Abstract
Background
In adolescents with type 1 diabetes, insulin resistance likely plays a role in the deterioration of metabolic control. In type 1 diabetes, addition of metformin to insulin therapy, to improve insulin sensitivity, has been assessed in a few trials involving few patients or in uncontrolled studies of short duration. No systematic reviews are available up to date to summarize the evidence about metformin addition to insulin therapy in adolescents with type 1 diabetes.
Objectives
To assess the effects of metformin added to insulin therapy for type 1 diabetes mellitus in adolescents.
Search methods
We searched The Cochrane Library, MEDLINE and EMBASE. We also searched databases of ongoing trials, reference lists of relevant reviews, and we contacted experts, authors and manufacturers.
Selection criteria
Any randomised controlled trial (RCT) of at least three months duration of treatment comparing metformin added to insulin therapy versus insulin therapy alone in adolescents with type 1 diabetes was included. Cross‐over and quasi‐randomised controlled trials were excluded.
Data collection and analysis
Two reviewers read all abstracts, assessed quality and extracted data independently. Authors were contacted for missing data.
Main results
Only two trials (60 participants) investigating the effect of metformin added to insulin therapy for three months in adolescents with poorly controlled type 1 diabetes could be included. Meta‐analysis was not possible due to the clinical and methodological heterogeneity of data. Both studies suggested that metformin treatment lowered glycosylated haemoglobin A1c (HbA1c) in adolescents with type 1 diabetes and poor metabolic control. Improvements in insulin sensitivity, body composition or serum lipids were not documented in either study, however, one study showed a decrease in insulin dosage by 10%. Adverse effects were mainly gastrointestinal in both studies and hypoglycaemia in one study. No data on health‐related quality of life, all‐cause mortality or morbidity are currently available.
Authors' conclusions
There is some evidence suggesting improvement of metabolic control in poorly controlled adolescents with type 1 diabetes, on addition of metformin to insulin therapy. Stronger evidence is required from larger studies, carried out over longer time periods to document the long‐term effects on metabolic control, health‐related quality of life as well as morbidity and mortality in those patients.
Plain language summary
Metformin added to insulin therapy for type 1 diabetes mellitus in adolescents
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. Metabolic control (glycaemic control, that is long‐term blood glucose levels as measured by glycosylated haemoglobin A1c (HbA1c)) often deteriorates during puberty in children with type 1 diabetes possibly due to the development of insulin resistance (insulin does not work effectively in the tissues anymore) and this creates a great need for alternative therapeutic strategies in those patients. We searched for randomised controlled trials of good quality that studied the effects of metformin added to insulin therapy for type 1 diabetes mellitus in adolescents on glycaemic control, insulin sensitivity, health‐related quality of life, side‐effects as well as effects on body weight, serum lipids and insulin dose. Only two trials (60 participants, three months treatment) could be included. Both studies suggested that metformin plus insulin treatment lowered HbA1c somewhat more than placebo plus insulin. Improvement in insulin sensitivity, body weight or serum lipids were not seen in either study. However, one study showed a small decrease in insulin dosage by 10%. Side effects were mainly gastrointestinal upset in both studies and hypoglycaemia (low blood sugar) in one study. There was no information on health‐related quality of life, costs, morbidity or mortality in either study.
Background
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (that is elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased. For a detailed overview of diabetes mellitus, please see under 'Additional information' in the information on the Metabolic and Endocrine Disorders Group in The Cochrane Library (see 'About', 'Cochrane Review Groups (CRGs)'). For an explanation of methodological terms, see the main glossary in The Cochrane Library.
Metabolic control often deteriorates during puberty in children with type 1 diabetes. Puberty is associated with marked insulin resistance. Moreover, weight gain is prevalent in adolescents with type 1 diabetes after attainment of final height, which might further impair insulin sensitivity. Insulin dosages are often increased to overcome the resistance to insulin, but the metabolic control, however, often deteriorates during the later stages of pubertal development (Mortensen 1998). Other factors contributing to insulin resistance in adolescents with type 1 diabetes are the increase of sex steroids in this period, the hyperglycaemia associated with noncompliance (Yki‐Jarvinen 1997), as well as the disturbance of the insulin‐like growth factor I (IGF‐I) to growth hormone (GH) axis, leading to elevated GH levels (Halldin 1998), resulting in impaired peripheral insulin sensitivity (Caprio 1994; Yki‐Jarvinen 1997).
Description of the intervention
Pubertal changes create a great need for alternative therapeutic strategies in adolescents with type 1 diabetes. One possibility, to accommodate for these changes, is the addition of a drug that improves insulin sensitivity. A candidate for this is metformin, the effect of which regarding insulin sensitivity has been documented (Sarnblad 2003). Metformin belongs to the biguanides class (Saenz 2005; Salpeter 2005). It has been proposed that metformin might increase insulin sensitivity in the liver by inhibiting hepatic gluconeogenesis and thereby reducing hepatic glucose production (Hamilton 2003). It has also been proposed that metformin decreases fatty acid oxidation and intestinal glucose absorption, but the contributions of these effects to the total antihyperglycaemic action is considered to be small (Meyer 2002). Metformin also seems to increase peripheral insulin sensitivity by enhancing glucose uptake in the muscle (Sarnblad 2003).
Adverse effects of the intervention
The reported side effects of metformin are: hypoglycaemia, lactic acidosis, poor compliance and gastrointestinal upset (Gin 1982; Hamilton 2003; Sarnblad 2003). One study (Meyer 2002) debated the appropriateness of metformin use for people with type 1 diabetes; given the potential for coexisting lactic acidosis and diabetic ketoacidosis, and that the minimal reduction of daily insulin requirements, does not equal the risk of severe hypoglycaemia. Furthermore, it is likely that the incidence of hypoglycaemia is much greater if more aggressive metabolic targets are applied. Despite the failure to observe diabetic ketoacidosis, the limited number and short period of observation do not permit the conclusion that metformin is safe in ketosis‐prone diabetic individuals (Meyer 2002). Therefore, the question of safety of metformin use in type 1 diabetes is still questionable (Aldasouqi 2003; Faichney 2003; Misbin 1998).
How the intervention might work
Metformin has mainly been used in adult patients with type 2 diabetes and several studies have shown beneficial effects on body weight, blood lipid levels and metabolic control (Howlet 1999; Mehnert 2001; UKDPS 1998). Moreover, randomised controlled trials with metformin, in adolescents with type 2 diabetes, noted an improvement in fasting plasma glucose (Jones 2002). Similarly, studies done in type 1 diabetes, demonstrated different combinations of the following: reduction of glycosylated haemoglobin A1c (HbA1c), increased insulin sensitivity, decreased dosage of insulin, decreased body mass index (BMI) and improvement of lipid profile (Hamilton 2003; Meyer 2002; Sarnblad 2003). The insulin‐sparing effect during metformin therapy in patients with type 1 diabetes has been reported to be around 25% (Golay 1995). It is reasonable to speculate that the main effect of metformin in adolescents with type 1 diabetes is associated with improved peripheral insulin sensitivity. This is in contrast to patients with type 2 diabetes, where the effect is mainly mediated by decreased hepatic glucose output (Hundal 2000).
Why it is important to do this review
In adolescents with type 1 diabetes, insulin resistance likely plays a role in the deterioration of metabolic control seen in this age group (Sarnblad 2003). In the Diabetes Control and Complications Trial (DCCT), adolescents achieved HbA1c levels that were on average 1% higher than in adults in both the conventional and intensive treatment groups, despite receiving more insulin (units per kilogram body weight) and having increased weight gain (DCCT 1994). This triad of high HbA1c, high insulin dosage, and weight gain suggests that the insulin administered was less effective in maintaining glycaemic control in the adolescent cohort. Therefore, oral agents used to treat type 2 diabetes may be a useful adjunctive therapy in individuals with type 1 diabetes and insulin resistance (Jones 2002). The biguanide, metformin, acts primarily by decreasing hepatic glucose output, but also affects insulin sensitivity. Both mechanisms may benefit the insulin‐resistant individual with type 1 diabetes (Sarnblad 2003).
In type 1 diabetes, addition of metformin to insulin therapy has been assessed in a few trials involving few patients or in uncontrolled studies of short duration (Gin 1982; Janssen 1991; Pagno 1983). These studies suggested a mean reduction in insulin resistance of 25% with a variation of 20% to 40%. Several other studies were reviewed by Daniel and Hagmeyer (Daniel 1997); most of them, however, were conducted before the introduction of HbA1c with small samples of mainly adult patients. Thus, the clinical interest of metformin in the treatment of type 1 diabetes has remained questionable. One study (Meyer 2002) showed that a small subset of type 1 diabetic patients benefited in terms of insulin dose reduction when metformin was added to insulin, however, questions about long‐term safety and efficacy in this patient population remained unanswered. Moreover, there have been conflicting reports from studies in adolescents with type 1 diabetes (Desmangles 2000; Hamilton 2003; Meyer 2002; Sarnblad 2003; Walravens 2000). The benefit was transient in one study (Walravens 2000) and negative in another (Desmangles 2000). The main drawback of these studies was the small sample size, and the lack of reporting on long‐term benefit and safety of adjunctive therapy in many of them. No systematic reviews are available up to date to summarize the evidence about metformin addition to insulin therapy in this cohort.
