Abstract
Background
Bone marrow stromal cells (BMSC) have promise in cartilage tissue engineering, but for their potential to be fully realised, the propensity to undergo hypertrophy must be mitigated. The literature contains diverging reports on the effect of parathyroid hormone (PTH) on BMSC differentiation. Cartilage tissue models can be heterogeneous, confounding efforts to improve media formulations.
Methods
Herein, we use a novel microwell platform (the Microwell-mesh) to manufacture hundreds of small-diameter homogeneous micro-pellets and use this high-resolution assay to quantify the influence of constant or intermittent PTH(1–34) medium supplementation on BMSC chondrogenesis and hypertrophy. Micro-pellets were manufactured from 5000 BMSC each and cultured in standard chondrogenic media supplemented with (1) no PTH, (2) intermittent PTH, or (3) constant PTH.
Results
Relative to control chondrogenic cultures, BMSC micro-pellets exposed to intermittent PTH had reduced hypertrophic gene expression following 1 week of culture, but this was accompanied by a loss in chondrogenesis by the second week of culture. Constant PTH treatment was detrimental to chondrogenic culture.
Conclusions
This study provides further clarity on the role of PTH on chondrogenic differentiation in vitro and suggests that while PTH may mitigate BMSC hypertrophy, it does so at the expense of chondrogenesis.
Keywords: Mesenchymal stem cell, Chondrogenesis, Hypertrophy, Parathyroid hormone, Microwell
Background
Articular cartilage is an avascular, aneural tissue with limited regenerative potential. This has motivated significant investment into cell-based therapies with potential to repair cartilage defects and delay or prevent osteoarthritis. Bone marrow stromal cells (BMSC, also known as mesenchymal stem cells) have emerged as a promising cell source for cartilage defect repair [1]. However, while BMSC appear to be able to form chondrocyte-like cells in vitro, these cells appear to engage an intrinsic endochondral differentiation program and yield mineralised hypertrophic tissue when implanted in vivo [2]. The search for compounds that mitigate BMSC hypertrophy is an active area of research, previously reviewed here [2, 3].
Parathyroid hormone (PTH) [4, 5] and its homologue, PTH-related protein (PTHrP) [6], are candidate molecules that may be useful to mitigate BMSC hypertrophy. PTH and PTHrP are expressed in different tissues, but share an amino-terminal sequence that binds and signals through a common G-protein coupled receptor, PTH/PTHrP type 1 receptor (PTH1R) [7]. As a result, an active 34-amino acid fragment of the PTH/PTHrP amino-terminus, PTH(1–34), is often used in studies. PTH1R is found on the surface of a variety of cell types including developing and mature chondrocytes and osteoblasts [8–10]. In previous studies with chondrocytes, PTH signalling was shown to increase type II collagen synthesis [11] and proteoglycan synthesis [11–16], while others, paradoxically, showed that PTH inhibited type II collagen synthesis [4, 17, 18]. In mesenchymal progenitor cell lines (C3H10T1/2), PTH appeared to play different roles during different stages of chondrogenesis, enhancing early chondrogenic differentiation while suppressing the late stages of chondrogenic maturation and osteogenesis [19]. In rats, PTH significantly increased aggrecan levels in chondrocytes isolated from day 17 and 18 embryos, but no increase was observed in chondrocytes harvested from embryos at day 20 or 21 of gestation [16]. These observations suggest that the stage of chondrogenesis may be important for PTH signalling outcomes.
PTH has been explored in BMSC differentiation cultures with varying results. Chen et al. reported that PTH-induced rabbit BMSC chondrogenesis resulted in better repair of articular cartilage injury in rabbits, compared with non-PTH-treated BMSC [20]. Zhang et al. observed a dose-dependent effect, whereby lower concentrations (1–10 nM) of PTH promoted BMSC chondrogenesis, while higher concentrations (> 100 nM) suppressed matrix production [18]. The timing of PTH dosing has also been reported to influence BMSC differentiation. In mildly adipogenic cultures, intermittent PTH treatment inhibited human BMSC adipogenesis, while constant PTH treatment did not [21]. Intermittent PTH treatment suppressed rat BMSC osteogenesis, while constant PTH treatment promoted osteogenesis [22]. Using a single injection in mice [6] and intermittent injection in humans [10], PTH was shown to improve osteogenic differentiation of BMSC, which may function through a mechanism that enhances BMP signalling [6]. In chondrogenic human BMSC pellet cultures, intermittent PTH treatment appeared to restore chondrogenic differentiation that was lost in cultures treated with constant PTH, also leading to reduced Indian hedgehog (IHH) expression and alkaline phosphatase (ALP) activity [23]. These findings suggest that a PTH treatment regimen may be important in controlling the differentiation of BMSC.
Traditional BMSC chondrogenic pellet cultures typically generate tissues that are physically large (2–3 mm diameter), causing steep diffusion gradients and result in heterogeneous tissue formation [24, 25]. Heterogeneous tissue can confound the study of subtle differences in differentiation outcomes [24, 25]. In these large diameter tissues, differentiation occurs at different rates depending on the spatial location of cells within the tissue. In previous work, we developed the Microwell-mesh [24], a high-throughput microwell platform that enables the manufacture of hundreds of homogeneous cartilage micro-pellets (sometimes referred to as microtissues) and maintains these micro-pellets in individual microwells over extended culture periods. As a result of their smaller size (0.5–1 mm diameter), relative to traditional pellet cultures, micro-pellets are more sensitive to differentiation signalling factors [26].
In this study, we used the Microwell-mesh platform to manufacture uniform BMSC micro-pellets and cultured them under three conditions: (1) control (chondrogenic medium with no PTH), (2) intermittent PTH (2.5 nM PTH(1–34), 6-h treatment every 48 h), and (3) constant PTH (2.5 nM PTH(1–34), continuous treatment, re-supplemented every 48 h). BMSC chondrogenesis was evaluated at 7 and 14 days by quantifying micro-pellet and medium glycosaminoglycan (GAG) content, micro-pellet histology, and chondrogenic/hypertrophic gene expression. Using this more sensitive micro-pellet culture approach, we aimed to clarify the influence of PTH on BMSC in vitro chondrogenesis.
