Abstract
Patients with COVID-19 frequently experience a coagulopathy associated with a high incidence of thrombotic events leading to poor outcomes. Here, biomarkers of coagulation (such as D-dimer, fibrinogen, platelet count), inflammation (such as interleukin-6) and immunity (such as lymphocyte count) as well as clinical scoring systems (such as SOFA, ISTH DIC and SIC score) can be helpful in predicting clinical course, need for hospital resources (such as ICU beds, intubation and ventilator therapy, and ECMO) and patient’s outcome in patients with COVID-19. However, therapeutic options are actually limited to unspecific supportive therapy. Whether viscoelastic testing can provide additional value in predicting clinical course, need for hospital resources and patient’s outcome or in guiding anticoagulation in COVID-19 associated coagulopathy is still incompletely understood and currently under investigation (eg, in the ROHOCO study). This paper summarizes what we know already about COVID-19 associated coagulopathy and – perhaps even more importantly – characterizes important knowledge gaps.
Keywords: Anticoagulation, coagulopathy, COVID-19, SARS-CoV-2, thrombosis
Introduction
After severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002, H1N1 influenza in 2009 and Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012, the SARS-CoV-2 pandemic is now challenging the world with COVID-19. Although most of the infected persons either have subclinical or mild clinical symptoms, a small patient population has severe disease manifestations of COVID-19. In particular, this applies for male patients older than 60 years and patients with comorbidities. Patients with poor outcome are characterized by a high incidence of COVID-19 associated coagulopathy, venous thrombosis, pulmonary embolism/thrombosis, and multiple organ failure.1
What do we know already about COVID-19-associated coagulopathy?
COVID-19 is associated with a high incidence of venous thrombosis and pulmonary embolism/thrombosis
Cui et al. reported an incidence of venous thromboembolism (VTE) of 25% (20/81) in critically ill patients with COVID-19 treated at the intensive care unit (ICU) which was at least two-times higher compared to other critically ill patients.2,3 Mortality in these patients was 40%. A D-dimer cutoff of ≥ 1.5 µg/mL (reference range <0.5 µg/mL) predicted VTE with a sensitivity of 85.0%, a specificity of 88.5% and a negative predictive value of 94.7%. A high incidence of VTE of 31% in 184 critically ill COVID-19 patients despite pharmacologic thromboprophylaxis was confirmed by Klok et al.4 Here, pulmonary embolism (PE) was with 81% the most frequent thrombotic complication. Llitjos et al. reported that VTE was even detected in 100% (8/8) of severe COVID-19 patients treated with prophylactic and in 56% (10/18) in patients with therapeutic anticoagulation.5 Even if VTE was observed most often in ICU patients,6 Lodigiani demonstrated that half of the VTE (overall 21%) were diagnosed already within 24 hours of hospital admission.7 Therefore, monitoring should be initiated early after hospital admission and should not be limited on critically ill COVID-19 patients treated at the ICU. However, COVID-19 patients receiving continuous renal replacement therapy or extracorporeal membrane oxygenation (ECMO) may even be at increased risk of VTE, PE and circuit clotting.8 Finally, Wichmann et al. detected VTE in 58% (7/12) of autopsies in COVID-19 patients and PE was the direct cause of death in 33% (4/12).9 This high incidence of pulmonary thrombosis and embolism in autopsies has recently been confirmed by other authors.10,11
Biomarkers can help predict the clinical course of COVID-19 patients
Gao et al. reported that D-dimer differentiated between COVID-19 patients with severe versus mild disease. The optimal threshold and area under the ROC curve of D-Dimer were 0.280 µg/mL and 0.750, respectively.12 Zhou et al. showed in their multivariable regression increasing odds of in-hospital death associated with older age (OR, 1.10, 95% CI, 1.03-1.17, per year increase; P = 0.0043), and D-dimer greater than 1 µg/mL (OR, 18.42, 95% CI, 2.64-128.55; P = 0.0033) on hospital admission.13 Zhang et al. reported an optimum cutoff value of D-dimer of ≥ 2.0 µg/mL within 24 hours after hospital admission to predict in-hospital mortality with a sensitivity of 92.3% and a specificity of 83.3% and a hazard ratio of 51.5 (95% CI, 12.9-206.7).14 Accordingly, the potential risk factors of older age and D-dimer ≥2 µg/mL may help clinicians to identify patients with poor prognosis at an early stage. Elevated D-dimers as a risk factor for Acute Respiratory Distress Syndrome (ARDS) and mortality have been confirmed by Tang et al. and Wu et al.15,16 Since most patients with severe COVID-19 are older than 60 years, it seems to be reasonable to use an age-adjusted D-dimer cutoff value (patient’s age x 10 µg/L).17–19 Notably, Tang et al. reported that patients with D-dimer >3 µg/mL (6fold of upper limit of normal) showed a significant reduction in 28-day mortality (32.8% vs 52.4%; P = 0.017) if treated with unfractionated heparin (UFH) or low molecular weight heparin (LMWH).20–22 Accordingly, D-dimer may be considered as a good biomarker for severe COVID-19 infection and the need for intensified (intermediate or therapeutic dose) and extended (to post hospital discharge) thromboprophylaxis, even though there is still no clear evidence available.23.24
Gao et al. reported that also IL-6 plasma concentration (reference range <7 pg/mL) differentiated between mild and severe groups of COVID-19 patients. The optimal threshold and area under the ROC curve of IL-6 were 24.3 pg/mL and 0.795, respectively.11 Notably, the area under the ROC curve of IL-6 combined with D-dimer was 0.840. The specificity of predicting the severity of COVID-19 by IL-6 and D-dimer tandem testing was up to 93.3%, while the sensitivity of IL-6 and D-dimer by parallel test in the severe COVID-19 was 96.4%. Accordingly, the combined d-dimer and IL-6 testing provides the highest sensitivity and specificity for early prediction of COVID-19 severity. The prognostic value of IL-6 has been confirmed by Ruan et al. who demonstrated that nonsurvivors had 1.7-times higher IL-6 values compared to survivors.25 Accordingly, Henry recommended tracking IL-6 before and during ECMO since patients with hyper-inflammation might not benefit but rather be harmed by ECMO therapy.26 Furthermore, IL-6 plasma concentrations are consistently elevated and inversely correlated with survival in children and adults during ECMO.27
Lymphopenia (lymphocyte count; median (IQR), 800 (600-1100) /µL; reference range, 1100-3200/µL) has been reported in 70.3% of hospitalized COVID-19 patients.28 Also lymphocyte count - as a biomarker of an exhausted adaptive immune system – is associated with COVID-19 severity. Patients who died from COVID-19 are reported to have had significantly lower lymphocyte counts than survivors.26 Tracking both lymphocyte count and IL-6 may reflect the balance between the innate and adaptive immune system in patients with severe COVID-19.