Objectives
To assess the effects of metformin added to insulin therapy for type 1 diabetes mellitus in adolescents.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials.
Inclusion criteria
Any randomised controlled trial of at least three months duration of treatment comparing metformin added to insulin therapy versus insulin therapy alone in adolescents with type 1 diabetes was included.
Exclusion criteria
Cross‐over and quasi‐randomised controlled trials.
Types of participants
Adolescents (between 12 and 20 years) diagnosed as having type 1 diabetes. To be consistent with changes in classification and diagnostic criteria of type 1 diabetes mellitus through the years, the diagnosis should have been established using the standard criteria valid at the time of the beginning of the trial (for example ADA 1999; WHO 1980; WHO 1985; WHO 1998). Ideally, diagnostic criteria should have been described. If necessary, authors' definition of type 1 diabetes mellitus were used. Diagnostic criteria were planned to be subjected to a sensitivity analysis.
Types of interventions
Metformin added to insulin therapy versus placebo added to insulin therapy.
Types of outcome measures
Primary outcomes
glycaemic control measured by glycosylated haemoglobin A1c (HbA1c) and postprandial glucose;
adverse effects of metformin (for example nausea, diarrhoea);
health‐related quality of life if measured by a validated instrument.
Secondary outcomes
change in insulin dose;
change of body mass index (BMI) or body weight or both;
change of serum lipids;
change in peripheral insulin sensitivity (assessed by a euglycaemic hyperinsulinaemic clamp);
costs of metformin therapy;
mortality and morbidity ‐ (all‐cause and diabetes related).
Covariates, effect modifiers and confounders
Patient compliance with treatment regimen, patient education, and duration of diabetes.
Timing of outcome measurement
We collected data at baseline and at the end of the study. Any length of follow up was included. The minimum duration of treatment was considered to be three months.
Search methods for identification of studies
Electronic searches
We used the following sources in the literature search for the identification of relevant trials:
The Cochrane Library (issue 2, 2008);
MEDLINE (until August 2008);
EMBASE (until August 2008).
The overall search strategy combined searches for type 1 diabetes and metformin, insulin therapy and adolescents, with searches for randomised controlled trials. The described search strategy (see Appendix 1 for a detailed search strategy) was used for MEDLINE. For use with EMBASE, The Cochrane Library and the other databases this strategy was slightly adapted.
When additional key words of relevance were detected during any of the electronic or other searches, electronic search strategies were modified to incorporate these terms.
We also searched databases of ongoing trials (latest access March 2008):
Current Controlled Trials (http://www.controlled‐trials.com ‐ with links to other databases of ongoing trials);
UK National Research Register (http://www.update‐software.com/National/nrr‐frame.html);
USA ‐ CenterWatch Clinical Trials Listing Service (http://www.CenterWatch.com/);
USA ‐ National Institutes of Health (http://clinicalstudies.info.nih.gov/);
Dutch Trial Register (Nederlands Trial Register) (http://www.trialregister.nl/).
Searching other resources
In addition, we hand searched abstracts of major diabetes conferences (American Association of Diabetes (ADA), European Association for the Study of Diabetes (EASD)). We also contacted pharmaceutical companies (Takeda, Bristol‐Myers Squibb, Lilly) for unpublished trial data of relevant trials. We planned to obtain full text translations or evaluations of all relevant non‐English articles or both.
We tried to identify additional studies published in different languages, by searching the reference lists of included trials and (systematic) reviews, meta‐analyses and health technology assessment reports noticed.
Data collection and analysis
Selection of studies
To determine the studies to be assessed further, two authors (SA and AA) independently scanned the abstract, titles or both sections of every record retrieved. All potentially relevant articles were investigated as full text. When a title or abstract could not be rejected with certainty, the full text of the article was obtained. Interrater agreement for study selection was measured using the kappa statistic (Cohen 1960). Differences were determined and it was planned that if these studies were later on included, the influence of the primary choice would be subjected to a sensitivity analysis. Articles were only rejected on initial screen if we could clearly determine from the title and abstract that the article was not a report of a randomised controlled trial, or the trial did not address the research question, or the trial was of less than three months duration. An adapted QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection was attached (Moher 1999).
Dealing with duplicate publications
In the case of duplicate publications and companion papers of a primary study, we intended to maximise yield of information by simultaneous evaluation of all available data. In cases of doubt, the original publication (usually the oldest version) would obtain priority.
Data extraction and management
For studies that fulfilled the inclusion criteria, two authors (SA and AA) independently abstracted relevant population and intervention characteristics using standard data extraction templates with any disagreements resolved by discussion. Any relevant missing information on the trial was sought from the original author(s) of the article, if required.
Assessment of risk of bias in included studies
Two authors (SA and AA) assessed each trial independently. Such quality was assessed using the criteria set out by Jadad and Schultz (Jadad 1996; Schultz 1995) as described in the Cochrane Handbook for Systemic Reviews of Interventions (Higgins 2008). Possible disagreement was resolved by consensus. Interrater agreement for the key quality indicator (concealment of allocation) was planned to be calculated using the kappa statistic (Cohen 1960).
The following criteria were used: (1) minimization of selection bias: a) Was the randomisation procedure adequate? b) Was the allocation concealment adequate? (2) minimization of performance bias: Were the patients and people administering the treatment blind to the intervention? (3) Minimization of attrition bias: a) Were withdrawals and drop‐outs described completely? b) Was analysis by intention‐to‐treat? (4) Minimization of detection bias: Were measures objective (glycosylated haemoglobin A1c (HB A1c), mortality) or were outcome assessors blind to the intervention? Based on these criteria, studies were subdivided into one of the following three categories: A ‐ all quality criteria met: low risk of bias. B ‐ one or more of the quality criteria only partly met: moderate risk of bias. C ‐ one or more criteria not met: high risk of bias.
Measures of treatment effect
We expected both event (dichotomous) data and continuous data.
Dichotomous data
For dichotomous data, we extracted numbers of participants experiencing an outcome and total number of participants randomised in each study arm. Dichotomous outcome data (for example side effects of metformin (yes/no)) were planned to be expressed as odds ratios (OR) or relative risk (RR) with 95% confidence intervals (CI). Where appropriate, the risk difference (RD) was intended to be calculated as well as the number needed to treat (NNT), taking into account baseline differences and time.
Continuous data
For continuous outcomes, means and standard deviations of the initial and final readings in each arm were extracted together with details of change if available. Change of a measure was calculated from baseline to the end of the study with a minimum duration of the intervention of three months. Continuous outcomes (for example metabolic control as measured by HbA1c, insulin dose, serum lipids, body mass index, insulin sensitivity) were planned to be expressed and calculated, as weighted mean differences of the change between treatment and control groups with 95% CI.
Time‐to‐event data
Time to event outcomes were planned to be expressed as hazard ratios (HR) with 95% confidence intervals (CI).
Dealing with missing data
Authors were contacted to provide relevant missing data, if feasible, and the impact of missing data was planned to be discussed. Missing data were planned to be quantified and characterised as pre‐randomisation, immediately post‐randomisation or drop‐out during the intervention period. Evaluation of important numerical data such as screened, eligible and randomised patients as well as intention‐to‐treat (ITT) and per‐protocol (PP) population were carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of last‐observation‐carried‐forward (LOCF), ITT and PP was planned to be critically appraised and compared to specification of primary outcome parameters.
Assessment of heterogeneity
In the event of substantial clinical or methodological or statistical heterogeneity, study results were not combined by means of meta‐analysis. Heterogeneity was planned to be identified by visual inspection of the forest plots, by using a standard χ2‐test and a significance level of α = 0.1, in view of the low power of such tests. Heterogeneity was intended to be specifically examined with I2 (Higgins 2002), where I2‐values of 50% and more indicated a substantial level of heterogeneity (Higgins 2003). When heterogeneity was found, we attempted to determine potential reasons for it by examining individual study characteristics (sensitivity analyses) and those of subgroups of the main body of evidence.
Assessment of reporting biases
We planned to use funnel plots in an exploratory data analysis to assess for the potential existence of small study and publication bias. There are a number of explanations for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to study size, poor methodological design of small studies and publication bias (Sterne 2001). Therefore, results would have been interpreted carefully (Lau 2006).
Data synthesis
We planned to summarise data statistically if they were available, sufficiently similar and of sufficient quality. Statistical analysis was planned to be performed according to the statistical guidelines referenced in the newest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). Differences between groups were planned to be pooled across studies by calculating a weighted treatment effect based on means for continuous data and either odds or risk ratios for dichotomous data using the generic inverse variance method.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to be mainly performed if one of the primary outcome parameters demonstrated statistically significant differences between intervention groups. In any other case subgroup analyses would be clearly marked as a hypothesis generating exercise.
Where possible, the impact of sex (male/female) was intended to be explored through subgroup analysis
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size:
repeating the analysis excluding unpublished studies;
repeating the analysis taking account of study quality, as specified above;
repeating the analysis excluding any very long or large studies to establish how much they dominate the results;
repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
The robustness of the results was also to be tested by repeating the analyses using different measures of effects size (risk difference, odds ratio etc.) and different statistical models (fixed and random effects models).