Methods
BMSC isolation and culture
BMSC isolation and characterisation were performed as previously described [25]. Briefly, 20 mL of bone marrow aspirate was collected from the iliac crest of fully informed and consenting healthy adult donors. The collection was approved by the Mater Misericordiae Ltd. Human Research Ethics Committee (EC00332) and the Queensland University of Technology Human Ethics Committee (EC00171, 1000000938). The bone marrow aspirate was diluted 1:1 with 2 mM EDTA/PBS and overlaid onto 15 mL of Ficoll-Paque PLUS (GE Healthcare). The tubes were centrifuged for 30 min at 400×g, and interface cells were collected, washed, and resuspended in low glucose Dulbecco’s modified Eagle’s medium (LG-DMEM; Thermo Fisher Scientific) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific), 10 ng/mL fibroblast growth factor-1 (FGF-1; PeproTech), and 100 U/mL penicillin/streptomycin (PenStrep; Thermo Fisher Scientific). Cells were seeded in T175 cm2 flasks (Nunc) and incubated overnight in a 20% O2 and 5% CO2 incubator at 37 °C. Media were exchanged the following day to enrich for plastic-adherent cells. Cells were subsequently grown in 2% O2 and 5% CO2 at 37 °C. When the cells reached 80–90% confluence, they were passaged using 0.25% Trypsin/EDTA (Thermo Fisher Scientific) and re-seeded at 1500 cells/cm2. All experiments were carried out with three different BMSC donors, referred to as donors 1, 2, and 3.
Fabrication and preparation of the Microwell-Mesh system
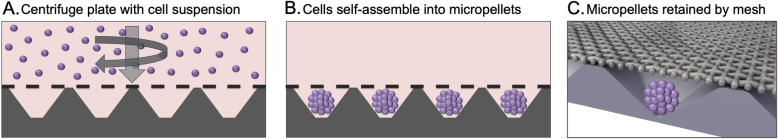
Figure 1 provides a schematic of how the Microwell-mesh enables the rapid manufacture and culture of hundreds of uniform micro-pellets. The process of fabricating the Microwell-mesh was detailed previously by our laboratory [24]. In brief, degassed polydimethylsiloxane polymer (PDMS, SYLGARD 184 Silicone Elastomer Kit; 10:1 polymer to crosslinker) was poured into a microwell mould and permitted to cure for 60 min at 80 °C. The resultant PDMS sheets had an array of wells measuring 2 mm × 2 mm with a depth of 0.8 mm. Priory wad punches (Amazon.com) were used to punch discs from the sheets, which fit snugly into tissue culture plate wells. A nylon mesh (36 μm square pore openings, part number CMN-0035; Amazon.com) was bound to the open face of PDMS discs with silicone glue (Selleys Aquarium Safe). This mesh pore size is large enough to allow single cells through, but small enough to entrap aggregated cell micro-pellets within discrete microwells. Inserts were secured to tissue culture plastic with a small dab of silicone glue. Sterilisation was carried out with 70% ethanol. To ensure efficient contact of all surfaces and removal of any air bubbles, 4 mL of 70% ethanol solution was added to each well and centrifuged for 5 min at 2000×g. Plates were then submerged in 70% ethanol for 1 h at room temperature, followed by 3 washes with sterile phosphate-buffered saline (PBS; Thermo Fisher Scientific). PBS was left in wells overnight to elute residual ethanol. Before use, inserts were washed with a sterile 5% Pluronic solution (F-127 Pluronic; Sigma-Aldrich) in PBS. Pluronic renders the PDMS surface non-adhesive and promotes cell aggregation [27]. The plates were centrifuged for 5 min at 2000×g upon addition of Pluronic solution. Wells were rinsed three times with PBS, and the plates were ready for use in cell culture.
Fig. 1.
The Microwell-mesh enables rapid manufacture of hundreds of uniform micro-pellets, simultaneously. a Using a plate centrifuge, BMSC are pelleted through the porous mesh into individual microwells. b BMSC self-assemble into micro-pellets after a few hours of culture. c The nylon mesh retains individual micro-pellets in discrete microwells over the culture period. Image C was slightly modified from an image originally provided by abpLearning (medical-animations.com, Australia and [26]) using SoftImage (Autodesk, Montreal, Canada) and gifted to the Doran Laboratory
Chondrogenic induction media
To induce chondrogenesis, BMSC were resuspended in chondrogenic medium composed of high glucose DMEM (HG-DMEM; Thermo Fisher Scientific), 1X GlutaMax (Thermo Fisher Scientific), 10 ng/mL transforming growth factor-beta 1 (TGF-β1, PeproTech), 100 nM dexamethasone (Sigma-Aldrich), 200 μM ascorbic acid 2-phosphate (Sigma-Aldrich), 100 μM sodium pyruvate (Thermo Fisher Scientific), 40 μg/mL L-proline (Sigma-Aldrich), 1% insulin-transferrin-selenium (ITS-X, Thermo Fisher Scientific), and 100 U/mL PenStrep (Thermo Fisher Scientific). In preliminary studies, we trialled constant PTH(1–34) (Sigma-Aldrich) at a concentration of 0, 1, 10, or 100 nM, captured microscopic images, performed histology, and live/dead viability staining (Thermo Fisher Scientific). We noted that continuously treated micro-pellets were reduced in size at all PTH concentrations, but appeared to have similar GAG staining (Alcian blue). Following this trial, all subsequent experiments were conducted with 2.5 nM PTH, as this concentration was used in previous work by Fischer et al. [23]. Cultures were maintained for 14 days, with a full medium exchange performed every 2 days. Three different PTH exposure protocols were compared: (1) control (chondrogenic medium with no PTH); (2) intermittent PTH with 2.5 nM PTH(1–34) added 6 h prior to media exchange, performed every 48 h; and (3) constant PTH supplementation with 2.5 nM PTH(1–34) replenished during each 48-h media exchange.
Assembly of Micro-pellets within the Microwell-Mesh platform
To prepare plates for seeding, 1 mL of chondrogenic induction medium was added to each well, and Microwell-mesh plates were centrifuged for 5 min at 2000×g to eliminate any air bubbles from the microwells. Experiments were performed in 6-well plates, and each well was seeded with 1.2 × 106 cells, yielding 5000 cells per microwell. BMSC were centrifuged for 5 min at 500×g to pellet the cells into microwells. Cultures were maintained at 2% O2 and 5% CO2 in a 37 °C incubator and media exchanged every other day (or 48 h). Our group previously showed that BMSC chondrogenesis was improved at 2% O2 [24, 25]. At the time of harvest, the mesh was removed from the Microwell-mesh disc with forceps and the micro-pellets were collected with a wide pipette tip. Aliquots of medium were collected at each medium exchange and stored at − 30 °C for later GAG quantification.