Clinical scores such as SOFA, SIC and ISTH DIC score predict mortality in COVID-19
Zhou et al. showed in their multivariable regression increasing odds of in-hospital death associated with older age (OR, 1.10, 95% CI, 1.03-1.17, per year increase; P = 0.0043), and higher Sequential Organ Failure Assessment (SOFA) score (OR, 5.65, 95% CI, 2.61-12.23; P < 0.0001). In combination with D-dimer >1 µg/mL this can help clinicians to identify COVID-19 patients with poor prognosis at an early stage.13 SOFA score as a part of the Sepsis-induced Coagulopathy (SIC) score can also identify COVID-19 patients which might benefit from intensified thromboprophylaxis. Here, Tang et al. reported that patients with SIC score ≥4 or D-dimer >3 µg/mL (6fold of upper limit of normal) showed a significant reduction in 28-day mortality (40.0% vs 64.2%, P = 0.029 and 32.8% vs 52.4%; P = 0.017, respectively) if treated with UFH or LMWH.20–22 Furthermore, 71.4% of COVID-19 nonsurvivors and 0.6% survivors met the ISTH DIC criteria during their hospital stay.15 Therefore, clinical scoring systems such as SOFA, SIC and ISTH DIC score can be helpful in predicting outcome in patients with COVID-19. However, clinical scores such as the ISTH DIC score have to be interpreted in the clinical context and as a dynamic process. Accordingly, not every patient with a DIC score ≥5 is suffering definitively from overt DIC.
What are the knowledge gaps?
Is COVID-19 associated coagulopathy different from sepsis-induced coagulopathy (SIC) and disseminated intravascular coagulation (DIC)?
Tang et al. reported that 71.4% of patients who die of COVID-19 are meeting ISTH criteria for disseminated intravascular coagulopathy (DIC) while only 0.6% of patients who survived meet these criteria.15,29 However, COVID-19 is characterized as a predominantly prothrombotic disease with elevated D-dimers (>1 µg/mL; OR, 18.42; 95% CI, 2.64-128.55; P=0.0033), higher Sequential Organ Failure Assessment (SOFA) score (OR, 5.65; 95% CI, 2.61-12.23; P<0.0001), high fibrinogen levels (median (IQR), 4.55 (3.66-5.17) g/L; reference range, 2.0-4.0 g/L), but only mildly decreased antithrombin levels (median (IQR), 91 (83-97)%; reference range, 80-120%) on ICU admission and microvascular thrombosis rather than a bleeding diathesis.12–16,30,31 However, pathophysiology of COVID-19-associated coagulopathy seems to be different from SIC and DIC caused by other infectious diseases.32–34
What are the differences between viral and bacterial sepsis-induced coagulopathy?
In 2016, the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) defined sepsis as life-threatening organ dysfunction caused by a dysregulated host response to infection. For clinical operationalization, organ dysfunction can be represented by an increase in the SOFA score of 2 points or more, which is associated with an in-hospital mortality greater than 10%.35 Early phase of bacterial sepsis is characterized by hypercoagulability due to tissue factor expression on circulating monocytes and microparticles, increased fibrinogen plasma concentration, platelet activation and subsequent dysfunction, and hypofibrinolysis/fibrinolysis shutdown.36,37 Tissue factor expression on circulating monocytes and microparticles is triggered by bacterial toxins (lipopolysaccharides) and other pathogen-associated molecular patterns (PAMPs) via NF-kappaB1 activation and subsequent induction of plasminogen activator inhibitor type 1 (PAI-1) and proinflammatory cytokines such as interleukin-6 (IL-6) (Figure 1).38–40 Tissue factor expression on circulating cells and microparticles, changes in clot firmness and hypofibrinolysis can be detected by thromboelastometry (Figure 2).41,42 On the one hand, this allows for discrimination between Systemic Inflammatory Response Syndrome (SIRS) and bacterial sepsis, and on the other hand, for prediction of mortality in sepsis.43–46 Furthermore, both cell-free DNA (cfDNA) and extracellular RNA (exRNA) are activating coagulation in sepsis. While higher exRNA levels correlated with a faster coagulation time (CT) and more stable clots (MCF), cfDNA correlated with a shorter CT but also less fibrinolysis (LI60) in thromboelastometry.47 Zuo et al. reported recently that neutrophil extracellular traps (NETs), including cell-free DNA, are elevated in severe COVID-19 patients receiving mechanical ventilation and strongly correlate with acute phase reactants, including CRP, D-dimer and LDH. These NETs have the potential to propagate inflammation and microvascular thrombosis.48 Whether extracellular RNA derived from SARS-CoV-2 contributes to the hypercoagulability seen in COVID-19 is not known. However, RNAemia is detected only in critically ill COVID-19 patients and is closely correlated with very high IL-6 levels (≥ 100 pg/mL; r = 0.902) and poor prognosis.49 In contrast to COVID-19, patients present with decreased plasma fibrinogen concentrations in some viral infections – in particular in hemorrhagic fevers such as Ebola or Dengue.50–53 Accordingly, viral infection does not change hemostasis in an uniform way. Dependent on the pathogen, it can result in hypo- or hypercoagulability with the clinical appearance of bleeding or thrombosis.
Figure 1.
