Abstract
Background
Carpal tunnel syndrome (CTS) is a condition where one of two main nerves in the wrist is compressed, which can lead to pain in the hand, wrist and sometimes arm, and numbness and tingling in the thumb, index and long finger. Splinting is usually offered to people with mild to moderate symptoms. However, the effectiveness and duration of the benefit of splinting for this condition remain unknown.
Objectives
To compare the effectiveness of splinting for carpal tunnel syndrome with no treatment, placebo or another non‐surgical intervention.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (10 January 2011), CENTRAL, NHSEED and DARE (The Cochrane Library 2011, Issue 4), MEDLINE (January 1966 to December 2011), EMBASE (January 1980 to January 2012), AMED (January 1985 to January 2012), and CINAHL Plus (January 1937 to January 2012), using no time limits. We searched the reference lists of all included trials and relevant reviews for further relevant studies.
Selection criteria
All randomised and quasi‐randomised trials comparing splinting with no treatment (or a placebo) or with other non‐surgical treatments were eligible for inclusion. We also included studies comparing one splint type or regimen versus another. We excluded studies comparing splinting with surgical treatment. There were no language restrictions. We included all patients diagnosed with carpal tunnel syndrome unless they had undergone surgical release.
Data collection and analysis
Two review authors independently selected trials for inclusion, and performed data extraction. Two authors also independently performed the assessment of risk of bias. We calculated measures of effect as risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CI) reported and statistical significance set at P < 0.05 for all outcome comparisons.
Main results
The review included 19 studies randomising 1190 participants with carpal tunnel syndrome. Two studies compared splinting with no treatment, five compared different splint designs, one compared different splint‐wearing regimens, seven compared splint delivered as a single intervention with another non‐surgical intervention, and five compared splint delivered alongside other non‐surgical interventions with another non‐surgical intervention. Only three studies reported concealing the allocation sequence, and only one reported blinding of participants. Three studies measured the primary outcome, short‐term overall improvement at three months or less. One low quality study with 80 wrists found that compared to no treatment, splints worn at night more than tripled the likelihood of reporting overall improvement at the end of four weeks of treatment (RR 3.86, 95% CI 2.29 to 6.51). However, the lack of patient blinding and unclear allocation concealment suggests this result should be interpreted with caution. A very low quality quasi‐randomised trial with 90 wrists found that wearing a neutral splint more than doubled the likelihood of reporting 'a lot or complete relief' at the end of two weeks of treatment compared with an extension splint (RR 2.43, 95% CI 1.12 to 5.28). The third study which measured short‐term overall improvement did not report outcome data separately per group. Nine studies measured adverse effects of splinting and all found either no or few participants reporting discomfort or swelling due to splinting; however, the precision of all RRs was very low. Differences between groups in the secondary outcomes ‐ symptoms, function, and neurophysiologic parameters ‐ were most commonly small with 95% CIs incorporating effects in either direction.
Authors' conclusions
Overall, there is limited evidence that a splint worn at night is more effective than no treatment in the short term, but there is insufficient evidence regarding the effectiveness and safety of one splint design or wearing regimen over others, and of splint over other non‐surgical interventions for CTS. More research is needed on the long‐term effects of this intervention for CTS.
Plain language summary
Splinting for carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is a condition where one of two main nerves in the wrist is compressed, which can lead to pain in the hand, wrist and sometimes arm, and numbness and tingling in the thumb, index and long finger. CTS is more common in women and older age groups. Many people undergo surgery to treat this condition, though sometimes other non‐surgical treatments, such as splinting, are offered. Splinting involves immobilisation of the wrist with a device that is worn over the wrist, which usually leaves the fingers and thumb free to move. We searched for study reports and found 19 randomised or quasi‐randomised controlled trials including 1190 participants overall that assessed the safety and benefit of splinting for people with CTS. The risk of bias of studies was low in some studies and unclear or high in others. One low quality study suggests that splinting at night leads to more overall improvement in the short term when compared to no treatment, but we cannot say from the evidence whether one splint design or wearing regimen is more effective than another, nor can we say that splinting is more effective than other non‐surgical interventions for CTS (for example exercises, oral steroids). Nine trials measured adverse effects of splinting and all found either no or few participants reported discomfort or swelling due to splinting. More research is needed to find out how effective and safe splinting is for people with carpal tunnel syndrome, particularly in the long term.
Summary of findings
Summary of findings for the main comparison. Splint versus no treatment for carpal tunnel syndrome.
| Splint versus no treatment for carpal tunnel syndrome | ||||||
| Patient or population: People with carpal tunnel syndrome Settings: Referred for possible carpal tunnel syndrome to EMG laboratory Intervention: Splint versus no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Splint versus no treatment | |||||
| Short‐term overall improvement (3 months or less) | Study population | RR 3.86 (2.29 to 6.51) | 80 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| 250 per 10001 | 965 per 1000 (572 to 1000) | |||||
| Adverse effects (difficulty falling asleep) | Study population | RR 7 (0.37 to 131.28) | 80 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| 0 per 10001 | 0 per 1000 (0 to 0) | |||||
|
Short‐term improvement in CTS symptoms (Levine questionnaire) (3 months or less) Scale: 0 to 5 |
The mean CTS symptom severity score (assessed using the Levine questionnaire) at the end of four weeks of treatment in the control group was 2.611 |
The mean CTS symptom severity score (assessed using the Levine questionnaire) at the end of four weeks of treatment in the intervention group was 1.07 lower4 (1.29 to 0.85 lower) | 80 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
|
Short‐term improvement in functional status (Levine questionnaire) (3 months or less) Scale: 0 to 5 |
The mean functional status score (assessed using the Levine questionnaire) at the end of four weeks of treatment in the control group was 2.031 |
The mean functional status score (assessed using the Levine questionnaire) at the end of four weeks of treatment in the intervention group was 0.55 lower4 (0.82 to 0.28 lower) | 80 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| Short‐term improvement in distal motor latency (ms) (3 months or less) | The mean short‐term improvement in distal motor latency at the end of four weeks of treatment in the control group was 4.47 ms1 |
The mean short‐term improvement in distal motor latency at the end of four weeks of treatment in the intervention group was 0.02 ms shorter5 (0.49 ms shorter to 0.45 ms longer) | 80 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| Short‐term improvement in sensory nerve conduction velocity (m/s) (3 months or less) | The mean short‐term improvement in sensory nerve conduction velocity at the end of four weeks of treatment in the control group was 37.92 m/s1 |
The mean short‐term improvement in sensory nerve conduction velocity at the end of four weeks of treatment in the intervention group was 0.72 m/s faster6 (5.85 m/s faster to 4.41 m/s slower) | 80 (1) | ⊕⊕⊝⊝ low2,3 | ||
| Short‐term improvement in sensory nerve action potential (µV) (3 months or less) | The mean short‐term improvement in sensory nerve action potential at the end of four weeks of treatment in the control group was 12.44 µV1 |
The mean short‐term improvement in sensory nerve action potential at the end of four weeks of treatment in the intervention group was 6.3 µV larger7 (0.6 to 12 larger) | 80 (1) | ⊕⊕⊝⊝ low2,3 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Assumed risk is based on the risk in the control group in the study conducted by Manente 2001
2 It was not clear if the allocation sequence was concealed, and participants, personnel and outcome assessors were not blind to treatment allocation 3 Participants only wore splint at night. The effect of full‐time or daytime splint‐wearing versus no treatment has not been investigated
4 Lower scores denote better outcome
5 Shorter latency denotes better outcome
6 Faster conduction velocity denotes better outcome
7 Larger action potential denotes better outcome
Background
Description of the condition
In the condition of carpal tunnel syndrome (CTS), the median nerve is irritated in the carpal tunnel. Symptoms of CTS include pain in the wrist and hand which can spread to the arm and paraesthesiae (numbness or tingling) in the thumb, index, middle and radial half of the ring finger (Atroshi 1999). Advanced CTS can result in thenar muscle weakness and atrophy (Keir 2005).The course of CTS is not predictable; some patients progress from intermittent paraesthesiae to more constant paraesthesiae, and eventual thenar atrophy while others experience intermittent exacerbation of sensory symptoms, with no symptoms in between, over many years, while other experience spontaneous (and lasting) remission (Braun 1989). There is no reliable data on the number of people who experience spontaneous remission, as such information is often based on assessment using nerve conduction studies, which have been found to correlate weakly with clinical outcomes (Hardoim 2009; Padua 1999; Resende 2003).
Results of a Swedish study suggest that the prevalence of CTS in the general population is 3.8% for clinically diagnosed cases and 2.7% for electrophysiologically confirmed cases (Atroshi 1999). Age and gender are associated with the incidence of CTS. People aged less than 25 years accounted for only 2.4% of patients presenting to Australian general practices between 2000 and 2009 with the condition, compared to people aged 45 to 64 years who accounted for 45.5% of these cases (Charles 2009). As for gender, 67% of CTS encounters at Australian general practices were attributable to females (Charles 2009). Females in their fourth and fifth decades have been found to suffer CTS four times more commonly than males (Atroshi 1999). CTS has been reported to occur more frequently in some professions, where there is frequent grasping, forceful grasping and flexed wrist postures, or exposure to vibration from hand‐held tools (Palmar 2007).
Description of the intervention
Treatment of CTS is either surgical or non‐surgical, however carpal tunnel release (CTR) is now the most common surgery in the United States, with more than 400,000 carpal tunnel releases performed annually, with an estimated total cost to the healthcare system of $2 billion (Concannon 2000; Huisstede 2010). Surgical treatment is usually offered to those with advanced CTS, who have constant symptoms, severe sensory disturbance or thenar motor weakness. Non‐surgical treatments are recommended as an initial treatment for those who have symptoms without evidence of denervation, cannot undergo surgery, or have intermittent symptoms of mild to moderate CTS. Non‐surgical treatment for CTS include wrist splinting, steroid injections into the carpal canal, exercises, yoga, therapeutic ultrasound, activity or ergonomic modification, oral medication and vitamins (Muller 2004).
Splinting creates immobilisation of the wrist joint by an external device. The splint usually leaves the fingers and thumb free to move and it may be worn at nighttime, or at night and during daytime activities that cause wrist motion. A thermoplastic splint may be custom‐fitted to the patient by an occupational therapist, or a softer, adjustable splint may be fitted and purchased. A specific soft splint that prevents the wrist from moving into flexion, and maintains the long and ring fingers in extension at the metacarpophalangeal (MCP) and interphalangeal joints, called the 'MANU' hand brace, is commercially made (De Angelis 2009; Manente 2001).
How the intervention might work
In patients with CTS, the rationale behind splinting of the wrist in a neutral position is that pressure on the median nerve as it passes through the carpal tunnel is increased in positions of wrist flexion and extension (Gelberman 1984). The pressure on the median nerve is lowest when the wrist is in a neutral position and this is where the splint holds the wrist, even when the patient is asleep and likely to flex their wrist without being able to correct themselves. The exact angle at which the wrist should be splinted has not been determined. The rationale for the MANU hand brace described above is that the different positions of the fingers are said to relieve carpal tunnel symptoms, by altering the shape of the carpal tunnel and moving the lumbricals distally out of the carpal tunnel, to decrease pressure on the median nerve (Manente 2001).
Why it is important to do this review
Following the publication of the original version of this review (O'Connor 2003), the evidence base for all non‐surgical interventions for CTS has grown, and a number of systematic reviews of these treatments have been published (Ashworth 2010;Gerritsen 2002b; Goodyear‐Smith 2004; Huisstede 2010; Muller 2004; Ono 2010; Piazzini 2007). The search in the most recent review (Huisstede 2010) was conducted up to January 2010 and found that the evidence base for many interventions, including splinting, remains incomplete. Cochrane systematic reviews of local steroid injection (Marshall 2007), surgical versus non‐surgical treatment (Verdugo 2008), different surgical treatment options (Scholten 2007), therapeutic ultrasound (Page 2012), and ergonomic interventions (O'Connor 2012) for CTS already exist, and up‐to‐date Cochrane systematic reviews of other non‐surgical interventions for CTS (e.g. splinting, exercises, oral drugs) are required. Given the personal and financial impact of CTS, there is a need to ascertain the efficacy of splinting for the treatment of CTS.
Objectives
To compare the effectiveness of splinting for CTS with no treatment, placebo, or another non‐surgical treatment for improving clinical outcome.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised and quasi‐randomised controlled trials were eligible for inclusion. We included studies comparing splinting with no treatment (or a placebo) or with other non‐surgical treatments. We also included studies comparing one splint type or regimen versus another. We excluded studies comparing splinting with surgical treatment (these are reviewed elsewhere, Verdugo 2008). There were no language restrictions.
Types of participants
All participants with a diagnosis of CTS, as defined by the authors of each study. We excluded participants who had previous surgery for CTS.
Types of interventions
We included all splinting interventions. Comparison interventions included no treatment, placebo and other non‐surgical interventions.
Types of outcome measures
The outcomes reported in this review have been modified from the original review (O'Connor 2003) to be consistent as possible with other Cochrane reviews for CTS (Marshall 2007; Scholten 2007; Verdugo 2008).
Primary outcomes
Short‐term overall improvement (any measure in which patients indicate the intensity of their complaints compared with baseline) (three months or less; reported as dichotomous outcome).
Secondary outcomes
Adverse effects.
Short‐term improvement in CTS symptoms (for example, pain, paraesthesia, nocturnal paraesthesia) (three months or less).
Short‐term improvement in functional ability or health‐related quality of life (three months or less).
Short‐term improvement in neurophysiologic parameters (three months or less).
Long‐term improvement in CTS symptoms (greater than three months).
Long‐term improvement in functional ability or health‐related quality of life (greater than three months).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Neuromuscular Disease Group Specialized Register (10 January 2011), CENTRAL, NHSEED and DARE (The Cochrane Library 2011, Issue 4), MEDLINE (January 1966 to December 2011), EMBASE (January 1980 to January 2012), AMED (January 1985 to January 2012), and CINAHL Plus (January 1937 to January 2012), using no time limits.
The detailed search strategies are MEDLINE (Appendix 1), EMBASE (Appendix 2), AMED (Appendix 3), CINAHL Plus (Appendix 4), and CENTRAL, NHSEED and DARE (Appendix 5).
Searching other resources
We searched the reference lists of all included trials and relevant reviews for further relevant studies.
Data collection and analysis
The review authors followed the recommended strategies for data collection and analysis as documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
Two review authors independently selected trials for possible inclusion against a predetermined checklist of inclusion criteria. We initially categorised studies into the following groups.
Possibly relevant ‐ studies that met the inclusion criteria and/or studies from which it was not possible to determine whether they met the criteria either from their title or abstract.
Excluded ‐ those clearly not meeting the inclusion criteria.
If a title or abstract appeared to meet the eligibility criteria for inclusion in the review, or we could not tell, we obtained a full text version of the article and two review authors independently assessed it to determine whether it met the inclusion criteria. Authors resolved any disagreement via discussion.
Data extraction and management
Two review authors independently extracted data using a standard data extraction form. Authors resolved any discrepancies by discussion. We pilot tested the data extraction form and modified it accordingly before use. In addition to risk of bias characteristics and study results, we also recorded the following details:
participant details, including demographic and inclusion/exclusion criteria;
types of interventions used and their comparison;
outcomes reported, including the type and timing of measures used.
One review author compiled all comparisons and entered outcome data into the Cochrane statistical software, Review Manager 5 (RevMan 5). At least one review author cross‐checked data. For trials that did not report the required data, one review author requested further information. When unsuccessful, we included the study in the review and fully described it, but did not include it in any meta‐analysis. An entry of this process was made in the notes section of the 'Characteristics of included studies' table.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in included studies using The Cochrane Collaboration's 'Risk of bias' tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We assessed the following items for risk of bias based on information extracted from reports of the included studies:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data (defined separately for data measured at three months or less, and after three months);
selective reporting;
other sources of bias (e.g. inappropriate unit of analysis).
The review authors rated each item as being at 'Low risk of bias', 'Unclear risk of bias' or 'High risk of bias'. We resolved any discrepancies through discussion.
Measures of treatment effect
We used Review Manager 5 software to perform data analysis. We expressed results as risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes and mean differences (MD) with 95% CI for continuous outcomes if the same measurement tool was used to measure the same outcome across separate studies. If necessary, we planned to summarise continuous outcomes using the standardised mean difference (SMD) when studies measured the same outcome but employed different measurement tools. We set statistical significance at P < 0.05 for primary and secondary outcome measures.
Unit of analysis issues
We sought information about the unit of randomisation used (i.e. wrists or participants, where participants with bilateral CTS received the same intervention for both wrists). In studies which randomised wrists, we sought information about whether each participant's wrist was allocated to different treatments, or whether there was no constraint that each participant's wrist be allocated to different treatments. Given that results for different wrists in participants with bilateral CTS are unlikely to be independent, we assessed how the investigators of studies which included participants with bilateral CTS took account of this dependence in their analyses (e.g. use of paired or matched analyses, generalised estimating equations). If this information was not reported, we contacted trialists for clarification. We also requested individual wrist outcome data from trialists to re‐analyse the data. If we were unable to obtain individual wrist outcome data, we had planned to estimate parameters (such as an intra‐class correlation coefficient) from studies that reported sufficient information to calculate this, and to use these estimates to adjust the results in other studies, following the advice provided in sections 16.3 and 16.4 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011c). If unable to adjust the outcome data, we included the data as reported by the trialists, and commented on the validity of such analyses.
Dealing with missing data
The review authors sought relevant missing information about study design and/or results from the study investigators, where possible.
Assessment of heterogeneity
We assessed clinical heterogeneity by determining whether the characteristics of participants, interventions, outcome measures and timing of outcome measurement were similar across studies. Statistical heterogeneity was assessed using the Chi2 statistic and the I2 test (Higgins 2002). We interpreted the I2 statistic using the following as an approximate guide:
0% to 40% might not be important heterogeneity;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% may represent considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
To assess publication bias, we intended to generate funnel plots if the review included at least 10 studies examining the same treatment comparison (Sterne 2011). To assess outcome reporting bias, we searched protocols of trials on the clinical trials register that is maintained by the US National Institute of Health at http://clinicaltrials.gov, and we searched protocols of trials published after July 1st 2005 using the Clinical Trial Register at the International Clinical Trials Registry Platform of the World Health Organization (http://apps.who.int/trialssearch), to compare with the corresponding published RCTs (Dwan 2008; Dwan 2011).
Data synthesis
We pooled results of studies with similar characteristics (participants, interventions, outcome measures and timing of outcome measurement) to provide estimates of the efficacy of splinting for CTS. Where we could not combine data, we presented a narrative synthesis of results. We carried out a meta‐analysis of pooled results using either a fixed‐effect or random‐effects model (depending on the level of clinical and methodological heterogeneity).
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses according to the severity of CTS symptoms and sex, since these factors may cause variations in outcomes. We defined subgroups as follows:
severity of CTS symptoms: early (E), intermediate (I) and advanced (A) symptoms (Szabo 1992);
sex: male, female.
Sensitivity analysis
We conducted sensitivity analyses for each element on the 'Risk of bias' table by excluding studies that had a high risk of bias. Sensitivity analyses were also conducted using the following filter.
Quality of diagnostic criteria: high (A), moderate (B) and low (C) quality (Rempel 1998).
Summary of findings
We created a 'Summary of findings' table for the main comparison of the review, splint versus no treatment, and only included results of studies which were randomised (rather than quasi‐randomised). We included in the table one effect estimate for each of our primary and secondary outcomes (see Types of outcome measures).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The search conducted up until 10 January 2012 identified a total of 389 records. Table 1 reports the number of hits retrieved by each search strategy. The number of records after removal of duplicates was 188. From these, we retrieved 35 full text papers for further examination. After screening the full text of the selected papers for eligibility, 19 studies (Arinci Incel 2005; Bardak 2009; Bilgici 2010;Brininger 2007; Burke 1994; Bye 2011, Celiker 2002; De Angelis 2009; de Entrambasaguas 2006, Garfinkel 1998; Kumnerddee 2010; Madjdinasab 2008; Manente 2001; Mishra 2006; Premoselli 2006; Sevim 2004; Walker 2000; Werner 2005; Zinnuroglu 2010) fulfilled the inclusion criteria. One study is currently being translated and is therefore awaiting assessment (Taspinar 2007). A flow diagram of the study selection process is presented in Figure 1.
1.
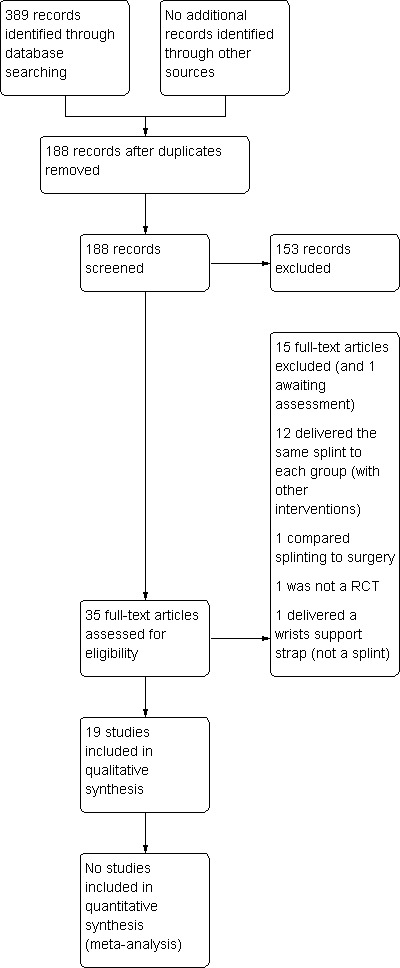
Study flow diagram.
Table 1
| Database | Period searched | Date searched | Number of hits |
| Cochrane Neuromuscular Disease Group specialised register | Issue 4, 2011 | 10 January 2012 | 61 |
| CENTRAL | Issue 4, 2011 | 10 January 2012 | 64 |
| MEDLINE | 1966 to December 2011 | 10 January 2012 | 93 |
| EMBASE | 1980 to January 2012 | 10 January 2012 | 79 |
| CINAHL Plus | 1937 to January 2012 | 10 January 2012 | 58 |
| AMED | 1985 to January 2012 | 10 January 2012 | 22 |
| DARE | Issue 4, 2011 | 10 January 2012 | 10 |
| NHSEED | Issue 4, 2011 | 10 January 2012 | 2 |
Included studies
Nineteen randomised controlled trials were included in this review, published between 1994 and 2011.
Participants
The 19 included studies comprised 1190 randomised participants, some with bilateral CTS thus comprising 1287 wrists. There were 179 men and 839 women, but the gender of 97 participants from Burke 1994 and de Entrambasaguas 2006 was not described. The mean age of participants in most studies was 40 to 50 years, and the duration of CTS symptoms was variable. All participants were screened for comorbidities that could affect the upper limb, while one study included patients with comorbid diabetes and rheumatoid arthritis (Walker 2000).
Interventions
Treatments were variable in duration, type and routine of splint wear. The duration of splint use ranged between two weeks of nocturnal use (Burke 1994), to one year of nocturnal use (Sevim 2004). The most common time frames were between two and four weeks, and the most commonly prescribed schedule was nocturnal wear. Some studies did not report the duration and schedule of splint wear, and only one study asked patients approximately how much they had worn their splint.
Splints were both custom made and commercially available, and all involved wrist support at angles of 'neutral' to 20° of wrist extension. Most splints did not describe joint involvement other than the wrist, except for the MANU hand brace developed by Manente 2001 (fingers two to five were splinted), and in some cases the MCP joints were also splinted in a 'neutral 'position.
Five studies had at least one study group in which a splint was delivered in conjunction with another non‐surgical intervention (Arinci Incel 2005; Bardak 2009; Bilgici 2010; Celiker 2002; Werner 2005).
Outcomes
The primary outcome, short‐term overall improvement using any measure where patients indicate the intensity of their complaints compared with baseline (over a period of three months or less), was reported in only three of the 19 studies (Brininger 2007, Burke 1994, Manente 2001). Adverse effects of splint and other non‐surgical interventions for CTS were reported in nine studies (Arinci Incel 2005; Bilgici 2010; Burke 1994; Celiker 2002; De Angelis 2009; Kumnerddee 2010; Manente 2001; Mishra 2006; Sevim 2004). The most commonly reported secondary outcomes were pain, using 10‐ or 100‐point visual analogue scales (VAS) and symptoms and self‐reported functional ability using the Levine Carpal Tunnel Syndrome Questionnaire (where higher scores on the symptom severity and functional status scores denote poorer outcome) (Levine 1993). Only three studies assessed outcomes at long‐term follow‐up (more than three months post‐treatment) (Bardak 2009, De Angelis 2009, Werner 2005).
Unit of analysis
In 10 studies (Arinci Incel 2005; Bilgici 2010; Burke 1994; Bye 2011; Celiker 2002; de Entrambasaguas 2006; Garfinkel 1998; Madjdinasab 2008; Mishra 2006; Walker 2000), some or all participants had bilateral CTS, where both wrists contributed to the analysis. In six of these studies (Arinci Incel 2005; Celiker 2002; Garfinkel 1998; Madjdinasab 2008; Mishra 2006; Walker 2000), randomisation occurred at the level of participants, where the same intervention was delivered to both wrists in participants with bilateral CTS. In Burke 1994, quasi‐randomisation of wrists occurred, where for all participants with bilateral CTS, each wrist received a different intervention. In Bye 2011, quasi‐randomisation of wrists occurred, where there was no constraint that each participants' wrist be allocated to different treatments. It was unclear in Bilgici 2010 or de Entrambasaguas 2006 whether participants with bilateral CTS received the same or different interventions for each wrist. All outcomes were analysed at the wrist‐level in Arinci Incel 2005, Bilgici 2010, Burke 1994, de Entrambasaguas 2006, Garfinkel 1998, Mishra 2006, and Walker 2000; all outcomes were analysed at the participant‐level in Madjdinasab 2008; some outcomes were analysed at the wrist‐level and others at the participant level in Celiker 2002; and the unit of analysis was unclear in Bye 2011. In eight of these studies (Bilgici 2010; Burke 1994; Bye 2011; Celiker 2002; de Entrambasaguas 2006; Garfinkel 1998; Madjdinasab 2008; Walker 2000), the trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful (so it is not clear whether a unit of analysis error occurred in these studies). However, personal communication with Arinci Incel 2005 and Mishra 2006 confirmed that the correlation between both wrists was not accounted for in the analysis (therefore a unit of analysis error occurred in these studies).
Excluded studies
In total, the review authors excluded 15 studies after review of the full publication. Reasons for exclusion of studies are given in the 'Characteristics of excluded studies' table. The main reason for exclusion was that the same splint was delivered to both groups, so a comparison of the effectiveness and safety of splinting over other non‐surgical interventions in those studies was not possible.
Risk of bias in included studies
For details of risk of bias in the included studies, see the 'Characteristics of included studies' tables and Figure 2.
2.
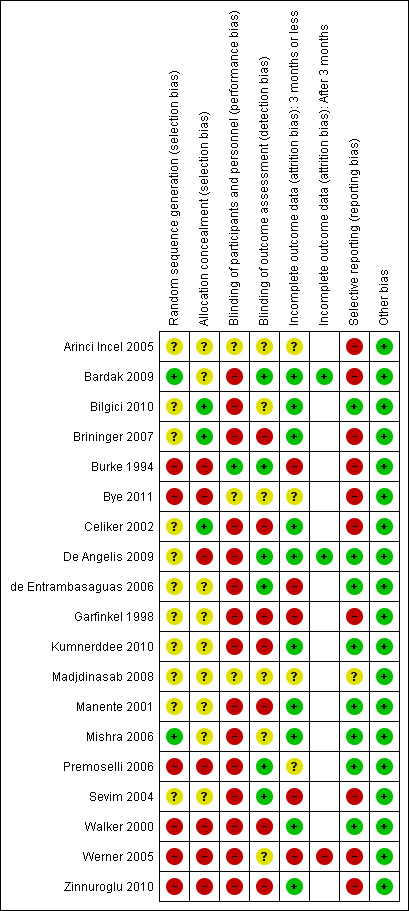
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Only two studies reported a method of random sequence generation that was deemed adequate and rated as being at low risk of bias (Bardak 2009; Mishra 2006). In six studies (Burke 1994; Bye 2011; Premoselli 2006; Walker 2000; Werner 2005; Zinnuroglu 2010), the method of allocation was a type of alternation (i.e. non‐random), so these studies were rated at high risk of selection bias (for both random sequence generation and allocation concealment domains). Only three studies described an adequate type of allocation concealment (Bilgici 2010; Brininger 2007; Celiker 2002).
Blinding
Blinding of patients was not possible in most cases where the difference between splint and not having a splint was obvious. Only one study (Burke 1994) reported patient blinding, as the different designs of splint worn were very similar. Assessors and/or clinicians were blinded in six studies (Bardak 2009; Burke 1994; De Angelis 2009; de Entrambasaguas 2006; Premoselli 2006; Sevim 2004).
Incomplete outcome data
Outcome data collected at three months or less was rated as being at low risk of bias in 10 studies (Bardak 2009; Bilgici 2010; Brininger 2007; Celiker 2002; De Angelis 2009; Kumnerddee 2010; Manente 2001; Mishra 2006; Walker 2000; Zinnuroglu 2010) as there were either no missing data or the amount and reasons for missing data were similar across groups. Of the three studies that assessed outcomes after three months post‐treatment (Bardak 2009; De Angelis 2009; Werner 2005) only Werner 2005 was rated as being at high risk of attrition bias, as more than half the participants did not complete outcome assessment at six months post‐treatment.
Selective reporting
Reporting bias was suspected in 10 studies (Arinci Incel 2005; Bardak 2009; Brininger 2007; Burke 1994; Bye 2011; Celiker 2002; Garfinkel 1998; Sevim 2004; Werner 2005; Zinnuroglu 2010), as outcomes were either reported in the Methods section of the publication but not reported in the Results section, or outcomes that were not statistically significant were not reported in full (that is, the study reported insufficient data for inclusion in a meta‐analysis). Our search of trial registries only identified a registry entry for one study (Brininger 2007), so our assessment of selective reporting is limited.
Other potential sources of bias
All studies were judged to be at low risk of other potential sources of bias.
Effects of interventions
See: Table 1
Splint versus no treatment
Two studies compared use of a splint with no treatment (Manente 2001; Premoselli 2006). Manente 2001 compared a splint worn at night for four weeks with no treatment, whereas Premoselli 2006 was a quasi‐randomised trial that compared a splint worn at night for six months with no treatment. Manente 2001 measured outcomes at the end of four weeks of treatment and Premoselli 2006 measured outcomes after the first three months and at the end of six months of treatment. We chose not to combine data in Manente 2001 at four weeks with data in Premoselli 2006 at three months because both studies were at high risk of performance bias due to non‐blinding of participants, Manente 2001 was at unclear risk of selection bias and Premoselli 2006 was rated as being at high risk of selection bias because the sequence used to allocate participants was not random. Therefore, we have provided a narrative description of the results.
Primary outcomes
1) Short‐term overall improvement (three months or less)
Reported as an outcome in Manente 2001 but not Premoselli 2006.
Manente 2001 measured short‐term overall improvement using the Subjects' Global Impression of Change Questionnaire (SGICQ) at the end of four weeks. Overall improvement was 3.86 times more likely in the hand brace group compared with no treatment (risk ratio (RR) 3.86, 95% confidence interval (CI) 2.29 to 6.51; Analysis 1.1). However, the lack of participant blinding may have biased results in favour of the splinting group, as participants' knowledge that they were receiving treatment may have influenced them to exaggerate their rating of improvement.
1.1. Analysis.
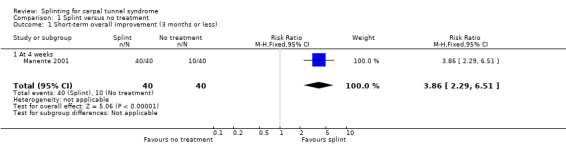
Comparison 1 Splint versus no treatment, Outcome 1 Short‐term overall improvement (3 months or less).