Glycosaminoglycan and DNA quantification
Micro-pellets were digested overnight in papain (1.6 U/mL, Sigma-Aldrich) in a 60 °C water bath. Digests were vortexed briefly and used for GAG and DNA quantification. The 1,9-dimethylmethylene blue (DMMB, Sigma-Aldrich) assay was used to quantify GAG as described previously [25]. Chondroitin sulfate from shark cartilage (Sigma-Aldrich) was used to generate a standard curve. DNA content was determined using a Quant-iT PicoGreen dsDNA assay kit as per the manufacturer’s protocol (Thermo Fisher Scientific).
Quantitative polymerase chain reaction
Micro-pellets were washed in PBS and stored in TRIzol (Thermo Fisher Scientific) at − 80 °C. RNA isolation was completed as per the manufacturer’s protocol followed by DNase I digest. RNA was quantified using a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific). RNA (1000 ng) was reverse-transcribed using the SuperScript III First-Strand Synthesis System for qPCR (Thermo Fisher Scientific) as described by the manufacturer. The master mix included 2X SYBR Green PCR Master Mix (Applied Biosystems), 200 nM of the forward and reverse primers, RNase-free water, and 1 μL of sample cDNA. The reactions were run in triplicate using 5 μL per well in a 384-well plate inside a Viia7 Real-Time PCR System (Applied Biosystems). The initial cycle was 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. A melt curve analysis was used to confirm the specificity of products. Gene expression data sets were analysed for statistically significant differences between conditions at each time using ANOVA. Primer set information is given in Table 1. Primers for ACAN, COL1A1, COL2A1, and BGLAP/PB-OST [28]; RPLP0 and COL10A1 [29]; RUNX2 [30]; and IHH [31] were as previously published in the literature. Primers for SOX9 were designed using Primer3Plus software [32]. RPLP0 was found to be stable across conditions and was used to standardise relative expression of chondrogenic, osteogenic, and hypertrophy genes.
Table 1.
Primers used for qPCR of human genes
| Gene | Sequence (5′ to 3′) | Amplicon size (bp) |
|---|---|---|
| ACAN |
F: TCGAGGACAGCGAGGCC R: TCGAGGGTGTAGCGTGTAGAGA |
85 |
| COL1A1 |
F: CAGCCGCTTCACCTACAGC R: TTTTGTATTCAATCACTGTCTTGCC |
83 |
| COL2A1 |
F: GGCAATAGCAGGTTCACGTACA R: CGATAACAGTCTTGCCCCACTT |
79 |
| RPLP0 |
F: TGTGGGCTCCAAGCAGATGCA R: GCAGCAGTTTCTCCAGAGCTGGG |
137 |
| COL10A1 |
F: ACTCCCAGCACGCAGAATCCA R: TGGGCCTTTTATGCCTGTGGGC |
132 |
| RUNX2 |
F: GGAGTGGACGAGGCAAGAGTTT R: AGCTTCTGTCTGTGCCTTCTGG |
133 |
| SOX9 |
F: ACTCCTCCTCCGGCATGAG R: GCTGCACGTCGGTTTTGG |
102 |
| IHH |
F: ATGAAGGCAAGATCGCTCG R: GATAGCCAGCGAGTTCAGG |
149 |
| BGLAP/PB-OST |
F: GAAGCCCAGCGGTGCA R: CACTACCTCGCTGCCCTCC |
70 |
Histology and immunohistochemistry
Micro-pellets were harvested at days 7 and 14 and fixed in 4% paraformaldehyde (PFA) for 15 min and frozen in Tissue-Tek OCT compound (Sakura Finetek). Samples were cryosectioned with a Leica Cryostat CM1850 (Leica) at 7 μm and collected onto poly-lysine-coated slides (Thermo Fisher Scientific) and stored in a freezer until further processing. The sectioned tissues were fixed for 15 min with 4% PFA and washed with PBS. Alcian blue staining was performed to visualise GAG present in the tissues. Sections were stained with 1% Alcian blue (Sigma-Aldrich) solution in 3% acetic acid (pH 2.5) for 30 min. Sections were rinsed with tap water and counterstained with Nuclear Fast Red for 5 min, rinsed with tap water, and mounted (CC/mount, Sigma-Aldrich) for imaging. Immunohistological staining was performed for collagen type I, type II, and type X. Sections were permeabilised for 5 min with 0.1% Triton X-100 and blocked for 30 min at room temperature (RT) with 10% normal goat serum (Invitrogen). Primary antibodies (Abcam) raised against collagen type I (1:800), type II (1:100), and type X (1:100) were diluted in 1% BSA/PBS-T (PBS-0.1% Tween-20), and sections were incubated with antibodies at 4 °C overnight. Sections were rinsed twice for 5 min with 0.025% Triton X-100/PBS. Slides were incubated with 0.3% H2O2 in 100% methanol for 15 min and rinsed twice with PBS. Sections were incubated for 60 min at room temperature with secondary antibodies, goat anti-rabbit IgG H&L (HRP; ab6721) or goat anti-mouse IgG H7L (HRP; ab97023; both 1:1000; Abcam), in 1% BSA/PBS, then washed twice with PBS. The DAB kit chromogen solution was applied for 8 min, and slides were rinsed thoroughly in water. Nuclei were stained with Nuclear Fast Red for 2 min and rinsed with water.
Data collection and analysis
Statistical analysis was performed using GraphPad Prism. Data were analysed using two-way ANOVA, and statistical significance was determined using Tukey’s test. Experiments were carried out with 3 unique biological donors. Results are represented as mean ± SD, n = 4, P < 0.05 unless otherwise noted.
Results
Intermittent and constant PTH treatment reduced Micro-pellet size
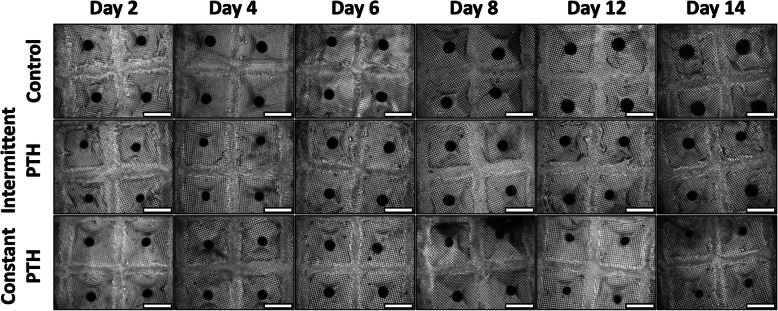
We initially tested micro-pellet induction cultures supplemented with constant PTH at concentrations of 0, 1, 10, and 100 nM. Microscopy imaging indicated that micro-pellet size was reduced with increasing concentrations of PTH; however, GAG staining was not affected and micro-pellets grown in continuous 2.5 nM PTH appeared viable (Supplementary Figure 1). Based on these data, subsequent experiments were completed with 2.5 nM PTH to maintain consistency with previous BMSC chondrogenic studies [23]. Following 14 days of chondrogenic induction culture, micro-pellets in the control (no PTH) condition increased in size over the culture period and were visibly larger than the intermittent and constant PTH treatment micro-pellets (Fig. 2). Micro-pellets exposed to intermittent PTH grew modestly in size over the 14-day culture, whereas micro-pellets in constant PTH culture grew until about 1 week and then appeared to decrease in size during the last week of culture.
