Abstract
Background
Pressure ulcers (also called pressure sores, bed sores and decubitus ulcers) are areas of tissue damage that occur in the elderly, malnourished or acutely ill, who cannot reposition themselves. Pressure ulcers impose a significant financial burden on health care systems and negatively affect quality of life. Wound cleansing is considered an important component of pressure ulcer care.
Objectives
This systematic review seeks to answer the following question: what is the effect of wound cleansing solutions and wound cleansing techniques on the rate of healing of pressure ulcers?
Search methods
For this third update, we searched the Cochrane Wounds Group Specialised Register (searched 3 January 2013); The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 12); Ovid MEDLINE (2010 to November Week 3 2012); Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations December 31, 2012); Ovid EMBASE (2010 to 2012 Week 52); and EBSCO CINAHL (2010 to 21 December 2012).
Selection criteria
Randomised controlled trials (RCTs) comparing wound cleansing with no wound cleansing, or different wound cleansing solutions, or different cleansing techniques, were eligible for inclusion if they reported an objective measure of pressure ulcer healing.
Data collection and analysis
Two review authors extracted data independently and resolved disagreements through discussion. A structured narrative summary of the included studies was conducted. For dichotomous outcomes, risk ratio (RR), plus 95% confidence intervals (CI) were calculated; for continuous outcomes, mean difference (MD), plus 95% CI were calculated. Meta analysis was not conducted because of the small number of diverse RCTs identified. Two review authors independently assessed each included study using the Cochrane Collaboration tool for assessing risk of bias.
Main results
One additional eligible study was identified from the updated searches, one study was added to the table of excluded studies. A total of three studies (169 participants) met the inclusion criteria for the review. No studies compared cleansing with no cleansing. Two studies compared different wound cleansing solutions. A statistically significant improvement in Pressure Sore Status Tool scores occurred for wounds cleansed with saline spray containing Aloe vera, silver chloride and decyl glucoside (Vulnopur) compared with isotonic saline (P value = 0.025), but no statistically significant change in healing was seen when water was compared with saline (RR 3.00, 95% CI 0.21 to 41.89). One study compared cleansing techniques; for pressure ulcers cleansed with pulsatile lavage, compared with sham (the lavage flow was directed into a wash basin positioned adjacent to the wound and not visible to the participants), there was a statistically significant reduction in ulcer volume at the end of the three week study period in the lavage group compared with the sham group (MD ‐6.60, 95% CI‐11.23, ‐1.97).
Authors' conclusions
We identified three small studies addressing cleansing of pressure ulcers. One reported a statistically significant improvement in pressure ulcer healing for wounds cleansed with saline spray containing Aloe vera, silver chloride and decyl glucoside (Vulnopur) compared with isotonic saline solution, a further study reported no statistically significant change in healing was seen when wounds were cleaned with water was compared with saline. A final study compared pulsatile lavage with sham and found a significantly greater reduction in ulcer volume at the end of the study period in the lavage group compared with the sham group. The authors conclude that there is no good trial evidence to support use of any particular wound cleansing solution or technique for pressure ulcers.
Keywords: Humans, Wound Healing, Aloe, Glucosides, Glucosides/therapeutic use, Pressure Ulcer, Pressure Ulcer/nursing, Randomized Controlled Trials as Topic, Silver Compounds, Silver Compounds/therapeutic use, Skin Care, Skin Care/methods, Sodium Chloride, Sodium Chloride/therapeutic use, Therapeutic Irrigation, Therapeutic Irrigation/methods
Plain language summary
Wound cleansing to help pressure ulcers heal.
Pressure ulcers (also called pressure sores, bed sores and decubitus ulcers) are areas of tissue damage that occur in the elderly, malnourished or acutely ill, who cannot reposition themselves. The three trials identified found no good evidence that cleansing pressure ulcers (bed sores) using a particular technique, or cleansing with a particular solution, helps healing. Very little research has studied the cleansing of pressure ulcers and therefore we are unable to draw any firm conclusions.
Background
Description of the condition
Pressure ulcers (also known as pressure sores, bed sores and decubitus ulcers) are localised areas of tissue damage caused by excess pressure, shearing or friction forces, which occur in those who cannot reposition themselves in order to relieve pressure on their bony prominences. This ability is often diminished in the very old, the malnourished and those with acute illness (Robertson 1990). In order to quantify the problem of pressure ulcers, prevalence studies and incidence studies have been conducted (Keelaghan 2008; Moore 2012; Schoonhoven 2006). It should be noted that the terms 'prevalence' and 'incidence' have different meanings and should not be used interchangeably. Prevalence refers to the number of people with a pressure ulcer at a point in time, or during a specific time period, while incidence concerns the rate at which new pressure ulcers develop in a defined population in a specific time period (Beaglehole 1993).
One cross‐sectional European study found that approximately 18% of hospital patients had a pressure ulcer (EPUAP 2002). An number of Irish studies have identified prevalence rates of between 10% ‐ 18.8% in acute and long tern care settings (Gallagher 2008; Moore 2000; Moore 2012). Reported incidence rates of pressure ulcers range from 2.2% to 66% in the UK, to 0% to 65.6% in the USA and Canada (Kaltenthaler 2001). These figures are influenced by the location and condition of the patient group (hospital compared with community setting, general hospital patients compared with those with fractured neck of femur) (Bridel 1996; Hanson 1993; Richardson 1981; Versluysen 1986). Pressure ulcers are more common in patient groups such as the elderly (Whittington 2000), those in orthopaedic settings (Versluysen 1986), and those who cannot reposition themselves (for example, younger patients with injuries to the spinal cord). Other medical conditions can also predispose the development of pressure ulcers (Schoonhoven 2002). Changing population demographics and the rise in the number of elderly patients in the future means that the number of people with pressure ulcers is likely to increase in the years ahead (Haalboom 2000). It is reasonable to suggest, therefore, that anything which improves ulcer healing outcomes will have a positive impact on both the individual and the health service as a whole (Thompson 1999).
The presence of a pressure ulcer impacts on the individual in many ways (Gorecki 2009). Pressure ulcers are painful (Gorecki 2009) and malodorous, especially when there is a large amount of dead tissue combined with anaerobic bacteria in the wound bed (Stotts 2001). Furthermore, pressure ulcers can exude profusely, particularly during the early inflammatory phase (Iocono 1998), and so require frequent changes of dressings (Rolstad 2000). It has been noted that the issues of concern for patients are pain, exudates, body image and worry about healing (Fox 2002), all of which alter an individual's quality of life (Gorecki 2009). In addition, it has been suggested that pressure ulcers also contribute to increased mortality (Kroger 2008; Redelings 2005).
Pressure ulcers are a significant financial burden to health care systems (Posnett 2009). The Touche Ross report (Touche Ross 1993) estimated the annual cost of treatment for pressure ulcers in the UK in 1993, at between £180 and £321 million, with the cost of prevention estimated at £180 to £755 million. More recently, Bennett 2004 explored the cost of pressure ulcer management and suggested that the total annual cost in the UK is £1.4 to 2.1 billion, or 4% of total healthcare expenditure. It is worth noting that costs of litigation or effects on quality of life, in terms of pain, depression and social isolation, were not included in these estimates. Therefore, at present, the precise economic impact of pressure ulcers has yet to be established (Posnett 2009).
Description of the intervention
The management of people with pressure ulcers involves a myriad of different interventions such as nutritional care (EPUAP/NPUAP 2009), pressure reducing/relieving surfaces (McInnes 2011), repositioning (Moore 2011) and skin and wound care (EPUAP/NPUAP 2009). Furthermore, in order to reduce the distress for individuals with pressure ulcers, it is essential that their wounds be managed successfully (Fox 2002). Following assessment of both the patient and the wound, the goal of management is to create the optimum local wound environment for healing (Rolstad 2000).
Selection of appropriate topical therapies (i.e. those applied to the skin) is widely believed to contribute to healing (Rolstad 2000). Available therapies include wound debridement (Ligresti 2007), the application of dressings (Ligresti 2007) and topical antimicrobial agents (O'Meara 2001). However there is little high quality research evidence to support the use of these therapies. Wound cleansing is regarded as an important component of pressure ulcer care (Hellewell 1997). It is assumed to be necessary to remove dead tissue and foreign bodies from wounds, and is usually undertaken before applying a dressing (Flanagan 1998a). However, there is uncertainty about what constitutes best practice (Fernandez 2012) and whether cleansing at all makes a difference. Clinicians and manufacturers recommend different solutions and methods of application, which is confusing (Fernandez 2012; Lawrence 1997; Lindholm 1999). Indeed, it is argued that wound cleansing practice is often based on past experience and ritual rather than the best available evidence (Carr 2006).
