled trial used to justify heparin and vitamin K antagonists (e.g., warfarin, phenprocoumon, and acenocoumarol) as treatment of DVT. Ironically, the study population consisted of patients clinically diagnosed with PE and the subset with DVT was not reported. The clinical PE diagnoses of the investigators were not confirmed by pulmonary angiograms or lung scans. This small study noted that patients who had survived symptomatic PE and then received anticoagulants had a significantly lower mortality from PE (i.e., 0/16 with anticoagulants versus 5/19 with placebo, P < 0.0007). Anticoagulated patients received three days of intravenous heparin concurrently with oral nicoumalone (Sinthrome), a vitamin K antagonist, for a total of 14 days of anticoagulation (Barritt 1960).
We now know that approximately 75% of those clinically diagnosed with PE do not have it (Ginsberg 1996; Hull 1986; Pioped 1990). Autopsy descriptions of the patients in Barritt and Jordan's study show that in four of the five deaths, severe underlying diseases (e.g., cerebral infarction and cavitary pneumonia with sepsis) caused the deaths, with PE only appearing as a contributing factor (Barritt 1960). Thus, the first trial was methodologically inadequate. Our purpose was to review subsequent randomized controlled trials, but not other forms of evidence, to determine the effectiveness of anticoagulants for the treatment of venous thromboembolism.
Objectives
(1) To examine the existing clinical trial evidence of the effect of anticoagulants (heparins and vitamin K antagonists) in patients with VTE on the incidence of fatal and non‐fatal pulmonary emboli (PE), and the recurrence or extension of deep vein thrombosis (DVT).
(2) To evaluate in those RCTs the all‐cause mortality and the risks of anticoagulant therapy for VTE, including all major bleeding events and anticoagulant‐induced clotting (heparin‐induced‐thrombocytopenia (HIT), and warfarin necrosis).
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials designed to compare anticoagulants versus non‐steroidal anti‐inflammatory drugs or placebo in the initial treatment of VTE (DVT or PE or both).
Types of participants
Patients with DVT (diagnosed by venography, ultrasonography, or any validated measurement) or PE (diagnosed by pulmonary angiography, ventilation/perfusion (V/Q) lung scan, or computerized tomography (CT) scan).
Types of interventions
The intervention of interest was the administration of anticoagulants (heparins or vitamin K antagonists). The comparison was non‐steroidal anti‐inflammatory drugs (NSAIDs) or placebo.
Types of outcome measures
Primary outcomes
mortality due to PE;
incidence of PE (defined by a V/Q lung scan showing at least one segmental defect not seen on the preceding scan, abnormal pulmonary angiography or CT, or fatal PE at autopsy);
incidence of recurrent DVT and extension of DVT or both.
Secondary outcomes
all‐cause mortality;
major hemorrhagic events (associated with a decreased hemoglobin concentration of > 2 g/dl, retroperitoneal or intracranial bleed, a transfusion of two or more units of blood);
fatal hemorrhagic events;
morbidity and mortality due to heparin induced thrombocytopenia with thrombosis (HIT).
Search methods for identification of studies
The Cochrane Peripheral Vascular Diseases (PVD) Group searched their Specialized Register (last searched 14 May 2008) and the Cochrane Central Register of Controlled Trials (CENTRAL) database (last searched Issue 2, 2008) for publications describing (or which might describe) randomized controlled trials of anticoagulants versus non‐steroidal anti‐inflammatory drugs or placebo in the initial treatment of venous thromboembolism (DVT and PE or both). See Appendix 1 and Appendix 2 for details of search strategies used to search CENTRAL.
In addition, DKC checked reference lists of studies identified for relevant randomized controlled trials and searched for unpublished randomized clinical trials through personal communication with colleagues and representatives of pharmaceutical companies.
Briefly, the Specialized Register of the Cochrane PVD Group has been constructed from regular electronic searches of MEDLINE (1966 onwards), EMBASE (1980 onwards), and CINAHL (1982 to date), and the Cochrane Central Register of Controlled Trials (CENTRAL), and through handsearching 38 relevant journals and numerous conference proceedings. The full list of journals and conference proceedings, as well as the search strategies for the electronic databases, are described in the 'Search strategies for the identification of studies' section within the editorial information about the Cochrane PVD Group (http://www.mrw.interscience.wiley.com/cochrane/clabout/articles/PVD/frame.html).
Data collection and analysis
Locating and selecting studies
DKC and JM independently assessed the titles and abstracts of all reports of trials identified by the electronic searches and obtained hard copies of the full text of possible trials that met the selection criteria. The review authors were not blinded to the journal, institution or results of the study. Titles and abstracts of non‐English articles were translated into English then assessed for inclusion. The full text of the article was translated into English if the title and abstract met the inclusion criteria. We reassessed studies with insufficient information when additional information became available from the trial authors. We resolved by consensus differences on whether trials met the inclusion criteria, and documented reasons for excluding studies.
Critical appraisal of studies
DKC and JM independently assessed the methodological quality of each trial, and any differences were resolved by discussion. Details of the randomization (sequence and concealment), blinding, and the number of patients lost to follow up were recorded. Both DKC and JM gave the trial a score for each item; A ‐ low risk of bias, B ‐ moderate risk of bias, and C ‐ high risk of bias (Cochrane Handbook).