Results
Description of studies
Results of the search
For an overview of the QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection see Figure 1.
1.
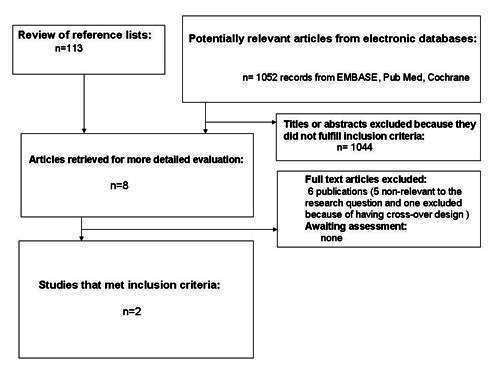
QUOROM (quality of reporting of meta‐analyses) flow‐chart of study selection
MEDLINE: 65 records were retrieved and assessed on basis of the title or abstract, or both (until end of August 2008). We identified eight studies which could not be assessed by scrutiny of the title and abstract, only. On assessing the full text, five were found to be non‐relevant, two were included in the final review and one was excluded.
EMBASE: 75 records were retrieved and assessed on basis of the title or abstract, or both (until end of August 2008), eight records were initially included for further reading (the same retrieved through MEDLINE). On assessing the full text, five were found non‐relevant, two were included in the final review and one was excluded.
The Cochrane Library: 65 records were retrieved and assessed on basis of the title or abstract, or both (until end of July 2008), four records were initially included for further reading, two were included in the final review (the same as from MEDLINE and EMBASE).
Databases of ongoing trials mentioned below: No relevant trials were detected.
Experts: We obtained one reference by corresponding with experts or authors. It was an abstract published in a conference proceeding and was later on excluded because the full text could not be accessed and the details of the methodology and results were inadequately presented (Walravens 2000).
Manufacturers: Takeda the developer of metformin, Bristol‐Myers Squibb, and Lilly did not report ongoing relevant trials or unpublished studies.
Hand searching (checking references of existing reviews, browsing the Internet, posters on congress etc.): Two references found by hand searching were excluded because of their non‐RCT design.
Interrater agreement Interrater agreement for study selection was 0.94. Differences in opinions were resolved through open discussion.
Missing data We contacted all authors for data clarification and missing data.
No unpublished data were available for analysis.
Included studies
Two studies with 60 participants, described in two articles were finally included in the review. Details are given in Characteristics of included studies. The two included studies were published as journal articles (Hamilton 2003, Sarnblad 2003).
Participants
In both studies, participants were poorly controlled adolescents with type 1 diabetes
Trial design
Both included studies were randomised, double‐blind, placebo‐controlled studies.
Outcome measures
In both studies, outcome were metabolic control as measured by glycosylated haemoglobin A1c (HbA1c) and blood glucose; furthermore insulin sensitivity was determined in one study by euglycaemic hyperinsulinaemic clamp (Sarnblad 2003), and in the other by frequently sampled blood glucose after intravenous glucose tolerance test (Hamilton 2003). Other outcome measures reported by both studies were the effect on insulin dosage, blood lipids, body mass index (BMI), waist circumference or side effects as gastrointestinal upset and hypoglycaemia.
Excluded studies
One study (Schatz 1975) was excluded because of having a cross‐over design, and it was not focused on adolescents (it included children from the age of 4 to 16 years). Another study (Walravens 2000) was excluded because although it was mentioned to be a randomised, placebo‐controlled, double‐blind study it was published as an abstract only. The authors were contacted, but did not respond to date.
Risk of bias in included studies
There was complete agreement among the two authors over the methodological quality of both included studies. The overall quality was roughly assessed on a three‐point scale according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008): both studies scored B (moderate risk of bias). A summary of risk of bias characteristics is given in Appendix 2.
Allocation
One study had both an adequate randomisation and allocation concealment (Hamilton 2003). In the other study (Sarnblad 2003), the method of randomisation and the concealing of allocation was unclear. The first author of the latter study did not respond to asking for additional data.
Blinding
Double‐blinding was stated in both studies, but there was no precise information about who actually was masked. There was no mention about blinding of outcome assessors in either study. There was no response from first authors of both studies about these conditions.
Incomplete outcome data
Drop‐out rates were relatively high in both studies (10% and 20%) in Hamilton's and Sarnblad 's studies, respectively. Neither study used intention‐to‐treat analysis. However, withdrawals, losses to follow‐up and drop‐outs were adequately described and reasons were mentioned in both studies.
Screened and randomised patients
Number of screened patients were not mentioned in either study, only the number of those randomised were reported.
Other potential sources of bias
Definition of primary and secondary endpoints
Both primary and secondary endpoints were defined in both studies. Both studies reported the effect of addition of metformin on glycosylated haemoglobin A1c, blood glucose and mentioned side‐effects of metformin. Secondary endpoints such as body mass index, insulin sensitivity and serum lipids were also assessed in both studies. However, there was no information about health‐related quality of life, costs, all‐cause or diabetes related mortality or morbidity in either study.
Power calculation
In Hamilton 2003 study, a planned sample size of 32 participants was estimated to give a power of 80% to detect a difference of change of 30% improvement in insulin sensitivity between metformin and placebo groups at a two‐sided 0.05 significance level. However, the actual sample size was smaller than that required (27 participants). In the Sarnblad 2003 study, an estimated sample size of 34 participants would give a power of 80% to detect a difference in HbA1c of 1% (with SD 1.0%) and a two‐sided 0.05 significance level. However, only 30 patients were randomised and only 26 of them completed the trial.
Compliance measures
Compliance was measured in both studies. Hamilton 2003 defined compliance as acceptable if less than 25% of the prescribed pills were returned at each assessment. On the other hand, Sarnblad 2003 defined poor compliance by the number of missed doses (10% of the total doses during the study period or more than seven consecutive days without treatment).
Funding
In the Hamilton 2003 study, grants were provided from the Hospital for Sick Children Research Institute and the Order of the Eastern Star of Ontario. Drug and placebo were provided by Aventis Pharma. In the Sarnblad 2003 study drug and placebo were provided by Merck AB, Pharma division.
Publication status
Both studies were published in peer reviewed journals.
Effects of interventions
Characteristics and results of the two included studies are shown in Appendix 3, Appendix 4, Appendix 5 and Appendix 6.
Primary outcomes
Glycaemic control
In one study (Hamilton 2003), glycosylated haemoglobin A1c (HbA1c) was 0.6% lower in the metformin group than in the placebo group (P < 0.035) after three months of therapy. Change of mean HbA1c at the end of study was ‐0.3 % (0.7) in the metformin group and 0.3 % (0.7) in the placebo group (P = 0.03). Mean change of fasting blood glucose was ‐0.9 mmol/L (3.8) in the metformin group and ‐0.5 mmol/L (3.2) in the placebo group (P = 0.04).
The effect on HbA1c was confirmed by Sarnblad 2003 who showed a change of mean HbA1c of ‐0.9% in the metformin group compared to ‐ 0.3% in the placebo group, after three months of metformin therapy. During the study period, the mean HbA1c value decreased from 9.6% (1.0) to 8.7% (1.5) in the metformin group, but remained unchanged (9.5% (1.2) to 9.2% (1.3)) in the placebo group. However, there was no significant change in mean glucose concentrations during steady‐state euglycaemic hyperinsulinaemic clamps in either group.
Adverse effects
The metformin group experienced more side effects compared to the placebo group in the Hamilton 2003 study (73% versus 47%). Two patients (13%) dropped out due to side effects in the metformin group versus one participant (7%) in the placebo group. Severe hypoglycaemia occurred in two patients (13% ) in the metformin group and one participant (7%) in the control group, while mild hypoglycaemia occurred more frequently in the metformin than in the placebo group after three months of therapy: mean 1.75 (0.8) versus mean 0.9 (0.4) events per patient and week, respectively (P = 0.03). Gastrointestinal upset occurred in nine patients (60%) in the metformin group (two of them reported as serious) versus five patients (33%) in the placebo group who had only mild gastrointestinal upset. No lactic acidosis or ketoacidosis occurred in any of the patients in either group. Sarnblad 2003 reported less side effects in the metformin group versus the placebo group (19% versus 43%). No hypoglycaemia or serious side effects were reported. Only one patient dropped out in the metformin group due to nausea.
Health‐related quality of life
We found no data for health‐related quality of life in either study.
Secondary outcomes
Change in insulin dose
Hamilton 2003 reported a significant change in the mean daily insulin dose in the metformin group in comparison to the placebo group after three months of metformin therapy of ‐0.14 (0.1) versus 0.02 (0.2), P = 0.01. However, Sarnblad 2003 did not find a significant difference between the metformin and placebo groups regarding the daily insulin dosage after three months of therapy (1.1 (0.3) versus 1.3 (0.2)).
Change of body mass index (BMI) or body weight
No significant changes in mean BMI in the metformin versus the placebo group after three months of metformin therapy were reported by Hamilton 2003 which was confirmed by Sarnblad 2003. There were also non‐significant changes in body weight, waist circumference or waist‐to‐hip ratio.
Change of serum lipids
Both studies reported non‐significant differences in serum lipids (triglycerides and cholesterol).