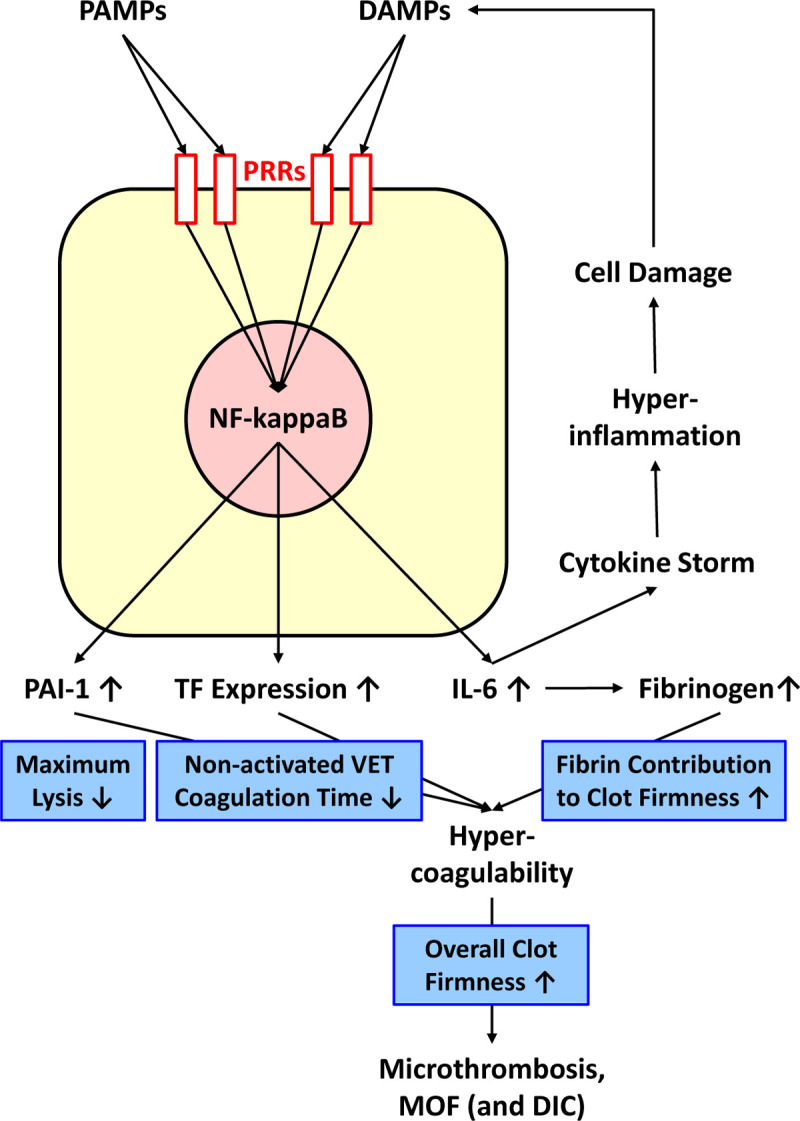
Pathophysiology of sepsis-induced coagulopathy (SIC)/COVID-19-associated coagulopathy and effects on viscoelastic testing variables (as shown in the in blue boxes). DAMPs, damage-associated molecular patterns; DIC, disseminated intravascular coagulation; IL-6, interleukin 6; MOF, multiple organ failure; NF-kappaB, nuclear factor kappa-light-chain-enhancer of activated B cells; PAI-1, plasminogen activator inhibitor type 1; PAMPs, pathogen-associated molecular patterns; PRRs, pattern recognition receptors; TF, tissue factor.
Figure 2.
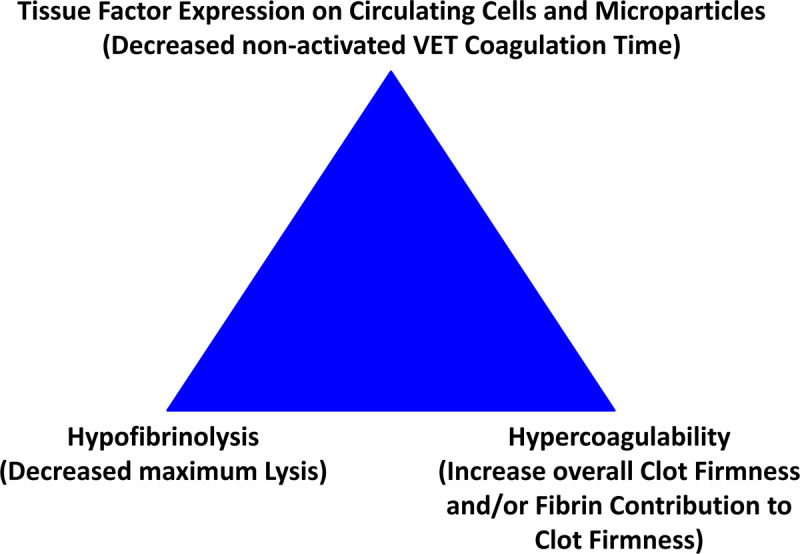
Thromboelastometry triad of thrombosis. VET, viscoelastic testing.
What is the role of the innate and adaptive immune system in COVID-19?
The innate immune system is based on physical (eg, epidermis and ciliated respiratory epithelium) and chemical (eg, gastric acid) barriers to infection, as well as on different cell types recognizing invading pathogens and activating antimicrobial immune response (dendritic cells, macrophages and granulocytes). It constitutes the first line of host defense during infection and therefore plays a crucial role in the early recognition and subsequent triggering of a proinflammatory response to invading pathogens. The innate immune response is relatively nonspecific and relies on recognition of evolutionarily conserved structures on pathogens, termed pathogen-associated molecular patterns (PAMPs), through a limited number of pattern recognition receptors (PRRs; eg, Toll-like receptors (TLRs)). In contrast, the adaptive immune system is responsible for the elimination of pathogens in the late phase of infection and in the generation of immunological memory. It is characterized by specificity developed by clonal gene rearrangements from a broad repertoire of antigen-specific receptors on lymphocytes. If the adaptive immune system is not able to clear the viruses, proinflammatory cytokines – in particular IL-6 – can cause host damage by triggering a vicious circle of systemic inflammatory response including pneumonia.54 The release of damage-associated molecular patterns (DAMPs) from the damaged tissues and cells can further stimulate PRR signaling and lead to a chain reaction culminating in viral sepsis if the infection is not cleared. This ‘cytokine storm’ can result in multiple organ failure and death.25,55
Which patient will benefit from anti-inflammatory drugs in COVID-19?