External validity was defined by characteristics of the participants (inclusion and exclusion criteria; clinical and laboratory diagnosis criteria; number of participants; age; sex; duration of follow up; duration of the study; and location of study); the interventions (type of heparin; route of administration; duration of heparin treatment; introduction of oral vitamin K antagonists; laboratory control of anticoagulation); and the outcomes.
Collection of data
DKC and JM independently extracted data using pre‐designed data extraction sheets, and the information was cross checked. Data from any studies published twice were extracted from the more complete study.
Data analysis
For each study, we summarized binary outcomes into relative risks, and continuous outcomes into weighted mean differences. Studies were not combined using meta‐analysis if they differed in the interventions under comparison.
Sensitivity analyses
We did not perform sensitivity analysis as only two studies were included in the systematic review.
Results
Description of studies
We identified twenty reports of studies using the search strategy. We excluded five trials on the basis of title and abstract and retrieved 15 articles comprising 11 studies for detailed evaluation. Of these, nine studies were excluded (11 articles; two duplicate references). (Please see Table of excluded studies for details.) Two studies met the inclusion criteria (Nielsen 1994; Ott 1988). The study by Nielsen and others (Nielsen 1994) was reported in three publications; one in Thrombosis Research (Nielsen 1994a), one in the Journal of Internal Medicine (Nielsen 1994b), and the other was an abstract (Nielsen 1985). We extracted data from both full papers. Please see Quorom statement for details of the screening process (Quorum).
Both included studies were conducted in Denmark. The paper by Ott and others (Ott 1988) was published in Danish and the information presented is based on a translation of the article.
We contacted over a hundred specialists in the field of venous thromboembolism, and eight pharmaceutical companies that manufacture anticoagulants (Barr Labs, Pharmacia, Aventis Pharmaceuticals, Eli Lilly, American Home Products Corporation (Wyeth), DuPont Pharmaceuticals, Organon Inc, LEO Pharma) for unpublished data. This exercise did not identify any additional trials.
Risk of bias in included studies
Quality assessment
Randomization and allocation concealment scoring system
A = Clearly adequate: Centralized randomization by telephone; randomization scheme controlled by pharmacy; numbered or coded identical containers administered sequentially; on‐site computer system which can only be accessed after entering the characteristics of an enrolled participant; sequentially numbered, sealed, opaque envelopes.
B = Possibly adequate: Sealed envelopes but not sequentially numbered or opaque; list of random numbers read by someone entering patient into trial (open list); a trial in which the description suggests adequate concealment, but other features are suspicious (e.g. markedly unequal controls and trial groups); stated random, but unable to obtain further details.
C = Clearly inadequate: Any allocation procedure transparent before assignment (e.g. an open list of random numbers, alternation, date of birth, day of the week, case record numbers).
D = Not described.
Ott 1988 evaluated 236 patients with DVT in a randomized trial of anticoagulants (heparin and warfarin) versus placebo. Only 23 of these patients met all the study entry criteria (venogram showed DVT; 60 to 80 years old; symptoms less than 14 days; able to ambulate; having no contraindications to anticoagulants; and willing to participate). The method of randomization was not stated. Twelve patients received placebo and 11 received anticoagulation therapy for three months. The treatment group received heparin 10,000 units subcutaneously three times on one day and oral warfarin for three months. The control group received saline 0.4 ml subcutaneously three times a day and oral placebo tablets. Both groups were treated with active mobilization, graduated compression stockings and nightly leg elevation. Three patients were excluded from the treatment group post randomization, one for bleeding risk due to hepatic cancer, one for poor compliance, and one for disease progression. In the placebo group, three patients were also excluded post randomization, one for bleeding risk due to colon cancer, and two for worsening symptoms. The trialists evaluated patients while on therapy and after 11 to 27 months (Ott 1988).
The main outcomes reported were signs of venous insufficiency and deaths. Data on fatal and non‐fatal DVT or PE and major hemorrhagic events were not available in the translation. The main limitation of the study is the high rate of loss to follow up post randomization, which precludes intention‐to‐treat analysis.
Nielsen 1994 evaluated 112 patients with DVT of whom 90 met the inclusion criteria (venogram showed DVT; symptoms less than six days; no signs of PE clinically or on chest X‐ray). Patients with clinical symptoms of PE and chest X‐ray infiltrates were excluded. Of the randomized patients, 48 received heparin and phenprocoumon (Marcoumar, Roche) for three months and 42 received phenylbutazone (a non‐steroidal anti‐inflammatory drug). Results were assessed while on treatment and after one year. Randomization was by sealed envelopes. In the anticoagulated group, heparin was administered for at least six days (10,000 units intravenous bolus followed by a continuous intravenous infusion of 40,000 units in 24 hours adjusted according to the Activated Partial Thromboplastin Time (APTT)). Phenprocoumon was started on the third day and continued for three months. The control group received phenylbutazone 200 mg three times a day on the first day followed by 100 mg three times a day for nine days. Both groups were actively mobilized and wore graduated compression stockings from the day of admission. Follow up was good with no losses to follow up.