Change in peripheral insulin sensitivity
Hamilton 2003 calculated insulin sensitivity according to the minimal model (MINMOD) formulas using the MINMOD computer software (Bergman 1989) and found no significant changes in mean insulin sensitivity, measured by frequently sampled glucose after intravenous glucose tolerance test, after three months of metformin therapy in the metformin versus the placebo group. At the end of the 12‐week study period, the change in insulin sensitivity was not statistically significantly different between the two groups. Similarly, Sarnblad 2003 calculated "M" as the amount of glucose infused during the last 60 min after a steady‐state was achieved, while "I" was measured as the mean insulin concentration during steady‐state after 60 min of glucose infusion. No significant change in M/I after three months were found using the euglycaemic hyperinsulinaemic clamp technique. There were no significant differences between the groups in either of the two clamps. Neither the M/I‐values nor the M‐values were significantly different between the groups at baseline or after three months. The M‐values were unchanged in both groups. M/I, however, increased significantly in the metformin group during the study (P < 0.05), but was unchanged in the placebo group. In the metformin group, change in insulin sensitivity (M/I) showed no association with initial HbA1c, insulin dosage or change in insulin dose. However, there was a significant positive association between change in insulin sensitivity and initial M/I (r = 0.77; P < 0.01), indicating that patients with lower initial insulin sensitivity benefited most from metformin treatment.
Costs
Not reported in either study.
Mortality and morbidity
We found no data for mortality or morbidity in either study.
Heterogeneity
Because only two studies could be included in the review, formal testing of heterogeneity was not performed. The two included studies were reasonably homogeneous with respect to the following: using oral metformin added to insulin therapy in adolescents with poorly controlled type 1 diabetes and assessing some common outcome measures: HbA1c, insulin sensitivity, blood glucose, body mass index, body weight, lipid profiles, and the presence of side effects such as gastrointestinal upset or hypoglycaemia.
However, the studies were clearly heterogenous with respect to the following:
Clinical Heterogeneity
1. Age: although both trials recruited adolescents, the age range of patients in the Sarnblad 2003 study was 14 to 20 years, while in the Hamilton 2003 study, the age range was 12 to 17 years.
2. Sex: the female/male ratio was 21/9 in one study (Sarnblad 2003) and 14/13 in the other (Hamilton 2003).
Methodological Heterogeneity
1. Dose of metformin differed in the included studies. In one study (Sarnblad 2003), the dose was 1000 mg twice daily, while in the other (Hamilton 2003), it differed according to the body weight: 1000 mg/day (500 mg twice daily) for those weighing less than 50 kg, 1500 mg/day for those weighing 50 to 75 kg, or 2000 mg/day for those weighing more than 75 kg.
2. Peripheral insulin sensitivity was measured differently in both studies. Sarnblad 2003 assessed it using the euglycaemic hyperinsulinaemic clamp while Hamilton 2003 used frequently sampled glucose after intravenous glucose tolerance test.
3. Blood glucose was estimated at different times in both studies. Sarnblad 2003 measured it during steady‐state while the euglycaemic hyperinsulinaemic clamp was being performed, while Hamilton 2003 measured it in the fasting state.
Measurements
1. BMI and body weight were presented as mean (SD) in one study (Hamilton 2003) and as median (range) in the other (Sarnblad 2003). 2. Values of peripheral insulin sensitivity were presented as geometric mean in one study (Hamilton 2003) and as median (range) in the other study (Sarnblad 2003). 3. HbA1c and insulin dose after three months of metformin therapy were presented in one study as mean (SD) (Sarnblad 2003), while the other one only mentioned the change in mean values and SD from baseline to end of study after three months of therapy (Hamilton 2003).
Compliance
Of the 27 participants who completed the Hamilton 2003 study, 11 (79%) of the metformin‐treated participants and 8 (62%) of the placebo‐treated participants were compliant with the the intake of tablets (less than 25% of the prescribed pills were returned at each assessment).
According to the Sarnblad 2003 definition of poor compliance (10% of the total doses during the study period or more than seven consecutive days without treatment), two patients receiving placebo but none of the metformin group showed poor compliance. There was no correlation between the number of missed doses and changes in HbA1c or daily dosage of insulin during this study.
Sensitivity analyses, sub‐group analyses, meta‐regression analyses, small study bias
No analysis was performed.
Publication bias
No unpublished studies were available for analysis. Funnel plots were not drawn because only two studies could be included.
Discussion
Summary of main results
In this systematic review, we found some evidence that metformin added to insulin therapy in adolescents with poorly controlled diabetes can lead to better glycaemic control, demonstrated by significantly decreased glycosylated haemoglobin A1c (HbA1c) in the two included studies and by decreased fasting blood glucose in the Hamilton 2003 study. However, there was no significant change in peripheral insulin sensitivity in both studies three months following the addition of metformin. The evidence related to side‐effects was conflicting. The metformin group experienced more side effects compared to the placebo group in the Hamilton 2003 study in the form of hypoglycaemia and gastrointestinal upset, while the opposite was demonstrated by the Sarnblad 2003 study with less side‐effects in metformin compared to the placebo group. There was no significant effect on body mass index or serum lipids in either study. There were no data on healt‐related quality of life, costs, all‐cause or disease‐specific mortality or morbidity in either study.
Overall completeness and applicability of evidence
Effect on glycaemic control
There is evidence from both studies, that addition of metformin improves metabolic control in the form of decrease of HbA1c, while decrease of blood glucose was evident in only one study. This improvement may have been attributable to the direct impact of metformin on peripheral tissues, but more likely was secondary to the effects on decreased hepatic glucose output (Hundal 2000). However, both trials were of short duration and it is the long‐term glycaemic control that should be monitored and is actually more important in clinical practice.
Effect on peripheral insulin sensitivity
Puberty is associated with marked insulin resistance, mainly affecting peripheral glucose utilisation and to a less extent fat metabolism. Insulin dosages are often increased to overcome the resistance to insulin, but the metabolic control, however, often deteriorates during the later stages of pubertal development. It has been proposed that metformin might increase insulin sensitivity in the liver by inhibiting hepatic gluconeogenesis and thereby reducing hepatic glucose production (Hamilton 2003). It may also decrease fatty acid oxidation and intestinal glucose absorption as well as enhance glucose uptake in the muscle (Sarnblad 2003).
However, both studies, included in this review, failed to demonstrate a statistically significant effect of metformin therapy on peripheral insulin sensitivity. Sarnblad 2003 used the euglycaemic hyperinsulinaemic clamp technique. They estimated the peripheral glucose uptake but not the hepatic glucose production and found an improved glucose uptake in the patients treated with metformin but not in the placebo group. They also observed that more insulin‐resistant patients benefited most from metformin treatment, as there was an association between initial M/I and both change in HbA1c and change in insulin sensitivity. These results emphasise that metformin effect on peripheral insulin sensitivity seems to be of importance for the obtained metabolic effect in insulin‐resistant adolescents with type 1 diabetes, although simultaneous effects on hepatic glucose production can not be excluded.
Hamilton 2003 used the frequently sampled i.v. glucose tolerance test, which was originally designed for participants with residual pancreatic insulin secretion. Although they used an insulin‐modified test, they experienced major methodological problems and their patients were not kept normoglycaemic overnight. Fasting blood glucose levels were thus negatively correlated to insulin sensitivity (SI), and glucose levels tended to increase during the end of the test resulting in a false high SI. The SD in the study population was much wider than anticipated and likely was related to difficulties in calculating SI in this population by the MINMOD analysis method (Bergman 1989). Ambient glucose at the start of the study correlated with SI; thus, for this test to be improved upon in type 1 diabetes, strict stabilization of blood glucose at the onset may be necessary. Further work to determine the most appropriate modifications of this technique in type 1 diabetes may make it a more reliable tool in this setting.
Effect on insulin dose
Hamilton 2003 reported a significant reduction in mean daily insulin dose after three months of metformin therapy ( ‐0.14 (0.1), P = 0.01). The insulin‐sparing effect during metformin therapy in patients with type 1 diabetes has been reported to be around 25% in previous studies probably due to the improvement of peripheral insulin sensitivity (Gin 1982; Janssen 1991; Pagno 1983).
On the contrary, Sarnblad 2003 did not find any reduced need for insulin three months after metformin therapy. This might be explained by the fact that the population was selected from adolescents with poorly controlled type 1 diabetes. A reduction of the insulin dosage was not the primary goal of the study. On the other hand, if the study had been longer and the effect on metabolic control sustained, effects on the daily dosage of insulin, might have been observed.
Potential biases in the review process
As far as we are aware of, no systematic review has been done with an exclusive focus on the value of addition of metformin to insulin therapy in adolescents with type 1 diabetes. One of the main strengths of this review is the rigourness and completeness of the search. However, it is remarkable that only two trials were retrieved by comprehensive searching (MEDLINE, EMBASE, The Cochrane Library, databases of ongoing trials as well as by hand searching) which emphasizes the deficiency of RCTs in this area. Second, the a‐priori decision to include randomised trials only with a duration of at least three months ensured a minimum level of quality, and at the same time, the relevance of the review to guide clinical practice. Third, we assessed many different outcomes in the review which enables the readers to judge by themselves what matters most for their own particular question. Finally, we think that the tables and the extensive provision of all outcome data and information related to quality and heterogeneity, makes the review transparent.