Secondary outcomes
1) Adverse effects
Reported as an outcome in Manente 2001 but not Premoselli 2006.
Three of 40 participants in the splint group compared with no participants in the no treatment group reported difficulty in falling asleep (RR 7.00, 95% CI 0.37 to 131.28; Analysis 1.2), and four of 40 participants in the splint group compared with no participants in the no treatment group reported transient paraesthesias after the splint was removed in the morning (RR 9.00, 95% CI 0.50 to 161.86; Analysis 1.2). The precision of these effect estimates was very low.
1.2. Analysis.
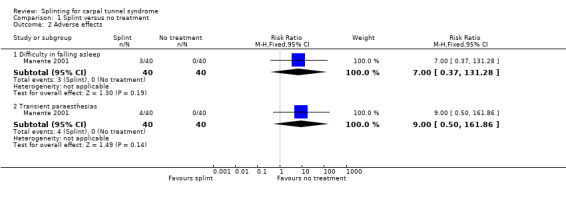
Comparison 1 Splint versus no treatment, Outcome 2 Adverse effects.
2) Short‐term improvement in CTS symptoms (three months or less)
Reported as an outcome in Manente 2001 and Premoselli 2006.
At the end of four weeks of treatment, Manente 2001 found that wrists receiving splint treatment had a symptom score (assessed using the Levine questionnaire (Levine 1993)) which was 1.07 points lower (denoting a better outcome) on a five‐point scale compared with wrists receiving no treatment (mean difference (MD) ‐1.07, 95% CI ‐1.29 to ‐0.85; Analysis 1.3).
1.3. Analysis.
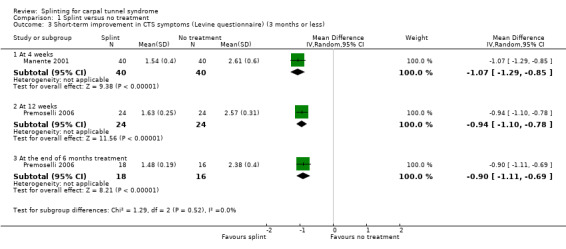
Comparison 1 Splint versus no treatment, Outcome 3 Short‐term improvement in CTS symptoms (Levine questionnaire) (3 months or less).
Premoselli 2006 found that compared with no treatment, wrists receiving a splint had: a symptom score (assessed using the Levine questionnaire (Levine 1993)) which was 0.94 points lower on a five‐point scale after three months of treatment (MD ‐0.94, 95% CI ‐1.10 to ‐0.78; Analysis 1.3) and 0.90 points lower at the end of six months treatment (MD ‐0.90, 95% CI ‐1.11 to ‐0.69; Analysis 1.3); time between the start of pressure and first manifestation of pain in the pressure‐provocative test which was 0.04 seconds more after three months splint treatment (MD 0.04, 95% CI ‐5.31 to 5.39; Analysis 1.4) and 8.25 seconds more at the end of six months treatment (MD 8.25, 95% CI 2.49 to 14.01; Analysis 1.4); and time between the start of pressure and first manifestation of pain in the Phalen test, which was 2.74 seconds more after three months treatment (MD 2.74, 95% CI ‐3.32 to 8.80; Analysis 1.5) and 6.20 seconds slower at the end of six months treatment (MD 6.20, 95% CI ‐1.44 to 13.84; Analysis 1.5). Only the differences between groups on the Levine questionnaire therefore were statistically significant, but all these effect estimates should be interpreted with caution due to the high risk of selection and performance bias in this study.
1.4. Analysis.
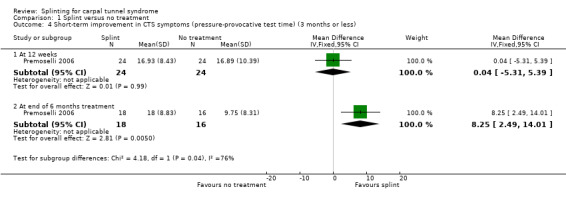
Comparison 1 Splint versus no treatment, Outcome 4 Short‐term improvement in CTS symptoms (pressure‐provocative test time) (3 months or less).
1.5. Analysis.
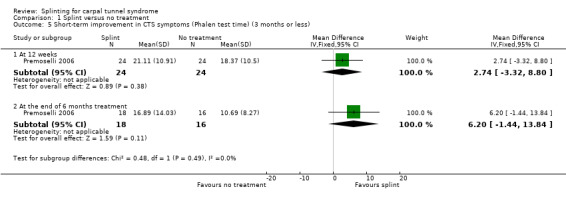
Comparison 1 Splint versus no treatment, Outcome 5 Short‐term improvement in CTS symptoms (Phalen test time) (3 months or less).
3) Short‐term improvement in functional ability or health‐related quality of life (three months or less)
Reported as an outcome in Manente 2001 and Premoselli 2006.
At the end of four weeks of treatment, Manente 2001 found that wrists receiving splint treatment had a functional status score (assessed using the Levine questionnaire (Levine 1993)) which was 0.55 points lower on a five‐point scale compared with wrists receiving no treatment (MD ‐0.55, 95% CI ‐0.82 to ‐0.28; Analysis 1.6).
1.6. Analysis.
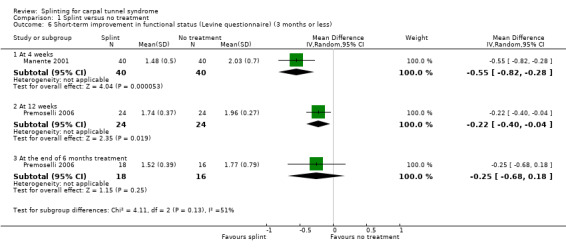
Comparison 1 Splint versus no treatment, Outcome 6 Short‐term improvement in functional status (Levine questionnaire) (3 months or less).
Premoselli 2006 found that wrists receiving splint treatment had a functional status score (assessed using the Levine questionnaire (Levine 1993)) which was 0.22 points lower on a five‐point scale after three months of treatment (MD ‐0.22, 95% CI ‐0.40 to ‐0.04; Analysis 1.6) and 0.25 points lower at the end of six months treatment (MD ‐0.25, 95% CI ‐0.68 to 0.18; Analysis 1.6) compared with wrists receiving no treatment. However, the high risk of selection bias and lack of participant blinding may have influenced participants' self‐reported functional ability.
4) Short‐term improvement in neurophysiologic parameters (three months or less)
Reported as an outcome in Manente 2001 and Premoselli 2006.
Manente 2001 found that at the end of four weeks of treatment, wrists receiving splint had distal motor latency that was 0.02 ms shorter (MD ‐0.02, 95% CI ‐0.49 to 0.45; Analysis 1.7), sensory nerve conduction velocity that was 0.72 m/s faster (MD ‐0.72, 95% CI ‐5.85 to 4.41; Analysis 1.8) and sensory nerve action potential that was 6.30 µV larger (MD 6.30, 95% CI 0.60 to 12.00; Analysis 1.9) compared with wrists receiving no treatment. The precision of each of these effect estimates was low and both positive and negative effects of treatment are plausible.
1.7. Analysis.
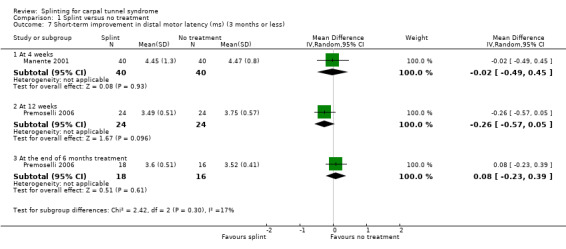
Comparison 1 Splint versus no treatment, Outcome 7 Short‐term improvement in distal motor latency (ms) (3 months or less).
1.8. Analysis.
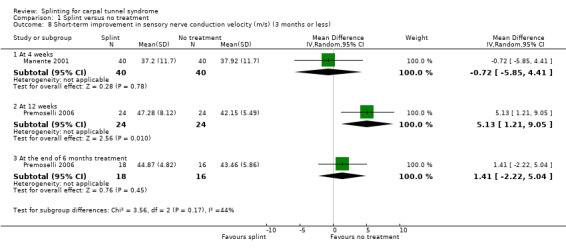
Comparison 1 Splint versus no treatment, Outcome 8 Short‐term improvement in sensory nerve conduction velocity (m/s) (3 months or less).
1.9. Analysis.
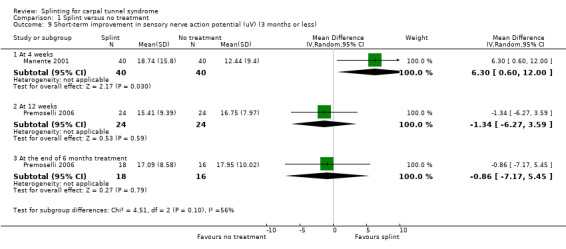
Comparison 1 Splint versus no treatment, Outcome 9 Short‐term improvement in sensory nerve action potential (uV) (3 months or less).
Premoselli 2006 found that when compared with no treatment, wrists receiving splint had distal motor latency that was 0.26 ms shorter after three months of treatment (MD ‐0.26, 95% CI ‐0.57 to 0.05; Analysis 1.7) and 0.08 ms longer at the end of six months of treatment (MD 0.08, 95% CI ‐0.23 to 0.39; Analysis 1.7); sensory nerve conduction velocity that was 5.13 m/s slower after three months of treatment (MD 5.13, 95% CI 1.21 to 9.05; Analysis 1.8) and 1.41 m/s slower at the end of six months of treatment (MD 1.41, 95% CI ‐2.22 to 5.04; Analysis 1.8); sensory nerve action potential that was 1.34 µV smaller after three months of treatment (MD ‐1.34, 95% CI ‐6.27 to 3.59; Analysis 1.9) and 0.86 µV smaller at the end of six months of treatment (MD ‐0.86, 95% CI ‐7.17 to 5.45; Analysis 1.9); distal sensory latency that was 0.26 ms shorter after three months of treatment (MD ‐0.26, 95% CI ‐0.47 to ‐0.05; Analysis 1.10) and 0.10 ms shorter at the end of six months of treatment (MD ‐0.10, 95% CI ‐0.37 to 0.17; Analysis 1.10); motor nerve conduction velocity that was 2.97 m/s slower after three months of treatment (MD 2.97, 95% CI 0.83 to 5.11; Analysis 1.11) and 1.70 m/s slower at the end of six months of treatment (MD 1.70, 95% CI ‐1.17 to 4.57; Analysis 1.11); and motor nerve action potential that was 2.21 mV smaller after three months of treatment (MD ‐2.21, 95% CI ‐4.47 to 0.05; Analysis 1.12) and 1.85 mV larger at the end of six months of treatment (MD 1.85, 95% CI ‐0.49 to 4.19; Analysis 1.12). All these effect estimates had relatively low precision, and even those that are statistically significant should be interpreted with caution due to the high risk of selection bias resulting from quasi‐randomisation.
1.10. Analysis.
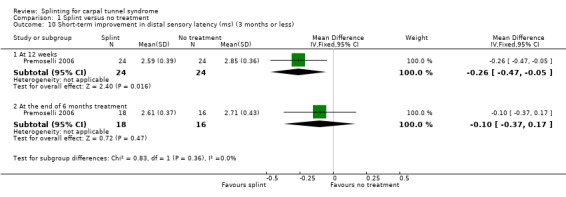
Comparison 1 Splint versus no treatment, Outcome 10 Short‐term improvement in distal sensory latency (ms) (3 months or less).
1.11. Analysis.
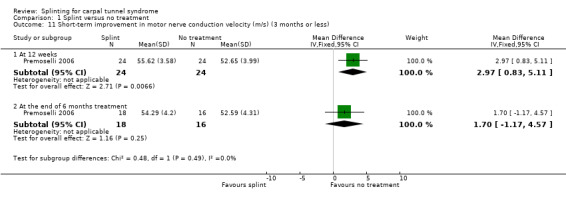
Comparison 1 Splint versus no treatment, Outcome 11 Short‐term improvement in motor nerve conduction velocity (m/s) (3 months or less).
1.12. Analysis.
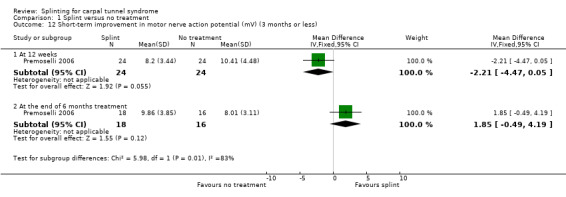
Comparison 1 Splint versus no treatment, Outcome 12 Short‐term improvement in motor nerve action potential (mV) (3 months or less).
5) Long‐term improvement in CTS symptoms (greater than three months)
Not reported as an outcome in Manente 2001 or Premoselli 2006.
6) Long‐term improvement in functional ability or health‐related quality of life (greater than three months)
Not reported as an outcome in Manente 2001 or Premoselli 2006.
Different splint designs
Five studies compared the effectiveness of different splint designs. Brininger 2007 compared fabricated neutral wrist and MCP splint to off‐the‐shelf wrist cock‐up splint, both worn for four weeks. Burke 1994 compared a splint worn at neutral with a splint worn at 20° extension, both worn for two months. Bye 2011 compared a MANU hand brace (which keeps the third and fourth finger in extension, and which was developed by Manente 2001) with a cock‐up splint (with a natural wrist angle), both worn for four weeks. De Angelis 2009 compared the CAMP TIELLE wrist splint with the MANU hand brace, both worn for three months. Zinnuroglu 2010 compared a carpal lock splint with a volar supporting orthosis, both worn for three months. It is unclear whether the correlation between wrists in participants with bilateral CTS in Burke 1994 and Bye 2011 was accounted for in the analysis in these studies, and attempts to retrieve individual wrist outcome data from the trialists were unsuccessful. Therefore, all outcome data reported in these two studies may be invalid due to a unit of analysis error. Without access to the individual wrist data, and without being able to estimate parameters such as the intraclass correlation coefficient from other studies included in the review, we did not attempt to adjust the results of these two studies. We have included the outcome data as reported by the trialists, but emphasise that results of these studies should be interpreted with caution, as the possible lack of adjustment may have produced overly narrow 95% CIs with artificially smaller P values (Higgins 2011c).
None of the studies were deemed sufficiently similar in terms of interventions delivered and outcome data, so no data were combined in a meta‐analysis, therefore we have provided a narrative description of the results.
Primary outcomes
1) Short‐term overall improvement (three months or less)
Reported as an outcome in Brininger 2007 and Burke 1994 and, but not Bye 2011, De Angelis 2009, or Zinnuroglu 2010.
Burke 1994 found that by using an ordinal scale (1 = not at all, 2 = a little, 3 = a lot, 4 = completely) and dichotomising the data into "a lot/complete relief" versus "none/little relief", wearing a neutral splint more than doubled the chance of reporting "a lot/complete relief" at two weeks when compared with wearing an extension splint (RR 2.43, 95% CI 1.12 to 5.28; Analysis 2.1). However, this result should be interpreted with caution as the study is at high risk of selection bias owing to use of a non‐random sequence to allocate participants, and the authors only reported results after two weeks of treatment and omitted reporting the results at the end of two months of treatment.
2.1. Analysis.
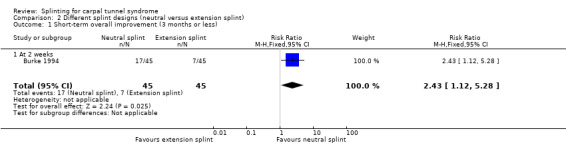
Comparison 2 Different splint designs (neutral versus extension splint), Outcome 1 Short‐term overall improvement (3 months or less).
Brininger 2007 reported measuring the proportion of participants reporting "no to occasional symptoms" at four weeks post‐treatment cessation. However, these data were not reported per intervention group, and attempts to obtain these data from the trialists were unsuccessful, so we were unable to calculate an effect estimate.
Secondary outcomes
1) Adverse effects
Reported as an outcome in Burke 1994 and De Angelis 2009, but not Brininger 2007, Bye 2011, or Zinnuroglu 2010.
Burke 1994 reported that many participants who wore the splint during the day indicated that it was restrictive and that this made it difficult to continue wearing it; however, the authors did not specify the number of participants who reported this complaint, and attempts to obtain these data from the trialists were unsuccessful.
In De Angelis 2009, six participants who wore the CAMP TIELLE splint experienced cutaneous intolerance to the splint, whereas no participants wearing the MANU hand brace experienced any adverse effects (RR 13.28, 95% CI 0.77 to 229.07; Analysis 3.1). However, the 95% CI of this RR is very wide and incorporates effects in either direction.
3.1. Analysis.
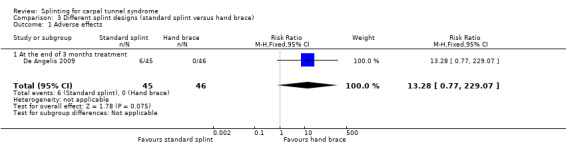
Comparison 3 Different splint designs (standard splint versus hand brace), Outcome 1 Adverse effects.
2) Short‐term improvement in CTS symptoms (three months or less)
Reported as an outcome in Brininger 2007, Burke 1994, Bye 2011, De Angelis 2009, and Zinnuroglu 2010.
Brininger 2007 reported that they measured short‐term symptom severity using the Levine questionnaire (Levine 1993) at the end of the four‐week treatment period, and at four weeks of follow‐up, but the only data reported were change from baseline to end of treatment or follow‐up for all intervention and control groups combined, and only F and P values were reported. Attempts to obtain these data from the trialists were unsuccessful, therefore we were unable to calculate an effect estimate.
Burke 1994 found that wrists wearing a neutral splint had an increased chance of nighttime symptom relief (RR 2.14, 95% CI 0.99 to 4.65; Analysis 2.2) and daytime symptom relief (RR 1.83, 95% CI 0.56 to 5.97; Analysis 2.3) after two weeks of treatment when compared with wrists wearing an extension splint. For the latter two outcomes, there was a large amount of missing data, primarily in the extension splint group, so these results should be interpreted with caution.
2.2. Analysis.
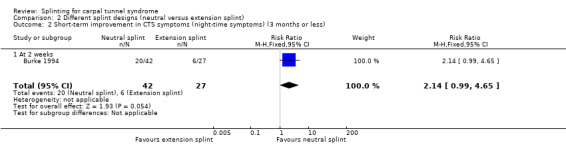
Comparison 2 Different splint designs (neutral versus extension splint), Outcome 2 Short‐term improvement in CTS symptoms (night‐time symptoms) (3 months or less).
2.3. Analysis.
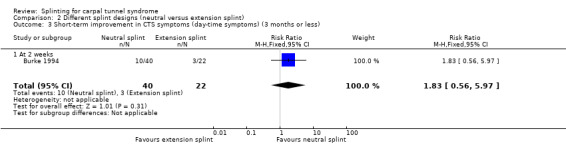
Comparison 2 Different splint designs (neutral versus extension splint), Outcome 3 Short‐term improvement in CTS symptoms (day‐time symptoms) (3 months or less).
Bye 2011 assessed pain on a 100‐point VAS and symptoms on a five‐point scale using the Levine questionnaire after two weeks and at the end of four weeks of treatment. However, it was not clear how many participants were allocated to each group, and attempts to obtain these data from the trialists were unsuccessful, so no data were entered into RevMan. The authors reported that there were only statistically significant differences between the MANU hand brace and wrist cock‐up splint on these outcomes.
At the end of three months of treatment, De Angelis 2009 found that wrists receiving the standard CAMP TIELLE wrist splint had a symptom score (as measured using the Italian‐translated Levine Questionnaire) that was 0.10 points higher on a five‐point scale (MD 0.10, 95% CI ‐0.20 to 0.40; Analysis 3.2), VAS pain that was 0.10 points lower on a 100‐point scale (MD ‐0.10, 95% CI ‐9.54 to 9.34; Analysis 3.3), and VAS paraesthesia that was 10.20 points higher on a 100‐point scale (MD 10.20, 95% CI ‐1.15 to 21.55; Analysis 3.4) compared with wrists receiving the MANU hand brace. None of these effect estimates had 95% CIs incorporating effects falling in one direction only.
3.2. Analysis.
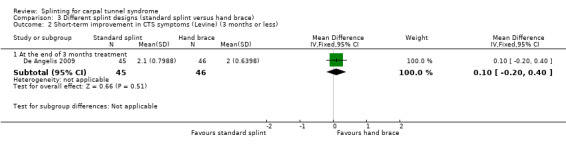
Comparison 3 Different splint designs (standard splint versus hand brace), Outcome 2 Short‐term improvement in CTS symptoms (Levine) (3 months or less).
3.3. Analysis.
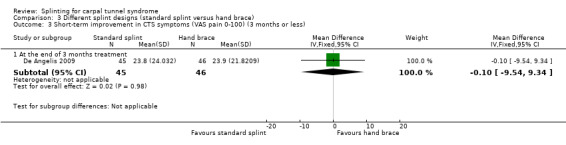
Comparison 3 Different splint designs (standard splint versus hand brace), Outcome 3 Short‐term improvement in CTS symptoms (VAS pain 0‐100) (3 months or less).
3.4. Analysis.
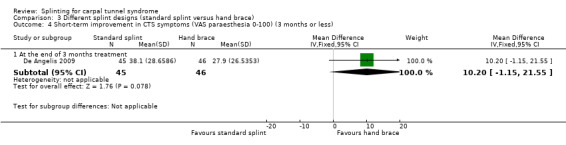
Comparison 3 Different splint designs (standard splint versus hand brace), Outcome 4 Short‐term improvement in CTS symptoms (VAS paraesthesia 0‐100) (3 months or less).
At the end of three months of treatment, Zinnuroglu 2010 found that wrists receiving the carpal lock splint had pain that was 1.10 points lower on a 10‐point VAS (MD ‐1.10, 95% CI ‐2.71 to 0.51; Analysis 4.1) and dysaesthesia that was 0.80 points lower on a 10‐point VAS (MD ‐0.80, 95% CI ‐2.33 to 0.73; Analysis 4.2) compared with wrists receiving the volar supporting orthosis. The 95% CIs suggest that small benefits of either intervention are plausible.
4.1. Analysis.
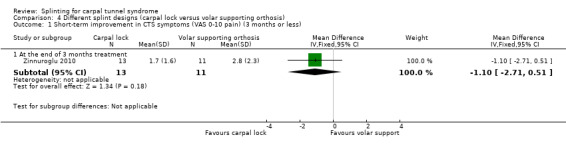
Comparison 4 Different splint designs (carpal lock versus volar supporting orthosis), Outcome 1 Short‐term improvement in CTS symptoms (VAS 0‐10 pain) (3 months or less).
4.2. Analysis.
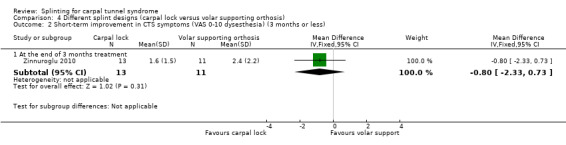
Comparison 4 Different splint designs (carpal lock versus volar supporting orthosis), Outcome 2 Short‐term improvement in CTS symptoms (VAS 0‐10 dysesthesia) (3 months or less).
3) Short‐term improvement in functional ability or health‐related quality of life (three months or less)
Reported as an outcome in Brininger 2007, Bye 2011, and De Angelis 2009, but not Burke 1994 or Zinnuroglu 2010.
Brininger 2007 reported that they measured short‐term functional status (using the Levine questionnaire (Levine 1993)), Moberg Pick‐Up test, grip strength, tip pinch strength, palmar pinch strength, and lateral pinch strength at the end of the four‐week treatment period, and at four weeks' follow‐up, but the only numerical data reported were change from baseline to end of treatment or follow‐up for all intervention and control groups combined, and for most outcomes only F and P values were reported. Attempts to obtain these data from the trialists were unsuccessful, therefore we could enter no data into RevMan 5.
Bye 2011 assessed function on a five‐point scale using the Levine questionnaire after two weeks and at the end of four weeks of treatment. However, no data were entered into RevMan as the number of participants in each group was not clear, and attempts to obtain these data from the trialists were unsuccessful. The authors reported that there were no statistically significant differences between the MANU hand brace and cock‐up splint for this outcome.
De Angelis 2009 found at the end of three months of treatment that functional status score as assessed using the Italian‐translated Levine Questionnaire was 0.20 points lower on a five‐point scale (MD ‐0.20, 95% CI ‐0.46 to 0.06; Analysis 3.5) in wrists receiving the CAMP TIELLE wrist splint compared with wrists receiving the MANU hand brace. The 95% CI suggests that small effects in either direction are plausible.
3.5. Analysis.
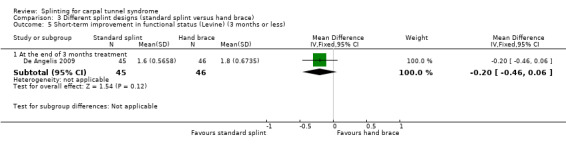
Comparison 3 Different splint designs (standard splint versus hand brace), Outcome 5 Short‐term improvement in functional status (Levine) (3 months or less).
4) Short‐term improvement in neurophysiologic parameters (three months or less)
Reported as an outcome in De Angelis 2009 and Zinnuroglu 2010, but not Brininger 2007, Burke 1994 or Bye 2011.
In De Angelis 2009, at the end of three months of treatment, wrists receiving the standard CAMP TIELLE wrist splint had a median nerve motor distal latency that was 0.10 ms longer (MD 0.10, 95% CI ‐0.34 to 0.54; Analysis 3.6), a sensory nerve conduction velocity that was 0.70 m/s faster (MD ‐0.70, 95% CI ‐3.56 to 2.16; Analysis 3.7) and a sensory nerve action potential (SNAP) amplitude that was 4 µV smaller (MD ‐4.00, 95% CI ‐8.10 to 0.10; Analysis 3.8) when compared with wrists receiving the MANU hand brace. The low precision of these effect estimates means that alternative effects cannot be ruled out.
3.6. Analysis.
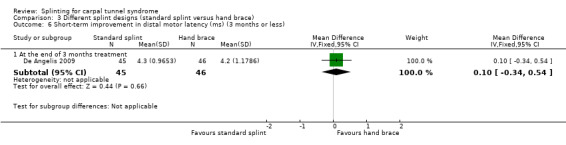
Comparison 3 Different splint designs (standard splint versus hand brace), Outcome 6 Short‐term improvement in distal motor latency (ms) (3 months or less).
3.7. Analysis.
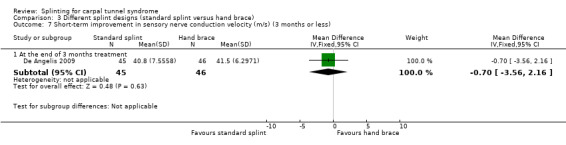
Comparison 3 Different splint designs (standard splint versus hand brace), Outcome 7 Short‐term improvement in sensory nerve conduction velocity (m/s) (3 months or less).
3.8. Analysis.
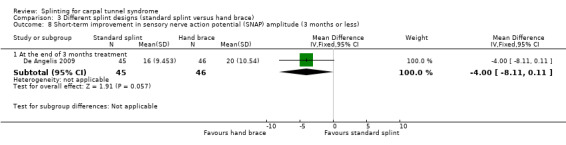
Comparison 3 Different splint designs (standard splint versus hand brace), Outcome 8 Short‐term improvement in sensory nerve action potential (SNAP) amplitude (3 months or less).
Zinnuroglu 2010 found at the end of three months of treatment that wrists wearing the carpal lock splint had a sensory conduction velocity of the second finger‐to‐wrist segment that was 0.30 m/s slower (MD 0.30, 95% CI ‐3.19 to 3.79; Analysis 4.3), and a sensory conduction velocity of the palm‐to‐wrist segment that was 1.30 m/s faster (MD ‐1.30, 95% CI ‐4.82 to 2.22; Analysis 4.4) compared with wrists receiving the volar supporting orthosis. However, the 95% CIs of these effect estimates incorporate effects in both directions.
4.3. Analysis.
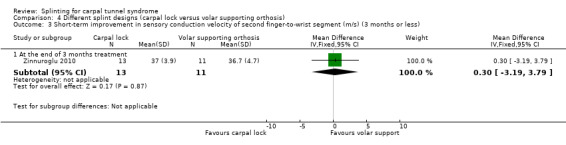
Comparison 4 Different splint designs (carpal lock versus volar supporting orthosis), Outcome 3 Short‐term improvement in sensory conduction velocity of second finger‐to‐wrist segment (m/s) (3 months or less).
4.4. Analysis.
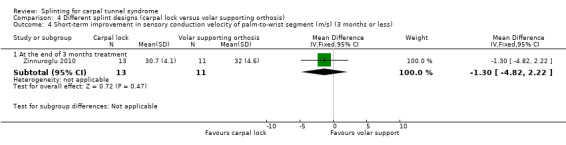
Comparison 4 Different splint designs (carpal lock versus volar supporting orthosis), Outcome 4 Short‐term improvement in sensory conduction velocity of palm‐to‐wrist segment (m/s) (3 months or less).
5) Long‐term improvement in CTS symptoms (greater than three months)
Reported as an outcome in De Angelis 2009, but not Brininger 2007, Burke 1994, Bye 2011, or Zinnuroglu 2010.
Six months post‐treatment, De Angelis 2009 found that wrists receiving the CAMP TIELLE wrist splint had a symptom score (as measured using the Italian‐translated Levine Questionnaire) that was 0.10 points lower on a five‐point scale (MD ‐0.10, 95% CI ‐0.47 to 0.27; Analysis 3.9), VAS pain that was 3.10 points lower on a 100‐point scale (MD ‐3.10, 95% CI ‐14.96 to 8.76; Analysis 3.10), and VAS paraesthesia that was 2.40 points higher on a 100‐point scale (MD 2.40, 95% CI ‐10.33 to 15.13; Analysis 3.11) compared with wrists receiving the MANU hand brace. The precision of both effect estimates was very low and does not rule out a beneficial effect of either intervention.
3.9. Analysis.
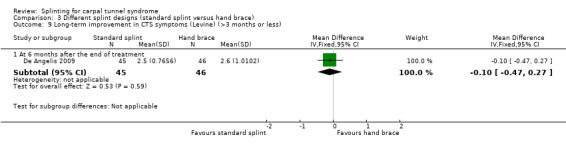
Comparison 3 Different splint designs (standard splint versus hand brace), Outcome 9 Long‐term improvement in CTS symptoms (Levine) (>3 months or less).
3.10. Analysis.
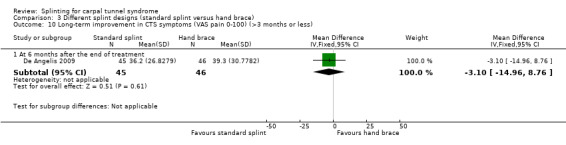
Comparison 3 Different splint designs (standard splint versus hand brace), Outcome 10 Long‐term improvement in CTS symptoms (VAS pain 0‐100) (>3 months or less).
3.11. Analysis.
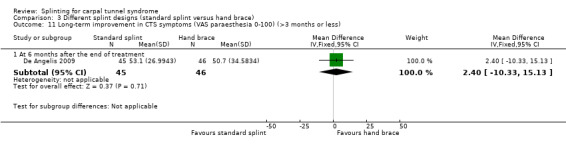
Comparison 3 Different splint designs (standard splint versus hand brace), Outcome 11 Long‐term improvement in CTS symptoms (VAS paraesthesia 0‐100) (>3 months or less).
6) Long‐term improvement in functional ability or health‐related quality of life (greater than three months)
Reported as an outcome in De Angelis 2009, but not Brininger 2007, Burke 1994, Bye 2011, or Zinnuroglu 2010.
At six months post‐treatment, De Angelis 2009 found that functional status score as assessed using the Italian‐translated Levine Questionnaire was 0.20 points lower on a five‐point scale (MD ‐0.20, 95% CI ‐0.57 to 0.17; Analysis 3.12) in wrists receiving the CAMP TIELLE wrist splint compared with wrists receiving the MANU hand brace. The 95% CIs suggest that a small beneficial effect of the MANU hand brace is plausible.