Primary outcomes were VTE on the basis of venogram at 30 days and lung scans at day 10 and day 60. These were fairly objective measures of the outcomes of interest and observer bias was minimized by blinding the radiologists who interpreted the venograms and lung scans to the clinical signs and treatment allocation. Secondary outcomes were DVT recurrence and PE symptoms within three months and death within 12 months. Nielsen 1994 scored A; Ott 1988 scored B (see "Characteristics of Included Studies" Table).
Effects of interventions
Primary outcomes
(see "Comparisons and Data" tables) The trial of Ott and colleagues reported no deaths due to PE (Ott 1988). In the study by Nielsen (Nielsen 1994) there was no significant difference in deaths due to PE between the anticoagulant‐treated group and the group treated with phenylbutazone (1/48 versus 0/42, relative risk (RR) 2.63; 95% confidence intervals (CI) 0.11 to 62.95). Similarly, there was no difference in the combined outcome PE, DVT progression, or return (5/11 of those anticoagulated versus 5/12 of those not anticoagulated, RR 1.09, 95% CI 0.43 to 2.77) in the study by Ott and others (Ott 1988), or in the recurrent DVT or DVT extension in the study by Nielsen and others (18/48 versus 22/42, RR 0.72, 95% CI 0.45 to 1.14) (Nielsen 1994).
Secondary outcomes
(see "Comparisons and Data" tables) There was no significant difference in the secondary outcomes all‐cause mortality and major hemorrhage in either study. Neither of the trials reported morbidity or mortality due to heparin‐induced thrombocytopenia (HIT) with thrombosis, or vitamin K antagonist necrosis (pathological cell death).
Discussion
Heparinoids and vitamin K antagonists have a clear rationale regarding prevention of death from PE. However, there is a paucity of properly designed RCTs comparing anticoagulants with placebo. These two randomized trials of anticoagulants versus no anticoagulants in DVT were inconclusive about the efficacy of heparin and oral vitamin K antagonists in the treatment of venous thromboembolism. The studies were properly designed. However, the randomised controlled trial evidence in this review is based on only two trials with a total of 113 participants having few primary outcomes. This severely limits the power to detect a real difference between the two interventions.
Authors' conclusions
Implications for practice.
The evidence from the two randomized controlled trials is limited and is inconclusive about whether anticoagulants are efficacious or safe in VTE. However, the use of anticoagulants in the treatment of VTE is widely accepted in clinical practice. Physicians have based their practice on their interpretation of secondary evidence such as trials comparing anticoagulation versus placebo after initial anticoagulation, short versus long term anticoagulation, rapid versus delayed initiation of anticoagulation, and low molecular weight heparin versus unfractionated heparin. The evidence from this review cannot be used to argue for or against a change in practice.
Implications for research.
In the absence of definitive randomized controlled trial evidence evaluating directly the safety and efficacy of anticoagulant therapy for VTE compared to placebo, the best way theoretically to answer the question would be to conduct a prospective randomised controlled trial. However, the impediments to conducting a definitive study of this type are likely to be insurmountable. First, the option of early mobilization and supportive care alone as an alternative to a widely‐established therapy for a potentially life‐threatening condition would most likely be unacceptable to institutional review boards and to prospective subjects. Second, a large number of subjects would be needed to detect all clinically important differences in safety and efficacy. To have an 80% chance of detecting a two‐fold difference in PE mortality (non‐anticoagulated versus anticoagulated) (e.g., between 1.2% and 0.6% (Gould 1999) in PE mortality), at an overall alpha level of 0.05, would need more than 8000 subjects to be recruited. Fewer subjects would however be required to detect other endpoints such as recurrence or extension of VTE. In light of the above, it unlikely that a definitive RCT along the lines described above will ever be conducted.
What's new
| Date | Event | Description |
|---|---|---|
| 14 May 2008 | New search has been performed | Searches re‐run. No new trials found. |
| 14 May 2008 | Amended | Converted to new review format. |
Acknowledgements
The Peripheral Vascular Diseases Group for assisting with literature searches.
The Consumer Network for assisting with the development of the plain language summary.
Prof David Bergqvist for acting as external peer reviewer.