General treatment with corticosteroids is not recommended in COVID-19 since it may delay virus clearance and promote superinfection.56 However, some authors recommend dexamethasone 20 mg/day for 5 days and then 10 mg/day for 5 days in patients with ARDS within 24 hours after ARDS diagnosis.28,57 The indication must be discussed with the ICU team. Russell et al. found no published evidence for or against the use of NSAIDs in COVID-19 patients. Meanwhile, there appeared to be some evidence that corticosteroids may be beneficial if utilized in the early acute phase of infection, however, conflicting evidence from the World Health Organization (WHO) surrounding corticosteroid use in certain viral infections means this evidence is not conclusive.58 Patients with ARDS and high IL-6 levels may benefit from tocilizumab, a monoclonal antibody targeting the IL-6 receptor, indicated in cytokine release syndrome (CRS).59 Several studies on the efficacy and safety of corticosteroids and tocilizumab in COVID-19 are running, actually (NCT04273321, NCT04325061, NCT04343729, NCT04306705, NCT04317092, NCT04320615, NCT04322773, NCT04332913).
What patient demographics affect the incidence of thrombosis in COVID-19 patients?
Ethnicity has major effects on thrombotic risk, with a 3-4-fold lower risk in Chinese compared to Caucasians and a significantly higher risk in African-Americans.60 Accordingly, Fox et al. reported autopsy finding of thrombosis and microangiopathy in the small vessels and capillaries of the lung that significantly contributed to death in African American patients with COVID-19 from New Orleans.61 However, it is still under debate how much social factor contribute to the increased mortality in African American patients with COVID-19.62 Furthermore, gene polymorphisms such as the PAI-1 polymorphism, the NF-kappaB1 promoter polymorphism and several cytokine gene polymorphisms have been show to impact complications and mortality in bacterial sepsis.63–67
Older age is a risk factors associated with the development of ARDS and progression from ARDS to death in COVID-19 (hazard ratio [HR], 3.26; 95% CI, 2.08-5.11; and HR, 6.17; 95% CI, 3.26-11.67, respectively).14 Despite an increasing number of case reports dealing with COVID-19-associated deaths in younger patients, older age has been confirmed as one of the most important risk factors for COVID-19-associated mortality in recent publications.68,69 There are many reasons for this increased mortality in patients older than 60 years including a higher likelihood of comorbidities (diabetes, obesity, chronic obstructive pulmonary disease, chronic heart disease, chronic renal disease, thrombosis), decreased organ function reserves, prolonged hospitalization times, generally weaker immune response (immunosensescence), and chronic inflammatory diseases. Older adults also experience persistent T cell exhaustion in part due to constant low-level inflammation, thought to be caused by accumulation of self-debris brought on by a decrease in the ability to clear them. This process, often called “inflammaging” is characterized by elevated baseline levels of the cytokines IL-6, IL-1, and TNF-alpha.70,71
About 60% of COVID-19 patients are male.72 Notably, transmembrane protease serine subtype 2 (TMPRSS2) is both the most frequently altered gene in primary prostate cancer and a critical factor enabling cellular infection by coronaviruses, including SARS-CoV-2. The modulation of its expression by sex steroids could contribute to the male predominance of severe infections and given that TMPRSS2 has no known indispensable functions, and inhibitors (eg, camostat) are available, it is an appealing target for prevention or treatment of respiratory viral infections.73 Two studies on the efficacy and safety of camostat therapy alone or in combination with hydroxychloroquine in COVID-19 are running, actually (NCT04321096 and NCT04338906).
What viscoelastic parameters may be helpful in guiding therapy in patients with COVID-19?
On the one hand, hypofibrinolysis/fibrinolysis shutdown (lysis index 60 min after CT; LI60 ≥ 96.5%) and hypocoagulability (decreased maximum clot firmness; MCF ≤55 mm) in thromboelastometry have been shown to predict increased mortality in bacterial sepsis.43–46 Furthermore, early platelet dysfunction predicts mortality in bacterial sepsis.37 Whether this applies for COVID-19 associated coagulopathy, too, is actually under clinical investigation. On the other hand, increased clot firmness amplitudes (A10 > 61.5 mm or MCF/MA > 68 mm and FIBTEM MCF >25 mm) in thromboelastometry/graphy have been shown to be predictive for thrombosis in adults and neonates undergoing cardiac and noncardiac surgery (Figure 2).74–78
Recently, first studies confirmed markedly hypercoagulable viscoelastic profiles characterized by increased clot firmness parameter (A5, A10, MCF or MA) in patients with COVID-19. Here, Almskog et al. showed in their study including 60 COVID-19 patients and 76 healthy subjects that thromboelastometry maximum clot firmness at hospital admission predicts the final level of care (regular ward or ward with specialized ventilation support).79 Patients who needed transfer to specialized wards showed already a more hypercoagulative state at hospital admission compared to patients who could be managed at the normal ward and did not require intubation and mechanical ventilation (EXTEM MCF, 76 vs 70 mm; P<0.01 (reference range, 50-72 mm) and FIBTEM MCF 33 vs 27 mm; P=0.04 (reference range, 9-25 mm)). Whether early thromboelastometry analysis at hospital admission can predict outcome (thrombosis and mortality) in COVID-19 will be analyzed at a later stage of this research project. Another prospective, multicenter (22), multinational (11) observational study on the predictive value of ROTEM analysis and standard coagulation tests in (500) hospitalized patients with COVID-19 (ROHOCO Study) is currently recruiting patients.
Mortus et al. assessed hemostasis in 21 critically ill COVID-19 patients with 19% requiring ECMO support and 86% requiring renal replacement therapy.80 All patients received deep vein thrombosis chemoprophylaxis on ICU admission and therapeutic anticoagulation (UFH or LMWH) for thrombotic complications. There were no statistically significant differences in prothrombin time, INR, partial thromboplastin time, or platelet levels between 10 patients with at least two thrombotic events vs 11 patients with fewer than two events. In contrast, innate TEG MA was significantly greater for the high event rate group than the low event rate group (mean [SD], 75 [7] mm vs 61 [21] mm; P = 0.01). Elevated MA (> 65 mm) was observed in 100% in the high event rate group vs 45% in the low event rate group. Innate TEG MA provided 100% sensitivity and 100% negative predictive value for multiple thromboembolic events. LY30 was decreased to 0.5% in the high event rate group.
Pavoni et al. demonstrated in 40 critically ill patients with COVID-19 that hypercoagulability in thromboelastometry persists in the first five days but it deceases ten days after, without returning to normal values.81 Antithrombin levels and platelet count did not decreased in these patients, but fibrinogen, FIBTEM MCF and IL-6 levels decrease in parallel during recovery (Figure 1). The good correlation between fibrinogen and IL-6 (r = 0.711; P = 0.003) and the procoagulant viscoelastic pattern in COVID-19 patients with acute respiratory distress syndrome was also confirmed by Ranucci et al.82 Accordingly, plasma fibrinogen concentration or FIBTEM MCF might be used as a surrogate for IL-6 when this test is not 24/7 or timely available.