3.12. Analysis.
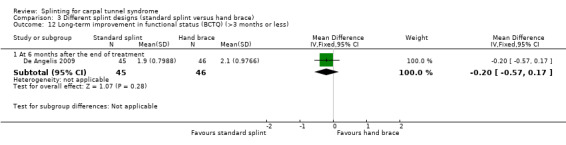
Comparison 3 Different splint designs (standard splint versus hand brace), Outcome 12 Long‐term improvement in functional status (BCTQ) (>3 months or less).
Different splint‐wearing regimens
One study investigated the effect of different splint‐wearing regimens. Walker 2000 compared a splint worn full time for six weeks with a splint worn only at nighttime for six weeks. It is unclear whether the correlation between wrists in participants with bilateral CTS in this study was accounted for in the analysis, and attempts to retrieve individual wrist outcome data from the trialists were unsuccessful. Therefore, all outcome data reported in this study may be invalid due to a unit of analysis error. Without access to the individual wrist data, and without being able to estimate parameters such as the intraclass correlation coefficient from other studies included in the review, we did not attempt to adjust the results of this study. We have included the outcome data as reported by the trialists, but emphasise that results of this study should be interpreted with caution, as the possible lack of adjustment may have produced overly narrow 95% CIs with artificially smaller P values (Higgins 2011c).
Primary outcomes
1) Short‐term overall improvement (three months or less)
Not reported as an outcome.
Secondary outcomes
1) Adverse effects
Not reported as an outcome.
2) Short‐term improvement in CTS symptoms (three months or less)
Walker 2000 found at the end of six weeks of treatment that wrists wearing a splint full time had a symptom score (as assessed using the Levine questionnaire) that was 0.21 points lower on a five‐point scale (MD ‐0.21, 95% CI ‐0.83 to 0.41; Analysis 5.1), though the precision of this effect estimate was low, and a small greater benefit of nighttime splint is plausible.
5.1. Analysis.
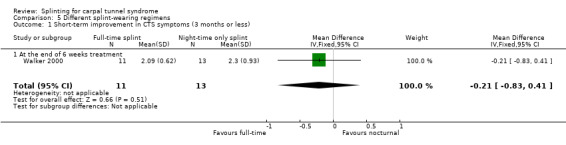
Comparison 5 Different splint‐wearing regimens, Outcome 1 Short‐term improvement in CTS symptoms (3 months or less).
3) Short‐term improvement in functional ability or health‐related quality of life (three months or less)
Wrists wearing a splint full time had a functional status score (as assessed using Levine questionnaire) that was also 0.21 points lower on a five‐point scale at the end of six weeks of treatment (MD ‐0.21, 95% CI ‐0.87 to 0.45; Analysis 5.2), though similar to the symptom score, the 95% CI incorporates an effect in both directions.
5.2. Analysis.
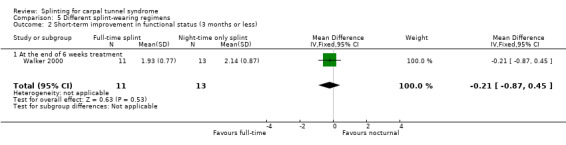
Comparison 5 Different splint‐wearing regimens, Outcome 2 Short‐term improvement in functional status (3 months or less).
4) Short‐term improvement in neurophysiologic parameters (three months or less)
In wrists wearing a splint full time, motor distal latency was 0.63 ms shorter (MD ‐0.63, 95% CI ‐2.05 to 0.79; Analysis 5.3) and sensory distal latency was 0.05 ms longer (MD 0.05, 95% CI ‐0.62 to 0.72; Analysis 5.4) than wrists wearing a splint at nighttime only, at the end of six weeks of treatment. No clear benefit of full‐time splint over nighttime splint was found though, as the precision of these effect estimates was low.
5.3. Analysis.
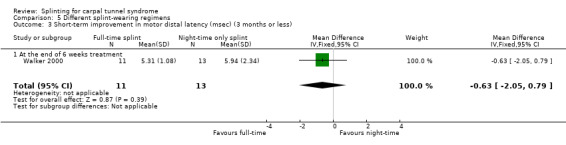
Comparison 5 Different splint‐wearing regimens, Outcome 3 Short‐term improvement in motor distal latency (msec) (3 months or less).
5.4. Analysis.
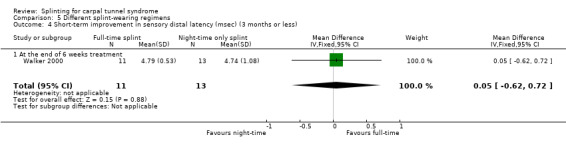
Comparison 5 Different splint‐wearing regimens, Outcome 4 Short‐term improvement in sensory distal latency (msec) (3 months or less).
5) Long‐term improvement in CTS symptoms (greater than three months)
Not reported as an outcome.
6) Long‐term improvement in functional ability or health‐related quality of life (greater than three months)
Not reported as an outcome.
Splint (single intervention) versus other non‐surgical intervention
Seven studies investigated this splint (delivered as a single intervention) versus another non‐surgical intervention. Brininger 2007 compared a fabricated neutral wrist and MCP splint with a fabricated neutral wrist and MCP splint plus nerve and tendon gliding exercises for four weeks, and to off‐the‐shelf wrist cock‐up splint plus nerve and tendon gliding exercises for four weeks. de Entrambasaguas 2006 compared splint versus steroid injection versus phonophoresis (the therapeutic application of ultrasound to enhance the absorption of topically applied analgesics and anti‐inflammatory agents), for four weeks. Garfinkel 1998 compared splint with yoga for eight weeks. Kumnerddee 2010 compared a neutral volar wrist splint worn at night only to acupuncture delivered twice a week for five weeks. Madjdinasab 2008 compared a splint worn for six weeks with 20 mg oral corticosteroid taken daily for two weeks. Mishra 2006 compared a splint worn for four weeks with 20 mg oral steroid taken for two weeks and 10 mg oral steroid taken for two weeks. Sevim 2004 compared a splint worn nightly for one year with proximal steroid injections and with distal steroid injections.
It is unclear whether the correlation between wrists in participants with bilateral CTS in de Entrambasaguas 2006, Garfinkel 1998, and Madjdinasab 2008 was accounted for in the analysis in these studies, whereas, the correlation between wrists was clearly not accounted for in Mishra 2006. Attempts to retrieve individual wrist outcome data from the trialists were unsuccessful. Therefore, all outcome data reported in these four studies are either clearly or possibly invalid due to a unit of analysis error. Without access to the individual wrist data, and without being able to estimate parameters such as the intraclass correlation coefficient from other studies included in the review, we did not attempt to adjust the results of these four studies. We have included the outcome data as reported by the trialists, but emphasise that results of these studies should be interpreted with caution, as the possible lack of adjustment may have produced overly narrow 95% CIs with artificially smaller P values in de Entrambasaguas 2006, Garfinkel 1998, and Mishra 2006, and overly wide 95% CIs in Madjdinasab 2008 (Higgins 2011c).
Only the studies conducted by Madjdinasab 2008 and Mishra 2006 were deemed sufficiently similar in terms of interventions and outcomes, but because the it was not clear whether allocation was concealed and there was a high risk of performance bias resulting from lack of patient blinding, we chose not to combine the data from these studies in a meta‐analysis. We have provided a narrative description of the results.
Primary outcomes
1) Short‐term overall improvement (three months or less)
Reported as an outcome in Brininger 2007, but not de Entrambasaguas 2006, Garfinkel 1998, Kumnerddee 2010, Madjdinasab 2008, Mishra 2006 or Sevim 2004.
Brininger 2007 reported that the proportion of participants reporting "no to occasional symptoms" at four weeks after treatment finished. However, these data were not reported per intervention group, and attempts to obtain these data from the trialists were unsuccessful, so we were unable to calculate an effect estimate.
Secondary outcomes
1) Adverse effects
Reported as an outcome in Kumnerddee 2010, Mishra 2006 and Sevim 2004, but not Brininger 2007, de Entrambasaguas 2006, Garfinkel 1998, or Madjdinasab 2008.
Kumnerddee 2010 found that no participants wearing a splint at night reported any adverse effects, whereas six participants receiving acupuncture experienced temporary skin bruising at the wrist or elbow due to small vessel damage (RR 0.08; 95% CI 0.004 to 1.31; Analysis 7.1).
7.1. Analysis.
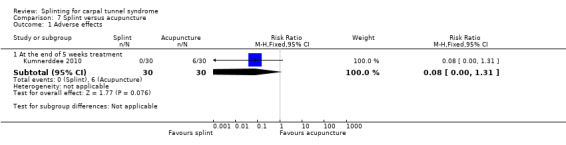
Comparison 7 Splint versus acupuncture, Outcome 1 Adverse effects.
Mishra 2006 reported that two participants in the splint group reported discomfort and swelling of the wrist and hands and that no participants in the oral steroids group experienced adverse effects (RR 4.86, 95% CI 0.24 to 97.86; Analysis 8.1). However, the precision of this effect estimate is very low and the 95% CIs incorporate effects in either direction.
8.1. Analysis.
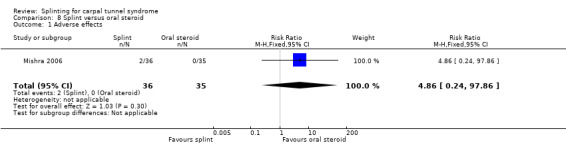
Comparison 8 Splint versus oral steroid, Outcome 1 Adverse effects.
Sevim 2004 recorded adverse effects but reported that "Of the 60 participants in the splint group, 9 wore the splints on average 1‐5 nights per week and were excluded. Twenty‐three from this group wore the splints less than 1 night per week and were considered to form a control group." Thus, as the randomisation schedule was manipulated, resulting in a high risk of attrition bias, we chose not to include any data from this study in the review.
2) Short‐term improvement in CTS symptoms (three months or less)
Reported as an outcome in Brininger 2007, de Entrambasaguas 2006, Garfinkel 1998, Kumnerddee 2010, Mishra 2006 and Sevim 2004, but not Madjdinasab 2008.
Brininger 2007 reported that they measured short‐term symptom severity using the Levine questionnaire (Levine 1993) at the end of the four‐week treatment period, and at four weeks of follow‐up, but the only data reported were change from baseline to end of treatment or follow‐up for all intervention and control groups combined, and only F and P values were reported. Attempts to obtain these data from the trialists were unsuccessful, therefore we were unable to calculate an effect estimate.
de Entrambasaguas 2006 was written in Spanish and reported measuring sensory symptoms (tingling, numbness, pain and autonomic manifestations) and Tinel's test, but numerical data could not be translated into English, and attempts to obtain these data from the trialists were unsuccessful, so no data were entered into RevMan. In the English abstract, the authors reported that there were no differences between treatments in the clinical parameters.
At the end of eight weeks of treatment, Garfinkel 1998 found that pain intensity for the previous week was 1.40 points higher on a 0 to 10 VAS in participants receiving splint compared with participants receiving yoga (MD 1.40, 95% CI 0.07 to 2.73; Analysis 6.1). Garfinkel 1998 also found fewer participants assigned to splints experienced improvement in sleep disturbance (RR 0.47, 95% CI 0.10 to 2.25; Analysis 6.2), improvement in Tinel's test (RR 0.47, 95% CI 0.13 to 1.66; Analysis 6.3), and improvement in Phalen's test (RR 0.19, 95% CI 0.05 to 0.78; Analysis 6.4) compared with participants receiving yoga. However, these results should be interpreted with caution as participants were not blind to treatment and their expectations regarding the potential effectiveness of yoga over splint may have biased their responses.
6.1. Analysis.
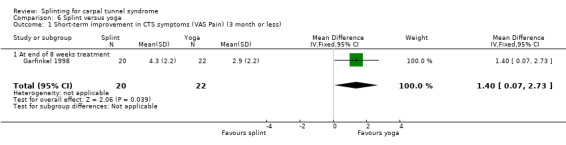
Comparison 6 Splint versus yoga, Outcome 1 Short‐term improvement in CTS symptoms (VAS Pain) (3 month or less).
6.2. Analysis.
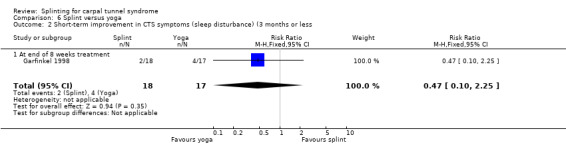
Comparison 6 Splint versus yoga, Outcome 2 Short‐term improvement in CTS symptoms (sleep disturbance) (3 months or less.
6.3. Analysis.
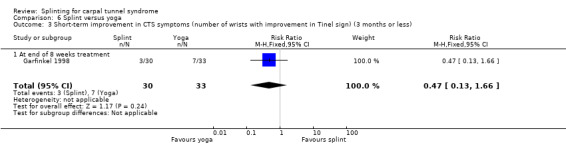
Comparison 6 Splint versus yoga, Outcome 3 Short‐term improvement in CTS symptoms (number of wrists with improvement in Tinel sign) (3 months or less).
6.4. Analysis.
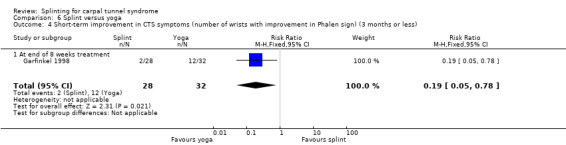
Comparison 6 Splint versus yoga, Outcome 4 Short‐term improvement in CTS symptoms (number of wrists with improvement in Phalen sign) (3 months or less).
At the end of five weeks treatment, Kumnerddee 2010 found that symptom severity (assessed using the Thai version of the Boston Carpal Tunnel Questionnaire (Levine 1993) was 0.09 points higher (worse) on a five‐point scale (MD 0.09; 95% CI ‐0.14 to 0.32; Analysis 7.2), and pain was 9.63 points higher on a 100 mm visual analogue scale (MD 9.63; 95% CI ‐0.01 to 19.27; Analysis 7.3) in the group receiving a night splint compared with the group receiving acupuncture. Both these effect estimates have 95% CIs incorporating effects in either direction, and should be interpreted with caution as participants were not blind to treatment and their expectations regarding the potential effectiveness of acupuncture over splint may have biased their responses.
7.2. Analysis.
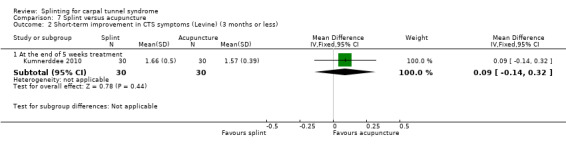
Comparison 7 Splint versus acupuncture, Outcome 2 Short‐term improvement in CTS symptoms (Levine) (3 months or less).
7.3. Analysis.
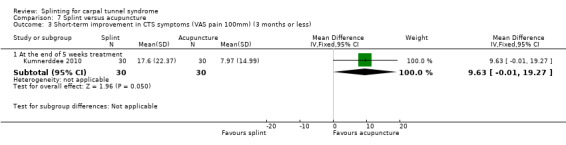
Comparison 7 Splint versus acupuncture, Outcome 3 Short‐term improvement in CTS symptoms (VAS pain 100mm) (3 months or less).
In Mishra 2006, symptom severity (assessed using the Levine questionnaire (Levine 1993)) was 0.21 points higher on a five‐point scale at the end of four weeks of treatment (MD 0.21, 95% CI ‐0.02 to 0.44; Analysis 8.2) and 0.25 points higher eight weeks post‐treatment (0.25, 95% CI ‐0.03 to 0.53; Analysis 8.2) in wrists receiving a splint compared with wrists receiving oral steroids. The 95% CIs of these effect estimates suggest that only very small benefits associated with using a splint over oral steroids are plausible.
8.2. Analysis.
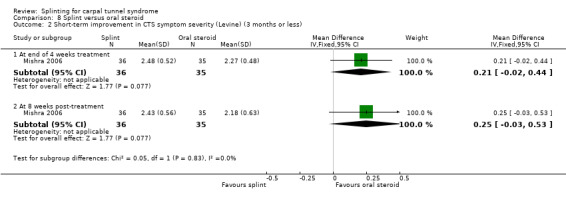
Comparison 8 Splint versus oral steroid, Outcome 2 Short‐term improvement in CTS symptom severity (Levine) (3 months or less).
Two clinicians in Sevim 2004 assessed the number and severity of neurologic symptoms (for example, numbness, pain, paraesthesia, swelling, sense of swelling, drying or colour change in the related hand; numbness, pain, paraesthesia of the forearm and arm; provocation of symptoms by housework, reading and driving; existence of nighttime symptoms; awakening due to nighttime symptoms; frequency of nighttime symptoms; numb hand upon awakening in the morning) at the end of 12 months of treatment (assessed at a mean 11 months, range nine to 14 months). However, no data were included in the review because of the high risk of attrition bias (described above under 'Adverse effects').
3) Short‐term improvement in functional ability or health‐related quality of life (three months or less)
Reported as an outcome in Brininger 2007, Garfinkel 1998, Kumnerddee 2010, and Mishra 2006, but not de Entrambasaguas 2006, Madjdinasab 2008 or Sevim 2004.
Brininger 2007 reported that they measured short‐term functional status (using the Levine questionnaire (Levine 1993)), Moberg Pick‐Up test, grip strength, tip pinch strength, palmar pinch strength, and lateral pinch strength at end of the four‐week treatment period, and at four weeks of follow‐up, but the only numerical data reported was change from baseline to end of treatment or follow‐up for all intervention and control groups combined, and for most outcomes only F and P values were reported. Attempts to obtain these data from the trialists were unsuccessful, therefore no data could be entered in RevMan 5.
Garfinkel 1998 found that the participants receiving splint treatment had a grip strength which was 3.10 mmHg better immediately after eight weeks of treatment than participants receiving yoga (MD 3.10, 95% CI ‐31.06 to 37.26; Analysis 6.5), though the precision of this estimate is very low and opposite effects of treatment cannot be ruled out.
6.5. Analysis.
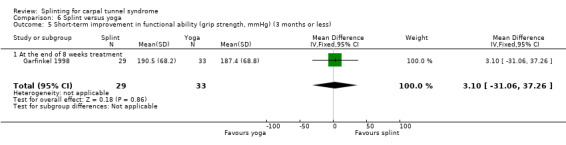
Comparison 6 Splint versus yoga, Outcome 5 Short‐term improvement in functional ability (grip strength, mmHg) (3 months or less).
At the end of five weeks of treatment, Kumnerddee 2010 found that functional status (assessed using the Thai version of the Boston Carpal Tunnel Questionnaire (Levine 1993) was 0.04 points higher (worse) on a 5‐point scale (MD 0.04; 95% CI ‐0.18 to 0.26; Analysis 7.4) in the group receiving a night splint compared with the group receiving acupuncture. This effect estimate has a 95% CI incorporating effects in either direction, and should be interpreted with caution as participants were not blind to treatment and their expectations regarding the potential effectiveness of acupuncture over splint may have biased their responses.
7.4. Analysis.
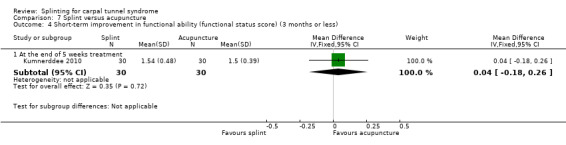
Comparison 7 Splint versus acupuncture, Outcome 4 Short‐term improvement in functional ability (functional status score) (3 months or less).
In Mishra 2006, functional status score (assessed using the Levine questionnaire (Levine 1993)) was 0.12 points higher on a five‐point scale at the end of four weeks of treatment (MD 0.12, 95% CI ‐0.05 to 0.29; Analysis 8.3) and 0.12 points higher eight weeks post‐treatment (0.12, 95% CI ‐0.06 to 0.30; Analysis 8.3) in wrists receiving a splint compared with wrists receiving oral steroids. Similar to the symptom score, the 95% CIs of these effect estimates suggest that only very small benefits associated with splint over oral steroid are plausible.
8.3. Analysis.
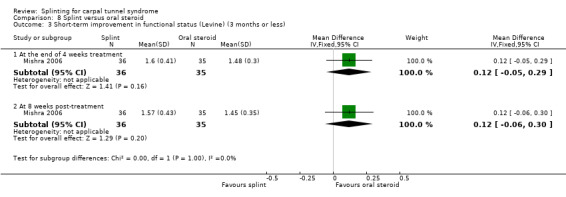
Comparison 8 Splint versus oral steroid, Outcome 3 Short‐term improvement in functional status (Levine) (3 months or less).
4) Short‐term improvement in neurophysiologic parameters (three months or less)
Reported as an outcome in de Entrambasaguas 2006, Garfinkel 1998, Madjdinasab 2008, Mishra 2006, and Sevim 2004, but not Brininger 2007 or Kumnerddee 2010.
de Entrambasaguas 2006 reported measuring nerve conduction, though no numerical data could be translated into English, and attempts to obtain these data from the trialists were unsuccessful, so no data were included in the review.
At the end of eight weeks of treatment, Garfinkel 1998 found participants receiving splint had a median nerve motor distal latency which was 0.25 ms longer (MD 0.25, 95% CI ‐0.37 to 0.87; Analysis 6.6) and a median nerve sensory distal latency which was 0.39 ms longer (MD 0.39, 95% CI ‐0.35 to 1.13; Analysis 6.7) compared with participants receiving yoga. However, the 95% CIs for these effect estimates incorporate both positive and negative effects of treatment.
6.6. Analysis.
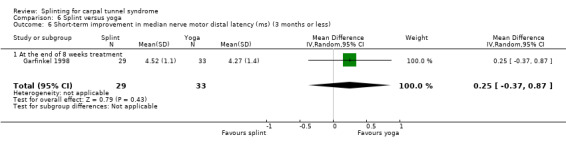
Comparison 6 Splint versus yoga, Outcome 6 Short‐term improvement in median nerve motor distal latency (ms) (3 months or less).
6.7. Analysis.
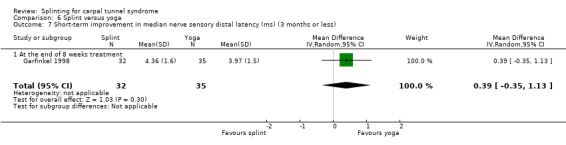
Comparison 6 Splint versus yoga, Outcome 7 Short‐term improvement in median nerve sensory distal latency (ms) (3 months or less).
Mishra 2006 found that wrists receiving splint had motor distal latency that was 0.14 ms longer at the end of four weeks of treatment (MD 0.14, 95% CI ‐0.18 to 0.46; Analysis 8.4) and 0.27 ms longer at eight weeks post‐treatment (MD 0.27, 95% CI ‐0.03 to 0.57; Analysis 8.4); sensory distal latency that was 0.33 ms longer at the end of four weeks of treatment (MD 0.33, 95% CI ‐0.06 to 0.72; Analysis 8.5) and 0.56 ms longer eight weeks post‐treatment (MD 0.56, 95% CI 0.28 to 0.84; Analysis 8.5); motor nerve conduction velocity that was 0.14 m/s faster at the end of four weeks of treatment (MD ‐0.14, 95% CI ‐3.84 to 3.56; Analysis 8.6) and 3.28 m/s faster at eight weeks post‐treatment (MD ‐3.28, 95% CI ‐6.35 to ‐0.21; Analysis 8.6); and sensory nerve conduction velocity that was 0.65 m/s slower at the end of four weeks of treatment (MD 0.65, 95% CI ‐3.02 to 4.32; Analysis 8.7) and 3.95 m/s faster at eight weeks post‐treatment (MD ‐3.95, 95% CI ‐7.60 to ‐0.30; Analysis 8.7).
8.4. Analysis.
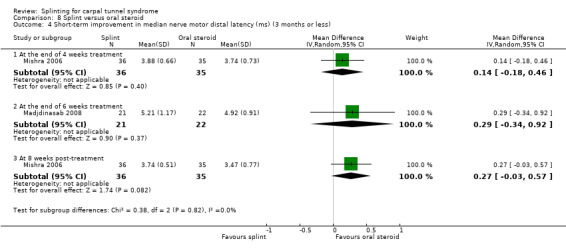
Comparison 8 Splint versus oral steroid, Outcome 4 Short‐term improvement in median nerve motor distal latency (ms) (3 months or less).
8.5. Analysis.
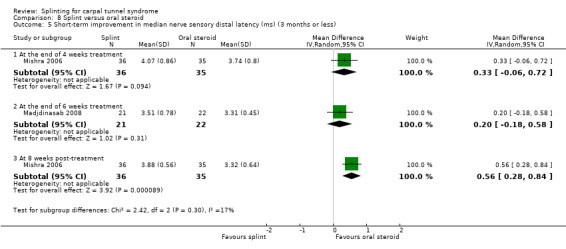
Comparison 8 Splint versus oral steroid, Outcome 5 Short‐term improvement in median nerve sensory distal latency (ms) (3 months or less).
8.6. Analysis.
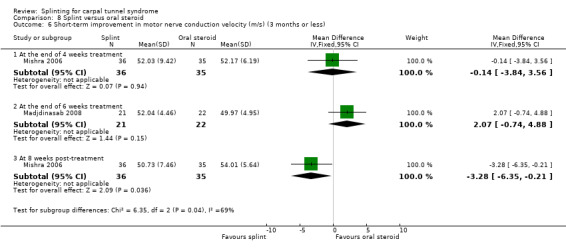
Comparison 8 Splint versus oral steroid, Outcome 6 Short‐term improvement in motor nerve conduction velocity (m/s) (3 months or less).
8.7. Analysis.
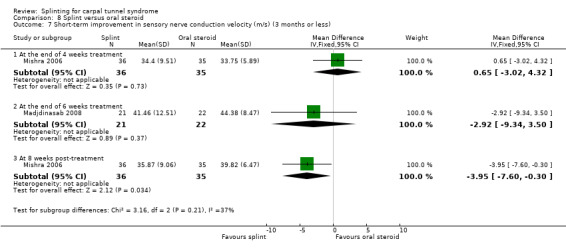
Comparison 8 Splint versus oral steroid, Outcome 7 Short‐term improvement in sensory nerve conduction velocity (m/s) (3 months or less).
At the end of six weeks of treatment, Madjdinasab 2008 found that participants receiving splints had a motor distal latency that was 0.29 ms longer (MD 0.29, 95% CI ‐0.34 to 0.92; Analysis 8.4) and sensory distal latency that was 0.20 ms longer (MD 0.20, 95% CI ‐0.18 to 0.58; Analysis 8.5), a motor nerve conduction velocity that was 2.07 m/s slower (MD 2.07, 95% CI ‐0.74 to 4.88; Analysis 8.6), and a sensory nerve conduction velocity that was 2.92 m/s faster (MD ‐2.92, 95% CI ‐9.34 to 3.50; Analysis 8.7) than participants receiving oral steroids. None of the 95% CIs of these effect estimates rule out the possibility for alternative effects of these interventions.
Sevim 2004 measured nerve conduction at the end of 12 months of treatment, but no data were included in the review because of the high risk of attrition bias (described above under 'Adverse effects').
5) Long‐term improvement in CTS symptoms (greater than three months)
Not reported as an outcome in Brininger 2007, de Entrambasaguas 2006, Garfinkel 1998, Kumnerddee 2010, Madjdinasab 2008, Mishra 2006 or Sevim 2004
6) Long‐term improvement in functional ability or health‐related quality of life (greater than three months)
Not reported as an outcome in Brininger 2007, de Entrambasaguas 2006, Garfinkel 1998, Kumnerddee 2010, Madjdinasab 2008, Mishra 2006 or Sevim 2004
Splint (as part of multiple interventions) versus other non‐surgical intervention
Five studies investigated splint delivered as part of a multi‐component intervention with another non‐surgical intervention. Arinci Incel 2005 compared splint plus nerve and tendon gliding exercises with gabapentin plus nerve and tendon gliding exercises for six months. Bardak 2009 compared splint plus steroid injection with splint plus steroid injection plus nerve and tendon gliding exercises or nerve and tendon gliding exercises alone for six weeks. Bilgici 2010 compared splint plus steroid injection with therapeutic ultrasound for four weeks. Celiker 2002 compared splint plus nonsteroidal anti‐inflammatory drug (NSAID) with local corticosteroid injection for eight weeks. Werner 2005 compared splint plus ergonomic education with ergonomic education alone for six weeks. Of the three interventions delivered in Bardak 2009, we only compared splint plus steroid injection with nerve and tendon gliding exercises, and splint plus steroid injection plus nerve and tendon gliding exercises with nerve and tendon gliding exercises (splint plus steroid injection compared with splint plus steroid injection plus nerve and tendon gliding exercises was not analysed in this review as splint was delivered to both groups in this comparison).
It is unclear whether the correlation between wrists in participants with bilateral CTS in Bilgici 2010 and Celiker 2002 was accounted for in the analysis in these studies, whereas the correlation between wrists was clearly not accounted for in Arinci Incel 2005. Attempts to retrieve individual wrist outcome data from the trialists were unsuccessful. Therefore, all outcome data reported in these three studies are either clearly or possibly invalid due to a unit of analysis error. Without access to the individual wrist data, and without being able to estimate parameters such as the intraclass correlation coefficient from other studies included in the review, we did not attempt to adjust the results of these three studies. We have included the outcome data as reported by the trialists, but emphasise that results of these studies should be interpreted with caution, as the possible lack of adjustment may have produced overly narrow 95% CIs with artificially smaller P values for all outcomes in Arinci Incel 2005 and Bilgici 2010, and for the outcomes, Tinel's test, Phalen's test, reverse Phalen's test, and neurophysiologic parameters (which were analysed at the wrist‐level) in Celiker 2002, and overly wide 95% CIs in Celiker 2002 for the VAS pain and Levine symptom severity score (which were analysed at the participant‐level) (Higgins 2011c).
None of the studies were deemed sufficiently similar in terms of interventions delivered and outcome data reported, so no data were combined in a meta‐analysis, thus we have provided a narrative description of the results.
Primary outcomes
1) Short‐term overall improvement (three months or less)
Not reported as an outcome in Arinci Incel 2005, Bardak 2009, Bilgici 2010, Celiker 2002 or Werner 2005.
Secondary outcomes
1) Adverse effects
Reported as an outcome in Arinci Incel 2005, Bilgici 2010, and Celiker 2002, but not Bardak 2009 or Werner 2005.
Arinci Incel 2005 reported that no major adverse effects were reported and "the most common complaints were somnolence and dizziness which faded in two to three weeks." However, the authors did not report how many of these common complaints occurred in each group.
Bilgici 2010 found some participants receiving splint plus local corticosteroid injection reported transient local injection pain (however, the number of participants reporting this was not reported), whereas no adverse effects due to ultrasound treatment were reported by participants.
In Celiker 2002, no participant in either group reported any complications or adverse effects.
2) Short‐term improvement in CTS symptoms (three months or less)
Reported as an outcome in Arinci Incel 2005, Bardak 2009, Bilgici 2010, Celiker 2002, and Werner 2005.
Arinci Incel 2005 measured pain using a VAS (0 to 10) and CTS symptoms using the Levine Questionnaire (Levine 1993) at the end of six months of treatment. However, means and SDs at this time point were only reported for the gabapentin plus nerve and tendon gliding exercises group (no data for the splint plus nerve and tendon gliding exercises were reported, and attempts to obtain these data from the trialists were unsuccessful). According to the authors, "There were no statistically significant differences for any of the post‐treatment measures between groups".