Why it is important to do this review
Fernandez 2012 previously conducted a systematic review of the effect of water as a wound cleanser however an exploration of the effect of cleansing technique was not a feature of this review. It is argued that wound cleansing has three elements: namely, the technique, the solution and the equipment (Young 1995). Techniques used include high pressure irrigation, swabbing, low pressure irrigation, showering, bathing, washing the affected area under a running solution, or total immersion in a whirlpool bath (also known as hydrotherapy) (Lawrence 1997; Lindholm 1999). Different cleansing solutions are also used; for example, normal saline, water, and antiseptic solutions (Angeras 1992). Furthermore, wound cleansing requires the use of equipment; for example, syringes, needles, catheters and pressurised canisters (Young 1995). Therefore, it is important to explore all components of the wound cleansing process, as the correct application of the solution may be as relevant as the solution itself (Morison 1989; Singer 1994). Consequently, it was decided to undertake a systematic review of the literature to summarise current evidence that could provide a contribution to relevant clinical guidelines. In addition, the review will inform research in this important area of patient care.
Objectives
To assess the effects of wound cleansing solutions and wound cleansing techniques on the healing rates of pressure ulcers.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing wound cleansing with no wound cleansing, or RCTs comparing different wound cleansing solutions or different wound cleansing techniques, were considered for the review. Controlled clinical trials (CCTs) were to be considered in the absence of RCTs.
Types of participants
Studies involving people of any age, in any health care setting, with existing pressure ulcers (defined as a break in the continuity of the skin, caused by pressure, shearing or friction forces) (Nixon 1999).
Types of interventions
For the purposes of this review, cleansing was defined as the application of fluid to the pressure ulcer to aid removal of exudate, debris and contaminants, but not the use of dressings or mechanical debridement (Towler 2001). Water was included if, within the relevant study, it had been compared with another solution.
Studies investigating the following comparisons were eligible for the review:
cleansing compared with no cleansing;
one cleansing solution compared with another;
one cleansing technique compared with another (e.g. irrigation, swabbing, soaking, immersion).
Types of outcome measures
Primary outcomes
Trials were considered if they reported at least one of the primary outcomes:
an objective measure of pressure ulcer healing, such as time to complete healing; absolute or percentage change in pressure ulcer area or volume over time; proportion of pressure ulcers healed at the completion of the trial period; or healing rate.
Secondary outcomes
procedural pain (using validated scales where reported);
ease of use of the method of cleansing.
Secondary outcomes were only reported from studies that also reported primary outcomes.
Search methods for identification of studies
The search methods section of the second update of this review can be found in Appendix 1.
Electronic searches
For this third review update we searched the following electronic databases:
Cochrane Wounds Group Specialised Register (searched 3 January 2013);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 12);
Ovid MEDLINE (2010 to November Week 3 2012);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations December 31, 2012);
Ovid EMBASE (2010 to 2012 Week 52); EBSCO CINAHL (2010 to December 2012).
The following search strategy was used in The Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor Sodium Chloride explode all trees #2 MeSH descriptor Sodium Hypochlorite explode all trees #3 MeSH descriptor Saline Solution, Hypertonic explode all trees #4 MeSH descriptor Iodophors explode all trees #5 MeSH descriptor Chlorhexidine explode all trees #6 MeSH descriptor Anti‐Infective Agents, Local explode all trees #7 MeSH descriptor Disinfectants explode all trees #8 MeSH descriptor Detergents explode all trees #9 MeSH descriptor Soaps explode all trees #10 MeSH descriptor Hydrogen Peroxide explode all trees #11 MeSH descriptor Benzoyl Peroxide explode all trees #12 MeSH descriptor Gentian Violet explode all trees #13 MeSH descriptor Water explode all trees #14 MeSH descriptor Alcohols explode all trees #15 MeSH descriptor Solutions explode all trees #16 normal saline or hypochlorit* or iodophor* or povidone or iodine or chlorhexidine or hibitane or betadine or antiseptic* or disinfectant* or antiseptic* or detergent* or soap* or “hydrogen peroxide” or “benzoyl peroxide” or “gentian violet” or eusol or dakin* or permanganate or water or "alcohol" or alcohols or solution* #17 MeSH descriptor Irrigation explode all trees #18 MeSH descriptor Baths explode all trees #19 MeSH descriptor Hydrotherapy explode all trees #20 (wound NEXT clean*) or (wound NEXT cleans*) #21 wash* or scrub* or swab* or shower* or bath* or soak* or irrigat* or whirlpool #22 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) #23 MeSH descriptor Pressure Ulcer explode all trees #24 pressure NEXT (ulcer* or sore*) #25 decubitus NEXT (ulcer* or sore*) #26 (bed NEXT sore*) or bedsore* #27 (#23 OR #24 OR #25 OR #26) #28 (#22 AND #27)
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2, Appendix 3 and Appendix 4 respectively. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2011). The Ovid EMBASE and EBSCO CINAHL searches was combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2013). There were no restrictions on the basis of date or language of publication.
Searching other resources
For the original review, we searched the bibliographies of all retrieved and relevant publications, identified through these strategies. Manufacturers of cleansing solutions, as identified in the British National Formulary (BNF 2003) and experts in the wound care field, namely: council members of the European Pressure Ulcer Advisory Panel, The European Wound Management Association, The National Pressure Ulcer Advisory Panel and the World Union of Wound Healing Societies, were contacted (by ZM) to identify any studies not located through the primary search, or to identify any further researchers involved in pressure ulcer research, whom the authors could contact directly.
Data collection and analysis
Selection of studies
Titles and, where available, abstracts of the studies were assessed by two review authors independently, for their eligibility for inclusion in the review. Full versions of potentially relevant studies were obtained and screened against the inclusion criteria by two review authors independently. Any differences in opinion were resolved by discussion and where necessary with reference to the Cochrane Wounds Group editorial base.
Data extraction and management
Data from included trials were extracted into pre‐prepared data extraction tables. Two review authors conducted data extraction independently and any differences in opinion were resolved by discussion and reference to the Cochrane Wounds Group editorial base. If data were missing from reports, the study authors were contacted to obtain the missing information. The following information was extracted specifically from trial reports:
Author; title; source; date of study; geographical location of study.
Care setting.
Type of wound.
Inclusion/exclusion criteria.
Sample size.
Patient characteristics (by treatment group).
Design details; study type; method of group allocation.
Intervention details; outcome measures.
Analysis; results; conclusions.
Assessment of risk of bias in included studies
Two review authors independently assessed each included study using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains: namely, sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance) (see Appendix 5) for details of criteria on which each judgement was based). Blinding and completeness of outcome data were assessed for each outcome separately. We then completed a risk of bias table for each eligible study, identifying wether we judged each component of the study to be low, unclear or high risk of bias. We have presented an assessment of risk of bias using a 'risk of bias summary figure' (see Figure 1) which presents all of the judgments in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give to the results of each study.
1.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Data synthesis
Initially, a structured narrative summary of the studies reviewed was conducted. Data were then entered into the Cochrane RevMan software. For dichotomous outcomes, risk ratio (RR), plus 95% confidence intervals (CI) were calculated; for continuous outcomes, mean difference (MD), plus 95% CI were calculated.
Results
Description of studies
The initial search identified 111 titles. In addition, 33 letters were written to wound care experts and drug companies supplying cleansing solutions and 13 replies were received (response rate of 40%). No further trials were identified through this process.
Following independent review of the abstracts by two review authors, 12 papers were judged to be eligible and full papers were obtained. Two review authors independently assessed the papers and applied the inclusion and exclusion criteria. There was complete agreement between the review authors and three papers were identified as meeting the inclusion criteria (see Characteristics of included studies). The Characteristics of excluded studies table summarises the nine studies which did not meet the inclusion criteria and were subsequently excluded from the review (Boykin 1989; Colombo 1983; Della Marchina 1997; Hartman 2002; Hinz 1986; Kucan 1981; Saydak 1990; Toba 1997; Van Der Cammen 1987).
For subsequent updates of this review six studies were identified as excluded studies (Anzai 1989; Bellingeri 2003; Burke 1998; Diekmann 1984; Liu 1999; Wang 1997) (Characteristics of excluded studies) and one additional study was included (Ho 2012). The study by Burke 1998 was excluded at this review stage because the ocutome measures employed were objective not subjective (defined by Burke 1998 as wounds improved or not improved), this is an exclusion criteria as we had previously stated that the we would only include studies reporting an objective measure of pressure ulcer healing, such as time to complete healing; absolute or percentage change in pressure ulcer area or volume over time; proportion of pressure ulcers healed at the completion of the trial period; or healing rate.