Spiezia et al. confirmed that COVID-19 patients admitted to the ICU for acute respiratory failure present a severe hypercoagulability (EXTEM MCF, 69 ± 6 mm vs 64 ± 5 mm; P = 0.0003 and FIBTEM MCF, 31 ± 9 mm vs 18 ± 6 mm; P < 0.0001) rather than a consumptive coagulopathy (no significantly decreased antithrombin level or platelet count) when compared to healthy controls.34 Panigada et al. reported the same observation that their thromboelastography results in critically ill COVID-19 patients support hypercoagulability together with a severe inflammatory state but are not consistent with acute DIC.83 Here, besides fibrinogen, coagulation factor VIII and von Willebrand factor antigen are increased as typical acute phase reactants to 140-380%, 300-400% and 200-500%, respectively.
Madathil et al. reported that critically ill COVID-19 patients receiving mechanical lung ventilation (and 27% additional ECMO support) presented fibrinolysis shutdown with 0% maximum lysis in EXTEM and FIBTEM despite high CRP and D-dimers.84 Accordingly, Wright et al. showed that fibrinolysis shutdown correlates to thromboembolic events and renal failure in severe COVID-19 infection.85 Here, the best predictive value was provided by the combination of elevated D-dimer (>2.6 µg/mL) and fibrinolysis shutdown (TEG LY30 = 0%). In patients presenting neither elevated D-dimer nor fibrinolysis shutdown, the incidence of venous thromboembolism was 0% and for renal failure with the need for dialysis 14%. In contrast, in patients presenting elevated D-dimer and fibrinolysis shutdown, the incidence of venous thromboembolism was 50% and for renal failure with the need for dialysis 80%.
Accordingly, thrombolytic therapy might be reasonable, in particular in patients with sudden deterioration of oxygenation and signs of pulmonary hypertension and right ventricular failure.86 Actually, the value of thrombolytic therapy with tissue plasminogen activator (tPA) is under clinical investigation (NCT04356833, NCT04357730).29,87 Notably, a FIBTEM MCF cutoff <13 mm provides a sensitivity of 94% and a specificity of 80% to predict bleeding complications in patients with acute ischemic stroke undergoing thrombolytic therapy with tPA.88 This might help selecting the right patients for thrombolytic therapy.
Furthermore, Maier et al. as well as Ranucci pointed out that anticoagulation with direct thrombin inhibitors (argatroban or bivalirudin) in COVID-19 patients with suspected heparin-induced thrombocytopenia or heparin resistance can result in falsely low results for plasma fibrinogen using the Clauss method (eg, 99 mg/dL instead of 590 mg/dL).89,90 In contrast, FIBTEM and TEG Functional Fibrinogen measurements still provide reliable clot firmness results under direct thrombin inhibitor therapy.
Accordingly, Rubulo et al. advised the use of viscoelastic point-of-care testing for all COVID-19 patients with severe pneumonia in their paper “technologies to optimize care of severe COVID-19 patients for health care providers challenged by limited resources”.91 Also the Chinese Society on Thrombosis and Haemostasis recommends to use viscoelastic testing in COVID-9 patients with coagulopathy to monitor hemostasis and anticoagulation in particular in patients requiring ECMO, in patients with heparin-induced thrombocytopenia requiring alternative anticoagulation, and in patients with bleeding complications requiring goal-directed replacement therapy.92 However, further data are needed to define the role of viscoelastic testing in the management of patients with thrombo-inflammation and severe COVID-19.93
Which (if any) patient with COVID-19 has an increased bleeding risk?
In contrast to viral hemorrhagic fever such as Dengue, there is only one report regarding hemorrhagic problem in patients with COVID-19. Here, Joob et al. presented observations from Thailand with 1 out of 41 COVID-19 patients presented mild bleeding (petechiae).94 Tang et al. reported that 57.1% of the nonsurvivors exhibited thrombocytopenia (33.3% had 50-100 platelets/nL and 23.8% had <50 platelets/nL).15 Accordingly, thrombocytopenia is a marker of severe disease and a prognostic marker of mortality in patients with COVID-19, and thus can serve as a clinical indicator of worsening illness during hospitalization.95 While thrombocytopenia is a key diagnostic component in DIC, the data from COVID-19 studies raises the questions of whether thrombocytopenia in COVID-19 is a part of sepsis-induced DIC and/or a direct platelet-viral interaction and if so, is this interaction beneficial for the host or for the virus, and what are the possible mechanisms? These questions are worth exploring further in clinical studies. Several COVID-19 patients even present thrombocytosis.83 Studies assessing platelet function on the one hand and the effect of antiplatelet drugs on outcome in COVID-19 patients on the other hand are actually under clinical investigation (NCT04365309, NCT04368377).96
However, restricted platelet transfusion has been shown to be associated with a significant lower mortality compared to liberal platelet transfusion in several settings.97–99 Furthermore, platelet transfusion might increase the proinflammatory response as well as the risk of VTE and PE in COVID-19.99,100 Therefore, platelet transfusion should be considered carefully in COVID-19 patients.
What is the ideal anticoagulation strategy for patients with COVID-19?
Due to the high incidence of VTE and PE, thromboprophylaxis is recommended in all hospitalized COVID-19 patients and should also be maintained for 7-14 days at home after hospital discharge in case of preexisting or persisting VTE risk factors.23,24 However, the optimal drug and dose for thromboprophylaxis in COVID-19 patients is actually unknown. LMWH or UFH is used in most centers.17,18 Since several patients with severe COVID-19 develop hypercoagulability with increased D-dimer and fibrinogen levels but only moderately decreased antithrombin levels, as well as VTE and PE even under thromboprophylaxis, some patients might benefit from intermediate-dose or therapeutic anticoagulation.5,6,12–18,22,29–31,101 Tang et al. reported that patients with SIC score ≥4 or D-dimer >3 µg/mL (6fold of upper limit of normal) showed a significant reduction in 28-day mortality (40.0% vs64.2%, P = 0.029 and 32.8% vs 52.4%; P = 0.017, respectively) if treated with UFH or LMWH. No difference in 28-day mortality was found between heparin users and nonusers in the overall population of severe COVID-19 patients (30.3% vs 29.7%, P = 0.910). Thromboprophylaxis was done by administering LMWH (40-60 mg enoxaparin/day) or UFH (10,000-15,000 U/day) for at least 7 days.20,21
Notably, DOAC patients treated with antiviral drugs show an alarming increase in DOAC plasma levels.24,102 Therefore, some authors recommend replacing DOACs by UFH or LMWH as long as antiviral therapy is necessary.24,102 Here, thromboelastometry can be helpful to detect supratherapeutic DOAC levels in a timely manner.103,104 Furthermore, it is not known, whether supplementation of antithrombin and/or thrombomodulin is helpful due to their anticoagulant and anti-inflammatory effect.33,105,106 Studies assessing the effect of antiplatelet drugs on hypercoagulability and outcome in COVID-19 patients are actually under clinical investigation (NCT04365309, NCT04368377).