Bardak 2009 found at the end of six weeks of treatment that wrists receiving splint plus steroid injection had a lower (better) symptom total score compared with wrists receiving nerve and tendon gliding exercises (MD 2.31, 95% CI 1.59 to 3.03; Analysis 9.1). The authors also found that fewer wrists receiving splint plus steroid injection had a positive Tinel's test (RR 1.41, 95% CI 0.84 to 2.35; Analysis 9.2), Phalen's test (RR 1.23, 95% CI 0.83 to 1.82; Analysis 9.3) and compression test (RR 1.28, 95% CI 0.65 to 2.53; Analysis 9.4) than wrists receiving nerve and tendon gliding exercises, whereas more wrists receiving splint plus steroid injection had a positive reverse Phalen's test (RR 1.02, 95% CI 0.59 to 1.79; Analysis 9.5). Further, at the end of six weeks of treatment, wrists receiving splint plus steroid injection plus nerve and tendon gliding exercises had a lower (better) symptom total score compared with wrists receiving nerve and tendon gliding exercises (MD ‐2.81, 95% CI ‐3.49 to ‐2.13; Analysis 10.1). At this time point fewer wrists receiving splint plus steroid injection plus nerve and tendon gliding exercises had a positive Phalen's test (RR 0.95, 95% CI 0.66 to 1.38; Analysis 10.2) than wrists receiving nerve and tendon gliding exercises, whereas more wrists receiving splint plus steroid injection plus nerve and tendon gliding exercises had a positive Tinel's test (RR 1.22, 95% CI 0.81 to 1.84; Analysis 10.3), reverse Phalen's test (RR 1.21, 95% CI 0.71 to 2.06; Analysis 10.4), and compression test (RR 1.42, 95% CI 0.80 to 2.51; Analysis 10.5). Of all these effect estimates, only the 95% CIs of the symptom total scores suggested that plausible values of effect were in one direction only.
9.1. Analysis.
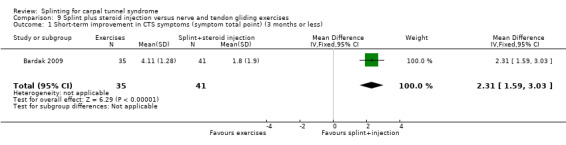
Comparison 9 Splint plus steroid injection versus nerve and tendon gliding exercises, Outcome 1 Short‐term improvement in CTS symptoms (symptom total point) (3 months or less).
9.2. Analysis.
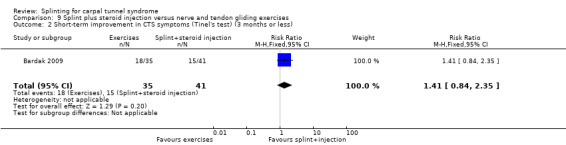
Comparison 9 Splint plus steroid injection versus nerve and tendon gliding exercises, Outcome 2 Short‐term improvement in CTS symptoms (Tinel's test) (3 months or less).
9.3. Analysis.
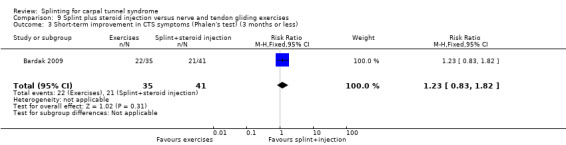
Comparison 9 Splint plus steroid injection versus nerve and tendon gliding exercises, Outcome 3 Short‐term improvement in CTS symptoms (Phalen's test) (3 months or less).
9.4. Analysis.
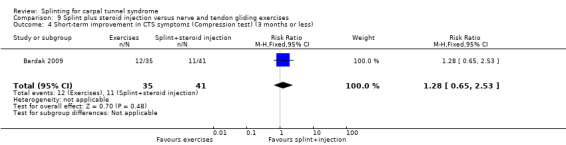
Comparison 9 Splint plus steroid injection versus nerve and tendon gliding exercises, Outcome 4 Short‐term improvement in CTS symptoms (Compression test) (3 months or less).
9.5. Analysis.
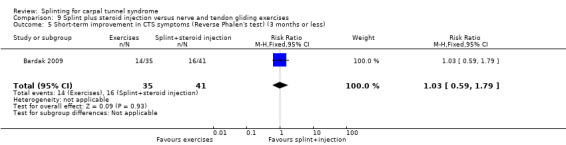
Comparison 9 Splint plus steroid injection versus nerve and tendon gliding exercises, Outcome 5 Short‐term improvement in CTS symptoms (Reverse Phalen's test) (3 months or less).
10.1. Analysis.
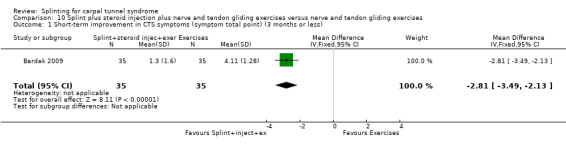
Comparison 10 Splint plus steroid injection plus nerve and tendon gliding exercises versus nerve and tendon gliding exercises, Outcome 1 Short‐term improvement in CTS symptoms (symptom total point) (3 months or less).
10.2. Analysis.
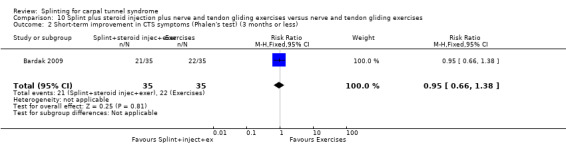
Comparison 10 Splint plus steroid injection plus nerve and tendon gliding exercises versus nerve and tendon gliding exercises, Outcome 2 Short‐term improvement in CTS symptoms (Phalen's test) (3 months or less).
10.3. Analysis.
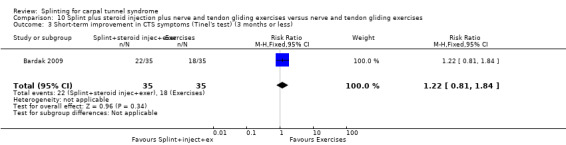
Comparison 10 Splint plus steroid injection plus nerve and tendon gliding exercises versus nerve and tendon gliding exercises, Outcome 3 Short‐term improvement in CTS symptoms (Tinel's test) (3 months or less).
10.4. Analysis.
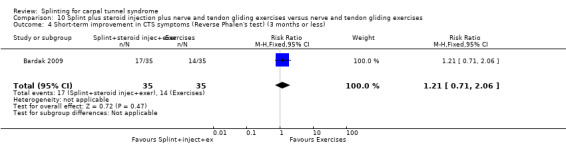
Comparison 10 Splint plus steroid injection plus nerve and tendon gliding exercises versus nerve and tendon gliding exercises, Outcome 4 Short‐term improvement in CTS symptoms (Reverse Phalen's test) (3 months or less).
10.5. Analysis.
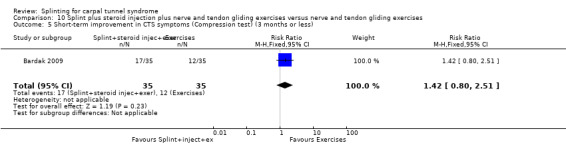
Comparison 10 Splint plus steroid injection plus nerve and tendon gliding exercises versus nerve and tendon gliding exercises, Outcome 5 Short‐term improvement in CTS symptoms (Compression test) (3 months or less).
Bilgici 2010 reported that wrists receiving splint plus local corticosteroid injection had a symptom severity score (measured using a Turkish‐validated version of the Levine questionnaire (Levine 1993)) that was 0.66 points higher at the end of four weeks of treatment (MD ‐0.66, 95% CI ‐1.89 to 0.57; Analysis 11.1), but 0.18 points lower four weeks post‐treatment (MD 0.18, 95% CI ‐0.45 to 0.81; Analysis 11.1), and pain (measured using a VAS; scale units not reported) that was 0.55 points higher at the end of four weeks of treatment (MD ‐0.55, 95% CI ‐2.17 to 1.07; Analysis 11.2) and 0.12 points higher four weeks post‐treatment (MD ‐0.12, 95% CI ‐1.39 to 1.15; Analysis 11.2), compared with wrists receiving therapeutic ultrasound. The precision of each of these effect estimates was low, and opposite effects of treatment are possible.
11.1. Analysis.
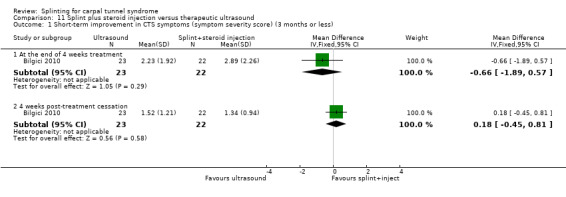
Comparison 11 Splint plus steroid injection versus therapeutic ultrasound, Outcome 1 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less).
11.2. Analysis.
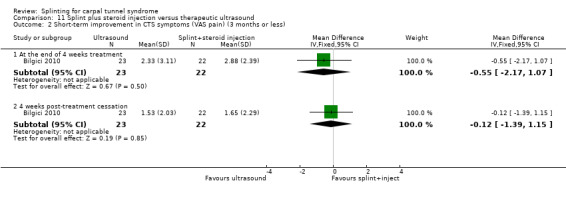
Comparison 11 Splint plus steroid injection versus therapeutic ultrasound, Outcome 2 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less).
In Celiker 2002, at the end of eight weeks of treatment, participants receiving splint plus NSAID had a VAS pain score that was 0.10 points lower on a 10‐point scale (MD ‐0.10, 95% CI ‐1.33 to 1.13; Analysis 12.1), and a Levine symptom score (Levine 1993) that was 0.10 points lower on a five‐point scale (MD ‐0.10, 95% CI ‐0.53 to 0.33; Analysis 12.2) compared with participants receiving local steroid injection. Celiker 2002 also found that at the end of eight weeks of treatment that fewer wrists receiving splint and NSAID had a positive Phalen's test (RR 0.18, 95% CI 0.01 to 3.34; Analysis 12.3), a positive reverse Phalen's test (RR 0.43, 95% CI 0.02 to 9.94; Analysis 12.4), and a positive Tinel's test (RR 0.44, 95% CI 0.10 to 1.89; Analysis 12.5) than wrists receiving local corticosteroid injection. However, the 95% CIs of these RRs were wide and incorporate effects in either direction
12.1. Analysis.
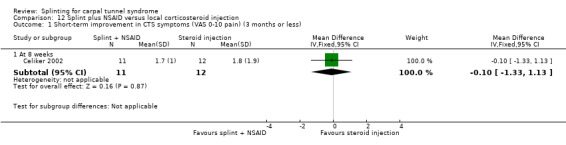
Comparison 12 Splint plus NSAID versus local corticosteroid injection, Outcome 1 Short‐term improvement in CTS symptoms (VAS 0‐10 pain) (3 months or less).
12.2. Analysis.
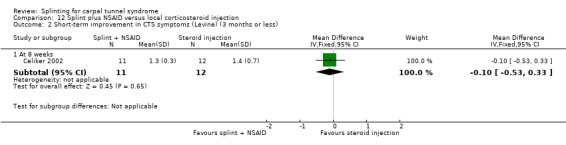
Comparison 12 Splint plus NSAID versus local corticosteroid injection, Outcome 2 Short‐term improvement in CTS symptoms (Levine) (3 months or less).
12.3. Analysis.
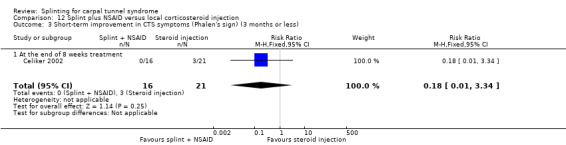
Comparison 12 Splint plus NSAID versus local corticosteroid injection, Outcome 3 Short‐term improvement in CTS symptoms (Phalen's sign) (3 months or less).
12.4. Analysis.
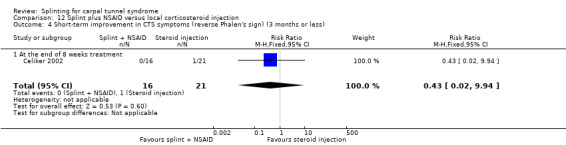
Comparison 12 Splint plus NSAID versus local corticosteroid injection, Outcome 4 Short‐term improvement in CTS symptoms (reverse Phalen's sign) (3 months or less).
12.5. Analysis.
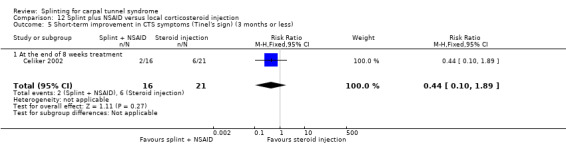
Comparison 12 Splint plus NSAID versus local corticosteroid injection, Outcome 5 Short‐term improvement in CTS symptoms (Tinel's sign) (3 months or less).
Werner 2005 measured symptoms using the Levine questionnaire, and elbow and forearm, and wrist, hand and finger discomfort using a 0 to10 VAS three months post‐treatment, but did not report the data because half the participants did not complete the questionnaires at this time point.
3) Short‐term improvement in functional ability or health‐related quality of life (three months or less)
Reported as an outcome in Arinci Incel 2005, Bardak 2009, and Bilgici 2010, but not Celiker 2002 or Werner 2005.
Arinci Incel 2005 measured functional status using the Boston Carpal Tunnel Questionnaire, grip strength (kg) and pinch strength (kg), and measured quality of life using the Short Form 36 Health Survey (SF‐36), all at the end of six months of treatment. However, means and SDs at this time point were only reported for the gabapentin plus nerve and tendon gliding exercises group (no data were reported for the splint plus nerve and tendon gliding exercises group and attempts to obtain these data from the trialists were unsuccessful). According to the authors, "There were no statistically significant differences for any of the post‐treatment measures between groups".
In Bardak 2009, at the end of six weeks of treatment, wrists receiving splint plus steroid injection had a lower (better) self‐reported functional status score (MD 4.20, 95% CI 1.88 to 6.52; Analysis 9.6) and better two‐point discrimination (MD 0.10, 95% CI ‐0.39 to 0.59; Analysis 9.7) than wrists receiving nerve and tendon gliding exercises. The authors also found that wrists receiving splint plus steroid injection plus nerve and tendon gliding exercises had a lower (better) self‐reported functional status score (MD ‐4.40, 95% CI ‐6.90 to ‐1.90; Analysis 10.6) and better two‐point discrimination (MD ‐0.30, 95% CI ‐0.70 to 0.10; Analysis 10.7) than wrists receiving nerve and tendon gliding exercises. Only the 95% CIs of the functional status scores suggested that plausible values of effect were in one direction only.
9.6. Analysis.
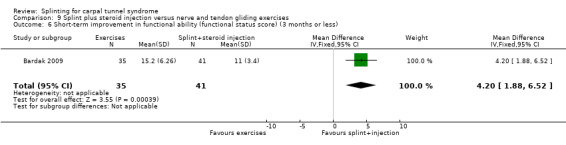
Comparison 9 Splint plus steroid injection versus nerve and tendon gliding exercises, Outcome 6 Short‐term improvement in functional ability (functional status score) (3 months or less).
9.7. Analysis.
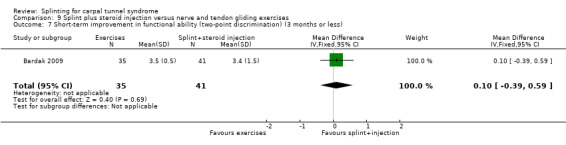
Comparison 9 Splint plus steroid injection versus nerve and tendon gliding exercises, Outcome 7 Short‐term improvement in functional ability (two‐point discrimination) (3 months or less).
10.6. Analysis.
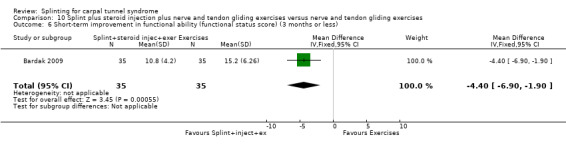
Comparison 10 Splint plus steroid injection plus nerve and tendon gliding exercises versus nerve and tendon gliding exercises, Outcome 6 Short‐term improvement in functional ability (functional status score) (3 months or less).
10.7. Analysis.
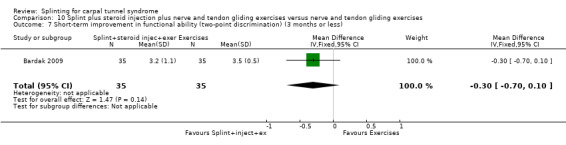
Comparison 10 Splint plus steroid injection plus nerve and tendon gliding exercises versus nerve and tendon gliding exercises, Outcome 7 Short‐term improvement in functional ability (two‐point discrimination) (3 months or less).
Bilgici 2010 reported that wrists receiving splint plus local corticosteroid injection had a functional status score (measured using a Turkish‐validated version of the Levine questionnaire (Levine 1993)) that was 0.81 points higher (worse) at the end of four weeks of treatment (MD ‐0.81, 95% CI ‐1.70 to 0.08; Analysis 11.3) and 0.24 points higher at four weeks post‐treatment (MD ‐0.24, 95% CI ‐1.01 to 0.53; Analysis 11.3); grip strength that was 2.80 mmHg worse at the end of four weeks of treatment (MD 2.80, 95% CI 1.01 to 4.59; Analysis 11.4) and 3.43 mmHg worse at four weeks post‐treatment (MD 3.43, 95% CI 1.71 to 5.15; Analysis 11.4); and two‐point discrimination that was 0.30 points worse at the end of four weeks of treatment (MD 0.30, 95% CI ‐0.49 to 1.09; Analysis 11.5) and 0.32 points worse at four weeks post‐treatment (MD 0.32, 95% CI ‐0.25 to 0.89; Analysis 11.5), compared with wrists receiving therapeutic ultrasound. Of all these effect estimates, only the grip strength results had 95% CIs that ruled out a null or alternative effect of treatment.
11.3. Analysis.
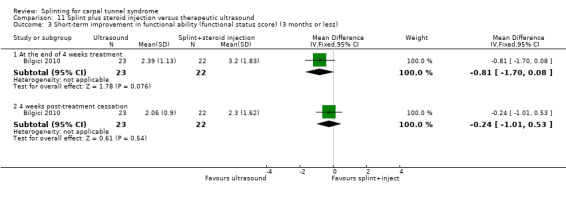
Comparison 11 Splint plus steroid injection versus therapeutic ultrasound, Outcome 3 Short‐term improvement in functional ability (functional status score) (3 months or less).
11.4. Analysis.
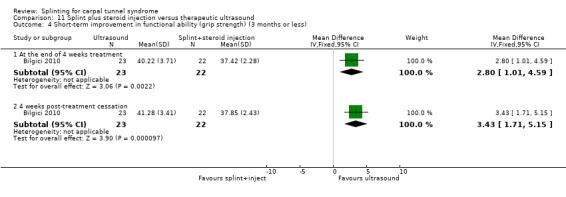
Comparison 11 Splint plus steroid injection versus therapeutic ultrasound, Outcome 4 Short‐term improvement in functional ability (grip strength) (3 months or less).
11.5. Analysis.
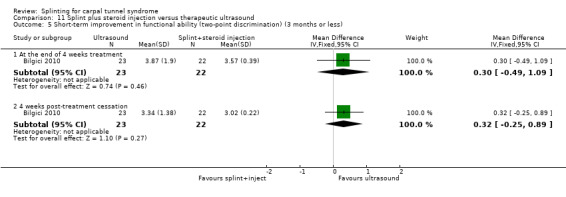
Comparison 11 Splint plus steroid injection versus therapeutic ultrasound, Outcome 5 Short‐term improvement in functional ability (two‐point discrimination) (3 months or less).
4) Short‐term improvement in neurophysiologic parameters (three months or less)
Reported as an outcome in Bilgici 2010, Celiker 2002, and Werner 2005, but not Arinci Incel 2005 or Bardak 2009.
Bilgici 2010 reported that wrists receiving splint plus local corticosteroid injection had a median nerve motor distal latency that was 0.05 msec longer at the end of four weeks of treatment (MD ‐0.05, 95% CI ‐0.55 to 0.45; Analysis 11.6) and 0.11 msec shorter at four weeks post‐treatment (MD 0.11, 95% CI ‐0.66 to 0.88; Analysis 11.6), and a sensory nerve conduction velocity that was 3.71 m/s faster at the end of four weeks of treatment (MD 3.71, 95% CI ‐0.45 to 7.87; Analysis 11.7) and 2.32 m/s faster at four weeks post‐treatment (MD 2.32, 95% CI ‐1.89 to 6.53; Analysis 11.7), compared with wrists receiving therapeutic ultrasound. The 95% CIs of all these effect estimate were wide and incorporate both null and opposite effects of treatment.
11.6. Analysis.
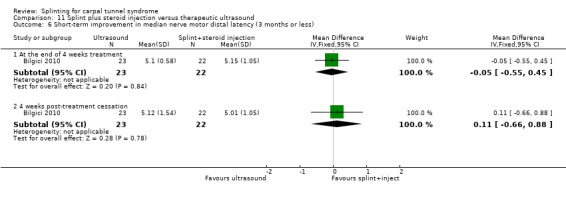
Comparison 11 Splint plus steroid injection versus therapeutic ultrasound, Outcome 6 Short‐term improvement in median nerve motor distal latency (3 months or less).
11.7. Analysis.
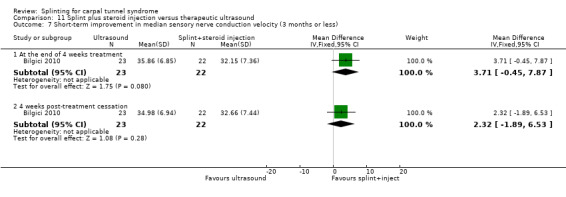
Comparison 11 Splint plus steroid injection versus therapeutic ultrasound, Outcome 7 Short‐term improvement in median sensory nerve conduction velocity (3 months or less).
Celiker 2002 found at the end of eight weeks of treatment that wrists receiving splint plus NSAID had a median nerve motor distal latency which was 0.10 ms longer (MD 0.10, 95% CI ‐0.42 to 0.62; Analysis 12.6) and a median nerve sensory distal latency that was 0.10 ms shorter (MD ‐0.10, 95% CI ‐0.53 to 0.33; Analysis 12.7) than wrists receiving local corticosteroid injection.
12.6. Analysis.
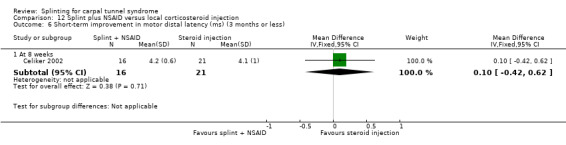
Comparison 12 Splint plus NSAID versus local corticosteroid injection, Outcome 6 Short‐term improvement in motor distal latency (ms) (3 months or less).
12.7. Analysis.
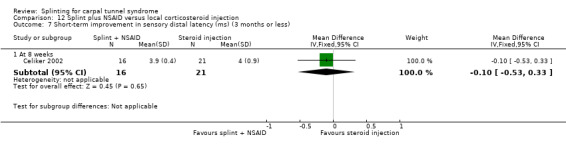
Comparison 12 Splint plus NSAID versus local corticosteroid injection, Outcome 7 Short‐term improvement in sensory distal latency (ms) (3 months or less).
Werner 2005 measured nerve conduction at three months post‐treatment, but did not report data at this time point because half the participants did not return for assessment.
5) Long‐term improvement in CTS symptoms (greater than three months)
Reported as an outcome in Bardak 2009 and Werner 2005, but not Arinci Incel 2005, Bilgici 2010, or Celiker 2002.
In Bardak 2009, more participants receiving splint plus steroid injection rated their satisfaction with treatment at 11 months post‐treatment as 'excellent/good' than participants receiving nerve and tendon gliding exercises (RR 1.92, 95% CI 1.05 to 3.49; Analysis 9.8). However, the high risk of bias associated with lack of patient blinding in this study suggests that these results should be interpreted with caution, as participants responses may have been based on their beliefs about the superiority of splint and steroid injection over nerve and tendon gliding exercises. Further, more participants receiving splint plus steroid injection plus nerve and tendon gliding exercises rated their satisfaction with treatment at 11 months post‐treatment as 'excellent/good' than participants receiving nerve and tendon gliding exercises (RR 1.47, 95% CI 0.99 to 2.19; Analysis 10.8), though the 95% CI incorporates opposite effects of treatment.
9.8. Analysis.
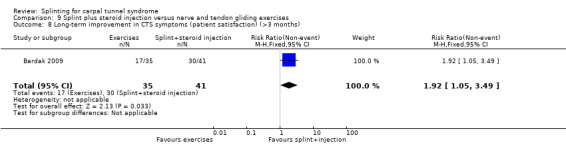
Comparison 9 Splint plus steroid injection versus nerve and tendon gliding exercises, Outcome 8 Long‐term improvement in CTS symptoms (patient satisfaction) (>3 months).
10.8. Analysis.
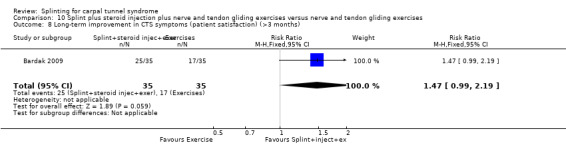
Comparison 10 Splint plus steroid injection plus nerve and tendon gliding exercises versus nerve and tendon gliding exercises, Outcome 8 Long‐term improvement in CTS symptoms (patient satisfaction) (>3 months).
At a mean of 12 months (range seven to 15) after the six‐week treatment period ended, Werner 2005 found that wrists receiving splint plus ergonomic education had a symptom score (as assessed using the Levine questionnaire (Levine 1993)) which was 0.33 points lower on a five‐point scale (MD ‐0.33, 95% CI ‐0.73 to 0.07; Analysis 13.1); VAS pain of the elbow and forearm which was 0.95 points lower on a 10‐point scale (MD ‐0.95, 95% CI ‐2.25 to 0.35; Analysis 13.2); and VAS pain of the wrist, hand and fingers that was 1.15 points lower on a 10‐point scale (MD ‐1.15, 95% CI ‐2.51 to 0.21; Analysis 13.3) compared with wrists receiving ergonomic education alone. The precision of these effect estimates was low and greater benefit of ergonomic education alone is plausible. The authors also measured these outcomes six months post‐treatment, but did not report the data because half the participants did not complete the questionnaires at this time point.
13.1. Analysis.
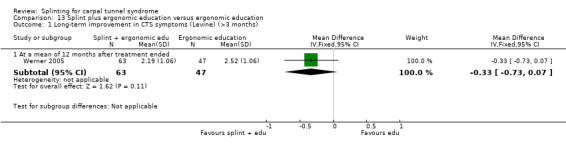
Comparison 13 Splint plus ergonomic education versus ergonomic education, Outcome 1 Long‐term improvement in CTS symptoms (Levine) (>3 months).
13.2. Analysis.
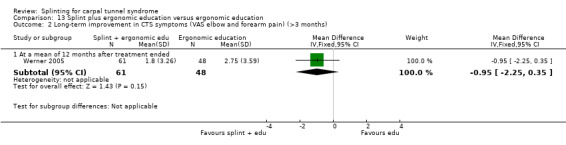
Comparison 13 Splint plus ergonomic education versus ergonomic education, Outcome 2 Long‐term improvement in CTS symptoms (VAS elbow and forearm pain) (>3 months).
13.3. Analysis.
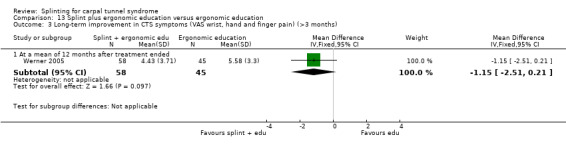
Comparison 13 Splint plus ergonomic education versus ergonomic education, Outcome 3 Long‐term improvement in CTS symptoms (VAS wrist, hand and finger pain) (>3 months).
6) Long‐term improvement in functional ability or health‐related quality of life (greater than three months)
Not reported as an outcome in Arinci Incel 2005, Bardak 2009, Bilgici 2010, Celiker 2002 or Werner 2005.
Discussion
Summary of main results
Overall there is limited evidence that a splint worn at night is more effective in the short term than no treatment, but insufficient evidence regarding the effectiveness and safety of one splint design or wearing regimen over others, and of splint over other non‐surgical interventions for CTS. Only three studies measured the primary outcome of the review, and only three studies measured outcomes at more than three months post‐treatment. The studies were heterogenous in the interventions delivered, risk of bias, and outcomes reported, which prevented any pooling of outcome data.
Two studies (one low quality and the other very low quality) comparing a splint worn at night with no treatment found that a splint was beneficial for the outcomes overall improvement, symptoms and self‐reported functional ability, but there were less clear differences between groups in neurophysiologic parameters (Manente 2001; Premoselli 2006). Five studies compared different types of splint designs, one study compared different splint‐wearing regimens, seven studies compared splint delivered alone with other non‐surgical interventions, and five studies compared splint delivered alongside other interventions with other non‐surgical interventions. Across each of these studies, any differences between groups detected in overall improvement, symptoms, function, and neurophysiologic parameters were often not statistically significant, were not precise, and may have resulted from methodological limitations of the studies, leading to a high risk of bias. Few adverse effects were reported in the studies that measured this outcome, but larger, more rigorous studies are needed to confirm the potential harms of splinting.
Overall completeness and applicability of evidence
Although different types of splints, treatment combinations and wearing schedules were investigated, the studies were heterogenous and unsuitable for comparison in a meta‐analysis. Therefore, the review could not define the most effective splint design, wrist position or wearing schedule. A further source of variation in treatment delivery was the amount of splint wear that each patient was prescribed and actually experienced. Three studies asked patients about hours of splint use and found them to be variable. The studies were not of high quality, but they found a trend that supported longer hours of splint wear leading to greater improvement in symptoms. The positive effects of splinting upon CTS symptoms observed by Werner 2005 were still present one year after treatment began. Werner 2005 had the longest follow‐up of all; most studies followed patients for between two and 10 weeks, which was to the endpoint of their treatment. These shorter studies may have failed to measure some of the therapeutic benefits of treatment, as CTS is a chronic rather than acute condition.
The trials comprised a high ratio of women to men, which is supported by epidemiological research into CTS (Atroshi 1999; Concannon 2000; Jablecki 2002; Szabo 1994). The sampling methods of the trials in this review varied, in that one study (Werner 2005) included 55 men and 57 women, from an occupational population. This sample of workers also included those with comorbidities such as diabetes and rheumatoid arthritis, while other trials excluded patients with these comorbidities. A further sampling method that varied between studies was that nine studies included patients with bilateral involvement and both hands were treated as part of the study, whereas the remaining studies treated one hand from each patient.
The primary outcome for the review, short‐term overall improvement (three months or less), was only measured in three studies (Brininger 2007, Burke 1994, Manente 2001). We selected this outcome as primary given its importance to patients, whereas the time point was chosen for pragmatic purposes, as based on our knowledge that few existing CTS trials measure outcomes beyond three months (and to be as consistent as possible with other Cochrane reviews on CTS (Marshall 2007; Scholten 2007; Verdugo 2008)). However, the natural history of CTS often extends over many years, so the results of this outcome may be limited in applicability. Only three of the included studies assessed any outcomes longer than three months (Bardak 2009; De Angelis 2009; Werner 2005), so it is essential that future splinting trials evaluate outcomes over a much longer, more clinically relevant time period. Further, in the interest of parsimony, we did not include any long‐term objective outcomes as outcomes of interest to the review. We recommend that objective outcomes be assessed in future long‐term splinting trials, and will consider their inclusion in future updates of this review.
This review only focused on trials comparing splinting with other non‐surgical interventions for CTS. An additional splinting study which may be of interest to people with CTS (but which we excluded because splinting was compared with surgery) is the study conducted by Gerritsen 2002a. The results of this study are summarised in the Cochrane review comparing surgical versus non‐surgical treatment for CTS (Verdugo 2008).
Quality of the evidence
The papers reviewed ranged between fulfilling none to five of the eight 'Risk of bias' criteria. Three studies only described adequate random sequence generation and allocation concealment. Given the nature of the interventions, blinding of patients was often not possible, with only one study reporting being able to blind patients to their treatment (Burke 1994). Lack of patient blinding may have influenced participants to exaggerate or under‐report their self‐reported symptoms and functional ability, and beliefs about their level of overall improvement. It is concerning that only six studies blinded outcome assessors (Bardak 2009; Burke 1994; De Angelis 2009; de Entrambasaguas 2006; Premoselli 2006; Sevim 2004), given that all studies assessing objective outcomes such as grip strength and neurophysiologic parameters should have been able to blind outcome assessors. The amount and reasons for attrition at three months or less was rated as being at low risk of bias for just over half the studies, with five studies being rated at high risk of attrition bias. Finally, 10 studies were suspected of selectively reporting outcomes, which is concerning given that selective outcome reporting of 'positive' or statistically significant trial results can bias the results and conclusions of a systematic review (Kirkham 2010).