It is clear that the main limitations are the missing and unclear data about the two trials. Corresponding authors were contacted via emails but no further data were submitted up‐to‐date. The heterogeneity of the studies hampered the performance of meta‐analysis, so we could not confirm the beneficial effects of metformin therapy on improvement of insulin sensitivity, decrease of insulin dose or ensure the safety of the tested drug regimen. Even the analysis of the effect on glycaemic control has to be interpreted with caution since the measurements of glycosylated haemoglobin were not standardised among studies and reference ranges demonstrated distinct dissimilarities. Moreover, many patient‐oriented outcomes like health‐related quality of life or diabetes complications and mortality were never investigated in high‐quality randomised clinical trials and so these important primary outcomes could not be assessed in this review.
Agreements and disagreements with other studies or reviews
Use of metformin, along with insulin therapy, has been studied less frequently in type 1 than in type 2 diabetes, but insulin‐sparing effects of metformin have been observed (Gin 1982; Golay 1995; Janssen 1991; Pagno 1983). Most of these studies have been small (Gin 1982), were uncontrolled (Janssen 1991), or were cross‐over trials of short duration (Pagno 1983). Even in one previous trial involving the administration of insulin by continuous subcutaneous insulin infusion (CSII), the duration of treatment was only three weeks (Bending 1987), and during this period, insulin resistance was not modified. Pagno et al. (Pagno 1983) showed the most marked reduction in insulin resistance in type 1diabetic patients using large doses of metformin (850 mg three times a day). However, the 25% reduction in insulin resistance observed during 24‐h euglycaemic clamp did not really correspond to insulin needs in clinical practice. In one study (Meyer 2002), the maximum effect of metformin in reducing insulin resistane was not seen until after four months of treatment, followed thereafter by a stabilization period. In contrast, previous studies showed that the insulin sparing effect in type 1 diabetes occurred after a few days (Coscelli 1984) or a few weeks of metformin use (Janssen 1991; Pagno 1983). Preliminary results of two studies examining metformin treatment in teens with type 1 diabetes have been presented (Desmangles 2000; Walravens 2000). The first, an open‐label, randomized study of five adolescents taking 500 to 1000 mg metformin daily found no improvement in HbA1c or a decrease in insulin dosage after six months (Desmangles 2000). This study was limited because it was small, uncontrolled, and used low metformin dosages. The second was a larger randomised controlled trial, with metformin administered as 500 mg twice daily for six months in 80 adolescents with poor metabolic control and type 1 diabetes (Walravens 2000).
Authors' conclusions
Implications for practice.
Metformin added to insulin therapy might be used in clinical practice in adolescents with type 1 diabetes who are poorly controlled and show evidence of insulin resistance, taking into consideration the side effects of metformin therapy while balancing the benefits and harms of therapy for individual patients.
Implications for research.
Metformin represents a novel adjunctive therapy worthy of further investigation, that may improve metabolic control in teens with type 1 diabetes. Further, larger studies, carried out over longer time periods, are recommended to document the long‐term safety and efficacy of this regimen. Health‐related quality of life, costs, all‐cause and disease‐specific mortality and morbidity are important patient‐oriented outcomes that should be reported in future studies to guide clinical practice.
What's new
| Date | Event | Description |
|---|---|---|
| 6 November 2008 | Amended | Converted to new review format. |
Acknowledgements
Special thanks to Prof. Jill Hamilton and Prof. Stefan Sarnblad for providing us with an abstract that could not be retrieved via electronic databases (Walravens 2000).
Appendices
Appendix 1. Search strategy
| Search terms |
| Unless otherwise stated, search terms were free text terms; exp = exploded MeSH: Medical Subject Heading (Medline medical index term); the dollar sign ($) stands for any character(s); the question mark (?) = substitute for one or no characters; ab = abstract; ti = titel; ot = original titel; pt = publication type; sh = MeSH: Medical subject heading (MEDLINE medical index term); adj = adjacency. I. Metformin: 1.exp Biguanides/ 2.(metformin$ or glucophag$ or biguanid$).ab,ti,ot. 3.1 or 2 II. Insulin therapy: 4.Insulin/ad, aa, tu, th [Administration & Dosage, Analogs & Derivatives, Therapeutic Use, Therapy] 5.(insulin$ adj6 (therap$ or treatment$ or intervention$)).ab,ti,ot. 6.4 or 5 III. Diabetes mellitus: 7.exp diabetes mellitus/ 8.diabet$.ab,ti,ot. 9.(IDDM or NIDDM or MODY or T1DM or T2DM).ab,ti,ot. 10.((typ$ 1 or typ$ 2) and diabet$).ab,ti,ot. 11.((typ$ I or typ$ II) and diabet$).ab,ti,ot. 12.insulin$ secret$ dysfunc$.ab,ti,ot. 13.impaired glucose toleran$.ab,ti,ot. 14.exp Glucose Intolerance/ 15.glucose intoleran$.ab,ti,ot. 16.exp Insulin Resistance/ 17.insulin$ resist$.ab,ti,ot. 18.(non insulin$ depend$ or noninsulin$ depend$ or non insulin?depend$ or noninsulin?depend$).ab,ti,ot. 19.(insulin$ depend$ or insulin?depend$).ab,ti,ot. 20.metabolic$ syndrom$.ab,ti,ot. 21.(pluri metabolic$ syndrom$ or plurimetabolic$ syndrom$).ab,ti,ot. 22.(late onset adj diabet$).ab,ti,ot. 23.(maturity onset adj diabet$).ab,ti,ot. 24.(juvenile adj diabet$).ab,ti,ot. 25.(syndrome X and diabet$).ab,ti,ot. 26.hyperinsulin$.ab,ti,ot. 27.insulin sensitiv$.ab,ti,ot. 28.or/7‐27 29.exp diabetes insipidus/ 30.diabet$ insipidus.ab,ti,ot. 31.29 or 30 32.28 not 31 IV. RCT/CCT (sensitive search): Part 1: 33.randomized controlled trial.pt. 34.controlled clinical trial.pt. 35.randomized controlled trials.sh. 36.random allocation.sh. 37.double‐blind method.sh. 38.single‐blind method.sh. 39.or/33‐38 Part 2: 40.clinical trial.pt. 41.exp clinical trials/ 42.(clinic$ adj25 trial$).ab,ti,ot. 43.((singl$ or doubl$ or trebl$ or tripl$) adj (mask$ or blind$)).ab,ti,ot. 44.placebos.sh. 45.placebo$.ab,ti,ot. 46.random$.ab,ti,ot. 47.research design.sh. 48.(latin adj square).ab,ti,ot. 49.or/40‐48 Part 3: 50.comparative study.pt. 51.exp evaluation studies/ 52.follow‐up studies.sh. 53.prospective studies.sh. 54.(control$ or prospectiv$ or volunteer$).ab,ti,ot. 55.cross‐over studies.sh. 56.or/50‐55 57.39 or 49 or 56 V. Meta‐analysis: 58.exp meta‐analysis/ 59.exp Review Literature/ 60.meta‐analysis.pt. 61.review.pt. 62.or/58‐61 63.letter.pt. 64.comment.pt. 65.editorial.pt. 66.historical‐article.pt. 67.or/63‐66 68.62 not 67 69.((systematic$ or quantitativ$ or methodologic$) adj (review$ or overview$)).ab,ti,ot. 70.meta?anal$.ab,ti,ot. 71.(integrativ$ research review$ or research integration$).ab,ti,ot. 72.quantitativ$ synthes$.ab,ti,ot. 73.(pooling$ or pooled analys$ or mantel$ haenszel$).ab,ti,ot. 74.(peto$ or der?simonian$ or fixed effect$ or random effect$).ab,ti,ot. 75.or/69‐74 76.68 or 75 VI. HTA: 77.exp Technology Assessment, Biomedical/ 78.HTA.ab,ti,ot. 79.(health technology adj6 assessment$).ab,ti,ot. 80.(biomedical adj6 technology assessment$).ab,ti,ot. 81.or/77‐80 VII. Metformin + Insulin + Diabetes mellitus: 82.3 and 6 and 32 VIII. RCT/CCT + Meta‐analysis + HTA: 83.57 or 76 or 81 IX. VII + VIII: 84.82 and 83 X. Polycystic ovary syndrome (excluded): 85.exp Polycystic Ovary Syndrome/ 86.84 not 85 XI. Adolescent: 87.exp Adolescent/ 88.exp Puberty/ 89.(adolescent$ or pubert$).ab,ti,ot. 90.or/87‐89 91. 86 and 90 92. limit 91 to animal 93. limit 91 to human 94. 92 not 93 95. 91 not 94 |
Appendix 2. Risk of bias
| Characteristic | Hamilton 2003 | Sarnblad 2003 |
| Intervention 1 (I1) / intervention 2 (I2) / control 1 (C1) | I1: oral metformin added to s.c. insulin C1: placebo added to s.c. insulin | I1: oral metformin added to s.c. insulin C1: placebo added to s.c. insulin |
| Randomised controlled clinical trial (RCT) | Y | Y |
| Non‐inferiority / equivalence trial | N | N |
| Controlled clinical trial | Y | Y |
| Design: parallel, crossover, factorial RCT | parallel | parallel |