Three eligible RCTs were identified, therefore, CCTs were not considered. The trials were published between 2001 and 2012. The first study looked at cleansing of chronic wounds (including pressure ulcers) with saline compared with cleansing with water, in the community care setting (Griffiths 2001). The second study looked at cleansing of pressure ulcers with isotonic saline compared with cleansing with saline spray containing Aloe vera, silver chloride and decyl glucoside (Vulnopur) in the hospital setting (Bellingeri 2004). The final study looked at cleansing of pressure ulcers with low‐pressure pulsatile lavage compared with sham (the lavage flow was directed into a washbasin positioned adjacent to the wound), in a spinal cord injury tertiary care center inpatient unit (Ho 2012). Overall, sample size was generally small which, consequently, is a major limitation of the studies; indeed the mean sample size was 53 (range 8 to 110).
Risk of bias in included studies
All studies detailed participant eligibility criteria and all stated that the participants were randomly allocated to the groups however only two studies reported an adequate method of generating the random sequence (Griffiths 2001; Ho 2012). The extent of allocation concealment was unclear for all three studies due to poor reporting.
Performance bias occurs where the awareness of which group the participant is in, rather than the intervention itself, actually affect the study outcomes. Reducing the risk of performance bias may be achieved through blinding participants and care givers. However, this remains a challenge in wound care, as many of the treatments used look different and are therefore difficult to disguise. None the less, Ho 2012 ensured that the participants were blind to the treatment allocation, however the treatment nurse knew which group each participant was in. Griffiths 2001 ensured that the outcome assessor was blinded to the treatment allocation, in addition the treatment nurse was also blinded (this was relatively easy to do as both solutions (saline and tap water) appeared identical). Bellingeri 2004 does not report whether the participant or the care giver were blinded to treatment allocation.
The risk of detection bias, that is systematic differences in how outcomes are assessed depending on which group the individual is allocated to, is reduced through the use of blinding of outcome assessment. Ho 2012 and Griffiths 2001 ensured that the outcome assessor was blind to the treatment allocation. However, Bellingeri 2004 did not report this information.
There were no drop outs in the study by Ho 2012, all ulcers being assessed at the final outcome assessment. Bellingeri 2004 reported that a per protocol analysis, instead of an intention to treat analysis, was undertaken, because it was not possible to assign a PSST score to patients withdrawn from the trial. Thus, only those who completed the study were included in the final analysis. Griffiths 2001, in their overall study, did not conduct intention to treat analysis as he excluded from the analysis 8 participants who were lost to follow up (four from each group) because they were admitted into hospital, did not adhere to the protocol, or declined to continue to participate.
Data on baseline comparability for prognostic factors were not clearly described in the studies of Griffiths 2001 and Bellingeri 2004. However, Ho 2012 reported that groups were equivalent at baseline.
Effects of interventions
How the results are presented and what the terms mean
Results for dichotomous variables are presented as risk ratio (RR) with 95% CI. Risk ratio is the rate of the event of interest (e.g. pressure ulcers healed) in the experimental group divided by the rate of this event in the control group and indicates the chances of pressure ulcer healing for people on the experimental treatment compared with the control treatment (Higgins 2011). Results for continuous variables are presented as mean difference (MD), with 95% CI. The mean difference measures the absolute difference between the mean value in two groups in a clinical trial (Higgins 2011). It estimates the amount by which the experimental intervention changes the outcome on average (for example, changes in pressure ulcer volume) compared with the control (Higgins 2011). Interpretation of the results is the same as RR except the point of no effect is 0 rather than 1 (Higgins 2011).
Comparison: cleansing compared with no cleansing
No trials were identified for this comparison.
Comparison: different cleansing solutions
Two trials were identified that compared different cleansing solutions (Bellingeri 2004; Griffiths 2001).
Saline Spray with aloe vera, silver chloride and decyl glucoside compared with isotonic saline
One RCT (Bellingeri 2004) enrolled 133 patients with pressure ulcers greater than Grade 1 (National Pressure Ulcer Advisory Panel scale, NPUAP 1989), seven of whom withdrew before the end of the trial because they were put onto antibiotics; use of antibiotics was an exclusion criterion. It is not known to which group or groups these seven participants were allocated and the trial author, in a personal communication, has not been able to obtain this information. Analysis was based on the 126 subjects who completed the trial.
The Pressure Sore Status Tool (PSST), developed in 1992 by Bates‐Jensen and colleagues, was used as an outcome measure in this study (Bates‐Jensen 1992). The tool uses 13 different items to assess pressure ulcer condition. All the items are scored with a Likert scale, giving a final value of between 13 and 65, with 13 indicating a healed ulcer.
There were 46 women and 28 men in the control group, with a median age of 73 years (range 62 to 83 years). Their wounds were cleansed with isotonic saline solution: PSST at baseline was 33 for this group, with a standard deviation (SD) of 10.3, minimum value (min) 15, maximum value (max) 52. There were 36 women and 23 men in the intervention group, with a median age of 74 years (range 56 to 84 years). Their wounds were cleansed with saline spray with Aloe vera, silver chloride and decyl glucoside (Vulnopur): mean PSST at baseline was 34.0, with SD 11.5, min 13.0, max 52.0. (Table 1 data presented in the study report with translated headings).
1. Bellingeri 2004 Table of results.
| Intervention | PSST Baseline | PSST Day 7 | PSST Day 14 | Total % Change |
| Isotonic saline solution (control) | mean 31.6 (SD 10.3, min 15.0, max 52.0) | 28.9 (SD 10.5, min 12.0, max 52.0). | 25.3 (SD 12.2, min 10.0, max 50.0). | ‐20.5 (SD 24.1, min ‐65.8, max 22.7). |
| Saline spray, Aloe vera, silver chloride and decyl glucoside (Vulnopur) (intervention) | mean 31.3 (SD 11.5, min 13.0 max 56.0) | 27.1 (SD 11.1, min 13.0, max 54.0). | 21.6 (SD 11.6, min 10.0, max 51,0). | ‐27.8 (SD 31.3, min ‐69.8, max 123.5). |
The authors did not describe how the cleansing was carried out, or identify the types of dressings used on the wounds after cleansing. The patients were followed up for a period of 14 days.
Primary outcome measure: percentage reduction in pressure sore status
The mean percentage change from baseline to day 14 in the control group was ‐20.5 (SD 24.1, min ‐65.8, max 22.7), while the mean percentage change in the Vulnopur spray group was ‐27.8 (SD 31.3, min 69.8, max 123.5). The data from this study were skewed and the trialists used non‐parametric tests that cannot be reproduced, because the raw data were not reported. As RevMan assumes a normal distribution, data have not been plotted graphically for this study, and we have accepted the trialists' analysis, which found that there was a statistically significant improvement in healing in the intervention group (P value = 0.025). The original author cannot confirm the group(s) from which the seven patients withdrew due to infection, however, the final group sizes are n = 59 in the Vulnopur group and n = 74 in the control group. If more of these patients withdrew from the Vulnopur group, the result would be biased towards that group.
Saline compared with tap water
Whilst the RCT comparing saline with tap water (Griffiths 2001), which enrolled 43 patients with 60 wounds, was intended to be analysed by intention to treat, eight patients were lost to follow up (four from each group) because they were admitted into hospital, did not adhere to the protocol, or declined to continue to participate therefore this was not analysis by intention to treat. Data analysis was, therefore, conducted on 35 patients with 49 wounds, eight of which were pressure ulcers. The data on pressure ulcers alone was made available by the trial authors, and this data forms the basis of what is reported in this review, i.e. 8 individuals with one pressure ulcer each.
The patients had chronic wounds of Grade 2 or 3, using Carville's definition (Carville 1995), and were receiving care in a community setting. According to Carville's definition, Grade 2 wounds have partial‐thickness skin loss (down to the epidermis and/or dermis), while Grade 3 wounds have full‐thickness skin loss (down to, but not through, the fascia) (Carville 1995). For the purpose of this review, data are presented on the eight pressure ulcers.
There were three men and three women in the intervention group (six wounds), with a mean age of 70.5 years (range 40 to 82 years). Their wounds were cleansed with tap water; the mean wound diameter size at baseline was 463 mm (range 59 mm to 826 mm). The control group consisted of one man and one woman (two wounds), with a mean age of 71 years (range 56 to 86 years). Their wounds were cleansed using saline; the mean wound size at baseline was 713 mm; range 535 mm to 790 mm.
Wound cleansing for both groups was conducted in a similar manner. The wounds were irrigated with either saline or water, delivered at room temperature via a 30 ml syringe and a 20 g cannula. The surrounding skin was patted dry and a clean dressing applied. It is not clear from the study how often the wounds were irrigated. A variety of topical dressings were used, including hydrocolloids and gels. A combination of hydrocolloid and gel or hydrocolloid alone was used topically on the intervention group, whereas either a hydrocolloid alone or a hydrocolloid and hydrocolloid paste was used in the control group. The participants were followed up for a period of six weeks.