Potential biases in the review process
We did not search databases containing unpublished research reports or dissertations so it is possible that some unpublished studies have not been included in the review. Also, while we included non‐English language studies conducted by Bye 2011, de Entrambasaguas 2006 and Zinnuroglu 2010, the translators may have missed some methodological detail regarding the risk of bias of the studies. It was also difficult to obtain relevant unpublished data from some of the authors of included studies. This had a considerable impact on our analysis of 10 studies which either committed, or possibly committed, unit of analysis errors (Arinci Incel 2005; Bilgici 2010; Burke 1994; Bye 2011; Celiker 2002; de Entrambasaguas 2006; Garfinkel 1998; Madjdinasab 2008; Mishra 2006; Walker 2000), as we were unable to re‐analyse the outcome data using methods that address the dependency of data.
Agreements and disagreements with other studies or reviews
The findings of this review are generally consistent with those of other systematic reviews of non‐surgical interventions for CTS, which conclude that splinting may be more effective than no treatment, but is not more or less effective than other non‐surgical interventions (Ashworth 2010; Gerritsen 2002b; Goodyear‐Smith 2004; Huisstede 2010; Muller 2004; Ono 2010; Piazzini 2007). However, in comparison to this review, the most recent systematic review by Huisstede 2010 did not include the studies conducted by Arinci Incel 2005, Bardak 2009, Bilgici 2010, Bye 2011, de Entrambasaguas 2006, Kumnerddee 2010, Madjdinasab 2008, or Zinnuroglu 2010. Based on the date we conducted our searches, to our knowledge the current review is the most comprehensive and up‐to‐date review of randomised trials assessing the efficacy of splinting for CTS.
Authors' conclusions
Implications for practice.
Overall, there is limited evidence that a splint worn at night is more effective in the short term than no treatment, but insufficient evidence regarding the effectiveness and safety of one splint design or wearing regimen over others, and insufficient evidence regarding the effectiveness and safety of splints over other non‐surgical interventions for CTS.
Implications for research.
There is a need for more evidence on the long‐term benefits of splinting. All future studies should attempt to blind participants, personnel, and outcome assessors where possible, and trialists should consider collecting data on overall improvement, adverse effects, CTS symptoms, function, and neurophysiologic parameters. If participants with bilateral CTS are included in the trial, trialists should use statistical methods which take the dependency between wrists into account, and report which statistical methods they used to achieve this. Finally, outcomes should be collected at long‐term follow‐up (that is, at least three months post‐treatment cessation).
Notes
This is one of six new reviews that will update the currently published review 'Non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome' (O'Connor 2003). When all six reviews are published we will withdraw the original review from publication. This review includes a new search, revised review question and selection criteria, updated methodology and an updated review team.
Acknowledgements
We thank Ruth Brassington, Louisa Dunn, Kate Jewitt, Carolyn Reid, Angela Gunn and Rachel Barton from the Cochrane Neuromuscular Disease Group for their assistance in devising the search strategy, helping to locate people to translate the non‐English trials and ongoing support for this review. We thank Shawn Marshall for his substantial contribution to the original version of this review. We thank Katherine Beringer from the School of Public Health and Preventive Medicine, Monash University, for her assistance in retrieving studies relevant to the review. We thank the translators for their assistance in translating abstracts and papers for the review. We thank the peer reviewers for their helpful comments.
We thank the following institutions for their support during the review:
School of Occupational Therapy, University of South Australia, Adelaide, Australia;
School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
Denise O'Connor is supported by an Australian National Health and Medical Research Council (NHMRC) Public Health Fellowship (#606726).
The editorial base of the Cochrane Neuromuscular Disease Group is supported by the MRC Centre for Neuromuscular Diseases.
Appendices
Appendix 1. MEDLINE (OvidSP) search strategy
1 randomized controlled trial.pt. (315877) 2 controlled clinical trial.pt. (83182) 3 randomized.ab. (221432) 4 placebo.ab. (127183) 5 drug therapy.fs. (1488786) 6 randomly.ab. (160369) 7 trial.ab. (228368) 8 groups.ab. (1061229) 9 or/1‐8 (2757907) 10 Carpal Tunnel Syndrome.tw. or Carpal Tunnel Syndrome/ (7168) 11 ((nerve entrapment or nerve compression or entrapment neuropath$) and carpal).mp. (952) 12 10 or 11 (7268) 13 SPLINTS/ (7065) 14 BRACES/ (4108) 15 (SPLINT$ or BRACE$ or WRIST SUPPORT$).tw. (13308) 16 or/13‐15 (19010) 17 9 and 12 and 16 (97) 18 remove duplicates from 17 (93)
Appendix 2. EMBASE (OvidSP) search strategy
1 crossover‐procedure/ (31558) 2 double‐blind procedure/ (102446) 3 randomized controlled trial/ (295130) 4 single‐blind procedure/ (14625) 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. (1049473) 6 or/1‐5 (1120564) 7 exp animals/ (1664443) 8 exp humans/ (12828730) 9 7 not (7 and 8) (1266349) 10 6 not 9 (1084181) 11 limit 10 to embase (880818) 12 carpal tunnel syndrome/ (9331) 13 carpal tunnel syndrome.mp. (9982) 14 ((nerve entrapment or nerve compression or entrapment neuropath$) and carpal).mp. (1553) 15 or/12‐14 (10081) 16 splint/ (6430) 17 brace/ (5397) 18 (splint$ or brace$ or wrist support$).tw. (14987) 19 or/16‐18 (21153) 20 11 and 15 and 19 (79) 21 remove duplicates from 20 (79)
Appendix 3. AMED (OvidSP) search strategy
1 Randomized controlled trials/ (1495) 2 Random allocation/ (302) 3 Double blind method/ (425) 4 Single‐Blind Method/ (24) 5 exp Clinical Trials/ (3146) 6 (clin$ adj25 trial$).tw. (5353) 7 ((singl$ or doubl$ or treb$ or trip$) adj25 (blind$ or mask$ or dummy)).tw. (2197) 8 placebos/ (516) 9 placebo$.tw. (2475) 10 random$.tw. (12456) 11 research design/ (1663) 12 Prospective Studies/ (417) 13 meta analysis/ (106) 14 (meta?analys$ or systematic review$).tw. (1749) 15 control$.tw. (27000) 16 (multicenter or multicentre).tw. (710) 17 ((study or studies or design$) adj25 (factorial or prospective or intervention or crossover or cross‐over or quasi‐experiment$)).tw. (9504) 18 or/1‐17 (41602) 19 carpal tunnel syndrome/ or carpal tunnel syndrome.tw. (443) 20 ((nerve entrapment or nerve compression or entrapment neuropath$) and carpal).mp. (53) 21 19 or 20 (444) 22 splints/ (98) 23 braces/ (170) 24 (splint$ or brace$ or wrist support$).tw. (1128) 25 or/22‐24 (1128) 26 18 and 21 and 25 (22) 27 remove duplicates from 26 (22)
Appendix 4. CINAHL (EBSCOhost) search strategy
S28 S18 and S24 and S27 58 S27 S25 or S26 3964 S26 splint* or brace* or wrist support* 3964 S25 (MH "Splints") 1476 S24 s19 or s20 or s21 or s22 or s23 1820 S23 entrapment neuropath* and carpal 41 S22 nerve compression and carpal 141 S21 nerve entrapment and carpal 51 S20 carpal tunnel syndrome 1813 S19 (MH "Carpal Tunnel Syndrome") 1591 S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 509020 S17 ABAB design* 72 S16 TI random* or AB random* 104569 S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 217146 S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 72504 S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 20589 S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 17178 S11 PT ("clinical trial" or "systematic review") 96175 S10 (MH "Factorial Design") 793 S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 165362 S8 (MH "Meta Analysis") 13350 S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 30 S6 (MH "Quasi‐Experimental Studies") 5120 S5 (MH "Placebos") 7223 S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 23000 S3 (MH "Clinical Trials+") 133287 S2 (MH "Crossover Design") 8720 S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 54473
Appendix 5. CENTRAL, NHSEED and DARE search strategy
#1"Carpal Tunnel Syndrome" #2("nerve entrapment" OR "nerve compression" OR "entrapment neuropath*") #3"median nerve entrapment" #4(#1 OR #2 OR #3) #5splint* or brace* or "wrist support*" #6(#4 AND #5)
Data and analyses
Comparison 1. Splint versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term overall improvement (3 months or less) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [2.29, 6.51] |
| 1.1 At 4 weeks | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [2.29, 6.51] |
| 2 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Difficulty in falling asleep | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 131.28] |
| 2.2 Transient paraesthesias | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.50, 161.86] |
| 3 Short‐term improvement in CTS symptoms (Levine questionnaire) (3 months or less) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 At 4 weeks | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.29, ‐0.85] |
| 3.2 At 12 weeks | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.10, ‐0.78] |
| 3.3 At the end of 6 months treatment | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.11, ‐0.69] |
| 4 Short‐term improvement in CTS symptoms (pressure‐provocative test time) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 12 weeks | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐5.31, 5.39] |
| 4.2 At end of 6 months treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 8.25 [2.49, 14.01] |
| 5 Short‐term improvement in CTS symptoms (Phalen test time) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 12 weeks | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 2.74 [‐3.32, 8.80] |
| 5.2 At the end of 6 months treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [‐1.44, 13.84] |
| 6 Short‐term improvement in functional status (Levine questionnaire) (3 months or less) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 At 4 weeks | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.82, ‐0.28] |
| 6.2 At 12 weeks | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.40, ‐0.04] |
| 6.3 At the end of 6 months treatment | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.68, 0.18] |
| 7 Short‐term improvement in distal motor latency (ms) (3 months or less) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 At 4 weeks | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.49, 0.45] |
| 7.2 At 12 weeks | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.57, 0.05] |
| 7.3 At the end of 6 months treatment | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.23, 0.39] |
| 8 Short‐term improvement in sensory nerve conduction velocity (m/s) (3 months or less) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 At 4 weeks | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐5.85, 4.41] |
| 8.2 At 12 weeks | 1 | 48 | Mean Difference (IV, Random, 95% CI) | 5.13 [1.21, 9.05] |
| 8.3 At the end of 6 months treatment | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 1.41 [‐2.22, 5.04] |
| 9 Short‐term improvement in sensory nerve action potential (uV) (3 months or less) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 At 4 weeks | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 6.30 [0.60, 12.00] |
| 9.2 At 12 weeks | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐6.27, 3.59] |
| 9.3 At the end of 6 months treatment | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐7.17, 5.45] |
| 10 Short‐term improvement in distal sensory latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 At 12 weeks | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.47, ‐0.05] |
| 10.2 At the end of 6 months treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.37, 0.17] |
| 11 Short‐term improvement in motor nerve conduction velocity (m/s) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 At 12 weeks | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 2.97 [0.83, 5.11] |
| 11.2 At the end of 6 months treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐1.17, 4.57] |
| 12 Short‐term improvement in motor nerve action potential (mV) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 At 12 weeks | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐2.21 [‐4.47, 0.05] |
| 12.2 At the end of 6 months treatment | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 1.85 [‐0.49, 4.19] |
Comparison 2. Different splint designs (neutral versus extension splint).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term overall improvement (3 months or less) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.12, 5.28] |
| 1.1 At 2 weeks | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.12, 5.28] |
| 2 Short‐term improvement in CTS symptoms (night‐time symptoms) (3 months or less) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.99, 4.65] |
| 2.1 At 2 weeks | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.99, 4.65] |
| 3 Short‐term improvement in CTS symptoms (day‐time symptoms) (3 months or less) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.56, 5.97] |
| 3.1 At 2 weeks | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.56, 5.97] |
Comparison 3. Different splint designs (standard splint versus hand brace).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.28 [0.77, 229.07] |
| 1.1 At the end of 3 months treatment | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.28 [0.77, 229.07] |
| 2 Short‐term improvement in CTS symptoms (Levine) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At the end of 3 months treatment | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.20, 0.40] |
| 3 Short‐term improvement in CTS symptoms (VAS pain 0‐100) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At the end of 3 months treatment | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐9.54, 9.34] |
| 4 Short‐term improvement in CTS symptoms (VAS paraesthesia 0‐100) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At the end of 3 months treatment | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | 10.20 [‐1.15, 21.55] |
| 5 Short‐term improvement in functional status (Levine) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At the end of 3 months treatment | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.46, 0.06] |
| 6 Short‐term improvement in distal motor latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 At the end of 3 months treatment | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.34, 0.54] |
| 7 Short‐term improvement in sensory nerve conduction velocity (m/s) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 At the end of 3 months treatment | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐3.56, 2.16] |
| 8 Short‐term improvement in sensory nerve action potential (SNAP) amplitude (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 At the end of 3 months treatment | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐8.11, 0.11] |
| 9 Long‐term improvement in CTS symptoms (Levine) (>3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 At 6 months after the end of treatment | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.47, 0.27] |
| 10 Long‐term improvement in CTS symptoms (VAS pain 0‐100) (>3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 At 6 months after the end of treatment | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐14.96, 8.76] |
| 11 Long‐term improvement in CTS symptoms (VAS paraesthesia 0‐100) (>3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 At 6 months after the end of treatment | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [‐10.33, 15.13] |
| 12 Long‐term improvement in functional status (BCTQ) (>3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 At 6 months after the end of treatment | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.57, 0.17] |
Comparison 4. Different splint designs (carpal lock versus volar supporting orthosis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (VAS 0‐10 pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At the end of 3 months treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.71, 0.51] |
| 2 Short‐term improvement in CTS symptoms (VAS 0‐10 dysesthesia) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At the end of 3 months treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐2.33, 0.73] |
| 3 Short‐term improvement in sensory conduction velocity of second finger‐to‐wrist segment (m/s) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At the end of 3 months treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐3.19, 3.79] |
| 4 Short‐term improvement in sensory conduction velocity of palm‐to‐wrist segment (m/s) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At the end of 3 months treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐4.82, 2.22] |
Comparison 5. Different splint‐wearing regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (3 months or less) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.83, 0.41] |
| 1.1 At the end of 6 weeks treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.83, 0.41] |
| 2 Short‐term improvement in functional status (3 months or less) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.87, 0.45] |
| 2.1 At the end of 6 weeks treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.87, 0.45] |
| 3 Short‐term improvement in motor distal latency (msec) (3 months or less) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐2.05, 0.79] |
| 3.1 At the end of 6 weeks treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐2.05, 0.79] |
| 4 Short‐term improvement in sensory distal latency (msec) (3 months or less) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.62, 0.72] |
| 4.1 At the end of 6 weeks treatment | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.62, 0.72] |
Comparison 6. Splint versus yoga.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (VAS Pain) (3 month or less) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.07, 2.73] |
| 1.1 At end of 8 weeks treatment | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.07, 2.73] |
| 2 Short‐term improvement in CTS symptoms (sleep disturbance) (3 months or less | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.10, 2.25] |
| 2.1 At end of 8 weeks treatment | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.10, 2.25] |
| 3 Short‐term improvement in CTS symptoms (number of wrists with improvement in Tinel sign) (3 months or less) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.66] |
| 3.1 At end of 8 weeks treatment | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.13, 1.66] |
| 4 Short‐term improvement in CTS symptoms (number of wrists with improvement in Phalen sign) (3 months or less) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.05, 0.78] |
| 4.1 At end of 8 weeks treatment | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.05, 0.78] |
| 5 Short‐term improvement in functional ability (grip strength, mmHg) (3 months or less) | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [‐31.06, 37.26] |
| 5.1 At the end of 8 weeks treatment | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [‐31.06, 37.26] |
| 6 Short‐term improvement in median nerve motor distal latency (ms) (3 months or less) | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.37, 0.87] |
| 6.1 At the end of 8 weeks treatment | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.37, 0.87] |
| 7 Short‐term improvement in median nerve sensory distal latency (ms) (3 months or less) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.35, 1.13] |
| 7.1 At the end of 8 weeks treatment | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.35, 1.13] |
Comparison 7. Splint versus acupuncture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At the end of 5 weeks treatment | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.31] |
| 2 Short‐term improvement in CTS symptoms (Levine) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At the end of 5 weeks treatment | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.14, 0.32] |
| 3 Short‐term improvement in CTS symptoms (VAS pain 100mm) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At the end of 5 weeks treatment | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 9.63 [‐0.01, 19.27] |
| 4 Short‐term improvement in functional ability (functional status score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At the end of 5 weeks treatment | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.18, 0.26] |
Comparison 8. Splint versus oral steroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.86 [0.24, 97.86] |
| 2 Short‐term improvement in CTS symptom severity (Levine) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At end of 4 weeks treatment | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.02, 0.44] |
| 2.2 At 8 weeks post‐treatment | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.03, 0.53] |
| 3 Short‐term improvement in functional status (Levine) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At the end of 4 weeks treatment | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.05, 0.29] |
| 3.2 At 8 weeks post‐treatment | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.06, 0.30] |
| 4 Short‐term improvement in median nerve motor distal latency (ms) (3 months or less) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 At the end of 4 weeks treatment | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.18, 0.46] |
| 4.2 At the end of 6 weeks treatment | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.34, 0.92] |
| 4.3 At 8 weeks post‐treatment | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.03, 0.57] |
| 5 Short‐term improvement in median nerve sensory distal latency (ms) (3 months or less) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 At the end of 4 weeks treatment | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.06, 0.72] |
| 5.2 At the end of 6 weeks treatment | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.18, 0.58] |
| 5.3 At 8 weeks post‐treatment | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.56 [0.28, 0.84] |
| 6 Short‐term improvement in motor nerve conduction velocity (m/s) (3 months or less) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 At the end of 4 weeks treatment | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐3.84, 3.56] |
| 6.2 At the end of 6 weeks treatment | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 2.07 [‐0.74, 4.88] |
| 6.3 At 8 weeks post‐treatment | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐3.28 [‐6.35, ‐0.21] |
| 7 Short‐term improvement in sensory nerve conduction velocity (m/s) (3 months or less) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 At the end of 4 weeks treatment | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [‐3.02, 4.32] |
| 7.2 At the end of 6 weeks treatment | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐2.92 [‐9.34, 3.50] |
| 7.3 At 8 weeks post‐treatment | 1 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐3.95 [‐7.60, ‐0.30] |
Comparison 9. Splint plus steroid injection versus nerve and tendon gliding exercises.
Comparison 10. Splint plus steroid injection plus nerve and tendon gliding exercises versus nerve and tendon gliding exercises.
Comparison 11. Splint plus steroid injection versus therapeutic ultrasound.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At the end of 4 weeks treatment | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐1.89, 0.57] |
| 1.2 4 weeks post‐treatment cessation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.45, 0.81] |
| 2 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At the end of 4 weeks treatment | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐2.17, 1.07] |
| 2.2 4 weeks post‐treatment cessation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐1.39, 1.15] |
| 3 Short‐term improvement in functional ability (functional status score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At the end of 4 weeks treatment | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.81 [‐1.70, 0.08] |
| 3.2 4 weeks post‐treatment cessation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐1.01, 0.53] |
| 4 Short‐term improvement in functional ability (grip strength) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At the end of 4 weeks treatment | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [1.01, 4.59] |
| 4.2 4 weeks post‐treatment cessation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 3.43 [1.71, 5.15] |
| 5 Short‐term improvement in functional ability (two‐point discrimination) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At the end of 4 weeks treatment | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.49, 1.09] |
| 5.2 4 weeks post‐treatment cessation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.25, 0.89] |
| 6 Short‐term improvement in median nerve motor distal latency (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 At the end of 4 weeks treatment | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.55, 0.45] |
| 6.2 4 weeks post‐treatment cessation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.66, 0.88] |
| 7 Short‐term improvement in median sensory nerve conduction velocity (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 At the end of 4 weeks treatment | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 3.71 [‐0.45, 7.87] |
| 7.2 4 weeks post‐treatment cessation | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 2.32 [‐1.89, 6.53] |
Comparison 12. Splint plus NSAID versus local corticosteroid injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (VAS 0‐10 pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 8 weeks | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.33, 1.13] |
| 2 Short‐term improvement in CTS symptoms (Levine) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 8 weeks | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.53, 0.33] |
| 3 Short‐term improvement in CTS symptoms (Phalen's sign) (3 months or less) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.34] |
| 3.1 At the end of 8 weeks treatment | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.34] |
| 4 Short‐term improvement in CTS symptoms (reverse Phalen's sign) (3 months or less) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.02, 9.94] |
| 4.1 At the end of 8 weeks treatment | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.02, 9.94] |
| 5 Short‐term improvement in CTS symptoms (Tinel's sign) (3 months or less) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.10, 1.89] |
| 5.1 At the end of 8 weeks treatment | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.10, 1.89] |
| 6 Short‐term improvement in motor distal latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 At 8 weeks | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.42, 0.62] |
| 7 Short‐term improvement in sensory distal latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 At 8 weeks | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.53, 0.33] |
Comparison 13. Splint plus ergonomic education versus ergonomic education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long‐term improvement in CTS symptoms (Levine) (>3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At a mean of 12 months after treatment ended | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.73, 0.07] |
| 2 Long‐term improvement in CTS symptoms (VAS elbow and forearm pain) (>3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At a mean of 12 months after treatment ended | 1 | 109 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐2.25, 0.35] |
| 3 Long‐term improvement in CTS symptoms (VAS wrist, hand and finger pain) (>3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At a mean of 12 months after treatment ended | 1 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐1.15 [‐2.51, 0.21] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arinci Incel 2005.
| Methods | Randomised controlled trial No blinding reported Ethics approval and informed consent obtained Randomisation occurred at the level of participants, where participants with bilateral CTS received the same intervention for both affected wrists. |
|
| Participants | Total N = 70 (115 wrists) randomised Intervention group N = 35 (60 wrists) randomised Control group N = 35 (55 wrists) randomised 0 males, 70 females Mean ± SD age: Intervention group: 49.94 ± 8.12 years Control group: 49.82 ± 7.01 years Inclusion criteria: 1. Diagnosis of CTS confirmed by electrodiagnostic studies performed on a Medelec Synergy v2.0; criteria were median sensory distal latency greater than 3.6 msec; prolonged in the wrist to palm segment, with or without prolongation of the distal motor latency by using supramaximal stimulation and surface electrodes. Exclusion criteria: 1. Concomitant polyneuropathy; 2. Neurologic or inflammatory arthritis conditions; 3. History of upper extremity fracture; 4. Previous use of gabapentin for any other reason. |
|
| Interventions | Intervention: gabapentin 1800 and 2400 mg/day, which was titrated progressively: 300 mg at bedtime on day 1, 300 mg twice on day 2 and 300 mg three times a day on day 3. The dosage was individualised for each participant between 1800 and 2400 mg as recommended. Duration of this treatment was 6 months. Control: splint‐exercise: participants were instructed to wear a neutral, volar wrist splint at night and during the day as much as possible for 6 months. Nerve and tendon gliding exercises as described by Tetton and Hunter were ordered to both groups. A brochure demonstrating hand positions of these exercises was given to participants. The exercises were repeated twice daily, 10 times at each session. |
|
| Outcomes | Outcomes assessed at baseline and at the end of 6 months of treatment:* 1. Grip strength (kg) using a Jamar hand dynamometer. The mean of 3 attempts was reported. 2. Pinch strength (kg) using a pinch meter. The mean of 3 attempts was reported. 3. Symptoms using carpal tunnel questionnaire (rates 11 items on ordinal scale 1: no difficulty with the activity, to 5: cannot perform the activity at all) 4. Hand function using carpal tunnel questionnaire (rates 8 items on ordinal scale 1: no difficulty with the activity, to 5: cannot perform the activity at all) 5. Pain using a 10 cm VAS. 6. Quality of life using the SF‐36. The units of measurement (i.e. scores calculated) for this outcome were not reported by the authors. 7. Adverse events: no reporting on how these events were recorded and how frequently this was done. |
|
| Notes | * Analysis was undertaken at the wrist‐level for all outcomes, though some participants in each group had bilateral CTS. Bilateral cases had the same intervention applied to each wrist. The trialists reported (via personal communication) that the correlation between both wrists was not accounted for in the analysis. Therefore, a unit of analysis error occurred. Numerical data were only reported for the gabapentin group at the end of six months of treatment, so no data from this study were entered into RevMan. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomised to 2 groups: splint‐exercise group and gabapentin‐exercise groups with 35 patients in each." Comment: Not enough information to determine the adequacy of the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Participants were randomised to 2 groups: splint‐exercise group and gabapentin‐exercise groups with 35 patients in each." Comment: not enough information to determine whether the allocation sequence was adequately concealed until interventions were assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: not reported, but due to the nature of the interventions (splint vs oral drug), it is unlikely that participants were not aware of which treatment they were allocated to |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not reported, and it would have been possible to blind the outcome assessors of the objective outcomes (grip and pinch strength) |
| Incomplete outcome data (attrition bias) 3 months or less | Unclear risk | Quote: "Seventy patients with CTS were enrolled in the study." Comment: the authors do not report that there were no withdrawals or losses to follow‐up, and do not indicate whether the data obtained at the end of 6 months treatment were based on the complete randomised sample |
| Selective reporting (reporting bias) | High risk | Comment: the authors only reported data at the end of 6 months of treatment for the gabapentin plus exercises group; the results for the splint group are only reported descriptively (i.e. indicating whether a 'significant' or 'non‐significant' difference existed between groups) |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Bardak 2009.
| Methods | Randomised controlled trial Blinded outcome assessors Ethics approval and informed consent obtained |
|
| Participants | Total n = 111 (111 wrists) randomised Intervention group 1 n = 41 (41 wrists) randomised; 41 (41 wrists) completed Intervention group 2 n = 35 (35 wrists) randomised; 35 (35 wrists) completed Intervention group 3 n = 35 (35 wrists) randomised; 35 (35 wrists) completed 3 males, 108 females Mean ± SD age: Intervention group 1: 33 ± 9.6 yrs Intervention group 2: 26 ± 10.3 yrs Intervention group 3: 22 ± 9.9 yrs Mean± SD duration of CTS symptoms: Intervention group 1: 13.3 ± 8.6 months Intervention group 2: 12.9 ± 8.8 months Intervention group 3: 19 ± 16.3 months Inclusion criteria: 1. Diagnosed according to Lundborg classification as intermediate stage CTS, characterised as nocturnal increase in the carpal tunnel tissue pressure Exclusion criteria: 1. Diagnosed according to Lundborg classification as early stage or late stage CTS 2. Had diabetes mellitus 3. Had thyroid diseases 4. Had rheumatoid arthritis 5. Had peripheral neuropathy 6. Had cervical radiculopathy 7. Had CTS with thenar atrophies 8. Were pregnant 9. Had history of steroid injections or splinting 10. Had bilateral CTS |
|
| Interventions | Intervention group 1: standard conservative treatment consisting of a neutral splint worn day and night for the first three weeks and then at night only for the next three weeks, and 3 mg betamethasone (steroid) injection into the carpal groove. Intervention group 2: standard conservative treatment (see above) plus tendon and median nerve gliding exercises performed at home 3 times a day with every particular exercise repeated 5 times for a period of 6 weeks (exercises were demonstrated by a physiotherapist initially and participants received a brochure describing the exercises, and were asked to complete the exercises at home with a weekly follow‐up with the physiotherapist to ensure the exercises were being performed properly). Intervention group 3: tendon and median nerve gliding exercises performed at home 3 times a day with every particular exercise repeated 5 times for a period of 6 weeks (see above) |
|
| Outcomes | Outcomes assessed at baseline and 8 weeks after treatment ended: 1. Symptom total point, calculated as the sum of five scores (scored as symptomatic = 1 point or asymptomatic = 0 points) for five symptoms (hand pain, tingling, numbness, nocturnal numbness, and interrupted sleep). The total score ranges from 0 to 5, with lower scores denoting fewer symptoms 2. Functional status score, calculated as the sum of seven scores for ability to perform seven daily living activities (writing, buttoning clothes, gripping a telephone receiver, opening jars, doing housework, carrying grocery bags, bathing), each scored as 1 = easy, 2 = somewhat difficult, 3 = moderately difficult, 4 = very difficult, 5 = impossible). The total score ranges from 7 to 35, with lower scores denoting better function. 3. Phalen's test 4. Tinel's test 5. Reverse Phalen's test 6. Compression test 7. Pain measured on a VAS (scale properties not reported)* 8. Static 2‐point discrimination (mm) performed on the pulp of the 3 radial digits, and the mean value was recorded 9. Patient satisfaction measured via telephone, where participants were asked to rate themselves as asymptomatic = good, symptomatic during difficult activities = fair or persistent symptoms after the treatment = poor (measured only at 11 months post‐treatment) |
|
| Notes | *No data reported on this outcome in the trial publication. Requests to obtain these data from the authors were unsuccessful. Only participants with unilateral CTS were included in the study, so a unit of analysis error resulting from the correlation between two wrists in bilateral CTS participants could not have occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For randomization of the patients into treatment groups, a biostatistician created a computer‐generated randomization list." Comment: the randomisation sequence was probably adequately concealed |
| Allocation concealment (selection bias) | Unclear risk | Quote: "According to this list, numbered, sealed envelopes containing one of the treatment groups were prepared. When patients entered the study, the corresponding envelope was opened and the enclosed card determined the treatment group" Comment: it is not clear whether the sealed, numbered envelopes were opaque, so it is not clear whether the allocation sequence was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Two investigators were assigned to this study. One of the investigators was blind to the therapy given to the patient and only evaluated the subjective symptoms, clinical examinations, and the functional status of the patient. These evaluations were carried out pretreatment and 8 weeks posttreatment. The second investigators was blind to the functional status and symptoms of the patients and only applied the treatment" Comment: participants and personnel delivering the intervention were probably not blind to treatment allocation, given the nature of the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two investigators were assigned to this study. One of the investigators was blind to the therapy given to the patient and only evaluated the subjective symptoms, clinical examinations, and the functional status of the patient. These evaluations were carried out pretreatment and 8 weeks posttreatment. The second investigators was blind to the functional status and symptoms of the patients and only applied the treatment" Comment: the outcome assessor was probably blind to treatment allocation |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: no drop‐outs or losses to follow‐up were reported in the trial publication, and the tables of outcome data clearly indicate that data reported are based on a complete samples of participants who were randomised |
| Incomplete outcome data (attrition bias) After 3 months | Low risk | Comment: no drop‐outs or losses to follow‐up were reported in the trial publication, and the tables of outcome data clearly indicate that data reported are based on a complete samples of participants who were randomised |
| Selective reporting (reporting bias) | High risk | Comment: all outcomes specified in the methods section of the publication were reported in the results section of the publication, except for the outcome, VAS pain. Further, no protocol or trial registry entry was identified, and it is not clear whether the outcome commonly measured in other CTS trials, nerve conduction, was measured as an outcome |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Bilgici 2010.