| Design: crossover study | ||
| Design: factorial study | ||
| Crossover study: wash‐out phase | ||
| Crossover study: carryover effect tested | ||
| Crossover study: period effect tested | ||
| Method of randomisation (specify) | Y: computer generated block random number table | ?? |
| Unit of randomisation (individuals, cluster ‐ specify) | Individual block randomization by sex and pubertal status | Individual stratified according to gender |
| Randomisation stratified for centres | N | N |
| Randomisation ratio | 1:1 | 1:1 |
| Concealment of allocation (specify) | Y clear adequate | ? |
| Stated blinding (open; single, double, triple blind) | double | double |
| Actual blinding: participant | ? | ? |
| Actual blinding: caregiver / treatment administrator | ? | ?? |
| Actual blinding: outcome assessor | ? | ? |
| Actual blinding: others | ? | ? |
| Blinding checked: participant | ? | ? |
| Blinding checked: caregiver / treatment administrator | ? | ? |
| Primary endpoint defined | N | Y (HbA1c) |
| [n] of primary endpoint(s) | not specified | 1 |
| [n] of secondary endpoints | not specified | 8 |
| Total [n] of endpoints | 13 | 9 |
| Prior publication of study design | ? | ? |
| Outcomes of prior and current publication identical | ? | ? |
| Power calculation | Y: <80%? | Y: <80%? |
| [n] participants per group calculated | I: 14 C: 13 | I: 16 C: 14 |
| Non‐inferiority trial: interval for equivalence specified | ||
| Intention‐to‐treat analysis (ITT) | N | N |
| Per‐protocol‐analysis | Y | Y |
| ITT defined | N | N |
| Missing data: last‐observation‐carried‐forward (LOCF) | N | N |
| Missing data: other methods | N | N |
| LOCF defined | N | N |
| [n] of screened participants (I1/ I2 / C1 / total) | ? | ? |
| [n] of randomised participants (for primary endpoint) | I1: 15 C1: 15 Total: 30 | I1: 16 C1: 14 Total: 30 |
| [n] of participants finishing the study | 27 | 24 |
| [n] of patients analysed (for primary endpoint) | 27 | 26 |
| Description of discontinuing participants | Y | Y |
| Drop‐outs (reasons explained) | Y | Y |
| Withdrawals (reasons explained) | Y | Y |
| Losses‐to‐follow‐up (reasons explained) | Y | Y |
| [n] of participants who discontinued | 3 | 6 |
| [%] discontinuation rate | 3/30= 10% | 6/30=20% |
| Discontinuation rate similar between groups | N I:1(6.7%) C:2 (13.4%) | N I:5/16: 31% C:1/14: 7% |
| [%] crossover between groups | N | N |
| Differences [n] calculated to analysed patients | 7 | 7 |
| Adjustment for multiple outcomes / repeated measurements | N | N |
| Baseline characteristics: clinically relevant differences | N | N |
| Treatment identical (apart from intervention) | Y | Y |
| Compliance measured | Y | Y |
| Other important covariates measured (specify) | N | N |
| Co‐morbidities measured | N | N |
| Co‐medications measured | N | N |
| Specific doubts about study quality | N | Y: method of randomisation not specified, allocation concealment not mentioned, attrition bias (drop‐out: 20%), detection bias (outcome assessors not mentioned to be blind) |
| Funding: commercial | Y: grants from the Hospital for Sick Children Research Institute and the Order of the Eastern Star of Ontario | N |
| Funding: non‐commercial | Y: Drug and Placebo were provided by Aventis Pharma | Y: Drug and Placebo were provided by Merck AB, Pharma division |
| Publication status: peer review journal | Y | Y |
| Publication status: journal supplement | N | N |
| Publication status: abstract | N | N |
| Publication status: other | N | N |
| Footnotes: Y = yes; N = no; ? = unclear I = intervention; C = control | ||
Appendix 3. Baseline characteristics
| Characteristic | Hamilton 2003 | Sarnblad 2003 |
| Intervention 1 (I1) / control 1 (C1) | I1: oral metformin and s.c. insulin C1: placebo and s.c. insulin | I1: oral metformin and s.c. insulin C1: placebo and s.c. insulin |
| [n] (I1/ C1 / total) | [n] I1: 14/ C1: 13/ total:27 out of 30 randomized ) | [n] I1: 16/ C1: 14/ total:30 ) |
| Sex [n,%] | 14 females( 52 %), 13 males ( 48%) | 21 females( 70 %), 9 males ( 30%) |
| Age [years] mean (SD) | I1: 15.9 (1.9) C1: 16.0 (1.7) | I1: 17.2 (1.7) C1: 16.9 (1.4) |
| Ethnic groups [%] | N: all Caucasians | ? |
| Duration of disease [years] mean (SD) | I1: 9.9 (4.4) C1: 7.0 (3.8) | I1: 9.1 (5.0) C1: 7.1 (3.0) |
| Body mass index [kg/m2] mean (SD) | I1: 22.8 (4.2) C1: 25.7 (2.9) | Median(range) I1: 26.2(18.6‐35.4) C1: 23.9 (17.0‐29.2) |
| Pharmaco‐naive patients [n,%] | ? | ? |
| HbA1c [%] mean (SD) | I1: 9.3 (1.4) C1: 8.6(0.8) | I1: 9.3 (1.1) C1: 9.3(1.4) |
| Insulin sensitivity | S1 (x 10‐4. min‐1. uU‐1. ml‐1) I: 1.7 (CI: 1.0‐2.6) C: 1.1 (CI 0.6‐2.2) | M/I (mg/m2 per min x uU/ml) Median (range) I: 1.5 (0.8 ‐ 4.2) C: 2.0 (0.2 ‐ 3.7) |
| Footnotes: Y = yes; N = no; ? = unclear; I = intervention; C = control | ||
Appendix 4. Adverse events
| Characteristic | Hamilton 2003 | Sarnblad 2003 |
| Intervention 1 (I1) / control 1 (C1) | I1: oral metformin and s.c. insulin (randomised[n]=15) C1: placebo and s.c. insulin (randomised[n]=15) | I1: oral metformin and s.c. insulin (randomised[n]=16) C1: placebo and s.c. insulin (randomised[n]=14) |
| [n] of participants who died | N | Not reported |
| [n] adverse events (I1/ I2 / C1 / total) | I:11 C: 7 Total: 18 | I: 3 C: 6 Total: 9 |
| [%] adverse events | I: 73% C: 47% | I: 19% C: 43% |
| [n] serious adverse events | I: 2 C: 2 | N |
| [%] serious adverse events | I: 13% C: 13% | N |
| [n] drop‐outs due to adverse events | I1: 2 C1: 1 Total:3 | I: 1 C: 0 |
| [%] drop‐outs due to adverse events | I1: 13% C1: 7% Total:20% | I: 6% C: 0% |
| [n] hypoglycaemic episodes | I:2 C:1 mild hypoglycemia after 3 months: I: Mean 1.75 (0.8) events/patient/week C: Mean 0.9 (0.4) events/patient/week | N |
| [%] hypoglycaemic episodes | I: 13% C: 7% | N |
| [n] severe hypoglycaemic episodes | I: 2 C:1 | N |
| [%] severe hypoglycaemic episodes | I: 13% C: 7% | N |
| [n] nocturnal hypoglycaemic episodes | ? | N |
| [%] nocturnal hypoglycaemic episodes | ? | N |
| [n] with symptoms | I: 2 C: 1 | N |
| [%] with symptoms | I: 13% C: 7% | N |
| [n] with GIT upset | I: 9 C: 5 | I: 3 C: 6 Total: 9 |
| [%] with GIT upset | I: 60% C: 33% | I: 19% C: 43% |
| [%] with severe GIT upset | I:13% C:0% | N |
| [n] with lactic acidosis | N | N |
| [%] with lactic acidosis | N | N |
| [%] with severe lactic acidosis | N | N |
| Footnotes: Y = yes; N = no; ? = unclear I = intervention; C = control | ||
Appendix 5. Primary outcome data
| Caracteristic | HbA1c (%) mean (SD) | Blood glucose mean (SD) | Side effects (%) | Quality of life |
| Hamilton 2003 | At baseline, mean HbA1C% was 9.3 (1.4) vs 8.6 (0.8) in metformin and placebo groups respectively. After 3 months of metformin therapy, change in mean HbA1C was: I: ‐0.3 (0.7)% C: 0.3 (0.7)% P=0.03 At the end of the study, HbA1c was 0.6% lower in the metformin group than in the placebo group (P<0.035) | Mean change of fasting blood glucose after 3 months of metformin therapy I: ‐0.9 (3.8) mmol/l C: ‐0.5 (3.2) mmol/l P=0.04 | I: 73% C: 47% | Not reported |
| Sarnblad 2003 | Mean change of HbA1C after 3 months of metformin therapy I: ‐0.9% C: ‐ 0.3% During the study period the mean HbA1c value decreased from 9.6 (1.0) to 8.7 (1.5)% (95% CI for the change: 21.6 to 20.1; P , 0.05) in the metformin group, but remained unchanged (9.5 (1.2) vs 9.2 (1.3)%; ns) in the placebo group | Change in mean glucose concentration during steady state of euglycemic hyperinsulinemic clamp I: unchanged C: unchanged | I: 19% C: 43% | Not reported |
| Footnotes: Y = yes; N = no; ? = unclear I = intervention; C = control | ||||
Appendix 6. Secondary outcome data
| Characteristic | Insulin dose IU/kg/d | BMI change(kg/m2) | Serum lipids | Insulin sensitivity | Costs | Mortality | Morbidity |
| Hamilton 2003 | Change in mean daily insulin dose after 3 months of metformin therapy I: –0.14 (0.1) C: 0.02 (0.2) p=0.01 | Change in mean BMI after 3 months of metformin therapy I: –0.05 (1.0) C: 0.2 (0.5) p=0.35 There was a trend to lower BMI in the metformin group at study end (P=0.15) | No change in either groups and no change between groups in serum triglycerides or cholesterol from baseline to 3 months after metformin therapy | Change in S1(x 10‐4 min ‐1. uU‐1. ml‐1) after 3 months of metformin therapy I: 2.6 (CI: 1.0‐4.1) C: 2.5 (CI: 1.9‐2.9) P= 0.26 Mean SI (95% CI) at onset of intervention was 1.35 (CI 0.57–2.51) min /uU/min, with no difference between the two groups. At the end of the 12‐week study period, the change in SI was not statistically significantly different between the two groups | ? | ? | ? |