Primary outcome measure: number of ulcers healed
Three wounds cleansed with tap water healed in the six‐week period, whereas none of the wounds cleansed with saline had healed at six weeks; risk ratio (RR) was 3.00 (95% confidence interval (CI) 0.21 to 41.89) (Analysis 2.1). The sample size was too small to rule out a treatment effect and therefore it is not possible to draw a firm conclusion.
2.1. Analysis.
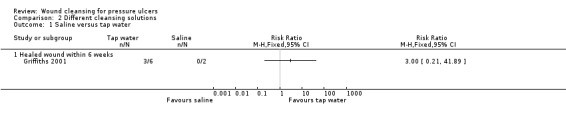
Comparison 2 Different cleansing solutions, Outcome 1 Saline versus tap water.
Comparison: different cleansing techniques
One trial was identified that compared different cleansing techniques (Ho 2012).
Pulsatile lavage compared with sham
One small RCT (Ho 2012) recruited inpatients who had spinal cord injury and were receiving standard wound care for Grade 3 and 4 pelvic (coccygeal, ischial, or trochanteric region) pressure ulcers. 28 males were included in the study and randomly allocated, using a computer‐generated randomisation table, to control group (sham; n=14 ) or to the intervention group (pulsatile lavage; n=14 ). The authors report that the two groups were equivalent at baseline.
In the intervention group, low‐pressure pulsatile lavage treatment with 1 litre of normal saline at 11 psi of pressure was applied to the pressure ulcer once daily. In the control group, the pressure ulcers received sham treatment, that is, the lavage flow was directed into a washbasin positioned adjacent to the wound and was not visible to the participants, yet the noise of the machine could be heard by the participant. Following pulsatile lavage or sham treatment, each study participant received standard wound care (moist wound care) and bed rest with regular turning, and use of a low air loss mattress for those with pelvic pressure ulcers.
The participants were followed up for a three week period and improvement in pressure ulcer condition was assessed by measuring changes in ulcer volume, length, width and depth of the pressure ulcers.
Primary outcome measure: improvement in pressure ulcer condition
The wounds in the pulsatile lavage group demonstrated a statistically significantly greater mean reduction in ulcer volume (‐4.9 cm3) than the wounds in the sham group (‐3.7 cm3) (MD ‐6.60, 95% CI ‐11.23 to ‐1.97) (Analysis 1.1). However, this is a small study and these between group differences would need to be confirmed in a larger study.
1.1. Analysis.

Comparison 1 Different cleansing techniques, Outcome 1 Pulsatile Lavage versus sham.
Discussion
No adequately powered RCT was identified that compared cleansing with no cleansing of pressure ulcers. Three small RCTs were identified, each exploring a different aspect of wound cleansing. This is interesting when one considers that 18% of hospitalised patients have a pressure ulcer (EPUAP 2002) and that wound cleansing, among many other interventions such as repositioning and nutritional support, is a routine component of the management of these wounds (Rolstad 2000).
Saline spray containing Aloe vera, silver chloride and decyl glucoside (Vulnopur) was found to significantly improve PSST scores when used to cleanse pressure ulcers compared with isotonic saline. Furthermore, in one small RCT, cleansing with pulsatile lavage yielded a greater mean reduction in ulcer volume, compared with sham. There was no statistically significant difference in healing of pressure ulcers that had cleansed with saline when compared with those cleansed with water.
Some methodological issues require consideration and limit the conclusions that can be drawn from this review. The existing studies are small and underpowered ‐ indeed the mean sample size was 53 (range 8 to 110) ‐ which restricts the certainty with which differences between groups can be identified as statistically significant.
Risk of bias due to methodological approaches used and potential risk of bias where poor reporting precluded the adequate assessment of bias are important considerations in this review. True allocation concealment prevents the researcher being aware which group, experimental or control, a participant is allocated to, ensuring that the participant is assigned to a specific study group by chance (Higgins 2011). Concealment of group allocation was inadequately described in all of the studies. It has been suggested that lack of a clear description of allocation concealment leads to bias in assessing the outcome of studies (Moher 2001); the size of the effect could be overestimated and so give a false impression of the value of the intervention. Studies without adequate allocation concealment tend to report larger estimates of effect when compared to those who describe adequate allocation concealment (Schulz 2000).
Blinding of the study is said to be complete if the investigators, participants, outcome assessor, and the data analyst do not know which group the participant is allocated to (Higgins 2011). Human behaviour is influenced by prior knowledge, thus, without blinding, there is a risk that the size of the effect may be overestimated, resulting in a bias in favour of the treatment (Day 2000). Therefore, blinding is considered important in the assessment of subjective outcomes, such as ease of use of a treatment, and in ensuring comparability of assessment and diagnostic interventions across all groups within a study (Higgins 2011). Blinding is difficult to achieve in wound care, however blinded outcome assessment was achieved in two trials (Griffiths 2001; Ho 2012), though information on blinding was not reported by Bellingeri 2004.
The rationale for using intention to treat analysis is two‐fold; it maintains treatment groups that are similar (apart from random variation) and therefore validates the use of randomisation, and allows for handling of protocol deviations, further protecting the randomisation process (Hollis 1999). Omitting those who do not complete the study from the final analysis may bias the outcomes of the study, because those who do not complete may do so because of adverse effects of the intervention (Montori 2001). Intention to treat analysis was conducted in one of the studies as there were no reported withdrawals (Ho 2012) and Bellingeri 2004 reported undertaking a per protocol analysis. Greenhalgh 1997 suggests that data should be analysed for all participants originally included in the study, even if they did not complete the trial. Failure to do this can lead to an overestimation of the size of the effect, usually in favour of the intervention. However, although Fergusson 2002 argues that there may be certain circumstances where it is possible to exclude patients from analysis after randomisation (in order to avoid bias and to minimise random error), well designed studies that adhere to a high standard of methodological rigour, should ensure that this rarely happens.
In three of the studies, information regarding prognostic factors was unclear. The CONSORT statement set out to identify the key criteria required of authors when reporting results of studies (Moher 2001). The rationale for the development of this statement is that, to understand the significance of an RCT, the reader must be able to comprehend all components of the study clearly. Therefore, in order to be able to do this, the studies must include all relevant information when they are published. For the reader, the ability to judge the quality of a study is severely hampered by a lack of baseline data. It is evident from the studies included in this review that important pieces of information are either not reported or are not adequately described.
In conclusion, there is little evidence available pertaining to wound cleansing for pressure ulcers. One study demonstrated a statistically significant difference in outcomes for wounds cleansed with saline spray containing Aloe vera, silver chloride and decyl glucoside (Vulnopur) compared to isotonic saline solution (Bellingeri 2004) however this study was poorly reported and at potential risk of bias. A small study demonstrated a statistically significant reduction in ulcer volume reduction in wounds cleansed with pulsatile lavage compared with those cleansed using sham pulsatile lavage (Ho 2012). It appears that there is no evidence supporting the use of water rather than saline as a wound cleansing solution. None of the trials were without some risk of bias.
Well designed, robust studies are required. Such trials need to be sufficiently powered, with all groups comparable at baseline, adequate allocation concealment and blinded outcome assessment, with an intention to treat analysis, before firm conclusions can be drawn.
Authors' conclusions
Implications for practice.
There have been no RCTs comparing the effects of cleansing pressure ulcers with not cleansing them. There is some evidence that cleansing pressure ulcers using a saline spray containing Aloe vera, silver chloride and decyl glucoside (Vulnopur) may be associated with improved healing however this evidence may be at risk of bias. There is also some evidence to suggest that pressure ulcer cleansing using pulsatile lavage may increase healing. Overall high quality, valid evidence about the effects of different approaches to cleansing pressure ulcers is scant.
Implications for research.
There is a need for further research in this area. It remains important that future studies be of sound methodological quality, incorporating the following:
true randomisation;
adequate allocation concealment;
blinded outcome assessment;
intention to treat analysis;
baseline comparability of groups;
adequate sample size; and
reporting of studies in accordance with the CONSORT guidelines (Moher 2001).
