Abstract
长链非编码RNA是一类长度超过200nt、不编码蛋白质的结构复杂的转录本,长链非编码RNA可与DNA、mRNA及蛋白质相互作用,通过多种机制调控基因表达,进而参与调控多种生物过程。研究表明,长链非编码RNA在神经系统发育和疾病发生中具有重要作用。该文就长链非编码RNA在缺氧缺血脑损伤中的作用作一综述。
Keywords: 长链非编码RNA, 缺氧缺血脑损伤
Abstract
Long non-coding RNAs (lncRNAs) are transcripts with a complex structure and a length of > 200 nt which are unable to encode proteins. The lncRNAs interact with DNA, mRNA, and proteins and regulate gene expression through various mechanisms, thus participating in the regulation of various biological processes. Studies have shown that lncRNAs play important roles in neural development and the pathogenesis of diseases. This article reviews the roles of lncRNAs in hypoxic-ischemic brain damage.
Keywords: Long non-coding RNA, Hypoxic-ischemic brain damage
长链非编码RNA(long noncoding RNA, lncRNA)可在转录前水平、转录水平和转录后水平调控基因表达,广泛参与机体几乎所有的生理病理过程[1-5]。脑组织是哺乳动物体内除生殖细胞外lncRNA含量最丰富、最多样的器官[6]。许多lncRNA特异性表达于神经细胞[7-8],且在脑组织不同分区的表达也存在差异[9],提示lncRNA可能在脑组织发育过程中起着重要作用。目前,lncRNA的研究尚处于起步阶段,关于lncRNA的功能及其分子机制尚有很多未知之谜。大量研究表明,lncRNA在许多疾病,包括神经系统疾病中异常表达,且与疾病的发生、发展密切相关[6-9]。缺氧缺血脑损伤的病理损伤机制非常复杂,损伤性分子与保护性分子并存,细胞凋亡、自噬、炎性反应、神经再生、血管再生等多种生物过程参与其中,涉及一系列基因的激活、表达和调控[10]。我们的近期研究发现,缺氧缺血脑损伤可明显改变新生鼠脑组织中lncRNA的表达[11],提示lncRNA可能在新生鼠缺氧缺血脑损伤的病理过程中具有重要作用。本文就lncRNA与缺氧缺血脑损伤关系的研究进展作一综述。
1. lncRNA概述
lncRNA是一类长度超过200 nt的功能性RNA分子,占全部非编码RNA的80%~90%[12]。lncRNA是生命科学领域中继microRNA之后的又一研究热点。与microRNA相比,lncRNA分子量更大、空间结构更复杂、作用机制更多样。lncRNA既能和DNA、mRNA还能和蛋白质相互作用(图 1),其作用机制包括:(1)直接与编码基因启动子区结合;(2)介导组蛋白修饰、染色质重构;(3)调控mRNA前体的可变剪接,或形成内源性siRNA分子;(4)调控转录因子活性或募集转录调控因子至启动子区,调控靶基因转录;(5)改变转录因子的亚细胞定位或作为结构组分与蛋白质形成核酸蛋白质复合体,进一步调节转录或调控蛋白活性;(6)作为小分子RNA的前体参与基因调控;(7)作为miRNA海绵,与miRNA靶基因竞争性结合,解除miRNA对其靶基因的抑制作用,升高靶基因的表达水平[5]。
1.