| Sarnblad 2003 | Change in mean daily insulin dose after 3 months of metformin therapy I: 0 (unchanged C: + 0.1 There was no significant difference between the metformin and placebo group regarding daily insulin dosage at baseline[1.1(0.3) vs 1.2(0.2)] or after 3 months of therapy [1.1(0.3) vs 1.3(0.2)] In neither of the groups did the daily insulin dosage change significantly | Median (range)change in BMI after 3 months of metformin therapy I: –0.2 C: ‐0.6 p=ns There was no change in median BMI(range) between metformin and placebo group at baseline [23.5 (18.6‐35.4) vs 23.9 (17.0‐29.2)] or after 3 months of metformin therapy [23.3(18.4‐34.4) vs 23.3 (17.7‐29.4) | No change in either groups and no change between groups in serum triglycerides or cholesterol from baseline to 3 months after metformin therapy | Change in M/I after 3 mo Median (range) I: 0.7 (p< 0.05) C: 0.3 There were no significant differences between the groups in either of the two clamps. Neither the M/I values nor the M values were significantly different between the groups at baseline or after 3 months. The M values were unchanged in both groups. M/I, however, increased significantly in the metformin group during the study (P < 0.05), but was unchanged in the placebo group. In the metformin group, change in insulin sensitivity (M/I) showed no association with initial HbA1c, insulin dosage or change in insulin dose. However, there was a significant positive association between change in insulin sensitivity and initial M/I (r = 0.77; P < 0.01), indicating that patients with lower initial insulin sensitivity benefited most from metformin treatment. | ? | ? | ? |
| Footnotes Y = yes; N = no; ? = unclear I = intervention; C = control S1: it is calculated according to minimal model (MINMOD) formulas using MINMOD computer software(Bergman 1989) M: the calculated amount of glucose infused during the last 60 min after a steady‐state was achieved I: mean insulin concentration during steady state after 60 min of glucose infusion | |||||||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hamilton 2003.
| Methods | DESIGN: Randomized double blind placebo controlled trial COUNTRY: Canada DURATION OF INTERVENTION: 3 months DURATION OF FOLLOW‐UP: 3 months RUN‐IN PERIOD: 2 months LANGUAGE OF PUBLICATION: English | |
| Participants | WHO PARTCIPATED: N= 27 adolescents SEX: 14 females, 13 males DISEASE: poorly controlled type 1 diabetes INCLUSION CRITERIA: AGE: 12‐17 years, PUBERTY: Tanner stage 2‐5, DURATION of DIABETES: > 3 years, METABOLIC CONTROL: HB A1c above 8% but <11% for the prior 6 months, daily dosage of insulin > 1 U/kg EXCLUSION CRITERIA: persistent nephropathy, proliferative retinopathy, recurrent ketoacidosis, recurrent severe hypoglycaemia, renal or hepatic dysfunction, another serious medical illness, known eating disorder, sexually active female unwilling to take birth control DIAGNOSTIC CRITERIA: Caucasians, typical diabetes symptoms and ketosis at onset, required insulin treatment from onset of diabetes SUBGROUPS: none CO‐MORBIDITIES: none CO‐MEDICATIONS: none | |
| Interventions | NUMBER OF STUDY CENTRES: one COUNTRY/ LOCATION: Canada, Toronto SETTING: Diabetes clinic at The Hospital for Sick Children INTERVENTION N= 14 patients SEX: 8 females, 6 males DESCRIPTION: s.c. insulin and oral metformin 500 mg/d for 1 week, which was increased by 500 mg/day each week to a maximum of 1000 mg/day (500 mg twice daily) for those weighing less than 50 kg, 1500 mg/day (one 1000 mg and one 500 mg dose) for those weighing 50 to 75 kg, or 2000 mg/day (1000 mg twice daily for those weighing more 75 kg CONTROL N= 14 patients, SEX: 10 females, 4 males DESCRIPTION: s.c. insulin and placebo TREATMENT BEFORE STUDY: s.c. insulin TITRATION PERIOD: none | |
| Outcomes | No subdivision into primary and secondary outcomes. Outcomes were measured on inclusion and at the end of the study
1. Glycemic control: HBA1c and fasting blood glucose
2. Side effects: hypoglycaemia, GIT (discomfort, vomiting)
3. Quality of life: not reported
4. Insulin dose: daily insulin dose/Kg
5. Weight: BMI, body weight
6. Serum lipids: serum triglycerides and cholesterol
7. Insulin sensitivity (by the frequently sampled intravenous glucose tolerance test [FSIGT])
8. Cost: not reported
9. Compliance : <25% of prescribed pills returned
10. Mortality: not reported
11. Morbidity: not reported SAFETY ASSESSMENTS: 1. renal functions 2. hepatic functions (alanine aminotransferase and aspartate aminotransferase) 3. complete blood count 4. lactate 5. mild symptomatic hypoglycemia 6. severe hypoglycemic episodes |
|
| Notes | Sponsor: grants from the Hospital for Sick Children Research Institute and the Order of the Eastern Star of Ontario. Drug and Placebo were provided by Aventis Pharma Author contacted: did not respond to date about missing data Study retreived:Medline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Sarnblad 2003.
| Methods | DESIGN: Randomized double blind placebo controlled trial COUNTRY: Sweden DURATION OF INTERVENTION: 3 months DURATION OF FOLLOW‐UP: 3 months RUN‐IN PERIOD: 1 month LANGUAGE OF PUBLICATION: English | |
| Participants | WHO PARTCIPATED: N= 30 adolescents SEX: 21 females, 9 males DISEASE: poorly controlled type 1 diabetes INCLUSION CRITERIA: Girls: 14‐20 years, Boys: 16‐20 years, HB A1c above 8%, daily dosage of insulin > 0.9 U/kg EXCLUSION CRITERIA: other diseases, other medications, persistent nephropathy DIAGNOSTIC CRITERIA: typical diabetes symptoms and ketosis at onset, required insulin treatment from onset of diabetes, late stages of pubertal development (Tanner 4‐5) SUBGROUPS: none CO‐MORBIDITIES: none CO‐MEDICATIONS: none | |
| Interventions | NUMBER OF STUDY CENTRES: five departments of Pediatrics COUNTRY/ LOCATION: central Sweden ( Eskilstuna, Falun, Karlstad, Vasteras and Orebro) SETTING: Pediatric outpatient departments INTERVENTION : N= 16 patients SEX: 11 females, 5 males DESCRIPTION: s.c. insulin and oral metformin 500 mg/d for 1 week, followed by 500 mg twice daily for 3 weeks, then 1000 mg twice daily for 8 weeks CONTROL: N= 14 patients, SEX: 10 females, 4 males, DESCRIPTION: s.c. insulin and placebo TREATMENT BEFORE STUDY: s.c. insulin TITRATION PERIOD: none | |
| Outcomes | 1. Glycemic control: HBA1c and blood glucose
2. Side effects: hypoglycaemia, GIT (discomfort, vomiting, abdominal pain), lactic acidosis, ketoacidosis
3. Quality of life: not reported
4. Insulin dose: daily insulin dose/Kg
5. Weight: BMI, body weight
6. Serum lipids: serum triglycerides and cholesterol
7. Insulin sensitivity (by euglycaemic hyperinsulinaemic clamp)
8. Cost: not reported
9. Compliance : <10% of total doses missed
10. Mortality: not reported
11. Morbidity: not reported SAFETY ASSESSMENTS: 1. renal functions 2. hepatic functions (alanine aminotransferase and aspartate aminotransferase) 3. complete blood count 4. lactate 5. mild symptomatic hypoglycemia 6. severe hypoglycemic episodes |
|
| Notes | Sponsor: Drugs and placebo were provided by Merck AB, Pharma division Authors contacted: Study retreived:Medline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Schatz 1975 | Schatz 1975 1. cross over study design 2. age range between 4 and 16, not all patients are adolescents 3. insulin regimen (type and dose)was not standardized in groups under study 4. Primary outcomes of the review were not assessed in the study |
| Walravens 2000 | Walravens 2000 Abstract in a conference proceedings, which does not reveal details verifying methodology or assuring the study quality. The full text could not be accessed through all databases. The authors were contacted but did not respond. |
Contributions of authors
SHEREEN ABDELGHAFFAR: Title registration, drafting the protocol, searching databases for studies, independent selection of studies for inclusion, independent data extraction, independent quality assessment of studies, drafting the review, data analysis and presentation.