| Methods | Randomised controlled trial No blinding reported Ethics approval and informed consent obtained It is unclear whether randomisation occurred at the level of participants or wrists, and whether all bilateral CTS participants received the same or different intervention for each wrist |
|
| Participants | Details of sampling frame: Total N randomised = 34 participants (49 wrists) randomised; 31 participants (45 wrists) completed Intervention group 1 N = 16 participants (24 wrists) randomised; 15 participants (23 wrists) completed Intervention group 2 N = 18 participants (25 wrists) randomised; 16 participants (22 wrists) completed Group 1: 5 males; 10 females Group 2: 4 males; 12 females Group‐specific sex only reported for participants who completed trial. Overall 24 women and 10 men were randomised Mean ± SD (range) age: Group 1: 47.33 ± 7.44 Group 2: 44.15 ± 9.30 Group‐specific age only reported for participants who completed trial Mean ± SD (range) duration of CTS symptoms: Group 1: 46.33 ± 34.04 months Group 2: 46.29 ± 61.36 months Group‐specific duration of symptoms only reported for participants who completed trial Inclusion criteria: 1. Had clinical symptoms and signs of CTS confirmed by standard electrodiagnosis, with no abnormalities in the radial or ulnar nerve. Exclusion criteria: 1. Had thenar atrophy or spontaneous activity (fibrillation potentials and positive sharp waves) on electromyographic examination of the abductor pollicis brevis muscle 2. Pregnant 3. Had previous wrist trauma 4. Had a history of steroid injection into the carpal tunnel 5. Had rheumatic diseases 6. Had cervical radiculopathy 7. Had diabetes or other pathologic conditions predisposing to peripheral neuropathies |
|
| Interventions | Group 1: ultrasound treatment delivered under water at a frequency of 3MHz and with an intensity of 1.5W/cm2 for five minutes, five times per week for four weeks. Group 2: local corticosteroid injection plus neutral‐positioned wrist splint worn as much as possible during the day and night for four weeks. Local corticosteroid injection was given using a 22‐gauge needle at the proximal part of the carpal tunnel to the wrist crease just medial to the tendons of the flexor radial muscle involving a single 4 mg dexamethasone injection without lidocaine |
|
| Outcomes | Outcomes assessed at baseline, at the end of the 4 week treatment period, and at four weeks post‐treatment. 1. Symptoms using the Turkish‐translated Boston Carpal Tunnel Questionnaire, calculated as the mean of 11 items scored from 1 (mildest) to 5 (most severe) 2. Pain using a VAS 3. Function using the Turkish‐translated Boston Carpal Tunnel Questionnaire, calculated as the mean of 8 items scored from 1 (no difficulty in the activity to 5 (cannot perform the activity at all) 4. Grip strength measured using a hand‐held dynamometer, where the participants positioning was standardised and the average force of 3 consecutive trials was calculated 5. 2‐point discrimination performed on the pulp of three radial digits and the mean recorded 6. Nerve conduction: median nerve motor distal latency (msec), median sensory nerve conduction velocity (m/sec) 7. Adverse effects |
|
| Notes | Analysis was undertaken at the wrist‐level for all outcomes, though some participants in each group had bilateral CTS. It is not clear whether bilateral CTS participants received the same intervention for both wrists. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomization was performed by using sequentially numbered and sealed opaque envelopes. Following the baseline assessment, patients were randomised to either ultrasound treatment (group A) or local corticosteroid injection plus splinting (group B)." Comment: no information on how the random sequence was generated was provided |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was performed by using sequentially numbered and sealed opaque envelopes. Following the baseline assessment, patients were randomised to either ultrasound treatment (group A) or local corticosteroid injection plus splinting (group B)." Comment: the allocation sequence was probably adequately generated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: due to the nature of the interventions delivered (ultrasound versus splint plus corticosteroid injection), it is unlikely that participants and personnel were unaware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All patients were examined by the same physician". Comment: the authors did not report whether the outcome assessor of objective outcomes was blind to treatment allocation |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "A total of 49 hands of 34 patients (24 women and 10 men) were enrolled in this study. 16 patients were randomly assigned to the group A, and 18 patients were randomly assigned to the group B. Three patients did not complete the 8 week follow‐up. One patient in group B did not allow to be injected into her hand after randomization. Two patients (one in each group), could not be reached and were lost to follow‐up. They were excluded from the study and data analysis. Thus, 15 patients (23 hands) in the Group A, and 16 patients (22 hands) in the Group B completed the follow‐up at 8 weeks" Quote: "The per‐protocol analyses included 45 hands". Comment: The overall amount of attrition, and reasons for this, is small and relatively similar across groups, and unlikely to have affected the results of outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes specified in the Methods section were reported in the Results section in sufficient detail to be included in a meta‐analysis |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Brininger 2007.
| Methods | Randomised controlled trial No blinding reported Ethics approval and informed consent obtained |
|
| Participants | Total N = 61 (61 wrists) randomised Intervention group 1 N =16 wrists randomised, 14 wrists completed Intervention group 2 N = 17 wrists randomised, 13 wrists completed Intervention group 3 N =16 wrists randomised, 11 wrists completed Intervention group 4 N =12 wrists randomised, 13 wrists completed 10 males, 51 females* Mean ± SD age:* Intervention group 1: 49.0 ± 15.4 yrs Intervention group 2: 51.9 ± 15.7 yrs Intervention group 3: 46.6 ± 12.9 yrs Intervention group 4: 50.1 ± 13.2 yrs Inclusion criteria: 1. At least 18 years of age 2. Positive Tinel's test or Phalen maneuver 3. Complaints of nocturnal numbness and tingling Exclusion criteria: 1. A neuropathy other than CTS in the past year 2. Pregnancy 3. Thenar atrophy 4. Steroid injection into the carpal canal in the past 3 months or a prior carpal tunnel release. |
|
| Interventions | Intervention group 1: Fabricated neutral wrist and MCP splint with no exercises (neutral wrist and MCP): Participants received a customised, fabricated wrist splint positioning the wrist in neutral (0°) and the MCP joints from 0° to 10° of flexion. Participants were instructed to wear the splint during their regularly scheduled sleep time for 4 weeks. Intervention group 2. Fabricated neutral wrist and MCP splint with tendon and nerve gliding exercises (neutral wrist and MCP‐exercise): Participants received a customised, fabricated wrist splint positioning the wrist in neutral (0°) and the MCP joints from 0° to 10° of flexion. Participants were instructed to wear the splint during their regularly scheduled sleep time for 4 weeks. In addition, participants received visual and verbal instructions on tendon and nerve gliding exercises. Participants were instructed to perform the exercises 3 to 5 times a day, with 10 repetitions in each position, and to hold each position for 5 seconds. Intervention group 3. Off‐the‐shelf, wrist cock‐up splint with no exercises (wrist cock‐up): Participants received a prefabricated, off‐the‐shelf wrist cock‐up splint that immobilised the wrist in 20° of extension. Participants were instructed to wear the splint during their regularly scheduled sleep time for 4 weeks. Intervention group 4. Off‐the‐shelf, wrist cock‐up splint with tendon and nerve gliding exercises (wrist cock‐up‐exercise): Participants received a prefabricated, off‐the‐shelf wrist cock‐up splint that immobilised the wrist in 20° of extension. Participants instructed to wear the splint during their regularly scheduled sleep time for 4 weeks. In addition, participants received visual and verbal instructions on tendon and nerve gliding exercises. Participants were instructed to perform the exercises 3 to 5 times a day, with 10 repetitions in each position, and to hold each position for 5 seconds. |
|
| Outcomes | Outcomes assessed at baseline and at the end of four weeks of treatment : 1. Symptoms using carpal tunnel questionnaire (rates 11 items on ordinal scale 1: no symptoms, to 5: most severe pain)** 2. Hand function using carpal tunnel questionnaire (rates 8 items on ordinal scale 1: no difficulty with the activity, to 5: unable to perform activity)** 3. Functional sensibility using the Moberg Pick‐up Test: participants are timed on how quickly they pick up an assortment of objects such as a coin, safety pin, and paper clip, and place them in a small box** 4. Grip strength using a hand‐held dynamometer: participants were given 3 opportunities to exert maximum force; the mean of 3 successive trials was recorded (higher scores indicate less impairment)** 5. Pinch strength using a reliable and accurate hand‐held pinch meter. Participants had 1 opportunity to exert maximum force with 3 types of pinch: tip pinch, lateral pinch, and palmar pinch (higher scores indicate less impairment)** 6. Satisfaction using an exit survey developed by the primary investigator that was designed to evaluate their level of satisfaction with the treatment provided (measured at four weeks after treatment ended only). No information on how this outcome is rated by participants and scored by outcome assessors.** |
|
| Notes | *Data only reported for participants completing treatment (n = 51) **Data only reported overall from baseline to end of treatment or follow‐up for all intervention and control groups combined, and often only in the form of F and P values. Thus, no data appropriate for meta‐analysis were entered into RevMan. The authors were contacted in order to retrieve these data, but efforts were unsuccessful. Interventions were only applied to one wrist per participant (even in bilateral CTS participants). Therefore, a unit of analysis error resulting from the correlation between two wrists in bilateral CTS participants could not have occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Random allocation was made after subjects gave their informed consent and baseline assessments were completed. Subjects were randomized into groups by selecting a sealed opaque envelope that contained a number corresponding to an intervention group." Comment: Probably done, but not enough information to determine the adequacy of the randomisation sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Random allocation was made after subjects gave their informed consent and baseline assessments were completed. Subjects were randomized into groups by selecting a sealed opaque envelope that contained a number corresponding to an intervention group." Comment: The allocation sequence was probably adequately concealed until interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Unlikely that participants would have been blinded to which treatment they were allocated to. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Another limitation was that the person (TLB) who administered the treatment and evaluated outcomes was not masked to subjects' group assignments, and that may have biased the results." Comment: The outcome assessor (who also administered the interventions) was probably not blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "Sixty‐one of 79 eligible patients enrolled in the study. Four subjects withdrew because: they had an injection or surgery (n = 2), developed an illness (n = 1), or moved out of the area (n = 1); 6 subjects were lost to follow‐up". Comment: There were drop‐outs and losses to follow‐up in each of the four groups, and these were detailed, and unlikely to have biased the results. |
| Selective reporting (reporting bias) | High risk | Comment: Majority of the outcomes are reported incompletely (e.g., only as F values or P values from an ANOVA), and cannot be entered into a meta‐analysis. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Burke 1994.
| Methods | Double‐blind quasi‐randomised controlled trial Blinded subjects and assessors* Ethics approval and informed consent obtained Quasi‐randomisation occurred at the level of wrists, where participants with bilateral CTS received a different intervention for each affected wrist. |
|
| Participants | Total N = 59 (90 wrists) randomised
Group 1 N = 45 wrists randomised
Group 2 N = 45 wrists randomised Gender of participants not reported. Age of participants not reported. Inclusion criteria: 1. Clinical diagnosis of CTS (hypoaesthesia or paraesthesiae in median nerve distribution, weakness or atrophy in abductor pollicis brevis or opponens pollicis) Exclusion criteria: 1. History of CTR surgery 2. Injection at wrist 3. Previous splint use |
|
| Interventions | Group 1: Wrist splint in neutral for two months Group 2: Wrist splint in 20° extension for two months Wearing regimen (day or night) was not controlled, though researchers emphasised to participants nightly use of splints. |
|
| Outcomes | Outcome assessed at two weeks and two months** 1. Symptom relief*** (overall, nocturnal, daytime) assessed using ordinal scale (1 = not at all, 2 = a little, 3 = a lot, 4 = completely) 2. Compliance**** (wore splint every night, most nights, some nights, never) 3. Adverse effects (any difficulty with splints) |
|
| Notes | Age and sex of participants not reported *Confirmed with author in personal communication **No data for any of the outcomes at two months were reported ***Dichotomised by trialists for analysis into 'a lot/complete relief' and 'none/little relief' ****Not a pre‐specified outcome of interest to this review Analysis was undertaken at the wrist‐level for all outcomes, though some participants in each group had bilateral CTS. Bilateral cases had a different intervention applied to each wrist. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Splinting was completed by alternating between extension and neutral. The order of splinting was dominate then non‐dominant hand. Thus, if the next splint on the alternating list was for neutral, the dominant hand would then receive the extension splint". Comment: A non‐random sequence (alternation) was used. |
| Allocation concealment (selection bias) | High risk | Comment: Alternate allocation was used therefore trial personnel and participants could predict the order of group assignments. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both splints were attached in the same way, and patients were not told of the difference in angle. No patients expressed an awareness of a difference between the two splints". Comment: Participants were probably blind to group assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both splints were attached in the same way, and patients were not told of the difference in angle. No patients expressed an awareness of a difference between the two splints". Comment: All outcomes (symptom relief; overall, nighttime, daytime) were self‐reported by participants who were blinded to group assignments |
| Incomplete outcome data (attrition bias) 3 months or less | High risk | Comment: Data missing for daytime and nighttime symptom relief assessed at two weeks. For daytime relief, 5 wrists were missing from the neutral splint group and 23 wrists were missing from the extension splint group. For nighttime relief, 3 wrists were missing from the neutral splint group and 18 wrists were missing from the extension splint group. There was a disproportionate number of wrists missing from the extension splint group compared with the neutral splint group and no explanation for missing data was provided. Results for symptom relief at daytime and nighttime could have been biased in favour of neutral splints. |
| Selective reporting (reporting bias) | High risk | Comment: There was selective reporting of outcomes assessed at two weeks due to incomplete data for outcomes assessed at two months. Nighttime and daytime compliance were measured but not reported. Participants were instructed to return to the clinic if they had any difficulty with the splints however, it is not stated whether this occurred for any cases. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Bye 2011.
| Methods | Quasi‐randomised controlled trial No blinding reported Unclear if ethics approval and informed consent obtained Randomisation occurred at the level of wrists, with no constraint that each participants' wrist be allocated to different treatments |
|
| Participants | Total N randomised = 12 (20 wrists) randomised Intervention group N = Not reported Control group N = Not reported 0 males, 12 females Mean ± SD (range) age: Group 1: 43.50 ± 10.9 years Group 2: 35.60 ± 5.79 years Inclusion criteria: 1. Housewives aged 30 to 65 years 2. Chronic hand or wrist pain with paraesthesia or numbness in at least one finger innervated by Median nerve 3. Nocturnal symptoms for at least 3 months 4. Mild to moderate CTS based on electrodiagnostic findings Exclusion criteria: 1. History of corticosteroid injection in wrist during the past three months 2. History of carpal tunnel surgery in the affected hand 3. History of wrist fracture in the affected hand 4. Pregnancy 5. Upper motor neuron problems |
|
| Interventions | Intervention group: MANU hand brace (keeps third and forth finger in extension, and which was developed by Manente 2001) at nights for 4 weeks Control group: Short 'Cock‐up' splint (with natural wrist angle) at nights for 4 weeks |
|
| Outcomes | Outcomes assessed at baseline, after two weeks, and at the end of four weeks of treatment: 1. Pain VAS was filled by the participant at the beginning of the study. The participant was instructed to mark a point for her diurnal pain and one for her nocturnal pain for every day during the study. The VAS was a 100 mm line in which 0 meant no pain and 100 meant the most possible pain, so each point corresponded to a number indicating severity of the pain. For each participant the numbers were added and their mean was considered as her mean pain. 2. Symptoms assessed using the Levine carpal tunnel questionnaire, with 11 questions, each with five answers rating from 1 (least) to 5 (most), so the highest possible score is 55. 3. Function assessed using the Levine carpal tunnel questionnaire, with 11 questions, each with five answers rating from 1 (least) to 5 (most), so the highest score possible score is 40. |
|
| Notes | Study written in Turkish and was translated into English by a translator recruited by the Neuromuscular Disease Review Group. Some participants in each group had bilateral CTS, however, the number of participants and wrists allocated to each group was not reported. Some bilateral CTS participants received the same intervention for both wrists while others received different interventions for each wrist. It is not clear if analysis was undertaken at the participant‐ or wrist‐level for outcomes, . Attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. As the sample size for each outcome is unclear, we could not include this outcome data in the Data and analyses section. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: Alternation was used, in that the first participant with mild symptoms was placed in MANU hand brace group and the next participant in the other group. Also, the first participant with moderate symptoms was placed in MANU hand brace group and the next participant in the other group. This was done to promote severity of the disease equality between groups. |
| Allocation concealment (selection bias) | High risk | Comment: As alternation was used, the personnel responsible for recruiting participants would be aware of what the next group participants would be allocated to, thus the allocation was not adequately concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: It is not clear whether participants and personnel were blind to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The authors did not mention if they had assessed how often and how the participants used the splints. Not enough information was provided in the paper to determine whether outcome assessors (who are the participants only as only self‐reported outcomes were used) were blind to treatment. |
| Incomplete outcome data (attrition bias) 3 months or less | Unclear risk | Comment: In this study, there was no explanation about excluded patients or percentage of participants appearing for follow‐up. It seems that all the patients who entered the study finished the study. However, it was not clear how many participants were allocated to each group. |
| Selective reporting (reporting bias) | High risk | Comment: The means and SDs of all outcomes were reported, but as the number of participants allocated to each group was not clear, it was not possible to enter these data into RevMan. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Celiker 2002.
| Methods | Randomised controlled trial No blinding Ethics approval and informed consent obtained Randomisation occurred at the level of participants, where participants with bilateral CTS received the same intervention for both affected wrists. |
|
| Participants | Total N = 23 (37 wrists) randomised Intervention group N = 11 (16 wrists) randomised Control group N = 12 (21 wrists) randomised 1 male, 22 females Mean ± SD (range) age: Intervention group 1: 49.6 ± 15.3 yrs Control group 2: 46.9 ± 10.0 yrs Mean ± SD duration of CTS symptoms: Intervention group 1: 6.9 ± 6.9 months (range 1 to 24 months) Control group 2: 8.5 + 16.4 months (range 1 to 60 months) Inclusion criteria: 1. Electrodiagnostic confirmation of unilateral or bilateral CTS Exclusion criteria: 1. Presence of thenar atrophy |
|
| Interventions | Intervention: Splinting and acemetacine 120 mg/day was received by participants. Lightweight, neutral‐positioned wrist splints were used just at night. The duration of splint‐wearing and NSAID taking was eight weeks Control: Local corticosteroid injection (40 mg methylprednisolone acetate (1 mL)). The point of entry was about 4 cm proximal to the wrist at the midline or just to the radial side of the palmaris longus. A 22‐guage needle was angled almost horizontally and passed its full length into the carpal tunnel without piercing either the tendon or the nerve. If median paraesthesias were elicited, the needle was repositioned, and then the steroid suspension was injected. The entire suspension was discharged under the transverse ligament. The number of injections received over the 8‐week study period was not reported. |
|
| Outcomes | Outcomes assessed at baseline and at two weeks and at the end of eight weeks of treatment: 1. Tinel's test 2. Phalen's test and reverse Phalen's test, which were both performed for a minimum of 60 sec. 3. Pain: measured using a VAS. The authors did not report the measurement units of this VAS, but based on the data reported, MP assumes that a 0 to 10 VAS was used. 4. Symptom severity using a Swedish translated version of the Levine CTS Symptom Severity Scale, with the mean of 11 items rated from 1 to 5 reported (higher scores denote worse symptoms). Outcomes assessed at baseline and at 8 weeks (end of treatment: 1. Nerve conduction: median nerve motor conduction velocity*, median nerve motor latency, median nerve sensory conduction velocity*, median nerve distal sensory latency, ulnar motor nerve conduction velocity*, ulnar motor nerve distal latency* 2. Adverse effects: the authors did not report how this outcome was recorded. |
|
| Notes | *Data not reported in the publication The results of neurophysiologic parameters that were reported in the publication were reported as both endpoint and change from baseline values. There were no differences between endpoint and change from baseline values in terms of statistical significance, so we chose to be consistent and only include endpoint values (as we included endpoint values where available for all other studies included in the review). Analysis was undertaken at the participant‐level for pain and symptom severity score, and at the wrist‐level for Tinel's test, Phalen's test, reverse Phalen's test, and nerve conduction studies, though some participants in each group had bilateral CTS. Bilateral cases had the same intervention applied to each wrist. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of the two groups by using sequentially numbered sealed opaque envelopes." Comment: Not enough information to determine the adequacy of the allocation sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly assigned to one of the two groups by using sequentially numbered sealed opaque envelopes." Comment: The allocation sequence was probably adequately concealed until interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This study was a prospective, unblinded, randomized clinical trial with an 8‐wk follow‐up." Comment: Due to the nature of the interventions, participants were probably aware of which treatment they received. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This study was a prospective, unblinded, randomized clinical trial with an 8‐wk follow‐up." Comment: Outcome assessors were probably aware of which treatment participants were allocated to. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: No withdrawals/losses to follow‐up were reported, and the tables of data indicate that the reported data were based on the complete randomised sample. |
| Selective reporting (reporting bias) | High risk | Comment: The outcomes of median nerve motor conduction velocity, median nerve sensory conduction velocity, ulnar motor nerve conduction velocity, and ulnar motor nerve distal latency were specified to have been measured at baseline and at 8 weeks follow‐up in the Methods section of the publication but were not reported in the Results section. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
De Angelis 2009.
| Methods | Randomised, single‐blind controlled trial Blinded assessors Ethics approval and informed consent obtained |
|
| Participants | Total N = 120 participants (120 wrists) randomised Intervention group N = 61 participants (61 wrists) randomised, 45 participants (45 wrists) completed Control group N = 59 participants (59 wrists) randomised, 46 (46 wrists) participants completed 17 males and 103 females randomised; 12 males and 79 females completed Mean ± SD age:* Intervention group: 46.3 ± 7.9 years Control group: 46.0 ± 11.8 years Inclusion criteria: 1. Pain, numbness and paraesthesias and/or hypoaesthesia in the median nerve distribution 2. Phalen test positive 3. CTS exclusive or predominant in one hand 4. Electrophysiological diagnosis of CTS Exclusion criteria: 1. Previous CTS surgery or intracarpal steroid injections 2. Rheumatoid arthritis 3. Clinical and electrophysiological signs of polyneuropathy 4. Wrist trauma 5. Coexisting cervical radiculopathies 6. Brachial plexopathies or more proximal median mononeuropathies 7. CTS related to systemic diseases 8. Fibromyalgia 9. Pregnancy 10. Age lesser than 18 years |
|
| Interventions | Intervention: The wrist splint CAMP TIELLE model 1.2 (TIELLE SpA, Milano, Italy) was worn every night for 3 months. The splint immobilised the wrist in a dorsiflexion position with an external angle of 30° and internal angle of 16°. Control: The hand brace MANU (developed by Manente 2001) was worn every night for 3 months. It was made of soft tissue without rigid components and consisted of: (i) a palmar strap with a Velcro adjustable fastening to tighten the distal heads of the second and fifth metacarpal bones; (ii) a triangular prism‐shaped pad positioned dorsal to digits II and V, producing slight stretching of digits III and IV; (iii) a dorsal strap connected to a wrist band with a Velcro adjustable fastening; and (iv) a component that connects and stabilizes the other parts. The hand brace did not impede thumb‐index finger pinch, thumb‐little finger opposition and wrist flexion and extension. |
|
| Outcomes | Outcomes assessed at baseline, at the end of three months treatment, and at six months after the end of treatment (i.e., 9 months from baseline): 1. Symptoms using the Italian version of the Boston Carpal Tunnel Questionnaire (rates 11 items on ordinal scale from 1 to 5; the verbal descriptors of the 1 to 5 scale were not reported)** 2. Function using the Italian version of the Boston Carpal Tunnel Questionnaire (rates 8 items on ordinal scale from 1 to 5; the verbal descriptors of the 1 to 5 scale were not reported)** 3. Pain using a 100 mm VAS (with the left sided verbal descriptor being ‘no pain’ and the right sided verbal descriptor being ‘worst pain’). Participants were instructed to place a mark on the line to report the intensity of the sensation being experienced. The pain score was identified by measuring the millimetres from the left end of the scale to the subject’s mark.** 4. Paraesthesias using a 100 mm VAS (with the left sided verbal descriptor being ‘no paresthesias' and the right sided verbal descriptor being ‘worst paresthesias'). Participants were instructed to place a mark on the line to report the intensity of the sensation being experienced. The pain score was identified by measuring the millimetres from the left end of the scale to the subject’s mark.** 6. Nerve conduction: median nerve distal motor latency (ms), median nerve sensory conduction velocity (m/s), and sensory nerve action potential (SNAP) amplitude (µV).** 7. Adverse effects: At the end of 3 months treatment participants were assessed for whether there were adverse effects to the treatment. No report on how this was assessed and recorded. |
|
| Notes | *Data reported only for participants completing trial (n = 91) **The authors reported outcome data as means and 95% CIs of the means. 95% CIs were converted to SDs using the RevMan calculator. In participants with bilateral CTS, the wrist with the most severe symptoms was chosen for treatment and evaluation, so a unit of analysis error resulting from the correlation between two wrists in bilateral CTS participants could not have occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomization was carried out by using a simple randomization process. Each subject meeting the inclusion and exclusion criteria for entering the trial has been randomly assigned to one of the two groups in a 1:1 ratio." Comment: Not enough information to determine the adequacy of the randomisation sequence generation. |
| Allocation concealment (selection bias) | High risk | Quote: "The randomization process of assigning individual subjects to their groups was performed by an unblinded investigator." Comment: The allocation sequence was not adequately concealed until interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "To optimize blinding, the patients were reminded, immediately before their visit, to reveal information regarding their treatment only to the treating physician." Comment: Participants were unlikely to have been unaware of which treatment they received. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The physicians who assisted the patient in filling the BCTQ and VAS scales and performed conduction velocities (MVDA and FP) were blinded for the allocated treatment, whereas the treating physician (AU) was not blinded. To optimize blinding, the patients were reminded, immediately before their visit, to reveal information regarding their treatment only to the treating physician." Comment: Outcome assessors were probably blind to treatment allocation. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "Twenty‐nine subjects were lost to follow‐up, 13 (44.8%) in the MANU group and 16 (55.2%) in the CAMP TIELLE group....As the withdrawal from the study at T1 and T2 was balanced in both groups, we chose to include in the statistical analysis only those subjects who completed the study. This has the advantage to compare longitudinally the same subjects without the need to extrapolate for missing data." Comment: The number of drop‐outs and reasons for these are clearly reported. It can be safely assumed that the data reported in tables are based on 91 participants (i.e., all of those who completed the study) as this is specified in the second figure in the publication. |
| Incomplete outcome data (attrition bias) After 3 months | Low risk | Quote: "Twenty‐nine subjects were lost to follow‐up, 13 (44.8%) in the MANU group and 16 (55.2%) in the CAMP TIELLE group....As the withdrawal from the study at T1 and T2 was balanced in both groups, we chose to include in the statistical analysis only those subjects who completed the study. This has the advantage to compare longitudinally the same subjects without the need to extrapolate for missing data." Comment: The number of drop‐outs and reasons for these are clearly reported. It can be safely assumed that the data reported in tables are based on 91 participants (i.e., all of those who completed the study) as this is specified in the second figure in the publication. |
| Selective reporting (reporting bias) | Low risk | Comment: All of the outcomes specified in the Methods section of the publication were reported in the pre‐specified way. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
de Entrambasaguas 2006.
| Methods | Randomised, single‐blind controlled trial Blinded outcome assessor Unclear if ethics approval and informed consent obtained It is unclear whether randomisation occurred at the level of participants or wrists, and whether all bilateral CTS participants received the same or different intervention for each wrist |
|
| Participants | Total N = 75 wrists randomised; 38 participants (52 wrists) completed (number of participants randomised not clear) Intervention group 1 N = 26 wrists randomised; 18 wrists completed Intervention group 2 N = 24 wrists randomised; 18 wrists completed Intervention group 3 N = 25 wrists randomised; 16 wrists completed Number of male wrists not clear, 44 females wrists Mean ± SD (range) age: 50.7 ± 10.3 (25 to 73) (group‐specific ages not reported) Inclusion criteria: 1. Mild CTS (increase of sensory or mixed latencies of the median nerve, regardless of the amplitude of potentials) or moderate CTS (criteria for mild CTS plus increase of distal motor latency of the median nerve) Exclusion criteria: 1. Severe CTS (absence or low amplitude of sensory or motor potentials, with presence of denervation or reinnervation on needle EMG). 2. CTS previously treated, surgically or otherwise. 3. Presence of any condition aetiologically related to CTS, with the exception of manual work. 4. Treatment being carried out at the time for whatever reason with anti‐inflammatory drugs. |
|
| Interventions | Intervention group 1: Splinting – each splint was modelled individually for each hand, and worn for 12 hours daily for four weeks; if uncomfortable, splint was adjusted. Intervention group 2: Steroid injection ‐ injection of 40 mg of triamcinolone with 10 mg of lidocaine Intervention group 3: Phonophoresis ‐ diclofenac gel was used to administer ultrasound pulses in 10‐minute sessions, five days per week, for four weeks |
|
| Outcomes | Outcomes assessed at baseline and one month after treatment ended: 1. Sensory symptoms: tingling, numbness, pain, autonomic manifestations (sweating of palms, changes in skin colour, subjective swelling or clumsiness) measured as 'better', 'worse' or 'no change'. 2. Physical exam: pinprick: median territory vs ulnar, abductor pollicis brevis muscle vs abductor digiti minimi, Tinel's test at the wrist. Each measured as 'better', 'worse' or 'no change' 3. Nerve conduction: sensory distal latency of median nerve (third digit‐wrist, longest), mixed median nerve (palm‐wrist, shortest) |
|
| Notes | Written in Spanish, and some details were translated by a translator recruited by the Neuromuscular Disease Review Group. Some participants had bilateral CTS, and analysis was undertaken at the wrist‐level for outcomes, However, it is not clear whether bilateral CTS participants received the same intervention for both wrists. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. Outcome data were not translated, so the only information regarding the results of this study comes from the English abstract: "The outcome of clinical parameters could not differentiate one treatment from another. Nerve conduction studies improved significantly in the steroid injection group when compared with the phonophoresis group, but not in the rest of the analysis. One nerve conduction parameter showed a minor significant improvement when compared with the basal study in the wrist splinting group. Phonophoresis had no effect on nerve conduction studies." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No information regarding how the random sequence was generated was reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information regarding the method of allocation was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Given the nature of the interventions (splint versus injection versus phonophoresis), it is likely that participants and personnel were aware of which treatment they received. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: The outcome assessor who rated symptoms, physical exam and nerve conducted was reported as being blind to treatment allocation. |
| Incomplete outcome data (attrition bias) 3 months or less | High risk | Comment: A flow chart details the number of wrists assigned to the each group, plus the number of wrists in each group where participants rejected treatment, did not show up, or were excluded because follow‐up was carried out by physicians not directly involved in the study or because participants did not follow instructions. The amount of loss to follow‐up and reasons for these were not equally balanced across the groups. |
| Selective reporting (reporting bias) | Low risk | Comment: According to the translator, all outcomes reported in the Methods section were fully reported in the Results section of the report. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Garfinkel 1998.
| Methods | Randomised, single‐blind, controlled trial Blinded assessors Ethics approval and informed consent obtained Randomisation occurred at the level of participants, where participants with bilateral CTS received the same intervention for both affected wrists. |
|
| Participants | Total N = 51 participants randomised
Intervention group N = 26 participants randomised; 22 participants (35 wrists) completed
Control group N = 25 participants randomised; 20 participants (32 wrists) completed 13 males; 28 females* Mean age: (SD not reported) Intervention 49 yrs Control 49 yrs Inclusion criteria: 1. Presence of 2 or more of the following: positive Tinel's test; positive Phalen's test; pain in median nerve distribution; sleep disturbance due to hand; numbness/paraesthesias in median nerve distribution 2. Abnormal electrophysiological findings 3. Subject agrees not to change medications, receive other new treatments or change work duties during trial Exclusion criteria: 1. Previous surgery for CTS 2. Rheumatoid arthritis or other recognised inflammatory arthritis 3. CTS related to systemic disease (hypothyroidism) 4. Pregnancy |
|
| Interventions | Intervention: Yoga for 1 to 1.5 hours twice weekly for eight weeks Control: Wrist splint to supplement current treatment for eight weeks |
|
| Outcomes | Outcomes assessed at the end of eight weeks of treatment: 1. Pain severity using visual analogue scale (0 to 10, with 10 denoting greatest level of pain) 2. Nocturnal wakening using ordinal scale (rated as worsened, same, improved) 3. Phalen's test (rated as worsened, same, improved) 4. Tinel's test (rated as worsened, same, improved) 5. Grip strength in mmHg using sphygmomanometer cuff (mean of 3 trials) 6. Nerve conduction: median motor and sensory distal latencies (in ms) 7. Patterns of paraesthesia and numbness (recorded on hand diagram)** |
|
| Notes | *1 missing participant for demographic data **No data reported for this outcome The results of pain severity, grip strength and neurophysiologic parameters were reported as both endpoint and change from baseline values. There were no differences between endpoint and change from baseline values in terms of statistical significance, so we chose to be consistent and only include endpoint values (as we included endpoint values where available for all other studies included in the review). Analysis was undertaken at the wrist‐level for all outcomes, though some participants in each group had bilateral CTS. Bilateral cases had the same intervention applied to each wrist. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomised into 2 groups by having them select sealed envelopes containing a group assignment". Comment: No information on how the random sequence was generated prior to putting these into envelopes was reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Sealed envelopes were used however they may not have been distributed according to a randomised sequence and it is unclear whether opaque envelopes were used. It is unclear whether participants or trial personnel could predict assignments. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants were aware of group assignments therefore self‐reported outcomes such as pain, nocturnal wakening, and patterns of paraesthesia and numbness may be biased. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The assessments all were conducted by 1 physician who was blinded to the patient's group assignment and the intervention". Comment: Participants were aware of group assignments which may have influenced their performance when outcomes such as grip strength, Phalen's test, and Tinel's test were measured however, nerve conduction studies were less likely to be compromised. |
| Incomplete outcome data (attrition bias) 3 months or less | High risk | Quote: "9 dropped out or were excluded". Comment: Four participants from the treatment group and five from the control group were not included in the analysis. No reasons were provided to explain these drop‐outs or exclusions |
| Selective reporting (reporting bias) | High risk | Comment: Patterns of paraesthesia and numbness were recorded on hand diagrams but no results of these measurements were reported. Results were reported for all other measurements. |
| Other bias | Low risk | Comment: The unit of analysis was at the level of the individual. It is apparent that some participants had bilateral involvement however these cases were equally distributed across the groups [intervention group: N = 22 (35 wrists), control group: N = 20 (32 wrists)]. |
Kumnerddee 2010.