Fig. 2.
Microscope images of micro-pellets in the microwell-mesh cultured over 14 days. Scale bar, 1 mm. Replicate images from BMSC donors 2 and 3 are shown in Supplementary Figures 2 and 3, respectively
Intermittent and constant PTH inhibit glycosaminoglycan production
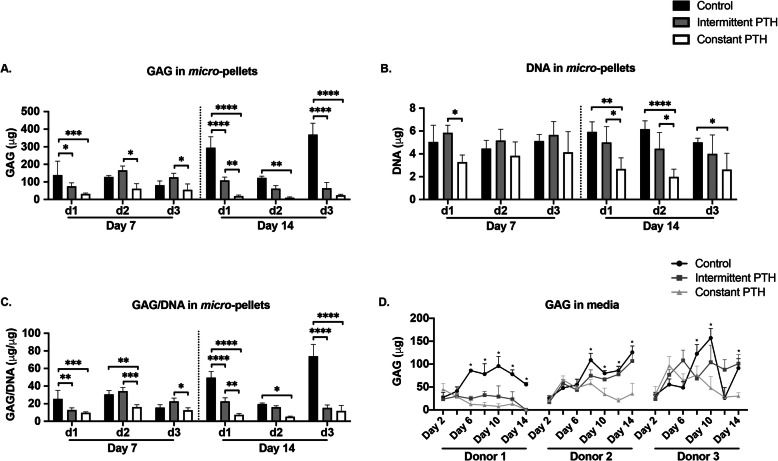
We compared the GAG and DNA content of micro-pellets in control, intermittent PTH, and constant PTH chondrogenic cultures (Fig. 3). At days 7 and 14, the GAG content in constant PTH micro-pellets was significantly lower than in control and intermittent PTH micro-pellets (Fig. 3a). At day 14, both intermittent PTH and constant PTH micro-pellets had reduced GAG content when compared with control micro-pellets, and this was statistically significant for 2 of 3 donors (Fig. 3a). DNA quantities were similar across conditions at day 7, while constant PTH micro-pellets had significantly less DNA than the control group at day 14 (Fig. 3b). When GAG was normalised to DNA, GAG/DNA was reduced following both intermittent PTH and constant PTH treatment, relative to control (Fig. 3c). Additionally, less GAG was present in the medium in both intermittent PTH and constant PTH cultures, relative to control cultures (Fig. 3d).
Fig. 3.
Quantification of GAG and DNA in micro-pellet cultures generated from three BMSC donors. a Quantities of GAG in micro-pellets at day 7 and day 14. b DNA quantities in micro-pellets at day 7 and day 14. c GAG normalised to DNA in micro-pellets. d Quantification of GAG secreted to the media by micro-pellets over a 14-day culture period. For a–c, data represent mean ± SD, n = 4, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. For d, data represent mean ± SD, n = 6, a single asterisk is used for all levels of significance when P < 0.05
Intermittent and constant PTH both inhibit extracellular matrix production
We analysed extracellular matrix makers associated with chondrogenesis (GAG and collagen II), hypertrophy (collagen X), and fibrogenesis (collagen I) histologically for control, intermittent PTH, and constant PTH conditions (Fig. 4). Alcian blue staining for GAG and immunohistochemistry staining for collagen II, X, and I were similar in micro-pellets from each condition on day 7, but notably reduced by constant PTH treatment on day 14 (Fig. 4). Neither intermittent PTH nor constant PTH improved BMSC chondrogenesis, relative to control, based on the staining of these markers.
Fig. 4.
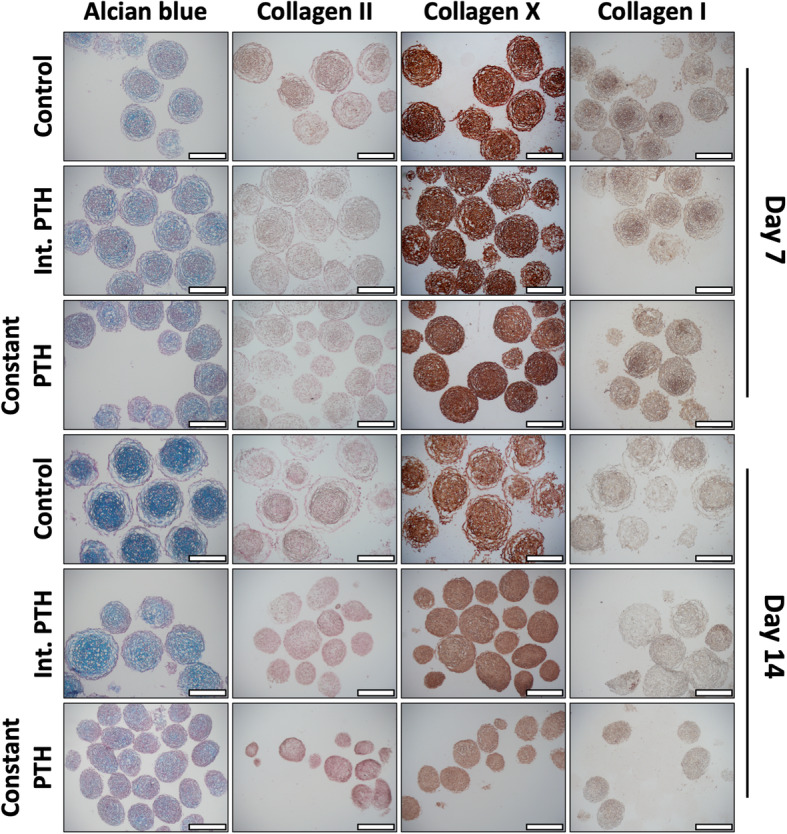
Micro-pellet sections stained for GAG (Alcian blue), collagen II, X, and I. Scale bar = 400 μm. Refer to Supplementary Figures 4 and 5 for replicate donor results
PTH decreased the expression of osteogenic, hypertrophic, and chondrogenic genes
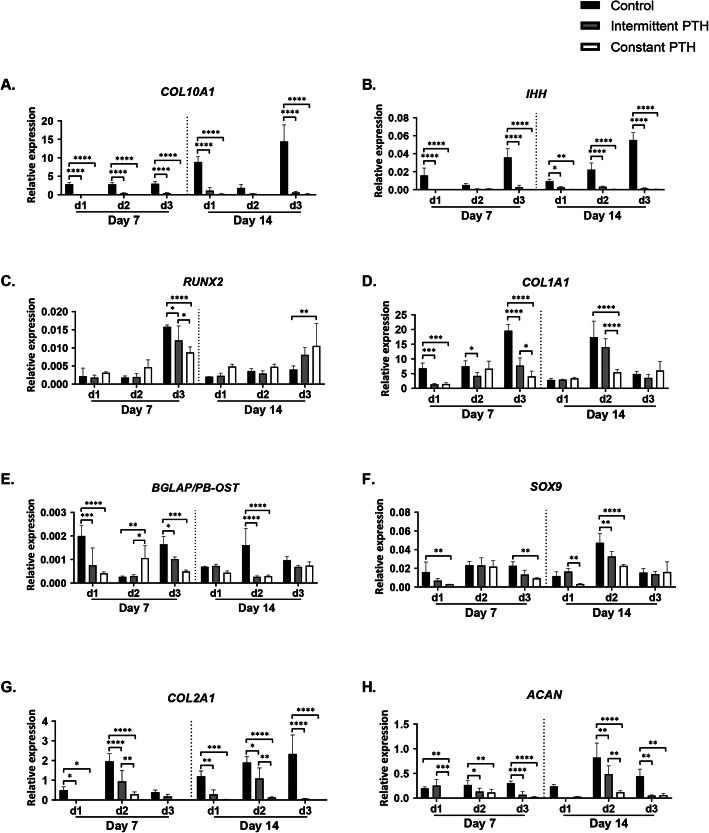
We assessed the expression of osteogenic, hypertrophic, and chondrogenic genes for control, intermittent PTH, and constant PTH conditions using qPCR (Fig. 5). PTH-treated micro-pellets had a decrease in hypertrophic marker expression (COL10A1 and IHH, Fig. 5a, b) at day 7 and day 14, compared with control. RUNX2, a master regulator of osteogenesis, expression was similar across conditions, although in one donor they were reduced at day 7 and increased at day 14 (Fig. 5c). PTH supplementation resulted in significantly lower levels of osteogenic markers COL1A1 and osteocalcin (BGLAP/PB-OST) in donors 1 and 3 at day 7, and donor 2 at day 14 (Fig. 5d, e). The expression of chondrogenic marker SOX9 (Fig. 5f) was generally similar between groups or reduced in the presence of PTH, while COL2A1 (Fig. 5g) and ACAN (Fig. 5h) were reduced by intermittent PTH or constant PTH, relative to control conditions without PTH. Overall, it appeared that both PTH conditions suppressed the undesirable expression of hypertrophic and osteogenic genes, as well as the desirable chondrogenic genes.