Appendices
Appendix 1. CENTRAL search strategy 1
| #1 | MeSH descriptor Venous Thrombosis explode all trees |
| #2 | MeSH descriptor Venous Thromboembolism explode all trees |
| #3 | MeSH descriptor Thrombosis explode all trees |
| #4 | MeSH descriptor Pulmonary Embolism explode all trees |
| #5 | thrombol* or thrombot* or thrombu* or thromboemb* |
| #6 | DVT or deep near (vein* or ven*) near thromb* |
| #7 | PE or Pulmonary near emb* |
| #8 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) |
| #9 | MeSH descriptor Anticoagulants explode all trees |
| #10 | anticoagulant* or unfractionated hep* or UFH or low molecular weight hep* or LMWH or warfarin |
| #11 | (#9 OR #10) |
| #12 | MeSH descriptor Anti‐Inflammatory Agents, Non‐Steroidal explode all trees |
| #13 | non near steroid* or non‐steroid* near inflamm* |
| #14 | (#12 OR #13) |
| #15 | (#8 AND #11 AND #14) |
Appendix 2. CENTRAL search strategy 2
| #1 | MeSH descriptor Venous Thrombosis explode all trees |
| #2 | MeSH descriptor Venous Thromboembolism explode all trees |
| #3 | MeSH descriptor Thrombosis explode all trees |
| #4 | MeSH descriptor Pulmonary Embolism explode all trees |
| #5 | thrombol* or thrombot* or thrombu* or thromboemb* |
| #6 | DVT or deep near (vein* or ven*) near thromb* |
| #7 | PE or Pulmonary near emb* |
| #8 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) |
| #9 | MeSH descriptor Anticoagulants explode all trees |
| #10 | anticoagulant* or unfractionated hep* or UFH or low molecular weight hep* or LMWH or warfarin |
| #11 | (#9 OR #10) |
| #12 | MeSH descriptor Placebos explode all trees |
| #13 | placebo* |
| #14 | (#12 OR #13) |
| #15 | (#8 AND #11 AND #14) |
Data and analyses
Comparison 1. Anticoagulant vs Phenylbutazone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fatal PE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Non‐fatal PE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Recurrent DVT/DVT extension (total) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Recurrent DVT/DVT extension (symptomatic) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Major hemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Fatal hemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 All‐cause mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 30 day venography ‐ distal veins ‐ progression or new lesions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Distal veins ‐ unchanged | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Distal veins ‐ regression | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Proximal veins ‐ progression or new lesions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Proximal veins ‐ unchanged | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 Proximal veins ‐ regression | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 V/Q at 10 days ‐ progression or new lesions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15 V/Q at 10 days ‐ unchanged | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16 V/Q at 10 days ‐ regression | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17 V/Q at 60 days ‐ progression or new lesions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18 V/Q at 60 days ‐ unchanged | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19 V/Q at 60 days ‐ regression | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20 Progression or new DVT at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21 Progression or new PE at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22 Progression or new VTE at 3 months ‐ DVT and PE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
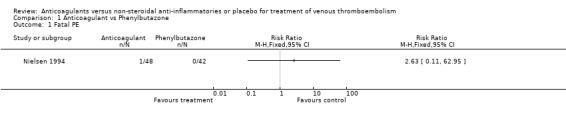
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 1 Fatal PE.
1.2. Analysis.
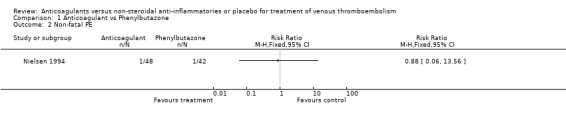
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 2 Non‐fatal PE.
1.3. Analysis.
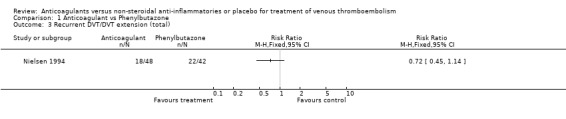
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 3 Recurrent DVT/DVT extension (total).
1.4. Analysis.
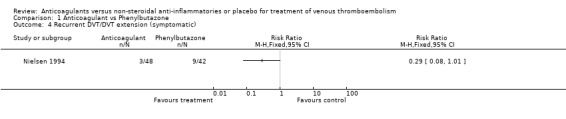
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 4 Recurrent DVT/DVT extension (symptomatic).
1.5. Analysis.
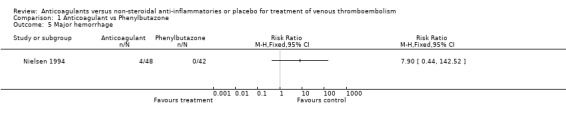
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 5 Major hemorrhage.
1.6. Analysis.
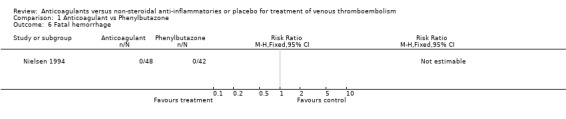
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 6 Fatal hemorrhage.
1.7. Analysis.
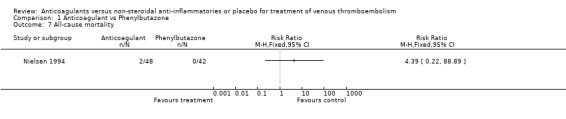
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 7 All‐cause mortality.
1.8. Analysis.
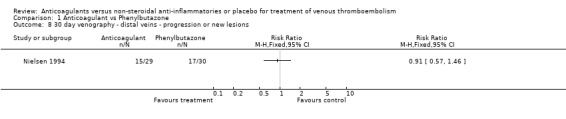
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 8 30 day venography ‐ distal veins ‐ progression or new lesions.
1.9. Analysis.
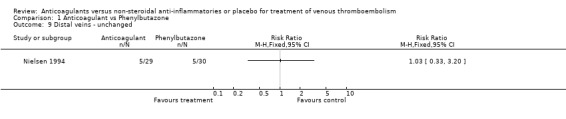
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 9 Distal veins ‐ unchanged.