| Methods | Randomised controlled trial No blinding reported Ethics approval and informed consent obtained |
|
| Participants | Total N = 61 participants (61 wrists )randomised Intervention group 1 N = 31 participants (31 wrists) randomised; 30 participants (30 wrists) completed Intervention group 2 N = 30 participants (30 wrists) randomised; 30 participants (30 wrists) completed 6 males, 54 females* Mean ± SD age:* Intervention group 1: 51.73 ± 8.92 years Intervention group 2: 50.37 ± 9.01 years Mean ± SD duration of CTS symptoms:* Intervention group 1: 8.32 ± 7.68 months Intervention group 2: 12.12 ± 15.71 months Inclusion criteria: 1. People with mild to moderate CTS Exclusion criteria: 1. Severe degree CTS 2. Peripheral neuropathy 3. Pregnancy 4. Tendinitis or arthralgia in wrist or hand 5. Obvious space occupying lesion at the wrist 6. Thenar muscle atrophy 7. History of local steroid injection 8. History of carpal tunnel surgery 9. Inability to discontinue analgesics 10. Unwillingness to participate in the present study. |
|
| Interventions | Intervention group 1: Prefabricated volar neutral wrist splint worn at night only for five weeks. The splint restricted flexion motion of the wrist by a metallic bar inserted within the volar aspect of the splint whereas the extension motion was relatively controlled by neoprene and Velcro strap over the dorsal aspect of the hand and forearm. Intervention group 2: Ten sessions of electro‐acupuncture were performed twice a week. Six acupoints including HeGu (LI 4), QuChi (LI 11), DaLing (PC 7), LaoGong (PC 8), and two BaXie points (EX‐UE9) were chosen in respect to the meridiens contributing to the affected area. All needles except EX‐UE9 points were connected with the SDZ‐II nerve and muscle stimulator (Hwato, Suzhou, China) generating 1 Hz continuous direct current for 30 minutes. |
|
| Outcomes | Outcomes assessed at baseline and at the end of five weeks treatment:** 1. Symptoms using the Thai version of the Boston Carpal Tunnel Questionnaire, calculated as the mean of 11 items scored from 1 (normal) to 5 (worst symptoms) 2. Pain using a 100mm visual analogue scale 3. Function using the Thai version of the Boston Carpal Tunnel Questionnaire, calculated as the mean of eight items scored from 1 (normal) to 5 (worst disability). 4. Adverse effects 5. Analgesic intake |
|
| Notes | *Data reported only for participants completing trial (n = 60) *The results of all outcomes were reported as both endpoint and change from baseline values. There were some differences between endpoint and change from baseline values in terms of statistical significance, though to minimise selective inclusion bias, we chose to be consistent and only include endpoint values (as we included endpoint values where available for all other studies included in the review). Sixty per cent of participants in the night splinting group had bilateral CTS, whereas 70% of participants in the acupuncture group had bilateral CTS. However, the trialists reported that "In case of bilateral CTS, only the more severe hand was evaluated", so a unit of analysis error resulting from the correlation between two wrists in bilateral CTS participants could not have occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Block randomization was carried out using Stata program version 10.0 (STATA Corp, LP. College Station, Tx) to allocate subjects into an acupuncture group (Acu) and a night splinting group (NS)." Comment: No information on how the random sequence was generated was reported. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Block randomization was carried out using Stata program version 10.0 (STATA Corp, LP. College Station, Tx) to allocate subjects into an acupuncture group (Acu) and a night splinting group (NS)." Comment: No information on how the random sequence was concealed from individuals responsible for allocating participants was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Pros and cons of all choices for the treatments of CTS were explained. Individuals who accepted both acupuncture and night splinting were asked to sign informed consent forms. Quote: " The present unblinded study may be at risk of assessment bias". Comment: Given the nature of the interventions delivered, it is unlikely that participants and personnel delivering the interventions could be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Pros and cons of all choices for the treatments of CTS were explained. Individuals who accepted both acupuncture and night splinting were asked to sign informed consent forms. Quote: " The present unblinded study may be at risk of assessment bias". Comment: Given the nature of the interventions delivered, it is unlikely that participants and personnel delivering the interventions could be blinded. Given that only self‐reported outcomes were measured, it is unlikely that outcome assessors (participants) could have been blinded. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "One subject in the NS [night splinting] group dropped out for operative treatment". Comment: Number of drop‐outs was small and reasons for this were reported, and are unlikely to have an impact on the results. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported in the methods section were reported fully in the results section of the publication. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Madjdinasab 2008.
| Methods | Double‐blind randomised controlled trial Not clear who was blinded (participants, personnel or outcome assessors) Ethics approval and informed consent obtained Randomisation occurred at the level of participants, where participants with bilateral CTS received the same intervention for both affected wrists. |
|
| Participants | Total N = 48 participants randomised Intervention group 1 N = 24 participants randomised Intervention group 2 N = 24 participants randomised 4 males, 44 females Mean age (SD not reported): Intervention group 1: 43 years Intervention group 2: 40 years Inclusion criteria: 1. Clinical diagnosis of CTS for at least one month 2. Participant has electrophysiological evidence of median neuropathy (defined as having two or more of the following: 1. Median nerve motor distal latency recording at abductor pollicis brevis and wrist stimulating greater than 4.4 ms; 2. Median nerve antidromic sensory peak latency recording at digit II greater than 3.5 ms; 3. Difference between antidromic median sensory latency and ulnar sensory latency at digit IV greater than 0.5 ms; 4. Antidromic latency difference more than 0.5 ms between median nerve at digit II and ulnar nerve at digit V; 5. The same distance of measurement). Exclusion criteria: 1. Patients with diabetes mellitus, trauma to wrist and deformity. 2. Any patient with evidence of generalised neuropathy /radiculopathy on electrodiagnostic study. 3. Patients with advanced CTS having wasting, marked weakness with marked axonal loss on nerve conduction study or nonstimulatable nerves. 4. Patients with a history of peptic ulcer. 5. Patients treated previously for CTS using medical or surgical therapy. 6. Pregnant women with CTS. 7. Patients with systemic disorders like rheumatoid arthritis, hypothyroidism, amyloidosis, etc. |
|
| Interventions | Intervention group 1: Commercially available splint worn at night and for as long as possible during the day for six weeks. Intervention group 2: Oral Prednisolone 20mg/day for two weeks Both groups were given advice to avoid extreme wrist flexion/extension, excessive hand movement and hand rest. |
|
| Outcomes | Outcomes assessed at baseline and at the end of six week treatment: 1. Nerve conduction: median and ulnar nerve sensory distal latency (ms), median and ulnar nerve motor distal latency (ms), median and ulnar nerve sensory conduction velocity, median and ulnar nerve motor conduction velocity |
|
| Notes | There were no self‐reported outcomes (e.g. symptoms, pain) or function outcomes reported as being measured in this study. The results of nerve conduction studies were reported as both endpoint and change from baseline values. There were no differences between endpoint and change from baseline values in terms of statistical significance, so we chose to be consistent and only include endpoint values (as we included endpoint values where available for all other studies included in the review). Analysis was undertaken at the participant‐level for all outcomes, though some participants in each group had bilateral CTS. Bilateral cases had the same intervention applied to each wrist. The trialists did not report the number of bilateral CTS participants in each group, or how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomly divided into two groups. Splint groups (N=24) used splint for six weeks; and steroid group (N=24) used oral Prednisolone 20mg/day for two weeks." Comment: No information on how the randomisation sequence was generated was reported. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "They were randomly divided into two groups. Splint groups (N=24) used splint for six weeks; and steroid group (N=24) used oral Prednisolone 20mg/day for two weeks." Comment: No information on how the randomisation sequence was adequately concealed was reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This double blind study was carried out in 48 idiopathic CTS patients". Comment: The authors report that this study was a double‐blind study, but do not indicate who specifically was blinded (participants, personnel delivering the treatment, or outcome assessors). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This double blind study was carried out in 48 idiopathic CTS patients". Comment: The authors report that this study was a double‐blind study, but do not indicate who specifically was blinded (participants, personnel delivering the treatment, or outcome assessors). |
| Incomplete outcome data (attrition bias) 3 months or less | Unclear risk | Quote: "In splint group three patients and in steroid group two patients did not complete the study and were eliminated."
Comment: 21/24 of the splint group completed assessments, and 22/24 of the prednisolone group completed assessments. The reasons for participants not completing the study were not reported, so it is not possible to determine whether the drop‐outs could have had an impact on the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: All outcomes reported in the Methods section of the publication were reported in the Results section of the publication. However, the only reported outcomes were electrophysiologic measures. Most other CTS RCTs measure symptoms and function too, and without access to a protocol for this study, we cannot determine whether those clinical outcomes were measured but not reported in the publication. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Manente 2001.
| Methods | Randomised controlled trial No blinding* Ethics approval and informed consent obtained |
|
| Participants | Total N = 83 participants (83 wrists) randomised; 80 participants (80 wrists) completed
Intervention group N = 41 participants (41 wrists) randomised; 40 participants (40 wrists) completed
Control group N = 42 participants (42 wrists) randomised; 40 participants (40 wrists) completed 11 males; 69 females Mean ± SD age: Intervention 46 ± 13 yrs Control 50 ± 13 yrs Inclusion criteria: 1. CTS symptoms (pain, numbness, paraesthesiae in median nerve distribution) exclusively or predominantly in one wrist 2. CTS signs (hypoaesthesia in median nerve distribution, thenar atrophy, positive Phalen's test) exclusively or predominantly in one wrist 3. At least one abnormal CTS electrodiagnostic study Exclusion criteria: 1. Previous carpal tunnel release 2. Rheumatoid arthritis 3. Systemic disease 4. Pregnancy 5. Polyneuropathy |
|
| Interventions | Intervention: Splint worn at night for four weeks Control: No treatment (asked to wait for an observational period of four weeks) for four weeks |
|
| Outcomes | Outcome assessed at two weeks and at the end of four weeks of treatment: 1. Symptoms using carpal tunnel questionnaire (rates 11 items on ordinal scale 1 to 5, with higher scores denoting worse symptoms) 2. Hand function using carpal tunnel questionnaire (rates 8 items on ordinal scale 1 to 5, with higher scores denoting worse symptoms) 3. Global impression of change (patient‐rated questionnaire rated in four categories: moderate or much improvement, minimal improvement, no change, worsening) (at 4 weeks only) 4. Nerve conduction: median motor distal latency (ms), median sensory conduction velocity (m/s), sensory nerve action potential amplitude (uV) (at 4 weeks only) 5. Changes of electrophysiological class of severity (4 weeks only) 6. Compliance and tolerability 7. Adverse effects |
|
| Notes | *Confirmed with author in personal communication. Only participants with unilateral CTS were included in the study, so a unit of analysis error resulting from the correlation between two wrists in bilateral CTS participants could not have occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomized into two groups by having them select sealed envelopes containing a group assignment". Comment: Insufficient information provided to determine whether adequate method used to generate random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were randomized into two groups by having them select sealed envelopes containing a group assignment". Comment: It is not specified whether envelopes were opaque or sequentially numbered and distributed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants, personnel, and outcome assessors were not blinded to treatment allocation. Assessment of symptoms, functional status, and global impression of change may be biased. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Outcome assessors were not blinded to treatment group assignments meaning results for nerve conduction studies may have been biased. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: Only one participant in the treatment group was lost to follow‐up and two participants in the control group were excluded after randomisation because they underwent surgery. This is unlikely to have introduced substantial bias in the comparison of outcomes for each group. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes stated in the methods section of the publication were reported in the results. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Mishra 2006.
| Methods | Randomised controlled trial No blinding Ethics approval and informed consent obtained Randomisation occurred at the level of participants, where participants with bilateral CTS received the same intervention for both affected wrists. |
|
| Participants | Total N = 40 (71 wrists) randomised and completed Intervention group N = 20 (36 wrists) randomised and completed Control group N = 20 (35 wrists) randomised and completed 7 males, 33 females Mean ± SD (range) age: Intervention group 1: 42.19 ± 9.39 (23 to 60) yrs Control group 2: 41.57 ± 9.26 (28 to 60) yrs Mean ± SD duration of CTS symptoms: Intervention group 1: 6.40 ± 7.09 months Control group 2: 6.31 ± 7.50 months Inclusion criteria: 1. Symptoms suggestive of CTS of at least 1‐month duration and electrophysiological evidence of median neuropathy at wrist Exclusion criteria: 1. Diabetes mellitus, trauma to wrist and deformity 2. Evidence of generalised neuropathy / radiculopathy on electrodiagnostic study 3. Advanced CTS having wasting, marked weakness with marked axonal loss on nerve conduction study or nonstimulatable nerves 4. History of peptic ulcer 5. Previous treatment for CTS using medical or surgical therapy 6. Pregnancy 7. Systemic disorders like rheumatoid arthritis, hypothyroidism, amyloidosis, etc |
|
| Interventions | Intervention: Commercially available carpal tunnel splint worn in the neutral position at night and as much as possible during the daytime for 4 weeks. In the case of bilateral symptoms, both hands were treated. Participants were also told not use additional medicines or other methods of treatment during the study period. Advice to avoid extremes of wrist flexion or extension, excessive hand movement and hand rest was provided. Control: Oral prednisolone 20 mg/day was taken for 2 weeks followed by 10 mg/day for another 2 weeks. Advice to avoid extremes of wrist flexion or extension, excessive hand movement and hand rest was provided. |
|
| Outcomes | Outcomes assessed before treatment and at the end of four weeks of treatment and at eight weeks post‐treatment: 1. Symptoms using carpal tunnel questionnaire (rates 11 items on ordinal scale 1: no symptoms, to 5: very severe symptoms) 2. Hand function using carpal tunnel questionnaire (rates 8 items on ordinal scale 1: no difficulty, to 5: very severe symptoms preventing the activity) 3. Nerve conduction: median nerve motor distal latency (msec), median nerve motor conduction velocity (metres/second), median nerve sensory distal latency (msec), median nerve sensory conduction velocity (metres/second) 4. Adverse effects: measured as the number of participants experiencing adverse effects (e.g., discomfort and swelling of the hands and wrist) |
|
| Notes | The results of all outcomes were reported as both endpoint and change from baseline values. There were no differences between endpoint and change from baseline values in terms of statistical significance, so we chose to be consistent and only include endpoint values (as we included endpoint values where available for all other studies included in the review). Analysis was undertaken at the wrist‐level for all outcomes, though some participants in each group had bilateral CTS. Bilateral cases had the same intervention applied to each wrist. The trialists confirmed (via personal communication) that the correlation between both wrists was not accounted for in the analysis. Therefore, a unit of analysis error occurred. Attempts to obtain the individual participant and wrist outcome data from the trialists were unsuccessful. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done using the table of random numbers." Comment: The randomisation sequence was probably adequately generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All patients were randomly allocated to one of the following two groups: 1. Splinting in neutral position. 2. Oral steroid. Randomization was done using the table of random numbers." Comment: Not enough information to determine whether the treatment allocation was adequately concealed until interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "A prospective randomised open‐label clinical and electrophysiological study of efficacy of splinting and oral steroids for the treatment of CTS was done." Comment: Participants were probably aware of which intervention they received. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Authors did not report any information on blinding of treatment allocation, so there is not enough information to determine whether outcome assessors were blind or not. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: No withdrawals, drop‐outs or losses to follow‐up were reported, and the authors indicated in the results tables that data was based on all 71 randomised wrists. |
| Selective reporting (reporting bias) | Low risk | Comment: All of the study's outcomes (pre‐specified in the Methods section of the study report) were reported in the pre‐specified way. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Premoselli 2006.
| Methods | Quasi‐randomised, single‐blind controlled trial Blinded assessor Ethics approval and informed consent obtained |
|
| Participants | Total N = 50 participants (50 wrists) randomised Intervention group N = 25 wrists randomised, 18 wrists completed trial Control group N = 25 wrists randomised, 16 wrists completed trial 5 males, 45 females Mean ± SD age: Intervention group: 53.1 ± 13.3 yrs Control group: 46.5 ± 13.8 yrs Inclusion criteria: 1. Compound motor action potential (CMAP) median nerve distal latency < 4.7 ms 2. Difference between median and ulnar sensory action potential latencies > 0.4 ms Exclusion criteria: 1. Diabetes 2. "Clear CTS" (i.e., not mild recent onset CTS, as measured using electromyographic measures) |
|
| Interventions | Intervention: Neutral custom‐moulded thermoplastic resin wrist splints were worn at nighttime only, for a minimum of 6 hours per night, for six months Control: No intervention |
|
| Outcomes | Outcomes assessed at baseline, at three months, and at the end of six months of treatment: 1. Nerve conduction: sensory action potential latency (ms), sensory action potential velocity (m/s), sensory action potential amplitude (µV), motor action potential latency (ms), motor action potential velocity (m/s), motor action potential amplitude (mV)* 2. Symptoms using carpal tunnel questionnaire (rates 11 items on ordinal scale 1: mildest pain, to 5: most severe pain)* 3. Hand function using carpal tunnel questionnaire (rates 8 items on ordinal scale 1: difficulty with activities, to 5: hindrance in performing an activity)* 4. Semeiotic testing using the Williams et al. (1992) pressure‐provocative test and the Phalen test. The time lapse between the moment of stimulation and the first manifestation of symptoms was assessed for each kind of test.* |
|
| Notes | *The results of all outcomes were reported as both endpoint and change from baseline values. There were some differences between endpoint and change from baseline values in terms of statistical significance, though to minimise selective inclusion bias, we chose to be consistent and only include endpoint values (as we included endpoint values where available for all other studies included in the review). Each participant contributed only one CTS‐affected wrist to the study, so a unit of analysis error resulting from the correlation between two wrists in bilateral CTS participants could not have occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The randomisation protocol was based on the last visit booking number (even or odd)." Comment: The trial authors used a non‐random component in the sequence generation process. |
| Allocation concealment (selection bias) | High risk | Quote: "The randomisation protocol was based on the last visit booking number (even or odd)." Comment: The trials authors did not adequately conceal the treatment allocation until interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Due to the nature of the interventions, it is unlikely that participants were not aware of which treatment they received (nighttime splint or no intervention). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examiner was blinded to treatment status (control or treatment)." Comment: Assessment of outcomes were probably done by a blinded assessor. |
| Incomplete outcome data (attrition bias) 3 months or less | Unclear risk | Quote: "Fifty patients (50 hands) were enrolled, of which 36 completed the study at 6 months." Quote: "At the three‐month follow‐up visit, 24/25 case patients and 24/25 control patients were evaluated." Quote: "At the six‐month follow‐up visit, 18 case group subjects and 16 control group subjects were evaluated." Comment: The numbers in these three quotes do not add up. In the abstract it says that 36 patients were available at 6 months follow‐up, but in the text, it says that 34 (18 + 16) patients were available at 6 months follow‐up. Therefore it is not clear how many participants were lost to follow‐up and the reasons for these. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes stated in the methods section of the publication were reported in their pre‐specified way. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Sevim 2004.
| Methods | Randomised, single‐blind controlled trial Blinded assessors Ethics approval and informed consent obtained |
|
| Participants | Total N = 120 participants (120 wrists) Intervention group 1 N =30 wrists randomised, 28 wrists completed Intervention group 2 N = 30 wrists randomised, 29 wrists completed Intervention group 3 N = 60 wrists randomised, 28 wrists completed Intervention group 4 N = 23 wrists completed* 16 males, 92 females** Mean ± SD (range) age:** Total sample: 46.27 ± 10.24 yrs (range 23 to 71 yrs) Intervention group 1: 43.89 ± 10.54 yrs (range not reported) Intervention group 2: 45.45 ± 11.60 yrs (range not reported) Intervention group 3: 49.71 ± 9.75 yrs (range not reported) Intervention group 4: 46.00 ± 7.90 yrs (range not reported) Mean ± SD (range) duration of CTS symptoms: Total sample: Range 5 months to 30 years (mean ± SD not reported) Inclusion criteria: 1. Referred to the electroneuromyography (ENMG) laboratory for the evaluation of CTS with symptoms including nocturnal paraesthesias, pain in the median nerve distribution during activity, or numbness in the median nerve distribution. 2. Abnormal median sensory nerve conduction values. Exclusion criteria: 1. Patients with secondary CTS (i.e. those with diabetes mellitus, hypothyroidism, rheumatic disease, previous wrist trauma) 1. Patients with coincident cervical radiculopathy or ulnar‐radial neuropathy 2. Patients younger than 18 years 3. Patients who had previous surgical treatment of CTS, used splints in the last 6 months or received steroid injections for CTS 4. Patients with a median motor distal latency longer than 6 ms on ENMG examination 5. Pregnant women. 6. Patients with a median nerve distal motor latency longer than the reference values underwent needle electromyography of the abductor pollicis brevis muscle, and those with fibrillation potentials, positive sharp waves or chronic neuropathic changes (decreased recruitment pattern, long duration or high amplitude of motor unit potentials) at needle electromyography were excluded 7. Patients with both normal motor and normal sensory conduction values |
|
| Interventions | Intervention group 1: Proximal steroid injection containing 3 mg betamethasone disodium phosphate and 3 mg betamethasone acetate suspension (Celestone Chronodose), mixed with 0.5 cc of a lidocaine HCl solution (Aritmal ampul 2%, 5 cc). The injection site was the volar side of the forearm 4 cm proximal to the wrist crease between the tendons of the radial flexor muscle; the long palmar muscle and the needle was inserted with an angle of 10º to 20º before injection of the solution. All the participants were injected once. Intervention group 2: Distal steroid injection containing 3 mg betamethasone disodium phosphate and 3 mg betamethasone acetate suspension (Celestone Chronodose), mixed with 0.5 cc of a lidocaine HCl solution (Aritmal ampul 2%, 5 cc). The needle was inserted at the anterior wrist flexion crease just near to ulnar side of the palmaris longus tendon and angulated 45º distally as well as 45º radially. All the participants were injected once. Intervention group 3: Splinting was performed by placing a standard lightweight wrist splint with a metal strip extending across the wrist to the midpalm region. The splint was bent so the wrist would be in neutral position (0° to 5° extended). The participants were instructed to wear the splints every night and to mark each night that they had worn the splints on a calendar. Splints were instructed to be worn every night until the 1‐year follow‐up (average 11 months, range 9 to 14). Intervention group 4: Control group formed by the subset of participants who were randomised to the splint group but who did not comply with wearing the splint 6 to 7 days per week during the 1 year treatment period (average 11 months, range 9 to 14), and instead wore the splint less than 1 night per week. |
|
| Outcomes | Outcomes assessed at baseline and at the end of 12 months treatment (average of 11 months post start of treatment, range 9 to 14 months): 1. Neurological symptoms measured by two clinicians using a structured questionnaire regarding possible symptoms of CTS: numbness, pain, paraesthesia, swelling, sense of swelling, drying or/and colour change in the related hand; numbness, pain, paraesthesia of the forearm and arm; provocation of symptoms by housework, reading and driving; existence of night symptoms; awakening due to night symptoms; frequency of night symptoms; numb hand upon awakening in morning; and mean duration of any symptom throughout the day. The severity of each symptom was graded from 0 to 3 (0, no symptom; 1, mild; 2, moderate; 3, severe). The sum of all complaint scores gave a total neurologic symptom score (NSS) for each participant. The authors do not indicate what the possible total NSS was. 2. Nerve conduction studies: median antidromic sensory nerve conduction studies of digits I, II and III (m/s), ulnar sensory nerve conduction study of digit V (m/s), median‐versus‐ulnar digit IV antidromic sensory distal latency difference (ms), mean antidromic median sensory action potential amplitude of the 3 digits (digits I, II and III) (uV)***, median motor nerve conduction (m/s)***, ulnar motor nerve conduction (m/s)***, median second lumbrical‐versus‐ulnar interossei distal motor latency (ms). 3. Adverse effects: the authors did not report how and when adverse effects were recorded. |
|
| Notes | *Control group was formed by the subset of participants who were randomised to the splint group but who did not comply with wearing the splint 6 to 7 days per week during the treatment period, and instead wore the splint less than one night per week. As a result, we chose not to include any outcome data from this study in the review, due to the high risk of bias associated with breaking the randomisation schedule. **Data only reported for participants available for follow‐up analysis (n = 108). ***Data not reported in the publication. Each participant contributed only one CTS‐affected wrist to the study, so a unit of analysis error resulting from the correlation between two wrists in bilateral CTS participants could not have occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of the 3 groups: splint group (60 patients), distal injection group (30 patients) and proximal injection group (30 patients)." Comment: Not enough information to determine the adequacy of the randomisation sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of the 3 groups: splint group (60 patients), distal injection group (30 patients) and proximal injection group (30 patients)." Comment: Not enough information to determine whether the allocation sequence was adequately concealed until interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Due to the nature of the interventions, participants and personnel were aware of which treatment they received. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two authors (HK and MA), blinded to the electrophysiologic findings and treatment methods of the patients throughout the study, assessed the patients using a structured questionnaire regarding possible symptoms of carpal tunnel syndrome." Quote: "Electrophysiological examinations were performed on the chosen hand of each patient before and after the treatment, by the same author (SS) who was blinded to treatment methods and historical data throughout the study." Comment: Outcome assessors were probably blind to treatment allocation. |
| Incomplete outcome data (attrition bias) 3 months or less | High risk | Quote: "At the end of 11 months (range, 9 to 14 months), contact with one patient from the proximal injection group and one from the distal injection group were lost for follow‐up. Another patient from the proximal injection group refused the electrophysiologic follow‐up examination. These 3 patients were dropped from the final analysis. Of the 60 participants in the splint group, 9 wore the splints on average 1‐5 nights per week and were excluded. Twenty‐three from this group wore the splints less than 1 night per week and were considered to form a control group. The remaining 28 patients wore the splints 6‐7 nights per week and they were taken as the properly used splint group. Thus, follow‐up evaluation was performed on 28 patients from the proximal injection group, 29 from the distal injection group, 28 from the splint group and 23 from the control group. These 108 participants were re‐evaluated by the same methods used at baseline and by the same physicians." Comment: Withdrawals and reasons for these were clearly reported. Participants who did not adhere to the splint protocol were either excluded from the analysis or entered into a 'control' group. The rationale for this method was not reported, and this 'as‐treated' analysis is likely to have biased the results |
| Selective reporting (reporting bias) | High risk | Comment: Median nerve motor distal latency, F wave latency, F wave persistency, median nerve compound action potential and median nerve sensory action potential amplitudes were not pre‐specified in the Methods section, but were reported in Results as being either significantly or non‐significantly reduced compared with baseline in the splint group (but no data were reported, and the result of this outcome for the remaining three groups was not reported at all). Ulnar sensory nerve conduction of digit V and ulnar motor nerve conduction were pre‐specified in the Methods section, but were not reported for any group in the Results. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Walker 2000.
| Methods | Quasi‐randomised controlled trial No blinding reported Ethics approval and informed consent obtained Randomisation occurred at the level of participants, where participants with bilateral CTS received the same intervention for both affected wrists. |
|
| Participants | Total N = 21 (30 wrists) randomised; 17 participants (24 wrists) completed
Group 1* N = 11 wrists completed
Group 2* N = 13 wrists completed 20 males; 1 female Mean ± SD age: Group 1: 60 ± 9 yrs Group 2: 61 ± 13 yrs Inclusion criteria: 1. Clinical diagnosis of CTS confirmed with electrodiagnostic studies 2. No previous treatment for CTS |
|
| Interventions | Group 1: Full time wear of wrist splint for 6 weeks Group 2: Nighttime only wear of wrist splint for 6 weeks |
|
| Outcomes | Outcome assessed at the end of six weeks of treatment: 1. Symptoms using carpal tunnel questionnaire (rates 11 items on ordinal scale 1 to 5, where 1 = mildest pain, 5 = most severe pain) 2. Hand function using carpal tunnel questionnaire (rates 8 items on ordinal scale 1 to 5, where 1 = no difficulty with activity, 5 = cannot perform activity at all) 3. Nerve conduction: median motor and sensory distal latencies (in ms) 4. Compliance (using questionnaire asking whether participants "always/usually wore", "sometimes wore" or "rarely/never wore" splint) 5. NSAID use |
|
| Notes | *Data only reported for participants completing treatment (n = 17 participants, 24 hands) The results of all outcomes were reported as both endpoint and change from baseline values. There were some differences between endpoint and change from baseline values in terms of statistical significance, though to minimise selective inclusion bias, we chose to be consistent and only include endpoint values (as we included endpoint values where available for all other studies included in the review). Analysis was undertaken at the wrist‐level for all outcomes, though some participants in each group had bilateral CTS. Bilateral cases had the same intervention applied to each wrist. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The randomization protocol was based on the last digit of the subject's Social Security number.." Comment: Allocation sequence was not truly random. |
| Allocation concealment (selection bias) | High risk | Comment: The last digit of the participant's Social Security number was used, therefore allocation was not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: The authors describe the trial as "unblinded". Participants were not blinded to splint‐wearing. Self‐administered questionnaires for the symptom severity scale, functional status scale, and splint‐wearing compliance for the last two weeks of the trial may have been influenced by the participant's knowledge of their own splint‐wearing behaviour. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "All electrodiagnostic testing was performed by or under the direct supervision of the principle investigator". Comment: Nerve conduction studies were not assessed blindly (personal communication with author). |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "Subjects were informed that steroid injections and surgery were also treatment options for CTS, and participation in this study did not prohibit them from seeking additional treatment, but they would be dropped from the study if they did so". Comment: One participant from each group was excluded because they had surgery or steroid injections. Losses from each group were balanced (two participants from each group) and unlikely to be a source of bias. |
| Selective reporting (reporting bias) | Low risk | Comment: All measures appear to be reported as described in the protocol of the trial publication. |
| Other bias | Low risk | Quote: "..subjects with bilateral involvement always received the same instructions for both hands...measures were taken for each hand". Comment: Participants were allocated to treatment groups (not hands) therefore those with bilateral involvement each contributed two hands to the analysis. The number of bilateral cases were similar in both treatment groups, so a unit‐of analysis error is unlikely to have occurred. |
Werner 2005.