In summary, COVID-19-associated coagulopathy is characterized by hypercoagulability and associated with a high incidence of VTE and PE and poor outcome in patients with COVID-19. Here, biomarkers of coagulation (such as D-dimer, fibrinogen, platelet count), inflammation (such as IL-6) and immunity (such as lymphocyte count) as well as clinical scoring systems (such as SOFA, ISTH DIC and SIC score) can be helpful in predicting clinical course, need for hospital resources (such as ICU beds, intubation and ventilator therapy, and ECMO) and patient’s outcome in patients with COVID-19. However, COVID-19 associated coagulopathy is still incompletely understood and therapeutic options are limited to unspecific supportive therapy. Whether viscoelastic and platelet function testing can provide additional value in predicting clinical course, need for hospital resources and patient’s outcome or in guiding anticoagulation in COVID-19 patients is actually under investigation (ROHOCO study).
FOOTNOTES
Glossary of Terms
- A5
- Amplitude of clot firmness 5 minutes after CT
- A10
- Amplitude of clot firmness 10 minutes after CT
- ARDS
- Acute Respiratory Distress Syndrome
- CI
- Confidence interval
- cfDNA
- Cell-free deoxyribonucleic acid
- COVID-19
- Coronavirus disease 2019
- CRP
- C-reactive protein
- CRS
- Cytokine release syndrome
- CT
- Coagulation time
- DAMPs
- Damage-associated molecular patterns
- DIC
- Disseminated intravascular coagulation
- DOACs
- Direct oral anticoagulants
- ECMO
- Extracorporeal membrane oxygenation
- exRNA
- Extracellular ribonucleic acid
- EXTEM
- Thromboelastometry assay with extrinsic activation
- FIBTEM
- Thromboelastometry assay to assess fibrin contribution to clot firmness
- HR
- Hazard ratio
- ICU
- Intensive care unit
- IL-6
- Interleukin-6
- INR
- International normalized ratio
- IQR
- Interquartile range
- ISTH
- International society on thrombosis and hemostasis
- LDH
- Lactate dehydrogenase
- LI60
- Lysis index 60 minutes after CT
- LMWH
- Low molecular weight heparin
- LY30
- Lysis 30 minutes after MA
- MA
- Maximum amplitude of clot firmness
- MCF
- Maximum clot firmness
- MERS-CoV
- Middle East respiratory syndrome coronavirus
- ML
- Maximum lysis
- MOF
- Multiple organ failure
- NETs
- Neutrophil extracellular traps
- NF-kappaB
- Nuclear factor kappa-light-chain-enhancer of activated B cells
- NSAIDs
- Nonsteroidal anti-inflammatory drugs
- OR
- Odds ratio
- PAI-1
- Plasminogen activator inhibitor type 1
- PAMPs
- pathogen-associated molecular patterns
- PE
- Pulmonary embolism
- PRRs
- Pattern recognition receptors
- ROHOCO
- ROTEM analysis and standard coagulation tests in hospitalized patients with COVID-19
- ROTEM
- Rotational thromboelastometry
- SARS-CoV
- Severe acute respiratory syndrome coronavirus
- SARS-CoV-2
- Severe acute respiratory syndrome coronavirus 2
- SD
- Standard deviation
- SIC
- Sepsis-induced coagulopathy
- SIRS
- Systemic inflammatory response syndrome
- SNPs
- Single nucleotide polymorphisms
- SOFA
- Sequential organ failure assessment
- TEG
- Thromboelastography
- TF
- Tissue factor
- TLRs
- Toll-like receptors
- TMPRSS2
- Transmembrane protease serine subtype 2
- tPA
- Tissue plasminogen activator
- UFH
- Unfractionated heparin
- VET
- Viscoelastic testing
- VTE
- Venous thromboembolism
- WHO
- World health organization
Funding Statement: No funding was provided for writing this manuscript (review paper).
Author Contribution Klaus Görlinger, MD: This author helped conceive the manuscript, perform the literature review, write the first draft, edit subsequent drafts, and designed figure 1 and 2.
Daniel Dirkmann, MD, PhD: This author helped review the manuscript, edit and modify drafts.
Ajay Gandhi, MD: This author helped perform the literature review, review the manuscript, edit and modify drafts.
Paolo Simioni, MD, PhD: This author helped review the manuscript, edit and modify drafts.
Approval of the final Manuscript
All authors read and approved the final manuscript.
Potential Conflicts of Interest Klaus Görlinger, MD Klaus Görlinger is the Medical Director of Tem Innovations GmbH, Munich, Germany.
Daniel Dirkmann, MD, PhD Daniel Dirkmann attended acute care diagnostics strategic advisory committee meetings of Instrumentation Laboratory, Bedford, MA.
Ajay Ganghi, MD Ajay Gandhi is head of clinical affairs of Instrumentation Laboratory India.
Paolo Simioni, MD, PhD Paolo Simioni attended hemostasis strategic advisory committee meetings of Instrumentation Laboratory, Bedford, MA.