1.10. Analysis.
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 10 Distal veins ‐ regression.
1.11. Analysis.
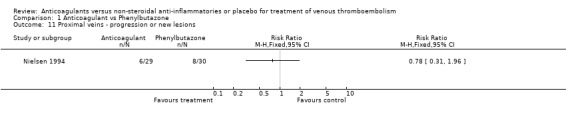
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 11 Proximal veins ‐ progression or new lesions.
1.12. Analysis.
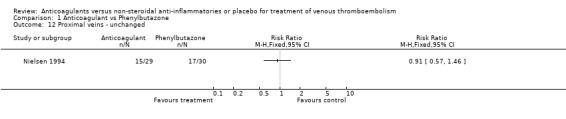
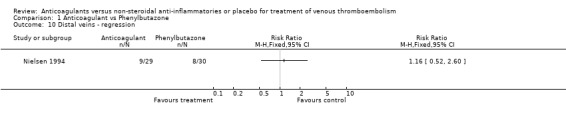
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 12 Proximal veins ‐ unchanged.
1.13. Analysis.
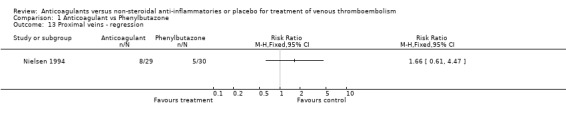
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 13 Proximal veins ‐ regression.
1.14. Analysis.
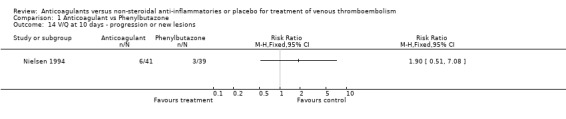
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 14 V/Q at 10 days ‐ progression or new lesions.
1.15. Analysis.
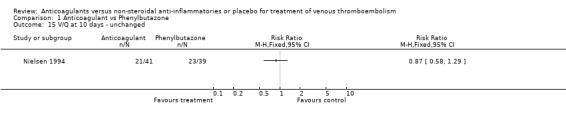
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 15 V/Q at 10 days ‐ unchanged.
1.16. Analysis.
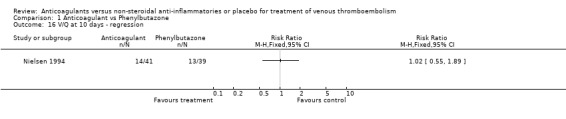
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 16 V/Q at 10 days ‐ regression.
1.17. Analysis.
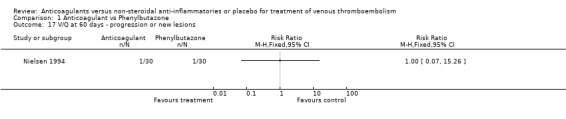
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 17 V/Q at 60 days ‐ progression or new lesions.
1.18. Analysis.
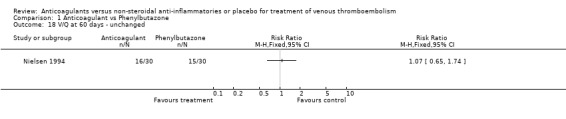
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 18 V/Q at 60 days ‐ unchanged.
1.19. Analysis.
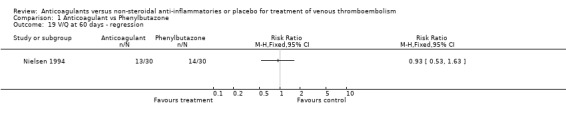
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 19 V/Q at 60 days ‐ regression.
1.20. Analysis.
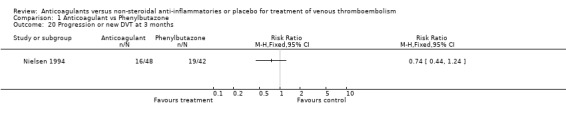
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 20 Progression or new DVT at 3 months.
1.21. Analysis.
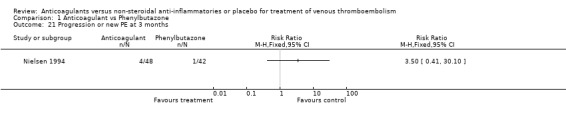
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 21 Progression or new PE at 3 months.
1.22. Analysis.
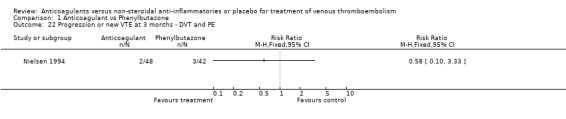
Comparison 1 Anticoagulant vs Phenylbutazone, Outcome 22 Progression or new VTE at 3 months ‐ DVT and PE.
Comparison 2. Anticoagulant vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fatal PE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Non‐fatal PE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Lung emboli / progression of DVT / return of DVT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Major hemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Fatal hemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 All‐cause mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
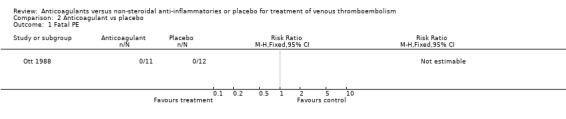
Comparison 2 Anticoagulant vs placebo, Outcome 1 Fatal PE.