Fig. 5.
qPCR analysis of micro-pellets generated from three BMSC donors. Relative gene expression analysis of aCOL10A1, bIHH, cRUNX2, dCOL1A1, eBGLAP/PB-OST, fSOX9, gCOL2A1, and hACAN. Micro-pellets supplemented with constant PTH demonstrated a decrease in hypertrophic and osteogenic markers that was accompanied by a decrease in chondrogenic markers SOX9, COL2A1, and ACAN (plotted as mean ± SD, n = 4, *P < 0.05)
Discussion
BMSC have shown potential as a cell source for cartilage tissue engineering. However, following BMSC chondrogenesis, hypertrophy occurs, yielding a bone-like tissue that is unsuitable for cartilage defect repair [2]. This hypertrophic process must be mitigated to allow the chondrocyte phenotype to persist, enabling these cells to produce and maintain a functional hyaline-like cartilage tissue. Many molecules have been investigated for their potential role in inhibiting hypertrophy (previously reviewed [2, 3]). PTH has previously been shown to inhibit the terminal differentiation of chondrocytes [5, 19], inhibit articular cartilage degeneration [33], and to promote the reversion of hypertrophic chondrocytes to a pre-hypertrophic proliferating phenotype [34]. PTH also reduced the expression of hypertrophy marker, collagen X, in BMSC from osteoarthritic patients [35]. Other studies suggest, however, that PTH inhibits type II collagen synthesis [4, 17, 18], an essential extracellular matrix component in articular cartilage. The utility of PTH in obstructing hypertrophy of BMSC cultured in chondrogenic induction medium in vitro remains unclear.
We posit that common large-diameter cartilage tissue models, which suffer large diffusion gradients and tissue heterogeneity, confound efforts to identify optimal BMSC chondrogenic induction protocols. In previous work, we demonstrated that it was possible to obtain more definitive insights into BMSC chondrogenesis, in vitro, if a more homogenous micro-pellet model system was used [24–26, 36]. In previous studies, it was reported that intermittent and constant PTH treatment of cells could result in profoundly alternate differentiation outcomes [18, 20–23]. In this study, we used a micro-pellet platform [24] with the aim of generating more definitive insights into how intermittent or constant PTH treatment influenced BMSC chondrogenesis and hypertrophy. We found that micro-pellets in chondrogenic differentiation media treated with intermittent PTH and constant PTH experienced a reduction in micro-pellet size by day 14, relative to control cultures, with the most drastic decrease observed with constant PTH. Based on histological evaluation, constant PTH showed a negative effect on chondrogenesis based on reduced staining of GAG and collagen II by day 14, while intermittent PTH appeared to have a lesser effect on these markers. A reduction in GAG production with either PTH treatment was also confirmed with GAG quantification assays. Based on gene expression, we found that both PTH treatments decreased the expression of hypertrophy markers COL10A1 and IHH, as well as osteogenic markers COL1A1 and osteocalcin (BGLAP/PB-OST). However, chondrogenic marker, COL2A1 and ACAN, expression also decreased. The expression of osteogenic transcription factor, RUNX2, and chondrogenic transcription factor, SOX9, was not drastically changed by PTH treatment conditions.
In Fischer et al. [23], prolonged exposure to PTHrP resulted in reduced cell proliferation at day 14. We similarly observe that constant PTH resulted in reduced DNA quantities compared to those found in control and intermittent PTH micro-pellets at day 14. To rule out cytotoxicity, we performed a live/dead assay on control and constant PTH micro-pellets. Both had strong green staining (live) with negligible red staining (dead), suggesting that PTH was not cytotoxic in our studies.