lncRNA的生物学功能示意图[5]
1转录干扰,2诱导染色体重建,3调控mRNA剪切,4产生内源性siRNAs,5调节蛋白质活性,6改变蛋白质定位,7结构组成成分,8小RNA前体,9海绵miRNA。
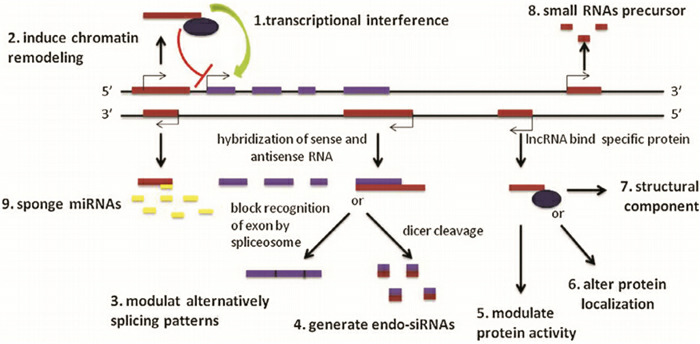
目前,长链非编码RNA的功能尚未完全阐明。研究表明,长链非编码RNA可通过多种形式参与染色质修饰、基因表达调控、细胞分化等生物学过程,不仅在物种进化、胚胎发育等生理过程,而且在癌症、心血管疾病、糖尿病、阿尔兹海默病等疾病发生发展中有着重要作用[13-15]。
2. lncRNA与凋亡
细胞凋亡是缺氧缺血脑损伤引起的神经元死亡的主要方式,凋亡在损伤后延迟发生,持续时间可长达数周,且经适当治疗可逆转凋亡的病理过程、改善预后,这使其成为治疗缺氧缺血脑损伤的重要靶点。我们既往研究[11]发现,lncRNA BC088414在新生鼠缺氧缺血脑组织中表达增加,采用siRNA抑制BC088414的表达可明显抑制凋亡相关蛋白肾上腺素能受体β2(β2-adrenergic receptor, Adrb2)和半胱氨酸蛋白酶6(caspase 6, Caspase 6)的表达,进而减少缺氧缺血引起的PC12神经细胞凋亡,意味着lncRNA BC088414参与调控缺氧缺血性神经细胞凋亡。在成年大鼠脑卒[16-17]和蛛网膜下腔出血动物模型中[18]的研究也显示,脑缺血、脑出血显著影响大脑皮层中lncRNA的表达,提示lncRNA可能参与脑卒中病理损伤及发生发展。进一步研究发现,脑缺血促使差异表达的lncRNA与染色质修饰蛋白Sin3a和coREST的结合能力增加,调控下游基因的表达。这些研究提示lncRNA可能通过与染色体修饰蛋白的相互作用在表观遗传水平介导脑卒中后基因表达的调控[19]。Mehta等[20]的研究证实,脑卒中引起成年大鼠脑组织中长链非编码RNA FosDT(Fos downstream transcript)表达上调,FosDT与Sin3a、coREST的结合能力增加,抑制其下游抗细胞凋亡基因GRIA2、NF-κB和突触可塑性相关基因GRIN1的表达,参与脑缺血性神经损伤。抑制FosDT表达,可解除REST对GRIA2、NFκB2和GRIN1基因表达的抑制作用,使脑梗死体积缩小,神经行为损害减轻[20]。可见,lncRNA在脑缺血性损伤细胞凋亡中起着重要作用,探讨细胞凋亡调控的lncRNA机制可能为深入阐明缺氧缺血脑损伤的发病及修复机制奠定基础。
3. lncRNA与自噬
自噬(autophagy)是一种进化保守的物质代谢过程,它将受损的细胞器、错误折叠的蛋白质等大分子转运至溶酶体中进行降解,既清除有害物质,又利于能源物质回收,起到维持细胞内稳态的作用[21]。研究表明,缺血或缺氧缺血可引起脑组织中自噬水平增加[21-26]。然而,在脑缺血或缺氧缺血性损伤中,自噬到底是促神经保护还是促神经损伤尚存在争议。有研究报道,自噬抑制剂渥曼青霉素和3-甲基腺嘌呤(3-methyladenine, 3-MA)可减少神经细胞凋亡,加速神经细胞坏死,而自噬激活剂雷帕霉素引起坏死细胞减少、脑损伤减轻[24]。然而,也有研究发现,自噬是缺氧缺血导致的神经元死亡的主要机制[21]。Puyal等[21]在新生大鼠MCAO动物模型中的研究表明,3-MA可显著降低新生大鼠脑组织中LC3Π水平,明显缩小脑梗死体积,意味着自噬是缺氧缺血神经细胞的损伤机制。目前有关lncRNA对自噬调控的研究较少。据报道,一种名为自噬促进因子(autophagy promoting factor, APF)的长链非编码RNA能够调控心肌梗塞中自噬的发生[27]。缺血再灌注后心肌细胞中APF表达显著增加。下调APF水平则抑制心肌细胞缺血再灌注损伤中自噬的激活,使心肌梗死体积显著缩小。APF参与心肌细胞缺氧再灌注损伤的机制可能是,APF与miR-188-3p结合,解除了miR-188-3p对自噬基因ATG7的抑制作用,进而促进细胞自噬,扩大心肌梗死体积。说明lncRNA在心肌缺血再灌注损伤中具有重要作用。lncRNA-PTENP1表达在人肝癌细胞中明显下降,过度表达PTENP1会提高PTEN表达水平,阻断PI3K/AKT信号通路的作用,解除其对自噬的抑制作用,诱导细胞自噬[28]。最近研究发现,3-MA可抑制糖尿病小鼠脑组织中lncRNA PVT1的表达,后者可通过介导自噬,抑制细胞凋亡,减轻神经认知功能损伤,提示lncRNA PVT1在神经细胞自噬调控中至关重要。可见lncRNA对缺氧缺血脑损伤中自噬的调控也有重要作用。
4. lncRNA与神经再生
成年哺乳动物脑室-脑室下区及海马齿状核的神经干细胞是脑损伤后神经修复的主要来源[29]。脑缺血可刺激脑室下区(subventricular zone, SVZ)神经干细胞增殖并迁移至受损区进行再生修复[30-31]。研究表明,有的lncRNA特异性表达于神经干细胞中,如lnc-pou3f3、Six3os和Dlxlas等,在神经干细胞分化成神经元及神经胶质细胞过程中起着重要作用[32]。Six3os参与调控神经干细胞分化为神经元和少突胶质细胞,而Dlxlas则在神经干细胞分化为神经元过程中至关重要;缺失Six3os基因使SVZ神经干细胞分化的神经元及少突胶质细胞减少,而神经胶质细胞增多[32]。lnc-pou3f3,又称Pnky,是一个进化保守的神经特异的核表达lncRNA,主要分布于脑室-脑室下区、皮层、小脑、嗅球和胚胎脑组织中[33]。Pnky通过与多聚嘧啶串结合蛋白1(polypyrimidine tract-binding protein 1, PTBP1)相互作用,调节下游与细胞表型相关基因的表达,进而促进神经干细胞分化为神经元[33]。PTBP1是一个公认的抑制神经元分化的RNA结合蛋白,在脑正常发育及脑肿瘤的发生中具有重要作用[34-35]。PTBP1还具有强大的细胞重编程功能,缺失PTBP1基因可使纤维母细胞直接转分化为神经元[36]。体外实验发现[33],敲除Pnky基因显著增加神经细胞数量;体内实验也证实[33],缺失pnky基因可明显增加胚胎及出生后脑皮质中新生神经元数目。可见,敲除pnky基因有利于神经前体细胞增殖,促进其分化为神经元,减少细胞死亡,改善脑缺血神经损伤。因此,lncRNA可能为寻找和探索缺氧缺血脑损伤治疗措施提供新思路。
5. lncRNA与炎症