What's new
| Date | Event | Description |
|---|---|---|
| 14 May 2013 | Amended | Amendment to citation Bellingeri 2004 as a result of feedback received. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 3 January 2013 | New citation required but conclusions have not changed | Third update no change to overall conclusions |
| 3 January 2013 | New search has been performed | New search, one additional study included (Ho 2012), two studies added to the table of excluded studies (Diekmann 1984, Burke 1998). |
| 23 March 2010 | New search has been performed | Second update ‐ risk of bias table added, new search, no new studies identified for inclusion, one study added to the table of excluded studies (Bellingeri 2003), conclusions unchanged. |
| 13 August 2008 | Amended | Converted to new review format. |
| 28 November 2007 | New search has been performed | First update ‐ for the first update of this review 3 further studies were excluded from the review, no new studies met the inclusion criteria. The conclusions of the review remain unchanged. |
| 12 August 2005 | New citation required and conclusions have changed | Substantive amendment This review was first published in the Cochrane Library in issue 4, 2005 with 3 included studies and 9 excluded studies. |
Acknowledgements
The authors would like to thank Susan O'Meara and Sally Bell‐Syer particularly, for their invaluable help, advice and support in the conduct of this review. The authors would also like to thank the Cochrane Wounds Group referees (Jacqui Fletcher, Nerys Woolacott) and Coordinating Editor (Nicky Cullum) for their comments on the review, Ruth Foxlee (TSC) for undertaking the searches for the update and Sally Stapley for support though the second update. Finally, the authors are grateful to Adrianna Castelli, Wu Taixing and Ikumi Iwama for their assistance with article translation and data extraction.
Appendices
Appendix 1. Search methods for the second review update ‐ 2010
Electronic searches
For this second review update we searched the following electronic databases:
Cochrane Wounds Group Specialised Register (Searched 26/3/10)
The Cochrane Central Register of Controlled Trials (CENTRAL) ‐ The Cochrane Library 2010 Issue 1
Ovid MEDLINE ‐ 2007 to March Week 2 2010
Ovid MEDLINE ‐ In‐Process & Other Non‐Indexed Citations (Searched 24/3/10)
Ovid EMBASE ‐ 2007 to 2010 Week 9
EBSCO CINAHL ‐ 2007 to March 26 2010
The following search strategy was used in The Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor Sodium Chloride explode all trees #2 MeSH descriptor Sodium Hypochlorite explode all trees #3 MeSH descriptor Saline Solution, Hypertonic explode all trees #4 MeSH descriptor Iodophors explode all trees #5 MeSH descriptor Chlorhexidine explode all trees #6 MeSH descriptor Anti‐Infective Agents, Local explode all trees #7 MeSH descriptor Disinfectants explode all trees #8 MeSH descriptor Detergents explode all trees #9 MeSH descriptor Soaps explode all trees #10 MeSH descriptor Hydrogen Peroxide explode all trees #11 MeSH descriptor Benzoyl Peroxide explode all trees #12 MeSH descriptor Gentian Violet explode all trees #13 MeSH descriptor Water explode all trees #14 MeSH descriptor Alcohols explode all trees #15 MeSH descriptor Solutions explode all trees #16 normal saline or hypochlorit* or iodophor* or povidone or iodine or chlorhexidine or hibitane or betadine or antiseptic* or disinfectant* or antiseptic* or detergent* or soap* or “hydrogen peroxide” or “benzoyl peroxide” or “gentian violet” or eusol or dakin* or permanganate or water or "alcohol" or alcohols or solution* #17 MeSH descriptor Irrigation explode all trees #18 MeSH descriptor Baths explode all trees #19 MeSH descriptor Hydrotherapy explode all trees #20 (wound NEXT clean*) or (wound NEXT cleans*) #21 wash* or scrub* or swab* or shower* or bath* or soak* or irrigat* or whirlpool #22 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) #23 MeSH descriptor Pressure Ulcer explode all trees #24 pressure NEXT (ulcer* or sore*) #25 decubitus NEXT (ulcer* or sore*) #26 (bed NEXT sore*) or bedsore* #27 (#23 OR #24 OR #25 OR #26) #28 (#22 AND #27)
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in appendices. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision). The Ovid EMBASE and EBSCO CINAHL searches was combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network. There were no restrictions on the basis of date or language of publication.
Searching other resources
For the original review, we searched the bibliographies of all retrieved and relevant publications, identified through these strategies. Drug companies who supply cleansing solutions, as identified in the British National Formulary (BNF 2003) and experts in the wound care field, namely: council members of the European Pressure Ulcer Advisory Panel, The European Wound Management Association, The National Pressure Ulcer Advisory Panel and the World Union of Wound Healing Societies, were contacted (by ZM) to identify any studies not located through the primary search, or to identify any further researchers involved in pressure ulcer research, whom the authors could contact directly.
Appendix 2. Ovid MEDLINE search strategy
1 exp Sodium Chloride/ 2 exp Sodium Hypochlorite/ 3 exp Saline Solution, Hypertonic/ 4 exp Iodophors/ 5 exp Chlorhexidine/ 6 exp Anti‐Infective Agents, Local/ 7 exp Disinfectants/ 8 exp Detergents/ 9 exp Soaps/ 10 exp Hydrogen Peroxide/ 11 exp Benzoyl Peroxide/ 12 exp Gentian Violet/ 13 exp Water/ 14 exp Alcohols/ 15 exp Solutions/ 16 (normal saline or hypochlorit$ or iodophor$ or povidone or iodine or chlorhexidine or hibitane or betadine or antiseptic$ or disinfectant$ or antiseptic$ or detergent$ or soap$ or hydrogen peroxide or benzoyl peroxide or gentian violet or eusol or dakin$ or permanganate or water or alcohol$1 or solution$).mp. 17 exp Irrigation/ 18 exp Baths/ 19 exp Hydrotherapy/ 20 (wound clean$ or wound cleans$).mp. 21 (wash$ or scrub$ or swab$ or shower$ or bath$ or soak$ or irrigat$ or whirlpool).mp. 22 or/1‐21 23 exp Pressure Ulcer/ 24 (pressure adj (ulcer$ or sore$)).mp. 25 (decubitus adj (ulcer$ or sore$)).mp. 26 (bed adj (ulcer$ or sore$)).mp. 27 or/23‐26 28 22 and 27
Appendix 3. Ovid EMBASE search strategy
1 exp Sodium Chloride/ 2 exp Sodium Hypochlorite/ 3 exp Saline Solution, Hypertonic/ 4 exp Iodophors/ 5 exp Chlorhexidine/ 6 exp Anti‐Infective Agents, Local/ 7 exp Disinfectants/ 8 exp Detergents/ 9 exp Soaps/ 10 exp Hydrogen Peroxide/ 11 exp Benzoyl Peroxide/ 12 exp Gentian Violet/ 13 exp Water/ 14 exp Alcohols/ 15 exp Solutions/ 16 (normal saline or hypochlorit$ or iodophor$ or povidone or iodine or chlorhexidine or hibitane or betadine or antiseptic$ or disinfectant$ or antiseptic$ or detergent$ or soap$ or hydrogen peroxide or benzoyl peroxide or gentian violet or eusol or dakin$ or permanganate or water or alcohol$1 or solution$).mp. 17 exp Irrigation/ 18 exp Baths/ 19 exp Hydrotherapy/ 20 (wound clean$ or wound cleans$).mp. 21 (wash$ or scrub$ or swab$ or shower$ or bath$ or soak$ or irrigat$ or whirlpool).mp. 22 or/1‐21 23 exp Pressure Ulcer/ 24 (pressure adj (ulcer$ or sore$)).mp. 25 (decubitus adj (ulcer$ or sore$)).mp. 26 (bed adj (ulcer$ or sore$)).mp. 27 or/23‐26 28 22 and 27
Appendix 4. EBSCO CINAHL search strategy
S28 S22 and S27 S27 S23 or S24 or S25 or S26 S26 TI (bed sore* or bedsore* ) or AB (bed sore* or bedsore*) S25 TI decubitus or AB decubitus S24 TI (pressure ulcer* or pressure sore*) or AB (pressure ulcer* or pressure sore*) S23 (MH "Pressure Ulcer") S22 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 S21 TI ( wash* or scrub* or swab* or shower* or bath* or soak* or irrigat* or whirlpool ) or AB ( wash* or scrub* or swab* or shower* or bath* or soak* or irrigat* or whirlpool ) S20 TI ( wound clean* or wound cleans* ) or AB ( wound clean* or wound cleans* ) S19 (MH "Hydrotherapy+") S18 (MH "Bathing and Baths") S17 (MH "Irrigation+") S16 AB ( normal saline or hypochlorit* or iodophor* or povidone or iodine or chlorhexidine or hibitane or betadine or antiseptic* or disinfectant* or antiseptic* or detergent* or soap* or hydrogen peroxide or benzoyl peroxide or gentian violet or eusol or dakin* or permanganate or water or alcohol*1 or solution* ) S15 TI ( normal saline or hypochlorit* or iodophor* or povidone or iodine or chlorhexidine or hibitane or betadine or antiseptic* or disinfectant* or antiseptic* or detergent* or soap* or hydrogen peroxide or benzoyl peroxide or gentian violet or eusol or dakin* or permanganate or water or alcohol*1 or solution* ) S14 (MH "Solutions+") S13 (MH "Alcohols+") S12 (MH "Water+") S11 (MH "Gentian Violet") S10 (MH "Hydrogen Peroxide") S9 (MH "Soaps") S8 (MH "Detergents+") S7 (MH "Disinfectants") S6 (MH "Antiinfective Agents, Local+") S5 (MH "Povidone‐Iodine") S4 (MH "Chlorhexidine") S3 (MH "Saline Solution, Hypertonic") S2 (MH "Sodium Hypochlorite") S1 (MH "Sodium Chloride+")
Appendix 5. The Cochrane Collaboration tool for assessing risk of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following.