| Methods | Quasi‐randomised single‐blind controlled trial Blinded assessors Ethics approval and informed consent obtained |
|
| Participants | Total N = 161 (161 wrists) randomised Intervention group N = 86 wrists randomised, 63 wrists completed Control group N = 75 wrists randomised, 49 wrists completed 55 males, 57 females* Mean ± SD (range) age:* Intervention group: 44.74 ± 1.02 (25.6 to 59.0) yrs Control group: 43.77 ± 1.44 (25.5 to 59.2) yrs Inclusion criteria: 1. Worker‐reported symptoms of numbness, tingling, burning, or pain in the wrist or the hand for more than a week or more than 3 times in the last 6 months 2. Hand diagram was suggestive of CTS; that is, there were symptoms of numbness, tingling, burning, or pain in the median nerve distribution Exclusion criteria: 1. Upper‐extremity musculoskeletal disorders secondary to acute trauma on or off the job 2. History of bilateral carpal tunnel release (CTR) surgery 3. Pregnancy |
|
| Interventions | Intervention: Customised wrist splints and ergonomic education ‐ participants were fitted with a custom wrist‐hand orthosis that maintained the wrist in a neutral posture, and was worn at night for 6 weeks. Participants received instructions in how to reduce ergonomic stressors in the work and home environments by viewing a 20‐minute video on CTS and ergonomic risk factors. Control: Ergonomic education alone via the same 20‐minute video on CTS and ergonomic risk factors presented to participants in the intervention group. |
|
| Outcomes | Outcomes assessed at baseline, and three**, six**, and a mean of 12 months (range 7 to 15 months) follow‐up (after the end of treatment): 1. Symptoms using carpal tunnel questionnaire (rates 11 items on ordinal scale 1: mildest, to 5: most severe) 2. Elbow and forearm, and wrist, hand and finger discomfort using a 30‐day worst‐discomfort rating on a 0 to 10 VAS 3. Surgical rates for CTS 4. Nerve conduction: median nerve sensory peak latency (msec), median nerve sensory amplitude (µv), median‐ulnar peak latency difference (msec) 5. Data in Occupational Health and Safety Administration logs, plant medical records, disability records, days of work missed due to upper extremity problems, and workers’ compensation status or work restrictions collected from computerised records*** 6. Splint usage and satisfaction (only in the intervention group) using a questionnaire administered at the end of the 6‐week treatment period |
|
| Notes | *Data only reported for participants completing treatment (n = 112) **According to the authors, half of the participants did not complete the questionnaires at 3 and 6 month follow‐up, so no data from these time points were reported. ***This was not a pre‐specified outcome of this review, so data were not entered into RevMan. In participants with bilateral CTS, the wrist with the most severe symptoms was chosen for treatment and evaluation, so a unit of analysis error resulting from the correlation between two wrists in bilateral CTS participants could not have occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Subjects were randomized to either a treatment or a control group, depending on whether the last digit of their Social Security number was odd or even." Comment: The trial authors used a non‐random component in the sequence generation process. |
| Allocation concealment (selection bias) | High risk | Quote: "Subjects were randomized to either a treatment or a control group, depending on whether the last digit of their Social Security number was odd or even. Subjects were not informed of the sequence for random allocation nor were they told to which group they were assigned until after consenting to participate." Comment: The trials authors did not adequately conceal the treatment allocation until interventions were assigned, as a non‐random process was used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Subjects were not blinded to their treatment, and the primary outcome measure was a self‐reported symptom severity score." Comment: Participants were probably not blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The nerve conduction data were collected at baseline and at the 12‐month follow‐up. Subjects reported to the medical department to have the testing done during regular work hours, and the person doing the testing was blinded to the treatment assignment." Comment: The outcome assessor of nerve conduction data was probably blinded, but it is not reported whether surgical rates and extraction of medical records were done by someone blinded to treatment assignment |
| Incomplete outcome data (attrition bias) 3 months or less | High risk | Quote: "Data collection was incomplete at the 3‐ and 6‐month follow‐up periods. Subjects were contacted by a study site coordinator and were reminded to fill out the questionnaire, but about half of the subjects did not complete the 3‐ or 6‐month questionnaires. The trend in outcome measures at 3 and 6 months was similar to the results at 12 months." Comment: Data not complete for all outcomes, with no explanation as to how this may have impacted on the data reported. |
| Incomplete outcome data (attrition bias) After 3 months | High risk | Quote: "The 12‐month follow‐up data are presented because they represent a more complete data set...The 12‐month follow‐up was actually a range of follow‐up times, with an average of 12 months and a range of 7 to 15 months." Comment: Data not complete for each outcome, with no explanation as to how this may have impacted on the data reported. |
| Selective reporting (reporting bias) | High risk | Comment: The results of the following outcomes were not reported, despite being mentioned as being collected in the Methods section of the trial: Occupational Health and Safety Administration logs, disability records, days of work missed due to upper extremity problems, and workers' compensation status or work restrictions collected from computerised records at baseline and 12 months' follow‐up, and nerve conduction data at 12 months' follow‐up. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Zinnuroglu 2010.
| Methods | Quasi‐randomised controlled trial No blinding Ethics approval and informed consent obtained |
|
| Participants | Total N = 27 (27 wrists) randomised; 24 participants (24 wrists) completed Intervention group N = 14 participants (14 wrists) randomised, 13 participants (13 wrists) completed Control group N = 13 participants (13 wrists) randomised, 11 participants (11 wrists) completed 1 male, 26 females Mean ± SD age:* Intervention group: 46.5 yrs (range 35 to 58 yrs) (SD not reported) Control group: 47.23 yrs (range 38 to 65 yrs) (SD not reported) Inclusion criteria: 1. Aged 35 to 65 years 2. Have mild or moderate idiopathic CTS Exclusion criteria: 1. Conditions that may cause secondary CTS, such as diabetes mellitus, rheumatoid arthritis, pregnancy, hypothyroiditis, renal insufficiency, fracture of carpal bones, ulna or radius, and peripheral neuropathy or any diseases which may cause peripheral neuropathy. |
|
| Interventions | Intervention: Carpal lock worn for two weeks continuous use followed by 2.5 months of nightly use. The wrist angle was adjusted to be in 15° of extension and the forearm was in neutral position. MCP joints were free to move, therefore finger and forearm movements were not restricted. Orthosis was fixed with Velcro straps which were on the forearm and palmar regions. Group 2: Volar supporting orthosis worn for two weeks continuous use followed by 2.5 months of nightly use. The orthosis was constructed with the same material as the carpal lock, but MPC joints, wrist and forearm movements were restricted and instead of dorsal support, volar support was used. Wrist and forearm angles were similar and MCP joints were in approximately 10° to 15° flexion. |
|
| Outcomes | Outcomes assessed before and at the end of three months treatment: 1. Pain using a 10 cm visual analogue scale 2. Dysaesthesia (numbness) using a 10 cm VAS 3. Nerve conduction: distal motor latency of the median nerve (msec), mixed nerve conduction of wrist‐elbow segment, sensory conduction velocity of second finger‐to‐wrist segment (metres/sec), sensory conduction velocity of palm‐to‐wrist segment (metres/sec), sensory conduction velocity of wrist‐elbow segment (metres/sec), latencies of the compound motor action potentials (mV), latencies of the sensory nerve action potentials (µV) |
|
| Notes | *Data only reported for participants completing treatment (n=24) In participants with bilateral CTS, only one wrist was randomly selected for treatment and evaluation, so a unit of analysis error resulting from the correlation between two wrists in bilateral CTS participants could not have occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Twenty‐seven patients with CTS were allocated into 2 groups, according to their date of admittance. All patients admitted during 1 week were assigned to the same group. In the following week, newly arriving patients were assigned to the second group. Similar numbers of patients were included in the two groups, as the numbers of weekly patient appointments were comparable." Comment: A non‐random allocation sequence was used. |
| Allocation concealment (selection bias) | High risk | Quote: "Twenty‐seven patients with CTS were allocated into 2 groups, according to their date of admittance. All patients admitted during 1 week were assigned to the same group. In the following week, newly arriving patients were assigned to the second group. Similar numbers of patients were included in the two groups, as the numbers of weekly patient appointments were comparable." Comment: A non‐random allocation sequence was used, so the allocation sequence was not able to be adequately concealed from individuals responsible for recruiting participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Another limitation of our study is that investigators were not blinded to the treatment protocol." Comment: Not enough information to determine whether participants were blinded to treatment allocation, but due to the nature of the interventions, it is unlikely that participants would not know which treatment they were receiving. Personnel were not blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Another limitation of our study is that investigators were not blinded to the treatment protocol." Comment: Outcome assessors were not blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "Two patients from the volar‐supporting orthosis group and another from the carpal lock group with bilateral CTS who did not comply with orthosis use as requested were excluded from the study." Comment: Withdrawals were reported by authors and the exclusion of these is not likely to significantly bias the results (their inclusion probably would have biased the results). |
| Selective reporting (reporting bias) | High risk | Quote: "There were no significant changes in distal motor latencies, mixed NCV values, or compound muscle action potential or sensory nerve action potential amplitudes." Comment: This is the only information the authors provide regarding these four outcomes (i.e., no means, SDs or P values were reported). Also, no data was reported on sensory conduction velocity of wrist‐elbow segment. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
CTS: carpal tunnel syndrome; EMG: Electromyography; MCP; metacarpopharyngeal; NCV: nerve conduction velocity; NSAID: nonsteroidal anti‐inflammatory drug; SD: standard deviation; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akalin 2002 | The same splint was applied to each group in this RCT (with other interventions) |
| Baysal 2006 | The same splint was applied to each group in this RCT (with other interventions) |
| Daniel 2000 | Not a RCT |
| Davis 1998 | The same splint was applied to each group in this RCT (with other interventions) |
| Eftekharsadat 2011 | The same splint was applied to each group in this RCT (with other interventions) |
| Ekim 2008 | The same splint was applied to each group in this RCT (with other interventions) |
| Evcik 2007 | The same splint was applied to each group in this RCT (with other interventions) |
| Gerritsen 2002a | Splinting is compared with surgery in this RCT |
| Gurcay 2009 | The same splint was applied to each group in this RCT |
| Heebner 2008 | The same splint was applied to each group in this RCT (with other interventions) |
| Kamanli 2011 | The same splint was applied to each group in this RCT (with other interventions) |
| Pinar 2005 | The same splint was applied to each group in this RCT (with other interventions) |
| Ruksen 2011 | The same splint was applied to each group in this RCT (with other interventions) |
| Weintraub 2000 | The effectiveness of a wrist support strap (not a splint) was investigated in this study |
| Yagci 2006 | Of the three groups, two received the same splint, and third received surgery, which is a not an eligible intervention for this review |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Taspinar 2007.
| Methods | Written in Turkish; requires translation |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Differences between protocol and review
This is a split review replacing the splinting interventions included in the previous review titled Non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome (O'Connor 2003).
In the review by O'Connor et al. (O'Connor 2003), types of outcome measures included in the review were as follows:
Primary outcome:
The primary outcome measure was improvement in clinical symptoms, such as pain and paraesthesiae, at least three months after the end of treatment.
Secondary outcome measures included: 1. improvement in functional status and/or health‐related quality of life parameters at least three months after treatment; 2. improvement in objective physical examination measures, such as grip, pinch strength, and sensory perception at least three months after treatment; 3. improvement in neurophysiological parameters after three months after treatment; 4. clinical improvement at less than three months of follow‐up; 5. clinical improvement at one year after treatment; 6. need for surgical release of the flexor retinaculum during follow‐up.
The outcomes reported in this review have been modified from the original review (O'Connor 2003) to make them as consistent as possible with other Cochrane reviews on CTS (Marshall 2007; Scholten 2007; Verdugo 2008).
Assessment for study risk of bias has been performed using The Cochrane Collaboration's 'Risk of bias' tool in this update of the review. We have included a 'Summary of findings' table.
Contributions of authors
MATTHEW PAGE (MP) was involved in the following stages of the review: design of the review (in collaboration with DOC); screening the search results (independently of, but in addition to NMW and DOC); organising retrieval of papers; screening retrieved papers against inclusion/exclusion criteria (independently of, but in addition to NMW and DOC); appraising the risk of bias of papers (independently of, but in addition to DOC and VP); extracting data from papers (independently of, but in addition to DOC, VP, and NMW); compiling the summary of comparisons, tables of included, excluded, awaiting and ongoing studies; entering data into RevMan; performing analysis of data; interpreting the findings (in collaboration with NMW, DOC and VP); writing of the review (in collaboration with DOC, VP and NMW).
NICOLA MASSY‐WESTROPP (NMW) was involved in the following stages of the review: screening the search results (independently of, but in addition to MP and DOC); organising retrieval of papers; screening retrieved papers against inclusion/exclusion criteria (independently of, but in addition to MP and DOC); appraising the risk of bias of papers (independently of, but in addition to MP, DOC and VP); extracting data from papers (independently of, but in addition to MP, DOC and VP); writing to study investigators for additional information; checking data entered into RevMan (independently, but in addition to DOC); interpreting the findings (in collaboration with MP, DOC and VP); writing of the review (in collaboration with MP, DOC, and VP).
DENISE O'CONNOR (DOC) was responsible for: design of the review (in collaboration with MP); developing the search strategy; screening the search results (independently of, but in addition to NMW and MP); screening retrieved papers against inclusion/exclusion criteria (independently of, but in addition to NMW and MP); appraising the risk of bias of papers (independently of, but in addition to NMW, MP and VP); extracting data from papers (independently of, but in addition to NMW, MP, and VP); checking data entered into RevMan by MP (independently, but in addition to NMW); interpreting the findings (in collaboration with NMW, MP and VP); contributing to the writing the review (with contribution from MP, VP and NMW).
VERONICA PITT (VP) was involved in the following stages of the review: extracting data from papers (independently of, but in addition to NMW, MP, and DOC); appraising the risk of bias of papers (independently of, but in addition to NMW, MP, and DOC); interpreting the findings (in collaboration with NMW, MP and DOC); contributing to the writing of the review (in collaboration with MP, DOC and NMW).
Sources of support
Internal sources
Australasian Cochrane Centre, Australia.
External sources
No sources of support supplied
Declarations of interest
The authors declare no competing commercial or copyright interests in this review.
New
References
References to studies included in this review
Arinci Incel 2005 {published data only}
- Arinci Incel N, Sezgin M, Aksit C, Sahin G. Effect of gabapentin in carpal tunnel syndrome: a controlled clinical trial [Karpal tunnel sendromunda gabapentin tedavisinin etkinligi: karsilastirmali klinik calisma]. Journal of Rheumatology and Medical Rehabilitation 2005;16(4):224‐30. [EMBASE: 2007022406] [Google Scholar]
Bardak 2009 {published data only}
- Bardak AN, Alp M, Erhan B, Paker N, Kaya B, Onal AE. Evaluation of the clinical efficacy of conservative treatment in the management of carpal tunnel syndrome. Advances in Therapy 2009;26(1):107‐16. [PUBMED: 19165436] [DOI] [PubMed] [Google Scholar]
Bilgici 2010 {published data only}
- Biligici A, Ulusoy H, Canturk F. The comparison of ultrasound treatment and local steroid injection plus splinting in the carpal tunnel syndrome: a randomized controlled trial. Bratislavske Lekarske Listy 2010;111(12):659‐65. [PUBMED: 21384736] [PubMed] [Google Scholar]
Brininger 2007 {published data only}
- Brininger TL, Rogers JC, Holm MB, Baker NA, Li ZM, Goitz RJ. Efficacy of a fabricated customized splint and tendon and nerve gliding exercises for the treatment of carpal tunnel syndrome: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88(11):1429‐35. [PUBMED: 17964883] [DOI] [PubMed] [Google Scholar]
Burke 1994 {published data only}
- Burke DT, Burke MM, Stewart GW, Cambre A. Splinting for carpal tunnel syndrome: in search of the optimal angle. Archives of Physical Medicine and Rehabilitation 1994;75(11):1241‐4. [PUBMED: 7979936] [DOI] [PubMed] [Google Scholar]
Bye 2011 {published data only}
- Bye R, Hajiaqai B, Frorough B. Comparison between efficacy of manu splint and cock‐up splint in carpal tunnel syndrome treatment. Journal of Babul University Medical Sciences 2011;13(1):51‐7. [Google Scholar]
Celiker 2002 {published data only}
- Celiker R, Arslan S, Inanici F. Corticosteroid injection vs. nonsteroidal antiinflammatory drug and splinting in carpal tunnel syndrome. American Journal of Physical Medicine and Rehabilitation 2002;81(3):182‐6. [PUBMED: 11989514] [DOI] [PubMed] [Google Scholar]
De Angelis 2009 {published data only}
- Angelis MV, Pierfelice F, Giovanni P, Staniscia T, Uncini A. Efficacy of a soft hand brace and a wrist splint for carpal tunnel syndrome: a randomized controlled study. Acta Neurologica Scandinavica 2009;119(1):68‐74. [PUBMED: 18638040] [DOI] [PubMed] [Google Scholar]
de Entrambasaguas 2006 {published data only}
- Entrambasaguas M, Mañez I, Girona G, Lopez‐Santoveña F, Poyatos YJ. Steroid injection, wrist splinting and phonophoresis in carpal tunnel syndrome [Infiltracion de esteroides, ferula de muneca fonoforesis en el syndrome del tunel carpiano]. Rehabilitacion 2006;40(4):193‐200. [Google Scholar]
Garfinkel 1998 {published data only}
- Garfinkel MS, Singhal A, Katz WA, Allan DA, Reshetar R, Schumacher HR Jr. Yoga‐based intervention for carpal tunnel syndrome: a randomized trial. JAMA 1998;280(18):1601‐3. [PUBMED: 9820263] [DOI] [PubMed] [Google Scholar]
Kumnerddee 2010 {published data only}
- Kumnerddee W, Kaewtong A. Efficacy of acupuncture versus night splinting for carpal tunnel syndrome: a randomized clinical trial. Journal of the Medical Association of Thailand 2010;93(12):1463‐9. [PubMed] [Google Scholar]
Madjdinasab 2008 {published data only}
- Madjdinasab N, Zadeh NS, Assarzadegan F, Ali AMA, Pipelzadeh M. Efficacy comparison of splint and oral steroid therapy in nerve conduction velocity and latency median nerve in carpal tunnel syndrome. Pakistan Journal of Medical Sciences 2008;24(5):725‐8. [EMBASE: 2008533657] [Google Scholar]
Manente 2001 {published data only}
- Manente G, Torrieri F, Blasio F, Staniscia T, Romano F, Uncini A. An innovative hand brace for carpal tunnel syndrome: a randomized controlled trial. Muscle and Nerve 2001;24(8):1020‐5. [PUBMED: 11439376] [DOI] [PubMed] [Google Scholar]
Mishra 2006 {published data only}
- Mishra S, Prabhakar S, Lal V, Modi M, Das CP, Khurana D. Efficacy of splinting and oral steroids in the treatment of carpal tunnel syndrome: a prospective randomized clinical and electrophysiological study. Neurology India 2006;54(3):286‐90. [DOI] [PubMed] [Google Scholar]
Premoselli 2006 {published data only}
- Premoselli S, Sioli P, Grossi A, Cerri C. Neutral wrist splinting in carpal tunnel syndrome: a 3‐ and 6‐month clinical and neurophysiologic follow‐up evaluation of night‐only splint therapy. Europa Medicophysica 2006;42(2):121‐6. [PUBMED: 16767058] [PubMed] [Google Scholar]
Sevim 2004 {published data only}
- Sevim S, Dogu O, Camdeviren H, Kaleagasi H, Aral M, Arslan E, et al. Long‐term effectiveness of steroid injections and splinting in mild and moderate carpal tunnel syndrome. Neurological Sciences 2004;25(2):48‐52. [PUBMED: 15221621] [DOI] [PubMed] [Google Scholar]
Walker 2000 {published data only}
- Walker WC, Metzler M, Cifu DX, Swartz Z. Neutral wrist splinting in carpal tunnel syndrome: a comparison of night‐only versus full‐time wear instructions. Archives of Physical Medicine and Rehabilitation 2000;81(4):424‐9. [PUBMED: 10768530] [DOI] [PubMed] [Google Scholar]
Werner 2005 {published data only}
- Werner RA, Franzblau A, Gell N. Randomized controlled trial of nocturnal splinting for active workers with symptoms of carpal tunnel syndrome. Archives of Physical Medicine and Rehabilitation 2005;86(1):1‐7. [PUBMED: 15640980] [DOI] [PubMed] [Google Scholar]
Zinnuroglu 2010 {published data only}
- Zinnuroglu M, Baspinar M, Beyazova M. Carpal lock and the volar supporting orthosis in mild and moderate carpal tunnel syndrome. American Journal of Physical Medicine and Rehabilitation 2010;89(9):759‐64. [PUBMED: 20581649] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akalin 2002 {published data only}
- Akalin E, El O, Peker O, Senocak O, Tamci S, Gülbahar S, et al. Treatment of carpal tunnel syndrome with nerve and tendon gliding exercises. American Journal of Physical Medicine & Rehabilitation 2002;81(2):108‐13. [PUBMED: 11807347] [DOI] [PubMed] [Google Scholar]
Baysal 2006 {published data only}
- Baysal O, Altay Z, Ozcan C, Ertem K, Yologlu S, Kayhan A. Comparison of three conservative treatment protocols in carpal tunnel syndrome. International Journal of Clinical Practice 2006;60(7):820‐8. [PUBMED: 16704676] [DOI] [PubMed] [Google Scholar]
Daniel 2000 {published data only}
- Daniel ES, Paul S. A comparison study of the volar wrist cock‐up splint and ulnar gutter splint in carpal tunnel syndrome. Occupational Therapy in Health Care 2000;12(4):79‐93. [DOI] [PubMed] [Google Scholar]
Davis 1998 {published data only}
- Davis PT, Hulbert JR, Kassak KM, Meyer JJ. Comparative efficacy of conservative medical and chiropractic treatments for carpal tunnel syndrome: a randomized clinical trial. Journal of Manipulative and Physiological Therapeutics 1998;21(5):317‐26. [PUBMED: 9627862] [PubMed] [Google Scholar]
Eftekharsadat 2011 {published data only}
- Eftekharsadat B, Shakouri SK, Shimia M, Rahbar M, Ghojazadeh M, Rashidi MR, et al. Effect of E. laciniata (L) ointment on mild and moderate carpal tunnel syndrome: a double‐blind, randomized clinical trial. Phytotherapy Research 2011;25(2):290‐5. [PUBMED: 20665472] [DOI] [PubMed] [Google Scholar]
Ekim 2008 {published data only}
- Ekim A, Colak E. Ultrasound treatment in carpal tunnel syndrome: a placebo controlled study. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2008;54(3):96‐101. [Google Scholar]
Evcik 2007 {published data only}
- Evcik D, Kavuncu V, Cakir T, Subasi V, Yaman M. Laser therapy in the treatment of carpal tunnel syndrome: a randomized controlled trial. Photomedicine and Laser Surgery 2007;25(1):34‐9. [PUBMED: 17352635] [DOI] [PubMed] [Google Scholar]
Gerritsen 2002a {published data only}
- Gerritsen AA, Vet HC, Scholten RJ, Bertelsmann FW, Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA 2002;288(10):1245‐51. [DOI] [PubMed] [Google Scholar]
Gurcay 2009 {published data only}
- Gurcay E, Unlu E, Gurcay AG, Tuncay R, Cakci A. Evaluation of the effect of local corticosteroid injection and anti‐inflammatory medication in carpal tunnel syndrome. Scottish Medical Journal 2009;54(1):4‐6. [PUBMED: 19291926] [DOI] [PubMed] [Google Scholar]
Heebner 2008 {published data only}
- Heebner ML, Roddey TS. The effects of neural mobilization in addition to standard care in persons with carpal tunnel syndrome from a community hospital. Journal of Hand Therapy 2008;21(3):229‐41. [DOI] [PubMed] [Google Scholar]
Kamanli 2011 {published data only}
- Kamanli A, Bezgincan M, Kaya A. Comparison of local steroid injection into carpal tunnel via proximal and distal approach in patients with carpal tunnel syndrome. Bratislavské lekárske listy 2011;112(6):337‐41. [PubMed] [Google Scholar]
Pinar 2005 {published data only}
- Pinar L, Enhos A, Ada S, Güngör N. Can we use nerve gliding exercises in women with carpal tunnel syndrome?. Advances in Therapy 2005;22(5):467‐75. [PUBMED: 16418156] [DOI] [PubMed] [Google Scholar]
Ruksen 2011 {published data only}
- Ruksen S, Oz B, Olmez N, Memis A. Comparison of clinical effectiveness of corticosteroid phonophoresis and local steroid injection treatment in carpal tunnel syndrome. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2011;57(3):119‐23. [Google Scholar]
Weintraub 2000 {published data only}
- Weintraub MI, Cole SP. Neuromagnetic treatment of pain in refractory carpal tunnel syndrome: an electrophysiological and placebo analysis. Journal of Back and Musculoskeletal Rehabilitation 2000;15(2‐3):77‐81. [DOI] [PubMed] [Google Scholar]
Yagci 2006 {published data only}
- Yağci I, Uçan H, Yilmaz L, Yağmurlu F, Keskin D, Bodur H. Comparison of splinting, splinting plus local steroid injection and surgery in carpal tunnel syndrome treatment. Turkish Journal of Physical Medicine and Rehabilitation 2006;52(2):55‐60. [Google Scholar]
References to studies awaiting assessment
Taspinar 2007 {published data only}
- Taşpınar Ş, Şahin F, Erçalık C, Kuran B, Barkut K, Çelik M, et al. Comparison of the efficacy of corticosteroid injection, night splint, and physiotherapy in diabetic carpal tunnel syndrome [Diyabetik karpal tünel sendromunda kortikosteroid enjeksiyonu, gece ateli ve fizik tedavinin etkinliğinin karşılaştırılması]. Turkish Journal of Physical Medicine and Rehabilitation 2007;53(2):54‐60. [Google Scholar]
Additional references
Ashworth 2010
- Ashworth N. Carpal tunnel syndrome. Clinical Evidence 2010;03:1114. [PUBMED: 21718565] [PMC free article] [PubMed] [Google Scholar]
Atroshi 1999
- Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999;282(2):153‐8. [PUBMED: 10411196] [DOI] [PubMed] [Google Scholar]
Braun 1989
- Braun RM, Davidson K, Doehr S. Provocative testing in the diagnosis of dynamic carpal tunnel syndrome. Journal of Hand Surgery (American Volume) 1989;14(2 Pt 1):195‐7. [PUBMED: 2703665] [DOI] [PubMed] [Google Scholar]
Charles 2009
- Charles J, Fahridin S, Britt H. Carpal tunnel syndrome. Australian Family Physician 2009;38(9):665. [PUBMED: 19893791] [PubMed] [Google Scholar]
Concannon 2000
- Concannon MJ, Brownfield ML, Puckett CL. The incidence of recurrence after endoscopic carpal tunnel release. Plastic and Reconstructive Surgery 2000;105(5):1662‐5. [PUBMED: 10809095] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Dwan 2008
- Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS ONE 2008;3(8):e3081. [PUBMED: 18769481] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwan 2011
- Dwan K, Altman DG, Cresswell L, Blundell M, Gamble CL, Williamson PR. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database of Systematic Reviews 2011;Issue 1:Art. No.: MR000031.DOI: 10.1002/14651858.MR000031.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gelberman 1984
- Gelberman RH, Szabo RM, Mortenson MM. Carpal tunnel pressures and wrist position in patients with Colles fractures. Journal of Trauma, Injury, Infection and Critical Care 1984;24(8):747‐9. [PUBMED: 6471140] [DOI] [PubMed] [Google Scholar]
Gerritsen 2002b
- Gerritsen AAM, Krom MCTFM, Struijs MA, Scholten RJPM, Vet HCW, Bouter LM. Conservative treatment options for carpal tunnel syndrome: a systematic review of randomised controlled trials. Journal of Neurology 2002;249(3):272‐80. [PUBMED: 11993525] [DOI] [PubMed] [Google Scholar]
Goodyear‐Smith 2004
- Goodyear‐Smith F, Arroll B. What can family physicians offer patients with carpal tunnel syndrome other than surgery? A systematic review of nonsurgical management. Annals of Family Medicine 2004;2(3):267‐73. [PUBMED: 15209206] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardoim 2009
- Hardoim DG, Oliveira GB, Kouyoumdjian JA. Carpal tunnel syndrome: long‐term nerve conduction studies in 261 hands. Arquivos de Neuro‐Psiquiatria 2009;67(1):69‐73. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG. Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Huisstede 2010
- Huisstede BM, Hoogvliet P, Randsdorp MS, Glerum S, Middelkoop M, Koes BW. Carpal tunnel syndrome. Part 1: Effectiveness of nonsurgical treatments ‐ a systematic review. Archives of Physical Medicine and Rehabilitation 2010;91(7):981‐1004. [PUBMED: 20599038] [DOI] [PubMed] [Google Scholar]
Jablecki 2002
- Jablecki CK, Andary MT, Floeter MK, Miller RG, Quartly CA, Vennix MJ, et al. Practice parameter: electrodiagnostic studies in carpal tunnel syndrome. Report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2002;58(11):1589‐92. [PUBMED: 12058083] [DOI] [PubMed] [Google Scholar]
Keir 2005
- Keir P, Rempel D. Pathomechanics of peripheral nerve loading. Journal of Hand Therapy 2005;18:259‐69. [PUBMED: 15891983] [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [PUBMED: 20156912] [DOI] [PubMed] [Google Scholar]
Levine 1993
- Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self‐administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. Journal of Bone and Joint Surgery. American Volume 1993;75(11):1585‐92. [PUBMED: 8245050] [DOI] [PubMed] [Google Scholar]
Marshall 2007
- Marshall SC, Tardif G, Ashworth NL. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD001554.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller 2004
- Muller M, Tsui D, Schnurr R, Biddulph‐Deisroth, Hard J, MacDermid J. Effectiveness of hand therapy interventions in primary management of carpal tunnel syndrome: a systematic review. Journal of Hand Therapy 2004;17(2):210‐28. [PUBMED: 15162107] [DOI] [PubMed] [Google Scholar]
O'Connor 2012
- O’Connor D, Page MJ, Marshall SC, Massy‐Westropp N. Ergonomic positioning or equipment for treating carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ono 2010
- Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. International Journal of General Medicine 2010;3:255‐61. [PUBMED: 20830201] [DOI] [PMC free article] [PubMed] [Google Scholar]
Padua 1999
- Padua L, Padua R, Lo Monaco M, Aprile I, Tonali P. Multiperspective assessment of carpal tunnel syndrome: a multicenter study. Italian CTS Study Group. Neurology 1999;53:1654‐9. [DOI] [PubMed] [Google Scholar]
Page 2012
- Page MJ, O’Connor D, Pitt V, Massy‐Westropp N. Therapeutic ultrasound for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009601] [DOI] [PubMed] [Google Scholar]
Palmar 2007
- Palmer K, Harris C, Coggon D. Carpal tunnel syndrome and its relation to occupation: a systematic literature review. Occupational Medicine 2007;57(1):57‐66. [DOI] [PubMed] [Google Scholar]
Piazzini 2007
- Muller M, Tsui D, Schnurr R, Biddulph‐Deisroth L, Hard J. Effectiveness of hand therapy interventions in primary management of carpal tunnel syndrome: a systematic review. Journal of Hand Therapy 2004;17(2):210‐28. [DOI] [PubMed] [Google Scholar]
Rempel 1998
- Rempel D, Evanoff B, Amadio PC, Krom M, Franklin G, Franzblau A, et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. American Journal of Public Health 1998;88(10):1447‐51. [PUBMED: 9772842] [DOI] [PMC free article] [PubMed] [Google Scholar]
Resende 2003
- Resende LA, Tahara A, Fonseca RG, Sardenberg T. The natural history of carpal tunnel syndrome: a study of 20 hands evaluated 4 to 9 years after initial diagnosis. Electromyography and Clinical Neurophysiology 2003;43:301‐4. [PubMed] [Google Scholar]
Scholten 2007
- Scholten RJPM, Molen AM, Uitdehaag BMJ, Bouter LM, Vet HCW. Surgical treatment options for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003905.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Szabo 1992
- Szabo RM, Madison M. Carpal tunnel syndrome. Orthopedic Clinics of North America 1992;23(1):103‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Szabo 1994
- Szabo RM, Steinberg DR. Nerve entrapment syndromes in the wrist. Journal of the American Academy of Orthopedic Surgeons 1994;2:115‐23. [DOI] [PubMed] [Google Scholar]
Verdugo 2008
- Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non‐surgical treatment for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD001552.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
O'Connor 2003
- O'Connor D, Marshall S, Massy‐Westropp N. Non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003219] [DOI] [PMC free article] [PubMed] [Google Scholar]