2.2. Analysis.
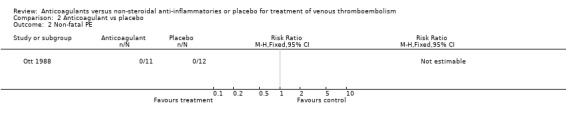
Comparison 2 Anticoagulant vs placebo, Outcome 2 Non‐fatal PE.
2.3. Analysis.
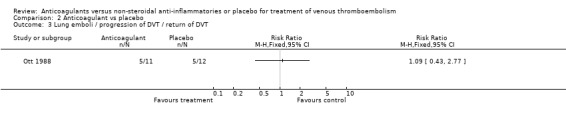
Comparison 2 Anticoagulant vs placebo, Outcome 3 Lung emboli / progression of DVT / return of DVT.
2.4. Analysis.
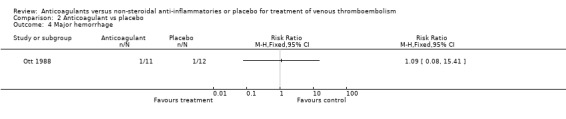
Comparison 2 Anticoagulant vs placebo, Outcome 4 Major hemorrhage.
2.5. Analysis.
Comparison 2 Anticoagulant vs placebo, Outcome 5 Fatal hemorrhage.
2.6. Analysis.
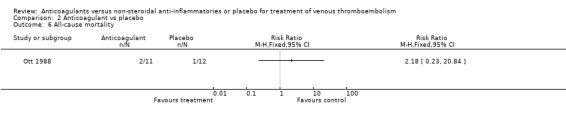
Comparison 2 Anticoagulant vs placebo, Outcome 6 All‐cause mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Nielsen 1994.
| Methods | Study design: randomized controlled trial using sealed envelopes. | |
| Participants | Country: Denmark. Setting: hospital. No: 90 patients; treatment group 48; control group 42. Age: treatment group (median) 57 years (range) 17 to 84; control group (median) 57 years (range) 20 to 82. Sex: treatment group males 30, females 18; control group males 28, females 14. Inclusion criteria: clinical signs of DVT; DVT confirmed by venogram; symptoms less than 6 days; no symptoms of PE. Exclusion criteria: clinical symptoms of PE, unable to be actively mobilized. |
|
| Interventions | Treatment group (48): heparin and phenprocoumon (Marcoumar, Roche). Control group (42): phenylbutazone (a non‐steroidal anti‐inflammatory drug). Duration: 3 months. |
|
| Outcomes | Results were assessed while on treatment and after one year. Primary outcomes: VTE on the basis of venogram at 30 days and lung scans at days 10 and 60. These were fairly objective measures of the outcomes of interest and observer bias was minimised by blinding the radiologists who interpreted the venograms and lung scans to the clinical signs and treatment allocation. Secondary outcomes: DVT recurrence and PE symptoms within 3 months and death within 12 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ott 1988.
| Methods | Study design: double blind randomized placebo‐ controlled trial. Method of randomization not stated. | |
| Participants | Country: Denmark. Setting: hospital, primarily geriatric and rehabilitation hospital. No: 23 patients; treatment group 11; control group 12. Age: treatment group 74 years (range) 64 to 78; control group 70.5 (range) 60 to 79. Sex: treatment group males 4, females 7; control group males 5, females 7. Inclusion criteria: DVT confirmed by venogram; age 60 to 80 years; symptoms less than or equal to 14 days; no contraindications to anticoagulants; able to be mobilized; willing to participate. Exclusion criteria: indication for fibrinolytic treatment; symptoms longer than 2 weeks; clinical symptoms of PE; contraindications to anticoagulants; absence of informed consent. |
|
| Interventions | Treatment group (11):anticoagulants (s.c. heparin followed by oral warfarin). Control group (12): s.c. saline followed by oral placebo tablets. |
|
| Outcomes | The main outcomes reported were signs of venous insufficiency and deaths. Data on fatal and nonfatal DVT/PE and major hemorrhagic events were not available in the translation. The main limitation of the study is the high rate of loss to follow up post randomization, which precludes intention‐to‐treat analysis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
DVT: deep vein thrombosis s.c.: subcutaneous PE: pulmonary embolism VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barritt 1960 | No lung scan or angiographic verification of PE. |
| Brandjes 1992 | No placebo or NSAID control group. |
| Butterman 1977 | DVT prophylaxis study. |
| Crowther 2000 | Condition of interest was warfarin‐induced coagulopathy, not venous thromboembolism |
| Kakkar 1968 | Abstract. Twenty one patients were randomized into three groups and received streptokinase, heparin or arvin. An additional six patients were 'untreated' but were not included in the original randomization scheme. |
| Kakkar 1969 | Anticoagulant versus thrombolysis. Full report of Kakkar 1968. |
| Lagerstedt 1985 | Both comparison groups received anticoagulants in the initial phase. |
| Levine 1993 | Abstract. Both groups received anticoagulants in initial phase. |
| Levine 1995 | Both comparison groups received anticoagulants in the initial phase. Full report of Levine 1993 |
| Moriau 1995 | Clinically relevant outcomes of DVT, PE or death not reported. Interventions under comparison were vitamin K antagonists versus vitamin K antagonists plus antiplatelet agent. |
| Schondorf 1975 | DVT prophylaxis study. |
DVT: deep vein thrombosis NSAID: non‐steroidal anti‐inflammatory PE: pulmonary embolism
Contributions of authors
DKC proposed the review, collaborated with JM to determine the objectives and methodology, assessed the titles and abstracts of all reports of trials, contacted anticoagulant researchers and pharmaceutical companies to find information concerning randomized venous thromboembolism (VTE) trials, assessed the methodological quality of each trial, extracted data, and wrote the first draft of the text.