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon)
High risk of bias
Any one of the following.
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
had extreme baseline imbalance; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Different cleansing techniques.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pulsatile Lavage versus sham | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Changes in Volume | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Different cleansing solutions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Saline versus tap water | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Healed wound within 6 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Griffiths 2001.
| Methods | RCT, allocation using random numbers table. Follow up for six weeks. | |
| Participants | Patients with chronic wounds of Grade 2 or Grade 3 receiving care in a community setting. 49 wounds, eight of which were pressure ulcers. | |
| Interventions | Intervention group: (n = 6) ulcers cleansed with tap water. Control group: (n = 2) ulcers cleansed with saline. A combination of hydrocolloid and gel or hydrocolloid alone was used topically on the intervention group, whereas either a hydrocolloid alone or a hydrocolloid and hydrocolloid paste was used in the control group. | |
| Outcomes | Sub group analysis not conducted. Three of the six wounds in the tap water group healed, whereas neither of the two wounds in the saline group healed in the study period. | |
| Notes | This study considered more than one type of wound: i.e. lacerations, venous ulcers and pressure ulcers, however, only the pressure ulcer data is reported here. Information regarding baseline comparability was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a random numbers table". |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Participants ‐ all outcomes | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) Blinding of personnel delivering intervention ‐ all outcomes | Low risk | The nurse performing the dressing was blinded. Quote: "solutions delivered in identical containers to the community nurses" |
| Blinding (performance bias and detection bias) Outcome assessors ‐ all outcomes | Low risk | Quote: "the project manager was blinded and undertook the assessment of the wound at baseline and at 6 weeks". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8 participants, 4 from each group were "withdrawn because they stopped participating, were admitted to hospital or did not adhere to the treatment". These participants were not included in the analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported were those outlined by the authors in the paper |
Bellingeri 2004.
| Methods | Multicentre RCT. Method of allocation unclear. Follow up 14 days. | |
| Participants | Elderly patients of both sexes, with ulcers of > Grade 1 NPUAP scale, dimensions of the ulcer within 10 cm x 10 cm, in patient admission or under home care assistance for greater than 24 hours. Control group: 46 females, 28 males, median age 73 years, range 62 to 83 years. Intervention group: 36 females, 23 males, median age 74 years, range 56 to 84 years. | |
| Interventions | Control group: cleansing with isotonic saline solution. Intervention group: cleansing with Saline spray with Aloe vera, silver chloride and decyl glucoside (Vulnopur). The authors did not describe the precise mechanism of application of the solutions. | |
| Outcomes | Mean percentage reduction in PSST at day 14: Vulnopur ‐22.7 (SD 31.3); isotonic saline ‐11.7 (SD 24.1) (P value =0.025). | |
| Notes | Data analysis conducted on 126 participants, seven participants withdrew from the trial (per protocol analysis carried out because it was not possible to assign a PSST score to those withdrawn from the trial). Information regarding baseline comparability was not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clearly stated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Participants ‐ all outcomes | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Blinding of personnel delivering intervention ‐ all outcomes | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) Outcome assessors ‐ all outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seven patients dropped out of the study because they started antibiotic therapy (per protocol analysis carried out). |
| Selective reporting (reporting bias) | Low risk | All outcomes reported were those outlined by the authors in the paper |
Ho 2012.
| Methods | RCT, Computer‐generated randomisation, follow up 3 weeks | |
| Participants | 28 inpatients who had SCI and were receiving standard wound care for stage III and IV pelvic(coccygeal, ischial, or trochanteric region) pressure ulcers. Age older than 18 years, with no preserved sensory function in the area of the pressure ulcers, clinically clean wound area (i.e., no necrotic tissue, no odour, and no exudate or minimal serosanguinous exudate only), No surrounding erythema or other evidence of cellulitis, no tunnelling, no actual or possible connection to body cavities, and no fistula, no malignancy or vascular disease associated with the area of tissue breakdown, no significant active systemic disease, such as heart disease, renal failure, diabetes, or end‐stage cancer, pressure ulcers with maximum diameters of 3 to 15 cm at recruitment into the study, no antibiotic therapy for 7 days before recruitment into the study | |
| Interventions | Intervention group: Daily low‐pressure pulsatile lavage treatment with 1 L of normal saline at 11 psi of pressure Control group: Daily sham treatment: The lavage flow was directed into a washbasin positioned adjacent to the wound and not visible to the participants |
|
| Outcomes | Baseline mean depth: Intervention: 2.4 (1.67 to 3.19) ‐ Control: 3.0 (2.16 to 3.84) Final mean depth: Intervention: 1.4 (0.59 to 2.20) ‐ Control: 2.5 (1.81 to 3.16) Baseline mean width: Intervention: 4.2 (3.15 to 5.15) ‐ Control: 4.5 (2.83 to 6.17) Final mean width, Intervention: 2.9 (1.92 to 3.78) ‐ Control: 3.0 (1.30 to 4.65) Baseline mean length: Intervention: 5.0 (3.92 to 6.02) ‐ Control: 5.1 (3.68 to 6.47) Final mean length, Intervention: 3.5 (2.47 to 4.54) ‐ Control: 4.9 (3.53 to 6.32) Baseline mean volume: Intervention: 10.7 (6.54 to 14.83) ‐ Control: 16.1 (10.56 to 21.69) Final mean volume: Intervention: 5.8 (2.79 to 8.79) ‐ Control: 12.4 (8.39 to 16.38) Difference between sample means: Depth (cm/wk) 0.24 (0.09 to 0.58) p<0.001 Width (cm/wk) 0.16 (0.06 to 0.39) p<0.0001 Length (cm/wk) 0.47 (0.18 to 1.12) p<0.0001 Volume (cm3/wk) 0.33 (0.13 to 0.80) p<0.001 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Participants ‐ all outcomes | Low risk | Quote: Participants were unaware of the intervention assignments |
| Blinding (performance bias and detection bias) Blinding of personnel delivering intervention ‐ all outcomes | Unclear risk | Quote: The research nurse administering the intervention was aware of the group assignments |
| Blinding (performance bias and detection bias) Outcome assessors ‐ all outcomes | Low risk | Quote: Assessors were unaware of the intervention assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None excluded |
| Selective reporting (reporting bias) | Low risk | all outcomes alluded to in the paper are reported on |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anzai 1989 | Study explores wound treatment, not wound cleansing. |
| Bellingeri 2003 | Insufficient information (author contacted but no response received). |
| Boykin 1989 | Study explores wound dressings, not wound cleansing. |
| Burke 1998 | Outcome measure are subjective not objective. |
| Colombo 1983 | Study explores wound dressings, not wound cleansing. |
| Della Marchina 1997 | Study explores wound dressings, not wound cleansing. |
| Diekmann 1984 | Study explores wound treatment, not wound cleansing. |
| Hartman 2002 | Study explores wound dressings, not wound cleansing. |
| Hinz 1986 | Study explores venous leg ulcers, not pressure ulcers. |
| Kucan 1981 | Study explores wound dressings, not wound cleansing. |
| Liu 1999 | Study explores wound dressings, not wound cleansing. |
| Saydak 1990 | Not an RCT, study explores wound dressings, not wound cleansing. |
| Toba 1997 | Study explores wound dressings, not wound cleansing. |
| Van Der Cammen 1987 | Study explores the prevention of pressure ulcers (i.e. no patient had a pressure ulcer at the start of the study). |
| Wang 1997 | Study explores wound treatment, not wound cleansing. |
Contributions of authors
Protocol and review development ‐ Zena Moore Commenting on draft of protocol and review ‐ Seamus Cowman Review of search results, abstracts of articles and full text ‐ Zena Moore, Seamus Cowman Review and data extraction of articles ‐ Zena Moore, Seamus Cowman, Writing the review and the subsequent updates ‐ Zena Moore.
Contributions of editorial base:
Nicky Cullum: edited the review, advised on methodology, interpretation and review content. Approved the final review and review update prior to submission. Sally Bell‐Syer: coordinated the editorial process. Advised on methodology, interpretation and content. Edited the review and the updated review. Ruth Foxlee: designed the search strategy, ran the searches and edited the search methods section for the update.