Unlike constant PTH, Fischer et al. [23] found evidence that intermittent PTHrP treatment might represent a potential means to improve chondrogenesis of BMSC. By contrast, the outcomes of our study do not support the use of intermittent or constant PTH treatment for improving BMSC chondrogenesis or inhibiting hypertrophy. While there are some differences in our culture methods [23], this study represents the closest study to our own, allowing for some comparison to be made. In the previous study, GAG and DNA production was increased with intermittent PTHrP treatment [23], whereas in our current study, these parameters were not improved. In the previous study, with intermittent PTH, the expression of COL2A1 was increased, COL10A1 was unchanged, and IHH was decreased [23], whereas in our study, we observed a downregulation of all three genes, amongst other genes. Like in our study, the previous study [23] observed a striking reduction in pellet growth and COL2A1 and COL10A1 expression in constant PTH conditions. There are two major differences between our studies, the first being the number of cells used to generate chondrogenic pellets: 5 × 105 cells per pellet in the previous study [23] and 5000 cells per micro-pellet in our study. The second was the duration of chondrogenic culture (6 weeks in the previous study [23] and 2 weeks in our study). In previous work, we demonstrated that pellets formed from 2 × 105 cells each suffer profound gradients and yield heterogenous tissues [25, 26]. In large-diameter pellets, differentiation appears to occur first at the peripheral edge of the pellet, with differentiation in the core being delayed, likely due to metabolite limitation in large-diameter tissues. By contrast, small-diameter micro-pellets appear to be relatively synchronised in differentiation across their diameter. As a result of this size difference, our micro-pellet study likely represents a more sensitive assay for BMSC chondrogenic response to PTH. Because of the large size of the chondrogenic pellets used in the previous study (5 × 105 cells per pellet [23]), it is likely that cellular response to PTHrP was muted within the core of the tissues, while being able to elicit a response by the cells near the surface of the pellet. The important influence of gradients on PTH signalling has been discussed in the tissue engineering context previously [37]. This is consistent with the authors’ observations, where there was a strong response at the surface of their pellets, and a muted response at the core [23]. In this case, averaged over a large pellet and a 6-week culture, they observed better chondrogenic outcomes with intermittent PTH. Conceptually, our micro-pellet is representative of the surface of their large pellet, and this thin and homogenous tissue did not benefit from similar intermittent PTH treatment.
It is possible that further optimisation of PTH administration in chondrogenic cultures may be required to apply this molecule in a meaningful way to improve chondrogenesis. PTH has been reported to induce early chondrogenesis while suppressing terminal differentiation of chondrocytes [19]. We observed that micro-pellets in the intermittent PTH condition grew in size in the first week of culture and then stopped increasing in size over the second week of culture, suggesting that BMSC differentiation stage may be an important factor to consider. It is possible that intermittent PTH has an anabolic effect on chondrogenesis in more primitive BMSC, which ceases, or becomes catabolic with extended PTH treatment, as the cells become more differentiated. Previous studies have reported that PTH exerts its function in osteogenesis by enhancing BMP signalling [6, 38]. Others have reported that antagonising BMP signalling can obstruct hypertrophy during BMSC chondrogenesis [39]. Using a combination of early intermittent PTH with other strategies, like BMP2 inhibition, may lead to improved protocols for BMSC chondrogenesis, but this will have to be trialled.
Conclusion
In our hands, up to 1 week of intermittent PTH treatment suppressed BMSC hypertrophic gene expression in chondrogenic cultures, but this benefit was countered by its hindering effect on chondrogenesis by 2 weeks of culture. It is possible that PTH may have an anti-hypertrophic effect on more primitive BMSC and a catabolic effect on BMSC as they become increasingly differentiated. To apply intermittent PTH in a useful manner in BMSC chondrogenic culture, a better understanding of its molecular mechanism in these cells during differentiation would be beneficial. Furthermore, combining early, short-term intermittent PTH treatment with other strategies, such as BMP signalling inhibition, could result in more optimal BMSC chondrogenic protocols.
Supplementary information
Additional file 1: Supplementary Figure 1. Concentration and live/dead assays of micro-pellets. Micro-pellets were treated with 1, 10 or 100 nM PTH or no PTH (control). A) Microscope images of micro-pellets within the microwell mesh at Day 14 of culture. Scale bar = 1 mm. B) Alcian blue staining of Day 14 micro-pellet sections with Nuclear Fast Red as a counterstain. Scale bar = 400 μm. C) Tissues were stained with LIVE/DEAD viability stain as per the manufacturer’s instructions (Thermo Fischer Scientific). LIVE/DEAD stain of control and PTH-treated (2.5 nM) micro-pellets at Day 14. Calcein-AM (green/live) and propidium iodide (red/dead) demonstrate relative viability in cultures without (control) or with PTH (2.5 nM) . Scale bar = 200 μm.
Additional file 2: Supplementary Figure 2. Microscope images of donor 2 micro-pellets within the microwell-mesh. Scale bar = 1 mm.
Additional file 3: Supplementary Figure 3. Microscope images of donor 3 micro-pellets within the microwell-mesh. Scale bar = 1 mm.
Additional file 4: Supplementary Figure 4. Alcian blue and collagen II, X and I staining of donor 2 micro-pellets. Scale bar = 400 μm.
Additional file 5: Supplementary Figure 5. Alcian blue and collagen II, X and I staining of donor 3 micro-pellets. Scale bar = 400 μm.
Acknowledgements
The Translational Research Institute (TRI) is supported by Therapeutic Innovation Australia (TIA). TIA is supported by the Australian Government through the National Collaborative Research Infrastructure Strategy (NCRIS) program. The authors thank the core facilities at the TRI, including the Flow Cytometry Core and the Microscopy Core. The authors thank the Mater Hospital for facilitating BMA collection and thank the volunteer donors.
Abbreviations
- ACAN
Aggrecan
- BGLAP/PB-OST
Osteocalcin
- BMSC
Bone marrow-derived stromal cells
- COL1A1
Collagen type I alpha 1 chain
- COL2A1
Collagen type II alpha 1 chain
- RPLP0
60S acidic ribosomal protein P0
- COL10A1
Collagen type X alpha 1 chain
- DMMB
1,9-Dimethylmethylene blue
- DNA
Deoxyribonucleic acid
- FBS
Fetal bovine serum
- FGF-1
Fibroblast growth factor-1
- GAG
Glycosaminoglycan
- HG-DMEM
High glucose DMEM
- ITS-X
Insulin-transferrin-selenium
- LG-DMEM
Low glucose Dulbecco’s modified Eagle’s medium
- PBS
Phosphate-buffered saline
- PDMS
Polydimethylsiloxane
- PenStrep
Penicillin/streptomycin
- PTH
Parathyroid hormone
- PTH1R
PTHrP receptor 1
- qRT-PCR
Quantitative real-time polymerase chain reaction
- RNA
Ribonucleic acid
- RUNX2
Runt-related transcription factor 2
- SOX9
SRY-Box transcription factor 9
- IHH
Indian hedgehog
- ssDNA
Single-stranded deoxyribonucleic acid
- TGF-β1
Transforming growth factor-beta 1
Authors’ contributions
EM, KF, JSP, MK, WBL, TJK, and MRD designed the research, analysed the data, and wrote the paper. EM, KF, and MRD performed the research. All authors read and approved the final manuscript.