炎症反应、兴奋性毒性和氧化应激是脑缺氧缺血损伤的主要机制。脑缺氧缺血发生后,形成大量细胞因子和趋化因子,参与脑缺血病理损伤[37]。TNF-α通过激活NF-κB,进而激活促炎因子的表达,在炎性反应中发挥重要的作用。Rapicavol等[38]在小鼠胚胎成纤维细胞MEF中研究发现,TNF-α可通过活化NF-κB使lncRNA Lethe表达显著增加,而上调的Lethe则与NF-κB结合,阻断其对靶基因的活化作用,进而减少炎症因子的产生。H3K27me3是染色体表达沉默的标志,脂多糖(lipopolysaccharide, LPS)刺激细胞,可引起促炎因子如E-选择素、血管黏附分子、白介素-6基因启动区的H3K27me3减少,进而激活转录,促进炎症。同时,LPS还可使lnc-IL7明显升高,上调的lnc-IL7可以维持H3K27me3水平,通过负反馈调节避免炎症基因的过度表达,减轻炎症反应[39]。这些研究结果提示,lncRNA可能是炎症信号通路中的重要调节因子,在炎性反应中起到重要作用。
6. lncRNA与血管再生
血脑屏障(blood brain barrier, BBB)是保证中枢神经系统正常生理功能的重要屏障系统[40]。脑微血管内皮细胞是BBB的核心组成成分。脑缺血损伤时,氧自由基、过氧化氢、各种炎症介质、细胞因子等大量释放,导致内皮细胞受损、血脑屏障通透性增加,形成脑水肿,加重脑损伤[40]。研究发现,氧糖剥夺处理可引起大鼠脑微血管内皮细胞中大量与血管新生相关的lncRNA异常表达[41],其中lncRNA MEG3与VEGFA呈负相关。过表达MEG3可通过抑制人脐静脉内皮细胞中VEGFA和notch1的表达,进而抑制脐静脉内皮细胞增殖、迁移和成管等血管形成能力[42]。这些结果提示lncRNA在脑缺血引起的血管内皮细胞损伤中有着重要作用。
7. 总结与展望
lncRNA在神经再生、细胞凋亡、炎性反应、细胞自噬和血管再生过程中有着重要作用,而这些细胞生物过程是脑缺氧缺血病理损伤的重要机制。因此,lncRNA可能在脑缺氧缺血损伤的发生发展中起着至关重要的作用。lncRNA在脑缺血损伤中的研究才刚刚起步,主要集中在成年鼠脑卒中方面,侧重于lncRNA与脑卒中的关联,且这些研究仅处于初步的数据积累阶段,较少对lncRNA在脑缺氧缺血损伤中的可能作用进行深入分析和探讨。因此,深入研究相关机制,有助于进一步阐明脑缺氧缺血损伤的发生发展过程,为预防和治疗缺氧缺血脑损伤提供新的思路。
Biography
赵凤艳, 女, 博士, 助理研究员。Email: mudz@scu.edu.cn
Funding Statement
国家自然科学基金(81300526,81270724);四川省科技厅基金(2016TD0002)
Contributor Information
赵 凤艳 (Feng-Yan ZHAO), Email: mudz@scu.edu.cn.
赵 凤艳 (Feng-Yan ZHAO), Email: mudz@scu.edu.cn.
References
- 1.Li J, Zhang M, An G, et al. LncRNA TUG1 acts as a tumor suppressor in human glioma by promoting cell apoptosis. Exp Biol Med (Maywood) 2016;241(6):644–649. doi: 10.1177/1535370215622708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang M, Gu H, Xu W, et al. Downregulation of lncRNA MALAT1 reduces cardiomyocyte apoptosis and improves left ventricular function in diabetic rats. Int J Cardiol. 2016;203:214–216. doi: 10.1016/j.ijcard.2015.10.136. [DOI] [PubMed] [Google Scholar]
- 3.Wu H, Liu J, Li W, et al. LncRNA-HOTAIR promotes TNFalpha production in cardiomyocytes of LPS-induced sepsis mice by activating NF-kappa B pathway. Biochem Biophys Res Commun. 2016;471(1):240–246. doi: 10.1016/j.bbrc.2016.01.117. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Wu P, Lin R, et al. LncRNA NALT interaction with NOTCH1 promoted cell proliferation in pediatric T cell acute lymphoblastic leukemia. Sci Rep. 2015;5:13749. doi: 10.1038/srep13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi X, Sun M, Liu H, et al. Long non-coding RNAs:a new frontier in the study of human diseases. Cancer Lett. 2013;339(2):159–166. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Goff LA, Groff AF, Sauvageau M, et al. Spatiotemporal expression and transcriptional perturbations by long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2015;112(22):6855–6862. doi: 10.1073/pnas.1411263112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aprea J, Prenninger S, Dori M, et al. Transcriptome sequencing during mouse brain development identifies long non-coding RNAs functionally involved in neurogenic commitment. EMBO J. 2013;32(24):3145–3160. doi: 10.1038/emboj.