Sources of support
Internal sources
The Faculty Board, Faculty of Nursing & Midwifery, RCSI, Dublin 2, Ireland.
Royal College of Surgeons in Ireland, Ireland.
External sources
Health Research Board, Ireland.
NIHR/Department of Health (England), (Cochrane Wounds Group), UK.
Declarations of interest
The author, Zena Moore, is a member of the medical advisory board of Systagenix Wound Management. The author, Zena Moore, has received an honorarium for speaking at professional meetings for KCI, ConvaTec, Systagenix Wound Management, Fanin Health Care and Smith & Nephew. Seamus Cowman ‐ none known.
Edited (no change to conclusions)
References
References to studies included in this review
Bellingeri 2004 {published data only}
- Bellingeri R, Attolini C, Fioretti O, Forma P, Traspedini M, Costa M, et al. Evaluation of the efficacy of a preparation for the cleansing of cutaneous injuries. Minerva Medica 2004;95(Suppl):1‐9. [Google Scholar]
Griffiths 2001 {published data only}
- Griffiths RD, Fernandez RS, Ussia CA. Is tap water a safe alternative to normal saline for wound irrigation in the community setting?. Journal of Wound Care 2001;10(10):407‐11. [DOI] [PubMed] [Google Scholar]
Ho 2012 {published data only}
- Ho CH, Bensitel T, Wang X, Bogie K M. Pulsatile lavage for the enhancement of pressure ulcer healing: a randomized controlled trial. Physical Therapy 2012;92(1):38‐48. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anzai 1989 {published data only}
- Anzai T, Shiratori A, Otomo E, Honda S, Yamamoto T, Nishikawa T, et al. Evaluation of Clinical Utility of NI‐009 on Various Cutaneous Ulcers: Comparative Study with Base. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1989;5(12):2585‐612. [Google Scholar]
Bellingeri 2003 {published data only}
- Bellingeri A, Forma O, Polignano R, Attolini R, Accardi S, Fabbri C, et al. Multi‐centre research on a cleanser for cutaneous wounds. Wound Repair and Regeneration 2003;11(5):A36 P.018. [Google Scholar]
Boykin 1989 {published data only}
- Boykin A, Winland‐Brown J. Pressure sores nursing management. Journal of Gerontological Nursing 1989;15(2232):252‐6. [DOI] [PubMed] [Google Scholar]
Burke 1998 {published data only}
- Burke DT, Ho CH, Saucier MA. Hydrotherapy effects on pressure ulcer healing. Archives of Physical Medicine and Rehabilitation 1997;78:1053. [DOI] [PubMed] [Google Scholar]
- Burke DT, Ho CHK, Saucier M, Stewart G. Effects of hydrotherapy on pressure ulcer healing. American Journal of Physical Medicine and Rehabilitation 1998;77(5):394‐8. [DOI] [PubMed] [Google Scholar]
Colombo 1983 {published data only}
- Colombo P. Topical chloroxidating solution in wounds treatment: a controlled trial. Acta Toxicologica et Therapeutica 1993;14(2):65‐72. [Google Scholar]
Della Marchina 1997 {published data only}
- Della Marchina M, Renzi G. Cleansing of wounds and cuts in an elderly patient with a new antiseptic preparation. Chronica Dermatologica 1997;7(6):873‐85. [Google Scholar]
Diekmann 1984 {published data only}
- Diekmann J M. Use of a dental irrigating device in the treatment of decubitus ulcers. Nursing Research 1984;33(5):303‐5. [PubMed] [Google Scholar]
Hartman 2002 {published data only}
- Hartman D, Coetzee JC. Two US practitioners' experience of using essential oils for wound care. Journal of Wound Care 2002;11(8):317‐20. [DOI] [PubMed] [Google Scholar]
Hinz 1986 {published data only}
- Hinz J, Hautzinger H, Stahl KW. Rationale for and results from a randomised, double blind trial of tetrachlorodecaoxygen anion complex in wound healing. Lancet 1986;1(8458):825‐8. [DOI] [PubMed] [Google Scholar]
Kucan 1981 {published data only}
- Kucan JO, Robson MC, Heggers JP, Ko F. Comparison of silver sulfadiazine, povidone‐iodine and physiologic saline in the treatment of chronic pressure ulcers. Journal of the American Geriatrics Society 1981;29(5):232‐5. [DOI] [PubMed] [Google Scholar]
Liu 1999 {published data only}
- Liu Y. Clinical effect on yousuo mixed solution to treat decubitus ulcer. Shanxi Nursing Journal 1999;13(4):174‐5. [Google Scholar]
Saydak 1990 {published data only}
- Saydak SJ. A pilot test of two methods for the treatment of pressure ulcers. Journal of Enterostomal Therapy 1990;17(3):140‐2. [DOI] [PubMed] [Google Scholar]
Toba 1997 {published data only}
- Toba K, Sudoh N, Nagano K, Eto N, Mizuno Y, Ouchi Y. Randomised prospective trial of gentian violet with dibutryl cAMP and povidone iodine with sugar as a treatment for pressure sores infected with methicillin resistant staphyloccocus aureus in elderly patients. Japanese Journal of Geriatrics 1997;34(7):577‐82. [DOI] [PubMed] [Google Scholar]
Van Der Cammen 1987 {published data only}
- Cammen TJM, O'Callaghan U, Whitefield M. Prevention of pressure sores, a comparison of new and old pressure sore treatments. British Journal of Clinical Practice 1987;41(11):1009‐11. [PubMed] [Google Scholar]
Wang 1997 {published data only}
- Wang Z, Ren J, Song J. Clinical and experimental study on the use of Chuangling solution to treat the ulcer. Shanxi Nursing Journal 1997;11(5):200‐3. [Google Scholar]
Additional references
Angeras 1992
- Angeral MH, Brandberg A, Falk A, Seeman T. Comparison between sterile saline and tap water for the cleansing of acute traumatic wounds. European Journal of Surgery 1992;158(6‐7):347‐50. [PubMed] [Google Scholar]
Bates‐Jensen 1992
- Bates‐Jensen BM, Vredevoe DL, Brecht M. Validity and reliability of the pressure sore status tool. Decubitus 1992;5(6):80S‐86S. [PubMed] [Google Scholar]
Beaglehole 1993
- Beaglehole R, Bonita R, Kjellstrom T. Measuring health and disease. Basic Epidemiology. Geneva: World Health Organisation, 1993:13‐20. [Google Scholar]
Bennett 2004
- Bennett G, Dealey C, Posnett J. The cost of pressure ulcers in the UK. Age and Ageing 2004;33(3):230‐5. [DOI] [PubMed] [Google Scholar]
BNF 2003
- BNF. 13.11 Skin Cleansers and Antiseptics. British National Formulary. Oxon: Pharmaceutical Press, 2003:575‐8. [Google Scholar]
Bridel 1996
- Bridel J, Banks S, Mitton C. The admission prevalence and hospital‐acquired incidence of pressure sores within a large teaching hospital during April 1994 to March 1995. Proceedings of the 5th European Conference on Advances in Wound Management. London: Macmillan, 1996.
Carr 2006
- Carr M. Wound Cleansing: sorely neglected?. Primary Intention: the Australian Journal of Wound Management 2006;14:150‐2, 154, 156‐7. [Google Scholar]
Carville 1995
- Carville K. Wound care manual. Wound care manual. Western Australia, Silver Chain Foundation, 1995. [Google Scholar]
Day 2000
- Day SJ, Altman DG. Statistics Notes: Blinding in clinical trials and other studies. British Medical Journal 2000;321(7259):504. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPUAP 2002
- European Pressure Ulcer Advisory Panel. Summary report on the prevalence of pressure ulcers. EPUAP Review 2002;4(2):49‐57. [Google Scholar]
EPUAP/NPUAP 2009
- European Pressure Ulcer Advisory Panel & National Pressure Ulcer Advisory Panel. Prevention and treatment of pressure ulcers: quick reference guide. National Pressure Ulcer Advisory Panel, Washington DC. 2009.