JM collaborated with DKC on the objectives and methodology and devised the search strategy, assessed the titles and abstracts of all reports of trials, assessed the methodological quality of each trial, extracted data, and edited and revised the text.
JCP determined the design characteristics of a proposed randomised trial of VTE therapy and wrote the implications for research.
Sources of support
Internal sources
No sources of support supplied
External sources
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
Declarations of interest
DKC withdrew warfarin from a patient with lower limb deep vein thrombosis on the grounds that the risk of bleeding in this case seemed to be higher than the benefit of anticoagulant treatment. The patient later died of pulmonary embolism, and DKC subsequently lost his medical license because of this case.
JM participated in this review purely out of interest in the subject. She learnt of the title independently through an advertisement by the Cochrane Peripheral Vascular Diseases Group for a co‐author. JM was then introduced to DKC by the Cochrane Peripheral Vascular Diseases Review Group Co‐ordinator. Therefore, JM has had no connection or involvement with DKC except discussing this review by e‐mail. JM's interest and commitment was to apply, without bias, Cochrane techniques and methodology in to carry out this systematic review in order to achieve accurate and objective results.
JCP has no competing interests.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Nielsen 1994 {published data only}
- Nielsen HK, Husted SE, Krusell L, Fasting H, Charles P, Hansen HH, et al. Anticoagulant therapy in deep venous thrombosis. A randomized controlled study. Thrombosis & Haemostasis 1985; Vol. 54, issue 1:233 Abstract No 01382. [DOI] [PubMed]
- Nielsen HK, Husted SE, Krusell LR, Fasting H, Charles P, Hansen HH. Silent pulmonary embolism in patients with deep venous thrombosis. Incidence and fate in a randomized, controlled trial of anticoagulation versus no anticoagulation. Journal of Internal Medicine 1994;235(5):457‐61. [DOI] [PubMed] [Google Scholar]
- Nielsen HK, Husted SE, Krusell LR, Fasting H, Charles P, Hansen HH, et al. Anticoagulant therapy in deep venous thrombosis. A randomized controlled study. Thrombosis Research 1994;73(3‐4):215‐26. [PMID: 8191414] [DOI] [PubMed] [Google Scholar]
Ott 1988 {published data only}
- Ott P, Eldrup E, Oxholm P. Value of anticoagulant therapy in deep venous thrombosis in the lower limb in elderly, mobilized patients. A double‐blind, placebo controlled study with open therapeutic guidance [Vaerdien af antikoagulansbehandling ved dyb venos trombose i underekstremiten hos den aeldre, mobiliserede patient]. Ugeskrift for Laeger 1988;150(4):218‐21. [PMID 3287734] [PubMed] [Google Scholar]
References to studies excluded from this review
Barritt 1960 {published data only}
- Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. The Lancet 1960;1:1309‐12. [DOI] [PubMed] [Google Scholar]
Brandjes 1992 {published data only}
- Brandjes DP, Heijboer H, Buller HR, Rijk H, Jagt M, Cate JW. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal‐vein thrombosis. New England Journal of Medicine 1992;327(21):1485‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Butterman 1977 {published data only}
- Buttermann G, Theisinger W, Weidenbach A, Hartung R, Welzel D, Pabst HW. Quantitative assessment of drug‐induced prophylaxis of postoperative thromboembolism. Comparison of frequencies of deep vein thrombosis and pulmonary embolism using acetylsalicylic‐acid, dextran, dihydroergotamine, low‐dose heparin and the fixed combination of heparin and dihydroergotamine (author's transl) [German] [Quantitative Bewertung der postoperativen Thromboembolieprophylaxe. Vergleichende Untersuchungen uber Thrombose‐ und Emboliehaufigkeiten unter Acetylsalicylsaure, Dextran, Dihydroergotamin, Heparin sowie der fixen Kombination von Heparin und Dihydroergotamin]. Medizinische Klinik 1977;72(40):1624‐38. [PubMed] [Google Scholar]
Crowther 2000 {published data only}
- Crowther MA, Julian J, McCarty D, Douketis J, Kovacs M, Biagoni L, et al. Treatment of warfarin‐associated coagulopathy with oral vitamin K: a randomised controlled trial. Lancet 2000;356(9241):1551‐3. [DOI] [PubMed] [Google Scholar]
Kakkar 1968 {published data only}
- Kakkar VV, Flanc C, O'Shea M, Flute P, Howe CT, Clarke MB. Treatment of deep‐vein thrombosis‐‐a random trial. British Journal of Surgery 1968;55(11):858. [PMID: 4879792] [PubMed] [Google Scholar]