Name of the Department and Institution the Work should be attributed
Department of Anesthesiology and Intensive Care Medicine, University Hospital Essen, University Duisburg-Essen, Hufelandstrasse 55, 45147 Essen, Germany
References
- 1.Kakodkar P, Kaka N, Baig MN. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19). Cureus. 2020;12:e7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malato A, Dentali F, Siragusa S, et al. The impact of deep vein thrombosis in critically ill patients: a meta-analysis of major clinical outcomes. Blood Transfus. 2015;13:559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodigiani C, Iapichino G, Carenzo L, et al. ; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms J, Tacquard C, Severac F, et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18:1517–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92:791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;1801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penaloza A, Roy PM, Kline J, et al. Performance of age-adjusted D-dimer cut-off to rule out pulmonary embolism. J Thromb Haemost. 2012;10:1291–1296. [DOI] [PubMed] [Google Scholar]
- 18.Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311:1117–1124. [DOI] [PubMed] [Google Scholar]
- 19.Nybo M, Hvas AM. Age-adjusted D-dimer cut-off in the diagnostic strategy for deep vein thrombosis: a systematic review. Scand J Clin Lab Invest. 2017;77:568–573. [DOI] [PubMed] [Google Scholar]
- 20.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohoon KP, Mahé G, Tafur AJ, Spyropoulos AC. Emergence of institutional antithrombotic protocols for coronavirus 2019. Res Pract Thromb Haemost. 2020;4:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marietta M, Ageno W, Artoni A, et al. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus. 2020;18:167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry BM. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risnes I, Wagner K, Ueland T, Mollnes T, Aukrust P, Svennevig J. Interleukin-6 may predict survival in extracorporeal membrane oxygenation treatment. Perfusion. 2008;23:173–178. [DOI] [PubMed] [Google Scholar]
- 28.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Hajizadeh N, Moore EE, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18:1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. [DOI] [PubMed] [Google Scholar]
- 32.Iba T, Levy JH, Warkentin TE, Thachil J, van der Poll T, Levi M; Scientific and Standardization Committee on DIC, and the Scientific and Standardization Committee on Perioperative and Critical Care of the International Society on Thrombosis and Haemostasis. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17:1989–1994. [DOI] [PubMed] [Google Scholar]
- 33.Iba T, Levy JH, Raj A, Warkentin TE. Advance in the management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Clin Med. 2019;8:728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiezia L, Boscolo A, Poletto F, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun H. The interaction between pathogens and the host coagulation system. Physiology (Bethesda). 2006;21:281–288. [DOI] [PubMed] [Google Scholar]
- 37.Adamzik M, Görlinger K, Peters J, Hartmann M. Whole blood impedance aggregometry as a biomarker for the diagnosis and prognosis of severe sepsis. Crit Care. 2012;16:R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang LX, Tang LM, Pan GQ, Yan CX, Lu L, Liang YF. Evaluation of correlation between NF-κB mediated PAI-1 gene and sepsis. Eur Rev Med Pharmacol Sci. 2017;21:30–36. [PubMed] [Google Scholar]
- 40.Mussbacher M, Salzmann M, Brostjan C, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front Immunol. 2019;10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartmann M, Ozlügedik S, Peters J. Thiopental inhibits lipopolysaccharide-induced tissue factor expression. Anesth Analg. 2009;109:109–113. [DOI] [PubMed] [Google Scholar]
- 42.Samuels JM, Moore HB, Moore EE. Coagulopathy in severe sepsis: interconnectivity of coagulation and the immune system. Surg Infect (Larchmt). 2018;19:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adamzik M, Eggmann M, Frey UH, et al. Comparison of thromboelastometry with procalcitonin, interleukin 6, and C-reactive protein as diagnostic tests for severe sepsis in critically ill adults. Crit Care. 2010;14:R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitt FCF, Manolov V, Morgenstern J, et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care. 2019;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adamzik M, Langemeier T, Frey UH, et al. Comparison of thrombelastometry with simplified acute physiology score II and sequential organ failure assessment scores for the prediction of 30-day survival: a cohort study. Shock. 2011;35:339–342. [DOI] [PubMed] [Google Scholar]
- 46.Boscolo A, Spiezia L, Campello E, et al. Whole-blood hypocoagulable profile correlates with a greater risk of death within 28 days in patients with severe sepsis. Korean J Anesthesiol. 2020;73:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneck E, Samara O, Koch C, et al. Plasma DNA and RNA differentially impact coagulation during abdominal sepsis-an explorative study. J Surg Res. 2017;210:231–243. [DOI] [PubMed] [Google Scholar]
- 48.Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5:138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piza FM, Corrêa TD, Marra AR, et al. Thromboelastometry analysis of thrombocytopenic dengue patients: a cross-sectional study. BMC Infect Dis. 2017;17:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smither SJ, O’Brien LM, Eastaugh L, et al. Haemostatic changes in five patients infected with ebola virus. Viruses. 2019;11:E647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fletcher TE, Leblebicioglu H, Bozkurt I, et al. Rotational thromboelastometry alongside conventional coagulation testing in patients with Crimean-Congo haemorrhagic fever: an observational cohort study. Lancet Infect Dis. 2019;19:862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scarlatescu E, Lance MD. Crimean-Congo haemorrhagic fever: test early with ROTEM? Lancet Infect Dis. 2019;19:796–797. [DOI] [PubMed] [Google Scholar]
- 54.Convertino I, Tuccori M, Ferraro S, et al. Exploring pharmacological approaches for managing cytokine storm associated with pneumonia and acute respiratory distress syndrome in COVID-19 patients. Crit Care. 2020;24:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouadma L, Lescure FX, Lucet JC, Yazdanpanah Y, Timsit JF. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020;46:579–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee KY, Rhim JW, Kang JH. Early preemptive immunomodulators (corticosteroids) for severe pneumonia patients infected with SARS-CoV-2. Clin Exp Pediatr. 2020;63:117–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russell B, Moss C, Rigg A, Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fogarty H, Townsend L, Ni Cheallaigh C, et al. COVID19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189:1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020:8681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim SJ, Bostwick W. Social vulnerability and racial inequality in COVID-19 deaths in Chicago. Health Educ Behav. 2020;47:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madách K, Aladzsity I, Szilágyi A, et al. 4G/5G polymorphism of PAI-1 gene is associated with multiple organ dysfunction and septic shock in pneumonia induced severe sepsis: prospective, observational, genetic study. Crit Care. 2010;14:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lorente L, Martín MM, Borreguero-León JM, et al. The 4G/4G genotype of PAI-1 polymorphism is associated with higher plasma PAI-1 concentrations and mortality in patients with severe sepsis. PLoS One. 2015;10:e0129565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adamzik M, Schäfer S, Frey UH, et al. The NFKB1 promoter polymorphism (-94ins/delATTG) alters nuclear translocation of NF-κB1 in monocytes after lipopolysaccharide stimulation and is associated with increased mortality in sepsis. Anesthesiology. 2013;118:123–133. [DOI] [PubMed] [Google Scholar]