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo H, Sun S, Li P, et al. Comprehensive characterization of 10571 mouse large intergenic noncoding RNAs from whole transcriptome sequencing. PLoS One. 2013;8(8):e70835. doi: 10.1371/journal.pone.0070835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabili MN, Trapnell C, Goff L, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25(18):1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon BJ, Reis C, Ho WM, et al. Neuroprotective strategies after neonatal hypoxic ischemic encephalopathy. Int J Mol Sci. 2015;16(9):22368–223401. doi: 10.3390/ijms160922368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao F, Qu Y, Liu J, et al. Microarray profiling and coexpression network analysis of lncRNAs and mRNAs in neonatal rats following hypoxic-ischemic brain damage. https://www.ncbi.nlm.nih.gov/pubmed/26349411. Sci Rep. 2015;5(3):370–371. doi: 10.1038/srep13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng SY, Lin L, Son BS, et al. Long noncoding RNAs in development and disease of the central nervous system. Trends Genet. 2013;29(8):461–468. doi: 10.1016/j.tig.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Zequn N, Xuemei Z, Wei L, et al. The role and potential mechanisms of LncRNA-TATDN1 on metastasis and invasion of non-small cell lung cancer. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4951283/ Oncotarget. 2016;7(14):18219–18228. doi: 10.18632/oncotarget.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu JY, Yao J, Li XM, et al. Pathogenic role of lncRNAMALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5:e1506. doi: 10.1038/cddis.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massone S, Vassallo I, Fiorino G, et al. 17A, a novel non-coding RNA, regulates GABA B alternative splicing and signaling in response to inflammatory stimuli and in Alzheimer disease. Neurobiol Dis. 2011;41(2):308–317. doi: 10.1016/j.nbd.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Dharap A, Nakka VP, Vemuganti R. Effect of focal ischemia on long noncoding RNAs. Stroke. 2012;43(10):2800–2802. doi: 10.1161/STROKEAHA.112.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Song G, Zhou L, et al. Compared analysis of lncRNA expression profiling in pdk1 gene knockout mice at two time points. Cell Physiol Biochem. 2013;32(5):1497–1508. doi: 10.1159/000356586. [DOI] [PubMed] [Google Scholar]