Fergusson 2002
- Fergusson D, Aaron SD, Guyatt G, Herbert P. Post‐randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;325(7365):652‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez 2012
- Fernandez R, Griffiths R, Ussia C. Water for wound cleansing. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD003861.pub3] [DOI] [PubMed] [Google Scholar]
Flanagan 1998a
- Flanagan M. Managing wounds. Access to clinical education; wound management. London: Churchill Livingstone, 1998:51‐66. [Google Scholar]
Fox 2002
- Fox C. Living with a pressure ulcer: a descriptive study of patients' experiences. British Journal of Community Nursing Wound Care Supplement 2002;10:12‐4. [DOI] [PubMed] [Google Scholar]
Gallagher 2008
- Gallagher P, Barry P, Hartigan I, McCluskey P, O'Connor K, O'Connor M. Prevalence of pressure ulcers in three university teaching hospitals in Ireland. Journal of Tissue Viability 2008;17:103‐9. [DOI] [PubMed] [Google Scholar]
Gorecki 2009
- Gorecki C, Brown JM, Nelson EA, Briggs M, Schoonhoven L, Dealey C, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. Journal of the American Geriatrics Society 2009;1(57):1175‐83. [DOI] [PubMed] [Google Scholar]
Greenhalgh 1997
- Greenhalgh T. How to read a paper: assessing the methodological quality of published papers. BMJ 1997;315:305‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haalboom 2000
- Haalboom JRE. Some remarks about overlays in the prevention and treatment of pressure ulcers. EPUAP Review 2000;2(2):67‐70. [Google Scholar]
Hanson 1993
- Hanson D, Langemo D, Olson B. The prevalence and incidence of pressure ulcers in home care: are patients at risk?. Journal of Home Health Care Practice 1993;5(3):25‐32. [Google Scholar]
Hellewell 1997
- Hellewell TB, Najor DA, Foresman PA, Rodeheaver GT. A cytotoxic evaluation of antimicrobial and non antimicrobial wound cleansers. Wounds 1997;9(1):15‐20. [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319:670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iocono 1998
- Iocono JA, Erlich HP, Gottrup F, Leaper DJ. The biology of healing. In: Leaper DJ, Harding KG editor(s). Wounds Biology and Management. Oxford: Oxford Medical Publications, 1998:10‐22. [Google Scholar]
Kaltenthaler 2001
- Kaltenthaler E, Whitfield MD, Walters SJ, Akehurst RL, Paisley S. UK, USA and Canada: how do their pressure ulcer prevalence and incidence data compare?. Journal of Wound Care 2001;10(1):530‐5. [DOI] [PubMed] [Google Scholar]
Keelaghan 2008
- Keelaghan E, Margolis D, Zhan M, Baumgarten M. Prevalence of pressure ulcers on hospital admission among nursing home residents transferred to the hospital. Wound Repair Regeration 2008;16(3):331‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kroger 2008
- Kröger K, Niebel W, Maier I, Stausberg J, Gerber V, Schwarzkopf A. Prevalence of Pressure Ulcers in Hospitalized Patients in Germany in 2005: Data from the Federal Statistical Office. Gerontology 2008;55(3):281‐7. [DOI] [PubMed] [Google Scholar]
Lawrence 1997
- Lawrence JC. Wound Irrigation. Journal of Wound Care 1997;6(1):23‐6. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ligresti 2007
- Ligresti C, Bo F. Wound bed preparation of difficult wounds: an evolution of the principles of TIME. International Wound Journal 2007;4(1):21‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindholm 1999
- Lindohlm C, Bergsten A, Berglund E. Chronic wounds and nursing care. Journal of Wound Care 1999;8(1):5‐10. [DOI] [PubMed] [Google Scholar]
McInnes 2011
- McInnes E, Dumville JC, Jammali‐Blasi A, Bell‐Syer SEM. Support surfaces for treating pressure ulcers. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009490] [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. CONSORT group. Journal of the American Medical Association 2001;285(15):1987‐91. [DOI] [PubMed] [Google Scholar]
Montori 2001
- Montori VM, Guyatt GH. Intention‐to‐treat principle. CMAJ 2001;165(10):1339‐41. [PMC free article] [PubMed] [Google Scholar]
Moore 2000
- Moore Z, Pitman S. Towards establishing a pressure sore prevention and management policy in an acute hospital setting. All Ireland Journal of Nursing and Midwifery 2000;1(1):7‐11. [Google Scholar]
Moore 2011
- Moore Z, Cowman S, Conroy RM. A randomised controlled clinical trial of repositioning, using the 30° tilt, for the prevention of pressure ulcers. Journal of Clinical Nursing 2011;20(17‐18):2633‐44. [DOI] [PubMed] [Google Scholar]
Moore 2012
- Moore Z, Cowman S. Pressure ulcer prevalence and prevention practices in care of the older person in the Republic of Ireland. Journal of Clinical Nursing 2012;21(3‐4):362‐71. [DOI] [PubMed] [Google Scholar]
Morison 1989
- Morison MJ. Wound cleansing‐‐which solution?. Professional Nurse 1989;4(5):220‐5. [PubMed] [Google Scholar]
Nixon 1999
- Nixon J, Smye S, Scott J, Bond S. The diagnosis of early pressure sores: report of the pilot study. Journal of Tissue Viability 1999;9(2):62‐6. [DOI] [PubMed] [Google Scholar]
NPUAP 1989
- National Pressure Ulcer Advisory Panel. Pressure ulcer incidence, economics, risk assessment. Consensus development conference statement. Decubitus 1989;2(2):24‐8. [PubMed] [Google Scholar]
O'Meara 2001
- O'Meara SM, Cullum NA, Majid M, Sheldon TA. Systematic review of antimicrobial agents used for chronic wounds. British Journal of Surgery 2001;88(1):4‐21. [DOI] [PubMed] [Google Scholar]
Posnett 2009
- Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health‐care providers in Europe. Journal of Wound Care 2009;18(4):154‐61. [DOI] [PubMed] [Google Scholar]
Redelings 2005
- Redelings MD, Lee NE, Sorvillo F. Pressure Ulcers: More Lethal Than We Thought?. Advances in Skin and Wound Care 2005;18:367‐72. [DOI] [PubMed] [Google Scholar]
Richardson 1981
- Richardson RR, Meyer PR. Prevalence and incidence of pressure sores in acute spinal cord injuries. Paraplegia 1981;19(4):235‐47. [DOI] [PubMed] [Google Scholar]
Robertson 1990
- Robertson J, Swain I, Gaywood I. The importance of pressure sores in total health care. Pressure sores, clinical practice and scientific approach. Bader DL. London: Macmillan Press, 1990:3‐13. [Google Scholar]
Rolstad 2000
- Rolstad BS, Ovington LG, Harris A. Principles of wound management. In: Bryant RA editor(s). Acute and chronic wounds nursing management. Second Edition. St. Louis, Missouri, USA: Mosby, 2000:85‐124. [Google Scholar]
Schoonhoven 2002
- Schoonhoven L, Defloor T, Grypdonck HHF. Incidence of pressure ulcers due to surgery. Journal of Clinical Nursing 2002;11(4):479‐87. [DOI] [PubMed] [Google Scholar]
Schoonhoven 2006
- Schoonhoven L, Bousema M, Buskens E. The prevalence and incidence of pressure ulcers in hospitalised patients in The Netherlands: A prospective inception cohort study. International Journal of Nursing Studies 2006;44(6):927‐35. [DOI] [PubMed] [Google Scholar]
Schulz 2000
- Schulz KF. Assessing allocation concealment and blinding in randomised controlled trials: why bother?. Evidence Based Medicine 2000;5:36‐8. [DOI] [PubMed] [Google Scholar]
SIGN 2013
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed February 7 2013).
Singer 1994
- Singer AJ, Hollander JE, Subramanian S, Malhotra AK, Villez PA. Pressure dynamics of varying irrigation techniques commonly used in the emergency department. Annals of Emergency Medicine 1994;24(1):36‐40. [DOI] [PubMed] [Google Scholar]
Stotts 2001
- Stotts N. Assessing a patient with a pressure ulcer. In: Morison M editor(s). The prevention and treatment of pressure ulcers. London: Mosby, 2001:99‐115. [Google Scholar]
Thompson 1999
- Thompson JS, Brooks RG. The economics of preventing and treating pressure ulcers: a pilot study. Journal of Wound Care 1999;8(6):312‐6. [DOI] [PubMed] [Google Scholar]
Touche Ross 1993
- Touche Ross. The costs of pressure sores. Touche Ross and Company. London, 1993.
Towler 2001
- Towler J. Cleansing traumatic wounds with swabs, water or saline. Journal of Wound Care 2001;10(6):231‐4. [DOI] [PubMed] [Google Scholar]
Versluysen 1986
- Versluysen M. How elderly patients with femoral fracture develop pressure sores in hospital. British Medical Journal 1986;292(6531):1311‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittington 2000
- Whittington K, Patrick M, Roberts J. A national study of pressure ulcer prevalence and incidence in acute care hospitals. Journal of Wound Care Nursing 2000;24(4):209‐15. [DOI] [PubMed] [Google Scholar]
Young 1995
- Young T. Common problems in wound care: wound cleansing. British Journal of Nursing 1995;4(5):286‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Moore 2008
- Moore Z, Cowman S. A systematic review of wound cleansing for pressure ulcers. Journal of Clinical Nursing 2008;17(15):1963‐72. [DOI] [PubMed] [Google Scholar]