Funding
MRD and TJK gratefully acknowledge project support from the National Health and Medicine Research Council (NHMRC) of Australia (project grant APP1083857) and NHMRC Fellowship support of MRD (APP1130013).
Availability of data and materials
Supporting data can be obtained from the corresponding author.
Ethics approval and consent to participate
Bone marrow aspirate was collected from the iliac crest of fully informed healthy adult donors who provided written consent. The collection was approved by the Mater Misericordiae Ltd. Human Research Ethics Committee (EC00332) and the Queensland University of Technology Human Ethics Committee (EC00171, 1000000938). All processes adhered to the National Health and Medical Research Council of Australia Human Ethics Guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ena Music, Email: ena.music@hdr.qut.edu.au.
Kathryn Futrega, Email: k.futrega@qut.edu.au.
James S. Palmer, Email: james.palmer@mater.org.au
Mackenzie Kinney, Email: mackenziekinney@gmail.com.
Bill Lott, Email: william.lott@qut.edu.au.
Travis J. Klein, Email: t2.klein@qut.edu.au
Michael R. Doran, Email: michael.doran@qut.edu.au
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13287-020-01820-6.
References
- 1.Johnstone B, et al. Tissue engineering for articular cartilage repair--the state of the art. Eur Cell Mater. 2013;25:248–267. doi: 10.22203/ecm.v025a18. [DOI] [PubMed] [Google Scholar]
- 2.Somoza RA, et al. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev. 2014;20(6):596–608. doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Studer D, et al. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. Eur Cell Mater. 2012;24:118–135. doi: 10.22203/ecm.v024a09. [DOI] [PubMed] [Google Scholar]
- 4.Harrington EK, et al. Parathyroid hormone/parathyroid hormone-related peptide modulates growth of avian sternal cartilage via chondrocytic proliferation. Anat Rec (Hoboken) 2007;290(2):155–167. doi: 10.1002/ar.20416. [DOI] [PubMed] [Google Scholar]
- 5.Chang JK, et al. Parathyroid hormone 1-34 inhibits terminal differentiation of human articular chondrocytes and osteoarthritis progression in rats. Arthritis Rheum. 2009;60(10):3049–3060. doi: 10.1002/art.24843. [DOI] [PubMed] [Google Scholar]
- 6.Yu B, et al. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. J Bone Miner Res. 2012;27(9):2001–2014. doi: 10.1002/jbmr.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juppner H, et al. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254(5034):1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 8.Qiu T, et al. PTH receptor signaling in osteoblasts regulates endochondral vascularization in maintenance of postnatal growth plate. J Bone Miner Res. 2015;30(2):309–317. doi: 10.1002/jbmr.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Q, et al. Expression of parathyroid hormone-related peptide (PthrP) and its receptor (PTH1R) during the histogenesis of cartilage and bone in the chicken mandibular process. J Anat. 2002;201(2):137–151. doi: 10.1046/j.1469-7580.2002.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, et al. Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum. 2008;58(12):3788–3797. doi: 10.1002/art.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa Y, et al. Effects of calcitonin and parathyroid hormone on calcification of primary cultures of chicken growth plate chondrocytes. J Bone Miner Res. 1997;12(3):356–366. doi: 10.1359/jbmr.1997.12.3.356. [DOI] [PubMed] [Google Scholar]
- 12.Takigawa M, et al. Polyamine and differentiation: induction of ornithine decarboxylase by parathyroid hormone is a good marker of differentiated chondrocytes. Proc Natl Acad Sci U S A. 1980;77(3):1481–1485. doi: 10.1073/pnas.77.3.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki F, Yoneda T, Shimomura Y. Calcitonin and parathyroid-hormone stimulation of acid mucopolysaccharide synthesis in cultured chondrocytes isolated from growth cartilage. FEBS Lett. 1976;70(1):155–158. doi: 10.1016/0014-5793(76)80747-8. [DOI] [PubMed] [Google Scholar]
- 14.Takigawa M, Takano T, Suzuki F. Effects of parathyroid hormone and cyclic AMP analogues on the activity of ornithine decarboxylase and expression of the differentiated phenotype of chondrocytes in culture. J Cell Physiol. 1981;106(2):259–268. doi: 10.1002/jcp.1041060212. [DOI] [PubMed] [Google Scholar]
- 15.Takigawa M, et al. Studies on chondrocytes from mandibular condylar cartilage, nasal septal cartilage, and spheno-occipital synchondrosis in culture. I. Morphology, growth, glycosaminoglycan synthesis, and responsiveness to bovine parathyroid hormone (1-34) J Dent Res. 1984;63(1):19–22. doi: 10.1177/00220345840630010201. [DOI] [PubMed] [Google Scholar]
- 16.Harvey AK, et al. Parathyroid hormone-(1-34) enhances aggrecan synthesis via an insulin-like growth factor-I pathway. J Biol Chem. 1999;274(33):23249–23255. doi: 10.1074/jbc.274.33.23249. [DOI] [PubMed] [Google Scholar]
- 17.Harrington EK, et al. PTH stimulated growth and decreased Col-X deposition are phosphotidylinositol-3,4,5 triphosphate kinase and mitogen activating protein kinase dependent in avian sterna. Anat Rec (Hoboken) 2010;293(2):225–234. doi: 10.1002/ar.21072. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Kumagai K, Saito T. Effect of parathyroid hormone on early chondrogenic differentiation from mesenchymal stem cells. J Orthop Surg Res. 2014;9:68. doi: 10.1186/s13018-014-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollnagel A, Ahrens M, Gross G. Parathyroid hormone enhances early and suppresses late stages of osteogenic and chondrogenic development in a BMP-dependent mesenchymal differentiation system (C3H10T1/2) J Bone Miner Res. 1997;12(12):1993–2004. doi: 10.1359/jbmr.1997.12.12.1993. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, et al. Parathyroid hormone-induced bone marrow mesenchymal stem cell chondrogenic differentiation and its repair of articular cartilage injury in rabbits. Med Sci Monit Basic Res. 2016;22:132–145. doi: 10.12659/MSMBR.900242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rickard DJ, et al. Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone. 2006;39(6):1361–1372. doi: 10.1016/j.bone.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Yang C, et al. Effects of continuous and pulsatile PTH treatments on rat bone marrow stromal cells. Biochem Biophys Res Commun. 2009;380(4):791–796. doi: 10.1016/j.bbrc.2009.01.167. [DOI] [PubMed] [Google Scholar]
- 23.Fischer J, et al. Role of PTHrP(1-34) pulse frequency versus pulse duration to enhance mesenchymal stromal cell chondrogenesis. J Cell Physiol. 2016;231(12):2673–2681. doi: 10.1002/jcp.25369. [DOI] [PubMed] [Google Scholar]
- 24.Futrega K, et al. The microwell-mesh: a novel device and protocol for the high throughput manufacturing of cartilage microtissues. Biomaterials. 2015;62:1–12. doi: 10.1016/j.biomaterials.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Markway BD, et al. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant. 2010;19(1):29–42. doi: 10.3727/096368909X478560. [DOI] [PubMed] [Google Scholar]
- 26.Futrega K, et al. Micro-pellet culture reveals that bone marrow mesenchymal stromal cell (BMSC) chondrogenic induction is triggered by a single day of TGF-beta1 exposure. bioRxiv 853556 [Preprint]. November 26, 2019 [cited 2020 May 19]. Available from: 10.1101/853556.