ABDELHAMID ATTIA: Drafting the protocol, independent selection of studies for inclusion, independent data extraction, independent quality assessment of studies, drafting the review, data analysis, assistance with statistics and data presentation.
Declarations of interest
None known.
New
References
References to studies included in this review
Hamilton 2003 {published data only}
- Hamilton J, Cummings E, Zdravkovic V, Finegood D, Daneman D. Metformin as an adjunct therapy in adolescents with type 1 diabetes and insulin resistance. Diabetes Care 2003;26:138‐43. [DOI] [PubMed] [Google Scholar]
Sarnblad 2003 {published data only}
- Sarnblad S, Kroon M, Aman J. Metformin as additional therapy in adolescents with poorly controlled type 1 diabetes: randomized placebo‐controlled trial with aspects on insulin sensitivity. European Journal of Endocrinology 2003;149:323‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Schatz 1975 {published data only}
- Schatz H, Winkler G, Jonatha EM, Pfeiffer EF. Studies on juvenile ‐type diabetes in children. Assessment of control under treatment with constant and variable doses of insulin with or without addition of biguanides. Diabete & Metabolisme 1975;1:211‐220. [PubMed] [Google Scholar]
Walravens 2000 {published data only (unpublished sought but not used)}
- Walravens PA, Chase PH, Klingensmith GJ, Ellison M, Cornell C, Monahan K. [Low dose metformin in adolescents with type 1 diabetes: a double‐blind, controlled study. (Abstract).]. Diabetes. 2000:49 A128.
Additional references
ADA 1999
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22 Suppl 1:S5‐19. [DOI] [PubMed] [Google Scholar]
Aldasouqi 2003
- Aldasouki SA, Duick DS. Safety issues on Metformin use. Diabetes Care 2003;26:3356‐7. [DOI] [PubMed] [Google Scholar]
Bending 1987
- Bending JJ, Collins AC, Keen H. Metformin increases response to insulin in insulin‐dependent diabetes. Diabet Med. 1987; Vol. 46:555A.
Bergman 1989
- Bergman RN. Lilly Lecture: Toward physiological understanding of glucose tolerance: minimal‐model approach (Review).. Diabetes 1989;38:1512‐1527. [DOI] [PubMed] [Google Scholar]
Caprio 1994
- Caprio S, Cline G, Boulware S, Permante C, Shulman GI, Sherwin RS, et al. Effects of puberty and diabetes on metabolism of insulin‐sensitive fuels. American Journal of Physiology 1994;266:E885–E91. [DOI] [PubMed] [Google Scholar]
Cohen 1960
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
Coscelli 1984
- Coscelli C, Palmari V, Saccardi F, Orsola A, Bonnora E. Effect of Metformin addition to insulin in type 1 diabetes mellitus.. Front Diabetes 1984;4:253‐257. [Google Scholar]
Daniel 1997
- Daniel JR, Hagmeyer KO. Metformin and insulin: is there a role for combination therapy?. Annals of Pharmacotherapy 1997;31:474–80. [DOI] [PubMed] [Google Scholar]
DCCT 1994
- DCCT Research Group. Effect of intensive diabetes treatment on the development and progression of long‐term complications in adolescents with insulin‐dependent diabetes mellitus. Diabetes Control and Complications Trial. Journal of pediatrics 1994;125:177–88. [DOI] [PubMed] [Google Scholar]
Desmangles 2000
- Desmangles J, Buchlis JG, Shine B, Quattrin T. Is Metformin a useful adjunct to insulin therapy in adolescents with type 1 diabetes in poor control?. Endocrine Society Meeting, Abstract 1833. 2000.
Faichney 2003
- Faichney JD, Tate PW. Metformin in type 1 diabetes: is this a good or bad idea?. Diabetes Care 2003;26(5):1655. [DOI] [PubMed] [Google Scholar]
Gin 1982
- Gin H, Slama G, Weissbrodt P, Poynard T, Vexiau P, Klein J, Tchobrousky G. Metformin reduces post‐prandial insulin needs in type 1 (insulin‐dependent) diabetic patients: assessment by the artificial pancreas. Diabetologia 1982;23:34–6. [DOI] [PubMed] [Google Scholar]
Golay 1995
- Golay A, Guillet‐Dauphine N, Fendel A, Juge C, Assal JP. The insulin‐sparing effect of metformin in insulin‐treated diabetic patients. Diabetes/Metabolism Reviews 1995;11:S63–S67. [DOI] [PubMed] [Google Scholar]
Halldin 1998
- Halldin MU, Tylleska¨r K, Hagena¨s L, Tuvemo T, Gustafsson J. Is growth hormone hypersecretion in diabetic adolescent girls also a daytime problem? growth hormone hypersecretion in diabetic adolescent girls also a daytime problem?. Clinical Endocrinology 1998;48:785–94. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.0 [updated February 2008]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2008. [Google Scholar]
Howlet 1999
- Howlett HCS, Bailey CJ. A risk‐benefit assessment of metformin in type 2 diabetes mellitus. Drug safety 1999;20:489‐503. [DOI] [PubMed] [Google Scholar]
Hundal 2000
- Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000;49:2063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad A, Moore A, Carrol D, et al. Assessing the quality of randomized trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Janssen 1991
- Janssen M, Rillaerts E, Leeuw I. Effects of metformin on haemorheology, lipid parameters and insulin resistance in insulin‐dependent diabetic patients (IDDM). Biomedicine & Pharmacotherapy 1991;45:363–7. [DOI] [PubMed] [Google Scholar]
Jones 2002
- Jones KL, Arslanian S, Peterokova VA, Park J‐S, Tomlinsson MJ. Effect of metformin in pediatric patients with type 2 diabetes. Diabetes Care 2002;25:89‐94. [DOI] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333:597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehnert 2001
- Mehnert H. Metformin, the rebirth of a biguanide: mechanism of action and place in the prevention and treatment of insulin resistance. Experimental and Clinical Endocrinology and Diabetes 2001;109:S259–S264. [DOI] [PubMed] [Google Scholar]
Meyer 2002
- Meyer L, Bohme P, Delbachian I, Lehert P, Cugnardey N, Drouin P, et al. The benefits of metformin therapy during continuous subcutaneous insulin infusion treatment of type 1 diabetic patients. Diabetes Care 2002;25:2153–8. [DOI] [PubMed] [Google Scholar]
Misbin 1998
- Misbin RI, Green L, Stadel BV, Gueriguian JL, Gubbi A, Fleming GA. Lactic acidosis in patients with diabetes treated with metformin (Letter). New England Journal of Medicine 1998;338:265‐6. [DOI] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354(9193):1896‐900. [DOI] [PubMed] [Google Scholar]
Mortensen 1998
- Mortensen HB, Robertson KJ, Aanstoot HJ, Danne T, Holl RW, Hougaard P, et al. Insulin management and metabolic control of type 1 diabetes mellitus in childhood and adolescence in 18 countries. Hvidore Study Group on Childhood Diabetes. Diabetic Medicine 1998;15:752‐9. [DOI] [PubMed] [Google Scholar]
Pagno 1983
- Pagano G, Tagliaferro V, Carta Q, Caselle M, Bozzo C, Vitelli F, et al. Metformin reduces insulin requirement in type 1 (insulin‐dependent) diabetes. Diabetologia 1983;24:351–4. [DOI] [PubMed] [Google Scholar]
Saenz 2005
- Saenz A, Fernandez‐Esteban I, Mataix A, Ausejo M, Roque M, Moher D. Metformin monotherapy for type 2 diabetes. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD002966.pub3] [DOI] [PubMed] [Google Scholar]
Salpeter 2005
- Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and non fatal lactic acidosis with Metformin use in type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD002967.pub2] [DOI] [PubMed] [Google Scholar]
Schultz 1995
- Schultz KF, Chalmers I, Hayes RJ, Altman DJ. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care; Meta‐analysis in Context. London: BMJ Publishing Group, 2001:189‐208. [Google Scholar]
UKDPS 1998
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–65. [PubMed] [Google Scholar]
WHO 1980
- WHO Expert Committee on Diabetes Mellitus. Second report. Technical Report Series 646. Geneva. WHO, 1980. [PubMed]
WHO 1985
- WHO Expert Committee on Diabetes Mellitus. World Health Organization, 1985. Technical Report Series 727. [PubMed]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its compliactions. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
Yki‐Jarvinen 1997
- Yki‐Jarvinen H, Makimattila S ‐Jarvinen H, Makimattila S. Insulin resistance due to hyperglycaemia: an adaptation protecting insulin‐sensitive tissues. Diabetologia 1997;40:S141‐S144. [DOI] [PubMed] [Google Scholar]