- 18.Zheng B, Liu H, Wang R, et al. Expression signatures of long non-coding RNAs in early brain injury following experimental subarachnoid hemorrhage. https://www.researchgate.net/publication/273702717_Expression_signatures_of_long_non-coding_RNAs_in_early_brain_injury_following_experimental_subarachnoid_hemorrhage. Mol Med Rep. 2015;12(1):967–973. doi: 10.3892/mmr.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dharap A, Pokrzywa C, Vemuganti R. Increased binding of stroke-induced long non-coding RNAs to the transcriptional corepressors Sin3A and coREST. ASN Neuro. 2013;5(4):283–289. doi: 10.1042/AN20130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta SL, Kim T, Vemuganti R. Long noncoding RNA FosDT promotes ischemic brain injury by interacting with RESTassociated chromatin-modifying proteins. J Neurosci. 2015;35(50):16443–16449. doi: 10.1523/JNEUROSCI.2943-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puyal J, Vaslin A, Mottier V, et al. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol. 2009;66(3):378–389. doi: 10.1002/ana.v66:3. [DOI] [PubMed] [Google Scholar]
- 22.Zhao G, Zhang W, Li L, et al. Pinocembrin protects the brain against ischemia-reperfusion injury and reverses the autophagy dysfunction in the penumbra area. Molecules. 2014;19(10):15786–15798. doi: 10.3390/molecules191015786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carloni S, Girelli S, Scopa C, et al. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxiaischemia. Autophagy. 2010;6(3):366–377. doi: 10.4161/auto.6.3.11261. [DOI] [PubMed] [Google Scholar]
- 24.Carloni S, Albertini MC, Galluzzi L, et al. Increased autophagy reduces endoplasmic reticulum stress after neonatal hypoxiaischemia:role of protein synthesis and autophagic pathways. Exp Neurol. 2014;255:103–112. doi: 10.1016/j.expneurol.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Gao C, Cai Y, Zhang X, et al. Ischemic preconditioning mediates neuroprotection against ischemia in mouse hippocampal CA1 neurons by inducing autophagy. PLoS One. 2015;10(9):e0137146. doi: 10.1371/journal.pone.0137146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ordoñez R, Fernández A, Prieto-Domínguez N, et al. Ceramide metabolism regulates autophagy and apoptotic cell death induced by melatonin in liver cancer cells. J Pineal Res. 2015;59(2):178–189. doi: 10.1111/jpi.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang K, Liu CY, Zhou LY, et al. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat Commun. 2015;6:6779. doi: 10.1038/ncomms7779. [DOI] [PubMed] [Google Scholar]
- 28.Chen CL, Tseng YW, Wu JC, et al. Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials. 2015;44:71–81. doi: 10.1016/j.biomaterials.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010;37(2):267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komitova M, Mattsson B, Johansson BB, et al. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36(6):1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- 31.Kojima T, Hirota Y, Ema M, et al. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. http://onlinelibrary.wiley.com/doi/10.1002/stem.306/full?globalMessage=0. Stem Cells. 2010;28(3):545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- 32.Ramos AD, Diaz A, Nellore A, et al. Integration of genomewide approaches identifies lncRNAs of adult neural stem cells and their progeny in vivo. Cell Stem Cell. 2013;12(5):616–628. doi: 10.1016/j.stem.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos AD, Andersen RE, Liu SJ, et al. The long noncoding RNA Pnky regulates neuronal differentiation of embryonic and postnatal neural stem cells. Cell Stem Cell. 2015;16(4):439–447. doi: 10.1016/j.stem.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makeyev EV, Zhang J, Carrasco MA, et al. The MicroRNA miR-124 promotes neuronal differentiation by triggering brainspecific alternative pre-mRNA splicing. Mol Cell. 2007;27(3):435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrarese R, Harsh GR 4th, Yadav AK, et al. Lineage-specific splicing of a brain-enriched alternative exon promotes glioblastoma progression. J Clin Invest. 2014;124(7):2861–2876. doi: 10.1172/JCI68836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue Y, Ouyang K, Huang J, et al. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell. 2013;152(1-2):82–96. doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng L, Wang Y, Liu J, et al. Pro-inflammatory cytokine network in peripheral inflammation response to cerebral ischemia. Neurosci Lett. 2013;548:4–9. doi: 10.1016/j.neulet.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 38.Rapicavoli NA, Qu K, Zhang J, et al. A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. https://archive.org/details/pubmed-PMC3721235. Elife. 2013;2:e00762. doi: 10.7554/eLife.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui H, Xie N, Tan Z, et al. The human long noncoding RNA lnc-IL7R regulates the inflammatory response. Eur J Immunol. 2014;44(7):2085–2095. doi: 10.1002/eji.201344126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruparelia AA, Oorschot V, Ramm G, et al.FLNC myofibrillar myopathy results from impaired autophagy and protein insufficiency[J].Hum Mol Genet, 2016, Mar 11.pii:ddw080.
- 41.Zhang J, Yuan L, Zhang X, et al. Altered long non-coding RNA transcriptomic profiles in brain microvascular endothelium after cerebral ischemia. https://www.researchgate.net/profile/Xuejing_Zhang7/publication/288855824_Altered_long_non-coding_RNA_transcriptomic_profiles_in_brain_microvascular_endothelium_after_cerebral_ischemia/links/56cf886208aeb52500c9aa6c.pdf?origin=publication_detail. Exp Neurol. 2015;277:162–170. doi: 10.1016/j.expneurol.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.刘娟, 邓志峰.调控脑卒中后血管新生lncRNAs的发现及其功能的初步探讨[D].南昌:南昌大学, 2014.