- 27.Chambers KF, et al. 3D cultures of prostate cancer cells cultured in a novel high-throughput culture platform are more resistant to chemotherapeutics compared to cells cultured in monolayer. PLoS One. 2014;9(11):e111029. doi: 10.1371/journal.pone.0111029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin I, et al. Enhanced cartilage tissue engineering by sequential exposure of chondrocytes to FGF-2 during 2D expansion and BMP-2 during 3D cultivation. J Cell Biochem. 2001;83(1):121–128. doi: 10.1002/jcb.1203. [DOI] [PubMed] [Google Scholar]
- 29.Ragni E, et al. What is beyond a qRT-PCR study on mesenchymal stem cell differentiation properties: how to choose the most reliable housekeeping genes. J Cell Mol Med. 2013;17(1):168–180. doi: 10.1111/j.1582-4934.2012.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selvamurugan N, et al. Transforming growth factor-beta 1 regulation of collagenase-3 expression in osteoblastic cells by cross-talk between the Smad and MAPK signaling pathways and their components, Smad2 and Runx2. J Biol Chem. 2004;279(18):19327–19334. doi: 10.1074/jbc.M314048200. [DOI] [PubMed] [Google Scholar]
- 31.Frisch J, et al. Determination of the chondrogenic differentiation processes in human bone marrow-derived mesenchymal stem cells genetically modified to overexpress transforming growth factor-beta via recombinant adeno-associated viral vectors. Hum Gene Ther. 2014;25(12):1050–1060. doi: 10.1089/hum.2014.091. [DOI] [PubMed] [Google Scholar]
- 32.Untergasser A, et al. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampson ER, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011;3(101):101ra93. doi: 10.1126/scitranslmed.3002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zerega B, et al. Parathyroid hormone [PTH(1-34)] and parathyroid hormone-related protein [PTHrP(1-34)] promote reversion of hypertrophic chondrocytes to a prehypertrophic proliferating phenotype and prevent terminal differentiation of osteoblast-like cells. J Bone Miner Res. 1999;14(8):1281–1289. doi: 10.1359/jbmr.1999.14.8.1281. [DOI] [PubMed] [Google Scholar]
- 35.Mwale F, et al. Effect of parathyroid hormone on type X and type II collagen expression in mesenchymal stem cells from osteoarthritic patients. Tissue Eng Part A. 2010;16(11):3449–3455. doi: 10.1089/ten.TEA.2010.0091. [DOI] [PubMed] [Google Scholar]
- 36.Babur BK, et al. The interplay between chondrocyte redifferentiation pellet size and oxygen concentration. PLoS One. 2013;8(3):e58865. doi: 10.1371/journal.pone.0058865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fahy N, et al. Parathyroid hormone-related protein gradients affect the progression of mesenchymal stem cell chondrogenesis and hypertrophy. Tissue Eng Part A. 2018;24(9–10):849–859. doi: 10.1089/ten.TEA.2017.0337. [DOI] [PubMed] [Google Scholar]
- 38.Chan GK, et al. Parathyroid hormone-related peptide interacts with bone morphogenetic protein 2 to increase osteoblastogenesis and decrease adipogenesis in pluripotent C3H10T 1/2 mesenchymal cells. Endocrinology. 2003;144(12):5511–5520. doi: 10.1210/en.2003-0273. [DOI] [PubMed] [Google Scholar]
- 39.Occhetta P, et al. Developmentally inspired programming of adult human mesenchymal stromal cells toward stable chondrogenesis. Proc Natl Acad Sci U S A. 2018;115(18):4625–4630. doi: 10.1073/pnas.1720658115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Figure 1. Concentration and live/dead assays of micro-pellets. Micro-pellets were treated with 1, 10 or 100 nM PTH or no PTH (control). A) Microscope images of micro-pellets within the microwell mesh at Day 14 of culture. Scale bar = 1 mm. B) Alcian blue staining of Day 14 micro-pellet sections with Nuclear Fast Red as a counterstain. Scale bar = 400 μm. C) Tissues were stained with LIVE/DEAD viability stain as per the manufacturer’s instructions (Thermo Fischer Scientific). LIVE/DEAD stain of control and PTH-treated (2.5 nM) micro-pellets at Day 14. Calcein-AM (green/live) and propidium iodide (red/dead) demonstrate relative viability in cultures without (control) or with PTH (2.5 nM) . Scale bar = 200 μm.
Additional file 2: Supplementary Figure 2. Microscope images of donor 2 micro-pellets within the microwell-mesh. Scale bar = 1 mm.
Additional file 3: Supplementary Figure 3. Microscope images of donor 3 micro-pellets within the microwell-mesh. Scale bar = 1 mm.
Additional file 4: Supplementary Figure 4. Alcian blue and collagen II, X and I staining of donor 2 micro-pellets. Scale bar = 400 μm.
Additional file 5: Supplementary Figure 5. Alcian blue and collagen II, X and I staining of donor 3 micro-pellets. Scale bar = 400 μm.
Data Availability Statement
Supporting data can be obtained from the corresponding author.