- 66.Schäfer ST, Gessner S, Scherag A, et al. Hydrocortisone fails to abolish NF-κB1 protein nuclear translocation in deletion allele carriers of the NFKB1 promoter polymorphism (-94ins/delATTG) and is associated with increased 30-day mortality in septic shock. PLoS One. 2014;9:e104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gu W, Zeng L, Zhou J, et al. Clinical relevance of 13 cytokine gene polymorphisms in Chinese major trauma patients. Intensive Care Med. 2010;36:1261–1265. [DOI] [PubMed] [Google Scholar]
- 68.Imam Z, Odish F, Gill I, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Banerjee A, Pasea L, Harris S, et al. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020;395:1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ventura MT, Casciaro M, Gangemi S, Buquicchio R. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy. 2017;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dimitrova-Karamfilova A, Patokova Y, Solarova T, Petrova I, Natchev G. Rotation thromboelastography for assessment of hypercoagulation and thrombosis in patients with cardiovascular diseases. J Life Sci. 2012;6:28–35. [Google Scholar]
- 75.Hincker A, Feit J, Sladen RN, Wagener G. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit Care. 2014;18:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zanetto A, Senzolo M, Vitale A, et al. Thromboelastometry hypercoagulable profiles and portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma. Dig Liver Dis. 2017;49:440–445. [DOI] [PubMed] [Google Scholar]
- 77.McCrath DJ, Cerboni E, Frumento RJ, Hirsh AL, Bennett-Guerrero E. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg. 2005;100:1576–1583. [DOI] [PubMed] [Google Scholar]
- 78.Faraoni D, Emani S, Halpin E, et al. Relationship between transfusion of blood products and the incidence of thrombotic complications in neonates and infants undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2017;31:1943–1948. [DOI] [PubMed] [Google Scholar]
- 79.Almskog LM, Wikman A, Svensson J, et al. Rotational thromboelastometry predicts care level in Covid-19. medRxiv Preprint. 2020. [Google Scholar]
- 80.Mortus JR, Manek SE, Brubaker LS, et al. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA Netw Open. 2020;3:e2011192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis. 2020;50:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Madathil R, Tabatabai A, Rabin J, et al. Thromboelastometry and D-dimer elevation in COVID-19. J Cardiothorac Vasc Anesth. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wright FL, Vogler TO, Moore EE, et al. Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231:193–203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost. 2020;18:1548–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choudhury R, Barrett CD, Moore HB, et al. Salvage use of tissue plasminogen activator (tPA) in the setting of acute respiratory distress syndrome (ARDS) due to COVID-19 in the USA: a Markov decision analysis. World J Emerg Surg. 2020;15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Campello E, Farina F, Spiezia L, et al. Thromboelastometry profiles in patients undergoing thrombolytic therapy for acute ischaemic stroke. Thromb Haemost. 2016;115:1231–1234. [DOI] [PubMed] [Google Scholar]
- 89.Maier CL, Barker NA, Sniecinski RM. Falsely low fibrinogen levels in COVID-19 patients on direct thrombin inhibitors. Anesth Analg. 2020;131:e117–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ranucci M. Fibrinogen levels in COVID-19 patients and their measure under direct thrombin inhibitors. Anesth Analg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rubulo F, Soliman-Aboumarie H, Filbey K, et al. Technologies to optimize the care of severe COVID-19 patients for healthcare providers challenged by limited resources. Anesth Analg. 2020:131351– 364.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song JC, Wang G, Zhang W, Zhang Y, Li WQ, Zhou Z; People’s Liberation Army Professional Committee of Critical Care Medicine, Chinese Society on Thrombosis and Haemostasis. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Mil Med Res. 2020;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.d’Alessandro E, Becker C, Bergmeier W, et al. ; Scientific Reviewer Committee. Thrombo-inflammation in cardiovascular disease: an expert consensus document from the third maastricht consensus conference on thrombosis. Thromb Haemost. 2020;120:538–564. [DOI] [PubMed] [Google Scholar]
- 94.Joob B, Wiwanitkit V. Hemorrhagic problem among the patients with COVID-19: clinical summary of 41 thai infected patients. Clin Appl Thromb Hemost. 2020;26:1076029620918308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warner MA, Chandran A, Frank RD, Kor DJ. Prophylactic platelet transfusions for critically ill patients with thrombocytopenia: a single-institution propensity-matched cohort study. Anesth Analg. 2019;128:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baharoglu MI, Cordonnier C, Al-Shahi Salman R, et al. ; PATCH Investigators. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016;387:2605–2613. [DOI] [PubMed] [Google Scholar]
- 99.Schmidt AE, Henrichs KF, Kirkley SA, Refaai MA, Blumberg N. Prophylactic preprocedure platelet transfusion is associated with increased risk of thrombosis and mortality. Am J Clin Pathol. 2017;149:87–94. [DOI] [PubMed] [Google Scholar]
- 100.Chen BZ, Xia R. Pro-inflammatory effects after platelet transfusion: a review. Vox Sang. 2020;115:349–357. [DOI] [PubMed] [Google Scholar]
- 101.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Testa S, Prandoni P, Paoletti O, et al. Direct oral anticoagulant plasma levels’ striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J Thromb Haemost. 2020;18:1320–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Henskens YMC, Gulpen AJW, van Oerle R, et al. Detecting clinically relevant rivaroxaban or dabigatran levels by routine coagulation tests or thromboelastography in a cohort of patients with atrial fibrillation. Thromb J. 2018;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schäfer ST, Wiederkehr T, Kammerer T, et al. Real-time detection and differentiation of direct oral anticoagulants (rivaroxaban and dabigatran) using modified thromboelastometric reagents. Thromb Res. 2020;190:103–111. [DOI] [PubMed] [Google Scholar]
- 105.Yokoyama N, Takaki S, Yokose M, et al. A question is “what are the optimal targets for anticoagulant therapies?”. J Intensive Care. 2020;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iba T, Thachil J. Present and future of anticoagulant therapy using antithrombin and thrombomodulin for sepsis-associated disseminated intravascular coagulation: a perspective from Japan. Int J Hematol. 2016;103:253–261. [DOI] [PubMed] [Google Scholar]