Kakkar 1969 {published data only}
- Kakkar VV, Flanc C, Howe CT, O'Shea M, Flute PT. Treatment of deep vein thrombosis. A trial of heparin, streptokinase, and arvin. British Medical Journal 1969;1(647):806‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lagerstedt 1985 {published data only}
- Lagerstedt CI, Olsson CG, Fagher BO, Oqvist BW, Albrechtsson U. Need for long‐term anticoagulant treatment in symptomatic calf‐vein thrombosis. Lancet 1985;2(8454):515‐8. [DOI] [PubMed] [Google Scholar]
Levine 1993 {published data only}
- Levine MN, Hrish J, Gent M, Turpie AG, Weitz J, Geerts W, et al. Optimal duration of oral anticoagulant therapy: a randomized trial comparing four weeks with three months of warfarin in patients with proximal deep vein thrombosis. Thrombosis & Haemostasis 1993;69(6):982 Abstract No 1581. [PubMed] [Google Scholar]
Levine 1995 {published data only}
- Levine MN, Hirsh J, Gent M, Turpie AG, Weitz J, Ginsburg J, et al. Optimal duration of oral anticoagulant therapy: a randomized trial comparing four weeks with three months of warfarin in patients with proximal deep vein thrombosis. Thrombosis & Haemostasis 1995;74(2):606‐11. [PubMed] [Google Scholar]
Moriau 1995 {published data only}
- Moriau M, Lavenne‐Pardonge E, Crasborn L, Frenckell R, Col‐Debeys C. The treatment of severe or recurrent deep venous thrombosis. Beneficial effect of the co‐administration of antiplatelet agents with or without rheological effects, and anticoagulants. Thrombosis Research 1995;78(6):469‐82. [DOI] [PubMed] [Google Scholar]
Schondorf 1975 {published data only}
- Schondorf TH, Hey D. Effectiveness of subcutaneous heparin in the prevention of thrombo‐embolism after major hip operations (author's transl) [German]. Deutsche Medizinische Wochenschrift 1975;100(40):2014‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Cochrane Handbook
- Clarke M, Oxman AD, editors. Assessment of study quality. Cochrane Reviewers' Handbook 4.1.2 [updated March 2001]; Section 6. In: The Cochrane Library [database on CDROM]. The Cochrane Collaboration. Oxford: Update Software. 2001, Issue 2.
Ginsberg 1996
- Ginsberg JS. Management of venous thromboembolism [Review]. New England Journal of Medicine 1996;335(24):1816‐28. [DOI] [PubMed] [Google Scholar]
Gould 1999
- Gould MK, Dembitzer, AD, Doyle RL, Hastie TJ, Garber AM. Low‐molecular‐weight‐heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis: A meta‐analysis of randomized, controlled trials. Annals of Internal Medicine 1999;130(10):800‐9. [DOI] [PubMed] [Google Scholar]
Hansson 1997
- Hansson PO, Welin L, Tibblin G, Eriksson H. Deep vein thrombosis and pulmonary embolism in the general population. 'The Study of Men Born in 1913'. Archives of Internal Medicine 1997;157(15):1665‐70. [PubMed] [Google Scholar]
Hull 1986
- Hull RD, Raskob GE, Hirsh J. The diagnosis of clinically suspected pulmonary embolism. Practical approaches. Chest 1986;89(5 Suppl):417S‐425S. [MEDLINE: ; NLM Unique identifier 3698720] [PubMed] [Google Scholar]
Nielsen 1985
Nielsen 1994a
- Nielsen HK, Husted SE, Krusell LR, Fasting H, Charles P, Hansen HH, et al. Anticoagulant therapy in deep venous thrombosis. A randomized controlled study. Thrombosis Research 1994;73(3‐4):215‐26. [PMID: 8191414] [DOI] [PubMed] [Google Scholar]
Nielsen 1994b
- Nielsen HK, Husted SE, Krusell LR, Fasting H, Charles P, Hansen HH. Silent pulmonary embolism in patients with deep venous thrombosis. Incidence and fate in a randomized, controlled trial of anticoagulation versus no anticoagulation. Journal of Internal Medicine 1994;235(5):457‐61. [DOI] [PubMed] [Google Scholar]
Nordstrom 1992
- Nordstrom M, Lindblad B, Bergqvist D, Kjellstrom T. A prospective study of the incidence of deep‐vein thrombosis within a defined urban population. Journal of Internal Medicine 1992;232(2):155‐60. [MEDLINE: ; NLM Unique identifier 1506812] [DOI] [PubMed] [Google Scholar]
Pioped 1990
- Anonymous. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). The PIOPED Investigators. JAMA 1990;263(20):2753‐9. [DOI] [PubMed] [Google Scholar]
Quorum
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [DOI] [PubMed] [Google Scholar]
Rubinstein 1988
- Rubinstein I, Murray D, Hoffstein V. Fatal pulmonary emboli in hospitalized patients. An autopsy study. Archives of Internal Medicine 1988;148(6):1425‐6. [PubMed] [Google Scholar]


